US20170333372A1 - Compositions for improvement of brain function - Google Patents
Compositions for improvement of brain function Download PDFInfo
- Publication number
- US20170333372A1 US20170333372A1 US15/447,974 US201715447974A US2017333372A1 US 20170333372 A1 US20170333372 A1 US 20170333372A1 US 201715447974 A US201715447974 A US 201715447974A US 2017333372 A1 US2017333372 A1 US 2017333372A1
- Authority
- US
- United States
- Prior art keywords
- lipase
- akg
- amylase
- protease
- alpha
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 128
- 230000006872 improvement Effects 0.000 title description 8
- 230000003925 brain function Effects 0.000 title description 3
- KPGXRSRHYNQIFN-UHFFFAOYSA-N 2-oxoglutaric acid Chemical compound OC(=O)CCC(=O)C(O)=O KPGXRSRHYNQIFN-UHFFFAOYSA-N 0.000 claims abstract description 277
- 108090001060 Lipase Proteins 0.000 claims abstract description 166
- 102000004882 Lipase Human genes 0.000 claims abstract description 166
- 239000004367 Lipase Substances 0.000 claims abstract description 166
- 235000019421 lipase Nutrition 0.000 claims abstract description 166
- 108091005804 Peptidases Proteins 0.000 claims abstract description 124
- 239000004365 Protease Substances 0.000 claims abstract description 114
- 108010065511 Amylases Proteins 0.000 claims abstract description 111
- 102000013142 Amylases Human genes 0.000 claims abstract description 111
- 235000019418 amylase Nutrition 0.000 claims abstract description 110
- 150000003839 salts Chemical class 0.000 claims abstract description 72
- HWXBTNAVRSUOJR-UHFFFAOYSA-N alpha-hydroxyglutaric acid Natural products OC(=O)C(O)CCC(O)=O HWXBTNAVRSUOJR-UHFFFAOYSA-N 0.000 claims abstract description 64
- 229940009533 alpha-ketoglutaric acid Drugs 0.000 claims abstract description 64
- 102000004190 Enzymes Human genes 0.000 claims abstract description 17
- 108090000790 Enzymes Proteins 0.000 claims abstract description 17
- 102000035195 Peptidases Human genes 0.000 claims description 123
- 238000000034 method Methods 0.000 claims description 66
- 238000011282 treatment Methods 0.000 claims description 63
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 46
- 229940088598 enzyme Drugs 0.000 claims description 16
- 201000010099 disease Diseases 0.000 claims description 6
- 229940025131 amylases Drugs 0.000 claims description 5
- 239000004382 Amylase Substances 0.000 abstract description 105
- 208000014674 injury Diseases 0.000 abstract description 19
- 208000015122 neurodegenerative disease Diseases 0.000 abstract description 18
- 230000008733 trauma Effects 0.000 abstract description 17
- 206010008874 Chronic Fatigue Syndrome Diseases 0.000 abstract description 13
- 208000029766 myalgic encephalomeyelitis/chronic fatigue syndrome Diseases 0.000 abstract description 13
- 230000000926 neurological effect Effects 0.000 abstract description 11
- 208000012902 Nervous system disease Diseases 0.000 abstract description 9
- 230000004770 neurodegeneration Effects 0.000 abstract description 6
- 208000025966 Neurological disease Diseases 0.000 abstract description 5
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 abstract 1
- 235000019419 proteases Nutrition 0.000 description 105
- 241001465754 Metazoa Species 0.000 description 50
- 208000035475 disorder Diseases 0.000 description 40
- 230000002265 prevention Effects 0.000 description 26
- 210000000225 synapse Anatomy 0.000 description 26
- 210000002504 synaptic vesicle Anatomy 0.000 description 26
- 210000004556 brain Anatomy 0.000 description 23
- 108010067035 Pancrelipase Proteins 0.000 description 21
- 210000000063 presynaptic terminal Anatomy 0.000 description 20
- 206010014950 Eosinophilia Diseases 0.000 description 19
- XRHVZWWRFMCBAZ-UHFFFAOYSA-L Endothal-disodium Chemical compound [Na+].[Na+].C1CC2C(C([O-])=O)C(C(=O)[O-])C1O2 XRHVZWWRFMCBAZ-UHFFFAOYSA-L 0.000 description 18
- 210000001320 hippocampus Anatomy 0.000 description 18
- 229940045258 pancrelipase Drugs 0.000 description 18
- 208000029028 brain injury Diseases 0.000 description 16
- 238000012360 testing method Methods 0.000 description 16
- 241000589513 Burkholderia cepacia Species 0.000 description 15
- 230000004766 neurogenesis Effects 0.000 description 15
- 208000010877 cognitive disease Diseases 0.000 description 14
- 239000013078 crystal Substances 0.000 description 14
- 239000003814 drug Substances 0.000 description 14
- 230000000971 hippocampal effect Effects 0.000 description 14
- 230000000946 synaptic effect Effects 0.000 description 14
- 241000981399 Aspergillus melleus Species 0.000 description 12
- 235000002247 Aspergillus oryzae Nutrition 0.000 description 12
- 240000006439 Aspergillus oryzae Species 0.000 description 12
- 241000699694 Gerbillinae Species 0.000 description 12
- 108010087173 bile salt-stimulated lipase Proteins 0.000 description 12
- 238000004519 manufacturing process Methods 0.000 description 12
- 208000020431 spinal cord injury Diseases 0.000 description 12
- 210000002569 neuron Anatomy 0.000 description 11
- 241000228212 Aspergillus Species 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 239000002858 neurotransmitter agent Substances 0.000 description 10
- 208000011580 syndromic disease Diseases 0.000 description 10
- 239000002775 capsule Substances 0.000 description 9
- 230000002538 fungal effect Effects 0.000 description 9
- 230000015654 memory Effects 0.000 description 9
- 230000001537 neural effect Effects 0.000 description 9
- 235000019833 protease Nutrition 0.000 description 9
- 208000024827 Alzheimer disease Diseases 0.000 description 8
- 208000020925 Bipolar disease Diseases 0.000 description 8
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 208000027061 mild cognitive impairment Diseases 0.000 description 8
- 210000005055 nestin Anatomy 0.000 description 8
- 210000001178 neural stem cell Anatomy 0.000 description 8
- 239000011734 sodium Substances 0.000 description 8
- 229910052708 sodium Inorganic materials 0.000 description 8
- 230000002195 synergetic effect Effects 0.000 description 8
- 208000026139 Memory disease Diseases 0.000 description 7
- 102000008730 Nestin Human genes 0.000 description 7
- 108010088225 Nestin Proteins 0.000 description 7
- 210000004027 cell Anatomy 0.000 description 7
- 230000001149 cognitive effect Effects 0.000 description 7
- 230000003920 cognitive function Effects 0.000 description 7
- 238000009826 distribution Methods 0.000 description 7
- 238000002474 experimental method Methods 0.000 description 7
- 230000002269 spontaneous effect Effects 0.000 description 7
- 102100035687 Bile salt-activated lipase Human genes 0.000 description 6
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 6
- 208000020358 Learning disease Diseases 0.000 description 6
- 208000019022 Mood disease Diseases 0.000 description 6
- 206010057430 Retinal injury Diseases 0.000 description 6
- 230000032683 aging Effects 0.000 description 6
- 230000001580 bacterial effect Effects 0.000 description 6
- 239000011575 calcium Substances 0.000 description 6
- 230000003931 cognitive performance Effects 0.000 description 6
- 235000015872 dietary supplement Nutrition 0.000 description 6
- 230000000302 ischemic effect Effects 0.000 description 6
- 230000013016 learning Effects 0.000 description 6
- 201000003723 learning disability Diseases 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 230000000813 microbial effect Effects 0.000 description 6
- 210000001525 retina Anatomy 0.000 description 6
- 241001453380 Burkholderia Species 0.000 description 5
- 208000023105 Huntington disease Diseases 0.000 description 5
- 208000018737 Parkinson disease Diseases 0.000 description 5
- 208000030886 Traumatic Brain injury Diseases 0.000 description 5
- 230000004075 alteration Effects 0.000 description 5
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 5
- 230000000875 corresponding effect Effects 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 235000018102 proteins Nutrition 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 230000009529 traumatic brain injury Effects 0.000 description 5
- KPGXRSRHYNQIFN-UHFFFAOYSA-L 2-oxoglutarate(2-) Chemical compound [O-]C(=O)CCC(=O)C([O-])=O KPGXRSRHYNQIFN-UHFFFAOYSA-L 0.000 description 4
- 208000019901 Anxiety disease Diseases 0.000 description 4
- 208000036640 Asperger disease Diseases 0.000 description 4
- 201000006062 Asperger syndrome Diseases 0.000 description 4
- 208000006096 Attention Deficit Disorder with Hyperactivity Diseases 0.000 description 4
- 206010003805 Autism Diseases 0.000 description 4
- 208000020706 Autistic disease Diseases 0.000 description 4
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 4
- 206010013654 Drug abuse Diseases 0.000 description 4
- 208000032382 Ischaemic stroke Diseases 0.000 description 4
- 208000027089 Parkinsonian disease Diseases 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 230000036506 anxiety Effects 0.000 description 4
- 230000006399 behavior Effects 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 208000028683 bipolar I disease Diseases 0.000 description 4
- 208000025307 bipolar depression Diseases 0.000 description 4
- 229910052791 calcium Inorganic materials 0.000 description 4
- 230000009514 concussion Effects 0.000 description 4
- 235000014113 dietary fatty acids Nutrition 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 210000003979 eosinophil Anatomy 0.000 description 4
- 206010015037 epilepsy Diseases 0.000 description 4
- 229930195729 fatty acid Natural products 0.000 description 4
- 239000000194 fatty acid Substances 0.000 description 4
- 150000004665 fatty acids Chemical class 0.000 description 4
- 230000009808 hippocampal neurogenesis Effects 0.000 description 4
- 230000000877 morphologic effect Effects 0.000 description 4
- 239000002417 nutraceutical Substances 0.000 description 4
- 235000021436 nutraceutical agent Nutrition 0.000 description 4
- 230000010412 perfusion Effects 0.000 description 4
- 235000020777 polyunsaturated fatty acids Nutrition 0.000 description 4
- 210000003538 post-synaptic density Anatomy 0.000 description 4
- 108010092804 postsynaptic density proteins Proteins 0.000 description 4
- 208000011117 substance-related disease Diseases 0.000 description 4
- 230000005062 synaptic transmission Effects 0.000 description 4
- SKSYZEHWGCTZPG-UHFFFAOYSA-N 5-methyl-4-phenyl-2-pyridin-2-yl-1h-pyrazol-3-one Chemical compound O=C1C(C=2C=CC=CC=2)=C(C)NN1C1=CC=CC=N1 SKSYZEHWGCTZPG-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 208000023275 Autoimmune disease Diseases 0.000 description 3
- 108010015598 Chromobacterium viscosum lipase Proteins 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical class CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 241000589516 Pseudomonas Species 0.000 description 3
- 241000303962 Rhizopus delemar Species 0.000 description 3
- 101000966369 Rhizopus oryzae Lipase Proteins 0.000 description 3
- 241000283984 Rodentia Species 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 229940024606 amino acid Drugs 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- LADYPAWUSNPKJF-UHFFFAOYSA-L calcium;2-oxopentanedioate Chemical compound [Ca+2].[O-]C(=O)CCC(=O)C([O-])=O LADYPAWUSNPKJF-UHFFFAOYSA-L 0.000 description 3
- 230000003197 catalytic effect Effects 0.000 description 3
- 230000019771 cognition Effects 0.000 description 3
- 230000002964 excitative effect Effects 0.000 description 3
- 230000028023 exocytosis Effects 0.000 description 3
- 235000013373 food additive Nutrition 0.000 description 3
- 239000002778 food additive Substances 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 210000003128 head Anatomy 0.000 description 3
- 108010033214 liprotamase lipase Proteins 0.000 description 3
- 230000001613 neoplastic effect Effects 0.000 description 3
- 229940116451 pancrelipase amylase Drugs 0.000 description 3
- 229940116449 pancrelipase protease Drugs 0.000 description 3
- 239000008363 phosphate buffer Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 201000000980 schizophrenia Diseases 0.000 description 3
- 108010079522 solysime Proteins 0.000 description 3
- 230000035882 stress Effects 0.000 description 3
- 201000008914 temporal lobe epilepsy Diseases 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 208000007848 Alcoholism Diseases 0.000 description 2
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 2
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 2
- 206010005003 Bladder cancer Diseases 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- 206010010741 Conjunctivitis Diseases 0.000 description 2
- 206010012289 Dementia Diseases 0.000 description 2
- 206010012438 Dermatitis atopic Diseases 0.000 description 2
- 206010064212 Eosinophilic oesophagitis Diseases 0.000 description 2
- 208000009329 Graft vs Host Disease Diseases 0.000 description 2
- 206010019196 Head injury Diseases 0.000 description 2
- 208000006968 Helminthiasis Diseases 0.000 description 2
- 208000017604 Hodgkin disease Diseases 0.000 description 2
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 2
- 238000001276 Kolmogorov–Smirnov test Methods 0.000 description 2
- 201000005099 Langerhans cell histiocytosis Diseases 0.000 description 2
- 208000009829 Lewy Body Disease Diseases 0.000 description 2
- 201000002832 Lewy body dementia Diseases 0.000 description 2
- 206010048911 Lissencephaly Diseases 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 241000699684 Meriones unguiculatus Species 0.000 description 2
- 208000005314 Multi-Infarct Dementia Diseases 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- 208000030852 Parasitic disease Diseases 0.000 description 2
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 2
- 208000028561 Primary cutaneous T-cell lymphoma Diseases 0.000 description 2
- 208000028017 Psychotic disease Diseases 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 208000009359 Sezary Syndrome Diseases 0.000 description 2
- 208000021386 Sjogren Syndrome Diseases 0.000 description 2
- 208000005718 Stomach Neoplasms Diseases 0.000 description 2
- 208000024770 Thyroid neoplasm Diseases 0.000 description 2
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 2
- 201000004810 Vascular dementia Diseases 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 230000036982 action potential Effects 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 230000016571 aggressive behavior Effects 0.000 description 2
- 206010001584 alcohol abuse Diseases 0.000 description 2
- 208000025746 alcohol use disease Diseases 0.000 description 2
- MBMBGCFOFBJSGT-KUBAVDMBSA-N all-cis-docosa-4,7,10,13,16,19-hexaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O MBMBGCFOFBJSGT-KUBAVDMBSA-N 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- 108090000637 alpha-Amylases Proteins 0.000 description 2
- 102000004139 alpha-Amylases Human genes 0.000 description 2
- 229940024171 alpha-amylase Drugs 0.000 description 2
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 238000011394 anticancer treatment Methods 0.000 description 2
- YZXBAPSDXZZRGB-DOFZRALJSA-N arachidonic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O YZXBAPSDXZZRGB-DOFZRALJSA-N 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 201000008937 atopic dermatitis Diseases 0.000 description 2
- 230000003542 behavioural effect Effects 0.000 description 2
- 210000001772 blood platelet Anatomy 0.000 description 2
- 208000030963 borderline personality disease Diseases 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 201000011510 cancer Diseases 0.000 description 2
- 206010008129 cerebral palsy Diseases 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- CVSVTCORWBXHQV-UHFFFAOYSA-N creatine Chemical compound NC(=[NH2+])N(C)CC([O-])=O CVSVTCORWBXHQV-UHFFFAOYSA-N 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 201000001981 dermatomyositis Diseases 0.000 description 2
- VTYGSWGUBDXCRA-UHFFFAOYSA-L dipotassium;2-oxopentanedioate Chemical compound [K+].[K+].[O-]C(=O)CCC(=O)C([O-])=O VTYGSWGUBDXCRA-UHFFFAOYSA-L 0.000 description 2
- 238000001493 electron microscopy Methods 0.000 description 2
- 231100000317 environmental toxin Toxicity 0.000 description 2
- 201000000708 eosinophilic esophagitis Diseases 0.000 description 2
- 238000004880 explosion Methods 0.000 description 2
- 239000000834 fixative Substances 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 206010017758 gastric cancer Diseases 0.000 description 2
- 208000024908 graft versus host disease Diseases 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 229910017053 inorganic salt Inorganic materials 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 208000028867 ischemia Diseases 0.000 description 2
- 229960003299 ketamine Drugs 0.000 description 2
- 208000014817 lissencephaly spectrum disease Diseases 0.000 description 2
- 201000007270 liver cancer Diseases 0.000 description 2
- 208000014018 liver neoplasm Diseases 0.000 description 2
- 238000011866 long-term treatment Methods 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 238000001000 micrograph Methods 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- 201000003631 narcolepsy Diseases 0.000 description 2
- 210000003463 organelle Anatomy 0.000 description 2
- -1 ornithine-AKG Chemical compound 0.000 description 2
- 230000036281 parasite infection Effects 0.000 description 2
- 208000014837 parasitic helminthiasis infectious disease Diseases 0.000 description 2
- 230000001575 pathological effect Effects 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 208000019899 phobic disease Diseases 0.000 description 2
- 230000003518 presynaptic effect Effects 0.000 description 2
- 210000001176 projection neuron Anatomy 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- LXNHXLLTXMVWPM-UHFFFAOYSA-N pyridoxine Chemical compound CC1=NC=C(CO)C(CO)=C1O LXNHXLLTXMVWPM-UHFFFAOYSA-N 0.000 description 2
- 238000004064 recycling Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 208000012672 seasonal affective disease Diseases 0.000 description 2
- 230000035939 shock Effects 0.000 description 2
- 208000019116 sleep disease Diseases 0.000 description 2
- 210000000278 spinal cord Anatomy 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 210000002784 stomach Anatomy 0.000 description 2
- 201000011549 stomach cancer Diseases 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 230000007470 synaptic degeneration Effects 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 201000002510 thyroid cancer Diseases 0.000 description 2
- 206010043778 thyroiditis Diseases 0.000 description 2
- 208000037816 tissue injury Diseases 0.000 description 2
- 230000000472 traumatic effect Effects 0.000 description 2
- 201000005112 urinary bladder cancer Diseases 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- 239000012114 Alexa Fluor 647 Substances 0.000 description 1
- 208000000044 Amnesia Diseases 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 125000002353 D-glucosyl group Chemical group C1([C@H](O)[C@@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 201000010374 Down Syndrome Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 229920002527 Glycogen Polymers 0.000 description 1
- 108010022901 Heparin Lyase Proteins 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241000579835 Merops Species 0.000 description 1
- 108010006035 Metalloproteases Proteins 0.000 description 1
- 102000005741 Metalloproteases Human genes 0.000 description 1
- MKYBYDHXWVHEJW-UHFFFAOYSA-N N-[1-oxo-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propan-2-yl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(C(C)NC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 MKYBYDHXWVHEJW-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 108010019160 Pancreatin Proteins 0.000 description 1
- 102100021904 Potassium-transporting ATPase alpha chain 1 Human genes 0.000 description 1
- 108010083204 Proton Pumps Proteins 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- COQLPRJCUIATTQ-UHFFFAOYSA-N Uranyl acetate Chemical compound O.O.O=[U]=O.CC(O)=O.CC(O)=O COQLPRJCUIATTQ-UHFFFAOYSA-N 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 230000009056 active transport Effects 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- JAZBEHYOTPTENJ-JLNKQSITSA-N all-cis-5,8,11,14,17-icosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O JAZBEHYOTPTENJ-JLNKQSITSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 210000004727 amygdala Anatomy 0.000 description 1
- 229940069428 antacid Drugs 0.000 description 1
- 239000003159 antacid agent Substances 0.000 description 1
- 229940114079 arachidonic acid Drugs 0.000 description 1
- 235000021342 arachidonic acid Nutrition 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 230000001174 ascending effect Effects 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 210000001130 astrocyte Anatomy 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical class CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 description 1
- 238000009227 behaviour therapy Methods 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- HOQPTLCRWVZIQZ-UHFFFAOYSA-H bis[[2-(5-hydroxy-4,7-dioxo-1,3,2$l^{2}-dioxaplumbepan-5-yl)acetyl]oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HOQPTLCRWVZIQZ-UHFFFAOYSA-H 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 208000015114 central nervous system disease Diseases 0.000 description 1
- 210000003710 cerebral cortex Anatomy 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229940045110 chitosan Drugs 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000003930 cognitive ability Effects 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 230000001447 compensatory effect Effects 0.000 description 1
- 238000007596 consolidation process Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 229960003624 creatine Drugs 0.000 description 1
- 239000006046 creatine Substances 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 210000001787 dendrite Anatomy 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 229940079919 digestives enzyme preparation Drugs 0.000 description 1
- 235000020669 docosahexaenoic acid Nutrition 0.000 description 1
- 229940090949 docosahexaenoic acid Drugs 0.000 description 1
- 230000035622 drinking Effects 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 235000020673 eicosapentaenoic acid Nutrition 0.000 description 1
- 229960005135 eicosapentaenoic acid Drugs 0.000 description 1
- JAZBEHYOTPTENJ-UHFFFAOYSA-N eicosapentaenoic acid Natural products CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O JAZBEHYOTPTENJ-UHFFFAOYSA-N 0.000 description 1
- 230000008451 emotion Effects 0.000 description 1
- 206010014599 encephalitis Diseases 0.000 description 1
- 230000012202 endocytosis Effects 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 230000021824 exploration behavior Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 230000008717 functional decline Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 229940096919 glycogen Drugs 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 210000001879 hippocampal ca1 region Anatomy 0.000 description 1
- 210000004295 hippocampal neuron Anatomy 0.000 description 1
- 235000003642 hunger Nutrition 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 1
- 238000003365 immunocytochemistry Methods 0.000 description 1
- 230000002055 immunohistochemical effect Effects 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000003199 leukotriene receptor blocking agent Substances 0.000 description 1
- 210000003715 limbic system Anatomy 0.000 description 1
- OYHQOLUKZRVURQ-AVQMFFATSA-N linoelaidic acid Chemical compound CCCCC\C=C\C\C=C\CCCCCCCC(O)=O OYHQOLUKZRVURQ-AVQMFFATSA-N 0.000 description 1
- OYHQOLUKZRVURQ-IXWMQOLASA-N linoleic acid Natural products CCCCC\C=C/C\C=C\CCCCCCCC(O)=O OYHQOLUKZRVURQ-IXWMQOLASA-N 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000007787 long-term memory Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000006984 memory degeneration Effects 0.000 description 1
- 208000023060 memory loss Diseases 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 238000013425 morphometry Methods 0.000 description 1
- 230000008450 motivation Effects 0.000 description 1
- 239000012120 mounting media Substances 0.000 description 1
- 210000005155 neural progenitor cell Anatomy 0.000 description 1
- 210000004498 neuroglial cell Anatomy 0.000 description 1
- 230000037420 neurological improvement Effects 0.000 description 1
- 230000003955 neuronal function Effects 0.000 description 1
- 230000019818 neurotransmitter uptake Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000001473 noxious effect Effects 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 229910000489 osmium tetroxide Inorganic materials 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 230000020477 pH reduction Effects 0.000 description 1
- 229940055695 pancreatin Drugs 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 230000001242 postsynaptic effect Effects 0.000 description 1
- 230000002360 prefrontal effect Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 210000004129 prosencephalon Anatomy 0.000 description 1
- 229940126409 proton pump inhibitor Drugs 0.000 description 1
- 239000000612 proton pump inhibitor Substances 0.000 description 1
- 210000002763 pyramidal cell Anatomy 0.000 description 1
- 235000008160 pyridoxine Nutrition 0.000 description 1
- 239000011677 pyridoxine Substances 0.000 description 1
- 238000004451 qualitative analysis Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 229960003254 reslizumab Drugs 0.000 description 1
- 238000011808 rodent model Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 230000006403 short-term memory Effects 0.000 description 1
- 230000006886 spatial memory Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 230000037351 starvation Effects 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000003319 supportive effect Effects 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 230000007607 synaptic alteration Effects 0.000 description 1
- 230000003956 synaptic plasticity Effects 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 229950003937 tolonium Drugs 0.000 description 1
- HNONEKILPDHFOL-UHFFFAOYSA-M tolonium chloride Chemical compound [Cl-].C1=C(C)C(N)=CC2=[S+]C3=CC(N(C)C)=CC=C3N=C21 HNONEKILPDHFOL-UHFFFAOYSA-M 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 230000007306 turnover Effects 0.000 description 1
- 229940011671 vitamin b6 Drugs 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/194—Carboxylic acids, e.g. valproic acid having two or more carboxyl groups, e.g. succinic, maleic or phthalic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/197—Carboxylic acids, e.g. valproic acid having an amino group the amino and the carboxyl groups being attached to the same acyclic carbon chain, e.g. gamma-aminobutyric acid [GABA], beta-alanine, epsilon-aminocaproic acid or pantothenic acid
- A61K31/198—Alpha-amino acids, e.g. alanine or edetic acid [EDTA]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/465—Hydrolases (3) acting on ester bonds (3.1), e.g. lipases, ribonucleases
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/47—Hydrolases (3) acting on glycosyl compounds (3.2), e.g. cellulases, lactases
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/48—Hydrolases (3) acting on peptide bonds (3.4)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/54—Mixtures of enzymes or proenzymes covered by more than a single one of groups A61K38/44 - A61K38/46 or A61K38/51 - A61K38/53
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y302/00—Hydrolases acting on glycosyl compounds, i.e. glycosylases (3.2)
- C12Y302/01—Glycosidases, i.e. enzymes hydrolysing O- and S-glycosyl compounds (3.2.1)
- C12Y302/01001—Alpha-amylase (3.2.1.1)
Definitions
- the present invention relates to the field of medicine and nutraceuticals, in particular the treatment of neurological and/or neurodegenerative disorders and improvement of brain function.
