WO2005027839A2 - Methods and reagents for the treatment of immunoinflammatory disorders - Google Patents
Methods and reagents for the treatment of immunoinflammatory disorders Download PDFInfo
- Publication number
- WO2005027839A2 WO2005027839A2 PCT/US2004/030210 US2004030210W WO2005027839A2 WO 2005027839 A2 WO2005027839 A2 WO 2005027839A2 US 2004030210 W US2004030210 W US 2004030210W WO 2005027839 A2 WO2005027839 A2 WO 2005027839A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- analog
- antihistamine
- composition
- patient
- administering
- Prior art date
Links
- MADRVGBADLFHMO-UHFFFAOYSA-N C(C1OCCNC1)Oc1cccc2c1CC=C2 Chemical compound C(C1OCCNC1)Oc1cccc2c1CC=C2 MADRVGBADLFHMO-UHFFFAOYSA-N 0.000 description 1
- XXPVSQRPGBUFKM-SAPNQHFASA-N COCCCC/C(/c(cc1)ccc1Cl)=N\OCCN Chemical compound COCCCC/C(/c(cc1)ccc1Cl)=N\OCCN XXPVSQRPGBUFKM-SAPNQHFASA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4172—Imidazole-alkanecarboxylic acids, e.g. histidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/473—Quinolines; Isoquinolines ortho- or peri-condensed with carbocyclic ring systems, e.g. acridines, phenanthridines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/5415—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame ortho- or peri-condensed with carbocyclic ring systems, e.g. phenothiazine, chlorpromazine, piroxicam
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/57—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of two carbon atoms, e.g. pregnane or progesterone
- A61K31/573—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of two carbon atoms, e.g. pregnane or progesterone substituted in position 21, e.g. cortisone, dexamethasone, prednisone or aldosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/12—Cyclic peptides, e.g. bacitracins; Polymyxins; Gramicidins S, C; Tyrocidins A, B or C
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/12—Cyclic peptides, e.g. bacitracins; Polymyxins; Gramicidins S, C; Tyrocidins A, B or C
- A61K38/13—Cyclosporins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
Definitions
- the invention relates to the treatment of immunoinflammatory disorders.
- Immunoinflammatory conditions are characterized by the inappropriate activation of the body's immune defenses. Rather than targeting infectious invaders, the immune response targets and damages the body's own tissues or transplanted tissues.
- the tissue targeted by the immune system varies with the disorder. For example, in multiple sclerosis, the immune response is directed against the neuronal tissue, while in Crohn's disease the digestive tract is targeted.
- Immunoinflammatory disorders affect millions of individuals and include conditions such as asthma, allergic intraocular inflammatory diseases, arthritis, atopic dermatitis, atopic eczema, diabetes, hemolytic anaemia, inflammatory dermatoses, inflammatory bowel or gastrointestinal disorders (e.g., Crohn's disease and ulcerative colitis), multiple sclerosis, myasthenia gravis, pruritis/inflammation, psoriasis, rheumatoid arthritis, cirrhosis, and systemic lupus erythematosus.
- Current treatment regimens for immunoinflammatory disorders typically rely on immunosuppressive agents. The effectiveness of these agents can vary and their use is often accompanied by adverse side effects.
- the invention features a method for treating an immunoinflammatory disease by administering to a patient in need thereof certain antihistamines, either alone or in combination with any of a number of additional agents. Accordingly, in one aspect, the invention features a method of treating an immunoinflammatory disease in a patient in need thereof by administering to the patient any one of certain antihistamines in an amount and for a duration to treat the disease. In another aspect, the invention features a pharmaceutical composition that includes an antihistamine and a corticosteroid.
- antihistamines are bromodiphenhydramine, clemizole, cyproheptadine, desloratadine, loratadine, thiethylperazine maleate, and promethazine
- corticosteroids are prednisolone, cortisone, dexamethasone, hydrocortisone, methylprednisolone, fluticasone, prednisone, triamcinolone, and diflorasone.
- the composition may be formulated for topical administration, or for systemic administration (e.g., oral administration).
- One or both of the drugs may be present in the composition in a low dosage or a high dose, each of which is defined herein.
- the invention features a method of decreasing proinflammatory cytokine secretion or production in a patient by administering to the patient an antihistamine and a corticosteroid simultaneously or within 14 days of each other in amounts sufficient to decrease proinflammatory cytokine secretion or production in the patient.
- the invention features a method for treating a patient diagnosed with or at risk of developing an immunoinflammatory disorder by administering to the patient an antihistamine and a corticosteroid simultaneously or within 14 days of each other in amounts sufficient to treat the patient.
- the invention features a kit that includes: (i) a composition that includes an antihistamine and a corticosteroid; and (ii) instructions for administering the composition to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- any of the above methods may include administration of one or more additional compounds (e.g., a glucocorticoid receptor modulator, NSAID, COX-2 inhibitor, DMARD, biologic, small molecule immunomodulator, xanthine, anticholinergic compound, beta receptor agonist, bronchodilator non-steroidal immunophilin-dependent immunosuppressant, vitamin D analog, psoralen, retinoid, or 5- amino salicylic acid.
- additional compounds e.g., a glucocorticoid receptor modulator, NSAID, COX-2 inhibitor, DMARD, biologic, small molecule immunomodulator, xanthine, anticholinergic compound, beta receptor agonist, bronchodilator non-steroidal immunophilin-
- the invention features a kit that includes: (i) an antihistamine; (ii) a corticosteroid; and (iii) instructions for administering the antihistamine and the corticosteroid to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a pharmaceutical composition that includes an antihistamine and ibudilast. The composition may be formulated for topical administration, or for systemic administration.
- the invention features a method of decreasing proinflammatory cytokine secretion or production in a patient by administering to the patient an antihistamine and ibudilast simultaneously or within 14 days of each other in amounts sufficient to decrease proinflammatory cytokine secretion or production in the patient.
- the invention features a method for treating a patient diagnosed with or at risk of developing an immunoinflammatory disorder by administering to the patient an antihistamine and ibudilast simultaneously or within 14 days of each other in amounts sufficient to treat the patient.
- the invention features a kit that includes: (i) a composition that includes an antihistamine and ibudilast; and (ii) instructions for administering the composition to a patient diagnosed with or at risk of developing an immunoinflammatory l disorder.
- the invention features a kit that includes: (i) an antihistamine; (ii) ibudilast; and (iii) instructions for administering the antihistamine and the ibudilast to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a pharmaceutical composition that includes an antihistamine and rolipram. The composition may be formulated for topical administration, or for systemic administration.
- the invention features a method of decreasing proinflammatory cytokine secretion or production in a patient by administering to the patient an antihistamine and rolipram simultaneously or within 14 days of each other in amounts sufficient to decrease proinflammatory cytokine secretion or production in the patient.
- the invention features a method for treating a patient diagnosed with or at risk of developing an immunoinflammatory disorder by administering to the patient an antihistamine and rolipram simultaneously or within 14 days of each other in amounts sufficient to treat the patient.
- the invention features a kit that includes: (i) a composition that includes an antihistamine and rolipram; and (ii) instructions for administering the composition to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a kit that includes: (i) an antihistamine; (ii) rolipram; and (iii) instructions for administering the antihistamine and the rolipram to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a pharmaceutical composition that includes an antihistamine and a tetra-substituted pyrimidopyrimidine.
- a particularly desirable tetra-substituted pyrimidopyrimidine is dipyridamole.
- the composition may be formulated for topical administration, or for systemic administration.
- the invention features a method of decreasing proinflammatory cytokine secretion or production in a patient by administering to the patient an antihistamine and a tetra-substituted pyrimidopyrimidine (e.g., dipyridamole) simultaneously or within 14 days of each other in amounts sufficient to decrease proinflammatory cytokine secretion or production in the patient.
- the invention features a method for treating a patient diagnosed with or at risk of developing an immunoinflammatory disorder by administering to the patient an antihistamine and a tetra-substituted pyrimidopyrimidine (e.g., dipyridamole) simultaneously or within 14 days of each other in amounts sufficient to treat the patient. .
- the invention features a kit that includes: (i) a composition that includes an antihistamine and a tetra-substituted pyrimidopyrimidine; and (ii) instructions for administering the composition to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a kit that includes: (i) an antihistamine; (ii) a tetra-substituted pyrimidopyrimidine; and (iii) instructions for administering the antihistamine and the tetra-substituted pyrimidopyrimidine to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a pharmaceutical composition that includes an antihistamine and a tricyclic or tetracyclic antidepressant.
- Particularly desirable tricyclic or tetracyclic antidepressants are nortryptiline, amoxapine, and desipramine.
- the antihistamine is not doxepin, while in another embodiment, the antidepressant is not doxepin.
- the composition may be formulated for topical administration, or for systemic administration.
- the invention features a method of decreasing proinflammatory cytokine secretion or production in a patient by administering to the patient an antihistamine and a tricyclic or tetracyclic antidepressant simultaneously or within 14 days of each other in amounts sufficient to decrease proinflammatory cytokine secretion or production in the patient.
- the antihistamine is not doxepin
- the antidepressant is not doxepin.
- the invention features a method for treating a patient diagnosed with or at risk of developing an immunoinflammatory disorder by administering to the patient an antihistamine and a tricyclic or tetracyclic antidepressant simultaneously or within 14 days of each other in amounts sufficient to treat the patient.
- the antihistamine is not doxepin, while in another embodiment, the antidepressant is not doxepin.
- the invention features a kit that includes: (i) a composition that includes an antihistamine and a tricyclic or tetracyclic antidepressant; and (ii) instructions for administering the composition to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the antihistamine is not doxepin, while in another embodiment, the antidepressant is not doxepin.
- the invention features a kit that includes: (i) an antihistamine; (ii) a tricyclic or tetracyclic antidepressant; and (iii) instructions for administering the antihistamine and the tricyclic or tetracyclic antidepressant to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a pharmaceutical composition that includes an antihistamine and a selective serotonin reirptake inhibitor (SSRI).
- SSRI selective serotonin reirptake inhibitor
- compositions may be formulated for topical administration, or for systemic administration (e.g., oral administration).
- the invention features a method of decreasing proinflammatory cytokine secretion or production in a patient by administering to the patient an antihistamine and an SSRI simultaneously or within 14 days of each other in amounts sufficient to decrease proinflammatory cytokine secretion or production in the patient.
- the invention features a method for treating a patient diagnosed with or at risk of developing an immunoinflammatory disorder by administering to the patient an antihistamine and an SSRI simultaneously or within 14 days of each other in amounts sufficient to treat the patient.
- the invention features a kit that includes: (i) a composition that includes an antihistamine and an SSRI; and (ii) instructions for administering the composition to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the invention features a kit that includes: (i) an antihistamine; (ii) an SSRI; and (iii) instructions for administering the antihistamine and the SSRI to a patient diagnosed with or at risk of developing an immunoinflammatory disorder.
- the compounds are administered within 10 days of each other, within, five days of each other, within twenty- four hours of each other, or even simultaneously.
- the compounds may be formulated together as a single composition, or may be formulated and administered separately.
- One or both compounds may be administered in a low dosage or in a high dosage, each of which is defined herein.
- a composition may include one or more additional compounds (e.g., a glucocorticoid receptor modulator, NSAID, COX-2 inhibitor, DMARD, biologic, small molecule immunomodulator, xanthine, anticholinergic compound, beta receptor agonist, bronchodilator non-steroidal immunophilin-dependent immunosuppressant, vitamin D analog, psoralen, retinoid, or 5- amino salicylic acid).
- additional compounds e.g., a glucocorticoid receptor modulator, NSAID, COX-2 inhibitor, DMARD, biologic, small molecule immunomodulator, xanthine, anticholinergic compound, beta receptor agonist, bronchodilator non-steroidal immunophilin-dependent immunosuppressant, vitamin D analog, psoralen, retinoid, or 5- amino salicylic acid.
- additional compounds e.g., a glucocorticoid receptor modulator, NSAID, COX-2 inhibitor,
- Combination therapies of the invention are especially useful for the treatment of immunoinflammatory disorders in combination with other anti-cytokine agents or agents that modulate the immune response to positively effect disease, such as agents that block the action of IL-6, IL-2, IL- 1 , IL- 12, IL- 15 , or TNF ⁇ (e.g., etanercept, infliximab, and adelimumab), and agents that influence cell adhesion.
- the combination therapy reduces the production of cytokines, etanercept or infliximab act on the remaining fraction of inflammatory cytokines, providing enhanced treatment.
- analogs of certain compounds may be employed in lieu of the compounds themselves.
- Analogs of antihistamines and other compounds are described herein.
- Structural analogs of a compound (e.g, ibudilast) or class of compound (e.g., antihistamines) do not need to have the same activity as the compound or class to which it is related.
- an SSRI analog does not necessarily inhibit serotonin reuptake.
- Immunoinflammatory disorders that may be treated by this method are provided herein, and include rheumatoid arthritis, Crohn's disease, ulcerative colitis, asthma, chronic obstructive pulmonary disease, polymylagia rheumatica, giant cell arteritis, systemic lupus erythematosus, atopic dermatitis, multiple sclerosis, myasthenia gravis, psoriasis, ankylosing spondylitis, and psoriatic arthritis.
- corticosteroid is meant any naturally occurring or synthetic compound characterized by a hydrogenated cyclopentanoperhydrophenanthrene ring system. Naturally occurring corticosteroids are generally produced by the adrenal cortex.
- Synthetic corticosteroids may be halogenated. Exemplary corticosteroids are described herein.
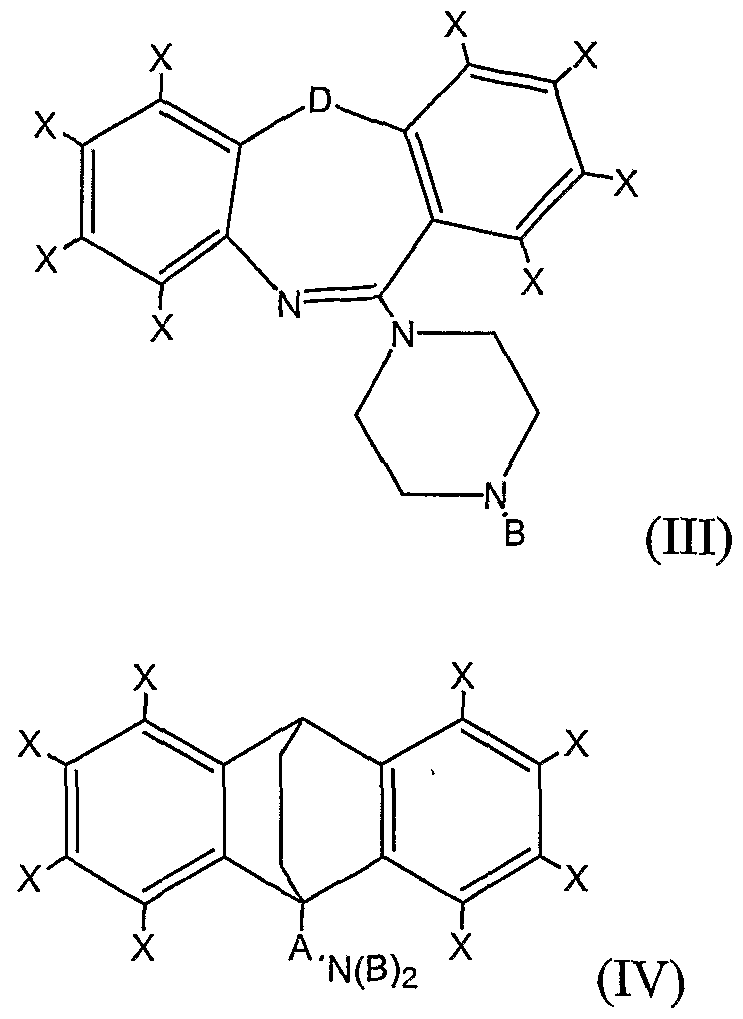
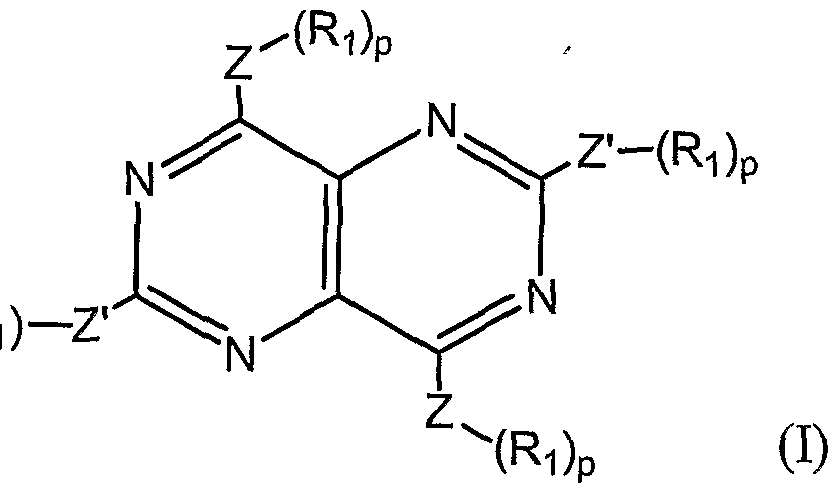
- trimer is meant a compound having one the formulas (I), (II), (III), or (IV):
- each X is, independently, H, Cl, F, Br, I, CH 3 , CF 3 , OH, OCH 3 , CH 2 CH 3 , or OCH 2 CH 3 ;
- Y is CH 2 , O, NH, S(O) 0-2 , (CH 2 ) 3 , (CH) 2 , CH 2 O, CH 2 NH, CHN, or CH 2 S;
- Z is C or S;
- A is a branched or unbranched, saturated or monounsaturated hydrocarbon chain having between 3 and 6 carbons, inclusive;
- each B is, independently, H, Cl, F, Br, I, CX 3 , CH 2 CH 3 , OCX 3 , or OCX 2 CX 3 ;
- D is CH 2 , O, NH, S(O) 0-2 .
