WO2013041844A2 - Antibodies, variable domains & chains tailored for human use - Google Patents
Antibodies, variable domains & chains tailored for human use Download PDFInfo
- Publication number
- WO2013041844A2 WO2013041844A2 PCT/GB2012/052296 GB2012052296W WO2013041844A2 WO 2013041844 A2 WO2013041844 A2 WO 2013041844A2 GB 2012052296 W GB2012052296 W GB 2012052296W WO 2013041844 A2 WO2013041844 A2 WO 2013041844A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- human
- gene segments
- gene
- vertebrate
- cell
- Prior art date
Links
- 241000282414 Homo sapiens Species 0.000 title claims abstract description 1966
- 241000251539 Vertebrata <Metazoa> Species 0.000 claims abstract description 535
- 238000000034 method Methods 0.000 claims abstract description 244
- 241000700159 Rattus Species 0.000 claims abstract description 206
- 229920001184 polypeptide Polymers 0.000 claims abstract description 22
- 102000004196 processed proteins & peptides Human genes 0.000 claims abstract description 22
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 22
- 108091028043 Nucleic acid sequence Proteins 0.000 claims abstract description 18
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 15
- 108090000623 proteins and genes Proteins 0.000 claims description 1415
- 210000004027 cell Anatomy 0.000 claims description 713
- 101150097493 D gene Proteins 0.000 claims description 257
- 230000035772 mutation Effects 0.000 claims description 228
- 241000699666 Mus <mouse, genus> Species 0.000 claims description 226
- 210000003917 human chromosome Anatomy 0.000 claims description 200
- 230000009261 transgenic effect Effects 0.000 claims description 162
- 108700005091 Immunoglobulin Genes Proteins 0.000 claims description 139
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 claims description 100
- 239000000427 antigen Substances 0.000 claims description 95
- 108091007433 antigens Proteins 0.000 claims description 95
- 102000036639 antigens Human genes 0.000 claims description 95
- 239000002773 nucleotide Substances 0.000 claims description 93
- 125000003729 nucleotide group Chemical group 0.000 claims description 93
- 108060003951 Immunoglobulin Proteins 0.000 claims description 88
- 102000018358 immunoglobulin Human genes 0.000 claims description 88
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 claims description 88
- 101001099888 Homo sapiens Ras-related protein Rab-3D Proteins 0.000 claims description 83
- 230000006798 recombination Effects 0.000 claims description 79
- 238000005215 recombination Methods 0.000 claims description 79
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 claims description 78
- 101150008942 J gene Proteins 0.000 claims description 76
- 210000003719 b-lymphocyte Anatomy 0.000 claims description 70
- 210000004602 germ cell Anatomy 0.000 claims description 62
- 150000001413 amino acids Chemical class 0.000 claims description 51
- 102000054767 gene variant Human genes 0.000 claims description 50
- 230000002708 enhancing effect Effects 0.000 claims description 44
- 229940113082 thymine Drugs 0.000 claims description 44
- 239000013598 vector Substances 0.000 claims description 44
- 229930024421 Adenine Natural products 0.000 claims description 41
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 claims description 41
- 229960000643 adenine Drugs 0.000 claims description 41
- 229940104302 cytosine Drugs 0.000 claims description 39
- 230000000295 complement effect Effects 0.000 claims description 35
- 101150117115 V gene Proteins 0.000 claims description 32
- 101150108210 IX gene Proteins 0.000 claims description 31
- 239000003814 drug Substances 0.000 claims description 30
- 238000011144 upstream manufacturing Methods 0.000 claims description 30
- 241000699670 Mus sp. Species 0.000 claims description 28
- 210000000349 chromosome Anatomy 0.000 claims description 28
- 238000004519 manufacturing process Methods 0.000 claims description 27
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 26
- 210000000628 antibody-producing cell Anatomy 0.000 claims description 25
- 230000014509 gene expression Effects 0.000 claims description 21
- 210000004408 hybridoma Anatomy 0.000 claims description 21
- 101150062031 L gene Proteins 0.000 claims description 20
- 230000001186 cumulative effect Effects 0.000 claims description 20
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 claims description 19
- 108020004705 Codon Proteins 0.000 claims description 18
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 claims description 17
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 claims description 17
- 101150101112 7 gene Proteins 0.000 claims description 16
- 108010008286 DNA nucleotidylexotransferase Proteins 0.000 claims description 16
- 102100033215 DNA nucleotidylexotransferase Human genes 0.000 claims description 16
- 201000010099 disease Diseases 0.000 claims description 16
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 16
- 210000004978 chinese hamster ovary cell Anatomy 0.000 claims description 14
- 108020004707 nucleic acids Proteins 0.000 claims description 14
- 102000039446 nucleic acids Human genes 0.000 claims description 14
- 150000007523 nucleic acids Chemical class 0.000 claims description 14
- 101150056393 H6 gene Proteins 0.000 claims description 13
- 238000003780 insertion Methods 0.000 claims description 13
- 230000037431 insertion Effects 0.000 claims description 13
- 102100038474 Ras-related protein Rab-3D Human genes 0.000 claims description 12
- 239000003085 diluting agent Substances 0.000 claims description 12
- 244000052769 pathogen Species 0.000 claims description 12
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 12
- 238000011814 C57BL/6N mouse Methods 0.000 claims description 11
- 101150042441 K gene Proteins 0.000 claims description 11
- 210000002459 blastocyst Anatomy 0.000 claims description 11
- 241000283984 Rodentia Species 0.000 claims description 10
- 239000013604 expression vector Substances 0.000 claims description 10
- 101150002004 lgg-2 gene Proteins 0.000 claims description 10
- 108700019146 Transgenes Proteins 0.000 claims description 9
- 101150090724 3 gene Proteins 0.000 claims description 7
- 101150033839 4 gene Proteins 0.000 claims description 7
- 101150096316 5 gene Proteins 0.000 claims description 7
- 206010069754 Acquired gene mutation Diseases 0.000 claims description 7
- 230000009824 affinity maturation Effects 0.000 claims description 7
- 208000015181 infectious disease Diseases 0.000 claims description 7
- 230000037439 somatic mutation Effects 0.000 claims description 7
- 208000035473 Communicable disease Diseases 0.000 claims description 6
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 claims description 6
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 claims description 6
- 101000800648 Mus musculus DNA nucleotidylexotransferase Proteins 0.000 claims description 6
- 230000004927 fusion Effects 0.000 claims description 6
- 101150118163 h gene Proteins 0.000 claims description 6
- 230000001717 pathogenic effect Effects 0.000 claims description 6
- 101000998951 Homo sapiens Immunoglobulin heavy variable 1-8 Proteins 0.000 claims description 5
- 102100036885 Immunoglobulin heavy variable 1-8 Human genes 0.000 claims description 5
- 230000001580 bacterial effect Effects 0.000 claims description 5
- 210000005260 human cell Anatomy 0.000 claims description 5
- 101001037153 Homo sapiens Immunoglobulin heavy variable 3-7 Proteins 0.000 claims description 4
- 101001037144 Homo sapiens Immunoglobulin heavy variable 3-9 Proteins 0.000 claims description 4
- 102100040231 Immunoglobulin heavy variable 3-7 Human genes 0.000 claims description 4
- 102100040234 Immunoglobulin heavy variable 3-9 Human genes 0.000 claims description 4
- 230000000692 anti-sense effect Effects 0.000 claims description 4
- 238000010367 cloning Methods 0.000 claims description 4
- 239000000203 mixture Substances 0.000 claims description 4
- 101000989060 Homo sapiens Immunoglobulin heavy variable 6-1 Proteins 0.000 claims description 3
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 claims description 3
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 claims description 3
- 102100029416 Immunoglobulin heavy variable 6-1 Human genes 0.000 claims description 3
- 102100029567 Immunoglobulin kappa light chain Human genes 0.000 claims description 3
- 101710189008 Immunoglobulin kappa light chain Proteins 0.000 claims description 3
- 244000052616 bacterial pathogen Species 0.000 claims description 3
- 210000004899 c-terminal region Anatomy 0.000 claims description 3
- 102000054766 genetic haplotypes Human genes 0.000 claims description 3
- 230000003053 immunization Effects 0.000 claims description 3
- 244000052613 viral pathogen Species 0.000 claims description 3
- 101000998952 Homo sapiens Immunoglobulin heavy variable 1-3 Proteins 0.000 claims description 2
- 101000839658 Homo sapiens Immunoglobulin heavy variable 3-66 Proteins 0.000 claims description 2
- 101000839686 Homo sapiens Immunoglobulin heavy variable 4-4 Proteins 0.000 claims description 2
- 101000989076 Homo sapiens Immunoglobulin heavy variable 4-61 Proteins 0.000 claims description 2
- 102100036886 Immunoglobulin heavy variable 1-3 Human genes 0.000 claims description 2
- 102100027821 Immunoglobulin heavy variable 3-66 Human genes 0.000 claims description 2
- 102100028308 Immunoglobulin heavy variable 4-4 Human genes 0.000 claims description 2
- 102100029419 Immunoglobulin heavy variable 4-61 Human genes 0.000 claims description 2
- 108010076504 Protein Sorting Signals Proteins 0.000 claims description 2
- 230000004069 differentiation Effects 0.000 claims description 2
- 230000002209 hydrophobic effect Effects 0.000 claims description 2
- 101150028074 2 gene Proteins 0.000 claims 7
- 101150039504 6 gene Proteins 0.000 claims 6
- 125000003275 alpha amino acid group Chemical group 0.000 claims 5
- 238000011321 prophylaxis Methods 0.000 claims 4
- 238000002560 therapeutic procedure Methods 0.000 claims 4
- 101100504121 Mus musculus Ighg gene Proteins 0.000 claims 2
- 101150072531 10 gene Proteins 0.000 claims 1
- 101150025032 13 gene Proteins 0.000 claims 1
- 101150076401 16 gene Proteins 0.000 claims 1
- 101150042997 21 gene Proteins 0.000 claims 1
- 101150029857 23 gene Proteins 0.000 claims 1
- 101150057657 27 gene Proteins 0.000 claims 1
- 101150044182 8 gene Proteins 0.000 claims 1
- 101150106774 9 gene Proteins 0.000 claims 1
- 238000011830 transgenic mouse model Methods 0.000 abstract description 9
- 229940124691 antibody therapeutics Drugs 0.000 abstract description 7
- 241000699660 Mus musculus Species 0.000 abstract description 5
- 230000000069 prophylactic effect Effects 0.000 abstract description 4
- 238000011824 transgenic rat model Methods 0.000 abstract description 3
- 230000000875 corresponding effect Effects 0.000 description 92
- 235000001014 amino acid Nutrition 0.000 description 38
- 108020004414 DNA Proteins 0.000 description 21
- 238000004458 analytical method Methods 0.000 description 18
- 101710143275 Single-stranded DNA cytosine deaminase Proteins 0.000 description 16
- 102100022433 Single-stranded DNA cytosine deaminase Human genes 0.000 description 16
- 239000000047 product Substances 0.000 description 16
- 241000894007 species Species 0.000 description 15
- 241000282412 Homo Species 0.000 description 11
- 238000013461 design Methods 0.000 description 11
- 238000001727 in vivo Methods 0.000 description 11
- 230000006870 function Effects 0.000 description 10
- 235000018102 proteins Nutrition 0.000 description 10
- 102000004169 proteins and genes Human genes 0.000 description 10
- 230000000392 somatic effect Effects 0.000 description 10
- 229940079593 drug Drugs 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 238000005516 engineering process Methods 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- 210000004436 artificial bacterial chromosome Anatomy 0.000 description 8
- 101100454808 Caenorhabditis elegans lgg-2 gene Proteins 0.000 description 7
- 108700005078 Synthetic Genes Proteins 0.000 description 7
- 230000027455 binding Effects 0.000 description 7
- 108700028369 Alleles Proteins 0.000 description 6
- 238000009826 distribution Methods 0.000 description 6
- 238000002649 immunization Methods 0.000 description 6
- 238000012163 sequencing technique Methods 0.000 description 6
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 239000012636 effector Substances 0.000 description 5
- 230000002068 genetic effect Effects 0.000 description 5
- 108020003175 receptors Proteins 0.000 description 5
- 102000005962 receptors Human genes 0.000 description 5
- UHDGCWIWMRVCDJ-UHFFFAOYSA-N 1-beta-D-Xylofuranosyl-NH-Cytosine Natural products O=C1N=C(N)C=CN1C1C(O)C(O)C(CO)O1 UHDGCWIWMRVCDJ-UHFFFAOYSA-N 0.000 description 4
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 4
- 101100217502 Caenorhabditis elegans lgg-3 gene Proteins 0.000 description 4
- UHDGCWIWMRVCDJ-PSQAKQOGSA-N Cytidine Natural products O=C1N=C(N)C=CN1[C@@H]1[C@@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-PSQAKQOGSA-N 0.000 description 4
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 4
- 238000003766 bioinformatics method Methods 0.000 description 4
- 230000024203 complement activation Effects 0.000 description 4
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 4
- UHDGCWIWMRVCDJ-ZAKLUEHWSA-N cytidine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-ZAKLUEHWSA-N 0.000 description 4
- 238000009510 drug design Methods 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 208000024191 minimally invasive lung adenocarcinoma Diseases 0.000 description 4
- 238000011160 research Methods 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 108020003589 5' Untranslated Regions Proteins 0.000 description 3
- 108010087819 Fc receptors Proteins 0.000 description 3
- 102000009109 Fc receptors Human genes 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 101001037145 Homo sapiens Immunoglobulin heavy variable 2-5 Proteins 0.000 description 3
- 101001037141 Homo sapiens Immunoglobulin heavy variable 3-21 Proteins 0.000 description 3
- 102100040235 Immunoglobulin heavy variable 2-5 Human genes 0.000 description 3
- 102100040217 Immunoglobulin heavy variable 3-21 Human genes 0.000 description 3
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 3
- 108020004511 Recombinant DNA Proteins 0.000 description 3
- 108091008874 T cell receptors Proteins 0.000 description 3
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 3
- 210000001106 artificial yeast chromosome Anatomy 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 238000010276 construction Methods 0.000 description 3
- 238000002823 phage display Methods 0.000 description 3
- 238000002702 ribosome display Methods 0.000 description 3
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 3
- 238000011740 C57BL/6 mouse Methods 0.000 description 2
- 108010034753 Complement Membrane Attack Complex Proteins 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 101000998950 Homo sapiens Immunoglobulin heavy variable 1-18 Proteins 0.000 description 2
- 101001037138 Homo sapiens Immunoglobulin heavy variable 3-11 Proteins 0.000 description 2
- 101001037137 Homo sapiens Immunoglobulin heavy variable 3-13 Proteins 0.000 description 2
- 101001037139 Homo sapiens Immunoglobulin heavy variable 3-30 Proteins 0.000 description 2
- 101000839684 Homo sapiens Immunoglobulin heavy variable 4-31 Proteins 0.000 description 2
- 101000839679 Homo sapiens Immunoglobulin heavy variable 4-39 Proteins 0.000 description 2
- 101000839781 Homo sapiens Immunoglobulin heavy variable 4-59 Proteins 0.000 description 2
- 102100036884 Immunoglobulin heavy variable 1-18 Human genes 0.000 description 2
- 102100040222 Immunoglobulin heavy variable 3-11 Human genes 0.000 description 2
- 102100040221 Immunoglobulin heavy variable 3-13 Human genes 0.000 description 2
- 102100040219 Immunoglobulin heavy variable 3-30 Human genes 0.000 description 2
- 102100028310 Immunoglobulin heavy variable 4-31 Human genes 0.000 description 2
- 102100028312 Immunoglobulin heavy variable 4-39 Human genes 0.000 description 2
- 102100028405 Immunoglobulin heavy variable 4-59 Human genes 0.000 description 2
- 208000030555 Pygmy Diseases 0.000 description 2
- 230000005888 antibody-dependent cellular phagocytosis Effects 0.000 description 2
- 101150010487 are gene Proteins 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 230000022534 cell killing Effects 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000002759 chromosomal effect Effects 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 210000001671 embryonic stem cell Anatomy 0.000 description 2
- 238000011577 humanized mouse model Methods 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000036737 immune function Effects 0.000 description 2
- 230000005847 immunogenicity Effects 0.000 description 2
- 206010022000 influenza Diseases 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 238000001823 molecular biology technique Methods 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- 238000002818 protein evolution Methods 0.000 description 2
- 230000007115 recruitment Effects 0.000 description 2
- 238000004064 recycling Methods 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- 101150086149 39 gene Proteins 0.000 description 1
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 1
- 244000303258 Annona diversifolia Species 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 108010032595 Antibody Binding Sites Proteins 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 244000175448 Citrus madurensis Species 0.000 description 1
- 235000004332 Citrus madurensis Nutrition 0.000 description 1
- 235000007438 Citrus mitis Nutrition 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 239000003155 DNA primer Substances 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 101150081594 FH14 gene Proteins 0.000 description 1
- 206010071602 Genetic polymorphism Diseases 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 101000998953 Homo sapiens Immunoglobulin heavy variable 1-2 Proteins 0.000 description 1
- 101000998949 Homo sapiens Immunoglobulin heavy variable 1-24 Proteins 0.000 description 1
- 241000725303 Human immunodeficiency virus Species 0.000 description 1
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 1
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 102100036887 Immunoglobulin heavy variable 1-2 Human genes 0.000 description 1
- 102100036890 Immunoglobulin heavy variable 1-24 Human genes 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 108091092195 Intron Proteins 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 101000755751 Mus musculus Single-stranded DNA cytosine deaminase Proteins 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 241000364051 Pima Species 0.000 description 1
- 102000018120 Recombinases Human genes 0.000 description 1
- 108010091086 Recombinases Proteins 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 208000018359 Systemic autoimmune disease Diseases 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 230000009615 deamination Effects 0.000 description 1
- 238000006481 deamination reaction Methods 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 238000012407 engineering method Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000012252 genetic analysis Methods 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 230000007614 genetic variation Effects 0.000 description 1
- 238000012268 genome sequencing Methods 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 239000012642 immune effector Substances 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000002998 immunogenetic effect Effects 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 230000001965 increasing effect Effects 0.000 description 1
- 101150102487 ine gene Proteins 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 238000005065 mining Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 102000054765 polymorphisms of proteins Human genes 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 229940043274 prophylactic drug Drugs 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000005204 segregation Methods 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000012916 structural analysis Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K67/00—Rearing or breeding animals, not otherwise provided for; New or modified breeds of animals
- A01K67/027—New or modified breeds of vertebrates
- A01K67/0275—Genetically modified vertebrates, e.g. transgenic
- A01K67/0278—Knock-in vertebrates, e.g. humanised vertebrates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
- A61P31/22—Antivirals for DNA viruses for herpes viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
- A61P33/06—Antimalarials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
- C07K16/085—Herpetoviridae, e.g. pseudorabies virus, Epstein-Barr virus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
- C07K16/085—Herpetoviridae, e.g. pseudorabies virus, Epstein-Barr virus
- C07K16/088—Varicella-zoster virus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
- C07K16/085—Herpetoviridae, e.g. pseudorabies virus, Epstein-Barr virus
- C07K16/089—Cytomegalovirus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
- C07K16/1018—Orthomyxoviridae, e.g. influenza virus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
- C07K16/1036—Retroviridae, e.g. leukemia viruses
- C07K16/1045—Lentiviridae, e.g. HIV, FIV, SIV
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
- C07K16/1203—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria
- C07K16/1217—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria from Neisseriaceae (F)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
- C07K16/1203—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria
- C07K16/1228—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria from Enterobacteriaceae (F), e.g. Citrobacter, Serratia, Proteus, Providencia, Morganella, Yersinia
- C07K16/1232—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria from Enterobacteriaceae (F), e.g. Citrobacter, Serratia, Proteus, Providencia, Morganella, Yersinia from Escherichia (G)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
- C07K16/1203—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria
- C07K16/1242—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria from Pasteurellaceae (F), e.g. Haemophilus influenza
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
- C07K16/461—Igs containing Ig-regions, -domains or -residues form different species
- C07K16/462—Igs containing a variable region (Fv) from one specie and a constant region (Fc) from another
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/8509—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells for producing genetically modified animals, e.g. transgenic
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2217/00—Genetically modified animals
- A01K2217/07—Animals genetically altered by homologous recombination
- A01K2217/072—Animals genetically altered by homologous recombination maintaining or altering function, i.e. knock in
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2217/00—Genetically modified animals
- A01K2217/15—Animals comprising multiple alterations of the genome, by transgenesis or homologous recombination, e.g. obtained by cross-breeding
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2227/00—Animals characterised by species
- A01K2227/10—Mammal
- A01K2227/105—Murine
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2267/00—Animals characterised by purpose
- A01K2267/01—Animal expressing industrially exogenous proteins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/20—Pseudochromosomes, minichrosomosomes
- C12N2800/204—Pseudochromosomes, minichrosomosomes of bacterial origin, e.g. BAC
Definitions
- the present invention relates to the provision of antibody therapeutics and prophylactics that are tailored specifically for human use.
- the present invention provides libraries, vertebrates and cells, such as transgenic mice or rats or transgenic mouse or rat cells. Furthermore, the invention relates to methods of using the vertebrates to isolate antibodies or nucleotide sequences encoding antibodies. Antibodies, heavy chains, polypeptides, nucleotide sequences, pharmaceutical compositions and uses are also provided by the invention.
- non-human vertebrates eg, mice and rats
- cells comprising transgenic immunoglobulin loci, such loci comprising human variable (V), diversity (D) and/or joining (J) segments, and optionally human constant regions.
- endogenous constant regions of the host vertebrate eg, mouse or rat constant regions
- the transgenic loci are provided in the transgenic loci.
- transgenic loci in the art include varying amounts of the human V(D) J repertoire.
- Existing transgenic immunoglobulin loci are based on a single human DNA source. The potential diversity of human antibody variable regions in non-human vertebrates bearing such transgenic loci is thus confined.
- the present invention has been developed from extensive bioinformatics analysis of natural antibody gene segment distributions across a myriad of different human populations and across more than two thousand samples from human individuals.
- the inventors have undertaken this huge task to more thoroughly understand and design non-human vertebrate systems and resultant antibodies to better address human medical therapeutics as a whole, as well as to enable rational design to address specific ethnic populations of humans.
- the inventors have constructed transgenic non-human vertebrates and isolated antibodies, antibody chains and cells expressing these in a way that yields products that utilise gene segments that have been purposely included on the basis of the human bioinformatics analysis.
- the examples illustrate worked experiments where the inventors isolated many cells and antibodies to this effect.
- the invention also relates to synthetically-extended & ethnically-diverse superhuman
- the present invention thus provides for novel and potentially expanded synthetic immunoglobulin diversities, thus providing a pool of diversity from which human antibody therapeutic leads can be selected.
- This expanded pool is useful when seeking to find antibodies with desirable characteristics, such as relatively high affinity to target antigen without the need for further affinity maturation (eg, using laborious in vitro techniques such as phage or ribosome display), or improved biophysical characteristics, or to address targets and new epitopes that have previously been difficult to address with antibodies are not reached by prior antibody binding sites.
- the invention also provides for diversity that is potentially biased towards variable gene usage common to members of a specific human population, which is useful for generating antibodies for treating and/or preventing diseases or conditions within such population. This ability to bias the antibody repertoire allows one to tailor antibody therapeutics with the aim of more effectively treating and/or preventing disease or medical conditions in specific human populations.
- the present inventors realised the possibility of providing immunoglobulin gene segments from disparate sources in transgenic loci, in order to provide for novel and potentially-expanded antibody diversities from which antibody therapeutics (and antibody tool reagents) could be generated. This- opens up the potential of transgenic human-mouse/rat technologies to the possibility of interrogating different and possibly larger antibody sequence-spaces than has hitherto been possible.
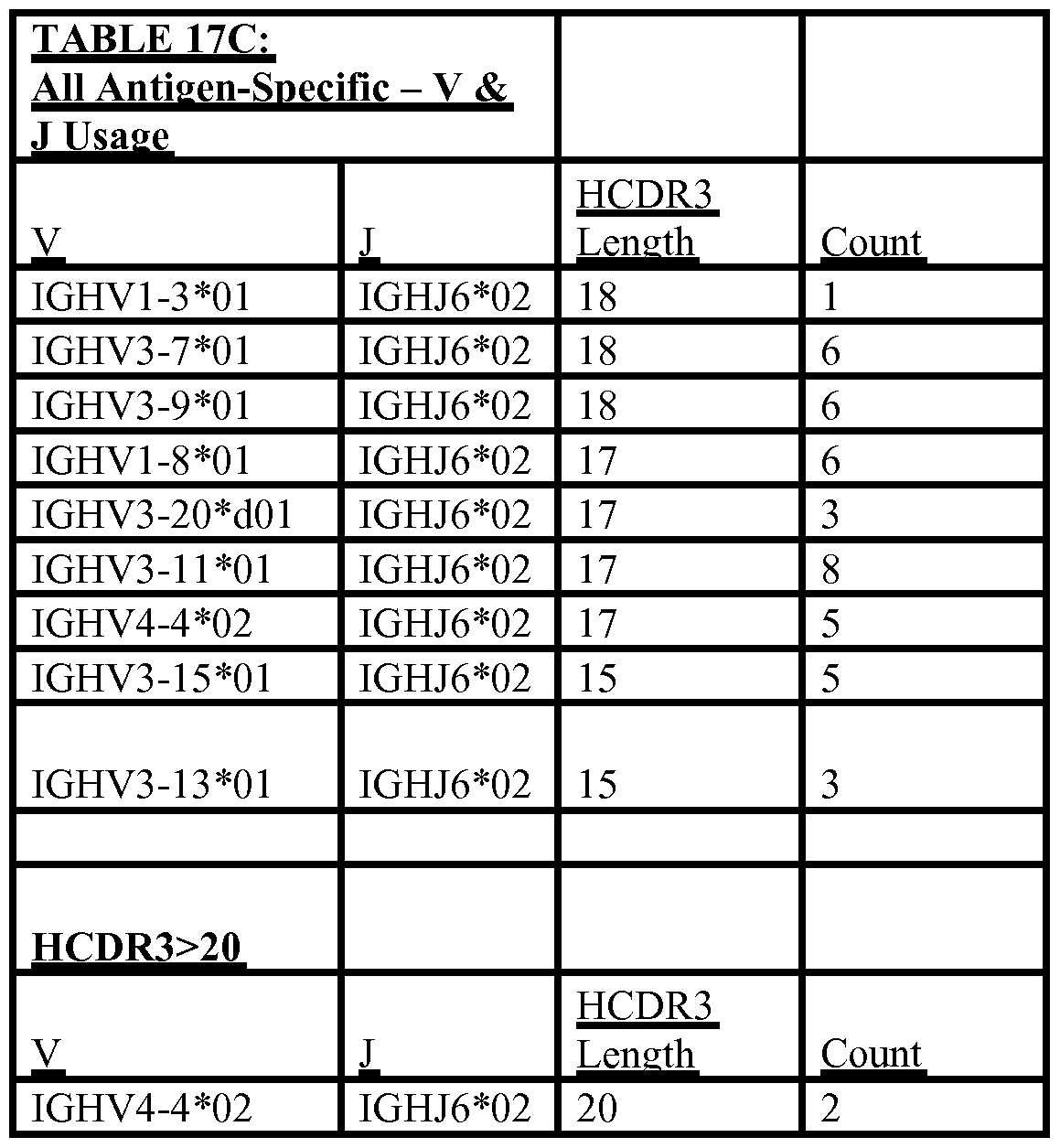
- HCDR3 length at least 20 amino acids
- naturally-occurring antibodies have been isolated from humans infected with infectious disease pathogens, such antibodies having a long HCDR3 length.
- Neutralising antibodies have been found in this respect.
- a long HCDR3 length would be desirable to address other antigens (eg, receptor clefts or enzyme active sites), not just limited to infectious disease pathogens, and thus the inventors realised the general desirability of the possibility of engineering transgenic loci to be able to produce long HCDR3 antibodies and heavy chains.
- the inventors through laborious execution of bioinformatics on in excess of 2000 human DNA samples via the 1000 Genomes project together with rational sequence choices, identified that the inclusion of the specific human gene segment variant JH6*02 is desirable for producing long HCDR3 antibodies and chains.
- antibodies are humanised with an arbitrary choice of human constant region (presumably derived from one (often unknown) ethnic population or non-naturally occurring) that does not function as well in patients of a different human ethnic population. This is important, since the constant region has the major role in providing antibody effector functions, eg, for antibody recycling, cellular and complement recruitment and for cell killing.
- a non-human vertebrate or vertebrate cell (optionally an ES cell or antibody-producing cell) comprising a genome having a superhuman immunoglobulin heavy chain human VH and/or D and/or J gene repertoire.
- a non-human vertebrate or vertebrate cell (optionally an ES cell or antibody-producing cell) comprising a genome having a superhuman immunoglobulin light chain human VL gene repertoire; optionally wherein the vertebrate or cell is according to the first configuration.
- a non-human vertebrate or vertebrate cell (optionally an ES cell or antibody-producing cell) whose genome comprises a transgenic immunoglobulin locus (eg, a heavy chain locus or a light chain locus), said locus comprising immunoglobulin gene segments according to the first and second human immunoglobulin gene segments (optionally V segments) as mentioned below operably connected upstream of an immunoglobulin constant region; optionally wherein the genome is homozygous for said transgenic immunoglobulin locus;
- a transgenic immunoglobulin locus eg, a heavy chain locus or a light chain locus
- said locus comprising immunoglobulin gene segments according to the first and second human immunoglobulin gene segments (optionally V segments) as mentioned below operably connected upstream of an immunoglobulin constant region; optionally wherein the genome is homozygous for said transgenic immunoglobulin locus;
- immunoglobulin locus comprises more than the natural human complement of functional V gene segments;
- immunoglobulin locus comprises more than the natural human complement of functional D gene segments;
- the immunoglobulin locus comprises more than the natural human complement of functional J gene segments.
- a transgenic non-human vertebrate eg, a mouse or rat
- vertebrate cell optionally an ES cell or antibody-producing cell
- the transgenic locus comprises one or more human immunoglobulin V gene segments, one or more human J gene segments and optionally one or more human D gene segments, a first (optionally a V segment) of said gene segments and a second (optionally a V segment) of said gene segments being different and derived from the genomes of first and second human individuals respectively, wherein the individuals are different; and optionally not related;
- immunoglobulin locus comprises more than the natural human complement of functional V gene segments;
- immunoglobulin locus comprises more than the natural human complement of functional D gene segments;
- immunoglobulin locus comprises more than the natural human complement of functional J gene segments.
