WO2012037526A2 - Nitric oxide oxidation catalysts - Google Patents
Nitric oxide oxidation catalysts Download PDFInfo
- Publication number
- WO2012037526A2 WO2012037526A2 PCT/US2011/052040 US2011052040W WO2012037526A2 WO 2012037526 A2 WO2012037526 A2 WO 2012037526A2 US 2011052040 W US2011052040 W US 2011052040W WO 2012037526 A2 WO2012037526 A2 WO 2012037526A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- metals
- catalyst
- group
- elements
- metallic element
- Prior art date
Links
- 239000003054 catalyst Substances 0.000 title claims abstract description 93
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 title claims abstract description 76
- 230000003647 oxidation Effects 0.000 title claims abstract description 44
- 238000007254 oxidation reaction Methods 0.000 title claims abstract description 44
- 229910052723 transition metal Inorganic materials 0.000 claims abstract description 21
- 150000003624 transition metals Chemical class 0.000 claims abstract description 20
- 229910052748 manganese Inorganic materials 0.000 claims abstract description 6
- 239000011572 manganese Substances 0.000 claims abstract description 6
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 claims abstract description 5
- 229910017052 cobalt Inorganic materials 0.000 claims abstract description 5
- 239000010941 cobalt Substances 0.000 claims abstract description 5
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims abstract description 5
- 229910052751 metal Inorganic materials 0.000 claims description 70
- 239000002184 metal Substances 0.000 claims description 40
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 33
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 22
- 239000000758 substrate Substances 0.000 claims description 18
- 229910052783 alkali metal Inorganic materials 0.000 claims description 16
- 150000001340 alkali metals Chemical class 0.000 claims description 16
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims description 16
- 150000001342 alkaline earth metals Chemical class 0.000 claims description 16
- 229910052747 lanthanoid Inorganic materials 0.000 claims description 16
- 150000002602 lanthanoids Chemical class 0.000 claims description 16
- 230000003197 catalytic effect Effects 0.000 claims description 14
- 150000002739 metals Chemical class 0.000 claims description 14
- CDBYLPFSWZWCQE-UHFFFAOYSA-L sodium carbonate Substances [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims description 14
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 claims description 13
- 150000003839 salts Chemical class 0.000 claims description 12
- 229910052697 platinum Inorganic materials 0.000 claims description 11
- 239000010457 zeolite Substances 0.000 claims description 11
- 238000000034 method Methods 0.000 claims description 9
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 claims description 8
- 229910000029 sodium carbonate Inorganic materials 0.000 claims description 7
- 229910021536 Zeolite Inorganic materials 0.000 claims description 6
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 claims description 6
- 230000000737 periodic effect Effects 0.000 claims description 6
- 230000007935 neutral effect Effects 0.000 claims description 4
- 230000001590 oxidative effect Effects 0.000 claims description 4
- 229910052763 palladium Inorganic materials 0.000 claims description 4
- VBIXEXWLHSRNKB-UHFFFAOYSA-N ammonium oxalate Chemical compound [NH4+].[NH4+].[O-]C(=O)C([O-])=O VBIXEXWLHSRNKB-UHFFFAOYSA-N 0.000 claims description 3
- 229910052782 aluminium Inorganic materials 0.000 claims description 2
- 229910052788 barium Inorganic materials 0.000 claims description 2
- 229910052791 calcium Inorganic materials 0.000 claims description 2
- 229910052804 chromium Inorganic materials 0.000 claims description 2
- 229910052733 gallium Inorganic materials 0.000 claims description 2
- 229910052735 hafnium Inorganic materials 0.000 claims description 2
- 229910052738 indium Inorganic materials 0.000 claims description 2
- 229910052758 niobium Inorganic materials 0.000 claims description 2
- 239000012266 salt solution Substances 0.000 claims description 2
- 229910052706 scandium Inorganic materials 0.000 claims description 2
- 229910052712 strontium Inorganic materials 0.000 claims description 2
- 229910052719 titanium Inorganic materials 0.000 claims description 2
- 229910052727 yttrium Inorganic materials 0.000 claims description 2
- 229910052725 zinc Inorganic materials 0.000 claims description 2
- 229910052726 zirconium Inorganic materials 0.000 claims description 2
- 238000001354 calcination Methods 0.000 claims 1
- 238000001035 drying Methods 0.000 claims 1
- 238000005406 washing Methods 0.000 claims 1
- 150000001875 compounds Chemical class 0.000 abstract description 8
- 239000010410 layer Substances 0.000 description 31
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 21
- UFMZWBIQTDUYBN-UHFFFAOYSA-N cobalt dinitrate Chemical compound [Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O UFMZWBIQTDUYBN-UHFFFAOYSA-N 0.000 description 16
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 15
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 12
- WGTYBPLFGIVFAS-UHFFFAOYSA-M tetramethylammonium hydroxide Chemical compound [OH-].C[N+](C)(C)C WGTYBPLFGIVFAS-UHFFFAOYSA-M 0.000 description 12
- 239000004372 Polyvinyl alcohol Substances 0.000 description 11
- 229920002451 polyvinyl alcohol Polymers 0.000 description 11
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 11
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 229930195733 hydrocarbon Natural products 0.000 description 8
- 150000002430 hydrocarbons Chemical class 0.000 description 8
- 230000008929 regeneration Effects 0.000 description 8
- 238000011069 regeneration method Methods 0.000 description 8
- 235000006408 oxalic acid Nutrition 0.000 description 7
- 229910021529 ammonia Inorganic materials 0.000 description 6
- 239000011449 brick Substances 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 239000013618 particulate matter Substances 0.000 description 6
- 238000002485 combustion reaction Methods 0.000 description 5
- -1 millerite compound Chemical class 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- 239000011248 coating agent Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 239000010970 precious metal Substances 0.000 description 4
- 239000004071 soot Substances 0.000 description 4
- 239000004215 Carbon black (E152) Substances 0.000 description 3
- 238000011045 prefiltration Methods 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 229920002125 Sokalan® Polymers 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- IWOUKMZUPDVPGQ-UHFFFAOYSA-N barium nitrate Inorganic materials [Ba+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O IWOUKMZUPDVPGQ-UHFFFAOYSA-N 0.000 description 2
- 238000000975 co-precipitation Methods 0.000 description 2
- 239000003344 environmental pollutant Substances 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 231100000719 pollutant Toxicity 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 238000006722 reduction reaction Methods 0.000 description 2
- DHEQXMRUPNDRPG-UHFFFAOYSA-N strontium nitrate Inorganic materials [Sr+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O DHEQXMRUPNDRPG-UHFFFAOYSA-N 0.000 description 2
- 229910052684 Cerium Inorganic materials 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical group OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 229910052688 Gadolinium Inorganic materials 0.000 description 1
- 229910052779 Neodymium Inorganic materials 0.000 description 1
- 239000012494 Quartz wool Substances 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- 229910052769 Ytterbium Inorganic materials 0.000 description 1
- 229910008334 ZrO(NO3)2 Inorganic materials 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 238000010531 catalytic reduction reaction Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- HSJPMRKMPBAUAU-UHFFFAOYSA-N cerium nitrate Inorganic materials [Ce+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O HSJPMRKMPBAUAU-UHFFFAOYSA-N 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 239000010416 ion conductor Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 229910052953 millerite Inorganic materials 0.000 description 1
- 229910003455 mixed metal oxide Inorganic materials 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- AHKZTVQIVOEVFO-UHFFFAOYSA-N oxide(2-) Chemical compound [O-2] AHKZTVQIVOEVFO-UHFFFAOYSA-N 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229940068984 polyvinyl alcohol Drugs 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- ZNCPFRVNHGOPAG-UHFFFAOYSA-L sodium oxalate Chemical compound [Na+].[Na+].[O-]C(=O)C([O-])=O ZNCPFRVNHGOPAG-UHFFFAOYSA-L 0.000 description 1
- 229940039790 sodium oxalate Drugs 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- WWNBZGLDODTKEM-UHFFFAOYSA-N sulfanylidenenickel Chemical class [Ni]=S WWNBZGLDODTKEM-UHFFFAOYSA-N 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/03—Precipitation; Co-precipitation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/70—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper
- B01J23/76—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36
- B01J23/825—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36 with gallium, indium or thallium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/70—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper
- B01J23/76—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36
- B01J23/83—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36 with rare earths or actinides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/19—Catalysts containing parts with different compositions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/024—Multiple impregnation or coating
- B01J37/0244—Coatings comprising several layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/202—Alkali metals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/204—Alkaline earth metals
- B01D2255/2042—Barium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/204—Alkaline earth metals
- B01D2255/2045—Calcium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/204—Alkaline earth metals
- B01D2255/2047—Magnesium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/206—Rare earth metals
- B01D2255/2063—Lanthanum
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20707—Titanium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20715—Zirconium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20746—Cobalt
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/209—Other metals
- B01D2255/2092—Aluminium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/50—Zeolites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/65—Catalysts not containing noble metals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/90—Physical characteristics of catalysts
- B01D2255/902—Multilayered catalyst
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/90—Physical characteristics of catalysts
- B01D2255/903—Multi-zoned catalysts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
- B01D53/9404—Removing only nitrogen compounds
- B01D53/9409—Nitrogen oxides
- B01D53/9413—Processes characterised by a specific catalyst
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2523/00—Constitutive chemical elements of heterogeneous catalysts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/08—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the faujasite type, e.g. type X or Y
- B01J29/084—Y-type faujasite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/40—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the pentasil type, e.g. types ZSM-5, ZSM-8 or ZSM-11, as exemplified by patent documents US3702886, GB1334243 and US3709979, respectively
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/70—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of types characterised by their specific structure not provided for in groups B01J29/08 - B01J29/65
- B01J29/7007—Zeolite Beta
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/024—Multiple impregnation or coating
- B01J37/0246—Coatings comprising a zeolite
Definitions
- Embodiments of the present invention are directed to metal oxide oxidation catalysts and more particularly nitric oxide oxidation catalysts containing mixed metal oxides. More particularly still, embodiments of the present invention are directed to engine exhaust catalysts containing a brown millerite compound modified with an additional transition metal.
