WO2012154564A1 - Fatty acid phenolic derivatives and their uses - Google Patents
Fatty acid phenolic derivatives and their uses Download PDFInfo
- Publication number
- WO2012154564A1 WO2012154564A1 PCT/US2012/036533 US2012036533W WO2012154564A1 WO 2012154564 A1 WO2012154564 A1 WO 2012154564A1 US 2012036533 W US2012036533 W US 2012036533W WO 2012154564 A1 WO2012154564 A1 WO 2012154564A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- independently
- optionally substituted
- compound
- disease
- Prior art date
Links
- 0 C*CN(C(CC1(C)C)=O)c(cc2)c1cc2C(C=Cc1ccc(*C)cc1)=O Chemical compound C*CN(C(CC1(C)C)=O)c(cc2)c1cc2C(C=Cc1ccc(*C)cc1)=O 0.000 description 20
- DGXROCKRBSATGS-UHFFFAOYSA-N CC(CCCCC1NNCC1)=O Chemical compound CC(CCCCC1NNCC1)=O DGXROCKRBSATGS-UHFFFAOYSA-N 0.000 description 2
- DQFBYFPFKXHELB-VAWYXSNFSA-N O=C(/C=C/c1ccccc1)c1ccccc1 Chemical compound O=C(/C=C/c1ccccc1)c1ccccc1 DQFBYFPFKXHELB-VAWYXSNFSA-N 0.000 description 2
- WGTSTVPYMZECLD-KUBAVDMBSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCCCC(C(O)=O)NC(c(cc1O)cc(O)c1O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCCCC(C(O)=O)NC(c(cc1O)cc(O)c1O)=O)=O WGTSTVPYMZECLD-KUBAVDMBSA-N 0.000 description 1
- IDMBWLZLKFRSTM-QQPOCBCUSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCCCC(C(O)=O)NC(c1ccc(/C=C/C(c2cc(C(C)(C)CCC3(C)C)c3cc2)=O)cc1)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCCCC(C(O)=O)NC(c1ccc(/C=C/C(c2cc(C(C)(C)CCC3(C)C)c3cc2)=O)cc1)=O)=O IDMBWLZLKFRSTM-QQPOCBCUSA-N 0.000 description 1
- JGCJEWMLWCNPBH-KUBAVDMBSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCOCCNC(Oc(cc1)cc2c1OC(c1ccccc1)=CC2=O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCOCCNC(Oc(cc1)cc2c1OC(c1ccccc1)=CC2=O)=O)=O JGCJEWMLWCNPBH-KUBAVDMBSA-N 0.000 description 1
- YFOMLVIULCIJNT-ZKFUEKSZSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCOCCNC(Oc(ccc(/C=C/C(CC(/C=C/c(cc1OC)ccc1O)=O)=O)c1)c1OC)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(NCCOCCNC(Oc(ccc(/C=C/C(CC(/C=C/c(cc1OC)ccc1O)=O)=O)c1)c1OC)=O)=O YFOMLVIULCIJNT-ZKFUEKSZSA-N 0.000 description 1
- RFFMKEMHOBBIPX-JLNKQSITSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCCCC(C(O)=O)NC(c(cc1O)cc(O)c1O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCCCC(C(O)=O)NC(c(cc1O)cc(O)c1O)=O)=O RFFMKEMHOBBIPX-JLNKQSITSA-N 0.000 description 1
- WDJXJGPZJFJPSE-JLNKQSITSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCOCCNC(c(cc1O)cc(O)c1O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCOCCNC(c(cc1O)cc(O)c1O)=O)=O WDJXJGPZJFJPSE-JLNKQSITSA-N 0.000 description 1
- LDJSAATVTNXGNX-XBXARRHUSA-N COCCOCOc(c(C1C2CC(C3)CC1CC3C2)c1)ccc1C(/C=C/c(cc1)ccc1C(O)=O)=O Chemical compound COCCOCOc(c(C1C2CC(C3)CC1CC3C2)c1)ccc1C(/C=C/c(cc1)ccc1C(O)=O)=O LDJSAATVTNXGNX-XBXARRHUSA-N 0.000 description 1
- DQKZBIYOJBTONX-KBXRYBNXSA-N COc(ccc(/C=C/C(CC(/C=C/c(cc1)cc(OC)c1O)=O)=O)c1)c1OC Chemical compound COc(ccc(/C=C/C(CC(/C=C/c(cc1)cc(OC)c1O)=O)=O)c1)c1OC DQKZBIYOJBTONX-KBXRYBNXSA-N 0.000 description 1
- VKDKQGICVQMVFN-VQHVLOKHSA-N Cc1ccc(/C=C/C(c(cc2)cc(C3C4CC(C5)CC3CC5C4)c2OCOCCOC)=O)cc1 Chemical compound Cc1ccc(/C=C/C(c(cc2)cc(C3C4CC(C5)CC3CC5C4)c2OCOCCOC)=O)cc1 VKDKQGICVQMVFN-VQHVLOKHSA-N 0.000 description 1
- GEGPABSGZQVUSF-UHFFFAOYSA-N O=C(Oc(cc1)ccc1N=O)Cl Chemical compound O=C(Oc(cc1)ccc1N=O)Cl GEGPABSGZQVUSF-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11C—FATTY ACIDS FROM FATS, OILS OR WAXES; CANDLES; FATS, OILS OR FATTY ACIDS BY CHEMICAL MODIFICATION OF FATS, OILS, OR FATTY ACIDS OBTAINED THEREFROM
- C11C3/00—Fats, oils, or fatty acids by chemical modification of fats, oils, or fatty acids obtained therefrom
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/115—Fatty acids or derivatives thereof; Fats or oils
- A23L33/12—Fatty acids or derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D311/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings
Definitions
- the invention relates to Fatty Acid Phenolic Derivatives; compositions comprising an effective amount of a Fatty Acid Phenolic Derivative; and methods for treating or preventing cancer and metabolic, autoimmune or neurodegenerative disorders, comprising the administration of an effective amount of a Fatty Acid Phenolic Derivative.
- All patents, patent applications, and publications cited herein are hereby incorporated by reference in their entireties.
- Oily cold water fish such as salmon, trout, herring, and tuna are the source of dietary marine omega-3 fatty acids, with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) being the key marine derived omega-3 fatty acids.
- Omega-3 fatty acids have previously been shown to improve insulin sensitivity and glucose tolerance in normoglycemic men and in obese individuals. Omega-3 fatty acids have also been shown to improve insulin resistance in obese and non-obese patients with an inflammatory phenotype. Lipid, glucose, and insulin metabolism have been shown to improve in overweight hypertensive subjects through treatment with omega-3 fatty acids.
- Omega-3 fatty acids have also been shown to decrease triglycerides and to reduce the risk for sudden death caused by cardiac arrhythmias in addition to improve mortality in patients at risk of a cardiovascular event. Omega-3 fatty acids have also been taken as dietary supplements part of therapy used to treat dyslipidemia, and anti-inflammatory properties. A higher intake of omega-3 fatty acids lower levels of circulating TNF-a and IL-6, two of the cytokines that are markedly increased during inflammation processes (Chapkin et al, Prostaglandins, Leukot Essent Fatty Acids 2009, 81, p. 187-191; Duda et al, Cardiovasc Res 2009, 84, p. 33-41).
- omega-3 fatty acids have also been shown to increase levels of the well-characterized anti-inflammatory cytokine IL-10 (Bradley et al, Obesity (Silver Spring) 2008, 16, p. 938-944). More recently, there is additional evidence that omega-3 fatty acids could play a significant role in oncology (Anderson et al, Lipids in Health and Disease 2009, 8, p.33; Bougnoux et al, Progress in Lipid Research 2010, 49, p. 76-86; Erickson et al, Prostaglandins, Leukotrienes and Essential Fatty Acids 2010, 82, p. 237-241).
- Both DHA and EPA are characterized as long chain fatty acids (aliphatic portion between 12-22 carbons).
- Medium chain fatty acids are characterized as those having the aliphatic portion between 6-12 carbons.
- Lipoic acid is a medium chain fatty acid found naturally in the body. It plays many important roles such as free radical scavenger, chelator to heavy metals and signal transduction mediator in various inflammatory and metabolic pathways, including the NF- ⁇ pathway (Shay, K. P. et al. Biochim. Biophys. Acta 2009, 1790, 1149-1160). Lipoic acid has been found to be useful in a number of chronic diseases that are associated with oxidative stress (for a review see Smith, A. R. et al Curr. Med. Chem.
- Lipoic acid has now been evaluated in the clinic for the treatment of diabetes (Morcos, M. et al Diabetes Res. Clin. Pract. 2001, 52, p. 175-183) and diabetic neuropathy (Mijnhout, G. S. et al Neth. J. Med. 2010, 110, p. 158-162). Lipoic acid has also been found to be potentially useful in treating cardiovascular diseases (Ghibu, S. et al, J. Cardiovasc. Pharmacol. 2009, 54, p. 391-8), Alzheimer's disease (Maczurek, A. et al, Adv. Drug Deliv. Rev. 2008, 60, p. 1463-70) and multiple sclerosis (Yadav, V. Multiple Sclerosis
- Gallic acid is a naturally occurring polyhydroxy phenolic compound found in gallnuts, grapes, certain vegetables, tea leaves and oak bark which has been shown to exhibit anti-oxidant, antimutagenic and anticarcinogenic effects.
- Gallic acid has demonstrated oral efficacy in an obesity rat model.
- HFD high fat diet
- animals dosed with gallic acid as an admix in the HDF chow had decreased liver weights as well as reduced adipose tissue (peritoneal fat and epidermal fat) weights when compared to the control animals on HFD alone.
- the HFD + Gallic acid fed rats also exhibited reduced serum triacylglycerol, insulin and lipid levels.
- gallic acid has also been shown in vitro to enhance insulin secretion in RINm5F cells through the upregulation of PDX-1 and Maf-A, two major beta-cell transcription factors.
- flavonoids are phenolic derivatives that have been shown to have beneficial anti-oxidant properties (for a comprehensive review, see: Flavonoids: Chemistry, Biochemistry and Applications 2006, Edited by Anderson, O. M. and Markham, K. R.). Quercetin, for instance, is a flavonoid that has been shown to have protective effects against ⁇ -cell damage in experimental streptozotocin (STZ)-induced diabetic rats (Corkun et al, Pharmacological Research 2005, 51, p. 117-123). Naringenin, epicatechin, myricetin, apigenin are some additional examples of naturally-occurring flavonoids that have some beneficial anti-diabetic properties (G. Brahmachari Opportunities, Challenges and Scope of Natural Products in Medicinal Chemistry 2011, p. 187-212).
- Curcumin is a phenolic derivative, belonging to the chalcone family of natural products, that has been shown to have many beneficial anti-oxidant and anti-inflammatory properties. Curcumin has been shown to protect islets of C57/BL6 mice against streptozotocin-induced oxidative stress (Meghana et al, Eur. J. Pharm. 2007, 577, p. 183- 191). It also has been shown to have antihyperglycemic effect and improved insulin sensitivity in diet-induced obese (DIO) Sprague Dawley rats (El-Moselhy et al, Food and Chemical Toxicology 2011, 49, p. 1129-1140).
- DIO diet-induced obese
- the mechanism of action may be attributed, in part, to the anti-inflammatory properties, as evident by attenuating TNF-a levels in these DIO rats.
- chalcone derivatives have also been shown to have significant anti-inflammatory properties. These include isoliquiritigenin, flavokawain A, cardomonin, butein, licochalcone A, xanthohumol, BMS 181156, AGN 193198 and MX 781 (Srinivasan et al J. Med. Chem. 2009, 52, p. 7228-7235).
- the anti-inflammatory properties can be attributed, in part, to the inhibition of NF- ⁇ through modification of its target(s), such as ⁇ , via a Michael addition onto the unsaturated enone moiety.
- the invention is based in part on the discovery of Fatty Acid Phenolic Derivatives and their demonstrated effects in achieving improved treatment that cannot be achieved by administering a Phenolic derivative or fatty acids alone or in combination.
- the Fatty Acid Phenolic Derivatives are designed to be stable in the plasma.
- the individual components i.e. fatty acid, phenolic derivative
- the action of various intracellular enzymes are then released by the action of various intracellular enzymes.
- novel Fatty Acid Phenolic Derivatives are useful in the treatment or prevention of metabolic disorders including atherosclerosis, dyslipidemia, coronary heart disease, hypercholesterolemia, Type 2 diabetes, elevated cholesterol, metabolic syndrome, diabetic nephropathy, IgA nephropathy, chronic kidney disease (CKD) and cardiovascular disease.
- metabolic disorders including atherosclerosis, dyslipidemia, coronary heart disease, hypercholesterolemia, Type 2 diabetes, elevated cholesterol, metabolic syndrome, diabetic nephropathy, IgA nephropathy, chronic kidney disease (CKD) and cardiovascular disease.
- autoimmune diseases such as rheumatoid arthritis, psoriasis, systemic lupus erythematosus, inflammatory bowel diseases (including colitis and Crohn's disease), respiratory diseases such as asthma, cystic fibrosis, COPD and neurodegenerative diseases such as multiple sclerosis, Parkinson's disease and Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis (ALS) and muscular dystrophy.
- autoimmune diseases such as rheumatoid arthritis, psoriasis, systemic lupus erythematosus, inflammatory bowel diseases (including colitis and Crohn's disease), respiratory diseases such as asthma, cystic fibrosis, COPD and neurodegenerative diseases such as multiple sclerosis, Parkinson's disease and Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis (ALS) and muscular dystrophy.
- autoimmune diseases such as rheumatoid arthritis, psoria
- the compounds described herein are also useful in treating a variety of cancer such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiple myeloma, seminoma, and cancer of the bladder, blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary, pancreas, prostate, testicle, spleen, small intestine, large intestine or stomach.
- cancer such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiple myeloma, seminoma
- cancer of the bladder blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary, pancrea
- a molecular conjugate which comprises a Phenolic derivative and a fatty acid wherein the fatty acid is selected from the group consisting of omega-3 fatty acids, fatty acids that are metabolized in vivo to omega-3 fatty acids, and lipoic acid, and the conjugate is capable of hydrolysis to produce free Phenolic derivative and free fatty acid.
- the fatty acid is selected from the group consisting of a//-cz ' s-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid, eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, tetracosahexaenoic acid and lipoic acid.
- the fatty acid is selected from eicosapentaenoic acid, docosahexaenoic acid and lipoic acid.
- the hydrolysis is enzymatic.
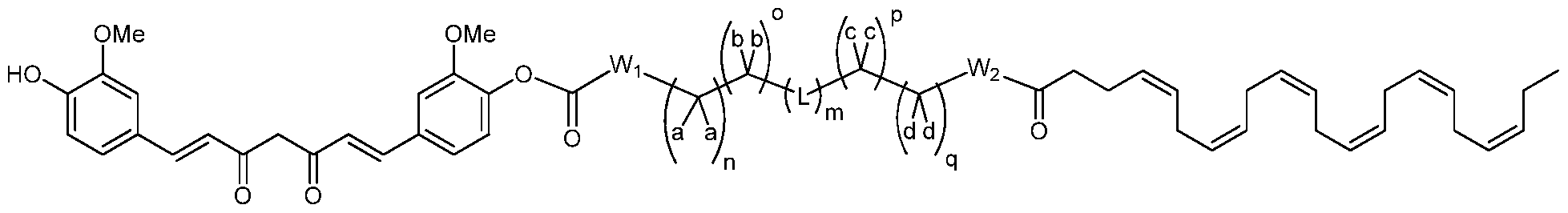
- R 7 , Rs and R9 are each independently selected from the group consisting of H, OH, OCH 3 , or OC(0)R' where R' is independently C 1 -C 3 alkyl, or a second molecule of gallic acid, each Wi,W 2 is independently null, O, S, NH, or NR, or Wi and W 2 can be taken together to form an optionally substituted imidazolidine or piperazine group; each a, b, c, d, is independently -H, -D, halogen, -CH 3 , -CF 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cyclo
- each R 6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)
- each Ri and R 2 is independently -H, -D, -C 1 -C4 alkyl, -halogen, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -
- W 3 is independently O or null
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group;
- Rio is independently H, OH, OR", R", or OC(0)R" where R" is independently C C 6 alkyl; each a, b, c, and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
- L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - C 6 cycloalkyl)-, a heterocycle, a heteroaryl,
- each R 6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C
- each Ri and R 2 is independently -H, -D, -C 1 -C 4 alkyl, -halogen, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(d-C 3 alkyl), -S(0)C C 3 alky
- W 3 is independently O or null
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group;
- Rio is independently H, OH, OR", R", or OC(0)R" where R" is independently d- C 6 alkyl; each a, b, c, and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
- L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - C 6 cycloalkyl)-, a heterocycle, a heteroaryl,
- each R 6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)
- each Ri and R 2 is independently -H, -D, -C 1 -C4 alkyl, -halogen, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(C C 3 alkyl), -S(0)
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
- L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - C 6 cycloalkyl)-, a heterocycle, a heteroaryl,
- each R 6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; L is independently null, -0-, -S-, -S(0)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - Cecycloalkyl)-, a heterocycle, a heteroaryl,
- each R 6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)
- each R5 is independently e, H or straight or branched C 1 -C 10 alkyl which can be optionally substituted with OH, NH 2 , C0 2 R, CONH 2 , phenyl, C 6 H 4 OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C 1 -C 3 alkyl, or straight or branched C 1 -C4 alkyl optionally substituted with -OH or halogen.
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; L is independently null, -0-, -S-, -S(0)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - Cecycloalkyl)-, a heterocycle, a heteroaryl,
- each R 6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
- L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - C 6 cycloalkyl)-, a heterocycle, a heteroaryl,
- each R 6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)
- any one or more of H may be substituted with a deuterium. It is also understood in Formula I, II, III, la, lb, Ilia and Illb that a methyl substituent can be substituted with a Ci-C 6 alkyl.
- compositions comprising at least one Fatty Acid Phenolic Derivative.
- the invention also includes pharmaceutical compositions that comprise an effective amount of a Fatty Acid Phenolic Derivative and a pharmaceutically acceptable carrier.
- the compositions are useful for treating or preventing a metabolic disorder, neurodegenerative diseases, and cancer.
- the invention includes a Fatty Acid Phenolic Derivative when provided as a pharmaceutically acceptable prodrug, a hydrate, a salt, such as a pharmaceutically acceptable salt, enantiomer, stereoisomer, or mixtures thereof.
- Metabolic disorders are a wide variety of medical disorders that interfere with a subject's metabolism. Metabolism is the process a subject's body uses to transform food into energy. Metabolism in a subject with a metabolic disorder is disrupted in some way. Autoimmune diseases arise from an overactive immune response of the body against tissues normally present in the body. Neurodegenerative diseases result from the deterioration of neurons or their myelin sheaths, which would eventually lead to a variety of CNS-related dysfunctions. The Fatty Acid Phenolic Derivatives possess the ability to treat or prevent metabolic disorders, autoimmune or neurodegenerative diseases.
- the Fatty Acid Phenolic Derivatives can also be used to treat a variety of cancer such as such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiple myeloma, seminoma, and cancer of the bladder, blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary, pancreas, prostate, testicle, spleen, small intestine, large intestine or stomach.
- cancer such as such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiple myeloma, seminoma, and cancer of the bladder, blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx,
- the Fatty Acid Phenolic Derivatives have been designed to bring together a Phenolic derivative and omega 3 fatty acids into a single molecular conjugate.
- the Fatty Acid Phenolic Derivatives have also been designed to bring together a Phenolic derivative and lipoic acid into a single molecular conjugate.
- the activity of the Fatty Acid Phenolic Derivatives is substantially greater than the sum of the components suggesting that the activity induced by the Fatty Acid Phenolic Derivatives is synergistic.
- Fatty Acid Phenolic Derivatives includes any and all possible isomers, stereoisomers, enantiomers, diastereomers, tautomers, pharmaceutically acceptable salts, hydrates, solvates, and prodrugs of the Fatty Acid Phenolic Derivatives described herein.
- aryl refers to cyclic, aromatic hydrocarbon groups that have 1 to 2 aromatic rings, including monocyclic or bicyclic groups such as phenyl, biphenyl or naphthyl. Where containing two aromatic rings (bicyclic, etc.), the aromatic rings of the aryl group may be joined at a single point (e.g., biphenyl), or fused (e.g., naphthyl).