- antihistamine is meant a compound that blocks the action of histamine.
- Classes of antihistamines include but are not limited to, ethanolamines, ethylenediamine, phenothiazine, alkylamines, piperazines, and piperidines
- SSRI is meant any member of the class of compounds that (i) inhibit the uptake of serotonin by neurons of the central nervous system, (ii) have an inhibition constant (Ki) of 10 nM or less, and (iii) a selectivity for serotonin over norepinephrine (i.e., the ratio of Ki(norepinephrine) over Ki(serotonin)) of greater than 100.
- SSRIs are administered in dosages of greater than 1 mg per day when used as antidepressants.
- SSRIs for use in the invention are fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, and venlafaxine.
- non-steroidal immunophilin-dependent immunosuppressant or “TSTsiDi” is meant any non-steroidal agent that decreases proinflammatory cytokine production or secretion, binds an immunophilin, or causes a down regulation of the proinflammatory reaction.
- NsIDIs include calcineurin inhibitors, such as cyclosporine, tacrolimus, ascomycin, pimecrolimus, as well as other agents (peptides, peptide fragments, chemically modified peptides, or peptide mimetics) that inhibit the phosphatase activity of calcineurin. NsIDIs also include rapamycin (sirolimus) and everolimus, which binds to an FK506-binding protein, FKBP-12, and block antigen-induced proliferation of white blood cells and cytokine secretion.
- calcineurin inhibitors such as cyclosporine, tacrolimus, ascomycin, pimecrolimus
- agents peptides, peptide fragments, chemically modified peptides, or peptide mimetics
- small molecule immunomodulator is meant a non-steroidal, non-NsIDI compound that decreases proinflammatory cytokine production or secretion, causes a down regulation of the proinflammatory reaction, or otherwise modulates the immune system in an immunophilin-independent manner.
- Examplary small molecule immunomodulators are p38 MAP kinase inhibitors such as VX 702 (Vertex Pharmaceuticals), SCIO 469 (Scios), doramapimod (Boehringer rngelheim), RO
- a "low dosage” is meant at least 5% less (e.g., at least 10%, 20%, 50%, 100%,
- a “high dosage” is meant at least 5% (e.g., at least 10%, 20%, 50%, 100%, 200%, or even 300%) more than the highest standard recommended dosage of a particular compound for treatment of any human disease or condition
- a “moderate dosage” is meant the dosage between the low dosage and the high dosage.
- treating is meant administering or prescribing a pharmaceutical composition for the treatment or prevention of an immunoinflammatory disease.
- patient is meant any animal (e.g., a human).
- an amount sufficient is meant the amount of a compound, in a combination of the invention, required to treat or prevent an immunoinflammatory disease in a clinically relevant manner.
- a sufficient amount of an active compound used to practice the present invention for therapeutic treatment of conditions caused by or contributing to an immunoinflammatory disease varies depending upon the manner of administration, the age, body weight, and general health of the patient. Ultimately, the prescribers will decide the appropriate amount and dosage regimen.
- an effective amount may can be that amount of compound in the combination of the invention that is safe and efficacious in the treatment of a patient having the immunoinflammatory disease over each agent alone as determined and approved by a regulatory authority (such as the U.S. Food and Drug Administration).
- a regulatory authority such as the U.S. Food and Drug Administration.
- more effective is meant that a method, composition, or kit exhibits greater efficacy, is less toxic, safer, more convenient, better tolerated, or less expensive, or provides more treatment satisfaction than another method, composition, or kit with which it is being compared. Efficacy may be measured by a skilled practitioner using any standard method that is appropriate for a given indication.
- the term "immunoinflammatory disorder” encompasses a variety of conditions, including autoimmune diseases, proliferative skin diseases, and inflammatory dermatoses.
- Immunoinflammatory disorders result in the destruction of healthy tissue by an inflammatory process, dysregulation of the immune system, and unwanted proliferation of cells.
- immunoinflammatory disorders are acne vulgaris; acute respiratory distress syndrome; Addison's disease; allergic rhinitis; allergic intraocular inflammatory diseases, ANCA-associated small-vessel vasculitis; ankylosing spondylitis; arthritis, asthma; atherosclerosis; atopic dermatitis; autoimmune hemolytic anemia; autoimmune hepatitis; Behcet's disease; Bell's palsy; bullous pemphigoid; cerebral ischaemia; chronic obstructive pulmonary disease; cirrhosis; Cogan's syndrome; contact cartatitis; COPD; Crohn's disease; Cushing's syndrome; dermatomyositis; diabetes mellitus; discoid lupus erythematosus; eosinophilic fasciitis; erythema nodosum; exfoliative dermatitis;
- Non-dermal inflammatory disorders include, for example, rheumatoid arthritis, hiflammatory bowel disease, asthma, and chronic obstructive pulmonary disease.
- Dermatoses include, for example, psoriasis, acute febrile neutrophilic dermatosis, eczema (e.g., histotic eczema, dyshidrotic eczema, vesicular palmoplantar eczema), balanitis circumscripta plasmacellularis, balanoposthitis, Behcet's disease, erythema annulare centrifugum, erythema dyschromicum perstans, erythema multiforme, granuloma annulare, lichen nitidus, lichen planus, lichen sclerosus et atrophicus, lichen simplex chronicus, lichen spinulosus, nummular dermatitis,
- proliferative skin disease is meant a benign or malignant disease that is characterized by accelerated cell division in the epidermis or dermis.
- proliferative skin diseases are psoriasis, atopic dermatitis, non-specific dermatitis, primary irritant contact dermatitis, allergic contact dermatitis, basal and squamous cell carcinomas of the skin, lamellar ichthyosis, epidermolytic hyperkeratosis, premalignatit keratosis, acne, and seborrheic dermatitis.
- a particular disease, disorder, or condition may be characterized as being both a proliferative skin disease and an inflammatory dermatosis.
- An example of such a disease is psoriasis.
- sustained release or controlled release is meant that the therapeutically active component is released from the formulation at a controlled rate such that therapeutically beneficial blood levels (but below toxic levels) of the component are maintained over an extended period of time ranging from e.g., about 12 to about 24 hours, thus, providing, for example, a 12 hour or a 24 hour dosage form.
- the number of atoms of a particular type in a substituent group is generally given as a range, e.g., an alkyl group containing from 1 to 7 carbon atoms or C 1- alkyl. Reference to such a range is intended to include specific references to groups having each of the integer number of atoms within the specified range.
- an alkyl group from 1 to 7 carbon atoms includes each of Ci, C 2 , C 3 , C , C 5 , C 6 , and C 7 .
- a C 1-7 heteroalkyl for example, includes from 1 to 7 carbon atoms in addition to one or more heteroatoms. Other numbers of atoms and other types of atoms may be indicated in a similar manner.
- alkyl and the prefix “alk-” are inclusive of both straight chain and branched chain groups and of cyclic groups, i.e., cycloalkyl.
- Cyclic groups can be monocyclic or polycyclic and preferably have from 3 to 6 ring carbon atoms, inclusive.
- Exemplary cyclic groups include cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl groups.
- the C ⁇ -7 alkyl group may be substituted or unsubstituted.
- substituents include alkoxy, aryloxy, sulfhydryl, alkylthio, arylthio, halide, hydroxyl, fluoroalkyl, perfluoralkyl, amino, aminoalkyl, disubstituted amino, quaternary amino, hydroxyalkyl, carboxyalkyl, and carboxyl groups.
- C ⁇ -7 alkyls include, without limitation, methyl; ethyl; n-propyl; isopropyl; cyclopropyl; cyclopropylmethyl; cyclopropylethyl; n-butyl; iso-butyl; sec-butyl; tert-butyl; cyclobutyl; cyclobutylmethyl; cyclobutylethyl; n-pentyl; cyclopentyl; cyclopentylmethyl; cyclopentylethyl; 1- methylbutyl; 2-methylbutyl; 3-methylbutyl; 2,2-dimethylpropyl; 1-ethylpropyl; 1 ,1- dimethylpropyl; 1,2-dimethylpropyl; 1-methylpentyl; 2-methylpentyl; 3-methyl ⁇ entyl; 4- methylpentyl; 1,1 -dimethylbutyl; 1,2-dimethylbutyl; 1,3
- C 2-7 alkenyl is meant a branched or unbranched hydrocarbon group containing one or more double bonds and having from 2 to 7 carbon atoms.
- a C 2 . alkenyl may optionally include monocyclic or polycyclic rings, in which each ring desirably has from three to six members.
- the C 2 - 7 alkenyl group may be substituted or unsubstituted.
- Exemplary substituents include alkoxy, aryloxy, sulfhydryl, alkylthio, .
- C 2-7 alkenyls include, without limitation, vinyl; allyl; 2-cyclopropyl-l-ethenyl; 1-propenyl; 1- butenyl; 2-butenyl; 3-butenyl; 2-methyl- 1-propenyl; 2-methyl-2-pro ⁇ enyl; 1-pentenyl; 2- pentenyl; 3-pentenyl; 4-pentenyl; 3-methyl-l-butenyl; 3-methyl-2-butenyl; 3-methyl-3- butenyl; 2-methyl- 1-butenyl; 2-methyl-2-butenyl; 2-methyl-3-butenyl; 2-ethyl-2- propenyl; 1 -methyl- 1-butenyl; l-methyl-2-butenyl; l-methyl-3-butenyl; 2-methyl-2- pentenyl; 3-methyl-2-pentenyl; 4-methyl-2-pentenyl; 2-methyl-3-pentenyl; 3-methyl-3- pentenyl; 4-methyl-2-pentenyl
- C 2- alkynyl is meant a branched or unbranched hydrocarbon group containing one or more triple bonds and having from 2 to 7 carbon atoms.
- a C 2-7 alkynyl may optionally include monocyclic, bicyclic, or tricyclic rings, in which each ring desirably has five or six members.
- the C -7 alkynyl group may be substituted or unsubstituted.
- substituents include alkoxy, aryloxy, sulfhydryl, alkylthio, arylthio, halide, hydroxy, fluoroalkyl, perfluoralkyl, amino, aminoalkyl, disubstituted amino, quaternary amino, hydroxyalkyl, carboxyalkyl, and carboxyl groups.
- C -7 alkynyls include, without limitation, ethynyl, 1-propynyl, 2-propynyl, 1 -butynyl, 2- butynyl, 3-butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 5-hexene-l-ynyl, 2- hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl; l-methyl-2-propynyl; l-methyl-2-butynyl; 1- methyl-3 -butynyl; 2-methyl-3 -butynyl; l,2-dimethyl-3 -butynyl; 2,2-dimethyl-3-butynyl; l-methyl-2-pentynyl; 2-methyl-3-pentynyl; l-methyl-4-pentynyl; 2-methyl-4-
- C 2-6 heterocyclyl is meant a stable 5- to 7-membered monocyclic or 7- to 14- membered bicyclic heterocyclic ring which is saturated partially unsaturated or unsaturated (aromatic), and which consists of 2 to 6 carbon atoms and 1, 2, 3 or 4 heteroatoms independently selected from the group consisting of N, O, and S and including any bicyclic group in which any of the above-defined heterocyclic rings is fused to a benzene ring.
- the heterocyclyl group may be substituted or unsubstituted.
- substituents include alkoxy, aryloxy, sulfhydryl, alkylthio, arylthio, halide, hydroxy, fluoroalkyl, perfluoralkyl, amino, aminoalkyl, disubstituted amino, quaternary amino, hydroxyalkyl, carboxyalkyl, and carboxyl groups.
- the nitrogen and sulfur heteroatoms may optionally be oxidized.
- the heterocyclic ring may be covalently attached via any heteroatom or carbon atom that results in a stable structure, e.g., an imidazolinyl ring may be linked at either of the ring-carbon atom positions or at the nitrogen atom.
- a nitrogen atom in the heterocycle may optionally be quatemized.
- Heterocycles include, without limitation, lH-indazole, 2- ⁇ yrrolidonyl, 2H,6H-l,5,2-dithiazinyl, 2H-pyrrolyl, 3H- indolyl, 4-piperidonyl, 4aH-carbazole, 4H-quinolizinyl, 6H-l,2,5-thiadiazinyl, acridinyl, azocinyl, benzimidazolyl, benzofuranyl, benzothiofuranyl, benzothiophenyl, benzoxazolyl, benzthiazolyl, benztriazolyl, benztetrazolyl, benzisoxazolyl, benzisothiazolyl, benzimidazalonyl, carbazolyl, 4aH-carbazolyl, b
- Preferred 5 to 10 membered heterocycles include, but are not limited to, pyridinyl, pyrimidinyl, triazinyl, furanyl, thienyl, thiazolyl, pyrrolyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, tetrazolyl, benzofuranyl, benzothiofuranyl, indolyl, benzimidazolyl, lH-indazolyl, oxazolidinyl, isoxazolidinyl, benzotriazolyl, benzisoxazolyl, oxindolyl, benzoxazolinyl, quinolinyl, and isoquinolinyl.
- Preferred 5 to 6 membered heterocycles include, without limitation, pyridinyl, pyrimidinyl, triazinyl, furanyl, thienyl, thiazolyl, pyrrolyl, piperazinyl, piperidinyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, 1, 4,5, 6-tetrahydro pyridinyl, and tetrazolyl.
- C 6-12 aryl is meant an aromatic group having a ring system comprised of carbon atoms with conjugated ⁇ electrons (e.g., phenyl). The aryl group has from 6 to 12 carbon atoms.
- Aryl groups may optionally include monocyclic, bicyclic, or tricyclic rings, in which each ring desirably has five or six members.
- the aryl group may be substituted or unsubstituted.
- Exemplary subsituents include alkyl, hydroxy, alkoxy, aryloxy, sulfhydryl, alkylthio, arylthio, halide, fluoroalkyl, carboxyl, hydroxyalkyl, carboxyalkyl, amino, aminoalkyl, monosubstituted amino, disubstituted amino, and quaternary amino groups.
