WO2015146928A1 - Heterocyclic compound - Google Patents
Heterocyclic compound Download PDFInfo
- Publication number
- WO2015146928A1 WO2015146928A1 PCT/JP2015/058777 JP2015058777W WO2015146928A1 WO 2015146928 A1 WO2015146928 A1 WO 2015146928A1 JP 2015058777 W JP2015058777 W JP 2015058777W WO 2015146928 A1 WO2015146928 A1 WO 2015146928A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ring
- group
- compound
- hydrogen atom
- optionally substituted
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D519/00—Heterocyclic compounds containing more than one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system not provided for in groups C07D453/00 or C07D455/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D495/04—Ortho-condensed systems
Definitions
- the present invention relates to a heterocyclic compound having an inhibitory action on protein kinase C (sometimes abbreviated as “PKC” in the present specification) and useful for the treatment of immune diseases, inflammatory diseases, and the like, and a medicament containing them It relates to compositions and the like.
- PKC protein kinase C
- T cells play an important role as part of an acquired immune mechanism that eliminates foreign antigens such as viruses and bacteria and cancer.
- foreign antigens such as viruses and bacteria and cancer.
- T cell activation is involved in the development of autoimmune diseases and causes adverse reactions to the human body such as rejection associated with transplantation. It is considered to be a promising therapeutic target in these diseases (Non-patent Document 1).
- the PKC family consists of at least 12 types and is known to be expressed in various tissues.
- the PKC family is expressed in immunocompetent cells such as T cells, B cells, and macrophages and plays an important role in various immune responses, and is therefore considered to be involved in immune diseases and inflammatory diseases (non-) Patent Document 4).
- PKC- ⁇ is a phosphorylase that is selectively expressed in T cells, plays a role in the activation of T cells, is responsible for the transmission of stimuli to T cell receptors into cells.
- Inhibition of PKC- ⁇ is known to suppress T cell activation (Non-patent Document 5), and PKC- ⁇ -deficient mice are used in multiple sclerosis models, inflammatory bowel disease models, grafts.
- PKC eg, PKC- ⁇
- organ transplantation such as kidney transplantation, liver transplantation, heart transplantation, lung transplantation, pancreatic transplantation, small intestine transplantation, rejection reaction in bone marrow transplantation, graft Anti-host disease, Behcet's disease, psoriasis vulgaris, pustular psoriasis, erythrodermic psoriasis, psoriatic arthritis, aplastic anemia, erythroblastosis, nephrotic syndrome, systemic myasthenia gravis, atopic dermatitis It becomes a prophylactic or therapeutic agent in various immune diseases and inflammatory diseases such as ulcerative colitis and asthma.
- R 1 is —NR 9 R 10 —, —NR 9 COR 9 —, —S (O) n R 12 — and the like (R 9 , R 10 and R 12 are each independently C 3-6 cycloalkyl, C 4-7 cycloalkylalkyl, n represents 0-2), cyano, C 1-4 haloalkyl, etc., or C 1-6 alkyl, C 3-6 cycloalkyl, etc.
- each , -NR 9 R 10 -, - NR 9 COR 9 -, - indicates may be substituted with etc.
- a 1 and A 2 independently represent N or CR 5 ;
- W represents ⁇ O, ⁇ S, —H or (—H, —H);
- X represents N or CR 1 ;
- Y represents —C (R Y ) 2 —, —N—R Y — or the like (R Y represents H or the like);
- R 2 represents H, halogen, cyano, C 1-4 alkyl, C 3-6 cycloalkyl and the like;
- B represents R 3 , NHR 3 and the like;
- R 3 represents C 1-10 alkyl or the like;
- R 4 represents H, —OR 10 , —COR 9 , halogen, C 1-6 alkyl, C 3-6 cycloalkyl and the like;
- R 5 represents H,
- R 1 and R 2 are independently H, halogen, —OR ′ (R ′ may be substituted with H, C 1-6 alkyl (—OR, —N (R) 2 , cyano, oxo, etc.). And cyano, C 1-10 aliphatic compounds (which may be substituted with halogen, —OR, —N (R) 2 , cyano, etc.), etc.]
- R ′ may be substituted with H, C 1-6 alkyl (—OR, —N (R) 2 , cyano, oxo, etc.).
- cyano C 1-10 aliphatic compounds (which may be substituted with halogen, —OR, —N (R) 2 , cyano, etc.), etc.]
- B represents an optionally substituted 5-membered aromatic heterocyclic ring
- R 1 and R 2 independently represent H, halogen, —OR ′, cyano, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds (halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl (halogen, —OR, —N ( R) 2 , —C (O) R, optionally substituted with cyano, oxo, etc.)) and the like;
- X represents C or N;
- R X is absent or represents H;
- C is an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8-12 bicyclic ring (0-5 heteroatoms) Having R represents H or C 1-6 al
- B represents an optionally substituted 6-membered aromatic heterocycle
- R 1 and R 2 independently represent H, halogen, —OR ′, cyano, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds ( optionally substituted with halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl, etc.) Etc .
- R 3 is absent or represents H, C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.)
- R 4 is an optionally substituted C 1-10 aliphatic compound, an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8 Represents
- B represents an optionally substituted 5-membered aromatic heterocyclic ring
- R 1 and R 2 independently represent H, halogen, cyano, —OR ′, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds (halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl (halogen, —OR, —N ( R) 2 , —C (O) R, optionally substituted with cyano, oxo, etc.))
- R 3 is absent or is H, C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.)
- R 4 is an optionally substituted C 1-10 aliphatic compound, an
- R 1 represents an optionally substituted phenyl or an optionally substituted aromatic heterocyclic group
- R 2 represents a hydrogen atom or a methyl group
- R 3 represents a 5- (R 5 ) -1H-imidazol-4-yl group or a 4- (R 5 ) -1H-imidazol-5-yl group
- R 4 represents a hydrogen atom, an amino group or a methylamino group
- R 5 represents a methyl group or an ethyl group
- X represents carbonyl or the like
- R 2 and R 3 represent a hydrogen atom, halogen, lower alkyl, imidazolyl, imidazolylmethyl or —N (R 4 ) —R 5
- R 4 and R 5 represent a hydrogen atom or lower alkyl, or combine with each other to form a 5- or 6-membered heterocyclic ring.
- An object of the present invention is to provide a compound having excellent PKC inhibitory activity and useful as a preventive or therapeutic agent for immune diseases, inflammatory diseases and the like.
- Ring A represents a nitrogen-containing unsaturated heterocyclic ring which may be further substituted
- Ring B represents a C 6-14 aromatic hydrocarbon ring which may be further substituted or an aromatic heterocyclic ring which may be further substituted
- L represents an optionally substituted C 1-2 alkylene
- X 1 represents N or CR 1
- X 2 represents N or CR 2
- X 3 represents N or CR 3
- X 4 represents N or CR 4
- R 1 , R 2 , R 3 and R 4 independently represent a hydrogen atom or a substituent
- X 5 represents CR 5a , CR 5b R 5c , NR 5d or a bond
- R 5a , R 5b , R 5c and R 5d independently represent a hydrogen atom or a substituent, or R 5b and R 5c may combine together to form an optionally substituted ring.
- X 6 represents CR 6a R 6b or NR 6c ;
- R 6a , R 6b and R 6c independently represent a hydrogen atom or a substituent, or R 6a and R 6b may together form an optionally substituted ring;
- R 7 represents a hydrogen atom or a substituent.
- a salt thereof hereinafter sometimes referred to as compound (I);
- the ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted, or may be further substituted, the formula (A):
- Ring A 1 represents an optionally further substituted 5- or 6-membered aromatic ring, or an optionally further substituted 5- or 6-membered non-aromatic unsaturated ring
- Ring A 2 represents an optionally substituted 5- or 6-membered aromatic ring, or an optionally substituted 5- or 6-membered non-aromatic unsaturated ring
- At least one of the ring constituent atoms of ring A 1 or ring A 2 is a nitrogen atom.
- Ring B is a benzene ring which may be further substituted, or a thiophene ring which may be further substituted or a pyridine ring which may be further substituted;
- L is C 1-2 alkylene;
- X 1 is N or CR 1 and R 1 is a hydrogen atom or a halogen atom;
- X 2 is N or CR 2 and R 2 is a hydrogen atom or a halogen atom;
- X 3 is N or CR 3 , R 3 is a hydrogen atom or a halogen atom;
- X 4 is N or CR 4 and R 4 is a hydrogen atom or a C 1-6 alkyl group;
- X 5 is CR 5b R 5c or a bond, and R 5b and R 5c are independently a hydrogen atom or a substituent;
- X 6 is CR 6a R 6b and R 6a and R 6b are independently a hydrogen atom or a substituent,
- Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group, or a halogen atom, hydroxy Selected from a group, an oxo group, an optionally substituted C 1-6 alkyl group, an optionally substituted C 1-6 alkoxy group, and an optionally substituted non-aromatic heterocyclic-oxy group;
- the ring A 1 may be further substituted with three substituents, the ring A 1 is a pyrrole ring, a pyrazole ring, an imidazole ring, a pyridine ring or an imidazoline ring, and the ring A 2 is a thiophene ring, a pyridine ring or a
- Compound (I) has excellent PKC (eg, PKC- ⁇ ) inhibitory activity and is useful as a preventive or therapeutic agent for immune diseases and inflammatory diseases.
- PKC eg, PKC- ⁇
- each substituent has the following definition.
- examples of the “halogen atom” include fluorine, chlorine, bromine and iodine.
- examples of the “C 1-6 alkyl group” include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 1-ethylpropyl, hexyl.
- Specific examples include methyl, chloromethyl, difluoromethyl, trichloromethyl, trifluoromethyl, ethyl, 2-bromoethyl, 2,2,2-trifluoroethyl, tetrafluoroethyl, pentafluoroethyl, propyl, 2,2- Difluoropropyl, 3,3,3-trifluoropropyl, isopropyl, butyl, 4,4,4-trifluorobutyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 5,5,5-tri Examples include fluoropentyl, hexyl, and 6,6,6-trifluorohexyl.
- examples of the “C 2-6 alkenyl group” include ethenyl, 1-propenyl, 2-propenyl, 2-methyl-1-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 3- Examples include methyl-2-butenyl, 1-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 4-methyl-3-pentenyl, 1-hexenyl, 3-hexenyl and 5-hexenyl.
- examples of the “C 2-6 alkynyl group” include ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-pentynyl, 2-pentynyl, 3- Examples include pentynyl, 4-pentynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 4-methyl-2-pentynyl.
- examples of the “C 3-10 cycloalkyl group” include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, bicyclo [2.2.1] heptyl, and bicyclo [2.2. 2] Octyl, bicyclo [3.2.1] octyl, and adamantyl.
- the "optionally halogenated C 3-10 also be cycloalkyl group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 3- A 10 cycloalkyl group.
- examples include cyclopropyl, 2,2-difluorocyclopropyl, 2,3-difluorocyclopropyl, cyclobutyl, difluorocyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
- examples of the “C 3-10 cycloalkenyl group” include cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, and cyclooctenyl.
- examples of the “C 6-14 aryl group” include phenyl, 1-naphthyl, 2-naphthyl, 1-anthryl, 2-anthryl, and 9-anthryl.
- examples of the “C 7-16 aralkyl group” include benzyl, phenethyl, naphthylmethyl, and phenylpropyl.
- examples of the “C 1-6 alkoxy group” include methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy, tert-butoxy, pentyloxy and hexyloxy.
- the "optionally halogenated C 1-6 alkoxy group” for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkoxy group is mentioned.
- Examples include methoxy, difluoromethoxy, trifluoromethoxy, ethoxy, 2,2,2-trifluoroethoxy, propoxy, isopropoxy, butoxy, 4,4,4-trifluorobutoxy, isobutoxy, sec-butoxy, pentyl.
- Examples include oxy and hexyloxy.
- examples of the “C 3-10 cycloalkyloxy group” include cyclopropyloxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, cycloheptyloxy, and cyclooctyloxy.
- examples of the “C 1-6 alkylthio group” include methylthio, ethylthio, propylthio, isopropylthio, butylthio, sec-butylthio, tert-butylthio, pentylthio and hexylthio.
- the "optionally halogenated C 1-6 alkylthio group optionally" for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkylthio group is mentioned.
- examples include methylthio, difluoromethylthio, trifluoromethylthio, ethylthio, propylthio, isopropylthio, butylthio, 4,4,4-trifluorobutylthio, pentylthio, hexylthio.
- examples of the “C 1-6 alkyl-carbonyl group” include acetyl, propanoyl, butanoyl, 2-methylpropanoyl, pentanoyl, 3-methylbutanoyl, 2-methylbutanoyl, 2,2- Examples include dimethylpropanoyl, hexanoyl, and heptanoyl.
- examples of the “ optionally halogenated C 1-6 alkyl-carbonyl group” include C 1 optionally having 1 to 7, preferably 1 to 5 halogen atoms.
- a -6 alkyl-carbonyl group is mentioned. Specific examples include acetyl, chloroacetyl, trifluoroacetyl, trichloroacetyl, propanoyl, butanoyl, pentanoyl and hexanoyl.
- examples of the “C 1-6 alkoxy-carbonyl group” include methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl, isobutoxycarbonyl, sec-butoxycarbonyl, tert-butoxycarbonyl, Examples include pentyloxycarbonyl and hexyloxycarbonyl.
- examples of the “C 6-14 aryl-carbonyl group” include benzoyl, 1-naphthoyl and 2-naphthoyl.
- examples of the “C 7-16 aralkyl-carbonyl group” include phenylacetyl and phenylpropionyl.
- examples of the “5- to 14-membered aromatic heterocyclic carbonyl group” include nicotinoyl, isonicotinoyl, thenoyl and furoyl.
- examples of the “3- to 14-membered non-aromatic heterocyclic carbonyl group” include morpholinylcarbonyl, piperidinylcarbonyl, and pyrrolidinylcarbonyl.
- examples of the “mono- or di-C 1-6 alkyl-carbamoyl group” include methylcarbamoyl, ethylcarbamoyl, dimethylcarbamoyl, diethylcarbamoyl, N-ethyl-N-methylcarbamoyl.
- examples of the “mono- or di-C 7-16 aralkyl-carbamoyl group” include benzylcarbamoyl and phenethylcarbamoyl.
- examples of the “C 1-6 alkylsulfonyl group” include methylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl, butylsulfonyl, sec-butylsulfonyl and tert-butylsulfonyl.
- the "optionally halogenated C 1-6 alkyl sulfonyl group” for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1- 6 alkylsulfonyl group is mentioned.
- examples include methylsulfonyl, difluoromethylsulfonyl, trifluoromethylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl, butylsulfonyl, 4,4,4-trifluorobutylsulfonyl, pentylsulfonyl, hexylsulfonyl.
- examples of the “C 6-14 arylsulfonyl group” include phenylsulfonyl, 1-naphthylsulfonyl and 2-naphthylsulfonyl.
- examples of the “substituent” include a halogen atom, a cyano group, a nitro group, an optionally substituted hydrocarbon group, an optionally substituted heterocyclic group, an acyl group, and a substituted group.
- An optionally substituted amino group an optionally substituted carbamoyl group, an optionally substituted thiocarbamoyl group, an optionally substituted sulfamoyl group, an optionally substituted hydroxy group, an optionally substituted sulfanyl ( SH) group and optionally substituted silyl group.
- examples of the “hydrocarbon group” include, for example, a C 1-6 alkyl group, a C 2-6 alkenyl group, Examples thereof include a C 2-6 alkynyl group, a C 3-10 cycloalkyl group, a C 3-10 cycloalkenyl group, a C 6-14 aryl group, and a C 7-16 aralkyl group.
- examples of the “optionally substituted hydrocarbon group” include a hydrocarbon group which may have a substituent selected from the following substituent group A.
- substituent group A (1) a halogen atom, (2) Nitro group, (3) a cyano group, (4) an oxo group, (5) a hydroxy group, (6) an optionally halogenated C 1-6 alkoxy group, (7) C 6-14 aryloxy group (eg, phenoxy, naphthoxy), (8) C 7-16 aralkyloxy group (eg, benzyloxy), (9) 5- to 14-membered aromatic heterocyclic oxy group (eg, pyridyloxy), (10) 3 to 14-membered non-aromatic heterocyclic oxy group (eg, morpholinyloxy, piperidinyloxy), (11) C 1-6 alkyl-carbonyloxy group (eg, acetoxy, propanoyloxy), (12) C 6-14 aryl-carbony
- the number of the substituents in the “optionally substituted hydrocarbon group” is, for example, 1 to 5, preferably 1 to 3. When the number of substituents is 2 or more, each substituent may be the same or different.
- examples of the “heterocyclic group” include, for example, a nitrogen atom, a sulfur atom and a ring atom other than a carbon atom.
- an aromatic heterocyclic group (ii) a non-aromatic heterocyclic group, and (iii) a 7 to 10-membered heterocyclic bridge group each containing 1 to 4 heteroatoms selected from oxygen atoms .
- the “aromatic heterocyclic group” (including the “5- to 14-membered aromatic heterocyclic group”) is, for example, selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom.
- 5- to 14-membered (preferably 5- to 10-membered) aromatic heterocyclic group containing 1 to 4 heteroatoms is, for example, selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom.
- 5- to 14-membered (preferably 5- to 10-membered) aromatic heterocyclic group containing 1 to 4 heteroatoms is, for example, selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom.
- Suitable examples of the “aromatic heterocyclic group” include thienyl, furyl, pyrrolyl, imidazolyl, pyrazolyl, thiazolyl, isothiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, 1,2,4-oxadiazolyl, 1 5-, 6-membered monocyclic aromatic heterocyclic groups such as 1,3,4-oxadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl, triazolyl, tetrazolyl, triazinyl; Benzothiophenyl, benzofuranyl, benzoimidazolyl, benzoxazolyl, benzisoxazolyl, benzothiazolyl, benzisothiazolyl, benzotriazolyl, imidazopyridinyl, thienopyri
- non-aromatic heterocyclic group examples include, for example, a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom.
- non-aromatic heterocyclic group containing 1 to 4 heteroatoms selected from Suitable examples of the “non-aromatic heterocyclic group” include aziridinyl, oxiranyl, thiylyl, azetidinyl, oxetanyl, thietanyl, tetrahydrothienyl, tetrahydrofuranyl, pyrrolinyl, pyrrolidinyl, imidazolinyl, imidazolidinyl, oxazolinyl, oxazolidinyl, pyrazolidinyl, pyrazolinyl, pyrazolinyl Thiazolinyl, thiazolidinyl, tetrahydroisothiazolyl, tetrahydrooxazolyl, tetrahydroisoxazolyl, piperidinyl, piperazinyl, t
- preferable examples of the “7 to 10-membered heterocyclic bridged ring group” include quinuclidinyl and 7-azabicyclo [2.2.1] heptanyl.
- examples of the “nitrogen-containing heterocyclic group” include those containing at least one nitrogen atom as a ring-constituting atom among the “heterocyclic groups”.
- examples of the “optionally substituted heterocyclic group” include a heterocyclic group which may have a substituent selected from the substituent group A described above.
- the number of substituents in the “optionally substituted heterocyclic group” is, for example, 1 to 3. When the number of substituents is 2 or more, each substituent may be the same or different.
- acyl group is, for example, “1 selected from a halogen atom, an optionally halogenated C 1-6 alkoxy group, a hydroxy group, a nitro group, a cyano group, an amino group, and a carbamoyl group.
- the “acyl group” also includes a hydrocarbon-sulfonyl group, a heterocyclic-sulfonyl group, a hydrocarbon-sulfinyl group, and a heterocyclic-sulfinyl group.
- the hydrocarbon-sulfonyl group is a sulfonyl group to which a hydrocarbon group is bonded
- the heterocyclic-sulfonyl group is a sulfonyl group to which a heterocyclic group is bonded
- the hydrocarbon-sulfinyl group is a hydrocarbon group.
- a sulfinyl group to which is bonded and a heterocyclic-sulfinyl group mean a sulfinyl group to which a heterocyclic group is bonded.
- the “acyl group” a formyl group, a carboxy group, a C 1-6 alkyl-carbonyl group, a C 2-6 alkenyl-carbonyl group (eg, crotonoyl), a C 3-10 cycloalkyl-carbonyl group ( Examples, cyclobutanecarbonyl, cyclopentanecarbonyl, cyclohexanecarbonyl, cycloheptanecarbonyl), C 3-10 cycloalkenyl-carbonyl group (eg, 2-cyclohexenecarbonyl), C 6-14 aryl-carbonyl group, C 7-16 aralkyl- Carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered
- Diallylcarbamoyl mono- or di-C 3-10 cycloalkyl-carbamoyl group (eg, cyclopropylcarbamoyl), mono- or di-C 6-14 aryl-carbamoyl group (eg, phenylcarbamoyl), mono- or Di-C 7-16 aralkyl-carbamoyl group, 5- to 14-membered aromatic heterocyclic carbamoyl group (eg, pyridylcarbamoyl), thiocarbamoyl group, mono- or di-C 1-6 alkyl-thiocarbamoyl group (eg, methylthio) Carbamoyl, N-ethyl-N-methyl Okarubamoiru), mono - or di -C 2-6 alkenyl - thiocarbamoyl group (e.g., diallyl thio carbamoyl), mono - or di cycl
- examples of the “optionally substituted amino group” include, for example, a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A” C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl Groups, mono- or di-C 1-6 alkyl-carbamoyl groups, mono- or di-C 7-16 aralkyl-carbamoyl groups, C 1-6 alkylsulfonyl groups and C 6- And an amino
- Suitable examples of the optionally substituted amino group include an amino group, a mono- or di- (optionally halogenated C 1-6 alkyl) amino group (eg, methylamino, trifluoromethylamino, Dimethylamino, ethylamino, diethylamino, propylamino, dibutylamino), mono- or di-C 2-6 alkenylamino groups (eg, diallylamino), mono- or di-C 3-10 cycloalkylamino groups (eg, Cyclopropylamino, cyclohexylamino), mono- or di-C 6-14 arylamino group (eg, phenylamino), mono- or di-C 7-16 aralkylamino group (eg, benzylamino, dibenzylamino), mono - or di - (optionally halogenated C 1-6 alkyl) - carbonyl amino group (e.g., a Chiru
- examples of the “optionally substituted carbamoyl group” include, for example, a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A” C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - 1 or 2 substituents selected from a carbamoyl group
- Suitable examples of the optionally substituted carbamoyl group include a carbamoyl group, a mono- or di-C 1-6 alkyl-carbamoyl group, a mono- or di-C 2-6 alkenyl-carbamoyl group (eg, diallylcarbamoyl group).
- Mono- or di-C 3-10 cycloalkyl-carbamoyl groups eg cyclopropylcarbamoyl, cyclohexylcarbamoyl
- mono- or di-C 6-14 aryl-carbamoyl groups eg phenylcarbamoyl
- mono- or Di-C 7-16 aralkyl-carbamoyl group mono- or di-C 1-6 alkyl-carbonyl-carbamoyl group (eg acetylcarbamoyl, propionylcarbamoyl), mono- or di-C 6-14 aryl-carbonyl-carbamoyl Groups (eg, benzoylcarbamoyl)
- a 5- to 14-membered aromatic heterocyclic carbamoyl group eg, pyridylcarbamoyl
- pyridylcarbamoyl pyridylcarb
- examples of the “optionally substituted thiocarbamoyl group” include, for example, “C 1-6 alkyl each optionally having 1 to 3 substituents selected from Substituent Group A” Group, C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7-16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - one or two location selected from a carbamoyl
- thiocarbamoyl group which may be substituted include a thiocarbamoyl group, a mono- or di-C 1-6 alkyl-thiocarbamoyl group (eg, methylthiocarbamoyl, ethylthiocarbamoyl, dimethylthiocarbamoyl, diethylthio).
- examples of the “optionally substituted sulfamoyl group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A”.
- the optionally substituted sulfamoyl group include sulfamoyl group, mono- or di-C 1-6 alkyl-sulfamoyl group (eg, methylsulfamoyl, ethylsulfamoyl, dimethylsulfamoyl, diethyl).
- examples of the “optionally substituted hydroxy group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A”.
- Suitable examples of the optionally substituted hydroxy group include a hydroxy group, a C 1-6 alkoxy group, a C 2-6 alkenyloxy group (eg, allyloxy, 2-butenyloxy, 2-pentenyloxy, 3-hexenyloxy).
- C 3-10 cycloalkyloxy group eg, cyclohexyloxy
- C 6-14 aryloxy group eg, phenoxy, naphthyloxy
- C 7-16 aralkyloxy group eg, benzyloxy, phenethyloxy
- C 1-6 alkyl-carbonyloxy group eg, acetyloxy, propionyloxy, butyryloxy, isobutyryloxy, pivaloyloxy
- C 6-14 aryl-carbonyloxy group eg, benzoyloxy
- C 7-16 aralkyl- A carbonyloxy group eg benzylcarbonyloxy)
- 5 to 14-membered aromatic heterocyclic carbonyloxy group e.g., nicotinoyl oxy
- 3 to 14-membered non-aromatic heterocyclic carbonyloxy group e.g., piperidinylcarbonyl oxy
- examples of the “optionally substituted sulfanyl group” include a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A”.
- C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group and 5 to Examples thereof include a sulfanyl group optionally having a substituent selected from a 14-membered aromatic heterocyclic group and a halogenated sulfanyl group.
- the optionally substituted sulfanyl group include a sulfanyl (—SH) group, a C 1-6 alkylthio group, a C 2-6 alkenylthio group (eg, allylthio, 2-butenylthio, 2-pentenylthio, 3-hexenylthio), C 3-10 cycloalkylthio group (eg, cyclohexylthio), C 6-14 arylthio group (eg, phenylthio, naphthylthio), C 7-16 aralkylthio group (eg, benzylthio, phenethylthio), C 1-6 alkyl-carbonylthio group (eg, acetylthio, propionylthio, butyrylthio, isobutyrylthio, pivaloylthio), C 6-14 aryl-carbonylthio group (eg, benzoylthio), 5-
- examples of the “optionally substituted silyl group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A”
- a silyl group optionally having 1 to 3 substituents selected from a C 2-6 alkenyl group, a C 3-10 cycloalkyl group, a C 6-14 aryl group and a C 7-16 aralkyl group ” Can be mentioned.
- Preferable examples of the optionally substituted silyl group include a tri-C 1-6 alkylsilyl group (eg, trimethylsilyl, tert-butyl (dimethyl) silyl).
- examples of the “C 1-6 alkylene group” include —CH 2 —, — (CH 2 ) 2 —, — (CH 2 ) 3 —, — (CH 2 ) 4 —, — (CH 2 ) 5 —, — (CH 2 ) 6 —, —CH (CH 3 ) —, —C (CH 3 ) 2 —, —CH (C 2 H 5 ) —, —CH (C 3 H 7 ) —, —CH (CH (CH 3 ) 2 ) —, — (CH (CH 3 )) 2 —, —CH 2 —CH (CH 3 ) —, —CH (CH 3 ) —CH 2 —, —CH 2 —CH 2 -C (CH 3) 2 - , - C (CH 3) 2 -CH 2 -CH 2 -, - CH 2 -CH 2 -CH 2 -C (CH 3) 2 -, - C (CH 3) 2
- examples of the “C 2-6 alkenylene group” include —CH ⁇ CH—, —CH 2 —CH ⁇ CH—, —CH ⁇ CH—CH 2 —, —C (CH 3 ) 2 —.
- examples of the “C 2-6 alkynylene group” include —C ⁇ C—, —CH 2 —C ⁇ C—, —C ⁇ C—CH 2 —, —C (CH 3 ) 2 —.
- examples of the “hydrocarbon ring” include a C 6-14 aromatic hydrocarbon ring, a C 3-10 cycloalkane, and a C 3-10 cycloalkene.
- examples of the “C 6-14 aromatic hydrocarbon ring” include benzene and naphthalene.
- examples of “C 3-10 cycloalkane” include cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, and cyclooctane.
- examples of “C 3-10 cycloalkene” include cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, and cyclooctene.
- examples of the “heterocycle” include aromatic heterocycles each containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom, and an oxygen atom in addition to a carbon atom as a ring-constituting atom. Non-aromatic heterocycles may be mentioned.
- the “aromatic heterocycle” is, for example, a 5- to 14-membered member containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring constituent atom ( Preferred is a 5- to 10-membered aromatic heterocyclic ring.
- aromatic heterocyclic ring examples include thiophene, furan, pyrrole, imidazole, pyrazole, thiazole, isothiazole, oxazole, isoxazole, pyridine, pyrazine, pyrimidine, pyridazine, 1,2,4-oxadi 5- to 6-membered monocyclic aromatic heterocycle such as azole, 1,3,4-oxadiazole, 1,2,4-thiadiazole, 1,3,4-thiadiazole, triazole, tetrazole, triazine; Benzothiophene, benzofuran, benzimidazole, benzoxazole, benzoisoxazole, benzothiazole, benzoisothiazole, benzotriazole, imidazopyridine, thienopyridine, furopyridine, pyrrolopyridine, pyrazolopyridine, oxazolopyridine, oxazolopyridine,
- non-aromatic heterocycle includes, for example, a 3 to 14 member containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring constituent atom. (Preferably 4 to 10 membered) non-aromatic heterocycle.
- non-aromatic heterocycle examples include aziridine, oxirane, thiirane, azetidine, oxetane, thietane, tetrahydrothiophene, tetrahydrofuran, pyrroline, pyrrolidine, imidazoline, imidazolidine, oxazoline, oxazolidine, pyrazoline, pyrazolidine, thiazoline.
- the “nitrogen-containing unsaturated heterocycle” of the “optionally substituted nitrogen-containing unsaturated heterocycle” includes one or more multiple bonds (eg, Those containing a double bond or a triple bond), and examples of the substituent include the above-mentioned “substituent”.
- the “nitrogen-containing unsaturated heterocycle” includes a nitrogen-containing aromatic heterocycle.
- C 1-2 alkylene of “optionally substituted C 1-2 alkylene” includes those having 1 or 2 carbon atoms in the above “C 1-6 alkylene group”.
- substituent include the above-mentioned “substituent”.
- examples of the “ring” of the “optionally substituted ring” include the above “hydrocarbon ring” and “heterocycle”, and the substituent includes the above “substituent”.
- the “aromatic ring” of the “optionally substituted 5- or 6-membered aromatic ring” includes a benzene ring and the above “aromatic heterocycle” having 5 or 6 carbon atoms.
- Examples of the substituent include the above-mentioned “substituent”.
- non-aromatic unsaturated ring of the “optionally substituted 5-membered or 6-membered non-aromatic unsaturated ring” is the carbon number of the above “C 3-10 cycloalkene”.
- heterocycle having 5 or 6 carbon atoms and containing one or more multiple bonds (eg, double bonds, triple bonds), and the substituents thereof.
- substituted can be mentioned.
- Ring A represents a nitrogen-containing unsaturated heterocyclic ring which may be further substituted.
- nitrogen-containing unsaturated heterocycle of the “optionally substituted nitrogen-containing unsaturated heterocycle” represented by ring A include monocyclic or bicyclic nitrogen-containing unsaturated heterocycles.
- Examples of the “substituent” of the “optionally substituted nitrogen-containing unsaturated heterocyclic ring” represented by ring A include a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, and an optionally substituted C A 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group (eg, methoxy), an optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyrani) Ruoxy).
- a halogen atom eg, fluorine atom
- a hydroxy group e.g, an oxo group
- an optionally substituted C A 1-6 alkyl group eg, methyl, ethyl
- an optionally substituted C 1-6 alkoxy group eg, methoxy
- an optionally substituted non-aromatic heterocyclic-oxy group eg, tetra
- Ring A is preferably further substituted, formula (A):
- Ring A 1 represents an optionally further substituted 5- or 6-membered aromatic ring, or an optionally further substituted 5- or 6-membered non-aromatic unsaturated ring
- Ring A 2 represents an optionally substituted 5- or 6-membered aromatic ring, or an optionally substituted 5- or 6-membered non-aromatic unsaturated ring
- At least one of the ring constituent atoms of ring A 1 or ring A 2 is a nitrogen atom.
- Ring A 1 is an imidazole ring
- ring A 2 is a pyridine ring
- formula (A) is
- the ring A 1 is an imidazoline ring and the ring A 2 is a pyridine ring.
- Examples of the “aromatic ring” of the “(further) optionally substituted 5-membered or 6-membered aromatic ring” represented by ring A 1 or ring A 2 include pyrrole ring, thiophene ring, pyrazole ring, imidazole ring, pyridine And a ring and a pyrimidine ring.
- Examples of the “non-aromatic unsaturated ring” of the “(further) optionally substituted 5- or 6-membered non-aromatic unsaturated ring” represented by ring A 1 or ring A 2 include imidazoline ring, tetrahydropyrazine A ring is mentioned.
- halogen atom eg, fluorine atom
- C 1-6 alkyl group eg, methyl, ethyl
- C A 1-6 alkoxy group eg, methoxy
- non-aromatic heterocyclic-oxy group eg, tetrahydropyranyloxy
- Ring A is more preferably a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1 to 3 (preferably 1 or 2) selected from a 1-6 alkoxy group (eg, methoxy) and an optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 which may be further substituted with a substituent is pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole ring, thiophene ring, pyridine It is a bicyclic nitrogen-containing unsaturated heterocycle represented by the formula (A) which is a ring or a pyrimidine
- ring A is preferably an optionally substituted monocyclic nitrogen-containing unsaturated heterocycle (eg, imidazolidine ring, imidazoline ring), more preferably, It is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group.
- monocyclic nitrogen-containing unsaturated heterocycle eg, imidazolidine ring, imidazoline ring
- It is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group.
- ring A includes any tautomer when tautomers (enol form and keto form) exist.
- Ring A is imidazo [4,5-b] pyridine, further substituted with hydroxy, the formula:
- Ring B represents a C 6-14 aromatic hydrocarbon ring which may be further substituted or an aromatic heterocyclic ring which may be further substituted.
- the “C 6-14 aromatic hydrocarbon ring” of the “ optionally substituted C 6-14 aromatic hydrocarbon ring” represented by ring B includes a benzene ring.
- Examples of the “aromatic heterocycle” of the “optionally substituted aromatic heterocycle” represented by ring B include a thiophene ring and a pyridine ring.
- a halogen atom eg, A fluorine atom, a chlorine atom
- a cyano group an optionally substituted C 1-6 alkyl group (eg, methyl), and an optionally substituted C 1-6 alkoxy group (eg, methoxy).
- Ring B is preferably a benzene ring which may be further substituted, or a thiophene ring or a pyridine ring which may be further substituted, more preferably a halogen atom (eg, fluorine atom, chlorine atom), 1 to 5 (preferably 1 to 5) selected from a cyano group, an optionally substituted C 1-6 alkyl group (eg, methyl) and an optionally substituted C 1-6 alkoxy group (eg, methoxy)
- a benzene ring which may be further substituted with three, more preferably 1 or 2) substituents, or a thiophene ring or a pyridine ring which may be further substituted respectively, and still more preferably, (1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms
- L represents C 1-2 alkylene which may be substituted.
- Examples of the “substituent” of the “optionally substituted C 1-2 alkylene” represented by L include a C 1-6 alkyl group (eg, methyl).
- L is preferably, C 1-2 alkylene (e.g., -CH 2 -, - CH 2 CH 2 -, - CH (CH 3) -) , more preferably an, -CH 2 - it is.
- X 1 represents N or CR 1
- X 2 represents N or CR 2
- X 3 represents N or CR 3
- X 4 represents N or CR 4
- R 1 , R 2 , R 3 and R 4 independently represent a hydrogen atom or a substituent.
- substituents include a halogen atom (eg, fluorine atom) and a C 1-6 alkyl group (eg, methyl).
- X 1 is preferably N or CR 1 (R 1 is a hydrogen atom or a halogen atom (eg, a fluorine atom)), more preferably N or CR 1 (R 1 is a hydrogen atom or A fluorine atom), and even more preferably CR 1 (R 1 is a hydrogen atom).
- X 2 is preferably N or CR 2 (R 2 is a hydrogen atom or a halogen atom (eg, fluorine atom)), more preferably N or CR 2 (R 2 is a hydrogen atom or A fluorine atom), and even more preferably CR 2 (R 2 is a hydrogen atom).
- X 3 is preferably N or CR 3 (R 3 is a hydrogen atom or a halogen atom (eg, fluorine atom)), and more preferably N or CR 3 (R 3 is a hydrogen atom or A fluorine atom), and even more preferably N or CR 3 (R 3 is a hydrogen atom).
- X 4 is preferably N or CR 4 (R 4 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl)), more preferably N or CR 4 (R 4 is A hydrogen atom or methyl), and even more preferably N or CR 4 (R 4 is a hydrogen atom).
- X 5 represents CR 5a , CR 5b R 5c , NR 5d or a bond
- R 5a , R 5b , R 5c and R 5d independently represent a hydrogen atom or a substituent, or R 5b And R 5c together may form an optionally substituted ring.
- R 5a , R 5b , R 5c or R 5d include an oxo group.
- R 5b and R 5c together include a C 3-10 cycloalkane ring (eg, cyclopentane ring).
- X 5 is preferably CR 5b R 5c (R 5b and R 5c are each independently a hydrogen atom or a substituent) or a bond, and more preferably CR 5b R 5c (R 5b and R 5c is a hydrogen atom.) Or a bond, and even more preferably a bond.
- X 6 represents CR 6a R 6b or NR 6c
- R 6a , R 6b and R 6c independently represent a hydrogen atom or a substituent, or R 6a and R 6b are substituted together May form a ring which may be
- R 6a , R 6b or R 6c include an optionally substituted C 1-6 alkyl group (eg, methyl).
- R 6a and R 6b together include a C 3-10 cycloalkane ring (eg, cyclopentane ring).
- X 6 is preferably CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom or a substituent), and more preferably CR 6a R 6b (R 6a and R 6b are Is independently a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl).), And even more preferably, CR 6a R 6b (R 6a and R 6b are independently And a C 1-6 alkyl group (eg, methyl) optionally substituted with a hydroxy group or a C 6-14 aryl group (eg, phenyl).
- X 5 is preferably CR 6a R 6b (where R 6a and R 6b are taken together to represent an optionally substituted C 3-10 cycloalkane ring (eg, And more preferably CR 6a R 6b (R 6a and R 6b together form a C 3-10 cycloalkane ring (eg, a cyclopentane ring). ).
- R 7 represents a hydrogen atom or a substituent.
- substituents include an optionally substituted C 1-6 alkyl group (eg, methyl).
- R 7 is preferably a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl), more preferably a hydrogen atom or a C 1-6 alkyl group (eg, methyl). Even more preferably, it is a hydrogen atom.
- Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted, or may be further substituted in the above formula (A)
- a bicyclic nitrogen-containing unsaturated heterocycle represented by Ring B is a benzene ring which may be further substituted, or a thiophene ring or a pyridine ring which may be further substituted respectively;
- L is C 1-2 alkylene (eg, —CH 2 —, —CH 2 CH 2 —, —CH (CH 3 ) —);
- X 1 is N or CR 1 (R 1 is a hydrogen atom or a halogen atom (eg, fluorine atom));
- X 2 is N or CR 2 (R 2 is a hydrogen atom or a halogen atom (eg, fluorine atom));
- X 3 is N or
- ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group Or a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group Further substituted with 1 to 3 (preferably 1 or 2) substituents selected from (eg, methoxy) and optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 is a pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazin
- ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group Or a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group Further substituted with 1 to 3 (preferably 1 or 2) substituents selected from (eg, methoxy) and optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 is a pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazin
- Ring A is a 1H-imidazo [4,5-b] pyridine ring further substituted with hydroxy or a 1H-pyrrolo [2,3-b] pyridine ring further substituted with methyl;
- Ring B is (1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms) and amino groups 1 to 5 (preferably 1 to 3, more preferably 1) selected from a C 1-6 alkyl group (eg, methyl) and (iv) a C 1-6 alkoxy group (eg, methoxy) Or a benzene ring optionally further substituted with 2) substituents, (2) a thiophene ring, or (3) a pyridine ring;
- L is —CH 2 —;
- X 1 is CR 1 (R 1 is a hydrogen atom);
- [Compound I-5] 1 to 3 (preferably 1 or 2) of ring A selected from a hydroxy group, an oxo group, a C 1-6 alkyl group (eg, methyl) and a C 1-6 alkoxy group (eg, methoxy) Ring A 1 which may be further substituted with a substituent is pyrrole ring, pyrazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole ring, thiophene ring, pyridine ring or pyrimidine A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the above formula (A) which is a ring; Ring B is (1) a benzene ring which may be further substituted with a C 1-6 alkyl group (eg, methyl) optionally substituted with 1 to 3 halogen atoms (eg, fluorine atom), (2) a
- compound (I) include, for example, the compounds of Examples 1 to 93. Among them, 2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2 , 3-Dihydroquinazolin-4 (1H) -one or a salt thereof (Example 14); 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one or a salt thereof Example 48; and 1-benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro -5H-pyrido [2,3-e] [1,4] dia
- salts include metal salts, ammonium salts, salts with organic bases, salts with inorganic acids, salts with organic acids, basic or acidic amino acids, and the like.
- examples include salts.
- the metal salt include alkali metal salts such as sodium salt and potassium salt; alkaline earth metal salts such as calcium salt, magnesium salt and barium salt; aluminum salt and the like.
- the salt with organic base include, for example, trimethylamine, triethylamine, pyridine, picoline, 2,6-lutidine, ethanolamine, diethanolamine, triethanolamine, cyclohexylamine, dicyclohexylamine, N, N′-dibenzyl.
- Examples include salts with ethylenediamine and the like.
- Preferable examples of the salt with inorganic acid include salts with hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid and the like.
- Preferable examples of the salt with organic acid include, for example, formic acid, acetic acid, trifluoroacetic acid, phthalic acid, fumaric acid, oxalic acid, tartaric acid, maleic acid, citric acid, succinic acid, malic acid, methanesulfonic acid, benzene And salts with sulfonic acid, p-toluenesulfonic acid and the like.
- salts with basic amino acids include salts with arginine, lysine, ornithine and the like
- salts with acidic amino acids include salts with aspartic acid, glutamic acid and the like. Is mentioned. Of these, pharmaceutically acceptable salts are preferred.
- inorganic salts such as alkali metal salts (eg, sodium salts, potassium salts, etc.), alkaline earth metal salts (eg, calcium salts, magnesium salts, etc.), ammonium salts
- a salt with an inorganic acid such as hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid, or acetic acid, phthalic acid, fumaric acid
- organic acids such as acid, tartaric acid, maleic acid, citric acid, succinic acid, methanesulfonic acid, benzenesulfonic acid, and p-toluenesulfonic acid.
- the raw materials and reagents used in each step in the following production method and the obtained compound may each form a salt.
- Examples of such salts include those similar to the salts of the aforementioned compound of the present invention.
- the compound obtained in each step is a free compound, it can be converted into a target salt by a method known per se.
- the compound obtained in each step is a salt, it can be converted into a free form or other types of desired salts by a method known per se.
- the compound obtained in each step remains in the reaction solution or is obtained as a crude product and can be used in the next reaction.
- the compound obtained in each step is concentrated from the reaction mixture according to a conventional method. , Crystallization, recrystallization, distillation, solvent extraction, fractional distillation, chromatography and the like, and can be isolated and / or purified.
- the reaction time may vary depending on the reagent and solvent to be used, but unless otherwise specified, is usually 1 minute to 48 hours, preferably 10 minutes to 8 hours.
- the reaction temperature may vary depending on the reagent and solvent to be used, but is usually ⁇ 78 ° C. to 300 ° C., preferably ⁇ 78 ° C. to 150 ° C., unless otherwise specified.
- the pressure may vary depending on the reagent and solvent used, but unless otherwise specified, is usually 1 to 20 atmospheres, preferably 1 to 3 atmospheres.
- a Microwave synthesizer such as an initiator manufactured by Biotage may be used.
- the reaction temperature may vary depending on the reagent and solvent to be used, but unless otherwise specified, is usually room temperature to 300 ° C., preferably 50 ° C. to 250 ° C.
- the reaction time may vary depending on the reagent and solvent to be used, but unless otherwise specified, is usually 1 minute to 48 hours, preferably 1 minute to 8 hours.
- the reagent is used in an amount of 0.5 equivalent to 20 equivalents, preferably 0.8 equivalent to 5 equivalents, relative to the substrate.
- the reagent is used in an amount of 0.001 equivalent to 1 equivalent, preferably 0.01 equivalent to 0.2 equivalent, relative to the substrate.
- the reagent also serves as a reaction solvent, the amount of solvent is used as the reagent.
- these reactions are performed without solvent or dissolved or suspended in a suitable solvent.
- the solvent include the solvents described in the examples or the following.
- Alcohols methanol, ethanol, tert-butyl alcohol, 2-methoxyethanol, etc .
- Ethers diethyl ether, diphenyl ether, tetrahydrofuran, 1,2-dimethoxyethane, etc .
- Aromatic hydrocarbons chlorobenzene, toluene, xylene, etc .
- Saturated hydrocarbons cyclohexane, hexane, etc .
- Amides N, N-dimethylformamide, N-methylpyrrolidone, etc .
- Halogenated hydrocarbons dichloromethane, carbon tetrachloride, etc .
- Nitriles acetonitrile, etc.
- Sulfoxides dimethyl sulfoxide and the like; Aromatic organic bases: pyridine, etc .; Acid anhydrides: acetic anhydride, etc .; Organic acids: formic acid, acetic acid, trifluoroacetic acid, etc .; Inorganic acids: hydrochloric acid, sulfuric acid, etc .; Esters: ethyl acetate and the like; Ketones: acetone, methyl ethyl ketone, etc .; water. Two or more of the above solvents may be mixed and used at an appropriate ratio.
- Inorganic bases sodium hydroxide, magnesium hydroxide, etc .
- Basic salts sodium carbonate, calcium carbonate, sodium bicarbonate, etc .
- Organic bases triethylamine, diethylamine, pyridine, 4-dimethylaminopyridine, N, N-dimethylaniline, 1,4-diazabicyclo [2.2.2] octane, 1,8-diazabicyclo [5.4.0]- 7-undecene, imidazole, piperidine and the like
- Metal alkoxides sodium ethoxide, potassium tert-butoxide and the like
- Alkali metal hydrides sodium hydride, etc .
- Metal amides sodium amide, lithium diisopropylamide, lithium hexamethyldisilazide, etc .
- Organic lithiums n-butyllithium and the
- an acid or an acidic catalyst is used in the reaction in each step, for example, the following acids and acidic catalysts, or acids and acidic catalysts described in the examples are used.
- Inorganic acids hydrochloric acid, sulfuric acid, nitric acid, hydrobromic acid, phosphoric acid, etc .
- Organic acids acetic acid, trifluoroacetic acid, citric acid, p-toluenesulfonic acid, 10-camphorsulfonic acid, etc .
- Lewis acid boron trifluoride diethyl ether complex, zinc iodide, anhydrous aluminum chloride, anhydrous zinc chloride, anhydrous iron chloride and the like.
- reaction in each step is a method known per se, for example, the 5th edition Experimental Chemistry Course, Volumes 13 to 19 (Edited by The Chemical Society of Japan); New Experimental Chemistry Course, Volumes 14 to 15 (Japan) Chemistry Society); Fine Organic Chemistry Revised 2nd Edition (L.F.Tietze, Th.Eicher, Nankodo); Revised Organic Personal Name Reaction, its mechanism and points (by Hideo Togo, Kodansha); ORGANIC SYNTHES Collective Volume I-VII ( John Wiley & Sons Inc); Modern Organic Synthesis in the Laboratory A Collection of Standard Exploratory Procedures (Jie Jack Li, UF by JIE Jack Li) IVERSITY publication); Comprehensive Heterocyclic Chemistry III, Vol.
- the protection or deprotection reaction of the functional group is carried out according to a method known per se, for example, “Protective Groups in Organic Synthesis, 4th Ed.” (Theodora W. Greene, Peter G. M., et al., Wiley-Interscience). The method described in Thimeme's 2004 “Protecting Groups 3rd Ed.” (By PJ Kocienski) or the like, or the method described in the examples.
- protecting groups for hydroxyl groups such as alcohol and phenolic hydroxyl groups
- ether-type protecting groups such as methoxymethyl ether, benzyl ether, t-butyldimethylsilyl ether, and tetrahydropyranyl ether
- carboxylate-type protecting groups such as acetate Sulfonic acid ester type protecting groups such as methanesulfonic acid ester
- carbonate ester type protecting groups such as t-butyl carbonate.
- protecting group for the carbonyl group of the aldehyde include an acetal-type protecting group such as dimethylacetal; and a cyclic acetal-type protecting group such as cyclic 1,3-dioxane.
- Examples of the protecting group for the carbonyl group of the ketone include a ketal-type protecting group such as dimethyl ketal; a cyclic ketal-type protecting group such as cyclic 1,3-dioxane; an oxime-type protecting group such as O-methyloxime; N, N— And hydrazone-type protecting groups such as dimethylhydrazone.
- Examples of the protecting group for carboxyl group include ester-type protecting groups such as methyl ester; amide-type protecting groups such as N, N-dimethylamide.
- thiol-protecting group examples include ether-type protecting groups such as benzylthioether; ester-type protecting groups such as thioacetate ester, thiocarbonate, and thiocarbamate.
- protecting groups for amino groups and aromatic heterocycles such as imidazole, pyrrole and indole include carbamate-type protecting groups such as benzylcarbamate; amide-type protecting groups such as acetamide; alkylamines such as N-triphenylmethylamine Type protecting groups, and sulfonamide type protecting groups such as methanesulfonamide.
- the protecting group can be removed by a method known per se, for example, acid, base, ultraviolet light, hydrazine, phenylhydrazine, sodium N-methyldithiocarbamate, tetrabutylammonium fluoride, palladium acetate, trialkylsilyl halide (for example, trimethylsilyl iodide). And trimethylsilyl bromide) or a reduction method.
- a method known per se for example, acid, base, ultraviolet light, hydrazine, phenylhydrazine, sodium N-methyldithiocarbamate, tetrabutylammonium fluoride, palladium acetate, trialkylsilyl halide (for example, trimethylsilyl iodide). And trimethylsilyl bromide) or a reduction method.
- the reducing agent used is lithium aluminum hydride, sodium triacetoxyborohydride, sodium cyanoborohydride, diisobutylaluminum hydride (DIBAL-H), sodium borohydride
- Metal hydrides such as hydrogenated triacetoxy boron tetramethylammonium; boranes such as borane tetrahydrofuran complex; Raney nickel; Raney cobalt; hydrogen; formic acid and the like.
- a catalyst such as palladium-carbon or a Lindlar catalyst.
- the oxidizing agent used includes peracids such as m-chloroperbenzoic acid (MCPBA), hydrogen peroxide and t-butyl hydroperoxide; tetrabutylammonium perchlorate, etc.
- Perchlorates such as sodium chlorate; Chlorites such as sodium chlorite; Periodic acids such as sodium periodate; High-valent iodine reagents such as iodosylbenzene; Manganese dioxide; Reagents having manganese such as potassium manganate; Leads such as lead tetraacetate; Reagents having chromium such as pyridinium chlorochromate (PCC), pyridinium dichromate (PDC), Jones reagent; N-bromosuccinimide (NBS) Halogen compounds such as oxygen; ozone; sulfur trioxide / pyridine complex; male tetroxide Um; selenium dioxide; 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and the like.
- PCC pyridinium chlorochromate
- PDC pyridinium dichromate
- NBS N-bromosuccinimide
- the radical initiator used is an azo compound such as azobisisobutyronitrile (AIBN); 4-4′-azobis-4-cyanopentanoic acid (ACPA) Water-soluble radical initiators such as triethylboron in the presence of air or oxygen, and benzoyl peroxide.
- AIBN azobisisobutyronitrile
- ACPA 4-4′-azobis-4-cyanopentanoic acid
- Water-soluble radical initiators such as triethylboron in the presence of air or oxygen, and benzoyl peroxide.
- the radical reaction reagent used include tributylstannane, tristrimethylsilylsilane, 1,1,2,2-tetraphenyldisilane, diphenylsilane, and samarium iodide.
- Examples of Wittig reagents used include alkylidene phosphoranes.
- the alkylidene phosphoranes can be prepared by a method known per se, for example, by reacting a phosphonium salt with a strong base.
- the reagents used include phosphonoacetate esters such as methyl dimethylphosphonoacetate and ethyl ethyl diethylphosphonoacetate; bases such as alkali metal hydrides and organolithiums Can be mentioned.
- examples of the reagent used include Lewis acid and acid chloride or an alkylating agent (eg, alkyl halides, alcohols, olefins, etc.).
- an organic acid or an inorganic acid can be used in place of the Lewis acid, and an acid anhydride such as acetic anhydride can be used in place of the acid chloride.
- a nucleophile eg, amines, imidazole, etc.
- a base eg, basic salts, organic bases, etc.
- a nucleophilic addition reaction with a carbanion In each step, a nucleophilic addition reaction with a carbanion, a nucleophilic 1,4-addition reaction with a carbanion (Michael addition reaction), or a nucleophilic substitution reaction with a carbanion, a base used to generate a carbanion Examples thereof include organic lithiums, metal alkoxides, inorganic bases, and organic bases.
- examples of the Grignard reagent include arylmagnesium halides such as phenylmagnesium bromide; alkylmagnesium halides such as methylmagnesium bromide.
- the Grignard reagent can be prepared by a method known per se, for example, by reacting alkyl halide or aryl halide with metal magnesium using ether or tetrahydrofuran as a solvent.
- reagents include an active methylene compound (eg, malonic acid, diethyl malonate, malononitrile, etc.) sandwiched between two electron-withdrawing groups and a base (eg, organic bases, Metal alkoxides and inorganic bases) are used.
- active methylene compound eg, malonic acid, diethyl malonate, malononitrile, etc.
- a base eg, organic bases, Metal alkoxides and inorganic bases
- phosphoryl chloride and an amide derivative eg, N, N-dimethylformamide, etc.
- examples of the azidation agent used include diphenylphosphoryl azide (DPPA), trimethylsilyl azide, and sodium azide.
- DPPA diphenylphosphoryl azide
- DBU 1,8-diazabicyclo [5.4.0] undec-7-ene
- examples of the reducing agent used include sodium triacetoxyborohydride, sodium cyanoborohydride, hydrogen, formic acid and the like.
- examples of the carbonyl compound used include paraformaldehyde, aldehydes such as acetaldehyde, and ketones such as cyclohexanone.
- examples of amines used include primary amines such as ammonia and methylamine; secondary amines such as dimethylamine and the like.
- azodicarboxylic acid esters eg, diethyl azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD), etc.
- triphenylphosphine eg, triphenylphosphine
- the reagents used include acyl halides such as acid chloride and acid bromide; acid anhydrides, active ester compounds, and sulfate ester compounds. And activated carboxylic acids.
- carboxylic acid activators include carbodiimide condensing agents such as 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (WSCD); 4- (4,6-dimethoxy-1,3,5- Triazine condensing agents such as triazin-2-yl) -4-methylmorpholinium chloride-n-hydrate (DMT-MM); carbonate condensing agents such as 1,1-carbonyldiimidazole (CDI); diphenyl Azide phosphate (DPPA); benzotriazol-1-yloxy-trisdimethylaminophosphonium salt (BOP reagent); 2-chloro-1-methyl-pyridinium iodide (Mukayama reagent); thionyl chloride; haloformates such as ethyl chloroformate Lower alkyl; O- (7-azabenzotriazol-1-yl) -N, N, N ′, N
- additives such as 1-hydroxybenzotriazole (HOBt), N-hydroxysuccinimide (HOSu), dimethylaminopyridine (DMAP) may be further added to the reaction.
- HOBt 1-hydroxybenzotriazole
- HOSu N-hydroxysuccinimide
- DMAP dimethylaminopyridine
- the metal catalyst used is palladium acetate (II), tetrakis (triphenylphosphine) palladium (0), dichlorobis (triphenylphosphine) palladium (II), dichlorobis (triethyl).
- Palladium compounds such as phosphine) palladium (II), tris (dibenzylideneacetone) dipalladium (0), 1,1′-bis (diphenylphosphino) ferrocenepalladium (II) chloride, palladium (II) acetate; tetrakis (tri Nickel compounds such as phenylphosphine) nickel (0); rhodium compounds such as tris (triphenylphosphine) rhodium (III) chloride; cobalt compounds; copper compounds such as copper oxide and copper iodide (I); platinum compounds and the like It is done. Furthermore, a base may be added to the reaction, and examples of such a base include inorganic bases and basic salts.
- diphosphorus pentasulfide is typically used as the thiocarbonylating agent.
- 2,4-bis (4-methoxyphenyl) is used.
- a reagent having a 1,3,2,4-dithiadiphosphetane-2,4-disulfide structure such as -1,3,2,4-dithiadiphosphetane-2,4-disulfide (Lawesson reagent) It may be used.
- halogenating agents used include N-iodosuccinimide, N-bromosuccinimide (NBS), N-chlorosuccinimide (NCS), bromine, sulfuryl chloride, etc. Is mentioned.
- the reaction can be accelerated by adding a radical initiator such as heat, light, benzoyl peroxide, or azobisisobutyronitrile to the reaction.
- the halogenating agent used is an acid halide of hydrohalic acid and an inorganic acid.
- bromination such as phosphorus chloride include 48% hydrobromic acid.
- a method of obtaining an alkyl halide from alcohol by the action of triphenylphosphine and carbon tetrachloride or carbon tetrabromide may be used.
- a method of synthesizing an alkyl halide through a two-step reaction in which an alcohol is converted into a sulfonate ester and then reacted with lithium bromide, lithium chloride, or sodium iodide may be used.
- examples of the reagent used include alkyl halides such as ethyl bromoacetate; phosphites such as triethyl phosphite and tri (isopropyl) phosphite.
- examples of the sulfonating agent used include methanesulfonyl chloride, p-toluenesulfonyl chloride, methanesulfonic acid anhydride, p-toluenesulfonic acid anhydride, and the like.
- each step when a hydrolysis reaction is performed, an acid or a base is used as a reagent.
- acid hydrolysis reaction of t-butyl ester is performed, formic acid or triethylsilane may be added in order to reductively trap the t-butyl cation produced as a by-product.
- examples of the dehydrating agent used include sulfuric acid, diphosphorus pentoxide, phosphorus oxychloride, N, N'-dicyclohexylcarbodiimide, alumina, and polyphosphoric acid.
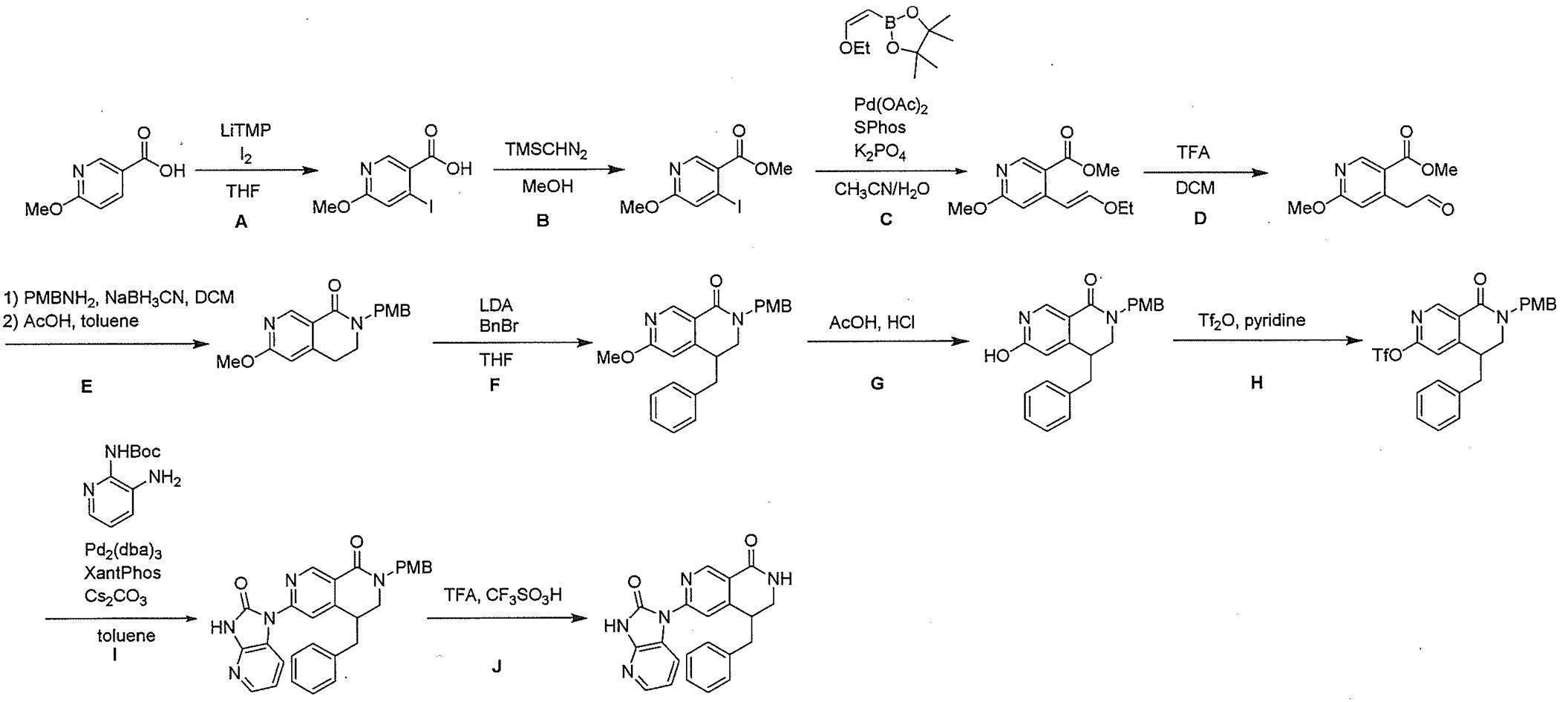
- Compound (I) can be produced by the method shown in the following scheme A or a method analogous thereto or the method described in Examples when X 4 is N and X 5 is a bond.
- LG represents a leaving group, and other symbols are as defined above.
- a leaving group represented by LG for example, a halogen atom (for example, a chlorine atom, a bromine atom, an iodine atom, etc.), an optionally substituted sulfonyloxy group (for example, 1 to 3 halogen atoms are substituted).
- a halogen atom for example, a chlorine atom, a bromine atom, an iodine atom, etc.
- an optionally substituted sulfonyloxy group for example, 1 to 3 halogen atoms are substituted.
- which may be C 1-6 alkylsulfonyloxy group (e.g., methanesulfonyloxy group, ethanesulfonyloxy group, etc.
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- Compound (II) used as a raw material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
- Compound (I) can be prepared by the method shown in the following scheme B when X 1 is CH, X 2 is CH, X 3 is CH, X 4 is CH, X 5 is a bond and X 6 is CH 2 or It can be produced by a similar method or a method described in Examples.
- Compound (VIII) can be produced by bromination reaction of compound (VII).
- bromination reagents include N-bromosuccinimide and azobisisobutyronitrile.
- Compound (XI) can be produced by a reduction reaction of compound (X). Examples of the reducing agent include borane-tetrahydrofuran complex.
- Compound (I) can be produced by a coupling reaction of compound (XI).
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- Compound (VI) used as a starting material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
- Compound (I) is a compound represented by the following scheme C when X 1 is CH, X 2 is CH, X 3 is CH, X 4 is CH, X 5 is a bond and X 6 is CCH 3 CH 3 or It can be produced by a method according to this or the method described in the Examples.
- Compound (XV) can be produced by nucleophilic substitution reaction of compound (XIV).
- nucleophilic substitution reagent include chloroacetonitrile.
- Compound (XVI) can be produced by subjecting compound (XV) to deprotection. Examples of the deprotecting reagent include thiourea.
- Compound (I) can be produced by a coupling reaction of compound (XVIII).
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- compound (XII) used as a starting material in this method a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
- Compound (I) is prepared by the method shown in the following scheme D or when X 1 is CH, X 2 is CH, X 3 is N, X 4 is CH, X 5 is a bond and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
- Compound (XXI) can be produced by a nucleophilic addition reaction of compound (XX). Examples of the nucleophile include paramethoxybenzylamine.
- Compound (XXIV) can be produced by chlorination reaction of compound (XXIII). Examples of the chlorinating reagent include phosphorus oxychloride.
- Compound (I) can be produced by a coupling reaction of compound (XXIV).
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- XIX used as a starting material in this method, a commercially available product may be used as it is, or it can be produced by a method known per se or a method analogous thereto.
- Compound (I) is prepared by the method shown in the following scheme E or when X 1 is CH, X 2 is N, X 3 is CH, X 4 is CH, X 5 is a bond, and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
- Compound (XXVI) can be produced by iodination reaction of compound (XXV).
- the iodination reagent include lithium tetramethylpiperidide and iodine.
- Compound (I) can be produced by a coupling reaction of compound (XXXIII) and a deprotection reaction of compound (XXXIV) obtained thereby.
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- Compound (XXV) used as a starting material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
- Compound (I) is prepared by the method shown in the following scheme F or when X 1 is N, X 2 is CH, X 3 is CH, X 4 is CH, X 5 is a bond, and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
- Compound (XXXVI) can be produced by iodination reaction of compound (XXXV).
- the iodination reagent include tetramethylpiperidine, normal butyl lithium, iodine and the like.
- Compound (I) can be produced by coupling reaction of compound (XLI) and deprotection reaction of compound (XLII) obtained thereby.
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- XXXV used as a starting material in this method, a commercially available product may be used as it is, or it can be produced by a method known per se or a method analogous thereto.
- Compound (I) can be prepared by the method shown in the following scheme G when X 1 is CH, X 2 is N, X 3 is N, X 4 is CH, X 5 is a bond and X 6 is CH 2 or It can be produced by a similar method or a method described in Examples.
- Compound (XLVI) can be produced by cyclization reaction and dehydration reaction of compound (XLV).
- the reagent include S-methylisothiourea.
- Compound (I) can be produced by a coupling reaction of compound (XLVII).
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
- Compound (I) is prepared by the method shown in Scheme H below or when X 1 is CH, X 2 is CH, X 3 is CH, X 4 is N, X 5 is CH 2 and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
- Compound (XLIX) can be produced by subjecting compound (XLVIII) to an alkylation reaction.
- the alkylating reagent include benzyl bromide.
- Compound (I) can be produced by a coupling reaction of compound (XLIX).
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
- Compound (I) can be prepared by the method shown in Scheme I below or when X 1 is CH, X 2 is CH, X 3 is N, X 4 is N, X 5 is CH 2 and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
- Compound (I) can be produced by a coupling reaction of compound (LI).
- Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
- a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
- the compound (I) thus obtained can be isolated and purified by known separation and purification means, for example, concentration, concentration under reduced pressure, solvent extraction, crystallization, recrystallization, transfer dissolution, chromatography, and the like.
- the compound of the present invention obtained by each of the above production methods can be isolated and purified by known means such as concentration, concentration under reduced pressure, solvent extraction, crystallization, recrystallization, transfer dissolution, chromatography and the like.
- each raw material compound used in each of the above production methods can be isolated and purified by the same known means as described above.
- the compound (I) produced by such a method can be isolated and purified by usual separation means such as recrystallization, distillation, chromatography and the like.
- compound (I) contains optical isomers, stereoisomers, positional isomers, and rotational isomers, these are also included as compound (I), and synthetic methods and separation methods known per se (for example, , Concentration, solvent extraction, column chromatography, recrystallization, etc.), each can be obtained as a single product.
- synthetic methods and separation methods known per se for example, , Concentration, solvent extraction, column chromatography, recrystallization, etc.
- the optical isomer can be produced by a method known per se. Specifically, an optical isomer is obtained by using an optically active synthetic intermediate or by optically resolving the final racemate according to a conventional method.
- a method known per se for example, fractional recrystallization method, chiral column method, diastereomer method and the like are used.
- Racemate and optically active compound for example, (+)-mandelic acid, ( ⁇ )-mandelic acid, (+)-tartaric acid, ( ⁇ )-tartaric acid, (+)-1-phenethylamine, (-)-1-phenethylamine, cinchonine, (-)-cinchonidine, brucine, etc.
- Racemate and optically active compound for example, (+)-mandelic acid, ( ⁇ )-mandelic acid, (+)-tartaric acid, ( ⁇ )-tartaric acid, (+)-1-phenethylamine, (-)-1-phenethylamine, cinchonine, (-)-cinchonidine, brucine, etc.
- Method 2) Chiral column method A method in which a racemate or a salt thereof is separated by applying to an optical isomer separation column (chiral column).
- a mixture of optical isomers is added to a chiral column such as ENANTIO-OVM (manufactured by Tosoh Corporation) or CHIRAL series (manufactured by Daicel Corporation), and water, various buffer solutions (eg, phosphate buffer) are added.
- buffer solutions eg, phosphate buffer
- an organic solvent eg, ethanol, methanol, isopropanol, acetonitrile, trifluoroacetic acid, diethylamine, etc.
- Diastereomer method A mixture of racemates is converted into a mixture of diastereomers by chemical reaction with an optically active reagent, and this is converted into a single substance through ordinary separation means (for example, fractional recrystallization, chromatography method, etc.). And then obtaining an optical isomer by separating the optically active reagent site by chemical treatment such as hydrolysis reaction.
- the compound (I) when the compound (I) has a hydroxy or a primary or secondary amino in the molecule, the compound and an optically active organic acid (for example, MTPA [ ⁇ -methoxy- ⁇ - (trifluoromethyl) phenylacetic acid], ( -)-Menthoxyacetic acid etc.) are subjected to a condensation reaction to obtain ester or amide diastereomers, respectively.
- an amide or ester diastereomer is obtained by subjecting the compound and an optically active amine or alcohol reagent to a condensation reaction. The separated diastereomer is converted into the optical isomer of the original compound by subjecting it to an acid hydrolysis or basic hydrolysis reaction.
- Compound (I) may be a crystal. Crystals of compound (I) can be produced by applying crystallization methods known per se to compound (I) for crystallization.
- the crystallization method include a crystallization method from a solution, a crystallization method from a vapor, a crystallization method from a melt, and the like.
- the “crystallization from solution” includes a state in which the compound is not saturated by changing factors related to the solubility of the compound (solvent composition, pH, temperature, ionic strength, redox state, etc.) or the amount of the solvent.
- a method of shifting from a supersaturated state to a supersaturated state is exemplified, and specific examples include a concentration method, a slow cooling method, a reaction method (diffusion method, electrolysis method), a hydrothermal growth method, and a flux method.
- solvent used examples include aromatic hydrocarbons (eg, benzene, toluene, xylene, etc.), halogenated hydrocarbons (eg, dichloromethane, chloroform, etc.), saturated hydrocarbons (eg, hexane, heptane, cyclohexane).
- aromatic hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, dichloromethane, chloroform, etc.
- saturated hydrocarbons eg, hexane, heptane, cyclohexane.
- Etc. ethers
- ethers eg, diethyl ether, diisopropyl ether, tetrahydrofuran, 1,4-dioxane, etc.
- nitriles eg, acetonitrile, etc.
- ketones eg, acetone, etc.
- sulfoxides eg, dimethyl sulfoxide, etc.
- Acid amides eg, N, N-dimethylformamide, etc.
- esters eg, ethyl acetate, etc.
- alcohols eg, methanol, ethanol, isopropyl alcohol, etc.
- water and the like eg, water and the like.
- solvents may be used alone or in admixture of two or more at an appropriate ratio (eg, 1: 1 to 1: 100 (volume ratio)).
- a seed crystal can also be used as needed.
- Examples of the “crystallization method from vapor” include a vaporization method (sealed tube method, air flow method), a gas phase reaction method, a chemical transport method, and the like.
- crystallization from the melt examples include normal freezing method (pulling method, temperature gradient method, Bridgman method), zone melting method (zone leveling method, float zone method), special growth method (VLS method). , Liquid phase epitaxy method) and the like.
- compound (I) is dissolved in a suitable solvent (eg, alcohol such as methanol, ethanol, etc.) at a temperature of 20 to 120 ° C., and the resulting solution is dissolved.
- a suitable solvent eg, alcohol such as methanol, ethanol, etc.
- Examples thereof include a method of cooling to a temperature or lower (for example, 0 to 50 ° C., preferably 0 to 20 ° C.).
- the crystals of the present invention thus obtained can be isolated by, for example, filtration.
- a method for analyzing the obtained crystal a crystal analysis method by powder X-ray diffraction is generally used.
- examples of the method for determining the crystal orientation include a mechanical method and an optical method.
- crystal of the present invention has high purity, high quality, low hygroscopicity, and is stored for a long time under normal conditions. Is very stable. In addition, it has excellent biological properties (eg, pharmacokinetics (absorbability, distribution, metabolism, excretion), expression of drug efficacy, etc.) and is extremely useful as a medicine.
- the specific optical rotation ([ ⁇ ] D ) is a ratio measured using, for example, an optical polarimeter (JASCO, P-1030 polarimeter (No. AP-2)) or the like.
- the melting point means a melting point measured using, for example, a trace melting point measuring device (Yanako, MP-500D type) or a DSC (Differential Scanning Calorimetry) apparatus (SEIKO, EXSTAR6000).
- Compound (I) may be used as a prodrug.
- a prodrug of compound (I) is a compound that is converted to compound (I) by a reaction with an enzyme, gastric acid, or the like under physiological conditions in vivo, that is, compound (I) that is enzymatically oxidized, reduced, hydrolyzed, etc.
- a compound in which amino of compound (I) is acylated, alkylated or phosphorylated for example, amino of compound (I) is eicosanoylated, alanylated, pentylaminocarbonylated, (5-methyl-2- Oxo-1,3-dioxolen-4-yl) methoxycarbonylation, tetrahydrofuranylation, pyrrolidylmethylation, pivaloyloxymethylation, tert-butylation, ethoxycarbonylation, tert-butoxycarbonylation, acetylation, Cyclopropylcarbonylated compounds, etc.); (2) Compound in which hydroxy of compound (I) is acylated, alkylated, phosphorylated, borated (for example, hydroxy of compound (I) is acetylated, palmitoylated, propanoylated, pivaloylated, succiny
- compound (I) and prodrugs thereof may be collectively abbreviated as “the compound of the present invention”.
- Compound (I) may be a hydrate, a non-hydrate, a solvate, or a non-solvate.
- Compounds labeled with isotopes eg, 3 H, 14 C, 35 S, 125 I, etc.
- a deuterium converter obtained by converting 1 H into 2 H (D) is also encompassed in compound (I).
- Tautomers are also encompassed in compound (I).
- Compound (I) may be a pharmaceutically acceptable cocrystal or cocrystal salt.
- co-crystals or co-crystal salts are two or more unique at room temperature, each having different physical properties (eg structure, melting point, heat of fusion, hygroscopicity, solubility and stability). It means a crystalline substance composed of a simple solid.
- the cocrystal or cocrystal salt can be produced according to a cocrystallization method known per se.
- Compound (I) may be used as a PET tracer.
- the compound of the present invention has an excellent PKC (particularly PKC- ⁇ ) inhibitory action, it is also useful as a safe pharmaceutical based on this action.
- the medicament of the present invention comprising the compound of the present invention is a PKC (in particular, a mouse, rat, hamster, rabbit, cat, dog, cow, sheep, monkey, human etc.). It can be used as a prophylactic or therapeutic agent for PKC- ⁇ ) -related diseases and T cell-related diseases, more specifically, as prophylactic or therapeutic agents for the diseases described in (1) to (5) below.
- Inflammatory diseases eg, acute pancreatitis, chronic pancreatitis, asthma, adult respiratory distress syndrome, chronic obstructive pulmonary disease (COPD), inflammatory bone disease, inflammatory lung disease, inflammatory bowel disease, celiac disease, hepatitis Systemic inflammatory response syndrome (SIRS), inflammation after surgery or trauma, pneumonia, nephritis, meningitis, cystitis, sore throat, gastric mucosal damage, meningitis, spondylitis, arthritis, dermatitis, chronic pneumonia , Bronchitis, pulmonary infarction, silicosis, pulmonary sarcoidosis, diabetic nephropathy, uveitis, purulent dysplasia, ventilation, etc.); (2) Immune disease (eg, rheumatoid arthritis), psoriasis (psoriasis), inflammatory bowel disease (eg, Crohn's disease, ulcerative colitis, etc.) , Sjogren's
- the medicament of the present invention is preferably an immune disease, inflammatory disease, bone or joint degenerative disease, central disease or neoplastic disease, more preferably graft-versus-host disease, aplasticity Anemia (aplastic ⁇ anemia), systemic lupus erythematosus, lupus nephritis, pemphigus, inflammatory bowel disease (preferably Crohn's disease) or ulcerative Colitis (ulcerative colitis), erythroblast ⁇ (pure red cell aplasia), myasthenia Gravis, asthma, vasculitis, ankylosing spondylitis (spondylarthritis ankylopoietica), rheumatoid arthritis (Rheumatoid arthritis), psoriasis (psoriasis), Sjogren's syndrome (Sjogren's syndrome), atopic dermatitis, Behcet's disease (Beh cet's syndrome, multiple sclerosis, Alzheimer's disease (
- prevention of the disease refers to, for example, a patient who has not developed the disease, which is expected to have a high risk of onset due to some factor related to the disease, or who has developed the subjective symptom. This means that a drug containing the compound of the present invention is administered to a patient who is not, or that a drug containing the compound of the present invention is administered to a patient who is concerned about recurrence of the disease after treatment of the disease.
- the medicament of the present invention has excellent pharmacokinetics (eg, blood drug half-life), low toxicity (eg, HERG inhibition, CYP inhibition, CYP induction), and reduction in drug interaction is observed.
- the compound of the present invention is mixed with a pharmacologically acceptable carrier as it is or according to a method known per se generally used in the preparation of pharmaceutical preparations to form a pharmaceutical composition, which is used as the pharmaceutical of the present invention. be able to.
- the medicament of the present invention is given orally or parenterally to mammals (eg, humans, monkeys, cows, horses, pigs, mice, rats, hamsters, rabbits, cats, dogs, sheep, goats, etc.). Safe to administer.
- the medicament containing the compound of the present invention is a pharmacologically acceptable compound of the present compound alone or with the compound of the present invention according to a method known per se as a method for producing a pharmaceutical preparation (eg, a method described in the Japanese Pharmacopoeia). It can be used as a pharmaceutical composition mixed with a carrier to be prepared.
- a method for producing a pharmaceutical preparation eg, a method described in the Japanese Pharmacopoeia
- It can be used as a pharmaceutical composition mixed with a carrier to be prepared.
- examples of the medicament containing the compound of the present invention include tablets (including sugar-coated tablets, film-coated tablets, sublingual tablets, orally disintegrating tablets, buccal tablets, etc.), pills, powders, granules, capsules (soft capsules, microcapsules).
- the content of the compound of the present invention in the medicament of the present invention is about 0.01% to about 100% by weight of the whole medicament.
- the dose of the compound of the present invention varies depending on the administration subject, administration route, disease and the like.
- the active ingredient (compound (I ) Of about 0.01 mg / kg body weight to about 500 mg / kg body weight, preferably about 0.1 mg / kg body weight to about 50 mg / kg body weight, more preferably about 1 mg / kg body weight to 30 mg / kg body weight. It may be administered once to several times a day.
- Examples of the pharmacologically acceptable carrier that may be used in the production of the medicament of the present invention include various organic or inorganic carrier substances commonly used as pharmaceutical materials.
- excipients and lubricants in solid preparations Binders and disintegrants, solvents in liquid preparations, solubilizers, suspending agents, tonicity agents, buffers and soothing agents.
- additives such as conventional preservatives, antioxidants, colorants, sweeteners, adsorbents, wetting agents and the like can be used in appropriate amounts.
- excipient examples include lactose, sucrose, D-mannitol, starch, corn starch, crystalline cellulose, light anhydrous silicic acid and the like.
- lubricant examples include magnesium stearate, calcium stearate, talc, colloidal silica and the like.
- binder examples include crystalline cellulose, sucrose, D-mannitol, dextrin, hydroxypropylcellulose, hydroxypropylmethylcellulose, polyvinylpyrrolidone, starch, sucrose, gelatin, methylcellulose, sodium carboxymethylcellulose and the like.
- Examples of the disintegrant include starch, carboxymethyl cellulose, carboxymethyl cellulose calcium, carboxymethyl starch sodium, L-hydroxypropyl cellulose, and the like.
- Examples of the solvent include water for injection, alcohol, propylene glycol, macrogol, sesame oil, corn oil, olive oil and the like.
- Examples of the solubilizer include polyethylene glycol, propylene glycol, D-mannitol, benzyl benzoate, ethanol, trisaminomethane, cholesterol, triethanolamine, sodium carbonate, sodium citrate and the like.
- suspending agent examples include surfactants such as stearyltriethanolamine, sodium lauryl sulfate, laurylaminopropionic acid, lecithin, benzalkonium chloride, benzethonium chloride, and glyceryl monostearate; And hydrophilic polymers such as sodium carboxymethylcellulose, methylcellulose, hydroxymethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, and the like.
- surfactants such as stearyltriethanolamine, sodium lauryl sulfate, laurylaminopropionic acid, lecithin, benzalkonium chloride, benzethonium chloride, and glyceryl monostearate
- hydrophilic polymers such as sodium carboxymethylcellulose, methylcellulose, hydroxymethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, and the like.
- Examples of the isotonic agent include glucose, D-sorbitol, sodium chloride, glycerin, D-mannitol and the like.
- Examples of the buffer include buffer solutions of phosphate, acetate, carbonate, citrate and the like.
- Examples of soothing agents include benzyl alcohol.
- Examples of the preservative include p-hydroxybenzoates, chlorobutanol, benzyl alcohol, phenylethyl alcohol, dehydroacetic acid, sorbic acid and the like.
- Examples of the antioxidant include sulfite, ascorbic acid, ⁇ -tocopherol and the like.
- the compound of the present invention can be used together with other drugs.
- the pharmaceutical used when the compound of the present invention is used in combination with another drug is referred to as “the combination agent of the present invention”.
- the compound of the present invention can be used in combination with the following drugs.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) (I) Classic NSAIDs Arcofenac, aceclofenac, sulindac, tolmetine, etodolac, fenoprofen, thiaprofenic acid, meclofenamic acid, meloxicam, teoxicam, lornoxicam, nabumetone, acetaminophen, phenacetin, ethenamide, sulpyrine, antipyrine, migrenin, aspirin, fefenamic acid, mefenamic acid Diclofenac sodium, loxoprofen sodium, phenylbutazone, indomethacin, ibuprofen, ketoprofen, naproxen, oxaprozin, flurbiprofen, fenbufen, pranoprofen, fructaphenine, piroxicam, epilisol, thiaramide hydrochloride, zal
- cyclooxygenase inhibitors COX-1 selective inhibitors, COX-2 selective inhibitors, etc.
- Salicylic acid derivatives eg, celecoxib, aspirin
- etoroxib etoroxib
- valdecoxib diclofenac
- indomethacin loxoprofen
- Nitric oxide free NSAIDs Iv) JAK inhibitors Tofacitinib (Tofacitinib), Ruxolitinib (Ruxolitinib) and the like.
- Anti-cytokine drug protein preparation
- TNF inhibitor etanercept TNF inhibitor etanercept, infliximab, adalimumab, certolizumab Pegor, golimumab, PASSTNF- ⁇ , soluble TNF- ⁇ receptor, TNF- ⁇ binding protein, anti-TNF- ⁇ antibody etc.
- Interleukin-1 inhibitor Anakinra interleukin-1 receptor antagonist
- soluble interleukin-1 receptor and the like.
- Interleukin-6 inhibitor Tocilizumab anti-interleukin-6 receptor antibody
- Iv Interleukin-10 drug Interleukin-10 and the like.
- V Interleukin-12 / 23 inhibitor Ustekinumab, briakinumab (anti-interleukin-12 / 23 antibody) and the like.
- II Non-protein preparation
- Ii Gene regulators Inhibitors of molecules related to signal transduction such as NF- ⁇ , NF- ⁇ B, IKK-1, IKK-2, AP-1.
- Iv TNF- ⁇ converting enzyme inhibitor
- VX-765 interleukin-1 ⁇ converting enzyme inhibitor
- Vi Interleukin-6 antagonist HMPL-004 and the like.
- Interleukin-8 inhibitor IL-8 antagonist Interleukin-8 inhibitor IL-8 antagonist, CXCR1 & CXCR2 antagonist, reparexin and the like.
- Chemokine antagonist CCR9 antagonist CCX-282, CCX-025), MCP-1 antagonist and the like.
- Ix Interleukin-2 receptor antagonist Denileukine, Defuchitox and the like.
- Therapeutic vaccines TNF- ⁇ vaccine and the like.
- Gene therapy drug Gene therapy drug for enhancing expression of genes having anti-inflammatory activity such as interleukin-4, interleukin-10, soluble interleukin-1 receptor, soluble TNF- ⁇ receptor .
- Immunomodulatory drugs immunosuppressive drugs
- Steroid drugs Dexamethasone, hexestrol, methimazole, betamethasone, triamcinolone, triamcinolone acetonide, fluocinonide, fluocinolone acetonide, prednisolone, methylprednisolone, cortisone acetate, hydrocortisone, fluorometholone, estriol propionate, estriol etc.
- Angiotensin converting enzyme inhibitor enalapril, captopril, ramipril, lisinopril, cilazapril, perindopril and the like.
- Angiotensin II receptor antagonist candesartan, cilexetil (TCV-116), valsartan, irbesartan, olmesartan, eprosartan and the like.
- (11) ⁇ receptor antagonist carvedilol, metoprolol, atenolol and the like.
- Antiplatelet drugs anticoagulants heparin, aspirin, warfarin and the like.
- HMG-CoA reductase inhibitor atorvastatin atorvastatin, simvastatin and the like.
- Contraceptives Sex hormones or derivatives thereof Progesterone or derivatives thereof (progesterone, 17 ⁇ -hydroxyprogesterone, medroxyprogesterone, medroxyprogesterone acetate, norethisterone, norethisterone enanthate, norethindrone, norethindrone acetate, norethinodrel, levonorgestrel , Norgestrel, etinodiol diacetate, desogestrel, norgestimate, guestden, progestin, etonogestrel, drospirenone, dienogest, trimegestone, nestron, chromadianone acetate, mifepristone, nomegestrol acetate, Org-30659, TX-525, EMM-310525) or Progesterone or its derivative and follicular hormone or its derivative (Ladiol, estradiol benzoate,
- T cell inhibitor Inosine monophosphate dehydrogenase (IMPDH) inhibitor Mycophenolate mofetil and the like.
- thalidomide v) cathepsin inhibitor
- MMPs matrix metalloprotease
- V-85546 Glucose-6-phosphate dehydrogenase inhibitor
- DHODH Dihydrorotate dehydrogenase
- PDEIV Phosphodiesterase IV
- X Phospholipase A2 inhibitor
- xi iNOS inhibitor VAS-203 and the like.
- Xii Microtubule stimulant paclitaxel and the like.
- Xiii Microtubule inhibitor Rheumacon and the like.
- Xiv MHC class II antagonist
- xv Prostacyclin agonist iloprost and the like.
- CD4 antagonist zanolimumab and the like.
- Xvii CD23 antagonist
- xviii LTB4 receptor antagonist DW-1305 and the like.
- Xix 5-lipoxygenase inhibitor zileuton and the like.
- Xx Cholinesterase inhibitor galantamine and the like.
- Glucosamine sulfate (xxxi) Amiprirose (xxxi) CD-20 inhibitor Rituximab, ibritumomab, tositumomab, ofatumuma and the like. (Xxxii) BAFF inhibitor belimumab, tabalumab, atacicept, A-623 and the like. (Xxxiii) CD52 inhibitor alemtuzumab and the like. (Xxxiv) IL-17 inhibitor secukinumab (AIN-457), LY-2439821, AMG827 and the like. (Xxxv) PDE4 inhibitor Roflumilast, Apremilast.
- concomitant drugs other than the above include, for example, antibacterial drugs, antifungal drugs, antiprotozoal drugs, antibiotics, antitussives and expectorants, sedatives, anesthetics, antiulcer drugs, antiarrhythmic drugs, antihypertensive diuretics, anticoagulants Drugs, tranquilizers, antipsychotics, antitumor drugs, antihyperlipidemic drugs, muscle relaxants, antiepileptic drugs, antidepressants, antiallergic drugs, cardiotonic drugs, antiarrhythmic drugs, vasodilators, vasoconstriction Drugs, antihypertensive diuretics, antidiabetics, narcotic antagonists, vitamins, vitamin derivatives, anti-asthma, frequent urinary / urinary incontinence, antidiarrheal, atopic dermatitis, allergic rhinitis, pressurization
- examples include drugs, endotoxin antagonists or antibodies, signal transduction inhibitors, inflammatory mediator action inhibitor
- Antibacterial drugs Sulfa drugs Sulfamethizol, sulfisoxazole, sulfamonomethoxine, sulfamethizol, salazosulfapyridine, sulfadiazine silver and the like.
- Quinoline antibacterial agents Nalidixic acid, pipemidic acid trihydrate, enoxacin, norfloxacin, ofloxacin, tosufloxacin tosylate, ciprofloxacin hydrochloride, lomefloxacin hydrochloride, sparfloxacin, fleroxacin and the like.
- Antituberculosis drugs Isoniazid, ethambutol (ethambutol hydrochloride), paraaminosalicylic acid (calcium paraaminosalicylate), pyrazinamide, etionamide, prothionamide, rifampicin, streptomycin sulfate, kanamycin sulfate, cycloserine and the like.
- Mycobacterial drugs Diaphenylsulfone, rifampicillin and the like.
- Antiviral drugs idoxuridine, acyclovir, vitarabine, ganciclovir, foscarnet and the like.
- Anti-HIV drugs zidovudine, didanosine, zarcitabine, indinavir sulfate ethanol adduct, ritonavir and the like.
- Antispirocheta drugs (viii) Antibiotics Tetracycline hydrochloride, ampicillin, piperacillin, gentamicin, dibekacin, cannendomycin, libidomycin, tobramycin, amikacin, fradiomycin, sisomycin, tetracycline, oxytetracycline, loritetracycline, doxycycline, doxycycline, doxycycline Piperacillin, ticarcillin, cephalothin, cefapirin, cephaloridine, cefaclor, cephalexin, cefloxazine, cefadroxyl, cefamandol, cephoam, cefuroxime, cefothium, cefothium hexetyl
- azole compounds [2-[(1R, 2R) -2- (2,4-difluorophenyl) -2-hydroxy- 1-methyl-3- (1H-1,2,4-triazol-1-yl) propyl] -4- [4- (2,2,3,3-tetrafluoro Propoxy) phenyl] -3- (2H, 4H) -1,2,4- triazolone, fluconazole, itraconazole, imipenem, meropenem, trimethoprim, sulfamethoxazole, etc.] and the like.
- Antifungal drugs Polyethylene antibiotics (eg, amphotericin B, nystatin, tricomycin) (Ii) griseofulvin, pyrrolnitrin, etc. (iii) cytosine antimetabolite (eg, flucytosine) (Iv) Imidazole derivatives (eg, econazole, clotrimazole, miconazole nitrate, bifonazole, croconazole) (V) Triazole derivatives (eg, fluconazole, itraconazole) (Vi) thiocarbamic acid derivatives (eg, trinaphthol) and the like.
- Polyethylene antibiotics eg, amphotericin B, nystatin, tricomycin
- cytosine antimetabolite eg, flucytosine
- Iv Imidazole derivatives (eg, econazole, clotrimazole, miconazole nitrate,
- Ephedrine hydrochloride noscapine hydrochloride, codeine phosphate, dihydrocodeine phosphate, isoproterenol hydrochloride, ephedrine hydrochloride, methylephedrine hydrochloride, noscapine hydrochloride, aloclamide, chlorfedianol, picoperidamine, cloperastine, protochlorol , Isoproterenol, salbutamol, tereptaline, oxypetebanol, morphine hydrochloride, dextropetrphan hydrobromide, oxycodone hydrochloride, dimorphan phosphate, tipipedin hibenzate, pentoxyberine citrate, clofedanol hydrochloride, benzonate, guaifenesin, Bromhexine hydrochloride, ambroxol hydrochloride, acetylcysteine,
- Anesthetic (6-1) Local anesthetic Cocaine hydrochloride, procaine hydrochloride, lidocaine, dibucaine hydrochloride, tetracaine hydrochloride, mepivacaine hydrochloride, bupivacaine hydrochloride, oxybuprocaine hydrochloride, ethyl aminobenzoate, oxesazein and the like.
- Anti-ulcer drugs Histidine hydrochloride, lansoprazole, metoclopramide, pirenzepine, cimetidine, ranitidine, famotidine, urogastrin, oxesasein, proglumide, omeprazole, sucralfate, sulpiride, cetraxate, gefarnate, aldioxa, tepregone, prostaglandin, etc.
- Arrhythmia drug (i) Sodium channel blocker (eg, quinidine, procainamide, disopyramide, azimarin, lidocaine, mexiletine, phenytoin), (Ii) ⁇ -blockers (eg, propranolol, alprenolol, bufetrol, hydrochloride, oxprenolol, atenolol, acebutolol, metoprolol, bisoprolol, pindolol, carteolol, arotinolol hydrochloride), (Iii) potassium channel blockers (eg, amiodarone), (Iv) Calcium channel blockers (eg, verapamil, diltiazem) and the like.
- Sodium channel blocker eg, quinidine, procainamide, disopyramide, azimarin, lidocaine, mexiletine, phenytoin
- ⁇ -blockers eg
- Muscle relaxants Pridinol, tubocurarine, pancuronium, tolperisone hydrochloride, chlorphenesin carbamate, baclofen, chlormezanone, mephenesin, clozoxazone, eperisone, tizanidine and the like.
- Antiepileptic drugs Phenytoin, ethosuximide, acetazolamide, chlordiazepoxide, tripetadione, carbamazepine, phenobarbital, primidone, sultiam, sodium valproate, clonazepam, diazepam, nitrazepam and the like.
- Antiallergic drugs diphenhydramine, chlorpheniramine, tripelenamine, methodiramine, clemizole, diphenylpyraline, methoxyphenamine, cromoglycate sodium, tranilast, repirinast, amlexanox, ibudilast, ketotifen, terfenadine, mequitazine, azelastine hydrochloride, epinastine hydrochloride , Pranlukast hydrate, seratrodast, etc.
- Cardiotonic drugs Transbioxocamphor, telephilol, aminophylline, ethylephrine, dopamine, dobutamine, denopamine, vesinaline, amrinone, pimobendan, ubidecarenone, digitoxin, digoxin, methyldigoxin, lanatoside C, G-strophantin and the like.
- Vasodilators Oxyfedrine, diltiazem, trazoline, hexobenzine, bamethane, clonidine, methyldopa, guanabenz and the like.
- Vasoconstricting agents dopamine, dobutamine denopamine and the like.
- Antihypertensive diuretics Hexamethonium bromide, pentolinium, mecamylamine, ecarazine, clonidine, diltiazem, nifedipine and the like.
- Antidiabetic drugs Tolbutamide, chlorpropamide, acetohexamide, glibenclamide, tolazamide, acarbose, epalrestat, troglitazone, glucagon, grimidine, glipzide, phenformin, pformin, metformin, and the like.
- narcotic antagonists levalorphan, nalolphine, naloxone or a salt thereof.
- Vitamin A Vitamin A1, Vitamin A2 and Retinol palmitate
- Vitamin D Vitamin D1, D2, D3, D4 and D5
- Vitamin E ⁇ -tocopherol, ⁇ -tocopherol, ⁇ -tocopherol, dl- ⁇ -tocopherol nicotinate
- Vitamin K vitamins K1, K2, K3 and K4
- Folic acid vitamin M
- Vitamin derivatives Various vitamin derivatives, for example, vitamin D3 derivatives such as 5,6-trans-cholecalciferol, 2,5-hydroxycholecalciferol, 1- ⁇ -hydroxycholecalciferol, 5,6-trans -Vitamin D2 derivatives such as ergocalciferol.
- vitamin D3 derivatives such as 5,6-trans-cholecalciferol, 2,5-hydroxycholecalciferol, 1- ⁇ -hydroxycholecalciferol, 5,6-trans -Vitamin D2 derivatives such as ergocalciferol.
- Anti-asthma drugs Isoprenaline hydrochloride, salbutamol sulfate, procaterol hydrochloride, terbutaline sulfate, trimethoquinol hydrochloride, tulobuterol hydrochloride, orciprenaline sulfate, fenoterol hydrobromide, ephedrine hydrochloride, iprotropium bromide, oxitropium bromide, bromide Flutropium, theophylline, aminophylline, sodium cromoglycate, tranilast, repirinast, amlexanone, ibudilast, ketotifen, terfenadine, mequitazine, azelastine, epinastine, ozagrel hydrochloride, pranlukast hydrate, seratrodast, dexamethasone, prednisolone hydrocolicone , Beclomethasone prop
- Atopic dermatitis therapeutic agent sodium cromoglycate and the like.
- Allergic rhinitis therapeutic agent Sodium cromoglycate, chlorpheniramine maleate, alimemazine tartrate, clemastine fumarate, homochlorcyclidine hydrochloride, fexofenadine, mequitazine and the like.
- the administration time of the compound of the present invention and the concomitant drug is not limited, and the compound of the present invention and the concomitant drug may be administered to the administration subject at the same time or may be administered with a time difference.
- the dose of the concomitant drug may be determined according to the dose used clinically, and can be appropriately selected depending on the administration subject, administration route, disease, combination and the like.
- the administration form of the combination is not particularly limited as long as the compound of the present invention and the concomitant drug are combined at the time of administration. Examples of such administration forms include (1) administration of a single preparation obtained by simultaneously formulating the compound of the present invention and a concomitant drug, and (2) obtained by separately formulating the compound of the present invention and the concomitant drug.
- the compounding ratio of the compound of the present invention and the concomitant drug in the concomitant drug of the present invention can be appropriately selected depending on the administration subject, administration route, disease and the like.
- the content of the compound of the present invention in the concomitant drug of the present invention varies depending on the form of the preparation, but is usually about 0.01 to 100% by weight, preferably about 0.1 to 50% by weight, based on the whole preparation, More preferably, it is about 0.5 to 20% by weight.
- the content of the concomitant drug in the concomitant drug of the present invention varies depending on the form of the preparation, but is usually about 0.01 to 100% by weight, preferably about 0.1 to 50% by weight, more preferably about the whole preparation. About 0.5 to 20% by weight.
- the content of additives such as carriers in the combination agent of the present invention varies depending on the form of the preparation, but is usually about 1 to 99.99% by weight, preferably about 10 to 90% by weight, based on the whole preparation. . The same content may be used when the compound of the present invention and the concomitant drug are formulated separately.
- the dose varies depending on the type of the compound of the present invention, administration route, symptom, patient age, etc.
- 1 kg body weight per day about 0.1 mg / kg body weight to about 50 mg / kg body weight, preferably about 1 mg / kg body weight to 30 mg / kg body weight per day as a free form of compound (I) may be administered once to several times a day. Good.
- the dosage is the type and content of compound (I), the dosage form, the duration of drug release, the animal to be administered (for example, mouse, rat, hamster, guinea pig) Mammals such as rabbits, cats, dogs, cows, horses, pigs, sheep, monkeys, humans, etc.), depending on the purpose of administration, for example, about 0.1 per week when applied by parenteral administration About 100 mg of compound (I) may be released from the dosage formulation.
- the animal to be administered for example, mouse, rat, hamster, guinea pig
- Mammals such as rabbits, cats, dogs, cows, horses, pigs, sheep, monkeys, humans, etc.
- about 100 mg of compound (I) may be released from the dosage formulation.
- the amount of the concomitant drug can be set as long as side effects do not become a problem.
- the daily dose as a concomitant drug varies depending on the degree of symptoms, age of the subject, sex, weight, sensitivity difference, timing of administration, interval, nature of the pharmaceutical preparation, formulation, type, type of active ingredient, etc.
- the amount of the drug is usually about 0.001 to 2000 mg per kg body weight of the mammal by oral administration, preferably about 0.01 to 500 mg, more preferably about 0.1 to 100 mg. This is usually administered in 1 to 4 divided doses per day.
- the compound of the present invention and the concomitant drug may be administered at the same time, or may be administered with a time difference.
- the time difference varies depending on the active ingredient, dosage form, and administration method to be administered.
- the concomitant drug when administering the concomitant drug first, within 1 minute to 3 days after administering the concomitant drug, preferably Examples include a method of administering the compound of the present invention within 10 minutes to 1 day, more preferably within 15 minutes to 1 hour.
- the concomitant drug is administered within 1 minute to 1 day, preferably within 10 minutes to 6 hours, more preferably within 15 minutes to 1 hour after the administration of the compound of the present invention. Is mentioned.
- room temperature usually indicates about 10 ° C. to about 35 ° C.
- the ratio shown in the mixed solvent is a volume ratio unless otherwise specified. Unless otherwise indicated, “%” indicates “% by weight”.
- silica gel column chromatography when NH is described, aminopropylsilane-bonded silica gel, when Diol is described, 3- (2,3-dihydroxypropoxy) propylsilane-bonded silica gel, when DiNH is described, N- (2-Aminoethyl) -3-aminopropylsilane bonded silica gel was used.
- HPLC high performance liquid chromatography
- octadecyl-bonded silica gel when it was described as C18, octadecyl-bonded silica gel was used.