- neurogenesis neural stem cells
- hippocampus One of the most active loci for adult neurogenesis is the hippocampus, which has a crucial role in memory and spatial navigation. Hippocampus is also one of the first regions suffering damage in Alzheimer's disease. Stimulation of adult neurogenesis has been subject to intensive research pursuing the hypothesis that a number of neurological disorders (in particular neurodegenerative disorders such as Parkinson's or Alzheimer's as well as depression) might be treatable in such manner.
- Eosinophils are white blood cells involved in defence against parasites, but are also involved in many neoplastic, autoimmune and allergic diseases. There are a number of treatments that lower eosinophile numbers, such as corticosteroids, mepoluzimab, reslizumab, leukotriene antagonists and imatinib, none of which is without drawbacks.
- salts of the invention may be inorganic or organic.
- preferable salts include sodium and calcium salts of alpha-ketoglutaric acid (denoted Na-AKG and Ca-AKG, respectively).
- Other preferable salts include potassium-AKG and magnesium-AKG as well as organic salts of alpha-ketoglutaric acid where the counter-ion is an amino-acid naturally occurring in proteins (such as arginine, leucine, isoleucine), pyridoxine, chitosan, creatine or ornithine.
- pharmaceutically acceptable salt encompasses any salts that are pharmaceutically acceptable in the sense that they are not toxic to the intended subject at the doses intended and are sufficiently stable and soluble for the intended purpose.
- the counter-ion of alpha-ketoglutarate in a pharmaceutically acceptable salt of the invention is not an active ingredient by itself, although it may in some cases be acceptable that the counter-ion is an active ingredient.
- lipase in the context of the present invention refers to enzymes that catalyze the hydrolytic release of fatty acids from triglycerides releasing fatty acids and glycerol, monoglycerides and/or diglycerides.
- a lipase catalyzes the release of long-chain or medium-chain polyunsaturated fatty acid (PUFA).
- long-chain may refer to fatty acids having tails longer than 12 carbons and “medium-chain” to fatty acids having tails of 6-12 carbons.
- polyunsaturated it is meant that there are at least two double bonds between the carbon atoms in the tail of polyunsaturated fatty acids.
- PUFA examples include but are not limited to linoleic acid, linoelaidic acid, ⁇ -linolenic acid, arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid. It is preferable that the activity of the lipase of the present invention is substantially independent of pH and that the lipase is active at a pH 6-8.
- lipase in the context of the present invention comprises lipases of any origin, including human, animal, plant and fungal, bacterial, eukaryotic and prokaryotic origin, irrespective of if the lipase has been produced by recombinant means or by non-recombinant means.
- the lipase is or has been made acid stable.
- Such acid stable lipase may be lipase-CLEC (cross-linked enzyme crystals).
- lipase-CLEC crystals and methods of their manufacture are known in the art, for instance from US2006/0121017, incorporated herein by reference. It is also possible to use non-acid stable lipases if the amount administered is increased to compensate for loss of activity in the stomach, the stomach acidity of the patient reduced by antacids, proton pump inhibitors or other suitable pharmacological means, or if the lipase is formulated in a protective enteric formulation well known in the art.
- protease in the context of the present invention refers to a proteinase, proteolytic enzyme or peptidase, which is an enzyme that catalyzes the splitting of interior amide peptide bonds in a protein. Specifically, proteases catalyze the conversion of proteins into smaller proteins/peptides and/or their component amino acids by cleaving the amide linkage between the carboxyl group of one amino acid and the amino group of another.
- Proteases are generally identified by their catalytic type, e.g., aspartic acid peptidases, cysteine (thiol) peptidases, metallopeptidases, serine peptidases, threonine peptidases, alkaline or semi-alkaline proteases, neutral and peptidases of unknown catalytic mechanism (see https://merops.sanger.ac.uk). Any catalytic type is encompassed by the term in the context of the present invention.
- protease in the context of the present invention comprises proteases of any origin, including human, animal, plant and fungal, bacterial, eukaryotic and prokaryotic origin, irrespective of if the protease has been produced by recombinant means or by non-recombinant means.
- amylaose in the context of the present invention refers to amylase enzymes having broad substrate specificity and catalyzing the hydrolysis of ⁇ -1,4-glucosidic linkages of starch, glycogen and related polysaccharides containing three or more ⁇ -1,4-linked D-glucose units yielding maltose, glucose and/or limit dextrins of 2-3 units.
- amylase in the context of the present invention comprises amylases of any origin, including human, animal, plant and fungal, bacterial, eukaryotic and prokaryotic origin, irrespective of if the amylase has been produced by recombinant means or by non-recombinant means.
- USP Unit refers to the United States Pharmacopoeia unit of enzyme activity present in an agent or composition.
- One USP Unit of lipase, protease or amylase is defined in Pancrelipase, USP, U.S. Pharmacopeia National Formulary, USP 24, pp. 1254-1255 (2000).
- Assays for lipase, protease and amylase are disclosed in that reference and are incorporated herein by reference.
- neurogenesis in the context of the present invention is taken to mean the process by which new neurons are formed from neural stem cells. In neurogenesis, there is active production of new neurons, astrocytes, glia, and other neural lineages from undifferentiated neural progenitor or stem cells.
- neurodegenerative disease in the context of the present invention is an umbrella term for any disease causing progressive loss of structure and/or function of neurons, with or without death of neurons.
- neurodegenerative diseases include but are not limited to Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis.
- eosinophilia in the context of the present invention encompasses both primary eosinophilia, as well as conditions where increased eosinophile numbers are a symptom.
- pancrelipose in the context of the present invention encompasses not only pancrelipase according to the normal meaning of the term in the art, but even similar preparations such as pancreatin, and similar preparations from any animal source.
- FIG. 1 illustrates increased adult neurogenesis detected by increased number of nestin-positive neurons per 1 mm of pyramidal layer length in hippocampal CA1 area by means of the present invention. Asterisks indicate statistically significant differences.
- FIG. 2 illustrates improved cognitive performance by means of the present invention, detected in the percentage of spontaneous alterations in a T-maze test. (+) denotes correct trials—animal visited left and right arms, ( ⁇ ) denotes incorrect trials—animal visited the same arm twice.
- FIG. 3A illustrates results from blood chemistry, specifically platelets 10 3 / ⁇ l. Letters a or c indicate statistically significant difference from control group (p ⁇ 0.05).
- FIG. 3B illustrates results from blood chemistry, specifically lymphocyte (%). Letters a or c indicate statistically significant difference from control group (p ⁇ 0.05).
- FIG. 3C illustrates results from blood chemistry, specifically eosinophils (%). Letters a or c indicate statistically significant difference from control group (p ⁇ 0.05).
- FIG. 3D illustrates results from blood chemistry, specifically monocytes (%). Letters a or c indicate statistically significant difference from control group (p ⁇ 0.05).
- FIG. 4 shows average number of synaptic terminals per 100 ⁇ m 2 of hippocampal CA1 zone in different treatment groups.
- Asterisk (*) denotes statistical significance compared to the control group (p ⁇ 0.05)
- FIG. 5 shows average area ( ⁇ m 2 ) of synaptic terminal in hippocampal CA1 zone in different treatment groups.
- FIG. 6A shows average number of synaptic vesicles per synapse in hippocampal CA1 zone.
- Asterisk (*) denotes statistical significance compared to the control group (p ⁇ 0.05).
- FIG. 6B shows average number of synaptic vesicles per 100 ⁇ m 2 of synaptic terminal in hippocampal CA1 zone.
- Asterisk (*) denotes statistical significance compared to the control group (p ⁇ 0.05).
- FIG. 7 shows average distance (nm) from synaptic vesicles to the active zone of presynaptic terminal.
- Asterisk (*) denotes statistical significance compared to the control group (p ⁇ 0.05).
- FIG. 8 shows average distance (nm) from vesicle to the to the nearest neighbor vesicle of presynaptic terminal.
- Asterisk (*) denotes statistical significance compared to the control group (p ⁇ 0.05).
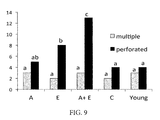
- FIG. 9 shows proportion (%) of perforated and multiple synapses in hippocampal CA1 zone. The remainder represents simple synapses.
- the letter on the gray and black bars describe statistic difference when p ⁇ 0.05. Columns with same letter are not statistically significantly different from each other.
- C denotes age-matched (old) control.
- FIG. 10 illustrates improved cognitive performance by means of the present invention, detected in the percentage of spontaneous alterations in a T-maze test (See Example 5).
- the test was performed at start (0 months) and repeated at 2 and 4 months.
- the fraction of correct trials is shown, with 95% confidence interval calculated by modified Wald method.
- the treatment at baseline (0) and treatment at 4 months are statistically significantly different at least at p ⁇ 0.05, since the 95% confidence intervals do not overlap.
- FIG. 11 shows the fraction of individual animals of Example 5 showing deteriorated or improved performance in the T-maze test at 2 and 4 months (results pooled) compared to their individual baseline performance.
- the fraction of animals in the group with improved performance is shown by the black columns, and the fraction with deteriorated performance is shown by the grey columns.
- the present invention provides the following items.
- AKG as well as a composition comprising lipase, protease and amylase had effects in neurogenesis and cognition.
- a combination of AKG and said enzymes had a synergistic effect in neurogenesis and in improving cognition.
- the combination also has a synergistic effect on synaptic morphology.
- the present invention discloses novel synergistic compositions comprising alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof (AKG), and one or more enzymes selected from a group consisting of a lipase, a protease and an amylase, the constituents being present at synergistically effective relative amounts.
- compositions have synergistic effect in promoting neurogenesis, improving synaptic morphology and reducing eosiniphil counts, enabling treatment of a number of conditions as explained in more detail below.
- the composition comprises a lipase but no protease or amylase.
- the composition comprises a protease but no lipase or amylase.
- the composition comprises an amylase but no protease or lipase.
- the composition comprises a lipase and a protease but no amylase.
- the composition comprises a lipase and an amylase but no protease.
- the composition comprises a protease and an amylase but no lipase.
- the composition comprises a lipase, a protease, and an amylase.
- Lipases, proteases and amylases (and combinations thereof) suitable for use in the composition of the invention are known in the art, see for instance US2006/012017, US2004/0057944, US2001/0046493 and U.S. Pat. No. 6,051,220, all incorporated herein by reference.
- composition may further comprise pharmaceutically or nutritionally acceptable excipients or additives.
- the composition may include additional active ingredients.
- the composition does not include additional active ingredients.
- composition is preferably for oral administration. It may be formulated in any manner well known in the art for oral administration of the substances that it comprises. If the formulation comprises non-acid stable lipase, it may be preferable to encapsulate at least this component in a protective enteric coating. Such coatings are well known in the art and found in products already in the market.
- the composition may be a food supplement, a nutraceutical, a pharmaceutical composition, a dietary supplement or a food additive.
- the composition is the first aspect may comprise a lipase, optionally in combination with a protease as described below, an amylase as described below or both.
- the lipase may be selected from a mammalian lipase, a microbial lipase, a bacterial lipase, a pancrelipase lipase (i.e. a lipase included in pancrelipase), a liprotamase lipase (i.e.
- a lipase included in liprotamase Pseudomonas lipase, human or other mammalian bile-salt stimulated lipase (BSSL) or bile-salt dependent lipase (BSDL), Rhizopus oryzae lipase, Chromobacterium viscosum lipase, Rhizopus delemar lipase, Burkholderia lipase, more preferably Burkholderia cepacia lipase, most preferably Burkholderia cepacia lipase as cross-linked lipase crystals.
- BSSL mammalian bile-salt stimulated lipase
- BSDL bile-salt dependent lipase
- Rhizopus oryzae lipase Rhizopus oryzae lipase
- Chromobacterium viscosum lipase Rhizopus delemar lipase
- Burkholderia lipase more preferably
- the composition may comprise 300-3000000 USP units of lipase per g AKG, preferably 3000-300000 USP units of lipase per g AKG, more preferably 6000-150000 USP units of lipase per g AKG and even more preferably 15000-60000 USP units of lipase per g AKG, and most preferably about 30000 USP units of lipase per g AKG.
- the composition of the first aspect may comprise a protease, optionally in combination with a lipase as described above, an amylase as described below or both.
- the protease may be selected from a mammalian protease, a microbial protease, a fungal protease, a pancrelipase protease (i.e. a protease included in pancrelipase), a liprotamase protease (i.e. a protease included in pancrelipase), an Aspergillus protease, most preferably Aspergillus melleus protease.
- the composition may comprise 200-2000000 USP units of protease per g AKG, preferably 2000-200000 USP units of protease per g AKG, more preferably 4000-100000 USP units of protease per g AKG and even more preferably 10000-40000 USP units of protease per g AKG, and most preferably about 20000 USP units of protease per g AKG.
- the composition of the first aspect may comprise an amylase, optionally in combination with a lipase as described above, a protease described above or both.
- the amylase may be selected from a mammalian amylase, a microbial amylase, a fungal amylase, a pancrelipase amylase (i.e. an amylase included in pancrelipase), a liprotamase amylase (i.e. an amylase included in liprotamase), an Aspergillus amylase, most preferably Aspergillus oryzae amylase.
- the composition may comprise 30.0-3000000 USP units of amylase per g AKG, preferably 300-300000 USP units of amylase per g AKG, more preferably 300-30000 USP units of amylase per g AKG and even more preferably 600-15000 USP units of amylase per g AKG, and most preferably about 3000 USP units of amylase per g AKG.
- composition of the first aspect may comprise 300-3000000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 200-2000000 USP units of Aspergillus melleus protease per g AKG and 30.0-3000000 USP units of Aspergillus oryzae amylase per g AKG.
- composition of the first aspect may comprise 3000-300000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 2000-200000 USP units of Aspergillus melleus protease per g AKG and 300-300000 USP units of Aspergillus oryzae amylase per g AKG.
- composition of the first aspect may comprise 6000-150000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 4000-100000 USP units of Aspergillus melleus protease per g AKG and 300-30000 USP units of Aspergillus oryzae amylase per g AKG.
- composition of the first aspect may comprise 15000-60000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 10000-40000 USP units of Aspergillus melleus protease per g AKG and 600-15000 USP units of Aspergillus oryzae amylase per g AKG.
- composition of the first aspect may comprise about 30000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), about 20000 USP units of Aspergillus melleus protease per g AKG and about 3000 USP units of Aspergillus oryzae amylase per g AKG.
- the composition of the first aspect may comprise liprotamase.
- the amount of liprotamase may be 300-300000 USP lipase units of liprotamase per g AKG, preferably 3000-150000 USP lipase units of liprotamase per g AKG, more preferably 15000-60000 USP lipase units of liprotamase per g AKG and most preferably about 30000 USP lipase units of liprotamase per g AKG.
- a composition with AKG and liprotamase may comprise Na-AKG and Ca-AKG in equal amounts in terms of weight.
- the composition of the first aspect may comprise pancrelipase.
- the amount of pancrelipase may be 300-300000 USP lipase units of pancrelipase per g AKG, preferably 3000-150000 USP lipase units of pancrelipase per g AKG, more preferably 1500-60000 USP lipase units of pancrelipase per g AKG and most preferably about 30000 USP lipase units of pancrelipase per g AKG.
- a composition with AKG and pancrelipase may comprise Na-AKG and Ca-AKG in equal amounts in terms of weight.
- the present invention also provides further embodiments of the composition of the first aspect in Table X appended after the Examples section.