- C 7 - 14 alkaryl is meant an alkyl substituted by an aryl group (e.g., benzyl, phenethyl, or 3,4-dichlorophenethyl) having from 7 to 14 carbon atoms.
- C 3 . 10 alkheterocyclyl is meant an alkyl substituted heterocyclic group having from 7 to 14 carbon atoms in addition to one or more heteroatoms (e.g., 3-furanylmethyl, 2-furanylmethyl, 3-tetrahydrofuranylmethyl, or 2-tetrahydrofuranylmethyl).
- C 1-7 heteroalkyl is meant a branched or unbranched alkyl, alkenyl, or alkynyl group having from 1 to 7 carbon atoms in addition to 1, 2, 3 or 4 heteroatoms independently selected from the group consisting of N, O, S, and P.
- Heteroalkyls include, without limitation, tertiary amines, secondary amines, ethers, thioethers, amides, thioamides, carbamates, thiocarbamates, hydrazones, i ines, phosphodiesters, phosphoramidates, sulfonamides, and disulfides.
- a heteroalkyl may optionally include monocyclic, bicyclic, or tricyclic rings, in which each ring desirably has three to six members.
- the heteroalkyl group may be substituted or unsubstituted.
- substituents include alkoxy, aryloxy, sulfhydryl, alkylthio, arylthio, halide, hydroxyl, fluoroalkyl, perfluoralkyl, amino, aminoalkyl, disubstituted amino, quaternary amino, hydroxyalkyl, hydroxyalkyl, carboxyalkyl, and carboxyl groups.
- acyl is meant a chemical moiety with the formula R-C(O)-, wherein R is selected from C 1-7 alkyl, C 2- alkenyl, C -7 alkynyl, C 2-6 heterocyclyl, C 6-12 aryl, C 7-14 alkaryl, C 3-10 alkheterocyclyl, or C 1-7 heteroalkyl.
- alkoxy is meant a chemical substituent of the fonnula -OR, wherein R is selected from C 1-7 alkyl, C 2-7 alkenyl, C 2- alkynyl, C 2 _6 heterocyclyl, C 6- i 2 aryl, C 7-14 alkaryl, C 3- ⁇ o alkheterocyclyl, or C 1-7 heteroalkyl.
- aryloxy is meant a chemical substituent of the formula -OR, wherein R is a
- -NRR' a chemical substituent of the formula -NRR', wherein the nitrogen atom is part of an amide bond (e.g., -C(O)-NRR') and wherein R and R' are each, independently, selected from C 1-7 alkyl, C 2-7 alkenyl, C -7 alkynyl, C 2-6 heterocyclyl, C 6- i 2 aryl, C 7- ⁇ 4 alkaryl, C 3- ⁇ 0 alkheterocyclyl, and C ⁇ -7 heteroalkyl, or -NRR' forms a C 2- 6 heterocyclyl ring, as defined above, but containing at least one nitrogen atom, such as piperidino, morpholino, and azabicyclo, among others.
- -NRR' forms a C 2- 6 heterocyclyl ring, as defined above, but containing at least one nitrogen atom, such as piperidino, morpholino, and azabicyclo, among others.
- halide is meant bromine, chlorine, iodine, or fluorine.
- fluoroalkyl is meant an alkyl group that is substituted with a fluorine.
- perfluoroalkyl is meant an alkyl group consisting of only carbon and fluorine atoms.
- carboxyalkyl is meant a chemical moiety with the formula -(R)-COOH, wherein R is selected from C J-7 alkyl, C 2- 7 alkenyl, C 2-7 alkynyl, C 2-6 heterocyclyl, C 6-12 aryl, C 7-14 alkaryl, C 3-10 alkheterocyclyl, or C 1-7 heteroalkyl.
- hydroxyalkyl is meant a chemical moiety with the formula -(R)-OH, wherein
- R is selected from C ⁇ -7 alkyl, C 2-7 alkenyl, C 2-7 alkynyl, C 2-6 heterocyclyl, C 6- ⁇ 2 aryl, C 7-I alkaryl, C 3-10 alkheterocyclyl, or C 1-7 heteroalkyl.
- alkylthio is meant a chemical substituent of the formula -SR, wherein R is selected from C 1-7 alkyl, C 2-7 alkenyl, C 2- alkynyl, C 2-6 heterocyclyl, C 6 -i 2 aryl, C -14 alkaryl, C 3-10 alkheterocyclyl, or C ⁇ -7 heteroalkyl.
- arylthio is meant a chemical substituent of the formula -SR, wherein R is a C ⁇ -12 aryl group.
- quaternary amino is meant a chemical substituent of the formula -(R)-N(R')(R")(R'") + , wherein R, R', R", and R'" are each independently an alkyl, alkenyl, alkynyl, or aryl group.
- R may be an alkyl group linking the quaternary amino nitrogen atom, as a substituent, to another moiety.
- the nitrogen atom, N is covalently attached to four carbon atoms of alkyl and/or aryl groups, resulting in a positive charge at the nitrogen atom.
- salts represent those salts which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and lower animals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio.
- Pharmaceutically acceptable salts are well known in the art.
- the salts can be prepared in situ during the final isolation and purification of the compounds of the invention, or separately by reacting the free base function with a suitable organic acid.
- Representative acid addition salts include acetate, adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate, !
- alkali or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like, as well as nontoxic ammonium, quaternary ammonium, and amine cations, including, but not limited to ammonium, tetramethylammonium, tetraethylammonium, methylamine, dimethylamine, trimethylamine, triethylamine, ethylamine, and the like.
- Compounds useful in the invention include those described herein in any of their pharmaceutically acceptable forms, including isomers such as diastereomers and enantiomers, salts, esters, amides, thioesters, solvates, and polymorphs thereof, as well as racemic mixtures and pure isomers of the compounds described herein.
- fexofenadine is meant the free base, as well as any pharmaceutically acceptable salt thereof (e.g., fexofenadine hydrochloride).
- any of the foregoing conditions may be treated by administration of an effective amount of an antihistamine or analog thereof, either alone or in combination with one or more additional agents.
- treatment of an immunoinflammatory disorder e.g., an inflammatory dermatosis, proliferative skin disease, organ transplant rejection, or graft versus host disease
- an antihistamine or analog thereof
- a corticosteroid is administered to a patient in need of such treatment.
- treatment of an immunoinflammatory disorder is perfonned by administering an antihistamine (or analog thereof) and a tricyclic or tetracyclic antidepressant to a patient in need of such treatment.
- treatment is performed by administering an antihistamine (or analog thereof) and a selective serotonin reuptake inhibitor to a patient suffering from any of the foregoing conditions.
- treatment is performed by administering to a patient in need of such treatment, in conjunction with an antihistamme or antihistamine analog, dipyridamole, ibudilast, rolipram, or an analog of any of these compounds.
- Exemplary routes of administration for the various embodiments can include, but are not limited to, topical, transdermal, and systemic administration (such as, intravenous, intramuscular, subcutaneous, inhalation, rectal, buccal, vaginal, intraperitoneal, intraarticular, ophthalmic or oral administration).
- systemic administration refers to all nondermal routes of administration, and specifically excludes topical and transdermal routes of administration. Any of the foregoing therapies may be administered with conventional pharmaceuticals useful for the treatment of immunoinflammatory disorders.
- Antihistamines are compounds that block the action of histamine.
- Classes of antihistamines include: (1) Ethanolamines (e.g., bromodiphenhydramine, carbinoxamine, clemastine, dimenhydrinate, diphenhydramine, diphenylpyraline, and doxylamine); (2) Ethylenediamines (e.g., pheniramine, pyrilamine, tripelennamine, and triprolidine); (3) Phenothiazines (e.g., diethazine, ethopropazine, methdilazine, promethazine, thiethylperazine, and trimeprazine); (4) Alkylamines (e.g., acrivastine, brompheniramine, chlorpheniramine, desbrompheniramine, dexchlorpheniramine, pyrrobutamine, and triprolidine); (5) Piperazines (e.g., buclizine,
- non-sedating and sedating antihistamines may be employed.
- Particularly desirable antihistamines for use in the methods, compositions, and kits of the invention are non-sedating antihistammes such as loratadine and desloratadine. Sedating antihistamines can also be used in the methods, compositions, and kits of the invention.
- Preferred sedating antihistamines are methods, compositions, and kits of the invention are azatadine, bromodiphenhydramine; chlorpheniramine; clemizole; cyproheptadine; dimenhydrinate; diphenhydramine; doxylamine; meclizine; promethazine; pyrilamine; thiethylperazine; and tripelennamine.
- antihistamines suitable for use in the methods and compositions of the invention are acrivastine; ahistan; antazoline; astemizole; azelastine (e.g., azelsatine hydrochloride); bamipine; bepotastine; bietanautine; brompheniramine (e.g., brompheniramine maleate); carbinoxamine (e.g., carbinoxamine maleate); cetirizine (e.g., cetirizine hydrochloride); cetoxime; chlorocyclizine; chloropyramine; chlorothen; chlorphenoxamine; cinnarizine; clemastine (e.g., clemastine fumarate); clobenzepam; clobenztropine; clocinizine; cyclizine (e.g., cyclizine hydrochloride; cyclizine lactate); deptropine; dexchlorpheniramine; de
- Antihistamine analogs include, without limitation, 10- piperazinylpropylphenothiazme; 4-(3 -(2-chlorophenothiazin- 10-yl)propyl)- 1 - piperazineethanol dihydrochloride; 1 -( 10-(3 -(4-methyl- 1 -piperazinyl)propyl)- 10H- phenothiazin-2-yl)-(9CI) 1-propanone; 3-methoxycyproheptadine; 4-(3-(2-Chloro-10H- phenothiazin- 10-yl)propyl)piperazine- 1 -ethanol hydrochloride; 10,11 -dihydro-5-(3-(4- ethoxycarbonyl-4-phenylpiperidino)propylidene)-5H-dibenzo(a,d)cycloheptene; aceprometa
- AD-0261 AHR- 5333; alinastine; arpromidine; ATI-19000; bermastine; bilastin; Bron-12; carebastine; chlorphenamine; clofurenadine; corsym; DF-1105501; DF-11062; DF-1111301; EL-301; elbanizine; F-7946T; F-9505; HE-90481 ; HE-90512; hivenyl; HSR-609; icotidine; KAA- 276; KY-234; lamiakast; LAS-36509; LAS-36674; levocetirizine; levoprotiline; metoclopramide; NIP-531; noberastine; oxatomide; PR-881-884A; quisultazine; rocastine; selenotifen; SK&F-94461; SODAS-HC; tagorizine;
- Standard recommended dosages Standard recommended dosages for several exemplary antihistamines are shown in Table 1. Other standard dosages are provided, e.g., in the Merck Manual of Diagnosis & Therapy (17th Ed. MH Beers et al., Merck & Co.) and Physicians' Desk Reference 2003 (57 th Ed. Medical Economics Staff et al., Medical Economics Co., 2002).
- Loratadine Loratadine is a tricyclic piperidine that acts as a selective peripheral histamine HI -receptor antagonist.
- loratadine and structural and functional analogs thereof such as piperidines, tricyclic piperidines, histamine Hl- receptor antagonists, are useful in the anti-immunoinflammatory combination of the invention for the treatment of immunoinflammatory disorders, transplanted organ rejection, and graft versus host disease.
- Loratadine functional and/or structural analogs include other HI -receptor antagonists, such as AHR-11325, acrivastine, antazoline, astemizole, azatadine, azelastine, bromopheniramine, carebastine, cetirizine, chlorpheniramine, chlorcyclizine, clemastine, cyproheptadine, descarboethoxyloratadine, dexchlorpheniramine, dimenhydrinate, diphenylpyraline, diphenhydramine, ebastine, fexofenadine, hydroxyzine ketotifen, lodoxamide, levocabastine, methdilazine, mequitazine, oxatomide, pheniramine pyrilamine, promethazine, pyrilamine, setastine, tazifylline, warmthlastine, terfenadine, trimeprazine, tripelennamine,
- Piperidine HI -receptor antagonists include loratadine, cyproheptadine hydrochloride (PERIACTIN), and phenindiamine tartrate (NOLAHIST).
- Piperazine Hl- receptor antagonists include hydroxyzine hydrochloride (ATARAX), hydroxyzine pamoate (VISTARIL), cyclizine hydrochloride (MAREZLNE), cyclizine lactate, and meclizine hydrochloride.
- Loratadine oral formulations include tablets, redi-tabs, and syrup. Loratadine tablets contain 10 mg micronized loratadine. Loratadine syrup contains 1 mg/ml micronized loratadine, and reditabs (rapidly-disintegrating tablets) contain 10 mg micronized loratadine in tablets that disintegrate quickly in the mouth. While suggested dosages will vary with a patient's condition, standard recommended dosages are provided below.
- Loratadine is typically administered once daily in a 10 mg dose, although other daily dosages useful in the anti-immunoinflammatory combination of the invention include 0.01-0.05 mg, 0.05-1 mg, 1-3 mg, 3-5 mg, 5-10 mg, 10-15 mg, 15-20 mg, 20-30 mg, and 30-40 mg.
- Loratadine is rapidly absorbed following oral administration. It is metabolized in the liver to descarboethoxyloratadme by cytochrome P450 3A4 and cytochrome P450 2D6. Loratadine metabolites are also useful in the anti-immunoinflammatory combination of the invention.
- one or more corticosteroid may be administered in a method of the invention or may be formulated with an antihistamine or analog thereof in a composition of the invention.
- Our data show that various antihistamines in combination with various corticosteroids are more effective in suppressing TNF ⁇ in vitro than either agent alone. Accordingly, this combination may be more effective in treating immunoinflammatory diseases, particularly those mediated by TNF ⁇ levels, than either the antihistamine or corticosteroid alone.
- Suitable corticosteroids include 11 -alpha, 17-alpha,21- trihydroxypregn-4-ene-3 ,20-dione; 11 -beta, 16-alpha, 17,21 -tetrahydroxypregn-4-ene- 3 ,20-dione; 11 -beta, 16-alpha, 17,21 -tetrahydroxypregn- 1 ,4-diene-3 ,20-dione; 11 -beta, 17- alpha,21 -trihydroxy-6-alpha-methylpregn-4-ene-3 ,20-dione; 11 -dehydrocorticosterone; 11 -deoxycortisol; 11 -hydroxy- l,4-androstadiene-3,17-dione; 11-ketotestosterone; 14- hydroxyandrost-4-ene-3,6, 17-trione; 15,17-dihydroxyprogesterone; 16- methylhydrocortisone; 17,21 -
- the dosage of corticosteroid administered is a dosage equivalent to a prednisolone dosage, as defined herein.
- a low dosage of a corticosteroid may be considered as the dosage equivalent to a low dosage of prednisolone.
- Steroid receptor modulators may be used as a substitute for or in addition to a corticosteroid in the methods, compositions, and kits of the invention.
- the invention features the combination of a tricyclic compound and a glucocorticoid receptor modulator or other steroid receptor modulator, and methods of treating immunoinflammatory disorders therewith.
- Glucocorticoid receptor modulators that may used in the methods, compositions, and kits of the invention include compounds described in U.S. Patent Nos. 6,380,207, 6,380,223, 6,448,405, 6,506,766, and 6,570,020, U.S. Patent Application Publication Nos.
- antihistamines in combination with ibudilast are more effective in suppressing TNF ⁇ in vitro than the agents alone. Accordingly, the combination of antihistamine or antihistamine analogs may be more effective in treating immunoinflammatory diseases, particulary those mediated by TNF ⁇ , than either agent alone.
- An antihistamine or an antihistamine analog may be administered or formulated with ibudilast or an ibudilast analog, defined by formula (V).