- the ratio of elution solvent indicates a volume ratio unless otherwise specified.
- the following abbreviations are used in the following examples.
- a peak from which H 2 O is eliminated may be observed as a fragment ion.
- a salt a free molecular ion peak or a fragment ion peak is usually observed.
- Ethyl 2-vinylnicotinate Ethyl 2-chloronicotinate (5.90 g), sodium bicarbonate (5.34 g) and 4,4,5,5-tetramethyl-2-vinyl-1,3,2-dioxaborolane ( Tetrakis (triphenylphosphine) palladium (0) (1.84 g) was added to a mixed solution of 5.14 g) of dimethoxyethane (20 mL) and water (2 mL) at room temperature. The reaction mixture was stirred at 80 ° C. under a nitrogen atmosphere overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate.
- the reaction mixture was cooled to room temperature, diluted with ethyl acetate, washed with 1M aqueous hydrochloric acid solution, saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The residue was purified by preparative liquid chromatography to give the title compound (11 mg).
- Example compounds prepared according to the above methods or methods similar thereto are shown in the following table. MS in the table indicates actual measurement.
- Test Example 1 PKC Theta Enzyme Inhibition Test
- the PKC theta enzyme inhibitory activity of the test compound was measured by the TR-FRET method. First, it was diluted with assay buffer (20 mM Tris-HCl (pH 7.5), 5 mM MgAcetate, 0.1 mM CaCl 2 , 1 mM DTT, 0.01% Tween 20, 0.05% BSA, 10% PKC Activator (Millipore)). A test compound was added in an amount of 2 ⁇ L to a 384 well plate.
- Formulation Example 1 (Manufacture of capsules) 1) 30 mg of the compound of Example 1 2) Fine powder cellulose 10 mg 3) Lactose 19 mg 4) Magnesium stearate 1 mg 60 mg total 1), 2), 3) and 4) are mixed and filled into gelatin capsules.
- Formulation Example 2 Manufacture of tablets
- the compound of the present invention has an excellent PKC inhibitory action and is useful as a preventive or therapeutic agent for immune diseases, inflammatory diseases and the like.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Provided is a compound, or a salt thereof, that has an excellent PKC inhibitory action, and that is useful as a prophylactic or therapeutic agent for immunological diseases, inflammatory diseases, etc. A compound represented by formula (I) (the symbols in the formula are as described in the Specification), or a salt thereof, has an excellent PKC inhibitory action, and is useful as a prophylactic or therapeutic agent for immunological diseases, inflammatory diseases, etc.
Description
本発明は、プロテインキナーゼC(本明細書中「PKC」と略記する場合がある)阻害作用を有し、免疫性疾患、炎症性疾患等の治療に有用な複素環化合物、それらを含有する医薬組成物などに関する。
The present invention relates to a heterocyclic compound having an inhibitory action on protein kinase C (sometimes abbreviated as “PKC” in the present specification) and useful for the treatment of immune diseases, inflammatory diseases, and the like, and a medicament containing them It relates to compositions and the like.
(発明の背景)
免疫システムにおいて、T細胞は、ウイルスや細菌などの外来抗原や癌を排除する獲得免疫機構の一部として重要な役割を果たしている。一方、過剰なT細胞の活性化は、自己免疫疾患の発症に関与することや、移植にともなう拒絶などの人体に対して有害な反応を引き起こすことが知られていることから、T細胞は、これらの疾患において有望な治療ターゲットであると考えられている(非特許文献1)。このことは、腎移植、肝移植、心移植、肺移植、膵移植、小腸移植などの臓器移植における拒絶反応、骨髄移植における拒絶反応、移植片対宿主病、ベーチェット病、尋常性乾癬、膿疱性乾癬、乾癬性紅皮症、関節症性乾癬、再生不良性貧血、赤芽球癆、ネフローゼ症候群、全身型重症筋無力症、アトピー性皮膚炎、潰瘍性大腸炎等の疾患において、T細胞の活性化を抑制する薬剤であるカルシニューリン阻害薬(TacrolimusおよびCyclosporin A)が、予防薬もしくは治療薬として日本において承認されていることからも明白である(非特許文献2および3)。
PKCファミリーは少なくとも12種類のサブタイプからなり、様々な組織で発現することが知られている。また、PKCファミリーはT細胞、B細胞やマクロファージなどの免疫担当細胞で発現し、種々の免疫反応に重要な役割を果たしていることから、免疫疾患や炎症性疾患に関与すると考えられている(非特許文献4)。
その中で、PKC-θは、T細胞に選択的に発現するリン酸化酵素であり、T細胞受容体に対する刺激の細胞内への伝達を担い、T細胞の活性化に重要な役割を果たしている。
PKC-θの阻害は、T細胞の活性化を抑制することが知られており(非特許文献5)、またPKC-θ欠損マウスは、多発性硬化症モデル、炎症性腸疾患モデル、移植片体宿主病モデル、慢性関節リウマチモデルおよび喘息モデルにおいて抵抗性であることが報告されている(非特許文献6~8)。
以上のことから、PKC(例、PKC-θ)阻害薬は、腎移植、肝移植、心移植、肺移植、膵移植、小腸移植などの臓器移植における拒絶反応、骨髄移植における拒絶反応、移植片対宿主病、ベーチェット病、尋常性乾癬、膿疱性乾癬、乾癬性紅皮症、関節症性乾癬、再生不良性貧血、赤芽球癆、ネフローゼ症候群、全身型重症筋無力症、アトピー性皮膚炎、潰瘍性大腸炎、喘息等の様々な免疫性疾患および炎症性疾患において予防薬もしくは治療薬になる。 (Background of the Invention)
In the immune system, T cells play an important role as part of an acquired immune mechanism that eliminates foreign antigens such as viruses and bacteria and cancer. On the other hand, it is known that excessive T cell activation is involved in the development of autoimmune diseases and causes adverse reactions to the human body such as rejection associated with transplantation. It is considered to be a promising therapeutic target in these diseases (Non-patent Document 1). This includes rejection in organ transplantation such as kidney transplantation, liver transplantation, heart transplantation, lung transplantation, pancreas transplantation, small intestine transplantation, rejection in bone marrow transplantation, graft-versus-host disease, Behcet's disease, psoriasis vulgaris, pustular In diseases such as psoriasis, psoriatic erythroderma, arthritic psoriasis, aplastic anemia, erythroblastosis, nephrotic syndrome, systemic myasthenia gravis, atopic dermatitis, ulcerative colitis, It is also clear from the fact that calcineurin inhibitors (Tacrolimus and Cyclosporin A), which are drugs that suppress activation, are approved in Japan as prophylactic or therapeutic agents (Non-patent Documents 2 and 3).
The PKC family consists of at least 12 types and is known to be expressed in various tissues. In addition, the PKC family is expressed in immunocompetent cells such as T cells, B cells, and macrophages and plays an important role in various immune responses, and is therefore considered to be involved in immune diseases and inflammatory diseases (non-) Patent Document 4).
Among them, PKC-θ is a phosphorylase that is selectively expressed in T cells, plays a role in the activation of T cells, is responsible for the transmission of stimuli to T cell receptors into cells. .
Inhibition of PKC-θ is known to suppress T cell activation (Non-patent Document 5), and PKC-θ-deficient mice are used in multiple sclerosis models, inflammatory bowel disease models, grafts. It has been reported to be resistant in somatic host disease models, rheumatoid arthritis models, and asthma models (Non-Patent Documents 6 to 8).
Based on the above, PKC (eg, PKC-θ) inhibitors are used for rejection in organ transplantation such as kidney transplantation, liver transplantation, heart transplantation, lung transplantation, pancreatic transplantation, small intestine transplantation, rejection reaction in bone marrow transplantation, graft Anti-host disease, Behcet's disease, psoriasis vulgaris, pustular psoriasis, erythrodermic psoriasis, psoriatic arthritis, aplastic anemia, erythroblastosis, nephrotic syndrome, systemic myasthenia gravis, atopic dermatitis It becomes a prophylactic or therapeutic agent in various immune diseases and inflammatory diseases such as ulcerative colitis and asthma.
免疫システムにおいて、T細胞は、ウイルスや細菌などの外来抗原や癌を排除する獲得免疫機構の一部として重要な役割を果たしている。一方、過剰なT細胞の活性化は、自己免疫疾患の発症に関与することや、移植にともなう拒絶などの人体に対して有害な反応を引き起こすことが知られていることから、T細胞は、これらの疾患において有望な治療ターゲットであると考えられている(非特許文献1)。このことは、腎移植、肝移植、心移植、肺移植、膵移植、小腸移植などの臓器移植における拒絶反応、骨髄移植における拒絶反応、移植片対宿主病、ベーチェット病、尋常性乾癬、膿疱性乾癬、乾癬性紅皮症、関節症性乾癬、再生不良性貧血、赤芽球癆、ネフローゼ症候群、全身型重症筋無力症、アトピー性皮膚炎、潰瘍性大腸炎等の疾患において、T細胞の活性化を抑制する薬剤であるカルシニューリン阻害薬(TacrolimusおよびCyclosporin A)が、予防薬もしくは治療薬として日本において承認されていることからも明白である(非特許文献2および3)。
PKCファミリーは少なくとも12種類のサブタイプからなり、様々な組織で発現することが知られている。また、PKCファミリーはT細胞、B細胞やマクロファージなどの免疫担当細胞で発現し、種々の免疫反応に重要な役割を果たしていることから、免疫疾患や炎症性疾患に関与すると考えられている(非特許文献4)。
その中で、PKC-θは、T細胞に選択的に発現するリン酸化酵素であり、T細胞受容体に対する刺激の細胞内への伝達を担い、T細胞の活性化に重要な役割を果たしている。
PKC-θの阻害は、T細胞の活性化を抑制することが知られており(非特許文献5)、またPKC-θ欠損マウスは、多発性硬化症モデル、炎症性腸疾患モデル、移植片体宿主病モデル、慢性関節リウマチモデルおよび喘息モデルにおいて抵抗性であることが報告されている(非特許文献6~8)。
以上のことから、PKC(例、PKC-θ)阻害薬は、腎移植、肝移植、心移植、肺移植、膵移植、小腸移植などの臓器移植における拒絶反応、骨髄移植における拒絶反応、移植片対宿主病、ベーチェット病、尋常性乾癬、膿疱性乾癬、乾癬性紅皮症、関節症性乾癬、再生不良性貧血、赤芽球癆、ネフローゼ症候群、全身型重症筋無力症、アトピー性皮膚炎、潰瘍性大腸炎、喘息等の様々な免疫性疾患および炎症性疾患において予防薬もしくは治療薬になる。 (Background of the Invention)
In the immune system, T cells play an important role as part of an acquired immune mechanism that eliminates foreign antigens such as viruses and bacteria and cancer. On the other hand, it is known that excessive T cell activation is involved in the development of autoimmune diseases and causes adverse reactions to the human body such as rejection associated with transplantation. It is considered to be a promising therapeutic target in these diseases (Non-patent Document 1). This includes rejection in organ transplantation such as kidney transplantation, liver transplantation, heart transplantation, lung transplantation, pancreas transplantation, small intestine transplantation, rejection in bone marrow transplantation, graft-versus-host disease, Behcet's disease, psoriasis vulgaris, pustular In diseases such as psoriasis, psoriatic erythroderma, arthritic psoriasis, aplastic anemia, erythroblastosis, nephrotic syndrome, systemic myasthenia gravis, atopic dermatitis, ulcerative colitis, It is also clear from the fact that calcineurin inhibitors (Tacrolimus and Cyclosporin A), which are drugs that suppress activation, are approved in Japan as prophylactic or therapeutic agents (Non-patent Documents 2 and 3).
The PKC family consists of at least 12 types and is known to be expressed in various tissues. In addition, the PKC family is expressed in immunocompetent cells such as T cells, B cells, and macrophages and plays an important role in various immune responses, and is therefore considered to be involved in immune diseases and inflammatory diseases (non-) Patent Document 4).
Among them, PKC-θ is a phosphorylase that is selectively expressed in T cells, plays a role in the activation of T cells, is responsible for the transmission of stimuli to T cell receptors into cells. .
Inhibition of PKC-θ is known to suppress T cell activation (Non-patent Document 5), and PKC-θ-deficient mice are used in multiple sclerosis models, inflammatory bowel disease models, grafts. It has been reported to be resistant in somatic host disease models, rheumatoid arthritis models, and asthma models (Non-Patent Documents 6 to 8).
Based on the above, PKC (eg, PKC-θ) inhibitors are used for rejection in organ transplantation such as kidney transplantation, liver transplantation, heart transplantation, lung transplantation, pancreatic transplantation, small intestine transplantation, rejection reaction in bone marrow transplantation, graft Anti-host disease, Behcet's disease, psoriasis vulgaris, pustular psoriasis, erythrodermic psoriasis, psoriatic arthritis, aplastic anemia, erythroblastosis, nephrotic syndrome, systemic myasthenia gravis, atopic dermatitis It becomes a prophylactic or therapeutic agent in various immune diseases and inflammatory diseases such as ulcerative colitis and asthma.
本明細書に記載の化合物と類似の構造を有する化合物としては、例えば、以下が挙げられる。
(1)下記式: Examples of the compound having a structure similar to the compound described in the present specification include the following.
(1) The following formula:
(1)下記式: Examples of the compound having a structure similar to the compound described in the present specification include the following.
(1) The following formula:
[式中、
R1は、-NR9R10-、-NR9COR9-、-S(O)nR12-等(R9、R10およびR12は、独立して、C3-6シクロアルキル、C4-7シクロアルキルアルキルを示し、nは0-2を示す)を示すか、シアノ、C1-4ハロアルキル等を示すか、またはC1-6アルキル、C3-6シクロアルキル等(各々、-NR9R10-、-NR9COR9-、-S(O)nR12-等で置換されていてもよい)を示し;
A1およびA2は、独立して、NまたはCR5を示し;
Wは、=O、=S、-Hまたは(-H、-H)を示し;
Xは、NまたはCR1を示し;
Yは、-C(RY)2-、-N-RY-等(RYはH等を示す)を示し;
R2は、H、ハロゲン、シアノ、C1-4アルキル、C3-6シクロアルキル等を示し;Bは、R3、NHR3等を示し;
R3は、C1-10アルキル等を示し;
R4は、H、-OR10、-COR9、ハロゲン、C1-6アルキル、C3-6シクロアルキル等を示し;
R5は、H、ハロゲン、C1-10アルキル、C2-10アルケニル、C3-6シクロアルキル等を示す。]
で示される化合物(特許文献1)。 [Where
R 1 is —NR 9 R 10 —, —NR 9 COR 9 —, —S (O) n R 12 — and the like (R 9 , R 10 and R 12 are each independently C 3-6 cycloalkyl, C 4-7 cycloalkylalkyl, n represents 0-2), cyano, C 1-4 haloalkyl, etc., or C 1-6 alkyl, C 3-6 cycloalkyl, etc. (each , -NR 9 R 10 -, - NR 9 COR 9 -, - indicates may be substituted with etc.) - S (O) n R 12;
A 1 and A 2 independently represent N or CR 5 ;
W represents ═O, ═S, —H or (—H, —H);
X represents N or CR 1 ;
Y represents —C (R Y ) 2 —, —N—R Y — or the like (R Y represents H or the like);
R 2 represents H, halogen, cyano, C 1-4 alkyl, C 3-6 cycloalkyl and the like; B represents R 3 , NHR 3 and the like;
R 3 represents C 1-10 alkyl or the like;
R 4 represents H, —OR 10 , —COR 9 , halogen, C 1-6 alkyl, C 3-6 cycloalkyl and the like;
R 5 represents H, halogen, C 1-10 alkyl, C 2-10 alkenyl, C 3-6 cycloalkyl or the like. ]
The compound shown by (patent document 1).
R1は、-NR9R10-、-NR9COR9-、-S(O)nR12-等(R9、R10およびR12は、独立して、C3-6シクロアルキル、C4-7シクロアルキルアルキルを示し、nは0-2を示す)を示すか、シアノ、C1-4ハロアルキル等を示すか、またはC1-6アルキル、C3-6シクロアルキル等(各々、-NR9R10-、-NR9COR9-、-S(O)nR12-等で置換されていてもよい)を示し;
A1およびA2は、独立して、NまたはCR5を示し;
Wは、=O、=S、-Hまたは(-H、-H)を示し;
Xは、NまたはCR1を示し;
Yは、-C(RY)2-、-N-RY-等(RYはH等を示す)を示し;
R2は、H、ハロゲン、シアノ、C1-4アルキル、C3-6シクロアルキル等を示し;Bは、R3、NHR3等を示し;
R3は、C1-10アルキル等を示し;
R4は、H、-OR10、-COR9、ハロゲン、C1-6アルキル、C3-6シクロアルキル等を示し;
R5は、H、ハロゲン、C1-10アルキル、C2-10アルケニル、C3-6シクロアルキル等を示す。]
で示される化合物(特許文献1)。 [Where
R 1 is —NR 9 R 10 —, —NR 9 COR 9 —, —S (O) n R 12 — and the like (R 9 , R 10 and R 12 are each independently C 3-6 cycloalkyl, C 4-7 cycloalkylalkyl, n represents 0-2), cyano, C 1-4 haloalkyl, etc., or C 1-6 alkyl, C 3-6 cycloalkyl, etc. (each , -NR 9 R 10 -, - NR 9 COR 9 -, - indicates may be substituted with etc.) - S (O) n R 12;
A 1 and A 2 independently represent N or CR 5 ;
W represents ═O, ═S, —H or (—H, —H);
X represents N or CR 1 ;
Y represents —C (R Y ) 2 —, —N—R Y — or the like (R Y represents H or the like);
R 2 represents H, halogen, cyano, C 1-4 alkyl, C 3-6 cycloalkyl and the like; B represents R 3 , NHR 3 and the like;
R 3 represents C 1-10 alkyl or the like;
R 4 represents H, —OR 10 , —COR 9 , halogen, C 1-6 alkyl, C 3-6 cycloalkyl and the like;
R 5 represents H, halogen, C 1-10 alkyl, C 2-10 alkenyl, C 3-6 cycloalkyl or the like. ]
The compound shown by (patent document 1).
(2)下記式:
(2) The following formula:
[式中、
Bは、置換されていてもよい6員芳香族複素環等を示し;
Cは、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
R1およびR2は、独立して、H、ハロゲン、-OR’(R’はH、C1-6アルキル(-OR、-N(R)2、シアノ、オキソ等で置換されていてもよい)を示す)、シアノ、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、シアノ等で置換されていてもよい)等を示す]
で示される化合物(特許文献2)。 [Where:
B represents an optionally substituted 6-membered aromatic heterocycle;
C is an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8-12 bicyclic ring (0-5 heteroatoms) Having
R 1 and R 2 are independently H, halogen, —OR ′ (R ′ may be substituted with H, C 1-6 alkyl (—OR, —N (R) 2 , cyano, oxo, etc.). And cyano, C 1-10 aliphatic compounds (which may be substituted with halogen, —OR, —N (R) 2 , cyano, etc.), etc.]
The compound shown by (patent document 2).
Bは、置換されていてもよい6員芳香族複素環等を示し;
Cは、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
R1およびR2は、独立して、H、ハロゲン、-OR’(R’はH、C1-6アルキル(-OR、-N(R)2、シアノ、オキソ等で置換されていてもよい)を示す)、シアノ、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、シアノ等で置換されていてもよい)等を示す]
で示される化合物(特許文献2)。 [Where:
B represents an optionally substituted 6-membered aromatic heterocycle;
C is an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8-12 bicyclic ring (0-5 heteroatoms) Having
R 1 and R 2 are independently H, halogen, —OR ′ (R ′ may be substituted with H, C 1-6 alkyl (—OR, —N (R) 2 , cyano, oxo, etc.). And cyano, C 1-10 aliphatic compounds (which may be substituted with halogen, —OR, —N (R) 2 , cyano, etc.), etc.]
The compound shown by (patent document 2).
(3)下記式:
(3) The following formula:
[式中、
Bは、置換されていてもよい5員芳香族複素環等を示し;
R1およびR2は、独立して、H、ハロゲン、-OR’、シアノ、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)、C3-8脂環式化合物(ハロゲン、-OR、-N(R)2、シアノ、オキソ、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)等で置換されていてもよい)等を示し;
Xは、CまたはNを示し;
RXは、存在しないかまたはHを示し;
Cは、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
Rは、HまたはC1-6アルキルを示し;
R’は、HまたはC1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示す]
で示される化合物(特許文献3)。 [Where:
B represents an optionally substituted 5-membered aromatic heterocyclic ring;
R 1 and R 2 independently represent H, halogen, —OR ′, cyano, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds (halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl (halogen, —OR, —N ( R) 2 , —C (O) R, optionally substituted with cyano, oxo, etc.)) and the like;
X represents C or N;
R X is absent or represents H;
C is an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8-12 bicyclic ring (0-5 heteroatoms) Having
R represents H or C 1-6 alkyl;
R ′ represents H or C 1-6 alkyl (which may be substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.)]
The compound shown by (patent document 3).
Bは、置換されていてもよい5員芳香族複素環等を示し;
R1およびR2は、独立して、H、ハロゲン、-OR’、シアノ、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)、C3-8脂環式化合物(ハロゲン、-OR、-N(R)2、シアノ、オキソ、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)等で置換されていてもよい)等を示し;
Xは、CまたはNを示し;
RXは、存在しないかまたはHを示し;
Cは、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
Rは、HまたはC1-6アルキルを示し;
R’は、HまたはC1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示す]
で示される化合物(特許文献3)。 [Where:
B represents an optionally substituted 5-membered aromatic heterocyclic ring;
R 1 and R 2 independently represent H, halogen, —OR ′, cyano, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds (halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl (halogen, —OR, —N ( R) 2 , —C (O) R, optionally substituted with cyano, oxo, etc.)) and the like;
X represents C or N;
R X is absent or represents H;
C is an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8-12 bicyclic ring (0-5 heteroatoms) Having
R represents H or C 1-6 alkyl;
R ′ represents H or C 1-6 alkyl (which may be substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.)]
The compound shown by (patent document 3).
(4)下記式:
(4) The following formula:
[式中、
Bは、置換されていてもよい6員芳香族複素環等を示し;
R1およびR2は、独立して、H、ハロゲン、-OR’、シアノ、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)、C3-8脂環式化合物(ハロゲン、-OR、-N(R)2、シアノ、オキソ、C1-6アルキル等で置換されていてもよい)等を示し;
R3は、存在しないか、またはH、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示し;
R4は、置換されていてもよいC1-10脂肪族化合物、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
Rは、HまたはC1-6アルキルを示し;
R’は、HまたはC1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示し;
Qは、N、OまたはSを示す]
で示される化合物(特許文献4)。 [Where:
B represents an optionally substituted 6-membered aromatic heterocycle;
R 1 and R 2 independently represent H, halogen, —OR ′, cyano, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds ( optionally substituted with halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl, etc.) Etc .;
R 3 is absent or represents H, C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.) ;
R 4 is an optionally substituted C 1-10 aliphatic compound, an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8 Represents a -12 bicyclic ring (having 0-5 heteroatoms);
R represents H or C 1-6 alkyl;
R ′ represents H or C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.);
Q represents N, O or S]
The compound shown by (patent document 4).
Bは、置換されていてもよい6員芳香族複素環等を示し;
R1およびR2は、独立して、H、ハロゲン、-OR’、シアノ、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)、C3-8脂環式化合物(ハロゲン、-OR、-N(R)2、シアノ、オキソ、C1-6アルキル等で置換されていてもよい)等を示し;
R3は、存在しないか、またはH、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示し;
R4は、置換されていてもよいC1-10脂肪族化合物、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
Rは、HまたはC1-6アルキルを示し;
R’は、HまたはC1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示し;
Qは、N、OまたはSを示す]
で示される化合物(特許文献4)。 [Where:
B represents an optionally substituted 6-membered aromatic heterocycle;
R 1 and R 2 independently represent H, halogen, —OR ′, cyano, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds ( optionally substituted with halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl, etc.) Etc .;
R 3 is absent or represents H, C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.) ;
R 4 is an optionally substituted C 1-10 aliphatic compound, an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8 Represents a -12 bicyclic ring (having 0-5 heteroatoms);
R represents H or C 1-6 alkyl;
R ′ represents H or C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.);
Q represents N, O or S]
The compound shown by (patent document 4).
(5)下記式:
(5) The following formula:
[式中、
Bは、置換されていてもよい5員芳香族複素環等を示し;
R1およびR2は、独立して、H、ハロゲン、シアノ、-OR’、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)、C3-8脂環式化合物(ハロゲン、-OR、-N(R)2、シアノ、オキソ、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)で置換されていてもよい)を示し;
R3は、存在しないか、または、H、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示し;
R4は、置換されていてもよいC1-10脂肪族化合物、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
Qは、N、OまたはSを示し;
RXは、存在しないかまたはHを示す]
で示される化合物(特許文献5)。 [Where:
B represents an optionally substituted 5-membered aromatic heterocyclic ring;
R 1 and R 2 independently represent H, halogen, cyano, —OR ′, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds (halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl (halogen, —OR, —N ( R) 2 , —C (O) R, optionally substituted with cyano, oxo, etc.))
R 3 is absent or is H, C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.) Show;
R 4 is an optionally substituted C 1-10 aliphatic compound, an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8 Represents a -12 bicyclic ring (having 0-5 heteroatoms);
Q represents N, O or S;
R X is absent or represents H]
The compound shown by (patent document 5).
Bは、置換されていてもよい5員芳香族複素環等を示し;
R1およびR2は、独立して、H、ハロゲン、シアノ、-OR’、C1-10脂肪族化合物(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)、C3-8脂環式化合物(ハロゲン、-OR、-N(R)2、シアノ、オキソ、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)で置換されていてもよい)を示し;
R3は、存在しないか、または、H、C1-6アルキル(ハロゲン、-OR、-N(R)2、-C(O)R、シアノ、オキソ等で置換されていてもよい)を示し;
R4は、置換されていてもよいC1-10脂肪族化合物、置換されていてもよい3-8員単環(0-3個のヘテロ原子を有する)、置換されていてもよいC8-12の二環性環(0-5個のヘテロ原子を有する)を示し;
Qは、N、OまたはSを示し;
RXは、存在しないかまたはHを示す]
で示される化合物(特許文献5)。 [Where:
B represents an optionally substituted 5-membered aromatic heterocyclic ring;
R 1 and R 2 independently represent H, halogen, cyano, —OR ′, C 1-10 aliphatic compound (halogen, —OR, —N (R) 2 , —C (O) R, cyano, Optionally substituted with oxo, etc.), C 3-8 alicyclic compounds (halogen, —OR, —N (R) 2 , cyano, oxo, C 1-6 alkyl (halogen, —OR, —N ( R) 2 , —C (O) R, optionally substituted with cyano, oxo, etc.))
R 3 is absent or is H, C 1-6 alkyl (optionally substituted with halogen, —OR, —N (R) 2 , —C (O) R, cyano, oxo, etc.) Show;
R 4 is an optionally substituted C 1-10 aliphatic compound, an optionally substituted 3-8 membered monocycle (having 0-3 heteroatoms), an optionally substituted C 8 Represents a -12 bicyclic ring (having 0-5 heteroatoms);
Q represents N, O or S;
R X is absent or represents H]
The compound shown by (patent document 5).
(6)下記式:
(6) The following formula:
[式中、
X1は、CHまたはNを示し;
X2は、CまたはNを示し;
Y1は、-(CR6aR6b)m-Z1-(CR7aR7b)n-を示し;
Y2は、-(CR8aR8b)p-Z2-(CR9aR9b)q-を示し;
Y3は、-(CR10aR10b)r-Z3-(CR11aR11b)s-を示し;
Y4は、-(CH2)t-Z4-を示し:
Z1-Z3は、独立して、結合、O、S、C(O)等を示し;
Z4は、結合、OまたはNを示し;
m、n、p、q、rおよびsは、独立して、0-6を示し;
tは、0-2を示し;
R6a-R11bは、独立して、結合、H、-OH、C1-3アルコキシまたはC1-3アルキルを示し;
R1、R2およびR3は、独立して、H、ハロゲン、アルコキシ、シアノ、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロシクロアルキル、置換されていてもよいヘテロアリールを示し;
R4は、H、O等を示し;
R5およびR13は、独立して、H、ハロゲン、OH、低級アルキル等を示し;
R14は、存在しないか、または置換されていてもよい低級シクロアルキル、置換されていてもよいフェニル等を示す]
で示される化合物(特許文献6)。 [Where:
X 1 represents CH or N;
X 2 represents C or N;
Y 1 represents — (CR 6a R 6b ) m —Z 1 — (CR 7a R 7b ) n —;
Y 2 represents — (CR 8a R 8b ) p —Z 2 — (CR 9a R 9b ) q —;
Y 3 represents — (CR 10a R 10b ) r —Z 3 — (CR 11a R 11b ) s —;
Y 4 represents — (CH 2 ) t —Z 4 —:
Z 1 -Z 3 independently represents a bond, O, S, C (O) or the like;
Z 4 represents a bond, O or N;
m, n, p, q, r and s independently represent 0-6;
t represents 0-2;
R 6a -R 11b independently represents a bond, H, —OH, C 1-3 alkoxy or C 1-3 alkyl;
R 1 , R 2 and R 3 are independently H, halogen, alkoxy, cyano, optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted heterocycloalkyl, substituted Represents optionally substituted heteroaryl;
R 4 represents H, O or the like;
R 5 and R 13 independently represent H, halogen, OH, lower alkyl, etc .;
R 14 represents a lower cycloalkyl which may be absent or substituted, a phenyl which may be substituted, etc.]
The compound shown by (patent document 6).
X1は、CHまたはNを示し;
X2は、CまたはNを示し;
Y1は、-(CR6aR6b)m-Z1-(CR7aR7b)n-を示し;
Y2は、-(CR8aR8b)p-Z2-(CR9aR9b)q-を示し;
Y3は、-(CR10aR10b)r-Z3-(CR11aR11b)s-を示し;
Y4は、-(CH2)t-Z4-を示し:
Z1-Z3は、独立して、結合、O、S、C(O)等を示し;
Z4は、結合、OまたはNを示し;
m、n、p、q、rおよびsは、独立して、0-6を示し;
tは、0-2を示し;
R6a-R11bは、独立して、結合、H、-OH、C1-3アルコキシまたはC1-3アルキルを示し;
R1、R2およびR3は、独立して、H、ハロゲン、アルコキシ、シアノ、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロシクロアルキル、置換されていてもよいヘテロアリールを示し;
R4は、H、O等を示し;
R5およびR13は、独立して、H、ハロゲン、OH、低級アルキル等を示し;
R14は、存在しないか、または置換されていてもよい低級シクロアルキル、置換されていてもよいフェニル等を示す]
で示される化合物(特許文献6)。 [Where:
X 1 represents CH or N;
X 2 represents C or N;
Y 1 represents — (CR 6a R 6b ) m —Z 1 — (CR 7a R 7b ) n —;
Y 2 represents — (CR 8a R 8b ) p —Z 2 — (CR 9a R 9b ) q —;
Y 3 represents — (CR 10a R 10b ) r —Z 3 — (CR 11a R 11b ) s —;
Y 4 represents — (CH 2 ) t —Z 4 —:
Z 1 -Z 3 independently represents a bond, O, S, C (O) or the like;
Z 4 represents a bond, O or N;
m, n, p, q, r and s independently represent 0-6;
t represents 0-2;
R 6a -R 11b independently represents a bond, H, —OH, C 1-3 alkoxy or C 1-3 alkyl;
R 1 , R 2 and R 3 are independently H, halogen, alkoxy, cyano, optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted heterocycloalkyl, substituted Represents optionally substituted heteroaryl;
R 4 represents H, O or the like;
R 5 and R 13 independently represent H, halogen, OH, lower alkyl, etc .;
R 14 represents a lower cycloalkyl which may be absent or substituted, a phenyl which may be substituted, etc.]
The compound shown by (patent document 6).
(7)下記式:
(7) The following formula:
[式中、
X1は、CHまたはNを示し;
X2は、CR13またはNを示し;
Y1は、-(CR6aR6b)m-Z1-(CR7aR7b)n-を示し;
Y2は、-(CR8aR8b)p-Z2-(CR9aR9b)q-を示し;
Y3は、-(CR10aR10b)r-Z3-(CR11aR11b)s-を示し;
Y4は、-(CH2)t-Z4-を示し;
Z1-Z3は、独立して、結合、O、S、C(O)等を示し;
Z4は、結合、OまたはNを示し;
m、n、p、q、rおよびsは、独立して、0-6を示し;
tは、0-2を示し;
R6a-R11bは、独立して、結合、H、-OH、C1-3アルコキシまたはC1-3アルキル等を示し;
R1、R2およびR3は、独立して、H、ハロゲン、置換されていてもよいアルコキシ、シアノ、置換されていてもよい低級シクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロシクロアルキル、置換されていてもよいヘテロアリール等を示し;
R4は、H、O、S、ハロゲン、シアノ、低級アルキル、低級アルケニル、アリール、低級ヘテロアリール等を示し;
R5およびR13は、独立して、H、ハロゲン、低級アルキル、低級アルケン等を示し;
X2がNのとき、R13は存在せず;
R14は、存在しないか、または低級シクロアルキル、フェニル(各々、C1-3アルキル等で置換されていてもよい)等を示し;
R16は、低級アルキル、カルボニル、ヘテロアリール等を示す]
で示される化合物(特許文献7)。 [Where:
X 1 represents CH or N;
X 2 represents CR 13 or N;
Y 1 represents — (CR 6a R 6b ) m —Z 1 — (CR 7a R 7b ) n —;
Y 2 represents — (CR 8a R 8b ) p —Z 2 — (CR 9a R 9b ) q —;
Y 3 represents — (CR 10a R 10b ) r —Z 3 — (CR 11a R 11b ) s —;
Y 4 represents — (CH 2 ) t —Z 4 —;
Z 1 -Z 3 independently represents a bond, O, S, C (O) or the like;
Z 4 represents a bond, O or N;
m, n, p, q, r and s independently represent 0-6;
t represents 0-2;
R 6a -R 11b are independently represents bond, H, -OH, a C 1-3 alkoxy or C 1-3 alkyl and the like;
R 1 , R 2 and R 3 are independently H, halogen, optionally substituted alkoxy, cyano, optionally substituted lower cycloalkyl, optionally substituted aryl, substituted A heterocycloalkyl, an optionally substituted heteroaryl, etc .;
R 4 represents H, O, S, halogen, cyano, lower alkyl, lower alkenyl, aryl, lower heteroaryl or the like;
R 5 and R 13 independently represent H, halogen, lower alkyl, lower alkene or the like;
When X 2 is N, R 13 is absent;
R 14 is absent or represents lower cycloalkyl, phenyl (which may each be substituted with C 1-3 alkyl or the like) and the like;
R 16 represents lower alkyl, carbonyl, heteroaryl or the like]
The compound shown by (patent document 7).
X1は、CHまたはNを示し;
X2は、CR13またはNを示し;
Y1は、-(CR6aR6b)m-Z1-(CR7aR7b)n-を示し;
Y2は、-(CR8aR8b)p-Z2-(CR9aR9b)q-を示し;
Y3は、-(CR10aR10b)r-Z3-(CR11aR11b)s-を示し;
Y4は、-(CH2)t-Z4-を示し;
Z1-Z3は、独立して、結合、O、S、C(O)等を示し;
Z4は、結合、OまたはNを示し;
m、n、p、q、rおよびsは、独立して、0-6を示し;
tは、0-2を示し;
R6a-R11bは、独立して、結合、H、-OH、C1-3アルコキシまたはC1-3アルキル等を示し;
R1、R2およびR3は、独立して、H、ハロゲン、置換されていてもよいアルコキシ、シアノ、置換されていてもよい低級シクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロシクロアルキル、置換されていてもよいヘテロアリール等を示し;
R4は、H、O、S、ハロゲン、シアノ、低級アルキル、低級アルケニル、アリール、低級ヘテロアリール等を示し;
R5およびR13は、独立して、H、ハロゲン、低級アルキル、低級アルケン等を示し;
X2がNのとき、R13は存在せず;
R14は、存在しないか、または低級シクロアルキル、フェニル(各々、C1-3アルキル等で置換されていてもよい)等を示し;
R16は、低級アルキル、カルボニル、ヘテロアリール等を示す]
で示される化合物(特許文献7)。 [Where:
X 1 represents CH or N;
X 2 represents CR 13 or N;
Y 1 represents — (CR 6a R 6b ) m —Z 1 — (CR 7a R 7b ) n —;
Y 2 represents — (CR 8a R 8b ) p —Z 2 — (CR 9a R 9b ) q —;
Y 3 represents — (CR 10a R 10b ) r —Z 3 — (CR 11a R 11b ) s —;
Y 4 represents — (CH 2 ) t —Z 4 —;
Z 1 -Z 3 independently represents a bond, O, S, C (O) or the like;
Z 4 represents a bond, O or N;
m, n, p, q, r and s independently represent 0-6;
t represents 0-2;
R 6a -R 11b are independently represents bond, H, -OH, a C 1-3 alkoxy or C 1-3 alkyl and the like;
R 1 , R 2 and R 3 are independently H, halogen, optionally substituted alkoxy, cyano, optionally substituted lower cycloalkyl, optionally substituted aryl, substituted A heterocycloalkyl, an optionally substituted heteroaryl, etc .;
R 4 represents H, O, S, halogen, cyano, lower alkyl, lower alkenyl, aryl, lower heteroaryl or the like;
R 5 and R 13 independently represent H, halogen, lower alkyl, lower alkene or the like;
When X 2 is N, R 13 is absent;
R 14 is absent or represents lower cycloalkyl, phenyl (which may each be substituted with C 1-3 alkyl or the like) and the like;
R 16 represents lower alkyl, carbonyl, heteroaryl or the like]
The compound shown by (patent document 7).
(8)下記式:
(8) The following formula:
で示される化合物(非特許文献9)。
The compound shown by (nonpatent literature 9).
(9)下記式:
(9) The following formula:
[式中、
R1は、置換されていてもよいフェニルまたは置換されていてもよい芳香族複素環基を示し;
R2は、水素原子またはメチル基を示し;
R3は、5-(R5)-1H-イミダゾール-4-イル基または4-(R5)-1H-イミダゾール-5-イル基を示し;
R4は、水素原子、アミノ基またはメチルアミノ基を示し;
R5は、メチル基またはエチル基を示す]
で示される化合物(特許文献8)。 [Where:
R 1 represents an optionally substituted phenyl or an optionally substituted aromatic heterocyclic group;
R 2 represents a hydrogen atom or a methyl group;
R 3 represents a 5- (R 5 ) -1H-imidazol-4-yl group or a 4- (R 5 ) -1H-imidazol-5-yl group;
R 4 represents a hydrogen atom, an amino group or a methylamino group;
R 5 represents a methyl group or an ethyl group]
The compound shown by (patent document 8).
R1は、置換されていてもよいフェニルまたは置換されていてもよい芳香族複素環基を示し;
R2は、水素原子またはメチル基を示し;
R3は、5-(R5)-1H-イミダゾール-4-イル基または4-(R5)-1H-イミダゾール-5-イル基を示し;
R4は、水素原子、アミノ基またはメチルアミノ基を示し;
R5は、メチル基またはエチル基を示す]
で示される化合物(特許文献8)。 [Where:
R 1 represents an optionally substituted phenyl or an optionally substituted aromatic heterocyclic group;
R 2 represents a hydrogen atom or a methyl group;
R 3 represents a 5- (R 5 ) -1H-imidazol-4-yl group or a 4- (R 5 ) -1H-imidazol-5-yl group;
R 4 represents a hydrogen atom, an amino group or a methylamino group;
R 5 represents a methyl group or an ethyl group]
The compound shown by (patent document 8).
(10)下記式:
(10) The following formula:
[式中、
Xは、カルボニル等を示し;
R2およびR3は、水素原子、ハロゲン、低級アルキル、イミダゾリル、イミダゾリルメチルまたは-N(R4)-R5を示し;
R4およびR5は、水素原子または低級アルキルを示すか、互いに結合して5員または6員の複素環を形成する]
で示される化合物(特許文献9)。 [Where:
X represents carbonyl or the like;
R 2 and R 3 represent a hydrogen atom, halogen, lower alkyl, imidazolyl, imidazolylmethyl or —N (R 4 ) —R 5 ;
R 4 and R 5 represent a hydrogen atom or lower alkyl, or combine with each other to form a 5- or 6-membered heterocyclic ring.
The compound shown by (patent document 9).
Xは、カルボニル等を示し;
R2およびR3は、水素原子、ハロゲン、低級アルキル、イミダゾリル、イミダゾリルメチルまたは-N(R4)-R5を示し;
R4およびR5は、水素原子または低級アルキルを示すか、互いに結合して5員または6員の複素環を形成する]
で示される化合物(特許文献9)。 [Where:
X represents carbonyl or the like;
R 2 and R 3 represent a hydrogen atom, halogen, lower alkyl, imidazolyl, imidazolylmethyl or —N (R 4 ) —R 5 ;
R 4 and R 5 represent a hydrogen atom or lower alkyl, or combine with each other to form a 5- or 6-membered heterocyclic ring.
The compound shown by (patent document 9).
本発明の目的は、優れたPKC阻害活性を有し、免疫性疾患、炎症性疾患等の予防または治療剤として有用な化合物を提供することである。
An object of the present invention is to provide a compound having excellent PKC inhibitory activity and useful as a preventive or therapeutic agent for immune diseases, inflammatory diseases and the like.
本発明者らは、上記課題を解決すべく鋭意検討した結果、下記の式で示される化合物またはその塩が優れたPKC阻害活性を有することを見出し、本発明を完成するに至った。
As a result of intensive studies to solve the above problems, the present inventors have found that a compound represented by the following formula or a salt thereof has an excellent PKC inhibitory activity, and has completed the present invention.
すなわち、本発明は、
[1]式(I): That is, the present invention
[1] Formula (I):
[1]式(I): That is, the present invention
[1] Formula (I):
[式中、
環Aは、さらに置換されていてもよい含窒素不飽和複素環を示し;
環Bは、さらに置換されていてもよいC6-14芳香族炭化水素環またはさらに置換されていてもよい芳香族複素環を示し;
Lは、置換されていてもよいC1-2アルキレンを示し;
X1は、NまたはCR1を示し;
X2は、NまたはCR2を示し;
X3は、NまたはCR3を示し;
X4は、NまたはCR4を示し;
R1、R2、R3およびR4は、独立して、水素原子または置換基を示し;
X5は、CR5a、CR5bR5c、NR5dまたは結合手を示し;
R5a、R5b、R5cおよびR5dは、独立して、水素原子または置換基を示すか、またはR5bとR5cは、一緒になって置換されていてもよい環を形成してもよく;
X6は、CR6aR6bまたはNR6cを示し;
R6a、R6bおよびR6cは、独立して、水素原子または置換基を示すか、またはR6aとR6bは、一緒になって置換されていてもよい環を形成してもよく;
R7は、水素原子または置換基を示す。]
で表される化合物またはその塩(以下、化合物(I)と称する場合がある);
[2]環Aが、さらに置換されていてもよい単環式の含窒素不飽和複素環であるか、または、さらに置換されていてもよい、式(A): [Where:
Ring A represents a nitrogen-containing unsaturated heterocyclic ring which may be further substituted;
Ring B represents a C 6-14 aromatic hydrocarbon ring which may be further substituted or an aromatic heterocyclic ring which may be further substituted;
L represents an optionally substituted C 1-2 alkylene;
X 1 represents N or CR 1 ;
X 2 represents N or CR 2 ;
X 3 represents N or CR 3 ;
X 4 represents N or CR 4 ;
R 1 , R 2 , R 3 and R 4 independently represent a hydrogen atom or a substituent;
X 5 represents CR 5a , CR 5b R 5c , NR 5d or a bond;
R 5a , R 5b , R 5c and R 5d independently represent a hydrogen atom or a substituent, or R 5b and R 5c may combine together to form an optionally substituted ring. Often;
X 6 represents CR 6a R 6b or NR 6c ;
R 6a , R 6b and R 6c independently represent a hydrogen atom or a substituent, or R 6a and R 6b may together form an optionally substituted ring;
R 7 represents a hydrogen atom or a substituent. ]
Or a salt thereof (hereinafter sometimes referred to as compound (I));
[2] The ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted, or may be further substituted, the formula (A):
環Aは、さらに置換されていてもよい含窒素不飽和複素環を示し;
環Bは、さらに置換されていてもよいC6-14芳香族炭化水素環またはさらに置換されていてもよい芳香族複素環を示し;
Lは、置換されていてもよいC1-2アルキレンを示し;
X1は、NまたはCR1を示し;
X2は、NまたはCR2を示し;
X3は、NまたはCR3を示し;
X4は、NまたはCR4を示し;
R1、R2、R3およびR4は、独立して、水素原子または置換基を示し;
X5は、CR5a、CR5bR5c、NR5dまたは結合手を示し;
R5a、R5b、R5cおよびR5dは、独立して、水素原子または置換基を示すか、またはR5bとR5cは、一緒になって置換されていてもよい環を形成してもよく;
X6は、CR6aR6bまたはNR6cを示し;
R6a、R6bおよびR6cは、独立して、水素原子または置換基を示すか、またはR6aとR6bは、一緒になって置換されていてもよい環を形成してもよく;
R7は、水素原子または置換基を示す。]
で表される化合物またはその塩(以下、化合物(I)と称する場合がある);
[2]環Aが、さらに置換されていてもよい単環式の含窒素不飽和複素環であるか、または、さらに置換されていてもよい、式(A): [Where:
Ring A represents a nitrogen-containing unsaturated heterocyclic ring which may be further substituted;
Ring B represents a C 6-14 aromatic hydrocarbon ring which may be further substituted or an aromatic heterocyclic ring which may be further substituted;
L represents an optionally substituted C 1-2 alkylene;
X 1 represents N or CR 1 ;
X 2 represents N or CR 2 ;
X 3 represents N or CR 3 ;
X 4 represents N or CR 4 ;
R 1 , R 2 , R 3 and R 4 independently represent a hydrogen atom or a substituent;
X 5 represents CR 5a , CR 5b R 5c , NR 5d or a bond;
R 5a , R 5b , R 5c and R 5d independently represent a hydrogen atom or a substituent, or R 5b and R 5c may combine together to form an optionally substituted ring. Often;
X 6 represents CR 6a R 6b or NR 6c ;
R 6a , R 6b and R 6c independently represent a hydrogen atom or a substituent, or R 6a and R 6b may together form an optionally substituted ring;
R 7 represents a hydrogen atom or a substituent. ]
Or a salt thereof (hereinafter sometimes referred to as compound (I));
[2] The ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted, or may be further substituted, the formula (A):
[式中、
環A1は、さらに置換されていてもよい5員または6員の芳香環、または、さらに置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A2は、置換されていてもよい5員または6員の芳香環、または、置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A1または環A2の環構成原子の少なくとも1個以上が窒素原子である。]
で表される二環式の含窒素不飽和複素環であり;
環Bが、さらに置換されていてもよいベンゼン環、あるいはさらに置換されていてもよいチオフェン環またはさらに置換されていてもよいピリジン環であり;
Lが、C1-2アルキレンであり;
X1が、NまたはCR1であり、R1が、水素原子またはハロゲン原子であり;
X2が、NまたはCR2であり、R2が、水素原子またはハロゲン原子であり;
X3が、NまたはCR3であり、R3が、水素原子またはハロゲン原子であり;
X4が、NまたはCR4であり、R4が、水素原子またはC1-6アルキル基であり;
X5が、CR5bR5cまたは結合手であり、R5bおよびR5cが、独立して、水素原子または置換基であり;
X6が、CR6aR6bであり、R6aおよびR6bが、独立して、水素原子または置換基であるか、または、R6aおよびR6bが一緒になって、置換されていてもよい環を形成し;
R7が、水素原子または置換されていてもよいC1-6アルキル基である;
[1]記載の化合物またはその塩;
[3]環Aが、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環であるか、または、ハロゲン原子、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基、置換されていてもよいC1-6アルコキシ基および置換されていてもよい非芳香族複素環-オキシ基から選ばれる1~3個の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、ピリジン環またはイミダゾリン環であり、環A2がチオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、
(1)(i)ハロゲン原子、(ii)シアノ基、(iii)ハロゲン原子またはアミノ基で置換されていてもよいC1-6アルキル基および(iv)C1-6アルコキシ基から選ばれる1~5個の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-、-CH2CH2-または-CH(CH3)-であり;
X1が、NまたはCR1であり、R1が、水素原子またはフッ素原子であり;
X2が、NまたはCR2であり、R2が、水素原子またはフッ素原子であり;
X3が、NまたはCR3であり、R3が、水素原子またはフッ素原子であり;
X4が、NまたはCR4であり、R4が、水素原子またはメチルであり;
X5が、CR5bR5cまたは結合手であり、R5bおよびR5cが、水素原子であり;
X6が、CR6aR6bであり、R6aおよびR6bが、水素原子、あるいは独立して、ヒドロキシ基またはC6-14アリール基で置換されていてもよいC1-6アルキル基であるか、または、R6aおよびR6bが一緒になって、C3-10シクロアルカン環を形成し;
R7が、水素原子またはC1-6アルキル基である;
[2]記載の化合物またはその塩;
[4]2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オンまたはその塩;
[5]4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オンまたはその塩;
[6]1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オンまたはその塩;
[7][1]に記載の化合物またはその塩を含有してなる医薬;
[8]プロテインキナーゼC阻害剤である、[7]記載の医薬;
[9]免疫性疾患および/または炎症性疾患の予防または治療剤である、[7]記載の医薬;
[10]炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療剤である、[7]記載の医薬;
[11]免疫性疾患および/または炎症性疾患の予防または治療に使用するための、[1]に記載の化合物またはその塩;
[12]炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療に使用するための、[1]に記載の化合物またはその塩;
[13][1]に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物におけるプロテインキナーゼCの阻害方法;
[14][1]に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物における免疫性疾患または炎症性疾患の予防または治療方法;
[15][1]に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物における炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療方法;
[16]免疫性疾患および/または炎症性疾患の予防または治療剤を製造するための、[1]に記載の化合物またはその塩の使用;
[17]炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療剤を製造するための、[1]に記載の化合物またはその塩の使用;
等に関する。 [Where:
Ring A 1 represents an optionally further substituted 5- or 6-membered aromatic ring, or an optionally further substituted 5- or 6-membered non-aromatic unsaturated ring;
Ring A 2 represents an optionally substituted 5- or 6-membered aromatic ring, or an optionally substituted 5- or 6-membered non-aromatic unsaturated ring;
At least one of the ring constituent atoms of ring A 1 or ring A 2 is a nitrogen atom. ]
A bicyclic nitrogen-containing unsaturated heterocycle represented by:
Ring B is a benzene ring which may be further substituted, or a thiophene ring which may be further substituted or a pyridine ring which may be further substituted;
L is C 1-2 alkylene;
X 1 is N or CR 1 and R 1 is a hydrogen atom or a halogen atom;
X 2 is N or CR 2 and R 2 is a hydrogen atom or a halogen atom;
X 3 is N or CR 3 , R 3 is a hydrogen atom or a halogen atom;
X 4 is N or CR 4 and R 4 is a hydrogen atom or a C 1-6 alkyl group;
X 5 is CR 5b R 5c or a bond, and R 5b and R 5c are independently a hydrogen atom or a substituent;
X 6 is CR 6a R 6b and R 6a and R 6b are independently a hydrogen atom or a substituent, or R 6a and R 6b may be substituted together to be substituted. Forming a ring;
R 7 is a hydrogen atom or an optionally substituted C 1-6 alkyl group;
[1] The compound according to [1] or a salt thereof;
[3] Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group, or a halogen atom, hydroxy Selected from a group, an oxo group, an optionally substituted C 1-6 alkyl group, an optionally substituted C 1-6 alkoxy group, and an optionally substituted non-aromatic heterocyclic-oxy group; The ring A 1 may be further substituted with three substituents, the ring A 1 is a pyrrole ring, a pyrazole ring, an imidazole ring, a pyridine ring or an imidazoline ring, and the ring A 2 is a thiophene ring, a pyridine ring or a pyrimidine ring A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the formula (A);
Ring B is
1 selected from (1) (i) a halogen atom, (ii) a cyano group, (iii) a C 1-6 alkyl group optionally substituted with a halogen atom or an amino group, and (iv) a C 1-6 alkoxy group A benzene ring optionally further substituted with up to 5 substituents,
(2) a thiophene ring, or (3) a pyridine ring;
L is, -CH 2 -, - CH 2 CH 2 - or -CH (CH 3) - and is,
X 1 is N or CR 1 and R 1 is a hydrogen atom or a fluorine atom;
X 2 is N or CR 2 and R 2 is a hydrogen atom or a fluorine atom;
X 3 is N or CR 3 , R 3 is a hydrogen atom or a fluorine atom;
X 4 is N or CR 4 and R 4 is a hydrogen atom or methyl;
X 5 is CR 5b R 5c or a bond, and R 5b and R 5c are hydrogen atoms;
X 6 is CR 6a R 6b , and R 6a and R 6b are a hydrogen atom, or independently, a C 1-6 alkyl group which may be substituted with a hydroxy group or a C 6-14 aryl group Or R 6a and R 6b together form a C 3-10 cycloalkane ring;
R 7 is a hydrogen atom or a C 1-6 alkyl group;
[2] The compound or a salt thereof according to [2];
[4] 2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl ) -2,3-dihydroquinazolin-4 (1H) -one or a salt thereof;
[5] 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one Or a salt thereof;
[6] 1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-pyrido [2,3-e] [1,4] diazepin-5-one or a salt thereof;
[7] A medicament comprising the compound or salt thereof according to [1];
[8] The medicament according to [7], which is a protein kinase C inhibitor;
[9] The medicament according to [7], which is a preventive or therapeutic agent for immune diseases and / or inflammatory diseases;
[10] The medicament according to [7], which is a prophylactic or therapeutic agent for inflammatory bowel disease, psoriasis or atopic dermatitis;
[11] The compound or salt thereof according to [1] for use in the prevention or treatment of immune diseases and / or inflammatory diseases;
[12] The compound or salt thereof according to [1] for use in the prevention or treatment of inflammatory bowel disease, psoriasis or atopic dermatitis;
[13] A method for inhibiting protein kinase C in a mammal, comprising administering an effective amount of the compound or salt thereof according to [1] to the mammal;
[14] A method for preventing or treating an immune disease or inflammatory disease in a mammal, comprising administering an effective amount of the compound or salt thereof according to [1] to the mammal;
[15] A method for preventing or treating inflammatory bowel disease, psoriasis or atopic dermatitis in a mammal, comprising administering an effective amount of the compound or salt thereof according to [1] to the mammal;
[16] Use of the compound or a salt thereof according to [1] for producing an agent for preventing or treating immune diseases and / or inflammatory diseases;
[17] Use of the compound or a salt thereof according to [1] for producing a preventive or therapeutic agent for inflammatory bowel disease, psoriasis or atopic dermatitis;
Etc.
環A1は、さらに置換されていてもよい5員または6員の芳香環、または、さらに置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A2は、置換されていてもよい5員または6員の芳香環、または、置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A1または環A2の環構成原子の少なくとも1個以上が窒素原子である。]
で表される二環式の含窒素不飽和複素環であり;
環Bが、さらに置換されていてもよいベンゼン環、あるいはさらに置換されていてもよいチオフェン環またはさらに置換されていてもよいピリジン環であり;
Lが、C1-2アルキレンであり;
X1が、NまたはCR1であり、R1が、水素原子またはハロゲン原子であり;
X2が、NまたはCR2であり、R2が、水素原子またはハロゲン原子であり;
X3が、NまたはCR3であり、R3が、水素原子またはハロゲン原子であり;
X4が、NまたはCR4であり、R4が、水素原子またはC1-6アルキル基であり;
X5が、CR5bR5cまたは結合手であり、R5bおよびR5cが、独立して、水素原子または置換基であり;
X6が、CR6aR6bであり、R6aおよびR6bが、独立して、水素原子または置換基であるか、または、R6aおよびR6bが一緒になって、置換されていてもよい環を形成し;
R7が、水素原子または置換されていてもよいC1-6アルキル基である;
[1]記載の化合物またはその塩;
[3]環Aが、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環であるか、または、ハロゲン原子、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基、置換されていてもよいC1-6アルコキシ基および置換されていてもよい非芳香族複素環-オキシ基から選ばれる1~3個の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、ピリジン環またはイミダゾリン環であり、環A2がチオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、
(1)(i)ハロゲン原子、(ii)シアノ基、(iii)ハロゲン原子またはアミノ基で置換されていてもよいC1-6アルキル基および(iv)C1-6アルコキシ基から選ばれる1~5個の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-、-CH2CH2-または-CH(CH3)-であり;
X1が、NまたはCR1であり、R1が、水素原子またはフッ素原子であり;
X2が、NまたはCR2であり、R2が、水素原子またはフッ素原子であり;
X3が、NまたはCR3であり、R3が、水素原子またはフッ素原子であり;
X4が、NまたはCR4であり、R4が、水素原子またはメチルであり;
X5が、CR5bR5cまたは結合手であり、R5bおよびR5cが、水素原子であり;
X6が、CR6aR6bであり、R6aおよびR6bが、水素原子、あるいは独立して、ヒドロキシ基またはC6-14アリール基で置換されていてもよいC1-6アルキル基であるか、または、R6aおよびR6bが一緒になって、C3-10シクロアルカン環を形成し;
R7が、水素原子またはC1-6アルキル基である;
[2]記載の化合物またはその塩;
[4]2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オンまたはその塩;
[5]4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オンまたはその塩;
[6]1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オンまたはその塩;
[7][1]に記載の化合物またはその塩を含有してなる医薬;
[8]プロテインキナーゼC阻害剤である、[7]記載の医薬;
[9]免疫性疾患および/または炎症性疾患の予防または治療剤である、[7]記載の医薬;
[10]炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療剤である、[7]記載の医薬;
[11]免疫性疾患および/または炎症性疾患の予防または治療に使用するための、[1]に記載の化合物またはその塩;
[12]炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療に使用するための、[1]に記載の化合物またはその塩;
[13][1]に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物におけるプロテインキナーゼCの阻害方法;
[14][1]に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物における免疫性疾患または炎症性疾患の予防または治療方法;
[15][1]に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物における炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療方法;
[16]免疫性疾患および/または炎症性疾患の予防または治療剤を製造するための、[1]に記載の化合物またはその塩の使用;
[17]炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療剤を製造するための、[1]に記載の化合物またはその塩の使用;
等に関する。 [Where:
Ring A 1 represents an optionally further substituted 5- or 6-membered aromatic ring, or an optionally further substituted 5- or 6-membered non-aromatic unsaturated ring;
Ring A 2 represents an optionally substituted 5- or 6-membered aromatic ring, or an optionally substituted 5- or 6-membered non-aromatic unsaturated ring;
At least one of the ring constituent atoms of ring A 1 or ring A 2 is a nitrogen atom. ]
A bicyclic nitrogen-containing unsaturated heterocycle represented by:
Ring B is a benzene ring which may be further substituted, or a thiophene ring which may be further substituted or a pyridine ring which may be further substituted;
L is C 1-2 alkylene;
X 1 is N or CR 1 and R 1 is a hydrogen atom or a halogen atom;
X 2 is N or CR 2 and R 2 is a hydrogen atom or a halogen atom;
X 3 is N or CR 3 , R 3 is a hydrogen atom or a halogen atom;
X 4 is N or CR 4 and R 4 is a hydrogen atom or a C 1-6 alkyl group;
X 5 is CR 5b R 5c or a bond, and R 5b and R 5c are independently a hydrogen atom or a substituent;
X 6 is CR 6a R 6b and R 6a and R 6b are independently a hydrogen atom or a substituent, or R 6a and R 6b may be substituted together to be substituted. Forming a ring;
R 7 is a hydrogen atom or an optionally substituted C 1-6 alkyl group;
[1] The compound according to [1] or a salt thereof;
[3] Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group, or a halogen atom, hydroxy Selected from a group, an oxo group, an optionally substituted C 1-6 alkyl group, an optionally substituted C 1-6 alkoxy group, and an optionally substituted non-aromatic heterocyclic-oxy group; The ring A 1 may be further substituted with three substituents, the ring A 1 is a pyrrole ring, a pyrazole ring, an imidazole ring, a pyridine ring or an imidazoline ring, and the ring A 2 is a thiophene ring, a pyridine ring or a pyrimidine ring A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the formula (A);
Ring B is
1 selected from (1) (i) a halogen atom, (ii) a cyano group, (iii) a C 1-6 alkyl group optionally substituted with a halogen atom or an amino group, and (iv) a C 1-6 alkoxy group A benzene ring optionally further substituted with up to 5 substituents,
(2) a thiophene ring, or (3) a pyridine ring;
L is, -CH 2 -, - CH 2 CH 2 - or -CH (CH 3) - and is,
X 1 is N or CR 1 and R 1 is a hydrogen atom or a fluorine atom;
X 2 is N or CR 2 and R 2 is a hydrogen atom or a fluorine atom;
X 3 is N or CR 3 , R 3 is a hydrogen atom or a fluorine atom;
X 4 is N or CR 4 and R 4 is a hydrogen atom or methyl;
X 5 is CR 5b R 5c or a bond, and R 5b and R 5c are hydrogen atoms;
X 6 is CR 6a R 6b , and R 6a and R 6b are a hydrogen atom, or independently, a C 1-6 alkyl group which may be substituted with a hydroxy group or a C 6-14 aryl group Or R 6a and R 6b together form a C 3-10 cycloalkane ring;
R 7 is a hydrogen atom or a C 1-6 alkyl group;
[2] The compound or a salt thereof according to [2];
[4] 2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl ) -2,3-dihydroquinazolin-4 (1H) -one or a salt thereof;
[5] 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one Or a salt thereof;
[6] 1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-pyrido [2,3-e] [1,4] diazepin-5-one or a salt thereof;
[7] A medicament comprising the compound or salt thereof according to [1];
[8] The medicament according to [7], which is a protein kinase C inhibitor;
[9] The medicament according to [7], which is a preventive or therapeutic agent for immune diseases and / or inflammatory diseases;
[10] The medicament according to [7], which is a prophylactic or therapeutic agent for inflammatory bowel disease, psoriasis or atopic dermatitis;
[11] The compound or salt thereof according to [1] for use in the prevention or treatment of immune diseases and / or inflammatory diseases;
[12] The compound or salt thereof according to [1] for use in the prevention or treatment of inflammatory bowel disease, psoriasis or atopic dermatitis;
[13] A method for inhibiting protein kinase C in a mammal, comprising administering an effective amount of the compound or salt thereof according to [1] to the mammal;
[14] A method for preventing or treating an immune disease or inflammatory disease in a mammal, comprising administering an effective amount of the compound or salt thereof according to [1] to the mammal;
[15] A method for preventing or treating inflammatory bowel disease, psoriasis or atopic dermatitis in a mammal, comprising administering an effective amount of the compound or salt thereof according to [1] to the mammal;
[16] Use of the compound or a salt thereof according to [1] for producing an agent for preventing or treating immune diseases and / or inflammatory diseases;
[17] Use of the compound or a salt thereof according to [1] for producing a preventive or therapeutic agent for inflammatory bowel disease, psoriasis or atopic dermatitis;
Etc.
化合物(I)は、優れたPKC(例、PKC-θ)阻害活性を有し、免疫性疾患や炎症性疾患等の予防または治療剤として有用である。
Compound (I) has excellent PKC (eg, PKC-θ) inhibitory activity and is useful as a preventive or therapeutic agent for immune diseases and inflammatory diseases.
(発明の詳細な説明)
以下、本明細書中で用いられる各置換基の定義について詳述する。特記しない限り各置換基は以下の定義を有する。
本明細書中、「ハロゲン原子」としては、例えば、フッ素、塩素、臭素、ヨウ素が挙げられる。
本明細書中、「C1-6アルキル基」としては、例えば、メチル、エチル、プロピル、イソプロピル、ブチル、イソブチル、sec-ブチル、tert-ブチル、ペンチル、イソペンチル、ネオペンチル、1-エチルプロピル、ヘキシル、イソヘキシル、1,1-ジメチルブチル、2,2-ジメチルブチル、3,3-ジメチルブチル、2-エチルブチルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキル基が挙げられる。具体例としては、メチル、クロロメチル、ジフルオロメチル、トリクロロメチル、トリフルオロメチル、エチル、2-ブロモエチル、2,2,2-トリフルオロエチル、テトラフルオロエチル、ペンタフルオロエチル、プロピル、2,2―ジフルオロプロピル、3,3,3-トリフルオロプロピル、イソプロピル、ブチル、4,4,4-トリフルオロブチル、イソブチル、sec-ブチル、tert-ブチル、ペンチル、イソペンチル、ネオペンチル、5,5,5-トリフルオロペンチル、ヘキシル、6,6,6-トリフルオロヘキシルが挙げられる。
本明細書中、「C2-6アルケニル基」としては、例えば、エテニル、1-プロペニル、2-プロペニル、2-メチル-1-プロペニル、1-ブテニル、2-ブテニル、3-ブテニル、3-メチル-2-ブテニル、1-ペンテニル、2-ペンテニル、3-ペンテニル、4-ペンテニル、4-メチル-3-ペンテニル、1-ヘキセニル、3-ヘキセニル、5-ヘキセニルが挙げられる。
本明細書中、「C2-6アルキニル基」としては、例えば、エチニル、1-プロピニル、2-プロピニル、1-ブチニル、2-ブチニル、3-ブチニル、1-ペンチニル、2-ペンチニル、3-ペンチニル、4-ペンチニル、1-ヘキシニル、2-ヘキシニル、3-ヘキシニル、4-ヘキシニル、5-ヘキシニル、4-メチル-2-ペンチニルが挙げられる。
本明細書中、「C3-10シクロアルキル基」としては、例えば、シクロプロピル、シクロブチル、シクロペンチル、シクロヘキシル、シクロヘプチル、シクロオクチル、ビシクロ[2.2.1]ヘプチル、ビシクロ[2.2.2]オクチル、ビシクロ[3.2.1]オクチル、アダマンチルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC3-10シクロアルキル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC3-10シクロアルキル基が挙げられる。具体例としては、シクロプロピル、2,2-ジフルオロシクロプロピル、2,3-ジフルオロシクロプロピル、シクロブチル、ジフルオロシクロブチル、シクロペンチル、シクロヘキシル、シクロヘプチル、シクロオクチルが挙げられる。
本明細書中、「C3-10シクロアルケニル基」としては、例えば、シクロプロペニル、シクロブテニル、シクロペンテニル、シクロヘキセニル、シクロヘプテニル、シクロオクテニルが挙げられる。
本明細書中、「C6-14アリール基」としては、例えば、フェニル、1-ナフチル、2-ナフチル、1-アントリル、2-アントリル、9-アントリルが挙げられる。
本明細書中、「C7-16アラルキル基」としては、例えば、ベンジル、フェネチル、ナフチルメチル、フェニルプロピルが挙げられる。 (Detailed description of the invention)
Hereinafter, the definition of each substituent used in the present specification will be described in detail. Unless otherwise specified, each substituent has the following definition.
In the present specification, examples of the “halogen atom” include fluorine, chlorine, bromine and iodine.
In the present specification, examples of the “C 1-6 alkyl group” include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 1-ethylpropyl, hexyl. , Isohexyl, 1,1-dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, 2-ethylbutyl.
In the present specification, the "optionally halogenated C 1-6 alkyl group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkyl group is mentioned. Specific examples include methyl, chloromethyl, difluoromethyl, trichloromethyl, trifluoromethyl, ethyl, 2-bromoethyl, 2,2,2-trifluoroethyl, tetrafluoroethyl, pentafluoroethyl, propyl, 2,2- Difluoropropyl, 3,3,3-trifluoropropyl, isopropyl, butyl, 4,4,4-trifluorobutyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 5,5,5-tri Examples include fluoropentyl, hexyl, and 6,6,6-trifluorohexyl.
In the present specification, examples of the “C 2-6 alkenyl group” include ethenyl, 1-propenyl, 2-propenyl, 2-methyl-1-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 3- Examples include methyl-2-butenyl, 1-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 4-methyl-3-pentenyl, 1-hexenyl, 3-hexenyl and 5-hexenyl.
In the present specification, examples of the “C 2-6 alkynyl group” include ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-pentynyl, 2-pentynyl, 3- Examples include pentynyl, 4-pentynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 4-methyl-2-pentynyl.
In the present specification, examples of the “C 3-10 cycloalkyl group” include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, bicyclo [2.2.1] heptyl, and bicyclo [2.2. 2] Octyl, bicyclo [3.2.1] octyl, and adamantyl.
In the present specification, the "optionally halogenated C 3-10 also be cycloalkyl group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 3- A 10 cycloalkyl group. Specific examples include cyclopropyl, 2,2-difluorocyclopropyl, 2,3-difluorocyclopropyl, cyclobutyl, difluorocyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
In the present specification, examples of the “C 3-10 cycloalkenyl group” include cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, and cyclooctenyl.
In the present specification, examples of the “C 6-14 aryl group” include phenyl, 1-naphthyl, 2-naphthyl, 1-anthryl, 2-anthryl, and 9-anthryl.
In the present specification, examples of the “C 7-16 aralkyl group” include benzyl, phenethyl, naphthylmethyl, and phenylpropyl.
以下、本明細書中で用いられる各置換基の定義について詳述する。特記しない限り各置換基は以下の定義を有する。
本明細書中、「ハロゲン原子」としては、例えば、フッ素、塩素、臭素、ヨウ素が挙げられる。
本明細書中、「C1-6アルキル基」としては、例えば、メチル、エチル、プロピル、イソプロピル、ブチル、イソブチル、sec-ブチル、tert-ブチル、ペンチル、イソペンチル、ネオペンチル、1-エチルプロピル、ヘキシル、イソヘキシル、1,1-ジメチルブチル、2,2-ジメチルブチル、3,3-ジメチルブチル、2-エチルブチルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキル基が挙げられる。具体例としては、メチル、クロロメチル、ジフルオロメチル、トリクロロメチル、トリフルオロメチル、エチル、2-ブロモエチル、2,2,2-トリフルオロエチル、テトラフルオロエチル、ペンタフルオロエチル、プロピル、2,2―ジフルオロプロピル、3,3,3-トリフルオロプロピル、イソプロピル、ブチル、4,4,4-トリフルオロブチル、イソブチル、sec-ブチル、tert-ブチル、ペンチル、イソペンチル、ネオペンチル、5,5,5-トリフルオロペンチル、ヘキシル、6,6,6-トリフルオロヘキシルが挙げられる。
本明細書中、「C2-6アルケニル基」としては、例えば、エテニル、1-プロペニル、2-プロペニル、2-メチル-1-プロペニル、1-ブテニル、2-ブテニル、3-ブテニル、3-メチル-2-ブテニル、1-ペンテニル、2-ペンテニル、3-ペンテニル、4-ペンテニル、4-メチル-3-ペンテニル、1-ヘキセニル、3-ヘキセニル、5-ヘキセニルが挙げられる。
本明細書中、「C2-6アルキニル基」としては、例えば、エチニル、1-プロピニル、2-プロピニル、1-ブチニル、2-ブチニル、3-ブチニル、1-ペンチニル、2-ペンチニル、3-ペンチニル、4-ペンチニル、1-ヘキシニル、2-ヘキシニル、3-ヘキシニル、4-ヘキシニル、5-ヘキシニル、4-メチル-2-ペンチニルが挙げられる。
本明細書中、「C3-10シクロアルキル基」としては、例えば、シクロプロピル、シクロブチル、シクロペンチル、シクロヘキシル、シクロヘプチル、シクロオクチル、ビシクロ[2.2.1]ヘプチル、ビシクロ[2.2.2]オクチル、ビシクロ[3.2.1]オクチル、アダマンチルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC3-10シクロアルキル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC3-10シクロアルキル基が挙げられる。具体例としては、シクロプロピル、2,2-ジフルオロシクロプロピル、2,3-ジフルオロシクロプロピル、シクロブチル、ジフルオロシクロブチル、シクロペンチル、シクロヘキシル、シクロヘプチル、シクロオクチルが挙げられる。
本明細書中、「C3-10シクロアルケニル基」としては、例えば、シクロプロペニル、シクロブテニル、シクロペンテニル、シクロヘキセニル、シクロヘプテニル、シクロオクテニルが挙げられる。
本明細書中、「C6-14アリール基」としては、例えば、フェニル、1-ナフチル、2-ナフチル、1-アントリル、2-アントリル、9-アントリルが挙げられる。
本明細書中、「C7-16アラルキル基」としては、例えば、ベンジル、フェネチル、ナフチルメチル、フェニルプロピルが挙げられる。 (Detailed description of the invention)
Hereinafter, the definition of each substituent used in the present specification will be described in detail. Unless otherwise specified, each substituent has the following definition.
In the present specification, examples of the “halogen atom” include fluorine, chlorine, bromine and iodine.
In the present specification, examples of the “C 1-6 alkyl group” include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 1-ethylpropyl, hexyl. , Isohexyl, 1,1-dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, 2-ethylbutyl.
In the present specification, the "optionally halogenated C 1-6 alkyl group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkyl group is mentioned. Specific examples include methyl, chloromethyl, difluoromethyl, trichloromethyl, trifluoromethyl, ethyl, 2-bromoethyl, 2,2,2-trifluoroethyl, tetrafluoroethyl, pentafluoroethyl, propyl, 2,2- Difluoropropyl, 3,3,3-trifluoropropyl, isopropyl, butyl, 4,4,4-trifluorobutyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 5,5,5-tri Examples include fluoropentyl, hexyl, and 6,6,6-trifluorohexyl.
In the present specification, examples of the “C 2-6 alkenyl group” include ethenyl, 1-propenyl, 2-propenyl, 2-methyl-1-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 3- Examples include methyl-2-butenyl, 1-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 4-methyl-3-pentenyl, 1-hexenyl, 3-hexenyl and 5-hexenyl.
In the present specification, examples of the “C 2-6 alkynyl group” include ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-pentynyl, 2-pentynyl, 3- Examples include pentynyl, 4-pentynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 4-methyl-2-pentynyl.
In the present specification, examples of the “C 3-10 cycloalkyl group” include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, bicyclo [2.2.1] heptyl, and bicyclo [2.2. 2] Octyl, bicyclo [3.2.1] octyl, and adamantyl.
In the present specification, the "optionally halogenated C 3-10 also be cycloalkyl group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 3- A 10 cycloalkyl group. Specific examples include cyclopropyl, 2,2-difluorocyclopropyl, 2,3-difluorocyclopropyl, cyclobutyl, difluorocyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
In the present specification, examples of the “C 3-10 cycloalkenyl group” include cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, and cyclooctenyl.
In the present specification, examples of the “C 6-14 aryl group” include phenyl, 1-naphthyl, 2-naphthyl, 1-anthryl, 2-anthryl, and 9-anthryl.
In the present specification, examples of the “C 7-16 aralkyl group” include benzyl, phenethyl, naphthylmethyl, and phenylpropyl.
本明細書中、「C1-6アルコキシ基」としては、例えば、メトキシ、エトキシ、プロポキシ、イソプロポキシ、ブトキシ、イソブトキシ、sec-ブトキシ、tert-ブトキシ、ペンチルオキシ、ヘキシルオキシが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルコキシ基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルコキシ基が挙げられる。具体例としては、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、エトキシ、2,2,2-トリフルオロエトキシ、プロポキシ、イソプロポキシ、ブトキシ、4,4,4-トリフルオロブトキシ、イソブトキシ、sec-ブトキシ、ペンチルオキシ、ヘキシルオキシが挙げられる。
本明細書中、「C3-10シクロアルキルオキシ基」としては、例えば、シクロプロピルオキシ、シクロブチルオキシ、シクロペンチルオキシ、シクロヘキシルオキシ、シクロヘプチルオキシ、シクロオクチルオキシが挙げられる。
本明細書中、「C1-6アルキルチオ基」としては、例えば、メチルチオ、エチルチオ、プロピルチオ、イソプロピルチオ、ブチルチオ、sec-ブチルチオ、tert-ブチルチオ、ペンチルチオ、ヘキシルチオが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキルチオ基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキルチオ基が挙げられる。具体例としては、メチルチオ、ジフルオロメチルチオ、トリフルオロメチルチオ、エチルチオ、プロピルチオ、イソプロピルチオ、ブチルチオ、4,4,4-トリフルオロブチルチオ、ペンチルチオ、ヘキシルチオが挙げられる。
本明細書中、「C1-6アルキル-カルボニル基」としては、例えば、アセチル、プロパノイル、ブタノイル、2-メチルプロパノイル、ペンタノイル、3-メチルブタノイル、2-メチルブタノイル、2,2-ジメチルプロパノイル、ヘキサノイル、ヘプタノイルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキル-カルボニル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキル-カルボニル基が挙げられる。具体例としては、アセチル、クロロアセチル、トリフルオロアセチル、トリクロロアセチル、プロパノイル、ブタノイル、ペンタノイル、ヘキサノイルが挙げられる。
本明細書中、「C1-6アルコキシ-カルボニル基」としては、例えば、メトキシカルボニル、エトキシカルボニル、プロポキシカルボニル、イソプロポキシカルボニル、ブトキシカルボニル、イソブトキシカルボニル、sec-ブトキシカルボニル、tert-ブトキシカルボニル、ペンチルオキシカルボニル、ヘキシルオキシカルボニルが挙げられる。
本明細書中、「C6-14アリール-カルボニル基」としては、例えば、ベンゾイル、1-ナフトイル、2-ナフトイルが挙げられる。
本明細書中、「C7-16アラルキル-カルボニル基」としては、例えば、フェニルアセチル、フェニルプロピオニルが挙げられる。
本明細書中、「5ないし14員芳香族複素環カルボニル基」としては、例えば、ニコチノイル、イソニコチノイル、テノイル、フロイルが挙げられる。
本明細書中、「3ないし14員非芳香族複素環カルボニル基」としては、例えば、モルホリニルカルボニル、ピペリジニルカルボニル、ピロリジニルカルボニルが挙げられる。 In the present specification, examples of the “C 1-6 alkoxy group” include methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy, tert-butoxy, pentyloxy and hexyloxy.
In the present specification, the "optionally halogenated C 1-6 alkoxy group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkoxy group is mentioned. Specific examples include methoxy, difluoromethoxy, trifluoromethoxy, ethoxy, 2,2,2-trifluoroethoxy, propoxy, isopropoxy, butoxy, 4,4,4-trifluorobutoxy, isobutoxy, sec-butoxy, pentyl. Examples include oxy and hexyloxy.
In the present specification, examples of the “C 3-10 cycloalkyloxy group” include cyclopropyloxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, cycloheptyloxy, and cyclooctyloxy.
In the present specification, examples of the “C 1-6 alkylthio group” include methylthio, ethylthio, propylthio, isopropylthio, butylthio, sec-butylthio, tert-butylthio, pentylthio and hexylthio.
In the present specification, the "optionally halogenated C 1-6 alkylthio group optionally", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkylthio group is mentioned. Specific examples include methylthio, difluoromethylthio, trifluoromethylthio, ethylthio, propylthio, isopropylthio, butylthio, 4,4,4-trifluorobutylthio, pentylthio, hexylthio.
In the present specification, examples of the “C 1-6 alkyl-carbonyl group” include acetyl, propanoyl, butanoyl, 2-methylpropanoyl, pentanoyl, 3-methylbutanoyl, 2-methylbutanoyl, 2,2- Examples include dimethylpropanoyl, hexanoyl, and heptanoyl.
In the present specification, examples of the “ optionally halogenated C 1-6 alkyl-carbonyl group” include C 1 optionally having 1 to 7, preferably 1 to 5 halogen atoms. A -6 alkyl-carbonyl group is mentioned. Specific examples include acetyl, chloroacetyl, trifluoroacetyl, trichloroacetyl, propanoyl, butanoyl, pentanoyl and hexanoyl.
In the present specification, examples of the “C 1-6 alkoxy-carbonyl group” include methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl, isobutoxycarbonyl, sec-butoxycarbonyl, tert-butoxycarbonyl, Examples include pentyloxycarbonyl and hexyloxycarbonyl.
In the present specification, examples of the “C 6-14 aryl-carbonyl group” include benzoyl, 1-naphthoyl and 2-naphthoyl.
In the present specification, examples of the “C 7-16 aralkyl-carbonyl group” include phenylacetyl and phenylpropionyl.
In the present specification, examples of the “5- to 14-membered aromatic heterocyclic carbonyl group” include nicotinoyl, isonicotinoyl, thenoyl and furoyl.
In the present specification, examples of the “3- to 14-membered non-aromatic heterocyclic carbonyl group” include morpholinylcarbonyl, piperidinylcarbonyl, and pyrrolidinylcarbonyl.
本明細書中、「ハロゲン化されていてもよいC1-6アルコキシ基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルコキシ基が挙げられる。具体例としては、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、エトキシ、2,2,2-トリフルオロエトキシ、プロポキシ、イソプロポキシ、ブトキシ、4,4,4-トリフルオロブトキシ、イソブトキシ、sec-ブトキシ、ペンチルオキシ、ヘキシルオキシが挙げられる。
本明細書中、「C3-10シクロアルキルオキシ基」としては、例えば、シクロプロピルオキシ、シクロブチルオキシ、シクロペンチルオキシ、シクロヘキシルオキシ、シクロヘプチルオキシ、シクロオクチルオキシが挙げられる。
本明細書中、「C1-6アルキルチオ基」としては、例えば、メチルチオ、エチルチオ、プロピルチオ、イソプロピルチオ、ブチルチオ、sec-ブチルチオ、tert-ブチルチオ、ペンチルチオ、ヘキシルチオが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキルチオ基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキルチオ基が挙げられる。具体例としては、メチルチオ、ジフルオロメチルチオ、トリフルオロメチルチオ、エチルチオ、プロピルチオ、イソプロピルチオ、ブチルチオ、4,4,4-トリフルオロブチルチオ、ペンチルチオ、ヘキシルチオが挙げられる。
本明細書中、「C1-6アルキル-カルボニル基」としては、例えば、アセチル、プロパノイル、ブタノイル、2-メチルプロパノイル、ペンタノイル、3-メチルブタノイル、2-メチルブタノイル、2,2-ジメチルプロパノイル、ヘキサノイル、ヘプタノイルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキル-カルボニル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキル-カルボニル基が挙げられる。具体例としては、アセチル、クロロアセチル、トリフルオロアセチル、トリクロロアセチル、プロパノイル、ブタノイル、ペンタノイル、ヘキサノイルが挙げられる。
本明細書中、「C1-6アルコキシ-カルボニル基」としては、例えば、メトキシカルボニル、エトキシカルボニル、プロポキシカルボニル、イソプロポキシカルボニル、ブトキシカルボニル、イソブトキシカルボニル、sec-ブトキシカルボニル、tert-ブトキシカルボニル、ペンチルオキシカルボニル、ヘキシルオキシカルボニルが挙げられる。
本明細書中、「C6-14アリール-カルボニル基」としては、例えば、ベンゾイル、1-ナフトイル、2-ナフトイルが挙げられる。
本明細書中、「C7-16アラルキル-カルボニル基」としては、例えば、フェニルアセチル、フェニルプロピオニルが挙げられる。
本明細書中、「5ないし14員芳香族複素環カルボニル基」としては、例えば、ニコチノイル、イソニコチノイル、テノイル、フロイルが挙げられる。
本明細書中、「3ないし14員非芳香族複素環カルボニル基」としては、例えば、モルホリニルカルボニル、ピペリジニルカルボニル、ピロリジニルカルボニルが挙げられる。 In the present specification, examples of the “C 1-6 alkoxy group” include methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy, tert-butoxy, pentyloxy and hexyloxy.
In the present specification, the "optionally halogenated C 1-6 alkoxy group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkoxy group is mentioned. Specific examples include methoxy, difluoromethoxy, trifluoromethoxy, ethoxy, 2,2,2-trifluoroethoxy, propoxy, isopropoxy, butoxy, 4,4,4-trifluorobutoxy, isobutoxy, sec-butoxy, pentyl. Examples include oxy and hexyloxy.
In the present specification, examples of the “C 3-10 cycloalkyloxy group” include cyclopropyloxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, cycloheptyloxy, and cyclooctyloxy.
In the present specification, examples of the “C 1-6 alkylthio group” include methylthio, ethylthio, propylthio, isopropylthio, butylthio, sec-butylthio, tert-butylthio, pentylthio and hexylthio.
In the present specification, the "optionally halogenated C 1-6 alkylthio group optionally", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1-6 An alkylthio group is mentioned. Specific examples include methylthio, difluoromethylthio, trifluoromethylthio, ethylthio, propylthio, isopropylthio, butylthio, 4,4,4-trifluorobutylthio, pentylthio, hexylthio.
In the present specification, examples of the “C 1-6 alkyl-carbonyl group” include acetyl, propanoyl, butanoyl, 2-methylpropanoyl, pentanoyl, 3-methylbutanoyl, 2-methylbutanoyl, 2,2- Examples include dimethylpropanoyl, hexanoyl, and heptanoyl.
In the present specification, examples of the “ optionally halogenated C 1-6 alkyl-carbonyl group” include C 1 optionally having 1 to 7, preferably 1 to 5 halogen atoms. A -6 alkyl-carbonyl group is mentioned. Specific examples include acetyl, chloroacetyl, trifluoroacetyl, trichloroacetyl, propanoyl, butanoyl, pentanoyl and hexanoyl.
In the present specification, examples of the “C 1-6 alkoxy-carbonyl group” include methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl, isobutoxycarbonyl, sec-butoxycarbonyl, tert-butoxycarbonyl, Examples include pentyloxycarbonyl and hexyloxycarbonyl.
In the present specification, examples of the “C 6-14 aryl-carbonyl group” include benzoyl, 1-naphthoyl and 2-naphthoyl.
In the present specification, examples of the “C 7-16 aralkyl-carbonyl group” include phenylacetyl and phenylpropionyl.
In the present specification, examples of the “5- to 14-membered aromatic heterocyclic carbonyl group” include nicotinoyl, isonicotinoyl, thenoyl and furoyl.
In the present specification, examples of the “3- to 14-membered non-aromatic heterocyclic carbonyl group” include morpholinylcarbonyl, piperidinylcarbonyl, and pyrrolidinylcarbonyl.
本明細書中、「モノ-またはジ-C1-6アルキル-カルバモイル基」としては、例えば、メチルカルバモイル、エチルカルバモイル、ジメチルカルバモイル、ジエチルカルバモイル、N-エチル-N-メチルカルバモイルが挙げられる。
本明細書中、「モノ-またはジ-C7-16アラルキル-カルバモイル基」としては、例えば、ベンジルカルバモイル、フェネチルカルバモイルが挙げられる。
本明細書中、「C1-6アルキルスルホニル基」としては、例えば、メチルスルホニル、エチルスルホニル、プロピルスルホニル、イソプロピルスルホニル、ブチルスルホニル、sec-ブチルスルホニル、tert-ブチルスルホニルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキルスルホニル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキルスルホニル基が挙げられる。具体例としては、メチルスルホニル、ジフルオロメチルスルホニル、トリフルオロメチルスルホニル、エチルスルホニル、プロピルスルホニル、イソプロピルスルホニル、ブチルスルホニル、4,4,4-トリフルオロブチルスルホニル、ペンチルスルホニル、ヘキシルスルホニルが挙げられる。
本明細書中、「C6-14アリールスルホニル基」としては、例えば、フェニルスルホニル、1-ナフチルスルホニル、2-ナフチルスルホニルが挙げられる。 In the present specification, examples of the “mono- or di-C 1-6 alkyl-carbamoyl group” include methylcarbamoyl, ethylcarbamoyl, dimethylcarbamoyl, diethylcarbamoyl, N-ethyl-N-methylcarbamoyl.
In the present specification, examples of the “mono- or di-C 7-16 aralkyl-carbamoyl group” include benzylcarbamoyl and phenethylcarbamoyl.
In the present specification, examples of the “C 1-6 alkylsulfonyl group” include methylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl, butylsulfonyl, sec-butylsulfonyl and tert-butylsulfonyl.
In the present specification, the "optionally halogenated C 1-6 alkyl sulfonyl group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1- 6 alkylsulfonyl group is mentioned. Specific examples include methylsulfonyl, difluoromethylsulfonyl, trifluoromethylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl, butylsulfonyl, 4,4,4-trifluorobutylsulfonyl, pentylsulfonyl, hexylsulfonyl.
In the present specification, examples of the “C 6-14 arylsulfonyl group” include phenylsulfonyl, 1-naphthylsulfonyl and 2-naphthylsulfonyl.
本明細書中、「モノ-またはジ-C7-16アラルキル-カルバモイル基」としては、例えば、ベンジルカルバモイル、フェネチルカルバモイルが挙げられる。
本明細書中、「C1-6アルキルスルホニル基」としては、例えば、メチルスルホニル、エチルスルホニル、プロピルスルホニル、イソプロピルスルホニル、ブチルスルホニル、sec-ブチルスルホニル、tert-ブチルスルホニルが挙げられる。
本明細書中、「ハロゲン化されていてもよいC1-6アルキルスルホニル基」としては、例えば、1ないし7個、好ましくは1ないし5個のハロゲン原子を有していてもよいC1-6アルキルスルホニル基が挙げられる。具体例としては、メチルスルホニル、ジフルオロメチルスルホニル、トリフルオロメチルスルホニル、エチルスルホニル、プロピルスルホニル、イソプロピルスルホニル、ブチルスルホニル、4,4,4-トリフルオロブチルスルホニル、ペンチルスルホニル、ヘキシルスルホニルが挙げられる。
本明細書中、「C6-14アリールスルホニル基」としては、例えば、フェニルスルホニル、1-ナフチルスルホニル、2-ナフチルスルホニルが挙げられる。 In the present specification, examples of the “mono- or di-C 1-6 alkyl-carbamoyl group” include methylcarbamoyl, ethylcarbamoyl, dimethylcarbamoyl, diethylcarbamoyl, N-ethyl-N-methylcarbamoyl.
In the present specification, examples of the “mono- or di-C 7-16 aralkyl-carbamoyl group” include benzylcarbamoyl and phenethylcarbamoyl.
In the present specification, examples of the “C 1-6 alkylsulfonyl group” include methylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl, butylsulfonyl, sec-butylsulfonyl and tert-butylsulfonyl.
In the present specification, the "optionally halogenated C 1-6 alkyl sulfonyl group", for example, 1 to 7, preferably which may have 1 to 5 halogen atoms C 1- 6 alkylsulfonyl group is mentioned. Specific examples include methylsulfonyl, difluoromethylsulfonyl, trifluoromethylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl, butylsulfonyl, 4,4,4-trifluorobutylsulfonyl, pentylsulfonyl, hexylsulfonyl.
In the present specification, examples of the “C 6-14 arylsulfonyl group” include phenylsulfonyl, 1-naphthylsulfonyl and 2-naphthylsulfonyl.
本明細書中、「置換基」としては、例えば、ハロゲン原子、シアノ基、ニトロ基、置換されていてもよい炭化水素基、置換されていてもよい複素環基、アシル基、置換されていてもよいアミノ基、置換されていてもよいカルバモイル基、置換されていてもよいチオカルバモイル基、置換されていてもよいスルファモイル基、置換されていてもよいヒドロキシ基、置換されていてもよいスルファニル(SH)基、置換されていてもよいシリル基が挙げられる。
本明細書中、「炭化水素基」(「置換されていてもよい炭化水素基」における「炭化水素基」を含む)としては、例えば、C1-6アルキル基、C2-6アルケニル基、C2-6アルキニル基、C3-10シクロアルキル基、C3-10シクロアルケニル基、C6-14アリール基、C7-16アラルキル基が挙げられる。 In the present specification, examples of the “substituent” include a halogen atom, a cyano group, a nitro group, an optionally substituted hydrocarbon group, an optionally substituted heterocyclic group, an acyl group, and a substituted group. An optionally substituted amino group, an optionally substituted carbamoyl group, an optionally substituted thiocarbamoyl group, an optionally substituted sulfamoyl group, an optionally substituted hydroxy group, an optionally substituted sulfanyl ( SH) group and optionally substituted silyl group.
In the present specification, examples of the “hydrocarbon group” (including the “hydrocarbon group” in the “optionally substituted hydrocarbon group”) include, for example, a C 1-6 alkyl group, a C 2-6 alkenyl group, Examples thereof include a C 2-6 alkynyl group, a C 3-10 cycloalkyl group, a C 3-10 cycloalkenyl group, a C 6-14 aryl group, and a C 7-16 aralkyl group.
本明細書中、「炭化水素基」(「置換されていてもよい炭化水素基」における「炭化水素基」を含む)としては、例えば、C1-6アルキル基、C2-6アルケニル基、C2-6アルキニル基、C3-10シクロアルキル基、C3-10シクロアルケニル基、C6-14アリール基、C7-16アラルキル基が挙げられる。 In the present specification, examples of the “substituent” include a halogen atom, a cyano group, a nitro group, an optionally substituted hydrocarbon group, an optionally substituted heterocyclic group, an acyl group, and a substituted group. An optionally substituted amino group, an optionally substituted carbamoyl group, an optionally substituted thiocarbamoyl group, an optionally substituted sulfamoyl group, an optionally substituted hydroxy group, an optionally substituted sulfanyl ( SH) group and optionally substituted silyl group.
In the present specification, examples of the “hydrocarbon group” (including the “hydrocarbon group” in the “optionally substituted hydrocarbon group”) include, for example, a C 1-6 alkyl group, a C 2-6 alkenyl group, Examples thereof include a C 2-6 alkynyl group, a C 3-10 cycloalkyl group, a C 3-10 cycloalkenyl group, a C 6-14 aryl group, and a C 7-16 aralkyl group.
本明細書中、「置換されていてもよい炭化水素基」としては、例えば、下記の置換基群Aから選ばれる置換基を有していてもよい炭化水素基が挙げられる。
[置換基群A]
(1)ハロゲン原子、
(2)ニトロ基、
(3)シアノ基、
(4)オキソ基、
(5)ヒドロキシ基、
(6)ハロゲン化されていてもよいC1-6アルコキシ基、
(7)C6-14アリールオキシ基(例、フェノキシ、ナフトキシ)、
(8)C7-16アラルキルオキシ基(例、ベンジルオキシ)、
(9)5ないし14員芳香族複素環オキシ基(例、ピリジルオキシ)、
(10)3ないし14員非芳香族複素環オキシ基(例、モルホリニルオキシ、ピペリジニルオキシ)、
(11)C1-6アルキル-カルボニルオキシ基(例、アセトキシ、プロパノイルオキシ)、
(12)C6-14アリール-カルボニルオキシ基(例、ベンゾイルオキシ、1-ナフトイルオキシ、2-ナフトイルオキシ)、
(13)C1-6アルコキシ-カルボニルオキシ基(例、メトキシカルボニルオキシ、エトキシカルボニルオキシ、プロポキシカルボニルオキシ、ブトキシカルボニルオキシ)、
(14)モノ-またはジ-C1-6アルキル-カルバモイルオキシ基(例、メチルカルバモイルオキシ、エチルカルバモイルオキシ、ジメチルカルバモイルオキシ、ジエチルカルバモイルオキシ)、
(15)C6-14アリール-カルバモイルオキシ基(例、フェニルカルバモイルオキシ、ナフチルカルバモイルオキシ)、
(16)5ないし14員芳香族複素環カルボニルオキシ基(例、ニコチノイルオキシ)、
(17)3ないし14員非芳香族複素環カルボニルオキシ基(例、モルホリニルカルボニルオキシ、ピペリジニルカルボニルオキシ)、
(18)ハロゲン化されていてもよいC1-6アルキルスルホニルオキシ基(例、メチルスルホニルオキシ、トリフルオロメチルスルホニルオキシ)、
(19)C1-6アルキル基で置換されていてもよいC6-14アリールスルホニルオキシ基(例、フェニルスルホニルオキシ、トルエンスルホニルオキシ)、
(20)ハロゲン化されていてもよいC1-6アルキルチオ基、
(21)5ないし14員芳香族複素環基、
(22)3ないし14員非芳香族複素環基、
(23)ホルミル基、
(24)カルボキシ基、
(25)ハロゲン化されていてもよいC1-6アルキル-カルボニル基、
(26)C6-14アリール-カルボニル基、
(27)5ないし14員芳香族複素環カルボニル基、
(28)3ないし14員非芳香族複素環カルボニル基、
(29)C1-6アルコキシ-カルボニル基、
(30)C6-14アリールオキシ-カルボニル基(例、フェニルオキシカルボニル、1-ナフチルオキシカルボニル、2-ナフチルオキシカルボニル)、
(31)C7-16アラルキルオキシ-カルボニル基(例、ベンジルオキシカルボニル、フェネチルオキシカルボニル)、
(32)カルバモイル基、
(33)チオカルバモイル基、
(34)モノ-またはジ-C1-6アルキル-カルバモイル基、
(35)C6-14アリール-カルバモイル基(例、フェニルカルバモイル)、
(36)5ないし14員芳香族複素環カルバモイル基(例、ピリジルカルバモイル、チエニルカルバモイル)、
(37)3ないし14員非芳香族複素環カルバモイル基(例、モルホリニルカルバモイル、ピペリジニルカルバモイル)、
(38)ハロゲン化されていてもよいC1-6アルキルスルホニル基、
(39)C6-14アリールスルホニル基、
(40)5ないし14員芳香族複素環スルホニル基(例、ピリジルスルホニル、チエニルスルホニル)、
(41)ハロゲン化されていてもよいC1-6アルキルスルフィニル基、
(42)C6-14アリールスルフィニル基(例、フェニルスルフィニル、1-ナフチルスルフィニル、2-ナフチルスルフィニル)、
(43)5ないし14員芳香族複素環スルフィニル基(例、ピリジルスルフィニル、チエニルスルフィニル)、
(44)アミノ基、
(45)モノ-またはジ-C1-6アルキルアミノ基(例、メチルアミノ、エチルアミノ、プロピルアミノ、イソプロピルアミノ、ブチルアミノ、ジメチルアミノ、ジエチルアミノ、ジプロピルアミノ、ジブチルアミノ、N-エチル-N-メチルアミノ)、
(46)モノ-またはジ-C6-14アリールアミノ基(例、フェニルアミノ)、
(47)5ないし14員芳香族複素環アミノ基(例、ピリジルアミノ)、
(48)C7-16アラルキルアミノ基(例、ベンジルアミノ)、
(49)ホルミルアミノ基、
(50)C1-6アルキル-カルボニルアミノ基(例、アセチルアミノ、プロパノイルアミノ、ブタノイルアミノ)、
(51)(C1-6アルキル)(C1-6アルキル-カルボニル)アミノ基(例、N-アセチル-N-メチルアミノ)、
(52)C6-14アリール-カルボニルアミノ基(例、フェニルカルボニルアミノ、ナフチルカルボニルアミノ)、
(53)C1-6アルコキシ-カルボニルアミノ基(例、メトキシカルボニルアミノ、エトキシカルボニルアミノ、プロポキシカルボニルアミノ、ブトキシカルボニルアミノ、tert-ブトキシカルボニルアミノ)、
(54)C7-16アラルキルオキシ-カルボニルアミノ基(例、ベンジルオキシカルボニルアミノ)、
(55)C1-6アルキルスルホニルアミノ基(例、メチルスルホニルアミノ、エチルスルホニルアミノ)、
(56)C1-6アルキル基で置換されていてもよいC6-14アリールスルホニルアミノ基(例、フェニルスルホニルアミノ、トルエンスルホニルアミノ)、
(57)ハロゲン化されていてもよいC1-6アルキル基、
(58)C2-6アルケニル基、
(59)C2-6アルキニル基、
(60)C3-10シクロアルキル基、
(61)C3-10シクロアルケニル基、及び
(62)C6-14アリール基。 In the present specification, examples of the “optionally substituted hydrocarbon group” include a hydrocarbon group which may have a substituent selected from the following substituent group A.