- a transgenic non-human vertebrate eg, a mouse or rat
- vertebrate cell eg. an ES cell or antibody-producing cell
- each locus comprising a plurality of human immunoglobulin gene segments operably connected upstream of a non-human vertebrate constant region for the production of a repertoire of chimaeric antibodies, or chimaeric light or heavy chains, having a non-human vertebrate constant region and a human variable region
- the first transgenic locus comprises one or more human immunoglobulin V gene segments, one or more human J gene segments and optionally one or more human D gene segments
- the second transgenic locus comprises one or more human immunoglobulin V gene segments, one or more human J gene segments and optionally one or more human D gene segments
- each immunoglobulin locus comprises more than the natural human
- each immunoglobulin locus comprises more than the natural human
- a method of constructing a cell eg, an ES cell according to the invention, the method comprising
- the gene segment(s) in step (b) are identified from an immunoglobulin gene database selected from the 1000 Genomes, Ensembl, Genbank and IMGT databases.
- Genbank is a reference to Genbank release number 185.0 or 191.0; the 1000 Genomes database is Phase 1, release v3, 16 th March 2012; the Ensembl database is assembly GRCh37.p8 (10/04/2012); the IMGT database is available at www.imgt.org .
- the first and second human individuals are members of first and second ethnic populations respectively, wherein the populations are different, optionally wherein the human immunoglobulin gene segment derived from the genome sequence of the second individual is low- frequency (optionally rare) within the second ethnic population.
- This configuration of the invention also provides a method of making a transgenic non-human vertebrate (eg, a mouse or rat), the method comprising
- an ES cell eg, a mouse C57BL/6N, C57BL/6J, 129S5 or 129Sv strain ES cell
- a donor non-human vertebrate blastocyst eg, a mouse C57BL/6N, C57BL/6J, 129S5 or 129Sv strain blastocyst
- the invention provides a method of isolating an antibody that binds a predetermined antigen (eg, a bacterial or viral pathogen antigen), the method comprising immunising a non-human vertebrate according to the invention.
- a predetermined antigen eg, a bacterial or viral pathogen antigen
- a first transgenic cell expresses a first antibody having a chain encoded by a first immunoglobulin gene, the gene comprising a first variable domain nucleotide sequence produced following recombination of a first human unrearranged immunoglobulin gene segment;
- a second transgenic cell expresses a second antibody having a chain encoded by a second immunoglobulin gene, the second gene comprising a second variable domain nucleotide sequence produced following recombination of a second human unrearranged immunoglobulin gene segment, the first and second antibodies being non-identical;
- the first and second gene segments are different and derived from the genome sequences of first and second human individuals respectively, wherein the individuals are different; and optionally not related; (d) wherein the cells are non-human vertebrate (eg, mouse or rat) cells.
- non-human vertebrate eg, mouse or rat
- the first and second human individuals are members of first and second ethnic populations respectively, wherein the populations are different; optionally wherein the ethnic populations are selected from those identified in the 1000 Genomes database.
- the second human immunoglobulin gene segment is a polymorphic variant of the first human immunoglobulin gene segment; optionally wherein the second gene segment is selected from the group consisting of a gene segment in any of Tables 1 to 7 and 9 to 14 below (eg, selected from Table 13 or Table 14), eg, the second gene segment is a polymorphic variant of VH1- 69.
- the heavy chain constant regions are gamma-type constant regions.
- the invention also provides an isolated nucleotide sequence encoding the antibody, optionally wherein the sequence is provided in an antibody expression vector, optionally in a host cell.
- the invention also provides a method of producing a human antibody, the method comprising replacing the non-human vertebrate constant regions of the antibody of the third configuration with human antibody constant regions.
- the invention also provides a pharmaceutical composition
- a pharmaceutical composition comprising an antibody according to the third configuration, or an antibody produced according to the method above and a diluent, excipient or carrier; optionally wherein the composition is provided in a container connected to an IV needle or syringe or in an IV bag.
- the invention also provides an antibody-producing cell that expresses the second antibody recited in any one of the configurations.
- the invention contemplates the combination of nucleotide sequences of first and second immunoglobulin gene segments (eg, two or more polymorphic variants of a particular human germline VH or VL gene segment) to provide a synthetic gene segment.
- synthetic gene segment is used, in one embodiment, to build a transgenic immunoglobulin locus, wherein the synthetic gene segment is provided in combination with one or more human variable and J regions (and optionally one or more human D regions) operably connected upstream of a constant region.
- the invention provides for superhuman gene segment diversity.
- sequences to be combined can be selected from gene segments that have been observed to be commonly used in human antibodies raised against a particular antigen (eg, a flu antigen, such as haemaglutinin).
- a flu antigen such as haemaglutinin
- the synthetic gene segment may recombine in vivo to produce an antibody that is well suited to the treatment and/or prevention of a disease or condition (eg, influenza) mediated by said antigen.
- a disease or condition eg, influenza
- a non-human vertebrate (optionally a mouse or a rat) or vertebrate cell whose genome comprises an immunoglobulin heavy chain locus comprising human gene segment JH6*02, one or more VH gene segments and one or more D gene segments upstream of a constant region; wherein the gene segments in the heavy chain locus are operably linked to the constant region thereof so that the mouse is capable of producing an antibody heavy chain produced by recombination of the human JH6*02 with a D segment and a VH segment.
- a non-human vertebrate cell (optionally a mouse cell or a rat cell) whose genome comprises an immunoglobulin heavy chain locus comprising human gene segment JH6*02, one or more VH gene segments and one or more D gene segments upstream of a constant region; wherein the gene segments in the heavy chain locus are operably linked to the constant region thereof for producing (eg, in a subsequent progeny cell) an antibody heavy chain produced by recombination of the human JH6*02 with a D segment and a VH segment.
- a heavy chain (eg, comprised by an antibody) isolated from a vertebrate of the invention wherein the heavy chain comprises a HCD 3 of at least 20 amino acids.
- a method for producing a heavy chain, VH domain or an antibody specific to a target antigen comprising immunizing a non-human vertebrate according to the invention with the antigen and isolating the heavy chain, VH domain or an antibody specific to a target antigen or a cell producing the heavy chain, VH domain or an antibody, wherein the heavy chain, VH domain or an antibody comprises a HCDR3 that is derived from the recombination of human JH6*02 with a VH gene segment and a D gene segment.
- a heavy chain, VH domain or an antibody produced by the method comprising immunizing a non-human vertebrate according to the invention with the antigen and isolating the heavy chain, VH domain or an antibody specific to a target antigen or a cell producing the heavy chain, VH domain or an antibody, wherein the heavy chain, VH domain or an antibody comprises a HCDR3 that is derived from the recombination of human JH6*02 with a VH gene segment and a
- a vector (eg, a CHO cell or HEK293 cell vector) comprising the nucleic acid; optionally wherein the vector is in a host cell (eg, a CHO cell or HEK293 cell).
- a pharmaceutical composition comprising the antibody, heavy chain or VH domain (eg, comprised by an antibody), together with a pharmaceutically-acceptable excipient, diluent or a medicament (eg, a further antigen-specific variable domain, heavy chain or antibody).
- the antibody, heavy chain or VH domain (eg, comprised by an antibody) as above for use in medicine.
- an antibody, heavy chain or VH domain eg, comprised by an antibody
- a method of producing an antibody heavy chain comprising
- variable domain (b) combining the variable domain with a human heavy chain constant region to produce an antibody heavy chain comprising (in N- to C-terminal direction) the variable domain and the constant region;
- human heavy chain constant region is an IGHGlref, IGHG2ref, IGHG2a, IGHG3ref, IGHG3a, IGHG3b, IGHG4ref or IGHG4a constant region.
- an antibody comprising a human heavy chain, the heavy chain comprising a variable domain that is specific for an antigen and a constant region that is an IGHGlref, IGHG2ref, IGHG2a, IGHG3ref, IGHG3a, IGHG3b, IGHG4ref or IGHG4a constant region .
- the variable domain comprises mouse-pattern AID somatic mutations.
- a polypeptide comprising (in N- to C- terminal direction) a leader sequence, a human variable domain that is specific for an antigen and a human constant region that is an IGHGlref, IGHG2ref, IGHG2a, IGHG3ref, IGHG3a, IGHG3b, IGHG4ref or IGHG4a constant region wherein (i) the leader sequence is not the native human variable domain leader sequence; and/or (ii) the variable domain comprises mouse AID-pattern somatic mutations and/or mouse Terminal deoxynucleotidyl transferase (TdT)- pattern junctional mutations.
- TdT Terminal deoxynucleotidyl transferase
- a vector (eg, a CHO cell or HEK293 cell vector) comprising a IGHGlref, IGHG2ref, IGHG2a, IGHG3ref, IGHG3a, IGHG3b, IGHG4ref or IGHG4a constant region nucleotide sequence that is 3' of a cloning site for the insertion of a human antibody heavy chain variable domain nucleotide sequence, such that upon insertion of such a variable domain sequence the vector comprises (in 5' to 3' direction) a promoter, a leader sequence, the variable domain sequence and the constant region sequence so that the vector is capable of expressing a human antibody heavy chain when present in a host cell.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 3 human variable region gene segments of the same type (eg, at least 3 human VH6-1 gene segments, at least 3 human JH6 gene segments, at least 3 human VK1-39 gene segments, at least 3 human D2-2 gene segments or at least 3 human JKI gene segments), wherein at least two of the human gene segments are variants that are not identical to each other.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 3 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) cis at the same Ig locus.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) trans at the same Ig locus; and optionally a third human gene segment of the same type, wherein the third gene segment is cis with one of said 2 different gene segments.
- a population of non-human vertebrates comprising a repertoire of human variable region gene segments, wherein the plurality comprises at least 2 human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), a first of said different gene segments is provided in the genome of a first vertebrate of the population, and a second of said different gene segments being provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second gene segment.
- the plurality comprises at least 2 human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments)
- a first of said different gene segments is provided in the genome of a first vertebrate
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), wherein the gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 3 human variable region gene segments of the same type (eg, at least 3 human VH6-1 gene segments, at least 3 human J H6 gene segments, at least 3 human VK1-39 gene segments, at least 3 human D2-2 gene segments or at least 3 human JKI gene segments), wherein at least two of the human gene segments are variants that are not identical to each other.
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) cis at the same Ig locus.
- a non-human vertebrate eg, a mouse or rat
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) trans at the same Ig locus; and optionally a third human gene segment of the same type, wherein the third gene segment is cis with one of said 2 different gene segments.
- a non-human vertebrate eg, a mouse or rat
- a method of providing an enhanced human immunoglobulin variable region gene segment repertoire comprising providing a population of non-human vertebrates (eg, a mouse or rat) comprising a repertoire of human variable region gene segments, wherein the method comprises providing at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VKI- 39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), wherein a first of said different gene segments is provided in the genome of a first vertebrate of the population, and a second of said different gene segments is provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second gene segment.
- a population of non-human vertebrates eg, a mouse or rat
- the method comprises providing at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), wherein the gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), wherein the gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations; optionally wherein at least 2 or 3 of said different gene segments are provided at the same Ig locus in said genome.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising first and second human Ig locus gene segments of the same type (eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments)
- the first gene segment is a gene segment selected from any one of Tables 1 and 9 to 14 (eg, selected from Table 13 or Table 14) (eg, IGHJ6-a)
- the second gene segment is the corresponding reference sequence.
- a population of non-human vertebrates comprising first and second human Ig locus gene segments of the same type (eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments), wherein the first gene segment is a gene segment selected from any one of Tables 1 and 9 to 14 (eg, selected from Table 13 or Table 14) (eg, IGHJ6-a) and the second gene segment is the corresponding reference sequence, wherein the first gene segment is provided in the genome of a first vertebrate of the population, and the second gene segment is provided in the genome of a second vertebrate of the population.
- first and second human Ig locus gene segments of the same type eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments
- the first gene segment is a gene segment selected from any one of Tables 1 and 9 to 14 (eg, selected from Table 13 or Table 14) (eg,
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising first and second human Ig locus gene segments of the same type (eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments), wherein the first gene segment is a gene segment selected from any one of Tables 1 and 9 to 14 (eg, selected from Table 13 or Table 14) (eg, IGHJ6-a) and the second gene segment is the corresponding reference sequence.
- first and second human Ig locus gene segments of the same type eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments
- the first gene segment is a gene segment selected from any one of Tables 1 and 9 to 14 (eg, selected from Table 13 or Table 14) (eg, IGHJ6-a) and the second gene segment is the corresponding reference sequence.
- the invention relates to human D gene segment variants as described further below.
- the invention relates to human V gene segment variants as described further below. In one aspect of this configuration, the invention relates to human J gene segment variants as described further below.
- Figures 1 to 3 Schematic illustrating a protocol for producing recombineered BAC vectors to add V gene segments into a mouse genome
- FIG. 4 Schematic illustrating a protocol for adding V gene segments to a mouse genome using sequential recombinase mediated cassette exchange (s MCE).
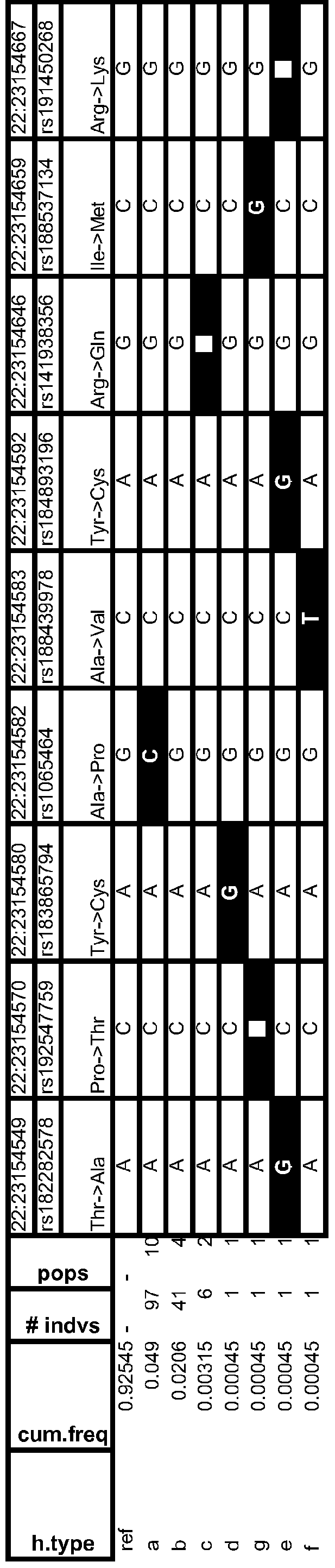
- Figure 5 Alignment of 13 IGHVl-69 variants showing the variable (V) coding region only. Nucleotides that differ from VH1-69 variant *01 are indicated at the appropriate position whereas identical nucleotides are marked with a dash. Where nucleotide changes result in amino acid differences, the encoded amino acid is shown above the corresponding triplet. Boxed regions correspond to CDR1, CDR2 and CDR3 as indicated.
- Figure 6 is a schematic illustrating gene segment diversity and the effect of including variant variants in cis according to the invention:-
- V4-4 can only be recombined within variant 1 to form for instance for instance V4-4-D-J6 or V4-4-D-J2 A .
- the variant V4-4 A can't be recombined with either J6 or J2 A from variant 1 and can only be joined with J-genes from variant 2 to form V4-4 A -D-J6 A and V4-4 A -D-J2.
- V4-4-J2/J6 complexity 4.
- (c) Supra mouse of the invention The variants are added in cis and thus can be recombined in every combination, expanding the repertoire.
- V4-4 can be combined with J6A, J6, J2A or J2 and similarly V4-4A can be recombined with these same J-genes.
- the V4-4-J6/J2 complexity 8 which in this simple example is double that of a person and 4X that of a mouse with a single variant.
- Figure 7 Alignment of human JH6*02 variants. Nucleotides that differ from JH6 *01 are indicated at the appropriate position whereas identical nucleotides are marked with a dash. Where nucleotide changes result in amino acid differences, the encoded amino acid is shown above. Accession numbers (eg, J00256) are shown to the left of the IMGT variant name.
- Figure 8 Alignment of JH sequences from various species.
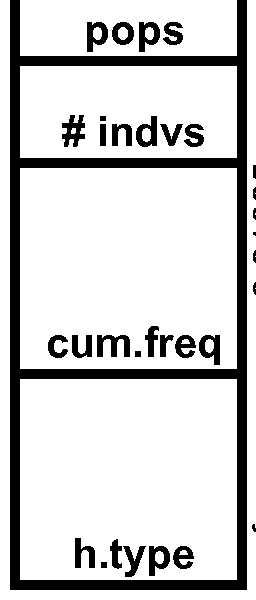
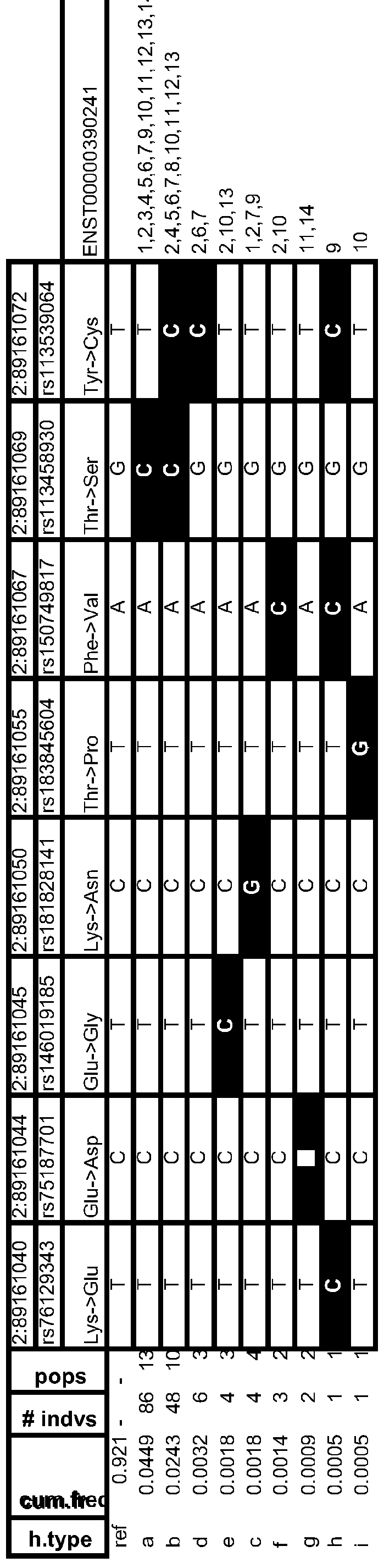
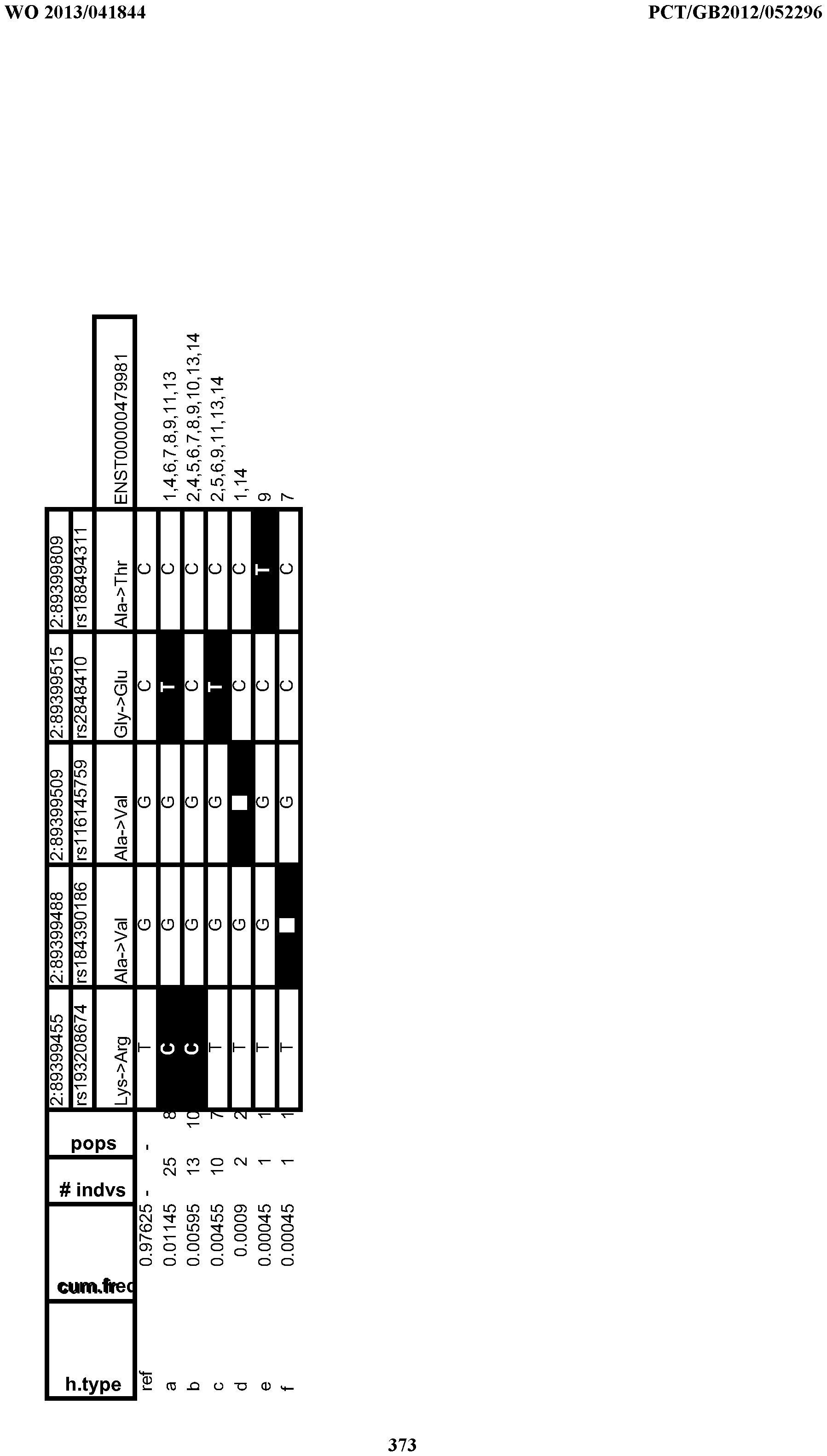
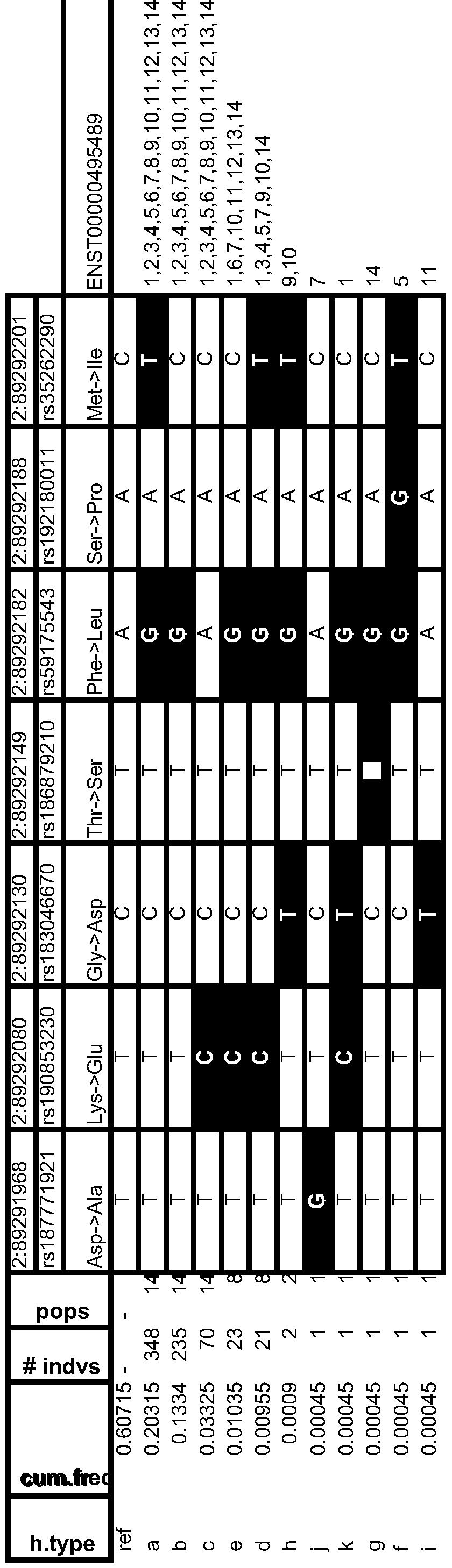
- Table 13 Variant Frequency Analyses & Human Population Distributions
- Table 14 Frequent Human Variant Distributions
- a suitable source of JH6*02 and other human DNA sequences for use in the invention will be readily apparent to the skilled person.
- a DNA sample from a consenting human donor (eg, a cheek swab sample as per the Example herein) from which can be obtained suitable DNA sequences for use in constructing a locus of the invention.
- Other sources of human DNA are commercially available, as will be known to the skilled person.
- the skilled person is able to construct gene segment sequence by referring to one or more databases of human Ig gene segment sequences disclosed herein.
- An example source for human V, D and J gene segments according to the invention are Bacterial Artificial Chromosomes (RPCI-11 BACs) obtained from Roswell Park Cancer Institute
- Male blood was obtained via a double-blind selection protocol. Male blood DNA was isolated from one randomly chosen donor (out of 10 male donors)".
- the invention relates to synthetically-extended & ethnically-diverse superhuman immunoglobulin gene repertoires.
- the human immunoglobulin repertoires are beyond those found in nature (ie, "Superhuman"), for example, they are more diverse than a natural human repertoire or they comprise combinations of human immunoglobulin gene segments from disparate sources in a way that is non-natural.
- the repertoires of the invention are "superhuman” immunoglobulin repertoires, and the invention relates to the application of these in transgenic cells and non-human vertebrates for utility in producing chimaeric antibodies (with the possibility of converting these into fully-human, isolated antibodies using recombinant DNA technology).
- the present invention thus provides for novel and potentially expanded synthetic immunoglobulin diversities, which provides for a pool of diversity from which antibody therapeutic leads (antibody therapeutics and antibody tool reagents) can be selected.
- This opens up the potential of transgenic human-mouse/rat technologies to the possibility of interrogating different and possibly larger antibody sequence- spaces than has hitherto been possible.
- the invention provides a SUPERHUMAN MOUSETM (aka SUPRA-MOUSETM) and a SUPERHUMAN RATTM (aka SUPRA-RATTM)
- SUPRA-RATTM aka SUPRA-RATTM
- the present inventors have realised the possibility of mining the huge genetics resources now available to the skilled person thanks to efforts such as the HapMap Project, 1000 Genomes Project and sundry other immunoglobulin gene databases (see below for more details).
- the inventors realised the application of these genome sequencing developments in the present invention to generate synthetically-produced and ethnically-diverse artificial immunoglobulin gene repertoires.
- the inventors realised that such repertoires are useful for the production of antibodies having improved affinity and/or biophysical characteristics, and/or wherein the range of epitope specificities produced by means of such repertoire is novel, provides for antibodies to epitopes that have hitherto been intractable by prior transgenic immunoglobulin loci or difficult to address.
- the present invention provides libraries, vertebrates and cells, such as transgenic mice or rats or transgenic mouse or rat cells. Furthermore, the invention relates to methods of using the vertebrates to isolate antibodies or nucleotide sequences encoding antibodies. Antibodies, nucleotide sequences, pharmaceutical compositions and uses are also provided by the invention.
- the present inventors have realized methods and antibody loci designs that harness the power of genetic variation analysis.
- the reference human genome provides a foundation for experimental work and genetic analysis of human samples.
- the reference human is a compilation of the genomes from a small number of individuals and for any one segment of the genome a high quality single reference genome for one of the two chromosomes is available. Because the reference genome was assembled from a series of very large insert clones, the identity of these clones is known.
- the 1000-Genomes Project has the objective of identifying the most frequent variations in the human genome.
- This public domain project involved sequencing the genomes of more than 1000 individuals from diverse ethnic groups, comparing these sequences to the reference and assembling a catalogue of variants. This has enabled the annotation of variants in coding regions, but because this sequence wasn't derived from large clones of DNA, the analysis of the sequence from diploid individuals can't discriminate the distribution of the variation between the maternal and paternally inherited chromosomes. Where more than one variant is identified in a protein coding gene, it is not possible to illuminate the distribution of the pattern of variants in each version of the protein.
- the 1000-Genome Project has sequenced mother-father-child trios. This allows one to "phase" the sequence variants, in other words identify blocks of sequence that are inherited from one or other parent and deconvolute the variants.
- the inventors' analysis of the 1000-genome data for the individual human coding segments of the C, V D and J genes from the heavy and light chains reveals that there is significant variation in these segments. Individuals will usually have two different heavy chain alleles and also different light chain alleles at both kappa and lambda loci. The repertoire of antibodies that can be generated from each allele will be different. This variation will contribute to a better or differing immune response to certain antigens.
- mice that have hitherto been generated with immunoglobulin heavy and light chain loci contain just one type of immunoglobulin locus. Even if these mice contain a full human heavy chain locus, the variation will be less than contained in a typical human because only one set of C, V, D and J genes are available, while a typical human would have two sets.
- the inventors have devised ways to improve on this limitation when constructing transgenic non- human vertebrates and cells for human antibody and variable region production in vivo.
- mice can be generated with two different loci, each engineered to have a different repertoire of V, D and J segments. This could be in a single mouse or two or more separate mouse strains and would be analogous to or beyond the repertoire found in a normal human. The engineering of such a mouse would go beyond the repertoire described in humanized mice to date which only have one set of alleles.
- JH gene segments eg, see the examples
- this addresses compatibility with human patients since the inventor's analysis has drawn out candidate variants that are naturally conserved and sometimes very prevalent amongst human ethnic populations. Additionally this enables one to tailor the configurations of the invention to provide for antibody-based drugs that better address specific human ethnic populations.
- loci and cells and vertebrates comprising these
- gene segments are provided in which gene segments from different human populations are used. This is desirable to increase antibody gene diversity to better address more diverse human patients.
- the gene segments are from first and second different human populations respectively, and thus the second gene segment is found in the second human population, but not so (or rarely) in the first human population. Rarely means, for example, that the gene segment is found in 5, 4, 3, 2, or 1 or zero individuals in the first population in the 1000 Genomes database.
- the first gene segment may be shown as present in a first population by reference to Table 13 or 14 herein
- the second gene segment may be shown as present in the second population by reference to Table 13 and not in the first population.
- the first gene segment may also be shown as being present in the second population by reference to Table 13 or 14.
- V gene segment In any configuration or aspect of the invention, where a V gene segment is used, this may be used optionally with the native leader sequence.
- genomic DNA eg, from BACs as in the examples
- the native leader will be used for each V gene segment incorporated into the locus and genomes of the invention.
- the skilled person may wish to inert a non-native leader sequence together with one or more of the V gene segments.
- this may be used optionally with the native 5' UTR sequence.
- genomic DNA eg, from BACs as in the examples
- native 5' UTR sequence will be used for each V gene segment incorporated into the locus and genomes of the invention.
- skilled person may wish to exclude the native 5' UTR sequence.
- the present invention provides, in a first configuration (a) Superhuman heavy chain gene repertoires
- a non-human vertebrate or vertebrate cell (optionally an ES cell or antibody-producing cell) comprising a genome having a superhuman immunoglobulin heavy chain human VH and/or D and/or J gene repertoire.
