WO2008090382A1 - Thiazole and oxazole derivatives for use in the treatment of prion diseases, cancer and conditions of the central nervous system as well as in the regulation of stem cells - Google Patents
Thiazole and oxazole derivatives for use in the treatment of prion diseases, cancer and conditions of the central nervous system as well as in the regulation of stem cells Download PDFInfo
- Publication number
- WO2008090382A1 WO2008090382A1 PCT/GB2008/050052 GB2008050052W WO2008090382A1 WO 2008090382 A1 WO2008090382 A1 WO 2008090382A1 GB 2008050052 W GB2008050052 W GB 2008050052W WO 2008090382 A1 WO2008090382 A1 WO 2008090382A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- optionally substituted
- compound according
- compound
- heterocyclyl
- halogen
- Prior art date
Links
- 0 **C1=C(*)N=C(*)*1 Chemical compound **C1=C(*)N=C(*)*1 0.000 description 2
- APNPQSIBFPYWKZ-UHFFFAOYSA-N CCN(CC)C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O Chemical compound CCN(CC)C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O APNPQSIBFPYWKZ-UHFFFAOYSA-N 0.000 description 1
- YSUHUYNMWRCKAI-UHFFFAOYSA-N CCN(CC)C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O Chemical compound CCN(CC)C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O YSUHUYNMWRCKAI-UHFFFAOYSA-N 0.000 description 1
- NQDVFZAZTZXSQX-UHFFFAOYSA-N CCN(CC)CCNC(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O Chemical compound CCN(CC)CCNC(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O NQDVFZAZTZXSQX-UHFFFAOYSA-N 0.000 description 1
- WDQKBUQPNOSCQU-UHFFFAOYSA-N CCN(CC)CCNC(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O Chemical compound CCN(CC)CCNC(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O WDQKBUQPNOSCQU-UHFFFAOYSA-N 0.000 description 1
- FMBSRXUMJKNNIY-UHFFFAOYSA-N COc(cc1)ccc1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O Chemical compound COc(cc1)ccc1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O FMBSRXUMJKNNIY-UHFFFAOYSA-N 0.000 description 1
- QJDLJIKAGUWYRA-UHFFFAOYSA-N COc(cc1)ccc1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O Chemical compound COc(cc1)ccc1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O QJDLJIKAGUWYRA-UHFFFAOYSA-N 0.000 description 1
- DWWAHZCLBQJWLP-UHFFFAOYSA-N COc(cccc1)c1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O Chemical compound COc(cccc1)c1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)=O DWWAHZCLBQJWLP-UHFFFAOYSA-N 0.000 description 1
- BZHNJODTAZKYPL-UHFFFAOYSA-N COc(cccc1)c1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O Chemical compound COc(cccc1)c1NC(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)=O BZHNJODTAZKYPL-UHFFFAOYSA-N 0.000 description 1
- YDTLDNNTCBXPPL-UHFFFAOYSA-N COc1cc(NC(c2c(-c3ccccc3)nc(-c3ccccc3)[o]2)=O)ccc1 Chemical compound COc1cc(NC(c2c(-c3ccccc3)nc(-c3ccccc3)[o]2)=O)ccc1 YDTLDNNTCBXPPL-UHFFFAOYSA-N 0.000 description 1
- GUGYTSWWIHHRJE-UHFFFAOYSA-N COc1cc(NC(c2c(-c3ccccc3)nc(-c3ccccc3)[s]2)=O)ccc1 Chemical compound COc1cc(NC(c2c(-c3ccccc3)nc(-c3ccccc3)[s]2)=O)ccc1 GUGYTSWWIHHRJE-UHFFFAOYSA-N 0.000 description 1
- VGCWYVIOOHYVDZ-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NCCN1CCOCC1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NCCN1CCOCC1 VGCWYVIOOHYVDZ-UHFFFAOYSA-N 0.000 description 1
- OLBZUIIGDJAKKL-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NCc1ccc[o]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NCc1ccc[o]1 OLBZUIIGDJAKKL-UHFFFAOYSA-N 0.000 description 1
- PUJYVAKOTNLCHB-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NCc1ccc[s]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NCc1ccc[s]1 PUJYVAKOTNLCHB-UHFFFAOYSA-N 0.000 description 1
- DQSPLSMHGCISPP-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NN1CCOCC1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)NN1CCOCC1 DQSPLSMHGCISPP-UHFFFAOYSA-N 0.000 description 1
- FPOWICIOIQVLNC-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1ccc[o]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1ccc[o]1 FPOWICIOIQVLNC-UHFFFAOYSA-N 0.000 description 1
- ZSDYEOGCBOMACU-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1ccc[s]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1ccc[s]1 ZSDYEOGCBOMACU-UHFFFAOYSA-N 0.000 description 1
- CWQSMVQLAOKYIS-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1ccccc1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1ccccc1 CWQSMVQLAOKYIS-UHFFFAOYSA-N 0.000 description 1
- ZGJVUMFDPXDYKY-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1cnc(cccc2)c2c1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1cnc(cccc2)c2c1 ZGJVUMFDPXDYKY-UHFFFAOYSA-N 0.000 description 1
- NTTPGUABTBUVCG-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1nc(cccc2)c2[s]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[o]1)Nc1nc(cccc2)c2[s]1 NTTPGUABTBUVCG-UHFFFAOYSA-N 0.000 description 1
- UFPUWGWMZVIBCH-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NCCN1CCOCC1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NCCN1CCOCC1 UFPUWGWMZVIBCH-UHFFFAOYSA-N 0.000 description 1
- FOCPGFSKRPTPTA-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NCc1ccc[o]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NCc1ccc[o]1 FOCPGFSKRPTPTA-UHFFFAOYSA-N 0.000 description 1
- HWRSYHKGJYJMIV-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NCc1ccc[s]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NCc1ccc[s]1 HWRSYHKGJYJMIV-UHFFFAOYSA-N 0.000 description 1
- PPDTZXYXLKMIDD-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NN1CCOCC1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)NN1CCOCC1 PPDTZXYXLKMIDD-UHFFFAOYSA-N 0.000 description 1
- CVHQRMNIUPWSAF-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1ccc[o]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1ccc[o]1 CVHQRMNIUPWSAF-UHFFFAOYSA-N 0.000 description 1
- USIZRKFHUVKBPY-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1ccc[s]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1ccc[s]1 USIZRKFHUVKBPY-UHFFFAOYSA-N 0.000 description 1
- KYLREEUTHHLYCP-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1ccccc1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1ccccc1 KYLREEUTHHLYCP-UHFFFAOYSA-N 0.000 description 1
- RWKKAZHGAWAQCN-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1cnc(cccc2)c2c1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1cnc(cccc2)c2c1 RWKKAZHGAWAQCN-UHFFFAOYSA-N 0.000 description 1
- XDAXVMMBNDIUQH-UHFFFAOYSA-N O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1nc2ccccc2[s]1 Chemical compound O=C(c1c(-c2ccccc2)nc(-c2ccccc2)[s]1)Nc1nc2ccccc2[s]1 XDAXVMMBNDIUQH-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D263/00—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings
- C07D263/02—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings
- C07D263/30—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D263/34—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D263/48—Nitrogen atoms not forming part of a nitro radical
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/02—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings
- C07D277/20—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D277/32—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D277/38—Nitrogen atoms
- C07D277/40—Unsubstituted amino or imino radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/02—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings
- C07D277/20—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D277/32—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D277/38—Nitrogen atoms
- C07D277/44—Acylated amino or imino radicals
- C07D277/46—Acylated amino or imino radicals by carboxylic acids, or sulfur or nitrogen analogues thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/10—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
Definitions
- This invention relates to compounds and their use in therapy, especially in the treatment of prion diseases and the regulation of stem cells.
- Prion diseases or transmissible spongiform encephalopathies (TSEs) are invariably fatal neurodegenerative disorders affecting humans and animals. As yet, no effective curative or prophylactic therapy exists.
- Prominent examples of prion diseases include bovine spongiform encephalopathy (BSE, cattle), scrapie (sheep), chronic wasting disorder (CWD, deer and elk) and transmissible mink encephalopathy (TME). Since a new variant of the human TSE Creutzfeldt-Jacob disease (vCJD) was discovered, thought to have been triggered by the consumption of contaminated beef products, prion diseases have been the focus of much research effort.
- BSE bovine spongiform encephalopathy
- CWD chronic wasting disorder
- TBE transmissible mink encephalopathy
- TSEs are associated with a post-translational conversion of the cell-surface glycosylphosphatidylinositol (GPI)-anchored protein PrP c (or PrP sen ) to a partially protease resistant isoform denoted PrP Sc (or PrP res ).
- GPI glycosylphosphatidylinositol
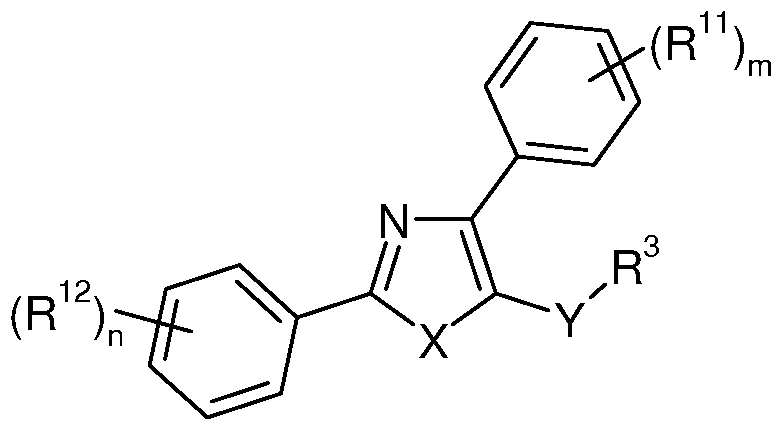
- R is selected from phenyl, 4-methoxyphenyl, thiophen-2-yl, cyclohexyl and isopropyl.
- X is oxygen or sulphur
- Y is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O) r , -N(R 5 )- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- R 1 and R 2 are selected from carbocyclyl and heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and the other is -Z-R 4 ;
- R 3 is selected from hydrogen; R 7 ; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- Z is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O) r , -N(R 5 )- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- R 4 is selected from hydrogen; R 7 ; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- R 5 is selected from R 6 , -OR 6 , -C(O)R 6 , -C(O)OR 6 and -S(O) 1 R 6 ;
- R 6 is selected from hydrogen; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or
- R 8 and R 9 are each independently hydrogen or R 10 ;
- R 10 is selected from hydrocarbyl and -(CH 2 ) k -heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy, C 1-6 alkyl and C 1-6 alkoxy;
- k is O, 1 , 2, 3, 4, 5 or 6;
- the invention also provides a pharmaceutical formulation comprising a compound of formula (I) and a pharmaceutically acceptable carrier or excipient.
- the invention relates to the use of a compound of formula (I), for the manufacture of a medicament for the treatment, prevention or delay of progression of a prion disease.
- a method of treating, preventing or delaying progression of a prion disease is also provided, which involves administering a therapeutically effective amount of a compound of the invention to a subject.
- Compounds of the invention may also be useful in the regulation of stem cells. Accordingly, in another aspect there is provided a method of regulating stem cell activity, which comprises contacting one or more stem cells with a compound of the invention.
- the compounds may also be useful in the treatment, prevention or delay of progression of cancer and diseases or conditions of the central nervous system, or in regenerative medicine.
- hydrocarbyl as used herein includes reference to moieties consisting exclusively of hydrogen and carbon atoms; such a moiety may comprise an aliphatic and/or an aromatic moiety. The moiety may comprise 1 , 2, 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15, 16, 17, 18, 19 or 20 carbon atoms.
- hydrocarbyl groups include C 1-6 alkyl (e.g. C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl); C 1-6 alkyl substituted by aryl (e.g.
- benzyl or by cycloalkyl (e.g cyclopropylmethyl); cycloalkyl (e.g. cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl); alkenyl (e.g. 2-butenyl); alkynyl (e.g. 2-butynyl); aryl (e.g. phenyl, naphthyl or fluorenyl) and the like.
- cycloalkyl e.g cyclopropylmethyl
- cycloalkyl e.g. cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl
- alkenyl e.g. 2-butenyl
- alkynyl e.g. 2-butynyl
- aryl e.g. phenyl, naphthyl or fluorenyl
- alkyl and C 1 ⁇ alkyl as used herein include reference to a straight or branched chain alkyl moiety having 1 , 2, 3, 4, 5 or 6 carbon atoms. This term includes reference to groups such as methyl, ethyl, propyl (n-propyl or isopropyl), butyl (n-butyl, sec-butyl or tert-butyl), pentyl, hexyl and the like. In particular, alkyl may have 1 , 2, 3 or 4 carbon atoms.
- alkenyl and C 2-6 alkenyl as used herein include reference to a straight or branched chain alkyl moiety having 2, 3, 4, 5 or 6 carbon atoms and having, in addition, at least one double bond, of either E or Z stereochemistry where applicable. This term includes reference to groups such as ethenyl, 2-propenyl, 1 -butenyl, 2-butenyl, 3- butenyl, 1 -pentenyl, 2-pentenyl, 3-pentenyl, 1 -hexenyl, 2-hexenyl and 3-hexenyl and the like.
- alkynyl and C 2-6 alkynyl as used herein include reference to a straight or branched chain alkyl moiety having 2, 3, 4, 5 or 6 carbon atoms and having, in addition, at least one triple bond. This term includes reference to groups such as ethynyl, 1 - propynyl, 2-propynyl, 1 -butynyl, 2-butynyl, 3-butynyl, 1 -pentynyl, 2-pentynyl, 3-pentynyl, 1 -hexynyl, 2-hexynyl and 3-hexynyl and the like.
- alkoxy and C 1-6 alkoxy as used herein include reference to -O-alkyl, wherein alkyl is straight or branched chain and comprises 1 , 2, 3, 4, 5 or 6 carbon atoms. In one class of embodiments, alkoxy has 1 , 2, 3 or 4 carbon atoms. This term includes reference to groups such as methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert- butoxy, pentoxy, hexoxy and the like.
- cycloalkyl as used herein includes reference to an alicyclic moiety having 3, 4, 5, 6, 7 or 8 carbon atoms.
- the group may be a bridged or polycyclic ring system. More often cycloalkyl groups are monocyclic. This term includes reference to groups such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, norbornyl, bicyclo[2.2.2]octyl and the like.
- cycloalkenyl as used herein includes reference to a non-aromatic cycloalkyl group having a double bond between one or more pairs of ring carbon atoms.
- the group may be a bridged or polycyclic ring system. More often cycloalkenyl groups are monocyclic. This term includes reference to groups such as cyclopentadienyl and the like.
- aryl as used herein includes reference to an aromatic ring system comprising 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 ring carbon atoms.
- Aryl is often phenyl but may be a polycyclic ring system, having two or more rings, at least one of which is aromatic. This term includes reference to groups such as phenyl, naphthyl, fluorenyl, azulenyl, indenyl, anthryl and the like.
- carbocyclyl as used herein includes reference to a saturated (e.g. cycloalkyl) or unsaturated (e.g. cycloalkenyl or aryl) ring moiety having 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 carbon ring atoms.
- carbocyclyl includes a 3- to 10- membered ring or ring system and, in particular, a 5- or 6-membered ring, which may be saturated or unsaturated.
- a carbocyclic moiety is, for example, selected from cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, norbomyl, bicyclo[2.2.2]octyl, phenyl, naphthyl, fluorenyl, azulenyl, indenyl, anthryl and the like.
- heterocyclyl as used herein includes reference to a saturated (e.g. heterocycloalkyl) or unsaturated (e.g. heteroaryl) heterocyclic ring moiety having from 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 ring atoms, at least one of which is selected from nitrogen, oxygen, phosphorus, silicon and sulphur.
- heterocyclyl includes a 3- to 10-membered ring or ring system and more particularly a 5- or 6- membered ring, which may be saturated or unsaturated.
- a heterocyclic moiety is, for example, selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofura- nyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrazinyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholin
- heterocycloalkyl as used herein includes reference to a saturated heterocyclic moiety having 3, 4, 5, 6 or 7 ring carbon atoms and 1 , 2, 3, 4 or 5 ring heteroatoms selected from nitrogen, oxygen, phosphorus and sulphur.
- the group may be a polycyclic ring system but more often is monocyclic.
- This term includes reference to groups such as azetidinyl, pyrrolidinyl, tetrahydrofuranyl, piperidinyl, oxiranyl, pyrazolidinyl, imidazolyl, indolizidinyl, piperazinyl, thiazolidinyl, morpholinyl, thiomorpholinyl, quinolizidinyl and the like.
- heteroaryl as used herein includes reference to an aromatic heterocyclic ring system having 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 ring atoms, at least one of which is selected from nitrogen, oxygen and sulphur.
- the group may be a polycyclic ring system, having two or more rings, at least one of which is aromatic, but is more often monocyclic.
- This term includes reference to groups such as pyrimidinyl, furanyl, benzo[b]thiophenyl, thiophenyl, pyrrolyl, imidazolyl, pyrrolidinyl, pyridinyl, benzo[b]furanyl, pyrazinyl, purinyl, indolyl, benzimidazolyl, quinolinyl, phenothiazinyl, triazinyl, phthalazinyl, 2H-chromenyl, oxazolyl, isoxazolyl, thiazolyl, isoindolyl, indazolyl, purinyl, isoquinolinyl, quinazolinyl, pteridinyl and the like.
- halogen as used herein includes reference to F, Cl, Br or I. In a particular, halogen may be F or Cl, of which F is more common. Substituted
- substituted as used herein in reference to a moiety means that one or more, especially up to 5, more especially 1 , 2 or 3, of the hydrogen atoms in said moiety are replaced independently of each other by the corresponding number of the described substituents.
- optionally substituted as used herein means substituted or unsubstituted.
- substituents are only at positions where they are chemically possible, the person skilled in the art being able to decide (either experimentally or theoretically) without inappropriate effort whether a particular substitution is possible.
- amino or hydroxy groups with free hydrogen may be unstable if bound to carbon atoms with unsaturated (e.g. olefinic) bonds.
- substituents described herein may themselves be substituted by any substituent, subject to the aforementioned restriction to appropriate substitutions as recognised by the skilled man.
- pharmaceutically acceptable includes reference to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings or animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio. This term includes acceptability for both human and veterinary purposes.
- X is oxygen or sulphur
- Y is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O) r , -N(R 5 )- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- R 1 and R 2 are selected from carbocyclyl and heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and the other is -Z-R 4 ;
- R 3 is selected from hydrogen; R 7 ; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- Z is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O) 1 -, -N(R 5 )- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- R 4 is selected from hydrogen; R 7 ; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- R 5 is selected from R 6 , -OR 6 , -C(O)R 6 , -C(O)OR 6 and -S(O) 1 R 6 ;
- R 6 is selected from hydrogen; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- R is selected from hydrocarbyl and -(CH 2 ) k -heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy, C 1-6 alkyl and C 1-6 alkoxy;
- k is O, 1 , 2, 3, 4, 5 or 6;
- I is O, 1 or 2;
- the compound is not 2,4-diphenyloxazol-5-ylamine or a compound of the following formulae:
- R is selected from phenyl, 4-methoxyphenyl, thiophen-2-yl, cyclohexyl and isopropyl.
- X is oxygen. In another class of compounds, X is sulphur.
- one of R 1 and R 2 is selected from carbocyclyl and heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and the other is -Z-R 4 , wherein Z is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O) r , -N(R 5 )- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and R 4 is selected from hydrogen; R 7 ; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 1 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and R 2 is -Z-R 4 .
- R 2 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and R 1 is -Z-R 4 .
- Z 1 , Z 2 , Z 3 , Z 4 and Z 5 are each independently selected from -O-, -C(O)-, -S(O),-, -N(R 4 )- and hydrocarbylene (e.g. C 1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 may be, for example, hydrocarbyl (e.g. C 1-6 alkyl, C 2-6 alkenyl or carbocyclyl) or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Z is a bond and R 4 is selected from C 1-6 alkyl (e.g. C 1 , C 2 , C 3 or C 4 alkyl), carbocyclyl and heterocyclyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 is phenyl, cyclopropyl or cyclohexyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert- butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 4 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 1 is phenyl.