[Substituent group A]
(1) a halogen atom,
(2) Nitro group,
(3) a cyano group,
(4) an oxo group,
(5) a hydroxy group,
(6) an optionally halogenated C 1-6 alkoxy group,
(7) C 6-14 aryloxy group (eg, phenoxy, naphthoxy),
(8) C 7-16 aralkyloxy group (eg, benzyloxy),
(9) 5- to 14-membered aromatic heterocyclic oxy group (eg, pyridyloxy),
(10) 3 to 14-membered non-aromatic heterocyclic oxy group (eg, morpholinyloxy, piperidinyloxy),
(11) C 1-6 alkyl-carbonyloxy group (eg, acetoxy, propanoyloxy),
(12) C 6-14 aryl-carbonyloxy group (eg, benzoyloxy, 1-naphthoyloxy, 2-naphthoyloxy),
(13) C 1-6 alkoxy-carbonyloxy group (eg, methoxycarbonyloxy, ethoxycarbonyloxy, propoxycarbonyloxy, butoxycarbonyloxy),
(14) mono- or di-C 1-6 alkyl-carbamoyloxy group (eg, methylcarbamoyloxy, ethylcarbamoyloxy, dimethylcarbamoyloxy, diethylcarbamoyloxy),
(15) C 6-14 aryl-carbamoyloxy group (eg, phenylcarbamoyloxy, naphthylcarbamoyloxy),
(16) a 5- to 14-membered aromatic heterocyclic carbonyloxy group (eg, nicotinoyloxy),
(17) 3 to 14-membered non-aromatic heterocyclic carbonyloxy group (eg, morpholinylcarbonyloxy, piperidinylcarbonyloxy),
(18) an optionally halogenated C 1-6 alkylsulfonyloxy group (eg, methylsulfonyloxy, trifluoromethylsulfonyloxy),
(19) a C 6-14 arylsulfonyloxy group (eg, phenylsulfonyloxy, toluenesulfonyloxy) optionally substituted with a C 1-6 alkyl group,
(20) an optionally halogenated C 1-6 alkylthio group,
(21) a 5- to 14-membered aromatic heterocyclic group,
(22) a 3- to 14-membered non-aromatic heterocyclic group,
(23) formyl group,
(24) a carboxy group,
(25) an optionally halogenated C 1-6 alkyl-carbonyl group,
(26) a C 6-14 aryl-carbonyl group,
(27) a 5- to 14-membered aromatic heterocyclic carbonyl group,
(28) a 3- to 14-membered non-aromatic heterocyclic carbonyl group,
(29) a C 1-6 alkoxy-carbonyl group,
(30) C 6-14 aryloxy-carbonyl group (eg, phenyloxycarbonyl, 1-naphthyloxycarbonyl, 2-naphthyloxycarbonyl),
(31) C 7-16 aralkyloxy-carbonyl group (eg, benzyloxycarbonyl, phenethyloxycarbonyl),
(32) a carbamoyl group,
(33) a thiocarbamoyl group,
(34) mono- or di-C 1-6 alkyl-carbamoyl group,
(35) C 6-14 aryl-carbamoyl group (eg, phenylcarbamoyl),
(36) 5- to 14-membered aromatic heterocyclic carbamoyl group (eg, pyridylcarbamoyl, thienylcarbamoyl),
(37) 3 to 14-membered non-aromatic heterocyclic carbamoyl group (eg, morpholinylcarbamoyl, piperidinylcarbamoyl),
(38) an optionally halogenated C 1-6 alkylsulfonyl group,
(39) a C 6-14 arylsulfonyl group,
(40) 5- to 14-membered aromatic heterocyclic sulfonyl group (eg, pyridylsulfonyl, thienylsulfonyl),
(41) an optionally halogenated C 1-6 alkylsulfinyl group,
(42) C 6-14 arylsulfinyl group (eg, phenylsulfinyl, 1-naphthylsulfinyl, 2-naphthylsulfinyl),
(43) a 5- to 14-membered aromatic heterocyclic sulfinyl group (eg, pyridylsulfinyl, thienylsulfinyl),
(44) an amino group,
(45) Mono- or di-C 1-6 alkylamino group (eg, methylamino, ethylamino, propylamino, isopropylamino, butylamino, dimethylamino, diethylamino, dipropylamino, dibutylamino, N-ethyl-N -Methylamino),
(46) mono- or di-C 6-14 arylamino group (eg, phenylamino),
(47) a 5- to 14-membered aromatic heterocyclic amino group (eg, pyridylamino),
(48) C 7-16 aralkylamino group (eg, benzylamino),
(49) formylamino group,
(50) C 1-6 alkyl-carbonylamino group (eg, acetylamino, propanoylamino, butanoylamino),
(51) (C 1-6 alkyl) (C 1-6 alkyl-carbonyl) amino group (eg, N-acetyl-N-methylamino),
(52) C 6-14 aryl-carbonylamino group (eg, phenylcarbonylamino, naphthylcarbonylamino),
(53) C 1-6 alkoxy-carbonylamino group (eg, methoxycarbonylamino, ethoxycarbonylamino, propoxycarbonylamino, butoxycarbonylamino, tert-butoxycarbonylamino),
(54) C 7-16 aralkyloxy-carbonylamino group (eg, benzyloxycarbonylamino),
(55) C 1-6 alkylsulfonylamino group (eg, methylsulfonylamino, ethylsulfonylamino),
(56) a C 6-14 arylsulfonylamino group (eg, phenylsulfonylamino, toluenesulfonylamino) optionally substituted with a C 1-6 alkyl group,
(57) an optionally halogenated C 1-6 alkyl group,
(58) a C 2-6 alkenyl group,
(59) C 2-6 alkynyl group,
(60) C 3-10 cycloalkyl group,
(61) a C 3-10 cycloalkenyl group, and (62) a C 6-14 aryl group.
[置換基群A]
(1)ハロゲン原子、
(2)ニトロ基、
(3)シアノ基、
(4)オキソ基、
(5)ヒドロキシ基、
(6)ハロゲン化されていてもよいC1-6アルコキシ基、
(7)C6-14アリールオキシ基(例、フェノキシ、ナフトキシ)、
(8)C7-16アラルキルオキシ基(例、ベンジルオキシ)、
(9)5ないし14員芳香族複素環オキシ基(例、ピリジルオキシ)、
(10)3ないし14員非芳香族複素環オキシ基(例、モルホリニルオキシ、ピペリジニルオキシ)、
(11)C1-6アルキル-カルボニルオキシ基(例、アセトキシ、プロパノイルオキシ)、
(12)C6-14アリール-カルボニルオキシ基(例、ベンゾイルオキシ、1-ナフトイルオキシ、2-ナフトイルオキシ)、
(13)C1-6アルコキシ-カルボニルオキシ基(例、メトキシカルボニルオキシ、エトキシカルボニルオキシ、プロポキシカルボニルオキシ、ブトキシカルボニルオキシ)、
(14)モノ-またはジ-C1-6アルキル-カルバモイルオキシ基(例、メチルカルバモイルオキシ、エチルカルバモイルオキシ、ジメチルカルバモイルオキシ、ジエチルカルバモイルオキシ)、
(15)C6-14アリール-カルバモイルオキシ基(例、フェニルカルバモイルオキシ、ナフチルカルバモイルオキシ)、
(16)5ないし14員芳香族複素環カルボニルオキシ基(例、ニコチノイルオキシ)、
(17)3ないし14員非芳香族複素環カルボニルオキシ基(例、モルホリニルカルボニルオキシ、ピペリジニルカルボニルオキシ)、
(18)ハロゲン化されていてもよいC1-6アルキルスルホニルオキシ基(例、メチルスルホニルオキシ、トリフルオロメチルスルホニルオキシ)、
(19)C1-6アルキル基で置換されていてもよいC6-14アリールスルホニルオキシ基(例、フェニルスルホニルオキシ、トルエンスルホニルオキシ)、
(20)ハロゲン化されていてもよいC1-6アルキルチオ基、
(21)5ないし14員芳香族複素環基、
(22)3ないし14員非芳香族複素環基、
(23)ホルミル基、
(24)カルボキシ基、
(25)ハロゲン化されていてもよいC1-6アルキル-カルボニル基、
(26)C6-14アリール-カルボニル基、
(27)5ないし14員芳香族複素環カルボニル基、
(28)3ないし14員非芳香族複素環カルボニル基、
(29)C1-6アルコキシ-カルボニル基、
(30)C6-14アリールオキシ-カルボニル基(例、フェニルオキシカルボニル、1-ナフチルオキシカルボニル、2-ナフチルオキシカルボニル)、
(31)C7-16アラルキルオキシ-カルボニル基(例、ベンジルオキシカルボニル、フェネチルオキシカルボニル)、
(32)カルバモイル基、
(33)チオカルバモイル基、
(34)モノ-またはジ-C1-6アルキル-カルバモイル基、
(35)C6-14アリール-カルバモイル基(例、フェニルカルバモイル)、
(36)5ないし14員芳香族複素環カルバモイル基(例、ピリジルカルバモイル、チエニルカルバモイル)、
(37)3ないし14員非芳香族複素環カルバモイル基(例、モルホリニルカルバモイル、ピペリジニルカルバモイル)、
(38)ハロゲン化されていてもよいC1-6アルキルスルホニル基、
(39)C6-14アリールスルホニル基、
(40)5ないし14員芳香族複素環スルホニル基(例、ピリジルスルホニル、チエニルスルホニル)、
(41)ハロゲン化されていてもよいC1-6アルキルスルフィニル基、
(42)C6-14アリールスルフィニル基(例、フェニルスルフィニル、1-ナフチルスルフィニル、2-ナフチルスルフィニル)、
(43)5ないし14員芳香族複素環スルフィニル基(例、ピリジルスルフィニル、チエニルスルフィニル)、
(44)アミノ基、
(45)モノ-またはジ-C1-6アルキルアミノ基(例、メチルアミノ、エチルアミノ、プロピルアミノ、イソプロピルアミノ、ブチルアミノ、ジメチルアミノ、ジエチルアミノ、ジプロピルアミノ、ジブチルアミノ、N-エチル-N-メチルアミノ)、
(46)モノ-またはジ-C6-14アリールアミノ基(例、フェニルアミノ)、
(47)5ないし14員芳香族複素環アミノ基(例、ピリジルアミノ)、
(48)C7-16アラルキルアミノ基(例、ベンジルアミノ)、
(49)ホルミルアミノ基、
(50)C1-6アルキル-カルボニルアミノ基(例、アセチルアミノ、プロパノイルアミノ、ブタノイルアミノ)、
(51)(C1-6アルキル)(C1-6アルキル-カルボニル)アミノ基(例、N-アセチル-N-メチルアミノ)、
(52)C6-14アリール-カルボニルアミノ基(例、フェニルカルボニルアミノ、ナフチルカルボニルアミノ)、
(53)C1-6アルコキシ-カルボニルアミノ基(例、メトキシカルボニルアミノ、エトキシカルボニルアミノ、プロポキシカルボニルアミノ、ブトキシカルボニルアミノ、tert-ブトキシカルボニルアミノ)、
(54)C7-16アラルキルオキシ-カルボニルアミノ基(例、ベンジルオキシカルボニルアミノ)、
(55)C1-6アルキルスルホニルアミノ基(例、メチルスルホニルアミノ、エチルスルホニルアミノ)、
(56)C1-6アルキル基で置換されていてもよいC6-14アリールスルホニルアミノ基(例、フェニルスルホニルアミノ、トルエンスルホニルアミノ)、
(57)ハロゲン化されていてもよいC1-6アルキル基、
(58)C2-6アルケニル基、
(59)C2-6アルキニル基、
(60)C3-10シクロアルキル基、
(61)C3-10シクロアルケニル基、及び
(62)C6-14アリール基。 In the present specification, examples of the “optionally substituted hydrocarbon group” include a hydrocarbon group which may have a substituent selected from the following substituent group A.
[Substituent group A]
(1) a halogen atom,
(2) Nitro group,
(3) a cyano group,
(4) an oxo group,
(5) a hydroxy group,
(6) an optionally halogenated C 1-6 alkoxy group,
(7) C 6-14 aryloxy group (eg, phenoxy, naphthoxy),
(8) C 7-16 aralkyloxy group (eg, benzyloxy),
(9) 5- to 14-membered aromatic heterocyclic oxy group (eg, pyridyloxy),
(10) 3 to 14-membered non-aromatic heterocyclic oxy group (eg, morpholinyloxy, piperidinyloxy),
(11) C 1-6 alkyl-carbonyloxy group (eg, acetoxy, propanoyloxy),
(12) C 6-14 aryl-carbonyloxy group (eg, benzoyloxy, 1-naphthoyloxy, 2-naphthoyloxy),
(13) C 1-6 alkoxy-carbonyloxy group (eg, methoxycarbonyloxy, ethoxycarbonyloxy, propoxycarbonyloxy, butoxycarbonyloxy),
(14) mono- or di-C 1-6 alkyl-carbamoyloxy group (eg, methylcarbamoyloxy, ethylcarbamoyloxy, dimethylcarbamoyloxy, diethylcarbamoyloxy),
(15) C 6-14 aryl-carbamoyloxy group (eg, phenylcarbamoyloxy, naphthylcarbamoyloxy),
(16) a 5- to 14-membered aromatic heterocyclic carbonyloxy group (eg, nicotinoyloxy),
(17) 3 to 14-membered non-aromatic heterocyclic carbonyloxy group (eg, morpholinylcarbonyloxy, piperidinylcarbonyloxy),
(18) an optionally halogenated C 1-6 alkylsulfonyloxy group (eg, methylsulfonyloxy, trifluoromethylsulfonyloxy),
(19) a C 6-14 arylsulfonyloxy group (eg, phenylsulfonyloxy, toluenesulfonyloxy) optionally substituted with a C 1-6 alkyl group,
(20) an optionally halogenated C 1-6 alkylthio group,
(21) a 5- to 14-membered aromatic heterocyclic group,
(22) a 3- to 14-membered non-aromatic heterocyclic group,
(23) formyl group,
(24) a carboxy group,
(25) an optionally halogenated C 1-6 alkyl-carbonyl group,
(26) a C 6-14 aryl-carbonyl group,
(27) a 5- to 14-membered aromatic heterocyclic carbonyl group,
(28) a 3- to 14-membered non-aromatic heterocyclic carbonyl group,
(29) a C 1-6 alkoxy-carbonyl group,
(30) C 6-14 aryloxy-carbonyl group (eg, phenyloxycarbonyl, 1-naphthyloxycarbonyl, 2-naphthyloxycarbonyl),
(31) C 7-16 aralkyloxy-carbonyl group (eg, benzyloxycarbonyl, phenethyloxycarbonyl),
(32) a carbamoyl group,
(33) a thiocarbamoyl group,
(34) mono- or di-C 1-6 alkyl-carbamoyl group,
(35) C 6-14 aryl-carbamoyl group (eg, phenylcarbamoyl),
(36) 5- to 14-membered aromatic heterocyclic carbamoyl group (eg, pyridylcarbamoyl, thienylcarbamoyl),
(37) 3 to 14-membered non-aromatic heterocyclic carbamoyl group (eg, morpholinylcarbamoyl, piperidinylcarbamoyl),
(38) an optionally halogenated C 1-6 alkylsulfonyl group,
(39) a C 6-14 arylsulfonyl group,
(40) 5- to 14-membered aromatic heterocyclic sulfonyl group (eg, pyridylsulfonyl, thienylsulfonyl),
(41) an optionally halogenated C 1-6 alkylsulfinyl group,
(42) C 6-14 arylsulfinyl group (eg, phenylsulfinyl, 1-naphthylsulfinyl, 2-naphthylsulfinyl),
(43) a 5- to 14-membered aromatic heterocyclic sulfinyl group (eg, pyridylsulfinyl, thienylsulfinyl),
(44) an amino group,
(45) Mono- or di-C 1-6 alkylamino group (eg, methylamino, ethylamino, propylamino, isopropylamino, butylamino, dimethylamino, diethylamino, dipropylamino, dibutylamino, N-ethyl-N -Methylamino),
(46) mono- or di-C 6-14 arylamino group (eg, phenylamino),
(47) a 5- to 14-membered aromatic heterocyclic amino group (eg, pyridylamino),
(48) C 7-16 aralkylamino group (eg, benzylamino),
(49) formylamino group,
(50) C 1-6 alkyl-carbonylamino group (eg, acetylamino, propanoylamino, butanoylamino),
(51) (C 1-6 alkyl) (C 1-6 alkyl-carbonyl) amino group (eg, N-acetyl-N-methylamino),
(52) C 6-14 aryl-carbonylamino group (eg, phenylcarbonylamino, naphthylcarbonylamino),
(53) C 1-6 alkoxy-carbonylamino group (eg, methoxycarbonylamino, ethoxycarbonylamino, propoxycarbonylamino, butoxycarbonylamino, tert-butoxycarbonylamino),
(54) C 7-16 aralkyloxy-carbonylamino group (eg, benzyloxycarbonylamino),
(55) C 1-6 alkylsulfonylamino group (eg, methylsulfonylamino, ethylsulfonylamino),
(56) a C 6-14 arylsulfonylamino group (eg, phenylsulfonylamino, toluenesulfonylamino) optionally substituted with a C 1-6 alkyl group,
(57) an optionally halogenated C 1-6 alkyl group,
(58) a C 2-6 alkenyl group,
(59) C 2-6 alkynyl group,
(60) C 3-10 cycloalkyl group,
(61) a C 3-10 cycloalkenyl group, and (62) a C 6-14 aryl group.
「置換されていてもよい炭化水素基」における上記置換基の数は、例えば、1ないし5個、好ましくは1ないし3個である。置換基数が2個以上の場合、各置換基は同一であっても異なっていてもよい。
本明細書中、「複素環基」(「置換されていてもよい複素環基」における「複素環基」を含む)としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子をそれぞれ含有する、(i)芳香族複素環基、(ii)非芳香族複素環基および(iii)7ないし10員複素架橋環基が挙げられる。 The number of the substituents in the “optionally substituted hydrocarbon group” is, for example, 1 to 5, preferably 1 to 3. When the number of substituents is 2 or more, each substituent may be the same or different.
In the present specification, examples of the “heterocyclic group” (including the “heterocyclic group” in the “optionally substituted heterocyclic group”) include, for example, a nitrogen atom, a sulfur atom and a ring atom other than a carbon atom. (I) an aromatic heterocyclic group, (ii) a non-aromatic heterocyclic group, and (iii) a 7 to 10-membered heterocyclic bridge group each containing 1 to 4 heteroatoms selected from oxygen atoms .
本明細書中、「複素環基」(「置換されていてもよい複素環基」における「複素環基」を含む)としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子をそれぞれ含有する、(i)芳香族複素環基、(ii)非芳香族複素環基および(iii)7ないし10員複素架橋環基が挙げられる。 The number of the substituents in the “optionally substituted hydrocarbon group” is, for example, 1 to 5, preferably 1 to 3. When the number of substituents is 2 or more, each substituent may be the same or different.
In the present specification, examples of the “heterocyclic group” (including the “heterocyclic group” in the “optionally substituted heterocyclic group”) include, for example, a nitrogen atom, a sulfur atom and a ring atom other than a carbon atom. (I) an aromatic heterocyclic group, (ii) a non-aromatic heterocyclic group, and (iii) a 7 to 10-membered heterocyclic bridge group each containing 1 to 4 heteroatoms selected from oxygen atoms .
本明細書中、「芳香族複素環基」(「5ないし14員芳香族複素環基」を含む)としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子を含有する5ないし14員(好ましくは5ないし10員)の芳香族複素環基が挙げられる。
該「芳香族複素環基」の好適な例としては、チエニル、フリル、ピロリル、イミダゾリル、ピラゾリル、チアゾリル、イソチアゾリル、オキサゾリル、イソオキサゾリル、ピリジル、ピラジニル、ピリミジニル、ピリダジニル、1,2,4-オキサジアゾリル、1,3,4-オキサジアゾリル、1,2,4-チアジアゾリル、1,3,4-チアジアゾリル、トリアゾリル、テトラゾリル、トリアジニルなどの5ないし6員単環式芳香族複素環基;
ベンゾチオフェニル、ベンゾフラニル、ベンゾイミダゾリル、ベンゾオキサゾリル、ベンゾイソオキサゾリル、ベンゾチアゾリル、ベンゾイソチアゾリル、ベンゾトリアゾリル、イミダゾピリジニル、チエノピリジニル、フロピリジニル、ピロロピリジニル、ピラゾロピリジニル、オキサゾロピリジニル、チアゾロピリジニル、イミダゾピラジニル、イミダゾピリミジニル、チエノピリミジニル、フロピリミジニル、ピロロピリミジニル、ピラゾロピリミジニル、オキサゾロピリミジニル、チアゾロピリミジニル、ピラゾロトリアジニル、ナフト[2,3-b]チエニル、フェノキサチイニル、インドリル、イソインドリル、1H-インダゾリル、プリニル、イソキノリル、キノリル、フタラジニル、ナフチリジニル、キノキサリニル、キナゾリニル、シンノリニル、カルバゾリル、β-カルボリニル、フェナントリジニル、アクリジニル、フェナジニル、フェノチアジニル、フェノキサジニルなどの8ないし14員縮合多環式(好ましくは2または3環式)芳香族複素環基が挙げられる。 In the present specification, the “aromatic heterocyclic group” (including the “5- to 14-membered aromatic heterocyclic group”) is, for example, selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom. And 5- to 14-membered (preferably 5- to 10-membered) aromatic heterocyclic group containing 1 to 4 heteroatoms.
Suitable examples of the “aromatic heterocyclic group” include thienyl, furyl, pyrrolyl, imidazolyl, pyrazolyl, thiazolyl, isothiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, 1,2,4-oxadiazolyl, 1 5-, 6-membered monocyclic aromatic heterocyclic groups such as 1,3,4-oxadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl, triazolyl, tetrazolyl, triazinyl;
Benzothiophenyl, benzofuranyl, benzoimidazolyl, benzoxazolyl, benzisoxazolyl, benzothiazolyl, benzisothiazolyl, benzotriazolyl, imidazopyridinyl, thienopyridinyl, furopyridinyl, pyrrolopyridinyl, pyrazolopyridinyl, oxazolo Pyridinyl, thiazolopyridinyl, imidazopyrazinyl, imidazopyrimidinyl, thienopyrimidinyl, furopyrimidinyl, pyrrolopyrimidinyl, pyrazolopyrimidinyl, oxazolopyrimidinyl, thiazolopyrimidinyl, pyrazolotriazinyl, naphtho [2,3 -B] thienyl, phenoxathiinyl, indolyl, isoindolyl, 1H-indazolyl, purinyl, isoquinolyl, quinolyl, phthalazinyl, naphthyridinyl, quinoxalinyl, quina And 8- to 14-membered condensed polycyclic (preferably 2 or 3 ring) aromatic heterocyclic groups such as linyl, cinnolinyl, carbazolyl, β-carbolinyl, phenanthridinyl, acridinyl, phenazinyl, phenothiazinyl, phenoxazinyl .
該「芳香族複素環基」の好適な例としては、チエニル、フリル、ピロリル、イミダゾリル、ピラゾリル、チアゾリル、イソチアゾリル、オキサゾリル、イソオキサゾリル、ピリジル、ピラジニル、ピリミジニル、ピリダジニル、1,2,4-オキサジアゾリル、1,3,4-オキサジアゾリル、1,2,4-チアジアゾリル、1,3,4-チアジアゾリル、トリアゾリル、テトラゾリル、トリアジニルなどの5ないし6員単環式芳香族複素環基;
ベンゾチオフェニル、ベンゾフラニル、ベンゾイミダゾリル、ベンゾオキサゾリル、ベンゾイソオキサゾリル、ベンゾチアゾリル、ベンゾイソチアゾリル、ベンゾトリアゾリル、イミダゾピリジニル、チエノピリジニル、フロピリジニル、ピロロピリジニル、ピラゾロピリジニル、オキサゾロピリジニル、チアゾロピリジニル、イミダゾピラジニル、イミダゾピリミジニル、チエノピリミジニル、フロピリミジニル、ピロロピリミジニル、ピラゾロピリミジニル、オキサゾロピリミジニル、チアゾロピリミジニル、ピラゾロトリアジニル、ナフト[2,3-b]チエニル、フェノキサチイニル、インドリル、イソインドリル、1H-インダゾリル、プリニル、イソキノリル、キノリル、フタラジニル、ナフチリジニル、キノキサリニル、キナゾリニル、シンノリニル、カルバゾリル、β-カルボリニル、フェナントリジニル、アクリジニル、フェナジニル、フェノチアジニル、フェノキサジニルなどの8ないし14員縮合多環式(好ましくは2または3環式)芳香族複素環基が挙げられる。 In the present specification, the “aromatic heterocyclic group” (including the “5- to 14-membered aromatic heterocyclic group”) is, for example, selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom. And 5- to 14-membered (preferably 5- to 10-membered) aromatic heterocyclic group containing 1 to 4 heteroatoms.
Suitable examples of the “aromatic heterocyclic group” include thienyl, furyl, pyrrolyl, imidazolyl, pyrazolyl, thiazolyl, isothiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, 1,2,4-oxadiazolyl, 1 5-, 6-membered monocyclic aromatic heterocyclic groups such as 1,3,4-oxadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl, triazolyl, tetrazolyl, triazinyl;
Benzothiophenyl, benzofuranyl, benzoimidazolyl, benzoxazolyl, benzisoxazolyl, benzothiazolyl, benzisothiazolyl, benzotriazolyl, imidazopyridinyl, thienopyridinyl, furopyridinyl, pyrrolopyridinyl, pyrazolopyridinyl, oxazolo Pyridinyl, thiazolopyridinyl, imidazopyrazinyl, imidazopyrimidinyl, thienopyrimidinyl, furopyrimidinyl, pyrrolopyrimidinyl, pyrazolopyrimidinyl, oxazolopyrimidinyl, thiazolopyrimidinyl, pyrazolotriazinyl, naphtho [2,3 -B] thienyl, phenoxathiinyl, indolyl, isoindolyl, 1H-indazolyl, purinyl, isoquinolyl, quinolyl, phthalazinyl, naphthyridinyl, quinoxalinyl, quina And 8- to 14-membered condensed polycyclic (preferably 2 or 3 ring) aromatic heterocyclic groups such as linyl, cinnolinyl, carbazolyl, β-carbolinyl, phenanthridinyl, acridinyl, phenazinyl, phenothiazinyl, phenoxazinyl .
本明細書中、「非芳香族複素環基」(「3ないし14員非芳香族複素環基」を含む)としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子を含有する3ないし14員(好ましくは4ないし10員)の非芳香族複素環基が挙げられる。
該「非芳香族複素環基」の好適な例としては、アジリジニル、オキシラニル、チイラニル、アゼチジニル、オキセタニル、チエタニル、テトラヒドロチエニル、テトラヒドロフラニル、ピロリニル、ピロリジニル、イミダゾリニル、イミダゾリジニル、オキサゾリニル、オキサゾリジニル、ピラゾリニル、ピラゾリジニル、チアゾリニル、チアゾリジニル、テトラヒドロイソチアゾリル、テトラヒドロオキサゾリル、テトラヒドロイソオキサゾリル、ピペリジニル、ピペラジニル、テトラヒドロピリジニル、ジヒドロピリジニル、ジヒドロチオピラニル、テトラヒドロピリミジニル、テトラヒドロピリダジニル、ジヒドロピラニル、テトラヒドロピラニル、テトラヒドロチオピラニル、モルホリニル、チオモルホリニル、アゼパニル、ジアゼパニル、アゼピニル、オキセパニル、アゾカニル、ジアゾカニルなどの3ないし8員単環式非芳香族複素環基;
ジヒドロベンゾフラニル、ジヒドロベンゾイミダゾリル、ジヒドロベンゾオキサゾリル、ジヒドロベンゾチアゾリル、ジヒドロベンゾイソチアゾリル、ジヒドロナフト[2,3-b]チエニル、テトラヒドロイソキノリル、テトラヒドロキノリル、4H-キノリジニル、インドリニル、イソインドリニル、テトラヒドロチエノ[2,3-c]ピリジニル、テトラヒドロベンゾアゼピニル、テトラヒドロキノキサリニル、テトラヒドロフェナントリジニル、ヘキサヒドロフェノチアジニル、ヘキサヒドロフェノキサジニル、テトラヒドロフタラジニル、テトラヒドロナフチリジニル、テトラヒドロキナゾリニル、テトラヒドロシンノリニル、テトラヒドロカルバゾリル、テトラヒドロ-β-カルボリニル、テトラヒドロアクリジニル、テトラヒドロフェナジニル、テトラヒドロチオキサンテニル、オクタヒドロイソキノリルなどの9ないし14員縮合多環式(好ましくは2または3環式)非芳香族複素環基が挙げられる。 In the present specification, examples of the “non-aromatic heterocyclic group” (including the “3- to 14-membered non-aromatic heterocyclic group”) include, for example, a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom. 3 to 14-membered (preferably 4 to 10-membered) non-aromatic heterocyclic group containing 1 to 4 heteroatoms selected from
Suitable examples of the “non-aromatic heterocyclic group” include aziridinyl, oxiranyl, thiylyl, azetidinyl, oxetanyl, thietanyl, tetrahydrothienyl, tetrahydrofuranyl, pyrrolinyl, pyrrolidinyl, imidazolinyl, imidazolidinyl, oxazolinyl, oxazolidinyl, pyrazolidinyl, pyrazolinyl, pyrazolinyl Thiazolinyl, thiazolidinyl, tetrahydroisothiazolyl, tetrahydrooxazolyl, tetrahydroisoxazolyl, piperidinyl, piperazinyl, tetrahydropyridinyl, dihydropyridinyl, dihydrothiopyranyl, tetrahydropyrimidinyl, tetrahydropyridazinyl, dihydropyrani , Tetrahydropyranyl, tetrahydrothiopyranyl, morpholinyl, thiomorpholinyl, azepanyl, diaze Cycloalkenyl, azepinyl, oxepanyl, azocanyl, 3 to 8-membered monocyclic non-aromatic heterocyclic group such as Jiazokaniru;
Dihydrobenzofuranyl, dihydrobenzimidazolyl, dihydrobenzoxazolyl, dihydrobenzothiazolyl, dihydrobenzoisothiazolyl, dihydronaphtho [2,3-b] thienyl, tetrahydroisoquinolyl, tetrahydroquinolyl, 4H-quinolidinyl, Indolinyl, isoindolinyl, tetrahydrothieno [2,3-c] pyridinyl, tetrahydrobenzoazepinyl, tetrahydroquinoxalinyl, tetrahydrophenanthridinyl, hexahydrophenothiazinyl, hexahydrophenoxazinyl, tetrahydrophthalazinyl, tetrahydro Naphthyridinyl, tetrahydroquinazolinyl, tetrahydrocinnolinyl, tetrahydrocarbazolyl, tetrahydro-β-carbolinyl, tetrahydroacridinyl, tetrahydro Rofenajiniru, tetrahydrothiophenyl key Sante alkenyl, 9 to 14 membered fused polycyclic, such as octahydro-isoquinolylmethyl (preferably 2 or tricyclic), and the non-aromatic heterocyclic group.
該「非芳香族複素環基」の好適な例としては、アジリジニル、オキシラニル、チイラニル、アゼチジニル、オキセタニル、チエタニル、テトラヒドロチエニル、テトラヒドロフラニル、ピロリニル、ピロリジニル、イミダゾリニル、イミダゾリジニル、オキサゾリニル、オキサゾリジニル、ピラゾリニル、ピラゾリジニル、チアゾリニル、チアゾリジニル、テトラヒドロイソチアゾリル、テトラヒドロオキサゾリル、テトラヒドロイソオキサゾリル、ピペリジニル、ピペラジニル、テトラヒドロピリジニル、ジヒドロピリジニル、ジヒドロチオピラニル、テトラヒドロピリミジニル、テトラヒドロピリダジニル、ジヒドロピラニル、テトラヒドロピラニル、テトラヒドロチオピラニル、モルホリニル、チオモルホリニル、アゼパニル、ジアゼパニル、アゼピニル、オキセパニル、アゾカニル、ジアゾカニルなどの3ないし8員単環式非芳香族複素環基;
ジヒドロベンゾフラニル、ジヒドロベンゾイミダゾリル、ジヒドロベンゾオキサゾリル、ジヒドロベンゾチアゾリル、ジヒドロベンゾイソチアゾリル、ジヒドロナフト[2,3-b]チエニル、テトラヒドロイソキノリル、テトラヒドロキノリル、4H-キノリジニル、インドリニル、イソインドリニル、テトラヒドロチエノ[2,3-c]ピリジニル、テトラヒドロベンゾアゼピニル、テトラヒドロキノキサリニル、テトラヒドロフェナントリジニル、ヘキサヒドロフェノチアジニル、ヘキサヒドロフェノキサジニル、テトラヒドロフタラジニル、テトラヒドロナフチリジニル、テトラヒドロキナゾリニル、テトラヒドロシンノリニル、テトラヒドロカルバゾリル、テトラヒドロ-β-カルボリニル、テトラヒドロアクリジニル、テトラヒドロフェナジニル、テトラヒドロチオキサンテニル、オクタヒドロイソキノリルなどの9ないし14員縮合多環式(好ましくは2または3環式)非芳香族複素環基が挙げられる。 In the present specification, examples of the “non-aromatic heterocyclic group” (including the “3- to 14-membered non-aromatic heterocyclic group”) include, for example, a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring-constituting atom. 3 to 14-membered (preferably 4 to 10-membered) non-aromatic heterocyclic group containing 1 to 4 heteroatoms selected from
Suitable examples of the “non-aromatic heterocyclic group” include aziridinyl, oxiranyl, thiylyl, azetidinyl, oxetanyl, thietanyl, tetrahydrothienyl, tetrahydrofuranyl, pyrrolinyl, pyrrolidinyl, imidazolinyl, imidazolidinyl, oxazolinyl, oxazolidinyl, pyrazolidinyl, pyrazolinyl, pyrazolinyl Thiazolinyl, thiazolidinyl, tetrahydroisothiazolyl, tetrahydrooxazolyl, tetrahydroisoxazolyl, piperidinyl, piperazinyl, tetrahydropyridinyl, dihydropyridinyl, dihydrothiopyranyl, tetrahydropyrimidinyl, tetrahydropyridazinyl, dihydropyrani , Tetrahydropyranyl, tetrahydrothiopyranyl, morpholinyl, thiomorpholinyl, azepanyl, diaze Cycloalkenyl, azepinyl, oxepanyl, azocanyl, 3 to 8-membered monocyclic non-aromatic heterocyclic group such as Jiazokaniru;
Dihydrobenzofuranyl, dihydrobenzimidazolyl, dihydrobenzoxazolyl, dihydrobenzothiazolyl, dihydrobenzoisothiazolyl, dihydronaphtho [2,3-b] thienyl, tetrahydroisoquinolyl, tetrahydroquinolyl, 4H-quinolidinyl, Indolinyl, isoindolinyl, tetrahydrothieno [2,3-c] pyridinyl, tetrahydrobenzoazepinyl, tetrahydroquinoxalinyl, tetrahydrophenanthridinyl, hexahydrophenothiazinyl, hexahydrophenoxazinyl, tetrahydrophthalazinyl, tetrahydro Naphthyridinyl, tetrahydroquinazolinyl, tetrahydrocinnolinyl, tetrahydrocarbazolyl, tetrahydro-β-carbolinyl, tetrahydroacridinyl, tetrahydro Rofenajiniru, tetrahydrothiophenyl key Sante alkenyl, 9 to 14 membered fused polycyclic, such as octahydro-isoquinolylmethyl (preferably 2 or tricyclic), and the non-aromatic heterocyclic group.
本明細書中、「7ないし10員複素架橋環基」の好適な例としては、キヌクリジニル、7-アザビシクロ[2.2.1]ヘプタニルが挙げられる。
本明細書中、「含窒素複素環基」としては、「複素環基」のうち、環構成原子として少なくとも1個以上の窒素原子を含有するものが挙げられる。
本明細書中、「置換されていてもよい複素環基」としては、例えば、前記した置換基群Aから選ばれる置換基を有していてもよい複素環基が挙げられる。
「置換されていてもよい複素環基」における置換基の数は、例えば、1ないし3個である。置換基数が2個以上の場合、各置換基は同一であっても異なっていてもよい。 In the present specification, preferable examples of the “7 to 10-membered heterocyclic bridged ring group” include quinuclidinyl and 7-azabicyclo [2.2.1] heptanyl.
In the present specification, examples of the “nitrogen-containing heterocyclic group” include those containing at least one nitrogen atom as a ring-constituting atom among the “heterocyclic groups”.
In the present specification, examples of the “optionally substituted heterocyclic group” include a heterocyclic group which may have a substituent selected from the substituent group A described above.
The number of substituents in the “optionally substituted heterocyclic group” is, for example, 1 to 3. When the number of substituents is 2 or more, each substituent may be the same or different.
本明細書中、「含窒素複素環基」としては、「複素環基」のうち、環構成原子として少なくとも1個以上の窒素原子を含有するものが挙げられる。
本明細書中、「置換されていてもよい複素環基」としては、例えば、前記した置換基群Aから選ばれる置換基を有していてもよい複素環基が挙げられる。
「置換されていてもよい複素環基」における置換基の数は、例えば、1ないし3個である。置換基数が2個以上の場合、各置換基は同一であっても異なっていてもよい。 In the present specification, preferable examples of the “7 to 10-membered heterocyclic bridged ring group” include quinuclidinyl and 7-azabicyclo [2.2.1] heptanyl.
In the present specification, examples of the “nitrogen-containing heterocyclic group” include those containing at least one nitrogen atom as a ring-constituting atom among the “heterocyclic groups”.
In the present specification, examples of the “optionally substituted heterocyclic group” include a heterocyclic group which may have a substituent selected from the substituent group A described above.
The number of substituents in the “optionally substituted heterocyclic group” is, for example, 1 to 3. When the number of substituents is 2 or more, each substituent may be the same or different.
本明細書中、「アシル基」としては、例えば、「ハロゲン原子、ハロゲン化されていてもよいC1-6アルコキシ基、ヒドロキシ基、ニトロ基、シアノ基、アミノ基およびカルバモイル基から選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C3-10シクロアルケニル基、C6-14アリール基、C7-16アラルキル基、5ないし14員芳香族複素環基および3ないし14員非芳香族複素環基から選ばれる1または2個の置換基」をそれぞれ有していてもよい、ホルミル基、カルボキシ基、カルバモイル基、チオカルバモイル基、スルフィノ基、スルホ基、スルファモイル基、ホスホノ基が挙げられる。
また、「アシル基」としては、炭化水素-スルホニル基、複素環-スルホニル基、炭化水素-スルフィニル基、複素環-スルフィニル基も挙げられる。
ここで、炭化水素-スルホニル基とは、炭化水素基が結合したスルホニル基を、複素環-スルホニル基とは、複素環基が結合したスルホニル基を、炭化水素-スルフィニル基とは、炭化水素基が結合したスルフィニル基を、複素環-スルフィニル基とは、複素環基が結合したスルフィニル基を、それぞれ意味する。
「アシル基」の好適な例としては、ホルミル基、カルボキシ基、C1-6アルキル-カルボニル基、C2-6アルケニル-カルボニル基(例、クロトノイル)、C3-10シクロアルキル-カルボニル基(例、シクロブタンカルボニル、シクロペンタンカルボニル、シクロヘキサンカルボニル、シクロヘプタンカルボニル)、C3-10シクロアルケニル-カルボニル基(例、2-シクロヘキセンカルボニル)、C6-14アリール-カルボニル基、C7-16アラルキル-カルボニル基、5ないし14員芳香族複素環カルボニル基、3ないし14員非芳香族複素環カルボニル基、C1-6アルコキシ-カルボニル基、C6-14アリールオキシ-カルボニル基(例、フェニルオキシカルボニル、ナフチルオキシカルボニル)、C7-16アラルキルオキシ-カルボニル基(例、ベンジルオキシカルボニル、フェネチルオキシカルボニル)、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基、モノ-またはジ-C2-6アルケニル-カルバモイル基(例、ジアリルカルバモイル)、モノ-またはジ-C3-10シクロアルキル-カルバモイル基(例、シクロプロピルカルバモイル)、モノ-またはジ-C6-14アリール-カルバモイル基(例、フェニルカルバモイル)、モノ-またはジ-C7-16アラルキル-カルバモイル基、5ないし14員芳香族複素環カルバモイル基(例、ピリジルカルバモイル)、チオカルバモイル基、モノ-またはジ-C1-6アルキル-チオカルバモイル基(例、メチルチオカルバモイル、N-エチル-N-メチルチオカルバモイル)、モノ-またはジ-C2-6アルケニル-チオカルバモイル基(例、ジアリルチオカルバモイル)、モノ-またはジ-C3-10シクロアルキル-チオカルバモイル基(例、シクロプロピルチオカルバモイル、シクロヘキシルチオカルバモイル)、モノ-またはジ-C6-14アリール-チオカルバモイル基(例、フェニルチオカルバモイル)、モノ-またはジ-C7-16アラルキル-チオカルバモイル基(例、ベンジルチオカルバモイル、フェネチルチオカルバモイル)、5ないし14員芳香族複素環チオカルバモイル基(例、ピリジルチオカルバモイル)、スルフィノ基、C1-6アルキルスルフィニル基(例、メチルスルフィニル、エチルスルフィニル)、スルホ基、C1-6アルキルスルホニル基、C6-14アリールスルホニル基、ホスホノ基、モノ-またはジ-C1-6アルキルホスホノ基(例、ジメチルホスホノ、ジエチルホスホノ、ジイソプロピルホスホノ、ジブチルホスホノ)が挙げられる。 In the present specification, the “acyl group” is, for example, “1 selected from a halogen atom, an optionally halogenated C 1-6 alkoxy group, a hydroxy group, a nitro group, a cyano group, an amino group, and a carbamoyl group. C 1-6 alkyl group, C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 3-10 cycloalkenyl group, C 6-14 aryl each optionally having 3 substituents Formyl optionally having 1 or 2 substituents selected from the group, a C 7-16 aralkyl group, a 5- to 14-membered aromatic heterocyclic group and a 3- to 14-membered non-aromatic heterocyclic group ” Group, carboxy group, carbamoyl group, thiocarbamoyl group, sulfino group, sulfo group, sulfamoyl group and phosphono group.
The “acyl group” also includes a hydrocarbon-sulfonyl group, a heterocyclic-sulfonyl group, a hydrocarbon-sulfinyl group, and a heterocyclic-sulfinyl group.
Here, the hydrocarbon-sulfonyl group is a sulfonyl group to which a hydrocarbon group is bonded, the heterocyclic-sulfonyl group is a sulfonyl group to which a heterocyclic group is bonded, and the hydrocarbon-sulfinyl group is a hydrocarbon group. A sulfinyl group to which is bonded and a heterocyclic-sulfinyl group mean a sulfinyl group to which a heterocyclic group is bonded.
As preferable examples of the “acyl group”, a formyl group, a carboxy group, a C 1-6 alkyl-carbonyl group, a C 2-6 alkenyl-carbonyl group (eg, crotonoyl), a C 3-10 cycloalkyl-carbonyl group ( Examples, cyclobutanecarbonyl, cyclopentanecarbonyl, cyclohexanecarbonyl, cycloheptanecarbonyl), C 3-10 cycloalkenyl-carbonyl group (eg, 2-cyclohexenecarbonyl), C 6-14 aryl-carbonyl group, C 7-16 aralkyl- Carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, C 6-14 aryloxy-carbonyl group (eg, phenyloxycarbonyl) , naphthyloxycarbonyl), C 7- 6 aralkyloxy - carbonyl group (e.g., benzyloxycarbonyl, phenethyloxycarbonyl carbonyl), a carbamoyl group, mono- - or di -C 1-6 alkyl - carbamoyl group, mono- - or di -C 2-6 alkenyl - carbamoyl group (e.g. , Diallylcarbamoyl), mono- or di-C 3-10 cycloalkyl-carbamoyl group (eg, cyclopropylcarbamoyl), mono- or di-C 6-14 aryl-carbamoyl group (eg, phenylcarbamoyl), mono- or Di-C 7-16 aralkyl-carbamoyl group, 5- to 14-membered aromatic heterocyclic carbamoyl group (eg, pyridylcarbamoyl), thiocarbamoyl group, mono- or di-C 1-6 alkyl-thiocarbamoyl group (eg, methylthio) Carbamoyl, N-ethyl-N-methyl Okarubamoiru), mono - or di -C 2-6 alkenyl - thiocarbamoyl group (e.g., diallyl thio carbamoyl), mono - or di -C 3-10 cycloalkyl - thiocarbamoyl group (e.g., cyclopropyl thiocarbamoyl, cyclohexyl Thiocarbamoyl), mono- or di-C 6-14 aryl-thiocarbamoyl group (eg, phenylthiocarbamoyl), mono- or di-C 7-16 aralkyl-thiocarbamoyl group (eg, benzylthiocarbamoyl, phenethylthiocarbamoyl) ) 5- to 14-membered aromatic heterocyclic thiocarbamoyl group (eg, pyridylthiocarbamoyl), sulfino group, C 1-6 alkylsulfinyl group (eg, methylsulfinyl, ethylsulfinyl), sulfo group, C 1-6 alkylsulfonyl group, C 6- 4 arylsulfonyl group, a phosphono group, a mono - or di -C 1-6 alkyl phosphono group (e.g., dimethylphosphono, diethylphosphono, diisopropylphosphono, dibutylphosphono) and the like.
また、「アシル基」としては、炭化水素-スルホニル基、複素環-スルホニル基、炭化水素-スルフィニル基、複素環-スルフィニル基も挙げられる。
ここで、炭化水素-スルホニル基とは、炭化水素基が結合したスルホニル基を、複素環-スルホニル基とは、複素環基が結合したスルホニル基を、炭化水素-スルフィニル基とは、炭化水素基が結合したスルフィニル基を、複素環-スルフィニル基とは、複素環基が結合したスルフィニル基を、それぞれ意味する。
「アシル基」の好適な例としては、ホルミル基、カルボキシ基、C1-6アルキル-カルボニル基、C2-6アルケニル-カルボニル基(例、クロトノイル)、C3-10シクロアルキル-カルボニル基(例、シクロブタンカルボニル、シクロペンタンカルボニル、シクロヘキサンカルボニル、シクロヘプタンカルボニル)、C3-10シクロアルケニル-カルボニル基(例、2-シクロヘキセンカルボニル)、C6-14アリール-カルボニル基、C7-16アラルキル-カルボニル基、5ないし14員芳香族複素環カルボニル基、3ないし14員非芳香族複素環カルボニル基、C1-6アルコキシ-カルボニル基、C6-14アリールオキシ-カルボニル基(例、フェニルオキシカルボニル、ナフチルオキシカルボニル)、C7-16アラルキルオキシ-カルボニル基(例、ベンジルオキシカルボニル、フェネチルオキシカルボニル)、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基、モノ-またはジ-C2-6アルケニル-カルバモイル基(例、ジアリルカルバモイル)、モノ-またはジ-C3-10シクロアルキル-カルバモイル基(例、シクロプロピルカルバモイル)、モノ-またはジ-C6-14アリール-カルバモイル基(例、フェニルカルバモイル)、モノ-またはジ-C7-16アラルキル-カルバモイル基、5ないし14員芳香族複素環カルバモイル基(例、ピリジルカルバモイル)、チオカルバモイル基、モノ-またはジ-C1-6アルキル-チオカルバモイル基(例、メチルチオカルバモイル、N-エチル-N-メチルチオカルバモイル)、モノ-またはジ-C2-6アルケニル-チオカルバモイル基(例、ジアリルチオカルバモイル)、モノ-またはジ-C3-10シクロアルキル-チオカルバモイル基(例、シクロプロピルチオカルバモイル、シクロヘキシルチオカルバモイル)、モノ-またはジ-C6-14アリール-チオカルバモイル基(例、フェニルチオカルバモイル)、モノ-またはジ-C7-16アラルキル-チオカルバモイル基(例、ベンジルチオカルバモイル、フェネチルチオカルバモイル)、5ないし14員芳香族複素環チオカルバモイル基(例、ピリジルチオカルバモイル)、スルフィノ基、C1-6アルキルスルフィニル基(例、メチルスルフィニル、エチルスルフィニル)、スルホ基、C1-6アルキルスルホニル基、C6-14アリールスルホニル基、ホスホノ基、モノ-またはジ-C1-6アルキルホスホノ基(例、ジメチルホスホノ、ジエチルホスホノ、ジイソプロピルホスホノ、ジブチルホスホノ)が挙げられる。 In the present specification, the “acyl group” is, for example, “1 selected from a halogen atom, an optionally halogenated C 1-6 alkoxy group, a hydroxy group, a nitro group, a cyano group, an amino group, and a carbamoyl group. C 1-6 alkyl group, C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 3-10 cycloalkenyl group, C 6-14 aryl each optionally having 3 substituents Formyl optionally having 1 or 2 substituents selected from the group, a C 7-16 aralkyl group, a 5- to 14-membered aromatic heterocyclic group and a 3- to 14-membered non-aromatic heterocyclic group ” Group, carboxy group, carbamoyl group, thiocarbamoyl group, sulfino group, sulfo group, sulfamoyl group and phosphono group.
The “acyl group” also includes a hydrocarbon-sulfonyl group, a heterocyclic-sulfonyl group, a hydrocarbon-sulfinyl group, and a heterocyclic-sulfinyl group.
Here, the hydrocarbon-sulfonyl group is a sulfonyl group to which a hydrocarbon group is bonded, the heterocyclic-sulfonyl group is a sulfonyl group to which a heterocyclic group is bonded, and the hydrocarbon-sulfinyl group is a hydrocarbon group. A sulfinyl group to which is bonded and a heterocyclic-sulfinyl group mean a sulfinyl group to which a heterocyclic group is bonded.
As preferable examples of the “acyl group”, a formyl group, a carboxy group, a C 1-6 alkyl-carbonyl group, a C 2-6 alkenyl-carbonyl group (eg, crotonoyl), a C 3-10 cycloalkyl-carbonyl group ( Examples, cyclobutanecarbonyl, cyclopentanecarbonyl, cyclohexanecarbonyl, cycloheptanecarbonyl), C 3-10 cycloalkenyl-carbonyl group (eg, 2-cyclohexenecarbonyl), C 6-14 aryl-carbonyl group, C 7-16 aralkyl- Carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, C 6-14 aryloxy-carbonyl group (eg, phenyloxycarbonyl) , naphthyloxycarbonyl), C 7- 6 aralkyloxy - carbonyl group (e.g., benzyloxycarbonyl, phenethyloxycarbonyl carbonyl), a carbamoyl group, mono- - or di -C 1-6 alkyl - carbamoyl group, mono- - or di -C 2-6 alkenyl - carbamoyl group (e.g. , Diallylcarbamoyl), mono- or di-C 3-10 cycloalkyl-carbamoyl group (eg, cyclopropylcarbamoyl), mono- or di-C 6-14 aryl-carbamoyl group (eg, phenylcarbamoyl), mono- or Di-C 7-16 aralkyl-carbamoyl group, 5- to 14-membered aromatic heterocyclic carbamoyl group (eg, pyridylcarbamoyl), thiocarbamoyl group, mono- or di-C 1-6 alkyl-thiocarbamoyl group (eg, methylthio) Carbamoyl, N-ethyl-N-methyl Okarubamoiru), mono - or di -C 2-6 alkenyl - thiocarbamoyl group (e.g., diallyl thio carbamoyl), mono - or di -C 3-10 cycloalkyl - thiocarbamoyl group (e.g., cyclopropyl thiocarbamoyl, cyclohexyl Thiocarbamoyl), mono- or di-C 6-14 aryl-thiocarbamoyl group (eg, phenylthiocarbamoyl), mono- or di-C 7-16 aralkyl-thiocarbamoyl group (eg, benzylthiocarbamoyl, phenethylthiocarbamoyl) ) 5- to 14-membered aromatic heterocyclic thiocarbamoyl group (eg, pyridylthiocarbamoyl), sulfino group, C 1-6 alkylsulfinyl group (eg, methylsulfinyl, ethylsulfinyl), sulfo group, C 1-6 alkylsulfonyl group, C 6- 4 arylsulfonyl group, a phosphono group, a mono - or di -C 1-6 alkyl phosphono group (e.g., dimethylphosphono, diethylphosphono, diisopropylphosphono, dibutylphosphono) and the like.
本明細書中、「置換されていてもよいアミノ基」としては、例えば、「置換基群Aから選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C6-14アリール基、C7-16アラルキル基、C1-6アルキル-カルボニル基、C6-14アリール-カルボニル基、C7-16アラルキル-カルボニル基、5ないし14員芳香族複素環カルボニル基、3ないし14員非芳香族複素環カルボニル基、C1-6アルコキシ-カルボニル基、5ないし14員芳香族複素環基、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基、モノ-またはジ-C7-16アラルキル-カルバモイル基、C1-6アルキルスルホニル基およびC6-14アリールスルホニル基から選ばれる1または2個の置換基」を有していてもよいアミノ基が挙げられる。
置換されていてもよいアミノ基の好適な例としては、アミノ基、モノ-またはジ-(ハロゲン化されていてもよいC1-6アルキル)アミノ基(例、メチルアミノ、トリフルオロメチルアミノ、ジメチルアミノ、エチルアミノ、ジエチルアミノ、プロピルアミノ、ジブチルアミノ)、モノ-またはジ-C2-6アルケニルアミノ基(例、ジアリルアミノ)、モノ-またはジ-C3-10シクロアルキルアミノ基(例、シクロプロピルアミノ、シクロヘキシルアミノ)、モノ-またはジ-C6-14アリールアミノ基(例、フェニルアミノ)、モノ-またはジ-C7-16アラルキルアミノ基(例、ベンジルアミノ、ジベンジルアミノ)、モノ-またはジ-(ハロゲン化されていてもよいC1-6アルキル)-カルボニルアミノ基(例、アセチルアミノ、プロピオニルアミノ)、モノ-またはジ-C6-14アリール-カルボニルアミノ基(例、ベンゾイルアミノ)、モノ-またはジ-C7-16アラルキル-カルボニルアミノ基(例、ベンジルカルボニルアミノ)、モノ-またはジ-5ないし14員芳香族複素環カルボニルアミノ基(例、ニコチノイルアミノ、イソニコチノイルアミノ)、モノ-またはジ-3ないし14員非芳香族複素環カルボニルアミノ基(例、ピペリジニルカルボニルアミノ)、モノ-またはジ-C1-6アルコキシ-カルボニルアミノ基(例、tert-ブトキシカルボニルアミノ)、5ないし14員芳香族複素環アミノ基(例、ピリジルアミノ)、カルバモイルアミノ基、(モノ-またはジ-C1-6アルキル-カルバモイル)アミノ基(例、メチルカルバモイルアミノ)、(モノ-またはジ-C7-16アラルキル-カルバモイル)アミノ基(例、ベンジルカルバモイルアミノ)、C1-6アルキルスルホニルアミノ基(例、メチルスルホニルアミノ、エチルスルホニルアミノ)、C6-14アリールスルホニルアミノ基(例、フェニルスルホニルアミノ)、(C1-6アルキル)(C1-6アルキル-カルボニル)アミノ基(例、N-アセチル-N-メチルアミノ)、(C1-6アルキル)(C6-14アリール-カルボニル)アミノ基(例、N-ベンゾイル-N-メチルアミノ)が挙げられる。 In the present specification, examples of the “optionally substituted amino group” include, for example, a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A” C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl Groups, mono- or di-C 1-6 alkyl-carbamoyl groups, mono- or di-C 7-16 aralkyl-carbamoyl groups, C 1-6 alkylsulfonyl groups and C 6- And an amino group optionally having 1 or 2 substituents selected from 14 arylsulfonyl groups.
Suitable examples of the optionally substituted amino group include an amino group, a mono- or di- (optionally halogenated C 1-6 alkyl) amino group (eg, methylamino, trifluoromethylamino, Dimethylamino, ethylamino, diethylamino, propylamino, dibutylamino), mono- or di-C 2-6 alkenylamino groups (eg, diallylamino), mono- or di-C 3-10 cycloalkylamino groups (eg, Cyclopropylamino, cyclohexylamino), mono- or di-C 6-14 arylamino group (eg, phenylamino), mono- or di-C 7-16 aralkylamino group (eg, benzylamino, dibenzylamino), mono - or di - (optionally halogenated C 1-6 alkyl) - carbonyl amino group (e.g., a Chiruamino, propionylamino), mono- - or di -C 6-14 aryl - carbonyl amino group (e.g., benzoylamino), mono - or di -C 7-16 aralkyl - carbonyl amino group (e.g., benzyl carbonyl amino), mono -Or di-5 to 14-membered aromatic heterocyclic carbonylamino group (eg, nicotinoylamino, isonicotinoylamino), mono- or di-3 to 14-membered non-aromatic heterocyclic carbonylamino group (eg, piperidyl) Nylcarbonylamino), mono- or di-C 1-6 alkoxy-carbonylamino group (eg, tert-butoxycarbonylamino), 5- to 14-membered aromatic heterocyclic amino group (eg, pyridylamino), carbamoylamino group, ( mono - or di -C 1-6 alkyl - carbamoyl) amino group (e.g., methylcarbamoyl Carbamoylamino), (mono - or di -C 7-16 aralkyl - carbamoyl) amino group (e.g., benzylcarbamoyl amino), C 1-6 alkylsulfonylamino group (e.g., methylsulfonylamino, ethylsulfonylamino), C 6 -14 arylsulfonylamino group (eg, phenylsulfonylamino), (C 1-6 alkyl) (C 1-6 alkyl-carbonyl) amino group (eg, N-acetyl-N-methylamino), (C 1-6 Alkyl) (C 6-14 aryl-carbonyl) amino group (eg, N-benzoyl-N-methylamino).
置換されていてもよいアミノ基の好適な例としては、アミノ基、モノ-またはジ-(ハロゲン化されていてもよいC1-6アルキル)アミノ基(例、メチルアミノ、トリフルオロメチルアミノ、ジメチルアミノ、エチルアミノ、ジエチルアミノ、プロピルアミノ、ジブチルアミノ)、モノ-またはジ-C2-6アルケニルアミノ基(例、ジアリルアミノ)、モノ-またはジ-C3-10シクロアルキルアミノ基(例、シクロプロピルアミノ、シクロヘキシルアミノ)、モノ-またはジ-C6-14アリールアミノ基(例、フェニルアミノ)、モノ-またはジ-C7-16アラルキルアミノ基(例、ベンジルアミノ、ジベンジルアミノ)、モノ-またはジ-(ハロゲン化されていてもよいC1-6アルキル)-カルボニルアミノ基(例、アセチルアミノ、プロピオニルアミノ)、モノ-またはジ-C6-14アリール-カルボニルアミノ基(例、ベンゾイルアミノ)、モノ-またはジ-C7-16アラルキル-カルボニルアミノ基(例、ベンジルカルボニルアミノ)、モノ-またはジ-5ないし14員芳香族複素環カルボニルアミノ基(例、ニコチノイルアミノ、イソニコチノイルアミノ)、モノ-またはジ-3ないし14員非芳香族複素環カルボニルアミノ基(例、ピペリジニルカルボニルアミノ)、モノ-またはジ-C1-6アルコキシ-カルボニルアミノ基(例、tert-ブトキシカルボニルアミノ)、5ないし14員芳香族複素環アミノ基(例、ピリジルアミノ)、カルバモイルアミノ基、(モノ-またはジ-C1-6アルキル-カルバモイル)アミノ基(例、メチルカルバモイルアミノ)、(モノ-またはジ-C7-16アラルキル-カルバモイル)アミノ基(例、ベンジルカルバモイルアミノ)、C1-6アルキルスルホニルアミノ基(例、メチルスルホニルアミノ、エチルスルホニルアミノ)、C6-14アリールスルホニルアミノ基(例、フェニルスルホニルアミノ)、(C1-6アルキル)(C1-6アルキル-カルボニル)アミノ基(例、N-アセチル-N-メチルアミノ)、(C1-6アルキル)(C6-14アリール-カルボニル)アミノ基(例、N-ベンゾイル-N-メチルアミノ)が挙げられる。 In the present specification, examples of the “optionally substituted amino group” include, for example, a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A” C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl Groups, mono- or di-C 1-6 alkyl-carbamoyl groups, mono- or di-C 7-16 aralkyl-carbamoyl groups, C 1-6 alkylsulfonyl groups and C 6- And an amino group optionally having 1 or 2 substituents selected from 14 arylsulfonyl groups.
Suitable examples of the optionally substituted amino group include an amino group, a mono- or di- (optionally halogenated C 1-6 alkyl) amino group (eg, methylamino, trifluoromethylamino, Dimethylamino, ethylamino, diethylamino, propylamino, dibutylamino), mono- or di-C 2-6 alkenylamino groups (eg, diallylamino), mono- or di-C 3-10 cycloalkylamino groups (eg, Cyclopropylamino, cyclohexylamino), mono- or di-C 6-14 arylamino group (eg, phenylamino), mono- or di-C 7-16 aralkylamino group (eg, benzylamino, dibenzylamino), mono - or di - (optionally halogenated C 1-6 alkyl) - carbonyl amino group (e.g., a Chiruamino, propionylamino), mono- - or di -C 6-14 aryl - carbonyl amino group (e.g., benzoylamino), mono - or di -C 7-16 aralkyl - carbonyl amino group (e.g., benzyl carbonyl amino), mono -Or di-5 to 14-membered aromatic heterocyclic carbonylamino group (eg, nicotinoylamino, isonicotinoylamino), mono- or di-3 to 14-membered non-aromatic heterocyclic carbonylamino group (eg, piperidyl) Nylcarbonylamino), mono- or di-C 1-6 alkoxy-carbonylamino group (eg, tert-butoxycarbonylamino), 5- to 14-membered aromatic heterocyclic amino group (eg, pyridylamino), carbamoylamino group, ( mono - or di -C 1-6 alkyl - carbamoyl) amino group (e.g., methylcarbamoyl Carbamoylamino), (mono - or di -C 7-16 aralkyl - carbamoyl) amino group (e.g., benzylcarbamoyl amino), C 1-6 alkylsulfonylamino group (e.g., methylsulfonylamino, ethylsulfonylamino), C 6 -14 arylsulfonylamino group (eg, phenylsulfonylamino), (C 1-6 alkyl) (C 1-6 alkyl-carbonyl) amino group (eg, N-acetyl-N-methylamino), (C 1-6 Alkyl) (C 6-14 aryl-carbonyl) amino group (eg, N-benzoyl-N-methylamino).
本明細書中、「置換されていてもよいカルバモイル基」としては、例えば、「置換基群Aから選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C6-14アリール基、C7-16アラルキル基、C1-6アルキル-カルボニル基、C6-14アリール-カルボニル基、C7-16アラルキル-カルボニル基、5ないし14員芳香族複素環カルボニル基、3ないし14員非芳香族複素環カルボニル基、C1-6アルコキシ-カルボニル基、5ないし14員芳香族複素環基、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基およびモノ-またはジ-C7-16アラルキル-カルバモイル基から選ばれる1または2個の置換基」を有していてもよいカルバモイル基が挙げられる。
置換されていてもよいカルバモイル基の好適な例としては、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基、モノ-またはジ-C2-6アルケニル-カルバモイル基(例、ジアリルカルバモイル)、モノ-またはジ-C3-10シクロアルキル-カルバモイル基(例、シクロプロピルカルバモイル、シクロヘキシルカルバモイル)、モノ-またはジ-C6-14アリール-カルバモイル基(例、フェニルカルバモイル)、モノ-またはジ-C7-16アラルキル-カルバモイル基、モノ-またはジ-C1-6アルキル-カルボニル-カルバモイル基(例、アセチルカルバモイル、プロピオニルカルバモイル)、モノ-またはジ-C6-14アリール-カルボニル-カルバモイル基(例、ベンゾイルカルバモイル)、5ないし14員芳香族複素環カルバモイル基(例、ピリジルカルバモイル)が挙げられる。 In the present specification, examples of the “optionally substituted carbamoyl group” include, for example, a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A” C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - 1 or 2 substituents selected from a carbamoyl group The have include a carbamoyl group which may.
Suitable examples of the optionally substituted carbamoyl group include a carbamoyl group, a mono- or di-C 1-6 alkyl-carbamoyl group, a mono- or di-C 2-6 alkenyl-carbamoyl group (eg, diallylcarbamoyl group). ), Mono- or di-C 3-10 cycloalkyl-carbamoyl groups (eg cyclopropylcarbamoyl, cyclohexylcarbamoyl), mono- or di-C 6-14 aryl-carbamoyl groups (eg phenylcarbamoyl), mono- or Di-C 7-16 aralkyl-carbamoyl group, mono- or di-C 1-6 alkyl-carbonyl-carbamoyl group (eg acetylcarbamoyl, propionylcarbamoyl), mono- or di-C 6-14 aryl-carbonyl-carbamoyl Groups (eg, benzoylcarbamoyl) A 5- to 14-membered aromatic heterocyclic carbamoyl group (eg, pyridylcarbamoyl) can be mentioned.
置換されていてもよいカルバモイル基の好適な例としては、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基、モノ-またはジ-C2-6アルケニル-カルバモイル基(例、ジアリルカルバモイル)、モノ-またはジ-C3-10シクロアルキル-カルバモイル基(例、シクロプロピルカルバモイル、シクロヘキシルカルバモイル)、モノ-またはジ-C6-14アリール-カルバモイル基(例、フェニルカルバモイル)、モノ-またはジ-C7-16アラルキル-カルバモイル基、モノ-またはジ-C1-6アルキル-カルボニル-カルバモイル基(例、アセチルカルバモイル、プロピオニルカルバモイル)、モノ-またはジ-C6-14アリール-カルボニル-カルバモイル基(例、ベンゾイルカルバモイル)、5ないし14員芳香族複素環カルバモイル基(例、ピリジルカルバモイル)が挙げられる。 In the present specification, examples of the “optionally substituted carbamoyl group” include, for example, a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A” C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - 1 or 2 substituents selected from a carbamoyl group The have include a carbamoyl group which may.
Suitable examples of the optionally substituted carbamoyl group include a carbamoyl group, a mono- or di-C 1-6 alkyl-carbamoyl group, a mono- or di-C 2-6 alkenyl-carbamoyl group (eg, diallylcarbamoyl group). ), Mono- or di-C 3-10 cycloalkyl-carbamoyl groups (eg cyclopropylcarbamoyl, cyclohexylcarbamoyl), mono- or di-C 6-14 aryl-carbamoyl groups (eg phenylcarbamoyl), mono- or Di-C 7-16 aralkyl-carbamoyl group, mono- or di-C 1-6 alkyl-carbonyl-carbamoyl group (eg acetylcarbamoyl, propionylcarbamoyl), mono- or di-C 6-14 aryl-carbonyl-carbamoyl Groups (eg, benzoylcarbamoyl) A 5- to 14-membered aromatic heterocyclic carbamoyl group (eg, pyridylcarbamoyl) can be mentioned.
本明細書中、「置換されていてもよいチオカルバモイル基」としては、例えば、「置換基群Aから選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C6-14アリール基、C7-16アラルキル基、C1-6アルキル-カルボニル基、C6-14アリール-カルボニル基、C7-16アラルキル-カルボニル基、5ないし14員芳香族複素環カルボニル基、3ないし14員非芳香族複素環カルボニル基、C1-6アルコキシ-カルボニル基、5ないし14員芳香族複素環基、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基およびモノ-またはジ-C7-16アラルキル-カルバモイル基から選ばれる1または2個の置換基」を有していてもよいチオカルバモイル基が挙げられる。
置換されていてもよいチオカルバモイル基の好適な例としては、チオカルバモイル基、モノ-またはジ-C1-6アルキル-チオカルバモイル基(例、メチルチオカルバモイル、エチルチオカルバモイル、ジメチルチオカルバモイル、ジエチルチオカルバモイル、N-エチル-N-メチルチオカルバモイル)、モノ-またはジ-C2-6アルケニル-チオカルバモイル基(例、ジアリルチオカルバモイル)、モノ-またはジ-C3-10シクロアルキル-チオカルバモイル基(例、シクロプロピルチオカルバモイル、シクロヘキシルチオカルバモイル)、モノ-またはジ-C6-14アリール-チオカルバモイル基(例、フェニルチオカルバモイル)、モノ-またはジ-C7-16アラルキル-チオカルバモイル基(例、ベンジルチオカルバモイル、フェネチルチオカルバモイル)、モノ-またはジ-C1-6アルキル-カルボニル-チオカルバモイル基(例、アセチルチオカルバモイル、プロピオニルチオカルバモイル)、モノ-またはジ-C6-14アリール-カルボニル-チオカルバモイル基(例、ベンゾイルチオカルバモイル)、5ないし14員芳香族複素環チオカルバモイル基(例、ピリジルチオカルバモイル)が挙げられる。 In the present specification, examples of the “optionally substituted thiocarbamoyl group” include, for example, “C 1-6 alkyl each optionally having 1 to 3 substituents selected from Substituent Group A” Group, C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7-16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - one or two location selected from a carbamoyl group Have a group "include good thiocarbamoyl group.
Suitable examples of the thiocarbamoyl group which may be substituted include a thiocarbamoyl group, a mono- or di-C 1-6 alkyl-thiocarbamoyl group (eg, methylthiocarbamoyl, ethylthiocarbamoyl, dimethylthiocarbamoyl, diethylthio). Carbamoyl, N-ethyl-N-methylthiocarbamoyl), mono- or di-C 2-6 alkenyl-thiocarbamoyl group (eg diallylthiocarbamoyl), mono- or di-C 3-10 cycloalkyl-thiocarbamoyl group ( Examples, cyclopropylthiocarbamoyl, cyclohexylthiocarbamoyl), mono- or di-C 6-14 aryl-thiocarbamoyl group (eg, phenylthiocarbamoyl), mono- or di-C 7-16 aralkyl-thiocarbamoyl group (eg, , Benzylthioca Bamoiru, phenethyl thio carbamoyl), mono - or di -C 1-6 alkyl - carbonyl - thiocarbamoyl group (e.g., acetyl thiocarbamoyl, propionylthio carbamoyl), mono - or di -C 6-14 aryl - carbonyl - thiocarbamoyl Groups (eg, benzoylthiocarbamoyl), 5- to 14-membered aromatic heterocyclic thiocarbamoyl groups (eg, pyridylthiocarbamoyl).
置換されていてもよいチオカルバモイル基の好適な例としては、チオカルバモイル基、モノ-またはジ-C1-6アルキル-チオカルバモイル基(例、メチルチオカルバモイル、エチルチオカルバモイル、ジメチルチオカルバモイル、ジエチルチオカルバモイル、N-エチル-N-メチルチオカルバモイル)、モノ-またはジ-C2-6アルケニル-チオカルバモイル基(例、ジアリルチオカルバモイル)、モノ-またはジ-C3-10シクロアルキル-チオカルバモイル基(例、シクロプロピルチオカルバモイル、シクロヘキシルチオカルバモイル)、モノ-またはジ-C6-14アリール-チオカルバモイル基(例、フェニルチオカルバモイル)、モノ-またはジ-C7-16アラルキル-チオカルバモイル基(例、ベンジルチオカルバモイル、フェネチルチオカルバモイル)、モノ-またはジ-C1-6アルキル-カルボニル-チオカルバモイル基(例、アセチルチオカルバモイル、プロピオニルチオカルバモイル)、モノ-またはジ-C6-14アリール-カルボニル-チオカルバモイル基(例、ベンゾイルチオカルバモイル)、5ないし14員芳香族複素環チオカルバモイル基(例、ピリジルチオカルバモイル)が挙げられる。 In the present specification, examples of the “optionally substituted thiocarbamoyl group” include, for example, “C 1-6 alkyl each optionally having 1 to 3 substituents selected from Substituent Group A” Group, C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7-16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - one or two location selected from a carbamoyl group Have a group "include good thiocarbamoyl group.
Suitable examples of the thiocarbamoyl group which may be substituted include a thiocarbamoyl group, a mono- or di-C 1-6 alkyl-thiocarbamoyl group (eg, methylthiocarbamoyl, ethylthiocarbamoyl, dimethylthiocarbamoyl, diethylthio). Carbamoyl, N-ethyl-N-methylthiocarbamoyl), mono- or di-C 2-6 alkenyl-thiocarbamoyl group (eg diallylthiocarbamoyl), mono- or di-C 3-10 cycloalkyl-thiocarbamoyl group ( Examples, cyclopropylthiocarbamoyl, cyclohexylthiocarbamoyl), mono- or di-C 6-14 aryl-thiocarbamoyl group (eg, phenylthiocarbamoyl), mono- or di-C 7-16 aralkyl-thiocarbamoyl group (eg, , Benzylthioca Bamoiru, phenethyl thio carbamoyl), mono - or di -C 1-6 alkyl - carbonyl - thiocarbamoyl group (e.g., acetyl thiocarbamoyl, propionylthio carbamoyl), mono - or di -C 6-14 aryl - carbonyl - thiocarbamoyl Groups (eg, benzoylthiocarbamoyl), 5- to 14-membered aromatic heterocyclic thiocarbamoyl groups (eg, pyridylthiocarbamoyl).
本明細書中、「置換されていてもよいスルファモイル基」としては、例えば、「置換基群Aから選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C6-14アリール基、C7-16アラルキル基、C1-6アルキル-カルボニル基、C6-14アリール-カルボニル基、C7-16アラルキル-カルボニル基、5ないし14員芳香族複素環カルボニル基、3ないし14員非芳香族複素環カルボニル基、C1-6アルコキシ-カルボニル基、5ないし14員芳香族複素環基、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基およびモノ-またはジ-C7-16アラルキル-カルバモイル基から選ばれる1または2個の置換基」を有していてもよいスルファモイル基が挙げられる。
置換されていてもよいスルファモイル基の好適な例としては、スルファモイル基、モノ-またはジ-C1-6アルキル-スルファモイル基(例、メチルスルファモイル、エチルスルファモイル、ジメチルスルファモイル、ジエチルスルファモイル、N-エチル-N-メチルスルファモイル)、モノ-またはジ-C2-6アルケニル-スルファモイル基(例、ジアリルスルファモイル)、モノ-またはジ-C3-10シクロアルキル-スルファモイル基(例、シクロプロピルスルファモイル、シクロヘキシルスルファモイル)、モノ-またはジ-C6-14アリール-スルファモイル基(例、フェニルスルファモイル)、モノ-またはジ-C7-16アラルキル-スルファモイル基(例、ベンジルスルファモイル、フェネチルスルファモイル)、モノ-またはジ-C1-6アルキル-カルボニル-スルファモイル基(例、アセチルスルファモイル、プロピオニルスルファモイル)、モノ-またはジ-C6-14アリール-カルボニル-スルファモイル基(例、ベンゾイルスルファモイル)、5ないし14員芳香族複素環スルファモイル基(例、ピリジルスルファモイル)が挙げられる。 In the present specification, examples of the “optionally substituted sulfamoyl group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A”. C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - 1 or 2 substituents selected from a carbamoyl group Have a "include sulfamoyl group.
Preferable examples of the optionally substituted sulfamoyl group include sulfamoyl group, mono- or di-C 1-6 alkyl-sulfamoyl group (eg, methylsulfamoyl, ethylsulfamoyl, dimethylsulfamoyl, diethyl). Sulfamoyl, N-ethyl-N-methylsulfamoyl), mono- or di-C 2-6 alkenyl-sulfamoyl groups (eg diallylsulfamoyl), mono- or di-C 3-10 cycloalkyl- Sulfamoyl group (eg, cyclopropylsulfamoyl, cyclohexylsulfamoyl), mono- or di-C 6-14 aryl-sulfamoyl group (eg, phenylsulfamoyl), mono- or di-C 7-16 aralkyl- Sulfamoyl group (eg, benzylsulfamoyl, phenethylsulfamoy) ), Mono - or di -C 1-6 alkyl - carbonyl - sulfamoyl group (e.g., acetyl sulfamoyl, propionitrile acylsulfamoyl), mono - or di -C 6-14 aryl - carbonyl - sulfamoyl group (e.g., benzoyl Sulfamoyl) and 5- to 14-membered aromatic heterocyclic sulfamoyl groups (eg, pyridylsulfamoyl).
置換されていてもよいスルファモイル基の好適な例としては、スルファモイル基、モノ-またはジ-C1-6アルキル-スルファモイル基(例、メチルスルファモイル、エチルスルファモイル、ジメチルスルファモイル、ジエチルスルファモイル、N-エチル-N-メチルスルファモイル)、モノ-またはジ-C2-6アルケニル-スルファモイル基(例、ジアリルスルファモイル)、モノ-またはジ-C3-10シクロアルキル-スルファモイル基(例、シクロプロピルスルファモイル、シクロヘキシルスルファモイル)、モノ-またはジ-C6-14アリール-スルファモイル基(例、フェニルスルファモイル)、モノ-またはジ-C7-16アラルキル-スルファモイル基(例、ベンジルスルファモイル、フェネチルスルファモイル)、モノ-またはジ-C1-6アルキル-カルボニル-スルファモイル基(例、アセチルスルファモイル、プロピオニルスルファモイル)、モノ-またはジ-C6-14アリール-カルボニル-スルファモイル基(例、ベンゾイルスルファモイル)、5ないし14員芳香族複素環スルファモイル基(例、ピリジルスルファモイル)が挙げられる。 In the present specification, examples of the “optionally substituted sulfamoyl group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A”. C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl group, mono - or di -C 1-6 alkyl - carbamoyl group and mono- - or di -C 7-16 aralkyl - 1 or 2 substituents selected from a carbamoyl group Have a "include sulfamoyl group.
Preferable examples of the optionally substituted sulfamoyl group include sulfamoyl group, mono- or di-C 1-6 alkyl-sulfamoyl group (eg, methylsulfamoyl, ethylsulfamoyl, dimethylsulfamoyl, diethyl). Sulfamoyl, N-ethyl-N-methylsulfamoyl), mono- or di-C 2-6 alkenyl-sulfamoyl groups (eg diallylsulfamoyl), mono- or di-C 3-10 cycloalkyl- Sulfamoyl group (eg, cyclopropylsulfamoyl, cyclohexylsulfamoyl), mono- or di-C 6-14 aryl-sulfamoyl group (eg, phenylsulfamoyl), mono- or di-C 7-16 aralkyl- Sulfamoyl group (eg, benzylsulfamoyl, phenethylsulfamoy) ), Mono - or di -C 1-6 alkyl - carbonyl - sulfamoyl group (e.g., acetyl sulfamoyl, propionitrile acylsulfamoyl), mono - or di -C 6-14 aryl - carbonyl - sulfamoyl group (e.g., benzoyl Sulfamoyl) and 5- to 14-membered aromatic heterocyclic sulfamoyl groups (eg, pyridylsulfamoyl).
本明細書中、「置換されていてもよいヒドロキシ基」としては、例えば、「置換基群Aから選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C6-14アリール基、C7-16アラルキル基、C1-6アルキル-カルボニル基、C6-14アリール-カルボニル基、C7-16アラルキル-カルボニル基、5ないし14員芳香族複素環カルボニル基、3ないし14員非芳香族複素環カルボニル基、C1-6アルコキシ-カルボニル基、5ないし14員芳香族複素環基、カルバモイル基、モノ-またはジ-C1-6アルキル-カルバモイル基、モノ-またはジ-C7-16アラルキル-カルバモイル基、C1-6アルキルスルホニル基およびC6-14アリールスルホニル基から選ばれる置換基」を有していてもよいヒドロキシ基が挙げられる。
置換されていてもよいヒドロキシ基の好適な例としては、ヒドロキシ基、C1-6アルコキシ基、C2-6アルケニルオキシ基(例、アリルオキシ、2-ブテニルオキシ、2-ペンテニルオキシ、3-ヘキセニルオキシ)、C3-10シクロアルキルオキシ基(例、シクロヘキシルオキシ)、C6-14アリールオキシ基(例、フェノキシ、ナフチルオキシ)、C7-16アラルキルオキシ基(例、ベンジルオキシ、フェネチルオキシ)、C1-6アルキル-カルボニルオキシ基(例、アセチルオキシ、プロピオニルオキシ、ブチリルオキシ、イソブチリルオキシ、ピバロイルオキシ)、C6-14アリール-カルボニルオキシ基(例、ベンゾイルオキシ)、C7-16アラルキル-カルボニルオキシ基(例、ベンジルカルボニルオキシ)、5ないし14員芳香族複素環カルボニルオキシ基(例、ニコチノイルオキシ)、3ないし14員非芳香族複素環カルボニルオキシ基(例、ピペリジニルカルボニルオキシ)、C1-6アルコキシ-カルボニルオキシ基(例、tert-ブトキシカルボニルオキシ)、5ないし14員芳香族複素環オキシ基(例、ピリジルオキシ)、カルバモイルオキシ基、C1-6アルキル-カルバモイルオキシ基(例、メチルカルバモイルオキシ)、C7-16アラルキル-カルバモイルオキシ基(例、ベンジルカルバモイルオキシ)、C1-6アルキルスルホニルオキシ基(例、メチルスルホニルオキシ、エチルスルホニルオキシ)、C6-14アリールスルホニルオキシ基(例、フェニルスルホニルオキシ)が挙げられる。 In the present specification, examples of the “optionally substituted hydroxy group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A”. C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl Groups, mono- or di-C 1-6 alkyl-carbamoyl groups, mono- or di-C 7-16 aralkyl-carbamoyl groups, C 1-6 alkylsulfonyl groups and C And a hydroxy group optionally having a substituent selected from 6-14 arylsulfonyl groups.
Suitable examples of the optionally substituted hydroxy group include a hydroxy group, a C 1-6 alkoxy group, a C 2-6 alkenyloxy group (eg, allyloxy, 2-butenyloxy, 2-pentenyloxy, 3-hexenyloxy). ), C 3-10 cycloalkyloxy group (eg, cyclohexyloxy), C 6-14 aryloxy group (eg, phenoxy, naphthyloxy), C 7-16 aralkyloxy group (eg, benzyloxy, phenethyloxy), C 1-6 alkyl-carbonyloxy group (eg, acetyloxy, propionyloxy, butyryloxy, isobutyryloxy, pivaloyloxy), C 6-14 aryl-carbonyloxy group (eg, benzoyloxy), C 7-16 aralkyl- A carbonyloxy group (eg benzylcarbonyloxy) ), 5 to 14-membered aromatic heterocyclic carbonyloxy group (e.g., nicotinoyl oxy), 3 to 14-membered non-aromatic heterocyclic carbonyloxy group (e.g., piperidinylcarbonyl oxy), C 1-6 alkoxy - carbonyl An oxy group (eg, tert-butoxycarbonyloxy), a 5- to 14-membered aromatic heterocyclic oxy group (eg, pyridyloxy), a carbamoyloxy group, a C 1-6 alkyl-carbamoyloxy group (eg, methylcarbamoyloxy), C 7-16 aralkyl-carbamoyloxy group (eg, benzylcarbamoyloxy), C 1-6 alkylsulfonyloxy group (eg, methylsulfonyloxy, ethylsulfonyloxy), C 6-14 arylsulfonyloxy group (eg, phenylsulfonyl) Oxy).
置換されていてもよいヒドロキシ基の好適な例としては、ヒドロキシ基、C1-6アルコキシ基、C2-6アルケニルオキシ基(例、アリルオキシ、2-ブテニルオキシ、2-ペンテニルオキシ、3-ヘキセニルオキシ)、C3-10シクロアルキルオキシ基(例、シクロヘキシルオキシ)、C6-14アリールオキシ基(例、フェノキシ、ナフチルオキシ)、C7-16アラルキルオキシ基(例、ベンジルオキシ、フェネチルオキシ)、C1-6アルキル-カルボニルオキシ基(例、アセチルオキシ、プロピオニルオキシ、ブチリルオキシ、イソブチリルオキシ、ピバロイルオキシ)、C6-14アリール-カルボニルオキシ基(例、ベンゾイルオキシ)、C7-16アラルキル-カルボニルオキシ基(例、ベンジルカルボニルオキシ)、5ないし14員芳香族複素環カルボニルオキシ基(例、ニコチノイルオキシ)、3ないし14員非芳香族複素環カルボニルオキシ基(例、ピペリジニルカルボニルオキシ)、C1-6アルコキシ-カルボニルオキシ基(例、tert-ブトキシカルボニルオキシ)、5ないし14員芳香族複素環オキシ基(例、ピリジルオキシ)、カルバモイルオキシ基、C1-6アルキル-カルバモイルオキシ基(例、メチルカルバモイルオキシ)、C7-16アラルキル-カルバモイルオキシ基(例、ベンジルカルバモイルオキシ)、C1-6アルキルスルホニルオキシ基(例、メチルスルホニルオキシ、エチルスルホニルオキシ)、C6-14アリールスルホニルオキシ基(例、フェニルスルホニルオキシ)が挙げられる。 In the present specification, examples of the “optionally substituted hydroxy group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A”. C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group, C 7 -16 aralkyl-carbonyl group, 5- to 14-membered aromatic heterocyclic carbonyl group, 3- to 14-membered non-aromatic heterocyclic carbonyl group, C 1-6 alkoxy-carbonyl group, 5- to 14-membered aromatic heterocyclic group, carbamoyl Groups, mono- or di-C 1-6 alkyl-carbamoyl groups, mono- or di-C 7-16 aralkyl-carbamoyl groups, C 1-6 alkylsulfonyl groups and C And a hydroxy group optionally having a substituent selected from 6-14 arylsulfonyl groups.
Suitable examples of the optionally substituted hydroxy group include a hydroxy group, a C 1-6 alkoxy group, a C 2-6 alkenyloxy group (eg, allyloxy, 2-butenyloxy, 2-pentenyloxy, 3-hexenyloxy). ), C 3-10 cycloalkyloxy group (eg, cyclohexyloxy), C 6-14 aryloxy group (eg, phenoxy, naphthyloxy), C 7-16 aralkyloxy group (eg, benzyloxy, phenethyloxy), C 1-6 alkyl-carbonyloxy group (eg, acetyloxy, propionyloxy, butyryloxy, isobutyryloxy, pivaloyloxy), C 6-14 aryl-carbonyloxy group (eg, benzoyloxy), C 7-16 aralkyl- A carbonyloxy group (eg benzylcarbonyloxy) ), 5 to 14-membered aromatic heterocyclic carbonyloxy group (e.g., nicotinoyl oxy), 3 to 14-membered non-aromatic heterocyclic carbonyloxy group (e.g., piperidinylcarbonyl oxy), C 1-6 alkoxy - carbonyl An oxy group (eg, tert-butoxycarbonyloxy), a 5- to 14-membered aromatic heterocyclic oxy group (eg, pyridyloxy), a carbamoyloxy group, a C 1-6 alkyl-carbamoyloxy group (eg, methylcarbamoyloxy), C 7-16 aralkyl-carbamoyloxy group (eg, benzylcarbamoyloxy), C 1-6 alkylsulfonyloxy group (eg, methylsulfonyloxy, ethylsulfonyloxy), C 6-14 arylsulfonyloxy group (eg, phenylsulfonyl) Oxy).
本明細書中、「置換されていてもよいスルファニル基」としては、例えば、「置換基群Aから選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C6-14アリール基、C7-16アラルキル基、C1-6アルキル-カルボニル基、C6-14アリール-カルボニル基および5ないし14員芳香族複素環基から選ばれる置換基」を有していてもよいスルファニル基、ハロゲン化されたスルファニル基が挙げられる。
置換されていてもよいスルファニル基の好適な例としては、スルファニル(-SH)基、C1-6アルキルチオ基、C2-6アルケニルチオ基(例、アリルチオ、2-ブテニルチオ、2-ペンテニルチオ、3-ヘキセニルチオ)、C3-10シクロアルキルチオ基(例、シクロヘキシルチオ)、C6-14アリールチオ基(例、フェニルチオ、ナフチルチオ)、C7-16アラルキルチオ基(例、ベンジルチオ、フェネチルチオ)、C1-6アルキル-カルボニルチオ基(例、アセチルチオ、プロピオニルチオ、ブチリルチオ、イソブチリルチオ、ピバロイルチオ)、C6-14アリール-カルボニルチオ基(例、ベンゾイルチオ)、5ないし14員芳香族複素環チオ基(例、ピリジルチオ)、ハロゲン化チオ基(例、ペンタフルオロチオ)が挙げられる。 In the present specification, examples of the “optionally substituted sulfanyl group” include a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A”. C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group and 5 to Examples thereof include a sulfanyl group optionally having a substituent selected from a 14-membered aromatic heterocyclic group and a halogenated sulfanyl group.
Preferable examples of the optionally substituted sulfanyl group include a sulfanyl (—SH) group, a C 1-6 alkylthio group, a C 2-6 alkenylthio group (eg, allylthio, 2-butenylthio, 2-pentenylthio, 3-hexenylthio), C 3-10 cycloalkylthio group (eg, cyclohexylthio), C 6-14 arylthio group (eg, phenylthio, naphthylthio), C 7-16 aralkylthio group (eg, benzylthio, phenethylthio), C 1-6 alkyl-carbonylthio group (eg, acetylthio, propionylthio, butyrylthio, isobutyrylthio, pivaloylthio), C 6-14 aryl-carbonylthio group (eg, benzoylthio), 5- to 14-membered aromatic heterocyclic thio group (Eg, pyridylthio), halogenated thio groups (eg, pentafluorothio) E).
置換されていてもよいスルファニル基の好適な例としては、スルファニル(-SH)基、C1-6アルキルチオ基、C2-6アルケニルチオ基(例、アリルチオ、2-ブテニルチオ、2-ペンテニルチオ、3-ヘキセニルチオ)、C3-10シクロアルキルチオ基(例、シクロヘキシルチオ)、C6-14アリールチオ基(例、フェニルチオ、ナフチルチオ)、C7-16アラルキルチオ基(例、ベンジルチオ、フェネチルチオ)、C1-6アルキル-カルボニルチオ基(例、アセチルチオ、プロピオニルチオ、ブチリルチオ、イソブチリルチオ、ピバロイルチオ)、C6-14アリール-カルボニルチオ基(例、ベンゾイルチオ)、5ないし14員芳香族複素環チオ基(例、ピリジルチオ)、ハロゲン化チオ基(例、ペンタフルオロチオ)が挙げられる。 In the present specification, examples of the “optionally substituted sulfanyl group” include a “C 1-6 alkyl group optionally having 1 to 3 substituents selected from the substituent group A”. C 2-6 alkenyl group, C 3-10 cycloalkyl group, C 6-14 aryl group, C 7-16 aralkyl group, C 1-6 alkyl-carbonyl group, C 6-14 aryl-carbonyl group and 5 to Examples thereof include a sulfanyl group optionally having a substituent selected from a 14-membered aromatic heterocyclic group and a halogenated sulfanyl group.
Preferable examples of the optionally substituted sulfanyl group include a sulfanyl (—SH) group, a C 1-6 alkylthio group, a C 2-6 alkenylthio group (eg, allylthio, 2-butenylthio, 2-pentenylthio, 3-hexenylthio), C 3-10 cycloalkylthio group (eg, cyclohexylthio), C 6-14 arylthio group (eg, phenylthio, naphthylthio), C 7-16 aralkylthio group (eg, benzylthio, phenethylthio), C 1-6 alkyl-carbonylthio group (eg, acetylthio, propionylthio, butyrylthio, isobutyrylthio, pivaloylthio), C 6-14 aryl-carbonylthio group (eg, benzoylthio), 5- to 14-membered aromatic heterocyclic thio group (Eg, pyridylthio), halogenated thio groups (eg, pentafluorothio) E).
本明細書中、「置換されていてもよいシリル基」としては、例えば、「置換基群Aから選ばれる1ないし3個の置換基をそれぞれ有していてもよい、C1-6アルキル基、C2-6アルケニル基、C3-10シクロアルキル基、C6-14アリール基およびC7-16アラルキル基から選ばれる1ないし3個の置換基」を有していてもよいシリル基が挙げられる。
置換されていてもよいシリル基の好適な例としては、トリ-C1-6アルキルシリル基(例、トリメチルシリル、tert-ブチル(ジメチル)シリル)が挙げられる。 In the present specification, examples of the “optionally substituted silyl group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A” A silyl group optionally having 1 to 3 substituents selected from a C 2-6 alkenyl group, a C 3-10 cycloalkyl group, a C 6-14 aryl group and a C 7-16 aralkyl group ” Can be mentioned.
Preferable examples of the optionally substituted silyl group include a tri-C 1-6 alkylsilyl group (eg, trimethylsilyl, tert-butyl (dimethyl) silyl).
置換されていてもよいシリル基の好適な例としては、トリ-C1-6アルキルシリル基(例、トリメチルシリル、tert-ブチル(ジメチル)シリル)が挙げられる。 In the present specification, examples of the “optionally substituted silyl group” include a “C 1-6 alkyl group each optionally having 1 to 3 substituents selected from the substituent group A” A silyl group optionally having 1 to 3 substituents selected from a C 2-6 alkenyl group, a C 3-10 cycloalkyl group, a C 6-14 aryl group and a C 7-16 aralkyl group ” Can be mentioned.
Preferable examples of the optionally substituted silyl group include a tri-C 1-6 alkylsilyl group (eg, trimethylsilyl, tert-butyl (dimethyl) silyl).
本明細書中、「C1-6アルキレン基」としては、例えば、-CH2-、-(CH2)2-、-(CH2)3-、-(CH2)4-、-(CH2)5-、-(CH2)6-、-CH(CH3)-、-C(CH3)2-、-CH(C2H5)-、-CH(C3H7)-、-CH(CH(CH3)2)-、-(CH(CH3))2-、-CH2-CH(CH3)-、-CH(CH3)-CH2-、-CH2-CH2-C(CH3)2-、-C(CH3)2-CH2-CH2-、-CH2-CH2-CH2-C(CH3)2-、-C(CH3)2-CH2-CH2-CH2-が挙げられる。
本明細書中、「C2-6アルケニレン基」としては、例えば、-CH=CH-、-CH2-CH=CH-、-CH=CH-CH2-、-C(CH3)2-CH=CH-、-CH=CH-C(CH3)2-、-CH2-CH=CH-CH2-、-CH2-CH2-CH=CH-、-CH=CH-CH2-CH2-、-CH=CH-CH=CH-、-CH=CH-CH2-CH2-CH2-、-CH2-CH2-CH2-CH=CH-が挙げられる。
本明細書中、「C2-6アルキニレン基」としては、例えば、-C≡C-、-CH2-C≡C-、-C≡C-CH2-、-C(CH3)2-C≡C-、-C≡C-C(CH3)2-、-CH2-C≡C-CH2-、-CH2-CH2-C≡C-、-C≡C-CH2-CH2-、-C≡C-C≡C-、-C≡C-CH2-CH2-CH2-、-CH2-CH2-CH2-C≡C-が挙げられる。 In this specification, examples of the “C 1-6 alkylene group” include —CH 2 —, — (CH 2 ) 2 —, — (CH 2 ) 3 —, — (CH 2 ) 4 —, — (CH 2 ) 5 —, — (CH 2 ) 6 —, —CH (CH 3 ) —, —C (CH 3 ) 2 —, —CH (C 2 H 5 ) —, —CH (C 3 H 7 ) —, —CH (CH (CH 3 ) 2 ) —, — (CH (CH 3 )) 2 —, —CH 2 —CH (CH 3 ) —, —CH (CH 3 ) —CH 2 —, —CH 2 —CH 2 -C (CH 3) 2 - , - C (CH 3) 2 -CH 2 -CH 2 -, - CH 2 -CH 2 -CH 2 -C (CH 3) 2 -, - C (CH 3) 2 And —CH 2 —CH 2 —CH 2 —.
In the present specification, examples of the “C 2-6 alkenylene group” include —CH═CH—, —CH 2 —CH═CH—, —CH═CH—CH 2 —, —C (CH 3 ) 2 —. CH═CH—, —CH═CH—C (CH 3 ) 2 —, —CH 2 —CH═CH—CH 2 —, —CH 2 —CH 2 —CH═CH—, —CH═CH—CH 2 — CH 2 -, - CH = CH -CH = CH -, - CH = CH-CH 2 -CH 2 -CH 2 -, - CH 2 -CH 2 -CH 2 -CH = CH- and the like.
In the present specification, examples of the “C 2-6 alkynylene group” include —C≡C—, —CH 2 —C≡C—, —C≡C—CH 2 —, —C (CH 3 ) 2 —. C≡C -, - C≡C-C ( CH 3) 2 -, - CH 2 -C≡C-CH 2 -, - CH 2 -CH 2 -C≡C -, - C≡C-CH 2 - CH 2 -, - C≡C-C≡C -, - C≡C-CH 2 -CH 2 -CH 2 -, - CH 2 -CH 2 -CH 2 -C≡C- , and the like.
本明細書中、「C2-6アルケニレン基」としては、例えば、-CH=CH-、-CH2-CH=CH-、-CH=CH-CH2-、-C(CH3)2-CH=CH-、-CH=CH-C(CH3)2-、-CH2-CH=CH-CH2-、-CH2-CH2-CH=CH-、-CH=CH-CH2-CH2-、-CH=CH-CH=CH-、-CH=CH-CH2-CH2-CH2-、-CH2-CH2-CH2-CH=CH-が挙げられる。
本明細書中、「C2-6アルキニレン基」としては、例えば、-C≡C-、-CH2-C≡C-、-C≡C-CH2-、-C(CH3)2-C≡C-、-C≡C-C(CH3)2-、-CH2-C≡C-CH2-、-CH2-CH2-C≡C-、-C≡C-CH2-CH2-、-C≡C-C≡C-、-C≡C-CH2-CH2-CH2-、-CH2-CH2-CH2-C≡C-が挙げられる。 In this specification, examples of the “C 1-6 alkylene group” include —CH 2 —, — (CH 2 ) 2 —, — (CH 2 ) 3 —, — (CH 2 ) 4 —, — (CH 2 ) 5 —, — (CH 2 ) 6 —, —CH (CH 3 ) —, —C (CH 3 ) 2 —, —CH (C 2 H 5 ) —, —CH (C 3 H 7 ) —, —CH (CH (CH 3 ) 2 ) —, — (CH (CH 3 )) 2 —, —CH 2 —CH (CH 3 ) —, —CH (CH 3 ) —CH 2 —, —CH 2 —CH 2 -C (CH 3) 2 - , - C (CH 3) 2 -CH 2 -CH 2 -, - CH 2 -CH 2 -CH 2 -C (CH 3) 2 -, - C (CH 3) 2 And —CH 2 —CH 2 —CH 2 —.
In the present specification, examples of the “C 2-6 alkenylene group” include —CH═CH—, —CH 2 —CH═CH—, —CH═CH—CH 2 —, —C (CH 3 ) 2 —. CH═CH—, —CH═CH—C (CH 3 ) 2 —, —CH 2 —CH═CH—CH 2 —, —CH 2 —CH 2 —CH═CH—, —CH═CH—CH 2 — CH 2 -, - CH = CH -CH = CH -, - CH = CH-CH 2 -CH 2 -CH 2 -, - CH 2 -CH 2 -CH 2 -CH = CH- and the like.
In the present specification, examples of the “C 2-6 alkynylene group” include —C≡C—, —CH 2 —C≡C—, —C≡C—CH 2 —, —C (CH 3 ) 2 —. C≡C -, - C≡C-C ( CH 3) 2 -, - CH 2 -C≡C-CH 2 -, - CH 2 -CH 2 -C≡C -, - C≡C-CH 2 - CH 2 -, - C≡C-C≡C -, - C≡C-CH 2 -CH 2 -CH 2 -, - CH 2 -CH 2 -CH 2 -C≡C- , and the like.
本明細書中、「炭化水素環」としては、例えば、C6-14芳香族炭化水素環、C3-10シクロアルカン、C3-10シクロアルケンが挙げられる。
本明細書中、「C6-14芳香族炭化水素環」としては、例えば、ベンゼン、ナフタレンが挙げられる。
本明細書中、「C3-10シクロアルカン」としては、例えば、シクロプロパン、シクロブタン、シクロペンタン、シクロヘキサン、シクロヘプタン、シクロオクタンが挙げられる。
本明細書中、「C3-10シクロアルケン」としては、例えば、シクロプロペン、シクロブテン、シクロペンテン、シクロヘキセン、シクロヘプテン、シクロオクテンが挙げられる。
本明細書中、「複素環」としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子をそれぞれ含有する、芳香族複素環および非芳香族複素環が挙げられる。 In the present specification, examples of the “hydrocarbon ring” include a C 6-14 aromatic hydrocarbon ring, a C 3-10 cycloalkane, and a C 3-10 cycloalkene.
In the present specification, examples of the “C 6-14 aromatic hydrocarbon ring” include benzene and naphthalene.
In the present specification, examples of “C 3-10 cycloalkane” include cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, and cyclooctane.
In the present specification, examples of “C 3-10 cycloalkene” include cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, and cyclooctene.
In the present specification, examples of the “heterocycle” include aromatic heterocycles each containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom, and an oxygen atom in addition to a carbon atom as a ring-constituting atom. Non-aromatic heterocycles may be mentioned.
本明細書中、「C6-14芳香族炭化水素環」としては、例えば、ベンゼン、ナフタレンが挙げられる。
本明細書中、「C3-10シクロアルカン」としては、例えば、シクロプロパン、シクロブタン、シクロペンタン、シクロヘキサン、シクロヘプタン、シクロオクタンが挙げられる。
本明細書中、「C3-10シクロアルケン」としては、例えば、シクロプロペン、シクロブテン、シクロペンテン、シクロヘキセン、シクロヘプテン、シクロオクテンが挙げられる。
本明細書中、「複素環」としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子をそれぞれ含有する、芳香族複素環および非芳香族複素環が挙げられる。 In the present specification, examples of the “hydrocarbon ring” include a C 6-14 aromatic hydrocarbon ring, a C 3-10 cycloalkane, and a C 3-10 cycloalkene.
In the present specification, examples of the “C 6-14 aromatic hydrocarbon ring” include benzene and naphthalene.
In the present specification, examples of “C 3-10 cycloalkane” include cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, and cyclooctane.
In the present specification, examples of “C 3-10 cycloalkene” include cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, and cyclooctene.
In the present specification, examples of the “heterocycle” include aromatic heterocycles each containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom, and an oxygen atom in addition to a carbon atom as a ring-constituting atom. Non-aromatic heterocycles may be mentioned.
