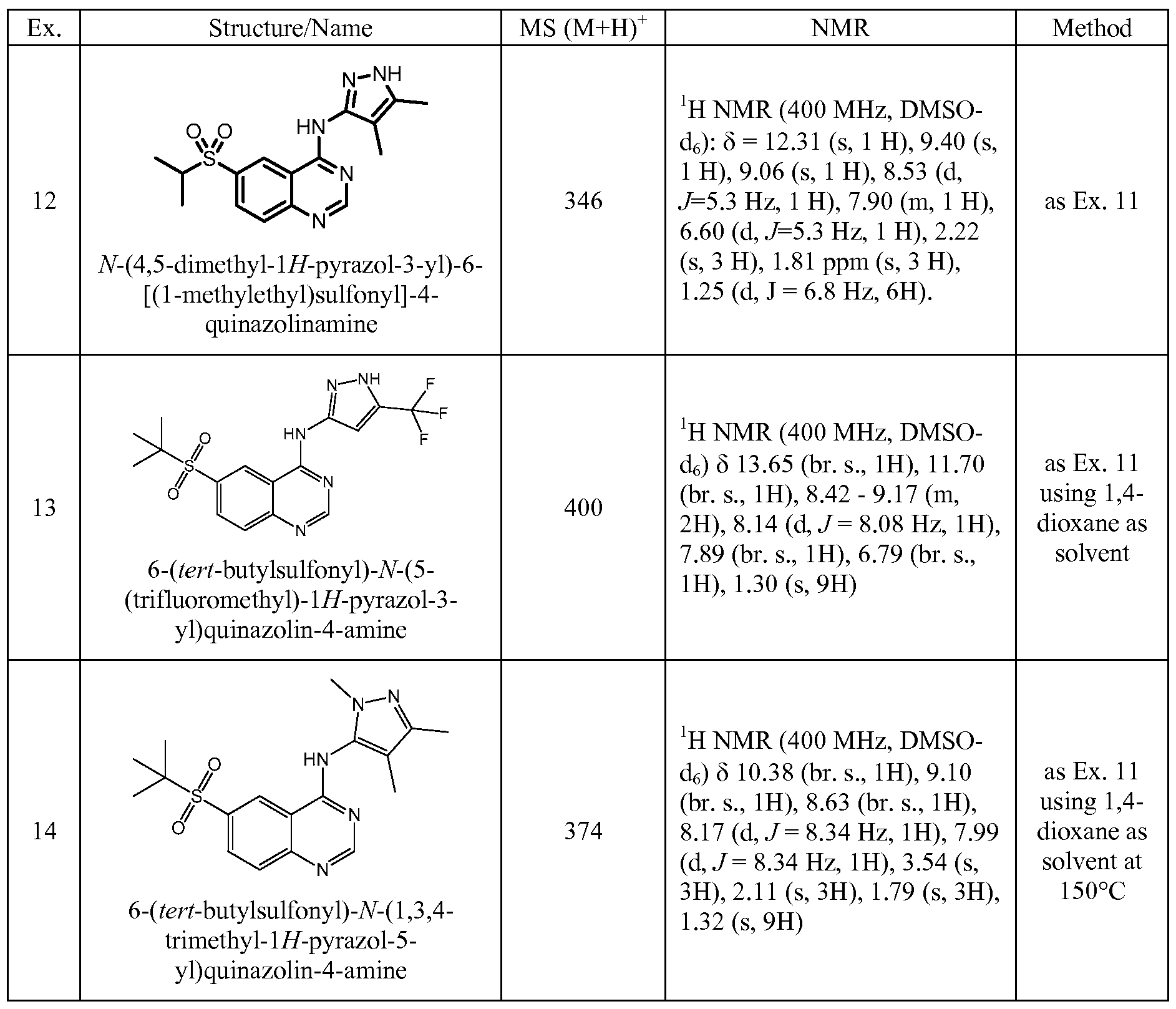
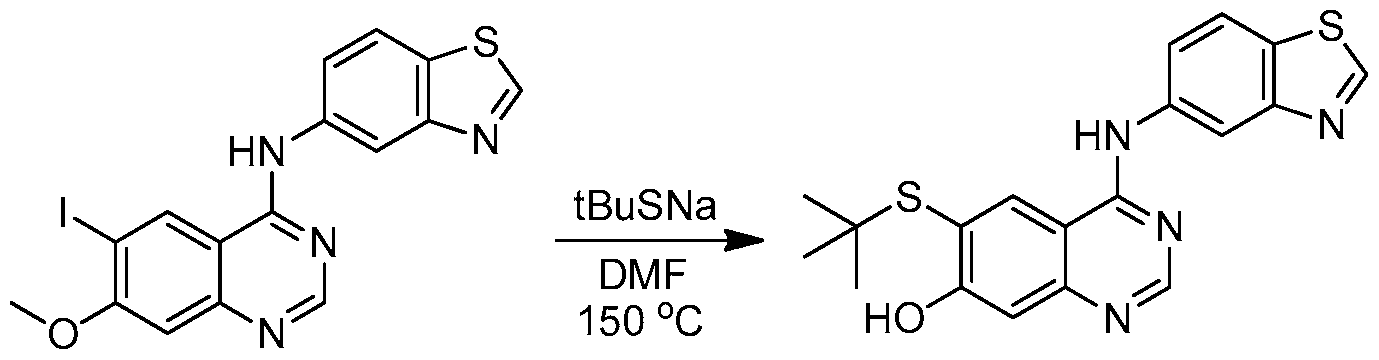
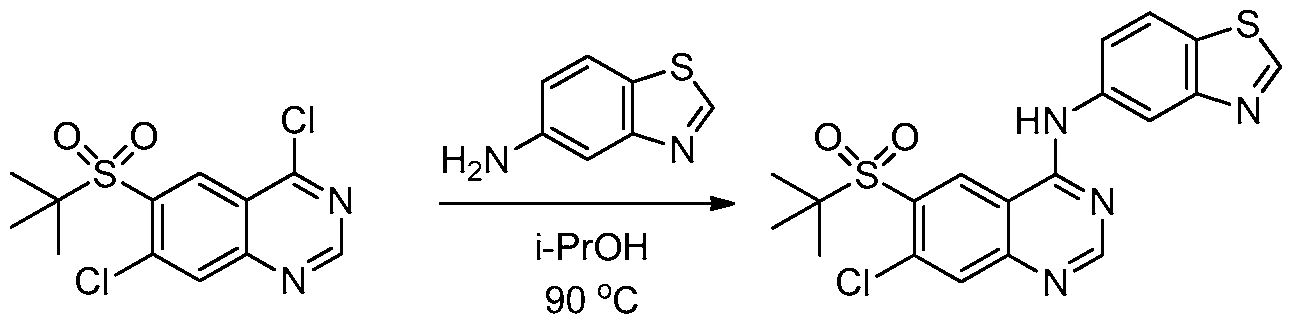
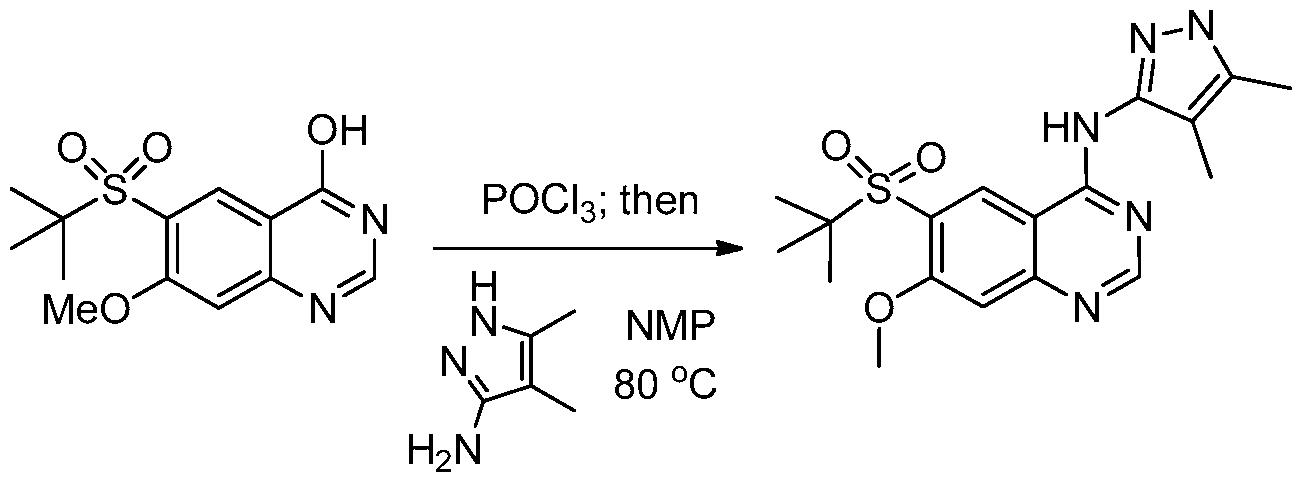
WO2013025958A1 - Amino quinazolines as kinase inhibitors - Google Patents
Amino quinazolines as kinase inhibitors Download PDFInfo
- Publication number
- WO2013025958A1 WO2013025958A1 PCT/US2012/051247 US2012051247W WO2013025958A1 WO 2013025958 A1 WO2013025958 A1 WO 2013025958A1 US 2012051247 W US2012051247 W US 2012051247W WO 2013025958 A1 WO2013025958 A1 WO 2013025958A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- alkoxy
- phenyl
- hydroxy
- group
- Prior art date
Links
- 229940043355 kinase inhibitor Drugs 0.000 title description 3
- 239000003757 phosphotransferase inhibitor Substances 0.000 title description 3
- CZAAKPFIWJXPQT-UHFFFAOYSA-N quinazolin-2-amine Chemical class C1=CC=CC2=NC(N)=NC=C21 CZAAKPFIWJXPQT-UHFFFAOYSA-N 0.000 title 1
- 150000001875 compounds Chemical class 0.000 claims abstract description 242
- 238000000034 method Methods 0.000 claims abstract description 37
- 125000000217 alkyl group Chemical group 0.000 claims description 482
- 125000003545 alkoxy group Chemical group 0.000 claims description 434
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 250
- 125000005843 halogen group Chemical group 0.000 claims description 146
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 137
- 150000003839 salts Chemical class 0.000 claims description 133
- 229910052736 halogen Inorganic materials 0.000 claims description 128
- -1 cyano, hydroxyl Chemical group 0.000 claims description 115
- 150000002367 halogens Chemical class 0.000 claims description 95
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 86
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 84
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 65
- 239000007787 solid Substances 0.000 claims description 65
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 62
- 102100022502 Receptor-interacting serine/threonine-protein kinase 2 Human genes 0.000 claims description 53
- 125000001424 substituent group Chemical group 0.000 claims description 52
- 125000001072 heteroaryl group Chemical group 0.000 claims description 51
- 125000005605 benzo group Chemical group 0.000 claims description 50
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 46
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 45
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 44
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 44
- LKFXYYLRIUSARI-UHFFFAOYSA-N 1,3-thiazol-5-amine Chemical compound NC1=CN=CS1 LKFXYYLRIUSARI-UHFFFAOYSA-N 0.000 claims description 42
- 101001109137 Homo sapiens Receptor-interacting serine/threonine-protein kinase 2 Proteins 0.000 claims description 42
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 37
- 229910052757 nitrogen Inorganic materials 0.000 claims description 36
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 35
- 125000003118 aryl group Chemical group 0.000 claims description 33
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 31
- 125000004448 alkyl carbonyl group Chemical group 0.000 claims description 30
- 125000004429 atom Chemical group 0.000 claims description 30
- 229910052760 oxygen Inorganic materials 0.000 claims description 30
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 claims description 29
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 29
- 125000003302 alkenyloxy group Chemical group 0.000 claims description 28
- 125000006568 (C4-C7) heterocycloalkyl group Chemical group 0.000 claims description 27
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims description 27
- 238000011282 treatment Methods 0.000 claims description 27
- 125000005842 heteroatom Chemical group 0.000 claims description 25
- 201000010099 disease Diseases 0.000 claims description 24
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 24
- 125000004076 pyridyl group Chemical group 0.000 claims description 24
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 claims description 22
- 229910052717 sulfur Inorganic materials 0.000 claims description 22
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 21
- 208000035475 disorder Diseases 0.000 claims description 20
- 239000008194 pharmaceutical composition Substances 0.000 claims description 19
- 230000001404 mediated effect Effects 0.000 claims description 18
- 239000001301 oxygen Substances 0.000 claims description 17
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 16
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 16
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 claims description 15
- 125000004399 C1-C4 alkenyl group Chemical group 0.000 claims description 14
- 125000000623 heterocyclic group Chemical group 0.000 claims description 14
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 claims description 14
- 125000006592 (C2-C3) alkenyl group Chemical group 0.000 claims description 13
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 13
- 208000009766 Blau syndrome Diseases 0.000 claims description 11
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 11
- 125000000446 sulfanediyl group Chemical group *S* 0.000 claims description 11
- 229910014585 C2-Ce Inorganic materials 0.000 claims description 10
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 claims description 10
- 125000004432 carbon atom Chemical group C* 0.000 claims description 10
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 10
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 claims description 9
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 9
- 125000006704 (C5-C6) cycloalkyl group Chemical group 0.000 claims description 8
- 229910052739 hydrogen Inorganic materials 0.000 claims description 8
- 230000005764 inhibitory process Effects 0.000 claims description 8
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 8
- 125000004786 difluoromethoxy group Chemical group [H]C(F)(F)O* 0.000 claims description 7
- CBOIHMRHGLHBPB-UHFFFAOYSA-N hydroxymethyl Chemical compound O[CH2] CBOIHMRHGLHBPB-UHFFFAOYSA-N 0.000 claims description 7
- 201000000306 sarcoidosis Diseases 0.000 claims description 7
- 125000004496 thiazol-5-yl group Chemical group S1C=NC=C1* 0.000 claims description 7
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 7
- 208000011231 Crohn disease Diseases 0.000 claims description 6
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 6
- 125000000000 cycloalkoxy group Chemical group 0.000 claims description 6
- 206010009900 Colitis ulcerative Diseases 0.000 claims description 5
- 201000006704 Ulcerative Colitis Diseases 0.000 claims description 5
- 206010046851 Uveitis Diseases 0.000 claims description 5
- 206010003246 arthritis Diseases 0.000 claims description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 5
- 210000000056 organ Anatomy 0.000 claims description 5
- 125000002373 5 membered heterocyclic group Chemical group 0.000 claims description 4
- 125000004008 6 membered carbocyclic group Chemical group 0.000 claims description 4
- 208000004930 Fatty Liver Diseases 0.000 claims description 4
- 208000019693 Lung disease Diseases 0.000 claims description 4
- 206010037660 Pyrexia Diseases 0.000 claims description 4
- 125000001153 fluoro group Chemical group F* 0.000 claims description 4
- 238000004519 manufacturing process Methods 0.000 claims description 4
- 230000002265 prevention Effects 0.000 claims description 4
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 3
- 108090000426 Caspase-1 Proteins 0.000 claims description 3
- 201000004624 Dermatitis Diseases 0.000 claims description 3
- 208000006673 asthma Diseases 0.000 claims description 3
- 239000003814 drug Substances 0.000 claims description 3
- BRTXNGOZQRLCTM-UHFFFAOYSA-N ethyl 5-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]sulfanylpentanoate Chemical compound N1=CN=C2C=C(OC)C(SCCCCC(=O)OCC)=CC2=C1NC1=CC=C(F)C(Cl)=C1 BRTXNGOZQRLCTM-UHFFFAOYSA-N 0.000 claims description 3
- HKYLKSMCVOFYTL-UHFFFAOYSA-N n-(6-tert-butylsulfonylquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC2=NC=NC3=CC=C(C=C32)S(=O)(=O)C(C)(C)C)=C1 HKYLKSMCVOFYTL-UHFFFAOYSA-N 0.000 claims description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 3
- 208000011580 syndromic disease Diseases 0.000 claims description 3
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 claims description 2
- XOOKKOAUOUOVEG-UHFFFAOYSA-N 3-methoxy-5-[(6-methylsulfanylquinazolin-4-yl)amino]phenol Chemical compound COC1=CC(O)=CC(NC=2C3=CC(SC)=CC=C3N=CN=2)=C1 XOOKKOAUOUOVEG-UHFFFAOYSA-N 0.000 claims description 2
- GHJHXKSZCJJGEF-UHFFFAOYSA-N 3-methoxy-5-[(6-methylsulfinylquinazolin-4-yl)amino]phenol Chemical compound COC1=CC(O)=CC(NC=2C3=CC(=CC=C3N=CN=2)S(C)=O)=C1 GHJHXKSZCJJGEF-UHFFFAOYSA-N 0.000 claims description 2
- OCEPZXZVPNPKJD-UHFFFAOYSA-N 6-methylsulfanyl-n-(4-phenylmethoxyphenyl)quinazolin-4-amine Chemical compound C12=CC(SC)=CC=C2N=CN=C1NC(C=C1)=CC=C1OCC1=CC=CC=C1 OCEPZXZVPNPKJD-UHFFFAOYSA-N 0.000 claims description 2
- DDAXBPLLWRSPCR-UHFFFAOYSA-N 6-methylsulfinyl-n-(4-phenylmethoxyphenyl)quinazolin-4-amine Chemical compound C12=CC(S(=O)C)=CC=C2N=CN=C1NC(C=C1)=CC=C1OCC1=CC=CC=C1 DDAXBPLLWRSPCR-UHFFFAOYSA-N 0.000 claims description 2
- ZUXSZSSULHQTPT-UHFFFAOYSA-N 6-methylsulfonyl-n-(4-phenylmethoxyphenyl)quinazolin-4-amine Chemical compound C12=CC(S(=O)(=O)C)=CC=C2N=CN=C1NC(C=C1)=CC=C1OCC1=CC=CC=C1 ZUXSZSSULHQTPT-UHFFFAOYSA-N 0.000 claims description 2
- 206010003827 Autoimmune hepatitis Diseases 0.000 claims description 2
- 208000009329 Graft vs Host Disease Diseases 0.000 claims description 2
- 206010063837 Reperfusion injury Diseases 0.000 claims description 2
- 206010069351 acute lung injury Diseases 0.000 claims description 2
- 125000003342 alkenyl group Chemical group 0.000 claims description 2
- 208000024908 graft versus host disease Diseases 0.000 claims description 2
- 230000002757 inflammatory effect Effects 0.000 claims description 2
- 208000012947 ischemia reperfusion injury Diseases 0.000 claims description 2
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 claims description 2
- 201000006417 multiple sclerosis Diseases 0.000 claims description 2
- ZLKQTCZFVNZQDO-UHFFFAOYSA-N n-(3-chloro-4-fluorophenyl)-7-methoxy-6-(trifluoromethylsulfonyl)quinazolin-4-amine Chemical compound C=12C=C(S(=O)(=O)C(F)(F)F)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 ZLKQTCZFVNZQDO-UHFFFAOYSA-N 0.000 claims description 2
- QNEMGKFYDABJKH-UHFFFAOYSA-N n-[(3,4-dichlorophenyl)methyl]-6-methylsulfanylquinazolin-4-amine Chemical compound C12=CC(SC)=CC=C2N=CN=C1NCC1=CC=C(Cl)C(Cl)=C1 QNEMGKFYDABJKH-UHFFFAOYSA-N 0.000 claims description 2
- UTDZNRMTLDCWFI-UHFFFAOYSA-N n-[(3,4-difluorophenyl)methyl]-6-methylsulfonylquinazolin-4-amine Chemical compound C12=CC(S(=O)(=O)C)=CC=C2N=CN=C1NCC1=CC=C(F)C(F)=C1 UTDZNRMTLDCWFI-UHFFFAOYSA-N 0.000 claims description 2
- OAZJURONLYELCO-UHFFFAOYSA-N n-[(3-fluoro-4-methoxyphenyl)methyl]-6-methylsulfanylquinazolin-4-amine Chemical compound C1=C(F)C(OC)=CC=C1CNC1=NC=NC2=CC=C(SC)C=C12 OAZJURONLYELCO-UHFFFAOYSA-N 0.000 claims description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 claims description 2
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 2
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims 1
- TVCQPRRPKVXBKS-UHFFFAOYSA-N n-(6-propan-2-ylsulfonylquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC2=NC=NC3=CC=C(C=C32)S(=O)(=O)C(C)C)=C1 TVCQPRRPKVXBKS-UHFFFAOYSA-N 0.000 claims 1
- 125000006299 oxetan-3-yl group Chemical group [H]C1([H])OC([H])([H])C1([H])* 0.000 claims 1
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 127
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 70
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 68
- 239000000243 solution Substances 0.000 description 68
- 239000000203 mixture Substances 0.000 description 48
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 47
- 238000006243 chemical reaction Methods 0.000 description 47
- 238000005160 1H NMR spectroscopy Methods 0.000 description 42
- 239000011541 reaction mixture Substances 0.000 description 42
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 39
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 36
- 235000019439 ethyl acetate Nutrition 0.000 description 34
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 31
- 239000002904 solvent Substances 0.000 description 26
- 239000000047 product Substances 0.000 description 24
- 238000004440 column chromatography Methods 0.000 description 23
- 239000013058 crude material Substances 0.000 description 21
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 20
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 19
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 19
- 239000000725 suspension Substances 0.000 description 18
- WYURNTSHIVDZCO-UHFFFAOYSA-N tetrahydrofuran Substances C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 18
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 15
- 241000700159 Rattus Species 0.000 description 15
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 15
- 238000002360 preparation method Methods 0.000 description 15
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 14
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 13
- 108010079933 Receptor-Interacting Protein Serine-Threonine Kinase 2 Proteins 0.000 description 13
- 210000004027 cell Anatomy 0.000 description 13
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 13
- 239000000543 intermediate Substances 0.000 description 12
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 12
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 12
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 12
- 239000011734 sodium Substances 0.000 description 12
- 229940124530 sulfonamide Drugs 0.000 description 12
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 11
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 11
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 10
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 10
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 10
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 10
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 10
- 108090000695 Cytokines Proteins 0.000 description 9
- 102000004127 Cytokines Human genes 0.000 description 9
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 9
- 239000003921 oil Substances 0.000 description 9
- BWDCBBZUYJDNJZ-UHFFFAOYSA-N quinazolin-7-ol Chemical compound C1=NC=NC2=CC(O)=CC=C21 BWDCBBZUYJDNJZ-UHFFFAOYSA-N 0.000 description 9
- 230000004044 response Effects 0.000 description 9
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 8
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 8
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 8
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 8
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 8
- 150000001412 amines Chemical class 0.000 description 8
- 239000000872 buffer Substances 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 239000012044 organic layer Substances 0.000 description 8
- 238000000746 purification Methods 0.000 description 8
- 239000000741 silica gel Substances 0.000 description 8
- 229910002027 silica gel Inorganic materials 0.000 description 8
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 8
- 0 Cc1c(*)c(*)cc(N(*)c2c(cc(*)c(*)c3)c3ncn2)c1* Chemical compound Cc1c(*)c(*)cc(N(*)c2c(cc(*)c(*)c3)c3ncn2)c1* 0.000 description 7
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 230000002378 acidificating effect Effects 0.000 description 7
- 239000012267 brine Substances 0.000 description 7
- 239000002552 dosage form Substances 0.000 description 7
- 239000000706 filtrate Substances 0.000 description 7
- 238000001914 filtration Methods 0.000 description 7
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 7
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 7
- 239000012453 solvate Substances 0.000 description 7
- 239000003643 water by type Substances 0.000 description 7
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 description 6
- 101001125026 Homo sapiens Nucleotide-binding oligomerization domain-containing protein 2 Proteins 0.000 description 6
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 6
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 6
- 238000005481 NMR spectroscopy Methods 0.000 description 6
- 102100029441 Nucleotide-binding oligomerization domain-containing protein 2 Human genes 0.000 description 6
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- 238000004587 chromatography analysis Methods 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
- 238000007796 conventional method Methods 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 238000004128 high performance liquid chromatography Methods 0.000 description 6
- 239000010410 layer Substances 0.000 description 6
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 6
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 6
- 229910000027 potassium carbonate Inorganic materials 0.000 description 6
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 6
- 229910052938 sodium sulfate Inorganic materials 0.000 description 6
- 235000011152 sodium sulphate Nutrition 0.000 description 6
- 239000011593 sulfur Substances 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- QAEDZJGFFMLHHQ-UHFFFAOYSA-N trifluoroacetic anhydride Chemical compound FC(F)(F)C(=O)OC(=O)C(F)(F)F QAEDZJGFFMLHHQ-UHFFFAOYSA-N 0.000 description 6
- BQIIWCWVXUAUOJ-UHFFFAOYSA-N 4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-ol Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)O)S(=O)(=O)C(C)(C)C)=C1 BQIIWCWVXUAUOJ-UHFFFAOYSA-N 0.000 description 5
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 5
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-diisopropylethylamine Substances CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 5
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 5
- 230000029936 alkylation Effects 0.000 description 5
- 238000005804 alkylation reaction Methods 0.000 description 5
- 238000003556 assay Methods 0.000 description 5
- 239000002585 base Substances 0.000 description 5
- 125000002619 bicyclic group Chemical group 0.000 description 5
- 239000000284 extract Substances 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 229960005205 prednisolone Drugs 0.000 description 5
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 5
- AHJBHKTZBNAQDR-UHFFFAOYSA-N quinazoline-6-sulfonamide Chemical compound N1=CN=CC2=CC(S(=O)(=O)N)=CC=C21 AHJBHKTZBNAQDR-UHFFFAOYSA-N 0.000 description 5
- 229920006395 saturated elastomer Polymers 0.000 description 5
- 230000011664 signaling Effects 0.000 description 5
- 239000000377 silicon dioxide Substances 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 238000006467 substitution reaction Methods 0.000 description 5
- 150000003457 sulfones Chemical group 0.000 description 5
- WMXCDAVJEZZYLT-UHFFFAOYSA-N tert-butylthiol Chemical compound CC(C)(C)S WMXCDAVJEZZYLT-UHFFFAOYSA-N 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- UJZYHMZRXGNDFB-UHFFFAOYSA-N 1,3-benzothiazol-5-amine Chemical compound NC1=CC=C2SC=NC2=C1 UJZYHMZRXGNDFB-UHFFFAOYSA-N 0.000 description 4
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 4
- FGOXXXONPZMOEC-UHFFFAOYSA-N 6-propan-2-ylsulfanyl-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=CC(SC(C)C)=CC=C21 FGOXXXONPZMOEC-UHFFFAOYSA-N 0.000 description 4
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 4
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 4
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- 229910019213 POCl3 Inorganic materials 0.000 description 4
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 238000005119 centrifugation Methods 0.000 description 4
- 239000003246 corticosteroid Substances 0.000 description 4
- 229960001334 corticosteroids Drugs 0.000 description 4
- 125000004122 cyclic group Chemical group 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 125000002541 furyl group Chemical group 0.000 description 4
- 150000004677 hydrates Chemical class 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 238000007912 intraperitoneal administration Methods 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 125000002950 monocyclic group Chemical group 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 229910052763 palladium Inorganic materials 0.000 description 4
- 238000007911 parenteral administration Methods 0.000 description 4
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 4
- 239000002244 precipitate Substances 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 4
- 229910052705 radium Inorganic materials 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000004007 reversed phase HPLC Methods 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 3
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 3
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 3
- KXOCTHJHIGLQKJ-UHFFFAOYSA-N 2-[[4,8-bis(azocan-1-yl)-2-[bis(2-hydroxyethyl)amino]pyrimido[5,4-d]pyrimidin-6-yl]-(2-hydroxyethyl)amino]ethanol Chemical compound C=12N=C(N(CCO)CCO)N=C(N3CCCCCCC3)C2=NC(N(CCO)CCO)=NC=1N1CCCCCCC1 KXOCTHJHIGLQKJ-UHFFFAOYSA-N 0.000 description 3
- CUYKNJBYIJFRCU-UHFFFAOYSA-N 3-aminopyridine Chemical compound NC1=CC=CN=C1 CUYKNJBYIJFRCU-UHFFFAOYSA-N 0.000 description 3
- JYVPUGQZJRFFAF-UHFFFAOYSA-N 4,5-dimethyl-1h-pyrazol-3-amine Chemical compound CC=1NN=C(N)C=1C JYVPUGQZJRFFAF-UHFFFAOYSA-N 0.000 description 3
- JBFWWIDMBIYZIG-UHFFFAOYSA-N 4-chloro-6-propan-2-ylsulfonylquinazoline Chemical compound N1=CN=C(Cl)C2=CC(S(=O)(=O)C(C)C)=CC=C21 JBFWWIDMBIYZIG-UHFFFAOYSA-N 0.000 description 3
- GVRRXASZZAKBMN-UHFFFAOYSA-N 4-chloroquinazoline Chemical compound C1=CC=C2C(Cl)=NC=NC2=C1 GVRRXASZZAKBMN-UHFFFAOYSA-N 0.000 description 3
- SEZQVJKLENTKBV-UHFFFAOYSA-N 4-methyl-3-[(6-methylsulfonylquinazolin-4-yl)amino]phenol Chemical compound CC1=CC=C(O)C=C1NC1=NC=NC2=CC=C(S(C)(=O)=O)C=C12 SEZQVJKLENTKBV-UHFFFAOYSA-N 0.000 description 3
- PUGXMZKDRVGIHC-UHFFFAOYSA-N 6-iodo-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=CC(I)=CC=C21 PUGXMZKDRVGIHC-UHFFFAOYSA-N 0.000 description 3
- MTLLYGIGUMMWBT-UHFFFAOYSA-N 6-methylsulfanyl-1h-quinazolin-4-one Chemical compound N1=CNC(=O)C2=CC(SC)=CC=C21 MTLLYGIGUMMWBT-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 3
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 239000004215 Carbon black (E152) Substances 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- 241000238631 Hexapoda Species 0.000 description 3
- 108091058560 IL8 Proteins 0.000 description 3
- 108090001005 Interleukin-6 Proteins 0.000 description 3
- 108090001007 Interleukin-8 Proteins 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 3
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 3
- 108091000080 Phosphotransferase Proteins 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Chemical compound CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 3
- 108700012920 TNF Proteins 0.000 description 3
- 229910021529 ammonia Inorganic materials 0.000 description 3
- 230000003110 anti-inflammatory effect Effects 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 150000001721 carbon Chemical group 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 239000012043 crude product Substances 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 208000037765 diseases and disorders Diseases 0.000 description 3
- 239000007884 disintegrant Substances 0.000 description 3
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 239000012458 free base Substances 0.000 description 3
- 229930195733 hydrocarbon Natural products 0.000 description 3
- 230000028709 inflammatory response Effects 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 150000007529 inorganic bases Chemical class 0.000 description 3
- 125000001786 isothiazolyl group Chemical group 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000000314 lubricant Substances 0.000 description 3
- 235000019359 magnesium stearate Nutrition 0.000 description 3
- 235000019341 magnesium sulphate Nutrition 0.000 description 3
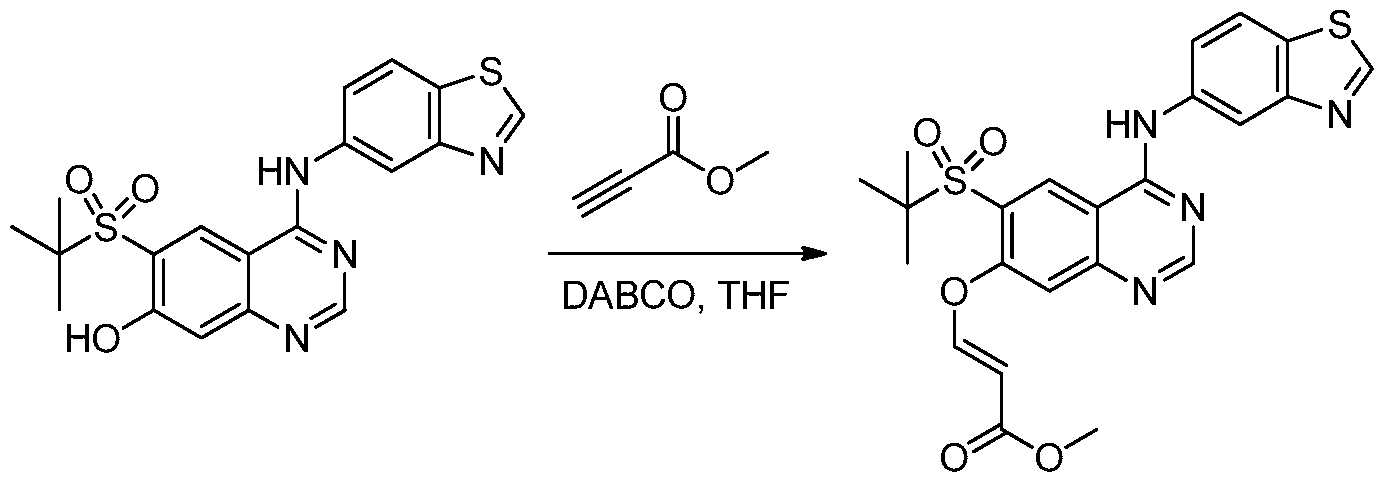
- OZFIBXQRPJRPPW-BQYQJAHWSA-N methyl (e)-3-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxyprop-2-enoate Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)O/C=C/C(=O)OC)=C1 OZFIBXQRPJRPPW-BQYQJAHWSA-N 0.000 description 3
- ACMVEAGHYONQFM-UHFFFAOYSA-N methyl 2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxyacetate Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)OCC(=O)OC)=C1 ACMVEAGHYONQFM-UHFFFAOYSA-N 0.000 description 3
- 229940016286 microcrystalline cellulose Drugs 0.000 description 3
- 239000008108 microcrystalline cellulose Substances 0.000 description 3
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 3
- DEMKOIXJPXTNIA-UHFFFAOYSA-N n-(6-tert-butylsulfanyl-7-methoxyquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)SC(C)(C)C)OC)=C1 DEMKOIXJPXTNIA-UHFFFAOYSA-N 0.000 description 3
- OEHMGZBZOPNPKA-UHFFFAOYSA-N n-(6-tert-butylsulfanylquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC2=NC=NC3=CC=C(C=C32)SC(C)(C)C)=C1 OEHMGZBZOPNPKA-UHFFFAOYSA-N 0.000 description 3
- 239000008184 oral solid dosage form Substances 0.000 description 3
- 150000007530 organic bases Chemical class 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 102000020233 phosphotransferase Human genes 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 125000004546 quinazolin-4-yl group Chemical group N1=CN=C(C2=CC=CC=C12)* 0.000 description 3
- 150000003254 radicals Chemical class 0.000 description 3
- 239000011347 resin Substances 0.000 description 3
- 229920005989 resin Polymers 0.000 description 3
- 125000006413 ring segment Chemical group 0.000 description 3
- 229910000029 sodium carbonate Inorganic materials 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 229940032147 starch Drugs 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 125000004434 sulfur atom Chemical group 0.000 description 3
- 125000001412 tetrahydropyranyl group Chemical group 0.000 description 3
- 125000001544 thienyl group Chemical group 0.000 description 3
- 150000003573 thiols Chemical class 0.000 description 3
- 238000011200 topical administration Methods 0.000 description 3
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 3
- 239000002451 tumor necrosis factor inhibitor Substances 0.000 description 3
- IHLOGGBZINFNAA-VOTSOKGWSA-N (e)-3-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxyprop-2-enoic acid Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)O\C=C\C(O)=O)S(=O)(=O)C(C)(C)C)=C1 IHLOGGBZINFNAA-VOTSOKGWSA-N 0.000 description 2
- JPRPJUMQRZTTED-UHFFFAOYSA-N 1,3-dioxolanyl Chemical group [CH]1OCCO1 JPRPJUMQRZTTED-UHFFFAOYSA-N 0.000 description 2
- ILWJAOPQHOZXAN-UHFFFAOYSA-N 1,3-dithianyl Chemical group [CH]1SCCCS1 ILWJAOPQHOZXAN-UHFFFAOYSA-N 0.000 description 2
- 125000005940 1,4-dioxanyl group Chemical group 0.000 description 2
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 2
- KJPALGOPKHOQHU-UHFFFAOYSA-N 2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxyacetamide Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)OCC(N)=O)S(=O)(=O)C(C)(C)C)=C1 KJPALGOPKHOQHU-UHFFFAOYSA-N 0.000 description 2
- HYWVUSOJGCTRPM-UHFFFAOYSA-N 2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxyacetic acid Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)OCC(O)=O)S(=O)(=O)C(C)(C)C)=C1 HYWVUSOJGCTRPM-UHFFFAOYSA-N 0.000 description 2
- KHRFZHMACJCYJV-UHFFFAOYSA-N 2-[4-(1,3-benzothiazol-5-ylamino)-7-methoxyquinazolin-6-yl]sulfanylethanol Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)SCCO)OC)=C1 KHRFZHMACJCYJV-UHFFFAOYSA-N 0.000 description 2
- MZLFZLVSRDZFFJ-UHFFFAOYSA-N 2-[4-(1,3-benzothiazol-5-ylamino)-7-methoxyquinazolin-6-yl]sulfonylethanol Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)CCO)OC)=C1 MZLFZLVSRDZFFJ-UHFFFAOYSA-N 0.000 description 2
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- SUBZTZVHVGYOPM-UHFFFAOYSA-N 2-chloro-5-fluoropyridine-3-carbonitrile Chemical compound FC1=CN=C(Cl)C(C#N)=C1 SUBZTZVHVGYOPM-UHFFFAOYSA-N 0.000 description 2
- KHRZSLCUROLAAR-UHFFFAOYSA-N 2-chloro-5-fluoropyridine-3-carboxamide Chemical compound NC(=O)C1=CC(F)=CN=C1Cl KHRZSLCUROLAAR-UHFFFAOYSA-N 0.000 description 2
- WMADTZFXZAITIR-UHFFFAOYSA-N 2-chloro-5-fluoropyridine-3-carboxylic acid Chemical compound OC(=O)C1=CC(F)=CN=C1Cl WMADTZFXZAITIR-UHFFFAOYSA-N 0.000 description 2
- XINPRUNXWSMHIA-UHFFFAOYSA-N 3-[4-(1,3-benzothiazol-5-ylamino)quinazolin-6-yl]sulfanyl-3-methylbutan-1-ol Chemical compound C1=C2SC=NC2=CC(NC2=NC=NC3=CC=C(C=C32)SC(C)(CCO)C)=C1 XINPRUNXWSMHIA-UHFFFAOYSA-N 0.000 description 2
- PZRBSRCAICQLLT-UHFFFAOYSA-N 3-[4-(1,3-benzothiazol-5-ylamino)quinazolin-6-yl]sulfonyl-3-methylbutan-1-ol Chemical compound C1=C2SC=NC2=CC(NC2=NC=NC3=CC=C(C=C32)S(=O)(=O)C(C)(CCO)C)=C1 PZRBSRCAICQLLT-UHFFFAOYSA-N 0.000 description 2
- KSBIPDJRJJHHFE-UHFFFAOYSA-N 3-amino-2-methylbut-2-enenitrile Chemical compound CC(N)=C(C)C#N KSBIPDJRJJHHFE-UHFFFAOYSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- VGUHUPXWUUVYHC-UHFFFAOYSA-N 4-chloro-6-iodo-7-methoxyquinazoline Chemical compound C1=NC(Cl)=C2C=C(I)C(OC)=CC2=N1 VGUHUPXWUUVYHC-UHFFFAOYSA-N 0.000 description 2
- BDAIUOPDSRAOKI-UHFFFAOYSA-N 4-chloro-6-iodoquinazoline Chemical compound C1=C(I)C=C2C(Cl)=NC=NC2=C1 BDAIUOPDSRAOKI-UHFFFAOYSA-N 0.000 description 2
- VWDXSIUWLLYHDE-UHFFFAOYSA-N 4-methyl-3-[(6-methylsulfanylquinazolin-4-yl)amino]phenol Chemical compound C12=CC(SC)=CC=C2N=CN=C1NC1=CC(O)=CC=C1C VWDXSIUWLLYHDE-UHFFFAOYSA-N 0.000 description 2
- FVHAXLFSGSMQSE-UHFFFAOYSA-N 6-iodo-7-methoxy-1h-quinazolin-4-one Chemical compound N1=CNC(=O)C2=C1C=C(OC)C(I)=C2 FVHAXLFSGSMQSE-UHFFFAOYSA-N 0.000 description 2
- 125000006164 6-membered heteroaryl group Chemical group 0.000 description 2
- CHRMMMLUWHPZAH-UHFFFAOYSA-N 7-methoxyquinazoline Chemical class C1=NC=NC2=CC(OC)=CC=C21 CHRMMMLUWHPZAH-UHFFFAOYSA-N 0.000 description 2
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 2
- 108010042708 Acetylmuramyl-Alanyl-Isoglutamine Proteins 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 208000011594 Autoinflammatory disease Diseases 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 2
- 102000021350 Caspase recruitment domains Human genes 0.000 description 2
- 108091011189 Caspase recruitment domains Proteins 0.000 description 2
- 229920002261 Corn starch Polymers 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 2
- XZWYTXMRWQJBGX-VXBMVYAYSA-N FLAG peptide Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)CC1=CC=C(O)C=C1 XZWYTXMRWQJBGX-VXBMVYAYSA-N 0.000 description 2
- 108010020195 FLAG peptide Proteins 0.000 description 2
- PNKUSGQVOMIXLU-UHFFFAOYSA-N Formamidine Chemical compound NC=N PNKUSGQVOMIXLU-UHFFFAOYSA-N 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- QZRGKCOWNLSUDK-UHFFFAOYSA-N Iodochlorine Chemical compound ICl QZRGKCOWNLSUDK-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 102100026888 Mitogen-activated protein kinase kinase kinase 7 Human genes 0.000 description 2
- 229920000881 Modified starch Polymers 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Chemical compound OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- 229910006124 SOCl2 Inorganic materials 0.000 description 2
- 240000006394 Sorghum bicolor Species 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- PZBFGYYEXUXCOF-UHFFFAOYSA-N TCEP Chemical compound OC(=O)CCP(CCC(O)=O)CCC(O)=O PZBFGYYEXUXCOF-UHFFFAOYSA-N 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- XXROGKLTLUQVRX-UHFFFAOYSA-N allyl alcohol Chemical compound OCC=C XXROGKLTLUQVRX-UHFFFAOYSA-N 0.000 description 2
- 235000001014 amino acid Nutrition 0.000 description 2
- 229940024606 amino acid Drugs 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 150000001448 anilines Chemical class 0.000 description 2
- 239000002260 anti-inflammatory agent Substances 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 125000002393 azetidinyl group Chemical group 0.000 description 2
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 2
- 235000010233 benzoic acid Nutrition 0.000 description 2
- LUFPJJNWMYZRQE-UHFFFAOYSA-N benzylsulfanylmethylbenzene Chemical compound C=1C=CC=CC=1CSCC1=CC=CC=C1 LUFPJJNWMYZRQE-UHFFFAOYSA-N 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 150000001723 carbon free-radicals Chemical class 0.000 description 2
- STNNHWPJRRODGI-UHFFFAOYSA-N carbonic acid;n,n-diethylethanamine Chemical compound [O-]C([O-])=O.CC[NH+](CC)CC.CC[NH+](CC)CC STNNHWPJRRODGI-UHFFFAOYSA-N 0.000 description 2
- 238000006555 catalytic reaction Methods 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 125000003016 chromanyl group Chemical group O1C(CCC2=CC=CC=C12)* 0.000 description 2
- 125000004230 chromenyl group Chemical group O1C(C=CC2=CC=CC=C12)* 0.000 description 2
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 125000004431 deuterium atom Chemical group 0.000 description 2
- 125000004852 dihydrofuranyl group Chemical group O1C(CC=C1)* 0.000 description 2
- 125000005043 dihydropyranyl group Chemical group O1C(CCC=C1)* 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 125000001188 haloalkyl group Chemical group 0.000 description 2
- 238000000265 homogenisation Methods 0.000 description 2
- IKDUDTNKRLTJSI-UHFFFAOYSA-N hydrazine hydrate Chemical compound O.NN IKDUDTNKRLTJSI-UHFFFAOYSA-N 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 125000002883 imidazolyl group Chemical group 0.000 description 2
- 125000003406 indolizinyl group Chemical group C=1(C=CN2C=CC=CC12)* 0.000 description 2
- 125000001041 indolyl group Chemical group 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000004941 influx Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 2
- 125000005956 isoquinolyl group Chemical group 0.000 description 2
- 125000000842 isoxazolyl group Chemical group 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- 239000012139 lysis buffer Substances 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- LRROFHLIWGYSEZ-UHFFFAOYSA-N methyl 2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxypropanoate Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)OC(C)C(=O)OC)=C1 LRROFHLIWGYSEZ-UHFFFAOYSA-N 0.000 description 2
- RXHOMYWGIHJLDD-UHFFFAOYSA-N methyl 2-amino-5-iodo-4-methoxybenzoate Chemical compound COC(=O)C1=CC(I)=C(OC)C=C1N RXHOMYWGIHJLDD-UHFFFAOYSA-N 0.000 description 2
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 2
- 230000000116 mitigating effect Effects 0.000 description 2
- 125000002757 morpholinyl group Chemical group 0.000 description 2
- 239000012452 mother liquor Substances 0.000 description 2
- BSOQXXWZTUDTEL-ZUYCGGNHSA-N muramyl dipeptide Chemical compound OC(=O)CC[C@H](C(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](C)O[C@H]1[C@H](O)[C@@H](CO)O[C@@H](O)[C@@H]1NC(C)=O BSOQXXWZTUDTEL-ZUYCGGNHSA-N 0.000 description 2
- SEGYZPWTTRMTSV-UHFFFAOYSA-N n-(4,5-dimethyl-1h-pyrazol-3-yl)formamide Chemical compound CC=1NN=C(NC=O)C=1C SEGYZPWTTRMTSV-UHFFFAOYSA-N 0.000 description 2
- UZXZRLICOHKBTM-UHFFFAOYSA-N n-(5-fluoro-1h-indazol-3-yl)-6-propan-2-ylsulfonylquinazolin-4-amine Chemical compound C1=C(F)C=C2C(NC3=NC=NC4=CC=C(C=C43)S(=O)(=O)C(C)C)=NNC2=C1 UZXZRLICOHKBTM-UHFFFAOYSA-N 0.000 description 2
- XOGMEWSQTVYLLA-UHFFFAOYSA-N n-(6-tert-butylsulfonyl-7-ethenoxyquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)OC=C)S(=O)(=O)C(C)(C)C)=C1 XOGMEWSQTVYLLA-UHFFFAOYSA-N 0.000 description 2
- XXAZQMDKRUYZBC-UHFFFAOYSA-N n-(6-tert-butylsulfonyl-7-ethylquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)CC)=C1 XXAZQMDKRUYZBC-UHFFFAOYSA-N 0.000 description 2
- SSJJZIPKSYXMSW-UHFFFAOYSA-N n-[6-(oxan-4-ylsulfonyl)quinazolin-4-yl]-1,3-benzothiazol-5-amine Chemical compound C=1C=C2N=CN=C(NC=3C=C4N=CSC4=CC=3)C2=CC=1S(=O)(=O)C1CCOCC1 SSJJZIPKSYXMSW-UHFFFAOYSA-N 0.000 description 2
- WNOHQWUHRHCOLN-UHFFFAOYSA-N n-[6-tert-butylsulfonyl-7-(2-methylsulfonylethoxy)quinazolin-4-yl]-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)OCCS(C)(=O)=O)S(=O)(=O)C(C)(C)C)=C1 WNOHQWUHRHCOLN-UHFFFAOYSA-N 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- 229910000069 nitrogen hydride Inorganic materials 0.000 description 2
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- 125000001715 oxadiazolyl group Chemical group 0.000 description 2
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 2
- 125000005968 oxazolinyl group Chemical group 0.000 description 2
- 125000002971 oxazolyl group Chemical group 0.000 description 2
- 125000003566 oxetanyl group Chemical group 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 238000000955 peptide mass fingerprinting Methods 0.000 description 2
- 239000003330 peritoneal dialysis fluid Substances 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- 125000004592 phthalazinyl group Chemical group C1(=NN=CC2=CC=CC=C12)* 0.000 description 2
- 125000004193 piperazinyl group Chemical group 0.000 description 2
- 125000003386 piperidinyl group Chemical group 0.000 description 2
- 230000010287 polarization Effects 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 2
- KJRCEJOSASVSRA-UHFFFAOYSA-N propane-2-thiol Chemical compound CC(C)S KJRCEJOSASVSRA-UHFFFAOYSA-N 0.000 description 2
- 125000001042 pteridinyl group Chemical group N1=C(N=CC2=NC=CN=C12)* 0.000 description 2
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 2
- 125000003373 pyrazinyl group Chemical group 0.000 description 2
- 125000002755 pyrazolinyl group Chemical group 0.000 description 2
- 125000002098 pyridazinyl group Chemical group 0.000 description 2
- 125000000714 pyrimidinyl group Chemical group 0.000 description 2
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 2
- 125000000168 pyrrolyl group Chemical group 0.000 description 2
- MAQSCXRYQVMPKA-UHFFFAOYSA-N quinazolin-7-yl trifluoromethanesulfonate Chemical compound C1=NC=NC2=CC(OS(=O)(=O)C(F)(F)F)=CC=C21 MAQSCXRYQVMPKA-UHFFFAOYSA-N 0.000 description 2
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 2
- 125000005493 quinolyl group Chemical group 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 238000007363 ring formation reaction Methods 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000012279 sodium borohydride Substances 0.000 description 2
- 229910000033 sodium borohydride Inorganic materials 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- HEMHJVSKTPXQMS-UHFFFAOYSA-M sodium hydroxide Inorganic materials [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 150000003456 sulfonamides Chemical class 0.000 description 2
- 150000003462 sulfoxides Chemical group 0.000 description 2
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical compound ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 238000007910 systemic administration Methods 0.000 description 2
- 108091008743 testicular receptors 4 Proteins 0.000 description 2
- 125000000147 tetrahydroquinolinyl group Chemical group N1(CCCC2=CC=CC=C12)* 0.000 description 2
- 125000003831 tetrazolyl group Chemical group 0.000 description 2
- 125000002769 thiazolinyl group Chemical group 0.000 description 2
- 125000000335 thiazolyl group Chemical group 0.000 description 2
- 125000004306 triazinyl group Chemical group 0.000 description 2
- 125000001425 triazolyl group Chemical group 0.000 description 2
- WJKHJLXJJJATHN-UHFFFAOYSA-N triflic anhydride Chemical compound FC(F)(F)S(=O)(=O)OS(=O)(=O)C(F)(F)F WJKHJLXJJJATHN-UHFFFAOYSA-N 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- ONDSBJMLAHVLMI-UHFFFAOYSA-N trimethylsilyldiazomethane Chemical compound C[Si](C)(C)[CH-][N+]#N ONDSBJMLAHVLMI-UHFFFAOYSA-N 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- BWHDROKFUHTORW-UHFFFAOYSA-N tritert-butylphosphane Chemical compound CC(C)(C)P(C(C)(C)C)C(C)(C)C BWHDROKFUHTORW-UHFFFAOYSA-N 0.000 description 2
- 241000701447 unidentified baculovirus Species 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- CXNIUSPIQKWYAI-UHFFFAOYSA-N xantphos Chemical compound C=12OC3=C(P(C=4C=CC=CC=4)C=4C=CC=CC=4)C=CC=C3C(C)(C)C2=CC=CC=1P(C=1C=CC=CC=1)C1=CC=CC=C1 CXNIUSPIQKWYAI-UHFFFAOYSA-N 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- GMKMEZVLHJARHF-UHFFFAOYSA-N (2R,6R)-form-2.6-Diaminoheptanedioic acid Natural products OC(=O)C(N)CCCC(N)C(O)=O GMKMEZVLHJARHF-UHFFFAOYSA-N 0.000 description 1
- WFFNANOPOVMPSU-CYBMUJFWSA-N (2r)-2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxypropan-1-ol Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)O[C@@H](CO)C)=C1 WFFNANOPOVMPSU-CYBMUJFWSA-N 0.000 description 1
- WWTBZEKOSBFBEM-SPWPXUSOSA-N (2s)-2-[[2-benzyl-3-[hydroxy-[(1r)-2-phenyl-1-(phenylmethoxycarbonylamino)ethyl]phosphoryl]propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)C(CP(O)(=O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 WWTBZEKOSBFBEM-SPWPXUSOSA-N 0.000 description 1
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 1
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 1
- MZEDHTHAYKNCDA-SNAWJCMRSA-N (e)-3-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]prop-2-en-1-ol Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)\C=C\CO)S(=O)(=O)C(C)(C)C)=C1 MZEDHTHAYKNCDA-SNAWJCMRSA-N 0.000 description 1
- DELJOESCKJGFML-RQOWECAXSA-N (z)-3-aminobut-2-enenitrile Chemical compound C\C(N)=C\C#N DELJOESCKJGFML-RQOWECAXSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 1
- IGERFAHWSHDDHX-UHFFFAOYSA-N 1,3-dioxanyl Chemical group [CH]1OCCCO1 IGERFAHWSHDDHX-UHFFFAOYSA-N 0.000 description 1
- KFHQOZXAFUKFNB-UHFFFAOYSA-N 1,3-oxathiolanyl Chemical group [CH]1OCCS1 KFHQOZXAFUKFNB-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- QMNUDYFKZYBWQX-UHFFFAOYSA-N 1H-quinazolin-4-one Chemical compound C1=CC=C2C(=O)N=CNC2=C1 QMNUDYFKZYBWQX-UHFFFAOYSA-N 0.000 description 1
- JVVRJMXHNUAPHW-UHFFFAOYSA-N 1h-pyrazol-5-amine Chemical compound NC=1C=CNN=1 JVVRJMXHNUAPHW-UHFFFAOYSA-N 0.000 description 1
- AVRPFRMDMNDIDH-UHFFFAOYSA-N 1h-quinazolin-2-one Chemical compound C1=CC=CC2=NC(O)=NC=C21 AVRPFRMDMNDIDH-UHFFFAOYSA-N 0.000 description 1
- HCSBTDBGTNZOAB-UHFFFAOYSA-N 2,3-dinitrobenzoic acid Chemical compound OC(=O)C1=CC=CC([N+]([O-])=O)=C1[N+]([O-])=O HCSBTDBGTNZOAB-UHFFFAOYSA-N 0.000 description 1
- WAUKSUUWTQZOQI-UHFFFAOYSA-N 2-(1,3-benzothiazol-5-ylamino)-7-methoxyquinazoline-6-sulfonyl chloride Chemical compound C1=C2SC=NC2=CC(NC=2N=C3C=C(C(=CC3=CN=2)S(Cl)(=O)=O)OC)=C1 WAUKSUUWTQZOQI-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- FCSBSGMKWOPSMX-UHFFFAOYSA-N 2-(thiolan-2-ylsulfinyl)thiolane Chemical compound C1CCSC1S(=O)C1CCCS1 FCSBSGMKWOPSMX-UHFFFAOYSA-N 0.000 description 1
- TWHOUWQNTGRQMY-UHFFFAOYSA-N 2-(thiolan-2-ylsulfonyl)thiolane Chemical compound C1CCSC1S(=O)(=O)C1CCCS1 TWHOUWQNTGRQMY-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- RTEHDLUIOYJIND-UHFFFAOYSA-N 2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxy-2-methylpropan-1-ol Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)OC(C)(CO)C)=C1 RTEHDLUIOYJIND-UHFFFAOYSA-N 0.000 description 1
- UHDOJINBFLDQJM-UHFFFAOYSA-N 2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxyethanol Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)OCCO)S(=O)(=O)C(C)(C)C)=C1 UHDOJINBFLDQJM-UHFFFAOYSA-N 0.000 description 1
- IYZUIOQTKXSNLZ-UHFFFAOYSA-N 2-[4-(1,3-benzothiazol-5-ylamino)quinazolin-6-yl]sulfonylethanol Chemical compound C1=C2SC=NC2=CC(NC2=NC=NC3=CC=C(C=C32)S(=O)(=O)CCO)=C1 IYZUIOQTKXSNLZ-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- QFXIIWGIPTWHOU-UHFFFAOYSA-N 2-[4-[(4,5-dimethyl-1h-pyrazol-3-yl)amino]-7-methoxyquinazolin-6-yl]sulfonylethanol Chemical compound C=12C=C(S(=O)(=O)CCO)C(OC)=CC2=NC=NC=1NC1=NNC(C)=C1C QFXIIWGIPTWHOU-UHFFFAOYSA-N 0.000 description 1
- HHNWXQCVWVVVQZ-UHFFFAOYSA-N 2-amino-4-methoxybenzoic acid Chemical compound COC1=CC=C(C(O)=O)C(N)=C1 HHNWXQCVWVVVQZ-UHFFFAOYSA-N 0.000 description 1
- JUIKUQOUMZUFQT-UHFFFAOYSA-N 2-bromoacetamide Chemical compound NC(=O)CBr JUIKUQOUMZUFQT-UHFFFAOYSA-N 0.000 description 1
- LDLCZOVUSADOIV-UHFFFAOYSA-N 2-bromoethanol Chemical compound OCCBr LDLCZOVUSADOIV-UHFFFAOYSA-N 0.000 description 1
- WMPTYRGXBUYONY-UHFFFAOYSA-N 2-chloroquinazoline Chemical compound C1=CC=CC2=NC(Cl)=NC=C21 WMPTYRGXBUYONY-UHFFFAOYSA-N 0.000 description 1
- FWMBEYDLDLJTDP-UHFFFAOYSA-N 2-iodoquinazoline Chemical class C1=CC=CC2=NC(I)=NC=C21 FWMBEYDLDLJTDP-UHFFFAOYSA-N 0.000 description 1
- ANTISGAVDLDNTA-UHFFFAOYSA-N 2-methoxyquinazolin-4-amine Chemical compound C1=CC=CC2=NC(OC)=NC(N)=C21 ANTISGAVDLDNTA-UHFFFAOYSA-N 0.000 description 1
- SLAMLWHELXOEJZ-UHFFFAOYSA-N 2-nitrobenzoic acid Chemical compound OC(=O)C1=CC=CC=C1[N+]([O-])=O SLAMLWHELXOEJZ-UHFFFAOYSA-N 0.000 description 1
- DWPYQDGDWBKJQL-UHFFFAOYSA-N 2-pyridin-4-ylethanol Chemical compound OCCC1=CC=NC=C1 DWPYQDGDWBKJQL-UHFFFAOYSA-N 0.000 description 1
- UMCMPZBLKLEWAF-BCTGSCMUSA-N 3-[(3-cholamidopropyl)dimethylammonio]propane-1-sulfonate Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 UMCMPZBLKLEWAF-BCTGSCMUSA-N 0.000 description 1
- ARURRWWRCKTFBE-UHFFFAOYSA-N 3-amino-2-methylprop-2-enenitrile Chemical compound NC=C(C)C#N ARURRWWRCKTFBE-UHFFFAOYSA-N 0.000 description 1
- NUNAWQZKZVVELQ-UHFFFAOYSA-N 3-amino-4-methylphenol Chemical compound CC1=CC=C(O)C=C1N NUNAWQZKZVVELQ-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-M 3-carboxy-2,3-dihydroxypropanoate Chemical compound OC(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-M 0.000 description 1
- ALKYHXVLJMQRLQ-UHFFFAOYSA-M 3-carboxynaphthalen-2-olate Chemical compound C1=CC=C2C=C(C([O-])=O)C(O)=CC2=C1 ALKYHXVLJMQRLQ-UHFFFAOYSA-M 0.000 description 1
- MCSXGCZMEPXKIW-UHFFFAOYSA-N 3-hydroxy-4-[(4-methyl-2-nitrophenyl)diazenyl]-N-(3-nitrophenyl)naphthalene-2-carboxamide Chemical class Cc1ccc(N=Nc2c(O)c(cc3ccccc23)C(=O)Nc2cccc(c2)[N+]([O-])=O)c(c1)[N+]([O-])=O MCSXGCZMEPXKIW-UHFFFAOYSA-N 0.000 description 1
- GBCGIJAYTBMFHI-UHFFFAOYSA-N 3-methyl-3-sulfanylbutan-1-ol Chemical compound CC(C)(S)CCO GBCGIJAYTBMFHI-UHFFFAOYSA-N 0.000 description 1
- ALYNCZNDIQEVRV-UHFFFAOYSA-M 4-aminobenzoate Chemical compound NC1=CC=C(C([O-])=O)C=C1 ALYNCZNDIQEVRV-UHFFFAOYSA-M 0.000 description 1
- CSFDTBRRIBJILD-UHFFFAOYSA-N 4-chloro-2-fluoroaniline Chemical compound NC1=CC=C(Cl)C=C1F CSFDTBRRIBJILD-UHFFFAOYSA-N 0.000 description 1
- NWMNIOBPSAXMMY-UHFFFAOYSA-N 4-chloro-6-methylsulfanylquinazoline Chemical compound N1=CN=C(Cl)C2=CC(SC)=CC=C21 NWMNIOBPSAXMMY-UHFFFAOYSA-N 0.000 description 1
- JVVRCYWZTJLJSG-UHFFFAOYSA-N 4-dimethylaminophenol Chemical compound CN(C)C1=CC=C(O)C=C1 JVVRCYWZTJLJSG-UHFFFAOYSA-N 0.000 description 1
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 1
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-dimethylaminopyridine Substances CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 1
- SJZRECIVHVDYJC-UHFFFAOYSA-N 4-hydroxybutyric acid Chemical compound OCCCC(O)=O SJZRECIVHVDYJC-UHFFFAOYSA-N 0.000 description 1
- 125000002471 4H-quinolizinyl group Chemical group C=1(C=CCN2C=CC=CC12)* 0.000 description 1
- WGHRQILGEUWYCX-UHFFFAOYSA-N 5-(methylthio)-2-nitrobenzoic acid Chemical compound CSC1=CC=C([N+]([O-])=O)C(C(O)=O)=C1 WGHRQILGEUWYCX-UHFFFAOYSA-N 0.000 description 1
- XDCUIJKNYICIIT-UHFFFAOYSA-N 5-[(6-tert-butylsulfonyl-7-methoxyquinazolin-4-yl)amino]-2-chlorophenol Chemical compound C=12C=C(S(=O)(=O)C(C)(C)C)C(OC)=CC2=NC=NC=1NC1=CC=C(Cl)C(O)=C1 XDCUIJKNYICIIT-UHFFFAOYSA-N 0.000 description 1
- CLATWDTWMVIPHK-UHFFFAOYSA-N 5-[2-[[3-[[4-(5-hydroxy-2-methylanilino)pyrimidin-2-yl]amino]benzoyl]amino]ethylcarbamoyl]-2-(3-hydroxy-6-oxoxanthen-9-yl)benzoic acid Chemical compound CC1=CC=C(O)C=C1NC1=CC=NC(NC=2C=C(C=CC=2)C(=O)NCCNC(=O)C=2C=C(C(=CC=2)C2=C3C=CC(=O)C=C3OC3=CC(O)=CC=C32)C(O)=O)=N1 CLATWDTWMVIPHK-UHFFFAOYSA-N 0.000 description 1
- UWLBVJRYQQUECL-UHFFFAOYSA-N 5-fluoro-1h-indazol-3-amine Chemical compound C1=C(F)C=C2C(N)=NNC2=C1 UWLBVJRYQQUECL-UHFFFAOYSA-N 0.000 description 1
- UFONWAPOQPJJLL-UHFFFAOYSA-N 5-fluoro-2h-pyrazolo[3,4-b]pyridin-3-amine Chemical compound C1=C(F)C=C2C(N)=NNC2=N1 UFONWAPOQPJJLL-UHFFFAOYSA-N 0.000 description 1
- 125000006163 5-membered heteroaryl group Chemical group 0.000 description 1
- BLJDQJLSUDXUGL-UHFFFAOYSA-N 6-iodoquinazoline Chemical compound N1=CN=CC2=CC(I)=CC=C21 BLJDQJLSUDXUGL-UHFFFAOYSA-N 0.000 description 1
- NHOGHVSHKSXFKK-UHFFFAOYSA-N 6-propan-2-ylsulfonyl-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=CC(S(=O)(=O)C(C)C)=CC=C21 NHOGHVSHKSXFKK-UHFFFAOYSA-N 0.000 description 1
- ODKCWJXVZKAMLX-UHFFFAOYSA-N 6-tert-butylsulfonyl-4,7-dichloroquinazoline Chemical compound N1=CN=C2C=C(Cl)C(S(=O)(=O)C(C)(C)C)=CC2=C1Cl ODKCWJXVZKAMLX-UHFFFAOYSA-N 0.000 description 1
- HTDAOJZPJPJZHK-UHFFFAOYSA-N 6-tert-butylsulfonyl-4-(4-chloro-2-fluoroanilino)quinazolin-7-ol Chemical compound N1=CN=C2C=C(O)C(S(=O)(=O)C(C)(C)C)=CC2=C1NC1=CC=C(Cl)C=C1F HTDAOJZPJPJZHK-UHFFFAOYSA-N 0.000 description 1
- SRCGLNSSSINORI-UHFFFAOYSA-N 6-tert-butylsulfonyl-4-chloro-7-methoxyquinazoline Chemical compound C1=NC(Cl)=C2C=C(S(=O)(=O)C(C)(C)C)C(OC)=CC2=N1 SRCGLNSSSINORI-UHFFFAOYSA-N 0.000 description 1
- UVRNALVUGHDPEX-UHFFFAOYSA-N 6-tert-butylsulfonyl-4-ethylsulfanylquinazolin-7-ol Chemical compound OC1=C(S(=O)(=O)C(C)(C)C)C=C2C(SCC)=NC=NC2=C1 UVRNALVUGHDPEX-UHFFFAOYSA-N 0.000 description 1
- BQTHLISEPIXVRZ-UHFFFAOYSA-N 6-tert-butylsulfonyl-7-chloro-n-(4,5-dimethyl-1h-pyrazol-3-yl)quinazolin-4-amine Chemical compound CC1=C(C)NN=C1NC1=NC=NC2=CC(Cl)=C(S(=O)(=O)C(C)(C)C)C=C12 BQTHLISEPIXVRZ-UHFFFAOYSA-N 0.000 description 1
- WEAMADQORMMOAF-UHFFFAOYSA-N 6-tert-butylsulfonyl-7-methoxy-n-(3-methyl-2h-indazol-6-yl)quinazolin-4-amine Chemical compound C1=C2C(C)=NNC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)OC)=C1 WEAMADQORMMOAF-UHFFFAOYSA-N 0.000 description 1
- JRAQIAOKZOOVOF-UHFFFAOYSA-N 6-tert-butylsulfonyl-n-(1h-indazol-6-yl)-7-methoxyquinazolin-4-amine Chemical compound C1=C2C=NNC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)OC)=C1 JRAQIAOKZOOVOF-UHFFFAOYSA-N 0.000 description 1
- YYCQIMDXTOTJBU-UHFFFAOYSA-N 6-tert-butylsulfonyl-n-(2,4,5-trimethylpyrazol-3-yl)quinazolin-4-amine Chemical compound CC1=NN(C)C(NC=2C3=CC(=CC=C3N=CN=2)S(=O)(=O)C(C)(C)C)=C1C YYCQIMDXTOTJBU-UHFFFAOYSA-N 0.000 description 1
- LLJFMVIGBWDCOB-UHFFFAOYSA-N 6-tert-butylsulfonyl-n-(4,5-dimethyl-1h-pyrazol-3-yl)-7-methoxyquinazolin-4-amine Chemical compound C=12C=C(S(=O)(=O)C(C)(C)C)C(OC)=CC2=NC=NC=1NC1=NNC(C)=C1C LLJFMVIGBWDCOB-UHFFFAOYSA-N 0.000 description 1
- BLQVQBRUHQELNJ-UHFFFAOYSA-N 6-tert-butylsulfonyl-n-(4-chloro-2-fluorophenyl)-7-methoxyquinazolin-4-amine Chemical compound C=12C=C(S(=O)(=O)C(C)(C)C)C(OC)=CC2=NC=NC=1NC1=CC=C(Cl)C=C1F BLQVQBRUHQELNJ-UHFFFAOYSA-N 0.000 description 1
- NGZONKIWOMPQFA-UHFFFAOYSA-N 6-tert-butylsulfonyl-n-(5-fluoro-1h-indazol-3-yl)quinazolin-4-amine Chemical compound C1=C(F)C=C2C(NC3=NC=NC4=CC=C(C=C43)S(=O)(=O)C(C)(C)C)=NNC2=C1 NGZONKIWOMPQFA-UHFFFAOYSA-N 0.000 description 1
- KZWFOONWYSBZKF-UHFFFAOYSA-N 6-tert-butylsulfonyl-n-[5-(trifluoromethyl)-1h-pyrazol-3-yl]quinazolin-4-amine Chemical compound C12=CC(S(=O)(=O)C(C)(C)C)=CC=C2N=CN=C1NC=1C=C(C(F)(F)F)NN=1 KZWFOONWYSBZKF-UHFFFAOYSA-N 0.000 description 1
- MJLGSGRUTPFEGE-UHFFFAOYSA-N 7-chloro-6-iodo-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=C1C=C(Cl)C(I)=C2 MJLGSGRUTPFEGE-UHFFFAOYSA-N 0.000 description 1
- HCCHEFLMILLAGQ-UHFFFAOYSA-N 7-ethylquinazoline Chemical compound C1=NC=NC2=CC(CC)=CC=C21 HCCHEFLMILLAGQ-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 102000002164 CARD domains Human genes 0.000 description 1
- 108050009503 CARD domains Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 102100035904 Caspase-1 Human genes 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- HZZVJAQRINQKSD-UHFFFAOYSA-N Clavulanic acid Natural products OC(=O)C1C(=CCO)OC2CC(=O)N21 HZZVJAQRINQKSD-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- DSLZVSRJTYRBFB-LLEIAEIESA-N D-glucaric acid Chemical compound OC(=O)[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O DSLZVSRJTYRBFB-LLEIAEIESA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 239000001692 EU approved anti-caking agent Substances 0.000 description 1
- 102100030011 Endoribonuclease Human genes 0.000 description 1
- 101710199605 Endoribonuclease Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 108010008165 Etanercept Proteins 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 239000007821 HATU Substances 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 101001052435 Homo sapiens Ubiquitin carboxyl-terminal hydrolase MINDY-3 Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 102000003777 Interleukin-1 beta Human genes 0.000 description 1
- 108090000193 Interleukin-1 beta Proteins 0.000 description 1
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 102000043136 MAP kinase family Human genes 0.000 description 1
- 108091054455 MAP kinase family Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 1
- 150000001204 N-oxides Chemical class 0.000 description 1
- 102000003945 NF-kappa B Human genes 0.000 description 1
- 108010057466 NF-kappa B Proteins 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 102000007399 Nuclear hormone receptor Human genes 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 229910002666 PdCl2 Inorganic materials 0.000 description 1
- 108010013639 Peptidoglycan Proteins 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 102000001253 Protein Kinase Human genes 0.000 description 1
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 102000013070 Receptor-Interacting Protein Serine-Threonine Kinase 2 Human genes 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- 101710113029 Serine/threonine-protein kinase Proteins 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 241000256251 Spodoptera frugiperda Species 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 238000006619 Stille reaction Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- 229920002253 Tannate Polymers 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 206010052779 Transplant rejections Diseases 0.000 description 1
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 1
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 102100024205 Ubiquitin carboxyl-terminal hydrolase MINDY-3 Human genes 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical compound C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 1
- ZZXDRXVIRVJQBT-UHFFFAOYSA-M Xylenesulfonate Chemical compound CC1=CC=CC(S([O-])(=O)=O)=C1C ZZXDRXVIRVJQBT-UHFFFAOYSA-M 0.000 description 1
- VMWJJLZSLPLGBU-UHFFFAOYSA-N [4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl] trifluoromethanesulfonate Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)OS(=O)(=O)C(F)(F)F)S(=O)(=O)C(C)(C)C)=C1 VMWJJLZSLPLGBU-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- IPBVNPXQWQGGJP-UHFFFAOYSA-N acetic acid phenyl ester Natural products CC(=O)OC1=CC=CC=C1 IPBVNPXQWQGGJP-UHFFFAOYSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000012042 active reagent Substances 0.000 description 1
- 150000001263 acyl chlorides Chemical class 0.000 description 1
- 229960002964 adalimumab Drugs 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- IAJILQKETJEXLJ-RSJOWCBRSA-N aldehydo-D-galacturonic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-RSJOWCBRSA-N 0.000 description 1
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 1
- 229910000272 alkali metal oxide Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910001860 alkaline earth metal hydroxide Inorganic materials 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- 150000001347 alkyl bromides Chemical class 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 229940061720 alpha hydroxy acid Drugs 0.000 description 1
- 150000001280 alpha hydroxy acids Chemical class 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- VZTDIZULWFCMLS-UHFFFAOYSA-N ammonium formate Chemical compound [NH4+].[O-]C=O VZTDIZULWFCMLS-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000011914 asymmetric synthesis Methods 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 230000035578 autophosphorylation Effects 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 150000007514 bases Chemical class 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 150000001559 benzoic acids Chemical class 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 1
- 125000003354 benzotriazolyl group Chemical group N1N=NC2=C1C=CC=C2* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- XAAHAAMILDNBPS-UHFFFAOYSA-L calcium hydrogenphosphate dihydrate Chemical compound O.O.[Ca+2].OP([O-])([O-])=O XAAHAAMILDNBPS-UHFFFAOYSA-L 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 239000007894 caplet Substances 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 238000007675 cardiac surgery Methods 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 150000005829 chemical entities Chemical class 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000005660 chlorination reaction Methods 0.000 description 1
- KVSASDOGYIBWTA-UHFFFAOYSA-N chloro benzoate Chemical compound ClOC(=O)C1=CC=CC=C1 KVSASDOGYIBWTA-UHFFFAOYSA-N 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229940090805 clavulanate Drugs 0.000 description 1
- HZZVJAQRINQKSD-PBFISZAISA-N clavulanic acid Chemical compound OC(=O)[C@H]1C(=C/CO)/O[C@@H]2CC(=O)N21 HZZVJAQRINQKSD-PBFISZAISA-N 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 229940126208 compound 22 Drugs 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- HCAJEUSONLESMK-UHFFFAOYSA-N cyclohexylsulfamic acid Chemical compound OS(=O)(=O)NC1CCCCC1 HCAJEUSONLESMK-UHFFFAOYSA-N 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 108091007930 cytoplasmic receptors Proteins 0.000 description 1
- 230000020335 dealkylation Effects 0.000 description 1
- 238000006900 dealkylation reaction Methods 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 1
- 229940027008 deltasone Drugs 0.000 description 1
- 238000000326 densiometry Methods 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 239000012973 diazabicyclooctane Substances 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 229940095079 dicalcium phosphate anhydrous Drugs 0.000 description 1
- ACYGYJFTZSAZKR-UHFFFAOYSA-J dicalcium;2-[2-[bis(carboxylatomethyl)amino]ethyl-(carboxylatomethyl)amino]acetate Chemical compound [Ca+2].[Ca+2].[O-]C(=O)CN(CC([O-])=O)CCN(CC([O-])=O)CC([O-])=O ACYGYJFTZSAZKR-UHFFFAOYSA-J 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- GPAYUJZHTULNBE-UHFFFAOYSA-N diphenylphosphine Chemical compound C=1C=CC=CC=1PC1=CC=CC=C1 GPAYUJZHTULNBE-UHFFFAOYSA-N 0.000 description 1
- 239000001177 diphosphate Substances 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000008482 dysregulation Effects 0.000 description 1
- 229940009662 edetate Drugs 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 229950005627 embonate Drugs 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 229940073621 enbrel Drugs 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229950000206 estolate Drugs 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-L ethane-1,2-disulfonate Chemical compound [O-]S(=O)(=O)CCS([O-])(=O)=O AFAXGSQYZLGZPG-UHFFFAOYSA-L 0.000 description 1
- NBVNURWDQZEWFF-UHFFFAOYSA-N ethyl 2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxy-2-methylpropanoate Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)OC(C)(C)C(=O)OCC)=C1 NBVNURWDQZEWFF-UHFFFAOYSA-N 0.000 description 1
- IOLQWGVDEFWYNP-UHFFFAOYSA-N ethyl 2-bromo-2-methylpropanoate Chemical compound CCOC(=O)C(C)(C)Br IOLQWGVDEFWYNP-UHFFFAOYSA-N 0.000 description 1
- DVCQFWNONVOLKG-UHFFFAOYSA-N ethyl 4-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]sulfanylbutanoate Chemical compound N1=CN=C2C=C(OC)C(SCCCC(=O)OCC)=CC2=C1NC1=CC=C(F)C(Cl)=C1 DVCQFWNONVOLKG-UHFFFAOYSA-N 0.000 description 1
- HRDJNXONCYKSGH-UHFFFAOYSA-N ethyl 4-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]sulfanylheptanoate Chemical compound N1=CN=C2C=C(OC)C(SC(CCC(=O)OCC)CCC)=CC2=C1NC1=CC=C(F)C(Cl)=C1 HRDJNXONCYKSGH-UHFFFAOYSA-N 0.000 description 1
- WPZDLCWJNUGCDH-UHFFFAOYSA-N ethyl 4-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]sulfonylheptanoate Chemical compound N1=CN=C2C=C(OC)C(S(=O)(=O)C(CCC(=O)OCC)CCC)=CC2=C1NC1=CC=C(F)C(Cl)=C1 WPZDLCWJNUGCDH-UHFFFAOYSA-N 0.000 description 1
- DNJIEGIFACGWOD-UHFFFAOYSA-N ethyl mercaptane Natural products CCS DNJIEGIFACGWOD-UHFFFAOYSA-N 0.000 description 1
- OJCSPXHYDFONPU-UHFFFAOYSA-N etoac etoac Chemical compound CCOC(C)=O.CCOC(C)=O OJCSPXHYDFONPU-UHFFFAOYSA-N 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 238000003818 flash chromatography Methods 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 229940044170 formate Drugs 0.000 description 1
- 229940050411 fumarate Drugs 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000001030 gas--liquid chromatography Methods 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 229960001731 gluceptate Drugs 0.000 description 1
- KWMLJOLKUYYJFJ-VFUOTHLCSA-N glucoheptonic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)C(O)=O KWMLJOLKUYYJFJ-VFUOTHLCSA-N 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 229940049906 glutamate Drugs 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 229960004275 glycolic acid Drugs 0.000 description 1
- 229960001743 golimumab Drugs 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 238000005658 halogenation reaction Methods 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-M hexanoate Chemical compound CCCCCC([O-])=O FUZZWVXGSFPDMH-UHFFFAOYSA-M 0.000 description 1
- 102000057608 human RIPK2 Human genes 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 229940048921 humira Drugs 0.000 description 1
- XGIHQYAWBCFNPY-AZOCGYLKSA-N hydrabamine Chemical compound C([C@@H]12)CC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC[C@@]1(C)CNCCNC[C@@]1(C)[C@@H]2CCC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC1 XGIHQYAWBCFNPY-AZOCGYLKSA-N 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 150000003840 hydrochlorides Chemical class 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 238000007327 hydrogenolysis reaction Methods 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- ILHIHKRJJMKBEE-UHFFFAOYSA-N hydroperoxyethane Chemical class CCOO ILHIHKRJJMKBEE-UHFFFAOYSA-N 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 125000002636 imidazolinyl group Chemical group 0.000 description 1
- 125000004857 imidazopyridinyl group Chemical group N1C(=NC2=C1C=CC=N2)* 0.000 description 1
- 230000037451 immune surveillance Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 125000003387 indolinyl group Chemical group N1(CCC2=CC=CC=C12)* 0.000 description 1
- 229960000598 infliximab Drugs 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 125000002346 iodo group Chemical group I* 0.000 description 1
- HVTICUPFWKNHNG-UHFFFAOYSA-N iodoethane Chemical compound CCI HVTICUPFWKNHNG-UHFFFAOYSA-N 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-N isobutanol Substances CC(C)CO ZXEKIIBDNHEJCQ-UHFFFAOYSA-N 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- 125000004594 isoindolinyl group Chemical group C1(NCC2=CC=CC=C12)* 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- FMKOJHQHASLBPH-UHFFFAOYSA-N isopropyl iodide Chemical compound CC(C)I FMKOJHQHASLBPH-UHFFFAOYSA-N 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N itaconic acid Chemical compound OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 229940099584 lactobionate Drugs 0.000 description 1
- JYTUSYBCFIZPBE-AMTLMPIISA-N lactobionic acid Chemical compound OC(=O)[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O JYTUSYBCFIZPBE-AMTLMPIISA-N 0.000 description 1
- 229940070765 laurate Drugs 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 239000012280 lithium aluminium hydride Substances 0.000 description 1
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- KBOPZPXVLCULAV-UHFFFAOYSA-N mesalamine Chemical compound NC1=CC=C(O)C(C(O)=O)=C1 KBOPZPXVLCULAV-UHFFFAOYSA-N 0.000 description 1
- 229960004963 mesalazine Drugs 0.000 description 1
- GMKMEZVLHJARHF-SYDPRGILSA-N meso-2,6-diaminopimelic acid Chemical compound [O-]C(=O)[C@@H]([NH3+])CCC[C@@H]([NH3+])C([O-])=O GMKMEZVLHJARHF-SYDPRGILSA-N 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- LRROFHLIWGYSEZ-CYBMUJFWSA-N methyl (2r)-2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxypropanoate Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)O[C@H](C)C(=O)OC)=C1 LRROFHLIWGYSEZ-CYBMUJFWSA-N 0.000 description 1
- LRROFHLIWGYSEZ-ZDUSSCGKSA-N methyl (2s)-2-[4-(1,3-benzothiazol-5-ylamino)-6-tert-butylsulfonylquinazolin-7-yl]oxypropanoate Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)O[C@@H](C)C(=O)OC)=C1 LRROFHLIWGYSEZ-ZDUSSCGKSA-N 0.000 description 1
- RUZLIIJDZBWWSA-INIZCTEOSA-N methyl 2-[[(1s)-1-(7-methyl-2-morpholin-4-yl-4-oxopyrido[1,2-a]pyrimidin-9-yl)ethyl]amino]benzoate Chemical group COC(=O)C1=CC=CC=C1N[C@@H](C)C1=CC(C)=CN2C(=O)C=C(N3CCOCC3)N=C12 RUZLIIJDZBWWSA-INIZCTEOSA-N 0.000 description 1
- ZGCFYZJEPDVXQD-UHFFFAOYSA-N methyl 2-amino-4-chloro-5-iodobenzoate Chemical compound COC(=O)C1=CC(I)=C(Cl)C=C1N ZGCFYZJEPDVXQD-UHFFFAOYSA-N 0.000 description 1
- CEKCJQBZVNIMLD-UHFFFAOYSA-N methyl 2-amino-4-methoxybenzoate Chemical compound COC(=O)C1=CC=C(OC)C=C1N CEKCJQBZVNIMLD-UHFFFAOYSA-N 0.000 description 1
- YDCHPLOFQATIDS-UHFFFAOYSA-N methyl 2-bromoacetate Chemical compound COC(=O)CBr YDCHPLOFQATIDS-UHFFFAOYSA-N 0.000 description 1
- ACEONLNNWKIPTM-UHFFFAOYSA-N methyl 2-bromopropanoate Chemical compound COC(=O)C(C)Br ACEONLNNWKIPTM-UHFFFAOYSA-N 0.000 description 1
- IZYBEMGNIUSSAX-UHFFFAOYSA-N methyl benzenecarboperoxoate Chemical compound COOC(=O)C1=CC=CC=C1 IZYBEMGNIUSSAX-UHFFFAOYSA-N 0.000 description 1
- 229940095102 methyl benzoate Drugs 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- IMAKHNTVDGLIRY-UHFFFAOYSA-N methyl prop-2-ynoate Chemical compound COC(=O)C#C IMAKHNTVDGLIRY-UHFFFAOYSA-N 0.000 description 1
- JZMJDSHXVKJFKW-UHFFFAOYSA-M methyl sulfate(1-) Chemical compound COS([O-])(=O)=O JZMJDSHXVKJFKW-UHFFFAOYSA-M 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000002062 molecular scaffold Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- SARKUXSGOQUHSM-UHFFFAOYSA-N n,4,5-trimethyl-1h-pyrazol-3-amine Chemical compound CNC1=NNC(C)=C1C SARKUXSGOQUHSM-UHFFFAOYSA-N 0.000 description 1
- DJUHCRXMBJTHCF-UHFFFAOYSA-N n-(4,5-dimethyl-1h-pyrazol-3-yl)-6-propan-2-ylsulfonylquinazolin-4-amine Chemical compound C12=CC(S(=O)(=O)C(C)C)=CC=C2N=CN=C1NC1=NNC(C)=C1C DJUHCRXMBJTHCF-UHFFFAOYSA-N 0.000 description 1
- IMVNDZNHQZYIOC-UHFFFAOYSA-N n-(5-fluoro-1h-indazol-3-yl)-6-iodoquinazolin-4-amine Chemical compound C1=C(I)C=C2C(NC3=NNC4=CC=C(C=C43)F)=NC=NC2=C1 IMVNDZNHQZYIOC-UHFFFAOYSA-N 0.000 description 1
- AGWDPBSUHIQOHL-UHFFFAOYSA-N n-(6-iodo-7-methoxyquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)I)OC)=C1 AGWDPBSUHIQOHL-UHFFFAOYSA-N 0.000 description 1
- SXKVPBAVQVMTMP-UHFFFAOYSA-N n-(6-tert-butylsulfinyl-7-methoxyquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)C(C)(C)C)OC)=C1 SXKVPBAVQVMTMP-UHFFFAOYSA-N 0.000 description 1
- GHBWUMFAKHRCHP-UHFFFAOYSA-N n-(6-tert-butylsulfonyl-7-ethenylquinazolin-4-yl)-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)C=C)S(=O)(=O)C(C)(C)C)=C1 GHBWUMFAKHRCHP-UHFFFAOYSA-N 0.000 description 1
- MZKXQFPUSVGNNS-UHFFFAOYSA-N n-[6-tert-butylsulfonyl-7-(2-methylsulfanylethoxy)quinazolin-4-yl]-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC=2N=CN=C3C=C(C(=CC3=2)S(=O)(=O)C(C)(C)C)OCCSC)=C1 MZKXQFPUSVGNNS-UHFFFAOYSA-N 0.000 description 1
- OQAUBIIENWLAFJ-UHFFFAOYSA-N n-[7-(2-bromoethoxy)-6-tert-butylsulfonylquinazolin-4-yl]-1,3-benzothiazol-5-amine Chemical compound C1=C2SC=NC2=CC(NC2=C3C=C(C(=CC3=NC=N2)OCCBr)S(=O)(=O)C(C)(C)C)=C1 OQAUBIIENWLAFJ-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- LAIZPRYFQUWUBN-UHFFFAOYSA-L nickel chloride hexahydrate Chemical compound O.O.O.O.O.O.[Cl-].[Cl-].[Ni+2] LAIZPRYFQUWUBN-UHFFFAOYSA-L 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000002414 normal-phase solid-phase extraction Methods 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 239000006186 oral dosage form Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 229940116315 oxalic acid Drugs 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- KHPXUQMNIQBQEV-UHFFFAOYSA-N oxaloacetic acid Chemical compound OC(=O)CC(=O)C(O)=O KHPXUQMNIQBQEV-UHFFFAOYSA-N 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- 229940023569 palmate Drugs 0.000 description 1
- 229940014662 pantothenate Drugs 0.000 description 1
- 235000019161 pantothenic acid Nutrition 0.000 description 1
- 239000011713 pantothenic acid Substances 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 238000010827 pathological analysis Methods 0.000 description 1
- HVAMZGADVCBITI-UHFFFAOYSA-M pent-4-enoate Chemical compound [O-]C(=O)CCC=C HVAMZGADVCBITI-UHFFFAOYSA-M 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- DYUMLJSJISTVPV-UHFFFAOYSA-N phenyl propanoate Chemical compound CCC(=O)OC1=CC=CC=C1 DYUMLJSJISTVPV-UHFFFAOYSA-N 0.000 description 1
- 229940049953 phenylacetate Drugs 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 125000004194 piperazin-1-yl group Chemical group [H]N1C([H])([H])C([H])([H])N(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000005936 piperidyl group Chemical group 0.000 description 1
- 229960005235 piperonyl butoxide Drugs 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- QPMDWIOUHQWKHV-ODZAUARKSA-M potassium;(z)-4-hydroxy-4-oxobut-2-enoate Chemical compound [K+].OC(=O)\C=C/C([O-])=O QPMDWIOUHQWKHV-ODZAUARKSA-M 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 238000000634 powder X-ray diffraction Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000008741 proinflammatory signaling process Effects 0.000 description 1
- KCXFHTAICRTXLI-UHFFFAOYSA-N propane-1-sulfonic acid Chemical compound CCCS(O)(=O)=O KCXFHTAICRTXLI-UHFFFAOYSA-N 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- UORVCLMRJXCDCP-UHFFFAOYSA-M propynoate Chemical compound [O-]C(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-M 0.000 description 1
- 125000006239 protecting group Chemical group 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 108060006633 protein kinase Proteins 0.000 description 1
- 150000003217 pyrazoles Chemical class 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- ILVXOBCQQYKLDS-UHFFFAOYSA-N pyridine N-oxide Chemical compound [O-][N+]1=CC=CC=C1 ILVXOBCQQYKLDS-UHFFFAOYSA-N 0.000 description 1
- JHHZLHWJQPUNKB-UHFFFAOYSA-N pyrrolidin-3-ol Chemical compound OC1CCNC1 JHHZLHWJQPUNKB-UHFFFAOYSA-N 0.000 description 1
- 229940076788 pyruvate Drugs 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- DRYRBWIFRVMRPV-UHFFFAOYSA-N quinazolin-4-amine Chemical class C1=CC=C2C(N)=NC=NC2=C1 DRYRBWIFRVMRPV-UHFFFAOYSA-N 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 229940116176 remicade Drugs 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 229940116351 sebacate Drugs 0.000 description 1
- CXMXRPHRNRROMY-UHFFFAOYSA-L sebacate(2-) Chemical compound [O-]C(=O)CCCCCCCCC([O-])=O CXMXRPHRNRROMY-UHFFFAOYSA-L 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229940068638 simponi Drugs 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 229940080313 sodium starch Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- TUZFIWBNGKNFPW-UHFFFAOYSA-M sodium;2-methylpropane-2-thiolate Chemical compound [Na+].CC(C)(C)[S-] TUZFIWBNGKNFPW-UHFFFAOYSA-M 0.000 description 1
- QJDUDPQVDAASMV-UHFFFAOYSA-M sodium;ethanethiolate Chemical compound [Na+].CC[S-] QJDUDPQVDAASMV-UHFFFAOYSA-M 0.000 description 1
- WGRULTCAYDOGQK-UHFFFAOYSA-M sodium;sodium;hydroxide Chemical compound [OH-].[Na].[Na+] WGRULTCAYDOGQK-UHFFFAOYSA-M 0.000 description 1
- 239000011877 solvent mixture Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- TYFQFVWCELRYAO-UHFFFAOYSA-L suberate(2-) Chemical compound [O-]C(=O)CCCCCCC([O-])=O TYFQFVWCELRYAO-UHFFFAOYSA-L 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- NCEXYHBECQHGNR-QZQOTICOSA-N sulfasalazine Chemical compound C1=C(O)C(C(=O)O)=CC(\N=N\C=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-QZQOTICOSA-N 0.000 description 1
- 229960001940 sulfasalazine Drugs 0.000 description 1
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 229950002757 teoclate Drugs 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 1
- 125000005958 tetrahydrothienyl group Chemical group 0.000 description 1
- 125000005247 tetrazinyl group Chemical group N1=NN=NC(=C1)* 0.000 description 1
- WROMPOXWARCANT-UHFFFAOYSA-N tfa trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.OC(=O)C(F)(F)F WROMPOXWARCANT-UHFFFAOYSA-N 0.000 description 1
- 125000002053 thietanyl group Chemical group 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-M toluene-4-sulfonate Chemical compound CC1=CC=C(S([O-])(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-M 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- QIWRFOJWQSSRJZ-UHFFFAOYSA-N tributyl(ethenyl)stannane Chemical compound CCCC[Sn](CCCC)(CCCC)C=C QIWRFOJWQSSRJZ-UHFFFAOYSA-N 0.000 description 1
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 1
- 229940070710 valerate Drugs 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229940071104 xylenesulfonate Drugs 0.000 description 1
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/70—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings condensed with carbocyclic rings or ring systems
- C07D239/72—Quinazolines; Hydrogenated quinazolines
- C07D239/86—Quinazolines; Hydrogenated quinazolines with hetero atoms directly attached in position 4
- C07D239/94—Nitrogen atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/14—Decongestants or antiallergics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
Definitions
- the present invention relates to quinazolyl amines that inhibit RIP2 kinase and methods of making and using the same. Specifically, the present invention relates to substituted quinazolyl amines as RIP2 kinase inhibitors.
- Receptor interacting protein-2 (RIP2) kinase which is also referred to as CARD3,
- RICK is a TKL family serine/threonine protein kinase involved in innate immune signaling.
- RIP2 kinase is composed of an N-terminal kinase domain and a C-terminal caspase-recruitment domain (CARD) linked via an intermediate (IM) region ((1998) J. Biol. Chem. 273, 12296-12300; (1998) Current Biology 8, 885-889; and (1998) J. Biol. Chem. 273, 16968-16975).
- CARD domain of RIP2 kinase mediates interaction with other CARD -containing proteins, such as NODI and NOD2 ((2000) J. Biol. Chem.
- NODI and NOD2 are cytoplasmic receptors which play a key role in innate immune surveillance. They recognize both gram positive and gram negative bacterial pathogens and are activated by specific peptidoglycan motifs, diaminopimelic acid (i.e., DAP) and muramyl dipeptide (MDP), respectively ((2007) J Immunol 178, 2380-2386).
- DAP diaminopimelic acid
- MDP muramyl dipeptide
- RIP2 kinase associates with NODI or NOD2 and appears to function principally as a molecular scaffold to bring together other kinases (TAK1, ⁇ / ⁇ / ⁇ ) involved in NF- ⁇ and mitogen-activated protein kinase activation ((2006) Nature Reviews Immunology 6, 9-20). RIP2 kinase undergoes a K63-linked
- a potent, selective, small molecule inhibitor of RIP2 kinase activity would block RIP2-dependent pro-inflammatory signaling and thereby provide a therapeutic benefit in autoinflammatory diseases characterized by increased and/or dysregulated RIP2 kinase activity.
- the invention is directed to quinazolyl amine compounds according to Formula (I):
- R 1 is H, -S0 2 (Ci-C 4 )alkyl, -CO(Ci-C 4 )alkyl, or (Ci-C 4 )alkyl;
- R 2 is - SR a , -SOR a , -S0 2 R a , -S0 2 NH 2 , or -S0 2 NR b R c ,
- R a is (Ci-C 6 )alkyl, halo(Ci-C 6 )alkyl, (C 3 -C 7 )cycloalkyl, 4-7 membered heterocycloalkyl, aryl, or heteroaryl, wherein:
- said (Ci-C 6 )alkyl is optionally substituted by one or two groups each independently selected from the group consisting of cyano, hydroxyl, (Ci-C 6 )alkoxy,
- (C 3 -C 7 )cycloalkyl, phenyl, (phenyl)(Ci-C 4 alkyl)amino-, 5-6 membered heteroaryl, 9-10 membered heteroaryl or 4-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of halogen, -CF 3 , hydroxyl, amino, ((Ci-C 4 )alkyl)amino-, ((Ci-C 4 )alkyl)((Ci-C 4 )alkyl)amino-, (Ci-C 4 )alkyl, phenyl(Ci-C 4 )alkyl-, hydroxy(Ci-C 4 )alkyl and (Ci-C 4 )alkoxy,
- said (C 3 -C 7 )cycloalkyl or 4-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of halogen, -CF 3 , hydroxyl, amino, ((Ci-C 4 )alkyl)amino-, ((Ci-C 4 )alkyl)((Ci-C 4 )alkyl)amino-, (Ci-C 4 )alkyl, phenyl(Ci-C 4 )alkyl-, hydroxy(Ci-C 4 )alkyl-, oxo and (Ci-C 4 )alkoxy, and
- said aryl or heteroaryl is optionally substituted by 1-3 groups each independently selected from the group consisting of halogen, -CF 3 , hydroxyl, amino,
- R b is (Ci-C6)alkyl or 4-7 membered heterocycloalkyl, wherein:
- said (Ci-C 6 )alkyl is optionally substituted by one or two groups each independently selected from the group consisting of hydroxyl, (Ci-C 6 )alkoxy,
- said 4-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of hydroxyl, amino, (Ci-C 4 )alkyl, (Ci-C 4 )alkoxycarbonyl-, hydroxy(Ci-C 4 )alkyl-, oxo and (Ci-C 4 )alkoxy, and
- R c is H, (Ci-C 4 )alkoxy or (Ci-C 6 )alkyl
- R b and R c taken together with the nitrogen atom to which they are attached form a 3-7 membered heterocycloalkyl group, optionally containing one or two additional ring heteroatoms each independently selected from nitrogen and oxygen, wherein said 3-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of (Ci-C 4 )alkyl, hydroxy, -C0 2 H and -CO(Ci-C 4 )alkyl;
- R 3 is H, halogen, hydroxy, (Ci-C 4 )alkyl-,(C 2 -C 4 )alkenyl-, halo(Ci-C 4 )alkyl-, hydroxy(C 2 -C 4 )alkenyl-, (Ci-C 4 )alkoxy-, (C 2 -C 4 )alkenyloxy-, halo(Ci-C 4 )alkoxy-,
- halo(Ci-C 4 )alkoxy(Ci-Ce)alkyl-, or halo(Ci-C 4 )alkoxy(C 2 -C 6 )alkoxy- groups contain 2 or 3 halo atoms,
- the (C 3 -C 6 )cycloalkyl moiety of the (C 3 -C 6 )cycloalkyl(Ci-C 4 )alkoxy- or (C 3 -Ce)cycloalkoxy- is optionally substituted by a group selected from the group consisting of cyano, halo, hydroxyl, (Ci-Ce)alkoxy and (Ci-C 4 )alkoxy(C 2 -C 6 )alkoxy, and wherein the 4-6 membered heterocycloalkyl moiety of the 4-6 membered heterocycloalkyl(Ci-C 4 )alkoxy-, or 4-6 membered-heterocycloalkyl-oxy- is optionally substituted by a group selected from the group consisting of cyano, halo, hydroxyl, (Ci-C 6 )alkoxy and (Ci-C 4 )alkoxy(C 2 -C 6 )alkoxy;
- Z is phenyl or aryl(Ci-C 4 )alkyl-, wherein in the phenyl group or the aryl moiety of the aryl(Ci-C 4 )alkyl- group is substituted by R 4 , R 5 , R 6 and R 7 , wherein:
- R 4 is H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy- is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; and
- each of R 5 , R 6 and R 7 is independently selected from the group consisting of H, hydroxyl, halogen, -CF 3 , hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; or
- Z is phenyl or pyridyl, substituted by R 8 , R 9 and R 10 , wherein:
- R 8 and R 9 are located on adjacent atoms and taken together with the atoms to which they are attached form a 5 -membered ring containing 1, 2 or 3 heteroatoms each independently selected from N, O and S, which 5 -membered ring is substituted by R 11 ; wherein one of R 10 or R 11 is H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, where the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4
- R 10 or R 11 is H, hydroxyl, halogen, -CF 3 , hydroxy(Ci-C 4 )alkyl, -C 4 )alkyl or (Ci-C 4 )alkoxy; or
- Z is pyrazolyl, having the formula: wherein:
- R is H, methyl or hydroxymethyl
- R 13 is methyl, trifluoromethyl or hydroxymethyl
- R 14 is H, OH, or (Ci-C 3 )alkyl; or R and R , taken together with the atoms to which they are attached, form a 6-membered ring substituted by R 15 and R 16 , wherein the 6-membered ring optionally contains 1 nitrogen atom;
- R 15 and R 16 are each independently selected from the group consisting of H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy,
- phenyl(Ci-C 4 )alkoxy hydroxyl, hydroxy(Ci-C 4 )alkyl-, and aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy;
- the compounds of Formula (I) do not include:
- the compounds according to Formula (I), or salts, particularly pharmaceutically acceptable salts, thereof, are inhibitors of RIP2 kinase.
- the present invention is also directed to a method of inhibiting RIP2 kinase which method comprises contacting a cell with a compound according to
- the invention is further directed to a method of treating a RIP2 kinase-mediated disease or disorder which comprises administering a therapeutically effective amount of a compound according to Formula (I), or a salt, particularly a pharmaceutically acceptable salt thereof, to a patient (a human or other mammal, particularly, a human) in need thereof.
- a RIP2 kinase-mediated diseases or disorders include uveitis, Crohn's disease, ulcerative colitis, early-onset and extra-intestinal inflammatory bowel disease and granulomateous disorders, such as sarcoidosis, Blau syndrome, early-onset sarcoidosis and Wegner's Granulomatosis.
- the present invention is further directed to a pharmaceutical composition
- a pharmaceutical composition comprising a compound according to Formula (I), or a salt, particularly a pharmaceutically acceptable salt, thereof and a pharmaceutically acceptable excipient.
- this invention is directed to a pharmaceutical composition for the treatment of a RIP2 kinase-mediated disease or disorder, where the composition comprises a compound according to Formula (I), or a salt, particularly a pharmaceutically acceptable salt, thereof and a pharmaceutically acceptable excipient.
- Figure 1 shows the combined cytokine response in rat whole blood samples obtained after pre-dosing rats with the compound of Example 4 or prednisolone, followed by dosing with LI 8-MDP.
- Figure 2 shows the combined cytokine response in rat whole blood samples obtained after pre-dosing rats with the compound of Example 6 or prednisolone, followed by dosing with LI 8-MDP.
- Figure 3 shows the combined cytokine response in rat whole blood samples obtained after pre-dosing rats with the compound of Example 16 or prednisolone, followed by dosing with LI 8-MDP.
- Figure 4 shows the combined cytokine response in rat whole blood samples obtained after pre-dosing rats with the compound of Example 21 or prednisolone, followed by dosing with L18-MDP.
- R When R is H, the compounds of this invention may exist as tautomers. However, when R 14 is (Ci-C3)alkyl, the compounds of this invention, may exist as either one of the regioisomers represented by Formula (I- A) or Formula (I-B), or as a mixture thereof.
- alkyls include, but are not limited to methyl (Me), ethyl (Et), n-propyl, isopropyl, n-butyl, s-butyl, isobutyl, t-butyl and pentyl.
- C 1 -C 4 alkyl refers to an alkyl group or moiety containing from 1 to 4 carbon atoms.
- alkyl When the term “alkyl” is used in combination with other substituent groups, such as “haloalkyl” or “hydroxyalkyl” or “arylalkyl”, the term “alkyl” is intended to encompass a divalent straight or branched-chain hydrocarbon radical.
- arylalkyl is intended to mean the radical -alkylaryl, wherein the alkyl moiety thereof is a divalent straight or branched-chain carbon radical and the aryl moiety thereof is as defined herein, and is represented by the bonding arrangement present in a benzyl group (-CH 2 -phenyl);
- halo(Ci-C 4 )alkyl or "(Ci-C 4 )haloalkyl” is intended to mean a radical having one or more halogen atoms, which may be the same or different, at one or more carbon atoms of an alkyl moiety containing from 1 to 4 carbon atoms, which a is straight or branched-chain carbon radical, and is represented by a trifluoromethyl group (-CF 3 ).
- cycloalkyl refers to a non-aromatic, saturated, cyclic hydrocarbon ring.
- (C 3 -Cv)cycloalkyl refers to a non-aromatic cyclic hydrocarbon ring having from three to eight ring carbon atoms.
- (C 3 -C8)cycloalkyl groups useful in the present invention include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and cycloheptyl.
- Alkoxy refers to a group containing an alkyl radical attached through an oxygen linking atom.
- (Ci-C 4 )alkoxy refers to a straight- or branched-chain
- hydrocarbon radical having at least 1 and up to 4 carbon atoms attached through an oxygen linking atom.
- exemplary "(Ci-C4)alkoxy” groups useful in the present invention include, but are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, s-butoxy, isobutoxy, and t-butoxy.
- Aryl represents a group or moiety comprising an aromatic, monocyclic or bicyclic hydrocarbon radical containing from 6 to 10 carbon ring atoms, which may be fused one or more cycloalkyl rings.
- aryl is phenyl.
- a heterocyclic group or moiety is a cyclic group or moiety having as ring members atoms of at least two different elements (carbon and one or more of nitrogen, oxygen and/or sulfur), which cyclic group or moiety may be saturated or partially unsaturated (non-aromatic; e.g., a heterocycloalkyl group or moiety) or fully unsaturated (aromatic; e.g., a heteroaryl group or moiety).
- Heterocycloalkyl represents a group or moiety comprising a non-aromatic, monocyclic or bicyclic radical, which is saturated or partially unsaturated, containing 3 to 10 ring atoms, unless otherwise specified, which includes 1 to 4 heteroatoms selected from nitrogen, oxygen and sulfur.
- heterocycloalkyls include, but are not limited to, azetidinyl, oxetanyl, pyrrolidyl (or pyrrolidinyl), piperidinyl, piperazinyl, morpholinyl, tetrahydro-2H-l,4-thiazinyl, tetrahydrofuryl (or tetrahydrofuranyl), dihydrofuryl, oxazolinyl, thiazolinyl, pyrazolinyl, tetrahydropyranyl, dihydropyranyl, 1,3-dioxolanyl, 1,3-dioxanyl, 1 ,4-dioxanyl, 1,3-oxathiolanyl, 1,3-oxathianyl, 1,3-dithianyl, azabicylo[3.2.1 ]octyl, azabicylo[3.3.1 ]non
- heterocycloalkyl groups include 4-membered heterocycloalkyl groups containing one heteroatom, such as oxetanyl, thietanyl and azetidinyl.
- heterocycloalkyl groups include 5-membered heterocycloalkyl groups containing one heteroatom selected from nitrogen, oxygen and sulfur and optionally containing one or two an additional nitrogen atoms, or optionally containing one additional oxygen or sulfur atom, such as pyrrolidyl (or pyrrolidinyl), tetrahydrofuryl (or tetrahydrofuranyl), tetrahydrothienyl, dihydrofuryl, oxazolinyl, thiazolinyl, imidazolinyl, pyrazolinyl, 1,3-dioxolanyl, and l,3-oxathiolan-2-on-yl.
- heterocycloalkyl groups include 5-membered heterocycloalkyl groups containing one heteroatom selected from nitrogen, oxygen and sulfur and optionally containing one or two an additional nitrogen atoms, or optionally containing one additional oxygen or sulfur atom, such as pyrrolidyl (or pyrrol
- heterocycloalkyl groups are 6-membered heterocycloalkyl groups containing one heteroatom selected from nitrogen, oxygen and sulfur and optionally containing one or two an additional nitrogen atoms or one additional oxygen or sulfur atom, such as piperidyl (or piperidinyl), piperazinyl, morpholinyl, thiomorpholinyl, l,ldioxoido-thiomorpholin-4-yl, tetrahydropyranyl, dihydropyranyl, tetrahydro-2H-l,4-thiazinyl, 1 ,4-dioxanyl, 1,3-oxathianyl, and 1,3-dithianyl.
- piperidyl or piperidinyl
- piperazinyl morpholinyl
- thiomorpholinyl thioxoido-thiomorpholin-4-yl
- tetrahydropyranyl dihydropyranyl, tetrahydro-2H
- Heteroaryl refers to a group or moiety comprising an aromatic monocyclic or bicyclic radical, containing 5 to 10 ring atoms, including 1 to 4 heteroatoms selected from nitrogen, oxygen and sulfur. This term also encompasses bicyclic heterocyclic-aryl compounds containing an aryl ring moiety fused to a heterocycloalkyl ring moiety, containing 5 to 10 ring atoms, including 1 to 4 heteroatoms selected from nitrogen, oxygen and sulfur.
- heteroaryls include, but are not limited to, thienyl, pyrrolyl, imidazolyl, pyrazolyl, furyl (or furanyl), isothiazolyl, isoxazolyl, oxazolyl, oxadiazolyl, thiazolyl, pyridyl (or pyridinyl), pyrazinyl, pyrimidinyl, pyridazinyl, triazinyl, tetrazinyl, triazolyl, tetrazolyl, benzo[£]thienyl, isobenzofuryl, 2,3-dihydrobenzofuryl, chromenyl, chromanyl, indolizinyl, isoindolyl, indolyl, indazolyl, purinyl, isoquinolyl, quinolyl, phthalazinyl, naphthridinyl, quinzolinyl,
- heteroaryl groups present in the compounds of this invention are 5-membered and/or 6-membered monocyclic heteroaryl groups. Selected
- 5- membered heteroaryl groups contain one nitrogen, oxygen or sulfur ring heteroatom, and optionally contain 1, 2 or 3 additional nitrogen ring atoms.
- Selected 6-membered heteroaryl groups contain 1, 2, 3 or 4 nitrogen ring heteroatoms.
- 6- membered heteroaryl groups include thienyl, pyrrolyl, imidazolyl, pyrazolyl, furyl (furanyl), isothiazolyl, isoxazolyl, oxazolyl, oxadiazolyl, thiazolyl, triazolyl and tetrazolyl or pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl and triazinyl.
- the heteroaryl groups present in the compounds of this invention are 9-membered or 10-membered monocyclic heteroaryl groups.
- Selected 9-10 membered heteroaryl groups contain one nitrogen, oxygen or sulfur ring heteroatom, and optionally contain 1, 2, 3 or 4 additional nitrogen ring atoms.
- heteroaryl groups include a
- 9- membered heteroaryl group which includes benzothienyl, benzofuranyl, indolyl, indolinyl, isoindolyl, isoindolinyl, indazolyl, indolizinyl, isobenzofuryl,
- heteroaryl groups include a
- 10- membered heteroaryl group which includes chromenyl, chromanyl, quinolyl, isoquinolyl, phthalazinyl, naphthridinyl, quinazolinyl, quinoxalinyl, 4H-quinolizinyl, tetrahydroquinolinyl, cinnolinyl, and pteridinyl.
- heterocyclic, heteroaryl, and heterocycloalkyl are intended to encompass stable heterocyclic groups where a ring nitrogen heteroatom is optionally oxidized (e.g., heterocyclic groups containing an N-oxide, such as pyridine-N-oxide) or where a ring sulfur heteroatom is optionally oxidized (e.g., heterocyclic groups containing sulfones or sulfoxide moieties, such as
- tetrahydrothienyl-1 -oxide a tetrahydrothienyl sulfoxide
- tetrahydrothienyl- 1,1 -dioxide a tetrahydrothienyl sulfone
- Hydroxo or hydroxyl is intended to mean the radical -OH.
- the terms "compound(s) of the invention” or “compound(s) of this invention” mean a compound of Formula (I), as defined above, in any form, i.e., any salt or non-salt form (e.g., as a free acid or base form, or as a salt, particularly a pharmaceutically acceptable salt thereof) and any physical form thereof (e.g., including non-solid forms (e.g., liquid or semi-solid forms), and solid forms (e.g., amorphous or crystalline forms, specific polymorphic forms, solvate forms, including hydrate forms (e.g., mono-, di- and hemi- hydrates)), and mixtures of various forms.
- any salt or non-salt form e.g., as a free acid or base form, or as a salt, particularly a pharmaceutically acceptable salt thereof
- any physical form thereof e.g., including non-solid forms (e.g., liquid or semi-solid forms), and solid forms (e.g., a
- the term "optionally substituted” indicates that a group (such as an alkyl, cycloalkyl, alkoxy, heterocycloalkyl, aryl, or heteroaryl group) or ring or moiety (such as a carbocyclic or heterocyclic ring or moiety) may be unsubstituted, or the group, ring or moiety may be substituted with one or more substituent(s) as defined.
- groups may be selected from a number of alternative groups, the selected groups may be the same or different.
- R 1 is H, -S0 2 (Ci-C 4 )alkyl, -CO(Ci-C 4 )alkyl, or (Ci-C 4 )alkyl;
- R 2 is - SR a , -SOR a , or -S0 2 R a , wherein R a is (Ci-C 6 )alkyl, halo(Ci-C 6 )alkyl, (C 3 -Cy)cycloalkyl, 4-7 membered heterocycloalkyl, aryl, or heteroaryl, wherein:
- said (Ci-C 6 )alkyl is optionally substituted by one or two groups each independently selected from cyano, hydroxyl, (Ci-C 6 )alkoxy, (Ci-C6)alkoxy(C2-C 6 )alkoxy, -C0 2 H, -C0 2 (Ci-C 4 )alkyl, -S0 2 (Ci-C 4 )alkyl, (C 3 -Cy)cycloalkyl, phenyl, 5-6 membered heteroaryl, 9-10 membered heteroaryl, 4-7 membered heterocycloalkyl and
- phenyl)(Ci-C 4 alkyl)amino-, 5-6 membered heteroaryl, 9-10 membered heteroaryl or 4-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from halogen, -CF 3 , hydroxyl, amino, ((Ci-C 4 )alkyl)amino-,
- said (C 3 -C 7 )cycloalkyl or 4-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from halogen, -CF 3 , hydroxyl, amino,
- said aryl or heteroaryl is optionally substituted by 1-3 groups each independently selected from halogen, -CF 3 , hydroxyl, amino, ((Ci-C 4 )alkyl)amino-,
- R 3 is H, halogen, hydroxy, (Ci-C 4 )alkyl-,(C 2 -C 4 )alkenyl-, halo(Ci-C 4 )alkyl-, (Ci-C 4 )alkoxy-, halo(Ci-C 4 )alkoxy-, (Ci-C 4 )alkoxy(Ci-Ce)alkyl-,
- halo(Ci-C 4 )alkoxy(Ci-Ce)alkyl-, or halo(Ci-C 4 )alkoxy(C 2 -Ce)alkoxy- groups contain 2 or 3 halo atoms; and wherein the (C 3 -C 6 )cycloalkyl moiety of the (C3-C 6 )cycloalkyl(Ci-C 4 )alkoxy- or (C3-C 6 )cycloalkoxy-, is optionally substituted by a group selected from cyano, halo, hydroxyl, (Ci-C 6 )alkoxy and (Ci-C 4 )alkoxy(C 2 -Ce)alkoxy;
- 4-6 membered-heterocycloalkyl moiety of the 4-6 membered- heterocycloalkyl(Ci-C 4 )alkoxy-, or 4-6 membered-heterocycloalkoxy- is optionally substituted by a group selected from cyano, halo, hydroxyl, (Ci-C 6 )alkoxy and
- Z is phenyl or aryl(Ci-C 4 )alkyl-, substituted by R 4 , R 5 , R 6 and R 7 , wherein:
- R 4 is H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy- is optionally substituted by 1-3 substituents each independently selected from halogen, -CF 3 , (Ci-C 4 )alkyl and
- each of R 5 , R 6 and R 7 is independently selected from H, hydroxyl, halogen, -CF 3 , hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; or
- Z is phenyl or pyridyl substituted by R 8 , R 9 and R 10 , wherein:
- R 8 and R 9 are located on adjacent atoms and taken together with the atoms to which they are attached form a 5-membered heterocyclic group containing 1, 2 or 3 heteroatoms each independently selected from N, O and S, which 5-membered heterocyclic group is substituted by R 11 ;
- R 10 or R 11 is H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, where the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; and
- R 10 or R 11 is H, hydroxyl, halogen, -CF 3 , hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl or (Ci-C 4 )alkoxy; or
- Z is pyrazolyl, having the formula:
- R 12 is H, methyl or hydroxymethyl; R is methyl, trifluoromethyl or hydroxymethyl;
- R 14 is H, OH, or (Ci-C 3 )alkyl
- R 12 and R 13 taken together with the atoms to which they are attached, form a 6 membered carbocyclic ring or heterocyclic ring substituted by R 15 and R 16 , wherein the heterocyclic ring contains 1 nitrogen atom;
- R 15 and R 16 are each independently selected from H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, and aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy.
- the compounds of Formula (I) do not include ethyl 5- ⁇ [4-[(3-chloro-4-fluorophenyl)amino]-7-(methoxy)-6-quinazolinyl]thio ⁇ pentanoate; ethyl 4- ⁇ [4-[(3-chloro-4-fluorophenyl)amino]-7-(methoxy)-6-quinazolinyl]thio ⁇ butanoate; ethyl 4- ⁇ [4-[(3-chloro-4-fluorophenyl)amino]-7-(methoxy)-6- quinazolinyl]thio ⁇ heptanoate; 7-(methoxy)-N-[l-(phenylmethyl)-lH-indazol-5-yl]-6- [(trifluoromethyl)sulfonyl]-4-quinazolinamine; or ethyl 4- ⁇ [4-
- R 1 is ⁇ . In other embodiments, R 1 is (Ci-C 3 )alkyl; specifically, -CH 3 or -CH 2 CH 3 . Generally, in the compounds of this invention, R 1 is H.
- R 2 is -SR a or -S0 2 R a . In a further embodiment, R 2 is -SOR a . In a still further embodiment, R 2 is -S0 2 R a .
- R a is an optionally substituted (Ci-C 6 )alkyl, (C 3 -C 6 )cycloalkyl, 4-6-membered heterocycloalkyl,
- (Ci-C 6 )alkyl is optionally substituted by one or two groups each independently selected from the group consisting of hydroxyl, (Ci-C 4 )alkoxy,
- R a is an unsubstituted (Ci-C 6 )alkyl or a (Ci-C 6 )alkyl substituted by one or two groups each independently selected from the group consisting of hydroxyl,
- R a is an optionally substituted (C 3 -C 6 )cycloalkyl, 4-6-membered heterocycloalkyl, 5-6-membered heteroaryl or phenyl group, wherein the
- (C 3 -C 6 )cycloalkyl, 4-6-membered heterocycloalkyl, 5-6-membered heteroaryl or phenyl is optionally substituted by 1-3 groups each independently selected from the group consisting of halogen, -CF 3 , hydroxyl, amino, (Ci-C 4 )alkyl, phenyl(Ci-C 4 )alkyl-,
- R a is a heterocycloalkyl or heteroaryl group
- the heterocycloalkyl or heteroaryl group is bonded to the sulfur atom of the -SR a , -SOR a or -S0 2 R a moiety by a ring carbon atom.
- R a is an optionally substituted (Ci-C 6 )alkyl
- said (Ci-C 6 )alkyl is optionally substituted by a group selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, (Ci-C 2 )alkoxy(C 2 -C 3 )alkoxy-, -S0 2 (Ci-C 2 )alkyl, and a group selected from the group consisting of (C 3 -C 6 )cycloalkyl (optionally substituted by (Ci-C 4 )alkyl or hydroxy(Ci-C 4 )alkyl), 4-6-membered heterocycloalkyl (optionally substituted by (Ci-C 4 )alkyl or halogen), 5-6-membered heteroaryl (optionally substituted by (Ci-C 4 )alkyl or hydroxy(Ci-C 4 )alkyl), phenyl, and 9-10-membered heteroaryl, and said (C 3 -Ce)cycloalkyl or 4-6-member
- any of said 5-6 membered heterocycloalkyl groups contains 1 heteroatom selected from N, O and S.
- R a is an optionally substituted (Ci-C 6 )alkyl
- said (Ci-C 6 )alkyl is optionally substituted by a group selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, and (Ci-C 2 )alkoxy(C 2 -C 3 )alkoxy-.
- R a is an optionally substituted (Ci-C 6 )alkyl or 5-6-membered heterocycloalkyl group, wherein:
- said (Ci-C 6 )alkyl is optionally substituted by a substituent selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, (Ci-C 2 )alkoxy(C 2 -C 3 )alkoxy-, amino,
- said 5-6 membered heterocycloalkyl is optionally substituted by 1 or 2 groups each independently selected from the group consisting of halogen, (Ci-C 4 )alkyl, and
- any of said 5-6 membered heterocycloalkyl contains 1 heteroatom selected from N, O and S.
- R a is halo(Ci-C 4 )alkyl containing 1-9 halogen atoms.
- R a is halo(Ci-C 2 )alkyl, specifically a halo(Ci-C 2 )alkyl containing 1-5 halogen atoms, and more specifically a halo(Ci-C 2 )alkyl containing 3 halogen atoms.
- R a is (Ci-C 6 )alkyl, optionally substituted by a substituent selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, and
- R a is a 5-6-membered heterocycloalkyl group optionally substituted by 1 or 2 independently selected (Ci-C 4 )alkyl groups.
- R a is an unsubstituted (Ci-Cs)alkyl. In a further embodiment of the compounds of this invention, R a is an unsubstituted (Ci-C 4 )alkyl group. In another embodiment, R a is a (Ci-C 5 )alkyl group substituted by a hydroxyl,
- R a is a (Ci-C 5 )alkyl substituted by one hydroxyl group. In yet another specific embodiment, R a is a tetrahydropyranyl group.
- R a is -CH 3 , -CH(CH 3 ) 2 , or -C(CH 3 ) 3 .
- R a is -CH 2 CH 2 OH or -C(CH 3 ) 2 CH 2 CH 2 OH.
- R a is tetrahydro-2H-pyran-4-yl.
- R b is (Ci-C 6 )alkyl or 4-7 membered heterocycloalkyl, wherein: said (Ci-C 6 )alkyl is optionally substituted by one or two groups each independently selected from the group consisting of hydroxyl, (Ci-C 6 )alkoxy,
- heterocycloalkyl wherein said 5-6 membered heteroaryl or 4-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of halogen, (Ci-C 4 )alkyl, hydroxy(Ci-C 4 )alkyl and (Ci-C 4 )alkoxy,
- said 4-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of hydroxyl, amino, (Ci-C 4 )alkyl, hydroxy(Ci-C 4 )alkyl-, oxo and (Ci-C 4 )alkoxy, and
- R c is H or (Ci-C 4 )alkyl
- R b and R c taken together with the nitrogen atom to which they are attached form a 5-7 membered heterocycloalkyl group, optionally containing one additional ring heteroatom selected from nitrogen and oxygen, wherein said 5-7 membered
- heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of (Ci-C 4 )alkyl, hydroxy, -C0 2 H and -CO(Ci-C 4 )alkyl.
- R b is (Ci-C 6 )alkyl and said (Ci-C 6 )alkyl is optionally substituted by lor 2 substituents each independently selected from the group consisting of hydroxyl, (Ci-C 4 )alkoxy, (Ci-C 4 )alkoxy(C 2 -C 4 )alkoxy-, (Ci-C 4 alkyl)amino-,
- R b is unsubstituted (Ci-C 6 )alkyl, particularly, R b is unsubstituted (Ci-C 4 )alkyl.
- R b is a (Ci-C 6 )alkyl substituted by lor 2 substituents each independently selected from the group consisting of hydroxyl,
- R b is a (Ci-C 4 )alkyl substituted by hydroxyl, (Ci-C 2 )alkoxy,
- R b is -CH 3 or -CH(CH 3 ) 2 . In another embodiment, R b is
- R b is -oxetan-3-yl, tetrahydro-2H-pyran-4-yl, -CH 2 -tetrahydro- 2H-pyran-4-yl, or -CH 2 CH 2 -lH-tetrazol-5-yl.
- R c is ⁇ or (Ci-C 4 )alkyl. In some specific embodiments, R c is -CH 3 . In other specific embodiments, R c is H.
- R b and R c taken together with the nitrogen atom to which they are attached form a 5-7 membered heterocycloalkyl group, optionally containing one additional ring heteroatom selected from nitrogen and oxygen, which 5-7 membered heterocycloalkyl is optionally substituted by 1-3 groups each independently selected from the group consisting of ((Ci-C 4 )alkyl, hydroxy, -CO 2 H and -CO(Ci-C 4 )alkyl.
- R b and R c taken together with the nitrogen atom to which they are attached form a 5-6-membered heterocycloalkyl, optionally containing 1 additional heteroatom selected from N and O, and optionally substituted by a hydroxyl, (Ci-C 4 )alkyl, carboxy or (Ci-C 4 )alkylcarbonyl- group.
- R b and R c taken together with the nitrogen atom to which they are attached form a morpholin-4-yl, 4-methylcarbonyl-piperazin-l-yl (that is, 4-acetyl-piperazin-l-yl), pyrrolidin-l-yl, 3-hydroxy-pyrrolidin-l-yl, or
- R 3 is H.
- R 3 is halogen, hydroxy, (Ci-C 3 )alkyl-,
- R 3 is halogen, hydroxy, (Ci-C 3 )alkyl-, (C 2 -C 3 )alkenyl-, halo(Ci-C 2 )alkyl-, hydroxy(C 2 -C 3 )alkenyl-, (Ci-C 4 )alkoxy-,
- R 3 is H or R 3 is halogen, hydroxy, (Ci-C 4 )alkyl-, (C 2 -C 4 )alkenyl-, halo(Ci-C 4 )alkyl-, (Ci-C 4 )alkoxy-, halo(Ci-C 4 )alkoxy-,
- R 3 is H, halogen, hydroxy, (Ci-C 3 )alkyl-, (C 2 -C 3 )alkenyl-, halo(Ci-C 2 )alkyl-, (Ci-C 4 )alkoxy-, halo(Ci-C 3 )alkoxy-, (C 5 -C 6 )cycloalkyl(Ci-C 3 )alkoxy-, 5-6-memebered-heterocycloalkyl-oxy-, (Ci-C 3 )alkoxy(Ci-C 4 )alkyl-,
- Z is phenyl substituted by R 4 , R 5 , R 6 and R 7 wherein:
- R 4 is ⁇ , halogen, cyano, (Ci-C4)alkyl, halo(Ci-C4)alkyl, (Ci-C4)alkoxy, hydroxyl, or hydroxy(Ci-C4)alkyl-;
- each of R 5 , R 6 and R 7 is independently selected from the group consisting of ⁇ , hydroxyl, halogen, hydroxy(Ci-C4)alkyl, (Ci-C4)alkyl, halo(Ci-C4)alkyl and
- Z is phenyl, substituted by 1 , 2 or 3 (more specifically, 1 or 2) substituents each independently selected from the group consisting of hydroxyl, halogen, hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy.
- Z is
- Z is 2-methyl-5-hydroxy-phenyl.
- Z is phenyl or pyridyl substituted by R 8 , R 9 and R 10 , wherein:
- R 8 and R 9 are located on adjacent atoms (carbon atoms) and taken together with the atoms to which they are attached form a 5-membered ring containing 1, 2 or 3 heteroatoms each independently selected from N, O and S, which 5-membered ring is substituted by
- R 11 wherein one of R 10 or R 11 is ⁇ , halogen, cyano, (Ci-C4)alkyl, halo(Ci-C4)alkyl,
- R 10 or R 11 is ⁇ , hydroxyl, halogen, -CF 3 , hydroxy(Ci-C4)alkyl,
- pyrazolyl having the formula: wherein: R is H, methyl or hydroxymethyl;
- R 13 is methyl, trifluoromethyl or hydroxymethyl
- R 14 is H, OH, or (Ci-C 3 )alkyl
- R 12 and R 13 taken together with the atoms to which they are attached, form a 6-membered ring substituted by R 15 and R 16 , wherein the 6-membered ring contains 1 nitrogen atom;
- R 15 and R 16 are each independently selected from the group consisting of H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy,
- phenyl(Ci-C 4 )alkoxy hydroxyl, hydroxy(Ci-C 4 )alkyl-, and aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (C C 4 )alkoxy.
- the 5-membered ring formed from R 8 and R 9 and the atoms to which they are attached may be non-aromatic (partially unsaturated) or aromatic (fully unsaturated). It will be further understood by one skilled in the art that the 6-membered ring formed from R 12 and R 13 and the atoms to which they are attached may be non-aromatic (partially unsaturated) or aromatic (fully unsaturated).
- Z is not phenyl or aryl(Ci-C 4 )alkyl-, wherein in the phenyl group or the aryl moiety of the aryl(Ci-C 4 )alkyl- group is substituted by R 4 , R 5 , R 6 and R 7 , wherein R 4 is H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl,
- hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy- is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; and each of R 5 , R 6 and R 7 is independently selected from the group consisting of H, hydroxyl, halogen, -CF 3 , hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy.
- Z is phenyl substituted by R 8 , R 9 and R 10 , wherein R 8 and R 9 are located on adjacent atoms and taken together with the atoms to which they are attached form a 5-membered ring containing 1, 2 or 3 heteroatoms each independently selected from N, O and S, which 5-membered ring is substituted by R 11 ; wherein R 10 and R 11 are each H or one of R 10 or R 11 is H, halogen, cyano, (Ci-C 4 )alkyl, -CF 3 , (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, where the phenyl moiety of said phenoxy or phenyl(Ci-C4)alkoxy is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3
- Z is benzothiazolyl, optionally substituted by 1-3 substituents independently selected from the group consisting of halogen, (Ci-C 4 )alkyl,
- Z is unsubstituted benzothiazolyl.
- Z is benzothiazolyl substituted by 1-3 substituents independently selected from the group consisting of halogen, (Ci-C 4 )alkyl,
- Z is benzo[ ⁇ i]thiazol-5-yl optionally substituted by chloro, fluoro, -CF 3 , methyl, or methoxy.
- Z is benzo[ ⁇ i]thiazol-5-yl.
- Z is pyridyl substituted by R 8 , R 9 and R 10 , wherein R 8 and R 9 are located on adjacent atoms (carbon atoms) and taken together with the atoms to which they are attached form a 5-membered ring containing 1, 2 or 3 heteroatoms each independently selected from N, O and S, which 5-membered ring is substituted by R 11 ; wherein one of R 10 or R 11 is H, halogen, cyano, (Ci-C 4 )alkyl, -CF 3 , (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, where the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3
- Z is pyrazolyl and R 12 is H or hydroxymethyl, R 13 is methyl or trifluoromethyl, and R 14 is H or methyl; or R 12 is H or methyl, R 13 is hydroxymethyl, and R 14 is H or methyl.
- Z is pyrazolyl, R 12 is H or methyl, R 13 is methyl or trifluoromethyl, and R 14 is OH.
- Z is pyrazolyl, R 12 is H or methyl, R is methyl or trifluoromethyl, and R is H or methyl.
- Z is pyrazolyl, R 12 and R 13 are both methyl, and R 14 is H.
- Z is 5-(trifluoromethyl)-lH-pyrazol-3-yl, l,3,4-trimethyl-lH-pyrazol-5-yl, or 4,5-dimethyl-lH-pyrazol-3-yl.
- Z is pyrazolyl, substituted by R 12 and R 13 wherein:
- R 12 and R 13 are located on adjacent carbon atoms and taken together with the atoms to which they are attached form a 6 membered carbocyclic ring or heterocyclic ring substituted by R 15 and R 16 ;
- R 15 is ⁇ , halogen, cyano, (Ci-C 4 )alkyl, -CF 3 , (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; and
- R 16 is ⁇ , hydroxyl, halogen, -CF 3 , hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl or
- the invention is directed to a compound according to Formula (I), wherein Z is a 9-membered bi-cyclic heteroaryl group, optionally substituted on either ring by halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl- or aminocarbonyl, wherein the 9-membered bi-cyclic heteroaryl group is an optionally substituted indazolyl or pyrazolo[3,4-£]pyridinyl, bonded to the amino (NR 1 ) moiety via a substitutable carbon ring atom of the 5-membered pyrazolyl ring moiety of the indazolyl or pyrazolo[3,4-3 ⁇ 4]pyridinyl group, or a salt, particularly a pharmaceutically acceptable salt, thereof.
- Z is a 9-membered bi-cyclic hetero
- Z is an optionally substituted indazolyl or
- pyrazolo[3,4-3 ⁇ 4]pyridinyl wherein the indazolyl or pyrazolo[3,4-3 ⁇ 4]pyridinyl is optionally substituted by hydroxyl, chloro, fluoro, -CF 3 , cyano, hydroxymethyl-, methyl, methoxy or aminocarbonyl.
- Z is 5 -fluoro- lH-indazol-3-yl, lH-indazol-6-yl or 3-methyl-lH-indazol-6-yl.
- Z is 5-fluoro-lH-indazol-3-yl.
- the invention is directed to a compound according to Formula (II):
- R 1 , R 2 , R 3 , R 12 and R 13 are as defined herein.
- the invention is directed to method of inhibiting RIP2 kinase comprising contacting a cell with a compound according to Formula (III):
- R 1 , R 2 and R 3 are as defined herein, and
- R Z1 is H, halogen, -CF 3 , (Ci-C 4 )alkyl or (Ci-C 4 )alkoxy; particularly, R Z1 is H or methyl;
- R Z2 is H, halogen, -CF 3 , (Ci-C 4 )alkyl or (Ci-C 4 )alkoxy;
- R Z3 is H, halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy- is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; and R is hydroxyl, hydroxy(Ci-C 4 )alkyl or (Ci-C 4 )alkoxy;
- the invention is directed to a compound of Formula (I), wherein:
- R 1 is H or (Ci-C 4 )alkyl
- R 2 is -SR a or -S0 2 R a
- R a is an optionally substituted (Ci-C 6 )alkyl
- said (Ci-C 6 )alkyl is optionally substituted by a groups selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, (Ci-C 2 )alkoxy(C 2 -C3)alkoxy-, -S0 2 (Ci-C 2 )alkyl, and a group selected from the group consisting of (C 3 -C 6 )cycloalkyl (optionally substituted by (Ci-C 4 )alkyl or hydroxy(Ci-C 4 )alkyl), 4-6-membered heterocycloalkyl (optionally substituted by (Ci-C 4 )alkyl or halogen), 5-6-membered heteroaryl (optionally substituted by (Ci-C 4 )alkyl or hydroxy(Ci-C 4 )alkyl), phenyl, and 9-10-membered heteroaryl, and said (C 3 -C 6 )cycloalkyl or 4-6-membere
- any of said 5-6 membered heterocycloalkyl groups contains 1 heteroatom selected from N, O and S;
- R 3 is H, halogen, hydroxy, (Ci-C 4 )alkyl-, (C 2 -C 4 )alkenyl-, halo(Ci-C 4 )alkyl-, (Ci-C 4 )alkoxy-, halo(Ci-C 4 )alkoxy-, (C3-C 6 )cycloalkyl(Ci-C 4 )alkoxy-, 5-6-memebered- heterocycloalkyl-oxy-, (Ci-C 4 )alkoxy(Ci-C 6 )alkyl-, (Ci-C 4 )alkoxy(C 2 -C 6 )alkoxy-, carboxy-(Ci-Ce)alkoxy-, (Ci-C 4 )alkoxycarbonyl(Ci-C 6 )alkoxy-, hydroxy(Ci-Ce)alkyl-, or hydroxy(C 2 -C 6 )alkoxy-;
- Z is phenyl, substituted by 1 , 2 or 3 substituents each independently selected from the group consisting of hydroxyl, halogen, hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl and
- R 8 and R 9 are located on adjacent atoms and taken together with the atoms to which they are attached form a 5 -membered ring containing 1, 2 or 3 heteroatoms each independently selected from N, O and S, which 5 -membered ring is substituted by R 11 ; wherein R 10 and R 11 are each H or one of R 10 or R 11 is H, halogen, cyano,
- R 12 is H or methyl
- R 13 is methyl or trifluoromethyl
- R 14 is H or methyl
- R 12 and R 13 are located on adjacent carbon atoms and taken together with the atoms to which they are attached form a 6-membered carbocyclic ring or heterocyclic ring substituted by R 15 and R 16 ;
- R 15 is H, halogen, cyano, (Ci-C 4 )alkyl, -CF 3 , (Ci-C 4 )alkoxy, phenoxy, phenyl(Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl-, or aminocarbonyl, wherein the phenyl moiety of said phenoxy or phenyl(Ci-C 4 )alkoxy is optionally substituted by 1-3 substituents each independently selected from the group consisting of halogen, -CF 3 , (Ci-C 4 )alkyl and (Ci-C 4 )alkoxy; and
- R 16 is H, hydroxyl, halogen, -CF 3 , hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl or
- R 2 is -S0 2 R a and/or R 3 is halogen, hydroxy, (Ci-C 4 )alkyl-,
- the invention is directed to a compound of Formula (I), wherein:
- R 1 is H;
- R 2 is -SR a , -SOR a or -S0 2 R a , wherein R a is a (Ci-C 6 )alkyl group, optionally substituted by a substituent selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, and (Ci-C 2 )alkoxy(C 2 -C 3 )alkoxy-,
- R a is a 5-6-membered heterocycloalkyl group optionally substituted by 1 or 2 independently selected (Ci-C 4 )alkyl groups;
- R 3 is halogen, hydroxy, (Ci-C 3 )alkyl-, (C 2 -C 3 )alkenyl-, halo(Ci-C 2 )alkyl-, hydroxy(C 2 -C 3 )alkenyl-, (Ci-C 4 )alkoxy-, (C 2 -C 3 )alkenyl-oxy-, halo(Ci-C 3 )alkoxy-, (C5-C 6 )cycloalkyl(Ci-C 3 )alkoxy-, 5-6-memebered-heterocycloalkyl-oxy-,
- Z is phenyl, substituted by 1 or 2 substituents each independently selected from the group consisting of hydroxyl, halogen, hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl and
- Z is benzothiazolyl, optionally substituted by 1-3 substituents independently selected from the group consisting of halogen, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl and
- R 12 is H or methyl
- R 13 is methyl or trifluoromethyl
- R 14 is H or methyl
- Z is a 9-membered bi-cyclic heteroaryl group, optionally substituted on either ring by halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl- or aminocarbonyl, wherein the 9-membered bi-cyclic heteroaryl group is an optionally substituted indazolyl or pyrazolo[3,4-£]pyridinyl, bonded to the amino (NR 1 ) moiety via a substitutable carbon ring atom of the 5-membered pyrazolyl ring moiety of the indazolyl or pyrazolo[3,4-£]pyridinyl group,
- the invention is directed to a compound of Formula (I), wherein:
- R 1 is H;
- R 2 is -SR a , -SOR a or -S0 2 R a , wherein R a is a (Ci-C 6 )alkyl group, optionally substituted by a substituent selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, and (Ci-C 2 )alkoxy(C 2 -C3)alkoxy-;
- R 3 is halogen, hydroxy, (Ci-C 3 )alkyl-, (C2-C 3 )alkenyl-, halo(Ci-C 2 )alkyl-, hydroxy(C 2 -C 3 )alkenyl-, (Ci-C4)alkoxy-, (C 2 -C 3 )alkenyl-oxy-, halo(Ci-C 3 )alkoxy-, (C5-C6)cycloalkyl(Ci-C 3 )alkoxy-, 5-6-memebered-heterocycloalkyl-oxy-,
- Z is benzothiazolyl, optionally substituted by 1-3 substituents independently selected from the group consisting of halogen, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl and
- R 12 is H or methyl
- R 13 is methyl or trifluoromethyl
- R 14 is H or methyl
- Z is a 9-membered bi-cyclic heteroaryl group, optionally substituted on either ring by halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl- or aminocarbonyl, wherein the 9-membered bi-cyclic heteroaryl group is an optionally substituted indazolyl or pyrazolo[3,4-£]pyridinyl, bonded to the amino (NR 1 ) moiety via a substitutable carbon ring atom of the 5-membered pyrazolyl ring moiety of the indazolyl or pyrazolo[3,4-£]pyridinyl group;
- the invention is directed to a compound of Formula (I), wherein:
- R 1 is H or -CH 2 CH 3 ; particularly, R 1 is H;
- R 2 is -SR a or -S0 2 R a , wherein:
- R a is a (Ci-C 6 )alkyl group, optionally substituted by a substituent selected from the group consisting of hydroxyl, (Ci-C 2 )alkoxy, and (Ci-C 2 )alkoxy(C 2 -C 3 )alkoxy-,
- R a is a 5-6-membered heterocycloalkyl group optionally substituted by 1 or 2 independently selected (Ci-C 4 )alkyl groups;
- R 3 is H, halogen, hydroxy, (Ci-C3)alkyl-, (C 2 -C 3 )alkenyl-, halo(Ci-C 2 )alkyl-, (Ci-C 4 )alkoxy-, halo(Ci-C 3 )alkoxy-, (C5-C6)cycloalkyl(Ci-C 3 )alkoxy-, 5-6-memebered heterocycloalkyl-oxy-, (Ci-C 3 )alkoxy(Ci-C 4 )alkyl-, (Ci-C 3 )alkoxy(C 2 -C 4 )alkoxy-, carboxy-(Ci-C 4 )alkoxy-, (Ci-C 3 )alkoxycarbonyl(Ci-C 4
- Z is phenyl, substituted by 1 or 2 substituents each independently selected from the group consisting of hydroxyl, halogen, hydroxy(Ci-C 4 )alkyl, (Ci-C 4 )alkyl and
- Z is benzothiazolyl, optionally substituted by 1-3 substituents independently selected from the group consisting of halogen, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl or
- R 12 is H or methyl
- R 13 is methyl or trifluoromethyl
- R 14 is H or methyl
- Z is a 9-membered bi-cyclic heteroaryl group, optionally substituted on either ring by halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, hydroxyl, hydroxy(Ci-C 4 )alkyl- or aminocarbonyl, wherein the 9-membered bi-cyclic heteroaryl group is an optionally substituted indazolyl or pyrazolo[3,4-£]pyridinyl, bonded to the amino (NH) moiety via a substitutable carbon ring atom of the 5-membered pyrazolyl ring moiety of the indazolyl or pyrazolo[3,4-£]pyridinyl group,
- the invention is directed to a compound of Formula (I), wherein:
- R 1 is H
- R 2 is -S0 2 R a ,wherein R a is an unsubstituted (Ci-C 5 )alkyl group or R a is a
- Z is benzothiazolyl, optionally substituted by a halogen, (Ci-C 4 )alkyl,
- R 12 is H or methyl
- R 13 is methyl or trifluoromethyl
- R 14 is H or methyl
- Z is a 9-membered bi-cyclic heteroaryl, optionally substituted on either ring by a halogen, cyano, (Ci-C 4 )alkyl, halo(Ci-C 4 )alkyl, (Ci-C 4 )alkoxy, hydroxyl,
- the 9-membered bi-cyclic heteroaryl is an optionally substituted indazolyl or pyrazolo[3,4-£]pyridinyl, bonded to the ammo (NR ⁇ /NH) moiety via a substitutable carbon ring atom of the 5-membered pyrazolyl ring moiety of the indazolyl or pyrazolo[3,4-£]pyridinyl group;
- R 1 is H or -CH 2 CH 3 ; particularly, R 1 is H;
- R 2 is -SR a or -S0 2 R a
- R a is -CH 3 , -CH(CH 3 ) 2 , -C(CH 3 ) 3 , -CH 2 CH 2 OH,
- Z is 2-methyl-4-hydroxy-phenyl, benzo[ ⁇ i]thiazol-5-yl or 5-fluoro-lH-indazol-3-yl, or Z is pyrazolyl, wherein R 12 is ⁇ or methyl, R 13 is methyl or trifluoromethyl, and R 14 is ⁇ or methyl,
- the invention is directed to a compound according to Formula (I) wherein:
- R 1 is ⁇
- R 2 is -S0 2 R a , and R a is -CH 3 , -CH(CH 3 ) 2 , or -C(CH 3 ) 3;
- Z is benzo[ ⁇ i]thiazol-5-yl or 5-fluoro-lH-indazol-3-yl,
- R 12 is ⁇ or methyl, R 13 is methyl or trifluoromethyl, and R 14 is ⁇ or methyl;
- R 1 is ⁇ ;
- R 2 is -SR a or -S0 2 R a , and R a is -CH 3 , -CH(CH 3 ) 2 , -C(CH 3 ) 3 , -CH 2 CH 2 OH, -C(CH 3 ) 2 CH 2 CH 2 OH, or tetrahydro-2H-pyran-4-yl;
- -OCH CH-C0 2 H
- -OCH CH-C0 2 CH 3
- -OCH CH-CONH 2 , -OH, -OCH 3 , -OCF 2 H, -OCH(CH 3 ) 2 , -OCH 2 CH 3 , -OCH 2 CF 3 , -OCH 2 CH 2 CH 3 , -0CH 2 CH 2 C1, -OCH 2 CH 2 Br, -OCH 2 CH 2 SCH 3 , -OCH 2 CH 2 S0 2 CH 3 , -OCH 2 CH 2 S0 2 CH(CH 3 ) 2 , -OCH 2 CH 2 OH,
- Z is 2-methyl-5 -hydroxy-phenyl, 2-fluoro-4-chloro-phenyl,
- the invention is further directed to a compound according to Formula (I) wherein:
- R 1 is ⁇
- R 2 is -S0 2 R a .
- R a is -CH 3 , -CH(CH 3 ) 2 , or -C(CH 3 ) 3 , or
- R a is -CH 2 CH 2 OH or -C(CH 3 ) 2 CH 2 CH 2 OH;
- -OCH CH-C0 2 H
- -OCH CH-C0 2 CH 3
- -OCH CH-CONH 2 , -OH, -OCH 3 , -OCF 2 H, -OCH(CH 3 ) 2 , -OCH 2 CH 3 , -OCH 2 CF 3 , -OCH 2 CH 2 CH 3 , -0CH 2 CH 2 C1, -OCH 2 CH 2 Br, -OCH 2 CH 2 SCH 3 , -OCH 2 CH 2 S0 2 CH 3 , -OCH 2 CH 2 S0 2 CH(CH 3 ) 2 , -OCH 2 CH 2 OH,
- Z is benzo[ ⁇ i]thiazol-5-yl, 5-(trifluoromethyl)-lH-pyrazol-3-yl, 1,3,4-trimethyl-lH- pyrazol-5-yl, 4,5-dimethyl-lH-pyrazol-3-yl, 5-fluoro-lH-indazol-3-yl, lH-indazol-6-yl or 3-methyl-lH-indazol-6-yl,
- Representative compounds of this invention include the compounds of Examples 1-80, specifically: 4-methy 1-3 - ⁇ [6-(methylthio)-4-quinazolinyl] amino ⁇ phenol,
- N- 1 ,3-benzothiazol-5-yl-6-[(l , 1 -dimethylethyl)sulfonyl]-7-ethyl-4-quinazolinamine N- 1 ,3-benzothiazol-5-yl-6-[(l , 1 -dimethylethyl)sulfonyl]-7-ethyl-4-quinazolinamine
- this invention is directed to N-l,3-benzothiazol-5-yl-6-[(l,l- dimethylethyl)sulfonyl]-4-quinazolinamine or a salt, particularly a pharmaceutically acceptable salt, thereof.
- This invention is also directed to N-l,3-benzothiazol-5-yl-6-[(l- methylethyl)sulfonyl]-4-quinazolinamine, or a salt, particularly a pharmaceutically acceptable salt, thereof.
- This invention is further directed to N-(6-(tert-butylsulfonyl)-7-methoxyquinazolin- 4-yl)benzo[ ⁇ i]thiazol-5 -amine or a salt, particularly a pharmaceutically acceptable salt, thereof.
- this invention is directed to 2-((4-(benzo[d]thiazol-5-ylamino)-6-(tert- butylsulfonyl)quinazolin-7-yl)oxy)ethanol or a salt, particularly a pharmaceutically acceptable salt, thereof.
- a compound of the invention includes a compound of Formula (I), or a salt thereof, particularly a pharmaceutically acceptable salt thereof.
- a compound of the invention includes a compound of Formula (I), particularly the specific compounds described herein, or a salt thereof, particularly a pharmaceutically acceptable salt thereof.
- the invention is directed to a method of inhibiting RIP2 kinase comprising contacting a cell with a compound of the invention.
- the invention is directed to a method of treating a RIP2 kinase-mediated disease or disorder comprising administering a therapeutically effective amount of a compound of the invention to a human in need thereof.
- the invention is still further directed to the use of a compound of the invention or a pharmaceutical composition comprising a compound of the invention to inhibit RIP2 kinase and/or treat a RIP2 kinase- mediated disease or disorder.
- the compounds according to Formula (I) may contain one or more asymmetric center (also referred to as a chiral center) and may, therefore, exist as individual enantiomers, diastereomers, or other stereoisomeric forms, or as mixtures thereof.
- Chiral centers such as a chiral carbon, or particularly, a chiral -SO- moiety, may also be present in the compounds of this invention.
- the stereochemistry of a chiral center present in a compound of this invention e.g., compound name
- the compound, compound name, or structure is intended to encompass all individual stereoisomers and all mixtures thereof.
- compounds according to Formula (I) containing one or more chiral center may be present as racemic mixtures, enantiomerically enriched mixtures, or as enantiomerically pure individual stereoisomers.
- each of (i?)-N-(6-(tert-butylsulfinyl)-7-methoxyquinazolin-4- yl)benzo [d]thiazol-5 -amine and (5)-N-(6-(tert-butylsulfinyl)-7-methoxyquinazolin-4- yl)benzo[ ⁇ i]thiazol-5 -amine are encompassed by the chemical name N-(6-(tert- butylsulfinyl)-7-methoxyquinazolin-4-yl)benzo[(i]thiazol-5-amine.
- Individual stereoisomers of a compound according to Formula (I) which contain one or more asymmetric center may be resolved by methods known to those skilled in the art. For example, such resolution may be carried out (1) by formation of diastereoisomeric salts, complexes or other derivatives; (2) by selective reaction with a stereoisomer-specific reagent, for example by enzymatic oxidation or reduction; or (3) by gas-liquid or liquid chromatography in a chiral environment, for example, on a chiral support such as silica with a bound chiral ligand or in the presence of a chiral solvent.
- stereoisomers may be synthesized by asymmetric synthesis using optically active reagents, substrates, catalysts or solvents, or by converting one enantiomer to the other by asymmetric transformation.
- a solid form of a compound of the invention may exist in crystalline forms, non-crystalline forms or a mixture thereof. Such crystalline forms may also exhibit polymorphism (i.e. the capacity to occur in different crystalline forms). These different crystalline forms are typically known as "polymorphs.” Polymorphs have the same chemical composition but differ in packing, geometrical arrangement, and other descriptive properties of the crystalline solid state. Polymorphs, therefore, may have different physical properties such as shape, density, hardness, deformability, stability, and dissolution properties. Polymorphs typically exhibit different melting points, IR spectra, and X-ray powder diffraction patterns, which may be used for identification. One of ordinary skill in the art will appreciate that different polymorphs may be produced, for example, by changing or adjusting the conditions used in crystallizing/recrystallizing the compound.
- Formula (I) are preferably pharmaceutically acceptable salts.
- Suitable pharmaceutically acceptable salts include those described by Berge, Bighley and Monkhouse J.Pharm.Sci (1977) 66, pp 1-19. Salts encompassed within the term “pharmaceutically acceptable salts" refer to non-toxic salts of the compounds of this invention.
- a desired salt form may be prepared by any suitable method known in the art, including treatment of the free base with an inorganic acid, such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like, or with an organic acid, such as acetic acid, trifluoroacetic acid, maleic acid, succinic acid, mandelic acid, fumaric acid, malonic acid, pyruvic acid, oxalic acid, glycolic acid, salicylic acid, and the like, or with a pyranosidyl acid, such as glucuronic acid or galacturonic acid, or with an alpha-hydroxy acid, such as citric acid or tartaric acid, or with an amino acid, such as aspartic acid or glutamic acid, or with an aromatic acid, such as benzoic acid or cinnamic acid, or with a sulfonic acid, such as /
- Suitable addition salts include acetate, p-aminobenzoate, ascorbate, aspartate, benzenesulfonate, benzoate, bicarbonate, bismethylenesalicylate, bisulfate, bitartrate, borate, calcium edetate, camsylate, carbonate, clavulanate, citrate, cyclohexylsulfamate, edetate, edisylate, estolate, esylate, ethanedisulfonate, ethanesulfonate, formate, fumarate, gluceptate, gluconate, glutamate, glycollate, glycollylarsanilate, hexylresorcinate, hydrabamine, hydrobromide, hydrochloride, dihydrochloride, hydrofumarate, hydrogen phosphate, hydroiodide, hydromaleate, hydrosuccinate, hydroxynaphthoate, isethionate,
- methylsulfate monopotassium maleate, mucate, napsylate, nitrate, N-methylglucamine, oxalate, oxaloacetate, pamoate (embonate), palmate, palmitate, pantothenate,
- phosphate/diphosphate pyruvate, polygalacturonate, propionate, saccharate, salicylate, stearate, subacetate, succinate, sulfate, tannate, tartrate, teoclate, tosylate, triethiodide, trifluoroacetate and valerate.
- exemplary acid addition salts include pyrosulfate, sulfite, bisulfite, decanoate, caprylate, acrylate, isobutyrate, caproate, heptanoate, propiolate, oxalate, malonate, suberate, sebacate, butyne-l,4-dioate, hexyne-l,6-dioate, chlorobenzoate, methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate, phenylacetate, phenylpropionate, phenylbutrate, lactate, ⁇ -hydroxybutyrate, mandelate, and sulfonates, such as xylenesulfonate, propanesulfonate, naphthalene- 1 -sulfonate and naphthalene-2-sulfonate.
- an inventive basic compound is isolated as a salt
- the corresponding free base form of that compound may be prepared by any suitable method known to the art, including treatment of the salt with an inorganic or organic base, suitably an inorganic or organic base having a higher pK a than the free base form of the compound.
- a desired salt may be prepared by any suitable method known to the art, including treatment of the free acid with an inorganic or organic base, such as an amine (primary, secondary, or tertiary), an alkali metal or alkaline earth metal hydroxide, or the like.
- an inorganic or organic base such as an amine (primary, secondary, or tertiary), an alkali metal or alkaline earth metal hydroxide, or the like.
- suitable salts include organic salts derived from amino acids such as glycine and arginine, ammonia, primary, secondary, and tertiary amines, and cyclic amines, such as N-methyl-D-glucamine, diethylamine, isopropylamine, trimethylamine, ethylene diamine, dicyclohexylamine, ethanolamine, piperidine, morpholine, and piperazine, as well as inorganic salts derived from sodium, calcium, potassium, magnesium, manganese, iron, copper, zinc, aluminum, and lithium.
- amino acids such as glycine and arginine
- ammonia such as glycine and arginine
- primary, secondary, and tertiary amines such as N-methyl-D-glucamine, diethylamine, isopropylamine, trimethylamine, ethylene diamine, dicyclohexylamine, ethanolamine, piperidine, morpholine, and piperazine
- Certain of the compounds of the invention may form salts with one or more equivalents of an acid (if the compound contains a basic moiety) or a base (if the compound contains an acidic moiety).
- the present invention includes within its scope all possible stoichiometric and non-stoichiometric salt forms.
- Compounds of the invention having both a basic and acidic moiety may be in the form of zwitterions, acid-addition salt of the basic moiety or base salts of the acidic moiety.
- This invention also provides for the conversion of one pharmaceutically acceptable salt of a compound of this invention into another pharmaceutically acceptable salt of a compound of this invention.
- pharmaceutically acceptable solvates may be formed wherein solvent molecules are incorporated into the crystalline lattice during crystallization.
- Solvates may involve nonaqueous solvents such as ethanol, isopropanol, DMSO, acetic acid, ethanolamine, and EtOAc, or they may involve water as the solvent that is incorporated into the crystalline lattice.
- Solvates wherein water is the solvent that is incorporated into the crystalline lattice are typically referred to as "hydrates.” Hydrates include stoichiometric hydrates as well as compositions containing variable amounts of water. The invention includes all such solvates, particularly hydrates. It is to be understood that the term "a salt, particularly a pharmaceutically acceptable salt, thereof, or hydrate thereof encompasses a salt of a compound of Formula (I), a pharmaceutically acceptable salt of a compound of Formula (I), a hydrate of a compound of Formula (I), a hydrate of a salt of a compound of Formula (I), and a hydrate of a pharmaceutically acceptable salt of a compound of Formula (I).
- the compounds of Formula (I) are intended for use in pharmaceutical compositions it will readily be understood that they are each preferably provided in substantially pure form, for example at least 60% pure, more suitably at least 75% pure and preferably at least 85%, especially at least 98% pure (% are on a weight for weight basis). Impure preparations of the compounds may be used for preparing the more pure forms used in the pharmaceutical compositions.
- the compounds of Formula (I) may be obtained by using synthetic procedures illustrated in the Schemes below or by drawing on the knowledge of a skilled organic chemist.
- the syntheses provided in these Schemes are applicable for producing compounds of the invention having a variety of different substituent groups employing appropriate precursors, which are suitably protected if needed, to achieve compatibility with the reactions outlined herein. Subsequent deprotection, where needed, affords compounds of the nature generally disclosed. While the Schemes are shown with compounds only of Formula (I), they are illustrative of processes that may be used to make the compounds of the invention.
- 4-Chloroquinazoline intermediates may be from the appropriately functionalized nitrobenzoic acid via reduction of the nitro group to an aniline followed by condensation with formamide or formimamide and cyclization to the 4-quinazolinone. Conversion to the chloroquinazoline could be accomplished with POCI3 or SOCl 2 at elevated temperatures.
- 6-alkylthioquinazo lines which could be final products, or further subjected to oxidation with Oxone to afford the corresponding sulfone final products.
- Sulfoxides may also be obtained by this route through carefully monitoring the reaction. Additionally, any sulfides at R 3 may be oxidized to sulfones in this manner.
- Substitution at C6 could also be installed prior to installation of the "A" moiety.
- a palladium catalyzed coupling of a thiol with the 6-iodoquinazolinone can provide a sulfide which can subsequently be oxidized to the sulfone.
- Chlorination with POCI 3 or SOCl 2 may provide the 4-chloroquinazoline.
- the sulfide at C6 could be installed while dealkylating C7 methoxyquinazoline with the appropriate sodium alkylthiolate.
- Final product may be obtained following alkylation of the resultant C7 hydroxyl and oxidation to the sulfone.
- the 7-ethylquinazoline could synthesized via a Stille coupling with the C7 triflate to provide the ethylenebenezene followed by hydrogeno lysis.
- the triflate can undergo a palladium catalyzed reaction to install the allylic alcohol.
- 5-Fluoro-lH-pyrazolo[3,4-3 ⁇ 4]pyridin-3-amine may be synthesized from 2-chloro-5- fluoro-3-pyridinecarboxylic acid. Formation of the acylchloride followed by the addition of ammonia can provide the amide which may be subsequently converted to the nitrile. Treatment with hydrazine may provide the azaindazole. Scheme 12
- 3,4-Dimethyl-lH-pyrazol-5-amine may be synthesized by alkylation of 3-amino-2- methylacrylonitrile followed by a cyclization with hydrazine.
- the pyrazolamine can be further methylated by reaction with formic acid followed by reduction with borohy dride .
- a groups may be installed via a C4 sulfide intermediate. Treatment of the 4-chloro-7-methoxyquinazo lines with sodium ethanethiolate provides the 4-ethylsulfide-7- hydroxyquinoazolines. These 4-alkythio-quinazoline intermediates may be directly treated with an excess of amine (A-NH 2 ) under acidic microwave conditions to provide final products. Alternatively, the intermediates could be alkylated at R 3 to afford the hydroxy ethyl ethers before undergoing reaction with amine (A-NH 2 ).
- Sulfonamides may be obtained through the sulfonyl chloride. Formation of the benzylthioether via a palladium catalyzed reaction at the 6-iodoquinazoline followed by treatment with NCS under acidic conditions provided the sulfonyl chloride. Treatment with an amine or ammonia affords the sulfonamide.
- the compounds of this invention may be particularly useful for treatment of RIP2 kinase-mediated diseases or disorders, particularly, uveitis, interleukin-1 converting enzyme (ICE, also known as Caspase-1) associated fever syndrome, dermatitis, acute lung injury, type 2 diabetes mellitus, arthritis (specifically rheumatoid arthritis), inflammatory bowel disorders (such as ulcerative colitis and Crohn's disease), early-onset and extraintestinal inflammatory bowel disease, prevention of ischemia reperfusion injury in solid organs (specifically kidney) in response ischemia induced by cardiac surgery, organ transplant, sepsis and other insults, liver diseases (non-alcohol steatohepatitis, alcohol steatohepatitis, and autoimmune hepatitis), allergic diseases (such as asthma), transplant reactions (such as graft versus host disease), autoimmune diseases (such as systemic lupus erythematosus, and multiple sclerosis), and granulomateous disorders (
- the compounds of this invention may be particularly useful in the treatment of uveitis, ICE fever, Blau Syndrome, early-onset sarcoidosis, ulcerative colitis, Crohn's disease, Wegener's granulamatosis and sarcoidosis.
- Treatment of RIP2 kinase-mediated diseases or disorders may be achieved using a compound of this invention as a monotherapy, or in dual or multiple combination therapy, particularly for the treatment of refractory cases, such as in combination with other anti-inflammatory and/or anti-TNF agents, which may be administered in
- the compounds of this invention may be administered in combination with corticosteroids and/or anti-TNF agents to treat Blau syndrome, early-onset sarcoidosis; or in combination with anti-TNF biologies or other anti-inflammatory biologies to treat Crohn's Disease; or in combination with 5 -ASA (mesalamine) or sulfasalazine to treat ulcerative colitis; or in combination with low-dose corticosteroids and/or methotrexate to treat Wegener's granulamatosis or sarcoidosis or interstitial pulmonary disease; or in combination with a biologic (e.g.
- anti-TNF anti-IL-6, etc.
- suitable anti-inflammatory agents include corticosteroids, particularly low-dose corticosteroids (such as Deltasone ® (prednisone)) and anti-inflammatory biologies (such as Acterma ® (anti-IL6R mAb) and Rituximab ® (anti-CD20
- anti-TNF agents include anti-TNF biologies (such as Enbrel® (etanecerpt)), Humira ® (adalimumab), Remicade ® (infliximab) and Simponi ® (golimumab)).
- Enbrel® etanecerpt
- Humira ® adalimumab
- Remicade ® infliximab
- Simponi ® golimumab
- This invention also provides a compound of Formula (I), or a salt thereof, particularly a pharmaceutically acceptable salt thereof, for use in therapy.
- This invention specifically provides for the use of a compound of Formula (I), or a pharmaceutically acceptable salt thereof, as an active therapeutic substance in the treatment of a RIP2 kinase-mediated disease or disorder, for example the diseases and disorders recited herein; more specifically, for use in the treatment of a disease mediated by inhibition of RIP2 kinase.
- the invention also provides for the use of a compound of Formula (I), or a salt thereof, particularly a pharmaceutically acceptable salt thereof, in the manufacture of a medicament for use in the treatment of a RIP2 kinase-mediated disease or disorder, for example the diseases and disorders recited herein.
- a therapeutically "effective amount” is intended to mean that amount of a compound that, when administered to a patient in need of such treatment, is sufficient to effect treatment, as defined herein.
- a therapeutically effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt thereof is a quantity of an inventive agent that, when administered to a human in need thereof, is sufficient to modulate or inhibit the activity of RIP2 kinase such that a disease condition which is mediated by that activity is reduced, alleviated or prevented.
- the amount of a given compound that will correspond to such an amount will vary depending upon factors such as the particular compound (e.g., the potency (pIC 5 o), efficacy (EC 50 ), and the biological half-life of the particular compound), disease condition and its severity, the identity (e.g., age, size and weight) of the patient in need of treatment, but can nevertheless be routinely determined by one skilled in the art.
- the particular compound e.g., the potency (pIC 5 o), efficacy (EC 50 ), and the biological half-life of the particular compound
- disease condition and its severity e.g., the identity of the patient in need of treatment, but can nevertheless be routinely determined by one skilled in the art.
- duration of treatment and the time period of administration (time period between dosages and the timing of the dosages, e.g., before/with/after meals) of the compound will vary according to the identity of the mammal in need of treatment (e.g., weight), the particular compound and its properties (e.g., pharmaceutical characteristics), disease or disorder and its severity and the specific composition and method being used, but can nevertheless be determined by one of skill in the art.
- Treating is intended to mean at least the mitigation of a disease or disorder in a patient.
- the methods of treatment for mitigation of a disease or disorder include the use of the compounds in this invention in any conventionally acceptable manner, for example for prevention, retardation, prophylaxis, therapy or cure of a mediated disease or disorder. Specific diseases and disorders that may be particularly susceptible to treatment using a compound of this invention are described herein.
- the compounds of the invention may be administered by any suitable route of administration, including both systemic administration and topical administration.
- Systemic administration includes oral administration, parenteral administration, transdermal administration, rectal administration, and administration by inhalation.
- Parenteral administration refers to routes of administration other than enteral, transdermal, or by inhalation, and is typically by injection or infusion.
- Parenteral administration includes intravenous, intramuscular, and subcutaneous injection or infusion.
- Inhalation refers to administration into the patient's lungs whether inhaled through the mouth or through the nasal passages.
- Topical administration includes application to the skin.
- the compounds of the invention may be administered once or according to a dosing regimen wherein a number of doses are administered at varying intervals of time for a given period of time. For example, doses may be administered one, two, three, or four times per day. Doses may be administered until the desired therapeutic effect is achieved or indefinitely to maintain the desired therapeutic effect. Suitable dosing regimens for a compound of the invention depend on the pharmacokinetic properties of that compound, such as absorption, distribution, and half-life, which can be determined by the skilled artisan.
- suitable dosing regimens including the duration such regimens are administered, for a compound of the invention depend on the disease or disorder being treated, the severity of the disease or disorder being treated, the age and physical condition of the patient being treated, the medical history of the patient to be treated, the nature of concurrent therapy, the desired therapeutic effect, and like factors within the knowledge and expertise of the skilled artisan. It will be further understood by such skilled artisans that suitable dosing regimens may require adjustment given an individual patient's response to the dosing regimen or over time as individual patient needs change.
- the compounds of the invention will be normally, but not necessarily, formulated into a pharmaceutical composition prior to administration to a patient. Accordingly, the invention also is directed to pharmaceutical compositions comprising a compound of the invention and a pharmaceutically acceptable excipient.
- compositions of the invention may be prepared and packaged in bulk form wherein an effective amount of a compound of the invention can be extracted and then given to the patient such as with powders, syrups, and solutions for injection.
- the pharmaceutical compositions of the invention may be prepared and packaged in unit dosage form.
- a dose of the pharmaceutical composition contains at least a therapeutically effective amount of a compound of this invention (i.e., a compound of Formula (I), or a salt, particularly a pharmaceutically acceptable salt, thereof).
- the pharmaceutical compositions may contain from 1 mg to 1000 mg of a compound of this invention.
- compositions of the invention typically contain one compound of the invention. However, in certain embodiments, the pharmaceutical compositions of the invention contain more than one compound of the invention. In addition, the pharmaceutical compositions of the invention may optionally further comprise one or more additional pharmaceutically active compounds.
- pharmaceutically acceptable excipient means a material, composition or vehicle involved in giving form or consistency to the composition.
- Each excipient must be compatible with the other ingredients of the pharmaceutical composition when commingled such that interactions which would substantially reduce the efficacy of the compound of the invention when administered to a patient and interactions which would result in pharmaceutical compositions that are not pharmaceutically acceptable are avoided.
- each excipient must of course be of sufficiently high purity to render it pharmaceutically acceptable.
- the compounds of the invention and the pharmaceutically acceptable excipient or excipients will typically be formulated into a dosage form adapted for administration to the patient by the desired route of administration.
- Conventional dosage forms include those adapted for (1) oral administration such as tablets, capsules, caplets, pills, troches, powders, syrups, elixirs, suspensions, solutions, emulsions, sachets, and cachets; (2) parenteral administration such as sterile solutions, suspensions, and powders for reconstitution; (3) transdermal administration such as transdermal patches; (4) rectal administration such as suppositories; (5) inhalation such as aerosols and solutions; and (6) topical administration such as creams, ointments, lotions, solutions, pastes, sprays, foams, and gels.
- Suitable pharmaceutically acceptable excipients will vary depending upon the particular dosage form chosen.
- suitable pharmaceutically acceptable excipients may be chosen for a particular function that they may serve in the composition.
- certain pharmaceutically acceptable excipients may be chosen for their ability to facilitate the production of uniform dosage forms.
- Certain pharmaceutically acceptable excipients may be chosen for their ability to facilitate the production of stable dosage forms.
- Certain pharmaceutically acceptable excipients may be chosen for their ability to facilitate the carrying or transporting the compound or compounds of the invention once administered to the patient from one organ, or portion of the body, to another organ, or portion of the body.
- Certain pharmaceutically acceptable excipients may be chosen for their ability to enhance patient compliance.
- Suitable pharmaceutically acceptable excipients include the following types of excipients: diluents, fillers, binders, disintegrants, lubricants, glidants, granulating agents, coating agents, wetting agents, solvents, co-solvents, suspending agents, emulsifiers, sweeteners, flavoring agents, flavor masking agents, coloring agents, anti-caking agents, humectants, chelating agents, plasticizers, viscosity increasing agents, antioxidants, preservatives, stabilizers, surfactants, and buffering agents.
- excipients may serve more than one function and may serve alternative functions depending on how much of the excipient is present in the formulation and what other ingredients are present in the formulation.
- Skilled artisans possess the knowledge and skill in the art to enable them to select suitable pharmaceutically acceptable excipients in appropriate amounts for use in the invention.
- resources that are available to the skilled artisan which describe pharmaceutically acceptable excipients and may be useful in selecting suitable pharmaceutically acceptable excipients. Examples include Remington's Pharmaceutical Sciences (Mack Publishing Company), The Handbook of Pharmaceutical Additives (Gower Publishing Limited), and The Handbook of Pharmaceutical Excipients (the American Pharmaceutical Association and the Pharmaceutical Press).
- compositions of the invention are prepared using techniques and methods known to those skilled in the art. Some of the methods commonly used in the art are described in Remington's Pharmaceutical Sciences (Mack Publishing Company).
- the invention is directed to a solid oral dosage form such as a tablet or capsule comprising an effective amount of a compound of the invention and a diluent or filler.
- Suitable diluents and fillers include lactose, sucrose, dextrose, mannitol, sorbitol, starch (e.g. corn starch, potato starch, and pre-gelatinized starch), cellulose and its derivatives (e.g. microcrystalline cellulose), calcium sulfate, and dibasic calcium phosphate.
- the oral solid dosage form may further comprise a binder. Suitable binders include starch (e.g.
- the oral solid dosage form may further comprise a disintegrant. Suitable disintegrants include crospovidone, sodium starch glycolate, croscarmelose, alginic acid, and sodium carboxymethyl cellulose.
- the oral solid dosage form may further comprise a lubricant. Suitable lubricants include stearic acid, magnesium stearate, calcium stearate, and talc.
- the invention also includes various deuterated forms of the compounds of
- Each available hydrogen atom attached to a carbon atom may be any available hydrogen atom attached to a carbon atom.
- deuterated pyrazole alkyl groups or deuterated alkyl-thioquinazolines or alkyl- sulfonylquinazo lines may be prepared by conventional techniques (see for example:
- ⁇ may be named as a tert-butylsulfonyl or as a (l,l-dimethylethyl)sulfonyl group/moiety.
- this program will name a structurally depicted compound as a tautomer of that compound. It is to be understood that any reference to a named compound or a structurally depicted compound is intended to encompass all tautomers of such compounds and any mixtures of tautomers thereof.
- Step 1 4-chloro-6-iodoquinazoline: 6-iodo-4(lH)-quinazolinone (10 g, 37 mmol) was weighed into a 250 mL flask. Thionyl chloride (100 mL, 1.4 mmol) and DMF (0.5 mL, 6.5 mmol) were added to give a grey suspension. The mixture was heated to reflux. Heating was continued for 6 h and then the mixture was cooled on ice bath for 1 h. A yellow solid precipitated and was collected by filtration to afford 8.6 g (77%) of the title compound.
- N-l,3-benzothiazol-5-yl-6-iodo-4-quinazolinamine To a solution of 4- chloro-6-iodoquinazoline (2.60 g, 8.95 mmol) in isopropanol (60 mL) was added 1,3- benzothiazol-5 -amine (1.479 g, 9.85 mmol). The mixture was then placed in oil bath preheated to 90°C. The reaction was complete in 30 min., and the solution was allowed to cool to room temperature. A yellow solid precipitated and was filtered and dried to provide 3.6 g (91%) of the title compound.
- Step 1 6-[(l-methylethyl)thio]-4(lH)-quinazolinone: To a solution of 6-iodo- 4(lH)-quinazolinone (3.0 g, 11.0 mmol), 2-propanethiol (1.1 mL, 12.1 mmol) and Et 3 N (4.6 mL, 33.1 mmol) in DMF (40 mL) was added Pd(Ph 3 P) 4 (1.27 g, 1.10 mmol) under nitrogen. The solution was stirred at 90 °C for 1 h. The reaction mixture was allowed to cool to rt and DMF was removed in vacuo.
- Step 3 4-chloro-6-[(l-methylethyl)sulfonyl]quinazoline: 6-[(l-Methylethyl)thio]- 4(lH)-quinazolinone (0.20 g, 0.91 mmol), DMF (0.1 mL) and thionyl chloride (5 mL) were place in a sealed tube and heated at 85°C for 5 h. LCMS showed 85% of starting material was converted to product. The solvent was removed in vacuo to provide the title compound (0.20 g crude material). MS (m/z): 271 ( ⁇ + ⁇ ).
- Step 1 7-chloro-6-iodo-4(lH)-quinazolinone: A solution of methyl 2-amino-4- chloro-5-iodobenzoate (3.4 g, 10.9 mmol) and imidoformamide (3.4 g, 32.7 mmol) in 2- methoxyethanol (15 mL) was stirred at 125°C for 7 h. The reaction mixture was allowed to cool to rt and the residue suspended in water. The solid was collected by filtration, washed with water and dried under vacuum (50 °C) to give 3.2 g of the title compound (96%). MS: m/z: 307 [M+H] + .
- Step 2 7-chloro-6-[(l,l-dimethylethyl)thio]-4(lH)-quinazolinone: To a solution of 6,7-3 ⁇ 4z5[(l,l-dimethylethyl)thio]-4(lH)-quinazolinone (500 mg, 1.6 mmol), 2-methyl-2- propanethiol (162 mg, 1.8 mmol), Et 3 N (0.68 mL, 4.9 mmol) in DMF (5 mL) was added Pd(Ph 3 P) 4 (189 mg, 0.16 mmol). The reaction mixture was stirred at 90°C for 1 h. Solvent was removed in vacuo. The crude material was purified by column chromatography (0 to 5% MeOH/CH 2 Cl 2 ) to provide 0.37 g of the title compound (84%). MS: m/z: 269 [M+H] + .
- Step 1 6-[(l,l-dimethylethyl)thio]-7-(methyloxy)-4(lH)-quinazolinone: To a solution of 6-iodo-7-(methyloxy)-4(lH)-quinazolinone(1.0 g, 3.3 mmol), 2-methyl-2- propanethiol (0.36 g, 4.0 mmol), Et 3 N (1.4 mL, 9.9 mmol) in DMF (5 mL) was added Pd(Ph 3 P)4 (0.38 g, 0.33 mmol). The reaction mixture was stirred at 90°C for 1 h. Solvent was removed in vacuo. The crude material was purified by column chromatography (0 to 5% MeOH/CH 2 Cl 2 ) to provide 0.90 g of the title compound (93%). MS:m/z: 265 [M+H] + .
- Step 3 4-chloro-6-[(l , 1 -dimethylethyl)sulfonyl]-7-(methyloxy)quinazoline:
- Stepl. 3-amino-2-methyl-2-butenenitrile To a suspension of NaH (11.7 g, 292 mmol) in toluene (100 mL) at 30°C was added a solution of (2Z)-3-amino-2-butenenitrile (20 g, 244 mmol) in toluene (400 mL) and the reaction mixture was stirred for 10 min. lodomethane (15.23 mL, 244 mmol) was added and the reaction was cooled with cold water to maintain a temperature of 40 °C. The reaction was then cooled to 30°C and stirred overnight. An orange solid formed and was collected via filtration washing with toluene.
- Step 2 3,4-dimethyl-lH-pyrazol-5-amine: To a solution of 3-amino-2-methyl-2- butenenitrile (1.0 g, 10.4 mmol) in ethanol (10.4 mL) was added hydrazine (0.60 mL, 10.4 mmol). The resulting mixture was heated to 75°C for 16 h open to atmosphere. The reaction was concentrated onto silica gel and purified via flash chromatography eluting with 0-10% MeOH in CH 2 C1 2 to give the title compound as a yellow oil (710 mg, 61%).
- Step 1 (4,5-dimethyl-lH-pyrazol-3-yl)formamide.
- a mixture of 4, 5 -dimethyl- 1H- pyrazol-3 -amine (1.92 g, 17.3 mmol) in formic acid (10 mL) was stirred under nitrogen at reflux for 2 h.
- the reaction mixture was cooled to rt and concentrated to yield the title compound as a solid.
- Step 2 N,4,5-trimethyl-lH-pyrazol-3-amine.
- a mixture of (4,5-dimethyl-lH- pyrazol-3-yl)formamide (2.47 g, 17.7 mmol) and BH 3 » THF (53.1 mL of a 1.0 M solution in THF, 53.1 mmol) was stirred under nitrogen at rt for 3 h. The mixture was then cooled to 0°C and quenched with MeOH (dropwise addition). The crude product was purified via column chromatography using 0-7% MeOH:CH 2 Cl 2 gradient, 80 g column to yield 0.70 g of the title compound as a colorless viscous oil.
- this reaction can be run in solvents other than 1 ,4-dioxane, including DMF, and at temperatures other than 60°C as appropriate for each substrate.
- N- 1 ,3-benzothiazol-5-yl-6-[(l , 1 -dimethylethyl)thio]-4-quinazolinamine (150 mg, 0.327 mmol) was dissolved in MeOH (10 mL) in a 20 mL scintillation vial. To this deep red solution was added oxone (300 mg, 0.488 mmol) and the reaction was stirred at rt. After 1 hr the reaction was treated with 5 mL of (5: 1 Na 2 S 2 0 3 /NaHC0 3 ) for 5 min then poured into 50mL sat. aq. bicarbonate solution.
- Step 1 3- ⁇ [4-(l,3-benzothiazol-5-ylamino)-6-quinazolinyl]thio ⁇ -3-methyl-l- butanol: To a solution of N-l,3-benzothiazol-5-yl-6-iodo-4-quinazolinamine (202 mg, 0.500 mmol), 3-mercapto-3-methyl-l-butanol (60 mg, 0.50 mmol), potassium tert-butoxide (168 mg, 1.50 mmol) and (oxydi-2,l-phenylene)bis(diphenyl phosphine) (27 mg, 0.05 mmol) in dioxane (4 mL) was added Pd 2 dba 3 (46 mg, 0.05 mmol) and the mixture was sparged with N 2 for 10 min.
- Cesium carbonate may also be used as the base in these coupling reactions.
- An equivalent of triethamine may also be added when the starting quinazoline is an HCl salt.
- EtOH and water (1 : 1) may also be used as the solvent mixture.
- the following example was prepared in a similar manner:
- Methyl 2-amino-5-iodo-4-methoxybenzoate Methyl 2-amino-4- (methyloxy) benzoate (3.78 g, 20.86 mmol) was dissolved in 25 mL of water, 15 mL of ethanol and 2.2 mL of concentrated HCl. A solution of ICl (1.1 mL, 21.9 mmol) in 3.8 mL concentrated HCl and 14 mL of water at 5 °C was added to the aniline solution. The reaction was stirred overnight and was then filtered to obtain 6.9 g of a light brown solid. MS: m/z: 308 [M+H] +
- Step 4 4-chloro-6-iodo-7-(methyloxy)quinazoline: 6-Iodo-7-(methyloxy)-4(lH)- quinazolinone (2.0 g, 6.6 mmol), POCl 3 (3.1 mL, 33.1 mmol) and DIPEA (6.9 mL, 40 mmol) were combined in DCE (50 mL) a round bottom flask. The reaction mixture was heated at 80°C for 5 h, followed by heating at 70°C for 10 h. The reaction mixture was allowed to cool to rt. A yellow solid was precipitated out. The solid was filtered. The solution was concentrated and neutralized with satd. NaHC0 3 , extracted with CH 2 CI 2 and dried over Na 2 S0 4 . The mixture was filtered, and the solvent was removed in vacuo.
- N-l,3-benzothiazol-5-yl-6-iodo-7-(methyloxy)-4-quinazolinamine To a solution of 4-chloro-6-iodo-7-(methyloxy)quinazoline (2.0 g, 5.4 mmol) in isopropanol (30 mL) was added l,3-benzothiazol-5-amine (1.2 g, 8.1 mmol). The suspension was heated in oil bath at 90°C (preheated). The reaction mixture stirred at this temperature for 30 min. A yellow solid precipitated out as the reaction mixture was allowed to cool to rt. The solid was filtered to provide 2.1 g of the title compound (77%, 93% pure). MS: m/z: All
- N-(6-(tert-butylthio)-7-methoxyquinazolin-4-yl)benzo [d]thiazol-5 -amine To a solution of N-l,3-benzothiazol-5-yl-6-iodo-7-(methyloxy)-4-quinazolinamine (2.1 g, 4.5 mmol), 2-methyl-2-propanethiol (483 mg, 5.35 mmol), Et 3 N (1.9 mL, 13.4 mmol) in DMF (5 mL) was added Pd(Ph 3 P) 4 (516 mg, 0.45 mmol). The reaction mixture was stirred at 90°C for 1 h. Most of DMF was removed in vacuo. The crude material was triturated with MeOH. The red solid was filtered and washed with Et 2 0 to provide 1.7 g of the title compound as an off white solid (96%). MS: m/z: 397 [M+H] + .
- Step 1 4-(benzo[ ⁇ i]thiazol-5-ylamino)-6-(tert-butylsulfonyl)quinazolin-7-yl trifluoromethanesulfonate: To an ice cooled solution of 4-(benzo[d]thiazol-5-ylamino)-6- (tert-butylsulfonyl)quinazolin-7-ol (346 mg, 0.83 mmol) in pyridine (6 mL) was slowly added triflic anhydride (421 ⁇ , 2.50 mmol). The reaction was warmed to rt over 5 min. The reaction mixture was triturated from CH 2 CI 2 .
- Step 2 N-(6-(tert-butylsulfonyl)-7-vinylquinazolin-4-yl)benzo [ ]thiazol-5 -amine : To a solution of 4-(benzo[ ⁇ i]thiazol-5-ylamino)-6-(tert-butylsulfonyl)quinazolin-7-yl trifluoromethanesulfonate (60.0 mg, 0.091 mmol) and vinyltri-n-butyltin (37.1 ⁇ , 0.126 mmol) in DMF (1.01 mL) was added PdCl 2 (dppf)-CH 2 Ci 2 adduct (18 mg, 0.02 mmol).
- reaction mixture was filtered through glass filter paper and concentrated in vacuo.
- the residue was purified via reverse phase chromatography (6% to 75% 0.1% TFA in MeCN in 0.1% TFA in water; 5um 30x150 mm Waters Sunfire column, 15 min gradient).
- the pure fraction was partitioned between EtOAc and satd. NaHCCh, the organic layer was washed with brine, then dried over MgS0 4 and concentrated in vacuo to obtain N-(6-(tert- butylsulfonyl)-7-ethylquinazolin-4-yl)benzo [ ]thiazol-5 -amine (7.5 mg, 19% yield).
- Step 1 2-((4-(benzo[d]thiazol-5-ylamino)-7-methoxyquinazolin-6-yl)thio)ethanol: To a flask was added N-(6-iodo-7-methoxyquinazolin-4-yl)benzo[d]thiazol-5 -amine (1 g, 2.30 mmol), Pd 2 dba 3 (0.21 g, 0.23 mmol), and Xantphos (0.13 g, 0.23 mmol) which was then evacuated and backfilled with nitrogen three times before DMF (15 ml), TEA (0.96 ml, 6.91 mmol), and mercaptoethanol (0.17 ml, 2.42 mmol) were added.
- 6-(tert-Butylsulfonyl)-4-(ethylthio)quinazolin-7-ol 144 mg, 0.44 mmol
- 4-chloro-2- fluoroaniline 0.49 mL, 4.4 mmol
- MeOH MeOH
- MP carbonate resin 400 mg, 3 eq. @ 3.28 mmol/gram loading
- Step 1 Methyl 2-((4-(benzo[d]thiazol-5-ylamino)-6-(tert-butylsulfonyl)quinazolin- 7-yl)oxy)acetate: A suspension of 4-(benzo[d]thiazol-5-ylamino)-6-(tert- butylsulfonyl)quinazolin-7-ol (350 mg, 0.84 mmol) and potassium carbonate (350 mg, 2.5 mmol) in DMF (4.4 mL) was stirred 2 minutes before adding methyl 2-bromoacetate (108 ⁇ , 1.14 mmol). The reaction was heated at 25°C for 6 h.
- Tablets are prepared using conventional methods and are formulated as follows:
- Lactose lOOmg Sodium starch glycollate 30mg
- Capsules are prepared using conventional methods and are formulated as follows: Ingredient Amount per tablet
- a fluorescent polarization based binding assay was developed to quantitate interaction of novel test compounds at the ATP binding pocket of RIPK2, by competition with a fluorescently labeled ATP competitive ligand.
- Full length FLAG His tagged RIPK2 was purified from a Baculovirus expression system and was used at a final assay concentration of twice the KDapparent.
- a fluorescent labeled ligand (5-( ⁇ [2-( ⁇ [3-( ⁇ 4-[(5- hydroxy-2-methylphenyl)amino]-2-pyrimidinyl ⁇ amino)phenyl]carbonyl ⁇ amino)ethyl] amino ⁇ carbonyl)-2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic acid, prepared as described in WO2011/120025) was used at a final assay concentration of 5nM. Both the enzyme and ligand were prepared in solutions in 50mM HEPES pH7.5, 150mM NaCl, lOmM MgCl 2 , ImM DTT, and lmM CHAPS.
- Test compounds were prepared in 100% DMSO and lOOnL was dispensed to individual wells of a multiwell plate. Next, 5ul RIPK2 was added to the test compounds at twice the final assay concentration, and incubated at rt for 10 min. Following the incubation, 5ul of the fluorescent labeled ligand solution, was added to each reaction, at twice the final assay concentration, and incubated at rt for at least 10 min. Finally, samples were read on an instrument capable of measuring fluorescent polarization. Test compound inhibition was expressed as percent (%) inhibition of internal assay controls. For concentration/dose response experiments, normalized data were fit and pICsoS determined using conventional techniques. The pICsoS are averaged to determine a mean value, for a minimum of 2 experiments.
- the compounds of Examples 1-80 exhibited a pIC 5 o between approximately 5.0 and 9.0.
- the compounds of Examples 4 and 16 inhibited RIP2 kinase in the above method with a mean pIC 5 o of approximately 7.5 and 8.5, respectively.
- the compounds of Examples 4, 6, 16, 21 , and 23 inhibited RIP2 kinase in the above method with a mean pICso in the range of
- RIPK2 receptor-interacting serine -threonine kinase 2
- cDNA was purchased from Invitrogen (Carlsbad, California, USA, Clone ID:IOH6368, RIPK2- pENTR 221).
- Gateway® LR cloning was used to site-specifically recombine RIPK2 downstream to an N-terminal FLAG-6His contained within the destination vector pDEST8- FLAG-His6 according to the protocol described by Invitrogen.
- Spodoptera frugiperda(Sf9) insect cells was performed using Cellfectin® (Invitrogen), according to the manufacturer's protocol.
- the lysate was decanted from the insoluble pellet and loaded at a linear flow rate of 16 cm/h onto a 55 mL FLAG-M2 affinity column (2.6 x 10.4 cm) that had been pre- equilibrated with 10 column volumes buffer A (50mM Tris (pH 8.0), 150mM NaCl,
- the purification process yielded 11.3 mg of total protein, with the RIPK2 present at 40% purity by gel densitometry scanning, and identity confirmed by peptide mass fingerprinting.
- the main contaminating proteins in the preparation were identified as lower molecular weight degraded species of RIPK2.
- lOOg cells (10 liter scale fermentation) were frozen, thawed, and re-suspended in 1L lysis buffer (50mM Tris HCL pH7.5, 250 mM NaCl, O. lmM TCEP, 3ml Protease inhibitor cocktail) and lysed by high pressure homogenization at 10,000 psi once (Avestin). The suspension was then clarified by centrifugation at 35,000g for 45 minutes at 4°C. The supernatant was collected by centrifugation and incubated with 5 ml anti-FLAG-M2 resin which was pre-equilibrated with buffer A (50mM Tris HCL pH7.5, 250 mM NaCl, O. lmM TCEP).
- buffer A 50mM Tris HCL pH7.5, 250 mM NaCl, O. lmM TCEP
- the resin was packed into two 25ml disposable columns. Each column was washed with 25ml buffer A and eluted with 10ml (buffer A + 200ug/ml Flag peptide). The elution pool was concentrated to 1ml and applied to a superdex 200 (16/60) sizing column.
- the efficacy of RIP2 inhibitors may also be evaluated in vivo in rodents.
- Intraperitoneal (i.p.) or intravenous (z ' .v.) administration of L18-MDP in mice has been shown to induce an inflammatory response through activation of the NOD2 signaling pathway (Rosenweig, H. L., et al. 2008. Journal of Leukocyte Biology 84:529-536).
- the level of the inflammatory response in the L18-MDP treated mice/rats is monitored using conventional techniques by measuring increases in cytokine levels (IL8, TNFa, IL6 and IL- ⁇ ) in serum and/or peritoneal lavage fluid and by measuring neutrophil influx into the peritoneal space (when L18-MDP is dosed i.p.).
- Inhibition of the L18-MDP induced inflammatory response in treated rodents may be shown by orally pre-dosing with selected compounds of this invention, then measuring and comparing cytokine levels (IL8, TNFa, IL6 and IL-1 ⁇ ) in serum and/or peritoneal lavage fluid and neutrophil influx into the peritoneal space (when L18-MDP is dosed i.p.) using conventional techniques.
- cytokine levels IL8, TNFa, IL6 and IL-1 ⁇
- rats were orally pre-dosed with a compound of Example 4, 6, 16, or 21, at a dose of 2 mg/kg or 10 mg/kg (8 rats) and with prednisolone (8 rats, used as a positive control), followed by dosing with L18-MDP (50 ⁇ g/rat) 0.25 h/min after pre- dosing.
- Combined cytokine levels IL8, TNFa, IL6 and IL- ⁇
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Immunology (AREA)
- Diabetes (AREA)
- Pulmonology (AREA)
- Rheumatology (AREA)
- Ophthalmology & Optometry (AREA)
- Gastroenterology & Hepatology (AREA)
- Transplantation (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Pain & Pain Management (AREA)
- Endocrinology (AREA)
- Obesity (AREA)
- Dermatology (AREA)
- Emergency Medicine (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
Description
Claims
Priority Applications (18)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP12824263.3A EP2744501B1 (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| MX2014001879A MX2014001879A (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors. |
| ES12824263.3T ES2615749T3 (en) | 2011-08-18 | 2012-08-17 | Aminoquinazolines as kinase inhibitors |
| CA2845630A CA2845630C (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| SG2014009526A SG2014009526A (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| CN201280050811.1A CN103874495B (en) | 2011-08-18 | 2012-08-17 | As the amido quinazoline of inhibitors of kinases |
| BR112014003693A BR112014003693A2 (en) | 2011-08-18 | 2012-08-17 | amino quinazolines as kinase inhibitors |
| NZ621314A NZ621314B2 (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| AU2012296411A AU2012296411B2 (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| EA201490474A EA025436B1 (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| US14/239,193 US9604938B2 (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| KR1020147006731A KR20140054246A (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| JP2014526237A JP6090801B2 (en) | 2011-08-18 | 2012-08-17 | Aminoquinazolines as kinase inhibitors |
| IL230902A IL230902A0 (en) | 2011-08-18 | 2014-02-10 | Amino quinazolines as kinase inhibitors |
| MA36820A MA35434B1 (en) | 2011-08-18 | 2014-03-12 | Amino-quinazolines as kinase inhibitors |
| US15/431,942 US9994529B2 (en) | 2011-08-18 | 2017-02-14 | Amino quinazolines as kinase inhibitors |
| US15/978,377 US20180258056A1 (en) | 2011-08-18 | 2018-05-14 | Amino quinazolines as kinase inhibitors |
| US16/286,920 US10717711B2 (en) | 2011-08-18 | 2019-02-27 | Amino quinazolines as kinase inhibitors |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161524925P | 2011-08-18 | 2011-08-18 | |
| US61/524,925 | 2011-08-18 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/239,193 A-371-Of-International US9604938B2 (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
| US15/431,942 Continuation US9994529B2 (en) | 2011-08-18 | 2017-02-14 | Amino quinazolines as kinase inhibitors |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013025958A1 true WO2013025958A1 (en) | 2013-02-21 |
Family
ID=47715493
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2012/051247 WO2013025958A1 (en) | 2011-08-18 | 2012-08-17 | Amino quinazolines as kinase inhibitors |
Country Status (21)
| Country | Link |
|---|---|
| US (4) | US9604938B2 (en) |
| EP (1) | EP2744501B1 (en) |
| JP (1) | JP6090801B2 (en) |
| KR (1) | KR20140054246A (en) |
| CN (1) | CN103874495B (en) |
| AU (1) | AU2012296411B2 (en) |
| BR (1) | BR112014003693A2 (en) |
| CA (1) | CA2845630C (en) |
| CL (1) | CL2014000398A1 (en) |
| CO (1) | CO6880068A2 (en) |
| CR (1) | CR20140079A (en) |
| DO (1) | DOP2014000033A (en) |
| EA (1) | EA025436B1 (en) |
| ES (1) | ES2615749T3 (en) |
| IL (1) | IL230902A0 (en) |
| MA (1) | MA35434B1 (en) |
| MX (1) | MX2014001879A (en) |
| PE (1) | PE20141408A1 (en) |
| SG (1) | SG2014009526A (en) |
| TW (1) | TWI547494B (en) |
| WO (1) | WO2013025958A1 (en) |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014043446A1 (en) | 2012-09-13 | 2014-03-20 | Glaxosmithkline Llc | Prodrugs of amino quinazoline kinase inhibitor |
| WO2014043437A1 (en) | 2012-09-13 | 2014-03-20 | Glaxosmithkline Llc | Amino-quinolines as kinase inhibitors |
| JP2014515730A (en) * | 2011-03-04 | 2014-07-03 | グラクソスミスクライン、インテレクチュアル、プロパティー、ナンバー2、リミテッド | Aminoquinolines as kinase inhibitors |
| WO2014128622A1 (en) | 2013-02-21 | 2014-08-28 | Glaxosmithkline Intellectual Property Development Limited | Quinazolines as kinase inhibitors |
| WO2014140299A1 (en) * | 2013-03-15 | 2014-09-18 | Oncodesign S.A | Macrocyclic rip2 kinase inhibitors |
| WO2016042087A1 (en) * | 2014-09-17 | 2016-03-24 | Oncodesign S.A. | Macrocyclic rip2 kinase inhibitors |
| WO2016172134A2 (en) | 2015-04-22 | 2016-10-27 | Glaxosmithkline Intellectual Property Development Limited | Novel compounds |
| WO2017046036A1 (en) | 2015-09-14 | 2017-03-23 | Glaxosmithkline Intellectual Property Development Limited | Compounds for the modulation of rip2 kinase activity |
| US9604938B2 (en) | 2011-08-18 | 2017-03-28 | Glaxosmithkline Intellectual Property Development Limited | Amino quinazolines as kinase inhibitors |
| WO2017182418A1 (en) | 2016-04-20 | 2017-10-26 | Glaxosmithkline Intellectual Property Development Limited | Conjugates comprising ripk2 inhibitors |
| WO2018130443A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2018130437A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2020043122A1 (en) * | 2018-08-28 | 2020-03-05 | 南京明德新药研发有限公司 | Quinazoline derivatives as rip2 kinase inhibitor |
| WO2020094613A1 (en) | 2018-11-06 | 2020-05-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Nod2 inhibitors for the treatment of hereditary periodic fevers |
| WO2022002818A1 (en) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2024059169A1 (en) * | 2022-09-14 | 2024-03-21 | Blueprint Medicines Corporation | Egfr inhibitors |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI730959B (en) | 2015-05-19 | 2021-06-21 | 英商葛蘭素史克智慧財產發展有限公司 | Heterocyclic amides as kinase inhibitors |
| JP7124266B2 (en) | 2020-05-28 | 2022-08-24 | 幸水 深谷 | Citrus fruit manufacturing method and citrus fruit |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993007124A1 (en) | 1991-09-30 | 1993-04-15 | Eisai Co., Ltd. | Nitrogenous heterocyclic compound |
| CA2086968A1 (en) | 1992-01-20 | 1993-07-21 | Andrew John Barker | Quinazoline derivatives |
| WO1996009294A1 (en) | 1994-09-19 | 1996-03-28 | The Wellcome Foundation Limited | Substituted heteroaromatic compounds and their use in medicine |
| US6046206A (en) | 1995-06-07 | 2000-04-04 | Cell Pathways, Inc. | Method of treating a patient having a precancerous lesions with amide quinazoline derivatives |
| EP1199070A2 (en) | 2000-10-20 | 2002-04-24 | Pfizer Limited | Use of PDE V inhibitors for improved fecundity in mammals |
| US6589758B1 (en) | 2000-05-19 | 2003-07-08 | Amgen Inc. | Crystal of a kinase-ligand complex and methods of use |
| WO2008033749A2 (en) | 2006-09-11 | 2008-03-20 | Curis, Inc. | Quinazoline based egfr inhibitors containing a zinc binding moiety |
| WO2008033747A2 (en) | 2006-09-11 | 2008-03-20 | Curis, Inc. | Multi-functional small molecules as anti-proliferative agents |
| WO2009080200A1 (en) | 2007-12-20 | 2009-07-02 | Bayer Schering Pharma Aktiengesellschaft | Novel sulphoximide-substituted quinoline and quinazoline derivatives as kinase inhibitors |
| WO2011011522A2 (en) | 2009-07-21 | 2011-01-27 | President And Fellows Of Harvard College | Potent small molecule inhibitors of autophagy, and methods of use thereof |
| WO2011120025A1 (en) | 2010-03-26 | 2011-09-29 | Glaxo Group Limited | Indazolyl-pyrimidines as kinase inhibitors |
Family Cites Families (79)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4916135A (en) | 1989-05-08 | 1990-04-10 | Hoechst Roussel Pharmaceuticals Inc. | N-heteroaryl-4-quinolinamines |
| US5710158A (en) | 1991-05-10 | 1998-01-20 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Aryl and heteroaryl quinazoline compounds which inhibit EGF and/or PDGF receptor tyrosine kinase |
| JP2994165B2 (en) * | 1992-06-26 | 1999-12-27 | ゼネカ・リミテッド | Quinazoline derivative, method for producing the same, and pharmaceutical preparation containing the quinazoline derivative for obtaining anticancer activity |
| US5747498A (en) | 1996-05-28 | 1998-05-05 | Pfizer Inc. | Alkynyl and azido-substituted 4-anilinoquinazolines |
| PT912559E (en) | 1996-07-13 | 2003-03-31 | Glaxo Group Ltd | HETEROCYCLIC COMPOUNDS FUSED AS PROTEIN INHIBITORS TYROSINE KINASE |
| WO1998005647A1 (en) | 1996-08-01 | 1998-02-12 | Dow Agrosciences Llc | Quinolinium derivatives having fungicidal activity |
| NZ334125A (en) | 1996-09-25 | 2000-10-27 | Zeneca Ltd | Quinoline derivatives inhibiting the effect of growth factors such as VEGF |
| UA73073C2 (en) | 1997-04-03 | 2005-06-15 | Уайт Холдінгз Корпорейшн | Substituted 3-cyan chinolines |
| RS49779B (en) * | 1998-01-12 | 2008-06-05 | Glaxo Group Limited, | Byciclic heteroaromatic compounds as protein tyrosine kinase inhibitors |
| EP1117653B1 (en) | 1998-10-01 | 2003-02-05 | AstraZeneca AB | Quinoline and quinazoline derivatives and their use as inhibitors of cytokine mediated diseases |
| GB2345486A (en) | 1999-01-11 | 2000-07-12 | Glaxo Group Ltd | Heteroaromatic protein tyrosine kinase inhibitors |
| CA2384291A1 (en) | 1999-09-21 | 2001-03-29 | Astrazeneca Ab | Quinazoline derivatives and their use as pharmaceuticals |
| WO2001055116A2 (en) | 2000-01-28 | 2001-08-02 | Astrazeneca Ab | Quinoline derivatives and their use as aurora 2 kinase inhibitors |
| US20030004174A9 (en) | 2000-02-17 | 2003-01-02 | Armistead David M. | Kinase inhibitors |
| US6521618B2 (en) | 2000-03-28 | 2003-02-18 | Wyeth | 3-cyanoquinolines, 3-cyano-1,6-naphthyridines, and 3-cyano-1,7-naphthyridines as protein kinase inhibitors |
| JP2004505964A (en) | 2000-08-09 | 2004-02-26 | アストラゼネカ アクチボラグ | Quinoline derivatives having VEGF inhibitory activity |
| US6548508B2 (en) | 2000-10-20 | 2003-04-15 | Pfizer, Inc. | Use of PDE V inhibitors for improved fecundity in mammals |
| GB0104422D0 (en) | 2001-02-22 | 2001-04-11 | Glaxo Group Ltd | Quinoline derivative |
| PE20020958A1 (en) | 2001-03-23 | 2002-11-14 | Bayer Corp | RHO-KINASE INHIBITORS |
| HN2002000067A (en) | 2001-03-23 | 2003-10-24 | Bayer Healthcare Llc | INHIBITORS OF THE RHO - QUINASA. |
| SE0101675D0 (en) | 2001-05-11 | 2001-05-11 | Astrazeneca Ab | Novel composition |
| US7115617B2 (en) | 2001-08-22 | 2006-10-03 | Amgen Inc. | Amino-substituted pyrimidinyl derivatives and methods of use |
| WO2003026664A1 (en) | 2001-09-26 | 2003-04-03 | Bayer Corporation | 2-phenylamino-4- (5-pyrazolylamino) -pyramidine derivatives as kinase inhibitors, in particular, src kinase inhibitors |
| CN100491372C (en) | 2001-12-24 | 2009-05-27 | 阿斯特拉曾尼卡有限公司 | Substituted quinazoline derivatives as inhibitors of aurora kinases |
| US7645878B2 (en) | 2002-03-22 | 2010-01-12 | Bayer Healthcare Llc | Process for preparing quinazoline Rho-kinase inhibitors and intermediates thereof |
| TWI275390B (en) | 2002-04-30 | 2007-03-11 | Wyeth Corp | Process for the preparation of 7-substituted-3- quinolinecarbonitriles |
| WO2004030672A1 (en) * | 2002-10-02 | 2004-04-15 | Merck Patent Gmbh | Use of 4 amino-quinazolines as anti cancer agents |
| US7262200B2 (en) | 2002-10-25 | 2007-08-28 | Vertex Pharmaceuticals Incorporated | Indazolinone compositions useful as kinase inhibitors |
| US20040122161A1 (en) | 2002-12-21 | 2004-06-24 | Paul Charles W. | Hot melt adhesive based on acrylic block copolymers |
| RU2357971C2 (en) | 2002-12-24 | 2009-06-10 | Астразенека Аб | Phosphonoxyquinazoline derivatives and their pharmaceutical application |
| TWI328009B (en) | 2003-05-21 | 2010-08-01 | Glaxo Group Ltd | Quinoline derivatives as phosphodiesterase inhibitors |
| JP2007501854A (en) | 2003-05-27 | 2007-02-01 | ファイザー・プロダクツ・インク | Quinazolines and pyrido [3,4-D] pyrimidines as receptor tyrosine kinase inhibitors |
| NZ544472A (en) | 2003-07-03 | 2009-04-30 | Myriad Genetics Inc | Compounds and therapeutical use thereof |
| CA2538884C (en) | 2003-09-16 | 2010-09-21 | Astrazeneca Ab | Quinazoline derivatives as tyrosine kinase inhibitors |
| EP1673085B1 (en) | 2003-09-26 | 2011-11-09 | Exelixis, Inc. | C-met modulators and methods of use |
| AU2005249503B2 (en) | 2003-11-10 | 2011-08-25 | Vertex Pharmaceuticals Incorporated | ICE inhibitors for the treatment of autoinflammatory diseases |
| EP1699780A1 (en) | 2003-12-23 | 2006-09-13 | Pfizer, Inc. | Novel quinoline derivatives |
| CN1934100A (en) | 2004-03-23 | 2007-03-21 | 万有制药株式会社 | Substituted quinazoline or pyridopyrimidine derivative |
| WO2005115145A2 (en) | 2004-05-20 | 2005-12-08 | Wyeth | Quinone substituted quinazoline and quinoline kinase inhibitors |
| EP1781293A1 (en) | 2004-06-04 | 2007-05-09 | Amphora Discovery Corporation | Quinoline- and isoquinoline-based compounds exhibiting atp-utilizing enzyme inhibitory activity, and compositions, and uses thereof |
| FR2873695A1 (en) | 2004-07-30 | 2006-02-03 | Palumed Sa | HYBRID MOLECULES QA OR Q IS AMINOQUINOLINE AND A IS AN ANTIBIOTIC OR A RESISTANCE INHIBITOR), THEIR SYNTHESIS AND USES THEREOF AS ANTIBACTERIAL AGENT |
| WO2006066955A1 (en) | 2004-12-22 | 2006-06-29 | Bayer Schering Pharma Aktiengesellschaft | Quinoline derivative, use and production thereof, and drug containing the same |
| US8258145B2 (en) | 2005-01-03 | 2012-09-04 | Myrexis, Inc. | Method of treating brain cancer |
| EP1874759A4 (en) | 2005-04-06 | 2009-07-15 | Exelixis Inc | C-met modulators and methods of use |
| JO2787B1 (en) | 2005-04-27 | 2014-03-15 | امجين إنك, | Substituted Amid derivatives & methods of use |
| EP1746096A1 (en) | 2005-07-15 | 2007-01-24 | 4Sc Ag | 2-Arylbenzothiazole analogues and uses thereof in the treatment of cancer |
| ITMI20052008A1 (en) | 2005-10-21 | 2007-04-22 | Ctg Pharma S R L | NEW ANTIMALARIC DERIVATIVES OF 4-AMINOCHINOLINA |
| FR2902100A1 (en) | 2006-06-13 | 2007-12-14 | Sanofi Aventis Sa | DUAL MOLECULES COMPRISING A PEROXYDIC DERIVATIVE, THEIR SYNTHESIS AND THEIR THERAPEUTIC APPLICATIONS |
| US7511063B2 (en) | 2006-08-16 | 2009-03-31 | Schering Corporation | High affinity quinoline-based kinase ligands |
| JP2008063278A (en) | 2006-09-07 | 2008-03-21 | Fujifilm Finechemicals Co Ltd | Method for producing 1-pyridin-4-yl-indole |
| US7977347B2 (en) | 2006-09-11 | 2011-07-12 | Curis, Inc. | Quinazoline based EGFR inhibitors |
| TW200829555A (en) | 2006-11-10 | 2008-07-16 | Astrazeneca Ab | Chemical compounds |
| US8148532B2 (en) | 2007-03-14 | 2012-04-03 | Guoqing Paul Chen | Spiro substituted compounds as angiogenesis inhibitors |
| US8163923B2 (en) | 2007-03-14 | 2012-04-24 | Advenchen Laboratories, Llc | Spiro substituted compounds as angiogenesis inhibitors |
| US20080234267A1 (en) | 2007-03-20 | 2008-09-25 | Karen Elizabeth Lackey | Compounds and Methods of Treatment |
| WO2008119771A2 (en) | 2007-03-30 | 2008-10-09 | Clanotech Ab | Quinoline-s-carboxylic acid derivatives as tyrosine kinase inhibitors |
| CN101663293B (en) | 2007-04-23 | 2013-07-31 | 塞诺菲-安万特股份有限公司 | Quinoline-carboxamide derivatives as p2y12 antagonists |
| PE20090717A1 (en) | 2007-05-18 | 2009-07-18 | Smithkline Beecham Corp | QUINOLINE DERIVATIVES AS PI3 KINASE INHIBITORS |
| WO2008150957A2 (en) | 2007-06-01 | 2008-12-11 | Wyeth | Treatment of imatinib resistant leukemia using 4-aminoquinoline-3-carbonitriles |
| CN101362719B (en) | 2007-08-06 | 2012-04-18 | 北京师范大学 | Chinolines derivates and composition containing thereof |
| US7939546B2 (en) | 2007-10-12 | 2011-05-10 | Supergen, Inc. | Quinoline derivatives for modulating DNA methylation |
| US7790746B2 (en) | 2007-10-12 | 2010-09-07 | Supergen, Inc. | Quinoline derivatives for modulating DNA methylation |
| US9028693B2 (en) | 2007-12-07 | 2015-05-12 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Apparatus for countercurrent chromatography |
| GB0801416D0 (en) | 2008-01-25 | 2008-03-05 | Piramed Ltd | Pharmaceutical compounds |
| WO2009111691A2 (en) | 2008-03-06 | 2009-09-11 | Genentech, Inc. | Combination therapy with c-met and egfr antagonists |
| CA2739302A1 (en) * | 2008-10-17 | 2010-04-22 | Brendan C. Bender | Treatment method |
| DE102008062566A1 (en) | 2008-12-16 | 2010-06-17 | Bayer Schering Pharma Aktiengesellschaft | Amino acid ester prodrugs and their use |
| WO2011112588A2 (en) | 2010-03-08 | 2011-09-15 | Case Western Reserve University | Compositions and methods for treating inflammatory disorders |
| WO2011120026A1 (en) | 2010-03-26 | 2011-09-29 | Glaxo Group Limited | Pyrazolyl-pyrimidines as kinase inhibitors |
| WO2011123609A1 (en) | 2010-03-31 | 2011-10-06 | Glaxo Group Limited | Imidazolyl-imidazoles as kinase inhibitors |
| WO2011140442A1 (en) | 2010-05-07 | 2011-11-10 | Glaxo Group Limited | Amino-quinolines as kinase inhibitors |
| UY33549A (en) | 2010-08-10 | 2012-01-31 | Glaxo Group Ltd | QUINOLIL AMINAS AS INHIBITING AGENTS OF KINASES |
| US8609672B2 (en) | 2010-08-27 | 2013-12-17 | University Of The Pacific | Piperazinylpyrimidine analogues as protein kinase inhibitors |
| EP2680886B1 (en) | 2011-02-28 | 2016-08-10 | Calitor Sciences, LLC | Substituted quinoline compounds |
| ES2609578T3 (en) | 2011-03-04 | 2017-04-21 | Glaxosmithkline Intellectual Property Development Limited | Amino-quinolines as kinase inhibitors |
| TWI547494B (en) * | 2011-08-18 | 2016-09-01 | 葛蘭素史克智慧財產發展有限公司 | Amino quinazolines as kinase inhibitors |
| AR092529A1 (en) | 2012-09-13 | 2015-04-22 | Glaxosmithkline Llc | AMINOQUINAZOLINE COMPOUND, PHARMACEUTICAL COMPOSITION THAT INCLUDES IT AND USE OF THIS COMPOSITE FOR THE PREPARATION OF A MEDICINAL PRODUCT |
| TW201425307A (en) | 2012-09-13 | 2014-07-01 | Glaxosmithkline Llc | Amino-quinolines as kinase inhibitors |
| TWI630203B (en) | 2013-02-21 | 2018-07-21 | 葛蘭素史克智慧財產發展有限公司 | Quinazolines as kinase inhibitors |
-
2012
- 2012-08-16 TW TW101129650A patent/TWI547494B/en not_active IP Right Cessation
- 2012-08-17 WO PCT/US2012/051247 patent/WO2013025958A1/en active Application Filing
- 2012-08-17 KR KR1020147006731A patent/KR20140054246A/en not_active Application Discontinuation
- 2012-08-17 BR BR112014003693A patent/BR112014003693A2/en not_active Application Discontinuation
- 2012-08-17 MX MX2014001879A patent/MX2014001879A/en unknown
- 2012-08-17 US US14/239,193 patent/US9604938B2/en active Active
- 2012-08-17 EA EA201490474A patent/EA025436B1/en not_active IP Right Cessation
- 2012-08-17 JP JP2014526237A patent/JP6090801B2/en not_active Expired - Fee Related
- 2012-08-17 CN CN201280050811.1A patent/CN103874495B/en not_active Expired - Fee Related
- 2012-08-17 PE PE2014000212A patent/PE20141408A1/en not_active Application Discontinuation
- 2012-08-17 SG SG2014009526A patent/SG2014009526A/en unknown
- 2012-08-17 AU AU2012296411A patent/AU2012296411B2/en not_active Ceased
- 2012-08-17 CA CA2845630A patent/CA2845630C/en not_active Expired - Fee Related
- 2012-08-17 EP EP12824263.3A patent/EP2744501B1/en active Active
- 2012-08-17 ES ES12824263.3T patent/ES2615749T3/en active Active
-
2014
- 2014-02-10 IL IL230902A patent/IL230902A0/en unknown
- 2014-02-14 CO CO14032108A patent/CO6880068A2/en active IP Right Grant
- 2014-02-18 CR CR20140079A patent/CR20140079A/en unknown
- 2014-02-18 DO DO2014000033A patent/DOP2014000033A/en unknown
- 2014-02-18 CL CL2014000398A patent/CL2014000398A1/en unknown
- 2014-03-12 MA MA36820A patent/MA35434B1/en unknown
-
2017
- 2017-02-14 US US15/431,942 patent/US9994529B2/en not_active Expired - Fee Related
-
2018
- 2018-05-14 US US15/978,377 patent/US20180258056A1/en not_active Abandoned
-
2019
- 2019-02-27 US US16/286,920 patent/US10717711B2/en not_active Expired - Fee Related
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993007124A1 (en) | 1991-09-30 | 1993-04-15 | Eisai Co., Ltd. | Nitrogenous heterocyclic compound |
| CA2086968A1 (en) | 1992-01-20 | 1993-07-21 | Andrew John Barker | Quinazoline derivatives |
| WO1996009294A1 (en) | 1994-09-19 | 1996-03-28 | The Wellcome Foundation Limited | Substituted heteroaromatic compounds and their use in medicine |
| US6046206A (en) | 1995-06-07 | 2000-04-04 | Cell Pathways, Inc. | Method of treating a patient having a precancerous lesions with amide quinazoline derivatives |
| US6589758B1 (en) | 2000-05-19 | 2003-07-08 | Amgen Inc. | Crystal of a kinase-ligand complex and methods of use |
| EP1199070A2 (en) | 2000-10-20 | 2002-04-24 | Pfizer Limited | Use of PDE V inhibitors for improved fecundity in mammals |
| WO2008033749A2 (en) | 2006-09-11 | 2008-03-20 | Curis, Inc. | Quinazoline based egfr inhibitors containing a zinc binding moiety |
| WO2008033747A2 (en) | 2006-09-11 | 2008-03-20 | Curis, Inc. | Multi-functional small molecules as anti-proliferative agents |
| WO2009080200A1 (en) | 2007-12-20 | 2009-07-02 | Bayer Schering Pharma Aktiengesellschaft | Novel sulphoximide-substituted quinoline and quinazoline derivatives as kinase inhibitors |
| WO2011011522A2 (en) | 2009-07-21 | 2011-01-27 | President And Fellows Of Harvard College | Potent small molecule inhibitors of autophagy, and methods of use thereof |
| WO2011120025A1 (en) | 2010-03-26 | 2011-09-29 | Glaxo Group Limited | Indazolyl-pyrimidines as kinase inhibitors |
Non-Patent Citations (39)
| Title |
|---|
| AM. J. HUM. GENET., vol. 70, 2002, pages 845 - 857 |
| AMERICAN JOURNAL OF OPHTHALMOLOGY, vol. 142, 2006, pages 1089 - 1092 |
| ARTHRITIS & RHEUMATISM, vol. 54, 2006, pages 3337 - 3344 |
| ARTHRITIS & RHEUMATISM, vol. 60, 2009, pages 1797 - 1803 |
| BERGE; BIGHLEY; MONKHOUSE, J.PHARM.SCI, vol. 66, 1977, pages 1 - 19 |
| BIOCHEM J404, 2007, pages 179 - 190 |
| BLOOD, vol. 105, 2005, pages 1195 - 1197 |
| BRITISH MEDICAL BULLETIN, vol. 87, 2008, pages 17 - 30 |
| CELLULAR SIGNALLING, vol. 18, 2006, pages 2223 - 2229 |
| CURRENT BIOLOGY, vol. 8, 1998, pages 885 - 889 |
| CURRENT RHEUMATOLOGY REPORTS, vol. 7, 2005, pages 427 - 433 |
| DIAGNOSTIC PATHOLOGY, vol. 4, 2009, pages 23 |
| EMBO JOURNAL, vol. 27, 2008, pages 373 - 383 |
| EMBO REPORTS, vol. 2, 2001, pages 736 - 742 |
| EUROPEAN JOURNAL OF HUMAN GENETICS, vol. 12, 2004, pages 206 - 212 |
| EUROPEAN JOURNAL OF HUMAN GENETICS, vol. 13, 2005, pages 742 - 747 |
| EXPERIMENTAL DERMATOLOGY, vol. 17, 2008, pages 1057 - 1058 |
| HUM. MOL. GENET, vol. 14, 2005, pages 1245 - 1250 |
| HUM. MOL. GENET., vol. 14, 2005, pages 935 - 941 |
| INFLAMMATORY BOWEL DISEASES, vol. 14, 2008, pages 295 - 302 |
| INFLAMMATORY BOWEL DISEASES, vol. 15, 2009, pages 1145 - 1154 |
| J. BIOL. CHEM., vol. 273, 1998, pages 12296 - 12300 |
| J. BIOL. CHEM., vol. 273, 1998, pages 16968 - 16975 |
| J. BIOL. CHEM., vol. 275, 2000, pages 27823 - 27831 |
| J. BIOL. CHEM., vol. 284, 2009, pages 19183 - 19188 |
| J. MED. CHEM., vol. 53, no. 3, 2010, pages 2000 - 2009 |
| JLMMUNOL, vol. 178, 2007, pages 2380 - 2386 |
| JOURNAL OF CLINICAL IMMUNOLOGY, vol. 29, 2009, pages 78 - 89 |
| JOURNAL OF RHEUMATOLOGY, vol. 32, 2005, pages 373 - 375 |
| MANON ET AL., J. MOL. BIOL., vol. 365, 2007, pages 160 - 174 |
| MANON ET AL.: "Solution Structure of NOD1 CARD and Mutational Analysis of its Interaction with the CARD of Downstream Kinase RICK", JOURNAL OF MOLECULAR BIOLOGY, vol. 365, no. 1, 2007, pages 160 - 174, XP005728215 * |
| MICROBES AND INFECTION, vol. 11, 2009, pages 912 - 918 |
| MUCOSAL IMMUNOLOGY, vol. 1, no. 1, 2008 |
| NATURE GENETICS, vol. 29, 2001, pages 19 - 20 |
| NATURE REVIEWS IMMUNOLOGY, vol. 6, 2006, pages 9 - 20 |
| RHEUMATOLOGY, vol. 49, 2010, pages 194 - 196 |
| ROSENWEIG, H. L. ET AL., JOURNAL OF LEUKOCYTE BIOLOGY, vol. 84, 2008, pages 529 - 536 |
| SARCOIDOSIS VASCULITIS AND DIFFUSE LUNG DISEASES, vol. 23, 2006, pages 23 - 29 |
| See also references of EP2744501A4 |
Cited By (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014515730A (en) * | 2011-03-04 | 2014-07-03 | グラクソスミスクライン、インテレクチュアル、プロパティー、ナンバー2、リミテッド | Aminoquinolines as kinase inhibitors |
| US10220030B2 (en) | 2011-03-04 | 2019-03-05 | Glaxosmithkline Intellectual Property Development Limited | Amino-quinolines as kinase inhibitors |
| US9604963B2 (en) | 2011-03-04 | 2017-03-28 | Glaxosmithkline Intellectual Property Development Limited | Amino-quinolines as kinase inhibitors |
| US9994529B2 (en) | 2011-08-18 | 2018-06-12 | Glaxosmithkline Intellectual Property Development Limited | Amino quinazolines as kinase inhibitors |
| US9604938B2 (en) | 2011-08-18 | 2017-03-28 | Glaxosmithkline Intellectual Property Development Limited | Amino quinazolines as kinase inhibitors |
| US10717711B2 (en) | 2011-08-18 | 2020-07-21 | Glaxosmithkline Intellectual Property Development Limited | Amino quinazolines as kinase inhibitors |
| US9216965B2 (en) | 2012-09-13 | 2015-12-22 | Glaxosmithkline Intellectual Property Development Limited | Amino-quinolines as kinase inhibitors |
| JP2015529681A (en) * | 2012-09-13 | 2015-10-08 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッドGlaxosmithkline Intellectual Property Development Limited | Prodrugs of aminoquinazoline kinase inhibitors |
| EP2895174A1 (en) * | 2012-09-13 | 2015-07-22 | GlaxoSmithKline Intellectual Property Development Limited | Prodrugs of amino quinazoline kinase inhibitor |
| EP3323819A1 (en) | 2012-09-13 | 2018-05-23 | GlaxoSmithKline Intellectual Property Development Limited | Prodrugs of amino quinazoline kinase inhibitor |
| EP2895167A4 (en) * | 2012-09-13 | 2016-03-16 | Glaxosmithkline Ip Dev Ltd | Amino-quinolines as kinase inhibitors |
| WO2014043446A1 (en) | 2012-09-13 | 2014-03-20 | Glaxosmithkline Llc | Prodrugs of amino quinazoline kinase inhibitor |
| EP2895174A4 (en) * | 2012-09-13 | 2016-03-30 | Glaxosmithkline Ip Dev Ltd | Prodrugs of amino quinazoline kinase inhibitor |
| US9695161B2 (en) | 2012-09-13 | 2017-07-04 | Glaxosmithkline Intellectual Property Development Limited | Prodrugs of amino quinazoline kinase inhibitor |
| US9586953B2 (en) | 2012-09-13 | 2017-03-07 | Glaxosmithkline Intellectual Property Development Limited | Prodrugs of amino quinazoline kinase inhibitor |
| WO2014043437A1 (en) | 2012-09-13 | 2014-03-20 | Glaxosmithkline Llc | Amino-quinolines as kinase inhibitors |
| CN105143208B (en) * | 2013-02-21 | 2017-09-26 | 葛兰素史密斯克莱知识产权发展有限公司 | It is used as the quinazoline of kinase inhibitor |
| US9650364B2 (en) | 2013-02-21 | 2017-05-16 | GlaxoSmithKline Intellectual Property Development Limted | Quinazolines as kinase inhibitors |
| WO2014128622A1 (en) | 2013-02-21 | 2014-08-28 | Glaxosmithkline Intellectual Property Development Limited | Quinazolines as kinase inhibitors |
| CN105143208A (en) * | 2013-02-21 | 2015-12-09 | 葛兰素史密斯克莱知识产权发展有限公司 | Quinazolines as kinase inhibitors |
| WO2014140299A1 (en) * | 2013-03-15 | 2014-09-18 | Oncodesign S.A | Macrocyclic rip2 kinase inhibitors |
| CN105228625A (en) * | 2013-03-15 | 2016-01-06 | 昂科迪塞恩股份有限公司 | Macro ring RIP2 inhibitors of kinases |
| WO2016042087A1 (en) * | 2014-09-17 | 2016-03-24 | Oncodesign S.A. | Macrocyclic rip2 kinase inhibitors |
| JP2017528481A (en) * | 2014-09-17 | 2017-09-28 | オンコデザイン エス.ア. | Macrocyclic RIP2 kinase inhibitor |
| US10676486B2 (en) | 2014-09-17 | 2020-06-09 | Oncodesign S.A. | Macrocyclic RIP2 kinase inhibitors |
| EA032872B1 (en) * | 2014-09-17 | 2019-07-31 | Онкодизайн С.А. | Macrocyclic rip2 kinase inhibitors |
| WO2016172134A2 (en) | 2015-04-22 | 2016-10-27 | Glaxosmithkline Intellectual Property Development Limited | Novel compounds |
| WO2017046036A1 (en) | 2015-09-14 | 2017-03-23 | Glaxosmithkline Intellectual Property Development Limited | Compounds for the modulation of rip2 kinase activity |
| CN109311867A (en) * | 2016-04-20 | 2019-02-05 | 葛兰素史克知识产权开发有限公司 | Conjugate comprising RIPK2 inhibitor |
| AU2017253560B2 (en) * | 2016-04-20 | 2019-03-21 | Glaxosmithkline Intellectual Property Development Limited | Conjugates comprising RIPK2 inhibitors |
| US20190119271A1 (en) * | 2016-04-20 | 2019-04-25 | Glaxosmithkline Intellectual Property Development Limited | Conjugates comprising ripk2 inhibitors |
| WO2017182418A1 (en) | 2016-04-20 | 2017-10-26 | Glaxosmithkline Intellectual Property Development Limited | Conjugates comprising ripk2 inhibitors |
| US10781205B2 (en) | 2016-04-20 | 2020-09-22 | Glaxosmithkline Intellectual Property Development Limited | Conjugates comprising RIPK2 inhibitors |
| WO2018130437A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2018130443A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2020043122A1 (en) * | 2018-08-28 | 2020-03-05 | 南京明德新药研发有限公司 | Quinazoline derivatives as rip2 kinase inhibitor |
| CN112638905A (en) * | 2018-08-28 | 2021-04-09 | 南京明德新药研发有限公司 | Quinazoline derivatives as RIP2 kinase inhibitors |
| WO2020094613A1 (en) | 2018-11-06 | 2020-05-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Nod2 inhibitors for the treatment of hereditary periodic fevers |
| WO2022002818A1 (en) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2024059169A1 (en) * | 2022-09-14 | 2024-03-21 | Blueprint Medicines Corporation | Egfr inhibitors |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10717711B2 (en) | Amino quinazolines as kinase inhibitors | |
| US10220030B2 (en) | Amino-quinolines as kinase inhibitors | |
| EP2603218B1 (en) | Quinolyl amines as kinase inhibitors | |
| AU2013315387B2 (en) | Amino-quinolines as kinase inhibitors | |
| PH12015500363B1 (en) | Prodrugs of amino quinazoline kinase inhibitor | |
| NZ621314B2 (en) | Amino quinazolines as kinase inhibitors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201280050811.1 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12824263 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 230902 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14032108 Country of ref document: CO Ref document number: 000212-2014 Country of ref document: PE |
|
| ENP | Entry into the national phase |
Ref document number: 2014526237 Country of ref document: JP Kind code of ref document: A Ref document number: 2845630 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14239193 Country of ref document: US Ref document number: MX/A/2014/001879 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014000398 Country of ref document: CL Ref document number: CR2014-000079 Country of ref document: CR |
|
| ENP | Entry into the national phase |
Ref document number: 2012296411 Country of ref document: AU Date of ref document: 20120817 Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2012824263 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012824263 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20147006731 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201490474 Country of ref document: EA |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014003693 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112014003693 Country of ref document: BR Kind code of ref document: A2 Effective date: 20140217 |