WO2010089060A2 - Particles for electrophoretic displays - Google Patents
Particles for electrophoretic displays Download PDFInfo
- Publication number
- WO2010089060A2 WO2010089060A2 PCT/EP2010/000552 EP2010000552W WO2010089060A2 WO 2010089060 A2 WO2010089060 A2 WO 2010089060A2 EP 2010000552 W EP2010000552 W EP 2010000552W WO 2010089060 A2 WO2010089060 A2 WO 2010089060A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- dye
- methacrylate
- particles
- monomer
- electrophoretic
- Prior art date
Links
- KHBSMYZKJKHZET-QZQOTICOSA-N CCN(CCOC(C)=O)c(cc1)ccc1/N=N/c(ccc([N+]([O-])=O)c1)c1Cl Chemical compound CCN(CCOC(C)=O)c(cc1)ccc1/N=N/c(ccc([N+]([O-])=O)c1)c1Cl KHBSMYZKJKHZET-QZQOTICOSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F20/00—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride, ester, amide, imide or nitrile thereof
- C08F20/62—Monocarboxylic acids having ten or more carbon atoms; Derivatives thereof
- C08F20/68—Esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
- C08F220/14—Methyl esters, e.g. methyl (meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2/00—Processes of polymerisation
- C08F2/12—Polymerisation in non-solvents
- C08F2/16—Aqueous medium
- C08F2/22—Emulsion polymerisation
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
- C08L33/10—Homopolymers or copolymers of methacrylic acid esters
- C08L33/12—Homopolymers or copolymers of methyl methacrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L35/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical, and containing at least one other carboxyl radical in the molecule, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L35/06—Copolymers with vinyl aromatic monomers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B69/00—Dyes not provided for by a single group of this subclass
- C09B69/10—Polymeric dyes; Reaction products of dyes with monomers or with macromolecular compounds
- C09B69/106—Polymeric dyes; Reaction products of dyes with monomers or with macromolecular compounds containing an azo dye
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/0056—Dyeing with polymeric dyes involving building the polymeric dyes on the fibres
- D06P1/006—Dyeing with polymeric dyes involving building the polymeric dyes on the fibres by using dyes with polymerisable groups, e.g. dye ---CH=CH2
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/165—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on translational movement of particles in a fluid under the influence of an applied field
- G02F1/166—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on translational movement of particles in a fluid under the influence of an applied field characterised by the electro-optical or magneto-optical effect
- G02F1/167—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on translational movement of particles in a fluid under the influence of an applied field characterised by the electro-optical or magneto-optical effect by electrophoresis
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
- C08F222/102—Esters of polyhydric alcohols or polyhydric phenols of dialcohols, e.g. ethylene glycol di(meth)acrylate or 1,4-butanediol dimethacrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/30—Nitriles
- C08F222/34—Vinylidene cyanide
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/36—Amides or imides
Definitions
- This invention relates to coloured polymer particles, preferably with surface functionality for charge retention, a process for their preparation, the use of these particles for the preparation of an electrophoretic device, colour electrophoretic displays comprising such particles, and new water-soluble dyes.
- EPDs Electrophoretic Displays
- One use of EPDs is for electronic paper. It is imperative that once an image is displayed, the image can be retained for a long period of time without further voltage being applied. Hence, this fulfils the requirements of low power use, and means an image can be visible until another image is required.
- An EPD generally comprises charged electrophoretic particles dispersed between two substrates, each comprising one or more electrodes.
- the space between the electrodes is filled with a dispersion medium which is a different colour to the colour of the particles. If a voltage is applied between the electrodes, charged particles move to the electrode of opposite polarity.
- the particles can cover the observer's side electrode, so that a colour identical to the colour of the particles is displayed when an image is observed from the observer's side. Any image can be observed using a multiplicity of pixels.
- EPDs Available technologies of EPDs include electronic paper, commercially used in electronic books. This application uses black and white or light colour.
- This application uses black and white or light colour.
- the main disadvantage of state of the art EPDs is the lack of a bright full colour system.
- the use of different coloured particles in a single pixel has been exemplified in recent patent literature (US 7,304,634, GB 2 438 436, US 2007/0268244), but all of these approaches require the use of complex cell structures and drive schemes.
- the object of this invention is to provide electro-optically active media for colour electrophoretic displays and specifically engineered coloured particles for use in such media.
- This object is solved by a process for the preparation of coloured polymer particles for use in electrophoretic devices comprising the steps of a) the reaction of at least one polymerisable dye, at least one monomer, at least one initiator, and optionally at least one charged co-monomer, and preferably b) washing and drying the coloured polymer particles, by these particles per se, by the use of these particles for the preparation of an electrophoretic device, by colour electrophoretic displays comprising such particles, and new water-soluble dyes.
- the subject matter of this invention relates specifically to the use of specifically engineered polymer particles and their dispersion in dielectric organic media to produce a composition preferably suitable as the electrically switchable component of a full colour e-paper or electrophoretic display.
- It also relates specifically to dispersions of the afore-mentioned polymer particles in dielectric organic media, which enable electrophoretic switching of the particles in an applied electric field.
- Advantages of the polymer particles according to the invention may be, in particular:
- the main advantages of the present invention are that it is possible to prepare particles of appropriate colours e.g. red, green and blue or a combination of cyan, magenta and yellow, and to be able to prepare coloured particles of a desired size and which have a high mono-dispersity, and which preferably incorporate a charge, to enable electrophoretic movement.
- appropriate colours e.g. red, green and blue or a combination of cyan, magenta and yellow
- the present process is a one-step reaction to provide coloured particles suitable for EPD enabling a cost effective production process.
- the dye can be specifically designed to give a desired suitable colour, e.g. cyan or red.
- the polymerisable group on the dye can be easily modified (e.g. methacrylate, acrylate, etc.) so that an appropriate dye monomer can react with other monomers to form the particle.
- Another major advantage is that preferably an emulsion polymerisation in aqueous solution can be used. This route gives excellent control over monodispersity, particle size with a small diameter range of sub-micron size for image quality. Use of water as a solvent gives obvious safety and environmental advantages over use of organic solvents.
- the present invention provides an easy way for the production of coloured polymeric particles, wherein charge and colour can be controlled independently from each other. It is especially advantageous that the inventive particles do not leach any colour into a non-polar solvent used as a carrier fluid in EPD even over a long time period.
- the present invention provides the opportunity to manipulate colour, charge, size, mono-dispersity etc. independently in order to produce particles with all the desired features for coloured EPD.
- An essential component of the present invention is a polymerisable dye.
- the polymerisable dyes may be solvent soluble or water soluble and they may be anionic, cationic or neutral.
- Preferably water soluble dyes are used.
- the function of the polymerisable dye is to colour the particle.
- the polymerisable dye consists of a chromophore, one or more polymerisable groups, optional linker groups (spacers), and optional groups to modify physical properties (like solubility, light fastness, etc.) and optionally charged group(s).
- the polymerisable dye preferably comprises a chromophoric group and a functional group or plurality of functional groups selected from polymerisable groups e.g. methacrylates, acrylates, methacrylamides, acrylonitriles, ⁇ - substituted acrylates, styrenes and vinyl ethers, vinyl esters, propenyl ethers, oxetanes and epoxys etc., in particular methacrylates and acrylates.
- the polymerised group may be attached directly to the chromophobe group or may be attached through a linker group.
- An example of a suitable linker group is an optionally substituted alkyl chain, a polyether alkyl chain, a cycloalkyl or aromatic ring, heteroaromatic ring or a combination thereof.
- the chromophobe group preferably comprises of conjugated aromatic (including heteroaromatic) and / or multiple bonds including: azo (including monoazo, bisazo, trisazo, linked azos etc), metallised azo, anthraquinone, pyrroline, phthalocyanine, polymethine, aryl-carbonium, triphendioxazine, diarylmethane, triarylmethane, anthraquinone, phthalocyanine, methine, polymethine, indoaniline, indophenol, stilbene, squarilium, aminoketone, xanthene, fluorone, acridene, quinolene, thiazole, azine, induline, nigrosine, oxazine, thiazine, indigoid, quinonioid, quinacridone, lactone, benzodifuranone, flavonol, chalone, polyene,
- Preferred chromophoric groups are azo groups (especially monoazo, and bisazo), anthraquinone and phthalocyanine groups.
- the polymerisable dye comprises a chromophoric group and one or more functional groups selected from an acrylate or methacrylate backbone.
- a polymerisable dye may contain a single chromophore, for example with bright yellow, magenta or cyan colours and self shade blacks. However, it may also contain mixed covalently attached chromophores for example to obtain a black colour, by covalently attached brown and blue or yellow, magenta and cyan. Green can be obtained by yellow and cyan etc. Extended conjugated chromophores can also be used to obtain some shades. For example, bis- and trisazo compounds can be used to obtain blacks and other duller shades (navy blue, brown, olive green, etc).
- Polymerisable dyes can also be used to obtain the correct particle shade; for example a black from single component mixtures of brown and blue or yellow, magenta and cyan pre-polymerised dyes.
- shades can be tuned for example by adding small quantities of separate polymerisable dyes to modify the colour of the particles (e.g. 95% yellow and 5% cyan to get a greener yellow shade).
- Modified polymerisable dyes from the application groups of reactive (anionic), direct (anionic), acidic (anionic) and basic
- Cationic polymerisable dyes contain a covalently attached group or groups which have a positive charge in the application or contain a positive charge in the chromophore group. They can be derived from protonation or quaternation of nitrogen, phosphorous, oxygen or sulphur atoms or groups containing them, for example heteroaromatic (thiazole, imidazole) delocalised nitrogen bases (guanidine etc).
- Associated anions preferably have a single charge and can preferably be halogen, preferably F ' , Cl “ , Br " , monobasic acid (oxo) anions, preferably acetate, propionate, lactate, methane sulphonate, p-toluenesulphonate, hydroxide, nitrate).
- halogen preferably F ' , Cl “ , Br "
- monobasic acid (oxo) anions preferably acetate, propionate, lactate, methane sulphonate, p-toluenesulphonate, hydroxide, nitrate).
