WO2012034403A1 - 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 - Google Patents
一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 Download PDFInfo
- Publication number
- WO2012034403A1 WO2012034403A1 PCT/CN2011/073810 CN2011073810W WO2012034403A1 WO 2012034403 A1 WO2012034403 A1 WO 2012034403A1 CN 2011073810 W CN2011073810 W CN 2011073810W WO 2012034403 A1 WO2012034403 A1 WO 2012034403A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- formula
- compound
- represented
- cyano
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/18—One oxygen or sulfur atom
- C07D231/20—One oxygen atom attached in position 3 or 5
- C07D231/22—One oxygen atom attached in position 3 or 5 with aryl radicals attached to ring nitrogen atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
Definitions
- R CF 3 , Cl, Br or OCH 2 CF 3
- A is N or CH
- n is an integer from 0 to 4.
- R 2 is halogen, cyano, d-Ce alkyl or halogenated dC 3 alkyl.
- R 1 is hydrogen, halogen, cyano, methyl or trifluoromethyl
- R 2 is a halogen or a cyano group.
- an isomer such as an optical isomer or a geometric isomer may be present, and the present invention encompasses various forms. Both the conformation and the mixture of isomers. Further, the present invention also encompasses various isomers other than the above within the scope of technical knowledge in the technical field. Further, although a chemical structure different from the above structural formula (I) may be formed depending on the kind of the isomer, as long as those skilled in the art can fully recognize that the isomer exists, it is apparent that Within the scope of the invention.
- A is N or CH
- R 1 is hydrogen, halogen, cyano, nitro, alkyl, haloalkyl, cyanoalkyl, alkenyl, haloalkenyl, block, halo block, alkoxy, cyanoalkoxy , alkoxy, alkylthio, alkylthio, alkylsulfonyl, alkylcarbonyl, alkylcarbonyl, alkoxycarbonyl, alkoxycarbonyl or aminoalkyl;
- R 1 is hydrogen, halogen, cyano, methyl or trifluoromethyl
- R 2 is a halogen or a cyano group.
- Step (1) The post-treatment of the compound represented by the structural formula (W) is: filtration, the filter cake is washed with a small amount of an organic solvent, and the filtrate is combined and concentrated.
- the hydrolysis reaction is carried out directly in the step (2) without isolation and purification.
- the post-treatment of the monofluoromethoxypyrazolecarboxylic acid represented by the structural formula (III) in the step (2) is: after evaporating the organic solvent, adding water, extracting with diethyl ether, and then adding acidic hydrochloric acid to the aqueous phase to make it acidic. A large amount of solid precipitated, filtered, washed with water, and dried by an infrared lamp to give the above formula (III) a solid containing a monofluoromethoxypyrazolecarboxylic acid.
- the base is an organic base, and the organic base is further preferably one selected from the group consisting of triethylamine, pyridine or 3-methylpyridine. Or a combination of two or more; the reaction temperature is -30 to 80 ° C, and more preferably -15 to 30 ° C.
- the volume of the organic base is 1 to 4 times (ml/g), preferably 1 to 2 times (ml/g), based on the mass of the monofluoromethoxypyrazolecarboxylic acid represented by the structural formula (III).
- the amount of the compound to be represented by IV) is 1:1 to 5, more preferably 1:1 to 2;
- the aprotic solvent is selected from the group consisting of tetrahydrofuran, acetonitrile, 1, 4-dioxane, diethyl ether, toluene One or a combination of two or more of dichloromethane or chloroform.
- the volume of the aprotic solvent is 5 to 60 times the mass of the 4H-benzo[1,3]oxazin-4-one compound containing the monofluoromethoxypyrazole represented by the structural formula ( ⁇ ) (ml/ g ) is preferably 5 to 20 times (ml/g).
- the present invention also provides the use of a monofluoroamidobenzamide compound containing a monofluoromethoxypyrazole represented by the formula (I) or an agriculturally applicable salt thereof, a pesticide preparation for controlling pests, and a control The method of pests.
- a pest control agent containing a fluoromethoxypyrazole-containing orthoformylbenzamide compound represented by the structural formula (I) of the present invention or an agriculturally applicable salt thereof can be used for pest control on agricultural crops, for example It is particularly useful as a control agent for various pests that is a problem in the field of agriculture and horticulture, that is, an agricultural and horticultural pest control agent, or a pest control agent that is parasitic on animals, that is, an animal parasite control agent.
- a pesticidal or acaricide for controlling plant parasitism such as two-spotted spider mites, red leafhopper, citrus scorpion, and whole claw scorpion.
- the agricultural and horticultural pest control agent containing the compound of the present invention is particularly effective for agricultural pests and the like. Further, the agricultural or horticultural pest control agent containing the fluoromethoxypyrazole-containing orthoformylaminobenzamide compound represented by the structural formula (I) of the present invention or an agriculturally applicable salt thereof Various resistant pests of an agent such as an organophosphorus agent, a carbamate agent, or a pyrethroid agent are effective.
- pest control agent containing the monofluoroamidobenzamide compound containing the monofluoromethoxypyrazole represented by the structural formula (I) of the present invention or an agriculturally applicable salt thereof may be mentioned.
- a pest control agent for agricultural and horticultural pest control such as plant parasitic mites, agricultural pests, and soil pests.
- Various forms of preparation are used, but as long as it is suitable for the purpose of the present invention, it can be made into all preparation forms which are generally used in the field.
- Examples of the adjuvant used in the preparation include diatomaceous earth, slaked lime, calcium carbonate, talc, white carbon, kaolin, bentonite, kaolinite and sericite mixture, clay, sodium carbonate, baking soda, thenardite, zeolite, starch.
- auxiliary agents can be used singly or in combination of two or more kinds as long as they do not deviate from the object of the present invention. Further, it may be appropriately selected from those known in the art in addition to the above-mentioned auxiliary agents.
- Various adjuvants commonly used such as extenders, thickeners, dustproofing agents, antifreeze agents, dispersion stabilizers, phytotoxicity reducing agents, and antifungal agents, can also be used.
- composition of the formula (I) represents a weight ratio of the compound and the various adjuvants, that is, the carrier, usually 0. 1: 99. 9 ⁇ 90: 10.
- these preparations can be used directly or diluted with a diluent to a prescribed concentration.
- Use various developing agents surfactants, vegetable oils, mineral oils, etc.) as needed.
- the application of the agricultural and horticultural pest control agent containing the compound represented by the structural formula (I) of the present invention varies depending on the meteorological conditions, the form of the preparation, the application period, the application site, the type of the pest or the occurrence state, and the like, and cannot be generalized.
- the application is generally carried out at a concentration of the active ingredient of 0.05 to 800 ppm, preferably 0.5 to 500 ppm, and the application amount per unit is 1 to 5000 g, preferably 10 to 1000 g per 1 hectare of the compound of the invention.
- the application of the agricultural and horticultural pest control agent containing the other preferred embodiment of the pest control agent of the present invention can be carried out according to the application of the above-mentioned pest control agent.
- the present invention also encompasses a method for controlling pests using such an application method, particularly a method for controlling plant parasitic mites and agricultural pests.
- the application of various preparations, or dilutions thereof, of the agricultural and horticultural pest control agent containing the compound of the formula (I) of the present invention can be generally carried out by a usual application method such as spreading, spraying, misting, misting.
- by mixing the above-mentioned active ingredients into the feed to the livestock it is also possible to hinder the occurrence and growth of pests, particularly harmful insects, in the excrement, and it is also possible to use the so-called ultra low volume.
- Administration in this method, may contain 100% of the active ingredient.
- the structural formula (I) according to the present invention can be used in combination with one or two or more kinds of conventional insecticidal, bactericidal or herbicidal pesticides, and exhibits more advantageous effects and functions.
- one or more mixed pest control compositions of the compound of the formula (I) and the other active ingredients of the pesticidal compound of the present invention may be used in combination or in combination, and the scope of application, the period of treatment of the drug, and the control activity may be applied. Wait for a good direction to improve.
- the compound of the present invention and other active ingredient compounds of the agricultural chemical may be used by mixing the separately prepared preparations at the time of dispersion, or may be used together as a preparation, and the present invention also encompasses such a mixed pest control composition. .
- the structural formula (I) of the present invention represents a compound and a preparation thereof, and has the following advantages:
- the present invention introduces a monofluoromethoxy group for the first time in a pyrazole ring, and the structure of the compound is novel;
- the compound of the present invention and its preparation have a broad spectrum of insecticidal activity: against lepidopteran pests (Plutella xylostella, Spodoptera frugiperda and Helicoverpa armigera), Hemipteran pests (Peach and aphid), and the same wing
- lepidopteran pests Plutella xylostella, Spodoptera frugiperda and Helicoverpa armigera
- Hemipteran pests Pieric and aphid
- the target pests rice planthopper
- the dipteran pests Lepidoptera: Trifolium
- the leaf-like pests Haorse genus
- the compound of the present invention and its preparation have an extremely high insecticidal activity: at a dose of 0.16 mg/L, it has a good effect on Plutella xylostella, Spodoptera frugiperda and Helicoverpa armigera; at a dose of 4 mg/L
- the lower aphids, trifolium and horseradish have also showed good results; at 20mg/L, the rice brown planthopper showed good effect;
- the compound of the present invention and its preparation have good safety, and are safe to some crops such as wheat, soybean, cotton, rice, etc., and are environmentally friendly;
- the compound of the present invention and its preparation have reasonable toxicity, ecotoxicity and environmental compatibility, and are low-toxic environmental friends. Good pesticides.
- the examples of the present invention are described below, but the present invention is not limited thereto, and a synthesis example of the compound of the present invention will be described first. detailed description
- the insecticidal activity evaluation test was carried out according to the following methods:
- test targets were Plutella xylostella, Spodopterem f rug i per da), Helicoverpa armigera, Aphis medicaginis ⁇ Myzus persicae ⁇ Ta lugens), Liriomyza trifolii (r /o ) and horseradish (Phaedon cochleariae).
- Mortality ( % ) number of live insects ⁇ ⁇ )0
- Potted cotton (2-3 leaf ages) was sprayed with the drug and allowed to dry. Put in the culture room and continue normal culture. After 5, 12, 19d, cut the leaves with scissors, place them in a 09cm plastic petri dish, connect the cotton bollworm larvae, put a piece of filter paper, and cover. Place in the observation room and check the results after 6 days. Mortality was calculated according to the above mortality calculation formula. Abnormal worms are also seen as dead. Compounds 3, 8, 10, 33, 36, 49, 51 in Table 1 gave 100% mortality at 20 mg/L. Compounds 8, 10, 33, and 51 gave 100% mortality at 4 mg/L.
- Potted cabbage (3-4 leaf ages) was sprayed with the drug and allowed to dry. Put in the culture room and continue normal culture. After 5, 12, 19d, use the nymphs respectively. Place in the observation room and check the results after 7-10d. Mortality is calculated according to the above mortality calculation formula. Abnormal worms are also seen as death. Compounds 3, 8, 10, 33, 51 in Index Table 1 achieved 100% mortality at 20 mg/L. Compounds 3, 8, 33, 51 gave at least 90% mortality at 4 mg/L.
- Example 12 Effect test on rice brown planthopper Nilaparvata lugens
- Potted cabbage (3-4 leaf ages) was sprayed with the drug and allowed to dry. Put in the culture room and continue normal culture. After 5, 12, 19d, cut the leaves with scissors, place them in a 09cm plastic petri dish, and connect the 2nd instar larvae of horseradish or Plutella xylostella, put a piece of filter paper, and cover. Place in the observation room and check the results after 6-7 days. Mortality was calculated according to the above mortality calculation formula. Abnormal worms are also seen as death. Compounds 3, 8, 10, 33, 36, 49, 51 in Table 1 gave 100% mortality at 20 mg/L. Compounds 3, 8, 33, 51 gave at least 90% mortality at 4 mg/L. Compound 8 gave at least 90% mortality at 0.8 mg/L. According to the above method, compound 8 and the known compound chlorantraniliprole were selected for killing the diamondback moth, cockroach and rice brown fly fruit as shown in Table 3.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Plural Heterocyclic Compounds (AREA)
Description
说明书
一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、 其合成方法及应用 技术领域
本发明提供了一种邻甲酰氨基苯甲酰胺类化合物。 背景技术
邻甲酰氨基苯甲酰胺类化合物 (鱼尼丁受体抑制剂类) 是近几年开发的防治无脊锥动物 害虫的有效杀虫剂。
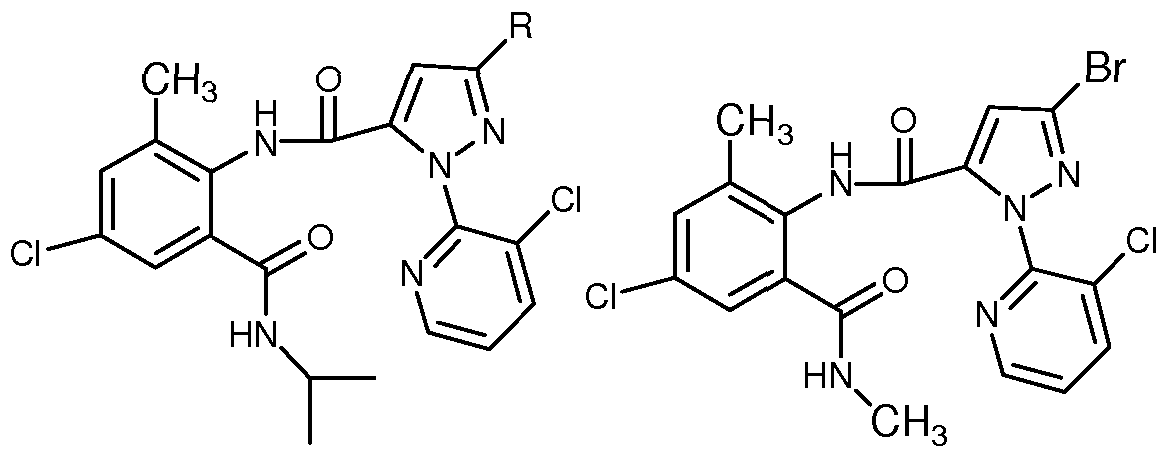
PCT专利申请 WO2003/015519 公开了如下具有杀虫活性的化合物:
R=CF3,Cl,Br或 OCH2CF3
PCT专利申请 WO2004/033468公开了如下具有杀虫活性的化合物:
PCT专利申请 WO2004/067528公开了如下具有杀虫活性的化合物:
PCT专利申请 WO2006/080311公开了如下具有杀虫活性的化合物:
PCT专利申请 WO2008/134969公开了如下具有杀虫活性的化合物:
上述 PCT专利申请中所有公开的化合物虽与本发明所述化合物有一定的相似之处, 但都没有记载具有相当于本发明所述式 ( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲 酰胺类化合物。 尽管用于防治无脊椎害虫的许多产品可以购买, 但仍然需要更有效、 低毒、 低成本、 对环境安全的新化合物。 发明内容
本发明的目的在于提供一种新的有害生物防除剂, 可防除在农业园艺领域中成为问题 的各种有害生物, 或寄生于动物的有害生物, 在低药量下具有对有害生物超高的防治效果。
为实现上述目的, 本发明提供如下技术手段:
其巾:
A为 N或 CH;
R1为氢、 卤素、 氰基、 硝基、 烷基、 卤代烷基、 氰基烷基、 链烯基、 卤代链烯基、 块基、 卤代块基、 烷氧基、 氰基烷氧基、 ^代烷氧基、 烷硫基、 ^代烷硫基、 烷基磺酰基、 烷基羰 基、 ^代烷基羰基、 烷氧基羰基、 ^代烷氧基羰基或氨基烷基;
R2为氢、 卤素、 烷基、 卤代烷基、 烷氧基、 卤代烷氧基或氰基;
R3为氢、 烷基或烷氧基;
R4为氢、 氰基、 烷基、 烷氧基、 烷氨基、 ^代烷基、 ^代烷氧基、 ^代烷氨基、 氰基烷 基、 环烷基、 ^代环烷基、 羟烷基、 烷氧羰基甲基、 烷氧基酰胺基、 烷基酰胺基、 ^代烷基 酰胺基、 氰基取代的烷基酰胺基或芳杂环甲基;
或者 R3和 R4与所连接的 N—起形成三元、 四元、 五元或六元环;
m为 0〜4的整数;
n为 0〜4的整数。 作为优选的方式, 结构式 (I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物 中:
R1为氢、 卤素、 氰基、 硝基、 d-Ce浣基、 d-Ce卤代烷基、 氰基 d-Ce烷基、 C2_C6链烯基、
C2_C6卤代链烯基、 C2-C6块基、 卤代 C3-C6块基、 ( -(:6烷氧基、 d-C6氰基烷氧基、 d-C6卤代烷 氧基、 d-Ce烷硫基、 d-Ce卤代烷硫基、 d-Ce烷基磺酰基、 CfCe烷基羰基、 卤代 CfCe烷基羰 基、 ( Ce烷氧基羰基、 ^代 d-C6烷氧基羰基或氨基 d-C6烷基;
R2为氢、 卤素、 d-Ce烷基、 卤代 d-Ce烷基、 d-Ce烷氧基、 卤代 CfCe烷氧基或氰基;
R3为氢或 烷基;
R4为氢、 氰基、 d-Ce烷基、 d-Ce烷氧基、 d-Ce烷氨基、 卤代 CfCe烷基、 卤代 CfCe烷氧 基、 卤代 ( -(:6烷氨基、 氰基 d-C6烷基、 (:3_(:6环烷基、 卤代(:3_(:6环烷基、 羟( -(:6烷基、 d-c6 烷氧羰基甲基、 (^-(:6烷氧基酰胺基、 d-c6烷基酰胺基、 ^代 d-c6烷基酰胺基、 氰基取代的 d-c6烷基酰胺基或芳杂环甲基;
m为 2;
n为 1。
作为进一步优选的方式, 结构式 (I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺 类化合物中:
A为 N;
R1为氢、 卤素、 氰基、 CfCe烷基、 d- 卤代烷基或 d- 烷基羰基;
R2为卤素、 氰基、 d-Ce烷基或卤代 d-C3烷基。
作为更进一步优选的方式, 结构式 (I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰 胺类化合物中:
R1为氢、 卤素、 氰基、 甲基或三氟甲基;
R2为卤素或氰基。
作为最为优选的方式, 结构式 (I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类 化合
在上述 A、 R R2、 R3、 R4和 R5基团中:
作为 R R2或 R4中的卤素或作为取代基的卤素, 可列举出, 氟、 氯、 溴或碘的各原子。 作为取代基的卤素的数可以为 1或 2以上, 在 2以上的场合, 各卤素可以相同也可以不同。 另外, 卤素的取代位置可以在任一位置。
R R2、 R3或 R4中的烷基或烷基部分, 可以是直链也可以是支链。 作为其具体例, 可列 举出, 甲基、 乙基、 丙基、 异丙基、 丁基、 叔丁基、 戊基、 己基这样的 d— 6的烷基等。
R1中的链烯基或链烯基部分,可以是直链或支链的任一一种。作为其具体例,可列举出, 乙烯基、 1-丙烯基、 烯丙基、 异丙烯基、 1-丁烯基、 1, 3-丁二烯基、 1-己烯基这样的 C2— 6的
R1中的块基或块基部分, 可以是直链或支链的任一一种。 作为其具体例, 可列举出乙块 基、 2-丁块基、 2-戊块基、 3-己块基这样的 C2— 6的块基等。
在上述结构式(I )表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物中, 有时存 在光学异构体、几何异构体这样的异构体, 本发明包含各异构体及异构体混合物这两者情况。 此外, 在该技术领域的技术常识的范围内, 本发明也包含上述以外的各种异构体。 另外, 虽 然有时因异构体的种类导致形成与上述结构式 (I )不同的化学结构的情况, 但只要是本领 域技术人员就能充分认识这是存在异构体的关系, 因此很明显是在本发明的范围内。
本发明还提供一种用于制备上述结构式(I )表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲 酰胺类化合物, 具有以下结构式 (Π ):
其中 A、 R1和 R2基团及!!!和!!与结构式 (I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲 酰胺类化合物中基团相对应, 即:
A为 N或 CH;
R1为氢、 卤素、 氰基、 硝基、 烷基、 卤代烷基、 氰基烷基、 链烯基、 卤代链烯基、 块基、 卤代块基、 烷氧基、 氰基烷氧基、 ^代烷氧基、 烷硫基、 ^代烷硫基、 烷基磺酰基、 烷基羰 基、 ^代烷基羰基、 烷氧基羰基、 ^代烷氧基羰基或氨基烷基;
R2为氢、 卤素、 烷基、 卤代烷基、 烷氧基、 卤代烷氧基或氰基;
m为 0〜4的整数;
