WO2005035514A2 - Modulators of atp-binding cassette transporters containing cycloalkyl or pyranyl groups - Google Patents
Modulators of atp-binding cassette transporters containing cycloalkyl or pyranyl groups Download PDFInfo
- Publication number
- WO2005035514A2 WO2005035514A2 PCT/US2004/033367 US2004033367W WO2005035514A2 WO 2005035514 A2 WO2005035514 A2 WO 2005035514A2 US 2004033367 W US2004033367 W US 2004033367W WO 2005035514 A2 WO2005035514 A2 WO 2005035514A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- ring
- aliphatic
- phenyl
- disease
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/87—Benzo [c] furans; Hydrogenated benzo [c] furans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/38—Heterocyclic compounds having sulfur as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/02—Drugs for disorders of the urinary system of urine or of the urinary tract, e.g. urine acidifiers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/04—Artificial tears; Irrigation solutions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
- A61P5/16—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4 for decreasing, blocking or antagonising the activity of the thyroid hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/18—Drugs for disorders of the endocrine system of the parathyroid hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/10—Antioedematous agents; Diuretics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/16—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
- C07C233/17—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
- C07C233/22—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom having the carbon atom of the carboxamide group bound to an acyclic carbon atom of a carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/57—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of rings other than six-membered aromatic rings
- C07C233/60—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of rings other than six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/64—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings
- C07C233/65—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atoms of the carboxamide groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/64—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings
- C07C233/67—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
- C07C233/68—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
- C07C233/73—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom of a carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/32—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton containing six-membered aromatic rings
- C07C235/34—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton containing six-membered aromatic rings having the nitrogen atoms of the carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/42—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/44—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring
- C07C235/46—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring having the nitrogen atoms of the carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/42—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/44—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring
- C07C235/48—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring having the nitrogen atom of at least one of the carboxamide groups bound to an acyclic carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C311/00—Amides of sulfonic acids, i.e. compounds having singly-bound oxygen atoms of sulfo groups replaced by nitrogen atoms, not being part of nitro or nitroso groups
- C07C311/15—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings
- C07C311/16—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the sulfonamide groups bound to hydrogen atoms or to an acyclic carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C311/00—Amides of sulfonic acids, i.e. compounds having singly-bound oxygen atoms of sulfo groups replaced by nitrogen atoms, not being part of nitro or nitroso groups
- C07C311/15—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings
- C07C311/16—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the sulfonamide groups bound to hydrogen atoms or to an acyclic carbon atom
- C07C311/17—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the sulfonamide groups bound to hydrogen atoms or to an acyclic carbon atom to an acyclic carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C323/00—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups
- C07C323/50—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton
- C07C323/51—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton having the sulfur atoms of the thio groups bound to acyclic carbon atoms of the carbon skeleton
- C07C323/60—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton having the sulfur atoms of the thio groups bound to acyclic carbon atoms of the carbon skeleton with the carbon atom of at least one of the carboxyl groups bound to nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/30—Indoles; Hydrogenated indoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to carbon atoms of the hetero ring
- C07D209/42—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/81—Amides; Imides
- C07D213/82—Amides; Imides in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/48—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
- C07D215/54—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen attached in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D217/00—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems
- C07D217/22—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the nitrogen-containing ring
- C07D217/26—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/54—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings condensed with carbocyclic rings or ring systems
- C07D231/56—Benzopyrazoles; Hydrogenated benzopyrazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D237/00—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings
- C07D237/26—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings condensed with carbocyclic rings or ring systems
- C07D237/28—Cinnolines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D261/00—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings
- C07D261/20—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/56—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D307/68—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/78—Benzo [b] furans; Hydrogenated benzo [b] furans
- C07D307/82—Benzo [b] furans; Hydrogenated benzo [b] furans with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the hetero ring
- C07D307/84—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
- C07D307/85—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen attached in position 2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/30—Hetero atoms other than halogen
- C07D333/32—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/52—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes
- C07D333/62—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the hetero ring
- C07D333/68—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
- C07D333/70—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen attached in position 2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D407/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00
- C07D407/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings
- C07D407/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/06—Systems containing only non-condensed rings with a five-membered ring
- C07C2601/08—Systems containing only non-condensed rings with a five-membered ring the ring being saturated
Definitions
- the present invention relates to modulators of ATP- Binding Cassette (“ABC”) transporters or fragments thereof, including CF Transmembrane Regulator (“CFTR”), compositions thereof, and methods therewith.
- the present invention also relates to methods of treating ABC transporter mediated diseases using such modulators.
- ABC transporters are a group of membrane transporter proteins that play a major role in the transport and protection of cells against a wide variety of pharmacological agents, potentially toxic drugs, and xenobiotics.
- ABC transporters are homologous membrane proteins that bind and use cellular adenosine triphosphate (ATP) for their specific activities.
- Some of these transporters were discovered as multidrug resistance proteins (like the MDR1-P glycoprotein, or the multidrug resistance protein, MRP1) , defending malignant cancer cells against chemotherapeutic agents.
- MRP1-P glycoprotein or the multidrug resistance protein, MRP1
- defending malignant cancer cells against chemotherapeutic agents Up until the present time, 48 Human ABC Transporters have been identified, and these have been arranged into 7 families based on their sequence identity and function.
- ABC transporters play a variety of important physiological roles within the body, as well as providing a defense against harmful compounds from the environment . Moreover they represent important potential drug targets both in their own right, as well as, because in many cases therapeutic drugs are also transported out of the target cell by these molecules.
- One of the members of the ABC transporter family namely, CFTR, is believed be the chloride channel responsible for cAMP-mediated chloride secretion in epithelial cells, and to play a key role in the secretion of chloride and maintenance of normal electrolyte transport throughout the body.
- CFTR is a protein of approximately 1480 amino acids made up of two repeated elements, each comprising six transmembrane segments and a nucleotide-binding domain.
- CFTR cystic fibrosis
- the other elements include the epithelial Na + channel, ENaC, Na + /2C1 " /K + co-transporter, Na + -K + -ATPase pump and the basolateral membrane K + channels, that are responsible for the uptake of chloride into the cell. [0010] These elements work together to achieve directional transport across the epithelium via their selective expression and localization within the cell. Chloride absorption takes place by the coordinated activity of: (i) ENaC and CFTR present on the apical membrane; and (ii) the Na + -K + -ATPase pump and Cl- channels expressed on the basolateral surface of the cell.
- COPD chronic obstructive pulmonary disease
- COPD dry eye disease
- Sj ⁇ gren's Syndrome Sj ⁇ gren's Syndrome.
- COPD chronic obstructive pulmonary disease
- Activators of mutant or wild-type CFTR offer a potential treatment of mucus hypersecretion and impaired mucociliary clearance that is common in COPD.
- CFTR dry eye disease
- CF and Sj ⁇ grens's syndrome Increasing anion secretion via CFTR would enhance fluid transport from the corneal endothelial cells and secretory glands surrounding the eye to increase corneal hydration.
- Sj ⁇ grens's syndrome is an autoimmune disease in which the immune system attacks moisture-producing glands throughout the body, including the eye, mouth, skin, respiratory tissue, liver, vagina, and gut. Symptoms include dry eye, mouth, and vagina, as well as lung disease. The disease is also associated with rheumatoid arthritis, systemic lupus, systemic sclerosis, and polymypositis/dermatomyositis . Defective protein trafficking is believed to cause the disease for which treatment options are limited. Modulators of CFTR activity may hydrate the various organs afflicted by the disease and help to elevate the associated symptoms.
- the diseases associated with the first class of ER malfunction are CF (due to misfolded ⁇ F508-CFTR) , hereditary emphysema (due to ⁇ l-antitrypsin; non Piz variants) , hereditary hemochromatosis, coagulation-fibrinolysis deficiencies, such as protein C deficiency, Type 1 hereditary angioedema, lipid processing deficiencies, such as familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia, lysosomal storage diseases, such as I- cell disease/pseudo-Hurler, mucopolysaccharidoses (due to lysosomal processing enzymes) , Sandhof/Tay-Sachs (due to ⁇ - hexosaminidase) , Crigler-Naj jar type II (due to UDP
- the diseases associated with the latter class of ER malfunction are glycanosis CDG type 1, hereditary emphysema (due to ⁇ l- antitrypsin (PiZ variant) , congenital hyperthyroidism, osteogenesis imperfecta (due to Type I, II, IV procollagen) , hereditary hypofibrinogenemia (due to fibrinogen) , ACT deficiency (due to c.l-antichymotrypsin) , Diabetes insipidus (Dl) , neurophyseal Dl (due to Vasopressin hormone/V2- receptor) , neprogenic Dl (due to aquaporin II) , Charcot-Marie Tooth syndrome (due to Peripheral myelin protein 22) , Perlizaeus-Merzbacher disease, neurodegenerative diseases such as Alzheimer's disease (due to APP and presenilins) , Parkinson's disease, amyotroph
- Diarrhea in barn animals and pets such as cows, pigs and horses, sheep, goats, cats and dogs, also known as scours, is a major cause of death in these animals. Diarrhea can result from any major transition, such as weaning or physical movement, as well as in response to a variety of bacterial or viral infections and generally occurs within the first few hours of the animal's life.
- the most common diarrheal causing bacteria is enterotoxogenic E-coli (ETEC) having the K99 pilus antigen.
- ETEC enterotoxogenic E-coli
- Common viral causes of diarrhea include rotavirus and coronavirus.
- Other infectious agents include cryptosporidium, giardia lamblia, and salmonella, among others.
- Symptoms of rotaviral infection include excretion of watery feces, dehydration and weakness. Coronavirus causes a more severe illness in the newborn animals, and has a higher mortality rate than rotaviral infection. Often, however, a young animal may be infected with more than one virus or with a combination of viral and bacterial microorganisms at one time. This dramatically increases the severity of the disease. [0022] Accordingly, there is a need for modulators of an ABC transporter activity, and compositions thereof, that can be used to modulate the activity of the ABC transporter in the cell membrane of a mammal . [0023] There is a need for methods of treating ABC transporter mediated diseases using such modulators of ABC transporter activity.
- ABC-transporter as used herein means an ABC-transporter protein or a fragment thereof comprising at least one binding domain, wherein said protein or fragment thereof is present in vivo or in vi tro.
- binding domain as used herein means a domain on the ABC-transporter that can bind to a modulator. See, e.g., Hwang, T. C. et al . , J . Gen. Physiol. (1998): 111(3), 477-90.
- CFTR cystic fibrosis transmembrane conductance regulator or a mutation thereof capable of regulator activity in part or full, including, but not limited to, ⁇ F508 CFTR and G551D CFTR (see, e.g., https://www.genet.sickkids.on.ca/cftr/, for CFTR mutations) .
- COPD chronic obstructive pulmonary disease and comprises chronic obstructive bronchitis, and emphysema.
- modulating as used herein means increasing or decreasing by a measurable amount.
- stable refers to compounds that are not substantially altered when subjected to conditions to allow for their production, detection, and preferably their recovery, purification, and use for one or more of the purposes disclosed herein.
- a stable compound or chemically feasible compound is one that is not substantially altered when kept at a temperature of 40°C or less, in the absence of moisture or other chemically reactive conditions, for at least a week.
- aliphatic or "aliphatic group”, as used herein, means a straight-chain (i.e., unbranched) or branched, substituted or unsubstituted hydrocarbon chain that is completely saturated or that contains one or more units of unsaturation, or a monocyclic, bicyclic, or tricyclic hydrocarbon that is completely saturated or that contains one or more units of unsaturation, but which is not aromatic (also referred to herein as “carbocycle” "cycloaliphatic” or “cycloalkyl” ) , that has a single point of attachment to the rest of the molecule.
- aliphatic groups contain 1-20 aliphatic carbon atoms, i.e., ( (C1-C20) alkyl) . In some embodiments, aliphatic groups contain 1-10 aliphatic carbon atoms, i.e., ( (C1-C10) alkyl) . In other embodiments, aliphatic groups contain 1-8 aliphatic carbon atoms, i.e., ( (C1-C8) alkyl .
- aliphatic groups contain 1-6 aliphatic carbon atoms, i.e., ( (C1-C6) alkyl, and in yet other embodiments aliphatic groups contain 1-4 aliphatic carbon atoms, i.e., ( (C1-C4) alkyl .
- cycloaliphatic refers to a monocyclic C3-C8 hydrocarbon or bicyclic or tricyclic C8-C12 hydrocarbon that is completely saturated or that contains one or more units of unsaturation, but which is not aromatic, that has a single point of attachment to the rest of the molecule wherein any individual ring in said bicyclic ring system has 3-7 members.
- Suitable aliphatic groups include, but are not limited to, linear or branched, substituted or unsubstituted alkyl, alkenyl, alkynyl groups and hybrids thereof such as (cycloalkyl) alkyl, (cycloalkenyl) alkyl or (cycloalkyl) alkenyl .
- heteroaliphatic means aliphatic groups wherein one or two carbon atoms are independently replaced by one or more of oxygen, sulfur, nitrogen, phosphorus, or silicon.
- Heteroaliphatic groups may be substituted or unsubstituted, branched or unbranched, cyclic or acyclic, and include “heterocycle” , “heterocyclyl” , “heterocycloaliphatic” , or “heterocyclic” groups. [0039] The term “heterocycle”, “heterocyclyl”,
- heterocycloaliphatic or “heterocyclic” as used herein means non-aromatic, monocyclic, bicyclic, or tricyclic ring systems in which one or more ring members is an independently selected heteroatom.
- the "heterocycle”, “heterocyclyl”, “heterocycloaliphatic”, or “heterocyclic” group has three to fourteen ring members in which one or more ring members is a heteroatom independently selected from oxygen, sulfur, nitrogen, or phosphorus, and each ring in the system contains 3 to 7 ring members .
- heteroatom means one or more of oxygen, sulfur, nitrogen, phosphorus, or silicon (including, any oxidized form of nitrogen, sulfur, phosphorus, or silicon; the quaternized form of any basic nitrogen or a substitutable nitrogen of a heterocyclic ring, for example N (as in 3,4- dihydro-2H-pyrrolyl) , NH (as in pyrrolidinyl) or NR + (as in N- substituted pyrrolidinyl)).
- alkoxy or “thioalkyl” , as used herein, refers to an alkyl group, as previously defined, attached to the principal carbon chain through an oxygen (“alkoxy”) or sulfur ( “thioalkyl” ) atom.