- water soluble cationic polymerisable dyes are listed in Table 2 (counter ion MeOSO 3 ; also preferably suitable are Cl “ , Br " , and acetate)
- Anionic polymerisable dyes contain a covalently attached group or groups which have a negative charge in the application and can be derived from deprotonation of an acidic group for example sulphonic, carboxylic, phosphonic acids.
- Associated cations preferably have a single charge and can be metallic (Li + , Na + , K + etc), charged nitrogen (NH 4 + , NEt 3 H + , NEt 4 + , NMe 4 + , imidazolium cation etc), positively charged phosphorous, sulphur etc.
- Preferred examples of water soluble anionic dyes are the Na + , NH 4 + , NEt 4 + salts of the acids. Another preferred example is
- Preferred dye acids are listed in Table 3.
- Preferred water dispersible neutral dyes are listed in Table 4.
- polymerisable water-soluble dye monomers such as the acrylate or methacrylate derivatives of cationic Basic Blue 41 (listed in Table 2 as numbers 1 and 2) and similar dyes, can be used.
- R1 ,R2,R3 alkyl, preferably C1-C4 alkyl
- R4 H or CH 3
- solvent soluble dyes such as commercially available Disperse Red 1 methacrylate.
- the cross-linked coloured polymer particles of the invention can be prepared in a simple 1-step reaction.
- the selection of the polymerisation conditions depends on the required size and size distribution of the particles. Adjustment of polymerization conditions is well known to someone skilled in the art.
- Emulsion polymerisation is a well known polymerisation process wherein barely water soluble monomers are emulsified in water by an emulsifier and polymerised by water-soluble initiators.
- the procedure by which an emulsion polymerisation is carried out has a profound effect upon the resulting particle size and polymer properties. Indeed, particles with quite different performance characteristics can be produced from the same reaction formulation by appropriate control of polymerisation process and conditions used.
- Comprehensive reviews of emulsion polymerisation conditions are given in "Emulsion polymerization"; van Herk, Alex; Gilbert, Bob;
- a batch emulsion polymerisation process is used wherein all reactants are completely added at the outset of the polymerisation process.
- Preferred changes which can be made in such cases are to the reaction temperature, reactor design and the type and speed of stirring.
- a batch emulsion polymerisation process is used for manufacture versus a semi-continuous batch process because of limited versatility and simple evaluations of reaction formulation.
- a surfactant- free emulsion copolymerisation using batch process is preferred.
- Protective colloids (water-soluble polymers) and surfactants are usually key formulation variables in emulsion polymerisation because of their impact on the intraparticle stability and particle size control but they may have a detrimental effect on the electrophoretic response
- water soluble dyes are used in emulsion polymerisation.
- a preferred way of incorporating water-insoluble dyes into particles is to use the so-called 'mini-emulsion polymerisation', as described in K. Landfester, Macromol. Rapid. Commun., 2001, 22, 896 - 936.
- a Mini-Emulsion Polymerisation forms small stable droplets by high shear (30-500 nm) in a system containing a dispersed phase, a continuous phase, a surfactant and an osmotic pressure agent (hydrophobe).
- the nano sized droplets formed by high shear mixing are considered to be individual nanoreactors. It is these droplets which are the primary location for initiation of polymerisation. It is due to an inability of the water-insoluble dyes to be transported through the water medium that allows incorporation of these severely hydrophobic components in the droplets, and hence the forming of particles. Stabilisation against coalescence is achieved by adding surfactant whereas stabilisation against diffusion is achieved by adding a highly monomer soluble and water insoluble agent. The aim is to initiate polymerisation in each of the stabilised droplets. High shear can be achieved using a rotor-stator or high pressure homogenisers to prepare mechanical emulsification or ultrasound.
- the polymerisation according to the invention is a free radical polymerisation.
- a monomer composition according to the invention comprises at least one polymerisable dye, at least one monomer, at least one initiator, and optionally at least one charged co-monomer.
- a monomer composition according to the invention comprises polymerisable dye, a monomer providing the basic structure, a crosslinking co-monomer, an ionic co-monomer and an initiator.
- the monomers described in the following for preparation of the polymeric particles can also be combined with the polymerisable dyes to produce a polymerisable dye-monomer mixture to be added to and/or incorporated in to the particles, for example as a core-shell effect so that there is more dye on the shell of the particles. Addition of a co-monomer seems advantageous in that it increases the amount of reactive groups available for polymerisation, the polymerisation proceeds faster with additional monomer.
- the monomers (and co-monomers) described in the following for preparation of the polymeric particles can be combined with the polymerisable dyes to produce a polymerisable dye/monomer mixture and/or the monomers can be incorporated stepwise into the polymerisable mixture to produce special effects, for example a core-shell effect so that there is more dye on the shell of the particles.
- Particularly preferable are monomers which are similar to the polymerisable dye, such as methyl methacrylate with disperse red 1 acrylate.
- the particles can be prepared from most monomer types, in particular methacrylates, acrylates, methacrylamides, acrylonitriles, ⁇ -substituted acrylates, styrenes and vinyl ethers, vinyl esters, propenyl ethers, oxetanes and epoxys but would typically be prepared from largest percentage to be monomer, then cross-linker, and include a charged monomer (e.g. quaternised monomer).
- methyl methacrylate and ethylene glycol dimethyl methacrylate as a cross-linker and 2-methacryloxy ethyl trimethyl ammonium chloride (MOTAC) as reactive charged monomer
- MOTAC 2-methacryloxy ethyl trimethyl ammonium chloride
- Methacrylic acid Methyl methacrylate (MMA), Ethyl methacrylate (EMA), n- Butyl methacrylate (BMA), 2-Aminoethyl methacrylate hydrochloride, AIIyI methacrylate, Benzyl methacrylate, 2-Butoxyethyl methacrylate, 2-(tert- Butylamino)ethyl methacrylate, Butyl methacrylate, terf-Butyl methacrylate, Caprolactone 2-(methacryloyloxy)ethyl ester, 3-Chloro-2-hydroxypropyl methacrylate, Cyclohexyl methacrylate, 2-(Diethylamino)ethyl methacrylate, Di(ethylene glycol) methyl ether methacrylate, 2-(Dimethylamino)ethy!
- Trimethylhexyl acrylate Preferably Methyl acrylate, Ethyl acrylate, Acrylic acid, and/or n-Butyl acrylate are used.
- Acrylamides Preferably Methyl acrylate, Ethyl acrylate, Acrylic acid, and/or n-Butyl acrylate are used.
- Vinylbenzoic acid 4-Vinylbenzoic acid, Vinylbenzyl chloride, 4-Vinylbenzyl chloride, (Vinylbenzyl)trimethylammonium chloride, 4-Vinylbiphenyl, 2- Vinylnaphthalene, 2-Vinylnaphthalene, Vinyl acetate, Vinyl benzoate, Vinyl 4-terf-butylbenzoate, Vinyl chloroformate, Vinyl chloroformate, Vinyl cinnamate, Vinyl decanoate, Vinyl neodecanoate, Vinyl neononanoate, Vinyl pivalate, Vinyl propionate, Vinyl stearate, Vinyl trifluoroacetate,
- monomers which may be used are those which have groups to help stabilisation of the particles, e.g. Poly(ethylene glycol) methyl ether acrylate, Poly(ethylene glycol) phenyl ether acrylate, lauryl methacrylate, Poly(ethylene glycol) methyl ether acrylate, Poly(propylene glycol) methyl ether acrylate, Lauryl acrylate and fluorinated monomers of above.
- Some of the monomers have groups for further reaction if so desired, e.g. Glycidyl ethacrylate, 2-Hydroxyethyl methacrylate.
- ethylene glycol dimethacrylate (EGDMA) 1 allyl methacrylate (ALMA), divinyl benzene, Bis[4-(vinyloxy)butyl] adipate, Bis[4-(vinyloxy)butyl] 1 ,6- hexanediylbiscarbamate, Bis[4-(vinyloxy)butyl] isophthalate, Bis[4- (vinyloxy)butyl] (methylenedi-4,1-phenylene)biscarbamate, Bis[4- (vinyloxy)butyl] succinate, Bis[4-(vinyloxy)butyl]terephthalate, Bis[4- (vinyloxymethyl)cyclohexylmethyl] glutarate, 1 ,4-Butanediol divinyl ether, 1 ,4-Butanediol vinyl ether, But
- Pentaerythritol triacrylate Polypropylene glycol) diacrylate, Poly(propylene glycol) dimethacrylate, 1 ,3,5-Triacryloylhexahydro-1 ,3,5-triazine, Tricyclo[5.2.1.0]decanedimethanol diacrylate, Trimethylolpropane benzoate diacrylate, Trimethylolpropane ethoxylate methyl ether diacrylate, Trimethylolpropane ethoxylate triacrylate, Trimethylolpropane triacrylate, Trimethylolpropane trimethacrylate, Tris[2-(acryloyloxy)ethyl] isocyanurate, Tri(propylene glycol) diacrylate.
- the monomer composition comprises at least one charged co- monomer.
- cationic monomers for particle stability and particle size control are 2-methacryloxy ethyl trimethyl ammonium chloride (MOTAC), acryloxy ethyl trimethyl ammonium chloride (AOTAC), [3- (Methacryloylamino)propyl]trimethylammonium chloride, [2- (Methacryloyloxy)ethyl]trimethylammonium methyl sulfate solution, tetraallyl ammonium chloride, diallyl dimethyl ammonium chloride, (Vinylbenzyl)trimethylammonium chloride.
- MOTAC 2-methacryloxy ethyl trimethyl ammonium chloride
- AOTAC acryloxy ethyl trimethyl ammonium chloride
- [3- (Methacryloylamino)propyl]trimethylammonium chloride [2- (Methacryloyloxy)ethyl]trimethylammoni
- MOTAC 2-methacryloxy ethyl trimethyl ammonium chloride
- AOTAC acryloxy ethyl trimethyl ammonium chloride
- [2-(Methacryloyloxy)ethyl]trimethylammonium methyl sulfate solution are used.