n为 0〜4的整数。
作为优选的方式, 结构式 (Π ) 表示的化合物中:
A为 N;
R1为氢、 卤素、 氰基、 硝基、 CrC6烷基、 CrC6卤代烷基、 氰基 CrC6烷基、 C2-C6链烯 基、 C2-C6卤代链烯基、 C2-C6块基、 卤代 C3-C6块基、 -C6烷氧基、 d-C6氰基烷氧基、 -C6 卤代烷氧基、 ^-^烷硫基、 C C6 ^代烷硫基、 CrC6烷基磺酰基、 CrC6烷基羰基、 ^代 CrC6 烷基羰基、 -C6烷氧基羰基、 ^代 -C6烷氧基羰基或氨基 -C6烷基;
R2为氢、 卤素、 CrC6烷基、 卤代 CrC6烷基、 CrC6烷氧基、 卤代 CrC6烷氧基或氰基; m为 2;
n为 1。
作为进一步优选的方式, 结构式 (Π ) 表示的化合物中:
R1为氢、 卤素、 氰基、 d-C6烷基、 d-C6卤代烷基或 d-C6烷基羰基;
R2为卤素、 氰基、 CfCe烷基或卤代 d-C3烷基。
作为更为优选的方式, 结构式 (Π ) 表示的化合物中:
R1为氢、 卤素、 氰基、 甲基或三氟甲基;
R2为卤素或氰基。
本发明还提供上述结构式(I )表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物 的合成方法。
一种结构式 (I ) 表示的的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物合成方法, 包括如下步骤:
( 1 ) 在非质子性溶剂中, 结构式 (VI) 表示的化合物与一氟一溴甲烷在缚酸剂作用下, 反应得到结构式 (W) 表示化合物;
(2) 在质子性溶剂中, 结构式 (W) 表示化合物在碱作用下水解得到结构式 (III) 表 示的含一氟甲氧基吡唑羧酸;
CHPF
昨
(VI) (VD) (III)
(3) 在非质子性溶剂中, 加入碱和烷基磺酰氯, 结构式 (III) 表示的含一氟甲氧基吡 唑羧酸和结构式 (V ) 表示的取代邻氨基苯甲酸反应得结构式 (Π ) 表示的含一氟甲氧基吡 唑的 4H-苯并 [1, 3]噁嗪 -4-酮类化合物;
( 4 ) 在非质子性溶剂中, 结构式 (Π )表示的含一氟甲氧基吡唑的 4Η-苯并 [1, 3]噁嗪 -4-酮类化合物和结构式 (IV)表示的化合物反应得结构式 (I )表示的含一氟甲氧基吡唑的 邻甲酰氨基苯甲酰胺类化合物;
(II) (IV) ( I )
结构式 (II) 至 (YD) 表示的化合物中 A、 R R2、 R3、 R4和 R5基团以及 m和 n的定义及 优选方式如上述结构式(I)表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物, 其中 结构式 (VI) 表示的化合物中!^为^— 6烷基。
上述结构式 (VI) 表示的化合物为公知化合物, 其制备方法可以参照 PCT 专利申请 WO03015519和 WO2006023783。
作为优选的实施方式, 前述合成方法步骤 (1) 中, 结构式 (VI)表示的化合物与一氟一 溴甲烷和缚酸剂的投料物质的量比为 1: 1.1〜2: 1.1〜2, 进一步优选 1: 1.1〜1.5: 1.1〜 1.5; 所述非质子性溶剂选自四氢呋喃、 乙腈、 1, 4-二氧六环或丙酮中的一种或两种以上 组合; 反应温度为 10〜90°C, 进一步优选 20〜80° (:。
步骤 (1) 结构式 (W)表示的化合物的后处理是: 过滤, 滤饼用少量的有机溶剂进行淋 洗, 滤液合并, 浓縮。 不必进行分离纯化直接进行步骤 (2) 所述水解反应。
作为优选的实施方式, 前述合成方法步骤(2) 中, 结构式 (W)表示化合物与碱的投料 物质的量比为 1: 1〜1.5, 进一步优选 1: 1〜1.2; 所述质子性溶剂为甲醇和 /或乙醇; 反 应温度为 10〜90°C, 进一步优选 20〜80° (:。
步骤 (2) 中结构式 (III) 表示的含一氟甲氧基吡唑羧酸的后处理是: 蒸除有机溶剂后, 加水, 用乙醚进行萃取, 然后水相中加稀盐酸使呈酸性, 大量固体析出, 过滤, 水洗, 红外 灯干燥, 得所述结构式 (III) 表示含一氟甲氧基吡唑羧酸固体。
作为优选的实施方式, 前述合成方法步骤(3) 中, 结构式 (III)表示的含一氟甲氧基吡 唑羧酸和结构式(V)表示的取代邻氨基苯甲酸及烷基磺酰氯的投料物质的量比为 1: 1〜1.5: 2〜3, 进一步优选 1: 1〜1.2: 2〜2.5; 所述烷基磺酰氯为甲基磺酰氯; 所述非质子性溶剂 选自四氢呋喃、 乙腈、 1, 4-二氧六环、 乙醚或甲苯中的一种或两种以上组合; 所述碱为 有机碱, 有机碱进一步优选自三乙胺、 吡啶或 3-甲基吡啶中的一种或两种以上组合; 反 应温度为 -30〜80°C, 进一步优选 -15〜30°C。 其中有机碱的体积用量为结构式(III)表示的 含一氟甲氧基吡唑羧酸质量的 1~4倍 (ml/g), 优选为 1~2倍 (ml/g)。
步骤(3) 的后处理为: 反应结束, 过滤, 滤饼先后用水、 有机溶剂与水的混合溶剂及少 量有机溶剂洗后得第一批固体; 滤液蒸除溶剂后, 加有机溶剂萃取, 无水硫酸钠干燥后, 过
滤, 浓縮, 用乙醇进行重结晶, 得第二批固体; 两批固体合并, 得所述结构式 (II) 表示的 化合物。
其中结构式 (V) 表示的取代邻氨基苯甲酸是一种已知化合物, 可以通过许多方法进行 制备, 可参照以下文献: Organic Synthesis, Coll.Vol.79,pl96(2002) ; Vol.l0,p23(2004) ; Adv.Heterocycl.Chem.1975, 18, 1 -58 ; Journal of the Brazilian Chemical Society 2001,12(3),273-324; Angew.Chem.Int.Ed.Engl.1980, 19,222-223。
作为优选的实施方式, 前述合成方法步骤(4 ) 中, 结构式 (Π )表示的含一氟甲氧基吡 唑的 4H-苯并 [ 1, 3]噁嗪 -4-酮类化合物和结构式(IV )表示的化合物的投料物质的量比为 1 : 1〜5, 进一步优选 1 : 1〜2; 所述非质子性溶剂选自四氢呋喃、 乙腈、 1, 4-二氧六环、 乙 醚、 甲苯、 二氯甲烷或氯仿中的一种或两种以上组合。 其中非质子性溶剂的体积用量为 结构式 (Π ) 表示的含一氟甲氧基吡唑的 4H-苯并 [ 1, 3]噁嗪 -4-酮类化合物质量的 5~60 倍 ( ml/g ) , 优选为 5~20倍 (ml/g)。
下面, 将本发明所涉
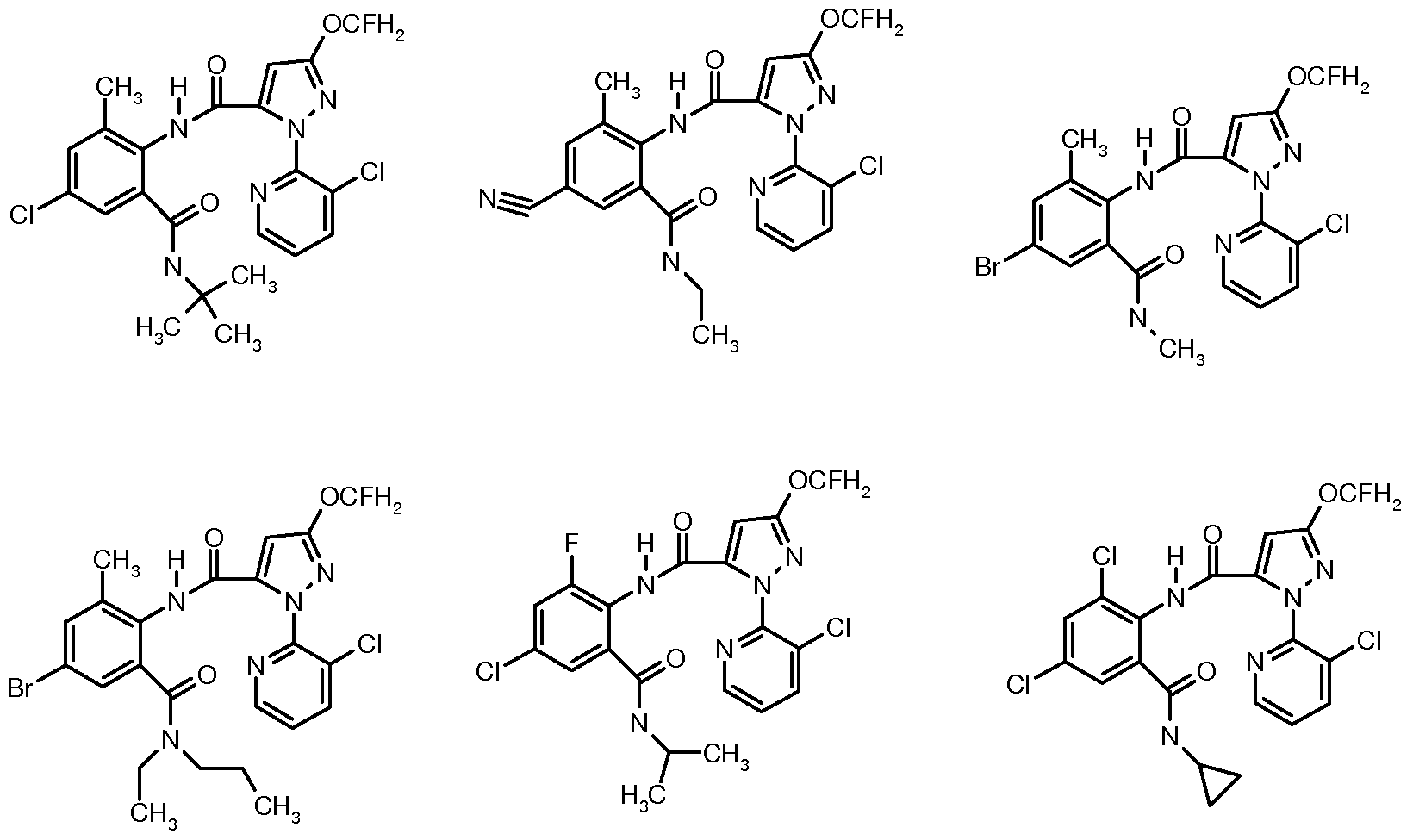
以下的索引表 1中采用的縮写如下: t是叔、 n是正、 i是异、 s是仲、 Me 是甲基、 Et是乙基、 Pr是丙基、 Bu是丁基;相应的 i-Pr是异丙基等。索引表 1中 R2=3_C1, n=l, R: 和 R4可以为独立的取代基, 也可以连接成一个整体。
以下索引表 2为索引表 1所述部分化合物的物化性能及核磁数据。 注:索引表 2所述的编号与索引表 1所述的化合物编号相对应。其中 s为单峰, d为双峰, t 为三重峰, q为四重峰, m为多重峰, brs为宽单峰。 索引表 2
编号 熔点 /°c ¾NMR (除非另有说明, 试剂均为 CDC13)
0. 95-0. 98 (m, 3H) , 1. 58-1. 62 (m, 2H) , 2. 19 (s, 3H) ,
3. 32-3. 37 (m, 2H) , 5. 83— 5. 96 (d, 2H) , 6. 12 (s, 1H) ,
1
6. 66 (s, 1H) , 7. 22 (s, 1H) , 7. 33-7. 37 (m, 1H) ,
7. 82-7. 84 (d, 1H) , 8· 45- 8· 46 (d, 1H) , 10. 00 (s, 1H)
1. 20-1. 22 (d, 6H) , 2. 18 (s, 3H) , 4. 15-4. 20 (m, 1H) ,
5. 83-5. 96 (d, 2H) , 5. 92-5. 94 (d, 1H) , 6. 71 (s, 1H) ,
2
7. 19-7. 36 (m, 3H) , 7· 82- 7· 84 (d, 1H) , 8. 46 (s, 1H) ,
10. 06 (s, 1H)
2. 16 (s, 3H) , 2. 67 (s, 3H) , 5. 85-5. 99 (d, 1H), 6. 93 (brs,
3 (实施例 2 ) 236-237 1H) , 7. 34 (s, 1H) , 7. 45 (s, 1H) , 7. 54-7. 57 (m, 1H) ,
8. 10-8. 47 (m, 3H) , 10. 18 (brs, 1H)
1. 16-1. 20 (t, 3H) , 2. 17 (s, 3H) , 3. 37-3. 44 (m, 2H) ,
4 5. 83-5. 96 (d, 2H) , 6. 15 (s, 1H) , 6. 75 (s, 1H) , 7. 19—7. 36
(m, 3H) , 7. 82-7. 84 (d, 1H) , 8. 44-8. 46 (m, 1H) ,
10. 00 (s, 1H)
0. 91-0. 95 (m, 3H) , 1. 34-1. 60 (m, 4H) , 2. 18 (s, 3H) ,
3. 34-3. 39 (m, 2H), 5. 82-5. 96 (d, 2H) , 6. 12 (s, 1H) ,
5 6. 71 (s, 1H) , 7. 19-7. 36 (m, 3H) , 7· 81— 7· 83 (d, 1H) ,
8. 44-8. 45 (d, 1H) ,
10. 00 (s, 1H)
1. 14-1. 96 (m, 10H) , 1. 59 (s, 3H) , 2. 18 (s, 3H) , 3. 85-3. 87 (d, 1H) , 5, 82-5. 96 (d, 2H) , 5. 96 (s, 1H), 6. 69 (s, 1H) ,
6 252-253
7. 19-7. 36 (m, 3H) , 7. 81-7· 83 (d, 1H) , 8. 44-8· 45 (d, 1H) , 10. 03 (s, 1H)
2. 34 (s, 3H) , 4. 54-4. 56 (d, 2H) , 5. 83-5. 97 (d, 2H) ,
6. 28-6. 64
7
(m, 3H) , 7. 34-7. 36 (d, 2H) , 7· 81- 7· 83 (d, 1H) ,
8. 44-8. 45 (d, 1H) , 9. 89 (s, 1H)
(Ηΐ 's)28 "6 '(Ηΐ 'P)ff S-Z "8
'(Ηΐ 'Ρ)98 "Z-S8 'ί '(HS '^) 9S 'ί-ίΖ 'ί ' (Η2 '^) Ζ9 "9-09 "9
'ΟΕ 'Ρ)96 -9-£8 "9
'ΟΕ 'Ρ)8ΐ ·卜 9ΐ '(HS 's)08 Τ '(HS ^)\Ζ 'Ζ 06ΐ 8ΐ
(Ηΐ 's)09 "6 '(Ηΐ 'Ρ)9 8- S 8 '(Ηΐ 'Ρ)98 "Ζ
'(Η2 'ω) S " -εε "Ζ '(HI 'S)99 "9 '(HI ^)ίΖ "9 '(ΙΕ'Ρ)
96 -9-£8 "9
ΐ
'(Η2 's)g Τ '(HS 's)T6 τ '(HS 's)T2 '(H9'S)S9"T
(Ηΐ 's)T6 ·6 '(Ηΐ 'Ρ) 9f ·8- ·8
' (Η2 'Ρ) 8 Ί-ZS 'ί ' (Η2 9S 'L-7Z Ί ' (Ηΐ 's) U ·9
9ΐ '(Η2 'Ρ)96 -9-£8 "9 ' (HS ^) Ζ ' (HS 's)TT '(H9's)^l
(Ηΐ 's)66 ·6
'(HT 's)9 ·8 '(Ηΐ 'Ρ)98 'ί ' (Η9 '^) 9S "Ζ-06 "9
9ΐ ' (Η2 'ΡΡ) S6 '9 ' (Η8 ΐθ -6ΐ Τ ' (HS 's) ΐθ ·Ζ
(Ηΐ 's) '6 '(Ηΐ 'P)S ·8- ·8 '(Ηΐ 'Ρ) 8 ·Ζ
' (Η^ 'ω) S "Ζ-06 "9 '(Η2 'Ρ)96 "9-28 "9 ' (Η^ '^) 9 SO Έ
'(Η£ 's)80 'Ζ '(HS 'ω)02 "ΐ-Ζΐ "ΐ ' (HS '^) 86 Ό-96 Ό
(Ηΐ 's)98 "6 '(Ηΐ 'P)S ·8- ·8 '(Ηΐ 'Ρ)
S8 "Ζ-Ϊ8 "Ζ '(Ηΐ 'ω)9ε " -2S "Ζ '(Ηΐ 's)90 "Ζ ' (Η2 's)96 "9
'(Η2 'Ρ)½ "9-Ϊ8 "9 '(HS 's)ZO Τ ' (HS 's)9Z 7 ' (HS 's)^07
(Ηΐ 's)OT Όΐ ' (Ηΐ 'Ρ) ff S-£ "8 ' (Ηΐ 'Ρ) 28 "Ζ-08 Ί ' (Η2
'冚)
9S "Ζ ' (HS 'ω) 20 "Ζ-86 "9 ' (Η2 'Ρ)06 "9-ΖΖ "9 ' (Η2 '^) 2ΐ w, 'm) z τ-ιζ τ '(HS 's)go τ '(m <IU)T61-WT
(Ηΐ 's)^8 "6 '(Ηΐ 'Ρ) ·8- ·8 '(Ηΐ 'Ρ)98 " -S8 Ί
' (HS 'ω) 9£ "Ζ ' (Η2 99 "9-69 ·9 ' (Η2 'Ρ)
ΐΐ
96 -9-£8 "9
'(Η 'Ρ)8Ζ · - ΐ · '(HS 's)T2 '(Η£ '冚)££ ·ΐ- 62 ·ΐ
(Ηΐ 'Ρ)00 Όΐ-96 ·6 '(Ηΐ 'Ρ)ε ·8- '(Ηΐ 'Ρ)
8 "Ζ-08 "Ζ '(Η ' εΉ6·9 '(Η2 'P)f6 "9-ΐ8 "9 ' (Η^ '^)
98ΐ- 8ΐ Οΐ
'(HS 's)207 '(Η2 'ω)ΐ9 "ΐ- ε 'ΐ ' (HS '^) 96 Ό-Τ6 Ό
(Ηΐ 's)20 Όΐ '(Ηΐ 'Ρ)9 ·8- ·8 ' (Ηΐ 'Ρ) 8 ·Ζ
'(Η2 'ω) ε "Ζ '(Ηΐ 's)89 "9 ' (Ηΐ 's) £ "9 ' (Η2 'Ρ)
6
96 -9-£8 "9
'(HS 's)6T 7 '(H9 )6S ·ΐ-εε ·ΐ ' (Η£ ' ) 06 ·0-68 Ό
(Ηΐ 's)OT Όΐ '(Ηΐ 'Ρ)9 8—
'(Ηΐ ·8-0ΐ ·8 ' (Η^ 'ω) 89 "Ζ ' (Ηΐ 's)S6 "9
822- LZZ
'(Η2 'Ρ)Ζ6 "9-^8 "9 ' (HS 's)9T 7 '(H6's)92 "ΐ :9Ρ— OSMI
L\
OT8C.0/llOZN3/X3d co o Ζ OAV
81
Ol8C.0/llOZN3/X3d co o Ζ OAV
6. 73-6. 74 (d, IH), 7. 27-7. 86 (m, 5H), 8. 44-8. 45 (d, IH) ,
7. 77-7. 79 (d, IH)
214-215
DMS0-d6 : 0. 81-0. 85 (m, 3H) , 1. 40-1. 45 (m, 2H) , 2. 16
(s, 3H) , 3. 06-3. 09 (m, 2H) , 5. 85— 5. 98 (d, 2H) , 6. 93 (s, IH) ,
7. 44-7. 59 (m, 3H) , 8. 10-8. 46 (m, 3H) , 10. 14 (s, IH)
0. 95-2. 22 (m, 10H) , 2. 11 (s, 3H) , 3. 32-3· 75 (m, 2H) ,
4. 62-5. 02 (m, 2H) , 5. 82-5. 95 (d, 2H) , 6. 91-7. 35 (m, 4H) ,
7. 80-7. 82 (d, IH) , 8. 42 (s, IH) , 9. 88 (s, IH)
0. 64-2. 43 (m, 14H) , 2. 05 (s, 3H) , 3. 39-3. 75 (m, 2H) ,
135-136
4. 69-5. 03 (m, 2H) , 5. 81-5. 94 (d, 2H) , 6. 88-7. 35 (m, 4H) ,
7. 80-7. 82 (d, IH) , 8. 42 (s, IH) , 9. 69 (s, IH)
2. 23 (s, 3H) , 2. 62 (s, 3H) , 2. 89 (s, 3H) , 5. 85— 5. 99 (d, 2H) , 6. 92 (s, IH) , 7. 58-8. 49 (m, 5H) , 10. 46 (s, IH)
DMS0-d6: . 89-0. 95 (m, 3H) , 1. 11-1. 97 (m, 9H) , 2. 20 (s, 3H) ,
3. 60-3. 62 (m, IH) , 5. 83-5. 97 (d, 2H) , 6· 67- 6· 68 (d, IH) , 7. 21-7. 37 (m, 3H) , 7· 82- 7· 84 (d, IH) , 8· 45- 8· 46 (d, IH) , 10. 05 (s, IH)
DMS0-d6: . 71-0. 79 (m, 3H) , 0· 99- 1· 79 (m, 9H) , 2. 22 (s, 3H) ,
252-253
3. 26-3. 33 (m, IH) , 5. 85- 5. 98 (d, 2H) , 6. 95- 6. 97 (d, IH), 7. 54-8. 45 (m, 5H) , 10. 05 (s, IH)
DMS0-d6: 1. 08-1. 74 (m, 10H) , 2. 22 (s, 3H) , 3. 59-3. 61 (m, IH) , 5. 86-5. 99 (d, 2H) , 6. 99 (s, IH) , 7. 56-8. 46 (m, 5H) ,
10. 38 (s, IH)
DMS0-d6 : 0. 83-0. 86 (m, 3H) , 1. 25-1. 45 (m, 6H) , 2. 21 (s, 3H) , 3. 11-3. 16 (m, 2H) , 5. 86—5. 99 (d, 2H) , 6. 95 (s, IH) , 7. 55-8. 47 (m, 6H) , 10. 42 (s, IH)
DMS0-d6: 1. 27 (s, 9H) , 2. 21 (s, 3H) , 5. 85— 5. 98 (d, 2H) ,
233-237
6. 96 (s, IH) , 7. 55-8. 47 (m, 6H) , 10. 33 (s, IH)
DMS0-d6: 2. 22 (s, 3H) , 4. 34-4. 36 (d, 2H) , 5· 86- 5· 99 (d, 2H) ,
142-143
6. 26-6. 37 (d, 2H) , 6. 92 (s, IH) , 7. 53- 8. 85 (m, 7H),
10. 41 (s, IH)
DMS0-d6 : l. 41-1. 50 (m, 4H) , 2. 30 (s, 3H) , 2. 91-3. 47 (m, 6H) , 5. 85-5. 98 (d, 2H) , 6. 97 (s, IH) , 7. 57- 7. 82 (m, 3H), 8. 13-8. 15 (d, IH) , 8· 46- 8· 47 (d, IH)
181-182
0. 91-0. 96 (m, 3H) , 1. 37-1. 64 (m, 2H) , 2. 03 (s, 3H) ,
2. 92-3. 39 (m, 4H) , 5. 81-5. 94 (d, 2H) , 6. 93-7. 35
(m, 4H) , 7. 80-7. 82 (d, IH) , 8. 42- 8. 43 (d, IH)
234. 5-24 1. 14-1. 96 (m, 10H) , 2. 36 (s, 3H) , 3. 82-3. 87 (m, IH) , 0 5. 83-5. 96 (d, 2H) , 6. 67 (s, IH) , 7. 33-7. 40 (m, 3H) ,
7. 81-7. 83 (d, IH) , 8· 44- 8· 46 (d, IH) , 10. 04 (s, IH)
223-224 2. 17 (s, 3H) , 2. 93-2. 94 (d, 3H) , 5· 83- 5· 96 (d, 2H) ,
6. 16 (s, IH) ,
99 τ
S9
(H2 'ω) ζζ τ-οζ τ (m <s)so 'ζ (m <ι")99 "ΐ-ΐ9 "ΐ 902-^02
(Ηΐ 's)06 "6 '(Ηΐ cP)Z S-Zf "8 '(Ηΐ 'Ρ) 28 "Ζ-08 "Ζ
' (Η2 'ω) 9£ ·Ζ- "Ζ ' (Ηΐ 's) 0ΐ "Ζ ' (Ηΐ 's) 6 ·9 ' (Η2 'Ρ)
f6 "9-08 "9
29
(Η ' ) 8 ε-εο ·ε '(HS 's)go 7 '(Η9 6ΐ ·ΐ- ε6·ο 99Τ-29Ϊ
(Ηΐ 'Ρ) 90 ΌΙ-fO Όΐ ' (Ηΐ 9 S- ·8
' (Ηΐ 'ω) S8 "Ζ-Ϊ8 "Ζ ' (HS 'ω) 0 ' ' (Ηΐ 'Ρ) £L '9-2 ·9
' (Η2 'Ρ) 96 "9-28 "9 ' (Ηΐ 'Ρ) 09 Τ-89 Τ ' (HS 's) 8ΐ ·Ζ
ΐ9 (Ηΐ 'Ρ) 6 ·ΐ- 06 ·ΐ '(Η6 'm)SL 'l-6fl ' (HS '^) S6 Ό-98 Ό
(Ηΐ 's)ZO Όΐ
(Ηΐ 'P)S ·8- ·8
(Η2 'Ρ)£8 "Ζ-Ϊ8 "Ζ '(Ηΐ 's) ·9
(Ηΐ 's)8T ·9 '(Η2 'Ρ)96 "9-28 "9 {wz 'm)i£ τ-ζ£ τ
09
'OK 's)9T ·Ζ (Η9 <ι")99 ·ΐ— ΐε ·ΐ (HS ' ΐ6 Ό-88 Ό
(Ηΐ 's)00 Όΐ '(Ηΐ 'Ρ)9 ·8- ·8 (Ηΐ 'Ρ) 8 "Ζ-Ϊ8 ·ί
(HS 'ω) If ' -εε ' '(HI 's^q)80 "9 {W, 'Ρ)96 "9-28 "9 9 "Ζ
69 (HS '^)£Ζ ·ΐ- 6ΐ ·ΐ
(Ηΐ 's)ZO Όΐ '(Ηΐ 'Ρ)9^ S-ff "8 ' (Ηΐ 'Ρ) S8 "Ζ-ΐ8 "Ζ
' (HS 'ω) S "Ζ ' (Ηΐ 's) fL ·9 ' (Ηΐ 's) 96 "9 ' (Η2 'Ρ)
96 "9-28 "9 9
89
(Ηΐ 'ω)6ΐ 'f-fl 'f '(HS 's) T τ '(Η9'Ρ)0Ζ ·ΐ-6ΐ ·ΐ "9ΐ2-9ΐ2
(Ηΐ 's)8T Όΐ
(Ηΐ 'P)ff S-Z ·8
(Η2 'Ρ)28 "Ζ-08 "Ζ (HS 'ω)9ε - - 6 '9 '(Ηΐ 'sjq)TO ·9
Ζ9 {W, 'Ρ)96 "9-28 "9 '(H6'S)9S ·ΐ Z-9ZZ
(Ηΐ 'sjq)T2 Όΐ '(Ηΐ 'Ρ)8 ·8- '(Ηΐ 'Ρ)9ΐ '8-εΐ "8 '(HS 'ω)69 " -SS ' ' (Ηΐ 's)06 "9 9
99 (Η2 'Ρ)86 "9-^8 "9 ' (HS 's)88 'Ζ ' (HS 's)297 :9Ρ- OSMI '9 -ε
(Ηΐ 's)W ·0ΐ '(Ηΐ 'Ρ)9 8— S 8 '(Ηΐ :s)8T ·8
:(Ηΐ 'Ρ)Οΐ ·8- 60 ·8 ' (HS '^) 89 'L-£ "Ζ '(Ηΐ :s)26 ·9
' (Η2 'Ρ) 86 "9-98 "9 ' (Η2 9ΐ ·ε- Όΐ '£ :(Η£ 's)
ΐ 'Ζ (m <lu) W\-ZZ '\ ' (HS 'ω) 98 Ό-28 Ό : 99
'P-OSHQ 1 Ζ
(Ηΐ 'Ρ)8 ·8- 9 ·8
(Ηΐ 'ΡΡ)9ΐ ·8- Π ·8 ' (Η£ ' ) £8 ·Ζ ' (Ηΐ 'Ρ) 9ΐ ·8- Π ·8
' (HS 'ω) S8 "Ζ-Ζ9 "Ζ ' (Ηΐ 's) Ζ6 ·9 ' (Η2 'Ρ) 86 "9-98 "9 ζ
(Η^ 'ω)οε -ε-ζο τ '(HS'S)9 '(Η '冚) ·Ϊ- 9 ·Ϊ:9Ρ- OS I LZ- 'ΟΙΖ
(Ηΐ 'Ρ)9 ·8- ·8
'(Ηΐ 'Ρ)ΖΙ "8-0ΐ "8 ' (Η£ ' ) 06 ·Ζ— ·Ζ '(Ηΐ 's)Z6 "9
S9 (Η2 'Ρ)66 "9-98 "9 ' (HS 's)29 Τ ' (HS 's)6T 7 :9Ρ- OSMI
(Ηΐ 's) ·0ΐ ' (Ηΐ 'Ρ) Lf ·8- 9 ·8 ' (Ηΐ 'Ρ) fl '8~ΖΙ ·8
(HS 'ω) S8 "Ζ-99 'ί ' (Ηΐ 's) £6 ·9 ' (Η2 'Ρ) Ζ6 "9-£8 "9 ' (Η 9
Ζ
ίΖ ·ε— ΐ6 ·Ζ ' (HS 's) 02 ·Ζ ' (Η9 ) W) ·Η8 Ό : 9Ρ- OSMI '0ΖΖ-ί\Ζ
(Ηΐ 's)86 ·6 (Ηΐ 'Ρ)
8- ·8
(Ηΐ 'Ρ) 8 Ί-Ζ8 'ί (Ηε 'ω)6ε '(ΗΤ S)0Z ·9
Ol8C.0/llOZN3/X3d co o ζ OAV
Ol8C.0/llOZN3/X3d co o Ζ OAV
(Ηΐ 's) l ·6
' (Ηΐ ' ) '(Ηΐ 'ω)^8 Ί-ZS 'ί ' (Η2 '^) Ιζ Ί~η 'ί
'(Ηΐ 's)92 'I '(Ηΐ 's) 8 '9 ' (Ηΐ 'Ρ) 80 ·9_90 ·9
'(Η2 'Ρ)96 "9-28 "9 ' (Ηΐ '^)^8 '£~Ζ8 '£ ' (Η2 '^) 06 "ΐ-Ζ8 "ΐ
'(Η£ ' ) ^ΐ- 99 ·ΐ '(Η2 'ω) "ΐ-^ε "ΐ '(Η£ ' )8ΐ ·ΐ- 80 ·ΐ S6
(Ηΐ 's)g0 Όΐ '(Ηΐ 'Ρ)9 ·8- ·8 (Ηΐ 'Ρ)ΖΙ ·8- Οΐ ·8
'(Ηΐ 's)Z6 "Ζ '(Ηΐ 'ω)89 "Ζ-99 "Ζ (Ηΐ 'P)S -L-£ 'I
'(Ηΐ 'P)2S ' -θε "Ζ '(HI 'S)96 "9 (Η2 'Ρ)66 "9-98 "9
'(Ηΐ ' )09·ε '(HS 's)027 (Η^ '^) l "ΐ-99 "ΐ
(Ηΐ 'ω)89 "ΐ-99 "ΐ '(Η9 "ΐ-ΖΟ "ΐ :9Ρ- OSMI 26
(Ηΐ 's)0T Όΐ '(Ηΐ 'Ρ)9 ·8- ·8
'(Ηΐ 'Ρ)ΖΙ "8-ΐΐ "8 '(Ηΐ 's)T0 '8 ' (Ηΐ 's)g '
(Η2 'ω) 9 " -½ "Ζ '(HT 's) 96 "9 ' (Η2 'Ρ) 86 "9-98 "9
'(Ηΐ 'ω)09 Τ-89 '£ '(HS 's)gT '(Η ) εΖ'ΐ-99 'ΐ
'(Ηΐ 'ω)89 "ΐ-99 "ΐ '(Η9 LZ "ΐ-ΖΟ "ΐ :9Ρ- OSMI ΐ6
(Ηΐ 's)Tg ΐ '(Η2 'ω)ΐ9 ·8- ^ "8
'(Ηΐ 'Ρ)06 "Ζ-88 "Ζ ' (Η£ ' ) 6£ ·Ζ '(Ηΐ 'ω)ΐΐ 'Z-80 "Ζ
'(Ηΐ 's)99 "9 '(Ηΐ 'P)fl -9-ΖΙ "9 '(Η2 'Ρ) 86 "9-98 "9
(Ηΐ ' )ΐΟ ·卜 66 Έ ' (Η2 'ω) ΖΟ 7-90 'Ζ ' (Η2 '^) 28 "ΐ-6Ζ "ΐ
'(Η2 'ω)2 "ΐ-Ζ9 "ΐ '(1Ε ·τ— '(Η2 ·ΐ- 02 ·ΐ 06
(Ηΐ 's)06 "6 '(Ηΐ 'Ρ) ·8
-£f ·8
'(ΗΪ 'ρ)^8 -L-ZS · ' ΟΕ '冚) 9ε ·ζ-οε ·Ζ '(Ηΐ 's)\Z 'I
(HT 's) 86 ·9 ' (Ηΐ 's)ZO "9 '(Η2 'P)f6 "9-ΐ8 "9 '(H6'S)SS "ΐ 68
(Ηΐ 's)0^ Όΐ
(Ηΐ 'P)S ·8- ·8 '(Ηΐ 'P)S8 "Ζ-Ϊ8 "Ζ '(Ηΐ
(Ηΐ 'Ρ) 02 "Ζ-8ΐ ·ί ' (Ηΐ 'Ρ) ΖΟ "Ζ-90 ·ί ' (Ηΐ 's) 26 ·9
'(Ηΐ 's)96 "9
'(Η2 'Ρ)96 "9-28 "9 ' (HS 's)9T '(H6'S)9S "ΐ 88
(Ηΐ 's)T2 ΐ '(Η2 'Ρ)ΐ9 "8-6^ "8
(Ηΐ 'Ρ)06 "Ζ-88 "Ζ ' (Η£ ' ) 9£ ·Ζ '(Ηΐ 'ω) 60 "Ζ-90 "Ζ
' (Ηΐ 's)g9 ·9
Ζ8
' (Ηΐ 's)60 "9 '(Η2 'Ρ)86 "9-98 "9 '(H6's)09 "ΐ
(Ηΐ „ ·8- ' (IE '冚) 8£ ·8—
1 (Η2 'ω) 8ΐ "8-80 "8 ' (Ηΐ '^)09 " -½ ' ' (Ηΐ 's)gO "Ζ
98 (Η2 'Ρ)00 "9-^8 "9 '(HS 's)9g '(H6'S)9S "ΐ :9Ρ- OSMI 29Τ-09Ϊ
(Ηΐ 's)9T Όΐ
'(Ηΐ 'Ρ)^ S-£ "8 '(Ηΐ 'P)S8 "Ζ-Ϊ8 "Ζ '(Ηΐ 's)T9
(Ηΐ 's)l 'I '(Ηΐ 'ω)9ε ' ' (Ηΐ 's)26 "9 ' (Ηΐ 's)86 "9
98 '(Η2 'Ρ)96 "9-28 "9 ' (HS 's)gT '(H6'S)9S "ΐ
(Ηΐ 's)T ·0ΐ '(Ηΐ 'Ρ) Lf ·8- 9 ·8
' (Ηΐ 'ω) Π ·8- Οΐ ·8 ' (Ηΐ 's) 8Ζ ' ' (Η2 89 "Ζ-ΐ9 '
'(Ηΐ 's)g6 ·9
(Ηΐ 's) 0 ·9 ' (Η2 'Ρ) 86 "9-98 "9 ' (Η2 Π - 80 '
^8
(Η2 'P)f6 '£-Z6 '£ '(HS 's)ZZ 'Ζ '(HS '^)92 "ΐ-9ΐ "ΐ :9Ρ- OSMI
Ol8C.0/llOZN3/X3d co o Ζ OAV
'(Ηΐ 's)L ' '(HS 's)U "Z '(Ηΐ 's)½ "9 '(HT 's)8 ·9
W, 'P)TS "9-92 "9 W, 'P)S8 "9-Ϊ8 "9 '(Η2 'P)Z9 ^-29 f£Z-Z£Z LOT
(Ηΐ 's)66 "6 '(Ηΐ 'Ρ)ε ·8- '(Ηΐ 'Ρ)28 "Ζ-08 "Ζ
'(Ηΐ '^)η 'ί ' (Η2 'ω) 90 "Ζ-86 "9 ' (Η2 'Ρ) S6 "9-08 "9
'0κ' )8ε·ε ' (Η2 'ω) go -ε-ε6 τ ' κ ΐ9 Ί- Ί
'(Ηΐ 8ΐ ·ΐ- ΐ ·ΐ ' (HS 'ω) 96 Ό-26 Ό '(Ηΐ 'ω)0 Ό-99 Ό SLI-LLI 90ΐ
(Ηΐ 's)69 "6 '(Ηΐ 'Ρ) ·8- ·8 '(Ηΐ 'P)S8 "Ζ-ΐ8 "Ζ '(Η2
<lu)gg "Ζ '(Ηΐ 's)S8 "9 '(Ηΐ 's)62 "9 ' (Η2 'P)f6 "9-ΐ8 "9
'(Η2 'ω)ΐε τ-9ζ τ 'ΟΕ'^ Ή ·ΐ ' (Hd) ε6 ·Ο-06 ·ο ΐΖΐ-ΟΖΐ 90ΐ
(Ηΐ 's)gg Όΐ '(Ηΐ 'Ρ)9 ·8- ·8
'ΟΕ 'Ρ)ΐΐ ·8— Οΐ ·8 '(Ηΐ 's)08 "Ζ '(Ηΐ '^)89 " -½ '
'(Ηΐ 's)^ · '(Ηΐ 's)20 "Ζ '(Η2 'Ρ) 86 "9-98 "9
'(Ηΐ 'ω)26 Τ '(Η9'Ρ)Μ)·ΐ-¾)·ΐ :9Ρ- OSMI 002-Ζ6Ϊ
(Ηΐ 's)99 '6 '(Ηΐ„·8- ·8 '(Ηΐ 'Ρ)
^8 Ί-ZS 'ί W, 'm)L£ " -9ε "Ζ ' (Ηΐ 's)08 "9 ' (Ηΐ 's)92 "9
'(Η2 'Ρ)96 "9-28 "9 ' (Η2 '^) ~η '£ ' (HS '^)9ΐ Ί-ΖΙ Ί Ζ8Ϊ-98Ϊ εοτ
{WZ 'Ρ)8 ·8- 9 ·8
' (Ηΐ 'Ρ) Ζ\ ·8- Οΐ ·8 ' (Ηΐ 's) 8Ζ "Ζ ' (Ηΐ 89 "Ζ-99 "Ζ
'(Ηΐ 's)9^ Ί '(Ηΐ 's)00 Ί '(Η2 'Ρ)66 "9-98 "9 ' (Ηΐ '^) ΐθ '
002-66Ϊ ΐθΐ '(Ηΐ '^)29 Ό-Ϊ9 Ό ' (Η2 '^) 99 Ό ' (Η2 '^) Ό :9Ρ- OSMI
(Ηΐ 's)02 Όΐ '(Ηΐ 'Ρ)6 ·8- 8 ·8 ' (Ηΐ 'Ρ) 9ΐ ·8- Π ·8
'(Ηΐ 's)6Z "Ζ '(Ηΐ 'ω)09 "Ζ-Ζ9 "Ζ ' (Ηΐ 's)9^ Ί
'(Ηΐ 's)Z6 "9 '(Η2 'Ρ)86 "9-98 "9 ' (HS 's)68 'Ζ ' (HS 's)Z97 ΟΟΐ
(Ηΐ 's)89 "6 '(Ηΐ 'Ρ)8 8- '(Ηΐ 'Ρ)\Ζ "8-0ΐ "8
'(Ηΐ 's)T "Ζ '(Ηΐ 'ω)29 "Ζ-99 "Ζ ' (Ηΐ 's)9^ 'ί ' (Ηΐ 's) 06 "9
'(Η2 'Ρ)86 "9-^8 "9 ' (HS 's)887 ' (HS 's)T9 :9Ρ- OSMI 66
(Ηΐ 's)02 ·0ΐ
'(Ηΐ 'Ρ)6 ·8- 8 ·8 '(Ηΐ 'Ρ)9ΐ ·8- ΐ ·8 '(Ηΐ
9 "Ζ-Ζ9 "Ζ '(Η2 's)8S "Ζ ' (Η2 '^)Ζ£ 'ί ' (Ηΐ 's)06 "9
'(Η2 'Ρ)ΐΟ "9-98 "9 ' (HS 's)087 ' (HS 's)Z9 'Ζ :9Ρ- OSMI 99Τ-99Ϊ 86
(Ηΐ 's)9S Όΐ '(Ηΐ 'P ·8- 9 ·8
' (Ηΐ 's) 82 ·8 ' (Ηΐ 'Ρ) Ζ\ ·8- Οΐ ·8 ' (Ηΐ 's) 6Ζ '
'(Ηΐ 'ω) 9 "Ζ-99 "Ζ '(Ηΐ 's)09 "Ζ ' (Ηΐ 's)86 "9
'(Η2 'Ρ)86 "9-98 "9 ' (HS 'Ρ) Ζ97-997 :9Ρ- OSMI Ζ6
(Ηΐ 's)OT Όΐ '(Ηΐ 'Ρ)6 8— 9 8
' (Η2 'Ρ) Π ·8- ΐ ΐ ·8 ' (Ηΐ 89 "Ζ-99 "Ζ ' (Ηΐ Lf Ί-ff Ί
'(Ηΐ 'ω)9ε -L-Z "Ζ '(Ηΐ 's)96 "9 ' (Η2 'Ρ)66 "9-98 "9
'(HS 'Ρ)697-897 '(HS 's)6T :9Ρ- OSMI 96
(Ηΐ 's)90 Όΐ '(Ηΐ 'Ρ)8 8— '(Ηΐ 's)8T "8
'(Ηΐ 'Ρ)Π ·8- Οΐ ·8 '(Ηΐ 's) l Ί ' (Ηΐ 's)T9 '
'(Ηΐ 'ω)89 " -½ "Ζ '(Ηΐ 's)S6 "9 ' (Η2 'Ρ)66 "9-98 "9
'(HS 'Ρ)Ζ97-99 '(H£'s)n,2 :9Ρ- OSMI 96
(Ηΐ 's)T \ '(Ηΐ 's)^8 "8
'(Ηΐ 'Ρ)29 '8-ΐ9 "8 '(Η2 ίΖ "8-6ΐ "8 ' (Ηΐ 'Ρ) S8 "Ζ-ΐ8 "Ζ
'(Ηΐ 'ω)99 "Ζ-Ϊ9 'ί '(Ηΐ 'ω)09 "Ζ ' (Ηΐ '^)ΖΖ 'LSI 'ί
'(Ηΐ 's)2 '9 '(Η2 'Ρ)ΐΟ "9-88 "9 ' (HS 'Ρ) 987-^87 :9Ρ- OSMI f6
OT8C.0/llOZN3/X3d co o Ζ OAV
7. 82-7. 84 (d, IH) , 8· 44- 8· 45 (d, IH) , 9. 35 (s, IH)
108 186-187 1. 28-1. 32 (t, 3H) , 4· 09— 4· 10 (d, 2H) , 4. 21-4. 26 (m, 2H) ,
5. 81-5. 96 (d, 2H) , 6. 69 (s, IH) , 6. 87 (s, IH) , 7. 26 (s, IH) , 7. 41-7. 47 (m, 2H) , 7. 82-7. 84 (m, IH) , 8· 41- 8· 42 (d, IH) , 9. 41 (s, IH)
109 122-123 1. 38 (s, 6H) , 2. 10 (s, 3H) , 2. 93 (s, 2H) , 5· 80- 5· 94 (d, 2H),
6. 20 (s, IH) , 6. 84 (s, IH) , 7. 26— 7. 34 (m, 3H) ,
7. 82-7. 84 (d, IH) , 8· 43- 8· 44 (d, IH) , 9. 68 (s, IH)
DMS0-d6: 0. 45-0. 48 (m, IH) , 0. 58-0. 65 (m, 3H) , 2. 21 (s, 3H) ,
110 148-149
2. 70-2. 71 (m, IH) , 5. 86— 5. 99 (d, 2H) , 6. 97 (s, IH) ,
7. 55-7. 58 (m, IH) , 7. 72 (s, IH) , 7. 84 (s, IH) ,
8. 10-8. 13 (d, IH) , 8. 46-8. 47 (m, 2H)
190-192 DMS0-d6: 1. 17 (d, 3H) , 2. 15 (s, 3H) , 3. 24-3. 27 (d, 3H) ,
111
3. 88-3. 94 (m, IH) , 5· 85— 5· 98 (d, 2H) , 6. 95 (s, IH) ,
7. 54-7. 57 (m, 2H) , 7. 73 (s, IH) , 7· 99— 8· 01 (d, IH),
8. 09-8. 11 (d, IH) , 8. 44-8· 46 (d, IH) , 10. 11 (s, IH)
153-155 1. 17-1. 21 (t, 3H) , 2. 15 (s, 3H) , 3. 38-3. 43 (m, 2H) ,
112
5. 82-5. 96
(d, 2H) , 6. 20 (s, IH) , 6. 70 (s, IH) , 7. 26— 7. 36 (m, IH) , 7. 58-7. 59 (d, 2H) , 7. 81-7· 83 (d, IH) , 8. 44-8· 46 (d, IH)
230-231 0. 93-0. 97 (t, 3H) , 1. 54-1. 59 (m, 2H) , 2. 15 (s, 3H) ,
113
3. 30-3. 35 (m, 2H) , 5. 82-5. 96 (d, 2H) , 6. 15 (s, IH) ,
6. 72 (s, IH) , 7. 26-7. 36 (m, IH) , 7· 51— 7· 59 (d, 2H) ,
7. 81-7. 83 (d, IH) , 8· 44- 8· 46 (d, IH) , 10. 07 (s, IH)
DMS0-d6: 2. 18 (s, 3H) , 2. 50 (s, 3H) , 2. 87-2. 90 (s, 3H) ,
114 230-231
5. 85-5. 98 (d, 2H) , 6. 91 (s, IH) , 7. 15— 7. 17 (d, IH) , 7. 45-
7. 47 (d, IH) , 7. 56-7. 59 (m, IH) , 8· 13- 8· 15 (d, IH) ,
8. 48-8. 49 (d, IH) , 10. 05 (s, IH)
DMS0-d6: 2. 09 (s, 3H) , 3. 85 (brs, IH) , 3. 97-4. 01 (m, 2H) ,
116
4. 59-4. 61 (m, 2H) , 5. 88-6. 01 (d, 2H) , 6. 48 (s, IH) ,
7. 23-7. 25
