WO2016167231A1 - 熱硬化性樹脂組成物およびホメオトロピック配向位相差フィルム - Google Patents
熱硬化性樹脂組成物およびホメオトロピック配向位相差フィルム Download PDFInfo
- Publication number
- WO2016167231A1 WO2016167231A1 PCT/JP2016/061746 JP2016061746W WO2016167231A1 WO 2016167231 A1 WO2016167231 A1 WO 2016167231A1 JP 2016061746 W JP2016061746 W JP 2016061746W WO 2016167231 A1 WO2016167231 A1 WO 2016167231A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- carbon atoms
- resin composition
- thermosetting resin
- component
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/14—Homopolymers or copolymers of esters of esters containing halogen, nitrogen, sulfur, or oxygen atoms in addition to the carboxy oxygen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/05—Alcohols; Metal alcoholates
- C08K5/053—Polyhydroxylic alcohols
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0025—Crosslinking or vulcanising agents; including accelerators
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/36—Sulfur-, selenium-, or tellurium-containing compounds
- C08K5/41—Compounds containing sulfur bound to oxygen
- C08K5/42—Sulfonic acids; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L101/00—Compositions of unspecified macromolecular compounds
- C08L101/02—Compositions of unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups
- C08L101/06—Compositions of unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups containing oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D133/00—Coating compositions based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Coating compositions based on derivatives of such polymers
- C09D133/04—Homopolymers or copolymers of esters
- C09D133/14—Homopolymers or copolymers of esters of esters containing halogen, nitrogen, sulfur or oxygen atoms in addition to the carboxy oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D201/00—Coating compositions based on unspecified macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D201/00—Coating compositions based on unspecified macromolecular compounds
- C09D201/02—Coating compositions based on unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups
- C09D201/06—Coating compositions based on unspecified macromolecular compounds characterised by the presence of specified groups, e.g. terminal or pendant functional groups containing oxygen atoms
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
- G02B5/3025—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state
- G02B5/3033—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
- G02B5/3025—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state
- G02B5/3033—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid
- G02B5/3041—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid comprising multiple thin layers, e.g. multilayer stacks
- G02B5/305—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid comprising multiple thin layers, e.g. multilayer stacks including organic materials, e.g. polymeric layers
Definitions
- the present invention relates to a thermosetting resin composition and a homeotropic alignment retardation film. More specifically, the present invention relates to a liquid crystal display (LCD), specifically a liquid crystal having positive dielectric anisotropy ( ⁇ + C plate (positive C plate) used to improve viewing angle characteristics of IPS liquid crystal display device (In-plane Switching LCD (IPS-LCD); in-plane orientation switching LCD) filled with ⁇ > 0)
- the present invention relates to a thermosetting resin composition and a homeotropic alignment phase difference film that are useful for producing a film.
- IPS-LCD is characterized by little change in luminance / color due to viewing angle because no vertical tilt of liquid crystal molecules occurs, but its weak point is that it is difficult to increase contrast ratio, luminance and response speed.
- a viewing angle compensation film is not used in an early IPS-LCD, and an inclination angle is not used in an IPS-LCD that does not use such a viewing angle compensation film. Due to the relatively large light leakage in the dark state, the contrast ratio is low.
- Patent Document 2 discloses an IPS-LCD compensation film using a + C plate and a + A plate (positive A plate).
- This document shows the following configuration of the liquid crystal display element described therein. 1) A liquid crystal layer having a horizontal alignment is sandwiched between both substrates supplied by electrodes capable of applying an electric field parallel to the surface of the liquid crystal layer. 2) One or more + A plates and + C plates are sandwiched between both polarizing plates. 3) The main optical axis of the + A plate is perpendicular to the main optical axis of the liquid crystal layer. 4) of the liquid crystal layer retardation value R LC, + C plate retardation value R + C, + A retardation value R + A plate is determined so as to satisfy the following equation.
- Patent Document 3 provides an IPS-LCD having high contrast characteristics at the front and tilt angles and low color shift by minimizing light leakage in the dark state at the tilt angles.
- An aimed IPS-LCD having a + A plate and a + C plate is disclosed.
- the + C plate is very useful as an optical compensation film for IPS-LCD because it can compensate for light leakage where the viewing angle of the polarizing plate is large.
- a polyimide solvent such as N-methyl-2-pyrrolidone for film preparation. For this reason, although it does not become a problem in a glass base material, when a base material is a film, there exists a problem of giving a damage to a base material at the time of alignment film formation.
- the vertical alignment film using polyimide requires firing at a high temperature, and there is a problem that the film substrate cannot withstand the high temperature. Accordingly, there is a need for a cured film forming composition that can form a highly reliable retardation material on a resin film such as a TAC or COP film and can form a + C plate by a simple method.
- Patent Documents 4 to 6 disclose As such a liquid crystal material, a side chain type liquid crystal polymer comprising a monomer unit (a) containing a liquid crystalline fragment side chain and a monomer unit (b) containing a non-liquid crystalline fragment side chain is disclosed.
- the polymer has a problem that it tends to become cloudy due to the unstable orientation of the liquid crystal.
- it has been disclosed to fix the alignment of the liquid crystal by adding a liquid crystal monomer and curing it by ultraviolet rays, but an ultraviolet irradiation step is required.
- JP-A-2-256603 Japanese Patent Laid-Open No. 11-133408 JP 2009-122715 A JP 2002-174725 A JP 2002-333642 A JP 2002-365635 A
- the present invention has been made in view of the above circumstances, and provides a thermosetting film that can be homeotropically aligned without using a vertical alignment film and has improved white turbidity (transparency) and surface tackiness.
- An object of the present invention is to provide a thermosetting resin composition and a homeotropic alignment retardation film.
- thermosetting resin composition can be obtained that can be homeotropically aligned without using a film, and can provide a cured film having good transparency and no surface tack.
- Thermosetting comprising a liquid crystalline side chain represented by formula (1), a polymer having a vertical alignment group and a thermally crosslinkable group, (B) a crosslinking agent, and (C) a solvent.
- Resin composition Wherein X is a COO group or OCO group, R is a hydrogen atom, a fluorine atom, a cyano group, an alkyl group having 1 to 6 carbon atoms, a hydroxy group, a carboxyl group, or an alkoxy group having 1 to 6 carbon atoms.
- N represents an integer of 1 to 10
- o and p represent an integer of 1 or 2.
- the thermally crosslinkable group is one or more selected from hydroxy group, carboxyl group, amino group, blocked isocyanate group, vinyl group, allyl group, (meth) acryl group, epoxy group, oxetanyl group and alkoxysilyl group.
- 1 thermosetting resin composition 3.
- Y 2 represents a single bond, an alkylene group having 1 to 15 carbon atoms or a —CH 2 —CH (OH) —CH 2 — group, or a divalent cyclic group selected from a benzene ring, a cyclohexane ring and a heterocyclic ring.
- Any hydrogen atom on the cyclic group may be substituted with Z;
- Y 3 represents a single bond or an alkylene group having 1 to 15 carbon atoms,
- Y 4 represents a single bond, a divalent cyclic group selected from a benzene ring, a cyclohexane ring or a heterocyclic ring, or a divalent organic group having a steroid skeleton having 17 to 30 carbon atoms.
- a hydrogen atom may be substituted with Z;
- Y 5 represents a divalent cyclic group selected from a benzene ring, a cyclohexane ring or a heterocyclic ring, and any hydrogen atom on these cyclic groups may be substituted with Z;
- m represents an integer of 0 to 4, and when m is 2 or more, Y 5 may be the same as or different from each other;
- Y 6 represents a hydrogen atom, an alkyl group having 1 to 18 carbon atoms, a fluorinated alkyl group having 1 to 18 carbon atoms, an alkoxy group having 1 to 18 carbon atoms or a fluorinated alkoxy group having 1 to 18 carbon atoms,
- Z represents an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluorinated alkyl group having 1 to 3 carbon atoms, a fluorinated alkoxy group having 1 to 3 carbon
- the alkylene group, —CH 2 —CH (OH) —CH 2 — group, divalent cyclic group, divalent organic group having a steroid skeleton, alkyl group and fluorinated alkyl group are: You may couple
- Y 1 , Y 2 and Y 4 are a single bond, Y 3 is an alkylene group having 1 to 15 carbon atoms, m is 0, and Y 6 is 1 to 18 carbon atoms.
- thermosetting resin composition of 3 wherein the total number of carbon atoms of Y 3 and Y 6 is 6 to 20, 5.
- the thermosetting resin composition according to any one of 1 to 4 wherein n represents an integer of 2 to 8, the o and p are 1, and the R is an alkoxy group having 1 to 6 carbon atoms, 6).
- D The thermosetting resin composition according to any one of 1 to 6, further comprising a crosslinking catalyst, 8).
- thermosetting resin composition according to any one of 1 to 7, which contains 1 to 100 parts by mass of the component (B) with respect to 100 parts by mass of the component (A).
- thermosetting resin composition containing 0.01 to 20 parts by mass of the component (D) with respect to a total of 100 parts by mass of the component (A) and the component (B), 10.
- a method for producing a homeotropic alignment retardation film wherein the thermosetting resin composition of any one of 1 to 9 is thermally cured and then allowed to stand for 24 hours or more after cooling.
- thermosetting resin composition of the present invention By using the thermosetting resin composition of the present invention, a homeotropic alignment retardation film can be produced simply by coating on a substrate and baking without using a vertical alignment film. Moreover, the obtained cured film is excellent in surface tackiness (no tack), has little white turbidity, and is excellent in transparency.
- thermosetting resin composition according to the present invention contains (A) a liquid crystal side chain, a polymer having a vertical alignment group and a thermally crosslinkable group, (B) a crosslinking agent, and (C) a solvent.
- the polymer of the component (A) used in the present invention is not particularly limited, but a monomer having an unsaturated double bond such as a (meth) acrylic acid ester compound, a vinyl compound, a styrene compound, and a maleimide compound is polymerized. The resulting copolymer is preferred.
- the polymer of the component (A) of the present invention is preferably an acrylic copolymer containing a liquid crystalline group, a vertical alignment group, and a thermally crosslinkable group in the side chain, and the main polymer constituting the acrylic copolymer is preferred.
- the skeleton of the chain or the type of other side chains There are no particular limitations on the skeleton of the chain or the type of other side chains.