- R 4 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the heterocyclyl group may be monocyclic or bicyclic, usually monocyclic.
- R 4 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoin
- R 4 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 4 is cyclohexyl, cyclopropyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Z is a bond and R 4 is phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Z is a bond and R 4 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the invention includes compounds in which R 1 and R 2 are each independently carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 1 and R 2 may be each independently cycloalkyl (e.g. cyclopropyl or cyclohexyl), aryl (e.g. phenyl) or heteroaryl (e.g. thiophenyl), any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 2 is carbocyclyl (e.g. phenyl) or heterocyclyl, and is substituted with at least one R 7 , wherein said R 7 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy, C 1-6 alkyl and C 1-6 alkoxy.
- R 2 is selected from cycloalkyl (e.g. cyclopropyl or cyclohexyl), cycloalkenyl (e.g. cyclopentadienyl), phenyl, furanyl, benzofuranyl, thiophenyl, isoxazolyl, quinolinyl, isoquinolinyl, quinoxazolinyl, benzothiazolyl and benzothiophenyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- cycloalkyl e.g. cyclopropyl or cyclohexyl
- cycloalkenyl e.g. cyclopentadienyl
- phenyl e.g. cyclopentadienyl
- phenyl e.g. cyclopentadienyl
- phenyl e.g. cyclopentadienyl
- R 1 and R 2 are each independently phenyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 . Of particular mention are compounds in which R 1 and R 2 are each phenyl.
- Y is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O) r , -N(R 5 )- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and R 3 is selected from hydrogen; R 7 ; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Y is a bond.
- Y is a linker having 1 to 10 in- chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O) r , -N(R 5 )- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Y comprises at least one -N(R 5 )- linkage.
- Y comprises an amide linkage, e.g. an -N(R 5 )C(O)- or -C(O)N(R 5 )- linkage.
- Y is a bond or is selected from the following linkers:
- Y 1 , Y 2 , Y 3 , Y 4 and Y 5 are each independently selected from -O-, -C(O)-, -S(O),-, -N(R 4 )- and hydrocarbylene (e.g. C 1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Y is -Y 1 - or -Y 1 -Y 2 -.
- -Y 1 -Y 2 - is -N(R 5 )C(O)- or -C(O)N(R 5 )-.
- R 5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 5 is hydrogen or C 1 ⁇ alkyl (e.g. C 1 , C 2 , C 3 or C 4 alkyl).
- Y is -Y 1 -Y 2 - Y 3 -.
- Y is -N(R 5 )C(O)-Y 3 - or -C(O)N(R 5 )-Y 3 -.
- R 5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 5 is hydrogen or C 1-6 alkyl (e.g. C 1 , C 2 , C 3 or C 4 alkyl).
- R 3 may be, for example, selected from hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is C 1-6 alkyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is C 1 , C 2 , C 3 or C 4 alkyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 3 is trifluoromethyl.
- R 3 is carbocyclyl (e.g. cycloalkyl or aryl) or heterocyclyl (e.g. heterocycloalkyl or heteroaryl), either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 3 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is phenyl or cyclohexyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- R 3 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 3 is phenyl.
- R 3 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the heterocyclyl group may be monocyclic or bicyclic, usually monocyclic.
- R 3 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoin
- R 3 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- R 3 is selected from C 1-6 alkyl, aryl and heteroaryl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is methyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is selected from methyl, trifluoromethyl and phenyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Y is -N(R 5 )C(O)- or -C(O)N(R 5 )-; and R 3 is selected from hydrogen, trifluoromethyl, -OR 8 , -C(O)R 8 , -C(O)OR 8 , -OC(O)R 8 , -S(O) 1 R 8 , amino, -C(O)N(R 8 )R 9 , -S(O) ⁇ N(R 8 )R 9 , -N(R 8 )S(O),R 8 , hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- n O, 1 , 2, 3, 4 or 5;
- n and n are each independently O, 1 , 2, 3, 4 or 5;
- Y 1 and Y 2 are each independently selected from -0-, -C(O)-, -S(O),-, -N(R 5 )- and hydrocarbylene (e.g. C 1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- hydrocarbylene e.g. C 1-5 alkylene
- Y 1 and Y 2 are each independently selected from -O-, -C(O)-, -S(O),-, -N(R 5 )- and hydrocarbylene (e.g. C 1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R 7 ;
- n and n are each independently O, 1 , 2, 3, 4 or 5;
- n O, 1 , 2, 3, 4 or 5;
- n and n are each independently O, 1 , 2, 3, 4 or 5;
- X is oxygen. In other embodiments, X is sulphur.
- the or each R 11 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- m is 0, 1 or 2. In a particular embodiment, m is 0.
- Z may be, for example, a bond.
- R 4 may be, for example, hydrocarbyl (e.g. C 1 -6 alkyl, C 2-6 alkenyl or carbocyclyl) or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- Z is a bond and R 4 is selected from carbocyclyl or heterocyclyl.
- R 4 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 4 is phenyl or cyclohexyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 4 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 1 is phenyl.
- R 4 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the heterocyclyl group may be monocyclic or bicyclic, usually monocyclic.
- R 4 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoin
- R 4 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 4 is cyclohexyl, cyclopropyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 12 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- n 0, 1 or 2.
- n 0.
- -Y 1 -Y 2 - may be, for example, -N(R 5 )C(O)- or -C(O)N(R 5 )-.
- R 5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 5 is hydrogen or C 1-6 alkyl (e.g. C 1 , C 2 , C 3 or C 4 alkyl).
- R 5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 5 is hydrogen or C 1 ⁇ alkyl (e.g. C 1 , C 2 , C 3 or C 4 alkyl).
- R 3 may be, for example, selected from hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 ; and -(CH 2 ) k -heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is C 1-6 alkyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is C 1 , C 2 , C 3 or C 4 alkyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 3 is trifluoromethyl.
- R 3 is carbocyclyl (e.g. cycloalkyl or aryl) or heterocyclyl (e.g. heterocycloalkyl or heteroaryl), either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- R 3 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is phenyl or cyclohexyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 3 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 3 is phenyl.
- R 3 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the heterocyclyl group may be monocyclic or bicyclic, usually monocyclic.
- R 3 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoin
- R 3 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- the or each R 7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C 1 , C 2 , C 3 or C 4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g.
- fluorine or chlorine atoms
- C 1 , C 2 , C 3 or C 4 alkoxy for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
- R 3 is selected from C 1-6 alkyl, aryl and heteroaryl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is methyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is selected from C 1- e alkyl (e.g. methyl) or phenyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- R 3 is trifluoromethyl or phenyl optionally substituted with 1 , 2, 3, 4 or 5 R 7 .
- each compound may be in the form of the free compound, an acid or base addition salt, or a prodrug.
- Compounds of the invention may be in the form of pharmaceutically acceptable salts.
- the pharmaceutically acceptable salts of the present disclosure can be synthesized from the parent compound which contains a basic or acidic moiety by conventional chemical methods. Generally, such salts can be prepared by reacting the free acid or base forms of these compounds with a stoichiometric amount of the appropriate base or acid in water or in an organic solvent, or in a mixture of the two; generally, nonaqueous media like ether, ethyl acetate, ethanol, isopropanol, or acetonitrile are preferred. Lists of suitable salts may be found in Remington's Pharmaceutical Sciences, 17th ed., Mack Publishing Company, Easton, Pa., US, 1985, p. 1418, the disclosure of which is hereby incorporated by reference; see also Stahl et al, Eds, "Handbook of Pharmaceutical Salts Properties Selection and Use", Verlag Helvetica Chimica Acta and Wiley- VCH, 2002.
- the invention thus includes pharmaceutically-acceptable salts of the disclosed compounds wherein the parent compound is modified by making acid or base salts thereof, for example the conventional non-toxic salts or the quaternary ammonium salts which are formed, e.g. from inorganic or organic acids or bases.
- acid addition salts include acetate, adipate, alginate, aspartate, benzoate, benzenesulfonate, bisulfate, butyrate, citrate, camphorate, camphorsulfonate, cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, fumarate, glucoheptanoate, glycerophosphate, hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethanesulfonate, lactate, maleate, methanesulfonate, 2- naphthalenesulfonate, nicotinate, oxalate, pamoate, pectinate, persulfate, 3- phenylpropionate, picrate, pivalate, propionate, succinate, tartrate, thiocyanate,
- Base salts include ammonium salts, alkali metal salts such as sodium and potassium salts, alkaline earth metal salts such as calcium and magnesium salts, salts with organic bases such as dicyclohexylamine salts, N-methyl-D-glucamine, and salts with amino acids such as arginine, lysine, and so forth.
- the basic nitrogen-containing groups may be quaternized with such agents as lower alkyl halides, such as methyl, ethyl, propyl, and butyl chloride, bromides and iodides; dialkyl sulfates like dimethyl, diethyl, dibutyl; and diamyl sulfates, long chain halides such as decyl, lauryl, myristyl and stearyl chlorides, bromides and iodides, aralkyl halides like benzyl and phenethyl bromides and others.
- lower alkyl halides such as methyl, ethyl, propyl, and butyl chloride, bromides and iodides
- dialkyl sulfates like dimethyl, diethyl, dibutyl
- diamyl sulfates long chain halides
- the invention includes prodrugs for the active pharmaceutical species of the invention, for example in which one or more functional groups are protected or derivatised but can be converted in vivo to the functional group, as in the case of esters of carboxylic acids convertible in vivo to the free acid, or in the case of protected amines, to the free amino group.
- prodrug represents in particular compounds which are rapidly transformed in vivo to the parent compound, for example, by hydrolysis in blood.
- Prodrugs therefore include drugs having a functional group which has been transformed into a reversible derivative thereof.
- such prodrugs are transformed to the active drug by hydrolysis.
- groups include carboxylic groups (reversible derivatives including esters, e.g. acyloxyalkyl esters and amides), alcohol groups (reversible derivatives including sulfates, phosphates and carboxylic acid esters), amine groups (reversible derivatives including amides, carbamates, imines and enamines) and carbonyl groups, e.g. aldehyde and ketone groups (reversible derivatives including imines, oximes, acetals/ketals, enol esters, oxazolidines and thiazoxolidines).
- Prodrugs also include compounds convertible to the active drug by an oxidative or reductive reaction.
- oxidative activation may be mentioned N- and O- dealkylation, oxidative deamination, N-oxidation and epoxidation.
- reductive activation may be mentioned azo reduction, sulfoxide reduction, disulfide reduction, bioreductive alkylation and nitro reduction.
- metabolic activations of prodrugs are nucleotide activation, phosphorylation activation and decarboxylation activation.
- the compounds of the disclosure may also contain one or more asymmetric carbon atoms and may therefore exhibit optical and/or diastereoisomerism. All diastereoisomers may be separated using conventional techniques, e.g. chromatography or fractional crystallisation. The various stereoisomers may be isolated by separation of a racemic or other mixture of the compounds using conventional, e.g. fractional crystallisation or HPLC, techniques. Alternatively the desired optical isomers may be made by reaction of the appropriate optically active starting materials under conditions which will not cause racemisation or epimerisation, or by derivatisation, for example with a homochiral acid followed by separation of the diastereomeric derivatives by conventional means (e.g. HPLC, chromatography over silica).
- HPLC chromatography over silica
- Geometric isomers may also exist in the compounds of the present disclosure.
- the present disclosure contemplates the various geometric isomers and mixtures thereof resulting from the arrangement of substituents around a carbon-carbon double bond and designates such isomers as of the Z or E configuration, wherein the term "Z” represents substituents on the same side of the carbon-carbon double bond and the term “E” represents substituents on opposite sides of the carbon-carbon double bond.
- the disclosure therefore includes all variant forms of the defined compounds, for example any tautomer or any pharmaceutically acceptable salt, ester, acid or other variant of the defined compounds and their tautomers as well as substances which, upon administration, are capable of providing directly or indirectly a compound as defined above or providing a species which is capable of existing in equilibrium with such a compound.
- a compound of the invention may be prepared according to the processes described herein. It will be understood that these processes are solely for the purpose of illustrating the invention and should not be construed as limiting. A process utilising similar or analogous reagents and/or conditions known to one skilled in the art may also be used to obtain a compound of the invention.
- Any mixtures of final products or intermediates obtained can be separated on the basis of the physico-chemical differences of the constituents, in a known manner, into the pure final products or intermediates, for example by chromatography, distillation, fractional crystallisation, or by the formation of a salt if appropriate or possible under the circumstances.
- the compounds of the invention will normally be administered orally, intravenously, subcutaneously, buccally, rectally, dermally, nasally, tracheally, bronchially, by any other parenteral route, as an oral or nasal spray or via inhalation,
- the compounds may be administered in the form of pharmaceutical preparations comprising prodrug or active compound either as a free compound or, for example, a pharmaceutically acceptable non-toxic organic or inorganic acid or base addition salt, in a pharmaceutically acceptable dosage form.
- the compositions may be administered at varying doses.
- the pharmaceutical compounds of the invention may be administered orally or parenterally ("parenterally” as used herein, refers to modes of administration which include intravenous, intramuscular, intraperitoneal, intrasternal, subcutaneous and intraarticular injection and infusion) to a host.
- parenterally refers to modes of administration which include intravenous, intramuscular, intraperitoneal, intrasternal, subcutaneous and intraarticular injection and infusion
- the compounds may be administered alone or as compositions in combination with pharmaceutically acceptable diluents, excipients or carriers.
- Actual dosage levels of active ingredients in the pharmaceutical compositions of this invention may be varied so as to obtain an amount of the active compound(s) that is effective to achieve the desired therapeutic response for a particular patient, compositions, and mode of administration.
- the selected dosage level will depend upon the activity of the particular compound, the route of administration, the severity of the condition being treated and the condition and prior medical history of the patient being treated. However, it is within the skill of the art to start doses of the compound at levels lower than required for to achieve the desired therapeutic effect and to gradually increase the dosage until the desired effect is achieved.
- an appropriate dosage level will generally be about 0.01 to 500 mg per kg patient body weight per day which can be administered in single or multiple doses.
- the dosage level is about 0.1 to about 250 mg/kg per day; more preferably about 0.5 to about 100 mg/kg per day.
- a suitable dosage level may be about 0.01 to 250 mg/kg per day, about 0.05 to 100 mg/kg per day, or about 0.1 to 50 mg/kg per day. Within this range the dosage may be 0.05 to 0.5, 0.5 to 5 or 5 to 50 mg/kg per day.
- the compositions may be provided in the form of tablets containing 1 .0 to 1000 milligrams of the active ingredient, particularly 1 .0, 5.0, 10.0, 15.0, 20.0, 25.0, 50.0, 75.0, 100.0, 150.0, 200.0, 250.0, 300.0, 400.0, 500.0, 600.0, 750.0, 800.0, 900.0 and 1000.0 milligrams of the active ingredient for the symptomatic adjustment of the dosage to the patient to be treated.
- the compounds may be administered on a regimen of 1 to 4 times per day, e.g. once or twice per day. The dosage regimen may be adjusted to provide the optimal therapeutic response.
- composition including a compound of the invention, in admixture with a pharmaceutically acceptable adjuvant, diluent or carrier.
- compositions of this invention for parenteral injection suitably comprise pharmaceutically acceptable sterile aqueous or nonaqueous solutions, dispersions, suspensions or emulsions as well as sterile powders for reconstitution into sterile injectable solutions or dispersions just prior to use.
- suitable aqueous and nonaqueous carriers, diluents, solvents or vehicles include water, ethanol, polyols (such as glycerol, propylene glycol, polyethylene glycol and the like), and suitable mixtures thereof, vegetable oils (such as olive oil) and injectable organic esters such as ethyl oleate.
- Proper fluidity can be maintained, for example, by the use of coating materials such as lecithin, by the maintenance of the required particle size in the case of dispersions and by the use of surfactants.
- compositions may also contain adjuvants such as preservative, wetting agents, emulsifying agents and dispersing agents. Prevention of the action of microorganisms may be ensured by the inclusion of various antibacterial and antifungal agents, for example, paraben, chlorobutanol or phenol sorbic acid. It may also be desirable to include isotonic agents such as sugars or sodium chloride, for example. Prolonged absorption of the injectable pharmaceutical form may be brought about by the inclusion of agents (for example aluminum monostearate and gelatin) which delay absorption.
- adjuvants such as preservative, wetting agents, emulsifying agents and dispersing agents.
- the absorption of the drug in order to prolong the effect of the drug, it is desirable to slow the absorption of the drug from subcutaneous or intramuscular injection. This may be accomplished by the use of a liquid suspension of crystalline or amorphous material with poor water solubility. The rate of absorption of the drug then depends upon its rate of dissolution which, in turn, may depend upon crystal size and crystalline form. Alternatively, delayed absorption of a parenterally administered drug form is accomplished by dissolving or suspending the drug in an oil vehicle.
- Injectable depot forms are suitably made by forming microencapsule matrices of the drug in biodegradable polymers, for example polylactide-polyglycolide. Depending upon the ratio of drug to polymer and the nature of the particular polymer employed, the rate of drug release can be controlled. Examples of other biodegradable polymers include poly(orthoesters) and poly(anhydrides). Depot injectable formulations may also prepared by entrapping the drug in liposomes or microemulsions which are compatible with body tissues.
- the injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable media just prior to use.
- Solid dosage forms for oral administration include capsules, tablets, pills, powders and granules.
- the active compound is typically mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or one or more: a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol and silicic acid; b) binders such as carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidone, sucrose and acacia; c) humectants such as glycerol; d) disintegrating agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates and sodium carbonate; e) solution retarding agents such as paraffin; f) absorption accelerators such as quaternary ammonium compounds; g) wetting agents such as cetyl alcohol and glycerol monostearate;
- the dosage form may also comprise buffering agents.
- Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycol, for example.
- oral formulations contain a dissolution aid.
- the dissolution aid is not limited as to its identity so long as it is pharmaceutically acceptable. Examples include nonionic surface active agents, such as sucrose fatty acid esters, glycerol fatty acid esters, sorbitan fatty acid esters (e.g.
- sorbitan trioleate polyethylene glycol, polyoxyethylene hydrogenated castor oil, polyoxyethylene sorbitan fatty acid esters, polyoxyethylene alkyl ethers, methoxypolyoxyethylene alkyl ethers, polyoxyethylene alkylphenyl ethers, polyethylene glycol fatty acid esters, polyoxyethylene alkylamines, polyoxyethylene alkyl thioethers, polyoxyethylene polyoxypropylene copolymers, polyoxyethylene glycerol fatty acid esters, pentaerythritol fatty acid esters, propylene glycol monofatty acid esters, polyoxyethylene propylene glycol monofatty acid esters, polyoxyethylene sorbitol fatty acid esters, fatty acid alkylolamides, and alkylamine oxides; bile acid and salts thereof (e.g.,
- ionic surface active agents such as sodium laurylsulfate, fatty acid soaps, alkylsulfonates, alkylphosphates, ether phosphates, fatty acid salts of basic amino acids; triethanolamine soap, and alkyl quaternary ammonium salts; and amphoteric surface active agents, such as betaines and aminocarboxylic acid salts.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally contain opacifying agents and may also be of a composition such that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, and/or in delayed fashion. Examples of embedding compositions include polymeric substances and waxes. The active compounds may also be in micro-encapsulated form, if appropriate, with one or more of the above-mentioned excipients.
- the active compounds may be in finely divided form, for example they may be micronised.
- Liquid dosage forms for oral administration include pharmaceutically acceptable emulsions, solutions, suspensions, syrups and elixirs.
- the liquid dosage forms may contain inert diluents commonly used in the art such as water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1 ,3-butylene glycol, dimethyl formamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan and mixtures thereof.
- inert diluents commonly used in the art such as water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol
- the oral compositions may also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring and perfuming agents.
- Suspensions in addition to the active compounds, may contain suspending agents such as ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar, and tragacanth and mixtures thereof.
- compositions for rectal or vaginal administration are preferably suppositories which can be prepared by mixing the compounds of this invention with suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at room temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
- suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at room temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
- Liposomes are generally derived from phospholipids or other lipid substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid crystals which are dispersed in an aqueous medium. Any non-toxic, physiologically acceptable and metabolisable lipid capable of forming liposomes can be used.