(m, IH) , 7. 57 (s, IH) , 7· 90- 7· 92 (d, IH) , 8. 12-8. 13 (m, IH) ,
8. 22 (s, IH)
4. 36-4. 37 (d, 2H), 5. 85-5· 98 (d, 2H), 6. 62 (s, IH), 6. 99 (brs,
117
IH), 7. 09 (m, 4H), 7. 91-7· 93 (d, IH), 8. 47-8· 53 (m, 2H)
201-203 2. 95-2. 96 (d, 3H), 5. 82-5· 95 (d, 2H), 6. 34 (brs, IH), 7. 19-7·
118
37 (m, 3H), 7. 86 (s, IH), 8. 44-8· 45 (d, IH)
142-146 1. 17-1. 20 (t, 3H) , 3. 36-3. 43 (m, 2H) , 5. 81-5. 94 (d, 2H) ,
119
6. 36 (brs, IH), 6. 66 (s, IH), 7. 15-7· 36 (m, 3H), 7. 83-7· 84 (d, 1H) , 8. 43-8. 44 (d, IH)
132-135 0. 95-0. 99 (t, 3H) , 1. 56-1. 60 (m, 2H) , 3. 33 (m, 2H) ,
120
5. 83-5. 96 (d, 2H), 6. 25 (brs, IH), 6. 63 (s, IH) ,
7. 13-7. 46 (m, 3H) , 7. 84-7· 86 (d, IH) , 8. 45-8· 46 (d, IH)
162-165 1. 22-1. 24 (d, 6H), 4. 16—4· 21 (m, IH), 5. 82—5· 95 (d, 2H) ,
121
6. 00-6. 02 (d, IH) , 6. 64 (s, IH) , 7. 13-7. 47 (m, 2H) ,
7. 84-7. 86 (d, IH) , 8. 45-8. 46 (d, IH) ,
186-189 0. 94-0. 96 (t, 3H) , 1. 35-1. 80 (m, 4H) , 3. 37-3. 42 (m, 2H) ,
122
5. 83-5. 96 (d, 2H), 6. 22 (brs, IH), 6. 63 (s, IH), 7. 21-7· 38 (m,
3H) , 7. 85-7. 86 (d, IH) , 8. 46-8· 47 (d, IH)
239-242 1. 42 (s, 9H), 5. 83-5. 96 (d, 2H), 5. 91 (s, IH), 6. 67 (s, IH), 7. 1
123
6-7. 38 (m, 3H) , 7. 84-7· 86 (d, IH) , 8. 47-8· 48 (d, IH)
150-154 0. 58-0. 62 (m, 2H), 0. 86-0. 91 (m, 2H), 5. 82-5. 96 (d, 2H), 6. 54
124
(s, IH), 6. 64 (s, IH), 7. 19-7· 47 (m, 3H), 7. 84-7· 86 (d, IH), 8. 46-8. 47 (d, IH) , 9. 96 (s, IH)
218-220 2. 83 (s, 3H), 3. 07 (s, 3H), 5. 83-5. 96 (d, 2H), 6. 90-7. 38 (m, 4H
125
) , 7. 84-7. 86 (d, IH) , 8. 46-8. 47 (d, IH) , 9. 90 (s, IH)
170-173 0. 90-0. 96 (dd, 3H) , 1. 11-1. 79 (m, 9H) , 5. 82-5. 96 (d, 2H) , 6. 6
127
0 (s, IH), 7. 21-7. 37 (m, 3H), 7. 83-7. 85 (d, IH), 8. 46-8. 47 (d,
IH)
223-227 1. 15-1. 97 (m, 10H), 3. 86-3. 88 (m, IH), 5. 82-5. 95 (d, 2H), 6. 0
128
3-6. 05 (d, IH), 6. 64 (s, IH), 7. 18—7. 37 (m, 3H), 7. 83—7. 85 (d, IH) , 8. 45-8. 46 (d, IH) , 10. 00 (s, IH)
152-155 4. 21-4. 22 (d, 2H) , 5. 81-5· 94 (d, 2H) , 6. 62 (s, IH) , 7. 27-7· 38
129
(m, 3H) , 7. 86-7. 88 (d, IH) , 8. 43-8· 44 (d, IH) ,
128-131 2. 2. 86 (s, 3H), 5. 83-5. 96 (d, 2H), 6. 44 (s, IH), 6. 88 (s, IH), 7
130
• 28-7. 88 (m, 4H) , 8. 45-8· 46 (d, IH)
175-178 1. 04-1. 07 (t, 3H), 3. 29-3. 36 (m, 2H), 5. 82-5. 95 (d, 2H), 6. 40
131
(s, IH) , 6. 96-7. 85 (m, 5H), 8. 42—8· 43 (d, IH), 9. 73 (s, IH)
179-181 0. 84-0. 85 (m, 3H), 3. 23-3. 28 (m, 2H), 5. 79-5· 93 (d, 2H), 6. 45
132
(brs, IH), 7. 27-7. 85 (m, 4H), 8. 42-8· 43 (d, IH), 9. 74 (s, IH)
215-219 1. 06-1. 07 (d, 6H), 4. 05-4. 10 (m, IH), 5. 82-5. 95 (d, 2H), 6. 25
133
-6. 27 (d, IH) , 6. 98 (s, IH) , 7. 34-7. 85 (m, 5H) , 8. 42-8· 43 (d, 1
H)
207-210 0. 85-0. 88 (m, 3H) , 1. 19-1. 43 (m, 4H) , 3. 26-3. 31 (m, 2H) , 5. 82
134
-5. 92
(d, 2H) , 6. 40 (brs, IH) , 6. 94 (s, IH) , 7. 33-7· 86 (m, 4H) , 8. 41- 8. 43 (d, IH)
124-126 1. 26 (s, 9H), 5. 78-5. 92 (d, 2H), 6. 25-6. 27 (d, IH), 7. 13-7. 60
135
(m, 4H) , 7. 81-7. 82 (d, IH) , 8. 40-8· 41 (d, IH) ,
0. 94-0. 97 (t, 3H) , 1. 54-1. 56 (m, 2H) , 2. 21 (s, 3H) ,
136
3. 32-3. 37
(m, 2H) , 5. 83-5. 96 (d, 2H) , 6. 14-6. 15 (m, IH) , 6. 64 (s, IH) , 7. 12-7. 16 (m, IH) , 7. 23-7· 29 (m, IH) , 7. 32-7· 35 (m, 2H) , 7. 81-7. 83 (d, IH) , 8. 44-8. 46 (d, IH) ,
2. 12 (s, 3H) , 2. 81 (s, 3H) , 3. 07 (s, 3H) , 5. 82— 5. 95 (d, 2H) ,
137
6. 74 (s, IH) , 7. 02-7. 04 (m, IH) , 7. 12-7. 13 (m, 2H) ,
7. 32-7. 35 (m, IH) , 7· 81- 7· 83 (d, IH) , 8· 44- 8· 46 (d, IH) , 9. 34 (s, IH)
3. 30-3. 71 (m, 8H), 5. 71-5· 84 (d, 2H), 6. 89-6· 94 (m, 3H), 7. 37
142 216-218
-7. 40 (m, IH) , 7. 85-7. 87 (d, IH) , 8. 46-8. 47 (dlH)
1. 37-1. 60 (m, 6H), 3. 19-3. 65 (m, 4H), 5. 78-5. 91 (d, 2H), 6. 85
143 208-210
-7. 37 (m, 4H), 7. 83-7· 85 (d, IH), 8. 44-8· 45 (d, IH)
1. 31-1. 32 (m, 3H), 4. 14—4. 16 (d, 2H), 4. 17—4. 28 (m, 4H), 5. 83
145 158-161
-5. 96
(d, 2H), 6. 59 (s, IH), 6. 74 (brs, IH), 7. 13-7· 34 (m, 3H), 7. 85- 7. 87 (d, 1H) , 8. 44-8. 45 (d, IH)
193-195 4. 56-4. 57 (d, 2H), 5. 83-5. 96 (d, 2H), 6. 30-6. 59 (m, 4H) , 7. 21
146
-7. 38 (m, 3H) , 7. 83-7. 85 (d, IH) , 8. 44-8. 45 (d, IH) , 9. 36 (s, 1
H)
169-173 0. 93-1. 66 (m, 8H), 3. 07——3. 51 (m, 4H), 5. 80—5. 93 (d, 2H), 6. 8
147
5-7. 36 (m, 4H) , 7. 82-7· 84 (d, IH) , 8. 44-8· 45 (d, IH)
130-134 0. 91-1. 66 (m, 10H), 2. 98-3. 50 (m, 2H), 5. 80-5. 94 (d, 2H), 6. 8
148
7-7. 36 (m, 4H) , 7. 82-7· 84 (d, IH) , 8. 44-8· 45 (d, IH)
195-196 0. 97-1. 00 (m, 3H) , 1. 42-1. 66 (m, 6H) , 3. 43-3. 48 (m, 2H) , 5. 90
149
-6. 03
(d, 2H), 6. 24 (brs, IH), 6. 69 (s, IH), 7. 28-7. 43 (m, 3H), 7. 91- 7. 93 (d, IH) , 8. 52-8. 54 (d, IH) , 10. 05 (brs, IH)
251-255 1. 00-1. 80 (m, 10H), 5. 79-5· 96 (d, 2H), 6. 86 (s, IH), 7. 35-7. 8
154
7 (m, 4H) , 8. 42-8. 43 (d, IH)
196-199 4. 45-4. 16 (d, 2H), 5. 82-5. 95 (d, 2H), 6. 72 (s, IH), 7. 19-7. 92
155
(m, 5H) , 8. 42-8. 43 (d, 2H) , 8. 94 (s, IH)
2. 98-3. 00 (s, 3H), 5. 87-6. 00 (d, 2H), 6. 37 (s, IH), 6. 67 (s, IH
161
) , 7. 23- 7. 40 (m, 4H) , 7. 88-7· 90 (d, IH) , 8. 49- 8. 50 (d, IH) , 10. 19 (s, IH)
1. 47 (s, 9H) , 5. 88-6. 01 (d, 2H) , 6. 01 (s, IH) , 6. 68 (s, IH) ,
163
7. 20-7. 38 (m, 4H), 7. 88-7· 89 (d, IH), 8. 51-8· 52 (d, IH) , 10. 07 (s, IH)
3. 32 (s, 3H), 3. 49 (s, 3H), 5. 84—5. 97 (d, 2H), 6. 74 (s, IH), 7. 3
180
1-7. 46
( m, 3H) , 7. 86-7. 88 (d, IH) , 8. 48-8. 49 (d, IH) 8. 95 (brs, IH)
129-132 2. 96-2. 97 (s, 3H), 5. 86-6· 00 (d, 2H), 6. 20 (brs, IH), 7. 02 (s,
182
IH) , 7. 23-7. 39 (m, 3H) , 7. 86-7· 88 (d, IH) , 8. 48-8· 49 (d, IH)
130-135 1. 19-1. 23 (t, 3H) , 3. 44-3· 49 (m, 2H) , 5. 90-6· 03 (d, 2H) , 6. 21
183
(brs,
IH), 7. 00 (s, IH), 7. 29-7. 43 (m, 3H), 7. 90-7· 92 (d, IH), 8. 52- 8. 53 (d, IH) , 9. 73 (brs, IH)
218-220 0. 90-0. 94 (m, 3H), 1. 49—1· 51 (m, 2H), 3. 30—3· 35 (m, 2H), 5. 83
184
-5. 96
(d, 2H), 6. 16 (brs, IH), 6. 99 (s, IH), 7. 19-7· 35 (m, 3H), 7. 82- 7. 84 (d, IH) , 8. 43-8. 45 (d, IH) , 9. 82 (brs, IH)
123-126 1. 13-1. 15 (d, 6H) , 4. 15—4. 21 (m, IH) , 5. 82—5. 95 (d, 2H) , 6. 94
185
-6. 96
( d, IH) , 7. 20-7. 36 (m, 3H) , 7. 83-7· 85 (d, IH) , 8. 43-8· 44 (d,
IH)
110-113 0. 86-0. 92 (m, 3H), 1. 27-1. 47 (m, 4H), 3. 34-3. 39 (m, 2H), 5. 83
186
-5. 96
(d, 2H), 6. 18 (brs, IH), 6. 98 (s, IH), 7. 20-7. 36 (m, 3H), 7. 83- 7. 85 (d, 1H) , 8. 44-8. 45 (d, IH)
239-243 1. 42 (s, 9H), 5. 80-5. 94 (d, 2H), 5. 97 (brs, IH), 7. 04-7· 33 (m,
187
4H) , 7. 80-7. 82 (d, IH) , 8· 41— 8· 42 (d, IH)
137-139 0. 54-0. 84 (m, 4H) , 2. 81-2· 83 (m, IH) , 5. 82-5· 96 (d, 2H) , 6. 31
188
(s, IH), 6. 99-7. 36 (m, 3H), 7. 83-7· 85 (d, IH), 8. 45-8· 47 (d, 1 H) , 9. 68 (brs, IH)
220-221 0. 86-0. 90 (m, 3H), 1. 30-1. 50 (m, 6H), 3. 32-3. 37 (m, 2H), 5. 82
189
-5. 96
(d, 2H), 6. 18 (brs, IH), 6. 94-7· 35 (m, 4H), 7. 82-7· 84 (d, IH) , 8. 44- 8. 45 (d, 1H) , 9. 72 (brs, IH)
0. 82-0. 84 (m, 3H) , 0. 91—1· 61 ( (m, 9H) , 3. 54—4· 20 (m, IH) , 5. 8
190
2-5. 95 (d, 2H), 7. 02-7. 34 (m, 4H), 7. 80-7. 82 (d, IH), 8. 42-8. 43 (d, IH) , 10. 00 (brs, IH)
226-228 1. 07-1. 86 (m, 10H), 3. 83-3. 85 (m, IH), 5. 82-5. 96 (d, 2H), 7. 0
200
3-7. 34 (m, 4H) , 7. 81-7· 83 (d, IH) , 8. 42-8· 43 (d, IH)
166-169 0. 92-1. 28 (m, 8H), 2. 85-3. 52 (m, 4H), 5. 82-5. 95 (d, 2H), 6. 99
201
-7. 33 (m, 4H) , 7. 81-7· 82 (d, IH) , 8. 42-8· 43 (d, IH)
109-113 0. 91-1. 63 (m, 10H) , 2. 86-3· 73 (m, 4H) , 5. 81-5· 94 (d, 2H) , 7. 0
202
1-7. 33 (m, 4H) , 7. 81-7· 83 (d, IH) , 8. 41-8· 42 (d, IH)
121-124 2. 71 (s, 3H), 3. 09 (s, 3H), 5. 82-5. 95 (d, 2H), 6. 98-7. 35 (m, 4H
203
) , 7. 83-7. 84 (d, IH) , 8. 44-8· 45 (d, IH)
219-221 1. 13-1. 72 (m, 6H), 2. 96-3. 05 (m, 2H), 3. 68-3. 70 (m, 2H), 5. 80
204
-5. 93
(d, 2H) , 6. 98-7. 32 (m, 4H) , 7. 80-7· 82 (d, IH) , 8. 41-8· 42 (d, 1
H)
171-174 0. 92-1. 28 (m, 8H), 2. 79-3. 39 (m, 4H), 5. 80-5. 93 (d, 2H), 6. 92
205
-7. 27 (m, 4H) , 7. 81-7· 82 (d, IH) , 8. 43-8· 44 (d, IH)
225-227 1. 10-1. 90 (m, 10H), 3. 83-3. 85 (m, IH), 5. 81-5. 95 (d, 2H), 6. 9
206
0-7. 36 (m, 4H) , 7. 82-7· 84 (d, IH) , 8. 44-8· 45 (d, IH)
223-225 0. 82-0. 84 (m, 3H), 0. 96-1. 54 ( (m, 9H), 3. 55-4. 15 (m, IH), 5. 8
207
1-5. 94 (d, 2H) , 6. 98-7. 34 (m, 4H) , 7. 81—7. 82 (d, IH) , 8. 43—8.
44 (d, IH)
214-216 0. 87-0. 90 (m, 3H), 1. 30-1· 51 (m, 6H), 3. 29-3· 34 (m, 2H) , 5. 81
208
-5. 95
(d, 2H), 6. 25 (brs, IH), 6. 91-7· 34 (m, 4H), 7. 82—7· 84 (d, IH) , 8. 44- 8. 45 (d, IH)
228-229 0. 48-0. 82 (m, 4H), 2. 75-2. 76 (m, IH), 5. 82-5. 95 (d, 2H), 6. 41
209
(s, IH), 6. 94-7. 36 (m, 3H), 7. 83-7· 85 (d, IH), 8. 45-8· 47 (d, 1 H) , 10. 01 (brs, IH)
215-219 1. 33 (s, 9H), 5. 82-5. 95 (d, 2H), 6. 04 (brs, IH), 7. 03-7· 35 (m,
210
4H) , 7. 82-7. 84 (d, IH) , 8. 44-8· 45 (d, IH)
180-183 0. 90-0. 94 (m, 3H), 1. 27-1. 48 (m, 4H), 3. 30-3. 35 (m, 2H), 5. 82
211
-5. 95
(d, 2H), 6. 27 (brs, IH), 6. 91 (s, IH), 7. 18—7· 32 (m, 3H), 7. 83- 7. 85 (d, 1H) , 8. 45-8. 46 (d, IH)
211-214 1. 13-1. 15 (d, 6H) , 4. 15—4. 21 (m, IH) , 5. 82—5. 95 (d, 2H) , 6. 94
212
-6. 96
( d, 1H) , 7. 20-7. 36 (m, 3H) , 7. 83-7· 85 (d, 1H) , 8. 43-8· 44 (d,
1H)
129-132 1. 14-1. 16 (d, 6H) , 4. 13-4. 18 (m, 1H) , 5. 82-5. 95 (d, 2H) , 6. 04
213
(brs,
1H), 6. 97 (s, 1H), 7. 14-7. 36 (m, 3H), 7. 83-7. 85 (d, 1H), 8. 45- 8. 46 (d, 1H)
168-170 2. 67 (s, 3H), 5. 86-5. 99 (d, 2H), 6. 99 (s, 1H), 7. 43-7. 56 (m, 3H
214
) , 8. 10-8. 12 (d, 1H) , 8. 46—8· 47 (d, 1H)
190-193 1. 19-1. 23 (t, 3H) , 3. 42-3. 48 (m, 2H) , 5. 89-6. 02 (d, 2H) , 6. 27
215
(brs,
1H), 6. 96 (s, 1H), 7. 26-7. 39 (m, 3H), 7. 90-7. 92 (d, 1H), 8. 52- 8. 53 (d, 1H) , 10. 13 (brs, 1H)
211-214 1. 13-1. 15 (d, 6H) , 4. 15—4. 21 (m, 1H) , 5. 82—5. 95 (d, 2H) , 6. 94
216
-6. 96
( d, 1H) , 7. 20-7. 36 (m, 3H) , 7. 83-7· 85 (d, 1H) , 8. 43-8· 44 (d,
1H)
218-220 0. 90-0. 94 (m, 3H), 1. 49—1· 51 (m, 2H), 3. 30—3· 35 (m, 2H), 5. 83
217
-5. 96
(d, 2H), 6. 16 (brs, 1H), 6. 99 (s, 1H), 7. 19-7· 35 (m, 3H), 7. 82- 7. 84 (d, 1H) , 8. 43-8. 45 (d, 1H) , 9. 82 (brs, 1H) 索引表 1和索引表 2中列举的本发明所述的结构式(I)表示的化合物均可根据本专利 说明书中所描述的合成方法及合成实施例 1-3所述的制备方法进行合成。
本发明还提供结构式 ( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物或 其农业上适用的盐的用途、 一种防治有害生物的农药制剂和一种防治有害生物的方法。
含有本发明所述结构式 ( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物 或其农业上适用的盐的有害生物防治剂可用于农作物上的害虫防治, 例如作为在农业园艺领 域中成为问题的各种有害生物的防治剂、 即农业园艺有害生物防治剂, 或寄生于动物的有害 生物防治剂、 即动物寄生生物防治剂特别有用。
作为农业园艺用有害生物防治剂, 例如作为杀虫、 杀螨剂, 是有用的, 具体地说, 对于 防治二斑叶螨、 红叶螨、 柑桔全爪螨、 苹果全爪螨等植物寄生性螨类; 小菜蛾、 甘蓝夜蛾、 斜纹夜蛾、 棉铃虫、 烟夜蛾、 烟毒蛾、 稻纵卷叶野螟、 稻褐带卷蛾、 苹果小卷蛾、 桃小实心 蛾、 梨小食心虫、 小地老虎、 马铃薯叶甲、 黄守瓜、 蚜虫类、 粉虱类、 蓟马类、 蝗虫类、 斑 潜蝇类等农业害虫类。 其中, 含有本发明化合物的农业园艺用有害生物防治剂, 对于农业害 虫类等特别有效。 另外, 含有本发明所述结构式 ( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨 基苯甲酰胺类化合物或其农业上适用的盐的农业园艺用有害生物防治剂, 对于防治对有机磷 剂、 氨基甲酸酯剂、 合成除虫菊酯剂等的药剂的各种抗性害虫有效。 进而, 本发明所述结构 式 ( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物或其农业上适用的盐因为
具有优异的渗透转移性, 所以通过使用含有本发明所述结构式 (I)表示的化合物的农业园艺 用有害生物防治剂来处理土壤, 在防治土壤有害昆虫类、 螨类等类的同时也可防治茎叶部的 害虫。
作为含有本发明所述结构式 ( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化 合物或其农业上适用的盐的有害生物防治剂的其他的优选形态, 可列举出综合防治上述植物 寄生性螨类、 农业害虫类、 土壤害虫类等的农业园艺用有害生物防治剂。
含有本发明所述结构式 ( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物 或其农业上适用的盐的农业园艺用有害生物防治剂, 通常混合该化合物与各种农药上的辅助 剂即载体, 形成粉剂、 颗粒剂、 颗粒可湿性粉剂、 可湿性粉剂、 水性悬浮剂、 油性悬浮剂、 水溶剂、 乳剂、 糊剂、 气雾剂、 微量散布剂等的各种形态的制剂来使用, 但只要适合本发明 的目的, 则可以制成通常在该领域中使用的所有制剂形态。 作为制剂中使用的辅助剂, 可以 列举出硅藻土、 消石灰、 碳酸钙、 滑石、 白炭墨、 高岭土、 膨润土、 高岭石和绢云母的混合 物、 粘土、 碳酸钠、 小苏打、 芒硝、 沸石、 淀粉等的固体载体; 水、 甲苯、 二甲苯、 溶剂石 脑油、 二噁烷、 丙酮、 异佛尔酮、 甲基异丁基酮、 氯苯、 环己烷、 二甲亚砜、 N,N-二甲基甲 酰胺、 二甲基乙酰胺、 N-甲基 -2-吡咯烷酮、 醇等的溶剂; 脂肪酸盐、 苯甲酸盐、 烷基磺基琥 珀酸盐、 二烷基磺基琥珀酸盐、 聚羧酸盐、 烷基硫酸酯盐、 烷基硫酸盐、 烷基芳基硫酸盐、 烷基二甘醇醚硫酸盐、 醇硫酸酯盐、 烷基磺酸盐、 烷基芳基磺酸盐、 芳基磺酸盐、 木质磺酸 盐、 烷基二苯基醚二磺酸盐、 聚苯乙烯磺酸盐、 烷基磷酸酯盐、 烷基芳基磷酸盐、 芳基磷酸 盐、 苯乙烯基芳基磷酸盐、 聚氧乙烯烷基醚磷酸盐、 聚氧乙烯烷基芳基磷酸酯盐、 萘磺酸甲 醛縮合物的盐这样的阴离子类的表面活性剂或展开剂; 脱水山梨糖醇脂肪酸酯、 甘油脂肪酸 酯、 脂肪酸聚甘油酯、 脂肪酸醇聚二醇醚、 乙块二醇、 块属醇、 氧化烯嵌段聚合物、 聚氧乙 烯烷基醚、 聚氧乙烯烷基芳基醚、 聚氧乙烯苯乙烯基芳基醚、 聚氧乙烯二醇烷基醚、 聚乙二 醇、 聚氧乙烯脂肪酸酯、 聚氧乙烯脱水山梨糖醇脂肪酸酯、 聚氧乙烯甘油脂肪酸酯、 聚氧乙 烯硬化蓖麻油、 聚氧丙烯脂肪酸酯这样的非离子类的表面活性剂、 展开剂; 橄榄油、 瓜哇木 棉油、 蓖麻油、 棕榈油、 山茶油、 椰子油、 芝麻油、 玉米油、 米糠油、 落花生油、 棉籽油、 大豆油、 菜籽油、 亚麻子油、 桐油、 液体石蜡等的植物油或矿物油等。 这些辅助剂的各种成 分, 只要不脱离本发明的目的, 可以适当选择 1种或 2种以上来使用。 另外, 也可以在上述 的辅助剂之外从该领域公知的物质中适当选择使用。 也可以使用例如增量剂、 增稠剂、 防尘 降剂、 防冻剂、 分散稳定剂、 药害减轻剂、 防霉剂等通常使用的各种辅助剂。
本发明所述结构式 ( I ) 表示化合物和各种辅助剂即载体的重量配合比例, 通常为 0. 1: 99. 9〜90: 10。 在实际使用这些制剂时, 可以直接使用, 或用稀释剂稀释至规定浓度后, 根
据需要添加各种展开剂 (表面活性剂、 植物油、 矿物油等) 使用。
含有本发明所述结构式 ( I ) 表示化合物的农业园艺用有害生物防治剂的施用, 根据气 象条件、 制剂形态、 施用时期、 施用场所、 病害虫的种类或发生状况等的不同而不同, 不能 一概而定, 但是一般以 0. 05〜800ppm, 优选为 0. 5〜500ppm的有效成分浓度来进行施用, 其 每单位的施用量是每 1公顷本发明化合物为 l〜5000g, 优选为 10〜1000g。 另外, 作为含有 本发明的有害生物防治剂的其他的优选形态的农业园艺用有害生物防治剂的施用, 可根据上 述有害生物防治剂的施用进行。 本发明也包含利用这样的施用方法的有害生物的防治方法, 特别是植物寄生性螨类、 农业害虫类的防治方法。
含有本发明所述结构式 ( I ) 表示化合物的农业园艺用有害生物防治剂的各种制剂、 或 其稀释物的施用, 通常可以利用一般进行的施用方法例如散布、 喷射、 喷雾 (misting)、 雾 化 (atomizing)、 撒粒、 水面施用法、 土壤施用 (混入、 灌注等)、 表面施用 (涂布、 粉衣、 被覆等)、 浸渍毒饵等来进行。 另外, 将上述有效成分混合至伺料中给予家畜, 也可以阻碍在 其排泄物中的害虫、 特别是有害昆虫的发生及生育, 另外可以利用所谓的超高浓度少量散步 法 (ultra low volume ) 施用, 在该方法中, 可以含有 100%的活性成分。
另外, 含有本发明所述结构式 ( I ) 表示化合物可与现有杀虫、 杀菌或除草的农药品种 的一种或二种或两种以上进行组合进行使用, 显示更有益的效果、 作用性。 特别是混用或合 用了本发明所述结构式 ( I ) 表示化合物和其他的农药有效成分化合物的 1种或 2种以上的 混合有害生物防治组合物, 可以将适用范围、 药剂处理的时期、 防治活性等向好的方向改良。 另外, 本发明化合物和其他的农药的有效成分化合物, 可以将分别制成的制剂在散布时混合 使用, 也可以将两者一起制成制剂使用, 本发明也包含这样的混合有害生物防治组合物。
本发明的所述结构式 ( I ) 表示化合物及其制剂, 具有以下优点:
( 1 ) 本发明首次在吡唑环引入一氟甲氧基, 化合物结构具有新颖性;
( 2 )本发明的化合物及其制剂具有广谱的杀虫活性: 对鳞翅目害虫(小菜蛾、 草地贪夜 蛾和棉铃虫)、 半翅目害虫 (桃蚜和苜蓿蚜)、 同翅目害虫 (水稻褐飞虱)、 双翅目害虫 (三叶 斑潜蝇) 以及叶甲类害虫 (辣根猿叶甲) 都表现出非常良好的活性;
( 3 ) 本发明的化合物及其制剂具有超高的杀虫活性: 在 0. 16mg/L剂量下对小菜蛾、 草 地贪夜蛾和棉铃虫都表现出很好的效果; 在 4mg/L剂量下对蚜虫、 三叶斑潜蝇和辣根猿叶甲 也都表现出很好的效果; 在 20mg/L剂量下对水稻褐飞虱表现出很好的效果;
( 4)本发明的化合物及其制剂具有很好的安全性, 对部分作物如小麦、 大豆、 棉花、 水 稻等安全性好, 并且对环境具有性;
( 5 )本发明的化合物及其制剂具有合理的毒性、 生态毒性和环境相容性, 属低毒环境友
好型农药。 下面记载本发明的实施例, 但是本发明不限定于此, 首先记载本发明化合物的合成例。 具体实施方式
实施例 1 N-[2- (叔丁基氨基甲酰基) -4-氯 -6-甲基-苯基] -1- (3-氯 -2-吡啶基 )-3-— 氟甲氧基 -1H-吡唑 -5-甲酰胺的合成 (化合物 8)
第一步: 3-—氟甲氧基 -1-(3_氯 -2-吡啶基) -1H-吡唑 -5-甲酸的合成
在 500ml 三口圆底烧瓶中, 依次加入 1-(3-氯 -2-吡啶基 )-3-羟基 -1H-吡唑 -5-甲酸乙酯 (13.35g, 0.05mol), 300ml 乙腈, 固体碳酸钾 (8.28g, 0.06mol), 一氟一溴甲烷 (8.48g, 0.075mol), 然后加热回流, 反应至原料完全消失, 冷却至室温, 过滤, 滤饼用乙腈 2*50ml 淋洗, 滤液浓縮后加 200ml 甲醇使之溶解, 然后在室温慢慢向其中滴加含氢氧化钠 (2.4g, 0.06mol)的 50ml水溶液, 室温搅拌约 30min后, 反应完全, 蒸除溶剂, 加水, 用乙醚 2*50ml 萃取, 水相加稀盐酸调 PH值显酸性, 体系有大量的白色固体生成, 过滤, 红外灯干燥, 得 3-—氟甲氧基 -1- (3-氯 -2-吡啶基) -1H-吡唑 -5-甲酸 11.05g,熔点 149-151°C,收率为 81.6%; ¾NMR (400MHz, DMSO— d6) δ5.84—5· 98 (d, 2H) , 6.74 (s, 1H) , 7.62—7· 65 (m, 1H) , 8. 19—8· 21 (d, 1H) , 8.53-8.54 (d, 1H), 13.65 (br, 1H); M(%) : 270(100)。
第二步: 6-氯 -2-[3_—氟甲氧基 -l-(3-氯 -2-B比啶基) - 1H-5-吡唑基 ]-8-甲基 -4H-[d] [1, 3]苯并噁嗪 -4-酮的合成
在 250ml 三口圆底烧瓶中, 3-—氟甲氧 -1-(3_氯 -2-吡啶基) -1H-吡唑 -5-甲酸 (3g, O. Ollmol) (实施例 1-合成第一步产物) , 2-氨基- 5-氯- 3-甲基苯甲酸 (2.04g, O. Ollmol), 乙腈 150ml,吡啶 7ml,搅拌使固体全部溶解,将体系冷却,在 _5°〜- 10°滴加甲基磺酰氯(2ml, 2.96g, 0.026mol) /乙腈 20ml溶液, 滴加完毕后, 保持在 _5°〜- 10°反应 lh, 然后自然升至 室温反应 3h,反应完全后,加水 30ml,搅拌 30min,过滤,滤饼先后用 2: 1乙腈 /水 (2*40ml)、 乙腈 (2*20ml) 及乙醚 (2*20ml) 淋洗, 得第一批固体; 滤液蒸除溶剂后, 加水, 用乙酸乙 酯 (3*40ml) 萃取, 有机相用无水硫酸钠干燥、 过滤、 浓縮后, 用乙醇进行重结晶, 得第二 批固体, 两批固体合并, 得 6-氯 -2-[3_—氟甲氧基 -1-(3_氯 -2-吡啶基) - 1H-5-吡唑基 ]-8- 甲基- 4H- [d] [1, 3]苯并噁嗪 -4-酮 4. 17g,熔点 206.0-207.5°C,收率为 84.22%; ¾NMR ( 400ΜΗζ , CDC13) ^1.81(s,3H),5.83-5.97(d,2H), 6.85(s,lH), 7.46-7.49(m,2H), 7.84-7.99(m,2H), 8.54-8.56(d, 1H); M(%) : 421.0(100)。
第三步: 2- (3-氯 -2-吡啶基 )-5-—氟甲氧基 -2H-吡唑- (2-叔丁氨基甲酰基 -4-氯 -6-甲基
-苯基) 3-甲酰胺的合成
在 50ml 单口圆底烧瓶中, 向 6-氯 -2- [3-—氟甲氧基 -1- (3-氯 -2-吡啶基) - 1H_5_吡唑 基] -8-甲基 _4H- [d] [1, 3]苯并噁嗪 -4-酮 (lg, 0. 00238mol ) (实施例 1_合成第二步产物) 的 四氢呋喃溶液中慢慢滴加叔丁胺 (0. 21g, 0. 00286mol ), 室温搅拌过夜后, 反应完全, 减 压蒸除四氢呋喃, 残余物中加少许乙醇, 搅拌数分钟后, 过滤, 得白色固体 1. 02 g, 即 为 2- (3-氯 -2-吡啶基 ) -5-—氟甲氧基 -2H-吡唑- (2-叔丁氨基甲酰基 -4-氯 -6-甲基-苯基) 3_ 甲 酰胺 , 熔 点 227.0-228.0°C , 收率 为 87· 6% ; ¾NMR ( 400MHz , DMS0_d6 ) ^ 1.26(s,9H),2.16(s,3H),5.84-5.98(d,2H), 6.93(s,lH), 7.26-7.58(m,4H), 8.10-8.13(m,lH), 8.45-8.46(d, 1H),10.10(S,1H); M (%): 492. 5 (100)。
实施例 2 N- [2- (甲氨基甲酰基) -4-氯- 6-甲基-苯基] -1- (3-氯- 2-吡啶基 ) -3-—氟甲 氧基 -1H-吡唑 -5-甲酰胺的合成 (化合物 3) 在 50ml 单口圆底烧瓶中, 向 6-氯 -2- [3-—氟甲氧基 -1- (3-氯 -2-吡啶基) - 1H_5_吡唑 基] -8-甲基 _4H- [d] [1, 3]苯并噁嗪 -4-酮 (lg, 0. 00238mol ) (实施例 1_合成第二步产物) 的 四氢呋喃溶液中加入甲胺盐酸盐(0. 19g, 0. 00286mol )及无水醋酸钠(0. 24g, 0. 00286mol ), 室温搅拌过夜后, 反应完全, 减压蒸除四氢呋喃, 残余物中加水, 用乙酸乙酯萃取, 有机 相用无水硫酸钠干燥, 过滤, 浓縮得粗品, 用乙醇进行重结晶得白色固体 0. 87 g, 即为 N- [2- (甲氨基甲酰基) -4-氯 -6-甲基-苯基] -1- (3_氯 -2-吡啶基 ) -3-—氟甲氧基 -1H-吡唑 -5- 甲酰胺,熔点 236.0-237.4°C,收率为 82. 7%; ¾NMR ( 400MHz , DMSO- d6) δ 2.18(s,3H), 2.93-2.94(d, 2H ), 5.83-5.96(d, 2H),6.16-6.17(d,lH), 6.70(s,lH), 7.20-7.36(m,4H), 7.82-7.84(d,lH), 8.44-8.45(d. 1H),9.96(S,1H); M (%): 450. 0 (100)。
实施例 3 N- [2- (乙氨基甲酰基) -4-氰基 -6-甲基-苯基] -1- (3-氯 -2-吡啶基 ) -3-—氟 甲氧基 -1H-吡唑 -5-甲酰胺的合成 (化合物 33) 第一步: 6-氰基 -2- [3_—氟甲氧基 -1- (3_氯 -2-吡啶基) - 1H-5-吡唑基 ] -8-甲基 -4H- [d] [1, 3]苯并噁嗪 -4-酮的合成
在 250ml三口圆底烧瓶中, 3-—氟甲氧基 -1- (3-氯 -2-吡啶基) -1H-吡唑 _5_甲酸 (5g, 0. 0185mol ) (实施例 1_合成第一步产物), 2-氨基 _5_氰基 _3_甲基苯甲酸(3. 24g, 0. 0185mol ), 乙腈 150ml,吡啶 15ml,搅拌使固体全部溶解,将体系冷却,在 _5°〜- 10°滴加甲基磺酰氯(4ml,
5.98g, 0.052mol) /乙腈 20ml溶液, 滴加完毕后, 保持在 _5°〜- 10°反应 lh, 然后自然升至 室温反应 3h,反应完全后,加水 30ml,搅拌 30min,过滤,滤饼先后用 2: 1乙腈 /水 (2*40ml)、 乙腈 (2*30ml) 及乙醚 (2*30ml) 淋洗, 得第一批固体; 滤液蒸除溶剂后, 加水, 用乙酸乙 酯 (3*40ml) 萃取, 有机相用无水硫酸钠干燥、 过滤、 浓縮后, 用乙醇进行重结晶, 得第二 批固体, 两批固体合并, 得淡黄色固体 4.17g, 即为 6-氰基 -2-[3_—氟甲氧基 -1-(3_氯 -2- 吡啶基) - 1H-5-吡唑基 ]-8-甲基 _4H-[d] [1,3]苯并噁嗪 -4-酮, 熔点 208.5-211.0°C, 收率为 54.87%; ¾NMR (400MHz, CDC13) δ 1.86(s,3H), 5.84-5.97(d,2H), 6.90(s,lH), 7.27-7.98(m,3H), 8.32(s,lH), 8.55-8.56(d,lH); M (%): 411.1(100)。
第二步: N-[2- (乙氨基甲酰基) -4-氰基 -6-甲基-苯基] -1- (3-氯 -2-吡啶基 )-3-—氟甲 氧基 -1H-吡唑 -5-甲酰胺的合成
