WO2006066769A2 - Papiere mit hohem füllstoffgehalt und hoher trockenfestigkeit - Google Patents
Papiere mit hohem füllstoffgehalt und hoher trockenfestigkeit Download PDFInfo
- Publication number
- WO2006066769A2 WO2006066769A2 PCT/EP2005/013430 EP2005013430W WO2006066769A2 WO 2006066769 A2 WO2006066769 A2 WO 2006066769A2 EP 2005013430 W EP2005013430 W EP 2005013430W WO 2006066769 A2 WO2006066769 A2 WO 2006066769A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- filler
- paper
- copolymers
- cationic
- mol
- Prior art date
Links
Classifications
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/33—Synthetic macromolecular compounds
- D21H17/34—Synthetic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D21H17/41—Synthetic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing ionic groups
- D21H17/44—Synthetic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing ionic groups cationic
- D21H17/45—Nitrogen-containing groups
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/21—Macromolecular organic compounds of natural origin; Derivatives thereof
- D21H17/24—Polysaccharides
- D21H17/28—Starch
- D21H17/29—Starch cationic
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/33—Synthetic macromolecular compounds
- D21H17/46—Synthetic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- D21H17/54—Synthetic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds obtained by reactions forming in the main chain of the macromolecule a linkage containing nitrogen
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/63—Inorganic compounds
- D21H17/67—Water-insoluble compounds, e.g. fillers, pigments
- D21H17/69—Water-insoluble compounds, e.g. fillers, pigments modified, e.g. by association with other compositions prior to incorporation in the pulp or paper
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/33—Synthetic macromolecular compounds
- D21H17/34—Synthetic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D21H17/41—Synthetic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing ionic groups
- D21H17/44—Synthetic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing ionic groups cationic
- D21H17/45—Nitrogen-containing groups
- D21H17/455—Nitrogen-containing groups comprising tertiary amine or being at least partially quaternised
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/33—Synthetic macromolecular compounds
- D21H17/46—Synthetic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- D21H17/54—Synthetic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds obtained by reactions forming in the main chain of the macromolecule a linkage containing nitrogen
- D21H17/55—Polyamides; Polyaminoamides; Polyester-amides
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/33—Synthetic macromolecular compounds
- D21H17/46—Synthetic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- D21H17/54—Synthetic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds obtained by reactions forming in the main chain of the macromolecule a linkage containing nitrogen
- D21H17/56—Polyamines; Polyimines; Polyester-imides
Definitions
- the present invention relates to a process for the production of papers with high filler content and high dry strength and the papers produced by this method.
- fillers are added to the fiber suspension, which is particularly advantageous when the filler is cheaper than the pulp.
- the addition or increased addition of filler can lead to a reduction of the fiber content and thus to a reduction in the production costs of the paper.
- Filler-containing papers or papers with a particularly high filler content are easier to dry than non-filler papers or papers with a lower filler content.
- the paper machine can be operated faster and with lower steam consumption, which both increases productivity and lowers costs.
- the filler slurry is added to the fiber suspension prior to passing it to the former of the paper machine.
- a retention aid or retention aid system is typically added to the filler / pulp suspension to retain as much filler as possible in the paper sheet.
- the addition of the filler to the paper gives the papermaker the opportunity to achieve numerous improvements in sheet properties. These include properties such as opacity, whiteness, feel and printability.
- the filler addition to the fiber suspension also entails disadvantages which can only be partially compensated by the addition of further paper auxiliaries.
- the amount of filler that can be used there are limits to the amount of filler that can be used.
- the strength properties of the paper are usually the most important parameters that limit the amount of filler in the paper.
- Other factors, such as filler retention, dewatering of the pulp suspension, and possibly increased need for chemicals in retention and sizing may also play a role here.
- the loss of strength properties of papers can in some cases be compensated in whole or in part by the use of dry and wet strength agents.
- a common procedure is the addition of cationic starch as dry strength in the pulp.
- synthetic dry and wet strength agents are used, for example, based on cationic or anionic polyacrylamides.
- the amount of addition and the strengthening effect are limited in most cases.
- the compensating effect in relation to the loss of strength due to an increase in the filler and thus also the realizable limited filler increase.
- not all strength properties are increased to the same extent and in some cases only insufficiently by the use of dry strength.
- An important example of this is the on-going work, which is only slightly influenced by the use of starch or synthetic dry strength compared to other strength parameters.
- the increase in the filler content in paper usually has a very strong negative impact on continuing work.
- the increase in the filler content leads to a decrease in the paper density and the thickness of the paper sheet at the same basis weight.
- the latter leads to a significant decrease in paper stiffness. In many cases, this decrease in paper stiffness can not be compensated for solely by the use of dry strength.
- additional measures such as the reduction of mechanical pressure in the press section in the calenders, in calenders or in the dryer section of the paper machine are necessary. The latter compensates the thickness loss by increasing the filler in whole or in part.
- amphoteric water-soluble polymers are added to aqueous suspensions of inorganic particles, wherein at least some of the polymers are adsorbed on the filler surface.
- the amphoteric polymers are preferably prepared by hydrolyzing copolymers of N-vinylformamide, acrylonitrile and acrylic acid in the presence of acids. They contain 20 to 90 mol% amidine units of the structure
- US-A 2002/0088579 describes the pretreatment of inorganic fillers with cationic, anionic and amphoteric (zwitterionic) polymers.
- the treatment consists in each case of at least two stages. It is recommended to first treat with a cationic polymer followed by treatment with an anionic polymer. In further steps, other cationic and anionic polymers can be adsorbed alternately again.
- the aqueous suspensions containing the pretreated filler particles are added to the stock in the production of filler-containing paper.
- the filler treatment leads to an improvement in various strength properties of the dried paper.
- WO 04/087818 describes aqueous slurries of finely divided fillers which are at least partially coated with polymers and which are obtainable by treating aqueous slurries of finely divided fillers with at least one water-soluble amphoteric copolymer which is obtainable by copolymerizing
- R 1 , R 2 H or C 1 - to C 6 -alkyl
- DE 103 34 133 A1 discloses aqueous compositions comprising at least one finely divided filler and at least one water-soluble amphoteric copolymer.
- polymerizate obtainable by copolymerizing a monomer mixture containing
- R 1 and R 2 independently of one another are H or C 1 - to C 6 -alkyl
- the monomer mixture contains at least one monomer b) or c) with at least one free acid group and / or one acid group in salt form
- the resulting papers should be distinguished by improved performance properties, especially good strength properties of the dried paper. These include, in particular, good dry tear lengths, tear propagation, flexural rigidity and internal strength. Furthermore, the papers produced should have a higher filler content than known from the prior art.