- Nitrogen oxides (NOx) abatement is one of the key concerns for lean burn engines such as diesel engines.
- One solution has been to oxidize the nitric oxide in the emission gases, as the oxidized NO 2 assists the oxidation of soot and promotes denox reactions in the downstream devices like SCR and LNT.
- Platinum based catalysts have been successfully used to oxidize the nitric oxide.
- platinum is a relatively high cost material.
- Embodiments of the present invention provide nitric oxide oxidation catalysts containing a brownmillerite compound modified with an additional transition metal.
- the brownmillerite compound is modified by the addition of Cobalt or Manganese.
- a nitric oxide oxidation catalyst has the general formula wherein A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements;
- B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series;
- C is a metallic element selected from the group consisting of the transition metals, wherein A, B, and C are different metals;
- x is a number from 1 to 5
- y is a number that renders the catalyst substantially charge neutral.
- the nitric oxide oxidation catalyst does not include a precious metal such as platinum, palladium, or gold.
- any of the above nitric oxide oxidation catalysts may be used as an engine exhaust catalyst and may be applied to a selected brick, zone, or layer in a multi-brick, multi-zoned, or multi-layered emission control systems to provide a boost in oxidation performance of the overall system and/or a cost reduction.
- a platinum based catalysts or other suitable catalysts may be included in a different one of the washcoat zones or layers. Zeolites may be added as a hydrocarbon absorbing component in any one of the bricks, zones, or layers.
- a method of producing an engine exhaust catalyst includes preparing a metal salt solution including Cobalt or Manganese and metal salts of the general formula:
- A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements; and B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series.
- the method also includes adding sodium hydroxide, sodium carbonate, or ammonium oxalate to precipitate the metal salts; oxidizing the metal salts with hydrogen peroxide; and separating the oxidized metal salts.
- FIGS. 1A-1 D are schematic representations of different engine exhaust systems in which embodiments of the present invention may be used.
- FIG. 2 is an illustration of a catalytic converter with a cut-away section that shows a substrate onto which emission control catalysts according to embodiments of the present invention are coated.
- FIGS. 3A-3D illustrate different configurations of a substrate for an emission control catalyst.
- FIGS 1A-1 D are schematic representations of different engine exhaust systems in which embodiments of the present invention may be used.
- the combustion process that occurs in an engine 102 produces harmful pollutants, such as CO, various hydrocarbons, particulate matter, and nitrogen oxides (NO x ), in an exhaust stream that is discharged through a tail pipe 108 of the exhaust system.
- harmful pollutants such as CO, various hydrocarbons, particulate matter, and nitrogen oxides (NO x )
- the exhaust stream from an engine 102 passes through a catalytic converter 104, before it is discharged into the atmosphere (environment) through a tail pipe 108.
- the catalytic converter 104 contains supported catalysts coated on a monolithic substrate that treat the exhaust stream from the engine 102.
- the exhaust stream is treated by way of various catalytic reactions that occur within the catalytic converter 104. These reactions include the oxidation of CO to form CO2, burning of hydrocarbons, and the conversion of NO to NO 2 .
- the exhaust stream from the engine 102 passes through a catalytic converter 104 and a particulate filter 106, before it is discharged into the atmosphere through a tail pipe 108.
- the catalytic converter 104 operates in the same manner as in the exhaust system of Figure 1A.
- the particulate filter 106 traps particulate matter that is in the exhaust stream, e.g., soot, liquid hydrocarbons, generally particulates in liquid form.
- the particulate filter 106 includes a supported catalyst coated thereon for the oxidation of NO and/or to aid in combustion of particulate matter.
- the exhaust stream from the engine 102 passes through a catalytic converter 104, a pre-filter catalyst 105 and a particulate filter 106, before it is discharged into the atmosphere through a tail pipe 108.
- the catalytic converter 104 operates in the same manner as in the exhaust system of Figure 1A.
- the pre-filter catalyst 105 includes a monolithic substrate and supported catalysts coated on the monolithic substrate for the oxidation of NO.
- the particulate filter 06 traps particulate matter that is in the exhaust stream, e.g., soot, liquid hydrocarbons, generally particulates in liquid form.
- the exhaust stream passes from the engine 102 through a catalytic converter 104, a particulate filter 106, a selective catalytic reduction (SCR) unit 107 and an ammonia slip catalyst 1 10, before it is discharged into the atmosphere through a tail pipe 108.
- the catalytic converter 104 operates in the same manner as in the exhaust system of Figure 1A.
- the particulate filter 106 traps particulate matter that is in the exhaust stream, e.g., soot, liquid hydrocarbons, generally particulates in liquid form.
- the particulate filter 106 includes a supported catalyst coated thereon for the oxidation of NO and/or to aid in combustion of particulate matter.
- the SCR unit 107 is provided to reduce the NO x species to N2.
- the SCR unit 107 may be ammonia/urea based or hydrocarbon based.
- the ammonia slip catalyst 1 10 is provided to reduce the amount of ammonia emissions through the tail pipe 108.
- An alternative configuration places the SCR unit 107 in front of the particulate filter 106.
- Alternative configurations of the exhaust system includes the provision of SCR unit 107 and the ammonia slip catalyst 1 10 in the exhaust system of Figure 1A or 1 C, and the provision of just the SCR unit 107, without the ammonia slip catalyst 1 10, in the exhaust system of Figures 1A, 1 B or 1 C.
- a NO x storage reduction (NSR) catalyst may be used in place of the SCR unit 107.
- N0 2 assisted oxidation As particulates get trapped in the particulate filter within the exhaust system of Figure 1 B, 1 C or 1 D, it becomes less effective and regeneration of the particulate filter becomes necessary.
- the regeneration of the particulate filter can be either passive or active. Passive regeneration occurs automatically in the presence of NO 2 . Thus, as the exhaust stream containing NO 2 passes through the particulate filter, passive regeneration occurs. During regeneration, the particulates get oxidized and NO 2 gets converted back to NO. In general, higher amounts of NO 2 improve the regeneration performance, and thus this process is commonly referred to as N0 2 assisted oxidation. However, too much N0 2 is not desirable because excess NO 2 is released into the atmosphere and N0 2 is considered to be a more harmful pollutant than NO.
- the NO used for regeneration can be formed in the engine during combustion, from NO oxidation in the catalytic converter 104, from NO oxidation in the pre-filter catalyst 105, and/or from NO oxidation in a catalyzed version of the particulate filter 106.