在 50ml单口圆底烧瓶中, 向 6-氰基 -2- [3-—氟甲氧基 -1- (3-氯 _2_吡啶基) - 1H_5_吡唑 基] -8-甲基 _4H-[d] [1,3]苯并噁嗪 -4-酮 (0.4g, 0.00097mol) (实施例 3_合成第一步产物) 的四氢呋喃溶液中慢慢滴加乙胺 (65-70%, 0.083g, 0.0012mol), 室温搅拌过夜后, 反应完 全,减压蒸除四氢呋喃,残余物中加少许乙醇,搅拌数分钟后,过滤,得浅灰色固体 0.35g, 即为 N-[2- (乙氨基甲酰基) -4-氰基 -6-甲基-苯基] -1-(3_氯 -2-吡啶基 )-3-—氟甲氧基 -1H- 吡唑 -5-甲酰胺, 熔点 151.0-152.5°C, 收率为 78.6%; ¾NMR ( 400MHz , DMSO- d6 ) ^i.23-1.27(m,3H),2.26(s,3H),3.45-3.48(m,2H),
5.84-5.98(d,2H),6.23(s,lH),6.64(s,lH),7.36-8.48(m,5H),10.52(s,lH); M (%): 457.1(100)。 以下实施例 4至实施例 6给出以本发明的化合物 ( I ) 作为活性物质组份, 加工配制几 种杀虫剂剂型的实际例子, 需要指出的是本发明并不仅仅局限在下述实例的范围内。 在这些 配方例子中, 所有的 "%"均指重量百分比。 实施例 4可湿性粉剂配方
将 15%的化合物(8) (索引表 1)、3%的木质素磺酸钠盐 (MQ)、2%的月桂醇聚氧乙烯醚 (JFC)、 40%的硅藻土和 40%的轻质碳酸钙充分地混合, 经超细粉碎机粉碎, 即得到 15%可湿性粉剂产
Π
ΡΠ。
实施例 5乳油配方
将 10%的化合物 (8) (索引表 1)、 5%的农乳 500号 (钙盐)、 5%的农乳 602号、 5%的 N- 甲基 -2-吡咯烷酮和 75%的二甲苯加热搅拌均匀, 即得 10%的乳油产品。
实施例 6水分散粒剂配方
将 60%的化合物(8 ) (索引表 1 )、 2%的聚乙烯吡咯烷酮、 12%的萘磺酸钠甲醛縮合物、 8% 的 N-甲基 -N-油酰基 -牛磺酸钠、 2%的羧甲基纤维素、 和 16%的高岭土均匀地混合, 粉碎, 再 加水捏合后, 加入 10— 100目筛网的造粒机中进行造粒, 然后再经干燥、 筛分 (筛网范围)。 即得 60%的水分散粒剂产品。 以下实施例 7至实施例 14给出下面给出使用本发明的化合物进行生物活性测定的实例, 需要指出的是本发明并不仅仅局限在下述实例的范围内。
杀虫活性评价试验根据下列方法进行:
待测化合物用丙酮 /甲醇 (1 : 1 ) 的混合溶剂溶解后, 用含有 0. 1%吐温 80的蒸馏水稀释 至所需的浓度。
试验靶标为小菜蛾 Plutella xylostella , 草地贪夜蛾 Spodoptem f rug i per da), 棉铃虫 iHeliothis armigera )、 苜精虫牙 ( Aphis medicaginis ^ 桃虫牙 {Myzus persicae ^ 水稻褐飞虱(
ta lugens)、三叶斑潜蝇( r /o )和辣根猿叶甲( Phaedon cochleariae 。
以下 "mg/L" 均指每毫克活性物 /升。
试验统计: 统计各个处理的死虫数和活虫数, 计算死亡率。
死亡率 (%) =试虫数 活虫数 χ Κ)0
试虫数 注: 生物活性测定实施例所述的编号与索引表 1和索引表 2所述的化合物编号相对应。 实施例 7对于小菜蛾 Plutella xylostella) 的效果试验
将甘蓝片剪下, 打孔成圆片, 然后浸于药液中 20s, 放于 09cm塑料培养皿内 (5片 /皿), 接小菜蛾 2龄幼虫 15头 /皿, 放一张滤纸, 加盖。 置于 26°C室内培养, 72h后检查结果。 试 验重复 4次。 以尖头镊子轻触虫体, 无反应视为死虫。根据上述死亡率计算公式计算死亡率。 异常虫也看作死亡。 索引表 1中化合物 1-27、 33-63、 85-86、 89、 93、 99-106、 110- 112在 4mg/L时得到了 100 %的死亡率。 另外化合物 1、 3、 4、 8、 33、 49-51、 57-58、 59、 83、 89、 101、 103、 106在 0. 16mg/L时得到了至少 80 %的死亡率。 实施例 8对于草地贪夜蛾 i Spodoptera frugiperda) 的效果试验
将盆栽棉花 (2-3张叶龄) 进行药剂喷雾处理, 晾干。 放入培养室继续正常培养。 在 5、 12、 19d后用剪刀将叶片剪下, 放于 09cm塑料培养皿内, 接草地贪夜蛾幼虫, 放一张滤纸, 加盖。 置于观察室内, 6d后检查结果。 根据上述死亡率计算公式计算死亡率。 异常虫也看作 死亡。 索引表 1中化合物 3、 8、 10、 33、 49、 50、 51在 4mg/L时得到了 100 %的死亡率。 化 合物 3、 8、 33、 51在 0. 8mg/L时得到了至少 90 %的死亡率。 化合物 8、 33、 51在 0. 16mg/L 时得到了至少 80 %的死亡率。 实施例 9对于棉铃虫 Heliothis armigera ) 的效果试验
将盆栽棉花 (2-3张叶龄) 进行药剂喷雾处理, 晾干。 放入培养室继续正常培养。 在 5、 12、 19d 后用剪刀将叶片剪下, 放于 09cm塑料培养皿内, 接棉铃虫幼虫, 放一张滤纸, 加 盖。 置于观察室内, 6d后检查结果。 根据上述死亡率计算公式计算死亡率。 异常虫也看作死 亡。 索引表 1中化合物 3 、 8、 10、 33、 36、 49、 51在 20mg/L时得到了 100 %的死亡率。 化 合物 8、 10、 33、 51在 4mg/L时得到了 100 %的死亡率。
化合物 8在 0. 16mg/L时得到了 100 %的死亡率。 实施例 10对于苜蓿蚜 phis medicaginis) 的效果试验
将蚕豆叶片剪去两端, 背面朝上放在小块棉花上, 置于培养皿内, 加少量水, 接苜蓿蚜 成蚜以产若蚜。 24h后去除成蚜, 继续培养 2d后将叶片在药液中浸润 5s后置于棉花上, 凉 干。 24h 后检查结果。 根据上述死亡率计算公式计算死亡率。 异常虫也看作死亡。 索引表 1 中化合物 3、 8、 10、 33-37、 39、 27、 47、 51、 83、 85、 89、 95、 101在 20mg/L时得到了至 少 90 %的死亡率。 化合物 3、 8、 33、 34、 35、 36、 51、 83、 101在 4mg/L时得到了至少 80 %的死亡率。 实施例 11 对于桃蚜 MyzUs persicae) 的效果试验
将盆栽甘蓝 (3-4张叶龄) 进行药剂喷雾处理, 晾干。 放入培养室继续正常培养。 在 5、 12、 19d 后用分别接入桃蚜若虫。 置于观察室内, 7-10d 后检查结果。 根据上述死亡率计算 公式计算死亡率。 异常虫也看作死亡。 索引表 1中化合物 3、 8、 10、 33、 51在 20mg/L时得 到了 100 %的死亡率。 化合物 3、 8、 33、 51在 4mg/L时得到了至少 90 %的死亡率。 实施例 12对于水稻褐飞虱 Nilaparvata lugens) 的效果试验
将水稻苗用白石英沙固定于培养皿内, 接用 C02麻醉 3龄中期若虫, 置于 POTTER喷雾塔
下喷雾。 喷雾后用透明塑料杯罩住, 标记后放于观察室内。 72h后检查结果。 试验重复 4次。 以尖头镊子轻触虫体, 无反应视为死虫。 根据上述死亡率计算公式计算死亡率。 异常虫也看 作死亡。 索引表 1中化合物 8、 33、 36、 51-53、 57、 58在 100mg/L时得到了至少 90 %的死 亡率。 化合物 8、 33、 51在 20mg/L时得到了至少 80 %的死亡率。 实施例 13 对于三叶斑潜蝇 iriomyza TrifoliD 的效果试验
将盆栽蚕豆 (3-4张叶龄) 进行药剂喷雾处理, 晾干。 放入培养室继续正常培养。 在 5、 12、 19d后用分别接入三叶斑潜蝇幼虫。 置于观察室内, 7d后检查结果。 根据上述死亡率计算 公式计算死亡率。 异常虫也看作死亡。 索引表 1中化合物 8、 9、 10、 33、 36、 49、 51在 20mg/L 时得到了 100 %的死亡率。 化合物 8、 10、 49、 51在 4mg/L时得到了 100%的死亡率。 实施例 14 对于辣根猿叶甲 Phaedon cochleariae) 的效果试验
将盆栽甘蓝 (3-4张叶龄) 进行药剂喷雾处理, 晾干。 放入培养室继续正常培养。 在 5、 12、 19d 后用剪刀将叶片剪下, 放于 09cm塑料培养皿内, 接辣根猿叶甲或小菜蛾 2龄幼虫, 放一张滤纸, 加盖。 置于观察室内, 6_7d后检查结果。 根据上述死亡率计算公式计算死亡率。 异常虫也看作死亡。 索引表 1中化合物 3、 8、 10、 33、 36、 49、 51在 20mg/L时得到了 100 %的死亡率。化合物 3、 8、 33、 51在 4mg/L时得到了至少 90%的死亡率。化合物 8在 0. 8mg/L 时得到了至少 90%的死亡率。 按照以上方法, 选取化合物 8和已知化合物氯虫酰胺进行了杀小菜蛾、 苜蓿蚜和水稻褐 飞 果见表 3。
氯虫酰胺 化合物 8和已知化合物氯虫酰胺的活性平行比较(死亡率, %) 浓度 浓度
化合物编号 小菜蛾 浓度 (mg/L) 苜蓿蚜 水稻褐飞虱
(mg/L) (mg/L)
0.8 100 20 100 100 100
8 (实施例 1 ) 0.16 100 4 90 20 80
0.032 80 0.8 30 4 0 氯虫酰胺 0.8 100 20 70 100 80
0.16 80 4 0 20 10
0.032 20 0.8 0 4 0 结论: 分别在 0. 16mg/L剂量下对鳞翅目害虫小菜蛾, 在 4mg/L剂量下对半翅目害虫苜蓿 蚜, 在 20mg/L剂量下对同翅目害虫水稻褐飞虱; 本发明化合物 8 (实施例 1 ) 的杀虫活性明 显优于已知化合物氯虫酰胺。
Claims
1、 一种结构式 (I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物或其农业 上适用的盐,
其巾:
A为 N或 CH;
R1为氢、 卤素、 氰基、 硝基、 烷基、 卤代烷基、 氰基烷基、 链烯基、 卤代链烯基、 块基、 卤代块基、 烷氧基、 氰基烷氧基、 ^代烷氧基、 烷硫基、 ^代烷硫基、 烷基磺酰基、 烷基羰 基、 ^代烷基羰基、 烷氧基羰基、 ^代烷氧基羰基或氨基烷基;
R2为氢、 卤素、 烷基、 卤代烷基、 烷氧基、 卤代烷氧基或氰基;
R3为氢、 烷基或烷氧基;
R4为氢、 氰基、 烷基、 烷氧基、 烷氨基、 ^代烷基、 ^代烷氧基、 ^代烷氨基、 氰基烷 基、 环烷基、 ^代环烷基、 羟烷基、 烷氧羰基甲基、 烷氧基酰胺基、 烷基酰胺基、 ^代烷基 酰胺基、 氰基取代的烷基酰胺基或芳杂环甲基;
或者 R3和 R4与所连接的 N—起形成三元、 四元、 五元或六元环;
m为 0〜4的整数;
n为 0〜4的整数。
2、按照权利要求 1所述的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物, 其特征在 于所述结构式 (I ) 中:
R1为氢、 卤素、 氰基、 硝基、 d-Ce浣基、 d-Ce卤代烷基、 氰基 d-Ce烷基、 C2_C6链烯基、
C2_C6卤代链烯基、 C2-C6块基、 卤代 C3-C6块基、 ( -(:6烷氧基、 d-C6氰基烷氧基、 d-C6卤代烷 氧基、 d-Ce烷硫基、 d-Ce卤代烷硫基、 d-Ce烷基磺酰基、 CfCe烷基羰基、 卤代 CfCe烷基羰 基、 ( Ce烷氧基羰基、 ^代 d-C6烷氧基羰基或氨基 d-C6烷基;
R2为氢、 卤素、 d-Ce烷基、 卤代 d-Ce烷基、 d-Ce烷氧基、 卤代 CfCe烷氧基或氰基;
R3为氢或 烷基;
R4为氢、 氰基、 d-Ce烷基、 d-Ce烷氧基、 d-Ce烷氨基、 卤代 CfCe烷基、 卤代 CfCe烷氧 基、 卤代 d-c6烷氨基、 氰基 d-c6烷基、 c3-c6环烷基、 卤代 c3-c6环烷基、 羟 -(:6烷基、 d-c6 烷氧羰基甲基、 (;-(:6烷氧基酰胺基、 d-c6烷基酰胺基、 ^代 d-c6烷基酰胺基、 氰基取代的 d-c6烷基酰胺基或芳杂环甲基;
m为 2;
n为 1。
3、按照权利要求 2所述的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物, 其特征在 于所述结构式 (I ) 中:
A为 N;
R1为氢、 卤素、 氰基、 d-C6烷基、 d-C6卤代烷基或 d-C6烷基羰基;
R2为卤素、 氰基、 CfCe烷基或卤代 d-C3烷基。
4、按照权利要求 3所述的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物, 其特征在 于所述结构式 (I ) 中:
R1为氢、 卤素、 氰基、 甲基或三氟甲基;
R2为卤素或氰基。
5、按照权利要求 4所述的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物, 其特征在 于所述的 I ) 化合物为:
其巾:
A为 N或 CH;
R1为氢、 卤素、 氰基、 硝基、 烷基、 卤代烷基、 氰基烷基、 链烯基、 卤代链烯基、 块基、 卤代块基、 烷氧基、 氰基烷氧基、 ^代烷氧基、 烷硫基、 ^代烷硫基、 烷基磺酰基、 烷基羰 基、 ^代烷基羰基、 烷氧基羰基、 ^代烷氧基羰基或氨基烷基;
R2为氢、 卤素、 烷基、 卤代烷基、 烷氧基、 卤代烷氧基或氰基;
m为 0〜4的整数;
n为 0〜4的整数。
7、按照权利要求 6所述的制备按照权利要求 1至 5之一所述的含一氟甲氧基吡唑的邻甲 酰氨基苯甲酰胺类化合物的中间体, 其特征在于结构式 (Π ) 中:
A为 N;
R1为氢、 卤素、 氰基、 硝基、 -Ce烷基、 d-C6卤代烷基、 氰基 d-C6烷基、 (:2-(:6链烯基、 C2_C6卤代链烯基、 C2-C6块基、 卤代 C3-C6块基、 d-C6烷氧基、 d-C6氰基烷氧基、 d-C6卤代烷 氧基、 (;-(:6烷硫基、 d-C6卤代烷硫基、 d-C6烷基磺酰基、 d-C6烷基羰基、 卤代 d-C6烷基羰 基、 d-C6烷氧基羰基、 ^代 d-C6烷氧基羰基或氨基 d-C6烷基;
R2为氢、 卤素、 CfCe烷基、 卤代 d-C6烷基、 ( -(:6烷氧基、 卤代 ( -(:6烷氧基或氰基; m为 2;
n为 1。
8、按照权利要求 7所述的制备按照权利要求 1至 5之一所述的含一氟甲氧基吡唑的邻甲 酰氨基苯甲酰胺类化合物的中间体, 其特征在于结构式 (Π ) 中:
R1为氢、 卤素、 氰基、 d-C6烷基、 d-C6卤代烷基或 d-C6烷基羰基;
R2为卤素、 氰基、 CfCe烷基或卤代 d-C3烷基。
9、按照权利要求 8所述的制备按照权利要求 1至 5之一所述的含一氟甲氧基吡唑的邻甲酰氨 基苯甲酰胺类化合物的中间体, 其特征在于结构式 (Π ) 中:
R1为氢、 卤素、 氰基、 甲基或三氟甲基;
R2为卤素或氰基。
10、 一种按照权利要求 1所述的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物合成 方法, 其特征在于按照如下步骤:
(1) 在非质子性溶剂中, 结构式 (VI) 表示的化合物与一氟一溴甲烷在缚酸剂作用下, 反应得到结构式 (W) 表示化合物;
(2) 在质子性溶剂中, 结构式 (W) 表示化合物在碱作用下水解得到结构式 (III) 表 示的含一氟甲氧基吡唑羧酸;
(3) 在非质子性溶剂中, 加入碱和烷基磺酰氯, 结构式 (III) 表示的含一氟甲氧基吡 唑羧酸和结构式 (V) 表示的取代邻氨基苯甲酸反应得结构式 (Π) 表示的含一氟甲氧基吡 唑的 4H-苯并 [1, 3]噁嗪 -4-酮类化合物;
(4) 在非质子性溶剂中, 结构式 (Π)表示的含一氟甲氧基吡唑的 4H-苯并 [1,3]噁嗪 -4-酮类化合物和结构式 (IV)表示的化合物反应得结构式 (I)表示的含一氟甲氧基吡唑的 邻甲酰氨基苯甲酰胺类化合物;
其中结构式 (Π)、 (111)、 (IV)、 (V)、 (VI) 和 (YD) 表示的化合物如下:
A、 R R2、 R3、 R4和 R5基团以及 m和 n的定义如权利要求 1。
11、 按照权利要求 10 所述的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物合成方 法, 其特征在于:
步骤(1) 中所述结构式(VI)表示的化合物与一氟一溴甲烷和缚酸剂的投料物质的量比 为 1: 1.1〜2: 1.1〜2, 所述非质子性溶剂选自四氢呋喃、 乙腈、 1, 4-二氧六环或丙酮中 的一种或两种以上组合, 反应温度为 10〜90°C;
步骤 (2) 中所述结构式 (W) 表示化合物与碱的投料物质的量比为 1: 1〜1.5, 所述质 子性溶剂为甲醇和 /或乙醇, 反应温度为 10〜90°C;
步骤(3) 中所述结构式 (III)表示的含一氟甲氧基吡唑羧酸和结构式 (V)表示的取代 邻氨基苯甲酸及烷基磺酰氯的投料物质的量比为 1: 1〜1. 5: 2〜3, 所述烷基磺酰氯为甲基 磺酰氯, 所述非质子性溶剂选自四氢呋喃、 乙腈、 1, 4-二氧六环、 乙醚或甲苯中的一种 或两种以上组合, 所述碱为有机碱, 反应温度为 -30〜80°C ;
步骤 (4) 中所述结构式 (Π ) 表示的含一氟甲氧基吡唑的 4H-苯并 [1, 3]噁嗪 -4-酮类化 合物和结构式(IV)表示的化合物的投料物质的量比为 1: 1〜5, 所述非质子性溶剂选自四 氢呋喃、 乙腈、 1, 4-二氧六环、 乙醚、 甲苯、 二氯甲烷或氯仿中的一种或两种以上组合。
12、按照权利要求 11所述的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物合成方法, 其特征在于:
步骤 (1 ) 中所述结构式 (VI) 表示的化合物与一氟一溴甲烷和缚酸剂的投料物质的量比 为 1: 1. 1〜1. 5: 1. 1〜1. 5, 反应温度为 20〜80°C;
步骤 (2) 中所述结构式 (VD) 表示化合物与碱的投料物质的量比为 1: 1〜1. 2, 反应温 度为 20〜80°C;
步骤(3) 中所述结构式 (III)表示的含一氟甲氧基吡唑羧酸和结构式 (V )表示的取代 邻氨基苯甲酸及烷基磺酰氯的投料物质的量比为 1: 1〜1. 2: 2〜2. 5, 所述有机碱选自三乙 胺、 吡啶或 3-甲基吡啶中的一种或两种以上组合, 反应温度为 -15〜30°C ;
步骤 (4) 中所述结构式 (Π ) 表示的含一氟甲氧基吡唑的 4H-苯并 [1, 3]噁嗪 -4-酮类化 合物和结构式 (IV) 表示的化合物的投料物质的量比为 1: 1〜2。
13、 一种如权利要求 1至 12之一所述的结构式( I )表示的含一氟甲氧基吡唑的邻甲酰 氨基苯甲酰胺类化合物或其农业上适用的盐的用途, 其特征在于结构(I )化合物用于农作物 上的害虫防治。
14、一种防治有害生物的农药制剂,其特征在于:含有按照权利要求 1所述的结构式( I ) 表示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物或其盐作为活性成分和农业、林业、 卫生上可接受的载体, 其中活性成分与载体的重量配比为 0. 1: 99. 9〜90: 10。
15、 一种防治有害生物的方法, 其特征在于: 将权利要求 14所述的农药制剂施于需要控 制的有害生物或其生长的介质上, 其中活性成分的有效量为每公顷 10克到 1000克。
16、 一种防治有害生物的方法, 其特在于: 含有按照权利要求 1所述的结构式 ( I ) 表 示的含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物与现有杀虫、 杀菌或除草的农药品种 的一种或二种或两种以上进行组合使用。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201010280882.3 | 2010-09-14 | ||
| CN 201010280882 CN101967139B (zh) | 2010-09-14 | 2010-09-14 | 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012034403A1 true WO2012034403A1 (zh) | 2012-03-22 |
Family
ID=43546375
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2011/073810 WO2012034403A1 (zh) | 2010-09-14 | 2011-05-09 | 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN101967139B (zh) |
| WO (1) | WO2012034403A1 (zh) |
Cited By (350)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014053403A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling insecticide resistant insects |
| WO2014053404A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053395A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Use of n-thio-anthranilamide compounds on cultivated plants |
| WO2014053407A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2014053406A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling ryanodine-modulator insecticide resistant insects |
| WO2014053405A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053401A2 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of improving plant health |
| WO2014079814A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079774A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079773A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079770A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079764A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079820A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Use of anthranilamide compounds for reducing insect-vectored viral infections |
| WO2014079841A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079804A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079813A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079766A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079772A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079752A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014090700A1 (en) | 2012-12-14 | 2014-06-19 | Basf Se | Malononitrile compounds for controlling animal pests |
| WO2014102244A1 (en) | 2012-12-27 | 2014-07-03 | Basf Se | 2-(pyridin-3-yl)-5-hetaryl-thiazole compounds carrying an imine or imine-derived substituent for combating invertebrate pests |
| WO2014170300A1 (en) | 2013-04-19 | 2014-10-23 | Basf Se | N-substituted acyl-imino-pyridine compounds and derivatives for combating animal pests |
| WO2014202751A1 (en) | 2013-06-21 | 2014-12-24 | Basf Se | Methods for controlling pests in soybean |
| WO2015007682A1 (en) | 2013-07-15 | 2015-01-22 | Basf Se | Pesticide compounds |
| WO2015040116A1 (en) | 2013-09-19 | 2015-03-26 | Basf Se | N-acylimino heterocyclic compounds |
| WO2015055757A1 (en) | 2013-10-18 | 2015-04-23 | Basf Se | Use of pesticidal active carboxamide derivative in soil and seed application and treatment methods |
| WO2015055497A1 (en) | 2013-10-16 | 2015-04-23 | Basf Se | Substituted pesticidal pyrazole compounds |
| JP2015517487A (ja) * | 2012-05-07 | 2015-06-22 | キョン ノン コーポレーション | カルバミン酸により置換されたジアミノアリール誘導体及びこれを含有する殺虫剤組成物 |
| WO2015091645A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | Azole compounds carrying an imine-derived substituent |
| WO2015091649A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | N-substituted imino heterocyclic compounds |
| WO2015104422A1 (en) | 2014-01-13 | 2015-07-16 | Basf Se | Dihydrothiophene compounds for controlling invertebrate pests |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| WO2017072039A1 (de) | 2015-10-26 | 2017-05-04 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| WO2017093214A1 (de) | 2015-12-03 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Mesolonische halogenierte 3-(acetyl)-1-[(1,3-thiazol-5-yl)methyl]-1h-imidazo[1,2-a]pyridin-4-ium-2-olat derivate und verwandte verbindungen als insektizide |
| WO2017093180A1 (de) | 2015-12-01 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017137338A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-(het)aryl-imidazolyl-carboxyamide als schädlingsbekämpfungsmittel |
| WO2017137339A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-oxyimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3210468A1 (de) | 2016-02-26 | 2017-08-30 | Bayer CropScience Aktiengesellschaft | Lösungsmittelfreie formulierungen von niedrig schmelzenden wirkstoffen |
| WO2017144341A1 (de) | 2016-02-23 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017157735A1 (de) | 2016-03-15 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2017157885A1 (de) | 2016-03-16 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | N-(cyanbenzyl)-6-(cyclopropylcarbonylamino)-4-(phenyl)-pyridin-2-carboxamid-derivate und verwandte verbindungen als pestizide pflanzenschutzmittel |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017174414A1 (de) | 2016-04-05 | 2017-10-12 | Bayer Cropscience Aktiengesellschaft | Naphthalin-derivate als schädlingsbekämpfungsmittel |
| WO2017178416A1 (en) | 2016-04-15 | 2017-10-19 | Bayer Animal Health Gmbh | Pyrazolopyrimidine derivatives |
| WO2017186536A1 (de) | 2016-04-25 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-alkylimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3241830A1 (de) | 2016-05-04 | 2017-11-08 | Bayer CropScience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| EP3245865A1 (en) | 2016-05-17 | 2017-11-22 | Bayer CropScience Aktiengesellschaft | Method for increasing yield in brassicaceae |
| WO2017198454A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in cotton |
| WO2017198449A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in brassicaceae |
| WO2017198450A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in maize |
| WO2017198452A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in soybean |
| WO2017198451A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in small grain cereals such as wheat and rice |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| WO2017198455A2 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in beta spp. plants |
| WO2017198453A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in potato, tomato or alfalfa |
| WO2018015289A1 (de) | 2016-07-19 | 2018-01-25 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018019937A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Formulation comprising a beneficial p. bilaii strain and talc for use in seed treatment |
| WO2018029102A1 (de) | 2016-08-10 | 2018-02-15 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-heterocyclyl-imidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3284739A1 (de) | 2017-07-19 | 2018-02-21 | Bayer CropScience Aktiengesellschaft | Substituierte (het)arylverbindungen als schädlingsbekämpfungsmittel |
| WO2018033455A1 (de) | 2016-08-15 | 2018-02-22 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018050825A1 (de) | 2016-09-19 | 2018-03-22 | Bayer Cropscience Aktiengesellschaft | Pyrazolo[1,5-a]pyridin- derivative und ihre verwendung als schädlingsbekämpfungsmittel |
| WO2018065292A1 (de) | 2016-10-06 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte bicyclische heterocyclen-derivate als schädlings-bekämpfungsmittel |
| WO2018065288A1 (de) | 2016-10-07 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-[2-phenyl-1-(sulfonylmethyl)vinyl]-imidazo[4,5-b]pyridin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel im pflanzenschutz |
| WO2018083288A1 (de) | 2016-11-07 | 2018-05-11 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2018087036A1 (en) | 2016-11-11 | 2018-05-17 | Bayer Animal Health Gmbh | New anthelmintic quinoline-3-carboxamide derivatives |
| WO2018095953A1 (de) | 2016-11-23 | 2018-05-31 | Bayer Cropscience Aktiengesellschaft | 2-[3-(alkylsulfonyl)-2h-indazol-2-yl]-3h-imidazo[4,5-b]pyridin-derivate und ähnliche verbindungen als schädlingsbekämpfungsmittel |
| WO2018104500A1 (en) | 2016-12-09 | 2018-06-14 | Bayer Cropscience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2018108791A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Cropscience Aktiengesellschaft | Thiadiazole derivatives as pesticides |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| WO2018108730A1 (de) | 2016-12-16 | 2018-06-21 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018130443A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018130437A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018138050A1 (de) | 2017-01-26 | 2018-08-02 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018141954A1 (de) | 2017-02-06 | 2018-08-09 | Bayer Aktiengesellschaft | Aryl- oder heteroaryl-substituierte imidazopyridinderivate und deren anwendung als schädlingsbekämpfungsmittel |