- the cell of the invention is an embryonic stem cell.
- the ES cell is derived from the mouse C57BL/6N, C57BL/6J, 129S5 or 129Sv strain.
- the non-human vertebrate is a rodent, suitably a mouse, and cells of the invention, are rodent cells or ES cells, suitably mouse ES cells.
- the ES cells of the present invention can be used to generate animals using techniques well known in the art, which comprise injection of the ES cell into a blastocyst followed by implantation of chimaeric blastocystys into females to produce offspring which can be bred and selected for homozygous recombinants having the required insertion.
- the invention relates to a transgenic animal comprised of ES cell-derived tissue and host embryo derived tissue. In one aspect the invention relates to genetically-altered subsequent generation animals, which include animals having a homozygous recombinants for the VDJ and/or VJ regions.
- the natural human immunoglobulin gene segment repertoire consists of (see eg, www.imgt.org 3 ⁇ 4:-
- V lambda total-75; functional-31 J lambda: total-7; functional-5
- the vertebrate or cell genome comprises a transgenic immunoglobulin heavy chain locus comprising a plurality of human immunoglobulin VH gene segments, one or more human D gene segments and one or more human J gene segments, wherein the plurality of VH gene segments consists of more than the natural human repertoire of functional VH gene segments; optionally wherein the genome is homozygous for said transgenic heavy chain locus.
- the VH gene repertoire consists of a plurality of VH gene segments derived from the genome sequence of a first human individual, supplemented with one or more different VH gene segments derived from the genome sequence of a second, different human individual.
- the D and J segments are derived from the genome sequence of the first human individual.
- the VH gene segments from the genome sequence of the second individual are selected from the VH gene segments listed in Table 1, 13 or 14. In this way, the locus provides a superhuman repertoire of D gene segments.
- the individuals are not related.
- Individuals are "not related" in the context of any configuration or aspect of the invention, for example, if one of the individuals does not appear in a family tree of the other individual in the same generation or going back one, two, three or four generations.
- are not related for example, if they do not share a common ancestor in the present generation or going back one, two, three or four generations.
- the transgenic locus comprises more than 41 functional human VH gene segment species, and thus more than the natural human functional repertoire.
- the locus comprises at least 42, 43, 44, 45, 46, 47, 48, 49 or 50 functional human VH gene segment species (eg, wherein the locus comprises the full functional VH repertoire of said first individual supplemented with one or more VH gene segments derived from the genome sequence of the second human individual and optionally with one or more VH gene segments derived from the genome sequence of a third human individual).
- the locus provides a superhuman repertoire of VH gene segments that is useful for generating a novel gene and antibody diversity for use in therapeutic and tool antibody selection.
- the transgenic locus comprises a first VH gene segment derived from the genome sequence of the first individual and a second VH gene segment derived from the genome sequence of the second individual, wherein the second VH gene segment is a polymorphic variant of the first VH gene segment.
- the VH gene segments are polymorphic variants of VHl-69 as illustrated in the examples below.
- the locus comprises a further polymorphic variant of the first VH gene segment (eg, a variant derived from the genome sequence of a third human individual). In this way, the locus provides a superhuman repertoire of VH gene segments.
- the genome (alternatively or additionally to the superhuman VH diversity) comprises a transgenic immunoglobulin heavy chain locus comprising a plurality of human immunoglobulin VH gene segments, a plurality of human D gene segments and one or more human J gene segments, wherein the plurality of D gene segments consists of more than the natural human repertoire of functional D gene segments.
- the genome is homozygous for said transgenic heavy chain locus.
- the D gene repertoire consists of a plurality of D gene segments derived from the genome sequence of a (or said) first human individual, supplemented with one or more different D gene segments derived from the genome sequence of a (or said) second, different human individual.
- the individuals are not related.
- the J segments are derived from the genome sequence of the first human individual.
- the D gene segments from the genome sequence of the second individual are selected from the D gene segments listed in Table 2, 13 or 14. In this way, the locus provides a superhuman repertoire of D gene segments.
- the transgenic locus comprises more than 23 functional human D gene segment species; optionally wherein the locus comprises at least 24, 25, 26, 27, 28, 29, 30 or 31 functional human D gene segment species (eg, wherein the locus comprises the full functional D repertoire of said first individual supplemented with one or more D gene segments derived from the genome sequence of the second human individual and optionally with one or more D gene segments derived from the genome sequence of a third human individual).
- the locus provides a superhuman repertoire of D gene segments.
- the transgenic locus comprises a first D gene segment derived from the genome sequence of the first individual and a second D gene segment derived from the genome sequence of the second individual, wherein the second D gene segment is a polymorphic variant of the first D gene segment.
- the locus comprises a further polymorphic variant of the first D gene segment (eg, a variant derived from the genome sequence of a third human individual). In this way, the locus provides a superhuman repertoire of D gene segments.
- the genome comprises a (or said) transgenic immunoglobulin heavy chain locus comprising a plurality of human immunoglobulin VH gene segments, one or more human D gene segments and a plurality of human JH gene segments, wherein the plurality of J gene segments consists of more than the natural human repertoire of functional J gene segments; optionally wherein the genome is homozygous for said transgenic heavy chain locus.
- the JH gene repertoire consists of a plurality of J gene segments derived from the genome sequence of a (or said) first human individual, supplemented with one or more different J gene segments derived from the genome sequence of a (or said) second, different human individual.
- the individuals are not related.
- D segments are derived from the genome sequence of the first human individual.
- the J gene segments from the genome sequence of the second individual are selected from the J gene segments listed in Table 3 13 or 14. In this way, the locus provides a superhuman repertoire of JH gene segments.
- the transgenic locus comprises more than 6 functional human JH gene segment segments.
- the locus comprises at least 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16 functional human JH gene segments (eg, wherein the locus comprises the full functional JH repertoire of said first individual supplemented with one or more J H gene segments derived from the genome sequence of the second human individual and optionally with one or more J H gene segments derived from the genome sequence of a third human individual).
- the locus provides a superhuman repertoire of JH gene segments.
- the transgenic locus comprises a first J H gene segment derived from the genome sequence of the first individual and a second JH gene segment derived from the genome sequence of the second individual, wherein the second J H gene segment is a polymorphic variant of the first JH gene segment.
- the locus comprises a further polymorphic variant of the first JH gene segment (eg, a variant derived from the genome sequence of a third human individual). In this way, the locus provides a superhuman repertoire of JH gene segments.
- the first configuration of the invention also provides:-
- a non-human vertebrate or vertebrate cell (optionally an ES cell or antibody-producing cell) comprising a genome having a superhuman immunoglobulin light chain human VL gene repertoire.
- the vertebrate or cell comprises a heavy chain transgene according to aspect (a) of the first configuration.
- superhuman diversity is provided in both the heavy and light chain immunoglobulin gene segments in the cell and vertebrate.
- the genome of the cell or vertebrate is homozygous for the heavy and light chain transgenes and endogenous antibody expression is inactivated.
- Such a vertebrate is useful for immunisation with a predetermined antigen to produce one or more selected antibodies that bind the antigen and have human variable regions resulting from recombination within the superhuman gene segment repertoire. This provides potentially for a novel antibody and gene sequence space from which to select therapeutic, prophylactic and tool antibodies.
- the vertebrate or cell genome comprises a transgenic immunoglobulin kappa light chain locus comprising a plurality of human immunoglobulin VK gene segments and one or more human J gene segments, wherein the plurality of VK gene segments consists of more than the natural human repertoire of functional VK gene segments; optionally wherein the genome is homozygous for said transgenic kappa light chain locus; and/or
- transgenic immunoglobulin lambda light chain locus comprising a plurality of human immunoglobulin V ⁇ gene segments and one or more human J gene segments, wherein the plurality of V ⁇ gene segments consists of more than the natural human repertoire of functional V ⁇ gene segments; optionally wherein the genome is homozygous for said transgenic lambda light chain locus.
- the locus provides a superhuman repertoire of VL gene segments.
- the VK gene repertoire consists of a plurality of VK gene segments derived from the genome sequence of a first human individual, supplemented with one or more VK gene segments derived from the genome sequence of a second, different human individual; optionally wherein the individuals are not related; optionally wherein the J segments are derived from the genome sequence of the first human individual; and optionally wherein the VK gene segments from the genome sequence of the second individual are selected from the VK gene segments listed in Table 4, 13 or 14; and
- the V ⁇ gene repertoire consists of a plurality of V ⁇ gene segments derived from the genome sequence of a first human individual, supplemented with one or more V ⁇ gene segments derived from the genome sequence of a second, different human individual; optionally wherein the individuals are not related; optionally wherein the J segments are derived from the genome sequence of the first human individual; and optionally wherein the V ⁇ gene segments from the genome sequence of the second individual are selected from the V ⁇ gene segments listed in Table 5, 13 or 14.
- the locus provides a superhuman repertoire of VL gene segments.
- the vertebrate or cell In one embodiment of the vertebrate or cell,
- the kappa light transgenic locus comprises more than 38 functional human VK gene segment species; optionally wherein the locus comprises at least 39, 40, 41, 42, 43, 44, 45, 46, 47 or 48 functional human VK gene segment species (eg, wherein the locus comprises the full functional VK repertoire of said first individual supplemented with one or more VK gene segments derived from the genome sequence of the second human individual and optionally with one or more VK gene segments derived from the genome sequence of a third human individual); and
- the lambda light transgenic locus comprises more than 31 functional human V ⁇ gene segment species; optionally wherein the locus comprises at least 32, 33, 34, 35, 36, 37, 38, 39, 40 or 41 functional human V ⁇ gene segment species (eg, wherein the locus comprises the full functional V ⁇ repertoire of said first individual supplemented with one or more V ⁇ gene segments derived from the genome sequence of the second human individual and optionally with one or more V ⁇ gene segments derived from the genome sequence of a third human individual).
- the locus provides a superhuman repertoire of VL gene segments.
- the kappa light transgenic locus comprises a first VK gene segment derived from the genome sequence of the first individual and a second VK gene segment derived from the genome sequence of the second individual, wherein the second VK gene segment is a polymorphic variant of the first VK gene segment; optionally wherein the locus comprises a further polymorphic variant of the first VK gene segment (eg, a variant derived from the genome sequence of a third human individual); and
- the lambda light transgenic locus comprises a first V ⁇ gene segment derived from the genome sequence of the first individual and a second V ⁇ gene segment derived from the genome sequence of the second individual, wherein the second V ⁇ gene segment is a polymorphic variant of the first V ⁇ gene segment; optionally wherein the locus comprises a further polymorphic variant of the first VX gene segment (eg, a variant derived from the genome sequence of a third human individual).
- the locus provides a superhuman repertoire of VL gene segments.
- the genome comprises a (or said) transgenic immunoglobulin light chain locus comprising a plurality of human immunoglobulin VL gene segments and a plurality of human JL gene segments, wherein the plurality of J gene segments consists of more than the natural human repertoire of functional J gene segments; optionally wherein the genome is homozygous for said transgenic heavy chain locus.
- the JK gene repertoire consists of a plurality of JK gene segments derived from the genome sequence of a (or said) first human individual, supplemented with one or more J K gene segments derived from the genome sequence of a (or said) second, different human individual; optionally wherein the individuals are not related; optionally wherein the VK segments are derived from the genome sequence of the first human individual; optionally wherein the J K gene segments from the genome sequence of the second individual are selected from the J K gene segments listed in Table 6, 13 or 14; and
- the JK gene repertoire consists of a plurality of iX gene segments derived from the genome sequence of a (or said) first human individual, supplemented with one or more iX gene segments derived from the genome sequence of a (or said) second, different human individual; optionally wherein the individuals are not related; optionally wherein the V ⁇ segments are derived from the genome sequence of the first human individual; optionally wherein the iX gene segments from the genome sequence of the second individual are selected from the iX gene segments listed in Table 7, 13 or 14.
- the locus provides a superhuman repertoire of J L gene segments.
- the transgenic light chain locus comprises more than 5 functional human J K gene segment species; optionally wherein the locus comprises at least 6, 7, 8, 9, 10, 11, 12, 13, 14 or 15 functional human JK gene segment species (eg, wherein the locus comprises the full functional J K repertoire of said first individual supplemented with one or more JK gene segments derived from the genome sequence of the second human individual and optionally with one or more J K gene segments derived from the genome sequence of a third human individual); and/or
- the transgenic light chain locus comprises more than 5 functional human iX gene segment species; optionally wherein the locus comprises at least 6, 7, 8, 9, 10, 11, 12, 13, 14 or 15 functional human iX gene segment species (eg, wherein the locus comprises the full functional iX repertoire of said first individual supplemented with one or more iX gene segments derived from the genome sequence of the second human individual and optionally with one or more iX gene segments derived from the genome sequence of a third human individual).
- the locus provides a superhuman repertoire of J L gene segments.
- the kappa light transgenic locus comprises a first JK gene segment derived from the genome sequence of the first individual and a second JK gene segment derived from the genome sequence of the second individual, wherein the second JK gene segment is a polymorphic variant of the first J K gene segment; optionally wherein the locus comprises a further polymorphic variant of the first J ⁇ gene segment (eg, a variant derived from the genome sequence of a third human individual); and
- the lambda light transgenic locus comprises a first iX gene segment derived from the genome sequence of the first individual and a second iX gene segment derived from the genome sequence of the second individual, wherein the second JK gene segment is a polymorphic variant of the first iX gene segment; optionally wherein the locus comprises a further polymorphic variant of the first iX gene segment (eg, a variant derived from the genome sequence of a third human individual).
- the locus provides a superhuman repertoire of J L gene segments.
- the present invention provides, in a second configuration
- a library of antibody-producing transgenic cells whose genomes collectively encode a repertoire of antibodies, wherein (a) a first transgenic cell expresses a first antibody having a chain (eg, heavy chain) encoded by a first immunoglobulin gene, the gene comprising a first variable domain nucleotide sequence produced following recombination of a first human unrearranged immunoglobulin gene segment (eg, a VH);
- a first transgenic cell expresses a first antibody having a chain (eg, heavy chain) encoded by a first immunoglobulin gene, the gene comprising a first variable domain nucleotide sequence produced following recombination of a first human unrearranged immunoglobulin gene segment (eg, a VH);
- a second transgenic cell expresses a second antibody having a chain (eg, a heavy chain) encoded by a second immunoglobulin gene, the second gene comprising a second variable domain nucleotide sequence produced following recombination of a second human unrearranged immunoglobulin gene segment (eg, a VH), the first and second antibodies being non-identical;
- the first and second gene segments are different and derived from the genome sequences of first and second human individuals respectively, wherein the individuals are different; and optionally not related;
- cells are non-human vertebrate (eg, mouse or rat) cells (eg, B-cells or hybridomas).
- the library is provided in vitro.
- the library is provided in vivo by one or a plurality of transgenic non-human vertebrates.
- the or each vertebrate is according to any aspect of the first configuration of the invention.
- the library encodes an antibody repertoire of from 10 to 10 9 antibodies, for example, 10, 20, 30, 40, 50, 100 or 1000 to 10 s ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 7 ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 s ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 s ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 4 antibodies.
- library encodes an antibody repertoire of at least 10 3 , 10 4 , 10 s , 10 s , 10 7 , 10 s , 10 9 ' or 10 10 antibodies.
- the first variable domain nucleotide sequence is produced following recombination of the first human unrearranged immunoglobulin gene segment with one or more other immunoglobulin gene segments (for example, human immunoglobulin gene segments).
- the first gene segment is a VH
- the first variable domain nucleotide sequence (a VH domain) is produced following recombination of the VH with a human D and JH segments in vivo, optionally with somatic hypermutation, in the first transgenic cell or an ancestor thereof.
- the first variable domain nucleotide sequence (a VL domain) is produced following recombination of the VL with a human JL segment in vivo, optionally with somatic hypermutation, in e first transgenic cell or an ancestor thereof.
- the second variable domain nucleotide sequence is produced following recombination of the second human unrearranged immunoglobulin gene segment with one or more other immunoglobulin gene segments (for example, human immunoglobulin gene segments).
- the second gene segment is a VH
- the second variable domain nucleotide sequence (a VH domain) is produced following recombination of the VH with a human D and JH segments in vivo, optionally with somatic hypermutation, in the second transgenic cell or an ancestor thereof.
- the second variable domain nucleotide sequence (a VL domain) is produced following recombination of the VL with a human JL segment in vivo, optionally with somatic hypermutation, in the second transgenic cell or an ancestor thereof.
- the first and second gene segments are respectively derived from genome sequences of first and second human individuals.
- a gene segment is isolated or cloned from a sample cell taken from said individual using standard molecular biology techniques as know to the skilled person.
- the sequence of the gene segment may be mutated (eg, by the introduction of up to 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 or 15 nucleotide changes) prior to use in the present invention.
- a gene segment is derived by identifying a candidate human immunoglobulin gene segment in a database (see guidance below) and a nucleotide sequence encoding a gene segment for use in the present invention is made by reference (eg, to be identical or a mutant with up to 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 or 15 nucleotide changes to the reference sequence) to the database sequence.
- a nucleotide sequence encoding a gene segment for use in the present invention is made by reference (eg, to be identical or a mutant with up to 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 or 15 nucleotide changes to the reference sequence) to the database sequence.
- the skilled person will be aware of methods of obtaining nucleotide sequences by reference to databases or by obtaining from cellular samples.
- the first and second human individuals are members of first and second ethnic populations respectively, wherein the populations are different. This, therefore, provides for superhuman gene diversity in transgenic loci, cells and vertebrates as per the invention.
- the ethnic populations are selected from those identified in the 1000 Genomes Project of database.
- Table 8 which provides details of the ethnic populations on which the 1000 Genomes database is based.
- the International HapMap Project discloses that goal of the HapMap Project: to determine the common patterns of DNA sequence variation in the human genome by determining the genotypes of one million or more sequence variants, their frequencies and the degree of association between them in DNA samples from populations with ancestry from parts of Africa, Asia and Europe.
- the relevant human populations of differing geographical ancestry include Yoruba, Japanese, Chinese, Northern European and Western European populations. More specifically:- Utah population with Northern or Western European ancestry (samples collected in 1980 by the
- a suitable sample of human populations from which the populations used in the present invention are selected is as follows:-
- each human population is selected from a population marked "(a)" above.
- each human population is selected from a population marked "(b)" above.
- each human population is selected from a population marked "(c)" above.
- the first and second ethnic populations are selected from the group consisting of an ethnic population with European ancestry, an ethnic population with East Asian, an ethnic population with West African ancestry, an ethnic population with Americas ancestry and an ethnic population with South Asian ancestry.
- the first and second ethnic populations are selected from the group consisting of an ethnic population with Northern European ancestry; or an ethnic population with Western European ancestry; or an ethnic population with Toscani ancestry; or an ethnic population with British ancestry; or an ethnic population with Icelandic ancestry; or an ethnic population with Finnish ancestry; or an ethnic population with Iberian ancestry; or an ethnic population with Japanese ancestry; or an ethnic population with Chinese ancestry; or an ethnic population Vietnamese ancestry; or an ethnic population with Yoruba ancestry; or an ethnic population with Luhya ancestry; or an ethnic population with Gambian ancestry; or an ethnic population with Malawian ancestry; or an ethnic population with Native American ancestry; or an ethnic population with Afro-Caribbean ancestry; or an ethnic population with Mexican ancestry; or an ethnic population with Puerto ican ancestry;
- the human immunoglobulin gene segment derived from the genome sequence of the second individual is low-frequency (optionally rare) within the second ethnic population.
- human immunoglobulin gene segment has a Minor Allele Frequency (MAF) (cumulative frequency) of between 0.5% - 5%, optionally less than 0.5%, in the second human population, eg, as in the 1000 Genomes database.
- MAF Minor Allele Frequency
- the first variable region nucleotide sequence is produced by recombination of the first human
- immunoglobulin gene segment with a first J gene segment and optionally a first D gene segment, wherein the first human immunoglobulin gene segment is a V gene segment and the V, D and J segments are derived from the first human population, optionally from the genome of one individual the first human populati
- the second variable region nucleotide sequence is produced by recombination of the second human immunoglobulin gene segment with a second J gene segment and optionally a second D gene segment, wherein the second human immunoglobulin gene segment is a V gene segment derived from the second population and the D and/or J segments are derived from the first human population, optionally the D and J gene segments being from the genome of one individual of the first human population.
- all of the D and J segments that have been recombined with the first and second V gene segments are D and J segments derived from the first human population, optionally the D and J gene segments being from the genome of one individual of the first human population.
- the second human immunoglobulin gene segment is a polymorphic variant of the first human immunoglobulin gene segment; optionally wherein the second gene segment is selected from the group consisting of a gene segment in any of Tables 1 to 7 and 9 to 14 (eg, selected from Table 13 or 14).
- the first and second human immunoglobulin gene segments are both (i) V H gene segments; (ii) D segments; (iii) J segments (optionally both J H segments, both J K segments or both ⁇ ⁇ segments); (iv) constant regions (optionally both a gamma constant region, optionally both a C gamma-1 constant region); (v) CHI regions; (vi) CH2 regions; or (vii) CH3 regions.
- the library is, for example, a naive and optionally has a library size of from 10 or 10 2 to 10 9 cells.
- the library has, for example, been selected against a predetermined antigen and optionally has a library size of from 10 or 10 2 to 10 9 cells.
- said first and second cells are progeny of first and second ancestor non-human vertebrate cells respectively, wherein the first ancestor cell comprises a genome comprising said first human immunoglobulin gene segment; and the second ancestor cell comprises a genome comprising said second human immunoglobulin gene segment.
- the invention further provides a library of antibody-producing transgenic cells whose genomes collectively encode a repertoire of antibodies, wherein the library comprises the first and second ancestor cells described above.
- the invention further provides a library of hybridoma cells produced by fusion of the library of the invention (eg, a B-cell library) with fusion partner cells and optionally has a library size of from 10 or 10 2 to 10 9 cells.
- a library size of from 10 or 10 2 to 10 9 cells.
- 10 or 10 2 to 10 9 cells For example, from 10, 20, 30, 40, 50, 100 or 1000 to 10 s ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 7 ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 s ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 s ; or 10, 20, 30, 40, 50, 100 or 1000 to 10 4 cells.
- Production of hybridomas is well known to the skilled person.
- fusion partners are SP2/0-gl4 (obtainable from ECACC), P3X63-Ag8.653 (obtainable from LGC Standards; C L-1580), NS1 and NS0 cells.
- PEG fusion or electrofusion can be carried out, as is conventional.
- the invention provides, in a third confieuration:- An isolated antibody having
- each variable domain of the antibody is a human variable domain.
- heavy chain constant regions are mu- or gamma-type constant regions.
- the invention also provides an isolated nucleotide sequence encoding the antibody of the third configuration, optionally wherein the sequence is provided in an antibody expression vector, optionally in a host cell.
- Suitable vectors are mammalian expression vectors (eg, CHO cell vectors or HEK293 cell vectors), yeast vectors (eg, a vector for expression in Picchia pastoris, or a bacterial expression vector, eg, a vector for E. coli expression.
- the invention also provides a method of producing a human antibody, the method comprising replacing the non-human vertebrate constant regions of the antibody of the third configuration with human antibody constant regions (eg, a C variant disclosed in table 13 or 18).
- human antibody constant regions eg, a C variant disclosed in table 13 or 18.
- the skilled person will be aware of standard molecular biology techniques to do this. For example, see Harlow, E. & Lane, D. 1998, 5 th edition, Antibodies: A Laboratory Manual, Cold Spring Harbor Lab. Press, Plainview, NY; and Pasqualini and Arap, Proceedings of the National Academy of Sciences (2004) 101:257-259 for standard immunisation.
- Joining of the variable regions of an antibody to a human constant region can be effected by techniques readily available in the art, such as using conventional recombinant DNA and NA technology as will be apparent to the skilled person. See e.g. Sambrook, J and Russell, D. (2001, 3'd edition) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- the method comprises further making a mutant or derivative of the antibody.
- the invention also provides a pharmaceutical composition
- a pharmaceutical composition comprising an antibody according to the third configuration, or a human antibody of the invention and a diluent, excipient or carrier;
- composition is provided in a container connected to an IV needle or syringe or in an IV bag.
- the invention also provides an antibody-producing cell (eg, a mammalian cell, eg, CHO or HEK293; a yeast cell, eg, P pastoris; a bacterial cell, eg, E coli; a B-cell; or a hybridoma) that expresses the second antibody of the third configuration or the isolated antibody of the invention.
- an antibody-producing cell eg, a mammalian cell, eg, CHO or HEK293; a yeast cell, eg, P pastoris; a bacterial cell, eg, E coli; a B-cell; or a hybridoma
- the first configuration of the invention also provides:-
- a non-human vertebrate or vertebrate cell (optionally an ES cell or antibody-producing cell) whose genome comprises a transgenic immunoglobulin locus (eg, a heavy chain locus or a light chain locus), said locus comprising immunoglobulin gene segments according to the first and second human immunoglobulin gene segments (optionally V segments) described above in connection with the third configuration.
- the gene segments are operably connected upstream of an immunoglobulin constant region; optionally wherein the genome is homozygous for said transgenic immunoglobulin locus.
- the immunoglobulin locus comprises more than the natural human complement of functional V gene segments; and/or
- immunoglobulin locus comprises more than the natural human complement of functional D gene segments;
- immunoglobulin locus comprises more than the natural human complement of functional J gene segments.
- the first configuration also provides:-
- a transgenic non-human vertebrate eg, a mouse or rat
- vertebrate cell eg. an ES cell or antibody-producing cell
- a transgenic immunoglobulin locus comprising a plurality of human immunoglobulin gene segments operably connected upstream of a non-human vertebrate constant region for the production of a repertoire of chimaeric antibodies, or chimaeric light or heavy chains, having a non-human vertebrate constant region and a human variable region
- the transgenic locus comprises one or more human immunoglobulin V gene segments, one or more human J gene segments and optionally one or more human D gene segments, a first (optionally a V segment) of said gene segments and a second (optionally a V segment) of said gene segments being different and derived from the genomes of first and second human individuals respectively, wherein the individuals are different; and optionally not related; optionally wherein the immunoglobulin locus comprises more than the natural human complement of functional V gene segments; and/or optionally where
- a superhuman immunoglobulin gene repertoire is provided in a transgenic non-human vertebrate or vertebrate cell according to the invention.
- the first configuration also provides:-
- a transgenic non-human vertebrate eg, a mouse or rat
- vertebrate cell eg. an ES cell or antibody-producing cell
- each locus comprising a plurality of human immunoglobulin gene segments operably connected upstream of a non-human vertebrate constant region for the production of a repertoire of chimaeric antibodies, or chimaeric light or heavy chains, having a non-human vertebrate constant region and a human variable region
- the first transgenic locus comprises one or more human immunoglobulin V gene segments, one or more human J gene segments and optionally one or more human D gene segments
- the second transgenic locus comprises one or more human immunoglobulin V gene segments, one or more human J gene segments and optionally one or more human D gene segments
- each immunoglobulin locus comprises more than the natural human
- each immunoglobulin locus comprises more than the natural human
- a superhuman immunoglobulin gene repertoire is provided in a transgenic non-human vertebrate or vertebrate cell according to the invention.
- the immunoglobulin gene segments are optionally as described for the third configuration.
- the genome optionally comprises a third immunoglobulin gene segment (optionally a V segment), the third gene segment being derived from a human individual that is different from the individual from which the first (and optionally also the second) gene segment is derived; optionally wherein the first, second and third gene segments are polymorphic variants of a human immunoglobulin gene segment (eg, VH1-69 - see the examples for further description).
- a human immunoglobulin gene segment eg, VH1-69 - see the examples for further description.
- the genome of the vertebrate or cell is optionally homozygous for the first, second and optional third gene segment, wherein a copy of the first, second and optional third gene segments are provided together on the same chromosome operably connected upstream of a common non-human vertebrate constant region.
- each first, second and optional third gene segment is a V gene segment.
- the library of the invention is provided by a collection of non-human vertebrates (optionally a collection of rodents, mice or rats); optionally, wherein a first member of said collection produces said first antibody but not said second antibody, and a second member of the collection produces said second antibody (but optionally not said first antibody). It is therefore contemplated to make non-human vertebrates where different human genomes have been used as a source for building the transgenic loci in the vertebrates.
- a first vertebrate comprises a transgenic heavy chain locus having gene segments only from a first (and optionally a second) human population or individual;
- a second vertebrate comprises a transgenic heavy chain locus having gene segments only from a third (and optionally a fourth) human population or individual; and optionally third and more vertebrates can be built similarly based on unique or overlapping human population genomes.
- the mixed population provides a collective pool of human immunoglobulin genes that is greater than found in a natural human repertoire. This is useful to extend the antibody and gene sequence space beyond those possible with prior transgenic mice and rats bearing human immunoglobulin loci. As explained above, these have been based on a single human genome.
- the collection of non-human vertebrates bear human immunoglobulin genes confined to human populations that are together grouped under the same population genus "(a)" mentioned above.
- This provides for a gene repertoire that is biased to producing human antibody variable regions prevalent in the population genus (a) and thus useful for generating antibody therapeutics/prophylactics for members of said population.
- gene segments from different human populations are provided in a single transgene according to the invention (not necessarily in a collection of vertebrates)
- the different human populations are for example together grouped under the same population genus "(a)" mentioned above.
- the invention also provides a repertoire of antibodies expressed from a library of cells according to the invention.
- the constant region of the transgenic locus is, in one example, an endogenous constant region of said vertebrate (eg, endogenous mouse or rat constant region, eg, from the same strain of mouse or rat as the non- human vertebrate itself).
- an endogenous constant region of said vertebrate eg, endogenous mouse or rat constant region, eg, from the same strain of mouse or rat as the non- human vertebrate itself.
- the invention also provides a method of constructing a cell (eg, an ES cell) according to the invention, the method comprising
- the cell comprises a heavy chain locus constructed according to steps (a) to (c) and/or a light chain locus (kappa and/or lambda loci) constructed according to steps (a) to (c).
- the cell is homozygous for the or each transgenic locus; optionally wherein antibody expression from loci endogenous to said cell has been inactivated. This is useful for confining the functional antibody gene repertoire, and thus antibody production, to antibodies bearing human variable regions.
- the gene segment(s) in step (b) are identified from an immunoglobulin gene database selected from the 1000 Genomes, Ensembl, Genbank and IMGT databases.
- the first and second human individuals are members of first and second ethnic populations respectively, wherein the populations are different, optionally wherein the human immunoglobulin gene segment derived from the genome sequence of the second individual is low- frequency (optionally rare) within the second ethnic population.
- the invention also provides a method of making a transgenic non-human vertebrate (eg, a mouse or rat), the method comprising
- an ES cell eg, a mouse C57BL/6N, C57BL/6J, 129S5 or 129Sv strain ES cell
- a donor non-human vertebrate blastocyst eg, a mouse C57BL/6N, C57BL/6J, 129S5 or 129Sv strain blastocyst
- the invention provides a transgenic non-human vertebrate (eg, a mouse or rat) made by the method or a progeny thereof.