- Ri and R 2 are each, independently, selected from H, C] -7 alkyl, C 2-7 alkenyl, C 2-7 alkynyl, C 2-6 heterocyclyl, C 6-12 aryl, C 7-14 alkaryl, C 3- ⁇ 0 alkheterocyclyl, and C 1 - 7 heteroalkyl;
- R 3 is selected from H, halide, alkoxy, and d -4 alkyl;
- R 4 is selected from H and acyl;
- R 5 is selected from H, halide, and C 1- alkyl;
- Re is selected from OH, alkoxy and amido;
- R 7 is selected from H, C ⁇ -7 alkyl, C 2-7 alkenyl, C 2- 7 alkyn
- Compounds of formula (V) include, the compounds described in U.S. Patent Nos. 3,850,941; 4,097,483; 4,578,392; 4,925,849; 4,994,453; and 5,296,490.
- Commercially available compounds of formula (V) include ibudilast and KC-764.
- the standard recommended dosage for the treatment of bronchial asthma is typically 10 mg of ibudilast twice daily, while in the case of cerebrovascular disorders, the standard recoomended dosage is 10 mg of ibudilast three times daily.
- KC-764 (CAS 94457-09-7) is reported to be a platelet aggregation inhibitor.
- KC-764 KC-764 and other compound of formula (V) can be prepared using the synthetic methods described in U.S. Patent Nos. 3,850,941; 4,097,483; 4,578,392; 4,925,849; 4,994,453; and 5,296,490.
- Rolipram We have discovered that antihistamines in combination with rolipram are more effective in suppressing TNF ⁇ in vitro than the agents alone. Accordingly, the combination of antihistamine or antihistamine analog in combination with rolipram or rolipram analogs may be more effective in treating immunoinflammatory diseases, particulary those mediated by TNF ⁇ , than either agent alone.
- an antihistamine or analog thereof is administered or formulated with rolipram (4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2- pyrrolidone) or an analog of rolipram.
- Rolipram analogs are described by formula (I) of U.S. Patent No. 4,193,926, hereby incorporated by reference.
- Tetra-substituted pyrimidopyrimidines We have discovered that antihistamines in combination with dipyridamole are more effective in suppressing TNF ⁇ in vitro than the agents alone. Accordingly, the combination of antihistamine or antihistamine analog in combination with a tetra- substituted pyrimidopyrimidines may be more effective in treating immunoinflammatory diseases, particulary those mediated by TNF ⁇ , than either agent alone.
- an anti stamine ot analog thereof is administered or formulated with a tetra-substituted pyrimidopyrimidine having the formula (VI):
- each is, independently, X, OH, N-alkyl (wherein the alkyl group has 1 to 20, more preferably 1-5, carbon atoms); a branched or unbranched alkyl group having 1 to 20, more preferably 1- 5, carbon atoms; or a heterocycle, preferably as defined in formula (Y), below.
- two Ri groups from a common Z or Z' atom, in combination with each other may represent -(CY 2 )k- in which k is an integer between 4 and 6, inclusive.
- Each Y is, independently, H, F, Cl, Br, or I.
- each Z is the same moiety
- each Z' is the same moiety
- Z and Z' are different moieties.
- Exemplary tetra-substituted pyrimidopyrimidines that are useful in the ' methods and compositions of this invention include 2,6-disubstituted 4,8- dibenzylaminopyrimido[5,4-d]pyrimidines.
- Particularly useful tetra-substituted pyrimidopyrimidines include dipyridamole (also known as 2,6-bis(diethanolamino)-4,8- dipiperidinopyrimido(5,4-d) ⁇ yrimidine); mopidamole; dipyridamole monoacetate; NU3026 (2,6-di-(2,2-dimethyl- 1 ,3-dioxolan-4-yl)-methoxy-4,8-di- piperidinopyrimidopyrimidme); NU3059 (2,6-bis-(2,3-dimethyoxypropoxy)-4,8-di- piperidrnopyrimidopyrimidine); NU3060 (2,6-bis[N,N-di(2-methoxy)ethyl]-4,6-di- piperidinopyrimidopyrimidine); and U3076 (2,6-bis(diethanolamino)-4,8-di-4- me
- Tricyclic and Tetracyclic Antidepressants We have discovered that antihistamines in combination with various tricyclic and tetracyclic antidepressants are more effective in suppressing TNF ⁇ in vitro than the agents alone. Accordingly, the combination of antihistamine or antihistamine analog in combination with tricyclic and tetracyclic antidepressants and their analogs may be more effective in treating immunoinflammatory diseases, particulary those mediated by TNF ⁇ , than either agent alone.
- an antihistamine or analog thereof is administered or formulated with a tricyclic or tetracyclic antidepressant, or an analog thereof.
- tricyclic or tetracyclic antidepressant analog is meant a compound having one the formulas (I), (II), (III), or (IV):
- each X is, mdependently, H, Cl, F, Br, I, CH 3 , CF 3 , OH, OCH 3 , CH 2 CH 3 , or OCH 2 CH 3 ;
- Y is CH 2 , O, NH, S(O)o -2 , (CH 2 ) 3 , (CH) 2 , CH 2 O, CH 2 NH, CHN, or CH 2 S;
- Z is C or S;
- A is a branched or unbranched, saturated or monounsaturated hydrocarbon chain having between 3 and 6 carbons, inclusive;
- each B is, independently, H, Cl, F, Br, I, CX 3 , CH 2 CH 3 , OCX 3 , or OCX 2 CX 3 ; and
- D is CH 2 , O, NH, S(O) 0-2 .
- each X is, independently, H, Cl, or F;
- Y is (CH 2 ) 25 Z is
- Tricyclic or tetracyclic antidepressants include 10-(4-methylpiperazin- 1 -yl)pyrido(4,3 -b)( 1 ,4)benzothiazepine; 11 -(4-methyl- 1 -piperazinyl)-5H- dibenzo(b,e)(l,4)diaze ⁇ ine; 5,10-dihydro-7-chloro-10-(2-(morpholino)ethyl)-l 1H- dibenzo(b,e)(l,4)diazepin-l 1-one; 2-(2-(7-hydroxy-4-dibenzo(b,f)(l,4)thiazepine-l 1-yl- 1 -piperazinyl)ethoxy)ethanol; 2-chloro- 11 -(4-methyl-
- antihistammes in combination with various SSRI's are more effective in suppressing TNF ⁇ in vitro than the agents alone. Accordingly, the combination of antihistamine or antihistamine analog in combination with SSRIs or their analogs may be more effective in treating immunoinflammatory diseases, particulary those mediated by TNF ⁇ , than either agent alone.
- an antihistamine or analog thereof is administered or formulated with an SSRI or an analog thereof.
- Suitable SSRIs and SSRI analogs include l,2,3,4-tetrahydro-N-methyl-4-phenyl-l-naphthylamine hydrochloride, 1 ,2,3 ,4-tetrahydro-N-methyl-4-phenyl-(E)- 1 -naphthylamine hydrochloride; N,N- dimethyl- 1 -phenyl- 1-phthalanpropylamine hydrochloride; gamma-(4- (trifluoromethyl)phenoxy)-benzenepropanamine hydrochloride; BP 554; citalopram; xitalopram hydrobromide; CP 53261; didesmethylcitalopram; escitalopram; escitalopram oxalate; femoxetine, fiuoxetine; fluoxetine hydrochloride; fluvoxamine; fluvoxamine maleate; indalpine, indeloxazine hydrochlor
- Citalopram HBr is a racemic bicyclic phthalane derivative designated ( ⁇ )- 1 -(3-dimethylamino ⁇ ro ⁇ yl)- 1 -(4-fluorophenyl)- 1 ,3 -dihydroisobenzofuran- 5-carbonitrile, HBr.
- Citalopram undergoes extensive metabolization; nor i -citalopram and nor 2 -citalo ⁇ ram are the main metabolites.
- Citalopram is available in 10 mg, 20 mg, and 40 mg tablets for oral administration.
- CELEXATM oral solution contains citalopram HBr equivalent to 2 mg/mL citalopram base.
- CELEXATM is typically administered at an initial dose of 20 mg once daily, generally with an increase to a dose of 40 mg/day. Dose increases typically occur in increments of 20 mg at intervals of no less than one week.
- Citalopram has the following structure:
- Structural analogs of citalopram are those having the formula:
- Ri and R 2 is independently selected from the group consisting of bromo, chloro, fluoro, trifluoromethyl, cyano and R-CO-, wherein R is C ⁇ -4 alkyl.
- Exemplary citalopram structural analogs are l-(4'-fluorophenyl)-l-(3-dimethylaminopropyl)-5- bromophthalane; l-(4'-chlorophenyl)-l-(3-dimethylaminopropyl)-5-chlorophthalane; 1- (4'-bromophenyl)-l-(3-dimethylaminopropyl)-5-chlorophthalane; l-(4'-fluorophenyl)-l-
- Clovoxamine Clovoxamine has the following structure:
- Structural analogs of clovoxamine are those having the formula:
- Hal is a chloro, bromo, or fluoro group and R is a cyano, methoxy, ethoxy, methoxymethyl, ethoxymethyl, methoxyethoxy, or cyanomethyl group.
- Exemplary clovoxamine structural analogs are 4'-chloro-5-ethoxyvalero ⁇ henone 0-(2-aminoethyl)oxime; 4'-chloro-5-(2-methoxyethoxy)valerophenone O-(2- aminoethyl)oxime; 4'-chloro-6-methoxycaprophenone O-(2-aminoethyl)oxime; 4'-chloro- 6-ethoxycaprophenone 0-(2-aminoethy ⁇ )oxime; 4'-bromo-5-(2- methoxyethoxy)valerophenone O-(2-aminoethyl)oxime; 4'-bromo-5- methoxyvalerophenone 0-(2-aminoethyl)oxime; 4'-chloro-6-cyanocaprophenone 0-(2- aminoethyl)oxime; 4'-chloro-5-cyanovalerophenone O-(2-amino
- Femoxetine has the following structure:
- Structural analogs of femoxetine are those having the formula:
- R t represents a C 1- alkyl or C 2- alkynyl group, or a phenyl group optionally substituted by C 1- alkyl, C ⁇ -4 alkylthio, C 1-4 alkoxy, bromo, chloro, fluoro, nitro, acylamino, methylsulfonyl, methylenedioxy, or tetrahydronaphthyl
- R 2 represents a C 1- alkyl or C 2-4 alkynyl group
- R 3 represents hydrogen, C 1-4 alkyl, C 1-4 alkoxy, trifluoroalkyl, hydroxy, bromo, chloro, fluoro, methylthio, or aralkyloxy.
- Exemplary femoxetine structural analogs are disclosed in Examples 7-67 of U.S. Patent No. 3,912,743, hereby incorporated by reference.
- Fluoxetine Fluoxetine hydrochloride (( ⁇ )-N-methyl-3-phenyl-3-[((alpha),(alpha),(alpha)- trifluoro-p-tolyl)oxy]propylamine hydrochloride) is sold as PROZAC in 10 mg, 20 mg, and 40 mg tablets for oral administration.
- the main metabolite of fluoxetine is nor- fluoxetine.
- Fluoxetine hydrochloride may also be administered as an oral solution equivalent to 20 mg/5 mL of fluoxetine.
- a delayed release formulation contains enteric- coated pellets of fluoxetine hydrochloride equivalent to 90 mg of fluoxetine .
- a dose of 20 mg/day, administered in the morning, is typically recommended as the initial dose.
- a dose increase may be considered after several weeks if no clinical improvement is observed. Doses above 20 mg/day may be administered on a once a day (morning) or twice a day schedule (e.g., morning and noon) and should not exceed a maximum dose of 80 mg/day.
- Fluoxetine has the following structure:
- Structural analogs of fluoxetine are those compounds having the formula: as well as pharmaceutically acceptable salts thereof, wherein each Ri is independently hydrogen or methyl; R is naphthyl or
- each of R 2 and R 3 is, independently, bromo, chloro, fluoro, trifluoromethyl, C ⁇ alkyl, C 1-3 alkoxy or C 3-4 alkenyl; and each of n and m is, independently, 0, 1 or 2.
- R is naphthyl, it can be either ⁇ -naphthyl or ⁇ -naphthyl.
- Exemplary fluoxetine structural analogs are 3-(p-isopropoxyphenoxy)-3- phenylpropylamine methanesulfonate, N,N-dirnethyl 3-(3 , ,4'-dimethoxyphenoxy)-3- phenylpropylamine p-hydroxybenzoate, N,N-dimethyl 3 -( ⁇ -naphthoxy)-3 - phenylpropylamine bromide, N,N-dimethyl 3-( ⁇ -naphthoxy)-3-phenyl-l- methylpropylamine iodide, 3-(2'-methyl-4 , ,5'-dichlorophenoxy)-3-phenylpropylamine nitrate, 3-(p-t-butylphenoxy)-3-phenylpropylamine glutarate, N-methyl 3-(2'-chloro-p- tolyloxy)-3 -phenyl- 1 -methylpropylamine lactate, 3-(
- Fluvoxamine maleate is supplied as 50 mg and 100 mg tablets. Treatment is typically initiated at 50 mg given once daily at bedtime, and then increased to 100 mg daily at bedtime after a few days, as tolerated. The effective daily dose usually lies between 100 and 200 mg, but may be administered up to a maximum of 300 mg. Fluvoxamine has the following structure:
- Structural analogs of fluvoxamine are those having the formula:
- Indalpine has the following structure:
- Structural analogs of indalpine are those having the formula:
- i is a hydrogen atom, a C 1 -C 4 alkyl group, or an aralkyl group of which the alkyl has 1 or 2 carbon atoms
- R 2 is hydrogen, C 1-4 alkyl, C ⁇ -4 alkoxy or C 1-4 alkylthio, chloro, bromo, fluoro, trifluoromethyl, nitro, hydroxy, or amino, the latter optionally substituted by one or two C 1-4 alkyl groups, an acyl group or a C 1-4 alkylsulfonyl group
- A represents -CO or -CH 2 - group
- n is 0, l or 2.
- indalpine structural analogs are indolyl-3 (piperidyl-4 methyl) ketone; (methoxy-5-indolyl-3) (piperidyl-4 methyl) ketone; (chloro-5-indolyl-3) (piperidyl-4 methyl) ketone; (indolyl-3)- l(piperidyl-4)-3 propanone, indolyl-3 piperidyl-4 ketone; (methyl- 1 indolyl-3) (piperidyl-4 methyl) ketone, (benzyl- 1 indolyl-3) (piperidyl-4 methyl) ketone; [(methoxy-5 indolyl-3)-2 ethyl]-piperidine, [(methyl- 1 indolyl-3)-2 ethyl]-4-piperidine; [(indolyl-3)-2 ethyl]-4 piperidine; (indolyl-3 methyl)-4 piperidine;
- Structural analogs of indeloxazine are those having the formula:
- Ri and R 3 each represents hydrogen, C ⁇ -4 alkyl, or phenyl;
- R 2 represents hydrogen, C ⁇ - alkyl, C 4-7 cycloalkyl, phenyl, or benzyl;
- one of the dotted lines means a single bond and the other means a double bond, or the tautomeric mixtures thereof.