- Table X The contents of table X are to be read as follows.
- Each line in table X corresponds to an embodiment. From the left, the first column denoted “#” shows the embodiment identification number. The second column from the left denoted “A” shows the lipase concentration range for that embodiment, with reference to tables Y and Z. The third column from the left denoted “B” shows the protease concentration range for that embodiment, with reference to tables Y and Z. The fourth column from the left denoted “C” shows the amylase concentration range for that embodiment, with reference to tables Y and Z.
- any endpoint in an embodiment may also be used to form the endpoint of an open range of an additional embodiment.
- value 2 in column A forms basis for an embodiment where the lipase amount is “at least base value/100”, and an embodiment where the lipase amount is “at least base value time 100” USP units/mmol AKG.
- value 2 in column A forms basis for an embodiment where the lipase amount is “less than base value/100”, and an embodiment where the lipase amount is “less than base value time 100” USP units/mmol AKG.
- embodiment 53 corresponds to lipase amount of base value/250 to base value times 250, protease amount of base value to base value times 5, and amylase amount of base value/25 to base value times 25.
- the embodiments of the first aspect of the invention disclosed in Table X with reference to Tables Z and Y comprise Burkholderia cepacia lipase as a lipase (preferably as cross-linked lipase crystals), Aspergillus melleus protease a protease and Aspergillus oryzae amylase as an amylase, to the extent said lipase, protease and amylase are present in the particular embodiment.
- the embodiments of the first aspect of the invention disclosed in Table X with reference to Tables Z and Y comprise Burkholderia cepacia lipase as the only lipase (preferably as cross-linked lipase crystals), Aspergillus melleus protease the only protease and/or Aspergillus oryzae amylase as the only amylase, to the extent said lipase, protease and amylase are present in the particular embodiment.
- the AKG component in the composition of the first aspect as disclosed above may consist of or comprise sodium alpha-ketoglutarate, calcium alpha-ketoglutarate or a combination thereof (preferably in equal amounts).
- the AKG component may also comprise or consist of another pharmaceutically acceptable inorganic salt of AKG including magnesium or potassium alpha-ketoglutarate, or a pharmaceutically acceptable organic salt of AKG, such as ornithine-AKG, arginine-AKG, chitosan-AKG, pyrodoxine-AKG, leucine-AKG, isoleucine-AKG or creatine-AKG, or a combination thereof.
- the present invention provides a composition according to the first aspect, for use as a medicament.
- composition according to the first aspect in the manufacture of a medicament is also provided.
- a method of treatment or prevention comprising administering to a subject in need thereof a composition according to the first aspect.
- the present invention provides a composition according to the first aspect, for use in the treatment or prevention of a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders.
- a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders.
- compositions according to the first aspect in the manufacture of a medicament for the treatment or prevention of a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders.
- a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders.
- a method of treatment or prevention of a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders comprising administering to a subject in need thereof a composition according to the first aspect.
- the present invention provides a composition according to the first aspect, for use in the treatment or prevention of a condition selected from: Alzheimer's disease, mild cognitive impairment (MCI), Parkinson's disease and Parkinsonian disorders, Huntington's disease, Amyotrophic Lateral Sclerosis, spinal ischemia, ischemic stroke, traumatic brain injury, traumatic spinal cord injury, cancer-related brain/spinal cord injury, schizophrenia and other psychoses, lissencephaly syndrome, depression, bipolar depression/disorder, chronic fatigue syndrome, anxiety syndromes/disorders, phobias, stress and related syndromes, cognitive function disorders, aggression, drug and alcohol abuse, obsessive compulsive behaviour syndromes, seasonal mood disorder, borderline personality disorder, cerebral palsy, life style drug abuse, multi-infarct dementia, Lewy body dementia, age related geriatric dementia, epilepsy and injury related to epilepsy, temporal lobe epilepsy, spinal cord injury, brain injury, brain surgery, trauma related brain/spinal cord injury, including
- composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of a condition selected from those disclosed above for the fourth aspect.
- a method of treatment or prevention of a condition selected from those disclosed above for the fourth aspect comprising administering to a subject in need thereof a composition according to the first aspect.
- the present invention provides a composition according to the first aspect, for use in the treatment or prevention of depression or chronic fatigue syndrome.
- composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of depression or chronic fatigue syndrome is also provided.
- a method of treatment or prevention of depression or chronic fatigue syndrome comprising administering to a subject in need thereof a composition according to the first aspect.
- the present invention provides a composition according to the first aspect, for use in the treatment or prevention of disorders involving eosinophilia.
- composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of disorders involving eosinophilia is also provided.
- a method of treatment or prevention of disorders involving eosinophilia comprising administering to a subject in need thereof a composition according to the first aspect.
- the present invention provides a composition according to the first aspect, for use in the treatment or prevention of allergic diseases including astma, atopic dermatitis, eosinophilic esophagitis and rhinoconjunctivitis; autoimmune diseases including primary biliary cirrhosis, Riedel invasive fibrous thyroiditis, dermatomyositis, systemic lupus erythematosus and Sjögren's syndrome; parasite infection-induced eosinophilia including helminth infection-induced eosinophilia; graft-versus-host disease; drug-induced eosinophilia; primary eosinophilia, neoplastic disease-induced eosinophilia including eosinophilia associated with primary cutaneous T-cell lymphoma, Sézary syndrome, Hodgkin's disease, Langerhans cell histiocytosis, thyroid cancer, stomach cancer, liver cancer
- composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of conditions disclosed above for the fourth aspect is also provided.
- composition according to the first aspect for use in a method comprising administering an amount of the composition containing 0.03-100 g AKG to a patient per day, 0.1-30 g AKG to a patient per day, 1-10 g AKG to a patient per day, or 2-6 g AKG to a patient per day.
- the eight aspect also provides a composition according to the first aspect, for use in a method comprising administering to a (preferably human) patient daily an amount of the composition containing the following amounts of AKG in mmol: 0.5-240, 1-120, 3-60, 5-36, 9-24, 0.5-3, 0.5-5, 0.5-9, 0.5-12, 1-5, 3-9, 3-12, 3-24, 1-5, 3-5, 1-3, 9-36, about 0.5, about 1, about 3, about 5, about 9, about 12, about 24, about 36, about 60, about 120 or about 240. Any range formed by any combination of the endpoints of the aforementioned ranges is also contemplated.
- Said administration may be oral.
- Said patient may be preferably human, in particular a human in need of such treatment.
- composition according to the first aspect in the manufacture of a food supplement, a dietary supplement, a nutraceutical or a food additive. Further, there is provided a use of a composition according to the first aspect as a dietary supplement, a food supplement, a nutraceutical or a food additive.
- the present invention provides alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use in the treatment or prevention of depression or chronic fatigue syndrome.
- alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment or prevention of depression or chronic fatigue syndrome is also provided.
- a method of treatment or prevention of depression or chronic fatigue syndrome comprising administering to a subject in need thereof alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, in an effective amount.
- the alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof according to the ninth aspect may be for use in a method comprising administering an amount of the alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof containing 0.03-100 g AKG to a patient per day, preferably 0.1-30 g AKG to a patient per day, more preferably, 1-10 g AKG to a patient per day, most preferably, 2-6 g AKG to a patient per day.
- the alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof according to the ninth aspect may be for use in a method comprising administering to a (preferably human) patient daily an amount of the composition containing the following amounts of AKG in mmol: 0.5-240, 1-120, 3-60, 5-36, 9-24, 0.5-3, 0.5-5, 0.5-9, 0.5-12, 1-5, 3-9, 3-12, 3-24, 1-5, 3-5, 1-3, 9-36, about 0.5, about 1, about 3, about 5, about 9, about 12, about 24, about 36, about 60, about 120 or about 240. Any range formed by any combination of the endpoints of the aforementioned ranges is also contemplated.
- Said administration is preferably oral.
- Said patient may be human, in particular a human in need of such treatment.
- the pharmaceutically acceptable salt may consist of or comprise a pharmaceutically acceptable inorganic salt of AKG such as sodium, calcium, magnesium or potassium alpha-ketoglutarate, or a pharmaceutically acceptable organic salt of AKG, such as ornithine-AKG, arginine-AKG, chitosan-AKG, pyrodoxine-AKG, leucine-AKG, isoleucine-AKG or creatine-AKG, or a combination thereof.
- a pharmaceutically acceptable inorganic salt of AKG such as sodium, calcium, magnesium or potassium alpha-ketoglutarate
- a pharmaceutically acceptable organic salt of AKG such as ornithine-AKG, arginine-AKG, chitosan-AKG, pyrodoxine-AKG, leucine-AKG, isoleucine-AKG or creatine-AKG, or a combination thereof.
- the present invention also provides alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use in a method of treatment or prevention of neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma, cognitive, learning and memory disorders, Alzheimer's disease, mild cognitive impairment (MCI), Parkinson's disease and Parkinsonian disorders, Huntington's disease, Amyotrophic Lateral Sclerosis, spinal ischemia, ischemic stroke, traumatic brain injury, traumatic spinal cord injury, cancer-related brain/spinal cord injury, schizophrenia and other psychoses, lissencephaly syndrome, depression, bipolar depression/disorder, chronic fatigue syndrome, anxiety syndromes/disorders, phobias, stress and related syndromes, cognitive function disorders, aggression, drug and alcohol abuse, obsessive compulsive behaviour syndromes, seasonal mood disorder, borderline personality disorder, cerebral palsy, life
- the method comprises administering a lipase but no protease or amylase.
- the method comprises administering a protease but no lipase or amylase.
- the method comprises administering an amylase but no protease or lipase.
- the method comprises administering a lipase and a protease but no amylase.
- the method comprises administering a lipase and an amylase but no protease.
- the method comprises administering a protease and an amylase but no lipase.
- the method comprises administering a lipase, a protease, and an amylase.
- the AKG, the lipase, the protease and the amylase administered in the method referred to in the tenth aspect may have same properties and features as specified for the components of the composition of the first aspect.
- the relative and absolute amounts of the AKG, the lipase, the protease and the amylase administered in the method referred to in the tenth aspect may be as specified for the composition of the first aspect and uses thereof.
- the method of the tenth aspect may comprise administering AKG to the subject at 0.03-100 g/day, 0.1-30 g per day, 1-10 g per day or 2-6 g per day.
- the method of the tenth aspect may comprise administering to a (preferably human) patient daily the following amount of AKG in mmol: 0.5-240, 1-120, 3-60, 5-36, 9-24, 0.5-3, 0.5-5, 0.5-9, 0.5-12, 1-5, 3-9, 3-12, 3-24, 1-5, 3-5, 1-3, 9-36, about 0.5, about 1, about 3, about 5, about 9, about 12, about 24, about 36, about 60, about 120 or about 240. Any range formed by any combination of the endpoints of the aforementioned ranges is also contemplated.
- the method referred to in the tenth aspect may comprise administering the AKG, the lipase, the protease and/or the amylase simultaneously, separately or consecutively.
- Said components may be administered in formulations comprising any combination of the substances.
- the method referred to in the tenth aspect comprises administering AKG and liprotamase simultaneously, separately or consecutively. Also preferably, the method referred to in the tenth aspect comprises administering AKG and pancrelipase simultaneously, separately or consecutively.
- Nestin is a marker for immature neurons.
- Nestin positive cells were found in the hippocampal layer of the pyramidal neurons. The somas of hippocampal cells displayed strong nestin staining. In some cells long processes that seem to be dendrites extended into deeper layers of the hippocampus, and these processes were nestin positive as well. In all of the CA fields of the hippocampus (CA1-CA3) these cells were structurally similar to pyramidal cells. The structural similarities between nestin positive cells and neurons allow regarding this type of cells as newly generated neurons.
- Results showed that while platelet, lymphocyte and monocyte counts were unaffected by the treatments, the eosinophile count was markedly reduced by the combination of AKG+ENZ, but not by AKG by itself or ENZ by itself. Again, the combination of AKG+ENZ showed a synergistic effect.
- hippocampus is one of the major components of the brain of humans and other vertebrates. It belongs to the limbic system and plays important roles in the consolidation of information from short-term memory to long-term memory and spatial memory and navigation, emotion and behavior formation, cognitive function. Hippocampus is very vulnerable to different noxious stimuli. Damage to the hippocampus can result from oxygen starvation (hypoxia), encephalitis, medial temporal lobe epilepsy, age-related disorders (Alzheimer's disease) and others. Hippocampus is one of the first regions of the brain to suffer damage; memory loss and disorientation are included among the early symptoms.
- hippocampal neurons are arranged in a specific spatial manner, which allows precise estimating of their quantity and quality.
- synapses There are many diseases which are accompanied with loss of synapses. It is worth to notice that both amount and structure of synapses are impaired in diseases as Alzheimer's disease, stroke, Down syndrome, schizophrenia and traumatic brain injury and stress. Some authors have observed synaptic loss during normal aging.
- the hippocampus is one of the brain regions that are prominently affected by neurodegeneration and functional decline even in what is still considered “normal aging”. Decreased neuronal amount, a decrease in the number of synaptic connections, intracellular pathology, all these facts suggest that the hippocampal formation may be especially vulnerable to the effects of aging. Morphological studies of the hippocampus in young and old rats have revealed that pyramidal neurons in old rats are smaller and contain fewer dendritic branches and spines. The density of presynaptic terminals per length unit of postsynaptic membrane is also lower.
- Each presynaptic terminal contains hundreds of synaptic vesicles filled with neurotransmitters.
- Synaptic vesicles are uniform organelles of 40 nm in diameter that constitute the central organelle for release and storage of a neurotransmitter that continuously undergoes an exo-endocytotic cycle. During this cycle vesicles change their positions within a presynaptic terminal and their number as well as spatial arrangement can provide insight into a neurotransmitter turnover.
- synaptic vesicle cycle consists of exocytosis followed by endocytosis and recycling.
- Synaptic vesicles are filled with neurotransmitters (NT) by active transport (neurotransmitter uptake) fueled by an electrochemical gradient established by a proton pump that acidifies the vesicle interior (vesicle acidification).
- NT neurotransmitters
- active transport neurotransmitter uptake
- electrochemical gradient established by a proton pump that acidifies the vesicle interior (vesicle acidification).
- synaptic vesicles are docked at the active zone, and primed by an ATP-dependent process that renders the vesicles competent to respond to a Ca 2+ -signal.
- an action potential depolarizes the presynaptic membrane, Ca 2+ channels open, causing a local increase in intracellular Ca 2+ at the active zone that triggers completion of the fusion reaction.
- Released neurotransmitters then bind to receptors associated with the postsynaptic density (PSD).
- PSD postsynaptic density
- the number of synaptic terminals per unit area (100 ⁇ m 2 ) in old control animals is three times lower compared with young animals, but after long time treatment with AKG and/or lipase+protease+amylase the number of synaptic contacts increased to 55% (AKG and ENZ) and to 47% (AKG+ENZ) ( FIG. 4 ).
- the area of synaptic terminals in old animals from all groups was three times greater than in young animals and was practically identical ( FIG. 6 ).
- the number of SVs per presynaptic terminal was increased in AKG, ENZ and AKG+ENZ groups of gerbils compared to control old animals ( FIG. 6A ), while the number of SVs per square units (100 ⁇ m 2 ) of terminal didn't differ from control, except for group 1 ( FIG. 6B ). This difference is due to the fact that the area of synaptic terminals in old animals from all groups was significantly greater than in young animals.
- vesicle clustering showed that SVs were also separated by larger distance: the average NND value estimated for control old animals was around 30% higher than for young ( FIG. 8 ). Only for the 3 group it was revealed significant decrease of NND compared with control old animals (group 4).
- Distance to the 1st nearest neighbor of each vesicle was used to measure the tendency of synaptic vesicles to form spatial clusters. Note that vesicle density is number of vesicles per 1 unit area, and the NND parameter is not the average vesicle-to-vesicle distance, but the average distance to the nearest neighbor vesicle, the comparison of these parameters describes the difference of spatial distribution of vesicles in synapse.
- perforated synapses Increase in the proportion of perforated synapses is thought to be more efficient in synaptic transmission and can be considered as compensatory mechanism in case of synaptic loss.
- Perforations have also been correlated with reactive synaptogenesis. Density of perforated synapses is coupled with recognition memory accuracy. Synapse remodeling is known as sign of neurological improvement, whereby the result is supportive for treatment and prophylaxis of neurological disorders in general and Alzheimer's in particular with the effective treatments.
- synaptic contacts such as its dimensions, shape, size of presynaptic terminal, SV density and distribution, are all related to the efficacy of the synaptic transmission.
- synaptic contacts are able to manifest modifications under inventive treatment conditions in animals.
- the decreased density of excitatory synapses in CA1 stratum radiatum of old animals compared with young ones is a sign of recovery after long-term treatment.
- Potentially deleterious morphological changes in the synapses, such as swelling of synaptic elements, depletion and rearrangement of SV pools, are accompanied by some apparently counter-balancing adaptive modifications: increase in perforations, which presumably could reflect strengthening of synaptic contacts, as well as relative increase in multiple spine boutons, which suggests reactive synaptogenesis.
- Example 1 The results demonstrate that the results in Example 1 were repeatable with different enzyme preparations and concentrations, that a discernible effect was beginning to show at 2 months of treatment and improvement continued further during 4 months of treatment.
- the measure allows discrimination of if there are individual shifts in the ability to pass the test over the course of treatment. Since the number of animals is large enough, the U-statistic, the deciding statistic of the Mann-Whitney test, is approximately Gaussian with known parameters, and the probability that either group is described with the same probabilistic law is less than 0.05, which proves that with the confidence level of 0.05 there are qualitative differences between two groups.
- Example 1 the animals were fed Labofeed B standard chow (from Labofeed company; Andrzej Morawski Feed Production Plant, Kcynia near. Bydgoszcz, Poland) supplemented with test compounds as specified in table 1.
- ENZ also denoted E, Enz or old lipase 600000 USP units/kg Enz
- AKG + ENZ also denoted A + E, 1% calcium AKG by weight AKG ⁇ Enz, old AKG + Enz
- Young controls also denoted Y, None Contr (young), Young
- the lipase, protease and amylase were obtained as capsules containing 30,000 USP units/capsule of lipase, 20,000 USP units/capsule of protease, and 3,000 USP units/capsule of amylase) prepared as shown in Table 2. Contents of 20 capsules were mixed per each kg of feed for the ENZ and AKG+ENZ groups.
- the capsules also contained filler up to a weight of 200 mg.
- Example 5 the animals were fed Labofeed B standard chow (from Labofeed company; Andrzej Morawski Feed Production Plant, Kcynia near. Bydgoszcz, Poland) supplemented with test compounds as specified in table 3.
- Example 5 Group Additive Age matched controls None AKG + ENZ (also denoted A + E) 1% calcium AKG by weight 1% sodium AKG by weight Total AKG ca. 114 mmol/kg lipase 200000 USP units/kg protease 750000 USP units/kg amylase 664000 USP units/kg
- Example 5 Determined Amount activity (mg/kg Manufacturer Component USP/mg fodder) Supplier designation Burkholderia 400 500 Amano Lipase PS cepacia lipase “Amano” SD Aspergillus 1500 500 Amano Protease DS-K melleus proteinase Aspergillus 3370 16850 Amano Amylase DS oryzae ⁇ - amylase
- the gerbils were housed in individual cages and divided into groups receiving different treatments (Table 1). Each cage was equipped with a drinking bottle.
- Gerbils (4-8 per cage) were housed in clear polycarbonate cages (48 ⁇ 27 ⁇ 20 cm) containing corn-cob bedding, paper towels for nesting material, and a tin can (13 cm long ⁇ 10 cm diameter) for enrichment.
- Lights were on from 07.00 to 19.00 h, and room temperature and humidity were maintained at approximately 23° C. and 30-70%, respectively.
- Control groups comprised 15 animals whereas the age-matched treatment group comprised 16 animals.
- the gerbils were anaesthetized with ketamine (100 mg/kg body weight i/m) and fixed by transcardial perfusion with 4% formaldehyde and 0.25% glutaraldehyde in 0.1M phosphate buffer. After perfusion the brains were isolated and separated for two hemispheres.
- Gerbils were anaesthetized with sublethal dose of ketamine (100 mg/kg body weight i/m) and fixed by transcardial perfusion with 4% formaldehyde and 0.25% glutaraldehyde in 0.1M phosphate buffer. After perfusion the brains were isolated and separated on two hemispheres.