- the invention also provides a population of such non-human vertebrates.
- the invention also provides a method of isolating an antibody that binds a predetermined antigen (eg, a bacterial or viral pathogen antigen), the method comprising
- This method optionally further comprises after step (e) the step of isolating from said B lymphocytes nucleic acid encoding said antibody that binds said antigen; optionally exchanging the heavy chain constant region nucleotide sequence of the antibody with a nucleotide sequence encoding a human or humanised heavy chain constant region and optionally affinity maturing the variable region of said antibody; and optionally inserting said nucleic acid into an expression vector and optionally a host.
- GenBank www.ncbi.nlm.nih.gov/genbank
- Bioinformatics tools for database manipulation are also readily available and known to the skilled person, eg, as publicly available from the 1000 Genomes Project/EBI (www.100Qgenomes.org)
- IMGT International ImMunoGeneTics Information System 9 ; M.-P. Lefranc, 2002;
- IMGT is an integrated information system that specializes in antibodies, T cell receptors, and MHC molecules of all vertebrate species. It provides a common portal to standardized data that include nucleotide and protein sequences, oligonucleotide primers, gene maps, genetic polymorphisms, specificities, and two-dimensional (2D) and three-dimensional (3D) structures.
- IMGT includes three sequence databases (IMGT/LIGM-DB, IMGT/MHC-DB, IMGT/PRIMERDB), one genome database (IMGT/GENE-DB), one 3D structure database (IMGT/3Dstructure-DB), and a range of web resources ("/MGf Marie-Paule page”) and interactive tools.
- V-BASE (I. M. Tomlinson, 2002;https://www.mrc-cpe.cam. ac.uk/vbase).
- V-BASE is a comprehensive directory of all human antibody germline variable region sequences compiled from more than one thousand published sequences. It includes a version of the alignment software DNAPLOT (developed by Hans-Helmar Althaus and Werner Muller) that allows the assignment of rearranged antibody V genes to their closest germline gene segments.
- Antibodies Structure and Sequence(A. C. R. Martin, 2002; htto:https://www. bioinf.ora.uk/abs). This page summarizes useful information on antibody structure and sequence. It provides a query interface to the Kabat antibody sequence data, general information on antibodies, crystal structures, and links to other antibody-related information. It also distributes an automated summary of all antibody structures deposited in the Protein Databank (PDB). Of particular interest is a thorough description and comparison of the various numbering schemes for antibody variable regions.
- PDB Protein Databank
- AAAAA AHo's Amazing Atlas of Antibody Anatomy (A. Honegger, 2001;
- This resource includes tools for structural analysis, modeling, and engineering. It adopts a unifying scheme for comprehensive structural alignment of antibody and T- cell-receptor sequences, and includes Excel macros for antibody analysis and graphical representation.
- Therapeutic Antibody Human Homology Project which aims to correlate clinical efficacy and antiimmunoglobulin responses with variable region sequences of therapeutic antibodies.
- the Antibody Resource Page (The Antibody Resource Page, 2000;
- HapMap The International HapMap Consortium. 2003;
- the HapMap Project is an international project that aims to compare the genetic sequences of different individuals to identify chromosomal regions containing shared genetic variants.
- the HapMap www site provides tools to identify chromosomal regions and the variant therein, with options to drill down to population level frequency data.
- This resource provides complete genomic sequence for 2500 unidentified individuals from one of 25 distinct population groups, with the aim of identifying genomic variants of >1%.
- the site provides the ability to interrogate data utilizing online tools (e.g. 'Variation Pattern Finder') and to download variant data for individual population groups.
- a low- frequency immunoglobulin gene segment is classed as one with 'Minor Allele Frequency' (MAF) (cumulative frequency) of between 0.5% - 5%, rare variants are those classed as having a MAF of less than 0.5% in a particular human population.
- MAF 'Minor Allele Frequency'
- germline refers to the canonical germline gene segment sequence.
- the inventors have devised a collection of candidate polymorphic antibody gene segment variants, eg, human variant JH gene segments (eg, see Example 4), that can be built into the design of transgenic heavy chain loci in mice for expressing increasingly diverse and new, synthetic repertoires of human variable regions.
- candidate polymorphic antibody gene segment variants eg, human variant JH gene segments (eg, see Example 4)
- the invention provides the following embodiments.
- the present invention provides in a fourth configuration-
- Antibodies with long HCDR3 have been shown to neutralise a variety of pathogens effectively including HIV, Influenza virus, malaria and Africa trypanosomes. Reference is also made to naturally-occurring Camelid (eg, llama or camel) heavy chain-only antibodies which bear long HCDR3s for reaching relatively inaccessible epitopes (see, eg, EP0937140). Long HCDR3s can form unique stable subdomains with extended loop structure that towers above the antibody surface to confer fine specificity. In some cases, the long HCD 3 itself is sufficient for epitope binding and neutralization (Liu, L et al; Journal of Virology. 2011.
- the unique structure of the long HCDR3 allows it to bind to cognate epitopes within inaccessible structure or extensive glycosylation on a pathogen surface.
- human peripheral blood there is around 3.5% of naive B antibodies or 1.9% of memory B IgG antibodies containing the HCDR3s with lengths of more than 24 amino acids (PLoS One. 2012;7(5):e36750. Epub 2012 May 9; "Human peripheral blood antibodies with long HCDR3s are established primarily at original recombination using a limited subset of germline genes"; Briney BS e al, incorporated herein by reference) (Fig. 1).
- the inventors chose in this configuration of the invention to include a human JH6 gene segment as a mandatory human gene segment in their IgH locus design.
- a human JH6 gene segment as a mandatory human gene segment in their IgH locus design.
- Several different naturally- occurring human JH6 variants are known (eg, JH6*01 to *04 as well as others; I MGT nomenclature). The inventors considered this when deciding upon which human JH6 variant should be included in the transgenic IgH locus design.
- FIG. 7 An alignment of some human JH6 variants is shown in Figure 7 (from www.imgt.org: dashes indicate identical nucleotides; nucleotide changes versus the *01 variant are shown by underlined nucleotides and corresponding amino acid changes are shown by underlined amino acids; Genbank accession numbers (release 185.0) are shown prefixed by J, X, M or A).
- the inventors used sequencing of human genomic DNA samples, inspection of public IgH DNA databases as well as informed choices on the basis of variant sequences as means to arrive at a rational choice of which JH6 variant to use.
- the 1000 Genomes database uses human JH6*03 as the reference sequence, which would be a possible choice for the skilled person wishing to construct a transgenic IgH locus.
- position 6 in JH6*03 is a tyrosine (Y) encoded by a TAC codon
- Y tyrosine
- G glycine
- GGT codon the glycine being present as a YYG motif, forming part of a larger YYGXDX motif.
- YYG and YYGXDX motifs are conserved across many vertebrate species (see Figures 7 & 8). This suggested to the inventors, therefore, that preservation of this motif might be desirable, which could guide the choice of JH6 variant for use in the present invention.
- AID mutation hotspots the cytidine being the substrate of AID
- these hotspots being the underlined motifs in the previous sentence.
- the inventors considered the impact of this and in doing so they considered possible mutants created by AID activity at the cytidine.
- JH6*02 decided specifically to use human JH6*02 as the mandatory human JH6 for their IgH locus design.
- JH6*01 was rejected as the mandatory JH6 gene segment since the nucleotide sequence GGG CAA (encoding G and Q) contains a GGCA motif which is an AID recognition hotspot.
- the inventors realised that JH6*04 also contains such a motif due to the presence of the sequence GGC AAA encoding G and K (positions 11 and 12 respectively).
- the *02 variant has a C instead of a G that is in the *01 variant, the C desirably being a synonymous change (ie, not changing the encoded amino acid sequence around the CDR3/FW4 junction) and also this does not provide a GGCA AID hotspot motif.
- the inventors therefore, decided that the mandatory JH6 should have this C base and this too pointed them to using the human JH6*02 variant.
- the only JH6 species included in the locus or genome is human JH6*02.
- the inventors obtained 9 anonymised DNA samples from cheek swabs of 9 consenting human adults. Sequencing was performed on IgH locus DNA to confirm natural JH6 variant usage. It was found that the genome of all 9 humans contained a JH6*02 variant gene segment. In 7 out of the 9 humans, the genome was homozygous for JH6*02 (ie, each chromosome 14 had JH6*02 as its JH6 gene segment in the IgH locus). The inventors also inspected the publicly-available sequence information from the genomes of well-known scientists Craig Venter and Jim Watson. Both of these genomes contain JH6*02 too. This indicated to the inventors that this variant is common in humans.
- the inventors constructed transgenic JH6*02-containing IgH loci in ES cells, generated transgenic non-human vertebrates from the ES cells (both naive and immunised with a range of different target antigen types), isolated antibodies and heavy chain sequences based on JH6*02 as well as B-cells expressing these and made hybridomas expressing antigen-specific antibodies that are based on the chosen JH6*02 variant.
- the chosen variant was preferably used over other JH gene segments in all settings (naive, immunised and antigen-specific) for the production of HCD 3 of at least 20 amino acids.
- the present invention provides an IgH locus including human JH6*02 (IMGT nomenclature) as a mandatory JH gene segment.
- the locus comprises non-human vertebrate (eg, mouse or rat) constant region gene segments downstream (ie, 3' of) the human JH6*02; and one or more VH gene segments (eg, a plurality of human VH gene segments) and one or more D gene segments (eg, a plurality of human D gene segments) upstream of (ie, 5' of) the human JH6*02.
- the locus is comprised by a vector (eg, a DNA vector, eg, a yeast artificial chromosome (YAC), BAC or PAC).
- Such a vector eg, YAC
- a non-human vertebrate eg, mouse or rat
- standard techniques eg, pronuclear injection
- IgH chains comprising at least one chain whose variable domain is a product of the recombination of human JH6*02 with a VH and a D gene segment.
- the locus (eg, with a completely human, rat or mouse constant region, or a human/mouse chimaeric constant region) can be provided in the genome of a non-human vertebrate (eg, mouse or rat) cell.
- the cell is an ES cell or an antibody-producing cell (eg, an isolated B-cell, an iPS cell or a hybridoma).
- the invention provides a non-human vertebrate (eg, a mouse or a rat) comprising an IgH locus of the invention which comprises a human JH6*02 gene segment, wherein the locus can express an IgH chain whose variable domain is a product of the recombination of human JH6*02 with a VH and a D gene segment.
- a non-human vertebrate eg, a mouse or a rat
- the locus can express an IgH chain whose variable domain is a product of the recombination of human JH6*02 with a VH and a D gene segment.
- the inventors have successfully produced such mice which produce such IgH chains with VH domains based on human JH6*02.
- the inventors isolated and sequenced IgH chains from the mice before (naive) and after (immunised) exposure to a range of target antigens and confirmed by comparison to IMGT IgH gene segment sequences that the isolated chains (and antibodies containing these) were produced based on JH6*02. Such chains were found in naive mice, as well as in antigen-specific antibodies from immunised mice.
- B-cells were isolated from immunised mice, wherein the B-cells express antibodies based on JH6*02 and hybridomas were generated from the B-cells, the hybridomas expressing antigen-specific antibodies based on JH6*02.
- the inventors therefore, provided the locus, vertebrate, cell and hybridoma of the invention based on the use of human JH6*02 and showed that antibodies based on JH6*02 and B-cells expressing these can be successfully produced and isolated following immunisation of the vertebrates, corresponding hybridomas being a good source of antibodies whose VH domains are based on JH6*02, eg for administration to a patient, eg, for human medicine. Furthermore, it was found possible to produce and isolated antigen-specific antibodies whose VH domains are based on JH6*02 and which had a relatively long HCDR3 (eg, 20 amino acids).
- a non-human vertebrate (optionally a mouse or a rat) or vertebrate cell whose genome comprises an immunoglobulin heavy chain locus comprising human gene segment JH6*02, one or more VH gene segments and one or more D gene segments upstream of a constant region; wherein the gene segments in the heavy chain locus are operably linked to the constant region thereof so that the mouse is capable of producing an antibody heavy chain produced by recombination of the human JH6*02 with a D segment and a VH segment.
- the invention provides
- the locus comprises a human JH6, eg, JH6*02.
- the invention also provides a HCDR3, VH domain, antibody heavy chain or antibody having a HCDR3 size of at least 20 amino acids.
- the HCDR3 or VH domain (or VH domain of the heavy chain or antibody) comprises mouse AID-pattern somatic hypermutations and/or mouse dTd-pattern mutations. This can be provided, for example, wherein VH domain is produced in a mouse comprising mouse AID and/or mouse TdT (eg, endogenous AID or TdT). See also Annu. Rev. Biochem. 2007. 76:1-22; Javier M. Di noisya and Michael S.
- the vertebrate is naive. In another embodiment, the vertebrate instead is immunised with a target antigen.
- the vertebrate or cell mentioned below is capable of so producing an antibody heavy chain upon immunisation with a target antigen.
- the vertebrate is an immunised vertebrate that produces antibody heavy chains specific for a target antigen and wherein the variable domains of the heavy chains are the product of recombination between a VH, D and JH6*02.
- the D is selected from human D3-3, D2-15, D3-9; D4-17; D3-10; D2-2; D5-24; D6-19; D3-22; D6-13; D5-12; Dl-26; Dl-20; D5-18; D3-16; D2-21; Dl-14; D7-27; Dili D6-25; D2-14 and D4-23 (eg, selected from D3-9*01; D4-17*01; D3-10*01; D2-2*02; D5- 24*01; D6-19*01; D3-22*01; D6-13*01; D5-12*01; Dl-26*01; Dl-20*01; D5-18*01; D3-16*02; D2-21*02; Dl-14*01; D7-27*02; Dl-l*01; D6-25*01; D2-15*01; and D4-23*01).
- the D is human D3-9 or D3-10.
- the HCD 3 length is at least
- the genome comprises additional human JH gene segments (eg, JH2, 3, 4 and 5 gene segments).
- the genome comprises an immunoglobulin light chain locus comprising one or more human V gene segments and one or more human J gene segments upstream of a constant region (eg, a human or a mouse lambda or kappa constant region).
- a constant region eg, a human or a mouse lambda or kappa constant region
- the locus comprises control elements, such as an ⁇ and 5 ⁇ between the J gene segment(s) and the constant region as is known by the skilled person.
- control elements such as an ⁇ and 5 ⁇ between the J gene segment(s) and the constant region as is known by the skilled person.
- a mouse ⁇ and 5 ⁇ is included in the heavy chain locus between the JH6*02 and the constant region (ie, in 5' to 3' order the locus comprises the JH6*02, ⁇ and 5 ⁇ and constant region).
- the ⁇ and 5 ⁇ are ⁇ and 5 ⁇ of a mouse 129-derived genome (eg, a 129Sv-derived genome, eg, 129Sv/EV (such as 129S7Sv/Ev (such as from AB2.1 or AB2.2 cells obtainable from Baylor College of Medicine, Texas, USA) or 129S6Sv/Ev))); in another example, the ⁇ and 5 ⁇ are ⁇ and 5 ⁇ of a mouse C57BL/6 -derived genome.
- the locus can be constructed in the IgH locus of the genome of a cell selected from AB2.1, AB2.2, VGF1, CJ7 and FH14.
- VGF1 cells were established and described in Auerbach W, Dunmore JH, Fairchild-Huntress V, et al; Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques 2000; 29:1024-8, 30, 32, incorporated herein by reference.
- the constant region (or at least a C ⁇ ; or C ⁇ and gamma constant regions thereof) is a constant region (or C ⁇ ; or C ⁇ and gamma constant regions thereof) is of a genome described in the paragraph immediately above.
- JH6*02 and other human DNA sequences will be readily apparent to the skilled person.
- a DNA sample from a consenting human donor (eg, a cheek swab sample as per the Example herein) from which can be obtained suitable DNA sequences for use in constructing a locus of the invention.
- Other sources of human DNA are commercially available, as will be known to the skilled person.
- the skilled person is able to construct gene segment sequence by referring to one or more databases of human Ig gene segment sequences disclosed herein.
- the immunised vertebrate produces an antibody heavy chain specific for a target antigen and wherein the variable domain of the heavy chain is the product of recombination between a VH, D and JH6*02 and wherein the HCDR3 length is at least 20 amino acids (eg, 20, 21, 23 or 24).
- a non-human vertebrate cell (optionally a mouse cell or a rat cell) whose genome comprises an immunoglobulin heavy chain locus comprising human gene segment JH6*02, one or more VH gene segments and one or more D gene segments upstream of a constant region; wherein the gene segments in the heavy chain locus are operably linked to the constant region thereof for producing (eg, in a subsequent progeny cell) an antibody heavy chain produced by recombination of the human JH6*02 with a D segment and a VH segment.
- the cell of clause 3 which is an ES cell capable of differentiation into a progeny antibody- producing cell that expresses said heavy chain.
- the heavy chain locus comprises a human JH6*02 recombination signal sequence (RSS) operably connected 5' to the JH6*02 gene segment.
- RSS recombination signal sequence
- the native RSS-JH6*02 sequence can be used to advantageously maintain the natural pairing between RSS and theis JH gene segment.
- the following sequence is used:- ggtttttgtggggteaggatggacattctgccattgtgattactactactactacggtatggacgtctggggccaagggaccacgg ⁇ tctcctcag (SEQ ID NO: 238)
- RSSs have a common architecture: 9mer (eg, first underlined sequence above) followed by a 22bp spacer and then a 7mer (eg, second underlined sequence above). Spacers are 23bp +/- 1 normally, while the 9 and 7mer are more conserved.
- JH6*02 is the only JH6-type gene segment in the genome.
- the locus comprises one, more or all human D gene segments D3-9; D4-17; D3-10; D2-2; D5-24; D6-19; D3-22; D6-13; D5-12; Dl-26; Dl-20; D5-18; D3-16; D2-21; Dl-14; D7-27; Dl-1; D6-25; D2-14; and D4-23.
- the locus comprises one, more or all of human D gene segments D3-9*01; D4- 17*01; D3-10*01; D2-2*02; D5-24*01; D6-19*01; D3-22*01; D6-13*01; D5-12*01; Dl-26*01; Dl-20*01; D5-18*01; D3-16*02; D2-21*02; Dl-14*01; D7-27*02; Dl-l*01; D6-25*01; D2-15*01; and D4-23*01.
- the vertebrate or cell of clause 10, wherein the locus comprises one, more or all human D gene segments D3-9, D3-10, D6-19, D4-17, D6-13, D3-22, D2-2, D2-25 and D3-3.
- the locus comprises one, more or all human D gene segments D3-9, D3-10, D6- 19, D4-17, D6-13 and D3-22 (for example one, more or all of D3-9*01, D3-10*01, D6-19*01, D4- 17*01, D6-13*01 and D3-22*01).
- D segments were found in naive repertoires to be productive in recombination with human JH6*02 to produce HCDR3s of at least 20 amino acids in length.
- the locus comprises one, more or all human D gene segments D3-10, D6-19 and Dl-26 (for example, one, more or all of D3-10*01, D6-19*01 and Dl-26*01). These D segments were found in immunised repertoires to be productive in recombination with human JH6*02 to produce HCDR3s of at least 20 amino acids in length.
- the locus comprises one, more or all human D gene segments D3-9 and D3-10 (for example, one, more or all of D3-9*01 and D3-10*01). These D segments were found in antigen-specific repertoires to be productive in recombination with human JH6*02 to produce HCDR3s of at least 20 amino acids in length.
- the 3'-most D gene segment is D7-27.
- the locus comprises all of human D gene segments from Dl-1 to D7-27 as present in a germline human IgH locus (eg, as shown in the IMGT database).
- the JH6*02 is in human germline configuration with respect to one, more or all of the ⁇ , 5 ⁇ and constant region (eg, C ⁇ ).
- the locus comprises one, more or all of IGHV gene segments selected from V3-21, V3-13, V3-7, V6-1, Vl-8, Vl-2, V7-4-1, Vl-3, Vl-18, V4-4, V3-9, V3-23, V3-11 and V3-20.
- the locus comprises one, more or all human IGHV gene segments V3-21, V3-13, V3-7, V6-1, Vl-8, Vl-2, V7-4-1, Vl-3, Vl-18, V4-4, V3-9, V3-23 (for example, one, more or all of V3-21*03, V3-13*01, V3-7*01, V6-l*01, Vl-8*01, Vl-2*02, V7-4-l*01, Vl-3*01, Vl-18*01, V4- 4*01, V3-9*01 and V3-23*04).
- These segments were found in naive repertoires to be productive in recombination with human JH6*02 to produce HCD 3s of at least 20 amino acids in length.
- the locus comprises one, more or all human IGHV gene segments V3-7, V3-11 and V4-4 (for example, one, more or all of V3-7*01, V3-ll*01 and V4-4*02). These segments were found in immunised repertoires to be productive in recombination with human JH6*02 to produce HCDR3s of at least 20 amino acids in length.
- the locus comprises one, more or all human IGHV gene segments V4-4, Vl-8, V3- 9, V3-11 and V3-20 (for example, one, more or all of V4-4*02, Vl-8*01, V3-9*01, V3-ll*01 and V3-20 (eg, *d01). These segments were found in antigen-specific repertoires to be productive in recombination with human JH6*02 to produce HCDR3s of at least 20 amino acids in length.
- the locus comprises one, more or all of human D3-9*01, D3-10*01, D6-19*01, D6-13*01, Dl-26*01, IGHV1-8*01, IGHV4-61*01, IGHV6- 1*01, IGHV4-4*02, IGHV1-3*01, IGHV3-66*03, IGHV3-7*01 and IGHV3-9*01.
- locus comprises one, more or all of human IGHV1-8*01, D3-9*01 and D3- 10*01. These gene segments were productive with JH6*02 to produce HCDR3s of at least 20 amino acids in more than 10 antibodies.
- An antibody-producing cell (eg, a B-cell) that is a progeny of the cell of any one of clauses 3 to 14, wherein the antibody-producing cell comprises a heavy chain locus comprising a rearranged variable region produced by recombination of human JH6*02 with a D segment and a VH segment (eg, JH6*02 with human VH3-11 (eg, VH3-11*01) and D3-9; VH3-20 (eg, VH3-20*01) and D3-10; VH4-4 (eg, VH4-4*02) and D3-10; VH3-9 (eg, VH3-9*01) and D3-10; or VH1-8 (eg, VH1-8*01) and D310).
- a heavy chain locus comprising a rearranged variable region produced by recombination of human JH6*02 with a D segment and a VH segment
- VH3-11 eg, VH3-11*01
- variable region would be the product of in vivo somatic hypermutation in a non-hman vertebrate or cell of the invention.
- a target antigen-specific antibody comprising a heavy chain that comprises a rearranged variable region produced by recombination of human JH6*02 with
- variable region would be the product of in vivo somatic hypermutation in a non-hman vertebrate or cell of the invention
- the HCDR3 length is at least 21, 22, 23, 24, 25, 26, 27, 28, 29 or 30 amino acids. Additionally, in one example the length is no more than 35, 34, 33, 32 or 31 amino acids. For example, the HCDR3 length is 20, 21, 22, 23 or 24 amino acids.
- a heavy chain (eg, comprised by an antibody) isolated from a vertebrate of any one of clauses 1, 2, 5 to 14 and 17 to 21 wherein the heavy chain comprises a HCD 3 of at least 20 amino acids.
- the heavy chain is chimaeric where the C region is non-human.
- the heavy chain is human where the C region is human.
- a heavy chain eg, comprised by an antibody whose VH variable domain is identical to the VH variable domain of the heavy chain of clause 22 or 23, and which comprises a human constant region or a human-mouse chimaeric constant region (eg, CHI is human and the other constant domains are mouse).
- a method for producing a heavy chain, VH domain or an antibody specific to a target antigen comprising immunizing a non-human vertebrate according to any one of clauses 1, 2, 5 to 14 and 17 to 21 with the antigen and isolating the heavy chain, VH domain or an antibody specific to a target antigen or a cell producing the heavy chain, VH domain or an antibody, wherein the heavy chain, VH domain or an antibody comprises a HCDR3 that is derived from the recombination of human JH6*02 with a VH gene segment and a D gene segment. 7.
- a method for producing a human heavy chain or antibody comprising carrying out the method of clause 26, wherein the constant region of the locus is a non-human vertebrate (eg, mouse or rat) constant region, and then replacing the non-human constant region of the isolated heavy chain or antibody with a human constant region (eg, by engineering of the nucleic acid encoding the antibody).
- the constant region of the locus is a non-human vertebrate (eg, mouse or rat) constant region, and then replacing the non-human constant region of the isolated heavy chain or antibody with a human constant region (eg, by engineering of the nucleic acid encoding the antibody).
- the HCD 3 length is at least 20 amino acids as herein described.
- a vector eg, a CHO cell or HEK293 cell vector comprising the nucleic acid of clause 30;
- a host cell eg, a CHO cell or HEK293 cell.
- a pharmaceutical composition comprising the antibody, heavy chain or VH domain (eg, comprised by an antibody) of any one of clauses 22 to 25 and 28, together with a
- pharmaceutically-acceptable excipient eg, a further antigen-specific variable domain, heavy chain or antibody.
- a medicament eg, a further antigen-specific variable domain, heavy chain or antibody.
- the antibody, heavy chain or VH domain eg, comprised by an antibody of any one of clauses 22 to 25 and 28 for use in medicine (eg, human medicine).
- the locus comprises the following human VH gene segments
- the locus comprises the following human VH gene segment
- the locus comprises the following human JH gene segment variants
- the locus comprises the following human D gene segments
- the present invention provides in a fifth confieuration:-
- Constant Regions Tailored to Human Use & Antibody Humanisation Additional rational design and bioinformatics has led the inventors to realise that specific human constant region variants are conserved across many diverse human populations. The inventors realised that this opens up the possibility of making a choice to humanise antibodies, chains and variable domains by using such specific constant regions in products, rather than arbitrarily choosing the human constant region (or a synthetic version of a human constant region). This aspect of the invention also enables one to tailor antibody-based drugs to specific human ethnic populations, thereby more closely matching drug to patient (and thus disease setting) than has hitherto been performed.
- antibodies are humanised with an arbitrary choice of human constant region (presumably derived from one (often unknown) ethnic population or non-naturally occurring) that does not function as well in patients of a different human ethnic population. This is important, since the constant region has the major role in providing antibody effector functions, eg, for antibody recycling, cellular and complement recruitment and for cell killing.
- human IgG sub-types IgGl, lgG2, gG3 and lgG4 exhibit differential capacity to recruit immune functions, such as antibody-dependent cellular cytotoxicity (ADCC, e.g., IgGl and lgG3), antibody-dependent cellular phagocytosis (ADCP, e.g., IgGl, lgG2, lgG3 and lgG4), and complement dependent cytotoxicity (CDC, e.g., IgGl, lgG3).
- ADCC antibody-dependent cellular cytotoxicity
- ADCP antibody-dependent cellular phagocytosis
- CDC complement dependent cytotoxicity
- Sub-type-specific engagement of such immune functions is based on selectivity for Fc receptors on distinct immune cells and the ability to bind Clq and activate the assembly of a membrane attack complex (MAC).
- MAC membrane attack complex
- FcYRI FcYRI
- FcYRIIa/b/c FcYRIIIa/b
- IgGl IgGl
- lgG2 restricted to the FcYRIIa 131H polymorphism
- lgG4 only has measurable affinity for FcYRI.
- the key contact residues for receptor binding have been mapped to the amino acid residues spanning the lower hinge and CH2 region.
- standard protein engineering techniques some success in enhancing or reducing the affinity of an antibody preparation for Fc receptors and the Clq component of complement has been achieved.
- lgG2 is least capable of binding the family of Fc receptors.
- lgG2 as the starting point, efforts have been made to find a mutant with diminished effector functions but which retains FcRn binding, prolonged stability, and low immunogenicity.
- Improved mutants of this nature may provide improved antibody therapeutics with retained safety.
- Human IgGl therapeutic antibodies that bind to cell surface targets are able to engage effector cells that may mediate cell lysis of the target cell by antibody-dependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC). These mechanisms occur through interaction of the CH2 region of the antibody Fc domain to Fcy receptors on immune effector cells or with Clq, the first component of the complement cascade.
- ADCC antibody-dependent cellular cytotoxicity
- CDC complement dependent cytotoxicity
- Table 19 shows the activities of different human gamma sub-types.
- the skilled person may choose accordingly to promote or dampen-down activity depending upon the disease setting in humans of interest.
- use of a human gamma-1 constant region is desirable when one wishes to isolated totally human heavy chains and antibodies that have relatively high complement activation activity by the classical pathway and FcYRl recognition in human patients. See also Mol Immunol. 2003 Dec;40(9):585-93; "Differential binding to human Fcgamma Rl la and Fcgamma Rllb receptors by human IgG wild type and mutant antibodies"; Armour KL et al, which is incorporated herein by reference.
- lgG2 constant regions are well suited to producing antibodies and heavy chains according to the invention for binding to cytokines or soluble targets in humans, since lgG2 is essentially FcYRl, Ill- silent, FcYRl la-active and has little Complement activity.
- IgGl constant regions have wide utility for human therapeutics, since IgGl antibodies and heavy chains are FcYRl, II, III- active and have complement activity. This can be enhanced by using a human gamma-1 constant region that has been activated by engineering as is known in the art.
- the work of the inventors has therefore identified a collection of human constant region of different isotypes from which an informed choice can be made when humanising chimaeric antibody chains (or conjugating V domains, such as dAbs or Camelid VHH, to constant regions).
- the collection was identified on the basis of bioinformatics analysis of the 1000 Genomes database, the inventors selecting constant region variants that are frequently occurring across several human ethnic populations, as well as those that appear with relatively high frequency within individual populations (as assessed by the number of individuals whose genomes comprise the variant).
- the inventors have provided a collection of human constant region variants that are naturally-occuring and which can be used when rationally designing antibodies, heavy chains and other antibody-based formats that bear a human constant region.
- this is useful when humanising chimaeric heavy chains to produce totally human chains in which both the variable and constant regions are human. This is useful for compatibility with human patients receiving antibody-based drugs.
- a method of producing an antibody heavy chain comprising
- an antigen-specific heavy chain variable domain eg, VH (such as a human VH or dAb) or VHH or a humanised heavy chain variable domain
- variable domain (b) combining the variable domain with a human heavy chain constant region to produce an antibody heavy chain comprising (in N- to C-terminal direction) the variable domain and the constant region;
- the human heavy chain constant region is an IGHAref, IGHAla, IGHA2a, IGHA2b, IGHGlref, IGHG2ref, IGHG2a, IGHG3ref, IGHG3a, IGHG3b, IGHG4ref, IGHG4a, IGHDref, IGHEref, IGHMref, IGHMa or IGHMb constant region.
- Step (b) can be carried out, eg, using recombinant DNA technology using the corresponding nucleotide sequences.
- genomic DNA or equivalent ie, having introns and exons and optionally also 5' UT sequences, eg, with native or a non-native leader sequence
- genomic DNA or equivalent ie, having introns and exons and optionally also 5' UT sequences, eg, with native or a non-native leader sequence
- an intronless sequence can be used, for example any of the "CDS" sequences disclosed as SEQ ID NO: 365 onwards herein (eg, with native or a non-native leader sequence).
