WO2011034631A1 - Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses - Google Patents
Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses Download PDFInfo
- Publication number
- WO2011034631A1 WO2011034631A1 PCT/US2010/027879 US2010027879W WO2011034631A1 WO 2011034631 A1 WO2011034631 A1 WO 2011034631A1 US 2010027879 W US2010027879 W US 2010027879W WO 2011034631 A1 WO2011034631 A1 WO 2011034631A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- nucleic acid
- fetal
- maternal
- dna
- fetal nucleic
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6804—Nucleic acid analysis using immunogens
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6806—Preparing nucleic acids for analysis, e.g. for polymerase chain reaction [PCR] assay
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6809—Methods for determination or identification of nucleic acids involving differential detection
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6879—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for sex determination
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6888—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for detection or identification of organisms
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/5308—Immunoassay; Biospecific binding assay; Materials therefor for analytes not provided for elsewhere, e.g. nucleic acids, uric acid, worms, mites
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/154—Methylation markers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/38—Pediatrics
- G01N2800/385—Congenital anomalies
Definitions
- the technology in part relates to prenatal diagnostics and enrichment methods.
- Non-invasive prenatal testing is becoming a field of rapidly growing interest. Early detection of pregnancy-related conditions, including complications during pregnancy and genetic defects of the fetus is of crucial importance, as it allows early medical intervention necessary for the safety of both the mother and the fetus. Prenatal diagnosis has been conducted using cells isolated from the fetus through procedures such as chorionic villus sampling (CVS) or amniocentesis. However, these conventional methods are invasive and present an apprecia ble risk to both the mother and the fetus. The National Health Service currently cites a miscarriage rate of between 1 and 2 per cent following the invasive amniocentesis and chorionic villus sampling (CVS) tests.
- CVS chorionic villus sampling
- Circulating cell free fetal nucleic acid has several advantages making it more applica ble for non-invasive prenatal testing. For example, cell free nucleic acid is present at higher levels than fetal cells and at concentrations sufficient for genetic analysis. Also, cffNA is cleared from the maternal bloodstream within hours after delivery, preventing contamination from previous pregnancies.
- Examples of prenatal tests performed by detecting fetal DNA in maternal plasma or serum include fetal rhesus D ( hD) genotyping (Lo et al., N. Engl. J. Med. 339:1734-1738, 1998), fetal sex determination (Costa et al., N. Engl. J. Med. 346: 1502, 2002), and diagnosis of several fetal disorders (Amicucci et al., Clin. Chem. 46:301-302, 2000; Saito et al., Lancet 356: 1170, 2000; and Chiu et al., Lancet 360:998-1000, 2002).
- the invention provides inter alia human epigenetic biomarkers that are useful for the noninvasive detection of fetal genetic traits, including, but not limited to, the presence or absence of fetal nucleic acid, the absolute or relative amount of fetal nucleic acid, fetal sex, and fetal chromosomal
- the human epigenetic biomarkers of the invention represent genomic DNA that display differential CpG methylation patterns between the fetus and mother.
- the compositions and processes of the invention allow for the detection and quantification of fetal nucleic acid in a maternal sample based on the methylation status of the nucleic acid in said sample. More specifically, the amount of fetal nucleic acid from a maternal sample can be determined relative to the total amount of nucleic acid present, thereby providing the percentage of fetal nucleic acid in the sample.
- the amount of fetal nucleic acid can be determined in a sequence-specific (or locus- specific) manner and with sufficient sensitivity to allow for accurate chromosomal dosage analysis (for example, to detect the presence or a bsence of a fetal aneuploidy).
- a method for enriching fetal nucleic acids from a maternal biological sample, based on differential methylation between fetal and maternal nucleic acid comprising the steps of: (a) binding a target nucleic acid, from a sample, and a control nucleic acid, from the sample, to a methylation-specific binding protein; and (b) eluting the bound nucleic acid based on methylation status, wherein differentially methylated nucleic acids elute at least partly into separate fractions.
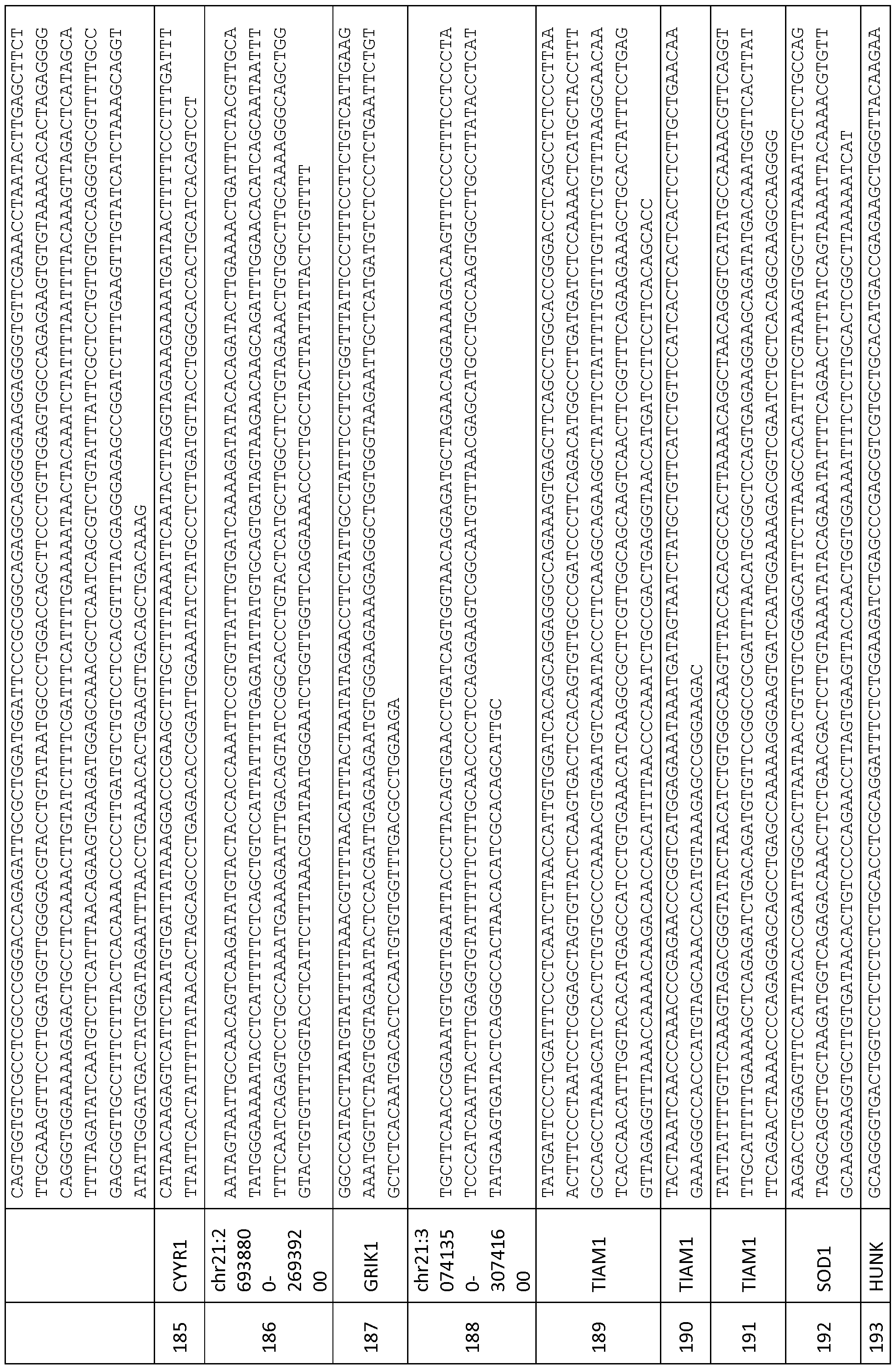
- the nucleic acid sequence includes one or more of the polynucleotide sequences of SEQ ID NOs: 1-261. SEQ ID NOs: 1-261 are provided in Tables 4A-4C.
- the invention includes the sequences of SEQ ID NOs: 1-261, and variations thereto.
- a control nucleic acid is not included in step (a).
- a method for enriching fetal nucleic acid from a maternal sample comprises the following steps: (a) obtaining a biological sample from a woman; (b) separating fetal and maternal nucleic acid based on the methylation status of a CpG-containing genomic sequence in the sample, wherein the genomic sequence from the fetus and the genomic sequence from the woman are differentially methylated, thereby distinguishing the genomic sequence from the woman and the genomic sequence from the fetus in the sample.
- the genomic sequence is at least 15 nucleotides in length, comprising at least one cytosine, further wherein the region consists of (1) a genomic locus selected from Tables 1A-1C; and (2) a DNA sequence of no more than 10 kb upstream and/or downstream from the locus.
- obtaining a biological sample from a woman is not meant to limit the scope of the invention. Said obtaining can refer to actually drawing a sample from a woman (e.g., a blood draw) or to receiving a sample from elsewhere (e.g., from a clinic or hospital) and performing the remaining steps of the method.
- a method for enriching fetal nucleic acid from a maternal sample comprises the following steps: (a) obtaining a biological sample from the woman; (b) digesting or removing maternal nucleic acid based on the methylation status of a CpG-containing genomic sequence in the sample, wherein the genomic sequence from the fetus and the genomic sequence from the woman are differentially methylated, thereby enriching for the genomic sequence from the fetus in the sample.
- Maternal nucleic acid may be digested using one or more methylation sensitive restriction enzymes that selectively digest or cleave maternal nucleic acid based on its methylation status.
- the genomic sequence is at least 15 nucleotides in length, comprising at least one cytosine, further wherein the region consists of (1) a genomic locus selected from Tables 1A-1C; and (2) a DNA sequence of no more than 10 kb upstream and/or downstream from the locus.
- a method for preparing nucleic acid having a nucleotide sequence of a fetal nucleic acid comprises the following steps: (a) providing a sample from a pregnant female; (b) separating fetal nucleic acid from maternal nucleic acid from the sample of the pregnant female according to a different methylation state between the fetal nucleic acid and the maternal nucleic acid counterpart, wherein the nucleotide sequence of the fetal nucleic acid comprises one or more CpG sites from one or more of the polynucleotide sequences of SEQ ID NOs: 1-261 within a polynucleotide sequence from a gene or locus that contains one of the polynucleotide sequences of SEQ ID NOs: 1-261; and (c) preparing nucleic acid comprising a nucleotide sequence of the fetal nucleic acid by an amplification process in which fetal nucleic acid separated in part (b
- a method for preparing nucleic acid having a nucleotide sequence of a fetal nucleic acid comprises the following steps: (a) providing a sample from a pregnant female; (b) digesting or removing maternal nucleic acid from the sample of the pregnant female according to a different methylation state between the fetal nucleic acid and the maternal nucleic acid counterpart, wherein the nucleotide sequence of the fetal nucleic acid comprises one or more CpG sites from one or more of the polynucleotide sequences of SEQ ID NOs: 1-261 within a polynucleotide sequence from a gene that contains one of the polynucleotide sequences of SEQ ID NOs: 1-261; and (c) preparing nucleic acid comprising a nucleotide sequence of the fetal nucleic acid.
- the preparing process of step (c) may be a hybridization process, a capture process, or an amplification process in which fetal nucleic acid separated in part (b) is utilized as a template.
- the maternal nucleic acid may be digested using one or more methylation sensitive restriction enzymes that selectively digest or cleave maternal nucleic acid based on its methylation status.
- the polynucleotide sequences of SEQ ID NOs: 1-261 may be within a polynucleotide sequence from a CpG island that contains one of the polynucleotide sequences of SEQ ID NOs: 1-261.
- polynucleotide sequences of SEQ ID NOs: 1-261 are further characterized in Tables 1-3 herein, including the identification of CpG islands that overlap with the polynucleotide sequences provided in SEQ ID NOs: 1-261.
- the nucleic acid prepared by part (c) is in solution.
- the method further comprises quantifying the fetal nucleic acid from the amplification process of step (c).
- a method for enriching fetal nucleic acid from a sample from a pregnant female with respect to maternal nucleic acid comprises the following steps: (a) providing a sample from a pregnant female; and (b) separating or capturing fetal nucleic acid from maternal nucleic acid from the sample of the pregnant female according to a different methylation state between the fetal nucleic acid and the maternal nucleic acid, wherein the nucleotide sequence of the fetal nucleic acid comprises one or more CpG sites from one or more of the polynucleotide sequences of SEQ ID NOs: 1-261 within a polynucleotide sequence from a gene that contains one of the
- polynucleotide sequences of SEQ ID NOs: 1-261 may be within a polynucleotide sequence from a CpG island that contains one of the polynucleotide sequences of SEQ ID NOs: 1-261.
- the polynucleotide sequences of SEQ ID NOs: 1-261 are characterized in Tables 1A-1C herein.
- the nucleic acid separated by part (b) is in solution.
- the method further comprises amplifying and/or quantifying the fetal nucleic acid from the separation process of step (b).
- a composition comprising an isolated nucleic acid from a fetus of a pregnant female, wherein the nucleotide sequence of the nucleic acid comprises one or more of the polynucleotide sequences of SEQ ID NOs: 1-261.
- the nucleotide sequence consists essentially of a nucleotide sequence of a gene, or portion thereof.
- the nucleotide sequence consists essentially of a nucleotide sequence of a CpG island, or portion thereof.
- the polynucleotide sequences of SEQ ID NOs: 1-261 are further characterized in Tables 1A-1C.
- the nucleic acid is in solution.
- the nucleic acid from the fetus is enriched relative to maternal nucleic acid.
- the composition further comprises an agent that binds to methylated nucleotides.
- the agent may be a methyl-CpG binding protein (MBD) or fragment thereof.
- a composition comprising an isolated nucleic acid from a fetus of a pregnant female, wherein the nucleotide sequence of the nucleic acid comprises one or more CpG sites from one or more of the polynucleotide sequences of SEQ ID NOs: 1-261 within a
- the nucleotide sequence of the nucleic acid comprises one or more CpG sites from one or more of the polynucleotide sequences of SEQ ID NOs: 1- 261 within a polynucleotide sequence from a CpG island, or portion thereof, that contains one of the polynucleotide sequences of SEQ ID NOs: 1-261.
- the polynucleotide sequences of SEQ ID NOs: 1-261 are further characterized in Tables 1A-1C.
- the nucleic acid is in solution.
- the nucleic acid from the fetus is enriched relative to maternal nucleic acid.
- Hyper- and hypomethylated nucleic acid sequences of the invention are identified in Tables 1A-1C.
- the composition further comprises an agent that binds to methylated nucleotides.
- the agent may be a methyl-CpG binding protein (MBD) or fragment thereof.
- a nucleotide sequence of the invention includes three or more of the CpG sites. In an embodiment, the nucleotide sequence includes five or more of the CpG sites. In an embodiment, the nucleotide sequence is from a gene region that comprises a P C2 domain (see Table 3). In an embodiment, the nucleotide sequence is from a gene region involved with development. For example, SOX14 - which is an epigenetic marker of the present invention (See Table 1) - is a member of the SOX (SRY-related HMG-box) family of transcription factors involved in the regulation of embryonic development and in the determination of cell fate.
- the genomic sequence from the woman is methylated and the genomic sequence from the fetus is unmethylated. In other embodiments, the genomic sequence from the woman is unmethylated and the genomic sequence from the fetus is methylated. In an embodiment, the genomic sequence from the fetus is hypermethylated relative to the genomic sequence from the mother.
- Fetal genomic sequences found to be hypermethylated relative to maternal genomic sequence are provided in SEQ ID NOs: 1-59, 90-163, 176, 179, 180, 184, 188, 189, 190, 191, 193, 195, 198, 199, 200, 201, 202, 203, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 221, 223, 225, 226, 231, 232, 233, 235, 239, 241, 257, 258, 259, and 261.
- the genomic sequence from the fetus is hypomethylated relative to the genomic sequence from the mother.
- Fetal genomic sequences found to be hypomethylated relative to maternal genomic sequence are provided in SEQ ID NOs: 60-85, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 177, 178, 181, 182, 183, 185, 186, 187, 192, 194, 196, 197, 204, 215, 216, 217, 218, 219, 220, 222, 224, 227, 228, 229, 230, 234, 236, 237, 238, 240, 242, 243, 244, 245, 246, 247, 248, 249, 250, 251, 252, 253, 254, 255, 256, and 260.
- Methylation sensitive restriction enzymes of the invention may be sensitive to hypo- or hyper- methylated nucleic acid.
- the fetal nucleic acid is extracellular nucleic acid. Generally the extracellular fetal nucleic acid is about 500, 400, 300, 250, 200 or 150 (or any number there between) nucleotide bases or less. In an embodiment, the digested maternal nucleic acid is less than about 90, 100, 110, 120, 130, 140 or 150 base pairs. In a related embodiment, the fetal nucleic acid is selectively amplified, captured or separated from or relative to the digested maternal nucleic acid based on size.
- PC primers may be designed to amplify nucleic acid greater than about 75, 80, 85, 90, 95, 100, 105, 110, 115 or 120 (or any number there between) base pairs thereby amplifying fetal nucleic acid and not digested maternal nucleic acid.
- the nucleic acid is subjected to fragmentation prior to the methods of the invention. Examples of methods of fragmenting nucleic acid, include but are not limited to sonication and restriction enzyme digestion.
- the fetal nucleic acid is derived from the placenta. In other embodiments the fetal nucleic acid is apoptotic.
- the present invention provides a method in which the sample is a member selected from the following: maternal whole blood, maternal plasma or serum, amniotic fluid, a chorionic villus sample, biopsy material from a pre-implantation embryo, fetal nucleated cells or fetal cellular remnants isolated from maternal blood, maternal urine, maternal saliva, washings of the female reproductive tract and a sample obtained by celocentesis or lung lavage.
- the biological sample is maternal blood.
- the biological sample is a chorionic villus sample.
- the maternal sample is enriched for fetal nucleic acid prior to the methods of the present invention. Examples of fetal enrichment methods are provided in PCT
- all nucleated and anucleated cell populations are removed from the sample prior to practicing the methods of the invention.
- the sample is collected, stored or transported in a manner known to the person of ordinary skill in the art to minimize degradation or the quality of fetal nucleic acid present in the sample.
- the sample can be from any animal, including but not limited, human, non-human, mammal, reptile, cattle, cat, dog, goat, swine, pig, monkey, ape, gorilla, bull, cow, bear, horse, sheep, poultry, mouse, rat, fish, dolphin, whale, and shark, or any animal or organism that may have a detectable pregnancy- associated disorder or chromosomal abnormality.
- the sample is treated with a reagent that differentially modifies methylated and unmethylated DNA.
- the reagent may comprise bisulfite; or the reagent may comprise one or more enzymes that preferentially cleave methylated DNA; or the reagent may comprise one or more enzymes that preferentially cleave unmethylated DNA.
- methylation sensitive restriction enzymes include, but are not limited to, Hhal and Hpall.
- the fetal nucleic acid is separated from the maternal nucleic acid by an agent that specifically binds to methylated nucleotides in the fetal nucleic acid. In an embodiment, the fetal nucleic acid is separated or removed from the maternal nucleic acid by an agent that specifically binds to methylated nucleotides in the maternal nucleic acid counterpart. In an embodiment, the agent that binds to methylated nucleotides is a methyl-CpG binding protein (MBD) or fragment thereof.
- MBD methyl-CpG binding protein
- a method for determining the amount or copy number of fetal DNA in a maternal sample that comprises differentially methylated maternal and fetal DNA.
- the method is performed by a) distinguishing between the maternal and fetal DNA based on differential methylation status; and b) quantifying the fetal DNA of step a).
- the method comprises a) digesting the maternal DNA in a maternal sample using one or more methylation sensitive restriction enzymes thereby enriching the fetal DNA; and b) determining the amount of fetal DNA from step a).
- the amount of fetal DNA can be used inter alia to confirm the presence or absence of fetal nucleic acid, determine fetal sex, diagnose fetal disease or a pregnancy-associated disorder, or be used in conjunction with other fetal diagnostic methods to improve sensitivity or specificity.
- the method for determining the amount of fetal DNA does not require the use of a polymorphic sequence. For example, an allelic ratio is not used to quantify the fetal DNA in step b).
- the method for determining the amount of fetal DNA does not require the treatment of DNA with bisulfite to convert cytosine residues to uracil.
- determining the amount of fetal DNA in step b) is done by introducing one or more competitors at known concentrations. In an embodiment, determining the amount of fetal DNA in step b) is done by T-PC , primer extension, sequencing or counting. In a related embodiment, the amount of nucleic acid is determined using BEAMing technology as described in US Patent Publication No. US20070065823. In a another related embodiment, the amount of nucleic acid is determined using the shotgun sequencing technology described in US Patent Publication No. US20090029377 (US Application No. 12/178,181), or variations thereof.
- the restriction efficiency is determined and the efficiency rate is used to further determine the amount of fetal DNA.
- Exemplary differentially methylated nucleic acids are provided in SEQ ID NOs: 1-261.
- a method for determining the concentration of fetal DNA in a maternal sample comprising a) determining the total amount of DNA present in the maternal sample; b) selectively digesting the maternal DNA in a maternal sample using one or more methylation sensitive restriction enzymes thereby enriching the fetal DNA; c) determining the amount of fetal DNA from step b); and d) comparing the amount of fetal DNA from step c) to the total amount of DNA from step a), thereby determining the concentration of fetal DNA in the maternal sample.
- the concentration of fetal DNA can be used inter alia in conjunction with other fetal diagnostic methods to improve sensitivity or specificity.
- the method for determining the amount of fetal DNA does not require the use of a polymorphic sequence. For example, an allelic ratio is not used to quantify the fetal DNA in step b).
- the method for determining the amount of fetal DNA does not require the treatment of DNA with bisulfite to convert cytosine residues to uracil.
- determining the amount of fetal DNA in step b) is done by introducing one or more competitors at known concentrations.
- determining the amount of fetal DNA in step b) is done by T-PC , sequencing or counting.
- the restriction efficiency is determined and used to further determine the amount of total DNA and fetal DNA.
- Exemplary differentially methylated nucleic acids are provided in SEQ ID NOs: 1-261.
- a method for determining the presence or absence of a fetal aneuploidy using fetal DNA from a maternal sample wherein the maternal sample comprises differentially methylated maternal and fetal DNA, comprising a) selectively digesting the maternal DNA in a maternal sample using one or more methylation sensitive restriction enzymes thereby enriching the fetal DNA; b) determining the amount of fetal DNA from a target chromosome; c) determining the amount of fetal DNA from a reference chromosome; and d) comparing the amount of fetal DNA from step b) to step c), wherein a biologically or statistically significant difference between the amount of target and reference fetal DNA is indicative of the presence of a fetal aneuploidy.
- the method for determining the amount of fetal DNA does not require the use of a polymorphic sequence. For example, an allelic ratio is not used to quantify the fetal DNA in step b). In an embodiment, the method for determining the amount of fetal DNA does not require the treatment of DNA with bisulfite to convert cytosine residues to uracil. In one embodiment, determining the amount of fetal DNA in steps b) and c) is done by introducing one or more competitors at known concentrations. In an embodiment, determining the amount of fetal DNA in steps b) and c) is done by RT-PCR, sequencing or counting.
- the amount of fetal DNA from a target chromosome determined in step b) is compared to a standard control, for example, the amount of fetal DNA from a target chromosome from euploid pregnancies.
- the restriction efficiency is determined and used to further determine the amount of fetal DNA from a target chromosome and from a reference chromosome.
- Exemplary differentially methylated nucleic acids are provided in SEQ ID NOs: 1-261.
- a method for detecting the presence or absence of a chromosomal abnormality by analyzing the amount or copy number of target nucleic acid and control nucleic acid from a sample of differentially methylated nucleic acids comprising the steps of: (a) enriching a target nucleic acid, from a sample, and a control nucleic acid, from the sample, based on its methylation state; (b) performing a copy number analysis of the enriched target nucleic acid in at least one of the fractions; (c) performing a copy number analysis of the enriched control nucleic acid in at least one of the fractions; (d) comparing the copy number from step (b) with the copy number from step (c); and (e) determining if a chromosomal abnormality exists based on the comparison in step (d), wherein the target nucleic acid and control nucleic acid have the same or substantially the same methylation status.
- a method for detecting the presence or absence of a chromosomal abnormality by analyzing the amount or copy number of target nucleic acid and control nucleic acid from a sample of differentially methylated nucleic acids comprising the steps of: (a) binding a target nucleic acid, from a sample, and a control nucleic acid, from the sample, to a binding agent; (b) eluting the bound nucleic acid based on methylation status, wherein differentially methylated nucleic acids elute at least partly into separate fractions; (c) performing a copy number analysis of the eluted target nucleic acid in at least one of the fractions; (d) performing a copy number analysis of the eluted control nucleic acid in at least one of the fractions; (e) comparing the copy number from step (c) with the copy number from step (d); and (f) determining if a chromosomal abnormality exists based on the comparison in step (e),
- a method for detecting the presence or absence of a chromosomal abnormality by analyzing the allelic ratio of target nucleic acid and control nucleic acid from a sample of differentially methylated nucleic acids comprising the steps of: (a) binding a target nucleic acid, from a sample, and a control nucleic acid, from the sample, to a binding agent; (b) eluting the bound nucleic acid based on methylation status, wherein differentially methylated nucleic acids elute at least partly into separate fractions; (c) performing an allelic ratio analysis of the eluted target nucleic acid in at least one of the fractions; (d) performing an allelic ratio analysis of the eluted control nucleic acid in at least one of the fractions; (e) comparing the allelic ratio from step c with the all from step d; and (f) determining if a chromosomal abnormality exists based on the comparison in step
- the amount of maternal nucleic acid is determined using the methylation-based methods of the invention.
- fetal nucleic acid can be separated (for example, digested using a methylation-sensitive enzyme) from the maternal nucleic acid in a sample, and the maternal nucleic acid can be quantified using the methods of the invention.
- the amount of maternal nucleic acid is determined, that amount can subtracted from the total amount of nucleic acid in a sample to determine the amount of fetal nucleic acid.
- the amount of fetal nucleic acid can be used to detect fetal traits, including fetal aneuploidy, as described herein.
- the methods may also be useful for detecting a pregnancy-associated disorder.
- the sample comprises fetal nucleic acid, or fetal nucleic acid and maternal nucleic acid.
- the fetal nucleic acid and the maternal nucleic acid may have a different methylation status. Nucleic acid species with a different methylation status can be differentiated by any method known in the art.
- the fetal nucleic acid is enriched by the selective digestion of maternal nucleic acid by a methylation sensitive restriction enzyme.
- the fetal nucleic acid is enriched by the selective digestion of maternal nucleic acid using two or more methylation sensitive restriction enzymes in the same assay.
- the target nucleic acid and control nucleic acid are both from the fetus.
- the average size of the fetal nucleic acid is about 100 bases to about 500 bases in length.
- the chromosomal abnormality is an aneuploidy, such as trisomy 21.
- the target nucleic acid is at least a portion of a chromosome which may be abnormal and the control nucleic acid is at least a portion of a chromosome which is very rarely abnormal.
- the control nucleic acid is from a chromosome other than chromosome 21 - preferably another autosome.
- the binding agent is a methylation-specific binding protein such as MBD-Fc.
- the enriched or eluted nucleic acid is amplified and/or quantified by any method known in the art.
- the fetal DNA is quantified using a method that does not require the use of a polymorphic sequence. For example, an allelic ratio is not used to quantify the fetal DNA.
- the method for quantifying the amount of fetal DNA does not require the treatment of DNA with bisulfite to convert cytosine residues to uracil.
- the methods of the invention include the additional step of determining the amount of one or more Y-chromosome-specific sequences in a sample.
- the amount of fetal nucleic acid in a sample as determined by using the methylation-based methods of the invention is compared to the amount of Y-chromosome nucleic acid present.
- Methods for differentiating nucleic acid based on methylation status include, but are not limited to, methylation sensitive capture, for example using, MBD2-Fc fragment; bisulfite conversion methods, for example, MSP (methylation-sensitive PC ), COBRA, methylation-sensitive single nucleotide primer extension (Ms-SNuPE) or Sequenom MassCLEAVETM technology; and the use of methylation sensitive restriction enzymes.
- MSP methylation-sensitive PC
- COBRA methylation-sensitive single nucleotide primer extension
- Sequenom MassCLEAVETM technology Sequenom MassCLEAVETM technology
- any method for differentiating nucleic acid based on methylation status can be used with the compositions and methods of the invention.
- methods of the invention may further comprise an amplification step.
- the amplification step can be performed by PCR, such as methylation-specific PCR.
- the amplification reaction is performed on single molecules, for example, by digital PCR, which is further described in US Patent Nos 6,143,496 and 6,440,706, both of which are hereby incorporated by reference.
- the method does not require amplification.
- the amount of enriched fetal DNA may be determined by counting the fetal DNA (or sequence tags attached thereto) with a flow cytometer or by sequencing means that do not require amplification.
- the amount of fetal DNA is determined by an amplification reaction that generates amplicons larger than the digested maternal nucleic acid, thereby further enriching the fetal nucleic acid.
- the fetal nucleic acid (alone or in combination with the maternal nucleic acid) comprises one or more detection moieties.
- the detection moiety may be any one or more of a compomer, sugar, peptide, protein, antibody, chemical compound (e.g., biotin), mass tag (e.g., metal ions or chemical groups), fluorescent tag, charge tag (e.g., such as polyamines or charged dyes) and hydrophobic tag.
- the detection moiety is a mass-distinguishable product (MDP) or part of an MDP detected by mass spectrometry.
- the detection moiety is a fluorescent tag or label that is detected by mass spectrometry.