- haloalkyl means alkyl, alkenyl or alkoxy, as the case may be, substituted with one or more halogen atoms.
- halogen means F, Cl, Br, or I.
- aryl used alone or as part of a larger moiety as in “aralkyl”, “aralkoxy”, or “aryloxyalkyl”, refers to monocyclic, bicyclic, and tricyclic ring systems having a total of five to fourteen ring members, wherein at least one ring in the system is aromatic and wherein each ring in the system contains 3 to 7 ring members.
- aryl may be used interchangeably with the term “aryl ring” .
- aryl also refers to heteroaryl ring systems as defined hereinbelow.
- heteroaryl used alone or as part of a larger moiety as in “heteroaralkyl” or “heteroarylalkoxy” , refers to monocyclic, bicyclic, and tricyclic ring systems having a total of five to fourteen ring members ' , wherein at least one ring in the system is aromatic, at least one ring in the system contains one or more heteroatoms, and wherein each ring in the system contains 3 to 7 ring members.
- heteroaryl may be used interchangeably with the term “heteroaryl ring” or the term “heteroaromatic” .
- An aryl (including aralkyl, aralkoxy, aryloxyalkyl and the like) or heteroaryl (including heteroaralkyl and heteroarylalkoxy and the like) group may contain one or more substituents.
- Optional substituents on the aliphatic group of R° are selected from NH 2 , NH (Cx ⁇ aliphatic) , N(C ⁇ - 4 aliphatic) 2 , halogen, C ⁇ _ 4 aliphatic, OH, 0 (C ⁇ -4 aliphatic) , N0 2 , CN, C0 2 H, C0 2 (C 1 . 4 aliphatic) , 0(haloC ⁇ - 4 aliphatic), or haloCi- aliphatic, wherein each of the foregoing C ⁇ _aliphatic groups of R° is unsubstituted.
- Optional substituents on the aliphatic group of R * are selected from NH 2 , NH(C 1-4 aliphatic), N(C1-C4 aliphatic) 2 , halogen, (C1-C4) aliphatic, OH, 0( (C1-C4) aliphatic) , N0 2 , CN, C0 2 H, C0 2 ( (C1-C4) aliphatic) , 0 (halo (C1-C4) aliphatic) , or halo ( (C1-C4) aliphatic) , wherein each of the foregoing (C1-C4) aliphatic groups of R * is unsubstituted .
- Optional substituents on the aliphatic group or the phenyl ring of R + are selected from NH 2 , NH ( (C1-C4) aliphatic) , N( (C1-C4) aliphatic) 2 , halogen, (C1-C4) aliphatic, OH, 0( (C1-C4) aliphatic) , N0 2 , CN, C0 2 H, C0 2 ( (C1-C4) aliphatic) , O (halo (C1-C4) aliphatic) , or halo ( (C1-C4) aliphatic) , wherein each of the foregoing Cx ⁇ aliphatic groups of R + is unsubstituted.
- alkylidene chain refers to a straight or branched carbon chain that may be fully saturated or have one or more units of unsaturation and has two points of attachment to the rest of the molecule.
- R° or R + , or any other variable similarly defined herein
- two independent occurrences of R° are taken together with the atom(s) to which each variable is bound to form a 3-8-membered cycloalkyl, heterocyclyl, aryl, or heteroaryl ring having 0-3 heteroatoms independently selected from nitrogen, oxygen, or sulfur.
- Exemplary rings that are formed when two independent occurrences of R° (or R + , or any other variable similarly defined herein) are taken together with the atom(s) to which each variable is bound include, but are not limited to the following: a) two independent occurrences of R° (or R + , or any other variable similarly defined herein) that are bound to the same atom and are taken together with that atom to form a ring, for example, N(R°) 2/ where both occurrences of R° are taken together with the nitrogen atom to form a piperidin-1- yl , piperazin-1-yl , or morpholin-4-yl group; and b) two independent occurrences of R° (or R + , or any other variable similarly defined herein) that are bound to different atoms and are taken together with both of those atoms to form a ring, for example where a phenyl group is substituted with two
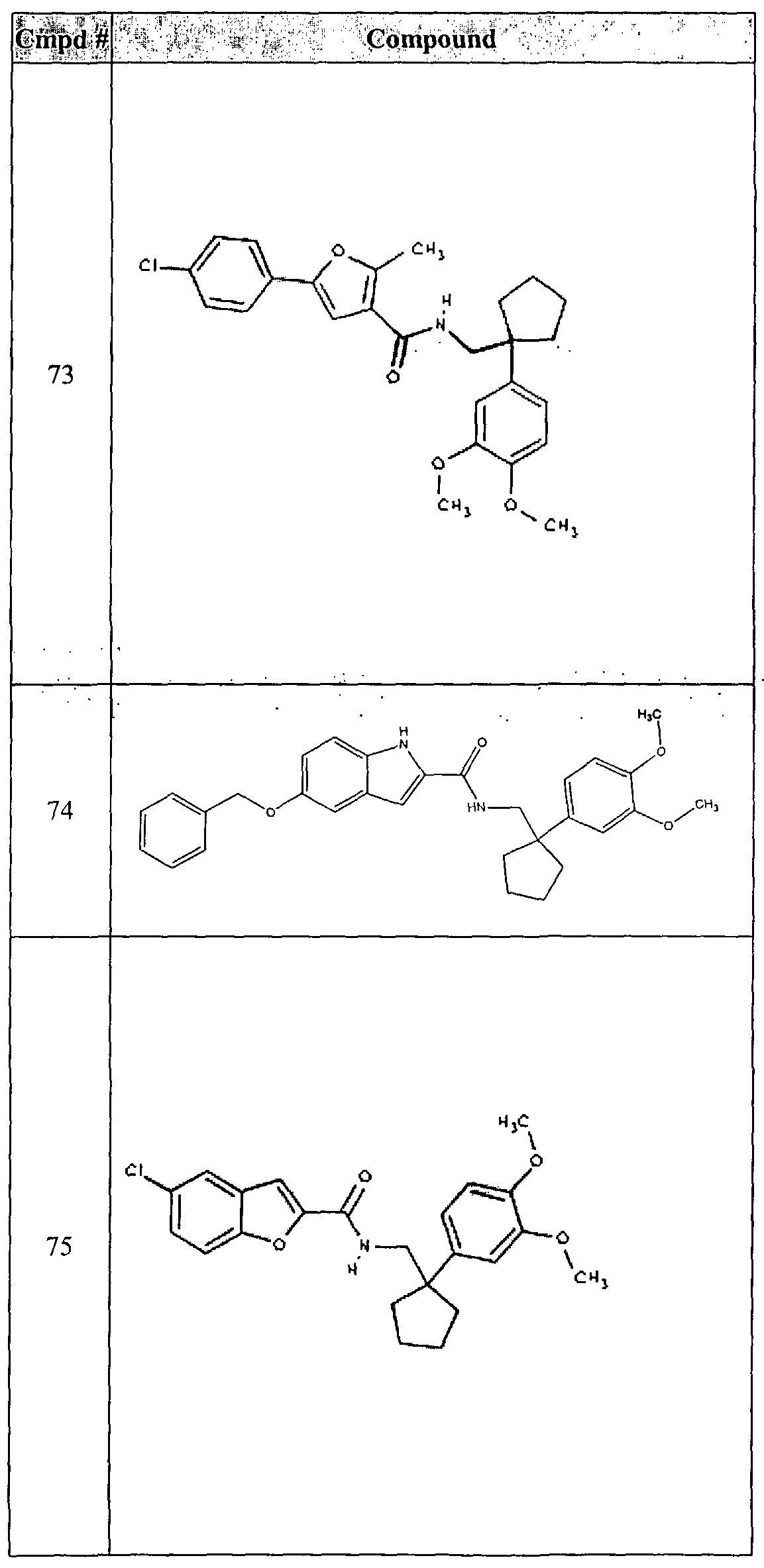
- the present invention relates to compounds of formula I:
- A is C(O) , or S0 2 ;
- R c and R D are independently selected from H, (C1-C4) alkyl , and aryl, or may be taken together to form a (C3-C8) cycloalkyl or heterocyclic;
- R E is H, (C1-C4) alkyl optionally substituted with a substituent selected from CN, N0 2 , CF 3 , 0CF 3 , OH, SR 6 , S(0)R 6 , S0 R 6 , COOH, COOR 6 , OR 6 or phenyl optionally substituted with
- R' B is aryl or heterocyclic
- L is (Cl-C6)alkylidene, -O- ( (C1-C6) alkylidene) , ( (Cl-6) alkylidene) -0- , or a bond, wherein up to two carbon atoms in said alkylidene in L are independently replaced with O, S, or N;
- W is aryl, heterocyclic, or (C5-C7) cycloalkyl ;
- m and n are independently 0 to 5;
- R B and R z are independently selected from R 1 , R 2 , R 3 , R 4 , or R5, wherein: R 1 is oxo, R 6 or ( (C1-C4) aliphatic) n -Y; n is 0 or 1 ;
- Y is halo, CN, N0 2 , CF 3 , OCF3 , OH, SR 6 , S(0)R 6 , S0 2 R 6 , NH 2 , NHR 6
- R 5 is a cycloaliphatic, aryl, heterocyclic, or heteroaryl ring, optionally comprising up to 3 R 1 substituents
- R 6 is H or aliphatic, wherein R 6 optionally comprises a R 7 substituent
- R 7 is a cycloaliphatic, aryl, heterocyclic, or heteroaryl ring, and each R 7 optionally comprises up to 2 substituents independently chosen from H, (C]_-Cg) -straight or branched alkyl, (C 2 -Cg) straight or branched alkenyl or alkynyl, 1 , 2-methylenedioxy, 1 , 2 -ethylenedioxy, or (CH2) n ⁇ Q;
- Q is selected from halo, CN, N0 2 , CF3 , OCF3 , OH, S-aliphatic, S (O)
- amino protecting group refers to a suitable chemical group that may be attached to a nitrogen atom.
- protecting refers to when the designated amino group is attached to a suitable chemical group (e.g., capping group). Examples of suitable amino capping groups are described in T.W. Greene et al . , Protective Groups in Organic Synthesis, 3d. Ed. , John Wiley and Sons (1999) ; L. Fieser and M. Fieser, Fieser and Fieser's Reagents for Organic Synthesis, John Wiley and Sons (1994) ; L. Paquette, ed. Encyclopedia of Reagents for Organic Synthesis, John Wiley and Sons (1995) and are exemplified in certain of the specific compounds used in this invention.
- the present invention provides a compound of formula II :
- A is C(0) or S0 2 ;
- R c and R D taken together form a 3-6 membered cycloalkyl ring or 4-pyranyl ring;
- R E is H, (C1-C4) alkyl optionally substituted with a substituent selected from (C1-C4) alkyl selected CN, N0 2 , CF3 , OCF3, OH, SR 6 , S(0)R 6 , S0 2 R 6 , COOH, COOR 6 , OR 6 or phenyl optionally substituted with R z ;
- B is phenyl;
- L is a bond
- W is a 5-14 membered monocyclic, bicyclic, or tricyclic heterocyclic or heteroaryl ring
- m and n are independently 0 to 5
- Z is diphenylmethyl wherein each phenyl has up to 5 R z is substituents
- R B and R z are independently selected from R 1 , R 2 , R 3 , R 4 , or R 5 , wherein: R 1 is oxo, R 6 or ( (C1-C4) aliphatic) n -Y; n is 0 or 1; Y is halo, CN, N0 2 , CF 3 , OCF 3 , OH, SR 6 , S(0)R 6 , S0 2 R 6 , NH 2 , NHR 6 , N(R 6 ) 2 , NR 6 R 8 , COOH, COOR 6 or OR 6 ; or two R 1 on adjacent ring atoms, taken together, form 1 , 2
- R E when R and R taken together form a cyclopentyl ring, R E is hydrogen, A is C(O), and ring W together with R z and m is diphenylmethyl, then ring B together with (R B ) n is not phenyl, 4-ethoxyphenyl, 4-butoxyphenyl, 4-isobutoxyphenyl, or 4 -methoxyphenyl .
- A is C(O) .
- A is S0 2 .
- R E is hydrogen.
- R E is C1-C4 alkyl .
- R c and R D taken together, form a 4-pyranyl ring.
- R c and R D taken together, form a 3-6 membered cycloalkyl ring.
- R c and R D taken together, form a 5-6 membered cycloalkyl ring.
- R c and R D taken together, form a 5-membered cycloalkyl ring.
- R c and R D taken together, form a 6-membered cycloalkyl ring.
- W is an optionally substituted indolyl, benzofuranyl , or benzothienyl .
- W is is indol-2- yl or indol-3-yl.
- W is benzofuran-2 -yl .
- W is benzothien-2-yl .
- W is an optionally substituted pyrazolyl or indazolyl .
- W is an optionally substituted pyrazol-3-yl or pyrazol-4-yl .
- W is an optionally substituted indazol-3-yl .
- W is an optionally substituted phenyl .
- W is an optionally substiuted six-membered heteroaromatic ring having up to three heteroatoms selected from O, S, or N.
- W is pyridyl .
- Z is diphenylmethyl.
- W is an optionally substituted ring selected from furanyl , thienyl , isoxazolyl, or pyrrolyl .
- W is an optionally substituted 10-12 membered bicyclic, heteroaromatic ring.
- W is an optionally substituted ring selected from quinolinyl or cinnolinyl.
- R c and R D each is methyl.
- R 8 is acetyl, arylsulfonyl or alkylsuifonyl .
- Another embodiment of the present invention provides a method of treating an ABC transporter mediated disease in a mammal, comprising the step of administering to said mammal a composition comprising a compound of the present invention or a pharmaceutically acceptable salt thereof.
- a preferred aspect of the present embodiment is where the ABC transporter mediated disease is selected from cystic fibrosis, hereditary emphysema, hereditary hemochromatosis, coagulation-cibrinolysis deficiencies, such as protein C deficiency, Type 1 hereditary angioedema, lipid processing deficiencies, such as familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia, lysosomal storage- diseases, such as I-cell disease/Pseudo-Hurler, secretory diarrhea or polycystic kidney disease, mucopolysaccharidoses , Sandhof/Tay-Sachs, Crigler-Naj jar type II, polyendocrinopathy/hyperinsulemia, Diabetes mellitus, Laron dwarfism, myleoperoxidase deficiency, primary hypoparathyroidism, melanoma, glycanosis CDG type 1, heredit
- An especially preferred method is where the disease is CF.