- the human heavy chain constant region is an IGHAref constant region.
- the human heavy chain constant region is an IGHAla constant region.
- the human heavy chain constant region is an IGHA2a constant region.
- the human heavy chain constant region is an IGHA2b constant region.
- the human heavy chain constant region is IGHGlref constant region.
- the human heavy chain constant region is an IGHG2ref constant region.
- the human heavy chain constant region is an IGHG2a constant region.
- the human heavy chain constant region is an IGHG3ref constant region.
- the human heavy chain constant region is an IGHG3a constant region.
- the human heavy chain constant region is an IGHG3b constant region.
- the human heavy chain constant region is an IGHG4ref constant region.
- the human heavy chain constant region is an IGHG4a constant region.
- the human heavy chain constant region is an IGHDref constant region.
- the human heavy chain constant region is an IGHEref constant region.
- the human heavy chain constant region is an IGHMref constant region.
- the human heavy chain constant region is an IGHMa constant region.
- the human heavy chain constant region is an IGHMb constant region.
- a derivative eg, a mutant or conjugate
- a toxic payload can be conjugated (eg, for oncology applications).
- one or more mutations can be introduced, as is known in the art, to inactivate or enhance Fc effector function.
- variable domain is a human variable domain.
- a human variable domain is, for example, the product of recombination in a transgenic non- human vertebrate of human VH, D and JH gene segments.
- the variable domain is identified using in vitro display technology from a human VH library, eg, using phage display, ribosome display or yeast display, as is known in the art.
- the variable domain is a humanised variable domain, eg, comprising human frameworks with non-human (eg, mouse or rat) CD s). Humanisation technology is conventional in the art, and will be readily known to the skilled person.
- variable domain has previously been selected from a non-human vertebrate that has been immunised with the antigen.
- the vertebrate (such as a mouse or rat) genome comprises a chimaeric heavy chain locus comprising a human variable region (human V, D and JH gene segments) operably connected upstream of a non-human vertebrate constant region so that the locus is able to rearrange for the expression of heavy chains comprising human variable domains and non- human vertebrate constant regions.
- a human variable region human V, D and JH gene segments
- variable domain is selected using an in vitro technology such as phage display, ribosome display or yeast display.
- the variable domain may be displayed with or without an constant region, provided that it is later combined with a human constant region as per the invention.
- an expression vector (Eg, a mammalian expression vector, such as a CHO or HEK293 vector) comprising a nucleotide sequence encoding the constant region; inserting a nucleotide sequence encoding the variable domain into the vector 5' of the constant region sequence; inserting the vector into a host cell and expressing the heavy chain by the host cell; the method further comprising isolating a heavy chain (eg, as part of an antibody) comprising the variable domain and the human constant region.
- an expression vector Eg, a mammalian expression vector, such as a CHO or HEK293 vector
- the vector comprises regulatory elements sufficient to effect expression of the heavy chain when the vector is harboured by a host cell, eg, a CHO or HEK293 cell.
- An antibody comprising a human heavy chain, the heavy chain comprising a variable domain that is specific for an antigen and a constant region that is an IGHAref, IGHAla, IGHA2a, IGHA2b, IGHGlref, IGHG2ref, IGHG2a, IGHG3ref, IGHG3a, IGHG3b, IGHG4ref, IGHG4a, IGHDref, IGHEref, IGHMref, IGHMa or IGHMb constant region.
- a polypeptide comprising (in N- to C- terminal direction) a leader sequence, a human variable domain that is specific for an antigen and a human constant region that is an IGHAref, IGHAla, IGHA2a, IGHA2b, IGHGlref, IGHG2ref, IGHG2a, IGHG3ref, IGHG3a, IGHG3b, IGHG4ref, IGHG4a, IGHDref, IGHEref, IGHMref, IGHMa or IGHMb constant region; wherein (i) the leader sequence is not the native human variable domain leader sequence (eg, the leader sequence is another human leader sequence or a non-human leader sequence); and/or (ii) the variable domain comprises mouse AID-pattern somatic mutations or mouse terminal deoxynucleotidyl transferase (TdT)- pattern junctional mutations.
- TdT mouse terminal deoxynucleotidyl
- the promoter sequence is a human IGK 3-15 promoter.
- variable domain comprises mouse AID-pattern somatic mutations and/or mouse terminal deoxynucleotidyl transferase (TdT)- pattern junctional mutations.
- one way, in any aspect of this configuration of the invention, to provide mouse AID-pattern somatic mutations and/or mouse terminal deoxynucleotidyl transferase (TdT)- pattern junctional mutations is to select a variable domain from a non-human vertebrate or cell.
- a vertebrate or cell as disclosed herein.
- a vector eg, a CHO cell or HEK293 cell vector
- comprising the nucleic acid of aspect 8, 9 or 10; optionally wherein the vector is in a host cell eg, a CHO cell or HEK293 cell.
- a pharmaceutical composition comprising the antibody or polypeptide of any one of aspects 6, 7 and 10, together with a pharmaceutically-acceptable excipient, diluent or a medicament (eg, a further antigen-specific variable domain, antibody chain or antibody).
- a pharmaceutically-acceptable excipient e.g, a further antigen-specific variable domain, antibody chain or antibody.
- the antibody or polypeptide of any one of aspects 6, 7 and 10 for use in treating and/or preventing a medical condition in a human patient.
- IGHAla constant region and the human population is selected from any population number 1-14;
- IGHA2a constant region and the human population is selected from any population number 1-14;
- (iV) IGHG2a constant region and the human population is selected from any population number 1-9 and 11-13;
- IGHG3b constant region and the human population is selected from any population number 1-8 and 11-13;
- IGHG4a constant region and the human population is selected from any population number 1-9 and 11-13;
- IGHMa constant region and the human population is selected from any population number 1-14; or
- IGHMb constant region and the human population is selected from any population number 1-14; Wherein the populations are numbered as follows (population labels being according to 1000
- a vector (eg, a CHO cell or HEK293 cell vector) comprising a IGHGlref, IGHG2ref, IGHG2a,
- IGHG3ref IGHG3a, IGHG3b, IGHG4ref or IGHG4a constant region nucleotide sequence that is 3' of a cloning site for the insertion of a human antibody heavy chain variable domain nucleotide sequence, such that upon insertion of such a variable domain sequence the vector comprises (in 5' to 3' direction) a promoter, a leader sequence, the variable domain sequence and the constant region sequence so that the vector is capable of expressing a human antibody heavy chain when present in a host cell.
- the present invention provides in a Sixth confieuration:-
- the inventors' analysis has revealed groupings of naturally-occurring human antibody gene segment variants as set out in Table 13 and Table 14. This revealed the possibility of producing transgenic genomes in non-human vertebrates and cells wherein the genomes contain more than the natural human complement of specific human gene segments. In one example, this can be achieved by providing more than the natural human complement of a specific gene segment type on one or both of the respective Ig locus (eg, one or both chromosomes harbouring IgH in a mouse genome or mouse cell genome).
- this configuration of the invention provides the following (as set out in numbered paragraphs):-
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 3 human variable region gene segments of the same type (eg, at least 3 human VH6-1 gene segments, at least 3 human J H6 gene segments, at least 3 human VK1-39 gene segments, at least 3 human D2-2 gene segments or at least 3 human JKI gene segments), wherein at least two of the human gene segments are variants that are not identical to each other.
- the genome comprises a variable region that comprises V, D and J gene segments (for the variable region of a heavy chain locus) or V and J gene segments (for the variable region of a light chain locus) upstream of a constant region for expression of heavy or light chains respectively.
- the skilled person can choose to provide more than the wild type human complement of a specific gene segment type by providing several copies of one variant type of the human gene segment.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 3 human variable region gene segments of the same type (eg, at least 3 human VH6-1 gene segments, at least 3 human J H6 gene segments, at least 3 human VK1-39 gene segments, at least 3 human D2-2 gene segments or at least 3 human JKI gene segments), wherein the human gene segments are identical variants.
- the genome comprises a variable region that comprises V, D and J gene segments (for the variable region of a heavy chain locus) or V and J gene segments (for the variable region of a light chain locus) upstream of a constant region for expression of heavy or light chains respectively.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 3 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) cis at the same Ig locus.
- the skilled person can choose to provide more than the wild type human complement of a specific gene segment type by providing several copies of one variant type of the human gene segment.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 non-endogenous variable region gene segments of the same variant type (eg, at least 2 human JH6*02 gene segments) cis at the same Ig locus.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) trans at the same Ig locus; and optionally a third human gene segment of the same type, wherein the third gene segment is cis with one of said 2 different gene segments.
- the skilled person can choose to provide more than the wild type human complement of a specific gene segment type by providing several copies of one variant type of the human gene segment.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different human variable region gene segments of the same variant type (eg, at least 2 human JH6*02 gene segments) trans at the same Ig locus; and optionally a third human gene segment of the same variant type, wherein the third gene segment is cis with one of said 2 different gene segments.
- a population of non-human vertebrates comprising a repertoire of human variable region gene segments, wherein the plurality comprises at least 2 human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), a first of said different gene segments is provided in the genome of a first vertebrate of the population, and a second of said different gene segments being provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second gene segment.
- the plurality comprises at least 2 human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments)
- a first of said different gene segments is provided in the genome of a first vertebrate
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), wherein the gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 3 human variable region gene segments of the same type (eg, at least 3 human VH6-1 gene segments, at least 3 human J H6 gene segments, at least 3 human VK1-39 gene segments, at least 3 human D2-2 gene segments or at least 3 human JKI gene segments), wherein at least two of the human gene segments are variants that are not identical to each other.
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) cis at the same Ig locus.
- a non-human vertebrate eg, a mouse or rat
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments) trans at the same Ig locus; and optionally a third human gene segment of the same type, wherein the third gene segment is cis with one of said 2 different gene segments.
- a non-human vertebrate eg, a mouse or rat
- a method of providing an enhanced human immunoglobulin variable region gene segment repertoire comprising providing a population of non-human vertebrates (eg, a mouse or rat) comprising a repertoire of human variable region gene segments, wherein the method comprises providing at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), wherein a first of said different gene segments is provided in the genome of a first vertebrate of the population, and a second of said different gene segments is provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second gene segment.
- a population of non-human vertebrates eg, a mouse or rat
- the method comprises providing at least 2 different human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human J H6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human JKI gene segments), wherein the gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- the gene segments are derived from the genome sequence of two or more different human individuals; optionally wherein the different human individuals are from different human populations.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 human variable region gene segments of the same type (eg, at least 2 human VH6-1 gene segments, at least 2 human JH6 gene segments, at least 2 human VK1-39 gene segments, at least 2 human D2-2 gene segments or at least 2 human J KI gene segments), wherein the gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations; optionally wherein at least 2 or 3 of said different gene segments are provided at the same Ig locus in said genome.
- a non-human vertebrate eg, a mouse or rat
- each of said gene segments occurs in 10 or more different human populations.
- each of said gene segments has a human frequency of 5% or greater (eg, 10, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, or 95% or greater).
- Frequency can, for example, be cumulative frequency in the 1000 Genomes database.
- each of said gene segments occurs in 10 or more different human populations.
- each of said gene segments occurs in the 1000 Genomes database in more than 50 individuals.
- each of said gene segments has a human frequency of 5% or greater (eg, 10, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, or 95% or greater); and (ii) occurs in 10 or more different human populations.
- Frequency can, for example, be cumulative frequency in the 1000 Genomes database.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising first and second human Ig locus gene segments of the same type (eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments), wherein the first gene segment is a gene segment selected from Table 14 (eg, IGHJ6-a) and the second gene segment is the corresponding reference sequence (eg, IGHJ6 ref; SEQ ID NO: 244).
- Table 14 lists commonly-occurring natural human variants. It can be seen that these occur across many human populations and thus usefully have wide applicability for human antibody- based drugs.
- the gene segments are provided as targeted insertions into an endogenous non- human vertebrate Ig locus.
- random integration eg, using YACs
- YACs YACs
- the genome comprises a variable region that comprises V, D and J gene segments (for the variable region of a heavy chain locus) or V and J gene segments (for the variable region of a light chain locus) upstream of a constant region for expression of heavy or light chains respectively.
- the invention enables the skilled person to select two or more different naturally-occurring human gene segment variants for combination into the genome of a non- human vertebrate or cell.
- a reference sequence need not be included. It may be desirable to use one or more rare gene segments to increase diversity of the repertoire. Additionally or alternatively, it may be desirable to include a mixture of frequent and rare variants of the same type to provide repertoire diversity. The variants may be chosen additionally or alternatively to tailor the gene segment inclusion to one or more specific human populations as indicated by the information provided in Table 13 or Table 14.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising first and second human Ig locus gene segments of the same type (eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments), wherein the gene segments are gene segments selected from Table 13 or Table 14; and optionally wherein one or more of the gene segments appears in Table 14 (eg, IGHJ6-a) or is a reference sequence (eg, IGHJ6 ref; SEQ. ID NO: 244).
- the gene segments are heavy chain gene segments and the non-human locus is an IgH locus.
- the gene segments are light chain (kappa or lambda) gene segments and the non-human locus is an IgL locus.
- the chromosomes are the same type (eg, both mouse chromosome 6 or rat chromosome 4).
- first gene segment is a gene segment selected from any one of Tables 1 to 7 and 9 to 14 (eg, selected from Table 13 or 14) and the second gene segment is the corresponding reference sequence.
- a population of non-human vertebrates comprising first and second human Ig locus gene segments of the same type (eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments), wherein the first gene segment is a gene segment selected from any one of Tables 1 to 7 and 9 to 14 (eg, Table 13 or 14) (eg, IGHJ6-a) and the second gene segment is the corresponding reference sequence (eg, SEQ. ID NO: 7), wherein the first gene segment is provided in the genome of a first vertebrate of the population, and the second gene segment is provided in the genome of a second vertebrate of the population.
- the gene segments are heavy chain gene segments and the non-human locus is an IgH locus.
- the gene segments are light chain (kappa or lambda) gene segments and the non-human locus is an IgL locus.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising first and second human Ig locus gene segments of the same type (eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments), wherein the first gene segment is a gene segment selected from any one of Tables 1 to 7 and 9 to 14 (eg, Table 13 or 14) (eg, IGHJ6-a) and the second gene segment is the corresponding reference sequence (eg, SEQ. ID NO: 7).
- first and second human Ig locus gene segments of the same type eg, first and second human JH6 gene segments; or first and second lgG2 gene segments; or first and second human J ⁇ 7 gene segments
- the first gene segment is a gene segment selected from any one of Tables 1 to 7 and 9 to 14 (eg, Table 13 or 14) (eg, IGHJ6-a) and the second
- a method of providing an enhanced human immunogolobulin gene segment repertoire comprising providing a population according to any one of paragraphs 30 to 33.
- the inventors identified gene segment variants from their analysis that are relatively prevalent in a small number of human populations, and not across many populations.
- the inventors realized that inclusion of one or more of such gene segments in the configurations of the invention (eg, in transgenic Ig loci, vertebrates and cells) would be useful for producing antibodies, Ig chains and variable domains that can address antigens (eg, disease- causing antigens or pathogens) to which the small number of human populations may become exposed.
- antigens eg, disease- causing antigens or pathogens
- Such products would be useful for treating and/or preventing disease or medical conditions in members of such a population.
- This aspect could also be useful for addressing infectious disease pathogens that may have been common in the small number of populations, but which in the future or relatively recently in evolution has become a more prevalent disease-causing pathogen in other human populations (ie, those not listed in Table 13 against the gene segment variant(s) in question).
- the inventors From the 1000 Genomes database the inventors have identified the gene segment variants listed in Table 20.
- one, more or all of the gene segments used in the present invention can be a gene segment listed in Table 20A, 20B, 20C or 20D.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 3 human JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6), wherein at least two of the human JH gene segments are variants that are not identical to each other.
- any cell of the invention is an isolated cell.
- An "isolated" cell is one that has been identified, separated and/or recovered from a component of its production environment (eg, naturally or recombinantly).
- the isolated cell is free of association with all other components from its production environment, eg, so that the cell can produce an antibody to an FDA-approvable or approved standard.
- Contaminant components of its production environment such as that resulting from recombinant transfected cells, are materials that would typically interfere with research, diagnostic or therapeutic uses for the resultant antibody, and may include enzymes, hormones, and other proteinaceous or non-proteinaceous solutes.
- the polypeptide will be purified: (1) to greater than 95% by weight of antibody as determined by, for example, the Lowry method, and in some embodiments, to greater than 99% by weight; (2) to a degree sufficient to obtain at least 15 residues of N-terminal or internal amino acid sequence by use of a spinning cup sequenator, or (3) to homogeneity by SDS-PAGE under non- reducing or reducing conditions using Coomassie blue or, preferably, silver stain. Ordinarily, however, an isolated cell will be prepared by at least one purification step.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 2 different non-endogenous JH gene segments (eg, human gene segments) of the same type (JH1, JH2, JH3, JH4, JH5 or JH6) cis at the same Ig (eg, IgH, eg, endogenous IgH, eg, mouse or rat IgH) locus.
- the genome comprises a human VH, D and JH repertoire comprising said different JH gene segments.
- the non-endogenous JH gene segments are non-mouse or non-rat, eg, human JH gene segments.
- one or more or all of the non-endogenous gene segments are synthetic.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 2 different human JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6) trans at the same Ig (eg, IgH, eg, endogenous IgH, eg, mouse or rat IgH) locus; and optionally a third human JH gene segments of the same type, wherein the third JH is cis with one of said 2 different JH gene segments.
- a population of non-human vertebrates comprising a repertoire of human JH gene segments, wherein the plurality comprises at least 2 different human JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6), a first of said different JH gene segments is provided in the genome of a first vertebrate of the population, and a second of said different JH gene segments being provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second JH gene segment.
- non-human vertebrates eg, mice or rats
- the plurality comprises at least 2 different human JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6)
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B- cell
- having a genome comprising at least 2 different non-endogenous (eg, human) JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6), wherein the JH gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations (eg, 3, 4, 5 or 6 generations).
- the non-endogenous JH gene segments are human JH gene segments.
- one or more or all of the non-endogenous gene segments are synthetic.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 3 human JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6), wherein at least two of the human JH gene segments are variants that are not identical to each other.
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous (eg, human) JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6) cis at the same Ig (eg, IgH, eg, endogenous IgH, eg, mouse or rat IgH) locus).
- the non-endogenous JH gene segments are non-mouse or non-rat, eg, human JH gene segments.
- one or more or all of the non-endogenous gene segments are synthetic.
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different human JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6) trans at the same Ig (eg, IgH, eg, endogenous IgH, eg, mouse or rat IgH) locus; and optionally a third human JH gene segments of the same type, wherein the third JH is cis with one of said 2 different JH gene segments.
- JH1, JH2, JH3, JH4, JH5 or JH6 trans at the same Ig (eg, IgH, eg, endogenous IgH, eg, mouse or rat IgH) locus
- a third human JH gene segments of the same type wherein the third JH is cis with one of said 2 different JH gene segments.
- a method of providing an enhanced human immunoglobulin JH gene segment repertoire comprising providing a population of non-human vertebrates (eg, a mouse or rat) comprising a repertoire of human JH gene segments, wherein the method comprises providing at least 2 different human JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6), wherein a first of said different J H gene segments is provided in the genome of a first vertebrate of the population, and a second of said different JH gene segments is provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second JH gene segment.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous (eg, human) JH gene segments of the same type (JH1, JH2, JH3, JH4, JH5 or JH6), wherein the JH gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations (eg, 3, 4, 5, or 6 generations).
- the non-endogenous JH gene segments are human JH gene segments.
- one or more or all of the non-endogenous gene segments are synthetic.
- At least 2 or 3 of said different gene segments are provided cis at the same Ig locus in said genome.
- the JH gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations (eg, 3, 4, 5, or 6 generations).
- the JH gene segments are derived from the genome sequence of two or more different human individuals; optionally wherein the different human individuals are from different human populations.
- the individuals are not genetically related (eg, going back 3, 4, 5, or 6 generations).
- At least one of the different JH segments is a synthetic mutant of a human germline JH gene segment.
- the invention also provides a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate (eg, a mouse or rat), the method comprising providing the vertebrate with a genome comprising at least 2 human JH gene segments of the same type (JH1, JH2, J H3, JH4, JH5 or JH6), wherein the JH gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations (eg, 3, 4, 5, or 6 generations); optionally wherein at least 2 or 3 of said different gene segments are provided at the same IgH locus in said genome.
- a non-human vertebrate eg, a mouse or rat
- the genome comprises a substantially complete functional repertoire of human JH gene segment types supplemented with one, two or more human JH gene segments, wherein said substantially complete functional repertoire and the supplementary JH gene segments are not found together in the germline genome of a human individual.
- the population comprises a substantially complete functional repertoire of human JH gene segment types supplemented with one, two or more human JH gene segments, wherein said substantially complete functional repertoire and the supplementary JH gene segments are not found together in the germline genome of a human individual.
- a non-human vertebrate eg, a mouse or rat
- a non-human cell eg, an ES cell or a B-cell
- a population of non-human vertebrates comprising a substantially complete functional repertoire of human JH gene segment types supplemented with one, two or more human JH gene segments, wherein said substantially complete functional repertoire and the supplementary JH gene segments are not found together in the germline genome of a human individual.
- a population of non-human vertebrates eg, mice or rats
- comprising a substantially complete functional repertoire of human JH gene segment types supplemented with one, two or more human JH gene segments wherein said substantially complete functional repertoire and the supplementary JH gene segments are not found together in the germline genome of a human individual.
- At least one of said JH gene segments is SEQ ID NO: 1, 2, 3 or 4.
- at least one of said JH gene segments is SEQ ID NO: 1 and at least one, two or more of said supplementary JH gene segments is a variant according to any example above.
- at least one of said JH gene segments is SEQ ID NO: 2 and at least one, two or more of said supplementary JH gene segments is a variant according to any one of the examples above.
- at least one of said JH gene segments is SEQ ID NO: 2 and at least one, two or more of said supplementary JH gene segments is a variant according to any one of the examples above.
- the non-human vertebrate or vertebrate cell of the invention comprises a genome that comprises VH, D and JH gene repertoires comprising human gene segments, the JH gene repertoire (eg, a human JH gene segment repertoire) comprising a plurality of JH1 gene segments provided by at least 2 different JH1 gene segments in cis at the same Ig locus in said genome;
- the JH gene repertoire eg, a human JH gene segment repertoire
- JH gene segments are derived from the genome sequence of two or more different human individuals.
- said at least 2 different JH gene segments are human gene segments or synthetic gene segments derived from human gene segments.
- the Ig locus is a IgH locus, eg, an endogenous locus, eg, a mouse or rat IgH locus.
- the non-human vertebrate or vertebrate cell of the invention comprises a genome that comprises VH, D and JH gene repertoires comprising human gene segments, the JH gene repertoire (eg, a human JH gene segment repertoire) comprising
- JH gene segments are derived from the genome sequence of two or three different human individuals;
- said at least 3 different JH gene segments are human gene segments or synthetic gene segments derived from human gene segments.
- the Ig locus is a IgH locus, eg, an endogenous locus, eg, a mouse or rat IgH locus.
- the different human individuals are from different human populations.
- the individuals are not genetically related (eg, Going back 3, 4, 5 or 6generations).
- at least one of the different JH segments is a synthetic mutant of a human germline JH gene segment.
- the vertebrate or cell genome comprises human VH, D and JH gene repertoires, the JH gene repertoire (eg, a human JH gene repertoire) comprising
- JH1 gene segments provided by at least 2 different human JH1 gene segments, optionally in cis at the same Ig locus in said genome;
- JH2 gene segments provided by at least 2 different human JH2 gene segments, optionally in cis at the same Ig locus in said genome;
- JH3 gene segments provided by at least 2 different human JH3 gene segments, optionally in cis at the same Ig locus in said genome;
- JH4 gene segments provided by at least 2 different human JH4 gene segments, optionally in cis at the same Ig locus in said genome;
- JH5 gene segments provided by at least 2 different human JH5 gene segments, optionally in cis at the same Ig locus in said genome;
- JH6 gene segments provided by at least 2 different human JH6 gene segments, optionally in cis at the same Ig locus in said genome;
- JH gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations (eg, 3, 4, 5 or 6 generations).
- said at least 2 different JH gene segments are human gene segments or synthetic gene segments derived from human gene segments.
- the Ig locus is a IgH locus, eg, an endogenous locus, eg, a mouse or rat IgH locus.
- the human individuals are from different human populations.
- An embodiment provides a vertebrate, cell or population of the invention whose genome comprises a plurality of JH5 gene segments, wherein the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a nucleotide mutation at one or more positions corresponding to positions
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a guanine at a position corresponding to position 106,330,067 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the variant comprises additionally a mutation at a position corresponding to (i) position 106,330,071 on human chromosome 14 (optionally the additional mutation being a guanine); (ii) position 106,330,066 on human chromosome 14 (optionally the additional mutation being a guanine); and/or (iii) position 106,330,068 on human chromosome 14 (optionally the additional mutation being a thymine).
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a guanine at a position corresponding to position 106,330,071 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the variant comprises additionally a mutation at a position corresponding to (i) position 106,330,063 on human chromosome 14 (optionally the additional mutation being an adenine); and/or (ii) position 106,330,067 on human chromosome 14 (optionally the additional mutation being a guanine).
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a cytosine at a position corresponding to position 106,330,045 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises an adenine at a position corresponding to position 106,330,044 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the variant comprises additionally a mutation at a position corresponding to (i) position
- 106.330.066 on human chromosome 14 (optionally the additional mutation being a guanine); and/or (ii) position 106,330,068 on human chromosome 14 (optionally the additional mutation being a thymine).
- the plurality comprises a human JH5 gene variant of SEQ I D NO: 1, wherein the variant comprises a guanine at a position corresponding to position 106,330,066 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the variant comprises additionally a mutation at a position corresponding to (i) position
- 106.330.067 on human chromosome 14 (optionally the additional mutation being a guanine); and/or (ii) position 106,330,068 on human chromosome 14 (optionally the additional mutation being a thymine).
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a thymine at a position corresponding to position 106,330,068 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the variant comprises additionally a mutation at a position corresponding to (i) position 106,330,067 on human chromosome 14 (optionally the additional mutation being a guanine); and/or (ii) position 106,330,066 on human chromosome 14 (optionally the additional mutation being a guanine).
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a cytosine at a position corresponding to position 106,330,027 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises an adenine at a position corresponding to position 106,330,024 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a thymine at a position corresponding to position 106,330,032 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises a thymine at a position corresponding to position 106,330,041 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the plurality comprises a human JH5 gene variant of SEQ ID NO: 1, wherein the variant comprises an adenine or thymine at a position corresponding to position 106,330,063 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the variant comprises additionally a mutation at a position corresponding to position 106,330,071 on human chromosome 14 (optionally the additional mutation being a guanine).
- the plurality comprises a human JH5 gene variant of SEQ ID NO: l,wherein the variant comprises a cytosine at a position corresponding to position 106,330,062 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 1.
- the genome comprises SEQ ID NO:l; optionally in cis at the same Ig locus as one, two or more of the variants.
- An embodiment provides a vertebrate, cell or population of the invention whose genome comprises a plurality of JH6 gene segments, wherein the plurality comprises a human JH6 gene variant of SEQ ID NO: 2, wherein the variant comprises a nucleotide mutation at one or more positions corresponding to positions
- the genome of the vertebrate, cell or population comprises a plurality of JH6 gene segments, wherein the plurality comprises a human JH6 gene variant of SEQ ID NO: 2, wherein the variant comprises a guanine at a position corresponding to position 106,329,435 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 2.
- the variant comprises additionally a mutation at a position corresponding to (i) position 106,329,468 on human chromosome 14 (optionally the additional mutation being a guanine); (ii) position 106,329,419 on human chromosome 14 (optionally the additional mutation being an adenine); (iii) position 106,329,434 on human chromosome 14 (optionally the additional mutation being a cytosine) and/or position 106,329,414 on human chromosome 14 (optionally the additional mutation being a guanine); (iv) position 106,329,426 on human chromosome 14 (optionally the additional mutation being an adenine); (v) position 106,329,413 on human chromosome 14 (optionally the additional mutation being an adenine); (vi) position 106,329,417 on human chromosome 14 (optionally the additional mutation being a thymine); (vii) position 106,
- the variant comprises additionally mutations at positions corresponding to position
- the vertebrate, cell or population optionally comprises a plurality of JH6 gene segments, wherein the plurality comprises a human JH6 gene variant of SEQ ID NO: 2, wherein the variant comprises a guanine at a position corresponding to position 106,329,468 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 2.
- the variant comprises additionally a mutation at a position corresponding to position 106,329,435 on human chromosome 14 (optionally the additional mutation being a guanine).
- the vertebrate, cell or population comprises a plurality of JH6 gene segments, wherein the plurality comprises a human JH6 gene variant of SEQ ID NO: 2, wherein the variant comprises a thymine at a position corresponding to position 106,329,417 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 2.
- the variant comprises additionally a mutation at a position corresponding to position 106,329,435 on human chromosome 14 (optionally the additional mutation being a guanine).
- the vertebrate, cell or population comprises a plurality of JH6 gene segments, wherein the plurality comprises a human JH6 gene variant of SEQ ID NO: 2, wherein the variant comprises a cytosine at a position corresponding to position 106,329,434 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 2.
- the variant comprises additionally a mutation at a position corresponding to (i) position 106,329,414 on human chromosome 14 (optionally the additional mutation being a guanine); and/or (ii) position 106,329,435 on human chromosome 14 (optionally the additional mutation being a guanine).
- the vertebrate, cell or population comprises a plurality of JH6 gene segments, wherein the plurality comprises a human JH6 gene variant of SEQ ID NO: 2, wherein the variant comprises a thymine at a position corresponding to position 106,329,411 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 2.
- the variant comprises additionally a mutation at a position corresponding to position 106,329,435 on human chromosome 14 (optionally the additional mutation being a guanine).
- the vertebrate, cell or population comprises a plurality of JH6 gene segments, wherein the plurality comprises a human JH6 gene variant that is an antisense sequence of a variant described above.
- the genome comprises SEQ ID NO:2; optionally cis at the same Ig locus as one, two or more of the JH6 variants.
- An embodiment provides a vertebrate, cell or population of the invention whose genome comprises a plurality of JH2 gene segments, wherein the plurality comprises a human JH2 gene variant of SEQ ID NO: 3, wherein the variant comprises a nucleotide mutation at one or more positions
- the vertebrate, cell or population comprises said plurality of JH2 gene segments, wherein the plurality comprises a human JH2 gene variant of SEQ ID NO: 3, wherein the variant comprises a guanine at a position corresponding to position 106,331,455 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 3.