- the detection moiety is at the 5' end of a detector oligonucleotide, the detection moiety is attached to a non-complementary region of a detector oligonucleotide, or the detection moiety is at the 5' terminus of a non-complementary sequence.
- the detection moiety is incorporated into or linked to an internal nucleotide or to a nucleotide at the 3' end of a detector oligonucleotide.
- one or more detection moieties are used either alone or in combination. See for example US Patent Applications US20080305479 and US20090111712.
- a detection moiety is cleaved by a restriction endonuclease, for example, as described in US Application No. 12/726,246.
- a specific target chromosome is labeled with a specific detection moiety and one or more non-target chromosomes are labeled with a different detection moiety, whereby the amount target chromsome can be compared to the amount of non- target chromosome.
- any one of the following sequencing technologies may be used: a primer extension method (e.g., iPLEX ® ; Sequenom, Inc.), direct DNA sequencing, restriction fragment length polymorphism (RFLP analysis), real-time PCR, for example using "STAR" (Scalable Transcription Analysis Routine) technology (see US Patent No.
- Nanopore-based methods may include sequencing nucleic acid using a nanopore, or counting nucleic acid molecules using a nanopore, for example, based on size wherein sequence information is not determined.
- the absolute copy number of one or more nucleic acids can be determined, for example, using mass spectrometry, a system that uses a competitive PCR approach for absolute copy number measurements. See for example, Ding C, Cantor CR (2003) A high-throughput gene expression analysis technique using competitive PCR and matrix-assisted laser desorption ionization time-of-flight MS. Proc Natl Acad Sci U S A 100:3059-3064, and US Patent Application No. 10/655762, which published as US Patent Publication No. 20040081993, both of which are hereby incorporated by reference.
- the amount of the genomic sequence is compared with a standard control, wherein an increase or decrease from the standard control indicates the presence or progression of a pregnancy-associated disorder.
- the amount of fetal nucleic acid may be compared to the total amount of DNA present in the sample.
- the amount of fetal nucleic acid from target chromosome may be compared to the amount of fetal nucleic acid from a reference chromosome.
- the reference chromosome is another autosome that has a low rate of aneuploidy.
- the ratio of target fetal nucleic acid to reference fetal nucleic acid may be compared to the same ratio from a normal, euploid pregnancy.
- a control ratio may be determined from a DNA sample obtained from a female carrying a healthy fetus who does not have a chromosomal abnormality.
- chromosome anomalies are known, one can also have standards that are indicative of a specific disease or condition.
- a panel of control DNAs that have been isolated from mothers who are known to carry a fetus with, for example, chromosome 13, 18, or 21 trisomy, and a mother who is pregnant with a fetus who does not have a chromosomal abnormality.
- the present invention provides a method in which the alleles from the target nucleic acid and control nucleic acid are differentiated by sequence variation.
- the sequence variation may be a single nucleotide polymorphism (SNP) or an insertion/deletion polymorphism.
- the fetal nucleic acid should comprise at least one high frequency heterozygous polymorphism (e.g., about 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%, 25% or more frequency rate), which allows the determination of the allelic-ratio of the nucleic acid in order to assess the presence or absence of the chromosomal abnormality.
- a list of exemplary SNPs is provided in Table 2, however, this does not represent a complete list of polymorphic alleles that can be used as part of the invention.
- any SNP meeting the following criteria may also be considered: (a) the SNP has a heterozygosity frequency greater than about 2% (preferably across a range of different populations), (b) the SNP is a heterozygous locus; and (c)(i) the SNP is within nucleic acid sequence described herein, or (c)(iii) the SNP is within about 5 to about 2000 base pairs of a SNP described herein (e.g., within about 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000, 1250, 1500, 1750 or 2000 base pairs of a SNP described herein).
- the sequence variation is a short tandem repeat (ST ) polymorphism.
- the sequence variation falls in a restriction site, whereby one allele is susceptible to digestion by a restriction enzyme and the one or more other alleles are not.
- the sequence variation is a methylation site.
- performing an allelic ratio analysis comprises determining the ratio of alleles of the target nucleic acid and control nucleic acid from the fetus of a pregnant woman by obtaining an nucleic acid-containing biological sample from the pregnant woman, wherein the biological sample contains fetal nucleic acid, partially or wholly separating the fetal nucleic acid from the maternal nucleic acid based on differential methylation, discriminating the alleles from the target nucleic acid and the control nucleic acid, followed by determination of the ratio of the alleles, and detecting the presence or absence of a chromosomal disorder in the fetus based on the ratio of alleles, wherein a ratio above or below a normal, euploid ratio is indicative of a chromosomal disorder.
- the target nucleic acid is from a suspected aneuploid chromosome (e.g., chromosome 21) and the control nucleic acid is from a euploid chromosome from a suspected aneup
- the present invention is combined with other fetal markers to detect the presence or a bsence of multiple chromosomal abnormalities, wherein the chromosomal abnormalities are selected from the following: trisomy 21, trisomy 18 and trisomy 13, or combinations thereof.
- the chromosomal disorder involves the X chromosome or the Y chromosome.
- the compositions or processes may be multiplexed in a single reaction.
- the amount of fetal nucleic acid may be determined at multiple loci across the genome.
- the amount of fetal nucleic acid may be determined at multiple loci on one or more target chromosomes (e.g., chromosomes 13, 18 or 21) and on one or more reference chromosomes. If an allelic ratio is being used, one or more alleles from Table 2 can be detected and discriminated simultaneously. When determining allelic ratios, multiplexing embodiments are particularly important when the genotype at a polymorphic locus is not known.
- the assay may not be informative. In one embodiment, greater than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 30, 35, 40, 50, 100, 200, 300 or 500, and any intermediate levels,
- polynucleotide sequences of the invention are enriched, separated and/or examined according the methods of the invention.
- detecting a chromosomal abnormality by analyzing the copy number of target nucleic acid and control nucleic acid less than 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, or 14 polynucleotide sequences may need to be analyzed to accurately detect the presence or absence of a chromosomal abnormality.
- the compositions or processes of the invention may be used to assay samples that have been divided into 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 30, 35, 40, 50, 100 or more replicates, or into single molecule equivalents.
- the present invention provides a method wherein a comparison step shows an increased risk of a fetus having a chromosomal disorder if the ratio of the alleles or absolute copy number of the target nucleic acid is higher or lower by 1 standard deviation from the standard control sequence.
- the comparison step shows an increased risk of a fetus having a chromosomal disorder if the ratio of the alleles or absolute copy number of the target nucleic acid is higher or lower by 2 standard deviation from the standard control sequence. In some other embodiments, the comparison step shows an increased risk of a fetus having a chromosomal disorder if the ratio of the alleles or absolute copy number of the target nucleic acid is higher or lower by 3 standard deviation from the standard control sequence. In some embodiments, the comparison step shows an increased risk of a fetus having a chromosomal disorder if the ratio of the alleles or absolute copy number of the target nucleic acid is higher or lower than a statistically significant standard deviation from the control. In one embodiment, the standard control is a maternal reference, and in an embodiment the standard control is a fetal reference chromosome (e.g., non-trisomic autosome).
- the standard control is a maternal reference
- the standard control is a fetal
- the methods of the invention may be combined with other methods for diagnosing a chromosomal abnormality.
- a noninvasive diagnostic method may require confirmation of the presence or absence of fetal nucleic acid, such as a sex test for a female fetus or to confirm an hD negative female fetus in an RhD negative mother.
- the compositions and methods of the invention may be used to determine the percentage of fetal nucleic acid in a maternal sample in order to enable another diagnostic method that requires the percentage of fetal nucleic acid be known. For example, does a sample meet certain threshold concentration
- the amount or concentration of fetal nucleic acid may be required to make a diagnose with a given sensitivity and specificity.
- the compositions and methods of the invention for detecting a chromosomal abnormality can be combined with other known methods thereby improving the overall sensitivity and specificity of the detection method.
- an increased risk for a chromosomal abnormality is based on the outcome or result(s) produced from the compositions or methods provided herein.
- An example of an outcome is a deviation from the euploid absolute copy number or allelic ratio, which indicates the presence of chromosomal aneuploidy. This increase or decrease in the absolute copy number or ratio from the standard control indicates an increased risk of having a fetus with a chromosomal abnormality (e.g., trisomy 21).
- Information pertaining to a method described herein, such as an outcome, result, or risk of trisomy or aneuploidy, for example, may be transfixed, renditioned, recorded and/or displayed in any suita ble medium.
- an outcome may be transfixed in a medium to save, store, share, commu nicate or otherwise analyze the outcome.
- a medium can be ta ngible (e.g., paper) or intangible (e.g., electronic medium), and examples of media include, but are not limited to, computer media, data bases, charts, patient charts, records, patient records, graphs and tables, a nd any other medium of expression.
- the information sometimes is stored and/or renditioned in computer reada ble form and sometimes is stored and organized in a data base.
- the information may be transferred from one location to another using a physical medium (e.g., paper) or a computer reada ble medium (e.g., optical and/or magnetic storage or transmission medium, floppy disk, hard disk, random access memory, computer processing unit, facsimile signal, satellite signal, transmission over an internet or transmission over the world-wide web).
- a physical medium e.g., paper
- a computer reada ble medium e.g., optical and/or magnetic storage or transmission medium, floppy disk, hard disk, random access memory, computer processing unit, facsimile signal, satellite signal, transmission over an internet or transmission over the world-wide web.
- a CpG island may be used as the CpG-containing genomic sequence in some cases, whereas in other cases the CpG-containing genomic sequence may not be a CpG island.
- the present invention provides a kit for performing the methods of the invention.
- One component of the kit is a methylation-sensitive binding agent.
- FIGURE 1 Shows the design of the recombinant M BD-Fc protein used to separate differentially methylated DNA.
- FIGURE 2 Shows the methyl-CpG-binding, antibody-like protein has a high affinity and high avidity to its "antigen", which is prefera bly DNA that is methylated at CpG di-nucleotides.
- FIGURE 3 Shows the methyl binding domain of M BD-FC binds all DNA molecules regardless of their methylation status. The strength of this protein/DNA interaction is defined by the level of DNA methylation. After binding genomic DNA, eluate solutions of increasing salt concentrations can be used to fractionate non-methylated a nd methylated DNA allowing for a controlled separation.
- FIGURE 4 Shows the experiment used to identify differentially methylated DNA from a fetus and mother using the recom binant M BD-Fc protein and a microarray.
- FIGURE 5 Shows typical results generated by Sequenom ® EpiTYPERTM method, which was used to validate the results generated from the experiment illustrated in Figure 4.
- FIGURE 6 Shows the correlation between the log ratios derived from microarray analysis (x axis) and methylation differences obtained by EpiTYPER analysis (y axis). Each data point represents the average for one region across all measured samples.
- the microarray analysis is comparative in nature because the highly methylated fraction of the maternal DNA is hybridized together with the highly methylated fraction of placenta DNA. Positive values indicate higher methylation of the placenta samples. In mass spectrometry each samples is measured individually. We first calculated difference in methylation by subtracting the maternal methylation values from the placenta methylation value. To compare the results with the microarray data we calculated the average of the differences for all maternal / placenta DNA pairs.
- FIGURE 8 Shown is the correlation between the number of gDNA molecules that were expected and the number of molecules measured by competitive PCR in combination with mass spectrometry analysis.
- DNA derived from whole blood (black plus signs) and commercially available fully methylated DNA(red crosses) in a 90 to 10 ratio.
- MBD-FC fusion protein to separate the non-methylated and the methylated fraction of DNA. Each fraction was subject to competitive PCR analysis with mass spectrometry readout.
- the method has been described earlier for the analysis of copy number variations and is commercially available for gene expression analysis. The approach allows absolute quantification of DNA molecules with the help of a synthetic oligonucleotides of know concentration.
- FIGURE 9A-9C Shown are bar graph plots of the methylation differences obtained from the microarray analysis (dark bars) and the mass spectrometry analysis (light grey bars) with respect to their genomic location.
- the x axis for each plot shows the chromosomal position of the region.
- the y axis depicts the log ration (in case of the microarrays) and the methylation differences (in case of the mass spectrometry results).
- each hybridization probe in the area is shown as a single black (or dark grey) bar.
- Bars showing values greater than zero indicate higher DNA methylation in the placenta samples compared to the maternal DNA. For some genes the differences are small (i.e. RBI or DSCR6) but still statistically significant. Those regions would be less suitable for a fetal DNA enrichment strategy.
- FIGURE 10 Shows one embodiment of the Fetal Quantifier Method. Maternal nucleic acid is selectively digested and the remaining fetal nucleic acid is quantified using a competitor of known concentration. In this schema, the analyte is separated and quantified by a mass spectromter.
- FIGURE 11 Shows one embodiment of the Methylation-Based Fetal Diagnostic Method.
- Maternal nucleic acid is selectively digested and the remaining fetal nucleic acid is quantified for three different chromosomes (13, 18 and 21).
- Parts 2 and 3 of the Figure illustrate the size distribution of the nucleic acid in the sample before and after digestion.
- the amplification reactions can be size-specific (e.g., greater than 100 base pair amplicons) such that they favor the longer, non-digested fetal nucleic acid over the digested maternal nucleic acid, thereby further enriching the fetal nucleic acid.
- the spectra at the bottom of the Figure show an increased amount of chromosome 21 fetal nucleic acid indicative of trisomy 21.
- FIGURE 12 Shows the total number of amplifiable genomic copies from four different DNA samples isolated from the blood of non-pregnant women. Each sample was diluted to contain approximately 2500, 1250, 625 or 313 copies per reaction. Each measurement was obtained by taking the mean DNA/competitor ratio obtained from two total copy number assays (ALB and RNAseP in Table X). As Figure 12 shows, the total copy number is accurate and stable across the different samples, thus validating the usefulness of the competitor-based approach.
- FIGURES 13A and B A model system was created that contained a constant number of maternal non- methylated DNA with varying amounts of male placental methylated DNA spiked-in. The samples were spiked with male placental amounts ranging from approximately 0 to 25% relative to the maternal non- methylated DNA. The fraction of placental DNA was calculated using the ratios obtained from the methylation assays ( Figure 13A) and the Y-chromosome marker ( Figure 13B) as compared to the total copy number assay. The methylation and Y-chromosome markers are provided in Table X.
- FIGURES 14 A and B Show the results of the total copy number assay from plasma samples.
- Figure 14A the copy number for each sample is shown. Two samples (no 25 and 26) have a significantly higher total copy number than all the other samples. A mean of approximately 1300 amplifiable copies/ml plasma was obtained (range 766-2055).
- Figure 14B shows a box-and-whisker plot of the given values, summarizing the results.
- FIGURES 15A and B The amount (or copy numbers) of fetal nucleic acid from 33 different plasma samples taken from pregnant women with male fetuses are plotted. The copy numbers obtained were calculated using the methylation markers and the Y-chromosome-specific markers using the assays provided in Table X. As can be seen in Figure 15B, the box-and-whisker plot of the given values indicated minimal difference between the two different measurements, thus validating the accuracy and stability of the method.
- FIGURE 16 Shows a paired correlation between the results obtained using the methylation markers versus the Y-chromosome marker from Figure 15A.
- FIGURE 17 Shows the digestion efficiency of the restriction enzymes using the ratio of digestion for the control versus the competitor and comparing this value to the mean total copy number assays. Apart from sample 26 all reactions indicate the efficiency to be above about 99%.
- FIGURE 18 Provides a specific method for calculating fetal DNA fraction (or concentration) in a sample using the Y-chromosome-specific markers for male pregnancies and the mean of the methylated fraction for all pregnancies (regardless of fetal sex).
- FIGURE 19 Provides a specific method for calculating fetal DNA fraction (or concentration) in a sample without the Y-chromosome-specific markers. Instead, only the Assays for Methylation Quantification were used to determine the concentration of fetal DNA.
- FIGURE 20 Shows a power calculation t-test for a simulated trisomy 21 diagnosis using the methods of the invention. The Figure shows the relationship between the coefficient of variation (CV) on the x-axis and the power to discriminate the assay populations using a simple t-test (y-axis). The data indicates that in 99% of all cases, one can discriminate the two population (euploid vs. aneuploid) on a significance level of 0.001 provided a CV of 5% or less.
- CV coefficient of variation
- pregnancy-associated disorder refers to any condition or disease that may affect a pregnant woman, the fetus, or both the woman and the fetus. Such a condition or disease may manifest its symptoms during a limited time period, e.g., during pregnancy or delivery, or may last the entire life span of the fetus following its birth.
- a pregnancy-associated disorder include ectopic pregnancy, preeclampsia, preterm labor, RhD incompatibility, fetal
- compositions and processes described herein are particularly useful for diagnosis, prognosis and monitoring of pregnancy-associated disorders associated with quantitative a bnormalities of fetal DNA in maternal plasma/serum, including but not limited to, preeclampsia (Lo et al., Clin. Chem. 45:184-188, 1999 and Zhong et al., Am. J. Obstet.
- Gynecol. 184:414-419, 2001 fetal trisomy (Lo et al., Clin. Chem. 45:1747-1751, 1999 and Zhong et al., Prenat. Diagn. 20:795-798, 2000) and hyperemesis gravidarum (Sekizawa et al., Clin. Chem. 47:2164- 2165, 2001).
- an elevated level of fetal nucleic acid in maternal blood (as compared to a normal pregnancy or pregnancies) may be indicative of a preeclamptic preganancy.
- the ability to enrich fetal nucleic from a maternal sample may prove particularly useful for the noninvasive prenatal diagnosis of autosomal recessive diseases such as the case when a mother and father share an identical disease causing mutation, an occurrence previously perceived as a challenge for maternal plasma-based non-trisomy prenatal diagnosis.
- chromosomal abnormality or "aneuploidy” as used herein refers to a deviation between the structure of the subject chromosome and a normal homologous chromosome.
- normal refers to the predominate karyotype or banding pattern found in healthy individuals of a particular species, for example, a euploid genome (in humans, 46XX or 46XY).
- a chromosomal abnormality can be numerical or structural, and includes but is not limited to aneuploidy, polyploidy, inversion, a trisomy, a monosomy, duplication, deletion, deletion of a part of a chromosome, addition, addition of a part of chromosome, insertion, a fragment of a chromosome, a region of a chromosome, chromosomal rearrangement, and translocation.
- Chromosomal abnormality may also refer to a state of chromosomal abnormality where a portion of one or more chromosomes is not an exact multiple of the usual haploid number due to, for example, chromosome translocation.
- Chromosomal translocation e.g.
- a chromosomal abnormality can be correlated with presence of a pathological condition or with a predisposition to develop a pathological condition.
- a chromosomal abnormality may be detected by quantitative analysis of nucleic acid.
- nucleic acid and “nucleic acid molecule” may be used interchangea bly throughout the disclosure.
- nucleic acids of any composition from, such as DNA (e.g., complementary DNA (cDNA), genomic DNA (gDNA) and the like), RNA (e.g., message RNA (mRNA), short inhibitory RNA (siRNA), ribosomal RNA (rRNA), tRNA, microRNA, RNA highly expressed by the fetus or placenta, and the like), and/or DNA or RNA analogs (e.g., containing base analogs, sugar analogs and/or a non-native backbone and the like), RNA/DNA hybrids and polyamide nucleic acids (PNAs), all of which can be in single- or dou ble-stranded form, and unless otherwise limited, can encompass known analogs of natural nucleotides that can function in a similar manner as naturally occurring nucleotides.
- DNA e.g., complementary DNA (cDNA), genomic DNA (gDNA) and the like
- RNA e.g., message RNA (mRNA), short inhibitory
- nucleic acids provided in SEQ ID NOs: 1-261 can be in any form useful for conducting processes herein (e.g., linear, circular, supercoiled, single-stranded, double-stranded and the like) or may include variations (e.g., insertions, deletions or substitutions) that do not alter their utility as part of the present invention.
- a nucleic acid may be, or may be from, a plasmid, phage, autonomously replicating sequence (ARS), centromere, artificial chromosome, chromosome, or other nucleic acid able to replicate or be replicated in vitro or in a host cell, a cell, a cell nucleus or cytoplasm of a cell in certain embodiments.
- a template nucleic acid in some embodiments can be from a single chromosome (e.g., a nucleic acid sample may be from one chromosome of a sample obtained from a diploid organism).
- nucleic acids containing known analogs of natural nucleotides that have similar binding properties as the reference nucleic acid and are metabolized in a manner similar to naturally occurring nucleotides Unless otherwise indicated, a particular nucleic acid sequence also implicitly encompasses conservatively modified variants thereof (e.g., degenerate codon substitutions), alleles, orthologs, single nucleotide polymorphisms (SNPs), and complementary sequences as well as the sequence explicitly indicated.
- degenerate codon substitutions may be achieved by generating sequences in which the third position of one or more selected (or all) codons is substituted with mixed-base and/or deoxyinosine residues (Batzer et al., Nucleic Acid Res. 19:5081 (1991); Ohtsuka et al., J. Biol. Chem. 260:2605-2608 (1985); and Rossolini et al., Mol. Cell. Probes 8:91- 98 (1994)).
- the term nucleic acid is used interchangeably with locus, gene, cDNA, and mRNA encoded by a gene.
- RNA or DNA synthesized from nucleotide analogs single-stranded ("sense” or “antisense”, “plus” strand or “minus” strand, "forward” reading frame or “reverse” reading frame) and double-stranded polynucleotides.
- Deoxyribonucleotides include deoxyadenosine, deoxycytidine, deoxyguanosine and deoxythymidine.
- the base cytosine is replaced with uracil.
- a template nucleic acid may be prepared using a nucleic acid obtained from a subject as a template.
- a "nucleic acid comprising one or more CpG sites” or a "CpG-containing genomic sequence” as used herein refers to a segment of DNA sequence at a defined location in the genome of an individual such as a human fetus or a pregnant woman.
- a "CpG-containing genomic sequence” is at least 15 nucleotides in length and contains at least one cytosine.
- it can be at least 30, 50, 80, 100, 150, 200, 250, or 300 nucleotides in length and contains at least 2, 5, 10, 15, 20, 25, or 30 cytosines.
- CpG-containing genomic sequence at a given location, e.g., within a region centering around a given genetic locus (see Tables 1A-1C), nucleotide sequence variations may exist from individual to individual and from allele to allele even for the same individual.
- a region centering around a defined genetic locus e.g., a CpG island
- Each of the upstream or downstream sequence (counting from the 5' or 3' boundary of the genetic locus, respectively) can be as long as 10 kb, in other cases may be as long as 5 kb, 2 kb, 1 kb, 500 bp, 200 bp, or 100 bp.
- a "CpG-containing genomic sequence” may encompass a nucleotide sequence transcribed or not transcribed for protein production, and the nucleotide sequence can be an inter-gene sequence, intra-gene sequence, protein-coding sequence, a non protein-coding sequence (such as a transcription promoter), or a combination thereof.
- a "methylated nucleotide” or a “methylated nucleotide base” refers to the presence of a methyl moiety on a nucleotide base, where the methyl moiety is not present in a recognized typical nucleotide base.
- cytosine does not contain a methyl moiety on its pyrimidine ring, but 5- methylcytosine contains a methyl moiety at position 5 of its pyrimidine ring. Therefore, cytosine is not a methylated nucleotide and 5-methylcytosine is a methylated nucleotide.
- thymine contains a methyl moiety at position 5 of its pyrimidine ring, however, for purposes herein, thymine is not considered a methylated nucleotide when present in DNA since thymine is a typical nucleotide base of DNA.
- Typical nucleoside bases for DNA are thymine, adenine, cytosine and guanine.
- Typical bases for NA are uracil, adenine, cytosine and guanine.
- a "methylation site" is the location in the target gene nucleic acid region where methylation has, or has the possibility of occurring. For example a location containing CpG is a methylation site wherein the cytosine may or may not be methylated.
- a "CpG site” or “methylation site” is a nucleotide within a nucleic acid that is susceptible to methylation either by natural occurring events in vivo or by an event instituted to chemically methylate the nucleotide in vitro.
- a "methylated nucleic acid molecule” refers to a nucleic acid molecule that contains one or more methylated nucleotides that is/are methylated.
- CpG island as used herein describes a segment of DNA sequence that comprises a functionally or structurally deviated CpG density.
- Yamada et al. (Genome Research 14:247-266, 2004) have described a set of standards for determining a CpG island: it must be at least 400 nucleotides in length, has a greater than 50% GC content, and an OCF/ECF ratio greater than 0.6.
- Others (Takai et al., Proc. Natl. Acad. Sci. U.S.A. 99:3740-3745, 2002) have defined a CpG island less stringently as a sequence at least 200 nucleotides in length, having a greater than 50% GC content, and an OCF/ECF ratio greater than 0.6.
- epigenetic state refers to any structural feature at a molecular level of a nucleic acid (e.g., DNA or RNA) other than the primary nucleotide sequence.
- a nucleic acid e.g., DNA or RNA
- the epigenetic state of a genomic DNA may include its secondary or tertiary structure determined or influenced by, e.g., its methylation pattern or its association with cellular proteins.
- methylation profile refers to the characteristics of a DNA segment at a particular genomic locus relevant to methylation. Such characteristics include, but are not limited to, whether any of the cytosine (C) residues within this DNA sequence are methylated, location of methylated C residue(s), percentage of methylated C at any particular stretch of residues, and allelic differences in methylation due to, e.g., difference in the origin of the alleles.
- methylation profile” or “methylation status” also refers to the relative or absolute concentration of methylated C or unmethylated C at any particular stretch of residues in a biological sample. For example, if the cytosine (C) residue(s) within a DNA sequence are methylated it may be referred to as "hypermethylated";
- cytosine (C) residue(s) within a DNA sequence are not methylated it may be referred to as "hypomethylated".
- the cytosine (C) residue(s) within a DNA sequence e.g., fetal nucleic acid
- the cytosine (C) residue(s) within a DNA sequence are methylated as compared to another sequence from a different region or from a different individual (e.g., relative to maternal nucleic acid)
- that sequence is considered hypermethylated compared to the other sequence.
- the cytosine (C) residue(s) within a DNA sequence are not methylated as compared to another sequence from a different region or from a different individual (e.g., the mother), that sequence is considered hypomethylated compared to the other sequence.
- sequences are said to be “differentially methylated", and more specifically, when the methylation status differs between mother and fetus, the sequences are considered “differentially methylated maternal and fetal
- agent that binds to methylated nucleotides refers to a substance that is capable of binding to methylated nucleic acid.
- the agent may be naturally-occurring or synthetic, and may be modified or unmodified. In one embodiment, the agent allows for the separation of different nucleic acid species according to their respective methylation states.
- An example of an agent that binds to methylated nucleotides is described in PCT Patent Application No. PCT/EP2005/012707, which published as WO06056480A2 and is hereby incorporated by reference.
- the described agent is a bifunctional polypeptide comprising the DNA-binding domain of a protein belonging to the family of Methyl-CpG binding proteins (MBDs) and an Fc portion of an antibody (see Figure 1).
- MBDs Methyl-CpG binding proteins
- the recombinant methyl-CpG-binding, antibody-like protein can preferably bind CpG methylated DNA in an antibody-like manner. That means, the methyl-CpG-binding, antibody-like protein has a high affinity and high avidity to its "antigen", which is preferably DNA that is methylated at CpG dinucleotides.
- the agent may also be a multivalent MBD (see Figure 2).
- polymorphism refers to a sequence variation within different alleles of the same genomic sequence.
- a sequence that contains a polymorphism is considered “polymorphic sequence”. Detection of one or more polymorphisms allows differentiation of different alleles of a single genomic sequence or between two or more individuals.
- polymorphic marker or “polymorphic sequence” refers to segments of genomic DNA that exhibit heritable variation in a DNA sequence between individuals.
- Such markers include, but are not limited to, single nucleotide polymorphisms (SNPs), restriction fragment length polymorphisms (RFLPs), short tandem repeats, such as di-, tri- or tetra-nucleotide repeats (STRs), and the like.
- SNPs single nucleotide polymorphisms
- RFLPs restriction fragment length polymorphisms
- STRs tetra-nucleotide repeats
- Polymorphic markers according to the present invention can be used to specifically differentiate between a maternal and paternal allele in the enriched fetal nucleic acid sample.
- single nucleotide polymorphism refers to the polynucleotide sequence variation present at a single nucleotide residue within different alleles of the same genomic sequence. This variation may occur within the coding region or non-coding region (i.e., in the promoter or intronic region) of a genomic sequence, if the genomic sequence is transcribed during protein production. Detection of one or more SNP allows differentiation of different alleles of a single genomic sequence or between two or more individuals.
- allele is one of several alternate forms of a gene or non-coding regions of DNA that occupy the same position on a chromosome.
- the term allele can be used to describe DNA from any organism including but not limited to bacteria, viruses, fungi, protozoa, molds, yeasts, plants, humans, non-humans, animals, and archeabacteria.
- ratio of the alleles or “allelic ratio” as used herein refer to the ratio of the population of one allele and the population of the other allele in a sample. In some trisomic cases, it is possible that a fetus may be tri-allelic for a particular locus. In such cases, the term “ratio of the alleles” refers to the ratio of the population of any one allele against one of the other alleles, or any one allele against the other two alleles.
- non-polymorphism-based quantitative method refers to a method for determining the amount of an analyte (e.g., total nucleic acid, Y-chromosome nucleic acid, or fetal nucleic acid) that does not require the use of a polymorphic marker or sequence. Although a polymorphism may be present in the sequence, said polymorphism is not required to quantify the sequence.
