WO2008045905A1 - Pyrrolydine derivatives as iap inhibitors - Google Patents
Pyrrolydine derivatives as iap inhibitors Download PDFInfo
- Publication number
- WO2008045905A1 WO2008045905A1 PCT/US2007/080875 US2007080875W WO2008045905A1 WO 2008045905 A1 WO2008045905 A1 WO 2008045905A1 US 2007080875 W US2007080875 W US 2007080875W WO 2008045905 A1 WO2008045905 A1 WO 2008045905A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methylamino
- pyrrolidin
- propionamide
- cyclohexyl
- oxo
- Prior art date
Links
- 0 C*NC(C)C(CC1)CCC1OC Chemical compound C*NC(C)C(CC1)CCC1OC 0.000 description 2
- DFTXIXIDLDGNJO-UHFFFAOYSA-N Bc1cc(C(CCC(OC)OC)=O)ccn1 Chemical compound Bc1cc(C(CCC(OC)OC)=O)ccn1 DFTXIXIDLDGNJO-UHFFFAOYSA-N 0.000 description 1
- LPAGFVYQRIESJQ-UHFFFAOYSA-N C1c(cccc2)c2NC1 Chemical compound C1c(cccc2)c2NC1 LPAGFVYQRIESJQ-UHFFFAOYSA-N 0.000 description 1
- QSUXZIPXYDQFCX-UHFFFAOYSA-N CC(C)(C)OC(NC(C1CCCCC1)C(O)=O)=O Chemical compound CC(C)(C)OC(NC(C1CCCCC1)C(O)=O)=O QSUXZIPXYDQFCX-UHFFFAOYSA-N 0.000 description 1
- JCDQPLUQIVNIEA-NAKRPHOHSA-N CC(C)(C)OC(N[C@@H](C1CCCCC1)C(N(CCC1)C[C@@H]1c1ccnc(N(CC2)c3c2cccc3)c1)=O)=O Chemical compound CC(C)(C)OC(N[C@@H](C1CCCCC1)C(N(CCC1)C[C@@H]1c1ccnc(N(CC2)c3c2cccc3)c1)=O)=O JCDQPLUQIVNIEA-NAKRPHOHSA-N 0.000 description 1
- PEVACMGRBUFIHD-UHFFFAOYSA-N CC(C)(c(cccc12)c1[n]1cc2Br)OC1=O Chemical compound CC(C)(c(cccc12)c1[n]1cc2Br)OC1=O PEVACMGRBUFIHD-UHFFFAOYSA-N 0.000 description 1
- FEILGAWKLAOZQV-NZVRHLSZSA-N CC(c(cc1)ccc1OC)N(CCC1)[C@@H]1c1cc(-c(c2c3c(C(C)(C)O4)ccc2)c[n]3C4=O)cnc1 Chemical compound CC(c(cc1)ccc1OC)N(CCC1)[C@@H]1c1cc(-c(c2c3c(C(C)(C)O4)ccc2)c[n]3C4=O)cnc1 FEILGAWKLAOZQV-NZVRHLSZSA-N 0.000 description 1
- OOGALSDKTFQSKW-UHFFFAOYSA-N CN(C(c1ccnc(Br)c1)=O)OC Chemical compound CN(C(c1ccnc(Br)c1)=O)OC OOGALSDKTFQSKW-UHFFFAOYSA-N 0.000 description 1
- VQIHFIHZAPEOHA-DYVFJYSZSA-N C[C@H](c(cc1)ccc1OC)N(CCC1)[C@@H]1c1ccnc(Br)c1 Chemical compound C[C@H](c(cc1)ccc1OC)N(CCC1)[C@@H]1c1ccnc(Br)c1 VQIHFIHZAPEOHA-DYVFJYSZSA-N 0.000 description 1
- CRYMBIGNEOPVMP-VGSWGCGISA-N C[C@H](c(cc1)ccc1OC)N(CCC1)[C@@H]1c1cncc(B2OC(C)(C)C(C)(C)O2)c1 Chemical compound C[C@H](c(cc1)ccc1OC)N(CCC1)[C@@H]1c1cncc(B2OC(C)(C)C(C)(C)O2)c1 CRYMBIGNEOPVMP-VGSWGCGISA-N 0.000 description 1
- VCKPRTQSWRLBTJ-MBXJOHMKSA-N O=C(CC/C=C1\OC1)c1ccnc(Br)c1 Chemical compound O=C(CC/C=C1\OC1)c1ccnc(Br)c1 VCKPRTQSWRLBTJ-MBXJOHMKSA-N 0.000 description 1
- YBTKGKVQEXAYEM-UHFFFAOYSA-N OC(c1ccnc(Br)c1)=O Chemical compound OC(c1ccnc(Br)c1)=O YBTKGKVQEXAYEM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/06—Dipeptides
- C07K5/06191—Dipeptides containing heteroatoms different from O, S, or N
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/05—Dipeptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/06—Dipeptides
Definitions
- the present invention relates generally to novel compounds that inhibit the binding of the Smac protein to Inhibitor of Apoptosis Proteins (IAPs). More specifically, the present invention includes novel compounds, novel compositions, methods of their use and methods of their manufacture, where such compounds are generally pharmacologically useful as agents in therapies whose mechanism of action rely on the inhibition of the Smac/IAP interaction, and more particularly useful in therapies for the treatment of proliferative diseases, including cancer.
- IAPs Apoptosis Proteins
- Programmed cell death plays a critical role in regulating cell number and in eliminating stressed or damaged cells from normal tissues. Indeed, the network of apoptotic signaling mechanisms inherent in most cell types provides a major barrier to the development and progression of human cancer. Since most commonly used radiation and chemo-therapies rely on activation of apoptotic pathways to kill cancer cells, tumor cells which are capable of evading programmed cell death often become resistant to treatment.
- Apoptosis signaling networks are classified as either intrinsic when mediated by death receptor-ligand interactions or extrinsic when mediated by cellular stress and mitochondrial permeabilization. Both pathways ultimately converge on individual Caspases. Once activated, Caspases cleave a number of cell death-related substrates, effecting destruction of the cell.
- Tumor cells have devised a number of strategies to circumvent apoptosis.
- One recently reported molecular mechanism involves the overexpression of members of the IAP (Inhibitor of Apoptosis) protein family.
- IAPs sabotage apoptosis by directly interacting with and neutralizing Caspases.
- the prototype IAPs, XIAP and clAP have three functional domains referred to as BIR 1 , 2 & 3 domains.
- BIR3 domain interacts directly with Caspase 9 and inhibits its ability to bind and cleave its natural substrate, Procaspase 3.
- Smac a proapoptotic mitochondrial protein
- DIABLO a proapoptotic mitochondrial protein
- Smac also known as DIABLO
- Binding of peptides derived from Smac has also been reported to trigger autocatalytic polyubiquitination and subsequent proteosome-mediated degradation of CIAP1.
- the present invention relates to therapeutic molecules that bind to the Smac binding pocket thereby promoting apoptosis in rapidly dividing cells. Such therapeutic molecules are useful for the treatment of proliferative diseases, including cancer.