- the aryl group may be optionally substituted by one or more substituents, e.g., 1 to 5 substituents, at any point of attachment. The substituents can themselves be optionally substituted.
- C 1 -C 3 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-3 carbon atoms.
- Examples of a C 1 -C 3 alkyl group include, but are not limited to, methyl, ethyl, propyl and isopropyl.
- C 1 -C 4 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-4 carbon atoms.
- Examples of a C 1 -C 4 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, isopropyl, isobutyl, sec-butyl and tert-butyl.
- C 1 -C5 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-5 carbon atoms.
- Examples of a C 1 -C5 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, pentyl, isopropyl, isobutyl, sec-butyl and tert-butyl, isopentyl and neopentyl.
- Ci-C 6 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-6 carbon atoms.
- Examples of a Ci-C 6 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, pentyl, hexyl, isopropyl, isobutyl, sec-butyl, tert-butyl, isopentyl, and neopentyl.
- cycloalkyl refers to a cyclic hydrocarbon containing 3-6 carbon atoms.
- examples of a cycloalkyl group include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
- any of the substitutable hydrogens on an alkyl or cycloalkyl can be substituted with halogen, C 1 -C 3 alkyl, hydroxyl, alkoxy and cyano groups.
- heterocycle refers to a cyclic hydrocarbon containing 3- 6 atoms wherein at least one of the atoms is an O, N, or S.
- heterocycles include, but are not limited to, aziridine, oxirane, thiirane, azetidine, oxetane, thietane, pyrrolidine, tetrahydrofuran, tetrahydrothiophene, piperidine, tetrahydropyran, thiane, imidazolidine, oxazolidine, thiazolidine, dioxolane, dithiolane, piperazine, oxazine, dithiane, and dioxane.
- heteroaryl refers to a monocyclic or bicyclic ring structure having 5 to 12 ring atoms wherein one or more of the ring atoms is a heteroatom, e.g. N, O or S and wherein one or more rings of the bicyclic ring structure is aromatic.
- heteroaryl are pyridyl, furyl, pyrrolyl, thienyl, thiazolyl, oxazolyl, imidazolyl, indolyl, tetrazolyl, benzofuryl, xanthenes and dihydroindole. It is understood that any of the substitutable hydrogens on a heteroaryl can be substituted with halogen, C 1 -C 3 alkyl, hydroxyl, alkoxy and cyano groups.
- any one of the side chains of the naturally occurring amino acids means a side chain of any one of the following amino acids: Isoleucine, Alanine, Leucine, Asparagine, Lysine, Aspartate, Methionine, Cysteine, Phenylalanine, Glutamate, Threonine, Glutamine, Tryptophan, Glycine, Valine, Proline, Arginine, Serine, Histidine and Tyrosine.
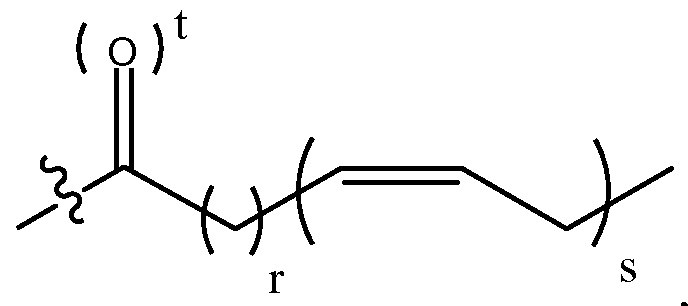
- fatty acid as used herein means an omega-3 fatty acid and fatty acids that are metabolized in vivo to omega-3 fatty acids.
- Non-limiting examples of fatty acids are a/7-cz ' s-7,10,13-hexadecatrienoic acid, a-linolenic acid (ALA or all-cis-9, 12,15- octadecatrienoic acid), stearidonic acid (STD or a/7-cz ' s-6,9,12,15-octadecatetraenoic acid), eicosatrienoic acid (ETE or all-cis- 11,14,17-eicosatrienoic acid), eicosatetraenoic acid (ETA or a//-cz ' s-8,l l,14,17-eicosatetraenoic acid), eicosapentaenoic acid (EPA or all-cis- 5,8,11, 14, 17-eicos
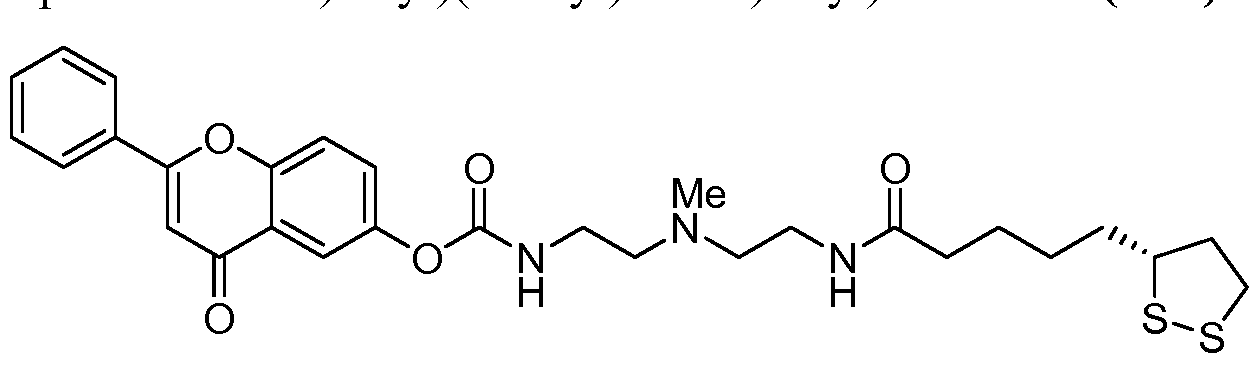
- phenolic derivative as used herein means the molecule known as gallic acid, flavonoid (such as quercetin, naringenin, epicatechin, myricetin, apigenin) or chalcone (such as curcumin, isoliquiritigenin, flavokawain A, cardomonin, butein, licochalcone A, xanthohumol, BMS 181156, AGN 193198 and MX 781) and any derivatives thereof. More comprehensive list of flavonoids and chalcones can be found in Flavonoids: Chemistry, Biochemistry and Applications 2006, Edited by Anderson, O. M. and Markham, K. R. and Srinivasan et al J. Med. Chem. 2009, 52, p. 7228-7235.
- a "subject” is a mammal, e.g., a human, mouse, rat, guinea pig, dog, cat, horse, cow, pig, or non-human primate, such as a monkey, chimpanzee, baboon or rhesus, and the terms “subject” and “patient” are used interchangeably herein.
- the invention also includes pharmaceutical compositions comprising an effective amount of a Fatty Acid Phenolic Derivative and a pharmaceutically acceptable carrier.
- the invention includes a Fatty Acid Phenolic Derivative when provided as a pharmaceutically acceptable prodrug, hydrate, salt, such as a pharmaceutically acceptable salt, enantiomers, stereoisomers, or mixtures thereof.
- Representative "pharmaceutically acceptable salts” include, e.g., water-soluble and water-insoluble salts, such as the acetate, amsonate (4,4-diaminostilbene-2, 2 - disulfonate), benzenesulfonate, benzonate, bicarbonate, bisulfate, bitartrate, borate, bromide, butyrate, calcium, calcium edetate, camsylate, carbonate, chloride, citrate, clavulariate, dihydrochloride, edetate, edisylate, estolate, esylate, fiunarate, gluceptate, gluconate, glutamate, glycollylarsanilate, hexafluorophosphate, hexylresorcinate, hydrabamine, hydrobromide, hydrochloride, hydroxynaphthoate, iodide, isothionate, lactate, lactobionate, la
- metabolic disease refers to disorders, diseases and syndromes involving dyslipidemia, and the terms metabolic disorder, metabolic disease, and metabolic syndrome are used interchangeably herein.
- an "effective amount" when used in connection with a fatty acid phenolic derivative is an amount effective for treating or preventing a metabolic disease.
- carrier encompasses carriers, excipients, and diluents and means a material, composition or vehicle, such as a liquid or solid filler, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting a pharmaceutical agent from one organ, or portion of the body, to another organ, or portion of the body.
- treating refers to improving at least one symptom of the subject's disorder. Treating can be curing, improving, or at least partially ameliorating the disorder.
- disorder is used in this disclosure to mean, and is used interchangeably with, the terms disease, condition, or illness, unless otherwise indicated.
- administer refers to either directly administering a compound or pharmaceutically acceptable salt of the compound or a composition to a subject, or administering a prodrug derivative or analog of the compound or pharmaceutically acceptable salt of the compound or composition to the subject, which can form an equivalent amount of active compound within the subject's body.
- prodrug means a compound which is convertible in vivo by metabolic means ⁇ e.g., by hydrolysis) to a fatty acid phenolic derivative.
- the following abbreviations are used herein and have the indicated definitions: Boc and BOC are tert-butoxycarbonyl, Boc 2 0 is di-tert-butyl dicarbonate, BSA is bovine serum albumin, CDI is 1 , ⁇ -carbonyldiimidazole, DCC is N,N-dicyclohexylcarbodiimide, DIEA is N,N-diisopropylethylamine, DMAP is 4-dimethylaminopyridine, DMEM is Dulbecco's Modified Eagle Medium, DMF is N,N-dimethylformamide, DOSS is sodium dioctyl sulfosuccinate, EDC and EDCI are l-ethyl-3-(3-d
- the present invention provides a molecular conjugate which comprises a phenolic derivative and a fatty acid covalently linked, wherein the fatty acid is selected from the group consisting of omega-3 fatty acids and fatty acids that are metabolized in vivo to omega-3 fatty acids, wherein the conjugate comprises is capable of hydrolysis to produce free phenolic derivative and free fatty acid.
- the fatty acid is selected from the group consisting of all- cz ' s-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid, eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, and tetracosahexaenoic acid.
- the fatty acid is selected from eicosapentaenoic acid and docosahexaenoic acid. In some embodiments, the fatty acid is selected from lipoic acid. In some embodiments, the hydrolysis is enzymatic.
- the present invention provides fatty acid phenolic derivatives according to Formula I, II, III, la, lb, Ilia and Illb:
- Gi,G 2 , Ri, R 2 , R3, R 4 , R5, Re, R7, Rs, R9, Rio, Wi, W 2 , W 3 , L, a, c, b, d, e, g, h, m, n, o, p, q, Z, r, s, t, u, v, w and z, are as defined above for Formula I, II, III, la, lb, Ilia and Illb.
- Gi is
- Gi is
- Gi is
- G 2 is
- G 2 is
- G 2 is [0065] In some embodiments, G 2 is
- G 2 is
- G 2 is
- W 3 is null.
- W 3 is O.
- one Z is
- one Z is
- one Z is
- one Z is
- one Z is
- one Z is
- one Z is
- one Z is and v is 2.
- one Z is and v is 6.
- one Z is and s is 3. [0080] In some embodiments, one Z is
- one Z is
- Z is and t is 1.
- Z is and t is 1.
- Wi is NH.
- W 2 is NH
- Wi is O.
- W 2 is O.
- Wi is null.
- W 2 is null.
- Wi and W 2 are each NH.
- Wi and W 2 are each null.
- Wi is O and W 2 is NH.
- Wi and W 2 are each NR, and R is CH 3 .
- m is 0.
- m is 1.
- n is 2.
- L is -S- or -S-S-.
- L is -0-.
- L is -C(O)-.
- L is heteroaryl
- L is heterocycle
- L is
- L is
- L is [0106] In some embodiments, L is
- L is
- L is ⁇ ⁇ ⁇ H2 ⁇ m ⁇ wherein m is 2.
- L is ⁇ ⁇ ⁇ H2 ⁇ m ⁇ wherein m is 3.
- L is
- L is
- L is
- L is N
- L is
- L is N
- one of n, o, p, and q is 1.
- two of n, o, p, and q are each 1.
- n, o, p, and q are each 1.
- one d is C(0)OR.
- r is 2 and s is 6.
- r is 3 and s is 5.
- t is 1.
- w 0.
- w is 1.
- Wi and W 2 are each NH, m is 0, n, and o are each 1, and p and q are each 0.
- Wi and W 2 are each NH, m is 1, n, o, p, and q are each 1, and L is O.
- Wi and W 2 are each NH, m is 1, n, o, p, and q are each 1, and L is
- Wi and W 2 are each NH, m is 1, n, o, p, and q are each 1, and L is -S-S-.
- Wi and W 2 are each NH, m is 1, n and o are each 0, p and q are each 1 , and L is
- Wi and W 2 are each NH, m is 1, k is O, n and o are each 0, p and q are each 1 , and L is
- Wi and W 2 are each NH, m is 1, n and o are each 1 , p and q are each 0, and L is
- Wi and W 2 are each NH, m is 1, k is 0, n is 1, o, p and q are each 0, and L is
- Wi and W 2 are each NH, m is 1, n, o, and p are each q is 1 , and L is
- Wi and W 2 are each NH, m is 1, k is 1, n, o, and p are each 1 , and L is
- Wi and W 2 are each NH, m is 1, n is 1, and o, p, and q are each 0, and L is
- Wi and W 2 are each NH, m is 1, k is 1, o, p, and q are each 0, and L is
- Wi and W 2 are each NH, m is 1, n, o, p, and q are each 1, and L is
- Wi and W 2 are each NH, m is 1, n, o, p, and q are each 1, and L is
- Wi and W 2 are each NH, m is 0, k is 1, o and p are each 1, and q is 0.
- Wi and W 2 are each NH, m is 0, n, o, p, and q are each 1. [0144] In some embodiments, Wi and W 2 are each NH, m is 0, n and o are each 1 , p and q are each 0, and each a is CH 3 .
- Wi and W 2 are each NH, m is 0, n and o are each 1 , p and q are each 0, and each b is CH 3 .
- Wi and W 2 are each NH, m is 1, n, o, p, and q are each 1, R 3 is H, and L is
- Wi and W 2 are each NH, m is 1, n, p and q are each 1, and o is 2, R 3 is H, and L is
- Wi and W 2 are each NH, m is 1, n, o, p are each 1, and q is 2, and L is
- Wi and W 2 are each NH, m is 1, n, o, p, and q are each 1, and L is
- Wi and W 2 are each NH, m is 1, n and p are each 1 , and o and q are each 0, and L is -C(O)-.
- Wi and W 2 are each NH, m is 1, n and p are each 1, and o, and q are each 0, and L is
- Wi and W 2 are each NH, m is 1 , n, o, p, q are each 1 , and L is
- Wi and W 2 are each NH, m is 1 , n, o, p , and q are each 1, h is 1 , and L is
- Wi and W 2 are each NH, m is 1 , n, o, p , and q are each 1, and L is-S-.
- W 1 and W 2 are each NH, m is 1, n, o, p are each 0, q is 1, one d is -C3 ⁇ 4, and L is
- Wi and W 2 are each NH, m is 2, n, o, p, and q are each 0, one L is
- m is 0, n, o, p, and q are each 0, and Wi and W2 are taken together to form an optionally substituted piperazine group.
- m is 1, n, o, p, and q are each 0, Wi and W 2 are each null, and L is
- m is 1, n and p are each 1, o and q are each 0, Wi and W 2 are each NH, and L is C 3 -C 6 cycloalkyl.
- m is 1, n is 1 , o, p, and q are each 0, Wi and W 2 are each NH, and L is C 3 -C 6 cycloalkyl.
- m is 1 , n, o, p, are each 0, q is 1 , Wi and W 2 are each NH, and L is C -C 6 cycloalkyl.
- m is 1, n, o, p, and q are each 0, Wi is NH, W 2 is null, and L is
- m is 1, n o, p, and q are each 0, Wi is null, W 2 is NH, and
- m is 1, n o, p, and q are each 0, Wi is NH, W 2 is null, and
- L is [0165] In some embodiments, m is 1, n o, p, and q are each 0, Wi is null, W 2 is NH, and L is
- m is 1, n is 1, o, p, and q are each 0, Wi is NH, W 2 is null, and L is
- m is 1, n, o, p, are each 0, q is 1, Wi is null, W 2 is NH, and L is
- m is 1, n, o, p, and q are each 0, Wi is NH, W 2 is null, and L is
- m is 1, n, o, p, and q are each 0, Wi is null, W 2 is NH, and L is
- m is 1, n is 1, o, p, and q are each 0, Wi is NH, W 2 is null, and L is
- m is 1, n, o, p, are each 0, q is 1, Wi is null, W 2 is NH, and
- m is 1, n is 1, o, p, and q are each 0, Wi is NH, W 2 is null,
- m is 1, n, o, p, are each 0, q is 1, Wi is null, W 2 is NH, and
- m is 1, n, o, p, q are each 0, Wi and W 2 is null, and L is
- m is 1, n, o, p, q are each 0, Wi and W 2 is null, and L is [0176] In some embodiments, m is 1, n, o, p, q are each 0, Wi is NH, W 2 is null, and L is
- m is 1, n, o, p, q are each 0, Wi is null, W 2 is NH, and L is
- m is 1, n, o, p, are each 0, q is 1, Wi and W 2 are each and NH, is null, L is
- m is 1, n, o, p, are each 0, q is 1, Wi and W 2 are each NH, is null, and L is a heteroaryl.
- r is 2
- s is 6
- t is 1.
- r is 3, s is 5 and t is 1.
- any one or more of H may be substituted with a deuterium. It is also understood in Formula I, II, III, la, lb, Ilia and Illb that a methyl substituent can be substituted with a Ci-C 6 alkyl.
- Also provided in the invention is a method for inhibiting, preventing, or treating inflammation or an inflammatory disease in a subject.
- the inflammation can be associated with an inflammatory disease or a disease where inflammation contributes to the disease.
- Inflammatory diseases can arise where there is an inflammation of the body tissue. These include local inflammatory responses and systemic inflammation. Examples of such diseases include, but are not limited to: organ transplant rejection; reoxygenation injury resulting from organ transplantation (see Grupp et ah, J. Mol. Cell Cardiol.
- Metabolic disease such as type II diabetes mellitus; the prevention of type I diabetes; dyslipidemia; hypertriglyceridemia; diabetic complications, including, but not limited to glaucoma, retinopathy, macula edema, nephropathy, such as microalbuminuria and progressive diabetic nephropathy, polyneuropathy, diabetic neuropathy, atherosclerotic coronary arterial disease, peripheral arterial disease, nonketotic hyperglycemichyperosmolar coma, mononeuropathies, autonomic neuropathy, joint problems, and a skin or mucous membrane complication, such as an infection, a shin spot, a candidal infection or necrobiosis lipoidica diabeticorum; immune-complex vasculitis, systemic lupus erythematosus; inflammatory diseases of the heart such as cardiomyopathy, ischemic heart disease hypercholesterolemia, and atherosclerosis; as well as various other diseases that can have significant inflammatory components,
- the inflammatory disease can also be a systemic inflammation of the body, exemplified by gram-positive or gram negative shock, hemorrhagic or anaphylactic shock, or shock induced by cancer chemotherapy in response to proinflammatory cytokines, e.g., shock associated with proinflammatory cytokines.
- shock can be induced, e.g., by a chemotherapeutic agent that is administered as a treatment for cancer.
- Other disorders include depression, obesity, allergic diseases, acute cardiovascular events, arrhythmia, prevention of sudden death.
- muscle wasting diseases such as Muscular Dystrophy including but not limited to Duchenne's Muscular Dystrophy, Becker Muscular Dystrophy, Emery- Dreifuss Muscular Dystrophy, Limb-Girdle Muscular Dystrophy, Facioscapulohumeral Muscular Dystrophy, Myotonic Dystrophy, Oculopharyngeal Muscular Dystrophy, Distal Muscular Dystrophy, Congential Muscular Dystrophy, Spinal Muscular Atrophy, and Spinal Bulbar Muscular Dystrophy.
- Muscular Dystrophy including but not limited to Duchenne's Muscular Dystrophy, Becker Muscular Dystrophy, Emery- Dreifuss Muscular Dystrophy, Limb-Girdle Muscular Dystrophy, Facioscapulohumeral Muscular Dystrophy, Myotonic Dystrophy, Oculopharyngeal Muscular Dystrophy, Distal Muscular Dystrophy, Con
- fatty acid phenolic derivatives include inflammatory myopathies such as dermatomositis, inclusion body myositis, and polymyositis, and cancer cachexia. Also inflammation that results from surgery and trauma can be treated with a Fatty Acid Phenolic Derivative.