本明細書中、「芳香族複素環」としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子を含有する5ないし14員(好ましくは5ないし10員)の芳香族複素環が挙げられる。該「芳香族複素環」の好適な例としては、チオフェン、フラン、ピロール、イミダゾール、ピラゾール、チアゾール、イソチアゾール、オキサゾール、イソオキサゾール、ピリジン、ピラジン、ピリミジン、ピリダジン、1,2,4-オキサジアゾール、1,3,4-オキサジアゾール、1,2,4-チアジアゾール、1,3,4-チアジアゾール、トリアゾール、テトラゾール、トリアジンなどの5ないし6員単環式芳香族複素環;
ベンゾチオフェン、ベンゾフラン、ベンゾイミダゾール、ベンゾオキサゾール、ベンゾイソオキサゾール、ベンゾチアゾール、ベンゾイソチアゾール、ベンゾトリアゾール、イミダゾピリジン、チエノピリジン、フロピリジン、ピロロピリジン、ピラゾロピリジン、オキサゾロピリジン、チアゾロピリジン、イミダゾピラジン、イミダゾピリミジン、チエノピリミジン、フロピリミジン、ピロロピリミジン、ピラゾロピリミジン、オキサゾロピリミジン、チアゾロピリミジン、ピラゾロピリミジン、ピラゾロトリアジン、ナフト[2,3-b]チオフェン、フェノキサチイン、インド-ル、イソインドール、1H-インダゾール、プリン、イソキノリン、キノリン、フタラジン、ナフチリジン、キノキサリン、キナゾリン、シンノリン、カルバゾール、β-カルボリン、フェナントリジン、アクリジン、フェナジン、フェノチアジン、フェノキサジンなどの8ないし14員縮合多環式(好ましくは2または3環式)芳香族複素環が挙げられる。 In the present specification, the “aromatic heterocycle” is, for example, a 5- to 14-membered member containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring constituent atom ( Preferred is a 5- to 10-membered aromatic heterocyclic ring. Preferable examples of the “aromatic heterocyclic ring” include thiophene, furan, pyrrole, imidazole, pyrazole, thiazole, isothiazole, oxazole, isoxazole, pyridine, pyrazine, pyrimidine, pyridazine, 1,2,4-oxadi 5- to 6-membered monocyclic aromatic heterocycle such as azole, 1,3,4-oxadiazole, 1,2,4-thiadiazole, 1,3,4-thiadiazole, triazole, tetrazole, triazine;
Benzothiophene, benzofuran, benzimidazole, benzoxazole, benzoisoxazole, benzothiazole, benzoisothiazole, benzotriazole, imidazopyridine, thienopyridine, furopyridine, pyrrolopyridine, pyrazolopyridine, oxazolopyridine, thiazolopyridine, imidazopyrazine, Imidazopyrimidine, thienopyrimidine, furopyrimidine, pyrrolopyrimidine, pyrazolopyrimidine, oxazolopyrimidine, thiazolopyrimidine, pyrazolopyrimidine, pyrazolotriazine, naphtho [2,3-b] thiophene, phenoxathiin, indol, Isoindole, 1H-indazole, purine, isoquinoline, quinoline, phthalazine, naphthyridine, quinoxaline, quinazoline, cinnoline, cal Tetrazole, beta-carboline, phenanthridine, acridine, phenazine, phenothiazine, 8 to 14 membered fused polycyclic, such as phenoxazine (preferably 2 or tricyclic) and aromatic heterocycle.
ベンゾチオフェン、ベンゾフラン、ベンゾイミダゾール、ベンゾオキサゾール、ベンゾイソオキサゾール、ベンゾチアゾール、ベンゾイソチアゾール、ベンゾトリアゾール、イミダゾピリジン、チエノピリジン、フロピリジン、ピロロピリジン、ピラゾロピリジン、オキサゾロピリジン、チアゾロピリジン、イミダゾピラジン、イミダゾピリミジン、チエノピリミジン、フロピリミジン、ピロロピリミジン、ピラゾロピリミジン、オキサゾロピリミジン、チアゾロピリミジン、ピラゾロピリミジン、ピラゾロトリアジン、ナフト[2,3-b]チオフェン、フェノキサチイン、インド-ル、イソインドール、1H-インダゾール、プリン、イソキノリン、キノリン、フタラジン、ナフチリジン、キノキサリン、キナゾリン、シンノリン、カルバゾール、β-カルボリン、フェナントリジン、アクリジン、フェナジン、フェノチアジン、フェノキサジンなどの8ないし14員縮合多環式(好ましくは2または3環式)芳香族複素環が挙げられる。 In the present specification, the “aromatic heterocycle” is, for example, a 5- to 14-membered member containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring constituent atom ( Preferred is a 5- to 10-membered aromatic heterocyclic ring. Preferable examples of the “aromatic heterocyclic ring” include thiophene, furan, pyrrole, imidazole, pyrazole, thiazole, isothiazole, oxazole, isoxazole, pyridine, pyrazine, pyrimidine, pyridazine, 1,2,4-oxadi 5- to 6-membered monocyclic aromatic heterocycle such as azole, 1,3,4-oxadiazole, 1,2,4-thiadiazole, 1,3,4-thiadiazole, triazole, tetrazole, triazine;
Benzothiophene, benzofuran, benzimidazole, benzoxazole, benzoisoxazole, benzothiazole, benzoisothiazole, benzotriazole, imidazopyridine, thienopyridine, furopyridine, pyrrolopyridine, pyrazolopyridine, oxazolopyridine, thiazolopyridine, imidazopyrazine, Imidazopyrimidine, thienopyrimidine, furopyrimidine, pyrrolopyrimidine, pyrazolopyrimidine, oxazolopyrimidine, thiazolopyrimidine, pyrazolopyrimidine, pyrazolotriazine, naphtho [2,3-b] thiophene, phenoxathiin, indol, Isoindole, 1H-indazole, purine, isoquinoline, quinoline, phthalazine, naphthyridine, quinoxaline, quinazoline, cinnoline, cal Tetrazole, beta-carboline, phenanthridine, acridine, phenazine, phenothiazine, 8 to 14 membered fused polycyclic, such as phenoxazine (preferably 2 or tricyclic) and aromatic heterocycle.
本明細書中、「非芳香族複素環」としては、例えば、環構成原子として炭素原子以外に窒素原子、硫黄原子および酸素原子から選ばれる1ないし4個のヘテロ原子を含有する3ないし14員(好ましくは4ないし10員)の非芳香族複素環が挙げられる。該「非芳香族複素環」の好適な例としては、アジリジン、オキシラン、チイラン、アゼチジン、オキセタン、チエタン、テトラヒドロチオフェン、テトラヒドロフラン、ピロリン、ピロリジン、イミダゾリン、イミダゾリジン、オキサゾリン、オキサゾリジン、ピラゾリン、ピラゾリジン、チアゾリン、チアゾリジン、テトラヒドロイソチアゾール、テトラヒドロオキサゾール、テトラヒドロイソオキサゾール、ピペリジン、ピペラジン、テトラヒドロピリジン、ジヒドロピリジン、ジヒドロチオピラン、テトラヒドロピリミジン、テトラヒドロピリダジン、ジヒドロピラン、テトラヒドロピラン、テトラヒドロチオピラン、モルホリン、チオモルホリン、アゼパニン、ジアゼパン、アゼピン、アゾカン、ジアゾカン、オキセパンなどの3ないし8員単環式非芳香族複素環;
ジヒドロベンゾフラン、ジヒドロベンゾイミダゾール、ジヒドロベンゾオキサゾール、ジヒドロベンゾチアゾール、ジヒドロベンゾイソチアゾール、ジヒドロナフト[2,3-b]チオフェン、テトラヒドロイソキノリン、テトラヒドロキノリン、4H-キノリジン、インドリン、イソインドリン、テトラヒドロチエノ[2,3-c]ピリジン、テトラヒドロベンゾアゼピン、テトラヒドロキノキサリン、テトラヒドロフェナントリジン、ヘキサヒドロフェノチアジン、ヘキサヒドロフェノキサジン、テトラヒドロフタラジン、テトラヒドロナフチリジン、テトラヒドロキナゾリン、テトラヒドロシンノリン、テトラヒドロカルバゾール、テトラヒドロ-β-カルボリン、テトラヒドロアクリジン、テトラヒドロフェナジン、テトラヒドロチオキサンテン、オクタヒドロイソキノリンなどの9ないし14員縮合多環式(好ましくは2または3環式)非芳香族複素環が挙げられる。
本明細書中、「含窒素複素環」としては、「複素環」のうち、環構成原子として少なくとも1個以上の窒素原子を含有するものが挙げられる。 In the present specification, the “non-aromatic heterocycle” includes, for example, a 3 to 14 member containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring constituent atom. (Preferably 4 to 10 membered) non-aromatic heterocycle. Suitable examples of the “non-aromatic heterocycle” include aziridine, oxirane, thiirane, azetidine, oxetane, thietane, tetrahydrothiophene, tetrahydrofuran, pyrroline, pyrrolidine, imidazoline, imidazolidine, oxazoline, oxazolidine, pyrazoline, pyrazolidine, thiazoline. , Thiazolidine, tetrahydroisothiazole, tetrahydrooxazole, tetrahydroisoxazole, piperidine, piperazine, tetrahydropyridine, dihydropyridine, dihydrothiopyran, tetrahydropyrimidine, tetrahydropyridazine, dihydropyran, tetrahydropyran, tetrahydrothiopyran, morpholine, thiomorpholine, azepanine, 3 types such as diazepan, azepine, azocan, diazocan, oxepane 8-membered monocyclic non-aromatic heterocyclic ring and;
Dihydrobenzofuran, dihydrobenzimidazole, dihydrobenzoxazole, dihydrobenzothiazole, dihydrobenzoisothiazole, dihydronaphtho [2,3-b] thiophene, tetrahydroisoquinoline, tetrahydroquinoline, 4H-quinolidine, indoline, isoindoline, tetrahydrothieno [2 , 3-c] pyridine, tetrahydrobenzazepine, tetrahydroquinoxaline, tetrahydrophenanthridine, hexahydrophenothiazine, hexahydrophenoxazine, tetrahydrophthalazine, tetrahydronaphthyridine, tetrahydroquinazoline, tetrahydrocinnoline, tetrahydrocarbazole, tetrahydro-β-carboline Tetrahydroacridine, tetrahydrophenazine, tetrahydrothi Xanthene, 9 to 14 membered fused polycyclic, such as octahydro-isoquinoline (preferably 2 or tricyclic) non-aromatic heterocyclic ring.
In the present specification, examples of the “nitrogen-containing heterocycle” include those containing at least one nitrogen atom as a ring-constituting atom among the “heterocycle”.
ジヒドロベンゾフラン、ジヒドロベンゾイミダゾール、ジヒドロベンゾオキサゾール、ジヒドロベンゾチアゾール、ジヒドロベンゾイソチアゾール、ジヒドロナフト[2,3-b]チオフェン、テトラヒドロイソキノリン、テトラヒドロキノリン、4H-キノリジン、インドリン、イソインドリン、テトラヒドロチエノ[2,3-c]ピリジン、テトラヒドロベンゾアゼピン、テトラヒドロキノキサリン、テトラヒドロフェナントリジン、ヘキサヒドロフェノチアジン、ヘキサヒドロフェノキサジン、テトラヒドロフタラジン、テトラヒドロナフチリジン、テトラヒドロキナゾリン、テトラヒドロシンノリン、テトラヒドロカルバゾール、テトラヒドロ-β-カルボリン、テトラヒドロアクリジン、テトラヒドロフェナジン、テトラヒドロチオキサンテン、オクタヒドロイソキノリンなどの9ないし14員縮合多環式(好ましくは2または3環式)非芳香族複素環が挙げられる。
本明細書中、「含窒素複素環」としては、「複素環」のうち、環構成原子として少なくとも1個以上の窒素原子を含有するものが挙げられる。 In the present specification, the “non-aromatic heterocycle” includes, for example, a 3 to 14 member containing 1 to 4 heteroatoms selected from a nitrogen atom, a sulfur atom and an oxygen atom in addition to a carbon atom as a ring constituent atom. (Preferably 4 to 10 membered) non-aromatic heterocycle. Suitable examples of the “non-aromatic heterocycle” include aziridine, oxirane, thiirane, azetidine, oxetane, thietane, tetrahydrothiophene, tetrahydrofuran, pyrroline, pyrrolidine, imidazoline, imidazolidine, oxazoline, oxazolidine, pyrazoline, pyrazolidine, thiazoline. , Thiazolidine, tetrahydroisothiazole, tetrahydrooxazole, tetrahydroisoxazole, piperidine, piperazine, tetrahydropyridine, dihydropyridine, dihydrothiopyran, tetrahydropyrimidine, tetrahydropyridazine, dihydropyran, tetrahydropyran, tetrahydrothiopyran, morpholine, thiomorpholine, azepanine, 3 types such as diazepan, azepine, azocan, diazocan, oxepane 8-membered monocyclic non-aromatic heterocyclic ring and;
Dihydrobenzofuran, dihydrobenzimidazole, dihydrobenzoxazole, dihydrobenzothiazole, dihydrobenzoisothiazole, dihydronaphtho [2,3-b] thiophene, tetrahydroisoquinoline, tetrahydroquinoline, 4H-quinolidine, indoline, isoindoline, tetrahydrothieno [2 , 3-c] pyridine, tetrahydrobenzazepine, tetrahydroquinoxaline, tetrahydrophenanthridine, hexahydrophenothiazine, hexahydrophenoxazine, tetrahydrophthalazine, tetrahydronaphthyridine, tetrahydroquinazoline, tetrahydrocinnoline, tetrahydrocarbazole, tetrahydro-β-carboline Tetrahydroacridine, tetrahydrophenazine, tetrahydrothi Xanthene, 9 to 14 membered fused polycyclic, such as octahydro-isoquinoline (preferably 2 or tricyclic) non-aromatic heterocyclic ring.
In the present specification, examples of the “nitrogen-containing heterocycle” include those containing at least one nitrogen atom as a ring-constituting atom among the “heterocycle”.
本明細書中、「置換されていてもよい含窒素不飽和複素環」の「含窒素不飽和複素環」としては、上記「含窒素複素環」のうち、1個以上の多重結合(例、二重結合、三重結合)を含有するものが挙げられ、その置換基としては、上記「置換基」が挙げられる。該「含窒素不飽和複素環」には、含窒素芳香族複素環も含まれる。
In the present specification, the “nitrogen-containing unsaturated heterocycle” of the “optionally substituted nitrogen-containing unsaturated heterocycle” includes one or more multiple bonds (eg, Those containing a double bond or a triple bond), and examples of the substituent include the above-mentioned “substituent”. The “nitrogen-containing unsaturated heterocycle” includes a nitrogen-containing aromatic heterocycle.
本明細書中、「置換されていてもよいC1-2アルキレン」の「C1-2アルキレン」としては、上記「C1-6アルキレン基」のうち、炭素数が1または2のものが挙げられ、その置換基としては、上記「置換基」が挙げられる。
In the present specification, “C 1-2 alkylene” of “optionally substituted C 1-2 alkylene” includes those having 1 or 2 carbon atoms in the above “C 1-6 alkylene group”. Examples of the substituent include the above-mentioned “substituent”.
本明細書中、「置換されていてもよい環」の「環」としては、上記「炭化水素環」および「複素環」が挙げられ、その置換基としては、上記「置換基」が挙げられる。
本明細書中、「置換されていてもよい5員または6員の芳香環」の「芳香環」としては、ベンゼン環および上記「芳香族複素環」のうち炭素数が5または6のものが挙げられ、その置換基としては、上記「置換基」が挙げられる。
本明細書中、「置換されていてもよい5員または6員の非芳香族不飽和環」の「非芳香族不飽和環」としては、上記「C3-10シクロアルケン」のうち炭素数が5または6のもの、上記「複素環」のうち、炭素数が5または6でかつ1個以上の多重結合(例、二重結合、三重結合)を含有するものが挙げられ、その置換基としては、上記「置換基」が挙げられる。 In the present specification, examples of the “ring” of the “optionally substituted ring” include the above “hydrocarbon ring” and “heterocycle”, and the substituent includes the above “substituent”. .
In the present specification, the “aromatic ring” of the “optionally substituted 5- or 6-membered aromatic ring” includes a benzene ring and the above “aromatic heterocycle” having 5 or 6 carbon atoms. Examples of the substituent include the above-mentioned “substituent”.
In the present specification, the “non-aromatic unsaturated ring” of the “optionally substituted 5-membered or 6-membered non-aromatic unsaturated ring” is the carbon number of the above “C 3-10 cycloalkene”. Of the above-mentioned “heterocycle” having 5 or 6 carbon atoms and containing one or more multiple bonds (eg, double bonds, triple bonds), and the substituents thereof. As the above, the above-mentioned “substituent” can be mentioned.
本明細書中、「置換されていてもよい5員または6員の芳香環」の「芳香環」としては、ベンゼン環および上記「芳香族複素環」のうち炭素数が5または6のものが挙げられ、その置換基としては、上記「置換基」が挙げられる。
本明細書中、「置換されていてもよい5員または6員の非芳香族不飽和環」の「非芳香族不飽和環」としては、上記「C3-10シクロアルケン」のうち炭素数が5または6のもの、上記「複素環」のうち、炭素数が5または6でかつ1個以上の多重結合(例、二重結合、三重結合)を含有するものが挙げられ、その置換基としては、上記「置換基」が挙げられる。 In the present specification, examples of the “ring” of the “optionally substituted ring” include the above “hydrocarbon ring” and “heterocycle”, and the substituent includes the above “substituent”. .
In the present specification, the “aromatic ring” of the “optionally substituted 5- or 6-membered aromatic ring” includes a benzene ring and the above “aromatic heterocycle” having 5 or 6 carbon atoms. Examples of the substituent include the above-mentioned “substituent”.
In the present specification, the “non-aromatic unsaturated ring” of the “optionally substituted 5-membered or 6-membered non-aromatic unsaturated ring” is the carbon number of the above “C 3-10 cycloalkene”. Of the above-mentioned “heterocycle” having 5 or 6 carbon atoms and containing one or more multiple bonds (eg, double bonds, triple bonds), and the substituents thereof. As the above, the above-mentioned “substituent” can be mentioned.
以下に、式(I)中の各記号の定義について詳述する。
環Aは、さらに置換されていてもよい含窒素不飽和複素環を示す。
環Aで示される「さらに置換されていてもよい含窒素不飽和複素環」の「含窒素不飽和複素環」としては、単環式または二環式の含窒素不飽和複素環が挙げられる。
環Aで示される「さらに置換されていてもよい含窒素不飽和複素環」の「置換基」としては、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)、置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)が挙げられる。 Below, the definition of each symbol in Formula (I) is explained in full detail.
Ring A represents a nitrogen-containing unsaturated heterocyclic ring which may be further substituted.
Examples of the “nitrogen-containing unsaturated heterocycle” of the “optionally substituted nitrogen-containing unsaturated heterocycle” represented by ring A include monocyclic or bicyclic nitrogen-containing unsaturated heterocycles.
Examples of the “substituent” of the “optionally substituted nitrogen-containing unsaturated heterocyclic ring” represented by ring A include a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, and an optionally substituted C A 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group (eg, methoxy), an optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyrani) Ruoxy).
環Aは、さらに置換されていてもよい含窒素不飽和複素環を示す。
環Aで示される「さらに置換されていてもよい含窒素不飽和複素環」の「含窒素不飽和複素環」としては、単環式または二環式の含窒素不飽和複素環が挙げられる。
環Aで示される「さらに置換されていてもよい含窒素不飽和複素環」の「置換基」としては、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)、置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)が挙げられる。 Below, the definition of each symbol in Formula (I) is explained in full detail.
Ring A represents a nitrogen-containing unsaturated heterocyclic ring which may be further substituted.
Examples of the “nitrogen-containing unsaturated heterocycle” of the “optionally substituted nitrogen-containing unsaturated heterocycle” represented by ring A include monocyclic or bicyclic nitrogen-containing unsaturated heterocycles.
Examples of the “substituent” of the “optionally substituted nitrogen-containing unsaturated heterocyclic ring” represented by ring A include a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, and an optionally substituted C A 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group (eg, methoxy), an optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyrani) Ruoxy).
環Aは、好ましくは、さらに置換されていてもよい、式(A):
Ring A is preferably further substituted, formula (A):
[式中、
環A1は、さらに置換されていてもよい5員または6員の芳香環、または、さらに置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A2は、置換されていてもよい5員または6員の芳香環、または、置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A1または環A2の環構成原子の少なくとも1個以上が窒素原子である。]
で表される二環式の含窒素不飽和複素環である。 [Where:
Ring A 1 represents an optionally further substituted 5- or 6-membered aromatic ring, or an optionally further substituted 5- or 6-membered non-aromatic unsaturated ring;
Ring A 2 represents an optionally substituted 5- or 6-membered aromatic ring, or an optionally substituted 5- or 6-membered non-aromatic unsaturated ring;
At least one of the ring constituent atoms of ring A 1 or ring A 2 is a nitrogen atom. ]
It is a bicyclic nitrogen-containing unsaturated heterocyclic ring represented by these.
環A1は、さらに置換されていてもよい5員または6員の芳香環、または、さらに置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A2は、置換されていてもよい5員または6員の芳香環、または、置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A1または環A2の環構成原子の少なくとも1個以上が窒素原子である。]
で表される二環式の含窒素不飽和複素環である。 [Where:
Ring A 1 represents an optionally further substituted 5- or 6-membered aromatic ring, or an optionally further substituted 5- or 6-membered non-aromatic unsaturated ring;
Ring A 2 represents an optionally substituted 5- or 6-membered aromatic ring, or an optionally substituted 5- or 6-membered non-aromatic unsaturated ring;
At least one of the ring constituent atoms of ring A 1 or ring A 2 is a nitrogen atom. ]
It is a bicyclic nitrogen-containing unsaturated heterocyclic ring represented by these.
ここで、本明細書中、上記式(A)において(環A1と環A2とが1つの共有の結合を有して二環式環を形成する場合)、該二環式環の形成に関与する環A1の結合多重度と環A2の結合多重度とは同じとする。例えば、式(A)が
Here, in the present specification, in the above formula (A) (when ring A 1 and ring A 2 have one shared bond to form a bicyclic ring), formation of the bicyclic ring It is assumed that the bond multiplicity of the ring A 1 and the bond multiplicity of the ring A 2 involved in is the same. For example, the formula (A) is
である場合、環A1はイミダゾール環であり、環A2はピリジン環であり、式(A)が
, Ring A 1 is an imidazole ring, ring A 2 is a pyridine ring, and formula (A) is
である場合、環A1はイミダゾリン環であり、環A2はピリジン環である。
The ring A 1 is an imidazoline ring and the ring A 2 is a pyridine ring.
環A1または環A2で示される「(さらに)置換されていてもよい5員または6員の芳香環」の「芳香環」としては、ピロール環、チオフェン環、ピラゾール環、イミダゾール環、ピリジン環、ピリミジン環が挙げられる。
環A1または環A2で示される「(さらに)置換されていてもよい5員または6員の非芳香族不飽和環」の「非芳香族不飽和環」としては、イミダゾリン環、テトラヒドロピラジン環が挙げられる。
環A1または環A2で示される「(さらに)置換されていてもよい5員または6員の芳香環」または「(さらに)置換されていてもよい5員または6員の不飽和環」の「置換基」としては、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)、置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)が挙げられる。 Examples of the “aromatic ring” of the “(further) optionally substituted 5-membered or 6-membered aromatic ring” represented by ring A 1 or ring A 2 include pyrrole ring, thiophene ring, pyrazole ring, imidazole ring, pyridine And a ring and a pyrimidine ring.
Examples of the “non-aromatic unsaturated ring” of the “(further) optionally substituted 5- or 6-membered non-aromatic unsaturated ring” represented by ring A 1 or ring A 2 include imidazoline ring, tetrahydropyrazine A ring is mentioned.
“(Further) optionally substituted 5- or 6-membered aromatic ring” or “(further) optionally substituted 5- or 6-membered unsaturated ring” represented by ring A 1 or ring A 2 As the “substituent”, a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C A 1-6 alkoxy group (eg, methoxy) and a non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) which may be substituted.
環A1または環A2で示される「(さらに)置換されていてもよい5員または6員の非芳香族不飽和環」の「非芳香族不飽和環」としては、イミダゾリン環、テトラヒドロピラジン環が挙げられる。
環A1または環A2で示される「(さらに)置換されていてもよい5員または6員の芳香環」または「(さらに)置換されていてもよい5員または6員の不飽和環」の「置換基」としては、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)、置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)が挙げられる。 Examples of the “aromatic ring” of the “(further) optionally substituted 5-membered or 6-membered aromatic ring” represented by ring A 1 or ring A 2 include pyrrole ring, thiophene ring, pyrazole ring, imidazole ring, pyridine And a ring and a pyrimidine ring.
Examples of the “non-aromatic unsaturated ring” of the “(further) optionally substituted 5- or 6-membered non-aromatic unsaturated ring” represented by ring A 1 or ring A 2 include imidazoline ring, tetrahydropyrazine A ring is mentioned.
“(Further) optionally substituted 5- or 6-membered aromatic ring” or “(further) optionally substituted 5- or 6-membered unsaturated ring” represented by ring A 1 or ring A 2 As the “substituent”, a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C A 1-6 alkoxy group (eg, methoxy) and a non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) which may be substituted.
環Aは、より好ましくは、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)および置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)から選ばれる1~3個(好ましくは、1または2個)の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、チオフェン環、ピリジン環、イミダゾリン環またはテトラヒドロピラジン環であり、環A2がピロール環、チオフェン環、ピリジン環またはピリミジン環である式(A)で表される二環式の含窒素不飽和複素環である。
環Aは、さらにより好ましくは、ヒドロキシ基でさらに置換された1H-イミダゾ[4,5-b]ピリジン環またはメチルでさらに置換された1H-ピロロ[2,3-b]ピリジン環である。 Ring A is more preferably a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1 to 3 (preferably 1 or 2) selected from a 1-6 alkoxy group (eg, methoxy) and an optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 which may be further substituted with a substituent is pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole ring, thiophene ring, pyridine It is a bicyclic nitrogen-containing unsaturated heterocycle represented by the formula (A) which is a ring or a pyrimidine ring.
Ring A is even more preferably a 1H-imidazo [4,5-b] pyridine ring further substituted with a hydroxy group or a 1H-pyrrolo [2,3-b] pyridine ring further substituted with methyl.
環Aは、さらにより好ましくは、ヒドロキシ基でさらに置換された1H-イミダゾ[4,5-b]ピリジン環またはメチルでさらに置換された1H-ピロロ[2,3-b]ピリジン環である。 Ring A is more preferably a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1 to 3 (preferably 1 or 2) selected from a 1-6 alkoxy group (eg, methoxy) and an optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 which may be further substituted with a substituent is pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole ring, thiophene ring, pyridine It is a bicyclic nitrogen-containing unsaturated heterocycle represented by the formula (A) which is a ring or a pyrimidine ring.
Ring A is even more preferably a 1H-imidazo [4,5-b] pyridine ring further substituted with a hydroxy group or a 1H-pyrrolo [2,3-b] pyridine ring further substituted with methyl.
本願発明の別の実施態様において、環Aは、好ましくは、さらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)であり、より好ましくは、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)である。
In another embodiment of the present invention, ring A is preferably an optionally substituted monocyclic nitrogen-containing unsaturated heterocycle (eg, imidazolidine ring, imidazoline ring), more preferably, It is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group.
なお、本明細書中、環Aは、互変異性体(エノール体とケト体)が存在する場合、いずれの互変異性体も含む。例えば、環Aが、ヒドロキシでさらに置換されたイミダゾ[4,5-b]ピリジンである場合、式:
In the present specification, ring A includes any tautomer when tautomers (enol form and keto form) exist. For example, when Ring A is imidazo [4,5-b] pyridine, further substituted with hydroxy, the formula:
で示される2つの互変異性体(エノール体(a)およびケト体(b))が存在し、環Aはいずれの互変異性体も含む。
There are two tautomers (enol form (a) and keto form (b)) shown by the following formula, and ring A includes both tautomers.
環Bは、さらに置換されていてもよいC6-14芳香族炭化水素環またはさらに置換されていてもよい芳香族複素環を示す。
環Bで示される「さらに置換されていてもよいC6-14芳香族炭化水素環」の「C6-14芳香族炭化水素環」としては、ベンゼン環が挙げられる。
環Bで示される「さらに置換されていてもよい芳香族複素環」の「芳香族複素環」としては、チオフェン環、ピリジン環が挙げられる。
環Bで示される「さらに置換されていてもよいC6-14芳香族炭化水素環」および「さらに置換されていてもよい芳香族複素環」の「置換基」としては、ハロゲン原子(例、フッ素原子、塩素原子)、シアノ基、置換されていてもよいC1-6アルキル基(例、メチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)が挙げられる。 Ring B represents a C 6-14 aromatic hydrocarbon ring which may be further substituted or an aromatic heterocyclic ring which may be further substituted.
The “C 6-14 aromatic hydrocarbon ring” of the “ optionally substituted C 6-14 aromatic hydrocarbon ring” represented by ring B includes a benzene ring.
Examples of the “aromatic heterocycle” of the “optionally substituted aromatic heterocycle” represented by ring B include a thiophene ring and a pyridine ring.
As the “substituent” of the “optionally substituted C 6-14 aromatic hydrocarbon ring” and the “optionally substituted aromatic heterocycle” represented by ring B, a halogen atom (eg, A fluorine atom, a chlorine atom), a cyano group, an optionally substituted C 1-6 alkyl group (eg, methyl), and an optionally substituted C 1-6 alkoxy group (eg, methoxy).
環Bで示される「さらに置換されていてもよいC6-14芳香族炭化水素環」の「C6-14芳香族炭化水素環」としては、ベンゼン環が挙げられる。
環Bで示される「さらに置換されていてもよい芳香族複素環」の「芳香族複素環」としては、チオフェン環、ピリジン環が挙げられる。
環Bで示される「さらに置換されていてもよいC6-14芳香族炭化水素環」および「さらに置換されていてもよい芳香族複素環」の「置換基」としては、ハロゲン原子(例、フッ素原子、塩素原子)、シアノ基、置換されていてもよいC1-6アルキル基(例、メチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)が挙げられる。 Ring B represents a C 6-14 aromatic hydrocarbon ring which may be further substituted or an aromatic heterocyclic ring which may be further substituted.
The “C 6-14 aromatic hydrocarbon ring” of the “ optionally substituted C 6-14 aromatic hydrocarbon ring” represented by ring B includes a benzene ring.
Examples of the “aromatic heterocycle” of the “optionally substituted aromatic heterocycle” represented by ring B include a thiophene ring and a pyridine ring.
As the “substituent” of the “optionally substituted C 6-14 aromatic hydrocarbon ring” and the “optionally substituted aromatic heterocycle” represented by ring B, a halogen atom (eg, A fluorine atom, a chlorine atom), a cyano group, an optionally substituted C 1-6 alkyl group (eg, methyl), and an optionally substituted C 1-6 alkoxy group (eg, methoxy).
環Bは、好ましくは、さらに置換されていてもよいベンゼン環、あるいはそれぞれさらに置換されていてもよいチオフェン環またはピリジン環であり、より好ましくは、ハロゲン原子(例、フッ素原子、塩素原子)、シアノ基、置換されていてもよいC1-6アルキル基(例、メチル)および置換されていてもよいC1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、あるいはそれぞれさらに置換されていてもよいチオフェン環またはピリジン環であり、さらにより好ましくは、
(1)(i)ハロゲン原子(例、フッ素原子、塩素原子)、(ii)シアノ基、(iii)ハロゲン原子(例、フッ素原子)およびアミノ基から選ばれる1~3個の置換基で置換されていてもよいC1-6アルキル基(例、メチル)および(iv)C1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
である。 Ring B is preferably a benzene ring which may be further substituted, or a thiophene ring or a pyridine ring which may be further substituted, more preferably a halogen atom (eg, fluorine atom, chlorine atom), 1 to 5 (preferably 1 to 5) selected from a cyano group, an optionally substituted C 1-6 alkyl group (eg, methyl) and an optionally substituted C 1-6 alkoxy group (eg, methoxy) A benzene ring which may be further substituted with three, more preferably 1 or 2) substituents, or a thiophene ring or a pyridine ring which may be further substituted respectively, and still more preferably,
(1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms) and amino groups 1 to 5 (preferably 1 to 3, more preferably 1) selected from a C 1-6 alkyl group (eg, methyl) and (iv) a C 1-6 alkoxy group (eg, methoxy) Or a benzene ring optionally further substituted with 2) substituents,
(2) a thiophene ring, or (3) a pyridine ring.
(1)(i)ハロゲン原子(例、フッ素原子、塩素原子)、(ii)シアノ基、(iii)ハロゲン原子(例、フッ素原子)およびアミノ基から選ばれる1~3個の置換基で置換されていてもよいC1-6アルキル基(例、メチル)および(iv)C1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
である。 Ring B is preferably a benzene ring which may be further substituted, or a thiophene ring or a pyridine ring which may be further substituted, more preferably a halogen atom (eg, fluorine atom, chlorine atom), 1 to 5 (preferably 1 to 5) selected from a cyano group, an optionally substituted C 1-6 alkyl group (eg, methyl) and an optionally substituted C 1-6 alkoxy group (eg, methoxy) A benzene ring which may be further substituted with three, more preferably 1 or 2) substituents, or a thiophene ring or a pyridine ring which may be further substituted respectively, and still more preferably,
(1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms) and amino groups 1 to 5 (preferably 1 to 3, more preferably 1) selected from a C 1-6 alkyl group (eg, methyl) and (iv) a C 1-6 alkoxy group (eg, methoxy) Or a benzene ring optionally further substituted with 2) substituents,
(2) a thiophene ring, or (3) a pyridine ring.
Lは、置換されていてもよいC1-2アルキレンを示す。
Lで示される「置換されていてもよいC1-2アルキレン」の「置換基」としては、C1-6アルキル基(例、メチル)が挙げられる。
Lは、好ましくは、C1-2アルキレン(例、-CH2-、-CH2CH2-、-CH(CH3)-)であり、より好ましくは、-CH2-である。 L represents C 1-2 alkylene which may be substituted.
Examples of the “substituent” of the “optionally substituted C 1-2 alkylene” represented by L include a C 1-6 alkyl group (eg, methyl).
L is preferably, C 1-2 alkylene (e.g., -CH 2 -, - CH 2 CH 2 -, - CH (CH 3) -) , more preferably an, -CH 2 - it is.
Lで示される「置換されていてもよいC1-2アルキレン」の「置換基」としては、C1-6アルキル基(例、メチル)が挙げられる。
Lは、好ましくは、C1-2アルキレン(例、-CH2-、-CH2CH2-、-CH(CH3)-)であり、より好ましくは、-CH2-である。 L represents C 1-2 alkylene which may be substituted.
Examples of the “substituent” of the “optionally substituted C 1-2 alkylene” represented by L include a C 1-6 alkyl group (eg, methyl).
L is preferably, C 1-2 alkylene (e.g., -CH 2 -, - CH 2 CH 2 -, - CH (CH 3) -) , more preferably an, -CH 2 - it is.
X1は、NまたはCR1を示し、X2は、NまたはCR2を示し、X3は、NまたはCR3を示し、X4は、NまたはCR4を示し、R1、R2、R3およびR4は、独立して、水素原子または置換基を示す。
R1、R2、R3またはR4で示される「置換基」としては、ハロゲン原子(例、フッ素原子)、C1-6アルキル基(例、メチル)が挙げられる。
X1は、好ましくは、NまたはCR1(R1は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり、より好ましくは、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり、さらにより好ましくは、CR1(R1は、水素原子である。)である。
X2は、好ましくは、NまたはCR2(R2は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり、より好ましくは、NまたはCR2(R2は、水素原子またはフッ素原子である。)であり、さらにより好ましくは、CR2(R2は、水素原子である。)である。
X3は、好ましくは、NまたはCR3(R3は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり、より好ましくは、NまたはCR3(R3は、水素原子またはフッ素原子である。)であり、さらにより好ましくは、NまたはCR3(R3は、水素原子である。)である。
X4は、好ましくは、NまたはCR4(R4は、水素原子またはC1-6アルキル基(例、メチル)である。)であり、より好ましくは、NまたはCR4(R4は、水素原子またはメチルである。)であり、さらにより好ましくは、NまたはCR4(R4は、水素原子である。)である。 X 1 represents N or CR 1 , X 2 represents N or CR 2 , X 3 represents N or CR 3 , X 4 represents N or CR 4 , R 1 , R 2 , R 3 and R 4 independently represent a hydrogen atom or a substituent.
Examples of the “substituent” represented by R 1 , R 2 , R 3 or R 4 include a halogen atom (eg, fluorine atom) and a C 1-6 alkyl group (eg, methyl).
X 1 is preferably N or CR 1 (R 1 is a hydrogen atom or a halogen atom (eg, a fluorine atom)), more preferably N or CR 1 (R 1 is a hydrogen atom or A fluorine atom), and even more preferably CR 1 (R 1 is a hydrogen atom).
X 2 is preferably N or CR 2 (R 2 is a hydrogen atom or a halogen atom (eg, fluorine atom)), more preferably N or CR 2 (R 2 is a hydrogen atom or A fluorine atom), and even more preferably CR 2 (R 2 is a hydrogen atom).
X 3 is preferably N or CR 3 (R 3 is a hydrogen atom or a halogen atom (eg, fluorine atom)), and more preferably N or CR 3 (R 3 is a hydrogen atom or A fluorine atom), and even more preferably N or CR 3 (R 3 is a hydrogen atom).
X 4 is preferably N or CR 4 (R 4 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl)), more preferably N or CR 4 (R 4 is A hydrogen atom or methyl), and even more preferably N or CR 4 (R 4 is a hydrogen atom).
R1、R2、R3またはR4で示される「置換基」としては、ハロゲン原子(例、フッ素原子)、C1-6アルキル基(例、メチル)が挙げられる。
X1は、好ましくは、NまたはCR1(R1は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり、より好ましくは、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり、さらにより好ましくは、CR1(R1は、水素原子である。)である。
X2は、好ましくは、NまたはCR2(R2は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり、より好ましくは、NまたはCR2(R2は、水素原子またはフッ素原子である。)であり、さらにより好ましくは、CR2(R2は、水素原子である。)である。
X3は、好ましくは、NまたはCR3(R3は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり、より好ましくは、NまたはCR3(R3は、水素原子またはフッ素原子である。)であり、さらにより好ましくは、NまたはCR3(R3は、水素原子である。)である。
X4は、好ましくは、NまたはCR4(R4は、水素原子またはC1-6アルキル基(例、メチル)である。)であり、より好ましくは、NまたはCR4(R4は、水素原子またはメチルである。)であり、さらにより好ましくは、NまたはCR4(R4は、水素原子である。)である。 X 1 represents N or CR 1 , X 2 represents N or CR 2 , X 3 represents N or CR 3 , X 4 represents N or CR 4 , R 1 , R 2 , R 3 and R 4 independently represent a hydrogen atom or a substituent.
Examples of the “substituent” represented by R 1 , R 2 , R 3 or R 4 include a halogen atom (eg, fluorine atom) and a C 1-6 alkyl group (eg, methyl).
X 1 is preferably N or CR 1 (R 1 is a hydrogen atom or a halogen atom (eg, a fluorine atom)), more preferably N or CR 1 (R 1 is a hydrogen atom or A fluorine atom), and even more preferably CR 1 (R 1 is a hydrogen atom).
X 2 is preferably N or CR 2 (R 2 is a hydrogen atom or a halogen atom (eg, fluorine atom)), more preferably N or CR 2 (R 2 is a hydrogen atom or A fluorine atom), and even more preferably CR 2 (R 2 is a hydrogen atom).
X 3 is preferably N or CR 3 (R 3 is a hydrogen atom or a halogen atom (eg, fluorine atom)), and more preferably N or CR 3 (R 3 is a hydrogen atom or A fluorine atom), and even more preferably N or CR 3 (R 3 is a hydrogen atom).
X 4 is preferably N or CR 4 (R 4 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl)), more preferably N or CR 4 (R 4 is A hydrogen atom or methyl), and even more preferably N or CR 4 (R 4 is a hydrogen atom).
X5は、CR5a、CR5bR5c、NR5dまたは結合手を示し、R5a、R5b、R5cおよびR5dは、独立して、水素原子または置換基を示すか、または、R5bとR5cは、一緒になって置換されていてもよい環を形成してもよい。
R5a、R5b、R5cまたはR5dで示される「置換基」としては、オキソ基が挙げられる。
R5bとR5cが一緒になって形成する「置換されていてもよい環」の「環」としては、C3-10シクロアルカン環(例、シクロペンタン環)が挙げられ、その「置換基」としては、ヒドロキシ基で置換されていてもよいC1-6アルキル基(例、メチル、ヒドロキシメチル)、カルバモイル基、ヒドロキシ基が挙げられる。
X5は、好ましくは、CR5bR5c(R5bおよびR5cは、独立して、水素原子または置換基である。)または結合手であり、より好ましくは、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり、さらにより好ましくは、結合手である。 X 5 represents CR 5a , CR 5b R 5c , NR 5d or a bond, and R 5a , R 5b , R 5c and R 5d independently represent a hydrogen atom or a substituent, or R 5b And R 5c together may form an optionally substituted ring.
Examples of the “substituent” represented by R 5a , R 5b , R 5c or R 5d include an oxo group.
Examples of the “ring” of the “optionally substituted ring” formed by R 5b and R 5c together include a C 3-10 cycloalkane ring (eg, cyclopentane ring). " Includes a C 1-6 alkyl group (eg, methyl, hydroxymethyl) optionally substituted with a hydroxy group, a carbamoyl group, and a hydroxy group.
X 5 is preferably CR 5b R 5c (R 5b and R 5c are each independently a hydrogen atom or a substituent) or a bond, and more preferably CR 5b R 5c (R 5b and R 5c is a hydrogen atom.) Or a bond, and even more preferably a bond.
R5a、R5b、R5cまたはR5dで示される「置換基」としては、オキソ基が挙げられる。
R5bとR5cが一緒になって形成する「置換されていてもよい環」の「環」としては、C3-10シクロアルカン環(例、シクロペンタン環)が挙げられ、その「置換基」としては、ヒドロキシ基で置換されていてもよいC1-6アルキル基(例、メチル、ヒドロキシメチル)、カルバモイル基、ヒドロキシ基が挙げられる。
X5は、好ましくは、CR5bR5c(R5bおよびR5cは、独立して、水素原子または置換基である。)または結合手であり、より好ましくは、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり、さらにより好ましくは、結合手である。 X 5 represents CR 5a , CR 5b R 5c , NR 5d or a bond, and R 5a , R 5b , R 5c and R 5d independently represent a hydrogen atom or a substituent, or R 5b And R 5c together may form an optionally substituted ring.
Examples of the “substituent” represented by R 5a , R 5b , R 5c or R 5d include an oxo group.
Examples of the “ring” of the “optionally substituted ring” formed by R 5b and R 5c together include a C 3-10 cycloalkane ring (eg, cyclopentane ring). " Includes a C 1-6 alkyl group (eg, methyl, hydroxymethyl) optionally substituted with a hydroxy group, a carbamoyl group, and a hydroxy group.
X 5 is preferably CR 5b R 5c (R 5b and R 5c are each independently a hydrogen atom or a substituent) or a bond, and more preferably CR 5b R 5c (R 5b and R 5c is a hydrogen atom.) Or a bond, and even more preferably a bond.
X6は、CR6aR6bまたはNR6cを示し、R6a、R6bおよびR6cは、独立して、水素原子または置換基を示すか、またはR6aとR6bは、一緒になって置換されていてもよい環を形成してもよい。
R6a、R6bまたはR6cで示される「置換基」としては、置換されていてもよいC1-6アルキル基(例、メチル)が挙げられる。
R6aとR6bが一緒になって形成する「置換されていてもよい環」の「環」としては、C3-10シクロアルカン環(例、シクロペンタン環)が挙げられ、その「置換基」としては、ヒドロキシ基で置換されていてもよいC1-6アルキル基(例、メチル、ヒドロキシメチル)、カルバモイル基、ヒドロキシ基が挙げられる。
X6は、好ましくは、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換基である。)であり、より好ましくは、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)である。)であり、さらにより好ましくは、CR6aR6b(R6aおよびR6bは、独立して、水素原子、あるいはヒドロキシ基またはC6-14アリール基(例、フェニル)で置換されていてもよいC1-6アルキル基(例、メチル)である。)である。
あるいは、本願発明の別の実施態様において、X5は、好ましくは、CR6aR6b(R6aおよびR6bは一緒になって、置換されていてもよいC3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり、より好ましくは、CR6aR6b(R6aおよびR6bは一緒になって、C3-10シクロアルカン環(例、シクロペンタン環)を形成する。)である。 X 6 represents CR 6a R 6b or NR 6c , and R 6a , R 6b and R 6c independently represent a hydrogen atom or a substituent, or R 6a and R 6b are substituted together May form a ring which may be
Examples of the “substituent” represented by R 6a , R 6b or R 6c include an optionally substituted C 1-6 alkyl group (eg, methyl).
Examples of the “ring” of the “optionally substituted ring” formed by R 6a and R 6b together include a C 3-10 cycloalkane ring (eg, cyclopentane ring). " Includes a C 1-6 alkyl group (eg, methyl, hydroxymethyl) optionally substituted with a hydroxy group, a carbamoyl group, and a hydroxy group.
X 6 is preferably CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom or a substituent), and more preferably CR 6a R 6b (R 6a and R 6b are Is independently a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl).), And even more preferably, CR 6a R 6b (R 6a and R 6b are independently And a C 1-6 alkyl group (eg, methyl) optionally substituted with a hydroxy group or a C 6-14 aryl group (eg, phenyl).
Alternatively, in another embodiment of the present invention, X 5 is preferably CR 6a R 6b (where R 6a and R 6b are taken together to represent an optionally substituted C 3-10 cycloalkane ring (eg, And more preferably CR 6a R 6b (R 6a and R 6b together form a C 3-10 cycloalkane ring (eg, a cyclopentane ring). ).
R6a、R6bまたはR6cで示される「置換基」としては、置換されていてもよいC1-6アルキル基(例、メチル)が挙げられる。
R6aとR6bが一緒になって形成する「置換されていてもよい環」の「環」としては、C3-10シクロアルカン環(例、シクロペンタン環)が挙げられ、その「置換基」としては、ヒドロキシ基で置換されていてもよいC1-6アルキル基(例、メチル、ヒドロキシメチル)、カルバモイル基、ヒドロキシ基が挙げられる。
X6は、好ましくは、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換基である。)であり、より好ましくは、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)である。)であり、さらにより好ましくは、CR6aR6b(R6aおよびR6bは、独立して、水素原子、あるいはヒドロキシ基またはC6-14アリール基(例、フェニル)で置換されていてもよいC1-6アルキル基(例、メチル)である。)である。
あるいは、本願発明の別の実施態様において、X5は、好ましくは、CR6aR6b(R6aおよびR6bは一緒になって、置換されていてもよいC3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり、より好ましくは、CR6aR6b(R6aおよびR6bは一緒になって、C3-10シクロアルカン環(例、シクロペンタン環)を形成する。)である。 X 6 represents CR 6a R 6b or NR 6c , and R 6a , R 6b and R 6c independently represent a hydrogen atom or a substituent, or R 6a and R 6b are substituted together May form a ring which may be
Examples of the “substituent” represented by R 6a , R 6b or R 6c include an optionally substituted C 1-6 alkyl group (eg, methyl).
Examples of the “ring” of the “optionally substituted ring” formed by R 6a and R 6b together include a C 3-10 cycloalkane ring (eg, cyclopentane ring). " Includes a C 1-6 alkyl group (eg, methyl, hydroxymethyl) optionally substituted with a hydroxy group, a carbamoyl group, and a hydroxy group.
X 6 is preferably CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom or a substituent), and more preferably CR 6a R 6b (R 6a and R 6b are Is independently a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl).), And even more preferably, CR 6a R 6b (R 6a and R 6b are independently And a C 1-6 alkyl group (eg, methyl) optionally substituted with a hydroxy group or a C 6-14 aryl group (eg, phenyl).
Alternatively, in another embodiment of the present invention, X 5 is preferably CR 6a R 6b (where R 6a and R 6b are taken together to represent an optionally substituted C 3-10 cycloalkane ring (eg, And more preferably CR 6a R 6b (R 6a and R 6b together form a C 3-10 cycloalkane ring (eg, a cyclopentane ring). ).
R7は、水素原子または置換基を示す。
R7で示される「置換基」としては、置換されていてもよいC1-6アルキル基(例、メチル)が挙げられる。
R7は、好ましくは、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)であり、より好ましくは、水素原子またはC1-6アルキル基(例、メチル)であり、さらにより好ましくは、水素原子である。 R 7 represents a hydrogen atom or a substituent.
Examples of the “substituent” represented by R 7 include an optionally substituted C 1-6 alkyl group (eg, methyl).
R 7 is preferably a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl), more preferably a hydrogen atom or a C 1-6 alkyl group (eg, methyl). Even more preferably, it is a hydrogen atom.
R7で示される「置換基」としては、置換されていてもよいC1-6アルキル基(例、メチル)が挙げられる。
R7は、好ましくは、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)であり、より好ましくは、水素原子またはC1-6アルキル基(例、メチル)であり、さらにより好ましくは、水素原子である。 R 7 represents a hydrogen atom or a substituent.
Examples of the “substituent” represented by R 7 include an optionally substituted C 1-6 alkyl group (eg, methyl).
R 7 is preferably a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl), more preferably a hydrogen atom or a C 1-6 alkyl group (eg, methyl). Even more preferably, it is a hydrogen atom.
化合物(I)の好適な例としては、以下の化合物が挙げられる。
[化合物I-1]
環Aが、さらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)であるか、または、さらに置換されていてもよい上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、さらに置換されていてもよいベンゼン環、あるいはそれぞれさらに置換されていてもよいチオフェン環またはピリジン環であり;
Lが、C1-2アルキレン(例、-CH2-、-CH2CH2-、-CH(CH3)-)であり;
X1が、NまたはCR1(R1は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり;
X2が、NまたはCR2(R2は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり;
X3が、NまたはCR3(R3は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり;
X4が、NまたはCR4(R4は、水素原子またはC1-6アルキル基(例、メチル)である。)であり;
X5が、CR5bR5c(R5bおよびR5cは、独立して、水素原子または置換基である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換基であるか、または、R6aおよびR6bが一緒になって、置換されていてもよい環を形成する。)であり;
R7が、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)である;
化合物(I)。 Preferable examples of compound (I) include the following compounds.
[Compound I-1]
Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted, or may be further substituted in the above formula (A) A bicyclic nitrogen-containing unsaturated heterocycle represented by
Ring B is a benzene ring which may be further substituted, or a thiophene ring or a pyridine ring which may be further substituted respectively;
L is C 1-2 alkylene (eg, —CH 2 —, —CH 2 CH 2 —, —CH (CH 3 ) —);
X 1 is N or CR 1 (R 1 is a hydrogen atom or a halogen atom (eg, fluorine atom));
X 2 is N or CR 2 (R 2 is a hydrogen atom or a halogen atom (eg, fluorine atom));
X 3 is N or CR 3 (R 3 is a hydrogen atom or a halogen atom (eg, fluorine atom));
X 4 is N or CR 4 (R 4 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl));
X 5 is CR 5b R 5c (R 5b and R 5c are each independently a hydrogen atom or a substituent) or a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom or a substituent, or R 6a and R 6b are taken together to form an optionally substituted ring. Forming);
R 7 is a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl);
Compound (I).
[化合物I-1]
環Aが、さらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)であるか、または、さらに置換されていてもよい上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、さらに置換されていてもよいベンゼン環、あるいはそれぞれさらに置換されていてもよいチオフェン環またはピリジン環であり;
Lが、C1-2アルキレン(例、-CH2-、-CH2CH2-、-CH(CH3)-)であり;
X1が、NまたはCR1(R1は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり;
X2が、NまたはCR2(R2は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり;
X3が、NまたはCR3(R3は、水素原子またはハロゲン原子(例、フッ素原子)である。)であり;
X4が、NまたはCR4(R4は、水素原子またはC1-6アルキル基(例、メチル)である。)であり;
X5が、CR5bR5c(R5bおよびR5cは、独立して、水素原子または置換基である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換基であるか、または、R6aおよびR6bが一緒になって、置換されていてもよい環を形成する。)であり;
R7が、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)である;
化合物(I)。 Preferable examples of compound (I) include the following compounds.
[Compound I-1]
Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted, or may be further substituted in the above formula (A) A bicyclic nitrogen-containing unsaturated heterocycle represented by
Ring B is a benzene ring which may be further substituted, or a thiophene ring or a pyridine ring which may be further substituted respectively;
L is C 1-2 alkylene (eg, —CH 2 —, —CH 2 CH 2 —, —CH (CH 3 ) —);
X 1 is N or CR 1 (R 1 is a hydrogen atom or a halogen atom (eg, fluorine atom));
X 2 is N or CR 2 (R 2 is a hydrogen atom or a halogen atom (eg, fluorine atom));
X 3 is N or CR 3 (R 3 is a hydrogen atom or a halogen atom (eg, fluorine atom));
X 4 is N or CR 4 (R 4 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl));
X 5 is CR 5b R 5c (R 5b and R 5c are each independently a hydrogen atom or a substituent) or a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom or a substituent, or R 6a and R 6b are taken together to form an optionally substituted ring. Forming);
R 7 is a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl);
Compound (I).
[化合物I-2]
環Aが、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)であるか、または、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)および置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)から選ばれる1~3個(好ましくは、1または2個)の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、チオフェン環、ピリジン環、イミダゾリン環またはテトラヒドロピラジン環であり、環A2がピロール環、チオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、ハロゲン原子(例、フッ素原子、塩素原子)、シアノ基、置換されていてもよいC1-6アルキル基(例、メチル)および置換されていてもよいC1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、あるいはそれぞれさらに置換されていてもよいチオフェン環またはピリジン環であり;
Lが、C1-2アルキレン(例、-CH2-、-CH2CH2-、-CH(CH3)-)であり;
X1が、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり;
X2が、NまたはCR2(R2は、水素原子またはフッ素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子またはフッ素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子またはメチルである。)であり;
X5が、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)であるか、または、R6aおよびR6bが一緒になって、置換されていてもよいC3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり;
R7が、水素原子またはC1-6アルキル基(例、メチル)である;
化合物(I)。 [Compound I-2]
Whether ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group Or a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group Further substituted with 1 to 3 (preferably 1 or 2) substituents selected from (eg, methoxy) and optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 is a pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the above formula (A) which is a ring, a thiophene ring, a pyridine ring or a pyrimidine ring;
Ring B is a halogen atom (eg, fluorine atom, chlorine atom), a cyano group, an optionally substituted C 1-6 alkyl group (eg, methyl) and an optionally substituted C 1-6 alkoxy group ( A benzene ring which may be further substituted with 1 to 5 (preferably 1 to 3, more preferably 1 or 2) substituents selected from eg methoxy), or each may be further substituted A thiophene ring or a pyridine ring;
L is C 1-2 alkylene (eg, —CH 2 —, —CH 2 CH 2 —, —CH (CH 3 ) —);
X 1 is N or CR 1 (R 1 is a hydrogen atom or a fluorine atom);
X 2 is N or CR 2 (R 2 is a hydrogen atom or a fluorine atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom or a fluorine atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom or methyl);
X 5 is CR 5b R 5c (R 5b and R 5c are hydrogen atoms) or a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl), or R 6a and R 6b 6b together form an optionally substituted C 3-10 cycloalkane ring (eg, cyclopentane ring));
R 7 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl);
Compound (I).
環Aが、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)であるか、または、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)および置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)から選ばれる1~3個(好ましくは、1または2個)の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、チオフェン環、ピリジン環、イミダゾリン環またはテトラヒドロピラジン環であり、環A2がピロール環、チオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、ハロゲン原子(例、フッ素原子、塩素原子)、シアノ基、置換されていてもよいC1-6アルキル基(例、メチル)および置換されていてもよいC1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、あるいはそれぞれさらに置換されていてもよいチオフェン環またはピリジン環であり;
Lが、C1-2アルキレン(例、-CH2-、-CH2CH2-、-CH(CH3)-)であり;
X1が、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり;
X2が、NまたはCR2(R2は、水素原子またはフッ素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子またはフッ素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子またはメチルである。)であり;
X5が、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、独立して、水素原子または置換されていてもよいC1-6アルキル基(例、メチル)であるか、または、R6aおよびR6bが一緒になって、置換されていてもよいC3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり;
R7が、水素原子またはC1-6アルキル基(例、メチル)である;
化合物(I)。 [Compound I-2]
Whether ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group Or a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group Further substituted with 1 to 3 (preferably 1 or 2) substituents selected from (eg, methoxy) and optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 is a pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the above formula (A) which is a ring, a thiophene ring, a pyridine ring or a pyrimidine ring;
Ring B is a halogen atom (eg, fluorine atom, chlorine atom), a cyano group, an optionally substituted C 1-6 alkyl group (eg, methyl) and an optionally substituted C 1-6 alkoxy group ( A benzene ring which may be further substituted with 1 to 5 (preferably 1 to 3, more preferably 1 or 2) substituents selected from eg methoxy), or each may be further substituted A thiophene ring or a pyridine ring;
L is C 1-2 alkylene (eg, —CH 2 —, —CH 2 CH 2 —, —CH (CH 3 ) —);
X 1 is N or CR 1 (R 1 is a hydrogen atom or a fluorine atom);
X 2 is N or CR 2 (R 2 is a hydrogen atom or a fluorine atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom or a fluorine atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom or methyl);
X 5 is CR 5b R 5c (R 5b and R 5c are hydrogen atoms) or a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom or an optionally substituted C 1-6 alkyl group (eg, methyl), or R 6a and R 6b 6b together form an optionally substituted C 3-10 cycloalkane ring (eg, cyclopentane ring));
R 7 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl);
Compound (I).
[化合物I-3]
環Aが、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)であるか、または、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)および置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)から選ばれる1~3個(好ましくは、1または2個)の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、チオフェン環、ピリジン環、イミダゾリン環またはテトラヒドロピラジン環であり、環A2がピロール環、チオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、
(1)(i)ハロゲン原子(例、フッ素原子、塩素原子)、(ii)シアノ基、(iii)ハロゲン原子(例、フッ素原子)およびアミノ基から選ばれる1~3個の置換基で置換されていてもよいC1-6アルキル基(例、メチル)および(iv)C1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-、-CH2CH2-または-CH(CH3)-であり;
X1が、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり;
X2が、NまたはCR2(R2は、水素原子またはフッ素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子またはフッ素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子またはメチルである。)であり;
X5が、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、水素原子、あるいは独立して、ヒドロキシ基またはC6-14アリール基(例、フェニル)で置換されていてもよいC1-6アルキル基(例、メチル)であるか、または、R6aおよびR6bが一緒になって、C3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり;
R7が、水素原子またはC1-6アルキル基(例、メチル)である;
化合物(I)。 [Compound I-3]
Whether ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group Or a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group Further substituted with 1 to 3 (preferably 1 or 2) substituents selected from (eg, methoxy) and optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 is a pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the above formula (A) which is a ring, a thiophene ring, a pyridine ring or a pyrimidine ring;
Ring B is
(1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms) and amino groups 1 to 5 (preferably 1 to 3, more preferably 1) selected from a C 1-6 alkyl group (eg, methyl) and (iv) a C 1-6 alkoxy group (eg, methoxy) Or a benzene ring optionally further substituted with 2) substituents,
(2) a thiophene ring, or (3) a pyridine ring;
L is, -CH 2 -, - CH 2 CH 2 - or -CH (CH 3) - and is,
X 1 is N or CR 1 (R 1 is a hydrogen atom or a fluorine atom);
X 2 is N or CR 2 (R 2 is a hydrogen atom or a fluorine atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom or a fluorine atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom or methyl);
X 5 is CR 5b R 5c (R 5b and R 5c are hydrogen atoms) or a bond;
X 6 represents CR 6a R 6b (R 6a and R 6b are each a hydrogen atom, or independently, a C 1-6 alkyl optionally substituted with a hydroxy group or a C 6-14 aryl group (eg, phenyl)) A group (eg, methyl) or R 6a and R 6b together form a C 3-10 cycloalkane ring (eg, a cyclopentane ring));
R 7 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl);
Compound (I).
環Aが、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環(例、イミダゾリジン環、イミダゾリン環)であるか、または、ハロゲン原子(例、フッ素原子)、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基(例、メチル、エチル)、置換されていてもよいC1-6アルコキシ基(例、メトキシ)および置換されていてもよい非芳香族複素環-オキシ基(例、テトラヒドロピラニルオキシ)から選ばれる1~3個(好ましくは、1または2個)の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、チオフェン環、ピリジン環、イミダゾリン環またはテトラヒドロピラジン環であり、環A2がピロール環、チオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、
(1)(i)ハロゲン原子(例、フッ素原子、塩素原子)、(ii)シアノ基、(iii)ハロゲン原子(例、フッ素原子)およびアミノ基から選ばれる1~3個の置換基で置換されていてもよいC1-6アルキル基(例、メチル)および(iv)C1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-、-CH2CH2-または-CH(CH3)-であり;
X1が、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり;
X2が、NまたはCR2(R2は、水素原子またはフッ素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子またはフッ素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子またはメチルである。)であり;
X5が、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、水素原子、あるいは独立して、ヒドロキシ基またはC6-14アリール基(例、フェニル)で置換されていてもよいC1-6アルキル基(例、メチル)であるか、または、R6aおよびR6bが一緒になって、C3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり;
R7が、水素原子またはC1-6アルキル基(例、メチル)である;
化合物(I)。 [Compound I-3]
Whether ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring (eg, imidazolidine ring, imidazoline ring) which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group Or a halogen atom (eg, fluorine atom), a hydroxy group, an oxo group, an optionally substituted C 1-6 alkyl group (eg, methyl, ethyl), an optionally substituted C 1-6 alkoxy group Further substituted with 1 to 3 (preferably 1 or 2) substituents selected from (eg, methoxy) and optionally substituted non-aromatic heterocyclic-oxy group (eg, tetrahydropyranyloxy) Ring A 1 is a pyrrole ring, pyrazole ring, imidazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the above formula (A) which is a ring, a thiophene ring, a pyridine ring or a pyrimidine ring;
Ring B is
(1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms) and amino groups 1 to 5 (preferably 1 to 3, more preferably 1) selected from a C 1-6 alkyl group (eg, methyl) and (iv) a C 1-6 alkoxy group (eg, methoxy) Or a benzene ring optionally further substituted with 2) substituents,
(2) a thiophene ring, or (3) a pyridine ring;
L is, -CH 2 -, - CH 2 CH 2 - or -CH (CH 3) - and is,
X 1 is N or CR 1 (R 1 is a hydrogen atom or a fluorine atom);
X 2 is N or CR 2 (R 2 is a hydrogen atom or a fluorine atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom or a fluorine atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom or methyl);
X 5 is CR 5b R 5c (R 5b and R 5c are hydrogen atoms) or a bond;
X 6 represents CR 6a R 6b (R 6a and R 6b are each a hydrogen atom, or independently, a C 1-6 alkyl optionally substituted with a hydroxy group or a C 6-14 aryl group (eg, phenyl)) A group (eg, methyl) or R 6a and R 6b together form a C 3-10 cycloalkane ring (eg, a cyclopentane ring));
R 7 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl);
Compound (I).
[化合物I-4]
環Aが、ヒドロキシでさらに置換された1H-イミダゾ[4,5-b]ピリジン環またはメチルでさらに置換された1H-ピロロ[2,3-b]ピリジン環であり;
環Bが、
(1)(i)ハロゲン原子(例、フッ素原子、塩素原子)、(ii)シアノ基、(iii)ハロゲン原子(例、フッ素原子)およびアミノ基から選ばれる1~3個の置換基で置換されていてもよいC1-6アルキル基(例、メチル)および(iv)C1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-であり;
X1が、CR1(R1は、水素原子である。)であり;
X2が、CR2(R2は、水素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子である。)であり;
X5が、結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、独立して、水素原子、あるいはヒドロキシ基またはC6-14アリール基(例、フェニル)で置換されていてもよいC1-6アルキル基(例、メチル)であるか、または、R6aおよびR6bが一緒になって、C3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり;
R7が、水素原子である;
化合物(I)。 [Compound I-4]
Ring A is a 1H-imidazo [4,5-b] pyridine ring further substituted with hydroxy or a 1H-pyrrolo [2,3-b] pyridine ring further substituted with methyl;
Ring B is
(1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms) and amino groups 1 to 5 (preferably 1 to 3, more preferably 1) selected from a C 1-6 alkyl group (eg, methyl) and (iv) a C 1-6 alkoxy group (eg, methoxy) Or a benzene ring optionally further substituted with 2) substituents,
(2) a thiophene ring, or (3) a pyridine ring;
L is —CH 2 —;
X 1 is CR 1 (R 1 is a hydrogen atom);
X 2 is CR 2 (R 2 is a hydrogen atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom);
X 5 is a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom, or a C 1-6 alkyl optionally substituted with a hydroxy group or a C 6-14 aryl group (eg, phenyl)) A group (eg, methyl) or R 6a and R 6b together form a C 3-10 cycloalkane ring (eg, a cyclopentane ring));
R 7 is a hydrogen atom;
Compound (I).
環Aが、ヒドロキシでさらに置換された1H-イミダゾ[4,5-b]ピリジン環またはメチルでさらに置換された1H-ピロロ[2,3-b]ピリジン環であり;
環Bが、
(1)(i)ハロゲン原子(例、フッ素原子、塩素原子)、(ii)シアノ基、(iii)ハロゲン原子(例、フッ素原子)およびアミノ基から選ばれる1~3個の置換基で置換されていてもよいC1-6アルキル基(例、メチル)および(iv)C1-6アルコキシ基(例、メトキシ)から選ばれる1~5個(好ましくは1~3個、より好ましくは1または2個)の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-であり;
X1が、CR1(R1は、水素原子である。)であり;
X2が、CR2(R2は、水素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子である。)であり;
X5が、結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、独立して、水素原子、あるいはヒドロキシ基またはC6-14アリール基(例、フェニル)で置換されていてもよいC1-6アルキル基(例、メチル)であるか、または、R6aおよびR6bが一緒になって、C3-10シクロアルカン環(例、シクロペンタン環)を形成する。)であり;
R7が、水素原子である;
化合物(I)。 [Compound I-4]
Ring A is a 1H-imidazo [4,5-b] pyridine ring further substituted with hydroxy or a 1H-pyrrolo [2,3-b] pyridine ring further substituted with methyl;
Ring B is
(1) (i) substituted with 1 to 3 substituents selected from halogen atoms (eg, fluorine atoms, chlorine atoms), (ii) cyano groups, (iii) halogen atoms (eg, fluorine atoms) and amino groups 1 to 5 (preferably 1 to 3, more preferably 1) selected from a C 1-6 alkyl group (eg, methyl) and (iv) a C 1-6 alkoxy group (eg, methoxy) Or a benzene ring optionally further substituted with 2) substituents,
(2) a thiophene ring, or (3) a pyridine ring;
L is —CH 2 —;
X 1 is CR 1 (R 1 is a hydrogen atom);
X 2 is CR 2 (R 2 is a hydrogen atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom);
X 5 is a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are each independently a hydrogen atom, or a C 1-6 alkyl optionally substituted with a hydroxy group or a C 6-14 aryl group (eg, phenyl)) A group (eg, methyl) or R 6a and R 6b together form a C 3-10 cycloalkane ring (eg, a cyclopentane ring));
R 7 is a hydrogen atom;
Compound (I).
[化合物I-5]
環Aが、ヒドロキシ基、オキソ基、C1-6アルキル基(例、メチル)およびC1-6アルコキシ基(例、メトキシ)から選ばれる1~3個(好ましくは、1または2個)の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、チオフェン環、ピリジン環、イミダゾリン環またはテトラヒドロピラジン環であり、環A2がピロール環、チオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、
(1)1~3個のハロゲン原子(例、フッ素原子)で置換されていてもよいC1-6アルキル基(例、メチル)でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-、-CH2CH2-または-CH(CH3)-であり;
X1が、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり;
X2が、NまたはCR2(R2は、水素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子またはメチルである。)であり;
X5が、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、水素原子、あるいは独立してC1-6アルキル基(例、メチル)である。)であり;
R7が、水素原子またはC1-6アルキル基(例、メチル)である;
化合物(I)。 [Compound I-5]
1 to 3 (preferably 1 or 2) of ring A selected from a hydroxy group, an oxo group, a C 1-6 alkyl group (eg, methyl) and a C 1-6 alkoxy group (eg, methoxy) Ring A 1 which may be further substituted with a substituent is pyrrole ring, pyrazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole ring, thiophene ring, pyridine ring or pyrimidine A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the above formula (A) which is a ring;
Ring B is
(1) a benzene ring which may be further substituted with a C 1-6 alkyl group (eg, methyl) optionally substituted with 1 to 3 halogen atoms (eg, fluorine atom),
(2) a thiophene ring, or (3) a pyridine ring;
L is, -CH 2 -, - CH 2 CH 2 - or -CH (CH 3) - and is,
X 1 is N or CR 1 (R 1 is a hydrogen atom or a fluorine atom);
X 2 is N or CR 2 (R 2 is a hydrogen atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom or methyl);
X 5 is CR 5b R 5c (R 5b and R 5c are hydrogen atoms) or a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are a hydrogen atom or independently a C 1-6 alkyl group (eg, methyl));
R 7 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl);
Compound (I).
環Aが、ヒドロキシ基、オキソ基、C1-6アルキル基(例、メチル)およびC1-6アルコキシ基(例、メトキシ)から選ばれる1~3個(好ましくは、1または2個)の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、チオフェン環、ピリジン環、イミダゾリン環またはテトラヒドロピラジン環であり、環A2がピロール環、チオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、
(1)1~3個のハロゲン原子(例、フッ素原子)で置換されていてもよいC1-6アルキル基(例、メチル)でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-、-CH2CH2-または-CH(CH3)-であり;
X1が、NまたはCR1(R1は、水素原子またはフッ素原子である。)であり;
X2が、NまたはCR2(R2は、水素原子である。)であり;
X3が、NまたはCR3(R3は、水素原子である。)であり;
X4が、NまたはCR4(R4は、水素原子またはメチルである。)であり;
X5が、CR5bR5c(R5bおよびR5cは、水素原子である。)または結合手であり;
X6が、CR6aR6b(R6aおよびR6bは、水素原子、あるいは独立してC1-6アルキル基(例、メチル)である。)であり;
R7が、水素原子またはC1-6アルキル基(例、メチル)である;
化合物(I)。 [Compound I-5]
1 to 3 (preferably 1 or 2) of ring A selected from a hydroxy group, an oxo group, a C 1-6 alkyl group (eg, methyl) and a C 1-6 alkoxy group (eg, methoxy) Ring A 1 which may be further substituted with a substituent is pyrrole ring, pyrazole ring, thiophene ring, pyridine ring, imidazoline ring or tetrahydropyrazine ring, and ring A 2 is pyrrole ring, thiophene ring, pyridine ring or pyrimidine A bicyclic nitrogen-containing unsaturated heterocyclic ring represented by the above formula (A) which is a ring;
Ring B is
(1) a benzene ring which may be further substituted with a C 1-6 alkyl group (eg, methyl) optionally substituted with 1 to 3 halogen atoms (eg, fluorine atom),
(2) a thiophene ring, or (3) a pyridine ring;
L is, -CH 2 -, - CH 2 CH 2 - or -CH (CH 3) - and is,
X 1 is N or CR 1 (R 1 is a hydrogen atom or a fluorine atom);
X 2 is N or CR 2 (R 2 is a hydrogen atom);
X 3 is N or CR 3 (R 3 is a hydrogen atom);
X 4 is N or CR 4 (R 4 is a hydrogen atom or methyl);
X 5 is CR 5b R 5c (R 5b and R 5c are hydrogen atoms) or a bond;
X 6 is CR 6a R 6b (R 6a and R 6b are a hydrogen atom or independently a C 1-6 alkyl group (eg, methyl));
R 7 is a hydrogen atom or a C 1-6 alkyl group (eg, methyl);
Compound (I).
化合物(I)の具体例としては、例えば、実施例1~93の化合物が挙げられ、中でも、
2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オンまたはその塩(実施例14);
4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オンまたはその塩(実施例48);および
1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オンまたはその塩(実施例88)
が好ましい。 Specific examples of compound (I) include, for example, the compounds of Examples 1 to 93. Among them,
2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2 , 3-Dihydroquinazolin-4 (1H) -one or a salt thereof (Example 14);
4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one or a salt thereof Example 48; and 1-benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro -5H-pyrido [2,3-e] [1,4] diazepin-5-one or a salt thereof (Example 88)
Is preferred.
2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オンまたはその塩(実施例14);
4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オンまたはその塩(実施例48);および
1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オンまたはその塩(実施例88)
が好ましい。 Specific examples of compound (I) include, for example, the compounds of Examples 1 to 93. Among them,
2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2 , 3-Dihydroquinazolin-4 (1H) -one or a salt thereof (Example 14);
4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one or a salt thereof Example 48; and 1-benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro -5H-pyrido [2,3-e] [1,4] diazepin-5-one or a salt thereof (Example 88)
Is preferred.
化合物(I)が塩である場合、そのような塩としては、例えば、金属塩、アンモニウム塩、有機塩基との塩、無機酸との塩、有機酸との塩、塩基性または酸性アミノ酸との塩等が挙げられる。金属塩の好適な例としては、例えば、ナトリウム塩、カリウム塩等のアルカリ金属塩;カルシウム塩、マグネシウム塩、バリウム塩等のアルカリ土類金属塩;アルミニウム塩等が挙げられる。有機塩基との塩の好適な例としては、例えば、トリメチルアミン、トリエチルアミン、ピリジン、ピコリン、2,6-ルチジン、エタノールアミン、ジエタノールアミン、トリエタノールアミン、シクロヘキシルアミン、ジシクロヘキシルアミン、N,N'-ジベンジルエチレンジアミン等との塩が挙げられる。無機酸との塩の好適な例としては、例えば、塩酸、臭化水素酸、硝酸、硫酸、リン酸等との塩が挙げられる。有機酸との塩の好適な例としては、例えば、ギ酸、酢酸、トリフルオロ酢酸、フタル酸、フマル酸、シュウ酸、酒石酸、マレイン酸、クエン酸、コハク酸、リンゴ酸、メタンスルホン酸、ベンゼンスルホン酸、p-トルエンスルホン酸等との塩が挙げられる。塩基性アミノ酸との塩の好適な例としては、例えば、アルギニン、リジン、オルニチン等との塩が挙げられ、酸性アミノ酸との塩の好適な例としては、例えば、アスパラギン酸、グルタミン酸等との塩が挙げられる。
このうち、薬学的に許容し得る塩が好ましい。例えば、化合物内に酸性官能基を有する場合には、アルカリ金属塩(例、ナトリウム塩、カリウム塩等)、アルカリ土類金属塩(例、カルシウム塩、マグネシウム塩等)等の無機塩、アンモニウム塩等、また、化合物内に塩基性官能基を有する場合には、例えば、塩酸、臭化水素酸、硝酸、硫酸、リン酸等の無機酸との塩、または酢酸、フタル酸、フマル酸、シュウ酸、酒石酸、マレイン酸、クエン酸、コハク酸、メタンスルホン酸、ベンゼンスルホン酸、p-トルエンスルホン酸等の有機酸との塩が挙げられる。 When compound (I) is a salt, examples of such salts include metal salts, ammonium salts, salts with organic bases, salts with inorganic acids, salts with organic acids, basic or acidic amino acids, and the like. Examples include salts. Preferable examples of the metal salt include alkali metal salts such as sodium salt and potassium salt; alkaline earth metal salts such as calcium salt, magnesium salt and barium salt; aluminum salt and the like. Preferable examples of the salt with organic base include, for example, trimethylamine, triethylamine, pyridine, picoline, 2,6-lutidine, ethanolamine, diethanolamine, triethanolamine, cyclohexylamine, dicyclohexylamine, N, N′-dibenzyl. Examples include salts with ethylenediamine and the like. Preferable examples of the salt with inorganic acid include salts with hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid and the like. Preferable examples of the salt with organic acid include, for example, formic acid, acetic acid, trifluoroacetic acid, phthalic acid, fumaric acid, oxalic acid, tartaric acid, maleic acid, citric acid, succinic acid, malic acid, methanesulfonic acid, benzene And salts with sulfonic acid, p-toluenesulfonic acid and the like. Preferable examples of salts with basic amino acids include salts with arginine, lysine, ornithine and the like, and preferable examples of salts with acidic amino acids include salts with aspartic acid, glutamic acid and the like. Is mentioned.
Of these, pharmaceutically acceptable salts are preferred. For example, when the compound has an acidic functional group, inorganic salts such as alkali metal salts (eg, sodium salts, potassium salts, etc.), alkaline earth metal salts (eg, calcium salts, magnesium salts, etc.), ammonium salts In addition, when the compound has a basic functional group, for example, a salt with an inorganic acid such as hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid, or acetic acid, phthalic acid, fumaric acid, And salts with organic acids such as acid, tartaric acid, maleic acid, citric acid, succinic acid, methanesulfonic acid, benzenesulfonic acid, and p-toluenesulfonic acid.
このうち、薬学的に許容し得る塩が好ましい。例えば、化合物内に酸性官能基を有する場合には、アルカリ金属塩(例、ナトリウム塩、カリウム塩等)、アルカリ土類金属塩(例、カルシウム塩、マグネシウム塩等)等の無機塩、アンモニウム塩等、また、化合物内に塩基性官能基を有する場合には、例えば、塩酸、臭化水素酸、硝酸、硫酸、リン酸等の無機酸との塩、または酢酸、フタル酸、フマル酸、シュウ酸、酒石酸、マレイン酸、クエン酸、コハク酸、メタンスルホン酸、ベンゼンスルホン酸、p-トルエンスルホン酸等の有機酸との塩が挙げられる。 When compound (I) is a salt, examples of such salts include metal salts, ammonium salts, salts with organic bases, salts with inorganic acids, salts with organic acids, basic or acidic amino acids, and the like. Examples include salts. Preferable examples of the metal salt include alkali metal salts such as sodium salt and potassium salt; alkaline earth metal salts such as calcium salt, magnesium salt and barium salt; aluminum salt and the like. Preferable examples of the salt with organic base include, for example, trimethylamine, triethylamine, pyridine, picoline, 2,6-lutidine, ethanolamine, diethanolamine, triethanolamine, cyclohexylamine, dicyclohexylamine, N, N′-dibenzyl. Examples include salts with ethylenediamine and the like. Preferable examples of the salt with inorganic acid include salts with hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid and the like. Preferable examples of the salt with organic acid include, for example, formic acid, acetic acid, trifluoroacetic acid, phthalic acid, fumaric acid, oxalic acid, tartaric acid, maleic acid, citric acid, succinic acid, malic acid, methanesulfonic acid, benzene And salts with sulfonic acid, p-toluenesulfonic acid and the like. Preferable examples of salts with basic amino acids include salts with arginine, lysine, ornithine and the like, and preferable examples of salts with acidic amino acids include salts with aspartic acid, glutamic acid and the like. Is mentioned.
Of these, pharmaceutically acceptable salts are preferred. For example, when the compound has an acidic functional group, inorganic salts such as alkali metal salts (eg, sodium salts, potassium salts, etc.), alkaline earth metal salts (eg, calcium salts, magnesium salts, etc.), ammonium salts In addition, when the compound has a basic functional group, for example, a salt with an inorganic acid such as hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid, or acetic acid, phthalic acid, fumaric acid, And salts with organic acids such as acid, tartaric acid, maleic acid, citric acid, succinic acid, methanesulfonic acid, benzenesulfonic acid, and p-toluenesulfonic acid.
本発明化合物の製造法について以下に説明する。
The production method of the compound of the present invention will be described below.
以下の製造方法における各工程で用いられた原料や試薬、ならびに得られた化合物は、それぞれ塩を形成していてもよい。このような塩としては、例えば、前述の本発明化合物の塩と同様のもの等が挙げられる。
The raw materials and reagents used in each step in the following production method and the obtained compound may each form a salt. Examples of such salts include those similar to the salts of the aforementioned compound of the present invention.
各工程で得られた化合物が遊離化合物である場合には、自体公知の方法により、目的とする塩に変換することができる。逆に各工程で得られた化合物が塩である場合には、自体公知の方法により、遊離体または目的とする他の種類の塩に変換することができる。
When the compound obtained in each step is a free compound, it can be converted into a target salt by a method known per se. On the contrary, when the compound obtained in each step is a salt, it can be converted into a free form or other types of desired salts by a method known per se.
各工程で得られた化合物は反応液のままか、または粗生成物として得た後に、次反応に用いることもできる、あるいは、各工程で得られた化合物を、常法に従って、反応混合物から濃縮、晶出、再結晶、蒸留、溶媒抽出、分溜、クロマトグラフィーなどの分離手段により単離および/または精製することができる。
The compound obtained in each step remains in the reaction solution or is obtained as a crude product and can be used in the next reaction. Alternatively, the compound obtained in each step is concentrated from the reaction mixture according to a conventional method. , Crystallization, recrystallization, distillation, solvent extraction, fractional distillation, chromatography and the like, and can be isolated and / or purified.
各工程の原料や試薬の化合物が市販されている場合には、市販品をそのまま用いることができる。
When the raw materials and reagent compounds for each step are commercially available, commercially available products can be used as they are.
各工程の反応において、反応時間は、用いる試薬や溶媒により異なり得るが、特に記載の無い場合、通常1分~48時間、好ましくは10分~8時間である。
In the reaction in each step, the reaction time may vary depending on the reagent and solvent to be used, but unless otherwise specified, is usually 1 minute to 48 hours, preferably 10 minutes to 8 hours.
各工程の反応において、反応温度は、用いる試薬や溶媒により異なり得るが、特に記載が無い場合、通常-78℃~300℃、好ましくは-78℃~150℃である。
In the reaction in each step, the reaction temperature may vary depending on the reagent and solvent to be used, but is usually −78 ° C. to 300 ° C., preferably −78 ° C. to 150 ° C., unless otherwise specified.
各工程の反応において、圧力は、用いる試薬や溶媒により異なり得るが、特に記載が無い場合、通常1気圧~20気圧、好ましくは1気圧~3気圧である。
In the reaction in each step, the pressure may vary depending on the reagent and solvent used, but unless otherwise specified, is usually 1 to 20 atmospheres, preferably 1 to 3 atmospheres.
各工程の反応において、例えば、Biotage社製InitiatorなどのMicrowave合成装置を用いることがある。反応温度は、用いる試薬や溶媒により異なり得るが、特に記載がない場合、通常室温~300℃、好ましくは50℃~250℃である。反応時間は、用いる試薬や溶媒により異なり得るが、特に記載の無い場合、通常1分~48時間、好ましくは1分~8時間である。
In the reaction of each step, for example, a Microwave synthesizer such as an initiator manufactured by Biotage may be used. The reaction temperature may vary depending on the reagent and solvent to be used, but unless otherwise specified, is usually room temperature to 300 ° C., preferably 50 ° C. to 250 ° C. The reaction time may vary depending on the reagent and solvent to be used, but unless otherwise specified, is usually 1 minute to 48 hours, preferably 1 minute to 8 hours.
各工程の反応において、試薬は、特に記載が無い場合、基質に対して0.5当量~20当量、好ましくは0.8当量~5当量が用いられる。試薬を触媒として使用する場合、試薬は基質に対して0.001当量~1当量、好ましくは0.01当量~0.2当量が用いられる。試薬が反応溶媒を兼ねる場合、試薬は溶媒量が用いられる。
In the reaction in each step, unless otherwise specified, the reagent is used in an amount of 0.5 equivalent to 20 equivalents, preferably 0.8 equivalent to 5 equivalents, relative to the substrate. When a reagent is used as a catalyst, the reagent is used in an amount of 0.001 equivalent to 1 equivalent, preferably 0.01 equivalent to 0.2 equivalent, relative to the substrate. When the reagent also serves as a reaction solvent, the amount of solvent is used as the reagent.
各工程の反応において、特に記載が無い場合、これらの反応は、無溶媒、あるいは適当な溶媒に溶解または懸濁して行われる。溶媒の具体例としては、実施例に記載されている溶媒、あるいは以下が挙げられる。
アルコール類:メタノール、エタノール、tert-ブチルアルコール、2-メトキシエタノールなど;
エーテル類:ジエチルエーテル、ジフェニルエーテル、テトラヒドロフラン、1,2-ジメトキシエタンなど;
芳香族炭化水素類:クロロベンゼン、トルエン、キシレンなど;
飽和炭化水素類:シクロヘキサン、ヘキサンなど;
アミド類:N,N-ジメチルホルムアミド、N-メチルピロリドンなど;
ハロゲン化炭化水素類:ジクロロメタン、四塩化炭素など;
ニトリル類:アセトニトリルなど;
スルホキシド類:ジメチルスルホキシドなど;
芳香族有機塩基類:ピリジンなど;
酸無水物類:無水酢酸など;
有機酸類:ギ酸、酢酸、トリフルオロ酢酸など;
無機酸類:塩酸、硫酸など;
エステル類:酢酸エチルなど;
ケトン類:アセトン、メチルエチルケトンなど;
水。
上記溶媒は、二種以上を適宜の割合で混合して用いてもよい。 In the reaction in each step, unless otherwise specified, these reactions are performed without solvent or dissolved or suspended in a suitable solvent. Specific examples of the solvent include the solvents described in the examples or the following.
Alcohols: methanol, ethanol, tert-butyl alcohol, 2-methoxyethanol, etc .;
Ethers: diethyl ether, diphenyl ether, tetrahydrofuran, 1,2-dimethoxyethane, etc .;
Aromatic hydrocarbons: chlorobenzene, toluene, xylene, etc .;
Saturated hydrocarbons: cyclohexane, hexane, etc .;
Amides: N, N-dimethylformamide, N-methylpyrrolidone, etc .;
Halogenated hydrocarbons: dichloromethane, carbon tetrachloride, etc .;
Nitriles: acetonitrile, etc.
Sulfoxides: dimethyl sulfoxide and the like;
Aromatic organic bases: pyridine, etc .;
Acid anhydrides: acetic anhydride, etc .;
Organic acids: formic acid, acetic acid, trifluoroacetic acid, etc .;
Inorganic acids: hydrochloric acid, sulfuric acid, etc .;
Esters: ethyl acetate and the like;
Ketones: acetone, methyl ethyl ketone, etc .;
water.
Two or more of the above solvents may be mixed and used at an appropriate ratio.
アルコール類:メタノール、エタノール、tert-ブチルアルコール、2-メトキシエタノールなど;
エーテル類:ジエチルエーテル、ジフェニルエーテル、テトラヒドロフラン、1,2-ジメトキシエタンなど;
芳香族炭化水素類:クロロベンゼン、トルエン、キシレンなど;
飽和炭化水素類:シクロヘキサン、ヘキサンなど;
アミド類:N,N-ジメチルホルムアミド、N-メチルピロリドンなど;
ハロゲン化炭化水素類:ジクロロメタン、四塩化炭素など;
ニトリル類:アセトニトリルなど;
スルホキシド類:ジメチルスルホキシドなど;
芳香族有機塩基類:ピリジンなど;
酸無水物類:無水酢酸など;
有機酸類:ギ酸、酢酸、トリフルオロ酢酸など;
無機酸類:塩酸、硫酸など;
エステル類:酢酸エチルなど;
ケトン類:アセトン、メチルエチルケトンなど;
水。
上記溶媒は、二種以上を適宜の割合で混合して用いてもよい。 In the reaction in each step, unless otherwise specified, these reactions are performed without solvent or dissolved or suspended in a suitable solvent. Specific examples of the solvent include the solvents described in the examples or the following.
Alcohols: methanol, ethanol, tert-butyl alcohol, 2-methoxyethanol, etc .;
Ethers: diethyl ether, diphenyl ether, tetrahydrofuran, 1,2-dimethoxyethane, etc .;
Aromatic hydrocarbons: chlorobenzene, toluene, xylene, etc .;
Saturated hydrocarbons: cyclohexane, hexane, etc .;
Amides: N, N-dimethylformamide, N-methylpyrrolidone, etc .;
Halogenated hydrocarbons: dichloromethane, carbon tetrachloride, etc .;
Nitriles: acetonitrile, etc.
Sulfoxides: dimethyl sulfoxide and the like;
Aromatic organic bases: pyridine, etc .;
Acid anhydrides: acetic anhydride, etc .;
Organic acids: formic acid, acetic acid, trifluoroacetic acid, etc .;
Inorganic acids: hydrochloric acid, sulfuric acid, etc .;
Esters: ethyl acetate and the like;
Ketones: acetone, methyl ethyl ketone, etc .;
water.
Two or more of the above solvents may be mixed and used at an appropriate ratio.
各工程の反応において塩基を用いる場合、例えば、以下に示す塩基、あるいは実施例に記載されている塩基が用いられる。
無機塩基類:水酸化ナトリウム、水酸化マグネシウムなど;
塩基性塩類:炭酸ナトリウム、炭酸カルシウム、炭酸水素ナトリウムなど;
有機塩基類:トリエチルアミン、ジエチルアミン、ピリジン、4-ジメチルアミノピリジン、N,N-ジメチルアニリン、1,4-ジアザビシクロ[2.2.2]オクタン、1,8-ジアザビシクロ[5.4.0]-7-ウンデセン、イミダゾール、ピペリジンなど;
金属アルコキシド類:ナトリウムエトキシド、カリウムtert-ブトキシドなど;
アルカリ金属水素化物類:水素化ナトリウムなど;
金属アミド類:ナトリウムアミド、リチウムジイソプロピルアミド、リチウムヘキサメチルジシラジドなど;
有機リチウム類:n-ブチルリチウムなど。 When a base is used in the reaction in each step, for example, the following bases or the bases described in the examples are used.
Inorganic bases: sodium hydroxide, magnesium hydroxide, etc .;
Basic salts: sodium carbonate, calcium carbonate, sodium bicarbonate, etc .;
Organic bases: triethylamine, diethylamine, pyridine, 4-dimethylaminopyridine, N, N-dimethylaniline, 1,4-diazabicyclo [2.2.2] octane, 1,8-diazabicyclo [5.4.0]- 7-undecene, imidazole, piperidine and the like;
Metal alkoxides: sodium ethoxide, potassium tert-butoxide and the like;
Alkali metal hydrides: sodium hydride, etc .;
Metal amides: sodium amide, lithium diisopropylamide, lithium hexamethyldisilazide, etc .;
Organic lithiums: n-butyllithium and the like.
無機塩基類:水酸化ナトリウム、水酸化マグネシウムなど;
塩基性塩類:炭酸ナトリウム、炭酸カルシウム、炭酸水素ナトリウムなど;
有機塩基類:トリエチルアミン、ジエチルアミン、ピリジン、4-ジメチルアミノピリジン、N,N-ジメチルアニリン、1,4-ジアザビシクロ[2.2.2]オクタン、1,8-ジアザビシクロ[5.4.0]-7-ウンデセン、イミダゾール、ピペリジンなど;
金属アルコキシド類:ナトリウムエトキシド、カリウムtert-ブトキシドなど;
アルカリ金属水素化物類:水素化ナトリウムなど;
金属アミド類:ナトリウムアミド、リチウムジイソプロピルアミド、リチウムヘキサメチルジシラジドなど;
有機リチウム類:n-ブチルリチウムなど。 When a base is used in the reaction in each step, for example, the following bases or the bases described in the examples are used.
Inorganic bases: sodium hydroxide, magnesium hydroxide, etc .;
Basic salts: sodium carbonate, calcium carbonate, sodium bicarbonate, etc .;
Organic bases: triethylamine, diethylamine, pyridine, 4-dimethylaminopyridine, N, N-dimethylaniline, 1,4-diazabicyclo [2.2.2] octane, 1,8-diazabicyclo [5.4.0]- 7-undecene, imidazole, piperidine and the like;
Metal alkoxides: sodium ethoxide, potassium tert-butoxide and the like;
Alkali metal hydrides: sodium hydride, etc .;
Metal amides: sodium amide, lithium diisopropylamide, lithium hexamethyldisilazide, etc .;
Organic lithiums: n-butyllithium and the like.
各工程の反応において酸または酸性触媒を用いる場合、例えば、以下に示す酸や酸性触媒、あるいは実施例に記載されている酸や酸性触媒が用いられる。
無機酸類:塩酸、硫酸、硝酸、臭化水素酸、リン酸など;
有機酸類:酢酸、トリフルオロ酢酸、クエン酸、p-トルエンスルホン酸、10-カンファースルホン酸など;
ルイス酸:三フッ化ホウ素ジエチルエーテル錯体、ヨウ化亜鉛、無水塩化アルミニウム、無水塩化亜鉛、無水塩化鉄など。 When an acid or an acidic catalyst is used in the reaction in each step, for example, the following acids and acidic catalysts, or acids and acidic catalysts described in the examples are used.
Inorganic acids: hydrochloric acid, sulfuric acid, nitric acid, hydrobromic acid, phosphoric acid, etc .;
Organic acids: acetic acid, trifluoroacetic acid, citric acid, p-toluenesulfonic acid, 10-camphorsulfonic acid, etc .;
Lewis acid: boron trifluoride diethyl ether complex, zinc iodide, anhydrous aluminum chloride, anhydrous zinc chloride, anhydrous iron chloride and the like.
無機酸類:塩酸、硫酸、硝酸、臭化水素酸、リン酸など;
有機酸類:酢酸、トリフルオロ酢酸、クエン酸、p-トルエンスルホン酸、10-カンファースルホン酸など;
ルイス酸:三フッ化ホウ素ジエチルエーテル錯体、ヨウ化亜鉛、無水塩化アルミニウム、無水塩化亜鉛、無水塩化鉄など。 When an acid or an acidic catalyst is used in the reaction in each step, for example, the following acids and acidic catalysts, or acids and acidic catalysts described in the examples are used.
Inorganic acids: hydrochloric acid, sulfuric acid, nitric acid, hydrobromic acid, phosphoric acid, etc .;
Organic acids: acetic acid, trifluoroacetic acid, citric acid, p-toluenesulfonic acid, 10-camphorsulfonic acid, etc .;
Lewis acid: boron trifluoride diethyl ether complex, zinc iodide, anhydrous aluminum chloride, anhydrous zinc chloride, anhydrous iron chloride and the like.
各工程の反応は、特に記載の無い限り、自体公知の方法、例えば、第5版実験化学講座、13巻~19巻(日本化学会編);新実験化学講座、14巻~15巻(日本化学会編);精密有機化学 改定第2版(L. F. Tietze,Th. Eicher、南江堂);改訂 有機人名反応 そのしくみとポイント(東郷秀雄著、講談社);ORGANIC SYNTHESES Collective Volume I~VII(John Wiley & Sons Inc);Modern Organic Synthesis in the Laboratory A Collection of Standard Experimental Procedures(Jie Jack Li著、OXFORD UNIVERSITY出版);Comprehensive Heterocyclic Chemistry III、Vol.1~Vol.14(エルゼビア・ジャパン株式会社);人名反応に学ぶ有機合成戦略(富岡清監訳、化学同人発行);コンプリヘンシブ・オーガニック・トランスフォーメーションズ(VCH Publishers Inc.)1989年刊などに記載された方法、あるいは実施例に記載された方法に準じて行われる。
Unless otherwise specified, the reaction in each step is a method known per se, for example, the 5th edition Experimental Chemistry Course, Volumes 13 to 19 (Edited by The Chemical Society of Japan); New Experimental Chemistry Course, Volumes 14 to 15 (Japan) Chemistry Society); Fine Organic Chemistry Revised 2nd Edition (L.F.Tietze, Th.Eicher, Nankodo); Revised Organic Personal Name Reaction, its mechanism and points (by Hideo Togo, Kodansha); ORGANIC SYNTHES Collective Volume I-VII ( John Wiley & Sons Inc); Modern Organic Synthesis in the Laboratory A Collection of Standard Exploratory Procedures (Jie Jack Li, UF by JIE Jack Li) IVERSITY publication); Comprehensive Heterocyclic Chemistry III, Vol. 1 to Vol. 14 (Elsevier Japan Co., Ltd.); Organic synthesis strategy learned from name reaction (translated by Kiyoshi Tomioka, published by Doujin Chemical); Comprehensive Organic Transformations (VCH Publishers Inc.) published in 1989, etc., Or it carries out according to the method described in the Example.
各工程において、官能基の保護または脱保護反応は、自体公知の方法、例えば、Wiley-Interscience社2007年刊「Protective Groups in Organic Synthesis, 4th Ed.」(Theodora W. Greene, Peter G. M. Wuts著);Thieme社2004年刊「Protecting Groups 3rd Ed.」(P.J.Kocienski著)などに記載された方法、あるいは実施例に記載された方法に準じて行われる。
アルコールなどの水酸基やフェノール性水酸基の保護基としては、例えば、メトキシメチルエーテル、ベンジルエーテル、t-ブチルジメチルシリルエーテル、テトラヒドロピラニルエーテルなどのエーテル型保護基;酢酸エステルなどのカルボン酸エステル型保護基;メタンスルホン酸エステルなどのスルホン酸エステル型保護基;t-ブチルカルボネートなどの炭酸エステル型保護基などが挙げられる。
アルデヒドのカルボニル基の保護基としては、例えば、ジメチルアセタールなどのアセタール型保護基;環状1,3-ジオキサンなどの環状アセタール型保護基などが挙げられる。
ケトンのカルボニル基の保護基としては、例えば、ジメチルケタールなどのケタール型保護基;環状1,3-ジオキサンなどの環状ケタール型保護基;O-メチルオキシムなどのオキシム型保護基;N,N-ジメチルヒドラゾンなどのヒドラゾン型保護基などが挙げられる。
カルボキシル基の保護基としては、例えば、メチルエステルなどのエステル型保護基;N,N-ジメチルアミドなどのアミド型保護基などが挙げられる。
チオールの保護基としては、例えば、ベンジルチオエーテルなどのエーテル型保護基;チオ酢酸エステル、チオカルボネート、チオカルバメートなどのエステル型保護基などが挙げられる。
アミノ基や、イミダゾール、ピロール、インドールなどの芳香族ヘテロ環の保護基としては、例えば、ベンジルカルバメートなどのカルバメート型保護基;アセトアミドなどのアミド型保護基;N-トリフェニルメチルアミンなどのアルキルアミン型保護基、メタンスルホンアミドなどのスルホンアミド型保護基などが挙げられる。
保護基の除去は、自体公知の方法、例えば、酸、塩基、紫外光、ヒドラジン、フェニルヒドラジン、N-メチルジチオカルバミン酸ナトリウム、テトラブチルアンモニウムフルオリド、酢酸パラジウム、トリアルキルシリルハライド(例えば、トリメチルシリルヨージド、トリメチルシリルブロミド)を使用する方法や還元法などを用いて行うことができる。 In each step, the protection or deprotection reaction of the functional group is carried out according to a method known per se, for example, “Protective Groups in Organic Synthesis, 4th Ed.” (Theodora W. Greene, Peter G. M., et al., Wiley-Interscience). The method described in Thimeme's 2004 “Protecting Groups 3rd Ed.” (By PJ Kocienski) or the like, or the method described in the examples.
Examples of protecting groups for hydroxyl groups such as alcohol and phenolic hydroxyl groups include ether-type protecting groups such as methoxymethyl ether, benzyl ether, t-butyldimethylsilyl ether, and tetrahydropyranyl ether; carboxylate-type protecting groups such as acetate Sulfonic acid ester type protecting groups such as methanesulfonic acid ester; carbonate ester type protecting groups such as t-butyl carbonate.
Examples of the protecting group for the carbonyl group of the aldehyde include an acetal-type protecting group such as dimethylacetal; and a cyclic acetal-type protecting group such as cyclic 1,3-dioxane.
Examples of the protecting group for the carbonyl group of the ketone include a ketal-type protecting group such as dimethyl ketal; a cyclic ketal-type protecting group such as cyclic 1,3-dioxane; an oxime-type protecting group such as O-methyloxime; N, N— And hydrazone-type protecting groups such as dimethylhydrazone.
Examples of the protecting group for carboxyl group include ester-type protecting groups such as methyl ester; amide-type protecting groups such as N, N-dimethylamide.
Examples of the thiol-protecting group include ether-type protecting groups such as benzylthioether; ester-type protecting groups such as thioacetate ester, thiocarbonate, and thiocarbamate.
Examples of protecting groups for amino groups and aromatic heterocycles such as imidazole, pyrrole and indole include carbamate-type protecting groups such as benzylcarbamate; amide-type protecting groups such as acetamide; alkylamines such as N-triphenylmethylamine Type protecting groups, and sulfonamide type protecting groups such as methanesulfonamide.
The protecting group can be removed by a method known per se, for example, acid, base, ultraviolet light, hydrazine, phenylhydrazine, sodium N-methyldithiocarbamate, tetrabutylammonium fluoride, palladium acetate, trialkylsilyl halide (for example, trimethylsilyl iodide). And trimethylsilyl bromide) or a reduction method.