- the weight average molecular weight of the polymer of component (A) is not particularly limited, but it exhibits good retardation, heat resistance by thermosetting and good tack, and handling properties by solvent solubility. In consideration, 1,000 to 200,000 is preferable, 2,000 to 150,000 is more preferable, and 3,000 to 100,000 is even more preferable.
- the weight average molecular weight in this invention is an average molecular weight obtained by standard polystyrene conversion by a high performance liquid chromatography analysis.
- the liquid crystalline side chain is not particularly limited as long as it is a nematic liquid crystalline side chain and the obtained thermosetting film has homeotropic alignment, and can be appropriately selected from known ones.
- the compound represented by the formula (1) is particularly preferable.
- X is a COO group or an OCO group, and a COO group is preferable.
- R represents a hydrogen atom, a fluorine atom, a cyano group, an alkyl group having 1 to 6 carbon atoms, a hydroxy group, a carboxyl group, or an alkoxy group having 1 to 6 carbon atoms, preferably an alkoxy group having 1 to 6 carbon atoms. It is a group.
- the alkyl group having 1 to 6 carbon atoms may be linear, branched or cyclic, and specific examples thereof include methyl, ethyl, n-propyl, isopropyl, cyclopropyl, n-butyl, isobutyl, and s-butyl.
- alkoxy group having 1 to 6 carbon atoms examples include a group in which an oxygen atom (—O—) is bonded to the above alkyl group having 1 to 6 carbon atoms.
- Specific examples thereof include methoxy, ethoxy, n-propoxy, iso Propoxy, n-butoxy, isobutoxy, s-butoxy, t-butoxy, n-pentoxy, 1-methyl-n-butoxy, 2-methyl-n-butoxy, 3-methyl-n-butoxy, 1,1-dimethyl- n-propoxy, 1,2-dimethyl-n-propoxy, 2,2-dimethyl-n-propoxy, 1-ethyl-n-propoxy, n-hexyloxy, 1-methyl-n-pentyloxy, 2-methyl- n-pentyloxy, 3-methyl-n-pentyloxy, 4-methyl-n-pentyloxy, 1,1-dimethyl-n-butoxy, 1,2-dimethyl-n-butoxy 1,3-di
- n is an integer of 1 to 10, preferably an integer of 2 to 8, and more preferably an integer of 4 to 8.
- o and p each represent an integer of 1 or 2, and 1 is preferable for both.
- the liquid crystalline side chain can be introduced into the polymer using, for example, a (meth) acrylic monomer having a liquid crystalline side chain, a vinyl monomer, a styrene monomer, a maleimide monomer, etc. As described above, it is preferable to introduce the polymer into the polymer using a (meth) acrylic monomer having a liquid crystalline side chain. These monomers can be produced by a known method.
- the vertical alignment group is not particularly limited, but a group containing a hydrocarbon group having about 6 to 20 carbon atoms is preferable, and specifically, a group represented by the formula (2) is preferable. It is.
- Y 1 is a single bond, or —O—, —CH 2 O—, —COO—, —OCO—, —NHCO—, —NH—CO—O—, and —NH—CO—NH.
- Y 2 represents a single bond, an alkylene group having 1 to 15 carbon atoms or a —CH 2 —CH (OH) —CH 2 — group, a benzene ring, a cyclohexane ring or a heterocyclic ring.
- any hydrogen atom on the cyclic group may be substituted with Z
- Y 3 represents a single bond or an alkylene group having 1 to 15 carbon atoms
- Y 4 represents ,
- Y 5 may be a benzene ring, cyclohexyl, or Represents a divalent cyclic group selected from a sun ring or a heterocyclic ring, an arbitrary hydrogen atom on these cyclic groups may be substituted with Z
- m represents an integer of 0 to 4
- m is 2 or more Y 5 may be the same as or different from each other
- Y 6 represents a hydrogen atom, an alkyl group having 1 to
- a fluorinated alkoxy group having 1 to 18 carbon atoms wherein Z is an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluorinated alkyl group having 1 to 3 carbon atoms, or 1 to 3 carbon atoms.
- 3 represents a fluorinated alkoxy group or a fluorine atom, and an alkylene group, an alkyl group, a fluorinated alkyl group, an alkoxy group and a fluorinated alkoxy group have 1 to 3 of the above bonds, unless the linking groups are adjacent to each other.
- the total number of carbon atoms of the substituents represented by Y 2 to Y 6 is 6 to 30.
- the alkylene group having 1 to 15 carbon atoms is obtained by removing one hydrogen atom from the alkyl group having 1 to 15 carbon atoms out of the alkyl group having 1 to 6 carbon atoms and the alkyl group having 1 to 18 carbon atoms described later.
- Specific examples thereof include methylene, ethylene, propylene, trimethylene, tetramethylene, pentamethylene, hexamethylene, heptamethylene, octamethylene groups and the like.
- heterocyclic ring examples include pyrrole ring, imidazole ring, oxazole ring, thiazole ring, pyrazole ring, pyridine ring, pyrimidine ring, quinoline ring, pyrazoline ring, isoquinoline ring, carbazole ring, purine ring, thiadiazole ring, pyridazine ring, Pyrazoline ring, triazine ring, pyrazolidine ring, triazole ring, pyrazine ring, benzimidazole ring, cinnoline ring, phenanthroline ring, indole ring, quinoxaline ring, benzothiazole ring, phenothiazine ring, oxadiazole ring, acridine ring, etc.
- pyrrole ring imidazole ring, pyrazole ring, pyridine ring, pyrimidine ring, pyrazoline ring, carbazole ring, pyridazine ring, pyrazoline ring, triazine ring, pyrazolidine ring, triazole ring, pyrazine ring, benzene 'S imidazole ring.
- divalent organic group having a steroid skeleton having 17 to 30 carbon atoms examples include cholesteryl, andosteryl, ⁇ -cholesteryl, epiandrosteryl, erygosteryl, estril, 11 ⁇ -hydroxymethylsteryl, 11 ⁇ -progesteryl, and lanosteryl.
- a divalent organic group having a structure in which two hydrogen atoms are removed from a structure selected from: mestranyl, methyltestosteryl, norethisteryl, pregnenolonyl, ⁇ -sitosteryl, stigmasteryl, testosteryl, and acetic acid cholesterol ester Specific examples include divalent organic groups shown below, but are not limited thereto.
- alkyl group having 1 to 18 carbon atoms examples include n-heptyl, 1-methyl-n-hexyl, 2-methyl-n-hexyl, 3-methyl-n, in addition to the alkyl groups exemplified for the above 1 to 6 carbon atoms.
- Examples of the fluorinated alkyl group having 1 to 18 carbon atoms include groups in which at least one hydrogen atom in the above alkyl group having 1 to 18 carbon atoms is substituted with a fluorine atom. Specific examples thereof include fluoromethyl and difluoromethyl.
- Trifluoromethyl pentafluoroethyl, 2,2,2-trifluoroethyl, heptafluoropropyl, 2,2,3,3,3-pentafluoropropyl, 2,2,3,3-tetrafluoropropyl, 2, , 2,2-trifluoro-1- (trifluoromethyl) ethyl, nonafluorobutyl, 4,4,4-trifluorobutyl, undecafluoropentyl, 2,2,3,3,4,4,5, 5,5-nonafluoropentyl, 2,2,3,3,4,4,5,5-octafluoropentyl, tridecafluorohexyl, 2,2,3 , 4,4,5,5,6,6,6-undecafluorohexyl, 2,2,3,3,4,5,5,6,6-decafluorohexyl, 3,3,4, 4,5,5,6,6,6-nonafluorohexyl and the like.
- fluorinated alkoxy group having 1 to 18 carbon atoms include a group in which an oxygen atom (—O—) is bonded to the above fluorinated alkyl group having 1 to 18 carbon atoms.
- examples of the alkyl group having 1 to 3 carbon atoms in Z include those having 1 to 3 carbon atoms among the groups exemplified for the above 1 to 6 carbon atoms, and examples of the alkoxy group having 1 to 3 carbon atoms include Among the groups exemplified as the alkoxy group having 1 to 6 carbon atoms, those having 1 to 3 carbon atoms can be mentioned.
- examples of the fluorinated alkyl group having 1 to 3 carbon atoms include the fluorinated alkyl groups having 1 to 18 carbon atoms.
- Examples of the group exemplified by the group include those having 1 to 3 carbon atoms, and examples of the fluorinated alkoxy group having 1 to 3 carbon atoms include carbon atoms among the groups exemplified as the fluorinated alkoxy group having 1 to 18 carbon atoms. Examples are those represented by formulas 1 to 3.
- Y 1 is preferably a single bond
- Y 2 is preferably a benzene ring or a cyclohexane ring
- Y 3 is preferably an alkylene group having 1 to 15 carbon atoms, More preferred is an alkylene group having 1 to 9
- Y 4 is preferably a benzene ring, a cyclohexane ring or a divalent organic group having a steroid skeleton having 17 to 30 carbon atoms
- Y 5 is a benzene ring or a cyclohexane ring.
- Y 6 is preferably an alkyl group having 1 to 18 carbon atoms, a fluorinated alkyl group having 1 to 10 carbon atoms, an alkoxy group having 1 to 18 carbon atoms or a fluorinated alkoxy group having 1 to 10 carbon atoms.
- An alkyl group having ⁇ 12 or an alkoxy group having 1 to 12 carbon atoms is more preferable, and an alkyl group having 1 to 9 carbon atoms or an alkoxy group having 1 to 9 carbon atoms is still more preferable.
- Y 4 is a divalent organic group having a steroid skeleton
- Y 6 is preferably a hydrogen atom.
- m is preferably from 0 to 3, more preferably from 0 to 2, and even more preferably 0 or 1 from the viewpoint of availability of raw materials and ease of synthesis.
- the alkylene group, the alkyl group, the fluorinated alkyl group, the alkoxy group, and the fluorinated alkoxy group may have 1 to 3 of the above-described linking groups therein unless the linking groups are adjacent to each other.
- Y 2 to Y 6 an alkylene group, —CH 2 —CH (OH) —CH 2 — group, divalent cyclic group, divalent organic group having a steroid skeleton, alkyl group and fluorinated alkyl group are: You may couple
- the vertical alignment group is preferably a group containing an alkyl group having 7 to 18 carbon atoms, particularly 8 to 15 carbon atoms.