- the hippocampus from another hemisphere of each animal was isolated and cut in 400- ⁇ m-thick transverse slices by a chopper (McIllwain tissue chopper, Great Britain). Slices were postfixed in fixative solution with 2.5% of glutaraldehyde for 1.5 h and then in 1% OsO4 for 1 h. Tissue slices were then dehydrated in an ascending series of ethanol followed by dry acetone and embedded in EPON resin according to official protocol. The sections were produced by ultramicrotome LKB-8800. Toluidine blue-stained semi-thin (1 ⁇ m) sections of hippocampus were used to localize hippocampal CA1 area. For electron microscopy, ultra-thin sections (70 nm) from the middle portion of CA1 stratum pyramidale and stratum radiatum were stained with uranyl acetate and lead citrate.
- the synaptic terminals of the investigated area revealed high degree of structural plasticity including modifications in the ratio of different forms of synaptic terminals (simple, perforated, multiple boutons).
- Perforated synapse was defined as a synapse with discontinuous PSD.
- the synapses with more than one spine contacting the same presynaptic terminal were classified as multiple spine boutons (MSB).
- x- and y-coordinates of SV profile centers and points tracing the active zone of a synapse were marked with UTHSCSA ImageTool software (version 3, University of Texas, San Antonio, Tex.; ftp:https://maxrad6.uthscsa.edu) on digitized micrographs, using the point tool.
- the shortest distance from a SV to the active zone profile (hence forth referred to as an active zone distance (AZD), as well as spatial proximity of SV profiles to each other (referred to as the nearest neighbor distance (NND)) were quantified using the nearest neighbor formalism with LoClust software as described (Nikonenko and Skibo, 2004).
- AZD and NND quantities were pooled together per group. For each experimental and control condition, 100 synapses containing SVs have been analyzed. Statistical analysis was performed using Statistica software (version 5, StatSoft, USA). Values are shown as mean ⁇ standard error of the mean (SEM). The two-tailed Kolmogorov-Smirnov test was used to assess the differences between samples (P ⁇ 0.05 was considered to indicate statistical significance).
- T Maze Spontaneous Alternation is a behavioral test for measuring exploratory behavior in animals, especially rodent models for CNS disorders. This test is based on the willingness of rodents to explore a new environment, i.e. they prefer to visit a new arm of the maze rather than a familiar arm. Many parts of the brain—including the hippocampus, septum, basal forebrain, and prefrontal cortex—are involved in this task.
- This protocol details a method for using a T-maze to assess the cognitive ability of rodents.
- the T-maze is an elevated or enclosed apparatus in the form of a T placed horizontally. Animals are started from the base of the T and allowed to choose one of the goal arms abutting the other end of the stem. If two trials are given in quick succession, on the second trial the rodent tends to choose the arm not visited before, reflecting memory of the first choice. This is called ‘spontaneous alternation’. Both spontaneous and rewarded alternation are very sensitive to dysfunction of the hippocampus, but other brain structures are also involved. Each trial should be completed in less than 2 min, but the total number of trials required will vary according to statistical and scientific requirements.
- Alternation reflects the motivation of the animal to explore its environment and locate the presence of resources such as food, water, mates or shelter. Animals do not need to be deprived of such resources to show alternation behavior; in this case it is called ‘spontaneous alternation’.
- Subjects were first placed in the start arm of the T Maze. Upon leaving the start arm, subjects chose between entering either the left or the right goal arm. With repeated trials, the animals showed less of a tendency to enter a previously visited arm. The percentage of alternation (number of turns in each goal arm) and total trial duration were recorded.
- Example 2 the animals were subjected to the test once.
- Example 5 the animals were subjected to the test on 4 consecutive days at the start of the experiment, and subsequently subjected to the test on 3 consecutive days after 2 months and 4 months of treatment.
- hematological parameters were studied using hematolyzer ABX Micros 60 OT (France) according standard methods.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Gastroenterology & Hepatology (AREA)
- Psychiatry (AREA)
- Psychology (AREA)
- Hospice & Palliative Care (AREA)
- Cardiology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Ophthalmology & Optometry (AREA)
- Pain & Pain Management (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Nutrition Science (AREA)
- Urology & Nephrology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Enzymes And Modification Thereof (AREA)
Abstract
Description
- The present invention relates to the field of medicine and nutraceuticals, in particular the treatment of neurological and/or neurodegenerative disorders and improvement of brain function.
- It has been known for some time now that formation of new neurons from neural stem cells (neurogenesis) continues throughout life in adult mammals including humans, although it has a clear tendency to decrease with age. One of the most active loci for adult neurogenesis is the hippocampus, which has a crucial role in memory and spatial navigation. Hippocampus is also one of the first regions suffering damage in Alzheimer's disease. Stimulation of adult neurogenesis has been subject to intensive research pursuing the hypothesis that a number of neurological disorders (in particular neurodegenerative disorders such as Parkinson's or Alzheimer's as well as depression) might be treatable in such manner.
- Eosinophils (or eosinophil granulocytes) are white blood cells involved in defence against parasites, but are also involved in many neoplastic, autoimmune and allergic diseases. There are a number of treatments that lower eosinophile numbers, such as corticosteroids, mepoluzimab, reslizumab, leukotriene antagonists and imatinib, none of which is without drawbacks.
- There is considerable unmet medical need for alternative and/or improved treatments of disorders involving eosinophilia, neurological and/or neurodegenerative disorders and for improvement of brain function.
- It is an object of the present invention to provide such treatments and means for such treatments.
- Throughout this application, unless specifically stated otherwise, the abbreviation AKG refers to alpha-ketoglutaric acid (see Formula I):
- as well as any pharmaceutically acceptable salts thereof. Such salts of the invention may be inorganic or organic. Examples of preferable salts include sodium and calcium salts of alpha-ketoglutaric acid (denoted Na-AKG and Ca-AKG, respectively). Other preferable salts include potassium-AKG and magnesium-AKG as well as organic salts of alpha-ketoglutaric acid where the counter-ion is an amino-acid naturally occurring in proteins (such as arginine, leucine, isoleucine), pyridoxine, chitosan, creatine or ornithine.
- The term pharmaceutically acceptable salt encompasses any salts that are pharmaceutically acceptable in the sense that they are not toxic to the intended subject at the doses intended and are sufficiently stable and soluble for the intended purpose. Preferably, the counter-ion of alpha-ketoglutarate in a pharmaceutically acceptable salt of the invention is not an active ingredient by itself, although it may in some cases be acceptable that the counter-ion is an active ingredient.
- The term lipase in the context of the present invention refers to enzymes that catalyze the hydrolytic release of fatty acids from triglycerides releasing fatty acids and glycerol, monoglycerides and/or diglycerides. Preferably, a lipase catalyzes the release of long-chain or medium-chain polyunsaturated fatty acid (PUFA). In this context “long-chain” may refer to fatty acids having tails longer than 12 carbons and “medium-chain” to fatty acids having tails of 6-12 carbons. By polyunsaturated it is meant that there are at least two double bonds between the carbon atoms in the tail of polyunsaturated fatty acids. Examples of PUFA include but are not limited to linoleic acid, linoelaidic acid, α-linolenic acid, arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid. It is preferable that the activity of the lipase of the present invention is substantially independent of pH and that the lipase is active at a pH 6-8.
- The term lipase in the context of the present invention comprises lipases of any origin, including human, animal, plant and fungal, bacterial, eukaryotic and prokaryotic origin, irrespective of if the lipase has been produced by recombinant means or by non-recombinant means.
- Preferably, the lipase is or has been made acid stable. Such acid stable lipase may be lipase-CLEC (cross-linked enzyme crystals). Such lipase-CLEC crystals and methods of their manufacture are known in the art, for instance from US2006/0121017, incorporated herein by reference. It is also possible to use non-acid stable lipases if the amount administered is increased to compensate for loss of activity in the stomach, the stomach acidity of the patient reduced by antacids, proton pump inhibitors or other suitable pharmacological means, or if the lipase is formulated in a protective enteric formulation well known in the art.
- The term protease in the context of the present invention refers to a proteinase, proteolytic enzyme or peptidase, which is an enzyme that catalyzes the splitting of interior amide peptide bonds in a protein. Specifically, proteases catalyze the conversion of proteins into smaller proteins/peptides and/or their component amino acids by cleaving the amide linkage between the carboxyl group of one amino acid and the amino group of another. Proteases are generally identified by their catalytic type, e.g., aspartic acid peptidases, cysteine (thiol) peptidases, metallopeptidases, serine peptidases, threonine peptidases, alkaline or semi-alkaline proteases, neutral and peptidases of unknown catalytic mechanism (see https://merops.sanger.ac.uk). Any catalytic type is encompassed by the term in the context of the present invention. The term protease in the context of the present invention comprises proteases of any origin, including human, animal, plant and fungal, bacterial, eukaryotic and prokaryotic origin, irrespective of if the protease has been produced by recombinant means or by non-recombinant means.
- The term amylaose in the context of the present invention refers to amylase enzymes having broad substrate specificity and catalyzing the hydrolysis of α-1,4-glucosidic linkages of starch, glycogen and related polysaccharides containing three or more α-1,4-linked D-glucose units yielding maltose, glucose and/or limit dextrins of 2-3 units. The term amylase in the context of the present invention comprises amylases of any origin, including human, animal, plant and fungal, bacterial, eukaryotic and prokaryotic origin, irrespective of if the amylase has been produced by recombinant means or by non-recombinant means.
- The term “USP Unit” refers to the United States Pharmacopoeia unit of enzyme activity present in an agent or composition. One USP Unit of lipase, protease or amylase is defined in Pancrelipase, USP, U.S. Pharmacopeia National Formulary, USP 24, pp. 1254-1255 (2000). Assays for lipase, protease and amylase are disclosed in that reference and are incorporated herein by reference.
- The term neurogenesis in the context of the present invention is taken to mean the process by which new neurons are formed from neural stem cells. In neurogenesis, there is active production of new neurons, astrocytes, glia, and other neural lineages from undifferentiated neural progenitor or stem cells.
- The term neurodegenerative disease in the context of the present invention is an umbrella term for any disease causing progressive loss of structure and/or function of neurons, with or without death of neurons. Examples of neurodegenerative diseases include but are not limited to Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis.
- The term eosinophilia in the context of the present invention encompasses both primary eosinophilia, as well as conditions where increased eosinophile numbers are a symptom.
- The term pancrelipose in the context of the present invention encompasses not only pancrelipase according to the normal meaning of the term in the art, but even similar preparations such as pancreatin, and similar preparations from any animal source.
- For explanation on the groups, please see Table 1 and Example 1.
-
FIG. 1 illustrates increased adult neurogenesis detected by increased number of nestin-positive neurons per 1 mm of pyramidal layer length in hippocampal CA1 area by means of the present invention. Asterisks indicate statistically significant differences. -
FIG. 2 illustrates improved cognitive performance by means of the present invention, detected in the percentage of spontaneous alterations in a T-maze test. (+) denotes correct trials—animal visited left and right arms, (−) denotes incorrect trials—animal visited the same arm twice. -
FIG. 3A illustrates results from blood chemistry, specificallyplatelets 103/μl. Letters a or c indicate statistically significant difference from control group (p<0.05). -
FIG. 3B illustrates results from blood chemistry, specifically lymphocyte (%). Letters a or c indicate statistically significant difference from control group (p<0.05). -
FIG. 3C illustrates results from blood chemistry, specifically eosinophils (%). Letters a or c indicate statistically significant difference from control group (p<0.05). -
FIG. 3D illustrates results from blood chemistry, specifically monocytes (%). Letters a or c indicate statistically significant difference from control group (p<0.05). -
FIG. 4 shows average number of synaptic terminals per 100 μm2 of hippocampal CA1 zone in different treatment groups. Asterisk (*) denotes statistical significance compared to the control group (p<0.05) -
FIG. 5 shows average area (μm2) of synaptic terminal in hippocampal CA1 zone in different treatment groups. -
FIG. 6A shows average number of synaptic vesicles per synapse in hippocampal CA1 zone. Asterisk (*) denotes statistical significance compared to the control group (p<0.05). -
FIG. 6B shows average number of synaptic vesicles per 100 μm2 of synaptic terminal in hippocampal CA1 zone. Asterisk (*) denotes statistical significance compared to the control group (p<0.05). -
FIG. 7 shows average distance (nm) from synaptic vesicles to the active zone of presynaptic terminal. Asterisk (*) denotes statistical significance compared to the control group (p<0.05). -
FIG. 8 shows average distance (nm) from vesicle to the to the nearest neighbor vesicle of presynaptic terminal. Asterisk (*) denotes statistical significance compared to the control group (p<0.05). -
FIG. 9 shows proportion (%) of perforated and multiple synapses in hippocampal CA1 zone. The remainder represents simple synapses. The letter on the gray and black bars describe statistic difference when p<0.05. Columns with same letter are not statistically significantly different from each other. C denotes age-matched (old) control. -
FIG. 10 illustrates improved cognitive performance by means of the present invention, detected in the percentage of spontaneous alterations in a T-maze test (See Example 5). The test was performed at start (0 months) and repeated at 2 and 4 months. The fraction of correct trials is shown, with 95% confidence interval calculated by modified Wald method. The treatment at baseline (0) and treatment at 4 months are statistically significantly different at least at p<0.05, since the 95% confidence intervals do not overlap. -
FIG. 11 shows the fraction of individual animals of Example 5 showing deteriorated or improved performance in the T-maze test at 2 and 4 months (results pooled) compared to their individual baseline performance. The fraction of animals in the group with improved performance is shown by the black columns, and the fraction with deteriorated performance is shown by the grey columns.Group 1=age-matched untreated controls,Group 2=treatment with AKG+enzymes. The difference is significant at p<0.05. - The present invention provides the following items.
- 1. A composition comprising alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof (AKG), and one or more enzymes selected from the group consisting of lipases, proteases and amylases.
- 2. The composition according to
item 1, comprising a lipase, a protease and/or an amylase in an amount according to an embodiment indicated in Table X with reference to Tables Y and Z. - 3. The composition according to
item - 4. The composition according to
item 3, wherein the lipase is a mammalian lipase. - 5. The composition according to
item 3, wherein the lipase is a microbial lipase. - 6. The composition according to
item 3, wherein the lipase is a bacterial lipase. - 7. The composition according to
item 3, wherein the lipase is a pancrelipase lipase. - 8. The composition according to
item 3, wherein the lipase is a liprotamase lipase. - 9. The composition according to
item 3 wherein the lipase is a Pseudomonas lipase. - 10. The composition according to
item 3, wherein the lipase is a human or other mammalian bile-salt stimulated lipase (BSSL), preferably human. - 11. The composition according to
item 3, wherein the lipase is a human or other mammalian bile-salt dependent lipase (BSDL), preferably human. - 12. The composition according to
item 3, wherein the lipase is a Rhizopus oryzae lipase. - 13. The composition according to
item 3, wherein the lipase is a Chromobacterium viscosum lipase. - 14. The composition according to
item 3, wherein the lipase is a Rhizopus delemar lipase. - 15. The composition according to
item 3, wherein the lipase is a Burkholderia lipase. - 16. The composition according to
item 15, wherein the lipase is a Burkholderia cepacia lipase. - 17. The composition according to
item 16, wherein the lipase is a Burkholderia cepacia lipase as cross-linked lipase crystals. - 18. The composition according to any of items 1-17, comprising a protease.
- 19. The composition according to
item 18, wherein the protease is a mammalian protease. - 20. The composition according to
item 18, wherein the protease is a microbial protease. - 21. The composition according to
item 18, wherein the protease is a fungal protease. - 22. The composition according to
item 18, wherein the protease is a pancrelipase protease. - 23. The composition according to
item 18, wherein the protease is a liprotamase protease - 24. The composition according to
item 18, wherein the protease is an Aspergillus protease. - 25. The composition according to item 24, wherein the protease is an Aspergillus melleus protease.
- 26. The composition according to any of items 1-25 comprising an amylase.
- 27. The composition according to item 26, wherein the amylase is a mammalian amylase.
- 28. The composition according to item 26, wherein the amylase is a microbial amylase.
- 29. The composition according to item 26, wherein the amylase is a fungal amylase.
- 30. The composition according to item 26, wherein the amylase is a pancrelipase amylase.
- 31. The composition according to item 26, wherein the amylase is a liprotamase amylase.
- 32. The composition according to item 26, wherein the amylase is an Aspergillus amylase.
- 33. The composition according to item 32, wherein the amylase is Aspergillus oryzae amylase.
- 34. The composition according to any of items 1-17, wherein the composition comprises 200-20000 USP units of lipase per mmol AKG.
- 35. The composition according to any of items 18-25 or 34, wherein the composition comprises 500-50000 USP units of protease per mmol AKG.
- 36. The composition according to any of items 26-33 or 34-35 wherein the composition comprises 200-20000 USP units of amylase per mmol AKG.
- 37. The composition according to
item 1, comprising 3000-300000 USP units of Burkholderia cepacia lipase per g AKG as cross-linked lipase crystals, 2000-200000 USP units of Aspergillus melleus protease per g AKG and 300-30000 USP units of Aspergillus oryzae amylase per g AKG. - 38. The composition according to
item 1, comprising liprotamase in an amount corresponding to 300-300000 USP lipase units of liprotamase per g AKG. - 39. The composition according to
item 1, comprising pancrelipase in an amount corresponding to 300-300000 USP lipase units of pancrelipase per g AKG. - 40. The composition according to any of the preceding items, wherein the composition comprises sodium alpha-ketoglutarate.
- 41. The composition according to any of the preceding items, wherein the composition comprises calcium alpha-ketoglutarate.
- 42. The composition according to any of the preceding items, for use as a medicament.
- 43. The composition according to any of the preceding items, for use in the treatment or prevention of a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders.
- 44. The composition according to any of the preceding items, for use in the treatment or prevention of a condition selected from: Alzheimer's disease, mild cognitive impairment (MCI), Parkinson's disease and Parkinsonian disorders, Huntington's disease, Amyotrophic Lateral Sclerosis, ischemic stroke, traumatic brain injury, depression, bipolar depression/disorder, chronic fatigue syndrome, anxiety syndromes/disorders, autism, Asperger's syndrome, attention deficit disorders, and disorders of cognitive performance or memory.
- 45. The composition for use according to item 44, for use in the treatment or prevention of depression or chronic fatigue syndrome.
- 46. The composition according to any of the preceding items, for use in a method comprising administering an amount of the composition containing 0.1-30 g AKG to a patient per day.
- 47. The composition according to any of items 1-45, for use in a method comprising administering to a human patient an amount of the composition containing 0.5-240 mmol AKG.
- 48. The composition according to item 47, for use in a method comprising administering an amount of the composition containing 0.5-24 mmol AKG to a patient per day.
- 49. The composition according to item 48, for use in a method comprising administering an amount of the composition containing 1-12 mmol AKG to a patient per day.
- 50. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use in the treatment or prevention of depression or chronic fatigue syndrome.
- 51. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use in a method of treatment or prevention of neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders, Alzheimer's disease, mild cognitive impairment (MCI), Parkinson's disease and Parkinsonian disorders, Huntington's disease, Amyotrophic Lateral Sclerosis, ischemic stroke, traumatic brain injury, depression, bipolar depression/disorder, chronic fatigue syndrome, anxiety syndromes/disorders, autism, Asperger's syndrome, attention deficit disorders, and disorders of cognitive performance or memory,
- the method comprising administering alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof and one or more enzymes selected from a lipase, a protease and an amylase thereof to a subject.
- 52. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 51, wherein the method comprises administering an amount of a lipase, a protease and/or an amylase relative to the amount AKG according to any of the embodiments indicated in Table X with reference to Tables Y and Z.
- 53. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 51 or 52, wherein the method comprises administering a lipase.
- 54. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a mammalian lipase.
- 55. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a microbial lipase.
- 56. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a bacterial lipase.
- 57. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a pancrelipase lipase.
- 58. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a liprotamase lipase.
- 59. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a Pseudomonas lipase.
- 60. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a human or other mammalian bile-salt stimulated lipase (BSSL), preferably human.
- 61. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a human or other mammalian bile-salt dependent lipase (BSDL), preferably human.
- 62. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a Rhizopus oryzae lipase.
- 63. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a Chromobacterium viscosum lipase.
- 64. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a Rhizopus delemar lipase.
- 65. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 53, wherein the lipase is a Burkholderia lipase.
- 66. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 65, wherein the lipase is a Burkholderia cepacia lipase.
- 67. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 66, wherein the lipase is a Burkholderia cepacia lipase as cross-linked lipase crystals.
- 68. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-67, wherein the method comprises administering a protease.
- 69. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 68, wherein the protease is a mammalian protease.