- the variant comprises additionally a mutation at a position corresponding to (i) position 106,331,453 on human chromosome 14 (optionally the additional mutation being an adenine); and/or (ii) position 106,331,409 on human chromosome 14 (optionally the additional mutation being an adenine); (iii) position 106,329,434 on human chromosome 14 (optionally the additional mutation being an adenine).
- the vertebrate, cell or population comprises a plurality of JH2 gene segments, wherein the plurality comprises a human JH2 gene variant of SEQ ID NO: 3, wherein the variant comprises an adenine at a position corresponding to position 106,331,453 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 3.
- the variant comprises additionally a mutation at a position corresponding to position 106,331,409 on human chromosome 14 (optionally the additional mutation being an adenine).
- the vertebrate, cell or population comprises a plurality of JH2 gene segments, wherein the plurality comprises a human JH2 gene variant of SEQ ID NO: 3, wherein the variant comprises an adenine at a position corresponding to position 106,331,409 on human chromosome 14; and optionally no further mutation from the sequence of SEQ ID NO: 3.
- the vertebrate, cell or population comprises a plurality of JH2 gene segments, wherein the plurality comprises a human JH2 gene variant that is an antisense sequence of a variant described above.
- the genome comprises SEQ ID NO:3; optionally cis at the same Ig locus as one, two or more of the JH2 variants.
- the vertebrate, cell or population genome comprises two or more different JH gene segments selected from SEQ ID NOs: 1 to 3 and variants described above; optionally wherein said JH gene segments are cis at the same immunoglobulin Ig locus.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 3 human D gene segments of the same type (eg, D2-2 gene segments), wherein at least two of the human D gene segments are variants that are not identical to each other (eg, D2-2ref and D2-2a).
- one or more or all of the variants are naturally-occurring human gene segments.
- one or more of the variants may be a synthetic variant of a human gene segment.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different non-endogenous D gene segments of the same type type (eg, D2-2ref and D2-2a) cis at the same Ig locus.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different human D gene segments of the same type (eg, D2-2ref and D2-2a) trans at the same Ig locus; and optionally a third human D gene segment (eg, (eg, D2-2ref, D2-2a or D2-2b) of the same type, wherein the third D is cis with one of said 2 different D gene segments.
- a population of non-human vertebrates comprising a repertoire of human D gene segments, wherein the plurality comprises at least 2 different human D gene segments of the same type (eg, D2-2 gene segments), a first of said different D gene segments (eg, D2-2ref) is provided in the genome of a first vertebrate of the population, and a second of said different D gene segment (eg, D2-2a) being provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second D gene segment.
- non-human vertebrates eg, mice or rats
- the plurality comprises at least 2 different human D gene segments of the same type (eg, D2-2 gene segments)
- a first of said different D gene segments eg, D2-2ref
- a second of said different D gene segment eg, D2-2a
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different non-endogenous D gene segments of the same type (eg, human D2-2 gene segments), wherein the D gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- vertebrate eg, a mouse or rat
- the method comprising providing the vertebrate with a genome comprising at least 3 human D gene segments of the same type (eg, D2-2 gene segments), wherein at least two of the human D gene segments are variants that are not identical to each other (eg, D2-2ref and D2-2a).
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous D gene segments of the same type (eg, human D2-2 gene segments) cis at the same Ig locus.
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different human D gene segments of the same type (eg, D2-2ref and D2-2a) trans at the same Ig locus; and optionally a third human D gene segment (eg, D2-2ref, D2-2a or D2-2b) of the same type, wherein the third D is cis with one of said 2 different D gene segments.
- a method of providing an enhanced human immunoglobulin D gene segment repertoire comprising providing a population of non-human vertebrates (eg, a mouse or rat) comprising a repertoire of human D gene segments, wherein the method comprises providing at least 2 different human D gene segments of the same type (eg, D2-2ref and D2-2a), wherein a first of said different D gene segments is provided in the genome of a first vertebrate of the population, and a second of said different D gene segments is provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second D gene segment.
- non-human vertebrates eg, a mouse or rat
- the method comprises providing at least 2 different human D gene segments of the same type (eg, D2-2ref and D2-2a), wherein a first of said different D gene segments is provided in the genome of a first vertebrate of the population, and a second of said different D gene segments is provided in the genome of a second vertebrate of the population
- vertebrate eg, a mouse or rat
- the method comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous D gene segments of the same type (eg, D2-2ref and D2-2a), wherein the D gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- vertebrate eg, a mouse or rat
- the method comprising providing the vertebrate with a genome comprising at least 2 human D gene segments of the same type (eg, D2-2ref and D2-2a), wherein the D gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations; optionally wherein at least 2 or 3 of said different gene segments are provided at the same IgH locus in said genome. 170.
- a non-human vertebrate eg, a mouse or rat
- a non-human cell eg, an ES cell or a B-cell
- having a genome comprising a substantially complete functional repertoire of human D gene segment types supplemented with one, two or more variant human D gene segments, wherein said substantially complete functional repertoire and the supplementary D gene segments are not found together in the germline genome of a human individual.
- a population of non-human vertebrates comprising a substantially
- D6-13ref and D6-13a or D3-9ref and D3-9a; or
- first and/or second D gene segment is present in two or more copies.
- the vertebrate, cell or population comprises one or more D segments selected from human D3-3, D2-15, D3-9; D4-17; D3-10; D2-2; D5-24; D6-19; D3-22; D6-13; D5-12; Dl-26; Dl-20; D5-18; D3-16; D2-21; Dl-14; D7-27; Dl-1; D6-25; D2-14 and D4-23 (eg, selected from D3- 9*01; D4-17*01; D3-10*01; D2-2*02; D5-24*01; D6-19*01; D3-22*01; D6-13*01; D5-12*01; Dl- 26*01; Dl-20*01; D5-18*01; D3-16*02; D2-21*02; Dl-14*01; D7-27*02; Dl-l*01; D6-25*01; D2-15*01; and D4-23*01), together with the reference sequence(s) of said selected segment(s) of said
- a non-human vertebrate or vertebrate cell according to clause 155 comprising a genome that comprises VH, D and JH gene repertoires comprising human gene segments, the D gene repertoire comprising one or more of a plurality of D2-2 gene segments provided by at least 2 different D2-2 gene segments in cis at the same Ig locus in said genome;
- a non-human vertebrate or vertebrate cell according to clause 155 comprising a genome that comprises VH, D and JH gene repertoires comprising human gene segments, the D gene repertoire comprising one or more of a plurality of D2-2 gene segments provided by at least 2 different D2-2 gene segments in trans in said genome;
- D7-27 gene segments provided by at least 2 different D7-27 gene segments in trans in said genome; optionally wherein the D gene segments are derived from the genome sequence of two or more different human individuals.
- a non-human vertebrate or vertebrate cell (optionally an ES cell or B-cell), according to clause 154, comprising a genome that comprises VH, D and JH gene repertoires comprising human gene segments, the D gene repertoire comprising one or more of a plurality of D2-2 gene segments provided by at least 3 different D2-2 gene segments;
- D4-4 gene segments provided by at least 3 different D4-4 gene segments
- D7-27 gene segments provided by at least 3 different D7-27 gene segments
- the D gene segments are derived from the genome sequence of two or three different human individuals; optionally wherein at least 2 or 3 of said different gene segments are provided in cis at the same Ig locus in said genome.
- a non-human vertebrate or vertebrate cell (optionally an ES cell or B-cell) according to clause 158, comprising a genome comprising human VH, D and JH gene repertoires, the D gene repertoire comprising of one or more of a plurality of D2-2 gene segments provided by at least 2 different D2-2 gene ; optionally in cis in said genome;
- D3-16 gene segments provided by at least 2 different D3-16 gene ; optionally in cis in said genome;
- D3-9 gene segments provided by at least 2 different D3-9 gene ; optionally in cis in said genome;
- D7-27 gene segments provided by at least 2 different D7-27 gene ; optionally in cis in said genome; wherein the D gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- one or more of the D gene segments is a variant of a human germline D gene segment, wherein the variant gene segment encodes an amino acid sequence that differs by 1, 2 or 3 amino acids from the corresponding amino acid sequence encoded by the human germline D gene segment, provided in that said amino acid sequence encoded by the variant does not include a stop codon when said corresponding amino acid sequence does not include a stop codon.
- variant and germline D gene segments encode the respective amino acid sequences in reading frame 2 (IMGT numbering). See Briney et al 2012.
- D segments are usable in all three reading frames.
- a variant of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or all of these human germline D gene segments is used.
- a human D2-2 gene segment ((optionally two copies and/or in homozygous state) comprising a thymine at a position corresponding to position 106,382,687 on human chromosome 14; and optionally no further mutation from the sequence of D2-2ref.
- a human D3-16 gene segment (optionally two copies and/or in homozygous state) comprising a cytosine at a position corresponding to position 106,361,515 on human chromosome 14; and optionally no further mutation from the sequence of D3-16a.
- the vertebrate, cell or population of clause 205 wherein the plurality comprises a human D6-13 gene segment (optionally two copies and/or in homozygous state) comprising a thymine at a position corresponding to position 106,367,013 on human chromosome 14; and optionally no further mutation from the sequence of D6-13ref. 207.
- a human D3-10 gene segment (optionally two copies and/or in homozygous state) comprising a thymine at a position corresponding to position 106,370,370 on human chromosome 14; and optionally no further mutation from the sequence of D3-10ref.
- a human D3-10 gene segment (optionally two copies and/or in homozygous state) comprising a cytosine at a position corresponding to position 106,370,370 on human chromosome 14; and optionally no further mutation from the sequence of D3-10a.
- a human D3-10 gene segment (optionally two copies and/or in homozygous state) comprising an adenine at a position corresponding to position 106,370,371 on human chromosome 14; and optionally no further mutation from the sequence of D3-10ref.
- a human D3-9 gene segment (optionally two copies and/or in homozygous state) comprising an adenine at a position corresponding to position 106,370,567 on human chromosome 14; and optionally no further mutation from the sequence of D3-9ref.
- a human D3-9 gene segment (optionally two copies and/or in homozygous state) comprising a thymine at a position corresponding to position 106,370,567 on human chromosome 14; and optionally no further mutation from the sequence of D3-9a.
- a human D2-8 gene segment (optionally two copies and/or in homozygous state) comprising a thymine at a position corresponding to position 106,373,085 on human chromosome 14; and optionally no further mutation from the sequence of D2-8b.
- a D4-4 gene segment (optionally two copies and/or in homozygous state) comprising a cytosine at a position corresponding to position 106,379,086 on human chromosome 14; and optionally no further mutation from the sequence of D4-4ref.
- a human D4-4 gene segment (optionally two copies and/or in homozygous state) comprising a cytosine at a position corresponding to position 106,379,089 on human chromosome 14; and optionally no further mutation from the sequence of D4-4ref or a cytosine at a position corresponding to position 106,379,086 on human chromosome 14.
- a D3-3 gene segment (optionally two copies and/or in homozygous state) comprising a thymine at a position corresponding to position 106,380,241 on human chromosome 14; and optionally no further mutation from the sequence of D3-3ref.
- a human D3-3 gene segment (optionally two copies and/or in homozygous state) comprising a cytosine at a position corresponding to position 106,380,241 on human chromosome 14; and optionally no further mutation from the sequence of D3-3a.
- a specific application of this configuration is the provision of multiple human JL gene segments ( and/or i ⁇ ) as follows (as set out in numbered paragraphs, starting at paragraph number 80).
- 80. A non-human vertebrate (eg, a mouse or rat) or a non-human vertebrate cell (eg, an ES cell or a B-cell) having a genome comprising at least 3 human JL gene segments of the same type (eg, JKI), wherein at least two of the human J L gene segments are variants that are not identical to each other.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different non-endogenous JL gene segments of the same type (eg, JKI) cis at the same Ig locus.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different human JL gene segments of the same type (eg, JKI) trans at the same Ig locus; and optionally a third human JL gene segment of the same type, wherein the third JL is cis with one of said 2 different JL gene segments.
- a population of non-human vertebrates comprising a repertoire of human JL gene segments, wherein the plurality comprises at least 2 different human JL gene segments of the same type (eg, JKI), a first of said different J L gene segments is provided in the genome of a first vertebrate of the population, and a second of said different JL gene segments being provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second JL gene segment.
- a non-human vertebrate eg, a mouse or rat
- a non-human vertebrate cell eg, an ES cell or a B-cell
- having a genome comprising at least 2 different non-endogenous JL gene segments of the same type (eg, JKI), wherein the JL gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 3 human JL gene segments of the same type (eg, JKI), wherein at least two of the human JL gene segments are variants that are not identical to each other.
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous JL gene segments of the same type (eg, JKI) cis at the same Ig locus.
- a method of enhancing the immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different human JL gene segments of the same type(eg, JKI) trans at the same Ig locus; and optionally a third human JL gene segment of the same type, wherein the third JL is cis with one of said 2 different J L gene segments.
- a method of providing an enhanced human immunoglobulin JL gene segment repertoire comprising providing a population of non-human vertebrates (eg, a mouse or rat) comprising a repertoire of human JL gene segments, wherein the method comprises providing at least 2 different human JL gene segments of the same type (eg, JKI), wherein a first of said different JL gene segments is provided in the genome of a first vertebrate of the population, and a second of said different J L gene segments is provided in the genome of a second vertebrate of the population, wherein the genome of the first vertebrate does not comprise the second JL gene segment.
- non-human vertebrates eg, a mouse or rat
- JKI human JL gene segments of the same type
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 different non-endogenous JL gene segments of the same type (eg, JKI), wherein the JL gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- JL gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations.
- JL gene segments are derived from the genome sequence of two or more different human individuals; optionally wherein the different human individuals are from different human populations.
- a method of enhancing the human immunoglobulin gene diversity of a non-human vertebrate comprising providing the vertebrate with a genome comprising at least 2 human J L gene segments of the same type (eg, JKI), wherein the J L gene segments are derived from the genome sequence of different human individuals that are not genetically related over at least 3 generations; optionally wherein at least 2 or 3 of said different gene segments are provided at the same IgL locus in said genome.
- the population of paragraph 83 wherein the population comprises a substantially complete functional repertoire of human J L gene segment types supplemented with one, two or more human JK and/or iX gene segments respectively, wherein said substantially complete functional repertoire and the supplementary gene segments are not found together in the germline genome of a human individual.
- a non-human vertebrate eg, a mouse or rat
- a non-human cell eg, an ES cell or a B-cell
- having a genome comprising a substantially complete functional repertoire of human JK and/or iX gene segment types supplemented with one, two or more human JK and/or iX gene segments respectively, wherein said substantially complete functional repertoire and the supplementary gene segments are not found together in the germline genome of a human individual.
- a population of non-human vertebrates comprising a substantially complete functional repertoire of human JK and/or iX gene segment types supplemented with one, two or more human JK and/or iX gene segments respectively, wherein said substantially complete functional repertoire and the supplementary gene segments are not found together in the germline genome of a human individual.
- a non-human vertebrate or vertebrate cell according to paragraph 81 comprising a genome that comprises VL and J L gene repertoires comprising human gene segments, the J L gene repertoire comprising a plurality of human JKI gene segments provided by at least 2 different human JKI gene segments in cis at the same Ig locus in said genome;
- a non-human vertebrate or vertebrate cell (optionally an ES cell or B-cell), according to paragraph 80, comprising a genome that comprises VL and J L gene repertoires comprising human gene segments, the J L gene repertoire comprising a plurality of human JKI gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human JKI gene segments;
- a plurality of human JK2 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human JKI gene segments;
- a plurality of human JK3 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human JKI gene segments;
- a plurality of human JK4 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human JKI gene segments;
- a plurality of human JK5 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human JKI gene segments;
- a plurality of human J ⁇ l gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human J ⁇ l gene segments;
- a plurality of human J ⁇ 2 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human J ⁇ 2 gene segments;
- a plurality of human J ⁇ 3 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human J ⁇ 3 gene segments;
- a plurality of human J ⁇ 4 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human J ⁇ 4 gene segments;
- a plurality of human J ⁇ 5 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human J ⁇ 5 gene segments;
- a plurality of human J ⁇ 6 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human J ⁇ 6 gene segments; or
- J ⁇ 7 gene segments provided by at least 3 (eg, 3, 4, 5, 6, or 7) different human J ⁇ 7 gene segments; optionally wherein the J L gene segments are derived from the genome sequence of two or three different human individuals; optionally wherein at least 2 or 3 of said different gene segments are provided in cis at the same Ig locus in said genome.
- a non-human vertebrate or vertebrate cell (optionally an ES cell or B-cell) according to paragraph 84, comprising a genome comprising human VL and J L gene repertoires, the J L gene repertoire comprising a plurality of human JKI gene segments provided by at least 2 different human JKI gene segments, optionally in cis at the same Ig locus in said genome;
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Virology (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Communicable Diseases (AREA)
- Tropical Medicine & Parasitology (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Environmental Sciences (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Animal Husbandry (AREA)
- Pulmonology (AREA)
- Biodiversity & Conservation Biology (AREA)
- AIDS & HIV (AREA)
- Hematology (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
Claims
Priority Applications (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES12772122.3T ES2612935T3 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains and chains adapted for use in humans |
| CA2846319A CA2846319A1 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains & chains tailored for human use |
| EP16189625.3A EP3128009B1 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains & chains tailored for human use |
| NZ623756A NZ623756B2 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains & chains tailored for human use |
| EP20188009.3A EP3839049A3 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains & chains tailored for human use |
| CN201280056727.0A CN104080916A (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains & chains tailored for human use |
| AU2012311286A AU2012311286B2 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains and chains tailored for human use |
| BR112014006390A BR112014006390A2 (en) | 2011-09-19 | 2012-09-18 | antibodies, variable domains and chains made especially for human use |
| JP2014531302A JP5813880B2 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains and chains made for human use |
| EP12772122.3A EP2758535B1 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains&chains tailored for human use |
| US14/052,259 US20140041067A1 (en) | 2011-09-19 | 2013-10-11 | Antibodies, variable domains & chains tailored for human use |
| HK14108036.3A HK1194757A1 (en) | 2011-09-19 | 2014-08-05 | Antibodies, variable domains & chains tailored for human use |
| US15/786,281 US20180030121A1 (en) | 2011-09-19 | 2017-10-17 | Antibodies, variable domains & chains tailored for human use |
Applications Claiming Priority (14)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB201116122A GB201116122D0 (en) | 2011-09-19 | 2011-09-19 | Synthetically-extended & ethnically diverse superhuman immunoglobulin gene repertoires |
| GB201116120A GB201116120D0 (en) | 2011-09-19 | 2011-09-19 | Manipulation of immunoglobulin gene diversity and multi antibody therapeutics, especially for infectious diseases |
| GB1116120.5 | 2011-09-19 | ||
| GB1116122.1 | 2011-09-19 | ||
| GBGB1203257.9A GB201203257D0 (en) | 2012-02-24 | 2012-02-24 | Animals, repertoires & methods |
| GB1203257.9 | 2012-02-24 | ||
| GB1204592.8 | 2012-03-15 | ||
| GBGB1204592.8A GB201204592D0 (en) | 2012-03-15 | 2012-03-15 | Animals, repertoires & methods |
| GBGB1205702.2A GB201205702D0 (en) | 2012-03-29 | 2012-03-29 | Animals,repertoires & methods |
| GB1205702.2 | 2012-03-29 | ||
| GB1208749.0 | 2012-05-18 | ||
| GBGB1208749.0A GB201208749D0 (en) | 2012-05-18 | 2012-05-18 | Synthetically-extended & ethnically-diverse superhuman immunoglobulin gene repertoires |
| GB1211692.7 | 2012-07-02 | ||
| GB201211692A GB201211692D0 (en) | 2012-07-02 | 2012-07-02 | Animals, repertories & methods |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/052,259 Continuation US20140041067A1 (en) | 2011-09-19 | 2013-10-11 | Antibodies, variable domains & chains tailored for human use |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| WO2013041844A2 true WO2013041844A2 (en) | 2013-03-28 |
| WO2013041844A3 WO2013041844A3 (en) | 2013-07-11 |
| WO2013041844A9 WO2013041844A9 (en) | 2014-11-27 |
Family
ID=46889370
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2012/052298 WO2013041846A2 (en) | 2011-09-19 | 2012-09-18 | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics |
| PCT/GB2012/052296 WO2013041844A2 (en) | 2011-09-19 | 2012-09-18 | Antibodies, variable domains & chains tailored for human use |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2012/052298 WO2013041846A2 (en) | 2011-09-19 | 2012-09-18 | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics |
Country Status (11)
| Country | Link |
|---|---|
| US (4) | US20140041067A1 (en) |
| EP (6) | EP3311661B1 (en) |
| JP (4) | JP2014533930A (en) |
| CN (4) | CN106995822B (en) |
| AU (3) | AU2012311286B2 (en) |
| BR (2) | BR112014006390A2 (en) |
| CA (2) | CA2846322A1 (en) |
| DK (2) | DK3311661T3 (en) |
| ES (1) | ES2612935T3 (en) |
| HK (1) | HK1194757A1 (en) |
| WO (2) | WO2013041846A2 (en) |
Cited By (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8945560B1 (en) | 2014-07-15 | 2015-02-03 | Kymab Limited | Method of treating rheumatoid arthritis using antibody to IL6R |
| US8980273B1 (en) | 2014-07-15 | 2015-03-17 | Kymab Limited | Method of treating atopic dermatitis or asthma using antibody to IL4RA |
| US8986691B1 (en) | 2014-07-15 | 2015-03-24 | Kymab Limited | Method of treating atopic dermatitis or asthma using antibody to IL4RA |
| US8986694B1 (en) | 2014-07-15 | 2015-03-24 | Kymab Limited | Targeting human nav1.7 variants for treatment of pain |
| US8992927B1 (en) | 2014-07-15 | 2015-03-31 | Kymab Limited | Targeting human NAV1.7 variants for treatment of pain |
| FR3011249A1 (en) * | 2013-10-01 | 2015-04-03 | Kymab Ltd | |
| US8999341B1 (en) | 2014-07-15 | 2015-04-07 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| NL2013554A (en) * | 2013-10-01 | 2015-04-07 | Kymab Ltd | Animal models and therapeutic molecules. |
| US9017678B1 (en) | 2014-07-15 | 2015-04-28 | Kymab Limited | Method of treating rheumatoid arthritis using antibody to IL6R |
| US9034332B1 (en) | 2014-07-15 | 2015-05-19 | Kymab Limited | Precision medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| US9040052B1 (en) | 2013-12-17 | 2015-05-26 | Kymab Limited | Precision Medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| US9045548B1 (en) | 2014-07-15 | 2015-06-02 | Kymab Limited | Precision Medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| US9045545B1 (en) | 2014-07-15 | 2015-06-02 | Kymab Limited | Precision medicine by targeting PD-L1 variants for treatment of cancer |
| US9051378B1 (en) | 2014-07-15 | 2015-06-09 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| US9062105B1 (en) | 2014-07-15 | 2015-06-23 | Kymab Limited | Precision Medicine by targeting VEGF-A variants for treatment of retinopathy |
| EP2886557A1 (en) * | 2013-12-17 | 2015-06-24 | Kymab Limited | Antibodies for use in treating conditions related to specific PCSK9 variants in specific patient populations |
| WO2015092394A1 (en) * | 2013-12-17 | 2015-06-25 | Kymab Limited | Antibodies for use in treating conditions related to specific pcsk9 variants in specific patients populations |
| US9067998B1 (en) | 2014-07-15 | 2015-06-30 | Kymab Limited | Targeting PD-1 variants for treatment of cancer |
| US9139648B1 (en) | 2014-07-15 | 2015-09-22 | Kymab Limited | Precision medicine by targeting human NAV1.9 variants for treatment of pain |
| US9150660B1 (en) | 2014-07-15 | 2015-10-06 | Kymab Limited | Precision Medicine by targeting human NAV1.8 variants for treatment of pain |
| DE202014010499U1 (en) | 2013-12-17 | 2015-10-20 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| EP2975059A1 (en) | 2014-07-15 | 2016-01-20 | Kymab Limited | Antibodies for use in treating conditions related to specific pcsk9 variants in specific patients populations |
| EP2975058A1 (en) * | 2014-07-15 | 2016-01-20 | Kymab Limited | Antibodies for use in treating conditions related to specific PCSK9 variants in specific patient populations |
| WO2016008899A1 (en) | 2014-07-15 | 2016-01-21 | Kymab Limited | Targeting human pcsk9 for cholesterol treatment |
| US9253965B2 (en) | 2012-03-28 | 2016-02-09 | Kymab Limited | Animal models and therapeutic molecules |