アルコールなどの水酸基やフェノール性水酸基の保護基としては、例えば、メトキシメチルエーテル、ベンジルエーテル、t-ブチルジメチルシリルエーテル、テトラヒドロピラニルエーテルなどのエーテル型保護基;酢酸エステルなどのカルボン酸エステル型保護基;メタンスルホン酸エステルなどのスルホン酸エステル型保護基;t-ブチルカルボネートなどの炭酸エステル型保護基などが挙げられる。
アルデヒドのカルボニル基の保護基としては、例えば、ジメチルアセタールなどのアセタール型保護基;環状1,3-ジオキサンなどの環状アセタール型保護基などが挙げられる。
ケトンのカルボニル基の保護基としては、例えば、ジメチルケタールなどのケタール型保護基;環状1,3-ジオキサンなどの環状ケタール型保護基;O-メチルオキシムなどのオキシム型保護基;N,N-ジメチルヒドラゾンなどのヒドラゾン型保護基などが挙げられる。
カルボキシル基の保護基としては、例えば、メチルエステルなどのエステル型保護基;N,N-ジメチルアミドなどのアミド型保護基などが挙げられる。
チオールの保護基としては、例えば、ベンジルチオエーテルなどのエーテル型保護基;チオ酢酸エステル、チオカルボネート、チオカルバメートなどのエステル型保護基などが挙げられる。
アミノ基や、イミダゾール、ピロール、インドールなどの芳香族ヘテロ環の保護基としては、例えば、ベンジルカルバメートなどのカルバメート型保護基;アセトアミドなどのアミド型保護基;N-トリフェニルメチルアミンなどのアルキルアミン型保護基、メタンスルホンアミドなどのスルホンアミド型保護基などが挙げられる。
保護基の除去は、自体公知の方法、例えば、酸、塩基、紫外光、ヒドラジン、フェニルヒドラジン、N-メチルジチオカルバミン酸ナトリウム、テトラブチルアンモニウムフルオリド、酢酸パラジウム、トリアルキルシリルハライド(例えば、トリメチルシリルヨージド、トリメチルシリルブロミド)を使用する方法や還元法などを用いて行うことができる。 In each step, the protection or deprotection reaction of the functional group is carried out according to a method known per se, for example, “Protective Groups in Organic Synthesis, 4th Ed.” (Theodora W. Greene, Peter G. M., et al., Wiley-Interscience). The method described in Thimeme's 2004 “Protecting Groups 3rd Ed.” (By PJ Kocienski) or the like, or the method described in the examples.
Examples of protecting groups for hydroxyl groups such as alcohol and phenolic hydroxyl groups include ether-type protecting groups such as methoxymethyl ether, benzyl ether, t-butyldimethylsilyl ether, and tetrahydropyranyl ether; carboxylate-type protecting groups such as acetate Sulfonic acid ester type protecting groups such as methanesulfonic acid ester; carbonate ester type protecting groups such as t-butyl carbonate.
Examples of the protecting group for the carbonyl group of the aldehyde include an acetal-type protecting group such as dimethylacetal; and a cyclic acetal-type protecting group such as cyclic 1,3-dioxane.
Examples of the protecting group for the carbonyl group of the ketone include a ketal-type protecting group such as dimethyl ketal; a cyclic ketal-type protecting group such as cyclic 1,3-dioxane; an oxime-type protecting group such as O-methyloxime; N, N— And hydrazone-type protecting groups such as dimethylhydrazone.
Examples of the protecting group for carboxyl group include ester-type protecting groups such as methyl ester; amide-type protecting groups such as N, N-dimethylamide.
Examples of the thiol-protecting group include ether-type protecting groups such as benzylthioether; ester-type protecting groups such as thioacetate ester, thiocarbonate, and thiocarbamate.
Examples of protecting groups for amino groups and aromatic heterocycles such as imidazole, pyrrole and indole include carbamate-type protecting groups such as benzylcarbamate; amide-type protecting groups such as acetamide; alkylamines such as N-triphenylmethylamine Type protecting groups, and sulfonamide type protecting groups such as methanesulfonamide.
The protecting group can be removed by a method known per se, for example, acid, base, ultraviolet light, hydrazine, phenylhydrazine, sodium N-methyldithiocarbamate, tetrabutylammonium fluoride, palladium acetate, trialkylsilyl halide (for example, trimethylsilyl iodide). And trimethylsilyl bromide) or a reduction method.
各工程において、還元反応を行う場合、使用される還元剤としては、水素化アルミニウムリチウム、水素化トリアセトキシホウ素ナトリウム、水素化シアノホウ素ナトリウム、水素化ジイソブチルアルミニウム(DIBAL-H)、水素化ホウ素ナトリウム、水素化トリアセトキシホウ素テトラメチルアンモニウムなどの金属水素化物類;ボランテトラヒドロフラン錯体などのボラン類;ラネーニッケル;ラネーコバルト;水素;ギ酸などが挙げられる。炭素-炭素二重結合あるいは三重結合を還元する場合は、パラジウム-カーボンやLindlar触媒などの触媒を用いる方法がある。
When performing the reduction reaction in each step, the reducing agent used is lithium aluminum hydride, sodium triacetoxyborohydride, sodium cyanoborohydride, diisobutylaluminum hydride (DIBAL-H), sodium borohydride Metal hydrides such as hydrogenated triacetoxy boron tetramethylammonium; boranes such as borane tetrahydrofuran complex; Raney nickel; Raney cobalt; hydrogen; formic acid and the like. In the case of reducing a carbon-carbon double bond or triple bond, there are methods using a catalyst such as palladium-carbon or a Lindlar catalyst.
各工程において、酸化反応を行う場合、使用される酸化剤としては、m-クロロ過安息香酸(MCPBA)、過酸化水素、t-ブチルヒドロペルオキシドなどの過酸類;過塩素酸テトラブチルアンモニウムなどの過塩素酸塩類;塩素酸ナトリウムなどの塩素酸塩類;亜塩素酸ナトリウムなどの亜塩素酸塩類;過ヨウ素酸ナトリウムなどの過ヨウ素酸類;ヨードシルベンゼンなどの高原子価ヨウ素試薬;二酸化マンガン、過マンガン酸カリウムなどのマンガンを有する試薬;四酢酸鉛などの鉛類;クロロクロム酸ピリジニウム(PCC)、二クロム酸ピリジニウム(PDC)、ジョーンズ試薬などのクロムを有する試薬;N-ブロモスクシンイミド(NBS)などのハロゲン化合物類;酸素;オゾン;三酸化硫黄・ピリジン錯体;四酸化オスミウム;二酸化セレン;2,3-ジクロロ-5,6-ジシアノ-1,4-ベンゾキノン(DDQ)などが挙げられる。
In each step, when an oxidation reaction is carried out, the oxidizing agent used includes peracids such as m-chloroperbenzoic acid (MCPBA), hydrogen peroxide and t-butyl hydroperoxide; tetrabutylammonium perchlorate, etc. Perchlorates; Chlorates such as sodium chlorate; Chlorites such as sodium chlorite; Periodic acids such as sodium periodate; High-valent iodine reagents such as iodosylbenzene; Manganese dioxide; Reagents having manganese such as potassium manganate; Leads such as lead tetraacetate; Reagents having chromium such as pyridinium chlorochromate (PCC), pyridinium dichromate (PDC), Jones reagent; N-bromosuccinimide (NBS) Halogen compounds such as oxygen; ozone; sulfur trioxide / pyridine complex; male tetroxide Um; selenium dioxide; 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and the like.
各工程において、ラジカル環化反応を行う場合、使用されるラジカル開始剤としては、アゾビスイソブチロニトリル(AIBN)などのアゾ化合物;4-4’-アゾビス-4-シアノペンタン酸(ACPA)などの水溶性ラジカル開始剤;空気あるいは酸素存在下でのトリエチルホウ素;過酸化ベンゾイルなどが挙げられる。また、使用されるラジカル反応試剤としては、トリブチルスタナン、トリストリメチルシリルシラン、1,1,2,2-テトラフェニルジシラン、ジフェニルシラン、ヨウ化サマリウムなどが挙げられる。
In each step, when a radical cyclization reaction is performed, the radical initiator used is an azo compound such as azobisisobutyronitrile (AIBN); 4-4′-azobis-4-cyanopentanoic acid (ACPA) Water-soluble radical initiators such as triethylboron in the presence of air or oxygen, and benzoyl peroxide. Examples of the radical reaction reagent used include tributylstannane, tristrimethylsilylsilane, 1,1,2,2-tetraphenyldisilane, diphenylsilane, and samarium iodide.
各工程において、Wittig反応を行う場合、使用されるWittig試薬としては、アルキリデンホスホラン類などが挙げられる。アルキリデンホスホラン類は、自体公知の方法、例えば、ホスホニウム塩と強塩基を反応させることで調製することができる。
In each step, when a Wittig reaction is performed, examples of Wittig reagents used include alkylidene phosphoranes. The alkylidene phosphoranes can be prepared by a method known per se, for example, by reacting a phosphonium salt with a strong base.
各工程において、Horner-Emmons反応を行う場合、使用される試薬としては、ジメチルホスホノ酢酸メチル、ジエチルホスホノ酢酸エチルなどのホスホノ酢酸エステル類;アルカリ金属水素化物類、有機リチウム類などの塩基が挙げられる。
In each step, when performing Horner-Emmons reaction, the reagents used include phosphonoacetate esters such as methyl dimethylphosphonoacetate and ethyl ethyl diethylphosphonoacetate; bases such as alkali metal hydrides and organolithiums Can be mentioned.
各工程において、Friedel-Crafts反応を行う場合、使用される試薬としては、ルイス酸と、酸クロリドあるいはアルキル化剤(例、ハロゲン化アルキル類、アルコール、オレフィン類など)が挙げられる。あるいは、ルイス酸の代わりに、有機酸や無機酸を用いることもでき、酸クロリドの代わりに、無水酢酸などの酸無水物を用いることもできる。
In each step, when the Friedel-Crafts reaction is performed, examples of the reagent used include Lewis acid and acid chloride or an alkylating agent (eg, alkyl halides, alcohols, olefins, etc.). Alternatively, an organic acid or an inorganic acid can be used in place of the Lewis acid, and an acid anhydride such as acetic anhydride can be used in place of the acid chloride.
各工程において、芳香族求核置換反応を行う場合、試薬としては、求核剤(例、アミン類、イミダゾールなど)と塩基(例、塩基性塩類、有機塩基類など)が用いられる。
In each step, when an aromatic nucleophilic substitution reaction is performed, a nucleophile (eg, amines, imidazole, etc.) and a base (eg, basic salts, organic bases, etc.) are used as reagents.
各工程において、カルボアニオンによる求核付加反応、カルボアニオンによる求核1,4-付加反応(Michael付加反応)、あるいはカルボアニオンによる求核置換反応を行う場合、カルボアニオンを発生するために用いる塩基としては、有機リチウム類、金属アルコキシド類、無機塩基類、有機塩基類などが挙げられる。
In each step, a nucleophilic addition reaction with a carbanion, a nucleophilic 1,4-addition reaction with a carbanion (Michael addition reaction), or a nucleophilic substitution reaction with a carbanion, a base used to generate a carbanion Examples thereof include organic lithiums, metal alkoxides, inorganic bases, and organic bases.
各工程において、Grignard反応を行う場合、Grignard試薬としては、フェニルマグネシウムブロミドなどのアリールマグネシウムハライド類;メチルマグネシウムブロミドなどのアルキルマグネシウムハライド類が挙げられる。Grignard試薬は、自体公知の方法、例えばエーテルあるいはテトラヒドロフランを溶媒として、ハロゲン化アルキルまたはハロゲン化アリールと、金属マグネシウムとを反応させることにより調製することができる。
In each step, when the Grignard reaction is performed, examples of the Grignard reagent include arylmagnesium halides such as phenylmagnesium bromide; alkylmagnesium halides such as methylmagnesium bromide. The Grignard reagent can be prepared by a method known per se, for example, by reacting alkyl halide or aryl halide with metal magnesium using ether or tetrahydrofuran as a solvent.
各工程において、Knoevenagel縮合反応を行う場合、試薬としては、二つの電子求引基に挟まれた活性メチレン化合物(例、マロン酸、マロン酸ジエチル、マロノニトリルなど)および塩基(例、有機塩基類、金属アルコキシド類、無機塩基類)が用いられる。
In each step, when the Knoevenagel condensation reaction is performed, reagents include an active methylene compound (eg, malonic acid, diethyl malonate, malononitrile, etc.) sandwiched between two electron-withdrawing groups and a base (eg, organic bases, Metal alkoxides and inorganic bases) are used.
各工程において、Vilsmeier-Haack反応を行う場合、試薬としては、塩化ホスホリルとアミド誘導体(例、N,N-ジメチルホルムアミドなど)が用いられる。
In each step, when performing the Vilsmeier-Haack reaction, phosphoryl chloride and an amide derivative (eg, N, N-dimethylformamide, etc.) are used as reagents.
各工程において、アルコール類、アルキルハライド類、スルホン酸エステル類のアジド化反応を行う場合、使用されるアジド化剤としては、ジフェニルホスホリルアジド(DPPA)、トリメチルシリルアジド、アジ化ナトリウムなどが挙げられる。例えば、アルコール類をアジド化する場合、ジフェニルホスホリルアジドと1,8-ジアザビシクロ[5.4.0]ウンデカ-7-エン(DBU)を用いる方法やトリメチルシリルアジドとルイス酸を用いる方法などがある。
In each step, when an azidation reaction of alcohols, alkyl halides, and sulfonates is performed, examples of the azidation agent used include diphenylphosphoryl azide (DPPA), trimethylsilyl azide, and sodium azide. For example, when an alcohol is azidated, there are a method using diphenylphosphoryl azide and 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU), a method using trimethylsilyl azide and a Lewis acid, and the like.
各工程において、還元的アミノ化反応を行う場合、使用される還元剤としては、水素化トリアセトキシホウ素ナトリウム、水素化シアノホウ素ナトリウム、水素、ギ酸などが挙げられる。基質がアミン化合物の場合は、使用されるカルボニル化合物としては、パラホルムアルデヒドの他、アセトアルデヒドなどのアルデヒド類、シクロヘキサノンなどのケトン類が挙げられる。基質がカルボニル化合物の場合は、使用されるアミン類としては、アンモニア、メチルアミンなどの1級アミン;ジメチルアミンなどの2級アミンなどが挙げられる。
In each step, when a reductive amination reaction is carried out, examples of the reducing agent used include sodium triacetoxyborohydride, sodium cyanoborohydride, hydrogen, formic acid and the like. When the substrate is an amine compound, examples of the carbonyl compound used include paraformaldehyde, aldehydes such as acetaldehyde, and ketones such as cyclohexanone. When the substrate is a carbonyl compound, examples of amines used include primary amines such as ammonia and methylamine; secondary amines such as dimethylamine and the like.
各工程において、光延反応を行う場合、試薬としては、アゾジカルボン酸エステル類(例、アゾジカルボン酸ジエチル(DEAD)、アゾジカルボン酸ジイソプロピル(DIAD)など)およびトリフェニルホスフィンが用いられる。
In each step, when Mitsunobu reaction is performed, azodicarboxylic acid esters (eg, diethyl azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD), etc.) and triphenylphosphine are used as reagents.
各工程において、エステル化反応、アミド化反応、あるいはウレア化反応を行う場合、使用される試薬としては、酸クロリド、酸ブロミドなどのハロゲン化アシル体;酸無水物、活性エステル体、硫酸エステル体など活性化されたカルボン酸類が挙げられる。カルボン酸の活性化剤としては、1-エチル-3-(3-ジメチルアミノプロピル)カルボジイミド塩酸塩(WSCD)などのカルボジイミド系縮合剤;4-(4,6-ジメトキシ-1,3,5-トリアジン-2-イル)-4-メチルモルホリニウムクロライド-n-ハイドレート(DMT-MM)などのトリアジン系縮合剤;1,1-カルボニルジイミダゾール(CDI)などの炭酸エステル系縮合剤;ジフェニルリン酸アジド(DPPA);ベンゾトリアゾール-1-イルオキシ-トリスジメチルアミノホスホニウム塩(BOP試薬);ヨウ化2-クロロ-1-メチル-ピリジニウム(向山試薬);塩化チオニル;クロロギ酸エチルなどのハロギ酸低級アルキル;O-(7-アザベンゾトリアゾール-1-イル)-N,N,N’,N’-テトラメチルウロニウムヘキサフルオロリン酸塩(HATU);硫酸;あるいはこれらの組み合わせなどが挙げられる。カルボジイミド系縮合剤を用いる場合、1-ヒドロキシベンゾトリアゾール(HOBt)、N-ヒドロキシコハク酸イミド(HOSu)、ジメチルアミノピリジン(DMAP)などの添加剤をさらに反応に加えてもよい。
In each step, when an esterification reaction, an amidation reaction, or a urealation reaction is performed, the reagents used include acyl halides such as acid chloride and acid bromide; acid anhydrides, active ester compounds, and sulfate ester compounds. And activated carboxylic acids. Examples of carboxylic acid activators include carbodiimide condensing agents such as 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (WSCD); 4- (4,6-dimethoxy-1,3,5- Triazine condensing agents such as triazin-2-yl) -4-methylmorpholinium chloride-n-hydrate (DMT-MM); carbonate condensing agents such as 1,1-carbonyldiimidazole (CDI); diphenyl Azide phosphate (DPPA); benzotriazol-1-yloxy-trisdimethylaminophosphonium salt (BOP reagent); 2-chloro-1-methyl-pyridinium iodide (Mukayama reagent); thionyl chloride; haloformates such as ethyl chloroformate Lower alkyl; O- (7-azabenzotriazol-1-yl) -N, N, N ′, N′— Tetramethyl uronium hexafluorophosphate (HATU); and the like, or combinations thereof; sulfate. When a carbodiimide condensing agent is used, additives such as 1-hydroxybenzotriazole (HOBt), N-hydroxysuccinimide (HOSu), dimethylaminopyridine (DMAP) may be further added to the reaction.
各工程において、カップリング反応を行う場合、使用される金属触媒としては、酢酸パラジウム(II)、テトラキス(トリフェニルホスフィン)パラジウム(0)、ジクロロビス(トリフェニルホスフィン)パラジウム(II)、ジクロロビス(トリエチルホスフィン)パラジウム(II)、トリス(ジベンジリデンアセトン)ジパラジウム(0)、塩化1,1’-ビス(ジフェニルホスフィノ)フェロセンパラジウム(II)、酢酸パラジウム(II)などのパラジウム化合物;テトラキス(トリフェニルホスフィン)ニッケル(0)などのニッケル化合物;塩化トリス(トリフェニルホスフィン)ロジウム(III)などのロジウム化合物;コバルト化合物;酸化銅、ヨウ化銅(I)などの銅化合物;白金化合物などが挙げられる。さらに反応に塩基を加えてもよく、このような塩基としては、無機塩基類、塩基性塩類などが挙げられる。
In each step, when a coupling reaction is performed, the metal catalyst used is palladium acetate (II), tetrakis (triphenylphosphine) palladium (0), dichlorobis (triphenylphosphine) palladium (II), dichlorobis (triethyl). Palladium compounds such as phosphine) palladium (II), tris (dibenzylideneacetone) dipalladium (0), 1,1′-bis (diphenylphosphino) ferrocenepalladium (II) chloride, palladium (II) acetate; tetrakis (tri Nickel compounds such as phenylphosphine) nickel (0); rhodium compounds such as tris (triphenylphosphine) rhodium (III) chloride; cobalt compounds; copper compounds such as copper oxide and copper iodide (I); platinum compounds and the like It is done. Furthermore, a base may be added to the reaction, and examples of such a base include inorganic bases and basic salts.
各工程において、チオカルボニル化反応を行う場合、チオカルボニル化剤としては、代表的には五硫化二リンが用いられるが、五硫化二リンの他に、2,4-ビス(4-メトキシフェニル-1,3,2,4-ジチアジホスフェタン-2,4-ジスルフィド(Lawesson試薬)などの1,3,2,4-ジチアジホスフェタン-2,4-ジスルフィド構造を持つ試薬を用いてもよい。
In each step, when a thiocarbonylation reaction is performed, diphosphorus pentasulfide is typically used as the thiocarbonylating agent. In addition to diphosphorus pentasulfide, 2,4-bis (4-methoxyphenyl) is used. A reagent having a 1,3,2,4-dithiadiphosphetane-2,4-disulfide structure such as -1,3,2,4-dithiadiphosphetane-2,4-disulfide (Lawesson reagent) It may be used.
各工程において、Wohl-Ziegler反応を行う場合、使用されるハロゲン化剤としては、N-ヨードコハク酸イミド、N-ブロモコハク酸イミド(NBS)、N-クロロコハク酸イミド(NCS)、臭素、塩化スルフリルなどが挙げられる。さらに、熱、光、過酸化ベンゾイル、アゾビスイソブチロニトリルなどのラジカル開始剤を反応に加えることで、反応を加速させることができる。
In each process, when the Wohl-Ziegler reaction is performed, halogenating agents used include N-iodosuccinimide, N-bromosuccinimide (NBS), N-chlorosuccinimide (NCS), bromine, sulfuryl chloride, etc. Is mentioned. Furthermore, the reaction can be accelerated by adding a radical initiator such as heat, light, benzoyl peroxide, or azobisisobutyronitrile to the reaction.
各工程において、ヒドロキシ基のハロゲン化反応を行う場合、使用されるハロゲン化剤としては、ハロゲン化水素酸と無機酸の酸ハロゲン化物、具体的には、塩素化では、塩酸、塩化チオニル、オキシ塩化リンなど、臭素化では、48%臭化水素酸などが挙げられる。また、トリフェニルホスフィンと四塩化炭素または四臭化炭素などとの作用により、アルコールからハロゲン化アルキル体を得る方法を用いてもよい。あるいは、アルコールをスルホン酸エステルに変換の後、臭化リチウム、塩化リチウムまたはヨウ化ナトリウムと反応させるような2段階の反応を経てハロゲン化アルキル体を合成する方法を用いてもよい。
In each step, when a halogenation reaction of a hydroxy group is performed, the halogenating agent used is an acid halide of hydrohalic acid and an inorganic acid. Specifically, in chlorination, hydrochloric acid, thionyl chloride, oxy Examples of bromination such as phosphorus chloride include 48% hydrobromic acid. Alternatively, a method of obtaining an alkyl halide from alcohol by the action of triphenylphosphine and carbon tetrachloride or carbon tetrabromide may be used. Alternatively, a method of synthesizing an alkyl halide through a two-step reaction in which an alcohol is converted into a sulfonate ester and then reacted with lithium bromide, lithium chloride, or sodium iodide may be used.
各工程において、Arbuzov反応を行う場合、使用される試薬としては、ブロモ酢酸エチルなどのハロゲン化アルキル類;トリエチルホスファイトやトリ(イソプロピル)ホスファイトなどのホスファイト類が挙げられる。
In each step, when performing an Arbuzov reaction, examples of the reagent used include alkyl halides such as ethyl bromoacetate; phosphites such as triethyl phosphite and tri (isopropyl) phosphite.
各工程において、スルホン酸エステル化反応を行う場合、使用されるスルホン化剤としては、メタンスルホニルクロリド、p-トルエンスルホニルクロリド、メタンスルホン酸無水物、p-トルエンスルホン酸無水物などが挙げられる。
In each step, when a sulfonic acid esterification reaction is performed, examples of the sulfonating agent used include methanesulfonyl chloride, p-toluenesulfonyl chloride, methanesulfonic acid anhydride, p-toluenesulfonic acid anhydride, and the like.
各工程において、加水分解反応を行う場合、試薬としては、酸または塩基が用いられる。また、t-ブチルエステルの酸加水分解反応を行う場合、副生するt-ブチルカチオンを還元的にトラップするためにギ酸やトリエチルシランなどを加えることがある。
In each step, when a hydrolysis reaction is performed, an acid or a base is used as a reagent. When acid hydrolysis reaction of t-butyl ester is performed, formic acid or triethylsilane may be added in order to reductively trap the t-butyl cation produced as a by-product.
各工程において、脱水反応を行う場合、使用される脱水剤としては、硫酸、五酸化二リン、オキシ塩化リン、N,N’-ジシクロヘキシルカルボジイミド、アルミナ、ポリリン酸などが挙げられる。
In each step, when a dehydration reaction is performed, examples of the dehydrating agent used include sulfuric acid, diphosphorus pentoxide, phosphorus oxychloride, N, N'-dicyclohexylcarbodiimide, alumina, and polyphosphoric acid.
化合物(I)は、X4がNおよびX5が結合手のとき、以下のスキームAに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) can be produced by the method shown in the following scheme A or a method analogous thereto or the method described in Examples when X 4 is N and X 5 is a bond.
[スキームA]
[Scheme A]
[式中、LGは脱離基を示し、その他の各記号は前記と同義を示す。]
LGで示される脱離基としては、例えば、ハロゲン原子(例えば、塩素原子、臭素原子、ヨウ素原子など)、置換されていてもよいスルホニルオキシ基(例えば、1ないし3個のハロゲン原子で置換されていてもよいC1-6アルキルスルホニルオキシ基(例えば、メタンスルホニルオキシ基、エタンスルホニルオキシ基、トリフルオロメタンスルホニルオキシ基など);1ないし3個のC1-6アルキル基で置換されていてもよいC6-14アリールスルホニルオキシ基(例えば、ベンゼンスルホニルオキシ基、p-トルエンスルホニルオキシ基など);C7-14アラルキルスルホニルオキシ基(例えば、ベンジルスルホニルオキシ基など)など)、[(オキシド)フェニル-λ4-スルファニリデン]ジメチルアンモニウム基などが挙げられる。
化合物(I)は、化合物(V)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(II)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein, LG represents a leaving group, and other symbols are as defined above. ]
As the leaving group represented by LG, for example, a halogen atom (for example, a chlorine atom, a bromine atom, an iodine atom, etc.), an optionally substituted sulfonyloxy group (for example, 1 to 3 halogen atoms are substituted). which may be C 1-6 alkylsulfonyloxy group (e.g., methanesulfonyloxy group, ethanesulfonyloxy group, etc. trifluoromethanesulfonyloxy group); 1 to be substituted with 3 C 1-6 alkyl groups Good C 6-14 arylsulfonyloxy group (eg, benzenesulfonyloxy group, p-toluenesulfonyloxy group, etc.); C 7-14 aralkylsulfonyloxy group (eg, benzylsulfonyloxy group, etc.), [(oxide) Phenyl-λ4-sulfanilidene] dimethylammonium group, etc. It is below.
Compound (I) can be produced by a coupling reaction of compound (V). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
Compound (II) used as a raw material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
LGで示される脱離基としては、例えば、ハロゲン原子(例えば、塩素原子、臭素原子、ヨウ素原子など)、置換されていてもよいスルホニルオキシ基(例えば、1ないし3個のハロゲン原子で置換されていてもよいC1-6アルキルスルホニルオキシ基(例えば、メタンスルホニルオキシ基、エタンスルホニルオキシ基、トリフルオロメタンスルホニルオキシ基など);1ないし3個のC1-6アルキル基で置換されていてもよいC6-14アリールスルホニルオキシ基(例えば、ベンゼンスルホニルオキシ基、p-トルエンスルホニルオキシ基など);C7-14アラルキルスルホニルオキシ基(例えば、ベンジルスルホニルオキシ基など)など)、[(オキシド)フェニル-λ4-スルファニリデン]ジメチルアンモニウム基などが挙げられる。
化合物(I)は、化合物(V)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(II)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein, LG represents a leaving group, and other symbols are as defined above. ]
As the leaving group represented by LG, for example, a halogen atom (for example, a chlorine atom, a bromine atom, an iodine atom, etc.), an optionally substituted sulfonyloxy group (for example, 1 to 3 halogen atoms are substituted). which may be C 1-6 alkylsulfonyloxy group (e.g., methanesulfonyloxy group, ethanesulfonyloxy group, etc. trifluoromethanesulfonyloxy group); 1 to be substituted with 3 C 1-6 alkyl groups Good C 6-14 arylsulfonyloxy group (eg, benzenesulfonyloxy group, p-toluenesulfonyloxy group, etc.); C 7-14 aralkylsulfonyloxy group (eg, benzylsulfonyloxy group, etc.), [(oxide) Phenyl-λ4-sulfanilidene] dimethylammonium group, etc. It is below.
Compound (I) can be produced by a coupling reaction of compound (V). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
Compound (II) used as a raw material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がCH、X2がCH、X3がCH、X4がCH、X5が結合手およびX6がCH2のとき、以下のスキームBに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) can be prepared by the method shown in the following scheme B when X 1 is CH, X 2 is CH, X 3 is CH, X 4 is CH, X 5 is a bond and X 6 is CH 2 or It can be produced by a similar method or a method described in Examples.
[スキームB]
[Scheme B]
[式中、各記号は前記と同義を示す。]
化合物(VIII)は、化合物(VII)のブロモ化反応により製造することができる。ブロモ化試薬としては、N-ブロモスクシンイミド及びアゾビスイソブチロニトリルなどが挙げられる。
化合物(XI)は、化合物(X)の還元反応により製造することができる。還元剤としては、ボラン-テトラヒドロフラン錯体などが挙げられる。
化合物(I)は、化合物(XI)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(VI)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein each symbol has the same meaning as described above. ]
Compound (VIII) can be produced by bromination reaction of compound (VII). Examples of bromination reagents include N-bromosuccinimide and azobisisobutyronitrile.
Compound (XI) can be produced by a reduction reaction of compound (X). Examples of the reducing agent include borane-tetrahydrofuran complex.
Compound (I) can be produced by a coupling reaction of compound (XI). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
Compound (VI) used as a starting material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
化合物(VIII)は、化合物(VII)のブロモ化反応により製造することができる。ブロモ化試薬としては、N-ブロモスクシンイミド及びアゾビスイソブチロニトリルなどが挙げられる。
化合物(XI)は、化合物(X)の還元反応により製造することができる。還元剤としては、ボラン-テトラヒドロフラン錯体などが挙げられる。
化合物(I)は、化合物(XI)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(VI)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein each symbol has the same meaning as described above. ]
Compound (VIII) can be produced by bromination reaction of compound (VII). Examples of bromination reagents include N-bromosuccinimide and azobisisobutyronitrile.
Compound (XI) can be produced by a reduction reaction of compound (X). Examples of the reducing agent include borane-tetrahydrofuran complex.
Compound (I) can be produced by a coupling reaction of compound (XI). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
Compound (VI) used as a starting material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がCH、X2がCH、X3がCH、X4がCH、X5が結合手およびX6がCCH3CH3のとき、以下のスキームCに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) is a compound represented by the following scheme C when X 1 is CH, X 2 is CH, X 3 is CH, X 4 is CH, X 5 is a bond and X 6 is CCH 3 CH 3 or It can be produced by a method according to this or the method described in the Examples.
[スキームC]
[Scheme C]
[式中、記号は前記と同義を示す。]
化合物(XV)は、化合物(XIV)の求核置換反応により製造することができる。求核置換試薬としては、クロロアセトニトリルなどが挙げられる。
化合物(XVI)は、化合物(XV)の脱保護反応により製造することができる。脱保護試薬としては、チオウレアなどが挙げられる。
化合物(I)は、化合物(XVIII)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XII)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XV) can be produced by nucleophilic substitution reaction of compound (XIV). Examples of the nucleophilic substitution reagent include chloroacetonitrile.
Compound (XVI) can be produced by subjecting compound (XV) to deprotection. Examples of the deprotecting reagent include thiourea.
Compound (I) can be produced by a coupling reaction of compound (XVIII). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As compound (XII) used as a starting material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
化合物(XV)は、化合物(XIV)の求核置換反応により製造することができる。求核置換試薬としては、クロロアセトニトリルなどが挙げられる。
化合物(XVI)は、化合物(XV)の脱保護反応により製造することができる。脱保護試薬としては、チオウレアなどが挙げられる。
化合物(I)は、化合物(XVIII)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XII)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XV) can be produced by nucleophilic substitution reaction of compound (XIV). Examples of the nucleophilic substitution reagent include chloroacetonitrile.
Compound (XVI) can be produced by subjecting compound (XV) to deprotection. Examples of the deprotecting reagent include thiourea.
Compound (I) can be produced by a coupling reaction of compound (XVIII). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As compound (XII) used as a starting material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がCH、X2がCH、X3がN、X4がCH、X5が結合手およびX6がCH2のとき、以下のスキームDに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) is prepared by the method shown in the following scheme D or when X 1 is CH, X 2 is CH, X 3 is N, X 4 is CH, X 5 is a bond and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
[スキームD]
[Scheme D]
[式中、記号は前記と同義を示す。]
化合物(XXI)は、化合物(XX)の求核付加反応により製造することができる。求核試薬としては、パラメトキシベンジルアミンなどが挙げられる。
化合物(XXIV)は、化合物(XXIII)のクロロ化反応により製造することができる。クロロ化試薬としては、オキシ塩化リンなどが挙げられる。
化合物(I)は、化合物(XXIV)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XIX)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XXI) can be produced by a nucleophilic addition reaction of compound (XX). Examples of the nucleophile include paramethoxybenzylamine.
Compound (XXIV) can be produced by chlorination reaction of compound (XXIII). Examples of the chlorinating reagent include phosphorus oxychloride.
Compound (I) can be produced by a coupling reaction of compound (XXIV). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XIX) used as a starting material in this method, a commercially available product may be used as it is, or it can be produced by a method known per se or a method analogous thereto.
化合物(XXI)は、化合物(XX)の求核付加反応により製造することができる。求核試薬としては、パラメトキシベンジルアミンなどが挙げられる。
化合物(XXIV)は、化合物(XXIII)のクロロ化反応により製造することができる。クロロ化試薬としては、オキシ塩化リンなどが挙げられる。
化合物(I)は、化合物(XXIV)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XIX)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XXI) can be produced by a nucleophilic addition reaction of compound (XX). Examples of the nucleophile include paramethoxybenzylamine.
Compound (XXIV) can be produced by chlorination reaction of compound (XXIII). Examples of the chlorinating reagent include phosphorus oxychloride.
Compound (I) can be produced by a coupling reaction of compound (XXIV). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XIX) used as a starting material in this method, a commercially available product may be used as it is, or it can be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がCH、X2がN、X3がCH、X4がCH、X5が結合手およびX6がCH2のとき、以下のスキームEに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) is prepared by the method shown in the following scheme E or when X 1 is CH, X 2 is N, X 3 is CH, X 4 is CH, X 5 is a bond, and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
[スキームE]
[Scheme E]
[式中、記号は前記と同義を示す。]
化合物(XXVI)は、化合物(XXV)のヨウ素化反応により製造することができる。ヨウ素化試薬としては、リチウムテトラメチルピペリジド及びヨウ素などが挙げられる。
化合物(I)は、化合物(XXXIII)のカップリング反応およびそれによって得られる化合物(XXXIV)の脱保護反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XXV)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XXVI) can be produced by iodination reaction of compound (XXV). Examples of the iodination reagent include lithium tetramethylpiperidide and iodine.
Compound (I) can be produced by a coupling reaction of compound (XXXIII) and a deprotection reaction of compound (XXXIV) obtained thereby. Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
Compound (XXV) used as a starting material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
化合物(XXVI)は、化合物(XXV)のヨウ素化反応により製造することができる。ヨウ素化試薬としては、リチウムテトラメチルピペリジド及びヨウ素などが挙げられる。
化合物(I)は、化合物(XXXIII)のカップリング反応およびそれによって得られる化合物(XXXIV)の脱保護反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XXV)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XXVI) can be produced by iodination reaction of compound (XXV). Examples of the iodination reagent include lithium tetramethylpiperidide and iodine.
Compound (I) can be produced by a coupling reaction of compound (XXXIII) and a deprotection reaction of compound (XXXIV) obtained thereby. Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
Compound (XXV) used as a starting material in this method may be a commercially available product, or can be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がN、X2がCH、X3がCH、X4がCH、X5が結合手およびX6がCH2のとき、以下のスキームFに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) is prepared by the method shown in the following scheme F or when X 1 is N, X 2 is CH, X 3 is CH, X 4 is CH, X 5 is a bond, and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
[スキームF]
[Scheme F]
[式中、記号は前記と同義を示す。]
化合物(XXXVI)は、化合物(XXXV)のヨウ素化反応により製造することができる。ヨウ素化試薬としては、テトラメチルピペリジン、ノルマルブチルリチウム及びヨウ素などが挙げられる。
化合物(I)は、化合物(XLI)のカップリング反応およびそれによって得られる化合物(XLII)の脱保護反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XXXV)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XXXVI) can be produced by iodination reaction of compound (XXXV). Examples of the iodination reagent include tetramethylpiperidine, normal butyl lithium, iodine and the like.
Compound (I) can be produced by coupling reaction of compound (XLI) and deprotection reaction of compound (XLII) obtained thereby. Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XXXV) used as a starting material in this method, a commercially available product may be used as it is, or it can be produced by a method known per se or a method analogous thereto.
化合物(XXXVI)は、化合物(XXXV)のヨウ素化反応により製造することができる。ヨウ素化試薬としては、テトラメチルピペリジン、ノルマルブチルリチウム及びヨウ素などが挙げられる。
化合物(I)は、化合物(XLI)のカップリング反応およびそれによって得られる化合物(XLII)の脱保護反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XXXV)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XXXVI) can be produced by iodination reaction of compound (XXXV). Examples of the iodination reagent include tetramethylpiperidine, normal butyl lithium, iodine and the like.
Compound (I) can be produced by coupling reaction of compound (XLI) and deprotection reaction of compound (XLII) obtained thereby. Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XXXV) used as a starting material in this method, a commercially available product may be used as it is, or it can be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がCH、X2がN、X3がN、X4がCH、X5が結合手およびX6がCH2のとき、以下のスキームGに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) can be prepared by the method shown in the following scheme G when X 1 is CH, X 2 is N, X 3 is N, X 4 is CH, X 5 is a bond and X 6 is CH 2 or It can be produced by a similar method or a method described in Examples.
[スキームG]
[Scheme G]
[式中、記号は前記と同義を示す。]
化合物(XLVI)は、化合物(XLV)の環化反応及び脱水反応により製造することができる。試薬としては、S-メチルイソチオウレア等が挙げられる。
化合物(I)は、化合物(XLVII)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XLIII)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XLVI) can be produced by cyclization reaction and dehydration reaction of compound (XLV). Examples of the reagent include S-methylisothiourea.
Compound (I) can be produced by a coupling reaction of compound (XLVII). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XLIII) used as a raw material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
化合物(XLVI)は、化合物(XLV)の環化反応及び脱水反応により製造することができる。試薬としては、S-メチルイソチオウレア等が挙げられる。
化合物(I)は、化合物(XLVII)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XLIII)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XLVI) can be produced by cyclization reaction and dehydration reaction of compound (XLV). Examples of the reagent include S-methylisothiourea.
Compound (I) can be produced by a coupling reaction of compound (XLVII). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XLIII) used as a raw material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がCH、X2がCH、X3がCH、X4がN、X5がCH2およびX6がCH2のとき、以下のスキームHに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) is prepared by the method shown in Scheme H below or when X 1 is CH, X 2 is CH, X 3 is CH, X 4 is N, X 5 is CH 2 and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
[スキームH]
[Scheme H]
[式中、記号は前記と同義を示す。]
化合物(XLIX)は、化合物(XLVIII)のアルキル化反応により製造することができる。アルキル化試薬としては、ベンジルブロマイドなどが挙げられる。
化合物(I)は、化合物(XLIX)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XLVIII)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XLIX) can be produced by subjecting compound (XLVIII) to an alkylation reaction. Examples of the alkylating reagent include benzyl bromide.
Compound (I) can be produced by a coupling reaction of compound (XLIX). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XLVIII) used as a starting material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
化合物(XLIX)は、化合物(XLVIII)のアルキル化反応により製造することができる。アルキル化試薬としては、ベンジルブロマイドなどが挙げられる。
化合物(I)は、化合物(XLIX)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(XLVIII)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (XLIX) can be produced by subjecting compound (XLVIII) to an alkylation reaction. Examples of the alkylating reagent include benzyl bromide.
Compound (I) can be produced by a coupling reaction of compound (XLIX). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (XLVIII) used as a starting material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
化合物(I)は、X1がCH、X2がCH、X3がN、X4がN、X5がCH2およびX6がCH2のとき、以下のスキームIに示す方法またはこれに準ずる方法もしくは実施例に記載の方法で製造することができる。
Compound (I) can be prepared by the method shown in Scheme I below or when X 1 is CH, X 2 is CH, X 3 is N, X 4 is N, X 5 is CH 2 and X 6 is CH 2. It can be produced by a similar method or a method described in Examples.
[スキームI]
[Scheme I]
[式中、記号は前記と同義を示す。]
化合物(I)は、化合物(LI)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(L)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (I) can be produced by a coupling reaction of compound (LI). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (L) used as a raw material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
化合物(I)は、化合物(LI)のカップリング反応により製造することができる。金属触媒とその配位子としては、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II)またはトリス(ジベンジリデンアセトン)二パラジウム(0)及び2-ジシクロヘキシルホスフィノ-2’,6’-ジイソプロポキシ-1,1’-ビフェニル等が挙げられる。
本法において原料として用いる化合物(L)は、市販品をそのまま用いてもよく、あるいは、それ自体公知あるいはそれに準じた方法により製造することもできる。 [Wherein the symbols are as defined above. ]
Compound (I) can be produced by a coupling reaction of compound (LI). Metal catalysts and their ligands include bis (di-tert-butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) or tris (dibenzylideneacetone) dipalladium (0) and 2-dicyclohexylphosphino- 2 ′, 6′-diisopropoxy-1,1′-biphenyl and the like.
As the compound (L) used as a raw material in this method, a commercially available product may be used as it is, or it may be produced by a method known per se or a method analogous thereto.
こうして得られる化合物(I)は、公知の分離精製手段、例えば、濃縮、減圧濃縮、溶媒抽出、晶出、再結晶、転溶、クロマトグラフィー等により、単離精製することができる。
The compound (I) thus obtained can be isolated and purified by known separation and purification means, for example, concentration, concentration under reduced pressure, solvent extraction, crystallization, recrystallization, transfer dissolution, chromatography, and the like.
上記の各製造法により得られる本発明化合物は、濃縮、減圧濃縮、溶媒抽出、晶出、再結晶、転溶、クロマトグラフィー等の公知の手段により単離精製することができる。また、上記の各製造法において用いられる各原料化合物は、前記と同様の公知の手段によって単離精製することができる。一方、これら原料化合物を単離することなく、そのまま反応混合物として、次の工程の原料として用いてもよい。
このような方法により生成した化合物(I)は、例えば、再結晶、蒸留、クロマトグラフィー等の通常の分離手段により単離、精製することができる。
化合物(I)が、光学異性体、立体異性体、位置異性体、回転異性体を含有する場合には、これらも化合物(I)として含有されるとともに、自体公知の合成手法、分離手法(例えば、濃縮、溶媒抽出、カラムクロマトグラフィー、再結晶等)によりそれぞれを単品として得ることができる。例えば、化合物(I)に光学異性体が存在する場合には、該化合物から分割された光学異性体も化合物(I)に包含される。 The compound of the present invention obtained by each of the above production methods can be isolated and purified by known means such as concentration, concentration under reduced pressure, solvent extraction, crystallization, recrystallization, transfer dissolution, chromatography and the like. Moreover, each raw material compound used in each of the above production methods can be isolated and purified by the same known means as described above. On the other hand, you may use these raw material compounds as a reaction mixture as it is as a raw material of the following process, without isolating.
The compound (I) produced by such a method can be isolated and purified by usual separation means such as recrystallization, distillation, chromatography and the like.
When compound (I) contains optical isomers, stereoisomers, positional isomers, and rotational isomers, these are also included as compound (I), and synthetic methods and separation methods known per se (for example, , Concentration, solvent extraction, column chromatography, recrystallization, etc.), each can be obtained as a single product. For example, when compound (I) has an optical isomer, the optical isomer resolved from the compound is also encompassed in compound (I).
このような方法により生成した化合物(I)は、例えば、再結晶、蒸留、クロマトグラフィー等の通常の分離手段により単離、精製することができる。
化合物(I)が、光学異性体、立体異性体、位置異性体、回転異性体を含有する場合には、これらも化合物(I)として含有されるとともに、自体公知の合成手法、分離手法(例えば、濃縮、溶媒抽出、カラムクロマトグラフィー、再結晶等)によりそれぞれを単品として得ることができる。例えば、化合物(I)に光学異性体が存在する場合には、該化合物から分割された光学異性体も化合物(I)に包含される。 The compound of the present invention obtained by each of the above production methods can be isolated and purified by known means such as concentration, concentration under reduced pressure, solvent extraction, crystallization, recrystallization, transfer dissolution, chromatography and the like. Moreover, each raw material compound used in each of the above production methods can be isolated and purified by the same known means as described above. On the other hand, you may use these raw material compounds as a reaction mixture as it is as a raw material of the following process, without isolating.
The compound (I) produced by such a method can be isolated and purified by usual separation means such as recrystallization, distillation, chromatography and the like.
When compound (I) contains optical isomers, stereoisomers, positional isomers, and rotational isomers, these are also included as compound (I), and synthetic methods and separation methods known per se (for example, , Concentration, solvent extraction, column chromatography, recrystallization, etc.), each can be obtained as a single product. For example, when compound (I) has an optical isomer, the optical isomer resolved from the compound is also encompassed in compound (I).
光学異性体は自体公知の方法により製造することができる。具体的には、光学活性な合成中間体を用いる、または、最終物のラセミ体を常法に従って光学分割することにより光学異性体を得る。
光学分割法としては、自体公知の方法、例えば、分別再結晶法、キラルカラム法、ジアステレオマー法等が用いられる。 The optical isomer can be produced by a method known per se. Specifically, an optical isomer is obtained by using an optically active synthetic intermediate or by optically resolving the final racemate according to a conventional method.
As the optical resolution method, a method known per se, for example, fractional recrystallization method, chiral column method, diastereomer method and the like are used.
光学分割法としては、自体公知の方法、例えば、分別再結晶法、キラルカラム法、ジアステレオマー法等が用いられる。 The optical isomer can be produced by a method known per se. Specifically, an optical isomer is obtained by using an optically active synthetic intermediate or by optically resolving the final racemate according to a conventional method.
As the optical resolution method, a method known per se, for example, fractional recrystallization method, chiral column method, diastereomer method and the like are used.
1)分別再結晶法
ラセミ体と光学活性な化合物(例えば、(+)-マンデル酸、(-)-マンデル酸、(+)-酒石酸、(-)-酒石酸、(+)-1-フェネチルアミン、(-)-1-フェネチルアミン、シンコニン、(-)-シンコニジン、ブルシン等)と塩を形成させ、これを分別再結晶法によって分離し、所望により、中和工程を経てフリーの光学異性体を得る方法。
2)キラルカラム法
ラセミ体またはその塩を光学異性体分離用カラム(キラルカラム)にかけて分離する方法。例えば、液体クロマトグラフィーの場合、ENANTIO-OVM(東ソー社製)あるいは、CHIRALシリーズ(ダイセル社製)等のキラルカラムに光学異性体の混合物を添加し、水、種々の緩衝液(例、リン酸緩衝液等)、有機溶媒(例、エタノール、メタノール、イソプロパノール、アセトニトリル、トリフルオロ酢酸、ジエチルアミン等)を単独あるいは混合した溶液として展開させることにより、光学異性体を分離する。また、例えば、ガスクロマトグラフィーの場合、CP-Chirasil-DeX CB(ジーエルサイエンス社製)等のキラルカラムを使用して分離する。
3)ジアステレオマー法
ラセミ体の混合物を光学活性な試薬と化学反応によってジアステレオマーの混合物とし、これを通常の分離手段(例えば、分別再結晶、クロマトグラフィー法等)等を経て単一物質とした後、加水分解反応等の化学的な処理により光学活性な試薬部位を切り離すことにより光学異性体を得る方法。例えば、化合物(I)が分子内にヒドロキシまたは1,2級アミノを有する場合、該化合物と光学活性な有機酸(例えば、MTPA〔α-メトキシ-α-(トリフルオロメチル)フェニル酢酸〕、(-)-メントキシ酢酸等)等とを縮合反応に付すことにより、それぞれエステル体またはアミド体のジアステレオマーが得られる。一方、化合物(I)がカルボン酸基を有する場合、該化合物と光学活性アミンまたはアルコール試薬とを縮合反応に付すことにより、それぞれアミド体またはエステル体のジアステレオマーが得られる。分離されたジアステレオマーは、酸加水分解あるいは塩基性加水分解反応に付すことにより、元の化合物の光学異性体に変換される。 1) Fractional recrystallization method Racemate and optically active compound (for example, (+)-mandelic acid, (−)-mandelic acid, (+)-tartaric acid, (−)-tartaric acid, (+)-1-phenethylamine, (-)-1-phenethylamine, cinchonine, (-)-cinchonidine, brucine, etc.) to form a salt, which is separated by fractional recrystallization, and if desired, a free optical isomer is obtained through a neutralization step. Method.
2) Chiral column method A method in which a racemate or a salt thereof is separated by applying to an optical isomer separation column (chiral column). For example, in the case of liquid chromatography, a mixture of optical isomers is added to a chiral column such as ENANTIO-OVM (manufactured by Tosoh Corporation) or CHIRAL series (manufactured by Daicel Corporation), and water, various buffer solutions (eg, phosphate buffer) are added. Liquid, etc.) and an organic solvent (eg, ethanol, methanol, isopropanol, acetonitrile, trifluoroacetic acid, diethylamine, etc.) are developed as a single or mixed solution to separate optical isomers. Further, for example, in the case of gas chromatography, separation is performed using a chiral column such as CP-Chirasil-DeX CB (manufactured by GL Sciences).
3) Diastereomer method A mixture of racemates is converted into a mixture of diastereomers by chemical reaction with an optically active reagent, and this is converted into a single substance through ordinary separation means (for example, fractional recrystallization, chromatography method, etc.). And then obtaining an optical isomer by separating the optically active reagent site by chemical treatment such as hydrolysis reaction. For example, when the compound (I) has a hydroxy or a primary or secondary amino in the molecule, the compound and an optically active organic acid (for example, MTPA [α-methoxy-α- (trifluoromethyl) phenylacetic acid], ( -)-Menthoxyacetic acid etc.) are subjected to a condensation reaction to obtain ester or amide diastereomers, respectively. On the other hand, when the compound (I) has a carboxylic acid group, an amide or ester diastereomer is obtained by subjecting the compound and an optically active amine or alcohol reagent to a condensation reaction. The separated diastereomer is converted into the optical isomer of the original compound by subjecting it to an acid hydrolysis or basic hydrolysis reaction.
ラセミ体と光学活性な化合物(例えば、(+)-マンデル酸、(-)-マンデル酸、(+)-酒石酸、(-)-酒石酸、(+)-1-フェネチルアミン、(-)-1-フェネチルアミン、シンコニン、(-)-シンコニジン、ブルシン等)と塩を形成させ、これを分別再結晶法によって分離し、所望により、中和工程を経てフリーの光学異性体を得る方法。
2)キラルカラム法
ラセミ体またはその塩を光学異性体分離用カラム(キラルカラム)にかけて分離する方法。例えば、液体クロマトグラフィーの場合、ENANTIO-OVM(東ソー社製)あるいは、CHIRALシリーズ(ダイセル社製)等のキラルカラムに光学異性体の混合物を添加し、水、種々の緩衝液(例、リン酸緩衝液等)、有機溶媒(例、エタノール、メタノール、イソプロパノール、アセトニトリル、トリフルオロ酢酸、ジエチルアミン等)を単独あるいは混合した溶液として展開させることにより、光学異性体を分離する。また、例えば、ガスクロマトグラフィーの場合、CP-Chirasil-DeX CB(ジーエルサイエンス社製)等のキラルカラムを使用して分離する。
3)ジアステレオマー法
ラセミ体の混合物を光学活性な試薬と化学反応によってジアステレオマーの混合物とし、これを通常の分離手段(例えば、分別再結晶、クロマトグラフィー法等)等を経て単一物質とした後、加水分解反応等の化学的な処理により光学活性な試薬部位を切り離すことにより光学異性体を得る方法。例えば、化合物(I)が分子内にヒドロキシまたは1,2級アミノを有する場合、該化合物と光学活性な有機酸(例えば、MTPA〔α-メトキシ-α-(トリフルオロメチル)フェニル酢酸〕、(-)-メントキシ酢酸等)等とを縮合反応に付すことにより、それぞれエステル体またはアミド体のジアステレオマーが得られる。一方、化合物(I)がカルボン酸基を有する場合、該化合物と光学活性アミンまたはアルコール試薬とを縮合反応に付すことにより、それぞれアミド体またはエステル体のジアステレオマーが得られる。分離されたジアステレオマーは、酸加水分解あるいは塩基性加水分解反応に付すことにより、元の化合物の光学異性体に変換される。 1) Fractional recrystallization method Racemate and optically active compound (for example, (+)-mandelic acid, (−)-mandelic acid, (+)-tartaric acid, (−)-tartaric acid, (+)-1-phenethylamine, (-)-1-phenethylamine, cinchonine, (-)-cinchonidine, brucine, etc.) to form a salt, which is separated by fractional recrystallization, and if desired, a free optical isomer is obtained through a neutralization step. Method.
2) Chiral column method A method in which a racemate or a salt thereof is separated by applying to an optical isomer separation column (chiral column). For example, in the case of liquid chromatography, a mixture of optical isomers is added to a chiral column such as ENANTIO-OVM (manufactured by Tosoh Corporation) or CHIRAL series (manufactured by Daicel Corporation), and water, various buffer solutions (eg, phosphate buffer) are added. Liquid, etc.) and an organic solvent (eg, ethanol, methanol, isopropanol, acetonitrile, trifluoroacetic acid, diethylamine, etc.) are developed as a single or mixed solution to separate optical isomers. Further, for example, in the case of gas chromatography, separation is performed using a chiral column such as CP-Chirasil-DeX CB (manufactured by GL Sciences).
3) Diastereomer method A mixture of racemates is converted into a mixture of diastereomers by chemical reaction with an optically active reagent, and this is converted into a single substance through ordinary separation means (for example, fractional recrystallization, chromatography method, etc.). And then obtaining an optical isomer by separating the optically active reagent site by chemical treatment such as hydrolysis reaction. For example, when the compound (I) has a hydroxy or a primary or secondary amino in the molecule, the compound and an optically active organic acid (for example, MTPA [α-methoxy-α- (trifluoromethyl) phenylacetic acid], ( -)-Menthoxyacetic acid etc.) are subjected to a condensation reaction to obtain ester or amide diastereomers, respectively. On the other hand, when the compound (I) has a carboxylic acid group, an amide or ester diastereomer is obtained by subjecting the compound and an optically active amine or alcohol reagent to a condensation reaction. The separated diastereomer is converted into the optical isomer of the original compound by subjecting it to an acid hydrolysis or basic hydrolysis reaction.
化合物(I)は、結晶であってもよい。
化合物(I)の結晶は、化合物(I)に自体公知の結晶化法を適用して、結晶化することによって製造することができる。
ここで、結晶化法としては、例えば、溶液からの結晶化法、蒸気からの結晶化法、溶融体からの結晶化法等が挙げられる。 Compound (I) may be a crystal.
Crystals of compound (I) can be produced by applying crystallization methods known per se to compound (I) for crystallization.
Here, examples of the crystallization method include a crystallization method from a solution, a crystallization method from a vapor, a crystallization method from a melt, and the like.
化合物(I)の結晶は、化合物(I)に自体公知の結晶化法を適用して、結晶化することによって製造することができる。
ここで、結晶化法としては、例えば、溶液からの結晶化法、蒸気からの結晶化法、溶融体からの結晶化法等が挙げられる。 Compound (I) may be a crystal.
Crystals of compound (I) can be produced by applying crystallization methods known per se to compound (I) for crystallization.
Here, examples of the crystallization method include a crystallization method from a solution, a crystallization method from a vapor, a crystallization method from a melt, and the like.
該「溶液からの結晶化法」としては、化合物の溶解度に関係する因子(溶媒組成、pH、温度、イオン強度、酸化還元状態等)または溶媒の量を変化させることによって、飽和していない状態から過飽和状態に移行させる方法が一般的であり、具体的には、例えば、濃縮法、徐冷法、反応法(拡散法、電解法)、水熱育成法、融剤法等が挙げられる。用いられる溶媒としては、例えば、芳香族炭化水素類(例、ベンゼン、トルエン、キシレン等)、ハロゲン化炭化水素類(例、ジクロロメタン、クロロホルム等)、飽和炭化水素類(例、ヘキサン、ヘプタン、シクロヘキサン等)、エーテル類(例、ジエチルエーテル、ジイソプロピルエーテル、テトラヒドロフラン、1,4-ジオキサン等)、ニトリル類(例、アセトニトリル等)、ケトン類(例、アセトン等)、スルホキシド類(例、ジメチルスルホキシド等)、酸アミド類(例、N,N-ジメチルホルムアミド等)、エステル類(例、酢酸エチル等)、アルコール類(例、メタノール、エタノール、イソプロピルアルコール等)、水等が挙げられる。これらの溶媒は単独あるいは二種以上を適当な割合(例、1:1ないし1:100(容積比))で混合して用いられる。必要に応じて種晶を使用することもできる。
The “crystallization from solution” includes a state in which the compound is not saturated by changing factors related to the solubility of the compound (solvent composition, pH, temperature, ionic strength, redox state, etc.) or the amount of the solvent. In general, a method of shifting from a supersaturated state to a supersaturated state is exemplified, and specific examples include a concentration method, a slow cooling method, a reaction method (diffusion method, electrolysis method), a hydrothermal growth method, and a flux method. Examples of the solvent used include aromatic hydrocarbons (eg, benzene, toluene, xylene, etc.), halogenated hydrocarbons (eg, dichloromethane, chloroform, etc.), saturated hydrocarbons (eg, hexane, heptane, cyclohexane). Etc.), ethers (eg, diethyl ether, diisopropyl ether, tetrahydrofuran, 1,4-dioxane, etc.), nitriles (eg, acetonitrile, etc.), ketones (eg, acetone, etc.), sulfoxides (eg, dimethyl sulfoxide, etc.) ), Acid amides (eg, N, N-dimethylformamide, etc.), esters (eg, ethyl acetate, etc.), alcohols (eg, methanol, ethanol, isopropyl alcohol, etc.), water and the like. These solvents may be used alone or in admixture of two or more at an appropriate ratio (eg, 1: 1 to 1: 100 (volume ratio)). A seed crystal can also be used as needed.
該「蒸気からの結晶化法」としては、例えば、気化法(封管法、気流法)、気相反応法、化学輸送法等が挙げられる。
Examples of the “crystallization method from vapor” include a vaporization method (sealed tube method, air flow method), a gas phase reaction method, a chemical transport method, and the like.
該「溶融体からの結晶化法」としては、例えば、ノルマルフリージング法(引上げ法、温度傾斜法、ブリッジマン法)、帯溶融法(ゾーンレベリング法、フロートゾーン法)、特殊成長法(VLS法、液相エピタキシー法)等が挙げられる。
Examples of the “crystallization from the melt” include normal freezing method (pulling method, temperature gradient method, Bridgman method), zone melting method (zone leveling method, float zone method), special growth method (VLS method). , Liquid phase epitaxy method) and the like.
結晶化法の好適な例としては、化合物(I)を20~120℃の温度下において、適当な溶媒(例、メタノール、エタノール等のアルコール類等)に溶解し、得られる溶液を溶解時の温度以下(例えば、0~50℃、好ましくは0~20℃)に冷却する方法等が挙げられる。
このようにして得られる本発明の結晶は、例えば、ろ過等によって単離することができる。
得られた結晶の解析方法としては、粉末X線回折による結晶解析の方法が一般的である。さらに、結晶の方位を決定する方法としては、例えば、機械的な方法または光学的な方法等も挙げられる。 As a preferred example of the crystallization method, compound (I) is dissolved in a suitable solvent (eg, alcohol such as methanol, ethanol, etc.) at a temperature of 20 to 120 ° C., and the resulting solution is dissolved. Examples thereof include a method of cooling to a temperature or lower (for example, 0 to 50 ° C., preferably 0 to 20 ° C.).
The crystals of the present invention thus obtained can be isolated by, for example, filtration.
As a method for analyzing the obtained crystal, a crystal analysis method by powder X-ray diffraction is generally used. Furthermore, examples of the method for determining the crystal orientation include a mechanical method and an optical method.
このようにして得られる本発明の結晶は、例えば、ろ過等によって単離することができる。
得られた結晶の解析方法としては、粉末X線回折による結晶解析の方法が一般的である。さらに、結晶の方位を決定する方法としては、例えば、機械的な方法または光学的な方法等も挙げられる。 As a preferred example of the crystallization method, compound (I) is dissolved in a suitable solvent (eg, alcohol such as methanol, ethanol, etc.) at a temperature of 20 to 120 ° C., and the resulting solution is dissolved. Examples thereof include a method of cooling to a temperature or lower (for example, 0 to 50 ° C., preferably 0 to 20 ° C.).
The crystals of the present invention thus obtained can be isolated by, for example, filtration.
As a method for analyzing the obtained crystal, a crystal analysis method by powder X-ray diffraction is generally used. Furthermore, examples of the method for determining the crystal orientation include a mechanical method and an optical method.
上記の製造法で得られる化合物(I)の結晶(以下、「本発明の結晶」と略記する)は、高純度、高品質であり、吸湿性が低く、通常条件下で長期間保存しても変質せず、安定性に極めて優れている。また、生物学的性質(例、体内動態(吸収性、分布、代謝、排泄)、薬効発現等)にも優れ、医薬として極めて有用である。
The crystal of compound (I) obtained by the above production method (hereinafter abbreviated as “crystal of the present invention”) has high purity, high quality, low hygroscopicity, and is stored for a long time under normal conditions. Is very stable. In addition, it has excellent biological properties (eg, pharmacokinetics (absorbability, distribution, metabolism, excretion), expression of drug efficacy, etc.) and is extremely useful as a medicine.
本明細書中、比旋光度([α]D)は、例えば、旋光度計(日本分光(JASCO)、P-1030型旋光計(No.AP-2))等を用いて測定される比旋光度を意味する。
本明細書中、融点は、例えば、微量融点測定器(ヤナコ、MP-500D型)またはDSC(示差走査熱量分析)装置(SEIKO,EXSTAR6000)等を用いて測定される融点を意味する。 In the present specification, the specific optical rotation ([α] D ) is a ratio measured using, for example, an optical polarimeter (JASCO, P-1030 polarimeter (No. AP-2)) or the like. Means optical rotation.
In the present specification, the melting point means a melting point measured using, for example, a trace melting point measuring device (Yanako, MP-500D type) or a DSC (Differential Scanning Calorimetry) apparatus (SEIKO, EXSTAR6000).
本明細書中、融点は、例えば、微量融点測定器(ヤナコ、MP-500D型)またはDSC(示差走査熱量分析)装置(SEIKO,EXSTAR6000)等を用いて測定される融点を意味する。 In the present specification, the specific optical rotation ([α] D ) is a ratio measured using, for example, an optical polarimeter (JASCO, P-1030 polarimeter (No. AP-2)) or the like. Means optical rotation.
In the present specification, the melting point means a melting point measured using, for example, a trace melting point measuring device (Yanako, MP-500D type) or a DSC (Differential Scanning Calorimetry) apparatus (SEIKO, EXSTAR6000).
化合物(I)はプロドラッグとして用いてもよい。化合物(I)のプロドラッグは、生体内における生理条件下で酵素や胃酸等による反応により化合物(I)に変換する化合物、すなわち酵素的に酸化、還元、加水分解等を起こして化合物(I)に変化する化合物、胃酸等により加水分解等を起こして化合物(I)に変化する化合物をいう。
化合物(I)のプロドラッグとしては、例えば、
(1)化合物(I)のアミノがアシル化、アルキル化、りん酸化された化合物(例えば、化合物(I)のアミノが、エイコサノイル化、アラニル化、ペンチルアミノカルボニル化、(5-メチル-2-オキソ-1,3-ジオキソレン-4-イル)メトキシカルボニル化、テトラヒドロフラニル化、ピロリジルメチル化、ピバロイルオキシメチル化、tert-ブチル化、エトキシカルボニル化、tert-ブトキシカルボニル化、アセチル化、シクロプロピルカルボニル化された化合物等);
(2)化合物(I)のヒドロキシが、アシル化、アルキル化、りん酸化、ホウ酸化された化合物(例えば、化合物(I)のヒドロキシが、アセチル化、パルミトイル化、プロパノイル化、ピバロイル化、スクシニル化、フマリル化、アラニル化、ジメチルアミノメチルカルボニル化された化合物等);
(3)化合物(I)のカルボキシが、エステル化、アミド化された化合物(例えば、化合物(I)のカルボキシが、エチルエステル化、フェニルエステル化、カルボキシメチルエステル化、ジメチルアミノメチルエステル化、ピバロイルオキシメチルエステル化、エトキシカルボニルオキシエチルエステル化、フタリジルエステル化、(5-メチル-2-オキソ-1,3-ジオキソレン-4-イル)メチルエステル化、シクロヘキシルオキシカルボニルエチルエステル化、メチルアミド化された化合物等);
等が挙げられる。これらの化合物は、自体公知の方法によって化合物(I)から製造することができる。
また、化合物(I)のプロドラッグは、広川書店1990年刊「医薬品の開発」第7巻分子設計163頁から198頁に記載されているような生理的条件で化合物(I)に変化するものであってもよい。 Compound (I) may be used as a prodrug. A prodrug of compound (I) is a compound that is converted to compound (I) by a reaction with an enzyme, gastric acid, or the like under physiological conditions in vivo, that is, compound (I) that is enzymatically oxidized, reduced, hydrolyzed, etc. A compound that changes to compound (I) by hydrolysis or the like due to gastric acid or the like.
As a prodrug of compound (I), for example,
(1) A compound in which amino of compound (I) is acylated, alkylated or phosphorylated (for example, amino of compound (I) is eicosanoylated, alanylated, pentylaminocarbonylated, (5-methyl-2- Oxo-1,3-dioxolen-4-yl) methoxycarbonylation, tetrahydrofuranylation, pyrrolidylmethylation, pivaloyloxymethylation, tert-butylation, ethoxycarbonylation, tert-butoxycarbonylation, acetylation, Cyclopropylcarbonylated compounds, etc.);
(2) Compound in which hydroxy of compound (I) is acylated, alkylated, phosphorylated, borated (for example, hydroxy of compound (I) is acetylated, palmitoylated, propanoylated, pivaloylated, succinylated , Fumarylated, alanylated, dimethylaminomethylcarbonylated compounds, etc.);
(3) Compound (I) in which carboxy is esterified or amidated (for example, compound (I) in which carboxy is ethyl esterified, phenyl esterified, carboxymethyl esterified, dimethylaminomethyl esterified, Valoyloxymethyl esterification, ethoxycarbonyloxyethyl esterification, phthalidyl esterification, (5-methyl-2-oxo-1,3-dioxolen-4-yl) methyl esterification, cyclohexyloxycarbonylethyl esterification, methylamide Compound etc.);
Etc. These compounds can be produced from compound (I) by a method known per se.
The prodrug of compound (I) changes to compound (I) under physiological conditions as described in Hirokawa Shoten, 1990, “Development of Drugs”, Volume 7, Molecular Design, pages 163 to 198. There may be.
化合物(I)のプロドラッグとしては、例えば、
(1)化合物(I)のアミノがアシル化、アルキル化、りん酸化された化合物(例えば、化合物(I)のアミノが、エイコサノイル化、アラニル化、ペンチルアミノカルボニル化、(5-メチル-2-オキソ-1,3-ジオキソレン-4-イル)メトキシカルボニル化、テトラヒドロフラニル化、ピロリジルメチル化、ピバロイルオキシメチル化、tert-ブチル化、エトキシカルボニル化、tert-ブトキシカルボニル化、アセチル化、シクロプロピルカルボニル化された化合物等);
(2)化合物(I)のヒドロキシが、アシル化、アルキル化、りん酸化、ホウ酸化された化合物(例えば、化合物(I)のヒドロキシが、アセチル化、パルミトイル化、プロパノイル化、ピバロイル化、スクシニル化、フマリル化、アラニル化、ジメチルアミノメチルカルボニル化された化合物等);
(3)化合物(I)のカルボキシが、エステル化、アミド化された化合物(例えば、化合物(I)のカルボキシが、エチルエステル化、フェニルエステル化、カルボキシメチルエステル化、ジメチルアミノメチルエステル化、ピバロイルオキシメチルエステル化、エトキシカルボニルオキシエチルエステル化、フタリジルエステル化、(5-メチル-2-オキソ-1,3-ジオキソレン-4-イル)メチルエステル化、シクロヘキシルオキシカルボニルエチルエステル化、メチルアミド化された化合物等);
等が挙げられる。これらの化合物は、自体公知の方法によって化合物(I)から製造することができる。
また、化合物(I)のプロドラッグは、広川書店1990年刊「医薬品の開発」第7巻分子設計163頁から198頁に記載されているような生理的条件で化合物(I)に変化するものであってもよい。 Compound (I) may be used as a prodrug. A prodrug of compound (I) is a compound that is converted to compound (I) by a reaction with an enzyme, gastric acid, or the like under physiological conditions in vivo, that is, compound (I) that is enzymatically oxidized, reduced, hydrolyzed, etc. A compound that changes to compound (I) by hydrolysis or the like due to gastric acid or the like.
As a prodrug of compound (I), for example,
(1) A compound in which amino of compound (I) is acylated, alkylated or phosphorylated (for example, amino of compound (I) is eicosanoylated, alanylated, pentylaminocarbonylated, (5-methyl-2- Oxo-1,3-dioxolen-4-yl) methoxycarbonylation, tetrahydrofuranylation, pyrrolidylmethylation, pivaloyloxymethylation, tert-butylation, ethoxycarbonylation, tert-butoxycarbonylation, acetylation, Cyclopropylcarbonylated compounds, etc.);
(2) Compound in which hydroxy of compound (I) is acylated, alkylated, phosphorylated, borated (for example, hydroxy of compound (I) is acetylated, palmitoylated, propanoylated, pivaloylated, succinylated , Fumarylated, alanylated, dimethylaminomethylcarbonylated compounds, etc.);
(3) Compound (I) in which carboxy is esterified or amidated (for example, compound (I) in which carboxy is ethyl esterified, phenyl esterified, carboxymethyl esterified, dimethylaminomethyl esterified, Valoyloxymethyl esterification, ethoxycarbonyloxyethyl esterification, phthalidyl esterification, (5-methyl-2-oxo-1,3-dioxolen-4-yl) methyl esterification, cyclohexyloxycarbonylethyl esterification, methylamide Compound etc.);
Etc. These compounds can be produced from compound (I) by a method known per se.
The prodrug of compound (I) changes to compound (I) under physiological conditions as described in Hirokawa Shoten, 1990, “Development of Drugs”, Volume 7, Molecular Design, pages 163 to 198. There may be.
本明細書中、化合物(I)、およびそのプロドラッグを纏めて「本発明化合物」と略記する場合がある。
In the present specification, compound (I) and prodrugs thereof may be collectively abbreviated as “the compound of the present invention”.
化合物(I)は、水和物、非水和物、溶媒和物、無溶媒和物のいずれであってもよい。
同位元素(例、3H、14C、35S、125I等)等で標識された化合物も、化合物(I)に包含される。
さらに、1Hを2H(D)に変換した重水素変換体も、化合物(I)に包含される。
互変異性体も、化合物(I)に包含される。
化合物(I)は、薬学的に許容され得る共結晶または共結晶塩であってもよい。ここで、共結晶または共結晶塩とは、各々が異なる物理的特性(例えば、構造、融点、融解熱、吸湿性、溶解性および安定性等)を持つ、室温で二種またはそれ以上の独特な固体から構成される結晶性物質を意味する。共結晶または共結晶塩は、自体公知の共結晶化法に従い製造することができる。
化合物(I)は、PETトレーサーとして用いてもよい。 Compound (I) may be a hydrate, a non-hydrate, a solvate, or a non-solvate.
Compounds labeled with isotopes (eg, 3 H, 14 C, 35 S, 125 I, etc.) are also encompassed in compound (I).
Further, a deuterium converter obtained by converting 1 H into 2 H (D) is also encompassed in compound (I).
Tautomers are also encompassed in compound (I).
Compound (I) may be a pharmaceutically acceptable cocrystal or cocrystal salt. Here, co-crystals or co-crystal salts are two or more unique at room temperature, each having different physical properties (eg structure, melting point, heat of fusion, hygroscopicity, solubility and stability). It means a crystalline substance composed of a simple solid. The cocrystal or cocrystal salt can be produced according to a cocrystallization method known per se.
Compound (I) may be used as a PET tracer.
同位元素(例、3H、14C、35S、125I等)等で標識された化合物も、化合物(I)に包含される。
さらに、1Hを2H(D)に変換した重水素変換体も、化合物(I)に包含される。
互変異性体も、化合物(I)に包含される。
化合物(I)は、薬学的に許容され得る共結晶または共結晶塩であってもよい。ここで、共結晶または共結晶塩とは、各々が異なる物理的特性(例えば、構造、融点、融解熱、吸湿性、溶解性および安定性等)を持つ、室温で二種またはそれ以上の独特な固体から構成される結晶性物質を意味する。共結晶または共結晶塩は、自体公知の共結晶化法に従い製造することができる。
化合物(I)は、PETトレーサーとして用いてもよい。 Compound (I) may be a hydrate, a non-hydrate, a solvate, or a non-solvate.
Compounds labeled with isotopes (eg, 3 H, 14 C, 35 S, 125 I, etc.) are also encompassed in compound (I).
Further, a deuterium converter obtained by converting 1 H into 2 H (D) is also encompassed in compound (I).
Tautomers are also encompassed in compound (I).
Compound (I) may be a pharmaceutically acceptable cocrystal or cocrystal salt. Here, co-crystals or co-crystal salts are two or more unique at room temperature, each having different physical properties (eg structure, melting point, heat of fusion, hygroscopicity, solubility and stability). It means a crystalline substance composed of a simple solid. The cocrystal or cocrystal salt can be produced according to a cocrystallization method known per se.
Compound (I) may be used as a PET tracer.
本発明化合物は、優れたPKC(特に、PKC-θ)阻害作用を有することから、この作用に基づく安全な医薬としても有用である。
例えば、本発明化合物を含有してなる本発明の医薬は、哺乳動物(例えば、マウス、ラット、ハムスター、ウサギ、ネコ、イヌ、ウシ、ヒツジ、サル、ヒト等)に対して、PKC(特に、PKC-θ)関連疾患、およびT細胞関連疾患の予防または治療剤、より具体的には、以下(1)~(5)に記載の疾患の予防または治療剤として用いることができる。
(1)炎症性疾患(例、急性膵炎、慢性膵炎、喘息、成人呼吸困難症候群、慢性閉塞性肺疾患(COPD)、炎症性骨疾患、炎症性肺疾患、炎症性腸疾患、セリアック病、肝炎、全身性炎症反応症候群(SIRS)、手術または外傷後の炎症、肺炎、腎炎、髄膜炎、膀胱炎、咽喉頭炎、胃粘膜損傷、髄膜炎、脊椎炎、関節炎、皮膚炎、慢性肺炎、気管支炎、肺梗塞、珪肺症、肺サルコイドーシス、糖尿病性腎症、ブドウ膜炎、化膿性汗腺炎、通風等);
(2)免疫性疾患(例、関節リウマチ(rheumatoid arthritis)、乾癬(psoriasis)、炎症性腸疾患(inflammatory bowel disease)(例、クローン病(Crohn's disease)、潰瘍性大腸炎(ulcerative colitis)等)、シェーグレン症候群(Sjogren's syndrome)、ベーチェット病(Behcet's syndrome)、多発性硬化症(multiple sclerosis)、全身性エリテマトーデス(systemic lupus erythematosus)、ループス腎炎(lupus nephritis)、円板状紅斑性狼瘡(Discoid lupus erythematosus)、キャッスルマン病(Castleman's disease)、強直性脊椎炎、多発性筋炎、皮膚筋炎(DM)、結節性多発性動脈炎(PN)、混合性結合性組織症(MCTD)、強皮症、深在性紅斑性狼瘡、慢性甲状腺炎、グレーブス病、自己免疫性胃炎、I型およびII型糖尿病、自己免疫性溶血性貧血、自己免疫性好中球減少症、血小板減少症、アトピー性皮膚炎、慢性活動性肝炎、重症筋無力症、移植片対宿主疾患、アジソン病、異常免疫応答、関節炎、皮膚炎、放射線皮膚炎、原発性胆汁性肝硬変(Primary Biliary Cirrhosis)等);
(3)骨または関節変性疾患(例、関節リウマチ、骨粗鬆症、変形性関節症等);
(4)腫瘍性疾患〔例、悪性腫瘍、血管新生緑内障、幼児性血管腫、多発性骨髄腫(multiple myeloma)、慢性肉腫、転移黒色腫、カポジ肉腫、血管増殖、悪液質(cachexia)、乳癌の転移等、癌(例、大腸癌(例、家族性大腸癌、遺伝性非ポリポーシス大腸癌、消化管間質腫瘍など)、肺癌(例、非小細胞肺癌、小細胞肺癌、悪性中皮腫など)、中皮腫、膵臓癌(例、膵管癌など)、胃癌(例、乳頭腺癌、粘液性腺癌、腺扁平上皮癌など)、乳癌(例、浸潤性乳管癌、非浸潤性乳管癌、炎症性乳癌など)、卵巣癌(例、上皮性卵巣癌、性腺外胚細胞腫瘍、卵巣性胚細胞腫瘍、卵巣低悪性度腫瘍など)、前立腺癌(prostate cancer)(例、ホルモン依存性前立腺癌、ホルモン非依存性前立腺癌など)、肝臓癌(例、原発性肝癌、肝外胆管癌など)、甲状腺癌(例、甲状腺髄様癌など)、腎臓癌(例、腎細胞癌、腎盂と尿管の移行上皮癌など)、子宮癌、脳腫瘍(例、松果体星細胞腫瘍、毛様細胞性星細胞腫、びまん性星細胞腫、退形成性星細胞腫など)、黒色腫(メラノーマ)、肉腫、膀胱癌、多発性骨髄腫を含む血液癌等、下垂体腺腫、神経膠腫、聴神経鞘腫、網膜肉腫、咽頭癌、喉頭癌、舌癌、胸腺腫、食道癌、十二指腸癌、結腸癌、直腸癌、肝細胞癌、膵内分泌腫瘍、胆管癌、胆嚢癌、陰茎癌、尿管癌、精巣腫瘍、外陰癌、子宮頚部癌、子宮体部癌、子宮肉腫、絨毛性疾患、膣癌、皮膚癌、菌状息肉症、基底細胞腫、軟部肉腫、悪性リンパ腫、ホジキン病、骨髄異形成症候群、成人T細胞白血病、慢性骨髄増殖性疾患、膵内分泌腫瘍、線維性組織球腫、平滑筋肉腫、横紋筋肉腫、原発不明癌など)、白血病(leukemia)(例、急性白血病(例、急性リンパ性白血病、急性骨髄性白血病など)、慢性白血病(例、慢性リンパ性白血病、慢性骨髄性白血病など)、骨髄形成症候群など)、子宮肉腫(例、子宮中胚葉性混合腫瘍、子宮平滑筋肉腫(uterine leiomyosarcoma)、子宮内膜間質腫瘍など)、骨髄線維症(myelofibrosis)など〕;
(5)中枢性疾患(例、統合失調症、アルツハイマー病(例、アルツハイマー型認知症))。 Since the compound of the present invention has an excellent PKC (particularly PKC-θ) inhibitory action, it is also useful as a safe pharmaceutical based on this action.
For example, the medicament of the present invention comprising the compound of the present invention is a PKC (in particular, a mouse, rat, hamster, rabbit, cat, dog, cow, sheep, monkey, human etc.). It can be used as a prophylactic or therapeutic agent for PKC-θ) -related diseases and T cell-related diseases, more specifically, as prophylactic or therapeutic agents for the diseases described in (1) to (5) below.
(1) Inflammatory diseases (eg, acute pancreatitis, chronic pancreatitis, asthma, adult respiratory distress syndrome, chronic obstructive pulmonary disease (COPD), inflammatory bone disease, inflammatory lung disease, inflammatory bowel disease, celiac disease, hepatitis Systemic inflammatory response syndrome (SIRS), inflammation after surgery or trauma, pneumonia, nephritis, meningitis, cystitis, sore throat, gastric mucosal damage, meningitis, spondylitis, arthritis, dermatitis, chronic pneumonia , Bronchitis, pulmonary infarction, silicosis, pulmonary sarcoidosis, diabetic nephropathy, uveitis, purulent dysplasia, ventilation, etc.);
(2) Immune disease (eg, rheumatoid arthritis), psoriasis (psoriasis), inflammatory bowel disease (eg, Crohn's disease, ulcerative colitis, etc.) , Sjogren's syndrome, Behcet's syndrome, multiple sclerosis, systemic lupus erythematosus, lupus nephritis, discoid lupus erythematosus ), Castleman's disease, ankylosing spondylitis, polymyositis, dermatomyositis (DM), nodular polyarteritis (PN), mixed connective tissue disease (MCTD), scleroderma, deep Lupus erythematosus, chronic thyroiditis, Graves disease, autoimmune gastritis, type I and type II diabetes, autoimmune hemolytic anemia, autoimmune neutropenia, platelets Adolescent, atopic dermatitis, chronic active hepatitis, myasthenia gravis, graft-versus-host disease, Addison's disease, abnormal immune response, arthritis, dermatitis, radiation dermatitis, primary biliary cirrhosis etc);
(3) bone or joint degenerative diseases (eg, rheumatoid arthritis, osteoporosis, osteoarthritis, etc.);
(4) Neoplastic disease [eg, malignant tumor, neovascular glaucoma, infantile hemangioma, multiple myeloma, chronic sarcoma, metastatic melanoma, Kaposi's sarcoma, vascular proliferation, cachexia, Cancer (eg, colorectal cancer (eg, familial colorectal cancer, hereditary non-polyposis colorectal cancer, gastrointestinal stromal tumor)), lung cancer (eg, non-small cell lung cancer, small cell lung cancer, malignant mesothelioma) ), Mesothelioma, pancreatic cancer (eg, pancreatic duct cancer), stomach cancer (eg, papillary adenocarcinoma, mucinous adenocarcinoma, adenosquamous carcinoma), breast cancer (eg, invasive ductal carcinoma, non-invasive) Breast cancer, inflammatory breast cancer, etc.), ovarian cancer (eg, epithelial ovarian cancer, extragonadal germ cell tumor, ovarian germ cell tumor, ovarian low-grade tumor, etc.), prostate cancer (eg, hormone) Dependent prostate cancer, hormone-independent prostate cancer, etc.), liver cancer (eg, primary liver cancer, extrahepatic bile duct cancer, etc.), thyroid gland (Eg, medullary thyroid cancer), kidney cancer (eg, renal cell carcinoma, transitional cell carcinoma of the renal pelvis and ureter), uterine cancer, brain tumor (eg, pineal gland cell tumor, ciliary cell stellate cell) Tumor, diffuse astrocytoma, anaplastic astrocytoma), melanoma, sarcoma, bladder cancer, blood cancer including multiple myeloma, pituitary adenoma, glioma, acoustic schwannoma, Retinal sarcoma, pharyngeal cancer, laryngeal cancer, tongue cancer, thymoma, esophageal cancer, duodenal cancer, colon cancer, rectal cancer, hepatocellular carcinoma, pancreatic endocrine tumor, bile duct cancer, gallbladder cancer, penile cancer, ureteral cancer, testicular tumor , Vulvar cancer, cervical cancer, uterine body cancer, uterine sarcoma, choriocarcinoma, vaginal cancer, skin cancer, mycosis fungoides, basal cell tumor, soft tissue sarcoma, malignant lymphoma, Hodgkin's disease, myelodysplastic syndrome, adult T cell leukemia, chronic myeloproliferative disorder, pancreatic endocrine tumor, fibrous histiocytoma, leiomyosarcoma, rhabdomyosarcoma, primary unknown Leukemia (eg, acute leukemia (eg, acute lymphocytic leukemia, acute myeloid leukemia)), chronic leukemia (eg, chronic lymphocytic leukemia, chronic myelogenous leukemia), myelogenic syndrome, etc.) Uterine sarcoma (eg, uterine mesoderm mixed tumor, uterine leiomyosarcoma, endometrial stromal tumor, etc.), myelofibrosis, etc.];
(5) Central diseases (eg, schizophrenia, Alzheimer's disease (eg, Alzheimer type dementia)).
例えば、本発明化合物を含有してなる本発明の医薬は、哺乳動物(例えば、マウス、ラット、ハムスター、ウサギ、ネコ、イヌ、ウシ、ヒツジ、サル、ヒト等)に対して、PKC(特に、PKC-θ)関連疾患、およびT細胞関連疾患の予防または治療剤、より具体的には、以下(1)~(5)に記載の疾患の予防または治療剤として用いることができる。
(1)炎症性疾患(例、急性膵炎、慢性膵炎、喘息、成人呼吸困難症候群、慢性閉塞性肺疾患(COPD)、炎症性骨疾患、炎症性肺疾患、炎症性腸疾患、セリアック病、肝炎、全身性炎症反応症候群(SIRS)、手術または外傷後の炎症、肺炎、腎炎、髄膜炎、膀胱炎、咽喉頭炎、胃粘膜損傷、髄膜炎、脊椎炎、関節炎、皮膚炎、慢性肺炎、気管支炎、肺梗塞、珪肺症、肺サルコイドーシス、糖尿病性腎症、ブドウ膜炎、化膿性汗腺炎、通風等);
(2)免疫性疾患(例、関節リウマチ(rheumatoid arthritis)、乾癬(psoriasis)、炎症性腸疾患(inflammatory bowel disease)(例、クローン病(Crohn's disease)、潰瘍性大腸炎(ulcerative colitis)等)、シェーグレン症候群(Sjogren's syndrome)、ベーチェット病(Behcet's syndrome)、多発性硬化症(multiple sclerosis)、全身性エリテマトーデス(systemic lupus erythematosus)、ループス腎炎(lupus nephritis)、円板状紅斑性狼瘡(Discoid lupus erythematosus)、キャッスルマン病(Castleman's disease)、強直性脊椎炎、多発性筋炎、皮膚筋炎(DM)、結節性多発性動脈炎(PN)、混合性結合性組織症(MCTD)、強皮症、深在性紅斑性狼瘡、慢性甲状腺炎、グレーブス病、自己免疫性胃炎、I型およびII型糖尿病、自己免疫性溶血性貧血、自己免疫性好中球減少症、血小板減少症、アトピー性皮膚炎、慢性活動性肝炎、重症筋無力症、移植片対宿主疾患、アジソン病、異常免疫応答、関節炎、皮膚炎、放射線皮膚炎、原発性胆汁性肝硬変(Primary Biliary Cirrhosis)等);
(3)骨または関節変性疾患(例、関節リウマチ、骨粗鬆症、変形性関節症等);
(4)腫瘍性疾患〔例、悪性腫瘍、血管新生緑内障、幼児性血管腫、多発性骨髄腫(multiple myeloma)、慢性肉腫、転移黒色腫、カポジ肉腫、血管増殖、悪液質(cachexia)、乳癌の転移等、癌(例、大腸癌(例、家族性大腸癌、遺伝性非ポリポーシス大腸癌、消化管間質腫瘍など)、肺癌(例、非小細胞肺癌、小細胞肺癌、悪性中皮腫など)、中皮腫、膵臓癌(例、膵管癌など)、胃癌(例、乳頭腺癌、粘液性腺癌、腺扁平上皮癌など)、乳癌(例、浸潤性乳管癌、非浸潤性乳管癌、炎症性乳癌など)、卵巣癌(例、上皮性卵巣癌、性腺外胚細胞腫瘍、卵巣性胚細胞腫瘍、卵巣低悪性度腫瘍など)、前立腺癌(prostate cancer)(例、ホルモン依存性前立腺癌、ホルモン非依存性前立腺癌など)、肝臓癌(例、原発性肝癌、肝外胆管癌など)、甲状腺癌(例、甲状腺髄様癌など)、腎臓癌(例、腎細胞癌、腎盂と尿管の移行上皮癌など)、子宮癌、脳腫瘍(例、松果体星細胞腫瘍、毛様細胞性星細胞腫、びまん性星細胞腫、退形成性星細胞腫など)、黒色腫(メラノーマ)、肉腫、膀胱癌、多発性骨髄腫を含む血液癌等、下垂体腺腫、神経膠腫、聴神経鞘腫、網膜肉腫、咽頭癌、喉頭癌、舌癌、胸腺腫、食道癌、十二指腸癌、結腸癌、直腸癌、肝細胞癌、膵内分泌腫瘍、胆管癌、胆嚢癌、陰茎癌、尿管癌、精巣腫瘍、外陰癌、子宮頚部癌、子宮体部癌、子宮肉腫、絨毛性疾患、膣癌、皮膚癌、菌状息肉症、基底細胞腫、軟部肉腫、悪性リンパ腫、ホジキン病、骨髄異形成症候群、成人T細胞白血病、慢性骨髄増殖性疾患、膵内分泌腫瘍、線維性組織球腫、平滑筋肉腫、横紋筋肉腫、原発不明癌など)、白血病(leukemia)(例、急性白血病(例、急性リンパ性白血病、急性骨髄性白血病など)、慢性白血病(例、慢性リンパ性白血病、慢性骨髄性白血病など)、骨髄形成症候群など)、子宮肉腫(例、子宮中胚葉性混合腫瘍、子宮平滑筋肉腫(uterine leiomyosarcoma)、子宮内膜間質腫瘍など)、骨髄線維症(myelofibrosis)など〕;
(5)中枢性疾患(例、統合失調症、アルツハイマー病(例、アルツハイマー型認知症))。 Since the compound of the present invention has an excellent PKC (particularly PKC-θ) inhibitory action, it is also useful as a safe pharmaceutical based on this action.
For example, the medicament of the present invention comprising the compound of the present invention is a PKC (in particular, a mouse, rat, hamster, rabbit, cat, dog, cow, sheep, monkey, human etc.). It can be used as a prophylactic or therapeutic agent for PKC-θ) -related diseases and T cell-related diseases, more specifically, as prophylactic or therapeutic agents for the diseases described in (1) to (5) below.
(1) Inflammatory diseases (eg, acute pancreatitis, chronic pancreatitis, asthma, adult respiratory distress syndrome, chronic obstructive pulmonary disease (COPD), inflammatory bone disease, inflammatory lung disease, inflammatory bowel disease, celiac disease, hepatitis Systemic inflammatory response syndrome (SIRS), inflammation after surgery or trauma, pneumonia, nephritis, meningitis, cystitis, sore throat, gastric mucosal damage, meningitis, spondylitis, arthritis, dermatitis, chronic pneumonia , Bronchitis, pulmonary infarction, silicosis, pulmonary sarcoidosis, diabetic nephropathy, uveitis, purulent dysplasia, ventilation, etc.);
(2) Immune disease (eg, rheumatoid arthritis), psoriasis (psoriasis), inflammatory bowel disease (eg, Crohn's disease, ulcerative colitis, etc.) , Sjogren's syndrome, Behcet's syndrome, multiple sclerosis, systemic lupus erythematosus, lupus nephritis, discoid lupus erythematosus ), Castleman's disease, ankylosing spondylitis, polymyositis, dermatomyositis (DM), nodular polyarteritis (PN), mixed connective tissue disease (MCTD), scleroderma, deep Lupus erythematosus, chronic thyroiditis, Graves disease, autoimmune gastritis, type I and type II diabetes, autoimmune hemolytic anemia, autoimmune neutropenia, platelets Adolescent, atopic dermatitis, chronic active hepatitis, myasthenia gravis, graft-versus-host disease, Addison's disease, abnormal immune response, arthritis, dermatitis, radiation dermatitis, primary biliary cirrhosis etc);
(3) bone or joint degenerative diseases (eg, rheumatoid arthritis, osteoporosis, osteoarthritis, etc.);
(4) Neoplastic disease [eg, malignant tumor, neovascular glaucoma, infantile hemangioma, multiple myeloma, chronic sarcoma, metastatic melanoma, Kaposi's sarcoma, vascular proliferation, cachexia, Cancer (eg, colorectal cancer (eg, familial colorectal cancer, hereditary non-polyposis colorectal cancer, gastrointestinal stromal tumor)), lung cancer (eg, non-small cell lung cancer, small cell lung cancer, malignant mesothelioma) ), Mesothelioma, pancreatic cancer (eg, pancreatic duct cancer), stomach cancer (eg, papillary adenocarcinoma, mucinous adenocarcinoma, adenosquamous carcinoma), breast cancer (eg, invasive ductal carcinoma, non-invasive) Breast cancer, inflammatory breast cancer, etc.), ovarian cancer (eg, epithelial ovarian cancer, extragonadal germ cell tumor, ovarian germ cell tumor, ovarian low-grade tumor, etc.), prostate cancer (eg, hormone) Dependent prostate cancer, hormone-independent prostate cancer, etc.), liver cancer (eg, primary liver cancer, extrahepatic bile duct cancer, etc.), thyroid gland (Eg, medullary thyroid cancer), kidney cancer (eg, renal cell carcinoma, transitional cell carcinoma of the renal pelvis and ureter), uterine cancer, brain tumor (eg, pineal gland cell tumor, ciliary cell stellate cell) Tumor, diffuse astrocytoma, anaplastic astrocytoma), melanoma, sarcoma, bladder cancer, blood cancer including multiple myeloma, pituitary adenoma, glioma, acoustic schwannoma, Retinal sarcoma, pharyngeal cancer, laryngeal cancer, tongue cancer, thymoma, esophageal cancer, duodenal cancer, colon cancer, rectal cancer, hepatocellular carcinoma, pancreatic endocrine tumor, bile duct cancer, gallbladder cancer, penile cancer, ureteral cancer, testicular tumor , Vulvar cancer, cervical cancer, uterine body cancer, uterine sarcoma, choriocarcinoma, vaginal cancer, skin cancer, mycosis fungoides, basal cell tumor, soft tissue sarcoma, malignant lymphoma, Hodgkin's disease, myelodysplastic syndrome, adult T cell leukemia, chronic myeloproliferative disorder, pancreatic endocrine tumor, fibrous histiocytoma, leiomyosarcoma, rhabdomyosarcoma, primary unknown Leukemia (eg, acute leukemia (eg, acute lymphocytic leukemia, acute myeloid leukemia)), chronic leukemia (eg, chronic lymphocytic leukemia, chronic myelogenous leukemia), myelogenic syndrome, etc.) Uterine sarcoma (eg, uterine mesoderm mixed tumor, uterine leiomyosarcoma, endometrial stromal tumor, etc.), myelofibrosis, etc.];
(5) Central diseases (eg, schizophrenia, Alzheimer's disease (eg, Alzheimer type dementia)).
本発明の医薬は、好ましくは、免疫性疾患、炎症性疾患、骨または関節変性疾患、中枢性疾患または腫瘍性疾患、さらに好ましくは、移植片対宿主病(graft versus host disease)、再生不良性貧血(aplastic anemia)、全身性エリテマトーデス(systemic lupus erythematosus)、ループス腎炎(lupus nephritis)、天疱瘡(pemphigus)、炎症性腸疾患(inflammatory bowel disease)(好ましくは、クローン病(Crohn's disease)または潰瘍性大腸炎(ulcerative colitis))、赤芽球癆(pure red cell aplasia)、重症筋無力症(Myasthenia Gravis)、喘息(asthma)、血管炎(vasculitis)、強直性脊椎炎(spondylarthritis ankylopoietica)、関節リウマチ(rheumatoid arthritis)、乾癬(psoriasis)、シェーグレン症候群(Sjogren's syndrome)、アトピー性皮膚炎(atopic dermatitis)、ベーチェット病(Behcet's syndrome)、多発性硬化症(multiple sclerosis)、アルツハイマー病(Alzheimer's disease)(好ましくは、アルツハイマー型認知症(dementia of Alzheimer type))、成人型呼吸窮迫症候群(adult respiratory distress syndrome)、セリアック病(celiac disease)、キャッスルマン病(Castleman's disease)、白血病(leukemia)、子宮平滑筋肉腫(uterine leiomyosarcoma)、前立腺癌(prostate cancer)、多発性骨髄腫(multiple myeloma)、悪液質(cachexia)、骨髄線維症(myelofibrosis)、ネフローゼ症候群(nephrotic syndrome)、または腎移植(kidney transplant)、肝移植(liver transplant)、心移植(cardiac transplant)、肺移植(lung transplant)、膵移植(pancreas transplant)、小腸移植(small bowel transplant)、造血細胞移植(hematopoietic stem cell transplant)などの移植における拒絶反応の予防または治療剤として用いることができる。
The medicament of the present invention is preferably an immune disease, inflammatory disease, bone or joint degenerative disease, central disease or neoplastic disease, more preferably graft-versus-host disease, aplasticity Anemia (aplastic 、 anemia), systemic lupus erythematosus, lupus nephritis, pemphigus, inflammatory bowel disease (preferably Crohn's disease) or ulcerative Colitis (ulcerative colitis), erythroblast 癆 (pure red cell aplasia), myasthenia Gravis, asthma, vasculitis, ankylosing spondylitis (spondylarthritis ankylopoietica), rheumatoid arthritis (Rheumatoid arthritis), psoriasis (psoriasis), Sjogren's syndrome (Sjogren's syndrome), atopic dermatitis, Behcet's disease (Beh cet's syndrome, multiple sclerosis, Alzheimer's disease (preferably dementia of Alzheimer type), adult respiratory distress syndrome (adult respiratory distress syndrome), celiac disease celiac disease, Castleman's disease, leukemia, uterine leiomyosarcoma, prostate cancer, multiple myeloma, cachexia, bone marrow Myelofibrosis, nephrotic syndrome, kidney transplant, liver transplant, liver transplant, cardiac transplant, lung transplant, pancreas transplant, small intestine Prediction of rejection in transplantation (small bowel transplant), hematopoietic stem cell transplant, etc. Or it can be used as a therapeutic agent.
ここで、上記疾患の「予防」とは、例えば、当該疾患に関連する何らかの因子により、発症の危険性が高いと予想される当該疾患を発症していない患者あるいは発症しているが自覚症状のない患者に対し、本発明の化合物を含む医薬を投与すること、あるいは当該疾患治療後、当該疾患の再発が懸念される患者に対し、本発明の化合物を含む医薬を投与することを意味する。
Here, “prevention” of the disease refers to, for example, a patient who has not developed the disease, which is expected to have a high risk of onset due to some factor related to the disease, or who has developed the subjective symptom. This means that a drug containing the compound of the present invention is administered to a patient who is not, or that a drug containing the compound of the present invention is administered to a patient who is concerned about recurrence of the disease after treatment of the disease.
本発明の医薬は、体内動態(例、血中薬物半減期)に優れ、毒性が低く(例、HERG阻害、CYP阻害、CYP誘導)、薬物相互作用の軽減が認められる。本発明化合物をそのまま、あるいは医薬製剤の製造法で一般的に用いられている自体公知の手段に従って、薬理学的に許容される担体と混合して医薬組成物とし、本発明の医薬として使用することができる。本発明の医薬は、哺乳動物(例えば、ヒト、サル、ウシ、ウマ、ブタ、マウス、ラット、ハムスター、ウサギ、ネコ、イヌ、ヒツジ、ヤギ等)に対して、経口的、または非経口的に安全に投与できる。
本発明の化合物を含有する医薬は、医薬製剤の製造法として自体公知の方法(例、日本薬局方記載の方法等)に従って、本発明化合物を単独で、または本発明化合物と薬理学的に許容される担体とを混合した医薬組成物として使用することができる。本発明の化合物を含有する医薬は、例えば錠剤(糖衣錠、フィルムコーティング錠、舌下錠、口腔内崩壊錠、バッカル錠等を含む)、丸剤、散剤、顆粒剤、カプセル剤(ソフトカプセル剤、マイクロカプセル剤を含む)、トローチ剤、シロップ剤、液剤、乳剤、懸濁剤、放出制御製剤(例、速放性製剤、徐放性製剤、徐放性マイクロカプセル剤)、エアゾール剤、フィルム剤(例、口腔内崩壊フィルム、口腔粘膜貼付フィルム)、注射剤(例、皮下注射剤、静脈内注射剤、筋肉内注射剤、腹腔内注射剤)、点滴剤、経皮吸収型製剤、クリーム剤、軟膏剤、ローション剤、貼付剤、坐剤(例、肛門坐剤、膣坐剤)、ペレット、経鼻剤、経肺剤(例、吸入剤)、点眼剤等として、経口的または非経口的(例、静脈内、筋肉内、皮下、臓器内、鼻腔内、皮内、点眼、脳内、直腸内、膣内、腹腔内、腫瘍内部、腫瘍の近位、病巣等)に安全に投与することができる。 The medicament of the present invention has excellent pharmacokinetics (eg, blood drug half-life), low toxicity (eg, HERG inhibition, CYP inhibition, CYP induction), and reduction in drug interaction is observed. The compound of the present invention is mixed with a pharmacologically acceptable carrier as it is or according to a method known per se generally used in the preparation of pharmaceutical preparations to form a pharmaceutical composition, which is used as the pharmaceutical of the present invention. be able to. The medicament of the present invention is given orally or parenterally to mammals (eg, humans, monkeys, cows, horses, pigs, mice, rats, hamsters, rabbits, cats, dogs, sheep, goats, etc.). Safe to administer.