- the compounds described herein are also useful in treating a variety of cancer such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiople myeloma, seminoma, and cancer of the bladder, blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary, pancreas, prostate, testicle, spleen, small intestine, large intestine or stomach.
- cancer such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiople myeloma, seminoma
- cancer of the bladder blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary
- Fatty Acid Phenolic Derivatives include fatty liver disease, non-alcoholic fatty liver disease, NASH (non-alcoholic steatohepatitis), Sarcopenia, Sjogren syndrome, Myasthenia gravis, and xerophthalmia.
- Also provided in the invention is a method for inhibiting, preventing, or treating a metabolic disease, or symptoms of a metabolic disease, in a subject.
- disorders include, but are not limited to atherosclerosis, dyslipidemia, hypertriglyceridemia, hypertension, heart failure, cardiac arrhythmias, low HDL levels, high LDL levels, sudden death, stable angina, coronary heart disease, acute myocardial infarction, secondary prevention of myocardial infarction, cardiomyopathy, endocarditis, type 2 diabetes, insulin resistance, impaired glucose tolerance, hypercholesterolemia, stroke, hyperlipidemia, hyperlipoproteinemia, chronic kidney disease, intermittent claudication, hyperphosphatemia, carotid atherosclerosis, peripheral arterial disease, diabetic nephropathy, hypercholesterolemia in HIV infection, acute coronary syndrome (ACS), non-alcoholic fatty liver disease, arterial occlusive diseases, cerebral arteriosclerosis, cerebrovascular disorders, myocardial ischemia, polycystic ovary
- the subject is administered an effective amount of a fatty acid phenolic derivative.
- the invention also includes pharmaceutical compositions useful for treating or preventing a metabolic disease, or for inhibiting a metabolic disease, or more than one of these activities.
- the compositions can be suitable for internal use and comprise an effective amount of a fatty acid phenolic derivative and a pharmaceutically acceptable carrier.
- the fatty acid phenolic derivatives are especially useful in that they demonstrate very low peripheral toxicity or no peripheral toxicity.
- the fatty acid phenolic derivative scan each be administered in amounts that are sufficient to treat or prevent a metabolic disease or prevent the development thereof in subjects.
- Administration of the fatty acid phenolic derivatives can be accomplished via any mode of administration for therapeutic agents. These modes include systemic or local administration such as oral, nasal, parenteral, transdermal, subcutaneous, vaginal, buccal, rectal or topical administration modes.
- compositions can be in solid, semi-solid or liquid dosage form, such as, for example, injectables, tablets, suppositories, pills, time-release capsules, elixirs, tinctures, emulsions, syrups, powders, liquids, suspensions, or the like, sometimes in unit dosages and consistent with conventional pharmaceutical practices.
- injectables tablets, suppositories, pills, time-release capsules, elixirs, tinctures, emulsions, syrups, powders, liquids, suspensions, or the like, sometimes in unit dosages and consistent with conventional pharmaceutical practices.
- they can also be administered in intravenous (both bolus and infusion), intraperitoneal, subcutaneous or intramuscular form, all using forms well known to those skilled in the pharmaceutical arts.
- Illustrative pharmaceutical compositions are tablets and gelatin capsules comprising a fatty acid phenolic derivative and a pharmaceutically acceptable carrier, such as: a) a diluent, e.g., purified water, triglyceride oils, such as hydrogenated or partially hydrogenated vegetable oil, or mixtures thereof, corn oil, olive oil, sunflower oil, safflower oil, fish oils, such as EPA or DHA, or their esters or triglycerides or mixtures thereof, omega- 3 fatty acids or derivatives thereof, lactose, dextrose, sucrose, mannitol, sorbitol, cellulose, sodium, saccharin, glucose and/or glycine; b) a lubricant, e.g., silica, talcum, stearic acid, its magnesium or calcium salt, sodium oleate, sodium stearate, magnesium stearate, sodium benzoate, sodium acetate, sodium chloride and/or polyethylene glyco
- Liquid, particularly injectable, compositions can, for example, be prepared by dissolution, dispersion, etc.
- the fatty acid phenolic derivative is dissolved in or mixed with a pharmaceutically acceptable solvent such as, for example, water, saline, aqueous dextrose, glycerol, ethanol, and the like, to thereby form an injectable isotonic solution or suspension.
- a pharmaceutically acceptable solvent such as, for example, water, saline, aqueous dextrose, glycerol, ethanol, and the like.
- Proteins such as albumin, chylomicron particles, or serum proteins can be used to solubilize the fatty acid phenolic derivative.
- the fatty acid phenolic derivatives can be also formulated as a suppository that can be prepared from fatty emulsions or suspensions; using polyalkylene glycols such as propylene glycol, as the carrier.
- the fatty acid phenolic derivatives can also be administered in the form of liposome delivery systems, such as small unilamellar vesicles, large unilamellar vesicles and multilamellar vesicles.
- Liposomes can be formed from a variety of phospholipids, containing cholesterol, stearylamine or phosphatidylcholines.
- a film of lipid components is hydrated with an aqueous solution of drug to a form lipid layer encapsulating the drug, as described in United States Patent No. 5,262,564, the contents of which are herein incorporated by reference in their entirety.
- the fatty acid phenolic derivatives can also be delivered by the use of monoclonal antibodies as individual carriers to which the fatty acid phenolic derivatives are coupled.
- the fatty acid phenolic derivatives can also be coupled with soluble polymers as targetable drug carriers.
- Such polymers can include polyvinylpyrrolidone, pyran copolymer, polyhydroxypropylmethacrylamide-phenol, polyhydroxyethylaspanamidephenol, or polyethyleneoxidepolylysme substituted with palmitoyl residues.
- fatty acid phenolic derivatives can be coupled to a class of biodegradable polymers useful in achieving controlled release of a drug, for example, polylactic acid, polyepsilon caprolactone, polyhydroxy butyric acid, polyorthoesters, polyacetals, polydihydropyrans, polycyanoacrylates and cross-linked or amphipathic block copolymers of hydrogels.
- fatty acid phenolic derivative is not covalently bound to a polymer, e.g., a polycarboxylic acid polymer, or a polyacrylate.
- Parenteral injectable administration is generally used for subcutaneous, intramuscular or intravenous injections and infusions.
- Injectables can be prepared in conventional forms, either as liquid solutions or suspensions or solid forms suitable for dissolving in liquid prior to injection.
- compositions can be prepared according to conventional mixing, granulating or coating methods, respectively, and the present pharmaceutical compositions can contain from about 0.1 % to about 90 %, from about 10 % to about 90 %, or from about 30 % to about 90 % of the fatty acid phenolic derivative by weight or volume.
- the dosage regimen utilizing the fatty acid phenolic derivative is selected in accordance with a variety of factors including type, species, age, weight, sex and medical condition of the patient; the severity of the condition to be treated; the route of administration; the renal or hepatic function of the patient; and the particular fatty acid phenolic derivative employed.
- a physician or veterinarian of ordinary skill in the art can readily determine and prescribe the effective amount of the drug required to prevent, counter or arrest the progress of the condition.
- Effective dosage amounts of the present invention when used for the indicated effects, range from about 20 mg to about 5,000 mg of the fatty acid phenolic derivative per day.
- Compositions for in vivo or in vitro use can contain about 20, 50, 75, 100, 150, 250, 500, 750, 1,000, 1,250, 2,500, 3,500, or 5,000 mg of the fatty acid phenolic derivative.
- the compositions are in the form of a tablet that can be scored.
- Effective plasma levels of the fatty acid phenolic derivative can range from about 5 ng/mL to 5,000 ng/mL.
- Appropriate dosages of the fatty acid phenolic derivatives can be determined as set forth in Goodman, L. S.; Gilman, A. The Pharmacological Basis of Therapeutics, 5th ed.; MacMillan: New York, 1975, pp. 201-226.
- Fatty acid phenolic derivatives can be administered in a single daily dose, or the total daily dosage can be administered in divided doses of two, three or four times daily. Furthermore, fatty acid phenolic derivatives can be administered in intranasal form via topical use of suitable intranasal vehicles, or via transdermal routes, using those forms of transdermal skin patches well known to those of ordinary skill in that art. To be administered in the form of a transdermal delivery system, the dosage administration can be continuous rather than intermittent throughout the dosage regimen.
- topical preparations include creams, ointments, lotions, aerosol sprays and gels, wherein the concentration of the fatty acid phenolic derivative ranges from about 0.1 % to about 15 %, w/w or w/v.
- R 9 , r and s are as defined above.
- the mono-BOC protected amine of the Formula B can be obtained from commercial sources or prepared according to the procedures outlined in Krapcho et al, Synthetic Communications 1990, 20, p. 2559-2564.
- the commercially available compound A can be triacetylated with acetic anhydride, subsequently this intermediate can then be amidated with the amine B using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH 2 C1 2 or dioxane to produce the coupled compound C.
- a coupling reagent such as DCC, CDI, EDC
- a tertiary amine base and/or catalyst e.g., DMAP
- R, r and s are as defined above.
- the acylated amine of the Formula F can be prepared using the procedures outlined in Andruszkiewicz et al, Synthetic Communications, 2008, 38, p. 905-913.
- Compound A can be can be triacetylated with acetic anhydride, and this resulting intermediate can then amidated with the amine F using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH 2 CI 2 or dioxane to produce the coupled compound G.
- a coupling reagent such as DCC, CDI, EDC
- a tertiary amine base and/or catalyst e.g., DMAP
- Activation of compound J with a coupling agent such as HATU in the presence of an amine such as DIEA followed by addition of a fatty acid of Formula D affords compounds of the formula K.
- the amine T can be prepared from the commercially available diamine according to the procedures outlined in Dahan et al, J. Org. Chem. 2007, 72, p. 2289-2296.
- Compound A can be triacetylated with acetic anhydride, subsequently this intermediate can then be amidated with the amine T using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to afford compound U.
- a coupling reagent such as DCC, CDI, EDC
- a tertiary amine base and/or catalyst e.g., DMAP
- the BOC group of compound U can be removed with acids such as TFA or HCl in a solvent such as CH 2 CI 2 or dioxane and the resulting amine can be coupled with a fatty acid of Formula D using HATU in the presence of an amine such as DIEA to afford compounds of the Formula V.
- the hydroxyl group in compound U can be further acylated or converted to an amino group by standard mesylation chemistry followed by displacement with sodium azide and hydrogenation over a catalyst such as Palladium on carbon.
- the amine can be further acylated or alkylated, followed by the removal of the BOC group.
- the resulting amine can be coupled with a fatty acid of the Formula D and subsequently hydrolysis of triacetyl groups using hydrazine hydrate, affords compounds of the Formula W.
- the commercially available amino acid esters EE can be coupled with a fatty acid of the Formula D using a coupling agent such as EDCI or HATU, followed by alkaline hydrolysis of the methyl ester to afford compounds of the Formula FF.
- Compounds of the Formula FF can be coupled with the commercially available BOC-amino acid derivatives GG using a coupling agent such as EDCI or HATU.
- the BOC group can be removed by treatment with acids such as TFA or HCl to afford compounds of the Formula HH which can then be coupled with compound A after triacetylation with acetic anhydride, with hydrolysis of the triacetyl groups under hydrazine hydrate as the final step to afford compounds of the Formula II.
- Compound A can be triacetylated with acetic anhydride, subsequently this intermediate can then be coupled with a BOC-protected diamine of the general formula DA to obtain the BOC-protected amide derivative. After treatment with HCI in dioxane, the resulting amine OO can be coupled with a fatty acid of the formula D and subsequently hydrolysis of triacetyl groups using hydrazine hydrate, affords compounds of the Formula PP.
- BOC-protected diamines are commercially available. Diamines DAI, DA2, DA3, and DA4
- DA1 DA2 DA3 and DA4 and derivatives thereof can be prepared according to the procedures outlined in the corresponding references: diamine DAI, Stocks et al, Bioorganic and Medicinal Chemistry Letters 2010, p. 7458; diamine DA2, Fritch et al, Bioorganic and Medicinal Chemistry Letters 2010, p. 6375; diamine DA3 and DA4, Moffat et al, J. Med. Chem. 2010, 53, p.8663- 8678), the disclosures of the foregoing references are incorporated herein in their entireties.
- R 10 , ml, r, s are as defined above.
- the mono-BOC protected diamine AA can be coupled with a fatty acid of the formula D using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB.
- a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB.
- a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB.
- a coupling reagent such as DCC, CDI, EDC
- a tertiary amine base and/or catalyst e.g., DMAP
- the mono-BOC protected diamine AA can be coupled with a fatty acid of the formula D using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB.
- a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB.
- a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB.
- a coupling reagent such as DCC, CDI, EDC
- a tertiary amine base and/or catalyst e.g., DMAP
- the reaction sequence shown in Scheme 7 can be repeated with a chalcone derivative such as curcumin BA.
- the mono-BOC protected diamine AA can be coupled with a fatty acid of the formula D using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB.
- a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP
- This is followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH 2 CI 2 or dioxane to produce the free amine salt AH.
- Compound BA is subjected to acylation with 4-nitrophenyl chloro formate to afford compounds of the Formula BB.
- Compounds of the Formula BB can then be reacted with compound AH in
- the purpose of this assay is to measure the ability of small molecules to inhibit the secretion of TNFa in cultured macrophages stimulated with lipopolysaccharide (LPS).
- LPS lipopolysaccharide
- Treatment of macrophages with LPS activates inflammatory cytokine pathways primarily through the TLR4-NF-KB signaling axis.
- the compounds of this invention inhibit the transcriptional activation of NF- ⁇ and thus decrease the production and release of TNFa.
- Dexamethasone a potent agonist of the glucocorticoid receptor is used a positive control for inhibition of TNFa release.
- Day 1 Seed RAW 264.7 macrophages into 96 well culture plates. Remove culture media from RAW 264.7 cell growing in a 75 mm 2 tissue culture flask (cells should be at -70% confluence) and add 10 ml of warmed complete growth media (DMEM + 10%FBS + IX pen/step). The cells are scraped into suspension using a sterile plate scraper and homogenized by pipetting up and down with a 10 ml serological pipette. The cell concentration is determined using a clinical hematoctyometer. Cells are then diluted to 150,000 cells per ml into growth media.
- DMEM + 10%FBS + IX pen/step warmed complete growth media
- the cell concentration is determined using a clinical hematoctyometer. Cells are then diluted to 150,000 cells per ml into growth media.
- test compound sample plate is prepared. Test compounds are prepared in growth media. Compounds are delivered to media from 1000X stocks in 100% DMSO (e.g. for a 10 ⁇ final concentration of test compound, deliver 2 ⁇ ⁇ of 10 mM test compound to 2 ml of media). At least 150 ⁇ of IX compound in media is added to 96 well sample plate.
- the perimeter wells of the 96 well plate are not used to avoid edge effects. Twelve sample wells are prepared with media plus 0.1% DMSO (these samples will serve as the vehicle controls; LPS-stimulated and non-stimulated. 10 ⁇ dexamethasone is used as a positive control). Culture plates are then returned to the growth incubator for 2 hours. Cells are stimulated afterwards by adding 25 ⁇ of 50 ng/ml LPS is added to every well (except the 6 unstimulated vehicle control wells: final concentration of 10 ng/ml LPS. Plates are returned to growth incubator for 3 hours. Afterwards, 100 ⁇ of media supernatant is removed and transferred to a 96 well v-bottom sample plate.
- the media supernatant plate is centrifuged for 5 minutes at 1000 rpm in a swing-bucket centrifuge, pelleting any cellular debris that may remain in supernatant. 80 ⁇ of supernatant is removed from sample plate and transferred to a fresh v-bottom 96 well plate.
- Cell viability is measured using Celltiter- glo kit. By measuring cell viability, a given compound's effects on TNFa secretion can show whether such effects are due to cytotoxicity or to inhibition of inflammatory signaling. 100 ⁇ of Celltiter-glo reagent are added to each well of the cell culture plate and afterwards measure the luminescence signal (CPS) of the plate is measured using the Victor 5 plate reader (0.3 second read; 60 second plate shaking prior to read). Cell viability of a given compound at a given concentration is computed as follows:
- TNFa secretion percent of control is plotted as a function of compound concentration using a four parameter dose-response curve fit equation (XLFIT Model # 205):
- RAW264.7 macrophages were seeded at a density of 100,000 cells/well in a 96- well plate in DMEM supplemented with 10% FBS and penicillin:streptomycin. 16 hours later, medium was aspirated and replaced with 90 ⁇ 11 of serum-free DMEM.
- the compounds of the invention were brought up in 100% ethanol to a concentration of lOOmM and then diluted 1 : 100 in 100% FBS for a stock solution consisting of ImM compound and 1%) ethanol. These stock solutions were then diluted 1 : 10 in FBS supplemented with 1% ethanol to generate a 100 ⁇ of the compounds of the invention.
- ⁇ ⁇ ⁇ was then added to the RAW246.7 cells to generate final concentrations 10 ⁇ of the compounds of the invention or 10 ⁇ each bioactive, along with vehicle only control.
- the compounds of the invention were allowed to pre-incubate for 2 hours before stimulation of 100 ng/ml LPS ( ⁇ of ⁇ g/ml LPS was added to each well). Following 3 hours of LPS stimulation, cells were washed once in lx PBS, aspirated dry, and flash frozen in liquid nitrogen. R A was then isolated and converted to cDNA using the Cells to cDNA kit (AMBION®) according to the manufacturer's protocol.
- HMOX transcript levels were then measured using Taqman primer/probe assay sets (APPLIED BIOSYSTEMS®), normalized to GAPDH using the deltaCt method, and the data expressed relative to vehicle only control.
- Figure 1 summarizes the HMOX activity of compound 1-1 in this particular assay.
- RAW 264.7 cells transfected with an NF-KB-driven luciferase reporter were plated in 96 well plates. Cells were treated with Vehicle (0.1% ethanol) or test compounds for 2 hours. As a positive control for inhibition of NFKB signaling, 6 wells were treated with 10 ⁇ dexamethasone. Cells were then challenged with 200 ng/mL LPS for 3 hours in the presence of test compounds. A subset of wells treated with vehicle remained unstimulated with LPS to determine the floor signal of the assay.
- NF- ⁇ driven luciferase activity was developed by addition of BriteLite luciferase kit (PERKIN ELMER®) and measured using a Victor V plate reader.
- NF- ⁇ activity (luciferase activity) for each treatment was normalized to Vehicle wells treated with LPS (% NF- ⁇ Response).
- AlamarBlue was used to monitor cell viability to ensure that inhibition of luciferase signal was not a result of compound cytotoxicity.
- the IC 50 of compound 1-1 and 1-3 were determined to ⁇ 50 ⁇ , while the IC 50 of gallic acid was determined to be > 200 ⁇ .
- mice Twelve-week-old male Zucker fa/fa rats and 8-week-old ob/ob (Lepob/ob) and ob/1 mice are given free access to food and water.
- a compound of the invention 120 mg/kg/day is dosed orally by gavage once per day.
- glucose 2.0 g/kg is administered by oral gavage (rats) or intraperitoneal injection (mice) after an overnight fast.
- Blood glucose and serum insulin concentrations are determined during oral glucose tolerance tests in Zucker fa/fa rats or fa/1 rats.
- insulin 2.0 U/kg
- Cholesterol, triglyceride, long- chain FFA, and ALT concentrations are measured in sera from fasting Zucker fa/fa rats.
- reaction was washed with saturated NH 4 C1, brine, and dried over Na 2 S0 4 and concentrated under reduced pressure.
- the resulting residue was purified by silica gel chromatography (0-60% gradient EtOAc in pentanes) to afford 5-((2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13, 16,19- hexaenamido)ethyl)carbamoyl)benzene-l,2,3-triyl triacetate (383 mg, 70% yield).
- tert-Butyl (2-(2-aminoethoxy)ethyl)carbamate was prepared according to the procedure outlined in WO 201 108521 1. The same experimental procedure outlined in example 5 was used to prepare 3,4,5-triacetoxybenzoic acid. This material, 3,4,5- triacetoxybenzoic acid (500 mg, 1.69 mmol) was taken up in 5 mL CH 2 CI 2 along with tert- butyl (2-(2-aminoethoxy)ethyl)carbamate (380 mg, 1.1 equivalents), HATU (835 mg, 1.3 equivalents) and DIE A (571 ul, 2.0 equivalents). The resulting reaction mixture was stirred at room temperature for 4 h.