- anionic monomers are sodium, potassium or triethylamine salts of methacrylic acid, Acrylic acid, 2-(Trifluoromethyl)acrylic acid, 3-(2- Furyl)acrylic acid, 3-(2-Thienyl)acrylic acid, 3-(Phenylthio)acrylic acid, Poly(acrylic acid) potassium salt, Poly(acrylic acid) sodium salt, Poly(acrylic acid), Poly(acrylic acid, sodium salt) solution, frans-3-(4- Methoxybenzoyl)acrylic acid, 2-Methoxycinnamic acid, 3-lndoleacrylic acid, 3-Methoxycinnamic acid, 4-lmidazoleacrylic acid, 4-Methoxycinnamic acid, Poly(styrene)-b/oc/f-poly(acry lie acid), Poly(acrylonitrile-co-butadiene-co- acrylic acid), dicarboxy terminated, Poly(acrylonitrile-co-butadiene-co-acrylic acid), dicar
- Biphenyl]-4-YI)-2-Oxoethyl]Acrylic Acid 2-(2-(2-Chloroanilino)-2-Oxoethyl)- 3-(4-Methoxyphenyl)Acrylic Acid, 2-(2-((2-Hydroxyethyl)Amino)-2-Oxoethyl)- 3-(4-Methoxyphenyl)Acrylic Acid, 2-(2-(Cyclohexylamino)-2-Oxoethyl)-3-(4- Methoxyphenyl)Acrylic Acid.
- a preferred monomer composition comprises methyl methacrylate and ethylene glycol dimethacrylate as a cross-linker and 2-methacryloxy ethyl trimethyl ammonium chloride (MOTAC) as reactive charged monomer.
- MOTAC 2-methacryloxy ethyl trimethyl ammonium chloride
- a water soluble initiator is used in the surfactant-free emulsion copolymerisation in order to control size, particle morphology and to reduce the residual monomers at the end of the reaction.
- examples are azo compounds or peroxide compounds, hydroperoxides or peracid esters.
- azo compounds are used, especially azobis(isobutylamidine) hydrochloride (AIBA) and similar compounds.
- the polymerisable composition of the invention usually comprises up to 10 %, preferably 0.005 - 10 %, especially 0.05 - 5 % by weight of dye, 50 - 95 %, preferably 70 - 90 %, by weight of monomer, 1 - 40 %, preferably 1 - 10 %, by weight of crosslinking monomer, 1 - 30 %, preferably 1 - 10 %, by weight of ionic monomer and 0.1 - 10 %, preferably 0.1 - 5 %, by weight of initiator, all percentages are based on the total weight of the polymerisable composition (except solvent).
- Cross-linked copolymer nanoparticles can be prepared by emulsifier-free copolymerization of methyl methacrylate (MMA) 1 ethylene glycol dimethacrylate (EGDMA), and a cationic comonomer, methacryloxy ethyl trimethyl ammonium chloride (MOTAC) using azobis(isobutylamidine) hydrochloride (AIBA) as an initiator.
- MMA methyl methacrylate
- EGDMA ethylene glycol dimethacrylate
- MOTAC methacryloxy ethyl trimethyl ammonium chloride
- AIBA azobis(isobutylamidine) hydrochloride
- emulsifier-free emulsion polymerizations are conducted using a batch process,
- Polymer particles prepared according to the invention are preferably spherical particles with a size (diameter) in the range of 50 - 1000 nm and preferably with a monodisperse size distribution.
- Preferred particle sizes are 50 - 600 nm, preferably 50 - 560 nm, especially 50 - 500 nm, even more preferred 100 - 400 nm.
- Particle sizes are determined by photon correlation spectroscopy of aqueous particle dispersions by a common apparatus such as a Malvern NanoZS particle analyser.
- the size of polymer particles in electrophoretic fluids may be different from sizes measured in aqueous dispersions because of the influence of solvents and/or surfactants.
- the polymer particles of the invention preferably have a particle size of 100 - 800 nm, especially 100 - 700 nm, preferably 150 - 700 nm are preferred.
- polymer particles having a particle size of 150 - 600 nm are particularly preferred.
- Particles of the invention are primarily designed for use in electrophoretic displays. So, further subjects of the invention are electrophoretic fluids and electrophoretic displays comprising A typical electrophoretic display preferably consists of the particles dispersed in a low polar or non-polar solvent along with additives to improve electrophoretic properties, such as stability and charge.
- Typical additives to improve the stability of the electrophoretic fluid are known to experts in the field and include (but are not limited to) the Brij, Span and Tween series of surfactants (Aldrich), the Solsperse, lrcosperse and Colorburst series
- any other additives to improve the electrophoretic properties can be incorporated provided they are soluble in the formulation medium, in particular thickening agents or polymer additives designed to minimise settling effects.
- the dispersion solvent can be chosen primarily on the basis of dielectric constant, refractive index, density and viscosity.
- a preferred solvent choice would display a low dielectric constant ( ⁇ 10, more preferably ⁇ 5), high volume resistivity (about 10 15 ohm-cm), a low viscosity (less than 5cst), low water solubility, a high boiling point (>80°C) and a refractive index and density similar to that of the particles. Tweaking these variables can be useful in order to change the behavior of the final application. For example, in a slow-switching application such as poster displays or shelf labels, it can be advantageous to have an increased viscosity to improve the lifetime of the image, at the cost of slower switching speeds.
- the preferred solvents are often non- polar hydrocarbon solvents such as the lsopar series (Exxon-Mobil), Norpar, Shell-Sol (Shell), Sol-Trol (Shell), naphtha, and other petroleum solvents, as well as long chain alkanes such as dodecane, tetradecane, decane and nonane). These tend to be low dielectric, low viscosity, and low density solvents.
- a density matched particle / solvent mixture will yield much improved settling/sedimentation characteristics and thus is desirable. For this reason, often it can be useful to add a halogenated solvent to enable density matching.
- Typical examples of such solvents are the Halocarbon oil series (Halocarbon products), or tetrachlorethylene, carbon tetrachloride, 1 ,2,4-trichlorobenzene and similar solvents.
- Halocarbon oil series Halocarbon products
- tetrachlorethylene carbon tetrachloride
- 1 ,2,4-trichlorobenzene and similar solvents.
- the negative aspect of many of these solvents is toxicity and environmental friendliness, and so in some cases it can also be beneficial to add additives to enhance stability to sedimentation rather than using such solvents.
- the preferred additives and solvents used in the formulation of the particles of the invention are OLOA11000 (Chevron Chemicals), lrcosperse 2153 (Lubrizol Ltd), and dodecane (Sigma Aldrich)
- electrophoretic fluids comprise a charged inorganic nanoparticle such as titania, alumina or barium sulphate, coated with a surface layer to promote good dispersibility in dielectric media and a dielectric fluid media.
- the solvents and additives used to disperse the particles are not limited to those used within the examples of this invention and many other solvents and/or dispersants can be used. Lists of suitable solvents and dispersants for electrophoretic displays can be found in existing literature, in particular WO 99/10767) and WO 2005/017046)
- the Electrophoretic fluid is then incorporated into an Electrophoretic display element by a variety of pixel architectures, such as can be found in C. M. Lampert, Displays; 2004, 25(5) published by Elsevier B.V., Amsterdam.
- Electrophoretic displays comprise typically, the electrophoretic display media in close combination with a monolithic or patterned backplane electrode structure, suitable for switching the pixels or patterned elements between the black and white optical states or their intermediate greyscale states.
- the electrophoretic particles according to the present invention are suitable for all known electrophoretic media and electrophoretic displays, e.g. flexible displays, one particle systems, two particle systems, dyed fluids, systems comprising microcapsules, microcup systems, air gap systems and others as described in C. M. Lampert, Displays; 2004, 25(5) published by Elsevier B.V., Amsterdam.
- flexible displays are dynamic keypads, e- paper watches, dynamic pricing and advertising, e-readers, Tollable displays, smart card media, product packaging, mobile phones, lab tops, display card, digital signage.
- the characterisation of the formulations was performed using a Malvern NanoZS particle analyser. This instrument measures the size of particles in dispersion and the zeta potential of an electrophoretic fluid.
- the Zeta potential (ZP) is derived from the real-time measurement of the electrophoretic mobility and thus is an indicator of the suitability of the fluid for use in electrophoretic applications.
- 2-Amino-6-methoxybenzothiazole (18.0 g) is stirred in a mixture of acetic acid (70 ml) and propionic acid (50 ml) at 5O 0 C. The resulting solution is 0 cooled to -1O 0 C. Nitrosylsulphuric acid solution (40 weight-% in sulphuric acid) (32.0 g) is added dropwise. This mixture is added to a stirred solution of N-ethyl-N-(2-hydroxyethyl) aniline and sulphamic acid (1.0 g) in acetic acid (25 ml) and ice/water (100 ml). After 20 minutes, the pH is raised to 4 by the dropwise addition of potassium hydroxide solution.
- a tarry residue is 5 formed; the mixture is stirred for a further 2 hours until the tar solidifies. This solid is collected, washed with water and then dissolved in alcohol and acetone to give a deep red solution. Hot water is added to precipitate a solid which is removed by filtration. The solid is washed with cold alcohol and dried (29.5 g, 83% yield) Mp 178-179 0 C 0
- Stage 2 The above hydroxy ethyl disperse dye (10.7 g) dye is stirred in methylene chloride (100 ml) and pyridine (20 ml). Methacrylic anhydride (10 ml) is added and the mixture is heated under reflux for 24 hours. On cooling to room temperature, water (5 ml) is added and the mixture is stirred for 2
- the spectra show ions at m/z 439 which corresponds with the cation for the proposed structure.