- Specific examples of the vertical alignment group include a hydrocarbon group having about 6 to 20 carbon atoms.
- Examples of the hydrocarbon group having 6 to 20 carbon atoms include a linear, branched or cyclic alkyl group having 6 to 20 carbon atoms or a hydrocarbon group having 6 to 20 carbon atoms including an aromatic group.
- a-1 examples include n-nonadecyl and n-eicosinyl groups in addition to the alkyl group having 6 to 18 carbon atoms exemplified for the alkyl group having 1 to 18 carbon atoms. Etc.
- Y 1 to Y 4 are a single bond, m is 2 or 3, and Y 5 is a benzene ring or a cyclohexane ring.
- a vertical alignment group (a-2) in which Y 6 is an alkyl group having 1 to 18 carbon atoms can also be suitably used.
- Specific examples of such a vertical alignment group (a-2) include groups represented by the following (a-2-1) to (a-2-6), but are not limited thereto. Absent.
- Y 1 to Y 3 are single bonds, and Y 4 has a steroid skeleton having 17 to 30 carbon atoms.
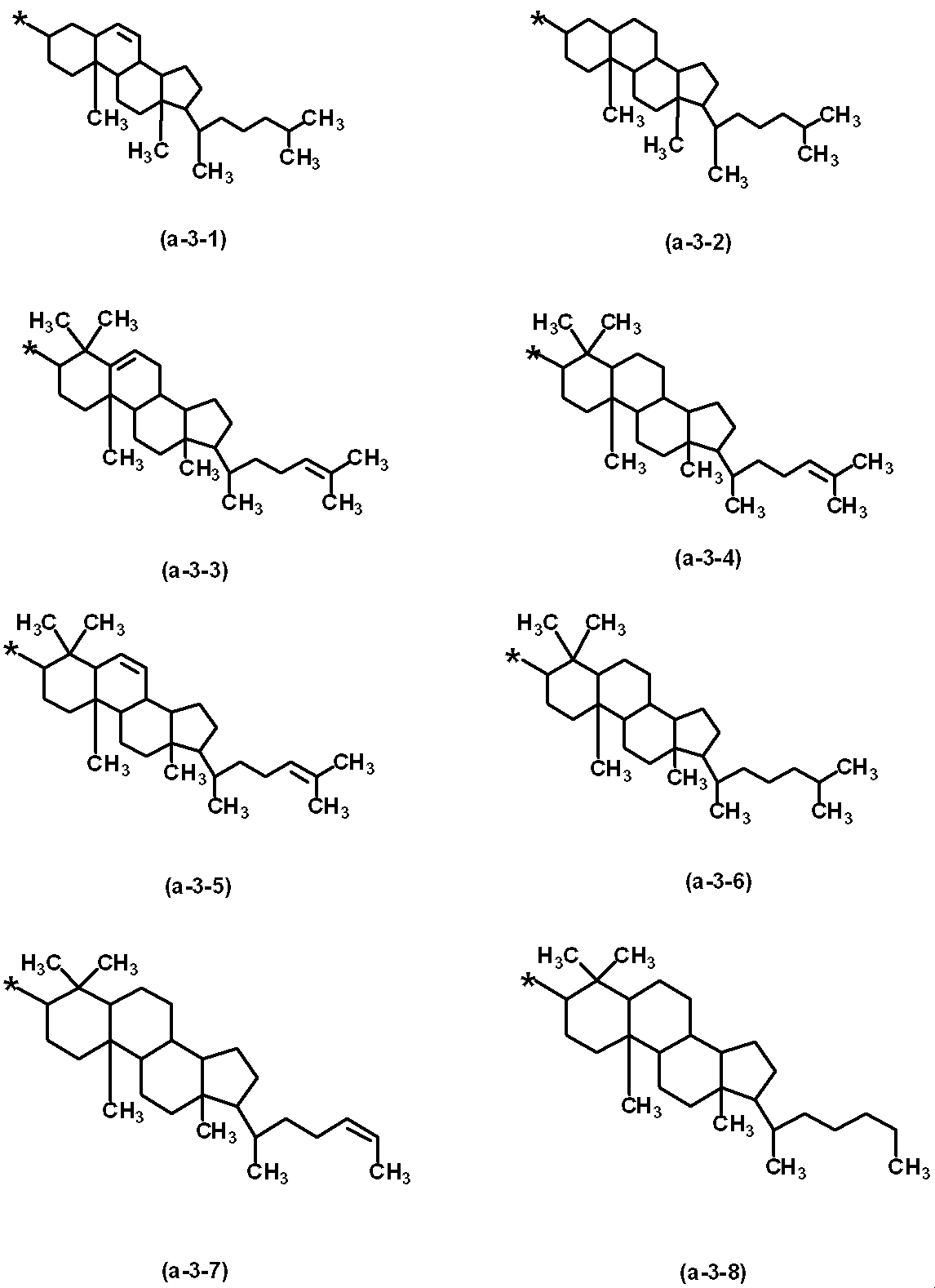
- the vertical alignment group (a-3) in which m is 0 and Y 6 is a hydrogen atom can also be suitably used. Examples of such a vertical alignment group (a-3) include, but are not limited to, groups represented by the following (a-3-1) to (a-3-8): .
- the vertical alignment group described above is introduced into the polymer using a monomer having an unsaturated double bond such as a (meth) acrylic monomer, a vinyl monomer, a styrene monomer, a maleimide monomer, or the like.
- a monomer having an unsaturated double bond such as a (meth) acrylic monomer, a vinyl monomer, a styrene monomer, a maleimide monomer, or the like.
- a monomer having an unsaturated double bond such as a (meth) acrylic monomer, a vinyl monomer, a styrene monomer, a maleimide monomer, or the like.
- a monomer having an unsaturated double bond such as a (meth) acrylic monomer, a vinyl monomer, a styrene monomer, a maleimide monomer, or the like.
- Specific monomers include alkyl esters of (meth) acrylic acid, alkyl vinyl ethers,
- the thermally crosslinkable group possessed by the polymer of component (A) used in the present invention includes a hydroxy group, a carboxyl group, an amino group, a blocked isocyanate group, a vinyl group, an allyl group, a (meth) acryl group, an epoxy group, and oxetanyl.
- Group and alkoxysilyl group Among these, a hydroxy group, a carboxyl group, an amino group, and an alkoxysilyl group are preferable from the viewpoint of forming a thermosetting film at a low temperature and in a short time.
- thermally crosslinkable groups can also be introduced into the polymer by using a monomer having an unsaturated double bond such as a (meth) acrylic monomer, a vinyl monomer, a styrene monomer, a maleimide monomer, or the like.
- a monomer having an unsaturated double bond such as a (meth) acrylic monomer, a vinyl monomer, a styrene monomer, a maleimide monomer, or the like.
- Examples of the monomer having a hydroxy group, a carboxyl group, an amino group, or an alkoxysilyl group as a thermally crosslinkable group include 2-hydroxyethyl acrylate, 2-hydroxyethyl methacrylate, 2-hydroxypropyl acrylate, 2-hydroxypropyl methacrylate, 4-hydroxybutyl acrylate, 4-hydroxybutyl methacrylate, 2,3-dihydroxypropyl acrylate, 2,3-dihydroxypropyl methacrylate, diethylene glycol monoacrylate, diethylene glycol monomethacrylate, caprolactone 2- (acryloyloxy) ethyl ester, caprolactone 2- ( Methacryloyloxy) ethyl ester, poly (ethylene glycol) ethyl ether acrylate, poly ( Tylene glycol) having hydroxy groups such as ethyl ether methacrylate, 5-acryloyloxy-6-hydroxynorbornene-2-carboxyl-6-lactone
- a monomer having a vertical alignment group As long as the effects of the present invention are not impaired, in addition to the above-mentioned monomer having a liquid crystalline side chain, a monomer having a vertical alignment group, and a monomer having a thermally crosslinkable group, it can be copolymerized with these monomers. Further, other monomers having no liquid crystalline side chain, vertical alignment group and heat crosslinkable group can be used in combination. Specific examples of other monomers include (meth) acrylic acid ester compounds, maleimide compounds, acrylamide compounds, acrylonitrile, maleic anhydride, styrene compounds and vinyl compounds.
- acrylate compound examples include methyl acrylate, ethyl acrylate, isopropyl acrylate, benzyl acrylate, naphthyl acrylate, anthryl acrylate, anthryl methyl acrylate, phenyl acrylate, glycidyl acrylate, 2,2,2-trifluoroethyl acrylate.
- methacrylic ester compound examples include methyl methacrylate, ethyl methacrylate, isopropyl methacrylate, benzyl methacrylate, naphthyl methacrylate, anthryl methacrylate, anthryl methyl methacrylate, phenyl methacrylate, glycidyl methacrylate, 2,2,2-trifluoroethyl methacrylate.
- maleimide compound examples include maleimide, N-methylmaleimide, N-ethylmaleimide and the like.
- styrene compound examples include styrene, methylstyrene, chlorostyrene, bromostyrene, and the like.
- vinyl compound examples include methyl vinyl ether, benzyl vinyl ether, vinyl naphthalene, vinyl carbazole, allyl glycidyl ether, 3-ethenyl-7-oxabicyclo [4.1.0] heptane, 1,2-epoxy-5-hexene. 1,7-octadiene monoepoxide and the like.
- the polymer of the component (A) used in the present invention is at least one selected from monomers having a liquid crystalline side chain, at least one selected from monomers having a vertical alignment group, and a monomer having a thermally crosslinkable group. It can be obtained by copolymerizing at least one selected with other monomers if desired. In this case, the amount of each monomer used is not particularly limited as long as the target liquid crystal polymer is obtained. However, the coating property of the liquid crystal polymer is ensured while exhibiting good vertical alignment properties and thermosetting properties.
- 3 to 90 mol% of a monomer having a liquid crystalline side chain 3 to 90 mol% of a monomer having a vertical alignment group, 3 to 90 mol% of a monomer having a thermally crosslinkable group, 0 It is preferably ⁇ 91 mol% of other monomers (however, the total of these monomers is 100 mol%).
- the polymerization method is not particularly limited, and examples thereof include a method of reacting each of the above monomers in a solvent at 50 to 110 ° C. in the presence of a polymerization initiator.