- the present invention relates to novel compounds of formula I:
- R 1 is H, C 1 -C 4 alkyl, C 2 -C 4 alkenyl, C 2 -C 4 alkynyl or C 3 -C 1 O cycloalkyl, which R 1 may be unsubstituted or substituted;
- R 2 is H, C 1 -C 4 alkyl, C 2 -C 4 alkenyl, C 2 -C 4 alkynyl, C 3 -C 10 cycloalkyl which R 2 may be unsubstituted or substituted;
- R 1 and R 2 may be taken together to form a ring or het
- R 3 and R 3 ' are independently H, CF 3 , C 2 F 5 , C 1 -C 4 alkyl, C 2 -C 4 alkenyl, C 2 -C 4 alkynyl, CH 2 -Z or R 2 and R 3 taken together with the nitrogen atom to which they are attached form het, wherein alkyl, alkenyl, alkynyl or het ring may be unsubstituted or substituted;
- Z is H, OH, F, Cl, CH 3 , CH 2 CI, CH 2 F or CH 2 OH;
- R 4 is C 0-1O alkyl, C 0-10 alkyl-C 3 . 10 cycloalkyl, C 0 . 10 alkyl-C 6 - 10 aryl, C 0 . 10 alkyl-het, wherein any carbon may be replaced with a heteroatom or group from the list N, O, S(O) n and any atom may be unsubstituted or substituted;
- A is a 6 membered heteroaryl ring or an 8-12 membered fused ring system that may include one 5-7 membered heterocyclic ring containing 1 , 2, or 3 heteroring atoms selected from N, O and S, which any position of the rings is unsubstituted or substituted with one or more Q's; r is 0, 1 , or 2;
- Q and Y are independently H, F, Cl, Br, I, Ci-C 10 alkyl, C 1 -C 10 alkoxy, aryl C 1 -C 10 alkoxy, OH, O-C r C 10 -alkyl, (CH 2 )( T6 -C 3 -C 7 cycloalkyl, aryl, aryl C 1 -C 10 alkyl, O-(CH 2 ) 0 - 6 aryl, (CH 2 ) r6 het, het, CKCH ⁇ ehet, -OR 11 , C(O)R 11 , -C(O)N(R 11 )(R 12 ), N(R 11 )(R 12 ) ⁇ R 11 , S(O)R 111 S(O) 2 R 11 , S(O) 2 -N(R 11 )(R 12 ), or NR 11 -S(O) 2 -(R 12 ), wherein alkyl, cycloalkyl
- X is aryl, C 3 -C 10 cycloalkyl, or het, substituted or unsubstituted, in which substituents on aryl, C 3 -C 10 cycloalkyl and het are alkyl, halo, lower alkoxy, NR 5 R 6 , CN, NO 2 or SR 5 ;
- R 5 and R 6 are independently H, F, Cl, Br, I, C 1 -C 1 0 alkyl, C 1 -C 1 0 alkoxy, aryl C1-C10 alkoxy, OH, O-C r C 10 -alkyl, (CH 2 ) 0 - 6 -C 3 -C 7 cycloalkyl, aryl, aryl C 1 -C 10 alkyl, O-(CH 2 ) 0 .
- R 11 and R 12 are independently H, C 1 -C 10 alkyl, (CH 2 ) 0 ⁇ -C 3 -C 7 cycloalkyl, (CH 2 ) 0 . 6 - (CH) 0 . 1 (aryl) 1 . 2 ,C(O)-C 1 -C 10 alkyl, -C(O)-(CH 2 ) 0 . 6 -C 3 -C 7 cycloalkyl, -C(O)-O-(CH 2 ) 0 . 6 -aryl, -C(O)- (CH 2 ) 0 . 6 -O-fluorenyl, C(O)-NH-(CH 2 ) 0 .
- R 9 , R 10 , and R 13 are independently hydrogen, lower alkyl, halogen substituted lower alkyl, aryl, aryl lower alkyl, halogen substituted aryl, halogen substituted aryl lower alkyl.
- the present invention also relates to pharmaceutical compositions comprising therapeutically effective amounts of compounds of Formula I, as defined hereinabove, or a pharmaceutically acceptable salt thereof, and a pharmaceutical carrier therefor.
- the present invention is directed to a method of treating a mammal, especially human, afflicted with a proliferative disease, especially those dependent on the binding of the smac protein to Inhibitor of Apoptosis Proteins (IAPs), such as cancer, which method comprises administering to said mammal in need of treatment an anti-proloferative effective amount of a compound of Formula I or a pharmaceutically acceptable salt thereof.
- IAPs Apoptosis Proteins
- the present invention is also directed to the manufacture of compounds of Formula I for use in the treatment of said diseases.
- Aryl is defined as an aromatic radical having 6 to 14 ring carbon atoms, and no ring heteroatoms.
- the aryl group may be monocyclic or fused bicyclic or tricyclic. It may be unsubstituted or substituted by one or more, preferably one or two, substituents, wherein the substituents are as described herein.
- the aryl moiety may be completely aromatic regardless of whether it is monocyclic or bicyclic. However, if it contains more than one ring, as defined herein, the term aryl includes moieties wherein at least one ring is completely aromatic while the other ring(s) may be partially unsaturated or saturated or completely aromatic.
- Preferred "aryl” is phenyl or naphthyl. The most preferred aryl is phenyl.
- Het refers to heteroaryl and heterocyclic compounds containing at least one S, O or N ring heteroatom. More specifically, “Het” is a 5-7 membered heterocyclic ring containing 1- 4 heteroatoms selected from N, O and S, or an 8-12 membered fused ring system including at least one 5-7 membered heterocyclic ring containing 1 , 2 or 3 heteroatoms selected from N, O, and S.
- Examples of het include unsubstituted and substituted pyrrolidyl, tetrahydrofuryl, tetrahydrothiofuryl, piperidyl, piperazyl, purinyl, tetrahydropyranyl, morpholino, 1 ,3-diazapanyl, 1 ,4-diazapanyl, 1 ,4- oxazepanyl, 1,4-oxathiapanyl, furyl, thienyl, pyrryl, pyrrolyl, pyrazolyl, triazolyl, tetrazolyl, indazolyl, oxadiazolyl, imidazolyl, pyrrolidyl, pyrrolidinyl, thiazolyl, oxazolyl, pyridyl, pyrazolyl, pyrazinyl, pyrimidinyl, isoxazolyl, pyraziny
- Heteroaryls are within the scope of the definition of het. Examples of heteroaryls are pyridyl, pyrimidinyl, quinolyl, thiazolyl and benzothiazolyl. The most preferred het are pyridyl, pyrimidinyl and thiazolyl. The het may be unsubstituted or substituted as described herein.
- halogen especially fluorine or chlorine, hydroxy, C 1 -C 4 alkyl, such as methyl and ethyl, C 1 -C 4 alkoxy, especially methoxy and ethoxy, nitro, -O-C(O)-C 1 -C 4 alkyl or -C(O)-O-C 1 -C 4 alkyl, SCN or nitro or on a nitrogen atom by C 1 -C 4 alkyl, especially methyl or ethyl, -O-C(O)-Ci-C 4 alkyl or -C(O)-O-C 1 -C 4 alkyl, such as carbomethoxy or carboethoxy.
- heterocyclic ring is a nitrogen-containing ring, such as aziridine, azetidine, azole, piperidine, piperazine, morphiline, pyrrole, pyrazole, thiazole, oxazole, pyridine, pyrimidine, isoxazole, and the like, wherein such het may be unsubstituted or substituted as defined hereinabove.
- nitrogen-containing ring such as aziridine, azetidine, azole, piperidine, piperazine, morphiline, pyrrole, pyrazole, thiazole, oxazole, pyridine, pyrimidine, isoxazole, and the like, wherein such het may be unsubstituted or substituted as defined hereinabove.
- Halogen is fluorine, chlorine, bromine or iodine, especially fluorine and chlorine.
- alkyl includes straight or branched chain alkyl, such as methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, n-pentyl and branched pentyl, n-hexyl and branched hexyl, and the like.
- a "cycloalkyl” group means C 3 to C 10 cycloalkyl having 3 to 10 ring carbon atoms and may be, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl or cyclooctyl, cyclononyl and the like.
- the cycloalkyl group may be monocyclic or fused bicyclic. It is preferred that it is monocyclic.
- the preferred cycloalkyl group is cyclopentyl or cyclohexyl. Most preferably, cycloalkyl is cyclohexyl.