- Exemplary indeloxazine structural analogs are 2-(7-indenyloxymethyl)-4- isopropylmorpholine; 4-butyl-2-(7-indenyloxymethyl)mo holine; 2-(7- indenyloxymethyl)-4-methylmorpholine; 4-ethyl-2-(7-mdenyloxymethyl)morpholine, 2- (7-indenyloxymethyl)-morpholine; 2-(7-indenyloxymethyl)-4-propylmorpholine; 4- cyclohexyl-2-(7-indenyloxymethyl)mor ⁇ holine; 4-benzyl-2-(7-indenyloxymethyl)- morpholine; 2-(7-indenyloxymethyl)-4-phenylmorpholine; 2-(4- indenyloxymethyl)morpholine; 2-(3-methyl-7-indenyloxymethyl)-morpholine; 4- isopropyl-2-(3-methyl-7-indenyloxymethyl)morpholine
- Milnacipram Milnacipran (IXEL T i M !V , Cypress Bioscience Inc.) has the chemical formula (Z)-l- diethylaminocarbonyl-2-aminoethyl-l -phenyl-cyclopropane)hydrochlorate, and is provided in 25 mg and 50 mg tablets for oral administration. It is typically administered in dosages of 25 mg once a day, 25 mg twice a day, or 50 mg twice a day for the treatment of severe depression. Milnacipram has the following structure:
- each R independently, represents hydrogen, bromo, chloro, fluoro, C ⁇ alkyl, C ⁇ -4 alkoxy, hydroxy, nitro or amino
- each of and R 2 independently, represents hydrogen, C 1-4 alkyl, C 6-12 aryl or C 7- 14 alkylaryl, optionally substituted, preferably in para position, by bromo, chloro, or fluoro, or Ri and R 2 together form a heterocycle having 5 or 6 members with the adjacent nitrogen atoms
- R 3 and R 4 represent hydrogen or a C 1 - 4 alkyl group or R 3 and R 4 form with the adjacent nitrogen atom a heterocycle having 5 or 6 members, optionally containing an additional heteroatom selected from nitrogen, sulphur, and oxygen.
- Exemplary milnacipram structural analogs are 1 -phenyl 1-aminocarbonyl 2- dimethylaminomethyl cyclopropane; 1 -phenyl 1-dimethylaminocarbonyl 2- dimethylaminomethyl cyclopropane; 1 -phenyl 1-ethylaminocarbonyl 2- dimethylaminomethyl cyclopropane; 1 -phenyl 1-diethylaminocarbonyl 2-aminomethyl cyclopropane; 1 -phenyl 2-dimethylaminomethyl N-(4'-chlorophenyl)cyclopropane carboxamide; 1 -phenyl 2-dimethylaminomethyl N-(4'-chlorobenzyl)cyclopropane carboxamide; 1 -phenyl 2-dimethylaminomethyl N-(2-phenylethyl)cyclopropane carboxamide; (3 ,4-dichloro- 1 -phenyl) 2-dimethyla
- Paroxetine Paroxetine hydrochloride ((-)- trans -4 R -(4'-fluoro ⁇ henyl)-3 S -[(3',4'- methylenedioxyphenoxy) methyl] piperidine hydrochloride hemihydrate) is provided as PAXILTM.
- Controlled-release tablets contain paroxetine hydrochloride equivalent to paroxetine in 12.5 mg, 25 mg, or 37.5 mg dosages.
- One layer of the tablet consists of a degradable barrier layer and the other contains the active material in a hydrophilic matrix.
- the recommended initial dose of PAXILTM is 25 mg/day. Some patients not responding to a 25 mg dose may benefit from dose increases, in 12.5 mg/day increments, up to a maximum of 62.5 mg/day. Dose changes typically occur at intervals of at least one week.
- Paroxetine has the following structure:
- Structural analogs of paroxetine are those having the formula:
- Ri represents hydrogen or a C alkyl group
- the fluorine atom may be in any of the available positions.
- Sertraline Sertraline (( 1 S-cis)-4-(3 ,4-dichlorophenyl)- 1 ,2,3 ,4-tetrahydro-N-methyl- 1 - nanphthalenamine hydrochloride) is provided as ZOLOFTTM in 25 mg, 50 mg and 100 mg tablets for oral administration. Because sertraline undergoes extensive metabolic transformation into a number of metabolites that may be therapeutically active, these metabolites may be substituted for sertraline in an anti-inflammatory combination of the invention. The metabolism of sertraline includes, for example, oxidative N- demethylation to yield N-desmethylsertraline (nor-sertraline). ZOLOFT is typically administered at a dose of 50 mg once daily. Sertraline has the following structure:
- Structural analogs of sertraline are those having the formula:
- Ri is selected from the group consisting of hydrogen and C alkyl
- R 2 is C alkyl
- X and Y are each selected from the group consisting of hydrogen, fluoro, chloro, bromo, trifluoromethyl, C ⁇ -3 alkoxy, and cyano
- W is selected from the group consisting of hydrogen, fluoro, chloro, bromo, trifluoromethyl and C ⁇ -3 alkoxy.
- Preferred sertraline analogs are in the cis-isomeric configuration.
- the term "cis-isomeric" refers to the relative orientation of the NR ⁇ R 2 and phenyl moieties on the cyclohexene ring (i.e. they are both oriented on the same side of the ring).
- each cis- compound has two optically active enantiomeric forms denoted (with reference to the 1 -carbon) as the cis-(lR) and cis-(lS) enantiomers.
- Sibutramine hydrochloride monohydrate Sibutramine hydrochloride monohydrate (MERIDIATM) is an orally administered agent for the treatment of obesity.
- Sibutramine hydrochloride is a racemic mixture of the (+) and (-) enantiomers of cyclobutanemethanamme, 1 -(4-chlorophenyl)- N, N-dimethyl- (alpha)-(2-methylpropyl)-, hydrochloride, monohydrate.
- Each MERIDIATM capsule contains 5 mg, 10 mg, or 15 mg of sibutramine hydrochloride monohydrate.
- the recommended starting dose of MERIDIATM is 10 mg administered once daily with or without food. If there is inadequate weight loss, the dose may be titrated after four weeks to a total of 15 mg once daily. The 5 mg dose is typically reserved for patients who do not tolerate the 10 mg dose.
- Zimeldine Zimeldine has the following structure:
- Structural analogs of zimeldine are those compounds having the formula:
- pyridine nucleus is bound in ortho-, meta- or para-position to the adjacent carbon atom and where Ri is selected from the group consisting of H, chloro, fluoro, and bromo.
- Ri is selected from the group consisting of H, chloro, fluoro, and bromo.
- Exemplary zimeldine analogs are (e)- and (z)- 3-(4'-bromophenyl-3-(2"-pyridyl)- dimethylallylamine; 3-(4'-bromophenyl)-3 -(3 "-pyridyl)-dimethylallylamine; 3 -(4'- bromophenyl)-3-(4"-pyridyl)-dimethylallylamine; and pharmaceutically acceptable salts of any thereof.
- Structural analogs of any of the above SSRIs are considered herein to be SSRI analogs and thus may be employed in any of the methods, compositions, and kits of the invention.
- Metabolites Pharmacologically active metabolites of any of the foregoing SSRIs can also be used in the methods, compositions, and kits of the invention.
- Exemplary metabolites are didesmethylcitalopram, desmethylcitalopram, desmethylsertraline, and norfluoxetine.
- Analogs Functional analogs of SSRIs can also be used in the methods, compositions, and kits of the invention. Exemplary SSRI functional analogs are provided below.
- SSRI analogs include SNRIs (selective serotonin norepinephrine reuptake inhibitors), which include venlafaxine, duloxetine, and 4-(2-fluorophenyl)-6-methyl-2- piperazinothieno [2,3-d] pyrimidine.
- Venlafaxine Venlafaxine hydrochloride is an antidepressant for oral administration. It is designated (R/S)-l-[2-(dimethylamino)- ! l-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride or ( ⁇ )- 1 - [(alpha)- [(dimethyl-amino)methyl]-p- methoxybenzyl] cyclohexanol hydrochloride.
- Compressed tablets contain venlafaxine hydrochloride equivalent to 25 mg, 37.5 mg, 50 mg, 75 mg, or 100 mg venlafaxine.
- the recommended starting dose for venlafaxine is 75 mg/day, administered in two or three divided doses, taken with food. Depending on tolerability and the need for further clinical effect, the dose may be increased to 150 mg/day. If desirable, the dose can be further increased up to 225 mg/day. When increasing the dose, increments of up to 75 mg/day are typically made at intervals of no less than four days.
- Venlafaxine has the following structure:
- Structural analogs of venlafaxine are those compounds having the formula:
- Ri is hydrogen or alkyl
- R 2 is C alkyl
- R 4 is hydrogen, C M alkyl, formyl or alkanoyl
- R 3 is hydrogen or C ⁇ _ alkyl
- R 5 and 5 are, independently, hydrogen, hydroxyl, C M alkyl, C alkoxy, C alkanoyloxy, cyano, nitro, alkylmercapto, amino, C M alkylamino, dialkylamino, C alkanamido, halo, trifluoromethyl or,,taken together, methylenedioxy
- n is 0, 1, 2, 3 or 4.
- Duloxetine has the following structure:
- Structural analogs of duloxetine are those compounds described by the formula disclosed in U.S. Patent No. 4,956,388, hereby incorporated by reference.
- Other SSRI analogs are 4-(2-fluorophenyl)-6-methyl-2-piperazinothieno [2,3-d] pyrimidine, l,2,3,4-tetrahydro-N-methyl-4-phenyl-l-naphthylamine hydrochloride; 1 ,2,3,4-tetrahydro-N-methyl-4-phenyl-(E)- 1 -naphthylamine hydrochloride; N,N- dimethyl-1 -phenyl- 1-phthalanpropylamine hydrochloride; gamma-(4- (trifluoromethyl)phenoxy)-benzenepropanamine hydrochloride; BP 554; CP 53261; O- desmethylvenlafaxine; WY 45,818; WY 45,881; N-(3-
- the suppression of cytokine secretion or production and the treatment of the immuninflammatory disorder may be achieved by administering, in addition to one or more of the compounds described above, one or more compounds selected from methotrexate, hydroxychloroquine, sulfasalazine, tacrolimus, sirolimus, mycophenolate mofetil, and/or methyl prednisolone.
- a hyperproliferative skin disease e.g., psoriasis
- topical agents including coal tar, calcipotriene, and/or corticosteroids.
- Nonsteroidal Immunophilin-Dependent Immunosuppr ess ants We have discovered that antihistamines in combination with various nonsteroidal immunophilin-dependent immunosupressants (NsIDIs) are more effective in suppressing TNF ⁇ in vitro than the agents alone. Accordingly, the combination of antihistamine or antihistamine analog in combination with immunophilin dependant immunosupressants and their analogs may be more effective in treating immunoinflammatory diseases, particulary those mediated by TNF ⁇ , than either agent alone.
- the NsIDI is cyclosporine, and is administered in an amount between 0.05 and 50 milligrams per kilogram per day (e.g., orally in an amount between 0.1 and 12 milligrams per kilogram per day).
- the NsIDI is tacrolimus and is administered in an amount between 0.0001-20 milligrams per kilogram per day (e.g., orally in an amount between 0.01-0.2 milligrams per kilogram per day).
- the NsIDI is rapamycin and is administered in an amount between 0.1-502 milligrams per day (e.g., at a single loading dose of 6 mg/day, followed by a 2 mg/day maintenance dose).
- the NsIDI is everolimus, administered at a dosage of 0.75-8 mg/day.
- the NsIDI is pimecrolimus, administered in an amount between 0.1 and 200 milligrams per day (e.g., as a 1% cream/twice a day to treat atopic dermatitis or 60 mg a day for the treatment of psoriasis), or the NsIDI is a calcineurin-binding peptide administered in an amount and frequency sufficient to treat the patient.
- Two or more NsIDIs can be administered contemporaneously.
- the immune system uses cellular effectors, such as B and T cells, to target infectious microbes and abnormal cell types while leaving normal cells intact.
- activated T cells damage healthy tissues.
- Calcineurin inhibitors e.g., cyclosporines, tacrolimus, pimecrolimus
- rapamycin target many types of immunoregulatory cells, including T cells and suppress the immune response in organ transplantation and autoimmune disorders.
- Cyclosporines are fungal metabolites that comprise a class of cyclic oligopeptides that act as immunosuppressants.
- Cyclosporine A is a hydrophobic cyclic polypeptide consisting of eleven amino acids. It binds and forms a complex with the mtracellular receptor cyclophilin. The cyclosporine/cyclophilin complex binds to and inhibits calcineurin, a Ca -calmodulin-dependent serine-threonine-specific protein phosphatase. Calcineurin mediates signal transduction events required for T-cell activation (reviewed in Schreiber et al., Cell 70:365-368, 1991).
- Cyclosporines and their functional and structural analogs suppress the T cell-dependent immune response by inhibiting antigen-triggered signal transduction. This inhibition decreases the expression of proinflammatory cytokines, such as IL-2.
- Many different cyclosporines e.g., cyclosporine A, B, C, D, E, F, G, H, and I
- Cyclosporine A is a commercially available under the trade name NEORAL from Novartis.
- Cyclosporine A structural and functional analogs include cyclosporines having one or more fluorinated amino acids (described, e.g., in U.S. Patent No. 5,227,467); cyclosporines having modified amino acids (described, e.g., in U.S.
- Cyclosporine analogs include, but are not limited to, D-Sar ( ⁇ -SMe) 3 Val 2 - DH-Cs (209-825), Allo-Thr-2-Cs, Norvaline-2-Cs, D-Ala(3-acetylamino)-8-Cs, Thr-2- Cs, and D-MeSer-3-Cs, D-Ser(O-CH 2 CH 2 -OH)-8-Cs, and D-Ser-8-Cs, which are described in Cruz et al. (Antimicrob. Agents Chemother. 44:143-149, 2000). Cyclosporines are highly hydrophobic and readily precipitate in the presence of water (e.g. on contact with body fluids).
- Cyclosporine microemulsion compositions are described in U.S. Patent Nos. 5,866,159, 5,916,589, 5,962,014, 5,962,017, 6,007,840, and 6,024,978. Cyclosporines can be administered either intravenously or orally, but oral administration is preferred. To overcome the hydrophobicity of cyclosporine A, an intravenous cyclosporme A is usually provided in an ethanol-polyoxyethylated castor oil vehicle that must be diluted prior to administration.
- Cyclosporme A may be provided, e.g., as a microemulsion in a 25 mg or 100 mg tablets, or in a 100 mg/ml oral solution (NEORAL).
- patient dosage of an oral cyclosporine varies according to the patient's condition, but some standard recommended dosages are provided herein.
- Patients undergoing organ transplant typically receive an initial dose of oral cyclosporine A in amounts between 12 and 15 mg/kg/day. Dosage is then gradually decreased by 5% per week until a 7-12 mg/kg/day maintenance dose is reached. For intravenous administration 2-6 mg/kg/day is preferred for most patients. For patients diagnosed as having Crohn's disease or ulcerative colitis, dosage amounts from 6-8 mg/kg/day are generally given.
- dosage amounts from 2.2-6.0 mg/kg/day are generally given.
- dosage amounts from 0.5-4 mg/kg/day are typical.
- a suggested dosing schedule is shown in Table 5.
- Other useful dosages include 0.5-5 mg/kg/day, 5-10 mg/kg/day, 10- 15 mg/kg/day, 15-20 mg/kg/day, or 20-25 mg/kg/day.
- cyclosporines are administered in combination with other immunosuppressive agents, such as glucocorticoids. Table 5
- Tacrolimus Tacrolimus (FK506) is an immunosuppressive agent that targets T cell intracellular signal transduction pathways.
- Tacrolimus binds to an intracellular protein FK506 binding protein (FKBP-12) that is not structurally related to cyclophilin (Harding et al. Nature 341 :758-7601, 1989; Siekienka et al. Nature 341:755-757, 1989; and Soltoff et al., J. Biol. Chem. 267: 17472-17477, 1992).
- FKBP-12 intracellular protein FK506 binding protein
- the FKBP/FK506 complex binds to calcineurin and inhibits calcineurin' s phosphatase activity.
- Tacrolimus is a macrolide antibiotic that is produced by Streptomyces tsukubaensis. It suppresses the immune system and prolongs the survival of transplanted organs. It is currently available in oral and injectable formulations. Tacrolimus capsules contain 0.5 mg, 1 mg, or 5 mg of anhydrous tacrolimus within a gelatin capsule shell.
- the injectable formulation contains 5 mg anhydrous tacrolimus in castor oil and alcohol that is diluted with 0.9% sodium chloride or 5% dextrose prior to injection. While oral administration is preferred, patients unable to take oral capsules may receive injectable tacrolimus.