- the present compositions in liposome form can contain, in addition to a compound of the present invention, stabilisers, preservatives, excipients and the like.
- the preferred lipids are the phospholipids and the phosphatidyl cholines (lecithins), both natural and synthetic. Methods to form liposomes are known in the art, for example, Prescott, Ed., Methods in Cell Biology, Volume XIV, Academic Press, New York, N.Y. (1976), p 33 et seq.
- Dosage forms for topical administration of a compound of this invention include powders, sprays, ointments and inhalants.
- the active compound is mixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives, buffers or propellants which may be required.
- Ophthalmic formulations, eye ointments, powders and solutions are also contemplated as being within the scope of this invention.
- Compounds of the invention may be useful in the therapy of a variety of diseases and conditions.
- the subject of said therapy may be a human or an animal.
- compounds of the invention may be useful in the treatment or prevention of prion diseases.
- Prion diseases are often characterized by symptoms of dementia or cognitive impairment.
- the prion disease may be inherited, infectious or sporadic. Examples of prion disease include Creutzfeldt-Jakob disease, kuru, Gerstmann-Straussler-Sheinker disease, fatal familial insomnia and transmissible spongiform encephalopathies (TSEs). Examples of TSEs include bovine spongiform encephalopathy (BSE), scrapie, chronic wasting disease (e.g. in deer or elk) and transmissible mink encephalopathy (TME).
- BSE bovine spongiform encephalopathy
- TBE transmissible mink encephalopathy
- the invention also provides a method of regulating stem cell activity, comprising contacting one or more types of stem cells with a compound of the invention. Said contacting generally takes place under conditions such that activity is regulated. In one embodiment, said contacting takes place in vitro.
- melting points were measured using a Bibby-Sterilin SMP10 melting point apparatus and are uncorrected. Accurate mass and nominal mass measurements were measured using a Waters-Micromass LCT electrospray mass spectrometer. Flash column chromatography was carried out using Fluorochem silica gel 60 A. All compounds were isolated in >95% purity unless otherwise stated (as determined by HPLC under two sets of conditions-HPLC 1 ; Luna 5 ⁇ C18, 150 x 4.6 mm, 5-95% acetonitrile (0.1 % TFA) in water (0.1 % TFA) over 4 min, 1 mL min '1 , 20 ⁇ L injection, detection at 256 nm, run time 10 min.
- Thiazole compounds 1c to 1x were prepared.
- the structures of compounds 1c and 1e to 1x are shown below:
- Compound 1d has the following structure:
- Acid chloride (0.33 mmol) was added to a solution of 1d (76 mg, 0.30 mmol) and DMAP (10 mg) in pyridine (3 mL). The reaction mixture was stirred at ambient temperature for 18 hours. All volatiles were removed under reduced pressure and the residue taken up in DCM (30 mL). This solution was washed thoroughly with 1 M HCI (4 x 30 mL) then sat. NaHCO 3 (2 x 30 mL), dried over MgSO 4 , filtered and evaporated to dryness to provide the product. Where necessary, further purification was carried out by flash column chromatography on silica gel. In the case of 1g, 1 r, the relevant acid chloride was not available commercially. These amide derivatives were prepared from the carboxylic acids, through in situ formation of the acid chloride followed by reaction with amine 1d in pyridine.
- SPR surface plasmon resonance
- SMB shed mediastinal blood
- SPR screening methodology Surface plasmon resonance (SPR) was carried out using a BIAcore 3000 (BIAcore, Uppsala, Sweden) equipped with a CM5 sensor chip (carboxymethylated dextran). The methodology was as reported previously (Touil, F.; Pratt, S. ; Mutter, R.; Chen, B., J. Pharm. Biomed. Anal. 2006, 40, 822-832). Interactions were measured with two forms of prion protein, full length human (huPrP c ) and full length murine (moPrP c ).
- Cells were grown in tissue culture treated plastic dishes in Medium 199 (phenol red free), supplemented with 10% newborn calf serum (heat inactivated), 5% foetal calf serum (heat inactivated) and penicillin-streptomycin at 10 mg L "1 at 37 °C in an atmosphere of 5% CO 2 in air at 95% relative humidity. Medium was changed every 3 rd or 4 th day, and every 7 days confluent cells were passaged using 0.05% trypsin and 0.002% EDTA at a split ratio of 4. To assess the effects of compounds cells were distributed into 96-well cluster plates at 3 x 10 4 cells per well and incubated for 24 h to allow for cell attachment.
- the compounds were diluted to 400 times the required concentration in DMSO as stock solutions then transferred, at a 20-fold dilution, into Hank's balanced salt solution. This solution was then transferred at a further 20-fold dilution into the cell medium. The cells were incubated with the compound-containing medium for 5 days.
- the reaction was stopped with 1 mM phenylmethylsulfonyl fluoride (PMSF) in 20 mM Tris-HCI-buffered saline (TBS), the membrane washed extensively with TBS, and immersed in 1 .8 M guanidine thiocyanate in TBS for 10 min at room temperature. After further washing with TBS the membrane was blocked using 5% fat-free milk powder in phosphate buffered saline (PBS), processed with 0.2 ⁇ g mL -1 mouse monoclonal anti-PrP 6H4 (Prionics) and developed using an ECL kit (Amersham Pharmacia Biotech).
- PMSF phenylmethylsulfonyl fluoride
- TBS Tris-HCI-buffered saline
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Compounds of the formula (I) are provided: Fomula (I) wherein X, Y, R1, R2 and R3 are as defined in the specification. The compounds may be useful in the treatment of various diseases and conditions, in particular prion diseases.
Description
THIAZOLE AND OXAZOLE DERIVATIVES FOR THE USE IN THE
TREATMENT OF PRION DISEASES, CANCER AND CONDITIONS OF THE
CENTRAL NERVOUS SYSTEM AS WELL AS IN THE REGULATION OF
STEM CELLS
Field of the Invention
This invention relates to compounds and their use in therapy, especially in the treatment of prion diseases and the regulation of stem cells.
Background to the Invention
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are invariably fatal neurodegenerative disorders affecting humans and animals. As yet, no effective curative or prophylactic therapy exists. Prominent examples of prion diseases include bovine spongiform encephalopathy (BSE, cattle), scrapie (sheep), chronic wasting disorder (CWD, deer and elk) and transmissible mink encephalopathy (TME). Since a new variant of the human TSE Creutzfeldt-Jacob disease (vCJD) was discovered, thought to have been triggered by the consumption of contaminated beef products, prion diseases have been the focus of much research effort. They represent a highly significant risk to public health due to transmission both to and between humans. TSEs are associated with a post-translational conversion of the cell-surface glycosylphosphatidylinositol (GPI)-anchored protein PrPc (or PrPsen) to a partially protease resistant isoform denoted PrPSc (or PrPres).
Lichtenberger et al (Bull. Soc. ChIm. Fr. 1956, 1 184-1 192) report the compound 2,4- diphenyloxazol-5-ylamine, i.e.:
Thompson et al ( Tetrahedron Lett. 2006, 47, 2361 -2364) report the synthesis of the following 5-aminothiazole compounds:
wherein, in each case, R is selected from phenyl, 4-methoxyphenyl, thiophen-2-yl, cyclohexyl and isopropyl.
Summary of the Invention
According to the present invention, there is provided a compound of the formula (I):
(I)
wherein
X is oxygen or sulphur;
Y is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7;
one of R1 and R2 is selected from carbocyclyl and heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7; and the other is -Z-R4;
R3 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
Z is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7;
R4 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
R5 is selected from R6, -OR6, -C(O)R6, -C(O)OR6 and -S(O)1R6;
R6 is selected from hydrogen; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or
5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
each R7 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10;
R8 and R9 are each independently hydrogen or R10;
R10 is selected from hydrocarbyl and -(CH2)k-heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy, C1-6 alkyl and C1-6 alkoxy;
k is O, 1 , 2, 3, 4, 5 or 6; and
Ms O, 1 or 2;
or a pharmaceutically acceptable salt or prodrug thereof.
The invention also provides a pharmaceutical formulation comprising a compound of formula (I) and a pharmaceutically acceptable carrier or excipient.
In a further aspect, the invention relates to the use of a compound of formula (I), for the manufacture of a medicament for the treatment, prevention or delay of progression of a prion disease. A method of treating, preventing or delaying progression of a prion disease is also provided, which involves administering a therapeutically effective amount of a compound of the invention to a subject.
Compounds of the invention may also be useful in the regulation of stem cells. Accordingly, in another aspect there is provided a method of regulating stem cell activity, which comprises contacting one or more stem cells with a compound of the invention. The compounds may also be useful in the treatment, prevention or delay of progression of cancer and diseases or conditions of the central nervous system, or in regenerative medicine.
Features, integers, characteristics, compounds, chemical moieties or groups described in conjunction with a particular aspect, embodiment or example of the invention are to be understood to be applicable to any other aspect, embodiment or example described herein unless incompatible therewith.
Description of Various Embodiments
Hydrocarbyl
The term "hydrocarbyl" as used herein includes reference to moieties consisting exclusively of hydrogen and carbon atoms; such a moiety may comprise an aliphatic and/or an aromatic moiety. The moiety may comprise 1 , 2, 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15, 16, 17, 18, 19 or 20 carbon atoms. Examples of hydrocarbyl groups include C1-6 alkyl (e.g. C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl); C1-6 alkyl substituted by aryl (e.g. benzyl) or by cycloalkyl (e.g cyclopropylmethyl); cycloalkyl (e.g. cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl); alkenyl (e.g. 2-butenyl); alkynyl (e.g. 2-butynyl); aryl (e.g. phenyl, naphthyl or fluorenyl) and the like.
Alkyl
The terms "alkyl" and "C1^ alkyl" as used herein include reference to a straight or branched chain alkyl moiety having 1 , 2, 3, 4, 5 or 6 carbon atoms. This term includes reference to groups such as methyl, ethyl, propyl (n-propyl or isopropyl), butyl (n-butyl, sec-butyl or tert-butyl), pentyl, hexyl and the like. In particular, alkyl may have 1 , 2, 3 or 4 carbon atoms.
Alkenyl
The terms "alkenyl" and "C2-6 alkenyl" as used herein include reference to a straight or branched chain alkyl moiety having 2, 3, 4, 5 or 6 carbon atoms and having, in addition, at least one double bond, of either E or Z stereochemistry where applicable. This term includes reference to groups such as ethenyl, 2-propenyl, 1 -butenyl, 2-butenyl, 3- butenyl, 1 -pentenyl, 2-pentenyl, 3-pentenyl, 1 -hexenyl, 2-hexenyl and 3-hexenyl and the like.
Alkynyl
The terms "alkynyl" and "C2-6 alkynyl" as used herein include reference to a straight or branched chain alkyl moiety having 2, 3, 4, 5 or 6 carbon atoms and having, in addition, at least one triple bond. This term includes reference to groups such as ethynyl, 1 - propynyl, 2-propynyl, 1 -butynyl, 2-butynyl, 3-butynyl, 1 -pentynyl, 2-pentynyl, 3-pentynyl, 1 -hexynyl, 2-hexynyl and 3-hexynyl and the like.
Alkoxy
The terms "alkoxy" and "C1-6 alkoxy" as used herein include reference to -O-alkyl, wherein alkyl is straight or branched chain and comprises 1 , 2, 3, 4, 5 or 6 carbon atoms. In one class of embodiments, alkoxy has 1 , 2, 3 or 4 carbon atoms. This term includes reference to groups such as methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert- butoxy, pentoxy, hexoxy and the like.
Cycloalkyl
The term "cycloalkyl" as used herein includes reference to an alicyclic moiety having 3, 4, 5, 6, 7 or 8 carbon atoms. The group may be a bridged or polycyclic ring system. More often cycloalkyl groups are monocyclic. This term includes reference to groups such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, norbornyl, bicyclo[2.2.2]octyl and the like.
Cycloalkenyl
The term "cycloalkenyl" as used herein includes reference to a non-aromatic cycloalkyl group having a double bond between one or more pairs of ring carbon atoms. The group
may be a bridged or polycyclic ring system. More often cycloalkenyl groups are monocyclic. This term includes reference to groups such as cyclopentadienyl and the like.
Aryl
The term "aryl" as used herein includes reference to an aromatic ring system comprising 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 ring carbon atoms. Aryl is often phenyl but may be a polycyclic ring system, having two or more rings, at least one of which is aromatic. This term includes reference to groups such as phenyl, naphthyl, fluorenyl, azulenyl, indenyl, anthryl and the like.
Carbocyclyl
The term "carbocyclyl" as used herein includes reference to a saturated (e.g. cycloalkyl) or unsaturated (e.g. cycloalkenyl or aryl) ring moiety having 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 carbon ring atoms. In particular, carbocyclyl includes a 3- to 10- membered ring or ring system and, in particular, a 5- or 6-membered ring, which may be saturated or unsaturated. A carbocyclic moiety is, for example, selected from cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, norbomyl, bicyclo[2.2.2]octyl, phenyl, naphthyl, fluorenyl, azulenyl, indenyl, anthryl and the like.
Heterocyclyl
The term "heterocyclyl" as used herein includes reference to a saturated (e.g. heterocycloalkyl) or unsaturated (e.g. heteroaryl) heterocyclic ring moiety having from 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 ring atoms, at least one of which is selected from nitrogen, oxygen, phosphorus, silicon and sulphur. In particular, heterocyclyl includes a 3- to 10-membered ring or ring system and more particularly a 5- or 6- membered ring, which may be saturated or unsaturated.
A heterocyclic moiety is, for example, selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofura- nyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrazinyl, pyrimidinyl, piperidyl, piperazinyl,
pyridazinyl, morpholinyl, thiomorpholinyl, especially thiomorpholino, indolizinyl, isoindolyl, 3/-/-indolyl, indolyl, benzimidazolyl, cumaryl, indazolyl, triazolyl, tetrazolyl, purinyl, AH- quinolizinyl, isoquinolyl, quinolyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl, octahydroisoquinolyl, benzofuranyl, dibenzofuranyl, benzothiophenyl, dibenzothiophenyl, phthalazinyl, naphthyridinyl, quinoxalyl, quinazolinyl, quinazolinyl, cinnolinyl, pteridinyl, carbazolyl, β-carbolinyl, phenanthridinyl, acridinyl, perimidinyl, phenanthrolinyl, furazanyl, phenazinyl, phenothiazinyl, phenoxazinyl, chromenyl, isochromanyl, chromanyl and the like.
Heterocycloalkyl
The term "heterocycloalkyl" as used herein includes reference to a saturated heterocyclic moiety having 3, 4, 5, 6 or 7 ring carbon atoms and 1 , 2, 3, 4 or 5 ring heteroatoms selected from nitrogen, oxygen, phosphorus and sulphur. The group may be a polycyclic ring system but more often is monocyclic. This term includes reference to groups such as azetidinyl, pyrrolidinyl, tetrahydrofuranyl, piperidinyl, oxiranyl, pyrazolidinyl, imidazolyl, indolizidinyl, piperazinyl, thiazolidinyl, morpholinyl, thiomorpholinyl, quinolizidinyl and the like.
Heteroaryl
The term "heteroaryl" as used herein includes reference to an aromatic heterocyclic ring system having 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, 15 or 16 ring atoms, at least one of which is selected from nitrogen, oxygen and sulphur. The group may be a polycyclic ring system, having two or more rings, at least one of which is aromatic, but is more often monocyclic. This term includes reference to groups such as pyrimidinyl, furanyl, benzo[b]thiophenyl, thiophenyl, pyrrolyl, imidazolyl, pyrrolidinyl, pyridinyl, benzo[b]furanyl, pyrazinyl, purinyl, indolyl, benzimidazolyl, quinolinyl, phenothiazinyl, triazinyl, phthalazinyl, 2H-chromenyl, oxazolyl, isoxazolyl, thiazolyl, isoindolyl, indazolyl, purinyl, isoquinolinyl, quinazolinyl, pteridinyl and the like.
Halogen
The term "halogen" as used herein includes reference to F, Cl, Br or I. In a particular, halogen may be F or Cl, of which F is more common.
Substituted
The term "substituted" as used herein in reference to a moiety means that one or more, especially up to 5, more especially 1 , 2 or 3, of the hydrogen atoms in said moiety are replaced independently of each other by the corresponding number of the described substituents. The term "optionally substituted" as used herein means substituted or unsubstituted.
It will, of course, be understood that substituents are only at positions where they are chemically possible, the person skilled in the art being able to decide (either experimentally or theoretically) without inappropriate effort whether a particular substitution is possible. For example, amino or hydroxy groups with free hydrogen may be unstable if bound to carbon atoms with unsaturated (e.g. olefinic) bonds. Additionally, it will of course be understood that the substituents described herein may themselves be substituted by any substituent, subject to the aforementioned restriction to appropriate substitutions as recognised by the skilled man.
Pharmaceutically acceptable
The term "pharmaceutically acceptable" as used herein includes reference to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings or animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio. This term includes acceptability for both human and veterinary purposes.
Independently
Where two or more moieties are described as being "each independently" selected from a list of atoms or groups, this means that the moieties may be the same or different. The identity of each moiety is therefore independent of the identities of the one or more other moieties.
Compounds
Compounds of the invention are of the formula (I):
wherein
X is oxygen or sulphur;
Y is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7;
one of R1 and R2 is selected from carbocyclyl and heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7; and the other is -Z-R4;
R3 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
Z is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)1-, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7;
R4 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
R5 is selected from R6, -OR6, -C(O)R6, -C(O)OR6 and -S(O)1R6;
R6 is selected from hydrogen; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
each R7 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O),R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10;
R and R are each independently hydrogen or R 10;
R is selected from hydrocarbyl and -(CH2)k-heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy, C1-6 alkyl and C1-6 alkoxy;
k is O, 1 , 2, 3, 4, 5 or 6; and
I is O, 1 or 2;
or a pharmaceutically acceptable salt or prodrug thereof.
In one embodiment, the compound is not 2,4-diphenyloxazol-5-ylamine or a compound of the following formulae:
wherein, in each case, R is selected from phenyl, 4-methoxyphenyl, thiophen-2-yl, cyclohexyl and isopropyl.
Further embodiments of the invention are described below. It will be appreciated that the features specified in each embodiment may be combined with other specified features, to provide yet further embodiments.
X
In one class of compounds, X is oxygen. In another class of compounds, X is sulphur.
R1 & R2
According to formula (I), one of R1 and R2 is selected from carbocyclyl and heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7; and the other is -Z-R4,
wherein Z is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7; and R4 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
In one embodiment, R1 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7; and R2 is -Z-R4.
In another embodiment, R2 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7; and R1 is -Z-R4.
Of mention are compounds in which Z is a bond or one of the following linkers:
Z1-; -Z1 -Z2-;
-Z1-Z2-Z3-; -Z1-Z2-Z3-Z4-; and -Z1-Z2-Z3-Z4-Z5-;
wherein Z1 , Z2, Z3, Z4 and Z5 are each independently selected from -O-, -C(O)-, -S(O),-, -N(R4)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7.
Of particular mention are compounds in which Z is a bond.
R4 may be, for example, hydrocarbyl (e.g. C1-6 alkyl, C2-6 alkenyl or carbocyclyl) or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In embodiments, Z is a bond and R4 is selected from C1-6 alkyl (e.g. C1 , C2, C3 or C4 alkyl), carbocyclyl and heterocyclyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
Where R4 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. For example, R4 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In a particular embodiment, R4 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R7. In embodiments, R4 is phenyl, cyclopropyl or cyclohexyl, either of which is optionally substituted with 1 , 2,
3, 4 or 5 R7. In this case, the or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert- butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a particular embodiment, R4 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. Of mention are compounds in which R1 is phenyl.
