WO2006089633A2 - Spiroketal-substituierte cyclische ketoenole - Google Patents
Spiroketal-substituierte cyclische ketoenole Download PDFInfo
- Publication number
- WO2006089633A2 WO2006089633A2 PCT/EP2006/001089 EP2006001089W WO2006089633A2 WO 2006089633 A2 WO2006089633 A2 WO 2006089633A2 EP 2006001089 W EP2006001089 W EP 2006001089W WO 2006089633 A2 WO2006089633 A2 WO 2006089633A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- alkoxy
- compounds
- substituted
- formula
- Prior art date
Links
- 0 CC(C*C*(C)C1)CC(*)CC1(C(OC(*)=O)=C1c2c(*)cc(C)c(N)c2*)OC1=O Chemical compound CC(C*C*(C)C1)CC(*)CC1(C(OC(*)=O)=C1c2c(*)cc(C)c(N)c2*)OC1=O 0.000 description 6
- NVRUDHSUWVUWDP-UHFFFAOYSA-O CC1[OH+]N=C(C)N1 Chemical compound CC1[OH+]N=C(C)N1 NVRUDHSUWVUWDP-UHFFFAOYSA-O 0.000 description 1
- WPHPAGOLIKGFKX-UHFFFAOYSA-N Cc(cc1)cc(C)c1C(C(NC1(CC2)CCC22OCCO2)=O)=C1O Chemical compound Cc(cc1)cc(C)c1C(C(NC1(CC2)CCC22OCCO2)=O)=C1O WPHPAGOLIKGFKX-UHFFFAOYSA-N 0.000 description 1
- OJXHIYZLQHWMTG-UHFFFAOYSA-N Cc1cc(C)c(CC(NC(CC2)(CCC22OCCO2)C(OC)=O)=O)c(C)c1 Chemical compound Cc1cc(C)c(CC(NC(CC2)(CCC22OCCO2)C(OC)=O)=O)c(C)c1 OJXHIYZLQHWMTG-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/10—Spiro-condensed systems
- C07D491/113—Spiro-condensed systems with two or more oxygen atoms as ring hetero atoms in the oxygen-containing ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/36—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings
- A01N43/38—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/12—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings condensed with a carbocyclic ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/24—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/24—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms
- A01N43/26—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings
- A01N43/28—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings with two hetero atoms in positions 1,3
- A01N43/30—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings with two hetero atoms in positions 1,3 with two oxygen atoms in positions 1,3, condensed with a carbocyclic ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/24—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms
- A01N43/32—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms six-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/36—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/90—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having two or more relevant hetero rings, condensed among themselves or with a common carbocyclic ring system
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/02—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having no bond to a nitrogen atom
- A01N47/06—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having no bond to a nitrogen atom containing —O—CO—O— groups; Thio analogues thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/08—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having one or more single bonds to nitrogen atoms
- A01N47/10—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof
- A01N47/16—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof the nitrogen atom being part of a heterocyclic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/10—Spiro-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D493/00—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system
- C07D493/02—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system in which the condensed system contains two hetero rings
- C07D493/10—Spiro-condensed systems
Definitions
- the present invention relates to novel spiroketalsubstituABLE cyclic ketoenols, a plurality of processes for their preparation and their use as pesticides and / or herbicides and / or microbicides.
- the invention also relates to selective herbicidal compositions which contain spiroketal-substituted cyclic ketoenols on the one hand and a crop plant compatibility-improving compound on the other hand.
- EP-A-0 262 399 and GB-A-2266 888 disclose similarly structured compounds (3-aryl-pyrrolidine-2,4-diones), of which, however, no herbicidal, insecticidal or acaricidal action has become known.
- Unsubstituted, bicyclic 3-arylpyrrolidine-2,4-dione derivatives (EP-A-355 599, EP-A-415 211 and JP-A-12-053 670 are known to have herbicidal, insecticidal or acaricidal activity and substituted monocyclic 3-arylpyrrolidine-2,4-dione derivatives (EP-A-377 893 and EP-A-442 077).
- EP-A-442 073 polycyclic 3-arylpyrrolidine-2,4-dione derivatives
- EP-A-456 063 EP-A-521 334, EP-A- 596 298, EP-A-613 884, EP-A-613 885, WO 94/01 997, WO 95/26 954, WO 95/20 572, EP-A-0 668 267, WO 96/25 395, WO 96 / 35,664, WO 97/01 535, WO 97/02 243, WO 97/36868, WO 97/43275, WO 98/05638, WO 98/06721, WO 98/25928, WO 99/24437, WO 99/43649 , WO 99/48869 and WO 99/55673, WO 01/17972, WO 01/23354, WO 01/74770, WO 03/0132
- ketal-substituted 1H-arylpyrrolidine-2,4-diones from WO 99/16748 and (spiro) ketal-substituted N-alkoxy-alkoxy-substituted aryl-pyrrolidinediones are known from JP-A-14 205 984 and Ito M. et al., Bioscience, Biotechnology and Biochemistry 67, 1230-1238, (2003).
- W is hydrogen, alkyl, alkenyl, alkynyl, halogen, alkoxy, alkenyloxy, haloalkyl, haloalkoxy or cyano,
- X is halogen, alkyl, alkenyl, alkynyl, alkoxy, alkenyloxy, alkylthio, alkylsulfinyl, alkylsulfonyl, haloalkyl, haloalkoxy, haloalkenyloxy, nitro or cyano,
- Y and Z independently of one another represent hydrogen, alkyl, alkenyl, alkynyl, alkoxy, halogen, halogenoalkyl, haloalkoxy, cyano, nitro or in each case optionally substituted aryl or hetaryl,
- a and B and the carbon atom to which they are attached are each optionally substituted by alkyl, haloalkyl, alkoxy, alkoxyalkyl or optionally substituted phenyl substituted five- to seven-membered ketal, thioketal or dithioketal, which may optionally be interrupted by another heteroatom,
- Q 1 , Q 2 independently of one another represent hydrogen, alkyl, haloalkyl or alkoxy
- G is hydrogen (a) or one of the groups
- E is a metal ion or an ammonium ion
- L is oxygen or sulfur
- M is oxygen or sulfur
- R 1 is in each case optionally halogen or cyano-substituted alkyl, alkenyl,
- R.2 represents in each case optionally halogen, cyano-substituted alkyl, alkenyl, alkoxyalkyl or polyalkoxyalkyl or represents optionally substituted cycloalkyl, phenyl or benzyl,
- R 4 and R 1 independently of one another are each optionally halogen-substituted alkyl, alkoxy, alkylamino, dialkylamino, alkylthio, alkenylthio or cycloalkylthio or in each case optionally substituted phenyl, benzyl, phenoxy or phenylthio,
- R.6 and R7 are each independently hydrogen, each optionally substituted by halogen or cyano alkyl, cycloalkyl, alkenyl, alkoxy, alkoxyalkyl, each optionally substituted phenyl or benzyl, or together with the N-atom to which they are attached , form an optionally oxygen or sulfur containing and optionally substituted cycle.
- the compounds of the formula (I) can also be present in different compositions as optical isomers or mixtures of isomers, which can optionally be separated in a customary manner. Both the pure isomers and the mixtures of isomers, their preparation and use and agents containing them are the subject of the present invention. However, in the following, for the sake of simplicity, compounds of the formula (I) are always referred to, although both the pure compounds and optionally also mixtures with different proportions of isomeric compounds are meant.
- A, B, G, Q, Q 2, W, X, Y and Z have the meaning given above.
- A, B, E, L, M, Ql, Q 2 , W, X 5 Y, Z, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 and R 7 have the meanings given above.
- A, B, Q, Q 2, W, X, Y and Z have the meanings given above,
- A, B, Q 1 , Q 2 , W, X, Y and Z have the meanings given above,
- R 8 is Alky 1 (preferably C 1 -C 6 -alky 1)
- A, B, Q, Q 2, W, X, Y and Z have the meanings given above,
- A, B 5 , Q 1, Q 2, W, X, Y, Z and R 1 have the meanings given above,
- Hal is halogen (especially chlorine or bromine)
- R 1 has the meaning given above
- R 1 and M have the meanings given above,
- Hal is halogen (especially chlorine or bromine),
- Me is a mono- or divalent metal (preferably an alkali metal or alkaline earth metal such as lithium, sodium, potassium, magnesium or calcium),
- Rl Q 5 5 RJL R12 independently represent hydrogen or alkyl (preferably Ci-Cs alkyl),
- D, G, Q 1 , Q 2 , W, X, Y and Z are as defined above. and A, B and the carbon atom to which they are attached for a
- novel compounds of the formula (I) have a good activity as pesticides, preferably as insecticides and / or acaricides and / or fungicides and / or herbicides and, moreover, are often very well tolerated by plants, in particular with respect to crop plants, are.
- the invention also relates to selective herbicidal compositions containing an effective content of a combination of active substances comprising as components (a 1 ) at least one compound of the formula (I) in which A, B 3 D, G, Q 1 , Q 2 , W, X, Y and Z have the abovementioned meaning
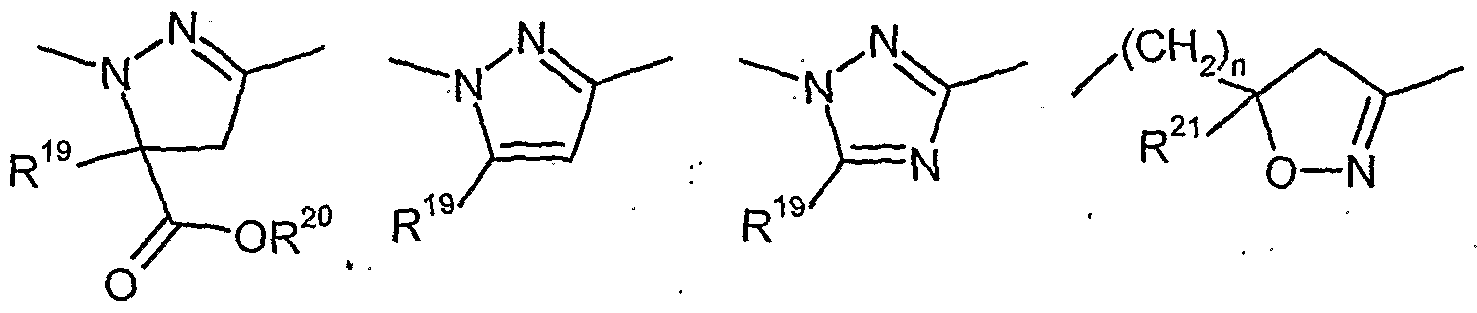
- n is a number 0, 1, 2, 3, 4 or 5
- a 1 represents one of the divalent heterocyclic groupings outlined below,
- n is a number 0, 1, 2, 3, 4 or 5
- a 2 represents alkanediyl having 1 or 2 carbon atoms which is optionally substituted by Q 1 -C 4 -alkyl and / or C 1 -C 4 -alkoxy-carbonyl and / or C 1 -C 4 -alkenyloxycarbonyl,
- R 14 represents hydroxyl, mercapto, amino, Ci-C ⁇ -alkoxy, Ci-C ö alkylthio, CrC ⁇ -alkylamino or di- (Ci-Gj-alkyO-amino group,
- C r C 6 - R 15 is hydroxy, mercapto, amino, C 1 -C 7 -alkoxy, C r C 6 alkylthio, d-Ce-alkenyloxy, C 1 -C 6 - Alkylamino or di- (C 1 -C 4 -alkyl) -amino,
- R 16 is optionally C 1 -C 4 -alkyl which is substituted by fluorine, chlorine and / or bromine,
- R 17 represents hydrogen, in each case optionally substituted by fluorine, chlorine and / or bromine-substituted Q- C ö alkyl, C 2 -C 6 alkenyl or C 2 -C 6 -AIkinyl, C-G t-alkoxy-Ci Gralkyl, Dioxolanyl-C r C 4 -alkyl, furyl, furyl-C 1 -C 4 -alkyl, thienyl, thiazolyl, piperidinyl, or phenyl optionally substituted by fluorine, chlorine and / or bromine or C 1 -C 4 -alkyl,
- R 18 is hydrogen, in each case optionally substituted by fluorine, chlorine and / or bromine C] - 'Ce-alkyl, C 2 -C 6 alkenyl or C 2 -C 6 alkynyl, Ci-C 4 alkoxy C r C 4 -alkyl, Dipxolanyl-Ci-C 4 - alkyl, furyl, furyl-Ci-C 4 -alkyl, thienyl, thiazolyl, piperidinyl, or optionally
- R 19 is hydrogen, cyano, halogen, or is in each case optionally substituted by fluorine, chlorine and / or bromine-substituted C 1 -C 6 -alkyl, C 3 -C 6 -cycloalkyl or phenyl,
- R 20 is hydrogen, in each case optionally substituted by hydroxy, cyano, halogen or Cj-G) - alkoxy-substituted C r C 6 alkyl, C 3 -C 6 cycloalkyl or tri-CCrC ⁇ alky ⁇ silyl,
- R 21 represents hydrogen, cyano, halogen or represents in each case optionally fluorine, chlorine and / or bromine-substituted C 1 -C 4 -alkyl, C 3 -C 6 -cycloalkyl or phenyl,
- X 1 represents nitro, cyano, halogen, C r C 4 alkyl, Q-CrHalogenalkyl, C r C 4 alkoxy or C 1 -C 4 - haloalkoxy,
- X 2 represents hydrogen, cyano, nitro, halogen, QQ-alkyl, C r C 4 haloalkyl, Ci-C,
- X 3 represents hydrogen, cyano, nitro, halogen, C r C alkyl 4 5 C r C 4 haloalkyl, C, -C 4 alkoxy or C r C is 4 haloalkoxy,
- t is a number 0, 1, 2, 3, 4 or 5
- v is a number 0, 1, 2, 3, 4 or 5
- R 22 is hydrogen or C 4 alkyl C r '
- R 23 is hydrogen or C r C 4 alkyl,.
- R 24 represents hydrogen, in each case optionally cyano-, halogen or Ci-C4 alkoxy-substituted Ci-COE--alkyl, C r C 6 alkoxy, C r C 6 alkylthio, C r C 6 -alkylamino or di- (C R C 4 -alkyl) -amino, or in each case optionally substituted by cyano, halogen or C 1 -C 4 -alkyl-substituted C 3 -C 6 -cycloalkyl, C 3 -C 6 -cycloalkyloxy, C 3 -C 6 -cycloalkylthio or C 3 -C 6 -cycloalkylamino,
- R 25 represents hydrogen, optionally substituted by cyano, hydroxy, halogen or Ci-C 4 alkoxy Cj-C ⁇ -alkyl, each optionally substituted by cyano or halogen substituted C 3 -C 6 - alkenyl or C 3 -C 6 alkynyl, or C 3 -C 6 -cycloalkyl optionally substituted by cyano, halogen or C 1 -C 6 -alkyl,
- R 26 is hydrogen, optionally substituted by cyano, hydroxy, halogen or Ci-C 4 - alkoxy Ci-Cö-alkyl, in each case optionally substituted by cyano or halogen C 3 -C 6 - alkenyl or C 3 -C 6 alkynyl, optionally by cyano, halogen or C r C 4 -alkyl-substituted C 3 -C 6 cycloalkyl, or optionally substituted by nitro, cyano, halogen, Ci-C4-alkyl, Ci-C4-haloalkyl, Ci-C 4 alkoxy or C r C 4 -haloalkoxy-substituted phenyl, or together with R 25 represents in each case optionally substituted by Ci-C 4 alkyl-substituted C 2 -C 6 -
- X is 4-C Ci 4 alkoxy or Ci-C 4 haloalkoxy stands for nitro, cyano, carboxy, carbamoyl, formyl, Sulfaraoyl, hydroxy, amino, halo, C r C 4 alkyl, Ci-C 4 haloalkyl, and
- X is nitro, cyano, carboxy, carbamoyl, formyl, sulfamoyl, hydroxy, amino, halo, C 1 -C 4 alkyl, C r C r haloalkyl ; Ci-C 4 alkoxy or C r C 4 haloalkoxy.
- W preferably represents hydrogen, Ci-Gg-alkyl, C 2 -CG-alkenyl, C 2 -CG-alkynyl, halogen, C 1 -C 6 -alkoxy, Ci-C 4 haloalkyl, CrC 4 -HalogenaIkoxy or cyano,
- X preferably represents halogen, C 1 -C 8 alkyl, C 2 -CG-alkenyl, C 2 -CG-AIkUIyI, C 1 -C 6 - haloalkyl, C 1 -CG -alkoxy, C3-Cg-alkenyloxy, C ⁇ Cg alkylthio, C r C 6 alkylsulfinyl, C ⁇ -CG-alkylsulfonyl, C1-C6 haloalkoxy, C3-Cg-Hal ⁇ genalkenyloxy, nitro or cyano,
- Y and Z are preferably each independently hydrogen, halogen, Cj-C ⁇ alkyl, C 2 - Cg alkenyl, C 2 -CG-AIkUVyL, C 1 -C 6 alkoxy, C 1 -C 6 haloalkyl, C 1 -C 6 -haloalkoxyl, cyano, C 2 -Cg -alkenyl, C 2 -Cg -alkynyl or for one of the (Het) -aryl radicals
- V 1 is preferably hydrogen, halogen, Cj-C ⁇ alkyl, C 1 -C 6 alkoxy, C 1 -C 6 - alkylthio, C j--Cö Alkylsulfmyl, C 1 -C 6 alkylsulfonyl, C j - C 1-4 haloalkyl, C 1 -C 4 haloalkoxy, nitro, cyano or in each case optionally mono- or polysubstituted by halogen, C 1 -C 6 -alkyl, C 1 -C 8 -alkoxy, C 1 -C 4 -haloalkyl, C 2 -C 4 -haloalkoxy, nitro or cyano-substituted phenyl, phenoxy, phenoxy-Ci-C4-alkyl, phenyl-C 1 -C 4 -alkoxy, phenylthio-C 1 -C 4 alkyl or phen
- V 1 and V 1 are preferably, independently of one another, hydrogen, halogen, C 1 -C 6 -alkyl, C 1 -C -alkoxy, C 1 -C 4 -haloalkyl or C 1 -C 4 -haloalkoxy,
- a and B and the carbon atom to which they are attached preferably represent an optionally mono- to tetrasubstituted by Ci-Cg-alkyl, Ci-C4-haloalkyl, Ci-C 6 alkoxy or C 1 -C 4 alkoxy C 1 -C 4 -alkyl-substituted five- to seven-membered ketal, thioketal or dithioketal, which may optionally be interrupted by another oxygen atom, sulfur atom or by the group N - y 4 ,
- V 4 is preferably hydrogen, Q-COE-alkyl, C 3 -C 6 alkenyl, or represents the groups -CO-V S, -CO 2 V 5, CO-NH-V 5 or CO-NH-OV 5 .
- V 5 is preferably C 1 -C 6 -alkyl
- D is preferably NH (1) or oxygen (2)
- Q 1 and Q 2 are preferably independently of one another represent hydrogen, C r C 6 alkyl, C 1 -C 2 - haloalkyl or C 1 -C 4 - ⁇ -alkoxy. , ⁇ .
- G is preferably hydrogen (a) or one of the groups
- E is a metal ion or an ammonium ion
- M is oxygen or sulfur
- RI preferably represents in each case optionally substituted by halogen or cyano, C ⁇ - C 2 o alkyl, C 2 -C 2 -alkenyl, C 1 -C 6 alkoxy-C 1 -C 6 alkyl, C ⁇ Cg alkylthio -Ci-Cs-alkyl or poly-C i -Cs-alkoxy-C 1 -C 6 -alkyl or C 3 -C 8 -cycloalkyl optionally substituted by halogen, C 1 -C 8 -alkyl or C 1 -C 8 -alkoxy, in which optionally one or two not directly adjacent methylene groups are replaced by oxygen and / or sulfur,
- halogen cyano, nitro, C 1 -C 8 alkyl, C 1 -C 8 alkoxy, C 1 -C 6 - haloalkyl, C 1 -C 6 haloalkoxy, C 1 -C 8 alkylthio or C C 1 -C 6 -alkylsulfonyl-substituted phenyl,
- phenyl-C 1 -C 6 -alkyl which is unsubstituted or substituted by halogen, nitro, cyano, C 1 -C 8 -alkyl, C 1 -C -alkoxy, C 1 -C 8 -halogenoalkyl or C 1 -C 9 -halogenoalkoxy,
- R 2 preferably represents in each case optionally halogen or cyano-substituted C 1 -.
- Css-Cg-cycloalkyl optionally substituted by halogen, C 1 -C 6 -alkyl or Cj-Cg-alkoxy or
- R-3 is preferably C 1 -C 8 -alkyl optionally substituted by halogen or in each case optionally halogen, C 1 -C 6 -alkyl, C 1 -C 8 -alkoxy, C 1 -C 4 -haloalkyl, C 1 -C 4 Haloalkoxy, cyano or nitro substituted phenyl or benzyl,
- R4 and RS are each, independently of one another, preferably C 1 -C -alkyl which is optionally substituted by halogen, C 1 -C 6 -alkoxy, C 1 -C 6 -alkylamino, di- (C 1 -C 6 -alkyl) ammo, C 1 -C 4 -alkyl -Cg-alkylthio or Cß-Cg-alkenylthio or for each optionally by halogen,
- R ⁇ and R? independently of one another preferably represent hydrogen, in each case optionally halogen- or cyano-substituted C 1 -C 6 -alkyl, C 3 -C 9 -cycloalkyl, C 1 -C -alkoxy, C 3 -C -alkenyl or C 1 -C 6 -alkoxy C 2 -C 6 -alkyl, each of which is optionally substituted by halogen, Cj-Cg-alkyl, C 1 -C 6 -haloalkyl or C 1 -C 8 -alkoxy-substituted phenyl or benzyl or together represent an optionally substituted by Cj-Cg-alkyl C3 -C6-alkylene radical in which optionally a methylene group is replaced by oxygen or sulfur.
- halogen is fluorine, chlorine, bromine and iodine, in particular fluorine, chlorine and bromine.
- W is particularly preferably hydrogen, chlorine, bromine, C 1 -C 4 -alkyl, C 2 -C 4 -alkenyl, C 2 -C 4 -alkynyl, C 1 -C 4 -alkoxy, C 1 -C 8 -haloalkyl or Cy-C -Halogenalkoxy
- X is more preferably chlorine ,.
- Y and Z are particularly preferably each independently hydrogen, fluorine, chlorine, bromine, C 1 -C 4 alkyl, C 2 -C 4 -AlkCHyI 5 C 2 -C 4 -AIkMyI, C 1 -C 6 -alkoxy, Ci C 4 -haloalkyl, C 1 -C 4 -haloalkoxy, cyano, C 2 -C 4 -alkenyl, C 2 -C 4 -alkyl or for one of the (het) -aryl radicals,
- V * particularly preferably represents hydrogen, fluorine, chlorine, bromine, C 1 -C 8 alkyl, C 1 -C 4 - alkoxy, C 1 -C 2 - haloalkyl, C 1 -C 2 -HaIOgBUaIkOXy, nitro, cyano or in each case, if appropriate, once to twice by fluorine, chlorine, bromine, C 1 -C 4 -alkyl, C 1 -C 4 -alkoxy, C 1 -
- V 1 and V 1 particularly preferably independently of one another represent hydrogen, fluorine, chlorine, bromine, C 1 -C 4 -alkyl, C 1 -C 4 -alkoxy, or C 1 -C 2 -haloalkoxy,
- a and B and the carbon atom, on. that they are attached particularly preferably represent an optionally mono- to trisubstituted by C r C 4 - ⁇ .lkyl, C r C3-haloalkyl, C 1 -C 4 -alkoxy or C 1 -C 4 -alkoxy-C 1 -C 2 -EIlCyI substituted five-, six- or sieve 'engliedr ⁇ ges ketal, which may be optionally interrupted by a further oxygen atom,
- D particularly preferably represents NH (1) or oxygen (2)
- Q 1 and Q 2 are particularly preferably independently of one another represent hydrogen, methyl, ethyl, trifluoromethyl, methoxy or ethoxy,
- G is particularly preferably hydrogen (a) or one of the groups
- E is a metal ion or an ammonium ion
- M is oxygen or sulfur
- R.1 particularly preferably represents in each case optionally mono- to trisubstituted by fluorine or chlorine-substituted Ci-Cig-alkyl, C2 -Ci6 alkenyl, C 1 -C 6 alkoxy-C ⁇ alkyl, C] 1 -Cg- Alkylthio-C ⁇ -C4-alkyl or poly-C i -Cg-alkoxy-Ci-C ⁇ alkyl or optionally mono- to. two times substituted by fluorine, chlorine, C ⁇ -Cs-alkyl or Ci-C5-alkoxy
- phenyl-C 1 -C 4 -alkyl optionally substituted once to twice by fluorine, chlorine, bromine, C 1 -C 4 -alkyl, C 1 -C 4 -alkoxy, C 1 -C 3 -haloalkyl or C 1 -C 3 -haloalkoxy, in each case optionally mono- to disubstituted by fluorine, chlorine, bromine or C 1 -C 4 -alkyl-substituted pyrazolyl, thiazolyl, pyridyl, pyrimidyl, furanyl or thienyl,
- R 2 particularly preferably represents in each case optionally monosubstituted to trisubstituted by fluorine or chlorine C 1 -C 16 -alkyl, C 2 -C 16 -alkenyl, C 1 -C 6 -alkoxy-C 2 -C 6 -alkyl or poly- C 1 -C 6 -alkoxy-C 2 -C 6 -alkyl,
- R 3 particularly preferably represents optionally mono- to trisubstituted by fluorine or chlorine-substituted C 1 -C 6 -alkyl or in each case optionally mono- to disubstituted by fluorine, chlorine, bromine, C 1 -C 4 -alkyl, C j ⁇ -alkoxy, Ci -C 2 -haloalkoxy, C 1 -C 2 -haloalkyl, cyano or nitro-substituted phenyl or benzyl,
- R 4 and R 1 independently of one another particularly preferably represent in each case optionally mono- to trisubstituted by fluorine or chlorine, C 1 -C 6 -alkyl, C 1 -C 6 -alkoxy, C 1 -C 6 -alkylamino, di- (C 1 -C 6 -alkyl) amino, C 1 -C 6 -alkylthio or C 3 -C 4 -alkenylthio, or in each case optionally mono- to disubstituted by fluorine, chlorine, bromine, nitro, cyano, C 1 -C 3 -alkoxy, C 1 -C 3 -haloalkoxy, C 1 -C 3 alkylthio, C 1 -C 3 haloalkylthio, C 1 -C 3 alkyl or C 1 -C 3 -halogenoalkyl-substituted phenyl, phenoxy or phenylthio,
- R 6 and R 7 independently of one another particularly preferably represent hydrogen, in each case optionally mono- to trisubstituted by fluorine or chlorine, C 1 -C 6 -alkyl, C 3 -C 6 -cycloalkyl, C 1 -C 6 -alkoxy, Cs-Cg- Alkenyl or C 1 -C 6 -Alkoxy ⁇ -Cö alkyl, each optionally optionally mono- to trisubstituted by fluorine, chlorine, bromine, Cj-C5-Halo genalkyl, Cj-C5-alkyl or C j -C5-alkoxy-substituted phenyl or benzyl, or together for an optionally C 2 -C 4 -alkyl-substituted C 3 -C 6 -alkylene radical, in which optionally a methylene group is replaced by oxygen or sulfur.
- halogen is fluorine, chlorine and bromine, in particular fluorine and chlorine.
- W is very particularly preferably hydrogen, chlorine, bromine, methyl, ethyl, vinyl, ethynyl, propynyl, methoxy, ethoxy or trifluoromethyl,
- X is very particularly preferably chlorine, bromine, methyl, ethyl, propyl, isopropyl, vinyl, ethynyl, propynyl, methoxy, ethoxy, trifluoromethyl, difluoromethoxy, trifluoromethoxy or cyano,
- Y and Z very particularly preferably independently of one another represent hydrogen, fluorine, chlorine, bromine, methyl, ethyl, vinyl, ethynyl, propynyl, methoxy, trifluoromethyl, trifluoromethoxy, cyano or a phenyl radical,
- V 1 very particularly preferably represents hydrogen, fluorine, chlorine, bromine, methyl, ethyl, n-propyl, isopropyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropoxy, trifluoromethyl or trifluoromethoxy,
- V 2 very particularly preferably represents hydrogen, fluorine, chlorine, methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy or trifluoromethyl,
- a and B and the carbon atom to which they are attached very particularly preferably represent an optionally mono- or disubstituted by methyl, ethyl, propyl, trifluoromethyl,
- Oxygen atom can be interrupted, D is very particularly preferably NH (I) or oxygen (2), ',.
- Q 1 and Q 2 very particularly preferably represent hydrogen
- G is very particularly preferably hydrogen (a) or one of the groups
- E is a metal ion or an ammonium ion
- M is oxygen or sulfur
- R.1 very particularly preferably represents in each case optionally monosubstituted to trisubstituted by fluorine or chlorine, C 1 -C 10 -alkyl, C 2 -C 10 -alkenyl, C 1 -C 4 -alkoxy-C 1 -C 2 -alkyl, C 1 C 4 -alkylthio-C 1 -C 2 -alkyl or C 3 -C 6 -cycloalkyl which is optionally monosubstituted by fluorine, chlorine, methyl, ethyl or methoxy,
- R.2 very particularly preferably represents in each case optionally mono- to trisubstituted by fluorine or chlorine, C ⁇ -C Q alkyl, C 2 -C 10 alkenyl or C 1 -C 4 alkoxy-C2-C4 alkyl,
- cyclopentyl or cyclohexyl or represents phenyl or benzyl which is optionally mono- to disubstituted by fluorine, chlorine, cyano, nitro, methyl, ethyl, methoxy, trifluoromethyl or trifluoromethoxy,
- R 1 very particularly preferably represents in each case optionally mono- to trisubstituted by fluorine or chlorine, methyl, ethyl, propyl or iso-propyl, or in each case optionally simply by fluorine, chlorine, bromine, methyl, ethyl, iso-propyl, teit-butyl, Methoxy, ethoxy, iso-propoxy, trifluoromethyl, trifluoromethoxy, cyano or nitro substituted phenyl,
- R.4 and R.5 are each independently very particularly preferably represent C 1 -C 4 alkoxy or C j - C ⁇ alkylthio, or represents in each case optionally monosubstituted by fluorine, chlorine, bromine, nitro,
- R.6 and R? independently of one another very particularly preferably represent hydrogen, C1-C4 alkyl, C 3 -C ⁇ 5 cycloalkyl, Ci-O / j alkoxy, C3-C 4 alkenyl or C 1 -C 4 -alkoxy-C2 C4-alkyl, in each case optionally once or twice by fluorine, chlorine, bromine, methyl,
- W is particularly preferably hydrogen, chlorine, bromine, methyl, ethyl or methoxy
- X is particularly preferably chlorine, bromine, methyl, ethyl or methoxy
- Y and Z are particularly preferably independently of one another hydrogen, chlorine, bromine, methyl or the radical
- radicals W or Z must not be hydrogen if X and Y are methyl
- a and B and the carbon atom to which they are bonded are particularly preferably a five- or six-membered ketal which is optionally mono- to disubstituted by methyl, ethyl, propyl, monochloromethyl or methoxymethyl.
- D is particularly preferably NH (1) or oxygen (2)
- Q 1 and Q 2 are particularly preferably hydrogen
- G is particularly preferably hydrogen (a) or one of the groups
- R 1 particularly preferably represents C 1 -C 10 -alkyl, C 1 -C 4 -alkoxy-C 1 -C 2 -alkyl, C 3 -C 6 -cycloalkyl,
- R ⁇ especially preferably represents C ⁇ -C ⁇ Q alkyl, C2-C ⁇ Q alkenyl, or benzyl,
- R 1 particularly preferably represents methyl
- R ⁇ and R? are particularly preferably together for a C5-C (5-alkylene radical in which optionally a methylene group is replaced by oxygen or sulfur.
- Saturated or unsaturated hydrocarbon radicals such as alkyl, alkanediyl or alkenyl may also be used in conjunction with heteroatoms, e.g. in alkoxy, as far as possible, in each case straight-chain or branched.
- optionally substituted radicals may be monosubstituted or polysubstituted, with multiple substituents the substituents being the same or different.
- Table 2 W. X, Y and Z as indicated in Table 1
- Table 3 W, X, Y and Z as given in Table 1
- Table 5 W, X, Y and Z as given in Table 1
- Table 7 W, X 5 Y and Z as indicated in Table 1
- Table 8 W, X, Y and Z as given in Table 1 AB 4 -O-CH-CH-CHrO-CH 3
- Table 10 W, X, Y and Z as given in Table 1
- Table 11 W, X, Y and Z as given in Table 1
- Table 12 W, X, Y and Z as given in Table 1
- Table 14 W, X, Y and Z as given in Table 1
- Table 15 W, X, Y and Z as given in Table 1
- Table 16 W, X, Y and Z as given in Table 1
- Table 18 W, X, Y and Z as shown in Table 17
- Table 20 W 5 X, Y and Z as given in Table 17
- Table 21 W, X, Y and Z as shown in Table 17
- Table 22 W, X, Y and Z as shown in Table 17
- Table 23 W, X, Y and Z as indicated in Table 17
- Table 27 W, X, Y and Z as indicated in Table 17
- Table 28 W, X, Y and Z are as shown in Table 17
- Table 30 W, X, Y and Z as shown in Table 17
- Table 31 W, X, Y and Z as shown in Table 17
- Table 32 W, X, Y and Z as indicated in Table 17
- a 1 preferably represents one of the divalerite / heterocyclic groupings outlined below
- n preferably represents the numbers 0, 1, 2, 3 or 4.
- a 2 preferably represents in each case optionally methyl, ethyl, methoxycarbonyl or ethoxycarbonyl or alloxycarbonyl-substituted methylene or ethylene.
- R 14 is preferably hydroxy, mercapto, amino, methoxy, ethoxy, n- or i-propoxy, n-, i-, s- or t-butoxy, methylthio, ethylthio, n- or i-propylthio, n-, i -, s- or t-butylthio, methylamino, ethylamino, n- or i-propylamino, n-, i-, s- or t-butylamino, dimethylamino or diethylamino.
- R 15 preferably represents hydroxyl, mercapto, amino, mefhoxy, ethoxy, n- or i-propoxy, n-, i-, s- or t-butoxy, 1-methylhexyloxy, allyloxy, 1-allyloxymethyl-ethoxy, methylthio, ethylthio , n- or i-propylthio, n-, i-, s- or t-butylthio, methylamino, ethylamino, n- or i-propylamino, n-, i-, s- or t-butylamino, dimethylamino or diethylamino.
- R 16 is preferably in each case optionally substituted by fluorine, chlorine and / or bromine substituted methyl, ethyl, n- or i-propyl.
- R 17 is preferably hydrogen, in each case optionally fluorine- and / or chlorine-substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, propenyl, butenyl, propynyl or butynyl, methoxymethyl, Ethoxymethyl, methoxyethyl, ethoxyethyl, dioxolanylmethyl, furyl, furylmethyl, thienyl, thiazolyl, piperidinyl, or optionally fluorine, chlorine,
- R 18 is preferably hydrogen, in each case optionally fluorine- and / or chlorine-substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, propenyl, butenyl, propynyl or butynyl, methoxymethyl, Ethoxymethyl, methoxyethyl, ethoxyethyl, dioxolanylmethyl, furyl, furylmethyl, thienyl, thiazolyl, piperidinyl, or optionally fluorine, chlorine,
- R 19 preferably represents hydrogen, cyano, fluorine, chlorine, bromine, or represents in each case optionally substituted by fluorine, chlorine and / or bromine-substituted methyl, ethyl, n- or i-propyl, cyclo pro ⁇ yl. Cyclobutyl, cyclopentyl, cyclohexyl or phenyl.
- R 20 is preferably hydrogen, optionally substituted by hydroxyl, cyano, fluorine, chlorine, methoxy, ethoxy, n- or i-propoxy substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t- butyl.
- R 21 preferably represents hydrogen, cyano, fluorine, chlorine, bromine, or represents in each case optionally fluorine, chlorine and / or bromine-substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t- Butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or phenyl.
- X 1 is preferably nitro, cyano, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, difluoromethyl, dichloromethyl, trifluoromethyl, trichloromethyl, chlorodifluoromethyl , Fluorodichloromethyl, methoxy, ethoxy, n- or i-propoxy, difluoromethoxy or trifluoromethoxy.
- X 2 is preferably hydrogen, nitro, cyano, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, difluoromethyl, dichloromethyl, trifluoromethyl, trichloromethyl, chlorodifluoromethyl , Fluorodichloromethyl, methoxy, ethoxy, n- or i-propoxy, difluoromethoxy or trifluoromethoxy.
- X 3 is preferably hydrogen, nitro, cyano, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, difluoromethyl, dichloromethyl, trifluoromethyl, trichloromethyl, chlorodifluoromethyl , Fluorodichloromethyl, methoxy, ethoxy, n- or i-propoxy, difluoromethoxy or trifluoromethoxy.
- t preferably stands for the numbers 0, 1, 2, 3 or 4.
- v preferably represents the numbers 0, 1, 2 or 3.
- R 22 is preferably hydrogen, methyl, ethyl, n- or i-propyl.
- R 23 is preferably hydrogen, methyl, E & yl, n- or i-propyl.
- R 24 is preferably hydrogen, respectively. optionally by cyano, fluorine; Chlorine, methoxy, ethoxy, n- or i-propoxy-substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, methoxy, ethoxy, n- or i-propoxy, n- , i-, s- or t-butoxy, methylthio, ethylthio, n- or i-propylthio, n-, i-, s- or t-butylthio,, methylamino, ethylamino, n- or i-propylamino, n-, i-, s- or t-butylamino, dimethyla
- R 25 is preferably hydrogen, in each case optionally cyano, hydroxyl, fluorine, chlorine, methoxy, ethoxy, n- or i-propoxy-substituted methyl, ethyl, n- or i-propyl, n-, i- or s-butyl, in each case optionally substituted by cyano, fluorine, chlorine or bromine substituted propenyl, butenyl, propynyl or butynyl, or in each case optionally substituted by cyano, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl.
- R 26 is preferably hydrogen, in each case optionally cyano, hydroxyl, fluorine, chlorine, methoxy, ethoxy, n- or i-propoxy-substituted methyl, ethyl, n- or i-propyl, n-, i- or s-butyl, in each case optionally substituted by cyano, fluorine, chlorine or bromine substituted propenyl, butenyl, propynyl or butynyl, in each case optionally substituted by cyano, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, cyclopropyl, cyclobutyl, cyclopentyl or
- Cyclohexyl or optionally by nitro, cyano, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, trifluoromethyl, methoxy, ethoxy, n- or i- Propoxy, di-fluoromethoxy or trifluoromethoxy-substituted phenyl, or together with R 25 for each optionally substituted by methyl or ethyl butane-1,4-diyl (trimethylene), pentane-1,5-diyL, 1-oxa-butan-1, 4-diyl or 3-oxa-pentane-l, 5-diyl.
- X 4 is preferably nitro, cyano, carboxy, carbamoyl, formyl, sulfamoyl, hydroxy, amino, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl , Trifluoromethyl, methoxy, ethoxy, n- or i-propoxy, difluoromethoxy or trifluoromethoxy.
- X 5 is preferably nitro, cyano, carboxy, carbamoyl, formyl, sulfamoyl, hydroxy, amino, fluorine, chlorine, bromine, methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl , Trifluoromethyl,
- herbicidal safeners particularly preferred compounds of the formula (Ha) according to the invention are listed in the table below. , , ' . , Table Examples of the compounds of the formula (Ha)
- herbicidal safeners particularly preferred compounds of formula (Ub) according to the invention are listed in the table below.
- herbicidal safeners particularly preferred compounds of the formula (Ho) according to the invention are listed in the table below.
- herbicidal safeners according to the invention very particularly preferred compounds of formula (Hd) are listed in the table below.
- Cropquintocet-mexyl, fenchlorazole-ethyl, isoxadifen-ethyl, mefenpyr-diethyl, furilazoles, fenclorim, cumyluron, dymron, dimepiperate and the compounds He-5 and IIe are considered as the crop plant compatibility-improving compound [component (b 1 )].
- 11 is most preferred, with cloquintocet-mexyl and mefenpyr-diethyl being particularly emphasized.
- the compounds of general formula (Ha) to be used according to the invention as safeners are known and / or can be prepared by processes known per se (cf., WO-A-91/07874, WO-A-95/07897).
- reaction scheme For example, according to process (G), use is made of 8,8'-propylenedioxy-3 - [(2,4-dichloro-6-methyl) -phenyl] -l-oxaspiro [4,5] decane-2,4-dione and methanthoethane phosphonic acid chloride (2,2,2-trifluoroethyl ester) as starting materials, the reaction can be represented by the following reaction scheme:
- A, B, QI, Q2, W, X, Y, Z and R ⁇ have the meanings given above,
- acylamino acid esters of the formula (II) are obtained, for example, if amino acid derivatives of the formula (XVI)
- A, B, Cr and Q 2 and R 1 have the abovementioned meaning
- W, X, Y and Z have the meanings given above, and U for a by Carbonklaregent michigangenzien such as carbonyldiimidazole,.
- Carbonyldiimide such as Dicyciohexylcarbondiimid
- Phosphorylierungsreagenzien such as POCl 3 , BOP-Cl
- halogenating agent such as thionyl chloride, oxalyl chloride, phosgene or chloroformate introduced leaving group
- A, B, Q! , Cp-, W, X, Y and Z have the meanings given above,
- the compounds of the formula (XVDI) are obtained, for example, by reacting 1-aminocyclohexanecarboxylic acids of the formula (XIX). , , , ,
- A, B, Ql and Q 2 have the meanings given above
- A, B, Q 1 , Q 2 , W, X, Y, Z and R 8 have the meanings given above,
- A, B, Q 1 and Q 2 have the meanings given above,
- A, B, Cr, Cr, W, X, Y and Z have the meanings given above,
- the compounds of formula (XXI) are also new.
- the compounds of formula (XX) are in part new and can be e.g. as described in EP-A-595 130.
- A, B, Q 1 , Q 2 , W, X, Y, Z and R 8 have the meanings given above,
- A, B, CA Q 2 and R 1 have the meanings given above,
- the l-hydroxy-S-alkoxy-cyclohexyl-carboxylic acid esters of the formula (XXII) are new. They are obtained, for example, by reacting substituted 1-hydroxy-4,4'-alkylidenyldioxy-cyclohexane-carbonitriles in the presence of acids, e.g. reacted according to Pinner with alcohols and the resulting l-hydroxy-4- ⁇ xo-cylohexancarbonklareester known from WO 99/16748 (Ex. XXI-I) ketalized again with diols.
- the cyanohydrin is obtained, for example, by reaction of substituted 4,4'-alkylidenyldioxycyclohexan-1-ones with hydrocyanic acid (see WO 99/16748).
- acid halides of the formula (IV) carboxylic anhydrides of Formula (V), chloroformate or chloroformic thioester of the formula (VI), chloro-monothioic acid esters or Chlordithioameisenhoffreester of formula (VI), sulfonyl chlorides of the formula (VTS), phosphorus compounds of the formula (IX) and metal hydroxides, metal alkoxides or amines of the formula (X ) and (XI) and isocyanates of the formula (XU) and carbamido acid chlorides of the formula (XST) and diols of the formula (XV) are generally known compounds. fertilize organic or inorganic chemistry.
- the compounds of formula 4 (XIV) are known in part from WO 99/16748.
- the process (A) is characterized in that compounds of the formula (IT) in which A, B, Q 1 , Q, W, X, Y, Z and R 1 have the abovementioned meanings, in the presence of a diluent and in the presence of a base of an intramolecular condensation.
- Diluents which can be used in the process (A) according to the invention are all inert organic solvents in relation to the reactants. Preference is given to using hydrocarbons, such as toluene and xylene, furthermore ethers, such as dibutyl ether, tetrahydrofuran, dioxane, glycol dimethyl ether and diglycol dimethyl ether, furthermore polar solvents, such as dimethyl sulfoxide, sulfolane, dimethylformamide and N-methylpyrrolidone, and also alcohols, such as methanol , Ethanol, propanol, iso-propanol, butanol, iso-butanol and tert-butanol.
- hydrocarbons such as toluene and xylene
- furthermore ethers such as dibutyl ether, tetrahydrofuran, dioxane, glycol dimethyl ether and diglycol dimethyl ether
- all customary proton acceptors can be used as the base (deprotonating agent).
- alkali metals such as sodium or potassium can be used.
- alkali metal and alkaline earth metal amides and hydrides such as sodium amide, sodium hydride and calcium hydride, and also alkali metal alkoxides, such as sodium methoxide, sodium ethylate and potassium tert-butoxide.
- the Reaktipnstemperatur can be varied in carrying out the process (A) according to the invention within a substantial range. In general, one works at temperatures between -75 ° C and 200 0 C, preferably between -50 0 C and 150 0 C.
- the process (A) according to the invention is generally carried out under atmospheric pressure.
- reaction component of the formula (H) and the deprotonating base are generally employed in equimolar to about twice-equimolar amounts. However, it is also possible to use one or the other component in a larger excess (up to 3 MpI).
- the process (B) is characterized in that compounds of the. Formula (IE) in which A, B, Q 1 , Q, W, X, Y, Z and R 8 have the meanings given above, intramolecularly condensed in the presence of a diluent and in the presence of a base.
- Diluents used in process (B) according to the invention are all organic solvents inert to the reactants. Preference is given to using hydrocarbons, such as toluene and xylene, furthermore ethers, such as dibutyl ether, tetrahydrofuran, dioxane, glycol dimethyl ether and diglycol dimethyl ether, as well as polar solvents, such as dimethyl sulfoxide, sulfolane, dimethylformamide and N-methylpyrrolidone. Furthermore, alcohols such as methanol, ethanol, propanol, iso-propanol, butanol, isobutanol and tert-butanol can be used.
- hydrocarbons such as toluene and xylene
- ethers such as dibutyl ether, tetrahydrofuran, dioxane, glycol dimethyl ether and diglycol dimethyl ether
- polar solvents such as di
- all conventional proton acceptors can be used in carrying out the process (B) according to the invention.
- phase transfer catalysts e.g. Triethylbenzylammonium chloride, tetrabutylammonium bromide
- TDA 1 tris (methoxyethoxyethy
- alkali metals such as sodium or potassium can be used.
- alkali metal and alkaline earth metal amides and hydrides such as sodium amide, sodium hydride and calcium hydride, and also alkali metal alkoxides, such as sodium methoxide, sodium ethoxide and potassium tert-butoxide can be used.
- the reaction temperature can be varied within a substantial range when carrying out the process (B) according to the invention. In general, one works at temperatures between -75 0 C and 200 0 C, preferably between -50 0 C and 15O 0 C.
- the erf ⁇ ndungshiele process (B) is generally carried out under atmospheric pressure.
- reaction components of the formula (EI) and the deprotonating bases are generally employed in approximately equimolar amounts. However, it is also possible to use one or the other component in a larger excess (up to 3 mol).
- the process (C ⁇ ) is characterized in that compounds of the formulas (IIa) to (I-2-a) are each reacted with carboxylic acid halide de ⁇ of the formula (IV), if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder.
- Suitable diluents for the process according to the invention (C ⁇ ) are all solvents which are inert to the acid halides.
- hydrocarbons such as benzene, benzene, toluene, xylene and tetralin, furthermore halogenated hydrocarbons, such as methylene chloride, chloroform, carbon tetrachloride, chlorobenzene and o-dichlorobenzene, furthermore ketones, such as acetone and methyl isopropyl ketone, furthermore ethers, such as diethyl ether, tetrahydrofuran and Dioxane, moreover carboxylic acid esters, such as ethyl acetate, and also strongly polar solvents, such as dimethylformamide, Diniethylsulfoxid and sulfolane. If the hydrolytic stability of the acid halide permits it, the reaction may also be carried out in the presence of water.
- Suitable acid binders in the reaction by the process (C ⁇ ) according to the invention are all customary acid acceptors.
- tertiary amines such as triethylamine, pyridine, diazabicyclooctane (DABCO), diazabicycloundecene (DBU), diazabicyclo-nonene (DBN), Hünig base and N
- the reaction temperature can be varied within a relatively wide range in the process according to the invention (C ⁇ ). In general, one works at temperatures between -20 0 C and +150 0 C, preferably between 0 0 C and 100 0 C.
- the starting materials of the formulas (IIa) to (I-2-a) and the carboxylic acid halide of the formula (IV) are generally each used in approximately equivalent amounts. However, it is also possible to use the carboxylic acid halide in a larger excess (up to 5 moles). The workup is carried out by conventional methods.
- the process (C ⁇ ) is characterized in that compounds of the formulas (I-I-a) to (I-2-a) are each reacted with carboxylic anhydrides of the formula (V), if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder.
- Diluents which may be used in the process (C ⁇ ) according to the invention are preferably those diluents which are also suitable when using acid halides.
- an excess carboxylic acid anhydride may also function as a diluent at the same time.
- Suitable acid binders which may be added in process (C ⁇ ) are preferably those acid binders which are also preferably used when using acid halides.
- the reaction temperature in the process (C ⁇ ) according to the invention can be varied within a relatively wide range. In general, it is carried out at temperatures between -20 0 C and + 15O 0 C 5, preferably between 0 0 C. and 100 0 C.
- the starting materials of the formulas (I-I-a) to (I-2-a) and the carboxylic anhydride of the formula (V) are generally used in approximately equivalent amounts. However, it is also possible to use the carboxylic acid anhydride in a larger excess (up to 5 moles). The workup is carried out by conventional methods.
- diluent and excess carboxylic anhydride and the resulting carboxylic acid are removed by distillation or by washing with an organic solvent or with water.
- the process (D) is characterized in that compounds of the formulas (I-I-a) to (I-2-a) are reacted in each case with chloroformates or chloroformic thioesters of the formula (VI), if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder.
- Suitable acid binders in the process (D) according to the invention are all customary acid acceptors. Preference is given to using tertiary amines, such as triethylamine, pyridine, DABCO, DBU, DBN, Hünig base and N, N-dimethylaniline, furthermore alkaline earth metal oxides, such as magnesium and calcium oxide, and also alkali metal and alkaline earth metal carbonates, such as sodium carbonate, potassium carbonate and calcium carbonate and alkali hydroxides such as sodium hydroxide and potassium hydroxide.
- tertiary amines such as triethylamine, pyridine, DABCO, DBU, DBN, Hünig base and N, N-dimethylaniline
- alkaline earth metal oxides such as magnesium and calcium oxide
- alkali metal and alkaline earth metal carbonates such as sodium carbonate, potassium carbonate and calcium carbonate and alkali hydroxides such as sodium hydroxide and potassium hydroxide.
- Suitable diluents for the process (D) according to the invention are all solvents which are inert to the chloroformic esters or chloroformic thioesters.
- hydrocarbons such as benzene, benzene, toluene, xylene and tetralin
- halogenated hydrocarbons such as methylene chloride, chloroform, carbon tetrachloride, chlorobenzene and o-dichlorobenzene
- ketones such as acetone and methyl isopropyl ether
- furthermore ethers such as diethyl ether, tetrahydrofuran and dioxane
- carboxylic esters such as ethyl acetate, and also nitriles, such as acetonitrile, and also strongly polar solvents, such as dimethylformamide, dimethyl sulfoxide and sulfolane.
- the reaction temperature can in
- the erf ⁇ ndungshiele process (D) is generally carried out under atmospheric pressure.
- the starting materials of the formulas (I-I-a) to (I-2-a) and the corresponding chloroformate or chloroformic thioester of the formula (VI) are generally each used in approximately equivalent amounts. However, it is also possible to use one or the other component in a larger excess (up to 2 mol).
- the workup is carried out by conventional methods. In general, the procedure is to remove precipitated salts and to concentrate the remaining reaction mixture by stripping off the diluent.
- the process (E) according to the invention is characterized in that compounds of the formulas (I-I-a) to (I-2-a) are each reacted with compounds of the formula (VIT) in the presence of a diluent and optionally in the presence of an acid binder.
- Suitable optionally added diluents are all inert polar organic solvents, such as ethers, amides, sulfones, sulfoxides, but also haloalkanes.
- the addition of strong deprotonating agents e.g. Sodium hydride or potassium tert-butylate the enolate salt of the compounds (I-l-a) to (I-2-a) can be dispensed with the further addition of acid binders.
- the bases used in process (E) are all customary proton acceptors. Preference is given to using alkali metal hydrides, alkali metal alcoholates, alkali metal or alkaline earth metal carbonates or bicarbonates or. Nitrogen bases. Examples include sodium hydride, sodium methoxide, sodium hydroxide, calcium hydroxide, potassium carbonate, sodium hydrogencarbonate, triethylamine, dibenzylamine, diisopropylamine, pyridine, quinoline, diazabicyclooctane (DABCO), Diazäbicyclononen (DBN) 'and diazabicycloundecene were. (DBU). , The reaction can be carried out at atmospheric pressure or under elevated pressure, preferably ⁇ works at atmospheric pressure. The workup is done by conventional methods.
- the process (F) according to the invention is characterized in that compounds of the formulas (I-I-a) to (I-2-a) are each reacted with sulfonyl chlorides of the formula (VIH), if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder.
- the process (F) is preferably carried out in the presence of a diluent.
- Suitable diluents are all inert polar organic solvents such as ethers, amides, ketones, carboxylic acid esters, nitriles, sulfones, sulfoxides or halogenated hydrocarbons such as methylene chloride.
- the addition of strong deprotonating agents represents the enolate salt of the compounds (I-I-a) to (I-2-a)
- the further addition of acid binders can be dispensed with.
- acid binders are used, conventional inorganic or organic bases are suitable; sodium hydroxide, sodium carbonate, potassium carbonate, pyridine and triethylamine may be mentioned by way of example.
- the reaction can be carried out at atmospheric pressure or under elevated pressure, preferably is carried out at atmospheric pressure.
- the workup is done by conventional methods.
- Process (G) according to the invention is characterized in that compounds of the formulas (I-I-a) to (I-2-a) are each reacted with phosphorus compounds of the formula (IX), if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder.
- the process (G) is preferably carried out in the presence of a diluent.
- Suitable diluents are all inert, polar organic solvents such as ethers, carboxylic esters, halogenated hydrocarbons, ketones, amides, nitriles, sulfones, sulfoxides, etc.
- acetonitrile dimethyl sulfoxide, tetrahydrofuran, dimethylformamide, methylene chloride are used.
- Suitable acid binders which may be added are customary inorganic or organic bases such as hydroxides, carbonates or amines. Exemplary sodium hydroxide, sodium carbonate, potassium carbonate, pyridine and triethylamine are listed.
- the reaction can be carried out at atmospheric pressure or under elevated pressure, preferably is carried out at atmospheric pressure.
- the workup is done by conventional methods of organic chemistry.
- the final products are preferably purified by crystallization, chromatographic purification or by so-called “distillation", i. Removal of volatiles purified in vacuo.
- the process (H) is characterized in that compounds of the formulas (IIa) to (I-2-a) are each reacted with metal hydroxides or metal alkoxides of the formula (X) or amines of the formula (XI), if appropriate in the presence of a diluent, implements.
- Suitable diluents in the process (H) according to the invention are preferably ethers such as tetrahydrofuran, dioxane, diethyl ether or else alcohols such as methanol, ethanol, isopropanol, but also water.
- the process (H) according to the invention is generally carried out under atmospheric pressure.
- the reaction temperature is generally between -20 0 C and 100 0 C, preferably between 0 0 C and 50 0 C.
- the process (I) according to the invention is characterized in that compounds of the formulas (IIa) to (I-2-a) are each reacted with (I ⁇ ) : compounds of the formula (XH), if appropriate in the presence of a diluent and if appropriate in the presence of a catalyst or (I ⁇ ) is reacted with compounds of the formula (XIH), if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder.
- the process (Ia) is preferably carried out in the presence of a diluent.
- Suitable diluents are all inert organic solvents, such as aromatic hydrocarbons, halogenated hydrocarbons, ethers, amides, nitriles, sulfones or sulfoxides.
- catalysts can be added to accelerate the reaction.
- organotin compounds e.g. Dibutylzmndilaurat be used.
- Possible diluents which are added are all inert polar organic solvents, such as ethers, carboxylic esters, nitriles, ketones, amides, sulfones, sulfoxides or halogenated hydrocarbons.
- the addition of strong deprotonating agents represents the enolate salt of the compound (I-I-a) to (I-2-a)
- the further addition of acid binders can be dispensed with.
- customary inorganic or organic bases are suitable, for example sodium hydroxide, sodium carbonate, potassium carbonate, triethylamine or pyridine.
- the reaction can be carried out at atmospheric pressure or under elevated pressure, preferably is carried out at atmospheric pressure.
- the workup is done by conventional methods.
- Suitable diluents are all inert organic solvents such as aromatic hydrocarbons, halogenated hydrocarbons ethers, esters, amides, nitriles, sulfones or sulfoxides.
- catalysts can be added to accelerate the reaction.
- acids such as e.g. p-toluenesulfonic acid, conc. Sulfuric acid but also Lewis acids such. Bortrifluoretherat be used.
- dehydrating conditions can be achieved both by azeotropic removal of the water and by addition of suitable dehydrating reagents, e.g. Formic acid orthoester, dimethoxypropane but also achieve molecular sieve.
- suitable dehydrating reagents e.g. Formic acid orthoester, dimethoxypropane but also achieve molecular sieve.
- the workup is done by the usual methods.
- the active compounds / active substance combinations according to the invention are suitable, with good plant compatibility, favorable toxicity to warm-blooded animals and good environmental compatibility, for protecting plants and plant organs, for increasing crop yields, improving the quality of the crop and for controlling animal pests, in particular insects, arachnids, heatminths, Nematodes and molluscs found in agriculture, horticulture, livestock, forestry, gardens and recreational facilities, in the protection of materials and materials and in the hygiene sector. They can preferably be used as crop protection agents. They are effective against normally sensitive and resistant species as well as against all or individual developmental stages.
- the above mentioned pests include:
- Anoplura e.g. Damalinia spp., Haematopinus spp., Linognathus spp., Pediculus spp., Trichodectes spp.
- arachnids e.g. Acarus siro, Aceria sheldoni,. Aculops spp., Acutus spp., Amblyomma spp., Argas spp., Boophilus spp., Brevipalpus spp., Bryobia praetiosa, Chorioptes spp., Dermanyssus gallinae, Eotetranychus spp., Epitrimerus pyri, Eutetranychus spp., Eriophyes spp., Hemitarsonemus Spp., Hyalomma spp., Ixodes spp., Latrodectus mactans, Metatetranychus spp., Oligonychus spp., Ornithodoros spp., Panonychus spp., Phyllocoptruta oleivora, Polyphagotarsonemus
- Gastropoda e.g. Arion spp., Biomphalaria spp., Bulinus spp., Deroceras spp., Galba spp., Lymnaea spp., Oncomelania spp., Succinea spp.
- helminths e.g. Ancylostoma duodenale, Ancylostoma ceylanicum, Acylostoma braziliensis, Ancylostoma spp., Ascaris lubricoides, Ascaris spp., Brugia malayi, Brugia timori, Bunostomum spp., Chabertia spp., Clonorchis spp., Cooperia spp., Dictocoelram spp, Dictyocaulus filaria, Diphyllobothrium latum , Dracunculus medinensis, Echinococcus granulosus, Echinococcus multilocularis, Enterobius vermicularis, Faciola spp., Haemonchus spp., Heterakis spp., Hymenolepis nana, Hyostrongulus spp., Loa Loa,
- protozoa such as Eimeria
- Eimeria protozoa
- Pteromalis spp Pyrilla spp., Quadraspidiotus spp., Quesada gigas, Rastrococcus spp., Rhopalosiphum spp., Saissetia spp., Scaphoides titanus, Schizaphis graminum, Selenaspidus articulatus, Sogata spp., Sogatella furcifera, Sogatodes spp., Stictocephala festina, Tenalaphara malayensis, Tinocallis caryaefoliae, Tomaspis spp., Toxoptera spp., Tnalleurodes vaporariorum, Trioza spp., Typhlocyba spp., Unaspis spp., Viteus vitifolii.
- Hymenoptera e.g. Diprion spp., Hoplocampa spp., Lasius spp., Monomorium pharaonis, Vespa spp.
- Orthoptera e.g. Acheta domesticus, Blatta orientalis, Blattella germanica, Gryllotalpa spp., Leucophaea maderae, Locusta spp., Melanoplus spp., Periplaneta americana, Schistocerca gregaria.
- siphonaptera e.g. Ceratophyllus spp., Xenopsylla cheopis.
- Symphyla e.g. Scutigerella Immaculata.
- Thysanoptera e.g. Basothrips biformis, Enneothrips flavens, Frankliniella spp., Heliothrips spp., Hercinothrips femoralis, Rhipiphorothrips cruentatus, Scirtothrips spp., Taeniothrips cardamoni, Thrips spp.
- Thysanura e.g. Lepisma saccharina.
- the plant parasitic nematodes include e.g. Aphelenchoides spp., Bursaphelenchus spp., Ditylenchus dipsaci, Globodera spp., Heterodera spp., Longidorus spp., Meloidogyne spp., Pratylenchus spp., Radopholus similis, Trichodorus spp., Tylenchulus semipenetrans, Xiphinema spp.
- Aphelenchoides spp. Bursaphelenchus spp.
- Ditylenchus dipsaci Globodera spp.
- Heterodera spp. Heterodera spp.
- Longidorus spp. Meloidogyne spp.
- Pratylenchus spp. Radopholus similis
- Trichodorus spp. Tylenchulus semipenetrans
- the compounds / active substance combinations according to the invention may optionally also be used in certain concentrations or application rates as herbicides, safeners, growth regulators or agents for improving plant properties, or as microbicides, for example as fungicides, antimycotics, bactericides, viricides (including anti-viral agents) or as agents MLO (Mycoplasma-like-organism) and RLO (Rickettsia-like-organism) are used. If appropriate, they can also be used as intermediates or precursors for the synthesis of further active ingredients.
- plants and parts of plants can be treated.
- plants are understood as meaning all plants and plant populations, such as desired and undesired wild plants or crop plants (including naturally occurring crop plants).
- Crop plants can be plants produced by conventional breeding and Optimization methods or by biotechnological and genetic engineering methods or combinations of these methods can be obtained, including the transgenic plants and including protected by plant breeders' rights or non-protectable plant varieties.
- Plant parts are to be understood as meaning all aboveground and subterranean parts and organs of the plants, such as shoot, leaf, flower and root, examples of which include leaves, needles, stems, stems, flowers, fruiting bodies, fruits and seeds, and roots, tubers and rhizomes.
- Crops also include crops as well as vegetative and generative propagation material, such as cuttings, tubers, rhizomes, cuttings and seeds.
- the erf ⁇ ndungssiee treatment of the plants and plant parts with the active ingredients / drug combinations is carried out directly or by affecting the environment, habitat or storage space according to the usual treatment methods, e.g. by dipping, spraying, evaporating, atomizing, spreading, spreading, injecting and propagating material, in particular in seeds, further by single or multilayer coating.
- the active compounds / active substance combinations can be converted into the customary formulations, such as solutions, emulsions, wettable powders, water- and oil-based suspensions, powders, dusts, pastes, soluble powders, soluble granules, scattering granules, suspension-emulsion concentrates, active substance-impregnated natural products, Active substance-impregnated synthetic substances, fertilizers and very fine encapsulation in.polymeren substances.
- formulations are prepared in a known manner, e.g. by mixing the active compounds / active substance combinations with extenders, ie liquid solvents and / or solid carriers, optionally with the use of surface-active agents, ie emulsifiers and / or dispersants and / or foam-forming agents.
- Aromatics such as xylene, toluene, or alkylnaphthalenes, chlorinated aromatics and chlorinated aliphatic hydrocarbons, such as chlorobenzenes, chloroethylenes or methylene chloride, aliphatic hydrocarbons, such as cyclohexane or paraffins, eg petroleum fractions, mineral and vegetable oils, alcohols, such as butanol or glycol and their ethers and esters, ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketor or cyanohexanone, strongly polar solvents such as dimethyl sulfoxide, and water.
- Suitable solid carriers are:
- Ammonium salts and ground natural minerals such as kaolins, clays, talc, chalk, quartz, attapulgite, montmorillonite or diatomaceous earth and synthetic minerals, such as finely divided silica, alumina and silicates, as solid carriers for granules kom-. in question: e.g. crushed and fractionated natural rocks such as calcite, marble, pumice, sepiolite, dolomite and synthetic granules of inorganic and organic flours and granules of organic material such as. Sawdust, coconut shells, corn cobs and tobacco stalks; suitable emulsifiers and / or foam formers are: e.g.
- nonionic and anionic emulsifiers such as polyoxyethylene fatty acid esters, polyoxyethylene fatty alcohol ethers, e.g. Alkylaryl polyglycol ethers, alkylsulfonates, alkyl sulfates, arylsulfonates and protein hydrolysates; suitable dispersants are: e.g. Lignin-sulphite liquors and methylcellulose.
- Adhesives such as carboxymethyl cellulose, natural and synthetic powdery, granular or latex polymers such as gum arabic, polyvinyl alcohol, polyvinyl acetate, and natural phospholipids such as cephalins and lecithins and synthetic phospholipids may be used in the formulations.
- Other additives may be mineral and vegetable oils.
- Dyes such as inorganic pigments, e.g. Iron oxide, titanium oxide, ferrocyan blue and organic dyes such as alizarin, azo and metal phthalocyanine dyes and trace nutrients such as salts of iron, manganese, boron, copper, cobalt, molybdenum and zinc.
- inorganic pigments e.g. Iron oxide, titanium oxide, ferrocyan blue and organic dyes such as alizarin, azo and metal phthalocyanine dyes and trace nutrients such as salts of iron, manganese, boron, copper, cobalt, molybdenum and zinc.
- the formulations generally contain between 0.1 and 95% by weight of active compound, preferably between 0.5 and 90%.
- the active substance / active substance combinations according to the invention can be present in its commercial formulations as well as in the formulations prepared from these formulations in admixture with other active ingredients such as insecticides, attractants, sterilants, bactericides, acaricides, nematicides, fungicides, growth-regulating substances, herbicides, safeners, fertilizers or semiochemicals.
- active ingredients such as insecticides, attractants, sterilants, bactericides, acaricides, nematicides, fungicides, growth-regulating substances, herbicides, safeners, fertilizers or semiochemicals.
- Particularly favorable mixing partners are e.g. the following:
- Buthiobate Burylamine; Calcium polysulfides; Capsiroycin; captafol; captan; carbendazim;
- carboxin carproparm ' d; carvones; chinomethionat; Chlobenthiazone; Chlorfenazole; chloroneb;
- chlorothalonil chlozolinate; Clozylacon; cyazofamid; cyflufenamid; cymoxanil; Cyproconazoles; cyprodinil; cyprofuram; Dagger G; debacarb; dichlofluanid; dichlone; dichlorophen;
- dimethomorph dimoxystrobin; diniconazole; Diniconazole-M; dinocap; diphenylamines;
- fenpropimorph ferbam; fluazinam; Flubenzimine; fludioxonil; flumetover; flumorph;
- fluoromides fluoxastrobin; fluquinconazole; flurprimidol; flusilazole; flusulfamide; flutolanil;
- flutriafol flutriafol; folpet; Fosetyl-Al; Fosetyl-sodium; fuberidazole; furalaxyl; furametpyr; Furcarbanil;
- iprobenfos iprodione; iprovalicarb; Irumamycin; isoprothiolane; Isovaledione; kasugamycin;
- M ⁇ talaxyl-M metconazole; methasulfocarb; MethfAiroxam; metiram; metominostrobin;
- phosdiphen phthalides; picoxystrobin; piperalin; Polyoxins; Polyoxorim; Probenazole; prochloraz;
- procymidone propamocarb; Propanosine-sodium; propiconazole; propineb; proquinazid;
- prothioconazole pyraclostrobin; Pyrazohos; pyrifenox; pyrimethanil; pyroquilon; Pyroxyfur; Pyrrolnitrine; Quinconazole; quinoxyfen; quintozene; Simeconazole; spiroxamine; Sul &r;
- tebuconazole tecloftalam; Tecnazene; Tetcyclacis; tetraconazole; thiabendazole; Thicyofen;
- Thifluzamide Thiophanate-methyl; thiram; Tioxymid; Tolclofos-methyl; tolylfluanid; Triadimefon; triadimenol; Triazbutil; triazoxide; Tricyclamide; Tricyclazole; tridemorph;
- trifloxystrobin triflumizole; triforine; triticonazole; Uniconazole; Validamycin A; vinclozolin; Zineb; ziram; zoxamide; (2S) -N- [2- [4 - [[3- (4-chlorophenyl) -2-propynyl] oxy] -3-methoxyphenyl] ethyl] -3-methyl-2 - [(methylsulfonyl) amino] - butanamide; I- (l-näphthalenyl) -lH- ⁇ yrrole-2,5-diones;
- Carbamates for example alanycarb, aldicarb, aldoxycarb, allyxycarb, aminocarb, bendiocarb, benzuracarb, bufencarb, butacarb, butocarboxime, butoxycarboxim, carbaryl, carbofuran, carbosulfan, cloethocarb, dimetilane, ethiofencarb, fenobucarb, fenothiocarb, formetanate, furathiocarb, isoprocarb , Metam-sodium, Methiocarb, Methomyl, Metolcarb, Oxamyl, Pirir ⁇ icarb, Promecarb, Propoxur, Thiodicarb, Thiofanox,
- Organophosphates for example acephates, azamethiphos, azinphos (-methyl, -ethyl), bromophos-ethyl, bromfenvinfos (-methyl), butathiofos, cadusafos, carbophenothion, chloroethoxyfos, chlorfenvinphos, chloroforms, chlorpyrifos (-methylethyl), coumaphos,
- Pyrethroids for example acrinathrin, allethrin (d-cis-trans, d-trans), beta-cyfluthrin, bifenthrin, bioallethrin, bioallethrin S-cyclopentyl isomer, bioethanomethrin, biopermethrin, bioresmethrin, chlovaporthrin, cis-cypermethrin, cis Resmethrin, cis-permethrin,
- Metaflumizone (BAS 3201)
- Acetylcholine receptor modulators are Acetylcholine receptor modulators
- GABA-driven chloride channel antagonists 5.1 organochlorines, for example, camphechlor, chlordane, endosulfan, gamma-HCH, HCH, heptachlor, Lindaüe, methoxychlor
- Fiproles for example, acetoprole, ethiprole, fipronil, pyrafluprole, pyriprole, vaniliprole
- Mectins for example avermectin, emamectin, emamectin benzoate, ivermectin, milbemydn
- Juvenile hormone mimetics for example, diofenolan, epofenonans, fenoxycarb, hydroprene, kinoprene,
- Diacylhydrazines for example chromafenozides, halofenozides, methoxyfenozides, tebufenozide inhibitors of chitin biosynthesis
- Benzoylureas for example bistrifluron, chlorofluazuron, diflubenzuron, fluazuron, flucycloxuron, fenphenoxuron, hexaflumuron, lufenuron, novaluron, noviflumuron, penfluron, teflubenzuron, triflumuron
- Organotin compounds for example azocyclotin, cyhexatin, fenbutatin oxides
- METFs for example Fenazaquin, Fenpyroximate, Pyrimidifen, Pyridaben, Tebufenpyrad, Tolfenpyrad as well
- Tetronic acids for example spirodiclofen, spiromesifen
- Carboxamides for example flonicamide Octopaminergic agonists, for example Amitraz
- Nereistoxin analogs for example thiocyclam hydrogen oxalate, thiosultap-sodium
- fumigants for example aluminum phosphides, methyl bromides, sulfuryl fluorides
- feed inhibitors for example cryolites, flonicamide, pymetrozines
- mite growth inhibitors for example clofentezine, etoxazole, hexythiazox
- the active compounds / active substance combinations according to the invention can furthermore be present when used as insecticides in their commercial formulations and in the forms of use prepared from these formulations in admixture with synergists.
- Synergists are compounds that increase the effect of the active ingredients without the added synergist itself having to be active.
- the active compounds / active substance combinations according to the invention can also be present in insecticides in their commercial formulations and in the formulations prepared from these formulations in mixtures with inhibitors, the degradation of the active ingredient after application in the environment of the plant, on the surface of plant parts or in plant Reduce tissue.
- the active substance content of the application forms prepared from the commercial formulations can vary within wide ranges.
- the active ingredient concentration of the use forms may be from 0.00000001 up to 95% by weight of active compound, preferably between 0.0000% and 1% by weight.
- the application is done in a custom forms adapted to the application forms.
- plants and their parts can be treated.
- wild species or plant species obtained by conventional biological breeding methods such as crossing or protoplast fusion
- plant cultivars and their parts are treated.
- transgenic plants and plant cultivars which have been obtained by genetic engineering methods if appropriate in combination with conventional methods (Genetically Modified Organisms), and parts thereof are treated.
- the terms "parts” or “parts of plants” or “plant parts” have been explained above.
- Plant varieties are understood as meaning plants having new traits which have been bred by conventional breeding, by mutagenesis or by recombinant DNA techniques. These can be varieties, biotypes and genotypes.
- the 'treatment of the invention can, depending on the plant species or plant cultivars, their location and growth conditions (soils, climate, vegetation period, diet) by, even superadditive ("synergistic") effects occur.
- superadditive superadditive
- reduced application rates and / or extensions of the spectrum of action and / or an increase in the effect of the substances and agents usable in the invention better plant growth, increased tolerance to high or low temperatures, increased tolerance to drought or to water or soil salt content, increased flowering power facilitated harvesting, acceleration of ripeness, higher crop yields, higher quality and / or higher nutritional value of the harvested products, higher shelf life and / or machinability of the harvested products, which go beyond the expected effects.
- plants or Pf ⁇ anzensorten include all plants that received genetic engineering material through the genetic modification, which gives these plants special beneficial properties ("traits"). Examples of such properties are better plant growth, increased tolerance to high or low temperatures, increased tolerance to dryness or to bottoms salt, increased flowering, easier harvesting, acceleration of ripeness, higher crop yields, higher quality and / or higher nutritional value of the harvested products , higher shelf life and / or workability of the harvested products.
- transgenic plants are the important crops such as cereals (wheat, rice), corn, soybeans, potatoes, sugar beets, tomatoes, peas and other vegetables, cotton, tobacco, oilseed rape and fruit plants (with the fruits apples, pears, citrus fruits and Grapes,), with particular emphasis on maize, soya, potato, cotton, tobacco and oilseed rape.
- Traits which are particularly emphasized are the increased defense of the plants against insects, arachnids, nematodes and snails by toxins produced in the plants, in particular those produced by the genetic material from Bacillus thuringiensis (eg by the genes Cry ⁇ A (a), Cry ⁇ A (b), Cry ⁇ A (c), CryllA, CrylDA, CryIIIB2, Cry9c Cry2Ab, Cry3Bb and CrylF and their combinations) in the plants be generated (hereinafter "Bt plants”). Traits also highlight the increased resistance of plants to fungi, bacteria and viruses by systemic acquired resistance (SAR) 5 systemin, phytoalexins, elicitors and resistance genes and correspondingly expressed proteins and toxins.
- SAR systemic acquired resistance
- Traits which are furthermore particularly emphasized are the increased tolerance of the plants to certain herbicidally active compounds, for example imidazolinones, sulfonylureas, glyphosate or phosphinotricin (eg "PAT" gene).
- the respectively the desired Traits can also occur in combinations with each other in the transgenic plants.
- “Bt plants” are maize varieties, cotton varieties, soybean varieties and potato varieties which are sold under the trade names YIELD GARD® (eg corn, cotton, soya), KnockOut® (eg maize), StarLink® (eg maize), Bollgard® ( Cotton), Nucotn® (cotton) and NewLeaf® (potato).
- herbicide-tolerant plants are maize varieties, cotton varieties and soybean varieties, which are sold under the trade names Roundup Ready® (tolerance to glyphosate eg corn, cotton, soy), Liberty Link® (tolerance to phosphinotricin, eg rapeseed), IMI® (tolerance against imidazolinone) and STS® (tolerance to sulfonylureas eg corn).
- Roundup Ready® tolerance to glyphosate eg corn, cotton, soy
- Liberty Link® tolerance to phosphinotricin, eg rapeseed
- IMI® tolerance against imidazolinone
- STS® tolerance to sulfonylureas eg corn
- Clearfield® varieties eg corn
- the listed plants can be treated particularly advantageously according to the invention with the compounds of the general formula I or the active substance mixtures according to the invention.
- the preferred ranges given above for the active compounds or mixtures also apply to the treatment of these plants.
- Particularly emphasized is the plant treatment with the compounds or mixtures specifically mentioned in the present text.
- the active compounds / drug combinations according to the invention not only act against plant, hygiene and storage pests, but also in the veterinary sector against animal parasites (ecto- and endoparasites) such as ticks, leather ticks, mange mites, running mites, flies (stinging and licking), parasitic fly larvae , Lice, hair pieces, feathers and fleas.
- animal parasites ecto- and endoparasites
- Anoplurida e.g. Haematopinus spp., Linognathus spp., Pedicutus spp., Phtirus spp., Solenopotes spp.
- Trimenopon spp. Menopon spp., Trinoton spp., Bovicola spp., Werneckiella spp., Lepikentron spp., Damalina spp., Trichodectes spp., Felicola spp.
- Nematocerina and Brachycerina eg Aedes spp., Anopheles spp., Culex spp., Simulium spp., Eusimulium spp., Phlebotomus spp., Lutzomyia spp., Culicoides spp., Chrysops spp., Hybomitra spp.
- Atylotus spp. Tabanus spp., Haematopota spp., Philipomyia spp., Bfaula spp., Musca spp., Hydrotaea spp., Stomoxys spp., Haematobia spp. Morellia spp., Fannia spp., Glossina spp.,.
- siphonaptrida e.g. Pulex spp., Ctenocephalides spp., Xenopsylla spp., Ceratophyllus spp.
- heteropterid e.g. Cimex spp., Triatoma spp., Rhodnius spp., Panstrongylus spp.
- Actinedida Prostigmata
- Acaridida Acaridida
- Acarapis spp. Ch ⁇ yletiella spp., Ornitrocheyletia spp., Myobia spp., Psorergates spp., Demodex spp., Trombicula spp., Listrophorus spp., Acarus spp., Tyrophagus spp., Caloglyphus spp., Hypodectes spp., Pterolichus spp , Psoroptes spp., Chorioptes spp., Otodectes spp., Sarcoptes spp., TSiotoedres spp., Knemidocoptes spp., Cytodites spp., Laminosioptes spp., Laminosioptes spp.
- the active compounds / active substance combinations of the formula (I) according to the invention are also suitable for controlling arthropods which are farm animals, such as e.g. Cattle, sheep, goats, horses, pigs, donkeys, camels, buffaloes, rabbits, chickens, turkeys, ducks, geese, bees, other pets such as e.g. Dogs, cats, caged birds, aquarium fish and so-called experimental animals, such. Hamsters, guinea pigs, rats and mice.
- arthropods are farm animals, such as e.g. Cattle, sheep, goats, horses, pigs, donkeys, camels, buffaloes, rabbits, chickens, turkeys, ducks, geese, bees, other pets such as e.g. Dogs, cats, caged birds, aquarium fish and so-called experimental animals, such. Hamsters, guinea pigs, rats and mice.
- the application of the active compounds / active compound combinations according to the invention takes place in the veterinary sector and animal husbandry in a known manner by enteral administration in the form of, for example, tablets, capsules, infusions, drenches, granules, pastes, boilies, the feedthrough method, suppositories, by parenteral Administration, for example by injections (intramuscular, subcutaneous, intravenous, intraperitoneal, etc.), implants, by nasal administration, dermal application in the form of e.g. Diving or swimming (Dipping), spraying, pouring and spot-on, washing, powdering and with the aid of active substance-containing shaped articles, such as collars, ear tags, tail tags, limb bands, holsters, marking devices, etc.
- enteral administration in the form of, for example, tablets, capsules, infusions, drenches, granules, pastes, boilies, the feedthrough method, suppositories
- parenteral Administration for example by injections
- the active compounds of the formula (I) can be used as formulations (for example powders, emulsions, flowable agents) which contain the active ingredients in an amount of from 1 to 80% by weight, directly or apply after 100 to 10,000 times dilution or use as a chemical bath.
- formulations for example powders, emulsions, flowable agents
- inventive compounds / drug combinations show a high insecticidal activity against insects that destroy industrial materials.
- insects By way of example and preferably without limiting however, the following insects are mentioned:
- Hymenoptera such as Sirex juveocus, Urocerus gigas, Urocerus gigas taignus, Urocerus augur;
- Termites such as Kalotermes flavicollis, Crypfotermes brevis, Heterotermes indicola, Reticulitermes flavipes, Reticulitermes santonensis, Reticulitermes lucifugus, Mastotermes darwiniensis, Zootermopsis nevadensis, Coptotermes formosanus;
- Non-living materials such as preferably plastics, adhesives, glues, papers and cardboard, leather, wood, wood processing products and paints.
- the material to be protected from insect attack is wood and woodworking products.
- the active compounds can be used as such, in the form of concentrates or generally customary formulations such as powders, granules, solutions, suspensions, emulsions or pastes.
- the formulations mentioned can be prepared in a manner known per se, e.g. by mixing the active compounds with at least one solvent or diluent, emulsifier, dispersing and / or binding or fixing agent, water repellent, optionally siccatives and UV stabilizers and optionally dyes and pigments and. other processing aids.
- the insecticidal agents or concentrates used for the protection of wood and wood-based materials contain the active ingredient according to the invention in a concentration of 0.0001 to 95 wt .-%, in particular 0.001 to 60 wt .-%.
- the amount of agents or concentrates used depends on the nature and occurrence of the insects and on the medium.
- the optimal amount used can be determined in each case by test series. In general, however, it is sufficiently 0.0001 to 20% by weight, preferably 0.001 to 10% by weight, of the active ingredient, based on the one to be protected
- the solvent and / or diluent used is an organic-chemical solvent or L ⁇ sungsmittelgemisch and / or an oily or oily high-volatile organic-chemical solvent or solvent mixture and / or a polar organic-chemical solvent or solvent mixture and / or water and optionally an emulsifier and / or wetting agent.
- Organic chemical solvents which are preferably oily or oil-type solvents with an evaporation number of above 35 and a flashpoint above 3O 0 C, preferably above 45 0 C inserted. 5
- water-insoluble, oily and oily solvents corresponding mineral oils or their aromatic fractions or mineral oil-containing solvent mixtures, preferably white spirit, petroleum and / or alkylbenzene are used.
- Mineral oils having a boiling range of 170 to 22O 0 C, white spirit having a boiling range of 170 to 220 0 C., spindle oil with a boiling range of 250 to 35O 0 C 5 petroleum or aromatics of boiling range from 160 to 280 0 C, oil of turpentine and Like. For use.
- the organic semi-volatile oily or oily solvents having an evaporation number above 35 and a flash point above 30O 0 C, preferably above 45 ° C, can partially replaced by light or medium volatile organic chemical solvents, with the proviso that the solvent mixture also has an evaporation number over 35 and a flashpoint above 3O 0 C, preferably above 45 0 C, and in that the insecticide fungicide mixture is soluble or emulsifiable in this solvent mixture is.
- a part of the organic-chemical solvent or solvent mixture or an aliphatic polar organic-chemical solvent or solvent mixture is replaced.
- aliphatic organic chemical solvents containing hydroxyl and / or ester and / or ether groups are used, for example glycol ethers, esters or the like. .
- organic-chemical binders are the water-dilutable and / or soluble or dispersible or emulsifiable synthetic resins and / or binding drying oils used in the organic-chemical solvents used, in particular binders consisting of or containing an acrylate resin, a vinyl resin, eg Polyvinyl acetate, polyester resin, polycondensation or polyaddition resin, polyurethane resin, alkyd resin or modified alkyd resin, phenolic resin, hydrocarbon resin such as indene Cumaronharz, silicone resin, drying vegetable and / or drying oils and / or physically drying binder based on a natural and / or synthetic resin used.
- binders consisting of or containing an acrylate resin, a vinyl resin, eg Polyvinyl acetate, polyester resin, polycondensation or polyaddition resin, polyurethane resin, alkyd resin or modified alkyd resin, phenolic resin, hydrocarbon resin such as indene Cumaronharz, silicone
- the synthetic resin used as the binder may be used in the form of an emulsion, dispersion or solution. Bitumen or bituminous substances up to 10% by weight can also be used as binders. In addition, dyestuffs, pigments, water repellents, scents and inhibitors or the like and the like can be used. According to the invention, at least one alkyd resin or modified alkyd resin and / or a drying vegetable oil is preferably present as the organic-chemical binder in the middle or in the concentrate. Alkyd resins with an oil content of more than 45% by weight, preferably 50 to 68% by weight, are preferably used according to the invention.
- the mentioned binder can be completely or partially replaced by a fixing agent (mixture) or a plasticizer (mixture). These additives are intended to prevent volatilization of the active ingredients and crystallization or failure. Preferably, they replace 0.01 to 30% of the binder (based on 100% of the binder used).
- the plasticizers are derived from the chemical classes of phthalic acid esters such as dibutyl, dioctyl or benzyl butyl phthalate, phosphoric esters such as tributyl phosphate, adipic acid esters such as di (2-ethylhexyl) adipate, stearates such as butyl stearate or amyl stearate, oleates such as butyl oleate, glycerol ethers or higher molecular weight glycol ethers, glycerol esters and p-toluenesulfonic acid ester.
- phthalic acid esters such as dibutyl, dioctyl or benzyl butyl phthalate
- phosphoric esters such as tributyl phosphate
- adipic acid esters such as di (2-ethylhexyl) adipate
- stearates such as butyl
- Fixing agents are chemically based on polyvinyl alkyl ethers such as e.g. Polyvinyl methyl ether or ketones such as benzophenone, ethylene benzophenone.
- Particularly suitable solvents or diluents are also water, optionally in admixture with one or more of the abovementioned organochemical solvents or diluents, emulsifiers and dispersants.
- wood protection is provided by large scale impregnation methods, e.g. Vacuum, double vacuum or printing process achieved.
- the ready-to-use agents may optionally contain further insecticides and optionally one or more fungicides.
- Especially preferred mixing partners which may insecticides such as chlorpyrifos, phoxim, Silafluofm, alphamethrin, cyfluthrin, cypermethrin, deltamethrin, permethrin, imidacloprid, NI-25, Flufenoxüron, hexaflumuron, transfluthrin, thiacloprid, Methoxyphe ⁇ oxide, triflumuron, clothianidine, spinosad, tefluthrin .
- insecticides such as chlorpyrifos, phoxim, Silafluofm, alphamethrin, cyfluthrin, cypermethrin, deltamethrin, permethrin, imidacloprid, NI-25, Flufenoxüron, hexaflumuron, transfluthrin, thiacloprid, Methoxyphe ⁇ oxide, triflumuron, clothianidine,
- fungicides such as epoxyconazoles, hexaconazoles, azaconazoles, propiconazoles, tebuconazoles, cyproconazoh, metconazoh, imazalil, dichlorofluid, tolylfluanid, 3-iodo-2-propynyl-butylcarbamate, N-octylisothiazolin-3-one and 4-dichloro-dichloro N-octylisothiazolin-3-one.
- the compounds according to the invention can be used to protect against the growth of objects, in particular hulls, sieves, nets, structures, wharfage systems and signal systems, which come into contact with seawater or brackish water.
- Butyl titanate, phenyl (bispyridine) bismuth chloride Tri-n-butyltin fluoride, manganese ethylene bis-thiocarbamate, zinc dimethyldithiocarbamate, zinc ethylene bis-thiocarbamate, zinc and copper salts of 2-pyridinethiol-1-oxide, bisdimethyldithiocarbamoylzinc ethylene bisthiocarbamate, zinc oxide, copper (I) ethylene bisdithiocarbamate, copper thiocyanate, copper naphthenate and tributyltin halides, or the concentration These compounds are decisively reduced.
- the ready-to-use antifouling paints may optionally contain other active substances, preferably algicides, fungicides, herbicides, molluscicides or other antifouling active ingredients.
- Suitable combination partners for the antifouling agents according to the invention are preferably: _ ?8th . ,
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Environmental Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Plant Pathology (AREA)
- Pest Control & Pesticides (AREA)
- Health & Medical Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Furan Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
- Heterocyclic Compounds That Contain Two Or More Ring Oxygen Atoms (AREA)
- Indole Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
Abstract
Description
Claims
Priority Applications (15)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DK06701829.1T DK1855529T3 (en) | 2005-02-22 | 2006-02-08 | SPIROKETAL-SUBSTITUTED CYCLIC KETOENOLS |
| JP2007555500A JP5095419B2 (ja) | 2005-02-22 | 2006-02-08 | スピロケタール置換環状ケトエノール類 |
| AP2007004096A AP2007004096A0 (en) | 2005-02-22 | 2006-02-08 | Spiroketal-substituted cyclic ketoenols |
| US11/884,887 US7897543B2 (en) | 2005-02-22 | 2006-02-08 | Spiroketal-substituted cyclic ketoenols |
| MX2007010111A MX2007010111A (es) | 2005-02-22 | 2006-02-08 | Cetoenoles ciclicos, substituidos por espirocetal. |
| EA200701772A EA012785B1 (ru) | 2005-02-22 | 2006-02-08 | Спирокетальзамещённые циклические кетоенолы |
| CN200680012797.0A CN101160049B (zh) | 2005-02-22 | 2006-02-08 | 螺酮缩醇取代的环状酮烯醇 |
| AU2006218154A AU2006218154B2 (en) | 2005-02-22 | 2006-02-08 | Spiroketal-substituted cyclic ketoenols |
| EP06701829.1A EP1855529B1 (de) | 2005-02-22 | 2006-02-08 | Spiroketal-substituierte cyclische ketoenole |
| ES06701829.1T ES2446240T3 (es) | 2005-02-22 | 2006-02-08 | Cetoenoles cíclicos, sustituidos con espirocetal |
| KR1020077020676A KR101351261B1 (ko) | 2005-02-22 | 2006-02-08 | 스피로케탈-치환된 사이클릭 케토에놀 |
| CA2597777A CA2597777C (en) | 2005-02-22 | 2006-02-08 | Spiroketal-substituted cyclic ketoenols |
| BRPI0607807A BRPI0607807B1 (pt) | 2005-02-22 | 2006-02-08 | cetoenóis cíclicos substituídos por espirocetal, seu processo de produção, seus usos e seus intermediários, agentes e seu processo de produção, processos para combate de parasitas, do crescimento indesejado de plantas e/ou de microorganismos indesejados |
| IL185253A IL185253A (en) | 2005-02-22 | 2007-08-14 | Cytoquenols are preserved in spirocetal, a process for their preparation, preparations containing them and methods for their use in the control of pests |
| US13/035,293 US20110190493A1 (en) | 2005-02-22 | 2011-02-25 | Spiroketal-Substituted Cyclic Ketoenols |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102005008021.9 | 2005-02-22 | ||
| DE102005008021A DE102005008021A1 (de) | 2005-02-22 | 2005-02-22 | Spiroketal-substituierte cyclische Ketoenole |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/035,293 Division US20110190493A1 (en) | 2005-02-22 | 2011-02-25 | Spiroketal-Substituted Cyclic Ketoenols |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2006089633A2 true WO2006089633A2 (de) | 2006-08-31 |
| WO2006089633A3 WO2006089633A3 (de) | 2006-11-23 |
Family
ID=36685717
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2006/001089 WO2006089633A2 (de) | 2005-02-22 | 2006-02-08 | Spiroketal-substituierte cyclische ketoenole |
Country Status (22)
| Country | Link |
|---|---|
| US (2) | US7897543B2 (de) |
| EP (1) | EP1855529B1 (de) |
| JP (1) | JP5095419B2 (de) |
| KR (1) | KR101351261B1 (de) |
| CN (5) | CN101885700A (de) |
| AP (1) | AP2007004096A0 (de) |
| AR (1) | AR053815A1 (de) |
| AU (1) | AU2006218154B2 (de) |
| BR (1) | BRPI0607807B1 (de) |
| CA (1) | CA2597777C (de) |
| DE (1) | DE102005008021A1 (de) |
| DK (1) | DK1855529T3 (de) |
| EA (1) | EA012785B1 (de) |
| ES (1) | ES2446240T3 (de) |
| IL (1) | IL185253A (de) |
| MA (1) | MA29324B1 (de) |
| MX (1) | MX2007010111A (de) |
| MY (1) | MY163974A (de) |
| TW (2) | TWI389640B (de) |
| UA (1) | UA88949C2 (de) |
| WO (1) | WO2006089633A2 (de) |
| ZA (1) | ZA200706980B (de) |
Cited By (330)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007073856A2 (de) | 2005-12-15 | 2007-07-05 | Bayer Cropscience Ag | 3'-alkoxy-spirocyclopentyl substituierte tetram- und tetronsäuren |
| WO2007096058A1 (de) | 2006-02-21 | 2007-08-30 | Bayer Cropscience Ag | Cycloalkyl-phenylsubstituierte cyclische ketoenole |
| WO2008067911A1 (de) * | 2006-12-04 | 2008-06-12 | Bayer Cropscience Ag | Biphenylsubstituierte spirocyclische ketoenole |
| JP2008137915A (ja) * | 2006-11-30 | 2008-06-19 | Sankyo Agro Kk | 除草性組成物 |
| DE102007001866A1 (de) | 2007-01-12 | 2008-07-17 | Bayer Cropscience Ag | Spirocyclische Tetronsäure-Derivate |
| EP2014169A1 (de) | 2007-07-09 | 2009-01-14 | Bayer CropScience AG | Wasserlösliche Konzentrate von 3-(2-Alkoxy-4-chlor-6-alkyl-phenyl)-substituierten Tetramaten und ihren korrespondierenden Enolen |
| EP2020413A1 (de) * | 2007-08-02 | 2009-02-04 | Bayer CropScience AG | Oxaspirocyclische-spiro-substituierte Tetram- und Tetronsäure-Derivate |
| EP2039248A1 (de) * | 2007-09-21 | 2009-03-25 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| WO2009039975A1 (de) | 2007-09-25 | 2009-04-02 | Bayer Cropscience Ag | Halogenalkoxyspirocyclische tetram- und tetronsäure-derivate |
| EP2103615A1 (de) | 2008-03-19 | 2009-09-23 | Bayer CropScience AG | 4'4'-Dioxaspiro-spirocyclisch substituierte Tetramate |
| EP2113172A1 (de) | 2008-04-28 | 2009-11-04 | Bayer CropScience AG | Verfahren zur verbesserten Nutzung des Produktionspotentials transgener Pflanzen |
| JP2009538844A (ja) * | 2006-06-02 | 2009-11-12 | バイエル・クロツプサイエンス・アクチエンゲゼルシヤフト | アルコキシアルキル置換環状ケトエノール |
| EP2127522A1 (de) | 2008-05-29 | 2009-12-02 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| CN101235023B (zh) * | 2008-03-04 | 2010-06-02 | 浙江大学 | 一种螺螨酯的合成方法 |
| WO2010086095A1 (en) | 2009-01-29 | 2010-08-05 | Bayer Cropscience Ag | Method for improved utilization of the production potential of transgenic plants introduction |
| WO2010094728A1 (en) | 2009-02-19 | 2010-08-26 | Bayer Cropscience Ag | Pesticide composition comprising a tetrazolyloxime derivative and a fungicide or an insecticide active substance |
| WO2010102758A2 (de) | 2009-03-11 | 2010-09-16 | Bayer Cropscience Ag | Halogenalkylmethylenoxy-phenyl-substituierte ketoenole |
| EP2264008A1 (de) | 2009-06-18 | 2010-12-22 | Bayer CropScience AG | Substituierte Enaminocarbonylverbindungen |
| WO2010149370A1 (en) | 2009-06-24 | 2010-12-29 | Bayer Cropscience Ag | Combinations of biological control agents and insecticides |
| EP2266399A1 (de) | 2006-05-12 | 2010-12-29 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Blattkäfer (Chrysomelidae) |
| WO2011009540A2 (en) | 2009-07-24 | 2011-01-27 | Bayer Cropscience Ag | Pesticidal carboxamides |
| DE102009028001A1 (de) | 2009-07-24 | 2011-01-27 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| WO2011020567A1 (de) | 2009-08-20 | 2011-02-24 | Bayer Cropscience Ag | 3-triazolylphenyl-substituierte sulfid-derivate als akarizide und insektizide |
| WO2011029506A1 (de) | 2009-08-20 | 2011-03-17 | Bayer Cropscience Ag | 3-[1-(3-haloalkyl)-triazolyl]-phenyl-sulfid-derivate als akarizide und insektizide |
| WO2011045224A1 (de) | 2009-10-12 | 2011-04-21 | Bayer Cropscience Ag | 1- (pyrid-3-yl) -pyrazole und 1- (pyrimid-5-yl) -pyrazole als schädlingsbekämpfungsmittel |
| WO2011045240A1 (de) | 2009-10-12 | 2011-04-21 | Bayer Cropscience Ag | Amide und thioamide als schädlingsbekämpfungsmittel |
| WO2011051151A1 (de) | 2009-10-26 | 2011-05-05 | Bayer Cropscience Ag | Neue feste form von 4-[[(6-chlorpyridin-3-yl)methyl](2,2-difluorethyl)amino]furan-2(5h)-on |
| WO2011051455A1 (en) | 2009-10-30 | 2011-05-05 | Bayer Cropscience Ag | Pesticidal heterocyclic compounds |
| WO2011054436A2 (de) | 2009-10-27 | 2011-05-12 | Bayer Cropscience Ag | Halogenalkyl-substituierte amide als insektizide und akarizide |
| WO2011061156A1 (en) | 2009-11-17 | 2011-05-26 | Bayer Cropscience Ag | Active compound combinations |
| WO2011076727A2 (en) | 2009-12-23 | 2011-06-30 | Bayer Cropscience Ag | Pesticidal compound mixtures |
| WO2011076726A2 (en) | 2009-12-23 | 2011-06-30 | Bayer Cropscience Ag | Pesticidal compound mixtures |
| WO2011076724A2 (en) | 2009-12-23 | 2011-06-30 | Bayer Cropscience Ag | Pesticidal compound mixtures |
| WO2011080211A1 (en) | 2009-12-28 | 2011-07-07 | Bayer Cropscience Ag | Pesticidal arylpyrrolidines |
| WO2011080044A2 (en) | 2009-12-16 | 2011-07-07 | Bayer Cropscience Ag | Active compound combinations |
| WO2010149274A3 (de) * | 2009-06-23 | 2011-07-21 | Bayer Cropscience Aktiengesellschaft | Verwendung von wirkstoffkombinationen mit insektiziden eigenschaften zur bekämpfung von tierischen schädlingen aus der familie der stinkwanzen |
| WO2011092147A1 (en) | 2010-01-29 | 2011-08-04 | Bayer Cropscience Ag | Method to reduce the frequency and/or intensity of blossom-end rot disorder in horticultural crops |
| WO2011098443A1 (de) | 2010-02-10 | 2011-08-18 | Bayer Cropscience Ag | Spiroheterocyclisch-substituierte tetramsäure-derivate |
| DE102010008642A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| WO2011098433A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft | Zyklische ketoenole zur therapie |
| WO2011098440A2 (de) | 2010-02-10 | 2011-08-18 | Bayer Cropscience Ag | Biphenylsubstituierte cyclische ketoenole |
| DE102010008643A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| WO2011131623A1 (de) | 2010-04-20 | 2011-10-27 | Bayer Cropscience Ag | Insektizide und/oder herbizide zusammensetzung mit verbesserter wirkung auf basis von spiroheterocyclisch-substituierten tetramsäure-derivaten |
| WO2011138285A1 (de) | 2010-05-05 | 2011-11-10 | Bayer Cropscience Ag | Thiazolderivate als schädlingsbekämpfungsmittel |
| CN102241590A (zh) * | 2011-05-17 | 2011-11-16 | 永农生物科学有限公司 | 一种螺环季酮酸类化合物关键中间体的合成方法 |
| US8067458B2 (en) | 2006-04-22 | 2011-11-29 | Bayer Cropscience Ag | Alkoxyalkyl-substituted cyclic ketoenols |
| WO2011157663A1 (de) | 2010-06-15 | 2011-12-22 | Bayer Cropscience Ag | Neue ortho-substituierte arylamid-derivate |
| WO2011157778A1 (de) | 2010-06-18 | 2011-12-22 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden eigenschaften |
| WO2012001068A2 (de) | 2010-07-02 | 2012-01-05 | Bayer Cropscience Ag | Insektizide oder akarizide formulierungen mit verbesserter verfügbarkeit auf pflanzenoberflächen |
| WO2012000902A1 (de) | 2010-06-29 | 2012-01-05 | Bayer Cropscience Ag | Verbesserte insektizide zusammensetzungen enthaltend cyclische carbonylamidine |
| WO2012000896A2 (de) | 2010-06-28 | 2012-01-05 | Bayer Cropscience Ag | Heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2012004326A1 (en) | 2010-07-08 | 2012-01-12 | Bayer Cropscience Ag | Pesticidal pyrroline derivatives |
| WO2012004293A2 (de) | 2010-07-08 | 2012-01-12 | Bayer Cropscience Ag | Insektizide und fungizide wirkstoffkombinationen |
| WO2012004208A1 (de) | 2010-07-09 | 2012-01-12 | Bayer Cropscience Ag | Anthranilsäurediamid-derivate als pestizide |
| WO2012007500A2 (de) | 2010-07-15 | 2012-01-19 | Bayer Cropscience Ag | Neue heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2012010509A2 (de) | 2010-07-20 | 2012-01-26 | Bayer Cropscience Ag | Gelköder zur bekämpfung von kriechenden schadinsekten |
| EP2422620A1 (de) | 2010-08-26 | 2012-02-29 | Bayer CropScience AG | Insektizide Wirkstoffkombinationen enthaltend Ethiprole und Pymetrozin |
| WO2012028583A1 (de) | 2010-09-03 | 2012-03-08 | Bayer Cropscience Ag | Deltamethrin enthaltende formulierungen |
| WO2012035011A1 (en) | 2010-09-15 | 2012-03-22 | Bayer Cropscience Ag | Pesticidal arylpyrrolidines |
| WO2012034957A1 (en) | 2010-09-15 | 2012-03-22 | Bayer Cropscience Ag | Pesticidal pyrroline n-oxide derivatives |
| WO2012038480A2 (en) | 2010-09-22 | 2012-03-29 | Bayer Cropscience Ag | Use of biological or chemical control agents for controlling insects and nematodes in resistant crops |
| WO2012045680A2 (de) | 2010-10-04 | 2012-04-12 | Bayer Cropscience Ag | Insektizide und fungizide wirkstoffkombinationen |
| WO2012045798A1 (en) | 2010-10-07 | 2012-04-12 | Bayer Cropscience Ag | Fungicide composition comprising a tetrazolyloxime derivative and a thiazolylpiperidine derivative |
| WO2012052412A1 (de) | 2010-10-22 | 2012-04-26 | Bayer Cropscience Ag | Neue heterocylische verbindungen als schädlingsbekämpfungsmittel |
| WO2012052490A1 (en) | 2010-10-21 | 2012-04-26 | Bayer Cropscience Ag | N-benzyl heterocyclic carboxamides |
| EP2446742A1 (de) | 2010-10-28 | 2012-05-02 | Bayer CropScience AG | Insektizide oder akarizide Zusammensetzungen enthaltend Mono- oder Disacchariden als Wirkungsverstärker |
| WO2012059497A1 (en) | 2010-11-02 | 2012-05-10 | Bayer Cropscience Ag | N-hetarylmethyl pyrazolylcarboxamides |
| WO2012065944A1 (en) | 2010-11-15 | 2012-05-24 | Bayer Cropscience Ag | N-aryl pyrazole(thio)carboxamides |
| WO2012072660A1 (en) | 2010-12-01 | 2012-06-07 | Bayer Cropscience Ag | Use of fluopyram for controlling nematodes in crops and for increasing yield |
| WO2012072489A1 (de) | 2010-11-29 | 2012-06-07 | Bayer Cropscience Ag | Alpha-beta-ungesättigte imine |
| WO2012076470A1 (en) | 2010-12-09 | 2012-06-14 | Bayer Cropscience Ag | Pesticidal mixtures with improved properties |
| WO2012076471A1 (en) | 2010-12-09 | 2012-06-14 | Bayer Cropscience Ag | Insecticidal mixtures with improved properties |
| WO2012082548A2 (en) | 2010-12-15 | 2012-06-21 | Syngenta Participations Ag | Soybean event syht0h2 and compositions and methods for detection thereof |
| WO2012080188A1 (de) | 2010-12-17 | 2012-06-21 | Bayer Cropscience Ag | Insektizid-wachs-partikel enthaltende zusammensetzung |
| DE102010063691A1 (de) | 2010-12-21 | 2012-06-21 | Bayer Animal Health Gmbh | Ektoparasitizide Wirkstoffkombinationen |
| US8211931B2 (en) | 2006-06-16 | 2012-07-03 | Bayer Cropscience Ag | Active compound combinations having insecticidal and acaricidal properties |
| WO2012101047A1 (de) | 2011-01-25 | 2012-08-02 | Bayer Cropscience Ag | Verfahren zur herstellung von 1-h-pyrrolidin-2,4-dion-derivaten |
| DE102011011040A1 (de) | 2011-02-08 | 2012-08-09 | Bayer Pharma Aktiengesellschaft | (5s,8s)-3-(4'-Chlor-3'-fluor-4-methylbiphenyl-3-yl)-4-hydroxy-8-methoxy-1-azaspiro[4.5]dec-3-en-2-on (Verbindung A) zur Therapie |
| US8247351B2 (en) | 2005-12-13 | 2012-08-21 | Bayer Cropscience Ag | Insecticidal compositions having improved effect |
| WO2012110464A1 (en) | 2011-02-17 | 2012-08-23 | Bayer Cropscience Ag | Use of sdhi fungicides on conventionally bred asr-tolerant, stem canker resistant and/or frog-eye leaf spot resistant soybean varieties |
| WO2012110519A1 (de) | 2011-02-17 | 2012-08-23 | Bayer Cropscience Ag | Substituierte 3-(biphenyl-3-yl)-8,8-difluor-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-one zur therapie und halogensubstituierte spirocyclische ketoenole |
| WO2012116960A1 (de) | 2011-03-01 | 2012-09-07 | Bayer Cropscience Ag | 2-acyloxy-pyrrolin-4-one |
| WO2012120105A1 (en) | 2011-03-10 | 2012-09-13 | Bayer Cropscience Ag | Use of lipochito-oligosaccharide compounds for safeguarding seed safety of treated seeds |
| WO2012119984A1 (de) | 2011-03-09 | 2012-09-13 | Bayer Cropscience Ag | Indol- und benzimidazolcarbonsäureamide als insektizide und akarizide |
| WO2012126766A1 (de) | 2011-03-18 | 2012-09-27 | Bayer Cropscience Ag | N- (3 -carbamoylphenyl) - 1h - pyrazol - 5 - carboxamid - derivate und ihre verwendung zur bekämpfung von tierischen schädlingen |
| WO2012136751A1 (en) | 2011-04-08 | 2012-10-11 | Basf Se | N-substituted hetero-bicyclic compounds and derivatives for combating animal pests |
| EP2535334A1 (de) | 2011-06-17 | 2012-12-19 | Bayer CropScience AG | Kristalline Modifikationen von Penflufen |
| EP2540163A1 (de) | 2011-06-30 | 2013-01-02 | Bayer CropScience AG | Nematozide N-Cyclopropylsulfonylamidderivate |
| WO2013010946A2 (en) | 2011-07-15 | 2013-01-24 | Basf Se | Pesticidal methods using substituted 3-pyridyl thiazole compounds and derivatives for combating animal pests i |
| WO2013014227A1 (en) | 2011-07-27 | 2013-01-31 | Bayer Intellectual Property Gmbh | Seed dressing for controlling phytopathogenic fungi |
| WO2013014126A1 (de) | 2011-07-26 | 2013-01-31 | Bayer Intellectual Property Gmbh | Veretherte laktatester, verfahren zu ihrer herstellung und ihre verwendung zur verbesserung der wirkung von pflanzenschutzmitteln |
| DE102011080406A1 (de) | 2011-08-04 | 2013-02-07 | Bayer Pharma AG | Substituierte 3-(Biphenyl-3-yl)-4-hydroxy-8-methoxy-1-azaspiro8[4.5]dec-3-en-2-one |
| DE102011080405A1 (de) | 2011-08-04 | 2013-02-07 | Bayer Pharma AG | Substituierte 3-(Biphenyl-3-yl)-8,8-difluor-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-one zur Therapie |
| WO2013024008A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | Aniline type compounds |
| US8389443B2 (en) | 2008-12-02 | 2013-03-05 | Bayer Cropscience Ag | Geminal alkoxy/alkylspirocyclic substituted tetramate derivatives |
| WO2013050433A1 (en) | 2011-10-05 | 2013-04-11 | Bayer Intellectual Property Gmbh | Pesticide preparation and process for producing the same |
| US8420608B2 (en) | 2008-11-14 | 2013-04-16 | Bayer Cropscience Ag | Active substance combinations with insecticides and acaricide properties |
| WO2013079601A1 (en) | 2011-12-02 | 2013-06-06 | Basf Se | Method and system for monitoring crops and/or infestation of crops with harmful organismus during storage |
| WO2013079600A1 (en) | 2011-12-02 | 2013-06-06 | Basf Se | Method and system for monitoring crops during storage |
| EP2604118A1 (de) | 2011-12-15 | 2013-06-19 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| EP2606726A1 (de) | 2011-12-21 | 2013-06-26 | Bayer CropScience AG | N-Arylamidine-substituierte trifluoroethylsulfid-Derivate als Akarizide und Insektizide |
| WO2013092522A1 (de) | 2011-12-20 | 2013-06-27 | Bayer Intellectual Property Gmbh | Neue insektizide aromatische amide |
| WO2013092868A1 (en) | 2011-12-21 | 2013-06-27 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013092943A1 (en) | 2011-12-23 | 2013-06-27 | Basf Se | Isothiazoline compounds for combating invertebrate pests |
| WO2013092519A1 (en) | 2011-12-19 | 2013-06-27 | Bayer Cropscience Ag | Use of anthranilic acid diamide derivatives for pest control in transgenic crops |
| WO2013107785A1 (en) | 2012-01-21 | 2013-07-25 | Bayer Intellectual Property Gmbh | Use of host defense inducers for controlling bacterial harmful organisms in useful plants |
| WO2013110612A1 (en) | 2012-01-26 | 2013-08-01 | Bayer Intellectual Property Gmbh | Phenyl-substituted ketoenols for controlling fish parasites |
| WO2013113789A1 (en) | 2012-02-02 | 2013-08-08 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| US8507537B2 (en) | 2006-10-25 | 2013-08-13 | Bayer Cropscience Ag | Trifluromethoxyphenyl-substituted tetramic acid derivatives pesticides and/or herbicides |
| WO2013135724A1 (en) | 2012-03-14 | 2013-09-19 | Bayer Intellectual Property Gmbh | Pesticidal arylpyrrolidines |
| WO2013144213A1 (en) | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyridinylidene compounds and derivatives for combating animal pests |
| WO2013144228A1 (en) | 2012-03-29 | 2013-10-03 | Basf Se | Pesticidal methods using heterocyclic compounds and derivatives for combating animal pests |
| WO2013144223A1 (en) | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyrimidinylidene compounds and derivatives for combating animal pests |
| WO2013149903A1 (en) | 2012-04-03 | 2013-10-10 | Basf Se | N- substituted hetero - bicyclic furanone derivatives for combating animal |
| WO2013149940A1 (en) | 2012-04-02 | 2013-10-10 | Basf Se | Acrylamide compounds for combating invertebrate pests |
| WO2013150115A1 (en) | 2012-04-05 | 2013-10-10 | Basf Se | N- substituted hetero - bicyclic compounds and derivatives for combating animal pests |
| WO2013164295A1 (en) | 2012-05-04 | 2013-11-07 | Basf Se | Substituted pyrazole-containing compounds and their use as pesticides |
| WO2013167633A1 (en) | 2012-05-09 | 2013-11-14 | Basf Se | Acrylamide compounds for combating invertebrate pests |
| WO2013171201A1 (de) | 2012-05-16 | 2013-11-21 | Bayer Cropscience Ag | Insektizide öl-in-wasser (o/w) formulierung |
| WO2013171199A1 (de) | 2012-05-16 | 2013-11-21 | Bayer Cropscience Ag | Insektizide wasser-in-öl (w/o) formulierung |
| WO2013174645A1 (en) | 2012-05-24 | 2013-11-28 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013174836A1 (en) | 2012-05-22 | 2013-11-28 | Bayer Cropscience Ag | Active compounds combinations comprising a lipo-chitooligosaccharide derivative and a nematicide, insecticidal or fungicidal compound |
| WO2013186089A2 (en) | 2012-06-14 | 2013-12-19 | Basf Se | Pesticidal methods using substituted 3-pyridyl thiazole compounds and derivatives for combating animal pests |
| WO2014019983A1 (en) | 2012-07-31 | 2014-02-06 | Bayer Cropscience Ag | Compositions comprising a pesticidal terpene mixture and an insecticide |
| WO2014026984A1 (de) | 2012-08-17 | 2014-02-20 | Bayer Cropscience Ag | Azaindolcarbonsäure- und -thiocarbonsäureamide als insektizide und akarizide |
| WO2014053405A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053395A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Use of n-thio-anthranilamide compounds on cultivated plants |
| WO2014053401A2 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of improving plant health |
| WO2014053404A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053450A1 (de) | 2012-10-02 | 2014-04-10 | Bayer Cropscience Ag | Heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2014053407A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2014053403A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling insecticide resistant insects |
| WO2014053406A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling ryanodine-modulator insecticide resistant insects |
| WO2014060381A1 (de) | 2012-10-18 | 2014-04-24 | Bayer Cropscience Ag | Heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2014067962A1 (de) | 2012-10-31 | 2014-05-08 | Bayer Cropscience Ag | Neue heterocylische verbindungen als schädlingsbekämpfungsmittel |
| WO2014072250A1 (en) | 2012-11-06 | 2014-05-15 | Bayer Cropscience Ag | Herbicidal combinations for tolerant soybean cultures |
| WO2014079820A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Use of anthranilamide compounds for reducing insect-vectored viral infections |
| WO2014079770A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079773A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079774A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079772A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079841A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079764A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079813A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079814A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079752A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079766A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079804A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014083088A2 (en) | 2012-11-30 | 2014-06-05 | Bayer Cropscience Ag | Binary fungicidal mixtures |
| WO2014083033A1 (en) | 2012-11-30 | 2014-06-05 | Bayer Cropsience Ag | Binary fungicidal or pesticidal mixture |
| WO2014086749A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising a biological control agent and an insecticide |
| WO2014086750A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising a biological control agent and an insecticide |
| WO2014086753A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising biological control agents |
| WO2014086759A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising biological control agents |
| WO2014086758A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising a biological control agent and an insecticide |
| WO2014090700A1 (en) | 2012-12-14 | 2014-06-19 | Basf Se | Malononitrile compounds for controlling animal pests |
| WO2014090765A1 (en) | 2012-12-12 | 2014-06-19 | Bayer Cropscience Ag | Use of 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfinyl)phenyl]-5-amino-3-trifluoromethyl)-1 h-1,2,4 tfia zole for controlling nematodes in nematode-resistant crops |
| WO2014095826A1 (en) | 2012-12-18 | 2014-06-26 | Bayer Cropscience Ag | Binary fungicidal and bactericidal combinations |
| WO2014096238A1 (en) | 2012-12-21 | 2014-06-26 | Basf Se | Cycloclavine and derivatives thereof for controlling invertebrate pests |
| WO2014102244A1 (en) | 2012-12-27 | 2014-07-03 | Basf Se | 2-(pyridin-3-yl)-5-hetaryl-thiazole compounds carrying an imine or imine-derived substituent for combating invertebrate pests |
| WO2014124373A1 (en) | 2013-02-11 | 2014-08-14 | Bayer Cropscience Lp | Compositions comprising gougerotin and an insecticide |
| WO2014122083A1 (de) | 2013-02-06 | 2014-08-14 | Bayer Cropscience Ag | Halogensubstituierte pyrazolderivate als schädlingsbekämpfungsmittel |
| WO2014124375A1 (en) | 2013-02-11 | 2014-08-14 | Bayer Cropscience Lp | Compositions comprising gougerotin and a biological control agent |
| US8816097B2 (en) | 2006-07-18 | 2014-08-26 | Bayer Cropscience Ag | Active ingredient combinations having insecticide and acaricide properties |
| WO2014128136A1 (en) | 2013-02-20 | 2014-08-28 | Basf Se | Anthranilamide compounds and their use as pesticides |
| WO2014140111A1 (en) | 2013-03-13 | 2014-09-18 | Bayer Cropscience Ag | Lawn growth-promoting agent and method of using same |
| WO2014139897A1 (en) | 2013-03-12 | 2014-09-18 | Bayer Cropscience Ag | Use of dithiine-tetracarboximides for controlling bacterial harmful organisms in useful plants |
| US8846946B2 (en) | 2008-12-02 | 2014-09-30 | Bayer Cropscience Ag | Germinal alkoxy/alkylspirocyclic substituted tetramate derivatives |
| WO2014170300A1 (en) | 2013-04-19 | 2014-10-23 | Basf Se | N-substituted acyl-imino-pyridine compounds and derivatives for combating animal pests |
| WO2014170313A1 (en) | 2013-04-19 | 2014-10-23 | Bayer Cropscience Ag | Active compound combinations having insecticidal properties |
| WO2014170364A1 (en) | 2013-04-19 | 2014-10-23 | Bayer Cropscience Ag | Binary insecticidal or pesticidal mixture |
| DE202014008418U1 (de) | 2014-02-19 | 2014-11-14 | Clariant International Ltd. | Schaumarme agrochemische Zusammensetzungen |
| DE202014008415U1 (de) | 2014-02-19 | 2014-11-25 | Clariant International Ltd. | Wässrige Adjuvant-Zusammensetzung zur Wirkungssteigerung von Elektrolyt-Wirkstoffen |
| WO2014202510A1 (de) | 2013-06-20 | 2014-12-24 | Bayer Cropscience Ag | Arylsulfid- und arylsulfoxid-derivate als akarizide und insektizide |
| WO2014202751A1 (en) | 2013-06-21 | 2014-12-24 | Basf Se | Methods for controlling pests in soybean |
| WO2014202505A1 (de) | 2013-06-20 | 2014-12-24 | Bayer Cropscience Ag | Arylsulfid- und arylsulfoxid-derivate als akarizide und insektizide |
| WO2015004028A1 (de) | 2013-07-08 | 2015-01-15 | Bayer Cropscience Ag | Sechsgliedrige c-n-verknüpfte arylsulfid- und arylsulfoxid- derivate als schädlingsbekämpfungsmittel |
| WO2015007682A1 (en) | 2013-07-15 | 2015-01-22 | Basf Se | Pesticide compounds |
| WO2015040116A1 (en) | 2013-09-19 | 2015-03-26 | Basf Se | N-acylimino heterocyclic compounds |
| US8993782B2 (en) | 2006-12-04 | 2015-03-31 | Bayer Cropscience Ag | Cis-alkoxyspirocyclic biphenyl-substituted tetramic acid derivatives |
| WO2015055757A1 (en) | 2013-10-18 | 2015-04-23 | Basf Se | Use of pesticidal active carboxamide derivative in soil and seed application and treatment methods |
| WO2015055497A1 (en) | 2013-10-16 | 2015-04-23 | Basf Se | Substituted pesticidal pyrazole compounds |
| WO2015091645A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | Azole compounds carrying an imine-derived substituent |
| WO2015091649A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | N-substituted imino heterocyclic compounds |
| WO2015101622A1 (de) | 2014-01-03 | 2015-07-09 | Bayer Cropscience Ag | Neue pyrazolyl-heteroarylamide als schädlingsbekämpfungsmittel |
| WO2015104422A1 (en) | 2014-01-13 | 2015-07-16 | Basf Se | Dihydrothiophene compounds for controlling invertebrate pests |
| DE102014018274A1 (de) | 2014-12-12 | 2015-07-30 | Clariant International Ltd. | Zuckertenside und deren Verwendung in agrochemischen Zusammensetzungen |
| WO2015160620A1 (en) | 2014-04-16 | 2015-10-22 | Bayer Cropscience Lp | Compositions comprising ningnanmycin and an insecticide |
| WO2015160618A1 (en) | 2014-04-16 | 2015-10-22 | Bayer Cropscience Lp | Compositions comprising ningnanmycin and a biological control agent |
| WO2016001129A1 (de) | 2014-07-01 | 2016-01-07 | Bayer Cropscience Aktiengesellschaft | Verbesserte insektizide zusammensetzungen |
| WO2016008830A1 (de) | 2014-07-15 | 2016-01-21 | Bayer Cropscience Aktiengesellschaft | Aryl-triazolyl-pyridine als schädlingsbekämpfungsmittel |
| DE102014012022A1 (de) | 2014-08-13 | 2016-02-18 | Clariant International Ltd. | Organische Ammoniumsalze von anionischen Pestiziden |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| WO2016106063A1 (en) | 2014-12-22 | 2016-06-30 | Bayer Corpscience Lp | Method for using a bacillus subtilis or bacillus pumilus strain to treat or prevent pineapple disease |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| EP3178320A1 (de) | 2015-12-11 | 2017-06-14 | Bayer CropScience AG | Flüssige fungizid-haltige formulierungen |
| WO2017121699A1 (de) | 2016-01-15 | 2017-07-20 | Bayer Cropscience Aktiengesellschaft | Verfahren zur herstellung von substituierten 2-aryl-ethanolen |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017186543A2 (en) | 2016-04-24 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Use of fluopyram and/or bacillus subtilis for controlling fusarium wilt in plants of the musaceae family |
| EP3243387A2 (de) | 2012-05-30 | 2017-11-15 | Bayer CropScience Aktiengesellschaft | Zusammensetzungen mit einem biologischen schädlingsbekämpfungsmittel und einem insektizid |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| EP3248465A1 (de) | 2016-05-25 | 2017-11-29 | Bayer CropScience Aktiengesellschaft | Agrochemische zusammensetzung polymerisches emulsionspulver enthaltend. |
| WO2018019676A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Active compound combinations and methods to protect the propagation material of plants |
| WO2018024659A1 (de) | 2016-08-04 | 2018-02-08 | Bayer Cropscience Aktiengesellschaft | Verfahren zur herstellung von spiroketal-substituierten cyclischen ketoenolen |
| EP3301092A2 (de) | 2018-01-26 | 2018-04-04 | Bayer CropScience Aktiengesellschaft | Verfahren zur herstellung von spiroketal-substituierten phenylacetylaminosäureestern und spiroketal-substituierten cyclischen ketoenolen |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| EP3363289A2 (de) | 2012-05-30 | 2018-08-22 | Bayer CropScience Aktiengesellschaft | Zusammensetzungen mit einem biologischen schädlingsbekämpfungsmittel und einem insektizid |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| WO2018210616A1 (de) | 2017-05-15 | 2018-11-22 | Bayer Cropscience Aktiengesellschaft | Verfahren zur herstellung von (4-halogen-2,6-dialkylphenyl)malononitrilen |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| EP3415007A1 (de) | 2017-06-12 | 2018-12-19 | Bayer AG | Ptz formulierungen mit niedrigem gehalt an desthio |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | SUBSTITUTED CYCLOPROPYL DERIVATIVES |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | SUBSTITUTED PYRIMIDINIUM COMPOUNDS AND DERIVATIVES FOR CONTROLLING HARMFUL ANIMALS |
| WO2019042932A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING RICE PARASITES IN RICE |
| EP3453706A1 (de) | 2017-09-08 | 2019-03-13 | Basf Se | Pestizide imidazolverbindungen |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS AYNT INSECTICIDE / ACARICIDE PROPERTIES |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| WO2019197620A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von speziellen insekten |
| WO2019197637A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Hochbeladene formulierungen mit insektiziden aus der ketoenol-klasse zur verwendung bei drip- und drench- applikationen |
| WO2019197615A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit fungiziden, insektiziden und akariziden eigenschaften |
| WO2019197623A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2019197232A1 (de) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Verfahren zur herstellung von 2,6-dialkylphenyl-essigsäuren |
| WO2019197617A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von tierischen schädlingen durch angiessen, tröpfchenapplikation. pflanzlochbehandlung oder furchenapplikation |
| WO2019197612A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von tierischen schädlingen durch angiessen oder tröpfchenapplikation |
| WO2019197616A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Verwendung von tetramsäurederivaten zur reduktion von nematodenpopulationen |
| WO2019197618A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von speziellen insekten |
| WO2019197652A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Feststoff-formulierung insektizider mischungen |
| WO2019197231A1 (de) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Verfahren zur herstellung von substituierten cyclohexanaminosäureestern und spiroketalsubstituierten cyclischen ketoenolen |
| WO2019201842A1 (de) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Verfahren zur herstellung von estern n-acylierter aminosäuren mit säurelabilen keto-schutzgruppenfunktionen |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2019224143A1 (de) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| EP3613736A1 (de) | 2018-08-22 | 2020-02-26 | Basf Se | Substituierte glutarimidderivate |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3628157A1 (de) | 2018-09-28 | 2020-04-01 | Basf Se | Verfahren zur kontrolle von insektizid-resistenten insekten und virusübertragung auf pflanzen |
| EP3628158A1 (de) | 2018-09-28 | 2020-04-01 | Basf Se | Pestizide zusammensetzung die eine mesoionische verbindung und ein biopestizid enthält |
| EP3628156A1 (de) | 2018-09-28 | 2020-04-01 | Basf Se | Verfahren zur bekämpfung von schädlingen von zuckerrohr-, zitrus-, raps- und kartoffelpflanzen |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| EP3643705A1 (de) | 2018-10-24 | 2020-04-29 | Basf Se | Pestizidverbindungen |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3696177A1 (de) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| EP3701796A1 (de) | 2019-08-08 | 2020-09-02 | Bayer AG | Wirkstoffkombinationen |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| US10772324B2 (en) | 2012-11-03 | 2020-09-15 | Clariant International Ltd. | Aqueous adjuvant-compositions |
| WO2020187871A1 (de) | 2019-03-19 | 2020-09-24 | Bayer Aktiengesellschaft | Stabilisierte formulierungen von thioketonen |
| US10813862B2 (en) | 2012-05-30 | 2020-10-27 | Clariant International Ltd. | Use of N-methyl-N-acylglucamines as solubilizers |
| CN111970928A (zh) * | 2018-04-13 | 2020-11-20 | 拜耳作物科学股份公司 | 油基悬浮浓缩剂 |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| US10864275B2 (en) | 2012-05-30 | 2020-12-15 | Clariant International Ltd. | N-methyl-N-acylglucamine-containing composition |
| WO2020254230A1 (de) | 2019-06-15 | 2020-12-24 | Bayer Aktiengesellschaft | Stabilisierte formulierungen von dithiocarbamaten |
| EP3766879A1 (de) | 2019-07-19 | 2021-01-20 | Basf Se | Pestizide pyrazolverbindungen |
| EP3769623A1 (de) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionische imidazolverbindungen und derivate zur bekämpfung von tierischen schädlingen |
| US10920080B2 (en) | 2015-10-09 | 2021-02-16 | Clariant International Ltd. | N-Alkyl glucamine-based universal pigment dispersions |
| EP3772955A1 (de) * | 2018-04-13 | 2021-02-17 | Bayer Aktiengesellschaft | Feststoff-formulierung insektizider mischungen |
| US10961484B2 (en) | 2015-10-09 | 2021-03-30 | Clariant International Ltd. | Compositions comprising sugar amine and fatty acid |
| WO2021130143A1 (en) | 2019-12-23 | 2021-07-01 | Basf Se | Enzyme enhanced root uptake of agrochemical active compound |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| WO2021170463A1 (en) | 2020-02-28 | 2021-09-02 | BASF Agro B.V. | Methods and uses of a mixture comprising alpha-cypermethrin and dinotefuran for controlling invertebrate pests in turf |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| WO2021219513A1 (en) | 2020-04-28 | 2021-11-04 | Basf Se | Pesticidal compounds |
| EP3909950A1 (de) | 2020-05-13 | 2021-11-17 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| US11220603B2 (en) | 2016-05-09 | 2022-01-11 | Clariant International Ltd. | Stabilizers for silicate paints |
| WO2022058522A1 (de) | 2020-09-20 | 2022-03-24 | Bayer Aktiengesellschaft | Stabilisierung von thioketonen auf oberflächen |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| EP4043444A1 (de) | 2021-02-11 | 2022-08-17 | Basf Se | Substituierte isoxazolinderivate |
| WO2022175318A1 (en) | 2021-02-19 | 2022-08-25 | Syngenta Crop Protection Ag | Insect and acarina pest control |
| WO2022175420A1 (en) | 2021-02-19 | 2022-08-25 | Syngenta Crop Protection Ag | Insect and acarina pest control |
| US11425904B2 (en) | 2014-04-23 | 2022-08-30 | Clariant International Ltd. | Use of aqueous drift-reducing compositions |
| WO2022200364A1 (en) | 2021-03-25 | 2022-09-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2022238575A1 (en) | 2021-05-14 | 2022-11-17 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2022238576A1 (en) | 2021-05-14 | 2022-11-17 | Syngenta Crop Protection Ag | Seed treatment compositions |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| WO2022268813A1 (en) | 2021-06-24 | 2022-12-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2022268815A1 (en) | 2021-06-24 | 2022-12-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023280999A1 (en) | 2021-07-07 | 2023-01-12 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| EP4119547A1 (de) | 2021-07-12 | 2023-01-18 | Basf Se | Triazolverbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2023021136A1 (en) | 2021-08-20 | 2023-02-23 | Syngenta Crop Protection Ag | Method of controlling pests on tea plants |
| EP4140995A1 (de) | 2021-08-27 | 2023-03-01 | Basf Se | Pyrazin verbindungen zur kontrolle von wirbellosen schädlingen |
| EP4140986A1 (de) | 2021-08-23 | 2023-03-01 | Basf Se | Pyrazolverbindungen zur bekämpfung von wirbellosen schädlingen |
| EP4151631A1 (de) | 2021-09-20 | 2023-03-22 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2023046853A1 (en) | 2021-09-23 | 2023-03-30 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| EP4194453A1 (de) | 2021-12-08 | 2023-06-14 | Basf Se | Pyrazinverbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2023105065A1 (en) | 2021-12-10 | 2023-06-15 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023105064A1 (en) | 2021-12-10 | 2023-06-15 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| EP4198033A1 (de) | 2021-12-14 | 2023-06-21 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| EP4198023A1 (de) | 2021-12-16 | 2023-06-21 | Basf Se | Pestizidaktive thiosemicarbazonverbindungen |
| US11700852B2 (en) | 2014-12-19 | 2023-07-18 | Clariant International Ltd | Aqueous electrolyte-containing adjuvant compositions, active ingredient-containing compositions and the use thereof |
| EP4238971A1 (de) | 2022-03-02 | 2023-09-06 | Basf Se | Substituierte isoxazolinderivate |
| WO2023180162A1 (de) | 2022-03-21 | 2023-09-28 | Bayer Aktiengesellschaft | Verfahren zur herstellung von ketonen |
| WO2023203038A1 (en) | 2022-04-19 | 2023-10-26 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2023208447A1 (en) | 2022-04-25 | 2023-11-02 | Basf Se | An emulsifiable concentrate having a (substituted) benzaldehyde-based solvent system |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
| EP4342885A1 (de) | 2022-09-20 | 2024-03-27 | Basf Se | N-(3-(aminomethyl)-phenyl)-5-(4-phenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-amin-derivate und ähnliche verbindungen als pestizide |
| EP4389210A1 (de) | 2022-12-21 | 2024-06-26 | Basf Se | Heteroarylverbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2024218261A1 (en) | 2023-04-18 | 2024-10-24 | Syngenta Crop Protection Ag | Method and composition for treating plant propagation materials |
| EP4455137A1 (de) | 2023-04-24 | 2024-10-30 | Basf Se | Pyrimidinverbindungen zur bekämpfung von wirbellosen schädlingen |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102004011006A1 (de) * | 2004-03-06 | 2005-09-22 | Bayer Cropscience Ag | Suspensionskonzentrate auf Ölbasis |
| DE102004053192A1 (de) * | 2004-11-04 | 2006-05-11 | Bayer Cropscience Ag | 2-Alkoxy-6-alkyl-phenyl substituierte spirocyclische Tetramsäure-Derivate |
| DE102004053191A1 (de) | 2004-11-04 | 2006-05-11 | Bayer Cropscience Ag | 2,6-Diethyl-4-methyl-phenyl substituierte Tetramsäure-Derivate |
| JP2011515422A (ja) * | 2008-03-27 | 2011-05-19 | バイエル・クロツプサイエンス・アクチエンゲゼルシヤフト | 昆虫およびアカハダニと戦うための土地上への灌水、小滴施用または浸漬施用によるテトロン酸誘導体の使用 |
| BR122017001862B1 (pt) * | 2009-08-05 | 2017-06-20 | Dow Global Technologies Llc | Antimicrobial composition synergistic |
| KR20120099396A (ko) * | 2009-09-09 | 2012-09-10 | 바이엘 크롭사이언스 아게 | 식물병원성 박테리아를 구제하기 위한 사이클릭 케토에놀의 용도 |
| JP6100264B2 (ja) * | 2011-09-16 | 2017-03-22 | バイエル・インテレクチュアル・プロパティ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツングBayer Intellectual Property GmbH | 植物の収量を向上させるための5−フェニル−2−イソオキサゾリン−3−カルボキシレート又は5−ベンジル−2−イソオキサゾリン−3−カルボキシレートの使用 |
| WO2017079719A1 (en) * | 2015-11-06 | 2017-05-11 | Regents Of The University Of Minnesota | Aromatic surfactants |
| US10743535B2 (en) | 2017-08-18 | 2020-08-18 | H&K Solutions Llc | Insecticide for flight-capable pests |
| WO2019197619A1 (de) * | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Suspensionskonzentrate auf ölbasis |
| CN114794131A (zh) * | 2022-05-23 | 2022-07-29 | 山东中新科农生物科技有限公司 | 一种含Spidoxamat和螺螨酯的杀螨组合物 |
| CN114931142B (zh) * | 2022-05-31 | 2024-06-28 | 山东中新科农生物科技有限公司 | 一种杀螨组合物及其制备方法和应用 |
Family Cites Families (75)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4021224A (en) | 1971-12-09 | 1977-05-03 | Stauffer Chemical Company | Herbicide compositions |
| US4186130A (en) | 1973-05-02 | 1980-01-29 | Stauffer Chemical Company | N-(haloalkanoyl) oxazolidines |
| MA19709A1 (fr) | 1982-02-17 | 1983-10-01 | Ciba Geigy Ag | Application de derives de quinoleine a la protection des plantes cultivees . |
| ATE103902T1 (de) | 1982-05-07 | 1994-04-15 | Ciba Geigy Ag | Verwendung von chinolinderivaten zum schuetzen von kulturpflanzen. |
| DE3525205A1 (de) | 1984-09-11 | 1986-03-20 | Hoechst Ag, 6230 Frankfurt | Pflanzenschuetzende mittel auf basis von 1,2,4-triazolderivaten sowie neue derivate des 1,2,4-triazols |
| DE3680212D1 (de) | 1985-02-14 | 1991-08-22 | Ciba Geigy Ag | Verwendung von chinolinderivaten zum schuetzen von kulturpflanzen. |
| US4925868A (en) | 1986-08-29 | 1990-05-15 | Takeda Chemical Industries, Ltd. | 4-Hydroxy-3-pyrrolin-2-ones and treatment of circulatory disorders therewith |
| DE3633840A1 (de) | 1986-10-04 | 1988-04-14 | Hoechst Ag | Phenylpyrazolcarbonsaeurederivate, ihre herstellung und verwendung als pflanzenwachstumsregulatoren und safener |
| DE3808896A1 (de) | 1988-03-17 | 1989-09-28 | Hoechst Ag | Pflanzenschuetzende mittel auf basis von pyrazolcarbonsaeurederivaten |
| DE3817192A1 (de) | 1988-05-20 | 1989-11-30 | Hoechst Ag | 1,2,4-triazolderivate enthaltende pflanzenschuetzende mittel sowie neue derivate des 1,2,4-triazols |
| US4985063A (en) | 1988-08-20 | 1991-01-15 | Bayer Aktiengesellschaft | 3-aryl-pyrrolidine-2,4-diones |
| ES2063108T3 (es) | 1989-01-07 | 1995-01-01 | Bayer Ag | Derivados de 3-aril-pirrolidin-2,4-diona. |
| DE3929087A1 (de) | 1989-09-01 | 1991-03-07 | Bayer Ag | 3-aryl-pyrrolidin-2,4-dion-derivate |
| DE4014420A1 (de) | 1989-09-23 | 1991-04-04 | Bayer Ag | 5h-furan-2-on-derivate |
| DE3939010A1 (de) | 1989-11-25 | 1991-05-29 | Hoechst Ag | Isoxazoline, verfahren zu ihrer herstellung und ihre verwendung als pflanzenschuetzende mittel |
| US5700758A (en) | 1989-11-30 | 1997-12-23 | Hoechst Aktiengesellschaft | Pyrazolines for protecting crop plants against herbicides |
| DE4032090A1 (de) | 1990-02-13 | 1991-08-14 | Bayer Ag | Polycyclische 3-aryl-pyrrolidin-2,4-dion-derivate |
| DE4107394A1 (de) | 1990-05-10 | 1991-11-14 | Bayer Ag | 1-h-3-aryl-pyrrolidin-2,4-dion-derivate |
| EP0492366B1 (de) | 1990-12-21 | 1997-03-26 | Hoechst Schering AgrEvo GmbH | Neue 5-Chlorchinolin-8-oxyalkancarbonsäurederivate, Verfahren zu ihrer Herstellung und ihre Verwendung als Antidots von Herbiziden |
| US5811374A (en) | 1991-02-07 | 1998-09-22 | Bayer Aktiengesellschaft | 3-aryl-pyrrolidine-2,4-dione derivatives |
| DE4121365A1 (de) | 1991-06-28 | 1993-01-14 | Bayer Ag | Substituierte 1-h-3-aryl-pyrrolidin-2,4-dion-derivate |
| DE4216814A1 (de) | 1991-07-16 | 1993-01-21 | Bayer Ag | 3-aryl-4-hydroxy-(delta)(pfeil hoch)3(pfeil hoch)-dihydrofuranon- und 3-aryl-4-hydroxy-(delta)(pfeil hoch)3(pfeil hoch)-dihydrothiophenon-derivate |
| GB9210393D0 (en) | 1992-05-15 | 1992-07-01 | Merck Sharp & Dohme | Therapeutic agents |
| TW259690B (de) | 1992-08-01 | 1995-10-11 | Hoechst Ag | |
| DE4236400A1 (de) * | 1992-10-28 | 1994-05-05 | Bayer Ag | N-Phenylacetaminonitrile |
| AU666040B2 (en) * | 1992-10-28 | 1996-01-25 | Bayer Aktiengesellschaft | Substituted 1-H-3-aryl-pyrrolidine-2,4-dione derivatives |
| DE4306259A1 (de) | 1993-03-01 | 1994-09-08 | Bayer Ag | Dialkyl-1-H-3-(2,4-dimethylphenyl)-pyrrolidin-2,4-dione, ihre Herstellung und ihre Verwendung |
| DE4306257A1 (de) | 1993-03-01 | 1994-09-08 | Bayer Ag | Substituierte 1-H-3-Phenyl-5-cycloalkylpyrrolidin-2,4-dione, ihre Herstellung und ihre Verwendung |
| US5407897A (en) | 1993-03-03 | 1995-04-18 | American Cyanamid Company | Method for safening herbicides in crops using substituted benzopyran and tetrahydronaphthalene compounds |
| JP3404747B2 (ja) | 1993-07-05 | 2003-05-12 | バイエル・アクチエンゲゼルシヤフト | 置換アリールケト−エノール型複素環式化合物 |
| DE4331448A1 (de) | 1993-09-16 | 1995-03-23 | Hoechst Schering Agrevo Gmbh | Substituierte Isoxazoline, Verfahren zu deren Herstellung, diese enthaltende Mittel und deren Verwendung als Safener |
| EP0647637B1 (de) | 1993-09-17 | 1999-01-27 | Bayer Ag | 3-Aryl-4-hydroxy-3-dihydrofuranon-Derivate |
| DE4425617A1 (de) | 1994-01-28 | 1995-08-03 | Bayer Ag | 1-H-3-Aryl-pyrrolidin-2,4-dion-Derivate |
| DE4431730A1 (de) | 1994-02-09 | 1995-08-10 | Bayer Ag | Substituierte 1H-3-Aryl-pyrrolidin-2,4-dion-Derivate |
| DE4410420A1 (de) | 1994-03-25 | 1995-09-28 | Bayer Ag | 3-Aryl-4-hydroxy- DELTA·3·-dihydrothiophenon-Derivate |
| AU2072695A (en) * | 1994-04-05 | 1995-10-23 | Bayer Aktiengesellschaft | Alkoxy-alkyl-substituted 1-h-3-aryl-pyrrolidine-2,4-diones used as herbicides and pesticides |
| AU4342096A (en) | 1994-12-23 | 1996-07-19 | Bayer Aktiengesellschaft | 3-aryl-tetronic acid derivatives, the production thereof and the use thereof as anti-parasitic agents |
| CN1154634C (zh) | 1995-02-13 | 2004-06-23 | 拜尔公司 | 作为除草剂和杀虫剂的2-苯基取代杂环1,3-酮烯醇 |
| WO1996035664A1 (de) | 1995-05-09 | 1996-11-14 | Bayer Aktiengesellschaft | Alkyl-dihalogenphenylsubstituierte ketoenole als schädlingsbekämpfungsmittel und herbizide |
| GB9510459D0 (en) | 1995-05-24 | 1995-07-19 | Zeneca Ltd | Bicyclic amines |
| RU2195449C2 (ru) | 1995-06-28 | 2002-12-27 | Байер Акциенгезельшафт | 2,4,5-тризамещенные фенилкетоенолы, промежуточные соединения для их получения, способ и средство для борьбы с насекомыми и паукообразными на их основе |
| BR9609301A (pt) | 1995-06-30 | 1999-05-25 | Bayer Ag | Cetoenois dialquil-halogenofenil-substituídos |
| US6140358A (en) | 1996-04-02 | 2000-10-31 | Bayer Aktiengesellschaft | Substituted phenyl keto enols as pesticides and herbicides |
| JP4306799B2 (ja) | 1996-05-10 | 2009-08-05 | バイエル アクチェンゲゼルシャフト | 新規な置換ピリジルケトエノール |
| DE19621522A1 (de) | 1996-05-29 | 1997-12-04 | Hoechst Schering Agrevo Gmbh | Neue N-Acylsulfonamide, neue Mischungen aus Herbiziden und Antidots und deren Verwendung |
| CN100339352C (zh) | 1996-08-05 | 2007-09-26 | 拜尔公司 | 2-和2,5-取代的苯基酮烯醇 |
| DE19632126A1 (de) * | 1996-08-09 | 1998-02-12 | Bayer Ag | Phenylsubstituierte cyclische Ketoenole |
| IL130058A0 (en) | 1996-11-26 | 2000-02-29 | Zeneca Ltd | 8-Azabicyclo(3.2.1)octane-8-azabicyclo(3.2.1)oct-6-ene-9-azabicyclo(3.3.1)nonane-9-aza-3-oxabicyclo (3.3.1)nonane- and 9-aza-3-thiabicyclo(3.3.1)nonane derivatives their preparation and their use as insecticides |
| US6391912B1 (en) | 1996-12-12 | 2002-05-21 | Bayer Aktiengesellschaft | Substituted phenylketoenols |
| DE19742492A1 (de) * | 1997-09-26 | 1999-04-01 | Bayer Ag | Spirocyclische Phenylketoenole |
| DE19742951A1 (de) | 1997-09-29 | 1999-04-15 | Hoechst Schering Agrevo Gmbh | Acylsulfamoylbenzoesäureamide, diese enthaltende nutzpflanzenschützende Mittel und Verfahren zu ihrer Herstellung |
| DE19749720A1 (de) | 1997-11-11 | 1999-05-12 | Bayer Ag | Neue substituierte Phenylketoenole |
| DE19808261A1 (de) | 1998-02-27 | 1999-10-28 | Bayer Ag | Arylphenylsubstituierte cyclische Ketoenole |
| DE19813354A1 (de) | 1998-03-26 | 1999-09-30 | Bayer Ag | Arylphenylsubstituierte cyclische Ketoenole |
| DE19818732A1 (de) | 1998-04-27 | 1999-10-28 | Bayer Ag | Arylphenylsubstituierte cyclische Ketoenole |
| DE19827855A1 (de) | 1998-06-23 | 1999-12-30 | Hoechst Schering Agrevo Gmbh | Kombinationen aus Herbiziden und Safenern |
| JP2000053670A (ja) | 1998-08-10 | 2000-02-22 | Ube Ind Ltd | アルコキシメチルフラノン誘導体及び有害生物防除剤 |
| PL202547B1 (pl) | 1999-09-07 | 2009-07-31 | Syngenta Participations Ag | Chwastobójczo działające związki heterocykliczne podstawione grupą fenylową, środek chwastobójczy i powstrzymujący wzrost roślin, selektywny środek chwastobójczy oraz sposób selektywnego zwalczania chwastów i traw w uprawach roślin użytkowych |
| DE19946625A1 (de) | 1999-09-29 | 2001-04-05 | Bayer Ag | Trifluormethylsubstituierte spirocyclische Ketoenole |
| DE10016544A1 (de) | 2000-04-03 | 2001-10-11 | Bayer Ag | C2-phenylsubstituierte Ketoenole |
| JP2002205984A (ja) * | 2000-05-11 | 2002-07-23 | Sankyo Co Ltd | N−置換スピロジヒドロピロール誘導体 |
| DE10139465A1 (de) | 2001-08-10 | 2003-02-20 | Bayer Cropscience Ag | Selektive Herbizide auf Basis von substituierten, cayclischen Ketoenolen und Safenern |
| CN1615310A (zh) | 2002-01-22 | 2005-05-11 | 辛根塔参与股份公司 | 可用作除草剂的苯基取代的杂环化合物 |
| DE10231333A1 (de) | 2002-07-11 | 2004-01-22 | Bayer Cropscience Ag | Cis-Alkoxysubstituierte spirocyclische 1-H-Pyrrolidin-2,4-dion-Derivate |
| DE10239479A1 (de) | 2002-08-28 | 2004-03-04 | Bayer Cropscience Ag | Substituierte spirocyclische Ketoenole |
| DE10301804A1 (de) | 2003-01-20 | 2004-07-29 | Bayer Cropscience Ag | 2,4-Dihalogen-6-(C2-C3-alkyl)-phenyl substituierte Tetramsäure-Derivate |
| DE10311300A1 (de) | 2003-03-14 | 2004-09-23 | Bayer Cropscience Ag | 2,4,6-Phenylsubstituierte cyclische Ketoenole |
| DE10326386A1 (de) | 2003-06-12 | 2004-12-30 | Bayer Cropscience Ag | N-Heterocyclyl-phenylsubstituierte cyclische Ketoenole |
| DE10351646A1 (de) | 2003-11-05 | 2005-06-09 | Bayer Cropscience Ag | 2-Halogen-6-alkyl-phenyl substituierte spirocyclische Tetramsäure-Derivate |
| DE10351647A1 (de) | 2003-11-05 | 2005-06-09 | Bayer Cropscience Ag | 2-Halogen-6-alkyl-phenyl substituierte Tetramsäure-Derivate |
| DE10354628A1 (de) | 2003-11-22 | 2005-06-16 | Bayer Cropscience Ag | 2-Ethyl-4,6-dimethyl-phenyl-substituierte Tetramsäure-Derivate |
| DE10354629A1 (de) | 2003-11-22 | 2005-06-30 | Bayer Cropscience Ag | 2-Ethyl-4,6-dimethyl-phenyl substituierte spirocyclische Tetramsäure-Derivate |
| DE102004001433A1 (de) | 2004-01-09 | 2005-08-18 | Bayer Cropscience Ag | cis-Alkoxyspiro-substituierte Tetramsäure-Derivate |
| DE102004014620A1 (de) | 2004-03-25 | 2005-10-06 | Bayer Cropscience Ag | 2,4,6-phenylsubstituierte cyclische Ketoenole |
| DE102004030753A1 (de) | 2004-06-25 | 2006-01-19 | Bayer Cropscience Ag | 3'-Alkoxy spirocyclische Tetram- und Tretronsäuren |
-
2005
- 2005-02-22 DE DE102005008021A patent/DE102005008021A1/de not_active Withdrawn
-
2006
- 2006-02-08 CN CN2010102161847A patent/CN101885700A/zh active Pending
- 2006-02-08 KR KR1020077020676A patent/KR101351261B1/ko active IP Right Grant
- 2006-02-08 DK DK06701829.1T patent/DK1855529T3/en active
- 2006-02-08 CN CN200680012797.0A patent/CN101160049B/zh active Active
- 2006-02-08 CN CN2010102161743A patent/CN101885719A/zh active Pending
- 2006-02-08 CN CN201010216182A patent/CN101863873A/zh active Pending
- 2006-02-08 EA EA200701772A patent/EA012785B1/ru unknown
- 2006-02-08 WO PCT/EP2006/001089 patent/WO2006089633A2/de active Application Filing
- 2006-02-08 AP AP2007004096A patent/AP2007004096A0/xx unknown
- 2006-02-08 MX MX2007010111A patent/MX2007010111A/es active IP Right Grant
- 2006-02-08 US US11/884,887 patent/US7897543B2/en active Active
- 2006-02-08 EP EP06701829.1A patent/EP1855529B1/de active Active
- 2006-02-08 BR BRPI0607807A patent/BRPI0607807B1/pt active IP Right Grant
- 2006-02-08 UA UAA200710559A patent/UA88949C2/ru unknown
- 2006-02-08 AU AU2006218154A patent/AU2006218154B2/en active Active
- 2006-02-08 JP JP2007555500A patent/JP5095419B2/ja active Active
- 2006-02-08 CN CN201410015606.2A patent/CN103755716A/zh active Pending
- 2006-02-08 ES ES06701829.1T patent/ES2446240T3/es active Active
- 2006-02-08 CA CA2597777A patent/CA2597777C/en active Active
- 2006-02-21 TW TW095105685A patent/TWI389640B/zh active
- 2006-02-21 TW TW102105081A patent/TWI475955B/zh active
- 2006-02-22 AR ARP060100639A patent/AR053815A1/es active IP Right Grant
- 2006-02-22 MY MYPI20060757A patent/MY163974A/en unknown
-
2007
- 2007-08-14 IL IL185253A patent/IL185253A/en active IP Right Grant
- 2007-08-17 ZA ZA200706980A patent/ZA200706980B/xx unknown
- 2007-09-10 MA MA30206A patent/MA29324B1/fr unknown
-
2011
- 2011-02-25 US US13/035,293 patent/US20110190493A1/en not_active Abandoned
Non-Patent Citations (1)
| Title |
|---|
| None |
Cited By (417)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8247351B2 (en) | 2005-12-13 | 2012-08-21 | Bayer Cropscience Ag | Insecticidal compositions having improved effect |
| WO2007073856A2 (de) | 2005-12-15 | 2007-07-05 | Bayer Cropscience Ag | 3'-alkoxy-spirocyclopentyl substituierte tetram- und tetronsäuren |
| US8039014B2 (en) | 2005-12-15 | 2011-10-18 | Bayer Cropscience Ag | 3′-alkoxyspirocyclopentyl-substituted tetramic and tetronic acids |
| EP2184275A1 (de) | 2006-02-21 | 2010-05-12 | Bayer CropScience AG | Cycloalkyl-phenylsubstituierte cyclische Ketoenole |
| WO2007096058A1 (de) | 2006-02-21 | 2007-08-30 | Bayer Cropscience Ag | Cycloalkyl-phenylsubstituierte cyclische ketoenole |
| EP2186791A1 (de) | 2006-02-21 | 2010-05-19 | Bayer CropScience AG | Cycloalkyl-phenylsubstituierte cyclische Ketoenole |
| EP2186805A1 (de) | 2006-02-21 | 2010-05-19 | Bayer CropScience AG | Cycloalkyl-phenylsubstituierte cyclische Ketoenole |
| US8067458B2 (en) | 2006-04-22 | 2011-11-29 | Bayer Cropscience Ag | Alkoxyalkyl-substituted cyclic ketoenols |
| EP2289316A1 (de) | 2006-05-12 | 2011-03-02 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Zwergzikaden (Cicadellidae) |
| EP2277377A1 (de) | 2006-05-12 | 2011-01-26 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Thripidae |
| EP2289319A1 (de) | 2006-05-12 | 2011-03-02 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Blattwespen (Tenthredinidae) |
| EP2266399A1 (de) | 2006-05-12 | 2010-12-29 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Blattkäfer (Chrysomelidae) |
| EP2277380A1 (de) | 2006-05-12 | 2011-01-26 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Rüsselkäfer (Curculionidae) |
| EP2289317A1 (de) | 2006-05-12 | 2011-03-02 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Gallmücken (Cecidomyiidae) |
| EP2277378A1 (de) | 2006-05-12 | 2011-01-26 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Fliegen (Diptera) |
| EP2277379A1 (de) | 2006-05-12 | 2011-01-26 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Fruchtfliegen (Tephritidae) |
| EP2289318A1 (de) | 2006-05-12 | 2011-03-02 | Bayer CropScience AG | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Familie der Wickler (Tortricidae) |
| JP2009538844A (ja) * | 2006-06-02 | 2009-11-12 | バイエル・クロツプサイエンス・アクチエンゲゼルシヤフト | アルコキシアルキル置換環状ケトエノール |
| US8173697B2 (en) | 2006-06-02 | 2012-05-08 | Bayer Cropscience Ag | Alkoxyalkyl-substituted cyclic keto-enols |
| US8658688B2 (en) | 2006-06-16 | 2014-02-25 | Bayer Cropscience Ag | Active compound combinations having insecticidal and acaricidal properties |
| US8211931B2 (en) | 2006-06-16 | 2012-07-03 | Bayer Cropscience Ag | Active compound combinations having insecticidal and acaricidal properties |
| EP3001905A1 (de) | 2006-06-16 | 2016-04-06 | Bayer Intellectual Property GmbH | Wirkstoffkombinationen mit insektiziden und akariziden eigenschaften |
| US8816097B2 (en) | 2006-07-18 | 2014-08-26 | Bayer Cropscience Ag | Active ingredient combinations having insecticide and acaricide properties |
| US8507537B2 (en) | 2006-10-25 | 2013-08-13 | Bayer Cropscience Ag | Trifluromethoxyphenyl-substituted tetramic acid derivatives pesticides and/or herbicides |
| JP2008137915A (ja) * | 2006-11-30 | 2008-06-19 | Sankyo Agro Kk | 除草性組成物 |
| JP2010511643A (ja) * | 2006-12-04 | 2010-04-15 | バイエル・クロツプサイエンス・アクチエンゲゼルシヤフト | ビフェニル置換されたスピロ環式ケトエノール |
| WO2008067911A1 (de) * | 2006-12-04 | 2008-06-12 | Bayer Cropscience Ag | Biphenylsubstituierte spirocyclische ketoenole |
| CN102408326A (zh) * | 2006-12-04 | 2012-04-11 | 拜尔农作物科学股份公司 | 联苯基取代的螺环酮-烯醇 |
| US9000189B2 (en) | 2006-12-04 | 2015-04-07 | Bayer Cropscience Ag | Biphenyl-substituted spirocyclic ketoenols |
| US8993782B2 (en) | 2006-12-04 | 2015-03-31 | Bayer Cropscience Ag | Cis-alkoxyspirocyclic biphenyl-substituted tetramic acid derivatives |
| CN102408326B (zh) * | 2006-12-04 | 2014-04-16 | 拜尔农作物科学股份公司 | 联苯基取代的螺环酮-烯醇 |
| US8247579B2 (en) | 2007-01-12 | 2012-08-21 | Bayer Cropscience Ag | Spirocyclic tetronic acid derivatives |
| DE102007001866A1 (de) | 2007-01-12 | 2008-07-17 | Bayer Cropscience Ag | Spirocyclische Tetronsäure-Derivate |
| EP2014169A1 (de) | 2007-07-09 | 2009-01-14 | Bayer CropScience AG | Wasserlösliche Konzentrate von 3-(2-Alkoxy-4-chlor-6-alkyl-phenyl)-substituierten Tetramaten und ihren korrespondierenden Enolen |
| WO2009015801A1 (de) | 2007-08-02 | 2009-02-05 | Bayer Cropscience Ag | Oxaspirocyclische-spiro-substituierte tetram- und tetronsäure-derivate |
| JP2010535161A (ja) * | 2007-08-02 | 2010-11-18 | バイエル・クロツプサイエンス・アクチエンゲゼルシヤフト | オキサスピロ環式スピロ置換テトラミン酸及びテトロン酸誘導体 |
| CN101772503B (zh) * | 2007-08-02 | 2013-07-03 | 拜尔农作物科学股份公司 | 氧杂螺环型螺取代的特特拉姆酸及特窗酸衍生物 |
| US8859466B2 (en) | 2007-08-02 | 2014-10-14 | Bayer Cropscience Ag | Oxaspirocyclic spiro-substituted tetramic acid and tetronic acid derivatives |
| EP2020413A1 (de) * | 2007-08-02 | 2009-02-04 | Bayer CropScience AG | Oxaspirocyclische-spiro-substituierte Tetram- und Tetronsäure-Derivate |
| CN101801196A (zh) * | 2007-09-21 | 2010-08-11 | 拜尔农作物科学股份公司 | 具有杀昆虫和杀螨性质的活性化合物结合物 |
| WO2009039951A3 (de) * | 2007-09-21 | 2009-12-23 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden eigenschaften |
| WO2009039951A2 (de) * | 2007-09-21 | 2009-04-02 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden eigenschaften |
| EP2039248A1 (de) * | 2007-09-21 | 2009-03-25 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| WO2009039975A1 (de) | 2007-09-25 | 2009-04-02 | Bayer Cropscience Ag | Halogenalkoxyspirocyclische tetram- und tetronsäure-derivate |
| CN101235023B (zh) * | 2008-03-04 | 2010-06-02 | 浙江大学 | 一种螺螨酯的合成方法 |
| EP2103615A1 (de) | 2008-03-19 | 2009-09-23 | Bayer CropScience AG | 4'4'-Dioxaspiro-spirocyclisch substituierte Tetramate |
| WO2009115262A1 (de) | 2008-03-19 | 2009-09-24 | Bayer Cropscience Ag | 4'4'-dioxaspiro-spirocyclisch substituierte tetramate |
| CN101977916A (zh) * | 2008-03-19 | 2011-02-16 | 拜耳作物科学公司 | 4'4'-二氧杂螺-螺环取代的特特拉姆酸化物 |
| US20110039700A1 (en) * | 2008-04-28 | 2011-02-17 | Reiner Fischer | Method for Improved Utilization of the Production Potential of Transgenic Plants Introduction |
| EP2113172A1 (de) | 2008-04-28 | 2009-11-04 | Bayer CropScience AG | Verfahren zur verbesserten Nutzung des Produktionspotentials transgener Pflanzen |
| EP2127522A1 (de) | 2008-05-29 | 2009-12-02 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| US8420608B2 (en) | 2008-11-14 | 2013-04-16 | Bayer Cropscience Ag | Active substance combinations with insecticides and acaricide properties |
| US8389443B2 (en) | 2008-12-02 | 2013-03-05 | Bayer Cropscience Ag | Geminal alkoxy/alkylspirocyclic substituted tetramate derivatives |
| US8846946B2 (en) | 2008-12-02 | 2014-09-30 | Bayer Cropscience Ag | Germinal alkoxy/alkylspirocyclic substituted tetramate derivatives |
| WO2010086095A1 (en) | 2009-01-29 | 2010-08-05 | Bayer Cropscience Ag | Method for improved utilization of the production potential of transgenic plants introduction |
| WO2010094728A1 (en) | 2009-02-19 | 2010-08-26 | Bayer Cropscience Ag | Pesticide composition comprising a tetrazolyloxime derivative and a fungicide or an insecticide active substance |
| EP3153503A1 (de) | 2009-03-11 | 2017-04-12 | Bayer Intellectual Property GmbH | Zwischenprodukte für halogenalkylmethylenoxy-phenyl-substituierte ketoenole |
| WO2010102758A2 (de) | 2009-03-11 | 2010-09-16 | Bayer Cropscience Ag | Halogenalkylmethylenoxy-phenyl-substituierte ketoenole |
| WO2010145764A1 (de) | 2009-06-18 | 2010-12-23 | Bayer Cropscience Ag | Substituierte enaminocarbonylverbindungen |
| US8343893B2 (en) | 2009-06-18 | 2013-01-01 | Bayer Cropscience Ag | Substituted enaminocarbonyl compounds |
| EP2264008A1 (de) | 2009-06-18 | 2010-12-22 | Bayer CropScience AG | Substituierte Enaminocarbonylverbindungen |
| WO2010149274A3 (de) * | 2009-06-23 | 2011-07-21 | Bayer Cropscience Aktiengesellschaft | Verwendung von wirkstoffkombinationen mit insektiziden eigenschaften zur bekämpfung von tierischen schädlingen aus der familie der stinkwanzen |
| WO2010149370A1 (en) | 2009-06-24 | 2010-12-29 | Bayer Cropscience Ag | Combinations of biological control agents and insecticides |
| EP2269455A1 (de) | 2009-06-24 | 2011-01-05 | Bayer CropScience AG | Kombinationen von biologischen Schädlingsbekämpfungsmittel und Insektiziden |
| DE102009028001A1 (de) | 2009-07-24 | 2011-01-27 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| WO2011009540A2 (en) | 2009-07-24 | 2011-01-27 | Bayer Cropscience Ag | Pesticidal carboxamides |
| WO2011020567A1 (de) | 2009-08-20 | 2011-02-24 | Bayer Cropscience Ag | 3-triazolylphenyl-substituierte sulfid-derivate als akarizide und insektizide |
| WO2011029506A1 (de) | 2009-08-20 | 2011-03-17 | Bayer Cropscience Ag | 3-[1-(3-haloalkyl)-triazolyl]-phenyl-sulfid-derivate als akarizide und insektizide |
| WO2011045224A1 (de) | 2009-10-12 | 2011-04-21 | Bayer Cropscience Ag | 1- (pyrid-3-yl) -pyrazole und 1- (pyrimid-5-yl) -pyrazole als schädlingsbekämpfungsmittel |
| WO2011045240A1 (de) | 2009-10-12 | 2011-04-21 | Bayer Cropscience Ag | Amide und thioamide als schädlingsbekämpfungsmittel |
| WO2011051151A1 (de) | 2009-10-26 | 2011-05-05 | Bayer Cropscience Ag | Neue feste form von 4-[[(6-chlorpyridin-3-yl)methyl](2,2-difluorethyl)amino]furan-2(5h)-on |
| WO2011054436A2 (de) | 2009-10-27 | 2011-05-12 | Bayer Cropscience Ag | Halogenalkyl-substituierte amide als insektizide und akarizide |
| WO2011051455A1 (en) | 2009-10-30 | 2011-05-05 | Bayer Cropscience Ag | Pesticidal heterocyclic compounds |
| WO2011061156A1 (en) | 2009-11-17 | 2011-05-26 | Bayer Cropscience Ag | Active compound combinations |
| WO2011080044A2 (en) | 2009-12-16 | 2011-07-07 | Bayer Cropscience Ag | Active compound combinations |
| WO2011076724A2 (en) | 2009-12-23 | 2011-06-30 | Bayer Cropscience Ag | Pesticidal compound mixtures |
| WO2011076726A2 (en) | 2009-12-23 | 2011-06-30 | Bayer Cropscience Ag | Pesticidal compound mixtures |
| WO2011076727A2 (en) | 2009-12-23 | 2011-06-30 | Bayer Cropscience Ag | Pesticidal compound mixtures |
| WO2011080211A1 (en) | 2009-12-28 | 2011-07-07 | Bayer Cropscience Ag | Pesticidal arylpyrrolidines |
| WO2011092147A1 (en) | 2010-01-29 | 2011-08-04 | Bayer Cropscience Ag | Method to reduce the frequency and/or intensity of blossom-end rot disorder in horticultural crops |
| WO2011098440A2 (de) | 2010-02-10 | 2011-08-18 | Bayer Cropscience Ag | Biphenylsubstituierte cyclische ketoenole |
| WO2011098443A1 (de) | 2010-02-10 | 2011-08-18 | Bayer Cropscience Ag | Spiroheterocyclisch-substituierte tetramsäure-derivate |
| WO2011098433A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft | Zyklische ketoenole zur therapie |
| DE102010008643A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| DE102010008644A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| DE102010008642A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| WO2011131623A1 (de) | 2010-04-20 | 2011-10-27 | Bayer Cropscience Ag | Insektizide und/oder herbizide zusammensetzung mit verbesserter wirkung auf basis von spiroheterocyclisch-substituierten tetramsäure-derivaten |
| WO2011138285A1 (de) | 2010-05-05 | 2011-11-10 | Bayer Cropscience Ag | Thiazolderivate als schädlingsbekämpfungsmittel |
| WO2011157663A1 (de) | 2010-06-15 | 2011-12-22 | Bayer Cropscience Ag | Neue ortho-substituierte arylamid-derivate |
| US8658800B2 (en) | 2010-06-15 | 2014-02-25 | Bayer Cropscience Ag | Ortho-substituted arylamide derivatives |
| US9968086B2 (en) | 2010-06-18 | 2018-05-15 | Bayer Intellectual Property Gmbh | Active ingredient combinations having insecticidal and acaricidal properties |
| WO2011157778A1 (de) | 2010-06-18 | 2011-12-22 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden eigenschaften |
| US9198424B2 (en) | 2010-06-18 | 2015-12-01 | Bayer Intellectual Property Gmbh | Active ingredient combinations having insecticidal and acaricidal properties |
| WO2012000896A2 (de) | 2010-06-28 | 2012-01-05 | Bayer Cropscience Ag | Heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2012000902A1 (de) | 2010-06-29 | 2012-01-05 | Bayer Cropscience Ag | Verbesserte insektizide zusammensetzungen enthaltend cyclische carbonylamidine |
| WO2012001068A2 (de) | 2010-07-02 | 2012-01-05 | Bayer Cropscience Ag | Insektizide oder akarizide formulierungen mit verbesserter verfügbarkeit auf pflanzenoberflächen |
| WO2012004293A2 (de) | 2010-07-08 | 2012-01-12 | Bayer Cropscience Ag | Insektizide und fungizide wirkstoffkombinationen |
| WO2012004326A1 (en) | 2010-07-08 | 2012-01-12 | Bayer Cropscience Ag | Pesticidal pyrroline derivatives |
| US9790202B2 (en) | 2010-07-09 | 2017-10-17 | Bayer Intellectual Property Gmbh | Anthranilamide derivatives as pesticides |
| WO2012004208A1 (de) | 2010-07-09 | 2012-01-12 | Bayer Cropscience Ag | Anthranilsäurediamid-derivate als pestizide |
| WO2012007500A2 (de) | 2010-07-15 | 2012-01-19 | Bayer Cropscience Ag | Neue heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2012010509A2 (de) | 2010-07-20 | 2012-01-26 | Bayer Cropscience Ag | Gelköder zur bekämpfung von kriechenden schadinsekten |
| EP2422620A1 (de) | 2010-08-26 | 2012-02-29 | Bayer CropScience AG | Insektizide Wirkstoffkombinationen enthaltend Ethiprole und Pymetrozin |
| WO2012028583A1 (de) | 2010-09-03 | 2012-03-08 | Bayer Cropscience Ag | Deltamethrin enthaltende formulierungen |
| WO2012034957A1 (en) | 2010-09-15 | 2012-03-22 | Bayer Cropscience Ag | Pesticidal pyrroline n-oxide derivatives |
| US9375000B2 (en) | 2010-09-15 | 2016-06-28 | Bayer Intellectual Property Gmbh | Pesticidal arylpyrrolidines |
| WO2012035011A1 (en) | 2010-09-15 | 2012-03-22 | Bayer Cropscience Ag | Pesticidal arylpyrrolidines |
| WO2012038480A2 (en) | 2010-09-22 | 2012-03-29 | Bayer Cropscience Ag | Use of biological or chemical control agents for controlling insects and nematodes in resistant crops |
| WO2012045680A2 (de) | 2010-10-04 | 2012-04-12 | Bayer Cropscience Ag | Insektizide und fungizide wirkstoffkombinationen |
| WO2012045798A1 (en) | 2010-10-07 | 2012-04-12 | Bayer Cropscience Ag | Fungicide composition comprising a tetrazolyloxime derivative and a thiazolylpiperidine derivative |
| WO2012052490A1 (en) | 2010-10-21 | 2012-04-26 | Bayer Cropscience Ag | N-benzyl heterocyclic carboxamides |
| WO2012052412A1 (de) | 2010-10-22 | 2012-04-26 | Bayer Cropscience Ag | Neue heterocylische verbindungen als schädlingsbekämpfungsmittel |
| US9173396B2 (en) | 2010-10-22 | 2015-11-03 | Bayer Intellectual Property Gmbh | Heterocyclic compounds as pesticides |
| EP2446742A1 (de) | 2010-10-28 | 2012-05-02 | Bayer CropScience AG | Insektizide oder akarizide Zusammensetzungen enthaltend Mono- oder Disacchariden als Wirkungsverstärker |
| WO2012059497A1 (en) | 2010-11-02 | 2012-05-10 | Bayer Cropscience Ag | N-hetarylmethyl pyrazolylcarboxamides |
| WO2012065944A1 (en) | 2010-11-15 | 2012-05-24 | Bayer Cropscience Ag | N-aryl pyrazole(thio)carboxamides |
| US9206137B2 (en) | 2010-11-15 | 2015-12-08 | Bayer Intellectual Property Gmbh | N-Aryl pyrazole(thio)carboxamides |
| US9055743B2 (en) | 2010-11-29 | 2015-06-16 | Bayer Intellectual Property Gmbh | Alpha, beta-unsaturated imines |
| WO2012072489A1 (de) | 2010-11-29 | 2012-06-07 | Bayer Cropscience Ag | Alpha-beta-ungesättigte imine |
| WO2012072660A1 (en) | 2010-12-01 | 2012-06-07 | Bayer Cropscience Ag | Use of fluopyram for controlling nematodes in crops and for increasing yield |
| WO2012076471A1 (en) | 2010-12-09 | 2012-06-14 | Bayer Cropscience Ag | Insecticidal mixtures with improved properties |
| WO2012076470A1 (en) | 2010-12-09 | 2012-06-14 | Bayer Cropscience Ag | Pesticidal mixtures with improved properties |
| WO2012082548A2 (en) | 2010-12-15 | 2012-06-21 | Syngenta Participations Ag | Soybean event syht0h2 and compositions and methods for detection thereof |
| WO2012080188A1 (de) | 2010-12-17 | 2012-06-21 | Bayer Cropscience Ag | Insektizid-wachs-partikel enthaltende zusammensetzung |
| WO2012084852A2 (de) | 2010-12-21 | 2012-06-28 | Bayer Animal Health Gmbh | Ektoparasitizide wirkstoffkombinationen |
| DE102010063691A1 (de) | 2010-12-21 | 2012-06-21 | Bayer Animal Health Gmbh | Ektoparasitizide Wirkstoffkombinationen |
| WO2012101047A1 (de) | 2011-01-25 | 2012-08-02 | Bayer Cropscience Ag | Verfahren zur herstellung von 1-h-pyrrolidin-2,4-dion-derivaten |
| EP3372580A1 (de) | 2011-01-25 | 2018-09-12 | Bayer CropScience Aktiengesellschaft | Verfahren zur herstellung von 1-h-pyrrolidin-2,4-dion-derivaten |
| DE102011011040A1 (de) | 2011-02-08 | 2012-08-09 | Bayer Pharma Aktiengesellschaft | (5s,8s)-3-(4'-Chlor-3'-fluor-4-methylbiphenyl-3-yl)-4-hydroxy-8-methoxy-1-azaspiro[4.5]dec-3-en-2-on (Verbindung A) zur Therapie |
| US8946124B2 (en) | 2011-02-17 | 2015-02-03 | Bayer Intellectual Property Gmbh | Substituted 3-(biphenyl-3-yl)-8,8-difluoro-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-ones for therapy and halogen-substituted spirocyclic ketoenols |
| WO2012110518A1 (de) | 2011-02-17 | 2012-08-23 | Bayer Pharma Aktiengesellschaft | Substituierte 3-(biphenyl-3-yl)-8,8-difluor-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-one zur therapie |
| WO2012110519A1 (de) | 2011-02-17 | 2012-08-23 | Bayer Cropscience Ag | Substituierte 3-(biphenyl-3-yl)-8,8-difluor-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-one zur therapie und halogensubstituierte spirocyclische ketoenole |
| WO2012110464A1 (en) | 2011-02-17 | 2012-08-23 | Bayer Cropscience Ag | Use of sdhi fungicides on conventionally bred asr-tolerant, stem canker resistant and/or frog-eye leaf spot resistant soybean varieties |
| US9204640B2 (en) | 2011-03-01 | 2015-12-08 | Bayer Intellectual Property Gmbh | 2-acyloxy-pyrrolin-4-ones |
| WO2012116960A1 (de) | 2011-03-01 | 2012-09-07 | Bayer Cropscience Ag | 2-acyloxy-pyrrolin-4-one |
| WO2012119984A1 (de) | 2011-03-09 | 2012-09-13 | Bayer Cropscience Ag | Indol- und benzimidazolcarbonsäureamide als insektizide und akarizide |
| US9107411B2 (en) | 2011-03-09 | 2015-08-18 | Bayer Intellectual Property Gmbh | Indolecarboxamides and benzimidazolecarboxamides as insecticides and acaricides |
| WO2012120105A1 (en) | 2011-03-10 | 2012-09-13 | Bayer Cropscience Ag | Use of lipochito-oligosaccharide compounds for safeguarding seed safety of treated seeds |
| WO2012126766A1 (de) | 2011-03-18 | 2012-09-27 | Bayer Cropscience Ag | N- (3 -carbamoylphenyl) - 1h - pyrazol - 5 - carboxamid - derivate und ihre verwendung zur bekämpfung von tierischen schädlingen |
| WO2012136751A1 (en) | 2011-04-08 | 2012-10-11 | Basf Se | N-substituted hetero-bicyclic compounds and derivatives for combating animal pests |
| CN102241590A (zh) * | 2011-05-17 | 2011-11-16 | 永农生物科学有限公司 | 一种螺环季酮酸类化合物关键中间体的合成方法 |
| EP2535334A1 (de) | 2011-06-17 | 2012-12-19 | Bayer CropScience AG | Kristalline Modifikationen von Penflufen |
| WO2013010758A1 (en) | 2011-06-30 | 2013-01-24 | Bayer Intellectual Property Gmbh | Nematocide n- cyclopropyl - sulfonylamide derivatives |
| EP2540163A1 (de) | 2011-06-30 | 2013-01-02 | Bayer CropScience AG | Nematozide N-Cyclopropylsulfonylamidderivate |
| WO2013010947A2 (en) | 2011-07-15 | 2013-01-24 | Basf Se | Pesticidal methods using substituted 3-pyridyl thiazole compounds and derivatives for combating animal pests ii |
| WO2013010946A2 (en) | 2011-07-15 | 2013-01-24 | Basf Se | Pesticidal methods using substituted 3-pyridyl thiazole compounds and derivatives for combating animal pests i |
| US9826734B2 (en) | 2011-07-26 | 2017-11-28 | Clariant International Ltd. | Etherified lactate esters, method for the production thereof and use thereof for enhancing the effect of plant protecting agents |
| WO2013014126A1 (de) | 2011-07-26 | 2013-01-31 | Bayer Intellectual Property Gmbh | Veretherte laktatester, verfahren zu ihrer herstellung und ihre verwendung zur verbesserung der wirkung von pflanzenschutzmitteln |
| WO2013014227A1 (en) | 2011-07-27 | 2013-01-31 | Bayer Intellectual Property Gmbh | Seed dressing for controlling phytopathogenic fungi |
| DE102011080406A1 (de) | 2011-08-04 | 2013-02-07 | Bayer Pharma AG | Substituierte 3-(Biphenyl-3-yl)-4-hydroxy-8-methoxy-1-azaspiro8[4.5]dec-3-en-2-one |
| WO2013017600A1 (de) | 2011-08-04 | 2013-02-07 | Bayer Intellectual Property Gmbh | Substituierte 3-(biphenyl-3-yl)-4-hydroxy-8-methoxy-1-azaspiro[4.5]dec-3-en-2-one |
| DE102011080405A1 (de) | 2011-08-04 | 2013-02-07 | Bayer Pharma AG | Substituierte 3-(Biphenyl-3-yl)-8,8-difluor-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-one zur Therapie |
| WO2013024008A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | Aniline type compounds |
| WO2013050433A1 (en) | 2011-10-05 | 2013-04-11 | Bayer Intellectual Property Gmbh | Pesticide preparation and process for producing the same |
| WO2013079600A1 (en) | 2011-12-02 | 2013-06-06 | Basf Se | Method and system for monitoring crops during storage |
| WO2013079601A1 (en) | 2011-12-02 | 2013-06-06 | Basf Se | Method and system for monitoring crops and/or infestation of crops with harmful organismus during storage |
| WO2013087709A1 (en) | 2011-12-15 | 2013-06-20 | Bayer Intellectual Property Gmbh | Active ingredient combinations having insecticidal and acaricidal properties |
| EP2604118A1 (de) | 2011-12-15 | 2013-06-19 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| WO2013092519A1 (en) | 2011-12-19 | 2013-06-27 | Bayer Cropscience Ag | Use of anthranilic acid diamide derivatives for pest control in transgenic crops |
| WO2013092522A1 (de) | 2011-12-20 | 2013-06-27 | Bayer Intellectual Property Gmbh | Neue insektizide aromatische amide |
| WO2013092350A1 (de) | 2011-12-21 | 2013-06-27 | Bayer Cropscience Ag | N-arylamidine-substituierte trifluoroethylsulfid-derivate als akarizide und insektizide |
| EP3395171A1 (de) | 2011-12-21 | 2018-10-31 | Bayer CropScience Aktiengesellschaft | N-arylamidine-substituierte trifluoroethylsulfid-derivate als akarizide und insektizide |
| WO2013092868A1 (en) | 2011-12-21 | 2013-06-27 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| EP2606726A1 (de) | 2011-12-21 | 2013-06-26 | Bayer CropScience AG | N-Arylamidine-substituierte trifluoroethylsulfid-Derivate als Akarizide und Insektizide |
| WO2013092943A1 (en) | 2011-12-23 | 2013-06-27 | Basf Se | Isothiazoline compounds for combating invertebrate pests |
| WO2013107785A1 (en) | 2012-01-21 | 2013-07-25 | Bayer Intellectual Property Gmbh | Use of host defense inducers for controlling bacterial harmful organisms in useful plants |
| WO2013110612A1 (en) | 2012-01-26 | 2013-08-01 | Bayer Intellectual Property Gmbh | Phenyl-substituted ketoenols for controlling fish parasites |
| WO2013113789A1 (en) | 2012-02-02 | 2013-08-08 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013135724A1 (en) | 2012-03-14 | 2013-09-19 | Bayer Intellectual Property Gmbh | Pesticidal arylpyrrolidines |
| WO2013144228A1 (en) | 2012-03-29 | 2013-10-03 | Basf Se | Pesticidal methods using heterocyclic compounds and derivatives for combating animal pests |
| WO2013144213A1 (en) | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyridinylidene compounds and derivatives for combating animal pests |
| WO2013144223A1 (en) | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyrimidinylidene compounds and derivatives for combating animal pests |
| WO2013149940A1 (en) | 2012-04-02 | 2013-10-10 | Basf Se | Acrylamide compounds for combating invertebrate pests |
| WO2013149903A1 (en) | 2012-04-03 | 2013-10-10 | Basf Se | N- substituted hetero - bicyclic furanone derivatives for combating animal |
| WO2013150115A1 (en) | 2012-04-05 | 2013-10-10 | Basf Se | N- substituted hetero - bicyclic compounds and derivatives for combating animal pests |
| WO2013164295A1 (en) | 2012-05-04 | 2013-11-07 | Basf Se | Substituted pyrazole-containing compounds and their use as pesticides |
| WO2013167633A1 (en) | 2012-05-09 | 2013-11-14 | Basf Se | Acrylamide compounds for combating invertebrate pests |
| WO2013171201A1 (de) | 2012-05-16 | 2013-11-21 | Bayer Cropscience Ag | Insektizide öl-in-wasser (o/w) formulierung |
| WO2013171199A1 (de) | 2012-05-16 | 2013-11-21 | Bayer Cropscience Ag | Insektizide wasser-in-öl (w/o) formulierung |
| WO2013174836A1 (en) | 2012-05-22 | 2013-11-28 | Bayer Cropscience Ag | Active compounds combinations comprising a lipo-chitooligosaccharide derivative and a nematicide, insecticidal or fungicidal compound |
| WO2013174645A1 (en) | 2012-05-24 | 2013-11-28 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| EP3243387A2 (de) | 2012-05-30 | 2017-11-15 | Bayer CropScience Aktiengesellschaft | Zusammensetzungen mit einem biologischen schädlingsbekämpfungsmittel und einem insektizid |
| EP3363289A2 (de) | 2012-05-30 | 2018-08-22 | Bayer CropScience Aktiengesellschaft | Zusammensetzungen mit einem biologischen schädlingsbekämpfungsmittel und einem insektizid |
| US10813862B2 (en) | 2012-05-30 | 2020-10-27 | Clariant International Ltd. | Use of N-methyl-N-acylglucamines as solubilizers |
| US10864275B2 (en) | 2012-05-30 | 2020-12-15 | Clariant International Ltd. | N-methyl-N-acylglucamine-containing composition |
| WO2013186089A2 (en) | 2012-06-14 | 2013-12-19 | Basf Se | Pesticidal methods using substituted 3-pyridyl thiazole compounds and derivatives for combating animal pests |
| EP3424322A1 (de) | 2012-07-31 | 2019-01-09 | Bayer CropScience Aktiengesellschaft | Zusammensetzungen mit einer pestizid-terpen-mischung und einem insektizid |
| WO2014019983A1 (en) | 2012-07-31 | 2014-02-06 | Bayer Cropscience Ag | Compositions comprising a pesticidal terpene mixture and an insecticide |
| WO2014026984A1 (de) | 2012-08-17 | 2014-02-20 | Bayer Cropscience Ag | Azaindolcarbonsäure- und -thiocarbonsäureamide als insektizide und akarizide |
| WO2014053404A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053405A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Pesticidally active mixtures comprising anthranilamide compounds |
| WO2014053395A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Use of n-thio-anthranilamide compounds on cultivated plants |
| WO2014053401A2 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of improving plant health |
| WO2014053407A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2014053403A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling insecticide resistant insects |
| WO2014053406A1 (en) | 2012-10-01 | 2014-04-10 | Basf Se | Method of controlling ryanodine-modulator insecticide resistant insects |
| WO2014053450A1 (de) | 2012-10-02 | 2014-04-10 | Bayer Cropscience Ag | Heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2014060381A1 (de) | 2012-10-18 | 2014-04-24 | Bayer Cropscience Ag | Heterocyclische verbindungen als schädlingsbekämpfungsmittel |
| WO2014067962A1 (de) | 2012-10-31 | 2014-05-08 | Bayer Cropscience Ag | Neue heterocylische verbindungen als schädlingsbekämpfungsmittel |
| US10772324B2 (en) | 2012-11-03 | 2020-09-15 | Clariant International Ltd. | Aqueous adjuvant-compositions |
| EP3387906A1 (de) | 2012-11-06 | 2018-10-17 | Bayer CropScience Aktiengesellschaft | Herbizidkombinationen für tolerante sojabohnenkulturen |
| WO2014072250A1 (en) | 2012-11-06 | 2014-05-15 | Bayer Cropscience Ag | Herbicidal combinations for tolerant soybean cultures |
| EP3369318A1 (de) | 2012-11-06 | 2018-09-05 | Bayer CropScience Aktiengesellschaft | Herbizidkombinationen für tolerante sojabohnenkulturen |
| WO2014079770A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079766A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079814A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079804A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079764A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079820A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Use of anthranilamide compounds for reducing insect-vectored viral infections |
| WO2014079841A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079772A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079774A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079773A1 (en) | 2012-11-22 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079813A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014079752A1 (en) | 2012-11-23 | 2014-05-30 | Basf Se | Pesticidal mixtures |
| WO2014083088A2 (en) | 2012-11-30 | 2014-06-05 | Bayer Cropscience Ag | Binary fungicidal mixtures |
| WO2014083033A1 (en) | 2012-11-30 | 2014-06-05 | Bayer Cropsience Ag | Binary fungicidal or pesticidal mixture |
| WO2014086758A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising a biological control agent and an insecticide |
| WO2014086759A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising biological control agents |
| WO2014086753A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising biological control agents |
| WO2014086750A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising a biological control agent and an insecticide |
| WO2014086749A2 (en) | 2012-12-03 | 2014-06-12 | Bayer Cropscience Ag | Composition comprising a biological control agent and an insecticide |
| WO2014090765A1 (en) | 2012-12-12 | 2014-06-19 | Bayer Cropscience Ag | Use of 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfinyl)phenyl]-5-amino-3-trifluoromethyl)-1 h-1,2,4 tfia zole for controlling nematodes in nematode-resistant crops |
| US10117430B2 (en) | 2012-12-14 | 2018-11-06 | Basf Se | Malononitrile compounds for controlling animal pests |
| WO2014090700A1 (en) | 2012-12-14 | 2014-06-19 | Basf Se | Malononitrile compounds for controlling animal pests |
| WO2014095826A1 (en) | 2012-12-18 | 2014-06-26 | Bayer Cropscience Ag | Binary fungicidal and bactericidal combinations |
| WO2014096238A1 (en) | 2012-12-21 | 2014-06-26 | Basf Se | Cycloclavine and derivatives thereof for controlling invertebrate pests |
| WO2014102244A1 (en) | 2012-12-27 | 2014-07-03 | Basf Se | 2-(pyridin-3-yl)-5-hetaryl-thiazole compounds carrying an imine or imine-derived substituent for combating invertebrate pests |
| WO2014122083A1 (de) | 2013-02-06 | 2014-08-14 | Bayer Cropscience Ag | Halogensubstituierte pyrazolderivate als schädlingsbekämpfungsmittel |
| WO2014124375A1 (en) | 2013-02-11 | 2014-08-14 | Bayer Cropscience Lp | Compositions comprising gougerotin and a biological control agent |
| WO2014124373A1 (en) | 2013-02-11 | 2014-08-14 | Bayer Cropscience Lp | Compositions comprising gougerotin and an insecticide |
| WO2014124361A1 (en) | 2013-02-11 | 2014-08-14 | Bayer Cropscience Lp | Compositions comprising a streptomyces-based biological control agent and another biological control agent |
| WO2014124379A1 (en) | 2013-02-11 | 2014-08-14 | Bayer Cropscience Lp | Compositions comprising a streptomyces-based biological control agent and an insecticide |
| WO2014128136A1 (en) | 2013-02-20 | 2014-08-28 | Basf Se | Anthranilamide compounds and their use as pesticides |
| WO2014139897A1 (en) | 2013-03-12 | 2014-09-18 | Bayer Cropscience Ag | Use of dithiine-tetracarboximides for controlling bacterial harmful organisms in useful plants |
| WO2014140111A1 (en) | 2013-03-13 | 2014-09-18 | Bayer Cropscience Ag | Lawn growth-promoting agent and method of using same |
| WO2014170364A1 (en) | 2013-04-19 | 2014-10-23 | Bayer Cropscience Ag | Binary insecticidal or pesticidal mixture |
| WO2014170300A1 (en) | 2013-04-19 | 2014-10-23 | Basf Se | N-substituted acyl-imino-pyridine compounds and derivatives for combating animal pests |
| WO2014170313A1 (en) | 2013-04-19 | 2014-10-23 | Bayer Cropscience Ag | Active compound combinations having insecticidal properties |
| WO2014202505A1 (de) | 2013-06-20 | 2014-12-24 | Bayer Cropscience Ag | Arylsulfid- und arylsulfoxid-derivate als akarizide und insektizide |
| WO2014202510A1 (de) | 2013-06-20 | 2014-12-24 | Bayer Cropscience Ag | Arylsulfid- und arylsulfoxid-derivate als akarizide und insektizide |
| WO2014202751A1 (en) | 2013-06-21 | 2014-12-24 | Basf Se | Methods for controlling pests in soybean |
| WO2015004028A1 (de) | 2013-07-08 | 2015-01-15 | Bayer Cropscience Ag | Sechsgliedrige c-n-verknüpfte arylsulfid- und arylsulfoxid- derivate als schädlingsbekämpfungsmittel |
| WO2015007682A1 (en) | 2013-07-15 | 2015-01-22 | Basf Se | Pesticide compounds |
| WO2015040116A1 (en) | 2013-09-19 | 2015-03-26 | Basf Se | N-acylimino heterocyclic compounds |
| WO2015055497A1 (en) | 2013-10-16 | 2015-04-23 | Basf Se | Substituted pesticidal pyrazole compounds |
| EP3456201A1 (de) | 2013-10-18 | 2019-03-20 | BASF Agrochemical Products B.V. | Verwendung von pestizidwirksamen carboxamidderivaten in der boden- und samenanwendung und behandlungsverfahren |
| WO2015055757A1 (en) | 2013-10-18 | 2015-04-23 | Basf Se | Use of pesticidal active carboxamide derivative in soil and seed application and treatment methods |
| WO2015091649A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | N-substituted imino heterocyclic compounds |
| WO2015091645A1 (en) | 2013-12-18 | 2015-06-25 | Basf Se | Azole compounds carrying an imine-derived substituent |
| WO2015101622A1 (de) | 2014-01-03 | 2015-07-09 | Bayer Cropscience Ag | Neue pyrazolyl-heteroarylamide als schädlingsbekämpfungsmittel |
| WO2015104422A1 (en) | 2014-01-13 | 2015-07-16 | Basf Se | Dihydrothiophene compounds for controlling invertebrate pests |
| DE202014008415U1 (de) | 2014-02-19 | 2014-11-25 | Clariant International Ltd. | Wässrige Adjuvant-Zusammensetzung zur Wirkungssteigerung von Elektrolyt-Wirkstoffen |
| DE202014008418U1 (de) | 2014-02-19 | 2014-11-14 | Clariant International Ltd. | Schaumarme agrochemische Zusammensetzungen |
| WO2015160620A1 (en) | 2014-04-16 | 2015-10-22 | Bayer Cropscience Lp | Compositions comprising ningnanmycin and an insecticide |
| WO2015160618A1 (en) | 2014-04-16 | 2015-10-22 | Bayer Cropscience Lp | Compositions comprising ningnanmycin and a biological control agent |
| US11425904B2 (en) | 2014-04-23 | 2022-08-30 | Clariant International Ltd. | Use of aqueous drift-reducing compositions |
| WO2016001129A1 (de) | 2014-07-01 | 2016-01-07 | Bayer Cropscience Aktiengesellschaft | Verbesserte insektizide zusammensetzungen |
| WO2016008830A1 (de) | 2014-07-15 | 2016-01-21 | Bayer Cropscience Aktiengesellschaft | Aryl-triazolyl-pyridine als schädlingsbekämpfungsmittel |
| DE102014012022A1 (de) | 2014-08-13 | 2016-02-18 | Clariant International Ltd. | Organische Ammoniumsalze von anionischen Pestiziden |
| EP3556211A1 (de) | 2014-08-13 | 2019-10-23 | Clariant International Ltd | Organische ammoniumsalze von anionischen pestiziden |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| DE102014018274A1 (de) | 2014-12-12 | 2015-07-30 | Clariant International Ltd. | Zuckertenside und deren Verwendung in agrochemischen Zusammensetzungen |
| US11700852B2 (en) | 2014-12-19 | 2023-07-18 | Clariant International Ltd | Aqueous electrolyte-containing adjuvant compositions, active ingredient-containing compositions and the use thereof |
| WO2016106063A1 (en) | 2014-12-22 | 2016-06-30 | Bayer Corpscience Lp | Method for using a bacillus subtilis or bacillus pumilus strain to treat or prevent pineapple disease |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| US10701937B2 (en) | 2015-02-11 | 2020-07-07 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| US10961484B2 (en) | 2015-10-09 | 2021-03-30 | Clariant International Ltd. | Compositions comprising sugar amine and fatty acid |
| US10920080B2 (en) | 2015-10-09 | 2021-02-16 | Clariant International Ltd. | N-Alkyl glucamine-based universal pigment dispersions |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| WO2017097882A1 (de) | 2015-12-11 | 2017-06-15 | Bayer Cropscience Aktiengesellschaft | Flüssige prothioconazol-haltige formulierungen |
| EP3178320A1 (de) | 2015-12-11 | 2017-06-14 | Bayer CropScience AG | Flüssige fungizid-haltige formulierungen |
| WO2017121699A1 (de) | 2016-01-15 | 2017-07-20 | Bayer Cropscience Aktiengesellschaft | Verfahren zur herstellung von substituierten 2-aryl-ethanolen |
| US10519085B2 (en) | 2016-01-15 | 2019-12-31 | Bayer Cropscience Aktiengesellschaft | Process for preparing substituted 2-arylethanols |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017186543A2 (en) | 2016-04-24 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Use of fluopyram and/or bacillus subtilis for controlling fusarium wilt in plants of the musaceae family |
| US11220603B2 (en) | 2016-05-09 | 2022-01-11 | Clariant International Ltd. | Stabilizers for silicate paints |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| US12108757B2 (en) | 2016-05-25 | 2024-10-08 | Bayer Cropscience Aktiengesellschaft | Agrochemical formulation based on emulsion polymers |
| WO2017202684A1 (en) | 2016-05-25 | 2017-11-30 | Bayer Cropscience Aktiengesellschaft | Agrochemical formulation based on emulsion polymers |
| EP3248465A1 (de) | 2016-05-25 | 2017-11-29 | Bayer CropScience Aktiengesellschaft | Agrochemische zusammensetzung polymerisches emulsionspulver enthaltend. |
| WO2018019676A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Active compound combinations and methods to protect the propagation material of plants |
| WO2018024659A1 (de) | 2016-08-04 | 2018-02-08 | Bayer Cropscience Aktiengesellschaft | Verfahren zur herstellung von spiroketal-substituierten cyclischen ketoenolen |
| US11220511B2 (en) | 2016-08-04 | 2022-01-11 | Bayer Cropscience Aktiengesellschaft | Process for preparing spiroketal-substituted cyclic ketoenols |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| EP3978504A1 (de) | 2017-03-31 | 2022-04-06 | Basf Se | Chirale 2,3-dihydrothiazolo[3,2-a]pyrimidine derivate zur bekämpfung von tierischen schädlingen |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| WO2018210616A1 (de) | 2017-05-15 | 2018-11-22 | Bayer Cropscience Aktiengesellschaft | Verfahren zur herstellung von (4-halogen-2,6-dialkylphenyl)malononitrilen |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| WO2018228885A1 (de) | 2017-06-12 | 2018-12-20 | Bayer Aktiengesellschaft | Stabilisierte prothioconazol-haltige formulierungen mit niedrigem gehalt an 2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-(1h-1,2,4-triazol-1-yl)propan-2-ol |
| EP3415007A1 (de) | 2017-06-12 | 2018-12-19 | Bayer AG | Ptz formulierungen mit niedrigem gehalt an desthio |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | SUBSTITUTED PYRIMIDINIUM COMPOUNDS AND DERIVATIVES FOR CONTROLLING HARMFUL ANIMALS |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | SUBSTITUTED CYCLOPROPYL DERIVATIVES |
| WO2019042932A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING RICE PARASITES IN RICE |
| WO2019043183A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING PESTS OF RICE IN RICE |
| EP3453706A1 (de) | 2017-09-08 | 2019-03-13 | Basf Se | Pestizide imidazolverbindungen |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS AYNT INSECTICIDE / ACARICIDE PROPERTIES |
| US11647750B2 (en) | 2017-10-18 | 2023-05-16 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| EP3301092A2 (de) | 2018-01-26 | 2018-04-04 | Bayer CropScience Aktiengesellschaft | Verfahren zur herstellung von spiroketal-substituierten phenylacetylaminosäureestern und spiroketal-substituierten cyclischen ketoenolen |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| US12006327B2 (en) | 2018-04-10 | 2024-06-11 | Bayer Aktiengesellschaft | Process for preparing substituted cyclohexane amino acid esters and spiroketal-substituted cyclic keto-enols |
| US11691938B2 (en) | 2018-04-10 | 2023-07-04 | Bayer Aktiengesellschaft | Process for preparing 2,6-dialkylphenylacetic acids |
| CN111954667A (zh) * | 2018-04-10 | 2020-11-17 | 拜耳公司 | 制备螺缩酮取代的环状酮烯醇的方法 |
| WO2019197232A1 (de) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Verfahren zur herstellung von 2,6-dialkylphenyl-essigsäuren |
| WO2019197231A1 (de) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Verfahren zur herstellung von substituierten cyclohexanaminosäureestern und spiroketalsubstituierten cyclischen ketoenolen |
| WO2019197618A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von speziellen insekten |
| WO2019197617A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von tierischen schädlingen durch angiessen, tröpfchenapplikation. pflanzlochbehandlung oder furchenapplikation |
| WO2019197620A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von speziellen insekten |
| CN111970928A (zh) * | 2018-04-13 | 2020-11-20 | 拜耳作物科学股份公司 | 油基悬浮浓缩剂 |
| US12127558B2 (en) | 2018-04-13 | 2024-10-29 | Bayer Aktiengesellschaft | Solid formulation of insecticidal mixtures |
| WO2019197652A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Feststoff-formulierung insektizider mischungen |
| US12089597B2 (en) | 2018-04-13 | 2024-09-17 | Bayer Aktiengesellschaft | Active ingredient combinations with insecticidal, nematicidal and acaricidal properties |
| WO2019197616A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Verwendung von tetramsäurederivaten zur reduktion von nematodenpopulationen |
| WO2019197612A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von tierischen schädlingen durch angiessen oder tröpfchenapplikation |
| IL277782B1 (en) * | 2018-04-13 | 2023-07-01 | Bayer Cropscience Ag | Use of tetramic acid derivatives for pest control through irrigation or drip |
| IL277782B2 (en) * | 2018-04-13 | 2023-11-01 | Bayer Cropscience Ag | Use of tetramic acid derivatives for pest control through irrigation or drip |
| WO2019197623A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| EP3772955A1 (de) * | 2018-04-13 | 2021-02-17 | Bayer Aktiengesellschaft | Feststoff-formulierung insektizider mischungen |
| WO2019197615A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit fungiziden, insektiziden und akariziden eigenschaften |
| WO2019197637A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Hochbeladene formulierungen mit insektiziden aus der ketoenol-klasse zur verwendung bei drip- und drench- applikationen |
| US11384061B2 (en) | 2018-04-17 | 2022-07-12 | Bayer Aktiengesellschaft | Process for preparing esters of N-acylated amino acids with acid-labile keto protective group functions |
| WO2019201842A1 (de) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Verfahren zur herstellung von estern n-acylierter aminosäuren mit säurelabilen keto-schutzgruppenfunktionen |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2019224143A1 (de) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| EP3613736A1 (de) | 2018-08-22 | 2020-02-26 | Basf Se | Substituierte glutarimidderivate |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020064408A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| EP3628157A1 (de) | 2018-09-28 | 2020-04-01 | Basf Se | Verfahren zur kontrolle von insektizid-resistenten insekten und virusübertragung auf pflanzen |
| EP3628158A1 (de) | 2018-09-28 | 2020-04-01 | Basf Se | Pestizide zusammensetzung die eine mesoionische verbindung und ein biopestizid enthält |
| EP3628156A1 (de) | 2018-09-28 | 2020-04-01 | Basf Se | Verfahren zur bekämpfung von schädlingen von zuckerrohr-, zitrus-, raps- und kartoffelpflanzen |
| WO2020064480A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| EP3643705A1 (de) | 2018-10-24 | 2020-04-29 | Basf Se | Pestizidverbindungen |
| WO2020083733A1 (en) | 2018-10-24 | 2020-04-30 | Basf Se | Pesticidal compounds |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3696177A1 (de) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| US20220132851A1 (en) * | 2019-03-01 | 2022-05-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020187871A1 (de) | 2019-03-19 | 2020-09-24 | Bayer Aktiengesellschaft | Stabilisierte formulierungen von thioketonen |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2020254230A1 (de) | 2019-06-15 | 2020-12-24 | Bayer Aktiengesellschaft | Stabilisierte formulierungen von dithiocarbamaten |
| EP3766879A1 (de) | 2019-07-19 | 2021-01-20 | Basf Se | Pestizide pyrazolverbindungen |
| WO2021013561A1 (en) | 2019-07-19 | 2021-01-28 | Basf Se | Pesticidal pyrazole and triazole derivatives |
| EP3769623A1 (de) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionische imidazolverbindungen und derivate zur bekämpfung von tierischen schädlingen |
| EP3701796A1 (de) | 2019-08-08 | 2020-09-02 | Bayer AG | Wirkstoffkombinationen |
| WO2021130143A1 (en) | 2019-12-23 | 2021-07-01 | Basf Se | Enzyme enhanced root uptake of agrochemical active compound |
| WO2021170463A1 (en) | 2020-02-28 | 2021-09-02 | BASF Agro B.V. | Methods and uses of a mixture comprising alpha-cypermethrin and dinotefuran for controlling invertebrate pests in turf |
| WO2021219513A1 (en) | 2020-04-28 | 2021-11-04 | Basf Se | Pesticidal compounds |
| EP3909950A1 (de) | 2020-05-13 | 2021-11-17 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2022058522A1 (de) | 2020-09-20 | 2022-03-24 | Bayer Aktiengesellschaft | Stabilisierung von thioketonen auf oberflächen |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| EP4043444A1 (de) | 2021-02-11 | 2022-08-17 | Basf Se | Substituierte isoxazolinderivate |
| WO2022175420A1 (en) | 2021-02-19 | 2022-08-25 | Syngenta Crop Protection Ag | Insect and acarina pest control |
| WO2022175318A1 (en) | 2021-02-19 | 2022-08-25 | Syngenta Crop Protection Ag | Insect and acarina pest control |
| WO2022200364A1 (en) | 2021-03-25 | 2022-09-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2022238576A1 (en) | 2021-05-14 | 2022-11-17 | Syngenta Crop Protection Ag | Seed treatment compositions |
| WO2022238575A1 (en) | 2021-05-14 | 2022-11-17 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| WO2022268815A1 (en) | 2021-06-24 | 2022-12-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2022268813A1 (en) | 2021-06-24 | 2022-12-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023280999A1 (en) | 2021-07-07 | 2023-01-12 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| EP4119547A1 (de) | 2021-07-12 | 2023-01-18 | Basf Se | Triazolverbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2023021136A1 (en) | 2021-08-20 | 2023-02-23 | Syngenta Crop Protection Ag | Method of controlling pests on tea plants |
| EP4140986A1 (de) | 2021-08-23 | 2023-03-01 | Basf Se | Pyrazolverbindungen zur bekämpfung von wirbellosen schädlingen |
| EP4140995A1 (de) | 2021-08-27 | 2023-03-01 | Basf Se | Pyrazin verbindungen zur kontrolle von wirbellosen schädlingen |
| EP4151631A1 (de) | 2021-09-20 | 2023-03-22 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2023046853A1 (en) | 2021-09-23 | 2023-03-30 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| EP4194453A1 (de) | 2021-12-08 | 2023-06-14 | Basf Se | Pyrazinverbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2023105065A1 (en) | 2021-12-10 | 2023-06-15 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023105064A1 (en) | 2021-12-10 | 2023-06-15 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| EP4198033A1 (de) | 2021-12-14 | 2023-06-21 | Basf Se | Heterocyclische verbindungen zur bekämpfung von wirbellosen schädlingen |
| EP4198023A1 (de) | 2021-12-16 | 2023-06-21 | Basf Se | Pestizidaktive thiosemicarbazonverbindungen |
| EP4238971A1 (de) | 2022-03-02 | 2023-09-06 | Basf Se | Substituierte isoxazolinderivate |
| WO2023180162A1 (de) | 2022-03-21 | 2023-09-28 | Bayer Aktiengesellschaft | Verfahren zur herstellung von ketonen |
| WO2023203038A1 (en) | 2022-04-19 | 2023-10-26 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2023208447A1 (en) | 2022-04-25 | 2023-11-02 | Basf Se | An emulsifiable concentrate having a (substituted) benzaldehyde-based solvent system |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
| EP4342885A1 (de) | 2022-09-20 | 2024-03-27 | Basf Se | N-(3-(aminomethyl)-phenyl)-5-(4-phenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-amin-derivate und ähnliche verbindungen als pestizide |
| EP4389210A1 (de) | 2022-12-21 | 2024-06-26 | Basf Se | Heteroarylverbindungen zur bekämpfung von wirbellosen schädlingen |
| WO2024218261A1 (en) | 2023-04-18 | 2024-10-24 | Syngenta Crop Protection Ag | Method and composition for treating plant propagation materials |
| EP4455137A1 (de) | 2023-04-24 | 2024-10-30 | Basf Se | Pyrimidinverbindungen zur bekämpfung von wirbellosen schädlingen |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DK1855529T3 (en) | SPIROKETAL-SUBSTITUTED CYCLIC KETOENOLS | |
| EP1791816B1 (de) | Jod-phenylsubstituierte cyclische ketoenole | |
| US8173697B2 (en) | Alkoxyalkyl-substituted cyclic keto-enols | |
| EP2013168A1 (de) | Alkoxyalkyl-substituierte cyclische ketoenole | |
| DE102005059891A1 (de) | 3'-Alkoxy-spirocyclopentyl substituierte Tetram- und Tetronsäuren | |
| DE102006007882A1 (de) | Cycloalkyl-phenylsubstituierte cyclische Ketoenole | |
| WO2006000355A1 (de) | 3'-alkoxy spirocyclische tetram- und tetronsäuren | |
| EP1638957A1 (de) | N-heterocyclyl-phenylsubstituierte cyclische ketoenole | |
| DE102005059892A1 (de) | Alkylthio-spirocyclische Tetramsäuren | |
| WO2007048545A2 (de) | Alkoxyalkyl spirocyclische tetram- und tetronsäuren | |
| MXPA06015186A (en) | 3'-alkoxy spirocyclic tetramic and tetronic acids | |
| BRPI0712498B1 (pt) | Alkoxyalkyl substituted cyclic ketoenols, their application, pesticides and / or herbicides and / or fungicides, their production process, agent, composition and methods of controlling animal pests and / or undesired vegetation and / or fungus, the growth of unwanted plants and of increasing the effectiveness of pesticides and / or herbicides |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2006701829 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 6147/DELNP/2007 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 185253 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2597777 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/a/2007/010111 Country of ref document: MX Ref document number: 2006218154 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12007501787 Country of ref document: PH Ref document number: 2007555500 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 07085915 Country of ref document: CO |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020077020676 Country of ref document: KR |
|
| ENP | Entry into the national phase |
Ref document number: 2006218154 Country of ref document: AU Date of ref document: 20060208 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2006218154 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1200701922 Country of ref document: VN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200701772 Country of ref document: EA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200680012797.0 Country of ref document: CN |
|
| WWP | Wipo information: published in national office |
Ref document number: 2006701829 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11884887 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: PI0607807 Country of ref document: BR Kind code of ref document: A2 |