- the cycloalkyl group may be fully saturated or partially unsaturated, although it is preferred that it is fully saturated. As defined herein, it excludes aryl groups.
- the cycloalkyl groups may be unsubstituted or substituted with any of the substituents defined below, preferably halo, hydroxy or C 1 -C 6 alkyl such as methyl.
- Such lipophillic substituents include a C 6 -C 30 alkyl which is saturated, monounsaturated, polyunsaturated, including methylene-interrupted polyene, phenyl, phenyl which is substituted by one or two C 1 -C 8 alkyl groups, C 5 -C 9 cycloalkyl, C 5 -C 9 cycloalkyl which is substituted by one or two C 1 - C 8 alkyl groups, -X ⁇ phenyl, -X ⁇ phenyl which is substituted in the phenyl ring by one or two C 1 -C 8 alkyl groups, X 1 -C 5 -Cg cycloalkyl Or X 1 -C 5 -Cg cycloalkyl which is substituted by one or two C 1 -C 8 alkyl groups; where X 1 is C 1 -C 24 alkyl which is saturated, monounsaturated or polyunsaturated and straight or branched chain.
- any of the above defined aryl, het, alkyl, alkenyl, alkynyl, or cycloalkyl may be unsubstituted or independently substituted by up to four, preferably one, two or three substituents, selected from the group consisting of: halo (such as Cl or Br); hydroxy; lower alkyl (such as C 1 -C 3 alkyl); lower alkyl which may be substituted with any of the substituents defined herein; lower alkenyl; lower alkynyl; lower alkanoyl; lower alkoxy (such as methoxy); aryl (such as phenyl or naphthyl); substituted aryl (such as fluoro phenyl or methoxy phenyl); aryl lower alkyl such as benzyl, amino, mono or di-lower alkyl (such as dimethylamino); lower alkanoyl amino acetylamino; amino lower alkoxy (
- R 8 and R 14 together with the N atom form a 3- to 8-membered heterocyclic ring containing a nitrogen heteroring atom and optionally one or two additional heteroring atoms selected from the group consisting of nitrogen, oxygen and sulfur (e.g. piperazinyl, pyrazinyl, lower alkyl-piperazinyl, pyridyl, indolyl, thiophenyl, thiazolyl, benzothiophenyl, pyrrolidinyl, piperidino or imidazolinyl) where the heterocyclic ring may be substituted with any of the substituents defined hereinabove.
- nitrogen, oxygen and sulfur e.g. piperazinyl, pyrazinyl, lower alkyl-piperazinyl, pyridyl, indolyl, thiophenyl, thiazolyl, benzothiophenyl, pyrrolidinyl, piperidino or imidazol
- alkyl, cycloalkyl, and aryl groups are independently unsubstituted or are substituted by lower alkyl, aryl, aryl lower alkyl, carboxy, lower carbalkoxy and especially halogen, -OH, -SH, -OCH 3 , -SCH 3 , -CN, -SCN or nitro.
- lower alkyl when used alone or in combination refers to alkyl containing 1-6 carbon atoms.
- the alkyl group may be branched or straight-chained, and is as defined hereinabove.
- lower alkenyl refers to a alkenyl group which contains 2-6 carbon atoms.
- An alkenyl group is a hydrocarbyl group containing at least one carbon-carbon double bond. As defined herein, it may be unsubstituted or substituted with the substituents described herein.
- the carbon-carbon double bonds may be between any two carbon atoms of the alkenyl group. It is preferred that it contains 1 or 2 carbon-carbon double bonds and more preferably one carbon-carbon double bond.
- the alkenyl group may be straight chained or branched.
- Examples include ethenyl, 1-propenyl, 2-propenyl, 1-butenyl, 2-butenyl, 2-methyl- 1-propenyl, 1 , 3-butadienyl, and the like.
- the preferred alkenyl group is ethenyl.
- lower alkynyl refers to an alkynyl group containing 2-6 carbon atoms.
- An alkynyl group is a hydrocarbyl group containing at least one carbon-carbon triple bond.
- the carbon-carbon triple bond may be between any two carbon atom of the alkynyl group. It is preferred that the alkynyl group contains 1 or 2 carbon-carbon triple bonds and more preferably one carbon-carbon triple bond.
- the alkynyl group may be straight chained or branched. Examples include ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl and the like.
- the preferred alkynyl group is ethynyl.
- aryl alkyl refers to a aryl group connected to the main chain by a bridging alkylene group. Examples include benzyl, phenethyl, naphthylmethyl, and the like. The preferred aryl alkyl is benzyl.
- cyano alkyl group refers to a cyano group connected to the main chain by a bridging alkylene group.
- alkyl aryl refers to an alkyl group bridged to the main chain through a phenylene group. Examples include methylphenyl, ethylphenyl, and the like.
- alkoxy refers to an alkyl group as defined herein, connected to the main chain by an oxygen atom. Examples include methoxy, ethoxy, and the like.
- lower thioalkyl refers to an alkyl group, as defined herein, connected to the main chain by a sulfur atom. Examples include thiomethyl (or mercapto methyl), thioethyl (mercapto ethyl) and the like.
- lower carbalkoxy or synonym thereto refers to an alkoxycarbonyl group, where the attachment to the main chain is through the aryl group (C(O)). Examples include methoxy carbonyl, ethoxy carbonyl, and the like.
- C 0 as used herein, as part of a definition of alkyl, as e.g., C o- io, refers to zero carbon atoms.
- C 0 -Ci 0 aryl alkyl means that the aryl group is bonded directly to the main chain (C 0 ) or that there is a C 1 -Ci 0 alkylene group bridging the main chain to an aryl group.
- (CH 2 W as part of definition of a larger group refers to a group that is not present (CH 2 ) 0 , or to a group that contains 1-6 carbon atoms (CH 2 ) L6 .
- Rn and Ri 2 is intended to mean one of the following (CH 2 )i. 6 -aryl, aryl, -CH(aryl) 2 or (CH 2 )i-e (CH) (aryl) 2 .
- n refers to number of substitutents on the pyrrolidinyl (tetrahydropyrrolyl) ring.
- n is defined as 0-7 and it determines the number of Q substituents on the pyrrolidinyl (tetrahydro-pyrrolyl) ring.
- Q can only be present at the 2, 3, 4, or 5 positions of the pyrrolidinyl ring, i.e., at the carbon atoms of the pyrrolidinyl ring. Except for carbon number 2 that can allow for one substitution, each of other carbon atoms are saturated and each of them may have two substituents thereon. When n is 7, then each of the carbon atoms are bonded with Q as defined herein. Each Q may be the same or different. However, when n is 6, then one of the seven possible substituents is H, and the other five are Q, which can be the same or different. Further, when n is 5, then two of the possible substitutents are H, and the other five are independently Q, as defined herein.
- n 4 When n is 4, then three of the seven possible substituents are H, and the remainder are Q independently as defined herein. Where n is 3, then four of the seven possible substituents are H, and the other three are Q as defined herein. When n is 2, then two of the seven possible substituent are Q, and the remainder are H. When n is 1 , then only one of the seven possible substituent is Q, and the remainder are H. Finally, when n is 0, all seven of the substituents are H.
- each of the Q substituents may be the same or they may be different.
- Any asymmetric carbon atom may be present in the (R)-, (S)- or (R,S)-configuration, preferably in the (R)- or (S)-configuration.
- the compounds may thus be present as mixtures of isomers or preferably as pure isomers, preferably as enantiomermally pure diastereomers or pure enantiomers.
- R 1 group is H and C 1 -C 4 alkyl especially methyl.
- R 1 may be unsubstituted or substituted and is most preferably unsubstituted.
- the most preferred values of R 1 is H, methyl and ethyl, and especially methyl or ethyl and most especially methyl.
- R 2 is preferably H or C 1 -C 4 alkyl, especially methyl.
- R 2 may be unsubstituted or substituted. It is most preferably unsubstituted. It is preferred that R 2 is hydrogen.
- R 3 and R 3 ' are, independently, preferably H or C 1 -C 4 alkyl especially hydrogen, methyl, or ethyl and most especially methyl or ethyl, and most especially methyl, which may be unsubstituted or substituted.
- R 3 may be unsubstituted or substituted as defined herein. It is preferred that it is unsubstituted methyl or H. In a most preferred embodiment one of R 3 and R 3 ' is H and the other is methyl.
- R 4 is preferably C 5 -C 7 cycloalkyl, especially cyclohexyl, or C 1 -C 4 alkyl, especially isopropyl .
- R 4 may be substituted or unsubstituted.
- Q is preferably H.
- A is a 6-membered heteroaryl or an 8-12 membered fused ring system that may include one 5-7 membered heterocyclic ring containing 1 , 2, or 3 heteroring atoms selected from N, O and S.
- A may be unsubstituted or substituted in any position with one or more Q's.
- Preferably A is pyridyl, pyrimidinyl, indolyl, benzothiazolyl, or quinolinyl.
- A may be unsubstituted or substituted. It is preferred that A is unsubstituted or substituted with lower alkyl such as methyl, or halo.
- X is aryl, C 3 -C 10 cycloalkyl, or het.
- X is quinolinyl, isoquinolyl, benzothiazolyl, pyridinyl, indolyl, benzoimidazolyl, naphthyl, benzo[1 ,3]dioxolyl, benzofurnayl, naphthyridine, pyrrolo[2,3b]pyridinyl, indanzolyl, benzotriazolyl, indazolyl, 2-oxobenzo-oxazolyl, or phenyl.
- X may be unsubstituted or substituted in any position with one or more Y.
- Y is halo especially F or Cl, lower alkyl, especially methyl, ethyl, t- butyl or isopropyl, said lower alkyl may be substituted such as trifluoromethyl, lower alkoxy such as methoxy, lower alkyl amino such as dimethyl amino.
- R 1 is H, C 1 -C 4 alkyl, which R 1 may be unsubstituted or substituted;
- R 2 is H, C 1 -C 4 alkyl, which R 2 may be unsubstituted or substituted;
- R 3 and R 3 ' are independently H, or C 1 -C 4 alkyl
- R 4 is C 5 -C 7 cycloalkyl, especially cyclohexyl, or C 1 -C 4 alkyl, especially isopropyl;
- A is a 6 membered heteroaryl ring or an 8-12 membered fused ring system that may include one 5-7 membered heterocyclic ring containing 1 , 2, or 3 heteroring atoms selected from N,
- Q and Y are independently H, F, Cl, Br, I 1 C 1 -C 10 alkyl, C 1 -C 10 alkoxy;
- X is aryl, C 3 -C 10 cycloalkyl, or het, which may be substituted or unsubstituted.
- a preferred embodiment is the compound of Formula I 1 or pharmaceutically acceptable salts thereof, wherein
- R 1 is H, or methyl
- R 2 is H, or methyl; one of R 3 and R 3 ' a is H and the other is methyl;
- R 4 is cyclohexyl, or isopropyl
- A is pyridyl, pyrimidinyl, indolyl, benzothiazolyl, or quinolinyl which may be unsubstituted or substituted with lower alkyl such as methyl, or halo;
- Q and Y are independently H, F or Cl, lower alkyl, especially methyl, ethyl, t- butyl or isopropyl, said lower alkyl may be substituted such as trifluoromethyl, lower alkoxy such as methoxy, lower alkyl amino such as dimethyl amino; and
- X is quinolinyl, isoquinolyl, benzothiazolyl, pyridinyl, indolyl, benzoimidazolyl, naphthyl, benzo[1 ,3]dioxolyl, benzofurnayl, naphthyridine, pyrrolo[2,3b]pyridinyl, indanzolyl, benzotriazolyl, indazolyl, 2-oxobenzo-oxazolyl, or phenyl, which may be substituted or unsubstituted.
- the active compounds of this invention may be prepared as described in the following reaction schemes. Unless otherwise indicated, R 1 , R 2 in the reaction schemes and discussion that follow, are as defined above.
- Scheme A illustrates a method for preparing compounds of the formula 3 by reacting a compound of the formula 1 (Int. Pat. Appl. WO2005097791A1), wherein R 1 ' is either fluorine or methyl, nitrogen could be in any position of the ring, with an excess compound of formula 2.
- the reaction is run in the presence of a palladium catalyst such as Pd 2 (dba) 3 , a ligand such as 2-(dicyclohexylphosphino)-biphenyl and a base such as potassium tert- butoxide in toluene at a rang of temperature of 70 0 C to 10O 0 C, but preferably at around 80 0 C.
- the reaction is typically run for a period of 3 hour up to 15 hours but preferably between 3 and 5 hours.
- N Scheme B illustrates a method for preparing compounds of the formula 5 by reacting a compound of the formula 1 (Int. Pat. Appl. WO2005097791A1), wherein R'-, is either fluorine or methyl, nitrogen could be in any position of the ring, with a compound of formula 4.
- the reaction typically run in the presence of a base such as potassium carbonate or cesium carbonate.
- a base such as potassium carbonate or cesium carbonate.
- CuI was employed as catalyst in the reaction.
- the solvent used may be NMP.
- the temperature of the reaction may vary from 180 0 C to 220 0 C for a period of 25 min to 60 min in a microwave reaction stove, preferably around 30 min.
- Scheme C illustrates a method of Suzuki coupling for preparing compounds of the formula 7 by reacting a compound of the formula 1 (Int. Pat. Appl. WO2005097791A1), wherein R'i is either fluorine or methyl, nitrogen could be in any position of the ring, with a compound of formula 6.
- the reaction typically run in the presence of Pd(O) such as Pd(Ph) 4 and base such as sodium carbonate, and in a solvent mixture of toluene, ethanol and water.
- the temperature of the reaction typically is 80 0 C.
- compounds of formula 1 may be transformed to boronic acid/ester and couple to heterocyclic bromides similar to formula 6. Table
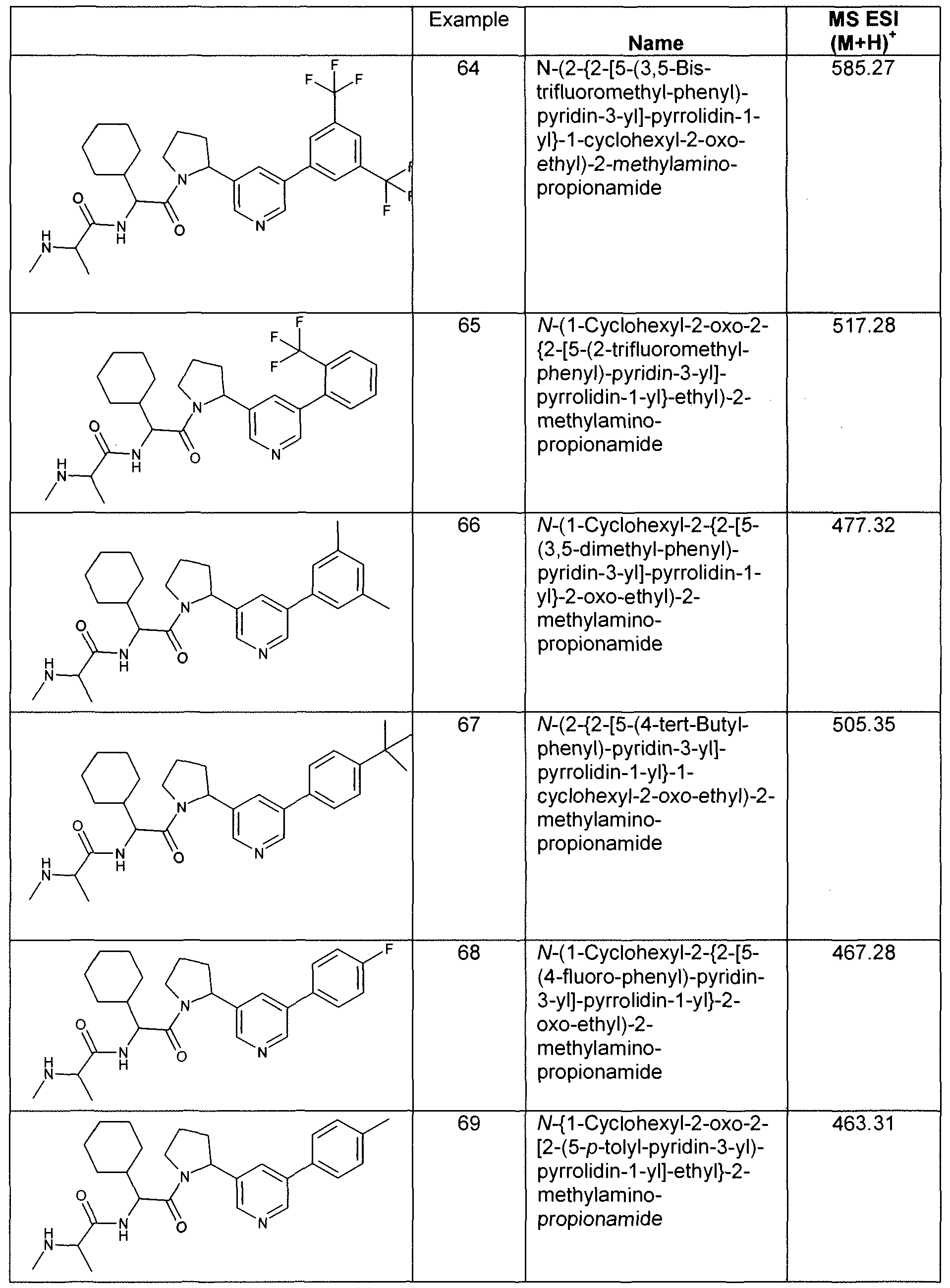
- Example 28 (S)-N-((S)-1-Cyclohexyl-2- ⁇ (S)-2-[2-[2-(1 H-indol-3-yl)-pyridin-4- yl]-pyrrolidin-1-yl ⁇ -2-oxo-ethyl)-2-methylamino-propionamide.
- reaction mixture is stirred at 85°C overnight, cooled to room temperature, filtered through a celite pad and concentrated down to give crude 3-(4, 4, 5, 5-tetramethyl-[1 , 3, 2]dioxaborolan-2-yl)-indole-1 -carboxylic acid tert-butyl ester (13), which is used in next step without further purification.
- the crude product was purified by reversed phase HPLC (Column: Waters Sunfire, 30 X 30 mm; Mobile phase: CH 3 CN 15% H 2 O 85% with 0.1% TFA to CH 3 CN 60% H 2 O 40% with 0.1% TFA by gradient in 11 minutes; Flow rate 40mL/minute; Detector: 215 nm UV) to give product as TFA salt which was dissolved in 30 ml_ of dichloromethane and basicfied by saturated sodium bicabonate to pH 8.
- an ELISA and a cell based assays are utilized.
- the remaining GST-BIR3 fusion protein is monitored by ELISA assay involving first, incubation with goat anti-GST antibodies followed by washing and incubation with alkaline phosphatase conjugated anti-goat antibodies. Signal is amplified using Attophos (Promega) and read with Cytoflour Ex 450nm/40 and Em 580nm.
- IC 50 1 S correspond to concentration of compound which displaces half of GST-BIR3 signal.
- the IC 50 for non-biotinylated Smac is 400 nM.
- the IC 50 values of compounds of Examples 1- 103 in the described ELISA assays ranged from ⁇ 0.001 - 10 ⁇ M.
- the ability of compounds to inhibit tumor cell growth in vitro is monitored using the CellTiter 96® AQ ueous Non-Radioactive Cell Proliferation Assay (Promega).
- This assay is composed of solutions of a novel tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3- carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] and an electron coupling reagent (phenazine methosulfate) PMS.
- MTS is bioreduced by cells into a formazan product, the absorbance of which is measured at 490nm.
- the conversion of MTS into the aqueous soluble formazan product is accomplished by dehydrogenase enzymes found in metabolically active cells.
- the quantity of formazan product as measured by the amount of 490nm absorbance is directly proportional to the number of living cells in culture.
- the IC 5 0 values of compounds described in Examples 1-103 in this cell assays ranged from O.001 - 50 ⁇ M.
- Tablets 1 comprising compounds of the formula (I)
- Tablets comprising, as active ingredient, 50 mg of any one of the compounds of formula (I) mentioned in the preceding Examples 1-103 of the following composition are prepared using routine method:
- the active ingredient is combined with part of the wheat starch, the lactose and the colloidal silica and the mixture pressed through a sieve.
- a further part of the wheat starch is mixed with 5-fold amount of water on a water bath to form a paste and the mixture made first is kneaded with this paste until a weakly plastic mass is formed.
- the dry granules are pressed through a sieve having a mesh size of 3 mm, mixed with a pre-sieved mixture (1 mm sieve) of the remaining corn starch, magnesium stearate and talcum and compressed to form slightly biconvex tablets.
- Tablets 2 comprising compounds of the formula (I)
- Tablets comprising, as active ingredient, 100 mg of any one of the compounds of formula (I) of Examples 1-103 are prepared with the following standard procedures:
- the active ingredient is mixed with the carrier materials and compressed by means of a tabletting machine (Korsch EKO 1 St Zindmesser 10 mm).
- Capsules comprising as active ingredient, 100 mg of any one of the compounds of formula (I) given in Examples 1-103, of the following composition are prepared according to standard procedures
- Manufacturing is done by mixing the components and filling them into hard gelatine capsules, size 1.
- active ingredient refers to a compound of Formula I-VII or a pharmaceutically acceptable salt thereof, as defined herein.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Veterinary Medicine (AREA)
- Biochemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Gastroenterology & Hepatology (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP07844058.3A EP2102229B1 (en) | 2006-10-12 | 2007-10-10 | Pyrrolydine derivatives as iap inhibitors |
| CA002666112A CA2666112A1 (en) | 2006-10-12 | 2007-10-10 | Pyrrolydine derivatives as iap inhibitors |
| JP2009532544A JP5190062B2 (en) | 2006-10-12 | 2007-10-10 | Pyrrolidine derivatives as IAP inhibitors |
| AU2007307763A AU2007307763A1 (en) | 2006-10-12 | 2007-10-10 | Pyrrolydine derivatives as IAP inhibitors |
| BRPI0719221-5A BRPI0719221A2 (en) | 2006-10-12 | 2007-10-10 | Pyrrolidine Derivatives as IAP Inhibitors |
| MX2009003834A MX2009003834A (en) | 2006-10-12 | 2007-10-10 | Pyrrolydine derivatives as iap inhibitors. |
| US12/445,435 US8044209B2 (en) | 2006-10-12 | 2007-10-10 | Pyrrolydine derivatives as IAP inhibitors |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US82923406P | 2006-10-12 | 2006-10-12 | |
| US60/829,234 | 2006-10-12 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2008045905A1 true WO2008045905A1 (en) | 2008-04-17 |
Family
ID=38984426
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2007/080875 WO2008045905A1 (en) | 2006-10-12 | 2007-10-10 | Pyrrolydine derivatives as iap inhibitors |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US8044209B2 (en) |
| EP (1) | EP2102229B1 (en) |
| JP (1) | JP5190062B2 (en) |
| KR (1) | KR20090065548A (en) |
| CN (1) | CN101595121A (en) |
| AU (1) | AU2007307763A1 (en) |
| BR (1) | BRPI0719221A2 (en) |
| CA (1) | CA2666112A1 (en) |
| MX (1) | MX2009003834A (en) |
| RU (1) | RU2009117701A (en) |
| WO (1) | WO2008045905A1 (en) |
Cited By (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008079735A1 (en) * | 2006-12-19 | 2008-07-03 | Genentech, Inc. | Imidazopyridine inhibitors of iap |
| WO2008134679A1 (en) * | 2007-04-30 | 2008-11-06 | Genentech, Inc. | Inhibitors of iap |
| US7547724B2 (en) | 2005-10-25 | 2009-06-16 | Aegera Therpeutics, Inc. | IAP BIR domain binding compounds |
| US7579320B2 (en) | 2006-03-16 | 2009-08-25 | Aegera Therapeutics, Inc. | IAP BIR domain binding compounds |
| WO2009140447A1 (en) | 2008-05-16 | 2009-11-19 | Novartis Ag | Immunomodulation by iap inhibitors |
| WO2010021934A3 (en) * | 2008-08-16 | 2010-06-10 | Genentech, Inc. | Azaindole inhibitors of iap |
| US7772177B2 (en) | 2005-05-18 | 2010-08-10 | Aegera Therapeutics, Inc. | BIR domain binding compounds |
| WO2011016576A1 (en) | 2009-08-04 | 2011-02-10 | Takeda Pharmaceutical Company Limited | Alanine derivatives as inhibitors of apoptosis proteins |
| EP2326631A2 (en) * | 2008-08-18 | 2011-06-01 | Yale University | Mif modulators |
| US8110681B2 (en) | 2006-03-17 | 2012-02-07 | The United States Of America As Represented By The Secretary, Department Of Health And Human Services | Compounds for the treatment of spinal muscular atrophy and other uses |
| US8163792B2 (en) | 2006-05-16 | 2012-04-24 | Pharmascience Inc. | IAP BIR domain binding compounds |
| WO2012080260A1 (en) | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| WO2012080271A1 (en) * | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| US8247557B2 (en) | 2005-12-19 | 2012-08-21 | Genentech, Inc. | IAP inhibitors |
| US8362068B2 (en) | 2009-12-18 | 2013-01-29 | Idenix Pharmaceuticals, Inc. | 5,5-fused arylene or heteroarylene hepatitis C virus inhibitors |
| WO2013103703A1 (en) * | 2012-01-03 | 2013-07-11 | Curis, Inc. | Inhibitors of iap |
| US8609845B2 (en) | 2004-12-20 | 2013-12-17 | Genentech, Inc. | Pyrrolidine inhibitors of IAP |
| WO2014060767A1 (en) * | 2012-10-19 | 2014-04-24 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| WO2014060770A1 (en) * | 2012-10-19 | 2014-04-24 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| WO2014060768A1 (en) * | 2012-10-19 | 2014-04-24 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US8835393B2 (en) | 2008-08-02 | 2014-09-16 | Genentech, Inc. | Inhibitors of IAP |
| US9018214B2 (en) | 2011-04-21 | 2015-04-28 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US9284350B2 (en) | 2010-02-12 | 2016-03-15 | Pharmascience Inc. | IAP BIR domain binding compounds |
| US9540322B2 (en) | 2008-08-18 | 2017-01-10 | Yale University | MIF modulators |
| US9643922B2 (en) | 2008-08-18 | 2017-05-09 | Yale University | MIF modulators |
| US9783538B2 (en) | 2013-12-20 | 2017-10-10 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US9980973B2 (en) | 2012-10-19 | 2018-05-29 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US10441654B2 (en) | 2014-01-24 | 2019-10-15 | Children's Hospital Of Eastern Ontario Research Institute Inc. | SMC combination therapy for the treatment of cancer |
| WO2020027225A1 (en) | 2018-07-31 | 2020-02-06 | ファイメクス株式会社 | Heterocyclic compound |
| WO2021020585A1 (en) | 2019-07-31 | 2021-02-04 | ファイメクス株式会社 | Heterocyclic compound |
| WO2021148396A1 (en) | 2020-01-20 | 2021-07-29 | Astrazeneca Ab | Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of cancer |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PE20110224A1 (en) * | 2006-08-02 | 2011-04-05 | Novartis Ag | PROCEDURE FOR THE SYNTHESIS OF A PEPTIDOMIMETIC OF Smac INHIBITOR OF IAP, AND INTERMEDIARY COMPOUNDS FOR THE SYNTHESIS OF THE SAME |
| MX2011001349A (en) * | 2008-08-04 | 2011-08-17 | Chidi Inc | Certain kynurenine-3-monooxygenase inhibitors, pharmaceutical compositions, and methods of use thereof. |
| US20100317593A1 (en) * | 2009-06-12 | 2010-12-16 | Astrazeneca Ab | 2,3-dihydro-1h-indene compounds |
| US8835458B2 (en) * | 2010-08-31 | 2014-09-16 | Hanmi Science Co., Ltd | Quinoline or quinazoline derivatives with apoptosis inducing activity on cells |
| JP2014094886A (en) * | 2011-02-28 | 2014-05-22 | Nippon Chemiphar Co Ltd | Gpr119 agonist |
| MX2014001088A (en) * | 2011-07-28 | 2015-03-19 | Chdi Foundation Inc | Certain kynurenine-3-monooxygenase inhibitors, pharmaceutical compositions, and methods of use thereof. |
| AU2012302144B2 (en) | 2011-08-30 | 2017-06-15 | Chdi Foundation, Inc. | Kynurenine-3-monooxygenase inhibitors, pharmaceutical compositions, and methods of use thereof |
| SG2014011654A (en) | 2011-08-30 | 2014-08-28 | Chdi Foundation Inc | Kynurenine-3-monooxygenase inhibitors, pharmaceutical compositions, and methods of use thereof |
| JP6324976B2 (en) * | 2012-10-19 | 2018-05-16 | アステックス、セラピューティックス、リミテッドAstex Therapeutics Limited | Bicyclic heterocyclic compounds and their use in therapy |
| JP6321020B2 (en) * | 2012-10-19 | 2018-05-09 | アステックス、セラピューティックス、リミテッドAstex Therapeutics Limited | Bicyclic heterocyclic compounds and their use in therapy |
| BR112017000922A2 (en) | 2014-07-17 | 2018-01-16 | Chdi Foundation, Inc. | methods and compositions for treating hiv-related disorders |
| KR20170004160A (en) * | 2015-07-01 | 2017-01-11 | 엘지전자 주식회사 | Mobile terminal and method for controlling the same |
| US12084472B2 (en) | 2015-12-18 | 2024-09-10 | Ardelyx, Inc. | Substituted 4-phenyl pyridine compounds as non-systemic TGR5 agonists |
| TWI773657B (en) | 2015-12-18 | 2022-08-11 | 美商亞德利克斯公司 | Substituted 4-phenyl pyridine compounds as non-systemic tgr5 agonists |
| JP7257397B2 (en) * | 2017-11-13 | 2023-04-13 | チア タイ ティエンチン ファーマシューティカル グループ カンパニー リミテッド | SMAC mimetics useful as IAP inhibitors and uses thereof |
| EP3936126A4 (en) * | 2019-03-07 | 2022-12-21 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Combination of iap inhibitor and immune checkpoint inhibitor |
| CN113748119B (en) * | 2019-05-10 | 2024-04-02 | 正大天晴药业集团股份有限公司 | Crystal of SMAC mimics used as IAP inhibitor and preparation method thereof |
| CN112521372B (en) * | 2019-09-18 | 2022-07-08 | 南京华威医药科技集团有限公司 | Apoptosis protein inhibitor and preparation method and application thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004007529A2 (en) * | 2002-07-15 | 2004-01-22 | The Trustees Of Princeton University | Iap binding compounds |
| WO2005097791A1 (en) * | 2004-04-07 | 2005-10-20 | Novartis Ag | Inhibitors of iap |
| WO2006014361A1 (en) * | 2004-07-02 | 2006-02-09 | Genentech, Inc. | Inhibitors of iap |
| WO2006069063A1 (en) * | 2004-12-20 | 2006-06-29 | Genentech, Inc. | Pyrrolidine inhibitors of iap |
| WO2007106192A2 (en) * | 2005-12-19 | 2007-09-20 | Genentech, Inc. | Inhibitors of iap |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TR200200767T1 (en) | 2000-05-23 | 2002-09-23 | Vertex Pharmaceuticals Incorporated | Caspase inhibitors and their use |
| CA2491041A1 (en) | 2002-07-02 | 2004-01-15 | Novartis Ag | Peptide inhibitors of smac protein binding to inhibitor of apoptosis proteins (iap) |
-
2007
- 2007-10-10 US US12/445,435 patent/US8044209B2/en not_active Expired - Fee Related
- 2007-10-10 WO PCT/US2007/080875 patent/WO2008045905A1/en active Application Filing
- 2007-10-10 EP EP07844058.3A patent/EP2102229B1/en not_active Not-in-force
- 2007-10-10 CA CA002666112A patent/CA2666112A1/en not_active Abandoned
- 2007-10-10 CN CNA2007800458268A patent/CN101595121A/en active Pending
- 2007-10-10 BR BRPI0719221-5A patent/BRPI0719221A2/en not_active Application Discontinuation
- 2007-10-10 JP JP2009532544A patent/JP5190062B2/en not_active Expired - Fee Related
- 2007-10-10 RU RU2009117701/04A patent/RU2009117701A/en not_active Application Discontinuation
- 2007-10-10 KR KR1020097009617A patent/KR20090065548A/en not_active Application Discontinuation
- 2007-10-10 AU AU2007307763A patent/AU2007307763A1/en not_active Abandoned
- 2007-10-10 MX MX2009003834A patent/MX2009003834A/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004007529A2 (en) * | 2002-07-15 | 2004-01-22 | The Trustees Of Princeton University | Iap binding compounds |
| WO2005097791A1 (en) * | 2004-04-07 | 2005-10-20 | Novartis Ag | Inhibitors of iap |
| WO2006014361A1 (en) * | 2004-07-02 | 2006-02-09 | Genentech, Inc. | Inhibitors of iap |
| WO2006069063A1 (en) * | 2004-12-20 | 2006-06-29 | Genentech, Inc. | Pyrrolidine inhibitors of iap |
| WO2007106192A2 (en) * | 2005-12-19 | 2007-09-20 | Genentech, Inc. | Inhibitors of iap |
Cited By (82)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8609845B2 (en) | 2004-12-20 | 2013-12-17 | Genentech, Inc. | Pyrrolidine inhibitors of IAP |
| US9040706B2 (en) | 2004-12-20 | 2015-05-26 | Genentech, Inc. | Pyrrolidine inhibitors of IAP |
| US7772177B2 (en) | 2005-05-18 | 2010-08-10 | Aegera Therapeutics, Inc. | BIR domain binding compounds |
| US8575113B2 (en) | 2005-05-18 | 2013-11-05 | Pharmascience Inc. | BIR domain binding compounds |
| US7547724B2 (en) | 2005-10-25 | 2009-06-16 | Aegera Therpeutics, Inc. | IAP BIR domain binding compounds |
| US7589118B2 (en) | 2005-10-25 | 2009-09-15 | Aegera Therapeutics, Inc. | IAP BIR domain binding compounds |
| US7795298B2 (en) | 2005-10-25 | 2010-09-14 | Aegera Therapeutics, Inc. | IAP BIR domain binding compounds |
| US8063095B2 (en) | 2005-10-25 | 2011-11-22 | Pharmascience Inc. | IAP BIR domain binding compounds |
| US8247557B2 (en) | 2005-12-19 | 2012-08-21 | Genentech, Inc. | IAP inhibitors |
| US7579320B2 (en) | 2006-03-16 | 2009-08-25 | Aegera Therapeutics, Inc. | IAP BIR domain binding compounds |
| US7645741B2 (en) | 2006-03-16 | 2010-01-12 | Aegera Therapeutics, Inc. | IAP BIR domain binding compounds |
| US8765681B2 (en) | 2006-03-16 | 2014-07-01 | Pharmascience Inc. | IAP BIR domain binding compounds |
| US9365614B2 (en) | 2006-03-16 | 2016-06-14 | Pharmascience Inc. | IAP BIR domain binding compounds |
| US8110681B2 (en) | 2006-03-17 | 2012-02-07 | The United States Of America As Represented By The Secretary, Department Of Health And Human Services | Compounds for the treatment of spinal muscular atrophy and other uses |
| US8648094B2 (en) | 2006-05-16 | 2014-02-11 | Pharmascience, Inc. | IAP BIR domain binding compounds |
| US8163792B2 (en) | 2006-05-16 | 2012-04-24 | Pharmascience Inc. | IAP BIR domain binding compounds |
| WO2008079735A1 (en) * | 2006-12-19 | 2008-07-03 | Genentech, Inc. | Imidazopyridine inhibitors of iap |
| JP2010513561A (en) * | 2006-12-19 | 2010-04-30 | ジェネンテック, インコーポレイテッド | IAP imidazopyridine inhibitors |
| US8907092B2 (en) | 2007-04-30 | 2014-12-09 | Genentech, Inc. | Inhibitors of IAP |
| WO2008134679A1 (en) * | 2007-04-30 | 2008-11-06 | Genentech, Inc. | Inhibitors of iap |
| US9750729B2 (en) | 2008-05-16 | 2017-09-05 | Dana-Farber Cancer Institute, Inc. | Immunomodulation by IAP inhibitors |
| EP3701947A1 (en) * | 2008-05-16 | 2020-09-02 | Novartis AG | Immunomodulation by iap inhibitors |
| US10786491B2 (en) | 2008-05-16 | 2020-09-29 | Novartis Ag | Immunomodulation by IAP inhibitors |
| JP2011524339A (en) * | 2008-05-16 | 2011-09-01 | ノバルティス アーゲー | Immune modulation by IAP inhibitors |
| JP2016094429A (en) * | 2008-05-16 | 2016-05-26 | ノバルティス アーゲー | Immunomodulation by iap inhibitors |
| US11382905B2 (en) | 2008-05-16 | 2022-07-12 | Novartis Ag | Immunomodulation by IAP inhibitors |
| WO2009140447A1 (en) | 2008-05-16 | 2009-11-19 | Novartis Ag | Immunomodulation by iap inhibitors |
| US8835393B2 (en) | 2008-08-02 | 2014-09-16 | Genentech, Inc. | Inhibitors of IAP |
| CN102124004A (en) * | 2008-08-16 | 2011-07-13 | 健泰科生物技术公司 | Azaindole inhibitors of iap |
| JP2012500272A (en) * | 2008-08-16 | 2012-01-05 | ジェネンテック, インコーポレイテッド | Azaindole inhibitors of IAP |
| WO2010021934A3 (en) * | 2008-08-16 | 2010-06-10 | Genentech, Inc. | Azaindole inhibitors of iap |
| EP2318401A2 (en) * | 2008-08-16 | 2011-05-11 | Genentech, Inc. | Azaindole inhibitors of iap |
| EP2318401A4 (en) * | 2008-08-16 | 2013-10-30 | Genentech Inc | Azaindole inhibitors of iap |
| US9643922B2 (en) | 2008-08-18 | 2017-05-09 | Yale University | MIF modulators |
| US11584717B2 (en) | 2008-08-18 | 2023-02-21 | Yale University | MIF modulators |
| EP2326631A4 (en) * | 2008-08-18 | 2012-03-21 | Univ Yale | Mif modulators |
| US10202343B2 (en) | 2008-08-18 | 2019-02-12 | Yale University | MIF modulators |
| US9540322B2 (en) | 2008-08-18 | 2017-01-10 | Yale University | MIF modulators |
| EP2326631A2 (en) * | 2008-08-18 | 2011-06-01 | Yale University | Mif modulators |
| JP2012500260A (en) * | 2008-08-18 | 2012-01-05 | イェール・ユニヴァーシティー | MIF modulator |
| WO2011016576A1 (en) | 2009-08-04 | 2011-02-10 | Takeda Pharmaceutical Company Limited | Alanine derivatives as inhibitors of apoptosis proteins |
| US9187496B2 (en) | 2009-12-18 | 2015-11-17 | Idenix Pharmaceuticals Llc | 5,5-fused arylene or heteroarylene hepatitis C virus inhibitors |
| US8362068B2 (en) | 2009-12-18 | 2013-01-29 | Idenix Pharmaceuticals, Inc. | 5,5-fused arylene or heteroarylene hepatitis C virus inhibitors |
| US9284350B2 (en) | 2010-02-12 | 2016-03-15 | Pharmascience Inc. | IAP BIR domain binding compounds |
| CN103261186A (en) * | 2010-12-13 | 2013-08-21 | 诺瓦提斯公司 | Dimeric IAP inhibitors |
| WO2012080271A1 (en) * | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| WO2012080260A1 (en) | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| US9018214B2 (en) | 2011-04-21 | 2015-04-28 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US11866428B2 (en) | 2011-04-21 | 2024-01-09 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US9676768B2 (en) | 2011-04-21 | 2017-06-13 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| TWI503318B (en) * | 2012-01-03 | 2015-10-11 | Genentech Inc | Inhibitors of iap |
| US9238675B2 (en) | 2012-01-03 | 2016-01-19 | Genentech, Inc. | Inhibitors of IAP |
| US9586991B2 (en) | 2012-01-03 | 2017-03-07 | Genentech, Inc. | Inhibitors of IAP |
| US11096982B2 (en) | 2012-01-03 | 2021-08-24 | Genentech, Inc. | Inhibitors of IAP |
| KR101917992B1 (en) | 2012-01-03 | 2018-11-13 | 쿠리스 인코퍼레이션 | Inhibitors of iap |
| KR101553792B1 (en) | 2012-01-03 | 2015-09-16 | 쿠리스 인코퍼레이션 | Inhibitors of iap |
| WO2013103703A1 (en) * | 2012-01-03 | 2013-07-11 | Curis, Inc. | Inhibitors of iap |
| CN104159897A (en) * | 2012-01-03 | 2014-11-19 | 库里斯公司 | IAP inhibitors |
| US8716236B2 (en) | 2012-01-03 | 2014-05-06 | Genentech, Inc. | Inhibitors of IAP |
| RU2728789C2 (en) * | 2012-01-03 | 2020-07-31 | Кьюрис, Инк. | Iap inhibitors |
| KR101855566B1 (en) | 2012-01-03 | 2018-05-04 | 쿠리스 인코퍼레이션 | Inhibitors of iap |
| US11963994B2 (en) | 2012-01-03 | 2024-04-23 | Genentech, Inc. | Inhibitors of IAP |
| WO2014060770A1 (en) * | 2012-10-19 | 2014-04-24 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| KR102298474B1 (en) | 2012-10-19 | 2021-09-03 | 아스텍스 테라퓨틱스 리미티드 | Bicyclic heterocycle compounds and their uses in therapy |
| WO2014060767A1 (en) * | 2012-10-19 | 2014-04-24 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| WO2014060768A1 (en) * | 2012-10-19 | 2014-04-24 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| KR20150072436A (en) * | 2012-10-19 | 2015-06-29 | 아스텍스 테라퓨틱스 리미티드 | Bicyclic heterocycle compounds and their uses in therapy |
| KR20150072434A (en) * | 2012-10-19 | 2015-06-29 | 아스텍스 테라퓨틱스 리미티드 | Bicyclic heterocycle compounds and their uses in therapy |
| US9663512B2 (en) | 2012-10-19 | 2017-05-30 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US9617283B2 (en) | 2012-10-19 | 2017-04-11 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US9980973B2 (en) | 2012-10-19 | 2018-05-29 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| KR102299832B1 (en) | 2012-10-19 | 2021-09-07 | 아스텍스 테라퓨틱스 리미티드 | Bicyclic heterocycle compounds and their uses in therapy |
| US9617248B2 (en) | 2012-10-19 | 2017-04-11 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US11225476B2 (en) | 2013-12-20 | 2022-01-18 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US9783538B2 (en) | 2013-12-20 | 2017-10-10 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US10618895B2 (en) | 2013-12-20 | 2020-04-14 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US11643410B2 (en) | 2013-12-20 | 2023-05-09 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| US10441654B2 (en) | 2014-01-24 | 2019-10-15 | Children's Hospital Of Eastern Ontario Research Institute Inc. | SMC combination therapy for the treatment of cancer |
| US11639354B2 (en) | 2018-07-31 | 2023-05-02 | Fimecs, Inc. | Heterocyclic compound |
| WO2020027225A1 (en) | 2018-07-31 | 2020-02-06 | ファイメクス株式会社 | Heterocyclic compound |
| WO2021020585A1 (en) | 2019-07-31 | 2021-02-04 | ファイメクス株式会社 | Heterocyclic compound |
| WO2021148396A1 (en) | 2020-01-20 | 2021-07-29 | Astrazeneca Ab | Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of cancer |
Also Published As
| Publication number | Publication date |
|---|---|
| US8044209B2 (en) | 2011-10-25 |
| US20110015232A1 (en) | 2011-01-20 |
| JP2010506847A (en) | 2010-03-04 |
| EP2102229B1 (en) | 2014-03-26 |
| RU2009117701A (en) | 2010-11-20 |
| AU2007307763A1 (en) | 2008-04-17 |
| MX2009003834A (en) | 2009-04-22 |
| KR20090065548A (en) | 2009-06-22 |
| JP5190062B2 (en) | 2013-04-24 |
| EP2102229A1 (en) | 2009-09-23 |
| CA2666112A1 (en) | 2008-04-17 |
| CN101595121A (en) | 2009-12-02 |
| BRPI0719221A2 (en) | 2014-03-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2102229B1 (en) | Pyrrolydine derivatives as iap inhibitors | |
| EP2537846B1 (en) | Intermediate compounds for preparing SMAC peptidomimetics | |
| KR100398944B1 (en) | Boronic acid esters and boronic acid compounds, synthesis methods and uses thereof | |
| EP1902065A2 (en) | Cyclic amide derivatives inhibitors of the inhibitor of apoptosis proteins, their preparation, and their use | |
| JP2012522000A (en) | Inhibitor of hepatitis C virus replication | |
| CA2930030A1 (en) | Macrocyclic compounds for inhibition of inhibitors of apoptosis | |
| EP2099769B1 (en) | 6-oxo.-1, 6-dihydropyrimidin-2-yls in the treatment of proliferative diseases |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200780045826.8 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07844058 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2666112 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007307763 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2009/003834 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2347/DELNP/2009 Country of ref document: IN |
|
| ENP | Entry into the national phase |
Ref document number: 2009532544 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007844058 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2007307763 Country of ref document: AU Date of ref document: 20071010 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020097009617 Country of ref document: KR |
|
| ENP | Entry into the national phase |
Ref document number: 2009117701 Country of ref document: RU Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12445435 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: PI0719221 Country of ref document: BR Kind code of ref document: A2 Effective date: 20090413 |