- Example 2 Preparation of blue polymethyl methacrylate (PMMA) particles containing Methacrylate derived from Basic Blue 41 Methyl methacrylate (95.0 g), ethylene glycol dimethacrylate (8.0 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (4.0 g), deionised water (900 g) and blue dye of Example 1 (1.0 g) are stirred at 300 rpm under an atmosphere of nitrogen in a 2 litre 3-neck flask at 7O 0 C. Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride
- a known quantity of the freeze dried particles is weighed into a fixed volume of dodecane and redispersed. The dispersion is centrifuged for 5 minutes at 1000 rpm. The supernatant is removed, filtered through a 0.1 micron PTFE (polytetrafluoroethylene) syringe filter. The remaining particles are added to a further fixed volume of dodecane. The above experimental is repeated as many times as required to determine whether dye is leaching from the particles.
- the supernatants are analysed by ultra-violet/visible spectrophometric analysis over a suitable range (typically 350-700nm) to determine if dye leaching is occurring. No dye is detected.
- Example 3 Preparation of blue polymethyl methacrylate (PMMA) particles containing Methacrylate derived from Basic Blue 41 Methyl methacrylate (7.13 g), ethylene glycol dimethacrylate (0.06 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (0.3 g), water (95.0 g) and blue dye of Example 1 (7.13 mg) are stirred at 400 rpm under an atmosphere of nitrogen in a 250 ml 3-neck flask at 7O 0 C. Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride (0.08 g) is added.
- PMMA polymethyl methacrylate
- the blue latex After 20 hours the blue latex is allowed to cool to room temperature, and is filtered through a 5 micron cloth. Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 248 nm and zeta potential of +70 mV. The suspension is freeze dried to give a fine blue powder (3).
- the above method is used to determine whether any dye is leaching from the particles. No leaching can be detected.
- the supernatants are colourless.
- Methyl methacrylate (7.13 g), ethylene glycol dimethacrylate (0.06 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (0.3 g), water (95.0 g) and blue dye of Example 1 (17.8 mg) are stirred at 400 rpm under an atmosphere of nitrogen in a 250 ml 3-neck flask at 7O 0 C. Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride (0.08 g) is added. After 20 hours the blue latex is allowed to cool to room temperature, and is filtered through a 5 micron cloth. Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 337 nm and zeta potential of +51 mV.
- the suspension is freeze dried to give a fine blue powder (4).
- the above method is used to determine whether any dye is leaching from the particles. No leaching can be detected.
- the supernatants are colourless.
- Example 5 Preparation of blue polymethyl methacrylate (PMMA) particles containing Methacrylate derived from Basic Blue 41 Methyl methacrylate (7.13 g), ethylene glycol dimethacrylate (0.06 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (0.3 g), water (95.0 g) and blue dye of Example 1 (35.6 mg) are stirred at 400 rpm under an atmosphere of nitrogen in a 250 ml 3-neck flask at 7O 0 C. Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride (0.08 g) is added.
- PMMA polymethyl methacrylate
- the blue latex After 20 hours the blue latex is allowed to cool to room temperature, and is filtered through a 5 micron cloth. Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 290 nm and zeta potential of +66 mV. The suspension is freeze dried to give a fine blue powder (5).
- the above method is used to determine whether any dye is leaching from the particles. No leaching can be detected.
- the supernatants are colourless.
- Methyl methacrylate (7.13 g), ethylene glycol dimethacrylate (0.06 g, [2- (methacryloyloxy) ethyl]-trimethyl ammonium methyl sulphate solution (MOTAMS in water (80%) (0.15 g), water (95.0 g) and blue dye of Example 1 (35.7 mg) are stirred at 400 rpm under an atmosphere of nitrogen in a 250 ml 3-neck flask at 7O 0 C.
- Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride (0.08 g) is added. After 20 hours the blue latex is allowed to cool to room temperature, and is filtered through a 5 micron cloth.
- Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 194 nm and zeta potential of +55 mV.
- the suspension is freeze dried to give a fine blue powder (6).
- the above method is used to determine whether any dye is leaching from the particles. No leaching can be detected.
- the supernatants are colourless.
- Example 7 Preparation of blue polymethyl methacrylate (PMMA) particles containing Methacrylate derived from Basic Blue 41 Methyl methacrylate (7.13 g), ethylene glycol dimethacrylate (0.06 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (0.3 g) and water (95.0 g) are stirred at 400 rpm under an atmosphere of nitrogen in a 250 ml 3-neck flask at 7O 0 C. Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride (0.08 g) is added. Blue dye of
- Example 1 (35.6 mg) is added after initiation. After 20 hours the blue latex is allowed to cool to room temperature, and is filtered through a 5 micron cloth. Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 183 nm and zeta potential of +69 mV. The suspension is freeze dried to give a fine blue powder (7).
- the above method is used to determine whether any dye is leaching from the particles. No leaching can be detected.
- the supernatants are colourless.
- Methyl methacrylate (7.13 g), ethylene glycol dimethacrylate (0.06 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (0.15 g), water (95.0 g) and blue dye of Example 1 (70.13 mg) are stirred at 400 rpm under an atmosphere of nitrogen in a 250 ml 3-neck flask at 7O 0 C. Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride (0.08 g) is added. After 20 hours the blue latex is allowed to cool to room temperature, and is filtered through a 5 micron cloth. Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 235 nm and zeta potential of +54 mV.
- the suspension is freeze dried to give a fine blue powder (8).
- the above method is used to determine whether any dye is leaching from the particles. No leaching can be detected.
- the supernatants are colourless.
- Example 9 Preparation of polymethyl methacrylate (PMMA) particles using Disperse Red 1 Methacrylate Methyl methacrylate (95.0 g), ethylene glycol dimethacrylate (8.0 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (4.0 g) and disperse red 1 methacrylate dye (Sigma-Aldrich) (250 mg) are stirred at 300 rpm under an atmosphere of nitrogen in a 2 litre 3- neck flask at 70 0 C. Initiator 2,2'-azobis (2-methylpropionamidine) dihydrochloride (1.0 g) is added.
- PMMA Polymethyl methacrylate
- the above method is used to determine whether any dye is leaching from the particles. No leaching can be detected.
- the supernatants are colourless.
- Example 10 Electrophoretic formulation containing PMMA incorporating Methacrylate of Basic Blue 41
- Example 11 Electrophoretic formulation containing PMMA incorporating Methacrylate of Basic Blue 41 (example 3) 0.3080 g of coloured PMMA of Example 3 is added to 0.00972 g of detergent lnfineum E (Infineum Corporation) in 0.96259 g of dodecane and vortex mixed. The resultant dispersion is then homogenised using an ultra- turrax T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath. The dispersion is then roller mixed overnight to yield a blue electrophoretic ink.
- Example 12 Electrophoretic formulation containing PMMA incorporating Methacrylate of Basic Blue 41 (example 4)
- Example 13 Electrophoretic formulation containing PMMA incorporating Methacrylate of Basic Blue 41 (example 5) 0.3040 g of coloured PMMA of Example 5 is added to 0.00970 g of detergent Infineum E (Infineum Corporation) in 0.9610 g of dodecane and vortex mixed. The resultant dispersion is then homogenised using an ultra- turrax T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath. The dispersion is then roller mixed overnight to yield a blue electrophoretic ink. Size (260 nm), Electrophoretic Mobility (0.07089 ⁇ mcm/Vs), ZP (+76 mV).
- Example 14 Electrophoretic formulation containing PMMA incorporating Methacrylate of Basic Blue 41 (example 7) 0.03070 g of coloured PMMA of Example 7 is added to 0.00989 g of detergent Infineum E (Infineum Corporation) in 0.98011 g of dodecane and vortex mixed. The resultant dispersion is then homogenised using an ultra- turrax T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath. The dispersion is then roller mixed overnight to yield a blue electrophoretic ink. Size (176 nm), Electrophoretic Mobility (0.06321 ⁇ mcm/Vs), ZP (+68 mV).
- Example 15 Electrophoretic formulation containing PMMA incorporating Disperse Red1 Methacrylate 2.014 g of coloured PMMA of Example 9 is added to 0.216 g of OLOA 11000 and 0.201 g of lrcosperse 2153 in 10.942 g of dodecane and vortex mixed. The resultant dispersion is then homogenised using an ultra-turrax T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath. The dispersion is then roller mixed overnight to yield a pink electrophoretic ink. Size (264 nm), Electrophoretic Mobility (0.04257 ⁇ mcm/Vs), ZP (+51 mV).
- Stage 2 Sodium nitrite solution (2N, 28.5 ml) is added to a cold stirred mixture of 4- chloro-2-nitroaniline (9.77 g, 0.0566 mol) in acetic acid/12N hydrochloric acid (75/25). After 2 hours, the resulting solution of diazonium salt is added to a solution of the above pyridone (11.0 g., 0.0566 mol) in water (prepared by dropwise addition of 2N sodium hydroxide to an aqueous suspension of the coupling component).
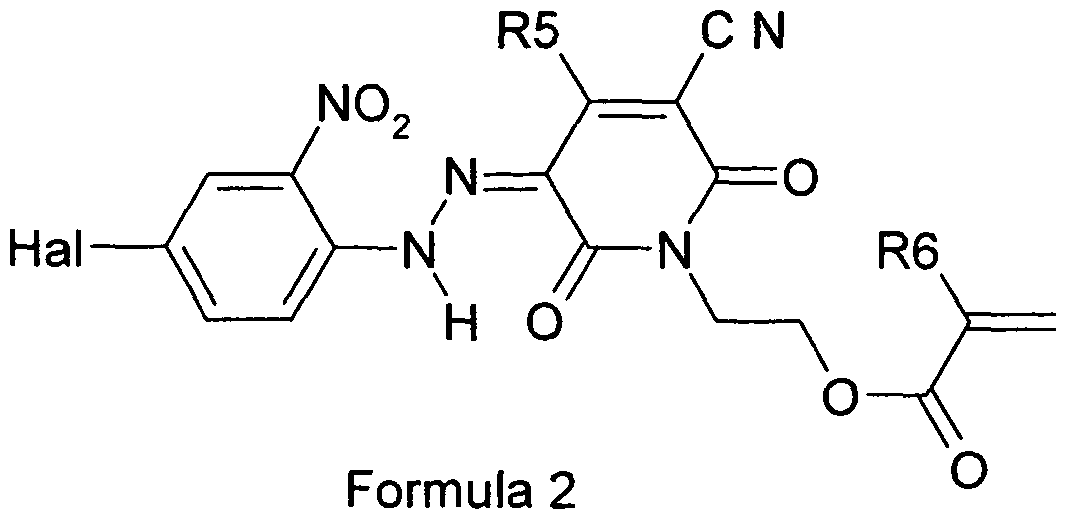
- Methacryloyl anhydride (17.9 g, 0.116 mol) is added to a stirred mixture of 5-(4-chloro-2-nitrophenylazo)-3-cyano-6-hydroxy-1-(2-hydroxyethyl)-4- methylpyrid-2-one (22 g, 058 mol) and pyridine (25 ml) in methylene chloride (200 ml).