- Active regeneration is carried out by heating up the particulate filter 106 and oxidizing the particulates. At higher temperatures, NO 2 assistance of the particulate oxidation becomes less important.
- the heating of the particulate filter 106 may be carried out in various ways known in the art. One way is to employ a fuel burner which heats the particulate filter 106 to particulate combustion temperatures. Another way is to increase the temperature of the exhaust stream by modifying the engine output when the particulate filter load reaches a predetermined level.
- the present invention provides catalysts that are to be used in the catalytic converter 104 shown in FIGS. 1A-1 D, or generally as catalysts in any vehicle emission control system, including as a diesel oxidation catalyst, a diesel filter catalyst, an ammonia-slip catalyst, an NSR catalyst, an SCR catalyst, or as a component of a three-way catalyst.
- the present invention further provides a vehicle emission control system, such as the ones shown in FIGS. 1A-1 D, comprising an emission control catalyst comprising a monolith and a supported catalyst coated on the monolith.
- FIG. 2 is an illustration of a catalytic converter with a cut-away section that shows a substrate 210 onto which supported metal catalysts are coated.
- the exploded view of the substrate 210 shows that the substrate 210 has a honeycomb structure comprising a plurality of channels into which washcoats containing supported metal catalysts are flowed in slurry form so as to form coating 220 on the substrate 210.
- FIGS. 3A-3D illustrate multi-layered, multi-zoned, and multi-brick embodiments of the present invention.
- coating 220 comprises two washcoat layers 221 , 223 on top of substrate 210.
- Washcoat layer 221 is the bottom layer that is disposed directly on top of the substrate 210.
- Washcoat layer 223 is the top layer that is in direct contact with the exhaust stream. Based on their positions relative to the exhaust stream, washcoat layer 223 encounters the exhaust stream before washcoat layer 221.
- coating 220 comprises three washcoat layers 221 , 222, 223 on top of substrate 210.
- Washcoat layer 221 is the bottom layer that is disposed directly on top of the substrate 210.
- Washcoat layer 223 is the top layer that is in direct contact with the exhaust stream.
- Washcoat layer 222 is the middle layer that is disposed in between washcoat layers 221 , 223. The middle layer is also referred to as a buffer layer. Based on their positions relative to the exhaust stream, washcoat layer 223 encounters the exhaust stream before washcoat layers 221 , 222, and washcoat layer 222 encounters the exhaust stream before washcoat layer 221.
- the substrate 210 is a single monolith that has two coating zones 21 OA, 210B. A first washcoat is coated onto a first zone 21 OA and a second washcoat is coated onto a second zone 210B.
- the substrate 210 includes first and second monoliths 231 , 232, which are physically separate monoliths. A first washcoat is coated onto the first monolith 231 and a second washcoat is coated onto the second monolith 232.
- Embodiments of the nitric oxide oxidation catalyst according to the present invention may be included in a washcoat that is applied to zones or layers of a multi-zoned or multi-layered system, such as the ones illustrated in FIGS. 3A- 3D.
- the washcoat containing the NOx oxidation catalyst is coated onto a downstream zone or layer, e.g., downstream zone or monolith in a two-zone , or two-brick system, the bottom layer in a two-layered system, or the middle layer or the bottom layer in a three-layered system.
- a platinum-based catalyst may be included in one of the other zones or layers.
- zeolites may be included in any one of the zones, bricks, or layers as a hydrocarbon absorbing component. Zeolites include at least one of ZSM-5 zeolite, beta zeolite, and Y zeolite.
- the nitric oxide oxidation catalyst contains an ion conducting brownmillerite compound modified with an additional transition metal, wherein the catalyst has the general formula wherein A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements;
- B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series;
- C is a metallic element selected from the group consisting of the transition metals, wherein A, B, and C are different metals;
- x is a number from 1 to 5
- y is a number that renders the catalyst substantially charge neutral.
- Exemplary metallic element A includes Ba, Sr, Mg and Ca.
- Exemplary metallic element B includes Lathanide series (such as Ce, Gd, Yb, Nd) and Zr, Ti, Hf, Ga, Cr, Sc, Al, Nb, Zn, In, Y, and mixtures thereof.
- Exemplary metallic element C includes Co and Mn.
- a portion of metallic elements A and/or B of the brownmillerite compound may be partially substituted with one or more metallic elements select from within each respective group of potential metallic elements.
- the brownmillerite structure may be modified as ⁇ 2 ⁇ 5 , AA'A"B 2 0 5 , ⁇ 2 ⁇ 5, A 2 BB'B"0 5 , AA'BBOs, AA'BB'CxO( 5+ y), and variations thereof, wherein A' and A" represent metallic elements which have been substituted for a portion of metallic element A, and B' and B" represent metallic element which have been substituted portion of metallic element B.
- Exemplary substituted brownmillerite compound include Ba 2 Ln x Zr 2- x0 5 and Sr 2 Ln x Zr2 -x 05. Additional exemplary Brown millerite compounds and their respective oxygen ion conductivities are shown in Table 1 below.
- Table 1 above is taken from Table 1 of K. Kendall, et al., "Recent developments in perovskite-based oxide ion conductors," Solid State Ionics 82 (1995) 215-223.
- the nitric oxide oxidation catalyst does not include a precious metal such as platinum or palladium.
- the nitric oxide oxidation catalyst may provide a lower cost alternative to precious metal oxidation catalysts. It is expected the non-precious metal nitric oxide oxidation catalyst disclosed herein will exhibit a similar level of nitric oxide oxidation performance.
- the transition metal in the nitric oxide oxidation catalyst may be present in molar ratio from 3:1 to 1 :3 of the total metal in the brownmillerite compound.
- the transition metal is present in a molar ratio of 2:1 to 1 :2 of the total metal in the brownmillerite compound.
- the nitric oxide oxidation catalyst may be synthesized using any suitable process.
- An exemplary synthesis method is co-precipitation method.
- the co-precipitation method includes dissolving suitable amounts of the transition metal salts and the brownmillerite metal salt precursors such as nitrates or acetates in water.
- Optional polymer surfactants such as Poly Vinyl Alcohol (“PVA”) or the sodium salt of Polyacrylic Acid (“Na-PAA”) may be added to the solution.
- PVA Poly Vinyl Alcohol
- Na-PAA sodium salt of Polyacrylic Acid
- the metal cations are then precipitated with sodium hydroxide, sodium carbonate, oxalic acid, sodium oxalate or ammonium oxalate, and optionally oxidized with hydrogen peroxide.
- the resulting metal precipitant is filtered and washed using deionized water 3 or 4 times. Finally, the metal precipitant is dried and calcined at 500°C for 2 hours.
- the NOx oxidation catalyst may be synthesized using the citric acid method.
- the transition metal and brownmillerite metal salt precursors are dissolved in water with 10% excess of molar amounts of citric acid.
- all or a portion of the citric acid may be substituted with EDTA or a mixture of PVA and sucrose. The mixture is stirred and heated until a viscous gel forms. The viscous gel is dried, processed, and calcined at 500°C for 2 hours.