| EP3369320A1 (de) | 2017-03-02 | 2018-09-05 | Bayer CropScience Aktiengesellschaft | Wirkstoff zur bekämpfung von wanzen |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| WO2018189077A1 (de) | 2017-04-12 | 2018-10-18 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018192872A1 (de) | 2017-04-21 | 2018-10-25 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018197257A1 (de) | 2017-04-24 | 2018-11-01 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018197401A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Animal Health Gmbh | New bicyclic pyrazole derivatives |
| WO2018197692A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Aktiengesellschaft | Heteroarylphenylaminoquinolines and analogues |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018202715A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylbenzylbenzimidazoles and analogues |
| WO2018202706A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylheteroaryloxyquinolines and analogues |
| WO2018202524A1 (de) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | 2-{[2-(phenyloxymethyl)pyridin-5-yl]oxy}-ethanamin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel z.b. für den pflanzenschutz |
| WO2018202712A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylphenoxyquinolines and analogues |
| WO2018202494A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202501A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202525A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | Phenoxyethanamine derivatives for controlling pests |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | SUBSTITUTED CYCLOPROPYL DERIVATIVES |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | SUBSTITUTED PYRIMIDINIUM COMPOUNDS AND DERIVATIVES FOR CONTROLLING HARMFUL ANIMALS |
| WO2019002132A1 (en) | 2017-06-30 | 2019-01-03 | Bayer Animal Health Gmbh | NEW AZAQUINOLINE DERIVATIVES |
| WO2019007887A1 (de) | 2017-07-06 | 2019-01-10 | Bayer Aktiengesellschaft | Insektizide und fungizide wirkstoffkombinationen |
| WO2019025341A1 (en) | 2017-08-04 | 2019-02-07 | Bayer Animal Health Gmbh | QUINOLINE DERIVATIVES FOR THE TREATMENT OF INFECTIONS BY HELMINTHES |
| WO2019035881A1 (en) | 2017-08-17 | 2019-02-21 | Bayer Cropscience Lp | DISPERSIBLE COMPOSITIONS IN LIQUID FERTILIZER AND ASSOCIATED METHODS |
| WO2019038195A1 (de) | 2017-08-22 | 2019-02-28 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019042932A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING RICE PARASITES IN RICE |
| EP3453706A1 (en) | 2017-09-08 | 2019-03-13 | Basf Se | Pesticidal imidazole compounds |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | AGENT FOR EXTENDED CONTROL OF ECTOPARASITES FOR ANIMAL |
| WO2019068572A1 (de) | 2017-10-04 | 2019-04-11 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| EP3473100A1 (en) | 2017-10-18 | 2019-04-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3473103A1 (de) | 2017-10-17 | 2019-04-24 | Bayer AG | Wässrige suspensionskonzentrate auf basis von 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinon |
| WO2019076751A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076749A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076750A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDE PROPERTIES7ACARICIDES |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS AYNT INSECTICIDE / ACARICIDE PROPERTIES |
| WO2019076754A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019092086A1 (en) | 2017-11-13 | 2019-05-16 | Bayer Aktiengesellschaft | Tetrazolylpropyl derivatives and their use as fungicides |
| WO2019105871A1 (de) | 2017-11-29 | 2019-06-06 | Bayer Aktiengesellschaft | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| WO2019105875A1 (en) | 2017-11-28 | 2019-06-06 | Bayer Aktiengesellschaft | Heterocyclic compounds as pesticides |
| WO2019122319A1 (en) | 2017-12-21 | 2019-06-27 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylheteroaryloxyquinolines and analogues |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019155066A1 (en) | 2018-02-12 | 2019-08-15 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019162174A1 (de) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019162228A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | 1-(5-substituted imidazol-1-yl)but-3-en derivatives and their use as fungicides |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019170626A1 (en) | 2018-03-08 | 2019-09-12 | Bayer Aktiengesellschaft | Use of heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides in plant protection |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019175046A1 (de) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| EP3545764A1 (en) | 2019-02-12 | 2019-10-02 | Bayer AG | Crystal form of 2-({2-fluoro-4-methyl-5-[(r)-(2,2,2-trifluoroethyl)sulfinyl]phenyl}imino)-3-(2,2,2- trifluoroethyl)-1,3-thiazolidin-4-one |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| WO2019197468A1 (en) | 2018-04-12 | 2019-10-17 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| WO2019197371A1 (en) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Oxadiazoline derivatives |
| WO2019197623A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2019197615A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit fungiziden, insektiziden und akariziden eigenschaften |
| WO2019201835A1 (en) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019202077A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019201921A1 (de) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019206799A1 (en) | 2018-04-25 | 2019-10-31 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| EP3564225A1 (en) | 2019-03-21 | 2019-11-06 | Bayer Aktiengesellschaft | Crystalline form of spiromesifen |
| WO2019215182A1 (en) | 2018-05-09 | 2019-11-14 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2019224143A1 (de) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| EP3586630A1 (en) | 2018-06-28 | 2020-01-01 | Bayer AG | Active compound combinations having insecticidal/acaricidal properties |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020005678A1 (en) | 2018-06-25 | 2020-01-02 | Bayer Cropscience Lp | Seed treatment method |
| WO2020002189A1 (de) | 2018-06-27 | 2020-01-02 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| WO2020007905A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020020816A1 (en) | 2018-07-26 | 2020-01-30 | Bayer Aktiengesellschaft | Novel triazole derivatives |
| WO2020021082A1 (en) | 2018-07-27 | 2020-01-30 | Bayer Aktiengesellschaft | Controlled release formulations for agrochemicals |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| WO2020020813A1 (en) | 2018-07-25 | 2020-01-30 | Bayer Aktiengesellschaft | Fungicidal active compound combinations |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020025650A1 (en) | 2018-07-31 | 2020-02-06 | Bayer Aktiengesellschaft | Controlled release formulations with lignin for agrochemicals |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| EP3608311A1 (en) | 2019-06-28 | 2020-02-12 | Bayer AG | Crystalline form a of n-[4-chloro-3-[(1-cyanocyclopropyl)carbamoyl]phenyl]-2-methyl-4-methylsulfonyl-5-(1,1,2,2,2-pentafluoroethyl)pyrazole-3-carboxamide |
| EP3613736A1 (en) | 2018-08-22 | 2020-02-26 | Basf Se | Substituted glutarimide derivatives |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3620052A1 (en) | 2018-12-12 | 2020-03-11 | Bayer Aktiengesellschaft | Use of phenoxypyridinyl-substituted (1h-1,2,4-triazol-1-yl)alcohols for controlling fungicidal diseases in maize |
| WO2020053282A1 (de) | 2018-09-13 | 2020-03-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020057939A1 (en) | 2018-09-17 | 2020-03-26 | Bayer Aktiengesellschaft | Use of the fungicide isoflucypram for controlling claviceps purpurea and reducing sclerotia in cereals |
| EP3628156A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method for controlling pests of sugarcane, citrus, rapeseed, and potato plants |
| EP3628158A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| EP3628157A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| WO2020070050A1 (en) | 2018-10-01 | 2020-04-09 | Bayer Aktiengesellschaft | Fungicidal 5-substituted imidazol-1-yl carbinol derivatives |
| EP3636644A1 (de) | 2018-10-11 | 2020-04-15 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2020078839A1 (de) | 2018-10-16 | 2020-04-23 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| WO2020079173A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Pyridylphenylaminoquinolines and analogues |
| WO2020079167A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Heteroarylaminoquinolines and analogues |
| WO2020079232A1 (en) | 2018-10-20 | 2020-04-23 | Bayer Aktiengesellschaft | Oxetanylphenoxyquinolines and analogues |
| EP3643705A1 (en) | 2018-10-24 | 2020-04-29 | Basf Se | Pesticidal compounds |
| EP3643711A1 (en) | 2018-10-24 | 2020-04-29 | Bayer Animal Health GmbH | New anthelmintic compounds |
| WO2020109391A1 (en) | 2018-11-28 | 2020-06-04 | Bayer Aktiengesellschaft | Pyridazine (thio)amides as fungicidal compounds |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020114934A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| WO2020114932A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP3669652A1 (en) | 2018-12-21 | 2020-06-24 | Bayer AG | Active compound combination |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020127780A1 (en) | 2018-12-20 | 2020-06-25 | Bayer Aktiengesellschaft | Heterocyclyl pyridazine as fungicidal compounds |
| WO2020127974A1 (en) | 2018-12-21 | 2020-06-25 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as new antifungal agents |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| EP3679792A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679790A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679789A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679791A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679793A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3696177A1 (en) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| EP3701796A1 (en) | 2019-08-08 | 2020-09-02 | Bayer AG | Active compound combinations |
| WO2020173860A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Fused bicyclic heterocycle derivatives as pesticides |
| WO2020173861A1 (de) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020178307A1 (en) | 2019-03-05 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3708565A1 (en) | 2020-03-04 | 2020-09-16 | Bayer AG | Pyrimidinyloxyphenylamidines and the use thereof as fungicides |
| WO2020182929A1 (en) | 2019-03-13 | 2020-09-17 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| WO2020187656A1 (en) | 2019-03-15 | 2020-09-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3725788A1 (en) | 2019-04-15 | 2020-10-21 | Bayer AG | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| WO2020225436A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading, uptake and rainfastness ulv formulations |
| WO2020225242A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020229398A1 (de) | 2019-05-14 | 2020-11-19 | Bayer Aktiengesellschaft | (1-alkenyl)-substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| WO2020231751A1 (en) | 2019-05-10 | 2020-11-19 | Bayer Cropscience Lp | Active compound combinations |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| EP3750888A1 (en) | 2019-06-12 | 2020-12-16 | Bayer Aktiengesellschaft | Crystalline form a of 1,4-dimethyl-2-[2-(pyridin-3-yl)-2h-indazol-5-yl]-1,2,4-triazolidine-3,5-dione |
| WO2020254492A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254486A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254487A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254494A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2020254488A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and use thereof as fungicides |
| WO2020254493A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Thienylhydroxyisoxazolines and derivatives thereof |
| WO2020254490A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Phenoxyphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254489A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Benzylphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020263812A1 (en) | 2019-06-24 | 2020-12-30 | Auburn University | A bacillus strain and methods of its use for plant growth promotion |
| WO2021001331A1 (en) | 2019-07-03 | 2021-01-07 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides and derivatives thereof as microbicides |
| WO2021001273A1 (de) | 2019-07-04 | 2021-01-07 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP3766879A1 (en) | 2019-07-19 | 2021-01-20 | Basf Se | Pesticidal pyrazole derivatives |
| EP3769623A1 (en) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2021013721A1 (de) | 2019-07-22 | 2021-01-28 | Bayer Aktiengesellschaft | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| WO2021013720A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021013719A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| EP3771714A1 (de) | 2019-07-30 | 2021-02-03 | Bayer AG | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| WO2021018839A1 (en) | 2019-07-30 | 2021-02-04 | Bayer Animal Health Gmbh | Isoquinoline derivatives and their use for the treatment of parasitic infections |
| WO2021048188A1 (de) | 2019-09-11 | 2021-03-18 | Bayer Aktiengesellschaft | Hochwirksame formulierungen auf basis von 2-[(2;4-dichlorphenyl)-m ethyl|-4,4'-dimethyl- 3-isoxazolidinone sowie vorauflaufherbiziden |
| WO2021058659A1 (en) | 2019-09-26 | 2021-04-01 | Bayer Aktiengesellschaft | Rnai-mediated pest control |
| WO2021069569A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069567A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069575A1 (en) | 2019-10-11 | 2021-04-15 | Bayer Animal Health Gmbh | Heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021089673A1 (de) | 2019-11-07 | 2021-05-14 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2021097162A1 (en) | 2019-11-13 | 2021-05-20 | Bayer Cropscience Lp | Beneficial combinations with paenibacillus |
| WO2021099303A1 (en) | 2019-11-18 | 2021-05-27 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021105091A1 (en) | 2019-11-25 | 2021-06-03 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021122986A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Thienyloxazolones and analogues |
| WO2021123051A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides, thiophene carboxylic acids and derivatives thereof |
| WO2021130143A1 (en) | 2019-12-23 | 2021-07-01 | Basf Se | Enzyme enhanced root uptake of agrochemical active compound |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| EP3845304A1 (en) | 2019-12-30 | 2021-07-07 | Bayer AG | Capsule suspension concentrates based on polyisocyanates and biodegradable amine based cross-linker |
| EP3868207A1 (de) | 2020-02-24 | 2021-08-25 | Bayer Aktiengesellschaft | Verkapselte pyrethroide mit verbesserter wirksamkeit bei boden- und blattanwendungen |
| WO2021165195A1 (en) | 2020-02-18 | 2021-08-26 | Bayer Aktiengesellschaft | Heteroaryl-triazole compounds as pesticides |
| WO2021170463A1 (en) | 2020-02-28 | 2021-09-02 | BASF Agro B.V. | Methods and uses of a mixture comprising alpha-cypermethrin and dinotefuran for controlling invertebrate pests in turf |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| WO2021204930A1 (en) | 2020-04-09 | 2021-10-14 | Bayer Animal Health Gmbh | Substituted condensed azines as anthelmintic compounds |
| WO2021209368A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209364A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209363A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209490A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Cyclaminephenylaminoquinolines as fungicides |
| WO2021209365A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209366A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021213978A1 (de) | 2020-04-21 | 2021-10-28 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2021219513A1 (en) | 2020-04-28 | 2021-11-04 | Basf Se | Pesticidal compounds |
| WO2021224323A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021224220A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Pyridine (thio)amides as fungicidal compounds |
| EP3909950A1 (en) | 2020-05-13 | 2021-11-17 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2021228734A1 (en) | 2020-05-12 | 2021-11-18 | Bayer Aktiengesellschaft | Triazine and pyrimidine (thio)amides as fungicidal compounds |
| WO2021233861A1 (en) | 2020-05-19 | 2021-11-25 | Bayer Aktiengesellschaft | Azabicyclic(thio)amides as fungicidal compounds |