- Another embodiment of the present invention provides a pharmaceutical composition
- a pharmaceutical composition comprising: a. a compound of the present invention; b. a pharmaceutically acceptable carrier; and c. an additional agent selected from a mucolytic agent, bronchodialator, an antibiotic, an anti-infective agent, an anti- inflammatory agent, CFTR modulator other than a compound of the present invention, or a nutritional agent.
- Another embodiment of the present invention provides a method of modulating ABC transporter activity, comprising the step of contacting said ABC transporter with a compound of the present invention.
- a preferred aspect of this embodiment is where the ABC transporter or a fragment thereof is in vivo. Another preferred aspect of this embodiment is where the ABC transporter or a fragment thereof is in vi tro .
- the present invention provides a method of increasing the number of functional ABC transporters in a membrane of a cell, comprising the step of contacting said cell with a compound of formula (I) .
- the term "functional ABC transporter" as used herein means an ABC transporter that is capable of transport activity.
- said functional ABC transporter is CFTR.
- Another embodiment of the present invention provides a kit for use in measuring the activity of a ABC transporter or a fragment thereof in a biological sample in vi tro or in vivo, comprising: a. a composition comprising a compound of the present invention; and b. instructions for: i) contacting said composition with the biological sample; and ii) measuring activity of said ABC transporter or fragment thereof .
- a preferred aspect of this embodiment is where the ABC transporter is CFTR.
- R c and R D are independently selected from H, C ⁇ _ 4 alkyl, and aryl, or may be taken together to form a (C3-C8) cycloalkyl; B is aryl; and Z is (C1-C6) alkyl , aryl, (Cl-C4alkyl)aryl, C5-C7cycloalkyl , or ( (Cl-4) alkyl) C 5 _ 7 cycloalkyl .
- a preferred embodiment of the present invention is where A is C(O) , and L is a bond.
- a preferred embodiment of the present invention is where R c and R D taken together form (C3-C8) cycloalkyl .
- a particularly preferred embodiment of the present invention is where R c and R D taken together form cyclopentyl.
- a preferred embodiment of the present invention is where R c and R D taken together form a heterocyclic. [0088] A particularly preferred embodiment of the present invention is where R c and R D taken together form pyranyl . In certain embodiments, R c and R D taken together form 4-pyranyl. [0089] Yet another particularly preferred embodiment of the present invention is where R c and R D are H. [0090] Yet another particularly preferred embodiment of the present invention is where R c and R D are methyl . [0091] A preferred embodiment of the present invention is where B is aryl.
- a particularly preferred embodiment of the present invention is where B is phenyl .
- a preferred embodiment of the present invention is where Z is aryl.
- Another preferred embodiment of the present invention is where Z is pyridinyl .
- Another preferred embodiment of the present invention is where Z is phenyl.
- Another preferred embodiment of the present invention is where Z is benzofuran.
- Another preferred embodiment of the present invention is where Z is benzothiophenyl .
- Another preferred embodiment of the present invention is where Z is indolyl .
- Another preferred embodiment of the present invention is where Z is pyrazolyl .
- Another preferred embodiment of the present invention is where Z is furanyl .
- Another preferred embodiment of the present invention is where Z is quinolinyl .
- Another preferred embodiment of the present invention is where Z is isoquinolinyl .
- Another preferred embodiment of the present invention is where Z is is cinnolinyl.
- An especially preferred embodiment of the present invention is where Z is benzofuranyl , and R c and R D taken together form cyclohexyl .
- Another especially preferred embodiment is where B is a substituted phenyl .
- An especially preferred embodiment of the present invention is a compound where Z is benzofuranyl , R c and R D taken together form cyclohexyl, and B is a substituted phenyl as represented by formula II:
- R z is independently selected from (C1-C4) alkyl, (C1-C4) alkoxy, and halo, particularly methyl, methoxy, F, or Cl; n is 0 to 4 ; R B is independently selected from halo, and (C1-C4) alkoxy; and m is 0 to 5.
- the compounds of the present invention can be prepared by methods well known in the art.
- An exemplary method of producing compounds of the present invention is shown below in Schemes 1-3.
- Scheme 1 illustrates an exemplary method for producing amine intermediates for compounds of the present invention wherein R c and R D cyclize to form a ring: i) XCH 2 (CH 2 )n ' X, NaH, THF (X is C , Br, I; n' is 1 to 4) ii) LiAlH 4 , ether [00111] An optionally substituted (R*) 2-phenylacetonitrile is reacted with an appropriate dihalo-alkyl compound, sodium hydride or the like, in THF or a similar solvent. The resulting spiro compound is reacted with lithium aluminum hydride or similar reducing reagent to provide the desired cyclic amine. [00112] Scheme 2 below illustrates- an exemplary method for producing amine intermediates for the present invention wherein R c and R D do not cyclize to form a ring. [00113] Scheme 2:
- Scheme 3 below illstrates an exemplary method for producing certain compounds of the present invention using the amine intermediates of, e.g., Scheme 1 and Scheme 2 above.
- the ABC transporter mediated disease is selected from Cystic fibrosis, COPD, Asthma, chronic pancreatitis, pneumonia, polycystic kidney disease, Hereditary emphysema, Hereditary hemochromatosis, Coagulation-Fibrinolysis deficiencies, such as Protein C deficiency, Type 1 hereditary angioedema, Lipid processing deficiencies, such as Familial hypercholesterolemia, Type 1 chylomicronemia, Abetalipoproteinemia, Lysosomal storage diseases, such as I- cell disease/Pseudo-Hurler, Mucopolysaccharidoses, Sandhof/Tay-Sachs, Crigler-Naj jar type II,
- Glycanosis CDG type 1 Hereditary emphysema, Congenital hyperthyroidism, Osteogenesis imperfecta, Hereditary hypofibrinogenemia, ACT deficiency, Diabetes insipidus (Dl) , Neurophyseal Dl , Neprogenic Dl , Charcot-Marie Tooth syndrome, Perlizaeus-Merzbacher disease, neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Amyotrophic lateral sclerosis, Progressive supranuclear plasy, Pick's disease, several polyglutamine neurological disorders such as Huntington, Spinocerebullar ataxia type I, Spinal and bulbar muscular atrophy, Dentatorubal pallidoluysian
- the ABC transporter mediated disease is cystic fibrosis.
- Another embodiment of the present invention provides a method of treating a disease selected from Cystic fibrosis, COPD, asthma, chronic pancreatitis, pneumonia, polycytic kidney disease, Hereditary emphysema, Hereditary hemochromatosis, Coagulation-Fibrinolysis deficiencies, such as Protein C deficiency, Type 1 hereditary angioedema, Lipid processing deficiencies, such as Familial hypercholesterolemia, Type 1 chylomicronemia, Abetalipoproteinemia, Lysosomal storage diseases, such as I- cell disease/Pseudo-Hurler, Mucopolysaccharidoses, Sandhof/Tay-Sachs, Crigler-Naj jar type II,
- Polyendocrinopathy/Hyperinsulemia Diabetes mellitus, Laron dwarfism, Myleoperoxidase deficiency, Primary hypoparathyroidism, Melanoma, Glycanosis CDG type 1, Hereditary emphysema, Congenital hyperthyroidism, Osteogenesis imperfecta, Hereditary hypofibrinogenemia, ACT deficiency, Diabetes insipidus (Dl), Neurophyseal Dl , Neprogenic Dl , Charcot-Marie Tooth syndrome, Perlizaeus-Merzbacher disease, neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Amyotrophic lateral sclerosis, Progressive supranuclear plasy, Pick's disease, several polyglutamine neurological disorders asuch as Huntington, Spinocerebullar ataxia type I, Spinal and bulbar muscular atrophy, Dentatorubal pallidoluysian, and Myotonic dystrophy, as well as
- the disease so treated is selected from Tangier's disease, stargardt disease 1, age related macular dystrophy 2, retinintis pigmentosa, bare lymphocyte syndrome, PFIC-3, anemia, progressive intrahepatic cholestasis-2, Dublin-Johnson syndrome, Pseudoxanthoma elasticum, cystic fibrosis, familial persistent hyperinsulinemic hyproglycemia of infancy, adrenolecukodystrophy, sitosterolemia, chronic obstructive pulmonary disease, asthma, disseminated bronchiectasis, chronic pancreatitis, male infertility, emphysema, or pneumonia .
- the ABC transporter mediated disease is secretory diarrhea, COPD, or polycystic kidney disease in a mammal.
- the present invention provides a method of treating cystic fibrosis or secretory diahrrea comprising the step of administering to said mammal a composition comprising the step of administering to said mammal a composition comprising a compound of the present invention, or a preferred embodiment thereof as set forth above. Most preferably, said disease is cystic fibrosis.
- the present invention provides a method of modulating CFTR activity in a cell membrane ("potentiating") of a mammal in need thereof, comprising the step of administering to said mammal a composition comprising a compound of the present invention as defined above.
- the preferred embodiments of the compounds of the present invention useful in potentiating the activity of CFTR include the preferred embodiments of the present invention described above.
- the present invention provides a method of increasing the number of functional ABC transporters in a membrane of a cell, comprising the step of contacting said cell with a compound of the present invention.
- the term "functional ABC transporter" as used herein means an ABC transporter that is capable of transport activity.
- said functional ABC transporter is CFTR.
- the preferred embodiments of compounds of the present invention useful in increasing the number of functional ABC transporters include preferred embodiments of compounsd of the present invention as described above.
- the present invention provides a method of modulating activity of an anion channel in vi tro or in vivo, comprising the step of contacting said channel with a compound of the present invention.
- said anion channel is a chloride channel or a bicarbonate channel. More preferably, said anion channel is a chloride channel .
- the present invention provides a method of treating an anion channel mediated disease in a mammal, comprising the step of administering to said mammal a composition comprising a compound according to the present invention.
- the present invention provides a pharmaceutical composition
- a pharmaceutical composition comprising: (i) a compound of the present invention as described above ; (ii) a pharmaceutically acceptable carrier; and (iii) an additional agent selected from a mucolytic agent, bronchodialator, an anti-biotic, an anti-infective agent, an anti-inflammatory agent, CFTR modulator other than a compound of the present invention, or a nutritional agent.
- Preferred embodiments of compounds the present invention in the above pharmaceutical composition are those as described above.
- the present invention provides a kit for use in measuring the activity of a ABC transporter or a fragment thereof in a biological sample in vi tro or in vivo, comprising: (i) a composition comprising a compound of the present invention; and (ii) instructions for: a) contacting the composition with the biological sample; b) measuring activity of said ABC transporter or a fragment thereof .
- the kit is useful in measuring the activity of CFTR.
- the compounds of this invention may be prepared in general by methods known to those skilled in the art for analogous compounds, as illustrated by the general schemes below, and the preparative examples that follow.
- Starting materials are commercially available from typical chemical reagent supply companies, such as, Aldrich Chemicals Co., Sigma Chemical Company, ChemBridge Corporation, and the like.
- structures depicted herein are also meant to include all stereochemical forms of the structure; i.e., the R and S configurations for each asymmetric center. Therefore, single stereochemical isomers as well as enantiomeric and diastereomeric mixtures of the present compounds are within the scope of the invention. Unless otherwise stated, structures depicted herein are also meant to include compounds that differ only in the presence of one or more isotopically enriched atoms. For example, compounds having the present structures except for the replacement of a hydrogen by a deuterium or tritium, or the replacement of a carbon by a 13 C- or 14 C-enriched carbon are within the scope of this invention.
- the present invention provides compounds that are useful as modulators of ABC transporters and thus are useful in treating a disease selected from Cystic fibrosis, COPD, chronic pancreatitis, pneumonia, polycystic kidney disease, Hereditary emphysema, Hereditary hemochromatosis, Coagulation-Fibrinolysis deficiencies, such as Protein C deficiency, Type 1 hereditary angioedema, Lipid processing deficiencies, such as Familial hypercholesterolemia, Type 1 chylomicronemia, Abetalipoproteinemia, Lysosomal storage diseases, such as I- cell disease/Pseudo-Hurler, Mucopolysaccharidoses, Sandhof/Tay-Sachs, Crigler-Naj jar type II,
- Polyendocrinopathy/Hyperinsulemia Diabetes mellitus, Laron dwarfism, Myleoperoxidase deficiency, Primary hypoparathyroidism, Melanoma, Glycanosis CDG type 1, Hereditary emphysema, Congenital hyperthyroidism, Osteogenesis imperfecta, Hereditary hypofibrinogenemia, ACT deficiency, Diabetes insipidus (Dl) , Neurophyseal Dl, Neprogenic Dl , Charcot-Marie Tooth syndrome, Perlizaeus-Merzbacher disease, neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Amyotrophic lateral sclerosis, Progressive supranuclear plasy, Pick's disease, several polyglutamine neurological disorders asuch as Huntington, Spinocerebullar ataxia type I, Spinal and bulbar muscular atrophy, Dentatorubal pallidoluysian, and Myotonic dystrophy, as well
- compositions comprising any of the compounds as described herein, and optionally comprise a pharmaceutically acceptable carrier, adjuvant or vehicle.
- these compositions optionally further comprise one or more additional therapeutic agents.
- certain of the compounds of present invention can exist in free' form for treatment, or where appropriate, as a pharmaceutically acceptable derivative thereof.
- a pharmaceutically acceptable derivative includes, but is not limited to, pharmaceutically acceptable salts, esters, salts of such esters, or any other adduct or derivative which upon administration to a patient in need is capable of providing, directly or indirectly, a compound as otherwise described herein, or a metabolite or residue thereof .
- pharmaceutically acceptable salt refers to those salts which are, within the scope of sound medical judgement, suitable for use in contact with the tissues of humans and lower animals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio.
- a “pharmaceutically acceptable salt” means any non-toxic salt or salt of an ester of a compound of this invention that, upon administration to a recipient, is capable of providing, either directly or indirectly, a compound of this invention or an inhibitorily active metabolite or residue thereof.
- compositions of this invention include those derived from suitable inorganic and organic acids and bases.
- Examples of pharmaceutically acceptable, nontoxic acid addition salts are salts of an amino group formed with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids such as acetic acid, oxalic acid, ⁇ maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by using other methods used in the art such as ion exchange.
- inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid
- organic acids such as acetic acid, oxalic acid, ⁇ maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by using other methods used in the art such as ion exchange.