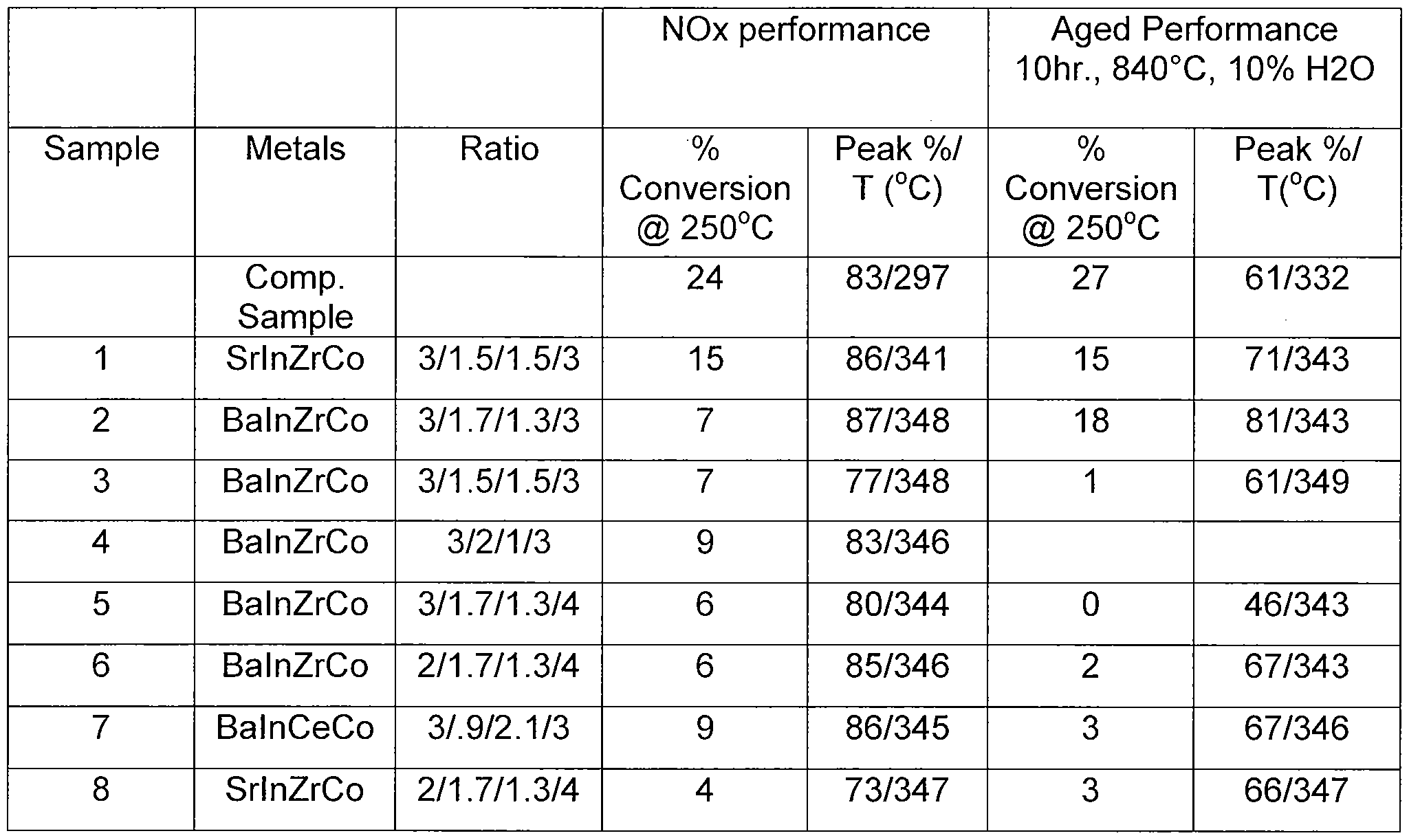
- nitric oxide oxidation catalyst A comparative study was carried out to observe the performance of nitric oxide oxidation catalyst.
- the performance of the nitric oxide oxidation catalyst was compared to a platinum/palladium catalyst, specifically, a 3/1.5% PtPd catalyst on AI 2 O 3 (Comp. Sample).
- the testing conditions included mixing 10mg catalyst with 90 mg of 100 mesh a-alumina and packing the mixture with quartz wool into a flow- through glass U-tube.
- the catalyst is exposed to a gas mixture of 450ppm NO, 10% O 2 , and the remaining balance He, flowing at a rate of 200 seem, in a furnace ramping to 350°C at 10°C/min.
- Table 2 shows the performance catalysts and aged catalysts.
- Table 2 shows the % conversion of NO at 250°C and the maximum peak % conversion at the measured temperature, e.g., sample 1 converted 15% NO at 250°C and had a maximum conversion of 86% NO at 341 °C.
- the nitric oxide oxidation catalysts of Samples 1-5 were prepared in the following manner: [0040] Sample 1 : Dissolve 2.7726g of Co(NO 3 ) 2 »6H 2 O, 1.1588 g Zr(C 2 H 3 02)2(OH)2, 2.0161 g Sr(N0 3 ) 2 , 1 .8620 g of ⁇ ( ⁇ 3 )3 ⁇ 5 ⁇ 2 0, and 0.5246 g of PVA (9,000-10,000 MW) in 50ml_ water whilst stirring. Add 9 mL 5M NaOH to reach pH 12. Add 1.68 mL 30% H 2 0 2 , 0.8663 g Oxalic Acid and 2.5 mL 5M NaOH to bring to pH up to 9. Filter, wash with 60mL water, dry and calcine for 2 hours at 500°C.
- Sample 2 Dissolve 2.3878 g of Co(NO 3 ) 2 »6H 2 O, 0.8647 g Zr(C 2 H 3 O 2 ) 2 (OH) 2 , 2.0956 g Ba(C 2 H 3 O 2 )2, 1 .8174 g of ⁇ ( ⁇ 3 ) 3 ⁇ 5 ⁇ 2 ⁇ , and 0.2259 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 6.5 mL 5M NaOH and 2mL 1 M Na 2 CO 3 to reach pH 10. Add 1.26 mL 30% H 2 O 2 , 2.05 g Oxalic Acid and 7.5 mL 5M NaOH to bring to pH up to 9.5. Filter, wash with 60mL water, dry and calcine for 2 hours at 500°C.
- Sample 3 Dissolve 2.3946 g of Co(NO 3 ) 2 »6H 2 O, 1.0009 g Zr(C 2 H 3 O2) 2 (OH)2, 2.1016 g Ba(C 2 H 3 O 2 ) 2 , 1.6082 g of ⁇ ( ⁇ 3 ) 3 ⁇ 5 ⁇ 2 ⁇ , and 0.2265 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 7 mL 5M NaOH and 2mL 1 M Na 2 CO 3 to reach pH 1 1. Add 1.26 mL 30% H 2 O 2 , 0.743 g Oxalic Acid and 2.5 mL 5M NaOH to bring to pH up to 10. Filter, wash with 60mL water, dry and calcine for 2 hours at 500°C.
- Sample 4 Dissolve 3.1712 g of Co(NO 3 ) 2 *6H 2 O, 0.8841 g Zr(C 2 H 3 O 2 ) 2 (OH) 2 , 2.7822 g Ba(C 2 H 3 O 2 )2, 2.1201 g of ⁇ ( ⁇ 3 ) 3 ⁇ 5 ⁇ 2 ⁇ , and 0.5998 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 10 mL 5M NaOH and to reach pH 10.5. Add 1.225 mL 30% H 2 O 2 and 12mL 1 M Na 2 CO 3 to reach a pH 1 1.5. Filter, wash with 80mL water, dry and calcine for 2 hours at 500°C.
- Sample 5 Dissolve 3.955 g of Co(NO 3 ) 2 «6H 2 O, 1.0751 g Zr(C 2 H 3 O 2 ) 2 (OH) 2 , 2.6036 g Ba(C 2 H 3 O 2 )2, 2.2580 g of ⁇ ( ⁇ 3 ) 3 ⁇ 5 ⁇ 2 ⁇ , and 0.6548 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 12 mL 5M NaOH and to reach pH 10. Add 1.53 mL 30% H 2 O 2 and 1 1.4 mL 1 M Na 2 CO 3 to reach a pH 1 1. Filter, wash with 80mL water, dry and calcine for 2 hours at 500°C.
- Sample 6 Dissolve 4.5478 g Co(NO 3 ) 2 »6H 2 O, 2.0420 g 1.8794 g Ba(NO 3 ) 2 , 2.6295 g ⁇ ( ⁇ 3 ) 3 ⁇ 5 ⁇ 2 ⁇ , 1.2355 g Zr(C 2 H 3 O 2 ) 2 (OH) 2 , and 0.3227 g PVA. Increase the pH with 20ml_ 25% TMAOH to a pH of 10.9. Add 2.2633 g oxalic acid, followed by 4.6 mL 25% TMAOH and1.76 mL 30% H 2 O 2 .
- Sample 7 Dissolve 6.2780 g Co(NO 3 ) 2 «6H 2 O, 1 .8794 g Ba(NO 3 ) 2 , 0.8433 g ⁇ ( ⁇ 3 ) 3 ⁇ 5 ⁇ 2 ⁇ , 2.1857 g Ce(NO 3 ) 3 »6H 2 O and 0.1980 g PVA in water. Increase the pH with 17mL of 25% TMAOH to a pH of 11.2. Add 1.3277 g oxalic acid, followed by 2.99 mL 30% H 2 O 2 . Reestablish a pH of 9 with 1 mL TMAOH.