- analyte e.g., total nucleic acid, Y-chromosome nucleic acid, or fetal nucleic acid
- non-polymorphism-based quantitative methods include, but are not limited to, T-PC , digital PCR, array-based methods, sequencing methods, nanopore-based methods, nucleic acid- bound bead-based counting methods and competitor-based methods wherein one or more competitors are introduced at a known concentration(s) to determine the amount of one or more analytes.
- some of the above exemplary methods may need to be actively modified or designed such that one or more polymorphisms are not interrogated.
- a bsolute amount or “copy number” as used herein refers to the amount or quantity of an analyte (e.g., total nucleic acid or fetal nucleic acid).
- analyte e.g., total nucleic acid or fetal nucleic acid.
- the present invention provides compositions and processes for determining the absolute amount of fetal nucleic acid in a mixed maternal sample.
- Absolute amount or copy number represents the number of molecules available for detection, and may be expressed as the genomic equivalents per unit.
- concentration refers to the amount or proportion of a substance in a mixture or solution (e.g., the amount of fetal nucleic acid in a maternal sample that comprises a mixture of maternal and fetal nucleic acid). The concentration may be expressed as a percentage, which is used to express how large/small one quantity is, relative to another quantity as a fraction of 100.
- Platforms for determining the quantity or amount of an analyte include, but are not limited to, mass spectrometery, digital PCR, sequencing by synthesis platforms (e.g., pyrosequencing), fluorescence spectroscopy and flow cytometry.
- sample refers to a specimen containing nucleic acid.
- samples include, but are not limited to, tissue, bodily fluid (for example, blood, serum, plasma, saliva, urine, tears, peritoneal fluid, ascitic fluid, vaginal secretion, breast fluid, breast milk, lymph fluid, cerebrospinal fluid or mucosa secretion), umbilical cord blood, chorionic villi, amniotic fluid, an embryo, a two-celled embryo, a four-celled embryo, an eight-celled embryo, a 16-celled embryo, a 32-celled embryo, a 64- celled embryo, a 128-celled embryo, a 256-celled embryo, a 512-celled embryo, a 1024-celled embryo, embryonic tissues, lymph fluid, cerebrospinal fluid, mucosa secretion, or other body exudate, fecal matter, an individual cell or extract of the such sources that contain the nucleic
- Fetal DNA can be obtained from sources including but not limited to maternal blood, maternal serum, maternal plasma, fetal cells, umbilical cord blood, chorionic villi, amniotic fluid, urine, saliva, lung lavage, cells or tissues.
- blood refers to a blood sample or preparation from a pregnant woman or a woman being tested for possible pregnancy.
- the term encompasses whole blood or any fractions of blood, such as serum and plasma as conventionally defined.
- bisulfite encompasses all types of bisulfites, such as sodium bisulfite, that are capable of chemically converting a cytosine (C) to a uracil (U) without chemically modifying a methylated cytosine and therefore can be used to differentially modify a DNA sequence based on the methylation status of the DNA.
- a reagent that "differentially modifies” methylated or non-methylated DNA As used herein, a reagent that "differentially modifies" methylated or non-methylated DNA
- processes may include, but are not limited to, chemical reactions (such as a C.fwdarw.U conversion by bisulfite) and enzymatic treatment (such as cleavage by a methylation-dependent endonuclease).
- an enzyme that preferentially cleaves or digests methylated DNA is one capable of cleaving or digesting a DNA molecule at a much higher efficiency when the DNA is methylated, whereas an enzyme that preferentially cleaves or digests unmethylated DNA exhibits a significantly higher efficiency when the DNA is not methylated.
- non-bisulfite-based method and “non-bisulfite-based quantitative method” as used herein refer to any method for quantifying methylated or non-methylated nucleic acid that does not require the use of bisulfite.
- the terms also refer to methods for preparing a nucleic acid to be quantified that do not require bisulfite treatment. Examples of non-bisulfite-based methods include, but are not limited to, methods for digesting nucleic acid using one or more methylation sensitive enzymes and methods for separating nucleic acid using agents that bind nucleic acid based on methylation status.
- methyl-sensitive enzymes and "methylation sensitive restriction enzymes” are DNA restriction endonucleases that are dependent on the methylation state of their DNA recognition site for activity. For example, there are methyl-sensitive enzymes that cleave or digest at their DNA recognition sequence only if it is not methylated. Thus, an unmethylated DNA sample will be cut into smaller fragments than a methylated DNA sample. Similarly, a hypermethylated DNA sample will not be cleaved. In contrast, there are methyl-sensitive enzymes that cleave at their DNA recognition sequence only if it is methylated. As used herein, the terms “cleave”, “cut” and “digest” are used interchangeably.
- target nucleic acid refers to a nucleic acid examined using the methods disclosed herein to determine if the nucleic acid is part of a pregnancy-related disorder or chromosomal abnormality.
- a target nucleic acid from chromosome 21 could be examined using the methods of the invention to detect Down's Syndrome.
- control nucleic acid refers to a nucleic acid used as a reference nucleic acid according to the methods disclosed herein to determine if the nucleic acid is part of a chromosomal abnormality.
- a control nucleic acid from a chromosome other than chromosome 21 (herein referred to as a "reference chromosome”) could be as a reference sequence to detect Down's Syndrome.
- the control sequence has a known or predetermined quantity.
- gene means the segment of DNA involved in producing a polypeptide chain; it includes regions preceding and following the coding region (leader and trailer) involved in the
- polypeptide polypeptide
- peptide protein
- protein protein
- amino acid polymers in which one or more amino acid residue is an artificial chemical mimetic of a corresponding naturally occurring amino acid, as well as to naturally occurring amino acid polymers and non-naturally occurring amino acid polymers.
- the terms encompass amino acid chains of any length, including full-length proteins (i.e., antigens), wherein the amino acid residues are linked by covalent peptide bonds.
- amino acid refers to naturally occurring and synthetic amino acids, as well as amino acid analogs and amino acid mimetics that function in a manner similar to the naturally occurring amino acids.
- Naturally occurring amino acids are those encoded by the genetic code, as well as those amino acids that are later modified, e.g., hydroxyproline, . gamma. -carboxyglutamate, and O-phosphoserine.
- Amino acids may be referred to herein by either the commonly known three letter symbols or by the one-letter symbols recommended by the lUPAC-IUB Biochemical Nomenclature Commission.
- Nucleotides likewise, may be referred to by their commonly accepted single-letter codes.
- Primer refers to oligonucleotides that can be used in an amplification method, such as a polymerase chain reaction (PC ), to amplify a nucleotide sequence based on the polynucleotide sequence corresponding to a particular genomic sequence, e.g., one located within the CpG island CGI137, PDE9A, or CGI009 on chromosome 21, in various methylation status. At least one of the PCR primers for amplification of a polynucleotide sequence is sequence-specific for the sequence.
- PC polymerase chain reaction
- template refers to any nucleic acid molecule that can be used for amplification in the invention.
- NA or DNA that is not naturally double stranded can be made into double stranded DNA so as to be used as template DNA.
- Any double stranded DNA or preparation containing multiple, different double stranded DNA molecules can be used as template DNA to amplify a locus or loci of interest contained in the template DNA.
- amplification reaction refers to a process for copying nucleic acid one or more times.
- the method of amplification includes but is not limited to polymerase chain reaction, self-sustained sequence reaction, ligase chain reaction, rapid amplification of cDNA ends, polymerase chain reaction and ligase chain reaction, Q-beta phage amplification, strand displacement amplification, or splice overlap extension polymerase chain reaction.
- a single molecule of nucleic acid is amplified, for example, by digital PCR.
- sensitivity refers to the number of true positives divided by the number of true positives plus the number of false negatives, where sensitivity (sens) may be within the range of 0 ⁇ sens ⁇ 1.
- method embodiments herein have the number of false negatives equaling zero or close to equaling zero, so that no subject is wrongly identified as not having at least one chromosome abnormality or other genetic disorder when they indeed have at least one chromosome abnormality or other genetic disorder.
- an assessment often is made of the ability of a prediction algorithm to classify negatives correctly, a complementary measurement to sensitivity.
- sensitivity refers to the number of true negatives divided by the number of true negatives plus the number of false positives, where sensitivity (spec) may be within the range of 0 ⁇ spec ⁇ 1.
- methods embodiments herein have the number of false positives equaling zero or close to equaling zero, so that no subject wrongly identified as having at least one chromosome abnormality other genetic disorder when they do not have the chromosome abnormality other genetic disorder being assessed.
- variable refers to a factor, quantity, or function of an algorithm that has a value or set of values.
- a variable may be the design of a set of amplified nucleic acid species, the number of sets of amplified nucleic acid species, percent fetal genetic contribution tested, percent maternal genetic contribution tested, type of chromosome abnormality assayed, type of genetic disorder assayed, type of sex-linked abnormalities assayed, the age of the mother and the like.
- independent refers to not being influenced or not being controlled by another.
- dependent refers to being influenced or controlled by another. For example, a particular chromosome and a trisomy event occurring for that particular chromosome that results in a viable being are variables that are dependent upon each other.
- One of skill in the art may use any type of method or prediction algorithm to give significance to the data of the present invention within an acceptable sensitivity and/or specificity.
- prediction algorithms such as Chi-squared test, z-test, t-test, ANOVA (analysis of variance), regression analysis, neural nets, fuzzy logic, Hidden Markov Models, multiple model state estimation, and the like may be used.
- One or more methods or prediction algorithms may be determined to give significance to the data having different independent and/or dependent varia bles of the present invention. And one or more methods or prediction algorithms may be determined not to give significance to the data having different independent and/or dependent varia bles of the present invention.
- prediction algorithms e.g., num ber of sets analyzed, types of nucleotide species in each set.
- these algorithms may be chosen to be tested. These algorithms can be trained with raw data. For each new raw data sample, the trained algorithms will assign a classification to that sample (i.e. trisomy or normal). Based on the classifications of the new raw data samples, the trained algorithms' performance may be assessed based on sensitivity and specificity. Finally, an algorithm with the highest sensitivity and/or specificity or com bination thereof may be identified.
- fetal nucleic acid in maternal plasma was first reported in 1997 and offers the possibility for non-invasive prenatal diagnosis simply through the analysis of a maternal blood sample (Lo et al., Lancet 350:485-487, 1997). To date, numerous potential clinical applications have been developed. In particular, quantitative abnormalities of fetal nucleic acid, for example DNA, concentrations in maternal plasma have been found to be associated with a num ber of pregnancy-associated disorders, including preecla mpsia, preterm la bor, antepartum hemorrhage, invasive placentation, fetal Down syndrome, and other fetal chromosomal aneuploidies. Hence, fetal nucleic acid analysis in maternal plasma represents a powerful mechanism for the monitoring of fetomaternal well-being.
- Methylation is an epigenetic phenomenon, which refers to processes that alter a phenotype without involving changes in the DNA sequence.
- the present inventors provides novel genomic polynucleotides that are differentially methylated between the fetal DNA from the fetus (e.g., from the placenta) and the maternal DNA from the mother, for example from peripheral blood cells. This discovery thus provides a new approach for distinguishing fetal and maternal genomic DNA and new methods for accurately quantifying fetal nucleic which may be used for non-invasive prenatal diagnosis.
- Practicing the invention utilizes routine techniques in the field of molecular biology.
- Basic texts disclosing the general methods of use in the invention include Sambrook and Russell, Molecular Cloning, A Laboratory Manual (3rd ed. 2001); Kriegler, Gene Transfer and Expression: A Laboratory Manual (1990); and Current Protocols in Molecular Biology (Ausubel et al., eds., 1994)).
- nucleic acids sizes are given in either kilobases (kb) or base pairs (bp). These are estimates derived from agarose or acrylamide gel electrophoresis, from sequenced nucleic acids, or from published DNA sequences.
- kb kilobases
- bp base pairs
- proteins sizes are given in kilodaltons (kDa) or amino acid residue numbers. Protein sizes are estimated from gel electrophoresis, from sequenced proteins, from derived amino acid sequences, or from published protein sequences.
- Oligonucleotides that are not commercially available can be chemically synthesized, e.g., according to the solid phase phosphoramidite triester method first described by Beaucage & Caruthers, Tetrahedron Lett. 22: 1859-1862 (1981), using an automated synthesizer, as described in Van Devanter et. al., Nucleic Acids Res. 12: 6159-6168 (1984). Purification of oligonucleotides is performed using any art-recognized strategy, e.g., native acrylamide gel electrophoresis or anion-exchange high performance liquid chromatography (HPLC) as described in Pearson & Reanier, J. Chrom. 255: 137-149 (1983).
- HPLC high performance liquid chromatography
- the present invention relates to separating, enriching and analyzing fetal DNA found in maternal blood as a non-invasive means to detect the presence and/or to monitor the progress of a pregnancy- associated condition or disorder.
- the first steps of practicing the invention are to obtain a blood sample from a pregnant woman and extract DNA from the sample.
- a blood sample is obtained from a pregnant woman at a gestational age suitable for testing using a method of the present invention.
- the suitable gestational age may vary depending on the disorder tested, as discussed below.
- Collection of blood from a woman is performed in accordance with the standard protocol hospitals or clinics generally follow.
- An appropriate amount of peripheral blood e.g., typically between 5-50 ml, is collected and may be stored according to standard procedure prior to further preparation.
- Blood samples may be collected, stored or transported in a manner known to the person of ordinary skill in the art to minimize degradation or the quality of nucleic acid present in the sample.
- the analysis of fetal DNA found in maternal blood may be performed using, e.g., the whole blood, serum, or plasma.
- the methods for preparing serum or plasma from maternal blood are well known among those of skill in the art.
- a pregnant woman's blood can be placed in a tu be containing EDTA or a specialized commercial product such as Vacutainer SST (Becton Dickinson, Franklin Lakes, N.J.) to prevent blood clotting, and plasma can then be obtained from whole blood through centrifugation.
- serum may be obtained with or without centrifugation-following blood clotting. If centrifugation is used then it is typically, though not exclusively, conducted at an appropriate speed, e.g., 1,500-3,000 times g.
- Plasma or serum may be subjected to additional centrifugation steps before being transferred to a fresh tube for DNA extraction.
- DNA may also be recovered from the cellular fraction, enriched in the buffy coat portion, which can be obtained following centrifugation of a whole blood sample from the woman and removal of the plasma.
- the sample may first be enriched or relatively enriched for fetal nucleic acid by one or more methods.
- the discrimination of fetal and maternal DNA can be performed using the compositions and processes of the present invention alone or in combination with other discriminating factors. Examples of these factors include, but are not limited to, single nucleotide differences between chromosome X and Y, chromosome Y-specific sequences, polymorphisms located elsewhere in the genome, size differences between fetal and maternal DNA and differences in methylation pattern between maternal and fetal tissues.
- Other methods for enriching a sample for a particular species of nucleic acid are described in PCT Patent Application Number PCT/US07/69991, filed May 30, 2007, PCT Patent Application Number
- maternal nucleic acid is selectively removed (either partially, substantially, almost completely or completely) from the sample.
- the methods provided herein offer an alternative approach for the enrichment of fetal DNA based on the methylation-specific separation of differentially methylated DNA. It has recently been discovered that many genes involved in developmental regulation are controlled through epigenetics in embryonic stem cells. Consequently, multiple genes can be expected to show differential DNA methylation between nucleic acid of fetal origin and maternal origin. Once these regions are identified, a technique to capture methylated DNA can be used to specifically enrich fetal DNA. For identification of differentially methylated regions, a novel approach was used to capture methylated DNA.
- MBD-FC methyl binding domain of MBD2
- MBD-FC Fc fragment of an antibody
- This fusion protein has several advantages over conventional methylation specific antibodies.
- the MBD-FC has a higher affinity to methylated DNA and it binds double stranded DNA. Most importantly the two proteins differ in the way they bind DNA.
- Methylation specific antibodies bind DNA stochastically, which means that only a binary answer can be obtained.
- the methyl binding domain of MBD-FC on the other hand binds all DNA molecules regardless of their methylation status. The strength of this protein - DNA interaction is defined by the level of DNA methylation.
- eluate solutions of increasing salt concentrations can be used to fractionate non-methylated and methylated DNA allowing for a more controlled separation (Gebhard C, Schwarzfischer L, Pham TH, Andreesen R, Mackensen A, Rehli M (2006) Rapid and sensitive detection of CpG-methylation using methyl-binding (MB)-PCR. Nucleic Acids Res 34:e82). Consequently this method, called Methyl-CpG immunoprecipitation (MCIP), cannot only enrich, but also fractionate genomic DNA according to methylation level, which is particularly helpful when the unmethylated DNA fraction should be investigated as well.
- MCIP Methyl-CpG immunoprecipit
- the invention also provides compositions and processes for determining the amount of fetal nucleic acid from a maternal sample.
- the invention allows for the enrichment of fetal nucleic acid regions in a maternal sample by selectively digesting nucleic acid from said maternal sample with an enzyme that selectively and completely or substantially digests the maternal nucleic acid to enrich the sample for at least one fetal nucleic acid region.
- the digestion efficiency is greater than about 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%.
- the amount of fetal nucleic acid can be determined by quantitative methods that do not require polymorphic sequences or bisulfite treatment, thereby, offering a solution that works equally well for female fetuses and across different ethnicities and preserves the low copy number fetal nucleic acid present in the sample.
- methyl-sensitive enzymes that preferentially or substantially cleave or digest at their DNA recognition sequence if it is non-methylated.
- an unmethylated DNA sample will be cut into smaller fragments than a methylated DNA sample.
- a hypermethylated DNA sample will not be cleaved.
- methyl-sensitive enzymes that cleave at their DNA recognition sequence only if it is methylated.
- Methyl-sensitive enzymes that digest unmethylated DNA suitable for use in methods of the invention include, but are not limited to, Hpall, Hhal, Maell, BstUI and Acil.
- An enzyme that can be used is Hpall that cuts only the unmethylated sequence CCGG.
- Another enzyme that can be used is Hhal that cuts only the unmethylated sequence GCGC. Both enzymes are available from New England BioLabs ® , Inc. Combinations of two or more methyl-sensitive enzymes that digest only unmethylated DNA can also be used.
- Suitable enzymes that digest only methylated DNA include, but are not limited to, Dpnl, which cuts at a recognition sequence GATC, and McrBC, which belongs to the family of AAA.sup.+ proteins and cuts DNA containing modified cytosines and cuts at recognition site 5' . . . Pu.sup.mC(N.sub.40-3000) Pu.sup.mC . . . 3' (New England BioLabs, Inc., Beverly, Mass.).
- methylation analysis procedures are known in the art, and can be used in conjunction with the present invention. These assays allow for determination of the methylation state of one or a plurality of CpG islands within a DNA sequence. In addition, the methods maybe used to quantify methylated nucleic acid. Such assays involve, among other techniques, DNA sequencing of bisulfite-treated DNA, PC (for sequence-specific amplification), Southern blot analysis, and use of methylation-sensitive restriction enzymes.
- Genomic sequencing is a technique that has been simplified for analysis of DNA methylation patterns and 5-methylcytosine distribution by using bisulfite treatment (Frommer et al., Proc. Natl. Acad. Sci. USA 89:1827-1831, 1992). Additionally, restriction enzyme digestion of PCR products amplified from bisulfite-converted DNA may be used, e.g., the method described by Sadri & Hornsby (Nucl. Acids Res. 24:5058-5059, 1996), or COBRA (Combined Bisulfite Restriction Analysis) (Xiong & Laird, Nucleic Acids Res. 25:2532-2534, 1997).
- COBRA analysis is a quantitative methylation assay useful for determining DNA methylation levels at specific gene loci in small amounts of genomic DNA (Xiong & Laird, Nucleic Acids Res. 25:2532-2534, 1997). Briefly, restriction enzyme digestion is used to reveal methylation-dependent sequence differences in PCR products of sodium bisulfite-treated DNA. Methylation-dependent sequence differences are first introduced into the genomic DNA by standard bisulfite treatment according to the procedure described by Frommer et al. (Proc. Natl. Acad. Sci. USA 89:1827-1831, 1992). PCR
- amplification of the bisulfite converted DNA is then performed using primers specific for the interested CpG islands, followed by restriction endonuclease digestion, gel electrophoresis, and detection using specific, labeled hybridization probes.
- Methylation levels in the original DNA sample are represented by the relative amounts of digested and undigested PCR product in a linearly quantitative fashion across a wide spectrum of DNA methylation levels.
- this technique can be reliably applied to DNA obtained from microdissected paraffin-embedded tissue samples.
- Typical reagents for COBRA analysis may include, but are not limited to: PCR primers for specific gene (or methylation-altered DNA sequence or CpG island); restriction enzyme and appropriate buffer; gene-hybridization oligo; control hybridization oligo; kinase labeling kit for oligo probe; and radioactive nucleotides.
- bisulfite conversion reagents may include: DNA denaturation buffer; sulfonation buffer; DNA recovery reagents or kits (e.g., precipitation, ultrafiltration, affinity column); desulfonation buffer; and DNA recovery components.
- the MethyLightTM assay is a high-throughput quantitative methylation assay that utilizes fluorescence- based real-time PCR (TaqMan.RTM.) technology that requires no further manipulations after the PCR step (Eads et al., Cancer Res. 59:2302-2306, 1999). Briefly, the MethyLight.TM. process begins with a mixed sample of genomic DNA that is converted, in a sodium bisulfite reaction, to a mixed pool of methylation-dependent sequence differences according to standard procedures (the bisulfite process converts unmethylated cytosine residues to uracil).
- Fluorescence-based PCR is then performed either in an "unbiased” (with primers that do not overlap known CpG methylation sites) PCR reaction, or in a “biased” (with PCR primers that overlap known CpG dinucleotides) reaction. Sequence discrimination can occur either at the level of the amplification process or at the level of the fluorescence detection process, or both.
- the MethyLight assay may be used as a quantitative test for methylation patterns in the genomic DNA sample, wherein sequence discrimination occurs at the level of probe hybridization.
- the PCR reaction provides for unbiased amplification in the presence of a fluorescent probe that overlaps a particular putative methylation site.
- An unbiased control for the amount of input DNA is provided by a reaction in which neither the primers, nor the probe overlie any CpG dinucleotides.
- a qualitative test for genomic methylation is achieved by probing of the biased PCR pool with either control oligonucleotides that do not "cover” known methylation sites (a fluorescence-based version of the "MSP" technique), or with oligonucleotides covering potential methylation sites.
- the MethyLight process can by used with a "TaqMan” probe in the amplification process.
- double-stranded genomic DNA is treated with sodium bisulfite and subjected to one of two sets of PCR reactions using TaqMan.RTM. probes; e.g., with either biased primers and TaqMan.RTM. probe, or unbiased primers and TaqMan.RTM. probe.
- the TaqMan.RTM. probe is dual-labeled with fluorescent "reporter” and "quencher” molecules, and is designed to be specific for a relatively high GC content region so that it melts out at about 10. degree. C. higher temperature in the PCR cycle than the forward or reverse primers. This allows the TaqMan.RTM.
- Taq polymerase enzymatically synthesizes a new strand during PCR, it will eventually reach the annealed TaqMan.RTM. probe.
- the Taq polymerase 5' to 3' endonuclease activity will then displace the TaqMan.RTM. probe by digesting it to release the fluorescent reporter molecule for quantitative detection of its now unquenched signal using a real-time fluorescent detection system.
- Typical reagents e.g., as might be found in a typical MethyLight.TM. -based kit
- MethyLight.TM. analysis may include, but are not limited to: PCR primers for specific gene (or methylation-altered DNA sequence or CpG island); TaqMan.RTM. probes; optimized PCR buffers and deoxynucleotides; and Taq polymerase.
- the Ms-SNuPE technique is a quantitative method for assessing methylation differences at specific CpG sites based on bisulfite treatment of DNA, followed by single-nucleotide primer extension (Gonzalgo & Jones, Nucleic Acids Res. 25:2529-2531, 1997).
- genomic DNA is reacted with sodium bisulfite to convert unmethylated cytosine to uracil while leaving 5-methylcytosine unchanged.
- Amplification of the desired target sequence is then performed using PCR primers specific for bisulfite-converted DNA, and the resulting product is isolated and used as a template for methylation analysis at the CpG site(s) of interest.
- Small amounts of DNA can be analyzed (e.g., microdissected pathology sections), and it avoids utilization of restriction enzymes for determining the methylation status at CpG sites.
- Typical reagents for Ms-SNuPE analysis may include, but are not limited to: PCR primers for specific gene (or methylation-altered DNA sequence or CpG island); optimized PCR buffers and deoxynucleotides; gel extraction kit; positive control primers; Ms-SNuPE primers for specific gene; reaction buffer (for the Ms-SNuPE reaction); and radioactive nucleotides.
- bisulfite conversion reagents may include: DNA denaturation buffer;
- DNA recovery regents or kit e.g., precipitation, ultrafiltration, affinity column
- desulfonation buffer e.g., DNA recovery buffer
- MSP methylation-specific PCR
- DNA is modified by sodium bisulfite converting all unmethylated, but not methylated cytosines to uracil, and subsequently amplified with primers specific for methylated versus umnethylated DNA.
- MSP requires only small quantities of DNA, is sensitive to 0.1% methylated alleles of a given CpG island locus, and can be performed on DNA extracted from paraffin-embedded samples.
- Typical reagents e.g., as might be found in a typical MSP-based kit
- MSP analysis may include, but are not limited to: methylated and unmethylated PCR primers for specific gene (or methylation-altered DNA sequence or CpG island), optimized PCR buffers and deoxynucleotides, and specific probes.
- the MCA technique is a method that can be used to screen for altered methylation patterns in genomic DNA, and to isolate specific sequences associated with these changes (Toyota et al., Cancer Res.
- restriction enzymes with different sensitivities to cytosine methylation in their recognition sites are used to digest genomic DNAs from primary tumors, cell lines, and normal tissues prior to arbitrarily primed PCR amplification. Fragments that show differential methylation are cloned and sequenced after resolving the PCR products on high-resolution polyacrylamide gels. The cloned fragments are then used as probes for Southern analysis to confirm differential methylation of these regions.
- Typical reagents for MCA analysis may include, but are not limited to: PCR primers for arbitrary priming Genomic DNA; PCR buffers and nucleotides, restriction enzymes and appropriate buffers; gene-hybridization oligos or probes; control hybridization oligos or probes.
- Another method for analyzing methylation sites is a primer extension assay, including an optimized PCR amplification reaction that produces amplified targets for subsequent primer extension genotyping analysis using mass spectrometry.
- the assay can also be done in multiplex. This method (particularly as it relates to genotyping single nucleotide polymorphisms) is described in detail in PCT publication WO05012578A1 and US publication US20050079521A1.
- the assay can be adopted to detect bisulfite introduced methylation dependent C to T sequence changes.
- multiplexed amplification reactions and multiplexed primer extension reactions e.g., multiplexed homogeneous primer mass extension (hME) assays
- hME primer mass extension
- DNA methylation analysis includes restriction landmark genomic scanning (RLGS, Costello et al., 2000), methylation-sensitive-representational difference analysis (MS-RDA), methylation-specific AP-PCR (MS-AP-PCR) and methyl-CpG binding domain column/segregation of partly melted molecules (MBD/SPM).
- RGS restriction landmark genomic scanning
- MS-RDA methylation-sensitive-representational difference analysis
- MS-AP-PCR methylation-specific AP-PCR
- MBD/SPM methyl-CpG binding domain column/segregation of partly melted molecules
- nucleic acid may be subjected to sequence-based analysis. Furthermore, once it is determined that one particular genomic sequence of fetal origin is hypermethylated or hypomethylated compared to the maternal counterpart, the amount of this fetal genomic sequence can be determined. Subsequently, this amount can be compared to a standard control value and serve as an indication for the potential of certain pregnancy- associated disorder.
- nucleic acid amplification is the enzymatic synthesis of nucleic acid amplicons (copies) which contain a sequence that is complementary to a nucleic acid sequence being amplified. Nucleic acid amplification is especially beneficial when the amount of target sequence present in a sample is very low.
- the sensitivity of an assay can be vastly improved, since fewer target sequences are needed at the beginning of the assay to better ensure detection of nucleic acid in the sample belonging to the organism or virus of interest.
- PCR polymerase chain reaction
- PCR is most usually carried out as an automated process with a thermostable enzyme. In this process, the temperature of the reaction mixture is cycled through a denaturing region, a primer annealing region, and an extension reaction region automatically. Machines specifically adapted for this purpose are commercially available.
- PCR amplification of a polynucleotide sequence is typically used in practicing the present invention
- amplification of a genomic sequence found in a maternal blood sample may be accomplished by any known method, such as ligase chain reaction (LCR), transcription-mediated amplification, and self-sustained sequence replication or nucleic acid sequence- based amplification (NASBA), each of which provides sufficient amplification.
- LCR ligase chain reaction
- NASBA nucleic acid sequence- based amplification
- More recently developed branched-DNA technology may also be used to qualitatively demonstrate the presence of a particular genomic sequence of the invention, which represents a particular methylation pattern, or to quantitatively determine the amount of this particular genomic sequence in the maternal blood.
- branched-DNA signal amplification for direct quantitation of nucleic acid sequences in clinical samples, see Nolte, Adv. Clin. Chem. 33:201-235, 1998.
- compositions and processes of the invention are also particularly useful when practiced with digital PCR.
- Digital PCR was first developed by Kalinina and colleagues (Kalinina et al., "Nanoliter scale PCR with TaqMan detection.” Nucleic Acids Research. 25; 1999-2004, (1997)) and further developed by Vogelstein and Kinzler (Digital PCR. Proc Natl Acad Sci U S A. 96; 9236-41, (1999)).