| WO2016023916A1 (en) | 2014-08-12 | 2016-02-18 | Kymab Limited | Treatment of disease using ligand binding to targets of interest |
| WO2016071701A1 (en) | 2014-11-07 | 2016-05-12 | Kymab Limited | Treatment of disease using ligand binding to targets of interest |
| DE202015009002U1 (en) | 2014-07-15 | 2016-08-18 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| US9434782B2 (en) | 2009-07-08 | 2016-09-06 | Kymab Limited | Animal models and therapeutic molecules |
| US9445581B2 (en) | 2012-03-28 | 2016-09-20 | Kymab Limited | Animal models and therapeutic molecules |
| US9516868B2 (en) | 2010-08-02 | 2016-12-13 | Regeneron Pharmaceuticals, Inc. | Mice that make VL binding proteins |
| JP2017502030A (en) * | 2013-12-17 | 2017-01-19 | カイマブ・リミテッド | Ligands that specifically bind to the target human target |
| JP2017505127A (en) * | 2014-01-24 | 2017-02-16 | チルドレンズ メディカル センター コーポレーション | High-throughput mouse model for antibody affinity optimization |
| US9622459B2 (en) | 2011-12-20 | 2017-04-18 | Regeneron Pharmaceuticals, Inc. | Humanized light chain mice |
| US9783593B2 (en) | 2013-05-02 | 2017-10-10 | Kymab Limited | Antibodies, variable domains and chains tailored for human use |
| US9783618B2 (en) | 2013-05-01 | 2017-10-10 | Kymab Limited | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics |
| US9788534B2 (en) | 2013-03-18 | 2017-10-17 | Kymab Limited | Animal models and therapeutic molecules |
| US9930871B2 (en) | 2013-02-20 | 2018-04-03 | Regeneron Pharmaceuticals, Inc. | Non-human animals with modified immunoglobulin heavy chain sequences |
| US9932398B2 (en) | 2011-10-17 | 2018-04-03 | Regeneron Pharmaceuticals, Inc. | Restricted immunoglobulin heavy chain mice |
| US9963716B2 (en) | 2011-09-26 | 2018-05-08 | Kymab Limited | Chimaeric surrogate light chains (SLC) comprising human VpreB |
| US10130081B2 (en) | 2011-08-05 | 2018-11-20 | Regeneron Pharmaceuticals, Inc. | Humanized universal light chain mice |
| WO2019037099A1 (en) * | 2017-08-25 | 2019-02-28 | Wenning Qin | Large scale modification of eukaryotic genome |
| US10238093B2 (en) | 2012-06-12 | 2019-03-26 | Regeneron Pharmaceuticals, Inc. | Humanized non-human animals with restricted immunoglobulin heavy chain loci |
| US10251377B2 (en) | 2012-03-28 | 2019-04-09 | Kymab Limited | Transgenic non-human vertebrate for the expression of class-switched, fully human, antibodies |
| US10667501B2 (en) | 2012-05-17 | 2020-06-02 | Kymab Limited | Transgenic non-human vertebrate for the in vivo production of dual specificity immunoglobulins or hypermutated heavy chain only immunoglobulins |
| WO2020169022A1 (en) * | 2019-02-18 | 2020-08-27 | Beijing Biocytogen Co., Ltd | Genetically modified non-human animals with humanized immunoglobulin locus |
| US10787522B2 (en) | 2014-03-21 | 2020-09-29 | Regeneron Pharmaceuticals, Inc. | VL antigen binding proteins exhibiting distinct binding characteristics |
| US10881085B2 (en) | 2014-03-21 | 2021-01-05 | Regeneron Pharmaceuticals, Inc. | Non-human animals that make single domain binding proteins |
| US11051497B2 (en) | 2011-09-19 | 2021-07-06 | Kymab Limited | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics |
| US11111314B2 (en) | 2015-03-19 | 2021-09-07 | Regeneron Pharmaceuticals, Inc. | Non-human animals that select for light chain variable regions that bind antigen |
| EP3476942B1 (en) * | 2017-10-27 | 2022-01-26 | Trianni, Inc. | Long germline dh genes and long hcdr3 antibodies |
| WO2022056276A1 (en) | 2020-09-11 | 2022-03-17 | Regeneron Pharmaceuticals, Inc. | Identification and production of antigen-specific antibodies |
| WO2022132943A1 (en) | 2020-12-16 | 2022-06-23 | Regeneron Pharmaceuticals, Inc. | Mice expressing humanized fc alpha receptors |
| US11707056B2 (en) | 2013-05-02 | 2023-07-25 | Kymab Limited | Animals, repertoires and methods |
| US11753479B2 (en) | 2014-03-04 | 2023-09-12 | Kymab Limited | Nucleic acids encoding anti-OX40L antibodies |
| US11779604B2 (en) | 2016-11-03 | 2023-10-10 | Kymab Limited | Antibodies, combinations comprising antibodies, biomarkers, uses and methods |
| EP4257599A3 (en) * | 2016-01-13 | 2024-01-17 | Regeneron Pharmaceuticals, Inc. | Rodents having an engineered heavy chain diversity region |
| EP4173480A4 (en) * | 2020-06-25 | 2024-04-17 | Humab Co. Ltd. | Heterozygous transgenic animal |
| US11997994B2 (en) | 2020-06-02 | 2024-06-04 | Biocytogen Pharmaceuticals (Beijing) Co., Ltd. | Genetically modified non-human animals with common light chain immunoglobulin locus |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3988649A1 (en) * | 2013-09-18 | 2022-04-27 | Kymab Limited | Methods, cells and organisms |
| US10513711B2 (en) * | 2014-08-13 | 2019-12-24 | Dupont Us Holding, Llc | Genetic targeting in non-conventional yeast using an RNA-guided endonuclease |
| EP3462853B1 (en) | 2016-06-03 | 2023-03-01 | Regeneron Pharmaceuticals, Inc. | Rodents expressing exogenous terminal deoxynucleotidyltransferase |
| CN107312863A (en) * | 2017-08-08 | 2017-11-03 | 深圳华大基因研究院 | The method that the Ig gene distributions of bone-marrow-derived lymphocyte are identified using digital pcr |
| CN107557367B (en) * | 2017-09-08 | 2020-11-03 | 北京大学人民医院 | IGHG1 gene mutant and application thereof |
| CN108486126A (en) * | 2018-03-27 | 2018-09-04 | 重庆金迈博生物科技有限公司 | A kind of nucleic acid molecules and its application in humanized antibody |
| CA3102441C (en) | 2018-06-08 | 2023-06-13 | Crystal Bioscience Inc. | Transgenic animal for producing diversified antibodies that have the same light chain i |
| MX2020013652A (en) * | 2018-06-14 | 2022-04-21 | Regeneron Pharma | Non-human animals capable of dh-dh rearrangement in the immunoglobulin heavy chain coding sequences. |
| EP4051700A1 (en) | 2020-12-23 | 2022-09-07 | Regeneron Pharmaceuticals, Inc. | Nucleic acids encoding anchor modified antibodies and uses thereof |
| JPWO2023090361A1 (en) * | 2021-11-16 | 2023-05-25 | ||
| WO2024075756A1 (en) * | 2022-10-05 | 2024-04-11 | 株式会社Logomix | Cell library and method for producing same |
| JP7212982B1 (en) | 2022-10-05 | 2023-01-26 | 株式会社Logomix | Cell library and its production method |
| TW202430558A (en) * | 2023-01-18 | 2024-08-01 | 美商基利科學股份有限公司 | Human immunoglobulin heavy chain long cdr3 transgene constructs and uses thereof |
| CN117736322B (en) * | 2024-02-21 | 2024-04-19 | 吉林大学 | Anti SYCE2 single-molecule antibody T2D and application thereof |
Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5859301A (en) | 1993-12-23 | 1999-01-12 | Dsm N.V. | Process for preparing alkanones and alkanols |
| US5939598A (en) | 1990-01-12 | 1999-08-17 | Abgenix, Inc. | Method of making transgenic mice lacking endogenous heavy chains |
| EP0937140A2 (en) | 1996-06-27 | 1999-08-25 | Vlaams Interuniversitair Instituut voor Biotechnologie vzw. | Recognition molecules interacting specifically with the active site or cleft of a target molecule |
| EP1034260A2 (en) | 1997-12-05 | 2000-09-13 | Europäisches Laboratorium Für Molekularbiologie (Embl) | Novel dna cloning method relying on the e. coli rece/rect recombination system |
| US6130364A (en) | 1995-03-29 | 2000-10-10 | Abgenix, Inc. | Production of antibodies using Cre-mediated site-specific recombination |
| EP1204740A1 (en) | 1999-07-09 | 2002-05-15 | The European Molecular Biology Laboratory | Methods and compositions for directed cloning and subcloning using homologous recombination |
| WO2002066630A1 (en) | 2001-02-16 | 2002-08-29 | Regeneron Pharmaceuticals, Inc. | Methods of modifying eukaryotic cells |
| US6586251B2 (en) | 2000-10-31 | 2003-07-01 | Regeneron Pharmaceuticals, Inc. | Methods of modifying eukaryotic cells |
| US6673986B1 (en) | 1990-01-12 | 2004-01-06 | Abgenix, Inc. | Generation of xenogeneic antibodies |
| EP1399559A2 (en) | 2001-06-21 | 2004-03-24 | The Babraham Institute | Mouse lambda light chain locus |
| US7501552B2 (en) | 1991-08-28 | 2009-03-10 | Medarex, Inc. | Transgenic non-human animals for producing chimeric antibodies |
| WO2009076464A2 (en) | 2007-12-10 | 2009-06-18 | Aliva Biopharmaceuticals, Inc. | Methods for sequential replacement of targeted region by homologous recombination |
| WO2009097006A2 (en) | 2007-08-10 | 2009-08-06 | Medarex, Inc. | Hco32 and hco27 and related examples |
| WO2009143472A2 (en) | 2008-05-23 | 2009-11-26 | Aliva Biopharmaceuticals, Inc. | Method of generating single vl domain antibodies in transgenic animals |
| WO2010039900A2 (en) | 2008-09-30 | 2010-04-08 | Aliva Biopharmaceuticals, Inc. | Non-human mammals for the production of chimeric antibodies |
| WO2011004192A1 (en) | 2009-07-08 | 2011-01-13 | Genome Research Limited | Animal models and therapeutic molecules |
| WO2011066501A1 (en) | 2009-11-30 | 2011-06-03 | Centocor Ortho Biotech Inc. | Antibody fc mutants with ablated effector functions |
| WO2012141798A1 (en) | 2011-02-25 | 2012-10-18 | Regeneron Pharmaceuticals, Inc. | Adam6 mice |
Family Cites Families (173)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1014946A (en) | 1910-06-16 | 1912-01-16 | Us Sherardizing Company | Alloy for coating with metal. |
| US1006439A (en) | 1911-03-11 | 1911-10-17 | Clarence Cosby | Can-shearing machine. |
| US5807715A (en) | 1984-08-27 | 1998-09-15 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and transformed mammalian lymphocyte cells for producing functional antigen-binding protein including chimeric immunoglobulin |
| US5169939A (en) | 1985-05-21 | 1992-12-08 | Massachusetts Institute Of Technology & Pres. & Fellows Of Harvard College | Chimeric antibodies |
| US4720449A (en) | 1985-06-03 | 1988-01-19 | Polaroid Corporation | Thermal imaging method |
| GB8823869D0 (en) | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| WO1991000906A1 (en) | 1989-07-12 | 1991-01-24 | Genetics Institute, Inc. | Chimeric and transgenic animals capable of producing human antibodies |
| US6657103B1 (en) | 1990-01-12 | 2003-12-02 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US6713610B1 (en) | 1990-01-12 | 2004-03-30 | Raju Kucherlapati | Human antibodies derived from immunized xenomice |
| US5633425A (en) * | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US6300129B1 (en) | 1990-08-29 | 2001-10-09 | Genpharm International | Transgenic non-human animals for producing heterologous antibodies |
| US5770429A (en) * | 1990-08-29 | 1998-06-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| AU8507191A (en) | 1990-08-29 | 1992-03-30 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US7084260B1 (en) | 1996-10-10 | 2006-08-01 | Genpharm International, Inc. | High affinity human antibodies and human antibodies against human antigens |
| US6255458B1 (en) | 1990-08-29 | 2001-07-03 | Genpharm International | High affinity human antibodies and human antibodies against digoxin |
| AU2515992A (en) | 1991-08-20 | 1993-03-16 | Genpharm International, Inc. | Gene targeting in animal cells using isogenic dna constructs |
| AU3328493A (en) | 1991-12-17 | 1993-07-19 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| JPH07509137A (en) | 1992-07-24 | 1995-10-12 | セル ジェネシス,インク. | Production of xenoantibodies |
| DE69330523D1 (en) | 1992-08-21 | 2001-09-06 | Vrije Universiteit Brussel Bru | IMMUNOGLOBULINE WITHOUT LIGHT CHAINS |
| DE4228162C1 (en) | 1992-08-25 | 1994-01-13 | Rajewsky Klaus Dr | Method for replacing homologous gene segments from mammals in the germline of non-human mammals |
| JP4242447B2 (en) | 1993-01-22 | 2009-03-25 | イミュネックス・コーポレーション | Detection and treatment of CD40 ligand gene mutations |
| JPH08509612A (en) | 1993-04-26 | 1996-10-15 | ジェンファーム インターナショナル インコーポレイテッド | Transgenic non-human animal capable of producing heterologous antibody |
| DE4331162A1 (en) | 1993-09-14 | 1995-03-16 | Bayer Ag | Process for the preparation of cyanine dyes |
| US7119248B1 (en) | 1994-04-12 | 2006-10-10 | Miltenyi Biotec Gmbh | Antibodies against epitopes with homology to self antigens, methods of preparation and applications thereof |
| WO1997049804A1 (en) | 1996-06-26 | 1997-12-31 | Baylor College Of Medicine | Chromosomal rearrangement by insertion of two recombination substrates |
| EP2305027B1 (en) * | 1996-12-03 | 2014-07-02 | Amgen Fremont Inc. | Transgenic mammals having human Ig loci including plural VH and Vkappa regions and antibodies produced therefrom |
| US6319906B1 (en) | 1996-12-31 | 2001-11-20 | Isis Pharmaceuticals | Oligonucleotide compositions and methods for the modulation of the expression of B7 protein |
| DK0979281T3 (en) | 1997-05-02 | 2005-11-21 | Genentech Inc | Process for the preparation of multispecific antibodies with heteromultimers and common components |
| US20020088019A1 (en) | 1997-09-02 | 2002-07-04 | Oron Yacoby-Zeevi | Methods of and pharmaceutical compositions for improving implantation of embryos |
| CA2306184C (en) | 1997-11-18 | 2007-05-15 | Pioneer Hi-Bred International, Inc. | Compositions and methods for genetic modification of plants |
| EP0939120A1 (en) | 1998-02-27 | 1999-09-01 | Gesellschaft für biotechnologische Forschung mbH (GBF) | Method for marker-free repetitive DNA expression cassette exchange in the genome of cells or parts of cells |
| GB9823930D0 (en) | 1998-11-03 | 1998-12-30 | Babraham Inst | Murine expression of human ig\ locus |
| US6914128B1 (en) | 1999-03-25 | 2005-07-05 | Abbott Gmbh & Co. Kg | Human antibodies that bind human IL-12 and methods for producing |
| CA2373221A1 (en) * | 1999-05-03 | 2000-11-30 | Yashwant M. Deo | Human antibodies to staphylococcus aureus |
| US6833268B1 (en) | 1999-06-10 | 2004-12-21 | Abgenix, Inc. | Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions |
| US7605238B2 (en) | 1999-08-24 | 2009-10-20 | Medarex, Inc. | Human CTLA-4 antibodies and their uses |
| CA2307503A1 (en) | 2000-05-02 | 2001-11-02 | Carlos F. Barbas Iii | Peptides for use as a vaccine or treatment for hiv infection |
| BR0110927A (en) * | 2000-05-19 | 2003-03-11 | Scancell Ltd | Antibody, nucleic acid, vector, cell, method of making an antibody, pharmaceutical composition, use of an antibody or nucleic acid, and method for treating or prophylaxis of cancer |
| JP2004504055A (en) | 2000-07-21 | 2004-02-12 | アメリカ合衆国 | Methods of DNA replacement, translocation and stacking in eukaryotic genomes |
| US7129084B2 (en) * | 2000-08-03 | 2006-10-31 | Therapeutic Human Polyclonals, Inc. | Production of humanized antibodies in transgenic animals |
| US8165246B1 (en) * | 2000-08-28 | 2012-04-24 | Alcatel Lucent | Training sequence for low latency LMS implementation |
| US7105348B2 (en) | 2000-10-31 | 2006-09-12 | Regeneron Pharmaceuticals, Inc. | Methods of modifying eukaryotic cells |
| EP2042594B1 (en) | 2000-11-17 | 2011-03-23 | Kyowa Hakko Kirin Co., Ltd. | Expression of xenogenous (human) immunoglobulins in cloned, transgenic ungulates |
| KR100857943B1 (en) * | 2000-11-30 | 2008-09-09 | 메다렉스, 인코포레이티드 | Transgenic Transchromosomal Rodents for Making Human Antibodies |
| US20030093818A1 (en) | 2000-12-19 | 2003-05-15 | Belmont Heather J. | Transgenic animals comprising a humanized immune system |
| AP2072A (en) * | 2001-01-05 | 2009-12-10 | Pfizer | Antibodies to insulin-like growth factor I receptor |
| FR2827302B1 (en) | 2001-07-13 | 2003-10-10 | Genoway | TRANSGENIC CELL AND ANIMAL MODELING HUMAN ANTIGENIC PRESENTATION AND USES THEREOF |
| US20030108925A1 (en) | 2001-10-05 | 2003-06-12 | U.S. Epa | Genetic testing for male factor infertility |
| US20060199204A1 (en) | 2001-10-05 | 2006-09-07 | U.S. Epa | Genetic testing for male factor infertility |
| US7390885B2 (en) * | 2001-11-26 | 2008-06-24 | Cell Matrix, Inc. | Humanized collagen antibodies and related methods |
| ES2645698T3 (en) | 2001-11-30 | 2017-12-07 | Amgen Fremont Inc. | Transgenic animals that carry human Ig light chain genes |
| AU2003214842A1 (en) | 2002-01-17 | 2003-09-02 | Albert Einstein College Of Medicine Of Yeshiva University | Mutations caused by activation-induced cytidine deaminase |
| US8877901B2 (en) | 2002-12-13 | 2014-11-04 | Immunomedics, Inc. | Camptothecin-binding moiety conjugates |
| AU2003250074B2 (en) | 2002-07-18 | 2010-09-09 | Merus N.V. | Recombinant production of mixtures of antibodies |
| CA2496233A1 (en) | 2002-09-09 | 2004-03-18 | California Institute Of Technology | Methods and compositions for the generation of humanized mice |
| KR20110017020A (en) * | 2002-10-17 | 2011-02-18 | 젠맵 에이/에스 | Human monoclonal antibodies against cd20 |
| US7700356B2 (en) | 2002-11-08 | 2010-04-20 | The United States Of America As Represented By The Secretary Of Agriculture | System for gene targeting and producing stable genomic transgene insertions |
| DE10251918A1 (en) | 2002-11-08 | 2004-05-19 | Horn, Carsten, Dipl.-Biochem. Dr. | Inheritable integration of DNA into germ-line invertebrate cells, useful for large-scale production of transgenic insects, includes post-transformational removal of mobilizable elements to increase stability |
| EP2258392A1 (en) | 2002-11-08 | 2010-12-08 | Ablynx N.V. | Method of administering therapeutic polypeptides |
| AR042145A1 (en) | 2002-11-27 | 2005-06-08 | Dow Agrociences Llc | IMMUNOGLOBULIN PRODUCTION IN PLANTS WITH A REDUCED FUCOCILATION |
| GB2398784B (en) | 2003-02-26 | 2005-07-27 | Babraham Inst | Removal and modification of the immunoglobulin constant region gene cluster of a non-human mammal |
| AU2004242614B2 (en) | 2003-05-30 | 2011-09-22 | Merus N.V. | Fab library for the preparation of anti vegf and anti rabies virus fabs |
| US20100069614A1 (en) | 2008-06-27 | 2010-03-18 | Merus B.V. | Antibody producing non-human mammals |
| WO2005001087A2 (en) | 2003-06-11 | 2005-01-06 | Regeneron Pharmaceuticals, Inc. | Methods of modifying genes in eukaryotic cells |
| GB2403475B (en) | 2003-07-01 | 2008-02-06 | Oxitec Ltd | Stable integrands |
| ES2473596T3 (en) * | 2003-07-15 | 2014-07-07 | Therapeutic Human Polyclonals, Inc. | Humanized immunoglobulin loci |
| US7663017B2 (en) | 2003-07-30 | 2010-02-16 | Institut Pasteur | Transgenic mice having a human major histocompatability complex (MHC) phenotype, experimental uses and applications |
| US20050153392A1 (en) | 2003-08-11 | 2005-07-14 | Roland Buelow | Transgenesis with humanized immunoglobulin loci |
| US7604994B2 (en) | 2003-09-03 | 2009-10-20 | Morphotek, Inc. | Genetically altered antibody-producing cell lines with improved antibody characteristics |
| US7205140B2 (en) | 2003-10-20 | 2007-04-17 | Campusgen Gmbh | Nucleotide sequence for creatinine deiminase and method of use |
| US20050191293A1 (en) | 2003-12-10 | 2005-09-01 | Shrikant Deshpande | IP-10 antibodies and their uses |
| US7625549B2 (en) * | 2004-03-19 | 2009-12-01 | Amgen Fremont Inc. | Determining the risk of human anti-human antibodies in transgenic mice |
| US20050260679A1 (en) | 2004-03-19 | 2005-11-24 | Sirid-Aimee Kellerman | Reducing the risk of human anti-human antibodies through V gene manipulation |
| NZ550106A (en) * | 2004-04-22 | 2009-06-26 | Kyowa Hakko Kirin Co Ltd | Transgenic animals and uses thereof |
| JP2006029459A (en) | 2004-07-16 | 2006-02-02 | Kayaba Ind Co Ltd | Buffer |
| RU2398882C2 (en) | 2004-07-22 | 2010-09-10 | Эрасмус Юниверсити Медикал Сентр Роттердам | METHOD OF PRODUCING ANTIGEN-BINDING Vh DOMAIN, APPLICATION THEREOF |
| FR2875239B1 (en) | 2004-09-10 | 2007-07-20 | Inst Necker Ass Loi De 1901 | METHOD FOR THE ACCELERATION OF SOMATIC MUTATIONS AND ITS APPLICATION IN PROTEOMICS |
| CN101076542A (en) | 2004-09-13 | 2007-11-21 | 伊沃詹尼克斯有限公司 | Antibodies specific for hepatocellular carcinoma and other carcinomas and uses thereof |
| WO2006044492A2 (en) | 2004-10-14 | 2006-04-27 | Ingenious Targeting Laboratory, Inc. | Methods for generating rat embryo-derived cell lines and genetic modification of rat genome |
| SI1802193T1 (en) | 2004-10-19 | 2014-08-29 | Regeneron Pharmaceuticals, Inc. | Method for generating a mouse homozygous for a genetic modification |
| WO2006055704A2 (en) | 2004-11-17 | 2006-05-26 | Curagen Corporation | Antibodies directed to ten-m proteins and uses thereof |
| EP3699191A1 (en) | 2004-12-21 | 2020-08-26 | MedImmune Limited | Antibodies directed to angiopoietin-2 and uses thereof |
| CA2604440A1 (en) | 2005-04-29 | 2006-11-09 | Inserm (Institut De La Sante Et De La Recherche Medicale) | Transgenic animals and methods of making recombinant antibodies |
| BRPI0520212A2 (en) | 2005-05-14 | 2009-08-18 | Univ Fudan | methods of generating a transgenic non-human vertebrate, and a recombinant vertebrate cell, cultured vertebrate cell, and nucleic acid |
| EP1780272A1 (en) | 2005-10-27 | 2007-05-02 | GSF-Forschungszentrum für Umwelt und Gesundheit GmbH | Method for enhancing somatic hypermutation, gene conversion and class switch recombination |
| GB0601513D0 (en) | 2006-01-25 | 2006-03-08 | Univ Erasmus Medical Ct | Binding molecules 3 |
| SG169348A1 (en) | 2006-01-25 | 2011-03-30 | Univ Erasmus Medical Ct | Generation of heavy-chain only antibodies in transgenic animals |
| US7462759B2 (en) | 2006-02-03 | 2008-12-09 | Pioneer Hi-Bred International, Inc. | Brittle stalk 2 gene family and related methods and uses |
| AU2007235496B2 (en) | 2006-03-31 | 2013-11-21 | E. R. Squibb & Sons, L.L.C. | Transgenic animals expressing chimeric antibodies for use in preparing human antibodies |
| SI2374818T1 (en) | 2006-06-02 | 2013-03-29 | Regeneron Pharmaceuticals, Inc. | High affinity antibodies to human IL-6 receptor |
| EP2409715A1 (en) | 2006-06-27 | 2012-01-25 | Sanofi Pasteur VaxDesign Corporation | Models for vaccine assessment |
| EP1878342A1 (en) | 2006-07-13 | 2008-01-16 | Institut Pasteur | Immunodeficient mice transgenic for HLA class I and HLA class II molecules and their uses |
| CN101501073A (en) | 2006-08-22 | 2009-08-05 | G2英弗勒美欣私人有限公司 | Method for producing antibodies |
| PL2769992T3 (en) | 2006-10-02 | 2021-08-02 | Regeneron Pharmaceuticals, Inc. | High affinity human antibodies to human IL-4 receptor |
| US7732195B2 (en) | 2006-11-01 | 2010-06-08 | Facet Biotech Corporation | Tethered vectors for cell surface immunoglobulin display |
| NO347649B1 (en) | 2006-12-14 | 2024-02-12 | Regeneron Pharma | Human antibody or antibody fragment that specifically binds human delta-like ligand 4 (hDII4), nucleic acid molecule that codes for such and vector and host-vector systems, as well as method for production, composition and use. |
| GB0700194D0 (en) | 2007-01-05 | 2007-02-14 | Univ Edinburgh | Humanisation of animals |
| NZ579010A (en) | 2007-02-20 | 2012-03-30 | Anaptysbio Inc | Somatic hypermutation systems |
| JP5642972B2 (en) | 2007-02-21 | 2014-12-17 | ユニバーシティー オブ マサチューセッツUniversity of Massachusetts | Human antibody against hepatitis C virus (HCV) and use thereof |
| WO2008118970A2 (en) | 2007-03-27 | 2008-10-02 | Sea Lane Biotechnologies, Llc | Constructs and libraries comprising antibody surrogate light chain sequences |
| GB0706628D0 (en) | 2007-04-04 | 2007-05-16 | Univ Erasmus | Germ-line manipulation 1 |
| NZ581396A (en) | 2007-06-01 | 2012-07-27 | Omt Inc | Compositions and methods for inhibiting endogenous immunoglobulin genes and producing transgenic human idiotype antibodies |
| WO2009013620A2 (en) | 2007-06-11 | 2009-01-29 | Erasmus University Medical Center Rotterdam | Homologous recombination |
| EP2178916B1 (en) | 2007-07-31 | 2014-12-17 | Regeneron Pharmaceuticals, Inc. | Human antibodies to human cd20 and method of using thereof |
| BRPI0815370B8 (en) | 2007-08-10 | 2021-05-25 | Regeneron Pharma | high affinity human antibodies against human neural growth factor and its use, nucleic acid molecule, expression vector, method for producing an anti-human ngf antibody, and pharmaceutical composition |
| ES2695999T3 (en) | 2007-10-12 | 2019-01-11 | Hoffmann La Roche | Expression of proteins from multiple nucleic acids |
| US8227577B2 (en) | 2007-12-21 | 2012-07-24 | Hoffman-La Roche Inc. | Bivalent, bispecific antibodies |
| JP2011510155A (en) | 2008-01-25 | 2011-03-31 | キャボット コーポレイション | Method for preparing modified color pigments |
| JP2011515098A (en) | 2008-03-26 | 2011-05-19 | アイティーアイ・スコットランド・リミテッド | Efficient insertion of DNA into embryonic stem cells |
| US8012714B2 (en) | 2008-04-14 | 2011-09-06 | Innovative Targeting Solutions, Inc. | Sequence diversity generation in immunoglobulins |
| CA2729095C (en) | 2008-06-27 | 2018-12-04 | Merus B.V. | Method for producing a transgenic murine mammal |
| JO3672B1 (en) * | 2008-12-15 | 2020-08-27 | Regeneron Pharma | High Affinity Human Antibodies to PCSK9 |
| MX2011007660A (en) | 2008-12-18 | 2011-08-17 | Kingdon Craig R | Non-human transgenic animals expressing humanised antibodies and use therof. |
| US9085795B2 (en) | 2009-02-04 | 2015-07-21 | Molecular Innovations, Inc. | Methods for screening candidate agents for modulating prorenin and renin, assays for detecting prorenin and antibodies |
| US20110305692A1 (en) | 2009-02-24 | 2011-12-15 | Glaxo Group Limited | Antigen-binding contructs |
| GB0905023D0 (en) * | 2009-03-24 | 2009-05-06 | Univ Erasmus Medical Ct | Binding molecules |
| EP2414507B1 (en) | 2009-04-03 | 2014-07-02 | Medical Research Council | Mutants of activation-induced cytidine deaminase (aid) and methods of use |
| CN102498210B (en) | 2009-06-26 | 2015-07-29 | 航道生物技术有限责任公司 | Substitute the expression of light chain |
| US9445581B2 (en) | 2012-03-28 | 2016-09-20 | Kymab Limited | Animal models and therapeutic molecules |
| WO2011008093A1 (en) | 2009-07-15 | 2011-01-20 | Aimm Therapeutics B.V. | Means and methods for producing high affinity antibodies |
| JO3182B1 (en) | 2009-07-29 | 2018-03-08 | Regeneron Pharma | High Affinity Human Antibodies to Human Angiopoietin-2 |
| US8586526B2 (en) | 2010-05-17 | 2013-11-19 | Sangamo Biosciences, Inc. | DNA-binding proteins and uses thereof |
| US8592644B2 (en) | 2009-08-13 | 2013-11-26 | Crystal Bioscience Inc. | Transgenic animal for production of antibodies having minimal CDRS |
| CN102050877B (en) * | 2009-10-30 | 2014-05-07 | 上海抗体药物国家工程研究中心有限公司 | Anti-human CD20 humanized antibody, preparation method and application thereof |
| US20120251552A1 (en) | 2009-11-05 | 2012-10-04 | Anaptysbio, Inc. | Methods of generating improved antigen-binding agents using chain shuffling and optionally somatic hypermutation |
| JP5796846B2 (en) | 2009-11-17 | 2015-10-21 | エスエービー エルエルシー | Human artificial chromosome vector |
| WO2011071957A1 (en) | 2009-12-07 | 2011-06-16 | Sea Lane Biotechnologies, Llc | Conjugates comprising an antibody surrogate scaffold with improved pharmacokinetic properties |
| RU2603102C2 (en) | 2009-12-10 | 2016-11-20 | Ридженерон Фармасьютикалз, Инк. | Mice that make heavy chain antibodies |
| JO3274B1 (en) * | 2009-12-24 | 2018-09-16 | Regeneron Pharma | Human Antibodies To Human Angiopoietin-Like Protein 4 |
| US20120021409A1 (en) | 2010-02-08 | 2012-01-26 | Regeneron Pharmaceuticals, Inc. | Common Light Chain Mouse |
| PL2505654T5 (en) | 2010-02-08 | 2020-11-30 | Regeneron Pharmaceuticals, Inc. | Common light chain mouse |
| CA2802591A1 (en) | 2010-06-17 | 2011-12-22 | Kymab Limited | Animal models and therapeutic molecules |
| ME02444B (en) | 2010-06-22 | 2016-09-20 | Regeneron Pharma | Mice expressing an immunoglobulin hybrid light chain |
| CN118791596A (en) | 2010-08-02 | 2024-10-18 | 瑞泽恩制药公司 | Mice producing binding proteins comprising VL domains |
| DK2606064T3 (en) | 2010-08-16 | 2015-04-20 | Novimmune Sa | Methods for generating multispecific and multivalent antibodies |
| JO3375B1 (en) | 2010-11-08 | 2019-03-13 | Regeneron Pharma | Human antibodies to human tnf-like ligand 1a (tl1a) |
| MA34818B1 (en) | 2010-12-22 | 2014-01-02 | Genentech Inc | ANTI-PCSK9 ANTIBODIES AND METHODS OF USE |
| CN103917650B (en) | 2011-08-05 | 2017-10-24 | 瑞泽恩制药公司 | The universal light chain mouse of humanization |
| ES2612935T3 (en) | 2011-09-19 | 2017-05-19 | Kymab Limited | Antibodies, variable domains and chains adapted for use in humans |
| EP3741862A1 (en) | 2011-09-19 | 2020-11-25 | Kymab Limited | Animals, repertoires & methods for the production of human antibodies |
| WO2013045916A1 (en) | 2011-09-26 | 2013-04-04 | Kymab Limited | Chimaeric surrogate light chains (slc) comprising human vpreb |
| WO2013059230A1 (en) | 2011-10-17 | 2013-04-25 | Regeneron Pharmaceuticals, Inc. | Restricted immunoglobulin heavy chain mice |
| KR102024958B1 (en) | 2011-10-20 | 2019-09-24 | 쓰리엠 이노베이티브 프로퍼티즈 컴파니 | Apodized broadband partial reflectors |
| US20130102031A1 (en) | 2011-10-25 | 2013-04-25 | Anaptysbio, Inc. | Use of somatic hypermutation to create insertion and deletion mutations in vitro |
| GB2496375A (en) | 2011-10-28 | 2013-05-15 | Kymab Ltd | A non-human assay vertebrate comprising human antibody loci and human epitope knock-in, and uses thereof |
| US20180295821A1 (en) | 2011-12-02 | 2018-10-18 | Kymab Limited | Transgenic Animals |
| US9253965B2 (en) | 2012-03-28 | 2016-02-09 | Kymab Limited | Animal models and therapeutic molecules |
| GB201122047D0 (en) | 2011-12-21 | 2012-02-01 | Kymab Ltd | Transgenic animals |
