US20150285770A1 - Jet assembly for use in detectors and other devices - Google Patents
Jet assembly for use in detectors and other devices Download PDFInfo
- Publication number
- US20150285770A1 US20150285770A1 US14/615,207 US201514615207A US2015285770A1 US 20150285770 A1 US20150285770 A1 US 20150285770A1 US 201514615207 A US201514615207 A US 201514615207A US 2015285770 A1 US2015285770 A1 US 2015285770A1
- Authority
- US
- United States
- Prior art keywords
- flow path
- fluid flow
- substantially inert
- flame
- examples
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 239000012530 fluid Substances 0.000 claims abstract description 224
- 239000000463 material Substances 0.000 claims abstract description 174
- 230000003197 catalytic effect Effects 0.000 claims description 138
- 239000007769 metal material Substances 0.000 claims description 124
- 238000004587 chromatography analysis Methods 0.000 claims description 104
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 96
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 53
- 229910044991 metal oxide Inorganic materials 0.000 claims description 53
- 150000004706 metal oxides Chemical class 0.000 claims description 53
- 229910052719 titanium Inorganic materials 0.000 claims description 53
- 239000010936 titanium Substances 0.000 claims description 53
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 claims description 48
- 229910052804 chromium Inorganic materials 0.000 claims description 48
- 239000011651 chromium Substances 0.000 claims description 48
- 229910052759 nickel Inorganic materials 0.000 claims description 48
- 229910052782 aluminium Inorganic materials 0.000 claims description 46
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 46
- 229910052727 yttrium Inorganic materials 0.000 claims description 46
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 claims description 46
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims description 44
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 claims description 44
- SIWVEOZUMHYXCS-UHFFFAOYSA-N oxo(oxoyttriooxy)yttrium Chemical compound O=[Y]O[Y]=O SIWVEOZUMHYXCS-UHFFFAOYSA-N 0.000 claims description 44
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 claims description 44
- 238000006555 catalytic reaction Methods 0.000 claims description 41
- 238000000034 method Methods 0.000 claims description 40
- 238000000576 coating method Methods 0.000 abstract description 33
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 abstract description 24
- 239000011248 coating agent Substances 0.000 abstract description 21
- 230000000712 assembly Effects 0.000 abstract description 9
- 238000000429 assembly Methods 0.000 abstract description 9
- 229910052751 metal Inorganic materials 0.000 abstract description 6
- 239000002184 metal Substances 0.000 abstract description 6
- 239000000377 silicon dioxide Substances 0.000 abstract description 5
- 229910045601 alloy Inorganic materials 0.000 description 119
- 239000000956 alloy Substances 0.000 description 119
- 239000011521 glass Substances 0.000 description 63
- 229910000856 hastalloy Inorganic materials 0.000 description 59
- 239000000523 sample Substances 0.000 description 51
- 229910001220 stainless steel Inorganic materials 0.000 description 46
- 239000010935 stainless steel Substances 0.000 description 45
- 229910001026 inconel Inorganic materials 0.000 description 37
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 32
- 229910000037 hydrogen sulfide Inorganic materials 0.000 description 30
- 239000005350 fused silica glass Substances 0.000 description 15
- 239000007789 gas Substances 0.000 description 14
- 238000006243 chemical reaction Methods 0.000 description 12
- 238000011084 recovery Methods 0.000 description 12
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 8
- 230000008901 benefit Effects 0.000 description 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 7
- 230000008878 coupling Effects 0.000 description 7
- 238000010168 coupling process Methods 0.000 description 7
- 238000005859 coupling reaction Methods 0.000 description 7
- 230000004044 response Effects 0.000 description 7
- 239000001257 hydrogen Substances 0.000 description 6
- 229910052739 hydrogen Inorganic materials 0.000 description 6
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 5
- 239000012491 analyte Substances 0.000 description 5
- BHEPBYXIRTUNPN-UHFFFAOYSA-N hydridophosphorus(.) (triplet) Chemical compound [PH] BHEPBYXIRTUNPN-UHFFFAOYSA-N 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 229910052717 sulfur Inorganic materials 0.000 description 5
- 239000011593 sulfur Substances 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 238000007747 plating Methods 0.000 description 4
- 238000005070 sampling Methods 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 229930192474 thiophene Natural products 0.000 description 4
- 229910000990 Ni alloy Inorganic materials 0.000 description 3
- 238000005219 brazing Methods 0.000 description 3
- 239000000788 chromium alloy Substances 0.000 description 3
- 238000004817 gas chromatography Methods 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 229910000623 nickel–chromium alloy Inorganic materials 0.000 description 3
- 238000003466 welding Methods 0.000 description 3
- 229910000599 Cr alloy Inorganic materials 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 239000012159 carrier gas Substances 0.000 description 2
- 229940060799 clarus Drugs 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 229910017464 nitrogen compound Inorganic materials 0.000 description 2
- 150000002830 nitrogen compounds Chemical class 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 229910000521 B alloy Inorganic materials 0.000 description 1
- 229910001339 C alloy Inorganic materials 0.000 description 1
- 230000005526 G1 to G0 transition Effects 0.000 description 1
- 229910001199 N alloy Inorganic materials 0.000 description 1
- 229910000796 S alloy Inorganic materials 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 229910001080 W alloy Inorganic materials 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000000567 combustion gas Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000005264 electron capture Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 239000003566 sealing material Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 150000003464 sulfur compounds Chemical class 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 239000012207 thread-locking agent Substances 0.000 description 1
- 150000003606 tin compounds Chemical class 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/64—Electrical detectors
- G01N30/68—Flame ionisation detectors
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/64—Electrical detectors
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/74—Optical detectors
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/22—Fuels; Explosives
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/64—Electrical detectors
- G01N2030/685—Electrical detectors flame photometry
Definitions
- Certain embodiments herein are directed to a jet assembly that includes a substantially inert fluid flow path.
- certain embodiments are directed to a flame photometric detector jet assembly that includes a substantially inert fluid flow path.
- chromatography systems use detectors that burn a sample in a flame.
- the sample can react with hot surfaces in the flame jet assembly, which can cause the analyte to render it difficult or impossible to detect certain analytes in the sample.
- a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert material is provided.
- the fluid flow path comprises a substantially inert metal material.
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the substantially inert metal material is present in a major amount. In other embodiments, the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof. In additional embodiments, the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In some embodiments, the substantially inert metal material comprises nickel. In other embodiments, the substantially inert metal material is a Hastelloy® alloy. In some embodiments, the substantially inert metal material comprises chromium. In certain embodiments, the substantially inert metal material is an Inconel® alloy. In additional embodiments, the substantially inert metal material is present in a non-coated form. In some embodiments, the substantially inert metal material is in a tube that is integral to the housing.
- a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic material present in an effective amount to deter catalysis in the fluid flow path is described.
- the fluid flow path comprises a non-catalytic metal material.
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the non-catalytic metal material is present in a major amount.
- the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof.
- the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the non-catalytic metal material comprises nickel.
- the non-catalytic metal material is a Hastelloy® alloy.
- the non-catalytic metal material comprises chromium.
- the non-catalytic metal material is an Inconel® alloy.
- the non-catalytic metal material is present in a non-coated form.
- the non-catalytic metal material is in a tube that is integral to the housing.
- a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic material present in an effective amount to deter catalysis in the fluid flow path is disclosed.
- the fluid flow path comprises a non-catalytic metal material.
- the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof. In other embodiments, the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional embodiments, the non-catalytic metal material comprises nickel. In further embodiments, the non-catalytic metal material is a Hastelloy® alloy. In some embodiments, the non-catalytic metal material comprises chromium. In some examples, the non-catalytic metal material is an Inconel® alloy. In additional examples, the non-catalytic metal material is present in a non-coated form. In further examples, the second tube is longer than the first tube to fluidically couple to the chromatography column. In other examples, the first tube comprises a non-catalytic material.
- a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert material is provided.
- the substantially inert material is a substantially inert metal material.
- the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof. In other examples, the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional examples, the substantially inert metal material comprises nickel. In certain examples, the substantially inert metal material is a Hastelloy® alloy. In further examples, the substantially inert metal material comprises chromium. In other examples, the substantially inert metal material is an Inconel® alloy. In some examples, the substantially inert metal material is present in a non-coated form. In additional examples, the second tube is longer than the first tube to fluidically couple to the chromatography column. In further examples, the first tube comprises a substantially inert metal material.
- a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis is described.
- the non-catalytic, non-glass material comprises titanium, aluminum, yttrium or combinations thereof. In other embodiments, the non-catalytic, non-glass material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional embodiments, the non-catalytic, non-glass material comprises nickel. In further embodiments, the non-catalytic, non-glass material is a Hastelloy® alloy. In some embodiments, the non-catalytic, non-glass material comprises chromium. In other embodiments, the non-catalytic, non-glass material is an Inconel® alloy. In additional embodiments, the non-catalytic, non-glass material is present in a non-coated form. In some embodiments, the non-catalytic, non-glass material is in a tube that is integral to the housing. In other embodiments, the housing is configured as a first tube.
- a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material is disclosed.
- the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium or combinations thereof. In other examples, the substantially inert non-glass, non-stainless steel material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional examples, the substantially inert non-glass, non-stainless steel material comprises nickel. In further examples, the substantially inert non-glass, non-stainless steel material is a Hastelloy® alloy. In some examples, the substantially inert non-glass, non-stainless steel material comprises chromium. In other examples, the substantially inert non-glass, non-stainless steel material is an Inconel® alloy.
- the substantially inert non-glass, non-stainless steel material is present in a non-coated form.
- the substantially inert non-glass, non-stainless steel material is in a tube that is integral to the housing.
- the housing is configured as a first tube.
- a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert material is provided.
- the substantially inert material is a substantially inert metal material.
- the substantially inert metal material is present in a major amount.
- the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof.
- the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the substantially inert metal material comprises nickel or chromium.
- a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic material present in an effective amount to deter catalysis in the fluid flow path is provided.
- the non-catalytic material is a non-catalytic metal material.
- the non-catalytic metal material is present in a major amount.
- the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof.
- the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the non-catalytic metal material comprises nickel or chromium.
- a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis is described.
- the non-catalytic metal oxide material is present in a major amount. In other embodiments, the non-catalytic metal oxide material comprises titanium, aluminum, yttrium or combinations thereof. In some embodiments, the non-catalytic metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional embodiments, the non-catalytic metal oxide material comprises nickel or chromium.
- a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material is disclosed.
- the substantially inert metal oxide material is present in a major amount.
- the substantially inert metal oxide material comprises titanium, aluminum, yttrium or combinations thereof.
- the substantially inert metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the substantially inert metal oxide material comprises nickel or chromium.
- a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert material, e.g., a substantially inert metal material, is provided.
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the substantially inert metal material is present in a major amount.
- the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof.
- the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the substantially inert metal material comprises nickel or chromium.
- a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic material, e.g., a non-catalytic metal material, present in an effective amount to deter catalysis in the fluid flow path
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the non-catalytic metal material is present in a major amount.
- the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof.
- the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the non-catalytic metal material comprises nickel or chromium.
- a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material is disclosed.
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the substantially inert metal oxide material is present in a major amount.
- the substantially inert metal oxide material comprises titanium, aluminum, yttrium or combinations thereof.
- the substantially inert metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the substantially inert metal oxide material comprises nickel or chromium.
- a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis is described.
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the non-catalytic metal oxide material is present in a major amount.
- the non-catalytic metal oxide material comprises titanium, aluminum, yttrium or combinations thereof.
- the non-catalytic metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the non-catalytic metal oxide material comprises nickel or chromium.
- a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the non-catalytic, non-glass material is present in a major amount.
- the non-catalytic, non-glass material comprises titanium, aluminum, yttrium or combinations thereof.
- the non-catalytic, non-glass material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the non-catalytic, non-glass material comprises nickel or chromium.
- a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material
- the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- the substantially inert non-glass, non-stainless steel material is present in a major amount.
- the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium or combinations thereof.
- the substantially inert non-glass, non-stainless steel material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof.
- the substantially inert non-glass, non-stainless steel material comprises nickel or chromium.
- a brazeless and weldless jet assembly for use in a flame detector.
- the jet assembly comprises a fluid flow path in a first tube, the fluid flow path constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, the first tube coupled to a housing through a coupler constructed and arranged to couple the first tube to the housing without using a braze or weld.
- the fluid flow path comprises a substantially inert material.
- the first tube comprises a stainless steel and the substantially inert material is coated onto the stainless steel.
- the substantially inert material is a silica coating.
- the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis.
- the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- the fluid flow path comprises a substantially inert non-glass, non-stainless steel material.
- the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis.
- the fluid flow path comprises a substantially inert metal oxide material.
- the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis.
- the fluid flow path comprises a silica coating.
- the coupler can be configured as a compressible ferrule.
- the housing can include a second tube and a fitting, in which the second tube engages threads on the fitting to compress the ferrule and couple the first tube to the second tube.
- FIG. 1 is a cross-section of a flame photometric detector, in accordance with certain examples
- FIG. 2 is a cross-section of a jet assembly including a fluid flow path, in accordance with certain examples
- FIG. 3 is a schematic of a jet assembly including an outer tube and an inner tube, in accordance with certain examples
- FIG. 4 is a cross-section of a jet assembly that includes external threads to couple to a detector assembly, in accordance with certain examples
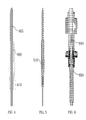
- FIG. 5 is an illustration of a jet assembly that includes a fitting for securely coupling the jet assembly to a detector assembly, in accordance with certain examples
- FIG. 6 is an illustration showing a jet assembly as part of a flame photometric detector, in accordance with certain examples
- FIG. 7A is an illustration showing a brazeless or weldless jet assembly, in accordance with certain examples.
- FIG. 7B is an expanded view of the coupler used to couple the inner and outer tubes of the jet assembly, in accordance with certain examples
- FIG. 8 is a schematic of a gas chromatography system, in accordance with certain examples.
- FIG. 9 is a chromatogram showing the absence of hydrogen sulfide when a conventional stainless steel fluid flow path is present in a jet assembly, in accordance with certain examples.