- the object was achieved by a process for the production of paper, paperboard and cardboard in the presence of an aqueous slurry of components containing finely divided fillers, wherein the finely divided fillers are at least partially coated with water-soluble amphoteric copolymers, wherein in addition to the aqueous slurry of finely divided fillers Components at least one cationic and / or amphoteric polymer containing as structural element no esters of unsaturated carboxylic acids with quaternized amino alcohols, the fiber suspension added before sheet formation.
- components comprising finely divided fillers are both finely divided fillers alone, i. in pure form or as a so-called fresh filler, as well as finely divided fillers containing raw materials such as the so-called Committee of coated paper, as well as mixtures in any composition thereof understood.
- the dosage of the cationic and / or amphoteric polymers may be at various points in the papermaking process. Conceivable is a dosage in the thick matter range, but also a dosage in the thin material of the fiber suspension. A split addition at different points in the manufacturing process is also possible.
- the at least one cationic and / or amphoteric polymer is added to the fiber suspension immediately after the addition of the aqueous slurry of components containing finely divided fillers. Immediately means that between the dosages of the components no further process step, i. no dosage of other paper auxiliaries or, for example, the action of shear forces on the suspension is.
- the cationic and / or amphoteric polymer contains no structural elements of esters of unsaturated carboxylic acids, for example C 3 -C 8 -carboxylic acids, with quaternized amino alcohols, for example N, N, N-trimethyl-ammonium-methanol.
- the cationic and / or amphoteric polymer is selected from
- Homopolymers and copolymers of vinylimidazoles, diallylalkylamines and allyldialkyamines these monomers being used in neutral form, as salts of acids or in quaternized form
- Homo- and copolymers of esters of unsaturated carboxylic acids with N 1 N- dialkylamino or N-Alkylaminalkoholen these monomers are used in neutral form or as salts of acids
- homo- and copolymers of amides of unsaturated carboxylic acids with N 1 N- Dialkyldiaminen or N- Alkyldiaminen these monomers are used in neutral form, as salts of acids or in quaternized form, condensation products of epichlorohydrin or bisepoxides with dialkylamines or polyamidoamines, polyethyleneimines, - graft products of ethyleneimines on amidoamines or polyamines, cationic starches and / or vinylamine units containing polymers.
- Typical representatives are, for example Catiofast® ® PR 8153 and PR 8154 ® Catiofast® the BASF Aktiengesellschaft, which are commonly used as fixatives in the paper industry.
- Polyethyleneimines are disclosed, for example, in WO 97/25367 and the literature cited therein.
- Graft products of ethyleneimines on amidoamines or polyamines are, for example, the nitrogen-containing condensation products described in German Offenlegungsschrift DE 24 34 816.
- Cationic starches are disclosed, for example, in Günther Tegge, Medicare und Medicarederivate, Behr's Verlag, Hamburg, 1984. These are, for example, potato starch, corn starch, wheat starch, rice starch, tapioca starch, sago starch, manioc starch and rye starch. These starches are e.g. reacted with 2,3- (epoxy) -propyltrimethyl ammonium chloride.
- Polymers containing vinylamine units are known, cf. US 4,421,602, US 5,334,287, EP-A 216,387, US 5,981,689, WO 00/63295, US 6,121,409 and US 6,132,558. They are prepared by hydrolysis of open-chain N-vinylcarboxylic acid amide units containing polymers. These polymers are e.g. obtainable by polymerizing N-vinylformamide, N-vinyl-N-methylformamide, N-vinylacetamide, N-vinyl-N-methylacetamide, N-vinyl-N-ethylacetamide and N-vinylpropionamide. The monomers mentioned can be polymerized either alone or together with other monomers. Preference is given to N-vinylformamide.
- Suitable monoethylenically unsaturated monomers which are copolymerized with the N-vinylcarboxamides are all compounds which can be copolymerized therewith.
- vinyl esters of saturated carboxylic acids of 1 to 6 carbon atoms such as vinyl formate, vinyl acetate, N-vinylpyrrolidone, vinyl propionate and vinyl butyrate and vinyl ethers such as C 1 to C 6 alkyl vinyl ethers, for example methyl or ethyl vinyl ether.
- Suitable comonomers are esters of alcohols having, for example, 1 to 6 carbon atoms, amides and nitriles of ethylenically unsaturated C 3 - to C 6 -carboxylic acids, for example methyl acrylate, methyl methacrylate, ethyl acrylate, ethyl methacrylate and dimethyl maleate, acrylamide and methacrylamide and acrylonitrile and methacrylonitrile.
- carboxylic acid esters are derived from glycols or polyalkylene glycols, in each case only one OH group esterified, for example hydroxyethyl acrylate, hydroxyethyl methacrylate, hydroxypropyl acrylate, hydroxybutyl acrylate, hydroxypropyl methacrylate, hydroxybutyl methacrylate and acrylic acid monoesters of polyalkylene glycols having a molecular weight of 500 to 10,000.
- esters of ethylenically unsaturated carboxylic acids with amino alcohols such as dimethylaminoethyl acrylate, dimethylaminoethyl methacrylate, diethylaminoethyl acrylate, diethylaminoethyl methacrylate, dimethylaminopropyl acrylate, dimethylaminopropyl methacrylate, diethylaminopropyl acrylate, dimethylaminobutyl acrylate and diethylaminobutyl acrylate.
- amino alcohols such as dimethylaminoethyl acrylate, dimethylaminoethyl methacrylate, diethylaminoethyl acrylate, diethylaminoethyl methacrylate, dimethylaminopropyl acrylate, dimethylaminobutyl acrylate and diethylaminobutyl acrylate.
- the basic acrylates can be used in the form of the free bases, the salts with mineral acids such as hydrochloric acid, sulfuric acid or nitric acid, the salts with organic acids such as formic acid, acetic acid, propionic acid or the sulfonic acids or in quaternized form.
- Suitable quaternizing agents are, for example, dimethyl sulfate, diethyl sulfate, methyl chloride, ethyl chloride or benzyl chloride.
- Suitable comonomers are amides of ethylenically unsaturated carboxylic acids such as acrylamide, methacrylamide and N-alkyl mono- and diamides of monoethylenically unsaturated carboxylic acids having alkyl radicals of 1 to 6 carbon atoms, e.g. N-methylacrylamide, N, N-dimethylacrylamide, N-methylmethacrylamide, N-ethylacrylamide, N-propylacrylamide and tert-butylacrylamide and basic (meth) acrylamides, such as e.g.
- N-vinylpyrrolidone N-vinylcaprolactam
- acrylonitrile methacrylonitrile
- N-vinylimidazole substituted N-vinylimidazoles, such as e.g. N-vinyl-2-methylimidazole, N-vinyl-4-methylimidazole, N-vinyl-5-methylimidazole, N-vinyl-2-ethylimidazole and N-vinylimidazolines
- N-vinylimidazoline N-vinyl-2-methylimidazo-Hn and N-vinyl-2-ethylimidazoline.
- N-vinylimidazoles and N-vinylimidazolines are used except in the form of the free bases also in mineral acids or organic acids neutralized or in quaternized form, the quaternization is preferably carried out with dimethyl sulfate, diethyl sulfate, methyl chloride or benzyl chloride. Also suitable are diallyldialkylammonium halides, e.g. Diallyldimethylammonium chloride.
- copolymers contain, for example
- the comonomers are preferably free of acid groups.
- the polymerization of the monomers is usually carried out in the presence of radical-forming polymerization initiators.
- the homopolymers and copolymers can be obtained by all known processes, for example by solution polymerization in water, alcohols, ethers or dimethylformamide or in mixtures of various solvents, by precipitation polymerization, reverse suspension polymerization (polymerizing an emulsion of a monomeric aqueous solution) Phase in an oil phase) and polymerizing a water-in-water emulsion, for example, in which one dissolves or emulsifies an aqueous monomer solution in an aqueous phase and polymerizes to form an aqueous dispersion of a water-soluble polymer, as described, for example, in WO 00/27893. Following the polymerization, the homopolymers and copolymers which contain copolymerized N-vinylcarboxamide units are partially or completely hydro
- the degree of hydrolysis being e.g. 1 to 100 mol%, preferably 25 to 100 mol%, particularly preferably 50 to 100 mol% and particularly preferably 70 to 100 mol%.
- the degree of hydrolysis corresponds to the content of the polymers of vinylamine groups in mol%.
- the hydrolysis of the above-described polymers is carried out by known methods by the action of acids (eg mineral acids such as sulfuric acid, hydrochloric acid or phosphoric acid, carboxylic acids such as formic acid or acetic acid, or sulfonic acids or Phsophonkla), bases or enzymes, such as in DE-A 31 28 478 and US 6,132,558.
- acids eg mineral acids such as sulfuric acid, hydrochloric acid or phosphoric acid, carboxylic acids such as formic acid or acetic acid, or sulfonic acids or Phsophonklaren
- bases or enzymes such as in DE-A 31 28 478 and US 6,132,558.
- the degree of hydrolysis of the homopolymers and copolymers used is 85 to 95 mol%.
- the degree of hydrolysis of the homopolymers is synonymous with the content of the polymers of vinylamine units.
- hydrolysis of the ester groups to form vinyl alcohol units may occur. This is especially the case when the hydrolysis of the copolymers risate in the presence of sodium hydroxide solution.
- Polymerized acrylonitrile is also chemically altered upon hydrolysis. In this case, for example, arise a-mid groups or carboxyl groups.
- the vinylamine units containing homo- and copolymers may optionally contain up to 20 mol% of amidine units, for example by reaction of formic acid with two adjacent amino groups or by intramolecular reaction of an amino group with an adjacent Amidgrup- example of copolymerized N-vinylformamide.
- the average molecular weights M w of the polymers containing vinylamine units are, for example, 500 to 10 million, preferably 750 to 5 million and particularly preferably 1 000 to 2 million g / mol (determined by light scattering).
- This molar mass range corresponds, for example, to K values of 30 to 150, preferably 60 to 100 (determined according to H. Fikentscher in 5% strength aqueous sodium chloride solution at 25 ° C., a pH of 7 and a polymer concentration of 0.5% by weight. ).
- Particular preference is given to using polymers comprising vinylamine units which have K values of from 85 to 95.
- the polymers containing vinylamine units have for example a charge density (determined at pH 7) of 0 to 18 meq / g, preferably of 5 to 18 meq / g and especially of 10 to 16 meq / g.
- the polymers containing vinylamine units are preferably used in salt-free form.
- Salt-free aqueous solutions of polymers comprising vinylamine units can be prepared, for example, from the above-described salt-containing polymer solutions by means of ultrafiltration on suitable membranes at separation limits of, for example, 1,000 to 500,000 daltons, preferably 10,000 to 300,000 daltons.
- Derivatives of polymers containing vinylamine units can also be used. It is thus possible, for example, to prepare a large number of suitable derivatives from the vinylamine units by amidation, alkylation, sulfonamide formation, urea formation, thiourea formation, carbamate formation, acylation, carboxymethylation, phosphonylation or Michael addition of the amino groups of the polymer.
- the polymers containing vinylamine units also include hydrolyzed graft polymers of, for example, N-vinylformamide on polyalkylene glycols, polyisocyanates vinyl acetate, polyvinyl alcohol, polyvinylformamides, polysaccharides such as starch, oligosaccharides or monosaccharides.
- the graft polymers can be obtained by free-radically polymerizing, for example, N-vinylformamide in aqueous medium in the presence of at least one of the stated grafting bases together with copolymerizable other monomers and then hydrolyzing the grafted vinylformamide units in a known manner to give vinylamine units.
- Preferred polymers containing vinylamine units are vinylamine homopolymers of N-vinylformamide having a degree of hydrolysis of from 1 to
- mol% preferably 25 to 100 mol%, and from 1 to 100 mol%, preferably from 25 to 100 mol%, of hydrolyzed copolymers of N-vinylformamide and vinyl formate, vinyl acetate, vinyl propionate, acrylonitrile, methyl acrylate, ethyl acrylate and / or methyl methacrylate having K values of 30 to 150, in particular 60 to 100.
- Particularly preferred in the process according to the invention are the aforementioned homopolymers of N-vinylformamide.
- Typical representatives of these homopolymers of N-vinylformamide are company known under the trade name Catiofast® ® VFH, Catiofast® ® VSH and Catiofast® ® VMP of BASF share.
- the cationic and / or amphoteric polymers to be used in the process according to the invention are particularly preferred in an amount of from 0.0001 to 1% by weight, based on the solids content of the paper stock suspension, preferably from 0.0005 to 0.5% by weight in an amount of 0.001 to 0.2 wt .-% and in particular in an amount of 0.005 to 0.1 wt .-%, each based on the solids content of the pulp suspension, added to the fiber suspension.
- papers prepared by the process according to the invention in addition to the increased filler content improved dry strength. This is especially characterized by properties such as dry breaking length, tear propagation, internal strength and bending stiffness.
- the paper gloss can also be significantly increased by the treatment according to the invention of the fillers. This is especially true for woody papers such as e.g. SC-paper.
- the gloss increase means an increase in paper quality, which allows the paper manufacturer to obtain a higher selling price.
- the finely divided fillers to be used in the process according to the invention are known from the literature. These are finely divided fillers which are at least partially coated with water-soluble amphoteric copolymers. Such aqueous slurries are known from JP-A 08059740, WO 04/087818 and the file reference DE 103 34 133 A1. These references are hereby incorporated by reference.
- the water-soluble amphoteric copolymers disclosed in these references have as common structural feature that they contain amide units, both five- and six-membered.
- finely divided fillers alone, i. in pure form or as a so-called fresh filler, as well as finely divided fillers containing raw materials such as the so-called committee of coated paper, as well as mixtures in any composition thereof understood by the term finely divided fillers components.
- aqueous slurries of 100% fresh filler are used in the process according to the invention.
- aqueous slurries can be used in the process according to the invention, the filler content is obtained to 100% from the Committee of coated paper. It does not matter whether it is the Committee of one or two sides coated paper.
- aqueous slurries of mixtures in any desired composition of fresh filler and scrap of coated paper are used.
- such a blend may consist of 90% fresh filler and 10% filler from the coated paper broke, each based on the filler content of the aqueous slurry.
- the ratio can also be reversed, namely fresh filler: filler from the coated paper broke of 10%: 90%.
- Examples of possible mixtures of fresh filler to filler from the coated paper committee are 15%: 85%, 20%: 80%, 30%: 70%, 40%: 60%, 50%: 50%, 60%: 40% , 70%: 30%, 80%: 20% and 85%: 15%.
- mixtures in any composition are possible.
- the mixing ratio is in the range of 15% (fresh filler) to 85% (filler of the coated paper broke) to 60% (fresh filler) of 40% (filler of the coated paper broke).
- the percentages in each case relate to the total filler content in the aqueous slurry.
- the filler base e.g. Calcium carbonates, which are present in the form of ground lime (GCC), lime, chalk, marble or in the form of precipitated calcium carbonate (PCC).
- GCC ground lime
- PCC precipitated calcium carbonate
- Talc, kaolin, bentonite, satin white, calcium sulfate, barium sulfate and titanium dioxide can likewise be used as fillers.
- the particle diameter of the fillers is preferably less than 2 ⁇ m, for example between 40 and 90% of the filler particles have a particle diameter of ⁇ 2 ⁇ m.
- the fillers are present as aqueous slurries.
- Precipitated calcium carbonate is usually present as an aqueous slurry in the absence of dispersants.
- an anionic dispersant for example polyacrylic acid with an average molecular weight M w of, for example, 1,000 to 40,000 daltons, is generally used. If the fillers contain a high solids content (eg 60% or more), the fillers are milled in the presence of an anionic dispersant.
- anionic dispersant it is used, for example, from 0.01 to 0.6% by weight, preferably from 0.2 to 0.5% by weight, for the preparation of aqueous filler slurries.
- the slurries dispersed in water in the presence of anionic dispersants contain, for example, 10-60% by weight, usually 15-50% by weight, of at least one filler.
- the water-soluble amphoteric polymers described in JP-A 08059740, WO 04/087818 and DE 103 34 133 A1 are added to the aqueous slurries. mixed. For example, from 0.1 to 5% by weight, based on fillers, of a water-soluble amphoteric polymer according to JP-A 08059740, WO 04/087818, and up to 1 to 60% by weight of at least one fine-particle filler containing aqueous slurry DE 103 34 133 A1 or enter an aqueous Anschläm- tion of a finely divided filler in an aqueous solution of an amphoteric polymer and mix the components each.
- This treatment of the aqueous slurry of particulate fillers with the amphoteric polymers can be carried out continuously or batchwise.
- the treatment of the fillers with the amphoteric polymer takes place in a continuous mode.
- the amphoteric polymer can be added as a dilute solution between the filler tank and the filler pump. The dilution and the shear forces in the filler pump guarantee a thorough mixing of the filler with the polymer.
- the finely divided fillers are at least partially coated or impregnated with the water-soluble amphoteric polymers.
- the solid content of the dilute polymer solution of the water-soluble amphoteric polymers may be between 20% by weight and 0.01% by weight.
- the treatment with the water-soluble amphoteric copolymers can be carried out, for example, by dissolving the broke of coated paper in the presence of the water-soluble amphoteric copolymers.
- the treatment with water-soluble amphoteric copolymers is carried out after dissolution of the coated paper committee.
- filler from the committee also finely divided fillers are obtained, which are at least partially coated or impregnated with water-soluble amphoteric copolymers.
- the filler sludge treated with the polymer passes directly into the thick material or the thin material of the paper machine. It is also conceivable that the treated filler be metered in both the thick and in the thin paper machine.
- the process according to the invention is suitable both for the production of wood-free papers and wood-containing papers.
- the process according to the invention leads to a significant increase in the filler content in the paper, without causing any significant losses in the paper properties, such as dry strength.
- the filler Content is increased without loss of strength by the addition of at least one cationic and / or amphoteric polymer.
- Suitable cellulose fibers are any of the common types, e.g. Cellulosic fibers of wood pulp and all annual plants obtained fibers.
- Wood pulp includes, for example, groundwood, thermo-mechanical pulp (TMP), chemo-thermo-mechanical pulp (CTMP), pressure groundwood, semi-pulp, high-yield pulp and refiner mechanical pulp (RMP) and waste paper.
- TMP thermo-mechanical pulp
- CMP chemo-thermo-mechanical pulp
- RMP refiner mechanical pulp
- pulps that can be used in bleached or unbleached form. Examples include sulphate, sulphite and soda pulps. Bleached pulps, also referred to as bleached kraft pulp, are preferably used.
- the fibers mentioned can be used alone or in a mixture.
- the present invention also provides papers coated in the presence of an aqueous slurry of finely divided fillers, wherein the finely divided fillers are at least partially coated with water-soluble amphoteric Copoly-, wherein in addition to the aqueous slurry of finely divided fillers containing components at least one cationic and / or amphoteric polymer are added to the fiber suspension prior to sheet formation.
- papers having a high filler content are understood in particular to be papers having a filler content of from 3 to 45% by weight, based on the solids content of the paper stock suspension, preferably from 10 to 45% by weight, more preferably from 15 to 40 wt .-% and particularly preferably from 20 to 35 wt .-%, each based on the solids content of the paper stock suspension having.
- the percentages in the examples are by weight unless otherwise indicated in the context.
- the electrophoretic mobility and the zeta potential were determined by laser optics.
- the samples were diluted with an aqueous KCl solution (eg 10 mmoles) to a concentration for the measurement of 1% by volume.
- the measuring instrument used was the Zetasizer 3000 HS from Malvern Instruments Ltd ..
- the molecular weights M w of the polymers were determined by means of static light scattering. The measurements were carried out at pH 7.6 in a 10 mmol aqueous saline solution.
- K values were determined according to H. Fikentscher, Cellulose Chemistry, Vol. 13, 48-64 and 71-74 (1932) in 1.0% aqueous brine at 25 ° C, pH 7, and polymer concentration of 0 , 1 wt .-% determined.
- the fillers used were precipitated chalk, precipitated calcium carbonate (PCC), ground chalk (GCC), kaolin or mixtures of the stated fillers.
- Five different copolymer-pretreated fillers were used in the examples of this invention.
- the structural composition of these copolymers was determined from the monomer mixture used, the degree of hydrolysis and using the calculation disclosed in the earlier German patent application with the file 103 34 133.1 and in WO 04/087818 based on the 13 C NMR spectroscopy. For this purpose, the signals of the C atoms were integrated.
- the solvent used was D 2 O.
- TMP Thermo-Mechanical Pulp
- groundwood was whisk-free in a ratio of 70/30 at a solids concentration of 4% in the laboratory pulper until a grinding degree of 60-65 was reached.
- the pH of the substance was in the range between 7 and 8.
- the milled substance was then diluted by the addition of water to a solids concentration of 0.35%.
- aqueous filler slurries of the pretreated fillers in combination with polymers containing vinylamine units in the production of filler-containing paper in each case 500 ml of the paper stock suspension were added and the slurries of the pretreated fillers and a vinylamine units were metered into this pulp containing polymer (Catio- fast® VMP).
- the metered amount of the polymers containing vinylamine units was in each case 0.1% of polymer, based on the solids content of the paper stock suspension.
- a cationic polyacrylamide retention aid (Polymin ® KE 2020) in this mixture.
- the metered amount of the retention agent was in each case 0.01% polymer, based on the solids content of the paper stock suspension.
- the amount of slurry was adjusted by means of several preliminary tests so that the amount of pretreated filler was about 20%.
- the paper sheets were each produced on a Rapid-Köthen sheet former according to ISO 5269/2 with a sheet weight of 80 g / m 2 and then dried for 7 minutes at 9O 0 C and then calendered with a line pressure of 200 N / cm.
- Comparative Examples 6 to 10 Paper sheets were prepared analogously to Examples 1 to 5 with the corresponding pretreated fillers. However, the addition of vinylamine-containing polymers was omitted.
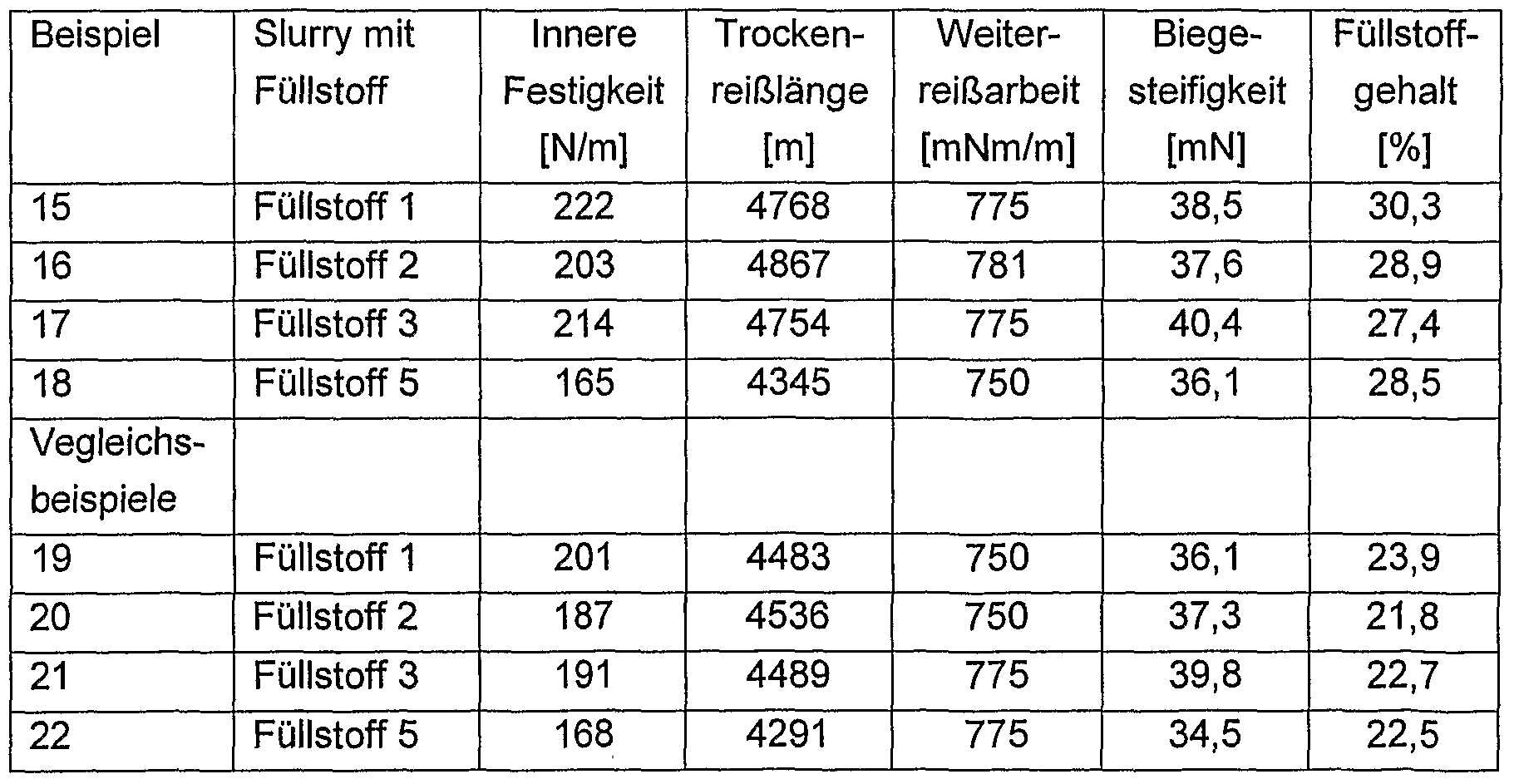
- a mixture of bleached birch sulphate and bleached pine sulphite was blotted open in a ratio of 70/30 at a solids concentration of 4% in the laboratory pulper until a freeness of 55-60 was reached.
- the opened substance is an optical brightener (Blankophor ® PSG) and a cationic starch (HICAT ® 5163 A) were then added.
- the digestion of the cationic starch was carried out as a 10% starch slurry in a jet cooker at 130 0 C and 1 minute residence time.
- the metered amount of the optical brightener was 0.5% commercial goods, based on the solids content of the paper stock suspension.
- the dosage of cationic starch was 0.5% starch, based on the solids content of the stock suspension.
- the pH of the substance was in the range between 7 and 8.
- the milled substance was then diluted by the addition of water to a solids concentration of 0.35%.
- the amount of slurry was adjusted by means of several preliminary tests so that the amount of pretreated filler was about 16%. 2
- the paper sheets were each produced on a Rapid-Köthen sheet former according to ISO 5269/2 with a sheet weight of 80 g / m 2 and then dried for 7 minutes at 90 0 C and then calendered with a line pressure of 200 N / cm.
- Paper sheets were prepared analogously to Examples 15 to 18 with the corresponding pretreated fillers. However, the addition of vinylamine-containing polymers has been omitted.
- a mixture of bleached chemical pulp and groundwood was blotted open in a ratio of 20/80 at a solids concentration of 4% in the laboratory pulper until a freeness of 55-60 was reached.
- the pH of the substance was in the range between 7 and 8.
- the milled substance was then diluted by the addition of water to a solids concentration of 0.35%.
- the amount of dosed filler slurry of the filler 2 and the untreated kaolin clay mixture was adjusted by means of several preliminary experiments so that the amount of filler 2 and untreated kaolin clay was about 20%.
- the total filler content was thus about 40%.
- the paper sheets were produced on a Rapid-Köthen sheet former according to ISO 5269/2 with a sheet weight of 80 g / m 2 and then dried for 7 minutes at 90 0 C and then calendered with a line pressure of 200 N / cm. Comparative Example 27
- Paper sheets were produced analogously to Example 26.
- the corresponding filler was untreated, ie free of amphoteric copolymers.
- the addition amount of the filler slurry in the sheet formation was increased so much that the equivalent filler content of the respective filler type of Example 26 was achieved.
- the double-coated woodfree paper having a basis weight of 104 g / m 2 used in the examples contained a total of 38.4% of filler according to analysis of the ashing data (500 ° C. for 2 hours in the ashing furnace). According to the paper manufacturer, the raw paper used for the production of the coated grade was produced with a filler content of about 23% (ground calcium carbonate, GCC). The weight on each side was 12 gsm. The coating pigment used was precipitated calcium carbonate. Examples 28-31
- a mixture of bleached birch sulphate and bleached pine sulphite was blotted open in a ratio of 70/30 at a solids concentration of 4% in the laboratory pulper until a freeness of 55-60 was reached.
- the whisker and the coated broke spread in the presence of the amphoteric copolymer were mixed in a 1: 1 ratio.
- the total material, an optical brightener (Blankophor ® PSG) and a cationic starch (HICAT ® 5163 A) were then added.
- the digestion of the cationic starch was carried out as a 10% strength starch in a jet cooker at 130 0 C and 1 minute residence time.
- the metered amount of the optical brightener was 0.5% commercial product, based on the solids content of the paper stock suspension.
- the dosage of cationic starch was 0.5% starch, based on the solids content of the stock suspension.
- the pH of the substance was in the range between 7 and 8. The total material was then diluted by the addition of water to a solids concentration of 0.35%.
- the metered amount of the retention agent was in each case in each case 0.01% polymer, based on the solids content of the paper stock suspension.
- the paper sheets were each produced on a Rapid-Köthen sheet former according to ISO 5269/2 with a sheet weight of 80 g / m 2 and then dried for 7 minutes at 90 0 C and then calendered with a line pressure of about 200 N / cm.
- 500 g of the coated paper were pitched in a laboratory pulper (Escher Wyss) with 12 liters of water (consistency 4%) for 10 minutes without stippings.
- the degree of grinding of the stock suspension was 65 Schopper Riegler. 5 g of a 12% strength aqueous solution of an amphoteric copolymer containing 40 mol% of vinylformamide units, 30 mol% of acrylic acid units and 30 mol% of vinylamine and amidine units having a molecular weight M w of approx. 500,000 too.
- a mixture of bleached birch sulphate and bleached pine sulphite was blasted in a ratio of 70/30 at a solids concentration of 4% in the laboratory pulper until a freeness of 55-60 was reached.
- the whisker and the coated broke spread in the presence of the amphoteric copolymer were mixed in a 1: 1 ratio.
- the total material, an optical brightener (Blankophor ® PSG) and a cationic starch (HICAT ® 5163 A) were then added.
- the digestion of the cationic starch was carried out as a 10% strength starch in a jet cooker at 130 0 C and 1 minute residence time.
- the metered amount of the optical brightener was 0.5% commercial goods, based on the solids content of the paper stock suspension.
- the dosage of cationic starch was 0.5% starch, based on the solids content of the stock suspension.
- the pH of the substance was in the range between 7 and 8. The total material was then diluted by addition of water to a solids concentration of 0.35%.
- the paper sheets were each produced on a Rapid-Köthen sheet former according to ISO 5269/2 with a sheet weight of 80 g / m 2 and then dried for 7 minutes at 90 0 C and then calendered with a line pressure of about 200 N / cm.
- a mixture of bleached birch sulphate and bleached pine sulphite was blotted open in a ratio of 70/30 at a solids concentration of 4% in the laboratory pulper until a freeness of 55-60 was reached.
- the whipped material was then mixed with the coated broke in the ratio 1: 1.
- the total material, an optical brightener (Blankophor ® PSG) was then added as a so-cationic starch (HICAT ® 5163 A).
- the digestion of the cationic starch was carried out as a 10% starch slurry in a jet cooker at 13O 0 C and 1 minute residence time.
- the metered amount of the optical brightener was 0.5% strength, based on the solids content of the paper stock suspension.
- the dosage of cationic starch was 0.5% starch, based on the solids content of the pulp suspension.
- the pH of the substance was in the range between 7 and 8.
- the total material was then diluted by the addition of water to a
- the paper sheets were each produced on a Rapid-Köthen sheet former according to ISO 5269/2 with a sheet weight of 80 g / m 2 and then dried for 7 minutes at 90 0 C and then calendered with a line pressure of about 200 N / cm.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Paper (AREA)
Abstract
Description
Claims
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA2590489A CA2590489C (en) | 2004-12-17 | 2005-12-14 | Papers with a high filler material content and high dry strength |
| US11/721,929 US8778139B2 (en) | 2004-12-17 | 2005-12-14 | Papers with a high filler material content and high dry strength |
| ES05819674.2T ES2554691T3 (es) | 2004-12-17 | 2005-12-14 | Papeles con un alto contenido de cargas y una elevada resistencia a la tracción en seco |
| JP2007545938A JP5130049B2 (ja) | 2004-12-17 | 2005-12-14 | 高い填料含量および高い乾燥強度を有する紙 |
| PL05819674T PL1828481T3 (pl) | 2004-12-17 | 2005-12-14 | Papiery o dużej zawartości wypełniaczy i wysokiej wytrzymałości na sucho |
| EP05819674.2A EP1828481B1 (de) | 2004-12-17 | 2005-12-14 | Papiere mit hohem f]llstoffgehalt und hoher trockenfestigkeit |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE200410061605 DE102004061605A1 (de) | 2004-12-17 | 2004-12-17 | Papiere mit hohem Füllstoffgehalt und hoher Trockenfestigkeit |
| DE102004061605.1 | 2004-12-17 | ||
| DE200510022799 DE102005022799A1 (de) | 2005-05-12 | 2005-05-12 | Papier mit hohem Füllstoffgehalt und hoher Trockenfestigkeit |
| DE102005022799.6 | 2005-05-12 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2006066769A2 true WO2006066769A2 (de) | 2006-06-29 |
| WO2006066769A3 WO2006066769A3 (de) | 2006-11-16 |
Family
ID=36602111
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2005/013430 WO2006066769A2 (de) | 2004-12-17 | 2005-12-14 | Papiere mit hohem füllstoffgehalt und hoher trockenfestigkeit |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US8778139B2 (de) |
| EP (1) | EP1828481B1 (de) |
| JP (1) | JP5130049B2 (de) |
| CA (1) | CA2590489C (de) |
| ES (1) | ES2554691T3 (de) |
| PL (1) | PL1828481T3 (de) |
| PT (1) | PT1828481E (de) |
| WO (1) | WO2006066769A2 (de) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006128814A1 (de) * | 2005-05-31 | 2006-12-07 | Basf Aktiengesellschaft | Polymer-pigment-hybride für die papierherstellung |
| CN102124161A (zh) * | 2008-08-18 | 2011-07-13 | 巴斯夫欧洲公司 | 增加纸,纸板和卡纸的干强度的方法 |
| US8382948B2 (en) | 2008-06-24 | 2013-02-26 | Basf Se | Production of paper |
| EP2367978B1 (de) | 2008-11-26 | 2019-10-09 | Nalco Company | Methode zur erhöhung des füllergehalts bei der papierherstellung |
| US10626558B2 (en) | 2015-08-06 | 2020-04-21 | Solenis Technologies, L.P. | Method for producing paper |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102004052957A1 (de) * | 2004-10-29 | 2006-05-04 | Basf Ag | Verfahren zur Herstellung von gekrepptem Papier |
| WO2009004080A2 (de) * | 2007-07-05 | 2009-01-08 | Basf Se | Verfahren zur herstellung von wässrigen anschlämmungen von feinteiligen füllstoffen und ihre verwendung zur herstellung von papieren mit hohem füllstoffgehalt und hoher trockenfestigkeit |
| FR2992981B1 (fr) | 2012-07-09 | 2014-07-04 | Snf Sas | Procede ameliore de fabrication de papier utilisant un polymere obtenu par degradation d'hofmann |
| FR3048436B1 (fr) | 2016-03-03 | 2018-03-23 | S.P.C.M. Sa | Procede de fabrication de papier et de carton |
| CN106868925A (zh) * | 2016-12-30 | 2017-06-20 | 芜湖市哈贝纸业有限公司 | 一种高强高填料纸张及其制备方法 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6033524A (en) * | 1997-11-24 | 2000-03-07 | Nalco Chemical Company | Selective retention of filling components and improved control of sheet properties by enhancing additive pretreatment |
| WO2004087818A1 (de) * | 2003-04-03 | 2004-10-14 | Basf Aktiengesellschaft | Wässrige anschlämmungen von feinteiligen füllstoffen, verfahren zu ihrer herstellung und ihre verwendung zur herstellung füllstoffhaltiger papiere |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3534273A1 (de) * | 1985-09-26 | 1987-04-02 | Basf Ag | Verfahren zur herstellung von vinylamin-einheiten enthaltenden wasserloeslichen copolymerisaten und deren verwendung als nass- und trockenverfestigungsmittel fuer papier |
| DE3842820A1 (de) * | 1988-12-20 | 1990-06-28 | Basf Ag | Verfahren zur herstellung von stabilen wasser-in-oel-emulsionen von hydrolysierten polymerisaten von n-vinylamiden und ihre verwendung |
| DE4001045A1 (de) * | 1990-01-16 | 1991-07-18 | Basf Ag | Verfahren zur herstellung von papier, pappe und karton |
| JP2960185B2 (ja) * | 1991-03-06 | 1999-10-06 | 三菱製紙株式会社 | 紙の製造方法 |
| JPH05106103A (ja) | 1991-10-16 | 1993-04-27 | Danaa Japan:Kk | 衣服のポケツト |
| JP3472352B2 (ja) | 1994-08-16 | 2003-12-02 | ハイモ株式会社 | 製紙用添加剤 |
| DE19617983A1 (de) * | 1996-05-06 | 1997-11-13 | Basf Ag | ß-Hydroxyalkylvinylamin-Einheiten enthaltende Polymerisate, Verfahren zu ihrer Herstellung und ihre Verwendung |
| DE19627553A1 (de) * | 1996-07-09 | 1998-01-15 | Basf Ag | Verfahren zur Herstellung von Papier und Karton |
| DE19654390A1 (de) * | 1996-12-27 | 1998-07-02 | Basf Ag | Verfahren zur Herstellung von Papier |
| DE19851024A1 (de) * | 1998-11-05 | 2000-05-11 | Basf Ag | Wäßrige Dispersionen von wasserlöslichen Polymerisaten von N-Vinylcarbonsäureamiden, Verfahren zu ihrer Herstellung und ihre Verwendung |
| SE521591C2 (sv) * | 1998-11-30 | 2003-11-18 | Sca Res Ab | Metod att framställa en partikel uppvisande beläggning av med varandra växelverkande polymerer och pappers -eller nonwovenprodukt innehållande partiklarna |
| FI117716B (fi) | 2000-04-18 | 2007-01-31 | Ciba Sc Holding Ag | Menetelmä täyteaineen esikäsittelemiseksi, modifioitu täyteaine ja sen käyttö |
| DE10162052A1 (de) * | 2001-12-17 | 2003-06-26 | Basf Ag | Verfahren zur Herstellung von Papier, Pappe und Karton |
| JP2004018323A (ja) | 2002-06-18 | 2004-01-22 | Nippon Paper Industries Co Ltd | 複合粒子の製造方法並びに高填料内添紙の製造方法 |
| JP2004018336A (ja) * | 2002-06-19 | 2004-01-22 | Nippon Paper Industries Co Ltd | 酸化チタン複合粒子の製造方法並びに填料内添紙の製造方法 |
| DE10334133A1 (de) | 2003-07-25 | 2005-02-24 | Basf Ag | Wässrige Zusammensetzung und deren Verwendung zur Papierherstellung |
-
2005
- 2005-12-14 EP EP05819674.2A patent/EP1828481B1/de active Active
- 2005-12-14 CA CA2590489A patent/CA2590489C/en active Active
- 2005-12-14 WO PCT/EP2005/013430 patent/WO2006066769A2/de active Application Filing
- 2005-12-14 PT PT58196742T patent/PT1828481E/pt unknown
- 2005-12-14 PL PL05819674T patent/PL1828481T3/pl unknown
- 2005-12-14 JP JP2007545938A patent/JP5130049B2/ja not_active Expired - Fee Related
- 2005-12-14 US US11/721,929 patent/US8778139B2/en active Active
- 2005-12-14 ES ES05819674.2T patent/ES2554691T3/es active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6033524A (en) * | 1997-11-24 | 2000-03-07 | Nalco Chemical Company | Selective retention of filling components and improved control of sheet properties by enhancing additive pretreatment |
| WO2004087818A1 (de) * | 2003-04-03 | 2004-10-14 | Basf Aktiengesellschaft | Wässrige anschlämmungen von feinteiligen füllstoffen, verfahren zu ihrer herstellung und ihre verwendung zur herstellung füllstoffhaltiger papiere |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006128814A1 (de) * | 2005-05-31 | 2006-12-07 | Basf Aktiengesellschaft | Polymer-pigment-hybride für die papierherstellung |
| US8343312B2 (en) | 2005-05-31 | 2013-01-01 | Basf Aktiengesellschaft | Polymer-pigment hybrids for use in papermaking |
| US8382948B2 (en) | 2008-06-24 | 2013-02-26 | Basf Se | Production of paper |
| CN102124161A (zh) * | 2008-08-18 | 2011-07-13 | 巴斯夫欧洲公司 | 增加纸,纸板和卡纸的干强度的方法 |
| EP2367978B1 (de) | 2008-11-26 | 2019-10-09 | Nalco Company | Methode zur erhöhung des füllergehalts bei der papierherstellung |
| US10626558B2 (en) | 2015-08-06 | 2020-04-21 | Solenis Technologies, L.P. | Method for producing paper |
Also Published As
| Publication number | Publication date |
|---|---|
| PL1828481T3 (pl) | 2016-03-31 |
| US20090272506A1 (en) | 2009-11-05 |
| CA2590489A1 (en) | 2006-06-29 |
| ES2554691T3 (es) | 2015-12-22 |
| PT1828481E (pt) | 2016-01-26 |
| CA2590489C (en) | 2015-02-10 |
| EP1828481A2 (de) | 2007-09-05 |
| WO2006066769A3 (de) | 2006-11-16 |
| JP2008524452A (ja) | 2008-07-10 |
| JP5130049B2 (ja) | 2013-01-30 |
| EP1828481B1 (de) | 2015-09-23 |
| US8778139B2 (en) | 2014-07-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2443284B1 (de) | Verfahren zur erhöhung der trockenfestigkeit von papier, pappe und karton | |
| EP2315875B1 (de) | Verfahren zur erhöhung der trockenfestigkeit von papier, pappe und karton | |
| EP2491177B1 (de) | Verfahren zur herstellung von papier, pappe und karton mit hoher trockenfestigkeit | |
| DE68917069T2 (de) | Trockenfestigkeitszusatz für Papier. | |
| EP2288750B1 (de) | Verfahren zur herstellung von papier, pappe und karton mit hoher trockenfestigkeit | |
| EP1819877B1 (de) | Verfahren zur herstellung von papier, pappe und karton mit hoher trockenfestigkeit | |
| EP1613703B1 (de) | Wässrige anschlämmungen von feinteiligen füllstoffen, verfahren zu ihrer herstellung und ihre verwendung zur herstellung füllstoffhaltiger papiere | |
| DE69408485T2 (de) | Verfahren zur Herstellung von Papier mit erhöhter Festigkeit im nassen und trockenen Zustand | |
| DE3644072A1 (de) | Beschwertes papier | |
| EP2920364B1 (de) | Emulgierung von alkenylbernsteinsäureanhydrid mit aminhaltigem homopolymer oder copolymer | |
| EP2393982B1 (de) | Verfahren zur herstellung von papier, pappe und karton mit hoher trockenfestigkeit | |
| EP1828481B1 (de) | Papiere mit hohem f]llstoffgehalt und hoher trockenfestigkeit | |
| WO2010026101A1 (de) | Verfahren zur herstellung von papier, pappe und karton unter verwendung von endo-beta-1,4-glucanasen als entwässerungsmittel | |
| EP0573458B1 (de) | Wässrige anschlämmungen von feinteiligen füllstoffen und ihre verwendung zur herstellung von füllstoffhaltigem papier | |
| DE69915070T2 (de) | Füllmittel mit modifizierter oberfläche zum leimen von papier | |
| EP1792010B1 (de) | Verfahren zur herstellung von papier, pappe und karton | |
| EP3332063B1 (de) | Verfahren zur herstellung von papier | |
| EP2723943B1 (de) | Verfahren zur herstellung von papier, pappe und karton | |
| WO2005083174A1 (de) | Wässrige dispersion von reaktivleimungsmitteln, verfahren zu ihrer herstellung und ihre verwendung | |
| EP1727938B1 (de) | Verfahren zur herstellung von papier, pappe und karton | |
| DE102004061605A1 (de) | Papiere mit hohem Füllstoffgehalt und hoher Trockenfestigkeit | |
| DE102005022799A1 (de) | Papier mit hohem Füllstoffgehalt und hoher Trockenfestigkeit | |
| DE2115409A1 (de) | Füllstoffe | |
| DE102006040771B3 (de) | Papiererzeugnis und Verfahren zu dessen Herstellung sowie dessen Verwendung | |
| WO2006136556A2 (de) | Verfahren zur herstellung von papier, pappe und karton |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KN KP KR KZ LC LK LR LS LT LU LV LY MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU LV MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005819674 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2590489 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11721929 Country of ref document: US Ref document number: 2007545938 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200580043366.6 Country of ref document: CN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWP | Wipo information: published in national office |
Ref document number: 2005819674 Country of ref document: EP |