Where R4 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The heterocyclyl group may be monocyclic or bicyclic, usually monocyclic. In particular, R4 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoindolyl, 3/-/-indolyl, indolyl, benzimidazolyl, cumaryl, indazolyl, triazolyl, tetrazolyl, purinyl, 4/-/-quinolizinyl, isoquinolyl, quinolyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl, octahydroisoquinolyl, dibenzofuranyl, benzothiophenyl, dibenzothiophenyl, phthalazinyl, naphthyridinyl, quinoxalyl, quinazolinyl, quinazolinyl, cinnolinyl, pteridinyl, carbazolyl, β- carbolinyl, phenanthridinyl, acridinyl, perimidinyl, phenanthrolinyl, furazanyl, phenazinyl, phenothiazinyl, phenoxazinyl, chromenyl, isochromanyl and chromanyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a further embodiment, R4 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-
butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a particular embodiment, R4 is cyclohexyl, cyclopropyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. Of particular mention are compounds in which Z is a bond and R4 is phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a particular embodiment, Z is a bond and R4 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. Thus, the invention includes compounds in which R1 and R2 are each independently carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. For example, R1 and R2 may be each independently cycloalkyl (e.g. cyclopropyl or cyclohexyl), aryl (e.g. phenyl) or heteroaryl (e.g. thiophenyl), any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a further embodiment, R2 is carbocyclyl (e.g. phenyl) or heterocyclyl, and is substituted with at least one R7, wherein said R7 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy, C1-6 alkyl and C1-6 alkoxy.
In a further embodiment, R2 is selected from cycloalkyl (e.g. cyclopropyl or cyclohexyl), cycloalkenyl (e.g. cyclopentadienyl), phenyl, furanyl, benzofuranyl, thiophenyl, isoxazolyl, quinolinyl, isoquinolinyl, quinoxazolinyl, benzothiazolyl and benzothiophenyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7;
In particular compounds, R1 and R2 are each independently phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7. Of particular mention are compounds in which R1 and R2 are each phenyl.
-Y-H3
In formula (I), Y is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7; and R3 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
In one embodiment, Y is a bond. In another embodiment, Y is a linker having 1 to 10 in- chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7. In certain compounds, Y comprises at least one -N(R5)- linkage. Of mention are compounds in which Y comprises an amide linkage, e.g. an -N(R5)C(O)- or -C(O)N(R5)- linkage.
In a further embodiment, Y is a bond or is selected from the following linkers:
-Y1-; -Y1 -Y2-; γ1 γ2 γ3 . _γ1_γ2_γ3_γ4_. and
-Y1 -Y2- Y3- Y4- Y5-;
wherein Y1 , Y2, Y3, Y4 and Y5 are each independently selected from -O-, -C(O)-, -S(O),-, -N(R4)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a further embodiment, Y is -Y1- or -Y1 -Y2-. In particular compounds, -Y1 -Y2- is -N(R5)C(O)- or -C(O)N(R5)-. R5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R5 is hydrogen or C1^ alkyl (e.g. C1 , C2, C3 or C4 alkyl).
In a further embodiment, Y is -Y1 -Y2- Y3-. In particular compounds, Y is -N(R5)C(O)-Y3- or -C(O)N(R5)-Y3-. R5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R5 is hydrogen or C1-6 alkyl (e.g. C1 , C2, C3 or C4 alkyl).
R3 may be, for example, selected from hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
In one embodiment, R3 is C1-6 alkyl optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R3 is C1 , C2, C3 or C4 alkyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. Of particular mention are compounds in which R3 is trifluoromethyl.
In another embodiment, R3 is carbocyclyl (e.g. cycloalkyl or aryl) or heterocyclyl (e.g. heterocycloalkyl or heteroaryl), either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
Where R3 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. For example, R3 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In a particular embodiment, R3 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R7. In embodiments,
R3 is phenyl or cyclohexyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In this case, the or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a particular embodiment, R3 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. Of mention are compounds in which R3 is phenyl.
Where R3 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The heterocyclyl group may be monocyclic or bicyclic, usually monocyclic. In particular, R3 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoindolyl, 3/-/-indolyl, indolyl, benzimidazolyl, cumaryl, indazolyl, triazolyl, tetrazolyl, purinyl, 4/-/-quinolizinyl, isoquinolyl, quinolyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl, octahydroisoquinolyl, dibenzofuranyl, benzothiophenyl, dibenzothiophenyl, phthalazinyl, naphthyridinyl, quinoxalyl, quinazolinyl, quinazolinyl, cinnolinyl, pteridinyl, carbazolyl, β- carbolinyl, phenanthridinyl, acridinyl, perimidinyl, phenanthrolinyl, furazanyl, phenazinyl, phenothiazinyl, phenoxazinyl, chromenyl, isochromanyl and chromanyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a further embodiment, R3 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a further embodiment, R3 is selected from C1-6 alkyl, aryl and heteroaryl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R3 is methyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. Of particular mention are compounds in which R3 is selected from methyl, trifluoromethyl and phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a further embodiment, Y is -N(R5)C(O)- or -C(O)N(R5)-; and R3 is selected from hydrogen, trifluoromethyl, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, amino, -C(O)N(R8)R9, -S(O)ιN(R8)R9, -N(R8)S(O),R8, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
Also of mention are compounds in which -Y-R3 is -N(R8)R9, for example amino.
In a particular embodiment, there is provided a compound of the formula (II):
(II) wherein
each R11 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
(III) wherein
R1 1 and R12 are each independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m and n are each independently O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
In a further embodiment, there is provided a compound of the formula (IV):
(IV)
wherein
Y1 and Y2 are each independently selected from -0-, -C(O)-, -S(O),-, -N(R5)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7.
each R11 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
In a particular embodiment, there is provided a compound of the formula (V):
(V)
wherein
Y1 and Y2 are each independently selected from -O-, -C(O)-, -S(O),-, -N(R5)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7;
R1 1 and R12 are each independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m and n are each independently O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
In a further embodiment, there is provided a compound of the formula (Vl):
wherein
each R11 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
In a further embodiment, there is provided a compound of the formula (VII):
(VII)
R1 1 and R12 are each independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m and n are each independently O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
In embodiments of formulae (II) to (VII), X is oxygen. In other embodiments, X is sulphur.
In compounds of the formulae (II) to (VII), the or each R11 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally
substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. In embodiments, m is 0, 1 or 2. In a particular embodiment, m is 0.
With regard to formulae (II), (IV) and (Vl), Z may be, for example, a bond. R4 may be, for example, hydrocarbyl (e.g. C1 -6 alkyl, C2-6 alkenyl or carbocyclyl) or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In embodiments, Z is a bond and R4 is selected from carbocyclyl or heterocyclyl.
Where R4 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. For example, R4 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In a particular embodiment, R4 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R7. In embodiments, R4 is phenyl or cyclohexyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In this case, the or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a particular embodiment, R4 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. Of mention are compounds in which R1 is phenyl.
Where R4 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The heterocyclyl group may be
monocyclic or bicyclic, usually monocyclic. In particular, R4 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoindolyl, 3/-/-indolyl, indolyl, benzimidazolyl, cumaryl, indazolyl, triazolyl, tetrazolyl, purinyl, 4/-/-quinolizinyl, isoquinolyl, quinolyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl, octahydroisoquinolyl, dibenzofuranyl, benzothiophenyl, dibenzothiophenyl, phthalazinyl, naphthyridinyl, quinoxalyl, quinazolinyl, quinazolinyl, cinnolinyl, pteridinyl, carbazolyl, β- carbolinyl, phenanthridinyl, acridinyl, perimidinyl, phenanthrolinyl, furazanyl, phenazinyl, phenothiazinyl, phenoxazinyl, chromenyl, isochromanyl and chromanyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a further embodiment, R4 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a particular embodiment, R4 is cyclohexyl, cyclopropyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
With regard to formulae (III), (V) and (VII), R12 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. In embodiments, n is 0, 1 or 2. In a particular embodiment, n is 0.
With regard to formulae (V) and (VII), -Y1 -Y2- may be, for example, -N(R5)C(O)- or -C(O)N(R5)-. R5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R5 is hydrogen or C1-6 alkyl (e.g. C1 , C2, C3 or C4 alkyl).
With regard to formulae (Vl) and (VII), R5 may be, for example, selected from hydrogen, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R5 is hydrogen or C1^ alkyl (e.g. C1 , C2, C3 or C4 alkyl).
With regard to each of formulae (II) to (VII), R3 may be, for example, selected from hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
In one embodiment, R3 is C1-6 alkyl optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R3 is C1 , C2, C3 or C4 alkyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. Of particular mention are compounds in which R3 is trifluoromethyl.
In another embodiment, R3 is carbocyclyl (e.g. cycloalkyl or aryl) or heterocyclyl (e.g. heterocycloalkyl or heteroaryl), either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
Where R3 is carbocyclyl, it may be, for example, cycloalkyl or aryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. For example, R3 may be cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl or naphthyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In a particular embodiment, R3 is aryl, in particular phenyl or naphthyl, and is optionally substituted with 1 , 2, 3, 4 or 5 R7. In embodiments, R3 is phenyl or cyclohexyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. In this case, the or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a particular embodiment, R3 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, sec- butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms. Of mention are compounds in which R3 is phenyl.
Where R3 is heterocyclyl, it may be, for example, heterocycloalkyl or heteroaryl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. The heterocyclyl group may be monocyclic or bicyclic, usually monocyclic. In particular, R3 may be selected from oxiranyl, azirinyl, 1 ,2-oxathiolanyl, imidazolyl, thienyl, furyl, tetrahydrofuryl, pyranyl, thiopyranyl, thianthrenyl, isobenzofuranyl, benzofuranyl, chromenyl, 2/-/-pyrrolyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, benzimidazolyl, pyrazolyl, pyrazinyl, pyrazolidinyl, thiazolyl, isothiazolyl, dithiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrimidinyl, piperidyl, piperazinyl, pyridazinyl, morpholinyl, thiomorpholinyl, indolizinyl, isoindolyl, 3/-/-indolyl, indolyl, benzimidazolyl, cumaryl, indazolyl, triazolyl, tetrazolyl, purinyl, 4/-/-quinolizinyl, isoquinolyl, quinolyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl, octahydroisoquinolyl, dibenzofuranyl, benzothiophenyl, dibenzothiophenyl, phthalazinyl, naphthyridinyl, quinoxalyl, quinazolinyl, quinazolinyl, cinnolinyl, pteridinyl, carbazolyl, β- carbolinyl, phenanthridinyl, acridinyl, perimidinyl, phenanthrolinyl, furazanyl, phenazinyl,
phenothiazinyl, phenoxazinyl, chromenyl, isochromanyl and chromanyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
In a further embodiment, R3 is heteroaryl (often monocyclic) optionally substituted with 1 , 2, 3, 4 or 5 R7. The or each R7 may be, for example, hydroxy, halogen (for example, chlorine or fluorine); C1 , C2, C3 or C4 alkyl, for example methyl, ethyl, propyl, isopropyl, n- butyl, sec-butyl or tert-butyl, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms, an example being trifluoromethyl; or C1 , C2, C3 or C4 alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy, butoxy, tert-butoxy, any of which is optionally substituted with 1 , 2, 3 or 4 halogen (e.g. fluorine or chlorine) atoms.
In a further embodiment, R3 is selected from C1-6 alkyl, aryl and heteroaryl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. Of mention are compounds in which R3 is methyl, phenyl, furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
Of mention are compounds, especially those of formula (VII) in which R3 is selected from C1-e alkyl (e.g. methyl) or phenyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7. Of particular mention are compounds of formula (VII) in which R3 is trifluoromethyl or phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
Also of mention are compounds in which -Y-R3 is -N(R8)R9, for example amino.
Examples of compounds of the invention include those shown below. It will of course be appreciated that, where appropriate, each compound may be in the form of the free compound, an acid or base addition salt, or a prodrug.
Compounds of the invention may be in the form of pharmaceutically acceptable salts. The pharmaceutically acceptable salts of the present disclosure can be synthesized from the parent compound which contains a basic or acidic moiety by conventional chemical methods. Generally, such salts can be prepared by reacting the free acid or base forms of these compounds with a stoichiometric amount of the appropriate base or acid in water or in an organic solvent, or in a mixture of the two; generally, nonaqueous media like ether, ethyl acetate, ethanol, isopropanol, or acetonitrile are preferred. Lists of suitable salts may be found in Remington's Pharmaceutical Sciences, 17th ed., Mack Publishing Company, Easton, Pa., US, 1985, p. 1418, the disclosure of which is hereby incorporated by reference; see also Stahl et al, Eds, "Handbook of Pharmaceutical Salts Properties Selection and Use", Verlag Helvetica Chimica Acta and Wiley- VCH, 2002.
The invention thus includes pharmaceutically-acceptable salts of the disclosed compounds wherein the parent compound is modified by making acid or base salts thereof, for example the conventional non-toxic salts or the quaternary ammonium salts which are formed, e.g. from inorganic or organic acids or bases. Examples of such acid addition salts include acetate, adipate, alginate, aspartate, benzoate, benzenesulfonate, bisulfate, butyrate, citrate, camphorate, camphorsulfonate, cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, fumarate, glucoheptanoate, glycerophosphate, hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethanesulfonate, lactate, maleate, methanesulfonate, 2- naphthalenesulfonate, nicotinate, oxalate, pamoate, pectinate, persulfate, 3- phenylpropionate, picrate, pivalate, propionate, succinate, tartrate, thiocyanate, tosylate, and undecanoate. Base salts include ammonium salts, alkali metal salts such as sodium and potassium salts, alkaline earth metal salts such as calcium and magnesium salts, salts with organic bases such as dicyclohexylamine salts, N-methyl-D-glucamine, and salts with amino acids such as arginine, lysine, and so forth. Also, the basic nitrogen-containing groups may be quaternized with such agents as lower alkyl halides, such as methyl, ethyl, propyl, and butyl chloride, bromides and iodides; dialkyl sulfates like dimethyl, diethyl, dibutyl; and diamyl sulfates, long chain halides such as decyl, lauryl, myristyl and stearyl chlorides, bromides and iodides, aralkyl halides like benzyl and phenethyl bromides and others.
The invention includes prodrugs for the active pharmaceutical species of the invention, for example in which one or more functional groups are protected or derivatised but can be converted in vivo to the functional group, as in the case of esters of carboxylic acids
convertible in vivo to the free acid, or in the case of protected amines, to the free amino group. The term "prodrug," as used herein, represents in particular compounds which are rapidly transformed in vivo to the parent compound, for example, by hydrolysis in blood. A thorough discussion is provided in T. Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, Vol. 14 of the A.C.S. Symposium Series, Edward B. Roche, ed., Bioreversible Carriers in Drug Design, American Pharmaceutical Association and Pergamon Press, 1987; H Bundgaard, ed, Design of Prodrugs, Elsevier, 1985; and Judkins, et al. Synthetic Communications, 26(23), 4351 -4367 (1996), each of which is incorporated herein by reference.
Prodrugs therefore include drugs having a functional group which has been transformed into a reversible derivative thereof. Typically, such prodrugs are transformed to the active drug by hydrolysis. Examples of such groups include carboxylic groups (reversible derivatives including esters, e.g. acyloxyalkyl esters and amides), alcohol groups (reversible derivatives including sulfates, phosphates and carboxylic acid esters), amine groups (reversible derivatives including amides, carbamates, imines and enamines) and carbonyl groups, e.g. aldehyde and ketone groups (reversible derivatives including imines, oximes, acetals/ketals, enol esters, oxazolidines and thiazoxolidines).
Prodrugs also include compounds convertible to the active drug by an oxidative or reductive reaction. As examples of oxidative activation may be mentioned N- and O- dealkylation, oxidative deamination, N-oxidation and epoxidation. As examples of reductive activation may be mentioned azo reduction, sulfoxide reduction, disulfide reduction, bioreductive alkylation and nitro reduction.
Also to be mentioned as metabolic activations of prodrugs are nucleotide activation, phosphorylation activation and decarboxylation activation. For additional information, see "The Organic Chemistry of Drug Design and Drug Action", R B Silverman (particularly Chapter 8, pages 497 to 546), incorporated herein by reference.
The use of protecting groups is fully described in 'Protective Groups in Organic Chemistry', edited by J W F McOmie, Plenum Press (1973), and 'Protective Groups in Organic Synthesis' , 2nd edition, T W Greene & P G M Wutz, Wiley-lnterscience (1991 ).
Thus, it will be appreciated by those skilled in the art that, although protected derivatives of compounds of the disclosure may not possess pharmacological activity as such, they
may be administered, for example parenterally or orally, and thereafter metabolised in the body to form compounds of the invention which are pharmacologically active. Such derivatives are therefore examples of "prodrugs". All prodrugs of the described compounds are included within the scope of the disclosure.
Some groups mentioned herein (especially those containing heteroatoms and conjugated bonds) may exist in tautomeric forms and all these tautomers are included in the scope of the disclosure. More generally, many species may exist in equilibrium, as for example in the case of organic acids and their counterpart anions; a reference herein to a species accordingly includes reference to all equilibrium forms thereof.
The compounds of the disclosure may also contain one or more asymmetric carbon atoms and may therefore exhibit optical and/or diastereoisomerism. All diastereoisomers may be separated using conventional techniques, e.g. chromatography or fractional crystallisation. The various stereoisomers may be isolated by separation of a racemic or other mixture of the compounds using conventional, e.g. fractional crystallisation or HPLC, techniques. Alternatively the desired optical isomers may be made by reaction of the appropriate optically active starting materials under conditions which will not cause racemisation or epimerisation, or by derivatisation, for example with a homochiral acid followed by separation of the diastereomeric derivatives by conventional means (e.g. HPLC, chromatography over silica). All stereoisomers are included within the scope of the disclosure. Where a single enantiomer or diasteromer is disclosed, the disclosure also covers the other enantiomers or diastereomers, and also racemates; in this regard, particular reference is made to the specific compounds listed herein.
Geometric isomers may also exist in the compounds of the present disclosure. The present disclosure contemplates the various geometric isomers and mixtures thereof resulting from the arrangement of substituents around a carbon-carbon double bond and designates such isomers as of the Z or E configuration, wherein the term "Z" represents substituents on the same side of the carbon-carbon double bond and the term "E" represents substituents on opposite sides of the carbon-carbon double bond.
The disclosure therefore includes all variant forms of the defined compounds, for example any tautomer or any pharmaceutically acceptable salt, ester, acid or other variant of the defined compounds and their tautomers as well as substances which, upon administration, are capable of providing directly or indirectly a compound as
defined above or providing a species which is capable of existing in equilibrium with such a compound.
Synthesis
A compound of the invention may be prepared according to the processes described herein. It will be understood that these processes are solely for the purpose of illustrating the invention and should not be construed as limiting. A process utilising similar or analogous reagents and/or conditions known to one skilled in the art may also be used to obtain a compound of the invention.
Any mixtures of final products or intermediates obtained can be separated on the basis of the physico-chemical differences of the constituents, in a known manner, into the pure final products or intermediates, for example by chromatography, distillation, fractional crystallisation, or by the formation of a salt if appropriate or possible under the circumstances.
Administration & Pharmaceutical Formulations
The compounds of the invention will normally be administered orally, intravenously, subcutaneously, buccally, rectally, dermally, nasally, tracheally, bronchially, by any other parenteral route, as an oral or nasal spray or via inhalation, The compounds may be administered in the form of pharmaceutical preparations comprising prodrug or active compound either as a free compound or, for example, a pharmaceutically acceptable non-toxic organic or inorganic acid or base addition salt, in a pharmaceutically acceptable dosage form. Depending upon the disorder and patient to be treated and the route of administration, the compositions may be administered at varying doses.
Typically, therefore, the pharmaceutical compounds of the invention may be administered orally or parenterally ("parenterally" as used herein, refers to modes of administration which include intravenous, intramuscular, intraperitoneal, intrasternal, subcutaneous and intraarticular injection and infusion) to a host. In the case of larger animals, such as humans, the compounds may be administered alone or as compositions in combination with pharmaceutically acceptable diluents, excipients or carriers.
Actual dosage levels of active ingredients in the pharmaceutical compositions of this invention may be varied so as to obtain an amount of the active compound(s) that is effective to achieve the desired therapeutic response for a particular patient, compositions, and mode of administration. The selected dosage level will depend upon the activity of the particular compound, the route of administration, the severity of the condition being treated and the condition and prior medical history of the patient being treated. However, it is within the skill of the art to start doses of the compound at levels lower than required for to achieve the desired therapeutic effect and to gradually increase the dosage until the desired effect is achieved.