- FIG. 10 is a chromatogram showing the presence of hydrogen sulfide when a titanium fluid flow path is present in a jet assembly, in accordance with certain examples
- FIG. 11A is chromatogram of H 2 S using a brazeless jet assembly including fused capillary insert
- FIG. 11A is a chromatogram of H 2 S using a brazeless jet assembly without the insert, in accordance with certain examples
- FIG. 12 is an overlay chromatogram of the chromatograms of FIGS. 11A and 11B , in accordance with certain examples;
- FIG. 13A is a chromatogram of H 2 S using a fused capillary insert with a jet assembly having a stainless steel fluid flow path
- FIG. 13B is a chromatogram showing no recovery of H 2 S when the fused capillary insert is removed, in accordance with certain examples
- FIGS. 14A-14C are chromatograms of thiophene when tested using a Silconert-2000 coated jet assembly, in accordance with certain examples
- FIGS. 15A-15C are chromatograms of thiophene when tested using a non silica coated jet assembly, in accordance with certain examples
- FIGS. 16A and 16B are chromatograms of H 2 S initial measurements using a Silconert-2000 coated jet assembly, in accordance with certain examples
- FIGS. 17A-17C are chromatograms of H 2 S measurements using the Silconet-2000 coated jet assembly, in accordance with certain examples.
- FIGS. 18A and 18B are chromatograms of H 2 S final measurements using a Silconert-2000 coated jet assembly, in accordance with certain examples.
- Certain examples described herein are directed to jet assemblies that include one or more materials in a fluid flow path that can render at least some portion of fluid flow path substantially inert or non-catalytic.
- the substantially inert material on one or more surfaces of the jet assembly that are exposed to sample, the sample that contacts or resides near the surfaces should not react with the surfaces to a substantial degree or unwanted reactions are not substantially catalyzed by the surfaces.
- the particular type and amount of material on or in the fluid flow path (or other components of the jet assembly) can vary, and different types of materials may be desirably present to render the surface substantially inert.
- the jet assemblies disclosed herein can be used with the injectors and injector inserts, if desired, that are described in U.S. 61/308,461.
- the jet assemblies can also be used with other devices and components commonly found in a chromatography system such as a gas chromatography system.
- the entire jet assembly can be produced from the substantially inert or non-catalytic materials.
- substantially inert materials are those materials that do not react with, catalyze or otherwise are affected by analytes in a sample stream.
- Non-catalytic materials are a materials that are not necessarily inert under all conditions, but they do not catalyze any reactions to a substantial degree under selected chromatographic conditions.
- a non-catalytic material has suitable properties such that it does not catalyze any reaction to a substantial degree during the time a sample is resident or exposed to the surface of the jet assembly.
- substantially inert materials and non-catalytic materials there can be overlap of substantially inert materials and non-catalytic materials since substantially inert materials also do not catalyze reactions to any substantial degree no matter the residence time of the sample near the surface of the jet assembly. Specific types and amounts of each of the materials are described in more detail below. To reduce overall cost, it may be desirable to include the substantially inert or non-catalytic materials only on surfaces that contact the sample, and other portions of the jet assembly can be produced using conventional materials such as stainless steel.
- Illustrative types of materials that can be substantially inert and/or non-catalytic include but are not limited to titanium, titanium oxide, yttrium, yttrium oxide, aluminum, aluminum oxide, nickel, nickel alloys, chromium, chromium alloys, nickel chromium alloys and the like.
- Desirable nickel alloys include, but are not limited to, a Hastelloy® A alloy, a Hastelloy® B alloy, a Hastelloy® B2 alloy, a Hastelloy® B3 alloy, a Hastelloy® B142T alloy, a Hastelloy® Hybrid-BC1 alloy, a Hastelloy® C alloy, a Hastelloy® C4 alloy, a Hastelloy® C22 alloy, a Hastelloy® C22HS alloy, a Hastelloy® C2000 alloy, a Hastelloy® C263 alloy, a Hastelloy® C276 alloy, a Hastelloy® D alloy, a Hastelloy® G alloy, a Hastelloy® G2 alloy, a Hastelloy® G3 alloy, a Hastelloy® G30 alloy, a Hastelloy® G50 alloy, a Hastelloy® H9M alloy, a Hastelloy® N alloy, a Hastelloy
- the substantially inert material or the non-catalytic material can be a nickel-chromium alloy such as an Inconel® 600 alloy, an Inconel® 625 alloy, an Inconel® 718 alloy or other Inconel® alloys commercially available from Special Metals Corporation (New Hartford, N.Y.). Combinations of these various materials can also be used. One or more of these materials can be present on a surface of the jet assembly such that exposure of the sample to the surface does not result in any unwanted chemical reactions.
- the substantially inert or non-catalytic materials can desirably be present on or in the fluid path of the jet assembly such as those used, for example in a flame photometric detector.
- a flame photometric detector FPD
- the flame photometric detector 100 includes an optical filter assembly 105 , a cap 110 , an O-ring 115 , an I-ring 120 , a window filter assembly 125 , a glass liner 130 , a flame jet 140 , an air inlet 150 , a hydrogen inlet 160 , a column fitting 170 and a photomultiplier tube 180 .
- gases other than hydrogen can be introduced into the assembly and devices other than a photomultiplier tube can be used to receive a signal from the optical filter assembly 105 .
- sample effluent from the column is mixed with hydrogen gas.
- the mixture can then be burned in the presence of air in the flame jet 140 .
- the FPD 100 is particularly useful for detecting sulfur, phosphorous, and tin compounds, which produce chemiluminescent reactions with emissions at wavelengths characteristic of the S 2 (or other sulfur species), Sn and HPo species. Trace amounts of liable reactive S 2 compounds can react with tubular internal metal parts of the stainless steel jet assembly.
- Heated metal part components will react with highly labile species such as hydrogen sulfide (H 2 S). H 2 S will react with any unprotected internal jet assembly part resulting in loss of sample and unsatisfactory results in gas chromatography-FPD applications.
- H 2 S hydrogen sulfide
- some ways to prevent undesired reactions in the jet assembly is to use sulfur resistant coating methods such as Sulfinert coating, inserting fused silica glass tubing into the jet, or adding gold plating to prevent loss of sample. While these coatings or materials can reduce losses, satisfactory performance is still not achieved with these methods in all cases. Sulfinert products are manufacturer recommended for maximum temperature use of 450 degrees Celsius, and it is difficult to uniformly coat a long small inner diameter tube such as required in a FPD jet construction. In addition, portions of the FPD jet assembly near the hydrogen air flame can surpass 450 degrees Celsius. Further, brazing or welding used in the jet assembly production process can compromise the coatings.
- Gold or other noble metal plating present the same type of limitations as for coatings; inadequate uniform plating of the long small inner tube diameter and temperature limitations near the flame as described for the inert coating processes. Additionally coatings and platings can degrade over time with temperature and exposure to chemicals.
- Use of fused silica tubes presents additional user steps at installation. The fused silica needs to be perfectly aligned to tip of the jet assembly. If placed slightly lower exposing any stainless steel surfaces then loss of samples will occur. If placed too high, it can interfere with the hydrogen air flame and add baseline noise to the chromatogram.
- the jet assemblies described herein can include a substantially inert material or a non-catalytic material in a fluid flow path to prevent, or reduce the likelihood, of reaction of sample analyte with the jet assembly or to prevent catalysis of reactions by the jet assembly.
- a schematic of a jet assembly is shown in FIG. 2 .
- the jet assembly 200 includes a housing 210 that surrounds or encloses a fluid flow path 220 .
- the housing 210 can be produced using stainless steels or other materials since the housing 210 typically does not contact the sample. If desired, however, the housing 210 can be produced from the substantially inert and/or non-catalytic materials described herein.
- the housing 210 can include suitable fittings to permit coupling of the jet assembly 200 to other components of a flame photometric detector or other device where the jet assembly is to be used.
- the fluid flow path 220 can be entirely inside the housing 210 , or as shown in FIG. 2 , only a portion of the fluid flow path 210 can be in the housing 210 .
- the fluid flow path typically is constructed and arranged such that it can be fluidically coupled to a chromatography column at an end 222 and can be fluidically coupled to a flame jet at an end 224 . As sample exits from a column (not shown) it enters the fluid flow path 220 at the end 222 .
- the sample can be mixed with air and hydrogen (or another combustion gas), and can then be burned in the flame to cause light emission of the sample.
- the surfaces of the fluid flow path 220 can include a substantially inert material or a non-catalytic material. Such materials can be coated on, or, desirably, the entire fluid flow path can be formed from the substantially inert material or non-catalytic material such that unwanted interferences from coatings flaking off will not result.
- the fluid flow path can be provided by producing a tube of the substantially inert material that can be coupled to a housing.
- a tube comprising titanium can be produced with a hollow channel in the tube to provide a fluid flow path.
- the tube can be coupled to the housing using adhesives, welding, brazing or the like.
- the tube can be inserted into the housing and can be brazed at a junction to the housing to retain the tube in place. The entire assembly can then be used as a jet assembly.
- the jet assembly can include an outer tube and an inner tube, where the inner tube provides the fluid flow path between a chromatography column and a flame jet.
- an outer tube 310 is shown as surrounding an upper part of an inner tube 320 .
- the inner tube 320 can be coupled to the outer tube 310 at a brazed junction 325 , though other methods of joining the two tubes can be used.
- the entire inner tube 310 can be produced from a substantially inert material or a non-catalytic material to prevent unwanted reactions of the sample with surfaces of the inner tube.
- the inner diameter of a tube that provides the fluid flow path can vary from about 0.015 inches to about 0.05 inches, e.g., about 0.028 to about 0.038 inches inner diameter.
- the outer diameter of a tube that provides the fluid flow path can vary from about 0.02 inches to about 0.2 inches.
- the cross-sectional shape of the fluid flow path can vary and may be, for example, circular, elliptical, triangular, rectangular or take other shapes.
- the outer tube can include a suitable fitting or ferrule to couple to other devices.
- a jet assembly housing 400 can include external threads 410 that can couple to internal threads of other components.
- the coupling of the threads can effectuate a fluid tight seal such that fluid entering the fluid flow path 405 does not escape or leak into other parts of the system.
- the housing can include a fitting 510 that can be engaged by a wrench or other tool a wrench to tighten and couple the jet assembly to another device.
- one or more gaskets, seals or the like can be included to assist in creation of a fluid tight seal, if desired. Referring to FIG.
- a jet assembly 610 that includes a fluid flow path comprising a substantially inert material or a non-catalytic material is shown as being coupled to a flame photometric detector by mating the threads of the jet assembly 610 with threads 620 of the detector housing.
- sealing materials such as adhesives, thread lockers or the like can be used to retain the two components to each others.
- a jet assembly 700 includes an outer tube 710 , an inner tube 740 , a ferrule 720 and a threaded coupling 730 .
- the inner tube 740 can be secured by the threads in the outer tube 710 , which itself can be coupled to the threaded coupling 730 .
- the coupling of the outer tube 710 to the threaded coupling 730 can act to compress the ferrule 720 in the region near the ferrule 720 , as shown in FIG. 7B .
- this compression is operative to secure the inner tube 740 in a selected or fixed position in the jet assembly 700 .
- the inner tube 740 comprises one or more of the materials described herein, e.g., a substantially inert metal material, a non-catalytic metal material present in an effective amount to deter catalysis, a non-catalytic, non-glass material present in an effective amount to deter catalysis, a substantially inert non-glass, non-stainless steel material, a non-catalytic metal oxide material present in an major amount to deter catalysis, a substantially inert metal oxide material and combinations and derivatives thereof.
- the inner tube 710 may include materials such as inert coatings, e.g., Sulfinert, etc.
- inert coatings e.g., Sulfinert
- the lack of brazing or welding in the jet assembly 700 permits the use of coatings that are typically compromised during the production steps of conventional jet assemblies.
- the brazeless or weldless embodiments described herein typically do not use high enough temperatures in the production process to effect the coatings.
- brazeless or weldless jet assemblies permit the inner tube to be comprised of other inert materials as well as solid metals and alloys, e.g., an inert coating on a stainless steel tube (for example, stainless steel 347 and the like), an inert coating on a substantially inert metal material, an inert coating on a non-catalytic metal material, an inert coating on a non-catalytic, non-glass material, a inert coating on a substantially inert non-glass, non-stainless steel material, an inert coating on a non-catalytic metal oxide material, an inert coating on a substantially inert metal oxide material, and combinations thereof. It will be within the ability of the person of ordinary skill in the art, given the benefit of this disclosure, to design and use suitable brazeless jet assemblies for an intended use.
- the coating may be, or may include, a silica coating including, but not limited to, a SulfinertTM coating, a SiltekTM coating, a SilcoKleanTM coating, a SilcoGuardTM coating, a SilcolloyTM coating, a SilcoNertTM coating such as, for example, SilcoNertTM 1000 and SilcoNertTM 2000 coatings or other suitable coatings including but not limited to a borosilicate such as, for example, an extruded borosilicate.
- a silica coating including, but not limited to, a SulfinertTM coating, a SiltekTM coating, a SilcoKleanTM coating, a SilcoGuardTM coating, a SilcolloyTM coating, a SilcoNertTM coating such as, for example, SilcoNertTM 1000 and SilcoNertTM 2000 coatings or other suitable coatings including but not limited to a borosilicate such as, for example, an extrude
- a glass lined tube e.g., a stainless steel glass lined tube can be used in the jet assembly or with the jet assembly.
- Suitable glass lined tubes will be readily selected by the person of ordinary skill in the art, given the benefit of this disclosure, and illustrative glass tubes may be obtained commercially from SGE Incorporated (Austin, Tex.).
- FIG. 8 an illustrative gas chromatography system that can include one or more of the devices described herein is shown in FIG. 8 .
- the system 800 includes a carrier gas supply 810 fluidically coupled to an injector 820 .
- the injector 820 can be fluidically coupled to a column 830 , which includes a stationary phase selected to separate the analytes in a sample.
- the injector 820 is typically coupled to the column 830 through one or more ferrules or fittings to provide a fluid tight seal between the injector 820 and the column 830 .
- the column 830 can take various forms and configurations including packed columns and open tubular or capillary columns.
- the column 830 can be housed in an oven 825 , which is configured to implement one or more temperature profiles during the separation run.
- the column 830 can also be fluidically coupled to a detector 840 .
- the analyte species can be provided to the detector 840 .