- tert-Butyl (2-((2-aminoethyl)(methyl)amino)ethyl)carbamate was prepared according to the procedure outlined in WO 2011085211. Following General Preparation B for synthesis of N-(2-((4Z,7Z,10Z,13Z,16Z,19 )-docosa-4,7,10,13,16,19- hexaenamido)ethoxy)ethyl)-3,4,5-trihydroxybenzamide, tert-butyl (2-((2- aminoethyl)(methyl)amino)ethyl)carbamate (404 mg, 1.1 equivalents) was used in the coupling reaction to to 3,4,5-triacetoxybenzoic acid (500 mg, 1.69 mmol).
- the generated intermediate namely 4- ((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l,6-dien-l-yl)-2-methoxyphenyl (4-nitrophenyl) carbonate, was used for the next step without isolation.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Mycology (AREA)
- Nutrition Science (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The invention relates to fatty acid phenolic derivatives; compositions comprising an effective amount of a fatty acid phenolic derivative; and methods for treating or preventing a metabolic disease comprising the administration of an effective amount of a fatty acid phenolic derivative.
Description
FATTY ACID PHENOLIC DERIVATIVES AND THEIR USES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to and the benefit of U.S. Provisional Application Serial No. 61/483,418 filed May 6, 2011, the disclosure of which is hereby incorporated by reference in its entirety.
FIELD OF THE INVENTION
[0002] The invention relates to Fatty Acid Phenolic Derivatives; compositions comprising an effective amount of a Fatty Acid Phenolic Derivative; and methods for treating or preventing cancer and metabolic, autoimmune or neurodegenerative disorders, comprising the administration of an effective amount of a Fatty Acid Phenolic Derivative. All patents, patent applications, and publications cited herein are hereby incorporated by reference in their entireties.
BACKGROUND OF THE INVENTION
[0003] Oily cold water fish, such as salmon, trout, herring, and tuna are the source of dietary marine omega-3 fatty acids, with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) being the key marine derived omega-3 fatty acids. Omega-3 fatty acids have previously been shown to improve insulin sensitivity and glucose tolerance in normoglycemic men and in obese individuals. Omega-3 fatty acids have also been shown to improve insulin resistance in obese and non-obese patients with an inflammatory phenotype. Lipid, glucose, and insulin metabolism have been shown to improve in overweight hypertensive subjects through treatment with omega-3 fatty acids. Omega-3 fatty acids (EPA/DHA) have also been shown to decrease triglycerides and to reduce the risk for sudden death caused by cardiac arrhythmias in addition to improve mortality in patients at risk of a cardiovascular event. Omega-3 fatty acids have also been taken as dietary supplements part of therapy used to treat dyslipidemia, and anti-inflammatory properties. A higher intake of omega-3 fatty acids lower levels of circulating TNF-a and IL-6, two of the cytokines that are markedly increased during inflammation processes (Chapkin et al, Prostaglandins, Leukot Essent Fatty Acids 2009, 81, p. 187-191; Duda et al, Cardiovasc Res 2009, 84, p. 33-41). In addition, a higher intake of omega-3 fatty acids has also been shown to increase levels of the
well-characterized anti-inflammatory cytokine IL-10 (Bradley et al, Obesity (Silver Spring) 2008, 16, p. 938-944). More recently, there is additional evidence that omega-3 fatty acids could play a significant role in oncology (Anderson et al, Lipids in Health and Disease 2009, 8, p.33; Bougnoux et al, Progress in Lipid Research 2010, 49, p. 76-86; Erickson et al, Prostaglandins, Leukotrienes and Essential Fatty Acids 2010, 82, p. 237-241). In a study using the xenograft model in nude mice, treatment with omega-3 fatty acids, such as DHA and EPA, resulted in breast tumor regression. Here, treatment with DHA/EPA appeared to increase the level of PTEN protein and attenuate the PI 3 kinase and Akt kinase activity as well as the expression of the anti-apoptotic proteins Bcl-2 and Bcl-XL in the breast tumors (Ghosh-Choudhury, T. et al. Breast Cancer Res. Treat. 2009, 118 (1), 213-228). Additional evidence supporting the use of omega-3 fatty acids in oncology also appeared in a recent study by Lim et al. showing that DHA/EPA could inhibit hepatocellular carcinoma cell growth, presumably by blocking β-catenin and cyclooxygenase-2 (Lim, K. et al. Mol. Cancer Ther. 2009, 8 (11), 3046-3055).
[0004] Both DHA and EPA are characterized as long chain fatty acids (aliphatic portion between 12-22 carbons). Medium chain fatty acids are characterized as those having the aliphatic portion between 6-12 carbons. Lipoic acid is a medium chain fatty acid found naturally in the body. It plays many important roles such as free radical scavenger, chelator to heavy metals and signal transduction mediator in various inflammatory and metabolic pathways, including the NF-κΒ pathway (Shay, K. P. et al. Biochim. Biophys. Acta 2009, 1790, 1149-1160). Lipoic acid has been found to be useful in a number of chronic diseases that are associated with oxidative stress (for a review see Smith, A. R. et al Curr. Med. Chem.
2004, 11, p. 1135-46). Lipoic acid has now been evaluated in the clinic for the treatment of diabetes (Morcos, M. et al Diabetes Res. Clin. Pract. 2001, 52, p. 175-183) and diabetic neuropathy (Mijnhout, G. S. et al Neth. J. Med. 2010, 110, p. 158-162). Lipoic acid has also been found to be potentially useful in treating cardiovascular diseases (Ghibu, S. et al, J. Cardiovasc. Pharmacol. 2009, 54, p. 391-8), Alzheimer's disease (Maczurek, A. et al, Adv. Drug Deliv. Rev. 2008, 60, p. 1463-70) and multiple sclerosis (Yadav, V. Multiple Sclerosis
2005, 11, p. 159-65; Salinthone, S. et al, Endocr. Metab. Immune Disord. Drug Targets 2008, S, p. 132-42).
[0005] Gallic acid is a naturally occurring polyhydroxy phenolic compound found in gallnuts, grapes, certain vegetables, tea leaves and oak bark which has been shown to exhibit
anti-oxidant, antimutagenic and anticarcinogenic effects. Gallic acid has demonstrated oral efficacy in an obesity rat model. In male Wistar rats on a high fat diet (HFD), animals dosed with gallic acid as an admix in the HDF chow had decreased liver weights as well as reduced adipose tissue (peritoneal fat and epidermal fat) weights when compared to the control animals on HFD alone. The HFD + Gallic acid fed rats also exhibited reduced serum triacylglycerol, insulin and lipid levels. (Yen, G-C and Hsu, C-L, British Journal of Nutrition, 2007, 98, 727-735) One possible mechanism of action of gallic acid involves the NF-kB signaling pathway through the inhibition of p300-mediated p65 acetylation, thereby restoring the association of IKBa with the p65 subunit of NF-κΒ. (Jung, M. G. et al, Mol Cancer Res, 2009, 7(12), 2011-2021) Gallic acid has also been shown in vitro to enhance insulin secretion in RINm5F cells through the upregulation of PDX-1 and Maf-A, two major beta-cell transcription factors. (Balasubramanyam, Muthuswamy et al, Phytotherapy Research, 2010, 24, 883-894). Treatment of glucose-treated THP-1 monocytes with gallic acid demonstrated decreased mRNA levels of anti-inflammatory cytokines such as IL-6, TNF-a, SOCS-3 and NADPH (Balasubramanyam, Muthuswamy et al, Cytokine, 2010, 49, 229-234). Chronic oxidative stress and inflammation have now been linked to the development and progression of a number of debilitating diseases beyond metabolic disease. Some of these diseases include renal failure, heart failure, atherosclerosis, osteoporosis, cancer, chronic obstructive pulmonary disease (COPD), Parkinson's disease and Alzheimer's disease.
[0006] In addition to gallic acid, flavonoids are phenolic derivatives that have been shown to have beneficial anti-oxidant properties (for a comprehensive review, see: Flavonoids: Chemistry, Biochemistry and Applications 2006, Edited by Anderson, O. M. and Markham, K. R.). Quercetin, for instance, is a flavonoid that has been shown to have protective effects against β-cell damage in experimental streptozotocin (STZ)-induced diabetic rats (Corkun et al, Pharmacological Research 2005, 51, p. 117-123). Naringenin, epicatechin, myricetin, apigenin are some additional examples of naturally-occurring flavonoids that have some beneficial anti-diabetic properties (G. Brahmachari Opportunities, Challenges and Scope of Natural Products in Medicinal Chemistry 2011, p. 187-212).
[0007] Curcumin is a phenolic derivative, belonging to the chalcone family of natural products, that has been shown to have many beneficial anti-oxidant and anti-inflammatory
properties. Curcumin has been shown to protect islets of C57/BL6 mice against streptozotocin-induced oxidative stress (Meghana et al, Eur. J. Pharm. 2007, 577, p. 183- 191). It also has been shown to have antihyperglycemic effect and improved insulin sensitivity in diet-induced obese (DIO) Sprague Dawley rats (El-Moselhy et al, Food and Chemical Toxicology 2011, 49, p. 1129-1140). The mechanism of action may be attributed, in part, to the anti-inflammatory properties, as evident by attenuating TNF-a levels in these DIO rats. In addition to curcumin, a variety of other chalcone derivatives have also been shown to have significant anti-inflammatory properties. These include isoliquiritigenin, flavokawain A, cardomonin, butein, licochalcone A, xanthohumol, BMS 181156, AGN 193198 and MX 781 (Srinivasan et al J. Med. Chem. 2009, 52, p. 7228-7235). For many of these chalcone derivatives, the anti-inflammatory properties can be attributed, in part, to the inhibition of NF-κΒ through modification of its target(s), such as ΙΚΚβ, via a Michael addition onto the unsaturated enone moiety.
[0008] The ability to provide the effects of fatty acids and phenolic derivatives such as gallic acid, flavonoids and chalcones in a synergistic way would provide benefits in treating a variety of cancer, metabolic, autoimmune and neurodegenerative diseases.
SUMMARY OF THE INVENTION
[0009] The invention is based in part on the discovery of Fatty Acid Phenolic Derivatives and their demonstrated effects in achieving improved treatment that cannot be achieved by administering a Phenolic derivative or fatty acids alone or in combination. The Fatty Acid Phenolic Derivatives are designed to be stable in the plasma. In target tissues, the individual components (i.e. fatty acid, phenolic derivative) are then released by the action of various intracellular enzymes. These novel Fatty Acid Phenolic Derivatives are useful in the treatment or prevention of metabolic disorders including atherosclerosis, dyslipidemia, coronary heart disease, hypercholesterolemia, Type 2 diabetes, elevated cholesterol, metabolic syndrome, diabetic nephropathy, IgA nephropathy, chronic kidney disease (CKD) and cardiovascular disease. In addition, they are useful in the treatment of autoimmune diseases such as rheumatoid arthritis, psoriasis, systemic lupus erythematosus, inflammatory bowel diseases (including colitis and Crohn's disease), respiratory diseases such as asthma, cystic fibrosis, COPD and neurodegenerative diseases such as multiple sclerosis, Parkinson's disease and Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis (ALS)
and muscular dystrophy. The compounds described herein are also useful in treating a variety of cancer such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiple myeloma, seminoma, and cancer of the bladder, blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary, pancreas, prostate, testicle, spleen, small intestine, large intestine or stomach.
[0010] Accordingly in one aspect, a molecular conjugate is described which comprises a Phenolic derivative and a fatty acid wherein the fatty acid is selected from the group consisting of omega-3 fatty acids, fatty acids that are metabolized in vivo to omega-3 fatty acids, and lipoic acid, and the conjugate is capable of hydrolysis to produce free Phenolic derivative and free fatty acid. In some embodiments, the fatty acid is selected from the group consisting of a//-cz's-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid, eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, tetracosahexaenoic acid and lipoic acid. In other embodiments, the fatty acid is selected from eicosapentaenoic acid, docosahexaenoic acid and lipoic acid. In some embodiments, the hydrolysis is enzymatic.
[0011] In another aspect, compounds of the Formula I are described:
Formula I and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers, and stereoisomers thereof; wherein
R7, Rs and R9 are each independently selected from the group consisting of H, OH, OCH3, or OC(0)R' where R' is independently C1-C3 alkyl, or a second molecule of gallic acid, each Wi,W2 is independently null, O, S, NH, or NR, or Wi and W2 can be taken together to form an optionally substituted imidazolidine or piperazine group; each a, b, c, d, is independently -H, -D, halogen, -CH3, -CF3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, q is independently 0, 1 or 2; each L is independently null, -0-, -C(0)-,-S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, - (C3-C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula I; each R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4;
each h is independently 1, 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1, 2 or 3; k is 0, 1, 2 or 3; z is 1, 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each Z is independently H,
provided that there is at least one of
in the compound; each r is independently 2, 3, or 7; each s is independently 3, 5, or 6; each t is independently 0 or 1 ; each v is independently 1, 2, or 6; each w is independently 0 or 1; each Ri and R2 is independently -H, -D, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4
alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each R5 is independently e, H or straight or branched Ci-Cio alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -Ci-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
[0012] In another aspect, compounds of the Formula II are described:
Formula II and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof; wherein Gi is
W3 is independently O or null;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group;
Rio is independently H, OH, OR", R", or OC(0)R" where R" is independently C C6 alkyl; each a, b, c, and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
- 12-
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula II; each R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1, 2 or 3; k is 0, 1, 2 or 3; z is 1, 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each Z is independently H,
in the compound;
each r is independently 2, 3, or each s is independently 3, 5, or each t is independently 0 or 1 ; each v is independently 1, 2, or each w is independently 0 or 1; each Ri and R2 is independently -H, -D, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(d-C3 alkyl), -S(0)C C3 alkyl, -S(0)2C C3 alkyl; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or argmme; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
[0013] In another aspect, compounds of the Formula III are described:
Formula III and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof;
wherein G2 is
W3 is independently O or null;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group;
Rio is independently H, OH, OR", R", or OC(0)R" where R" is independently d- C6 alkyl; each a, b, c, and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
- 17-
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula III; each R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1, 2 or 3; k is 0, 1, 2 or 3; z is 1, 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR,
and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each Z is independently H,
provided that there is at least one of
in the compound; each r is independently 2, 3, or 7; each s is independently 3, 5, or 6; each t is independently 0 or 1 ; each v is independently 1, 2, or 6; each w is independently 0 or 1; each Ri and R2 is independently -H, -D, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2C C3 alkyl; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids;
each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
[0014] In another aspect, compounds of the Formula la are described:
Formula la and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
-22-
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1, 2 or 3; k is 0, 1, 2 or 3; z is 1, 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR,
and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, CeH4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
[0015] In another aspect, compounds of the Formula lb are described:
Formula lb and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(0)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1, 2 or 3; k is 0, 1, 2 or 3; z is 1, 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR,
and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole;
each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
[0016] In another aspect, compounds of the Formula Ilia are described:
Formula Ilia and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(0)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1, 2 or 3; k is 0, 1, 2 or 3; z is 1, 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR,
and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, CeH4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
[0017] In another aspect, compounds of the Formula Illb are described:
Formula Illb and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
-31 -
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1, 2 or 3; k is 0, 1, 2 or 3; z is 1, 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR,
and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
[0018] In Formula I, II, III, la, lb, Ilia and Mb any one or more of H may be substituted with a deuterium. It is also understood in Formula I, II, III, la, lb, Ilia and Illb that a methyl substituent can be substituted with a Ci-C6 alkyl.
[0019] Also described are pharmaceutical formulations comprising at least one Fatty Acid Phenolic Derivative.
[0020] Also described herein are methods of treating a disease susceptible to treatment with a Fatty Acid Phenolic Derivative in a patient in need thereof by administering to the patient an effective amount of a Fatty Acid Phenolic Derivative.
[0021] Also described herein are methods of treating metabolic disorders or autoimmune disease or neurodegenerative diseases by administering to a patient in need thereof an effective amount of a Fatty Acid Phenolic Derivative.
[0022] Also described herein are methods of treating neurodegenerative diseases by administering to a patient in need thereof an effective amount of a Fatty Acid Phenolic Derivative.
[0023] Also described herein are methods of treating cancer by administering to a patient in need thereof an effective amount of a Fatty Acid Phenolic Derivative.
[0024] The invention also includes pharmaceutical compositions that comprise an effective amount of a Fatty Acid Phenolic Derivative and a pharmaceutically acceptable carrier. The compositions are useful for treating or preventing a metabolic disorder,
neurodegenerative diseases, and cancer. The invention includes a Fatty Acid Phenolic Derivative when provided as a pharmaceutically acceptable prodrug, a hydrate, a salt, such as a pharmaceutically acceptable salt, enantiomer, stereoisomer, or mixtures thereof.
[0025] The details of the invention are set forth in the accompanying description below. Although methods and materials similar or equivalent to those described herein can be used in the practice or testing of the present invention, illustrative methods and materials are now described. Other features, objects, and advantages of the invention will be apparent from the description and from the claims. In the specification and the appended claims, the singular forms also include the plural unless the context clearly dictates otherwise. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. All patents and publications cited in this specification are incorporated herein by reference in their entireties.
DETAILED DESCRIPTION OF THE INVENTION
[0026] Metabolic disorders are a wide variety of medical disorders that interfere with a subject's metabolism. Metabolism is the process a subject's body uses to transform food into energy. Metabolism in a subject with a metabolic disorder is disrupted in some way. Autoimmune diseases arise from an overactive immune response of the body against tissues normally present in the body. Neurodegenerative diseases result from the deterioration of neurons or their myelin sheaths, which would eventually lead to a variety of CNS-related dysfunctions. The Fatty Acid Phenolic Derivatives possess the ability to treat or prevent metabolic disorders, autoimmune or neurodegenerative diseases. In addition, the Fatty Acid Phenolic Derivatives can also be used to treat a variety of cancer such as such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiple myeloma, seminoma, and cancer of the bladder, blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary, pancreas, prostate, testicle, spleen, small intestine, large intestine or stomach.
[0027] The Fatty Acid Phenolic Derivatives have been designed to bring together a Phenolic derivative and omega 3 fatty acids into a single molecular conjugate. In addition, the Fatty Acid Phenolic Derivatives have also been designed to bring together a Phenolic derivative and lipoic acid into a single molecular conjugate. The activity of the Fatty Acid
Phenolic Derivatives is substantially greater than the sum of the components suggesting that the activity induced by the Fatty Acid Phenolic Derivatives is synergistic.
DEFINITIONS
[0028] The following definitions are used in connection with the Fatty Acid Phenolic derivative:
[0029] The term "Fatty Acid Phenolic Derivatives" includes any and all possible isomers, stereoisomers, enantiomers, diastereomers, tautomers, pharmaceutically acceptable salts, hydrates, solvates, and prodrugs of the Fatty Acid Phenolic Derivatives described herein.
[0030] The articles "a" and "an" are used in this disclosure to refer to one or more than one (i.e., to at least one) of the grammatical object of the article. By way of example, "an element" means one element or more than one element.
[0031] The term "and/or" is used in this disclosure to mean either "and" or "or" unless indicated otherwise.
[0032] Unless otherwise specifically defined, the term "aryl" refers to cyclic, aromatic hydrocarbon groups that have 1 to 2 aromatic rings, including monocyclic or bicyclic groups such as phenyl, biphenyl or naphthyl. Where containing two aromatic rings (bicyclic, etc.), the aromatic rings of the aryl group may be joined at a single point (e.g., biphenyl), or fused (e.g., naphthyl). The aryl group may be optionally substituted by one or more substituents, e.g., 1 to 5 substituents, at any point of attachment. The substituents can themselves be optionally substituted.
[0033] "C1-C3 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-3 carbon atoms. Examples of a C1-C3 alkyl group include, but are not limited to, methyl, ethyl, propyl and isopropyl.
[0034] "C1-C4 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-4 carbon atoms. Examples of a C1-C4 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, isopropyl, isobutyl, sec-butyl and tert-butyl.
[0035] "C1-C5 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-5 carbon atoms. Examples of a C1-C5 alkyl group include, but are not limited
to, methyl, ethyl, propyl, butyl, pentyl, isopropyl, isobutyl, sec-butyl and tert-butyl, isopentyl and neopentyl.
[0036] "Ci-C6 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-6 carbon atoms. Examples of a Ci-C6 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, pentyl, hexyl, isopropyl, isobutyl, sec-butyl, tert-butyl, isopentyl, and neopentyl.