In certain embodiments, an appropriate dosage level will generally be about 0.01 to 500 mg per kg patient body weight per day which can be administered in single or multiple doses. In a particular embodiment, the dosage level is about 0.1 to about 250 mg/kg per day; more preferably about 0.5 to about 100 mg/kg per day. A suitable dosage level may be about 0.01 to 250 mg/kg per day, about 0.05 to 100 mg/kg per day, or about 0.1 to 50 mg/kg per day. Within this range the dosage may be 0.05 to 0.5, 0.5 to 5 or 5 to 50 mg/kg per day. For oral administration, the compositions may be provided in the form of tablets containing 1 .0 to 1000 milligrams of the active ingredient, particularly 1 .0, 5.0, 10.0, 15.0, 20.0, 25.0, 50.0, 75.0, 100.0, 150.0, 200.0, 250.0, 300.0, 400.0, 500.0, 600.0, 750.0, 800.0, 900.0 and 1000.0 milligrams of the active ingredient for the symptomatic adjustment of the dosage to the patient to be treated. The compounds may be administered on a regimen of 1 to 4 times per day, e.g. once or twice per day. The dosage regimen may be adjusted to provide the optimal therapeutic response.
According to a further aspect of the invention there is thus provided a pharmaceutical composition including a compound of the invention, in admixture with a pharmaceutically acceptable adjuvant, diluent or carrier.
Pharmaceutical compositions of this invention for parenteral injection suitably comprise pharmaceutically acceptable sterile aqueous or nonaqueous solutions, dispersions, suspensions or emulsions as well as sterile powders for reconstitution into sterile injectable solutions or dispersions just prior to use. Examples of suitable aqueous and nonaqueous carriers, diluents, solvents or vehicles include water, ethanol, polyols (such as glycerol, propylene glycol, polyethylene glycol and the like), and suitable mixtures thereof, vegetable oils (such as olive oil) and injectable organic esters such as ethyl oleate. Proper fluidity can be maintained, for example, by the use of coating materials
such as lecithin, by the maintenance of the required particle size in the case of dispersions and by the use of surfactants.
These compositions may also contain adjuvants such as preservative, wetting agents, emulsifying agents and dispersing agents. Prevention of the action of microorganisms may be ensured by the inclusion of various antibacterial and antifungal agents, for example, paraben, chlorobutanol or phenol sorbic acid. It may also be desirable to include isotonic agents such as sugars or sodium chloride, for example. Prolonged absorption of the injectable pharmaceutical form may be brought about by the inclusion of agents (for example aluminum monostearate and gelatin) which delay absorption.
In some cases, in order to prolong the effect of the drug, it is desirable to slow the absorption of the drug from subcutaneous or intramuscular injection. This may be accomplished by the use of a liquid suspension of crystalline or amorphous material with poor water solubility. The rate of absorption of the drug then depends upon its rate of dissolution which, in turn, may depend upon crystal size and crystalline form. Alternatively, delayed absorption of a parenterally administered drug form is accomplished by dissolving or suspending the drug in an oil vehicle.
Injectable depot forms are suitably made by forming microencapsule matrices of the drug in biodegradable polymers, for example polylactide-polyglycolide. Depending upon the ratio of drug to polymer and the nature of the particular polymer employed, the rate of drug release can be controlled. Examples of other biodegradable polymers include poly(orthoesters) and poly(anhydrides). Depot injectable formulations may also prepared by entrapping the drug in liposomes or microemulsions which are compatible with body tissues. The injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable media just prior to use.
Solid dosage forms for oral administration include capsules, tablets, pills, powders and granules. In such solid dosage forms, the active compound is typically mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or one or more: a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol and silicic acid; b) binders such as carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidone, sucrose and acacia; c)
humectants such as glycerol; d) disintegrating agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates and sodium carbonate; e) solution retarding agents such as paraffin; f) absorption accelerators such as quaternary ammonium compounds; g) wetting agents such as cetyl alcohol and glycerol monostearate; h) absorbents such as kaolin and bentonite clay and i) lubricants such as talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate and mixtures thereof. In the case of capsules, tablets and pills, the dosage form may also comprise buffering agents. Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycol, for example.
Suitably, oral formulations contain a dissolution aid. The dissolution aid is not limited as to its identity so long as it is pharmaceutically acceptable. Examples include nonionic surface active agents, such as sucrose fatty acid esters, glycerol fatty acid esters, sorbitan fatty acid esters (e.g. sorbitan trioleate), polyethylene glycol, polyoxyethylene hydrogenated castor oil, polyoxyethylene sorbitan fatty acid esters, polyoxyethylene alkyl ethers, methoxypolyoxyethylene alkyl ethers, polyoxyethylene alkylphenyl ethers, polyethylene glycol fatty acid esters, polyoxyethylene alkylamines, polyoxyethylene alkyl thioethers, polyoxyethylene polyoxypropylene copolymers, polyoxyethylene glycerol fatty acid esters, pentaerythritol fatty acid esters, propylene glycol monofatty acid esters, polyoxyethylene propylene glycol monofatty acid esters, polyoxyethylene sorbitol fatty acid esters, fatty acid alkylolamides, and alkylamine oxides; bile acid and salts thereof (e.g. chenodeoxycholic acid, cholic acid, deoxycholic acid, dehydrocholic acid and salts thereof, and glycine or taurine conjugate thereof); ionic surface active agents, such as sodium laurylsulfate, fatty acid soaps, alkylsulfonates, alkylphosphates, ether phosphates, fatty acid salts of basic amino acids; triethanolamine soap, and alkyl quaternary ammonium salts; and amphoteric surface active agents, such as betaines and aminocarboxylic acid salts.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally contain opacifying agents and may also be of a composition such that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, and/or in delayed fashion. Examples of embedding compositions include polymeric substances and waxes.
The active compounds may also be in micro-encapsulated form, if appropriate, with one or more of the above-mentioned excipients.
The active compounds may be in finely divided form, for example they may be micronised.
Liquid dosage forms for oral administration include pharmaceutically acceptable emulsions, solutions, suspensions, syrups and elixirs. In addition to the active compounds, the liquid dosage forms may contain inert diluents commonly used in the art such as water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1 ,3-butylene glycol, dimethyl formamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan and mixtures thereof. Besides inert diluents, the oral compositions may also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring and perfuming agents. Suspensions, in addition to the active compounds, may contain suspending agents such as ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar, and tragacanth and mixtures thereof.
Compositions for rectal or vaginal administration are preferably suppositories which can be prepared by mixing the compounds of this invention with suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at room temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
Compounds of the present invention can also be administered in the form of liposomes. As is known in the art, liposomes are generally derived from phospholipids or other lipid substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid crystals which are dispersed in an aqueous medium. Any non-toxic, physiologically acceptable and metabolisable lipid capable of forming liposomes can be used. The present compositions in liposome form can contain, in addition to a compound of the present invention, stabilisers, preservatives, excipients and the like. The preferred lipids are the phospholipids and the phosphatidyl cholines (lecithins), both natural and synthetic.
Methods to form liposomes are known in the art, for example, Prescott, Ed., Methods in Cell Biology, Volume XIV, Academic Press, New York, N.Y. (1976), p 33 et seq.
Dosage forms for topical administration of a compound of this invention include powders, sprays, ointments and inhalants. The active compound is mixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives, buffers or propellants which may be required. Ophthalmic formulations, eye ointments, powders and solutions are also contemplated as being within the scope of this invention.
Use
Compounds of the invention may be useful in the therapy of a variety of diseases and conditions. The subject of said therapy may be a human or an animal. In particular, compounds of the invention may be useful in the treatment or prevention of prion diseases. Prion diseases are often characterized by symptoms of dementia or cognitive impairment. The prion disease may be inherited, infectious or sporadic. Examples of prion disease include Creutzfeldt-Jakob disease, kuru, Gerstmann-Straussler-Sheinker disease, fatal familial insomnia and transmissible spongiform encephalopathies (TSEs). Examples of TSEs include bovine spongiform encephalopathy (BSE), scrapie, chronic wasting disease (e.g. in deer or elk) and transmissible mink encephalopathy (TME).
The compounds may also be useful in the regulation of stem cells. Thus, the invention also provides a method of regulating stem cell activity, comprising contacting one or more types of stem cells with a compound of the invention. Said contacting generally takes place under conditions such that activity is regulated. In one embodiment, said contacting takes place in vitro.
The following Examples illustrate the invention.
In the Examples, melting points were measured using a Bibby-Sterilin SMP10 melting point apparatus and are uncorrected. Accurate mass and nominal mass measurements were measured using a Waters-Micromass LCT electrospray mass spectrometer. Flash column chromatography was carried out using Fluorochem silica gel 60 A. All compounds were isolated in >95% purity unless otherwise stated (as determined by HPLC under two sets of conditions-HPLC 1 ; Luna 5 μ C18, 150 x 4.6 mm, 5-95% acetonitrile (0.1 % TFA) in water (0.1 % TFA) over 4 min, 1 mL min'1 , 20 μL injection,
detection at 256 nm, run time 10 min. HPLC 2; Altima HP 3 μ C18 EPS, 150 x 4.6 mm, 35-98% acetonitrile (0.1 % TFA) in water (0.1 % TFA) over 4 min, 1 .0 mL min'1 , 20 μL injection, detection at 256 nm, run time 1 1 min). All reagents were purchased directly from commercial sources and used as supplied.
Example 1 : Thiazoles
Thiazole compounds 1c to 1x were prepared. The structures of compounds 1c and 1e to 1x are shown below:
Compounds 1c and 1d were obtained as described in Thompson, M. J.; Heal, W.; Chen, B. Tetrahedron Lett. 2006, 47, 2361-2364. Full 1 H NMR. 13C NMR and IR spectral data for these compounds are given in the supplementary information section of that paper.
Compounds 1e to 1x were prepared from compound 1d, as shown in Scheme 1 :
1e-1x Scheme 1 . Reagents and conditions: (a) (COCI)2, DCM, room temp., 80 min; (b) 1d, pyridine, room temp., 18 h; (c) pyridine, DMAP, 1d, room temp., 18 h.
Acid chloride (0.33 mmol) was added to a solution of 1d (76 mg, 0.30 mmol) and DMAP (10 mg) in pyridine (3 mL). The reaction mixture was stirred at ambient temperature for 18 hours. All volatiles were removed under reduced pressure and the residue taken up in DCM (30 mL). This solution was washed thoroughly with 1 M HCI (4 x 30 mL) then sat. NaHCO3 (2 x 30 mL), dried over MgSO4, filtered and evaporated to dryness to provide the product. Where necessary, further purification was carried out by flash column chromatography on silica gel. In the case of 1g, 1 r, the relevant acid chloride was not available commercially. These amide derivatives were prepared from the carboxylic acids, through in situ formation of the acid chloride followed by reaction with amine 1d in pyridine.
N-(2,4-Diphenylthiazol-5-yl)-benzamide (1e).
Yield 74%; mp 181-183° C; m/z (ES); HRMS, 357 ([M+H]+); HRMS, found 357.1047 (C22H17N2OS, [M+H]+, requires 357.1062).
N-(2,4-Diphenylthiazol-5-yl)-4-fluorobenzamide (1f).
Yield 86%; mp 201-203° C; m/z (ES), 375 ([M+H]+); HRMS, found 375.0961 (C22H16FN2OS, [M+H]+, requires 375.0967).
N-(2,4-Diphenylthiazol-5-yl)-4-methoxybenzamide (1g).
A stirred suspension of p-anisic acid (44 mg, 0.29 mmol) in DCM (3.0 mL) was treated with oxalyl chloride (26.2 μL, 0.30 mmol) and DMF (40 μL), at which point effervescence was observed. A homogeneous solution was gradually obtained as the reaction took place. After 80 min the reaction mixture was concentrated under reduced pressure and dried further under high vacuum (with periodical warming) to yield the crystalline, crude acid chloride. 2,4-Diphenyl-5-aminothiazole (70 mg, 0.28 mmol), pyridine (3.0 mL) and DMAP (10 mg) were added, and the resultant orange solution stirred at ambient temperature for 18 hours. The pyridine was removed under vacuum and the residue taken up in DCM (40 mL) then washed with 1 M HCI (4 x 50 mL) followed by sat.
NaHCO3 (2 x 40 mL). The organic layer was evaporated and the residue purified by flash column chromatography on silica gel, eluted with 60-75-90% DCM-hexane, to provide 1e as a yellowish solid (13 mg, 12%). m/z (ES), 409 ([M+Na]+); HRMS, found
409.1006 (C23H18N2NaO2S, [M+Na]+, requires 409.0987).
N-(2,4-Diphenylthiazol-5-yl)-2-trifluoromethylbenzamide (1 h).
Yield 83%; mp 230-231 ° C (dec); m/z (ES), 425 ([M+H]+); HRMS, found 425.0938 (C23H16F3N2OS, [M+H]+, requires 425.0935).
N-(2,4-Diphenylthiazol-5-yl)-3-trifluoromethylbenzamide (1 i).
Yield 83%; mp 154-157° C (dec); m/z (ES), 425 ([M+H]+); HRMS, found 425.0926 (C23H16F3N2OS, [M+H]+, requires 425.0935).
N-(2,4-Diphenylthiazol-5-yl)-4-trifluoromethylbenzamide (1j).
Yield 79%; mp 201-203° C (dec); m/z (ES), 425 ([M+H]+); HRMS, found 425.0949 (C23H16F3N2OS, [M+H]+, requires 425.0935).
N-(2,4-Diphenylthiazol-5-yl)nicotinamide (1 k).
Yield 69%; mp 184-185° C (dec); m/z (ES), 358 ([M+H]+); HRMS, found 358.1000 (C21 H16N3OS, [M+H]+, requires 358.1014).
N-(2,4-Diphenylthiazol-5-yl)isonicotinamide (11).
Yield 75%; mp 214-215° C (dec); m/z (El), 357 (M+); HRMS, found 357.0922 (C21 H15N3OS, M+, requires 357.0936).
Quinoline-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1 m).
Yield 65%; mp 229-230° C; m/z (ES), 408 ([M+H]+); HRMS, found 408.1 155 (C25H18N3OS, [M+H]+, requires 408.1 171 ).
Furan-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1 n).
Yield 51 %; m/z (ES), 346 ([M+H]+); HRMS, found 347.0868 (C20H15N2O2S, [M+H]+, requires 347.0854).
5-Nitrofuran-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1 o).
Yield 9%; m/z (ES), 392 ([M+H]+); HRMS, found 392.0710 (C20H14N3O4S, [M+H]+, requires 392.0705).
lsoxazole-5-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1 p).
Yield 74%; mp 191 ° C (dec); m/z (ES), 348 ([M+H]+); HRMS, found 348.0790 (C19H14N3O2S, [M+H]+, requires 348.0807).
Thiophene-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1q).
Yield 82%; mp 161 ° C (dec); m/z (ES), 363 ([M+H]+); HRMS, found 363.0615 (C20H15N2OS2, [M+H]+, requires 363.0626).
5-Methylthiophene-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1 r).
5-Methylthiophene-2-carboxylic acid (213 mg, 1 .50 mmol) was suspended in DCM (5.0 mL) then oxalyl chloride (131 μL, 1 .50 mmol) and DMF-DCM (1 :9, 20 μL) was added. After stirring at ambient temperature for 1 h, a solution of 2,4-diphenyl-5-aminothiazole (252 mg, 1 .00 mmol) in pyridine (5.0 mL) was added and stirring of the resultant mixture continued at ambient temperature for 18 hours. The reaction mixture was diluted with DCM (60 mL) and washed successively with 1 M HCI (4 x 40 mL) and sat. NaHCO3 (2 x 40 mL). The organic layer was dried over MgSO4, filtered and evaporated giving crude material, which was purified by flash column chromatography on silica gel, eluted with 60-75% DCM-hexane, yielding 1 r as an off-white solid (252 mg, 67%). mp 1900 C; m/z (El), 376 (M+); HRMS, found 376.0695 (C21 H16N2OS2, M+, requires 376.0704).
Benzo[j8]thiophene-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1s).
Yield 83%; mp 209-210° C (dec); m/z (ES), 413 ([M+H]+); HRMS, found 413.0780 (C24H17N2OS2, [M+H]+, requires 413.0782).
Benzofuran-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1t).
Yield 86%; mp 196-197° C; m/z (ES), 397 ([M+H]+); HRMS, found 397.1005 (C24H17N2O2S, [M+H]+, requires 397.101 1 ).
Benzothiazole-2-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1 u).
Yield 27%; mp 227° C; m/z (ES), 414 ([M+H]+); HRMS, found 414.0748 (C23H16N3OS2, [M+H]+, requires 414.0735).
Benzo[/8]thiophene-5-carboxylic acid (2,4-diphenylthiazol-5-yl)amide (1v).
Yield 59%; mp 205-206° C (dec); m/z (ES), 413 ([M+H]+); HRMS, found 413.0788 (C24H17N2OS2, [M+H]+, requires 413.0782).
Cyclopropanecarboxylic acid (2,4-diphenylthiazol-5-yl)amide (1w).
Yield 61 %; mp 215-216° C; m/z (ES), 343 ([M+Na]+ HRMS, found 343.0893 (C19H16NaN2OS, [M+Na]+, requires 343.0881 ).
2-Propylpentanoic acid (2,4-diphenylthiazol-5-yl)amide (1x).
A stirred solution of 2-propylpentanoic acid (218 μL, 200 mg, 1 .39 mmol) in DCM (3 mL) was treated with oxalyl chloride (122 μL, 177 mg, 1 .39 mmol) and DMF-DCM (1 :4, 20 μL). After stirring for 1 h, the reaction mixture was evaporated to dryness and further dried under high vacuum to yield the crude acid chloride. 1d (70 mg, 0.28 mmol) in pyridine (3 mL) was added, followed by DMAP (10 mg), and the solution stirred at ambient temperature for 18 h. All volatiles were removed under reduced pressure and the residue taken up in DCM (40 mL). This solution was washed thoroughly with 1 M HCI (3 x 40 mL) then sat. NaHCO3 (3 x 40 mL) and evaporated. Purification by flash column chromatography on silica gel, eluted with 40-50% DCM-hexane, afforded the bis-amide product, 2,4-Diphenyl-5-[Λ/,Λ/-bis(2-propylpentanoyl)amino]thiazole (57 mg, 41 %), which crystallised as a pale orange solid on scratching. A portion of this material (47 mg) was suspended in 2-propanol (2.0 mL), then tetraethylammonium hydroxide, 20% w/v aqueous solution (146 μL, 2.0 mmol) was added. Dissolution of the starting material was observed as hydrolysis took place. After 2.5 hours the mixture was diluted with 1 M HCI (50 mL) and DCM (50 mL). The organic layer was separated, dried over MgSO4, filtered and evaporated. Purification by flash column chromatography on silica gel, eluted with 50-65% DCM-hexane, yielded 1x as a white, crystalline solid (26 mg, 74%). m/z (ES), 379 ([M+H]+); HRMS, found 379.1841 (C23H27N2OS, [M+H]+, requires 379.1844).
Example 2: Oxazoles
The following oxazole compounds were prepared:
Compound 2a was obtained as described in Thompson, M. J.; Heal, W.; Chen, B. Tetrahedron Lett. 2006, 47, 2361 -2364. Full 1 H NMR. 13C NMR and IR spectral data for this compound are given in the supplementary information section of that paper.
Compound 2c was prepared according to Scheme 2:
2c
Scheme 2
2-(Benzoylamino)-2-phenylglycinonitrile hydrochloride (3.89 g, 14.3 mmol) was partitioned between DCM (70 mL) and water (50 mL) and sodium carbonate added portionwise, with thorough mixing, until the aqueous layer was basic to universal indicator paper (pH 10). The organic layer was separated, dried over MgSO4 and filtered. The filtrate (-100 mL) was added slowly to a solution of triphosgene (4.23 g, 14.3 mmol) in DCM (30 mL). A precipitate began to appear during this addition. After stirring for 15 minutes, te/t-butanol (30 mL) was added cautiously. A homogeneous solution resulted after 2-3 minutes, at which point stirring was continued for an additional 5 minutes. The reaction was quenched by addition of 0.1 M K2CO3 (200 mL) and the organic layer
separated, washed with further 0.1 M K2CO3 (2 x 150 mL), dried over MgSO4. The crude material was purified by flash column chromatography on silica gel, eluted with 60-90- 100% DCM-hexane, giving 2c as an off-white foam (1 .07 g, 22%). mp 1500 C (dec); m/z (El"), 335 (M"); HRMS, found 335.1396 (C20H20N2O3, M", requires 335.1396).