- Sample 8 Dissolve 5.0363 g Co(NO 3 ) 2 *6H 2 O, 1.8313 g Sr(NO 3 ) 2 , 2.9123 g ⁇ ( ⁇ 3 ) 3 ⁇ 5 ⁇ 2 ⁇ , 1 .3006 ZrO(NO3)2 in water. Add 1.945 mL 30% H 2 O 2 , 6mL 25% TMAOH and 0.8339g PVA. Add 18mL TMAOH to pH 10.8, followed by 1.861 g oxalic acid to a pH of 9.4. Filter, dry and calcine for 2 hours at 500°C.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Catalysts (AREA)
- Exhaust Gas Treatment By Means Of Catalyst (AREA)
Abstract
A nitric oxide oxidation catalyst contains a brownmillerite compound modified with an additional transition metal. In one embodiment, the additional transition metal is selected from cobalt or manganese. The nitric oxide oxidation catalyst may be used as an engine exhaust catalyst.
Description
NITRIC OXIDE OXIDATION CATALYSTS
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0001] Embodiments of the present invention are directed to metal oxide oxidation catalysts and more particularly nitric oxide oxidation catalysts containing mixed metal oxides. More particularly still, embodiments of the present invention are directed to engine exhaust catalysts containing a brown millerite compound modified with an additional transition metal.
2. Description of the Related Art
[0002] Nitrogen oxides (NOx) abatement is one of the key concerns for lean burn engines such as diesel engines. One solution has been to oxidize the nitric oxide in the emission gases, as the oxidized NO2 assists the oxidation of soot and promotes denox reactions in the downstream devices like SCR and LNT. Platinum based catalysts have been successfully used to oxidize the nitric oxide. However, platinum is a relatively high cost material.
[0003] In this respect, catalyst developers are continually exploring ways to use alternative metals that are less costly. Therefore is, therefore, suitable non- precious group metals for use as catalysts.
SUMMARY OF THE INVENTION
[0004] Embodiments of the present invention provide nitric oxide oxidation catalysts containing a brownmillerite compound modified with an additional transition metal. In one embodiment, the brownmillerite compound is modified by the addition of Cobalt or Manganese.
[0005] A nitric oxide oxidation catalyst according to another embodiment has the general formula
wherein A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements;
B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series;
C is a metallic element selected from the group consisting of the transition metals, wherein A, B, and C are different metals;
x is a number from 1 to 5, and
y is a number that renders the catalyst substantially charge neutral.
[0006] In another variation, the nitric oxide oxidation catalyst does not include a precious metal such as platinum, palladium, or gold.
[0007] In another embodiment, any of the above nitric oxide oxidation catalysts may be used as an engine exhaust catalyst and may be applied to a selected brick, zone, or layer in a multi-brick, multi-zoned, or multi-layered emission control systems to provide a boost in oxidation performance of the overall system and/or a cost reduction. In yet another embodiment, a platinum based catalysts or other suitable catalysts may be included in a different one of the washcoat zones or layers. Zeolites may be added as a hydrocarbon absorbing component in any one of the bricks, zones, or layers.
[0008] A method of producing an engine exhaust catalyst includes preparing a metal salt solution including Cobalt or Manganese and metal salts of the general formula:
A2B205
wherein A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements; and B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series. The method also includes adding sodium hydroxide, sodium carbonate, or ammonium oxalate to precipitate the metal
salts; oxidizing the metal salts with hydrogen peroxide; and separating the oxidized metal salts.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] So that the manner in which the above recited features of the present invention can be understood in detail, a more particular description of the invention, briefly summarized above, may be had by reference to embodiments, some of which are illustrated in the appended drawings. It is to be noted, however, that the appended drawings illustrate only typical embodiments of this invention and are therefore not to be considered limiting of its scope, for the invention may admit to other equally effective embodiments.
[0010] FIGS. 1A-1 D are schematic representations of different engine exhaust systems in which embodiments of the present invention may be used.
[0011] FIG. 2 is an illustration of a catalytic converter with a cut-away section that shows a substrate onto which emission control catalysts according to embodiments of the present invention are coated.
[0012] FIGS. 3A-3D illustrate different configurations of a substrate for an emission control catalyst.
DETAILED DESCRIPTION
[0013] In the following, reference is made to embodiments of the invention. However, it should be understood that the invention is not limited to specific described embodiments. Instead, any combination of the following features and elements, whether related to different embodiments or not, is contemplated to implement and practice the invention. Furthermore, in various embodiments the invention provides numerous advantages over the prior art. However, although embodiments of the invention may achieve advantages over other possible solutions and/or over the prior art, whether or not a particular advantage is achieved by a given embodiment is not limiting of the invention. Thus, the following aspects, features, embodiments and advantages are merely illustrative and are not
considered elements or limitations of the appended claims except where explicitly recited in the claims. Likewise, reference to "the invention" shall not be construed as a generalization of any inventive subject matter disclosed herein and shall not be considered to be an element or limitation of the appended claims except where explicitly recited in the claims.
[0014] Figures 1A-1 D are schematic representations of different engine exhaust systems in which embodiments of the present invention may be used. The combustion process that occurs in an engine 102 produces harmful pollutants, such as CO, various hydrocarbons, particulate matter, and nitrogen oxides (NOx), in an exhaust stream that is discharged through a tail pipe 108 of the exhaust system.
[0015] In the exhaust system of Figure 1A, the exhaust stream from an engine 102 passes through a catalytic converter 104, before it is discharged into the atmosphere (environment) through a tail pipe 108. The catalytic converter 104 contains supported catalysts coated on a monolithic substrate that treat the exhaust stream from the engine 102. The exhaust stream is treated by way of various catalytic reactions that occur within the catalytic converter 104. These reactions include the oxidation of CO to form CO2, burning of hydrocarbons, and the conversion of NO to NO2.
[0016] In the exhaust system of Figure 1 B, the exhaust stream from the engine 102 passes through a catalytic converter 104 and a particulate filter 106, before it is discharged into the atmosphere through a tail pipe 108. The catalytic converter 104 operates in the same manner as in the exhaust system of Figure 1A. The particulate filter 106 traps particulate matter that is in the exhaust stream, e.g., soot, liquid hydrocarbons, generally particulates in liquid form. In an optional configuration, the particulate filter 106 includes a supported catalyst coated thereon for the oxidation of NO and/or to aid in combustion of particulate matter.
[0017] In the exhaust system of Figure 1 C, the exhaust stream from the engine 102 passes through a catalytic converter 104, a pre-filter catalyst 105 and a particulate filter 106, before it is discharged into the atmosphere through a tail pipe 108. The catalytic converter 104 operates in the same manner as in the exhaust
system of Figure 1A. The pre-filter catalyst 105 includes a monolithic substrate and supported catalysts coated on the monolithic substrate for the oxidation of NO. The particulate filter 06 traps particulate matter that is in the exhaust stream, e.g., soot, liquid hydrocarbons, generally particulates in liquid form.
[0018] In the exhaust system of Figure 1 D, the exhaust stream passes from the engine 102 through a catalytic converter 104, a particulate filter 106, a selective catalytic reduction (SCR) unit 107 and an ammonia slip catalyst 1 10, before it is discharged into the atmosphere through a tail pipe 108. The catalytic converter 104 operates in the same manner as in the exhaust system of Figure 1A. The particulate filter 106 traps particulate matter that is in the exhaust stream, e.g., soot, liquid hydrocarbons, generally particulates in liquid form. In an optional configuration, the particulate filter 106 includes a supported catalyst coated thereon for the oxidation of NO and/or to aid in combustion of particulate matter. The SCR unit 107 is provided to reduce the NOx species to N2. The SCR unit 107 may be ammonia/urea based or hydrocarbon based. The ammonia slip catalyst 1 10 is provided to reduce the amount of ammonia emissions through the tail pipe 108. An alternative configuration places the SCR unit 107 in front of the particulate filter 106.
[0019] Alternative configurations of the exhaust system includes the provision of SCR unit 107 and the ammonia slip catalyst 1 10 in the exhaust system of Figure 1A or 1 C, and the provision of just the SCR unit 107, without the ammonia slip catalyst 1 10, in the exhaust system of Figures 1A, 1 B or 1 C. As a further alternative, a NOx storage reduction (NSR) catalyst may be used in place of the SCR unit 107.