| EP3915371A1 (en) | 2020-11-04 | 2021-12-01 | Bayer AG | Active compound combinations and fungicide compositions comprising those |
| EP3915971A1 (en) | 2020-12-16 | 2021-12-01 | Bayer Aktiengesellschaft | Phenyl-s(o)n-phenylamidines and the use thereof as fungicides |
| WO2021245087A1 (en) | 2020-06-04 | 2021-12-09 | Bayer Aktiengesellschaft | Heterocyclyl pyrimidines and triazines as novel fungicides |
| WO2021249995A1 (en) | 2020-06-10 | 2021-12-16 | Bayer Aktiengesellschaft | Azabicyclyl-substituted heterocycles as fungicides |
| WO2021255091A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as fungicides |
| WO2021255071A1 (en) | 2020-06-18 | 2021-12-23 | Bayer Aktiengesellschaft | 3-(pyridazin-4-yl)-5,6-dihydro-4h-1,2,4-oxadiazine derivatives as fungicides for crop protection |
| WO2021255089A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines and 1,3,4-oxadiazole pyridines as fungicides |
| WO2021255169A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021255170A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| EP3929189A1 (en) | 2020-06-25 | 2021-12-29 | Bayer Animal Health GmbH | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021260017A1 (en) | 2020-06-26 | 2021-12-30 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates comprising biodegradable ester groups |
| WO2022002818A1 (de) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2022033991A1 (de) | 2020-08-13 | 2022-02-17 | Bayer Aktiengesellschaft | 5-amino substituierte triazole als schädlingsbekämpfungsmittel |
| WO2022053453A1 (de) | 2020-09-09 | 2022-03-17 | Bayer Aktiengesellschaft | Azolcarboxamide als schädlingsbekämpfungsmittel |
| WO2022058327A1 (en) | 2020-09-15 | 2022-03-24 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| EP3974414A1 (de) | 2020-09-25 | 2022-03-30 | Bayer AG | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| EP3994985A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| EP3994991A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| EP3994992A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| EP3994993A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| EP3994994A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994989A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, rainfastness and uptake properties |
| EP3994990A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and uptake properties |
| EP3994995A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994986A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| EP3994987A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift and uptake properties |
| EP3994988A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and rainfastness properties |
| WO2022129196A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | Heterobicycle substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129188A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | 1,2,4-oxadiazol-3-yl pyrimidines as fungicides |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022152728A1 (de) | 2021-01-15 | 2022-07-21 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP4036083A1 (de) | 2021-02-02 | 2022-08-03 | Bayer Aktiengesellschaft | 5-oxy substituierte hetereozyklen, als schädlingsbekämpfungsmittel |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| EP4043444A1 (en) | 2021-02-11 | 2022-08-17 | Basf Se | Substituted isoxazoline derivatives |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022233777A1 (en) | 2021-05-06 | 2022-11-10 | Bayer Aktiengesellschaft | Alkylamide substituted, annulated imidazoles and use thereof as insecticides |
| WO2022238194A1 (de) | 2021-05-10 | 2022-11-17 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| WO2022238391A1 (de) | 2021-05-12 | 2022-11-17 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| EP4119547A1 (en) | 2021-07-12 | 2023-01-18 | Basf Se | Triazole compounds for the control of invertebrate pests |
| WO2023017120A1 (en) | 2021-08-13 | 2023-02-16 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| EP4140986A1 (en) | 2021-08-23 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4140995A1 (en) | 2021-08-27 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| WO2023025682A1 (en) | 2021-08-25 | 2023-03-02 | Bayer Aktiengesellschaft | Novel pyrazinyl-triazole compounds as pesticides |
| EP4144739A1 (de) | 2021-09-02 | 2023-03-08 | Bayer Aktiengesellschaft | Anellierte pyrazole als schädlingsbekämpfungsmittel |
| EP4148052A1 (en) | 2021-09-09 | 2023-03-15 | Bayer Animal Health GmbH | New quinoline derivatives |
| EP4151631A1 (en) | 2021-09-20 | 2023-03-22 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2023078915A1 (en) | 2021-11-03 | 2023-05-11 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether (thio)amides as fungicidal compounds |
| WO2023092050A1 (en) | 2021-11-20 | 2023-05-25 | Bayer Cropscience Lp | Beneficial combinations with recombinant bacillus cells expressing a serine protease |
| WO2023099445A1 (en) | 2021-11-30 | 2023-06-08 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether oxadiazines as fungicidal compounds |
| EP4194453A1 (en) | 2021-12-08 | 2023-06-14 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4198023A1 (en) | 2021-12-16 | 2023-06-21 | Basf Se | Pesticidally active thiosemicarbazone compounds |
| EP4198033A1 (en) | 2021-12-14 | 2023-06-21 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2023110656A1 (en) | 2021-12-15 | 2023-06-22 | Bayer Aktiengesellschaft | Spectroscopic solution for non-destructive quantification of one or more chemical substances in a matrix comprising coating and bulk material in a sample, such as coated seeds, using multivariate data analysis |
| EP4238971A1 (en) | 2022-03-02 | 2023-09-06 | Basf Se | Substituted isoxazoline derivatives |
| EP4265110A1 (en) | 2022-04-20 | 2023-10-25 | Bayer AG | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023205602A1 (en) | 2022-04-18 | 2023-10-26 | Basf Corporation | High-load agricultural formulations and methods of making same |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2023208447A1 (en) | 2022-04-25 | 2023-11-02 | Basf Se | An emulsifiable concentrate having a (substituted) benzaldehyde-based solvent system |
| WO2023213670A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine |
| WO2023213626A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms |
| WO2023217619A1 (en) | 2022-05-07 | 2023-11-16 | Bayer Aktiengesellschaft | Low drift aqueous liquid formulations for low, medium, and high spray volume application |
| WO2023237444A1 (en) | 2022-06-06 | 2023-12-14 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295683A1 (en) | 2022-06-21 | 2023-12-27 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295688A1 (en) | 2022-09-28 | 2023-12-27 | Bayer Aktiengesellschaft | Active compound combination |
| WO2024013015A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024013016A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
| EP4342885A1 (en) | 2022-09-20 | 2024-03-27 | Basf Se | N-(3-(aminomethyl)-phenyl)-5-(4-phenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-amine derivatives and similar compounds as pesticides |
| WO2024068519A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068473A1 (de) | 2022-09-27 | 2024-04-04 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| WO2024068520A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068518A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068517A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| EP4353082A1 (en) | 2022-10-14 | 2024-04-17 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024104643A1 (en) | 2022-11-17 | 2024-05-23 | Bayer Aktiengesellschaft | Use of isotianil for controlling plasmodiophora brassica |
| EP4389210A1 (en) | 2022-12-21 | 2024-06-26 | Basf Se | Heteroaryl compounds for the control of invertebrate pests |
| WO2024170472A1 (en) | 2023-02-16 | 2024-08-22 | Bayer Aktiengesellschaft | Herbicidal mixtures |
| WO2024213752A1 (en) | 2023-04-14 | 2024-10-17 | Elanco Animal Health Gmbh | Long-term prevention and/or treatment of a disease by slo-1 inhibitors |
| EP4455137A1 (en) | 2023-04-24 | 2024-10-30 | Basf Se | Pyrimidine compounds for the control of invertebrate pests |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102285963B (zh) * | 2010-06-21 | 2014-04-09 | 中国中化股份有限公司 | 3-甲氧基吡唑酰胺类化合物及其应用 |
| CN101967139B (zh) * | 2010-09-14 | 2013-06-05 | 中化蓝天集团有限公司 | 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 |
| CN102907438A (zh) * | 2012-11-06 | 2013-02-06 | 中化蓝天集团有限公司 | 含有zj3757和吡蚜酮的杀虫组合物 |
| CN102919260B (zh) * | 2012-11-06 | 2015-05-27 | 中化蓝天集团有限公司 | 含有zj3757和新烟碱类杀虫剂的杀虫组合物 |
| CN103467380B (zh) * | 2013-09-29 | 2015-06-24 | 南开大学 | 一类取代苯基吡唑酰胺衍生物及其制备方法和应用 |
| CN105669643A (zh) * | 2013-12-05 | 2016-06-15 | 江西天人生态股份有限公司 | 一类基于鱼泥丁受体的邻甲酰氨基苯甲酰胺衍生物及其制备方法和用途 |
| CN105557710A (zh) * | 2014-10-14 | 2016-05-11 | 陕西美邦农药有限公司 | 一种含氟氧虫酰胺与生物源类的农药组合物 |
| CN105557696A (zh) * | 2014-10-14 | 2016-05-11 | 陕西美邦农药有限公司 | 一种含氟氧虫酰胺的高效杀虫组合物 |
| CN105580830A (zh) * | 2014-10-22 | 2016-05-18 | 陕西美邦农药有限公司 | 一种含氟氧虫酰胺的杀虫组合物 |
| CN105801485B (zh) * | 2014-12-29 | 2018-04-24 | 浙江省化工研究院有限公司 | 一类苯基连吡唑酰胺衍生物、其制备方法及应用 |
| CN104945326B (zh) * | 2015-06-24 | 2017-03-01 | 安徽农业大学 | 一种双吡唑酰胺类衍生物及其制备方法及在防治小菜蛾中的应用 |
| CN105130969A (zh) * | 2015-09-06 | 2015-12-09 | 青岛科技大学 | 一种n-呋喃甲基酰胺类杀虫剂 |
| CN105061412A (zh) * | 2015-09-06 | 2015-11-18 | 青岛科技大学 | 一种含氟n-呋喃甲基酰胺类化合物及其应用 |
| CN105218517A (zh) * | 2015-09-11 | 2016-01-06 | 江苏中旗作物保护股份有限公司 | N-(氰基环丙基)苯甲酰胺类化合物及其应用 |
| CN105541794A (zh) * | 2016-01-08 | 2016-05-04 | 南开大学 | 含有七氟异丙基的吡啶基吡唑酰胺类衍生物及其应用 |
| CN107759561B (zh) * | 2016-08-18 | 2021-04-02 | 江苏中旗科技股份有限公司 | 一种具有杀虫活性的邻甲酰氨基苯甲酰胺类化合物及其应用 |
| CN106831752B (zh) * | 2017-01-10 | 2019-06-21 | 青岛科技大学 | 一种含噻二唑四氟丙氧基吡啶连吡唑酰胺类化合物 |
| CN109928928A (zh) * | 2017-12-15 | 2019-06-25 | 南开大学 | 一类含n-苯基吡唑的双酰胺类衍生物及其制备方法和应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004046129A2 (en) * | 2002-11-15 | 2004-06-03 | E.I. Du Pont De Nemours And Company | Novel anthranilamide insecticides |
| WO2005118552A2 (en) * | 2004-04-13 | 2005-12-15 | E.I. Dupont De Nemours And Company | Anthranilamide insecticides |
| CN101298435A (zh) * | 2007-04-30 | 2008-11-05 | 中国中化集团公司 | 邻甲酰氨基苯甲酰胺类化合物及其应用 |
| CN101967139A (zh) * | 2010-09-14 | 2011-02-09 | 中化蓝天集团有限公司 | 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY142967A (en) * | 2001-08-13 | 2011-01-31 | Du Pont | Method for controlling particular insect pests by applying anthranilamide compounds |
| TWI363756B (en) * | 2004-12-07 | 2012-05-11 | Du Pont | Method for preparing n-phenylpyrazole-1-carboxamides |
| WO2008134969A1 (fr) * | 2007-04-30 | 2008-11-13 | Sinochem Corporation | Composés benzamides et leurs applications |
-
2010
- 2010-09-14 CN CN 201010280882 patent/CN101967139B/zh active Active
-
2011
- 2011-05-09 WO PCT/CN2011/073810 patent/WO2012034403A1/zh active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004046129A2 (en) * | 2002-11-15 | 2004-06-03 | E.I. Du Pont De Nemours And Company | Novel anthranilamide insecticides |
| WO2005118552A2 (en) * | 2004-04-13 | 2005-12-15 | E.I. Dupont De Nemours And Company | Anthranilamide insecticides |
| CN101298435A (zh) * | 2007-04-30 | 2008-11-05 | 中国中化集团公司 | 邻甲酰氨基苯甲酰胺类化合物及其应用 |
| CN101967139A (zh) * | 2010-09-14 | 2011-02-09 | 中化蓝天集团有限公司 | 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 |
Cited By (400)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015517487A (ja) * | 2012-05-07 | 2015-06-22 | キョン ノン コーポレーション | カルバミン酸により置換されたジアミノアリール誘導体及びこれを含有する殺虫剤組成物 |
| WO2014053404A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053395A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Use of n-thio-anthranilamide compounds on cultivated plants |
| WO2014053407A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2014053406A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling ryanodine-modulator insecticide resistant insects |
| WO2014053405A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053401A2 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of improving plant health |
| WO2014053403A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling insecticide resistant insects |
| WO2014079773A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079774A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079770A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079764A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079820A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Use of anthranilamide compounds for reducing insect-vectored viral infections |
| WO2014079841A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079804A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079814A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079766A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079772A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079813A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079752A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014090700A1 (en) | 2012-12-14 | 2014-06-19 | Basf Se | Malononitrile compounds for controlling animal pests |
| US10117430B2 (en) | 2012-12-14 | 2018-11-06 | Basf Se | Malononitrile compounds for controlling animal pests |
| WO2014102244A1 (en) | 2012-12-27 | 2014-07-03 | Basf Se | 2-(pyridin-3-yl)-5-hetaryl-thiazole compounds carrying an imine or imine-derived substituent for combating invertebrate pests |
| WO2014170300A1 (en) | 2013-04-19 | 2014-10-23 | Basf Se | N-substituted acyl-imino-pyridine compounds and derivatives for combating animal pests |
| WO2014202751A1 (en) | 2013-06-21 | 2014-12-24 | Basf Se | Methods for controlling pests in soybean |
| WO2015007682A1 (en) | 2013-07-15 | 2015-01-22 | Basf Se | Pesticide compounds |
| WO2015040116A1 (en) | 2013-09-19 | 2015-03-26 | Basf Se | N-acylimino heterocyclic compounds |
| WO2015055497A1 (en) | 2013-10-16 | 2015-04-23 | Basf Se | Substituted pesticidal pyrazole compounds |
| WO2015055757A1 (en) | 2013-10-18 | 2015-04-23 | Basf Se | Use of pesticidal active carboxamide derivative in soil and seed application and treatment methods |
| EP3456201A1 (en) | 2013-10-18 | 2019-03-20 | BASF Agrochemical Products B.V. | Use of pesticidal active carboxamide derivative in soil and seed application and treatment meth-ods |
| WO2015091645A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | Azole compounds carrying an imine-derived substituent |
| WO2015091649A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | N-substituted imino heterocyclic compounds |
| WO2015104422A1 (en) | 2014-01-13 | 2015-07-16 | Basf Se | Dihydrothiophene compounds for controlling invertebrate pests |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| US10701937B2 (en) | 2015-02-11 | 2020-07-07 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| WO2017072039A1 (de) | 2015-10-26 | 2017-05-04 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| WO2017093180A1 (de) | 2015-12-01 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017093214A1 (de) | 2015-12-03 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Mesolonische halogenierte 3-(acetyl)-1-[(1,3-thiazol-5-yl)methyl]-1h-imidazo[1,2-a]pyridin-4-ium-2-olat derivate und verwandte verbindungen als insektizide |
| WO2017137339A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-oxyimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| WO2017137338A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-(het)aryl-imidazolyl-carboxyamide als schädlingsbekämpfungsmittel |
| WO2017144341A1 (de) | 2016-02-23 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017144497A1 (de) | 2016-02-26 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Lösungsmittelfreie formulierungen von niedrig schmelzenden wirkstoffen |
| EP3210468A1 (de) | 2016-02-26 | 2017-08-30 | Bayer CropScience Aktiengesellschaft | Lösungsmittelfreie formulierungen von niedrig schmelzenden wirkstoffen |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017157735A1 (de) | 2016-03-15 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2017157885A1 (de) | 2016-03-16 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | N-(cyanbenzyl)-6-(cyclopropylcarbonylamino)-4-(phenyl)-pyridin-2-carboxamid-derivate und verwandte verbindungen als pestizide pflanzenschutzmittel |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017174414A1 (de) | 2016-04-05 | 2017-10-12 | Bayer Cropscience Aktiengesellschaft | Naphthalin-derivate als schädlingsbekämpfungsmittel |
| WO2017178416A1 (en) | 2016-04-15 | 2017-10-19 | Bayer Animal Health Gmbh | Pyrazolopyrimidine derivatives |
| WO2017186536A1 (de) | 2016-04-25 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-alkylimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3241830A1 (de) | 2016-05-04 | 2017-11-08 | Bayer CropScience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017198450A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in maize |
| WO2017198449A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in brassicaceae |