Compounds 2d to 2w were synthesised according to Scheme 3:
2d-2w
Scheme 3. Reagents and conditions: (a) NaH, THF, room temp., 5 min, then RCOCI, THF, room temp., 10 min; (b) RCOCI, DMAP, DIPEA, room temp., 2 h; (c) TFAA (20%) in DCM, room temp., 18 h.
To a suspension of sodium hydride (60% dispersion in mineral oil, 0.65 mmol) in dry THF (3.0 mL) was added a solution of 2c (0.59 mmol) in dry THF (4.0 mL). The after 5 minutes the reaction mixture was clear and a solution of acid chloride (0.65 mmol) was added. The reaction was deemed complete by TLC after 10 minutes. The reaction was quenched by the cautious addition of water followed by the removal of all volatiles under reduced pressure. The aqueous mixture remaining was extracted thoroughly with DCM. The combined organic phases were combined, dried over MgSO4 and concentrated to a volume of 50 mL under reduced pressure. TFA was added to a concentration of 20% and the mixture stirred at ambient temperature for 18 hours. Removal of all volatiles under reduced pressure gave the crude product, which was recrystallised from hot n- hexane-ethyl acetate.
N-(2,4-Diphenyloxazol-5-yl)benzamide (2d).
Yield 72%; mp 196-197° C; m/z (ES), 341 ([M+H]+); HRMS, found 341 .1289 (C22H17N2O2, [M+H]+, requires 341 .1290).
N-(2,4-Diphenyloxazol-5-yl)-4-fluorobenzamide (2e).
Yield 81 %; mp 245-247° C; m/z (ES), 359 ([M+H]+); HRMS, found 359.1 188 (C22H16N2O2F, [M+H]+, requires 359.1 196).
N-(2,4-Diphenyloxazol-5-yl)-4-methoxybenzamide (2f) (DMAP procedure).
2,4-Diphenyl-5-Λ/-Boc-aminooxazole 2c (100 mg, 0.30 mmol) was dissolved in dry DCM (3.0 mL) then Λ/,Λ/-diisopropylethylamine (58 μL, 0.33 mmol) and DMAP (~7 mg, 20 mol %) were added followed by 2-furoyl chloride (33 μL, 0.33 mmol). The reaction was deemed complete by TLC after 150 minutes' stirring at ambient temperature. TFA (0.75 mL) was then added and stirring continued at ambient temperature for 18 h. DCM (40 mL) was added and the solution washed with water (2 x 40 mL) then dried over MgSO4. Further purification was achieved by flash column chromatography on silica gel, eluted with 0-1 % MeOH-DCM, to provide 2f as an off-white foam (76 mg, 77%). mp 224-225° C; m/z (ES), 371 ([M+H]+); HRMS, found 371 .1383 (C23H19N2O3, [M+H]+, requires 371 .1396).
N-(2,4-Diphenyloxazol-5-yl)-2-trifluoromethylbenzamide (2g).
Yield 71 %; mp 201-202° C; m/z (ES), 409 ([M+H]+); HRMS, found 409.1 151 (C23H16F3N2O2, [M+H]+, requires 409.1 164).
N-(2,4-Diphenyloxazol-5-yl)-3-trifluoromethylbenzamide (2h).
Yield 54%; mp 213-215° C; m/z (ES), 409 ([M+H]+); HRMS, found 409.1 180 (C23H16F3N2O2, [M+H]+, requires 409.1 164).
N-(2,4-Diphenyloxazol-5-yl)-4-trifluoromethylbenzamide (2i).
Yield 31 %; mp 220-221 ° C; m/z (ES), 409 ([M+H]+); HRMS, found 409.1 149 (C23H16F3N2O2, [M+H]+, requires 409.1 164).
N-(2,4-Diphenyloxazol-5-yl)nicotinamide (2j).
Yield 14%; mp 146-149° C; m/z (ES), 342 ([M+H]+); HRMS, found 342.1235 (C21 H16N3O2, [M+H]+, requires 342.1234).
N-(2,4-Diphenyloxazol-5-yl)isonicotinamide (2k).
Yield 59%; mp 201-204° C (dec); m/z (ES), 342 ([M+H]+); HRMS, found 342.1245 (C21 H16N3O2, [M+H]+, requires 342.1243).
Quinoline-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2I).
Yield 27%; mp 180° C (dec); m/z (ES), 392 ([M+H]+); HRMS, found 392.1384 (C25H18N3O2, [M+H]+, requires 392.1399).
Furan-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2m).
Yield 39%; mp 84-86° C; m/z (ES), 331 ([M+H]+); HRMS, found 331 .1093 (C20H15N2O3, [M+H]+, requires 331 .1083).
5-Nitrofuran-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2n).
Yield 19%; mp 184-186° C; m/z (ES), 376 ([M+H]+); HRMS, found 376.0934 (C20H14N3O5, [M+H]+, requires 376.0933).
lsoxazole-5-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2o).
Purification by flash column chromatography (n-hexane-ethyl acetate, 4:1 ) gave 2o (58.2 mg, 23%) mp 151-153° C; m/z (ES), 332 ([M+H]+); HRMS, found 332.1023 (C19H14N3O3, [M+H]+, requires 332.1035).
Thiophene-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2p).
Yield 79%; mp 194-196° C; m/z (ES), 347 ([M+H]+); HRMS, found 347.0851 (C20H15N2O2S, [M+H]+, requires 347.0854).
5-Methylthiophene-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2q).
Yield 56%; mp 194° C; m/z (ES), 361 ([M+H]+); HRMS, found 361 .1007 (C21 H17N2O2S, [M+H]+, requires 361 .101 1 ).
Benzo[/8]thiophene-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2r).
Yield 18%; mp 218-220° C; m/z (ES), 397 ([M+H]+); HRMS, found 397.1001 (C24H17N2O2S, [M+H]+, requires 397.101 1 ).
Benzofuran-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2s).
Yield 55%; mp 198-199° C; m/z (ES), 381 ([M+H]+); HRMS, found 381 .1250 (C24H17N2O3, [M+H]+, requires 381 .1239).
Benzothiazole-2-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2t).
Yield 47%; mp 195-196° C; m/z (ES), 398 ([M+H]+); HRMS, found 398.0977 (C23H16N3O2S, [M+H]+, requires 398.0963).
Benzo[β]thiophene-5-carboxylic acid (2,4-diphenyloxazol-5-yl)amide (2u).
Yield 65%; mp 195-200° C; m/z (ES), 397 ([M+H]+); HRMS, found 397.1029 (C24H17N2O2S, [M+H]+, requires 397.101 1 ).
Cyclopropanecarboxylic acid (2,4-diphenyloxazol-5-yl)amide (2v).
Yield 54%; mp 183-185° C; m/z (ES), 305 ([M+H]+); HRMS, found 305.1278 (C19H17N2O2, [M+H]+, requires 305.1290).
2-Propylpentanoic acid (2,4-diphenyloxazol-5-yl)amide (2w).
Yield 38%; mp 138° C; m/z (ES), 363 ([M+H]+); HRMS, found 363.2066 (C23H27N2O2, [M+H]+, requires 363.2073).
Example 3: Activity assay
The activity of various compounds of the invention towards prion proteins was assessed using surface plasmon resonance (SPR) and shed mediastinal blood (SMB) cells.
SPR screening methodology
Surface plasmon resonance (SPR) was carried out using a BIAcore 3000 (BIAcore, Uppsala, Sweden) equipped with a CM5 sensor chip (carboxymethylated dextran). The methodology was as reported previously (Touil, F.; Pratt, S. ; Mutter, R.; Chen, B., J. Pharm. Biomed. Anal. 2006, 40, 822-832). Interactions were measured with two forms of prion protein, full length human (huPrPc) and full length murine (moPrPc). Compounds were screened at 40 μM in running buffer (10 mM sodium phosphate, pH 7.4, 150 mM NaCI, 3.4 mM EDTA, 0.005% (v/v) surfactant P20) containing 6.5% DMSO. DMSO calibration using buffer samples containing 5.5-7.5% DMSO was carried out to correct for solvent effects. Compounds producing a response of more than 2.5 response units (RU) were considered to be binders.
SMB cell screening methodology
Compounds were screened for inhibition of PrPSc formation in SMB cells of mesodermal origin, the procedure used being based upon that reported by Rudyk, H.; Vasiljevic, S.; Hennion, R. M. ; Birkett, C. R.; Hope, J.; Gilbert, I. H., J. Gen. Virol. 2000, 81, 1 155-1 164. A persistently infected mouse cell line (SMB), cloned originally from scrapie infected mouse brain but of non-neuronal origin (Clarke, M. C ; Haig, D. A.., Nature 1970, 225, 100-101 ) was used. Cells were grown in tissue culture treated plastic dishes in Medium 199 (phenol red free), supplemented with 10% newborn calf serum (heat inactivated), 5% foetal calf serum (heat inactivated) and penicillin-streptomycin at 10 mg L"1 at 37 °C in an atmosphere of 5% CO2 in air at 95% relative humidity. Medium was changed every 3rd or 4th day, and every 7 days confluent cells were passaged using 0.05% trypsin and 0.002% EDTA at a split ratio of 4. To assess the effects of compounds cells were distributed into 96-well cluster plates at 3 x 104 cells per well and incubated for 24 h to allow for cell attachment. The compounds were diluted to 400 times the required concentration in DMSO as stock solutions then transferred, at a 20-fold dilution, into Hank's balanced salt solution. This solution was then transferred at a further 20-fold dilution into the cell medium. The cells were incubated with the compound-containing medium for 5 days.
After 5 days cell viability was assessed by the MTT assay following the standard protocol supplied with the reagent (Sigma). For dot blot analyses cells were extracted using lysis buffer (10 mM Tris-HCI [pH 7.6], 100 mM NaCI, 1 OmM EDTA, 0.5% v/v NP40 and 0.5% w/v sodium deoxycholate), and the content of the well loaded onto a nitrocellulose membrane (0.45 μm) under gentle vacuum at a total cellular protein concentration of
approximately 30-40 μg/well (determined by the Bradford assay following the protocol supplied with the reagent - Sigma). The membrane was air dried and subjected to 75 μg mL-1 proteinase K digestion for 1 h at 37 °C. The reaction was stopped with 1 mM phenylmethylsulfonyl fluoride (PMSF) in 20 mM Tris-HCI-buffered saline (TBS), the membrane washed extensively with TBS, and immersed in 1 .8 M guanidine thiocyanate in TBS for 10 min at room temperature. After further washing with TBS the membrane was blocked using 5% fat-free milk powder in phosphate buffered saline (PBS), processed with 0.2 μg mL-1 mouse monoclonal anti-PrP 6H4 (Prionics) and developed using an ECL kit (Amersham Pharmacia Biotech).
Each experiment was carried out in triplicate and an average value for PrPSc concentration calculated, relative to an untreated control, together with a standard deviation. Compounds were initially screened at 1 and 10 μM and were considered to be active if PrPSc levels were reduced to less than 70% of that of the untreated control after 5 days' exposure. Where an acceptable dose-response curve was observed, EC50 values were determined.
Results
Compounds of the invention were found to bind to PrPc and showed potent inhibition of PrPSc formation. In the case of exemplary compounds, their IC50S were found to range from 1 .5 to 20 μM.
Claims
1 . A compound of formula (I):
(I)
wherein
X is oxygen or sulphur;
Y is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7;
one of R1 and R2 is selected from carbocyclyl and heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7; and the other is -Z-R4;
R3 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
Z is a bond or a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)r, -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7;
R4 is selected from hydrogen; R7; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
R5 is selected from R6, -OR6, -C(O)R6, -C(O)OR6 and -S(O)1R6;
R6 is selected from hydrogen; hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7; each R7 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10;
R8 and R9 are each independently hydrogen or R10;
R10 is selected from hydrocarbyl and -(CH2)k-heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy, C1-6 alkyl and C1-6 alkoxy;
k is O, 1 , 2, 3, 4, 5 or 6; and
I is O, 1 or 2;
or a pharmaceutically acceptable salt or prodrug thereof;
for use in the treatment, prevention or delay of progression of a prion disease.
2. A compound according to claim 1 , wherein R1 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7; and R2 is -Z-R4.
3. A compound according to claim 2, wherein R1 is carbocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
4. A compound according to claim 3, wherein R1 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
5. A compound according to claim 4, wherein R1 is phenyl.
6. A compound according to any preceding claim, wherein Z is a bond.
7. A compound according to any preceding claim, wherein R4 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
8. A compound according to any of claims 2 to 5, wherein R2 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
9. A compound according to claim 8, wherein R2 is phenyl, furanyl, benzofuranyl, thiophenyl or isoxazole, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
10. A compound according to any preceding claim, wherein Y is a linker having 1 to 10 in-chain atoms and comprising one or more linkages selected from -O-, -C(O)-, -S(O)n -N(R5)- and hydrocarbylene optionally substituted with 1 , 2, 3, 4 or 5 R7.
1 1 . A compound according to claim 10, wherein Y comprises an amide linkage.
12. A compound according to claim 10 or claim 1 1 , wherein Y is selected from the following linkers:
-Y1-; -Y1 -Y2-; γ1 γ2 γ3 . _γ1_γ2_γ3_γ4_. and
-Y1 -Y2- Y3- Y4- Y5-;
wherein Y1 , Y2, Y3, Y4 and Y5 are each independently selected from -O-, -C(O)-,
-S(O)n -N(R5)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7.
13. A compound according to claim 12, wherein Y is -Y1- or -Y1 -Y2-.
14. A compound according to claim 13, wherein Y is -N(R5)C(O)- or -C(O)N(R5)-.
15. A compound according to claim 14, wherein R5 is hydrogen or C1-6 alkyl.
16. A compound according to claim 1 , which is of the formula (II):
each R11 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O),R8, -N(R8)R9,
-C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
17. A compound according to claim 16, which is of the formula
each R12 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
n is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
18. A compound according to claim 1 , which is of the formula (IV):
(IV)
wherein
Y1 and Y2 are each independently selected from -O-, -C(O)-, -S(O),-, -N(R5)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7.
each R11 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O),R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
19. A compound according to claim 18, which is of the formula (V):
(V) wherein
Y1 and Y2 are each independently selected from -0-, -C(O)-, -S(O),-, -N(R5)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7; R1 1 and R12 are each independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m and n are each independently O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
20. A compound according to claim 1 , which is of the formula (Vl):
(Vl)
wherein
Y1 and Y2 are each independently selected from -0-, -C(O)-, -S(O),-, -N(R5)- and hydrocarbylene (e.g. C1-5 alkylene) optionally substituted with 1 , 2, 3, 4 or 5 R7.
each R11 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O),R8, -N(R8)R9,
-C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
m is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
21 . A compound according to claim 20, which is of the formula (VII):
(VII)
each R12 is independently selected from halogen, trifluoromethyl, cyano, nitro, oxo, =NR8, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, -N(R8)R9, -C(O)N(R8)R9, -S(O),N(R8)R9 and R10; and
n is O, 1 , 2, 3, 4 or 5;
or a pharmaceutically acceptable salt or prodrug thereof.
22. A compound according to claim 20 or claim 21 , wherein R5 is hydrogen.
23. A compound according to any preceding claim, wherein R3 is selected from hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
24. A compound according to claim 23, wherein R3 is C1-6 alkyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
25. A compound according to claim 23, wherein R3 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
26. A compound according to claim 25, wherein R3 is carbocyclyl (e.g. aryl or cycloalkyl) optionally substituted with 1 , 2, 3, 4 or 5 R7.
27. A compound according to claim 26, wherein R3 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
28. A compound according to claim 25, wherein R3 is heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
29. A compound according to claim 28, wherein R3 is furanyl, benzofuranyl, thiophenyl or isoxazolyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7.
30. A compound according to any preceding claim, wherein X is oxygen.
31 . A compound according to any of claims 1 to 29, wherein X is sulphur.
32. A compound according to any preceding claim, wherein the disease is selected from Creutzfeldt-Jakob disease, kuru, Gerstmann-Straussler-Sheinker disease, fatal familial insomnia and transmissible spongiform encephalopathies (TSEs).
33. A compound according to claim 32, wherein the disease is selected from bovine spongiform encephalopathy (BSE), scrapie, chronic wasting disease and transmissible mink encephalopathy (TME).
34. A compound of claim 1 , independent of use, wherein:
R1 is carbocyclyl or heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 R7;
R2 is selected from cycloalkyl, cycloalkenyl, phenyl, furanyl, benzofuranyl, thiophenyl, isoxazolyl, quinolinyl, isoquinolinyl, quinoxazolinyl, benzothiazolyl and benzothiophenyl, any of which is optionally substituted with 1 , 2, 3, 4 or 5 R7;
Y is -N(R5)C(O)- or -C(O)N(R5)-; and R3 is selected from hydrogen, trifluoromethyl, -OR8, -C(O)R8, -C(O)OR8, -OC(O)R8, -S(O)1R8, amino, -C(O)N(R8)R9, -S(O),N(R8)R9, -N(R8)S(O),R8, hydrocarbyl optionally substituted with 1 , 2, 3, 4 or 5 R7; and -(CH2)k-heterocyclyl optionally substituted with 1 , 2, 3, 4 or 5 R7;
or -Y-R3 is -N(R8)R9; and
R10 is selected from hydrocarbyl and -(CH2)k-heterocyclyl, either of which is optionally substituted with 1 , 2, 3, 4 or 5 substituents independently selected from halogen, cyano, amino, hydroxy and C1-6 alkyl; with the proviso that the compound is not 2,4-diphenyloxazol-5-ylamine or a compound of one of the following formulae:
wherein, in each case, R is selected from phenyl, 4-methoxyphenyl, thiophen-2-yl, cyclohexyl.
35. A compound according to claim 34, wherein R1 is as defined in any of claims 2 to 5.
36. A compound according to claim 35, wherein R1 is phenyl optionally substituted with 1 , 2, 3, 4 or 5 R7.
37. A compound according to any of claims 34 to 36, wherein R3 is as defined in any of claims 23 to 29.
38. A compound selected from:
and pharmaceutically acceptable salts or prodrugs thereof:
39. A compound of any of claims 34 to 38, for therapeutic use.
40. A pharmaceutical formulation comprising a compound of any of claims 34 to 38.
41 . A formulation according to claim 40, which comprises a pharmaceutically acceptable carrier or excipient.
42. Use of a compound of formula (I) as defined in any of claims 1 to 31 , for the manufacture of a medicament for the treatment, prevention or delay of progression of a prion disease.
43. A method of treating, preventing or delaying the progression of a prion disease in a subject, comprising administering a therapeutically effective amount of a compound of any of claims 1 to 31 .
44. A method according to claim 39, wherein the disease is as defined in claim 32 or claim 33.
45. A method of regulating stem cell activity, which comprises contacting one or more stem cells with a compound of formula (I) as defined in any of claims 1 to 31 .
46. A method according to claim 45, wherein said contacting takes place in vitro.
47. Use of a compound of formula (I) as defined in any of claims 1 to 31 , for regulating stem cell activity.
48. Use according to claim 47, for regulating stem cell activity in vitro.
49. Use of a compound of formula (I) as defined in any of claims 1 to 31 , for the manufacture of a medicament for the treatment, prevention or delay of progression of cancer or a disease or condition of the central nervous system.
50. Use of a compound of formula (I) as defined in any of claims 1 to 31 , for the manufacture of a medicament for use in regenerative medicine.
51 . Use according to claim 49 or claim 50, wherein the compound is a compound of any of claims 34 to 38.
52. A compound of formula (I) as defined in any of claims 1 to 31 , for use in the treatment, prevention or delay of progression of cancer or a disease or condition of the central nervous system.