- 70. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 68, wherein the protease is a microbial protease.
- 71. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 68, wherein the protease is a fungal protease.
- 72. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 68, wherein the protease is a pancrelipase protease.
- 73. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 68, wherein the protease is a liprotamase protease
- 74. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 68, wherein the protease is an Aspergillus protease.
- 75. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 74, wherein the protease is an Aspergillus melleus protease.
- 76. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-75, wherein the method comprises administering an amylase.
- 77. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 76, wherein the amylase is a mammalian amylase.
- 78. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 76, wherein the amylase is a microbial amylase.
- 79. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 76, wherein the amylase is a fungal amylase.
- 80. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 76, wherein the amylase is a pancrelipase amylase.
- 81. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 76, wherein the amylase is a liprotamase amylase.
- 82. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 76, wherein the amylase is an Aspergillus amylase.
- 83. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 82, wherein the amylase is Aspergillus oryzae amylase.
- 84. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-83, wherein the method comprises administering 200-20000 USP units of lipase per mmol AKG.
- 85. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-84, wherein the method comprises administering 500-5000 USP units of protease per mmol AKG.
- 86. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-85, wherein the method comprises administering 200-20000 USP units of amylase per mmol AKG.
- 87. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 51, wherein the method comprises administering 3000-300000 USP units of Burkholderia cepacia lipase per g AKG as cross-linked lipase crystals, 2000-200000 USP units of Aspergillus melleus protease per g AKG and 300-30000 USP units of Aspergillus oryzae amylase per g AKG.
- 88. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 51, wherein the method comprises administering liprotamase in an amount corresponding to 300-300000 USP lipase units of liprotamase per g AKG.
- 89. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 51, wherein the method comprises administering pancrelipase in an amount corresponding to 300-300000 USP lipase units of pancrelipase per g AKG.
- 90. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-89, wherein the method comprises administering sodium alpha-ketoglutarate.
- 91. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-90, wherein the method comprises administering calcium alpha-ketoglutarate.
- 92. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-91, wherein the method comprises administering AKG to the subject at 0.03-100 g/day.
- 93. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to any of items 51-91, wherein the method comprises administering 0.5-240 mmol AKG per day to the subject.
- 94. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according item 93, wherein the method comprises administering 0.5-24 mmol AKG per day to the subject.
- 95. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 94, wherein the method comprises administering 1-12 mmol AKG per day to the subject.
- 96. Alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use according to item 95, wherein the method comprises administering 3-9 mmol AKG per day to the subject.
- The inventors have found that AKG as well as a composition comprising lipase, protease and amylase had effects in neurogenesis and cognition. A combination of AKG and said enzymes had a synergistic effect in neurogenesis and in improving cognition. The combination also has a synergistic effect on synaptic morphology.
- In a first aspect, the present invention discloses novel synergistic compositions comprising alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof (AKG), and one or more enzymes selected from a group consisting of a lipase, a protease and an amylase, the constituents being present at synergistically effective relative amounts.
- Such compositions have synergistic effect in promoting neurogenesis, improving synaptic morphology and reducing eosiniphil counts, enabling treatment of a number of conditions as explained in more detail below.
- In certain embodiments, the composition comprises a lipase but no protease or amylase.
- In certain embodiments, the composition comprises a protease but no lipase or amylase.
- In certain embodiments, the composition comprises an amylase but no protease or lipase.
- In certain embodiments, the composition comprises a lipase and a protease but no amylase.
- In certain embodiments, the composition comprises a lipase and an amylase but no protease.
- In certain embodiments, the composition comprises a protease and an amylase but no lipase.
- In certain embodiments, the composition comprises a lipase, a protease, and an amylase.
- Lipases, proteases and amylases (and combinations thereof) suitable for use in the composition of the invention are known in the art, see for instance US2006/012017, US2004/0057944, US2001/0046493 and U.S. Pat. No. 6,051,220, all incorporated herein by reference.
- The composition may further comprise pharmaceutically or nutritionally acceptable excipients or additives. Optionally, the composition may include additional active ingredients. Preferably, the composition does not include additional active ingredients.
- The composition is preferably for oral administration. It may be formulated in any manner well known in the art for oral administration of the substances that it comprises. If the formulation comprises non-acid stable lipase, it may be preferable to encapsulate at least this component in a protective enteric coating. Such coatings are well known in the art and found in products already in the market.
- The composition may be a food supplement, a nutraceutical, a pharmaceutical composition, a dietary supplement or a food additive.
- The composition is the first aspect may comprise a lipase, optionally in combination with a protease as described below, an amylase as described below or both. The lipase may be selected from a mammalian lipase, a microbial lipase, a bacterial lipase, a pancrelipase lipase (i.e. a lipase included in pancrelipase), a liprotamase lipase (i.e. a lipase included in liprotamase), Pseudomonas lipase, human or other mammalian bile-salt stimulated lipase (BSSL) or bile-salt dependent lipase (BSDL), Rhizopus oryzae lipase, Chromobacterium viscosum lipase, Rhizopus delemar lipase, Burkholderia lipase, more preferably Burkholderia cepacia lipase, most preferably Burkholderia cepacia lipase as cross-linked lipase crystals.
- The composition may comprise 300-3000000 USP units of lipase per g AKG, preferably 3000-300000 USP units of lipase per g AKG, more preferably 6000-150000 USP units of lipase per g AKG and even more preferably 15000-60000 USP units of lipase per g AKG, and most preferably about 30000 USP units of lipase per g AKG.
- The composition of the first aspect may comprise a protease, optionally in combination with a lipase as described above, an amylase as described below or both. The protease may be selected from a mammalian protease, a microbial protease, a fungal protease, a pancrelipase protease (i.e. a protease included in pancrelipase), a liprotamase protease (i.e. a protease included in pancrelipase), an Aspergillus protease, most preferably Aspergillus melleus protease.
- The composition may comprise 200-2000000 USP units of protease per g AKG, preferably 2000-200000 USP units of protease per g AKG, more preferably 4000-100000 USP units of protease per g AKG and even more preferably 10000-40000 USP units of protease per g AKG, and most preferably about 20000 USP units of protease per g AKG.
- The composition of the first aspect may comprise an amylase, optionally in combination with a lipase as described above, a protease described above or both. The amylase may be selected from a mammalian amylase, a microbial amylase, a fungal amylase, a pancrelipase amylase (i.e. an amylase included in pancrelipase), a liprotamase amylase (i.e. an amylase included in liprotamase), an Aspergillus amylase, most preferably Aspergillus oryzae amylase.
- The composition may comprise 30.0-3000000 USP units of amylase per g AKG, preferably 300-300000 USP units of amylase per g AKG, more preferably 300-30000 USP units of amylase per g AKG and even more preferably 600-15000 USP units of amylase per g AKG, and most preferably about 3000 USP units of amylase per g AKG.
- The composition of the first aspect may comprise 300-3000000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 200-2000000 USP units of Aspergillus melleus protease per g AKG and 30.0-3000000 USP units of Aspergillus oryzae amylase per g AKG.
- The composition of the first aspect may comprise 3000-300000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 2000-200000 USP units of Aspergillus melleus protease per g AKG and 300-300000 USP units of Aspergillus oryzae amylase per g AKG.
- The composition of the first aspect may comprise 6000-150000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 4000-100000 USP units of Aspergillus melleus protease per g AKG and 300-30000 USP units of Aspergillus oryzae amylase per g AKG.
- The composition of the first aspect may comprise 15000-60000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), 10000-40000 USP units of Aspergillus melleus protease per g AKG and 600-15000 USP units of Aspergillus oryzae amylase per g AKG.
- The composition of the first aspect may comprise about 30000 USP units of Burkholderia cepacia lipase per g AKG (preferably as cross-linked lipase crystals), about 20000 USP units of Aspergillus melleus protease per g AKG and about 3000 USP units of Aspergillus oryzae amylase per g AKG.
- The composition of the first aspect may comprise liprotamase. The amount of liprotamase may be 300-300000 USP lipase units of liprotamase per g AKG, preferably 3000-150000 USP lipase units of liprotamase per g AKG, more preferably 15000-60000 USP lipase units of liprotamase per g AKG and most preferably about 30000 USP lipase units of liprotamase per g AKG. A composition with AKG and liprotamase may comprise Na-AKG and Ca-AKG in equal amounts in terms of weight.
- The composition of the first aspect may comprise pancrelipase. The amount of pancrelipase may be 300-300000 USP lipase units of pancrelipase per g AKG, preferably 3000-150000 USP lipase units of pancrelipase per g AKG, more preferably 1500-60000 USP lipase units of pancrelipase per g AKG and most preferably about 30000 USP lipase units of pancrelipase per g AKG. A composition with AKG and pancrelipase may comprise Na-AKG and Ca-AKG in equal amounts in terms of weight.
- The present invention also provides further embodiments of the composition of the first aspect in Table X appended after the Examples section. The contents of table X are to be read as follows.
- Each line in table X corresponds to an embodiment. From the left, the first column denoted “#” shows the embodiment identification number. The second column from the left denoted “A” shows the lipase concentration range for that embodiment, with reference to tables Y and Z. The third column from the left denoted “B” shows the protease concentration range for that embodiment, with reference to tables Y and Z. The fourth column from the left denoted “C” shows the amylase concentration range for that embodiment, with reference to tables Y and Z.
-
TABLE Y Interpretation of columns A, B and C in Table X: Value in column A, B or C in Table X Corresponding amount range 1 base value/250 to base value times 2502 base value/100 to base value times 1003 base value/25 to base value times 254 base value/10 to base value times 105 base value/5 to base value times 56 base value to base value times 57 base value/5 to base value 8 base value to base value times 259 base value/25 to base value 10 base value times 0 (=not present) - It is also contemplated that any of the endpoints of ranges of any embodiment in Table X can be combined with any other endpoint of a range (in the same column) of another embodiment in Table X.
- For instance two embodiments with
values base value times 100, (3) base value/25 tobase value times 100 or (4) base value/100 to base value/25. - Any endpoint in an embodiment may also be used to form the endpoint of an open range of an additional embodiment. For instance,
value 2 in column A forms basis for an embodiment where the lipase amount is “at least base value/100”, and an embodiment where the lipase amount is “at leastbase value time 100” USP units/mmol AKG. Similarly,value 2 in column A forms basis for an embodiment where the lipase amount is “less than base value/100”, and an embodiment where the lipase amount is “less thanbase value time 100” USP units/mmol AKG. -
TABLE Z Base values for interpretation of columns A, B and C in Table X (with reference to Table Y) Feature Base value (USP units/mmol AKG) Lipase, column A in Table X 2000 Protease, column B in Table X 5000 Amylase, column C in Table X 2000 - To facilitate understanding of how Table X is to be read, the following example is provided: Embodiment 53 in Table X corresponds to the following values: Column A=1, Column B=6, Column C=3.
- From Table Y it can thus be deduced that embodiment 53 corresponds to lipase amount of base value/250 to
base value times 250, protease amount of base value tobase value times 5, and amylase amount of base value/25 to base value times 25. - Base values can be found in Table Z, whereby embodiment 53 corresponds to:
-
- Lipase amount: 2000/250 to 2000
times 250 USP units/mmol AKG=8-500,000 USP units lipase/mmol AKG - Protease amount: 5000 to 5000
times 5 USP units/mmol AKG=5,000-25,000 USP units protease/mmol AKG - Amylase amount: 2000/25 to 2000
times 25 USP units/mmol AKG=8-50,000 USP units amylase/mmol AKG
- Lipase amount: 2000/250 to 2000
- Preferably, the embodiments of the first aspect of the invention disclosed in Table X with reference to Tables Z and Y comprise Burkholderia cepacia lipase as a lipase (preferably as cross-linked lipase crystals), Aspergillus melleus protease a protease and Aspergillus oryzae amylase as an amylase, to the extent said lipase, protease and amylase are present in the particular embodiment.
- More preferably, the embodiments of the first aspect of the invention disclosed in Table X with reference to Tables Z and Y comprise Burkholderia cepacia lipase as the only lipase (preferably as cross-linked lipase crystals), Aspergillus melleus protease the only protease and/or Aspergillus oryzae amylase as the only amylase, to the extent said lipase, protease and amylase are present in the particular embodiment.
- The AKG component in the composition of the first aspect as disclosed above (including the embodiments in Table X) may consist of or comprise sodium alpha-ketoglutarate, calcium alpha-ketoglutarate or a combination thereof (preferably in equal amounts). The AKG component may also comprise or consist of another pharmaceutically acceptable inorganic salt of AKG including magnesium or potassium alpha-ketoglutarate, or a pharmaceutically acceptable organic salt of AKG, such as ornithine-AKG, arginine-AKG, chitosan-AKG, pyrodoxine-AKG, leucine-AKG, isoleucine-AKG or creatine-AKG, or a combination thereof.
- In a second aspect, the present invention provides a composition according to the first aspect, for use as a medicament.
- Use of a composition according to the first aspect in the manufacture of a medicament is also provided.
- Additionally, there is provided a method of treatment or prevention comprising administering to a subject in need thereof a composition according to the first aspect.
- In a third aspect, the present invention provides a composition according to the first aspect, for use in the treatment or prevention of a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders.
- Use of a composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders.
- Additionally, there is provided a method of treatment or prevention of a neurological disorder selected from neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma and cognitive, learning and memory disorders comprising administering to a subject in need thereof a composition according to the first aspect.
- In a fourth aspect, the present invention provides a composition according to the first aspect, for use in the treatment or prevention of a condition selected from: Alzheimer's disease, mild cognitive impairment (MCI), Parkinson's disease and Parkinsonian disorders, Huntington's disease, Amyotrophic Lateral Sclerosis, spinal ischemia, ischemic stroke, traumatic brain injury, traumatic spinal cord injury, cancer-related brain/spinal cord injury, schizophrenia and other psychoses, lissencephaly syndrome, depression, bipolar depression/disorder, chronic fatigue syndrome, anxiety syndromes/disorders, phobias, stress and related syndromes, cognitive function disorders, aggression, drug and alcohol abuse, obsessive compulsive behaviour syndromes, seasonal mood disorder, borderline personality disorder, cerebral palsy, life style drug abuse, multi-infarct dementia, Lewy body dementia, age related geriatric dementia, epilepsy and injury related to epilepsy, temporal lobe epilepsy, spinal cord injury, brain injury, brain surgery, trauma related brain/spinal cord injury, including concussion related brain injury, brain injury due to repeated concussion or repeated trauma to the head, and brain injury caused by shock wave from an explosion, anti-cancer treatment related brain/spinal cord tissue injury, infection and inflammation related brain/spinal cord injury, environmental toxin related brain/spinal cord injury, multiple sclerosis, autism, Asperger's syndrome, attention deficit disorders, narcolepsy, sleep disorders, and disorders of cognitive performance or memory.
- Use of a composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of a condition selected from those disclosed above for the fourth aspect.
- Additionally, there is provided a method of treatment or prevention of a condition selected from those disclosed above for the fourth aspect, comprising administering to a subject in need thereof a composition according to the first aspect.
- In a fifth aspect, the present invention provides a composition according to the first aspect, for use in the treatment or prevention of depression or chronic fatigue syndrome.
- Use of a composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of depression or chronic fatigue syndrome is also provided.
- Additionally, there is provided a method of treatment or prevention of depression or chronic fatigue syndrome comprising administering to a subject in need thereof a composition according to the first aspect.
- In a sixth aspect, the present invention provides a composition according to the first aspect, for use in the treatment or prevention of disorders involving eosinophilia.
- Use of a composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of disorders involving eosinophilia is also provided.
- Additionally, there is provided a method of treatment or prevention of disorders involving eosinophilia comprising administering to a subject in need thereof a composition according to the first aspect.
- In a seventh aspect, the present invention provides a composition according to the first aspect, for use in the treatment or prevention of allergic diseases including astma, atopic dermatitis, eosinophilic esophagitis and rhinoconjunctivitis; autoimmune diseases including primary biliary cirrhosis, Riedel invasive fibrous thyroiditis, dermatomyositis, systemic lupus erythematosus and Sjögren's syndrome; parasite infection-induced eosinophilia including helminth infection-induced eosinophilia; graft-versus-host disease; drug-induced eosinophilia; primary eosinophilia, neoplastic disease-induced eosinophilia including eosinophilia associated with primary cutaneous T-cell lymphoma, Sézary syndrome, Hodgkin's disease, Langerhans cell histiocytosis, thyroid cancer, stomach cancer, liver cancer and bladder cancer.
- Use of a composition according to the first aspect in the manufacture of a medicament for the treatment or prevention of conditions disclosed above for the fourth aspect is also provided.
- Additionally, there is provided a method of treatment or prevention of conditions disclosed above for the fourth aspect, comprising administering to a subject in need thereof a composition according to the first aspect.
- In an eight aspect, there is provided a composition according to the first aspect, for use in a method comprising administering an amount of the composition containing 0.03-100 g AKG to a patient per day, 0.1-30 g AKG to a patient per day, 1-10 g AKG to a patient per day, or 2-6 g AKG to a patient per day.
- The eight aspect also provides a composition according to the first aspect, for use in a method comprising administering to a (preferably human) patient daily an amount of the composition containing the following amounts of AKG in mmol: 0.5-240, 1-120, 3-60, 5-36, 9-24, 0.5-3, 0.5-5, 0.5-9, 0.5-12, 1-5, 3-9, 3-12, 3-24, 1-5, 3-5, 1-3, 9-36, about 0.5, about 1, about 3, about 5, about 9, about 12, about 24, about 36, about 60, about 120 or about 240. Any range formed by any combination of the endpoints of the aforementioned ranges is also contemplated.
- Said administration may be oral. Said patient may be preferably human, in particular a human in need of such treatment.
- There is also provided a use of a composition according to the first aspect in the manufacture of a food supplement, a dietary supplement, a nutraceutical or a food additive. Further, there is provided a use of a composition according to the first aspect as a dietary supplement, a food supplement, a nutraceutical or a food additive.
- In a ninth aspect, the present invention provides alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use in the treatment or prevention of depression or chronic fatigue syndrome.
- Use of alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment or prevention of depression or chronic fatigue syndrome is also provided.
- Additionally, there is provided a method of treatment or prevention of depression or chronic fatigue syndrome comprising administering to a subject in need thereof alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, in an effective amount.
- The alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof according to the ninth aspect may be for use in a method comprising administering an amount of the alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof containing 0.03-100 g AKG to a patient per day, preferably 0.1-30 g AKG to a patient per day, more preferably, 1-10 g AKG to a patient per day, most preferably, 2-6 g AKG to a patient per day.
- The alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof according to the ninth aspect may be for use in a method comprising administering to a (preferably human) patient daily an amount of the composition containing the following amounts of AKG in mmol: 0.5-240, 1-120, 3-60, 5-36, 9-24, 0.5-3, 0.5-5, 0.5-9, 0.5-12, 1-5, 3-9, 3-12, 3-24, 1-5, 3-5, 1-3, 9-36, about 0.5, about 1, about 3, about 5, about 9, about 12, about 24, about 36, about 60, about 120 or about 240. Any range formed by any combination of the endpoints of the aforementioned ranges is also contemplated.
- Said administration is preferably oral. Said patient may be human, in particular a human in need of such treatment.
- The pharmaceutically acceptable salt may consist of or comprise a pharmaceutically acceptable inorganic salt of AKG such as sodium, calcium, magnesium or potassium alpha-ketoglutarate, or a pharmaceutically acceptable organic salt of AKG, such as ornithine-AKG, arginine-AKG, chitosan-AKG, pyrodoxine-AKG, leucine-AKG, isoleucine-AKG or creatine-AKG, or a combination thereof.