| MY173936A (en) | 2011-12-20 | 2020-02-27 | Regeneron Pharma | Humanized light chain mice field of invention |
| BR112014018843B1 (en) | 2012-02-01 | 2024-02-20 | Regeneron Pharmaceuticals, Inc | METHODS FOR OBTAINING A MOUSE, FOR OBTAINING A GENETICALLY MODIFIED MOUSE, FOR PRODUCING A PROTEIN AND FOR MODIFYING AN IMMUNOGLOBULIN HEAVY CHAIN LOCUS OF A MOUSE |
| NZ714516A (en) | 2012-03-02 | 2017-06-30 | Regeneron Pharma | Human antibodies to clostridium difficile toxins |
| RU2683514C2 (en) | 2012-03-06 | 2019-03-28 | Регенерон Фармасьютикалз, Инк. | Common light chain mouse |
| MY173376A (en) | 2012-03-16 | 2020-01-21 | Regeneron Pharma | Mice that produce antigen?binding proteins with ph?dependent binding characteristics |
| SG11201405059XA (en) | 2012-03-28 | 2014-09-26 | Kymab Ltd | Transgenic non-human vertebrate for the expression of class - switched, fully human, antibodies |
| GB2502127A (en) | 2012-05-17 | 2013-11-20 | Kymab Ltd | Multivalent antibodies and in vivo methods for their production |
| US10251377B2 (en) | 2012-03-28 | 2019-04-09 | Kymab Limited | Transgenic non-human vertebrate for the expression of class-switched, fully human, antibodies |
| JO3820B1 (en) | 2012-05-03 | 2021-01-31 | Regeneron Pharma | Human antibodies to fel d1 and methods of use thereof |
| MA37663B1 (en) | 2012-05-25 | 2019-12-31 | Univ California | Methods and compositions for modifying rna-directed target DNA and modulating rna-directed transcription |
| KR20150027793A (en) | 2012-06-12 | 2015-03-12 | 리제너론 파마슈티칼스 인코포레이티드 | Humanized non-human animals with restricted immunoglobulin heavy chain loci |
| US8962913B2 (en) | 2012-06-18 | 2015-02-24 | Regeneron Pharmaceuticals, Inc. | Humanized IL-7 rodents |
| RU2721275C2 (en) | 2012-12-12 | 2020-05-18 | Те Брод Инститьют, Инк. | Delivery, construction and optimization of systems, methods and compositions for sequence manipulation and use in therapy |
| EP2840892B1 (en) | 2013-02-20 | 2018-04-18 | Regeneron Pharmaceuticals, Inc. | Non-human animals with modified immunoglobulin heavy chain sequences |
| US10993420B2 (en) | 2013-03-15 | 2021-05-04 | Erasmus University Medical Center | Production of heavy chain only antibodies in transgenic mammals |
| US9788534B2 (en) | 2013-03-18 | 2017-10-17 | Kymab Limited | Animal models and therapeutic molecules |
| US9783618B2 (en) | 2013-05-01 | 2017-10-10 | Kymab Limited | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics |
| US20150033372A1 (en) | 2013-05-01 | 2015-01-29 | Kymab Limited | Human VpreB & Chimaeric Surrogate Light Chains in Transgenic Non-Human Vertebrates |
| US11707056B2 (en) | 2013-05-02 | 2023-07-25 | Kymab Limited | Animals, repertoires and methods |
| US9783593B2 (en) | 2013-05-02 | 2017-10-10 | Kymab Limited | Antibodies, variable domains and chains tailored for human use |
| US20140331339A1 (en) | 2013-05-03 | 2014-11-06 | Kymab Limited | Transgenic Non-Human Assay Vertebrates, Assays and Kits |
| US20140331344A1 (en) | 2013-05-03 | 2014-11-06 | Kymab Ltd. | Transgenic Animals |
| EP3794941B1 (en) | 2013-10-01 | 2024-09-25 | Kymab Limited | Animal models and therapeutic molecules |
| GB201710984D0 (en) | 2017-07-07 | 2017-08-23 | Kymab Ltd | Cells, vertebrates, populations & methods |
-
2012
- 2012-09-18 ES ES12772122.3T patent/ES2612935T3/en active Active
- 2012-09-18 BR BR112014006390A patent/BR112014006390A2/en not_active Application Discontinuation
- 2012-09-18 EP EP17196235.0A patent/EP3311661B1/en active Active
- 2012-09-18 CN CN201610883894.2A patent/CN106995822B/en active Active
- 2012-09-18 JP JP2014531304A patent/JP2014533930A/en active Pending
- 2012-09-18 CN CN201280056528.XA patent/CN103945689B/en active Active
- 2012-09-18 CA CA2846322A patent/CA2846322A1/en not_active Abandoned
- 2012-09-18 AU AU2012311286A patent/AU2012311286B2/en not_active Expired - Fee Related
- 2012-09-18 BR BR112014006394A patent/BR112014006394A2/en not_active IP Right Cessation
- 2012-09-18 DK DK17196235.0T patent/DK3311661T3/en active
- 2012-09-18 EP EP12772122.3A patent/EP2758535B1/en not_active Revoked
- 2012-09-18 EP EP22168117.4A patent/EP4091442A1/en active Pending
- 2012-09-18 CN CN201280056727.0A patent/CN104080916A/en active Pending
- 2012-09-18 AU AU2012311288A patent/AU2012311288B2/en not_active Ceased
- 2012-09-18 WO PCT/GB2012/052298 patent/WO2013041846A2/en active Application Filing
- 2012-09-18 DK DK12762378.3T patent/DK2757875T3/en active
- 2012-09-18 CA CA2846319A patent/CA2846319A1/en not_active Abandoned
- 2012-09-18 EP EP16189625.3A patent/EP3128009B1/en active Active
- 2012-09-18 CN CN201610821299.6A patent/CN107056936B/en active Active
- 2012-09-18 WO PCT/GB2012/052296 patent/WO2013041844A2/en active Application Filing
- 2012-09-18 EP EP12762378.3A patent/EP2757875B2/en active Active
- 2012-09-18 EP EP20188009.3A patent/EP3839049A3/en not_active Withdrawn
- 2012-09-18 JP JP2014531302A patent/JP5813880B2/en active Active
-
2013
- 2013-10-11 US US14/052,259 patent/US20140041067A1/en not_active Abandoned
-
2014
- 2014-03-19 US US14/220,074 patent/US11051497B2/en active Active
- 2014-08-05 HK HK14108036.3A patent/HK1194757A1/en not_active IP Right Cessation
-
2015
- 2015-09-16 JP JP2015182782A patent/JP6324355B2/en active Active
-
2017
- 2017-02-08 JP JP2017021028A patent/JP7067868B2/en active Active
- 2017-10-17 US US15/786,281 patent/US20180030121A1/en active Pending
- 2017-11-03 AU AU2017254949A patent/AU2017254949A1/en not_active Abandoned
-
2021
- 2021-07-06 US US17/368,266 patent/US20220000085A1/en active Pending
Patent Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5939598A (en) | 1990-01-12 | 1999-08-17 | Abgenix, Inc. | Method of making transgenic mice lacking endogenous heavy chains |
| US6673986B1 (en) | 1990-01-12 | 2004-01-06 | Abgenix, Inc. | Generation of xenogeneic antibodies |
| US7501552B2 (en) | 1991-08-28 | 2009-03-10 | Medarex, Inc. | Transgenic non-human animals for producing chimeric antibodies |
| US5859301A (en) | 1993-12-23 | 1999-01-12 | Dsm N.V. | Process for preparing alkanones and alkanols |
| US6130364A (en) | 1995-03-29 | 2000-10-10 | Abgenix, Inc. | Production of antibodies using Cre-mediated site-specific recombination |
| EP0937140A2 (en) | 1996-06-27 | 1999-08-25 | Vlaams Interuniversitair Instituut voor Biotechnologie vzw. | Recognition molecules interacting specifically with the active site or cleft of a target molecule |
| EP1034260A2 (en) | 1997-12-05 | 2000-09-13 | Europäisches Laboratorium Für Molekularbiologie (Embl) | Novel dna cloning method relying on the e. coli rece/rect recombination system |
| EP1204740A1 (en) | 1999-07-09 | 2002-05-15 | The European Molecular Biology Laboratory | Methods and compositions for directed cloning and subcloning using homologous recombination |
| US6586251B2 (en) | 2000-10-31 | 2003-07-01 | Regeneron Pharmaceuticals, Inc. | Methods of modifying eukaryotic cells |
| WO2002066630A1 (en) | 2001-02-16 | 2002-08-29 | Regeneron Pharmaceuticals, Inc. | Methods of modifying eukaryotic cells |
| EP1399559A2 (en) | 2001-06-21 | 2004-03-24 | The Babraham Institute | Mouse lambda light chain locus |
| WO2009097006A2 (en) | 2007-08-10 | 2009-08-06 | Medarex, Inc. | Hco32 and hco27 and related examples |
| WO2009076464A2 (en) | 2007-12-10 | 2009-06-18 | Aliva Biopharmaceuticals, Inc. | Methods for sequential replacement of targeted region by homologous recombination |
| WO2009143472A2 (en) | 2008-05-23 | 2009-11-26 | Aliva Biopharmaceuticals, Inc. | Method of generating single vl domain antibodies in transgenic animals |
| WO2010039900A2 (en) | 2008-09-30 | 2010-04-08 | Aliva Biopharmaceuticals, Inc. | Non-human mammals for the production of chimeric antibodies |
| WO2011004192A1 (en) | 2009-07-08 | 2011-01-13 | Genome Research Limited | Animal models and therapeutic molecules |
| WO2011066501A1 (en) | 2009-11-30 | 2011-06-03 | Centocor Ortho Biotech Inc. | Antibody fc mutants with ablated effector functions |
| WO2012141798A1 (en) | 2011-02-25 | 2012-10-18 | Regeneron Pharmaceuticals, Inc. | Adam6 mice |
Non-Patent Citations (30)
| Title |
|---|
| "HapMap", 2003, THE INTERNATIONAL HAPMAP CONSORTIUM |
| ANNU. REV. BIOCHEM., vol. 76, 2007, pages 1 - 22 |
| AUERBACH W; DUNMORE JH; FAIRCHILD-HUNTRESS V ET AL.: "Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines", BIOTECHNIQUES, vol. 29, 2000, pages 1024 - 8 |
| BENNY K C LO: "Methods in Molecular BiologyTM", HUMAN PRESS, article "Antibody Engineering Methods and Protocols" |
| CAMACHO C.; COULOURIS G.; AVAGYAN V.; MA N.; PAPADOPOULOS J.; BEALER K.; MADDEN T.L.: "BLAST+: architecture and applications.", BMC BIOINFORMATICS, vol. 10, 2008, pages 421, Retrieved from the Internet <URL:https://www.ncbi.nlm.nih.gov/pubmed/20003500> |
| CURR OPIN IMMUNOL., vol. 7, no. 2, April 1995 (1995-04-01), pages 248 - 54 |
| GENOMES PROJECT CONSORTIUM, 2010, Retrieved from the Internet <URL:https://www.1000genomes.org> |
| H.HAGA ET AL., JAPANESE SNP DATABASE, 2002, Retrieved from the Internet <URL:https://snp.ims.u-tokyo.ac.ip/index.html> |
| HARLOW, E.; LANE, D.: "Antibodies: A Laboratory Manual, 5th edition,", 1998, COLD SPRING HARBOR LAB. PRESS |
| J MOL BIOL., vol. 270, no. 4, 25 July 1997 (1997-07-25), pages 587 - 97 |
| J. SALDANHA, HUMANIZATION BYDESIGN, 2000 |
| JAVIER M. DI NOIA; MICHAEL S. NEUBERGER, MOLECULAR MECHANISMS OF ANTIBODY SOMATIC HYPERMUTATION |
| KABAT, E. A.; WU, T. T.; PERRY, H.; GOTTESMAN, K.; FOELLER, C.: "Sequences of Proteins of Immunological Interest, 5th ed.,", 1991, NIH PUBLICATION NO. 91- 3242 |
| LIU, L ET AL., JOURNAL OF VIROLOGY, vol. 85, 2011, pages 8467 - 8476 |
| M. R. CLARK, MIKE'S IMMUNOGLOBULIN STRUCTURE/FUNCTION, 2001, Retrieved from the Internet <URL:http:llwww.path.cam.ac.uk/~mrc7/mikeimages.htm/> |
| MARIE-PAULE LEFRANC; GÉRARD LEFRANC: "The Immunoglobulin Facts Book", 2001, ACADEMIC PRESS, ISBN: 0-12-441351-X, pages: 3pp, 104, XP003035047 |
| MOL IMMUNOL., vol. 40, no. 9, December 2003 (2003-12-01), pages 585 - 93 |
| N A ROSENBERG ET AL., SCIENCE, vol. 298, no. 5602, 20 December 2002 (2002-12-20), pages 2342 - 2343 |
| N. WHITELEGG; A. R. REES: "WAM?Web Antibody Modeling", 2001, CENTRE FOR PROTEIN ANALYSIS AND DESIGN AT THE UNIVERSITY OF BATH |
| NAT REV GENET., vol. 2, no. 10, October 2001 (2001-10-01), pages 769 - 79 |
| NATURE, vol. 426, no. 6968, 18 December 2003 (2003-12-18), pages 789 - 96 |
| OSOEGAWA K; MAMMOSER AG; WU C; FRENGEN E; ZENG C; CATANESE JJ; DE JONG PJ: "A bacterial artificial chromosome library for sequencing the complete human genome", GENOME RES., vol. 11, no. 3, March 2001 (2001-03-01), pages 483 - 96, XP002310986, DOI: doi:10.1101/gr.169601 |
| OSOEGAWA, K.; WOON, P.Y.; ZHAO, B.; FRENGEN, E.; TATENO, M.; CATANESE, J.J; JONG, P.J.: "An Improved Approach for Construction of Bacterial Artificial Chromosome Libraries", GENOMICS, vol. 52, 1998, pages 1 - 8, XP004449066, DOI: doi:10.1006/geno.1998.5423 |
| PASQUALINI; ARAP, PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES, vol. 101, 2004, pages 257 - 259 |
| PLOS ONE, vol. 6, no. 3, 30 March 2011 (2011-03-30), pages EL6857 |
| PLOS ONE., vol. 7, no. 5, 9 May 2012 (2012-05-09), pages E36750 |
| ROGOZIN; DIAZ: "Cutting Edge: DGYW/WRCH Is a Better Predictor of Mutability at G:C Bases in lg Hypermutation Than the Widely Accepted RGYW/WRCY Motif and Probably Reflects a Two-Step Activation-Induced Cytidine Deaminase-Triggered Process", JOURNAL OF IMMUNOLOGY, vol. 172, no. 6, 15 March 2004 (2004-03-15), pages 3382 - 3384 |
| SAMBROOK, J; RUSSELL, D.: "Molecular Cloning: A Laboratory Manual, 3'd edition", 2001, COLD SPRING HARBOR LAB. PRESS |
| See also references of EP2758535A2 |
| THE ANTIBODY RESOURCE PAGE, 2000 |
Cited By (129)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9447177B2 (en) | 2009-07-08 | 2016-09-20 | Kymab Limited | Transgenic mouse homozygous for chimeric IgH locus |
| US9434782B2 (en) | 2009-07-08 | 2016-09-06 | Kymab Limited | Animal models and therapeutic molecules |
| US10064398B2 (en) | 2009-07-08 | 2018-09-04 | Kymab Limited | Animal models and therapeutic molecules |
| US11812731B2 (en) | 2009-07-08 | 2023-11-14 | Kymab Ltd. | Animal models and therapeutic molecules |
| US11606941B2 (en) | 2009-07-08 | 2023-03-21 | Kymab Limited | Animal models and therapeutic molecules |
| US11564380B2 (en) | 2009-07-08 | 2023-01-31 | Kymab Limited | Animal models and therapeutic molecules |
| US10165763B2 (en) | 2009-07-08 | 2019-01-01 | Kymab Limited | Animal models and therapeutic molecules |
| US9505827B2 (en) | 2009-07-08 | 2016-11-29 | Kymab Limited | Animal models and therapeutic molecules |
| US9504236B2 (en) | 2009-07-08 | 2016-11-29 | Kymab Limited | Animal models and therapeutic molecules |
| US10954310B2 (en) | 2010-08-02 | 2021-03-23 | Regeneran Pharmaceuticals, Inc. | Mice that make VL binding proteins |
| US9686970B2 (en) | 2010-08-02 | 2017-06-27 | Regeneron Pharmaceuticals, Inc. | Mice that make VL binding proteins |
| US9516868B2 (en) | 2010-08-02 | 2016-12-13 | Regeneron Pharmaceuticals, Inc. | Mice that make VL binding proteins |
| US10130081B2 (en) | 2011-08-05 | 2018-11-20 | Regeneron Pharmaceuticals, Inc. | Humanized universal light chain mice |
| US11357217B2 (en) | 2011-08-05 | 2022-06-14 | Regeneron Pharmaceuticals, Inc. | Humanized universal light chain mice |
| US11051497B2 (en) | 2011-09-19 | 2021-07-06 | Kymab Limited | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics |
| US9963716B2 (en) | 2011-09-26 | 2018-05-08 | Kymab Limited | Chimaeric surrogate light chains (SLC) comprising human VpreB |
| US9932398B2 (en) | 2011-10-17 | 2018-04-03 | Regeneron Pharmaceuticals, Inc. | Restricted immunoglobulin heavy chain mice |
| US10246509B2 (en) | 2011-10-17 | 2019-04-02 | Regeneron Pharmaceuticals, Inc. | Restricted immunoglobulin heavy chain mice |
| US11261248B2 (en) | 2011-10-17 | 2022-03-01 | Regeneron Pharmaceuticals, Inc. | Restricted immunoglobulin heavy chain mice |
| US9622459B2 (en) | 2011-12-20 | 2017-04-18 | Regeneron Pharmaceuticals, Inc. | Humanized light chain mice |
| US10561124B2 (en) | 2011-12-20 | 2020-02-18 | Regeneron Pharmaceuticals, Inc. | Humanized light chain mice |
| US9706759B2 (en) | 2011-12-20 | 2017-07-18 | Regeneron Pharmaceuticals, Inc. | Humanized light chain mice |
| US11612151B2 (en) | 2011-12-20 | 2023-03-28 | Regeneron Pharmaceuticals, Inc. | Humanized light chain mice |
| US11617357B2 (en) | 2011-12-20 | 2023-04-04 | Regeneron Pharmaceuticals, Inc. | Humanized light chain mice |
| US11297811B2 (en) | 2012-03-28 | 2022-04-12 | Kymab Limited | Transgenic non-human vertebrate for the expression of class-switched, fully human, antibodies |
| US9924705B2 (en) | 2012-03-28 | 2018-03-27 | Kymab Limited | Animal models and therapeutic molecules |
| US9938357B2 (en) | 2012-03-28 | 2018-04-10 | Kymab Limited | Animal models and therapeutic molecules |
| US9938358B2 (en) | 2012-03-28 | 2018-04-10 | Kymab Limited | Animal models and therapeutic molecules |
| US10774155B2 (en) | 2012-03-28 | 2020-09-15 | Kymab Limited | Animal models and therapeutic molecules |
| US9896516B2 (en) | 2012-03-28 | 2018-02-20 | Kymab Limited | Animal models and therapeutic molecules |
| US9445581B2 (en) | 2012-03-28 | 2016-09-20 | Kymab Limited | Animal models and therapeutic molecules |
| US10251377B2 (en) | 2012-03-28 | 2019-04-09 | Kymab Limited | Transgenic non-human vertebrate for the expression of class-switched, fully human, antibodies |
| US9253965B2 (en) | 2012-03-28 | 2016-02-09 | Kymab Limited | Animal models and therapeutic molecules |
| US10667501B2 (en) | 2012-05-17 | 2020-06-02 | Kymab Limited | Transgenic non-human vertebrate for the in vivo production of dual specificity immunoglobulins or hypermutated heavy chain only immunoglobulins |
| US10542735B2 (en) | 2012-06-12 | 2020-01-28 | Regerneron Pharmaceuticals, Inc. | Humanized non-human animals with restricted immunoglobulin heavy chain loci |
| US11666040B2 (en) | 2012-06-12 | 2023-06-06 | Regeneron Pharmaceuticals, Inc. | Humanized non-human animals with restricted immunoglobulin heavy chain loci |
| US11559050B2 (en) | 2012-06-12 | 2023-01-24 | Regeneron Pharmaceuticals, Inc. | Humanized non-human animals with restricted immunoglobulin heavy chain loci |
| US10238093B2 (en) | 2012-06-12 | 2019-03-26 | Regeneron Pharmaceuticals, Inc. | Humanized non-human animals with restricted immunoglobulin heavy chain loci |
| US9930871B2 (en) | 2013-02-20 | 2018-04-03 | Regeneron Pharmaceuticals, Inc. | Non-human animals with modified immunoglobulin heavy chain sequences |
| US9788534B2 (en) | 2013-03-18 | 2017-10-17 | Kymab Limited | Animal models and therapeutic molecules |
| US10226033B2 (en) | 2013-03-18 | 2019-03-12 | Kymab Limited | Animal models and therapeutic molecules |
| US11297810B2 (en) | 2013-03-18 | 2022-04-12 | Kymab Limited | Animal models and therapeutic molecules |
| US9783618B2 (en) | 2013-05-01 | 2017-10-10 | Kymab Limited | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics |
| US11707056B2 (en) | 2013-05-02 | 2023-07-25 | Kymab Limited | Animals, repertoires and methods |
| US10730930B2 (en) | 2013-05-02 | 2020-08-04 | Kymab Limited | Antibodies, variable domains and chains tailored for human use |
| US11820810B2 (en) | 2013-05-02 | 2023-11-21 | Kymab Limited | Antibodies, variable domains and chains tailored for human use |
| US9783593B2 (en) | 2013-05-02 | 2017-10-10 | Kymab Limited | Antibodies, variable domains and chains tailored for human use |
| JP2021087442A (en) * | 2013-10-01 | 2021-06-10 | カイマブ・リミテッド | Animal models and therapeutic molecules |
| US10149462B2 (en) | 2013-10-01 | 2018-12-11 | Kymab Limited | Animal models and therapeutic molecules |
| NL2015773A (en) * | 2013-10-01 | 2016-09-22 | Kymab Ltd | Animal models and therapeutic molecules. |
| JP7179106B2 (en) | 2013-10-01 | 2022-11-28 | カイマブ・リミテッド | Animal models and therapeutic molecules |
| JP7133902B2 (en) | 2013-10-01 | 2022-09-09 | カイマブ・リミテッド | Animal models and therapeutic molecules |
| NL2013554A (en) * | 2013-10-01 | 2015-04-07 | Kymab Ltd | Animal models and therapeutic molecules. |
| CN105683365A (en) * | 2013-10-01 | 2016-06-15 | 科马布有限公司 | Animal models and therapeutic molecules |
| EP3794941A1 (en) | 2013-10-01 | 2021-03-24 | Kymab Limited | Animal models and therapeutic molecules |
| FR3011249A1 (en) * | 2013-10-01 | 2015-04-03 | Kymab Ltd | |
| US10966412B2 (en) | 2013-10-01 | 2021-04-06 | Kymab Limited | Animal models and therapeutic molecules |
| US11399522B2 (en) | 2013-10-01 | 2022-08-02 | Kymab Limited | Animal models and therapeutic molecules |
| WO2015049517A3 (en) * | 2013-10-01 | 2015-08-06 | Kymab Limited | Animal models and therapeutic molecules |
| EP2886558A1 (en) * | 2013-12-17 | 2015-06-24 | Kymab Limited | Antibodies for use in treating conditions related to specific PCSK9 variants in specific patient populations |
| US9040052B1 (en) | 2013-12-17 | 2015-05-26 | Kymab Limited | Precision Medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| JP2017502030A (en) * | 2013-12-17 | 2017-01-19 | カイマブ・リミテッド | Ligands that specifically bind to the target human target |
| US10611849B2 (en) | 2013-12-17 | 2020-04-07 | Kymab Limited | Precision medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| US10618971B2 (en) | 2013-12-17 | 2020-04-14 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| EP2886557A1 (en) * | 2013-12-17 | 2015-06-24 | Kymab Limited | Antibodies for use in treating conditions related to specific PCSK9 variants in specific patient populations |
| WO2015092394A1 (en) * | 2013-12-17 | 2015-06-25 | Kymab Limited | Antibodies for use in treating conditions related to specific pcsk9 variants in specific patients populations |
| DE202014010421U1 (en) | 2013-12-17 | 2015-11-12 | Kymab Limited | Human goals |
| DE202014010499U1 (en) | 2013-12-17 | 2015-10-20 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| WO2015092393A3 (en) * | 2013-12-17 | 2015-09-11 | Kymab Limited | Ligands specifically binding to human targets of interest |
| US11434305B2 (en) | 2013-12-17 | 2022-09-06 | Kymab Limited | Precision medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| JP2017505127A (en) * | 2014-01-24 | 2017-02-16 | チルドレンズ メディカル センター コーポレーション | High-throughput mouse model for antibody affinity optimization |
| US11974553B2 (en) | 2014-01-24 | 2024-05-07 | Children's Medical Center Corporation | High-throughput mouse model for optimizing antibody affinities |
| US11753479B2 (en) | 2014-03-04 | 2023-09-12 | Kymab Limited | Nucleic acids encoding anti-OX40L antibodies |
| US11773175B2 (en) | 2014-03-04 | 2023-10-03 | Kymab Limited | Antibodies, uses and methods |
| US10881085B2 (en) | 2014-03-21 | 2021-01-05 | Regeneron Pharmaceuticals, Inc. | Non-human animals that make single domain binding proteins |
| US10787522B2 (en) | 2014-03-21 | 2020-09-29 | Regeneron Pharmaceuticals, Inc. | VL antigen binding proteins exhibiting distinct binding characteristics |
| WO2016008899A1 (en) | 2014-07-15 | 2016-01-21 | Kymab Limited | Targeting human pcsk9 for cholesterol treatment |
| US9139648B1 (en) | 2014-07-15 | 2015-09-22 | Kymab Limited | Precision medicine by targeting human NAV1.9 variants for treatment of pain |
| EP3332790A1 (en) | 2014-07-15 | 2018-06-13 | Kymab Limited | Antibodies for use in treating conditions related to specific pcsk9 variants in specific patients populations |
| US9914769B2 (en) | 2014-07-15 | 2018-03-13 | Kymab Limited | Precision medicine for cholesterol treatment |
| US9439963B2 (en) | 2014-07-15 | 2016-09-13 | Kymab Limited | Methods of treating anaemia |
| US9428578B2 (en) | 2014-07-15 | 2016-08-30 | Kymab Limited | Methods of treating anaemia |
| US10618955B2 (en) | 2014-07-15 | 2020-04-14 | Kymab Limited | Methods for treating neurodegenerative disease using anti-PD-1 antibodies |
| DE202015009006U1 (en) | 2014-07-15 | 2016-08-19 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| DE202015009007U1 (en) | 2014-07-15 | 2016-08-19 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| US10711059B2 (en) | 2014-07-15 | 2020-07-14 | Kymab Limited | Methods for treating neurodegenerative diseases using anti-PD-L1 antibodies |
| DE202015009002U1 (en) | 2014-07-15 | 2016-08-18 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| US8980273B1 (en) | 2014-07-15 | 2015-03-17 | Kymab Limited | Method of treating atopic dermatitis or asthma using antibody to IL4RA |
| US9416180B1 (en) | 2014-07-15 | 2016-08-16 | Kymab Limited | Precision medicine by targeting VEGF-A variants for treatment of diabetic macular edema and branch retinal vein occlusion |
| US9394568B2 (en) | 2014-07-15 | 2016-07-19 | Kymab Limited | Methods of treating anaemia |
| DE202015008988U1 (en) | 2014-07-15 | 2016-06-30 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| DE202015008974U1 (en) | 2014-07-15 | 2016-06-30 | Kymab Limited | Targeting of human PCSK9 for cholesterol treatment |
| US8986691B1 (en) | 2014-07-15 | 2015-03-24 | Kymab Limited | Method of treating atopic dermatitis or asthma using antibody to IL4RA |
| US9303089B2 (en) | 2014-07-15 | 2016-04-05 | Kymab Limited | Methods of treating anaemia |
| US8986694B1 (en) | 2014-07-15 | 2015-03-24 | Kymab Limited | Targeting human nav1.7 variants for treatment of pain |
| US8945560B1 (en) | 2014-07-15 | 2015-02-03 | Kymab Limited | Method of treating rheumatoid arthritis using antibody to IL6R |
| US8992927B1 (en) | 2014-07-15 | 2015-03-31 | Kymab Limited | Targeting human NAV1.7 variants for treatment of pain |
| US8999341B1 (en) | 2014-07-15 | 2015-04-07 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| EP2975058A1 (en) * | 2014-07-15 | 2016-01-20 | Kymab Limited | Antibodies for use in treating conditions related to specific PCSK9 variants in specific patient populations |
| US9017678B1 (en) | 2014-07-15 | 2015-04-28 | Kymab Limited | Method of treating rheumatoid arthritis using antibody to IL6R |
| EP2975059A1 (en) | 2014-07-15 | 2016-01-20 | Kymab Limited | Antibodies for use in treating conditions related to specific pcsk9 variants in specific patients populations |
| US9187562B1 (en) | 2014-07-15 | 2015-11-17 | Kymab Limited | Methods for treating anaemia |
| US9150660B1 (en) | 2014-07-15 | 2015-10-06 | Kymab Limited | Precision Medicine by targeting human NAV1.8 variants for treatment of pain |
| US9023359B1 (en) | 2014-07-15 | 2015-05-05 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| US9034332B1 (en) | 2014-07-15 | 2015-05-19 | Kymab Limited | Precision medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| US9067998B1 (en) | 2014-07-15 | 2015-06-30 | Kymab Limited | Targeting PD-1 variants for treatment of cancer |
| US9068012B1 (en) | 2014-07-15 | 2015-06-30 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| US9062105B1 (en) | 2014-07-15 | 2015-06-23 | Kymab Limited | Precision Medicine by targeting VEGF-A variants for treatment of retinopathy |
| US9034331B1 (en) | 2014-07-15 | 2015-05-19 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| US11555066B2 (en) | 2014-07-15 | 2023-01-17 | Kymab Limited | Precision medicine for cholesterol treatment |
| US9051378B1 (en) | 2014-07-15 | 2015-06-09 | Kymab Limited | Targeting rare human PCSK9 variants for cholesterol treatment |
| US9045545B1 (en) | 2014-07-15 | 2015-06-02 | Kymab Limited | Precision medicine by targeting PD-L1 variants for treatment of cancer |
| US9045548B1 (en) | 2014-07-15 | 2015-06-02 | Kymab Limited | Precision Medicine by targeting rare human PCSK9 variants for cholesterol treatment |
| WO2016023916A1 (en) | 2014-08-12 | 2016-02-18 | Kymab Limited | Treatment of disease using ligand binding to targets of interest |
| WO2016071701A1 (en) | 2014-11-07 | 2016-05-12 | Kymab Limited | Treatment of disease using ligand binding to targets of interest |
| US11111314B2 (en) | 2015-03-19 | 2021-09-07 | Regeneron Pharmaceuticals, Inc. | Non-human animals that select for light chain variable regions that bind antigen |
| EP4257599A3 (en) * | 2016-01-13 | 2024-01-17 | Regeneron Pharmaceuticals, Inc. | Rodents having an engineered heavy chain diversity region |
| US11779604B2 (en) | 2016-11-03 | 2023-10-10 | Kymab Limited | Antibodies, combinations comprising antibodies, biomarkers, uses and methods |
| WO2019037099A1 (en) * | 2017-08-25 | 2019-02-28 | Wenning Qin | Large scale modification of eukaryotic genome |
| US11946063B2 (en) | 2017-10-27 | 2024-04-02 | Trianni, Inc. | Long germline DH genes and long HCDR3 antibodies |
| EP3476942B1 (en) * | 2017-10-27 | 2022-01-26 | Trianni, Inc. | Long germline dh genes and long hcdr3 antibodies |
| US11730151B2 (en) | 2019-02-18 | 2023-08-22 | Biocytogen Pharmaceuticals (Beijing) Co., Ltd. | Genetically modified non-human animals with humanized immunoglobulin locus |
| EP3927832A4 (en) * | 2019-02-18 | 2022-11-30 | Biocytogen Pharmaceuticals (Beijing) Co., Ltd. | Genetically modified non-human animals with humanized immunoglobulin locus |
| WO2020169022A1 (en) * | 2019-02-18 | 2020-08-27 | Beijing Biocytogen Co., Ltd | Genetically modified non-human animals with humanized immunoglobulin locus |
| US12004495B2 (en) | 2019-02-18 | 2024-06-11 | Biocytogen Pharmaceuticals (Beijing) Co., Ltd. | Genetically modified non-human animals with humanized immunoglobulin locus |
| US11997994B2 (en) | 2020-06-02 | 2024-06-04 | Biocytogen Pharmaceuticals (Beijing) Co., Ltd. | Genetically modified non-human animals with common light chain immunoglobulin locus |
| EP4173480A4 (en) * | 2020-06-25 | 2024-04-17 | Humab Co. Ltd. | Heterozygous transgenic animal |
| WO2022056276A1 (en) | 2020-09-11 | 2022-03-17 | Regeneron Pharmaceuticals, Inc. | Identification and production of antigen-specific antibodies |
| WO2022132943A1 (en) | 2020-12-16 | 2022-06-23 | Regeneron Pharmaceuticals, Inc. | Mice expressing humanized fc alpha receptors |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11820810B2 (en) | Antibodies, variable domains and chains tailored for human use | |
| US20220000085A1 (en) | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics | |
| US9783618B2 (en) | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics | |
| NZ623745B2 (en) | Manipulation of immunoglobulin gene diversity and multi-antibody therapeutics | |
| NZ623756B2 (en) | Antibodies, variable domains & chains tailored for human use |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12772122 Country of ref document: EP Kind code of ref document: A2 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2846319 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2014531302 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2012772122 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012772122 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2012311286 Country of ref document: AU Date of ref document: 20120918 Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014006390 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112014006390 Country of ref document: BR Kind code of ref document: A2 Effective date: 20140318 |