- the detector 840 can take various forms including, but not limited to a flame ionization detector, a thermal conductivity detector, a thermionic detector, a nitrogen phosphorous detector, an electron capture detector, an atomic emission detector, a flame photometric detector, a photoionization detector or a mass spectrometer. Where the detector takes the form of a mass spectrometer, a single mass spectrometer can be present or multiple mass spectrometers can be present.
- surfaces of these detectors that come into contact with the sample can include a substantially inert material or a non-catalytic material as described herein.
- a tube comprising a fluid flow path that is substantially inert in a flame ionization detector, a nitrogen phosphorous detector or other detectors to reduce the likelihood that hot surfaces of the detectors will react with or catalyze a reaction.
- the temperature of the oven 825 may be raised to a starting temperature to permit the column 830 to warm up to that temperature.
- a sample can be injected through the injector 820 . Carrier gas from the gas source 810 will sweep the sample into the fluid flow path of the injector 820 .
- Sample can enter the column 830 where it will be separated into individual analytes. These separated analytes will elute from the column 830 and be provided to the detector 840 for detection.
- the sample can pass through a fluid flow path comprising a substantially inert material or a non-catalytic material prior to or after being mixed with air and a gas such as hydrogen.
- the burned sample can emit light which is detected by optics and/or electronics in the detector.
- FID flame ionization detector
- FID flame ionization detector
- the sample can be burned and ionized, and the resulting current can be measured.
- a nitrogen-phosphorous detector operates similar to an FID, but is more sensitive toward phosphorous and nitrogen compounds.
- the NPD detector can measure large ion currents produced in the presence of phosphorous and nitrogen compounds.
- Other detectors that include a jet assembly or a flame may particularly benefit by the inclusion of a fluid flow path comprising a substantially inert or non-catalytic material.
- a jet assembly for use in a detector can include a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert metal material.
- the detector can be a FPD, a FID or a NPD or other detector.
- the substantially inert metal material can be present in a major amount.
- the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- the substantially inert metal material is present in a non-coated form. In other examples, the substantially inert metal material is in a tube that is integral to the housing.
- a jet assembly can include a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the detector can be a FPD, a FID or a NPD or other detector.
- the non-catalytic metal material is present in a major amount.
- the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- the non-catalytic metal material is present in a non-coated form. In other embodiments, the non-catalytic metal material is in a tube that is integral to the housing.
- a jet assembly that includes a first tube and a second tube can be used.
- the first tube can be configured to couple to a detector assembly.
- the second tube can be inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the detector assembly can be a FPD, a FID or a NPD assembly or other detector assembly.
- the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- the non-catalytic metal material is present in a non-coated form.
- the second tube is longer than the first tube to fluidically couple to the chromatography column.
- the first tube comprises a non-catalytic material.
- a jet assembly can be used that includes a first tube configured to couple to a detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert metal material.
- the detector assembly can be a FPD, a FID or a NPD assembly or other detector assembly.
- the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- the substantially inert metal material is present in a non-coated form.
- the second tube is longer than the first tube to fluidically couple to the chromatography column.
- the first tube comprises a substantially inert metal material.
- a jet assembly can be used that includes a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- the jet assembly can be used in a FPD, a FID or a NPD or other detector.
- the non-catalytic, non-glass material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- the non-catalytic, non-glass material is present in a non-coated form.
- the non-catalytic, non-glass material is in a tube that is integral to the housing.
- the housing is configured as a first tube.
- a jet assembly can be used that includes a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material.
- the jet assembly can be used in a FPD, a FID or a NPD or other detector.
- the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- the substantially inert non-glass, non-stainless steel material is present in a non-coated form.
- the substantially inert non-glass, non-stainless steel material is in a tube that is integral to the housing.
- the housing is configured as a first tube.
- a jet assembly insert can be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal material.
- the jet assembly insert can be used in a FPD, a FID or a NPD or other detector.
- the substantially inert metal material is present in a major amount.
- the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a jet assembly insert can be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the jet assembly insert can be used in a FPD, a FID or a NPD or other detector.
- the non-catalytic metal material is present in a major amount.
- the non-catalytic metal material titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a jet assembly insert can be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis.
- the jet assembly insert can be used in a FPD, a FID or a NPD or other detector.
- the non-catalytic metal oxide material is present in a major amount.
- the non-catalytic metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a jet assembly insert ca be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material.
- the jet assembly insert can be used in a FPD, a FID or a NPD or other detector.
- the substantially inert metal oxide material is present in a major amount.
- the substantially inert metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a flame detector can be used that includes a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal material.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- the substantially inert metal material is present in a major amount.
- the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a flame detector can be used that includes a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- the non-catalytic metal material is present in a major amount.
- the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- the substantially inert metal oxide material is present in a major amount.
- the substantially inert metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- the non-catalytic metal oxide material is present in a major amount.
- the non-catalytic metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- the non-catalytic, non-glass material is present in a major amount.
- the non-catalytic, non-glass material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- the substantially inert non-glass, non-stainless steel material is present in a major amount.
- the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert metal material.
- the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly comprising a first tube configured to couple to a flame photometric detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly comprising a first tube configured to couple to a flame photometric detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert metal material.
- the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- the non-catalytic, non-glass material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material.
- the non-glass, non-stainless steel material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal material.
- the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis.
- the non-catalytic metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material.
- the substantially inert metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal material.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material.
- the flame detector can be a FPD, a FID or a NPD or other detector.
- a method of facilitating chromatographic analysis includes providing a jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert metal material.
- a method of facilitating chromatographic analysis can include providing a jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- a method of facilitating chromatographic analysis can include providing a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- a method of facilitating chromatographic analysis can include providing a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert metal material.
- a method of facilitating chromatographic analysis can include providing a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- a method of facilitating chromatographic analysis can include providing a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material.
- a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal material.
- a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis.
- a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material.
- a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal material.
- a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material.
- a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis.
- a method of facilitating chromatographic analysis can include providing a flame photometric detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- a method of facilitating chromatographic analysis can include providing a flame photometric detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material.
- the fluid flow paths can include titanium, yttrium, aluminum, nickel, chromium, a nickel alloy, a chromium alloy, a nickel chromium alloy, Hastelloy® alloys, Inconel® alloys, combinations thereof, or other materials. Certain specific examples are described below to illustrate further the novel technology described herein
- a flame photometric detector including a jet assembly with a conventional stainless steel fluid flow path was compared a flame photometric detector including a jet assembly with a titanium flow path.
- the titanium flow path was provided using a titanium inner tube that was inserted into the housing and brazed at a single site.
- the test equipment was a Kin-Tek Model 491-MB gas standards generator, and a VICI gas sampling valve injecting 1 ppm of Hydrogen sulfide gas was used to test the recovery.
- the gas sampling valve was connected to a Perkin Elmer model Clarus 500 Gas Chromatograph equipped with an FPD detector and programmable pneumatic control. The sample including hydrogen sulfide (H 2 S) was injected and analyzed using each of the FPD detectors.
- H 2 S peak was absent (see FIG. 9 ). H 2 S appeared to react with the stainless steel in the conventional jet assembly. Where the titanium fluid flow path was present, the H 2 S peak was observed (see FIG. 10 ), thus confirming that a substantially inert fluid flow path is suitable for use with species such as H 2 S.
- a brazeless jet assembly was tested for its ability to recover H 2 S.
- the test equipment was a Kin-Tek Model 491-MB gas standards generator, and a VICI gas sampling valve injecting 1 ppm of Hydrogen sulfide gas was used to test the recovery.
- the gas sampling valve was connected to a Perkin Elmer model Clarus 500 Gas Chromatograph equipped with an FPD detector and programmable pneumatic control.
- a jet assembly was tested first with a fused silica capillary tube installed all the way to the top of the jet assembly flush with the tip of the jet.
- the inner tube was produced using titanium.
- the H 2 S sample would travel out of the fused silica column without any exposure to the steel jet assembly. This configuration should yield the maximum ideal response for H 2 S.
- the H 2 S sample is not exposed to any metal surface of the jet assembly avoiding any reaction and not incurring any substantial loss of H 2 S sample.
- the results using this fused silica capillary tube were used as a control for the comparison of the percent recovery for H 2 S.
- the H 2 S FPD chromatogram response was recorded in the form of peak area ( FIG. 11A ).
- the fused silica capillary tube was then removed from the jet assembly allowing the H 2 S sample to be exposed to and travel inside the length of the jet assembly.
- the H 2 S FPD chromatogram response again is recorded in the form of peak area ( FIG. 11B ).
- the chromatograms were overlaid for comparison, as shown in FIG. 12 .
- the recovery was then calculated as percentage of peak area response (fused silica to top of jet) divided by peak area response (no fused silica) times 100. A good recovery result will yield a minimum 90% or greater. The calculated recovery was greater than 90%.
- FIG. 13A An H 2 S response is shown in FIG. 13A , where the stainless steel jet assembly included a fused silica insert. When the fused silica inert was removed from the jet assembly, there was no to minimal response and no recovery of the H 2 S ( FIG. 13B ). All H 2 S sample was lost due to exposure to the unprotected stainless steel jet body.
- a jet assembly was produced from a 1/16 inch outer diameter, 0.034 inch inner diameter swaged inner tube.
- the inner jet tube was produced using 347 anticorrosion stainless steel (High Nickel, Chromium content, doped with tantalum) and was coated with Silconert-2000.
- the jet assembly was tested using thiophene and the conditions listed in Examples 1 and 2. The results are shown in FIGS. 14A-14C (three separate runs). Chromatograms for an old jet are provided in FIGS. 15A-15C for comparison.
- the new FPD jet measured 5.25 ⁇ 10 ⁇ 12 grams Sulfur/second which, is 1.9 times over the published minimum detectable quantity.
- the old jet using the exact same method and standard, was 6.64 ⁇ 10 ⁇ 12 grams sulfur/sec., which is 1.5 times the minimum detectable quantity.
- the retention times for the two jets were about the same, e.g., 1-2 minutes for thiophene.
- FIGS. 16A and 16B are chromatograms showing the initial recovery of the jet at the initial injections using a control (fused silica tube and fused silica tube removed). 745 injections of H 2 S were then made. Illustrative chromatograms are shown in FIGS. 17A-17C . Measurements were then made with same the control (fused silica tube and fused silica tube removed) after the 745 injections to determine the recovery (see FIGS. 18A and 18B ). Even after the 745 injections, the recovery of the coated jet assembly was substantially the same as the recovery upon initial injection. These results are consistent with the coated jet assembly providing a long usable life and minimal to no degradation during operation.
Landscapes
- Physics & Mathematics (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Pathology (AREA)
- Food Science & Technology (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Investigating, Analyzing Materials By Fluorescence Or Luminescence (AREA)
- Other Investigation Or Analysis Of Materials By Electrical Means (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Jet Pumps And Other Pumps (AREA)
Abstract
Description
- This application claims priority to, and the benefit of, U.S. Provisional Application No. 61/308,499 filed on Feb. 26, 2010, the entire disclosure of which is hereby incorporated herein by reference for all purposes.
- This application is related to commonly owned provisional application No. 61/308,461 filed on Feb. 26, 2010, the entire disclosure of which is hereby incorporated herein by reference for all purposes.
- Certain embodiments herein are directed to a jet assembly that includes a substantially inert fluid flow path. In particular, certain embodiments are directed to a flame photometric detector jet assembly that includes a substantially inert fluid flow path.
- Many chromatography systems use detectors that burn a sample in a flame. In some instances, the sample can react with hot surfaces in the flame jet assembly, which can cause the analyte to render it difficult or impossible to detect certain analytes in the sample.
- In an aspect, a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert material is provided. In some examples, the fluid flow path comprises a substantially inert metal material. In other examples, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In certain embodiments, the substantially inert metal material is present in a major amount. In other embodiments, the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof. In additional embodiments, the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In some embodiments, the substantially inert metal material comprises nickel. In other embodiments, the substantially inert metal material is a Hastelloy® alloy. In some embodiments, the substantially inert metal material comprises chromium. In certain embodiments, the substantially inert metal material is an Inconel® alloy. In additional embodiments, the substantially inert metal material is present in a non-coated form. In some embodiments, the substantially inert metal material is in a tube that is integral to the housing.
- In another aspect, a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic material present in an effective amount to deter catalysis in the fluid flow path is described. In certain examples, the fluid flow path comprises a non-catalytic metal material. In other examples, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In certain examples, the non-catalytic metal material is present in a major amount. In other examples, the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof. In additional examples, the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In further examples, the non-catalytic metal material comprises nickel. In yet other examples, the non-catalytic metal material is a Hastelloy® alloy. In some examples, the non-catalytic metal material comprises chromium. In additional examples, the non-catalytic metal material is an Inconel® alloy. In further examples, the non-catalytic metal material is present in a non-coated form. In some examples, the non-catalytic metal material is in a tube that is integral to the housing.
- In an additional aspect, a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic material present in an effective amount to deter catalysis in the fluid flow path is disclosed. In certain examples, the fluid flow path comprises a non-catalytic metal material.
- In certain embodiments, the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof. In other embodiments, the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional embodiments, the non-catalytic metal material comprises nickel. In further embodiments, the non-catalytic metal material is a Hastelloy® alloy. In some embodiments, the non-catalytic metal material comprises chromium. In some examples, the non-catalytic metal material is an Inconel® alloy. In additional examples, the non-catalytic metal material is present in a non-coated form. In further examples, the second tube is longer than the first tube to fluidically couple to the chromatography column. In other examples, the first tube comprises a non-catalytic material.
- In another aspect, a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert material is provided. In certain embodiments, the substantially inert material is a substantially inert metal material.
- In some examples, the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof. In other examples, the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional examples, the substantially inert metal material comprises nickel. In certain examples, the substantially inert metal material is a Hastelloy® alloy. In further examples, the substantially inert metal material comprises chromium. In other examples, the substantially inert metal material is an Inconel® alloy. In some examples, the substantially inert metal material is present in a non-coated form. In additional examples, the second tube is longer than the first tube to fluidically couple to the chromatography column. In further examples, the first tube comprises a substantially inert metal material.
- In an additional aspect, a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis is described.