[0037] The term "cycloalkyl" refers to a cyclic hydrocarbon containing 3-6 carbon atoms. Examples of a cycloalkyl group include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
[0038] It is understood that any of the substitutable hydrogens on an alkyl or cycloalkyl can be substituted with halogen, C1-C3 alkyl, hydroxyl, alkoxy and cyano groups.
[0039] The term "heterocycle" as used herein refers to a cyclic hydrocarbon containing 3- 6 atoms wherein at least one of the atoms is an O, N, or S. Examples of heterocycles include, but are not limited to, aziridine, oxirane, thiirane, azetidine, oxetane, thietane, pyrrolidine, tetrahydrofuran, tetrahydrothiophene, piperidine, tetrahydropyran, thiane, imidazolidine, oxazolidine, thiazolidine, dioxolane, dithiolane, piperazine, oxazine, dithiane, and dioxane.
[0040] The term "heteroaryl" as used herein refers to a monocyclic or bicyclic ring structure having 5 to 12 ring atoms wherein one or more of the ring atoms is a heteroatom, e.g. N, O or S and wherein one or more rings of the bicyclic ring structure is aromatic. Some examples of heteroaryl are pyridyl, furyl, pyrrolyl, thienyl, thiazolyl, oxazolyl, imidazolyl, indolyl, tetrazolyl, benzofuryl, xanthenes and dihydroindole. It is understood that any of the substitutable hydrogens on a heteroaryl can be substituted with halogen, C1-C3 alkyl, hydroxyl, alkoxy and cyano groups.
[0041] The term "any one of the side chains of the naturally occurring amino acids" as used herein means a side chain of any one of the following amino acids: Isoleucine, Alanine, Leucine, Asparagine, Lysine, Aspartate, Methionine, Cysteine, Phenylalanine, Glutamate, Threonine, Glutamine, Tryptophan, Glycine, Valine, Proline, Arginine, Serine, Histidine and Tyrosine.
[0042] The term "fatty acid" as used herein means an omega-3 fatty acid and fatty acids that are metabolized in vivo to omega-3 fatty acids. Non-limiting examples of fatty acids are
a/7-cz's-7,10,13-hexadecatrienoic acid, a-linolenic acid (ALA or all-cis-9, 12,15- octadecatrienoic acid), stearidonic acid (STD or a/7-cz's-6,9,12,15-octadecatetraenoic acid), eicosatrienoic acid (ETE or all-cis- 11,14,17-eicosatrienoic acid), eicosatetraenoic acid (ETA or a//-cz's-8,l l,14,17-eicosatetraenoic acid), eicosapentaenoic acid (EPA or all-cis- 5,8,11, 14, 17-eicosapentaenoic acid), docosapentaenoic acid (DP A, clupanodonic acid or all- cz's-7,10,13,16,19-docosapentaenoic acid), docosahexaenoic acid (DHA or all-cis- 4,7,10,13,16,19-docosahexaenoic acid), tetracosapentaenoic acid (a/7-cz's-9,12,15,18,21- docosahexaenoic acid), tetracosahexaenoic acid (nisinic acid or a/7-cz's-6,9,12,15,18,21- tetracosenoic acid) or lipoic acid.
[0043] The term "phenolic derivative" as used herein means the molecule known as gallic acid, flavonoid (such as quercetin, naringenin, epicatechin, myricetin, apigenin) or chalcone (such as curcumin, isoliquiritigenin, flavokawain A, cardomonin, butein, licochalcone A, xanthohumol, BMS 181156, AGN 193198 and MX 781) and any derivatives thereof. More comprehensive list of flavonoids and chalcones can be found in Flavonoids: Chemistry, Biochemistry and Applications 2006, Edited by Anderson, O. M. and Markham, K. R. and Srinivasan et al J. Med. Chem. 2009, 52, p. 7228-7235.
[0044] A "subject" is a mammal, e.g., a human, mouse, rat, guinea pig, dog, cat, horse, cow, pig, or non-human primate, such as a monkey, chimpanzee, baboon or rhesus, and the terms "subject" and "patient" are used interchangeably herein.
[0045] The invention also includes pharmaceutical compositions comprising an effective amount of a Fatty Acid Phenolic Derivative and a pharmaceutically acceptable carrier. The invention includes a Fatty Acid Phenolic Derivative when provided as a pharmaceutically acceptable prodrug, hydrate, salt, such as a pharmaceutically acceptable salt, enantiomers, stereoisomers, or mixtures thereof.
[0046] Representative "pharmaceutically acceptable salts" include, e.g., water-soluble and water-insoluble salts, such as the acetate, amsonate (4,4-diaminostilbene-2, 2 - disulfonate), benzenesulfonate, benzonate, bicarbonate, bisulfate, bitartrate, borate, bromide, butyrate, calcium, calcium edetate, camsylate, carbonate, chloride, citrate, clavulariate, dihydrochloride, edetate, edisylate, estolate, esylate, fiunarate, gluceptate, gluconate, glutamate, glycollylarsanilate, hexafluorophosphate, hexylresorcinate, hydrabamine, hydrobromide, hydrochloride, hydroxynaphthoate, iodide, isothionate, lactate, lactobionate,
laurate, magnesium, malate, maleate, mandelate, mesylate, methylbromide, methylnitrate, methylsulfate, mucate, napsylate, nitrate, N-methylglucamine ammonium salt, 3-hydroxy-2- naphthoate, oleate, oxalate, palmitate, pamoate (l,l-methene-bis-2-hydroxy-3 -naphthoate, einbonate), pantothenate, phosphate/diphosphate, picrate, polygalacturonate, propionate, p-toluenesulfonate, salicylate, stearate, subacetate, succinate, sulfate, sulfosalicylate, suramate, tannate, tartrate, teoclate, tosylate, triethiodide and valerate salts.
[0047] The term "metabolic disease" as used herein refers to disorders, diseases and syndromes involving dyslipidemia, and the terms metabolic disorder, metabolic disease, and metabolic syndrome are used interchangeably herein.
[0048] An "effective amount" when used in connection with a fatty acid phenolic derivative is an amount effective for treating or preventing a metabolic disease.
[0049] The term "carrier", as used in this disclosure, encompasses carriers, excipients, and diluents and means a material, composition or vehicle, such as a liquid or solid filler, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting a pharmaceutical agent from one organ, or portion of the body, to another organ, or portion of the body.
[0050] The term "treating", with regard to a subject, refers to improving at least one symptom of the subject's disorder. Treating can be curing, improving, or at least partially ameliorating the disorder.
[0051] The term "disorder" is used in this disclosure to mean, and is used interchangeably with, the terms disease, condition, or illness, unless otherwise indicated.
[0052] The term "administer", "administering", or "administration" as used in this disclosure refers to either directly administering a compound or pharmaceutically acceptable salt of the compound or a composition to a subject, or administering a prodrug derivative or analog of the compound or pharmaceutically acceptable salt of the compound or composition to the subject, which can form an equivalent amount of active compound within the subject's body.
[0053] The term "prodrug," as used in this disclosure, means a compound which is convertible in vivo by metabolic means {e.g., by hydrolysis) to a fatty acid phenolic derivative.
[0054] The following abbreviations are used herein and have the indicated definitions: Boc and BOC are tert-butoxycarbonyl, Boc20 is di-tert-butyl dicarbonate, BSA is bovine serum albumin, CDI is 1 , Γ-carbonyldiimidazole, DCC is N,N-dicyclohexylcarbodiimide, DIEA is N,N-diisopropylethylamine, DMAP is 4-dimethylaminopyridine, DMEM is Dulbecco's Modified Eagle Medium, DMF is N,N-dimethylformamide, DOSS is sodium dioctyl sulfosuccinate, EDC and EDCI are l-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, ELISA is enzyme-linked immunosorbent assay, EtOAc is ethyl acetate, FBS is fetal bovine serum, h is hour, HATU is 2-(7-aza-lH-benzotriazole-l-yl)-l, 1,3,3- tetramethyluronium hexafluorophosphate, HIV is human immunodeficiency virus, HPMC is hydroxypropyl methylcellulose, oxone is potassium peroxymonosulfate, Pd/C is palladium on carbon, TFA is trifluoroacetic acid, TGPS is tocopherol propylene glycol succinate, and THF is tetrahydrofuran.
COMPOUNDS
[0055] Accordingly in one aspect, the present invention provides a molecular conjugate which comprises a phenolic derivative and a fatty acid covalently linked, wherein the fatty acid is selected from the group consisting of omega-3 fatty acids and fatty acids that are metabolized in vivo to omega-3 fatty acids, wherein the conjugate comprises is capable of hydrolysis to produce free phenolic derivative and free fatty acid.
[0056] In some embodiments, the fatty acid is selected from the group consisting of all- cz's-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid, eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, and tetracosahexaenoic acid. In other embodiments, the fatty acid is selected from eicosapentaenoic acid and docosahexaenoic acid. In some embodiments, the fatty acid is selected from lipoic acid. In some embodiments, the hydrolysis is enzymatic.
[0057] In another aspect, the present invention provides fatty acid phenolic derivatives according to Formula I, II, III, la, lb, Ilia and Illb:
Formula Ilia
Formula Illb
and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof; wherein
Gi,G2, Ri, R2, R3, R4, R5, Re, R7, Rs, R9, Rio, Wi, W2 , W3, L, a, c, b, d, e, g, h, m, n, o, p, q, Z, r, s, t, u, v, w and z, are as defined above for Formula I, II, III, la, lb, Ilia and Illb.
[0058] In some embodiments, Gi is
[0059] In some embodiments, Gi
[0060] In some embodiments, Gi is
[0061] In some embodiments, Gi is
[0062] In some embodiments, G2 is
[0063] In some embodiments, G2 is
[0066] In some embodiments, G2 is
[0068] In some embodiments, W3 is null.
[0069] In some embodiments, W3 is O.
[0070] In some embodiments, one Z is
[0071] In some embodiments, one Z is
and r is 7.
and s is 3.
and s is 5.
and s is 6.
and v is 1.
[0081] In other embodiments, one Z is
[0084] In some embodiments, Wi is NH.
[0085] In some embodiments, W2 is NH.
[0086] In some embodiments, Wi is O.
[0087] In some embodiments, W2 is O.
[0088] In some embodiments, Wi is null.
[0089] In some embodiments, W2 is null.
[0090] In some embodiments, Wi and W2 are each NH.
[0091] In some embodiments, Wi and W2 are each null.
[0092] In some embodiments, Wi is O and W2 is NH.
[0093] In some embodiments, Wi and W2 are each NR, and R is CH3.
[0094] In some embodiments, m is 0.
[0095] In other embodiments, m is 1.
[0096] In other embodiments, m is 2.
[0097] In some embodiments, L is -S- or -S-S-.
[0098] In some embodiments, L is -0-.
[0100] In some embodiments, L is -C(O)-.
[0101] In some embodiments, L is heteroaryl.
[0102] In some embodiments, L is heterocycle.
[0107] In some embodiments, L is
R3
[0109] In some embodiments, L is ^ ^ ^H2^m ^ wherein m is 2.
R3
[0110] In some embodiments, L is ^ ^ ^H2^m ^ wherein m is 3.
[0112] In some embodiments, L is
[0113] In some embodiments, L is
[0115] In some embodiments, L is
[0116] In some embodiments, L is
[0118] In other embodiments, one of n, o, p, and q is 1.
[0119] In some embodiments, two of n, o, p, and q are each 1.
[0120] In other embodiments, three of n, o, p, and q are each 1.
[0121] In some embodiments n, o, p, and q are each 1.
[0122] In some embodiments, one d is C(0)OR.
[0123] In some embodiments, r is 2 and s is 6.
[0124] In some embodiments, r is 3 and s is 5.
[0125] In some embodiments, t is 1.
[0126] In some embodiments, w is 0.
[0127] In some embodiments, w is 1.
[0128] In some embodiments, Wi and W2 are each NH, m is 0, n, and o are each 1, and p and q are each 0.
[0129] In some embodiments, Wi and W2 are each NH, m is 1, n, o, p, and q are each 1, and L is O.
[0131] In some embodiments, Wi and W2 are each NH, m is 1, n, o, p, and q are each 1, and L is -S-S-.
[0132] In some embodiments, Wi and W2 are each NH, m is 1, n and o are each 0, p and q are each 1 , and L is
[0133] In some embodiments, Wi and W2 are each NH, m is 1, k is O, n and o are each 0, p and q are each 1 , and L is
[0134] In some embodiments, Wi and W2 are each NH, m is 1, n and o are each 1 , p and q are each 0, and L is
[0135] In some embodiments, Wi and W2 are each NH, m is 1, k is 0, n is 1, o, p and q are each 0, and L is
In some embodiments, Wi and W2 are each NH, m is 1, k is 1, n, o, and p are each 1 , and L is
[0138] In some embodiments, Wi and W2 are each NH, m is 1, n is 1, and o, p, and q are each 0, and L is
[0140] In some embodiments, Wi and W2 are each NH, m is 1, n, o, p, and q are each 1, and L is
[0142] In some embodiments, Wi and W2 are each NH, m is 0, k is 1, o and p are each 1, and q is 0.
[0143] In some embodiments, Wi and W2 are each NH, m is 0, n, o, p, and q are each 1.
[0144] In some embodiments, Wi and W2 are each NH, m is 0, n and o are each 1 , p and q are each 0, and each a is CH3.
[0145] In some embodiments, Wi and W2 are each NH, m is 0, n and o are each 1 , p and q are each 0, and each b is CH3.
[0146] In some embodiments, Wi and W2 are each NH, m is 1, n, o, p, and q are each 1, R3 is H, and L is
R3
xV.
[0147] In some embodiments, Wi and W2 are each NH, m is 1, n, p and q are each 1, and o is 2, R3 is H, and L is
R3
xV.
[0148] In some embodiments, Wi and W2 are each NH, m is 1, n, o, p are each 1, and q is 2, and L is
R3
xV.
[0149] In some embodiments, Wi and W2 are each NH, m is 1, n, o, p, and q are each 1, and L is
N R3R3
(?H2)g
[0150] In some embodiments, Wi and W2 are each NH, m is 1, n and p are each 1 , and o and q are each 0, and L is -C(O)-.
[0151] In some embodiments, Wi and W2 are each NH, m is 1, n and p are each 1, and o, and q are each 0, and L is
[0152] In some embodiments, Wi and W2 are each NH, m is 1 , n, o, p, q are each 1 , and L is
R
O'
[0153] In some embodiments, Wi and W2 are each NH, m is 1 , n, o, p , and q are each 1, h is 1 , and L is
[0154] In some embodiments, Wi and W2 are each NH, m is 1 , n, o, p , and q are each 1, and L is-S-.
[0155] In some embodiments, W1 and W2 are each NH, m is 1, n, o, p are each 0, q is 1, one d is -C¾, and L is
[0156] In some embodiments, Wi and W2 are each NH, m is 2, n, o, p, and q are each 0, one L is
[0157] In some embodiments, m is 0, n, o, p, and q are each 0, and Wi and W2 are taken together to form an optionally substituted piperazine group.
[0159] In some embodiments, m is 1, n and p are each 1, o and q are each 0, Wi and W2 are each NH, and L is C3-C6 cycloalkyl.
[0160] In some embodiments, m is 1, n is 1 , o, p, and q are each 0, Wi and W2 are each NH, and L is C3-C6 cycloalkyl.
[0161] In some embodiments, m is 1 , n, o, p, are each 0, q is 1 , Wi and W2 are each NH, and L is C -C6 cycloalkyl.
[0163] In some embodiments, m is 1, n o, p, and q are each 0, Wi is null, W2 is NH, and
[0164] In some embodiments, m is 1, n o, p, and q are each 0, Wi is NH, W2 is null, and
[0166] In some embodiments, m is 1, n is 1, o, p, and q are each 0, Wi is NH, W2 is null, and L is
(R6)m1
[0168] In some embodiments, m is 1, n, o, p, and q are each 0, Wi is NH, W2 is null, and L is
[0169] In some embodiments, m is 1, n, o, p, and q are each 0, Wi is null, W2 is NH, and L is
[0175] In some embodiments, m is 1, n, o, p, q are each 0, Wi and W2 is null, and L is
[0176] In some embodiments, m is 1, n, o, p, q are each 0, Wi is NH, W2 is null, and L is
[0177] In some embodiments, m is 1, n, o, p, q are each 0, Wi is null, W2 is NH, and L is
[0178] In some embodiments, m is 1, n, o, p, are each 0, q is 1, Wi and W2 are each and NH, is null, L is
[0179] In some embodiments, m is 1, n, o, p, are each 0, q is 1, Wi and W2 are each NH, is null, and L is a heteroaryl.
[0180] In some of the foregoing embodiments, r is 2, s is 6 and t is 1.
[0181] In some of the foregoing embodiments, r is 3, s is 5 and t is 1.
[0182] In Formula I, II, III, la, lb, Ilia and Illb any one or more of H may be substituted with a deuterium. It is also understood in Formula I, II, III, la, lb, Ilia and Illb that a methyl substituent can be substituted with a Ci-C6 alkyl.