- the reaction solvent is not particularly limited as long as it has the ability to dissolve each monomer and polymerization initiator to be used. Specific examples thereof include those listed below as the solvent of component (C). Can be used.
- the polymer of the component (A) obtained by the above reaction is usually in a solution state, but can be used as it is as a polymer (solution) of the component (A) as described later.
- the polymer solution obtained by the above method is poured into stirred diethyl ether or water to reprecipitate, and the generated precipitate is filtered and washed, and then dried at normal temperature or under reduced pressure at normal temperature or under heat.
- a powdery polymer may be used. By this operation, the remaining polymerization initiator and unreacted monomer can be removed, and as a result, high-purity polymer powder can be obtained. If the purification is insufficient in one operation, the obtained powder may be redissolved in a solvent and the above operation may be repeated.
- the purified polymer powder may be used as it is, or may be used in the form of a solution redissolved in the solvent of component (C) described later.
- the polymer of component (A) may be a mixture of a plurality of types of polymers.
- examples of the obtained polymer of the component (A) include those represented by the following formula.
- R 1 represents a hydrogen atom or a methyl group
- R 2 represents an alkyl group having a thermally crosslinkable group
- R 3 represents an alkyl group having 1 to 5 carbon atoms
- z 1 to z 4 represent mol% of the monomer unit
- z 1 to z 3 each independently represents a number of 3 to 90
- the crosslinking agent of component (B) in the thermosetting resin composition of the present invention is not particularly limited as long as it is a group that forms a crosslink with the thermally crosslinkable group of the polymer of component (A),
- a crosslinking agent having a methylol group or an alkoxymethyl group is preferred.
- the compound having these groups include methylol compounds such as alkoxymethylated glycoluril, alkoxymethylated benzoguanamine, and alkoxymethylated melamine.
- alkoxymethylated glycoluril examples include, for example, 1,3,4,6-tetrakis (methoxymethyl) glycoluril, 1,3,4,6-tetrakis (butoxymethyl) glycoluril, 1,3,4 , 6-tetrakis (hydroxymethyl) glycoluril, 1,3-bis (hydroxymethyl) urea, 1,1,3,3-tetrakis (butoxymethyl) urea, 1,1,3,3-tetrakis (methoxymethyl) Examples include urea, 1,3-bis (hydroxymethyl) -4,5-dihydroxy-2-imidazolinone, 1,3-bis (methoxymethyl) -4,5-dimethoxy-2-imidazolinone and the like.
- a glycoluril compound (trade name: Cymel (registered trademark) 1170, Powder Link (registered trademark) 1174) manufactured by Nippon Cytec Industries, Ltd., and methylated urea resin (product) Name: UFR (registered trademark) 65), butylated urea resin (trade names: UFR (registered trademark) 300, U-VAN10S60, U-VAN10R, U-VAN11HV), urea / formaldehyde resin (manufactured by DIC Corporation) High condensation type, trade name: Becamine (registered trademark) J-300S, P-955, N) and the like.
- alkoxymethylated benzoguanamine examples include tetramethoxymethyl benzoguanamine. These are also commercially available, for example, manufactured by Nippon Cytec Industries Co., Ltd. (trade name: Cymel (registered trademark) 1123), manufactured by Sanwa Chemical Co., Ltd. (trade name: Nicalac (registered trademark) BX-4000). BX-37, BL-60, BX-55H) and the like.
- alkoxymethylated melamine examples include, for example, hexamethoxymethylmelamine. These are also available as commercial products.
- methoxymethyl type melamine compounds (trade names: Cymel (registered trademark) 300, 301, 303, 350) manufactured by Nippon Cytec Industries, Ltd.
- butoxymethyl type melamine compounds (Product names: My Coat (registered trademark) 506, 508), methoxymethyl type melamine compound manufactured by Sanwa Chemical Co., Ltd.
- the crosslinking agent used in the present invention may be a compound obtained by condensing a melamine compound, urea compound, glycoluril compound and benzoguanamine compound in which the hydrogen atom of the amino group is substituted with a methylol group or an alkoxymethyl group.
- Specific examples of such compounds include high molecular weight compounds prepared from melamine compounds and benzoguanamine compounds described in US Pat. No. 6,323,310.
- crosslinking agent of component (B) hydroxymethyl groups (ie, methylol groups) or alkoxymethyl groups such as N-hydroxymethylacrylamide, N-methoxymethylmethacrylamide, N-ethoxymethylacrylamide, N-butoxymethylmethacrylamide, etc.
- hydroxymethyl groups ie, methylol groups
- alkoxymethyl groups such as N-hydroxymethylacrylamide, N-methoxymethylmethacrylamide, N-ethoxymethylacrylamide, N-butoxymethylmethacrylamide, etc.
- Polymer crosslinkers prepared using substituted acrylamide or methacrylamide compounds can also be used.
- Examples of such a polymer cross-linking agent include poly (N-butoxymethylacrylamide), a copolymer of N-butoxymethylacrylamide and styrene, a copolymer of N-hydroxymethylmethacrylamide and methylmethacrylate, N- Examples thereof include a copolymer of ethoxymethyl methacrylamide and benzyl methacrylate, a copolymer of N-butoxymethyl acrylamide, benzyl methacrylate and 2-hydroxypropyl methacrylate.
- the weight average molecular weight of the polymer crosslinking agent is not particularly limited, but is usually about 1,000 to 500,000, preferably 2,000 to 200,000, more preferably 3,000 to 150,000. More preferably, it is 3,000 to 50,000.
- the various crosslinking agents described above can be used alone or in combination of two or more.
- the content of the crosslinking agent as the component (B) in the thermosetting resin composition of the present invention is (A) in consideration of the vertical alignment property and heat resistance of the resulting cured film, as well as surface tackiness and transparency.
- the amount is preferably 1 to 100 parts by weight, more preferably 5 to 50 parts by weight, based on 100 parts by weight of the component polymer.
- the solvent of the component (C) used in the thermosetting resin composition of the present invention the polymer of the component (A) described above, the crosslinking agent of the component (B), and other components used as necessary described later.
- the type and structure are not particularly limited.
- the solvent include alcohols such as methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, 2-methyl-1-butanol and n-pentanol; glycols such as diethylene glycol and propylene glycol; Glycol ethers such as ethylene glycol monomethyl ether, ethylene glycol monoethyl ether, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, propylene glycol monomethyl ether, propylene glycol monoethyl ether, propylene glycol propyl ether; methyl cellosolve acetate, ethyl cellosolve acetate, propylene Glycol monomethyl ether acetate, propylene glycol pro Glycol esters such as ether acetate; aromatic hydrocarbons such as toluene and xylene; methyl ethyl ketone, isobutyl; glycol
- thermosetting resin composition of the present invention When a cured film is formed on a resin film using the thermosetting resin composition of the present invention, methanol, ethanol, n-propanol, isopropanol, n-butanol, 2-methyl which does not adversely affect the substrate film It is preferable to use 1-butanol, 2-heptanone, isobutyl methyl ketone, diethylene glycol, propylene glycol, propylene glycol monomethyl ether, propylene glycol monomethyl ether acetate and the like.
- thermosetting resin composition of the present invention may contain a crosslinking catalyst as the component (D) for the purpose of accelerating the thermosetting reaction of the polymer in addition to the components (A) to (C).
- a crosslinking catalyst it is preferable to use an acid or a thermal acid generator that generates an acid by thermal decomposition at a temperature during heat treatment (for example, about 80 to 250 ° C.).
- the acid include hydrochloric acid or a salt thereof; methanesulfonic acid, ethanesulfonic acid, propanesulfonic acid, butanesulfonic acid, pentanesulfonic acid, octanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, camphorsulfonic acid, Trifluoromethanesulfonic acid, p-phenolsulfonic acid, 2-naphthalenesulfonic acid, mesitylenesulfonic acid, p-xylene-2-sulfonic acid, m-xylene-2-sulfonic acid, 4-ethylbenzenesulfonic acid, 1H, 1H, 2H , 2H-perfluorooctanesulfonic acid, perfluoro (2-ethoxyethane) sulfonic acid, pentafluoroethanesulfonic acid, nonafluor
- thermal acid generator examples include bis (tosyloxy) ethane, bis (tosyloxy) propane, bis (tosyloxy) butane, p-nitrobenzyl tosylate, o-nitrobenzyl tosylate, 1,2,3-phenylenetris (Methyl sulfonate), p-toluenesulfonic acid pyridinium salt, p-toluenesulfonic acid morphonium salt, p-toluenesulfonic acid ethyl ester, p-toluenesulfonic acid propyl ester, p-toluenesulfonic acid butyl ester, p-toluenesulfonic acid Isobutyl ester, p-toluenesulfonic acid methyl ester, p-toluenesulfonic acid phenethyl ester, cyanomethyl p-
- thermosetting resin composition of this invention content of (D) component in the thermosetting resin composition of this invention is not specifically limited, The thermosetting of the cured film obtained, surface tackiness, and transparency, and preservation
- 0.01 to 20 parts by mass is preferable, and 0.1 to 15 parts by mass is more preferable, with respect to 100 parts by mass in total of the polymer of component (A) and the crosslinking agent of component (B). 0.5 to 10 parts by mass is even more preferable.
- thermosetting resin composition of the present invention is, as necessary, an adhesion improver, a silane coupling agent, a surfactant, a rheology modifier, a pigment, a dye, a storage, as long as the effects of the present invention are not impaired.
- Stabilizers, antifoaming agents, antioxidants and the like can be contained.
- thermosetting resin composition of the present invention may be prepared from any of (A) component polymer, (B) component crosslinking agent, (C) component solvent, (D) component crosslinking catalyst, etc. Can be prepared by mixing in order.
- the component (A) dissolved in the component (C) solvent is mixed with the component (B) and the component (D) used as necessary at a predetermined ratio, and the mixture is homogeneous.
- Examples of the method include a solution.
- the polymer solution of the component (A) obtained by polymerization reaction or the like can be used as it is, but in this case, (A) It is good also as a uniform solution by adding (B) component and the (D) component used as needed to the polymer solution of a component similarly to the above.
- a solvent of component (C) may be added for the purpose of adjusting the concentration.
- the solvent used for the polymer solution of component (A) and the solvent for concentration adjustment may be the same or different.