[0020] As particulates get trapped in the particulate filter within the exhaust system of Figure 1 B, 1 C or 1 D, it becomes less effective and regeneration of the particulate filter becomes necessary. The regeneration of the particulate filter can be either passive or active. Passive regeneration occurs automatically in the presence of NO2. Thus, as the exhaust stream containing NO2 passes through the particulate filter, passive regeneration occurs. During regeneration, the particulates get oxidized and NO2 gets converted back to NO. In general, higher amounts of NO2 improve the regeneration performance, and thus this process is commonly
referred to as N02 assisted oxidation. However, too much N02 is not desirable because excess NO2 is released into the atmosphere and N02 is considered to be a more harmful pollutant than NO. The NO used for regeneration can be formed in the engine during combustion, from NO oxidation in the catalytic converter 104, from NO oxidation in the pre-filter catalyst 105, and/or from NO oxidation in a catalyzed version of the particulate filter 106.
[0021] Active regeneration is carried out by heating up the particulate filter 106 and oxidizing the particulates. At higher temperatures, NO2 assistance of the particulate oxidation becomes less important. The heating of the particulate filter 106 may be carried out in various ways known in the art. One way is to employ a fuel burner which heats the particulate filter 106 to particulate combustion temperatures. Another way is to increase the temperature of the exhaust stream by modifying the engine output when the particulate filter load reaches a predetermined level.
[0022] The present invention provides catalysts that are to be used in the catalytic converter 104 shown in FIGS. 1A-1 D, or generally as catalysts in any vehicle emission control system, including as a diesel oxidation catalyst, a diesel filter catalyst, an ammonia-slip catalyst, an NSR catalyst, an SCR catalyst, or as a component of a three-way catalyst. The present invention further provides a vehicle emission control system, such as the ones shown in FIGS. 1A-1 D, comprising an emission control catalyst comprising a monolith and a supported catalyst coated on the monolith.
[0023] FIG. 2 is an illustration of a catalytic converter with a cut-away section that shows a substrate 210 onto which supported metal catalysts are coated. The exploded view of the substrate 210 shows that the substrate 210 has a honeycomb structure comprising a plurality of channels into which washcoats containing supported metal catalysts are flowed in slurry form so as to form coating 220 on the substrate 210.
[0024] In one embodiment of the present invention, a single layer of washcoat containing one or more supported metal catalysts is coated on substrate 210.
FIGS. 3A-3D illustrate multi-layered, multi-zoned, and multi-brick embodiments of the present invention. In the embodiment of FIG. 3A, coating 220 comprises two washcoat layers 221 , 223 on top of substrate 210. Washcoat layer 221 is the bottom layer that is disposed directly on top of the substrate 210. Washcoat layer 223 is the top layer that is in direct contact with the exhaust stream. Based on their positions relative to the exhaust stream, washcoat layer 223 encounters the exhaust stream before washcoat layer 221.
[0025] In the embodiment of FIG. 3B, coating 220 comprises three washcoat layers 221 , 222, 223 on top of substrate 210. Washcoat layer 221 is the bottom layer that is disposed directly on top of the substrate 210. Washcoat layer 223 is the top layer that is in direct contact with the exhaust stream. Washcoat layer 222 is the middle layer that is disposed in between washcoat layers 221 , 223. The middle layer is also referred to as a buffer layer. Based on their positions relative to the exhaust stream, washcoat layer 223 encounters the exhaust stream before washcoat layers 221 , 222, and washcoat layer 222 encounters the exhaust stream before washcoat layer 221.
[0026] In the embodiment of FIG. 3C, the substrate 210 is a single monolith that has two coating zones 21 OA, 210B. A first washcoat is coated onto a first zone 21 OA and a second washcoat is coated onto a second zone 210B. In the embodiment of FIG. 3D, the substrate 210 includes first and second monoliths 231 , 232, which are physically separate monoliths. A first washcoat is coated onto the first monolith 231 and a second washcoat is coated onto the second monolith 232.
[0027] Embodiments of the nitric oxide oxidation catalyst according to the present invention may be included in a washcoat that is applied to zones or layers of a multi-zoned or multi-layered system, such as the ones illustrated in FIGS. 3A- 3D. In these embodiments, the washcoat containing the NOx oxidation catalyst is coated onto a downstream zone or layer, e.g., downstream zone or monolith in a two-zone , or two-brick system, the bottom layer in a two-layered system, or the middle layer or the bottom layer in a three-layered system. A platinum-based catalyst may be included in one of the other zones or layers. Also, zeolites may be included in any one of the zones, bricks, or layers as a hydrocarbon absorbing
component. Zeolites include at least one of ZSM-5 zeolite, beta zeolite, and Y zeolite.
[0028] The nitric oxide oxidation catalyst contains an ion conducting brownmillerite compound modified with an additional transition metal, wherein the catalyst has the general formula
wherein A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements;
B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series;
C is a metallic element selected from the group consisting of the transition metals, wherein A, B, and C are different metals;
x is a number from 1 to 5, and
y is a number that renders the catalyst substantially charge neutral.
[0029] Exemplary metallic element A includes Ba, Sr, Mg and Ca. Exemplary metallic element B includes Lathanide series (such as Ce, Gd, Yb, Nd) and Zr, Ti, Hf, Ga, Cr, Sc, Al, Nb, Zn, In, Y, and mixtures thereof. Exemplary metallic element C includes Co and Mn.
[0030] In another embodiment, a portion of metallic elements A and/or B of the brownmillerite compound may be partially substituted with one or more metallic elements select from within each respective group of potential metallic elements. In this respect, the brownmillerite structure may be modified as ΑΑΈ2Ο5, AA'A"B205, Α2ΒΒΌ5, A2BB'B"05, AA'BBOs, AA'BB'CxO(5+y), and variations thereof, wherein A' and A" represent metallic elements which have been substituted for a portion of metallic element A, and B' and B" represent metallic element which have been substituted portion of metallic element B. Exemplary substituted brownmillerite compound include Ba2LnxZr2-x05 and Sr2LnxZr2-x05. Additional exemplary
Brown millerite compounds and their respective oxygen ion conductivities are shown in Table 1 below.
[0031] Table 1 above is taken from Table 1 of K. Kendall, et al., "Recent developments in perovskite-based oxide ion conductors," Solid State Ionics 82 (1995) 215-223.
[0032] In one embodiment, the nitric oxide oxidation catalyst does not include a precious metal such as platinum or palladium. In this respect, the nitric oxide oxidation catalyst may provide a lower cost alternative to precious metal oxidation
catalysts. It is expected the non-precious metal nitric oxide oxidation catalyst disclosed herein will exhibit a similar level of nitric oxide oxidation performance.
[0033] The transition metal in the nitric oxide oxidation catalyst may be present in molar ratio from 3:1 to 1 :3 of the total metal in the brownmillerite compound. Preferably, the transition metal is present in a molar ratio of 2:1 to 1 :2 of the total metal in the brownmillerite compound.
[0034] The nitric oxide oxidation catalyst may be synthesized using any suitable process. An exemplary synthesis method is co-precipitation method. In general, the co-precipitation method includes dissolving suitable amounts of the transition metal salts and the brownmillerite metal salt precursors such as nitrates or acetates in water. Optional polymer surfactants such as Poly Vinyl Alcohol ("PVA") or the sodium salt of Polyacrylic Acid ("Na-PAA") may be added to the solution. The metal cations are then precipitated with sodium hydroxide, sodium carbonate, oxalic acid, sodium oxalate or ammonium oxalate, and optionally oxidized with hydrogen peroxide. The resulting metal precipitant is filtered and washed using deionized water 3 or 4 times. Finally, the metal precipitant is dried and calcined at 500°C for 2 hours.
[0035] Alternatively, the NOx oxidation catalyst may be synthesized using the citric acid method. In general, the transition metal and brownmillerite metal salt precursors are dissolved in water with 10% excess of molar amounts of citric acid. Optionally, all or a portion of the citric acid may be substituted with EDTA or a mixture of PVA and sucrose. The mixture is stirred and heated until a viscous gel forms. The viscous gel is dried, processed, and calcined at 500°C for 2 hours.