53. A compound of formula (I) as defined in any of claims 1 to 31 , for use in regenerative medicine.
54. A compound according to claim 52 or claim 53, wherein the compound is a compound of any of claims 34 to 38.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0701426A GB0701426D0 (en) | 2007-01-25 | 2007-01-25 | Compounds and their use |
| GB0701426.9 | 2007-01-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2008090382A1 true WO2008090382A1 (en) | 2008-07-31 |
Family
ID=37872765
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2008/050052 WO2008090382A1 (en) | 2007-01-25 | 2008-01-25 | Thiazole and oxazole derivatives for use in the treatment of prion diseases, cancer and conditions of the central nervous system as well as in the regulation of stem cells |
Country Status (2)
| Country | Link |
|---|---|
| GB (1) | GB0701426D0 (en) |
| WO (1) | WO2008090382A1 (en) |
Cited By (87)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102008062878A1 (en) | 2008-12-17 | 2010-06-24 | Aicuris Gmbh & Co. Kg | Substituted furancarboxamides and their use |
| DE102008062863A1 (en) | 2008-12-17 | 2010-06-24 | Aicuris Gmbh & Co. Kg | Substituted (thiophenyl-carbonyl) imidazolidinones and their use |
| WO2010139982A1 (en) * | 2009-06-02 | 2010-12-09 | The University Of Sheffield | Indole derivatives for the stimulation of stem cell proliferation |
| EP2410858A1 (en) * | 2009-03-23 | 2012-02-01 | Merck Sharp & Dohme Corp. | P2x3 receptor antagonists for treatment of pain |
| EP2427191A1 (en) * | 2009-05-05 | 2012-03-14 | Dow AgroSciences LLC | Pesticidal compositions |
| US8314089B2 (en) | 2008-03-17 | 2012-11-20 | Aicuris Gmbh & Co. Kg | Substituted pyrazolamides and their use |
| US8399682B2 (en) | 2008-03-17 | 2013-03-19 | Aicuris Gmbh & Co. Kg | Substituted (pyrazolylcarbonyl)imidazolidinones and their use |
| US8436001B2 (en) | 2010-04-07 | 2013-05-07 | F. Hoffmann-La Roche Ag | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| JP2013522376A (en) * | 2010-03-24 | 2013-06-13 | メディカル ユニバーシティー オブ サウス カロライナ | Compositions and methods for treating degenerative diseases |
| EP2603216A1 (en) * | 2010-08-11 | 2013-06-19 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| EP2606889A1 (en) * | 2011-12-21 | 2013-06-26 | Telik, Inc. | Substituted Thiazoles as VEGFR2 kinase Inhibitors |
| US8524751B2 (en) | 2009-03-09 | 2013-09-03 | GlaxoSmithKline Intellecutual Property Development | 4-oxadiazol-2-YL-indazoles as inhibitors of P13 kinases |
| US8536169B2 (en) | 2008-06-05 | 2013-09-17 | Glaxo Group Limited | Compounds |
| US8575162B2 (en) | 2009-04-30 | 2013-11-05 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| US8614206B2 (en) | 2011-09-27 | 2013-12-24 | F. Hoffmann-La Roche Ag | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| WO2013184476A3 (en) * | 2012-06-04 | 2014-01-30 | Dow Agrosciences Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| CN103596938A (en) * | 2011-05-23 | 2014-02-19 | 默克专利有限公司 | Thiazole derivatives |
| US8658635B2 (en) | 2008-06-05 | 2014-02-25 | Glaxosmithkline Intellectual Property Development Limited | Benzpyrazol derivatives as inhibitors of PI3 kinases |
| WO2013184480A3 (en) * | 2012-06-04 | 2014-02-27 | Dow Agrosciences Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| WO2013184475A3 (en) * | 2012-06-04 | 2014-02-27 | Dow Agrosciences Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| US8765746B2 (en) | 2010-10-13 | 2014-07-01 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8765743B2 (en) | 2008-06-05 | 2014-07-01 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| US8796271B2 (en) | 2010-08-11 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8796314B2 (en) | 2009-01-30 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8815271B2 (en) | 2010-11-03 | 2014-08-26 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| WO2014160197A1 (en) * | 2013-03-13 | 2014-10-02 | Musc Foundation For Research Development | Thiazole compounds, compositions and methods for treatment of degenerative diseases and disorders |
| US8901153B2 (en) | 2012-04-27 | 2014-12-02 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| WO2015031710A1 (en) * | 2013-08-29 | 2015-03-05 | Baylor College Of Medicine | Compositions and methods for the treatment of metabolic and body weight related disorders |
| US8993576B2 (en) | 2010-10-27 | 2015-03-31 | Glaxo Group Limited | 6-(1H-indol-4-yl)-4-(5-{[4-1-methylethyl)-1-piperazinyl]methyl}-1,3-oxazol-2-yl)-1H-indazole hemi succinate salt, polymorphs and pharmaceutical compositions thereof |
| US9024031B1 (en) | 2014-08-19 | 2015-05-05 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029555B1 (en) | 2014-07-31 | 2015-05-12 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029556B1 (en) | 2014-07-31 | 2015-05-12 | Dow Argosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029554B1 (en) | 2013-10-17 | 2015-05-12 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9029411B2 (en) | 2008-01-25 | 2015-05-12 | Millennium Pharmaceuticals, Inc. | Thiophenes and uses thereof |
| US9044017B2 (en) | 2013-10-17 | 2015-06-02 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9062038B2 (en) | 2010-08-11 | 2015-06-23 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US9085564B2 (en) | 2013-10-17 | 2015-07-21 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9085552B1 (en) | 2014-09-12 | 2015-07-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9090601B2 (en) | 2009-01-30 | 2015-07-28 | Millennium Pharmaceuticals, Inc. | Thiazole derivatives |
| US9102655B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9102654B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9108946B2 (en) | 2013-10-17 | 2015-08-18 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9137998B2 (en) | 2013-10-22 | 2015-09-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9139589B2 (en) | 2009-01-30 | 2015-09-22 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US9144241B2 (en) | 2013-10-22 | 2015-09-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9149040B2 (en) | 2013-10-22 | 2015-10-06 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9174962B2 (en) | 2013-10-17 | 2015-11-03 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9199964B1 (en) | 2014-07-31 | 2015-12-01 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9282739B2 (en) | 2012-04-27 | 2016-03-15 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9282740B2 (en) | 2013-10-22 | 2016-03-15 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9295258B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9295260B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| CN105541753A (en) * | 2015-12-17 | 2016-05-04 | 浙江工业大学 | Carboxamide derivative containing thiazole ring and preparation method and application thereof |
| US9445597B2 (en) | 2013-10-22 | 2016-09-20 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9474276B2 (en) | 2013-10-22 | 2016-10-25 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9491944B2 (en) | 2013-10-22 | 2016-11-15 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9497967B2 (en) | 2013-10-22 | 2016-11-22 | Doe AgroSciences LLC | Synergistic pesticidal compositions and related methods |
| US9497966B2 (en) | 2013-10-22 | 2016-11-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9549560B2 (en) | 2013-10-22 | 2017-01-24 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9655365B2 (en) | 2011-10-26 | 2017-05-23 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9708288B2 (en) | 2012-04-27 | 2017-07-18 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9713613B2 (en) | 2007-02-02 | 2017-07-25 | Motonari Uesugi | Methods and compositions for the treatment of cancer and related hyperproliferative disorders |
| US9771379B2 (en) | 2015-09-24 | 2017-09-26 | Pfizer Inc. | N-(2-(2-amino-6-substituted-4,4a,5,6-tetrahydropyrano[3,4-d][1,3]OXAZIN-8a(8H)-yl)-thiazol-4-yl) amides |
| US9788545B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9788546B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9801383B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9801376B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9808008B2 (en) | 2013-10-22 | 2017-11-07 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9975886B1 (en) | 2017-01-23 | 2018-05-22 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US10100033B2 (en) | 2016-12-29 | 2018-10-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US10233155B2 (en) | 2016-12-29 | 2019-03-19 | Dow Agrosciences Llc | Processes for the preparation of pesticide compounds |
| US10328064B2 (en) | 2014-12-23 | 2019-06-25 | Fgh Biotech, Inc. | Compositions of fatostatin based heterocyclic compounds and uses thereof |
| US10538487B2 (en) | 2015-02-16 | 2020-01-21 | The University Of Queensland | Sulfonylureas and related compounds and use of same |
| US10774064B2 (en) | 2016-06-02 | 2020-09-15 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| WO2021214019A1 (en) | 2020-04-24 | 2021-10-28 | Bayer Aktiengesellschaft | Substituted aminothiazoles as dgkzeta inhibitors for immune activation |
| US11339142B2 (en) | 2016-09-07 | 2022-05-24 | Fgh Biotech, Inc. | Di-substituted pyrazole compounds for the treatment of diseases |
| US11370776B2 (en) | 2017-07-07 | 2022-06-28 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US11465992B2 (en) | 2017-07-07 | 2022-10-11 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11497738B2 (en) | 2016-04-29 | 2022-11-15 | Fgh Biotech, Inc. | Di-substituted pyrazole compounds for the treatment of diseases |
| US11518739B2 (en) | 2017-08-15 | 2022-12-06 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11542255B2 (en) | 2017-08-15 | 2023-01-03 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US11613542B2 (en) | 2017-08-15 | 2023-03-28 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US11773058B2 (en) | 2017-08-15 | 2023-10-03 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11905252B2 (en) | 2018-03-02 | 2024-02-20 | Inflazome Limited | Compounds |
| US11926600B2 (en) | 2017-08-15 | 2024-03-12 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US11993586B2 (en) | 2018-10-22 | 2024-05-28 | Novartis Ag | Crystalline forms of potassium channel modulators |
| US12012392B2 (en) | 2017-11-09 | 2024-06-18 | Inflazome Limited | Sulfonamide carboxamide compounds |
Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4113731A (en) * | 1975-08-27 | 1978-09-12 | Gruppo Lepetit S.P.A. | Fused isoquinoline derivatives |
| EP0761658A1 (en) * | 1995-09-11 | 1997-03-12 | Nihon Nohyaku Co., Ltd. | Azole-phenyl urea derivatives as ACAT inhibitors and their production |
| WO2000002871A1 (en) * | 1998-07-10 | 2000-01-20 | Merck & Co., Inc. | Novel angiogenesis inhibitors |
| WO2001004116A2 (en) * | 1999-07-09 | 2001-01-18 | Ortho-Mcneil Pharmaceutical, Inc. | Neurotrophic pyrrolidines and piperidines, and related compositions containing them |
| WO2001074811A2 (en) * | 2000-03-30 | 2001-10-11 | Takeda Chemical Industries, Ltd. | Substituted 1,3-thiazole compounds, their production and use |
| EP1205478A1 (en) * | 1999-08-06 | 2002-05-15 | Takeda Chemical Industries, Ltd. | p38MAP KINASE INHIBITORS |
| EP1256578A1 (en) * | 2001-05-11 | 2002-11-13 | Pfizer Products Inc. | Thiazole derivatives and their use as cdk inhibitors |
| US20030191167A1 (en) * | 2002-03-28 | 2003-10-09 | Fabrice Vergne | (4,2-Disubstituted-thiazol-5-yl)amine compounds as PDE7 inhibitors |
| WO2004033439A1 (en) * | 2002-10-09 | 2004-04-22 | Pfizer Products Inc. | Thiazole compounds for the treatment of neurodegenerative disorders |
| WO2005026137A2 (en) * | 2003-09-06 | 2005-03-24 | Vertex Pharmaceuticals Incorporated | Modulators of atp-binding cassette transporters |
| WO2005040139A2 (en) * | 2003-10-23 | 2005-05-06 | Ab Science | 2-aminoaryloxazole compounds as tyrosine kinase inhibitors |
| WO2005049018A1 (en) * | 2003-11-14 | 2005-06-02 | Vertex Pharmaceuticals Incorporated | Thiazoles and oxazoles useful as modulators of atp-binding cassette transporters |
| WO2005073225A1 (en) * | 2004-01-30 | 2005-08-11 | Ab Science | 2-(3-substituted-aryl)amino-4-aryl-thiazoles as tyrosine kinase inhibitors |
| WO2005097114A2 (en) * | 2004-04-01 | 2005-10-20 | Pfizer Products Inc. | Thiazole sulfonamide compounds for the treatment of neurodegenerative disorders |
| EP1700856A1 (en) * | 2003-12-26 | 2006-09-13 | Kyowa Hakko Kogyo Co., Ltd. | Thiazole derivative |
| WO2006114274A1 (en) * | 2005-04-26 | 2006-11-02 | Glaxo Group Limited | Oxazole and thiazole compounds and their use in the treatment of pge2 mediated disorders |
| WO2006137658A1 (en) * | 2005-06-20 | 2006-12-28 | Dongbu Hitek Co., Ltd. | New substituted 1,3-thiazole derivatives or pharmaceutically acceptable salts thereof having immunosuppression and inflammation inhibitory acitivity, intermediate compounds or pharmaceutically acceptable salts thereof, a process for the preparation thereof, and pharmaceutical composition comprising the same |
| EP1845081A1 (en) * | 2005-02-01 | 2007-10-17 | Takeda Pharmaceutical Company Limited | Amide compound |
-
2007
- 2007-01-25 GB GB0701426A patent/GB0701426D0/en not_active Ceased
-
2008
- 2008-01-25 WO PCT/GB2008/050052 patent/WO2008090382A1/en active Application Filing
Patent Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4113731A (en) * | 1975-08-27 | 1978-09-12 | Gruppo Lepetit S.P.A. | Fused isoquinoline derivatives |
| EP0761658A1 (en) * | 1995-09-11 | 1997-03-12 | Nihon Nohyaku Co., Ltd. | Azole-phenyl urea derivatives as ACAT inhibitors and their production |
| WO2000002871A1 (en) * | 1998-07-10 | 2000-01-20 | Merck & Co., Inc. | Novel angiogenesis inhibitors |
| WO2001004116A2 (en) * | 1999-07-09 | 2001-01-18 | Ortho-Mcneil Pharmaceutical, Inc. | Neurotrophic pyrrolidines and piperidines, and related compositions containing them |
| EP1205478A1 (en) * | 1999-08-06 | 2002-05-15 | Takeda Chemical Industries, Ltd. | p38MAP KINASE INHIBITORS |
| WO2001074811A2 (en) * | 2000-03-30 | 2001-10-11 | Takeda Chemical Industries, Ltd. | Substituted 1,3-thiazole compounds, their production and use |
| EP1256578A1 (en) * | 2001-05-11 | 2002-11-13 | Pfizer Products Inc. | Thiazole derivatives and their use as cdk inhibitors |
| US20030191167A1 (en) * | 2002-03-28 | 2003-10-09 | Fabrice Vergne | (4,2-Disubstituted-thiazol-5-yl)amine compounds as PDE7 inhibitors |
| WO2004033439A1 (en) * | 2002-10-09 | 2004-04-22 | Pfizer Products Inc. | Thiazole compounds for the treatment of neurodegenerative disorders |
| WO2005026137A2 (en) * | 2003-09-06 | 2005-03-24 | Vertex Pharmaceuticals Incorporated | Modulators of atp-binding cassette transporters |
| WO2005040139A2 (en) * | 2003-10-23 | 2005-05-06 | Ab Science | 2-aminoaryloxazole compounds as tyrosine kinase inhibitors |
| WO2005049018A1 (en) * | 2003-11-14 | 2005-06-02 | Vertex Pharmaceuticals Incorporated | Thiazoles and oxazoles useful as modulators of atp-binding cassette transporters |
| EP1700856A1 (en) * | 2003-12-26 | 2006-09-13 | Kyowa Hakko Kogyo Co., Ltd. | Thiazole derivative |
| WO2005073225A1 (en) * | 2004-01-30 | 2005-08-11 | Ab Science | 2-(3-substituted-aryl)amino-4-aryl-thiazoles as tyrosine kinase inhibitors |
| WO2005097114A2 (en) * | 2004-04-01 | 2005-10-20 | Pfizer Products Inc. | Thiazole sulfonamide compounds for the treatment of neurodegenerative disorders |
| EP1845081A1 (en) * | 2005-02-01 | 2007-10-17 | Takeda Pharmaceutical Company Limited | Amide compound |
| WO2006114274A1 (en) * | 2005-04-26 | 2006-11-02 | Glaxo Group Limited | Oxazole and thiazole compounds and their use in the treatment of pge2 mediated disorders |
| WO2006137658A1 (en) * | 2005-06-20 | 2006-12-28 | Dongbu Hitek Co., Ltd. | New substituted 1,3-thiazole derivatives or pharmaceutically acceptable salts thereof having immunosuppression and inflammation inhibitory acitivity, intermediate compounds or pharmaceutically acceptable salts thereof, a process for the preparation thereof, and pharmaceutical composition comprising the same |
Non-Patent Citations (6)
| Title |
|---|
| DATABASE CA [online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 2006, BALYA, A. G. ET AL: "Convenient method for addition of azolyl fragments to the 5 position of the thiazole ring", XP002477298, retrieved from STN Database accession no. 2007:165324 * |
| FRALEY ET AL, BIOORGANIC MEDICINAL CHEMISTRY LETTERS (EN), vol. 13, no. 18, 2003, pages 2973 - 2976, XP002477266 * |
| HEAL ET AL, JOURNAL OF MEDICINAL CHEMISTRY, vol. 50, 17 February 2007 (2007-02-17), pages 1347 - 1353, XP002477267 * |
| KATRIZKY ET AL: "Convenient Synthesis of Novel N-Substituted-5-aminothiazole Derivatives", JOURNAL OF ORGANIC CHEMISTRY (EN), vol. 65, no. 23, 2000, pages 8077 - 8079, XP002477265 * |
| THOMPSON ET AL: "Synthesis of 5-aminothiazoles as building blocks for library synthesis", TETRAHEDRON LETTERS, vol. 47, 17 February 2006 (2006-02-17), pages 2361 - 2364, XP002477306 * |
| ZHURNAL ORGANICHNOI TA FARMATSEVTICHNOI KHIMII , 4(4), 49-53 CODEN: ZOFKAM, 2006 * |
Cited By (183)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9713613B2 (en) | 2007-02-02 | 2017-07-25 | Motonari Uesugi | Methods and compositions for the treatment of cancer and related hyperproliferative disorders |
| US9029411B2 (en) | 2008-01-25 | 2015-05-12 | Millennium Pharmaceuticals, Inc. | Thiophenes and uses thereof |
| US8314089B2 (en) | 2008-03-17 | 2012-11-20 | Aicuris Gmbh & Co. Kg | Substituted pyrazolamides and their use |
| US8399682B2 (en) | 2008-03-17 | 2013-03-19 | Aicuris Gmbh & Co. Kg | Substituted (pyrazolylcarbonyl)imidazolidinones and their use |
| US8536169B2 (en) | 2008-06-05 | 2013-09-17 | Glaxo Group Limited | Compounds |
| US8765743B2 (en) | 2008-06-05 | 2014-07-01 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| US8658635B2 (en) | 2008-06-05 | 2014-02-25 | Glaxosmithkline Intellectual Property Development Limited | Benzpyrazol derivatives as inhibitors of PI3 kinases |
| WO2010075962A1 (en) | 2008-12-17 | 2010-07-08 | Aicuris Gmbh & Co. Kg | Substituted (thiophenyl-carbonyl)imidazolidinones, and use thereof |
| DE102008062863A1 (en) | 2008-12-17 | 2010-06-24 | Aicuris Gmbh & Co. Kg | Substituted (thiophenyl-carbonyl) imidazolidinones and their use |
| DE102008062878A1 (en) | 2008-12-17 | 2010-06-24 | Aicuris Gmbh & Co. Kg | Substituted furancarboxamides and their use |
| US8324268B2 (en) | 2008-12-17 | 2012-12-04 | Aicuris Gmbh & Co. Kg | Substituted furancarboxamides, and use thereof |
| US8546438B2 (en) | 2008-12-17 | 2013-10-01 | Kai Thede | Substituted (thiophenyl-carbonyl)imidazolidinones, and use thereof |
| WO2010075957A1 (en) | 2008-12-17 | 2010-07-08 | Aicuris Gmbh & Co. Kg | Substituted furancarboxamides, and use thereof |
| US9139589B2 (en) | 2009-01-30 | 2015-09-22 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US9090601B2 (en) | 2009-01-30 | 2015-07-28 | Millennium Pharmaceuticals, Inc. | Thiazole derivatives |
| US8796314B2 (en) | 2009-01-30 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8524751B2 (en) | 2009-03-09 | 2013-09-03 | GlaxoSmithKline Intellecutual Property Development | 4-oxadiazol-2-YL-indazoles as inhibitors of P13 kinases |
| US8946231B2 (en) | 2009-03-23 | 2015-02-03 | Merck Sharp & Dohme Corp. | P2X3, receptor antagonists for treatment of pain |
| EP2410858A4 (en) * | 2009-03-23 | 2012-08-22 | Merck Sharp & Dohme | P2x3 receptor antagonists for treatment of pain |
| EP2410858A1 (en) * | 2009-03-23 | 2012-02-01 | Merck Sharp & Dohme Corp. | P2x3 receptor antagonists for treatment of pain |
| US8586583B2 (en) | 2009-04-30 | 2013-11-19 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| US8609657B2 (en) | 2009-04-30 | 2013-12-17 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| US8586590B2 (en) | 2009-04-30 | 2013-11-19 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| US10383879B2 (en) | 2009-04-30 | 2019-08-20 | Glaxo Group Limited | Compounds |