- the prepared thermosetting resin composition is preferably used after being filtered using a filter having a pore diameter of about 0.2 ⁇ m.
- thermosetting resin composition of the present invention described above is applied directly on a substrate (without using an alignment film), and then dried by heating with a hot plate or an oven to obtain a homeotropically aligned cured film (single layer).
- a layer-coated homeotropic alignment film) can be formed.
- the substrate include a silicon / silicon dioxide coated substrate, a silicon nitride substrate, a substrate coated with metal (aluminum, molybdenum, chromium, etc.), a glass substrate, a quartz substrate, an ITO substrate, and triacetyl cellulose (TAC).
- resin film substrates such as films, polycarbonate (PC) films, cycloolefin polymer (COP) films, cycloolefin copolymer (COC) films, polyethylene terephthalate (PET) films, acrylic films, and polyethylene films.
- the coating method is not particularly limited, and various known coating methods such as bar coating, spin coating, flow coating, roll coating, slit coating, spin coating following slit, inkjet coating, and printing may be used. it can.
- the conditions for heat drying are not particularly limited as long as the crosslinking reaction by the crosslinking agent proceeds, and for example, 60 to 200 ° C., preferably 70 to 160 ° C., 0.4 to 60 minutes, preferably 0.5 to What is necessary is just to select suitably from the range for 10 minutes. In addition, it is preferable to cool to about 20 to 30 ° C. and leave it for about 24 hours after heat drying.
- the thickness of the cured film to be obtained is appropriately selected in consideration of the level difference of the substrate to be used and the optical and electrical properties, but is usually 0.5 to 100 ⁇ m.
- the thus obtained single layer coating type homeotropic alignment film of the present invention may be peeled off from the substrate and used as a film, or may be used as a liquid crystal alignment layer formed on the substrate.
- Such a single-layer coating type homeotropic alignment film is a material having optical characteristics suitable for applications such as display devices and recording materials, and in particular, optical compensation films such as polarizing plates for liquid crystal displays and retardation plates. Can be suitably used.
- Synthesis Example 2 Synthesis of Polymer P2 (Component A) 6Be 6.5 g, LAA 2.5 g, HEMA 1.5 g, and AIBN 0.17 g were dissolved in CH 18.9 g and reacted at 75 ° C. for 16 hours to obtain an acrylic copolymer solution P2. (Solid content concentration 35 mass%) was obtained. Mn of the obtained acrylic copolymer was 15,300 and Mw was 33,200.
- Examples 1-1 to 1-4, Comparative Examples 1-1 to 1-3 Components (A) to (D) were mixed in the proportions shown in Table 1 to prepare thermosetting resin compositions of Examples 1-1 to 1-4 and Comparative Examples 1-1 to 1-3.
- P1 to P3 represent the usage amount (g) of the acrylic copolymer solution
- P4 of the component (B) represents the usage amount (g) of the acrylic copolymer solution.
- thermosetting resin composition prepared in Examples 1-1 to 1-4 and Comparative Examples 1-1 to 1-3 was applied on a COP film having a thickness of 25 ⁇ m with a wet film thickness of 50 ⁇ m using a bar coater. did. Thereafter, each was heated and dried in a heat circulation oven at a temperature of 110 ° C. for 120 seconds to form a thermosetting film on the COP film, and then allowed to stand at 23 ° C. for 24 hours to obtain a laminated film.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Polymers & Plastics (AREA)
- Medicinal Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Materials Engineering (AREA)
- Engineering & Computer Science (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Polarising Elements (AREA)
- Liquid Crystal (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Paints Or Removers (AREA)
Abstract
Description
例えば、特許文献1に開示されるように、提案初期のIPS-LCDでは、視野角の補償フィルムが使用されておらず、こうした視野角の補償フィルムを使用していないIPS-LCDでは、傾斜角の暗状態における相対的に大きな光漏れのため、低いコントラスト比の値を示すという短所がある。
1)液晶層面に平行な電場が印加できる電極により供給される両基板の間に水平配向を有する液晶層が挟まれている。
2)一枚以上の+Aプレートと+Cプレートが両偏光板に挟まれている。
3)+Aプレートの主光軸は、液晶層の主光軸に垂直である。
4)液晶層の位相差値RLC、+Cプレートの位相差値R+C、+Aプレートの位相差値R+Aは、次式を満たすように決められる。
RLC:R+C:R+A≒1:0.5:0.25
5)+Aプレートと+Cプレートの位相差値に対する偏光板の保護フィルムの厚み方向の位相差値の関係は示されていない(TAC、COP、PNB)。
また、従来提案されているポリイミドを用いた垂直配向膜と重合性液晶を用いる方法では、膜作製にあたりN-メチル-2-ピロリドン等のポリイミドの溶剤を使用する必要がある。このためガラス基材では問題にならないが、基材がフィルムの場合、配向膜形成時に基材にダメージを与えてしまうという問題がある。その上、ポリイミドを用いた垂直配向膜では、高温での焼成が必要となり、フィルム基材が高温に耐えられないという問題がある。
したがって、TAC、COPフィルム等の樹脂フィルム上でも高信頼の位相差材を形成することができ、簡便な方法で+Cプレートを形成可能な硬化膜形成組成物が求められている。
また、この問題を改善するために液晶モノマーを添加して紫外線硬化させることで液晶の配向を固定化することが開示されているが、紫外線照射工程が必要であった。
1. (A)式(1)で示される液晶性側鎖、垂直配向性基および熱架橋性基を有するポリマー、(B)架橋剤、並びに(C)溶剤を含有することを特徴とする熱硬化性樹脂組成物、
2. 前記熱架橋性基が、ヒドロキシ基、カルボキシル基、アミノ基、ブロックイソシアネート基、ビニル基、アリル基、(メタ)アクリル基、エポキシ基、オキセタニル基およびアルコキシシリル基から選ばれる1種または2種以上である1の熱硬化性樹脂組成物、
3. 前記垂直配向性基が、式(2)で表される1または2の熱硬化性樹脂組成物、
Y2は、単結合、炭素数1~15のアルキレン基または-CH2-CH(OH)-CH2-基を表すか、ベンゼン環、シクロヘキサン環または複素環から選ばれる2価の環状基を表し、前記環状基上の任意の水素原子が、Zで置換されていてもよく、
Y3は、単結合または炭素数1~15のアルキレン基を表し、
Y4は、単結合、ベンゼン環、シクロヘキサン環もしくは複素環から選ばれる2価の環状基、または炭素数17~30のステロイド骨格を有する2価の有機基を表し、前記環状基上の任意の水素原子が、Zで置換されていてもよく、
Y5は、ベンゼン環、シクロヘキサン環または複素環から選ばれる2価の環状基を表し、これらの環状基上の任意の水素原子が、Zで置換されていてもよく、
mは、0~4の整数を表し、mが2以上の場合、Y5は互いに同一でも異なっていてもよく、
Y6は、水素原子、炭素数1~18のアルキル基、炭素数1~18のフッ化アルキル基、炭素数1~18のアルコキシ基または炭素数1~18のフッ化アルコキシ基を表し、
Zは、炭素数1~3のアルキル基、炭素数1~3のアルコキシ基、炭素数1~3のフッ化アルキル基、炭素数1~3のフッ化アルコキシ基またはフッ素原子を表し、
前記アルキレン基、アルキル基、フッ化アルキル基、アルコキシ基およびフッ化アルコキシ基は、結合基同士が隣り合わない限り、その中に1~3の前記結合基を有していてもよく、
前記Y2~Y6において、アルキレン基、-CH2-CH(OH)-CH2-基、2価の環状基、ステロイド骨格を有する2価の有機基、アルキル基およびフッ化アルキル基は、それらに隣接する基と前記結合基を介して結合していてもよい。
ただし、Y2~Y6が表す置換基の総炭素数は、6~30である。)
4. 前記Y1、Y2およびY4が、単結合であり、前記Y3が、炭素数1~15のアルキレン基であり、前記mが、0であり、前記Y6が、炭素数1~18のアルキル基であり、前記Y3およびY6の総炭素数が、6~20である3の熱硬化性樹脂組成物、
5. 前記nが、2~8の整数を表し、前記oおよびpが、1であり、前記Rが、炭素数1~6のアルコキシ基である1~4のいずれかの熱硬化性樹脂組成物、
6. 前記(B)成分が、メチロール基またはアルコキシメチル基を有する架橋剤である1~5のいずれかの熱硬化性樹脂組成物、
7. (D)架橋触媒をさらに含有する1~6のいずれかの熱硬化性樹脂組成物、
8. 前記(A)成分100質量部に対し、前記(B)成分1~100質量部含有する1~7のいずれかの熱硬化性樹脂組成物、
9. 前記(A)成分および(B)成分の合計100質量部に対し、前記(D)成分0.01~20質量部含有する7または8の熱硬化性樹脂組成物、
10. 1~9のいずれかの熱硬化性樹脂組成物を熱硬化させて形成されるホメオトロピック配向位相差フィルム、
11. 1~9のいずれかの熱硬化性樹脂組成物を熱硬化させた後、冷却後24時間以上放置するホメオトロピック配向位相差フィルムの製造方法
を提供する。
また、得られた硬化膜は、表面タック性に優れ(タックがなく)、白濁が少なく透明性にも優れている。
本発明に係る熱硬化性樹脂組成物は、(A)液晶性側鎖、垂直配向性基および熱架橋性基を有するポリマー、(B)架橋剤、並びに(C)溶剤を含有する。
本発明で用いる(A)成分のポリマーは特に限定されるものではないが、(メタ)アクリル酸エステル化合物、ビニル化合物、スチレン化合物、マレイミド化合物等の不飽和二重結合を有するモノマーを重合して得られる共重合体が好ましい。
特に、本発明の(A)成分のポリマーは、側鎖に、液晶性基、垂直配向性基および熱架橋性基を含むアクリル共重合体が好ましく、アクリル共重合体を構成する高分子の主鎖の骨格や、その他の側鎖の種類などは特に限定されない。
なお、本発明における重量平均分子量は、高速液体クロマトグラフィー分析による標準ポリスチレン換算で得られる平均分子量である。
Rは、水素原子、フッ素原子、シアノ基、炭素数1~6のアルキル基、ヒドロキシ基、カルボキシル基、または炭素数1~6のアルコキシ基を表すが、好ましくは、炭素数1~6のアルコキシ基である。
炭素数1~6のアルキル基は、鎖状、分岐状、環状のいずれでもよく、その具体例としては、メチル、エチル、n-プロピル、イソプロピル、シクロプロピル、n-ブチル、イソブチル、s-ブチル、t-ブチル、シクロブチル、1-メチル-シクロプロピル、2-メチル-シクロプロピル、n-ペンチル、1-メチル-n-ブチル、2-メチル-n-ブチル、3-メチル-n-ブチル、1,1-ジメチル-n-プロピル、1,2-ジメチル-n-プロピル、2,2-ジメチル-n-プロピル、1-エチル-n-プロピル、シクロペンチル、1-メチル-シクロブチル、2-メチル-シクロブチル、3-メチル-シクロブチル、1,2-ジメチル-シクロプロピル、2,3-ジメチル-シクロプロピル、1-エチル-シクロプロピル、2-エチル-シクロプロピル、n-ヘキシル、1-メチル-n-ペンチル、2-メチル-n-ペンチル、3-メチル-n-ペンチル、4-メチル-n-ペンチル、1,1-ジメチル-n-ブチル、1,2-ジメチル-n-ブチル、1,3-ジメチル-n-ブチル、2,2-ジメチル-n-ブチル、2,3-ジメチル-n-ブチル、3,3-ジメチル-n-ブチル、1-エチル-n-ブチル、2-エチル-n-ブチル、1,1,2-トリメチル-n-プロピル、1,2,2-トリメチル-n-プロピル、1-エチル-1-メチル-n-プロピル、1-エチル-2-メチル-n-プロピル、シクロヘキシル、1-メチル-シクロペンチル、2-メチル-シクロペンチル、3-メチル-シクロペンチル、1-エチル-シクロブチル、2-エチル-シクロブチル、3-エチル-シクロブチル、1,2-ジメチル-シクロブチル、1,3-ジメチル-シクロブチル、2,2-ジメチル-シクロブチル、2,3-ジメチル-シクロブチル、2,4-ジメチル-シクロブチル、3,3-ジメチル-シクロブチル、1-n-プロピル-シクロプロピル、2-n-プロピル-シクロプロピル、1-イソプロピル-シクロプロピル、2-イソプロピル-シクロプロピル、1,2,2-トリメチル-シクロプロピル、1,2,3-トリメチル-シクロプロピル、2,2,3-トリメチル-シクロプロピル、1-エチル-2-メチル-シクロプロピル、2-エチル-1-メチル-シクロプロピル、2-エチル-2-メチル-シクロプロピル、2-エチル-3-メチル-シクロプロピル基等が挙げられる。
oおよびpは、1または2の整数を表すが、いずれも1が好ましい。
なお、これらのモノマーは公知の方法により製造することができる。
複素環の具体例としては、ピロール環、イミダゾール環、オキサゾール環、チアゾール環、ピラゾール環、ピリジン環、ピリミジン環、キノリン環、ピラゾリン環、イソキノリン環、カルバゾール環、プリン環、チアジアゾール環、ピリダジン環、ピラゾリン環、トリアジン環、ピラゾリジン環、トリアゾール環、ピラジン環、ベンズイミダゾール環、シンノリン環、フェナントロリン環、インドール環、キノキサリン環、ベンゾチアゾール環、フェノチアジン環、オキサジアゾール環、アクリジン環等が挙げられ、これらの中でも、ピロール環、イミダゾール環、ピラゾール環、ピリジン環、ピリミジン環、ピラゾリン環、カルバゾール環、ピリダジン環、ピラゾリン環、トリアジン環、ピラゾリジン環、トリアゾール環、ピラジン環、ベンズイミダゾール環が好ましい。
炭素数1~18のフッ化アルキル基としては、上記炭素数1~18のアルキル基における少なくとも1つの水素原子をフッ素原子で置換した基が挙げられ、その具体例としては、フルオロメチル、ジフルオロメチル、トリフルオロメチル、ペンタフルオロエチル、2,2,2-トリフルオロエチル、ヘプタフルオロプロピル、2,2,3,3,3-ペンタフルオロプロピル、2,2,3,3-テトラフルオロプロピル、2,2,2-トリフルオロ-1-(トリフルオロメチル)エチル、ノナフルオロブチル、4,4,4-トリフルオロブチル、ウンデカフルオロペンチル、2,2,3,3,4,4,5,5,5-ノナフルオロペンチル、2,2,3,3,4,4,5,5-オクタフルオロペンチル、トリデカフルオロヘキシル、2,2,3,3,4,4,5,5,6,6,6-ウンデカフルオロヘキシル、2,2,3,3,4,4,5,5,6,6-デカフルオロヘキシル、3,3,4,4,5,5,6,6,6-ノナフルオロヘキシル等が挙げられる。
ただし、Y4がステロイド骨格を有する2価の有機基である場合は、Y6は水素原子が好ましい。
また、原料の入手性や合成の容易さ等の点から、mは0~3が好ましく、0~2がより好ましく、0または1がより一層好ましい。
特に、得られる液晶ポリマーの垂直配向性および塗布性等を考慮すると、垂直配向性基は、炭素数7~18、特に8~15のアルキル基を含む基が好ましい。
具体的な垂直配向性基としては、例えば炭素数6~20程度の炭化水素基が挙げられる。炭素数6~20の炭化水素基としては、直鎖状、分岐状または環状の炭素数6~20のアルキル基または芳香族基を含む炭素数6~20の炭化水素基が挙げられる。
したがって上記式(2)において、上記Y1、Y2およびY4が、単結合であり、Y3が、単結合または炭素数1~15のアルキレン基(好ましくは炭素数1~15のアルキレン基)であり、mが、0であり、Y6が、炭素数1~18のアルキル基であり、Y3およびY6の総炭素数が6~20のアルキル基(a-1)が好ましく、総炭素数が7~18のアルキル基がより好ましく、総炭素数が8~15のアルキル基がより一層好ましい。
このような垂直配向性基(a-1)の具体例としては、上述した炭素数1~18のアルキル基で例示した炭素数6~18のアルキル基に加え、n-ノナデシル、n-エイコシニル基等が挙げられる。
このような垂直配向性基(a-2)の具体例としては、下記(a-2-1)~(a-2-6)で示される基が挙げられるが、これらに限定されるものではない。
このような垂直配向性基(a-3)としては、例えば、下記(a-3-1)~(a-3-8)で示される基が挙げられるが、これらに限定されるものではない。
具体的なモノマーとしては、(メタ)アクリル酸のアルキルエステル、アルキルビニルエーテル、2-アルキルスチレン、3-アルキルスチレン、4-アルキルスチレン、N-アルキルマレイミドで、当該アルキル基が炭素数6~20のものが挙げられる。
これらのモノマーは、公知の方法により製造することができ、また市販品として入手可能なものもある。
なお、上記式(2)で表される垂直配向性基を有する(メタ)アクリル系モノマーを用いてポリマー中に垂直配向性基を導入する場合、その垂直配向性側鎖は下記式(2′)で示される。
これらの中でも、低温かつ短時間で熱硬化膜を形成するという点から、ヒドロキシ基、カルボキシル基、アミノ基、アルコキシシリル基が好ましい。
その他のモノマーの具体例としては、(メタ)アクリル酸エステル化合物、マレイミド化合物、アクリルアミド化合物、アクリロニトリル、マレイン酸無水物、スチレン化合物およびビニル化合物等が挙げられる。
アクリル酸エステル化合物の具体例としては、メチルアクリレート、エチルアクリレート、イソプロピルアクリレート、ベンジルアクリレート、ナフチルアクリレート、アントリルアクリレート、アントリルメチルアクリレート、フェニルアクリレート、グリシジルアクリレート、2,2,2-トリフルオロエチルアクリレート、tert-ブチルアクリレート、シクロヘキシルアクリレート、イソボルニルアクリレート、2-メトキシエチルアクリレート、メトキシトリエチレングリコールアクリレート、2-エトキシエチルアクリレート、テトラヒドロフルフリルアクリレート、3-メトキシブチルアクリレート等が挙げられる。
スチレン化合物の具体例としては、スチレン、メチルスチレン、クロロスチレン、ブロモスチレン等が挙げられる。
ビニル化合物の具体例としては、メチルビニルエーテル、ベンジルビニルエーテル、ビニルナフタレン、ビニルカルバゾール、アリルグリシジルエーテル、3-エテニル-7-オキサビシクロ[4.1.0]ヘプタン、1,2-エポキシ-5-ヘキセン、1,7-オクタジエンモノエポキサイド等が挙げられる。
この場合、各モノマーの使用量は、目的とする液晶ポリマーが得られる限り特に限定されるものではないが、良好な垂直配向性および熱硬化性を発揮させつつ、液晶ポリマーの塗布性を確保することなどを考慮すると、全モノマー中、液晶性側鎖を有するモノマー3~90モル%、垂直配向性基を有するモノマー3~90モル%、熱架橋性基を有するモノマー3~90モル%、0~91モル%のその他のモノマー(ただし、これらモノマーの合計は100モル%である)であることが好ましい。
反応溶媒は、使用する各モノマーおよび重合開始剤の溶解能を有するものであれば特に限定されるものではなく、その具体例としては、後述する(C)成分の溶剤で列挙するものを好適に使用できる。
また、上記方法で得られたポリマー溶液を、撹拌したジエチルエーテルや水等に投入して再沈殿させ、生成した沈殿物をろ過・洗浄した後、常圧または減圧下で、常温または加熱乾燥し、粉体状のポリマーとしてもよい。この操作により、残存する重合開始剤や、未反応モノマーを除去することができ、その結果、純度の高いポリマー粉体を得ることができる。なお、一度の操作では精製が不十分である場合、得られた粉体を溶剤に再溶解させ、上記の操作を繰り返し行えばよい。
精製したポリマー粉末は、そのまま用いても、後述する(C)成分の溶剤に再溶解した溶液形態で用いてもよい。
また、本発明においては、(A)成分のポリマーは、複数種のポリマーの混合物であってもよい。