[0036] A comparative study was carried out to observe the performance of nitric oxide oxidation catalyst. The performance of the nitric oxide oxidation catalyst was compared to a platinum/palladium catalyst, specifically, a 3/1.5% PtPd catalyst on AI2O3 (Comp. Sample). The testing conditions included mixing 10mg catalyst with 90 mg of 100 mesh a-alumina and packing the mixture with quartz wool into a flow- through glass U-tube. The catalyst is exposed to a gas mixture of 450ppm NO, 10% O2, and the remaining balance He, flowing at a rate of 200 seem, in a furnace
ramping to 350°C at 10°C/min. Table 2 below shows the performance catalysts and aged catalysts.
TABLE 2
[0037] Table 2 shows the % conversion of NO at 250°C and the maximum peak % conversion at the measured temperature, e.g., sample 1 converted 15% NO at 250°C and had a maximum conversion of 86% NO at 341 °C.
[0038] Although the data above show the fresh NO oxidation catalysts did not quite match the performance of the fresh platinum catalyst at low temperature, i.e., 250°C, the mixed oxide catalysts were shown to be sufficiently effective for oxidizing NO while possessing a lower cost advantage. Additionally, some aged NOx catalysts such as sample 1 showed good aging resistance.
[0039] In general, the nitric oxide oxidation catalysts of Samples 1-5 were prepared in the following manner:
[0040] Sample 1 : Dissolve 2.7726g of Co(NO3)2»6H2O, 1.1588 g Zr(C2H302)2(OH)2, 2.0161 g Sr(N03)2, 1 .8620 g of Ιη(ΝΟ3)3·5Η20, and 0.5246 g of PVA (9,000-10,000 MW) in 50ml_ water whilst stirring. Add 9 mL 5M NaOH to reach pH 12. Add 1.68 mL 30% H202, 0.8663 g Oxalic Acid and 2.5 mL 5M NaOH to bring to pH up to 9. Filter, wash with 60mL water, dry and calcine for 2 hours at 500°C.
[0041] Sample 2: Dissolve 2.3878 g of Co(NO3)2»6H2O, 0.8647 g Zr(C2H3O2)2(OH)2, 2.0956 g Ba(C2H3O2)2, 1 .8174 g of Ιη(ΝΟ3)3·5Η2Ο, and 0.2259 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 6.5 mL 5M NaOH and 2mL 1 M Na2CO3 to reach pH 10. Add 1.26 mL 30% H2O2, 2.05 g Oxalic Acid and 7.5 mL 5M NaOH to bring to pH up to 9.5. Filter, wash with 60mL water, dry and calcine for 2 hours at 500°C.
[0042] Sample 3: Dissolve 2.3946 g of Co(NO3)2»6H2O, 1.0009 g Zr(C2H3O2)2(OH)2, 2.1016 g Ba(C2H3O2)2, 1.6082 g of Ιη(ΝΟ3)3·5Η2Ο, and 0.2265 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 7 mL 5M NaOH and 2mL 1 M Na2CO3 to reach pH 1 1. Add 1.26 mL 30% H2O2, 0.743 g Oxalic Acid and 2.5 mL 5M NaOH to bring to pH up to 10. Filter, wash with 60mL water, dry and calcine for 2 hours at 500°C.
[0043] Sample 4: Dissolve 3.1712 g of Co(NO3)2*6H2O, 0.8841 g Zr(C2H3O2)2(OH)2, 2.7822 g Ba(C2H3O2)2, 2.1201 g of Ιη(ΝΟ3)3·5Η2Ο, and 0.5998 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 10 mL 5M NaOH and to reach pH 10.5. Add 1.225 mL 30% H2O2 and 12mL 1 M Na2CO3 to reach a pH 1 1.5. Filter, wash with 80mL water, dry and calcine for 2 hours at 500°C.
[0044] Sample 5: Dissolve 3.955 g of Co(NO3)2«6H2O, 1.0751 g Zr(C2H3O2)2(OH)2, 2.6036 g Ba(C2H3O2)2, 2.2580 g of Ιη(ΝΟ3)3·5Η2Ο, and 0.6548 g of PVA (9,000-10,000 MW) in 50mL water whilst stirring. Add 12 mL 5M NaOH and to reach pH 10. Add 1.53 mL 30% H2O2 and 1 1.4 mL 1 M Na2CO3 to reach a pH 1 1. Filter, wash with 80mL water, dry and calcine for 2 hours at 500°C.
[0045] Sample 6: Dissolve 4.5478 g Co(NO3)2»6H2O, 2.0420 g 1.8794 g Ba(NO3)2, 2.6295 g Ιη(ΝΟ3)3·5Η2Ο, 1.2355 g Zr(C2H3O2)2(OH)2, and 0.3227 g
PVA. Increase the pH with 20ml_ 25% TMAOH to a pH of 10.9. Add 2.2633 g oxalic acid, followed by 4.6 mL 25% TMAOH and1.76 mL 30% H2O2.
[0046] Sample 7: Dissolve 6.2780 g Co(NO3)2«6H2O, 1 .8794 g Ba(NO3)2, 0.8433 g Ιη(ΝΟ3)3·5Η2Ο, 2.1857 g Ce(NO3)3»6H2O and 0.1980 g PVA in water. Increase the pH with 17mL of 25% TMAOH to a pH of 11.2. Add 1.3277 g oxalic acid, followed by 2.99 mL 30% H2O2. Reestablish a pH of 9 with 1 mL TMAOH.
[0047] Sample 8: Dissolve 5.0363 g Co(NO3)2*6H2O, 1.8313 g Sr(NO3)2, 2.9123 g Ιη(ΝΟ3)3·5Η2Ο, 1 .3006 ZrO(NO3)2 in water. Add 1.945 mL 30% H2O2, 6mL 25% TMAOH and 0.8339g PVA. Add 18mL TMAOH to pH 10.8, followed by 1.861 g oxalic acid to a pH of 9.4. Filter, dry and calcine for 2 hours at 500°C.
[0048] While particular embodiments according to the invention have been illustrated and described above, those skilled in the art understand that the invention can take a variety of forms and embodiments within the scope of the appended claims.
Claims
1. A nitric oxide oxidation catalyst having the general formula
A2B2CxO(5+y), wherein
A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals, and the Lanthanide Series of the lUPAC Periodic Table of the Elements;
B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series;
C is a metallic element selected from the group consisting of the transition metals, wherein A, B, and C are different metals;
x is a number from 1 to 5, and
y is a number that renders the catalyst substantially charge neutral.
2 The catalyst of claim 1 , wherein the catalyst is of the general formula A2BB'CxO(5+y), wherein B' comprises substituted metal for a portion of B and is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series.
3. The catalyst of claim 1 , wherein the catalyst is of the general formula AA'B2CxO(5+y), wherein A' comprises substituted metal for a portion of A and is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series.
4. The catalyst of claim 1 , wherein the catalyst is of the general formula AA'BB'CxO(5+y), wherein A' comprises substituted metal for a portion of A and is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series, and wherein B' comprises substituted metal for a portion of B and is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series.
5. The catalyst of claim 1 , wherein C is selected from Cobalt or Manganese.
6. The catalyst of claim 1 , wherein A is selected from the group consisting of Ba, Sr, and Ca.
7. The catalyst of claim 1 , wherein B is selected from the group consisting of Lathanide series, and Zr, Ti, Hf, Ga, Cr, Sc, Al, Nb, Zn, In, Y.
8. The catalyst of claim 1 , wherein A and B are not platinum or palladium.
9. An engine exhaust system, comprising:
a catalytic oxidation reactor having a nitric oxide oxidation catalyst supported on a substrate, wherein the engine exhaust catalyst has the general formula:
A2B2CxO(5+y), wherein A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements;
B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series;
C is a metallic element selected from the group consisting of the transition metals, wherein A, B, and C are different metals;
x is a number from 1 to 5, and
y is a number that renders the catalyst substantially charge neutral.
10. The system of claim 9, wherein the substrate comprises multiple washcoat zones or layers, wherein the nitric oxide oxidation catalyst is included in one of the washcoat zones or layers.
1 1. The system of claim 10, further comprising a platinum-based catalyst included in one of the washcoat zones or layers that is different from the zone or layer containing the nitric oxide oxidation catalyst.
12. The system of claim 1 1 , wherein the zone or layer containing the nitric oxide oxidation catalyst encounters an engine exhaust flow after the platinum containing zone or layer.