- salts include adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryl sulfate, malate, maleate, malonate, methanesulfonate, 2-naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate, pamoate, pectinate,
- Salts derived from appropriate bases include alkali metal, alkaline earth metal, ammonium and N+(C1- 4alkyl)4 salts.
- This invention also envisions the quaternization of any basic nitrogen-containing groups of the compounds disclosed herein. Water or oil-soluble or dispersable products may be obtained by such quaternization.
- Representative alkali or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like.
- Further pharmaceutically acceptable salts include, when appropriate, nontoxic ammonium, quaternary ammonium, and amine cations formed using counterions such as halide, hydroxide, carboxylate, sulfate, phosphate, nitrate, loweralkyl sulfonate and aryl sulfonate.
- the pharmaceutically acceptable compositions of the present invention additionally comprise a pharmaceutically acceptable carrier, adjuvant, or vehicle, which, as used herein; includes any and all solvents, ' diluents, or other liquid vehicle, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants and the like, as suited to the particular dosage form desired.
- Remington's Pharmaceutical Sciences, Sixteenth Edition, E. W. Martin (Mack Publishing Co., Easton, Pa., 1980) discloses various carriers used in formulating pharmaceutically acceptable compositions and known techniques for the preparation thereof.
- any conventional carrier medium is incompatible with the compounds of the invention, such as by producing any undesirable biological effect or otherwise interacting in a deleterious manner with any other component (s) of the pharmaceutically acceptable composition, its use is contemplated to be within the scope of this invention.
- materials which can serve as pharmaceutically acceptable carriers include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, or potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes, such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, polyacrylates, waxes, polyethylene-polyoxypropylene-block polymers, wool fat, sugars such as lactose, glucose and sucrose; starches such as corn starch and potato starch; cellulose and its derivatives such as sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered tragacanth; malt; gelatin; talc
- the present invention provides a method of treating a condition, disease, or disorder implicated by ABC transporter activity.
- the present invention provides a method of treating a condition, disease, or disorder implicated by a deficiency of ABC transporter activity, the method comprising administering a composition comprising a compound of Formula I to a subject, preferably a mammal, in need thereof.
- the present invention provides a method of treating cystic fibrosis, hereditary emphysema, hereditary hemochromatosis, coagulation- cibrinolysis deficiencies, such as protein C deficiency, Type 1 hereditary angioedema, lipid processing deficiencies, such as familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia, lysosomal storage diseases, such as I- cell disease/pseudo-Hurler, secretory diarrhea or polycystic kidney disease, mucopolysaccharidoses , Sandhof/Tay-Sachs , Crigler-Naj jar type II, polyendocrinopathy/hyperinsulemia, Diabetes mellitus, Laron dwarfism, myleoperoxidase deficiency, primary hypoparathyroidism, melanoma, glycanosis CDG type 1, hereditary e
- the present invention provides a method of treating cystic fibrosis comprising the step of administering to said mammal a composition comprising the step of administering to said mammal an effective amount of a composition comprising a compound of the present invention.
- an "effective amount" of the compound or pharmaceutically acceptable composition is that amount effective for treating or lessening the severity of one or more of cystic fibrosis, hereditary emphysema, hereditary hemochromatosis, coagulation-cibrinolysis deficiencies, such as protein C deficiency, Type 1 hereditary angioedema, lipid processing deficiencies, such as familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia, lysosomal storage diseases, such as I- cell disease/pseudo-Hurler, secretory diarrhea or polycystic kidney disease, mucopolysaccharidoses, Sandhof/Tay-Sachs, Crigler-Naj jar type II, polyendocrinopathy/hyperinsulemia, Diabetes mellitus, Laron dwarfism, myleoperoxidase deficiency, primary hypoparathyroidism, melanoma,
- the compounds and compositions, according to the method of the present invention may be administered using any amount and any route of administration effective for treating or lessening the severity of one or more of cystic fibrosis, hereditary emphysema, hereditary hemochromatosis, coagulation- cibrinolysis deficiencies, such as protein C deficiency, Type 1 hereditary angioedema, lipid processing deficiencies, such as familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia, lysosomal storage diseases, such as I- cell disease/pseudo-Hurler, secretory diarrhea or polycystic kidney disease, mucopolysaccharidoses, Sandhof/Tay-Sachs, Crigler-Naj jar type II, polyendocrinopathy/hyperinsulemia, Diabetes mellitus, Laron dwarfism, myleoperoxidase deficiency, primary hypoparathyroidism
- the exact amount required will vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the infection, the particular agent, its mode of administration, and the like.
- the compounds of the invention are preferably formulated in dosage unit form for ease of administration and uniformity of dosage.
- dosage unit form refers to a physically discrete unit of agent appropriate for the patient to be treated. It will be understood, however, that the total daily usage of the compounds and compositions of the present invention will be decided by the attending physician within the scope of sound medical judgment.
- the specific effective dose level for any particular patient or organism will depend upon a variety of factors including the disorder being treated and the severity of the disorder; the activity of the specific compound employed; the specific composition employed; the age, body weight, general health, sex and diet of the patient; the time of administration, route of administration, and rate of excretion of the specific compound employed; the duration of the treatment; drugs used in combination or coincidental with the specific compound employed, and like factors well known in the medical arts.
- patient means an animal, preferably a mammal, and most preferably a human.
- compositions of this invention can be administered to humans and other animals orally, rectally, parenterally, intracisternally, intravaginally, intraperitoneally, topically (as by powders, ointments, or drops) , bucally, as an oral or nasal spray, or the like, depending on the severity of the infection being treated.
- the compounds of the invention may be administered orally or parenterally at dosage levels of about 0.01 mg/kg to about 50 mg/kg and preferably from about 1 mg/kg to about 25 mg/kg, of subject body weight per day, one or more times a day, to obtain the desired therapeutic effect.
- Liquid dosage forms for oral administration include, but are not limited to, pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups and elixirs.
- the liquid dosage forms may contain inert diluents commonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
- inert diluents commonly used in the art such as, for example, water or other solvents,
- the oral compositions can also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- injectable preparations for example, sterile injectable aqueous or oleaginous suspensions may be formulated according to the known art using suitable dispersing or wetting agents and suspending agents.
- the sterile injectable preparation may also be a sterile injectable solution, suspension or emulsion in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3- butanediol.
- acceptable vehicles and solvents that may be employed are water, Ringer's solution, U.S.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil can be employed including synthetic mono- or diglycerides .
- fatty acids such as oleic acid are used in the preparation of injectables .
- the injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter, or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium prior to use.
- sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium prior to use.
- delayed absorption of a parenterally administered compound form is accomplished by dissolving or suspending the compound in an oil vehicle.
- injectable depot forms are made by forming microencapsule matrices of the compound in biodegradable polymers such as polylactide-polyglycolide. Depending upon the ratio of compound to polymer and the nature of the particular polymer employed, the rate of compound release can be controlled. Examples of other biodegradable polymers include poly (orthoesters) and poly (anhydrides) . Depot injectable formulations are also prepared by entrapping the compound in liposomes or microemulsions that are compatible with body tissues .
- compositions for rectal or vaginal administration are preferably suppositories which can be prepared by mixing the compounds of this invention with suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound .
- suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound .
- Solid dosage forms for oral administration include capsules, tablets, pills, powders, and granules.
- the active compound is mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol, and silicic acid, b) binders such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol, d) disintegrating agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate, e) solution retarding agents such as paraffin, f) absorption accelerators such as quaternary ammonium compounds, g) wetting agents such as, for example, cetyl alcohol
- the dosage form may also comprise buffering agents.
- Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient (s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner.
- embedding compositions examples include polymeric substances and waxes. Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polethylene glycols and the like.
- the active compounds can also be in microencapsulated form with one or more excipients as noted above.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings, release controlling coatings and other coatings well known in the pharmaceutical formulating art.
- the active compound may be admixed with at least one inert diluent such as sucrose, lactose or starch.
- inert diluent such as sucrose, lactose or starch.
- Such dosage forms may also comprise, as is normal practice, additional substances other than inert diluents, e.g., tableting lubricants and other tableting aids such a magnesium stearate and microcrystalline cellulose.
- the dosage forms may-- also comprise buffering agents. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient (s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner.
- Dosage forms for topical or transdermal administration of a compound of this invention include ointments, pastes, creams, lotions, gels, powders, solutions, sprays, inhalants or patches.
- the active component is admixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives or buffers as may be required.
- Ophthalmic formulation, eardrops, and eye drops are also contemplated as being within the scope of this invention.
- the present invention contemplates the use of transdermal patches, which have the added advantage of providing controlled delivery of a compound to the body.
- Such dosage forms are prepared by dissolving or dispensing the compound in the proper medium.
- Absorption enhancers can also be used to increase the flux of the compound across the skin.
- the rate can be controlled by either providing a rate controlling membrane or by dispersing the compound in a polymer matrix or gel .
- the compounds of the invention are useful as modulators of ABC transporters.
- the compounds and compositions are particularly useful for treating or lessening the severity of a disease, condition, or disorder where hyperactivity or inactivity of ABC transporters is implicated in the disease, condition, or disorder.
- hyperactivity or inactivity of an ABC transporter is implicated in a particular disease, condition, or disorder
- the disease, condition, or disorder may also be referred to as a "ABC transporter-mediated disease, condition or disorder”.
- the present invention provides a method for treating or lessening the severity of a disease, condition, or disorder where hyperactivity or inactivity of an ABC transporter is implicated in the disease state.
- the activity of a compound utilized in this invention as a modulator of an ABC transporter may be assayed according to methods described generally in the art and in the Examples herein.
- the compounds and pharmaceutically acceptable compositions of the present invention can be employed in combination therapies, that is, the compounds and pharmaceutically acceptable compositions can be administered concurrently with, prior to, or subsequent to, one or more other desired therapeutics or medical procedures.
- the particular combination of therapies (therapeutics or procedures) to employ in a combination regimen will take into account compatibility of the desired therapeutics and/or procedures and the desired therapeutic effect to be achieved. It will also be appreciated that the therapies employed may achieve a desired effect for the same disorder (for example, an inventive compound may be administered concurrently with another agent used to treat the same disorder) , or they may achieve different effects (e.g., control of any adverse effects).
- additional therapeutic agents that are normally administered to treat or prevent a particular disease, or condition are known as "appropriate for the disease, or condition, being treated” .
- the amount of additional therapeutic agent present in the compositions of this invention will be no more than the amount that would normally be administered in a composition comprising that therapeutic agent as the only active agent.
- the amount of additional therapeutic agent in the presently disclosed compositions will range from about 50% to 100% of the amount normally present in a composition comprising that agent as the only therapeutically active agent .
- the compounds of this invention or pharmaceutically acceptable compositions thereof may also be incorporated into compositions for coating an implantable medical device, such as prostheses, artificial valves, vascular grafts, stents and catheters.
- the present invention in another aspect, includes a composition for coating an implantable device comprising a compound of the present invention as described generally above, and in classes and subclasses herein, and a carrier suitable for coating said implantable device.
- the present invention includes an implantable device coated with a composition comprising a compound of the present invention as described generally above, and in classes and subclasses herein, and a - I l l
- coatings and the general preparation of coated implantable devices are described in US Patents 6,099,562; 5,886,026; and 5,304,121.
- the coatings are typically biocompatible polymeric materials such as a hydrogel polymer, poly ethyldisiloxane, polycaprolactone, polyethylene glycol, polylactic acid, ethylene vinyl acetate, and mixtures thereof.
- the coatings may optionally be further covered by a suitable topcoat of fluorosilicone, polysaccarides, polyethylene glycol, phospholipids or combinations thereof to impart controlled release characteristics in the composition.
- Another aspect of the invention relates to modulating ABC transporter activity in a biological sample or a patient (e.g., in vitro or in vivo), which method comprises administering to the patient, or contacting said biological sample with a compound of the present invention or a composition comprising said compound.
- biological sample includes, without limitation, cell cultures or extracts thereof; biopsied material obtained from a mammal or extracts thereof; and blood, saliva, urine, feces, semen, tears, or other body fluids or extracts thereof.
- Modulation of ABC transporter activity in a biological sample is useful for a variety of purposes that are known to one of skill in the art.
- a method of modulating activity of an anion channel in vitro or in vivo comprising the step of contacting said channel with a compound of the present invention.
- the anion channel is a chloride channel or a bicarbonate channel .
- the anion channel is a chloride channel .
- the present invention provides a method of increasing the number of functional ABC transporters in a membrane of a cell, comprising the step of contacting said cell with a compound of the present invention.
- the term "functional ABC transporter” as used herein means an ABC transporter that is capable of transport activity.
- said functional ABC transporter is CFTR.
- the activity of the ABC transporter is measured by measuring the transmembrane voltage potential .
- Means for measuring the voltage potential across a membrane in the biological sample may employ any of the known methods in the art, such as optical membrane potential assay or other electrophysiological methods .
- the optical membrane potential assay utilizes voltage-sensitive FRET sensors described by Gonzalez and Tsien (See, Gonzalez, J. E. and R. Y.
- These voltage sensitive assays are based on the change in fluorescence resonant energy transfer (FRET) between the membrane-soluble, voltage-sensitive dye, DiSBAC2(3), and a fluorescent phospholipid, CC2-DMPE, which is attached to the outer leaflet of the plasma membrane and acts as a FRET donor.
- FRET fluorescence resonant energy transfer
- CC2-DMPE fluorescent phospholipid
- Changes in membrane potential (Vm) cause the negatively charged DiSBAC2(3) to redistribute across the plasma membrane and the amount of energy transfer from CC2-DMPE changes accordingly.
- the changes in fluorescence emission can be monitored using VIPRTM II, which is an integrated liquid handler and fluorescent detector designed to conduct cell- based screens in 96- or 384-well microtiter plates.
- the present invention provides a kit for use in measuring the activity of a ABC transporter or a fragment thereof in a biological sample in vitro or in vivo comprising (i) a composition comprising a compound of the present invention; and (ii) instructions for a) contacting the composition with the biological sample and b) measuring activity of said ABC transporter or a fragment thereof.
- the kit further comprises instructions for a) contacting an additional composition with the biological sample; b) measuring the activity of said ABC transporter or a fragment thereof in the presence of said additional compound, and c) comparing the activity of the ABC transporter in the presence of the additional compound with the density of the ABC transporter in the presence of a composition of the present invention.
- the kit is used to measure the density of CFTR.
- the reaction mixture was cooled to room temperature and quenched with the slow addition of methanol.
- the reaction mixture was evaporated to dryness and purified by column chromatography on silica gel to yield a pale yellow oil (3.65 g, 14.9 mmol, 52.8 %) .
- the resulting 1- (3 , 4-dimethoxy- phenyl) -cyclohexanecarbonitrile (2.00 g, 8.15 mmol) was dissolved in dry ether (40 mL) and cooled to 0°C under an atmosphere of nitrogen. Lithium aluminum hydride (8.15 mL, IM in ether) was slowly added and the reaction mixture was allowed to warm to room temperature and stirred for 16 hours.
- reaction mixture was cooled to 0°C and quenched with 0.34 mL water, 0.34 mL of 15 % sodium hydroxide, and then an additional 1.4 mL of water.
- the reaction mixture was then filtered through celite, washed with water and a saturated aqueous sodium chloride solution. The filtrate was evaporated to dryness to give a colorless oil (1.91 g, 7.66 mmol, 94.0 %) .
- ESI-MS m/z calc. 249.2, found 250.2 (M+l) + . Retention time of 1.76 minutes.
- reaction mixture was evaporated to dryness and purified by column chromatography on silica gel using a gradient of 0-40 % ethyl acetate in hexanes. The pure fractions were combined and evaporated to dryness to yield a white solid (0.426 g, 1.09 mmol, 37.7 %) .
- ESI-MS m/z calc. 390.2, found 391.2 (M+l) + . Retention time of 3.94 minutes. ⁇ .
- Benzofuran-2 -carboxylic acid (1-benzo [1, 3] dioxol-5-yl- cyclopentylmethyl) -amide.
- C- (1-Benzo [1,3] dioxol-5-yl -cyclopentyl) -methylamine (43.8 mg, 0.200 mmol)
- benzofuran-2 -carbonyl chloride (36.1 mg, 0.200 mmol) were dissolved in 2 mL of 1,4-dioxane containing triethylamine (83.6 ⁇ L, 0.600 mmol).
- Phenethylamine (72.7 mg, 0.600 mmol) and O- (7-azabenzotriazol-l-yl) -N, N, N ' , N' - tetramethyluronium hexafluorophosphate (304 mg, 0.800 mmol) was added and the solution was allowed to stir for 16 hours. The mixture was then purified by reverse phase preparative liquid chromatography to yield the pure product (2.9 mg, 0.0093 mmol, 1.6 %) ESI-MS m/z calc. 310.2, found 311.2 (M+l) + . Retention time of 3.40 minutes.
- 2-Fluoro-nicotinic acid (84.7 mg, 0.-600 mmol, 3 -methyl- . butan-1-ol (52.9 mg, 0.600 mmol) and potassium bis (trimethylsilyl) amide (478 mg, 2.40 mmol) were combined in 0.6 mL of N, N-dimethylformamide and subjected to microwave irradiation for 3 minutes at 180 °C.
- lH-Indazole-3 -carboxylic acid (32.4 mg, 0.200 mmol) and C- [1- (3, 4-Dimethoxy-phenyl) -cyclopentyl] -methylamine (47.1 g, 0.200 mmol) were dissolved in acetonitrile (1 mL) containing triethylamine (83.6 ⁇ L, 0.600 mmol).
- Bath Solution #1 (in mM) NaCl 160, KCl 4.5, CaCl 2 2, MgCl 2 1, HEPES 10, pH 7.4 with NaOH.
- Chloride-free bath solution Chloride salts in Bath Solution #1 are substituted with gluconate salts.
- CC2-DMPE Prepared as a 10 mM stock solution in DMSO and stored at - 20°C.
- DiSBAC 2 (3) Prepared as a 10 M stock in DMSO and stored at -20°C.
- NIH3T3 mouse fibroblasts stably expressing ⁇ F508- CFTR are used for optical measurements of membrane potential.
- the cells are maintained at 37 °C in 5% C0 2 and 90 % humidity in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 10 % fetal bovine serum, 1 X NEAA, ⁇ -ME, 1 X pen/strep, and 25 mM HEPES in 175 cm 2 culture flasks.
- the cells were seeded at 30,000/well in 384- well matrigel -coated plates and cultured for 2 hrs at 37 °C before culturing at 27 °C for 24 hrs. for the potentiator assay.
- the cells are cultured at 27 °C or 37 °C with and without compounds for 16 - 24 hours)
- the FRT epithelia demonstrated resistances of 4 K ⁇ / cm 2 or more.
- the solutions were maintained at 27 °C and bubbled with air.
- the electrode offset potential and fluid resistance were corrected using a cell -free insert.
- the current reflects the flow of Cl " through ⁇ F508-CFTR expressed in the apical membrane.
- the I sc was digitally acquired using an MPIOOA-CE interface and AcqKJiowledge software (v3.2.6; BIOPAC Systems, Santa Barbara, CA) .
- Typical protocol utilized a basolateral to apical membrane Cl " concentration gradient. To set up this gradient, normal ringer was used on the basolateral membrane, whereas apical NaCl was replaced by equimolar sodium gluconate
- Typical protocol utilized a basolateral to apical membrane Cl " concentration gradient. To set up this gradient, normal ringers was used on the basolateral membrane and was permeabilized with nystatin (360 ⁇ g/ml), whereas apical NaCl was replaced by equimolar sodium gluconate (titrated to pH 7.4 with NaOH) to give a large Cl " concentration gradient across the epithelium. All experiments were performed 30 min after nystatin permeabilization. Forskolin (10 ⁇ M) and all test compounds were added to both sides of the cell culture inserts. The efficacy of the putative ⁇ F508-CFTR potentiators was compared to that of the known potentiator, genistein.
- Basolateral solution in mM: NaCl (135), CaCl 2 (1.2), MgCl 2 (1.2), K 2 HP0 4 (2.4), KHP0 4 (0.6), N-2-hydroxyethylpiperazine-N' -2- ethanesulfonic acid (HEPES) (10) , and dextrose (10) .
- the solution was titrated to pH 7.4 with NaOH.
- Apical solution. in mM: Same as basolateral solution with NaCl replaced with Na Gluconate (135) .
- FRT Fisher rat epithelial cells expressing ⁇ F508- CFTR (FRT ⁇ F508"CFTR ) were used for Ussing chamber experiments for the putative ⁇ F508-CFTR modulators identified from our optical assays.
- the cells were cultured on Costar Snapwell cell culture inserts and cultured for five days at 37 °C and 5% C0 2 in Coon's modified Ham's F-12 medium supplemented with 5% fetal calf serum, 100 U/ml penicillin, and 100 ⁇ g/ml streptomycin.
- the cells Prior to use for characterizing the potentiator activity of compounds, the cells were incubated at 27 °C for 16 - 48 hrs- to correct for the ⁇ F508-CFTR. To determine the activity of corrections compounds, the cells were incubated at 27 °C or 37 °C with and without the compounds for 24 hours.
- the cells were incubated with 10 ⁇ M of the test compound for 24 hours at 37°C and the current density was compared to the 27°C and 37°C controls (% activity) . Prior to recording, the cells were washed 3X with extracellular recording medium to remove any remaining test compound. Preincubation with 10 ⁇ M of correction compounds significantly increased the cAMP- and genistein-dependent current compared to the 37°C controls.
- Intracellular solution in mM Cs-aspartate (90) , CsCl (50), MgCl 2 (1), HEPES (10), and 240 ⁇ g/ml amphotericin-B (pH adjusted to 7.35 with CsOH) .
- ⁇ IH3T3 mouse fibroblasts stably expressing ⁇ F508- CFTR are used for whole-cell recordings.
- the cells are maintained at 37 °C in 5% C0 2 and 90 % humidity in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 10 % fetal bovine serum, 1 X NEAA, ⁇ -ME, 1 X pen/strep, and 25 mM HEPES in 175 cm 2 culture flasks.
- the nonspecific phosphatase inhibitor F " (10 mM NaF) was added to the bath solution. Under these recording conditions, channel activity remained constant throughout the duration of the patch recording (up to 60 min) . Currents produced by positive charge moving from the intra- to extracellular solutions (anions moving in the opposite direction) are shown as positive currents.
- the pipette potential (V p ) was maintained at 80 mV.
- Extracellular solution (in mM) NMDG (150) , aspartic acid (150) , CaCl 2 (5) , MgCl 2 (2) , and HEPES (10) (pH adjusted to 7.35 with Tris base) .
- Intracellular solution in mM
- NMDG-C1 150
- MgCl 2 (2) EGTA (5)
- TES TES
- Tris base 1
- NIH3T3 mouse fibroblasts stably expressing ⁇ F508- CFTR are used for excised-membrane patch-clamp recordings.
- the cells are maintained at 37 °C in 5% C0 2 and 90 % humidity in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 10 % fetal bovine serum, 1 X NEAA, ⁇ -ME, 1 X pen/strep, and 25 mM HEPES in 175 cm 2 culture flasks.
- 2,500 - 5,000 cells were seeded on poly-L-lysine-coated glass coverslips and cultured for 24 - 48 hrs at 27 °C before use.
- Compounds of the invention demonstrated activity as modulators of ATP binding cassette transporters, specifically CFTR.
- the level of cAMP in FRT cells following 0.5 ⁇ M forskolin or test compound application was determined using a commercially available chemiluminiscent immunoassay system for mammalian cells called Tropix ® (Applied Biosystems, Bedford, MA) . Briefly, FRT cells were incubated for 15 minutes with a test compound in the presence and absence of 0.5 ⁇ M forskolin. The compounds were aspirated and the cells were then lysed and transferred along with the lysis buffer to a 96-well Tropix ® ELISA plate. A cAMP-Alk Phos conjugate is then added to the assay plate, followed by the addition of cAMP anti-body. After several wash and aspiration steps, Sapphire blue II solution is added and the fluorescence emission is read on the Topcount fluorescence reader, and the cAMP concentrations were determined using a cAMP standard curve that was present in each plate.
- Tropix ® chemiluminiscent immunoassay system for mammalian cells
- the example teaches that compounds can have potentiator activity without having an accompanying increase in cAMP concentrations.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Diabetes (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Hematology (AREA)
- Endocrinology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Urology & Nephrology (AREA)
- Ophthalmology & Optometry (AREA)
- Obesity (AREA)
- Psychology (AREA)
- Dermatology (AREA)
- Pulmonology (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
- Emergency Medicine (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pyridine Compounds (AREA)
- Heterocyclic Compounds Containing Sulfur Atoms (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2006534421A JP2007509044A (en) | 2003-10-08 | 2004-10-08 | Modulator of ATP binding cassette transporter containing cycloalkylpyranyl group |
| EP04794652A EP1680411A2 (en) | 2003-10-08 | 2004-10-08 | Modulators of atp-binding cassette transporters containing cycloalkyl or pyranyl groups |
| AU2004279855A AU2004279855A1 (en) | 2003-10-08 | 2004-10-08 | Modulators of ATP-binding cassette transporters containing cycloalkyl or pyranyl groups |
| MXPA06004005A MXPA06004005A (en) | 2003-10-08 | 2004-10-08 | Modulators of atp-binding cassette transporters containing cycloalkyl or pyranyl groups. |
| NZ546365A NZ546365A (en) | 2003-10-08 | 2004-10-08 | Modulators of ATP-binding cassette transporters containing cycloalkyl or pyranyl groups |
| CA002540978A CA2540978A1 (en) | 2003-10-08 | 2004-10-08 | Modulators of atp-binding cassette transporters containing cycloalkyl or pyranyl groups |
| IL174820A IL174820A0 (en) | 2003-10-08 | 2006-04-05 | Aryl and heterocyclic compounds containing cycloalkyl or pyranyl groups and pharmaceutical compositions containing the same |
| NO20062040A NO20062040L (en) | 2003-10-08 | 2006-05-08 | Modulators for atp binding cassette transporters containing cycloalkyl or pyranyl groups |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US50964203P | 2003-10-08 | 2003-10-08 | |
| US60/509,642 | 2003-10-08 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2005035514A2 true WO2005035514A2 (en) | 2005-04-21 |
| WO2005035514A3 WO2005035514A3 (en) | 2005-07-14 |
Family
ID=34435000
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2004/033367 WO2005035514A2 (en) | 2003-10-08 | 2004-10-08 | Modulators of atp-binding cassette transporters containing cycloalkyl or pyranyl groups |
Country Status (14)
| Country | Link |
|---|---|
| US (4) | US7598412B2 (en) |
| EP (1) | EP1680411A2 (en) |
| JP (1) | JP2007509044A (en) |
| KR (1) | KR20060121909A (en) |
| CN (1) | CN1886393A (en) |
| AU (1) | AU2004279855A1 (en) |
| CA (1) | CA2540978A1 (en) |
| IL (1) | IL174820A0 (en) |
| MX (1) | MXPA06004005A (en) |
| NO (1) | NO20062040L (en) |
| NZ (1) | NZ546365A (en) |
| RU (1) | RU2006115602A (en) |
| WO (1) | WO2005035514A2 (en) |
| ZA (1) | ZA200603515B (en) |
Cited By (69)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006002421A2 (en) | 2004-06-24 | 2006-01-05 | Vertex Pharmaceuticals Incorporated | Modulators of atp-binding cassette transporters |
| WO2007075946A1 (en) * | 2005-12-27 | 2007-07-05 | Vertex Pharmaceuticals Incorporated | Compounds useful in cftr assays and methods therewith |
| EP1976513A2 (en) * | 2006-01-06 | 2008-10-08 | Sepracor, Inc. | Cycloalkylamines as monoamine reuptake inhibitors |
| WO2009150137A2 (en) | 2008-06-10 | 2009-12-17 | Novartis Ag | Organic compounds |
| EP2149551A1 (en) | 2008-07-30 | 2010-02-03 | Bayer Schering Pharma AG | N-(indol-3-ylalkyl)-(hetero)arylamid derivatives as modulators of EP2 receptors |
| EP2149554A1 (en) | 2008-07-30 | 2010-02-03 | Bayer Schering Pharma Aktiengesellschaft | Indolyamides as modulators for an EP2 receptor |
| EP2149552A1 (en) | 2008-07-30 | 2010-02-03 | Bayer Schering Pharma AG | 5,6 substituted benzamide derivatives as modulators of EP2 receptors |
| WO2010102809A1 (en) * | 2009-03-12 | 2010-09-16 | Grünenthal GmbH | Substituted nicotinamides as kcnq2/3 modulators |
| US7833999B2 (en) | 2004-05-25 | 2010-11-16 | Sanofi-Aventis | Tetrahydroisoquinoline sulfonamide derivatives, the preparation thereof, and the use of the same in therapeutics |
| WO2011048525A1 (en) | 2009-10-20 | 2011-04-28 | Pfizer Inc. | Novel heteroaryl imidazoles and heteroaryl triazoles as gamma-secretase modulators |
| WO2011050325A1 (en) | 2009-10-22 | 2011-04-28 | Vertex Pharmaceuticals Incorporated | Compositions for treatment of cystic fibrosis and other chronic diseases |
| EP2332933A1 (en) | 2007-05-07 | 2011-06-15 | Novartis AG | Epithelial sodium channel (ENaC) inhibitors |
| US8008481B2 (en) | 2006-03-31 | 2011-08-30 | Ericsson Anna M | Indazole compounds |
| WO2011119984A1 (en) | 2010-03-25 | 2011-09-29 | Vertex Pharmaceuticals Incorporated | Solid forms of (r)-1(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihyderoxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide |
| WO2011127241A2 (en) | 2010-04-07 | 2011-10-13 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyriodin-2-yl)benzoic acid and administration thereof |
| WO2011127290A2 (en) | 2010-04-07 | 2011-10-13 | Vertex Pharmaceuticals Incorporated | Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid |
| WO2011133751A2 (en) | 2010-04-22 | 2011-10-27 | Vertex Pharmaceuticals Incorporated | Process of producing cycloalkylcarboxamido-indole compounds |
| WO2012027247A2 (en) | 2010-08-23 | 2012-03-01 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition of (r)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxy propyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration therof |
| WO2012035158A1 (en) | 2010-09-17 | 2012-03-22 | Novartis Ag | Pyrazine derivatives as enac blockers |
| EP2444120A1 (en) | 2007-12-10 | 2012-04-25 | Novartis AG | Spirocyclic amiloride analogues as ENac blockers |
| US8207342B2 (en) | 2009-03-10 | 2012-06-26 | Gruenenthal Gmbh | Substituted 3-amino-2-mercaptoquinolines as KCNQ2/3 modulators |
| US8247573B2 (en) | 2009-03-12 | 2012-08-21 | Gruenenthal Gmbh | Substituted N-(2-mercaptopyridin-3-yl)amides as KCNQ2/3 modulators |
| US8268349B2 (en) | 2003-08-28 | 2012-09-18 | Abbott Laboratories | Solid pharmaceutical dosage form |
| WO2012170061A1 (en) | 2011-06-08 | 2012-12-13 | Vertex Pharmaceuticals Incorporated | Formulations of (r)-1-(2,2-diflurobenzo[d][1,3] dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methyl propan-2-yl)-1h-indol-5-yl)cyclopropanecarboxamide |
| US8354427B2 (en) | 2004-06-24 | 2013-01-15 | Vertex Pharmaceutical Incorporated | Modulators of ATP-binding cassette transporters |
| US8377952B2 (en) | 2003-08-28 | 2013-02-19 | Abbott Laboratories | Solid pharmaceutical dosage formulation |
| US8399673B2 (en) | 2009-03-12 | 2013-03-19 | Gruenenthal Gmbh | Substituted 2-mercaptoquinoline-3-carboxamides as KCNQ2/3 modulators |
| US8410274B2 (en) | 2005-12-28 | 2013-04-02 | Vertex Pharmaceuticals | Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| EP2578571A1 (en) | 2007-11-16 | 2013-04-10 | Vertex Pharmaceuticals Incorporated | Isoquinoline modulators of ATP-binding cassette transporters |
| WO2013070961A1 (en) | 2011-11-08 | 2013-05-16 | Vertex Pharmaceuticals Incorporated | Modulators of atp-binding cassette transporters |
| US8470852B2 (en) | 2010-08-27 | 2013-06-25 | Gruenenthal Gmbh | Substituted 2-amino-quinoline-3-carboxamides as KCNQ2/3 modulators |
| EP2615085A1 (en) | 2008-03-31 | 2013-07-17 | Vertex Pharmaceuticals Incorporated | Pyridyl derivatives as CFTR modulators |
| WO2013112804A1 (en) | 2012-01-25 | 2013-08-01 | Vertex Pharmaceuticals Incorporated | Formulations of 3-(6-(1-(2.2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid |
| WO2013140319A1 (en) | 2012-03-19 | 2013-09-26 | Novartis Ag | Crystalline form of a succinate salt |
| EP2502914A3 (en) * | 2004-06-24 | 2013-12-04 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US8618129B2 (en) | 2010-09-01 | 2013-12-31 | Gruenenthal Gmbh | Substituted 1-oxo-dihydroisoquinoline-3-carboxamides as KCNQ2/3 modulators |
| US8653102B2 (en) | 2010-08-27 | 2014-02-18 | Gruenenthal Gmbh | Substituted 2-oxo- and 2-thioxo-dihydroquinoline-3-carboxamides as KCNQ2/3 modulators |
| US8653101B2 (en) | 2010-08-27 | 2014-02-18 | Gruenenthal Gmbh | Substituted 2-oxy-quinoline-3-carboxamides as KCNQ2/3 modulators |
| WO2014071122A1 (en) | 2012-11-02 | 2014-05-08 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cftr mediated diseases |
| US8802700B2 (en) | 2010-12-10 | 2014-08-12 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-Binding Cassette transporters |
| US9133204B2 (en) | 2007-07-19 | 2015-09-15 | H. Lundbeck A/S | 5-membered heterocyclic amides and related compounds |
| WO2015160787A1 (en) | 2014-04-15 | 2015-10-22 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| WO2016057572A1 (en) | 2014-10-06 | 2016-04-14 | Mark Thomas Miller | Modulators of cystic fibrosis transmembrane conductance regulator |
| EP3013341A4 (en) * | 2013-06-26 | 2017-02-08 | Proteostasis Therapeutics, Inc. | Methods of modulating cftr activity |
| EP3036220A4 (en) * | 2013-08-21 | 2017-04-12 | Alios Biopharma, Inc. | Antiviral compounds |
| EP3170818A1 (en) | 2007-12-07 | 2017-05-24 | Vertex Pharmaceuticals Incorporated | Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid |
| US9701639B2 (en) | 2014-10-07 | 2017-07-11 | Vertex Pharmaceuticals Incorporated | Co-crystals of modulators of cystic fibrosis transmembrane conductance regulator |
| US9732080B2 (en) | 2006-11-03 | 2017-08-15 | Vertex Pharmaceuticals Incorporated | Azaindole derivatives as CFTR modulators |
| US9745292B2 (en) | 2014-03-13 | 2017-08-29 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US9751839B2 (en) | 2009-03-20 | 2017-09-05 | Vertex Pharmaceuticals Incorporated | Process for making modulators of cystic fibrosis transmembrane conductance regulator |
| US9758510B2 (en) | 2006-04-07 | 2017-09-12 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US9790219B2 (en) | 2014-03-13 | 2017-10-17 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US9974781B2 (en) | 2006-04-07 | 2018-05-22 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US10022352B2 (en) | 2006-04-07 | 2018-07-17 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US10058546B2 (en) | 2012-07-16 | 2018-08-28 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof |
| US10081621B2 (en) | 2010-03-25 | 2018-09-25 | Vertex Pharmaceuticals Incorporated | Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide |
| US10174014B2 (en) | 2014-06-19 | 2019-01-08 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US10272046B2 (en) | 2012-02-27 | 2019-04-30 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| US10344023B2 (en) | 2014-12-23 | 2019-07-09 | Proteostasis Therapeutics, Inc. | Derivatives of 3-heteroarylisoxazol-5-carboxylic amide useful for the treatment of inter alia cystic fibrosis |
| US10392372B2 (en) | 2014-12-23 | 2019-08-27 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US10392378B2 (en) | 2014-12-23 | 2019-08-27 | Proteostasis Therapeutics, Inc. | Derivatives of 5-phenyl- or 5-heteroarylathiazol-2-carboxylic amide useful for the treatment of inter alia cystic fibrosis |
| US10550106B2 (en) | 2015-10-06 | 2020-02-04 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for modulating CFTR |
| US10548878B2 (en) | 2015-07-24 | 2020-02-04 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods of increasing CFTR activity |
| US10646481B2 (en) | 2008-08-13 | 2020-05-12 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| US10662207B2 (en) | 2016-04-07 | 2020-05-26 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for modulating CFTR |
| US10738011B2 (en) | 2014-12-23 | 2020-08-11 | Proteostasis Therapeutics, Inc. | Derivatives of 5-(hetero)arylpyrazol-3-carboxylic amide or 1-(hetero)aryltriazol-4-carboxylic amide useful for the treatment of inter alia cystic fibrosis |
| US10899751B2 (en) | 2016-06-21 | 2021-01-26 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| WO2021032934A1 (en) * | 2019-08-21 | 2021-02-25 | Kalvista Pharmaceuticals Limited | Enzyme inhibitors |
| US11014935B2 (en) | 2012-08-23 | 2021-05-25 | Janssen Biopharma, Inc. | Compounds for the treatment of paramyxovirus viral infections |
Families Citing this family (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ZA200602755B (en) | 2003-09-06 | 2007-06-27 | Vertex Pharma | Modulators of ATP-binding cassette transporters |
| ATE437640T1 (en) * | 2003-11-14 | 2009-08-15 | Vertex Pharma | THIAZOLES AND OXAZOLES AS MODULATORS OF ATP BINDING CASSETTE TRANSPORTERS |
| US7977322B2 (en) | 2004-08-20 | 2011-07-12 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| FR2866886B1 (en) * | 2004-02-26 | 2007-08-31 | Sanofi Synthelabo | ARYL-AND HETEROARYL-AKYLCARBAMATES DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| KR101494067B1 (en) * | 2004-03-05 | 2015-02-16 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | Methods for treating disorders or diseases associated with hyperlipidemia and hypercholesterolemia while minimizing side-effects |
| EP1912983B1 (en) | 2005-08-11 | 2011-06-08 | Vertex Pharmaceuticals, Inc. | Modulators of cystic fibrosis transmembrane conductance regulator |
| JP5317184B2 (en) | 2005-11-08 | 2013-10-16 | バーテックス ファーマシューティカルズ インコーポレイテッド | ATP binding cassette transporter modulator |
| US7671221B2 (en) * | 2005-12-28 | 2010-03-02 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-Binding Cassette transporters |
| AU2006336504C9 (en) * | 2005-12-28 | 2015-05-14 | Vertex Pharmaceuticals Incorporated | 1-(benzo [D] [1,3] dioxol-5-yl) -N- (phenyl) cyclopropane- carboxamide derivatives and related compounds as modulators of ATP-Binding Cassette transporters for the treatment of Cystic Fibrosis |
| EP2789606B1 (en) | 2007-05-09 | 2017-11-15 | Vertex Pharmaceuticals Incorporated | Modulators of CFTR |
| WO2009038913A2 (en) | 2007-08-24 | 2009-03-26 | Vertex Pharmaceuticals Incorporated | Isothiazolopyridinones useful for the treatment of (inter alia) cystic fibrosis |
| LT2639223T (en) * | 2007-12-07 | 2017-06-26 | Vertex Pharmaceuticals Incorporated | Process for producing cycloalkylcarboxiamido-pyridine benzoic acids |
| CN101998854A (en) * | 2007-12-07 | 2011-03-30 | 沃泰克斯药物股份有限公司 | Formulations of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cycklopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid |
| US20100036130A1 (en) * | 2007-12-07 | 2010-02-11 | Vertex Pharmaceuticals Incorporated | Processes for producing cycloalkylcarboxamido-pyridine benzoic acids |
| US8299099B2 (en) | 2008-02-28 | 2012-10-30 | Vertex Pharmaceuticals Incorporated | Heteroaryl derivatives as CFTR modulators |
| JP2012504143A (en) | 2008-09-29 | 2012-02-16 | バーテックス ファーマシューティカルズ インコーポレイテッド | 3- (6- (1- (2,2-difluorobenzo [d] [1,3] dioxol-5-yl) cyclopropanecarboxamido) -3-methylpyridin-2-yl) benzoic acid dosage unit |
| ES2483690T3 (en) * | 2008-10-23 | 2014-08-07 | Vertex Pharmaceuticals Incorporated | Modulators of cystic fibrosis transmembrane conductance regulator |
| US9175006B2 (en) * | 2009-06-17 | 2015-11-03 | Board Of Regents, The University Of Texas System | Compositions and methods for cyclofructans as separation agents |
| US8674108B2 (en) | 2012-04-20 | 2014-03-18 | Vertex Pharmaceuticals Incorporated | Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| JP6238969B2 (en) | 2012-05-08 | 2017-11-29 | エアロミクス・インコーポレイテッドAeromics,Inc. | New method |
| RU2691951C2 (en) | 2013-11-06 | 2019-06-19 | Аэромикс, Инк. | Novel compounds |
| KR102280372B1 (en) | 2013-11-12 | 2021-07-22 | 버텍스 파마슈티칼스 인코포레이티드 | Process of preparing pharmaceutical compositions for the treatment of cftr mediated diseases |
| WO2016069891A1 (en) | 2014-10-31 | 2016-05-06 | Abbvie Inc. | Substituted tetrahydropyrans and method of use |
| ES2882656T3 (en) | 2014-11-18 | 2021-12-02 | Vertex Pharma | Process for High Performance Liquid Chromatography High Performance Testing |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2846438A (en) * | 1954-03-15 | 1958-08-05 | Olin Mathieson | Nu-(beta-diethylaminoethyl) isonicotinamide |
| GB874206A (en) * | 1956-09-05 | 1961-08-02 | Knoll Ag | Basic derivatives of salicylamide |
| WO2003051877A1 (en) * | 2001-12-18 | 2003-06-26 | Bayer Corporation | 2-substituted pyrrolo[2.1-a]isoquinolines against cancer |
| WO2003062248A2 (en) * | 2002-01-18 | 2003-07-31 | Merck & Co., Inc. | N-(benzyl)aminoalkylcarboxylates, phosphinates, phosphonates and tetrazoles as edg receptor agonists |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2665440B1 (en) * | 1990-07-31 | 1994-02-04 | Lipha | NOVEL SUBSTITUTED CYCLOALKYLSULFONAMIDES, METHODS OF PREPARATION AND MEDICAMENTS CONTAINING THEM. |