- the application of digital PCR for use with fetal diagnostics was first described by Cantor et al. (PCT Patent Publication No. WO05023091A2) and subsequently described by Quake et al. (US Patent Publication No. US
- Digital PCR takes advantage of nucleic acid (DNA, cDNA or NA) amplification on a single molecule level, and offers a highly sensitive method for quantifying low copy number nucleic acid.
- Fluidigm ® Corporation offers systems for the digital analysis of nucleic acids.
- a primer extension reaction operates, for example, by discriminating the SNP alleles by the incorporation of
- the primer is extended with a polymerase.
- the primer extended SNP can be detected physically by mass spectrometry or by a tagging moiety such as biotin.
- the SNP site is only extended by a complementary deoxynucleotide or dideoxynucleotide that is either tagged by a specific label or generates a primer extension product with a specific mass, the SNP alleles can be discriminated and quantified.
- Reverse transcribed and amplified nucleic acids may be modified nucleic acids.
- Modified nucleic acids can include nucleotide analogs, and in certain embodiments include a detectable label and/or a capture agent.
- detectable labels include without limitation fluorophores, radioisotopes, colormetric agents, light emitting agents, chemiluminescent agents, light scattering agents, enzymes and the like.
- capture agents include without limitation an agent from a binding pair selected from antibody/antigen, antibody/antibody, antibody/antibody fragment, antibody/antibody receptor, antibody/protein A or protein G, hapten/anti-hapten, biotin/avidin, biotin/streptavidin, folic acid/folate binding protein, vitamin B12/intrinsic factor, chemical reactive group/complementary chemical reactive group (e.g., sulfhydryl/maleimide, sulfhydryl/haloacetyl derivative, amine/isotriocyanate,
- agent from a binding pair selected from antibody/antigen, antibody/antibody, antibody/antibody fragment, antibody/antibody receptor, antibody/protein A or protein G, hapten/anti-hapten, biotin/avidin, biotin/streptavidin, folic acid/folate binding protein, vitamin B12/intrinsic factor, chemical reactive group/complementary chemical reactive group (e.g., sulf
- Modified nucleic acids having a capture agent can be immobilized to a solid support in certain embodiments
- Mass spectrometry is a particularly effective method for the detection of a polynucleotide of the invention, for example a PCR amplicon, a primer extension product or a detector probe that is cleaved from a target nucleic acid.
- the presence of the polynucleotide sequence is verified by comparing the mass of the detected signal with the expected mass of the polynucleotide of interest.
- the relative signal strength, e.g., mass peak on a spectra, for a particular polynucleotide sequence indicates the relative population of a specific allele, thus enabling calculation of the allele ratio directly from the data.
- Sequencing technologies are improving in terms of throughput and cost. Sequencing technologies, such as that achievable on the 454 platform (Roche) (Margulies, M. et al. 2005 Nature 437, 376-380), lllumina Genome Analyzer (or Solexa platform) or SOLiD System (Applied Biosystems) or the Helicos True Single Molecule DNA sequencing technology (Harris T D et al. 2008 Science, 320, 106-109), the single molecule, real-time (SMRT.TM.) technology of Pacific Biosciences, and nanopore sequencing (Soni GV and Meller A. 2007 Clin Chem 53: 1996-2001), allow the sequencing of many nucleic acid molecules isolated from a specimen at high orders of multiplexing in a parallel fashion (Dear Brief Funct Genomic Proteomic 2003; 1: 397-416).
- Each of these platforms allow sequencing of clonally expanded or non-amplified single molecules of nucleic acid fragments.
- Certain platforms involve, for example, (i) sequencing by ligation of dye- modified probes (including cyclic ligation and cleavage), (ii) pyrosequencing, and (iii) single-molecule sequencing.
- Nucleotide sequence species, amplification nucleic acid species and detectable products generated there from can be considered a "study nucleic acid" for purposes of analyzing a nucleotide sequence by such sequence analysis platforms.
- Sequencing by ligation is a nucleic acid sequencing method that relies on the sensitivity of DNA ligase to base-pairing mismatch.
- DNA ligase joins together ends of DNA that are correctly base paired. Combining the ability of DNA ligase to join together only correctly base paired DNA ends, with mixed pools of fluorescently labeled oligonucleotides or primers, enables sequence determination by fluorescence detection.
- Longer sequence reads may be obtained by including primers containing cleavable linkages that can be cleaved after label identification. Cleavage at the linker removes the label and regenerates the 5' phosphate on the end of the ligated primer, preparing the primer for another round of ligation.
- primers may be labeled with more than one fluorescent label (e.g., 1 fluorescent label, 2, 3, or 4 fluorescent labels).
- Clonal bead populations can be prepared in emulsion microreactors containing study nucleic acid ("template"), amplification reaction components, beads and primers. After amplification, templates are denatured and bead enrichment is performed to separate beads with extended templates from undesired beads (e.g., beads with no extended templates). The template on the selected beads undergoes a 3' modification to allow covalent bonding to the slide, and modified beads can be deposited onto a glass slide. Deposition chambers offer the ability to segment a slide into one, four or eight chambers during the bead loading process.
- primers hybridize to the adapter sequence.
- a set of four color dye-labeled probes competes for ligation to the sequencing primer. Specificity of probe ligation is achieved by interrogating every 4th and 5th base during the ligation series. Five to seven rounds of ligation, detection and cleavage record the color at every 5th position with the number of rounds determined by the type of library used. Following each round of ligation, a new complimentary primer offset by one base in the 5' direction is laid down for another series of ligations. Primer reset and ligation rounds (5-7 ligation cycles per round) are repeated sequentially five times to generate 25-35 base pairs of sequence for a single tag. With mate-paired sequencing, this process is repeated for a second tag.
- Such a system can be used to exponentially amplify amplification products generated by a process described herein, e.g., by ligating a heterologous nucleic acid to the first amplification product generated by a process described herein and performing emulsion amplification using the same or a different solid support originally used to generate the first amplification product.
- Such a system also may be used to analyze amplification products directly generated by a process described herein by bypassing an exponential amplification process and directly sorting the solid supports described herein on the glass slide.
- Pyrosequencing is a nucleic acid sequencing method based on sequencing by synthesis, which relies on detection of a pyrophosphate released on nucleotide incorporation.
- sequencing by synthesis involves synthesizing, one nucleotide at a time, a DNA strand complimentary to the strand whose sequence is being sought.
- Study nucleic acids may be immobilized to a solid support, hybridized with a sequencing primer, incubated with DNA polymerase, ATP sulfurylase, luciferase, apyrase, adenosine 5' phosphsulfate and luciferin. Nucleotide solutions are sequentially added and removed.
- nucleotide Correct incorporation of a nucleotide releases a pyrophosphate, which interacts with ATP sulfurylase and produces ATP in the presence of adenosine 5' phosphsulfate, fueling the luciferin reaction, which produces a chemiluminescent signal allowing sequence determination.
- An example of a system that can be used by a person of ordinary skill based on pyrosequencing generally involves the following steps: ligating an adaptor nucleic acid to a study nucleic acid and hybridizing the study nucleic acid to a bead; amplifying a nucleotide sequence in the study nucleic acid in an emulsion; sorting beads using a picoliter multiwell solid support; and sequencing amplified nucleotide sequences by pyrosequencing methodology (e.g., Nakano et al., "Single-molecule PC using water-in-oil emulsion;" Journal of Biotechnology 102: 117-124 (2003)).
- Such a system can be used to exponentially amplify amplification products generated by a process described herein, e.g., by ligating a heterologous nucleic acid to the first amplification product generated by a process described herein.
- Certain single-molecule sequencing embodiments are based on the principal of sequencing by synthesis, and utilize single-pair Fluorescence Resonance Energy Transfer (single pair FRET) as a mechanism by which photons are emitted as a result of successful nucleotide incorporation.
- the emitted photons often are detected using intensified or high sensitivity cooled charge-couple-devices in conjunction with total internal reflection microscopy (TIRM). Photons are only emitted when the introduced reaction solution contains the correct nucleotide for incorporation into the growing nucleic acid chain that is synthesized as a result of the sequencing process.
- TIRM total internal reflection microscopy
- FRET FRET based single-molecule sequencing
- energy is transferred between two fluorescent dyes, sometimes polymethine cyanine dyes Cy3 and Cy5, through long-range dipole interactions.
- the donor is excited at its specific excitation wavelength and the excited state energy is transferred, non-radiatively to the acceptor dye, which in turn becomes excited.
- the acceptor dye eventually returns to the ground state by radiative emission of a photon.
- the two dyes used in the energy transfer process represent the "single pair", in single pair FRET. Cy3 often is used as the donor fluorophore and often is incorporated as the first labeled nucleotide.
- Cy5 often is used as the acceptor fluorophore and is used as the nucleotide label for successive nucleotide additions after incorporation of a first Cy3 labeled nucleotide.
- the fluorophores generally are within 10 nanometers of each for energy transfer to occur successfully.
- An example of a system that can be used based on single-molecule sequencing generally involves hybridizing a primer to a study nucleic acid to generate a complex; associating the complex with a solid phase; iteratively extending the primer by a nucleotide tagged with a fluorescent molecule; and capturing an image of fluorescence resonance energy transfer signals after each iteration (e.g., U.S. Patent No. 7,169,314; Braslavsky et al., PNAS 100(7): 3960-3964 (2003)).
- Such a system can be used to directly sequence amplification products generated by processes described herein.
- the released linear amplification product can be hybridized to a primer that contains sequences complementary to immobilized capture sequences present on a solid support, a bead or glass slide for example. Hybridization of the primer-released linear amplification product complexes with the immobilized capture sequences, immobilizes released linear amplification products to solid supports for single pair FRET based sequencing by synthesis.
- the primer often is fluorescent, so that an initial reference image of the surface of the slide with immobilized nucleic acids can be generated. The initial reference image is useful for determining locations at which true nucleotide incorporation is occurring. Fluorescence signals detected in array locations not initially identified in the "primer only" reference image are discarded as non-specific fluorescence.
- the bound nucleic acids often are sequenced in parallel by the iterative steps of, a) polymerase extension in the presence of one fluorescently labeled nucleotide, b) detection of fluorescence using appropriate microscopy, TIRM for example, c) removal of fluorescent nucleotide, and d) return to step a with a different fluorescently labeled nucleotide.
- nucleotide sequencing may be by solid phase single nucleotide sequencing methods and processes.
- Solid phase single nucleotide sequencing methods involve contacting sample nucleic acid and solid support under conditions in which a single molecule of sample nucleic acid hybridizes to a single molecule of a solid support. Such conditions can include providing the solid support molecules and a single molecule of sample nucleic acid in a "microreactor.” Such conditions also can include providing a mixture in which the sample nucleic acid molecule can hybridize to solid phase nucleic acid on the solid support.
- nanopore sequencing detection methods include (a) contacting a nucleic acid for sequencing ("base nucleic acid,” e.g., linked probe molecule) with sequence-specific detectors, under conditions in which the detectors specifically hybridize to substantially complementary subsequences of the base nucleic acid; (b) detecting signals from the detectors and (c) determining the sequence of the base nucleic acid according to the signals detected.
- the detectors hybridized to the base nucleic acid are disassociated from the base nucleic acid (e.g., sequentially dissociated) when the detectors interfere with a nanopore structure as the base nucleic acid passes through a pore, and the detectors disassociated from the base sequence are detected.
- a detector disassociated from a base nucleic acid emits a detectable signal, and the detector hybridized to the base nucleic acid emits a different detectable signal or no detectable signal.
- nucleotides in a nucleic acid e.g., linked probe molecule
- nucleotide representatives specific nucleotide sequences corresponding to specific nucleotides
- nucleotide representatives may be arranged in a binary or higher order arrangement (e.g., Soni and Meller, Clinical Chemistry 53(11): 1996-2001 (2007)).
- a nucleic acid is not expanded, does not give rise to an expanded nucleic acid, and directly serves a base nucleic acid (e.g., a linked probe molecule serves as a non-expanded base nucleic acid), and detectors are directly contacted with the base nucleic acid.
- a first detector may hybridize to a first subsequence and a second detector may hybridize to a second subsequence, where the first detector and second detector each have detectable labels that can be distinguished from one another, and where the signals from the first detector and second detector can be distinguished from one another when the detectors are disassociated from the base nucleic acid.
- detectors include a region that hybridizes to the base nucleic acid (e.g., two regions), which can be about 3 to about 100 nucleotides in length (e.g., about 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 30, 35, 40, 50, 55, 60, 65, 70, 75, 80, 85, 90, or 95 nucleotides in length).
- a detector also may include one or more regions of nucleotides that do not hybridize to the base nucleic acid.
- a detector is a molecular beacon.
- a detector often comprises one or more detectable labels independently selected from those described herein.
- Each detectable label can be detected by any convenient detection process capable of detecting a signal generated by each label (e.g., magnetic, electric, chemical, optical and the like).
- a CD camera can be used to detect signals from one or more distinguishable quantum dots linked to a detector.
- reads may be used to construct a larger nucleotide sequence, which can be facilitated by identifying overlapping sequences in different reads and by using identification sequences in the reads.
- sequence analysis methods and software for constructing larger sequences from reads are known to the person of ordinary skill (e.g., Venter et al., Science 291: 1304-1351 (2001)).
- Specific reads, partial nucleotide sequence constructs, and full nucleotide sequence constructs may be compared between nucleotide sequences within a sample nucleic acid (i.e., internal comparison) or may be compared with a reference sequence (i.e., reference comparison) in certain sequence analysis embodiments.
- nucleic acid species in a plurality of nucleic acids e.g., nucleotide sequence species, amplified nucleic acid species and detectable products generated from the foregoing.
- Multiplexing refers to the simultaneous detection of more than one nucleic acid species.
- General methods for performing multiplexed reactions in conjunction with mass spectrometry are known (see, e.g., U.S. Pat. Nos. 6,043,031, 5,547,835 and International PCT application No. WO 97/37041).
- Multiplexing provides an advantage that a plurality of nucleic acid species (e.g., some having different sequence variations) can be identified in as few as a single mass spectrum, as compared to having to perform a separate mass spectrometry analysis for each individual target nucleic acid species.
- Methods provided herein lend themselves to high-throughput, highly- automated processes for analyzing sequence variations with high speed and accuracy, in some embodiments. In some embodiments, methods herein may be multiplexed at high levels in a single reaction.
- the number of nucleic acid species multiplexed include, without limitation, about 1 to about 500 (e.g., about 1-3, 3-5, 5-7, 7-9, 9-11, 11-13, 13-15, 15-17, 17-19, 19-21, 21-23, 23- 25, 25-27, 27-29, 29-31, 31-33, 33-35, 35-37, 37-39, 39-41, 41-43, 43-45, 45-47, 47-49, 49-51, 51-53, 53- 55, 55-57, 57-59, 59-61, 61-63, 63-65, 65-67, 67-69, 69-71, 71-73, 73-75, 75-77, 77-79, 79-81, 81-83, 83- 85, 85-87, 87-89, 89-91, 91-93, 93-95, 95-97, 97-101, 101-103, 103-105, 105-107, 107-109,
- Design methods for achieving resolved mass spectra with multiplexed assays can include primer and oligonucleotide design methods and reaction design methods. See, for example, the multiplex schemes provided in Tables X and Y.
- primer and oligonucleotide design in multiplexed assays the same general guidelines for primer design applies for uniplexed reactions, such as avoiding false priming and primer dimers, only more primers are involved for multiplex reactions.
- analyte peaks in the mass spectra for one assay are sufficiently resolved from a product of any assay with which that assay is multiplexed, including pausing peaks and any other by-product peaks.
- multiplex analysis may be adapted to mass spectrometric detection of chromosome abnormalities, for example.
- multiplex analysis may be adapted to various single nucleotide or nanopore based sequencing methods described herein. Commercially produced micro-reaction chambers or devices or arrays or chips may be used to facilitate multiplex analysis, and are commercially available.
- Some methods rely on measuring the ratio of maternal to paternally inherited alleles to detect fetal chromosomal aneuploidies from maternal plasma.
- a diploid set yields a 1:1 ratio while trisomies can be detected as a 2:1 ratio. Detection of this difference is impaired by statistical sampling due to the low abundance of fetal DNA, presence of excess maternal DNA in the plasma sample and variability of the measurement technique. The latter is addressed by using methods with high measurement precision, like digital PCR or mass spectrometry.
- Enriching the fetal fraction of cell free DNA in a sample is currently achieved by either depleting maternal DNA through size exclusion or focusing on fetal-specific nucleic acids, like fetal-expressed RNA.
- fetal DNA Another differentiating feature of fetal DNA is its DNA methylation pattern.
- novel compositions and methods for accurately quantifying fetal nucleic acid based on differential methylation between a fetus and mother rely on sensitive absolute copy number analysis to quantify the fetal nucleic acid portion of a maternal sample, thereby allowing for the prenatal detection of fetal traits.
- the methods of the invention have identified approximately 3000 CpG rich regions in the genome that are differentially methylated between maternal and fetal DNA. The selected regions showed highly conserved differential methylation across all measured samples.
- the set of regions is enriched for genes important in developmental regulation, indicating that epigenetic regulation of these areas is a biologically relevant and consistent process (see Table 3).
- Enrichment of fetal DNA can now be achieved by using our MBD-FC protein to capture all cell free DNA and then elute the highly methylated DNA fraction with high salt concentrations. Using the low salt eluate fractions, the MBD-FC is equally capable of enriching non-methylated fetal DNA.
- the present invention provides 63 confirmed genomic regions on chromosomes 13, 18 and 21 with low maternal and high fetal methylation levels. After capturing these regions, SNPs can be used to determine the aforementioned allele ratios. When high frequency SNPs are used around 10 markers have to be measured to achieve a high confidence of finding at least one SNP where the parents have opposite homozygote genotypes and the child has a heterozygote genotype.
- a method for chromosomal abnormality detection utilizes absolute copy number quantification.
- a diploid chromosome set will show the same number of copies for differentially methylated regions across all chromosomes, but, for example, a trisomy 21 sample would show 1.5 times more copies for differentially methylated regions on chromosome 21.
- Normalization of the genomic DNA amounts for a diploid chromosome set can be achieved by using unaltered autosomes as reference (also provided herein - see Table IB). Comparable to other approaches, a single marker is less likely to be sufficient for detection of this difference, because the overall copy numbers are low. Typically there are approximately 100 to 200 copies of fetal DNA from 1 ml of maternal plasma at 10 to 12 weeks of gestation. However, the methods of the present invention offer a redundancy of detectable markers that enables highly reliable discrimination of diploid versus aneuploid chromosome sets.
- detection of a chromosome abnormality refers to identification of an imbalance of chromosomes by processing data arising from detecting sets of amplified nucleic acid species, nucleotide sequence species, or a detectable product generated from the foregoing (collectively “detectable product”). Any suitable detection device and method can be used to distinguish one or more sets of detectable products, as addressed herein.
- An outcome pertaining to the presence or absence of a chromosome abnormality can be expressed in any suitable form, including, without limitation, probability (e.g., odds ratio, p-value), likelihood, percentage, value over a threshold, or risk factor, associated with the presence of a chromosome abnormality for a subject or sample.
- An outcome may be provided with one or more of sensitivity, specificity, standard deviation, coefficient of variation (CV) and/or confidence level, or combinations of the foregoing, in certain embodiments.
- Detection of a chromosome abnormality based on one or more sets of detectable products may be identified based on one or more calculated variables, including, but not limited to, sensitivity, specificity, standard deviation, coefficient of variation (CV), a threshold, confidence level, score, probability and/or a combination thereof.
- CV coefficient of variation
- a threshold a threshold
- confidence level a threshold
- probability a combination thereof.
- the number of sets selected for a diagnostic method, and/or (ii) the particular nucleotide sequence species of each set selected for a diagnostic method is determined in part or in full according to one or more of such calculated variables.
- one or more of sensitivity, specificity and/or confidence level are expressed as a percentage.
- the percentage independently for each variable, is greater than about 90% (e.g., about 90, 91, 92, 93, 94, 95, 96, 97, 98 or 99%, or greater than 99% (e.g., about 99.5%, or greater, a bout 99.9% or greater, about 99.95% or greater, about 99.99% or greater)).
- Coefficient of variation in some embodiments is expressed as a percentage, and sometimes the percentage is about 10% or less (e.g., a bout 10, 9, 8, 7, 6, 5, 4, 3, 2 or 1%, or less than 1% (e.g., about 0.5% or less, about 0.1% or less, about 0.05% or less, about 0.01% or less)).
- a probability (e.g., that a particular outcome determined by an algorithm is not due to chance) in certain embodiments is expressed as a p- value, and sometimes the p-value is about 0.05 or less (e.g., about 0.05, 0.04, 0.03, 0.02 or 0.01, or less than 0.01 (e.g., about 0.001 or less, about 0.0001 or less, about 0.00001 or less, about 0.000001 or less)).
- scoring or a score may refer to calculating the probability that a particular chromosome abnormality is actually present or a bsent in a subject/sample.
- the value of a score may be used to determine for example the variation, difference, or ratio of amplified nucleic detectable product that may correspond to the actual chromosome abnormality. For example, calculating a positive score from detectable products can lead to an identification of a chromosome abnormality, which is particularly relevant to analysis of single samples.
- simulated (or simulation) data can aid data processing for example by training an algorithm or testing an algorithm.
- Simulated data may for instance involve hypothetical various samples of different concentrations of fetal and maternal nucleic acid in serum, plasma and the like.
- Simulated data may be based on what might be expected from a real population or may be skewed to test an algorithm and/or to assign a correct classification based on a simulated data set.
- Simulated data also is referred to herein as "virtual" data.
- Fetal/maternal contributions within a sample can be simulated as a table or array of numbers (for example, as a list of peaks corresponding to the mass signals of cleavage products of a reference biomolecule or amplified nucleic acid sequence), as a mass spectrum, as a pattern of bands on a gel, or as a representation of any technique that measures mass distribution. Simulations can be performed in most instances by a computer program.
- One possible step in using a simulated data set is to evaluate the confidence of the identified results, i.e. how well the selected positives/negatives match the sample and whether there are additional variations.
- a common approach is to calculate the probability value (p-value) which estimates the probability of a random sample having better score than the selected one. As p-value calculations can be prohibitive in certain circumstances, an empirical model may be assessed, in which it is assumed that at least one sample matches a reference sample (with or without resolved variations). Alternatively other distributions such as Poisson distribution can be used to describe the probability distribution.
- an algorithm can assign a confidence value to the true positives, true negatives, false positives and false negatives calculated.
- the assignment of a likelihood of the occurrence of a chromosome abnormality can also be based on a certain probability model.
- Simulated data often is generated in an in silico process.
- the term "in silico” refers to research and experiments performed using a computer. In silico methods include, but are not limited to, molecular modeling studies, karyotyping, genetic calculations, biomolecular docking experiments, and virtual representations of molecular structures and/or processes, such as molecular interactions.
- a "data processing routine" refers to a process, that can be embodied in software, that determines the biological significance of acquired data (i.e., the ultimate results of an assay). For example, a data processing routine can determine the amount of each nucleotide sequence species based upon the data collected. A data processing routine also may control an instrument and/or a data collection routine based upon results determined. A data processing routine and a data collection routine often are integrated and provide feed back to operate data acquisition by the instrument, and hence provide assay-based judging methods provided herein.
- software refers to computer readable program instructions that, when executed by a computer, perform computer operations.
- software is provided on a program product containing program instructions recorded on a computer readable medium, including, but not limited to, magnetic media including floppy disks, hard disks, and magnetic tape; and optical media including CD-ROM discs, DVD discs, magneto-optical discs, and other such media on which the program instructions can be recorded.
- true positive refers to a subject correctly diagnosed as having a chromosome abnormality.
- false positive refers to a subject wrongly identified as having a chromosome abnormality.
- true negative refers to a subject correctly identified as not having a chromosome abnormality.
- false negative refers to a subject wrongly identified as not having a chromosome abnormality.
- Two measures of performance for any given method can be calculated based on the ratios of these occurrences: (i) a sensitivity value, the fraction of predicted positives that are correctly identified as being positives (e.g., the fraction of nucleotide sequence sets correctly identified by level comparison
- Example 1 the Applicants used a new fusion protein that captures methylated DNA in combination with CpG Island array to identify genomic regions that are differentially methylated between fetal placenta tissue and maternal blood.
- a stringent statistical approach was used to only select regions which show little variation between the samples, and hence suggest an underlying biological mechanism.
- Eighty-five differentially methylated genomic regions predominantly located on chromosomes 13, 18 and 21 were validated.
- a quantitative mass spectrometry based approach was used that interrogated 261 PC amplicons covering these 85 regions. The results are in very good concordance (95% confirmation), proving the feasibility of the approach.
- the Applicants provide an innovative approach for aneuploidy testing, which relies on the measurement of absolute copy numbers rather than allele ratios.
- genomic DNA from maternal buffy coat and corresponding placental tissue was first extracted.
- M BD-FC was used to capture the methylated fraction of each DNA sample. See Figures 1-3.
- the two tissue fractions were labeled with different fluorescent dyes and hybridized to an Agilent ® CpG Island microarray. See Figure 4. This was done to identify differentially methylated regions that could be utilized for prenatal diagnoses. Therefore, two criteria were employed to select genomic regions as potential enrichment markers: the observed methylation difference had to be present in all tested sample pairs, and the region had to be more than 200 bp in length.
- Genomic DNA (gDNA) from maternal buffy coat and placental tissue was prepared using the QIAamp DNA Mini KitTM and QIAamp DNA Blood Mini KitTM, respectively, from Qiagen ® (Hilden, Germany).
- gDNA was quantified using the NanoDrop ND 1000TM spectrophotometer (Thermo Fisher ® , Waltham, MA,USA).
- Ultrasonication of 2.5 ⁇ g DNA in 500 ⁇ TE buffer to a mean fragment size of 300- 500 bp was carried out with the Branson Digital Sonifier 450TM (Danbury, CT, USA) using the following settings: amplitude 20%, sonication time 110 seconds, pulse on/pulse off time 1.4/0.6 seconds.
- Fragment range was monitored using gel electrophoresis.
- Sonicated DNA (2 ⁇ g) was added to the washed MBD-Fc beads in 2 ml Buffer A and rotated for 3 hours at 4°C. Beads were centrifuged to recover unbound DNA fragments (300 mM fraction) and subsequently washed twice with 600 ⁇ of buffers containing increasing NaCI concentrations (400, 500, 550, 600, and 1000 mM). The flow through of each wash step was collected in separate tubes and desalted using a MinElute PCR Purification KitTM (Qiagen ® ). In parallel, 200 ng sonicated input DNA was processed as a control using the MinElute PC Purification KitTM (Qiagen ® ).
- the 600 mM and 1M NaCI fractions (enriched methylated DNA) for each sample were combined and labeled with either Alexa Fluor 555- aha-dCTP (maternal) or Alexa Fluor 647-aha-dCTP (placental) using the BioPrime Total Genomic Labeling SystemTM (Invitrogen ® , Carlsbad, CA, USA).
- the labeling reaction was carried out according to the manufacturer's manual.
- Genomic DNA sodium bisulfite conversion was performed using EZ-96 DNA Methylation KitTM
- Sequenom's MassARRAY ® System was used to perform quantitative methylation analysis. This system utilizes matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry in combination with RNA base specific cleavage (Sequenom ® MassCLEAVETM). A detectable pattern is then analyzed for methylation status. PCR primers were designed using Sequenom ® EpiDESIGNERTM
- MassARRAYTM Compact MALDI-TOF (Sequenom ® , San Diego) and methylation ratios were generated by the EpiTYPERTM software vl.O (Sequenom ® , San Diego). Statistical analysis
- Groups that contained less than 4 probes were excluded from the analysis. For groups including four or five probes, all probes were used in a paired t-test. For Groups with six or more probes, a sliding window test consisting of five probes at a time was used, whereby the window was moved by one probe increments. Each test sample was compared to the control sample and the p- values were recorded. Genomic regions were selected as being differentially methylated if eight out of ten samples showed a p value ⁇ 0.01, or if six out of ten samples showed a p value ⁇ 0.001.
- genomic regions were classified as being not differentially methylated when the group showed less than eight samples with a p value ⁇ 0.01 and less than six samples with a p value ⁇ 0.001. Samples that didn't fall in either category were excluded from the analysis. For a subset of genomic regions that have been identified as differentially methylated, the results were confirmed using quantitative methylation analysis.
- Go analysis was performed using the online GOstat tool (https://gostat.wehi.edu.au/cgibin/- goStat.pl). P values were calculated using Fisher's exact test.
- a standard sample was used, in which the methylated DNA fraction of monocytes was hybridized against itself. This standard provided a reference for the variability of fluorescent measurements in a genomic region. Differentially methylated regions were then identified by comparing the log ratios of each of the ten placental/maternal samples against this standard. Because the goal of this study was to identify markers that allow the reliable separation of maternal and fetal DNA, the target selection was limited to genes that showed a stable, consistent methylation difference over a contiguous stretch of genomic DNA. This focused the analysis on genomic regions where multiple probes indicated differential methylation. The selection was also limited to target regions where all samples showed differential methylation, excluding those with strong inter- individual differences. Two of the samples showed generally lower log ratios in the microarray analysis. Because a paired test was used for target selection, this did not negatively impact the results.
- a GO analysis of the set of differentially methylated genes reveals that this set is significantly enriched for functions important during development.
- Example 2 describes a non-invasive approach for detecting the amount of fetal nucleic acid present in a maternal sample (herein referred to as the "Fetal Quantifier Method"), which may be used to detect or confirm fetal traits (e.g., fetal sex of hD compatibility), or diagnose chromosomal abnormalities such as Trisomy 21 (both of which are herein referred to as the "Methylation-Based Fetal Diagnostic Method”).