- the initial dose should be administered no sooner than six hours after transplant by continuous intravenous infusion.
- Tacrolimus and tacrolimus analogs are described by Tanaka et al., (J. Am. Chem. Soc, 109:5031, 1987) and in U.S. Patent Nos. 4,894,366, 4,929,611, and 4,956,352.
- F 506-related compounds including FR-900520, FR-900523, and FR-900525, are described in U.S. Patent No.
- Patients being treated for rheumatoid arthritis typically receive 1-3 mg/day oral tacrolimus.
- 0.01-0.15 mg/kg/day of oral tacrolimus is administered to a patient.
- Atopic dermatitis can be treated twice a day by applying a cream having 0.03-0.1% tacrolimus to the affected area.
- Patients receiving oral tacrolimus capsules typically receive the first dose no sooner than six hours after transplant, or eight to twelve hours after intravenous tacrolimus infusion was discontinued.
- tacrolimus dosages include 0.005-0.01 mg/kg/day, 0.01- 0.03 mg/kg/day, 0.03-0.05 mg/kg/day, 0.05-0.07 mg/kg/day, 0.07-0.10 mg/kg/day, 0.10- 0.25 mg/kg/day, or 0.25-0.5 mg/kg/day.
- Tacrolimus is extensively metabolized by the mixed- function oxidase system, in particular, by the cytochrome P-450 system. The primary mechanism of metabolism is demethylation and hydroxylation. While various tacrolimus metabolites are likely to exhibit immunosuppressive biological activity, the 13-demethyl metabolite is reported to have the same activity as tacrolimus.
- Pimecrolimus Pimecrolimus is the 33-epi-chloro derivative of the macrolactam ascomyin. Pimecrolimus structural and functional analogs are described in U.S. Patent No. 6,384,073. Pimecrolimus is particularly useful for the treatment of atopic dermatitis. Pimecrolimus is currently available as a 1% cream. Suggested dosing schedule for pimecrolimus is shown at Table 5. While individual dosing will vary with the patient's condition, some standard recommended dosages are provided below. Oral pimecrolimus can be given for the treatment of psoriasis or rheumatoid arthritis in amounts of 40-60 mg/day.
- pimecrolimus For the treatment of Crohn's disease or ulcerative colitis amounts of 80-160 mg/day pimecrolimus can be given. Patients having an organ transplant can be administered 160-240 mg/day of pimecrolimus. Patients diagnosed as having systemic lupus erythamatosus can be administered 40-120 mg/day of pimecrolimus. Other useful dosages of pimecrolimus include 0.5-5 mg/day, 5-10 mg/day, 10-30 mg/day, 40-80 mg/day, 80-120 mg/day, or even 120-200 mg/day.
- Rapamycin Rapamycin is a cyclic lactone produced by Streptomyces hygros copious.
- Rapamycin is an immunosuppressive agent that inhibits T cell activation and proliferation. Like cyclosporines and tacrolimus, rapamycin forms a complex with the immunophilin FKBP-12, but the rapamycin-FKBP-12 complex does not inhibit calcineurin phosphatase activity.
- the rapamycin immunophilin complex binds to and inhibits the mammalian kinase target of rapamycin (mTOR).
- mTOR is a kinase that is required for cell-cycle progression. Inhibition of mTOR kinase activity blocks T cell activation and proinflammatory cytokine secretion.
- Rapamycin structural and functional analogs include mono- and diacylated rapamycin derivatives (U.S. PatentNo.
- Rapamycin is currently available for oral administration in liquid and tablet formulations.
- RAPAMUNE liquid contains 1 mg/mL rapamycin that is diluted in water or orange juice prior to administration. Tablets containing 1 or 2 mg of rapamycin are also available. Rapamycin is preferably given once daily as soon as possible after transplantation. It is absorbed rapidly and completely after oral administration.
- rapamycin typically varies according to the patient's condition, but some standard recommended dosages are provided below.
- the initial loading dose for rapamycin is 6 mg.
- Subsequent maintenance doses of 0.5-2 mg/day are typical.
- a loading dose of 3 mg, 5 mg, 10 mg, 15 mg, 20 mg, or 25 mg can be used with a 1 mg, 3 mg, 5 mg, 7 mg, or 10 mg per day maintenance dose.
- rapamycin dosages are typically adjusted based on body surface area; 9 9 • generally a 3 mg/m /day loading dose and a 1 mg/m /day maintenance dose is used.
- Peptide Moieties Peptides, peptide mimetics, peptide fragments, either natural, synthetic or chemically modified, that impair the calcineurin-mediated dephosphorylation and nuclear translocation of NFAT are suitable for use in practicing the invention.
- Examples of peptides that act as calcineurin inhibitors by inhibiting the NFAT activation and the NFAT transcription factor are described, e.g., by Aramburu et al., Science 285:2129- 2133, 1999) and Aramburu et al., Mol. Cell 1 :627-637, 1998).
- these agents are useful in the methods of the invention.
- the invention features methods for suppressing secretion of proinflammatory cytokines as a means for treating an immunoinflammatory disorder, proliferative skin disease, organ transplant rejection, or graft versus host disease.
- the compounds are administered within 10 days of each other, within five days of each other, within twenty-four hours of each other, or simultaneously.
- the compounds may be formulated together as a single composition, or may be formulated and administered separately.
- One or both compounds may be administered in a low dosage or in a high dosage, each of which is defined herein.
- NSAID e.g., naproxen sodium, diclofenac sodium, diclofenac potassium, aspirin, sulindac, diflunisal, piroxicam, indomethacin, ibuprofen, nabumetone, choline magnesium trisalicylate, sodium salicylate, salicylsalicylic acid, fenoprofen, flurbiprofen, ketoprofen, meclofenamate sodium, meloxicam, oxaprozin, sulindac, and tolmetin), COX-2 inhibitor (e.g., rofecoxib, celecoxib, valdecoxib, and lumiracoxib), glucocorticoid receptor modulator, or DMARD.
- COX-2 inhibitor e.g., rofecoxib, celecoxib, valdecoxib, and lumiracoxib
- glucocorticoid receptor modulator e.g.,
- Combination therapies of the invention are especially useful for the treatment of immunoinflammatory disorders in combination with other agents - either biologies or small molecules - that modulate the immune response to positively affect disease.
- agents include those that deplete key inflammatory cells, influence cell adhesion, or influence cytokines involved in immune response.
- This last category includes both agents that mimic or increase the action of anti-inflammatory cytokines such as IL-10, as well as agents inhibit the activity of pro- inflammatory cytokines such as IL-6, IL-1, IL-2, IL-12, IL-15 or TNF ⁇ .
- Agents that inhibit TNF ⁇ include etanercept, adelimumab, infliximab, and CDP-870.
- the combination therapy reduces the production of cytokines, etanercept or infliximab act on the remaining fraction of inflammatory cytokines, providing enhanced treatment.
- Small molecule immunodulators include, e.g., p38 MAP kinase inhibitors such as VX 702, SCIO 469, doramapimod, RO 30201195, SCIO 323, TACE inhibitors such as DPC 333, ICE inhibitors such as pranalcasan, and IMPDH inhibitors such as mycophenolate and merimepodib.
- Treatment may be performed alone or in conjunction with another therapy and may be provided at home, the doctor's office, a clinic, a hospital's outpatient department, or a hospital. Treatment optionally begins at a hospital so that the doctor can observe the therapy's effects closely and make any adjustments that are needed, or it may begin on an outpatient basis.
- the duration of the therapy depends on the type of disease or disorder being treated, the age and condition of the patient, the stage and type of the patient's disease, and how the patient responds to the treatment. Additionally, a person having a greater risk of developing an inflammatory disease (e.g., a person who is undergoing age-related hormonal changes) may receive treatment to inhibit or delay the onset of symptoms.
- Routes of administration for the various embodiments include, but are not limited to, topical, transdermal, nasal, and systemic administration (such as, intravenous, intramuscular, subcutaneous, inhalation, rectal, buccal, vaginal, intraperitoneal, intraarticular, ophthalmic or oral administration).
- systemic administration refers to all nondermal routes of administration, and specifically excludes topical and transdermal routes of administration.
- the dosage and frequency of administration of each component of the combination can be controlled independently. For example, one compound may be administered three times per day, while the second compound may be administered once per day.
- Combination therapy may be given in on-and-off cycles that include rest periods so that the patient's body has a chance to recover from any as yet unforeseen side effects.
- the compounds may also be formulated together such that one administration delivers both compounds.
- the methods, compositions, and kits of the invention are more effective than other methods, compositions, and kits.
- “more effective” is meant that a method, composition, or kit exhibits greater efficacy, is less toxic, safer, more convenient, better tolerated, or less expensive, or provides more treatment satisfaction than another method, composition, or kit with which it is being compared.
- the methods, compositions, and kits of the invention are used for the treatment of chronic obstructive pulmonary disease (COPD).
- COPD chronic obstructive pulmonary disease
- one or more agents typically used to treat COPD may be used as a substitute for or in addition to a corticosteroid in the methods, compositions, and kits of the invention.
- Such agents include xanthines (e.g., theophylline), anticholinergic compounds (e.g., ipratropium, tiotropium), biologies, small molecule immunomodulators, and beta receptor agonists/bronchdilators (e.g., ibuterol sulfate, bitolterol mesylate, epinephrine, formoterol fumarate, isoproteronol, levalbuterol hydrochloride, metaproterenol sulfate, pirbuterol scetate, salmeterol xinafoate, and terbutaline).
- xanthines e.g., theophylline
- anticholinergic compounds e.g., ipratropium, tiotropium
- biologies e.g., beta receptor agonists/bronchdilators
- beta receptor agonists/bronchdilators e.g.
- Psoriasis The methods, compositions, and kits of the invention may be used for the treatment of psoriasis. If desired, one or more antipsoriatic agents typically used to treat psoriasis may be used as a substitute for or in addition to a corticosteroid in the methods, compositions, and kits of the invention.
- Such agents include biologies (e.g., alefacept, inflixamab, adelimumab, efalizumab, etanercept, and CDP-870), small molecule immunomodulators (e.g., VX 702, SCIO 469, doramapimod, RO 30201195, SCIO 323, DPC 333, pranalcasan, mycophenolate, and merimepodib), non-steroidal immunophilin- dependent immunosuppressants (e.g., cyclosporine, tacrolimus, pimecrolimus, and ISAtx247), vitamin D analogs (e.g., calcipotriene, calcipotriol), psoralens (e.g., methoxsalen), retinoids (e.g., acitretin, tazoretene), DMARDs (e.g., methotrexate), and anthralin.
- biologies e.
- the invention features the combination of a tricyclic compound and an antipsoriatic agent, and methods of treating psoriasis therewith.
- Inflammatory Bowel Disease The methods, compositions, and kits of the invention may be used for the treatment of inflammatory bowel disease. If desired, one or more agents typically used to treat inflammatory bowel disease may be used as a substitute for or in addition to a corticosteroid in the methods, compositions, and kits of the invention.
- Such agents include biologies (e.g., inflixamab, adelimumab, and CDP-870), small molecule immunomodulators (e.g., VX 702, SCIO 469, doramapimod, RO 30201195, SCIO 323, DPC 333, pranalcasan, mycophenolate, and merimepodib), non-steroidal immunophilin- dependent immunosuppressants (e.g., cyclosporine, tacrolimus, pimecrolimus, and
- the invention features the combination of a tricyclic compound and any of the foregoing agents, and methods of treating inflammatory bowel disease therewith.
- Rheumatoid Arthritis The methods, compositions, and kits of the invention may be used for the treatment of rheumatoid arthritis. If desired, one or more agents typically used to treat rheumatoid arthritis may be used as a substitute for or in addition to a corticosteroid in the methods, compositions, and kits of the invention.
- Such agents include NSAIDs (e.g., naproxen sodium, diclofenac sodium, diclofenac potassium, aspirin, sulindac, diflunisal, piroxicam, indomethacin, ibuprofen, nabumetone, choline magnesium trisalicylate, sodium salicylate, salicylsalicylic acid (salsalate), fenoprofen, flurbiprofen, ketoprofen, meclofenamate sodium, meloxicam, oxaprozin, sulindac, and tolmetin), COX-2 inhibitors (e.g., rofecoxib, celecoxib, valdecoxib, and lumiracoxib), biologies (e.g., inflixamab, adelimumab, etanercept, CDP-870, rituximab, and atlizumab), small molecule immunomodulators (e.g.,
- the invention features the combination of a tricyclic compound with any of the foregoing agents, and methods of treating rheumatoid arthritis therewith.
- compositions, and kits of the invention may be used for the treatment of asthma.
- agents typically used to treat asthma may be used as a substitute for or in addition to a corticosteroid in the methods, compositions, and kits of the invention.
- Such agents include beta 2 agonists/bronchodilators/leukotriene modifiers (e.g., zafirlukast, montelukast, and zileuton), biologies (e.g., omalizumab), small molecule immunomodulators, anticholinergic compounds, xanthines, ephedrine, guaifenesin, cromolyn sodium, nedocromil sodium, and potassium iodide.
- the invention features the combination of a tricyclic compound and any of the foregoing agents, and methods of treating asthma therewith.
- Suitable modes of administration include topical or transdermal, oral, rectal, intravenous, intramuscular, subcutaneous, inhalation, vaginal, intraperitoneal (IP), intraarticular, and ophthalmic.
- the invention can also be provided as components of a pharmaceutical pack.
- the two drugs can be formulated together or separately and in individual dosage amounts.
- Administration of each component of the combination may be by any suitable means that is effective for the treatment of an immunoinflammatory disorder, proliferative skin disease, organ transplant rejection, or graft versus host disease.
- Compounds are admixed with a suitable carrier substance, and are generally present in an amount of 0.1-95% by weight of the total weight of the composition.
- the composition may be provided in a dosage form that is suitable for oral, parenteral (e.g., intravenous, intramuscular, subcutaneous), rectal, transdermal, nasal, vaginal, inhalant, or ocular administration.
- parenteral e.g., intravenous, intramuscular, subcutaneous
- rectal transdermal
- nasal vaginal
- inhalant or ocular administration.
- the composition may be in form of, e.g., tablets, capsules, pills, powders, granulates, suspensions, emulsions, solutions, gels including hydrogels, pastes, ointments, creams, plasters, drenches, delivery devices, suppositories, enemas, injectables, implants, sprays, or aerosols.
- compositions may be formulated according to conventional pharmaceutical practice (see, e.g., Remington: The Science and Practice of Pharmacy, (20th ed.) ed. A.R. Gennaro, 2000, Lippincott Williams & Wilkins, Philadelphia, PA. and Encyclopedia of Pharmaceutical Technology, eds. J. Swarbrick and J. C. Boylan, 1988-2002, Marcel Dekker, New York).
- Pharmaceutical compositions according to the invention may be formulated to release the active compound substantially immediately upon administration or at any predetermined time period after administration, using controlled release formulations. Administration of compounds in controlled release formulations is useful where the compound, either alone or in combination, has (i) a narrow therapeutic index (e.g.
- the difference between the plasma concentration leading to harmful side effects or toxic reactions and the plasma concentration leading to a therapeutic effect is small; generally, the therapeutic index, Tl, is defined as the ratio of median lethal dose (LD 50 ) to median effective dose (ED 5 o)); (ii) a narrow absorption window in the gastro-intestinal tract; or (iii) a short biological half-life, so that frequent dosing during a day is required in order to sustain the plasma level at a therapeutic level.
- Many strategies can be pursued to obtain controlled release in which the rate of release outweighs the rate of metabolism of the therapeutic compound.
- controlled release can be obtained by the appropriate selection of formulation parameters and ingredients, including, e.g., appropriate controlled release compositions and coatings.