- the mixture is stirred under reflux for 24 hours, forming a solution after ca. 5 hr. After cooling to 2O 0 C water (20ml) is added, with stirring, followed by more water (200 ml) after a further 2 hours.
- Example 17 Preparation of yellow polystyrene (PS) particles containing disperse dye of Example 16 by mini-emulsion polymerisation Styrene (5.0 g), divinyl benzene (0.5 g), hexadecane (0.1 g), polyethyleneglycol methyl ether methacrylate (mw 475) (1.0 g), sodium dodecyl sulphate (100 mg), initiator Vazo 67 (2,2'-Azobis(2- methylbutyronitrile) (126 mg) and yellow dye of Example 16 (50 mg) are shaken until soluble and then deionised water (50 g) is added.
- PS yellow polystyrene
- a magnetic stirrer bar is added.
- the mixture is evacuated and purged with nitrogen three times using a low power ultrasonic bath on a stirrer hotplate.
- the stirrer bar is removed.
- the flask is put into an ice bath and the tip of a sonic probe (Branson 450) is inserted into the mixture.
- a nitrogen flow is maintained over the mixture.
- the mixture is sonicated for 3 minutes at 150 Watts, under an atmosphere of nitrogen. A stable yellow emulsion is formed.
- the flask is transferred to a hot oil bath, pre-heated to 75 0 C, a magnetic stirrer bar is added the flask and the contents are stirred on a stirrer hot plate/oil bath over night.
- the mixture is allowed to cool to room temperature and is filtered through a 5 micron cloth.
- the latex is cleaned with water and acetone to remove any unreacted monomers using a centrifuge to separate particles and supernatant.
- Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 206 nm.
- the suspension is freeze dried to give a fine yellow powder (17).
- Example 18 Preparation of yellow polystyrene (PS) particles containing disperse dye of Example 16 by mini-emulsion polymerisation
- Styrene (5.0 g), divinyl benzene (0.5 g), hexadecane (0.1 g), polyethyleneglycol methyl ether methacrylate mw 475 (1.0 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (100 mg), sodium dodecyl sulphate (100 mg), initiator Vazo 67 (2,2'- Azobis(2-methylbutyronitrile) (126 mg) and yellow pyridine methacrylate dye of Example 16 (50 mg) are shaken until soluble and then deionised water (50 g) is added. A magnetic stirrer bar is added.
- the mixture is evacuated and purged with nitrogen three times using a low power ultrasonic bath on a stirrer hotplate.
- the stirrer bar is removed.
- the flask is put into an ice bath and the tip of a sonic probe (Branson 450) is inserted into the mixture.
- a nitrogen flow is maintained over the mixture.
- the mixture is sonicated for 3 minutes at 150 Watts, under an atmosphere of nitrogen. A stable yellow emulsion is formed.
- the flask is transferred to a hot oil bath, pre-heated to 75 0 C, a magnetic stirrer bar is added the flask and the contents are stirred on a stirrer hot plate/oil bath over night.
- the mixture is allowed to cool to room temperature and is filtered through a 5 micron cloth.
- the latex is cleaned with water and acetone to remove any unreacted monomers using a centrifuge to separate particles and supernatant. Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 205 nm and zeta potential of +63 mV. The suspension is freeze dried to give a fine yellow powder (18).
- Example 19 Preparation of yellow polystyrene (PS) particles containing disperse dye of Example 16 by mini-emulsion polymerisation Styrene (5.0 g), divinyl benzene (0.5 g), hexadecane (0.1 g), polyethyleneglycol methyl ether methacrylate mw 475 (1.0 g), [2- (methacryloyloxy)ethyl]-trimethyl ammonium chloride solution (75% in water) (0.5 g), cetyl trimethyl ammonium bromide (100 mg), initiator Vazo 67 (2,2'-Azobis(2-methylbutyronitrile) (126 mg) and yellow pyridine methacrylate dye of Example 16 (50 mg) are shaken until soluble and then deionised water (50 g) is added.
- Styrene 5.0 g
- divinyl benzene 0.5 g
- hexadecane 0.1 g
- a magnetic stirrer bar is added.
- the mixture is evacuated and purged with nitrogen three times using a low power ultrasonic bath on a stirrer hotplate.
- the stirrer bar is removed.
- the flask is put into an ice bath and the tip of a sonic probe (Branson 450) is inserted into the mixture.
- a nitrogen flow is maintained over the mixture.
- the mixture is sonicated for 3 minutes at 150 Watts, under an atmosphere of nitrogen. A stable yellow emulsion is formed.
- the flask is transferred to a hot oil bath, pre-heated to 75 0 C 1 a magnetic stirrer bar is added the flask and the contents are stirred on a stirrer hot plate/oil bath over night.
- the mixture is allowed to cool to room temperature and is filtered through a 5 micron cloth.
- the latex is cleaned with water and acetone to remove any unreacted monomers using a centrifuge to separate particles and supernatant.
- Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 169 nm.
- the suspension is freeze dried to give a fine yellow powder (19).
- Example 20 Preparation of yellow polystyrene (PS) particles containing disperse dye of Example 16 by mini-emulsion polymerisation
- Styrene (5.0 g), divinyl benzene (0.5 g), hexadecane (0.1 g), polyethyleneglycol methyl ether methacrylate mw 475 (1.0 g), ), vinylbenzyl trimethyl ammonium chloride solution (250 mg), cetyl trimethyl ammonium bromide (100 mg), initiator Vazo 67 (2,2'-Azobis(2-methylbutyronitrile) (126 mg) and yellow pyridine methacrylate dye of Example 16 (50 mg) are shaken until soluble and then deionised water (50 g) is added. A magnetic stirrer bar is added.
- the mixture is evacuated and purged with nitrogen three times using a low power ultrasonic bath on a stirrer hotplate.
- the stirrer bar is removed.
- the flask is put into an ice bath and the tip of a sonic probe (Branson 450) is inserted into the mixture.
- a nitrogen flow is maintained over the mixture.
- the mixture is sonicated for 3 minutes at 150 Watts, under an atmosphere of nitrogen. A stable yellow emulsion is formed.
- the flask is transferred to a hot oil bath, pre-heated to 75 0 C, a magnetic stirrer bar is added the flask and the contents are stirred on a stirrer hot plate/oil bath over night.
- the mixture is allowed to cool to room temperature and is filtered through a 5 micron cloth.
- the latex is cleaned with water and acetone to remove any unreacted monomers using a centrifuge to separate particles and supernatant. Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 173 nm. The suspension is freeze dried to give a fine yellow powder (20).
- Example 21 Preparation of yellow polystyrene (PS) particles containing disperse dye of Example 16 by mini-emulsion polymerisation
- Styrene (5.0 g), divinyl benzene (0.5 g), hexadecane (0.1 g), polyethyleneglycol methyl ether methacrylate mw 475 (1.0 g), vinylbenzyl trimethyl ammonium chloride solution (500 mg), cetyl trimethyl ammonium bromide (50 mg), initiator Vazo 67 (2,2'-Azobis(2-methylbutyronitrile) (60 mg) and yellow pyridine methacrylate dye of Example 16 (75 mg) are shaken until soluble and then deionised water (50 g) is added. A magnetic stirrer bar is added.
- the mixture is evacuated and purged with nitrogen three times using a low power ultrasonic bath on a stirrer hotplate.
- the stirrer bar is removed.
- the flask is put into an ice bath and the tip of a sonic probe (Branson 450) is inserted into the mixture.
- a nitrogen flow is maintained over the mixture.
- the mixture is sonicated for 3 minutes at 150 Watts, under an atmosphere of nitrogen. A stable yellow emulsion is formed.
- the flask is transferred to a hot oil bath, pre-heated to 75 0 C, a magnetic stirrer bar is added the flask and the contents are stirred on a stirrer hot plate/oil bath over night.
- the mixture is allowed to cool to room temperature and is filtered through a 5 micron cloth.
- the latex is cleaned with water and acetone to remove any unreacted monomers using a centrifuge to separate particles and supernatant.
- Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 180 nm.
- the suspension is freeze dried to give a fine yellow powder (21).
- Example 22 Preparation of cationic co-monomer for use in particles of example 23
- Example 23 Preparation of yellow polystyrene (PS) particles containing disperse dye of Example 16 by mini-emulsion polymerisation
- Methyl methacrylate 5.0 g
- ethylene glycol dimethacrylate 0.5 g
- hexadecane 0.1 g
- polyethyleneglycol methyl ether methacrylate mw 2080 50 wt% in water
- initiator Vazo 67 (2,2'-Azobis(2- methylbutyronitrile)
- yellow pyridine methacrylate dye of Example 16 (50 mg) and co-monomer of Example 22 100 mg
- a magnetic stirrer bar is added.
- the mixture is evacuated and purged with nitrogen three times using a low power ultrasonic bath on a stirrer hotplate.
- the stirrer bar is removed.
- the flask is put into an ice bath and the tip of a sonic probe (Branson 450) is inserted into the mixture.
- a nitrogen flow is maintained over the mixture.
- the mixture is sonicated for 3 minutes at 150 Watts, under an atmosphere of nitrogen.
- a stable yellow emulsion is formed.
- the flask is transferred to a hot oil bath, pre-heated to 75 0 C, a magnetic stirrer bar is added the flask and the contents are stirred on a stirrer hot plate/oil bath over night.
- the mixture is allowed to cool to room temperature and is filtered through a 5 micron cloth.