The medicament containing the compound of the present invention is a pharmacologically acceptable compound of the present compound alone or with the compound of the present invention according to a method known per se as a method for producing a pharmaceutical preparation (eg, a method described in the Japanese Pharmacopoeia). It can be used as a pharmaceutical composition mixed with a carrier to be prepared. Examples of the medicament containing the compound of the present invention include tablets (including sugar-coated tablets, film-coated tablets, sublingual tablets, orally disintegrating tablets, buccal tablets, etc.), pills, powders, granules, capsules (soft capsules, microcapsules). Capsules), lozenges, syrups, solutions, emulsions, suspensions, controlled release formulations (eg, immediate release formulations, sustained release formulations, sustained release microcapsules), aerosols, films ( Eg, orally disintegrating film, oral mucosa adhesive film), injection (eg, subcutaneous injection, intravenous injection, intramuscular injection, intraperitoneal injection), instillation, transdermal preparation, cream, Ointments, lotions, patches, suppositories (eg, rectal suppositories, vaginal suppositories), pellets, nasal agents, pulmonary agents (eg, inhalants), eye drops, etc., orally or parenterally (Eg, intravenous, intramuscular, subcutaneous, internal organ, nasal cavity , Intradermal, instillation, intracerebral, intrarectal, intravaginal, intraperitoneal, tumor interior, proximal tumors, can be safely administered into a lesion, etc.).
本発明の化合物を含有する医薬は、医薬製剤の製造法として自体公知の方法(例、日本薬局方記載の方法等)に従って、本発明化合物を単独で、または本発明化合物と薬理学的に許容される担体とを混合した医薬組成物として使用することができる。本発明の化合物を含有する医薬は、例えば錠剤(糖衣錠、フィルムコーティング錠、舌下錠、口腔内崩壊錠、バッカル錠等を含む)、丸剤、散剤、顆粒剤、カプセル剤(ソフトカプセル剤、マイクロカプセル剤を含む)、トローチ剤、シロップ剤、液剤、乳剤、懸濁剤、放出制御製剤(例、速放性製剤、徐放性製剤、徐放性マイクロカプセル剤)、エアゾール剤、フィルム剤(例、口腔内崩壊フィルム、口腔粘膜貼付フィルム)、注射剤(例、皮下注射剤、静脈内注射剤、筋肉内注射剤、腹腔内注射剤)、点滴剤、経皮吸収型製剤、クリーム剤、軟膏剤、ローション剤、貼付剤、坐剤(例、肛門坐剤、膣坐剤)、ペレット、経鼻剤、経肺剤(例、吸入剤)、点眼剤等として、経口的または非経口的(例、静脈内、筋肉内、皮下、臓器内、鼻腔内、皮内、点眼、脳内、直腸内、膣内、腹腔内、腫瘍内部、腫瘍の近位、病巣等)に安全に投与することができる。 The medicament of the present invention has excellent pharmacokinetics (eg, blood drug half-life), low toxicity (eg, HERG inhibition, CYP inhibition, CYP induction), and reduction in drug interaction is observed. The compound of the present invention is mixed with a pharmacologically acceptable carrier as it is or according to a method known per se generally used in the preparation of pharmaceutical preparations to form a pharmaceutical composition, which is used as the pharmaceutical of the present invention. be able to. The medicament of the present invention is given orally or parenterally to mammals (eg, humans, monkeys, cows, horses, pigs, mice, rats, hamsters, rabbits, cats, dogs, sheep, goats, etc.). Safe to administer.
The medicament containing the compound of the present invention is a pharmacologically acceptable compound of the present compound alone or with the compound of the present invention according to a method known per se as a method for producing a pharmaceutical preparation (eg, a method described in the Japanese Pharmacopoeia). It can be used as a pharmaceutical composition mixed with a carrier to be prepared. Examples of the medicament containing the compound of the present invention include tablets (including sugar-coated tablets, film-coated tablets, sublingual tablets, orally disintegrating tablets, buccal tablets, etc.), pills, powders, granules, capsules (soft capsules, microcapsules). Capsules), lozenges, syrups, solutions, emulsions, suspensions, controlled release formulations (eg, immediate release formulations, sustained release formulations, sustained release microcapsules), aerosols, films ( Eg, orally disintegrating film, oral mucosa adhesive film), injection (eg, subcutaneous injection, intravenous injection, intramuscular injection, intraperitoneal injection), instillation, transdermal preparation, cream, Ointments, lotions, patches, suppositories (eg, rectal suppositories, vaginal suppositories), pellets, nasal agents, pulmonary agents (eg, inhalants), eye drops, etc., orally or parenterally (Eg, intravenous, intramuscular, subcutaneous, internal organ, nasal cavity , Intradermal, instillation, intracerebral, intrarectal, intravaginal, intraperitoneal, tumor interior, proximal tumors, can be safely administered into a lesion, etc.).
本発明化合物の、本発明の医薬中の含有量は、医薬全体の約0.01重量%~約100重量%である。
本発明化合物の投与量は、投与対象、投与ルート、疾患等により異なるが、例えば、移植片対宿主病の患者(体重約60kg)に対し、経口剤として、1日当たり、有効成分(化合物(I)のフリー体)として約0.01mg/kg体重~約500mg/kg体重、好ましくは約0.1mg/kg体重~約50mg/kg体重、さらに好ましくは約1mg/kg体重~30mg/kg体重を、1日1回~数回に分けて投与すればよい。 The content of the compound of the present invention in the medicament of the present invention is about 0.01% to about 100% by weight of the whole medicament.
The dose of the compound of the present invention varies depending on the administration subject, administration route, disease and the like. For example, the active ingredient (compound (I ) Of about 0.01 mg / kg body weight to about 500 mg / kg body weight, preferably about 0.1 mg / kg body weight to about 50 mg / kg body weight, more preferably about 1 mg / kg body weight to 30 mg / kg body weight. It may be administered once to several times a day.
本発明化合物の投与量は、投与対象、投与ルート、疾患等により異なるが、例えば、移植片対宿主病の患者(体重約60kg)に対し、経口剤として、1日当たり、有効成分(化合物(I)のフリー体)として約0.01mg/kg体重~約500mg/kg体重、好ましくは約0.1mg/kg体重~約50mg/kg体重、さらに好ましくは約1mg/kg体重~30mg/kg体重を、1日1回~数回に分けて投与すればよい。 The content of the compound of the present invention in the medicament of the present invention is about 0.01% to about 100% by weight of the whole medicament.
The dose of the compound of the present invention varies depending on the administration subject, administration route, disease and the like. For example, the active ingredient (compound (I ) Of about 0.01 mg / kg body weight to about 500 mg / kg body weight, preferably about 0.1 mg / kg body weight to about 50 mg / kg body weight, more preferably about 1 mg / kg body weight to 30 mg / kg body weight. It may be administered once to several times a day.
本発明の医薬の製造に用いられてもよい薬理学的に許容される担体としては、医薬素材として慣用の各種有機あるいは無機担体物質が挙げられ、例えば、固形製剤における賦形剤、滑沢剤、結合剤および崩壊剤、あるいは液状製剤における溶剤、溶解補助剤、懸濁化剤、等張化剤、緩衝剤および無痛化剤等が挙げられる。更に必要に応じ、通常の防腐剤、抗酸化剤、着色剤、甘味剤、吸着剤、湿潤剤等の添加物を適宜、適量用いることもできる。
Examples of the pharmacologically acceptable carrier that may be used in the production of the medicament of the present invention include various organic or inorganic carrier substances commonly used as pharmaceutical materials. For example, excipients and lubricants in solid preparations , Binders and disintegrants, solvents in liquid preparations, solubilizers, suspending agents, tonicity agents, buffers and soothing agents. If necessary, additives such as conventional preservatives, antioxidants, colorants, sweeteners, adsorbents, wetting agents and the like can be used in appropriate amounts.
賦形剤としては、例えば、乳糖、白糖、D-マンニトール、デンプン、コーンスターチ、結晶セルロース、軽質無水ケイ酸等が挙げられる。
滑沢剤としては、例えば、ステアリン酸マグネシウム、ステアリン酸カルシウム、タルク、コロイドシリカ等が挙げられる。
結合剤としては、例えば、結晶セルロース、白糖、D-マンニトール、デキストリン、ヒドロキシプロピルセルロース、ヒドロキシプロピルメチルセルロース、ポリビニルピロリドン、デンプン、ショ糖、ゼラチン、メチルセルロース、カルボキシメチルセルロースナトリウム等が挙げられる。
崩壊剤としては、例えば、デンプン、カルボキシメチルセルロース、カルボキシメチルセルロースカルシウム、カルボキシメチルスターチナトリウム、L-ヒドロキシプロピルセルロース等が挙げられる。
溶剤としては、例えば、注射用水、アルコール、プロピレングリコール、マクロゴール、ゴマ油、トウモロコシ油、オリーブ油等が挙げられる。
溶解補助剤としては、例えば、ポリエチレングリコール、プロピレングリコール、D-マンニトール、安息香酸ベンジル、エタノール、トリスアミノメタン、コレステロール、トリエタノールアミン、炭酸ナトリウム、クエン酸ナトリウム等が挙げられる。
懸濁化剤としては、例えば、ステアリルトリエタノールアミン、ラウリル硫酸ナトリウム、ラウリルアミノプロピオン酸、レシチン、塩化ベンザルコニウム、塩化ベンゼトニウム、モノステアリン酸グリセリン等の界面活性剤;例えば、ポリビニルアルコール、ポリビニルピロリドン、カルボキシメチルセルロースナトリウム、メチルセルロース、ヒドロキシメチルセルロース、ヒドロキシエチルセルロース、ヒドロキシプロピルセルロース等の親水性高分子等が挙げられる。 Examples of the excipient include lactose, sucrose, D-mannitol, starch, corn starch, crystalline cellulose, light anhydrous silicic acid and the like.
Examples of the lubricant include magnesium stearate, calcium stearate, talc, colloidal silica and the like.
Examples of the binder include crystalline cellulose, sucrose, D-mannitol, dextrin, hydroxypropylcellulose, hydroxypropylmethylcellulose, polyvinylpyrrolidone, starch, sucrose, gelatin, methylcellulose, sodium carboxymethylcellulose and the like.
Examples of the disintegrant include starch, carboxymethyl cellulose, carboxymethyl cellulose calcium, carboxymethyl starch sodium, L-hydroxypropyl cellulose, and the like.
Examples of the solvent include water for injection, alcohol, propylene glycol, macrogol, sesame oil, corn oil, olive oil and the like.
Examples of the solubilizer include polyethylene glycol, propylene glycol, D-mannitol, benzyl benzoate, ethanol, trisaminomethane, cholesterol, triethanolamine, sodium carbonate, sodium citrate and the like.
Examples of the suspending agent include surfactants such as stearyltriethanolamine, sodium lauryl sulfate, laurylaminopropionic acid, lecithin, benzalkonium chloride, benzethonium chloride, and glyceryl monostearate; And hydrophilic polymers such as sodium carboxymethylcellulose, methylcellulose, hydroxymethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, and the like.
滑沢剤としては、例えば、ステアリン酸マグネシウム、ステアリン酸カルシウム、タルク、コロイドシリカ等が挙げられる。
結合剤としては、例えば、結晶セルロース、白糖、D-マンニトール、デキストリン、ヒドロキシプロピルセルロース、ヒドロキシプロピルメチルセルロース、ポリビニルピロリドン、デンプン、ショ糖、ゼラチン、メチルセルロース、カルボキシメチルセルロースナトリウム等が挙げられる。
崩壊剤としては、例えば、デンプン、カルボキシメチルセルロース、カルボキシメチルセルロースカルシウム、カルボキシメチルスターチナトリウム、L-ヒドロキシプロピルセルロース等が挙げられる。
溶剤としては、例えば、注射用水、アルコール、プロピレングリコール、マクロゴール、ゴマ油、トウモロコシ油、オリーブ油等が挙げられる。
溶解補助剤としては、例えば、ポリエチレングリコール、プロピレングリコール、D-マンニトール、安息香酸ベンジル、エタノール、トリスアミノメタン、コレステロール、トリエタノールアミン、炭酸ナトリウム、クエン酸ナトリウム等が挙げられる。
懸濁化剤としては、例えば、ステアリルトリエタノールアミン、ラウリル硫酸ナトリウム、ラウリルアミノプロピオン酸、レシチン、塩化ベンザルコニウム、塩化ベンゼトニウム、モノステアリン酸グリセリン等の界面活性剤;例えば、ポリビニルアルコール、ポリビニルピロリドン、カルボキシメチルセルロースナトリウム、メチルセルロース、ヒドロキシメチルセルロース、ヒドロキシエチルセルロース、ヒドロキシプロピルセルロース等の親水性高分子等が挙げられる。 Examples of the excipient include lactose, sucrose, D-mannitol, starch, corn starch, crystalline cellulose, light anhydrous silicic acid and the like.
Examples of the lubricant include magnesium stearate, calcium stearate, talc, colloidal silica and the like.
Examples of the binder include crystalline cellulose, sucrose, D-mannitol, dextrin, hydroxypropylcellulose, hydroxypropylmethylcellulose, polyvinylpyrrolidone, starch, sucrose, gelatin, methylcellulose, sodium carboxymethylcellulose and the like.
Examples of the disintegrant include starch, carboxymethyl cellulose, carboxymethyl cellulose calcium, carboxymethyl starch sodium, L-hydroxypropyl cellulose, and the like.
Examples of the solvent include water for injection, alcohol, propylene glycol, macrogol, sesame oil, corn oil, olive oil and the like.
Examples of the solubilizer include polyethylene glycol, propylene glycol, D-mannitol, benzyl benzoate, ethanol, trisaminomethane, cholesterol, triethanolamine, sodium carbonate, sodium citrate and the like.
Examples of the suspending agent include surfactants such as stearyltriethanolamine, sodium lauryl sulfate, laurylaminopropionic acid, lecithin, benzalkonium chloride, benzethonium chloride, and glyceryl monostearate; And hydrophilic polymers such as sodium carboxymethylcellulose, methylcellulose, hydroxymethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, and the like.
等張化剤としては、例えば、ブドウ糖、D-ソルビトール、塩化ナトリウム、グリセリン、D-マンニトール等が挙げられる。
緩衝剤としては、例えば、リン酸塩、酢酸塩、炭酸塩、クエン酸塩等の緩衝液等が挙げられる。
無痛化剤としては、例えば、ベンジルアルコール等が挙げられる。
防腐剤としては、例えば、パラオキシ安息香酸エステル類、クロロブタノール、ベンジルアルコール、フェニルエチルアルコール、デヒドロ酢酸、ソルビン酸等が挙げられる。
抗酸化剤としては、例えば、亜硫酸塩、アスコルビン酸、α-トコフェロール等が挙げられる。 Examples of the isotonic agent include glucose, D-sorbitol, sodium chloride, glycerin, D-mannitol and the like.
Examples of the buffer include buffer solutions of phosphate, acetate, carbonate, citrate and the like.
Examples of soothing agents include benzyl alcohol.
Examples of the preservative include p-hydroxybenzoates, chlorobutanol, benzyl alcohol, phenylethyl alcohol, dehydroacetic acid, sorbic acid and the like.
Examples of the antioxidant include sulfite, ascorbic acid, α-tocopherol and the like.
緩衝剤としては、例えば、リン酸塩、酢酸塩、炭酸塩、クエン酸塩等の緩衝液等が挙げられる。
無痛化剤としては、例えば、ベンジルアルコール等が挙げられる。
防腐剤としては、例えば、パラオキシ安息香酸エステル類、クロロブタノール、ベンジルアルコール、フェニルエチルアルコール、デヒドロ酢酸、ソルビン酸等が挙げられる。
抗酸化剤としては、例えば、亜硫酸塩、アスコルビン酸、α-トコフェロール等が挙げられる。 Examples of the isotonic agent include glucose, D-sorbitol, sodium chloride, glycerin, D-mannitol and the like.
Examples of the buffer include buffer solutions of phosphate, acetate, carbonate, citrate and the like.
Examples of soothing agents include benzyl alcohol.
Examples of the preservative include p-hydroxybenzoates, chlorobutanol, benzyl alcohol, phenylethyl alcohol, dehydroacetic acid, sorbic acid and the like.
Examples of the antioxidant include sulfite, ascorbic acid, α-tocopherol and the like.
各種疾患の予防・治療に際し、本発明化合物は、他の薬剤と共に用いることもできる。以下、本発明化合物と他の薬物の併用時に使用する医薬を「本発明の併用剤」と称する。
例えば、本発明化合物は、以下の薬物と併用することができる。
(1)非ステロイド性抗炎症薬(NSAIDs)
(i)Classical NSAIDs
アルコフェナク、アセクロフェナク、スリンダク、トルメチン、エトドラク、フェノプロフェン、チアプロフェン酸、メクロフェナム酸、メロキシカム、テオキシカム、ロルノキシカム、ナブメトン、アセトアミノフェン、フェナセチン、エテンザミド、スルピリン、アンチピリン、ミグレニン、アスピリン、メフェナム酸、フルフェナム酸、ジクロフェナックナトリウム、ロキソプロフェンナトリウム、フェニルブタゾン、インドメタシン、イブプロフェン、ケトプロフェン、ナプロキセン、オキサプロジン、フルルビプロフェン、フェンブフェン、プラノプロフェン、フロクタフェニン、ピロキシカム、エピリゾール、塩酸チアラミド、ザルトプロフェン、メシル酸ガベキサート、メシル酸カモスタット、ウリナスタチン、コルヒチン、プロベネシド、スルフィンピラゾン、ベンズブロマロン、アロプリノール、金チオリンゴ酸ナトリウム、ヒアルロン酸ナトリウム、サリチル酸ナトリウム、塩酸モルヒネ、サリチル酸、アトロピン、スコポラミン、モルヒネ、ペチジン、レボルファノール、オキシモルフォンまたはその塩等。
(ii)シクロオキシゲナーゼ抑制薬(COX-1選択的阻害薬、COX-2選択的阻害薬等)
サリチル酸誘導体(例、セレコキシブ、アスピリン)、エトリコキシブ、バルデコキシブ、ジクロフェナック、インドメタシン、ロキソプロフェン等。
(iii)Nitric oxide遊離型NSAIDs
(iv)JAK阻害薬
トファシチニブ(Tofacitinib)、ルキソリチニブ(Ruxolitinib)等。 In the prevention and treatment of various diseases, the compound of the present invention can be used together with other drugs. Hereinafter, the pharmaceutical used when the compound of the present invention is used in combination with another drug is referred to as “the combination agent of the present invention”.
For example, the compound of the present invention can be used in combination with the following drugs.
(1) Nonsteroidal anti-inflammatory drugs (NSAIDs)
(I) Classic NSAIDs
Arcofenac, aceclofenac, sulindac, tolmetine, etodolac, fenoprofen, thiaprofenic acid, meclofenamic acid, meloxicam, teoxicam, lornoxicam, nabumetone, acetaminophen, phenacetin, ethenamide, sulpyrine, antipyrine, migrenin, aspirin, fefenamic acid, mefenamic acid Diclofenac sodium, loxoprofen sodium, phenylbutazone, indomethacin, ibuprofen, ketoprofen, naproxen, oxaprozin, flurbiprofen, fenbufen, pranoprofen, fructaphenine, piroxicam, epilisol, thiaramide hydrochloride, zaltoprofen, gabexate mesilate, camostat mesylate, Urinastatin, colchicine, Robeneshido, sulfinpyrazone, benzbromarone, allopurinol, sodium gold thiomalate, sodium hyaluronate, sodium salicylate, morphine hydrochloride, salicylic acid, atropine, scopolamine, morphine, pethidine, levorphanol, oxymorphone or a salt thereof.
(Ii) cyclooxygenase inhibitors (COX-1 selective inhibitors, COX-2 selective inhibitors, etc.)
Salicylic acid derivatives (eg, celecoxib, aspirin), etoroxib, valdecoxib, diclofenac, indomethacin, loxoprofen, etc.
(Iii) Nitric oxide free NSAIDs
(Iv) JAK inhibitors Tofacitinib (Tofacitinib), Ruxolitinib (Ruxolitinib) and the like.
例えば、本発明化合物は、以下の薬物と併用することができる。
(1)非ステロイド性抗炎症薬(NSAIDs)
(i)Classical NSAIDs
アルコフェナク、アセクロフェナク、スリンダク、トルメチン、エトドラク、フェノプロフェン、チアプロフェン酸、メクロフェナム酸、メロキシカム、テオキシカム、ロルノキシカム、ナブメトン、アセトアミノフェン、フェナセチン、エテンザミド、スルピリン、アンチピリン、ミグレニン、アスピリン、メフェナム酸、フルフェナム酸、ジクロフェナックナトリウム、ロキソプロフェンナトリウム、フェニルブタゾン、インドメタシン、イブプロフェン、ケトプロフェン、ナプロキセン、オキサプロジン、フルルビプロフェン、フェンブフェン、プラノプロフェン、フロクタフェニン、ピロキシカム、エピリゾール、塩酸チアラミド、ザルトプロフェン、メシル酸ガベキサート、メシル酸カモスタット、ウリナスタチン、コルヒチン、プロベネシド、スルフィンピラゾン、ベンズブロマロン、アロプリノール、金チオリンゴ酸ナトリウム、ヒアルロン酸ナトリウム、サリチル酸ナトリウム、塩酸モルヒネ、サリチル酸、アトロピン、スコポラミン、モルヒネ、ペチジン、レボルファノール、オキシモルフォンまたはその塩等。
(ii)シクロオキシゲナーゼ抑制薬(COX-1選択的阻害薬、COX-2選択的阻害薬等)
サリチル酸誘導体(例、セレコキシブ、アスピリン)、エトリコキシブ、バルデコキシブ、ジクロフェナック、インドメタシン、ロキソプロフェン等。
(iii)Nitric oxide遊離型NSAIDs
(iv)JAK阻害薬
トファシチニブ(Tofacitinib)、ルキソリチニブ(Ruxolitinib)等。 In the prevention and treatment of various diseases, the compound of the present invention can be used together with other drugs. Hereinafter, the pharmaceutical used when the compound of the present invention is used in combination with another drug is referred to as “the combination agent of the present invention”.
For example, the compound of the present invention can be used in combination with the following drugs.
(1) Nonsteroidal anti-inflammatory drugs (NSAIDs)
(I) Classic NSAIDs
Arcofenac, aceclofenac, sulindac, tolmetine, etodolac, fenoprofen, thiaprofenic acid, meclofenamic acid, meloxicam, teoxicam, lornoxicam, nabumetone, acetaminophen, phenacetin, ethenamide, sulpyrine, antipyrine, migrenin, aspirin, fefenamic acid, mefenamic acid Diclofenac sodium, loxoprofen sodium, phenylbutazone, indomethacin, ibuprofen, ketoprofen, naproxen, oxaprozin, flurbiprofen, fenbufen, pranoprofen, fructaphenine, piroxicam, epilisol, thiaramide hydrochloride, zaltoprofen, gabexate mesilate, camostat mesylate, Urinastatin, colchicine, Robeneshido, sulfinpyrazone, benzbromarone, allopurinol, sodium gold thiomalate, sodium hyaluronate, sodium salicylate, morphine hydrochloride, salicylic acid, atropine, scopolamine, morphine, pethidine, levorphanol, oxymorphone or a salt thereof.
(Ii) cyclooxygenase inhibitors (COX-1 selective inhibitors, COX-2 selective inhibitors, etc.)
Salicylic acid derivatives (eg, celecoxib, aspirin), etoroxib, valdecoxib, diclofenac, indomethacin, loxoprofen, etc.
(Iii) Nitric oxide free NSAIDs
(Iv) JAK inhibitors Tofacitinib (Tofacitinib), Ruxolitinib (Ruxolitinib) and the like.
(2)疾患修飾性抗リウマチ薬(DMARDs)
(i)金製剤
Auranofin等。
(ii)ペニシラミン
D-ペニシラミン。
(iii)アミノサルチル酸製剤
スルファサラジン、メサラミン、オルサラジン、バルサラジド。
(iv)抗マラリア薬
クロロキン等。
(v)ピリミジン合成阻害薬
レフルノマイド等。
(vi)プログラフ (2) Disease-modifying anti-rheumatic drugs (DMARDs)
(I) Gold formulation Auranofin et al.
(Ii) Penicillamine D-penicillamine.
(Iii) Amino salicylic acid preparation Sulfasalazine, mesalamine, olsalazine, balsalazide.
(Iv) Antimalarial drug chloroquine and the like.
(V) pyrimidine synthesis inhibitor leflunomide and the like.
(Vi) Prograph
(i)金製剤
Auranofin等。
(ii)ペニシラミン
D-ペニシラミン。
(iii)アミノサルチル酸製剤
スルファサラジン、メサラミン、オルサラジン、バルサラジド。
(iv)抗マラリア薬
クロロキン等。
(v)ピリミジン合成阻害薬
レフルノマイド等。
(vi)プログラフ (2) Disease-modifying anti-rheumatic drugs (DMARDs)
(I) Gold formulation Auranofin et al.
(Ii) Penicillamine D-penicillamine.
(Iii) Amino salicylic acid preparation Sulfasalazine, mesalamine, olsalazine, balsalazide.
(Iv) Antimalarial drug chloroquine and the like.
(V) pyrimidine synthesis inhibitor leflunomide and the like.
(Vi) Prograph
(3)抗サイトカイン薬
(I)タンパク質製剤
(i)TNF阻害薬
エタナーセプト、インフリキシマブ、アダリムマブ、セルトリズマブ ペゴール、ゴリムマブ、PASSTNF-α、可溶性TNF-α受容体、TNF-α結合蛋白、抗TNF-α抗体等。
(ii)インターロイキン-1阻害薬
アナキンラ(インターロイキン-1受容体拮抗薬)、可溶性インターロイキン-1受容体等。
(iii)インターロイキン-6阻害薬
トシリズマブ(抗インターロイキン-6受容体抗体)、抗インターロイキン-6抗体等。
(iv)インターロイキン-10薬
インターロイキン-10等。
(v)インターロイキン-12/23阻害薬
ウステキヌマブ、ブリアキヌマブ(抗インターロイキン-12/23抗体)等。
(II)非タンパク質製剤
(i)MAPK阻害薬
BMS-582949等。
(ii)遺伝子調節薬
NF-κ、NF-κB、IKK-1、IKK-2、AP-1等シグナル伝達に関係する分子の阻害薬等。
(iii)サイトカイン産生抑制薬
イグラチモド、テトミラスト等。
(iv)TNF-α変換酵素阻害薬
(v)インターロイキン-1β変換酵素阻害薬
VX-765等。
(vi)インターロイキン-6拮抗薬
HMPL-004等。
(vii)インターロイキン-8阻害薬
IL-8拮抗薬、CXCR1 & CXCR2拮抗薬、レパレキシン等。
(viii)ケモカイン拮抗薬
CCR9拮抗薬(CCX-282、CCX-025)、MCP-1拮抗薬等。
(ix)インターロイキン-2受容体拮抗薬
デニロイキン、ディフチトックス等。
(x)Therapeutic vaccines
TNF-αワクチン等。
(xi)遺伝子治療薬
インターロイキン-4、インターロイキン-10、可溶性インターロイキン-1受容体、可溶性TNF-α受容体等抗炎症作用を有する遺伝子の発現を亢進させることを目的とした遺伝子治療薬。
(xii)アンチセンス化合物
ISIS-104838等。 (3) Anti-cytokine drug (I) protein preparation (i) TNF inhibitor etanercept, infliximab, adalimumab, certolizumab Pegor, golimumab, PASSTNF-α, soluble TNF-α receptor, TNF-α binding protein, anti-TNF-α antibody etc.
(Ii) Interleukin-1 inhibitor Anakinra (interleukin-1 receptor antagonist), soluble interleukin-1 receptor and the like.
(Iii) Interleukin-6 inhibitor Tocilizumab (anti-interleukin-6 receptor antibody), anti-interleukin-6 antibody and the like.
(Iv) Interleukin-10 drug Interleukin-10 and the like.
(V) Interleukin-12 / 23 inhibitor Ustekinumab, briakinumab (anti-interleukin-12 / 23 antibody) and the like.
(II) Non-protein preparation (i) MAPK inhibitor BMS-582949 and the like.
(Ii) Gene regulators Inhibitors of molecules related to signal transduction such as NF-κ, NF-κB, IKK-1, IKK-2, AP-1.
(Iii) Cytokine production inhibitor iguratimod, tetomilast and the like.
(Iv) TNF-α converting enzyme inhibitor (v) interleukin-1β converting enzyme inhibitor VX-765 and the like.
(Vi) Interleukin-6 antagonist HMPL-004 and the like.
(Vii) Interleukin-8 inhibitor IL-8 antagonist, CXCR1 & CXCR2 antagonist, reparexin and the like.
(Viii) Chemokine antagonist CCR9 antagonist (CCX-282, CCX-025), MCP-1 antagonist and the like.
(Ix) Interleukin-2 receptor antagonist Denileukine, Defuchitox and the like.
(X) Therapeutic vaccines
TNF-α vaccine and the like.
(Xi) Gene therapy drug Gene therapy drug for enhancing expression of genes having anti-inflammatory activity such as interleukin-4, interleukin-10, soluble interleukin-1 receptor, soluble TNF-α receptor .
(Xii) Antisense compound ISIS-104838 and the like.
(I)タンパク質製剤
(i)TNF阻害薬
エタナーセプト、インフリキシマブ、アダリムマブ、セルトリズマブ ペゴール、ゴリムマブ、PASSTNF-α、可溶性TNF-α受容体、TNF-α結合蛋白、抗TNF-α抗体等。
(ii)インターロイキン-1阻害薬
アナキンラ(インターロイキン-1受容体拮抗薬)、可溶性インターロイキン-1受容体等。
(iii)インターロイキン-6阻害薬
トシリズマブ(抗インターロイキン-6受容体抗体)、抗インターロイキン-6抗体等。
(iv)インターロイキン-10薬
インターロイキン-10等。
(v)インターロイキン-12/23阻害薬
ウステキヌマブ、ブリアキヌマブ(抗インターロイキン-12/23抗体)等。
(II)非タンパク質製剤
(i)MAPK阻害薬
BMS-582949等。
(ii)遺伝子調節薬
NF-κ、NF-κB、IKK-1、IKK-2、AP-1等シグナル伝達に関係する分子の阻害薬等。
(iii)サイトカイン産生抑制薬
イグラチモド、テトミラスト等。
(iv)TNF-α変換酵素阻害薬
(v)インターロイキン-1β変換酵素阻害薬
VX-765等。
(vi)インターロイキン-6拮抗薬
HMPL-004等。
(vii)インターロイキン-8阻害薬
IL-8拮抗薬、CXCR1 & CXCR2拮抗薬、レパレキシン等。
(viii)ケモカイン拮抗薬
CCR9拮抗薬(CCX-282、CCX-025)、MCP-1拮抗薬等。
(ix)インターロイキン-2受容体拮抗薬
デニロイキン、ディフチトックス等。
(x)Therapeutic vaccines
TNF-αワクチン等。
(xi)遺伝子治療薬
インターロイキン-4、インターロイキン-10、可溶性インターロイキン-1受容体、可溶性TNF-α受容体等抗炎症作用を有する遺伝子の発現を亢進させることを目的とした遺伝子治療薬。
(xii)アンチセンス化合物
ISIS-104838等。 (3) Anti-cytokine drug (I) protein preparation (i) TNF inhibitor etanercept, infliximab, adalimumab, certolizumab Pegor, golimumab, PASSTNF-α, soluble TNF-α receptor, TNF-α binding protein, anti-TNF-α antibody etc.
(Ii) Interleukin-1 inhibitor Anakinra (interleukin-1 receptor antagonist), soluble interleukin-1 receptor and the like.
(Iii) Interleukin-6 inhibitor Tocilizumab (anti-interleukin-6 receptor antibody), anti-interleukin-6 antibody and the like.
(Iv) Interleukin-10 drug Interleukin-10 and the like.
(V) Interleukin-12 / 23 inhibitor Ustekinumab, briakinumab (anti-interleukin-12 / 23 antibody) and the like.
(II) Non-protein preparation (i) MAPK inhibitor BMS-582949 and the like.
(Ii) Gene regulators Inhibitors of molecules related to signal transduction such as NF-κ, NF-κB, IKK-1, IKK-2, AP-1.
(Iii) Cytokine production inhibitor iguratimod, tetomilast and the like.
(Iv) TNF-α converting enzyme inhibitor (v) interleukin-1β converting enzyme inhibitor VX-765 and the like.
(Vi) Interleukin-6 antagonist HMPL-004 and the like.
(Vii) Interleukin-8 inhibitor IL-8 antagonist, CXCR1 & CXCR2 antagonist, reparexin and the like.
(Viii) Chemokine antagonist CCR9 antagonist (CCX-282, CCX-025), MCP-1 antagonist and the like.
(Ix) Interleukin-2 receptor antagonist Denileukine, Defuchitox and the like.
(X) Therapeutic vaccines
TNF-α vaccine and the like.
(Xi) Gene therapy drug Gene therapy drug for enhancing expression of genes having anti-inflammatory activity such as interleukin-4, interleukin-10, soluble interleukin-1 receptor, soluble TNF-α receptor .
(Xii) Antisense compound ISIS-104838 and the like.
(4)インテグリン阻害薬
ナタリズマブ、ベドリズマブ、AJM300、TRK-170、E-6007等。 (4) Integrin inhibitors Natalizumab, vedolizumab, AJM300, TRK-170, E-6007, etc.
ナタリズマブ、ベドリズマブ、AJM300、TRK-170、E-6007等。 (4) Integrin inhibitors Natalizumab, vedolizumab, AJM300, TRK-170, E-6007, etc.
(5)免疫調節薬(免疫抑制薬)
メトトレキサート、シクロフォスファミド、MX-68、アチプリモド ディハイドロクロライド、BMS-188667、CKD-461、リメクソロン、シクロスポリン、タクロリムス、グスペリムス、シロリムス、エベロリムス、アザチオプリン、抗リンパ血清、乾燥スルホ化免疫グロブリン、エリスロポイエチン、コロニー刺激因子、インターロイキン、インターフェロン等。 (5) Immunomodulatory drugs (immunosuppressive drugs)
Methotrexate, cyclophosphamide, MX-68, atiprimod dihydrochloride, BMS-188667, CKD-461, limexolone, cyclosporine, tacrolimus, gusperimus, sirolimus, everolimus, azathioprine, anti-lymph serum, dry sulfonated immunoglobulin, erythropoi Etine, colony stimulating factor, interleukin, interferon, etc.
メトトレキサート、シクロフォスファミド、MX-68、アチプリモド ディハイドロクロライド、BMS-188667、CKD-461、リメクソロン、シクロスポリン、タクロリムス、グスペリムス、シロリムス、エベロリムス、アザチオプリン、抗リンパ血清、乾燥スルホ化免疫グロブリン、エリスロポイエチン、コロニー刺激因子、インターロイキン、インターフェロン等。 (5) Immunomodulatory drugs (immunosuppressive drugs)
Methotrexate, cyclophosphamide, MX-68, atiprimod dihydrochloride, BMS-188667, CKD-461, limexolone, cyclosporine, tacrolimus, gusperimus, sirolimus, everolimus, azathioprine, anti-lymph serum, dry sulfonated immunoglobulin, erythropoi Etine, colony stimulating factor, interleukin, interferon, etc.
(6)ステロイド薬
デキサメサゾン、ヘキセストロール、メチマゾール、ベタメサゾン、トリアムシノロン、トリアムシノロンアセトニド、フルオシノニド、フルオシノロンアセトニド、プレドニゾロン、メチルプレドニゾロン、酢酸コルチゾン、ヒドロコルチゾン、フルオロメトロン、プロピオン酸ベクロメタゾン、エストリオール、ブデソニド等。 (6) Steroid drugs Dexamethasone, hexestrol, methimazole, betamethasone, triamcinolone, triamcinolone acetonide, fluocinonide, fluocinolone acetonide, prednisolone, methylprednisolone, cortisone acetate, hydrocortisone, fluorometholone, estriol propionate, estriol etc.
デキサメサゾン、ヘキセストロール、メチマゾール、ベタメサゾン、トリアムシノロン、トリアムシノロンアセトニド、フルオシノニド、フルオシノロンアセトニド、プレドニゾロン、メチルプレドニゾロン、酢酸コルチゾン、ヒドロコルチゾン、フルオロメトロン、プロピオン酸ベクロメタゾン、エストリオール、ブデソニド等。 (6) Steroid drugs Dexamethasone, hexestrol, methimazole, betamethasone, triamcinolone, triamcinolone acetonide, fluocinonide, fluocinolone acetonide, prednisolone, methylprednisolone, cortisone acetate, hydrocortisone, fluorometholone, estriol propionate, estriol etc.
(7)アンジオテンシン変換酵素阻害薬
エナラプリル、カプトプリル、ラミプリル、リシノプリル、シラザプリル、ペリンドプリル等。 (7) Angiotensin converting enzyme inhibitor enalapril, captopril, ramipril, lisinopril, cilazapril, perindopril and the like.
エナラプリル、カプトプリル、ラミプリル、リシノプリル、シラザプリル、ペリンドプリル等。 (7) Angiotensin converting enzyme inhibitor enalapril, captopril, ramipril, lisinopril, cilazapril, perindopril and the like.
(8)アンジオテンシンII受容体拮抗薬
カンデサルタン、シレキセチル(TCV-116)、バルサルタン、イルベサルタン、オルメサルタン、エプロサルタン等。 (8) Angiotensin II receptor antagonist candesartan, cilexetil (TCV-116), valsartan, irbesartan, olmesartan, eprosartan and the like.
カンデサルタン、シレキセチル(TCV-116)、バルサルタン、イルベサルタン、オルメサルタン、エプロサルタン等。 (8) Angiotensin II receptor antagonist candesartan, cilexetil (TCV-116), valsartan, irbesartan, olmesartan, eprosartan and the like.
(9)利尿薬
ヒドロクロロチアジド、スピロノラクトン、フロセミド、インダパミド、ベンドロフルアジド、シクロペンチアジド等。 (9) Diuretics Hydrochlorothiazide, spironolactone, furosemide, indapamide, bendrofluazide, cyclopenthiazide and the like.
ヒドロクロロチアジド、スピロノラクトン、フロセミド、インダパミド、ベンドロフルアジド、シクロペンチアジド等。 (9) Diuretics Hydrochlorothiazide, spironolactone, furosemide, indapamide, bendrofluazide, cyclopenthiazide and the like.
(10)強心薬
ジゴキシン、ドブタミン等。 (10) Cardiotonic drugs Digoxin, dobutamine and the like.
ジゴキシン、ドブタミン等。 (10) Cardiotonic drugs Digoxin, dobutamine and the like.
(11)β受容体拮抗薬
カルベジロール、メトプロロール、アテノロール等。 (11) β receptor antagonist carvedilol, metoprolol, atenolol and the like.
カルベジロール、メトプロロール、アテノロール等。 (11) β receptor antagonist carvedilol, metoprolol, atenolol and the like.
(12)Ca感受性増強薬
MCC-135等。 (12) Ca sensitivity enhancer MCC-135 and the like.
MCC-135等。 (12) Ca sensitivity enhancer MCC-135 and the like.
(13)Caチャネル拮抗薬
ニフェジピン、ジルチアゼム、ベラパミル等。 (13) Ca channel antagonist nifedipine, diltiazem, verapamil and the like.
ニフェジピン、ジルチアゼム、ベラパミル等。 (13) Ca channel antagonist nifedipine, diltiazem, verapamil and the like.
(14)抗血小板薬、抗凝固薬
ヘパリン、アスピリン、ワルファリン等。 (14) Antiplatelet drugs, anticoagulants heparin, aspirin, warfarin and the like.
ヘパリン、アスピリン、ワルファリン等。 (14) Antiplatelet drugs, anticoagulants heparin, aspirin, warfarin and the like.
(15)HMG-CoA還元酵素阻害薬
アトロバスタチン、シンバスタチン等。 (15) HMG-CoA reductase inhibitor atorvastatin, simvastatin and the like.
アトロバスタチン、シンバスタチン等。 (15) HMG-CoA reductase inhibitor atorvastatin, simvastatin and the like.
(16)避妊薬
(i)性ホルモンまたはその誘導体
黄体ホルモンまたはその誘導体(プロゲステロン、17α-ヒドロキシプロゲステロン、メドロキシプロゲステロン、酢酸メドロキシプロゲステロン、ノルエチステロン、ノルエチステロンエナンタート、ノルエチンドロン、酢酸ノルエチンドロン、ノルエチノドレル、レボノルゲストレル、ノルゲストレル、二酢酸エチノジオール、デソゲストレル、ノルゲスチメート、ゲストデン、プロゲスチン、エトノゲストレル、ドロスピレノン、ジエノゲスト、トリメゲストン、ネストロン、酢酸クロマジノン、ミフェプリストン、酢酸ノメゲストロル、Org-30659、TX-525、EMM-310525)あるいは黄体ホルモンまたはその誘導体と卵胞ホルモンまたはその誘導体(エストラジオール、安息香酸エストラジオール、エストラジオールシピオネート、エストラジオールジプロピオナート、エストラジオールエナンタート、エストラジオールヘキサヒドロベンゾアート、エストラジオールフェニルプロピオナート、エストラジオールウンデカノアート、吉草酸エストラジオール、エストロン、エチニルエストラジオール、メストラノール)との合剤等。
(ii)抗卵胞ホルモン薬
オルメロキシフェン、ミフェプリストン、Org-33628等。
(iii)殺精子薬
ウシェルセル等。 (16) Contraceptives (i) Sex hormones or derivatives thereof Progesterone or derivatives thereof (progesterone, 17α-hydroxyprogesterone, medroxyprogesterone, medroxyprogesterone acetate, norethisterone, norethisterone enanthate, norethindrone, norethindrone acetate, norethinodrel, levonorgestrel , Norgestrel, etinodiol diacetate, desogestrel, norgestimate, guestden, progestin, etonogestrel, drospirenone, dienogest, trimegestone, nestron, chromadianone acetate, mifepristone, nomegestrol acetate, Org-30659, TX-525, EMM-310525) or Progesterone or its derivative and follicular hormone or its derivative (Ladiol, estradiol benzoate, estradiol cypionate, estradiol dipropionate, estradiol enanthate, estradiol hexahydrobenzoate, estradiol phenylpropionate, estradiol undecanoate, estradiol valerate, estrone, ethinyl estradiol, mestranol) Agents etc.
(Ii) Anti-follicular hormone drugs Olmeroxifene, mifepristone, Org-33628 and the like.
(Iii) Spermicide Uchel cell and the like.
(i)性ホルモンまたはその誘導体
黄体ホルモンまたはその誘導体(プロゲステロン、17α-ヒドロキシプロゲステロン、メドロキシプロゲステロン、酢酸メドロキシプロゲステロン、ノルエチステロン、ノルエチステロンエナンタート、ノルエチンドロン、酢酸ノルエチンドロン、ノルエチノドレル、レボノルゲストレル、ノルゲストレル、二酢酸エチノジオール、デソゲストレル、ノルゲスチメート、ゲストデン、プロゲスチン、エトノゲストレル、ドロスピレノン、ジエノゲスト、トリメゲストン、ネストロン、酢酸クロマジノン、ミフェプリストン、酢酸ノメゲストロル、Org-30659、TX-525、EMM-310525)あるいは黄体ホルモンまたはその誘導体と卵胞ホルモンまたはその誘導体(エストラジオール、安息香酸エストラジオール、エストラジオールシピオネート、エストラジオールジプロピオナート、エストラジオールエナンタート、エストラジオールヘキサヒドロベンゾアート、エストラジオールフェニルプロピオナート、エストラジオールウンデカノアート、吉草酸エストラジオール、エストロン、エチニルエストラジオール、メストラノール)との合剤等。
(ii)抗卵胞ホルモン薬
オルメロキシフェン、ミフェプリストン、Org-33628等。
(iii)殺精子薬
ウシェルセル等。 (16) Contraceptives (i) Sex hormones or derivatives thereof Progesterone or derivatives thereof (progesterone, 17α-hydroxyprogesterone, medroxyprogesterone, medroxyprogesterone acetate, norethisterone, norethisterone enanthate, norethindrone, norethindrone acetate, norethinodrel, levonorgestrel , Norgestrel, etinodiol diacetate, desogestrel, norgestimate, guestden, progestin, etonogestrel, drospirenone, dienogest, trimegestone, nestron, chromadianone acetate, mifepristone, nomegestrol acetate, Org-30659, TX-525, EMM-310525) or Progesterone or its derivative and follicular hormone or its derivative (Ladiol, estradiol benzoate, estradiol cypionate, estradiol dipropionate, estradiol enanthate, estradiol hexahydrobenzoate, estradiol phenylpropionate, estradiol undecanoate, estradiol valerate, estrone, ethinyl estradiol, mestranol) Agents etc.
(Ii) Anti-follicular hormone drugs Olmeroxifene, mifepristone, Org-33628 and the like.
(Iii) Spermicide Uchel cell and the like.
(17)その他
(i)T細胞阻害薬
(ii)イノシン一リン酸脱水素酵素(IMPDH)阻害薬
マイコフェノレート モフェチル等。
(iii)接着分子阻害薬
ISIS-2302、セレクチン阻害薬、ELAM-1、VCAM-1、ICAM-1等。
(iv)サリドマイド
(v)カテプシン阻害薬
(vi)マトリックスメタロプロテアーゼ(MMPs)阻害薬
V-85546等。
(vii)グルコース-6-リン酸脱水素酵素阻害薬
(viii)Dihydroorotate脱水素酵素(DHODH)阻害薬
(ix)ホスホジエステラーゼIV(PDEIV)阻害薬
ロフルミラスト、CG-1088等。
(x)ホスホリパーゼA2阻害薬
(xi)iNOS阻害薬
VAS-203等。
(xii)Microtuble刺激薬
パクリタキセル等。
(xiii)Microtuble阻害薬
リューマコン等。
(xiv)MHCクラスII拮抗薬
(xv)Prostacyclin作働薬
イロプロスト等。
(xvi)CD4拮抗薬
ザノリムマブ等。
(xvii)CD23拮抗薬
(xviii)LTB4受容体拮抗薬
DW-1305等。
(xix)5-リポキシゲナーゼ阻害薬
ジリュートン等。
(xx)コリンエステラーゼ阻害薬
ガランタミン等。
(xxi)チロシンキナーゼ阻害薬
Tyk2阻害薬(WO2010142752)等。
(xxii)カテプシンB阻害薬
(xxiii)Adenosine deaminase阻害薬
ペントスタチン等。
(xxiv)骨形成刺激薬
(xxv)ジペプチジルペプチダーゼ阻害薬
(xxvi)コラーゲン作働薬
(xxvii)Capsaicinクリーム
(xxviii)ヒアルロン酸誘導体
シンビスク(hylan G-F 20)、オルソビスク等。
(xxix)硫酸グルコサミン
(xxx)アミプリローゼ
(xxxi)CD-20阻害薬
リツキシマブ、イブリツモマブ、トシツモマブ、オファツマブ等。
(xxxii)BAFF阻害薬
ベリムマブ、タバルマブ、アタシセプト、A-623等。
(xxxiii)CD52阻害薬
アレムツズマブ等。
(xxxiv)IL-17阻害薬
セクキヌマブ(AIN-457)、LY-2439821、AMG827等。
(xxxv)PDE4阻害薬
Roflumilast、Apremilast。
(xxxvi)ヒトリンパ球・胸腺細胞抗体
抗ヒト胸腺細胞ウサギ免疫グロブリン、抗ヒト胸腺細胞ウマ免疫グロブリン等。
(xxxvii)抗CD3抗体
visilizumab等。
(xxxviii)抗CD25抗体
バシリキシマブ、daclizumab等。
(xxxix)CTLA-4-Ig
アバタセプト、ベラタセプト等。 (17) Others (i) T cell inhibitor (ii) Inosine monophosphate dehydrogenase (IMPDH) inhibitor Mycophenolate mofetil and the like.
(Iii) Adhesion molecule inhibitor ISIS-2302, selectin inhibitor, ELAM-1, VCAM-1, ICAM-1, etc.
(Iv) thalidomide (v) cathepsin inhibitor (vi) matrix metalloprotease (MMPs) inhibitor V-85546 and the like.
(Vii) Glucose-6-phosphate dehydrogenase inhibitor (viii) Dihydrorotate dehydrogenase (DHODH) inhibitor (ix) Phosphodiesterase IV (PDEIV) inhibitor Roflumilast, CG-1088 and the like.
(X) Phospholipase A2 inhibitor (xi) iNOS inhibitor VAS-203 and the like.
(Xii) Microtubule stimulant paclitaxel and the like.
(Xiii) Microtubule inhibitor Rheumacon and the like.
(Xiv) MHC class II antagonist (xv) Prostacyclin agonist iloprost and the like.
(Xvi) CD4 antagonist zanolimumab and the like.
(Xvii) CD23 antagonist (xviii) LTB4 receptor antagonist DW-1305 and the like.
(Xix) 5-lipoxygenase inhibitor zileuton and the like.
(Xx) Cholinesterase inhibitor galantamine and the like.
(Xxi) tyrosine kinase inhibitor Tyk2 inhibitor (WO20101422752) and the like.
(Xxii) Cathepsin B inhibitor (xxiii) Adenosine deaminase inhibitor Pentostatin and the like.
(Xxiv) Osteogenic stimulant (xxv) Dipeptidyl peptidase inhibitor (xxvi) Collagen agonist (xxvii) Capsaicin cream (xxviii) Hyaluronic acid derivative Synbisque (hylan GF 20), Orthobisque and the like.
(Xxix) Glucosamine sulfate (xxx) Amiprirose (xxxi) CD-20 inhibitor Rituximab, ibritumomab, tositumomab, ofatumuma and the like.
(Xxxii) BAFF inhibitor belimumab, tabalumab, atacicept, A-623 and the like.
(Xxxiii) CD52 inhibitor alemtuzumab and the like.
(Xxxiv) IL-17 inhibitor secukinumab (AIN-457), LY-2439821, AMG827 and the like.
(Xxxv) PDE4 inhibitor Roflumilast, Apremilast.
(Xxxvi) Human lymphocyte / thymocyte antibody Anti-human thymocyte rabbit immunoglobulin, anti-human thymocyte equine immunoglobulin and the like.
(Xxxvii) anti-CD3 antibody visilizumab and the like.
(Xxxviii) anti-CD25 antibody basiliximab, daclizumab and the like.
(Xxxix) CTLA-4-Ig
Abatacept, Beratacept, etc.
(i)T細胞阻害薬
(ii)イノシン一リン酸脱水素酵素(IMPDH)阻害薬
マイコフェノレート モフェチル等。
(iii)接着分子阻害薬
ISIS-2302、セレクチン阻害薬、ELAM-1、VCAM-1、ICAM-1等。
(iv)サリドマイド
(v)カテプシン阻害薬
(vi)マトリックスメタロプロテアーゼ(MMPs)阻害薬
V-85546等。
(vii)グルコース-6-リン酸脱水素酵素阻害薬
(viii)Dihydroorotate脱水素酵素(DHODH)阻害薬
(ix)ホスホジエステラーゼIV(PDEIV)阻害薬
ロフルミラスト、CG-1088等。
(x)ホスホリパーゼA2阻害薬
(xi)iNOS阻害薬
VAS-203等。
(xii)Microtuble刺激薬
パクリタキセル等。
(xiii)Microtuble阻害薬
リューマコン等。
(xiv)MHCクラスII拮抗薬
(xv)Prostacyclin作働薬
イロプロスト等。
(xvi)CD4拮抗薬
ザノリムマブ等。
(xvii)CD23拮抗薬
(xviii)LTB4受容体拮抗薬
DW-1305等。
(xix)5-リポキシゲナーゼ阻害薬
ジリュートン等。
(xx)コリンエステラーゼ阻害薬
ガランタミン等。
(xxi)チロシンキナーゼ阻害薬
Tyk2阻害薬(WO2010142752)等。
(xxii)カテプシンB阻害薬
(xxiii)Adenosine deaminase阻害薬
ペントスタチン等。
(xxiv)骨形成刺激薬
(xxv)ジペプチジルペプチダーゼ阻害薬
(xxvi)コラーゲン作働薬
(xxvii)Capsaicinクリーム
(xxviii)ヒアルロン酸誘導体
シンビスク(hylan G-F 20)、オルソビスク等。
(xxix)硫酸グルコサミン
(xxx)アミプリローゼ
(xxxi)CD-20阻害薬
リツキシマブ、イブリツモマブ、トシツモマブ、オファツマブ等。
(xxxii)BAFF阻害薬
ベリムマブ、タバルマブ、アタシセプト、A-623等。
(xxxiii)CD52阻害薬
アレムツズマブ等。
(xxxiv)IL-17阻害薬
セクキヌマブ(AIN-457)、LY-2439821、AMG827等。
(xxxv)PDE4阻害薬
Roflumilast、Apremilast。
(xxxvi)ヒトリンパ球・胸腺細胞抗体
抗ヒト胸腺細胞ウサギ免疫グロブリン、抗ヒト胸腺細胞ウマ免疫グロブリン等。
(xxxvii)抗CD3抗体
visilizumab等。
(xxxviii)抗CD25抗体
バシリキシマブ、daclizumab等。
(xxxix)CTLA-4-Ig
アバタセプト、ベラタセプト等。 (17) Others (i) T cell inhibitor (ii) Inosine monophosphate dehydrogenase (IMPDH) inhibitor Mycophenolate mofetil and the like.
(Iii) Adhesion molecule inhibitor ISIS-2302, selectin inhibitor, ELAM-1, VCAM-1, ICAM-1, etc.
(Iv) thalidomide (v) cathepsin inhibitor (vi) matrix metalloprotease (MMPs) inhibitor V-85546 and the like.
(Vii) Glucose-6-phosphate dehydrogenase inhibitor (viii) Dihydrorotate dehydrogenase (DHODH) inhibitor (ix) Phosphodiesterase IV (PDEIV) inhibitor Roflumilast, CG-1088 and the like.
(X) Phospholipase A2 inhibitor (xi) iNOS inhibitor VAS-203 and the like.
(Xii) Microtubule stimulant paclitaxel and the like.
(Xiii) Microtubule inhibitor Rheumacon and the like.
(Xiv) MHC class II antagonist (xv) Prostacyclin agonist iloprost and the like.
(Xvi) CD4 antagonist zanolimumab and the like.
(Xvii) CD23 antagonist (xviii) LTB4 receptor antagonist DW-1305 and the like.
(Xix) 5-lipoxygenase inhibitor zileuton and the like.
(Xx) Cholinesterase inhibitor galantamine and the like.
(Xxi) tyrosine kinase inhibitor Tyk2 inhibitor (WO20101422752) and the like.
(Xxii) Cathepsin B inhibitor (xxiii) Adenosine deaminase inhibitor Pentostatin and the like.
(Xxiv) Osteogenic stimulant (xxv) Dipeptidyl peptidase inhibitor (xxvi) Collagen agonist (xxvii) Capsaicin cream (xxviii) Hyaluronic acid derivative Synbisque (hylan GF 20), Orthobisque and the like.
(Xxix) Glucosamine sulfate (xxx) Amiprirose (xxxi) CD-20 inhibitor Rituximab, ibritumomab, tositumomab, ofatumuma and the like.
(Xxxii) BAFF inhibitor belimumab, tabalumab, atacicept, A-623 and the like.
(Xxxiii) CD52 inhibitor alemtuzumab and the like.
(Xxxiv) IL-17 inhibitor secukinumab (AIN-457), LY-2439821, AMG827 and the like.
(Xxxv) PDE4 inhibitor Roflumilast, Apremilast.
(Xxxvi) Human lymphocyte / thymocyte antibody Anti-human thymocyte rabbit immunoglobulin, anti-human thymocyte equine immunoglobulin and the like.
(Xxxvii) anti-CD3 antibody visilizumab and the like.
(Xxxviii) anti-CD25 antibody basiliximab, daclizumab and the like.
(Xxxix) CTLA-4-Ig
Abatacept, Beratacept, etc.
上記以外の併用薬物としては、例えば、抗菌薬、抗真菌薬、抗原虫薬、抗生物質、鎮咳・去たん薬、鎮静薬、麻酔薬、抗潰瘍薬、不整脈治療薬、降圧利尿薬、抗凝血薬、精神安定薬、抗精神病薬、抗腫瘍薬、抗高脂血症薬、筋弛緩薬、抗てんかん薬、抗うつ薬、抗アレルギー薬、強心薬、不整脈治療薬、血管拡張薬、血管収縮薬、降圧利尿薬、糖尿病治療薬、麻薬拮抗薬、ビタミン薬、ビタミン誘導体、抗喘息薬、頻尿・尿失禁治療薬、止痒薬、アトピー性皮膚炎治療薬、アレルギー性鼻炎治療薬、昇圧薬、エンドトキシン拮抗薬あるいは抗体、シグナル伝達阻害薬、炎症性メディエーター作用抑制薬、炎症性メディエーター作用抑制抗体、抗炎症性メディエーター作用抑制薬、抗炎症性メディエーター作用抑制抗体等が挙げられる。具体的には、以下のものが挙げられる。
Examples of concomitant drugs other than the above include, for example, antibacterial drugs, antifungal drugs, antiprotozoal drugs, antibiotics, antitussives and expectorants, sedatives, anesthetics, antiulcer drugs, antiarrhythmic drugs, antihypertensive diuretics, anticoagulants Drugs, tranquilizers, antipsychotics, antitumor drugs, antihyperlipidemic drugs, muscle relaxants, antiepileptic drugs, antidepressants, antiallergic drugs, cardiotonic drugs, antiarrhythmic drugs, vasodilators, vasoconstriction Drugs, antihypertensive diuretics, antidiabetics, narcotic antagonists, vitamins, vitamin derivatives, anti-asthma, frequent urinary / urinary incontinence, antidiarrheal, atopic dermatitis, allergic rhinitis, pressurization Examples include drugs, endotoxin antagonists or antibodies, signal transduction inhibitors, inflammatory mediator action inhibitors, inflammatory mediator action inhibitory antibodies, anti-inflammatory mediator action inhibitory drugs, anti-inflammatory mediator action inhibitory antibodies, and the like. Specific examples include the following.
(1)抗菌薬
(i)サルファ剤
スルファメチゾール、スルフィソキサゾール、スルファモノメトキシン、スルファメチゾール、サラゾスルファピリジン、スルファジアジン銀等。
(ii)キノリン系抗菌薬
ナリジクス酸、ピペミド酸三水和物、エノキサシン、ノルフロキサシン、オフロキサシン、トシル酸トスフロキサシン、塩酸シプロフロキサシン、塩酸ロメフロキサシン、スパルフロキサシン、フレロキサシン等。
(iii)抗結核薬
イソニアジド、エタンブトール(塩酸エタンブトール)、パラアミノサリチル酸(パラアミノサリチル酸カルシウム)、ピラジナミド、エチオナミド、プロチオナミド、リファンピシン、硫酸ストレプトマイシン、硫酸カナマイシン、サイクロセリン等。
(iv)抗酸菌薬
ジアフェニルスルホン、リファンピシリン等。
(v)抗ウイルス薬
イドクスウリジン、アシクロビル、ビタラビン、ガンシクロビル、ホスカルネット等。
(vi)抗HIV薬
ジドブジン、ジダノシン、ザルシタビン、硫酸インジナビルエタノール付加物、リトナビル等。
(vii)抗スピロヘータ薬
(viii)抗生物質
塩酸テトラサイクリン、アンピシリン、ピペラシリン、ゲンタマイシン、ジベカシン、カネンドマイシン、リビドマイシン、トブラマイシン、アミカシン、フラジオマイシン、シソマイシン、テトラサイクリン、オキシテトラサイクリン、ロリテトラサイクリン、ドキシサイクリン、アンピシリン、ピペラシリン、チカルシリン、セファロチン、セファピリン、セファロリジン、セファクロル、セファレキシン、セフロキサジン、セファドロキシル、セファマンドール、セフォトアム、セフロキシム、セフォチアム、セフォチアムヘキセチル、セフロキシムアキセチル、セフジニル、セフジトレンピボキシル、セフタジジム、セフピラミド、セフスロジン、セフメノキシム、セフポドキシムプロキセチル、セフピロム、セファゾプラン、セフェピム、セフスロジン、セフメノキシム、セフメタゾール、セフミノクス、セフォキシチン、セフブペラゾン、ラタモキナセフ、フロモキセフ、セファゾリン、セフォタキシム、セフォペラゾン、セフチゾキシム、モキサラクタム、チエナマイシン、スルファゼシン、アズスレオナムまたはそれらの塩、グリセオフルビン、ランカシジン類〔ジャーナル・オブ・アンチバイオティックス(J.Antibiotics),38,877-885(1985)〕、アゾール系化合物〔2-〔(1R,2R)-2-(2,4-ジフルオロフェニル)-2-ヒドロキシ-1-メチル-3-(1H-1,2,4-トリアゾール-1-イル)プロピル〕-4-〔4-(2,2,3,3-テトラフルオロプロポキシ)フェニル〕-3-(2H,4H)-1,2,4-トリアゾロン、フルコナゾール、イトラコナゾール、イミペネム、メロペネム、トリメトプリム、スルファメトキサゾール等〕等。 (1) Antibacterial drugs (i) Sulfa drugs Sulfamethizol, sulfisoxazole, sulfamonomethoxine, sulfamethizol, salazosulfapyridine, sulfadiazine silver and the like.
(Ii) Quinoline antibacterial agents Nalidixic acid, pipemidic acid trihydrate, enoxacin, norfloxacin, ofloxacin, tosufloxacin tosylate, ciprofloxacin hydrochloride, lomefloxacin hydrochloride, sparfloxacin, fleroxacin and the like.
(Iii) Antituberculosis drugs Isoniazid, ethambutol (ethambutol hydrochloride), paraaminosalicylic acid (calcium paraaminosalicylate), pyrazinamide, etionamide, prothionamide, rifampicin, streptomycin sulfate, kanamycin sulfate, cycloserine and the like.
(Iv) Mycobacterial drugs Diaphenylsulfone, rifampicillin and the like.
(V) Antiviral drugs idoxuridine, acyclovir, vitarabine, ganciclovir, foscarnet and the like.
(Vi) Anti-HIV drugs zidovudine, didanosine, zarcitabine, indinavir sulfate ethanol adduct, ritonavir and the like.
(Vii) Antispirocheta drugs (viii) Antibiotics Tetracycline hydrochloride, ampicillin, piperacillin, gentamicin, dibekacin, cannendomycin, libidomycin, tobramycin, amikacin, fradiomycin, sisomycin, tetracycline, oxytetracycline, loritetracycline, doxycycline, doxycycline, doxycycline Piperacillin, ticarcillin, cephalothin, cefapirin, cephaloridine, cefaclor, cephalexin, cefloxazine, cefadroxyl, cefamandol, cephoam, cefuroxime, cefothium, cefothium hexetyl, cefuroxime xetilyl, cefdinir ceftefirfide , Cefmenoxime, cefpodoxime Proxetyl, cefpirom, cefazoplan, cefepime, cefsulosin, cefmenoxime, cefmetazole, cefminox, cefoxitin, cefbuperazone, latamoquinacef, flomoxef, cefazoline, cefotaxime, cefoperamine, ceftizoxime, Of Antibiotics (J. Antibiotics), 38,877-885 (1985)], azole compounds [2-[(1R, 2R) -2- (2,4-difluorophenyl) -2-hydroxy- 1-methyl-3- (1H-1,2,4-triazol-1-yl) propyl] -4- [4- (2,2,3,3-tetrafluoro Propoxy) phenyl] -3- (2H, 4H) -1,2,4- triazolone, fluconazole, itraconazole, imipenem, meropenem, trimethoprim, sulfamethoxazole, etc.] and the like.
(i)サルファ剤
スルファメチゾール、スルフィソキサゾール、スルファモノメトキシン、スルファメチゾール、サラゾスルファピリジン、スルファジアジン銀等。
(ii)キノリン系抗菌薬
ナリジクス酸、ピペミド酸三水和物、エノキサシン、ノルフロキサシン、オフロキサシン、トシル酸トスフロキサシン、塩酸シプロフロキサシン、塩酸ロメフロキサシン、スパルフロキサシン、フレロキサシン等。
(iii)抗結核薬
イソニアジド、エタンブトール(塩酸エタンブトール)、パラアミノサリチル酸(パラアミノサリチル酸カルシウム)、ピラジナミド、エチオナミド、プロチオナミド、リファンピシン、硫酸ストレプトマイシン、硫酸カナマイシン、サイクロセリン等。
(iv)抗酸菌薬
ジアフェニルスルホン、リファンピシリン等。
(v)抗ウイルス薬
イドクスウリジン、アシクロビル、ビタラビン、ガンシクロビル、ホスカルネット等。
(vi)抗HIV薬
ジドブジン、ジダノシン、ザルシタビン、硫酸インジナビルエタノール付加物、リトナビル等。
(vii)抗スピロヘータ薬
(viii)抗生物質
塩酸テトラサイクリン、アンピシリン、ピペラシリン、ゲンタマイシン、ジベカシン、カネンドマイシン、リビドマイシン、トブラマイシン、アミカシン、フラジオマイシン、シソマイシン、テトラサイクリン、オキシテトラサイクリン、ロリテトラサイクリン、ドキシサイクリン、アンピシリン、ピペラシリン、チカルシリン、セファロチン、セファピリン、セファロリジン、セファクロル、セファレキシン、セフロキサジン、セファドロキシル、セファマンドール、セフォトアム、セフロキシム、セフォチアム、セフォチアムヘキセチル、セフロキシムアキセチル、セフジニル、セフジトレンピボキシル、セフタジジム、セフピラミド、セフスロジン、セフメノキシム、セフポドキシムプロキセチル、セフピロム、セファゾプラン、セフェピム、セフスロジン、セフメノキシム、セフメタゾール、セフミノクス、セフォキシチン、セフブペラゾン、ラタモキナセフ、フロモキセフ、セファゾリン、セフォタキシム、セフォペラゾン、セフチゾキシム、モキサラクタム、チエナマイシン、スルファゼシン、アズスレオナムまたはそれらの塩、グリセオフルビン、ランカシジン類〔ジャーナル・オブ・アンチバイオティックス(J.Antibiotics),38,877-885(1985)〕、アゾール系化合物〔2-〔(1R,2R)-2-(2,4-ジフルオロフェニル)-2-ヒドロキシ-1-メチル-3-(1H-1,2,4-トリアゾール-1-イル)プロピル〕-4-〔4-(2,2,3,3-テトラフルオロプロポキシ)フェニル〕-3-(2H,4H)-1,2,4-トリアゾロン、フルコナゾール、イトラコナゾール、イミペネム、メロペネム、トリメトプリム、スルファメトキサゾール等〕等。 (1) Antibacterial drugs (i) Sulfa drugs Sulfamethizol, sulfisoxazole, sulfamonomethoxine, sulfamethizol, salazosulfapyridine, sulfadiazine silver and the like.
(Ii) Quinoline antibacterial agents Nalidixic acid, pipemidic acid trihydrate, enoxacin, norfloxacin, ofloxacin, tosufloxacin tosylate, ciprofloxacin hydrochloride, lomefloxacin hydrochloride, sparfloxacin, fleroxacin and the like.
(Iii) Antituberculosis drugs Isoniazid, ethambutol (ethambutol hydrochloride), paraaminosalicylic acid (calcium paraaminosalicylate), pyrazinamide, etionamide, prothionamide, rifampicin, streptomycin sulfate, kanamycin sulfate, cycloserine and the like.
(Iv) Mycobacterial drugs Diaphenylsulfone, rifampicillin and the like.
(V) Antiviral drugs idoxuridine, acyclovir, vitarabine, ganciclovir, foscarnet and the like.
(Vi) Anti-HIV drugs zidovudine, didanosine, zarcitabine, indinavir sulfate ethanol adduct, ritonavir and the like.
(Vii) Antispirocheta drugs (viii) Antibiotics Tetracycline hydrochloride, ampicillin, piperacillin, gentamicin, dibekacin, cannendomycin, libidomycin, tobramycin, amikacin, fradiomycin, sisomycin, tetracycline, oxytetracycline, loritetracycline, doxycycline, doxycycline, doxycycline Piperacillin, ticarcillin, cephalothin, cefapirin, cephaloridine, cefaclor, cephalexin, cefloxazine, cefadroxyl, cefamandol, cephoam, cefuroxime, cefothium, cefothium hexetyl, cefuroxime xetilyl, cefdinir ceftefirfide , Cefmenoxime, cefpodoxime Proxetyl, cefpirom, cefazoplan, cefepime, cefsulosin, cefmenoxime, cefmetazole, cefminox, cefoxitin, cefbuperazone, latamoquinacef, flomoxef, cefazoline, cefotaxime, cefoperamine, ceftizoxime, Of Antibiotics (J. Antibiotics), 38,877-885 (1985)], azole compounds [2-[(1R, 2R) -2- (2,4-difluorophenyl) -2-hydroxy- 1-methyl-3- (1H-1,2,4-triazol-1-yl) propyl] -4- [4- (2,2,3,3-tetrafluoro Propoxy) phenyl] -3- (2H, 4H) -1,2,4- triazolone, fluconazole, itraconazole, imipenem, meropenem, trimethoprim, sulfamethoxazole, etc.] and the like.
(2)抗真菌薬
(i)ポリエチレン系抗生物質(例、アムホテリシンB、ナイスタチン、トリコマイシン)
(ii)グリセオフルビン、ピロールニトリン等
(iii)シトシン代謝拮抗薬(例、フルシトシン)
(iv)イミダゾール誘導体(例、エコナゾール、クロトリマゾール、硝酸ミコナゾール、ビホナゾール、クロコナゾール)
(v)トリアゾール誘導体(例、フルコナゾール、イトラコナゾール)
(vi)チオカルバミン酸誘導体(例、トリナフトール)等。 (2) Antifungal drugs (i) Polyethylene antibiotics (eg, amphotericin B, nystatin, tricomycin)
(Ii) griseofulvin, pyrrolnitrin, etc. (iii) cytosine antimetabolite (eg, flucytosine)
(Iv) Imidazole derivatives (eg, econazole, clotrimazole, miconazole nitrate, bifonazole, croconazole)
(V) Triazole derivatives (eg, fluconazole, itraconazole)
(Vi) thiocarbamic acid derivatives (eg, trinaphthol) and the like.
(i)ポリエチレン系抗生物質(例、アムホテリシンB、ナイスタチン、トリコマイシン)
(ii)グリセオフルビン、ピロールニトリン等
(iii)シトシン代謝拮抗薬(例、フルシトシン)
(iv)イミダゾール誘導体(例、エコナゾール、クロトリマゾール、硝酸ミコナゾール、ビホナゾール、クロコナゾール)
(v)トリアゾール誘導体(例、フルコナゾール、イトラコナゾール)
(vi)チオカルバミン酸誘導体(例、トリナフトール)等。 (2) Antifungal drugs (i) Polyethylene antibiotics (eg, amphotericin B, nystatin, tricomycin)
(Ii) griseofulvin, pyrrolnitrin, etc. (iii) cytosine antimetabolite (eg, flucytosine)
(Iv) Imidazole derivatives (eg, econazole, clotrimazole, miconazole nitrate, bifonazole, croconazole)
(V) Triazole derivatives (eg, fluconazole, itraconazole)
(Vi) thiocarbamic acid derivatives (eg, trinaphthol) and the like.
(3)抗原虫薬
メトロニダゾール、チニダゾール、クエン酸ジエチルカルバマジン、塩酸キニーネ、硫酸キニーネ等。 (3) Antiprotozoal drugs Metronidazole, tinidazole, diethylcarbamazine citrate, quinine hydrochloride, quinine sulfate and the like.
メトロニダゾール、チニダゾール、クエン酸ジエチルカルバマジン、塩酸キニーネ、硫酸キニーネ等。 (3) Antiprotozoal drugs Metronidazole, tinidazole, diethylcarbamazine citrate, quinine hydrochloride, quinine sulfate and the like.
(4)鎮咳・去たん薬
塩酸エフェドリン、塩酸ノスカピン、リン酸コデイン、リン酸ジヒドロコデイン、塩酸イソプロテレノール、塩酸エフェドリン、塩酸メチルエフェドリン、塩酸ノスカピン、アロクラマイド、クロルフェジアノール、ピコペリダミン、クロペラスチン、プロトキロール、イソプロテレノール、サルブタモール、テレプタリン、オキシペテバノール、塩酸モルヒネ、臭化水素酸デキストロペトルファン、塩酸オキシコドン、リン酸ジモルファン、ヒベンズ酸チペピジン、クエン酸ペントキシベリン、塩酸クロフェダノール、ベンゾナテート、グアイフェネシン、塩酸ブロムヘキシン、塩酸アンブロキソール、アセチルシステイン、塩酸エチルシステイン、カルボシステイン等。 (4) Antitussive / Antidepressant Ephedrine hydrochloride, noscapine hydrochloride, codeine phosphate, dihydrocodeine phosphate, isoproterenol hydrochloride, ephedrine hydrochloride, methylephedrine hydrochloride, noscapine hydrochloride, aloclamide, chlorfedianol, picoperidamine, cloperastine, protochlorol , Isoproterenol, salbutamol, tereptaline, oxypetebanol, morphine hydrochloride, dextropetrphan hydrobromide, oxycodone hydrochloride, dimorphan phosphate, tipipedin hibenzate, pentoxyberine citrate, clofedanol hydrochloride, benzonate, guaifenesin, Bromhexine hydrochloride, ambroxol hydrochloride, acetylcysteine, ethylcysteine hydrochloride, carbocysteine, etc.
塩酸エフェドリン、塩酸ノスカピン、リン酸コデイン、リン酸ジヒドロコデイン、塩酸イソプロテレノール、塩酸エフェドリン、塩酸メチルエフェドリン、塩酸ノスカピン、アロクラマイド、クロルフェジアノール、ピコペリダミン、クロペラスチン、プロトキロール、イソプロテレノール、サルブタモール、テレプタリン、オキシペテバノール、塩酸モルヒネ、臭化水素酸デキストロペトルファン、塩酸オキシコドン、リン酸ジモルファン、ヒベンズ酸チペピジン、クエン酸ペントキシベリン、塩酸クロフェダノール、ベンゾナテート、グアイフェネシン、塩酸ブロムヘキシン、塩酸アンブロキソール、アセチルシステイン、塩酸エチルシステイン、カルボシステイン等。 (4) Antitussive / Antidepressant Ephedrine hydrochloride, noscapine hydrochloride, codeine phosphate, dihydrocodeine phosphate, isoproterenol hydrochloride, ephedrine hydrochloride, methylephedrine hydrochloride, noscapine hydrochloride, aloclamide, chlorfedianol, picoperidamine, cloperastine, protochlorol , Isoproterenol, salbutamol, tereptaline, oxypetebanol, morphine hydrochloride, dextropetrphan hydrobromide, oxycodone hydrochloride, dimorphan phosphate, tipipedin hibenzate, pentoxyberine citrate, clofedanol hydrochloride, benzonate, guaifenesin, Bromhexine hydrochloride, ambroxol hydrochloride, acetylcysteine, ethylcysteine hydrochloride, carbocysteine, etc.
(5)鎮静薬
塩酸クロルプロマジン、硫酸アトロピン、フェノバルビタール、バルビタール、アモバルビタール、ペントバルビタール、チオペンタールナトリウム、チアミラールナトリウム、ニトラゼパム、エスタゾラム、フルラザパム、ハロキサゾラム、トリアゾラム、フルニトラゼパム、ブロムワレリル尿素、抱水クロラール、トリクロホスナトリウム等。 (5) Sedatives Chlorpromazine hydrochloride, atropine sulfate, phenobarbital, barbital, amobarbital, pentobarbital, thiopental sodium, thiamylal sodium, nitrazepam, estazolam, flurazapam, haloxazolam, triazolam, flunitrazepam, bromvaleryl urea, chlorphos chloral trihydrate Sodium etc.
塩酸クロルプロマジン、硫酸アトロピン、フェノバルビタール、バルビタール、アモバルビタール、ペントバルビタール、チオペンタールナトリウム、チアミラールナトリウム、ニトラゼパム、エスタゾラム、フルラザパム、ハロキサゾラム、トリアゾラム、フルニトラゼパム、ブロムワレリル尿素、抱水クロラール、トリクロホスナトリウム等。 (5) Sedatives Chlorpromazine hydrochloride, atropine sulfate, phenobarbital, barbital, amobarbital, pentobarbital, thiopental sodium, thiamylal sodium, nitrazepam, estazolam, flurazapam, haloxazolam, triazolam, flunitrazepam, bromvaleryl urea, chlorphos chloral trihydrate Sodium etc.
(6)麻酔薬
(6-1)局所麻酔薬
塩酸コカイン、塩酸プロカイン、リドカイン、塩酸ジブカイン、塩酸テトラカイン、塩酸メピバカイン、塩酸ブピバカイン、塩酸オキシブプロカイン、アミノ安息香酸エチル、オキセサゼイン等。
(6-2)全身麻酔薬
(i)吸入麻酔薬(例、エーテル、ハロタン、亜酸化窒素、インフルラン、エンフルラン)、
(ii)静脈麻酔薬(例、塩酸ケタミン、ドロペリドール、チオペンタールナトリウム、チアミラールナトリウム、ペントバルビタール)等。 (6) Anesthetic (6-1) Local anesthetic Cocaine hydrochloride, procaine hydrochloride, lidocaine, dibucaine hydrochloride, tetracaine hydrochloride, mepivacaine hydrochloride, bupivacaine hydrochloride, oxybuprocaine hydrochloride, ethyl aminobenzoate, oxesazein and the like.
(6-2) General anesthetic (i) Inhalation anesthetic (eg, ether, halothane, nitrous oxide, influrane, enflurane),
(Ii) intravenous anesthetics (eg, ketamine hydrochloride, droperidol, sodium thiopental, thiamylal sodium, pentobarbital) and the like.
(6-1)局所麻酔薬
塩酸コカイン、塩酸プロカイン、リドカイン、塩酸ジブカイン、塩酸テトラカイン、塩酸メピバカイン、塩酸ブピバカイン、塩酸オキシブプロカイン、アミノ安息香酸エチル、オキセサゼイン等。
(6-2)全身麻酔薬
(i)吸入麻酔薬(例、エーテル、ハロタン、亜酸化窒素、インフルラン、エンフルラン)、
(ii)静脈麻酔薬(例、塩酸ケタミン、ドロペリドール、チオペンタールナトリウム、チアミラールナトリウム、ペントバルビタール)等。 (6) Anesthetic (6-1) Local anesthetic Cocaine hydrochloride, procaine hydrochloride, lidocaine, dibucaine hydrochloride, tetracaine hydrochloride, mepivacaine hydrochloride, bupivacaine hydrochloride, oxybuprocaine hydrochloride, ethyl aminobenzoate, oxesazein and the like.
(6-2) General anesthetic (i) Inhalation anesthetic (eg, ether, halothane, nitrous oxide, influrane, enflurane),
(Ii) intravenous anesthetics (eg, ketamine hydrochloride, droperidol, sodium thiopental, thiamylal sodium, pentobarbital) and the like.
(7)抗潰瘍薬
塩酸ヒスチジン、ランソプラゾール、メトクロプラミド、ピレンゼピン、シメチジン、ラニチジン、ファモチジン、ウロガストリン、オキセサゼイン、プログルミド、オメプラゾール、スクラルファート、スルピリド、セトラキサート、ゲファルナート、アルジオキサ、テプレノン、プロスタグランジン等。 (7) Anti-ulcer drugs Histidine hydrochloride, lansoprazole, metoclopramide, pirenzepine, cimetidine, ranitidine, famotidine, urogastrin, oxesasein, proglumide, omeprazole, sucralfate, sulpiride, cetraxate, gefarnate, aldioxa, tepregone, prostaglandin, etc.
塩酸ヒスチジン、ランソプラゾール、メトクロプラミド、ピレンゼピン、シメチジン、ラニチジン、ファモチジン、ウロガストリン、オキセサゼイン、プログルミド、オメプラゾール、スクラルファート、スルピリド、セトラキサート、ゲファルナート、アルジオキサ、テプレノン、プロスタグランジン等。 (7) Anti-ulcer drugs Histidine hydrochloride, lansoprazole, metoclopramide, pirenzepine, cimetidine, ranitidine, famotidine, urogastrin, oxesasein, proglumide, omeprazole, sucralfate, sulpiride, cetraxate, gefarnate, aldioxa, tepregone, prostaglandin, etc.
(8)不整脈治療薬
(i)ナトリウムチャンネル遮断薬(例、キニジン、プロカインアミド、ジソピラミド、アジマリン、リドカイン、メキシレチン、フェニトイン)、
(ii)β遮断薬(例、プロプラノロール、アルプレノロール、塩酸ブフェトロール、オクスプレノロール、アテノロール、アセブトロール、メトプロロール、ビソプロロール、ピンドロール、カルテオロール、塩酸アロチノロール)、
(iii)カリウムチャンネル遮断薬(例、アミオダロン)、
(iv)カルシウムチャンネル遮断薬(例、ベラパミル、ジルチアゼム)等。 (8) Arrhythmia drug (i) Sodium channel blocker (eg, quinidine, procainamide, disopyramide, azimarin, lidocaine, mexiletine, phenytoin),
(Ii) β-blockers (eg, propranolol, alprenolol, bufetrol, hydrochloride, oxprenolol, atenolol, acebutolol, metoprolol, bisoprolol, pindolol, carteolol, arotinolol hydrochloride),
(Iii) potassium channel blockers (eg, amiodarone),
(Iv) Calcium channel blockers (eg, verapamil, diltiazem) and the like.
(i)ナトリウムチャンネル遮断薬(例、キニジン、プロカインアミド、ジソピラミド、アジマリン、リドカイン、メキシレチン、フェニトイン)、
(ii)β遮断薬(例、プロプラノロール、アルプレノロール、塩酸ブフェトロール、オクスプレノロール、アテノロール、アセブトロール、メトプロロール、ビソプロロール、ピンドロール、カルテオロール、塩酸アロチノロール)、
(iii)カリウムチャンネル遮断薬(例、アミオダロン)、
(iv)カルシウムチャンネル遮断薬(例、ベラパミル、ジルチアゼム)等。 (8) Arrhythmia drug (i) Sodium channel blocker (eg, quinidine, procainamide, disopyramide, azimarin, lidocaine, mexiletine, phenytoin),
(Ii) β-blockers (eg, propranolol, alprenolol, bufetrol, hydrochloride, oxprenolol, atenolol, acebutolol, metoprolol, bisoprolol, pindolol, carteolol, arotinolol hydrochloride),
(Iii) potassium channel blockers (eg, amiodarone),
(Iv) Calcium channel blockers (eg, verapamil, diltiazem) and the like.
(9)降圧利尿薬
ヘキサメトニウムブロミド、塩酸クロニジン、ヒドロクロロチアジド、トリクロルメチアジド、フロセミド、エタクリン酸、ブメタニド、メフルシド、アゾセミド、スピロノラクトン、カンレノ酸カリウム、トリアムテレン、アミロリド、アセタゾラミド、D-マンニトール、イソソルビド、アミノフィリン等。 (9) Antihypertensive diuretic hexamethonium bromide, clonidine hydrochloride, hydrochlorothiazide, trichloromethiazide, furosemide, ethacrynic acid, bumetanide, mefluside, azosemide, spironolactone, potassium canrenoate, triamterene, amiloride, acetazolamide, D-mannitol, isosorbide, aminosorbide etc.
ヘキサメトニウムブロミド、塩酸クロニジン、ヒドロクロロチアジド、トリクロルメチアジド、フロセミド、エタクリン酸、ブメタニド、メフルシド、アゾセミド、スピロノラクトン、カンレノ酸カリウム、トリアムテレン、アミロリド、アセタゾラミド、D-マンニトール、イソソルビド、アミノフィリン等。 (9) Antihypertensive diuretic hexamethonium bromide, clonidine hydrochloride, hydrochlorothiazide, trichloromethiazide, furosemide, ethacrynic acid, bumetanide, mefluside, azosemide, spironolactone, potassium canrenoate, triamterene, amiloride, acetazolamide, D-mannitol, isosorbide, aminosorbide etc.
(10)抗凝血薬
ヘパリンナトリウム、クエン酸ナトリウム、活性化プロテインC、組織因子経路阻害剤、アンチトロンビンIII、ダルテパリンナトリウム、ワルファリンカリウム、アルガトロバン、ガベキサート、クエン酸ナトリウム、オザグレルナトリウム、イコサペンタ酸エチル、ベラプロストナトリウム、アルプロスタジル、塩酸チクロピジン、ペントキシフィリン、ジピリダモール、チソキナーゼ、ウロキナーゼ、ストレプトキナーゼ等。 (10) Anticoagulant heparin sodium, sodium citrate, activated protein C, tissue factor pathway inhibitor, antithrombin III, dalteparin sodium, warfarin potassium, argatroban, gabexate, sodium citrate, ozagrel sodium, icosapentarate, Beraprost sodium, alprostadil, ticlopidine hydrochloride, pentoxifylline, dipyridamole, tisokinase, urokinase, streptokinase and the like.
ヘパリンナトリウム、クエン酸ナトリウム、活性化プロテインC、組織因子経路阻害剤、アンチトロンビンIII、ダルテパリンナトリウム、ワルファリンカリウム、アルガトロバン、ガベキサート、クエン酸ナトリウム、オザグレルナトリウム、イコサペンタ酸エチル、ベラプロストナトリウム、アルプロスタジル、塩酸チクロピジン、ペントキシフィリン、ジピリダモール、チソキナーゼ、ウロキナーゼ、ストレプトキナーゼ等。 (10) Anticoagulant heparin sodium, sodium citrate, activated protein C, tissue factor pathway inhibitor, antithrombin III, dalteparin sodium, warfarin potassium, argatroban, gabexate, sodium citrate, ozagrel sodium, icosapentarate, Beraprost sodium, alprostadil, ticlopidine hydrochloride, pentoxifylline, dipyridamole, tisokinase, urokinase, streptokinase and the like.
(11)精神安定薬
ジアゼパム、ロラゼパム、オキサゼパム、クロルジアゼポキシド、メダゼパム、オキサゾラム、クロキサゾラム、クロチアゼパム、ブロマゼパム、エチゾラム、フルジアゼパム、ヒドロキシジン等。 (11) Tranquilizers Diazepam, lorazepam, oxazepam, chlordiazepoxide, medazepam, oxazolam, cloxazolam, clothiazepam, bromazepam, etizolam, fludiazepam, hydroxyzine and the like.
ジアゼパム、ロラゼパム、オキサゼパム、クロルジアゼポキシド、メダゼパム、オキサゾラム、クロキサゾラム、クロチアゼパム、ブロマゼパム、エチゾラム、フルジアゼパム、ヒドロキシジン等。 (11) Tranquilizers Diazepam, lorazepam, oxazepam, chlordiazepoxide, medazepam, oxazolam, cloxazolam, clothiazepam, bromazepam, etizolam, fludiazepam, hydroxyzine and the like.
(12)抗精神病薬
塩酸クロルプロマジン、プロクロルペラジン、トリフロペラジン、塩酸チオリダジン、マレイン酸ペルフェナジン、エナント酸フルフェナジン、マレイン酸プロクロルペラジン、マレイン酸レボメプロマジン、塩酸プロメタジン、ハロペリドール、ブロムペリドール、スピペロン、レセルピン、塩酸クロカプラミン、スルピリド、ゾテピン等。 (12) Antipsychotics Chlorpromazine hydrochloride, prochlorperazine, trifluoroperazine, thioridazine hydrochloride, perphenazine maleate, fluphenazine enanthate, prochlorperazine maleate, levomepromazine maleate, promethazine hydrochloride, haloperidol, bromperidol , Spiperone, reserpine, clocapramine hydrochloride, sulpiride, zotepine and the like.
塩酸クロルプロマジン、プロクロルペラジン、トリフロペラジン、塩酸チオリダジン、マレイン酸ペルフェナジン、エナント酸フルフェナジン、マレイン酸プロクロルペラジン、マレイン酸レボメプロマジン、塩酸プロメタジン、ハロペリドール、ブロムペリドール、スピペロン、レセルピン、塩酸クロカプラミン、スルピリド、ゾテピン等。 (12) Antipsychotics Chlorpromazine hydrochloride, prochlorperazine, trifluoroperazine, thioridazine hydrochloride, perphenazine maleate, fluphenazine enanthate, prochlorperazine maleate, levomepromazine maleate, promethazine hydrochloride, haloperidol, bromperidol , Spiperone, reserpine, clocapramine hydrochloride, sulpiride, zotepine and the like.
(13)抗腫瘍薬
6-O-(N-クロロアセチルカルバモイル)フマギロール、ブレオマイシン、メトトレキサート、アクチノマイシンD、マイトマイシンC、ダウノルビシン、アドリアマイシン、ネオカルチノスタチン、シトシンアラビノシド、フルオロウラシル、テトラヒドロフリル-5-フルオロウラシル、ピシバニール、レンチナン、レバミゾール、ベスタチン、アジメキソン、グリチルリチン、塩酸ドキソルビシン、塩酸アクラルビシン、塩酸ブレオマイシン、硫酸ヘプロマイシン、硫酸ビンクリスチン、硫酸ビンブラスチン、塩酸イリノテカン、シクロフォスファミド、メルファラン、ズスルファン、チオテパ、塩酸プロカルバジン、シスプラチン、アザチオプリン、メルカプトプリン、テガフール、カルモフール、シタラビン、メチルテストステロン、プロピオン酸テストステロン、エナント酸テストステロン、メピチオスタン、ホスフェストロール、酢酸クロルマジノン、酢酸リュープロレリン、酢酸ブセレリン、ボルテゾミブ、レナリドミド、クロファラビン、アザシチジン、イマチニブ等。 (13) Anti-tumor drug 6-O- (N-chloroacetylcarbamoyl) fumagillol, bleomycin, methotrexate, actinomycin D, mitomycin C, daunorubicin, adriamycin, neocartinostatin, cytosine arabinoside, fluorouracil, tetrahydrofuryl-5 -Fluorouracil, picibanil, lentinan, levamisole, bestatin, azimexone, glycyrrhizin, doxorubicin hydrochloride, aclarubicin hydrochloride, bleomycin hydrochloride, hepromycin sulfate, vincristine sulfate, vinblastine sulfate, irinotecan, cyclophosphamide, melphalan, dusulfan hydrochloride, thiotepa, procarbazine hydrochloride , Cisplatin, azathioprine, mercaptopurine, tegafur, carmofur, cytarabine , Methyltestosterone, testosterone propionate, testosterone enanthate, mepithiostan, phosfestol, chlormadinone acetate, leuprorelin acetate, buserelin acetate, bortezomib, lenalidomide, clofarabine, azacitidine, imatinib, etc.
6-O-(N-クロロアセチルカルバモイル)フマギロール、ブレオマイシン、メトトレキサート、アクチノマイシンD、マイトマイシンC、ダウノルビシン、アドリアマイシン、ネオカルチノスタチン、シトシンアラビノシド、フルオロウラシル、テトラヒドロフリル-5-フルオロウラシル、ピシバニール、レンチナン、レバミゾール、ベスタチン、アジメキソン、グリチルリチン、塩酸ドキソルビシン、塩酸アクラルビシン、塩酸ブレオマイシン、硫酸ヘプロマイシン、硫酸ビンクリスチン、硫酸ビンブラスチン、塩酸イリノテカン、シクロフォスファミド、メルファラン、ズスルファン、チオテパ、塩酸プロカルバジン、シスプラチン、アザチオプリン、メルカプトプリン、テガフール、カルモフール、シタラビン、メチルテストステロン、プロピオン酸テストステロン、エナント酸テストステロン、メピチオスタン、ホスフェストロール、酢酸クロルマジノン、酢酸リュープロレリン、酢酸ブセレリン、ボルテゾミブ、レナリドミド、クロファラビン、アザシチジン、イマチニブ等。 (13) Anti-tumor drug 6-O- (N-chloroacetylcarbamoyl) fumagillol, bleomycin, methotrexate, actinomycin D, mitomycin C, daunorubicin, adriamycin, neocartinostatin, cytosine arabinoside, fluorouracil, tetrahydrofuryl-5 -Fluorouracil, picibanil, lentinan, levamisole, bestatin, azimexone, glycyrrhizin, doxorubicin hydrochloride, aclarubicin hydrochloride, bleomycin hydrochloride, hepromycin sulfate, vincristine sulfate, vinblastine sulfate, irinotecan, cyclophosphamide, melphalan, dusulfan hydrochloride, thiotepa, procarbazine hydrochloride , Cisplatin, azathioprine, mercaptopurine, tegafur, carmofur, cytarabine , Methyltestosterone, testosterone propionate, testosterone enanthate, mepithiostan, phosfestol, chlormadinone acetate, leuprorelin acetate, buserelin acetate, bortezomib, lenalidomide, clofarabine, azacitidine, imatinib, etc.
(14)抗高脂血症薬
クロフィブラート、2-クロロ-3-〔4-(2-メチル-2-フェニルプロポキシ)フェニル〕プロピオン酸エチル〔Chem.Pharm.Bull,38,2792-2796(1990)〕、プラバスタチン、シンバスタチン、プロブコール、ベザフィブラート、クリノフィブラート、ニコモール、コレスチラミン、デキストラン硫酸ナトリウム等。 (14) Antihyperlipidemic drug clofibrate, ethyl 2-chloro-3- [4- (2-methyl-2-phenylpropoxy) phenyl] propionate [Chem. Pharm. Bull, 38, 2792-2996 (1990)], pravastatin, simvastatin, probucol, bezafibrate, clinofibrate, nicomol, cholestyramine, dextran sulfate sodium and the like.
クロフィブラート、2-クロロ-3-〔4-(2-メチル-2-フェニルプロポキシ)フェニル〕プロピオン酸エチル〔Chem.Pharm.Bull,38,2792-2796(1990)〕、プラバスタチン、シンバスタチン、プロブコール、ベザフィブラート、クリノフィブラート、ニコモール、コレスチラミン、デキストラン硫酸ナトリウム等。 (14) Antihyperlipidemic drug clofibrate, ethyl 2-chloro-3- [4- (2-methyl-2-phenylpropoxy) phenyl] propionate [Chem. Pharm. Bull, 38, 2792-2996 (1990)], pravastatin, simvastatin, probucol, bezafibrate, clinofibrate, nicomol, cholestyramine, dextran sulfate sodium and the like.
(15)筋弛緩薬
プリジノール、ツボクラリン、パンクロニウム、塩酸トルペリゾン、カルバミン酸クロルフェネシン、バクロフェン、クロルメザノン、メフェネシン、クロゾキサゾン、エペリゾン、チザニジン等。 (15) Muscle relaxants Pridinol, tubocurarine, pancuronium, tolperisone hydrochloride, chlorphenesin carbamate, baclofen, chlormezanone, mephenesin, clozoxazone, eperisone, tizanidine and the like.
プリジノール、ツボクラリン、パンクロニウム、塩酸トルペリゾン、カルバミン酸クロルフェネシン、バクロフェン、クロルメザノン、メフェネシン、クロゾキサゾン、エペリゾン、チザニジン等。 (15) Muscle relaxants Pridinol, tubocurarine, pancuronium, tolperisone hydrochloride, chlorphenesin carbamate, baclofen, chlormezanone, mephenesin, clozoxazone, eperisone, tizanidine and the like.
(16)抗てんかん薬
フェニトイン、エトサクシミド、アセタゾラミド、クロルジアゼポキシド、トリペタジオン、カルバマゼピン、フェノバルビタール、プリミドン、スルチアム、バルプロ酸ナトリウム、クロナゼパム、ジアゼパム、ニトラゼパム等。 (16) Antiepileptic drugs Phenytoin, ethosuximide, acetazolamide, chlordiazepoxide, tripetadione, carbamazepine, phenobarbital, primidone, sultiam, sodium valproate, clonazepam, diazepam, nitrazepam and the like.
フェニトイン、エトサクシミド、アセタゾラミド、クロルジアゼポキシド、トリペタジオン、カルバマゼピン、フェノバルビタール、プリミドン、スルチアム、バルプロ酸ナトリウム、クロナゼパム、ジアゼパム、ニトラゼパム等。 (16) Antiepileptic drugs Phenytoin, ethosuximide, acetazolamide, chlordiazepoxide, tripetadione, carbamazepine, phenobarbital, primidone, sultiam, sodium valproate, clonazepam, diazepam, nitrazepam and the like.
(17)抗うつ薬
イミプラミン、クロミプラミン、ノキシプチリン、フェネルジン、塩酸アミトリプチリン、塩酸ノルトリプチリン、アモキサピン、塩酸ミアンセリン、塩酸マプロチリン、スルピリド、マレイン酸フルボキサミン、塩酸トラゾドン等。 (17) Antidepressant imipramine, clomipramine, noxiptylline, phenelzine, amitriptyline hydrochloride, nortriptyline hydrochloride, amoxapine, mianserin hydrochloride, maprotiline hydrochloride, sulpiride, fluvoxamine maleate, trazodone hydrochloride, and the like.
イミプラミン、クロミプラミン、ノキシプチリン、フェネルジン、塩酸アミトリプチリン、塩酸ノルトリプチリン、アモキサピン、塩酸ミアンセリン、塩酸マプロチリン、スルピリド、マレイン酸フルボキサミン、塩酸トラゾドン等。 (17) Antidepressant imipramine, clomipramine, noxiptylline, phenelzine, amitriptyline hydrochloride, nortriptyline hydrochloride, amoxapine, mianserin hydrochloride, maprotiline hydrochloride, sulpiride, fluvoxamine maleate, trazodone hydrochloride, and the like.
(18)抗アレルギー薬
ジフェンヒドラミン、クロルフェニラミン、トリペレナミン、メトジラミン、クレミゾール、ジフェニルピラリン、メトキシフェナミン、クロモグリク酸ナトリウム、トラニラスト、レピリナスト、アンレキサノクス、イブジラスト、ケトチフェン、テルフェナジン、メキタジン、塩酸アゼラスチン、エピナスチン、塩酸オザグレル、プランルカスト水和物、セラトロダスト等。 (18) Antiallergic drugs diphenhydramine, chlorpheniramine, tripelenamine, methodiramine, clemizole, diphenylpyraline, methoxyphenamine, cromoglycate sodium, tranilast, repirinast, amlexanox, ibudilast, ketotifen, terfenadine, mequitazine, azelastine hydrochloride, epinastine hydrochloride , Pranlukast hydrate, seratrodast, etc.
ジフェンヒドラミン、クロルフェニラミン、トリペレナミン、メトジラミン、クレミゾール、ジフェニルピラリン、メトキシフェナミン、クロモグリク酸ナトリウム、トラニラスト、レピリナスト、アンレキサノクス、イブジラスト、ケトチフェン、テルフェナジン、メキタジン、塩酸アゼラスチン、エピナスチン、塩酸オザグレル、プランルカスト水和物、セラトロダスト等。 (18) Antiallergic drugs diphenhydramine, chlorpheniramine, tripelenamine, methodiramine, clemizole, diphenylpyraline, methoxyphenamine, cromoglycate sodium, tranilast, repirinast, amlexanox, ibudilast, ketotifen, terfenadine, mequitazine, azelastine hydrochloride, epinastine hydrochloride , Pranlukast hydrate, seratrodast, etc.
(19)強心薬
トランスバイオキソカンファー、テレフィロール、アミノフィリン、エチレフリン、ドパミン、ドブタミン、デノパミン、ベシナリン、アムリノン、ピモベンダン、ユビデカレノン、ジギトキシン、ジゴキシン、メチルジゴキシン、ラナトシドC、G-ストロファンチン等。 (19) Cardiotonic drugs Transbioxocamphor, telephilol, aminophylline, ethylephrine, dopamine, dobutamine, denopamine, vesinaline, amrinone, pimobendan, ubidecarenone, digitoxin, digoxin, methyldigoxin, lanatoside C, G-strophantin and the like.
トランスバイオキソカンファー、テレフィロール、アミノフィリン、エチレフリン、ドパミン、ドブタミン、デノパミン、ベシナリン、アムリノン、ピモベンダン、ユビデカレノン、ジギトキシン、ジゴキシン、メチルジゴキシン、ラナトシドC、G-ストロファンチン等。 (19) Cardiotonic drugs Transbioxocamphor, telephilol, aminophylline, ethylephrine, dopamine, dobutamine, denopamine, vesinaline, amrinone, pimobendan, ubidecarenone, digitoxin, digoxin, methyldigoxin, lanatoside C, G-strophantin and the like.
(20)血管拡張薬
オキシフェドリン、ジルチアゼム、トラゾリン、ヘキソベンジン、バメタン、クロニジン、メチルドパ、グアナベンズ等。 (20) Vasodilators Oxyfedrine, diltiazem, trazoline, hexobenzine, bamethane, clonidine, methyldopa, guanabenz and the like.
オキシフェドリン、ジルチアゼム、トラゾリン、ヘキソベンジン、バメタン、クロニジン、メチルドパ、グアナベンズ等。 (20) Vasodilators Oxyfedrine, diltiazem, trazoline, hexobenzine, bamethane, clonidine, methyldopa, guanabenz and the like.
(21)血管収縮薬
ドパミン、ドブタミンデノパミン等。 (21) Vasoconstricting agents dopamine, dobutamine denopamine and the like.
ドパミン、ドブタミンデノパミン等。 (21) Vasoconstricting agents dopamine, dobutamine denopamine and the like.
(22)降圧利尿薬
ヘキサメトニウムブロミド、ペントリニウム、メカミルアミン、エカラジン、クロニジン、ジルチアゼム、ニフェジピン等。 (22) Antihypertensive diuretics Hexamethonium bromide, pentolinium, mecamylamine, ecarazine, clonidine, diltiazem, nifedipine and the like.