[0183] In other illustrative embodiments, compounds of Formula I, II, and III are as set forth below:
N-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)-3 ,4,5- trihydroxybenzamide (1-1)
3 ,4,5 -trihydroxy-N-(2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)benzamide (1-2)
N-(2-((2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)(methyl)amino)ethyl)-3,4,5-trihydroxybenzamide (1-3)
3 ,4,5-trihydroxy-N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)benzamide (1-4)
N-(2-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethoxy)ethyl)- -trihydroxybenzamide (1-5)
3 ,4,5 -trihydroxy-N-(2-(2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethoxy)ethyl)benzamide (1-6)
-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)-2-(3 ,4,5- trihydroxybenzamido)hexanoic acid (1-7)
-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)-6-(3 ,4,5- trihydroxybenzamido)hexanoic acid (1-9)
2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17-pentaenamido)-6-(3 ,4,5 - trihydroxybenzamido)hexanoic acid (1-10)
N-(2-((2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)disulfanyl)ethyl)-3,4,5-trihydroxybenzamide (I-ll)
3 ,4,5-trihydroxy-N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)benzamide (1-12)
(R)-N-(2-(5-(l,2-dithiolan-3-yl)pentanamido)ethyl)-3,4,5-trihydroxybenzamide (1-13)
(R)-N-(2-((2-(5 -( 1 ,2-dithiolan-3 -yl)pentanamido)ethyl)(methy l)amino)ethyl)-3 ,4,5 - trihydroxybenzamide (1-14)
(R)-N-(2-(2-(5-(l,2-dithiolan-3-yl)pentanamido)ethoxy)ethyl)-3,4,5-trihydroxybenzamide (I-
15)
2-(5-((R)-l ,2-dithiolan-3-yl)pentanamido)-6-(3,4,5-trihydroxybenzamido)hexanoic acid (I-
4-oxo-2-phenyl-4H-chromen-6-yl (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)carbamate (I- 18)
4-oxo-2-phenyl-4H-chromen-6-yl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)carbamate (I- 19)
(R)-4-oxo-2-phenyl-4H-chromen-6-yl (2-(5-(l,2-dithiolan-3-yl)pentanamido)ethyl)carbamate
-20)
-oxo-2-phenyl-4H-chromen-6-yl (2-((2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)(methyl)amino)ethyl)carbamate (1-21)
4-oxo-2-phenyl-4H-chromen-6-yl (2-((2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (1-22)
(R)-4-oxo-2-phenyl-4H-chromen-6-yl (2-((2-(5-(l ,2-dithiolan-3- yl)pentanamido)ethyl)(methyl)amino)ethyl)carbamate (1-23)
-oxo-2-phenyl-4H-chromen-6-yl (2-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethoxy)ethyl)carbamate (1-24)
4-oxo-2-phenyl-4H-chromen-6-yl (2-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethoxy)ethyl)carbamate (1-25)
-oxo-2-phenyl-4H-chromen-6-yl (2-(2-(5-(l ,2-dithiolan-3- yl)pentanamido)ethoxy)ethyl)carbamate (1-26)
-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)-2-((((4-oxo-2-phenyl- -chromen-6-yl)oxy)carbonyl)amino)hexanoic acid (1-27)
6-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)-2-((((4-oxo-2-phenyl-4H- chromen-6-yl)oxy)carbonyl)amino)hexanoic acid (1-28)
6-(5-((R)-l,2-dithiolan-3-yl)pentanamido)-2-((((4-oxo-2-phenyl-4H-chromen-6- yl)oxy)carbonyl)amino)hexanoic acid (1-29)
-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)-6-((((4-oxo-2-phenyl- -chromen-6-yl)oxy)carbonyl)amino)hexanoic acid (1-30)
2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)-6-((((4-oxo-2-phenyl-4H- chromen-6-yl)oxy)carbonyl)amino)hexanoic acid (1-31)
2-(5-((R)-l,2-dithiolan-3-yl)pentanamido)-6-((((4-oxo-2-phenyl-4H-chromen-6- yl)oxy)carbonyl)amino)hexanoic acid (1-32)
-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl (2- -docosa-4,7, 10,13,16,19-hexaenamido)ethyl)carbamate (1-33)
-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)carbamate (1-34)
-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl
(2-(5-((R)- 1 ,2-dithiolan-3-yl)pentanamido)ethyl)carbamate (1-35)
(2-((2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)(methyl)amino)ethyl)carbamate (1-36)
4-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl
(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (1-37)
4-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl
(2-((2-(5-((R)- 1 ,2-dithiolan-3-yl)pentanamido)ethyl)(methyl)amino)ethyl)carbamate (1-38)
4-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl
(2-(2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethoxy)ethyl)carbamate (1-39)
4-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl (2-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l ,14,17-pentaenamido)ethoxy)ethyl)carbamate (1-40)
-(( 1 E,6E)-7-(4-hydroxy-3 -methoxyphenyl)-3 ,5 -dioxohepta- 1 ,6-dien- 1 -yl)-2-methoxyphenyl -(2-(5-((R)- 1 ,2-dithiolan-3-yl)pentanamido)ethoxy)ethyl)carbamate (1-41)
(2-((2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10, 13,16, 19- hexaenamido)ethyl)disulfanyl)ethyl)carbamate (1-42)
N-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10, 13,16, 19-hexaenamido)ethyl)-4-((E)-3-oxo- 3-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-l-en-l-yl)benzamide (1-43)
N-(2-((5Z,8Z,l lZ,14Z, 17Z)-icosa-5,8, l l ,14, 17-pentaenamido)ethyl)-4-((E)-3-oxo-3- (5 ,5 ,8,8-tetramethyl-5 ,6,7,8-tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamide (1-44)
(R,E)-N-(2-(5-(l ,2-dithiolan-3-yl)pentanamido)ethyl)-4-(3-oxo-3-(5,5,8,8-tetramethyl- 5 ,6,7,8-tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamide (1-45)
N-(2-((2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)(methyl)amino)ethyl)-4-((E)-3-oxo-3-(5, 5,8, 8-tetramethyl-5, 6,7,8- tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamide (1-46)
N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)-4-((E)-3-oxo-3-(5,5,8,8-tetramethyl-5,6,7,8- tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamide (1-47)
(R,E)-N-(2-((2-(5-(l,2-dithiolan-3-yl)pentanamido)ethyl)(methyl)amino)ethyl)-4-(3-oxo-3- (5 ,5 ,8,8-tetramethyl-5 ,6,7,8-tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamide (1-48)
N-(2-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethoxy)ethyl)-4- ((E)-3-oxo-3-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-l-en-l- -49)
N-(2-(2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethoxy)ethyl)-4-((E)-3-oxo- 3-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-l-en-l-yl)benzamide (1-50)
(R,E)-N-(2-(2-(5-(l ,2-dithiolan-3-yl)pentanamido)ethoxy)ethyl)-4-(3-oxo-3-(5,5,8,8- tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-l-en-l-yl)benzamide (1-51)
6-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10, 13,16, 19-hexaenamido)-2-(4-((E)-3-oxo-3- (5 ,5 ,8,8-tetramethyl-5 ,6,7,8-tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamido)hexanoic acid (1-52)
6-((5Z,8Z, l lZ, 14Z, 17Z)-icosa-5,8,l l ,14, 17-pentaenamido)-2-(4-((E)-3-oxo-3-(5,5,8,8- tetramethyl-5 ,6,7,8-tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamido)hexanoic acid (I-
53)
6-(5-((R)-l ,2-dithiolan-3-yl)pentanamido)-2-(4-((E)-3-oxo-3-(5,5,8,8-tetramethyl-5,6,7,8- tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamido)hexanoic acid (1-54)
2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10, 13,16, 19-hexaenamido)-6-(4-((E)-3-oxo-3- (5 ,5 ,8,8-tetramethyl-5 ,6,7,8-tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamido)hexanoic acid (1-55)
2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)-6-(4-((E)-3-oxo-3-(5,5,8,8- tetramethyl-5 ,6,7,8-tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamido)hexanoic acid (I-
2-(5-((R)-l,2-dithiolan-3-yl)pentanamido)-6-(4-((E)-3-oxo-3-(5,5,8,8-tetramethyl-5,6,7,8- tetrahydronaphthalen-2-yl)prop- 1 -en- 1 -yl)benzamido)hexanoic acid (1-57)
METHODS FOR USING FATTY ACID PHENOLIC DERIVATIVES
[0184] Also provided in the invention is a method for inhibiting, preventing, or treating inflammation or an inflammatory disease in a subject. The inflammation can be associated with an inflammatory disease or a disease where inflammation contributes to the disease. Inflammatory diseases can arise where there is an inflammation of the body tissue. These include local inflammatory responses and systemic inflammation. Examples of such diseases include, but are not limited to: organ transplant rejection; reoxygenation injury resulting from organ transplantation (see Grupp et ah, J. Mol. Cell Cardiol. 31 : 297-303 (1999)) including, but not limited to, transplantation of the following organs: heart, lung, liver and kidney; chronic inflammatory diseases of the joints, including arthritis, rheumatoid arthritis, osteoarthritis and bone diseases associated with increased bone resorption; inflammatory bowel diseases such as ileitis, ulcerative colitis, Barrett's syndrome, and Crohn's disease; inflammatory lung diseases such as asthma, adult respiratory distress syndrome, chronic obstructive airway disease, and cystic fibrosis; inflammatory diseases of the eye including corneal dystrophy, trachoma, onchocerciasis, uveitis, sympathetic ophthalmitis and endophthalmitis; chronic inflammatory diseases of the gum, including gingivitis and periodontitis; chronic kidney disease (CKD) including focal segmented glomerulosclerosis, nephrotic syndrome, reflux uropathy or polycystic kidney disease; IgA nephropathy; inflammatory diseases of the kidney including uremic complications, glomerulonephritis and nephrosis; inflammatory diseases of the skin including sclerodermatitis, psoriasis and eczema; inflammatory diseases of the central nervous system, including chronic demyelinating diseases of the nervous system, multiple sclerosis, AIDS-related
neurodegeneration and Alzheimer's disease, infectious meningitis, encephalomyelitis, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and viral or autoimmune encephalitis. Metabolic disease such as type II diabetes mellitus; the prevention of type I diabetes; dyslipidemia; hypertriglyceridemia; diabetic complications, including, but not limited to glaucoma, retinopathy, macula edema, nephropathy, such as microalbuminuria and progressive diabetic nephropathy, polyneuropathy, diabetic neuropathy, atherosclerotic coronary arterial disease, peripheral arterial disease, nonketotic hyperglycemichyperosmolar coma, mononeuropathies, autonomic neuropathy, joint problems, and a skin or mucous membrane complication, such as an infection, a shin spot, a candidal infection or necrobiosis lipoidica diabeticorum; immune-complex vasculitis, systemic lupus erythematosus; inflammatory diseases of the heart such as cardiomyopathy, ischemic heart disease hypercholesterolemia, and atherosclerosis; as well as various other diseases that can have significant inflammatory components, including preeclampsia; chronic liver failure, brain and spinal cord trauma, and cancer. The inflammatory disease can also be a systemic inflammation of the body, exemplified by gram-positive or gram negative shock, hemorrhagic or anaphylactic shock, or shock induced by cancer chemotherapy in response to proinflammatory cytokines, e.g., shock associated with proinflammatory cytokines. Such shock can be induced, e.g., by a chemotherapeutic agent that is administered as a treatment for cancer. Other disorders include depression, obesity, allergic diseases, acute cardiovascular events, arrhythmia, prevention of sudden death.
[0185] In some embodiments, other diseases susceptible to treatment with a Fatty Acid Phenolic Derivative are muscle wasting diseases such as Muscular Dystrophy including but not limited to Duchenne's Muscular Dystrophy, Becker Muscular Dystrophy, Emery- Dreifuss Muscular Dystrophy, Limb-Girdle Muscular Dystrophy, Facioscapulohumeral Muscular Dystrophy, Myotonic Dystrophy, Oculopharyngeal Muscular Dystrophy, Distal Muscular Dystrophy, Congential Muscular Dystrophy, Spinal Muscular Atrophy, and Spinal Bulbar Muscular Dystrophy. Other diseases that can be treated with fatty acid phenolic derivatives include inflammatory myopathies such as dermatomositis, inclusion body myositis, and polymyositis, and cancer cachexia. Also inflammation that results from surgery and trauma can be treated with a Fatty Acid Phenolic Derivative. The compounds described herein are also useful in treating a variety of cancer such as carcinoma, sarcoma, lymphoma, leukemia, melanoma, mesothelioma, multiople myeloma, seminoma, and cancer
of the bladder, blood, bone, brain, breast, central nervous system, colon, endometrium, esophagus, genitourinary tract, head, larynx, liver, lung, neck, ovary, pancreas, prostate, testicle, spleen, small intestine, large intestine or stomach. Still other diseases that can be treated with Fatty Acid Phenolic Derivatives include fatty liver disease, non-alcoholic fatty liver disease, NASH (non-alcoholic steatohepatitis), Sarcopenia, Sjogren syndrome, Myasthenia gravis, and xerophthalmia.
[0186] Also provided in the invention is a method for inhibiting, preventing, or treating a metabolic disease, or symptoms of a metabolic disease, in a subject. Examples of such disorders include, but are not limited to atherosclerosis, dyslipidemia, hypertriglyceridemia, hypertension, heart failure, cardiac arrhythmias, low HDL levels, high LDL levels, sudden death, stable angina, coronary heart disease, acute myocardial infarction, secondary prevention of myocardial infarction, cardiomyopathy, endocarditis, type 2 diabetes, insulin resistance, impaired glucose tolerance, hypercholesterolemia, stroke, hyperlipidemia, hyperlipoproteinemia, chronic kidney disease, intermittent claudication, hyperphosphatemia, carotid atherosclerosis, peripheral arterial disease, diabetic nephropathy, hypercholesterolemia in HIV infection, acute coronary syndrome (ACS), non-alcoholic fatty liver disease, arterial occlusive diseases, cerebral arteriosclerosis, cerebrovascular disorders, myocardial ischemia, polycystic ovary syndrome and diabetic autonomic neuropathy.
[0187] In some embodiments, the subject is administered an effective amount of a fatty acid phenolic derivative.
[0188] The invention also includes pharmaceutical compositions useful for treating or preventing a metabolic disease, or for inhibiting a metabolic disease, or more than one of these activities. The compositions can be suitable for internal use and comprise an effective amount of a fatty acid phenolic derivative and a pharmaceutically acceptable carrier. The fatty acid phenolic derivatives are especially useful in that they demonstrate very low peripheral toxicity or no peripheral toxicity.
[0189] The fatty acid phenolic derivative scan each be administered in amounts that are sufficient to treat or prevent a metabolic disease or prevent the development thereof in subjects.
[0190] Administration of the fatty acid phenolic derivatives can be accomplished via any mode of administration for therapeutic agents. These modes include systemic or local
administration such as oral, nasal, parenteral, transdermal, subcutaneous, vaginal, buccal, rectal or topical administration modes.
[0191] Depending on the intended mode of administration, the compositions can be in solid, semi-solid or liquid dosage form, such as, for example, injectables, tablets, suppositories, pills, time-release capsules, elixirs, tinctures, emulsions, syrups, powders, liquids, suspensions, or the like, sometimes in unit dosages and consistent with conventional pharmaceutical practices. Likewise, they can also be administered in intravenous (both bolus and infusion), intraperitoneal, subcutaneous or intramuscular form, all using forms well known to those skilled in the pharmaceutical arts.
[0192] Illustrative pharmaceutical compositions are tablets and gelatin capsules comprising a fatty acid phenolic derivative and a pharmaceutically acceptable carrier, such as: a) a diluent, e.g., purified water, triglyceride oils, such as hydrogenated or partially hydrogenated vegetable oil, or mixtures thereof, corn oil, olive oil, sunflower oil, safflower oil, fish oils, such as EPA or DHA, or their esters or triglycerides or mixtures thereof, omega- 3 fatty acids or derivatives thereof, lactose, dextrose, sucrose, mannitol, sorbitol, cellulose, sodium, saccharin, glucose and/or glycine; b) a lubricant, e.g., silica, talcum, stearic acid, its magnesium or calcium salt, sodium oleate, sodium stearate, magnesium stearate, sodium benzoate, sodium acetate, sodium chloride and/or polyethylene glycol; for tablets also; c) a binder, e.g. , magnesium aluminum silicate, starch paste, gelatin, tragacanth, methylcellulose, sodium carboxymethylcellulose, magnesium carbonate, natural sugars such as glucose or beta-lactose, corn sweeteners, natural and synthetic gums such as acacia, tragacanth or sodium alginate, waxes and/or polyvinylpyrrolidone, if desired; d) a disintegrant, e.g., starches, agar, methyl cellulose, bentonite, xanthan gum, alginic acid or its sodium salt, or effervescent mixtures; e) absorbent, colorant, flavorant and sweetener; f) an emulsifier or dispersing agent, such as Tween 80, Labrasol, HPMC, DOSS, caproyl 909, labrafac, labrafil, peceol, transcutol, capmul MCM, capmul PG-12, captex 355, gelucire, vitamin E TGPS or other acceptable emulsifier; and/or g) an agent that enhances absorption of the compound such as cyclodextrin, hydroxypropyl-cyclodextrin, PEG400, PEG200.
[0193] Liquid, particularly injectable, compositions can, for example, be prepared by dissolution, dispersion, etc. For example, the fatty acid phenolic derivative is dissolved in or mixed with a pharmaceutically acceptable solvent such as, for example, water, saline, aqueous dextrose, glycerol, ethanol, and the like, to thereby form an injectable isotonic
solution or suspension. Proteins such as albumin, chylomicron particles, or serum proteins can be used to solubilize the fatty acid phenolic derivative.
[0194] The fatty acid phenolic derivatives can be also formulated as a suppository that can be prepared from fatty emulsions or suspensions; using polyalkylene glycols such as propylene glycol, as the carrier.
[0195] The fatty acid phenolic derivatives can also be administered in the form of liposome delivery systems, such as small unilamellar vesicles, large unilamellar vesicles and multilamellar vesicles. Liposomes can be formed from a variety of phospholipids, containing cholesterol, stearylamine or phosphatidylcholines. In some embodiments, a film of lipid components is hydrated with an aqueous solution of drug to a form lipid layer encapsulating the drug, as described in United States Patent No. 5,262,564, the contents of which are herein incorporated by reference in their entirety.
[0196] The fatty acid phenolic derivatives can also be delivered by the use of monoclonal antibodies as individual carriers to which the fatty acid phenolic derivatives are coupled. The fatty acid phenolic derivatives can also be coupled with soluble polymers as targetable drug carriers. Such polymers can include polyvinylpyrrolidone, pyran copolymer, polyhydroxypropylmethacrylamide-phenol, polyhydroxyethylaspanamidephenol, or polyethyleneoxidepolylysme substituted with palmitoyl residues. Furthermore, the fatty acid phenolic derivatives can be coupled to a class of biodegradable polymers useful in achieving controlled release of a drug, for example, polylactic acid, polyepsilon caprolactone, polyhydroxy butyric acid, polyorthoesters, polyacetals, polydihydropyrans, polycyanoacrylates and cross-linked or amphipathic block copolymers of hydrogels. In one embodiment, fatty acid phenolic derivative is not covalently bound to a polymer, e.g., a polycarboxylic acid polymer, or a polyacrylate.
[0197] Parenteral injectable administration is generally used for subcutaneous, intramuscular or intravenous injections and infusions. Injectables can be prepared in conventional forms, either as liquid solutions or suspensions or solid forms suitable for dissolving in liquid prior to injection.
[0198] Compositions can be prepared according to conventional mixing, granulating or coating methods, respectively, and the present pharmaceutical compositions can contain from
about 0.1 % to about 90 %, from about 10 % to about 90 %, or from about 30 % to about 90 % of the fatty acid phenolic derivative by weight or volume.
[0199] The dosage regimen utilizing the fatty acid phenolic derivative is selected in accordance with a variety of factors including type, species, age, weight, sex and medical condition of the patient; the severity of the condition to be treated; the route of administration; the renal or hepatic function of the patient; and the particular fatty acid phenolic derivative employed. A physician or veterinarian of ordinary skill in the art can readily determine and prescribe the effective amount of the drug required to prevent, counter or arrest the progress of the condition.
[0200] Effective dosage amounts of the present invention, when used for the indicated effects, range from about 20 mg to about 5,000 mg of the fatty acid phenolic derivative per day. Compositions for in vivo or in vitro use can contain about 20, 50, 75, 100, 150, 250, 500, 750, 1,000, 1,250, 2,500, 3,500, or 5,000 mg of the fatty acid phenolic derivative. In one embodiment, the compositions are in the form of a tablet that can be scored. Effective plasma levels of the fatty acid phenolic derivative can range from about 5 ng/mL to 5,000 ng/mL. Appropriate dosages of the fatty acid phenolic derivatives can be determined as set forth in Goodman, L. S.; Gilman, A. The Pharmacological Basis of Therapeutics, 5th ed.; MacMillan: New York, 1975, pp. 201-226.
[0201] Fatty acid phenolic derivatives can be administered in a single daily dose, or the total daily dosage can be administered in divided doses of two, three or four times daily. Furthermore, fatty acid phenolic derivatives can be administered in intranasal form via topical use of suitable intranasal vehicles, or via transdermal routes, using those forms of transdermal skin patches well known to those of ordinary skill in that art. To be administered in the form of a transdermal delivery system, the dosage administration can be continuous rather than intermittent throughout the dosage regimen. Other illustrative topical preparations include creams, ointments, lotions, aerosol sprays and gels, wherein the concentration of the fatty acid phenolic derivative ranges from about 0.1 % to about 15 %, w/w or w/v.
METHODS OF MAKING
Methods for making the fatty acid phenolic derivatives
[0202] Examples of synthetic pathways useful for making fatty acid phenolic derivatives of Formula I, II and III are set forth in the Examples below and generalized in Schemes 1- 10.
Scheme 1
wherein R9, r and s are as defined above.
[0203] The mono-BOC protected amine of the Formula B can be obtained from commercial sources or prepared according to the procedures outlined in Krapcho et al, Synthetic Communications 1990, 20, p. 2559-2564. The commercially available compound A can be triacetylated with acetic anhydride, subsequently this intermediate can then be amidated with the amine B using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH2C12 or dioxane to produce the coupled compound C. Activation of compound C with a coupling agent such as HATU in the presence of an amine such as DIEA followed by addition of a fatty acid of Formula D, and subsequently hydrolysis of triacetyl groups using hydrazine hydrate, affords compounds of the Formula E. To those skilled in the art, lipoic acid can be substituted for fatty acid D in this and subsequent schemes.
Scheme 2
wherein R, r and s are as defined above.
[0204] The acylated amine of the Formula F can be prepared using the procedures outlined in Andruszkiewicz et al, Synthetic Communications, 2008, 38, p. 905-913. Compound A can be can be triacetylated with acetic anhydride, and this resulting intermediate can then amidated with the amine F using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH2CI2 or dioxane to produce the coupled compound G. Activation of compound G with a coupling agent such as HATU in the presence of an amine such as DIEA followed by addition of a fatty acid of Formula D, and subsequently hydrolysis of triacetyl groups using hydrazine hydrate, affords compounds of the Formula H.
Scheme 3
wherein r and s are as defined above.
[0205] Compound A can be triacetylated with acetic anhydride, subsequently this intermediate can then be amidated with the corresponding amine I (where i = 0, 1, 2 or 3) using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH2C12 or dioxane to produce the coupled compound J. Activation of compound J with a coupling agent such as HATU in the presence of an amine such as DIEA followed by addition of a fatty acid of Formula D affords compounds of the formula K. Hydrolysis of the ester under basic conditions such as NaOH or LiOH produces the corresponding acid, which can be coupled with glycidyl. Subsequently hydrolysis of triacetyl groups using hydrazine hydrate, affords compounds of the Formula L.