- the latex is cleaned with water and acetone to remove any unreacted monomers using a centrifuge to separate particles and supernatant.
- Analysis using a Malvern Zetasizer shows a highly disperse latex with a particle size of 236 nm.
- the suspension is freeze dried to give a fine yellow powder (23).
- Example 24 Electrophoretic formulation containing PS incorporating Yellow Dye 0.02990 g of coloured PS of Example 17 is added to 0.00966 g of detergent lnfineum E (Infineum Corporation) in 0.95665 g of dodecane and vortex mixed. The resultant dispersion is then homogenised using an ultra-turrax T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath. The dispersion is then roller mixed overnight to yield a yellow electrophoretic ink.
- Example 25 Electrophoretic formulation containing PS incorporating Yellow Dye
- Example 18 0.01320 g of coloured PS of Example 18 is added to 0.00481 g of detergent Infineum E (Infineum Corporation) in 0.47689 g of dodecane and vortex mixed. The resultant dispersion is then homogenised using an ultra-turrax T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath. The dispersion is then roller mixed overnight to yield a yellow electrophoretic ink. Size (312nm), Electrophoretic Mobility (0.07511 ⁇ mcm/Vs), ZP (+81 mV).
- Example 26 Electrophoretic formulation containing PS incorporating Yellow Dye
- T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath.
- the dispersion is then roller mixed overnight to yield a yellow electrophoretic ink.
- Example 27 Electrophoretic formulation containing PS incorporating
- T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath.
- the dispersion is then roller mixed overnight to yield a yellow electrophoretic ink.
- Example 28 Electrophoretic formulation containing PS incorporating
- T25 homogeniser for 15 minutes and sonicated for a further 30 minutes in an Ultrawave ultrasonic bath.
- the dispersion is then roller mixed overnight to yield a yellow electrophoretic ink.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Structural Engineering (AREA)
- Textile Engineering (AREA)
- Nonlinear Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Molecular Biology (AREA)
- Electrochemistry (AREA)
- Toxicology (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Electrochromic Elements, Electrophoresis, Or Variable Reflection Or Absorption Elements (AREA)
- Processes Of Treating Macromolecular Substances (AREA)
- Polymerisation Methods In General (AREA)
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2010800068964A CN102307914A (en) | 2009-02-09 | 2010-01-29 | Particles for electrophoretic displays |
| JP2011548584A JP2012517487A (en) | 2009-02-09 | 2010-01-29 | Particles for electrophoretic displays |
| EP10703005.8A EP2393851B1 (en) | 2009-02-09 | 2010-01-29 | Coloured particles for electrophoretic displays |
| US13/148,381 US8593719B2 (en) | 2009-02-09 | 2010-01-29 | Particles for electrophoretic displays |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09001776 | 2009-02-09 | ||
| EP09001776.5 | 2009-02-09 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2010089060A2 true WO2010089060A2 (en) | 2010-08-12 |
| WO2010089060A3 WO2010089060A3 (en) | 2010-10-07 |
Family
ID=42174195
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2010/000552 WO2010089060A2 (en) | 2009-02-09 | 2010-01-29 | Particles for electrophoretic displays |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US8593719B2 (en) |
| EP (1) | EP2393851B1 (en) |
| JP (1) | JP2012517487A (en) |
| KR (1) | KR20110127196A (en) |
| CN (1) | CN102307914A (en) |
| TW (1) | TW201037017A (en) |
| WO (1) | WO2010089060A2 (en) |
Cited By (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011154104A1 (en) | 2010-06-07 | 2011-12-15 | Merck Patent Gmbh | White reflective polymer particles |
| WO2011154103A1 (en) | 2010-06-07 | 2011-12-15 | Merck Patent Gmbh | Coloured polymer particles |
| WO2012058256A1 (en) * | 2010-10-29 | 2012-05-03 | Rohm And Haas Company | A thermoplastic composition, method of producing the same, and articles made therefrom |
| WO2012072218A1 (en) | 2010-11-30 | 2012-06-07 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2012088345A1 (en) * | 2010-12-22 | 2012-06-28 | Rohm And Haas Company | A thermoplastic composition, method of procucing the same, and articles made therefrom |
| CN102618062A (en) * | 2011-02-01 | 2012-08-01 | 上海安诺其纺织化工股份有限公司 | Polymerizable yellow azo dye |
| EP2513718A1 (en) * | 2009-12-18 | 2012-10-24 | Sun Chemical Corporation | Colored fluids for electrowetting, electrofluidic, and electrophoretic technologies |
| WO2012152409A1 (en) | 2011-05-09 | 2012-11-15 | Merck Patent Gmbh | Reactive mesogen based polymer particles |
| WO2012152392A1 (en) | 2011-05-09 | 2012-11-15 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013079158A1 (en) | 2011-11-30 | 2013-06-06 | Merck Patent Gmbh | Electrophoretic fluids |
| WO2013079146A1 (en) | 2011-11-30 | 2013-06-06 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US20130208344A1 (en) * | 2010-08-07 | 2013-08-15 | Merck Patent Gmbh | Particles for electrophoretic displays |
| CN103319909A (en) * | 2013-06-19 | 2013-09-25 | 大连福思达专用化学有限公司 | Dyestuff with polymerizable group and preparation of microspheres containing dyestuff |
| WO2013139427A1 (en) * | 2012-03-23 | 2013-09-26 | Merck Patent Gmbh | Particles for electrowetting displays |
| WO2013149714A1 (en) | 2012-04-04 | 2013-10-10 | Merck Patent Gmbh | Particles for electrophoretic displays comprising a core and a random - copolymer coating |
| WO2013170936A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013170938A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013170932A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013170937A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013189580A1 (en) | 2012-06-22 | 2013-12-27 | Merck Patent Gmbh | Electrophoretic fluid |
| WO2014019650A1 (en) | 2012-08-01 | 2014-02-06 | Merck Patent Gmbh | Electrophoretic fluids |
| WO2014019651A1 (en) | 2012-08-01 | 2014-02-06 | Merck Patent Gmbh | Electrophoretic fluids |
| WO2014198373A1 (en) | 2013-06-12 | 2014-12-18 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2015120950A1 (en) | 2014-02-13 | 2015-08-20 | Merck Patent Gmbh | Reactive mesogen based polymer particles |
| KR101854692B1 (en) * | 2011-09-01 | 2018-05-04 | 엘지디스플레이 주식회사 | Color electrophoretic display using the same and method for manufacturing electrophoretic display |
| US9977309B2 (en) | 2013-12-19 | 2018-05-22 | Merck Patent Gmbh | Electrophoretic fluid |
| US10007166B2 (en) | 2013-08-30 | 2018-06-26 | Hewlett-Packard Development Company, L.P. | Electronic inks |
| US10233308B2 (en) | 2013-08-30 | 2019-03-19 | Sun Chemical Corporation | Colored fluids for display devices |
| US10308744B2 (en) | 2013-06-12 | 2019-06-04 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US10435566B2 (en) | 2014-12-19 | 2019-10-08 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2021037951A1 (en) | 2019-08-29 | 2021-03-04 | Merck Patent Gmbh | Electrophoretic fluid |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103205139B (en) * | 2012-01-16 | 2014-10-22 | 清华大学 | Polymerizable dye monomers, color polymer emulsion and preparation methods thereof |
| JP5981729B2 (en) | 2012-02-27 | 2016-08-31 | イー インク コーポレイション | Electrophoretic particles, electrophoretic particle dispersion, display medium, and display device |
| JP6114574B2 (en) * | 2013-02-28 | 2017-04-12 | イー インク コーポレイション | Electrophoretic particles, electrophoretic particle dispersion, display medium, and display device |
| WO2014166583A1 (en) * | 2013-04-12 | 2014-10-16 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US9834683B2 (en) | 2013-08-29 | 2017-12-05 | Canon Kabushiki Kaisha | Compound having azo skeleton structure, pigment dispersant, pigment composition, pigment dispersion, and toner |
| CN104031200B (en) | 2014-06-03 | 2016-06-15 | 京东方科技集团股份有限公司 | A kind of high molecular dye compound and photosensitive polymer combination thereof and application |
| WO2018110709A1 (en) * | 2016-12-15 | 2018-06-21 | 積水化学工業株式会社 | Colored latex particles and immunoassay reagent using same |
| CA3145983A1 (en) * | 2019-07-12 | 2021-01-21 | Gc Corporation | Antibacterial polymer particles, composition, and article |
Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4613559A (en) | 1985-04-01 | 1986-09-23 | Xerox Corporation | Process for colored toner compositions with controlled charges thereon |
| US5380362A (en) | 1993-07-16 | 1995-01-10 | Copytele, Inc. | Suspension for use in electrophoretic image display systems |
| US5403518A (en) | 1993-12-02 | 1995-04-04 | Copytele, Inc. | Formulations for improved electrophoretic display suspensions and related methods |
| US5607864A (en) | 1992-04-07 | 1997-03-04 | Societe Prolabo | Fluorescent latices having very low detection thresholds for fluorescent emission |
| US5716855A (en) | 1993-07-12 | 1998-02-10 | Societe Prolabo | Fluorescent latex containing at least two fluorochromes, process for producing it and application thereof |
| US5783614A (en) | 1997-02-21 | 1998-07-21 | Copytele, Inc. | Polymeric-coated dielectric particles and formulation and method for preparing same |