- In some embodiments, the non-catalytic, non-glass material comprises titanium, aluminum, yttrium or combinations thereof. In other embodiments, the non-catalytic, non-glass material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional embodiments, the non-catalytic, non-glass material comprises nickel. In further embodiments, the non-catalytic, non-glass material is a Hastelloy® alloy. In some embodiments, the non-catalytic, non-glass material comprises chromium. In other embodiments, the non-catalytic, non-glass material is an Inconel® alloy. In additional embodiments, the non-catalytic, non-glass material is present in a non-coated form. In some embodiments, the non-catalytic, non-glass material is in a tube that is integral to the housing. In other embodiments, the housing is configured as a first tube.
- In another aspect, a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material is disclosed.
- In certain examples, the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium or combinations thereof. In other examples, the substantially inert non-glass, non-stainless steel material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional examples, the substantially inert non-glass, non-stainless steel material comprises nickel. In further examples, the substantially inert non-glass, non-stainless steel material is a Hastelloy® alloy. In some examples, the substantially inert non-glass, non-stainless steel material comprises chromium. In other examples, the substantially inert non-glass, non-stainless steel material is an Inconel® alloy. In additional examples, the substantially inert non-glass, non-stainless steel material is present in a non-coated form. In certain examples, the substantially inert non-glass, non-stainless steel material is in a tube that is integral to the housing. In other examples, the housing is configured as a first tube.
- In an additional aspect, a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert material is provided. In some examples, the substantially inert material is a substantially inert metal material.
- In certain embodiments, the substantially inert metal material is present in a major amount. In other embodiments, the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof. In additional embodiments, the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In further embodiments, the substantially inert metal material comprises nickel or chromium.
- In another aspect, a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic material present in an effective amount to deter catalysis in the fluid flow path is provided. In some examples, the non-catalytic material is a non-catalytic metal material.
- In certain examples, the non-catalytic metal material is present in a major amount. In other examples, the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof. In additional examples, the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In other examples, the non-catalytic metal material comprises nickel or chromium.
- In an additional aspect, a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis is described.
- In certain embodiments, the non-catalytic metal oxide material is present in a major amount. In other embodiments, the non-catalytic metal oxide material comprises titanium, aluminum, yttrium or combinations thereof. In some embodiments, the non-catalytic metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional embodiments, the non-catalytic metal oxide material comprises nickel or chromium.
- In another aspect, a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material is disclosed.
- In certain examples, the substantially inert metal oxide material is present in a major amount. In other examples, the substantially inert metal oxide material comprises titanium, aluminum, yttrium or combinations thereof. In additional examples, the substantially inert metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In further examples, the substantially inert metal oxide material comprises nickel or chromium.
- In an additional aspect, a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert material, e.g., a substantially inert metal material, is provided. In some examples, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In certain embodiments, the substantially inert metal material is present in a major amount. In other embodiments, the substantially inert metal material comprises titanium, aluminum, yttrium or combinations thereof. In some embodiments, the substantially inert metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In additional embodiments, the substantially inert metal material comprises nickel or chromium.
- In another aspect, a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic material, e.g., a non-catalytic metal material, present in an effective amount to deter catalysis in the fluid flow path is described. In some embodiments, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In certain examples, the non-catalytic metal material is present in a major amount. In other examples, the non-catalytic metal material comprises titanium, aluminum, yttrium or combinations thereof. In additional examples, the non-catalytic metal material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In some examples, the non-catalytic metal material comprises nickel or chromium.
- In an additional aspect, a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material is disclosed. In certain embodiments, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In some examples, the substantially inert metal oxide material is present in a major amount. In additional examples, the substantially inert metal oxide material comprises titanium, aluminum, yttrium or combinations thereof. In other examples, the substantially inert metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In further examples, the substantially inert metal oxide material comprises nickel or chromium.
- In another aspect, a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis is described. In certain examples, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In certain embodiments, the non-catalytic metal oxide material is present in a major amount. In other embodiments, the non-catalytic metal oxide material comprises titanium, aluminum, yttrium or combinations thereof. In additional embodiments, the non-catalytic metal oxide material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In further embodiments, the non-catalytic metal oxide material comprises nickel or chromium.
- In an additional aspect, a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis is provided. In some examples, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In certain examples, the non-catalytic, non-glass material is present in a major amount. In other examples, the non-catalytic, non-glass material comprises titanium, aluminum, yttrium or combinations thereof. In additional examples, the non-catalytic, non-glass material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In some examples, the non-catalytic, non-glass material comprises nickel or chromium.
- In another aspect, a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material is disclosed. In certain examples, the flame detector can be a flame photometric detector, a flame ionization detector, a nitrogen-phosphorous detector or other flame based detectors.
- In some embodiments, the substantially inert non-glass, non-stainless steel material is present in a major amount. In other embodiments, the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium or combinations thereof. In additional embodiments, the substantially inert non-glass, non-stainless steel material comprises titanium oxide, aluminum oxide, yttrium oxide or combinations thereof. In further embodiments, the substantially inert non-glass, non-stainless steel material comprises nickel or chromium.
- In another aspect, a brazeless and weldless jet assembly for use in a flame detector is provided. In some embodiments, the jet assembly comprises a fluid flow path in a first tube, the fluid flow path constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, the first tube coupled to a housing through a coupler constructed and arranged to couple the first tube to the housing without using a braze or weld.
- In certain examples, the fluid flow path comprises a substantially inert material. In some examples, the first tube comprises a stainless steel and the substantially inert material is coated onto the stainless steel. In additional examples, the substantially inert material is a silica coating. In other examples, the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis. In further examples, the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis. In certain embodiments, the fluid flow path comprises a substantially inert non-glass, non-stainless steel material. In additional embodiments, the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis. In other embodiments, the fluid flow path comprises a substantially inert metal oxide material. In some embodiments, the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis. In certain examples, the fluid flow path comprises a silica coating. In other examples, the coupler can be configured as a compressible ferrule. In some examples, the housing can include a second tube and a fitting, in which the second tube engages threads on the fitting to compress the ferrule and couple the first tube to the second tube.
- Additional features, aspect, examples and embodiments are described in more detail below.
- Certain embodiments are described with reference to the figures in which:
-
FIG. 1 is a cross-section of a flame photometric detector, in accordance with certain examples; -
FIG. 2 is a cross-section of a jet assembly including a fluid flow path, in accordance with certain examples; -
FIG. 3 is a schematic of a jet assembly including an outer tube and an inner tube, in accordance with certain examples; -
FIG. 4 is a cross-section of a jet assembly that includes external threads to couple to a detector assembly, in accordance with certain examples; -
FIG. 5 is an illustration of a jet assembly that includes a fitting for securely coupling the jet assembly to a detector assembly, in accordance with certain examples; -
FIG. 6 is an illustration showing a jet assembly as part of a flame photometric detector, in accordance with certain examples; -
FIG. 7A is an illustration showing a brazeless or weldless jet assembly, in accordance with certain examples; -
FIG. 7B is an expanded view of the coupler used to couple the inner and outer tubes of the jet assembly, in accordance with certain examples; -
FIG. 8 is a schematic of a gas chromatography system, in accordance with certain examples; -
FIG. 9 is a chromatogram showing the absence of hydrogen sulfide when a conventional stainless steel fluid flow path is present in a jet assembly, in accordance with certain examples; -
FIG. 10 is a chromatogram showing the presence of hydrogen sulfide when a titanium fluid flow path is present in a jet assembly, in accordance with certain examples; -
FIG. 11A is chromatogram of H2S using a brazeless jet assembly including fused capillary insert, andFIG. 11A is a chromatogram of H2S using a brazeless jet assembly without the insert, in accordance with certain examples; -
FIG. 12 is an overlay chromatogram of the chromatograms ofFIGS. 11A and 11B , in accordance with certain examples; -
FIG. 13A is a chromatogram of H2S using a fused capillary insert with a jet assembly having a stainless steel fluid flow path, andFIG. 13B is a chromatogram showing no recovery of H2S when the fused capillary insert is removed, in accordance with certain examples; -
FIGS. 14A-14C are chromatograms of thiophene when tested using a Silconert-2000 coated jet assembly, in accordance with certain examples; -
FIGS. 15A-15C are chromatograms of thiophene when tested using a non silica coated jet assembly, in accordance with certain examples; -
FIGS. 16A and 16B are chromatograms of H2S initial measurements using a Silconert-2000 coated jet assembly, in accordance with certain examples; -
FIGS. 17A-17C are chromatograms of H2S measurements using the Silconet-2000 coated jet assembly, in accordance with certain examples; and -
FIGS. 18A and 18B are chromatograms of H2S final measurements using a Silconert-2000 coated jet assembly, in accordance with certain examples. - It will be recognized by the person of ordinary skill in the art, given the benefit of this disclosure, that certain dimensions or features in the figures may have been enlarged, distorted or shown in an otherwise unconventional or non-proportional manner to provide a more user friendly version of the figures. Where dimensions are specified in the description below, the dimensions are provided for illustrative purposes only.
- Certain examples described herein are directed to jet assemblies that include one or more materials in a fluid flow path that can render at least some portion of fluid flow path substantially inert or non-catalytic. By including the substantially inert material on one or more surfaces of the jet assembly that are exposed to sample, the sample that contacts or resides near the surfaces should not react with the surfaces to a substantial degree or unwanted reactions are not substantially catalyzed by the surfaces. As described in more detail below, the particular type and amount of material on or in the fluid flow path (or other components of the jet assembly) can vary, and different types of materials may be desirably present to render the surface substantially inert.
- In certain examples, the jet assemblies disclosed herein can be used with the injectors and injector inserts, if desired, that are described in U.S. 61/308,461. In addition, the jet assemblies can also be used with other devices and components commonly found in a chromatography system such as a gas chromatography system. Certain reference is made herein to one component being “inside” of another component. Where such reference is made, it is not intended to imply or mean that the entire component be inside the other component. Depending on the exact configuration of the device, all of one component may be positioned inside another component or a selected portion of one component may be positioned inside another component.
- In certain embodiments, the entire jet assembly, if desired, can be produced from the substantially inert or non-catalytic materials. As described in more detail below, substantially inert materials are those materials that do not react with, catalyze or otherwise are affected by analytes in a sample stream. Non-catalytic materials are a materials that are not necessarily inert under all conditions, but they do not catalyze any reactions to a substantial degree under selected chromatographic conditions. For example, a non-catalytic material has suitable properties such that it does not catalyze any reaction to a substantial degree during the time a sample is resident or exposed to the surface of the jet assembly. There can be overlap of substantially inert materials and non-catalytic materials since substantially inert materials also do not catalyze reactions to any substantial degree no matter the residence time of the sample near the surface of the jet assembly. Specific types and amounts of each of the materials are described in more detail below. To reduce overall cost, it may be desirable to include the substantially inert or non-catalytic materials only on surfaces that contact the sample, and other portions of the jet assembly can be produced using conventional materials such as stainless steel. Illustrative types of materials that can be substantially inert and/or non-catalytic include but are not limited to titanium, titanium oxide, yttrium, yttrium oxide, aluminum, aluminum oxide, nickel, nickel alloys, chromium, chromium alloys, nickel chromium alloys and the like. Desirable nickel alloys include, but are not limited to, a Hastelloy® A alloy, a Hastelloy® B alloy, a Hastelloy® B2 alloy, a Hastelloy® B3 alloy, a Hastelloy® B142T alloy, a Hastelloy® Hybrid-BC1 alloy, a Hastelloy® C alloy, a Hastelloy® C4 alloy, a Hastelloy® C22 alloy, a Hastelloy® C22HS alloy, a Hastelloy® C2000 alloy, a Hastelloy® C263 alloy, a Hastelloy® C276 alloy, a Hastelloy® D alloy, a Hastelloy® G alloy, a Hastelloy® G2 alloy, a Hastelloy® G3 alloy, a Hastelloy® G30 alloy, a Hastelloy® G50 alloy, a Hastelloy® H9M alloy, a Hastelloy® N alloy, a Hastelloy® R235 alloy, a Hastelloy® S alloy, a Hastelloy® W alloy, a Hastelloy® X alloy and other Hastelloy® alloys or Haynes alloys commercially available from Haynes International, Inc. (Kokomo, Ind.). In some examples, the substantially inert material or the non-catalytic material can be a nickel-chromium alloy such as an Inconel® 600 alloy, an Inconel® 625 alloy, an Inconel® 718 alloy or other Inconel® alloys commercially available from Special Metals Corporation (New Hartford, N.Y.). Combinations of these various materials can also be used. One or more of these materials can be present on a surface of the jet assembly such that exposure of the sample to the surface does not result in any unwanted chemical reactions.
- In certain embodiments, the substantially inert or non-catalytic materials can desirably be present on or in the fluid path of the jet assembly such as those used, for example in a flame photometric detector. Referring to
FIG. 1 , one illustration of a flame photometric detector (FPD) is shown. Theflame photometric detector 100 includes anoptical filter assembly 105, acap 110, an O-ring 115, an I-ring 120, awindow filter assembly 125, aglass liner 130, aflame jet 140, anair inlet 150, ahydrogen inlet 160, a column fitting 170 and aphotomultiplier tube 180. It will be recognized by the person of ordinary skill in the art, given the benefit of this disclosure, that gases other than hydrogen can be introduced into the assembly and devices other than a photomultiplier tube can be used to receive a signal from theoptical filter assembly 105. In operation of theFPD 100, sample effluent from the column is mixed with hydrogen gas. The mixture can then be burned in the presence of air in theflame jet 140. TheFPD 100 is particularly useful for detecting sulfur, phosphorous, and tin compounds, which produce chemiluminescent reactions with emissions at wavelengths characteristic of the S2 (or other sulfur species), Sn and HPo species. Trace amounts of liable reactive S2 compounds can react with tubular internal metal parts of the stainless steel jet assembly. Heated metal part components will react with highly labile species such as hydrogen sulfide (H2S). H2S will react with any unprotected internal jet assembly part resulting in loss of sample and unsatisfactory results in gas chromatography-FPD applications. - In certain examples, some ways to prevent undesired reactions in the jet assembly is to use sulfur resistant coating methods such as Sulfinert coating, inserting fused silica glass tubing into the jet, or adding gold plating to prevent loss of sample. While these coatings or materials can reduce losses, satisfactory performance is still not achieved with these methods in all cases. Sulfinert products are manufacturer recommended for maximum temperature use of 450 degrees Celsius, and it is difficult to uniformly coat a long small inner diameter tube such as required in a FPD jet construction. In addition, portions of the FPD jet assembly near the hydrogen air flame can surpass 450 degrees Celsius. Further, brazing or welding used in the jet assembly production process can compromise the coatings. Gold or other noble metal plating present the same type of limitations as for coatings; inadequate uniform plating of the long small inner tube diameter and temperature limitations near the flame as described for the inert coating processes. Additionally coatings and platings can degrade over time with temperature and exposure to chemicals. Use of fused silica tubes presents additional user steps at installation. The fused silica needs to be perfectly aligned to tip of the jet assembly. If placed slightly lower exposing any stainless steel surfaces then loss of samples will occur. If placed too high, it can interfere with the hydrogen air flame and add baseline noise to the chromatogram.
- In certain embodiments, the jet assemblies described herein can include a substantially inert material or a non-catalytic material in a fluid flow path to prevent, or reduce the likelihood, of reaction of sample analyte with the jet assembly or to prevent catalysis of reactions by the jet assembly. A schematic of a jet assembly is shown in
FIG. 2 . Thejet assembly 200 includes ahousing 210 that surrounds or encloses afluid flow path 220. In certain embodiments, thehousing 210 can be produced using stainless steels or other materials since thehousing 210 typically does not contact the sample. If desired, however, thehousing 210 can be produced from the substantially inert and/or non-catalytic materials described herein. Thehousing 210 can include suitable fittings to permit coupling of thejet assembly 200 to other components of a flame photometric detector or other device where the jet assembly is to be used. Thefluid flow path 220 can be entirely inside thehousing 210, or as shown inFIG. 2 , only a portion of thefluid flow path 210 can be in thehousing 210. The fluid flow path typically is constructed and arranged such that it can be fluidically coupled to a chromatography column at anend 222 and can be fluidically coupled to a flame jet at anend 224. As sample exits from a column (not shown) it enters thefluid flow path 220 at theend 222. The sample can be mixed with air and hydrogen (or another combustion gas), and can then be burned in the flame to cause light emission of the sample. The emitted light can be detected and used to ascertain how much of a particular analyte is present. In some examples, the surfaces of thefluid flow path 220 can include a substantially inert material or a non-catalytic material. Such materials can be coated on, or, desirably, the entire fluid flow path can be formed from the substantially inert material or non-catalytic material such that unwanted interferences from coatings flaking off will not result. - In certain embodiments, the fluid flow path can be provided by producing a tube of the substantially inert material that can be coupled to a housing. For example, a tube comprising titanium can be produced with a hollow channel in the tube to provide a fluid flow path. The tube can be coupled to the housing using adhesives, welding, brazing or the like. In some examples, the tube can be inserted into the housing and can be brazed at a junction to the housing to retain the tube in place. The entire assembly can then be used as a jet assembly.
- In certain examples, the jet assembly can include an outer tube and an inner tube, where the inner tube provides the fluid flow path between a chromatography column and a flame jet. Referring to
FIG. 3 , anouter tube 310 is shown as surrounding an upper part of aninner tube 320. Theinner tube 320 can be coupled to theouter tube 310 at a brazedjunction 325, though other methods of joining the two tubes can be used. The entireinner tube 310 can be produced from a substantially inert material or a non-catalytic material to prevent unwanted reactions of the sample with surfaces of the inner tube. - In certain embodiments, the inner diameter of a tube that provides the fluid flow path can vary from about 0.015 inches to about 0.05 inches, e.g., about 0.028 to about 0.038 inches inner diameter. In some examples, the outer diameter of a tube that provides the fluid flow path can vary from about 0.02 inches to about 0.2 inches. The cross-sectional shape of the fluid flow path can vary and may be, for example, circular, elliptical, triangular, rectangular or take other shapes.
- In some embodiments, the outer tube can include a suitable fitting or ferrule to couple to other devices. Referring to
FIG. 4 , ajet assembly housing 400 can includeexternal threads 410 that can couple to internal threads of other components. In some embodiments, the coupling of the threads can effectuate a fluid tight seal such that fluid entering thefluid flow path 405 does not escape or leak into other parts of the system. In some examples and referring toFIG. 5 , the housing can include a fitting 510 that can be engaged by a wrench or other tool a wrench to tighten and couple the jet assembly to another device. In addition, one or more gaskets, seals or the like can be included to assist in creation of a fluid tight seal, if desired. Referring toFIG. 6 , ajet assembly 610 that includes a fluid flow path comprising a substantially inert material or a non-catalytic material is shown as being coupled to a flame photometric detector by mating the threads of thejet assembly 610 withthreads 620 of the detector housing. If desired, sealing materials such as adhesives, thread lockers or the like can be used to retain the two components to each others. - In certain embodiments, the jet assembly can be brazeless or weldless such that no braze or weld is present in the jet assembly to hold the inner and outer tubes together. Referring to
FIGS. 7A and 7B , a jet assembly 700 includes anouter tube 710, aninner tube 740, aferrule 720 and a threadedcoupling 730. Theinner tube 740 can be secured by the threads in theouter tube 710, which itself can be coupled to the threadedcoupling 730. The coupling of theouter tube 710 to the threadedcoupling 730 can act to compress theferrule 720 in the region near theferrule 720, as shown inFIG. 7B . In certain examples, this compression is operative to secure theinner tube 740 in a selected or fixed position in the jet assembly 700. In some examples, theinner tube 740 comprises one or more of the materials described herein, e.g., a substantially inert metal material, a non-catalytic metal material present in an effective amount to deter catalysis, a non-catalytic, non-glass material present in an effective amount to deter catalysis, a substantially inert non-glass, non-stainless steel material, a non-catalytic metal oxide material present in an major amount to deter catalysis, a substantially inert metal oxide material and combinations and derivatives thereof. In other examples, theinner tube 710 may include materials such as inert coatings, e.g., Sulfinert, etc. For example, the lack of brazing or welding in the jet assembly 700 permits the use of coatings that are typically compromised during the production steps of conventional jet assemblies. The brazeless or weldless embodiments described herein typically do not use high enough temperatures in the production process to effect the coatings. Such brazeless or weldless jet assemblies permit the inner tube to be comprised of other inert materials as well as solid metals and alloys, e.g., an inert coating on a stainless steel tube (for example, stainless steel 347 and the like), an inert coating on a substantially inert metal material, an inert coating on a non-catalytic metal material, an inert coating on a non-catalytic, non-glass material, a inert coating on a substantially inert non-glass, non-stainless steel material, an inert coating on a non-catalytic metal oxide material, an inert coating on a substantially inert metal oxide material, and combinations thereof. It will be within the ability of the person of ordinary skill in the art, given the benefit of this disclosure, to design and use suitable brazeless jet assemblies for an intended use. - In certain embodiments where a coating is present in a brazeless or weldless jet assembly, the coating may be, or may include, a silica coating including, but not limited to, a Sulfinert™ coating, a Siltek™ coating, a SilcoKlean™ coating, a SilcoGuard™ coating, a Silcolloy™ coating, a SilcoNert™ coating such as, for example, SilcoNert™ 1000 and SilcoNert™ 2000 coatings or other suitable coatings including but not limited to a borosilicate such as, for example, an extruded borosilicate. In some examples, a glass lined tube, e.g., a stainless steel glass lined tube can be used in the jet assembly or with the jet assembly. Suitable glass lined tubes will be readily selected by the person of ordinary skill in the art, given the benefit of this disclosure, and illustrative glass tubes may be obtained commercially from SGE Incorporated (Austin, Tex.).
- In certain examples, an illustrative gas chromatography system that can include one or more of the devices described herein is shown in
FIG. 8 . Thesystem 800 includes acarrier gas supply 810 fluidically coupled to aninjector 820. Theinjector 820 can be fluidically coupled to acolumn 830, which includes a stationary phase selected to separate the analytes in a sample. Theinjector 820 is typically coupled to thecolumn 830 through one or more ferrules or fittings to provide a fluid tight seal between theinjector 820 and thecolumn 830. Thecolumn 830 can take various forms and configurations including packed columns and open tubular or capillary columns. Thecolumn 830 can be housed in anoven 825, which is configured to implement one or more temperature profiles during the separation run. Thecolumn 830 can also be fluidically coupled to adetector 840. As analyte species elute from thecolumn 830, the analyte species can be provided to thedetector 840. Thedetector 840 can take various forms including, but not limited to a flame ionization detector, a thermal conductivity detector, a thermionic detector, a nitrogen phosphorous detector, an electron capture detector, an atomic emission detector, a flame photometric detector, a photoionization detector or a mass spectrometer. Where the detector takes the form of a mass spectrometer, a single mass spectrometer can be present or multiple mass spectrometers can be present. If desired, surfaces of these detectors that come into contact with the sample can include a substantially inert material or a non-catalytic material as described herein. For example, it may be desirable to include a tube comprising a fluid flow path that is substantially inert in a flame ionization detector, a nitrogen phosphorous detector or other detectors to reduce the likelihood that hot surfaces of the detectors will react with or catalyze a reaction. In use of thesystem 800 shown inFIG. 8 , the temperature of theoven 825 may be raised to a starting temperature to permit thecolumn 830 to warm up to that temperature. A sample can be injected through theinjector 820. Carrier gas from thegas source 810 will sweep the sample into the fluid flow path of theinjector 820. Sample can enter thecolumn 830 where it will be separated into individual analytes. These separated analytes will elute from thecolumn 830 and be provided to thedetector 840 for detection. Where a FPD detector is used, the sample can pass through a fluid flow path comprising a substantially inert material or a non-catalytic material prior to or after being mixed with air and a gas such as hydrogen. The burned sample can emit light which is detected by optics and/or electronics in the detector. Where a flame ionization detector (FID) is used, the sample can be burned and ionized, and the resulting current can be measured. A nitrogen-phosphorous detector (NPD) operates similar to an FID, but is more sensitive toward phosphorous and nitrogen compounds. The NPD detector can measure large ion currents produced in the presence of phosphorous and nitrogen compounds. Other detectors that include a jet assembly or a flame may particularly benefit by the inclusion of a fluid flow path comprising a substantially inert or non-catalytic material. - In certain embodiments, a jet assembly for use in a detector can include a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert metal material. In some examples, the detector can be a FPD, a FID or a NPD or other detector. In certain embodiments, the substantially inert metal material can be present in a major amount. In some examples, the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof. In some examples, the substantially inert metal material is present in a non-coated form. In other examples, the substantially inert metal material is in a tube that is integral to the housing.
- In other embodiments, a jet assembly can include a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In some embodiments, the detector can be a FPD, a FID or a NPD or other detector. In certain embodiments, the non-catalytic metal material is present in a major amount. In other embodiments, the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof. In some embodiments, the non-catalytic metal material is present in a non-coated form. In other embodiments, the non-catalytic metal material is in a tube that is integral to the housing.
- In certain examples, a jet assembly that includes a first tube and a second tube can be used. In some examples, the first tube can be configured to couple to a detector assembly. In other examples, the second tube can be inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In some examples, the detector assembly can be a FPD, a FID or a NPD assembly or other detector assembly. In certain examples, the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof. In some examples, the non-catalytic metal material is present in a non-coated form. In other examples, the second tube is longer than the first tube to fluidically couple to the chromatography column. In additional examples, the first tube comprises a non-catalytic material.
- In certain embodiments, a jet assembly can be used that includes a first tube configured to couple to a detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert metal material. In some embodiments, the detector assembly can be a FPD, a FID or a NPD assembly or other detector assembly. In other embodiments, the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof. In certain embodiments, the substantially inert metal material is present in a non-coated form. In other embodiments, the second tube is longer than the first tube to fluidically couple to the chromatography column. In additional embodiments, the first tube comprises a substantially inert metal material.
- In certain examples, a jet assembly can be used that includes a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis. In some examples, the jet assembly can be used in a FPD, a FID or a NPD or other detector. In other examples, the non-catalytic, non-glass material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof. In certain examples, the non-catalytic, non-glass material is present in a non-coated form. In other examples, the non-catalytic, non-glass material is in a tube that is integral to the housing. In some examples, the housing is configured as a first tube.
- In certain embodiments, a jet assembly can be used that includes a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material. In some embodiments, the jet assembly can be used in a FPD, a FID or a NPD or other detector. In other embodiments, the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof. In additional examples, the substantially inert non-glass, non-stainless steel material is present in a non-coated form. In some examples, the substantially inert non-glass, non-stainless steel material is in a tube that is integral to the housing. In other examples, the housing is configured as a first tube.
- In certain examples, a jet assembly insert can be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal material. In some embodiments, the jet assembly insert can be used in a FPD, a FID or a NPD or other detector. In other examples, the substantially inert metal material is present in a major amount. In some examples, the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain embodiments, a jet assembly insert can be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In some examples, the jet assembly insert can be used in a FPD, a FID or a NPD or other detector. In other embodiments, the non-catalytic metal material is present in a major amount. In certain embodiments, the non-catalytic metal material titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain examples, a jet assembly insert can be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis. In some examples, the jet assembly insert can be used in a FPD, a FID or a NPD or other detector. In other examples, the non-catalytic metal oxide material is present in a major amount. In some examples, the non-catalytic metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain embodiments, a jet assembly insert ca be used that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material. In some embodiments, the jet assembly insert can be used in a FPD, a FID or a NPD or other detector. In other embodiments, the substantially inert metal oxide material is present in a major amount. In some embodiments, the substantially inert metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain examples, a flame detector can be used that includes a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal material. In some embodiments, the flame detector can be a FPD, a FID or a NPD or other detector. In certain embodiments, the substantially inert metal material is present in a major amount. In other examples, the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain embodiments, a flame detector can be used that includes a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In some embodiments, the flame detector can be a FPD, a FID or a NPD or other detector. In other embodiments, the non-catalytic metal material is present in a major amount. In additional embodiment, the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain examples, a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material. In some examples, the flame detector can be a FPD, a FID or a NPD or other detector. In other examples, the substantially inert metal oxide material is present in a major amount. In certain examples, the substantially inert metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain embodiments, a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis. In other embodiments, the flame detector can be a FPD, a FID or a NPD or other detector. In some embodiments, the non-catalytic metal oxide material is present in a major amount. In additional embodiments, the non-catalytic metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain examples, a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis. In other examples, the flame detector can be a FPD, a FID or a NPD or other detector. In some examples, the non-catalytic, non-glass material is present in a major amount. In additional examples, the non-catalytic, non-glass material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain embodiments, a flame detector can include a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material. In other embodiments, the flame detector can be a FPD, a FID or a NPD or other detector. In some embodiments, the substantially inert non-glass, non-stainless steel material is present in a major amount. In additional embodiments, the substantially inert non-glass, non-stainless steel material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In some examples, a kit can include a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert metal material. In additional examples, the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In other examples, a kit can include a jet assembly for use in a flame detector, the jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In some examples, the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In additional examples, a kit can include a jet assembly comprising a first tube configured to couple to a flame photometric detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In some examples, the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In some examples, a kit can include a jet assembly comprising a first tube configured to couple to a flame photometric detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert metal material. In other examples, the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In certain examples, a kit can include a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis. In some examples, the non-catalytic, non-glass material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In other examples, a kit can include a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material. In certain examples, the non-glass, non-stainless steel material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In additional examples, a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal material. In other examples, the substantially inert metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In further examples, a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In other examples, the non-catalytic metal material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In some embodiments, a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis. In other examples, the non-catalytic metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In other embodiments, a kit can include a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material. In other examples, the substantially inert metal oxide material comprises titanium, aluminum, yttrium, titanium oxide, aluminum oxide, yttrium oxide, nickel, chromium, a Hastelloy® alloy, an Inconel® alloy, other alloys commonly available from Haynes International, Inc. or combinations thereof.
- In additional embodiments, a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal material. In other embodiments, the flame detector can be a FPD, a FID or a NPD or other detector.
- In some embodiments, a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path. In other embodiments, the flame detector can be a FPD, a FID or a NPD or other detector.
- In certain examples, a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material. In other examples, the flame detector can be a FPD, a FID or a NPD or other detector.
- In certain embodiments, a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis. In certain examples, the flame detector can be a FPD, a FID or a NPD or other detector.
- In some embodiments, a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis. In certain embodiments, the flame detector can be a FPD, a FID or a NPD or other detector.
- In other embodiments, a kit can include a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material. In some examples, the flame detector can be a FPD, a FID or a NPD or other detector.
- In certain embodiments, a method of facilitating chromatographic analysis includes providing a jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a substantially inert metal material.
- In other embodiments, a method of facilitating chromatographic analysis can include providing a jet assembly comprising a fluid flow path in a housing, in which the fluid flow path is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- In additional embodiments, a method of facilitating chromatographic analysis can include providing a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- In some embodiments, a method of facilitating chromatographic analysis can include providing a jet assembly comprising a first tube configured to couple to a flame detector assembly, and a second tube inside the first tube, in which the second tube comprises a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column to receive sample from the chromatography column, and in which the fluid flow path comprises a major amount of a substantially inert metal material.
- In certain examples, a method of facilitating chromatographic analysis can include providing a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- In other examples, a method of facilitating chromatographic analysis can include providing a jet assembly comprising a fluid flow path inside a housing, in which the fluid flow path comprises, in which the fluid flow path comprises a substantially inert non-glass, non-stainless steel material.
- In additional examples, a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal material.
- In some examples, a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path of comprises a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- In further examples, a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a non-catalytic metal oxide material present in an major amount to deter catalysis.
- In certain embodiments, a method of facilitating chromatographic analysis can include providing a jet assembly insert that is constructed and arranged to couple to a housing of a jet assembly, the jet assembly insert comprising a fluid flow path that is configured to be fluidically coupled to a chromatography column, in which the fluid flow path comprises a substantially inert metal oxide material.
- In other embodiments, a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal material.
- In additional embodiments, a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal material present in an effective amount to deter catalysis in the fluid flow path.
- In certain examples, a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert metal oxide material.
- In other examples, a method of facilitating chromatographic analysis can include providing a flame detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic metal oxide material present in an major amount to deter catalysis.
- In some examples, a method of facilitating chromatographic analysis can include providing a flame photometric detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a non-catalytic, non-glass material present in an effective amount to deter catalysis.
- In additional examples, a method of facilitating chromatographic analysis can include providing a flame photometric detector comprising a flame jet, and a fluid flow path that is constructed and arranged to be fluidically coupled to a chromatography column at one end and to the flame jet at an opposite end, the fluid flow path comprising a substantially inert non-glass, non-stainless steel material.
- In certain embodiments of the jet assemblies, the fluid flow paths can include titanium, yttrium, aluminum, nickel, chromium, a nickel alloy, a chromium alloy, a nickel chromium alloy, Hastelloy® alloys, Inconel® alloys, combinations thereof, or other materials. Certain specific examples are described below to illustrate further the novel technology described herein
- A flame photometric detector including a jet assembly with a conventional stainless steel fluid flow path was compared a flame photometric detector including a jet assembly with a titanium flow path. The titanium flow path was provided using a titanium inner tube that was inserted into the housing and brazed at a single site. The test equipment was a Kin-Tek Model 491-MB gas standards generator, and a VICI gas sampling valve injecting 1 ppm of Hydrogen sulfide gas was used to test the recovery. The gas sampling valve was connected to a Perkin
Elmer model Clarus 500 Gas Chromatograph equipped with an FPD detector and programmable pneumatic control. The sample including hydrogen sulfide (H2S) was injected and analyzed using each of the FPD detectors. Where the conventional jet assembly was present, the H2S peak was absent (seeFIG. 9 ). H2S appeared to react with the stainless steel in the conventional jet assembly. Where the titanium fluid flow path was present, the H2S peak was observed (seeFIG. 10 ), thus confirming that a substantially inert fluid flow path is suitable for use with species such as H2S. - A brazeless jet assembly was tested for its ability to recover H2S. The test equipment was a Kin-Tek Model 491-MB gas standards generator, and a VICI gas sampling valve injecting 1 ppm of Hydrogen sulfide gas was used to test the recovery. The gas sampling valve was connected to a Perkin
Elmer model Clarus 500 Gas Chromatograph equipped with an FPD detector and programmable pneumatic control. - A jet assembly was tested first with a fused silica capillary tube installed all the way to the top of the jet assembly flush with the tip of the jet. The inner tube was produced using titanium. The H2S sample would travel out of the fused silica column without any exposure to the steel jet assembly. This configuration should yield the maximum ideal response for H2S. The H2S sample is not exposed to any metal surface of the jet assembly avoiding any reaction and not incurring any substantial loss of H2S sample. The results using this fused silica capillary tube were used as a control for the comparison of the percent recovery for H2S. The H2S FPD chromatogram response was recorded in the form of peak area (
FIG. 11A ). - The fused silica capillary tube was then removed from the jet assembly allowing the H2S sample to be exposed to and travel inside the length of the jet assembly. The H2S FPD chromatogram response again is recorded in the form of peak area (
FIG. 11B ). The chromatograms were overlaid for comparison, as shown inFIG. 12 . The recovery was then calculated as percentage of peak area response (fused silica to top of jet) divided by peak area response (no fused silica) times 100. A good recovery result will yield a minimum 90% or greater. The calculated recovery was greater than 90%. - For comparison purposes, a similar experiment was conducted using a stainless steel jet assembly. An H2S response is shown in
FIG. 13A , where the stainless steel jet assembly included a fused silica insert. When the fused silica inert was removed from the jet assembly, there was no to minimal response and no recovery of the H2S (FIG. 13B ). All H2S sample was lost due to exposure to the unprotected stainless steel jet body. - A jet assembly was produced from a 1/16 inch outer diameter, 0.034 inch inner diameter swaged inner tube. The inner jet tube was produced using 347 anticorrosion stainless steel (High Nickel, Chromium content, doped with tantalum) and was coated with Silconert-2000.
- The jet assembly was tested using thiophene and the conditions listed in Examples 1 and 2. The results are shown in
FIGS. 14A-14C (three separate runs). Chromatograms for an old jet are provided inFIGS. 15A-15C for comparison. The new FPD jet measured 5.25×10−12 grams Sulfur/second which, is 1.9 times over the published minimum detectable quantity. The old jet, using the exact same method and standard, was 6.64×10−12 grams sulfur/sec., which is 1.5 times the minimum detectable quantity. The retention times for the two jets were about the same, e.g., 1-2 minutes for thiophene. These results are consistent with the Silconert-2000 coated jet assembly providing suitable results for use with sulfur compounds. - The jet assembly of Example 3 was tested using H2S.
FIGS. 16A and 16B are chromatograms showing the initial recovery of the jet at the initial injections using a control (fused silica tube and fused silica tube removed). 745 injections of H2S were then made. Illustrative chromatograms are shown inFIGS. 17A-17C . Measurements were then made with same the control (fused silica tube and fused silica tube removed) after the 745 injections to determine the recovery (seeFIGS. 18A and 18B ). Even after the 745 injections, the recovery of the coated jet assembly was substantially the same as the recovery upon initial injection. These results are consistent with the coated jet assembly providing a long usable life and minimal to no degradation during operation. - When introducing elements of the examples disclosed herein, the articles “a,” “an,” “the” and “said” are intended to mean that there are one or more of the elements. The terms “comprising,” “including” and “having” are intended to be open-ended and mean that there may be additional elements other than the listed elements. It will be recognized by the person of ordinary skill in the art, given the benefit of this disclosure, that various components of the examples can be interchanged or substituted with various components in other examples.
- Although certain aspects, examples and embodiments have been described above, it will be recognized by the person of ordinary skill in the art, given the benefit of this disclosure, that additions, substitutions, modifications, and alterations of the disclosed illustrative aspects, examples and embodiments are possible.
Claims (20)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/615,207 US20150285770A1 (en) | 2010-02-26 | 2015-02-05 | Jet assembly for use in detectors and other devices |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US30849910P | 2010-02-26 | 2010-02-26 | |
| US13/035,938 US8951471B2 (en) | 2010-02-26 | 2011-02-26 | Jet assembly for use in detectors and other devices |
| US14/615,207 US20150285770A1 (en) | 2010-02-26 | 2015-02-05 | Jet assembly for use in detectors and other devices |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/035,938 Continuation US8951471B2 (en) | 2010-02-26 | 2011-02-26 | Jet assembly for use in detectors and other devices |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20150285770A1 true US20150285770A1 (en) | 2015-10-08 |
Family
ID=44505378
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/035,938 Active 2031-11-24 US8951471B2 (en) | 2010-02-26 | 2011-02-26 | Jet assembly for use in detectors and other devices |
| US14/615,207 Abandoned US20150285770A1 (en) | 2010-02-26 | 2015-02-05 | Jet assembly for use in detectors and other devices |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/035,938 Active 2031-11-24 US8951471B2 (en) | 2010-02-26 | 2011-02-26 | Jet assembly for use in detectors and other devices |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US8951471B2 (en) |
| EP (1) | EP2539033A4 (en) |
| CN (1) | CN203244808U (en) |
| AU (1) | AU2011220414B2 (en) |
| CA (1) | CA2790829C (en) |
| SG (1) | SG183177A1 (en) |
| WO (1) | WO2011106747A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020150126A1 (en) * | 2019-01-14 | 2020-07-23 | Agilent Technologies, Inc. | Versatile tube-free jet for gc detector |
| USD917321S1 (en) | 2019-05-13 | 2021-04-27 | Agilent Technologies, Inc. | Gas chromatography detector jet |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2539033A4 (en) * | 2010-02-26 | 2015-01-21 | Perkinelmer Health Sci Inc | Jet assembly for use in detectors and other devices |
Citations (82)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US586383A (en) * | 1897-07-13 | Key-fastener | ||
| US595670A (en) * | 1897-12-14 | Coat and umbrella suspending rack | ||
| US1406572A (en) * | 1921-03-19 | 1922-02-14 | Foster Machine Co | Stop motion for winding machines |
| US1451795A (en) * | 1922-03-20 | 1923-04-17 | Thompson Charles | Direction signal for automobiles |
| US1558594A (en) * | 1924-01-11 | 1925-10-27 | Candee & Company L | Testing machine |
| US2037066A (en) * | 1933-07-10 | 1936-04-14 | Reliable Electric Co | Connecter |
| US2664779A (en) * | 1950-06-13 | 1954-01-05 | John U White | Flame analyzer and flame source therefor |