これらの基を有する化合物としては、例えば、アルコキシメチル化グリコールウリル、アルコキシメチル化ベンゾグアナミンおよびアルコキシメチル化メラミン等のメチロール化合物などが挙げられる。
これらは市販品としても入手可能であり、例えば、日本サイテックインダストリーズ(株)製グリコールウリル化合物(商品名:サイメル(登録商標)1170、パウダーリンク(登録商標)1174)、同メチル化尿素樹脂(商品名:UFR(登録商標)65)、同ブチル化尿素樹脂(商品名:UFR(登録商標)300、U-VAN10S60、U-VAN10R、U-VAN11HV)、DIC(株)製尿素/ホルムアルデヒド系樹脂(高縮合型、商品名:ベッカミン(登録商標)J-300S、同P-955、同N)等が挙げられる。
これらも市販品として入手可能であり、例えば、日本サイテックインダストリーズ(株)製(商品名:サイメル(登録商標)1123)、(株)三和ケミカル製(商品名:ニカラック(登録商標)BX-4000、同BX-37、同BL-60、同BX-55H)等が挙げられる。
これらも市販品として入手可能であり、例えば、日本サイテックインダストリーズ(株)製メトキシメチルタイプメラミン化合物(商品名:サイメル(登録商標)300、同301、同303、同350)、ブトキシメチルタイプメラミン化合物(商品名:マイコート(登録商標)506、同508)、(株)三和ケミカル製メトキシメチルタイプメラミン化合物(商品名:ニカラック(登録商標)MW-30、同MW-22、同MW-11、同MS-001、同MX-002、同MX-730、同MX-750、同MX-035)、ブトキシメチルタイプメラミン化合物(商品名:ニカラック(登録商標)MX-45、同MX-410、同MX-302)等が挙げられる。
そのような化合物の具体例としては、米国特許第6323310号明細書に記載されているメラミン化合物およびベンゾグアナミン化合物から製造される高分子量の化合物が挙げられる。
ポリマー架橋剤の重量平均分子量は特に限定されるものではないが、通常、1,000~500,000程度であり、好ましくは2,000~200,000、より好ましくは3,000~150,000、より一層好ましくは3,000~50,000である。
なお、ポリマー架橋剤は、合成後のポリマー溶液のまま用いてもよい。
上述した各種架橋剤は、単独でまたは2種以上を組み合わせて使用することができる。
なお、(A)成分や(B)成分としてポリマー溶液を用いる場合、それに含まれる溶剤と、(C)成分の溶剤との合計量が上記の固形分濃度となる量であることが好ましい。
架橋触媒としては、酸や、加熱処理時の温度(例えば、80~250℃程度)で熱分解して酸を発生する熱酸発生剤を用いることが好ましい。
調製法の一例としては、(C)成分の溶剤に溶解した(A)成分の溶液に、(B)成分および必要に応じて用いられる(D)成分等を所定の割合で混合し、均一な溶液とする方法が挙げられる。
なお、調製された熱硬化性樹脂組成物は、孔径0.2μm程度のフィルタなどを用いて濾過した後に用いることが好ましい。
基板の具体例としては、シリコン/二酸化シリコン被覆基板、シリコンナイトライド基板、金属(アルミニウム、モリブデン、クロム等)が被覆された基板、ガラス基板、石英基板、ITO基板や、トリアセチルセルロース(TAC)フィルム、ポリカーボネート(PC)フィルム、シクロオレフィンポリマー(COP)フィルム、シクロオレフィンコポリマー(COC)フィルム、ポリエチレンテレフタレート(PET)フィルム、アクリルフィルム、ポリエチレンフィルム等の樹脂フィルム基板などが挙げられる。
塗布法としては、特に限定されるものではなく、バーコート、回転塗布、流し塗布、ロール塗布、スリット塗布、スリットに続いた回転塗布、インクジェット塗布、印刷等の公知の各種塗布法を用いることができる。
なお、加熱乾燥後、20~30℃程度に冷却し、24時間程度放置することが好ましい。
このような単層塗布型ホメオトロピック配向膜は、表示装置や記録材料等の用途に好適な光学特性を有する材料であり、特に、液晶ディスプレイ用の偏光板や、位相差板等の光学補償フィルムとして好適に用いることができる。
[平均分子量測定]
装置:(株)島津製作所製 高速液体クロマトグラフ装置 Prominence
カラム:Shodex(登録商標) KF-803L+KF-804L
カラム温度:40℃
溶媒:テトラヒドロフラン(流量1mL/分)
検出器:RI
検量線:標準ポリスチレン
[膜厚測定]
装置:(株)ミツトヨ製マイクロメータ MEQ-30
[Rth,Δnの評価]
装置:Axometrics社製 Axoscan
Rth:ホメオトロピック液晶層の厚さ方向のリタデーション値
Rth={(Nx+Ny)/2-Nz}×d[nm]
液晶層面内の最大屈折率を示す方向の屈折率:Nx
それと直交する方向の屈折率:Ny
厚さ方向の屈折率:Nz
液晶層の厚さ:d(nm)
Δn:厚さ方向屈折率NzからNxとNyの平均値を引いたもの
以下で用いる略記号の意味は、次のとおりである。
(A)成分:ポリマー原料および重合触媒
LAA:ラウリルアクリレート
HEMA:2-ヒドロキシエチルメタクリレート
6Be:4-メトキシフェニル 4-((6-(アクリロイルオキシ)ヘキシル)オキシ)ベンゾエート
MMA:メチルメタクリレート
AIBN:α,α′-アゾビスイソブチロニトリル
CYM303:ヘキサメトキシメチルメラミン(CYMEL303、日本サイテックインダストリーズ(株)製)
BMAA:N-ブトキシメチルアクリルアミド
PBMAA:ポリ(N-ブトキシメチルアクリルアミド)
PM:プロピレングリコールモノメチルエーテル
CH:シクロヘキサノン
PTSA:p-トルエンスルホン酸・一水和物
国際公開第2013/133078号の合成例3記載の方法で合成した6Be7.0g、LAA(東京化成工業(株)製)2.0g、HEMA(東京化成工業(株)製)1.0g、重合触媒としてAIBN(和光純薬工業(株)製)0.17gをCH18.9gに溶解し、75℃で16時間反応させてアクリル共重合体溶液P1(固形分濃度35質量%)を得た。得られたアクリル共重合体の数平均分子量(以下、Mnと称す。)は16,500、重量平均分子量(以下、Mwと称す。)は35,800であった。
6Be6.5g、LAA2.5g、HEMA1.5g、AIBN0.17gをCH18.9gに溶解し、75℃で16時間反応させてアクリル共重合体溶液P2(固形分濃度35質量%)を得た。得られたアクリル共重合体のMnは15,300、Mwは33,200であった。
6Be7.0g、MMA2.0g、HEMA1.0g、AIBN0.17gをCH18.9gに溶解し、75℃で16時間反応させてアクリル共重合体溶液P3(固形分濃度35質量%)を得た。得られたアクリル共重合体のMnは16,900、Mwは36,500であった。
BMAA(東京化成工業(株)製)25.0g、AIBN1.04gをPM48.4gに溶解し、85℃で20時間反応させてアクリル共重合体溶液P4(固形分濃度35質量%)を得た。得られたアクリル共重合体のMnは4,800、Mwは3,100であった。
表1に示す割合にて(A)~(D)成分を混合し、実施例1-1~1-4、比較例1-1~1-3の各熱硬化性樹脂組成物を調製した。なお、(A)成分におけるP1~P3は、アクリル共重合体溶液の使用量(g)を表し、(B)成分のうちP4は、アクリル共重合体溶液の使用量(g)を表す。
実施例1-1~1-4および比較例1-1~1-3で調製した各熱硬化性樹脂組成物を、25μm厚のCOPフィルム上にバーコーターを用いてウェット膜厚50μmにて塗布した。その後、それぞれについて温度110℃で120秒間、熱循環式オーブン中で加熱乾燥を行い、COPフィルム上に熱硬化膜を形成した後、23℃で24時間放置して積層フィルムを得た。
実施例2-1~2-4,比較例2-1~2-3で作製した積層フィルムの厚みを測定した。測定値からCOPフィルムの厚みを引いた値を熱硬化膜の膜厚とした。
実施例2-1~2-4および比較例2-1~2-3で作製した積層フィルムの進相軸のRthを測定した。得られたRthを膜厚で割った値を熱硬化膜のΔnとした。
実施例2-1~2-4および比較例2-1~2-3で作製した積層フィルムを目視にて観察し白濁の有無を判定した。
実施例2-1~2-4および比較例2-1~2-3で作製した積層フィルムの熱硬化膜表面に指を接触させ、表面に接触跡が残るか否かを判定した。接触跡が残ったものをタックありとした。
上記各評価結果を表2に併せて示す。
一方、(B)成分を含まない比較例2-1および2-2の塗膜は白濁し、タックがあることが確認された。そのため、Rthの評価に至らなかった。
また、垂直配向性基を有しないポリマーを用いた比較例2-3の硬化膜では透明でタックのない塗膜が得られたものの、十分なRthが得られていないことがわかる。
Claims (11)
- 前記熱架橋性基が、ヒドロキシ基、カルボキシル基、アミノ基、ブロックイソシアネート基、ビニル基、アリル基、(メタ)アクリル基、エポキシ基、オキセタニル基およびアルコキシシリル基から選ばれる1種または2種以上である請求項1記載の熱硬化性樹脂組成物。
- 前記垂直配向性基が、式(2)で表される請求項1または2記載の熱硬化性樹脂組成物。
Y2は、単結合、炭素数1~15のアルキレン基または-CH2-CH(OH)-CH2-基を表すか、ベンゼン環、シクロヘキサン環または複素環から選ばれる2価の環状基を表し、前記環状基上の任意の水素原子が、Zで置換されていてもよく、
Y3は、単結合または炭素数1~15のアルキレン基を表し、
Y4は、単結合、ベンゼン環、シクロヘキサン環もしくは複素環から選ばれる2価の環状基、または炭素数17~30のステロイド骨格を有する2価の有機基を表し、前記環状基上の任意の水素原子が、Zで置換されていてもよく、
Y5は、ベンゼン環、シクロヘキサン環または複素環から選ばれる2価の環状基を表し、これらの環状基上の任意の水素原子が、Zで置換されていてもよく、
mは、0~4の整数を表し、mが2以上の場合、Y5は互いに同一でも異なっていてもよく、
Y6は、水素原子、炭素数1~18のアルキル基、炭素数1~18のフッ化アルキル基、炭素数1~18のアルコキシ基または炭素数1~18のフッ化アルコキシ基を表し、
Zは、炭素数1~3のアルキル基、炭素数1~3のアルコキシ基、炭素数1~3のフッ化アルキル基、炭素数1~3のフッ化アルコキシ基またはフッ素原子を表し、
前記アルキレン基、アルキル基、フッ化アルキル基、アルコキシ基およびフッ化アルコキシ基は、結合基同士が隣り合わない限り、その中に1~3の前記結合基を有していてもよく、
前記Y2~Y6において、アルキレン基、-CH2-CH(OH)-CH2-基、2価の環状基、ステロイド骨格を有する2価の有機基、アルキル基およびフッ化アルキル基は、それらに隣接する基と前記結合基を介して結合していてもよい。
ただし、Y2~Y6が表す置換基の総炭素数は、6~30である。) - 前記Y1、Y2およびY4が、単結合であり、前記Y3が、炭素数1~15のアルキレン基であり、前記mが、0であり、前記Y6が、炭素数1~18のアルキル基であり、前記Y3およびY6の総炭素数が、6~20である請求項3記載の熱硬化性樹脂組成物。
- 前記nが、2~8の整数を表し、前記oおよびpが、1であり、前記Rが、炭素数1~6のアルコキシ基である請求項1~4のいずれか1項記載の熱硬化性樹脂組成物。
- 前記(B)成分が、メチロール基またはアルコキシメチル基を有する架橋剤である請求項1~5のいずれか1項記載の熱硬化性樹脂組成物。
- (D)架橋触媒をさらに含有する請求項1~6のいずれか1項記載の熱硬化性樹脂組成物。
- 前記(A)成分100質量部に対し、前記(B)成分1~100質量部含有する請求項1~7のいずれか1項記載の熱硬化性樹脂組成物。
- 前記(A)成分および(B)成分の合計100質量部に対し、前記(D)成分0.01~20質量部含有する請求項7または8記載の熱硬化性樹脂組成物。
- 請求項1~9のいずれか1項記載の熱硬化性樹脂組成物を熱硬化させて形成されるホメオトロピック配向位相差フィルム。
- 請求項1~9のいずれか1項記載の熱硬化性樹脂組成物を熱硬化させた後、冷却後24時間以上放置するホメオトロピック配向位相差フィルムの製造方法。
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201680028417.6A CN107532006B (zh) | 2015-04-17 | 2016-04-12 | 热固化性树脂组合物和垂直取向相位差膜 |