| WO1999010767A1 (en) | 1997-08-28 | 1999-03-04 | E-Ink Corporation | Electrophoretic displays and materials |
| US6194488B1 (en) | 1997-08-22 | 2001-02-27 | Copytele, Inc. | Method for making polymer-coated pigment particles using initiator-treated pigments |
| US6822782B2 (en) | 2001-05-15 | 2004-11-23 | E Ink Corporation | Electrophoretic particles and processes for the production thereof |
| EP1491941A2 (en) | 2003-06-24 | 2004-12-29 | Seiko Epson Corporation | Electrophoretic dispersion, electrophoretic display device, method of manufacturing electrophoretic display device, and electronic system |
| WO2005017046A2 (en) | 2003-08-08 | 2005-02-24 | Sipix Imaging, Inc. | Fluorinated dyes or colorants and their uses |
| US6956690B2 (en) | 2003-02-06 | 2005-10-18 | Sipix Imaging, Inc. | Electrophoretic display with a bi-modal particle system |
| US20050267263A1 (en) | 2004-05-31 | 2005-12-01 | Canon Kabushiki Kaisha | Electrophoretic particles and production process thereof |
| US7038655B2 (en) | 1999-05-03 | 2006-05-02 | E Ink Corporation | Electrophoretic ink composed of particles with field dependent mobilities |
| US7052766B2 (en) | 2002-01-03 | 2006-05-30 | Sipix Imaging, Inc. | Functionalized halogenated polymers for microencapsulation |
| US7110162B2 (en) | 2002-01-03 | 2006-09-19 | Sipix Imaging, Inc. | Electrophoretic dispersion with a fluorinated solvent and a charge controlling agent |
| WO2006126120A1 (en) | 2005-05-27 | 2006-11-30 | Koninklijke Philips Electronics N.V. | Robust multi particle system for color electrophoretic displays with very low driving voltages comprising a low amount of electrolytes |
| US7170670B2 (en) | 2001-04-02 | 2007-01-30 | E Ink Corporation | Electrophoretic medium and display with improved image stability |
| WO2007048721A1 (en) | 2005-10-26 | 2007-05-03 | Ciba Holding Inc. | Coloured particles for electrophoretic displays |
| US7226550B2 (en) | 2002-10-10 | 2007-06-05 | Sipix Imaging, Inc. | Electrophoretic dispersions |
| US7236290B1 (en) | 2000-07-25 | 2007-06-26 | E Ink Corporation | Electrophoretic medium with improved stability |
| US7247379B2 (en) | 1997-08-28 | 2007-07-24 | E Ink Corporation | Electrophoretic particles, and processes for the production thereof |
| US7277218B2 (en) | 2003-11-04 | 2007-10-02 | Sipix Imaging, Inc. | Electrophoretic compositions |
| US20070268244A1 (en) | 2006-05-19 | 2007-11-22 | Xerox Corporation | Electrophoretic display and method of displaying images |
| GB2438436A (en) | 2006-05-24 | 2007-11-28 | Lg Philips Lcd Co Ltd | Electronic ink panel having three types of electrophoretic particles each responsive to a different voltage level |
| US7304634B2 (en) | 1995-07-20 | 2007-12-04 | E Ink Corporation | Rear electrode structures for electrophoretic displays |
| US20070297038A1 (en) | 2006-06-23 | 2007-12-27 | Xerox Corporation | Electrophoretic display medium containing solvent resistant emulsion aggregation particles |
| WO2008003604A2 (en) | 2006-07-05 | 2008-01-10 | Ciba Holding Inc. | Coloured organic electrophoretic particles |
| WO2008003619A2 (en) | 2006-07-06 | 2008-01-10 | Ciba Holding Inc. | Encapsulated dispersions comprising electrophoretically mobile organic colorants |
| US20080013156A1 (en) | 2006-07-13 | 2008-01-17 | E Ink Corporation | Particles for use in electrophoretic displays |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2668158B1 (en) | 1990-10-22 | 1994-05-06 | Thomson Csf | CROSSLINKABLE POLYMER FOR NONLINEAR OPTICAL APPLICATIONS. |
| EP0600064A1 (en) * | 1992-06-19 | 1994-06-08 | F. Hoffmann-La Roche Ag | Optical non-linear polymers |
| US5521271A (en) * | 1994-09-29 | 1996-05-28 | Minnesota Mining And Manufacturing Company | Liquid toners with hydrocarbon solvents |
| JP3736221B2 (en) * | 1998-08-28 | 2006-01-18 | 凸版印刷株式会社 | Color filter and liquid crystal display device having the same |
| US6372838B1 (en) * | 2000-06-28 | 2002-04-16 | 3M Innovative Properties Company | Fine latex and seed method of making |
| US6384124B1 (en) * | 2000-06-28 | 2002-05-07 | 3M Innovative Properties Company | Non-film-forming electrophoretic latexes in fluorocarbon solvents |
| US7123319B2 (en) * | 2000-12-14 | 2006-10-17 | Koninklijke Philips Electronics N.V. | Liquid crystal display laminate and method of manufacturing such comprising a stratified-phase-separated composite |
| US7100162B2 (en) | 2002-06-20 | 2006-08-29 | Hewlett-Packard Development Company, L.P. | System and method for process management |
| JP4708736B2 (en) * | 2004-05-31 | 2011-06-22 | キヤノン株式会社 | Electrophoretic particles and method for producing the same |
| US7868059B2 (en) * | 2007-07-27 | 2011-01-11 | Hewlett-Packard Development Company, L.P. | Polymerizable dye-monomer conjugates for encapsulating pigment particles |
-
2010
- 2010-01-29 EP EP10703005.8A patent/EP2393851B1/en active Active
- 2010-01-29 CN CN2010800068964A patent/CN102307914A/en active Pending
- 2010-01-29 WO PCT/EP2010/000552 patent/WO2010089060A2/en active Application Filing
- 2010-01-29 KR KR1020117021070A patent/KR20110127196A/en not_active Application Discontinuation
- 2010-01-29 JP JP2011548584A patent/JP2012517487A/en active Pending
- 2010-01-29 US US13/148,381 patent/US8593719B2/en active Active
- 2010-02-09 TW TW099104007A patent/TW201037017A/en unknown
Patent Citations (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4613559A (en) | 1985-04-01 | 1986-09-23 | Xerox Corporation | Process for colored toner compositions with controlled charges thereon |
| US5607864A (en) | 1992-04-07 | 1997-03-04 | Societe Prolabo | Fluorescent latices having very low detection thresholds for fluorescent emission |
| US5716855A (en) | 1993-07-12 | 1998-02-10 | Societe Prolabo | Fluorescent latex containing at least two fluorochromes, process for producing it and application thereof |
| US5380362A (en) | 1993-07-16 | 1995-01-10 | Copytele, Inc. | Suspension for use in electrophoretic image display systems |
| US5403518A (en) | 1993-12-02 | 1995-04-04 | Copytele, Inc. | Formulations for improved electrophoretic display suspensions and related methods |
| US7304634B2 (en) | 1995-07-20 | 2007-12-04 | E Ink Corporation | Rear electrode structures for electrophoretic displays |
| US5783614A (en) | 1997-02-21 | 1998-07-21 | Copytele, Inc. | Polymeric-coated dielectric particles and formulation and method for preparing same |
| US6194488B1 (en) | 1997-08-22 | 2001-02-27 | Copytele, Inc. | Method for making polymer-coated pigment particles using initiator-treated pigments |
| WO1999010767A1 (en) | 1997-08-28 | 1999-03-04 | E-Ink Corporation | Electrophoretic displays and materials |
| US7247379B2 (en) | 1997-08-28 | 2007-07-24 | E Ink Corporation | Electrophoretic particles, and processes for the production thereof |
| US7038655B2 (en) | 1999-05-03 | 2006-05-02 | E Ink Corporation | Electrophoretic ink composed of particles with field dependent mobilities |
| US7236290B1 (en) | 2000-07-25 | 2007-06-26 | E Ink Corporation | Electrophoretic medium with improved stability |
| US7170670B2 (en) | 2001-04-02 | 2007-01-30 | E Ink Corporation | Electrophoretic medium and display with improved image stability |
| US6822782B2 (en) | 2001-05-15 | 2004-11-23 | E Ink Corporation | Electrophoretic particles and processes for the production thereof |
| US20070128352A1 (en) | 2001-05-15 | 2007-06-07 | E Ink Corporation | Electrophoretic particles and processes for the production thereof |
| US7052766B2 (en) | 2002-01-03 | 2006-05-30 | Sipix Imaging, Inc. | Functionalized halogenated polymers for microencapsulation |
| US7110162B2 (en) | 2002-01-03 | 2006-09-19 | Sipix Imaging, Inc. | Electrophoretic dispersion with a fluorinated solvent and a charge controlling agent |
| US7226550B2 (en) | 2002-10-10 | 2007-06-05 | Sipix Imaging, Inc. | Electrophoretic dispersions |
| US6956690B2 (en) | 2003-02-06 | 2005-10-18 | Sipix Imaging, Inc. | Electrophoretic display with a bi-modal particle system |
| EP1491941A2 (en) | 2003-06-24 | 2004-12-29 | Seiko Epson Corporation | Electrophoretic dispersion, electrophoretic display device, method of manufacturing electrophoretic display device, and electronic system |
| WO2005017046A2 (en) | 2003-08-08 | 2005-02-24 | Sipix Imaging, Inc. | Fluorinated dyes or colorants and their uses |
| US7277218B2 (en) | 2003-11-04 | 2007-10-02 | Sipix Imaging, Inc. | Electrophoretic compositions |
| US20050267263A1 (en) | 2004-05-31 | 2005-12-01 | Canon Kabushiki Kaisha | Electrophoretic particles and production process thereof |
| WO2006126120A1 (en) | 2005-05-27 | 2006-11-30 | Koninklijke Philips Electronics N.V. | Robust multi particle system for color electrophoretic displays with very low driving voltages comprising a low amount of electrolytes |
| WO2007048721A1 (en) | 2005-10-26 | 2007-05-03 | Ciba Holding Inc. | Coloured particles for electrophoretic displays |
| US20070268244A1 (en) | 2006-05-19 | 2007-11-22 | Xerox Corporation | Electrophoretic display and method of displaying images |
| GB2438436A (en) | 2006-05-24 | 2007-11-28 | Lg Philips Lcd Co Ltd | Electronic ink panel having three types of electrophoretic particles each responsive to a different voltage level |