13. The system of claim 10, wherein at least one of the zones or layers includes zeolites.
14. The system of claim 13, wherein the zeolites include at least one of ZSM-5 zeolite, beta zeolite, and Y zeolite.
15. A method of producing a nitric oxide oxidation catalyst comprising:
preparing a metal salt solution including Cobalt or Manganese and metal salts of the general formula:
A2B205
wherein A is a metallic element selected from the group consisting of elements from Alkali metals, Alkaline earth metals and the Lanthanide Series of the lUPAC Periodic Table of the Elements; and B is a metallic element selected from the group consisting of elements from the Alkali metals, Alkaline earth metals, transition metals, poor metals, and the Lanthanide Series;
adding sodium hydroxide, sodium carbonate, or ammonium oxalate to precipitate the metal salts;
oxidizing the metal salts with hydrogen peroxide; and
separating the oxidized metal salts.
16. The method of claim 15, further comprising washing the separated metal salts and then drying; and calcining the separated metal salts that have been washed and dried.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US38404110P | 2010-09-17 | 2010-09-17 | |
| US61/384,041 | 2010-09-17 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2012037526A2 true WO2012037526A2 (en) | 2012-03-22 |
| WO2012037526A3 WO2012037526A3 (en) | 2012-06-21 |
Family
ID=45832280
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/052040 WO2012037526A2 (en) | 2010-09-17 | 2011-09-16 | Nitric oxide oxidation catalysts |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012037526A2 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103801286A (en) * | 2012-11-14 | 2014-05-21 | 上海纳米技术及应用国家工程研究中心有限公司 | Supported chromic oxide catalyst as well as preparation and application thereof |
| CN103894185A (en) * | 2014-04-01 | 2014-07-02 | 北京工业大学 | Method for preparing selective catalytic reduction (SCR) denitration catalyst by taking TiO2-ZnO as composite carrier |
| EP2835171A1 (en) * | 2013-08-08 | 2015-02-11 | Technical University of Denmark | Method and system for the purification of exhaust gas with an electrochemical cell |
| CN107847864A (en) * | 2015-06-18 | 2018-03-27 | 庄信万丰股份有限公司 | There is no before ASC DOC in the system with SCR catalyst and with the gas extraction system for the ASC for serving as DOC |
| CN108246305A (en) * | 2018-02-06 | 2018-07-06 | 北京阳光欣禾科技有限公司 | It is a kind of for catalyst for selective oxidation of denitrating flue gas and preparation method thereof |
| US10927465B2 (en) | 2016-11-21 | 2021-02-23 | Toyota Motor Engineering & Manufacturing North America, Inc. | Brownmillerite oxides for oxygen evolution catalyst |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4160013A (en) * | 1976-08-05 | 1979-07-03 | University Of Southern California | Process for the oxidation of nitric oxide to nitrogen dioxide |
| JPH06170224A (en) * | 1992-12-07 | 1994-06-21 | Tosoh Corp | Brownmillerite type nox decomposing catalyst and its production |
| US7631488B2 (en) * | 2006-10-27 | 2009-12-15 | Postech Foundation | Oxidation catalyst for removing fine soot particulates from exhaust gases and method of removing fine soot particulates using the same |
| US20100086458A1 (en) * | 2008-10-03 | 2010-04-08 | Gm Global Technology Operations, Inc. | Method and architecture for oxidizing nitric oxide in exhaust gas from hydrocarbon fuel source with a fuel lean combustion mixture |
-
2011
- 2011-09-16 WO PCT/US2011/052040 patent/WO2012037526A2/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4160013A (en) * | 1976-08-05 | 1979-07-03 | University Of Southern California | Process for the oxidation of nitric oxide to nitrogen dioxide |
| JPH06170224A (en) * | 1992-12-07 | 1994-06-21 | Tosoh Corp | Brownmillerite type nox decomposing catalyst and its production |
| US7631488B2 (en) * | 2006-10-27 | 2009-12-15 | Postech Foundation | Oxidation catalyst for removing fine soot particulates from exhaust gases and method of removing fine soot particulates using the same |
| US20100086458A1 (en) * | 2008-10-03 | 2010-04-08 | Gm Global Technology Operations, Inc. | Method and architecture for oxidizing nitric oxide in exhaust gas from hydrocarbon fuel source with a fuel lean combustion mixture |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103801286A (en) * | 2012-11-14 | 2014-05-21 | 上海纳米技术及应用国家工程研究中心有限公司 | Supported chromic oxide catalyst as well as preparation and application thereof |
| EP2835171A1 (en) * | 2013-08-08 | 2015-02-11 | Technical University of Denmark | Method and system for the purification of exhaust gas with an electrochemical cell |
| WO2015018926A1 (en) * | 2013-08-08 | 2015-02-12 | Danmarks Tekniske Universitet | Method and system for the purification of exhaust gas with an electrochemical cell |
| CN103894185A (en) * | 2014-04-01 | 2014-07-02 | 北京工业大学 | Method for preparing selective catalytic reduction (SCR) denitration catalyst by taking TiO2-ZnO as composite carrier |
| CN107847864A (en) * | 2015-06-18 | 2018-03-27 | 庄信万丰股份有限公司 | There is no before ASC DOC in the system with SCR catalyst and with the gas extraction system for the ASC for serving as DOC |
| US10927465B2 (en) | 2016-11-21 | 2021-02-23 | Toyota Motor Engineering & Manufacturing North America, Inc. | Brownmillerite oxides for oxygen evolution catalyst |
| CN108246305A (en) * | 2018-02-06 | 2018-07-06 | 北京阳光欣禾科技有限公司 | It is a kind of for catalyst for selective oxidation of denitrating flue gas and preparation method thereof |
| CN108246305B (en) * | 2018-02-06 | 2021-05-25 | 北京阳光欣禾科技有限公司 | Selective oxidation catalyst for flue gas denitration and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2012037526A3 (en) | 2012-06-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9457344B2 (en) | Mixed phase oxide catalysts | |
| JP6474809B2 (en) | Ammonia slip catalyst | |
| JP6386962B2 (en) | Emission treatment system comprising an ammonia generation catalyst and an SCR catalyst | |
| JP6755306B2 (en) | Catalytic filter with soot catalyst and SCR catalyst | |
| CN101528324B (en) | Ce-ZR-R-O catalysts, articles comprising the Ce-ZR-R-O catalysts and methods of making and using the Ce-ZR-R-O catalysts | |
| EP3194053B1 (en) | Exhaust system with a modified lean noxtrap | |
| ES2542510T3 (en) | CHA copper zeolite catalysts | |
| CZ2005148A3 (en) | Compression ignition engine, motor vehicle, method for operating such a compression ignition engine and method for increasing rate of catalyzed reaction | |
| WO2011082357A2 (en) | Engine exhaust catalysts doped with bismuth or manganese | |
| WO2012037526A2 (en) | Nitric oxide oxidation catalysts | |
| KR20160055244A (en) | EXHAUST SYSTEM WITH A MODIFIED LEAN NOx TRAP | |
| WO2006013998A1 (en) | Process for catalytic reduction of nitrogen oxides | |
| CN110785546A (en) | Exhaust gas purification system | |
| WO2020230076A1 (en) | Exhaust system including paticulate filter with oxidation zone capable of generating no2 under lean conditions | |
| WO2012030127A2 (en) | Catalyst for selective catalytic reduction, with improved durability | |
| JP3925015B2 (en) | Internal combustion engine exhaust gas purification device, purification method, and purification catalyst | |
| JP6182036B2 (en) | Exhaust gas treatment catalyst, exhaust gas treatment device, and method for producing exhaust gas treatment catalyst | |
| EP4252890A1 (en) | Catalyst article for oxidation, adsorption and desorption reactions | |
| JP5925668B2 (en) | Exhaust gas purification catalyst | |
| JP2004209386A (en) | Method and catalyst therefor for reducing nitrogen oxide catalytically | |
| JP2001198463A (en) | Catalyst for exhaust-gas purification | |
| JPH10113560A (en) | Exhaust gas purifying catalyst and its manufacture | |
| JP2003071249A (en) | Method for catalytic reduction of nitrogen oxides and catalyst therefor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11826061 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 32PN | Ep: public notification in the ep bulletin as address of the adressee cannot be established |
Free format text: NOTING OF LOSS OF RIGHTS PURSUANT TO RULE 112(1) EPC (EPO FORM 1205A DATED 26/07/2013) |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11826061 Country of ref document: EP Kind code of ref document: A2 |