| US2714833A (en) * | 1950-04-19 | 1955-08-09 | Beckman Instruments Inc | Burner structure for producing spectral flames |
| US3025141A (en) * | 1958-08-21 | 1962-03-13 | Drager Otto H | Process and apparatus for the detection of halogen-containing compounds in air |
| US3086848A (en) * | 1960-05-23 | 1963-04-23 | Phillips Petroleum Co | Gas analyzer |
| US3088808A (en) * | 1957-10-28 | 1963-05-07 | Pennsalt Chemicals Corp | Flame photometry |
| US3205711A (en) * | 1963-04-11 | 1965-09-14 | Microtek Instr Inc | Sample injection in gas chromatography |
| US3213747A (en) * | 1961-01-19 | 1965-10-26 | Drager Otto H | Process for detecting phosphorous and/or sulphur in a gas |
| US3298785A (en) * | 1962-01-13 | 1967-01-17 | Heraeus Schott Quarzschmelze | Burner and method of use |
| US3355950A (en) * | 1965-07-01 | 1967-12-05 | Prec Sampling Corp | Chromatographic sample injection apparatus |
| US3372000A (en) * | 1964-02-27 | 1968-03-05 | Beckman Instruments Inc | Flame ionization detector |
| US3384457A (en) * | 1963-12-04 | 1968-05-21 | Gen Am Transport | Ionization detector and sampling system |
| US3399039A (en) * | 1965-03-22 | 1968-08-27 | Varian Associates | Flame ionization detector |
| US3451780A (en) * | 1966-02-18 | 1969-06-24 | Shell Oil Co | Flame ionization detector |
| US3455657A (en) * | 1965-10-14 | 1969-07-15 | Bodenseewerk Perkin Elmer Co | Flame ionization detector |
| US3489498A (en) * | 1965-11-05 | 1970-01-13 | Melpar Inc | Flame photometric detector with improved specificity to sulfur and phosphorus |
| US3545936A (en) * | 1966-10-01 | 1970-12-08 | Bodenseewerk Perkin Elmer Co | Flame ionization detector |
| US3547588A (en) * | 1968-01-12 | 1970-12-15 | Sumitomo Chemical Co | Gaschromatographic determination of minute amount of organophosphorus compounds |
| US3567391A (en) * | 1969-09-22 | 1971-03-02 | North American Rockwell | Analysis for biochemical oxygen demand |
| US3585003A (en) * | 1967-04-06 | 1971-06-15 | Varian Associates | Ionization detector for gas chromatography |
| US3661533A (en) * | 1969-09-22 | 1972-05-09 | Tracor | Adjustable apparatus for flame ionization and flame emission detection |
| US3677709A (en) * | 1969-07-14 | 1972-07-18 | Hewlett Packard Gmbh | Flame ionization detection apparatus and method |
| US3690146A (en) * | 1970-09-10 | 1972-09-12 | Hartmann & Braun Ag | Device for metering a particular quantity of fluid |
| US3698643A (en) * | 1971-01-15 | 1972-10-17 | Owens Illinois Inc | Inert gas shield for atomic absorption or flame emission spectrometer |
| US3767363A (en) * | 1970-08-06 | 1973-10-23 | Hartmann & Braun Ag | Flame ionization detection |
| US3803290A (en) * | 1970-08-31 | 1974-04-09 | Chlortrol Inc | Waste extraction process |
| US3806250A (en) * | 1971-02-05 | 1974-04-23 | Pye Ltd | Nebuliser assemblies for flame spectrometry |
| US3840343A (en) * | 1971-11-30 | 1974-10-08 | Hewlett Packard Gmbh | Flame detector |
| US3874592A (en) * | 1971-12-15 | 1975-04-01 | Texaco Development Corp | Burner for the partial oxidation of hydrocarbons to synthesis gas |
| US3877819A (en) * | 1968-02-21 | 1975-04-15 | Us Navy | Apparatus for detection of phosphorus or sulfur containing vapors or aerosols |
| US3879126A (en) * | 1972-03-08 | 1975-04-22 | Varian Associates | Flame photometric detector employing premixed hydrogen and oxygen gases |
| US3984205A (en) * | 1975-09-10 | 1976-10-05 | The Foxboro Company | Flame ionization detector |
| JPS526596A (en) * | 1975-07-04 | 1977-01-19 | Hitachi Ltd | Detector for gas chromatograph |
| US4097239A (en) * | 1977-02-28 | 1978-06-27 | Varian Associates, Inc. | Two-flame burner for flame photometric detection |
| US4111554A (en) * | 1975-02-18 | 1978-09-05 | Compagnie Francaise De Raffinage | Process for the specific quantitative detection of sulfur compounds and apparatus for carrying out this process |
| US4119404A (en) * | 1977-07-05 | 1978-10-10 | Core Laboratories, Inc. | Apparatus and method for sour gas analysis |
| US4167334A (en) * | 1978-03-30 | 1979-09-11 | Phillips Petroleum Co. | Flame head for flame photometric detector used in gas chromatography |
| JPS54130087A (en) * | 1978-03-31 | 1979-10-09 | Hitachi Ltd | Thermal ionization detector |
| US4182740A (en) * | 1976-03-01 | 1980-01-08 | Varian Associates, Inc. | Flame ionization detector |
| US4466943A (en) * | 1979-11-28 | 1984-08-21 | Nissan Motor Co., Ltd. | Flame photometric detector analyzer |
| US4473171A (en) * | 1981-04-16 | 1984-09-25 | Kennecott Corporation | Nozzle construction for jacketed pressure vessels |
| US4525138A (en) * | 1983-10-28 | 1985-06-25 | Union Carbide Corporation | Flame signal enhancer for post-mixed burner |
| US4596463A (en) * | 1983-11-22 | 1986-06-24 | Errol Akomer | Atomic spectroscopy surface burner |
| US4793556A (en) * | 1984-12-21 | 1988-12-27 | National Research Development Corporation | Method of and apparatus for the nebulization of liquids and liquid suspensions |
| US4798805A (en) * | 1985-04-05 | 1989-01-17 | Geoservices, Societe Anonyme | Apparatus and process for pyrolysis and analysis of samples containing organic matter |
| US4842825A (en) * | 1986-06-19 | 1989-06-27 | Ruska Laboratories, Inc. | Apparatus for determining chemical structure |
| US4999162A (en) * | 1988-08-26 | 1991-03-12 | Varian Associates, Inc. | High temperature flame jet for gas chromatography |
| US5083004A (en) * | 1989-05-09 | 1992-01-21 | Varian Associates, Inc. | Spectroscopic plasma torch for microwave induced plasmas |
| JPH04157361A (en) * | 1990-10-20 | 1992-05-29 | Toshinori Saito | Super-critical fluid chromatograph |
| US5227135A (en) * | 1988-11-25 | 1993-07-13 | Sievers Research, Inc. | Apparatus for simultaneous measurement of sulfur and non-sulfur containing compounds |
| US5246868A (en) * | 1987-10-26 | 1993-09-21 | Research Corporation Technologies, Inc. | Infrared emission detection |
| US5338488A (en) * | 1992-09-10 | 1994-08-16 | Council Of Scientific Research | Process for the production of synthesis gas by oxidative converson of methane (or natural gas) using composite catalyst containing transitional and alkine earth metal oxides |
| US5473162A (en) * | 1987-10-26 | 1995-12-05 | Baylor University | Infrared emission detection of a gas |
| US5501981A (en) * | 1988-11-25 | 1996-03-26 | Sievers Instruments, Inc. | Method and apparatus for chemiluminescent detection of sulfur |
| US5576626A (en) * | 1995-01-17 | 1996-11-19 | Microsensor Technology, Inc. | Compact and low fuel consumption flame ionization detector with flame tip on diffuser |
| US5578271A (en) * | 1995-03-01 | 1996-11-26 | O.I. Corporation | Tandem photoionization detector and halogen specific detector |
| JPH0961358A (en) * | 1995-08-28 | 1997-03-07 | Shimadzu Corp | Flame photometric detector |
| US5614417A (en) * | 1993-10-07 | 1997-03-25 | Kubala; Sidney W. | Sulfur chemiluminescence detection method |
| US5696378A (en) * | 1996-03-20 | 1997-12-09 | Baylor University | High accuracy determination of chlorine content by isotope dilution flame infrared emission spectrometry (ID-FIRE) |
| JPH10111246A (en) * | 1996-10-08 | 1998-04-28 | Shimadzu Corp | Nozzle for flame photometric detector and its manufacture |
| US6130095A (en) * | 1992-01-23 | 2000-10-10 | Sievers Instruments, Inc. | Method for the measurement of sulfur compounds |
| US6187226B1 (en) * | 1995-03-14 | 2001-02-13 | Bechtel Bwxt Idaho, Llc | Thermal device and method for production of carbon monoxide and hydrogen by thermal dissociation of hydrocarbon gases |
| US6238622B1 (en) * | 1997-12-05 | 2001-05-29 | Rosemount Analytical Inc. | Flame ionization detector |
| US20020024672A1 (en) * | 2000-07-11 | 2002-02-28 | Shimadzu Corporation | Flame photometric detector |
| US20020050097A1 (en) * | 1997-09-05 | 2002-05-02 | Fournier Donald J. | Method and apparatus for producing reformed gases |
| US20020121370A1 (en) * | 2000-12-08 | 2002-09-05 | Schlumberger Technology Corporation | Method and apparatus for hydrogen sulfide monitoring |
| US6620624B1 (en) * | 2000-08-10 | 2003-09-16 | Okazaki National Research Institutes | Mass spectrometry interface, a mass spectrometer and a mass spectrometry |
| US20030211631A1 (en) * | 1998-10-09 | 2003-11-13 | Cameron Skinner | Microfluidic devices connected to capillaries with minimal dead volume |
| US20040173579A1 (en) * | 2003-03-07 | 2004-09-09 | Carr Jeffrey W. | Apparatus and method for non-contact cleaning of a surface |
| US20040195379A1 (en) * | 2003-03-11 | 2004-10-07 | Trent Rance | Spray nozzle suitable for use in hot corrosive environments and method of use |
| US20050178747A1 (en) * | 2004-01-13 | 2005-08-18 | Shimadzu Corporation | Flame photometric detector of gas chromatograph |
| US20050226765A1 (en) * | 2000-09-02 | 2005-10-13 | Phlogiston Scientific Limited | Analyte detection system |
| US20050287033A1 (en) * | 2004-06-25 | 2005-12-29 | University Technologies International Inc. | Micro flame detector and method for gas chromatography |
| US20060213875A1 (en) * | 2005-03-25 | 2006-09-28 | Shimadzu Corporation | Flame photometric detector of gas chromatograph |
| US20060275174A1 (en) * | 2005-05-13 | 2006-12-07 | Horiba, Ltd. | Transportable measuring apparatus utilizing hydrogen flame |
| US20120022287A1 (en) * | 2009-03-23 | 2012-01-26 | Bala Subramaniam | Spray Process for Selective Oxidation |
| US8951471B2 (en) * | 2010-02-26 | 2015-02-10 | Perkinelmer Health Sciences, Inc. | Jet assembly for use in detectors and other devices |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH465271A (en) | 1963-10-02 | 1968-11-15 | Ceskoslovenska Akademie Ved | Column closure for column chromatograph |
| GB1335347A (en) | 1970-01-24 | 1973-10-24 | Becker Delft Nv | Gas chromatography apparatus |
| GB1406572A (en) | 1973-03-29 | 1975-09-17 | Foxboro Co | Flame ionization detector |
| GB2037066B (en) * | 1978-10-09 | 1983-02-16 | Simpson C | Flame ionisation detector and method of use thereof |
| US5811665A (en) * | 1996-09-11 | 1998-09-22 | Alanex Corporation | Large scale sorption-driven solid state chromatography |
| US6579459B2 (en) * | 1996-11-13 | 2003-06-17 | Transgenomic, Inc. | System and method for performing polynucleotide separations using liquid chromatography |
| NL1012127C2 (en) * | 1999-05-21 | 2000-11-23 | Sgt Exploitatie Bv | Assembly for desorbing sampling tubes, as well as an adapter and sampling tubes apparently intended for such an assembly, as well as a kit of parts for forming such an assembly. |
| US6907796B2 (en) * | 2001-05-30 | 2005-06-21 | Gerstel Systemtechnik Gmbh & Co. Kg | Temperature-controlled injector for a chemical analysis unit |
| US6601438B2 (en) * | 2001-09-28 | 2003-08-05 | Systec, Inc. | Apparatus for conducting high-temperature liquid chromotography analysis |
| US20040236083A1 (en) * | 2003-05-14 | 2004-11-25 | Libert Sharon Ann | Protein separation column |
| WO2005071396A1 (en) * | 2004-01-23 | 2005-08-04 | Waters Investments Limited | A sample injector system for liquid chromatography |
| US7384457B2 (en) * | 2005-10-21 | 2008-06-10 | Agilent Technologies, Inc. | Seal for gas chromatography |
| US20070181479A1 (en) * | 2005-11-21 | 2007-08-09 | Pentax Corporation | Column and method of manufacturing the column |
-
2011
- 2011-02-26 EP EP11748211.7A patent/EP2539033A4/en active Pending
- 2011-02-26 AU AU2011220414A patent/AU2011220414B2/en not_active Ceased
- 2011-02-26 US US13/035,938 patent/US8951471B2/en active Active
- 2011-02-26 SG SG2012057873A patent/SG183177A1/en unknown
- 2011-02-26 CN CN2011900003550U patent/CN203244808U/en not_active Expired - Lifetime
- 2011-02-26 CA CA2790829A patent/CA2790829C/en active Active
- 2011-02-26 WO PCT/US2011/026385 patent/WO2011106747A1/en active Application Filing
-
2015
- 2015-02-05 US US14/615,207 patent/US20150285770A1/en not_active Abandoned
Patent Citations (82)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US586383A (en) * | 1897-07-13 | Key-fastener | ||
| US595670A (en) * | 1897-12-14 | Coat and umbrella suspending rack | ||
| US1406572A (en) * | 1921-03-19 | 1922-02-14 | Foster Machine Co | Stop motion for winding machines |
| US1451795A (en) * | 1922-03-20 | 1923-04-17 | Thompson Charles | Direction signal for automobiles |
| US1558594A (en) * | 1924-01-11 | 1925-10-27 | Candee & Company L | Testing machine |
| US2037066A (en) * | 1933-07-10 | 1936-04-14 | Reliable Electric Co | Connecter |
| US2714833A (en) * | 1950-04-19 | 1955-08-09 | Beckman Instruments Inc | Burner structure for producing spectral flames |
| US2664779A (en) * | 1950-06-13 | 1954-01-05 | John U White | Flame analyzer and flame source therefor |
| US3088808A (en) * | 1957-10-28 | 1963-05-07 | Pennsalt Chemicals Corp | Flame photometry |
| US3025141A (en) * | 1958-08-21 | 1962-03-13 | Drager Otto H | Process and apparatus for the detection of halogen-containing compounds in air |
| US3086848A (en) * | 1960-05-23 | 1963-04-23 | Phillips Petroleum Co | Gas analyzer |
| US3213747A (en) * | 1961-01-19 | 1965-10-26 | Drager Otto H | Process for detecting phosphorous and/or sulphur in a gas |
| US3298785A (en) * | 1962-01-13 | 1967-01-17 | Heraeus Schott Quarzschmelze | Burner and method of use |
| US3205711A (en) * | 1963-04-11 | 1965-09-14 | Microtek Instr Inc | Sample injection in gas chromatography |
| US3384457A (en) * | 1963-12-04 | 1968-05-21 | Gen Am Transport | Ionization detector and sampling system |
| US3372000A (en) * | 1964-02-27 | 1968-03-05 | Beckman Instruments Inc | Flame ionization detector |
| US3399039A (en) * | 1965-03-22 | 1968-08-27 | Varian Associates | Flame ionization detector |
| US3355950A (en) * | 1965-07-01 | 1967-12-05 | Prec Sampling Corp | Chromatographic sample injection apparatus |
| US3455657A (en) * | 1965-10-14 | 1969-07-15 | Bodenseewerk Perkin Elmer Co | Flame ionization detector |
| US3489498A (en) * | 1965-11-05 | 1970-01-13 | Melpar Inc | Flame photometric detector with improved specificity to sulfur and phosphorus |
| US3451780A (en) * | 1966-02-18 | 1969-06-24 | Shell Oil Co | Flame ionization detector |
| US3545936A (en) * | 1966-10-01 | 1970-12-08 | Bodenseewerk Perkin Elmer Co | Flame ionization detector |
| US3585003A (en) * | 1967-04-06 | 1971-06-15 | Varian Associates | Ionization detector for gas chromatography |
| US3547588A (en) * | 1968-01-12 | 1970-12-15 | Sumitomo Chemical Co | Gaschromatographic determination of minute amount of organophosphorus compounds |
| US3877819A (en) * | 1968-02-21 | 1975-04-15 | Us Navy | Apparatus for detection of phosphorus or sulfur containing vapors or aerosols |
| US3677709A (en) * | 1969-07-14 | 1972-07-18 | Hewlett Packard Gmbh | Flame ionization detection apparatus and method |
| US3567391A (en) * | 1969-09-22 | 1971-03-02 | North American Rockwell | Analysis for biochemical oxygen demand |
| US3661533A (en) * | 1969-09-22 | 1972-05-09 | Tracor | Adjustable apparatus for flame ionization and flame emission detection |
| US3767363A (en) * | 1970-08-06 | 1973-10-23 | Hartmann & Braun Ag | Flame ionization detection |
| US3803290A (en) * | 1970-08-31 | 1974-04-09 | Chlortrol Inc | Waste extraction process |
| US3690146A (en) * | 1970-09-10 | 1972-09-12 | Hartmann & Braun Ag | Device for metering a particular quantity of fluid |
| US3698643A (en) * | 1971-01-15 | 1972-10-17 | Owens Illinois Inc | Inert gas shield for atomic absorption or flame emission spectrometer |
| US3806250A (en) * | 1971-02-05 | 1974-04-23 | Pye Ltd | Nebuliser assemblies for flame spectrometry |
| US3840343A (en) * | 1971-11-30 | 1974-10-08 | Hewlett Packard Gmbh | Flame detector |
| US3874592A (en) * | 1971-12-15 | 1975-04-01 | Texaco Development Corp | Burner for the partial oxidation of hydrocarbons to synthesis gas |
| US3879126A (en) * | 1972-03-08 | 1975-04-22 | Varian Associates | Flame photometric detector employing premixed hydrogen and oxygen gases |
| US4111554A (en) * | 1975-02-18 | 1978-09-05 | Compagnie Francaise De Raffinage | Process for the specific quantitative detection of sulfur compounds and apparatus for carrying out this process |
| JPS526596A (en) * | 1975-07-04 | 1977-01-19 | Hitachi Ltd | Detector for gas chromatograph |
| US3984205A (en) * | 1975-09-10 | 1976-10-05 | The Foxboro Company | Flame ionization detector |
| US4182740A (en) * | 1976-03-01 | 1980-01-08 | Varian Associates, Inc. | Flame ionization detector |
| US4097239A (en) * | 1977-02-28 | 1978-06-27 | Varian Associates, Inc. | Two-flame burner for flame photometric detection |
| US4119404A (en) * | 1977-07-05 | 1978-10-10 | Core Laboratories, Inc. | Apparatus and method for sour gas analysis |
| US4167334A (en) * | 1978-03-30 | 1979-09-11 | Phillips Petroleum Co. | Flame head for flame photometric detector used in gas chromatography |
| JPS54130087A (en) * | 1978-03-31 | 1979-10-09 | Hitachi Ltd | Thermal ionization detector |
| US4466943A (en) * | 1979-11-28 | 1984-08-21 | Nissan Motor Co., Ltd. | Flame photometric detector analyzer |
| US4473171A (en) * | 1981-04-16 | 1984-09-25 | Kennecott Corporation | Nozzle construction for jacketed pressure vessels |
| US4525138A (en) * | 1983-10-28 | 1985-06-25 | Union Carbide Corporation | Flame signal enhancer for post-mixed burner |
| US4596463A (en) * | 1983-11-22 | 1986-06-24 | Errol Akomer | Atomic spectroscopy surface burner |
| US4793556A (en) * | 1984-12-21 | 1988-12-27 | National Research Development Corporation | Method of and apparatus for the nebulization of liquids and liquid suspensions |
| US4798805A (en) * | 1985-04-05 | 1989-01-17 | Geoservices, Societe Anonyme | Apparatus and process for pyrolysis and analysis of samples containing organic matter |
| US4842825A (en) * | 1986-06-19 | 1989-06-27 | Ruska Laboratories, Inc. | Apparatus for determining chemical structure |
| US5246868A (en) * | 1987-10-26 | 1993-09-21 | Research Corporation Technologies, Inc. | Infrared emission detection |
| US5473162A (en) * | 1987-10-26 | 1995-12-05 | Baylor University | Infrared emission detection of a gas |
| US4999162A (en) * | 1988-08-26 | 1991-03-12 | Varian Associates, Inc. | High temperature flame jet for gas chromatography |
| US5227135A (en) * | 1988-11-25 | 1993-07-13 | Sievers Research, Inc. | Apparatus for simultaneous measurement of sulfur and non-sulfur containing compounds |
| US5501981A (en) * | 1988-11-25 | 1996-03-26 | Sievers Instruments, Inc. | Method and apparatus for chemiluminescent detection of sulfur |
| US5083004A (en) * | 1989-05-09 | 1992-01-21 | Varian Associates, Inc. | Spectroscopic plasma torch for microwave induced plasmas |
| JPH04157361A (en) * | 1990-10-20 | 1992-05-29 | Toshinori Saito | Super-critical fluid chromatograph |
| US6130095A (en) * | 1992-01-23 | 2000-10-10 | Sievers Instruments, Inc. | Method for the measurement of sulfur compounds |
| US5338488A (en) * | 1992-09-10 | 1994-08-16 | Council Of Scientific Research | Process for the production of synthesis gas by oxidative converson of methane (or natural gas) using composite catalyst containing transitional and alkine earth metal oxides |
| US5614417A (en) * | 1993-10-07 | 1997-03-25 | Kubala; Sidney W. | Sulfur chemiluminescence detection method |
| US5576626A (en) * | 1995-01-17 | 1996-11-19 | Microsensor Technology, Inc. | Compact and low fuel consumption flame ionization detector with flame tip on diffuser |
| US5578271A (en) * | 1995-03-01 | 1996-11-26 | O.I. Corporation | Tandem photoionization detector and halogen specific detector |
| US6187226B1 (en) * | 1995-03-14 | 2001-02-13 | Bechtel Bwxt Idaho, Llc | Thermal device and method for production of carbon monoxide and hydrogen by thermal dissociation of hydrocarbon gases |
| JPH0961358A (en) * | 1995-08-28 | 1997-03-07 | Shimadzu Corp | Flame photometric detector |
| US5696378A (en) * | 1996-03-20 | 1997-12-09 | Baylor University | High accuracy determination of chlorine content by isotope dilution flame infrared emission spectrometry (ID-FIRE) |
| JPH10111246A (en) * | 1996-10-08 | 1998-04-28 | Shimadzu Corp | Nozzle for flame photometric detector and its manufacture |
| US20020050097A1 (en) * | 1997-09-05 | 2002-05-02 | Fournier Donald J. | Method and apparatus for producing reformed gases |
| US6238622B1 (en) * | 1997-12-05 | 2001-05-29 | Rosemount Analytical Inc. | Flame ionization detector |
| US20030211631A1 (en) * | 1998-10-09 | 2003-11-13 | Cameron Skinner | Microfluidic devices connected to capillaries with minimal dead volume |
| US20020024672A1 (en) * | 2000-07-11 | 2002-02-28 | Shimadzu Corporation | Flame photometric detector |
| US6620624B1 (en) * | 2000-08-10 | 2003-09-16 | Okazaki National Research Institutes | Mass spectrometry interface, a mass spectrometer and a mass spectrometry |
| US20050226765A1 (en) * | 2000-09-02 | 2005-10-13 | Phlogiston Scientific Limited | Analyte detection system |
| US20020121370A1 (en) * | 2000-12-08 | 2002-09-05 | Schlumberger Technology Corporation | Method and apparatus for hydrogen sulfide monitoring |
| US20040173579A1 (en) * | 2003-03-07 | 2004-09-09 | Carr Jeffrey W. | Apparatus and method for non-contact cleaning of a surface |
| US20040195379A1 (en) * | 2003-03-11 | 2004-10-07 | Trent Rance | Spray nozzle suitable for use in hot corrosive environments and method of use |
| US20050178747A1 (en) * | 2004-01-13 | 2005-08-18 | Shimadzu Corporation | Flame photometric detector of gas chromatograph |
| US20050287033A1 (en) * | 2004-06-25 | 2005-12-29 | University Technologies International Inc. | Micro flame detector and method for gas chromatography |
| US20060213875A1 (en) * | 2005-03-25 | 2006-09-28 | Shimadzu Corporation | Flame photometric detector of gas chromatograph |
| US20060275174A1 (en) * | 2005-05-13 | 2006-12-07 | Horiba, Ltd. | Transportable measuring apparatus utilizing hydrogen flame |
| US20120022287A1 (en) * | 2009-03-23 | 2012-01-26 | Bala Subramaniam | Spray Process for Selective Oxidation |
| US8951471B2 (en) * | 2010-02-26 | 2015-02-10 | Perkinelmer Health Sciences, Inc. | Jet assembly for use in detectors and other devices |
Non-Patent Citations (7)
| Title |
|---|
| 52-6596 * |
| Clarus 600 GC Hardware Guide, publication date November 2006, 441 pages. * |
| Dziurdzia, B. et al, Measurement Science and Technology 2008, 19, Article 55206, 6 pages. * |
| Groeger, W. et al, Review of Scientific Instruments 1988, 59, 1971-1979. * |
| Jiang, G. B. et al, Analytical Chemistry 1991, 63, 1506-1509. * |
| Micheli, A. L. et al, Journal of the American Ceramic Society 1992, 75, 709-711. * |
| Yarita, T. et al, Journal of Chromatography A 2002, 976, 387-391. * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020150126A1 (en) * | 2019-01-14 | 2020-07-23 | Agilent Technologies, Inc. | Versatile tube-free jet for gc detector |
| CN113994206A (en) * | 2019-01-14 | 2022-01-28 | 安捷伦科技有限公司 | Universal tubeless nozzle for GC detector |
| US12117424B2 (en) | 2019-01-14 | 2024-10-15 | Agilent Technologies, Inc. | Versatile tube-free jet for gas chromatography detector having a conical inlet skirt |
| US12130266B2 (en) | 2019-01-14 | 2024-10-29 | Agilent Technologies, Inc | Versatile tube-free jet for gas chromatography detector having a conical inlet skirt |
| USD917321S1 (en) | 2019-05-13 | 2021-04-27 | Agilent Technologies, Inc. | Gas chromatography detector jet |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2539033A4 (en) | 2015-01-21 |
| CN203244808U (en) | 2013-10-23 |
| AU2011220414B2 (en) | 2016-09-15 |
| US20110211993A1 (en) | 2011-09-01 |
| WO2011106747A1 (en) | 2011-09-01 |
| SG183177A1 (en) | 2012-09-27 |
| CA2790829A1 (en) | 2011-09-01 |
| EP2539033A1 (en) | 2013-01-02 |
| AU2011220414A1 (en) | 2012-10-18 |
| CA2790829C (en) | 2017-10-03 |
| US8951471B2 (en) | 2015-02-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20210319998A1 (en) | Fluid chromatography injectors and injector inserts | |
| Merritt et al. | Performance and optimization of a combustion interface for isotope ratio monitoring gas chromatography/mass spectrometry | |
| US20150285770A1 (en) | Jet assembly for use in detectors and other devices | |
| US20120228872A1 (en) | Fitting component, ferrule and nut | |
| US8890534B2 (en) | Surface ionization detector | |
| US5289003A (en) | Probe for thermospray mass spectrometry | |
| Holmstrand et al. | Compound‐specific bromine isotope analysis of brominated diphenyl ethers using gas chromatography multiple collector/inductively coupled plasma mass spectrometry | |
| US9824876B2 (en) | Fluid chromatography injectors and injector inserts | |
| Colón et al. | Detectors in modern gas chromatography | |
| US8196449B2 (en) | Micro discharge device capable of low voltage discharges in a variety of carrier gases for detection and/or ionization | |
| US8913239B2 (en) | Apparatus and method for quenching-resistant multiple flame photometric detector | |
| JP6460452B2 (en) | GC column connection with flat connection to the meshing device | |
| EP2543995B1 (en) | Helium ionization detector | |
| de Zeeuw et al. | Simplifying the setup for vacuum‐outlet GC: Using a restriction inside the injection port | |
| Gary | Examination of a helium highly efficient microwave-induced plasma as an element-selective detector for supercritical fluid chromatography | |
| US20100209302A1 (en) | Apparatus With A Connection Between Two Capillaries | |
| US20240125745A1 (en) | Chromatography column adaptor and use for fluidic connections | |
| WO2024192627A1 (en) | Flame-based detectors with protected ignitor | |
| WO2024192625A1 (en) | Flame-based detectors with ignition promoter port | |
| JP3158049U (en) | Gas chromatograph | |
| Bramston-Cook | Primer on Flame Ionization Detectors | |
| GC et al. | Development of a Miniature Gas Chromatograph (µCAD) with |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: FINAL REJECTION MAILED |
|
| STCV | Information on status: appeal procedure |
Free format text: NOTICE OF APPEAL FILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: FINAL REJECTION MAILED |
|
| STCV | Information on status: appeal procedure |
Free format text: ON APPEAL -- AWAITING DECISION BY THE BOARD OF APPEALS |
|
| STCV | Information on status: appeal procedure |
Free format text: BOARD OF APPEALS DECISION RENDERED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: DOCKETED NEW CASE - READY FOR EXAMINATION |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| AS | Assignment |
Owner name: OWL ROCK CAPITAL CORPORATION, NEW YORK Free format text: SECURITY INTEREST;ASSIGNOR:PERKINELMER U.S. LLC;REEL/FRAME:066839/0109 Effective date: 20230313 |
|
| AS | Assignment |
Owner name: PERKINELMER U.S. LLC, CONNECTICUT Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:PERKINELMER HEALTH SCIENCES INC.;REEL/FRAME:063172/0900 Effective date: 20230313 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO PAY ISSUE FEE |
|
| AS | Assignment |
Owner name: PERKINELMER HEALTH SCIENCES, INC., MASSACHUSETTS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:MANNINO, ROSARIO;REEL/FRAME:064093/0597 Effective date: 20110429 |