Scheme 4
wherein r and s are as defined above.
[0206] The amine T can be prepared from the commercially available diamine according to the procedures outlined in Dahan et al, J. Org. Chem. 2007, 72, p. 2289-2296. Compound A can be triacetylated with acetic anhydride, subsequently this intermediate can then be amidated with the amine T using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to afford compound U. The BOC group of compound U can be removed with acids such as TFA or HCl in a solvent such as CH2CI2 or dioxane and the resulting amine can be coupled with a fatty acid of Formula D using HATU in the presence of an amine such as DIEA to afford compounds of the Formula V. To those familiar in the art, the hydroxyl group in compound U can be further acylated or converted to an amino group by standard mesylation chemistry followed by displacement with sodium azide and hydrogenation over a catalyst such as Palladium on carbon. The amine can be further acylated or alkylated, followed by the removal of the BOC group. The resulting amine can be coupled with a fatty acid of the Formula D and subsequently hydrolysis of triacetyl groups using hydrazine hydrate, affords compounds of the Formula W.
Scheme 5
wherein e, r and s are as defined above.
[0207] The commercially available amino acid esters EE can be coupled with a fatty acid of the Formula D using a coupling agent such as EDCI or HATU, followed by alkaline hydrolysis of the methyl ester to afford compounds of the Formula FF. Compounds of the Formula FF can be coupled with the commercially available BOC-amino acid derivatives GG using a coupling agent such as EDCI or HATU. The BOC group can be removed by treatment with acids such as TFA or HCl to afford compounds of the Formula HH which can then be coupled with compound A after triacetylation with acetic anhydride, with hydrolysis of the triacetyl groups under hydrazine hydrate as the final step to afford compounds of the Formula II.
Scheme 6
wherein r and s are as defined above.
[0208] Compound A can be triacetylated with acetic anhydride, subsequently this intermediate can then be coupled with a BOC-protected diamine of the general formula DA to obtain the BOC-protected amide derivative. After treatment with HCI in dioxane, the resulting amine OO can be coupled with a fatty acid of the formula D and subsequently hydrolysis of triacetyl groups using hydrazine hydrate, affords compounds of the Formula PP. A variety of BOC-protected diamines are commercially available. Diamines DAI, DA2, DA3, and DA4
DA1 DA2 DA3 and DA4 and derivatives thereof, can be prepared according to the procedures outlined in the corresponding references: diamine DAI, Stocks et al, Bioorganic and Medicinal Chemistry Letters 2010, p. 7458; diamine DA2, Fritch et al, Bioorganic and Medicinal Chemistry Letters 2010, p. 6375; diamine DA3 and DA4, Moffat et al, J. Med. Chem. 2010, 53, p.8663- 8678), the disclosures of the foregoing references are incorporated herein in their entireties. Detailed procedures to prepare a variety of mono-protected diamines can also be found in the
following references: WO 2004092172, WO 2004092171, and WO 2004092173, the disclosures of which are incorporated by reference herein in their entireties.
Scheme 7
[0209] The mono-BOC protected diamine AA can be coupled with a fatty acid of the formula D using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB. This is followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH2CI2 or dioxane to produce the free amine salt, which is subjected to acylation with 4-nitrophenyl chloro formate to afford compounds of the Formula AC. Compounds of the Formula AC can then be reacted with a flavonoid of the general structure AD in the presence of DIEA to afford products of the Formula AE. To those familiar in the art, the reaction shown in Scheme 7 can be repeated with any of the amines shown in Schemes 1-6.
Scheme 8
wherein R10, ml, r, s, are as defined above.
[0210] The mono-BOC protected diamine AA can be coupled with a fatty acid of the formula D using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB. This is followed by deprotection of the BOC group with acids such as TFA or HC1 in a solvent such as CH2CI2 or dioxane to produce the free amine salt, which is subjected to acylation with 4-nitrophenyl chloro formate to afford compounds of the Formula AC. Compounds of the Formula AC can then be reacted with a flavonoid of the general structure AF in the presence of DIEA to afford products of the Formula AG. To those familiar in the art, the reaction shown in Scheme 7 can be repeated with any of the amines shown in Schemes 1-6.
Scheme 9
[0211] To those familiar in the art, the reaction sequence shown in Scheme 7 can be repeated with a chalcone derivative such as curcumin BA. The mono-BOC protected diamine AA can be coupled with a fatty acid of the formula D using a coupling reagent such as DCC,
CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, to form AB. This is followed by deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH2CI2 or dioxane to produce the free amine salt AH. Compound BA is subjected to acylation with 4-nitrophenyl chloro formate to afford compounds of the Formula BB. Compounds of the Formula BB can then be reacted with compound AH in the presence of DIEA to afford products of the Formula BC.
Scheme 10
[0212] Through the use of coupling reagents such as HOBT, EDC, or optionally DCC, CDI, with tertiary amine base/catalyst, e.g. DMAP, chalcone CA can be coupled to mono- BOC protected diamine to give compound CB. The procedure can also apply to any of the amines shown in Scheme 1-6, which can be obtained from commercial sources or prepared according to the procedures outlined in Krapcho et al, Synthetic Communications 1990, 20, p. 2559-2564. Next, deprotection of the BOC group with acids such as TFA or HCl in a solvent such as CH2CI2 or dioxane to produce the free amine salt, which is subjected to coupling with a fatty acid of the formula D using above-mentioned coupling reagents to afford the Formula CD. To those familiar in the art, the reaction shown in Scheme 10 can be repeated with any of the amines shown in Schemes 1-6; in addition, the chalcone CA can be substituted with the following chalcone derivatives CE and CF:
[0213] The disclosure is further illustrated by the following examples, which are not to be construed as limiting this disclosure in scope or spirit to the specific procedures herein described. It is to be understood that the examples are provided to illustrate certain embodiments and that no limitation to the scope of the disclosure is intended thereby. It is to be further understood that resort may be had to various other embodiments, modifications, and equivalents thereof which may suggest themselves to those skilled in the art without departing from the spirit of the present disclosure and/or scope of the appended claims.
Example 1
TNFa Release Assay in RAW 264.7 Macrophages
[0214] The purpose of this assay is to measure the ability of small molecules to inhibit the secretion of TNFa in cultured macrophages stimulated with lipopolysaccharide (LPS). Treatment of macrophages with LPS activates inflammatory cytokine pathways primarily through the TLR4-NF-KB signaling axis. The compounds of this invention inhibit the transcriptional activation of NF-κΒ and thus decrease the production and release of TNFa. Dexamethasone, a potent agonist of the glucocorticoid receptor is used a positive control for inhibition of TNFa release.
[0215] Day 1 : Seed RAW 264.7 macrophages into 96 well culture plates. Remove culture media from RAW 264.7 cell growing in a 75 mm2 tissue culture flask (cells should be at -70% confluence) and add 10 ml of warmed complete growth media (DMEM + 10%FBS + IX pen/step). The cells are scraped into suspension using a sterile plate scraper and homogenized by pipetting up and down with a 10 ml serological pipette. The cell concentration is determined using a clinical hematoctyometer. Cells are then diluted to 150,000 cells per ml into growth media. The diluted cells are then transferred to a sterile reagent reservoir and 100 μΐ of cell suspension is pipetted into each well of a 96 well culture plate using a multichannel pipette (15,000 cells/well). Plates are then incubated at 37°C under normal tissue culture growth conditions (37°C, humidified C02 chamber).
[0216] Day 2: The test compound sample plate is prepared. Test compounds are prepared in growth media. Compounds are delivered to media from 1000X stocks in 100% DMSO (e.g. for a 10 μΜ final concentration of test compound, deliver 2 μΐ^ of 10 mM test compound to 2 ml of media). At least 150 μΐ of IX compound in media is added to 96 well sample plate. Note: the perimeter wells of the 96 well plate are not used to avoid edge effects. Twelve sample wells are prepared with media plus 0.1% DMSO (these samples will serve as the vehicle controls; LPS-stimulated and non-stimulated. 10 μΜ dexamethasone is used as a positive control). Culture plates are then returned to the growth incubator for 2 hours. Cells are stimulated afterwards by adding 25 μΐ of 50 ng/ml LPS is added to every well (except the 6 unstimulated vehicle control wells: final concentration of 10 ng/ml LPS. Plates are returned to growth incubator for 3 hours. Afterwards, 100 μΐ of media supernatant is removed and transferred to a 96 well v-bottom sample plate. The media supernatant plate is centrifuged for 5 minutes at 1000 rpm in a swing-bucket centrifuge, pelleting any cellular debris that may remain in supernatant. 80 μΐ of supernatant is removed from sample plate and transferred to a fresh v-bottom 96 well plate. Cell viability is measured using Celltiter- glo kit. By measuring cell viability, a given compound's effects on TNFa secretion can show whether such effects are due to cytotoxicity or to inhibition of inflammatory signaling. 100 μΐ of Celltiter-glo reagent are added to each well of the cell culture plate and afterwards measure the luminescence signal (CPS) of the plate is measured using the Victor 5 plate reader (0.3 second read; 60 second plate shaking prior to read). Cell viability of a given compound at a given concentration is computed as follows:
Cell viability = CPS Sample/(Average CPS unstimulated controls)* 100
Mouse TNFa ELISA
[0217] Place 20 μΐ of media supernatant in each well for TNFa ELISA. Follow Invitrogen/Biosource manufacture's protocol for the mouse TNFa ELISA. Chromogen development is typically conducted for 20-30 minutes as described in the manufacturer's protocol. After addition of stop solution, OD 450 nm is measured using the Victor 5 plate reader (0.1 second/well scan). The TNFa secretion percent of control is then determined by using the formula:
100 X (OD 450 nm Sample X ) - (Average OD 450 nm unstimulated vehicle controls)
(Average OD 450 nm LPS stimulated vehicle controls) - (Average OD 450 nm unstimulated vehicle controls)
[0218] For each test compound, TNFa secretion percent of control is plotted as a function of compound concentration using a four parameter dose-response curve fit equation (XLFIT Model # 205):
fit = (A+((B-A)/(l+((C/x)AD))))
inv = (C/((((B-A)/(y-A))-l)-(l/D)))
res = (y-fit)
Example 2
Effects of the Compounds of the Invention on HMOX Levels in RAW 264.7
Macrophages
[0219] RAW264.7 macrophages were seeded at a density of 100,000 cells/well in a 96- well plate in DMEM supplemented with 10% FBS and penicillin:streptomycin. 16 hours later, medium was aspirated and replaced with 90μίΛνε11 of serum-free DMEM. The compounds of the invention were brought up in 100% ethanol to a concentration of lOOmM and then diluted 1 : 100 in 100% FBS for a stock solution consisting of ImM compound and 1%) ethanol. These stock solutions were then diluted 1 : 10 in FBS supplemented with 1% ethanol to generate a 100 μΜ of the compounds of the invention. Ι ΟμΙ^ was then added to the RAW246.7 cells to generate final concentrations 10μΜ of the compounds of the invention or 10μΜ each bioactive, along with vehicle only control. The compounds of the invention were allowed to pre-incubate for 2 hours before stimulation of 100 ng/ml LPS (ΙΟμί of ^g/ml LPS was added to each well). Following 3 hours of LPS stimulation, cells were washed once in lx PBS, aspirated dry, and flash frozen in liquid nitrogen. R A was then isolated and converted to cDNA using the Cells to cDNA kit (AMBION®) according to the manufacturer's protocol. HMOX transcript levels were then measured using Taqman primer/probe assay sets (APPLIED BIOSYSTEMS®), normalized to GAPDH using the deltaCt method, and the data expressed relative to vehicle only control. Figure 1 summarizes the HMOX activity of compound 1-1 in this particular assay.
Figure 1
Compound 1-1
uM
Example 3
Activity of the compounds of the invention in an NF-KB-driven luciferase reporter
[0220] RAW 264.7 cells transfected with an NF-KB-driven luciferase reporter were plated in 96 well plates. Cells were treated with Vehicle (0.1% ethanol) or test compounds for 2 hours. As a positive control for inhibition of NFKB signaling, 6 wells were treated with 10 μΜ dexamethasone. Cells were then challenged with 200 ng/mL LPS for 3 hours in the presence of test compounds. A subset of wells treated with vehicle remained unstimulated with LPS to determine the floor signal of the assay. NF-κΒ driven luciferase activity was developed by addition of BriteLite luciferase kit (PERKIN ELMER®) and measured using a Victor V plate reader. NF-κΒ activity (luciferase activity) for each treatment was normalized to Vehicle wells treated with LPS (% NF-κΒ Response). AlamarBlue was used to monitor cell viability to ensure that inhibition of luciferase signal was not a result of compound cytotoxicity. In this assay, the IC50 of compound 1-1 and 1-3 were determined to < 50 μΜ, while the IC50 of gallic acid was determined to be > 200 μΜ.
Example 4
In vivo effects of compounds of the invention in an LPS-challenge TNFa mouse model
[0221] To measure the effects of compounds on TNFa secretion in vivo, Male Swiss Webster mice (n = 10 animals per group) are dosed by oral gavage with each test compound. All compounds are formulated in an aqueous solution of 0.5% carboxymethylcellulose and 0.05% TWEEN-80 (Vehicle). One hour after compound dosing, animals are treated with 0.2 mg/kg LPS (lipopolysaccharide) by intraperitoneal (IP) injection. Ninety minutes after LPS challenge, mice are anesthetized and bled by cardiac puncture into serum separator tubes (with sodium heparin). Bleeds are allowed to clot at room temperature for 2 hours, and tubes are then spun for 20 minutes at 2,000 xg. Serum is harvested from tubes (100-150 μΐ per animal) and frozen at -70 °C. TNFa serum levels are measured using commercially available TNFa ELISA kits (*p < 0.05 using a 2-tailed t-test).
Example 5
In vivo effects of compounds of the invention in Zucker fatty rats and ob/ob mice
[0222] Twelve-week-old male Zucker fa/fa rats and 8-week-old ob/ob (Lepob/ob) and ob/1 mice are given free access to food and water. A compound of the invention (120 mg/kg/day) is dosed orally by gavage once per day. For glucose tolerance tests, glucose (2.0 g/kg) is administered by oral gavage (rats) or intraperitoneal injection (mice) after an overnight fast. Blood glucose and serum insulin concentrations are determined during oral glucose tolerance tests in Zucker fa/fa rats or fa/1 rats. For insulin tolerance tests, insulin (2.0 U/kg) is injected intraperitoneally after an overnight fast. Cholesterol, triglyceride, long- chain FFA, and ALT concentrations are measured in sera from fasting Zucker fa/fa rats.
Compounds
[0223] The following non-limiting compound examples serve to illustrate further embodiments of the fatty acid phenolic derivatives. It is to be understood that any embodiments listed in the Examples section are embodiments of the fatty acid phenolic derivatives and, as such, are suitable for use in the methods and compositions described above.
Example 6
Preparation of N-(2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19- hexaenamido)ethyl)-3,4,5-trihydroxybenzamide:
[0224] To a solution of gallic acid (3,4,5-trihydroxybenzoic acid, 3.4 g, 0.02 mol) in acetic anhydride (11.3 mL, 6.0 equivalents) was added pyridine (2 mL, 1.25 equivalents) at room temperature. The resulting reaction mixture was stirred at room temperature for 4 hours. Upon completion, the reaction was quenched with 10 mL of cold 1M H3PO4. The aqueous mixture was extracted with EtOAc (10 mL x 3). The combined organics were washed with brine, saturated NaHC03, water, dried over Na2S04 and concentrated under reduced pressure to give 3,4,5-triacetoxybenzoic acid as white solid in (4 g, 67% yield). This material was then used in next step without further purification.
[0225] (4Z,7Z, 10Z, 13Z, 16Z, 19Z)-N-(2-Aminoethyl)docosa-4,7, 10,13,16,19-hexaenamide was prepared according to the procedures outlined in WO 2011106688. To 3,4,5- triacetoxybenzoic acid (250 mg, 0.84 mmol) in 3 mL CH2C12, was added (4Z,7Z, 10Z, 13Z, 16Z, 19Z)-N-(2-aminoethyl)docosa-4,7, 10,13,16,19-hexaenamide (312.8 mg, 0.84 mmol), followed by EDC (212 mg, 1.3 equivalents), HOBT (167 mg, 1.3 equivalents), and TEA (triethylamine, 230ul, 2.0 equivalents). The resulting reaction mixture was stirred under N2, at room temperature for 5 hours. Upon completion, reaction was washed with saturated NH4C1, brine, and dried over Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (0-60% gradient EtOAc in pentanes) to afford 5-((2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13, 16,19- hexaenamido)ethyl)carbamoyl)benzene-l,2,3-triyl triacetate (383 mg, 70% yield).
[0226] To 5-((2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl) carbamoyl)benzene-l,2,3-triyl triacetate (149 mg, 0.23 mmol) in 5 mL acetonitrile, was added hydrazine monohydrate (25 ul, 3.0 equivalents). The resulting reaction mixture was stirred for 20 minutes. Enough acetic acid was then added to adjust the pH=3.0. The crude reaction was diluted with 20 mL EtOAc, and the organic layer washed with water (20 mL x 4), brine, dried over Na2S04 and concentrated under reduced pressure to provide N-(2- ((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)-3 ,4,5- trihydroxybenzamide (115 mg, 95.6% yield ) without further purification. MS (EI) calculated for C3iH42N205: 522.68; found 523.3 [M+H]+.
Example 7
Preparation of 3,4,5-trihydroxy-N-(2-((5Z,8Z,llZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)benzamide:
[0227] Following General Preparation A, (5Z,8Z,1 lZ,14Z,17Z)-N-(2-aminoethyl)icosa- 5,8,11, 14, 17-pentaenamide (291 mg, 0.84 mmol) was used in the amide coupling to 3,4,5- triacetoxybenzoic acid (250 mg, 1.0 equivalent) to give 3,4,5-trihydroxy-N-(2- ((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)benzamide (21 mg). MS calculated for C29H4oN205: 496.64; found 497.3 [M+H] +.
Example 8
Preparation of N-(2-(2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19- hexaenamido)ethoxy)ethyl)-3,4,5-trihydroxybenzamide:
[0228] tert-Butyl (2-(2-aminoethoxy)ethyl)carbamate was prepared according to the procedure outlined in WO 201 108521 1. The same experimental procedure outlined in example 5 was used to prepare 3,4,5-triacetoxybenzoic acid. This material, 3,4,5- triacetoxybenzoic acid (500 mg, 1.69 mmol) was taken up in 5 mL CH2CI2 along with tert- butyl (2-(2-aminoethoxy)ethyl)carbamate (380 mg, 1.1 equivalents), HATU (835 mg, 1.3 equivalents) and DIE A (571 ul, 2.0 equivalents). The resulting reaction mixture was stirred at room temperature for 4 h. The organic layer was then washed with saturated NH4CI, brine, dried (Na2S04) and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (0-60% gradient EtOAc in pentanes) to afford 5-((2-(2-((tert- butoxycarbonyl)amino)ethoxy)ethyl)carbamoyl)benzene-l ,2,3-triyl triacetate.
[0229] To 5-((2-(2-((fert-butoxycarbonyl)amino)ethoxy)ethyl)carbamoyl)benzene-l ,2,3- triyl triacetate (221 mg, 0.46 mmol) was added 4 N HC1 in dioxanes (4 equivalents) and reaction stirred for 20 minutes. Next, the reaction was diluted with EtOAc and concentrated under reduced pressure to afford the HC1 salt of 5-((2-(2- aminoethoxy)ethyl)carbamoyl)benzene-l ,2,3-triyl triacetate .
[0230] The HC1 salt of 5-((2-(2-aminoethoxy)ethyl)carbamoyl)benzene-l ,2,3-triyl triacetate (176 mg, 0.46 mmol) which was taken up in 5 mL CH2C12 along with (4Z,7Z,10Z, 13Z, 16Z,19Z)-docosa-4,7, 10,13, 16,19-hexaenoic acid (124 mg, 0.9 equivalent), HATU (208 mg, 1.3 equivalents) and DIEA ( 213 ul, 3.0 equivalents). The resulting reaction mixture was stirred at room temperature for 5 hours. The organic layer was then washed with saturated NH4C1, brine, dried (Na2S04) and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (0-60% gradient EtOAc in pentanes) to afford 5-((2-(2-((4Z,7Z,10Z,13Z, 16Z,19Z)-docosa-4,7, 10,13, 16,19- hexaenamido)ethoxy)ethyl)carbamoyl)-benzene-l,2,3-triyl triacetate. This material was then subjected to the de-acetylation procedure detailed in example 5 to afford N-(2-(2- ((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10, 13,16, 19-hexaenamido)ethoxy)ethyl)-3 ,4,5- trihydroxybenzamide (80 mg, 65%> yield). MS calculated for C33H46N2O6: 566.73; found 567.3 [M+H] +.