| US5281714A (en) * | 1990-08-16 | 1994-01-25 | American Home Products Corporation | N,N',N'-trisubstituted-5-bisaminomethylene-1,3-dioxane-4,6-dione inhibitors of acyl-CoA: cholesterol-acyl transferase |
| US6632836B1 (en) * | 1998-10-30 | 2003-10-14 | Merck & Co., Inc. | Carbocyclic potassium channel inhibitors |
| FR2796070B1 (en) * | 1999-07-06 | 2003-02-21 | Lipha | BENZODIAZEPINES DERIVATIVES FOR USE IN THE TREATMENT OF DYSLIPIDEMIA, ATHEROSCLEROSIS AND DIABETES, PHARMACEUTICAL COMPOSITIONS CONTAINING THEM AND METHODS OF PREPARATION |
| AUPQ309399A0 (en) * | 1999-09-28 | 1999-10-21 | Fujisawa Pharmaceutical Co., Ltd. | Benzothiazoline derivatives |
| JP2003519120A (en) * | 1999-12-27 | 2003-06-17 | オーソーマクニール ファーマシューティカル, インコーポレイテッド | Substituted aminoalkylamide derivatives as antagonists of follicle-stimulating hormone |
| US6451814B1 (en) * | 2000-07-17 | 2002-09-17 | Wyeth | Heterocyclic β-3 adrenergic receptor agonists |
| US6908934B2 (en) * | 2001-06-11 | 2005-06-21 | Merck & Co., Inc. | Therapeutic compounds for treating dyslipidemic conditions |
| PA8557501A1 (en) * | 2001-11-12 | 2003-06-30 | Pfizer Prod Inc | BENZAMIDA, HETEROARILAMIDA AND INVESTED AMIDAS |
| TW200307539A (en) * | 2002-02-01 | 2003-12-16 | Bristol Myers Squibb Co | Cycloalkyl inhibitors of potassium channel function |
-
2004
- 2004-10-08 EP EP04794652A patent/EP1680411A2/en not_active Withdrawn
- 2004-10-08 WO PCT/US2004/033367 patent/WO2005035514A2/en active Application Filing
- 2004-10-08 MX MXPA06004005A patent/MXPA06004005A/en not_active Application Discontinuation
- 2004-10-08 RU RU2006115602/04A patent/RU2006115602A/en not_active Application Discontinuation
- 2004-10-08 US US10/961,485 patent/US7598412B2/en active Active
- 2004-10-08 NZ NZ546365A patent/NZ546365A/en unknown
- 2004-10-08 KR KR1020067006836A patent/KR20060121909A/en not_active Application Discontinuation
- 2004-10-08 CA CA002540978A patent/CA2540978A1/en not_active Abandoned
- 2004-10-08 ZA ZA200603515A patent/ZA200603515B/en unknown
- 2004-10-08 JP JP2006534421A patent/JP2007509044A/en active Pending
- 2004-10-08 CN CNA2004800355802A patent/CN1886393A/en active Pending
- 2004-10-08 AU AU2004279855A patent/AU2004279855A1/en not_active Abandoned
-
2006
- 2006-04-05 IL IL174820A patent/IL174820A0/en unknown
- 2006-05-08 NO NO20062040A patent/NO20062040L/en not_active Application Discontinuation
-
2009
- 2009-08-14 US US12/541,196 patent/US20100125090A1/en not_active Abandoned
-
2011
- 2011-03-09 US US13/043,895 patent/US20120035179A1/en not_active Abandoned
-
2012
- 2012-06-27 US US13/534,796 patent/US20130012536A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2846438A (en) * | 1954-03-15 | 1958-08-05 | Olin Mathieson | Nu-(beta-diethylaminoethyl) isonicotinamide |
| GB874206A (en) * | 1956-09-05 | 1961-08-02 | Knoll Ag | Basic derivatives of salicylamide |
| WO2003051877A1 (en) * | 2001-12-18 | 2003-06-26 | Bayer Corporation | 2-substituted pyrrolo[2.1-a]isoquinolines against cancer |
| WO2003062248A2 (en) * | 2002-01-18 | 2003-07-31 | Merck & Co., Inc. | N-(benzyl)aminoalkylcarboxylates, phosphinates, phosphonates and tetrazoles as edg receptor agonists |
Non-Patent Citations (6)
| Title |
|---|
| A.L. MNDZHOYAN ET AL.: "synthesis of 1-diphenylmethyl-4,6,7-substituted-1,2,3,4 -Tetrahydroisoquinolines and their analogs" CHEM. HETERO CYCL. COMPD (ENGL. TRANS), vol. 6, 1970, pages 1560-1563, XP008046560 & A.L. MNDZHOYAN ET AL: KHIM GETEROTSIKL, vol. 6, 1970, page 1670, * |
| CLARK C. ETAL: "Anticonvulsant Activity of some 4-Aminobenzamides" J. MED CHEM., vol. 27, no. 6, 1984, - 1984 pages 779-782, XP002327312 * |
| N.J. HARPER ET AL.: "1-(3,4-dichlorobenzamidomethyl)cyclohexyl dimethylamine and Related Compounds as Potential Analgesics" JOURNAL OF MEDICINAL CHEMISTRY, vol. 17, no. 11, 1974, pages 1188-1193, XP002327310 * |
| SHOUWU MIAO ET AL.: "Benzamide Derivatives as Blockers of Kv1.3 Ion Channel" BIOORGANIC MEDICINAL CHEMISTRY LETTERS, vol. 13, 2003, pages 1161-1164, XP002327311 * |
| TERRY S. PURCHASE ET AL.: "INhibitors of Acyl-CoA:Cholesterol Acyltransferase: Novel Trisubstituted Ureas as Hypoholesterolemic Agents" BIOORGANIC AND MEDICINAL CHEMISTRY, vol. 5, no. 4, 1997, pages 739-747, XP002327309 GREAT BRITAIN * |
| TONGHUI MA ET AL.: "High-Affinity Activators of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Chloride Conductance Identified by High-throughput Screening" THE JOURNAL OF BIOLOGICAL CHEMISTRY, vol. 277, no. 40, 4 October 2002 (2002-10-04), pages 37235-37241, XP002327308 USA * |
Cited By (140)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8377952B2 (en) | 2003-08-28 | 2013-02-19 | Abbott Laboratories | Solid pharmaceutical dosage formulation |
| US8333990B2 (en) | 2003-08-28 | 2012-12-18 | Abbott Laboratories | Solid pharmaceutical dosage form |
| US8309613B2 (en) | 2003-08-28 | 2012-11-13 | Abbvie Inc. | Solid pharmaceutical dosage form |
| US8268349B2 (en) | 2003-08-28 | 2012-09-18 | Abbott Laboratories | Solid pharmaceutical dosage form |
| US8399015B2 (en) | 2003-08-28 | 2013-03-19 | Abbvie Inc. | Solid pharmaceutical dosage form |
| US8691878B2 (en) | 2003-08-28 | 2014-04-08 | Abbvie Inc. | Solid pharmaceutical dosage form |
| US7833999B2 (en) | 2004-05-25 | 2010-11-16 | Sanofi-Aventis | Tetrahydroisoquinoline sulfonamide derivatives, the preparation thereof, and the use of the same in therapeutics |
| US8524700B2 (en) | 2004-05-25 | 2013-09-03 | Sanofi | Tetrahydroisoquinoline sulfonamide derivatives, the preparation thereof, and the use of the same in therapeutics |
| US8273733B2 (en) | 2004-05-25 | 2012-09-25 | Sanofi | Tetrahydroisoquinoline sulfonamide derivatives, the preparation thereof, and the use of the same in therapeutics |
| US8324242B2 (en) | 2004-06-24 | 2012-12-04 | Vertex Pharmaceutical Incorporated | Modulators of ATP-binding cassette transporters |
| WO2006002421A2 (en) | 2004-06-24 | 2006-01-05 | Vertex Pharmaceuticals Incorporated | Modulators of atp-binding cassette transporters |
| US8354427B2 (en) | 2004-06-24 | 2013-01-15 | Vertex Pharmaceutical Incorporated | Modulators of ATP-binding cassette transporters |
| EP2502914A3 (en) * | 2004-06-24 | 2013-12-04 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US8629162B2 (en) | 2004-06-24 | 2014-01-14 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US9090619B2 (en) | 2004-06-24 | 2015-07-28 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| WO2006002421A3 (en) * | 2004-06-24 | 2006-09-21 | Vertex Pharma | Modulators of atp-binding cassette transporters |
| US8829204B2 (en) | 2004-06-24 | 2014-09-09 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US8741925B2 (en) | 2004-06-24 | 2014-06-03 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US8101767B2 (en) | 2004-06-24 | 2012-01-24 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| EP2530075A3 (en) * | 2004-06-24 | 2014-12-24 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US10662192B2 (en) | 2004-06-24 | 2020-05-26 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US8614327B2 (en) | 2004-06-24 | 2013-12-24 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| WO2007075946A1 (en) * | 2005-12-27 | 2007-07-05 | Vertex Pharmaceuticals Incorporated | Compounds useful in cftr assays and methods therewith |
| US9139530B2 (en) | 2005-12-28 | 2015-09-22 | Vertex Pharmaceuticals Incorporated | Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US9931334B2 (en) | 2005-12-28 | 2018-04-03 | Vertex Pharmaceuticals Incorporated | Solid forms of N[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US10537565B2 (en) | 2005-12-28 | 2020-01-21 | Vertex Pharmaceuticals Incorporated | Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US9670163B2 (en) | 2005-12-28 | 2017-06-06 | Vertex Pharmaceuticals Incorporated | Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US8410274B2 (en) | 2005-12-28 | 2013-04-02 | Vertex Pharmaceuticals | Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US11291662B2 (en) | 2005-12-28 | 2022-04-05 | Vertex Pharmaceuticals Incorporated | Solid forms of n-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US8754224B2 (en) | 2005-12-28 | 2014-06-17 | Vertex Pharmaceuticals Incorporated | Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US8877975B2 (en) | 2006-01-06 | 2014-11-04 | Sunovion Pharmaceuticals Inc. | Cycloalkylamines as monoamine reuptake inhibitors |
| EP1976513A2 (en) * | 2006-01-06 | 2008-10-08 | Sepracor, Inc. | Cycloalkylamines as monoamine reuptake inhibitors |
| EP1976513A4 (en) * | 2006-01-06 | 2012-04-18 | Sepracor Inc | Cycloalkylamines as monoamine reuptake inhibitors |
| US9868718B2 (en) | 2006-01-06 | 2018-01-16 | Sunovion Pharmaceuticals Inc. | Cycloalkylamines as monoamine reuptake inhibitors |
| JP2013209390A (en) * | 2006-01-06 | 2013-10-10 | Sunovion Pharmaceuticals Inc | Cycloalkylamine as monoamine reuptake inhibitor |
| US10562878B2 (en) | 2006-01-06 | 2020-02-18 | Sunovion Pharamceuticals Inc. | Cycloalkylamines as monoamine reuptake inhibitors |
| US8008481B2 (en) | 2006-03-31 | 2011-08-30 | Ericsson Anna M | Indazole compounds |
| US10022352B2 (en) | 2006-04-07 | 2018-07-17 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US11639347B2 (en) | 2006-04-07 | 2023-05-02 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US10987348B2 (en) | 2006-04-07 | 2021-04-27 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US10975061B2 (en) | 2006-04-07 | 2021-04-13 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US10239867B2 (en) | 2006-04-07 | 2019-03-26 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US9758510B2 (en) | 2006-04-07 | 2017-09-12 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US9974781B2 (en) | 2006-04-07 | 2018-05-22 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| US9732080B2 (en) | 2006-11-03 | 2017-08-15 | Vertex Pharmaceuticals Incorporated | Azaindole derivatives as CFTR modulators |
| EP2332933A1 (en) | 2007-05-07 | 2011-06-15 | Novartis AG | Epithelial sodium channel (ENaC) inhibitors |
| US9133204B2 (en) | 2007-07-19 | 2015-09-15 | H. Lundbeck A/S | 5-membered heterocyclic amides and related compounds |
| EP2578571A1 (en) | 2007-11-16 | 2013-04-10 | Vertex Pharmaceuticals Incorporated | Isoquinoline modulators of ATP-binding cassette transporters |
| EP3012250A1 (en) | 2007-11-16 | 2016-04-27 | Vertex Pharmaceuticals Incorporated | Isoquinoline modulators of atp-binding cassette transporters |
| EP3683218A1 (en) | 2007-12-07 | 2020-07-22 | Vertex Pharmaceuticals Incorporated | Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid |
| EP3170818A1 (en) | 2007-12-07 | 2017-05-24 | Vertex Pharmaceuticals Incorporated | Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid |
| EP2444120A1 (en) | 2007-12-10 | 2012-04-25 | Novartis AG | Spirocyclic amiloride analogues as ENac blockers |
| EP2520574A1 (en) | 2007-12-10 | 2012-11-07 | Novartis AG | Amiloride analogues substituted on the cyclic guanidine moiety as ENaC blockers for treating respiratory diseases |
| EP2980077A1 (en) | 2008-03-31 | 2016-02-03 | Vertex Pharmaceuticals Incorporated | Pyridyl derivatives as cftr modulators |
| EP2615085A1 (en) | 2008-03-31 | 2013-07-17 | Vertex Pharmaceuticals Incorporated | Pyridyl derivatives as CFTR modulators |
| WO2009150137A2 (en) | 2008-06-10 | 2009-12-17 | Novartis Ag | Organic compounds |
| EP2149552A1 (en) | 2008-07-30 | 2010-02-03 | Bayer Schering Pharma AG | 5,6 substituted benzamide derivatives as modulators of EP2 receptors |
| EP2149554A1 (en) | 2008-07-30 | 2010-02-03 | Bayer Schering Pharma Aktiengesellschaft | Indolyamides as modulators for an EP2 receptor |
| EP2149551A1 (en) | 2008-07-30 | 2010-02-03 | Bayer Schering Pharma AG | N-(indol-3-ylalkyl)-(hetero)arylamid derivatives as modulators of EP2 receptors |
| US11564916B2 (en) | 2008-08-13 | 2023-01-31 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| US10646481B2 (en) | 2008-08-13 | 2020-05-12 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| US8207342B2 (en) | 2009-03-10 | 2012-06-26 | Gruenenthal Gmbh | Substituted 3-amino-2-mercaptoquinolines as KCNQ2/3 modulators |
| US8586755B2 (en) | 2009-03-12 | 2013-11-19 | Grünenthal GmbH | Substituted nicotinamides as KCNQ2/3 modulators |
| US8178684B2 (en) | 2009-03-12 | 2012-05-15 | Gruenenthal Gmbh | Substituted nicotinamides as KCNQ2/3 modulators |
| TWI475020B (en) * | 2009-03-12 | 2015-03-01 | The substituted nicotine amide as a KCNQ2 / 3 modifier | |
| US8247573B2 (en) | 2009-03-12 | 2012-08-21 | Gruenenthal Gmbh | Substituted N-(2-mercaptopyridin-3-yl)amides as KCNQ2/3 modulators |
| WO2010102809A1 (en) * | 2009-03-12 | 2010-09-16 | Grünenthal GmbH | Substituted nicotinamides as kcnq2/3 modulators |
| US8399673B2 (en) | 2009-03-12 | 2013-03-19 | Gruenenthal Gmbh | Substituted 2-mercaptoquinoline-3-carboxamides as KCNQ2/3 modulators |
| CN102369188A (en) * | 2009-03-12 | 2012-03-07 | 格吕伦塔尔有限公司 | Substituted nicotinamides as KCNQ2/3 modulators |