| US20070297038A1 (en) | 2006-06-23 | 2007-12-27 | Xerox Corporation | Electrophoretic display medium containing solvent resistant emulsion aggregation particles |
| WO2008003604A2 (en) | 2006-07-05 | 2008-01-10 | Ciba Holding Inc. | Coloured organic electrophoretic particles |
| WO2008003619A2 (en) | 2006-07-06 | 2008-01-10 | Ciba Holding Inc. | Encapsulated dispersions comprising electrophoretically mobile organic colorants |
| US20080013156A1 (en) | 2006-07-13 | 2008-01-17 | E Ink Corporation | Particles for use in electrophoretic displays |
Non-Patent Citations (5)
| Title |
|---|
| "Colour Index", 1982, THE SOCIETY OF DYERS AND COLOURISTS |
| C. M. LAMPERT: "Displays", vol. 25, 2004, ELSEVIER B.V. |
| J. NANOSCI. NANOTECHN., vol. 6, no. 11, 2006, pages 3450 - 3454 |
| K. LANDFESTER, MACROMOL. RAPID. COMMUN., vol. 22, 2001, pages 896 - 936 |
| VAN HERK, ALEX; GILBERT, BOB: "Emulsion polymerization", EINDHOVEN UNIVERSITY OF TECHNOLOGY |
Cited By (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2513718A4 (en) * | 2009-12-18 | 2013-08-28 | Sun Chemical Corp | Colored fluids for electrowetting, electrofluidic, and electrophoretic technologies |
| EP2513718A1 (en) * | 2009-12-18 | 2012-10-24 | Sun Chemical Corporation | Colored fluids for electrowetting, electrofluidic, and electrophoretic technologies |
| WO2011154103A1 (en) | 2010-06-07 | 2011-12-15 | Merck Patent Gmbh | Coloured polymer particles |
| US8901219B2 (en) | 2010-06-07 | 2014-12-02 | Merck Patent Gmbh | Coloured polymer particles |
| US8906998B2 (en) | 2010-06-07 | 2014-12-09 | Merck Patent Gmbh | White reflective polymer particles |
| WO2011154104A1 (en) | 2010-06-07 | 2011-12-15 | Merck Patent Gmbh | White reflective polymer particles |
| US9487611B2 (en) * | 2010-08-07 | 2016-11-08 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US20130208344A1 (en) * | 2010-08-07 | 2013-08-15 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US9493647B2 (en) | 2010-10-29 | 2016-11-15 | Rohm And Haas Company | Thermoplastic composition, method of producing the same, and articles made therefrom |
| CN103282430B (en) * | 2010-10-29 | 2016-07-13 | 罗姆及哈斯公司 | Thermoplastic compounds, its preparation method and goods prepared therefrom |
| CN103282430A (en) * | 2010-10-29 | 2013-09-04 | 罗姆及哈斯公司 | A thermoplastic composition, method of producing the same, and articles made therefrom |
| WO2012058256A1 (en) * | 2010-10-29 | 2012-05-03 | Rohm And Haas Company | A thermoplastic composition, method of producing the same, and articles made therefrom |
| US9182615B2 (en) | 2010-11-30 | 2015-11-10 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2012072218A1 (en) | 2010-11-30 | 2012-06-07 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2012088345A1 (en) * | 2010-12-22 | 2012-06-28 | Rohm And Haas Company | A thermoplastic composition, method of procucing the same, and articles made therefrom |
| US9376562B2 (en) | 2010-12-22 | 2016-06-28 | Rohm And Haas Company | Thermoplastic composition, method of producing the same, and articles made therefrom |
| CN102618062B (en) * | 2011-02-01 | 2014-09-03 | 上海安诺其纺织化工股份有限公司 | Polymerizable yellow azo dye |
| CN102618062A (en) * | 2011-02-01 | 2012-08-01 | 上海安诺其纺织化工股份有限公司 | Polymerizable yellow azo dye |
| US9598587B2 (en) | 2011-05-09 | 2017-03-21 | Merck Patent Gmbh | Reactive mesogen based polymer particles |
| US20140084216A1 (en) * | 2011-05-09 | 2014-03-27 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US9109160B2 (en) | 2011-05-09 | 2015-08-18 | Merck Patent Gmbh | Reactive mesogen based polymer particles |
| WO2012152409A1 (en) | 2011-05-09 | 2012-11-15 | Merck Patent Gmbh | Reactive mesogen based polymer particles |
| CN103547655A (en) * | 2011-05-09 | 2014-01-29 | 默克专利有限公司 | Reactive mesogen based polymer particles |
| WO2012152392A1 (en) | 2011-05-09 | 2012-11-15 | Merck Patent Gmbh | Particles for electrophoretic displays |
| JP2014519534A (en) * | 2011-05-09 | 2014-08-14 | メルク パテント ゲーエムベーハー | Polymer particles based on reactive mesogens |
| CN103547655B (en) * | 2011-05-09 | 2015-06-03 | 默克专利有限公司 | Reactive mesogen based polymer particles |
| KR101854692B1 (en) * | 2011-09-01 | 2018-05-04 | 엘지디스플레이 주식회사 | Color electrophoretic display using the same and method for manufacturing electrophoretic display |
| WO2013079158A1 (en) | 2011-11-30 | 2013-06-06 | Merck Patent Gmbh | Electrophoretic fluids |
| WO2013079146A1 (en) | 2011-11-30 | 2013-06-06 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US9383621B2 (en) | 2011-11-30 | 2016-07-05 | Merck Patent Gmbh | Electrophoretic fluids |
| US9152006B2 (en) | 2011-11-30 | 2015-10-06 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013139427A1 (en) * | 2012-03-23 | 2013-09-26 | Merck Patent Gmbh | Particles for electrowetting displays |
| WO2013149714A1 (en) | 2012-04-04 | 2013-10-10 | Merck Patent Gmbh | Particles for electrophoretic displays comprising a core and a random - copolymer coating |
| US20150092262A1 (en) * | 2012-04-04 | 2015-04-02 | Merck Patent Gmbh | Particles for electrophoretic displays comprising a core and a random-copolymer coating |
| US10126625B2 (en) | 2012-04-04 | 2018-11-13 | Merck Patent Gmbh | Particles for electrophoretic displays comprising a core and a random-copolymer coating |
| US9651846B2 (en) | 2012-05-14 | 2017-05-16 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US9645416B2 (en) | 2012-05-14 | 2017-05-09 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013170936A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013170938A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013170937A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2013170932A1 (en) | 2012-05-14 | 2013-11-21 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US9588357B2 (en) | 2012-05-14 | 2017-03-07 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US10048562B2 (en) | 2012-06-22 | 2018-08-14 | Merck Patent Gmbh | Electrophoretic fluid |
| WO2013189580A1 (en) | 2012-06-22 | 2013-12-27 | Merck Patent Gmbh | Electrophoretic fluid |
| WO2014019650A1 (en) | 2012-08-01 | 2014-02-06 | Merck Patent Gmbh | Electrophoretic fluids |
| US10353265B2 (en) | 2012-08-01 | 2019-07-16 | Merck Patent Gmbh | Electrophoretic fluids |
| US10007165B2 (en) | 2012-08-01 | 2018-06-26 | Merck Patent Gmbh | Electrophoretic fluids |
| WO2014019651A1 (en) | 2012-08-01 | 2014-02-06 | Merck Patent Gmbh | Electrophoretic fluids |
| US10106686B2 (en) | 2013-06-12 | 2018-10-23 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2014198373A1 (en) | 2013-06-12 | 2014-12-18 | Merck Patent Gmbh | Particles for electrophoretic displays |
| US10308744B2 (en) | 2013-06-12 | 2019-06-04 | Merck Patent Gmbh | Particles for electrophoretic displays |
| CN103319909A (en) * | 2013-06-19 | 2013-09-25 | 大连福思达专用化学有限公司 | Dyestuff with polymerizable group and preparation of microspheres containing dyestuff |
| US10233308B2 (en) | 2013-08-30 | 2019-03-19 | Sun Chemical Corporation | Colored fluids for display devices |
| US10007166B2 (en) | 2013-08-30 | 2018-06-26 | Hewlett-Packard Development Company, L.P. | Electronic inks |
| US9977309B2 (en) | 2013-12-19 | 2018-05-22 | Merck Patent Gmbh | Electrophoretic fluid |
| WO2015120950A1 (en) | 2014-02-13 | 2015-08-20 | Merck Patent Gmbh | Reactive mesogen based polymer particles |
| US9796926B2 (en) | 2014-02-13 | 2017-10-24 | Merck Patent Gmbh | Reactive mesogen based polymer particles |
| US10435566B2 (en) | 2014-12-19 | 2019-10-08 | Merck Patent Gmbh | Particles for electrophoretic displays |
| WO2021037951A1 (en) | 2019-08-29 | 2021-03-04 | Merck Patent Gmbh | Electrophoretic fluid |
Also Published As
| Publication number | Publication date |
|---|---|
| US8593719B2 (en) | 2013-11-26 |
| EP2393851B1 (en) | 2018-02-28 |
| JP2012517487A (en) | 2012-08-02 |
| EP2393851A2 (en) | 2011-12-14 |
| US20110306742A1 (en) | 2011-12-15 |
| KR20110127196A (en) | 2011-11-24 |
| CN102307914A (en) | 2012-01-04 |
| WO2010089060A3 (en) | 2010-10-07 |
| TW201037017A (en) | 2010-10-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2393851B1 (en) | Coloured particles for electrophoretic displays | |
| US8743451B2 (en) | Coloured particles for electrophoretic displays | |
| EP2393849B1 (en) | Coloured particles for electrophoretic displays | |
| US8462423B2 (en) | Coloured particles for electrophoretic displays | |
| KR101982356B1 (en) | Particles for electrophoretic displays | |
| EP2850489B1 (en) | Particles for electrophoretic displays | |
| EP3233944B1 (en) | Particles for electrophoretic displays | |
| WO2012152392A1 (en) | Particles for electrophoretic displays | |
| WO2013170934A1 (en) | Particles for electrophoretic displays | |
| US9908963B2 (en) | Particles for electrophoretic displays |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201080006896.4 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10703005 Country of ref document: EP Kind code of ref document: A2 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010703005 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011548584 Country of ref document: JP Ref document number: 13148381 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20117021070 Country of ref document: KR Kind code of ref document: A |