Example 9
Preparation of N-(2-((2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19- hexaenamido)ethyl)(methyl)amino)ethyl)-3,4,5-trihydroxybenzamide:
[0231] tert-Butyl (2-((2-aminoethyl)(methyl)amino)ethyl)carbamate was prepared according to the procedure outlined in WO 2011085211. Following General Preparation B for synthesis of N-(2-(2-((4Z,7Z,10Z,13Z,16Z,19 )-docosa-4,7,10,13,16,19- hexaenamido)ethoxy)ethyl)-3,4,5-trihydroxybenzamide, tert-butyl (2-((2- aminoethyl)(methyl)amino)ethyl)carbamate (404 mg, 1.1 equivalents) was used in the coupling reaction to to 3,4,5-triacetoxybenzoic acid (500 mg, 1.69 mmol). Final product, N- (2-((2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)(methyl)amino)ethyl)-3,4,5-trihydroxybenzamide (lOOmg) was isolated. MS calculated for C34H49N305: 579.77; found 580.4 [M+H] +.
Example 10
Preparation of 4-((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l,6-dien-l- yl)-2-methoxyphenyl (2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19- hexaenamido)ethyl)carbamate :
[0232] To (lE,6E)-l,7-bis(4-hydroxy-3-methoxyphenyl)hepta-l,6-diene-3,5-dione (curcumin, 1 g, 2.71 mmol) in 70 mL CH2CI2, 4-nitrophenyl carbonochloridate (547 mg, 1 equivalent) was added, followed by dropwise addition of DIEA (927 μί, 2 equivalents). The reaction was stirred at room temperature for 4 hours. The generated intermediate, namely 4- ((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l,6-dien-l-yl)-2-methoxyphenyl (4-nitrophenyl) carbonate, was used for the next step without isolation.
[0233] To the reaction, (4Z,7Z,10Z,13Z,16Z,19Z)-N-(2-aminoethyl)docosa- 4,7,10,13,16,19-hexaenamide (1.5 g, 1.5 equivalents) was added, followed by DIEA (927 μί, 2 equivalents). After 3 hours, the reaction was washed with saturated NH4C1, brine, dried over Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography or preparative TLC (95% CH2C12, 5% MeOH) to give 4-((lE,6E)- 7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l,6-dien-l-yl)-2-methoxyphenyl (2- ((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)carbamate. MS (EI) calcd for C46H56N208: 764.95; found 811.5 [M+2Na]~.
Example 11
Preparation of 4-oxo-2-phenyl-4H-chromen-6-yl (2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa- -hexaenamido)ethyl)carbamate:
[0234] The same procedure outlined in example 10 was used, substituting 6-hydroxy-2- phenyl-4H-chromen-4-one (1 g, 4.19 mmol) for curcumin. The final product was purified by silica gel chromatography (95% CH2CI2, 5% MeOH) to afford 4-oxo-2-phenyl-4H-chromen- 6-yl (2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)carbamate. MS (EI) calcd for C4oH46N205: 634.80; found 657.4 [M+Na]+.
[0235] The present invention is not to be limited in scope by the specific embodiments disclosed in the examples which are intended as illustrations of a few aspects of the invention and any embodiments that are functionally equivalent are within the scope of this invention. Indeed, various modifications of the invention in addition to those shown and described herein will become apparent to those skilled in the art and are intended to fall within the scope of the appended claims.
EQUIVALENTS
[0236] Those skilled in the art will recognize, or be able to ascertain, using no more than routine experimentation, numerous equivalents to the specific embodiments described specifically herein. Such equivalents are intended to be encompassed in the scope of the following claims.
Claims
1. A molecular conjugate comprising a phenolic derivative and a fatty acid selected from omega-3 fatty acids or fatty acids metabolized in vivo into omega-3 fatty acids.
2. A compound of the Formula I:
Formula I or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer, or a stereoisomer thereof; wherein
R7, R8 and R9 are each independently selected from the group consisting of H, OH, OCH3, or OC(0)R' where R' is independently C1-C3 alkyl, or a second molecule of gallic acid, each Wi,W2 is independently null, O, S, NH, or NR, or Wi and W2 can be taken together to form an optionally substituted imidazolidine or piperazine group; each a, b, c, d, is independently -H, -D, halogen, -CH3, -CF3, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, 0, p, q is independently 0, 1 or 2; each L is independently null, -0-, -C(0)-,-S-, -S(O)-, -S(0)2-, -S-S-, -(C C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula I; each ¾ is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl),
-N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1 , 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1 , 2 or 3; k is 0, 1, 2 or 3; z is 1 , 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each Z is independently H,
-99-
provided that there is at least one of
in the compound; each r is independently 2, 3, or 7; each s is independently 3, 5, or 6; each t is independently 0 or 1 ; each v is independently 1 , 2, or 6; each w is independently 0 or 1 ; each Ri and R2 is independently -H, -D, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)C C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci- C3 alkyl), -S(0)C C3 alkyl, -S(0)2C C3 alkyl; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine;
each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
3. A compound of the Formula II:
Formula II or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer or a stereoisomer thereof; wherein Gi is
W3 is independently O or null;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group;
Rio is independently H, OH, OR", R", or OC(0)R" where R" is independently Ci-C6 alkyl; each a, b, c, and d is independently -H, -D, -C¾, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula II; each I¾ is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1 , 2, 3 or 4;
each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1 , 2 or 3; k is 0, 1, 2 or 3; z is 1 , 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each Z is independently H,
in the compound;
each r is independently 2, 3, or each s is independently 3, 5, or each t is independently 0 or 1 ; each v is independently 1 , 2, or each w is independently 0 or 1 ;
each Ri and R2 is independently -H, -D, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci- C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -Ci-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
4. A compound of the Formula III:
Formula III or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer or a stereoisomer thereof; wherein G2 is
W3 is independently O or null;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group;
Rio is independently H, OH, OR", R", or OC(0)R" where R" is independently Ci-C6 alkyl; each a, b, c, and d is independently -H, -D, -C¾, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, 0, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula III; each ¾ is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl),
-N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1 , 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1 , 2 or 3; k is 0, 1, 2 or 3; z is 1 , 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole; each Z is independently H,
- 110-
provided that there is at least one of
in the compound; each r is independently 2, 3, or 7; each s is independently 3, 5, or 6; each t is independently 0 or 1 ; each v is independently 1 , 2, or 6; each w is independently 0 or 1 ; each Ri and R2 is independently -H, -D, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)C C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci- C3 alkyl), -S(0)C C3 alkyl, -S(0)2C C3 alkyl; each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine;
- I l l -
each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
5. A compound of the Formula la:
Formula la or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer or a stereoisomer thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -C¾, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, 0, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each ¾ is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl),
-N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1 , 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1 , 2 or 3; k is 0, 1, 2 or 3; z is 1 , 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole;
each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
Formula lb or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer or a stereoisomer thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -C¾, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, 0, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each ¾ is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl),
-N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1 , 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1 , 2 or 3; k is 0, 1, 2 or 3; z is 1 , 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole;
each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
7. A compound of the Formula Ilia:
Formula Ilia or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer or a stereoisomer thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -C¾, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, 0, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(C C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each ¾ is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl),
-N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1 , 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1 , 2 or 3; k is 0, 1, 2 or 3; z is 1 , 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole;
each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
8. A compound of the Formula Illb:
Formula Illb or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer or a stereoisomer thereof;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group; each a, b, c, and d is independently -H, -D, -C¾, -OCH3, -OCH2CH3, -C(0)OR, -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, 0, p, and q is independently 0, 1 or 2;
L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula la; each ¾ is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl),
-N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; each g is independently 2, 3 or 4; each h is independently 1 , 2, 3 or 4; each m is independently 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; each ml is independently 0, 1 , 2 or 3; k is 0, 1, 2 or 3; z is 1 , 2 or 3; each R4 is independently H, optionally substituted Ci-C6 alkyl, or -C(CH2OH)2; wherein a methylene unit of the Ci-C6 alkyl can be optionally substituted for either O or NR, and in NR4R4, both R4 when taken together with the nitrogen to which they are attached can form a heterocyclic ring such as a pyrrolidine, piperidine, morpholine, piperazine or pyrrole;
each R5 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with -OH or halogen.
9. A compound of claim 5 selected from a group consisting of
N-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)-3,4,5- trihydroxybenzamide (1-1)
N-(2-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethoxy)ethyl)- 3,4,5-trihydroxybenzamide (1-5)
6-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16, 19-hexaenamido)-2-(3,4,5- trihydroxybenzamido)hexanoic acid (1-7)
2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16, 19-hexaenamido)-6-(3,4,5- trihydroxybenzamido)hexanoic acid (1-9)
A compound of claim 6 selected from a group consisting of
3,4,5-trihydroxy-N-(2-((5Z,8Z, 1 1 Z, 14Z, 17Z)-icosa-5 ,8, 1 1 ,14,17- pentaenamido)ethyl)benzamide (1-2)
3,4,5-trihydroxy-N-(2-((2-((5Z,8Z, 1 1Z, 14Z, 17Z)-icosa-5,8, 1 1 , 14,17- pentaenamido)ethyl)(methyl)amino)ethyl)benzamide (1-4)
3,4,5-trihydroxy-N-(2-(2-((5Z,8Z, 1 1 Z, 14Z, 17Z)-icosa-5,8 , 1 1 , 14,17- pentaenamido)ethoxy)ethyl)benzamide (1-6)
6-((5Z,8Z, 1 1 Z, 14Z, 17Z)-icosa-5,8 , 1 1 ,14,17-pentaenamido)-2-(3,4,5- trihydroxybenzamido)hexanoic acid (1-8)
2-((5Z,8Z, 1 1 Z, 14Z, 17Z)-icosa-5,8 , 1 1 ,14,17-pentaenamido)-6-(3,4,5- trihydroxybenzamido)hexanoic acid (1-10)
1 1. A compound of claim 7 selected from a group consisting of
4-((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l ,6-dien-l-yl)-2-methoxyphenyl (2- ((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10, 13,16,19-hexaenamido)ethyl)carbamate (1-33)
4-((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l,6-dien-l-yl)-2-methoxyphenyl (2- ((2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)(methyl)amino)ethyl)carbamate (1-36)
4-((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l,6-dien-l-yl)-2-methoxyphenyl (2- (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethoxy)ethyl)carbamate (I- 39)
12. A compound of claim 8 selected from a group consisting of
4-((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l,6-dien-l-yl)-2- methoxyphenyl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 1 1,14,17- pentaenamido)ethyl)carbamate (1-34)
4-((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l ,6-dien-l-yl)-2- methoxyphenyl (2-((2-((5Z,8Z, 1 1 Z, 14Z, 17Z)-icosa-5,8 , 1 1 ,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (1-37)
4-((lE,6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxohepta-l ,6-dien-l-yl)-2- methoxyphenyl (2-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1 ,14,17- pentaenamido)ethoxy)ethyl)carbamate (1-40)
13. A pharmaceutical composition comprising a compound of claim 1 or 2 and a
pharmaceutically acceptable carrier.
14. A pharmaceutical composition comprising a compound of claim 9 and a
pharmaceutically acceptable carrier.
15. A pharmaceutical composition comprising a compound of claim 10 and a
pharmaceutically acceptable carrier.
16. A pharmaceutical composition comprising a compound of claim 1 1 and a
pharmaceutically acceptable carrier.
17. A pharmaceutical composition comprising a compound of claim 12 and a
pharmaceutically acceptable carrier.
18. A method for treating a metabolic disease comprising administering to a patient in need thereof an effective amount of a molecular conjugate of claim 1.
19. The method of claim 18, wherein the metabolic disease is selected from diabetic
nephropathy, chronic kidney disease (CKD), atherosclerosis, dyslipidemia, coronary
heart disease, hypercholesterolemia, Type 2 diabetes, elevated cholesterol, metabolic syndrome, polycystic ovary syndrome and cardiovascular disease.
20. A method for treating a metabolic disease comprising administering to a patient in need thereof an effective amount of a molecular conjugate of claim 2.
21. The method of claim 20, wherein the metabolic disease is selected from diabetic
nephropathy, chronic kidney disease (CKD), hypertriglyceridemia, hypercholesterolemia, fatty liver disease, atherosclerosis, coronary heart disease, Type 2 diabetes, diabetic neuropathy, diabetic retinopathy, metabolic syndrome, polycystic ovary syndrome and cardiovascular disease.
22. A method for treating a metabolic disease comprising administering to a patient in need thereof an effective amount of a molecular conjugate of claim 9.
23. The method of claim 22, wherein the metabolic disease is selected from diabetic
nephropathy, chronic kidney disease (CKD), hypertriglyceridemia, hypercholesterolemia, fatty liver disease, atherosclerosis, coronary heart disease, Type 2 diabetes, diabetic neuropathy, diabetic retinopathy, metabolic syndrome, polycystic ovary syndrome and cardiovascular disease.
24. A method for treating a metabolic disease comprising administering to a patient in need thereof an effective amount of a molecular conjugate of claim 10.
25. The method of claim 24, wherein the metabolic disease is selected from diabetic
nephropathy, chronic kidney disease (CKD), hypertriglyceridemia, hypercholesterolemia, fatty liver disease, atherosclerosis, coronary heart disease, Type 2 diabetes, diabetic neuropathy, diabetic retinopathy, metabolic syndrome, polycystic ovary syndrome and cardiovascular disease.
26. A method for treating a metabolic disease comprising administering to a patient in need thereof an effective amount of a molecular conjugate of claim 11.
27. The method of claim 26, wherein the metabolic disease is selected from diabetic nephropathy, chronic kidney disease (CKD), hypertriglyceridemia, hypercholesterolemia, fatty liver disease, atherosclerosis, coronary heart disease, Type 2 diabetes, diabetic neuropathy, diabetic retinopathy, metabolic syndrome, polycystic ovary syndrome and cardiovascular disease.
28. A method for treating a metabolic disease comprising administering to a patient in need thereof an effective amount of a molecular conjugate of claim 12.
29. The method of claim 28, wherein the metabolic disease is selected from diabetic
nephropathy, chronic kidney disease (CKD), hypertriglyceridemia, hypercholesterolemia, fatty liver disease, atherosclerosis, coronary heart disease, Type 2 diabetes, diabetic neuropathy, diabetic retinopathy, metabolic syndrome, polycystic ovary syndrome and cardiovascular disease.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161483418P | 2011-05-06 | 2011-05-06 | |
| US61/483,418 | 2011-05-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012154564A1 true WO2012154564A1 (en) | 2012-11-15 |
Family
ID=47139544
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2012/036533 WO2012154564A1 (en) | 2011-05-06 | 2012-05-04 | Fatty acid phenolic derivatives and their uses |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012154564A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2753174A4 (en) * | 2011-09-01 | 2015-05-20 | Xiangping Qian | Certain chemical entities, compositions, and methods |
| US10251845B2 (en) | 2014-11-26 | 2019-04-09 | Catabasis Pharmaceuticals, Inc. | Fatty acid cysteamine conjugates and their use as activators of autophagy |
| CN110156736A (en) * | 2019-06-24 | 2019-08-23 | 沈阳药科大学 | Daidzein carbamate prodrugs, its salt and its preparation and application |
| WO2019245273A1 (en) * | 2018-06-19 | 2019-12-26 | 송양헌 | Novel naringenin/lipoic acid conjugate compound and use thereof |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6180666B1 (en) * | 1997-09-05 | 2001-01-30 | Anmax, Inc. | Use of gallic acid esters to increase bioavailability of orally administered pharmaceutical compounds |
| US20040254357A1 (en) * | 2002-12-19 | 2004-12-16 | Zaloga Gary P. | Fatty acid phenolic conjugates |
| WO2010006085A1 (en) * | 2008-07-08 | 2010-01-14 | Catabasis Pharmaceuticals, Inc. | Fatty acid acetylated salicylates and their uses |
-
2012
- 2012-05-04 WO PCT/US2012/036533 patent/WO2012154564A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6180666B1 (en) * | 1997-09-05 | 2001-01-30 | Anmax, Inc. | Use of gallic acid esters to increase bioavailability of orally administered pharmaceutical compounds |
| US20040254357A1 (en) * | 2002-12-19 | 2004-12-16 | Zaloga Gary P. | Fatty acid phenolic conjugates |
| WO2010006085A1 (en) * | 2008-07-08 | 2010-01-14 | Catabasis Pharmaceuticals, Inc. | Fatty acid acetylated salicylates and their uses |
Non-Patent Citations (1)
| Title |
|---|
| SEVRIOUKOVA ET AL.: "Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir", PNAS, vol. 107, no. 43, 2010, pages 18422 - 18427 * |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2753174A4 (en) * | 2011-09-01 | 2015-05-20 | Xiangping Qian | Certain chemical entities, compositions, and methods |
| US9328081B2 (en) | 2011-09-01 | 2016-05-03 | Neupharma, Inc. | Certain chemical entities, compositions, and methods |
| US9707202B2 (en) | 2011-09-01 | 2017-07-18 | Neupharma, Inc. | Certain chemical entities, compositions, and methods |
| US10251845B2 (en) | 2014-11-26 | 2019-04-09 | Catabasis Pharmaceuticals, Inc. | Fatty acid cysteamine conjugates and their use as activators of autophagy |
| WO2019245273A1 (en) * | 2018-06-19 | 2019-12-26 | 송양헌 | Novel naringenin/lipoic acid conjugate compound and use thereof |
| KR20190143551A (en) * | 2018-06-19 | 2019-12-31 | 송양헌 | Novel lipoic acid/naringenin compounds and use thereof |
| KR102077919B1 (en) | 2018-06-19 | 2020-02-17 | 송양헌 | Novel lipoic acid/naringenin compounds and use thereof |
| CN110156736A (en) * | 2019-06-24 | 2019-08-23 | 沈阳药科大学 | Daidzein carbamate prodrugs, its salt and its preparation and application |
| CN110156736B (en) * | 2019-06-24 | 2022-09-13 | 沈阳药科大学 | Daidzein carbamate prodrug, salt thereof, preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DK2521447T3 (en) | Fatty Acid Fumarates and Their Uses | |
| EP2701509A1 (en) | Fatty acid guanidine and salicylate guanidine derivatives and their uses | |
| CA2772618C (en) | Fatty acid niacin conjugates and their uses | |
| WO2012115695A1 (en) | Bis-fatty acid conjugates and their uses | |
| WO2011106688A1 (en) | Bis-fatty acid conjugates and their uses | |
| US9029548B2 (en) | Fatty acid lenalidomide derivatives and their uses | |
| WO2013166176A1 (en) | Fatty acid conjugates of statin and fxr agonists; compositions and method of uses | |
| US20110213028A1 (en) | Fatty acid mycophenolate derivatives and their uses | |
| WO2012154554A1 (en) | Fatty acid triterpene derivatives and their uses | |
| WO2011044138A1 (en) | Lipoic acid acylated salicylate derivatives and their uses | |
| US20130045939A1 (en) | Fatty acid macrolide derivatives and their uses | |
| WO2011109681A1 (en) | Fatty acid cox inhibitor derivatives and their uses | |
| WO2012154564A1 (en) | Fatty acid phenolic derivatives and their uses | |
| US20110082202A1 (en) | Fatty acid acifran derivatives and their uses | |
| US20110082156A1 (en) | Fatty acid acipimox derivatives and their uses | |
| US20120252810A1 (en) | Fatty acid non-flushing niacin derivatives and their uses | |
| US20110212958A1 (en) | Fatty acid raloxifene derivatives and their uses | |
| WO2012161798A1 (en) | Fatty acid gamma aminobutyric acid (gaba) conjugates and their uses | |
| WO2013033602A2 (en) | Fatty acid amides, compositions and methods of use | |
| RU2588256C2 (en) | Fatty acid fumarate derivatives and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12782893 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12782893 Country of ref document: EP Kind code of ref document: A1 |