| WO2017198452A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in soybean |
| WO2017198453A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in potato, tomato or alfalfa |
| EP3245865A1 (en) | 2016-05-17 | 2017-11-22 | Bayer CropScience Aktiengesellschaft | Method for increasing yield in brassicaceae |
| WO2017198454A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in cotton |
| WO2017198451A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in small grain cereals such as wheat and rice |
| WO2017198455A2 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in beta spp. plants |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| WO2018015289A1 (de) | 2016-07-19 | 2018-01-25 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018019937A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Formulation comprising a beneficial p. bilaii strain and talc for use in seed treatment |
| WO2018029102A1 (de) | 2016-08-10 | 2018-02-15 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-heterocyclyl-imidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| WO2018033455A1 (de) | 2016-08-15 | 2018-02-22 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018050825A1 (de) | 2016-09-19 | 2018-03-22 | Bayer Cropscience Aktiengesellschaft | Pyrazolo[1,5-a]pyridin- derivative und ihre verwendung als schädlingsbekämpfungsmittel |
| WO2018065292A1 (de) | 2016-10-06 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte bicyclische heterocyclen-derivate als schädlings-bekämpfungsmittel |
| WO2018065288A1 (de) | 2016-10-07 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-[2-phenyl-1-(sulfonylmethyl)vinyl]-imidazo[4,5-b]pyridin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel im pflanzenschutz |
| WO2018083288A1 (de) | 2016-11-07 | 2018-05-11 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2018087036A1 (en) | 2016-11-11 | 2018-05-17 | Bayer Animal Health Gmbh | New anthelmintic quinoline-3-carboxamide derivatives |
| US10889573B2 (en) | 2016-11-11 | 2021-01-12 | Bayer Animal Health Gmbh | Anthelmintic quinoline-3-carboxamide derivatives |
| US11505545B2 (en) | 2016-11-11 | 2022-11-22 | Bayer Animal Health Gmbh | Anthelmintic quinoline-3-carboxamide derivatives |
| WO2018095953A1 (de) | 2016-11-23 | 2018-05-31 | Bayer Cropscience Aktiengesellschaft | 2-[3-(alkylsulfonyl)-2h-indazol-2-yl]-3h-imidazo[4,5-b]pyridin-derivate und ähnliche verbindungen als schädlingsbekämpfungsmittel |
| WO2018104500A1 (en) | 2016-12-09 | 2018-06-14 | Bayer Cropscience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2018108791A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Cropscience Aktiengesellschaft | Thiadiazole derivatives as pesticides |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| WO2018108730A1 (de) | 2016-12-16 | 2018-06-21 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018130443A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018130437A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018138050A1 (de) | 2017-01-26 | 2018-08-02 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018141954A1 (de) | 2017-02-06 | 2018-08-09 | Bayer Aktiengesellschaft | Aryl- oder heteroaryl-substituierte imidazopyridinderivate und deren anwendung als schädlingsbekämpfungsmittel |
| EP3369320A1 (de) | 2017-03-02 | 2018-09-05 | Bayer CropScience Aktiengesellschaft | Wirkstoff zur bekämpfung von wanzen |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| EP3978504A1 (en) | 2017-03-31 | 2022-04-06 | Basf Se | Chiral 2,3-dihydrothiazolo[3,2-a]pyrimidine derivatives for combating animal pests |
| WO2018189077A1 (de) | 2017-04-12 | 2018-10-18 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018192872A1 (de) | 2017-04-21 | 2018-10-25 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018197257A1 (de) | 2017-04-24 | 2018-11-01 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018197692A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Aktiengesellschaft | Heteroarylphenylaminoquinolines and analogues |
| US11130768B2 (en) | 2017-04-27 | 2021-09-28 | Bayer Animal Health Gmbh | Bicyclic pyrazole derivatives |
| WO2018197401A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Animal Health Gmbh | New bicyclic pyrazole derivatives |
| WO2018202494A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202501A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202712A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylphenoxyquinolines and analogues |
| WO2018202706A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylheteroaryloxyquinolines and analogues |
| WO2018202715A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylbenzylbenzimidazoles and analogues |
| WO2018202524A1 (de) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | 2-{[2-(phenyloxymethyl)pyridin-5-yl]oxy}-ethanamin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel z.b. für den pflanzenschutz |
| WO2018202525A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | Phenoxyethanamine derivatives for controlling pests |
| US11827616B2 (en) | 2017-05-04 | 2023-11-28 | Discovery Purchaser Corporation | Heterocyclic compounds as pesticides |
| EP3400801A1 (en) | 2017-05-10 | 2018-11-14 | Bayer CropScience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | SUBSTITUTED PYRIMIDINIUM COMPOUNDS AND DERIVATIVES FOR CONTROLLING HARMFUL ANIMALS |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | SUBSTITUTED CYCLOPROPYL DERIVATIVES |
| WO2019002132A1 (en) | 2017-06-30 | 2019-01-03 | Bayer Animal Health Gmbh | NEW AZAQUINOLINE DERIVATIVES |
| WO2019007887A1 (de) | 2017-07-06 | 2019-01-10 | Bayer Aktiengesellschaft | Insektizide und fungizide wirkstoffkombinationen |
| EP3284739A1 (de) | 2017-07-19 | 2018-02-21 | Bayer CropScience Aktiengesellschaft | Substituierte (het)arylverbindungen als schädlingsbekämpfungsmittel |
| WO2019025341A1 (en) | 2017-08-04 | 2019-02-07 | Bayer Animal Health Gmbh | QUINOLINE DERIVATIVES FOR THE TREATMENT OF INFECTIONS BY HELMINTHES |
| WO2019035881A1 (en) | 2017-08-17 | 2019-02-21 | Bayer Cropscience Lp | DISPERSIBLE COMPOSITIONS IN LIQUID FERTILIZER AND ASSOCIATED METHODS |
| WO2019038195A1 (de) | 2017-08-22 | 2019-02-28 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019043183A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING PESTS OF RICE IN RICE |
| WO2019042932A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING RICE PARASITES IN RICE |
| EP3453706A1 (en) | 2017-09-08 | 2019-03-13 | Basf Se | Pesticidal imidazole compounds |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | AGENT FOR EXTENDED CONTROL OF ECTOPARASITES FOR ANIMAL |
| WO2019068572A1 (de) | 2017-10-04 | 2019-04-11 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| EP3473103A1 (de) | 2017-10-17 | 2019-04-24 | Bayer AG | Wässrige suspensionskonzentrate auf basis von 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinon |
| WO2019076744A1 (de) | 2017-10-17 | 2019-04-25 | Bayer Aktiengesellschaft | Wässrige suspensionskonzentrate auf basis von 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinone |
| WO2019076750A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDE PROPERTIES7ACARICIDES |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS AYNT INSECTICIDE / ACARICIDE PROPERTIES |
| WO2019076754A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| EP3473100A1 (en) | 2017-10-18 | 2019-04-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076751A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076749A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019092086A1 (en) | 2017-11-13 | 2019-05-16 | Bayer Aktiengesellschaft | Tetrazolylpropyl derivatives and their use as fungicides |
| WO2019105875A1 (en) | 2017-11-28 | 2019-06-06 | Bayer Aktiengesellschaft | Heterocyclic compounds as pesticides |
| WO2019105871A1 (de) | 2017-11-29 | 2019-06-06 | Bayer Aktiengesellschaft | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019122319A1 (en) | 2017-12-21 | 2019-06-27 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylheteroaryloxyquinolines and analogues |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| WO2019155066A1 (en) | 2018-02-12 | 2019-08-15 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019162228A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | 1-(5-substituted imidazol-1-yl)but-3-en derivatives and their use as fungicides |
| WO2019162174A1 (de) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019170626A1 (en) | 2018-03-08 | 2019-09-12 | Bayer Aktiengesellschaft | Use of heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides in plant protection |
| WO2019175045A1 (de) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019175046A1 (de) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| WO2019197371A1 (en) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Oxadiazoline derivatives |
| WO2019197468A1 (en) | 2018-04-12 | 2019-10-17 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| EP3904350A1 (en) | 2018-04-12 | 2021-11-03 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| EP3904349A2 (en) | 2018-04-12 | 2021-11-03 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}heterocyclyl amide derivatives and similar compounds as pesticides |
| WO2019197615A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit fungiziden, insektiziden und akariziden eigenschaften |
| WO2019197623A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2019201835A1 (en) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019202077A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019201921A1 (de) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| EP3919486A1 (en) | 2018-04-25 | 2021-12-08 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019206799A1 (en) | 2018-04-25 | 2019-10-31 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019215182A1 (en) | 2018-05-09 | 2019-11-14 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2019224143A1 (de) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2020005678A1 (en) | 2018-06-25 | 2020-01-02 | Bayer Cropscience Lp | Seed treatment method |
| WO2020002189A1 (de) | 2018-06-27 | 2020-01-02 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| EP3586630A1 (en) | 2018-06-28 | 2020-01-01 | Bayer AG | Active compound combinations having insecticidal/acaricidal properties |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020007904A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007902A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007905A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| US11884643B2 (en) | 2018-07-05 | 2024-01-30 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| US11952359B2 (en) | 2018-07-05 | 2024-04-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| WO2020020813A1 (en) | 2018-07-25 | 2020-01-30 | Bayer Aktiengesellschaft | Fungicidal active compound combinations |
| WO2020020816A1 (en) | 2018-07-26 | 2020-01-30 | Bayer Aktiengesellschaft | Novel triazole derivatives |
| WO2020021082A1 (en) | 2018-07-27 | 2020-01-30 | Bayer Aktiengesellschaft | Controlled release formulations for agrochemicals |
| WO2020025650A1 (en) | 2018-07-31 | 2020-02-06 | Bayer Aktiengesellschaft | Controlled release formulations with lignin for agrochemicals |
| EP3613736A1 (en) | 2018-08-22 | 2020-02-26 | Basf Se | Substituted glutarimide derivatives |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020053282A1 (de) | 2018-09-13 | 2020-03-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020057939A1 (en) | 2018-09-17 | 2020-03-26 | Bayer Aktiengesellschaft | Use of the fungicide isoflucypram for controlling claviceps purpurea and reducing sclerotia in cereals |
| WO2020064480A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| WO2020064408A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| EP3628157A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| EP3628158A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| EP3628156A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method for controlling pests of sugarcane, citrus, rapeseed, and potato plants |
| WO2020070050A1 (en) | 2018-10-01 | 2020-04-09 | Bayer Aktiengesellschaft | Fungicidal 5-substituted imidazol-1-yl carbinol derivatives |
| EP3636644A1 (de) | 2018-10-11 | 2020-04-15 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2020078839A1 (de) | 2018-10-16 | 2020-04-23 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| WO2020079167A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Heteroarylaminoquinolines and analogues |
| WO2020079173A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Pyridylphenylaminoquinolines and analogues |
| WO2020079232A1 (en) | 2018-10-20 | 2020-04-23 | Bayer Aktiengesellschaft | Oxetanylphenoxyquinolines and analogues |
| WO2020083733A1 (en) | 2018-10-24 | 2020-04-30 | Basf Se | Pesticidal compounds |
| WO2020083971A2 (en) | 2018-10-24 | 2020-04-30 | Bayer Animal Health Gmbh | New anthelmintic compounds |
| EP3643711A1 (en) | 2018-10-24 | 2020-04-29 | Bayer Animal Health GmbH | New anthelmintic compounds |
| EP3643705A1 (en) | 2018-10-24 | 2020-04-29 | Basf Se | Pesticidal compounds |
| WO2020109391A1 (en) | 2018-11-28 | 2020-06-04 | Bayer Aktiengesellschaft | Pyridazine (thio)amides as fungicidal compounds |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020114932A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| WO2020114934A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP3620052A1 (en) | 2018-12-12 | 2020-03-11 | Bayer Aktiengesellschaft | Use of phenoxypyridinyl-substituted (1h-1,2,4-triazol-1-yl)alcohols for controlling fungicidal diseases in maize |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020127780A1 (en) | 2018-12-20 | 2020-06-25 | Bayer Aktiengesellschaft | Heterocyclyl pyridazine as fungicidal compounds |
| EP3669652A1 (en) | 2018-12-21 | 2020-06-24 | Bayer AG | Active compound combination |
| WO2020127974A1 (en) | 2018-12-21 | 2020-06-25 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as new antifungal agents |
| EP3679792A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679793A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679791A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679789A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679790A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3696177A1 (en) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| EP3545764A1 (en) | 2019-02-12 | 2019-10-02 | Bayer AG | Crystal form of 2-({2-fluoro-4-methyl-5-[(r)-(2,2,2-trifluoroethyl)sulfinyl]phenyl}imino)-3-(2,2,2- trifluoroethyl)-1,3-thiazolidin-4-one |
| WO2020173861A1 (de) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020173860A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Fused bicyclic heterocycle derivatives as pesticides |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020178307A1 (en) | 2019-03-05 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020182929A1 (en) | 2019-03-13 | 2020-09-17 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| WO2020187656A1 (en) | 2019-03-15 | 2020-09-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3564225A1 (en) | 2019-03-21 | 2019-11-06 | Bayer Aktiengesellschaft | Crystalline form of spiromesifen |
| WO2020212235A1 (en) | 2019-04-15 | 2020-10-22 | Bayer Animal Health Gmbh | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| EP3725788A1 (en) | 2019-04-15 | 2020-10-21 | Bayer AG | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| WO2020225436A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading, uptake and rainfastness ulv formulations |
| WO2020225440A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading and rainfastness ulv formulations |
| WO2020225437A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Ulv formulations with enhanced uptake |
| WO2020225435A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading and uptake ulv formulations |
| WO2020225434A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading ulv formulations for agrochemical compounds ii |
| WO2020225438A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High uptake and rainfastness ulv formulations |
| WO2020225242A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020225439A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Ulv formulations with enhanced rainfastness |
| WO2020225428A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading ulv formulations for insecticides |
| WO2020231751A1 (en) | 2019-05-10 | 2020-11-19 | Bayer Cropscience Lp | Active compound combinations |
| WO2020229398A1 (de) | 2019-05-14 | 2020-11-19 | Bayer Aktiengesellschaft | (1-alkenyl)-substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| EP3750888A1 (en) | 2019-06-12 | 2020-12-16 | Bayer Aktiengesellschaft | Crystalline form a of 1,4-dimethyl-2-[2-(pyridin-3-yl)-2h-indazol-5-yl]-1,2,4-triazolidine-3,5-dione |
| WO2020254494A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2020254492A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254486A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254487A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254488A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and use thereof as fungicides |