- In an tenth aspect, the present invention also provides alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof, for use in a method of treatment or prevention of neurodegenerative disorders, neural stem cell disorders, neural progenitor disorders, ischemic disorders, neurological traumas, affective disorders, neuropsychiatric disorders, degenerative diseases of the retina, retinal injury/trauma, cognitive, learning and memory disorders, Alzheimer's disease, mild cognitive impairment (MCI), Parkinson's disease and Parkinsonian disorders, Huntington's disease, Amyotrophic Lateral Sclerosis, spinal ischemia, ischemic stroke, traumatic brain injury, traumatic spinal cord injury, cancer-related brain/spinal cord injury, schizophrenia and other psychoses, lissencephaly syndrome, depression, bipolar depression/disorder, chronic fatigue syndrome, anxiety syndromes/disorders, phobias, stress and related syndromes, cognitive function disorders, aggression, drug and alcohol abuse, obsessive compulsive behaviour syndromes, seasonal mood disorder, borderline personality disorder, cerebral palsy, life style drug abuse, multi-infarct dementia, Lewy body dementia, age related geriatric dementia, epilepsy and injury related to epilepsy, temporal lobe epilepsy, spinal cord injury, brain injury, brain surgery, trauma related brain/spinal cord injury, including concussion related brain injury, brain injury due to repeated concussion or repeated trauma to the head, and brain injury caused by shock wave from an explosion, anti-cancer treatment related brain/spinal cord tissue injury, infection and inflammation related brain/spinal cord injury, environmental toxin related brain/spinal cord injury, multiple sclerosis, autism, Asperger's syndrome, attention deficit disorders, narcolepsy, sleep disorders, disorders of cognitive performance or memory, a disorder involving eosinophilia, astma, atopic dermatitis, eosinophilic esophagitis and rhinoconjunctivitis; autoimmune diseases including primary biliary cirrhosis, Riedel invasive fibrous thyroiditis, dermatomyositis, systemic lupus erythematosus and Sjögren's syndrome; parasite infection-induced eosinophilia including helminth infection-induced eosinophilia; graft-versus-host disease; drug-induced eosinophilia; primary eosinophilia, neoplastic disease-induced eosinophilia including eosinophilia associated with primary cutaneous T-cell lymphoma, Sézary syndrome, Hodgkin's disease, Langerhans cell histiocytosis, thyroid cancer, stomach cancer, liver cancer and bladder cancer, the method comprising administering alpha-ketoglutaric acid or a pharmaceutically acceptable salt thereof and one or more of a lipase, a protease and an amylase to a subject.
- In certain embodiments, the method comprises administering a lipase but no protease or amylase.
- In certain embodiments, the method comprises administering a protease but no lipase or amylase.
- In certain embodiments, the method comprises administering an amylase but no protease or lipase.
- In certain embodiments, the method comprises administering a lipase and a protease but no amylase.
- In certain embodiments, the method comprises administering a lipase and an amylase but no protease.
- In certain embodiments, the method comprises administering a protease and an amylase but no lipase.
- In certain embodiments, the method comprises administering a lipase, a protease, and an amylase.
- The AKG, the lipase, the protease and the amylase administered in the method referred to in the tenth aspect may have same properties and features as specified for the components of the composition of the first aspect.
- The relative and absolute amounts of the AKG, the lipase, the protease and the amylase administered in the method referred to in the tenth aspect may be as specified for the composition of the first aspect and uses thereof.
- The method of the tenth aspect may comprise administering AKG to the subject at 0.03-100 g/day, 0.1-30 g per day, 1-10 g per day or 2-6 g per day.
- In particular the method of the tenth aspect may comprise administering to a (preferably human) patient daily the following amount of AKG in mmol: 0.5-240, 1-120, 3-60, 5-36, 9-24, 0.5-3, 0.5-5, 0.5-9, 0.5-12, 1-5, 3-9, 3-12, 3-24, 1-5, 3-5, 1-3, 9-36, about 0.5, about 1, about 3, about 5, about 9, about 12, about 24, about 36, about 60, about 120 or about 240. Any range formed by any combination of the endpoints of the aforementioned ranges is also contemplated.
- The method referred to in the tenth aspect may comprise administering the AKG, the lipase, the protease and/or the amylase simultaneously, separately or consecutively. Said components may be administered in formulations comprising any combination of the substances.
- Preferably, the method referred to in the tenth aspect comprises administering AKG and liprotamase simultaneously, separately or consecutively. Also preferably, the method referred to in the tenth aspect comprises administering AKG and pancrelipase simultaneously, separately or consecutively.
- All references are hereby incorporated in their entirety. The following examples are not to be construed as limiting. The term “comprising” is used inclusively i.e. in the sense of “including, but not limited to”.
- After the course of treatment as specified under Materials and Methods, the animals were sacrificed and the brains were analysed histologically to quantitate hippocampal neurogenesis.
- Nestin is a marker for immature neurons. Nestin positive cells were found in the hippocampal layer of the pyramidal neurons. The somas of hippocampal cells displayed strong nestin staining. In some cells long processes that seem to be dendrites extended into deeper layers of the hippocampus, and these processes were nestin positive as well. In all of the CA fields of the hippocampus (CA1-CA3) these cells were structurally similar to pyramidal cells. The structural similarities between nestin positive cells and neurons allow regarding this type of cells as newly generated neurons.
- The results indicated that both AKG and ENZ increased hippocampal neurogenesis compared to age matched controls (=“old”) (see
FIG. 1 ). Note in particular, that the combination of AKG+ENZ was even more effective and resulted in a synergistic increase in neurogenesis (AKG+ENZ group was statistically significantly different from both AKG and ENZ groups.) The level of neurogenesis obtained by the combination therapy brought the level of neurogenesis in the old animals to the level of the young animals. - Conclusion: treatment with AKG, ENZ or AKG+ENZ increase neurogenesis. The combination of AKG+ENZ has a synergistic effect.
- During the course of the treatment, the animals were subjected to T-maze test measuring cognitive function and/or memory as specified under Materials and Methods.
- Intriguingly, the results correlate perfectly with the increase in neurogenesis seen in Example 1 (see
FIG. 2 ). This suggests that the neurogenesis resulted in improved cognitive function. - Irrespective of the mechanism, the experiments shows that all of AKG, ENZ and AKG+ENZ improved cognition in old animals, with superior effect from the combination AKG+ENZ.
- Blood samples were analysed to determine effects in blood parameters as detailed under Materials and Methods.
- Results (
FIG. 3 ) showed that while platelet, lymphocyte and monocyte counts were unaffected by the treatments, the eosinophile count was markedly reduced by the combination of AKG+ENZ, but not by AKG by itself or ENZ by itself. Again, the combination of AKG+ENZ showed a synergistic effect. - We have chosen hippocampus as an object of our investigation because it is one of the major components of the brain of humans and other vertebrates. It belongs to the limbic system and plays important roles in the consolidation of information from short-term memory to long-term memory and spatial memory and navigation, emotion and behavior formation, cognitive function. Hippocampus is very vulnerable to different noxious stimuli. Damage to the hippocampus can result from oxygen starvation (hypoxia), encephalitis, medial temporal lobe epilepsy, age-related disorders (Alzheimer's disease) and others. Hippocampus is one of the first regions of the brain to suffer damage; memory loss and disorientation are included among the early symptoms.
- It is worth to notice that hippocampal neurons are arranged in a specific spatial manner, which allows precise estimating of their quantity and quality.
- Taken together, all these features (functional role, high vulnerability, neurons' arrangement) make hippocampus one of the most suitable systems for neurological research.
- There are many diseases which are accompanied with loss of synapses. It is worth to notice that both amount and structure of synapses are impaired in diseases as Alzheimer's disease, stroke, Down syndrome, schizophrenia and traumatic brain injury and stress. Some authors have observed synaptic loss during normal aging.
- Anatomic and functional studies indicate that there is decrease in the human brain volume and weight in individuals ≧60 yr old. It remains controversial whether the loss of brain volume represents neuronal loss, shrinkage, or both. Neuron loss does appear to occur in specific regions of the brain including the hippocampus, cerebral cortex, and amygdala.
- The hippocampus is one of the brain regions that are prominently affected by neurodegeneration and functional decline even in what is still considered “normal aging”. Decreased neuronal amount, a decrease in the number of synaptic connections, intracellular pathology, all these facts suggest that the hippocampal formation may be especially vulnerable to the effects of aging. Morphological studies of the hippocampus in young and old rats have revealed that pyramidal neurons in old rats are smaller and contain fewer dendritic branches and spines. The density of presynaptic terminals per length unit of postsynaptic membrane is also lower.
- Each presynaptic terminal contains hundreds of synaptic vesicles filled with neurotransmitters. Synaptic vesicles are uniform organelles of 40 nm in diameter that constitute the central organelle for release and storage of a neurotransmitter that continuously undergoes an exo-endocytotic cycle. During this cycle vesicles change their positions within a presynaptic terminal and their number as well as spatial arrangement can provide insight into a neurotransmitter turnover.
- When an action potential depolarizes the presynaptic plasma membrane, Ca2+-channels open, and Ca2+ flows into the terminal to trigger the exocytosis of synaptic vesicles, thereby releasing their neurotransmitters into the synaptic cleft.
- The synaptic vesicle cycle consists of exocytosis followed by endocytosis and recycling. Synaptic vesicles are filled with neurotransmitters (NT) by active transport (neurotransmitter uptake) fueled by an electrochemical gradient established by a proton pump that acidifies the vesicle interior (vesicle acidification).
- In preparation to synaptic exocytosis, synaptic vesicles are docked at the active zone, and primed by an ATP-dependent process that renders the vesicles competent to respond to a Ca2+-signal. When an action potential depolarizes the presynaptic membrane, Ca2+ channels open, causing a local increase in intracellular Ca2+ at the active zone that triggers completion of the fusion reaction. Released neurotransmitters then bind to receptors associated with the postsynaptic density (PSD).
- In the middle portion of the CA1 stratum radiatum analyzed in the study, the great majority of synaptic inputs are excitatory and they preferentially terminate on the dendritic shaft. Taking into consideration these aspects, we could focus our analyses on the pool of excitatory CA1 spine synapses.
- The number of synaptic terminals per unit area (100 μm2) in old control animals is three times lower compared with young animals, but after long time treatment with AKG and/or lipase+protease+amylase the number of synaptic contacts increased to 55% (AKG and ENZ) and to 47% (AKG+ENZ) (
FIG. 4 ). The area of synaptic terminals in old animals from all groups was three times greater than in young animals and was practically identical (FIG. 6 ). - Decrease in the number of SV per terminal in old animals compared with younger could also point at impairment of SV recycling process. It has been shown in many studies that synaptic vesicles proteins change their phosphorylation level under physiological and pathological conditions.
- The number of SVs per presynaptic terminal was increased in AKG, ENZ and AKG+ENZ groups of gerbils compared to control old animals (
FIG. 6A ), while the number of SVs per square units (100 μm2) of terminal didn't differ from control, except for group 1 (FIG. 6B ). This difference is due to the fact that the area of synaptic terminals in old animals from all groups was significantly greater than in young animals. - It is worth to notice that the group of young controls represents the physiological state of hippocampus, where synaptogenesis and synthesis of neurotransmitter is neither impaired with aging or some pathology, nor stimulated with any external factors. The number of vesicles in synapse tightly correlates with quantity of neurotransmitter, and this overshoot observed can be explained by stimulated by treatment-induced synthesis of neurotransmitter.
- In the hippocampal synapses of the control old gerbils and of gerbils from groups 1 (AKG) and 2 (Enzymes) changes in spatial distribution of synaptic vesicles including the increase of a distance from a vesicle to the active zone were revealed (
FIG. 7 ). These parameters are significantly lower in gerbils from 3 group (AKG+Enzymes) and are only slightly larger compared to those of young animals. There is a differentiation of synaptic vesicles' pools in dependence of their distance to active zone. The nearest pool of vesicles is designed to immediate release from pre-synaptic terminal and the fastest neurotransmission. Thereby, the reduction of average distance from synaptic vesicle to active zone is the sign of synaptic transmission improvement. - The analysis of vesicle clustering showed that SVs were also separated by larger distance: the average NND value estimated for control old animals was around 30% higher than for young (
FIG. 8 ). Only for the 3 group it was revealed significant decrease of NND compared with control old animals (group 4). Distance to the 1st nearest neighbor of each vesicle was used to measure the tendency of synaptic vesicles to form spatial clusters. Note that vesicle density is number of vesicles per 1 unit area, and the NND parameter is not the average vesicle-to-vesicle distance, but the average distance to the nearest neighbor vesicle, the comparison of these parameters describes the difference of spatial distribution of vesicles in synapse. - The long term treatments caused pronounced structural modifications of the types of synaptic terminals. An important increase in the perforated synapses amount in
group FIG. 9 ) was observed. - Increase in the proportion of perforated synapses is thought to be more efficient in synaptic transmission and can be considered as compensatory mechanism in case of synaptic loss. Perforations have also been correlated with reactive synaptogenesis. Density of perforated synapses is coupled with recognition memory accuracy. Synapse remodeling is known as sign of neurological improvement, whereby the result is supportive for treatment and prophylaxis of neurological disorders in general and Alzheimer's in particular with the effective treatments.
- The present study indicates that morphological alteration of synapses associated with synaptic plasticity might in fact relate to mechanisms of activation under altered physiological and pathological conditions. The parameters of synaptic contacts such as its dimensions, shape, size of presynaptic terminal, SV density and distribution, are all related to the efficacy of the synaptic transmission.
- In summary, we describe here a number of morphological alterations within synaptic networks that are consequences of aging. We found that synaptic contacts are able to manifest modifications under inventive treatment conditions in animals. The decreased density of excitatory synapses in CA1 stratum radiatum of old animals compared with young ones is a sign of recovery after long-term treatment. Potentially deleterious morphological changes in the synapses, such as swelling of synaptic elements, depletion and rearrangement of SV pools, are accompanied by some apparently counter-balancing adaptive modifications: increase in perforations, which presumably could reflect strengthening of synaptic contacts, as well as relative increase in multiple spine boutons, which suggests reactive synaptogenesis.
- In the hippocampal synapses of the old gerbils there are changes in spatial distribution of synaptic vesicles including the increase of a distance from a vesicle to the active zone and to the nearest neighbour vesicle.
- Redistribution of different types of synaptic terminals takes place with the increase in perforated and multiple synapse numbers.
- It is possible that these treatment-induced plastic changes might play a role in the mechanism of improvement of hippocampal neuronal function demonstrated in T-maze.
- A repeat study using aged Mongolian Gerbils in the lines of Example 1 was undertaken, with modification of the enzyme components as specified in Materials and Methods.
- In this experiment, there were two groups, control and treatment with AKG+enzymes. Behavioral analysis with T-maze was performed at 0, 2 and 4 months. As shown in
FIG. 10 , the treatment group improved its performance already at 2 months, with further significant improvement at 4 months, while there was no clear trend for the control group. - The results demonstrate that the results in Example 1 were repeatable with different enzyme preparations and concentrations, that a discernible effect was beginning to show at 2 months of treatment and improvement continued further during 4 months of treatment.
- Statistics was calculated for each animal, both in the control and experimental groups. The difference between the percentage of tasks solved in the first trial and the mean of the percentages of tasks solved in two consequent trials was compared (
FIG. 11 ). The individual animals in the experimental group (group 1) tend to demonstrate an increase of the number of solved tasks per test more often and, in cases where increase is seen, to have greater increases than the animals in the control group. The gerbils in the first group in 67.7% of cases demonstrate improved performance at 2 and 4 months (pooled results) compared to performance compared to performance at baseline. In contrast, only 29% in the control group (Group 2) showed improved performance (FIG. 11 ). - In conclusion animals without any treatment tend to show worse results over time (possibly due to aging) but treatment animals tend to show improved performance over time, demonstrating a cognitive performance-enhancing effect of the treatment.
- The measure allows discrimination of if there are individual shifts in the ability to pass the test over the course of treatment. Since the number of animals is large enough, the U-statistic, the deciding statistic of the Mann-Whitney test, is approximately Gaussian with known parameters, and the probability that either group is described with the same probabilistic law is less than 0.05, which proves that with the confidence level of 0.05 there are qualitative differences between two groups.
- In Example 1. the animals were fed Labofeed B standard chow (from Labofeed company; Andrzej Morawski Feed Production Plant, Kcynia near. Bydgoszcz, Poland) supplemented with test compounds as specified in table 1.
-
TABLE 1 Treatment groups, example 1 Group Additive Age-matched controls (also denoted None C, Contr (old), old controlor C(L)) AKG (also denoted A or old AKG) 1% calcium AKG by weight 1% sodium AKG by weight Total AKG approx. 114 mmol/kg ENZ (also denoted E, Enz or old lipase 600000 USP units/kg Enz) protease 400000 USP units/kg amylase 60000 USP units/kg AKG + ENZ (also denoted A + E, 1% calcium AKG by weight AKG − Enz, old AKG + Enz) 1% sodium AKG by weight lipase 600000 USP units/kg protease 400000 USP units/kg amylase 60000 USP units/kg Young controls (also denoted Y, None Contr (young), Young) - The lipase, protease and amylase were obtained as capsules containing 30,000 USP units/capsule of lipase, 20,000 USP units/capsule of protease, and 3,000 USP units/capsule of amylase) prepared as shown in Table 2. Contents of 20 capsules were mixed per each kg of feed for the ENZ and AKG+ENZ groups.
-
TABLE 2 Capsules with lipase, protease and amylase Amount (USP Amount Component units/capsule) (mg/capsule) Supplier Cat no Burkholderia 30,000 13.1 Sigma Aldrich 534641 cepacia lipase Aspergillus 20,000 14.3 Sigma Aldrich P4032 melleus proteinase Aspergillus 3,000 30.0 Sigma Aldrich 10065 oryzae α- amylase - The capsules also contained filler up to a weight of 200 mg.
- In Example 5, the animals were fed Labofeed B standard chow (from Labofeed company; Andrzej Morawski Feed Production Plant, Kcynia near. Bydgoszcz, Poland) supplemented with test compounds as specified in table 3.
-
TABLE 3 Treatment groups, Example 5 Group Additive Age matched controls None AKG + ENZ (also denoted A + E) 1% calcium AKG by weight 1% sodium AKG by weight Total AKG ca. 114 mmol/kg lipase 200000 USP units/kg protease 750000 USP units/kg amylase 664000 USP units/kg - The enzymes in table 3 are further specified in Table 4.
-
TABLE 4 Specification of lipase, protease and amylase in Example 5 Determined Amount activity (mg/kg Manufacturer Component USP/mg fodder) Supplier designation Burkholderia 400 500 Amano Lipase PS cepacia lipase “Amano” SD Aspergillus 1500 500 Amano Protease DS-K melleus proteinase Aspergillus 3370 16850 Amano Amylase DS oryzae α- amylase - For long-lasting feeding (during 6 month) 75 old male Mongolian gerbils, randomly selected, were used. Those selected were approximately 1.5 years of age at start of the experiment with the mean body weight of 88.0±6 g and 2 years of age at experiment finish. Additionally, 6 male gerbils of “young”-adult (6 month) of age were used for additional “age-control” (denoted “Control (young)”, or “Young” or “Y” in the graphs).
- The gerbils were housed in individual cages and divided into groups receiving different treatments (Table 1). Each cage was equipped with a drinking bottle.
- Gerbils (4-8 per cage) were housed in clear polycarbonate cages (48×27×20 cm) containing corn-cob bedding, paper towels for nesting material, and a tin can (13 cm long×10 cm diameter) for enrichment.
- Food (individual for each group, Tables 1-2) and water were available ad lib, and provided once per day.
- Lights were on from 07.00 to 19.00 h, and room temperature and humidity were maintained at approximately 23° C. and 30-70%, respectively.
- All gerbils were acclimated to cages for a week before the start of the experiment.
- Animal care was similar to Examples 1-4 above with the exception that only two groups were included and treated in accordance with Tables 3-4.
- Control groups comprised 15 animals whereas the age-matched treatment group comprised 16 animals.
- At the end of the experiment, the gerbils were anaesthetized with ketamine (100 mg/kg body weight i/m) and fixed by transcardial perfusion with 4% formaldehyde and 0.25% glutaraldehyde in 0.1M phosphate buffer. After perfusion the brains were isolated and separated for two hemispheres.
- Samplings for immunohistochemistry (one hemisphere of each animal) were postfixed overnight in the same fixative at +4° C. The next day they were cut in 50-μm-thick frontal slices by a vibratome Vibroslice 752M (Campden Instruments Ltd, Great Britain). Brain slices were washed out with 0.1M phosphate buffer pH 7.4 and treated in blocking solution containing 1% normal goat serum and 0.3% Triton X-100. Goat monoclonal anti-nestin antibodies (1:500) (Santa Cruz biotechnology, inc, USA) were used for detection proliferating immature neuronal cells, which express the neural specific intermediate filament nestin. Slices were incubated with primary antibodies during 16 hours at +4° C. After washing slices were incubated with anti-goat secondary antibodies conjugated with Alexa Fluor 647 (1:1000) (Molecular probes, USA) for 1.5 h at room temperature. Then slices were washed out, placed on histological slides and mounted with Fluorescence Mounting Media (Dako, Denmark). Images of hippocampal tissue were taken by confocal FV1000-BX61WI microscope (Olympus, Japan). Qualitative analysis of nestin-positive cells distribution in CA1 hippocampal area was realized for each experimental group.