| JP2012520247A (en) * | 2009-03-12 | 2012-09-06 | グリュネンタール・ゲゼルシャフト・ミト・ベシュレンクテル・ハフツング | Substituted nicotinamides as KCNQ2 / 3 modulators |
| US9751839B2 (en) | 2009-03-20 | 2017-09-05 | Vertex Pharmaceuticals Incorporated | Process for making modulators of cystic fibrosis transmembrane conductance regulator |
| WO2011048525A1 (en) | 2009-10-20 | 2011-04-28 | Pfizer Inc. | Novel heteroaryl imidazoles and heteroaryl triazoles as gamma-secretase modulators |
| WO2011050325A1 (en) | 2009-10-22 | 2011-04-28 | Vertex Pharmaceuticals Incorporated | Compositions for treatment of cystic fibrosis and other chronic diseases |
| EP2813227A1 (en) | 2009-10-22 | 2014-12-17 | Vertex Pharmaceuticals Incorporated | Compositions for treatment of cystic fibrosis and other chronic diseases |
| US10906891B2 (en) | 2010-03-25 | 2021-02-02 | Vertex Pharmaceuticals Incoporated | Solid forms of (R)-1(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide |
| US11578062B2 (en) | 2010-03-25 | 2023-02-14 | Vertex Pharmaceuticals Incorporated | Solid forms of (R)-1(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide |
| US10081621B2 (en) | 2010-03-25 | 2018-09-25 | Vertex Pharmaceuticals Incorporated | Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide |
| EP4253381A2 (en) | 2010-03-25 | 2023-10-04 | Vertex Pharmaceuticals Incorporated | Solid forms of (r)-1-(2,2-difluorobenzo[d][1,3]d ioxol-5-yl)-n-(1-(2,3-dihydroxyp ropyl)-6-fluoro-2-(1-hydroxy- 2-methylpropan-2-yl)-1h-indol- 5-yl) cyclopropanecarboxamide |
| WO2011119984A1 (en) | 2010-03-25 | 2011-09-29 | Vertex Pharmaceuticals Incorporated | Solid forms of (r)-1(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihyderoxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide |
| EP3835297A1 (en) | 2010-03-25 | 2021-06-16 | Vertex Pharmaceuticals Incorporated | Synthesis and intermediates of (r)-1(2,2 -difluorobenzo[d][1,3]dioxol-5yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2yl)-1h-indol-5yl)cyclopropanecarboxamide |
| EP2826776A1 (en) | 2010-03-25 | 2015-01-21 | Vertex Pharmaceuticals Incorporated | Solid amorphous form of (R)-1(2,2-difluorobenzo(D)(1,3)dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)-cyclopropanecarboxamide |
| EP3181561A1 (en) | 2010-03-25 | 2017-06-21 | Vertex Pharmaceuticals Incorporated | Synthetic intermediate of (r)-1(2,2 -difluorobenzo[d][1,3]dioxol-5yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2yl)-1h-indol-5yl)cyclopropanecarboxamide |
| WO2011127241A2 (en) | 2010-04-07 | 2011-10-13 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyriodin-2-yl)benzoic acid and administration thereof |
| WO2011127290A2 (en) | 2010-04-07 | 2011-10-13 | Vertex Pharmaceuticals Incorporated | Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid |
| EP4005559A1 (en) | 2010-04-07 | 2022-06-01 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyriodin-2-yl)benzoic acid and administration thereof |
| EP3150198A1 (en) | 2010-04-07 | 2017-04-05 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyriodin-2-yl)benzoic acid and administration thereof |
| EP3045452A1 (en) | 2010-04-22 | 2016-07-20 | Vertex Pharmaceuticals Inc. | Process of producing cycloalkylcarboxamido-indole compounds |
| EP3381899A1 (en) | 2010-04-22 | 2018-10-03 | Vertex Pharmaceuticals Incorporated | Intermediate compound for process of producing cycloalkylcarboxamido-indole compounds |
| WO2011133751A2 (en) | 2010-04-22 | 2011-10-27 | Vertex Pharmaceuticals Incorporated | Process of producing cycloalkylcarboxamido-indole compounds |
| US10071979B2 (en) | 2010-04-22 | 2018-09-11 | Vertex Pharmaceuticals Incorporated | Process of producing cycloalkylcarboxamido-indole compounds |
| WO2012027247A2 (en) | 2010-08-23 | 2012-03-01 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition of (r)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxy propyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration therof |
| US9073862B2 (en) | 2010-08-27 | 2015-07-07 | Gruenenthal Gmbh | Substituted 2-oxy-quinoline-3-carboxamides as KCNQ2/3 modulators |
| US8653102B2 (en) | 2010-08-27 | 2014-02-18 | Gruenenthal Gmbh | Substituted 2-oxo- and 2-thioxo-dihydroquinoline-3-carboxamides as KCNQ2/3 modulators |
| US8653101B2 (en) | 2010-08-27 | 2014-02-18 | Gruenenthal Gmbh | Substituted 2-oxy-quinoline-3-carboxamides as KCNQ2/3 modulators |
| US8470852B2 (en) | 2010-08-27 | 2013-06-25 | Gruenenthal Gmbh | Substituted 2-amino-quinoline-3-carboxamides as KCNQ2/3 modulators |
| US8618129B2 (en) | 2010-09-01 | 2013-12-31 | Gruenenthal Gmbh | Substituted 1-oxo-dihydroisoquinoline-3-carboxamides as KCNQ2/3 modulators |
| WO2012035158A1 (en) | 2010-09-17 | 2012-03-22 | Novartis Ag | Pyrazine derivatives as enac blockers |
| US8802700B2 (en) | 2010-12-10 | 2014-08-12 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-Binding Cassette transporters |
| WO2012170061A1 (en) | 2011-06-08 | 2012-12-13 | Vertex Pharmaceuticals Incorporated | Formulations of (r)-1-(2,2-diflurobenzo[d][1,3] dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methyl propan-2-yl)-1h-indol-5-yl)cyclopropanecarboxamide |
| WO2013070961A1 (en) | 2011-11-08 | 2013-05-16 | Vertex Pharmaceuticals Incorporated | Modulators of atp-binding cassette transporters |
| WO2013112804A1 (en) | 2012-01-25 | 2013-08-01 | Vertex Pharmaceuticals Incorporated | Formulations of 3-(6-(1-(2.2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid |
| US11752106B2 (en) | 2012-02-27 | 2023-09-12 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| US10272046B2 (en) | 2012-02-27 | 2019-04-30 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| US11147770B2 (en) | 2012-02-27 | 2021-10-19 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| WO2013140319A1 (en) | 2012-03-19 | 2013-09-26 | Novartis Ag | Crystalline form of a succinate salt |
| US10058546B2 (en) | 2012-07-16 | 2018-08-28 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof |
| US11014935B2 (en) | 2012-08-23 | 2021-05-25 | Janssen Biopharma, Inc. | Compounds for the treatment of paramyxovirus viral infections |
| EP3470063A1 (en) | 2012-11-02 | 2019-04-17 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cftr mediated diseases |
| WO2014071122A1 (en) | 2012-11-02 | 2014-05-08 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cftr mediated diseases |
| EP3013341A4 (en) * | 2013-06-26 | 2017-02-08 | Proteostasis Therapeutics, Inc. | Methods of modulating cftr activity |
| EP3036220A4 (en) * | 2013-08-21 | 2017-04-12 | Alios Biopharma, Inc. | Antiviral compounds |
| US11021444B2 (en) | 2013-08-21 | 2021-06-01 | Janssen Biopharma, Inc. | Antiviral compounds |
| AU2014308991B2 (en) * | 2013-08-21 | 2019-02-14 | Janssen Biopharma, Inc. | Antiviral compounds |
| US9745292B2 (en) | 2014-03-13 | 2017-08-29 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US9790219B2 (en) | 2014-03-13 | 2017-10-17 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US10017503B2 (en) | 2014-03-13 | 2018-07-10 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| EP3424534A1 (en) | 2014-04-15 | 2019-01-09 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| EP4223294A1 (en) | 2014-04-15 | 2023-08-09 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| US11951212B2 (en) | 2014-04-15 | 2024-04-09 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| US10980746B2 (en) | 2014-04-15 | 2021-04-20 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| WO2015160787A1 (en) | 2014-04-15 | 2015-10-22 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| US10206877B2 (en) | 2014-04-15 | 2019-02-19 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| EP3925607A1 (en) | 2014-04-15 | 2021-12-22 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions for the treatment of cystic fibrosis transmembrane conductance regulator mediated diseases |
| US10738040B2 (en) | 2014-06-19 | 2020-08-11 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US10174014B2 (en) | 2014-06-19 | 2019-01-08 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| WO2016057572A1 (en) | 2014-10-06 | 2016-04-14 | Mark Thomas Miller | Modulators of cystic fibrosis transmembrane conductance regulator |
| US9701639B2 (en) | 2014-10-07 | 2017-07-11 | Vertex Pharmaceuticals Incorporated | Co-crystals of modulators of cystic fibrosis transmembrane conductance regulator |
| US10738011B2 (en) | 2014-12-23 | 2020-08-11 | Proteostasis Therapeutics, Inc. | Derivatives of 5-(hetero)arylpyrazol-3-carboxylic amide or 1-(hetero)aryltriazol-4-carboxylic amide useful for the treatment of inter alia cystic fibrosis |
| US10392378B2 (en) | 2014-12-23 | 2019-08-27 | Proteostasis Therapeutics, Inc. | Derivatives of 5-phenyl- or 5-heteroarylathiazol-2-carboxylic amide useful for the treatment of inter alia cystic fibrosis |
| US11098035B2 (en) | 2014-12-23 | 2021-08-24 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US10392372B2 (en) | 2014-12-23 | 2019-08-27 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| US10344023B2 (en) | 2014-12-23 | 2019-07-09 | Proteostasis Therapeutics, Inc. | Derivatives of 3-heteroarylisoxazol-5-carboxylic amide useful for the treatment of inter alia cystic fibrosis |
| US11083709B2 (en) | 2015-07-24 | 2021-08-10 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods of increasing CFTR activity |
| US10548878B2 (en) | 2015-07-24 | 2020-02-04 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods of increasing CFTR activity |
| US11136313B2 (en) | 2015-10-06 | 2021-10-05 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for modulating CFTR |
| US10550106B2 (en) | 2015-10-06 | 2020-02-04 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for modulating CFTR |
| US11248010B2 (en) | 2016-04-07 | 2022-02-15 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for modulating CFTR |
| US10662207B2 (en) | 2016-04-07 | 2020-05-26 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for modulating CFTR |
| US10899751B2 (en) | 2016-06-21 | 2021-01-26 | Proteostasis Therapeutics, Inc. | Compounds, compositions, and methods for increasing CFTR activity |
| WO2021032934A1 (en) * | 2019-08-21 | 2021-02-25 | Kalvista Pharmaceuticals Limited | Enzyme inhibitors |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20060121909A (en) | 2006-11-29 |
| AU2004279855A1 (en) | 2005-04-21 |
| US20100125090A1 (en) | 2010-05-20 |
| CN1886393A (en) | 2006-12-27 |
| US20130012536A1 (en) | 2013-01-10 |
| CA2540978A1 (en) | 2005-04-21 |
| WO2005035514A3 (en) | 2005-07-14 |
| MXPA06004005A (en) | 2006-06-28 |
| NO20062040L (en) | 2006-06-06 |
| RU2006115602A (en) | 2007-11-20 |
| IL174820A0 (en) | 2006-08-20 |
| US20120035179A1 (en) | 2012-02-09 |
| ZA200603515B (en) | 2007-11-28 |
| JP2007509044A (en) | 2007-04-12 |
| US7598412B2 (en) | 2009-10-06 |
| EP1680411A2 (en) | 2006-07-19 |
| US20050148648A1 (en) | 2005-07-07 |
| NZ546365A (en) | 2010-01-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7598412B2 (en) | Modulators of ATP-binding cassette transporters | |
| US8232302B2 (en) | Thiazoles and oxazoles useful as modulators of ATP-binding cassette transporters | |
| EP2363128B1 (en) | Indole modulators of ATP-binding cassette transporters | |
| US9102672B2 (en) | Azaindole derivatives as CFTR modulators | |
| EP3219709B1 (en) | Intermediate compound of modulators of atp-binding cassette transporters | |
| US20090105272A1 (en) | Prodrugs of modulators of ABC transporters | |
| EP2118103A2 (en) | Azaindole derivatives as cftr modulators | |
| WO2005026137A2 (en) | Modulators of atp-binding cassette transporters | |
| EP2271622A2 (en) | Heteroaryl derivatives as cftr modulators | |
| AU2015228930B2 (en) | Heteroaryl derivatives as CFTR modulators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200480035580.2 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2540978 Country of ref document: CA |
|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2004794652 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 546365 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 174820 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020067006836 Country of ref document: KR Ref document number: 2006534421 Country of ref document: JP Ref document number: PA/A/2006/004005 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2004279855 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200603515 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1196/KOLNP/2006 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006115602 Country of ref document: RU |
|
| ENP | Entry into the national phase |
Ref document number: 2004279855 Country of ref document: AU Date of ref document: 20041008 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004279855 Country of ref document: AU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004794652 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020067006836 Country of ref document: KR |