- Solid Dosage Forms for Oral Use Formulations for oral use include tablets containing the active ingredient(s) in a mixture with non-toxic pharmaceutically acceptable excipients. These excipients may be, for example, inert diluents or fillers (e.g., sucrose and sorbitol), lubricating agents, glidants, and antiadhesives (e.g., magnesium stearate, zinc stearate, stearic acid, silicas, hydrogenated vegetable oils, or talc).
- excipients may be, for example, inert diluents or fillers (e.g., sucrose and sorbitol), lubricating agents, glidants, and antiadhesives (e.g., magnesium stearate, zinc stearate, stearic acid, silicas, hydrogenated vegetable oils, or talc).
- the two compounds may be mixed together in a tablet or other vehicle, or may be partitioned.
- the first compound is contained on the inside of the tablet, and the second compound is on the outside, such that a substantial portion of the second compound is released prior to the release of the first compound.
- Formulations for oral use may also be provided as chewable tablets, or as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium.
- Topical Formulations For compositions adapted for topical use, a topical vehicle containing from between 0.1% to 25% (w/w) or more of antihistamine or analog and/or additional agent, preferably from between 0.1% to 10% (w/w), more preferably from between 0.05% to 4% (w/w) active agent.
- the cream can be applied one to four times daily, or as needed.
- a topical vehicle will contain from between 0.01% to 5% (w/w), preferably from between 0.01% to 2% (w/w), more preferably from between 0.01% to 1% (w/w) prednisolone.
- the topical vehicle containing a compound of anthistamine or antihistamine analog, and/or the additional agent is preferably applied to the site of discomfort on the subject.
- a cream may be applied to the hands of a subject suffering from arthritic fingers, while topical eye drops may be applied to an eye of a subject to treat uveitis.
- a low dosage as defined herein
- these dosages will vary depending on the health and condition of the patient. Thus, a moderate dosage or even a high dosage of one or both agents can be used.
- Administration of each drug in the combination can, mdependently, be one to four times daily for one day to one year, and may even be for the life of the patient. Chronic, long- term administration will be indicated in many cases.
- the compounds of the invention are also useful as screening tools.
- Single agents and combinations of the invention can be employed in antiproliferative or mechanistic assays to determine whether other combinations, or single agents are as effective as the combination in inhibiting the proliferation of proinflammatory cytokines using assays generally known in the art, e.g., TNF ⁇ , IL-2, etc., specific, non-limiting examples of which are described in the Examples section.
- candidate compounds are combined with a compound from with either the antihistamine (or antihistamine analog) or the additional agents described herein, applied to stimulated PBMCs, and at after a suitable time, the cells are examined for anitproliferative activity, TNF ⁇ or other assays for proinflammatory cytokine secretion.
- the relative effects of the combinations versus each other, and versus the single agents are compared, and effective compounds and combinations are identified.
- the screening method can be used for comparing the activity of novel single agents or new combinations of agents (novel or known) for relative activity in the assays.
- the combinations of the invention are also useful tools in elucidating mechanistic information about the biological pathways involved in inflammation or novel targets. Such information can lead to the development of new combinations or single agents (mechanistic and/or structural analogs of either the antihistamine or companion compound) for inhibiting proinflammatory cytokine secretion. Methods known in the art to determine biological pathways can be used to determine the pathway, or network of pathways affected by contacting cells stimulated to produce proinflammatory cytokines with the compounds of the invention.
- Such methods can include, analyzing cellular constituents that are expressed or repressed after contact with the compounds of the invention as compared to untreated, positive or negative control compounds, and/or new single agents and combinations, or analyzing some other metabolic activity of the cell such as enzyme activity, nutrient uptake, and proliferation.
- Cellular components analyzed can include gene transcripts, and protein expression.
- Suitable methods can include standard biochemistry techniques, radiolabeling the compounds of the invention (e,.g., 14 C or 3 H labeling), and observing the compounds binding to proteins, e.g. using 2d gels, gene expression profiling. Once identified, such compounds can be used in in vivo models to furtfier validate the tool or develop new anti-inflammatory agents.
- TNF ⁇ Secretion Assay The effects of test compound combinations on TNF ⁇ secretion were assayed in white blood cells from human buffy coat stimulated with phorbol 12-myristate 13-acetate and ionomycin as follows. Human white blood cells from buffy coat were diluted 1 :50 in media (RPMI; Gibco BRL, #11875-085), 10% fetal bovine serum (Gibco BRL, #25140- 097), 2% penicillin/streptomycin (Gibco BRL, #15140-122)) and 50 ⁇ L of the diluted white blood cells was placed in each well of the assay plate. Drugs were added to the indicated concentration.
- the plate was centrifuged and the supernatant transferred to a white opaque polystyrene 384-well plate (NalgeNunc, Maxisorb) coated with an anti-TNF ⁇ antibody (PharMingen, #551220). After a two-hour incubation, the plate was washed (Tecan Powerwasher 384) with PBS containing 0.1% Tween 20 and incubated for one additional hour with biotin labeled anti-TNFa antibody (PharMingen, #554511) and HRP coupled to streptavidin (PharMingen, #13047E). The plate was then washed again with 0.1% Tween 20/PBS. An HRP-luminescent substrate was added to each well, and the light intensity of each well was measured using a plate luminometer.
- IL-2 Secretion Assay The effects of test compound combinations on IL-2 secretion were assayed in white blood cells from human buffy coat stimulated with phorbol 12-myristate 13- acetate, as follows. Human white blood cells from buffy coat were diluted 1:50 in media (RPMI; Gibco BRL, #11875-085), 10% fetal bovine serum (Gibco BRL, #25140-097), 2% penicillin/streptomycin (Gibco BRL, #15140-122)) and 50 ⁇ L of the diluted white blood cells was placed in each well of the final assay plate created in the above section.
- media RPMI; Gibco BRL, #11875-085
- 10% fetal bovine serum Gibco BRL, #25140-097
- 2% penicillin/streptomycin Gibco BRL, #15140-122
- the plate was centrifuged and the supernatant was transferred to a white opaque 384-well plate (NalgeNunc, MAXISORB) coated with an anti-IL-2 antibody (PharMingen, #555051). After a two-hour incubation, the plate was washed (Tecan Powerwasher 384) with PBS containing 0.1% Tween 2O and incubated for an additional one hour with a biotin labeled anti-IL-2 antibody (Endogen, M600B) and horse radish peroxidase coupled to streptavidin (PharMingen, #13047E). The plate was then washed again with 0.1% Tween 20/PBS, and an HRP-luminescent substrate was added to each well. Light intensity was then measured using a plate luminometer.
- %I percent inhibition (avg. untreated wells - treated well)/(avg. untreated wells)] x 100
- the average untreated well value (avg. untreated wells) is the arithmetic mean of 40 wells from the same assay plate treated with vehicle alone. Negative inhibition values result from local variations in treated wells as compared to untreated wells.
- Tables 6-79 results of various combinations of compounds described on the reduction of TNF ⁇ secretion are shown in Tables 6-79, while the results of various combinations on the reduction of IL-2 secretion are shown in Tables 80-115.
- the effects of varying concentrations of single compound or when used in combination with another compound is shown in individual tables.
- Table 6 shows the effects of varying concentrations of prednisolone and a combination of desloratadine and prednisolone. These results were compared to control wells. These wells were stimulated with phorbol 12-myristate 13 -acetate and ionomycin, but did not receive desloratadine or prednisolone. The effects of the agents alone and in combination are shown as percent inhibition of TNF ⁇ secretion.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Rheumatology (AREA)
- Neurology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Dermatology (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Cardiology (AREA)
- Transplantation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Description
Claims
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2004273880A AU2004273880A1 (en) | 2003-09-15 | 2004-09-15 | Methods and reagents for the treatment of immunoinflammatory disorders |
| CA002537989A CA2537989A1 (en) | 2003-09-15 | 2004-09-15 | Methods and reagents for the treatment of immunoinflammatory disorders |
| BRPI0414435-0A BRPI0414435A (en) | 2003-09-15 | 2004-09-15 | methods and reagents for the treatment of immunoinflammatory disorders |
| MXPA06002929A MXPA06002929A (en) | 2003-09-15 | 2004-09-15 | Dynamic process control. |
| JP2006526998A JP2007516217A (en) | 2003-09-15 | 2004-09-15 | Methods and reagents for the treatment of immune inflammatory disorders |
| EP04784162A EP1670427A4 (en) | 2003-09-15 | 2004-09-15 | Methods and reagents for the treatment of immunoinflammatory disorders |
| IL174185A IL174185A0 (en) | 2003-09-15 | 2006-03-08 | Methods and reagents for the treatment of immunoinflammatory disorders |
| NO20061239A NO20061239L (en) | 2003-09-15 | 2006-03-17 | Methods and Reagents for the Treatment of Immune Inflammatory Disorders |
| IS8410A IS8410A (en) | 2003-09-15 | 2006-04-12 | Methods and Reagents for the Treatment of Immune Inflammatory Disorders |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US50302603P | 2003-09-15 | 2003-09-15 | |
| US60/503,026 | 2003-09-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2005027839A2 true WO2005027839A2 (en) | 2005-03-31 |
| WO2005027839A3 WO2005027839A3 (en) | 2007-06-28 |
Family
ID=34375301
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2004/030210 WO2005027839A2 (en) | 2003-09-15 | 2004-09-15 | Methods and reagents for the treatment of immunoinflammatory disorders |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US20050192261A1 (en) |
| EP (1) | EP1670427A4 (en) |
| JP (1) | JP2007516217A (en) |
| KR (1) | KR20060089725A (en) |
| CN (1) | CN101102760A (en) |
| AR (1) | AR047841A1 (en) |
| AU (1) | AU2004273880A1 (en) |
| BR (1) | BRPI0414435A (en) |
| CA (1) | CA2537989A1 (en) |
| IL (1) | IL174185A0 (en) |
| IS (1) | IS8410A (en) |
| MX (1) | MXPA06002929A (en) |
| NO (1) | NO20061239L (en) |
| RU (1) | RU2006112587A (en) |
| SG (1) | SG146653A1 (en) |
| TW (1) | TW200522932A (en) |
| WO (1) | WO2005027839A2 (en) |
| ZA (1) | ZA200601973B (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1689390A2 (en) * | 2003-11-21 | 2006-08-16 | CombinatoRx, Incorporated | Methods and reagents for the treatment of inflammatory disorders |
| WO2006114115A1 (en) * | 2005-04-26 | 2006-11-02 | Trion Pharma Gmbh | Combination of antibodies and glucocorticoids for treating cancer |
| WO2007103468A2 (en) * | 2006-03-07 | 2007-09-13 | Vertex Pharmaceuticals Incorporated | Use of vx-702 for treating rheumatoid arthritis |
| WO2008090331A1 (en) * | 2007-01-22 | 2008-07-31 | Imperial Innovations Limited | Serotonin reuptake inhibitors for treating arthritis |
| EP1956906A2 (en) * | 2005-11-09 | 2008-08-20 | CombinatoRx, Incorporated | Methods, compositions, and kits for the treatment of medical conditions |
| WO2008127975A2 (en) * | 2007-04-11 | 2008-10-23 | Alcon Research, Ltd. | Use of an inhibitor of tnfa plus an antihistamine to treat allergic rhinitis and allergic conjunctivitis |
| JP2009507009A (en) * | 2005-09-01 | 2009-02-19 | バイオリポックス エービー | Liposome compositions containing antihistamines and corticosteroids and their use for the manufacture of a medicament for the treatment of rhinitis and related diseases |
| US7534806B2 (en) | 2004-12-06 | 2009-05-19 | Avigen, Inc. | Method for treating neuropathic pain and associated syndromes |
| US8071073B2 (en) | 2004-11-24 | 2011-12-06 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| WO2013083569A1 (en) | 2011-12-07 | 2013-06-13 | Teva Branded Pharmaceutical Products R&D, Inc. | Nasal formulation |
| WO2014085884A1 (en) | 2012-12-03 | 2014-06-12 | Ems S.A. | Pharmaceutical composition comprising desloratadine and prednisolone and use thereof |
| US8758816B2 (en) | 2004-11-24 | 2014-06-24 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| US9517241B2 (en) | 2012-12-28 | 2016-12-13 | The Regents Of The University Of Michigan | Amelioration of intestinal fibrosis and treatment of Crohn's disease |
| RU2627424C1 (en) * | 2016-11-03 | 2017-08-08 | Лонг Шенг Фарма Лимитед | Pharmaceutical preparation for rheumatological diseases treatment |
| US10064817B2 (en) | 2004-11-24 | 2018-09-04 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6765001B2 (en) * | 2001-12-21 | 2004-07-20 | Medicis Pharmaceutical Corporation | Compositions and methods for enhancing corticosteroid delivery |
| GB2389530B (en) * | 2002-06-14 | 2007-01-10 | Cipla Ltd | Pharmaceutical compositions |
| US7704527B2 (en) * | 2002-10-25 | 2010-04-27 | Collegium Pharmaceutical, Inc. | Modified release compositions of milnacipran |
| MXPA05008649A (en) * | 2003-02-14 | 2005-11-23 | Combinatorx Inc | Combination therapy for the treatment of immunoinflammatory disorders. |
| JP5021621B2 (en) | 2005-04-13 | 2012-09-12 | アスション ファーマ エー/エス | Beta-2 adrenergic receptor agonist for treating cutaneous connective tissue disease |
| US20070179121A1 (en) * | 2006-02-02 | 2007-08-02 | Plott R T | Method of treating pediatric patients with corticosteroids |
| BRPI0712381A2 (en) * | 2006-06-06 | 2012-07-10 | Avigen Inc | substituted pyrazolo [1,5-a] pyridine compounds and their methods of use |
| US9205080B2 (en) * | 2006-11-16 | 2015-12-08 | Transderm, Inc. | Methods of treating keratin hyperproliferation disorders using mTOR inhibitors |
| US7536799B2 (en) * | 2007-06-07 | 2009-05-26 | Keson Industries | Chalk line apparatus with strategically located chalk fill opening |
| US8940730B2 (en) | 2007-09-18 | 2015-01-27 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and compositions of treating a Flaviviridae family viral infection |
| JP5715820B2 (en) * | 2007-09-18 | 2015-05-13 | スタンフォード ユニバーシティー | Methods for treating infections with Flaviviridae family viruses and compositions for treating infections with Flaviviridae family viruses |
| US9101628B2 (en) * | 2007-09-18 | 2015-08-11 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and composition of treating a flaviviridae family viral infection |
| US9149463B2 (en) * | 2007-09-18 | 2015-10-06 | The Board Of Trustees Of The Leland Standford Junior University | Methods and compositions of treating a Flaviviridae family viral infection |