- All the statistical data were estimated with STATISTICA ver.7.0 (StatSoft, USA). The two-tailed Kolmogorov-Smirnov test was used to assess the differences between samples (p<0.05 was considered to indicate statistical significance). SE (standard error) was used as error bars.
- Gerbils were anaesthetized with sublethal dose of ketamine (100 mg/kg body weight i/m) and fixed by transcardial perfusion with 4% formaldehyde and 0.25% glutaraldehyde in 0.1M phosphate buffer. After perfusion the brains were isolated and separated on two hemispheres.
- One hemisphere of each animal was used for immunohistochemical assay.
- The hippocampus from another hemisphere of each animal was isolated and cut in 400-μm-thick transverse slices by a chopper (McIllwain tissue chopper, Great Britain). Slices were postfixed in fixative solution with 2.5% of glutaraldehyde for 1.5 h and then in 1% OsO4 for 1 h. Tissue slices were then dehydrated in an ascending series of ethanol followed by dry acetone and embedded in EPON resin according to official protocol. The sections were produced by ultramicrotome LKB-8800. Toluidine blue-stained semi-thin (1 μm) sections of hippocampus were used to localize hippocampal CA1 area. For electron microscopy, ultra-thin sections (70 nm) from the middle portion of CA1 stratum pyramidale and stratum radiatum were stained with uranyl acetate and lead citrate.
- The images were taken with JEM-100CX (Jeol, Japan) transmission electron microscope at magnification of ×10 000. Calculations were provided for three animals from each group:
-
- Group 1 (AKG)
- Group 2 (Lipase+protease+amylase)
- Group 3 (AKG+Lipase+protease+amylase)
- Group 4 (old control)
- Group (Young)
- The synaptic terminals of the investigated area revealed high degree of structural plasticity including modifications in the ratio of different forms of synaptic terminals (simple, perforated, multiple boutons). Perforated synapse was defined as a synapse with discontinuous PSD. The synapses with more than one spine contacting the same presynaptic terminal were classified as multiple spine boutons (MSB).
- For the analysis of SV's distribution, x- and y-coordinates of SV profile centers and points tracing the active zone of a synapse were marked with UTHSCSA ImageTool software (
version 3, University of Texas, San Antonio, Tex.; ftp:https://maxrad6.uthscsa.edu) on digitized micrographs, using the point tool. The shortest distance from a SV to the active zone profile (hence forth referred to as an active zone distance (AZD), as well as spatial proximity of SV profiles to each other (referred to as the nearest neighbor distance (NND)) were quantified using the nearest neighbor formalism with LoClust software as described (Nikonenko and Skibo, 2004). - AZD and NND quantities were pooled together per group. For each experimental and control condition, 100 synapses containing SVs have been analyzed. Statistical analysis was performed using Statistica software (
version 5, StatSoft, USA). Values are shown as mean±standard error of the mean (SEM). The two-tailed Kolmogorov-Smirnov test was used to assess the differences between samples (P<0.05 was considered to indicate statistical significance). - Estimations of synapse density were carried out on single photos by counting all asymmetric spine synapses (determined by the presence of a spine head with prominent PSD and docked synaptic vesicles (SV) in the active zone of a presynaptic terminal) on the micrograph surface limited by the counting frame (36 mkm2 test area) followed by calculation of the number of synapses per surface unit area.
- T Maze Spontaneous Alternation is a behavioral test for measuring exploratory behavior in animals, especially rodent models for CNS disorders. This test is based on the willingness of rodents to explore a new environment, i.e. they prefer to visit a new arm of the maze rather than a familiar arm. Many parts of the brain—including the hippocampus, septum, basal forebrain, and prefrontal cortex—are involved in this task.
- This protocol details a method for using a T-maze to assess the cognitive ability of rodents. The T-maze is an elevated or enclosed apparatus in the form of a T placed horizontally. Animals are started from the base of the T and allowed to choose one of the goal arms abutting the other end of the stem. If two trials are given in quick succession, on the second trial the rodent tends to choose the arm not visited before, reflecting memory of the first choice. This is called ‘spontaneous alternation’. Both spontaneous and rewarded alternation are very sensitive to dysfunction of the hippocampus, but other brain structures are also involved. Each trial should be completed in less than 2 min, but the total number of trials required will vary according to statistical and scientific requirements. Alternation reflects the motivation of the animal to explore its environment and locate the presence of resources such as food, water, mates or shelter. Animals do not need to be deprived of such resources to show alternation behavior; in this case it is called ‘spontaneous alternation’.
- Subjects were first placed in the start arm of the T Maze. Upon leaving the start arm, subjects chose between entering either the left or the right goal arm. With repeated trials, the animals showed less of a tendency to enter a previously visited arm. The percentage of alternation (number of turns in each goal arm) and total trial duration were recorded.
- In Example 2, the animals were subjected to the test once.
- In Example 5, the animals were subjected to the test on 4 consecutive days at the start of the experiment, and subsequently subjected to the test on 3 consecutive days after 2 months and 4 months of treatment.
- At the end of the study, hematological parameters were studied using
hematolyzer ABX Micros 60 OT (France) according standard methods. -
TABLE X # A B C 1 1 1 1 2 1 1 2 3 1 1 3 4 1 1 4 5 1 1 5 6 1 1 6 7 1 1 7 8 1 1 8 9 1 1 9 10 1 1 10 11 1 2 1 12 1 2 2 13 1 2 3 14 1 2 4 15 1 2 5 16 1 2 6 17 1 2 7 18 1 2 8 19 1 2 9 20 1 2 10 21 1 3 1 22 1 3 2 23 1 3 3 24 1 3 4 25 1 3 5 26 1 3 6 27 1 3 7 28 1 3 8 29 1 3 9 30 1 3 10 31 1 4 1 32 1 4 2 33 1 4 3 34 1 4 4 35 1 4 5 36 1 4 6 37 1 4 7 38 1 4 8 39 1 4 9 40 1 4 10 41 1 5 1 42 1 5 2 43 1 5 3 44 1 5 4 45 1 5 5 46 1 5 6 47 1 5 7 48 1 5 8 49 1 5 9 50 1 5 10 51 1 6 1 52 1 6 2 53 1 6 3 54 1 6 4 55 1 6 5 56 1 6 6 57 1 6 7 58 1 6 8 59 1 6 9 60 1 6 10 61 1 7 1 62 1 7 2 63 1 7 3 64 1 7 4 65 1 7 5 66 1 7 6 67 1 7 7 68 1 7 8 69 1 7 9 70 1 7 10 71 1 8 1 72 1 8 2 73 1 8 3 74 1 8 4 75 1 8 5 76 1 8 6 77 1 8 7 78 1 8 8 79 1 8 9 80 1 8 10 81 1 9 1 82 1 9 2 83 1 9 3 84 1 9 4 85 1 9 5 86 1 9 6 87 1 9 7 88 1 9 8 89 1 9 9 90 1 9 10 91 1 10 1 92 1 10 2 93 1 10 3 94 1 10 4 95 1 10 5 96 1 10 6 97 1 10 7 98 1 10 8 99 1 10 9 100 1 10 10 101 2 1 1 102 2 1 2 103 2 1 3 104 2 1 4 105 2 1 5 106 2 1 6 107 2 1 7 108 2 1 8 109 2 1 9 110 2 1 10 111 2 2 1 112 2 2 2 113 2 2 3 114 2 2 4 115 2 2 5 116 2 2 6 117 2 2 7 118 2 2 8 119 2 2 9 120 2 2 10 121 2 3 1 122 2 3 2 123 2 3 3 124 2 3 4 125 2 3 5 126 2 3 6 127 2 3 7 128 2 3 8 129 2 3 9 130 2 3 10 131 2 4 1 132 2 4 2 133 2 4 3 134 2 4 4 135 2 4 5 136 2 4 6 137 2 4 7 138 2 4 8 139 2 4 9 140 2 4 10 141 2 5 1 142 2 5 2 143 2 5 3 144 2 5 4 145 2 5 5 146 2 5 6 147 2 5 7 148 2 5 8 149 2 5 9 150 2 5 10 151 2 6 1 152 2 6 2 153 2 6 3 154 2 6 4 155 2 6 5 156 2 6 6 157 2 6 7 158 2 6 8 159 2 6 9 160 2 6 10 161 2 7 1 162 2 7 2 163 2 7 3 164 2 7 4 165 2 7 5 166 2 7 6 167 2 7 7 168 2 7 8 169 2 7 9 170 2 7 10 171 2 8 1 172 2 8 2 173 2 8 3 174 2 8 4 175 2 8 5 176 2 8 6 177 2 8 7 178 2 8 8 179 2 8 9 180 2 8 10 181 2 9 1 182 2 9 2 183 2 9 3 184 2 9 4 185 2 9 5 186 2 9 6 187 2 9 7 188 2 9 8 189 2 9 9 190 2 9 10 191 2 10 1 192 2 10 2 193 2 10 3 194 2 10 4 195 2 10 5 196 2 10 6 197 2 10 7 198 2 10 8 199 2 10 9 200 2 10 10 201 3 1 1 202 3 1 2 203 3 1 3 204 3 1 4 205 3 1 5 206 3 1 6 207 3 1 7 208 3 1 8 209 3 1 9 210 3 1 10 211 3 2 1 212 3 2 2 213 3 2 3 214 3 2 4 215 3 2 5 216 3 2 6 217 3 2 7 218 3 2 8 219 3 2 9 220 3 2 10 221 3 3 1 222 3 3 2 223 3 3 3 224 3 3 4 225 3 3 5 226 3 3 6 227 3 3 7 228 3 3 8 229 3 3 9 230 3 3 10 231 3 4 1 232 3 4 2 233 3 4 3 234 3 4 4 235 3 4 5 236 3 4 6 237 3 4 7 238 3 4 8 239 3 4 9 240 3 4 10 241 3 5 1 242 3 5 2 243 3 5 3 244 3 5 4 245 3 5 5 246 3 5 6 247 3 5 7 248 3 5 8 249 3 5 9 250 3 5 10 251 3 6 1 252 3 6 2 253 3 6 3 254 3 6 4 255 3 6 5 256 3 6 6 257 3 6 7 258 3 6 8 259 3 6 9 260 3 6 10 261 3 7 1 262 3 7 2 263 3 7 3 264 3 7 4 265 3 7 5 266 3 7 6 267 3 7 7 268 3 7 8 269 3 7 9 270 3 7 10 271 3 8 1 272 3 8 2 273 3 8 3 274 3 8 4 275 3 8 5 276 3 8 6 277 3 8 7 278 3 8 8 279 3 8 9 280 3 8 10 281 3 9 1 282 3 9 2 283 3 9 3 284 3 9 4 285 3 9 5 286 3 9 6 287 3 9 7 288 3 9 8 289 3 9 9 290 3 9 10 291 3 10 1 292 3 10 2 293 3 10 3 294 3 10 4 295 3 10 5 296 3 10 6 297 3 10 7 298 3 10 8 299 3 10 9 300 3 10 10 301 4 1 1 302 4 1 2 303 4 1 3 304 4 1 4 305 4 1 5 306 4 1 6 307 4 1 7 308 4 1 8 309 4 1 9 310 4 1 10 311 4 2 1 312 4 2 2 313 4 2 3 314 4 2 4 315 4 2 5 316 4 2 6 317 4 2 7 318 4 2 8 319 4 2 9 320 4 2 10 321 4 3 1 322 4 3 2 323 4 3 3 324 4 3 4 325 4 3 5 326 4 3 6 327 4 3 7 328 4 3 8 329 4 3 9 330 4 3 10 331 4 4 1 332 4 4 2 333 4 4 3 334 4 4 4 335 4 4 5 336 4 4 6 337 4 4 7 338 4 4 8 339 4 4 9 340 4 4 10 341 4 5 1 342 4 5 2 343 4 5 3 344 4 5 4 345 4 5 5 346 4 5 6 347 4 5 7 348 4 5 8 349 4 5 9 350 4 5 10 351 4 6 1 352 4 6 2 353 4 6 3 354 4 6 4 355 4 6 5 356 4 6 6 357 4 6 7 358 4 6 8 359 4 6 9 360 4 6 10 361 4 7 1 362 4 7 2 363 4 7 3 364 4 7 4 365 4 7 5 366 4 7 6 367 4 7 7 368 4 7 8 369 4 7 9 370 4 7 10 371 4 8 1 372 4 8 2 373 4 8 3 374 4 8 4 375 4 8 5 376 4 8 6 377 4 8 7 378 4 8 8 379 4 8 9 380 4 8 10 381 4 9 1 382 4 9 2 383 4 9 3 384 4 9 4 385 4 9 5 386 4 9 6 387 4 9 7 388 4 9 8 389 4 9 9 390 4 9 10 391 4 10 1 392 4 10 2 393 4 10 3 394 4 10 4 395 4 10 5 396 4 10 6 397 4 10 7 398 4 10 8 399 4 10 9 400 4 10 10 401 5 1 1 402 5 1 2 403 5 1 3 404 5 1 4 405 5 1 5 406 5 1 6 407 5 1 7 408 5 1 8 409 5 1 9 410 5 1 10 411 5 2 1 412 5 2 2 413 5 2 3 414 5 2 4 415 5 2 5 416 5 2 6 417 5 2 7 418 5 2 8 419 5 2 9 420 5 2 10 421 5 3 1 422 5 3 2 423 5 3 3 424 5 3 4 425 5 3 5 426 5 3 6 427 5 3 7 428 5 3 8 429 5 3 9 430 5 3 10 431 5 4 1 432 5 4 2 433 5 4 3 434 5 4 4 435 5 4 5 436 5 4 6 437 5 4 7 438 5 4 8 439 5 4 9 440 5 4 10 441 5 5 1 442 5 5 2 443 5 5 3 444 5 5 4 445 5 5 5 446 5 5 6 447 5 5 7 448 5 5 8 449 5 5 9 450 5 5 10 451 5 6 1 452 5 6 2 453 5 6 3 454 5 6 4 455 5 6 5 456 5 6 6 457 5 6 7 458 5 6 8 459 5 6 9 460 5 6 10 461 5 7 1 462 5 7 2 463 5 7 3 464 5 7 4 465 5 7 5 466 5 7 6 467 5 7 7 468 5 7 8 469 5 7 9 470 5 7 10 471 5 8 1 472 5 8 2 473 5 8 3 474 5 8 4 475 5 8 5 476 5 8 6 477 5 8 7 478 5 8 8 479 5 8 9 480 5 8 10 481 5 9 1 482 5 9 2 483 5 9 3 484 5 9 4 485 5 9 5 486 5 9 6 487 5 9 7 488 5 9 8 489 5 9 9 490 5 9 10 491 5 10 1 492 5 10 2 493 5 10 3 494 5 10 4 495 5 10 5 496 5 10 6 497 5 10 7 498 5 10 8 499 5 10 9 500 5 10 10 501 6 1 1 502 6 1 2 503 6 1 3 504 6 1 4 505 6 1 5 506 6 1 6 507 6 1 7 508 6 1 8 509 6 1 9 510 6 1 10 511 6 2 1 512 6 2 2 513 6 2 3 514 6 2 4 515 6 2 5 516 6 2 6 517 6 2 7 518 6 2 8 519 6 2 9 520 6 2 10 521 6 3 1 522 6 3 2 523 6 3 3 524 6 3 4 525 6 3 5 526 6 3 6 527 6 3 7 528 6 3 8 529 6 3 9 530 6 3 10 531 6 4 1 532 6 4 2 533 6 4 3 534 6 4 4 535 6 4 5 536 6 4 6 537 6 4 7 538 6 4 8 539 6 4 9 540 6 4 10 541 6 5 1 542 6 5 2 543 6 5 3 544 6 5 4 545 6 5 5 546 6 5 6 547 6 5 7 548 6 5 8 549 6 5 9 550 6 5 10 551 6 6 1 552 6 6 2 553 6 6 3 554 6 6 4 555 6 6 5 556 6 6 6 557 6 6 7 558 6 6 8 559 6 6 9 560 6 6 10 561 6 7 1 562 6 7 2 563 6 7 3 564 6 7 4 565 6 7 5 566 6 7 6 567 6 7 7 568 6 7 8 569 6 7 9 570 6 7 10 571 6 8 1 572 6 8 2 573 6 8 3 574 6 8 4 575 6 8 5 576 6 8 6 577 6 8 7 578 6 8 8 579 6 8 9 580 6 8 10 581 6 9 1 582 6 9 2 583 6 9 3 584 6 9 4 585 6 9 5 586 6 9 6 587 6 9 7 588 6 9 8 589 6 9 9 590 6 9 10 591 6 10 1 592 6 10 2 593 6 10 3 594 6 10 4 595 6 10 5 596 6 10 6 597 6 10 7 598 6 10 8 599 6 10 9 600 6 10 10 601 7 1 1 602 7 1 2 603 7 1 3 604 7 1 4 605 7 1 5 606 7 1 6 607 7 1 7 608 7 1 8 609 7 1 9 610 7 1 10 611 7 2 1 612 7 2 2 613 7 2 3 614 7 2 4 615 7 2 5 616 7 2 6 617 7 2 7 618 7 2 8 619 7 2 9 620 7 2 10 621 7 3 1 622 7 3 2 623 7 3 3 624 7 3 4 625 7 3 5 626 7 3 6 627 7 3 7 628 7 3 8 629 7 3 9 630 7 3 10 631 7 4 1 632 7 4 2 633 7 4 3 634 7 4 4 635 7 4 5 636 7 4 6 637 7 4 7 638 7 4 8 639 7 4 9 640 7 4 10 641 7 5 1 642 7 5 2 643 7 5 3 644 7 5 4 645 7 5 5 646 7 5 6 647 7 5 7 648 7 5 8 649 7 5 9 650 7 5 10 651 7 6 1 652 7 6 2 653 7 6 3 654 7 6 4 655 7 6 5 656 7 6 6 657 7 6 7 658 7 6 8 659 7 6 9 660 7 6 10 661 7 7 1 662 7 7 2 663 7 7 3 664 7 7 4 665 7 7 5 666 7 7 6 667 7 7 7 668 7 7 8 669 7 7 9 670 7 7 10 671 7 8 1 672 7 8 2 673 7 8 3 674 7 8 4 675 7 8 5 676 7 8 6 677 7 8 7 678 7 8 8 679 7 8 9 680 7 8 10 681 7 9 1 682 7 9 2 683 7 9 3 684 7 9 4 685 7 9 5 686 7 9 6 687 7 9 7 688 7 9 8 689 7 9 9 690 7 9 10 691 7 10 1 692 7 10 2 693 7 10 3 694 7 10 4 695 7 10 5 696 7 10 6 697 7 10 7 698 7 10 8 699 7 10 9 700 7 10 10 701 8 1 1 702 8 1 2 703 8 1 3 704 8 1 4 705 8 1 5 706 8 1 6 707 8 1 7 708 8 1 8 709 8 1 9 710 8 1 10 711 8 2 1 712 8 2 2 713 8 2 3 714 8 2 4 715 8 2 5 716 8 2 6 717 8 2 7 718 8 2 8 719 8 2 9 720 8 2 10 721 8 3 1 722 8 3 2 723 8 3 3 724 8 3 4 725 8 3 5 726 8 3 6 727 8 3 7 728 8 3 8 729 8 3 9 730 8 3 10 731 8 4 1 732 8 4 2 733 8 4 3 734 8 4 4 735 8 4 5 736 8 4 6 737 8 4 7 738 8 4 8 739 8 4 9 740 8 4 10 741 8 5 1 742 8 5 2 743 8 5 3 744 8 5 4 745 8 5 5 746 8 5 6 747 8 5 7 748 8 5 8 749 8 5 9 750 8 5 10 751 8 6 1 752 8 6 2 753 8 6 3 754 8 6 4 755 8 6 5 756 8 6 6 757 8 6 7 758 8 6 8 759 8 6 9 760 8 6 10 761 8 7 1 762 8 7 2 763 8 7 3 764 8 7 4 765 8 7 5 766 8 7 6 767 8 7 7 768 8 7 8 769 8 7 9 770 8 7 10 771 8 8 1 772 8 8 2 773 8 8 3 774 8 8 4 775 8 8 5 776 8 8 6 777 8 8 7 778 8 8 8 779 8 8 9 780 8 8 10 781 8 9 1 782 8 9 2 783 8 9 3 784 8 9 4 785 8 9 5 786 8 9 6 787 8 9 7 788 8 9 8 789 8 9 9 790 8 9 10 791 8 10 1 792 8 10 2 793 8 10 3 794 8 10 4 795 8 10 5 796 8 10 6 797 8 10 7 798 8 10 8 799 8 10 9 800 8 10 10 801 9 1 1 802 9 1 2 803 9 1 3 804 9 1 4 805 9 1 5 806 9 1 6 807 9 1 7 808 9 1 8 809 9 1 9 810 9 1 10 811 9 2 1 812 9 2 2 813 9 2 3 814 9 2 4 815 9 2 5 816 9 2 6 817 9 2 7 818 9 2 8 819 9 2 9 820 9 2 10 821 9 3 1 822 9 3 2 823 9 3 3 824 9 3 4 825 9 3 5 826 9 3 6 827 9 3 7 828 9 3 8 829 9 3 9 830 9 3 10 831 9 4 1 832 9 4 2 833 9 4 3 834 9 4 4 835 9 4 5 836 9 4 6 837 9 4 7 838 9 4 8 839 9 4 9 840 9 4 10 841 9 5 1 842 9 5 2 843 9 5 3 844 9 5 4 845 9 5 5 846 9 5 6 847 9 5 7 848 9 5 8 849 9 5 9 850 9 5 10 851 9 6 1 852 9 6 2 853 9 6 3 854 9 6 4 855 9 6 5 856 9 6 6 857 9 6 7 858 9 6 8 859 9 6 9 860 9 6 10 861 9 7 1 862 9 7 2 863 9 7 3 864 9 7 4 865 9 7 5 866 9 7 6 867 9 7 7 868 9 7 8 869 9 7 9 870 9 7 10 871 9 8 1 872 9 8 2 873 9 8 3 874 9 8 4 875 9 8 5 876 9 8 6 877 9 8 7 878 9 8 8 879 9 8 9 880 9 8 10 881 9 9 1 882 9 9 2 883 9 9 3 884 9 9 4 885 9 9 5 886 9 9 6 887 9 9 7 888 9 9 8 889 9 9 9 890 9 9 10 891 9 10 1 892 9 10 2 893 9 10 3 894 9 10 4 895 9 10 5 896 9 10 6 897 9 10 7 898 9 10 8 899 9 10 9 900 9 10 10 901 10 1 1 902 10 1 2 903 10 1 3 904 10 1 4 905 10 1 5 906 10 1 6 907 10 1 7 908 10 1 8 909 10 1 9 910 10 1 10 911 10 2 1 912 10 2 2 913 10 2 3 914 10 2 4 915 10 2 5 916 10 2 6 917 10 2 7 918 10 2 8 919 10 2 9 920 10 2 10 921 10 3 1 922 10 3 2 923 10 3 3 924 10 3 4 925 10 3 5 926 10 3 6 927 10 3 7 928 10 3 8 929 10 3 9 930 10 3 10 931 10 4 1 932 10 4 2 933 10 4 3 934 10 4 4 935 10 4 5 936 10 4 6 937 10 4 7 938 10 4 8 939 10 4 9 940 10 4 10 941 10 5 1 942 10 5 2 943 10 5 3 944 10 5 4 945 10 5 5 946 10 5 6 947 10 5 7 948 10 5 8 949 10 5 9 950 10 5 10 951 10 6 1 952 10 6 2 953 10 6 3 954 10 6 4 955 10 6 5 956 10 6 6 957 10 6 7 958 10 6 8 959 10 6 9 960 10 6 10 961 10 7 1 962 10 7 2 963 10 7 3 964 10 7 4 965 10 7 5 966 10 7 6 967 10 7 7 968 10 7 8 969 10 7 9 970 10 7 10 971 10 8 1 972 10 8 2 973 10 8 3 974 10 8 4 975 10 8 5 976 10 8 6 977 10 8 7 978 10 8 8 979 10 8 9 980 10 8 10 981 10 9 1 982 10 9 2 983 10 9 3 984 10 9 4 985 10 9 5 986 10 9 6 987 10 9 7 988 10 9 8 989 10 9 9 990 10 9 10 991 10 10 1 992 10 10 2 993 10 10 3 994 10 10 4 995 10 10 5 996 10 10 6 997 10 10 7 998 10 10 8 999 10 10 9