ヘキサメトニウムブロミド、ペントリニウム、メカミルアミン、エカラジン、クロニジン、ジルチアゼム、ニフェジピン等。 (22) Antihypertensive diuretics Hexamethonium bromide, pentolinium, mecamylamine, ecarazine, clonidine, diltiazem, nifedipine and the like.
(23)糖尿病治療薬
トルブタミド、クロルプロパミド、アセトヘキサミド、グリベンクラミド、トラザミド、アカルボース、エパルレスタット、トログリタゾン、グルカゴン、グリミジン、グリプジド、フェンフォルミン、プフォルミン、メトフォルミン等。 (23) Antidiabetic drugs Tolbutamide, chlorpropamide, acetohexamide, glibenclamide, tolazamide, acarbose, epalrestat, troglitazone, glucagon, grimidine, glipzide, phenformin, pformin, metformin, and the like.
トルブタミド、クロルプロパミド、アセトヘキサミド、グリベンクラミド、トラザミド、アカルボース、エパルレスタット、トログリタゾン、グルカゴン、グリミジン、グリプジド、フェンフォルミン、プフォルミン、メトフォルミン等。 (23) Antidiabetic drugs Tolbutamide, chlorpropamide, acetohexamide, glibenclamide, tolazamide, acarbose, epalrestat, troglitazone, glucagon, grimidine, glipzide, phenformin, pformin, metformin, and the like.
(24)麻薬拮抗薬
レバロルファン、ナロルフィン、ナロキソンまたはその塩等。 (24) narcotic antagonists levalorphan, nalolphine, naloxone or a salt thereof.
レバロルファン、ナロルフィン、ナロキソンまたはその塩等。 (24) narcotic antagonists levalorphan, nalolphine, naloxone or a salt thereof.
(25)脂溶性ビタミン薬
(i)ビタミンA類:ビタミンA1、ビタミンA2およびパルミチン酸レチノール
(ii)ビタミンD類:ビタミンD1、D2、D3、D4およびD5
(iii)ビタミンE類:α-トコフェロール、β-トコフェロール、γ-トコフェロール、δ-トコフェロール、ニコチン酸dl-α-トコフェロール
(iv)ビタミンK類:ビタミンK1、K2、K3およびK4
(v)葉酸(ビタミンM)、ホリナートカルシウム等。 (25) Fat-soluble vitamin drugs (i) Vitamin A: Vitamin A1, Vitamin A2 and Retinol palmitate (ii) Vitamin D: Vitamin D1, D2, D3, D4 and D5
(Iii) Vitamin E: α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol, dl-α-tocopherol nicotinate (iv) Vitamin K: vitamins K1, K2, K3 and K4
(V) Folic acid (vitamin M), folinate calcium and the like.
(i)ビタミンA類:ビタミンA1、ビタミンA2およびパルミチン酸レチノール
(ii)ビタミンD類:ビタミンD1、D2、D3、D4およびD5
(iii)ビタミンE類:α-トコフェロール、β-トコフェロール、γ-トコフェロール、δ-トコフェロール、ニコチン酸dl-α-トコフェロール
(iv)ビタミンK類:ビタミンK1、K2、K3およびK4
(v)葉酸(ビタミンM)、ホリナートカルシウム等。 (25) Fat-soluble vitamin drugs (i) Vitamin A: Vitamin A1, Vitamin A2 and Retinol palmitate (ii) Vitamin D: Vitamin D1, D2, D3, D4 and D5
(Iii) Vitamin E: α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol, dl-α-tocopherol nicotinate (iv) Vitamin K: vitamins K1, K2, K3 and K4
(V) Folic acid (vitamin M), folinate calcium and the like.
(26)ビタミン誘導体
ビタミンの各種誘導体、例えば、5,6-トランス-コレカルシフェロール、2,5-ヒドロキシコレカルシフェロール、1-α-ヒドロキシコレカルシフェロール等のビタミンD3誘導体、5,6-トランス-エルゴカルシフェロール等のビタミンD2誘導体等。 (26) Vitamin derivatives Various vitamin derivatives, for example, vitamin D3 derivatives such as 5,6-trans-cholecalciferol, 2,5-hydroxycholecalciferol, 1-α-hydroxycholecalciferol, 5,6-trans -Vitamin D2 derivatives such as ergocalciferol.
ビタミンの各種誘導体、例えば、5,6-トランス-コレカルシフェロール、2,5-ヒドロキシコレカルシフェロール、1-α-ヒドロキシコレカルシフェロール等のビタミンD3誘導体、5,6-トランス-エルゴカルシフェロール等のビタミンD2誘導体等。 (26) Vitamin derivatives Various vitamin derivatives, for example, vitamin D3 derivatives such as 5,6-trans-cholecalciferol, 2,5-hydroxycholecalciferol, 1-α-hydroxycholecalciferol, 5,6-trans -Vitamin D2 derivatives such as ergocalciferol.
(27)抗喘息薬
塩酸イソプレナリン、硫酸サルブタモール、塩酸プロカテロール、硫酸テルブタリン、塩酸トリメトキノール、塩酸ツロブテロール、硫酸オルシプレナリン、臭化水素酸フェノテロール、塩酸エフェドリン、臭化イプロトロピウム、臭化オキシトロピウム、臭化フルトロピウム、テオフィリン、アミノフィリン、クロモグリク酸ナトリウム、トラニラスト、レピリナスト、アンレキサノン、イブジラスト、ケトチフェン、テルフェナジン、メキタジン、アゼラスチン、エピナスチン、塩酸オザグレル、プランルカスト水和物、セラトロダスト、デキサメタゾン、プレドニゾロン、ヒドロコルチゾン、コハク酸ヒドロコルチゾンナトリウム、プロピオン酸ベクロメタゾン等。 (27) Anti-asthma drugs Isoprenaline hydrochloride, salbutamol sulfate, procaterol hydrochloride, terbutaline sulfate, trimethoquinol hydrochloride, tulobuterol hydrochloride, orciprenaline sulfate, fenoterol hydrobromide, ephedrine hydrochloride, iprotropium bromide, oxitropium bromide, bromide Flutropium, theophylline, aminophylline, sodium cromoglycate, tranilast, repirinast, amlexanone, ibudilast, ketotifen, terfenadine, mequitazine, azelastine, epinastine, ozagrel hydrochloride, pranlukast hydrate, seratrodast, dexamethasone, prednisolone hydrocolicone , Beclomethasone propionate and the like.
塩酸イソプレナリン、硫酸サルブタモール、塩酸プロカテロール、硫酸テルブタリン、塩酸トリメトキノール、塩酸ツロブテロール、硫酸オルシプレナリン、臭化水素酸フェノテロール、塩酸エフェドリン、臭化イプロトロピウム、臭化オキシトロピウム、臭化フルトロピウム、テオフィリン、アミノフィリン、クロモグリク酸ナトリウム、トラニラスト、レピリナスト、アンレキサノン、イブジラスト、ケトチフェン、テルフェナジン、メキタジン、アゼラスチン、エピナスチン、塩酸オザグレル、プランルカスト水和物、セラトロダスト、デキサメタゾン、プレドニゾロン、ヒドロコルチゾン、コハク酸ヒドロコルチゾンナトリウム、プロピオン酸ベクロメタゾン等。 (27) Anti-asthma drugs Isoprenaline hydrochloride, salbutamol sulfate, procaterol hydrochloride, terbutaline sulfate, trimethoquinol hydrochloride, tulobuterol hydrochloride, orciprenaline sulfate, fenoterol hydrobromide, ephedrine hydrochloride, iprotropium bromide, oxitropium bromide, bromide Flutropium, theophylline, aminophylline, sodium cromoglycate, tranilast, repirinast, amlexanone, ibudilast, ketotifen, terfenadine, mequitazine, azelastine, epinastine, ozagrel hydrochloride, pranlukast hydrate, seratrodast, dexamethasone, prednisolone hydrocolicone , Beclomethasone propionate and the like.
(28)頻尿・尿失禁治療薬
塩酸フラボキサート等。 (28) Frequent urine and urinary incontinence treatment flavoxate hydrochloride and the like.
塩酸フラボキサート等。 (28) Frequent urine and urinary incontinence treatment flavoxate hydrochloride and the like.
(29)アトピー性皮膚炎治療薬
クロモグリク酸ナトリウム等。 (29) Atopic dermatitis therapeutic agent sodium cromoglycate and the like.
クロモグリク酸ナトリウム等。 (29) Atopic dermatitis therapeutic agent sodium cromoglycate and the like.
(30)アレルギー性鼻炎治療薬
クロモグリク酸ナトリウム、マレイン酸クロルフェニラミン、酒石酸アリメマジン、フマル酸クレマスチン、塩酸ホモクロルシクリジン、フェキソフェナジン、メキタジン等。 (30) Allergic rhinitis therapeutic agent Sodium cromoglycate, chlorpheniramine maleate, alimemazine tartrate, clemastine fumarate, homochlorcyclidine hydrochloride, fexofenadine, mequitazine and the like.
クロモグリク酸ナトリウム、マレイン酸クロルフェニラミン、酒石酸アリメマジン、フマル酸クレマスチン、塩酸ホモクロルシクリジン、フェキソフェナジン、メキタジン等。 (30) Allergic rhinitis therapeutic agent Sodium cromoglycate, chlorpheniramine maleate, alimemazine tartrate, clemastine fumarate, homochlorcyclidine hydrochloride, fexofenadine, mequitazine and the like.
(31)昇圧薬
ドパミン、ドブタミン、デノパミン、ジギトキシン、ジゴキシン、メチルジゴキシン、ラナトシドC、G-ストロファンチン等。 (31) Pressor drugs Dopamine, dobutamine, denopamine, digitoxin, digoxin, methyldigoxin, lanatoside C, G-strophantin and the like.
ドパミン、ドブタミン、デノパミン、ジギトキシン、ジゴキシン、メチルジゴキシン、ラナトシドC、G-ストロファンチン等。 (31) Pressor drugs Dopamine, dobutamine, denopamine, digitoxin, digoxin, methyldigoxin, lanatoside C, G-strophantin and the like.
(32)造血幹細胞移植前治療薬
ブスルファン、フルダラビン、クラドリビン、エトポシド。 (32) Hematopoietic stem cell pre-transplant drug busulfan, fludarabine, cladribine, etoposide.
ブスルファン、フルダラビン、クラドリビン、エトポシド。 (32) Hematopoietic stem cell pre-transplant drug busulfan, fludarabine, cladribine, etoposide.
(33)その他
ヒドロキシカム、ダイアセリン、メゲストロール酢酸、ニセロゴリン、クロファジミン、エトレチナート、ビスフォン酸、デフィブロタイド、プロスタグランジン類等。 (33) Others Hydroxycam, diacerine, megestrol acetic acid, falselogolin, clofazimine, etretinate, bisphonic acid, defibrotide, prostaglandins and the like.
ヒドロキシカム、ダイアセリン、メゲストロール酢酸、ニセロゴリン、クロファジミン、エトレチナート、ビスフォン酸、デフィブロタイド、プロスタグランジン類等。 (33) Others Hydroxycam, diacerine, megestrol acetic acid, falselogolin, clofazimine, etretinate, bisphonic acid, defibrotide, prostaglandins and the like.
併用に際しては、本発明化合物と併用薬物の投与時期は限定されず、本発明化合物および併用薬物を、投与対象に対し、同時に投与してもよいし、時間差をおいて投与してもよい。併用薬物の投与量は、臨床上用いられている投与量に準ずればよく、投与対象、投与ルート、疾患、組み合わせ等により適宜選択することができる。
併用の投与形態は、特に限定されず、投与時に、本発明化合物と併用薬物とが組み合わされていればよい。このような投与形態としては、例えば、(1)本発明化合物および併用薬物を同時に製剤化して得られる単一の製剤の投与、(2)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の同一投与経路での同時投与、(3)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の同一投与経路での時間差をおいての投与、(4)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の異なる投与経路での同時投与、(5)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の異なる投与経路での時間差をおいての投与(例えば、本発明化合物を投与した後の併用薬物の投与、またはその逆の順序での投与)等が挙げられる。
本発明の併用剤における本発明化合物および併用薬物との配合比は、投与対象、投与ルート、疾患等により適宜選択することができる。
例えば、本発明の併用剤における本発明化合物の含有量は、製剤の形態によって相違するが、通常製剤全体に対して約0.01~100重量%、好ましくは約0.1~50重量%、さらに好ましくは約0.5~20重量%程度である。 In the combined use, the administration time of the compound of the present invention and the concomitant drug is not limited, and the compound of the present invention and the concomitant drug may be administered to the administration subject at the same time or may be administered with a time difference. The dose of the concomitant drug may be determined according to the dose used clinically, and can be appropriately selected depending on the administration subject, administration route, disease, combination and the like.
The administration form of the combination is not particularly limited as long as the compound of the present invention and the concomitant drug are combined at the time of administration. Examples of such administration forms include (1) administration of a single preparation obtained by simultaneously formulating the compound of the present invention and a concomitant drug, and (2) obtained by separately formulating the compound of the present invention and the concomitant drug. Simultaneous administration of the two preparations by the same administration route, (3) administration of the two preparations obtained by separately formulating the compound of the present invention and the concomitant drug at different time intervals by the same administration route, (4) Simultaneous administration of two types of preparations obtained by separately formulating the compound of the present invention and a concomitant drug by different administration routes, (5) Two types of preparations obtained by formulating the compound of the present invention and a concomitant drug separately Administration with a time difference in the administration route (for example, administration of a concomitant drug after administration of the compound of the present invention, or administration in the reverse order) and the like.
The compounding ratio of the compound of the present invention and the concomitant drug in the concomitant drug of the present invention can be appropriately selected depending on the administration subject, administration route, disease and the like.
For example, the content of the compound of the present invention in the concomitant drug of the present invention varies depending on the form of the preparation, but is usually about 0.01 to 100% by weight, preferably about 0.1 to 50% by weight, based on the whole preparation, More preferably, it is about 0.5 to 20% by weight.
併用の投与形態は、特に限定されず、投与時に、本発明化合物と併用薬物とが組み合わされていればよい。このような投与形態としては、例えば、(1)本発明化合物および併用薬物を同時に製剤化して得られる単一の製剤の投与、(2)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の同一投与経路での同時投与、(3)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の同一投与経路での時間差をおいての投与、(4)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の異なる投与経路での同時投与、(5)本発明化合物および併用薬物を別々に製剤化して得られる2種の製剤の異なる投与経路での時間差をおいての投与(例えば、本発明化合物を投与した後の併用薬物の投与、またはその逆の順序での投与)等が挙げられる。
本発明の併用剤における本発明化合物および併用薬物との配合比は、投与対象、投与ルート、疾患等により適宜選択することができる。
例えば、本発明の併用剤における本発明化合物の含有量は、製剤の形態によって相違するが、通常製剤全体に対して約0.01~100重量%、好ましくは約0.1~50重量%、さらに好ましくは約0.5~20重量%程度である。 In the combined use, the administration time of the compound of the present invention and the concomitant drug is not limited, and the compound of the present invention and the concomitant drug may be administered to the administration subject at the same time or may be administered with a time difference. The dose of the concomitant drug may be determined according to the dose used clinically, and can be appropriately selected depending on the administration subject, administration route, disease, combination and the like.
The administration form of the combination is not particularly limited as long as the compound of the present invention and the concomitant drug are combined at the time of administration. Examples of such administration forms include (1) administration of a single preparation obtained by simultaneously formulating the compound of the present invention and a concomitant drug, and (2) obtained by separately formulating the compound of the present invention and the concomitant drug. Simultaneous administration of the two preparations by the same administration route, (3) administration of the two preparations obtained by separately formulating the compound of the present invention and the concomitant drug at different time intervals by the same administration route, (4) Simultaneous administration of two types of preparations obtained by separately formulating the compound of the present invention and a concomitant drug by different administration routes, (5) Two types of preparations obtained by formulating the compound of the present invention and a concomitant drug separately Administration with a time difference in the administration route (for example, administration of a concomitant drug after administration of the compound of the present invention, or administration in the reverse order) and the like.
The compounding ratio of the compound of the present invention and the concomitant drug in the concomitant drug of the present invention can be appropriately selected depending on the administration subject, administration route, disease and the like.
For example, the content of the compound of the present invention in the concomitant drug of the present invention varies depending on the form of the preparation, but is usually about 0.01 to 100% by weight, preferably about 0.1 to 50% by weight, based on the whole preparation, More preferably, it is about 0.5 to 20% by weight.
本発明の併用剤における併用薬物の含有量は、製剤の形態によって相違するが、通常製剤全体に対して約0.01~100重量%、好ましくは約0.1~50重量%、さらに好ましくは約0.5~20重量%程度である。
本発明の併用剤における担体等の添加剤の含有量は、製剤の形態によって相違するが、通常製剤全体に対して約1~99.99重量%、好ましくは約10~90重量%程度である。
また、本発明化合物および併用薬物をそれぞれ別々に製剤化する場合も同様の含有量でよい。
投与量は本発明化合物の種類、投与ルート、症状、患者の年令等によっても異なるが、例えば、移植片対宿主病の患者(体重約60kg)に経口的に投与する場合、1日当たり体重1kgあたり化合物(I)のフリー体として約0.1mg/kg体重~約50mg/kg体重、好ましくは約1mg/kg体重~30mg/kg体重を、1日1回~数回に分けて投与すればよい。
本発明の医薬組成物が徐放性製剤である場合の投与量は、化合物(I)の種類と含量、剤形、薬物放出の持続時間、投与対象動物(例えば、マウス、ラット、ハムスター、モルモット、ウサギ、ネコ、イヌ、ウシ、ウマ、ブタ、ヒツジ、サル、ヒト等の哺乳動物)、投与目的により種々異なるが、例えば、非経口投与により適用する場合には、1週間に約0.1から約100mgの化合物(I)が投与製剤から放出されるようにすればよい。 The content of the concomitant drug in the concomitant drug of the present invention varies depending on the form of the preparation, but is usually about 0.01 to 100% by weight, preferably about 0.1 to 50% by weight, more preferably about the whole preparation. About 0.5 to 20% by weight.
The content of additives such as carriers in the combination agent of the present invention varies depending on the form of the preparation, but is usually about 1 to 99.99% by weight, preferably about 10 to 90% by weight, based on the whole preparation. .
The same content may be used when the compound of the present invention and the concomitant drug are formulated separately.
The dose varies depending on the type of the compound of the present invention, administration route, symptom, patient age, etc. For example, when administered orally to a graft-versus-host disease patient (body weight of about 60 kg), 1 kg body weight per day About 0.1 mg / kg body weight to about 50 mg / kg body weight, preferably about 1 mg / kg body weight to 30 mg / kg body weight per day as a free form of compound (I) may be administered once to several times a day. Good.
When the pharmaceutical composition of the present invention is a sustained-release preparation, the dosage is the type and content of compound (I), the dosage form, the duration of drug release, the animal to be administered (for example, mouse, rat, hamster, guinea pig) Mammals such as rabbits, cats, dogs, cows, horses, pigs, sheep, monkeys, humans, etc.), depending on the purpose of administration, for example, about 0.1 per week when applied by parenteral administration About 100 mg of compound (I) may be released from the dosage formulation.
本発明の併用剤における担体等の添加剤の含有量は、製剤の形態によって相違するが、通常製剤全体に対して約1~99.99重量%、好ましくは約10~90重量%程度である。
また、本発明化合物および併用薬物をそれぞれ別々に製剤化する場合も同様の含有量でよい。
投与量は本発明化合物の種類、投与ルート、症状、患者の年令等によっても異なるが、例えば、移植片対宿主病の患者(体重約60kg)に経口的に投与する場合、1日当たり体重1kgあたり化合物(I)のフリー体として約0.1mg/kg体重~約50mg/kg体重、好ましくは約1mg/kg体重~30mg/kg体重を、1日1回~数回に分けて投与すればよい。
本発明の医薬組成物が徐放性製剤である場合の投与量は、化合物(I)の種類と含量、剤形、薬物放出の持続時間、投与対象動物(例えば、マウス、ラット、ハムスター、モルモット、ウサギ、ネコ、イヌ、ウシ、ウマ、ブタ、ヒツジ、サル、ヒト等の哺乳動物)、投与目的により種々異なるが、例えば、非経口投与により適用する場合には、1週間に約0.1から約100mgの化合物(I)が投与製剤から放出されるようにすればよい。 The content of the concomitant drug in the concomitant drug of the present invention varies depending on the form of the preparation, but is usually about 0.01 to 100% by weight, preferably about 0.1 to 50% by weight, more preferably about the whole preparation. About 0.5 to 20% by weight.
The content of additives such as carriers in the combination agent of the present invention varies depending on the form of the preparation, but is usually about 1 to 99.99% by weight, preferably about 10 to 90% by weight, based on the whole preparation. .
The same content may be used when the compound of the present invention and the concomitant drug are formulated separately.
The dose varies depending on the type of the compound of the present invention, administration route, symptom, patient age, etc. For example, when administered orally to a graft-versus-host disease patient (body weight of about 60 kg), 1 kg body weight per day About 0.1 mg / kg body weight to about 50 mg / kg body weight, preferably about 1 mg / kg body weight to 30 mg / kg body weight per day as a free form of compound (I) may be administered once to several times a day. Good.
When the pharmaceutical composition of the present invention is a sustained-release preparation, the dosage is the type and content of compound (I), the dosage form, the duration of drug release, the animal to be administered (for example, mouse, rat, hamster, guinea pig) Mammals such as rabbits, cats, dogs, cows, horses, pigs, sheep, monkeys, humans, etc.), depending on the purpose of administration, for example, about 0.1 per week when applied by parenteral administration About 100 mg of compound (I) may be released from the dosage formulation.
併用薬物は、副作用が問題とならない範囲でどのような量を設定することも可能である。併用薬物としての一日投与量は、症状の程度、投与対象の年齢、性別、体重、感受性差、投与の時期、間隔、医薬製剤の性質、調剤、種類、有効成分の種類等によって異なり、特に限定されないが、薬物の量として通常、例えば、経口投与で哺乳動物1kg体重あたり約0.001~2000mg、好ましくは約0.01~500mg、さらに好ましくは、約0.1~100mg程度であり、これを通常1日1~4回に分けて投与する。
本発明の併用剤を投与するに際しては、本発明化合物と併用薬物とを同時期に投与してもよいし、時間差をおいて投与してもよい。時間差をおいて投与する場合、時間差は投与する有効成分、剤形、投与方法により異なるが、例えば、併用薬物を先に投与する場合、併用薬物を投与した後1分~3日以内、好ましくは10分~1日以内、より好ましくは15分~1時間以内に本発明化合物を投与する方法が挙げられる。本発明化合物を先に投与する場合、本発明化合物を投与した後、1分~1日以内、好ましくは10分~6時間以内、より好ましくは15分~1時間以内に併用薬物を投与する方法が挙げられる。 The amount of the concomitant drug can be set as long as side effects do not become a problem. The daily dose as a concomitant drug varies depending on the degree of symptoms, age of the subject, sex, weight, sensitivity difference, timing of administration, interval, nature of the pharmaceutical preparation, formulation, type, type of active ingredient, etc. Although not limited, the amount of the drug is usually about 0.001 to 2000 mg per kg body weight of the mammal by oral administration, preferably about 0.01 to 500 mg, more preferably about 0.1 to 100 mg. This is usually administered in 1 to 4 divided doses per day.
When administering the concomitant drug of the present invention, the compound of the present invention and the concomitant drug may be administered at the same time, or may be administered with a time difference. When administered at a time difference, the time difference varies depending on the active ingredient, dosage form, and administration method to be administered. For example, when administering the concomitant drug first, within 1 minute to 3 days after administering the concomitant drug, preferably Examples include a method of administering the compound of the present invention within 10 minutes to 1 day, more preferably within 15 minutes to 1 hour. In the case where the compound of the present invention is administered first, the concomitant drug is administered within 1 minute to 1 day, preferably within 10 minutes to 6 hours, more preferably within 15 minutes to 1 hour after the administration of the compound of the present invention. Is mentioned.
本発明の併用剤を投与するに際しては、本発明化合物と併用薬物とを同時期に投与してもよいし、時間差をおいて投与してもよい。時間差をおいて投与する場合、時間差は投与する有効成分、剤形、投与方法により異なるが、例えば、併用薬物を先に投与する場合、併用薬物を投与した後1分~3日以内、好ましくは10分~1日以内、より好ましくは15分~1時間以内に本発明化合物を投与する方法が挙げられる。本発明化合物を先に投与する場合、本発明化合物を投与した後、1分~1日以内、好ましくは10分~6時間以内、より好ましくは15分~1時間以内に併用薬物を投与する方法が挙げられる。 The amount of the concomitant drug can be set as long as side effects do not become a problem. The daily dose as a concomitant drug varies depending on the degree of symptoms, age of the subject, sex, weight, sensitivity difference, timing of administration, interval, nature of the pharmaceutical preparation, formulation, type, type of active ingredient, etc. Although not limited, the amount of the drug is usually about 0.001 to 2000 mg per kg body weight of the mammal by oral administration, preferably about 0.01 to 500 mg, more preferably about 0.1 to 100 mg. This is usually administered in 1 to 4 divided doses per day.
When administering the concomitant drug of the present invention, the compound of the present invention and the concomitant drug may be administered at the same time, or may be administered with a time difference. When administered at a time difference, the time difference varies depending on the active ingredient, dosage form, and administration method to be administered. For example, when administering the concomitant drug first, within 1 minute to 3 days after administering the concomitant drug, preferably Examples include a method of administering the compound of the present invention within 10 minutes to 1 day, more preferably within 15 minutes to 1 hour. In the case where the compound of the present invention is administered first, the concomitant drug is administered within 1 minute to 1 day, preferably within 10 minutes to 6 hours, more preferably within 15 minutes to 1 hour after the administration of the compound of the present invention. Is mentioned.
本発明は、更に以下の実施例、試験例および製剤例によって詳しく説明されるが、これらは本発明を限定するものではなく、また本発明の範囲を逸脱しない範囲で変化させてもよい。
以下の実施例中の「室温」は通常約 10 ℃ないし約 35 ℃を示す。混合溶媒において示した比は、特に断らない限り容量比を示す。%は、特に断らない限り重量%を示す。
シリカゲルカラムクロマトグラフィーにおいて、NHと記載した場合は、アミノプロピルシラン結合シリカゲル、Diolと記載した場合は、3-(2,3-ジヒドロキシプロポキシ)プロピルシラン結合シリカゲル、DiNHと記載した場合は、N-(2-アミノエチル)-3-アミノプロピルシラン結合シリカゲルを用いた。HPLC (高速液体クロマトグラフィー) において、C18と記載した場合は、オクタデシル結合シリカゲルを用いた。溶出溶媒の比は、特に断らない限り容量比を示す。
以下の実施例においては下記の略号を使用する。
Mp:融点
MS:マススペクトル
[M+H]+、[M-H]-:分子イオンピーク
M:モル濃度
N:規定
CDCl3:重クロロホルム
DMSO-d6:重ジメチルスルホキシド
1H NMR:プロトン核磁気共鳴
LC/MS:液体クロマトグラフ質量分析計
ESI:ElectroSpray Ionization、エレクトロスプレーイオン化
APCI:Atomospheric Pressure Chemical Ionization、大気圧化学イオン化
THF:テトラヒドロフラン
DME:1,2-ジメトキシエタン
DMF:N,N-ジメチルホルムアミド
DMA:N,N-ジメチルアセトアミド
HATU:2-(7-アザベンゾトリアゾール-1-イル)-1,1,3,3-テトラメチルウロニウムヘキサフルオロホスフェート
HOBt:1-ヒドロキシベンゾトリアゾール The present invention is further explained in detail by the following examples, test examples and formulation examples, which are not intended to limit the present invention, and may be changed without departing from the scope of the present invention.
In the following examples, “room temperature” usually indicates about 10 ° C. to about 35 ° C. The ratio shown in the mixed solvent is a volume ratio unless otherwise specified. Unless otherwise indicated, “%” indicates “% by weight”.
In silica gel column chromatography, when NH is described, aminopropylsilane-bonded silica gel, when Diol is described, 3- (2,3-dihydroxypropoxy) propylsilane-bonded silica gel, when DiNH is described, N- (2-Aminoethyl) -3-aminopropylsilane bonded silica gel was used. In HPLC (high performance liquid chromatography), when it was described as C18, octadecyl-bonded silica gel was used. The ratio of elution solvent indicates a volume ratio unless otherwise specified.
The following abbreviations are used in the following examples.
Mp: melting point
MS: Mass spectrum
[M + H] + , [MH] − : Molecular ion peak
M: Molar concentration
N: Regulation
CDCl 3 : Deuterated chloroform
DMSO-d 6 : Heavy dimethyl sulfoxide
1 H NMR: proton nuclear magnetic resonance
LC / MS: Liquid chromatograph mass spectrometer
ESI: ElectroSpray Ionization
APCI: Atomospheric Pressure Chemical Ionization
THF: tetrahydrofuran
DME: 1,2-dimethoxyethane
DMF: N, N-dimethylformamide
DMA: N, N-dimethylacetamide
HATU: 2- (7-azabenzotriazol-1-yl) -1,1,3,3-tetramethyluronium hexafluorophosphate
HOBt: 1-hydroxybenzotriazole
以下の実施例中の「室温」は通常約 10 ℃ないし約 35 ℃を示す。混合溶媒において示した比は、特に断らない限り容量比を示す。%は、特に断らない限り重量%を示す。
シリカゲルカラムクロマトグラフィーにおいて、NHと記載した場合は、アミノプロピルシラン結合シリカゲル、Diolと記載した場合は、3-(2,3-ジヒドロキシプロポキシ)プロピルシラン結合シリカゲル、DiNHと記載した場合は、N-(2-アミノエチル)-3-アミノプロピルシラン結合シリカゲルを用いた。HPLC (高速液体クロマトグラフィー) において、C18と記載した場合は、オクタデシル結合シリカゲルを用いた。溶出溶媒の比は、特に断らない限り容量比を示す。
以下の実施例においては下記の略号を使用する。
Mp:融点
MS:マススペクトル
[M+H]+、[M-H]-:分子イオンピーク
M:モル濃度
N:規定
CDCl3:重クロロホルム
DMSO-d6:重ジメチルスルホキシド
1H NMR:プロトン核磁気共鳴
LC/MS:液体クロマトグラフ質量分析計
ESI:ElectroSpray Ionization、エレクトロスプレーイオン化
APCI:Atomospheric Pressure Chemical Ionization、大気圧化学イオン化
THF:テトラヒドロフラン
DME:1,2-ジメトキシエタン
DMF:N,N-ジメチルホルムアミド
DMA:N,N-ジメチルアセトアミド
HATU:2-(7-アザベンゾトリアゾール-1-イル)-1,1,3,3-テトラメチルウロニウムヘキサフルオロホスフェート
HOBt:1-ヒドロキシベンゾトリアゾール The present invention is further explained in detail by the following examples, test examples and formulation examples, which are not intended to limit the present invention, and may be changed without departing from the scope of the present invention.
In the following examples, “room temperature” usually indicates about 10 ° C. to about 35 ° C. The ratio shown in the mixed solvent is a volume ratio unless otherwise specified. Unless otherwise indicated, “%” indicates “% by weight”.
In silica gel column chromatography, when NH is described, aminopropylsilane-bonded silica gel, when Diol is described, 3- (2,3-dihydroxypropoxy) propylsilane-bonded silica gel, when DiNH is described, N- (2-Aminoethyl) -3-aminopropylsilane bonded silica gel was used. In HPLC (high performance liquid chromatography), when it was described as C18, octadecyl-bonded silica gel was used. The ratio of elution solvent indicates a volume ratio unless otherwise specified.
The following abbreviations are used in the following examples.
Mp: melting point
MS: Mass spectrum
[M + H] + , [MH] − : Molecular ion peak
M: Molar concentration
N: Regulation
CDCl 3 : Deuterated chloroform
DMSO-d 6 : Heavy dimethyl sulfoxide
1 H NMR: proton nuclear magnetic resonance
LC / MS: Liquid chromatograph mass spectrometer
ESI: ElectroSpray Ionization
APCI: Atomospheric Pressure Chemical Ionization
THF: tetrahydrofuran
DME: 1,2-dimethoxyethane
DMF: N, N-dimethylformamide
DMA: N, N-dimethylacetamide
HATU: 2- (7-azabenzotriazol-1-yl) -1,1,3,3-tetramethyluronium hexafluorophosphate
HOBt: 1-hydroxybenzotriazole
1H NMRはフーリエ変換型NMRで測定した。解析にはACD/SpecManager (商品名) などを用いた。水酸基やアミノ基などのプロトンが非常に緩やかなピークについては記載していない。
MSは、LC/MSにより測定した。イオン化法としては、ESI法、または、APCI法を用いた。データは実測値 (found) を記載した。通常、分子イオンピークが観測されるが、tert-ブトキシカルボニル基 を有する化合物の場合、フラグメントイオンとして、tert-ブトキシカルボニル基あるいはtert-ブチル基が脱離したピークが観測されることもある。また、水酸基を有する化合物の場合、フラグメントイオンとして、H2Oが脱離したピークが観測されることもある。塩の場合は、通常、フリー体の分子イオンピークもしくはフラグメントイオンピークが観測される。 1 H NMR was measured by Fourier transform NMR. For analysis, ACD / SpecManager (trade name) was used. Peaks with very gentle protons such as hydroxyl groups and amino groups are not described.
MS was measured by LC / MS. As the ionization method, the ESI method or the APCI method was used. The data is the actual measurement (found). Usually, a molecular ion peak is observed, but in the case of a compound having a tert-butoxycarbonyl group, a peak from which a tert-butoxycarbonyl group or a tert-butyl group is eliminated may be observed as a fragment ion. In the case of a compound having a hydroxyl group, a peak from which H 2 O is eliminated may be observed as a fragment ion. In the case of a salt, a free molecular ion peak or a fragment ion peak is usually observed.
MSは、LC/MSにより測定した。イオン化法としては、ESI法、または、APCI法を用いた。データは実測値 (found) を記載した。通常、分子イオンピークが観測されるが、tert-ブトキシカルボニル基 を有する化合物の場合、フラグメントイオンとして、tert-ブトキシカルボニル基あるいはtert-ブチル基が脱離したピークが観測されることもある。また、水酸基を有する化合物の場合、フラグメントイオンとして、H2Oが脱離したピークが観測されることもある。塩の場合は、通常、フリー体の分子イオンピークもしくはフラグメントイオンピークが観測される。 1 H NMR was measured by Fourier transform NMR. For analysis, ACD / SpecManager (trade name) was used. Peaks with very gentle protons such as hydroxyl groups and amino groups are not described.
MS was measured by LC / MS. As the ionization method, the ESI method or the APCI method was used. The data is the actual measurement (found). Usually, a molecular ion peak is observed, but in the case of a compound having a tert-butoxycarbonyl group, a peak from which a tert-butoxycarbonyl group or a tert-butyl group is eliminated may be observed as a fragment ion. In the case of a compound having a hydroxyl group, a peak from which H 2 O is eliminated may be observed as a fragment ion. In the case of a salt, a free molecular ion peak or a fragment ion peak is usually observed.
実施例14
2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オン Example 14
2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2 , 3-Dihydroquinazolin-4 (1H) -one
2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オン Example 14
2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2 , 3-Dihydroquinazolin-4 (1H) -one
A)(R)-4-ブロモ-2-((1-フェニルエチル)アミノ)ベンズアミド
4-ブロモ-2-フルオロベンゾニトリル (1.5 g) のDMSO (10 mL) 溶液に(R)-1-フェニルエタンアミン(1.2 g) 及び炭酸カリウム (2.1 g) を加え、100℃で終夜攪拌した。反応混合物を室温まで冷却した後、反応混合物に過酸化水素水(1.3 g) を加え、室温で2時間攪拌した。反応混合物を水に加えた後、析出物をろ取し、減圧下で乾燥し、標題化合物(2.2 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.22-1.50 (3H, m), 4.55-4.77 (1H, m), 6.18-8.96 (11H, m). A) (R) -4-Bromo-2-((1-phenylethyl) amino) benzamide (R) -1-phenyl in a solution of 4-bromo-2-fluorobenzonitrile (1.5 g) in DMSO (10 mL) Ethaneamine (1.2 g) and potassium carbonate (2.1 g) were added, and the mixture was stirred at 100 ° C. overnight. After the reaction mixture was cooled to room temperature, hydrogen peroxide (1.3 g) was added to the reaction mixture, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was added to water, and the precipitate was collected by filtration and dried under reduced pressure to give the title compound (2.2 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.22-1.50 (3H, m), 4.55-4.77 (1H, m), 6.18-8.96 (11H, m).
4-ブロモ-2-フルオロベンゾニトリル (1.5 g) のDMSO (10 mL) 溶液に(R)-1-フェニルエタンアミン(1.2 g) 及び炭酸カリウム (2.1 g) を加え、100℃で終夜攪拌した。反応混合物を室温まで冷却した後、反応混合物に過酸化水素水(1.3 g) を加え、室温で2時間攪拌した。反応混合物を水に加えた後、析出物をろ取し、減圧下で乾燥し、標題化合物(2.2 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.22-1.50 (3H, m), 4.55-4.77 (1H, m), 6.18-8.96 (11H, m). A) (R) -4-Bromo-2-((1-phenylethyl) amino) benzamide (R) -1-phenyl in a solution of 4-bromo-2-fluorobenzonitrile (1.5 g) in DMSO (10 mL) Ethaneamine (1.2 g) and potassium carbonate (2.1 g) were added, and the mixture was stirred at 100 ° C. overnight. After the reaction mixture was cooled to room temperature, hydrogen peroxide (1.3 g) was added to the reaction mixture, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was added to water, and the precipitate was collected by filtration and dried under reduced pressure to give the title compound (2.2 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.22-1.50 (3H, m), 4.55-4.77 (1H, m), 6.18-8.96 (11H, m).
B)(R)-7-ブロモ-2,2-ジメチル-1-(1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オン
(R)-4-ブロモ-2-((1-フェニルエチル)アミノ)ベンズアミド (1.0 g) のアセトン (20 mL) 溶液に2-メトキシプロパ-1-エン (1.48 mL) 及びメタンスルホン酸 (30 mg) を加え、室温で1時間攪拌した。溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物(800 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.51 (3H, s), 1.63 (3H, s), 1.72 (3H, d, J = 6.8 Hz), 5.21 (1H, q, J = 6.8 Hz), 6.40 (1H, d, J = 1.5 Hz), 6.80 (1H, dd, J = 8.1, 1.7 Hz), 7.18-7.30 (2H, m), 7.36-7.40 (3H, m), 7.61 (1H, d, J = 8.3 Hz), 8.30 (1H, s). B) (R) -7-Bromo-2,2-dimethyl-1- (1-phenylethyl) -2,3-dihydroquinazolin-4 (1H) -one (R) -4-Bromo-2-(( 2-Methoxyprop-1-ene (1.48 mL) and methanesulfonic acid (30 mg) were added to a solution of 1-phenylethyl) amino) benzamide (1.0 g) in acetone (20 mL), and the mixture was stirred at room temperature for 1 hour. The solvent was distilled off under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (800 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.51 (3H, s), 1.63 (3H, s), 1.72 (3H, d, J = 6.8 Hz), 5.21 (1H, q, J = 6.8 Hz) , 6.40 (1H, d, J = 1.5 Hz), 6.80 (1H, dd, J = 8.1, 1.7 Hz), 7.18-7.30 (2H, m), 7.36-7.40 (3H, m), 7.61 (1H, d , J = 8.3 Hz), 8.30 (1H, s).
(R)-4-ブロモ-2-((1-フェニルエチル)アミノ)ベンズアミド (1.0 g) のアセトン (20 mL) 溶液に2-メトキシプロパ-1-エン (1.48 mL) 及びメタンスルホン酸 (30 mg) を加え、室温で1時間攪拌した。溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物(800 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.51 (3H, s), 1.63 (3H, s), 1.72 (3H, d, J = 6.8 Hz), 5.21 (1H, q, J = 6.8 Hz), 6.40 (1H, d, J = 1.5 Hz), 6.80 (1H, dd, J = 8.1, 1.7 Hz), 7.18-7.30 (2H, m), 7.36-7.40 (3H, m), 7.61 (1H, d, J = 8.3 Hz), 8.30 (1H, s). B) (R) -7-Bromo-2,2-dimethyl-1- (1-phenylethyl) -2,3-dihydroquinazolin-4 (1H) -one (R) -4-Bromo-2-(( 2-Methoxyprop-1-ene (1.48 mL) and methanesulfonic acid (30 mg) were added to a solution of 1-phenylethyl) amino) benzamide (1.0 g) in acetone (20 mL), and the mixture was stirred at room temperature for 1 hour. The solvent was distilled off under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (800 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.51 (3H, s), 1.63 (3H, s), 1.72 (3H, d, J = 6.8 Hz), 5.21 (1H, q, J = 6.8 Hz) , 6.40 (1H, d, J = 1.5 Hz), 6.80 (1H, dd, J = 8.1, 1.7 Hz), 7.18-7.30 (2H, m), 7.36-7.40 (3H, m), 7.61 (1H, d , J = 8.3 Hz), 8.30 (1H, s).
C) 2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オン
tert-ブチル (3-アミノピリジン-2-イル)カルバマート (105 mg)およびナトリウム tert-ブトキシド (80 mg) のtert-ブチルアルコール (3 mL) 溶液にトリス(ジベンジリデンアセトン)二パラジウム(0) (38.2 mg) 及び2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (78 mg)を加え、100 ℃で3時間撹拌した。反応混合物をろ過し、ろ液を減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、標題化合物 (103 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.57 (3H, s), 1.66-1.81 (6H, m), 5.23 (1H, q, J = 7.1 Hz), 5.95 (1H, d, J = 6.8 Hz), 6.32 (1H, d, J = 1.9 Hz), 6.72 (1H, dd, J = 7.9, 5.3 Hz), 6.86 (1H, dd, J = 8.3, 1.9 Hz), 7.30-7.49 (5H, m), 7.79-7.94 (2H, m), 8.30 (1H, s), 11.79 (1H, s).
MS (ESI+): [M+H]+414.3. C) 2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2,3-Dihydroquinazolin-4 (1H) -one tert-butyl (3-aminopyridin-2-yl) carbamate (105 mg) and sodium tert-butoxide (80 mg) in tert-butyl alcohol (3 mL) Add tris (dibenzylideneacetone) dipalladium (0) (38.2 mg) and 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1'-biphenyl (78 mg) to the solution at 100 ° C. Stir for 3 hours. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) to give the title compound (103 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.57 (3H, s), 1.66-1.81 (6H, m), 5.23 (1H, q, J = 7.1 Hz), 5.95 (1H, d, J = 6.8 Hz), 6.32 (1H, d, J = 1.9 Hz), 6.72 (1H, dd, J = 7.9, 5.3 Hz), 6.86 (1H, dd, J = 8.3, 1.9 Hz), 7.30-7.49 (5H, m ), 7.79-7.94 (2H, m), 8.30 (1H, s), 11.79 (1H, s).
MS (ESI +): [M + H] + 414.3.
tert-ブチル (3-アミノピリジン-2-イル)カルバマート (105 mg)およびナトリウム tert-ブトキシド (80 mg) のtert-ブチルアルコール (3 mL) 溶液にトリス(ジベンジリデンアセトン)二パラジウム(0) (38.2 mg) 及び2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (78 mg)を加え、100 ℃で3時間撹拌した。反応混合物をろ過し、ろ液を減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、標題化合物 (103 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.57 (3H, s), 1.66-1.81 (6H, m), 5.23 (1H, q, J = 7.1 Hz), 5.95 (1H, d, J = 6.8 Hz), 6.32 (1H, d, J = 1.9 Hz), 6.72 (1H, dd, J = 7.9, 5.3 Hz), 6.86 (1H, dd, J = 8.3, 1.9 Hz), 7.30-7.49 (5H, m), 7.79-7.94 (2H, m), 8.30 (1H, s), 11.79 (1H, s).
MS (ESI+): [M+H]+414.3. C) 2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2,3-Dihydroquinazolin-4 (1H) -one tert-butyl (3-aminopyridin-2-yl) carbamate (105 mg) and sodium tert-butoxide (80 mg) in tert-butyl alcohol (3 mL) Add tris (dibenzylideneacetone) dipalladium (0) (38.2 mg) and 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1'-biphenyl (78 mg) to the solution at 100 ° C. Stir for 3 hours. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) to give the title compound (103 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.57 (3H, s), 1.66-1.81 (6H, m), 5.23 (1H, q, J = 7.1 Hz), 5.95 (1H, d, J = 6.8 Hz), 6.32 (1H, d, J = 1.9 Hz), 6.72 (1H, dd, J = 7.9, 5.3 Hz), 6.86 (1H, dd, J = 8.3, 1.9 Hz), 7.30-7.49 (5H, m ), 7.79-7.94 (2H, m), 8.30 (1H, s), 11.79 (1H, s).
MS (ESI +): [M + H] + 414.3.
実施例48
4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オン Example 48
4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one
4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オン Example 48
4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one
A) メチル 4-ブロモ-2-メチルベンゾアート
4-ブロモ-2-メチル安息香酸 (50.55 g) のメタノール (500 mL) 溶液に塩化チオニル (68.6 mL) を滴下し、反応混合物を還流条件下で2.5時間攪拌した。反応混合物を減圧下で濃縮し、残渣を炭酸水素ナトリウム水溶液で希釈し、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去し、標題化合物 (58.6 g) を得た。
1H NMR (300 MHz, CDCl3) δ 2.58 (3H, s), 3.88 (3H, s), 7.35-7.40 (1H, m), 7.42 (1H, s), 7.78 (1H, d, J = 8.3 Hz). A) Methyl 4-bromo-2-methylbenzoate To a solution of 4-bromo-2-methylbenzoic acid (50.55 g) in methanol (500 mL) was added dropwise thionyl chloride (68.6 mL), and the reaction mixture was refluxed. Stir for 2.5 hours. The reaction mixture was concentrated under reduced pressure, and the residue was diluted with aqueous sodium bicarbonate and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give the title compound (58.6 g).
1 H NMR (300 MHz, CDCl 3 ) δ 2.58 (3H, s), 3.88 (3H, s), 7.35-7.40 (1H, m), 7.42 (1H, s), 7.78 (1H, d, J = 8.3 Hz).
4-ブロモ-2-メチル安息香酸 (50.55 g) のメタノール (500 mL) 溶液に塩化チオニル (68.6 mL) を滴下し、反応混合物を還流条件下で2.5時間攪拌した。反応混合物を減圧下で濃縮し、残渣を炭酸水素ナトリウム水溶液で希釈し、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去し、標題化合物 (58.6 g) を得た。
1H NMR (300 MHz, CDCl3) δ 2.58 (3H, s), 3.88 (3H, s), 7.35-7.40 (1H, m), 7.42 (1H, s), 7.78 (1H, d, J = 8.3 Hz). A) Methyl 4-bromo-2-methylbenzoate To a solution of 4-bromo-2-methylbenzoic acid (50.55 g) in methanol (500 mL) was added dropwise thionyl chloride (68.6 mL), and the reaction mixture was refluxed. Stir for 2.5 hours. The reaction mixture was concentrated under reduced pressure, and the residue was diluted with aqueous sodium bicarbonate and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give the title compound (58.6 g).
1 H NMR (300 MHz, CDCl 3 ) δ 2.58 (3H, s), 3.88 (3H, s), 7.35-7.40 (1H, m), 7.42 (1H, s), 7.78 (1H, d, J = 8.3 Hz).
B) メチル 4-ブロモ-2-(ブロモメチル)ベンゾアート
メチル 4-ブロモ-2-メチルベンゾアート (26.6 g) のトリフルオロメチルベンゼン (100 mL) 溶液にアゾビスイソブチロニトリル (0.955 g) 及びN-ブロモスクシンイミド(21.7 g) を加え、反応混合物を70℃で窒素雰囲気下、3時間攪拌した。固体をろ別し、ろ液を減圧下で濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、ジイソプロピルエーテル及びヘキサンで洗浄し、標題化合物 (29.5 g) を得た。
1H NMR (300 MHz, CDCl3) δ 3.94 (3H, s), 4.90 (2H, s), 7.51 (1H, dd, J = 8.5, 2.1 Hz), 7.63 (1H, d, J = 1.9 Hz), 7.84 (1H, d, J = 8.7 Hz). B) Methyl 4-bromo-2- (bromomethyl) benzoate A solution of methyl 4-bromo-2-methylbenzoate (26.6 g) in trifluoromethylbenzene (100 mL) and azobisisobutyronitrile (0.955 g) and N-bromosuccinimide (21.7 g) was added and the reaction mixture was stirred at 70 ° C. under a nitrogen atmosphere for 3 hours. The solid was filtered off and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) and washed with diisopropyl ether and hexane to give the title compound (29.5 g).
1 H NMR (300 MHz, CDCl 3 ) δ 3.94 (3H, s), 4.90 (2H, s), 7.51 (1H, dd, J = 8.5, 2.1 Hz), 7.63 (1H, d, J = 1.9 Hz) , 7.84 (1H, d, J = 8.7 Hz).
メチル 4-ブロモ-2-メチルベンゾアート (26.6 g) のトリフルオロメチルベンゼン (100 mL) 溶液にアゾビスイソブチロニトリル (0.955 g) 及びN-ブロモスクシンイミド(21.7 g) を加え、反応混合物を70℃で窒素雰囲気下、3時間攪拌した。固体をろ別し、ろ液を減圧下で濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、ジイソプロピルエーテル及びヘキサンで洗浄し、標題化合物 (29.5 g) を得た。
1H NMR (300 MHz, CDCl3) δ 3.94 (3H, s), 4.90 (2H, s), 7.51 (1H, dd, J = 8.5, 2.1 Hz), 7.63 (1H, d, J = 1.9 Hz), 7.84 (1H, d, J = 8.7 Hz). B) Methyl 4-bromo-2- (bromomethyl) benzoate A solution of methyl 4-bromo-2-methylbenzoate (26.6 g) in trifluoromethylbenzene (100 mL) and azobisisobutyronitrile (0.955 g) and N-bromosuccinimide (21.7 g) was added and the reaction mixture was stirred at 70 ° C. under a nitrogen atmosphere for 3 hours. The solid was filtered off and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) and washed with diisopropyl ether and hexane to give the title compound (29.5 g).
1 H NMR (300 MHz, CDCl 3 ) δ 3.94 (3H, s), 4.90 (2H, s), 7.51 (1H, dd, J = 8.5, 2.1 Hz), 7.63 (1H, d, J = 1.9 Hz) , 7.84 (1H, d, J = 8.7 Hz).
C) メチル 4-ブロモ-2-(シアノメチル)ベンゾアート
メチル 4-ブロモ-2-(ブロモメチル)ベンゾアート(46 g) のアセトニトリル (300 mL) 溶液にトリメチルシランカルボニトリル (40.0 mL) を加えた。反応混合物を室温下で10分攪拌し、1Mフッ化テトラ-n-ブチルアンモニウムTHF溶液(299 mL)を加えた。反応混合物を減圧下で濃縮した。残渣に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、ジイソプロピルエーテルとヘキサンから再結晶し、標題化合物 (23.5 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 3.87 (3H, s), 4.26 (2H, s), 7.72-7.78 (1H, m), 7.83 (1H, d, J = 1.9 Hz), 7.90 (1H, d, J = 8.3 Hz). C) Methyl 4-bromo-2- (cyanomethyl) benzoate To a solution of methyl 4-bromo-2- (bromomethyl) benzoate (46 g) in acetonitrile (300 mL) was added trimethylsilanecarbonitrile (40.0 mL). The reaction mixture was stirred at room temperature for 10 minutes, and 1M tetra-n-butylammonium fluoride THF solution (299 mL) was added. The reaction mixture was concentrated under reduced pressure. Water was added to the residue and extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) and recrystallized from diisopropyl ether and hexane to obtain the title compound (23.5 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.87 (3H, s), 4.26 (2H, s), 7.72-7.78 (1H, m), 7.83 (1H, d, J = 1.9 Hz), 7.90 ( (1H, d, J = 8.3 Hz).
メチル 4-ブロモ-2-(ブロモメチル)ベンゾアート(46 g) のアセトニトリル (300 mL) 溶液にトリメチルシランカルボニトリル (40.0 mL) を加えた。反応混合物を室温下で10分攪拌し、1Mフッ化テトラ-n-ブチルアンモニウムTHF溶液(299 mL)を加えた。反応混合物を減圧下で濃縮した。残渣に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、ジイソプロピルエーテルとヘキサンから再結晶し、標題化合物 (23.5 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 3.87 (3H, s), 4.26 (2H, s), 7.72-7.78 (1H, m), 7.83 (1H, d, J = 1.9 Hz), 7.90 (1H, d, J = 8.3 Hz). C) Methyl 4-bromo-2- (cyanomethyl) benzoate To a solution of methyl 4-bromo-2- (bromomethyl) benzoate (46 g) in acetonitrile (300 mL) was added trimethylsilanecarbonitrile (40.0 mL). The reaction mixture was stirred at room temperature for 10 minutes, and 1M tetra-n-butylammonium fluoride THF solution (299 mL) was added. The reaction mixture was concentrated under reduced pressure. Water was added to the residue and extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) and recrystallized from diisopropyl ether and hexane to obtain the title compound (23.5 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.87 (3H, s), 4.26 (2H, s), 7.72-7.78 (1H, m), 7.83 (1H, d, J = 1.9 Hz), 7.90 ( (1H, d, J = 8.3 Hz).
D) メチル 4-ブロモ-2-(1-シアノ-2-フェニルエチル)ベンゾアート
メチル 4-ブロモ-2-(シアノメチル)ベンゾアート(8.0 g) 及びベンジルブロミド (4.11 mL) のTHF (100 mL) 溶液に1.3MリチウムヘキサメチルジシラジドTHF溶液(26.6 mL) を0℃で滴下した。反応混合物を室温で3時間攪拌した。反応混合物に1M塩酸を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (10.7 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 3.16-3.24 (2H, m), 3.87 (3H, s), 5.22 (1H, dd, J = 8.5, 6.6 Hz), 7.20-7.39 (6H, m), 7.71-7.77 (1H, m), 7.82-7.85 (1H, m). D) Methyl 4-bromo-2- (1-cyano-2-phenylethyl) benzoate Methyl 4-bromo-2- (cyanomethyl) benzoate (8.0 g) and benzyl bromide (4.11 mL) in THF (100 mL) To the solution, 1.3M lithium hexamethyldisilazide THF solution (26.6 mL) was added dropwise at 0 ° C. The reaction mixture was stirred at room temperature for 3 hours. 1M Hydrochloric acid was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (10.7 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.16-3.24 (2H, m), 3.87 (3H, s), 5.22 (1H, dd, J = 8.5, 6.6 Hz), 7.20-7.39 (6H, m ), 7.71-7.77 (1H, m), 7.82-7.85 (1H, m).
メチル 4-ブロモ-2-(シアノメチル)ベンゾアート(8.0 g) 及びベンジルブロミド (4.11 mL) のTHF (100 mL) 溶液に1.3MリチウムヘキサメチルジシラジドTHF溶液(26.6 mL) を0℃で滴下した。反応混合物を室温で3時間攪拌した。反応混合物に1M塩酸を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (10.7 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 3.16-3.24 (2H, m), 3.87 (3H, s), 5.22 (1H, dd, J = 8.5, 6.6 Hz), 7.20-7.39 (6H, m), 7.71-7.77 (1H, m), 7.82-7.85 (1H, m). D) Methyl 4-bromo-2- (1-cyano-2-phenylethyl) benzoate Methyl 4-bromo-2- (cyanomethyl) benzoate (8.0 g) and benzyl bromide (4.11 mL) in THF (100 mL) To the solution, 1.3M lithium hexamethyldisilazide THF solution (26.6 mL) was added dropwise at 0 ° C. The reaction mixture was stirred at room temperature for 3 hours. 1M Hydrochloric acid was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (10.7 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.16-3.24 (2H, m), 3.87 (3H, s), 5.22 (1H, dd, J = 8.5, 6.6 Hz), 7.20-7.39 (6H, m ), 7.71-7.77 (1H, m), 7.82-7.85 (1H, m).
E) 4-ベンジル-6-ブロモ-3,4-ジヒドロイソキノリン-1(2H)-オン
メチル 4-ブロモ-2-(1-シアノ-2-フェニルエチル)ベンゾアート (3.0 g) のTHF (10 mL) 溶液に1.1MボランTHF錯体 THF溶液 (23.77 mL) を室温で加えた。反応混合物を窒素雰囲気下、70℃で2時間攪拌した。反応混合物に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (710 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 2.67-2.82 (1H, m), 2.83-2.95 (1H, m), 2.99-3.22 (2H, m), 3.34-3.42 (1H, m), 7.14-7.38 (5H, m), 7.48 (1H, d, J = 1.9 Hz), 7.56 (1H, dd, J = 8.3, 1.9 Hz), 7.79 (1H, d, J = 8.3 Hz), 7.89-8.00 (1H, m). E) 4-Benzyl-6-bromo-3,4-dihydroisoquinolin-1 (2H) -one methyl 4-bromo-2- (1-cyano-2-phenylethyl) benzoate (3.0 g) in THF (10 To the solution, 1.1 M borane THF complex THF solution (23.77 mL) was added at room temperature. The reaction mixture was stirred at 70 ° C. for 2 hours under nitrogen atmosphere. A saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (710 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 2.67-2.82 (1H, m), 2.83-2.95 (1H, m), 2.99-3.22 (2H, m), 3.34-3.42 (1H, m), 7.14 -7.38 (5H, m), 7.48 (1H, d, J = 1.9 Hz), 7.56 (1H, dd, J = 8.3, 1.9 Hz), 7.79 (1H, d, J = 8.3 Hz), 7.89-8.00 ( 1H, m).
メチル 4-ブロモ-2-(1-シアノ-2-フェニルエチル)ベンゾアート (3.0 g) のTHF (10 mL) 溶液に1.1MボランTHF錯体 THF溶液 (23.77 mL) を室温で加えた。反応混合物を窒素雰囲気下、70℃で2時間攪拌した。反応混合物に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (710 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 2.67-2.82 (1H, m), 2.83-2.95 (1H, m), 2.99-3.22 (2H, m), 3.34-3.42 (1H, m), 7.14-7.38 (5H, m), 7.48 (1H, d, J = 1.9 Hz), 7.56 (1H, dd, J = 8.3, 1.9 Hz), 7.79 (1H, d, J = 8.3 Hz), 7.89-8.00 (1H, m). E) 4-Benzyl-6-bromo-3,4-dihydroisoquinolin-1 (2H) -one methyl 4-bromo-2- (1-cyano-2-phenylethyl) benzoate (3.0 g) in THF (10 To the solution, 1.1 M borane THF complex THF solution (23.77 mL) was added at room temperature. The reaction mixture was stirred at 70 ° C. for 2 hours under nitrogen atmosphere. A saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (710 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 2.67-2.82 (1H, m), 2.83-2.95 (1H, m), 2.99-3.22 (2H, m), 3.34-3.42 (1H, m), 7.14 -7.38 (5H, m), 7.48 (1H, d, J = 1.9 Hz), 7.56 (1H, dd, J = 8.3, 1.9 Hz), 7.79 (1H, d, J = 8.3 Hz), 7.89-8.00 ( 1H, m).
F) 4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オン
4-ベンジル-6-ブロモ-3,4-ジヒドロイソキノリン-1(2H)-オン (1500 mg)、tert-ブチル (3-アミノピリジン-2-イル)カルバマート (1092 mg)、ナトリウム tert-ブトキシド (912 mg)、トリス(ジベンジリデンアセトン)二パラジウム(0) (434 mg)および2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (885 mg) のtert-ブチルアルコール (5 mL) 溶液を、100 ℃で終夜撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、標題化合物 (330 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 2.91 (2H, d, J = 9.1 Hz), 3.30 (2H, s), 3.43-3.60 (1H, m), 6.81-7.11 (2H, m), 7.19-7.45 (5H, m), 7.48-7.69 (1H, m), 7.90-8.12 (3H, m), 11.91 (1H, s).
MS (ESI+): [M+H]+371.2. F) 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one 4 -Benzyl-6-bromo-3,4-dihydroisoquinolin-1 (2H) -one (1500 mg), tert-butyl (3-aminopyridin-2-yl) carbamate (1092 mg), sodium tert-butoxide (912 mg), tris (dibenzylideneacetone) dipalladium (0) (434 mg) and 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1'-biphenyl (885 mg) in tert-butyl alcohol (5 mL) The solution was stirred at 100 ° C. overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) to give the title compound (330 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 2.91 (2H, d, J = 9.1 Hz), 3.30 (2H, s), 3.43-3.60 (1H, m), 6.81-7.11 (2H, m), 7.19-7.45 (5H, m), 7.48-7.69 (1H, m), 7.90-8.12 (3H, m), 11.91 (1H, s).
MS (ESI +): [M + H] + 371.2.
4-ベンジル-6-ブロモ-3,4-ジヒドロイソキノリン-1(2H)-オン (1500 mg)、tert-ブチル (3-アミノピリジン-2-イル)カルバマート (1092 mg)、ナトリウム tert-ブトキシド (912 mg)、トリス(ジベンジリデンアセトン)二パラジウム(0) (434 mg)および2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (885 mg) のtert-ブチルアルコール (5 mL) 溶液を、100 ℃で終夜撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、標題化合物 (330 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 2.91 (2H, d, J = 9.1 Hz), 3.30 (2H, s), 3.43-3.60 (1H, m), 6.81-7.11 (2H, m), 7.19-7.45 (5H, m), 7.48-7.69 (1H, m), 7.90-8.12 (3H, m), 11.91 (1H, s).
MS (ESI+): [M+H]+371.2. F) 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one 4 -Benzyl-6-bromo-3,4-dihydroisoquinolin-1 (2H) -one (1500 mg), tert-butyl (3-aminopyridin-2-yl) carbamate (1092 mg), sodium tert-butoxide (912 mg), tris (dibenzylideneacetone) dipalladium (0) (434 mg) and 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1'-biphenyl (885 mg) in tert-butyl alcohol (5 mL) The solution was stirred at 100 ° C. overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) to give the title compound (330 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 2.91 (2H, d, J = 9.1 Hz), 3.30 (2H, s), 3.43-3.60 (1H, m), 6.81-7.11 (2H, m), 7.19-7.45 (5H, m), 7.48-7.69 (1H, m), 7.90-8.12 (3H, m), 11.91 (1H, s).
MS (ESI +): [M + H] + 371.2.
実施例67
4-ベンジル-3,3-ジメチル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オン Example 67
4-Benzyl-3,3-dimethyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinoline-1 (2H )-on
4-ベンジル-3,3-ジメチル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オン Example 67
4-Benzyl-3,3-dimethyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinoline-1 (2H )-on
A) メチル 2-(3-ブロモフェニル)-3-フェニルプロパノアート
ジイソプロピルアミン(4.24 g) のテトラヒドロフラン (50 mL) 溶液にノルマルブチルリチウムの2.5Mヘキサン溶液 (16.8 mL) を-78℃で窒素雰囲気下加え、-78℃で30 分撹拌した。反応混合物にメチル (3-ブロモフェニル)アセタート(9.16 g) のテトラヒドロフラン (100 mL) 溶液を-78℃で窒素雰囲気下加え、1時間攪拌した。反応混合物にベンジルブロマイド (7.18 g) を-78℃で窒素雰囲気下加え、室温で16時間撹拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (9.40 g) を得た。
1H NMR (400 MHz, CDCl3) δ 2.90-3.05 (1H, m), 3.30-3.45 (1H, m), 3.62 (3H, s), 3.75-3.85 (1H, m), 7.10 (2H, d, J = 6.8 Hz), 7.15-7.30 (5H, m), 7.35-7.45 (1H, m), 7.47 (1H, s). A) Methyl 2- (3-bromophenyl) -3-phenylpropanoate Diisopropylamine (4.24 g) in tetrahydrofuran (50 mL) was diluted with normal butyl lithium in 2.5 M hexane (16.8 mL) at -78 ° C and nitrogen. It added under atmosphere and stirred at -78 degreeC for 30 minutes. A solution of methyl (3-bromophenyl) acetate (9.16 g) in tetrahydrofuran (100 mL) was added to the reaction mixture at −78 ° C. in a nitrogen atmosphere, and the mixture was stirred for 1 hr. To the reaction mixture, benzyl bromide (7.18 g) was added at −78 ° C. under a nitrogen atmosphere, and the mixture was stirred at room temperature for 16 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (9.40 g).
1 H NMR (400 MHz, CDCl 3 ) δ 2.90-3.05 (1H, m), 3.30-3.45 (1H, m), 3.62 (3H, s), 3.75-3.85 (1H, m), 7.10 (2H, d , J = 6.8 Hz), 7.15-7.30 (5H, m), 7.35-7.45 (1H, m), 7.47 (1H, s).
ジイソプロピルアミン(4.24 g) のテトラヒドロフラン (50 mL) 溶液にノルマルブチルリチウムの2.5Mヘキサン溶液 (16.8 mL) を-78℃で窒素雰囲気下加え、-78℃で30 分撹拌した。反応混合物にメチル (3-ブロモフェニル)アセタート(9.16 g) のテトラヒドロフラン (100 mL) 溶液を-78℃で窒素雰囲気下加え、1時間攪拌した。反応混合物にベンジルブロマイド (7.18 g) を-78℃で窒素雰囲気下加え、室温で16時間撹拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (9.40 g) を得た。
1H NMR (400 MHz, CDCl3) δ 2.90-3.05 (1H, m), 3.30-3.45 (1H, m), 3.62 (3H, s), 3.75-3.85 (1H, m), 7.10 (2H, d, J = 6.8 Hz), 7.15-7.30 (5H, m), 7.35-7.45 (1H, m), 7.47 (1H, s). A) Methyl 2- (3-bromophenyl) -3-phenylpropanoate Diisopropylamine (4.24 g) in tetrahydrofuran (50 mL) was diluted with normal butyl lithium in 2.5 M hexane (16.8 mL) at -78 ° C and nitrogen. It added under atmosphere and stirred at -78 degreeC for 30 minutes. A solution of methyl (3-bromophenyl) acetate (9.16 g) in tetrahydrofuran (100 mL) was added to the reaction mixture at −78 ° C. in a nitrogen atmosphere, and the mixture was stirred for 1 hr. To the reaction mixture, benzyl bromide (7.18 g) was added at −78 ° C. under a nitrogen atmosphere, and the mixture was stirred at room temperature for 16 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (9.40 g).
1 H NMR (400 MHz, CDCl 3 ) δ 2.90-3.05 (1H, m), 3.30-3.45 (1H, m), 3.62 (3H, s), 3.75-3.85 (1H, m), 7.10 (2H, d , J = 6.8 Hz), 7.15-7.30 (5H, m), 7.35-7.45 (1H, m), 7.47 (1H, s).
B) 3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-オール
メチル 2-(3-ブロモフェニル)-3-フェニルプロパノアート(9.40 g) のテトラヒドロフラン(150 mL) 溶液にメチルマグネシウムブロマイドの3Mジエチルエーテル溶液(40.0 mL)を氷冷下で窒素雰囲気下加え、室温で16時間撹拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮し、標題化合物 (9.1 g) を得た。
1H NMR (400 MHz, CDCl3) δ 1.23 (3H, s), 1.30 (3H, s), 2.80-2.90 (1H, m), 2.91-3.00 (1H, m), 3.25-3.35 (1H, m), 6.97 (2H, d, J = 7.2 Hz), 7.00-7.20 (5H, m), 7.25-7.33 (1H, m), 7.37 (1H, s). B) 3- (3-Bromophenyl) -2-methyl-4-phenylbutan-2-ol methyl 2- (3-bromophenyl) -3-phenylpropanoate (9.40 g) in tetrahydrofuran (150 mL) To the solution, 3M diethyl ether solution (40.0 mL) of methylmagnesium bromide was added under a nitrogen atmosphere under ice cooling, and the mixture was stirred at room temperature for 16 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give the title compound (9.1 g).
1 H NMR (400 MHz, CDCl 3 ) δ 1.23 (3H, s), 1.30 (3H, s), 2.80-2.90 (1H, m), 2.91-3.00 (1H, m), 3.25-3.35 (1H, m ), 6.97 (2H, d, J = 7.2 Hz), 7.00-7.20 (5H, m), 7.25-7.33 (1H, m), 7.37 (1H, s).
メチル 2-(3-ブロモフェニル)-3-フェニルプロパノアート(9.40 g) のテトラヒドロフラン(150 mL) 溶液にメチルマグネシウムブロマイドの3Mジエチルエーテル溶液(40.0 mL)を氷冷下で窒素雰囲気下加え、室温で16時間撹拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮し、標題化合物 (9.1 g) を得た。
1H NMR (400 MHz, CDCl3) δ 1.23 (3H, s), 1.30 (3H, s), 2.80-2.90 (1H, m), 2.91-3.00 (1H, m), 3.25-3.35 (1H, m), 6.97 (2H, d, J = 7.2 Hz), 7.00-7.20 (5H, m), 7.25-7.33 (1H, m), 7.37 (1H, s). B) 3- (3-Bromophenyl) -2-methyl-4-phenylbutan-2-ol methyl 2- (3-bromophenyl) -3-phenylpropanoate (9.40 g) in tetrahydrofuran (150 mL) To the solution, 3M diethyl ether solution (40.0 mL) of methylmagnesium bromide was added under a nitrogen atmosphere under ice cooling, and the mixture was stirred at room temperature for 16 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give the title compound (9.1 g).
1 H NMR (400 MHz, CDCl 3 ) δ 1.23 (3H, s), 1.30 (3H, s), 2.80-2.90 (1H, m), 2.91-3.00 (1H, m), 3.25-3.35 (1H, m ), 6.97 (2H, d, J = 7.2 Hz), 7.00-7.20 (5H, m), 7.25-7.33 (1H, m), 7.37 (1H, s).
C) N-[3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-イル]-2-クロロアセトアミド
3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-オール (8.10 g)およびクロロアセトニトリル (19.3 g) の酢酸 (4.57 g) 溶液に濃硫酸 (10.2 g) を-10℃で窒素雰囲気下滴下した。反応混合物を室温で窒素雰囲気下、5時間撹拌した。反応混合物に氷水を加え、酢酸エチルで抽出した。有機層を飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮した。残渣をペンタンで洗浄し、標題化合物 (9.9 g) を得た。
MS (ESI+): [M+H]+395.9 C) N- [3- (3-Bromophenyl) -2-methyl-4-phenylbutan-2-yl] -2-chloroacetamide 3- (3-Bromophenyl) -2-methyl-4-phenylbutane- To a solution of 2-ol (8.10 g) and chloroacetonitrile (19.3 g) in acetic acid (4.57 g), concentrated sulfuric acid (10.2 g) was added dropwise at −10 ° C. under a nitrogen atmosphere. The reaction mixture was stirred at room temperature under a nitrogen atmosphere for 5 hours. Ice water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was washed with pentane to obtain the title compound (9.9 g).
MS (ESI +): [M + H] + 395.9
3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-オール (8.10 g)およびクロロアセトニトリル (19.3 g) の酢酸 (4.57 g) 溶液に濃硫酸 (10.2 g) を-10℃で窒素雰囲気下滴下した。反応混合物を室温で窒素雰囲気下、5時間撹拌した。反応混合物に氷水を加え、酢酸エチルで抽出した。有機層を飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮した。残渣をペンタンで洗浄し、標題化合物 (9.9 g) を得た。
MS (ESI+): [M+H]+395.9 C) N- [3- (3-Bromophenyl) -2-methyl-4-phenylbutan-2-yl] -2-chloroacetamide 3- (3-Bromophenyl) -2-methyl-4-phenylbutane- To a solution of 2-ol (8.10 g) and chloroacetonitrile (19.3 g) in acetic acid (4.57 g), concentrated sulfuric acid (10.2 g) was added dropwise at −10 ° C. under a nitrogen atmosphere. The reaction mixture was stirred at room temperature under a nitrogen atmosphere for 5 hours. Ice water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was washed with pentane to obtain the title compound (9.9 g).
MS (ESI +): [M + H] + 395.9
D) 3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-アミン
N-[3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-イル]-2-クロロアセトアミド (9.90 g) とチオ尿素 (2.28 g) の酢酸 (20 mL) 及び エタノール (100 mL) の混合溶液を還流条件下、窒素雰囲気下で16時間撹拌した。反応混合物を室温まで冷却し、析出物をろ過し、エタノールで洗浄した。ろ液を減圧下濃縮し、残渣に2M水酸化ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮し、標題化合物 (7.1 g) を得た。
MS (ESI+): [M+H]+319.7. D) 3- (3-Bromophenyl) -2-methyl-4-phenylbutan-2-amine N- [3- (3-bromophenyl) -2-methyl-4-phenylbutan-2-yl] -2 -A mixed solution of -chloroacetamide (9.90 g) and thiourea (2.28 g) in acetic acid (20 mL) and ethanol (100 mL) was stirred under refluxing nitrogen atmosphere for 16 hours. The reaction mixture was cooled to room temperature and the precipitate was filtered and washed with ethanol. The filtrate was concentrated under reduced pressure, 2M aqueous sodium hydroxide solution was added to the residue, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the title compound (7.1 g).
MS (ESI +): [M + H] + 319.7.
N-[3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-イル]-2-クロロアセトアミド (9.90 g) とチオ尿素 (2.28 g) の酢酸 (20 mL) 及び エタノール (100 mL) の混合溶液を還流条件下、窒素雰囲気下で16時間撹拌した。反応混合物を室温まで冷却し、析出物をろ過し、エタノールで洗浄した。ろ液を減圧下濃縮し、残渣に2M水酸化ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮し、標題化合物 (7.1 g) を得た。
MS (ESI+): [M+H]+319.7. D) 3- (3-Bromophenyl) -2-methyl-4-phenylbutan-2-amine N- [3- (3-bromophenyl) -2-methyl-4-phenylbutan-2-yl] -2 -A mixed solution of -chloroacetamide (9.90 g) and thiourea (2.28 g) in acetic acid (20 mL) and ethanol (100 mL) was stirred under refluxing nitrogen atmosphere for 16 hours. The reaction mixture was cooled to room temperature and the precipitate was filtered and washed with ethanol. The filtrate was concentrated under reduced pressure, 2M aqueous sodium hydroxide solution was added to the residue, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the title compound (7.1 g).
MS (ESI +): [M + H] + 319.7.
E) 4-ベンジル-6-ブロモ-3,3-ジメチル-1,2,3,4-テトラヒドロイソキノリン
3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-アミン (4.00 g) のジクロロエタン (42 mL) 溶液にパラホルムアルデヒド(756 mg) 及びトリフルオロ酢酸 (18 mL) を室温で加えた。反応混合物を還流条件下、窒素雰囲気下で16時間撹拌した。反応混合物を室温まで冷却し、減圧下で溶媒を除去した。残渣に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮し、標題化合物を含む粗精製物 (4.0 g) を得た。
MS (ESI+): [M+H]+331.7. E) 4-Benzyl-6-bromo-3,3-dimethyl-1,2,3,4-tetrahydroisoquinoline 3- (3-bromophenyl) -2-methyl-4-phenylbutan-2-amine (4.00 g ) In dichloroethane (42 mL) was added paraformaldehyde (756 mg) and trifluoroacetic acid (18 mL) at room temperature. The reaction mixture was stirred at reflux under a nitrogen atmosphere for 16 hours. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. To the residue was added saturated aqueous sodium hydrogen carbonate solution, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product (4.0 g) containing the title compound.
MS (ESI +): [M + H] + 331.7.
3-(3-ブロモフェニル)-2-メチル-4-フェニルブタン-2-アミン (4.00 g) のジクロロエタン (42 mL) 溶液にパラホルムアルデヒド(756 mg) 及びトリフルオロ酢酸 (18 mL) を室温で加えた。反応混合物を還流条件下、窒素雰囲気下で16時間撹拌した。反応混合物を室温まで冷却し、減圧下で溶媒を除去した。残渣に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮し、標題化合物を含む粗精製物 (4.0 g) を得た。
MS (ESI+): [M+H]+331.7. E) 4-Benzyl-6-bromo-3,3-dimethyl-1,2,3,4-tetrahydroisoquinoline 3- (3-bromophenyl) -2-methyl-4-phenylbutan-2-amine (4.00 g ) In dichloroethane (42 mL) was added paraformaldehyde (756 mg) and trifluoroacetic acid (18 mL) at room temperature. The reaction mixture was stirred at reflux under a nitrogen atmosphere for 16 hours. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. To the residue was added saturated aqueous sodium hydrogen carbonate solution, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product (4.0 g) containing the title compound.
MS (ESI +): [M + H] + 331.7.
F) 4-ベンジル-6-ブロモ-3,3-ジメチル-3,4-ジヒドロイソキノリン-1(2H)-オン
Eで得られた4-ベンジル-6-ブロモ-3,3-ジメチル-1,2,3,4-テトラヒドロイソキノリンを含む粗精製物 (4.00 g) のジクロロメタン (10 mL) 及び水 (50 mL) の混合溶液にヨードシルベンゼン (3.19 g) 及び臭化カリウム (1.44 g) を室温で加えた。反応混合物を室温で16時間撹拌した。反応混合物に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (800 mg) を得た。
MS (ESI+): [M+H]+345.8. F) 4-Benzyl-6-bromo-3,3-dimethyl-1,4-dimethyl-1,4-dihydroisoquinolin-1 (2H) -one obtained from E To a mixed solution of crude purified product (4.00 g) containing 2,3,4-tetrahydroisoquinoline (4.00 g) in dichloromethane (10 mL) and water (50 mL) was added iodosylbenzene (3.19 g) and potassium bromide (1.44 g) at room temperature. Added in. The reaction mixture was stirred at room temperature for 16 hours. A saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (800 mg).
MS (ESI +): [M + H] + 345.8.
Eで得られた4-ベンジル-6-ブロモ-3,3-ジメチル-1,2,3,4-テトラヒドロイソキノリンを含む粗精製物 (4.00 g) のジクロロメタン (10 mL) 及び水 (50 mL) の混合溶液にヨードシルベンゼン (3.19 g) 及び臭化カリウム (1.44 g) を室温で加えた。反応混合物を室温で16時間撹拌した。反応混合物に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (800 mg) を得た。
MS (ESI+): [M+H]+345.8. F) 4-Benzyl-6-bromo-3,3-dimethyl-1,4-dimethyl-1,4-dihydroisoquinolin-1 (2H) -one obtained from E To a mixed solution of crude purified product (4.00 g) containing 2,3,4-tetrahydroisoquinoline (4.00 g) in dichloromethane (10 mL) and water (50 mL) was added iodosylbenzene (3.19 g) and potassium bromide (1.44 g) at room temperature. Added in. The reaction mixture was stirred at room temperature for 16 hours. A saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (800 mg).
MS (ESI +): [M + H] + 345.8.
G) 4-ベンジル-3,3-ジメチル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オン
4-ベンジル-6-ブロモ-3,3-ジメチル-3,4-ジヒドロイソキノリン-1(2H)-オン (200 mg)、tert-ブチル (3-アミノピリジン-2-イル)カルバマート (182 mg)、ナトリウムtert-ブトキシド (279 mg)、トリス(ジベンジリデンアセトン)二パラジウム(0) (53 mg)および2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (54 mg) のtert-ブチルアルコール (5 mL) 溶液を、110 ℃で窒素雰囲気下16時間撹拌した。反応混合物を室温まで冷却し、反応混合物を水に加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、ペンタン及び酢酸エチルで洗浄することで、標題化合物 (43 mg) を得た。
1H NMR (400 MHz, CDCl3): δ 1.30 (3H, s), 1.54 (3H, s), 2.70-2.80 (1H, m), 2.90-3.00 (1H, m), 3.25-3.35 (1H, m), 5.95 (1H, brs), 6.15 (1H, dd, J = 8.0, 1.2 Hz), 6.58 (1H, d, J = 2.0 Hz), 6.85 (1H, dd, J = 8.0, 5.3 Hz), 6.89-6.97 (2H, m), 7.19-7.26 (3H, m), 7.61 (1H, dd, J= 8.3, 2.0 Hz), 8.04 (1H, dd, J = 5.3, 1.2 Hz), 8.25 (1H, d, J = 8.3 Hz), 9.54 (1H, brs). G) 4-Benzyl-3,3-dimethyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinoline-1 (2H) -one 4-benzyl-6-bromo-3,3-dimethyl-3,4-dihydroisoquinolin-1 (2H) -one (200 mg), tert-butyl (3-aminopyridin-2-yl) Carbamate (182 mg), sodium tert-butoxide (279 mg), tris (dibenzylideneacetone) dipalladium (0) (53 mg) and 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1 A solution of '-biphenyl (54 mg) in tert-butyl alcohol (5 mL) was stirred at 110 ° C. under a nitrogen atmosphere for 16 hours. The reaction mixture was cooled to room temperature, the reaction mixture was added to water and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) and washed with pentane and ethyl acetate to give the title compound (43 mg).
1 H NMR (400 MHz, CDCl 3 ): δ 1.30 (3H, s), 1.54 (3H, s), 2.70-2.80 (1H, m), 2.90-3.00 (1H, m), 3.25-3.35 (1H, m), 5.95 (1H, brs), 6.15 (1H, dd, J = 8.0, 1.2 Hz), 6.58 (1H, d, J = 2.0 Hz), 6.85 (1H, dd, J = 8.0, 5.3 Hz), 6.89-6.97 (2H, m), 7.19-7.26 (3H, m), 7.61 (1H, dd, J = 8.3, 2.0 Hz), 8.04 (1H, dd, J = 5.3, 1.2 Hz), 8.25 (1H, d, J = 8.3 Hz), 9.54 (1H, brs).
4-ベンジル-6-ブロモ-3,3-ジメチル-3,4-ジヒドロイソキノリン-1(2H)-オン (200 mg)、tert-ブチル (3-アミノピリジン-2-イル)カルバマート (182 mg)、ナトリウムtert-ブトキシド (279 mg)、トリス(ジベンジリデンアセトン)二パラジウム(0) (53 mg)および2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (54 mg) のtert-ブチルアルコール (5 mL) 溶液を、110 ℃で窒素雰囲気下16時間撹拌した。反応混合物を室温まで冷却し、反応混合物を水に加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、ペンタン及び酢酸エチルで洗浄することで、標題化合物 (43 mg) を得た。
1H NMR (400 MHz, CDCl3): δ 1.30 (3H, s), 1.54 (3H, s), 2.70-2.80 (1H, m), 2.90-3.00 (1H, m), 3.25-3.35 (1H, m), 5.95 (1H, brs), 6.15 (1H, dd, J = 8.0, 1.2 Hz), 6.58 (1H, d, J = 2.0 Hz), 6.85 (1H, dd, J = 8.0, 5.3 Hz), 6.89-6.97 (2H, m), 7.19-7.26 (3H, m), 7.61 (1H, dd, J= 8.3, 2.0 Hz), 8.04 (1H, dd, J = 5.3, 1.2 Hz), 8.25 (1H, d, J = 8.3 Hz), 9.54 (1H, brs). G) 4-Benzyl-3,3-dimethyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinoline-1 (2H) -one 4-benzyl-6-bromo-3,3-dimethyl-3,4-dihydroisoquinolin-1 (2H) -one (200 mg), tert-butyl (3-aminopyridin-2-yl) Carbamate (182 mg), sodium tert-butoxide (279 mg), tris (dibenzylideneacetone) dipalladium (0) (53 mg) and 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1 A solution of '-biphenyl (54 mg) in tert-butyl alcohol (5 mL) was stirred at 110 ° C. under a nitrogen atmosphere for 16 hours. The reaction mixture was cooled to room temperature, the reaction mixture was added to water and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) and washed with pentane and ethyl acetate to give the title compound (43 mg).
1 H NMR (400 MHz, CDCl 3 ): δ 1.30 (3H, s), 1.54 (3H, s), 2.70-2.80 (1H, m), 2.90-3.00 (1H, m), 3.25-3.35 (1H, m), 5.95 (1H, brs), 6.15 (1H, dd, J = 8.0, 1.2 Hz), 6.58 (1H, d, J = 2.0 Hz), 6.85 (1H, dd, J = 8.0, 5.3 Hz), 6.89-6.97 (2H, m), 7.19-7.26 (3H, m), 7.61 (1H, dd, J = 8.3, 2.0 Hz), 8.04 (1H, dd, J = 5.3, 1.2 Hz), 8.25 (1H, d, J = 8.3 Hz), 9.54 (1H, brs).
実施例69
8-ベンジル-2-(1H-ピロロ[2,3-b]ピリジン-3-イル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン Example 69
8-Benzyl-2- (1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one
8-ベンジル-2-(1H-ピロロ[2,3-b]ピリジン-3-イル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン Example 69
8-Benzyl-2- (1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one
A) エチル 2-ビニルニコチナート
エチル 2-クロロニコチナート (5.90 g)、炭酸水素ナトリウム (5.34 g)および4,4,5,5-テトラメチル-2-ビニル-1,3,2-ジオキサボロラン (5.14 g) のジメトキシエタン (20 mL) 及び水 (2 mL) の混合溶液にテトラキス(トリフェニルホスフィン)パラジウム(0) (1.84 g)を室温で加えた。反応混合物を80℃で窒素雰囲気下、終夜撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (4.75 g) を得た。
MS (ESI+): [M+H]+178.2. A) Ethyl 2-vinylnicotinate Ethyl 2-chloronicotinate (5.90 g), sodium bicarbonate (5.34 g) and 4,4,5,5-tetramethyl-2-vinyl-1,3,2-dioxaborolane ( Tetrakis (triphenylphosphine) palladium (0) (1.84 g) was added to a mixed solution of 5.14 g) of dimethoxyethane (20 mL) and water (2 mL) at room temperature. The reaction mixture was stirred at 80 ° C. under a nitrogen atmosphere overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (4.75 g).
MS (ESI +): [M + H] + 178.2.
エチル 2-クロロニコチナート (5.90 g)、炭酸水素ナトリウム (5.34 g)および4,4,5,5-テトラメチル-2-ビニル-1,3,2-ジオキサボロラン (5.14 g) のジメトキシエタン (20 mL) 及び水 (2 mL) の混合溶液にテトラキス(トリフェニルホスフィン)パラジウム(0) (1.84 g)を室温で加えた。反応混合物を80℃で窒素雰囲気下、終夜撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (4.75 g) を得た。
MS (ESI+): [M+H]+178.2. A) Ethyl 2-vinylnicotinate Ethyl 2-chloronicotinate (5.90 g), sodium bicarbonate (5.34 g) and 4,4,5,5-tetramethyl-2-vinyl-1,3,2-dioxaborolane ( Tetrakis (triphenylphosphine) palladium (0) (1.84 g) was added to a mixed solution of 5.14 g) of dimethoxyethane (20 mL) and water (2 mL) at room temperature. The reaction mixture was stirred at 80 ° C. under a nitrogen atmosphere overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (4.75 g).
MS (ESI +): [M + H] + 178.2.
B) 6-(4-メトキシベンジル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン
エチル 2-ビニルニコチナート (1.04 g) および4-メトキシベンジルアミン (0.886 g) のジメチルアセトアミド (5 mL) 溶液をマイクロウェーブ照射下、180℃で30分撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (3.40 g) を得た。
MS (ESI+): [M+H]+269.2. B) of 6- (4-methoxybenzyl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one ethyl 2-vinylnicotinate (1.04 g) and 4-methoxybenzylamine (0.886 g) The dimethylacetamide (5 mL) solution was stirred at 180 ° C. for 30 minutes under microwave irradiation. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (3.40 g).
MS (ESI +): [M + H] + 269.2.
エチル 2-ビニルニコチナート (1.04 g) および4-メトキシベンジルアミン (0.886 g) のジメチルアセトアミド (5 mL) 溶液をマイクロウェーブ照射下、180℃で30分撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (3.40 g) を得た。
MS (ESI+): [M+H]+269.2. B) of 6- (4-methoxybenzyl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one ethyl 2-vinylnicotinate (1.04 g) and 4-methoxybenzylamine (0.886 g) The dimethylacetamide (5 mL) solution was stirred at 180 ° C. for 30 minutes under microwave irradiation. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (3.40 g).
MS (ESI +): [M + H] + 269.2.
C) 8-ベンジル-6-(4-メトキシベンジル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン
6-(4-メトキシベンジル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン (650 mg) のテトラヒドロフラン (10 mL) 溶液にリチウムヘキサメチルジシラジドの1Mテトラヒドロフラン溶液(2.91 mL)を-78℃で加えた。反応混合物を-78℃で窒素雰囲気下、10分撹拌した。反応混合物にベンジルブロマイド (497 mg) を-78℃で加え、室温で窒素雰囲気下1時間攪拌した。反応混合物にリチウムジイソプロピルアミドの1Mテトラヒドロフラン溶液(2.88 mL)を-78℃で加え、室温で窒素雰囲気下終夜撹拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (375 mg) を得た。
MS (ESI+): [M+H]+359.3. C) 8-Benzyl-6- (4-methoxybenzyl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one 6- (4-methoxybenzyl) -7,8-dihydro-1, To a solution of 6-naphthyridin-5 (6H) -one (650 mg) in tetrahydrofuran (10 mL) was added 1M tetrahydrofuran solution (2.91 mL) of lithium hexamethyldisilazide at -78 ° C. The reaction mixture was stirred at −78 ° C. under a nitrogen atmosphere for 10 minutes. Benzyl bromide (497 mg) was added to the reaction mixture at −78 ° C., and the mixture was stirred at room temperature under a nitrogen atmosphere for 1 hour. To the reaction mixture was added lithium diisopropylamide in 1M tetrahydrofuran (2.88 mL) at -78 ° C, and the mixture was stirred at room temperature overnight under a nitrogen atmosphere. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (375 mg).
MS (ESI +): [M + H] + 359.3.
6-(4-メトキシベンジル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン (650 mg) のテトラヒドロフラン (10 mL) 溶液にリチウムヘキサメチルジシラジドの1Mテトラヒドロフラン溶液(2.91 mL)を-78℃で加えた。反応混合物を-78℃で窒素雰囲気下、10分撹拌した。反応混合物にベンジルブロマイド (497 mg) を-78℃で加え、室温で窒素雰囲気下1時間攪拌した。反応混合物にリチウムジイソプロピルアミドの1Mテトラヒドロフラン溶液(2.88 mL)を-78℃で加え、室温で窒素雰囲気下終夜撹拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (375 mg) を得た。
MS (ESI+): [M+H]+359.3. C) 8-Benzyl-6- (4-methoxybenzyl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one 6- (4-methoxybenzyl) -7,8-dihydro-1, To a solution of 6-naphthyridin-5 (6H) -one (650 mg) in tetrahydrofuran (10 mL) was added 1M tetrahydrofuran solution (2.91 mL) of lithium hexamethyldisilazide at -78 ° C. The reaction mixture was stirred at −78 ° C. under a nitrogen atmosphere for 10 minutes. Benzyl bromide (497 mg) was added to the reaction mixture at −78 ° C., and the mixture was stirred at room temperature under a nitrogen atmosphere for 1 hour. To the reaction mixture was added lithium diisopropylamide in 1M tetrahydrofuran (2.88 mL) at -78 ° C, and the mixture was stirred at room temperature overnight under a nitrogen atmosphere. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (375 mg).
MS (ESI +): [M + H] + 359.3.
D) 8-ベンジル-6-(4-メトキシベンジル)-5-オキソ-5,6,7,8-テトラヒドロ-1,6-ナフチリジン-1-オキシド
8-ベンジル-6-(4-メトキシベンジル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン(375mg) の酢酸エチル (10 mL) 溶液にメタクロロ過安息香酸 (284 mg) を氷冷下で加えた。反応混合物を室温で終夜撹拌した。反応混合物に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(390 mg) を得た。
MS (ESI+): [M+H]+375.2. D) 8-Benzyl-6- (4-methoxybenzyl) -5-oxo-5,6,7,8-tetrahydro-1,6-naphthyridine-1-oxide 8-benzyl-6- (4-methoxybenzyl) To a solution of -7,8-dihydro-1,6-naphthyridin-5 (6H) -one (375 mg) in ethyl acetate (10 mL) was added metachloroperbenzoic acid (284 mg) under ice cooling. The reaction mixture was stirred at room temperature overnight. A saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (390 mg).
MS (ESI +): [M + H] + 375.2.
8-ベンジル-6-(4-メトキシベンジル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン(375mg) の酢酸エチル (10 mL) 溶液にメタクロロ過安息香酸 (284 mg) を氷冷下で加えた。反応混合物を室温で終夜撹拌した。反応混合物に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(390 mg) を得た。
MS (ESI+): [M+H]+375.2. D) 8-Benzyl-6- (4-methoxybenzyl) -5-oxo-5,6,7,8-tetrahydro-1,6-naphthyridine-1-oxide 8-benzyl-6- (4-methoxybenzyl) To a solution of -7,8-dihydro-1,6-naphthyridin-5 (6H) -one (375 mg) in ethyl acetate (10 mL) was added metachloroperbenzoic acid (284 mg) under ice cooling. The reaction mixture was stirred at room temperature overnight. A saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (390 mg).
MS (ESI +): [M + H] + 375.2.
E) 8-ベンジル-2-クロロ-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン
8-ベンジル-6-(4-メトキシベンジル)-5-オキソ-5,6,7,8-テトラヒドロ-1,6-ナフチリジン-1-オキシド (390 mg) とオキシ塩化リン (2 mL) の混合物を80℃で2時間撹拌した。反応混合物を水に加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(55 mg) を得た。
MS (ESI+): [M+H]+273.2, 275.2. E) 8-Benzyl-2-chloro-7,8-dihydro-1,6-naphthyridin-5 (6H) -one 8-benzyl-6- (4-methoxybenzyl) -5-oxo-5,6,7 , 8-Tetrahydro-1,6-naphthyridine-1-oxide (390 mg) and phosphorus oxychloride (2 mL) were stirred at 80 ° C. for 2 hours. The reaction mixture was added to water and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (55 mg).
MS (ESI +): [M + H] + 273.2, 275.2.
8-ベンジル-6-(4-メトキシベンジル)-5-オキソ-5,6,7,8-テトラヒドロ-1,6-ナフチリジン-1-オキシド (390 mg) とオキシ塩化リン (2 mL) の混合物を80℃で2時間撹拌した。反応混合物を水に加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(55 mg) を得た。
MS (ESI+): [M+H]+273.2, 275.2. E) 8-Benzyl-2-chloro-7,8-dihydro-1,6-naphthyridin-5 (6H) -one 8-benzyl-6- (4-methoxybenzyl) -5-oxo-5,6,7 , 8-Tetrahydro-1,6-naphthyridine-1-oxide (390 mg) and phosphorus oxychloride (2 mL) were stirred at 80 ° C. for 2 hours. The reaction mixture was added to water and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (55 mg).
MS (ESI +): [M + H] + 273.2, 275.2.
F) 8-ベンジル-2-(1-(フェニルスルホニル)-1H-ピロロ[2,3-b]ピリジン-3-イル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン
8-ベンジル-2-クロロ-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン (55 mg)、炭酸水素ナトリウム (34 mg)および1-(フェニルスルホニル)-3-(4,4,5,5-テトラメチル-1,3,2-ジオキサボロラン-2-イル)-1H-ピロロ[2,3-b]ピリジン (77 mg) のジメトキシエタン (2 mL) 及び水 (0.2 mL) の混合溶液にテトラキス(トリフェニルホスフィン)パラジウム(0) (23 mg)を室温で加えた。反応混合物を90℃で窒素雰囲気下、終夜撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(37 mg) を得た。
MS (ESI+): [M+H]+495.3. F) 8-Benzyl-2- (1- (phenylsulfonyl) -1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridine-5 (6H)- On 8-benzyl-2-chloro-7,8-dihydro-1,6-naphthyridin-5 (6H) -one (55 mg), sodium bicarbonate (34 mg) and 1- (phenylsulfonyl) -3- ( 4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl) -1H-pyrrolo [2,3-b] pyridine (77 mg) in dimethoxyethane (2 mL) and water (0.2 tetrakis (triphenylphosphine) palladium (0) (23 mg) was added to a mixed solution at room temperature. The reaction mixture was stirred at 90 ° C. under a nitrogen atmosphere overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (37 mg).
MS (ESI +): [M + H] + 495.3.
8-ベンジル-2-クロロ-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン (55 mg)、炭酸水素ナトリウム (34 mg)および1-(フェニルスルホニル)-3-(4,4,5,5-テトラメチル-1,3,2-ジオキサボロラン-2-イル)-1H-ピロロ[2,3-b]ピリジン (77 mg) のジメトキシエタン (2 mL) 及び水 (0.2 mL) の混合溶液にテトラキス(トリフェニルホスフィン)パラジウム(0) (23 mg)を室温で加えた。反応混合物を90℃で窒素雰囲気下、終夜撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(37 mg) を得た。
MS (ESI+): [M+H]+495.3. F) 8-Benzyl-2- (1- (phenylsulfonyl) -1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridine-5 (6H)- On 8-benzyl-2-chloro-7,8-dihydro-1,6-naphthyridin-5 (6H) -one (55 mg), sodium bicarbonate (34 mg) and 1- (phenylsulfonyl) -3- ( 4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl) -1H-pyrrolo [2,3-b] pyridine (77 mg) in dimethoxyethane (2 mL) and water (0.2 tetrakis (triphenylphosphine) palladium (0) (23 mg) was added to a mixed solution at room temperature. The reaction mixture was stirred at 90 ° C. under a nitrogen atmosphere overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (37 mg).
MS (ESI +): [M + H] + 495.3.
G) 8-ベンジル-2-(1H-ピロロ[2,3-b]ピリジン-3-イル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン
8-ベンジル-2-(1-(フェニルスルホニル)-1H-ピロロ[2,3-b]ピリジン-3-イル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン(37 mg) のテトラヒドロフラン (3 mL)及びメタノール (1 mL) の混合溶液に2M水酸化ナトリウム水溶液(0.075 mL)を室温で加え、40℃で2時間撹拌した。反応混合物に8M水酸化ナトリウム水溶液(0.281 mL)を室温で加え、60℃で1時間攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をジイソプロピルエーテル及び酢酸エチルで洗浄し、標題化合物 (11 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 2.78-2.96 (1H, m), 3.12-3.27 (1H, m), 3.34-3.52 (3H, m), 7.14-7.42 (6H, m), 7.93 (2H, d, J = 8.3 Hz), 8.13 (1H, d, J = 8.3 Hz), 8.29 (1H, dd, J = 4.5, 1.5 Hz), 8.39 (1H, s), 8.71 (1H, dd, J = 7.9, 1.5 Hz), 12.19 (1H, brs). G) 8-Benzyl-2- (1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one 8-benzyl-2- (1- (phenylsulfonyl) -1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one (37 mg) in tetrahydrofuran ( 3M) and methanol (1 mL) were mixed with 2M aqueous sodium hydroxide solution (0.075 mL) at room temperature, and stirred at 40 ° C. for 2 hours. To the reaction mixture was added 8M aqueous sodium hydroxide solution (0.281 mL) at room temperature, and the mixture was stirred at 60 ° C. for 1 hr. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was washed with diisopropyl ether and ethyl acetate to give the title compound (11 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 2.78-2.96 (1H, m), 3.12-3.27 (1H, m), 3.34-3.52 (3H, m), 7.14-7.42 (6H, m), 7.93 (2H, d, J = 8.3 Hz), 8.13 (1H, d, J = 8.3 Hz), 8.29 (1H, dd, J = 4.5, 1.5 Hz), 8.39 (1H, s), 8.71 (1H, dd, J = 7.9, 1.5 Hz), 12.19 (1H, brs).
8-ベンジル-2-(1-(フェニルスルホニル)-1H-ピロロ[2,3-b]ピリジン-3-イル)-7,8-ジヒドロ-1,6-ナフチリジン-5(6H)-オン(37 mg) のテトラヒドロフラン (3 mL)及びメタノール (1 mL) の混合溶液に2M水酸化ナトリウム水溶液(0.075 mL)を室温で加え、40℃で2時間撹拌した。反応混合物に8M水酸化ナトリウム水溶液(0.281 mL)を室温で加え、60℃で1時間攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下濃縮した。残渣をジイソプロピルエーテル及び酢酸エチルで洗浄し、標題化合物 (11 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 2.78-2.96 (1H, m), 3.12-3.27 (1H, m), 3.34-3.52 (3H, m), 7.14-7.42 (6H, m), 7.93 (2H, d, J = 8.3 Hz), 8.13 (1H, d, J = 8.3 Hz), 8.29 (1H, dd, J = 4.5, 1.5 Hz), 8.39 (1H, s), 8.71 (1H, dd, J = 7.9, 1.5 Hz), 12.19 (1H, brs). G) 8-Benzyl-2- (1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one 8-benzyl-2- (1- (phenylsulfonyl) -1H-pyrrolo [2,3-b] pyridin-3-yl) -7,8-dihydro-1,6-naphthyridin-5 (6H) -one (37 mg) in tetrahydrofuran ( 3M) and methanol (1 mL) were mixed with 2M aqueous sodium hydroxide solution (0.075 mL) at room temperature, and stirred at 40 ° C. for 2 hours. To the reaction mixture was added 8M aqueous sodium hydroxide solution (0.281 mL) at room temperature, and the mixture was stirred at 60 ° C. for 1 hr. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was washed with diisopropyl ether and ethyl acetate to give the title compound (11 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 2.78-2.96 (1H, m), 3.12-3.27 (1H, m), 3.34-3.52 (3H, m), 7.14-7.42 (6H, m), 7.93 (2H, d, J = 8.3 Hz), 8.13 (1H, d, J = 8.3 Hz), 8.29 (1H, dd, J = 4.5, 1.5 Hz), 8.39 (1H, s), 8.71 (1H, dd, J = 7.9, 1.5 Hz), 12.19 (1H, brs).
実施例75
4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン Example 75
4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydro-2,7-naphthyridine-1 (2H) -on
4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン Example 75
4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydro-2,7-naphthyridine-1 (2H) -on
A) 4-ヨード-6-メトキシピリジン-3-カルボン酸
2,2,6,6-テトラメチルピペリジンのテトラヒドロフラン (100 mL) 溶液にノルマルブチルリチウムの2.5Mヘキサン溶液(39.2 mL)を-78℃で窒素雰囲気下加えた。反応混合物を-78℃で1時間攪拌した。反応混合物を6-メトキシピリジン-3-カルボン酸 (5.00 g) のテトラヒドロフラン (50 mL) 溶液に窒素雰囲気下-78℃で加え、-78℃で2時間攪拌した。反応混合物をヨウ素 (24.9 g) のテトラヒドロフラン (50 mL) 溶液に-78℃で窒素雰囲気下加え、室温で16時間攪拌した。反応混合物に水を加え、減圧下テトラヒドロフランを除去した。水相をメチルテトラブチルエーテルで洗浄した後、1M塩酸水溶液でpHを4に調節し、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去し、標題化合物を含む粗精製物 (5.40 g) を得た。
MS (ESI+): [M+H]+279.6. A) 4-Iodo-6-methoxypyridine-3-carboxylic acid 2,2,6,6-tetramethylpiperidine in tetrahydrofuran (100 mL) solution with 2.5M hexane solution of normal butyl lithium (39.2 mL) at -78 ° C In a nitrogen atmosphere. The reaction mixture was stirred at -78 ° C for 1 hour. The reaction mixture was added to a solution of 6-methoxypyridine-3-carboxylic acid (5.00 g) in tetrahydrofuran (50 mL) at −78 ° C. under a nitrogen atmosphere, and the mixture was stirred at −78 ° C. for 2 hours. The reaction mixture was added to a solution of iodine (24.9 g) in tetrahydrofuran (50 mL) at −78 ° C. under a nitrogen atmosphere, and the mixture was stirred at room temperature for 16 hr. Water was added to the reaction mixture, and tetrahydrofuran was removed under reduced pressure. The aqueous phase was washed with methyl tetrabutyl ether, adjusted to pH 4 with 1M aqueous hydrochloric acid, and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to give a crude product (5.40 g) containing the title compound.
MS (ESI +): [M + H] + 279.6.
2,2,6,6-テトラメチルピペリジンのテトラヒドロフラン (100 mL) 溶液にノルマルブチルリチウムの2.5Mヘキサン溶液(39.2 mL)を-78℃で窒素雰囲気下加えた。反応混合物を-78℃で1時間攪拌した。反応混合物を6-メトキシピリジン-3-カルボン酸 (5.00 g) のテトラヒドロフラン (50 mL) 溶液に窒素雰囲気下-78℃で加え、-78℃で2時間攪拌した。反応混合物をヨウ素 (24.9 g) のテトラヒドロフラン (50 mL) 溶液に-78℃で窒素雰囲気下加え、室温で16時間攪拌した。反応混合物に水を加え、減圧下テトラヒドロフランを除去した。水相をメチルテトラブチルエーテルで洗浄した後、1M塩酸水溶液でpHを4に調節し、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去し、標題化合物を含む粗精製物 (5.40 g) を得た。
MS (ESI+): [M+H]+279.6. A) 4-Iodo-6-methoxypyridine-3-carboxylic acid 2,2,6,6-tetramethylpiperidine in tetrahydrofuran (100 mL) solution with 2.5M hexane solution of normal butyl lithium (39.2 mL) at -78 ° C In a nitrogen atmosphere. The reaction mixture was stirred at -78 ° C for 1 hour. The reaction mixture was added to a solution of 6-methoxypyridine-3-carboxylic acid (5.00 g) in tetrahydrofuran (50 mL) at −78 ° C. under a nitrogen atmosphere, and the mixture was stirred at −78 ° C. for 2 hours. The reaction mixture was added to a solution of iodine (24.9 g) in tetrahydrofuran (50 mL) at −78 ° C. under a nitrogen atmosphere, and the mixture was stirred at room temperature for 16 hr. Water was added to the reaction mixture, and tetrahydrofuran was removed under reduced pressure. The aqueous phase was washed with methyl tetrabutyl ether, adjusted to pH 4 with 1M aqueous hydrochloric acid, and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to give a crude product (5.40 g) containing the title compound.
MS (ESI +): [M + H] + 279.6.
B) メチル 4-ヨード-6-メトキシピリジン-3-カルボキシラート
Aで得られた4-ヨード-6-メトキシピリジン-3-カルボン酸を含む粗精製物 (950 mg) のメタノール (30 mL) 溶液にトリメチルシリルジアゾメタンの2.0Mヘキサン溶液(10.0 mL)を氷冷下で加え、室温で16時間攪拌した。反応混合物に酢酸 (2 mL) を加え、減圧下溶媒を除去した。残渣に飽和炭酸水素ナトリウム水溶液と酢酸エチルを加え、有機層を濃縮した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物を含む粗精製物 (700 mg) を得た。
MS (ESI+): [M+H]+293.7. B) Methyl 4-iodo-6-methoxypyridine-3-carboxylate solution of 4-iodo-6-methoxypyridine-3-carboxylic acid obtained with A (950 mg) in methanol (30 mL) To the solution was added 2.0M hexane solution (10.0 mL) of trimethylsilyldiazomethane under ice-cooling, and the mixture was stirred at room temperature for 16 hours. Acetic acid (2 mL) was added to the reaction mixture, and the solvent was removed under reduced pressure. A saturated aqueous sodium hydrogen carbonate solution and ethyl acetate were added to the residue, and the organic layer was concentrated. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to obtain a crude product (700 mg) containing the title compound.
MS (ESI +): [M + H] + 293.7.
Aで得られた4-ヨード-6-メトキシピリジン-3-カルボン酸を含む粗精製物 (950 mg) のメタノール (30 mL) 溶液にトリメチルシリルジアゾメタンの2.0Mヘキサン溶液(10.0 mL)を氷冷下で加え、室温で16時間攪拌した。反応混合物に酢酸 (2 mL) を加え、減圧下溶媒を除去した。残渣に飽和炭酸水素ナトリウム水溶液と酢酸エチルを加え、有機層を濃縮した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物を含む粗精製物 (700 mg) を得た。
MS (ESI+): [M+H]+293.7. B) Methyl 4-iodo-6-methoxypyridine-3-carboxylate solution of 4-iodo-6-methoxypyridine-3-carboxylic acid obtained with A (950 mg) in methanol (30 mL) To the solution was added 2.0M hexane solution (10.0 mL) of trimethylsilyldiazomethane under ice-cooling, and the mixture was stirred at room temperature for 16 hours. Acetic acid (2 mL) was added to the reaction mixture, and the solvent was removed under reduced pressure. A saturated aqueous sodium hydrogen carbonate solution and ethyl acetate were added to the residue, and the organic layer was concentrated. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to obtain a crude product (700 mg) containing the title compound.
MS (ESI +): [M + H] + 293.7.
C) メチル 4-[(E)-2-エトキシエテニル]-6-メトキシピリジン-3-カルボキシラート
メチル 4-ヨード-6-メトキシピリジン-3-カルボキシラート (7.20 g)、(Z)-エトキシエテンボロン酸ピナコールエステル (5.37 g)、2-ジシクロヘキシルホスフィノ-2',6'-ジメトキシビフェニル(1.01 g)、酢酸パラジウム(II) (276 mg)およびリン酸カリウム (10.4 g)を含むアセトニトリル (90 mL) と水 (60 mL) の混合溶液を窒素雰囲気下、2時間還流条件下で攪拌した。反応混合物に水を加え、減圧下でアセトニトリルを除去し、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (5.10 g) を得た。
1H NMR (300 MHz, CDCl3) δ 1.36 (3H, t, J = 6.8 Hz), 3.88 (3H, s), 3.93-4.02 (5H, m), 6.69 (1H, s), 6.77 (1H, d, J = 12.8 Hz), 7.12 (1H, d, J = 13.2 Hz), 8.73 (1H, s). C) Methyl 4-[(E) -2-ethoxyethenyl] -6-methoxypyridine-3-carboxylate Methyl 4-iodo-6-methoxypyridine-3-carboxylate (7.20 g), (Z) -ethoxy Etheneboronic acid pinacol ester (5.37 g), 2-dicyclohexylphosphino-2 ', 6'-dimethoxybiphenyl (1.01 g), palladium (II) acetate (276 mg) and acetonitrile containing potassium phosphate (10.4 g) ( A mixed solution of 90 mL) and water (60 mL) was stirred under reflux conditions for 2 hours under a nitrogen atmosphere. Water was added to the reaction mixture, acetonitrile was removed under reduced pressure, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (5.10 g).
1 H NMR (300 MHz, CDCl 3 ) δ 1.36 (3H, t, J = 6.8 Hz), 3.88 (3H, s), 3.93-4.02 (5H, m), 6.69 (1H, s), 6.77 (1H, d, J = 12.8 Hz), 7.12 (1H, d, J = 13.2 Hz), 8.73 (1H, s).
メチル 4-ヨード-6-メトキシピリジン-3-カルボキシラート (7.20 g)、(Z)-エトキシエテンボロン酸ピナコールエステル (5.37 g)、2-ジシクロヘキシルホスフィノ-2',6'-ジメトキシビフェニル(1.01 g)、酢酸パラジウム(II) (276 mg)およびリン酸カリウム (10.4 g)を含むアセトニトリル (90 mL) と水 (60 mL) の混合溶液を窒素雰囲気下、2時間還流条件下で攪拌した。反応混合物に水を加え、減圧下でアセトニトリルを除去し、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (5.10 g) を得た。
1H NMR (300 MHz, CDCl3) δ 1.36 (3H, t, J = 6.8 Hz), 3.88 (3H, s), 3.93-4.02 (5H, m), 6.69 (1H, s), 6.77 (1H, d, J = 12.8 Hz), 7.12 (1H, d, J = 13.2 Hz), 8.73 (1H, s). C) Methyl 4-[(E) -2-ethoxyethenyl] -6-methoxypyridine-3-carboxylate Methyl 4-iodo-6-methoxypyridine-3-carboxylate (7.20 g), (Z) -ethoxy Etheneboronic acid pinacol ester (5.37 g), 2-dicyclohexylphosphino-2 ', 6'-dimethoxybiphenyl (1.01 g), palladium (II) acetate (276 mg) and acetonitrile containing potassium phosphate (10.4 g) ( A mixed solution of 90 mL) and water (60 mL) was stirred under reflux conditions for 2 hours under a nitrogen atmosphere. Water was added to the reaction mixture, acetonitrile was removed under reduced pressure, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (5.10 g).
1 H NMR (300 MHz, CDCl 3 ) δ 1.36 (3H, t, J = 6.8 Hz), 3.88 (3H, s), 3.93-4.02 (5H, m), 6.69 (1H, s), 6.77 (1H, d, J = 12.8 Hz), 7.12 (1H, d, J = 13.2 Hz), 8.73 (1H, s).
D) メチル 6-メトキシ-4-(2-オキソエチル)ピリジン-3-カルボキシラート
メチル 4-[(E)-2-エトキシエテニル]-6-メトキシピリジン-3-カルボキシラート (5.10 g) のトリフルオロ酢酸 (100 mL) 及びジクロロメタン (100 mL) の混合溶液を25℃で5時間攪拌した。減圧下で溶媒を除去し、標題化合物を含む粗精製物 (5.30 g) を得た。
MS (ESI+): [M+H]+209.8. D) Methyl 6-methoxy-4- (2-oxoethyl) pyridine-3-carboxylate Methyl 4-[(E) -2-ethoxyethenyl] -6-methoxypyridine-3-carboxylate (5.10 g) A mixed solution of fluoroacetic acid (100 mL) and dichloromethane (100 mL) was stirred at 25 ° C. for 5 hours. The solvent was removed under reduced pressure to obtain a crude product (5.30 g) containing the title compound.
MS (ESI +): [M + H] + 209.8.
メチル 4-[(E)-2-エトキシエテニル]-6-メトキシピリジン-3-カルボキシラート (5.10 g) のトリフルオロ酢酸 (100 mL) 及びジクロロメタン (100 mL) の混合溶液を25℃で5時間攪拌した。減圧下で溶媒を除去し、標題化合物を含む粗精製物 (5.30 g) を得た。
MS (ESI+): [M+H]+209.8. D) Methyl 6-methoxy-4- (2-oxoethyl) pyridine-3-carboxylate Methyl 4-[(E) -2-ethoxyethenyl] -6-methoxypyridine-3-carboxylate (5.10 g) A mixed solution of fluoroacetic acid (100 mL) and dichloromethane (100 mL) was stirred at 25 ° C. for 5 hours. The solvent was removed under reduced pressure to obtain a crude product (5.30 g) containing the title compound.
MS (ESI +): [M + H] + 209.8.
E) 6-メトキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン
Dで得た粗精製物 (5.30 g) を含むジクロロメタン (200 mL) 溶液に4-メトキシベンジルアミン (6.85 g) を加え、25℃で3時間攪拌した。反応混合物にシアノ水素化ホウ素ナトリウム (3.15 g) を加え、室温で16時間攪拌した。ジクロロメタンを減圧下で除去し、残渣にトルエン (100 mL) 及び酢酸 (3 mL) を加え、還流条件下で3時間攪拌した。室温まで冷却し、酢酸エチルで希釈し、水、飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) 及び分取液体クロマトグラフィーで精製し、標題化合物 (1.90 g) を得た。
MS (ESI+): [M+H]+298.8. E) Dichloromethane (200 mL) containing the crude product (5.30 g) obtained from 6-methoxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one D 4-methoxybenzylamine (6.85 g) was added to the solution, and the mixture was stirred at 25 ° C. for 3 hours. Sodium cyanoborohydride (3.15 g) was added to the reaction mixture, and the mixture was stirred at room temperature for 16 hours. Dichloromethane was removed under reduced pressure, toluene (100 mL) and acetic acid (3 mL) were added to the residue, and the mixture was stirred under reflux conditions for 3 hours. The mixture was cooled to room temperature, diluted with ethyl acetate, washed with water, saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) and preparative liquid chromatography to obtain the title compound (1.90 g).
MS (ESI +): [M + H] + 298.8.
Dで得た粗精製物 (5.30 g) を含むジクロロメタン (200 mL) 溶液に4-メトキシベンジルアミン (6.85 g) を加え、25℃で3時間攪拌した。反応混合物にシアノ水素化ホウ素ナトリウム (3.15 g) を加え、室温で16時間攪拌した。ジクロロメタンを減圧下で除去し、残渣にトルエン (100 mL) 及び酢酸 (3 mL) を加え、還流条件下で3時間攪拌した。室温まで冷却し、酢酸エチルで希釈し、水、飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) 及び分取液体クロマトグラフィーで精製し、標題化合物 (1.90 g) を得た。
MS (ESI+): [M+H]+298.8. E) Dichloromethane (200 mL) containing the crude product (5.30 g) obtained from 6-methoxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one D 4-methoxybenzylamine (6.85 g) was added to the solution, and the mixture was stirred at 25 ° C. for 3 hours. Sodium cyanoborohydride (3.15 g) was added to the reaction mixture, and the mixture was stirred at room temperature for 16 hours. Dichloromethane was removed under reduced pressure, toluene (100 mL) and acetic acid (3 mL) were added to the residue, and the mixture was stirred under reflux conditions for 3 hours. The mixture was cooled to room temperature, diluted with ethyl acetate, washed with water, saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) and preparative liquid chromatography to obtain the title compound (1.90 g).
MS (ESI +): [M + H] + 298.8.
F) 4-ベンジル-6-メトキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン
6-メトキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン(1.50 g) のテトラヒドロフラン (25 mL) 溶液にリチウムジイソプロピルアミドの2.0Mテトラヒドロフラン溶液(3.0 mL)を窒素雰囲気下-78℃で加えた。反応混合物を-78℃で30分撹拌し、ベンジルブロマイド(940 mL) を滴下し、室温下で16時間攪拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (1.25g) を得た。
MS (ESI+): [M+H]+389.0. F) 4-Benzyl-6-methoxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one 6-methoxy-2- (4-methoxybenzyl)- To a solution of 3,4-dihydro-2,7-naphthyridin-1 (2H) -one (1.50 g) in tetrahydrofuran (25 mL) was added a 2.0M solution of lithium diisopropylamide in tetrahydrofuran (3.0 mL) at -78 ° C under a nitrogen atmosphere. added. The reaction mixture was stirred at −78 ° C. for 30 minutes, benzyl bromide (940 mL) was added dropwise, and the mixture was stirred at room temperature for 16 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to obtain the title compound (1.25 g).
MS (ESI +): [M + H] + 389.0.
6-メトキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン(1.50 g) のテトラヒドロフラン (25 mL) 溶液にリチウムジイソプロピルアミドの2.0Mテトラヒドロフラン溶液(3.0 mL)を窒素雰囲気下-78℃で加えた。反応混合物を-78℃で30分撹拌し、ベンジルブロマイド(940 mL) を滴下し、室温下で16時間攪拌した。反応混合物に飽和塩化アンモニウム水溶液を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (1.25g) を得た。
MS (ESI+): [M+H]+389.0. F) 4-Benzyl-6-methoxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one 6-methoxy-2- (4-methoxybenzyl)- To a solution of 3,4-dihydro-2,7-naphthyridin-1 (2H) -one (1.50 g) in tetrahydrofuran (25 mL) was added a 2.0M solution of lithium diisopropylamide in tetrahydrofuran (3.0 mL) at -78 ° C under a nitrogen atmosphere. added. The reaction mixture was stirred at −78 ° C. for 30 minutes, benzyl bromide (940 mL) was added dropwise, and the mixture was stirred at room temperature for 16 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to obtain the title compound (1.25 g).
MS (ESI +): [M + H] + 389.0.
G) 4-ベンジル-6-ヒドロキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン
4-ベンジル-6-メトキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン (1.50 g) の酢酸 (12 mL) 溶液に36%塩酸水溶液(6 mL) を加え、還流条件下で16時間撹拌した。反応混合物を室温まで冷却し、溶媒を減圧下で除去した。残渣に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去し、標題化合物を含む粗精製物を得た。
MS (ESI+): [M+H]+254.8. G) 4-Benzyl-6-hydroxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one 4-benzyl-6-methoxy-2- (4- To a solution of (methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one (1.50 g) in acetic acid (12 mL) was added 36% aqueous hydrochloric acid (6 mL), and the mixture was refluxed for 16 Stir for hours. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. To the residue was added saturated aqueous sodium hydrogen carbonate solution, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give a crude product containing the title compound.
MS (ESI +): [M + H] + 254.8.
4-ベンジル-6-メトキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン (1.50 g) の酢酸 (12 mL) 溶液に36%塩酸水溶液(6 mL) を加え、還流条件下で16時間撹拌した。反応混合物を室温まで冷却し、溶媒を減圧下で除去した。残渣に飽和炭酸水素ナトリウム水溶液を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去し、標題化合物を含む粗精製物を得た。
MS (ESI+): [M+H]+254.8. G) 4-Benzyl-6-hydroxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one 4-benzyl-6-methoxy-2- (4- To a solution of (methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one (1.50 g) in acetic acid (12 mL) was added 36% aqueous hydrochloric acid (6 mL), and the mixture was refluxed for 16 Stir for hours. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. To the residue was added saturated aqueous sodium hydrogen carbonate solution, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give a crude product containing the title compound.
MS (ESI +): [M + H] + 254.8.
H) 5-ベンジル-7-(4-メトキシベンジル)-8-オキソ-5,6,7,8-テトラヒドロ-2,7-ナフチリジン-3-イルトリフルオロメタンスルホナート
Gで得た4-ベンジル-6-ヒドロキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オンを含む粗精製物 (600 mg) とピリジン (379 mg) のジクロロメタン (20 mL) 溶液に無水トリフルオロメタンスルホン酸 (677 mg) を氷冷下で加え、室温で16時間撹拌した。減圧下で溶媒を除去し、残渣をジクロロメタンに溶解した。有機層を1M塩酸水溶液、飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (380 mg) を得た。
MS (ESI+): [M+H]+507.0. H) 4-Benzyl-7- (4-methoxybenzyl) -8-oxo-5,6,7,8-tetrahydro-2,7-naphthyridin-3-yltrifluoromethanesulfonate G A crude product (600 mg) containing 6-hydroxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one and pyridine (379 mg) in dichloromethane (20 mL) To the solution was added trifluoromethanesulfonic anhydride (677 mg) under ice cooling, and the mixture was stirred at room temperature for 16 hours. The solvent was removed under reduced pressure and the residue was dissolved in dichloromethane. The organic layer was washed with 1M aqueous hydrochloric acid solution, saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (380 mg).
MS (ESI +): [M + H] + 507.0.
Gで得た4-ベンジル-6-ヒドロキシ-2-(4-メトキシベンジル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オンを含む粗精製物 (600 mg) とピリジン (379 mg) のジクロロメタン (20 mL) 溶液に無水トリフルオロメタンスルホン酸 (677 mg) を氷冷下で加え、室温で16時間撹拌した。減圧下で溶媒を除去し、残渣をジクロロメタンに溶解した。有機層を1M塩酸水溶液、飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (380 mg) を得た。
MS (ESI+): [M+H]+507.0. H) 4-Benzyl-7- (4-methoxybenzyl) -8-oxo-5,6,7,8-tetrahydro-2,7-naphthyridin-3-yltrifluoromethanesulfonate G A crude product (600 mg) containing 6-hydroxy-2- (4-methoxybenzyl) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one and pyridine (379 mg) in dichloromethane (20 mL) To the solution was added trifluoromethanesulfonic anhydride (677 mg) under ice cooling, and the mixture was stirred at room temperature for 16 hours. The solvent was removed under reduced pressure and the residue was dissolved in dichloromethane. The organic layer was washed with 1M aqueous hydrochloric acid solution, saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (380 mg).
MS (ESI +): [M + H] + 507.0.
I) 4-ベンジル-2-(4-メトキシベンジル)-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン
5-ベンジル-7-(4-メトキシベンジル)-8-オキソ-5,6,7,8-テトラヒドロ-2,7-ナフチリジン-3-イルトリフルオロメタンスルホナート (200 mg)、tert-ブチル (3-アミノピリジン-2-イル)カルバマート (100 mg)、トリス(ジベンジリデンアセトン)ジパラジウム(0) (37 mg)、キサントホス (23 mg) および炭酸セシウム (193 mg) を含むトルエン (5 mL) 溶液を還流条件下で窒素雰囲気下16時間撹拌した。反応混合物を室温まで冷却し、酢酸エチルで希釈し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去し、標題化合物を含む粗精製物 (250 mg) を得た。
MS (ESI+): [M+H]+492.0. I) 4-Benzyl-2- (4-methoxybenzyl) -6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydro -2,7-naphthyridin-1 (2H) -one 5-benzyl-7- (4-methoxybenzyl) -8-oxo-5,6,7,8-tetrahydro-2,7-naphthyridin-3-yltrifluor Lomethanesulfonate (200 mg), tert-butyl (3-aminopyridin-2-yl) carbamate (100 mg), tris (dibenzylideneacetone) dipalladium (0) (37 mg), xanthophos (23 mg) and carbonic acid A toluene (5 mL) solution containing cesium (193 mg) was stirred under a nitrogen atmosphere for 16 hours under reflux conditions. The reaction mixture was cooled to room temperature, diluted with ethyl acetate, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give a crude product (250 mg) containing the title compound. Obtained.
MS (ESI +): [M + H] + 492.0.
5-ベンジル-7-(4-メトキシベンジル)-8-オキソ-5,6,7,8-テトラヒドロ-2,7-ナフチリジン-3-イルトリフルオロメタンスルホナート (200 mg)、tert-ブチル (3-アミノピリジン-2-イル)カルバマート (100 mg)、トリス(ジベンジリデンアセトン)ジパラジウム(0) (37 mg)、キサントホス (23 mg) および炭酸セシウム (193 mg) を含むトルエン (5 mL) 溶液を還流条件下で窒素雰囲気下16時間撹拌した。反応混合物を室温まで冷却し、酢酸エチルで希釈し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去し、標題化合物を含む粗精製物 (250 mg) を得た。
MS (ESI+): [M+H]+492.0. I) 4-Benzyl-2- (4-methoxybenzyl) -6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydro -2,7-naphthyridin-1 (2H) -one 5-benzyl-7- (4-methoxybenzyl) -8-oxo-5,6,7,8-tetrahydro-2,7-naphthyridin-3-yltrifluor Lomethanesulfonate (200 mg), tert-butyl (3-aminopyridin-2-yl) carbamate (100 mg), tris (dibenzylideneacetone) dipalladium (0) (37 mg), xanthophos (23 mg) and carbonic acid A toluene (5 mL) solution containing cesium (193 mg) was stirred under a nitrogen atmosphere for 16 hours under reflux conditions. The reaction mixture was cooled to room temperature, diluted with ethyl acetate, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give a crude product (250 mg) containing the title compound. Obtained.
MS (ESI +): [M + H] + 492.0.
J) 4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オン
Iで得られた4-ベンジル-2-(4-メトキシベンジル)-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オンを含む粗精製物 (250 mg) のトリフルオロ酢酸 (4 mL) 及びトリフルオロメタンスルホン酸 (1 mL) の混合溶液を還流条件下で3時間撹拌した。反応混合物を室温まで冷却し、溶媒を減圧下で除去した。残渣をジクロロメタン(50 mL) に溶解し、飽和炭酸水素ナトリウム水溶液および飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣を分取液体クロマトグラフィーで精製し、標題化合物 (23 mg) を得た。
1H NMR (400 MHz, DMSO-d6)δ 2.78-2.87 (1H, m), 2.94-3.02 (1H, m), 3.09-3.16 (1H, m), 3.22-3.35 (2H, m), 7.12 (1H, dd, J = 7.6, 5.2 Hz), 7.20-7.35 (5H, m), 8.04-8.08 (2H, m), 8.13 (1H, s), 8.34 (1H, d, J = 8.0 Hz), 8.93 (1H, s), 11.96 (1H, brs). J) 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydro-2,7-naphthyridine-1 ( 2H) -one I obtained 4-benzyl-2- (4-methoxybenzyl) -6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl ) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one in a crude product (250 mg) mixed with trifluoroacetic acid (4 mL) and trifluoromethanesulfonic acid (1 mL) Stir for 3 hours under reflux conditions. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. The residue was dissolved in dichloromethane (50 mL), washed with saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by preparative liquid chromatography to give the title compound (23 mg).
1 H NMR (400 MHz, DMSO-d 6 ) δ 2.78-2.87 (1H, m), 2.94-3.02 (1H, m), 3.09-3.16 (1H, m), 3.22-3.35 (2H, m), 7.12 (1H, dd, J = 7.6, 5.2 Hz), 7.20-7.35 (5H, m), 8.04-8.08 (2H, m), 8.13 (1H, s), 8.34 (1H, d, J = 8.0 Hz), 8.93 (1H, s), 11.96 (1H, brs).
Iで得られた4-ベンジル-2-(4-メトキシベンジル)-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロ-2,7-ナフチリジン-1(2H)-オンを含む粗精製物 (250 mg) のトリフルオロ酢酸 (4 mL) 及びトリフルオロメタンスルホン酸 (1 mL) の混合溶液を還流条件下で3時間撹拌した。反応混合物を室温まで冷却し、溶媒を減圧下で除去した。残渣をジクロロメタン(50 mL) に溶解し、飽和炭酸水素ナトリウム水溶液および飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣を分取液体クロマトグラフィーで精製し、標題化合物 (23 mg) を得た。
1H NMR (400 MHz, DMSO-d6)δ 2.78-2.87 (1H, m), 2.94-3.02 (1H, m), 3.09-3.16 (1H, m), 3.22-3.35 (2H, m), 7.12 (1H, dd, J = 7.6, 5.2 Hz), 7.20-7.35 (5H, m), 8.04-8.08 (2H, m), 8.13 (1H, s), 8.34 (1H, d, J = 8.0 Hz), 8.93 (1H, s), 11.96 (1H, brs). J) 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydro-2,7-naphthyridine-1 ( 2H) -one I obtained 4-benzyl-2- (4-methoxybenzyl) -6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl ) -3,4-dihydro-2,7-naphthyridin-1 (2H) -one in a crude product (250 mg) mixed with trifluoroacetic acid (4 mL) and trifluoromethanesulfonic acid (1 mL) Stir for 3 hours under reflux conditions. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. The residue was dissolved in dichloromethane (50 mL), washed with saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by preparative liquid chromatography to give the title compound (23 mg).
1 H NMR (400 MHz, DMSO-d 6 ) δ 2.78-2.87 (1H, m), 2.94-3.02 (1H, m), 3.09-3.16 (1H, m), 3.22-3.35 (2H, m), 7.12 (1H, dd, J = 7.6, 5.2 Hz), 7.20-7.35 (5H, m), 8.04-8.08 (2H, m), 8.13 (1H, s), 8.34 (1H, d, J = 8.0 Hz), 8.93 (1H, s), 11.96 (1H, brs).
実施例77
8-ベンジル-2-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オン Example 77
8-Benzyl-2- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -7,8-dihydropyrido [4,3-d] pyrimidine-5 ( 6H) -ON
8-ベンジル-2-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オン Example 77
8-Benzyl-2- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -7,8-dihydropyrido [4,3-d] pyrimidine-5 ( 6H) -ON
A) tert-ブチル 5-ベンジル-2,4-ジオキソピペリジン-1-カルボキシラート
tert-ブチル 2,4-ジオキソピペリジン-1-カルボキシラート (500 mg) 及びベンジルブロマイド (1.61 g) のテトラヒドロフラン (30 mL) 溶液にリチウムヘキサメチルジシラジドの1.0Mテトラヒドロフラン溶液(5.9 mL)を窒素雰囲気下、-20℃で加えた。反応混合物を-20℃で3時間撹拌した。反応混合物に飽和塩化アンモニウム水溶液を氷冷下で加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (150 mg) を得た。
MS (ESI+): [M+Na]+325.8. A) tert-butyl 5-benzyl-2,4-dioxopiperidine-1-carboxylate tert-butyl 2,4-dioxopiperidine-1-carboxylate (500 mg) and benzyl bromide (1.61 g) in tetrahydrofuran ( 30 mL) A solution of lithium hexamethyldisilazide in 1.0M tetrahydrofuran (5.9 mL) was added to the solution at −20 ° C. under a nitrogen atmosphere. The reaction mixture was stirred at −20 ° C. for 3 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture under ice cooling, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (150 mg).
MS (ESI +): [M + Na] + 325.8.
tert-ブチル 2,4-ジオキソピペリジン-1-カルボキシラート (500 mg) 及びベンジルブロマイド (1.61 g) のテトラヒドロフラン (30 mL) 溶液にリチウムヘキサメチルジシラジドの1.0Mテトラヒドロフラン溶液(5.9 mL)を窒素雰囲気下、-20℃で加えた。反応混合物を-20℃で3時間撹拌した。反応混合物に飽和塩化アンモニウム水溶液を氷冷下で加え、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (150 mg) を得た。
MS (ESI+): [M+Na]+325.8. A) tert-butyl 5-benzyl-2,4-dioxopiperidine-1-carboxylate tert-butyl 2,4-dioxopiperidine-1-carboxylate (500 mg) and benzyl bromide (1.61 g) in tetrahydrofuran ( 30 mL) A solution of lithium hexamethyldisilazide in 1.0M tetrahydrofuran (5.9 mL) was added to the solution at −20 ° C. under a nitrogen atmosphere. The reaction mixture was stirred at −20 ° C. for 3 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture under ice cooling, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (150 mg).
MS (ESI +): [M + Na] + 325.8.
B) 5-ベンジル-3-[(ジメチルアミノ)メチリデン]ピペリジン-2,4-ジオン
tert-ブチル 5-ベンジル-2,4-ジオキソピペリジン-1-カルボキシラート (3.00 g) のN,N-ジメチルホルムアミドジメチルアセタール (20 mL) 溶液を50℃で16時間撹拌した。反応混合物を室温まで冷却し、減圧下溶媒を除去し、標題化合物を含む粗精製物 (3.10 g) を得た。
MS (ESI+): [M+H]+258.9. B) 5-benzyl-3-[(dimethylamino) methylidene] piperidine-2,4-dione tert-butyl 5-benzyl-2,4-dioxopiperidine-1-carboxylate (3.00 g) of N, N- The dimethylformamide dimethyl acetal (20 mL) solution was stirred at 50 ° C. for 16 hours. The reaction mixture was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a crude product (3.10 g) containing the title compound.
MS (ESI +): [M + H] + 258.9.
tert-ブチル 5-ベンジル-2,4-ジオキソピペリジン-1-カルボキシラート (3.00 g) のN,N-ジメチルホルムアミドジメチルアセタール (20 mL) 溶液を50℃で16時間撹拌した。反応混合物を室温まで冷却し、減圧下溶媒を除去し、標題化合物を含む粗精製物 (3.10 g) を得た。
MS (ESI+): [M+H]+258.9. B) 5-benzyl-3-[(dimethylamino) methylidene] piperidine-2,4-dione tert-butyl 5-benzyl-2,4-dioxopiperidine-1-carboxylate (3.00 g) of N, N- The dimethylformamide dimethyl acetal (20 mL) solution was stirred at 50 ° C. for 16 hours. The reaction mixture was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a crude product (3.10 g) containing the title compound.
MS (ESI +): [M + H] + 258.9.
C) 8-ベンジル-2-(メチルスルファニル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オン
Bで得られた5-ベンジル-3-[(ジメチルアミノ)メチリデン]ピペリジン-2,4-ジオンを含む粗精製物(400 mg)、メチルカルバムイミドチオアート0.5硫酸塩 (583 mg) 及び酢酸ナトリウムのN,N-ジメチルホルムアミド (5 mL) 溶液を80℃で窒素雰囲気下、16時間撹拌した。反応混合物を室温まで冷却し、水を加え希釈し、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (150 mg) を得た。
MS (ESI+): [M+H]+285.8. C) 5-Benzyl-3-[(dimethylamino) methylidene obtained from 8-benzyl-2- (methylsulfanyl) -7,8-dihydropyrido [4,3-d] pyrimidin-5 (6H) -one B ] A solution of piperidine-2,4-dione (400 mg), methylcarbamimidothioate 0.5sulfate (583 mg) and sodium acetate in N, N-dimethylformamide (5 mL) at 80 ° C. The mixture was stirred for 16 hours under a nitrogen atmosphere. The reaction mixture was cooled to room temperature, diluted by adding water, and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (150 mg).
MS (ESI +): [M + H] + 285.8.
Bで得られた5-ベンジル-3-[(ジメチルアミノ)メチリデン]ピペリジン-2,4-ジオンを含む粗精製物(400 mg)、メチルカルバムイミドチオアート0.5硫酸塩 (583 mg) 及び酢酸ナトリウムのN,N-ジメチルホルムアミド (5 mL) 溶液を80℃で窒素雰囲気下、16時間撹拌した。反応混合物を室温まで冷却し、水を加え希釈し、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ペンタン/酢酸エチル) で精製し、標題化合物 (150 mg) を得た。
MS (ESI+): [M+H]+285.8. C) 5-Benzyl-3-[(dimethylamino) methylidene obtained from 8-benzyl-2- (methylsulfanyl) -7,8-dihydropyrido [4,3-d] pyrimidin-5 (6H) -one B ] A solution of piperidine-2,4-dione (400 mg), methylcarbamimidothioate 0.5sulfate (583 mg) and sodium acetate in N, N-dimethylformamide (5 mL) at 80 ° C. The mixture was stirred for 16 hours under a nitrogen atmosphere. The reaction mixture was cooled to room temperature, diluted by adding water, and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (pentane / ethyl acetate) to give the title compound (150 mg).
MS (ESI +): [M + H] + 285.8.
D) 8-ベンジル-2-(メチルスルホニル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オン
8-ベンジル-2-(メチルスルファニル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オン (80 mg) のジクロロメタン (5 mL) 溶液にメタクロロ過安息香酸 (150 mg) を氷冷下で加え、室温で16時間撹拌した。反応混合物をジクロロメタン (30 mL) で希釈し、飽和チオ硫酸ナトリウム水溶液、飽和炭酸水素ナトリウム水溶液及び飽和塩化ナトリウム水溶液で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下で溶媒を除去し、標題化合物を含む粗精製物 (100 mg) を得た。
MS (ESI+): [M+Na]+339.8. D) 8-Benzyl-2- (methylsulfonyl) -7,8-dihydropyrido [4,3-d] pyrimidin-5 (6H) -one 8-benzyl-2- (methylsulfanyl) -7,8-dihydropyrido [ To a solution of 4,3-d] pyrimidin-5 (6H) -one (80 mg) in dichloromethane (5 mL) was added metachloroperbenzoic acid (150 mg) under ice cooling, and the mixture was stirred at room temperature for 16 hours. Dilute the reaction mixture with dichloromethane (30 mL), wash with saturated aqueous sodium thiosulfate, saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride, dry over anhydrous magnesium sulfate, remove the solvent under reduced pressure and remove the title compound. A crudely purified product (100 mg) was obtained.
MS (ESI +): [M + Na] + 339.8.
8-ベンジル-2-(メチルスルファニル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オン (80 mg) のジクロロメタン (5 mL) 溶液にメタクロロ過安息香酸 (150 mg) を氷冷下で加え、室温で16時間撹拌した。反応混合物をジクロロメタン (30 mL) で希釈し、飽和チオ硫酸ナトリウム水溶液、飽和炭酸水素ナトリウム水溶液及び飽和塩化ナトリウム水溶液で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下で溶媒を除去し、標題化合物を含む粗精製物 (100 mg) を得た。
MS (ESI+): [M+Na]+339.8. D) 8-Benzyl-2- (methylsulfonyl) -7,8-dihydropyrido [4,3-d] pyrimidin-5 (6H) -one 8-benzyl-2- (methylsulfanyl) -7,8-dihydropyrido [ To a solution of 4,3-d] pyrimidin-5 (6H) -one (80 mg) in dichloromethane (5 mL) was added metachloroperbenzoic acid (150 mg) under ice cooling, and the mixture was stirred at room temperature for 16 hours. Dilute the reaction mixture with dichloromethane (30 mL), wash with saturated aqueous sodium thiosulfate, saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride, dry over anhydrous magnesium sulfate, remove the solvent under reduced pressure and remove the title compound. A crudely purified product (100 mg) was obtained.
MS (ESI +): [M + Na] + 339.8.
E) 8-ベンジル-2-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オン
Dで得られた8-ベンジル-2-(メチルスルホニル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オンを含む粗精製物 (180 mg)、tert-ブチル(3-アミノピリジン-2-イル)カルバマート (180 mg)およびジイソプロピルエチルアミン (300 mg) のジオキサン (10 mL) 溶液を還流条件下、窒素雰囲気下16時間撹拌した。反応混合物を室温まで冷却し、酢酸エチルを加え希釈し、1M塩酸水溶液、飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下で溶媒を除去した。残渣を分取液体クロマトグラフィーで精製し、標題化合物 (11 mg) を得た。
1H NMR (400 MHz, DMSO-d6)δ 2.86-2.94 (1H, m), 3.26-3.37 (2H, m), 3.38-3.55 (2H, m), 7.05 (1H, dd, J = 8.0, 1.2 Hz), 7.22-7.30 (3H, m), 7.31-7.37 (2H, m), 7.79 (1H, dd, J = 8.0, 1.2 Hz), 8.05 (1H, dd, J = 5.2, 1.2 Hz), 8.24 (1H, brs), 9.15 (1H, s), 11.98 (1H, brs). E) 8-Benzyl-2- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -7,8-dihydropyrido [4,3-d] pyrimidine- Crude product containing 8-benzyl-2- (methylsulfonyl) -7,8-dihydropyrido [4,3-d] pyrimidin-5 (6H) -one obtained from 5 (6H) -one D (180 mg ), Tert-butyl (3-aminopyridin-2-yl) carbamate (180 mg) and diisopropylethylamine (300 mg) in dioxane (10 mL) were stirred under refluxing conditions for 16 hours under a nitrogen atmosphere. The reaction mixture was cooled to room temperature, diluted with ethyl acetate, washed with 1M aqueous hydrochloric acid solution, saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The residue was purified by preparative liquid chromatography to give the title compound (11 mg).
1 H NMR (400 MHz, DMSO-d 6 ) δ 2.86-2.94 (1H, m), 3.26-3.37 (2H, m), 3.38-3.55 (2H, m), 7.05 (1H, dd, J = 8.0, 1.2 Hz), 7.22-7.30 (3H, m), 7.31-7.37 (2H, m), 7.79 (1H, dd, J = 8.0, 1.2 Hz), 8.05 (1H, dd, J = 5.2, 1.2 Hz), 8.24 (1H, brs), 9.15 (1H, s), 11.98 (1H, brs).
Dで得られた8-ベンジル-2-(メチルスルホニル)-7,8-ジヒドロピリド[4,3-d]ピリミジン-5(6H)-オンを含む粗精製物 (180 mg)、tert-ブチル(3-アミノピリジン-2-イル)カルバマート (180 mg)およびジイソプロピルエチルアミン (300 mg) のジオキサン (10 mL) 溶液を還流条件下、窒素雰囲気下16時間撹拌した。反応混合物を室温まで冷却し、酢酸エチルを加え希釈し、1M塩酸水溶液、飽和炭酸水素ナトリウム水溶液及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、減圧下で溶媒を除去した。残渣を分取液体クロマトグラフィーで精製し、標題化合物 (11 mg) を得た。
1H NMR (400 MHz, DMSO-d6)δ 2.86-2.94 (1H, m), 3.26-3.37 (2H, m), 3.38-3.55 (2H, m), 7.05 (1H, dd, J = 8.0, 1.2 Hz), 7.22-7.30 (3H, m), 7.31-7.37 (2H, m), 7.79 (1H, dd, J = 8.0, 1.2 Hz), 8.05 (1H, dd, J = 5.2, 1.2 Hz), 8.24 (1H, brs), 9.15 (1H, s), 11.98 (1H, brs). E) 8-Benzyl-2- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -7,8-dihydropyrido [4,3-d] pyrimidine- Crude product containing 8-benzyl-2- (methylsulfonyl) -7,8-dihydropyrido [4,3-d] pyrimidin-5 (6H) -one obtained from 5 (6H) -one D (180 mg ), Tert-butyl (3-aminopyridin-2-yl) carbamate (180 mg) and diisopropylethylamine (300 mg) in dioxane (10 mL) were stirred under refluxing conditions for 16 hours under a nitrogen atmosphere. The reaction mixture was cooled to room temperature, diluted with ethyl acetate, washed with 1M aqueous hydrochloric acid solution, saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The residue was purified by preparative liquid chromatography to give the title compound (11 mg).
1 H NMR (400 MHz, DMSO-d 6 ) δ 2.86-2.94 (1H, m), 3.26-3.37 (2H, m), 3.38-3.55 (2H, m), 7.05 (1H, dd, J = 8.0, 1.2 Hz), 7.22-7.30 (3H, m), 7.31-7.37 (2H, m), 7.79 (1H, dd, J = 8.0, 1.2 Hz), 8.05 (1H, dd, J = 5.2, 1.2 Hz), 8.24 (1H, brs), 9.15 (1H, s), 11.98 (1H, brs).
実施例78
1-ベンジル-7-(4-メトキシ-7H-ピロロ[2,3-d]ピリミジン-5-イル)-2,2-ジメチル-2,3-ジヒドロキナゾリン-4(1H)-オン Example 78
1-Benzyl-7- (4-methoxy-7H-pyrrolo [2,3-d] pyrimidin-5-yl) -2,2-dimethyl-2,3-dihydroquinazolin-4 (1H) -one
1-ベンジル-7-(4-メトキシ-7H-ピロロ[2,3-d]ピリミジン-5-イル)-2,2-ジメチル-2,3-ジヒドロキナゾリン-4(1H)-オン Example 78
1-Benzyl-7- (4-methoxy-7H-pyrrolo [2,3-d] pyrimidin-5-yl) -2,2-dimethyl-2,3-dihydroquinazolin-4 (1H) -one
A) 5-ブロモ-4-クロロ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン
5-ブロモ-4-クロロ-7H-ピロロ[2,3-d]ピリミジン (10 g) のDMF (143 mL) 溶液に氷冷下で水素化ナトリウム (2.07 g) を加えた。反応混合物を氷冷下で30分攪拌した。2-トリメチルシリルエチルオキシメチルクロリド(9.14 mL) を氷冷下で反応混合物に加え、室温で3時間攪拌した。反応混合物を水で希釈し、室温で4時間攪拌した。析出物をろ取し、水で洗浄し、標題化合物 (15.5 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 0.09 (9H, s), 0.79-0.88 (2H, m), 3.49-3.57 (2H, m), 5.62 (2H, s), 8.16 (1H, s), 8.73 (1H, s). A) 5-Bromo-4-chloro-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine 5-bromo-4-chloro-7H-pyrrolo [2,3 To a solution of -d] pyrimidine (10 g) in DMF (143 mL) was added sodium hydride (2.07 g) under ice-cooling. The reaction mixture was stirred for 30 minutes under ice cooling. 2-Trimethylsilylethyloxymethyl chloride (9.14 mL) was added to the reaction mixture under ice cooling, and the mixture was stirred at room temperature for 3 hours. The reaction mixture was diluted with water and stirred at room temperature for 4 hours. The precipitate was collected by filtration and washed with water to give the title compound (15.5 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 0.09 (9H, s), 0.79-0.88 (2H, m), 3.49-3.57 (2H, m), 5.62 (2H, s), 8.16 (1H, s ), 8.73 (1H, s).
5-ブロモ-4-クロロ-7H-ピロロ[2,3-d]ピリミジン (10 g) のDMF (143 mL) 溶液に氷冷下で水素化ナトリウム (2.07 g) を加えた。反応混合物を氷冷下で30分攪拌した。2-トリメチルシリルエチルオキシメチルクロリド(9.14 mL) を氷冷下で反応混合物に加え、室温で3時間攪拌した。反応混合物を水で希釈し、室温で4時間攪拌した。析出物をろ取し、水で洗浄し、標題化合物 (15.5 g) を得た。
1H NMR (300 MHz, DMSO-d6) δ 0.09 (9H, s), 0.79-0.88 (2H, m), 3.49-3.57 (2H, m), 5.62 (2H, s), 8.16 (1H, s), 8.73 (1H, s). A) 5-Bromo-4-chloro-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine 5-bromo-4-chloro-7H-pyrrolo [2,3 To a solution of -d] pyrimidine (10 g) in DMF (143 mL) was added sodium hydride (2.07 g) under ice-cooling. The reaction mixture was stirred for 30 minutes under ice cooling. 2-Trimethylsilylethyloxymethyl chloride (9.14 mL) was added to the reaction mixture under ice cooling, and the mixture was stirred at room temperature for 3 hours. The reaction mixture was diluted with water and stirred at room temperature for 4 hours. The precipitate was collected by filtration and washed with water to give the title compound (15.5 g).
1 H NMR (300 MHz, DMSO-d 6 ) δ 0.09 (9H, s), 0.79-0.88 (2H, m), 3.49-3.57 (2H, m), 5.62 (2H, s), 8.16 (1H, s ), 8.73 (1H, s).
B) 5-ブロモ-4-メトキシ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン
5-ブロモ-4-クロロ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン (1.00 g) のTHF (28 mL) 溶液に28%ナトリウムメトキシドメタノール溶液 (0.638 g) を窒素雰囲気下、氷冷下で加えた。反応混合物を窒素雰囲気下、室温で3時間攪拌した。反応混合物を酢酸エチルで希釈し、水及び飽和食塩水で洗浄後、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(830 mg) を得た。
1H NMR (300 MHz, CDCl3) δ 0.05 (9H, s), 0.86-0.96 (2H, m), 3.47-3.57 (2H, m), 4.15 (3H, s), 5.57 (2H, s), 7.20 (1H, s), 8.46 (1H, s). B) 5-Bromo-4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine 5-bromo-4-chloro-7-((2- ( To a solution of trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine (1.00 g) in THF (28 mL) was added 28% sodium methoxide methanol solution (0.638 g) under a nitrogen atmosphere under ice cooling. added. The reaction mixture was stirred at room temperature for 3 hours under nitrogen atmosphere. The reaction mixture was diluted with ethyl acetate, washed with water and saturated brine, and dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (830 mg).
1 H NMR (300 MHz, CDCl 3 ) δ 0.05 (9H, s), 0.86-0.96 (2H, m), 3.47-3.57 (2H, m), 4.15 (3H, s), 5.57 (2H, s), 7.20 (1H, s), 8.46 (1H, s).
5-ブロモ-4-クロロ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン (1.00 g) のTHF (28 mL) 溶液に28%ナトリウムメトキシドメタノール溶液 (0.638 g) を窒素雰囲気下、氷冷下で加えた。反応混合物を窒素雰囲気下、室温で3時間攪拌した。反応混合物を酢酸エチルで希釈し、水及び飽和食塩水で洗浄後、無水硫酸ナトリウムで乾燥し、溶媒を減圧下留去した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(830 mg) を得た。
1H NMR (300 MHz, CDCl3) δ 0.05 (9H, s), 0.86-0.96 (2H, m), 3.47-3.57 (2H, m), 4.15 (3H, s), 5.57 (2H, s), 7.20 (1H, s), 8.46 (1H, s). B) 5-Bromo-4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine 5-bromo-4-chloro-7-((2- ( To a solution of trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine (1.00 g) in THF (28 mL) was added 28% sodium methoxide methanol solution (0.638 g) under a nitrogen atmosphere under ice cooling. added. The reaction mixture was stirred at room temperature for 3 hours under nitrogen atmosphere. The reaction mixture was diluted with ethyl acetate, washed with water and saturated brine, and dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (830 mg).
1 H NMR (300 MHz, CDCl 3 ) δ 0.05 (9H, s), 0.86-0.96 (2H, m), 3.47-3.57 (2H, m), 4.15 (3H, s), 5.57 (2H, s), 7.20 (1H, s), 8.46 (1H, s).
C) 2-(ベンジルアミノ)-4-(4-メトキシ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン-5-イル)安息香酸
2-(ベンジルアミノ)-4-ブロモベンズアミド (500 mg)、ビス(ピナコラト)二ホウ素 (624 mg)およびカリウムアセタート(482 mg) のDME (20 mL) 溶液に窒素雰囲気下でジクロロ[1,1'-ビス(ジフェニルホスフィノ)フェロセン]パラジウム (120 mg) を加えた。反応混合物を窒素雰囲気下、80℃で終夜攪拌した後、反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。得られた粗生成物、5-ブロモ-4-メトキシ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン (100 mg)、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II) (219.8 mg)および炭酸カリウム (116 mg) のDME (5 mL)及び水 (0.5 mL) の混合溶液をマイクロウェーブ照射下100℃で1時間攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (8 mg) を得た。
1H NMR (300 MHz, CDCl3) δ 0.05 (9H, s), 0.86-0.98 (2H, m), 3.51-3.62 (2H, m), 3.99 (3H, s), 4.57 (2H, s), 5.64 (2H, s), 6.93 (1H, dd, J = 8.3, 1.5 Hz), 7.11 (1H, d, J = 1.1 Hz), 7.26-7.44 (7H, m), 8.02 (1H, d, J = 8.3 Hz), 8.51 (1H, s). C) 2- (Benzylamino) -4- (4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidin-5-yl) benzoic acid 2- Dichloro [1,1 'in a DME (20 mL) solution of (benzylamino) -4-bromobenzamide (500 mg), bis (pinacolato) diboron (624 mg) and potassium acetate (482 mg) under a nitrogen atmosphere -Bis (diphenylphosphino) ferrocene] palladium (120 mg) was added. The reaction mixture was stirred at 80 ° C. overnight under a nitrogen atmosphere, water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The resulting crude product, 5-bromo-4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine (100 mg), bis (di-tert -Butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) (219.8 mg) and potassium carbonate (116 mg) in DME (5 mL) and water (0.5 mL) at 100 ° C under microwave irradiation Stir for 1 hour. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (8 mg).
1 H NMR (300 MHz, CDCl 3 ) δ 0.05 (9H, s), 0.86-0.98 (2H, m), 3.51-3.62 (2H, m), 3.99 (3H, s), 4.57 (2H, s), 5.64 (2H, s), 6.93 (1H, dd, J = 8.3, 1.5 Hz), 7.11 (1H, d, J = 1.1 Hz), 7.26-7.44 (7H, m), 8.02 (1H, d, J = 8.3 Hz), 8.51 (1H, s).
2-(ベンジルアミノ)-4-ブロモベンズアミド (500 mg)、ビス(ピナコラト)二ホウ素 (624 mg)およびカリウムアセタート(482 mg) のDME (20 mL) 溶液に窒素雰囲気下でジクロロ[1,1'-ビス(ジフェニルホスフィノ)フェロセン]パラジウム (120 mg) を加えた。反応混合物を窒素雰囲気下、80℃で終夜攪拌した後、反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。得られた粗生成物、5-ブロモ-4-メトキシ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン (100 mg)、ビス(ジ-tert-ブチル(4-ジメチルアミノフェニル)ホスフィン)ジクロロパラジウム(II) (219.8 mg)および炭酸カリウム (116 mg) のDME (5 mL)及び水 (0.5 mL) の混合溶液をマイクロウェーブ照射下100℃で1時間攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (ヘキサン/酢酸エチル) で精製し、標題化合物 (8 mg) を得た。
1H NMR (300 MHz, CDCl3) δ 0.05 (9H, s), 0.86-0.98 (2H, m), 3.51-3.62 (2H, m), 3.99 (3H, s), 4.57 (2H, s), 5.64 (2H, s), 6.93 (1H, dd, J = 8.3, 1.5 Hz), 7.11 (1H, d, J = 1.1 Hz), 7.26-7.44 (7H, m), 8.02 (1H, d, J = 8.3 Hz), 8.51 (1H, s). C) 2- (Benzylamino) -4- (4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidin-5-yl) benzoic acid 2- Dichloro [1,1 'in a DME (20 mL) solution of (benzylamino) -4-bromobenzamide (500 mg), bis (pinacolato) diboron (624 mg) and potassium acetate (482 mg) under a nitrogen atmosphere -Bis (diphenylphosphino) ferrocene] palladium (120 mg) was added. The reaction mixture was stirred at 80 ° C. overnight under a nitrogen atmosphere, water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The resulting crude product, 5-bromo-4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidine (100 mg), bis (di-tert -Butyl (4-dimethylaminophenyl) phosphine) dichloropalladium (II) (219.8 mg) and potassium carbonate (116 mg) in DME (5 mL) and water (0.5 mL) at 100 ° C under microwave irradiation Stir for 1 hour. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (hexane / ethyl acetate) to give the title compound (8 mg).
1 H NMR (300 MHz, CDCl 3 ) δ 0.05 (9H, s), 0.86-0.98 (2H, m), 3.51-3.62 (2H, m), 3.99 (3H, s), 4.57 (2H, s), 5.64 (2H, s), 6.93 (1H, dd, J = 8.3, 1.5 Hz), 7.11 (1H, d, J = 1.1 Hz), 7.26-7.44 (7H, m), 8.02 (1H, d, J = 8.3 Hz), 8.51 (1H, s).
D) 1-ベンジル-7-(4-メトキシ-7H-ピロロ[2,3-d]ピリミジン-5-イル)-2,2-ジメチル-2,3-ジヒドロキナゾリン-4(1H)-オン
2-(ベンジルアミノ)-4-(4-メトキシ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン-5-イル)安息香酸 (8 mg) のTFA (0.1 mL) 溶液を室温で2時間攪拌した。溶媒を減圧下で留去した後、残渣に8規定アンモニア-メタノール溶液(0.20 mL)を室温で加えた。反応混合物を室温で3時間攪拌した。溶媒を減圧下で留去した。残渣と1H-ベンゾ[d][1,2,3]トリアゾール-1-オールアンモニア塩(8.13 mg) のDMF (2 mL) 溶液に1-エチル-3-(3-ジメチルアミノプロピル)カルボジイミド ヒドロクロリド (8.29 mg) を室温で加えた。反応混合物を室温で終夜攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣とp-トルエンスルホン酸一水和物(8 mg) のアセトン (2 mL) 溶液に2-メトキシプロパ-1-エン (2 mL) を水冷下で滴下した。反応混合物を室温で5分攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、標題化合物 (3.1 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.49 (6H, s), 3.83 (3H, s), 4.62 (2H, s), 6.91 (1H, s), 7.00-7.09 (1H, m), 7.14-7.24 (1H, m), 7.26-7.42 (4H, m), 7.51 (1H, s), 7.72 (1H, d, J = 7.9 Hz), 8.13 (1H, s), 8.36 (1H, s), 12.28 (1H, brs).
MS (ESI+): [M+H]+414.3. D) 1-Benzyl-7- (4-methoxy-7H-pyrrolo [2,3-d] pyrimidin-5-yl) -2,2-dimethyl-2,3-dihydroquinazolin-4 (1H) -one 2 Of (benzylamino) -4- (4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidin-5-yl) benzoic acid (8 mg) The TFA (0.1 mL) solution was stirred at room temperature for 2 hours. The solvent was evaporated under reduced pressure, and 8N ammonia-methanol solution (0.20 mL) was added to the residue at room temperature. The reaction mixture was stirred at room temperature for 3 hours. The solvent was distilled off under reduced pressure. Add 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride to a solution of the residue and 1H-benzo [d] [1,2,3] triazol-1-ol ammonia salt (8.13 mg) in DMF (2 mL). (8.29 mg) was added at room temperature. The reaction mixture was stirred at room temperature overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. To a solution of the residue and p-toluenesulfonic acid monohydrate (8 mg) in acetone (2 mL), 2-methoxyprop-1-ene (2 mL) was added dropwise under water cooling. The reaction mixture was stirred at room temperature for 5 minutes. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) to give the title compound (3.1 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.49 (6H, s), 3.83 (3H, s), 4.62 (2H, s), 6.91 (1H, s), 7.00-7.09 (1H, m), 7.14-7.24 (1H, m), 7.26-7.42 (4H, m), 7.51 (1H, s), 7.72 (1H, d, J = 7.9 Hz), 8.13 (1H, s), 8.36 (1H, s) , 12.28 (1H, brs).
MS (ESI +): [M + H] + 414.3.
2-(ベンジルアミノ)-4-(4-メトキシ-7-((2-(トリメチルシリル)エトキシ)メチル)-7H-ピロロ[2,3-d]ピリミジン-5-イル)安息香酸 (8 mg) のTFA (0.1 mL) 溶液を室温で2時間攪拌した。溶媒を減圧下で留去した後、残渣に8規定アンモニア-メタノール溶液(0.20 mL)を室温で加えた。反応混合物を室温で3時間攪拌した。溶媒を減圧下で留去した。残渣と1H-ベンゾ[d][1,2,3]トリアゾール-1-オールアンモニア塩(8.13 mg) のDMF (2 mL) 溶液に1-エチル-3-(3-ジメチルアミノプロピル)カルボジイミド ヒドロクロリド (8.29 mg) を室温で加えた。反応混合物を室温で終夜攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣とp-トルエンスルホン酸一水和物(8 mg) のアセトン (2 mL) 溶液に2-メトキシプロパ-1-エン (2 mL) を水冷下で滴下した。反応混合物を室温で5分攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水及び飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をシリカゲルクロマトグラフィー (酢酸エチル/メタノール) で精製し、標題化合物 (3.1 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 1.49 (6H, s), 3.83 (3H, s), 4.62 (2H, s), 6.91 (1H, s), 7.00-7.09 (1H, m), 7.14-7.24 (1H, m), 7.26-7.42 (4H, m), 7.51 (1H, s), 7.72 (1H, d, J = 7.9 Hz), 8.13 (1H, s), 8.36 (1H, s), 12.28 (1H, brs).
MS (ESI+): [M+H]+414.3. D) 1-Benzyl-7- (4-methoxy-7H-pyrrolo [2,3-d] pyrimidin-5-yl) -2,2-dimethyl-2,3-dihydroquinazolin-4 (1H) -one 2 Of (benzylamino) -4- (4-methoxy-7-((2- (trimethylsilyl) ethoxy) methyl) -7H-pyrrolo [2,3-d] pyrimidin-5-yl) benzoic acid (8 mg) The TFA (0.1 mL) solution was stirred at room temperature for 2 hours. The solvent was evaporated under reduced pressure, and 8N ammonia-methanol solution (0.20 mL) was added to the residue at room temperature. The reaction mixture was stirred at room temperature for 3 hours. The solvent was distilled off under reduced pressure. Add 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride to a solution of the residue and 1H-benzo [d] [1,2,3] triazol-1-ol ammonia salt (8.13 mg) in DMF (2 mL). (8.29 mg) was added at room temperature. The reaction mixture was stirred at room temperature overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. To a solution of the residue and p-toluenesulfonic acid monohydrate (8 mg) in acetone (2 mL), 2-methoxyprop-1-ene (2 mL) was added dropwise under water cooling. The reaction mixture was stirred at room temperature for 5 minutes. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography (ethyl acetate / methanol) to give the title compound (3.1 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 1.49 (6H, s), 3.83 (3H, s), 4.62 (2H, s), 6.91 (1H, s), 7.00-7.09 (1H, m), 7.14-7.24 (1H, m), 7.26-7.42 (4H, m), 7.51 (1H, s), 7.72 (1H, d, J = 7.9 Hz), 8.13 (1H, s), 8.36 (1H, s) , 12.28 (1H, brs).
MS (ESI +): [M + H] + 414.3.
実施例81
1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-1,4-ベンゾジアゼピン-5-オン Example 81
1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-1,4- Benzodiazepine-5-one
1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-1,4-ベンゾジアゼピン-5-オン Example 81
1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-1,4- Benzodiazepine-5-one
8-ブロモ-3,4-ジヒドロ-1H-ベンゾ[e][1,4]ジアゼピン-5(2H)-オン (150 mg) のトルエン(5 mL) 溶液にトリエチルアミン(0.099 mL) 及びブロモメチルベンゼン(117 mg) を室温で加えた。反応混合物を窒素雰囲気下、60℃で終夜攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去することで標題化合物を含む粗精製物 (80 mg) を得た。得られた粗精製物、tert-ブチル (3-アミノピリジン-2-イル)カルバマート (61 mg)、ナトリウム tert-ブトキシド (46 mg)、トリス(ジベンジリデンアセトン)二パラジウム(0) (22 mg)および2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (45 mg) のtert-ブチルアルコール (5 mL) 溶液を、アルゴン雰囲気下、100 ℃で3時間撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をNHシリカゲルクロマトグラフィー(酢酸エチル/メタノール) で精製し、標題化合物(34 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 3.34 (4H, s), 4.46 (2H, s), 6.61 (1H, dd, J = 7.7, 1.3 Hz), 6.85 (1H, dd, J = 7.9, 5.3 Hz), 6.95 (1H, d, J = 1.9 Hz), 7.07 (1H, dd, J = 8.3, 1.9 Hz), 7.21-7.46 (5H, m), 7.68 (1H, d, J = 8.3 Hz), 7.94 (1H, dd, J = 5.1, 1.3 Hz), 8.30 (1H, brs), 11.80 (1H, brs). 8-Bromo-3,4-dihydro-1H-benzo [e] [1,4] diazepin-5 (2H) -one (150 mg) in toluene (5 mL) in triethylamine (0.099 mL) and bromomethylbenzene (117 mg) was added at room temperature. The reaction mixture was stirred at 60 ° C. overnight under a nitrogen atmosphere. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give a crude product (80 mg) containing the title compound. Crude product obtained, tert-butyl (3-aminopyridin-2-yl) carbamate (61 mg), sodium tert-butoxide (46 mg), tris (dibenzylideneacetone) dipalladium (0) (22 mg) And 2-dicyclohexylphosphino-2 ′, 6′-diisopropoxy-1,1′-biphenyl (45 mg) in tert-butyl alcohol (5 mL) were stirred at 100 ° C. for 3 hours under an argon atmosphere. . Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH silica gel chromatography (ethyl acetate / methanol) to give the title compound (34 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.34 (4H, s), 4.46 (2H, s), 6.61 (1H, dd, J = 7.7, 1.3 Hz), 6.85 (1H, dd, J = 7.9 , 5.3 Hz), 6.95 (1H, d, J = 1.9 Hz), 7.07 (1H, dd, J = 8.3, 1.9 Hz), 7.21-7.46 (5H, m), 7.68 (1H, d, J = 8.3 Hz) ), 7.94 (1H, dd, J = 5.1, 1.3 Hz), 8.30 (1H, brs), 11.80 (1H, brs).
1H NMR (300 MHz, DMSO-d6) δ 3.34 (4H, s), 4.46 (2H, s), 6.61 (1H, dd, J = 7.7, 1.3 Hz), 6.85 (1H, dd, J = 7.9, 5.3 Hz), 6.95 (1H, d, J = 1.9 Hz), 7.07 (1H, dd, J = 8.3, 1.9 Hz), 7.21-7.46 (5H, m), 7.68 (1H, d, J = 8.3 Hz), 7.94 (1H, dd, J = 5.1, 1.3 Hz), 8.30 (1H, brs), 11.80 (1H, brs). 8-Bromo-3,4-dihydro-1H-benzo [e] [1,4] diazepin-5 (2H) -one (150 mg) in toluene (5 mL) in triethylamine (0.099 mL) and bromomethylbenzene (117 mg) was added at room temperature. The reaction mixture was stirred at 60 ° C. overnight under a nitrogen atmosphere. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give a crude product (80 mg) containing the title compound. Crude product obtained, tert-butyl (3-aminopyridin-2-yl) carbamate (61 mg), sodium tert-butoxide (46 mg), tris (dibenzylideneacetone) dipalladium (0) (22 mg) And 2-dicyclohexylphosphino-2 ′, 6′-diisopropoxy-1,1′-biphenyl (45 mg) in tert-butyl alcohol (5 mL) were stirred at 100 ° C. for 3 hours under an argon atmosphere. . Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH silica gel chromatography (ethyl acetate / methanol) to give the title compound (34 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.34 (4H, s), 4.46 (2H, s), 6.61 (1H, dd, J = 7.7, 1.3 Hz), 6.85 (1H, dd, J = 7.9 , 5.3 Hz), 6.95 (1H, d, J = 1.9 Hz), 7.07 (1H, dd, J = 8.3, 1.9 Hz), 7.21-7.46 (5H, m), 7.68 (1H, d, J = 8.3 Hz) ), 7.94 (1H, dd, J = 5.1, 1.3 Hz), 8.30 (1H, brs), 11.80 (1H, brs).
実施例88
1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オン Example 88
1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-pyrido [2, 3-e] [1,4] diazepine-5-one
1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オン Example 88
1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-pyrido [2, 3-e] [1,4] diazepine-5-one
A) 1-ベンジル-8-クロロ-3,4-ジヒドロ-1H-ピリド[2,3-e][1,4]ジアゼピン-5(2H)-オン
2,6-ジクロロニコチン酸 (384 mg)、N-ベンジルエタン-1,2-ジアミン (330 mg)およびトリエチルアミン (0.279 mL) のDMF (3 mL) 溶液に、ヘキサフルオロリン酸(ベンゾトリアゾール-1-イルオキシ)トリピロリジノホスホニウムのDMF (2 mL) 溶液を加えた。反応混合物を室温で終夜攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水と飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。得られた残渣をDMF (3 mL) に溶解し、炭酸カリウム (553 mg) を加えた。反応混合物を100 ℃で終夜攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(100 mg) を得た。
1H NMR (300 MHz, CDCl3) δ 3.58 (4H, d, J = 8.3 Hz), 4.95 (2H, s), 6.38 (1H, brs), 6.73 (1H, d, J = 8.3 Hz), 7.26-7.41 (5H, m), 8.31 (1H, d, J = 8.3 Hz). A) 1-Benzyl-8-chloro-3,4-dihydro-1H-pyrido [2,3-e] [1,4] diazepin-5 (2H) -one 2,6-dichloronicotinic acid (384 mg) , N-benzylethane-1,2-diamine (330 mg) and triethylamine (0.279 mL) in DMF (3 mL) were added hexafluorophosphoric acid (benzotriazol-1-yloxy) tripyrrolidinophosphonium DMF (2 mL) The solution was added. The reaction mixture was stirred at room temperature overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The obtained residue was dissolved in DMF (3 mL), and potassium carbonate (553 mg) was added. The reaction mixture was stirred at 100 ° C. overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (100 mg).
1 H NMR (300 MHz, CDCl 3 ) δ 3.58 (4H, d, J = 8.3 Hz), 4.95 (2H, s), 6.38 (1H, brs), 6.73 (1H, d, J = 8.3 Hz), 7.26 -7.41 (5H, m), 8.31 (1H, d, J = 8.3 Hz).
2,6-ジクロロニコチン酸 (384 mg)、N-ベンジルエタン-1,2-ジアミン (330 mg)およびトリエチルアミン (0.279 mL) のDMF (3 mL) 溶液に、ヘキサフルオロリン酸(ベンゾトリアゾール-1-イルオキシ)トリピロリジノホスホニウムのDMF (2 mL) 溶液を加えた。反応混合物を室温で終夜攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水と飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。得られた残渣をDMF (3 mL) に溶解し、炭酸カリウム (553 mg) を加えた。反応混合物を100 ℃で終夜攪拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をNHシリカゲルクロマトグラフィー(ヘキサン/酢酸エチル) で精製し、標題化合物(100 mg) を得た。
1H NMR (300 MHz, CDCl3) δ 3.58 (4H, d, J = 8.3 Hz), 4.95 (2H, s), 6.38 (1H, brs), 6.73 (1H, d, J = 8.3 Hz), 7.26-7.41 (5H, m), 8.31 (1H, d, J = 8.3 Hz). A) 1-Benzyl-8-chloro-3,4-dihydro-1H-pyrido [2,3-e] [1,4] diazepin-5 (2H) -one 2,6-dichloronicotinic acid (384 mg) , N-benzylethane-1,2-diamine (330 mg) and triethylamine (0.279 mL) in DMF (3 mL) were added hexafluorophosphoric acid (benzotriazol-1-yloxy) tripyrrolidinophosphonium DMF (2 mL) The solution was added. The reaction mixture was stirred at room temperature overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The obtained residue was dissolved in DMF (3 mL), and potassium carbonate (553 mg) was added. The reaction mixture was stirred at 100 ° C. overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with water and dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by NH silica gel chromatography (hexane / ethyl acetate) to give the title compound (100 mg).
1 H NMR (300 MHz, CDCl 3 ) δ 3.58 (4H, d, J = 8.3 Hz), 4.95 (2H, s), 6.38 (1H, brs), 6.73 (1H, d, J = 8.3 Hz), 7.26 -7.41 (5H, m), 8.31 (1H, d, J = 8.3 Hz).
B) 1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オン
1-ベンジル-8-クロロ-3,4-ジヒドロ-1H-ピリド[2,3-e][1,4]ジアゼピン-5(2H)-オン (100 mg)、tert-ブチル(3-アミノピリジン-2-イル)カルバマート (87 mg)、ナトリウム tert-ブトキシド (67 mg)、トリス(ジベンジリデンアセトン)二パラジウム(0) (32 mg)および2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (65 mg) のtert-ブチルアルコール (5 mL) 溶液を、アルゴン雰囲気下、100 ℃で3時間撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をNHシリカゲルクロマトグラフィー(酢酸エチル/メタノール) で精製し、酢酸エチルで洗浄して標題化合物 (10 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 3.41-3.54 (2H, m), 3.61-3.73 (2H, m), 4.93 (2H, s), 6.52 (1H, dd, J = 7.9, 5.3 Hz), 7.20-7.33 (3H, m), 7.34-7.42 (2H, m), 7.43-7.53 (2H, m), 7.88 (1H, dd, J = 5.1, 1.3 Hz), 8.23-8.32 (1H, m), 8.35 (1H, d, J = 8.7 Hz), 11.92 (1H, brs). B) 1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-pyrido [ 2,3-e] [1,4] diazepine-5-one 1-benzyl-8-chloro-3,4-dihydro-1H-pyrido [2,3-e] [1,4] diazepine-5 (2H ) -One (100 mg), tert-butyl (3-aminopyridin-2-yl) carbamate (87 mg), sodium tert-butoxide (67 mg), tris (dibenzylideneacetone) dipalladium (0) (32 mg ) And 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1'-biphenyl (65 mg) in tert-butyl alcohol (5 mL) and stirred at 100 ° C for 3 hours under argon atmosphere did. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH silica gel chromatography (ethyl acetate / methanol) and washed with ethyl acetate to give the title compound (10 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.41-3.54 (2H, m), 3.61-3.73 (2H, m), 4.93 (2H, s), 6.52 (1H, dd, J = 7.9, 5.3 Hz ), 7.20-7.33 (3H, m), 7.34-7.42 (2H, m), 7.43-7.53 (2H, m), 7.88 (1H, dd, J = 5.1, 1.3 Hz), 8.23-8.32 (1H, m ), 8.35 (1H, d, J = 8.7 Hz), 11.92 (1H, brs).
1-ベンジル-8-クロロ-3,4-ジヒドロ-1H-ピリド[2,3-e][1,4]ジアゼピン-5(2H)-オン (100 mg)、tert-ブチル(3-アミノピリジン-2-イル)カルバマート (87 mg)、ナトリウム tert-ブトキシド (67 mg)、トリス(ジベンジリデンアセトン)二パラジウム(0) (32 mg)および2-ジシクロヘキシルホスフィノ-2',6'-ジイソプロポキシ-1,1'-ビフェニル (65 mg) のtert-ブチルアルコール (5 mL) 溶液を、アルゴン雰囲気下、100 ℃で3時間撹拌した。反応混合物に水を加え、酢酸エチルで抽出した。有機層を分離し、飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥し、溶媒を減圧下留去した。残渣をNHシリカゲルクロマトグラフィー(酢酸エチル/メタノール) で精製し、酢酸エチルで洗浄して標題化合物 (10 mg) を得た。
1H NMR (300 MHz, DMSO-d6) δ 3.41-3.54 (2H, m), 3.61-3.73 (2H, m), 4.93 (2H, s), 6.52 (1H, dd, J = 7.9, 5.3 Hz), 7.20-7.33 (3H, m), 7.34-7.42 (2H, m), 7.43-7.53 (2H, m), 7.88 (1H, dd, J = 5.1, 1.3 Hz), 8.23-8.32 (1H, m), 8.35 (1H, d, J = 8.7 Hz), 11.92 (1H, brs). B) 1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-pyrido [ 2,3-e] [1,4] diazepine-5-one 1-benzyl-8-chloro-3,4-dihydro-1H-pyrido [2,3-e] [1,4] diazepine-5 (2H ) -One (100 mg), tert-butyl (3-aminopyridin-2-yl) carbamate (87 mg), sodium tert-butoxide (67 mg), tris (dibenzylideneacetone) dipalladium (0) (32 mg ) And 2-dicyclohexylphosphino-2 ', 6'-diisopropoxy-1,1'-biphenyl (65 mg) in tert-butyl alcohol (5 mL) and stirred at 100 ° C for 3 hours under argon atmosphere did. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH silica gel chromatography (ethyl acetate / methanol) and washed with ethyl acetate to give the title compound (10 mg).
1 H NMR (300 MHz, DMSO-d 6 ) δ 3.41-3.54 (2H, m), 3.61-3.73 (2H, m), 4.93 (2H, s), 6.52 (1H, dd, J = 7.9, 5.3 Hz ), 7.20-7.33 (3H, m), 7.34-7.42 (2H, m), 7.43-7.53 (2H, m), 7.88 (1H, dd, J = 5.1, 1.3 Hz), 8.23-8.32 (1H, m ), 8.35 (1H, d, J = 8.7 Hz), 11.92 (1H, brs).
上記の方法またはそれらに準じた方法に従って製造した実施例化合物を以下の表に示す。表中のMSは実測値を示す。
Example compounds prepared according to the above methods or methods similar thereto are shown in the following table. MS in the table indicates actual measurement.
上記の方法またはそれらに準じた方法に従って製造した参考例化合物を以下の表に示す。表中のMSは実測値を示す。
Reference compounds prepared according to the above methods or methods similar thereto are shown in the following table. MS in the table indicates actual measurement.
試験例1 PKCシータ酵素阻害試験
被検化合物のPKCシータ酵素阻害活性はTR-FRET法にて測定した。まず、アッセイバッファー(20mM Tris-HCl(pH7.5)、5mM MgAcetate、0.1mM CaCl2、1mM DTT、0.01% Tween20、0.05% BSA、10% PKC Activator(ミリポア))で希釈した被検化合物を384ウェルプレートに2μLずつ添加した。次に、PKCシータを3pM、蛍光標識ペプチド基質(Fluorescein-PKC Substrate、ライフテクノロジーズ)を1.2μMとなるようアッセイバッファーで希釈した溶液を2μLずつ添加し、その後、60μMとなるようアッセイバッファーで調製したATP溶液を2μLずつ添加することにより酵素反応を開始した。室温にて1時間反応させた後、20mM EDTA、0.6nM テルビウム標識抗リン酸化セリン抗体(ライフテクノロジーズ)となるよう調製したTR-FRET Dilution Buffer(ライフテクノロジーズ)を6μLずつ添加した。室温にて1時間静置した後、プレートリーダーEnvision(パーキンエルマー)にて蛍光強度(励起波長340nm、蛍光波長520nm、delay time 100マイクロ秒)を測定した。各化合物の阻害活性は、酵素なしのウェルの蛍光強度を100%阻害とする相対活性値として算出した。その結果を表12に示す。 Test Example 1 PKC Theta Enzyme Inhibition Test The PKC theta enzyme inhibitory activity of the test compound was measured by the TR-FRET method. First, it was diluted with assay buffer (20 mM Tris-HCl (pH 7.5), 5 mM MgAcetate, 0.1 mM CaCl 2 , 1 mM DTT, 0.01% Tween 20, 0.05% BSA, 10% PKC Activator (Millipore)). A test compound was added in an amount of 2 μL to a 384 well plate. Next, add 2 μL each of the PKC theta 3 pM and the fluorescence-labeled peptide substrate (Fluorescein-PKC Substrate, Life Technologies) diluted to 1.2 μM with the assay buffer, and then prepare with the assay buffer to 60 μM. The enzyme reaction was started by adding 2 μL of the prepared ATP solution. After reacting at room temperature for 1 hour, 6 μL of TR-FRET Dilution Buffer (Life Technologies) prepared to become 20 mM EDTA, 0.6 nM terbium-labeled anti-phosphorylated serine antibody (Life Technologies) was added. After standing at room temperature for 1 hour, fluorescence intensity (excitation wavelength: 340 nm, fluorescence wavelength: 520 nm, delay time: 100 microseconds) was measured with a plate reader Envision (Perkin Elmer). The inhibitory activity of each compound was calculated as a relative activity value with 100% inhibition of the fluorescence intensity of wells without enzyme. The results are shown in Table 12.
被検化合物のPKCシータ酵素阻害活性はTR-FRET法にて測定した。まず、アッセイバッファー(20mM Tris-HCl(pH7.5)、5mM MgAcetate、0.1mM CaCl2、1mM DTT、0.01% Tween20、0.05% BSA、10% PKC Activator(ミリポア))で希釈した被検化合物を384ウェルプレートに2μLずつ添加した。次に、PKCシータを3pM、蛍光標識ペプチド基質(Fluorescein-PKC Substrate、ライフテクノロジーズ)を1.2μMとなるようアッセイバッファーで希釈した溶液を2μLずつ添加し、その後、60μMとなるようアッセイバッファーで調製したATP溶液を2μLずつ添加することにより酵素反応を開始した。室温にて1時間反応させた後、20mM EDTA、0.6nM テルビウム標識抗リン酸化セリン抗体(ライフテクノロジーズ)となるよう調製したTR-FRET Dilution Buffer(ライフテクノロジーズ)を6μLずつ添加した。室温にて1時間静置した後、プレートリーダーEnvision(パーキンエルマー)にて蛍光強度(励起波長340nm、蛍光波長520nm、delay time 100マイクロ秒)を測定した。各化合物の阻害活性は、酵素なしのウェルの蛍光強度を100%阻害とする相対活性値として算出した。その結果を表12に示す。 Test Example 1 PKC Theta Enzyme Inhibition Test The PKC theta enzyme inhibitory activity of the test compound was measured by the TR-FRET method. First, it was diluted with assay buffer (20 mM Tris-HCl (pH 7.5), 5 mM MgAcetate, 0.1 mM CaCl 2 , 1 mM DTT, 0.01% Tween 20, 0.05% BSA, 10% PKC Activator (Millipore)). A test compound was added in an amount of 2 μL to a 384 well plate. Next, add 2 μL each of the PKC theta 3 pM and the fluorescence-labeled peptide substrate (Fluorescein-PKC Substrate, Life Technologies) diluted to 1.2 μM with the assay buffer, and then prepare with the assay buffer to 60 μM. The enzyme reaction was started by adding 2 μL of the prepared ATP solution. After reacting at room temperature for 1 hour, 6 μL of TR-FRET Dilution Buffer (Life Technologies) prepared to become 20 mM EDTA, 0.6 nM terbium-labeled anti-phosphorylated serine antibody (Life Technologies) was added. After standing at room temperature for 1 hour, fluorescence intensity (excitation wavelength: 340 nm, fluorescence wavelength: 520 nm, delay time: 100 microseconds) was measured with a plate reader Envision (Perkin Elmer). The inhibitory activity of each compound was calculated as a relative activity value with 100% inhibition of the fluorescence intensity of wells without enzyme. The results are shown in Table 12.
製剤例1(カプセルの製造)
1)実施例1の化合物 30 mg
2)微粉末セルロース 10 mg
3)乳糖 19 mg
4)ステアリン酸マグネシウム 1 mg
計 60 mg
1)、2)、3)および4)を混合して、ゼラチンカプセルに充填する。 Formulation Example 1 (Manufacture of capsules)
1) 30 mg of the compound of Example 1
2) Fine powder cellulose 10 mg
3) Lactose 19 mg
4) Magnesium stearate 1 mg
60 mg total
1), 2), 3) and 4) are mixed and filled into gelatin capsules.
1)実施例1の化合物 30 mg
2)微粉末セルロース 10 mg
3)乳糖 19 mg
4)ステアリン酸マグネシウム 1 mg
計 60 mg
1)、2)、3)および4)を混合して、ゼラチンカプセルに充填する。 Formulation Example 1 (Manufacture of capsules)
1) 30 mg of the compound of Example 1
2) Fine powder cellulose 10 mg
3) Lactose 19 mg
4) Magnesium stearate 1 mg
60 mg total
1), 2), 3) and 4) are mixed and filled into gelatin capsules.
製剤例2(錠剤の製造)
1)実施例1の化合物 30 g
2)乳糖 50 g
3)トウモロコシデンプン 15 g
4)カルボキシメチルセルロースカルシウム 44 g
5)ステアリン酸マグネシウム 1 g
1000錠 計 140 g
1)、2)、3)の全量および30gの4)を水で練合し、真空乾燥後、整粒を行う。この整粒末に14gの4)および1gの5)を混合し、打錠機により打錠する。このようにして、1錠あたり実施例1の化合物30mgを含有する錠剤1000錠を得る。 Formulation Example 2 (Manufacture of tablets)
1) Compound of Example 1 30 g
2) Lactose 50 g
3) Corn starch 15 g
4) Carboxymethylcellulose calcium 44 g
5) Magnesium stearate 1 g
1000 tablets total 140 g
The total amount of 1), 2) and 3) and 30 g of 4) are kneaded with water, and after vacuum drying, the particles are sized. 14 g of 4) and 1 g of 5) are mixed with the sized powder, and tableted with a tableting machine. In this way, 1000 tablets containing 30 mg of the compound of Example 1 per tablet are obtained.
1)実施例1の化合物 30 g
2)乳糖 50 g
3)トウモロコシデンプン 15 g
4)カルボキシメチルセルロースカルシウム 44 g
5)ステアリン酸マグネシウム 1 g
1000錠 計 140 g
1)、2)、3)の全量および30gの4)を水で練合し、真空乾燥後、整粒を行う。この整粒末に14gの4)および1gの5)を混合し、打錠機により打錠する。このようにして、1錠あたり実施例1の化合物30mgを含有する錠剤1000錠を得る。 Formulation Example 2 (Manufacture of tablets)
1) Compound of Example 1 30 g
2) Lactose 50 g
3) Corn starch 15 g
4) Carboxymethylcellulose calcium 44 g
5) Magnesium stearate 1 g
1000 tablets total 140 g
The total amount of 1), 2) and 3) and 30 g of 4) are kneaded with water, and after vacuum drying, the particles are sized. 14 g of 4) and 1 g of 5) are mixed with the sized powder, and tableted with a tableting machine. In this way, 1000 tablets containing 30 mg of the compound of Example 1 per tablet are obtained.
本発明化合物は、優れたPKC阻害作用を有し、免疫性疾患、炎症性疾患等の予防または治療剤として有用である。
The compound of the present invention has an excellent PKC inhibitory action and is useful as a preventive or therapeutic agent for immune diseases, inflammatory diseases and the like.
本出願は、日本で出願された特願2014-060896を基礎としており、その内容は本明細書にすべて包含されるものである。
This application is based on Japanese Patent Application No. 2014-060896 filed in Japan, the contents of which are incorporated in full herein.
Claims (17)
- 式(I):
環Aは、さらに置換されていてもよい含窒素不飽和複素環を示し;
環Bは、さらに置換されていてもよいC6-14芳香族炭化水素環またはさらに置換されていてもよい芳香族複素環を示し;
Lは、置換されていてもよいC1-2アルキレンを示し;
X1は、NまたはCR1を示し;
X2は、NまたはCR2を示し;
X3は、NまたはCR3を示し;
X4は、NまたはCR4を示し;
R1、R2、R3およびR4は、独立して、水素原子または置換基を示し;
X5は、CR5a、CR5bR5c、NR5dまたは結合手を示し;
R5a、R5b、R5cおよびR5dは、独立して、水素原子または置換基を示すか、またはR5bとR5cは、一緒になって置換されていてもよい環を形成してもよく;
X6は、CR6aR6bまたはNR6cを示し;
R6a、R6bおよびR6cは、独立して、水素原子または置換基を示すか、またはR6aとR6bは、一緒になって置換されていてもよい環を形成してもよく;
R7は、水素原子または置換基を示す。]
で表される化合物またはその塩。 Formula (I):
Ring A represents a nitrogen-containing unsaturated heterocyclic ring which may be further substituted;
Ring B represents a C 6-14 aromatic hydrocarbon ring which may be further substituted or an aromatic heterocyclic ring which may be further substituted;
L represents an optionally substituted C 1-2 alkylene;
X 1 represents N or CR 1 ;
X 2 represents N or CR 2 ;
X 3 represents N or CR 3 ;
X 4 represents N or CR 4 ;
R 1 , R 2 , R 3 and R 4 independently represent a hydrogen atom or a substituent;
X 5 represents CR 5a , CR 5b R 5c , NR 5d or a bond;
R 5a , R 5b , R 5c and R 5d independently represent a hydrogen atom or a substituent, or R 5b and R 5c may combine together to form an optionally substituted ring. Often;
X 6 represents CR 6a R 6b or NR 6c ;
R 6a , R 6b and R 6c independently represent a hydrogen atom or a substituent, or R 6a and R 6b may together form an optionally substituted ring;
R 7 represents a hydrogen atom or a substituent. ]
Or a salt thereof. - 環Aが、さらに置換されていてもよい単環式の含窒素不飽和複素環であるか、または、さらに置換されていてもよい、式(A):
環A1は、さらに置換されていてもよい5員または6員の芳香環、または、さらに置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A2は、置換されていてもよい5員または6員の芳香環、または、置換されていてもよい5員または6員の非芳香族不飽和環を示し、
環A1または環A2の環構成原子の少なくとも1個以上が窒素原子である。]
で表される二環式の含窒素不飽和複素環であり;
環Bが、さらに置換されていてもよいベンゼン環、あるいはさらに置換されていてもよいチオフェン環またはさらに置換されていてもよいピリジン環であり;
Lが、C1-2アルキレンであり;
X1が、NまたはCR1であり、R1が、水素原子またはハロゲン原子であり;
X2が、NまたはCR2であり、R2が、水素原子またはハロゲン原子であり;
X3が、NまたはCR3であり、R3が、水素原子またはハロゲン原子であり;
X4が、NまたはCR4であり、R4が、水素原子またはC1-6アルキル基であり;
X5が、CR5bR5cまたは結合手であり、R5bおよびR5cが、独立して、水素原子または置換基であり;
X6が、CR6aR6bであり、R6aおよびR6bが、独立して、水素原子または置換基であるか、または、R6aおよびR6bが一緒になって、置換されていてもよい環を形成し;
R7が、水素原子または置換されていてもよいC1-6アルキル基である;
請求項1記載の化合物またはその塩。 Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted, or may be further substituted, Formula (A):
Ring A 1 represents an optionally further substituted 5- or 6-membered aromatic ring, or an optionally further substituted 5- or 6-membered non-aromatic unsaturated ring;
Ring A 2 represents an optionally substituted 5- or 6-membered aromatic ring, or an optionally substituted 5- or 6-membered non-aromatic unsaturated ring;
At least one of the ring constituent atoms of ring A 1 or ring A 2 is a nitrogen atom. ]
A bicyclic nitrogen-containing unsaturated heterocycle represented by:
Ring B is a benzene ring which may be further substituted, or a thiophene ring which may be further substituted or a pyridine ring which may be further substituted;
L is C 1-2 alkylene;
X 1 is N or CR 1 and R 1 is a hydrogen atom or a halogen atom;
X 2 is N or CR 2 and R 2 is a hydrogen atom or a halogen atom;
X 3 is N or CR 3 , R 3 is a hydrogen atom or a halogen atom;
X 4 is N or CR 4 and R 4 is a hydrogen atom or a C 1-6 alkyl group;
X 5 is CR 5b R 5c or a bond, and R 5b and R 5c are independently a hydrogen atom or a substituent;
X 6 is CR 6a R 6b and R 6a and R 6b are independently a hydrogen atom or a substituent, or R 6a and R 6b may be substituted together to be substituted. Forming a ring;
R 7 is a hydrogen atom or an optionally substituted C 1-6 alkyl group;
The compound according to claim 1 or a salt thereof. - 環Aが、ヒドロキシ基およびオキソ基から選ばれる1または2個の置換基でさらに置換されていてもよい単環式の含窒素不飽和複素環であるか、または、ハロゲン原子、ヒドロキシ基、オキソ基、置換されていてもよいC1-6アルキル基、置換されていてもよいC1-6アルコキシ基および置換されていてもよい非芳香族複素環-オキシ基から選ばれる1~3個の置換基でさらに置換されていてもよい、環A1がピロール環、ピラゾール環、イミダゾール環、ピリジン環またはイミダゾリン環であり、環A2がチオフェン環、ピリジン環またはピリミジン環である上記式(A)で表される二環式の含窒素不飽和複素環であり;
環Bが、
(1)(i)ハロゲン原子、(ii)シアノ基、(iii)ハロゲン原子またはアミノ基で置換されていてもよいC1-6アルキル基および(iv)C1-6アルコキシ基から選ばれる1~5個の置換基でさらに置換されていてもよいベンゼン環、
(2)チオフェン環、または
(3)ピリジン環
であり;
Lが、-CH2-、-CH2CH2-または-CH(CH3)-であり;
X1が、NまたはCR1であり、R1が、水素原子またはフッ素原子であり;
X2が、NまたはCR2であり、R2が、水素原子またはフッ素原子であり;
X3が、NまたはCR3であり、R3が、水素原子またはフッ素原子であり;
X4が、NまたはCR4であり、R4が、水素原子またはメチルであり;
X5が、CR5bR5cまたは結合手であり、R5bおよびR5cが、水素原子であり;
X6が、CR6aR6bであり、R6aおよびR6bが、水素原子、あるいは独立して、ヒドロキシ基またはC6-14アリール基で置換されていてもよいC1-6アルキル基であるか、または、R6aおよびR6bが一緒になって、C3-10シクロアルカン環を形成し;
R7が、水素原子またはC1-6アルキル基である;
請求項2記載の化合物またはその塩。 Ring A is a monocyclic nitrogen-containing unsaturated heterocyclic ring which may be further substituted with one or two substituents selected from a hydroxy group and an oxo group, or a halogen atom, a hydroxy group, an oxo group 1 to 3 groups selected from a group, an optionally substituted C 1-6 alkyl group, an optionally substituted C 1-6 alkoxy group and an optionally substituted non-aromatic heterocyclic-oxy group may be further substituted with a substituent, ring a 1 is a pyrrole ring, a pyrazole ring, an imidazole ring, a pyridine ring or an imidazoline ring, ring a 2 is thiophene ring, the formula is a pyridine ring or a pyrimidine ring (a Is a bicyclic nitrogen-containing unsaturated heterocycle represented by:
Ring B is
1 selected from (1) (i) a halogen atom, (ii) a cyano group, (iii) a C 1-6 alkyl group optionally substituted with a halogen atom or an amino group, and (iv) a C 1-6 alkoxy group A benzene ring optionally further substituted with up to 5 substituents,
(2) a thiophene ring, or (3) a pyridine ring;
L is, -CH 2 -, - CH 2 CH 2 - or -CH (CH 3) - and is,
X 1 is N or CR 1 and R 1 is a hydrogen atom or a fluorine atom;
X 2 is N or CR 2 and R 2 is a hydrogen atom or a fluorine atom;
X 3 is N or CR 3 , R 3 is a hydrogen atom or a fluorine atom;
X 4 is N or CR 4 and R 4 is a hydrogen atom or methyl;
X 5 is CR 5b R 5c or a bond, and R 5b and R 5c are hydrogen atoms;
X 6 is CR 6a R 6b , and R 6a and R 6b are a hydrogen atom, or independently, a C 1-6 alkyl group which may be substituted with a hydroxy group or a C 6-14 aryl group Or R 6a and R 6b together form a C 3-10 cycloalkane ring;
R 7 is a hydrogen atom or a C 1-6 alkyl group;
The compound according to claim 2 or a salt thereof. - 2,2-ジメチル-7-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1-((1R)-1-フェニルエチル)-2,3-ジヒドロキナゾリン-4(1H)-オンまたはその塩。 2,2-Dimethyl-7- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1-((1R) -1-phenylethyl) -2 , 3-Dihydroquinazolin-4 (1H) -one or a salt thereof.
- 4-ベンジル-6-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-3,4-ジヒドロイソキノリン-1(2H)-オンまたはその塩。 4-Benzyl-6- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -3,4-dihydroisoquinolin-1 (2H) -one or a salt thereof .
- 1-ベンジル-8-(2-オキソ-2,3-ジヒドロ-1H-イミダゾ[4,5-b]ピリジン-1-イル)-1,2,3,4-テトラヒドロ-5H-ピリド[2,3-e][1,4]ジアゼピン-5-オンまたはその塩。 1-Benzyl-8- (2-oxo-2,3-dihydro-1H-imidazo [4,5-b] pyridin-1-yl) -1,2,3,4-tetrahydro-5H-pyrido [2, 3-e] [1,4] diazepin-5-one or a salt thereof.
- 請求項1に記載の化合物またはその塩を含有してなる医薬。 A medicament comprising the compound according to claim 1 or a salt thereof.
- プロテインキナーゼC阻害剤である、請求項7記載の医薬。 The medicament according to claim 7, which is a protein kinase C inhibitor.
- 免疫性疾患および/または炎症性疾患の予防または治療剤である、請求項7記載の医薬。 The medicament according to claim 7, which is a preventive or therapeutic agent for immune diseases and / or inflammatory diseases.
- 炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療剤である、請求項7記載の医薬。 The medicament according to claim 7, which is a prophylactic or therapeutic agent for inflammatory bowel disease, psoriasis or atopic dermatitis.
- 免疫性疾患および/または炎症性疾患の予防または治療に使用するための、請求項1に記載の化合物またはその塩。 The compound or a salt thereof according to claim 1, for use in the prevention or treatment of immune diseases and / or inflammatory diseases.
- 炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療に使用するための、請求項1に記載の化合物またはその塩。 The compound or a salt thereof according to claim 1, for use in the prevention or treatment of inflammatory bowel disease, psoriasis or atopic dermatitis.
- 請求項1に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物におけるプロテインキナーゼCの阻害方法。 A method for inhibiting protein kinase C in a mammal, comprising administering an effective amount of the compound or salt thereof according to claim 1 to the mammal.
- 請求項1に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物における免疫性疾患および/または炎症性疾患の予防または治療方法。 A method for preventing or treating an immune disease and / or an inflammatory disease in a mammal, comprising administering an effective amount of the compound according to claim 1 or a salt thereof to the mammal.
- 請求項1に記載の化合物またはその塩を哺乳動物に有効量投与することを特徴とする、該哺乳動物における炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療方法。 A method for preventing or treating inflammatory bowel disease, psoriasis or atopic dermatitis in a mammal, comprising administering an effective amount of the compound according to claim 1 or a salt thereof to the mammal.
- 免疫性疾患および/または炎症性疾患の予防または治療剤を製造するための、請求項1に記載の化合物またはその塩の使用。 Use of the compound according to claim 1 or a salt thereof for producing a prophylactic or therapeutic agent for immune diseases and / or inflammatory diseases.
- 炎症性腸疾患、乾癬またはアトピー性皮膚炎の予防または治療剤を製造するための、請求項1に記載の化合物またはその塩の使用。 Use of the compound or a salt thereof according to claim 1 for producing a preventive or therapeutic agent for inflammatory bowel disease, psoriasis or atopic dermatitis.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-060896 | 2014-03-24 | ||
| JP2014060896 | 2014-03-24 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015146928A1 true WO2015146928A1 (en) | 2015-10-01 |
Family
ID=54195441
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/058777 WO2015146928A1 (en) | 2014-03-24 | 2015-03-23 | Heterocyclic compound |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2015146928A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020108619A1 (en) * | 2018-11-30 | 2020-06-04 | 上海迪诺医药科技有限公司 | Mnk inhibitor |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010521459A (en) * | 2007-03-13 | 2010-06-24 | メルク・シャープ・エンド・ドーム・コーポレイション | Inhibitors of Janus kinase and / or 3-phosphoinositide dependent protein kinase-1 |
| JP2011510990A (en) * | 2008-02-01 | 2011-04-07 | アイアールエム・リミテッド・ライアビリティ・カンパニー | Compounds and compositions as kinase inhibitors |
| JP2013509438A (en) * | 2009-10-29 | 2013-03-14 | ジェノスコ | Kinase inhibitor |
| JP2013525303A (en) * | 2010-04-16 | 2013-06-20 | アッヴィ・インコーポレイテッド | Phthalazine- (2H) -one kinase inhibitor |
-
2015
- 2015-03-23 WO PCT/JP2015/058777 patent/WO2015146928A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010521459A (en) * | 2007-03-13 | 2010-06-24 | メルク・シャープ・エンド・ドーム・コーポレイション | Inhibitors of Janus kinase and / or 3-phosphoinositide dependent protein kinase-1 |
| JP2011510990A (en) * | 2008-02-01 | 2011-04-07 | アイアールエム・リミテッド・ライアビリティ・カンパニー | Compounds and compositions as kinase inhibitors |
| JP2013509438A (en) * | 2009-10-29 | 2013-03-14 | ジェノスコ | Kinase inhibitor |
| JP2013525303A (en) * | 2010-04-16 | 2013-06-20 | アッヴィ・インコーポレイテッド | Phthalazine- (2H) -one kinase inhibitor |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020108619A1 (en) * | 2018-11-30 | 2020-06-04 | 上海迪诺医药科技有限公司 | Mnk inhibitor |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6412148B2 (en) | Pyrazole for the treatment of autoimmune diseases | |
| JP6556146B2 (en) | Heterocyclic compounds | |
| JP6611363B2 (en) | Heterocyclic compounds and their use as Retinoid-related Orphan Receptor (ROR) gamma-T inhibitors | |
| JP6689297B2 (en) | Heterocyclic compound | |
| JP6411342B2 (en) | Amide compounds | |
| WO2018052058A1 (en) | Heterocyclic compound | |
| US10357484B2 (en) | Heterocyclic compound | |
| WO2016199943A1 (en) | Heterocyclic compounds | |
| JPWO2015016206A1 (en) | Heterocyclic compounds | |
| WO2017033946A1 (en) | Heterocyclic compound | |
| JPWO2015002231A1 (en) | Heterocyclic compounds | |
| WO2015146929A1 (en) | Heterocyclic compound | |
| WO2014181813A1 (en) | Heterocyclic compound | |
| WO2016047678A1 (en) | Heterocyclic compound | |
| WO2020027225A1 (en) | Heterocyclic compound | |
| WO2015146928A1 (en) | Heterocyclic compound |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15769203 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15769203 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |