WO2005111018A1 - Pyridazinone derivatives, methods for producing them and their use as pharmaceuticals - Google Patents
Pyridazinone derivatives, methods for producing them and their use as pharmaceuticals Download PDFInfo
- Publication number
- WO2005111018A1 WO2005111018A1 PCT/EP2005/005346 EP2005005346W WO2005111018A1 WO 2005111018 A1 WO2005111018 A1 WO 2005111018A1 EP 2005005346 W EP2005005346 W EP 2005005346W WO 2005111018 A1 WO2005111018 A1 WO 2005111018A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- pyridazin
- heteroaryl
- heterocyclyl
- phenyl
- Prior art date
Links
- 0 *C(C(*)=NN1)=C(*)C1=O Chemical compound *C(C(*)=NN1)=C(*)C1=O 0.000 description 12
- CBXXIVIZFKXCKL-UHFFFAOYSA-N C[n]1ncc(-c2c(C3=CC(C(C=C4)=CNC4=O)=NNC3=O)[nH]c3ccccc23)c1 Chemical compound C[n]1ncc(-c2c(C3=CC(C(C=C4)=CNC4=O)=NNC3=O)[nH]c3ccccc23)c1 CBXXIVIZFKXCKL-UHFFFAOYSA-N 0.000 description 1
- YRCVBORQKQLWCJ-UHFFFAOYSA-N Cc(cc(cc1C)C(C=C2c([nH]c3c4cccc3)c4C(C(N3)=O)=CNC3=O)=NNC2=O)c1O Chemical compound Cc(cc(cc1C)C(C=C2c([nH]c3c4cccc3)c4C(C(N3)=O)=CNC3=O)=NNC2=O)c1O YRCVBORQKQLWCJ-UHFFFAOYSA-N 0.000 description 1
- OAJNKGCBKABRSW-UHFFFAOYSA-N Cc(cc(cc1C)C(C=C2c3cc4cc(C(OC)=O)ncc4[nH]3)=NNC2=O)c1O Chemical compound Cc(cc(cc1C)C(C=C2c3cc4cc(C(OC)=O)ncc4[nH]3)=NNC2=O)c1O OAJNKGCBKABRSW-UHFFFAOYSA-N 0.000 description 1
- TXLUYGGVRXCGFC-UHFFFAOYSA-N Cc(cc(cc1C)C(C=C2c3cc4ncccc4[nH]3)=NNC2=O)c1O Chemical compound Cc(cc(cc1C)C(C=C2c3cc4ncccc4[nH]3)=NNC2=O)c1O TXLUYGGVRXCGFC-UHFFFAOYSA-N 0.000 description 1
- NZJQZEXVEHZAMI-UHFFFAOYSA-N O=C(C(c([nH]c1c2)cc1ccc2Cl)=C1)NN=C1c1ccncc1 Chemical compound O=C(C(c([nH]c1c2)cc1ccc2Cl)=C1)NN=C1c1ccncc1 NZJQZEXVEHZAMI-UHFFFAOYSA-N 0.000 description 1
- MQWWZCBYYIUPRV-UHFFFAOYSA-N O=C(C(c1cc(cccc2)c2[nH]1)=C1)NN=C1C(C(N1)=O)=CNC1=O Chemical compound O=C(C(c1cc(cccc2)c2[nH]1)=C1)NN=C1C(C(N1)=O)=CNC1=O MQWWZCBYYIUPRV-UHFFFAOYSA-N 0.000 description 1
- BPDXPNKIMDKPOE-UHFFFAOYSA-N O=C(C(c1ccn[nH]1)=C1)NN=C1c1ccncc1 Chemical compound O=C(C(c1ccn[nH]1)=C1)NN=C1c1ccncc1 BPDXPNKIMDKPOE-UHFFFAOYSA-N 0.000 description 1
- SHZAHBYJWOABCM-ZHACJKMWSA-N O=C1C(c2c(/C=C/c3ccccc3)c(cccc3)c3[nH]2)=CC(C2=CCNC=C2)=NC2=[N]=C12 Chemical compound O=C1C(c2c(/C=C/c3ccccc3)c(cccc3)c3[nH]2)=CC(C2=CCNC=C2)=NC2=[N]=C12 SHZAHBYJWOABCM-ZHACJKMWSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
Definitions
- the present invention relates to compounds according to the general formula (I), with the definitions of the substituents X, R 1 and R 2 given below in the text, as well as their physiologically acceptable salts, methods for producing these compounds and their use as pharmaceuticals.
- These compounds are kinase inhibitors, in particular inhibitors of the kinase GSK- 3 ⁇ (glycogen synthase kinase-3 ⁇ ).
- WO 04/046117 discloses pyridazinone derivatives, which can be employed for the inhibition of GSK-3 ⁇ . They differ from the compounds of the present invention in the substitution of the pyridazinone cycle, since at position 4 of the cycle there is an amido group defined as substituent instead of a heteroaryl substituent such as pyrrole or indole.
- the International Application PCT-EP 05/002179 also discloses pyridazinone derivatives, which can be employed for the inhibition of GSK-3 ⁇ . In contrast to the compounds of the present invention, those compounds are substituted at position 4 of the pyridazinone cycle by a benzimidazole group.
- US-A 2002/0119963 discloses imidazole derivatives having activity for inhibiting CDK5, CDK2 and GSK-3.
- the imidazole derivatives are substituted via the first nitrogen atom, among others, with a 3-8-membered hydrocyclylalkyl or a 5-14-membered hydroaryl group, but a pyridazinone group is not explicitly disclosed therein. Additionally, the imidazole derivative is substituted with an amino group, which is further substituted with a carboxyl group. Therefore, it is evident, that the pyridazinone derivatives of the present invention are not disclosed by US-A 2002/0119963. Compounds as such explicitly disclosed by US 2002/0119963 are no subject of the present invention.
- WO 03/059891 discloses pyridazinone derivatives that are useful for treating diseases and conditions caused or exacerbated by unregulated p38 MAP Kinase activity and/or TNF activity.
- the compounds described therein can be used, for example, for the treatment of inflammatory conditions, diabetes, Alzheimer's disease or cancer. They differ from the compounds of the present invention in the substitution of the pyridazinone cycle, since the nitrogen at position 2 of the cycle is mostly substituted with alky, aryl or heteroaryl and at position 4 of the cycle there is no heteroaryl group (such as pyrrole or indole) defined as substituent.
- JP-A 09 216883 discloses pyridazinone derivatives which can be used to treat heart failure or high blood pressure.
- the pyridazinone derivatives described therein obligatorily have a pyrazolo[1 ,5-a]pyridine substituent in position 6, which is in turn substituted in position 2 by aryl, preferably phenyl.
- the pyridazinone ring itself is additionally substituted in position 2 by substituents such as hydrogen, lower alkyl or a heterocycle, while position 4 has substituents such as hydrogen, acyl, cyano, Heterocyclyl such as tetrazolyl, amino or a protected amino group.
- substituent in position 4 is a heterocycle, it preferably has 3 to 8 ring members and is saturated. However, a heterocyclic group is not included in the preferred substituents in position 4 of the pyridazinone ring of the compounds disclosed in this document. Compounds as such explicitly disclosed in JP-A 09 215883 are no subject of the present invention.
- the object of the present invention is to provide compounds showing this ability.
- X is a residue selected from the group consisting of:
- A is CR 3 or N
- B is CR 4 or N;
- D is CR 5 or N;
- E is CR 6 or N
- R 1 is halogen; unsubstituted or at least monosubstituted CrC ⁇ 0 -alkyl, where the substituents are selected from the group consisting of: halogen, CN, N0 2 , -OR 7 , -C(0)R 7 , -C(0)OR 7 , -0-C(0)R 7 , -NR 7 R 8 , -NR 8 C(0)R 7 , -C(0)NR 7 R 8 , -NR 8 C(S)R 7 , -C(S)NR 7 R 8 , -SR 7 , -S(0)R 7 , - S0 2 R 7 , -NR 8 S0 2 R 7 , -S0 2 NR 7 R 8 , -0-S0 2 R 7 , -SO2-O-R 7 , aryl, heteroaryl, heterocyclyl, trifluoromethyl and trifluoromethoxy, and aryl, heterocyclyl and heteroaryl may in turn be at least mono
- R 2 is hydrogen or C C ⁇ o-alkyl
- R 3 is selected from the group consisting of: hydrogen, halogen, -CN, -N0 2 , R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , -C(0)R 8 , -C(0)OR 8 , -0-C(0)R 8 , -NR 7 R 8 , -NR 7 C(0)R 8 , -C(0)NR 7 R 8 , -NR 7 C(S)R 8 , -C(S)NR 7 R 8 , -SR 8 , -S(0)R 8 , -S0 2 R 8 , -NR 7 S0 2 R 8 , -S0 2 NR 7 R 8 , -0-S0 2 R 8 , -SO2-O-R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and the CH 2
- R 4 is selected from the group consisting of: hydrogen, halogen, -CN, -N0 2 , R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , -C(0)R 8 , -C(0)OR 8 , -0-C(0)R 8 , -NR 7 R 8 , -NR 7 C(0)R 8 , -C(0)NR 7 R 8 , -NR 7 C(S)R 8 , -C(S)NR 7 R 8 , -SR 8 , -S(0)R 8 , -S0 2 R 8 , -NR 7 S0 2 R 8 , -S0 2 NR 7 R 8 , -0-S0 2 R 8 , -S0 2 -0-R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, aryl, heterocyclyl
- R 5 is selected from the group consisting of: hydrogen, halogen, -CN, -N0 2 , R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , -C(0)R 8 , -C(0)OR 8 , -0-C(0)R 8 , -NR 7 R 8 , -NR 7 C(0)R 8 , -C(0)NR 7 R 8 , -NR 7 C(S)R 8 , -C(S)NR 7 R 8 , -SR 8 , -S(0)R 8 , -S0 2 R 8 , -NR 7 S0 2 R 8 , -S0 2 NR 7 R 8 , -0-S0 2 R 8 , -S0 2 -0-R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, aryl, heterocyclyl
- R 7 is H; or unsubstituted or at least monosubstituted Ci-Cio-alkyl, C-2-C 1 0- alkenyl, C 2 -C ⁇ o-alkyinyl, aryl-(C ⁇ -C 6 -alkyl)-, heterocyclyl-(d-Ce-alkyl)- , heteroaryl-(C ⁇ -C 6 -alkyl)-, heterocyclyl, aryl or heteroaryl, where the substituents are selected from the group consisting of: heteroaryl, heterocyclyl, aryl, halogen, -OH, oxo, C C ⁇ o-alkyl, C1-C10- alkoxy, (d-C ⁇ o-alkyl)thio-, -C(0)OH, -C(0)0-(d-C 6 -alkyl), -C(0)NH 2 , trifluoromethyl, trifluoromethoxy, -CN, -NH 2 , -NH
- R 9 is selected from the group consisting of: hydrogen, halogen, -CN, -N0 2 , R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , -C(0)R 8 , -C(0)OR 8 , -0-C(0)R 8 , -NR 7 R 8 , -NR 7 C(0)R 8 , -C(0)NR 7 R 8 , -NR 7 C(S)R 8 , -C(S)NR 7 R 8 , -SR 8 , -S(0)R 8 , -S0 2 R 8 , -NR 7 SO 2 R 8 , -S0 2 NR 7 R 8 , -0-S0 2 R 8 , -S0 2 -0-R 8 , aryl, heteroaryl, heterocyclyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, aryl, heterocyclyl and heteroaryl may in turn
- R 10 is unsubstituted or at least monosubstituted Ci-Cio-alkyl, aryl-(C ⁇ -C- 6 - alkyl)-, heterocyclyl-(C ⁇ -C 6 -alkyl)-, heteroaryl-(CrC 6 -alkyl)-, C 2 -C 10 - alkenyl or C -C ⁇ o-alkyinyl, where the substituents are selected from the group consisting of: heteroaryl, heterocyclyl, aryl, halogen, -OH, oxo, C ⁇ -C ⁇ o-alkoxy, (d- C ⁇ o-alkyl)thio-, -C(0)OH, -C(0)0-(d-C 6 -alkyl), -C(0)NH 2 , trifluoromethyl, trifluoromethoxy, -CN, -NH 2 , -NH(C ⁇ -C ⁇ 0 -alkyl) and -N(C ⁇ -C
- Heteroaryl is a 5 to 10-membered, aromatic, mono- or bicyclic heterocycle containing one or more heteroatoms selected from the group consisting of N, O and S;
- Aryl is a 6 to 10-membered, aromatic mono- or bicyclus
- Heterocyclyl is a 4- to 10-membered, non-aromatic, mono- or bicyclic heterocycle containing one or more heteroatoms selected from the group consisting of N, O and S, or a physiologically acceptable salt thereof, with the provisio that R 1 is not unsubstituted or at least monosubstituted pyrazolo[1 ,5-a]pyridinyl.
- a substituent for example aryl
- a substituent for example aryl
- a group of further substituents for example, d-dralkyl, d-C 3 -alkoxy, halogen etc.
- the selection from the group of further substituents is independently from each other.
- all combinations of further substituents are comprised in the case of, for example, a double-substitution of aryl.
- aryl may be substituted twice with ethyl, aryl may be mono-substituted with methyl or ethoxy, respectively, aryl may be mono- substituted with ethyl or fluoro, respectively, aryl may be substituted twice with methoxy, etc..
- Alkyl, alkenyl and alkynyl residues may be linear or branched, acyclic or cyclic. This also applies when they are part of other groups, for example in alkoxy groups (d-Cio-alkyl-0-), alkoxycarbonyl groups or amino groups, or when they are substituted.
- alkyl groups are: methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl. This comprises both the n-isomers of these residues and isopropyl, isobutyl, isopentyl, sec-butyl, tert-butyl, neopentyl, 3,3-dimethylbutyl etc.
- alkyl here also includes unsubstituted alkyl residues as well as alkyl residues which are substituted by one or more, for example one, two, three or four, identical or different residues, for example aryl, heteroaryl, alkoxy or halogen. The substituents may be present in any desired position of the alkyl group.
- alkyl here also expressly includes cycloalkyl residues and cycloalkyl-alkyl-residues (alkyl substituted by cycloalkyl), where cycloalkyl contains at least three carbon atoms.
- cycloalkyl residues examples are cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and cyclooctyl. All cycloalkyl groups may be unsubstituted or optionally substituted by one or more further residues, as exemplified above in the case of the alkyl groups.
- alkenyl and alkynyl groups are the vinyl residue, the 1-propenyl residue, the 2-propenyl residue (allyl residue), the 2-butenyl residue, the 2-methyl- 2-propenyl residue, the 3-methyl-2-butenyl residue, the ethynyl residue, the 2- propynyl residue (propargyl residue), the 2-butynyl residue or the 3-butynyl residue.
- alkenyl here also expressly includes cycloalkenyl residues and cycloalkenyl-alkyl-residues (alkyl substituted by cycloalkenyl) containing at least three carbon atoms.
- Examples for cycloalkenyl residues are cyclopentenyl, cyclohexenyl, cycloheptenyl and cyclooctenyl.
- the alkenyl residues may have 1 to 3 conjugated or unconjugated double bonds (thus also alk-dienyl- as well as alk-trienyl-residues), preferably one double bond in a straight or branched chain; the same applies to alkynyl residues in respect of triple bonds.
- the alkenyl and alkinyl residues may be unsubstituted or optionally substituted by one or more further residues, as exemplified above in the case of the alkyl groups.
- the above-mentioned aryl, heteroaryl and heterocyclic residues may be unsubstituted or may carry one or more, for example one, two, three or four of the substituents indicated in the above definition, which substituents may be in any desired position.
- the substituent may be in the 2-position, the 3-position or the 4- position, in disubstituted phenyl residues the substituents may be in 2,3-position, 2,4-position, 2,5-position, 2,6-position, 3,4-position or 3,5-position.
- the substituents may be in 2,3,4-position, 2,3,5-position, 2,3,6- position, 2,4,5-position, 2,4,6-position or 3,4,5-position.
- the substituents may be in the 2,3,4,5-position, the 2,3,4,6- position, or the 2,3,5, 6-position.
- divalent residues may be attached to the adjacent groups by any ring carbon atom.
- a phenylene residue these may be in 1 ,2-position (ortho-phenylene), 1 ,3-position (meta-phenylene) or 1 ,4-position (para-phenylene).
- 5-membered ring aromatics containing one heteroatom such as, for example, thiophene or furan
- the two free bonds may be in 2,3-position, 2,4-position, 2,5-position or 3,4-position.
- a divalent residue derived from pyridine may be a 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-pyridinediyl residue.
- the present invention includes all positional isomers, i.e., in the case of a 2,3-pyridinediyl residue, for example, it includes the compound in which the one adjacent group is present in the 2-position and the other adjacent group is present in the 3-position as well as the compound in which the one adjacent group is present in the 3-position and the other adjacent group is present in the 2-position.
- heteroaryl residues, heteroarylene residues, heterocyclyl residues, heterocyclylen residues and rings which are formed by two groups bonded to a nitrogen are preferably derived from completely saturated, partially unsaturated or completely unsaturated heterocycles (i.e. heterocycloalkanes, heterocycloalkenes, heteroaromatics), which contain one, two, three or four heteroatoms, which may be identical or different; more preferably they are derived from heterocycles which contain one, two, or three, in particular one or two, heteroatoms, which may be identical or different.
- the heterocycles may be monocyclic or polycyclic, for example monocyclic, bicyclic or tricyclic.
- the rings Preferably they are monocyclic or bicyclic.
- the rings preferably are 5- membered rings, 6-membered rings or 7-membered rings.
- polycyclic heterocycles containing two or more heteroatoms they may all be within the same cycle or within different cycles.
- heteroaryl is a residue derived from mono- or bicyclic aromatic heterocycles.
- each of the above heteroaryls includes for its second cycle also its saturated form (perhydro form) or its partially unsaturated form (for example in the dihydro form or the tetrahydro form) in case the respective forms are known and stable.
- heteroaryl as used herein comprises therefore, for example, bicyclic residues in which both cycles are aromatic as well as bicyclic residues in which only one cycle is aromatic.
- heteroaryl are: 3H-indolinyl, 2(1 H)-quinolinonyl, 4-oxo-1 ,4-dihydroquinolinyl, 2H-1-oxoisoquinolyl, 1 ,2- dihydroquinolinyl, 3,4-dihydroquinolinyl, 1 ,2-dihydroisoquinolinyl, 3,4-dihydro- isoquinolinyl, chromonyl, chromanyl, 1 ,3-benzodioxolyl, oxindolyl, 1 ,2,3,4- tetrahydroisoquinolinyl, 1 ,2,3,4- tetrahydroquinolinyl, 5,6-dihydroquinolyl, 5,6- dihydroisoquinolyl, 5,6,7,8-tetrahydroquinolinyl or 5,6,7,8-tetrahydroisoquinolyl, According to the present invention
- Non-aromatic heterocycles comprise in the following especially heterocycloalkanes (completely saturated heterocycles) as well as heterocycloalkenes (partially unsaturated heterocycles).
- heterocycloalkenes there are also included compounds having two or more double bonds, which may optionally be conjugated.
- heterocyclyl examples include: pyrrolidinyl, piperidinyl, piperazinyl, imidazolidinyl, pyrazolidinyl, isothiazolidinyl, thiazolidinyl, isoxazolidinyl, oxazolidinyl, tetrahydrofuranyl, tetrahydrothiophenyl 1 ,3-dioxolanyl, 1 ,4-dioxinyl, pyranyl, thiopyranyl, tetrahydro-1 ,2-oxazinyl, tetrahydro-1 ,3-oxazinyl, morpholinyl, thiomorpholinyl, 1 ,2-thiazinyl, 1 ,3-thiazinyl, 1 ,4-thiazinyl, azepinyl, 1 ,2-diazepinyl, 1 ,3-diazepinyl, 1 ,4
- Substituents which may be derived from these heterocycles may be attached via any suitable carbon atom.
- Residues derived from nitrogen heterocycles may carry a hydrogen atom or a substituent on a ring nitrogen atom, and examples include pyrrole, imidazole, pyrrolidine, morpholine, piperazine residues, etc.
- Those nitrogen heterocyclic residues may also be attached via a ring nitrogen atom, in particular if the respective heterocyclic residue is bonded to a carbon atom.
- Suitable nitrogen heterocycles may also be present as N-oxides or as quarternary salts containing a counterion which is derived from a physiologically acceptable acid.
- Pyridyl residues for example, may be present as pyridine-N-oxides.
- Suitable sulfur-containing heterocycles may be present as S-oxid or S-S-dioxid.
- aryl is a residue derived from mono- or bicyclic aromatics, where the cycle does not contain any heteroatoms.
- the term aryl includes for its second cycle also its saturated form (perhydro form) or its partially unsaturated form (for example in the dihydro form or the tetrahydro form) in case the respective forms are known and stable.
- the term aryl as used herein comprises therefore, for example, bicyclic residues in which both cycles are aromatic as well as bicyclic residues in which only one cycle is aromatic.
- heteroaryl examples include: phenyl, naphthyl, indanyl, 1 ,2- dihydronaphthenyl, 1 ,4-dihydronaphthenyl, indenyl or 1 ,2,3,4-tetrahydronaphth yl.
- Arylalkyl (such as aryl-(C ⁇ -C 6 -alkyl)- ) means an alkyl residue (such as Ci-C ⁇ - alkyl), which in turn is substituted by an aryl residue.
- Heteroarylalkyl (such as heteroaryl-(C ⁇ -C 6 -alkyl)- ) means an alkyl residue (such as Ci-C ⁇ -alkyl), which in turn is substituted by a heteroaryl residue.
- Heterocyclylalkyl (such as heterocyclyl- (Ci-C ⁇ -alkyl)- ) means an alkyl residue (such as CrC ⁇ -alky!), which in turn is substituted by a heterocyclyl residue.
- Such arylalkyl, heteroarylalkyl or heterocyclylalkyl residues may themselves be a substituent of another substituent or fragment (such as heterocyclyl-(C ⁇ -C 6 -alkyl)-NH-), which means that a substituent or fragment (such as -NH-) in turn is substituted by a heterocyclylalkyl residue (such as heterocyclyl-(C ⁇ -C 6 -alkyl)- ).
- an alkyl residue include examples such as H 2 N-(C ⁇ -C6-alkyl)-, (C ⁇ -C 6 -alkyl)HN-(C ⁇ -C 6 - alkyl)- or (C ⁇ -C 6 -alkyl) 2 N-(C ⁇ -Ce-alkyl)- , which means an alkyl residue (such as d- Ce-alkyl), which in turn is substituted by -NH 2 , -NH(C C 6 -alkyl) or -N(C ⁇ -C 6 -alkyl) 2 , respectively.
- a residue such as (C ⁇ -C 6 -alkyl)HN-(C ⁇ -C 6 -alkyl)- may itself be a substituent of another substituent or fragment (such as (d-C 6 -alkyl)HN- (C ⁇ -C 6 -alkyl)-0-), which means that a substituent or fragment (such as -0-) in turn is substituted by a substituted alkyl residue (such as (C ⁇ -C 6 -alkyl)HN-(C ⁇ -C 6 -alkyl)- ).
- substituent or fragment such as -0
- Halogen is fluorine, chlorine, bromine or iodine, preferably fluorine, chlorine or bromine, most preferably fluorine or chlorine.
- the present invention includes all stereoisomeric forms of the compounds of the formula (I). Centers of asymmetry that are present in the compounds of formula (I) all independently of one another have S configuration or R configuration.
- the invention includes all possible enantiomers and diastereomers and mixtures of two or more stereoisomers, for example mixtures of enantiomers and/or diastereomers, in all ratios.
- compounds according to the present invention which may exist as enantiomers may be present in enantiomerically pure form, both as levorotatory and as dextrorotatory antipodes, in the form of racemates and in the form of mixtures of the two enantiomers in all ratios.
- the invention includes both: the cis form and the trans form as well as mixtures of these forms in all ratios. All these forms are an object of the present invention.
- the preparation of individual stereoisomers may be carried out, if desired, by separation of a mixture by customary methods, for example by chromatography or crystallization, by the use of stereochemicaUy uniform starting materials for the synthesis or by stereoselective synthesis.
- a derivatization may be carried out before a separation of stereoisomers.
- the separation of a mixture of stereoisomers may be carried out at the stage of the compounds of the formula (I) or at the stage of an intermediate during the synthesis.
- the present invention also includes all tautomeric forms of the compounds of formula (I), in particular keto-enol tautomerism, i.e. the respective compounds may be present either in their keto form or in their enol form or in mixtures thereof in all ratios.
- the invention also comprises their corresponding physiologically or toxicologically acceptable salts.
- Physiologically acceptable salts are particularly suitable for medical applications, due to their greater solubility in water compared with the starting or base compounds. Said salts must have a physiologically acceptable anion or cation.
- Suitable physiologically acceptable acid addition salts of the compounds of the invention are salts of inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, metaphosphoric acid, nitric acid, sulfonic acid and sulfuric acid and also of organic acids such as, for example, acetic acid, theophyllinacetic acid, methylene-bis-b-oxynaphthoic acid, benzenesulfonic acid, benzoic acid, citric acid, ethanesuifonic acid, salicylic acid, fumaric acid, gluconic acid, glycolic acid, isethionic acid, lactic acid, lactobionic acid, maleic acid, malic acid, methanesulfonic acid, succinic acid, p-toluenesul
- Suitable pharmaceutically acceptable basic salts are ammonium salts, alkali metal salts (such as sodium salts and potassium salts) and alkaline earth metal salts (such as magnesium salts and calcium salts). Salts having a pharmaceutically unacceptable anion are likewise included within the scope of the present invention as useful intermediates for preparing or purifying pharmaceutically acceptable salts and/or for use in nontherapeutic applications, for example in-vitro applications.
- the invention also includes, in addition to the salt forms mentioned, inner salts or betaines (zwitterions).
- the respective salts according to the formula (I) may be obtained by customary methods which are known to the person skilled in the art like, for example by contacting these with an organic or inorganic acid or base in a solvent or dispersant, or by anion exchange or cation exchange with other salts.
- the present invention furthermore includes all solvates of compounds of the formula (I), for example hydrates or adducts with alcohols, active metabolites of the compounds of the formula (I), and also derivatives, which contain physiologically tolerable and cleavable groups, for example esters or amides.
- physiologically functional derivative used herein relates to any physiologically acceptable derivative of an inventive compound of the formula I, for example an ester which on administration to a mammal, for example humans, is capable of forming (directly or indirectly) a compound of the formula I or an active metabolite thereof.
- the physiologically functional derivatives also include prodrugs of the compounds of the invention.
- prodrugs may be metabolized in vivo to a compound of the invention.
- These prodrugs may or may not be active themselves and are also object of the present invention.
- the compounds of the invention may also be present in various polymorphous forms, for example as amorphous and crystalline polymorphous forms. All polymorphous forms of the compounds of the invention are included within the scope of the invention and are another aspect of the invention.
- Preferred compounds of the formula (I) are those compounds in which one or more, including all, of the above-mentioned substituents R 1 to R 10 , A, B, D, E, X, aryl, heteroaryl and heterocyclyl of the formula (I) independently from each other have the following meanings (definitions), with all possible (if defined) combinations of the preferred meanings, the more preferred meanings, the much more preferred meanings, the particularly preferred meanings or the exceptionally preferred meanings, also in combination with substituents having their basic meanings, being a subject of the present invention.
- X is preferably a residue selected from the group consisting of:
- X is more preferably a residue selected from the group consisting of: , and unsubstituted and at least monosubstituted pyrrolyl and triazolyl where the substituents are selected from the group consisting of: halogen, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , -C(0)R 8 , -C(0)NR 8 H, phenyl, heteroaryl, heterocyclyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, phenyl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with Ci-C ⁇ -alkyI, C ⁇ -C 6 -alkoxy, oxo, halogen, trifluoromethyl, trifluoromethoxy or -OH; and each of said residues is bound to the pyridazinone fragment via the carbonatom being in ⁇ -position to the NH-fragment
- A is preferably CR 3 ;
- B is preferably CR 4 ;
- D is preferably CR 5 ;
- E is preferably CR 6 ; Unless each of the substituents A, B, D and E has its preferred meaning, preferably only two of the substituents A, B, D and E are N; more preferably only one of the substituents A, B, D and E is N;
- R 1 is preferably: unsubstituted or at least monosubstituted Ci-C ⁇ -alkyI, where the substituents are selected from the group consisting of: fluoro, chloro, -OH, C ⁇ -C 6 -alkoxy, -NH 2 , -NH(d-C 6 -alkyl), -N(d-C 6 -alkyl) 2 , heterocyclyl-(C ⁇ -C 6 -alkyl)-NH-, aryl-(C ⁇ -C 6 -alkyl)-NH-, heterocyclyl, aryl and heteroaryl, and the aryl-, heterocyclyl- and heteroaryl-fragments of said substituents may in turn be at least monosubstituted with d-C 4 -alkyl, C ⁇ -C 4 -alkoxy, fluor, chloro, trifluoromethyl, trifluoromethoxy or -OH; or unsubstituted or at least monosubstitute
- R 1 is more preferably: unsubstituted or at least monosubstituted phenyl, pyridinyl, pyrimidinyl, pyrazolyl, thiophenyl, oxazolyl, isoxazolyl, dihydropyridinonyl, imidazolyl, 1 H-pyrrolo[2,3-b]pyridinyl, benzo[b]thiophenyl, benzodioxolyl or thiazolo[3,2- b][1 ,2,4]-triazolyl, where the substituents are selected from the group consisting of: halogen, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 7 , -C(0)R 7 , -C(0)OR 7 , -NR 7 H, - NR 7 (CrC 6 -alkyl), -C(0)NR 7 H, -SR 7 , aryl, hetero
- R 1 is much more prefarably: unsubstituted or at least monosubstituted phenyl, pyridinyl, pyrimidinyl, pyrazolyl, thiophenyl, oxazolyl, isoxazolyl, dihydropyridinonyl, imidazolyl, 1 H-pyrrolo[2,3-b]pyridinyl benzo[b]thiophenyl, benzodioxolyl or thiazolo[3,2- b][1 ,2,4]-triazolyl, where the substituents are selected from the group consisting of: halogen, Ci-Ce-alkyl, phenyl-(d-C 6 -alkyl)-, H 2 N-(C ⁇ -C 6 -alkyl)-, (C C 6 -alkyl)HN-(C ⁇ - Ce-alkyl)-, (C ⁇ -C 6 -alkyl) 2 N-(C ⁇
- R 1 is particularly preferred: unsubstituted or at least monosubstituted phenyl, thiophenyl, pyrazolyl, pyridinyl or pyrimidinyl, where the substituents are selected from the group consisting of: C ⁇ -C - alkyl, -OH, C ⁇ -C 4 -alkoxy, (C C 4 -alkyl)thio-, (d-C 4 -alkyl)HN-(C ⁇ -C 4 -alkyl)-, H 2 N-(C ⁇ -C 4 -alkyl)-, trifluoromethyl, trifluoromethoxy and -NH(C C -alkyl), and -NH(C ⁇ -C -alkyl) may in turn be at least monosubstituted with phenyl, piperazinyl, piperidinyl or morpholinyl.
- R >1 is exceptionally preferred: pyridin-4-yl, 2-ethylamino-pyrimidin-4-yl, 3,5-dimethyl-4-hydroxyphenyl, 2- (1 -phenylethylamino)-pyrimidin-4-yl, 2-(2-morpholin-4-ylethylamino)-pyrimi- din-4-yl, 2-methylamino-pyrimidin-4-yl, 6-methyl-2-(2-morpholin-4-ylethyl- amino)-pyrimidin-4-yl, 3-methoxy-4-hydroxy-phenyl, 2-methylsulfanyl-pyri- midin-4-yl, 4-butylamino-pyrimidin-4-yl, 3-hydroxy-phenyl, thiophen-3-yl, 1 H- pyrazol-4-yl, 4-hydroxy-3-methoxy-5-methylaminomethyl-phenyl, 4-hydroxy- phenyl or 2,6-dimethyl-pyrimidin-4-yl;
- R 2 is preferably hydrogen or Ci-C ⁇ -alkyI; R 2 is particularly preferred hydrogen.
- R 3 is preferably selected from the group consisting of: hydrogen, halogen, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(0)R 8 , -C(0)OR 8 , -NR 8 H, -NR 8 (d-C 6 -alkyl), -C(0)NR 8 H, -SR 8 , -S0 2 NR 8 H, -SO 2 R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, aryl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with Ci-C ⁇ -alkyI, C ⁇ -C 6 -alkoxy, oxo, halogen, trifluoromethyl, trifluoromethoxy or -OH;
- R 3 is more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(0)OH, -C(0)0-(d-C 6 -alkyl), -C(0)NR 8 H, difluoromethyl, trifluoromethyl and trifluoromethoxy,
- R >3 is much more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, d-C 6 -alkyl, heterocyclyl-(C ⁇ -C 6 -alkyl)-, heteroaryl-(d-Ce-alkyl)-, phenyl-(C C 6 -alkyl)-, H 2 N-(C ⁇ -C 6 -alkyl)-, (d-C 6 - alkyl)HN-(C C 6 -alkyl)-, (C ⁇ -C 6 -alkyl) 2 N-(C ⁇ -C 6 -alkyl)-, -OH, C C 6 -alkoxy, heterocyclyl-(Ci-C 6 -alkyl)-0-, heteroaryl-(Ci-C 6 -alkyl)-0-, phenyl-(C C 6 - alkyl)-0-, H 2 N-(C C 6 -alkyl)-0-, (d
- R 3 is particularly preferred selected from the group consisting of: hydrogen, fluoro, chloro, -C(0)OH, 2-dimethylamino-ethoxy, 2-diethylamino- ethoxy, methoxy, ethoxy, 2-piperidin-1 -yl-ethoxy, piperidin-1-ylmethyl, 2- piperidin-1 -yl-ethoxy, 2-(4-methyl-piperazin-1 -yl)-ethoxy, 4-methyl-pipera- zin-1 -ylmethyl, 2-morpholin-4-yl-ethoxy, 4-cyclopropyl-piperazin-1 -ylmethyl, (1 -cyclopropyl-piperidin-4-ylamino)-methyl, 2-carboxy-pyrrolidin-1 -ylmethyl, [(1 -cyclopropyl-piperidin-4-yl)methylamino]-methyl, morpholin-4-ylmethyl, 1 - (1 -cyclopropyl-piperidin-4
- R 3 is exceptionally preferred selected from the group consisting of: hydrogen;
- R 4 is preferably selected from the group consisting of: hydrogen, halogen, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(0)R 8 , -C(0)OR 8 , -NR 8 H, -NR 8 (d-C 6 -alkyl), -C(0)NR 8 H, -SR 8 , -S0 2 NR 8 H, -SO 2 R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, aryl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with Ci-Ce-alkyl, Ci-Ce-alkoxy, oxo, halogen, trifluoromethyl, trifluoromethoxy or -OH;
- R 4 is more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(0)OH, -C(0)0-(C Ce-alkyl), -C(0)NR 8 H, difluoromethyl, trifluoromethyl and trifluoromethoxy,
- R 4 is much more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, CrC 6 -alkyl, heterocyclyl-(d-C 6 -alkyl)-, heteroaryl-(C ⁇ -Ce-alkyl)-, phenyl-(C C 6 -alkyl)-, H 2 N-(C ⁇ -C 6 -alkyl)-, (d-C 6 - alkyl)HN-(C ⁇ -Ce-alkyl)-, (d-Ce-alky sN- ⁇ rCe-alkyl)-, -OH, C ⁇ -C 6 -alkoxy, heterocyclyl-(Ci-C 6 -alkyl)-0-, heteroaryl-(Ci-C 6 -alkyl)-0-, phenyl-(d-C 6 - alkyl)-0-, H 2 N-(Ci-C 6 -alkyl)-0-, (Ci-C
- R 4 is particularly preferred selected from the group consisting of: hydrogen, fluoro, chloro, -C(0)OH, 2-dimethylamino-ethoxy, 2-diethylamino- ethoxy, methoxy, ethoxy, 2-piperidin-1-yl-ethoxy, piperidin-1 -ylmethyl, 2- piperidin-1 -yl-ethoxy, 2-(4-methyl-piperazin-1 -yl)-ethoxy, 4-methyl-pipera- zin-1 -ylmethyl, 2-morpholin-4-yl-ethoxy, 4-cyclopropyl-piperazin-1 -ylmethyl, (1 -cyclopropyl-piperidin-4-ylamino)-methyl, 2-carboxy-pyrrolidin-1 -ylmethyl, [(1 -cyclopropyl-piperidin-4-yl)methylamino]-methyl, morpholin-4-ylmethyl, 1 - (1-cyclopropyl-piperidin-4-
- R 5 is preferably selected from the group consisting of: hydrogen, halogen, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(0)R 8 , -C(0)OR 8 , -NR 8 H, -NR 8 (C ⁇ -C 6 -alkyl), -C(0)NR 8 H, -SR 8 , -S0 2 NR 8 H, -S0 2 R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, aryl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with CrC ⁇ -alky!, Ci-Ce-alkoxy, oxo, halogen, trifluormethyl, trifluoromethoxy or -OH;
- R 5 is more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(0)OH, -C(O)0-(C ⁇ -C 6 -alkyl), -C(0)NR 8 H, difluoromethyl, trifluoromethyl and trifluoromethoxy,
- R 5 is much more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, Ci-C ⁇ -alkyI, heterocyclyl-(C ⁇ -C 6 -alkyl)-, heteroaryl-(CrCe-alkyl)-, phenyl-(d-Ce-alkyl)-, H 2 N-(C ⁇ -C 6 -alkyl)-, (d-C 6 - alkyl)HN-(d-Ce-alkyl)-, (C ⁇ -C 6 -alkyl) 2 N-(C ⁇ -C 6 -alkyl)-, -OH, C ⁇ -C 6 -alkoxy, heterocyclyl-(C ⁇ -C 6 -alkyl)-0-, heteroaryl-(C ⁇ -C 6 -alkyl)-0-, phenyl-(C ⁇ -C 6 - alkyl)-0-, H 2 N-(d-C 6 -alkyl)
- R 5 is particularly preferred selected from the group consisting of: hydrogen, fluoro, chloro, -C(0)OH, 2-dimethylamino-ethoxy, 2-diethylamino- ethoxy, methoxy, ethoxy, 2-piperidin-1 -yl-ethoxy, piperidin-1 -ylmethyl, 2- piperidin-1 -yl-ethoxy, 2-(4-methyl-piperazin-1 -yl)-ethoxy, 4-methyl-pipera- zin-1 -ylmethyl, 2-morpholin-4-yl-ethoxy, 4-cyclopropyl-piperazin-1 -ylmethyl, (1 -cyclopropyl-piperidin-4-ylamino)-methyl, 2-carboxy-pyrrolidin-1 -ylmethyl, [(1 -cyclopropyl-piperidin-4-yl)methylamino]-methyl, morpholin-4-ylmethyl, 1 - (1 -cyclopropyl-piperidin
- R 6 is preferably selected from the group consisting of: hydrogen, halogen, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(O)R 8 , -C(0)OR 8 , -NR 8 H, -NR 8 (d-C 6 -alkyl), -C(0)NR 8 H, -SR 8 , -S0 2 NR 8 H, -S0 2 R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and the CH 2 -fragments, aryl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with C ⁇ -C 6 -alkyl, Ci-Ce-alkoxy, oxo, halogen, trifluoromethyl, trifluoromethoxy or -OH;
- R 6 is more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, R 10 , -(CH 2 ) n -NR 7 R 8 where n is 1 to 3, -OR 8 , - C(O)OH, -C(0)0-(Ci-C 6 -alkyl), -C(0)NR 8 H, difluoromethyl, trifluoromethyl and trifluoromethoxy,
- R 6 is much more preferably selected from the group consisting of: hydrogen, fluoro, chloro, -CN, Ci-Ce-alkyl, heterocyclyl-(C ⁇ -C 6 -alkyl)-, heteroaryl-(C Ce-alkyl)-, phenyl-(d-C 6 -alkyl)-, H 2 N-(C ⁇ -C 6 -alkyl)-, (Ci-Ce- alkyl)HN-(d-Ce-alkyl)-, (C ⁇ -C 6 -alkyl) 2 N-(CrC 6 -alkyl)-, -OH, Ci-Ce-alkoxy, heterocyclyl-(C ⁇ -C 6 -alkyl)-O-, heteroaryl-(d-C 6 -alkyl)-0-, phenyl-(C Ce- alkyl)-0-, H 2 N-(Ci-C 6 -alkyl)-0-, (Ci-C 6
- R 6 is particularly preferred selected from the group consisting of: hydrogen, fluoro, chloro, -C(0)OH, 2-dimethylamino-ethoxy, 2-diethylamino- ethoxy, methoxy, ethoxy, 2-piperidin-1-yl-ethoxy, piperidin-1 -ylmethyl, 2- piperidin-1 -yl-ethoxy, 2-(4-methyl-piperazin-1 -yl)-ethoxy, 4-methyl-pipera- zin-1 -ylmethyl, 2-morpholin-4-yl-ethoxy, 4-cyclopropyl-piperazin-1 -ylmethyl, (1 -cyclopropyl-piperidin-4-ylamino)-methyl, 2-carboxy-pyrrolidin-1 -ylmethyl, [(1 -cyclopropyl-piperidin-4-yl)methylamino]-methyl, mo holin-4-ylmethyl, 1 - (1-cyclopropyl-piperidin-4
- R 6 is exceptionally preferred hydrogen
- R 7 is preferably: H; or unsubstituted or at least monosubstituted C ⁇ -C 6 -alkyl, phenyl-(d-C 6 - alkyl)-, heterocyclyl-(C ⁇ -C 6 -alkyl)-, heteroaryl-(C ⁇ -C 6 -alkyl)-, heterocyclyl, phenyl or heteroaryl, where the substituents are selected from the group consisting of: fluoro, chloro, -OH, Ci-Ce-alkyl, C ⁇ -C 6 -alkoxy, -C(0)OH, -C(0)0-(d-C 3 -alkyl), - C(0)NH 2 , trifluoromethyl, trifluoromethoxy, -NH 2 , -NH(C ⁇ -C 6 -alkyl) and - N(C ⁇ -C 6 -alkyl) 2 , and the Ci-Ce-alkyl-fragments of said substituents may in turn be
- R 7 is more preferably: unsubstituted or at least monosubstituted C C ⁇ -alkyl, heterocyclyl or heterocyclyl-(C ⁇ -C 6 -alkyl)-, where the substituents are selected from the group consisting of: -OH, - C(0)OH, Ci-Ce-alkoxy, d-C 6 -alkyl, -NH 2 , -NH(C ⁇ -C 6 -alkyl) and -N(d-C 6 - alkyl) 2l and the Ci-C ⁇ -alkyl-fragments of said substituents may in turn be at least monosubstituted with d-C 3 -alkyl, d-C 3 -alkoxy, -C(0)OH, trifluoromethyl, trifluoromethoxy, fluoro, chloro or -OH;
- R 7 is particularly preferred: unsubstituted or at least monosubstituted d-d-alkyl, where the substituents are selected from the group consisting of: morpholinyl, piperazinyl, piperidinyl, imidazolyl, pyrrolidinyl, -C(O)OH, -NH 2 , -NH(C ⁇ -C 6 -alkyl) and -N(d-C 6 -alkyl) 2 , and morpholinyl, piperazinyl, piperidinyl, imidazolyl and pyrrolidinyl may in turn be monosubstituted with d-C 3 -alkyl, d-C 3 -alkoxy, trifluoromethyl, trifluoromethoxy, -C(0)OH, fluoro, chloro or -OH; or morpholinyl, piperazinyl, piperidinyl, imidazolyl or pyrrolidinyl, which may in
- R >8 is preferably:
- R 8 is more preferably: unsubstituted or at least monosubstituted Ci-Ce-alkyl, heterocyclyl or heterocyclyl-(C ⁇ -C 6 -alkyl)-, where the substituents are selected from the group consisting of: -OH, - C(0)OH, Ci-Ce-alkoxy, C ⁇ -C 6 -alkyl, -NH 2 , -NH(C ⁇ -C 6 -alkyl) and -N(d-C 6 - alkyl) 2 , and the C ⁇ -C 6 -alkyl-fragments of said substituents may in turn be at least monosubstituted with d-C 3 -alkyl, d-C 3 -alkoxy, -C(0)OH, trifluoromethyl, trifluoromethoxy, fluoro, chloro or -OH;
- R 8 is particularly preferred: unsubstituted or at least monosubstituted d-C -alkyl, where the substituents are selected from the group consisting of: morpholinyl, piperazinyl, piperidinyl, imidazolyl, pyrrolidinyl, -C(0)OH, -NH 2 , -NH(d-Ce-alkyl) and -N(C ⁇ -C 6 -alkyl) 2 , and morpholinyl, piperazinyl, piperidinyl, imidazolyl and pyrrolidinyl may in turn be monosubstituted with d-C 3 -alkyl, d-C 3 -alkoxy, trifluoromethyl, trifluoromethoxy, -C(0)OH, fluoro, chloro or -OH; or morpholinyl, piperazinyl, piperidinyl, imidazolyl or pyrrolidinyl, which may in turn be
- R is more preferably selected from the group consisting of: hydrogen; halogen; -C(0)-(CrC 3 -alkyl); (C ⁇ -Ce-alkyl)thio-; trifluoromethyl; trifluoromethoxy; unsubstituted and at least monosubstituted Ci-Ce-alkyl and C 2 -C 6 -alkenyl, where the substituents are selected from the group consisting of: heteroaryl, heterocyclyl, phenyl, -OH, Ci-Ce-alkoxy, -NH 2 , -NH(C ⁇ -C 6 -alkyl) and -N(d- C 6 -alkyl) 2 , and phenyl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with CrC 3 -alkyl, d-CValkoxy, -CO-(C ⁇ -C 3 -alkyl), fluoro, chloro, trifluoromethyl, tri
- R 9 is much more preferably selected from the group consisting of: hydrogen; bromo; chloro; -C(0)-(Ci-C 3 -alkyl); unsubstituted and at least monosubstituted d-C 4 -alkyl and C 2 -C -alkenyl, where the substituents are selected from the group consisting of: phenyl azetidinyl, pyridinyl, pyrazolyl, pyrimidinyl, morpholinyl, piperazinyl, piperidinyl, imidazolyl, pyrrolidinyl, -NH 2 , -NH(CrC 6 -alkyl) and -N(d-C 6 - alkyl) 2 , and phenyl, azetidinyl, pyridinyl, pyrazolyl, pyrimidinyl, morpholinyl, piperazinyl, piperidinyl, imidazolyl
- R 9 is particularly preferred: hydrogen, chloro, acetyl, methyl, ethyl, isobutyl, 1 -methyl-1 H-pyrazol-4-yl, morpholin-4-ylmethyl and phenyl;
- R 10 is preferably: unsubstituted or at least monosubstituted CrC 6 -alkyl, phenyl-(C ⁇ -C 6 -alkyl)-, heterocyclyl-(C ⁇ -C 6 -alkyl)-, heteroaryl-(C ⁇ -C 6 -alkyl)- or C 2 -C 6 -alkenyl, where the substituents are selected from the group consisting of: halogen, - d-Ce-alkyl, Ci-Ce-alkoxy, -OH, -C(0)-Ci-C 3 -alkyl, -C(0)OH, -C(0)0-(d-C 3 - alkyl), -C(0)NH 2 , -NH 2 , -NH(C ⁇ -C 6 -alkyl) and -N(C ⁇ -C 6 -alkyl) 2 , and the C C 6 -alkyl-fragments of said substituents may in turn be at least monosub
- R 10 is particularly preferred: unsubstituted or at least monosubstituted d-C -alkyl or C 2 -C 4 -alkenyl, where the substituents are selected from the group consisting of: phenyl, azetidinyl, pyridinyl, morpholinyl, piperazinyl, piperidinyl, imidazolyl, pyrrolidinyl, -C(0)OH, C ⁇ -C 3 -alkoxy, -NH 2 , -NH(C C 3 -alkyl) and -N(d-C 3 - alkyl) 2 , and phenyl, azetidinyl, pyridinyl, morpholinyl, piperazinyl, piperidinyl, imidazolyl and pyrrolidinyl may in turn be monosubstituted with d-C- 3 -alkyl, d-C 3 -alkoxy, triflu
- Heteroaryl is preferably imidazolyl, thiophenyl, furanyl, oxazolyl, isoxazolyl, pyridinyl, pyrimidinyl, pyrazolyl, benzo[b]thiophenyl, thiazolo[3,2-b][1 ,2,4]-triazolyl, pyrrolyl, chinolinyl, isochinolinyl, 1 ,2,3,4-tetrahydrochinolinyl, benzimidazolyl, indolyl or 1 ,3-benzodioxolyl; heteroaryl is particularly preferred pyridinyl, pyrazolyl, thiophenyl or pyrimidinyl;
- Aryl is preferably naphthyl, indanyl or phenyl; aryl is particularly preferred phenyl.
- Heterocyclyl is preferably acetidinyl, azepanyl, thiomorpholinyl, 1 ,4-oxazepanyl, azocanyl, 3-azabicyclo[3.2.2]nonyl, 6-azybicyclo[3.2.1]octyl, dihydropyridinonyl, pyrimidindionyl, 4-oxo-azepanyl, 1 ,4-diazepanyl, tetrahydrofuranyl, 1 ,3-dioxolanyl, morpholinyl, pyrrolidinyl, piperazinyl or piperidinyl; heterocyclyl is particularly preferred piperidinyl, morpholinyl or piperazinyl;
- R 1 to R 10 examples are: i) R 1 to R 10 , A, B, D, E, X heteroaryl, heterocyclyl and aryl have each its preferred meaning; or
- R 1 has its preferred meaning and all other substituents have their basic meaning
- R 2 has its particularly preferred meaning and all other substituents have their basic meaning; or iv) R 3 to R 6 have each its preferred meaning and all other substituents have their basic meaning; or
- R 7 and R 8 have each its preferred meaning and all other substituents have their basic meaning
- R 9 has its preferred meaning and all other substituents have their basic meaning
- R 10 has its preferred meaning and all other substituents have their basic meaning
- A has its preferred meaning and all other substituents have their basic meaning
- x) D has its preferred meaning and all other substituents have their basic meaning
- Heteroaryl has its preferred meaning and all other substituents have their basic meaning
- xvi) Heterocyclyl has its preferred meaning and all other substituents have their basic meaning
- Aryl has its preferred meaning and all other substituents have their basic meaning
- R 1 to R 10 , X heteroaryl, heterocyclyl and aryl have each its preferred meaning and A, B, D and E have their basic meaning, where only two of them may be N; or
- R 1 , R 3 , R 4 , R 5 , R 6 and R 9 have each its more preferred meaning
- R 7 , R 8 , R 10 , heteroaryl, heterocyclyl and aryl have each its preferred meaning
- R 2 and X have each its particularly preferred meaning
- A, B, D and E have their basic meaning, where only one of them may be N; or
- R 1 , R 3 , R 4 , R 5 , R 6 and R 9 have each its much more preferred meaning, heterocyclyl and heteroaryl have each its preferred meaning, R 2 and X have each its particularly preferred meaning and A, B, D and E have their basic meaning, where only one of them may be N; or
- R 2 , R 3 , R 4 , R 5 , R 6 , R 9 and X have each its particularly preferred meaning, R 1 has its exceptionally preferred meaning; B, D and E have each its preferred meaning and A has its basic meaning; or
- R 2 , R 4 , R 5 , R 9 and X have each its particularly preferred meaning
- R 1 , R 3 and R 6 have each its exceptionally preferred meaning
- B, D and E have each its preferred meaning and A has its basic meaning; or
- R 2 , R 3 , R 4 , R 5 , R 6 , R 9 and X have each its particularly preferred meaning, R 1 has its exceptionally preferred meaning and A, B, D and E have their basic meaning, where only two of them may be N; or
- R 2 , R 3 , R 4 , R 5 , R 6 , R 9 and X have each its particularly preferred meaning
- R 1 has its much more preferred meaning
- B, D, E, heteroaryl and heterocyclyl have each its preferred meaning and A has its basic meaning
- R 2 , R 9 and X have each its particularly preferred meaning
- R 3 , R 4 , R 5 and R 6 have each its much more preferred meaning
- R 1 has its exceptionally preferred meaning
- B, D, E, heteroaryl and heterocyclyl have each its preferred meaning and A has its basic meaning
- R 2 , R 3 , R 4 , R 5 , R 6 and X have each its particularly preferred meaning
- R 9 has its much more preferred meaning
- R 1 has its exceptionally preferred meaning
- B, D, E, heteroaryl and heterocyclyl have each its preferred meaning
- A has its basic meaning
- R 2 , R 3 , R 4 , R 5 , R 6 , R 8 and R 10 have each its particularly preferred meaning, R 9 has its much more preferred meaning, X has its more preferred meaning, R 1 has its exceptionally preferred meaning, B, D, E, heteroaryl and heterocyclyl have each its preferred meaning and A has its basic meaning; or
- R 1 and R 9 have each its much more preferred meaning
- R 3 and R 6 have each its exceptionally preferred meaning
- R 2 , R 4 , R 5 and X have each its particularly preferred meaning
- A, B, D, E, heterocyclyl and heteroaryl have each its preferred meaning
- R 1 and R 9 have each its much more preferred meaning
- X has its more preferred meaning
- R 3 , R 4 , R 5 , R 6 , aryl, heterocyclyl and heteroaryl have each its preferred meaning
- R 2 , R 8 and R 10 have each its particularly preferred meaning
- A, B, D and E have their basic meaning, where only two of them may be N; or
- R 3 , R 4 , R 5 , R 6 and R 9 have each its much more preferred meaning
- heteroaryl and heterocyclyl have each its preferred meaning
- R 1 , R 2 and X have each its particularly preferred meaning
- A, B, D and E have their basic meaning, where only one of them may be N; or
- R 1 , R 3 , R 4 , R 5 , R 6 and R 9 have each its much more preferred meaning
- aryl, heterocyclyl and heteroaryl have each its preferred meaning
- R 2 , R 7 , R 10 and X have each its particularly preferred meaning and A, B, D and E have their basic meaning, where only one of them may be N;
- xxxii) R 1 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 and R 9 have each its more preferred meaning
- X has its particularly preferred meaning
- R 2 , R 10 , heteroaryl, heterocyclyl and aryl have each its preferred meaning and A, B, D and E have their basic meaning, where only one of them may be N; or
- R 1 has its more preferred meaning
- R 9 has its much more preferred meaning
- R 7 , R 8 , R 10 , heteroaryl, heterocyclyl and aryl have each its preferred meaning
- R 3 and R 6 have each its exceptionally preferred meaning
- R 2 , R 4 , R 5 and X have each its particularly preferred meaning
- A, B, D and E have their basic meaning, where only one of them may be N; or
- R 2 to R 10 X heteroaryl, heterocyclyl and aryl have each its preferred meaning and R 1 , A, B, D and E have their basic meaning, where only two of them may be N; or
- R 1 , R 2 , R 7 to R 10 , X heteroaryl, heterocyclyl and aryl have each its preferred meaning and R 3 to R 6 , A, B, D and E have their basic meaning, where only two of them may be N; or
- the preferred compounds according to formula (I) are not limited to the above examples. Furthermore, all combinations of each substituent in its basic meaning with the preferred meanings, the more preferred meanings, the much more preferred meanings, the particularly preferred meanings or the exceptionally preferred meanings of the other substituents or all combinations of the preferred meanings, the more preferred meanings, the much more preferred meanings, the particularly preferred meanings or the exceptionally preferred meanings of the respective substituents, which are not exemplified above, are also a subject of the present invention. It is self-evident, that this is only the case, if the definitions of the respective substituents allow such a combination.
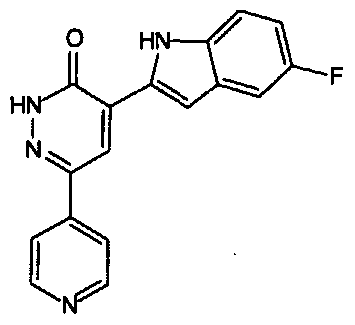
- Most preferred compounds according to the general formula (I) are selected from the group consisting of: 4-(5-chloro-1 H-indol-2-yl)-6-pyridin-4-yl-2H-pyridazin-3-one, 6-(4-hydroxy-3,5- dimethyl-phenyl)-4-(5-trifluoromethyl-1 H-indol-2-yl)-2H-pyridazin-3- one; 4-(3- phenyl-1 H-indol-2-yl)-6-pyridin-4-yl-2H-pyridazin-3-one, 4-(3-ethyl-1 H-indol-2-yl)-6- pyridin-4-yl-2H-pyridazin-3-one, 4-(3-morpholin-4-ylmethyl-1 H-indol-2-yl)-6-pyridin- 4-yl-2H-pyridazin-3-one, 4-(3-acetyl-1 H-indol-2-
- X is a residue selected from the group consisting of:
- A is CR 3 or N
- B is CR 4 or N
- D is CR 5 or N
- E is CR 6 or N
- R 1 is halogen; unsubstituted or at least monosubstituted CrCio-alkyl, where the substituents are selected from the group consisting of: halogen, -CN, -N0 2 , -OR 7 , -C(0)R 7 , -C(0)OR 7 , -0-C(0)R 7 , -NR 7 R 8 , -NR 8 C(0)R 7 , -C(0)NR 7 R 8 , -NR 8 C(S)R 7 , -C(S)NR 7 R 8 , -SR 7 , -S(0)R 7 , - S0 2 R 7 , -NR 8 S0 2 R 7 , -S0 2 NR 7 R 8 , -0-S0 2 R 7 , -S0 2 -O-R 7 , aryl, heteroaryl, heterocyclyl, trifluoromethyl and trifluoromethoxy, and aryl, heterocyclyl and heteroaryl may in turn
- R 2 is hydrogen or C Cio-alkyl
- R 3 , R 4 , R 5 and R 6 are independently from each other selected from the group consisting of: hydrogen, halogen, -CN, N0 2 , R 10 , -OR 8 , -C(0)R 8 , -C(0)OR 8 , -O- C(0)R 8 , -NR 7 R 8 , -NR 7 C(0)R 8 , -C(0)NR 7 R 8 , -NR 7 C(S)R 8 , - C(S)NR 7 R 8 , -SR 8 , -S(O)R 8 , -SO 2 R 8 , -NR 7 S0 2 R 8 , -S0 2 NR 7 R 8 , -O- S0 R 8 , -SO2-O-R 8 , aryl, heteroaryl, heterocyclyl, difluoromethyl, trifluoromethyl and trifluoromethoxy, and aryl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with
- R 9 is selected from the group consisting of: hydrogen, halogen, -CN, -N0 2 , R 10 , -OR 8 , -C(0)R 8 , -C(0)OR 8 , -O- C(0)R 8 , -NR 7 R 8 , -NR 7 C(0)R 8 , -C(0)NR 7 R 8 , -NR 7 C(S)R 8 , - C(S)NR 7 R 8 , -SR 8 , -S(0)R 8 , -S0 2 R 8 , -NR 7 S0 2 R 8 , -S0 2 NR 7 R 8 , -O- S0 2 R 8 , -S0 2 -0-R 8 , aryl, heteroaryl, heterocyclyl, trifluoromethyl and trifluoromethoxy, and aryl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with C ⁇ -C- 6 -alkyl, Ci-Ce-
- R 10 is unsubstituted or at least monosubstituted Ci-Cio-alkyl, C 2 -C ⁇ o- alkenyl or C 2 -C ⁇ o-alkyinyl, where the substituents are selected from the group consisting of: heteroaryl, heterocyclyl, aryl, halogen, -OH, oxo, C ⁇ -C ⁇ 0 -alkoxy, (d- C 10 -alkyl)thio-, -C(0)OH, -C(0)0-(d-C 6 -alkyl), -C(0)NH 2 , trifluoromethyl, trifluoromethoxy; -CN, -NH 2 , -NH(d-C ⁇ o-alkyl) and -N(C ⁇ -C ⁇ o-alkyl) 2 , and aryl, heterocyclyl and heteroaryl may in turn be at least monosubstituted with C ⁇ -C 6 -alkyl, Ci-Ce-alkoxy
- Heteroaryl is a 5 to 10-membered, aromatic, mono- or bicyclic heterocycle containing one or more heteroatoms selected from the group consisting of N, O and S;
- Aryl is a 6 to 10-membered, aromatic mono- or bicyclus
- Heterocyclyl is a 4- to 10-membered, non-aromatic, mono- or bicyclic heterocycle containing one or more heteroatoms selected from the group consisting of N, O and S; or a physiologically acceptable salt thereof.
- the substituent R is not unsubstituted or at least monosubstituted pyrazolo[1 ,5-a]pyridinyl.
- X is a residue selected from the group consisting of:
- A is CR 3 or N
- B is CR 4 or N
- D is CR 5 or N
- E is CR 6 or N
- R 1 is: unsubstituted or at least monosubstituted phenyl, pyridinyl, pyrimidinyl, pyrazolyl, thiophenyl, oxazolyl, isoxazolyl, 1 H-pyrrolo[2,3- bjpyridinyl, benzo[b]thiophenyl, benzodioxolyl or thiazolo[3,2- b][1 ,2,4]-triazolyl, where the substituents are selected from the group consisting of: halogen, Ci-Ce-alkyl , phenyl-(CrC 6 -alkyl)-, -OH, C ⁇ -C 6 -alkoxy, (C C 6 -alkyl)thio-, -O-phenyl, -NH 2 , -N(CrC 6 -alkyl) 2 , -NH(d-Ce-alkyl), H 2 N-(C
- R 2 is hydrogen
- R 3 , R 4 , R 5 and R 6 are independently from each other selected from the group consisting of: hydrogen, fluoro, chloro, bromo, Ci-Ce-alkyl, heterocyclyl-(d-C 6 - alkyl)-, H 2 N-(d-C 6 -alkyl)-, (C ⁇ -C 6 -alkyl)HN-(C ⁇ -C 6 -alkyl)-, (d-C ⁇ - alkyl) 2 N-(C ⁇ -C 6 -alkyl)-, -OH, CrC 6 -alkoxy, heterocyclyl-(d-C 6 -alkyl)- 0-, H 2 N-(Ci-C 6 -alkyl)-0-, (Ci-C 6 -alkyl)HN-(Ci-C 6 -alkyl)-0-, (C C ⁇ - alkyl) 2 N-(Ci-C 6 -alkyl)-0-, -C(0)
- R 9 is selected from the group consisting of: hydrogen; chloro; iodo; bromo; -C(0)-(d-C 3 -alkyl); unsubstituted and at least monosubstituted d-d-alkyl and C 2 -C 4 - alkenyl, where the substituents are selected from the group consisting of: phenyl azetidinyl, pyridinyl, morpholinyl, piperazinyl, piperidinyl, imidazolyl, pyrrolidinyl, -NH 2 , -NH(C ⁇ -C- 6 -alkyl) and -N(C ⁇ -C 6 -alkyl) 2 , and phenyl, azetidinyl, pyridinyl, morpholinyl, piperazinyl, piperidinyl, imidazolyl and pyrrolidinyl may in turn be monosubstituted with d- Q
- Heteroaryl is imidazolyl, thiophenyl, furanyl, oxazolyl, isoxazolyl, pyridinyl, pyrimidinyl, pyrazolyl, benzo[b]thiophenyl, thiazolo[3,2-b][1 ,2,4]-triazolyl, pyrrolyl, chinolinyl, isochinolinyl, 1 ,2,3,4-tetrahydrochinolinyl, benzoimidazolyl, indolyl or 1 ,3-benzodioxolyl;
- Heterocyclyl is acetidinyl, azepanyl, 4-oxo-azepanyl, 1 ,4-diazepanyl, tetrahydrofuranyl, 1 ,3-dioxolanyl, morpholinyl, pyrrolidinyl, piperazinyl or piperidinyl; or a physiologically acceptable salt thereof.
- X , pyrolyl, imidazolyl, pyrazolyl
- Y 2 H or a suitable protecting group, preferably tert-butoxycarbonyl (Boc)
- a compound of the formula I is obtained from intermediates II by metal catalysed coupling and elimination of the methylgroup.
- M may be for example B(OH) 2 , B(OC C ⁇ o-alkyl) 2 , Sn(C ⁇ -C ⁇ 0 -alkyl) 3 , Zn(C ⁇ -C ⁇ 0 - alkyl).
- Intermediates II and M-X are either commercially available or are prepared by procedures known to a person skilled in the art.
- Y 2 is a protecting group
- said group is removed using methods known by a person skilled in the art.
- Elimination of the methylgroup in step b) from compounds of the formula III can be carried out using any suitable reagent known by a person skilled in the art.
- A, B, D, E, R1 and R 9 can be modified after the metal catalysed coupling.
- R 1 Cl, Br, I
- it can be exchanged by palladium Suzuki or Stille coupling.
- R 9 H
- Cl, Br and I may in turn be exchanged with other substituents being defined for R 9 by standard metal catalysed procedures known to a person skilled in the art.
- Y 2 H or a suitable protecting groups, preferably tert-butoxycarbonyl (Boc)
- A, B, D, E, R 1 and R 9 can be modified after the metal catalysed coupling.
- R 1 Cl, Br, I it can be exchanged by palladium Suzuki or Stille coupling.
- R 9 H
- Cl, Br and I may in turn be exchanged with other substituents being defined for R 9 by standard metal catalysed procedures known to a person skilled in the art.
- step a) compound VII is reacted with a suitable hydrazine, followed by conversion with a suitable acetimidic acid ester in steb b) to obtain a compound of formula (VIII).
- the compounds of the formula (I) can be purified by customary purification procedures, for example by recrystallization or chromatography.
- the starting compounds for the preparation of the compounds of the formula (I) are commercially available or can be prepared according to or analogously to literature procedures.
- the compounds obtained with the above-mentioned synthesis methods are a further object of the present invention.
- Subject of the present invention is also the use of compounds according to the general formula (I) as pharmaceuticals or medicaments, respectively.
- the substituents X, R 1 and R 2 (as well as all further substituents defined by the before-mentioned substituents) the same explanations as laid out above in the context with the compounds as such apply.
- the compounds of general formula (I) are kinase inhibitors and can therefore be employed for the treatment of diseases, which may result from an abnormal activity of kinases.
- abnormal kinase activity there may be mentioned, for example, that of PI3K, AkT, GSK-3 ⁇ and the like.
- compounds according to the present invention can be used for the inhibition of the kinase GSK-3 ⁇ .
- This effect is particularly relevant for the treatment of metabolic diseases such as type II diabetes or neurodegenerative diseases such as Alzheimer's disease.
- compounds according to the general formula (I) have an inhibitory effect in respect of the phosphorylation of the tau-protein. This effect is particularly relevant for the treatment of neurodegenerative diseases such as Alzheimer's disease.
- diseases which can be treated with the compounds according to the present invention, include: neurodegenerative diseases, strokes, cranial and spinal traumas and peripheral neuropathies, obesity, metabolic diseases, type II diabetes, essential hypertension, atherosclerotic cardiovascular diseases, polycystic ovary syndrome, syndrome X or immunodeficiency.
- Neurodegenerative diseases are preferably: Alzheimer's disease, Parkinson's disease, frontoparietal dementia, corticobasal degeneration and Pick's disease.
- Compounds according to the present invention are preferably employed for the treatment of metabolic diseases, in particular of type II diabetes.
- the compounds according to the general formula (I) are preferably employed for the treatment of neurodegenerative diseases, in particular of Alzheimer's disease.
- the item treatment also includes prophylaxis, therapy or curing of the above-mentioned diseases.
- the compounds of the formula (I) can be administered to animals, preferably to mammals, and in particular to humans.
- the compounds of the formula (I) can be administered as pharmaceuticals by themselves, in mixtures with one another or in mixtures with other pharmaceuticals or in the form of pharmaceutical preparations.
- Further subjects of the present invention therefore also are the use of the compounds of the formula (I) for preparing one or more medicaments for prophylaxis and/or treatment of the before-mentioned diseases, pharmaceutical preparations (or pharmaceutical compositions) comprising an effective dose of at least one compound of the formula (I) as well as pharmaceutical preparations comprising an effective dose of at least one compound of the formula (I) for prophylaxis and/or treatment of the before-mentioned diseases
- the amount of a compound according to formula (I) which is required in order to attain the desired biological effect depends on a number of factors, for example the specific compound selected, the intended use, the type of administration and the clinical state of the patient.
- the daily dose is in the range from 0.3 mg to 100 mg (typically from 3 mg to 50 mg) per day per kilogram of body weight, for example 3-10 mg/kg/day.
- An intravenous dose can be, for example, in the range from 0.3 mg to 1.0 mg/kg and can be administered in a suitable manner as an infusion of 10 ng to 100 ng per kilogram per minute.
- Suitable infusion solutions for these purposes may contain, for example, from 0.1 ng to 10 mg, typically from 1 ng to 10 mg per milliliter.
- Individual doses may contain, for example, from 1 mg to 10 g of the active compound.
- ampoules for injections can contain, for example, from 1 mg to 100 mg
- orally administerable individual dose formulations such as, for example, tablets or capsules can contain, for example, from 1.0 to 1000 mg, typically from 10 to 600 mg.
- the abovementioned masses relate to the mass of the free compound on which the salt is based.
- the compound used for the prophylaxis or therapy of the abovementioned conditions may be the compounds according to formula (I) themselves, but they are preferably present in the form of a pharmaceutical composition together with an acceptable carrier.
- the carrier must be naturally acceptable, in the sense that it is compatible with the other ingredients of said composition and is not harmful to the patient's health.
- the carrier may be a solid or a liquid or both and is preferably formulated with the compound as an individual dose, for example as a tablet which may contain from 0.05% to 95% by weight of the active compound.
- Further pharmaceutically active substances may also be present, including further compounds according to formula (I).
- the pharmaceutical compositions of the invention may be prepared according to any of the known pharmaceutical methods which essentially comprise mixing the ingredients with pharmacologically acceptable carriers and/or excipients.
- the pharmaceutical preparations can also contain additives.
- additives can be employed, for example: fillers, binders, lubricants, wetting agents, stabilizers, emulsifiers, dispersants, preservatives, sweeteners, colorants, flavorings, aromatizers, thickeners, diluents, buffer substances, solvents, solubilizers, agents for achieving a depot effect, salts for altering the osmotic pressure, coating agents or antioxidants.
- compositions of the invention may be in form of a pill, tablet, lozenge, coated tablet, granule, capsule, hard or soft gelatin capsule, aqueous solution, alcoholic solution, oily solution, syrup, emulsion suspension pastille suppository, solution for injection or infusion, ointment, tincture, cream, lotion, powder, spray, transdermal therapeutic systems, nasal spray, aerosol mixture, microcapsule, implant, rod or plaster.
- Pharmaceutical compositions of the invention are those which are suitable for oral, rectal, topical, peroral (e.g. sublingual) and parenteral (e.g.
- Suitable enteric coatings include cellulose acetate phthalate, polyvinyl acetate phthalate, hydroxypropyl-methylcellulose phthalate and anionic polymers of methacrylic acid and methyl methacrylate.
- Suitable pharmaceutical compounds for oral administration may be present in separate units as, for example, capsules, cachets, lozenges or tablets, which in each case contain a particular amount of the compound according to formula (I); as powders ( gelatin capsules or cachets) or granules; as solution or suspension in an aqueous or nonaqueous liquid; or as an oil-in-water or water-in-oil emulsion.
- said compositions can be prepared according to any suitable pharmaceutical method which includes a step in which the active compound and the carrier (which may comprise one or more additional components) are contacted.
- compositions are prepared by uniform and homogeneous mixing of the active compound with a liquid and/or finely dispersed solid carrier, after which the product is shaped, if necessary.
- a tablet for example, may be prepared by pressing or shaping a powder or granules of the compound, where appropriate with one or more additional components. Pressed tablets can be prepared by tableting the compound in free-flowing form, for example a powder or granules, mixed, where appropriate, with a binder, lubricant, inert diluent and/or one or more surface active/dispersing agents in a suitable machine.
- Shaped tablets can be prepared by shaping the pulverulent compound, moistened with an inert liquid diluent, in a suitable machine.
- diluents can be used, for example, starch, cellulose, saccharose, lactose or silica.
- the pharmaceutical compositions of the invention may also comprise substances other than diluents, for example one or more lubricants such as magnesium stearate or talc, a coloring, a coating (sugar-coated tablets) or a varnish.
- compositions which are suitable for peroral (sublingual) administration include lozenges which contain a compound according to formula (I) with a flavoring, usually sucrose and gum arabic or tragacanth, and pastilles which comprise the compound in an inert base such as gelatin and glycerol or sucrose and gum arabic.
- Suitable pharmaceutical compositions for parenteral administration preferably comprise sterile aqueous preparations of a compound according to formula (I) which are preferably isotonic with the blood of the intended recipient. These preparations are preferably administered intravenously, although they may also be administered subcutaneously, intramuscularly or intradermally as an injection. Said preparations may preferably be prepared by mixing the compound with water and rendering the obtained solution sterile and isotonic with the blood. Injectable compositions of the invention generally contain from 0.1 to 5% by weight of the active compound.
- sterile compositions for parenteral administration may be preferably solutions which are aqueous or non aqueous, suspensions or emulsions.
- solvent or vehicle there may be used water, propylene glycol, polyethylene glycol, vegetable oils, in particular olive oil, organic esters for injection, for example ethyl oleate or other suitable organic solvents.
- These compositions may also contain adjuvants, in particular wetting, isotonizing, emulsifying, dispersing and stabilizing mediums.
- the sterilization may be carried out in several ways, for example by an aseptic filtration, by incorporating sterilizing agents into the composition, by irradiation or by heating. They may also be prepared in the form of sterile solid compositions which may be dissolved at the time of use in sterile water or in any other sterile medium for injection.
- Suitable pharmaceutical compositions for rectal administration are preferably present as individual dose suppositories. These may be prepared by mixing a compound according to formula (I) with one or more conventional solid carriers, for example cocoa butter, and shaping the resulting mixture.
- Suitable pharmaceutical compositions for topical application to the skin are preferably present as ointment, cream, lotion, paste, spray, aerosol or oil.
- Carriers which may be used are petroleum jelly, lanolin, polyethylene glycols, alcohols and combinations of two or more of these substances.
- the active compound is present at a concentration of from 0.1 to 15%, for example from 0.5 to 2%, by weight of the composition.
- Transdermal administration is also possible.
- Suitable pharmaceutical compositions for transdermal administration may be present as individual patches which are suitable for long-term close contact with the epidermis of the patient.
- patches suitably contain the active compound in an optionally buffered aqueous solution, dissolved and/or dispersed in an adhesive or dispersed in a polymer.
- a suitable active compound concentration is from approx. 1% to 35%, preferably approx. 3% to 15%.
- a particular possibility is the release of the active compound by electro- transport or iontophoresis, as described, for example, in Pharmaceutical Research, 2(6): 318 (1986).
- Gelatin capsules containing a dose of 50 mg of active product and having the following composition are prepared according to the usual technique:
- Tablets containing a dose of 50 mg of active product and having the following composition are prepared according to the usual technique:
- a solution for injection containing 10 mg of active product and having the following composition is prepared:
- Another subject of the present invention is the combination of compounds of the formula (I) with other pharmaceutically active substances not covered by formula (I).
- the compounds of the formula (I) are distinguished by beneficial actions on the metabolism of lipids, and they are particularly suitable for weight reduction and, after weight reduction, for maintaining a reduced weight in mammals and as anorectic agents.
- the compounds are distinguished by their low toxicity and their few side effects.
- the compounds may be employed alone or in combination with other weight- reducing or anorectic active compounds.
- Further anorectic active compounds of this kind are mentioned, for example, in the Rote Liste, Chapter 01 under weight- reducing agents/appetite suppressants, and may also include those active compounds which increase the energy turnover of the organism and thus lead to weight reduction or else those which influence the general metabolism of said organism such that increased calorie intake does not cause an enlargement of the fat depots and a normal calorie intake causes a reduction in the fat depots of said organism.
- the compounds are suitable for the prophylaxis and, in particular, for the treatment of problems of excess weight or obesity.
- the compounds of formula (I) have a beneficial effect on the glucose metabolism, they particularly lower the blood-sugar level and can be used for treatment of type I and type II diabetes.
- the compounds can therefore be used alone or in combination with other blood-sugar lowering active compounds (antidiabetics).
- the compounds of the formula I may be administered in combination with one or more further pharmacologically active substances which may be selected, for example, from the group consisting of antidiabetics, antiadipose agents, blood-pressure-lowering active compounds, lipid reducers and active compounds for the treatment and/or prevention of complications caused by diabetes or associated with diabetes.
- Suitable antidiabetics include insulins, amylin, GLP-1 and GLP-2 derivatives such as, for example, those disclosed by Novo Nordisk A/S in WO 98/08871 and also oral hypoglycemic active compounds.
- Said oral hypoglycemic active compounds preferably include sulfonyl ureas, biguanidines, meglitinides, oxadiazolidinediones, thiazolidinediones, glucosidase inhibitors, glucagon receptor antagonists, GLP-1 agonists, potassium channel openers such as, for example, those disclosed by Novo Nordisk A S in WO 97/26265 and WO 99/03861 , insulin sensitizers, activators of insulin receptor kinase, inhibitors of liver enzymes involved in the stimulation of gluconeogenesis and/or glycogenolysis, for example glycogen phosphorase inhibitors, modulators of glucose uptake and glucose elimination, lipid metabolism-modifying compounds such as antihyperlipidemic active compounds and antilipidemic active compounds, for example HMGCoA-reductase inhibitors, inhibitors of cholesterol transport/cholesterol uptake, inhibitors of the reabsorption of bile acid or inhibitors of microsomal
- the present compounds are administered in combination with insulin.
- the compounds of the invention are administered in combination with a sulfonylurea such as, for example, tolbutamide, glibenclamide, glimepiride, glipizide, gliquidone, glisoxepide, glibornuride or gliclazide.
- a sulfonylurea such as, for example, tolbutamide, glibenclamide, glimepiride, glipizide, gliquidone, glisoxepide, glibornuride or gliclazide.
- the compounds of the present invention are administered in combination with a biguanidine such as, for example, metformin.
- the compounds of the present invention are administered in combination with a meglitinide such as, for example, repaglinide.
- the compounds of the present invention are administered in combination with a thiazolidinedione such as, for example, troglitazone, ciglitazone, pioglitazone, rosiglitazone or the compounds disclosed by Dr. Reddy's Research Foundation in WO 97/41097, in particular 5-[[4-[(3,4-dihydro-3-methyl-4-oxo-2- quinazolinylmethoxy]phenyl]methyl]-2,4-thiazolidinedione.
- a thiazolidinedione such as, for example, troglitazone, ciglitazone, pioglitazone, rosiglitazone or the compounds disclosed by Dr. Reddy's Research Foundation in WO 97/41097, in particular 5-[[4-[(3,4-dihydro-3-methyl-4-oxo-2- quinazolinylmethoxy]phenyl]methyl]-2,4-thiazolidinedione
- the compounds of the present invention are administered in combination with an ⁇ -glucosidase inhibitor such as, for example, miglitol or acarbose.
- an ⁇ -glucosidase inhibitor such as, for example, miglitol or acarbose.
- the compounds of the present invention are administered in combination with an active compound which acts on the ATP-dependent potassium channel of the beta cells, such as, for example, tolbutamide, glibenclamide, glimepiride, glipizide, gliclazide or repaglinide.
- an active compound which acts on the ATP-dependent potassium channel of the beta cells such as, for example, tolbutamide, glibenclamide, glimepiride, glipizide, gliclazide or repaglinide.
- the compounds of the present invention are administered in combination with an antihyperlipidemic active compound or an antilipidemic active compound such as, for example, cholestyramine, colestipol, clofibrate, fenofibrate, gemfibrozil, lovastatin, pravastatin, simvastatin, atorvastatin, cerivastatin, fluvastatin, probucol, ezetimibe or dextrothyroxine.
- an antihyperlipidemic active compound or an antilipidemic active compound such as, for example, cholestyramine, colestipol, clofibrate, fenofibrate, gemfibrozil, lovastatin, pravastatin, simvastatin, atorvastatin, cerivastatin, fluvastatin, probucol, ezetimibe or dextrothyroxine.
- the compounds of the present invention are administered in combination with more than one of the aforementioned compounds, for example in combination with a sulfonylurea and metformin, a sulfonylurea and acarbose, repaglinide and metformin, insulin and a sulfonylurea, insulin and metformin, insulin and troglitazone, insulin and lovastatin, etc.
- the compounds of the invention may be administered in combination with one or more antiadipose agents or appetite-controlling active compounds.
- Such active compounds may be selected from the group consisting of CART agonists, NPY antagonists, MC4 agonists, orexin antagonists, H3 agonists, TNF agonists, CRF agonists, CRF BP antagonists, urocortin agonists, ⁇ 3 agonists, MSH (melanocyte-stimulating hormone) agonists, CCK agonists, serotonin re- uptake inhibitors, mixed serotonin and noradrenalin reuptake inhibitors, 5HT modulators, MAO inhibitors, bombesin agonists, galanin antagonists, growth hormone, growth-hormone-releasing compounds, TRH agonists, uncoupling protein 2 or 3 modulators, leptin agonists, dopamine agonists (bromocriptine, doprexin), lipase/amylase inhibitors, cannabinoid receptor 1 antagonists, modulators of acylation-stimulating protein (ASP), PPAR modulators, RXR modulators
- the antiadipose agent is leptin or modified leptin. In another embodiment, the antiadipose agent is dexamphetamine or amphetamine. In another embodiment, the antiadipose agent is fenfluramine or dexfenfluramine. In yet another embodiment, the antiadipose agent is sibutramine or the mono- and bis-demethylated active metabolite of sibutramine. In another embodiment, the antiadipose agent is orlistate. In another embodiment, the antiadipose agent is mazindol, diethylpropione or phentermine.
- antihypertensive active compounds examples include betablockers such as alprenolol, atenol, timolol, pindolol, propanolol and metoprolol, ACE (angiotensin-converting enzyme) inhibitors such as, for example, benazepril, captopril, enalapril, fosinopril, lisinopril, quinapril and rampril, calcium channel blockers such as nifedipine, felodipine, nicardipine, isradipine, nimodipine, diltiazem and verapamil, and also alphablockers such as doxazosin, urapidil, prazosin and terazosin.
- betablockers such as alprenolol, atenol, timolol, pindolol, propanolol and metoprolol
- ACE angiotens
- the cold (- 75°C) reaction mixture is subsequently added at a cold (-75°C) solution of 18,3 g iodine in 400 ml THF (tetrahydrofuran) and stirred at -75°C for 1.5 h.
- the reaction is quenched by adding 80 ml methanol /THF (1 :1) at -75°C and 300 ml saturated aqueous NaHC0 3 at room temperature and extracted with dichloromethane.
- the organic layer is ished with 5% aqueous Na 2 S 0 3 and saturated sodium chloride solution, dried (MgS0 4 ) and the solvens removed.
- the crude product is purified by chromatography on silica gel to yield 14,4 g of 4-iodo-3-methoxy-6-pyridin-4-yl- pyridazine.
- Argon is passed for 30 min through a suspension of 55 mg 4-iodo-3-methoxy-6- pyridin-4-yl-pyridazine, 55 mg 1-(tert-butoxycarbonyl)indole-2-boronic acid, 53,5 mg potassium carbonate, and 18,5 mg triphenylphosphine in 0,8 ml DME (dimethoxyethane) and 0,7 ml water. 4 mg palladium (II) acetate is added and the mixture stirred under reflux for 3h.
- the solution is diluted into DMF (dimethylformamide)/water and directly purified by HPLC (high performance liquid chromatography) on a RP18 (reversed phase 18) column giving 4,5mg 4-(1 H-lndol-2-yl)-6-pyridin-4-yl-2H-pyridazin-3-one.
- 6-Chloro-4-iodo-3-methoxy-pyridazine is synthesized following a procedure described in the literature (Mojovic, Ljubica; Turek, Alain; Pie, Nelly; Dorsy, Muriel; Ndzi, Bruno; Queguiner, Guy; Tetrahydron, 1995, 52, p10417)
- Argon is passed for 30 min through a suspension of 135 mg 6-chloro-4-iodo-3- methoxy-pyridazine, 130,5 mg 1-(tert-butoxycarbonyl)indole-2-boronic acid, 152 mg potassium carbonate, and 52,5 mg triphenylphosphine in 2,2 ml DME and 1 ,1 ml water. 11 ,2 mg palladium (II) acetate is added and the mixture stirred under reflux for 3h.
- Argon is passed for 30 min through a suspension of 70 mg 6-chloro-4-iodo-3- methoxy-pyridazine, 53 mg 4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenol, 59,4 mg potassium carbonate, and 20,1 mg triphenylphosphine in 0,8 ml DME and 0,7 ml water. 4,4 mg palladium (II) acetate is added and the mixture stirred under reflux for 3h.
- Argon is passed for 30 min through a suspension of 333 mg 4-iodo-3-methoxy-6- pyridin-4-yl-pyridazine, 380 mg 3-methyl-2-(4,4,5,5-tetramethyl-[1 ,3,2]dioxa- borolan-2-yl)-indole-1 -carboxylic acid tert- butyl ester, 294 mg potassium carbonate, and 111 ,6 mg triphenylphosphine in 4,8 ml DME and 2,4 ml water. 24 mg palladium (II) acetate is added and the mixture stirred under reflux for 3h.
- a solution of 23.5 ml acetaldehyde in 50 ml tetrahydrofuran is cooled to -75°C and added to the reaction.
- the solution is stirred at -75°C for 90 min, then a mixture of 25 ml concentrated aqueous HCI, 100 ml ethanol and 125 ml tetrahydrofuran is added and the mixture is allowed to warm to room temperature.
- 60 ml of saturated aqueous sodium bicarbonate is slowly added, and the tetrahydrofuran is removed under reduced pressure.
- the resulting aqueous phase is extracted 3 times with dichloromethane.
- the organic phase is dried (MgS0 ), filtered and evaporated under reduced pressure.
- Argon is passed for 30 min through a suspension of 270 mg 3-iodo-2-(3-methoxy- 6-pyridin-4-yl-pyridazin-4-yl)-1 H-indole TFA (trifluoroacetic acid) salt, 73 mg phenylboronic Acid, 220 mg potassium carbonate and 52.4 mg triphenylphosphine in 2 ml DME and 1 ml water. 11 ,2 mg palladium (II) acetate is added and the mixture stirred under reflux for 5h. The product is isolated by extraction with ethyl acetate and purified by chromatography on silica gel. Yield 64 mg LC-MS (ES+) 379 (M+H)+.
- the reaction is acidified with 4 mL of a solution made from 10% aqueous KHS0 (60 mL) and concentrated H 2 S0 4 (2 mL). After stirring for 15 min, the layers are separated and the organics ished with water (15 mL) and brine (15 mL) successively, dried (Na2S0 ), and the solvent removed under reduced pressure to yield a partially solidified material. The material is ished with hot hexanes (5 mL) and filtered.
- a solution of 65.9 ml propionaldehyde in 200 ml tetrahydrofuran is cooled to -75°C and added to the reaction.
- the solution is stirred at -75°C for 90 min, then a mixture of 55 ml concentrated aqueous HCI, 220 ml ethanol and 275 ml tetrahydrofuran is added and the mixture is allowed to warm to room temperature.
- 150 ml of saturated aqueous sodium bicarbonate is slowly added, and the tetrahydrofuran is removed under reduced pressure.
- the resulting aqueous phase is extracted 3 times with dichloromethane.
- the organic phase is dried (MgS0 4 ), filtered and evaporated under reduced pressure.
- Example 26 is synthesized similarly to example 25 by using NCS (N- chlorosuccinimid) instead of NIS Yield 42 mg.
- Example 27 6-Pyr idi n-4-y l-4-(3-vi ny 1-1 H-i ndol-2-yl)-2H-pyr idazi n-3-one
- 6-(2-Piperidylethyl)-2-(3-methoxy-6-pyridin-4-yl-pyridazin-4-yl)-indole-1 - carboxylic acid tert-butyl ester 54 mg of a mixture of 6-(2-oxoethyl)-2-(3-methoxy-6-pyridin-4-yl-pyridazin-4-yl)- indole-1 -carboxylic acid tert-butyl ester and 6-(2-oxoethyl)-2-(3-oxo-6-pyridin-4-yl- 2,3-dihydro-pyridazin-4-yl)indole-1 -carboxylic acid tert-butyl ester are dissolved in 9 mL dichloroethane.
- This compound is prepared as described for example 9c by using 1 - pentenylboronic Acid
- reaction mixture is stirred for 60 minutes at -78 °C, allowed to warm to 0°C and quenched by addition of sat. aq. NH 4 CI-solution.
- the reaction mixture is diluted with dichloromethane, washed with sat. aq. NH 4 CI-solution and brine, dried (MgS0 4 ) and concentrated in vacuo. 1.96 g (97%) crude 6-formyl-2-[6-(4-hydroxy-3,5-dimethyl-phenyl)-3-methoxy- pyridazin-4-yl]-indole-1 -carboxylic acid tert-butyl ester are obtained.
- n-butyllithium solution (1.6M in hexane) is added at -75°C to a 0.5 M solution of zinc chloride in THF and the resulting mixture stirred for 30 min at room temperature.
- This solution is added via a syringe to a solution of 107 mg 3-iodo-2- (3-methoxy-6-pyridin-4-yl-pyridazin-4-yl)-1 H-indole (9b) and 10mg 1 ,1 '- bis(diphenylphosphino)ferrocene-palladium(ll)dichloride (1 :1 complex with CH 2 CI 2 ) in THF and stirred for 4 h at 70°C.
- reaction solution is filtered through a silica gel cartridge, eluting with dichloromethane. The solvent is removed under reduced pressure.
- product is purified by preparative RP-HPLC eluting with a gradient of 0-100% acetonitrile in water (+0.01% trifluoroacetic acid). Yield 142 mg.
- This compound is prepared from 500 mg of 6-chloro-4-iodo-3-methoxy-pyridazine analogously to example 2. Yield 200 mg.
- reaction mixture is stirred for 1 hour at 0°C, quenched with water/1 N HCI (5.5 mL each) and stirred until two clear phases appear.
- the aq. phase is extracted twice with EtOAc, the combined org. phases dried (MgS0 4 ) and the solvent removed in vacuo. This affords 1.45 g (92%) crude product.
- Example 138 4-(3-Bromo-1H-indol-2-yl)-6-(4-hydroxy-3,5-dimethyl-phenyl)-2H-pyridazin-3- one
- Example 140 4-[3-(2-Methoxy-pyridin-3-yl)-1H-indol-2-yl]-6-pyridin-4-yl-2H-pyridazin-3-one
- Example 165 is prepared analogously example 164
- This compound is prepared, using 48 mg of 2-(3-oxo-6-pyridin-4-yl-2,3-dihydro- pyridazin-4-yl)-1 H-indole-5-carboxylic acid, analogously to example 174. Yield 37 mg.
- Example 1 8 4-[3-(1 -Methyl-1 H-pyrazol-4-yl)-1 H-indol-2-yl]-6-(4-methyl-thiophen-3-yl)-2H- pyridazin-3-one
- Example 192 4-[6-(3-Aza-bicyclo[3.2.2]non-3-ylmethyl)-1 H-indol-2-yl]-6-(4-hydroxy-3,5- dimethyl-phenyl)-2H-pyridazin-3-one
- Example 194 4-[3-(1 -Methyl-1 H-pyrazol-4-yl)-1 H-indol-2-yl]-6-thiophen-3-yl-2H-pyridazin-3- one
- GSK- ⁇ activity is measured using human recombinant GSK-3 ⁇ and a primed (pre- phosphorylated) substrate peptide (derived from glycogen synthase and containing the phosphorylation sites 3a, b, and c) on basis of the AlphaScreen technology in 384-well plate format (small volume plate, white, GREINER).
- a primed substrate peptide derived from glycogen synthase and containing the phosphorylation sites 3a, b, and c
- 384-well plate format small volume plate, white, GREINER
- phospho-glycogen synthase peptide 34 nM in kinase buffer (20 mM Hepes, pH 7,4, 10 mM MgCI 2 , 200 mM EDTA (ethylenediaminotetraacetate), 1 mM DTT (dithiotriol), 0,1 mg/ml BSA (bovine serum albumin), 10 ⁇ M ATP (adenosinetriphosphate)) are incubated at room temperature for 60 min.
Landscapes
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Health & Medical Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Diabetes (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Immunology (AREA)
- Endocrinology (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Emergency Medicine (AREA)
- Child & Adolescent Psychology (AREA)
- Psychology (AREA)
- Vascular Medicine (AREA)
- Urology & Nephrology (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Reproductive Health (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
Claims
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05745652.7A EP1753743B1 (en) | 2004-05-18 | 2005-05-17 | Pyridazinone derivatives, methods for producing them and their use as pharmaceuticals |
| JP2007517071A JP2007538035A (en) | 2004-05-18 | 2005-05-17 | Pyridazinone derivatives, processes for their preparation and their use as medicaments |
| US11/552,283 US7709466B2 (en) | 2004-05-18 | 2006-10-24 | Pyridazinone derivatives, methods for their production and their use as pharmaceuticals |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP04011734A EP1604988A1 (en) | 2004-05-18 | 2004-05-18 | Pyridazinone derivatives, methods for producing them and their use as pharmaceuticals |
| EP04011734.3 | 2004-05-18 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/552,283 Continuation US7709466B2 (en) | 2004-05-18 | 2006-10-24 | Pyridazinone derivatives, methods for their production and their use as pharmaceuticals |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2005111018A1 true WO2005111018A1 (en) | 2005-11-24 |
Family
ID=34925032
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2005/005346 WO2005111018A1 (en) | 2004-05-18 | 2005-05-17 | Pyridazinone derivatives, methods for producing them and their use as pharmaceuticals |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US7709466B2 (en) |
| EP (2) | EP1604988A1 (en) |
| JP (1) | JP2007538035A (en) |
| AR (1) | AR049103A1 (en) |
| TW (1) | TW200607804A (en) |
| UY (1) | UY28902A1 (en) |
| WO (1) | WO2005111018A1 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008016123A1 (en) * | 2006-08-03 | 2008-02-07 | Takeda Pharmaceutical Company Limited | GSK-3β INHIBITOR |
| WO2009021740A2 (en) | 2007-08-15 | 2009-02-19 | Sanofis-Aventis | Substituted tetrahydronaphthalenes, process for the preparation thereof and the use thereof as medicaments |
| WO2009032124A1 (en) * | 2007-08-29 | 2009-03-12 | Schering Corporation | Substituted indole derivatives and methods of use thereof |
| WO2010003624A2 (en) | 2008-07-09 | 2010-01-14 | Sanofi-Aventis | Heterocyclic compounds, processes for their preparation, medicaments comprising these compounds, and the use thereof |
| WO2010068601A1 (en) | 2008-12-08 | 2010-06-17 | Sanofi-Aventis | A crystalline heteroaromatic fluoroglycoside hydrate, processes for making, methods of use and pharmaceutical compositions thereof |
| WO2011023754A1 (en) | 2009-08-26 | 2011-03-03 | Sanofi-Aventis | Novel crystalline heteroaromatic fluoroglycoside hydrates, pharmaceuticals comprising these compounds and their use |
| WO2011107494A1 (en) | 2010-03-03 | 2011-09-09 | Sanofi | Novel aromatic glycoside derivatives, medicaments containing said compounds, and the use thereof |
| WO2011157827A1 (en) | 2010-06-18 | 2011-12-22 | Sanofi | Azolopyridin-3-one derivatives as inhibitors of lipases and phospholipases |
| WO2011161030A1 (en) | 2010-06-21 | 2011-12-29 | Sanofi | Heterocyclic substituted methoxyphenyl derivatives having an oxo group, method for producing same, and use thereof as gpr40 receptor modulators |
| WO2012004270A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | Spirocyclically substituted 1,3-propane dioxide derivatives, methods for the production thereof and use of the same as medicament |
| WO2012004269A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | (2-aryloxy-acetylamino)-phenyl-propionic acid derivatives, method for producing same and use thereof as pharmaceuticals |
| WO2012010413A1 (en) | 2010-07-05 | 2012-01-26 | Sanofi | Aryloxy-alkylene substituted hydroxyphenyl hexynoic acids, methods for the production thereof and use of the same as medicament |
| US8143305B2 (en) | 2007-08-29 | 2012-03-27 | Schering Corporation | 2,3-substituted indole derivatives for treating viral infections |
| US8207216B2 (en) | 2006-12-19 | 2012-06-26 | The Board Of Trustees Of The University Of Illinois | Benzofuran-3-yl(indol-3-yl) maleimides as potent GSK3 inhibitors |
| WO2012120055A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120052A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives substituted with carbocycles or heterocycles, method for producing same, drugs containing said compounds, and use thereof |
| WO2012120053A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Branched oxathiazine derivatives, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120056A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Tetrasubstituted oxathiazine derivatives, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| WO2012120054A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| US8268803B2 (en) | 2006-12-22 | 2012-09-18 | Merck Sharp & Dohme Corp. | 5, 6-ring annulated indole derivatives and use thereof |
| US8377928B2 (en) | 2007-11-16 | 2013-02-19 | Merck Sharp & Dohme Corp. | 3-aminosulfonyl substituted indole derivatives and methods of use thereof |
| EP2567959A1 (en) | 2011-09-12 | 2013-03-13 | Sanofi | 6-(4-Hydroxy-phenyl)-3-styryl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013037390A1 (en) | 2011-09-12 | 2013-03-21 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| US8404845B2 (en) | 2007-08-29 | 2013-03-26 | Merck Sharp & Dohme Corp. | 2,3-substituted azaindole derivatives for treating viral infections |
| WO2013045413A1 (en) | 2011-09-27 | 2013-04-04 | Sanofi | 6-(4-hydroxy-phenyl)-3-alkyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013083991A1 (en) * | 2011-12-06 | 2013-06-13 | Imperial Innovations Limited | Novel compounds and their use in therapy |
| US8546420B2 (en) | 2006-12-22 | 2013-10-01 | Merck Sharp & Dohme Corp. | 4, 5-ring annulated indole derivatives for treating or preventing of HCV and related viral infections |
| US8557848B2 (en) | 2006-12-22 | 2013-10-15 | Merck Sharp & Dohme Corp. | 4,5-ring annulated indole derivatives for treating or preventing of HCV and related viral infections |
| US8765757B2 (en) | 2007-11-16 | 2014-07-01 | Merck Sharp & Dohme Corp. | 3-heterocyclic substituted indole derivatives and methods of use thereof |
| US8901139B2 (en) | 2008-06-13 | 2014-12-02 | Merck Sharp & Dohme Corp. | Tricyclic indole derivatives and methods of use thereof |
| US20180185362A1 (en) * | 2016-12-29 | 2018-07-05 | Viamet Pharmaceuticals (NC), Inc. | Metalloenzyme inhibitor compounds |
| US10538511B2 (en) | 2016-12-29 | 2020-01-21 | Selenity Therapeutics (Bermuda), Ltd. | Metalloenzyme inhibitor compounds |
| US11787785B2 (en) | 2021-03-15 | 2023-10-17 | Novartis Ag | Pyrazolopyridine derivatives and uses thereof |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016057931A1 (en) | 2014-10-10 | 2016-04-14 | The Research Foundation For The State University Of New York | Trifluoromethoxylation of arenes via intramolecular trifluoromethoxy group migration |
| TW202332436A (en) | 2017-04-18 | 2023-08-16 | 美商塞爾基因定量細胞研究公司 | Therapeutic compounds |
| WO2019002183A1 (en) * | 2017-06-26 | 2019-01-03 | Merck Patent Gmbh | Method for producing substituted nitrogen-containing heterocycles |
| CN110506708B (en) * | 2019-09-24 | 2021-12-17 | 深圳大学 | Mouse model for Alzheimer's disease and evaluation method |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1997026265A1 (en) | 1996-01-17 | 1997-07-24 | Novo Nordisk A/S | Fused 1,2,4-thiadiazine and fused 1,4-thiazine derivatives, their preparation and use |
| JPH09216883A (en) | 1996-02-09 | 1997-08-19 | Fujisawa Pharmaceut Co Ltd | Pyrazolopyridine compound and medicine containing the same compound |
| WO1997041097A2 (en) | 1996-12-31 | 1997-11-06 | Dr. Reddy's Research Foundation | Novel heterocyclic compounds process for their preparation and pharmaceutical compositions containing them and their use in the treatment of diabetes and related diseases |
| WO1998008871A1 (en) | 1996-08-30 | 1998-03-05 | Novo Nordisk A/S | Glp-1 derivatives |
| WO1999003861A1 (en) | 1997-07-16 | 1999-01-28 | Novo Nordisk A/S | Fused 1,2,4-thiadiazine derivatives, their preparation and use |
| WO2002022605A1 (en) * | 2000-09-15 | 2002-03-21 | Vertex Pharmaceuticals Incorporated | Pyrazole compounds useful as protein kinase inhibitors |
| WO2002066020A2 (en) * | 2001-02-21 | 2002-08-29 | Can-Fite Biopharma Ltd. | Modulation of gsk-3beta activity and its different uses |
| US20020119963A1 (en) * | 2000-07-31 | 2002-08-29 | Sanner Mark A. | Imidazole derivatives |
| WO2003020276A1 (en) | 2001-08-30 | 2003-03-13 | Merck & Co., Inc. | Tyrosine kinase inhibitors |
| WO2003059891A1 (en) | 2002-01-18 | 2003-07-24 | Pharmacia Corporation | Substituted pyridazinones as inhibitors of p38 |
| WO2004046117A1 (en) | 2002-11-19 | 2004-06-03 | Aventis Pharma Deutschland Gmbh | Pyridazinone derivatives as gsk-3beta inhibitors |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HU190412B (en) | 1981-09-17 | 1986-09-29 | Warner-Lambert Co,Us | Process for producing substituted 4,5-dihiydro-6-bracket-substituted-bracket closed-phenyl-3-bracket-2h-bracket closed-pyridazinones and 6-bracket-substituted-bracket closed-phenyl-3-bracket-2h-bracket closed-pyridazinones |
| US4353905A (en) | 1981-09-17 | 1982-10-12 | Warner-Lambert Company | Substituted 4,5-dihydro-6-[4-(1H-imidazol-1-yl)phenyl]-3(2H)-pyridazinones and 6-[4-(1H-imidazol-1-yl)phenyl]-3(2H)-pyridazinones |
| US4734415A (en) | 1982-08-13 | 1988-03-29 | Warner-Lambert Company | Substituted 4,5-dihydro-6-(substituted)-phenyl-3(2H)-pyridazinones and 6-(substituted) phenyl-3(2H)-pyridazinones |
| DE4324580A1 (en) | 1993-07-22 | 1995-01-26 | Thomae Gmbh Dr K | Bicyclic heterocycles, pharmaceutical compositions containing them and methods for their preparation |
| CA2307111C (en) * | 1997-11-19 | 2009-06-30 | Kowa Co., Ltd. | Novel pyridazine derivatives and drugs containing the same as the active ingredient |
| RU2328495C2 (en) * | 2003-03-07 | 2008-07-10 | Кова Ко., Лтд. | Benzofuran derivatives |
| ES2232306B1 (en) * | 2003-11-10 | 2006-08-01 | Almirall Prodesfarma, S.A. | NEW DERIVATIVES OF PIRIDAZIN-3 (2H) -ONA. |
| EP1714654A4 (en) * | 2004-02-09 | 2011-02-16 | Nissan Chemical Ind Ltd | Drug for inhibiting vascular intimal hyperplasia |
| DE102004010207A1 (en) * | 2004-03-02 | 2005-09-15 | Aventis Pharma S.A. | New 4-benzimidazolyl-3(2H)-pyridazinone derivatives are kinase inhibitors, especially useful for treatment of cancer |
| DE102004010194A1 (en) | 2004-03-02 | 2005-10-13 | Aventis Pharma Deutschland Gmbh | 4-Benzimidazol-2-yl-pyridazin-3-one derivatives, their preparation and use in medicaments |
| NZ565190A (en) * | 2005-07-21 | 2010-02-26 | Hoffmann La Roche | Pyridazinone derivatives as thyroid hormone receptor agonists |
-
2004
- 2004-05-18 EP EP04011734A patent/EP1604988A1/en not_active Withdrawn
-
2005
- 2005-05-17 JP JP2007517071A patent/JP2007538035A/en not_active Abandoned
- 2005-05-17 WO PCT/EP2005/005346 patent/WO2005111018A1/en not_active Application Discontinuation
- 2005-05-17 TW TW094115844A patent/TW200607804A/en unknown
- 2005-05-17 UY UY28902A patent/UY28902A1/en not_active Application Discontinuation
- 2005-05-17 AR ARP050102027A patent/AR049103A1/en unknown
- 2005-05-17 EP EP05745652.7A patent/EP1753743B1/en not_active Not-in-force
-
2006
- 2006-10-24 US US11/552,283 patent/US7709466B2/en not_active Expired - Fee Related
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1997026265A1 (en) | 1996-01-17 | 1997-07-24 | Novo Nordisk A/S | Fused 1,2,4-thiadiazine and fused 1,4-thiazine derivatives, their preparation and use |
| JPH09216883A (en) | 1996-02-09 | 1997-08-19 | Fujisawa Pharmaceut Co Ltd | Pyrazolopyridine compound and medicine containing the same compound |
| WO1998008871A1 (en) | 1996-08-30 | 1998-03-05 | Novo Nordisk A/S | Glp-1 derivatives |
| WO1997041097A2 (en) | 1996-12-31 | 1997-11-06 | Dr. Reddy's Research Foundation | Novel heterocyclic compounds process for their preparation and pharmaceutical compositions containing them and their use in the treatment of diabetes and related diseases |
| WO1999003861A1 (en) | 1997-07-16 | 1999-01-28 | Novo Nordisk A/S | Fused 1,2,4-thiadiazine derivatives, their preparation and use |
| US20020119963A1 (en) * | 2000-07-31 | 2002-08-29 | Sanner Mark A. | Imidazole derivatives |
| WO2002022605A1 (en) * | 2000-09-15 | 2002-03-21 | Vertex Pharmaceuticals Incorporated | Pyrazole compounds useful as protein kinase inhibitors |
| WO2002066020A2 (en) * | 2001-02-21 | 2002-08-29 | Can-Fite Biopharma Ltd. | Modulation of gsk-3beta activity and its different uses |
| WO2003020276A1 (en) | 2001-08-30 | 2003-03-13 | Merck & Co., Inc. | Tyrosine kinase inhibitors |
| WO2003059891A1 (en) | 2002-01-18 | 2003-07-24 | Pharmacia Corporation | Substituted pyridazinones as inhibitors of p38 |
| WO2004046117A1 (en) | 2002-11-19 | 2004-06-03 | Aventis Pharma Deutschland Gmbh | Pyridazinone derivatives as gsk-3beta inhibitors |
Non-Patent Citations (10)
| Title |
|---|
| "Organic Reactions", JOHN WILEY & SONS |
| "Remington: The Science and Practice of Pharmacy", 1995, MACK PUBLISHING CO. |
| C. CHEN; D. LIEBERMAN; R. D. LARSEN; T. R. VERHOEVEN; P. J. REIDER, J. ORG. CHEM., vol. 62, 1997, pages 2676 - 2677 |
| HENRIKSON, AM. J. PHYSIOL., vol. 284, 2003, pages 892 - 900 |
| HOUBEN-WEYL: "Methoden der Organischen Chemie", THIEME-VERLAG |
| I. PARROT ET AL., SYNTHESIS, vol. 7, 1999, pages 1163 - 1168 |
| MOJOVIC, LJUBICA; TUREK, ALAIN; PLE, NELLY; DORSY, MURIEL; NDZI, BRUNO; QUEGUINER, GUY, TETRAHYDRON, vol. 52, 1995, pages 10417 |
| PATENT ABSTRACTS OF JAPAN vol. 1997, no. 12 25 December 1997 (1997-12-25) * |
| PHARMACEUTICAL RESEARCH, vol. 2, no. 6, 1986, pages 318 |
| S.E. NIKOULINA, DIABETES, vol. 51, 2002, pages 2190 - 2198 |
Cited By (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008016123A1 (en) * | 2006-08-03 | 2008-02-07 | Takeda Pharmaceutical Company Limited | GSK-3β INHIBITOR |
| US8492378B2 (en) | 2006-08-03 | 2013-07-23 | Takeda Pharmaceutical Company Limited | GSK-3β inhibitor |
| US8207216B2 (en) | 2006-12-19 | 2012-06-26 | The Board Of Trustees Of The University Of Illinois | Benzofuran-3-yl(indol-3-yl) maleimides as potent GSK3 inhibitors |
| US8557848B2 (en) | 2006-12-22 | 2013-10-15 | Merck Sharp & Dohme Corp. | 4,5-ring annulated indole derivatives for treating or preventing of HCV and related viral infections |
| US8546420B2 (en) | 2006-12-22 | 2013-10-01 | Merck Sharp & Dohme Corp. | 4, 5-ring annulated indole derivatives for treating or preventing of HCV and related viral infections |
| US8268803B2 (en) | 2006-12-22 | 2012-09-18 | Merck Sharp & Dohme Corp. | 5, 6-ring annulated indole derivatives and use thereof |
| WO2009021740A2 (en) | 2007-08-15 | 2009-02-19 | Sanofis-Aventis | Substituted tetrahydronaphthalenes, process for the preparation thereof and the use thereof as medicaments |
| US8143305B2 (en) | 2007-08-29 | 2012-03-27 | Schering Corporation | 2,3-substituted indole derivatives for treating viral infections |
| WO2009032124A1 (en) * | 2007-08-29 | 2009-03-12 | Schering Corporation | Substituted indole derivatives and methods of use thereof |
| US8614229B2 (en) | 2007-08-29 | 2013-12-24 | Merck Sharp & Dohme Corp. | Substituted indole derivatives and methods of use thereof |
| US8404845B2 (en) | 2007-08-29 | 2013-03-26 | Merck Sharp & Dohme Corp. | 2,3-substituted azaindole derivatives for treating viral infections |
| US8765757B2 (en) | 2007-11-16 | 2014-07-01 | Merck Sharp & Dohme Corp. | 3-heterocyclic substituted indole derivatives and methods of use thereof |
| US8377928B2 (en) | 2007-11-16 | 2013-02-19 | Merck Sharp & Dohme Corp. | 3-aminosulfonyl substituted indole derivatives and methods of use thereof |
| US8901139B2 (en) | 2008-06-13 | 2014-12-02 | Merck Sharp & Dohme Corp. | Tricyclic indole derivatives and methods of use thereof |
| WO2010003624A2 (en) | 2008-07-09 | 2010-01-14 | Sanofi-Aventis | Heterocyclic compounds, processes for their preparation, medicaments comprising these compounds, and the use thereof |
| WO2010068601A1 (en) | 2008-12-08 | 2010-06-17 | Sanofi-Aventis | A crystalline heteroaromatic fluoroglycoside hydrate, processes for making, methods of use and pharmaceutical compositions thereof |
| WO2011023754A1 (en) | 2009-08-26 | 2011-03-03 | Sanofi-Aventis | Novel crystalline heteroaromatic fluoroglycoside hydrates, pharmaceuticals comprising these compounds and their use |
| WO2011107494A1 (en) | 2010-03-03 | 2011-09-09 | Sanofi | Novel aromatic glycoside derivatives, medicaments containing said compounds, and the use thereof |
| WO2011157827A1 (en) | 2010-06-18 | 2011-12-22 | Sanofi | Azolopyridin-3-one derivatives as inhibitors of lipases and phospholipases |
| WO2011161030A1 (en) | 2010-06-21 | 2011-12-29 | Sanofi | Heterocyclic substituted methoxyphenyl derivatives having an oxo group, method for producing same, and use thereof as gpr40 receptor modulators |
| WO2012004270A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | Spirocyclically substituted 1,3-propane dioxide derivatives, methods for the production thereof and use of the same as medicament |
| WO2012010413A1 (en) | 2010-07-05 | 2012-01-26 | Sanofi | Aryloxy-alkylene substituted hydroxyphenyl hexynoic acids, methods for the production thereof and use of the same as medicament |
| WO2012004269A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | (2-aryloxy-acetylamino)-phenyl-propionic acid derivatives, method for producing same and use thereof as pharmaceuticals |
| WO2012120054A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120056A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Tetrasubstituted oxathiazine derivatives, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| WO2012120053A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Branched oxathiazine derivatives, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120052A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives substituted with carbocycles or heterocycles, method for producing same, drugs containing said compounds, and use thereof |
| WO2012120055A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| EP2567959A1 (en) | 2011-09-12 | 2013-03-13 | Sanofi | 6-(4-Hydroxy-phenyl)-3-styryl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013037390A1 (en) | 2011-09-12 | 2013-03-21 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013045413A1 (en) | 2011-09-27 | 2013-04-04 | Sanofi | 6-(4-hydroxy-phenyl)-3-alkyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013083991A1 (en) * | 2011-12-06 | 2013-06-13 | Imperial Innovations Limited | Novel compounds and their use in therapy |
| US20180185362A1 (en) * | 2016-12-29 | 2018-07-05 | Viamet Pharmaceuticals (NC), Inc. | Metalloenzyme inhibitor compounds |
| WO2018125800A2 (en) | 2016-12-29 | 2018-07-05 | Viamet Pharmaceuticals (Bermuda), Ltd. | Metalloenzyme inhibitor compounds |
| US10085984B2 (en) * | 2016-12-29 | 2018-10-02 | Viamet Pharmaceuticals (Bermuda), Ltd. | Metalloenzyme inhibitor compounds |
| US10538511B2 (en) | 2016-12-29 | 2020-01-21 | Selenity Therapeutics (Bermuda), Ltd. | Metalloenzyme inhibitor compounds |
| EP3562487A4 (en) * | 2016-12-29 | 2020-08-26 | Selenity Therapeutics (Bermuda), Ltd. | Metalloenzyme inhibitor compounds |
| US11040034B2 (en) | 2016-12-29 | 2021-06-22 | Phasebio Pharmaceuticals, Inc. | Metalloenzyme inhibitor compounds |
| US11136309B2 (en) | 2016-12-29 | 2021-10-05 | Phasebio Pharmaceuticals, Inc. | Metalloenzyme inhibitor compounds |
| US11919883B2 (en) | 2016-12-29 | 2024-03-05 | Ji Xing Pharmaceuticals Hong Kong Limited | Metalloenzyme inhibitor compounds |
| US11787785B2 (en) | 2021-03-15 | 2023-10-17 | Novartis Ag | Pyrazolopyridine derivatives and uses thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1753743A1 (en) | 2007-02-21 |
| TW200607804A (en) | 2006-03-01 |
| US7709466B2 (en) | 2010-05-04 |
| UY28902A1 (en) | 2005-12-30 |
| EP1753743B1 (en) | 2015-03-04 |
| AR049103A1 (en) | 2006-06-28 |
| US20070088041A1 (en) | 2007-04-19 |
| EP1604988A1 (en) | 2005-12-14 |
| JP2007538035A (en) | 2007-12-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1753743B1 (en) | Pyridazinone derivatives, methods for producing them and their use as pharmaceuticals | |
| US7727989B2 (en) | 4-benzimidazol-2-yl-pyridazine-3-one-derivatives, production and use thereof in medicaments | |
| US7968546B2 (en) | Pyridazinone kinase inhibitors | |
| EP1581505B1 (en) | Pyridazinone derivatives as gsk-3beta inhibitors | |
| US7754713B2 (en) | 4-benzimidazol-2-ylpyridazin-3-one derivatives | |
| KR20040016828A (en) | Indazolyl-substituted pyrroline compounds as kinase inhibitors | |
| EP2250169A1 (en) | Pyrido [4, 3-d]pyrimidinone derivatives as kinase inhibitors | |
| EP2459558A2 (en) | Compounds and compositions as syk kinase inhibitors | |
| US20040176377A1 (en) | Novel pyridazinone derivatives as pharmaceuticals and pharmaceutical compositions containing them | |
| CN117430606A (en) | Sulfonamide PARP7 inhibitors | |
| MXPA06009874A (en) | Novel 4-benzimidazol-2-ylpyridazin-3-one derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 11552283 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005745652 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007517071 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Country of ref document: DE |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005745652 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 11552283 Country of ref document: US |