- Figure 10 shows one embodiment of the Fetal Quantifier Method
- Figure 11 shows one
- Both processes use fetal DNA obtained from a maternal sample.
- the sample comprises maternal and fetal nucleic acid that is differentially methylated.
- the sample may be maternal plasma or serum.
- Fetal DNA comprises approximately 2-30% of the total DNA in maternal plasma.
- the actual amount of fetal contribution to the total nucleic acid present in a sample varies from pregnancy to pregnancy and can change based on a number of factors, including, but not limited to, gestational age, the mother's health and the fetus' health.
- the technical challenge posed by analysis of fetal DNA in maternal plasma lies in the need to be able to discriminate the fetal DNA from the co-existing background maternal DNA.
- the methods of the present invention exploit such differences, for example, the differential methylation that is observed between fetal and maternal DNA, as a means to enrich for the relatively small percentage of fetal DNA present in a sample from the mother.
- the non-invasive nature of the approach provides a major advantage over conventional methods of prenatal diagnosis such as, amniocentesis, chronic villus sampling and cordocentesis, which are associated with a small but finite risk of fetal loss.
- the method is not dependent on fetal cells being in any particular cell phase, the method provides a rapid detection means to determine the presence and also the nature of the chromosomal abnormality. Further, the approach is sex-independent (i.e., does not require the presence of a Y-chromosome) and polymorphic-independent (i.e., an allelic ratio is not determined).
- the compositions and methods of the invention represent improved universal, noninvasive approaches for accurately determining the amount of fetal nucleic acid present in a maternal sample.
- the present invention takes advantage of the presence of circulating, cell free fetal nucleic acid (ccfDNA) in maternal plasma or serum.
- ccfDNA circulating, cell free fetal nucleic acid
- the methods of the invention should only consume a small portion of the limited available fetal DNA. For example, less than 50%, 40%, 30%, 25%, 20%, 15%, 10%, 5% or less of the sample.
- the approach should preferably be developed in a multiplex assay format in which one or more (preferably all) of the following assays are included: • Assays for the detection of total amount of genomic equivalents present in the sample, i.e., assays recognizing both maternal and fetal DNA species;
- a target-specific, competitor oligonucleotide that is identical, or substantially identical, to the target sequence apart from a distinguishable feature of the competitor, such as a difference in one or more nucleotides relative to the target sequence.
- This oligonucleotide when added into the PC reaction will be co-amplified with the target and a ratio obtained between these two PCR amplicons will indicate the number of target specific DNA sequences (e.g., fetal DNA from a specific locus) present in the maternal sample.
- the amplicon lengths should preferably be of similar length in order not to skew the
- Differentially methylated targets can be selected from Tables 1A-1C or from any other targets known to be differentially methylated between mother and fetus. These targets can be hypomethylated in DNA isolated from non-pregnant women and hypermethylated in samples obtained from fetal samples. These assays will serve as controls for the restriction efficiency.
- fetal fraction of the amplifiable genomes fetal concentration or percentage
- Differences in copy number between fetally-derived DNA sequences for example, between fetal chromosome 21 and a reference chromosome such as chromosome 3).
- methylation sensitive restriction enzymes for example, Hhal and Hpall.
- Genomic Amplification- PCR was performed in a total volume of 50 ul by adding PCR reagents (Buffer, dNTPs, primers and polymerase). Exemplary PCR and extend primers are provided below. In addition, synthetic competitor oligonucleotide was added at known concentrations.
- the primer extension products can be simultaneously separated and detected using Matrix Assisted Laser Desorption/lonization, Time-Of-Flight (MALDI-TOF) mass spectrometry on the MassARRAY ® Analyzer Compact. Following this separation and detection, SEQUENOM's proprietary software automatically analyzes the data.
- MALDI-TOF Time-Of-Flight
- Targets were selected in housekeeping genes not located on the chromosomes 13, 18, 21, X or Y.
- the targets should be in a single copy gene and not contain any recognition sites for the methylation sensitive restriction enzymes.
- Targets specific for the Y-chromosome were selected, with no similar or paralog sequences elsewhere in the genome.
- the targets should preferably be in a single copy gene and not contain any recognition sites for the methylation sensitive restriction enzyme(s).
- Underlined sequences are PCR primer sites, and italic nucleotide(s) is the site for the single-base extend primer and bold letter (C) is the nucleotide extended on human DNA.
- Targets were selected in regions known to be differentially methylated between maternal and fetal DNA. Sequences were selected to contain several restriction sites for methylation sensitive enzymes. For this study the Hhal (GCGC) and Hpall (CCGG) enzymes were used.
- Underlined sequences are PCR primer sites, italic is the site for the single base extend primer and bold letter (C) is the nucleotide extended on human DNA, lower case letter are recognition sites for the methylation sensitive restriction enzymes.
- Targets were selected in regions known not to be methylated in any tissue to be investigated.
- Sequences were selected to contain no more than one site for each restriction enzyme to be used.
- the sensitivity and accuracy of the present invention was measured using both a model system and clinical samples.
- a multiplex assay was run that contains 2 assays for total copy number quantification, 3 assays for methylation quantification, 1 assay specific for chromosome Y and 1 digestion control assay. See Table X.
- Another multiplex scheme with additional assays is provided in Table Y.
- a model system was developed to simulate DNA samples isolated from plasma. These samples contained a constant number of maternal non-methylated DNA and were spiked with different amounts of male placental methylated DNA. The samples were spiked with amounts ranging from approximately 0 to 25% relative to the maternal non-methylated DNA. The results are shown in Figures 13A and B. The fraction of placental DNA was calculated using the ratios obtained from the methylation assays ( Figure 13A), the S Y markers ( Figure 13B) and the total copy number assays. The primer sequences for the methylation assays (TBX), Y-chromosome assays (SRY) and total copy number (APOE) are provided above. The model system demonstrated that the methylation-based method performed equal to the Y-chromosome method (SRY markers), thus validating the methylation- based method as a sex-independent fetal quantifier.
- Figure 14A The results from the total copy number quantification can be seen in Figures 14A and B.
- Figure 14A the copy number for each sample is shown. Two samples (nos. 25 and 26) have a significantly higher total copy number than all the other samples. In general, a mean of approximately 1300 amplifiable copies/ml plasma was obtained (range 766-2055).
- Figure 14B shows a box-and-whisker plot of the given values, summarizing the results.
- Figures 15A and B the numbers of fetal copies for each sample are plotted. As all samples were from male pregnancies. The copy numbers obtained can be calculated using either the methylation or the Y- chromosome-specific markers. As can be seen in Figure 15B, the box-and-whisker plot of the given values indicated minimal difference between the two different measurements.
- Mass spectra analysis was done using Typer 4 (a Sequenom software product). The peak height (signal over noise) for each individual DNA analyte and competitor assay was determined and exported for further analysis.
- the total number of molecules present for each amplicon was calculated by dividing the DNA specific peak by the competitor specific peak to give a ratio. (The "DNA” Peak in Figures 18 and 19 can be thought of as the analyte peak for a given assay). Since the number of competitor molecules added into the reaction is known, the total number of DNA molecules can be determined by multiplying the ratio by the number of added competitor molecules.
- the fetal DNA fraction (or concentration) in each sample was calculated using the Y-chromosome- specific markers for male pregnancies and the mean of the methylated fraction for all pregnancies.
- the ratio was obtained by dividing the analyte (DNA) peak by the competitor peak and multiplying this ratio by the number of competitor molecules added into the reaction. This value was divided by a similar ratio obtained from the total number of amplifiable genome equivalents determination (using the Assay(s) for Total Amount). See Figure 18. Since the total amount of nucleic acid present in a sample is a sum of maternal and fetal nucleic acid, the fetal contribution can be considered to be a fraction of the larger, background maternal contribution.
- a first simple power calculation was performed that assumes a measurement system that uses 20 markers from chromosome 21, and 20 markers from one or more other autosomes. Starting with 100 copies of fetal DNA, a measurement standard deviation of 25 copies and the probability for a type I error to be lower than 0.001, it was found that the methods of the invention will be able to differentiate a diploid from a triploid chromosome set in 99.5% of all cases.
- the practical implementation of such an approach could for example be achieved using mass spectrometry, a system that uses a competitive PCR approach for absolute copy number measurements.
- the method can run 20 assays in a single reaction and has been shown to have a standard deviation in repeated measurements of around 3 to 5%.
- FIG. 8 shows the effectiveness of MBD- FC protein (a methyl-binding agent) for capturing and thereby separating methylated DNA in the presence of an excess of unmethylated DNA (see Figure 8).
- a second statistical power analysis was performed to assess the predictive power of an embodiment of the Methylation-Based Fetal Diagnostic Method described herein.
- the simulation was designed to demonstrate the likelihood of differentiating a group of trisomic chromosome 21 specific markers from a group of reference markers (for example, autosomes excluding chromosome 21). Many parameters influence the ability to discriminate the two populations of markers reliably. For the present simulation, values were chosen for each parameter that have been shown to be the most likely to occur based on experimentation. The following parameters and respective values were used:
- Average methylation percentage in a target region for fetal DNA 80%
- differentially-methylated targets were selected for further analysis based upon previous microarray analysis. See Example 1 for a description of the microarray analysis.
- DM s differentially methylated regions
- Regions were selected for EpiTYPER confirmation based upon being hypermethylated in placenta relative to PBMC.
- regions were chosen based upon statistical significance with regions designed beginning with the most significant and working downward in terms of significance.
- the microarray screen uncovered only a subset of DMRs located on chromosome 21.
- the coverage of chromosome 21 by the microarray was insufficient. Therefore a further analysis was completed to examine all 356 CpG islands on chromosome 21 using the standard settings of the UCSC genome browser. As shown in Table 1C below, some of these targets overlapped with those already examined in Table 1A. More specifically, CpG sites located on chromosome 21 including ⁇ 1000bp upstream and downstream of each CpG was investigated using Sequenom's EpiTYPER ® technology. See Example 1, "Validation using Sequenom 9 EpiTYPERTM" for a description of Sequenom's EpiTYPER ® technology.
- Tables IB and 1C provide a description of the different targets, including their location and whether they were analyzed during the different phases of analysis, namely microarray analysis, EpiTYPER 8 analysis and EpiTYPER 73 analysis. A “YES” indicates it was analyzed and a “NO” indicates it was not analyzed. The definition of each column in Table IB and 1C is listed below.
- Region Name Each region is named by the gene(s) residing within the area defined or nearby.
- Regions where no gene name is listed but rather only contain a locus have no refseq genes in near proximity.
- Gene Region For those regions contained either in close proximity to or within a gene, the gene region further explains the relationship of this region to the nearby gene.
- Chrom The chromosome on which the DMR is located using the hgl8 build of the UCSC genome browser.
- PBMC Alexa Fluor 555-aha-dCTP
- Alexa Fluor 647-aha-dCTP placental
- PBMC peripheral blood mononuclear cells
- EpiTYPER 73 Samples Describes whether this region was subsequently analyzed using EpiTYPER technology in a sample cohort consisting of 73 paired samples of placenta and PBMC. All regions selected for analysis in this second sample cohort were selected based on the results from the experimentation described in the EpiTYPER 8 column. More specifically, the regions in this additional cohort exhibited a methylation profile similar to that determined in the EpiTYPER 8 Samples analysis. For example, all of the regions listed in Tables 1B-1C exhibit different levels of DNA methylation in a significant portion of the examined CpG dinucleotides within the defined region. Differential DNA methylation of CpG sites was determined using a paired T Test with those sites considered differentially methylated if the p-value (when comparing placental tissue to PBMC) is p ⁇ 0.05.
- Regions labeled as "hypermethylation” are more methylated within the designated region in placenta samples relative to PBMC and "hypomethylation" are more methylated within the designated region in PBMC samples.
- chr13 1 11595459- chr13 group00385 chr13 1 11595578 1 1 1595955 0.87 0.06 0.2 0.14 HYPERMETHYLATION
- chr13 1 11755805- chr13 group00390 chr13 1 11756337 1 1 1756593 0.71 0.12 0.34 0.22 HYPERMETHYLATION
- chr13 1 11757885- chr13 group00391 chr13 1 11759856 1 1 1760045 0.86 0.1 1 0.36 0.25 HYPERMETHYLATION
- chr12 1 18515877- chr12 group00801 chr12 1 18516189 1 18517435 1.1 0.06 0.25 0.19 HYPERMETHYLATION
- rs4433898 rs34497518; rs35135773; rs6566677; rs57425572; rs36026929; rs34666288; rs10627137; rs35943684; rs9964226; rs4892054; rs9964397; rs4606820; rs12966677;
- rs2236618 rs1 1908971 ; rs9975039; rs6517135; rs2009130; rs1005573; rs1122807; rs10653491 ; rs10653077; rs35086972; rs28588289; rs7509766; rs622161 14; rs35561747;
- rs2843956 rs55941652; rs56020428; rs56251824; rs13051109; rs13051 1 1 1 ; rs3833348; rs7510136; rs743289; rs5843690; rs33915227; rs11402829; rs2843723; rs8128138;
- GCTTTGGATTTATCCTCA GGCTAAATCCCTCCTGAAACATGAAACTGAAACAAAGCCCTGAACCCCCTCAGGCTGAAAAGACAAACCCCGCCTGAGGCCGG TCCCGCTCCCCACCTGGAGGGACCCAATTCTGGGCGCCTTCTGGCGACGGTCCCTGCTAGGGACGCTGCGCTCTCCGAGTGCGAGTTTTCGCCAAACTGATAA
- AAAGAAT GAAGT C AT GCCCCGGCCTGCACCC GGGAAAC T GC AC AC AGC G
- AAAGAT C GC C AC T GAGAT AAAGAGC T GAAAGC TATTCCCCAATTCAGC
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Engineering & Computer Science (AREA)
- Analytical Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Physics & Mathematics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Pathology (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Medicinal Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Tropical Medicine & Parasitology (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Enzymes And Modification Thereof (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
Claims
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN3139DEN2012 IN2012DN03139A (en) | 2009-09-16 | 2010-03-18 | |
| EP10817598.5A EP2478119B1 (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
| CA2774342A CA2774342C (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| EP20155147.0A EP3722440A1 (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| AU2010295968A AU2010295968B2 (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
| ES10817598.5T ES2650666T3 (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for fetal nucleic acid-based enrichment of a maternal sample useful for non-invasive prenatal diagnoses |
| JP2012529756A JP5873434B2 (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for enrichment based on methylation of fetal nucleic acids from maternal samples useful for non-invasive prenatal diagnosis |
| EP17182863.5A EP3330382B1 (en) | 2009-09-16 | 2010-03-18 | Processes for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| CN2010800527486A CN102648292A (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/561,241 | 2009-09-16 | ||
| US12/561,241 US8476013B2 (en) | 2008-09-16 | 2009-09-16 | Processes and compositions for methylation-based acid enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011034631A1 true WO2011034631A1 (en) | 2011-03-24 |
Family
ID=43759623
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/027879 WO2011034631A1 (en) | 2009-09-16 | 2010-03-18 | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
Country Status (10)
| Country | Link |
|---|---|
| US (7) | US8476013B2 (en) |
| EP (3) | EP3722440A1 (en) |
| JP (7) | JP5873434B2 (en) |
| CN (1) | CN102648292A (en) |
| AU (1) | AU2010295968B2 (en) |
| CA (3) | CA3024967C (en) |
| ES (1) | ES2650666T3 (en) |
| HK (1) | HK1254596A1 (en) |
| IN (1) | IN2012DN03139A (en) |
| WO (1) | WO2011034631A1 (en) |
Cited By (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102676513A (en) * | 2012-05-28 | 2012-09-19 | 武汉大学 | Fetal epigenetic marker and application thereof |
| WO2012149339A3 (en) * | 2011-04-29 | 2013-03-14 | Sequenom, Inc. | Quantification of a minority nucleic acid species |
| WO2013052907A2 (en) | 2011-10-06 | 2013-04-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013052913A2 (en) | 2011-10-06 | 2013-04-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013055817A1 (en) | 2011-10-11 | 2013-04-18 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013177086A1 (en) | 2012-05-21 | 2013-11-28 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013192562A1 (en) | 2012-06-22 | 2013-12-27 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014011928A1 (en) | 2012-07-13 | 2014-01-16 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| US8688388B2 (en) | 2011-10-11 | 2014-04-01 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014055790A2 (en) | 2012-10-04 | 2014-04-10 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014055774A1 (en) | 2012-10-04 | 2014-04-10 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014116598A2 (en) | 2013-01-25 | 2014-07-31 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014164838A1 (en) | 2013-03-12 | 2014-10-09 | Sequenom, Inc. | Quantification of cell-specific nucleic acid markers having a particular methylation state |
| WO2014165596A1 (en) | 2013-04-03 | 2014-10-09 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014168711A1 (en) | 2013-03-13 | 2014-10-16 | Sequenom, Inc. | Primers for dna methylation analysis |
| WO2014182726A2 (en) | 2013-05-07 | 2014-11-13 | Sequenom, Inc. | Genetic markers for macular degeneration disorder treatment |
| US8962247B2 (en) | 2008-09-16 | 2015-02-24 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
| WO2015051163A2 (en) | 2013-10-04 | 2015-04-09 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2015054080A1 (en) | 2013-10-07 | 2015-04-16 | Sequenom, Inc. | Methods and processes for non-invasive assessment of chromosome alterations |
| US9127312B2 (en) | 2011-02-09 | 2015-09-08 | Bio-Rad Laboratories, Inc. | Analysis of nucleic acids |
| WO2015138774A1 (en) * | 2014-03-13 | 2015-09-17 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP2942400A1 (en) | 2014-05-09 | 2015-11-11 | Lifecodexx AG | Multiplex detection of DNA that originates from a specific cell-type |
| EP2942401A1 (en) | 2014-05-09 | 2015-11-11 | Lifecodexx AG | Detection of DNA that originates from a specific cell-type |
| WO2015169947A1 (en) * | 2014-05-09 | 2015-11-12 | Lifecodexx Ag | Detection of dna that originates from a specific cell-type and related methods |
| WO2015183872A1 (en) | 2014-05-30 | 2015-12-03 | Sequenom, Inc. | Chromosome representation determinations |
| US9305756B2 (en) | 2013-03-13 | 2016-04-05 | Agena Bioscience, Inc. | Preparation enhancements and methods of use for MALDI mass spectrometry |
| US9310378B2 (en) | 2008-01-15 | 2016-04-12 | Agena Bioscience, Inc. | Compositions and processes for improved mass spectrometry analysis |
| WO2016057901A1 (en) | 2014-10-10 | 2016-04-14 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP2900837A4 (en) * | 2012-09-26 | 2016-06-01 | Agency Science Tech & Res | Biomarkers for down syndrome prenatal diagnosis |
| US9367663B2 (en) | 2011-10-06 | 2016-06-14 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US9605313B2 (en) | 2012-03-02 | 2017-03-28 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3168309A1 (en) | 2015-11-10 | 2017-05-17 | Lifecodexx AG | Detection of foetal chromosomal aneuploidies using dna regions that are differentially methylated between the foetus and the pregnant female |
| WO2017205826A1 (en) | 2016-05-27 | 2017-11-30 | Sequenom, Inc. | Methods for detecting genetic variations |
| WO2018022906A1 (en) | 2016-07-27 | 2018-02-01 | Sequenom, Inc. | Methods for non-invasive assessment of genomic instability |
| WO2018022890A1 (en) | 2016-07-27 | 2018-02-01 | Sequenom, Inc. | Genetic copy number alteration classifications |
| US9920361B2 (en) | 2012-05-21 | 2018-03-20 | Sequenom, Inc. | Methods and compositions for analyzing nucleic acid |
| US9926593B2 (en) | 2009-12-22 | 2018-03-27 | Sequenom, Inc. | Processes and kits for identifying aneuploidy |
| US9984198B2 (en) | 2011-10-06 | 2018-05-29 | Sequenom, Inc. | Reducing sequence read count error in assessment of complex genetic variations |
| WO2018136881A1 (en) | 2017-01-20 | 2018-07-26 | Sequenom, Inc. | Sequencing adapter manufacture and use |
| WO2018136888A1 (en) | 2017-01-20 | 2018-07-26 | Sequenom, Inc. | Methods for non-invasive assessment of genetic alterations |
| WO2018136882A1 (en) | 2017-01-20 | 2018-07-26 | Sequenom, Inc. | Methods for non-invasive assessment of copy number alterations |
| WO2018140521A1 (en) | 2017-01-24 | 2018-08-02 | Sequenom, Inc. | Methods and processes for assessment of genetic variations |
| WO2018170511A1 (en) | 2017-03-17 | 2018-09-20 | Sequenom, Inc. | Methods and processes for assessment of genetic mosaicism |
| US10196681B2 (en) | 2011-10-06 | 2019-02-05 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2019140201A1 (en) | 2018-01-12 | 2019-07-18 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| EP3540076A1 (en) | 2013-06-21 | 2019-09-18 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10424394B2 (en) | 2011-10-06 | 2019-09-24 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10504613B2 (en) | 2012-12-20 | 2019-12-10 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3578670A1 (en) | 2013-05-24 | 2019-12-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10738358B2 (en) | 2008-09-16 | 2020-08-11 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| WO2020206143A1 (en) | 2019-04-05 | 2020-10-08 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| EP3760739A1 (en) | 2014-07-30 | 2021-01-06 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2021072037A1 (en) | 2019-10-09 | 2021-04-15 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| CN112662761A (en) * | 2020-03-05 | 2021-04-16 | 博尔诚(北京)科技有限公司 | Probe composition for detecting 3 parenchymal organ tumors |
| WO2021087491A1 (en) | 2019-10-31 | 2021-05-06 | Sequenom, Inc. | Application of mosaicism ratio in multifetal gestations and personalized risk assessment |
| US11004537B2 (en) | 2011-06-24 | 2021-05-11 | Sequenom, Inc. | Methods and processes for non invasive assessment of a genetic variation |
| WO2021262805A1 (en) | 2020-06-24 | 2021-12-30 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| US11299780B2 (en) | 2016-07-15 | 2022-04-12 | The Regents Of The University Of California | Methods of producing nucleic acid libraries |
| WO2022076574A1 (en) | 2020-10-08 | 2022-04-14 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| US11629345B2 (en) | 2018-06-06 | 2023-04-18 | The Regents Of The University Of California | Methods of producing nucleic acid libraries and compositions and kits for practicing same |
| WO2023086967A1 (en) * | 2021-11-12 | 2023-05-19 | Guardant Health, Inc. | Method of analysis of methylated dna-binding proteins |
| US11697849B2 (en) | 2012-01-20 | 2023-07-11 | Sequenom, Inc. | Methods for non-invasive assessment of fetal genetic variations that factor experimental conditions |
| US11952616B2 (en) | 2016-12-22 | 2024-04-09 | Guardant Health, Inc. | Methods and systems for analyzing nucleic acid molecules |
| WO2024186778A1 (en) | 2023-03-03 | 2024-09-12 | Laboratory Corporation Of America Holdings | Methods and systems for positive cfdna screening on genetic variations using mosaicism ratio |
Families Citing this family (116)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11111544B2 (en) | 2005-07-29 | 2021-09-07 | Natera, Inc. | System and method for cleaning noisy genetic data and determining chromosome copy number |
| US9424392B2 (en) | 2005-11-26 | 2016-08-23 | Natera, Inc. | System and method for cleaning noisy genetic data from target individuals using genetic data from genetically related individuals |
| AU2009223671B2 (en) * | 2008-03-11 | 2014-11-27 | Sequenom, Inc. | Nucleic acid-based tests for prenatal gender determination |
| CN102216456A (en) * | 2008-09-16 | 2011-10-12 | 塞昆纳姆股份有限公司 | Methods and compositions for methylation-based enrichment of fetal nucleic acid in maternal samples for non-invasive prenatal diagnosis |
| US8825412B2 (en) | 2010-05-18 | 2014-09-02 | Natera, Inc. | Methods for non-invasive prenatal ploidy calling |
| US9315857B2 (en) | 2009-12-15 | 2016-04-19 | Cellular Research, Inc. | Digital counting of individual molecules by stochastic attachment of diverse label-tags |
| US8835358B2 (en) | 2009-12-15 | 2014-09-16 | Cellular Research, Inc. | Digital counting of individual molecules by stochastic attachment of diverse labels |
| CN102892899A (en) * | 2010-01-26 | 2013-01-23 | Nipd遗传学有限公司 | Methods and compositions for noninvasive prenatal diagnosis of fetal aneuploidies |
| US11332785B2 (en) | 2010-05-18 | 2022-05-17 | Natera, Inc. | Methods for non-invasive prenatal ploidy calling |
| US11322224B2 (en) | 2010-05-18 | 2022-05-03 | Natera, Inc. | Methods for non-invasive prenatal ploidy calling |
| US11408031B2 (en) | 2010-05-18 | 2022-08-09 | Natera, Inc. | Methods for non-invasive prenatal paternity testing |
| US10316362B2 (en) | 2010-05-18 | 2019-06-11 | Natera, Inc. | Methods for simultaneous amplification of target loci |
| US20190010543A1 (en) | 2010-05-18 | 2019-01-10 | Natera, Inc. | Methods for simultaneous amplification of target loci |
| US9677118B2 (en) | 2014-04-21 | 2017-06-13 | Natera, Inc. | Methods for simultaneous amplification of target loci |
| US11326208B2 (en) | 2010-05-18 | 2022-05-10 | Natera, Inc. | Methods for nested PCR amplification of cell-free DNA |
| US11332793B2 (en) | 2010-05-18 | 2022-05-17 | Natera, Inc. | Methods for simultaneous amplification of target loci |
| US11339429B2 (en) | 2010-05-18 | 2022-05-24 | Natera, Inc. | Methods for non-invasive prenatal ploidy calling |
| US11939634B2 (en) | 2010-05-18 | 2024-03-26 | Natera, Inc. | Methods for simultaneous amplification of target loci |
| US11031095B2 (en) | 2010-08-06 | 2021-06-08 | Ariosa Diagnostics, Inc. | Assay systems for determination of fetal copy number variation |
| US20120190557A1 (en) * | 2011-01-25 | 2012-07-26 | Aria Diagnostics, Inc. | Risk calculation for evaluation of fetal aneuploidy |
| US8700338B2 (en) | 2011-01-25 | 2014-04-15 | Ariosa Diagnosis, Inc. | Risk calculation for evaluation of fetal aneuploidy |
| US20140342940A1 (en) | 2011-01-25 | 2014-11-20 | Ariosa Diagnostics, Inc. | Detection of Target Nucleic Acids using Hybridization |
| US10533223B2 (en) | 2010-08-06 | 2020-01-14 | Ariosa Diagnostics, Inc. | Detection of target nucleic acids using hybridization |
| US20130261003A1 (en) | 2010-08-06 | 2013-10-03 | Ariosa Diagnostics, In. | Ligation-based detection of genetic variants |
| US11203786B2 (en) | 2010-08-06 | 2021-12-21 | Ariosa Diagnostics, Inc. | Detection of target nucleic acids using hybridization |
| US20120034603A1 (en) | 2010-08-06 | 2012-02-09 | Tandem Diagnostics, Inc. | Ligation-based detection of genetic variants |
| US20130040375A1 (en) | 2011-08-08 | 2013-02-14 | Tandem Diagnotics, Inc. | Assay systems for genetic analysis |
| US10167508B2 (en) | 2010-08-06 | 2019-01-01 | Ariosa Diagnostics, Inc. | Detection of genetic abnormalities |
| EP2668605B1 (en) * | 2011-01-25 | 2019-03-06 | Ariosa Diagnostics, Inc. | Risk calculation for evaluation of fetal aneuploidy |
| US9994897B2 (en) | 2013-03-08 | 2018-06-12 | Ariosa Diagnostics, Inc. | Non-invasive fetal sex determination |
| US11270781B2 (en) | 2011-01-25 | 2022-03-08 | Ariosa Diagnostics, Inc. | Statistical analysis for non-invasive sex chromosome aneuploidy determination |
| US10131947B2 (en) | 2011-01-25 | 2018-11-20 | Ariosa Diagnostics, Inc. | Noninvasive detection of fetal aneuploidy in egg donor pregnancies |
| US8756020B2 (en) | 2011-01-25 | 2014-06-17 | Ariosa Diagnostics, Inc. | Enhanced risk probabilities using biomolecule estimations |
| CN103608818B (en) | 2011-02-09 | 2017-12-08 | 纳特拉公司 | The antenatal ploidy identification device of Noninvasive |
| US12097495B2 (en) * | 2011-02-18 | 2024-09-24 | Bio-Rad Laboratories, Inc. | Methods and compositions for detecting genetic material |
| CN106011237B (en) * | 2011-02-24 | 2019-12-13 | 香港中文大学 | Molecular testing of multiple pregnancies |
| US8712697B2 (en) | 2011-09-07 | 2014-04-29 | Ariosa Diagnostics, Inc. | Determination of copy number variations using binomial probability calculations |
| SG11201404243WA (en) | 2012-01-26 | 2014-08-28 | Nugen Technologies Inc | Compositions and methods for targeted nucleic acid sequence enrichment and high efficiency library generation |
| EP2820174B1 (en) | 2012-02-27 | 2019-12-25 | The University of North Carolina at Chapel Hill | Methods and uses for molecular tags |
| AU2013226081B2 (en) | 2012-02-27 | 2018-06-14 | Becton, Dickinson And Company | Compositions and kits for molecular counting |
| WO2013130857A1 (en) * | 2012-02-29 | 2013-09-06 | Bio Dx, Inc. | Defining diagnostic and therapeutic targets of conserved fetal dna in maternal circulating blood |
| US9127317B2 (en) | 2012-03-02 | 2015-09-08 | Winthrop-University Hospital | Method for using probe based PCR detection to measure the levels of circulating demethylated β cell derived DNA as a measure of β cell loss in diabetes |
| ES2945311T3 (en) * | 2012-03-26 | 2023-06-30 | Univ Johns Hopkins | Rapid detection of aneuploidy |
| US10289800B2 (en) | 2012-05-21 | 2019-05-14 | Ariosa Diagnostics, Inc. | Processes for calculating phased fetal genomic sequences |
| US20150011396A1 (en) * | 2012-07-09 | 2015-01-08 | Benjamin G. Schroeder | Methods for creating directional bisulfite-converted nucleic acid libraries for next generation sequencing |
| WO2014015269A1 (en) | 2012-07-19 | 2014-01-23 | Ariosa Diagnostics, Inc. | Multiplexed sequential ligation-based detection of genetic variants |
| US20140100126A1 (en) | 2012-08-17 | 2014-04-10 | Natera, Inc. | Method for Non-Invasive Prenatal Testing Using Parental Mosaicism Data |
| WO2014089368A1 (en) * | 2012-12-05 | 2014-06-12 | Bio-Rad Laboratories, Inc | Methods for polymerase chain reaction copy number variation assays |
| EP3842542A1 (en) | 2013-08-28 | 2021-06-30 | Becton, Dickinson and Company | Massively parallel single cell analysis |
| EP3055676A1 (en) | 2013-10-07 | 2016-08-17 | Cellular Research, Inc. | Methods and systems for digitally counting features on arrays |
| AU2014333776B2 (en) * | 2013-10-11 | 2021-01-28 | Cellectis | Methods and kits for detecting nucleic acid sequences of interest using DNA-binding protein domain |
| CN109971852A (en) | 2014-04-21 | 2019-07-05 | 纳特拉公司 | Detect the mutation and ploidy in chromosome segment |
| KR102696857B1 (en) | 2014-07-25 | 2024-08-19 | 유니버시티 오브 워싱톤 | Methods of determining tissues and/or cell types giving rise to cell-free dna, and methods of identifying a disease or disorder using same |
| US10174383B2 (en) | 2014-08-13 | 2019-01-08 | Vanadis Diagnostics | Method of estimating the amount of a methylated locus in a sample |
| ES2803079T3 (en) * | 2014-09-12 | 2021-01-22 | Illumina Inc | Methods to detect the presence of polymer subunits using chemiluminescence |
| EP3209403A4 (en) * | 2014-10-24 | 2018-09-12 | Abbott Molecular Inc. | Enrichment of small nucleic acids |
| EP3766988B1 (en) | 2015-02-19 | 2024-02-14 | Becton, Dickinson and Company | High-throughput single-cell analysis combining proteomic and genomic information |
| WO2016138496A1 (en) | 2015-02-27 | 2016-09-01 | Cellular Research, Inc. | Spatially addressable molecular barcoding |
| KR101546366B1 (en) | 2015-03-16 | 2015-08-25 | 의료법인 제일의료재단 | Composition for detecing fetal epigenetic markers and detecting method thereof |
| EP3277843A2 (en) | 2015-03-30 | 2018-02-07 | Cellular Research, Inc. | Methods and compositions for combinatorial barcoding |
| CN107580632B (en) | 2015-04-23 | 2021-12-28 | 贝克顿迪金森公司 | Methods and compositions for whole transcriptome amplification |
| WO2016183106A1 (en) | 2015-05-11 | 2016-11-17 | Natera, Inc. | Methods and compositions for determining ploidy |
| DK3666902T3 (en) | 2015-05-22 | 2024-09-09 | Medicover Public Co Ltd | MULTIPLEX PARALLEL ANALYSIS OF TARGETED GENOMIC REGIONS FOR NON-INVASIVE PRENATAL TESTING |
| US11124823B2 (en) | 2015-06-01 | 2021-09-21 | Becton, Dickinson And Company | Methods for RNA quantification |
| US10619186B2 (en) | 2015-09-11 | 2020-04-14 | Cellular Research, Inc. | Methods and compositions for library normalization |
| US10822643B2 (en) | 2016-05-02 | 2020-11-03 | Cellular Research, Inc. | Accurate molecular barcoding |
| US10301677B2 (en) | 2016-05-25 | 2019-05-28 | Cellular Research, Inc. | Normalization of nucleic acid libraries |
| EP3465502B1 (en) | 2016-05-26 | 2024-04-10 | Becton, Dickinson and Company | Molecular label counting adjustment methods |
| US10202641B2 (en) | 2016-05-31 | 2019-02-12 | Cellular Research, Inc. | Error correction in amplification of samples |
| US10640763B2 (en) | 2016-05-31 | 2020-05-05 | Cellular Research, Inc. | Molecular indexing of internal sequences |
| KR102363716B1 (en) | 2016-09-26 | 2022-02-18 | 셀룰러 리서치, 인크. | Determination of protein expression using reagents having barcoded oligonucleotide sequences |
| US11854666B2 (en) | 2016-09-29 | 2023-12-26 | Myriad Women's Health, Inc. | Noninvasive prenatal screening using dynamic iterative depth optimization |
| US11485996B2 (en) | 2016-10-04 | 2022-11-01 | Natera, Inc. | Methods for characterizing copy number variation using proximity-litigation sequencing |
| EP3529377B1 (en) | 2016-10-19 | 2023-04-05 | The Chinese University Of Hong Kong | Gestational age assessment by methylation and size profiling of maternal plasma dna |
| WO2018089377A1 (en) | 2016-11-08 | 2018-05-17 | Cellular Research, Inc. | Methods for cell label classification |
| KR102722820B1 (en) | 2016-11-08 | 2024-10-29 | 벡톤 디킨슨 앤드 컴퍼니 | Expression profile classification method |
| US10011870B2 (en) | 2016-12-07 | 2018-07-03 | Natera, Inc. | Compositions and methods for identifying nucleic acid molecules |
| ES2961580T3 (en) | 2017-01-13 | 2024-03-12 | Cellular Res Inc | Hydrophilic coating of fluid channels |
| CN110382708A (en) | 2017-02-01 | 2019-10-25 | 赛卢拉研究公司 | Selective amplification is carried out using blocking property oligonucleotides |
| WO2018204367A1 (en) * | 2017-05-01 | 2018-11-08 | The Board Of Trustees Of The Leland Stanford Junior University | Profiling of dna methylation using magnetoresistive biosensor array |
| KR20220124280A (en) | 2017-06-05 | 2022-09-13 | 백톤 디킨슨 앤드 컴퍼니 | Sample indexing for single cells |
| CN107451419B (en) * | 2017-07-14 | 2020-01-24 | 浙江大学 | Method for generating simplified DNA methylation sequencing data by computer program simulation |
| CN111051511A (en) * | 2017-08-04 | 2020-04-21 | 十亿至一公司 | Target-associated molecules for characterization associated with biological targets |
| US11519024B2 (en) | 2017-08-04 | 2022-12-06 | Billiontoone, Inc. | Homologous genomic regions for characterization associated with biological targets |
| EP3486330A1 (en) | 2017-11-21 | 2019-05-22 | Ricoh Company, Ltd. | Device for measuring ranges of copy numbers |
| JP6447765B1 (en) * | 2017-11-21 | 2019-01-09 | 株式会社リコー | Inspection device and device |
| US10426424B2 (en) | 2017-11-21 | 2019-10-01 | General Electric Company | System and method for generating and performing imaging protocol simulations |
| JP2021506342A (en) | 2017-12-14 | 2021-02-22 | ティーエーアイ ダイアグノスティックス インコーポレイテッドTai Diagnostics,Inc. | Evaluation of Graft Conformity for Transplantation |
| WO2019126209A1 (en) | 2017-12-19 | 2019-06-27 | Cellular Research, Inc. | Particles associated with oligonucleotides |
| EP3752636A4 (en) | 2018-02-15 | 2022-01-26 | Thrive Earlier Detection Corp. | Barcoded molecular standards |
| EP3768856A1 (en) * | 2018-03-19 | 2021-01-27 | Illumina, Inc. | Methods and compositions for selective cleavage of nucleic acids with recombinant nucleases |
| AU2019251504A1 (en) | 2018-04-14 | 2020-08-13 | Natera, Inc. | Methods for cancer detection and monitoring by means of personalized detection of circulating tumor DNA |
| EP3788170A1 (en) | 2018-05-03 | 2021-03-10 | Becton, Dickinson and Company | Molecular barcoding on opposite transcript ends |
| US11773441B2 (en) | 2018-05-03 | 2023-10-03 | Becton, Dickinson And Company | High throughput multiomics sample analysis |
| US11525159B2 (en) | 2018-07-03 | 2022-12-13 | Natera, Inc. | Methods for detection of donor-derived cell-free DNA |
| JP2022511398A (en) | 2018-10-01 | 2022-01-31 | ベクトン・ディキンソン・アンド・カンパニー | Determining the 5'transcription sequence |
| EP3877520A1 (en) | 2018-11-08 | 2021-09-15 | Becton Dickinson and Company | Whole transcriptome analysis of single cells using random priming |
| CN113195717A (en) | 2018-12-13 | 2021-07-30 | 贝克顿迪金森公司 | Selective extension in single cell whole transcriptome analysis |
| US11371076B2 (en) | 2019-01-16 | 2022-06-28 | Becton, Dickinson And Company | Polymerase chain reaction normalization through primer titration |
| EP4242322B1 (en) | 2019-01-23 | 2024-08-21 | Becton, Dickinson and Company | Oligonucleotides associated with antibodies |
| EP3924506A1 (en) | 2019-02-14 | 2021-12-22 | Becton Dickinson and Company | Hybrid targeted and whole transcriptome amplification |
| US20220262462A1 (en) | 2019-04-10 | 2022-08-18 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Computational filtering of methylated sequence data for predictive modeling |
| WO2020214642A1 (en) | 2019-04-19 | 2020-10-22 | Becton, Dickinson And Company | Methods of associating phenotypical data and single cell sequencing data |
| US11939622B2 (en) | 2019-07-22 | 2024-03-26 | Becton, Dickinson And Company | Single cell chromatin immunoprecipitation sequencing assay |
| JP2022550131A (en) | 2019-09-30 | 2022-11-30 | ガーダント ヘルス, インコーポレイテッド | Compositions and methods for analyzing cell-free DNA in methylation partitioning assays |
| JP7522189B2 (en) | 2019-11-08 | 2024-07-24 | ベクトン・ディキンソン・アンド・カンパニー | Use of Random Priming to Obtain Full-Length V(D)J Information for Immune Repertoire Sequencing |
| US11649497B2 (en) | 2020-01-13 | 2023-05-16 | Becton, Dickinson And Company | Methods and compositions for quantitation of proteins and RNA |
| US11661625B2 (en) | 2020-05-14 | 2023-05-30 | Becton, Dickinson And Company | Primers for immune repertoire profiling |
| CN111593119A (en) * | 2020-06-28 | 2020-08-28 | 成都市妇女儿童中心医院 | Probe and kit for detecting free DNA beta-thalassemia mutation of fetus in maternal blood |
| KR102332540B1 (en) * | 2020-07-02 | 2021-11-29 | 의료법인 성광의료재단 | Down syndrome diagnosis method using epigenetic marker specific for Down syndrome |
| US11932901B2 (en) | 2020-07-13 | 2024-03-19 | Becton, Dickinson And Company | Target enrichment using nucleic acid probes for scRNAseq |
| JP2023544624A (en) * | 2020-10-09 | 2023-10-24 | タカラ バイオ ユーエスエー, インコーポレイテッド | Methods and compositions for preparing nucleic acids for genetic analysis |
| US11739443B2 (en) | 2020-11-20 | 2023-08-29 | Becton, Dickinson And Company | Profiling of highly expressed and lowly expressed proteins |
| CA3179883A1 (en) | 2020-12-02 | 2022-06-09 | Illumina Software, Inc. | System and method for detection of genetic alterations |
| CN113061653A (en) * | 2021-05-07 | 2021-07-02 | 济南国科医工科技发展有限公司 | PCR amplification composition for prenatal noninvasive detection of trisomy 21 syndrome and detection kit |
| CN117420314B (en) * | 2023-10-23 | 2024-10-25 | 昆明医科大学 | Application of vitamin K dependent protein Z |
Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5547835A (en) | 1993-01-07 | 1996-08-20 | Sequenom, Inc. | DNA sequencing by mass spectrometry |
| WO1997037041A2 (en) | 1996-03-18 | 1997-10-09 | Sequenom, Inc. | Dna sequencing by mass spectrometry |
| US5786146A (en) | 1996-06-03 | 1998-07-28 | The Johns Hopkins University School Of Medicine | Method of detection of methylated nucleic acid using agents which modify unmethylated cytosine and distinguishing modified methylated and non-methylated nucleic acids |
| US6043031A (en) | 1995-03-17 | 2000-03-28 | Sequenom, Inc. | DNA diagnostics based on mass spectrometry |
| US6143496A (en) | 1997-04-17 | 2000-11-07 | Cytonix Corporation | Method of sampling, amplifying and quantifying segment of nucleic acid, polymerase chain reaction assembly having nanoliter-sized sample chambers, and method of filling assembly |
| US6258540B1 (en) | 1997-03-04 | 2001-07-10 | Isis Innovation Limited | Non-invasive prenatal diagnosis |
| US6440706B1 (en) | 1999-08-02 | 2002-08-27 | Johns Hopkins University | Digital amplification |
| US20030211522A1 (en) * | 2002-01-18 | 2003-11-13 | Landes Gregory M. | Methods for fetal DNA detection and allele quantitation |
| US6723513B2 (en) | 1998-12-23 | 2004-04-20 | Lingvitae As | Sequencing method using magnifying tags |
| US20040081993A1 (en) | 2002-09-06 | 2004-04-29 | The Trustees Of Boston University | Quantification of gene expression |
| WO2005012578A1 (en) | 2003-07-31 | 2005-02-10 | Sequenom, Inc. | Methods for high level multiplexed polymerase chain reactions and homogeneous mass extension reactions for genotyping of polymorphisms |
| WO2005023091A2 (en) | 2003-09-05 | 2005-03-17 | The Trustees Of Boston University | Method for non-invasive prenatal diagnosis |
| US20050164241A1 (en) | 2003-10-16 | 2005-07-28 | Sinuhe Hahn | Non-invasive detection of fetal genetic traits |
| US6927028B2 (en) | 2001-08-31 | 2005-08-09 | Chinese University Of Hong Kong | Non-invasive methods for detecting non-host DNA in a host using epigenetic differences between the host and non-host DNA |
| WO2006056480A2 (en) | 2004-11-29 | 2006-06-01 | Klinikum Der Universität Regensburg | Means and methods for detecting methylated dna |
| US7081339B2 (en) | 2002-04-12 | 2006-07-25 | Primera Biosystems, Inc. | Methods for variation detection |
| US20060252071A1 (en) | 2005-03-18 | 2006-11-09 | The Chinese University Of Hong Kong | Method for the detection of chromosomal aneuploidies |
| US7169314B2 (en) | 1999-06-28 | 2007-01-30 | California Institute Of Technology | Microfabricated elastomeric valve and pump systems |
| US20070065823A1 (en) | 2003-07-05 | 2007-03-22 | Devin Dressman | Method and compositions for detection and enumeration of genetic variations |
| US20070202525A1 (en) | 2006-02-02 | 2007-08-30 | The Board Of Trustees Of The Leland Stanford Junior University | Non-invasive fetal genetic screening by digital analysis |
| WO2007132166A2 (en) * | 2006-05-03 | 2007-11-22 | The Chinese University Of Hong Kong | New fetal methylation markers |
| US20070275402A1 (en) * | 2006-05-03 | 2007-11-29 | The Chinese University Of Hong Kong | Novel markers for prenatal diagnosis and monitoring |
| WO2007140417A2 (en) | 2006-05-31 | 2007-12-06 | Sequenom, Inc. | Methods and compositions for the extraction and amplification of nucleic acid from a sample |
| US20080305479A1 (en) | 2006-12-05 | 2008-12-11 | Sequenom, Inc. | Detection and quantification of biomolecules using mass spectrometry |
| WO2008157264A2 (en) | 2007-06-15 | 2008-12-24 | Sequenom, Inc. | Combined methods for the detection of chromosomal aneuploidy |
| US20090031169A1 (en) | 2004-10-22 | 2009-01-29 | International Business Machines Corporation | Self-Repairing Of Microprocessor Array Structures |
| US20090029377A1 (en) | 2007-07-23 | 2009-01-29 | The Chinese University Of Hong Kong | Diagnosing fetal chromosomal aneuploidy using massively parallel genomic sequencing |
| WO2009032781A2 (en) | 2007-08-29 | 2009-03-12 | Sequenom, Inc. | Methods and compositions for universal size-specific polymerase chain reaction |
| US20090111712A1 (en) | 2006-12-05 | 2009-04-30 | Sequenom, Inc. | Detection and quantification of biomolecules using mass spectrometry |
| US20100279295A1 (en) | 2009-03-18 | 2010-11-04 | Sequenom, Inc. | Use of thermostable endonucleases for generating reporter molecules |
Family Cites Families (237)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2187108A (en) | 1938-05-27 | 1940-01-16 | Du Pont | Process of purifying lead nitrate solutions |
| JPS5727375B2 (en) | 1973-06-01 | 1982-06-10 | ||
| US4179337A (en) | 1973-07-20 | 1979-12-18 | Davis Frank F | Non-immunogenic polypeptides |
| DE2712230C2 (en) | 1977-03-19 | 1985-05-23 | Bayer Ag, 5090 Leverkusen | Branched segment polymers |
| US4109496A (en) | 1977-12-20 | 1978-08-29 | Norris Industries | Trapped key mechanism |
| US4458066A (en) * | 1980-02-29 | 1984-07-03 | University Patents, Inc. | Process for preparing polynucleotides |
| US4469863A (en) | 1980-11-12 | 1984-09-04 | Ts O Paul O P | Nonionic nucleic acid alkyl and aryl phosphonates and processes for manufacture and use thereof |
| JPS58129411A (en) | 1982-01-27 | 1983-08-02 | Nippon Kogaku Kk <Nikon> | Rear focus conversion lens |
| US4522811A (en) * | 1982-07-08 | 1985-06-11 | Syntex (U.S.A.) Inc. | Serial injection of muramyldipeptides and liposomes enhances the anti-infective activity of muramyldipeptides |
| GB8311018D0 (en) * | 1983-04-22 | 1983-05-25 | Amersham Int Plc | Detecting mutations in dna |
| US4683195A (en) * | 1986-01-30 | 1987-07-28 | Cetus Corporation | Process for amplifying, detecting, and/or-cloning nucleic acid sequences |
| US5656493A (en) | 1985-03-28 | 1997-08-12 | The Perkin-Elmer Corporation | System for automated performance of the polymerase chain reaction |
| US4965188A (en) | 1986-08-22 | 1990-10-23 | Cetus Corporation | Process for amplifying, detecting, and/or cloning nucleic acid sequences using a thermostable enzyme |
| US4683202A (en) * | 1985-03-28 | 1987-07-28 | Cetus Corporation | Process for amplifying nucleic acid sequences |
| US4676980A (en) * | 1985-09-23 | 1987-06-30 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Target specific cross-linked heteroantibodies |
| US4868103A (en) | 1986-02-19 | 1989-09-19 | Enzo Biochem, Inc. | Analyte detection by means of energy transfer |
| DE3751873T2 (en) | 1986-04-09 | 1997-02-13 | Genzyme Corp | Genetically transformed animals that secrete a desired protein in milk |
| US4851331A (en) * | 1986-05-16 | 1989-07-25 | Allied Corporation | Method and kit for polynucleotide assay including primer-dependant DNA polymerase |
| US5202231A (en) * | 1987-04-01 | 1993-04-13 | Drmanac Radoje T | Method of sequencing of genomes by hybridization of oligonucleotide probes |
| US5525464A (en) | 1987-04-01 | 1996-06-11 | Hyseq, Inc. | Method of sequencing by hybridization of oligonucleotide probes |
| US4873316A (en) | 1987-06-23 | 1989-10-10 | Biogen, Inc. | Isolation of exogenous recombinant proteins from the milk of transgenic mammals |
| US5080891A (en) * | 1987-08-03 | 1992-01-14 | Ddi Pharmaceuticals, Inc. | Conjugates of superoxide dismutase coupled to high molecular weight polyalkylene glycols |
| US5048530A (en) | 1988-08-17 | 1991-09-17 | Robert Hurwitz | Method of using an amniocentesis needle with improved sonographic visibility |
| US5720928A (en) * | 1988-09-15 | 1998-02-24 | New York University | Image processing and analysis of individual nucleic acid molecules |
| WO1990006952A1 (en) | 1988-12-22 | 1990-06-28 | Kirin-Amgen, Inc. | Chemically modified granulocyte colony stimulating factor |
| US5075212A (en) | 1989-03-27 | 1991-12-24 | University Of Patents, Inc. | Methods of detecting picornaviruses in biological fluids and tissues |
| US5143854A (en) * | 1989-06-07 | 1992-09-01 | Affymax Technologies N.V. | Large scale photolithographic solid phase synthesis of polypeptides and receptor binding screening thereof |
| US5800992A (en) * | 1989-06-07 | 1998-09-01 | Fodor; Stephen P.A. | Method of detecting nucleic acids |
| US5766849A (en) * | 1989-07-11 | 1998-06-16 | Gen-Probe Incorporated | Methods of amplifying nucleic acids using promoter-containing primer sequence |
| GB2236186B (en) * | 1989-08-22 | 1994-01-05 | Finnigan Mat Gmbh | Process and device for laser desorption of analyte molecular ions, especially of biomolecules |
| AU635844B2 (en) | 1989-11-06 | 1993-04-01 | Cell Genesys, Inc. | Production of proteins using homologous recombination |
| US5641628A (en) * | 1989-11-13 | 1997-06-24 | Children's Medical Center Corporation | Non-invasive method for isolation and detection of fetal DNA |
| US5272071A (en) | 1989-12-22 | 1993-12-21 | Applied Research Systems Ars Holding N.V. | Method for the modification of the expression characteristics of an endogenous gene of a given cell line |
| JPH05503423A (en) * | 1990-01-12 | 1993-06-10 | スクリップス クリニック アンド リサーチ ファウンデーション | Nucleic acid enzyme for DNA cutting |
| US6013431A (en) * | 1990-02-16 | 2000-01-11 | Molecular Tool, Inc. | Method for determining specific nucleotide variations by primer extension in the presence of mixture of labeled nucleotides and terminators |
| US5321131A (en) * | 1990-03-08 | 1994-06-14 | Hybridon, Inc. | Site-specific functionalization of oligodeoxynucleotides for non-radioactive labelling |
| US5210015A (en) * | 1990-08-06 | 1993-05-11 | Hoffman-La Roche Inc. | Homogeneous assay system using the nuclease activity of a nucleic acid polymerase |
| US6004744A (en) | 1991-03-05 | 1999-12-21 | Molecular Tool, Inc. | Method for determining nucleotide identity through extension of immobilized primer |
| DK51092D0 (en) | 1991-05-24 | 1992-04-15 | Ole Buchardt | OLIGONUCLEOTIDE ANALOGUE DESCRIBED BY PEN, MONOMERIC SYNTHONES AND PROCEDURES FOR PREPARING THEREOF, AND APPLICATIONS THEREOF |
| US5719262A (en) * | 1993-11-22 | 1998-02-17 | Buchardt, Deceased; Ole | Peptide nucleic acids having amino acid side chains |
| DE4214112A1 (en) * | 1991-08-02 | 1993-02-04 | Europ Lab Molekularbiolog | NEW METHOD FOR SEQUENCING NUCLEIC ACIDS |
| US5700922A (en) | 1991-12-24 | 1997-12-23 | Isis Pharmaceuticals, Inc. | PNA-DNA-PNA chimeric macromolecules |
| CA2087413A1 (en) * | 1992-01-17 | 1993-07-18 | Joseph R. Lakowicz | Fluorescent energy transfer immunoassay |
| GB9208733D0 (en) | 1992-04-22 | 1992-06-10 | Medical Res Council | Dna sequencing method |
| GB9211979D0 (en) * | 1992-06-05 | 1992-07-15 | Buchard Ole | Uses of nucleic acid analogues |
| CA2140542A1 (en) * | 1992-07-27 | 1994-02-03 | Abeysingle Padmapriya | Oligonucleotide alkylphosphonothioates |
| WO1994010300A1 (en) | 1992-10-30 | 1994-05-11 | The General Hospital Corporation | Interaction trap system for isolating novel proteins |
| US6194144B1 (en) * | 1993-01-07 | 2001-02-27 | Sequenom, Inc. | DNA sequencing by mass spectrometry |
| CA2154358A1 (en) * | 1993-01-21 | 1994-08-04 | Hybridon, Inc. | Integrated oligonucleotides |
| US6156501A (en) | 1993-10-26 | 2000-12-05 | Affymetrix, Inc. | Arrays of modified nucleic acid probes and methods of use |
| US6045996A (en) * | 1993-10-26 | 2000-04-04 | Affymetrix, Inc. | Hybridization assays on oligonucleotide arrays |
| WO1995014108A1 (en) | 1993-11-17 | 1995-05-26 | Amersham International Plc | Primer extension mass spectroscopy nucleic acid sequencing method |
| WO1995021271A1 (en) | 1994-02-07 | 1995-08-10 | Molecular Tool, Inc. | Ligase/polymerase-mediated genetic bit analysistm of single nucleotide polymorphisms and its use in genetic analysis |
| US5637684A (en) * | 1994-02-23 | 1997-06-10 | Isis Pharmaceuticals, Inc. | Phosphoramidate and phosphorothioamidate oligomeric compounds |
| ATE248224T1 (en) | 1994-04-25 | 2003-09-15 | Avitech Diagnostics Inc | DETERMINATION OF MUTATIONS BY CLIVATION WITH RESOLVASE |
| US5851770A (en) | 1994-04-25 | 1998-12-22 | Variagenics, Inc. | Detection of mismatches by resolvase cleavage using a magnetic bead support |
| US5834189A (en) | 1994-07-08 | 1998-11-10 | Visible Genetics Inc. | Method for evaluation of polymorphic genetic sequences, and the use thereof in identification of HLA types |
| PT773991E (en) * | 1994-07-15 | 2004-10-29 | Cephalon Inc | ACAPPLE EXPRESSED BY BACULOVIRUS |
| US5849483A (en) | 1994-07-28 | 1998-12-15 | Ig Laboratories, Inc. | High throughput screening method for sequences or genetic alterations in nucleic acids |
| US5589330A (en) | 1994-07-28 | 1996-12-31 | Genzyme Corporation | High-throughput screening method for sequence or genetic alterations in nucleic acids using elution and sequencing of complementary oligonucleotides |
| US5662813A (en) * | 1994-10-21 | 1997-09-02 | Bioseparations, Inc. | Method for separation of nucleated fetal erythrocytes from maternal blood samples |
| US5807718A (en) | 1994-12-02 | 1998-09-15 | The Scripps Research Institute | Enzymatic DNA molecules |
| MX9705172A (en) | 1995-01-09 | 1997-10-31 | Boehringer Ingelheim Int | Il-2r-associated polypeptide and dna molecules coding therefor. |
| US6239273B1 (en) * | 1995-02-27 | 2001-05-29 | Affymetrix, Inc. | Printing molecular library arrays |
| DE19515552A1 (en) | 1995-04-27 | 1996-10-31 | Europ Lab Molekularbiolog | Simultaneous sequencing of nucleic acids |
| US5962674A (en) | 1995-06-01 | 1999-10-05 | Hybridon, Inc. | Synthesis of oligonucleotides containing alkylphosphonate internucleoside linkages |
| US5614622A (en) * | 1995-06-01 | 1997-03-25 | Hybridon, Inc. | 5-pentenoyl moiety as a nucleoside-amino protecting group, 4-pentenoyl-protected nucleotide synthons, and related oligonucleotide syntheses |
| US5955599A (en) | 1995-06-01 | 1999-09-21 | Hybridon, Inc. | Process for making oligonucleotides containing o- and s- methylphosphotriester internucleoside linkages |
| US6140482A (en) | 1995-06-01 | 2000-10-31 | Hybridon, Inc. | Primary phosphoramidate internucleoside linkages and oligonucleotides containing the same |
| US5981186A (en) | 1995-06-30 | 1999-11-09 | Visible Genetics, Inc. | Method and apparatus for DNA-sequencing using reduced number of sequencing mixtures |
| US5637683A (en) * | 1995-07-13 | 1997-06-10 | Cornell Research Foundation, Inc. | Nucleic acid analog with amide linkage and method of making that analog |
| US5652356A (en) * | 1995-08-17 | 1997-07-29 | Hybridon, Inc. | Inverted chimeric and hybrid oligonucleotides |
| JP3193301B2 (en) * | 1995-09-14 | 2001-07-30 | 麒麟麦酒株式会社 | Bioactive protein p160 |
| US5869242A (en) * | 1995-09-18 | 1999-02-09 | Myriad Genetics, Inc. | Mass spectrometry to assess DNA sequence polymorphisms |
| GB9519638D0 (en) * | 1995-09-26 | 1995-11-29 | Dynal As | Method |
| EP0886681A1 (en) | 1996-03-04 | 1998-12-30 | Genetrace Systems, Inc. | Methods of screening nucleic acids using mass spectrometry |
| US6759217B2 (en) | 1996-03-26 | 2004-07-06 | Oncomedx, Inc. | Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer |
| CA2250118C (en) | 1996-03-26 | 2009-09-29 | Michael S. Kopreski | Method enabling use of extracellular rna extracted from plasma or serum to detect, monitor or evaluate cancer |
| WO1997040181A1 (en) * | 1996-04-25 | 1997-10-30 | Spectrametrix Inc. | Analyte assay using particulate labels |
| US5928906A (en) * | 1996-05-09 | 1999-07-27 | Sequenom, Inc. | Process for direct sequencing during template amplification |
| CA2258511A1 (en) | 1996-06-14 | 1997-12-18 | Sarnoff Corporation | Method for polynucleotide sequencing |
| AU4089397A (en) | 1996-08-26 | 1998-03-19 | Hybridon, Inc. | Improved reagents and process for synthesis of oligonucleotides containing phosphorodithioate internucleoside linkages |
| US5849546A (en) | 1996-09-13 | 1998-12-15 | Epicentre Technologies Corporation | Methods for using mutant RNA polymerases with reduced discrimination between non-canonical and canonical nucleoside triphosphates |
| US5886165A (en) * | 1996-09-24 | 1999-03-23 | Hybridon, Inc. | Mixed backbone antisense oligonucleotides containing 2'-5'-ribonucleotide- and 3'-5'-deoxyribonucleotides segments |
| GB9620209D0 (en) * | 1996-09-27 | 1996-11-13 | Cemu Bioteknik Ab | Method of sequencing DNA |
| US6057134A (en) | 1996-10-07 | 2000-05-02 | Ambion, Inc. | Modulating the efficiency of nucleic acid amplification reactions with 3' modified oligonucleotides |
| US6140053A (en) | 1996-11-06 | 2000-10-31 | Sequenom, Inc. | DNA sequencing by mass spectrometry via exonuclease degradation |
| US7285422B1 (en) * | 1997-01-23 | 2007-10-23 | Sequenom, Inc. | Systems and methods for preparing and analyzing low volume analyte array elements |
| US6024925A (en) * | 1997-01-23 | 2000-02-15 | Sequenom, Inc. | Systems and methods for preparing low volume analyte array elements |
| CA2268740C (en) | 1996-11-06 | 2010-07-20 | Sequenom, Inc. | High density immobilization of nucleic acids |
| EP0963997B1 (en) | 1996-11-18 | 2003-02-19 | Takeshi Imanishi | Novel nucleotide analogues |
| US6017702A (en) * | 1996-12-05 | 2000-01-25 | The Perkin-Elmer Corporation | Chain-termination type nucleic acid sequencing method including 2'-deoxyuridine-5'-triphosphate |
| US5876934A (en) * | 1996-12-18 | 1999-03-02 | Pharmacia Biotech Inc. | DNA sequencing method |
| US6046005A (en) * | 1997-01-15 | 2000-04-04 | Incyte Pharmaceuticals, Inc. | Nucleic acid sequencing with solid phase capturable terminators comprising a cleavable linking group |
| US20010051341A1 (en) | 1997-03-04 | 2001-12-13 | Isis Innovation Limited | Non-invasive prenatal diagnosis |
| JP3756313B2 (en) | 1997-03-07 | 2006-03-15 | 武 今西 | Novel bicyclonucleosides and oligonucleotide analogues |
| US5849497A (en) | 1997-04-03 | 1998-12-15 | The Research Foundation Of State University Of New York | Specific inhibition of the polymerase chain reaction using a non-extendable oligonucleotide blocker |
| US5739314A (en) * | 1997-04-25 | 1998-04-14 | Hybridon, Inc. | Method for synthesizing 2'-O-substituted pyrimidine nucleosides |
| EP2216416B1 (en) * | 1997-05-30 | 2012-05-09 | TrovaGene, Inc. | Methods for Detection of Nucleic Acid Sequences in Urine |
| JP2001511529A (en) * | 1997-07-25 | 2001-08-14 | アフィメトリックス インコーポレイテッド | Method and apparatus for providing a bioinformatics database |
| JP2001514907A (en) * | 1997-08-15 | 2001-09-18 | アフィメトリックス・インコーポレーテッド | Polymorph detection using cluster analysis |
| DE19754482A1 (en) * | 1997-11-27 | 1999-07-01 | Epigenomics Gmbh | Process for making complex DNA methylation fingerprints |
| US5998143A (en) | 1997-12-05 | 1999-12-07 | The Perkin-Elmer Corporation | Cycle sequencing thermal profiles |
| ATE224458T1 (en) | 1998-02-04 | 2002-10-15 | Variagenics Inc | MISMATCH DETECTION TECHNIQUES |
| SG71920A1 (en) * | 1998-04-27 | 2000-04-18 | Texas Instruments Inc | Method and apparatus for driving a polyphase brushless dc motor |
| US6183958B1 (en) * | 1998-05-06 | 2001-02-06 | Variagenics, Inc. | Probes for variance detection |
| US6723564B2 (en) | 1998-05-07 | 2004-04-20 | Sequenom, Inc. | IR MALDI mass spectrometry of nucleic acids using liquid matrices |
| US20030022207A1 (en) * | 1998-10-16 | 2003-01-30 | Solexa, Ltd. | Arrayed polynucleotides and their use in genome analysis |
| US6140054A (en) | 1998-09-30 | 2000-10-31 | University Of Utah Research Foundation | Multiplex genotyping using fluorescent hybridization probes |
| US6610492B1 (en) | 1998-10-01 | 2003-08-26 | Variagenics, Inc. | Base-modified nucleotides and cleavage of polynucleotides incorporating them |
| US6142681A (en) | 1999-02-22 | 2000-11-07 | Vialogy Corporation | Method and apparatus for interpreting hybridized bioelectronic DNA microarray patterns using self-scaling convergent reverberant dynamics |
| US6136541A (en) | 1999-02-22 | 2000-10-24 | Vialogy Corporation | Method and apparatus for analyzing hybridized biochip patterns using resonance interactions employing quantum expressor functions |
| DK1163250T3 (en) | 1999-03-24 | 2006-11-13 | Exiqon As | Improved synthesis of [2.2.1] bicyclonucleosides |
| US6368834B1 (en) * | 1999-04-06 | 2002-04-09 | Genome Technologies, Llc | PCR genome walking with synthetic primer |
| DE60020830T2 (en) | 1999-04-30 | 2006-07-27 | Sequenom, Inc., San Diego | DIAGNOSTIC SEQUENCING THROUGH A COMBINATION OF SPECIFIC CLASSIFICATION AND MASS SPECTROMETRY |
| KR20010001577A (en) | 1999-06-07 | 2001-01-05 | 윤종용 | Process for Preparing Oligopeptidenucleotide Probe Using Polymeric Photoacid Generator |
| AU6629200A (en) | 1999-08-25 | 2001-03-19 | Philip P Garner | Alpha-helical peptide nucleic acid alpha-pna |
| US20050287592A1 (en) | 2000-08-29 | 2005-12-29 | Yeda Research And Development Co. Ltd. | Template-dependent nucleic acid polymerization using oligonucleotide triphosphates building blocks |
| US6274320B1 (en) | 1999-09-16 | 2001-08-14 | Curagen Corporation | Method of sequencing a nucleic acid |
| US6448010B1 (en) | 1999-10-06 | 2002-09-10 | Amersham Pharmacia Biotech, Inc. | Method for detecting mutations using arrayed primer extension |
| AU1075701A (en) | 1999-10-08 | 2001-04-23 | Protogene Laboratories, Inc. | Method and apparatus for performing large numbers of reactions using array assembly |
| US6221600B1 (en) | 1999-10-08 | 2001-04-24 | Board Of Regents, The University Of Texas System | Combinatorial oligonucleotide PCR: a method for rapid, global expression analysis |
| US6297016B1 (en) | 1999-10-08 | 2001-10-02 | Applera Corporation | Template-dependent ligation with PNA-DNA chimeric probes |
| US7332275B2 (en) | 1999-10-13 | 2008-02-19 | Sequenom, Inc. | Methods for detecting methylated nucleotides |
| US6709816B1 (en) | 1999-10-18 | 2004-03-23 | Affymetrix, Inc. | Identification of alleles |
| AU2001268468A1 (en) | 2000-06-13 | 2001-12-24 | The Trustees Of Boston University | Use of nucleotide analogs in the analysis of oligonucleotide mixtures and in highly multiplexed nucleic acid sequencing |
| WO2002018616A1 (en) | 2000-09-01 | 2002-03-07 | Hitachi Chemical Co., Ltd. | Adjusting the efficiency of nucleic acid template amplification by attenuated pcr with template-mimic oligonucleotides |
| US6664056B2 (en) | 2000-10-17 | 2003-12-16 | The Chinese University Of Hong Kong | Non-invasive prenatal monitoring |
| US6929911B2 (en) | 2000-11-01 | 2005-08-16 | The Board Of Trustees Of The Leland Stanford Junior University | Method for determining genetic affiliation, substructure and gene flow within human populations |
| US20060136142A1 (en) * | 2000-11-02 | 2006-06-22 | Kurt Berlin | Systems, methods and computer program products for guiding selection of a therapeutic treatment regimen based on the methylation status of the DNA |
| DE10061348C2 (en) * | 2000-12-06 | 2002-10-24 | Epigenomics Ag | Method for the quantification of cytosine methylations in complex amplified genomic DNA |
| EP1229128A1 (en) * | 2001-01-31 | 2002-08-07 | Boehringer Mannheim Gmbh | New method for genotype determination |
| DE10112515B4 (en) * | 2001-03-09 | 2004-02-12 | Epigenomics Ag | Method for the detection of cytosine methylation patterns with high sensitivity |
| JP2004524044A (en) * | 2001-04-20 | 2004-08-12 | カロリンスカ イノベイションズ アクチボラゲット | High-throughput genome analysis method using microarray with restriction site tag |
| US20030054386A1 (en) | 2001-06-22 | 2003-03-20 | Stylianos Antonarakis | Method for detecting diseases caused by chromosomal imbalances |
| DE10130800B4 (en) * | 2001-06-22 | 2005-06-23 | Epigenomics Ag | Method for the detection of cytosine methylation with high sensitivity |
| US20050037388A1 (en) * | 2001-06-22 | 2005-02-17 | University Of Geneva | Method for detecting diseases caused by chromosomal imbalances |
| WO2003080645A2 (en) * | 2001-07-01 | 2003-10-02 | Keck Graduate Institute | Amplification of nucleic acid fragments using nicking agents |
| CA2455731C (en) * | 2001-07-25 | 2012-02-07 | Oncomedx, Inc. | Methods for evaluating pathologic conditions using extracellular rna |
| EA006066B1 (en) * | 2001-08-20 | 2005-08-25 | Такара Био Инк. | Nucleic acid amplification methods |
| DE10201138B4 (en) * | 2002-01-08 | 2005-03-10 | Epigenomics Ag | Method for the detection of cytosine methylation patterns by exponential ligation of hybridized probe oligonucleotides (MLA) |
| MXPA04008472A (en) | 2002-03-01 | 2005-09-20 | Ravgen Inc | Rapid analysis of variations in a genome. |
| US6977162B2 (en) | 2002-03-01 | 2005-12-20 | Ravgen, Inc. | Rapid analysis of variations in a genome |
| EP1487999B1 (en) | 2002-03-15 | 2006-12-27 | Epigenomics AG | Discovery and diagnostic methods using 5-methylcytosine dna glycosylase |
| US6908755B2 (en) * | 2002-03-21 | 2005-06-21 | Ken-Shwo Dai | Human megakaryocyte-associated tyrosine kinase (MATK)-related gene variant associated with lung cancers |
| EP1488003A1 (en) | 2002-03-25 | 2004-12-22 | Epigenomics AG | Method for the analysis of methylation patterns within nucleic acids by means of mass spectrometry |
| US7442506B2 (en) | 2002-05-08 | 2008-10-28 | Ravgen, Inc. | Methods for detection of genetic disorders |
| US20030211483A1 (en) * | 2002-05-09 | 2003-11-13 | Schroeder Benjamin G. | Methods for the enrichment of low-abundance polynucleotides |
| WO2004013284A2 (en) | 2002-08-02 | 2004-02-12 | Tufts University | A method to assess genomic dna methylation using high-performance liquid chromatography-electospray ionization mass spectrometry |
| US7820378B2 (en) * | 2002-11-27 | 2010-10-26 | Sequenom, Inc. | Fragmentation-based methods and systems for sequence variation detection and discovery |
| EP1583846B1 (en) | 2003-01-17 | 2011-11-16 | The Chinese University Of Hong Kong | Circulating mrna as diagnostic markers for pregnancy-related disorders |
| ES2396245T3 (en) | 2003-01-29 | 2013-02-20 | 454 Life Sciences Corporation | Nucleic Acid Amplification and Sequencing Method |
| AU2003900944A0 (en) | 2003-02-28 | 2003-03-13 | The University Of Queensland | Cell isolation & enrichment |
| AU2003268333A1 (en) | 2003-02-28 | 2004-09-28 | Ravgen, Inc. | Methods for detection of genetic disorders |
| US8394582B2 (en) | 2003-03-05 | 2013-03-12 | Genetic Technologies, Inc | Identification of fetal DNA and fetal cell markers in maternal plasma or serum |
| US20050009059A1 (en) * | 2003-05-07 | 2005-01-13 | Affymetrix, Inc. | Analysis of methylation status using oligonucleotide arrays |
| AU2003263660A1 (en) | 2003-08-29 | 2005-03-16 | Pantarhei Bioscience B.V. | Prenatal diagnosis of down syndrome by detection of fetal rna markers in maternal blood |
| DE602004018009D1 (en) * | 2003-09-22 | 2009-01-08 | Trisogen Biotechnology Ltd Par | PROVIDING PROCEDURES FOR DETERMINING A CHANGE IN A LOCUS COPIAL AMOUNT |
| JP2007508017A (en) | 2003-10-08 | 2007-04-05 | ザ トラスティーズ オブ ボストン ユニバーシティ | Methods for prenatal diagnosis of chromosomal abnormalities |
| JP4917891B2 (en) | 2003-10-21 | 2012-04-18 | オリオン ゲノミクス エルエルシー | Method of differential enzymatic fragmentation |
| AU2004286845A1 (en) | 2003-10-30 | 2005-05-19 | Tufts-New England Medical Center | Prenatal diagnosis using cell-free fetal DNA in amniotic fluid |
| US20070111233A1 (en) * | 2003-10-30 | 2007-05-17 | Bianchi Diana W | Prenatal diagnosis using cell-free fetal DNA in amniotic fluid |
| CA2556981C (en) * | 2004-02-18 | 2015-10-13 | The Trustees Of Boston University | Method for detecting and quantifying rare mutations or polymorphisms |
| US20100216153A1 (en) | 2004-02-27 | 2010-08-26 | Helicos Biosciences Corporation | Methods for detecting fetal nucleic acids and diagnosing fetal abnormalities |
| JP4492156B2 (en) | 2004-03-03 | 2010-06-30 | ニプロ株式会社 | Protein containing serum albumin domain |
| US7608394B2 (en) | 2004-03-26 | 2009-10-27 | Sequenom, Inc. | Methods and compositions for phenotype identification based on nucleic acid methylation |
| WO2005098050A2 (en) | 2004-03-26 | 2005-10-20 | Sequenom, Inc. | Base specific cleavage of methylation-specific amplification products in combination with mass analysis |
| US7468249B2 (en) | 2004-05-05 | 2008-12-23 | Biocept, Inc. | Detection of chromosomal disorders |
| EP1765994B1 (en) | 2004-06-01 | 2009-11-18 | The University of North Carolina at Chapel Hill | Reconstituted histone methyltransferase complex and methods of identifying modulators thereof |
| US7709194B2 (en) * | 2004-06-04 | 2010-05-04 | The Chinese University Of Hong Kong | Marker for prenatal diagnosis and monitoring |
| WO2006026027A2 (en) | 2004-07-30 | 2006-03-09 | Whitehead Institute For Biomedical Research | Markers of alterations in the y chromosome and uses therefor |
| JP2008513031A (en) * | 2004-09-20 | 2008-05-01 | プロテオジェニックス, インコーポレイテッド | Diagnosis of fetal aneuploidy |
| JP5774805B2 (en) | 2004-11-29 | 2015-09-09 | セクエノム,インコーポレイティド | Method and kit for detecting methylated DNA |
| CN102010902B (en) | 2005-03-18 | 2012-05-23 | 香港中文大学 | Markers for prenatal diagnosis and monitoring |
| US20070048755A1 (en) * | 2005-03-22 | 2007-03-01 | New York University | Assay for determining the sex of primates |
| CA2617738A1 (en) | 2005-08-02 | 2007-02-08 | Sequenom, Inc. | Methods and compositions for disease prognosis based on nucleic acid methylation |
| US20070122823A1 (en) | 2005-09-01 | 2007-05-31 | Bianchi Diana W | Amniotic fluid cell-free fetal DNA fragment size pattern for prenatal diagnosis |
| EP2351858B1 (en) * | 2006-02-28 | 2014-12-31 | University of Louisville Research Foundation | Detecting fetal chromosomal abnormalities using tandem single nucleotide polymorphisms |
| AU2007223102A1 (en) * | 2006-03-06 | 2007-09-13 | The Trustees Of Columbia University In The City Of New York | Specific amplification of fetal DNA sequences from a mixed, fetal-maternal source |
| WO2007121276A2 (en) | 2006-04-12 | 2007-10-25 | Biocept, Inc. | Enrichment of circulating fetal dna |
| WO2007147074A2 (en) * | 2006-06-14 | 2007-12-21 | Living Microsystems, Inc. | Use of highly parallel snp genotyping for fetal diagnosis |
| AU2007260750A1 (en) | 2006-06-16 | 2007-12-21 | Sequenom, Inc. | Methods and compositions for the amplification, detection and quantification of nucleic acid from a sample |
| US8262900B2 (en) * | 2006-12-14 | 2012-09-11 | Life Technologies Corporation | Methods and apparatus for measuring analytes using large scale FET arrays |
| US8173370B2 (en) | 2007-02-08 | 2012-05-08 | Sequenom, Inc. | Nucleic acid-based tests for RHD typing, gender determination and nucleic acid quantification |
| WO2008103761A2 (en) | 2007-02-20 | 2008-08-28 | Sequenom, Inc. | Methods and compositions for cancer diagnosis and treatment based on nucleic acid methylation |
| GB0703996D0 (en) | 2007-03-01 | 2007-04-11 | Oxitec Ltd | Nucleic acid detection |
| CN101641452B (en) | 2007-03-26 | 2013-10-23 | 塞昆纳姆股份有限公司 | Restriction endonuclease enhanced polymorphic sequence detection |
| WO2009032779A2 (en) | 2007-08-29 | 2009-03-12 | Sequenom, Inc. | Methods and compositions for the size-specific seperation of nucleic acid from a sample |
| US8748100B2 (en) * | 2007-08-30 | 2014-06-10 | The Chinese University Of Hong Kong | Methods and kits for selectively amplifying, detecting or quantifying target DNA with specific end sequences |
| WO2009039507A2 (en) * | 2007-09-21 | 2009-03-26 | Biocept, Inc. | Identification and isolation of fetal cells and nucleic acid |
| ES2460896T3 (en) * | 2007-10-04 | 2014-05-14 | Commonwealth Scientific And Industrial Research Organisation | Nucleic acid amplification |
| EA201000427A1 (en) | 2007-10-04 | 2010-10-29 | Хэлсион Молекулар | SEQUENCYING NUCLEIC ACID POLYMERS USING ELECTRON MICROSCOPY |
| US8093063B2 (en) * | 2007-11-29 | 2012-01-10 | Quest Diagnostics Investments Incorporated | Assay for detecting genetic abnormalities in genomic nucleic acids |
| EP2620511B1 (en) | 2008-01-17 | 2018-02-28 | Sequenom, Inc. | Single molecule nucleic acid sequence analysis processes |
| AU2009223671B2 (en) | 2008-03-11 | 2014-11-27 | Sequenom, Inc. | Nucleic acid-based tests for prenatal gender determination |
| EP2276858A4 (en) | 2008-03-26 | 2011-10-05 | Sequenom Inc | Restriction endonuclease enhanced polymorphic sequence detection |
| WO2010017214A1 (en) | 2008-08-04 | 2010-02-11 | Gene Security Network, Inc. | Methods for allele calling and ploidy calling |
| EP2307540B1 (en) | 2008-07-07 | 2017-04-19 | Oxford Nanopore Technologies Limited | Enzyme-pore constructs |
| CN102216456A (en) | 2008-09-16 | 2011-10-12 | 塞昆纳姆股份有限公司 | Methods and compositions for methylation-based enrichment of fetal nucleic acid in maternal samples for non-invasive prenatal diagnosis |
| US8476013B2 (en) | 2008-09-16 | 2013-07-02 | Sequenom, Inc. | Processes and compositions for methylation-based acid enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| US8962247B2 (en) | 2008-09-16 | 2015-02-24 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
| PL2334812T3 (en) | 2008-09-20 | 2017-06-30 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US20100240054A1 (en) | 2008-09-22 | 2010-09-23 | Biocept, Inc. | Identification and isolation of fetal cells and nucleic acid |
| EP2730662A1 (en) * | 2008-11-12 | 2014-05-14 | Caris Life Sciences Luxembourg Holdings | Methods and systems of using exosomes for determining phenotypes |
| WO2010065470A2 (en) | 2008-12-01 | 2010-06-10 | Consumer Genetics, Inc. | Compositions and methods for detecting background male dna during fetal sex determination |
| EP3211095B1 (en) | 2009-04-03 | 2019-01-02 | Sequenom, Inc. | Nucleic acid preparation compositions and methods |
| US8211735B2 (en) * | 2009-06-08 | 2012-07-03 | International Business Machines Corporation | Nano/microwire solar cell fabricated by nano/microsphere lithography |
| US8825412B2 (en) | 2010-05-18 | 2014-09-02 | Natera, Inc. | Methods for non-invasive prenatal ploidy calling |
| US8563242B2 (en) | 2009-08-11 | 2013-10-22 | The Chinese University Of Hong Kong | Method for detecting chromosomal aneuploidy |
| AU2015252141A1 (en) | 2009-09-16 | 2015-12-03 | Sequenom Center For Molecular Medicine | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
| AU2010315037B9 (en) | 2009-11-05 | 2015-04-23 | Sequenom, Inc. | Fetal genomic analysis from a maternal biological sample |
| EA034241B1 (en) | 2009-11-06 | 2020-01-21 | Те Чайниз Юниверсити Ов Гонконг | Method for performing prenatal diagnosis of a sequence imbalance |
| CA2785020C (en) | 2009-12-22 | 2020-08-25 | Sequenom, Inc. | Processes and kits for identifying aneuploidy |
| US8700341B2 (en) | 2010-01-19 | 2014-04-15 | Verinata Health, Inc. | Partition defined detection methods |
| PL3260555T3 (en) | 2010-01-19 | 2019-05-31 | Verinata Health Inc | Novel protocol for preparing sequencing libraries |
| CN102892899A (en) | 2010-01-26 | 2013-01-23 | Nipd遗传学有限公司 | Methods and compositions for noninvasive prenatal diagnosis of fetal aneuploidies |
| US8940487B2 (en) | 2010-02-09 | 2015-01-27 | UmiTaq Bio | Methods and compositions for universal detection of nucleic acids |
| WO2011142836A2 (en) | 2010-05-14 | 2011-11-17 | Fluidigm Corporation | Assays for the detection of genotype, mutations, and/or aneuploidy |
| EP2569453B1 (en) | 2010-05-14 | 2015-12-16 | Fluidigm Corporation | Nucleic acid isolation methods |
| US8603742B2 (en) | 2010-07-06 | 2013-12-10 | University of Pittsburgh—of the Commonwealth System of Higher Education | Methods for the diagnosis of fetal disease |
| WO2012012703A2 (en) | 2010-07-23 | 2012-01-26 | Esoterix Genetic Laboratories, Llc | Identification of differentially represented fetal or maternal genomic regions and uses thereof |
| AU2011348267A1 (en) | 2010-12-23 | 2013-08-01 | Sequenom, Inc. | Fetal genetic variation detection |
| WO2012118745A1 (en) | 2011-02-28 | 2012-09-07 | Arnold Oliphant | Assay systems for detection of aneuploidy and sex determination |
| EP3378954B1 (en) | 2011-04-29 | 2021-02-17 | Sequenom, Inc. | Quantification of a minority nucleic acid species |
| WO2013019361A1 (en) | 2011-07-07 | 2013-02-07 | Life Technologies Corporation | Sequencing methods |
| DK2764459T3 (en) | 2011-10-06 | 2021-08-23 | Sequenom Inc | METHODS AND PROCESSES FOR NON-INVASIVE ASSESSMENT OF GENETIC VARIATIONS |
| US9367663B2 (en) | 2011-10-06 | 2016-06-14 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013055817A1 (en) | 2011-10-11 | 2013-04-18 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013131021A1 (en) | 2012-03-02 | 2013-09-06 | Sequenom Inc. | Methods and processes for non-invasive assessment of genetic variations |
| ES2902401T3 (en) | 2012-05-21 | 2022-03-28 | Sequenom Inc | Methods and processes for the non-invasive evaluation of genetic variations |
| US9920361B2 (en) | 2012-05-21 | 2018-03-20 | Sequenom, Inc. | Methods and compositions for analyzing nucleic acid |
| US20150284783A1 (en) | 2012-05-21 | 2015-10-08 | Agena Bioscience, Inc. | Methods and compositions for analyzing nucleic acid |
| CA2878979C (en) | 2012-07-13 | 2021-09-14 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| WO2014168711A1 (en) | 2013-03-13 | 2014-10-16 | Sequenom, Inc. | Primers for dna methylation analysis |
| EP3117011B1 (en) | 2014-03-13 | 2020-05-06 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| CN106544407B (en) | 2015-09-18 | 2019-11-08 | 广州华大基因医学检验所有限公司 | The method for determining donor source cfDNA ratio in receptor cfDNA sample |
| JP6447765B1 (en) | 2017-11-21 | 2019-01-09 | 株式会社リコー | Inspection device and device |
-
2009
- 2009-09-16 US US12/561,241 patent/US8476013B2/en active Active
-
2010
- 2010-03-18 CA CA3024967A patent/CA3024967C/en active Active
- 2010-03-18 JP JP2012529756A patent/JP5873434B2/en not_active Expired - Fee Related
- 2010-03-18 EP EP20155147.0A patent/EP3722440A1/en active Pending
- 2010-03-18 CN CN2010800527486A patent/CN102648292A/en active Pending
- 2010-03-18 ES ES10817598.5T patent/ES2650666T3/en active Active
- 2010-03-18 EP EP17182863.5A patent/EP3330382B1/en active Active
- 2010-03-18 CA CA2774342A patent/CA2774342C/en active Active
- 2010-03-18 AU AU2010295968A patent/AU2010295968B2/en active Active
- 2010-03-18 IN IN3139DEN2012 patent/IN2012DN03139A/en unknown
- 2010-03-18 EP EP10817598.5A patent/EP2478119B1/en active Active
- 2010-03-18 WO PCT/US2010/027879 patent/WO2011034631A1/en active Application Filing
- 2010-03-18 CA CA3122552A patent/CA3122552A1/en active Pending
-
2012
- 2012-06-13 US US13/517,532 patent/US20130150249A1/en not_active Abandoned
- 2012-06-13 US US13/495,975 patent/US20120277119A1/en not_active Abandoned
- 2012-06-13 US US13/517,508 patent/US20130143211A1/en not_active Abandoned
-
2013
- 2013-03-13 US US13/801,384 patent/US20130296180A1/en not_active Abandoned
-
2015
- 2015-01-14 JP JP2015005024A patent/JP2015091262A/en not_active Withdrawn
- 2015-06-10 US US14/735,477 patent/US10738358B2/en active Active
- 2015-10-01 JP JP2015195591A patent/JP6039034B2/en active Active
-
2016
- 2016-10-07 JP JP2016199141A patent/JP6513622B2/en active Active
-
2017
- 2017-12-18 JP JP2017241844A patent/JP2018038438A/en not_active Withdrawn
-
2018
- 2018-10-22 HK HK18113518.6A patent/HK1254596A1/en unknown
-
2020
- 2020-06-29 US US16/915,173 patent/US20200362414A1/en active Pending
- 2020-08-03 JP JP2020131448A patent/JP2020174678A/en not_active Withdrawn
-
2023
- 2023-01-20 JP JP2023007221A patent/JP2023033606A/en active Pending
Patent Citations (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5547835A (en) | 1993-01-07 | 1996-08-20 | Sequenom, Inc. | DNA sequencing by mass spectrometry |
| US6043031A (en) | 1995-03-17 | 2000-03-28 | Sequenom, Inc. | DNA diagnostics based on mass spectrometry |
| WO1997037041A2 (en) | 1996-03-18 | 1997-10-09 | Sequenom, Inc. | Dna sequencing by mass spectrometry |
| US5786146A (en) | 1996-06-03 | 1998-07-28 | The Johns Hopkins University School Of Medicine | Method of detection of methylated nucleic acid using agents which modify unmethylated cytosine and distinguishing modified methylated and non-methylated nucleic acids |
| US6258540B1 (en) | 1997-03-04 | 2001-07-10 | Isis Innovation Limited | Non-invasive prenatal diagnosis |