| JP2017512534A JP6747433B2 (ja) | 2015-04-17 | 2016-04-12 | 熱硬化性樹脂組成物およびホメオトロピック配向位相差フィルム |
| KR1020177032369A KR102511460B1 (ko) | 2015-04-17 | 2016-04-12 | 열경화성 수지 조성물 및 호메오트로픽 배향 위상차 필름 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015084906 | 2015-04-17 | ||
| JP2015-084906 | 2015-04-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016167231A1 true WO2016167231A1 (ja) | 2016-10-20 |
Family
ID=57126563
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2016/061746 WO2016167231A1 (ja) | 2015-04-17 | 2016-04-12 | 熱硬化性樹脂組成物およびホメオトロピック配向位相差フィルム |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP6747433B2 (ja) |
| KR (1) | KR102511460B1 (ja) |
| CN (1) | CN107532006B (ja) |
| WO (1) | WO2016167231A1 (ja) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018009150A (ja) * | 2016-06-28 | 2018-01-18 | 大日本印刷株式会社 | 側鎖型液晶ポリマー、液晶組成物、位相差フィルム、位相差フィルムの製造方法、転写用積層体、光学部材、光学部材の製造方法、及び表示装置 |
| CN110167980A (zh) * | 2017-01-13 | 2019-08-23 | 日产化学株式会社 | 固化膜形成用组合物、取向材及相位差材 |
| US11390810B2 (en) | 2017-09-27 | 2022-07-19 | Dai Nippon Printing Co., Ltd. | Liquid crystal composition, retardation film, method for producing retardation film, transfer laminate, optical member, method for producing optical member, and display device |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102705605B1 (ko) * | 2018-12-19 | 2024-09-10 | 삼성에스디아이 주식회사 | 광학 필름 및 이를 포함하는 광학표시장치 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000327924A (ja) * | 1999-05-25 | 2000-11-28 | Nitto Denko Corp | 液晶ポリマー組成物、位相差板および楕円偏光板 |
| JP2007217656A (ja) * | 2006-01-23 | 2007-08-30 | Fujifilm Corp | 組成物、位相差板、液晶表示装置および、位相差板の製造方法 |
| WO2015019962A1 (ja) * | 2013-08-09 | 2015-02-12 | 日産化学工業株式会社 | 硬化膜形成組成物、配向材および位相差材 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2857889B2 (ja) | 1988-11-04 | 1999-02-17 | 富士写真フイルム株式会社 | 液晶表示装置 |
| JP3204182B2 (ja) | 1997-10-24 | 2001-09-04 | 日本電気株式会社 | 横電界方式の液晶表示装置 |
| JP3788734B2 (ja) | 2000-12-06 | 2006-06-21 | 日東電工株式会社 | ホメオトロピック配向液晶フィルムの製造方法およびホメオトロピック配向液晶フィルム |
| JP4174192B2 (ja) | 2001-05-08 | 2008-10-29 | 日東電工株式会社 | ホメオトロピック配向液晶性組成物、ホメオトロピック配向液晶フィルムの製造方法およびホメオトロピック配向液晶フィルム |
| JP3899482B2 (ja) | 2001-06-11 | 2007-03-28 | 日東電工株式会社 | ホメオトロピック配向液晶フィルムの製造方法およびホメオトロピック配向液晶フィルム |
| JP2003002927A (ja) * | 2001-06-18 | 2003-01-08 | Nitto Denko Corp | 側鎖型液晶ポリマー、液晶性組成物、ホメオトロピック配向液晶フィルムの製造方法およびホメオトロピック配向液晶フィルム |
| KR100677050B1 (ko) | 2003-10-22 | 2007-01-31 | 주식회사 엘지화학 | +a-플레이트와 +c-플레이트를 이용한 시야각보상필름을 포함하는 면상 스위칭 액정 표시장치 |
| JP2006220770A (ja) * | 2005-02-08 | 2006-08-24 | Nippon Oil Corp | 液晶フィルムおよび液晶表示素子 |
-
2016
- 2016-04-12 CN CN201680028417.6A patent/CN107532006B/zh active Active
- 2016-04-12 JP JP2017512534A patent/JP6747433B2/ja active Active
- 2016-04-12 WO PCT/JP2016/061746 patent/WO2016167231A1/ja active Application Filing
- 2016-04-12 KR KR1020177032369A patent/KR102511460B1/ko active IP Right Grant
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000327924A (ja) * | 1999-05-25 | 2000-11-28 | Nitto Denko Corp | 液晶ポリマー組成物、位相差板および楕円偏光板 |
| JP2007217656A (ja) * | 2006-01-23 | 2007-08-30 | Fujifilm Corp | 組成物、位相差板、液晶表示装置および、位相差板の製造方法 |
| WO2015019962A1 (ja) * | 2013-08-09 | 2015-02-12 | 日産化学工業株式会社 | 硬化膜形成組成物、配向材および位相差材 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018009150A (ja) * | 2016-06-28 | 2018-01-18 | 大日本印刷株式会社 | 側鎖型液晶ポリマー、液晶組成物、位相差フィルム、位相差フィルムの製造方法、転写用積層体、光学部材、光学部材の製造方法、及び表示装置 |
| US11332670B2 (en) | 2016-06-28 | 2022-05-17 | Dai Nippon Printing Co., Ltd. | Side-chain liquid crystal polymer, liquid crystal composition, retardation film, method for producing retardation film, transfer laminate, optical member, method for producing optical member, and display device |
| CN110167980A (zh) * | 2017-01-13 | 2019-08-23 | 日产化学株式会社 | 固化膜形成用组合物、取向材及相位差材 |
| CN110167980B (zh) * | 2017-01-13 | 2022-09-20 | 日产化学株式会社 | 固化膜形成用组合物、取向材及相位差材 |
| US11390810B2 (en) | 2017-09-27 | 2022-07-19 | Dai Nippon Printing Co., Ltd. | Liquid crystal composition, retardation film, method for producing retardation film, transfer laminate, optical member, method for producing optical member, and display device |
Also Published As
| Publication number | Publication date |
|---|---|
| KR102511460B1 (ko) | 2023-03-17 |
| JPWO2016167231A1 (ja) | 2018-03-01 |
| KR20170137813A (ko) | 2017-12-13 |
| JP6747433B2 (ja) | 2020-08-26 |
| CN107532006B (zh) | 2021-06-25 |
| CN107532006A (zh) | 2018-01-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9206355B2 (en) | Polymerizable liquid crystal compound, polymerizable liquid crystal composition, and optically anisotropic body | |
| KR102246723B1 (ko) | 경화막 형성조성물, 배향재 및 위상차재 | |
| JP6747433B2 (ja) | 熱硬化性樹脂組成物およびホメオトロピック配向位相差フィルム | |
| TWI636069B (zh) | 具有光配向性之熱硬化性組成物、配向層、附有配向層之基材、相位差板及裝置 | |
| CN110408159B (zh) | 固化膜形成用组合物、取向材料和相位差材料 | |
| JP2023061970A (ja) | 硬化膜形成組成物、配向材及び位相差材 | |
| JP6558074B2 (ja) | 熱硬化性樹脂組成物および位相差フィルム | |
| TWI761424B (zh) | 硬化膜形成組成物、定向材料及相位差材料 | |
| CN107207641B (zh) | 固化膜形成用组合物、取向材料和相位差材料 | |
| JP7249271B2 (ja) | 硬化膜形成組成物、配向材および位相差材 | |
| TWI653285B (zh) | 硬化膜形成組成物、配向材及相位差材 | |
| JP7569016B2 (ja) | 硬化膜形成組成物、配向材および位相差材 | |
| CN113874470B (zh) | 固化膜形成用组合物、取向构件和相位差构件 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16780022 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2017512534 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20177032369 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16780022 Country of ref document: EP Kind code of ref document: A1 |