| US10624898B2 (en) | 2009-04-30 | 2020-04-21 | Glaxo Group Limited | Compounds |
| US10946025B2 (en) | 2009-04-30 | 2021-03-16 | Glaxo Group Limited | Compounds |
| US8580797B2 (en) | 2009-04-30 | 2013-11-12 | Glaxo Smith Kline Intellectual Property Development Limited | Compounds |
| US8575162B2 (en) | 2009-04-30 | 2013-11-05 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| EP2614825A1 (en) * | 2009-05-05 | 2013-07-17 | Dow AgroSciences LLC | Process for the preparation of thiazole derivatives |
| EP2427191A1 (en) * | 2009-05-05 | 2012-03-14 | Dow AgroSciences LLC | Pesticidal compositions |
| JP2012526123A (en) * | 2009-05-05 | 2012-10-25 | ダウ アグロサイエンシィズ エルエルシー | Insecticidal composition |
| US8853246B2 (en) | 2009-05-05 | 2014-10-07 | Dow Agrosciences, Llc. | Pesticidal compositions |
| US9357780B2 (en) | 2009-05-05 | 2016-06-07 | Dow Agrosciences Llc | Pesticidal compositions |
| EP2427191A4 (en) * | 2009-05-05 | 2012-10-17 | Dow Agrosciences Llc | Pesticidal compositions |
| KR101808866B1 (en) * | 2009-05-05 | 2017-12-13 | 다우 아그로사이언시즈 엘엘씨 | Pesticidal compositions |
| US8350044B2 (en) | 2009-05-05 | 2013-01-08 | Dow Agrosciences, Llc. | Pesticidal compositions |
| CN105017240A (en) * | 2009-05-05 | 2015-11-04 | 陶氏益农公司 | Pesticidal compositions |
| CN102458403A (en) * | 2009-05-05 | 2012-05-16 | 陶氏益农公司 | Pesticidal compositions |
| EP2614826A1 (en) * | 2009-05-05 | 2013-07-17 | Dow AgroSciences LLC | Process for preparation of thiazole derivatives |
| US9006446B2 (en) | 2009-05-05 | 2015-04-14 | Dow Agrosciences Llc | Pesticidal compositions |
| WO2010139982A1 (en) * | 2009-06-02 | 2010-12-09 | The University Of Sheffield | Indole derivatives for the stimulation of stem cell proliferation |
| JP2016188257A (en) * | 2010-03-24 | 2016-11-04 | メディカル ユニバーシティー オブ サウス カロライナMedical University of South Carolina | Compositions and methods for treatment of degenerative diseases |
| JP2013522376A (en) * | 2010-03-24 | 2013-06-13 | メディカル ユニバーシティー オブ サウス カロライナ | Compositions and methods for treating degenerative diseases |
| US9573943B2 (en) | 2010-04-07 | 2017-02-21 | Genentech, Inc. | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| US8669361B2 (en) | 2010-04-07 | 2014-03-11 | F. Hoffmann-La Roche Ag | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| US8436001B2 (en) | 2010-04-07 | 2013-05-07 | F. Hoffmann-La Roche Ag | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| EP2603216A1 (en) * | 2010-08-11 | 2013-06-19 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8796268B2 (en) | 2010-08-11 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8859768B2 (en) | 2010-08-11 | 2014-10-14 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| EP2603216A4 (en) * | 2010-08-11 | 2013-12-18 | Millennium Pharm Inc | Heteroaryls and uses thereof |
| US9062038B2 (en) | 2010-08-11 | 2015-06-23 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8796271B2 (en) | 2010-08-11 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8765746B2 (en) | 2010-10-13 | 2014-07-01 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8993576B2 (en) | 2010-10-27 | 2015-03-31 | Glaxo Group Limited | 6-(1H-indol-4-yl)-4-(5-{[4-1-methylethyl)-1-piperazinyl]methyl}-1,3-oxazol-2-yl)-1H-indazole hemi succinate salt, polymorphs and pharmaceutical compositions thereof |
| US8815271B2 (en) | 2010-11-03 | 2014-08-26 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| US9422278B2 (en) | 2010-11-03 | 2016-08-23 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| CN103596938B (en) * | 2011-05-23 | 2016-12-28 | 默克专利有限公司 | Thiazole |
| JP2014518885A (en) * | 2011-05-23 | 2014-08-07 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Thiazole derivative |
| CN103596938A (en) * | 2011-05-23 | 2014-02-19 | 默克专利有限公司 | Thiazole derivatives |
| US9505746B2 (en) | 2011-09-27 | 2016-11-29 | Genentech, Inc. | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| US8614206B2 (en) | 2011-09-27 | 2013-12-24 | F. Hoffmann-La Roche Ag | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| US9655365B2 (en) | 2011-10-26 | 2017-05-23 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US10165775B2 (en) | 2011-10-26 | 2019-01-01 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| EP2606889A1 (en) * | 2011-12-21 | 2013-06-26 | Telik, Inc. | Substituted Thiazoles as VEGFR2 kinase Inhibitors |
| US8901153B2 (en) | 2012-04-27 | 2014-12-02 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| US9591857B2 (en) | 2012-04-27 | 2017-03-14 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9708288B2 (en) | 2012-04-27 | 2017-07-18 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9282739B2 (en) | 2012-04-27 | 2016-03-15 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| AU2013272011B2 (en) * | 2012-06-04 | 2016-03-03 | Corteva Agriscience Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| RU2647853C2 (en) * | 2012-06-04 | 2018-03-21 | ДАУ АГРОСАЙЕНСИЗ ЭлЭлСи | Methods for producing certain 2-(pyridine-3-yl)thiazoles |
| WO2013184476A3 (en) * | 2012-06-04 | 2014-01-30 | Dow Agrosciences Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| JP2015523984A (en) * | 2012-06-04 | 2015-08-20 | ダウ アグロサイエンシィズ エルエルシー | Process for the preparation of certain 2- (pyridin-3-yl) thiazoles |
| WO2013184480A3 (en) * | 2012-06-04 | 2014-02-27 | Dow Agrosciences Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| WO2013184475A3 (en) * | 2012-06-04 | 2014-02-27 | Dow Agrosciences Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| CN104603131A (en) * | 2012-06-04 | 2015-05-06 | 美国陶氏益农公司 | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| RU2649000C2 (en) * | 2012-06-04 | 2018-03-29 | ДАУ АГРОСАЙЕНСИЗ ЭлЭлСи | Method of producing some 2-(pyridin-3-yl)thiazoles |
| RU2647851C2 (en) * | 2012-06-04 | 2018-03-21 | ДАУ АГРОСАЙЕНСИЗ ЭлЭлСи | Methods of producing some 2-(pyridin-3-yl)thiazoles |
| JP2015520189A (en) * | 2012-06-04 | 2015-07-16 | ダウ アグロサイエンシィズ エルエルシー | Process for the preparation of certain 2- (pyridin-3-yl) thiazoles |
| CN104540821A (en) * | 2012-06-04 | 2015-04-22 | 美国陶氏益农公司 | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| US9108952B2 (en) | 2012-06-04 | 2015-08-18 | Dow Agrosciences Llc | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| US8993769B2 (en) | 2012-06-04 | 2015-03-31 | Dow Agrosciences, Llc. | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| CN104507935A (en) * | 2012-06-04 | 2015-04-08 | 美国陶氏益农公司 | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| US9198428B2 (en) | 2012-06-04 | 2015-12-01 | Dow Agrosciences, Llc. | Processes to produce certain 2-(pyridine-3-yl)thiazoles |
| CN104540821B (en) * | 2012-06-04 | 2017-07-18 | 美国陶氏益农公司 | The method for preparing some 2 (base of pyridine 3) thiazoles |
| US9850218B2 (en) | 2013-03-13 | 2017-12-26 | Musc Foundation For Research Development | Thiazole compounds, compositions and methods for treatment of degenerative diseases and disorders |
| WO2014160197A1 (en) * | 2013-03-13 | 2014-10-02 | Musc Foundation For Research Development | Thiazole compounds, compositions and methods for treatment of degenerative diseases and disorders |
| WO2015031710A1 (en) * | 2013-08-29 | 2015-03-05 | Baylor College Of Medicine | Compositions and methods for the treatment of metabolic and body weight related disorders |
| US9670164B2 (en) | 2013-10-17 | 2017-06-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9447048B2 (en) | 2013-10-17 | 2016-09-20 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9108946B2 (en) | 2013-10-17 | 2015-08-18 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9085564B2 (en) | 2013-10-17 | 2015-07-21 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9796682B2 (en) | 2013-10-17 | 2017-10-24 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9723839B2 (en) | 2013-10-17 | 2017-08-08 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9102655B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9199942B2 (en) | 2013-10-17 | 2015-12-01 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9126974B2 (en) | 2013-10-17 | 2015-09-08 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US10315999B2 (en) | 2013-10-17 | 2019-06-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9414594B2 (en) | 2013-10-17 | 2016-08-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9044017B2 (en) | 2013-10-17 | 2015-06-02 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9670178B2 (en) | 2013-10-17 | 2017-06-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9434712B2 (en) | 2013-10-17 | 2016-09-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9433215B2 (en) | 2013-10-17 | 2016-09-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9255082B2 (en) | 2013-10-17 | 2016-02-09 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9174962B2 (en) | 2013-10-17 | 2015-11-03 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9661849B2 (en) | 2013-10-17 | 2017-05-30 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9029554B1 (en) | 2013-10-17 | 2015-05-12 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9862702B2 (en) | 2013-10-17 | 2018-01-09 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9255083B2 (en) | 2013-10-17 | 2016-02-09 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9901095B2 (en) | 2013-10-17 | 2018-02-27 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9988356B2 (en) | 2013-10-17 | 2018-06-05 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9908864B2 (en) | 2013-10-17 | 2018-03-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9102654B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9260396B2 (en) | 2013-10-17 | 2016-02-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9540342B2 (en) | 2013-10-17 | 2017-01-10 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9550751B2 (en) | 2013-10-17 | 2017-01-24 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9580405B2 (en) | 2013-10-17 | 2017-02-28 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9137998B2 (en) | 2013-10-22 | 2015-09-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9144241B2 (en) | 2013-10-22 | 2015-09-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9549560B2 (en) | 2013-10-22 | 2017-01-24 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9149040B2 (en) | 2013-10-22 | 2015-10-06 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9523100B2 (en) | 2013-10-22 | 2016-12-20 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9497966B2 (en) | 2013-10-22 | 2016-11-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9497967B2 (en) | 2013-10-22 | 2016-11-22 | Doe AgroSciences LLC | Synergistic pesticidal compositions and related methods |
| US9491944B2 (en) | 2013-10-22 | 2016-11-15 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9474276B2 (en) | 2013-10-22 | 2016-10-25 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9445597B2 (en) | 2013-10-22 | 2016-09-20 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9801383B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| USRE48057E1 (en) | 2013-10-22 | 2020-06-23 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9808008B2 (en) | 2013-10-22 | 2017-11-07 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9295260B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9295258B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9801376B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9788545B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9788546B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9282740B2 (en) | 2013-10-22 | 2016-03-15 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9029555B1 (en) | 2014-07-31 | 2015-05-12 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9255081B1 (en) | 2014-07-31 | 2016-02-09 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9199964B1 (en) | 2014-07-31 | 2015-12-01 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029556B1 (en) | 2014-07-31 | 2015-05-12 | Dow Argosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9840490B2 (en) | 2014-07-31 | 2017-12-12 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9249122B1 (en) | 2014-07-31 | 2016-02-02 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US10035786B2 (en) | 2014-07-31 | 2018-07-31 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1h-pyrazol-1-yl)pyridine |
| US9573931B2 (en) | 2014-07-31 | 2017-02-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9371310B2 (en) | 2014-07-31 | 2016-06-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9611247B2 (en) | 2014-07-31 | 2017-04-04 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9580403B2 (en) | 2014-07-31 | 2017-02-28 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9024031B1 (en) | 2014-08-19 | 2015-05-05 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US10005758B2 (en) | 2014-08-19 | 2018-06-26 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9522900B2 (en) | 2014-08-19 | 2016-12-20 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9115115B1 (en) | 2014-08-19 | 2015-08-25 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9809570B2 (en) | 2014-08-19 | 2017-11-07 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9663489B2 (en) | 2014-09-12 | 2017-05-30 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9422265B2 (en) | 2014-09-12 | 2016-08-23 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9896430B2 (en) | 2014-09-12 | 2018-02-20 | Dow Agrosciences Llc | Process for the preparation of 3-(3-CHLORO-1H-pyrazol-1-yl)pyridine |
| US9085552B1 (en) | 2014-09-12 | 2015-07-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9156813B1 (en) | 2014-09-12 | 2015-10-13 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US10328064B2 (en) | 2014-12-23 | 2019-06-25 | Fgh Biotech, Inc. | Compositions of fatostatin based heterocyclic compounds and uses thereof |
| US11130731B2 (en) | 2015-02-16 | 2021-09-28 | The Provost, Fellows, Foundation Scholars, And The Other Members Of Board, Of The College Of The Holy And Undivided Trinity Of Queen Elizabeth Near Dublin | Sulfonylureas and related compounds and use of same |
| US10538487B2 (en) | 2015-02-16 | 2020-01-21 | The University Of Queensland | Sulfonylureas and related compounds and use of same |
| US9771379B2 (en) | 2015-09-24 | 2017-09-26 | Pfizer Inc. | N-(2-(2-amino-6-substituted-4,4a,5,6-tetrahydropyrano[3,4-d][1,3]OXAZIN-8a(8H)-yl)-thiazol-4-yl) amides |
| CN105541753A (en) * | 2015-12-17 | 2016-05-04 | 浙江工业大学 | Carboxamide derivative containing thiazole ring and preparation method and application thereof |
| US11497738B2 (en) | 2016-04-29 | 2022-11-15 | Fgh Biotech, Inc. | Di-substituted pyrazole compounds for the treatment of diseases |
| US10774064B2 (en) | 2016-06-02 | 2020-09-15 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US11339142B2 (en) | 2016-09-07 | 2022-05-24 | Fgh Biotech, Inc. | Di-substituted pyrazole compounds for the treatment of diseases |
| US10233155B2 (en) | 2016-12-29 | 2019-03-19 | Dow Agrosciences Llc | Processes for the preparation of pesticide compounds |
| US10100033B2 (en) | 2016-12-29 | 2018-10-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9975886B1 (en) | 2017-01-23 | 2018-05-22 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US10351553B2 (en) | 2017-01-23 | 2019-07-16 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US10717728B2 (en) | 2017-01-23 | 2020-07-21 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US11981667B2 (en) | 2017-07-07 | 2024-05-14 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11370776B2 (en) | 2017-07-07 | 2022-06-28 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US11465992B2 (en) | 2017-07-07 | 2022-10-11 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11773058B2 (en) | 2017-08-15 | 2023-10-03 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11518739B2 (en) | 2017-08-15 | 2022-12-06 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11542255B2 (en) | 2017-08-15 | 2023-01-03 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US11613542B2 (en) | 2017-08-15 | 2023-03-28 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US11926600B2 (en) | 2017-08-15 | 2024-03-12 | Inflazome Limited | Sulfonylureas and sulfonylthioureas as NLRP3 inhibitors |
| US12012392B2 (en) | 2017-11-09 | 2024-06-18 | Inflazome Limited | Sulfonamide carboxamide compounds |
| US11905252B2 (en) | 2018-03-02 | 2024-02-20 | Inflazome Limited | Compounds |
| US11993586B2 (en) | 2018-10-22 | 2024-05-28 | Novartis Ag | Crystalline forms of potassium channel modulators |
| WO2021214020A1 (en) | 2020-04-24 | 2021-10-28 | Bayer Aktiengesellschaft | Substituted aminothiazoles as dgkzeta inhibitors for immune activation |
| US11964953B2 (en) | 2020-04-24 | 2024-04-23 | Bayer Aktiengesellschaft | Substituted aminothiazoles as DGKzeta inhibitors for immune activation |
| WO2021214019A1 (en) | 2020-04-24 | 2021-10-28 | Bayer Aktiengesellschaft | Substituted aminothiazoles as dgkzeta inhibitors for immune activation |
Also Published As
| Publication number | Publication date |
|---|---|
| GB0701426D0 (en) | 2007-03-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2008090382A1 (en) | Thiazole and oxazole derivatives for use in the treatment of prion diseases, cancer and conditions of the central nervous system as well as in the regulation of stem cells | |
| AU2016299485B2 (en) | 1,3,4-oxadiazole amide derivative compound as histone deacetylase 6 inhibitor, and pharmaceutical composition containing same | |
| US20080221161A1 (en) | Heterocyclic modulators of tgr5 for treatment of disease | |
| AU776053B2 (en) | Diazepan derivatives or salts thereof | |
| TW200827346A (en) | Chemical compounds | |
| TW201124378A (en) | Indole compounds and pharmaceutical use thereof | |
| WO2008067219A2 (en) | Quinazolinone modulators of tgr5 | |
| WO2003074525A1 (en) | Nitrogen-containing heterocyclic compound | |
| AU2021200919A1 (en) | MCT4 inhibitors for treating disease | |
| JP2012509940A (en) | Substituted octahydrocyclopenta [c] pyrrol-4-amines as calcium channel blockers | |
| WO2008040974A1 (en) | Indoles for use as dpp-iv inhibitors | |
| TWI729443B (en) | 1,3,4-oxadiazole derivative compounds as histone deacetylase 6 inhibitor, and pharmaceutical composition comprising the same | |
| JPH01250370A (en) | Novel amino acid imide derivative, its production and use thereof | |
| EP2178371B1 (en) | Heterocyclic modulators of cannabinoid receptors | |
| DE69717044T2 (en) | Flavone derivatives, processes for their preparation and pharmaceutical compositions containing them | |
| AU2015207867A1 (en) | Compounds and methods for the treatment of pain and other diseases | |
| AU2004228452A2 (en) | Azabicyclo derivatives as muscarinic receptor antagonists | |
| JPH10508311A (en) | Spiro [heterocycle-imidazo [1,2-a] indeno [1,2-e] pyrazin] -4'-ones, their preparation and pharmaceuticals containing them | |
| JPS5877852A (en) | Amide compound and manufacture | |
| JP2022552158A (en) | Indazole carboxamides as kinase inhibitors | |
| CA2137605A1 (en) | Combination preparations comprising a quinoxaline and a nucleoside | |
| TWI258469B (en) | Aryl-substituted alicyclic compounds and pharmaceutical composition containing the same | |
| DE10252102A1 (en) | New 3-(piperidino- or -piperazino-alkyl)-indole derivatives, are 5HT-1A and/or 5HT-1D agonists and 5-HT reuptake inhibitors, useful e.g. for treating anxiety, depression or neurodegenerative diseases | |
| CN100540536C (en) | Phenylpyridylpiperazcompounds compounds, their preparation method and the pharmaceutical composition that comprises them | |
| HUT70148A (en) | Isoquinolone derivatives as techykinin receptor antagonists, process for producing thereof and pharmaceutical compositions comprising them |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 08702135 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 08702135 Country of ref document: EP Kind code of ref document: A1 |