| US6143496A (en) | 1997-04-17 | 2000-11-07 | Cytonix Corporation | Method of sampling, amplifying and quantifying segment of nucleic acid, polymerase chain reaction assembly having nanoliter-sized sample chambers, and method of filling assembly |
| US6723513B2 (en) | 1998-12-23 | 2004-04-20 | Lingvitae As | Sequencing method using magnifying tags |
| US7169314B2 (en) | 1999-06-28 | 2007-01-30 | California Institute Of Technology | Microfabricated elastomeric valve and pump systems |
| US6440706B1 (en) | 1999-08-02 | 2002-08-27 | Johns Hopkins University | Digital amplification |
| US6927028B2 (en) | 2001-08-31 | 2005-08-09 | Chinese University Of Hong Kong | Non-invasive methods for detecting non-host DNA in a host using epigenetic differences between the host and non-host DNA |
| US20030211522A1 (en) * | 2002-01-18 | 2003-11-13 | Landes Gregory M. | Methods for fetal DNA detection and allele quantitation |
| US7081339B2 (en) | 2002-04-12 | 2006-07-25 | Primera Biosystems, Inc. | Methods for variation detection |
| US20040081993A1 (en) | 2002-09-06 | 2004-04-29 | The Trustees Of Boston University | Quantification of gene expression |
| US20070065823A1 (en) | 2003-07-05 | 2007-03-22 | Devin Dressman | Method and compositions for detection and enumeration of genetic variations |
| WO2005012578A1 (en) | 2003-07-31 | 2005-02-10 | Sequenom, Inc. | Methods for high level multiplexed polymerase chain reactions and homogeneous mass extension reactions for genotyping of polymorphisms |
| US20050079521A1 (en) | 2003-07-31 | 2005-04-14 | Martin Beaulieu | Methods for high level multiplexed polymerase chain reactions and homogeneous mass extension reactions |
| US20070207466A1 (en) | 2003-09-05 | 2007-09-06 | The Trustees Of Boston University | Method for non-invasive prenatal diagnosis |
| WO2005023091A2 (en) | 2003-09-05 | 2005-03-17 | The Trustees Of Boston University | Method for non-invasive prenatal diagnosis |
| US20050164241A1 (en) | 2003-10-16 | 2005-07-28 | Sinuhe Hahn | Non-invasive detection of fetal genetic traits |
| US20090031169A1 (en) | 2004-10-22 | 2009-01-29 | International Business Machines Corporation | Self-Repairing Of Microprocessor Array Structures |
| WO2006056480A2 (en) | 2004-11-29 | 2006-06-01 | Klinikum Der Universität Regensburg | Means and methods for detecting methylated dna |
| US20060252071A1 (en) | 2005-03-18 | 2006-11-09 | The Chinese University Of Hong Kong | Method for the detection of chromosomal aneuploidies |
| US20070202525A1 (en) | 2006-02-02 | 2007-08-30 | The Board Of Trustees Of The Leland Stanford Junior University | Non-invasive fetal genetic screening by digital analysis |
| WO2007132166A2 (en) * | 2006-05-03 | 2007-11-22 | The Chinese University Of Hong Kong | New fetal methylation markers |
| US20070275402A1 (en) * | 2006-05-03 | 2007-11-29 | The Chinese University Of Hong Kong | Novel markers for prenatal diagnosis and monitoring |
| WO2007140417A2 (en) | 2006-05-31 | 2007-12-06 | Sequenom, Inc. | Methods and compositions for the extraction and amplification of nucleic acid from a sample |
| US20080305479A1 (en) | 2006-12-05 | 2008-12-11 | Sequenom, Inc. | Detection and quantification of biomolecules using mass spectrometry |
| US20090111712A1 (en) | 2006-12-05 | 2009-04-30 | Sequenom, Inc. | Detection and quantification of biomolecules using mass spectrometry |
| WO2008157264A2 (en) | 2007-06-15 | 2008-12-24 | Sequenom, Inc. | Combined methods for the detection of chromosomal aneuploidy |
| US20090029377A1 (en) | 2007-07-23 | 2009-01-29 | The Chinese University Of Hong Kong | Diagnosing fetal chromosomal aneuploidy using massively parallel genomic sequencing |
| WO2009032781A2 (en) | 2007-08-29 | 2009-03-12 | Sequenom, Inc. | Methods and compositions for universal size-specific polymerase chain reaction |
| US20100279295A1 (en) | 2009-03-18 | 2010-11-04 | Sequenom, Inc. | Use of thermostable endonucleases for generating reporter molecules |
Non-Patent Citations (60)
| Title |
|---|
| AMICUCCI ET AL., CLIN. CHEM., vol. 46, 2000, pages 301 - 302 |
| AUSUBEL ET AL.,: "Current Protocols in Molecular Biology", 1994 |
| BATZER ET AL., NUCLEIC ACID RES., vol. 19, 1991, pages 5081 |
| BEAUCAGE; CARUTHERS, TETRAHEDRON LETT., vol. 22, 1981, pages 1859 - 1862 |
| BRASLAVSKY ET AL., PNAS, vol. 100, no. 7, 2003, pages 3960 - 3964 |
| CHIU ET AL., LANCET, vol. 360, 2002, pages 998 - 1000 |
| COLELLA ET AL., BIOTECHNIQUES, vol. 35, no. L, July 2003 (2003-07-01), pages 146 - 50 |
| COSTA ET AL., N. ENGL. J. MED., vol. 346, 2002, pages 1502 |
| DEAR BRIEF FUNCT GENOMIC PROTEOMIC, vol. 1, 2003, pages 397 - 416 |
| DING C; CANTOR CR: "A high-throughput gene expression analysis technique using competitive PCR and matrix-assisted laser desorption ionization time-of-flight MS", PROC NATL ACAD SCI U S A, vol. 100, 2003, pages 3059 - 3064, XP002556773, DOI: doi:10.1073/pnas.0630494100 |
| DUPONT JM; TOST J; JAMMES H; GUT IG, ANAL BIOCHEM, vol. 333, no. 1, October 2004 (2004-10-01), pages 119 - 27 |
| EADS ET AL., CANCER RES., vol. 59, 1999, pages 2302 - 2306 |
| EHRICH M ET AL.: "Cytosine methylation profiling of cancer cell lines", PROC NATL ACAD SCI USA, vol. 105, 2008, pages 4844 - 48 |
| EHRICH M ET AL.: "Quantitative high-throughput analysis of DNA methylation patterns by base specific cleavage and mass spectrometry", PROC NATL ACAD SCI USA, vol. 102, 2005, pages 15785 - 15790, XP055083781, DOI: doi:10.1073/pnas.0507816102 |
| EHRICH M; NELSON MR; STANSSENS P; ZABEAU M; LILOGLOU T; XINARIANOS G; CANTOR CR; FIELD JK; VAN DEN BOOM D: "Quantitative high-throughput analysis of DNA methylation patterns by base specific cleavage and mass spectrometry", PROC NATL ACAD SCI U S A, vol. 102, 2005, pages 15785 - 15790, XP055083781, DOI: doi:10.1073/pnas.0507816102 |
| FROMMER ET AL., PROC. NATL. ACAD. SCI. USA, vol. 89, 1992, pages 1827 - 1831 |
| GEBHARD C; SCHWARZFISCHER L; PHAM TH; ANDREESEN R; MACKENSEN A; REHLI M: "Rapid and sensitive detection of CpG-methylation using methyl-binding (MB)-PCR", NUCLEIC ACIDS RES, vol. 34, 2006, pages E82 |
| GEBHARD C; SCHWARZFISCHER L; PHAM TH; SCHILLING E; KLUG M; ANDREESEN R; REHLI M: "Genomewide profiling of CpG methylation identifies novel targets of aberrant hypermethylation in myeloid leukemia", CANCER RES, vol. 66, 2006, pages 6118 - 6128, XP002663139, DOI: doi:10.1158/0008-5472.CAN-06-0376 |
| GONZALGO; JONES, NUCLEIC ACIDS RES., vol. 25, 1997, pages 2529 - 2531 |
| HARRIS T D ET AL., SCIENCE, vol. 320, 2008, pages 106 - 109 |
| HERMAN ET AL., PROC. NAT. ACAD. SCI. USA, vol. 93, 1996, pages 9821 - 9826 |
| INNIS ET AL.: "PCR Protocols: A Guide to Methods and Applications", 1990, ACADEMIC PRESS, INC |
| JURINKE, C.; OETH, P.; VAN DEN BOOM, D.: "MALDI-TOF mass spectrometry: a versatile tool for high-performance DNA analysis", MOL. BIOTECHNOL, vol. 26, 2004, pages 147 - 164 |
| KALININA ET AL.: "Nanoliter scale PCR with TaqMan detection", NUCLEIC ACIDS RESEARCH, vol. 25, 1997, pages 1999 - 2004, XP002471289, DOI: doi:10.1093/nar/25.10.1999 |
| KRIEGLER: "Gene Transfer and Expression: A Laboratory Manual", 1990 |
| LAIRD, P.W., NATURE REVIEWS CANCER, vol. 3, 2003, pages 253 - 266 |
| LEE TI ET AL.: "Control of developmental regulators by Polycomb in human embryonic stem cells", CELL, vol. 125, 2006, pages 301 - 313, XP055221189, DOI: doi:10.1016/j.cell.2006.02.043 |
| LO ET AL., CLIN. CHEM., vol. 45, 1999, pages 1747 - 1751 |
| LO ET AL., CLIN. CHEM., vol. 45, 1999, pages 184 - 188 |
| LO ET AL., LANCET, vol. 350, 1997, pages 485 - 487 |
| LO ET AL., N. ENGL. J. MED., vol. 339, 1998, pages 1734 - 1738 |
| LO; 350 ET AL., LANCET, 1997, pages 485 - 487 |
| MARGULIES, M. ET AL., NATURE, vol. 437, 2005, pages 376 - 380 |
| NAKANO ET AL.: "Single-molecule PCR using water-in-oil emulsion", JOURNAL OF BIOTECHNOLOGY, vol. 102, 2003, pages 117 - 124, XP002399942, DOI: doi:10.1016/S0168-1656(03)00023-3 |
| NOLTE, ADV. CLIN. CHEM., vol. 33, 1998, pages 201 - 235 |
| OETH, P. ET AL.: "iPLEX Assay: Increased Plexing Efficiency and Flexibility for MassARRAY® System through single base primer extension with mass-modified Terminators", SEQUENOM APPLICATION NOTE, 2005 |
| OHTSUKA ET AL., J. BIOL. CHEM., vol. 260, 1985, pages 2605 - 2608 |
| OLD RW.: "Candidate epigenetic biomarkers for non-invasive prenatal diagnosis of Down syndrome.", REPROD. BIOMED., vol. 15, no. 2, 2007, pages 227 - 235, XP008144463 * |
| PEARSON; REANIER, J. CHROM., vol. 255, 1983, pages 137 - 149 |
| ROSSOLINI ET AL., MOL. CELL. PROBES, vol. 8, 1994, pages 91 - 98 |
| SADRI; HOMSBY, NUCL. ACIDS RES., vol. 24, 1996, pages 5058 - 5059 |
| SAITO ET AL., LANCET, vol. 356, 2000, pages 1170 |
| SAMBROOK ET AL.: "Molecular Biology: A laboratory Approach", 1989, COLD SPRING HARBOR |
| SAMBROOK; RUSSELL: "Molecular Cloning, A Laboratory Manual 3rd ed.", 2001 |
| SAMBROOK; RUSSELL: "Molecular Cloning: A Laboratory Manual", 2001 |
| SEKIZAWA ET AL., CLIN. CHEM., vol. 47, 2001, pages 2164 - 2165 |
| SONI GV; MELLER A, CLIN CHEM, vol. 53, 2007, pages 1996 - 2001 |
| SONI; MELLER, CLINICAL CHEMISTRY, vol. 53, no. 11, 2007, pages 1996 - 2001 |
| TAKAI ET AL., PROC. NATL. ACAD. SCI. U.S.A., vol. 99, 2002, pages 3740 - 3745 |
| TOOKE N; PETTERSSON M., IVDT., November 2004 (2004-11-01), pages 41 |
| TOYOTA ET AL., CANCER RES., vol. 59, 1999, pages 2307 - 12 |
| UHLMANN, K. ET AL., ELECTROPHORESIS, vol. 23, 2002, pages 4072 - 4079 |
| VAN DEVANTER, NUCLEIC ACIDS RES., vol. 12, 1984, pages 6159 - 6168 |
| VENTER ET AL., SCIENCE, vol. 291, 2001, pages 1304 - 1351 |
| VOGELSTEIN; KINZLER, DIGITAL PCR. PROC NATL ACAD SCI USA., vol. 96, 1999, pages 9236 - 41 |
| WALD; HACKSHAW, PRENAT DIAGN, vol. 17, no. 9, 1997, pages 921 - 9 |
| XIONG; LAIRD, NUCLEIC ACIDS RES., vol. 25, 1997, pages 2532 - 2534 |
| YAMADA ET AL., GENOME RESEARCH, vol. 14, 2004, pages 247 - 266 |
| ZHONG ET AL., AM. J. OBSTET. GYNECOL., vol. 184, 2001, pages 414 - 419 |
| ZHONG ET AL., PRENAT. DIAGN., vol. 20, 2000, pages 795 - 798 |
Cited By (136)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12077819B2 (en) | 2008-01-15 | 2024-09-03 | Agena Bioscience, Inc. | Compositions and processes for improved mass spectrometry analysis |
| US10329612B2 (en) | 2008-01-15 | 2019-06-25 | Agena Bioscience, Inc. | Compositions and processes for improved mass spectrometry analysis |
| US9873912B2 (en) | 2008-01-15 | 2018-01-23 | Agena Bioscience, Inc. | Compositions and processes for improved mass spectrometry analysis |
| US9310378B2 (en) | 2008-01-15 | 2016-04-12 | Agena Bioscience, Inc. | Compositions and processes for improved mass spectrometry analysis |
| US8962247B2 (en) | 2008-09-16 | 2015-02-24 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
| US10612086B2 (en) | 2008-09-16 | 2020-04-07 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| US10738358B2 (en) | 2008-09-16 | 2020-08-11 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| US9926593B2 (en) | 2009-12-22 | 2018-03-27 | Sequenom, Inc. | Processes and kits for identifying aneuploidy |
| US11180799B2 (en) | 2009-12-22 | 2021-11-23 | Sequenom, Inc. | Processes and kits for identifying aneuploidy |
| US10167509B2 (en) | 2011-02-09 | 2019-01-01 | Bio-Rad Laboratories, Inc. | Analysis of nucleic acids |
| US9127312B2 (en) | 2011-02-09 | 2015-09-08 | Bio-Rad Laboratories, Inc. | Analysis of nucleic acids |
| US11499181B2 (en) | 2011-02-09 | 2022-11-15 | Bio-Rad Laboratories, Inc. | Analysis of nucleic acids |
| EP3378954A1 (en) * | 2011-04-29 | 2018-09-26 | Sequenom, Inc. | Quantification of a minority nucleic acid species |
| AU2012249531B2 (en) * | 2011-04-29 | 2017-06-29 | Sequenom, Inc. | Quantification of a minority nucleic acid species |
| CN103717750B (en) * | 2011-04-29 | 2017-03-08 | 塞昆纳姆股份有限公司 | The quantitation of minority nucleic acid substances |
| CN103717750A (en) * | 2011-04-29 | 2014-04-09 | 塞昆纳姆股份有限公司 | Quantification of a minority nucleic acid species |
| WO2012149339A3 (en) * | 2011-04-29 | 2013-03-14 | Sequenom, Inc. | Quantification of a minority nucleic acid species |
| US11004537B2 (en) | 2011-06-24 | 2021-05-11 | Sequenom, Inc. | Methods and processes for non invasive assessment of a genetic variation |
| US9984198B2 (en) | 2011-10-06 | 2018-05-29 | Sequenom, Inc. | Reducing sequence read count error in assessment of complex genetic variations |
| US10196681B2 (en) | 2011-10-06 | 2019-02-05 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10323268B2 (en) | 2011-10-06 | 2019-06-18 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013052913A2 (en) | 2011-10-06 | 2013-04-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11492659B2 (en) | 2011-10-06 | 2022-11-08 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11437121B2 (en) | 2011-10-06 | 2022-09-06 | Sequenom, Inc. | Methods and processes for non-invasive detection of a microduplication or a microdeletion with reduced sequence read count error |
| WO2013052907A2 (en) | 2011-10-06 | 2013-04-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3922731A2 (en) | 2011-10-06 | 2021-12-15 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10424394B2 (en) | 2011-10-06 | 2019-09-24 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US9367663B2 (en) | 2011-10-06 | 2016-06-14 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11001884B2 (en) | 2011-10-06 | 2021-05-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11560586B2 (en) | 2011-10-06 | 2023-01-24 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US8688388B2 (en) | 2011-10-11 | 2014-04-01 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3243908A1 (en) | 2011-10-11 | 2017-11-15 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013055817A1 (en) | 2011-10-11 | 2013-04-18 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11697849B2 (en) | 2012-01-20 | 2023-07-11 | Sequenom, Inc. | Methods for non-invasive assessment of fetal genetic variations that factor experimental conditions |
| EP3401399A1 (en) | 2012-03-02 | 2018-11-14 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US9605313B2 (en) | 2012-03-02 | 2017-03-28 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10738359B2 (en) | 2012-03-02 | 2020-08-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11312997B2 (en) | 2012-03-02 | 2022-04-26 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP4155401A1 (en) | 2012-03-02 | 2023-03-29 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3757210A1 (en) | 2012-03-02 | 2020-12-30 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US9920361B2 (en) | 2012-05-21 | 2018-03-20 | Sequenom, Inc. | Methods and compositions for analyzing nucleic acid |
| US11306354B2 (en) | 2012-05-21 | 2022-04-19 | Sequenom, Inc. | Methods and compositions for analyzing nucleic acid |
| EP3978621A1 (en) | 2012-05-21 | 2022-04-06 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3663409A1 (en) | 2012-05-21 | 2020-06-10 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013177086A1 (en) | 2012-05-21 | 2013-11-28 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP4276194A2 (en) | 2012-05-21 | 2023-11-15 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| CN102676513A (en) * | 2012-05-28 | 2012-09-19 | 武汉大学 | Fetal epigenetic marker and application thereof |
| US10497461B2 (en) | 2012-06-22 | 2019-12-03 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2013192562A1 (en) | 2012-06-22 | 2013-12-27 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3473731A1 (en) | 2012-06-22 | 2019-04-24 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP4137579A1 (en) | 2012-06-22 | 2023-02-22 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11332791B2 (en) | 2012-07-13 | 2022-05-17 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| WO2014011928A1 (en) | 2012-07-13 | 2014-01-16 | Sequenom, Inc. | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses |
| EP2900837A4 (en) * | 2012-09-26 | 2016-06-01 | Agency Science Tech & Res | Biomarkers for down syndrome prenatal diagnosis |
| EP3591067A1 (en) | 2012-10-04 | 2020-01-08 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014055790A2 (en) | 2012-10-04 | 2014-04-10 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014055774A1 (en) | 2012-10-04 | 2014-04-10 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10482994B2 (en) | 2012-10-04 | 2019-11-19 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US12112832B2 (en) | 2012-10-04 | 2024-10-08 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP4009329A1 (en) | 2012-10-04 | 2022-06-08 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10504613B2 (en) | 2012-12-20 | 2019-12-10 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10497462B2 (en) | 2013-01-25 | 2019-12-03 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP4261828A2 (en) | 2013-01-25 | 2023-10-18 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014116598A2 (en) | 2013-01-25 | 2014-07-31 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014164838A1 (en) | 2013-03-12 | 2014-10-09 | Sequenom, Inc. | Quantification of cell-specific nucleic acid markers having a particular methylation state |
| US11060145B2 (en) | 2013-03-13 | 2021-07-13 | Sequenom, Inc. | Methods and compositions for identifying presence or absence of hypermethylation or hypomethylation locus |
| US10204771B2 (en) | 2013-03-13 | 2019-02-12 | Agena Bioscience, Inc. | Preparation enhancements and methods of use for MALDI mass spectrometry |
| US9305756B2 (en) | 2013-03-13 | 2016-04-05 | Agena Bioscience, Inc. | Preparation enhancements and methods of use for MALDI mass spectrometry |
| WO2014168711A1 (en) | 2013-03-13 | 2014-10-16 | Sequenom, Inc. | Primers for dna methylation analysis |
| EP3597774A1 (en) | 2013-03-13 | 2020-01-22 | Sequenom, Inc. | Primers for dna methylation analysis |
| US10930368B2 (en) | 2013-04-03 | 2021-02-23 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP4187543A1 (en) | 2013-04-03 | 2023-05-31 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014165596A1 (en) | 2013-04-03 | 2014-10-09 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2014182726A2 (en) | 2013-05-07 | 2014-11-13 | Sequenom, Inc. | Genetic markers for macular degeneration disorder treatment |
| US10699800B2 (en) | 2013-05-24 | 2020-06-30 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11462298B2 (en) | 2013-05-24 | 2022-10-04 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3578670A1 (en) | 2013-05-24 | 2019-12-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP3540076A1 (en) | 2013-06-21 | 2019-09-18 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10622094B2 (en) | 2013-06-21 | 2020-04-14 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| EP4258269A2 (en) | 2013-10-04 | 2023-10-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10964409B2 (en) | 2013-10-04 | 2021-03-30 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2015051163A2 (en) | 2013-10-04 | 2015-04-09 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2015054080A1 (en) | 2013-10-07 | 2015-04-16 | Sequenom, Inc. | Methods and processes for non-invasive assessment of chromosome alterations |
| EP3851539A1 (en) | 2013-10-07 | 2021-07-21 | Sequenom, Inc. | Methods and processes for non-invasive assessment of chromosome alterations |
| US11929146B2 (en) | 2013-10-07 | 2024-03-12 | Sequenom, Inc. | Systems for non-invasive assessment of chromosome alterations using changes in subsequence mappability |
| EP3495496A1 (en) | 2013-10-07 | 2019-06-12 | Sequenom, Inc. | Methods and processes for non-invasive assessment of chromosome alterations |
| EP3736344A1 (en) * | 2014-03-13 | 2020-11-11 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2015138774A1 (en) * | 2014-03-13 | 2015-09-17 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11365447B2 (en) | 2014-03-13 | 2022-06-21 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US10017818B2 (en) | 2014-05-09 | 2018-07-10 | Lifecodexx Ag | Multiplex detection of DNA that originates from a specific cell-type |
| EP2942401A1 (en) | 2014-05-09 | 2015-11-11 | Lifecodexx AG | Detection of DNA that originates from a specific cell-type |
| US9822413B2 (en) | 2014-05-09 | 2017-11-21 | Lifecodexx Ag | Multiplex detection of DNA that originates from a specific cell-type |
| US10801067B2 (en) | 2014-05-09 | 2020-10-13 | Eurofins Lifecodexx Gmbh | Detection of DNA that originates from a specific cell-type and related methods |
| US11965207B2 (en) | 2014-05-09 | 2024-04-23 | Eurofins Lifecodexx Gmbh | Detection of DNA that originates from a specific cell-type and related methods |
| US9822412B2 (en) | 2014-05-09 | 2017-11-21 | Lifecodexx Ag | Detection of DNA that originates from a specific cell-type |
| WO2015169947A1 (en) * | 2014-05-09 | 2015-11-12 | Lifecodexx Ag | Detection of dna that originates from a specific cell-type and related methods |
| EP3521454A1 (en) | 2014-05-09 | 2019-08-07 | LifeCodexx AG | Detection of dna that originates from a specific cell-type and related methods |
| EP2942400A1 (en) | 2014-05-09 | 2015-11-11 | Lifecodexx AG | Multiplex detection of DNA that originates from a specific cell-type |
| US11773443B2 (en) | 2014-05-09 | 2023-10-03 | Eurofins Lifecodexx Gmbh | Multiplex detection of DNA that originates from a specific cell-type |
| WO2015183872A1 (en) | 2014-05-30 | 2015-12-03 | Sequenom, Inc. | Chromosome representation determinations |
| EP3598452A1 (en) | 2014-05-30 | 2020-01-22 | Sequenom, Inc. | Chromosome representation determinations |
| EP3760739A1 (en) | 2014-07-30 | 2021-01-06 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11783911B2 (en) | 2014-07-30 | 2023-10-10 | Sequenom, Inc | Methods and processes for non-invasive assessment of genetic variations |
| EP3730629A1 (en) | 2014-10-10 | 2020-10-28 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| WO2016057901A1 (en) | 2014-10-10 | 2016-04-14 | Sequenom, Inc. | Methods and processes for non-invasive assessment of genetic variations |
| US11753684B2 (en) | 2015-11-10 | 2023-09-12 | Eurofins Lifecodexx Gmbh | Detection of fetal chromosomal aneuploidies using DNA regions that are differentially methylated between the fetus and the pregnant female |
| EP3168309A1 (en) | 2015-11-10 | 2017-05-17 | Lifecodexx AG | Detection of foetal chromosomal aneuploidies using dna regions that are differentially methylated between the foetus and the pregnant female |
| WO2017081047A1 (en) | 2015-11-10 | 2017-05-18 | Lifecodexx Ag | Detection of foetal chromosomal aneuploidies using dna regions that are differentially methylated between the foetus and the pregnant female |
| EP4043581A1 (en) | 2016-05-27 | 2022-08-17 | Sequenom, Inc. | Method for generating a paralog assay system |
| WO2017205826A1 (en) | 2016-05-27 | 2017-11-30 | Sequenom, Inc. | Methods for detecting genetic variations |
| US11299780B2 (en) | 2016-07-15 | 2022-04-12 | The Regents Of The University Of California | Methods of producing nucleic acid libraries |
| WO2018022890A1 (en) | 2016-07-27 | 2018-02-01 | Sequenom, Inc. | Genetic copy number alteration classifications |
| US11515003B2 (en) | 2016-07-27 | 2022-11-29 | Sequenom, Inc. | Copy number alteration and reference genome mapping |
| US11200963B2 (en) | 2016-07-27 | 2021-12-14 | Sequenom, Inc. | Genetic copy number alteration classifications |
| WO2018022906A1 (en) | 2016-07-27 | 2018-02-01 | Sequenom, Inc. | Methods for non-invasive assessment of genomic instability |
| US11952616B2 (en) | 2016-12-22 | 2024-04-09 | Guardant Health, Inc. | Methods and systems for analyzing nucleic acid molecules |
| WO2018136888A1 (en) | 2017-01-20 | 2018-07-26 | Sequenom, Inc. | Methods for non-invasive assessment of genetic alterations |
| WO2018136881A1 (en) | 2017-01-20 | 2018-07-26 | Sequenom, Inc. | Sequencing adapter manufacture and use |
| EP4235676A2 (en) | 2017-01-20 | 2023-08-30 | Sequenom, Inc. | Methods for non-invasive assessment of genetic alterations |
| WO2018136882A1 (en) | 2017-01-20 | 2018-07-26 | Sequenom, Inc. | Methods for non-invasive assessment of copy number alterations |
| US11694768B2 (en) | 2017-01-24 | 2023-07-04 | Sequenom, Inc. | Methods and processes for assessment of genetic variations |
| WO2018140521A1 (en) | 2017-01-24 | 2018-08-02 | Sequenom, Inc. | Methods and processes for assessment of genetic variations |
| WO2018170511A1 (en) | 2017-03-17 | 2018-09-20 | Sequenom, Inc. | Methods and processes for assessment of genetic mosaicism |
| EP3998350A1 (en) | 2017-03-17 | 2022-05-18 | Sequenom, Inc. | Methods and processes for assessment of genetic mosaicism |
| WO2019140201A1 (en) | 2018-01-12 | 2019-07-18 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| US11584929B2 (en) | 2018-01-12 | 2023-02-21 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| US11629345B2 (en) | 2018-06-06 | 2023-04-18 | The Regents Of The University Of California | Methods of producing nucleic acid libraries and compositions and kits for practicing same |
| WO2020206143A1 (en) | 2019-04-05 | 2020-10-08 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| WO2021072037A1 (en) | 2019-10-09 | 2021-04-15 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| WO2021087491A1 (en) | 2019-10-31 | 2021-05-06 | Sequenom, Inc. | Application of mosaicism ratio in multifetal gestations and personalized risk assessment |
| CN112662761A (en) * | 2020-03-05 | 2021-04-16 | 博尔诚(北京)科技有限公司 | Probe composition for detecting 3 parenchymal organ tumors |
| WO2021262805A1 (en) | 2020-06-24 | 2021-12-30 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| EP4428244A2 (en) | 2020-06-24 | 2024-09-11 | Claret Bioscience, LLC | Methods and compositions for analyzing nucleic acid |
| WO2022076574A1 (en) | 2020-10-08 | 2022-04-14 | Claret Bioscience, Llc | Methods and compositions for analyzing nucleic acid |
| WO2023086967A1 (en) * | 2021-11-12 | 2023-05-19 | Guardant Health, Inc. | Method of analysis of methylated dna-binding proteins |
| WO2024186778A1 (en) | 2023-03-03 | 2024-09-12 | Laboratory Corporation Of America Holdings | Methods and systems for positive cfdna screening on genetic variations using mosaicism ratio |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20200362414A1 (en) | Processes and Compositions for Methylation-Based Enrichment of Fetal Nucleic Acid From a Maternal Sample Useful for Non-Invasive Prenatal Diagnoses | |
| US10612086B2 (en) | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses | |
| EP2329021B1 (en) | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses | |
| CA2878979C (en) | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non-invasive prenatal diagnoses | |
| AU2017251674B2 (en) | Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201080052748.6 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10817598 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2774342 Country of ref document: CA Ref document number: 2012529756 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010295968 Country of ref document: AU |
|
| REEP | Request for entry into the european phase |
Ref document number: 2010817598 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 3139/DELNP/2012 Country of ref document: IN Ref document number: 2010817598 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2010295968 Country of ref document: AU Date of ref document: 20100318 Kind code of ref document: A |