| US20100092479A1 (en) * | 2008-08-18 | 2010-04-15 | Combinatorx (Singapore) Pte. Ltd. | Compositions and methods for treatment of viral diseases |
| WO2010048264A2 (en) * | 2008-10-23 | 2010-04-29 | Combinatorx, Incorporated | Methods and compositions for the treatment of immunoinflammatory disorders |
| WO2010107739A2 (en) * | 2009-03-18 | 2010-09-23 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and compositions of treating a flaviviridae family viral infection |
| EA016240B1 (en) * | 2009-10-13 | 2012-03-30 | Федеральное Государственное Учреждение "Государственный Научный Центр Дерматовенерологии Федерального Агентства По Высокотехнологичной Медицинской Помощи" | Method for forecasting effectiveness of treating psoriasis patients by infliximab |
| EP2705847B1 (en) * | 2012-09-05 | 2014-07-02 | PSoriasis+Creams Sweden AB | Composition for treating psoriasis |
| CN103830208A (en) * | 2012-11-26 | 2014-06-04 | 天津金耀集团有限公司 | H1-receptor-antagonist-containing inhalation preparation |
| CN103830728A (en) * | 2012-11-26 | 2014-06-04 | 天津金耀集团有限公司 | Compound inhalation medicine of ciclesonide and H1 receptor antagonist |
| CN103830730A (en) * | 2012-11-26 | 2014-06-04 | 天津金耀集团有限公司 | Budesonide/H1 receptor antagonist compound inhalant |
| CN103830731A (en) * | 2012-11-26 | 2014-06-04 | 天津金耀集团有限公司 | Glucocorticoid/H1 receptor antagonist compound inhalation composition |
| AU2014209141B2 (en) | 2013-01-24 | 2018-05-10 | Palvella Therapeutics, Inc. | Compositions for transdermal delivery of mTOR inhibitors |
| US20170342136A1 (en) * | 2015-01-12 | 2017-11-30 | Agency For Science, Technology And Research | Monoclonal antibody against muramyl peptides in prevention and treatment of immune-mediated diseases |
| CN104940179B (en) * | 2015-05-29 | 2017-10-27 | 中国人民解放军第二军医大学 | Application of the bagodryl hydrochloride in Experiment on therapy Autoimmune Encephalomyelitis medicine is prepared |
| KR20230129590A (en) * | 2015-06-30 | 2023-09-08 | 아이거 그룹 인터내셔널, 인코포레이티드 | Use of Chloroquine and Clemizole Compounds for Treatment of Inflammatory and Cancerous Conditions |
| WO2018129364A1 (en) | 2017-01-06 | 2018-07-12 | Palvella Therapeutics Llc | Anhydrous compositions of mtor inhibitors and methods of use |
| WO2019178516A1 (en) * | 2018-03-16 | 2019-09-19 | SEN-JAM Pharmaceutical LLC | Methods and compositions to treat enteropathic arthritis |
| EP3817743A4 (en) | 2018-07-02 | 2022-07-06 | Palvella Therapeutics, Inc. | Anhydrous compositions of mtor inhibitors and methods of use |
| EP4236949A4 (en) * | 2020-10-30 | 2024-09-25 | Univ Leland Stanford Junior | Drugs targeting inflammation for the treatment of osteoarthritis and other inflammatory diseases |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3231468A (en) * | 1962-07-02 | 1966-01-25 | Merck & Co Inc | Dexamethasone-cyproheptadine oral antiflammatory compositions |
| US3419655A (en) * | 1967-03-17 | 1968-12-31 | American Home Prod | Treatment of inflammations by administering a cycloleucyl compound |
| US4444780A (en) * | 1982-08-30 | 1984-04-24 | Ortho Pharmaceutical Corporation | Method for treating atopic dermatitis |
| US5166149A (en) * | 1989-09-08 | 1992-11-24 | Chemex Pharmaceuticals, Inc. | Methotrexate compositions and methods of treatment using same |
| US5030634A (en) * | 1990-03-29 | 1991-07-09 | Krumdieck Carlos L | 10-deazaaminopterin: a new arthritis remittive drug |
| ATE227124T1 (en) * | 1994-10-05 | 2002-11-15 | Cari Loder | TREATMENT OF MULTIPLE SCLERosis (MS) AND OTHER DEMYELINIZATION DISEASES USING LOFEPRAMINE IN COMBINATION WITH L-PHENYLALAMIN, TYROSINE OR TRYPTOPHAN AND POSSIBLE. A VITAMIN B12 COMPOUND |
| WO1996020712A1 (en) * | 1994-12-30 | 1996-07-11 | American Home Products Corporation | Clear non-alcoholic hydrocortisone solutions |
| US5736537A (en) * | 1995-09-12 | 1998-04-07 | Estee Lauder, Inc. | Dehydroep:androsterone sailcylate useful against skin atrophy |
| EP0780127A1 (en) * | 1995-12-19 | 1997-06-25 | The Procter & Gamble Company | A nasal spray containing a steroid and a antihistamine |
| JPH11511758A (en) * | 1996-06-04 | 1999-10-12 | ザ、プロクター、エンド、ギャンブル、カンパニー | Intranasal spray containing nasal steroids and antihistamines |
| IN188720B (en) * | 1997-11-06 | 2002-11-02 | Panacea Biotec Ltd | |
| US6140337A (en) * | 1997-12-23 | 2000-10-31 | Schering Corporation | Methods for the treatment of mental disorders |
| US6423721B1 (en) * | 1998-09-10 | 2002-07-23 | Schering Corporation | Methods and compositions for treating sinusitis, otitis media and other related disorders using antihistamines |
| WO2000035454A1 (en) * | 1998-12-18 | 2000-06-22 | Du Pont Pharmaceuticals Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| JP2001510485A (en) * | 1999-03-01 | 2001-07-31 | シェーリング コーポレイション | Compositions and methods for treating atopic dermatitis, angioedema and other disorders using antihistamines and glucocorticoids |
| GB0023220D0 (en) * | 2000-09-21 | 2000-11-01 | Arakis Ltd | Corticosteroid formulation |
| US20020006961A1 (en) * | 1999-05-14 | 2002-01-17 | Katz Stanley E. | Method and composition for treating mammalian nasal and sinus diseases caused by inflammatory response |
| US20020061281A1 (en) * | 1999-07-06 | 2002-05-23 | Osbakken Robert S. | Aerosolized anti-infectives, anti-inflammatories, and decongestants for the treatment of sinusitis |
| WO2001026658A2 (en) * | 1999-10-08 | 2001-04-19 | Schering Corporation | Topical nasal treatment using desloratadine |
| WO2001076600A2 (en) * | 2000-04-07 | 2001-10-18 | Schering Corporation | Inhibition of cytokine generation |
| US6488937B1 (en) * | 2000-08-23 | 2002-12-03 | William Smits | Allergy treatment method using a rapid immunotherapy protocol |
| WO2002028862A2 (en) * | 2000-10-02 | 2002-04-11 | Emory University | Triptolide analogs for the treatment of autoimmune and inflammatory disorders |
| US6599914B2 (en) * | 2001-04-24 | 2003-07-29 | Schering Corporation | Inhibition of cytokine generation |
| US7034059B2 (en) * | 2001-07-02 | 2006-04-25 | Sepracor Inc. | Methods of using norfluoxetine |
| WO2003020274A1 (en) * | 2001-08-30 | 2003-03-13 | Aventis Pharmaceuticals Inc. | Treatment of atopic dermatitis |
| US20040220153A1 (en) * | 2002-09-24 | 2004-11-04 | Jost-Price Edward Roydon | Methods and reagents for the treatment of diseases and disorders associated with increased levels of proinflammatory cytokines |
| MXPA05008649A (en) * | 2003-02-14 | 2005-11-23 | Combinatorx Inc | Combination therapy for the treatment of immunoinflammatory disorders. |
-
2004
- 2004-09-14 US US10/940,902 patent/US20050192261A1/en not_active Abandoned
- 2004-09-14 TW TW093127736A patent/TW200522932A/en unknown
- 2004-09-15 JP JP2006526998A patent/JP2007516217A/en active Pending
- 2004-09-15 AU AU2004273880A patent/AU2004273880A1/en not_active Abandoned
- 2004-09-15 AR ARP040103302A patent/AR047841A1/en unknown
- 2004-09-15 RU RU2006112587/15A patent/RU2006112587A/en not_active Application Discontinuation
- 2004-09-15 BR BRPI0414435-0A patent/BRPI0414435A/en not_active IP Right Cessation
- 2004-09-15 ZA ZA200601973A patent/ZA200601973B/en unknown
- 2004-09-15 WO PCT/US2004/030210 patent/WO2005027839A2/en active Application Filing
- 2004-09-15 KR KR1020067006026A patent/KR20060089725A/en not_active Application Discontinuation
- 2004-09-15 EP EP04784162A patent/EP1670427A4/en not_active Withdrawn
- 2004-09-15 MX MXPA06002929A patent/MXPA06002929A/en unknown
- 2004-09-15 CN CNA2004800336483A patent/CN101102760A/en active Pending
- 2004-09-15 SG SG200806907-2A patent/SG146653A1/en unknown
- 2004-09-15 CA CA002537989A patent/CA2537989A1/en not_active Abandoned
-
2006
- 2006-03-08 IL IL174185A patent/IL174185A0/en unknown
- 2006-03-17 NO NO20061239A patent/NO20061239L/en not_active Application Discontinuation
- 2006-04-12 IS IS8410A patent/IS8410A/en unknown
Non-Patent Citations (1)
| Title |
|---|
| See references of EP1670427A4 * |
Cited By (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1689390A4 (en) * | 2003-11-21 | 2008-03-12 | Combinatorx Inc | Methods and reagents for the treatment of inflammatory disorders |
| EP1689390A2 (en) * | 2003-11-21 | 2006-08-16 | CombinatoRx, Incorporated | Methods and reagents for the treatment of inflammatory disorders |
| US7709495B2 (en) | 2003-11-21 | 2010-05-04 | Combinatorx, Incorporated | Methods and reagents for the treatment of inflammatory disorders |
| US9919050B2 (en) | 2004-11-24 | 2018-03-20 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine |
| US10064817B2 (en) | 2004-11-24 | 2018-09-04 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| US8758816B2 (en) | 2004-11-24 | 2014-06-24 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| US8518919B2 (en) | 2004-11-24 | 2013-08-27 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| US8071073B2 (en) | 2004-11-24 | 2011-12-06 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| US7534806B2 (en) | 2004-12-06 | 2009-05-19 | Avigen, Inc. | Method for treating neuropathic pain and associated syndromes |
| US10071158B2 (en) | 2005-04-26 | 2018-09-11 | Lindis Biotech Gmbh | Combination of the application of antibodies for immunostimulation together with glucocorticoids |
| US10576149B2 (en) | 2005-04-26 | 2020-03-03 | Lindis Biotech Gmbh | Combination of the application of antibodies for immunostimulation together with glucocorticoids |
| WO2006114115A1 (en) * | 2005-04-26 | 2006-11-02 | Trion Pharma Gmbh | Combination of antibodies and glucocorticoids for treating cancer |
| JP2009507009A (en) * | 2005-09-01 | 2009-02-19 | バイオリポックス エービー | Liposome compositions containing antihistamines and corticosteroids and their use for the manufacture of a medicament for the treatment of rhinitis and related diseases |
| US8067433B2 (en) | 2005-11-09 | 2011-11-29 | Zalicus Inc. | Methods, compositions, and kits for the treatment of ophthalmic disorders |
| US8258153B2 (en) | 2005-11-09 | 2012-09-04 | Zalicus, Inc. | Methods, compositions, and kits for the treatment of ophthalmic disorders |
| JP2009514969A (en) * | 2005-11-09 | 2009-04-09 | コンビナトアールエックス インコーポレーティッド | Methods, compositions, and kits for treating medical conditions |
| JP2013231047A (en) * | 2005-11-09 | 2013-11-14 | Zalicus Inc | Method, composition, and kit for treatment of medical condition |
| EP1956906A4 (en) * | 2005-11-09 | 2009-12-30 | Combinatorx Inc | Methods, compositions, and kits for the treatment of medical conditions |
| EP1956906A2 (en) * | 2005-11-09 | 2008-08-20 | CombinatoRx, Incorporated | Methods, compositions, and kits for the treatment of medical conditions |
| WO2007103468A2 (en) * | 2006-03-07 | 2007-09-13 | Vertex Pharmaceuticals Incorporated | Use of vx-702 for treating rheumatoid arthritis |
| WO2007103468A3 (en) * | 2006-03-07 | 2008-05-29 | Vertex Pharma | Use of vx-702 for treating rheumatoid arthritis |
| WO2008090331A1 (en) * | 2007-01-22 | 2008-07-31 | Imperial Innovations Limited | Serotonin reuptake inhibitors for treating arthritis |
| WO2008127975A3 (en) * | 2007-04-11 | 2009-07-30 | Alcon Res Ltd | Use of an inhibitor of tnfa plus an antihistamine to treat allergic rhinitis and allergic conjunctivitis |
| WO2008127975A2 (en) * | 2007-04-11 | 2008-10-23 | Alcon Research, Ltd. | Use of an inhibitor of tnfa plus an antihistamine to treat allergic rhinitis and allergic conjunctivitis |
| WO2013083569A1 (en) | 2011-12-07 | 2013-06-13 | Teva Branded Pharmaceutical Products R&D, Inc. | Nasal formulation |
| WO2014085884A1 (en) | 2012-12-03 | 2014-06-12 | Ems S.A. | Pharmaceutical composition comprising desloratadine and prednisolone and use thereof |
| US9517241B2 (en) | 2012-12-28 | 2016-12-13 | The Regents Of The University Of Michigan | Amelioration of intestinal fibrosis and treatment of Crohn's disease |
| RU2627424C1 (en) * | 2016-11-03 | 2017-08-08 | Лонг Шенг Фарма Лимитед | Pharmaceutical preparation for rheumatological diseases treatment |
Also Published As
| Publication number | Publication date |
|---|---|
| US20050192261A1 (en) | 2005-09-01 |
| EP1670427A4 (en) | 2009-01-07 |
| AR047841A1 (en) | 2006-03-01 |
| CA2537989A1 (en) | 2005-03-31 |
| EP1670427A2 (en) | 2006-06-21 |
| NO20061239L (en) | 2006-06-08 |
| RU2006112587A (en) | 2007-10-27 |
| IL174185A0 (en) | 2006-08-01 |
| TW200522932A (en) | 2005-07-16 |
| CN101102760A (en) | 2008-01-09 |
| JP2007516217A (en) | 2007-06-21 |
| SG146653A1 (en) | 2008-10-30 |
| MXPA06002929A (en) | 2006-06-14 |
| ZA200601973B (en) | 2008-08-27 |
| KR20060089725A (en) | 2006-08-09 |
| WO2005027839A3 (en) | 2007-06-28 |
| AU2004273880A1 (en) | 2005-03-31 |
| IS8410A (en) | 2006-04-12 |
| BRPI0414435A (en) | 2006-11-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20050192261A1 (en) | Methods and reagents for the treatment of immunoinflammatory disorders | |
| ZA200603116B (en) | Methods and reagents for the treatment of immunoinflammatory disorders | |
| AU2006311577B2 (en) | Methods, compositions, and kits for the treatment of medical conditions | |
| US20040224876A1 (en) | Combination therapy for the treatment of immunoinflammatory disorders | |
| US20050112199A1 (en) | Therapeutic regimens for administering drug combinations | |
| US20040229849A1 (en) | Methods and reagents for the treatment of diseases and disorders associated with increased levels of proinflammatory cytokines | |
| CA2509526A1 (en) | Methods and reagents for the treatment of diseases and disorders associated with increased levels of proinflammatory cytokines | |
| WO2005030132A2 (en) | Therapeutic regimens for administering drug combinations | |
| MXPA06004258A (en) | Methods and reagents for the treatment of immunoinflammatory disorders | |
| MX2007016114A (en) | Combination therapy for the treatment of immunoinflammatory disorders |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200480033648.3 Country of ref document: CN |
|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BW BY BZ CA CH CN CO CR CU CZ DK DM DZ EC EE EG ES FI GB GD GE GM HR HU ID IL IN IS JP KE KG KP KZ LC LK LR LS LT LU LV MA MD MK MN MW MX MZ NA NI NO NZ PG PH PL PT RO RU SC SD SE SG SK SY TJ TM TN TR TT TZ UA UG US UZ VN YU ZA ZM |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): BW GH GM KE LS MW MZ NA SD SZ TZ UG ZM ZW AM AZ BY KG MD RU TJ TM AT BE BG CH CY DE DK EE ES FI FR GB GR HU IE IT MC NL PL PT RO SE SI SK TR BF CF CG CI CM GA GN GQ GW ML MR SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2537989 Country of ref document: CA Ref document number: 2004273880 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 545737 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 174185 Country of ref document: IL Ref document number: 200601973 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006526998 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020067006026 Country of ref document: KR |
|
| ENP | Entry into the national phase |
Ref document number: 2004273880 Country of ref document: AU Date of ref document: 20040915 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004273880 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2004784162 Country of ref document: EP Ref document number: 1270/CHENP/2006 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006112587 Country of ref document: RU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004784162 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: PI0414435 Country of ref document: BR |