| WO2020254493A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Thienylhydroxyisoxazolines and derivatives thereof |
| WO2020254490A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Phenoxyphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254489A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Benzylphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020263812A1 (en) | 2019-06-24 | 2020-12-30 | Auburn University | A bacillus strain and methods of its use for plant growth promotion |
| EP3608311A1 (en) | 2019-06-28 | 2020-02-12 | Bayer AG | Crystalline form a of n-[4-chloro-3-[(1-cyanocyclopropyl)carbamoyl]phenyl]-2-methyl-4-methylsulfonyl-5-(1,1,2,2,2-pentafluoroethyl)pyrazole-3-carboxamide |
| WO2021001331A1 (en) | 2019-07-03 | 2021-01-07 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides and derivatives thereof as microbicides |
| WO2021001273A1 (de) | 2019-07-04 | 2021-01-07 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP4378314A2 (de) | 2019-07-04 | 2024-06-05 | Bayer AG | Herbizide zusammensetzungen |
| EP3766879A1 (en) | 2019-07-19 | 2021-01-20 | Basf Se | Pesticidal pyrazole derivatives |
| WO2021013561A1 (en) | 2019-07-19 | 2021-01-28 | Basf Se | Pesticidal pyrazole and triazole derivatives |
| WO2021013721A1 (de) | 2019-07-22 | 2021-01-28 | Bayer Aktiengesellschaft | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| EP3769623A1 (en) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2021013719A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021013720A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021018839A1 (en) | 2019-07-30 | 2021-02-04 | Bayer Animal Health Gmbh | Isoquinoline derivatives and their use for the treatment of parasitic infections |
| EP3771714A1 (de) | 2019-07-30 | 2021-02-03 | Bayer AG | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| EP3701796A1 (en) | 2019-08-08 | 2020-09-02 | Bayer AG | Active compound combinations |
| WO2021048188A1 (de) | 2019-09-11 | 2021-03-18 | Bayer Aktiengesellschaft | Hochwirksame formulierungen auf basis von 2-[(2;4-dichlorphenyl)-m ethyl|-4,4'-dimethyl- 3-isoxazolidinone sowie vorauflaufherbiziden |
| WO2021058659A1 (en) | 2019-09-26 | 2021-04-01 | Bayer Aktiengesellschaft | Rnai-mediated pest control |
| WO2021069567A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069569A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069575A1 (en) | 2019-10-11 | 2021-04-15 | Bayer Animal Health Gmbh | Heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021089673A1 (de) | 2019-11-07 | 2021-05-14 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2021097162A1 (en) | 2019-11-13 | 2021-05-20 | Bayer Cropscience Lp | Beneficial combinations with paenibacillus |
| WO2021099303A1 (en) | 2019-11-18 | 2021-05-27 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021105091A1 (en) | 2019-11-25 | 2021-06-03 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021122986A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Thienyloxazolones and analogues |
| WO2021123051A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides, thiophene carboxylic acids and derivatives thereof |
| WO2021130143A1 (en) | 2019-12-23 | 2021-07-01 | Basf Se | Enzyme enhanced root uptake of agrochemical active compound |
| WO2021136758A1 (en) | 2019-12-30 | 2021-07-08 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates based on polyurea shell material containing polyfunctional aminocarboxylic esters |
| EP3845304A1 (en) | 2019-12-30 | 2021-07-07 | Bayer AG | Capsule suspension concentrates based on polyisocyanates and biodegradable amine based cross-linker |
| WO2021165195A1 (en) | 2020-02-18 | 2021-08-26 | Bayer Aktiengesellschaft | Heteroaryl-triazole compounds as pesticides |
| EP3868207A1 (de) | 2020-02-24 | 2021-08-25 | Bayer Aktiengesellschaft | Verkapselte pyrethroide mit verbesserter wirksamkeit bei boden- und blattanwendungen |
| WO2021170527A1 (de) | 2020-02-24 | 2021-09-02 | Bayer Aktiengesellschaft | Verkapselte pyrethroide mit verbesserter wirksamkeit bei boden- und blattanwendungen |
| WO2021170463A1 (en) | 2020-02-28 | 2021-09-02 | BASF Agro B.V. | Methods and uses of a mixture comprising alpha-cypermethrin and dinotefuran for controlling invertebrate pests in turf |
| EP3708565A1 (en) | 2020-03-04 | 2020-09-16 | Bayer AG | Pyrimidinyloxyphenylamidines and the use thereof as fungicides |
| WO2021204930A1 (en) | 2020-04-09 | 2021-10-14 | Bayer Animal Health Gmbh | Substituted condensed azines as anthelmintic compounds |
| WO2021209365A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209368A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209366A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209364A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209490A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Cyclaminephenylaminoquinolines as fungicides |
| WO2021209363A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021213978A1 (de) | 2020-04-21 | 2021-10-28 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2021219513A1 (en) | 2020-04-28 | 2021-11-04 | Basf Se | Pesticidal compounds |
| WO2021224220A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Pyridine (thio)amides as fungicidal compounds |
| WO2021224323A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021228734A1 (en) | 2020-05-12 | 2021-11-18 | Bayer Aktiengesellschaft | Triazine and pyrimidine (thio)amides as fungicidal compounds |
| EP3909950A1 (en) | 2020-05-13 | 2021-11-17 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2021233861A1 (en) | 2020-05-19 | 2021-11-25 | Bayer Aktiengesellschaft | Azabicyclic(thio)amides as fungicidal compounds |
| WO2021245087A1 (en) | 2020-06-04 | 2021-12-09 | Bayer Aktiengesellschaft | Heterocyclyl pyrimidines and triazines as novel fungicides |
| WO2021249995A1 (en) | 2020-06-10 | 2021-12-16 | Bayer Aktiengesellschaft | Azabicyclyl-substituted heterocycles as fungicides |
| WO2021255071A1 (en) | 2020-06-18 | 2021-12-23 | Bayer Aktiengesellschaft | 3-(pyridazin-4-yl)-5,6-dihydro-4h-1,2,4-oxadiazine derivatives as fungicides for crop protection |
| WO2021255169A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021255089A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines and 1,3,4-oxadiazole pyridines as fungicides |
| WO2021255091A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as fungicides |
| WO2021255170A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021259997A1 (en) | 2020-06-25 | 2021-12-30 | Bayer Animal Health Gmbh | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| EP3929189A1 (en) | 2020-06-25 | 2021-12-29 | Bayer Animal Health GmbH | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021260017A1 (en) | 2020-06-26 | 2021-12-30 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates comprising biodegradable ester groups |
| WO2022002818A1 (de) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2022033991A1 (de) | 2020-08-13 | 2022-02-17 | Bayer Aktiengesellschaft | 5-amino substituierte triazole als schädlingsbekämpfungsmittel |
| WO2022053453A1 (de) | 2020-09-09 | 2022-03-17 | Bayer Aktiengesellschaft | Azolcarboxamide als schädlingsbekämpfungsmittel |
| WO2022058327A1 (en) | 2020-09-15 | 2022-03-24 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| EP3974414A1 (de) | 2020-09-25 | 2022-03-30 | Bayer AG | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| EP3915371A1 (en) | 2020-11-04 | 2021-12-01 | Bayer AG | Active compound combinations and fungicide compositions comprising those |
| WO2022096685A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| WO2022096690A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading and uptake properties |
| EP3994989A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, rainfastness and uptake properties |
| EP3994990A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and uptake properties |
| EP3994995A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994986A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| EP3994987A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift and uptake properties |
| EP3994988A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and rainfastness properties |
| WO2022096695A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| WO2022096691A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, uptake and rainfastness properties |
| WO2022096692A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| EP3994992A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| EP3994993A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| WO2022096687A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and uptake properties |
| WO2022096686A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| WO2022096693A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| WO2022096694A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| WO2022096688A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading and rainfastness properties |
| WO2022096696A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994994A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994985A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| EP3994991A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| EP3915971A1 (en) | 2020-12-16 | 2021-12-01 | Bayer Aktiengesellschaft | Phenyl-s(o)n-phenylamidines and the use thereof as fungicides |
| WO2022129196A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | Heterobicycle substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129188A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | 1,2,4-oxadiazol-3-yl pyrimidines as fungicides |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022152728A1 (de) | 2021-01-15 | 2022-07-21 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP4036083A1 (de) | 2021-02-02 | 2022-08-03 | Bayer Aktiengesellschaft | 5-oxy substituierte hetereozyklen, als schädlingsbekämpfungsmittel |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| EP4043444A1 (en) | 2021-02-11 | 2022-08-17 | Basf Se | Substituted isoxazoline derivatives |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022233777A1 (en) | 2021-05-06 | 2022-11-10 | Bayer Aktiengesellschaft | Alkylamide substituted, annulated imidazoles and use thereof as insecticides |
| WO2022238194A1 (de) | 2021-05-10 | 2022-11-17 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| WO2022238391A1 (de) | 2021-05-12 | 2022-11-17 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| EP4119547A1 (en) | 2021-07-12 | 2023-01-18 | Basf Se | Triazole compounds for the control of invertebrate pests |
| WO2023017120A1 (en) | 2021-08-13 | 2023-02-16 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| EP4140986A1 (en) | 2021-08-23 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| WO2023025682A1 (en) | 2021-08-25 | 2023-03-02 | Bayer Aktiengesellschaft | Novel pyrazinyl-triazole compounds as pesticides |
| EP4140995A1 (en) | 2021-08-27 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4144739A1 (de) | 2021-09-02 | 2023-03-08 | Bayer Aktiengesellschaft | Anellierte pyrazole als schädlingsbekämpfungsmittel |
| EP4148052A1 (en) | 2021-09-09 | 2023-03-15 | Bayer Animal Health GmbH | New quinoline derivatives |
| WO2023036821A1 (en) | 2021-09-09 | 2023-03-16 | Bayer Animal Health Gmbh | New quinoline derivatives |
| EP4151631A1 (en) | 2021-09-20 | 2023-03-22 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2023078915A1 (en) | 2021-11-03 | 2023-05-11 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether (thio)amides as fungicidal compounds |
| WO2023092050A1 (en) | 2021-11-20 | 2023-05-25 | Bayer Cropscience Lp | Beneficial combinations with recombinant bacillus cells expressing a serine protease |
| WO2023099445A1 (en) | 2021-11-30 | 2023-06-08 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether oxadiazines as fungicidal compounds |
| EP4194453A1 (en) | 2021-12-08 | 2023-06-14 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4198033A1 (en) | 2021-12-14 | 2023-06-21 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2023110656A1 (en) | 2021-12-15 | 2023-06-22 | Bayer Aktiengesellschaft | Spectroscopic solution for non-destructive quantification of one or more chemical substances in a matrix comprising coating and bulk material in a sample, such as coated seeds, using multivariate data analysis |
| EP4198023A1 (en) | 2021-12-16 | 2023-06-21 | Basf Se | Pesticidally active thiosemicarbazone compounds |
| EP4238971A1 (en) | 2022-03-02 | 2023-09-06 | Basf Se | Substituted isoxazoline derivatives |
| WO2023205602A1 (en) | 2022-04-18 | 2023-10-26 | Basf Corporation | High-load agricultural formulations and methods of making same |
| EP4265110A1 (en) | 2022-04-20 | 2023-10-25 | Bayer AG | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023203009A1 (en) | 2022-04-20 | 2023-10-26 | Bayer Aktiengesellschaft | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2023208447A1 (en) | 2022-04-25 | 2023-11-02 | Basf Se | An emulsifiable concentrate having a (substituted) benzaldehyde-based solvent system |
| WO2023213670A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine |
| WO2023213626A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms |
| WO2023217619A1 (en) | 2022-05-07 | 2023-11-16 | Bayer Aktiengesellschaft | Low drift aqueous liquid formulations for low, medium, and high spray volume application |
| WO2023237444A1 (en) | 2022-06-06 | 2023-12-14 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295683A1 (en) | 2022-06-21 | 2023-12-27 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| WO2024013016A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024013015A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
| EP4342885A1 (en) | 2022-09-20 | 2024-03-27 | Basf Se | N-(3-(aminomethyl)-phenyl)-5-(4-phenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-amine derivatives and similar compounds as pesticides |
| WO2024068473A1 (de) | 2022-09-27 | 2024-04-04 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| EP4295688A1 (en) | 2022-09-28 | 2023-12-27 | Bayer Aktiengesellschaft | Active compound combination |
| WO2024068520A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068518A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068517A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068519A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| EP4353082A1 (en) | 2022-10-14 | 2024-04-17 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024104643A1 (en) | 2022-11-17 | 2024-05-23 | Bayer Aktiengesellschaft | Use of isotianil for controlling plasmodiophora brassica |
| EP4389210A1 (en) | 2022-12-21 | 2024-06-26 | Basf Se | Heteroaryl compounds for the control of invertebrate pests |
| WO2024170472A1 (en) | 2023-02-16 | 2024-08-22 | Bayer Aktiengesellschaft | Herbicidal mixtures |
| WO2024213752A1 (en) | 2023-04-14 | 2024-10-17 | Elanco Animal Health Gmbh | Long-term prevention and/or treatment of a disease by slo-1 inhibitors |
| EP4455137A1 (en) | 2023-04-24 | 2024-10-30 | Basf Se | Pyrimidine compounds for the control of invertebrate pests |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101967139B (zh) | 2013-06-05 |
| CN101967139A (zh) | 2011-02-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2012034403A1 (zh) | 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 | |
| US11540516B2 (en) | M-diamide compound and preparation method therefor and use thereof | |
| CN107074781B (zh) | 嘧啶酮化合物 | |
| WO2017075910A1 (zh) | 吡唑酮类化合物或其盐、制备方法、除草剂组合物及用途 | |
| WO2019080224A1 (zh) | 一种吡唑酮类化合物或其盐、除草剂组合物及用途 | |
| WO2009116558A1 (ja) | 1-フェニル-5-ジフルオロメチルピラゾール-4-カルボキサミド誘導体及びこれを有効成分とする除草剤 | |
| EA022864B1 (ru) | Производные 6-ацил-1,2,4-триазин-3,5-диона и гербициды | |
| WO2006022225A1 (ja) | 光学活性フタルアミド誘導体及び農園芸用殺虫剤並びにその使用方法 | |
| WO2009131237A1 (ja) | 有害節足動物防除組成物および縮合複素環化合物 | |
| UA126056C2 (uk) | Піридазинонові гербіциди | |
| WO2020177778A1 (zh) | 1-吡啶基吡唑酰胺类化合物及其制备方法与应用 | |
| CN112661665B (zh) | 一种酰胺类化合物及其制备方法和应用 | |
| WO2019080226A1 (zh) | 取代的苯甲酰基二酮腈类化合物或其互变异构体、盐、制备方法、除草组合物及应用 | |
| CN110128352A (zh) | 哒嗪醇类化合物及其衍生物、制备方法、除草组合物和应用 | |
| US10781177B2 (en) | Pyridine compound and use thereof | |
| CN110054596B (zh) | 一种取代噁二唑类化合物及其应用 | |
| CN108084108A (zh) | 一种5-氯苯并噁唑衍生物及其制备方法、除草组合物和应用 | |
| EA024229B1 (ru) | Соединения, регулирующие рост растений | |
| JP2004508309A (ja) | 除草活性を持った5−ベンジルオキシメチル−1,2−イソオキサゾリン誘導体 | |
| WO2019080227A1 (zh) | 取代的苯甲酰基异恶唑类化合物或其互变异构体、盐、制备方法、除草组合物及应用 | |
| CN110878081B (zh) | 吡啶环取代的哒嗪醇类化合物及其衍生物、制备方法、除草组合物和应用 | |
| CN112390727B (zh) | 一种羧酸肟酯类化合物及用途 | |
| CN111072568B (zh) | 含偶氮结构的苯基吡唑类化合物及其制备方法和应用 | |
| WO2019033590A1 (zh) | 取代的苯甲酰基环己二酮类化合物或其互变异构体、盐、制备方法、除草组合物及应用 | |
| CN112174889A (zh) | 取代的苯甲酰类化合物及其在农业中的应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11824473 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11824473 Country of ref document: EP Kind code of ref document: A1 |