Claims (2)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/447,974 US20170333372A1 (en) | 2012-09-19 | 2017-03-02 | Compositions for improvement of brain function |
| US17/163,065 US20210154160A1 (en) | 2012-09-19 | 2021-01-29 | Method of Treating a Disorder of Cognitive Performance or Memory |
| US18/494,126 US20240065993A1 (en) | 2012-09-19 | 2023-10-25 | Methods of treatment for functional decline in hippocampus, for improving recognition memory accuracy, and for improving hippocampal neuronal function |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261703163P | 2012-09-19 | 2012-09-19 | |
| SE1200567-4 | 2012-09-19 | ||
| SE1200567 | 2012-09-19 | ||
| PCT/SE2013/051098 WO2014046603A1 (en) | 2012-09-19 | 2013-09-19 | Compositions for improvement of brain function |
| US201514428841A | 2015-03-17 | 2015-03-17 | |
| US15/447,974 US20170333372A1 (en) | 2012-09-19 | 2017-03-02 | Compositions for improvement of brain function |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/428,841 Division US9592211B2 (en) | 2012-09-19 | 2013-09-19 | Compositions for improvement of brain function |
| PCT/SE2013/051098 Division WO2014046603A1 (en) | 2012-09-19 | 2013-09-19 | Compositions for improvement of brain function |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/163,065 Division US20210154160A1 (en) | 2012-09-19 | 2021-01-29 | Method of Treating a Disorder of Cognitive Performance or Memory |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20170333372A1 true US20170333372A1 (en) | 2017-11-23 |
Family
ID=49304297
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/428,841 Active US9592211B2 (en) | 2012-09-19 | 2013-09-19 | Compositions for improvement of brain function |
| US15/447,974 Abandoned US20170333372A1 (en) | 2012-09-19 | 2017-03-02 | Compositions for improvement of brain function |
| US17/163,065 Abandoned US20210154160A1 (en) | 2012-09-19 | 2021-01-29 | Method of Treating a Disorder of Cognitive Performance or Memory |
| US18/494,126 Pending US20240065993A1 (en) | 2012-09-19 | 2023-10-25 | Methods of treatment for functional decline in hippocampus, for improving recognition memory accuracy, and for improving hippocampal neuronal function |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/428,841 Active US9592211B2 (en) | 2012-09-19 | 2013-09-19 | Compositions for improvement of brain function |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/163,065 Abandoned US20210154160A1 (en) | 2012-09-19 | 2021-01-29 | Method of Treating a Disorder of Cognitive Performance or Memory |
| US18/494,126 Pending US20240065993A1 (en) | 2012-09-19 | 2023-10-25 | Methods of treatment for functional decline in hippocampus, for improving recognition memory accuracy, and for improving hippocampal neuronal function |
Country Status (13)
| Country | Link |
|---|---|
| US (4) | US9592211B2 (en) |
| EP (2) | EP2897604B1 (en) |
| JP (2) | JP6263540B2 (en) |
| KR (1) | KR102198633B1 (en) |
| CN (1) | CN104797248B (en) |
| AU (1) | AU2013318686B2 (en) |
| CA (1) | CA2884592C (en) |
| DK (2) | DK2897604T3 (en) |
| ES (1) | ES2681343T3 (en) |
| FI (1) | FI3398594T3 (en) |
| HK (1) | HK1207566A1 (en) |
| PL (1) | PL2897604T3 (en) |
| WO (1) | WO2014046603A1 (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FI3398594T3 (en) * | 2012-09-19 | 2024-09-23 | Grespo Ab | Compositions for improvement of brain function |
| WO2016083399A1 (en) * | 2014-11-24 | 2016-06-02 | Forschungsgesellschaft Für Arbeitsphysiologie Und Arbeitsschutz E. V. | Alpha-ketoglutarate in combination with glutamate dehydrogenase for treating hyperammonemia |
| WO2019038655A1 (en) * | 2017-08-21 | 2019-02-28 | The Regents Of The University Of California | Compositions and methods for treating neurodegenerative diseases |
| WO2020068705A1 (en) | 2018-09-25 | 2020-04-02 | Ponce De Leon Health Designated Activity Company | Process of making calcium alpha-ketoglutarate |
| WO2022040022A1 (en) * | 2020-08-18 | 2022-02-24 | St. Jude Children's Research Hospital, Inc. | Use of amylase or maltose to treat or prevent neurodegeneration |
| WO2023200281A1 (en) * | 2022-04-15 | 2023-10-19 | 재단법인대구경북과학기술원 | Unagglomerated dissolving composition for diagnosis of alzheimer's disease or mild cognitive impairment, and diagnostic method using same |
| CN117530940A (en) * | 2023-10-11 | 2024-02-09 | 四川大学华西第二医院 | Application of alpha-ketoglutarate in preparation of medicines for promoting myelin repair and improving neuroinflammation |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2711436B2 (en) | 1995-02-22 | 1998-02-10 | マルホ株式会社 | Allergy remedies and allergic foods |
| US6051220A (en) | 1995-05-31 | 2000-04-18 | Medzyme N.V. And Simon Lodewijk Scharpe | Composition to improve digestibility and utilization of nutrients |
| US6372473B1 (en) | 1997-05-28 | 2002-04-16 | Human Genome Sciences, Inc. | Tissue plasminogen activator-like protease |
| US6537969B1 (en) | 1997-10-24 | 2003-03-25 | John P. Blass | Nutritional supplement for cerebral metabolic insufficiencies |
| US20010046493A1 (en) | 2000-02-24 | 2001-11-29 | Alex Margolin | Lipase-containing composition and methods of use thereof |
| US20070053895A1 (en) * | 2000-08-14 | 2007-03-08 | Fallon Joan M | Method of treating and diagnosing parkinsons disease and related dysautonomic disorders |
| ATE292985T1 (en) | 2001-01-09 | 2005-04-15 | Louis Johan Wagenaar | USE OF DEXPANTHENOL IN CONTACT LENS CARE COMPOSITIONS |
| AR032392A1 (en) | 2001-01-19 | 2003-11-05 | Solvay Pharm Gmbh | ENZYMES MIX, PHARMACEUTICAL PREPARATION AND USE OF PREPARED SAID. |
| US6689810B2 (en) | 2001-08-21 | 2004-02-10 | Cellular Sciences, Inc. | Method for treating pulmonary disease states in mammals by altering indigenous in vivo levels of nitric oxide |
| US8076373B2 (en) | 2001-09-11 | 2011-12-13 | North Cell Pharmacetical | Method for treating mammalian diseases and injuries caused by the over-expression of peroxynitrite |
| US7122578B2 (en) | 2001-09-11 | 2006-10-17 | Alain Martin | Method and composition for treating mammalian diseases and injuries which cause pain, erythema, swelling, crusting, ischemia scarring and excess white blood cell infiltration |
| US7241456B2 (en) | 2002-10-25 | 2007-07-10 | Australian Importers Ltd. | Formulations for topical delivery of bioactive substances and methods for their use |
| JP4265997B2 (en) | 2004-07-14 | 2009-05-20 | 富士通マイクロエレクトロニクス株式会社 | Semiconductor device and manufacturing method thereof |
| PL369552A1 (en) * | 2004-08-12 | 2006-02-20 | Sgp & Sons Ab | Pharmaceutical or/and nutrient or/and laboratory preparation for increasing, supporting the functioning of nerve cells and nervous system as well as application of pharmaceutical or/and nutrient or/and laboratory preparation in protection of the functions |
| DK1809320T3 (en) | 2004-10-14 | 2010-11-15 | Cystic Fibrosis Foundation The | Preparations containing lipase, protease and amylase for the treatment of pancreatic insufficiency |
| AU2005316295B2 (en) | 2004-12-17 | 2012-03-22 | Alan B. Cash | Method for extending lifespan and delaying the onset of age-related disease |
| KR20080102372A (en) * | 2006-01-19 | 2008-11-25 | 엔트레스 에이비 | Method of diagnosis and method of treatment |
| PL226695B1 (en) | 2006-07-03 | 2017-08-31 | Danuta Kruszewska | Agent for preventing and/or inhibiting the colonization of Helicobater pylori and its application |
| PL1915913T3 (en) * | 2006-10-23 | 2016-06-30 | Nestec Sa | Taste and flavour modulation by biotransformation in milk products |
| TWI609693B (en) * | 2007-02-20 | 2018-01-01 | 艾泰醫藥有限公司 | Stable digestive enzyme compositions |
| EP2318035B1 (en) | 2008-07-01 | 2019-06-12 | Curemark, Llc | Methods and compositions for the treatment of symptoms of neurological and mental health disorders |
| US20100092447A1 (en) * | 2008-10-03 | 2010-04-15 | Fallon Joan M | Methods and compositions for the treatment of symptoms of prion diseases |
| SG10201402289VA (en) | 2009-05-11 | 2014-07-30 | Berg Llc | Methods for treatment of disease using an epimetabolic shifter (coenzyme q10) |
| CN101884631A (en) | 2009-05-12 | 2010-11-17 | 复旦大学 | Anti-tumor medicament based on alpha ketoglutaric acid and derivatives thereof |
| CN102010890B (en) * | 2009-09-08 | 2013-05-22 | 北京迪迈医学诊断技术有限公司 | Kit for determining mitochondria aspartate aminotransferase |
| US20110064712A1 (en) * | 2009-09-16 | 2011-03-17 | Daniel Moses Amato | Dietary Supplement Compositions and Methods of Making and Using the Same |
| WO2011163319A2 (en) | 2010-06-22 | 2011-12-29 | Cash Alan B | Activation of amp-protein activated kinase by oxaloacetate compounds |
| CA2808978A1 (en) | 2010-08-23 | 2012-03-01 | Shmuel Ben-Sasson | Compositions for gastric delivery of active agents |
| WO2012078798A1 (en) | 2010-12-07 | 2012-06-14 | Bananalogix, Inc. | Methods of processing organic matter and compositions thereof |
| FI3398594T3 (en) * | 2012-09-19 | 2024-09-23 | Grespo Ab | Compositions for improvement of brain function |
-
2013
- 2013-09-19 FI FIEP18178948.8T patent/FI3398594T3/en active
- 2013-09-19 CA CA2884592A patent/CA2884592C/en active Active
- 2013-09-19 WO PCT/SE2013/051098 patent/WO2014046603A1/en active Application Filing
- 2013-09-19 AU AU2013318686A patent/AU2013318686B2/en active Active
- 2013-09-19 CN CN201380058821.4A patent/CN104797248B/en active Active
- 2013-09-19 JP JP2015533015A patent/JP6263540B2/en active Active
- 2013-09-19 ES ES13773433.1T patent/ES2681343T3/en active Active
- 2013-09-19 KR KR1020157009161A patent/KR102198633B1/en active IP Right Grant
- 2013-09-19 EP EP13773433.1A patent/EP2897604B1/en active Active
- 2013-09-19 EP EP18178948.8A patent/EP3398594B1/en active Active
- 2013-09-19 DK DK13773433.1T patent/DK2897604T3/en active
- 2013-09-19 DK DK18178948.8T patent/DK3398594T3/en active
- 2013-09-19 PL PL13773433T patent/PL2897604T3/en unknown
- 2013-09-19 US US14/428,841 patent/US9592211B2/en active Active
-
2015
- 2015-08-24 HK HK15108187.9A patent/HK1207566A1/en unknown
-
2017
- 2017-03-02 US US15/447,974 patent/US20170333372A1/en not_active Abandoned
- 2017-10-25 JP JP2017205991A patent/JP2018048175A/en active Pending
-
2021
- 2021-01-29 US US17/163,065 patent/US20210154160A1/en not_active Abandoned
-
2023
- 2023-10-25 US US18/494,126 patent/US20240065993A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| DK2897604T3 (en) | 2018-08-06 |
| CA2884592C (en) | 2021-08-31 |
| EP3398594A1 (en) | 2018-11-07 |
| US20150297544A1 (en) | 2015-10-22 |
| FI3398594T3 (en) | 2024-09-23 |
| KR20150058292A (en) | 2015-05-28 |
| DK3398594T3 (en) | 2024-09-23 |
| CN104797248B (en) | 2017-10-31 |
| WO2014046603A1 (en) | 2014-03-27 |
| EP3398594B1 (en) | 2024-07-10 |
| EP2897604B1 (en) | 2018-07-04 |
| KR102198633B1 (en) | 2021-01-05 |
| EP2897604A1 (en) | 2015-07-29 |
| AU2013318686A1 (en) | 2015-04-02 |
| JP2018048175A (en) | 2018-03-29 |
| US9592211B2 (en) | 2017-03-14 |
| JP6263540B2 (en) | 2018-01-17 |
| US20210154160A1 (en) | 2021-05-27 |
| ES2681343T3 (en) | 2018-09-12 |
| HK1207566A1 (en) | 2016-02-05 |
| CN104797248A (en) | 2015-07-22 |
| AU2013318686B2 (en) | 2017-11-30 |
| US20240065993A1 (en) | 2024-02-29 |
| PL2897604T3 (en) | 2018-10-31 |
| CA2884592A1 (en) | 2014-03-27 |
| JP2015529245A (en) | 2015-10-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240065993A1 (en) | Methods of treatment for functional decline in hippocampus, for improving recognition memory accuracy, and for improving hippocampal neuronal function | |
| Wu et al. | BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in a transgenic mouse model of Alzheimer's disease | |
| Haji et al. | TNF-α-mediated anxiety in a mouse model of multiple sclerosis | |
| Mouton-Liger et al. | PINK1/Parkin-dependent mitochondrial surveillance: from pleiotropy to Parkinson's disease | |
| Berríos-Cárcamo et al. | Oxidative stress and neuroinflammation as a pivot in drug abuse. A focus on the therapeutic potential of antioxidant and anti-inflammatory agents and biomolecules | |
| Baltan et al. | Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity | |
| Dexter et al. | Parkinson disease: from pathology to molecular disease mechanisms | |
| Labrie et al. | The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia | |
| Micsenyi et al. | Lysosomal membrane permeability stimulates protein aggregate formation in neurons of a lysosomal disease | |
| Bonfiglio et al. | Prophylactic versus therapeutic fingolimod: restoration of presynaptic defects in mice suffering from experimental autoimmune encephalomyelitis | |
| de Bartolomeis et al. | Rational and translational implications of D-amino acids for treatment-resistant schizophrenia: from neurobiology to the clinics | |
| Wang et al. | Nicotinamide adenine dinucleotide prevents neuroaxonal degeneration induced by manganese in cochlear organotypic cultures | |
| Cole-Edwards et al. | c-Jun N-terminal kinase activation responses induced by hippocampal kindling are mediated by reactive astrocytes | |
| Zhu et al. | Neuronal nitric oxide synthase contributes to pentylenetetrazole-kindling-induced hippocampal neurogenesis | |
| Jin et al. | Lanthanum damages learning and memory and suppresses astrocyte–neuron lactate shuttle in rat hippocampus | |
| Ahmed et al. | Psychopharmacological treatment of neurocognitive deficits in people with schizophrenia: a review of old and new targets | |
| Anashkina et al. | Molecular mechanisms of aberrant neuroplasticity in autism spectrum disorders | |
| Peng et al. | Thymopentin (TP-5) prevents lipopolysaccharide-induced neuroinflammation and dopaminergic neuron injury by inhibiting the NF-κB/NLRP3 signaling pathway | |
| Karanikas | The Gordian knot of the immune-redox systems’ interactions in psychosis | |
| LIBERONA R et al. | Neurobiological Aspects of Rett Syndrome | |
| Imai et al. | OPEN ACCESS EDITED BY | |
| Wei et al. | Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) via regulating the synaptic plasticity in hippocampus | |
| Sirinupong et al. | Effect of Edible Bird’s Nest on Protecting against Cognitive Deficit and Ameliorating Beta‐Amyloid in Hippocampal Rats’ Model of Cerebral Ischemia | |
| Venkatesh | The Tam | |
| WO2022217035A1 (en) | Activators of integrated stress response pathway for protection against ferroptosis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: GRESPO AB, SWEDEN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:PIERZYNOWSKI, STEFAN;REEL/FRAME:041862/0422 Effective date: 20150512 |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: AWAITING RESPONSE FOR INFORMALITY, FEE DEFICIENCY OR CRF ACTION |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: DOCKETED NEW CASE - READY FOR EXAMINATION |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |
|
| STCC | Information on status: application revival |
Free format text: WITHDRAWN ABANDONMENT, AWAITING EXAMINER ACTION |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: FINAL REJECTION MAILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE AFTER FINAL ACTION FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: ADVISORY ACTION MAILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: DOCKETED NEW CASE - READY FOR EXAMINATION |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |