US20120228176A1 - Multi-dose nutritional supplements - Google Patents
Multi-dose nutritional supplements Download PDFInfo
- Publication number
- US20120228176A1 US20120228176A1 US13/169,712 US201113169712A US2012228176A1 US 20120228176 A1 US20120228176 A1 US 20120228176A1 US 201113169712 A US201113169712 A US 201113169712A US 2012228176 A1 US2012228176 A1 US 2012228176A1
- Authority
- US
- United States
- Prior art keywords
- time
- supplement
- predetermined period
- dose package
- nutritional
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J7/00—Devices for administering medicines orally, e.g. spoons; Pill counting devices; Arrangements for time indication or reminder for taking medicine
- A61J7/04—Arrangements for time indication or reminder for taking medicine, e.g. programmed dispensers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
- A61J1/03—Containers specially adapted for medical or pharmaceutical purposes for pills or tablets
- A61J1/035—Blister-type containers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J7/00—Devices for administering medicines orally, e.g. spoons; Pill counting devices; Arrangements for time indication or reminder for taking medicine
- A61J7/04—Arrangements for time indication or reminder for taking medicine, e.g. programmed dispensers
- A61J7/0409—Arrangements for time indication or reminder for taking medicine, e.g. programmed dispensers with timers
- A61J7/0454—Arrangements for time indication or reminder for taking medicine, e.g. programmed dispensers with timers for dispensing of multiple drugs
Definitions
- the present invention relates to nutritional supplements and, in particular, nutritional supplements having varying dosages over a predetermined time period of time depending upon when the particular supplement is intended to be consumed within such predetermined time period.
- Nutritional supplements are commonly used for the purpose of maintaining good health.
- Nutritional supplements can include vitamins, ‘minerals’, amino acids, fiber, herbs or other botanicals, etc., which are traditionally sold in packages where all the unit dosages of a particular supplement are the same.
- Nutritional supplements can include vitamins, which are defined as any of various organic substances that are essential in minute quantities to the nutrition of most animals. Vitamins act especially as coenzymes and precursors of coenzymes in the regulation of metabolic processes, but do not provide energy or serve as building units. There are both fat-soluble vitamins (for example, Vitamin E), and water-soluble vitamins (for example, Vitamin C).
- Nutritional supplements can also include “minerals”, which are a chemical element required by living organisms to support the biochemical reactions of metabolism, and maintain optimal health. These required chemical elements can be are naturally present in food (e.g., calcium in milk), or can be added to food (e.g., iodized salt) or included in a dietary supplement.
- Nutritional supplements can also include neutraceuticals, which can be a fortified food that provides health or medical benefits in addition to its basic nutritional value.
- Nutritional supplements may further include, as vitamins, minerals, neutraceuticals, or in additional to those categories of supplements, soluble and insoluble fiber, fatty acids, herbs, plants, amino acids and digestive enzymes or any other substance or compound having or promoting a health benefit to consumers.
- nutritional supplements as well as their components, such as vitamins, minerals, neutraceuticals, etc. are traditionally sold in packages where all the unit dosages of a particular supplement are the same.
- multi-dose packages of nutritional supplements are provided that are designed for regular and continuous use by a specific target (i.e., consumer) over a reoccurring predetermined period of time, such as a day, month, season, year, or other defined time period.
- the multi-dose packaging of nutritional supplements contains supplements that vary in dosage and that are each intended to be taken at a specific time during the predetermined period of time.
- Each supplement in the series is tailored to contain unit dosages of nutritional supplements that are designed to address specific needs (e.g., psychological needs) of an individual during the predetermined period of time at which the supplement is intended to be ingested.
- the multi-dose package may contain at least a first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement, where the amount of the nutritional supplement in the first unit dosage differs from the amount of the nutritional supplement in the second unit dosage.
- the multi-dose packages may be prepackages or tailored for the specific needs of an individual with specific conditions having a varying need over time.
- the subject invention provides devices, systems and methods for meeting the nutritional needs of a subject.
- the nutritional needs of a subject may vary over time.
- representative implementations of the subject multi-dose nutritional supplement packages are described in detail.
- FIG. 1 illustrates one example of a weekly multi-dose package of the invention.
- FIG. 2 illustrates another example of a weekly multi-dose package of the invention.
- FIG. 3 illustrates one example of a twenty-eight day multi-dose package of the invention.
- FIG. 4 illustrates one example of a six month multi-dose package of the invention.
- the present invention relates to multi-dose packages of nutritional supplements designed for regular and continuous use by a specific target (i.e., consumer) over a reoccurring predetermined period of time (e.g., a day, month, season, year, or other defined time period).
- the multi-dose packaging of nutritional supplements contains supplements that vary in dosage and that are each intended to be taken at a specific time during the predetermined period of time.
- Each supplement in the series is tailored to contain unit dosages of nutritional supplements that are designed to address specific needs of an individual during the predetermined period of time at which each supplement is intended to be ingested.
- a “supplement” or a “nutritional supplement” is a vitamin, mineral, neutraceutical, enzyme, including digestive enzymes, or other ingestible substance having or promoting a health benefit or providing treatment for a particular condition.
- a “vitamin” is any of various organic substances that are essential in minute quantities to the nutrition of most animals. Vitamins act especially as coenzymes and precursors of coenzymes in the regulation of metabolic processes, but do not provide energy or serve as building units.
- Vitamins can include, but are not limited to, Vitamin A (Retinol), Vitamin B1 (Thiamine), Vitamin B2 (Riboflavin), Vitamin B3 (Niacin), Vitamin B5 (Pantothenic acid), Vitamin B6 (Pyridoxine), Vitamin B7 (Biotin), Vitamin B9 (Folic acid), Vitamin B12 (Cobalamins), Vitamin C (Ascorbic acid), Vitamin D (Calciferol), Vitamin E (Tocopherol), and Vitamin K1 (Phylloquinone).
- a “mineral” is a chemical element required by living organisms to support the biochemical reactions of metabolism, and maintain optimal health. These required chemical elements can be are naturally present in food (e.g., calcium in milk), or can be added to food (e.g., iodized salt) or included in a dietary supplement. Minerals can include but are not limited to: potassium, chlorine, sodium, calcium, phosphorus, magnesium, zinc, iron, manganese, copper, iodine, selenium, and molybdenum.
- a “neutraceutical” is a dietary supplement or a fortified food that provides health or medical benefits in addition to its basic nutritional value.
- a “dietary supplement” is a product taken orally that contains one or more nutrients that are intended to supplement one's diet and are not considered food, specifically according to the Dietary Supplements Health and Education Act.
- a dietary supplement can contain one or more vitamins, minerals, fiber, fatty acids, herbs or other botanicals, or amino acids, or a concentrate, constituent, metabolite, extract, or combination of these.
- Fatty acids can include “essential fatty acids,” which are fatty acids that humans and other animals cannot synthesize, but must obtain in the diet because they are required for certain biological processes.
- the two essential fatty acids for humans include alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid).
- Other fatty acids that can be used include gamma-linolenic acid (an omega-6 fatty acid), lauric acid (a saturated fatty acid), and palmitoleic acid (a monounsaturated fatty acid).
- a “supplement” or a “nutritional supplement” generally includes substances that do not require a prescription.
- the multi-dose nutritional supplement packages of the subject invention may, however, in some implementations, include pharmacological agents that require a prescription.
- the multi-dose nutritional supplement package of the invention may contain a plurality of unit dosage forms of a nutritional supplement that meets a nutritional need of the subject.
- the term “multi-dose” is meant to be a package containing more than one dose of a nutritional supplement.
- the multi-dose nutritional supplement package of the invention may be sold in unit dosage to be taken over a certain period of time, such as a day, week, a monthly or a given season of the year.
- unit dosage form refers to physically discrete units of a nutritional supplement(s) suitable as unitary dosages for human and animal subjects, each unit containing a predetermined quantity of a nutritional supplement(s) calculated in an amount sufficient to produce the desired effect.
- the specifications for the unit dosage forms of a given nutritional supplement employed in the practice of the present invention depend on, for example, the particular nutritional supplement employed and the effect to be achieved, the pharmacodynamics associated with the particular nutritional supplement in the subject, etc.
- the multi-dose package of the invention is tailored for use over a certain period of time by a certain type of consumer, such as a man, woman, child of a certain age. It is well known that the nutritional needs of individuals vary predetermined times depending upon the age and gender of a particular consumer, as well as the consumer's lifestyle. For example, during a given month period, the nutritional needs of both men and women vary based upon hormonal changes that are known to occur in both men and women. Further, the nutritional needs of both genders vary over the course of year depending upon the season and the exposure to sunlight, for example, which may vary by the physical location of the individual consumers (i.e., regions of the country or the world) but also by the local diet of the consumers. Further, sedentary individuals require different types of supplements than active or highly athletic consumer.
- the tailored multi-dose package can contain unit dosage forms in the appropriate number, in the appropriate dosage(s), and in the appropriate packaging (e.g., daily, monthly packaging, weekly packaging, etc.) for meeting a specific request, a specific nutritional need, or an anticipated nutritional need of a particular subject or target consumer.
- Parameters that can be used to determine the appropriate dosages for a particular subject can include, in addition the recommended dosages of a nutritional supplement, the subject's age, height, weight, gender, body mass index (BMI), known medical conditions, location of residence, time of the year, local diet, lifestyles and activity levels.
- BMI body mass index
- the appropriate dosage of a nutritional supplement in some instances can be more or in some instances can be less than the “recommended” guidelines, e.g., as published by the FDA, depending upon the parameters.
- An “effective amount” of a nutritional supplement, or an amount effective for meeting a subject's nutritional needs or nutritional requests may vary somewhat from subject to subject, and may depend upon factors such as, but not limited to, the age of the subject, the health of the subject, the medical conditions of the subject, the form of the nutritional supplement, the route and method of delivery, etc.
- a multi-dose package of the invention may be tailored for use by a menstruating woman to be taken over a 28 day period, commencing on the first day of her menses cycle.
- the dosages of the multi-dose package of may appear as follows, varying in the amount of dosages of each supplement, as illustrated below:
- “other” may include constant or varying dosages of any of the following: Vitamin A, C, D, and K, Thiamin (B1), Riboflavin (B2), Niacin (B3), Folic Acid, B12, Biotin, Pantothenic Acid, Iron, Phosphorus, Iodine, Zinc, Selenium, Cooper, Chromium, Molybdenum, Sodium, Chloride, Potassium, Boron, Nickel, Silicon, Tin and Vanadium.
- Vitamin A, C, D, and K Thiamin (B1), Riboflavin (B2), Niacin (B3), Folic Acid, B12, Biotin, Pantothenic Acid, Iron, Phosphorus, Iodine, Zinc, Selenium, Cooper, Chromium, Molybdenum, Sodium, Chloride, Potassium, Boron, Nickel, Silicon, Tin and Vanadium.
- the “other” may include constant dosages in the amounts set for below for each supplement:
- the multi-dose package may contain one or more supplements to be taken each day.
- the package will contain at least a first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement, where the amount of the nutritional supplement in the first unit dosage differs from the amount of the nutritional supplement in the second unit dosage.
- the package can include a third, fourth, fifth, etc. different dosage of a nutritional supplement.
- each unit dosage in a package could be different.
- every unit dosage of the nutritional supplement could be different, such that the monthly package contained 30 different unit dosages, with a different unit dosage for each day.
- the ratio of the difference between the first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement can be any suitable ratio, such as a ratio of from 1:1.1 to 1:1,000, including from 1:2 to 1:10, such as from 1:3 to 1:5.
- the package can also include two or more nutritional supplements.
- the first nutritional supplement dosage and the second nutritional supplement dosage are formed as separate units (e.g., are in two different pills); in other implementations one or more nutritional supplement dosages can be combined into a single unit or pill.
- Multiple dosage units of one or more nutritional supplements may be packaged in a single container, e.g., a single blister pack, tube, bottle, vial, container, and the like, or one or more dosage units or types of dosage units may be individually packaged and/or individually labeled such that certain packages may have more than one container of a nutritional supplement or of different nutritional supplements.
- a monthly package of nutritional supplements may be packaged in container with separate sections for each week, and/or separate sections for different nutritional supplements, i.e., a monthly package can have 30 unit dosages, with each unit dosage contained in a separate molded compartment, or blister pack.
- a seasonal package for example, can be packaged in a tube with a separate section for each week, or month, etc.
- Indicia may be further included on or in connection with each unit dosage indicating when, during the predetermined period of time, each supplement should be taken.
- the unit dosages may be marked by color, by number, or any other means to indicate to a consumer when a particular supplement dosage should be taken.
- Implementations of the multi-dose nutritional supplement package can include multi-dose packages formed for any suitable period of time, such as a daily package, a weekly package, a monthly package, a seasonal package, a 3-month or 90 day package, a yearly package, etc.
- season it is meant a time of year such as winter, spring, summer or fall.
- a season of the year can therefore be defined by months of the year (e.g., the winter months can include December, January, and February; the spring can include March, April and May; the summer can include June, July, August; and the fall can include September, October, November).
- season is not so limited, however, as conditions typical of a particular season can obviously vary according to the geographic location of a subject (e.g., the winter months are prolonged in the northern regions of the northern hemispheres, and the winter occurs in the “summer” months in the southern hemisphere, etc.) Seasons can be defined by variations in the environment such as the average, minimum or maximum ambient temperature, humidity, barometric pressure, the presence or absence of allergens (e.g., pollen) in the air, mold count, air quality, the average amount of available sunlight or the time of sunrise or sunset, the amount of precipitation, etc.
- allergens e.g., pollen
- the onset, duration, and termination of a season can be defined by variables that include changes in indoor environments (e.g., inside homes or office buildings), such as changes in humidity, temperature, allergens present in the air, degree of air filtration, etc. Seasons can in other implementations be defined by variations in behavior of a subject, such as variations in the amount of time spent indoors or outdoors, variations in activity level, diet, etc.
- the onset, duration, and termination of a season can also be defined by measured changes in physiological parameters of a subject, such as but not limited to changes in hormone levels, changes in mood, changes in blood pressure, etc.
- a season can be defined simply by the period of time in which a subject is noted to have or is suspected to have an exacerbation of a condition.
- a multi-dose nutritional supplement package of the subject invention can include daily discrete unit or continuous unit doses wherein the total number of daily units present in the package may be equal to the total number of days, or a multiple thereof, in implementations in which more than one nutritional supplement is being administered.
- a seasonal package may include from 30 to 540 doses, such as from 60 to 180 doses, or 90 to 120 doses ( FIG. 1A ).
- the package may have daily unit doses in the form of oral dosage forms such as tablets, capsules, and the like.
- a seasonal package can include discrete or continuous unit doses in the form of a transdermal patch, or an injectable dose for parenteral administration, which can have, for example, dosage units which are effective for one or more days, such as for a week, or a month, or more.
- a multi-dose nutritional supplement package of the subject invention can be a monthly package that includes daily discrete or continuous unit doses wherein the total number of daily units present in the monthly package may be equal to the total number of days in the month, or a multiple thereof, in implementations in which more than one nutritional supplement is being administered.
- a monthly package may include from 30 to 210 doses, such as from 60 to 180 doses, or 90 to 120 doses.
- the package may include, for example daily unit doses in the form of tablets, capsules and the like.
- a monthly package can include discrete or continuous unit doses in the form of a transdermal patch, or an injectable dose, which can have, for example, dosage units which are effective for one or more days, such as for a week, or two weeks, or a month, or more.
- Implementations may include daily discrete or continuous unit doses wherein the total number of daily units may be equal to the total number of days in a given period of time, e.g., a month, 3 months, a given season, a year, and the like, in the form of a package.
- implementations may include daily discrete or continuous unit doses wherein the total number of daily units may be equal to the total number of days of a season in a year, e.g., in the form of a seasonal package.
- Such a seasonal package may include a plurality of unit dosage forms having the same or different dosages of a nutritional supplement.
- implementations may include a monthly nutritional supplement package wherein the dosage of nutritional supplement of certain unit dosage forms of the package to be administered to a subject throughout the year.
- a multi-dose nutritional supplement package can have any suitable shape, e.g., a rectangular or square box, a cylindrical tube, a rectangle, square, or circular blister package, etc.
- the packages can be formed as a single unit (e.g., one monthly or seasonal pack), organized in sections (e.g., by week, or month, etc.).
- a monthly or seasonal package can be manufactured as a single unit blister pack with individual months separated by lines, by color, by perforations, etc.
- a yearly or seasonal package can also be a series of tubes or vials which are connected, such that the package can be manufactured as a single unit. The individual vials can be separated by tabs or links that can be cut as needed.
- a multi-dose package or can be a series of blister packs, for example, attached by a hinge, etc, or on a detachable ring such that a subject can discard one month's card when the dosages have all been administered.
- the multi-dose package can have a base to fit a card, e.g., a monthly card, with a flip-top cover. In this implementation, a user can discard one month's card when the dosages have all been administered and replace it with a new card fitted into the base.
- Implementations of the nutritional supplement package can also be enclosed in an outer case of any suitable shape, such as circular, rectangular, square, cylindrical, etc.
- the case can be unitary, i.e., made of a single piece of material.
- a case may be made of more than one separate piece (e.g., a base and a cover).
- the cover can be attached in any suitable manner such as with a hinge, or snap closure, etc.
- the case or cover can be made with any suitable material (e.g., plastic).
- a kit can be provided which may include a holder for an individual blister pack, or individual tube, i.e., a blister pack containing medication for a particular month.
- the holder can be made of any suitable material for holding a portion of a seasonal or yearly pack, e.g., a rectangular holder configured to hold a monthly blister pack in the shape of a rectangle.
- the packages can further be demarcated or labeled to identify the month in which the medication is to be taken, the particular day of the month, the day of the week, time of day, etc.
- the packages may have one or more lines or circles indicating the one or more dosages to be taken in a given day, e.g., on some days a subject may take only one medication, and on other days, a subject may take more than one medication.
- the packages can be color-coded, such that the dose for a particular day or time of day, or week, can be easily determined. For example, a blister package with multiple weeks can have alternating rows of two different colors to allow a subject to readily distinguish one week from another.
- a seasonal package may have weeks where the subject takes only one medication, and weeks where two medications are to be taken, and these weeks can be distinguished by printing the background of the package in two different colors to allow a subject to readily distinguish the days and weeks where two medications are to be taken. Multiple variations are possible depending on the complexity of the dosing schedule and number of nutritional supplements to be taken.
- FIGS. 1-4 Examples of single dosage packages that may be sold in different predetermined amounts are illustrated in FIGS. 1-4 .
- FIGS. 1-4 provide only examples of unit dosages packaged to be taken over a predetermined period of time.
- the illustrations are not intended to limit the daily dosages to a single dose or single supplement, nor are the figures intended to limit the predetermined time periods over which the dosages are to be taken to those time periods illustrated in the figures.
- the illustrations offer various optional examples of indicia that may be included on the physical supplements to indicate when the pills are to be taken.
- the indicia may, for example, include color coding, numbering or indication as to a day of the week.
- the color coding may indicate changes in dose amounts or a certain time when a given supplement should be taken over the predetermined period.
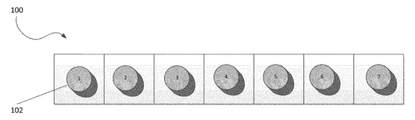
- FIG. 1 illustrates one example of a weekly multi-dose package 100 of the invention.
- the weekly multi-dose package 100 as illustrated in FIG. 1 shows seven unit dosages 102 , one to be taken on each day of the week.
- the seven unit dosages 102 may be optionally marked by numbers 1 through 7, illustrating which pills should be taken on which day of the week.
- FIG. 2 illustrates another example of a weekly multi-dose package 200 of the multi-dose nutritional supplement 202 of the invention.
- each dose 202 may be marked with the day of the week for which the respective pill 202 is to be taken.
- the doses or pills 202 may be color-coded to indicate which day to take in particular pill 202 or in which order to take the pills 202 .
- the doses 202 are coded by the colors of the rainbow, in which case the user may start with the first color and end with the last color in the series.
- FIG. 3 illustrates one example of a 28-day multi-dose package 300 of the invention.
- the example of FIG. 3 illustrates four rows 302 , 304 , 306 and 308 of pills 302 , each row including a week's dose of supplements.
- the target consumer may be a woman and the supplements may be designed to be taken in accordance with her menstrual cycle.
- the variations in the colors of the pills 302 may illustrate different dosages to be taken at different times.
- the pills 302 may be further marked with indicia such as color coding, numbers, or days of the week to indicate on what day a particular pill is intended to be taken during the 28-day pre-determined period.
- FIG. 4 illustrates one example of six month multi-dose package 402 of the invention.
- the example may include any number of months 412 and may, in one implementation, be designed to respond to seasonal changes.
- the months 412 may be perforated around the side edges 420 and the bottom edges 430 so that the individual months may be broken away from the other months 412 .
- the months 412 may contain multiple rows 404 , 406 , 408 , 410 of pills 402 to be taken during each day of the month 412 .
- the pills may be color-coded to indicate when the pills 402 are to be taken during the month or to indicate pills of different dosage amounts.
- the pills 402 may also be numbered or may be marked with indicators as to the day upon which each pill 402 is to be taken.
- the nutritional supplement may be incorporated into a variety of formulations for administration.
- the above example represents only one example of a nutritional supplement that may be administered to a subject.
- the supplement and types of formulations may vary greatly based upon the target consumer and the specific nutritional needs the supplements are intended to address.
- the example formulation above could administer additional iron during menstruation.
- the amount of vitamin D may further vary.
- the supplement may be further designed to additionally promote colon health, in which case, the supplement could include probiotics.
- the nutritional supplements of the invention may be designed for regular and continuous use by health consumers that desire to accommodate the known varying nutritional needs of individuals based upon gender, age, and common and/or naturally reoccurring physiological changes that occur over time based upon hormonal cycles, predetermined time periods, seasonal changes, diet and health and activity levels of certain subjects.
- the nutritional supplement may be formulated into compositions by combination with appropriate, pharmaceutically acceptable carriers.
- pharmaceutically acceptable carrier is meant a component such as a carrier, diluent, excipient, and the like of a composition that is compatible with the particular nutritional supplement and other optional ingredients of the subject nutritional supplement compositions in that a pharmaceutically acceptable carrier may be combined with the nutritional supplement without eliminating the biological or therapeutically effective activity of the nutritional supplement, and is suitable for use in subjects as provided in this application without undue adverse side effects (such as toxicity, irritation, allergic response, and death). Side effects are “undue” when their risk outweighs the benefit provided by the nutritional supplement.
- Non-limiting examples of pharmaceutically acceptable components include, but are not limited to, any of the standard pharmaceutical carriers such as phosphate buffered saline solutions, water, emulsions such as oil/water emulsions or water/oil emulsions, microemulsions, and various types of wetting agents.
- the nutritional supplement employed in the subject methods may be formulated into preparations in solid, semi-solid (e.g., gel), or liquid forms, such as tablets, capsules, powders, granules, solutions.
- administration of a nutritional supplement may be achieved in various ways, including, but not limited to, oral, buccal (e.g. sub-lingual), topical, or transdermal administration.
- a given nutritional supplement may be administered via a transdermal patch or film system such as or analogous to that described, e.g., in U.S. Pat. Nos. 6,503,532; 5,302,395; 5,262,165; 5,248,501; 5,232,702; 5,230,896; 5,227,169; 5,212,199; 5,202,125; 5,173,302; 5,154,922; 5,139,786; 5,122,383; 5,023,252; 4,978,532; 5,324,521; 5,306,503; 5,302,395; 5,296,230; 5,286,491; 5,252,334; 5,248,501; 5,230,896; 5,227,169; 5,212,199; 5,202,125; 5,173,302; 5,171,576; 5,139,786; 5,133,972; 5,122,383; 5,120,546; 5,118,509; 5,077,054; 5,066,494; 5,049,3
- implementations may include formulations for oral administration that may be formulated using pharmaceutically acceptable carriers well known in the art in dosages suitable for oral administration.
- Such carriers enable the pharmaceutical formulations to be formulated in unit dosage forms as tablets, pills, powder, dragees, capsules, liquids, lozenges, gels, syrups, slurries, suspensions, etc., suitable for ingestion by the patient.
- Pharmaceutical preparations for oral use may be obtained through combination of at least one nutritional supplement with a solid excipient, optionally grinding a resulting mixture, and processing the mixture of granules, after adding suitable additional compounds, if desired, to obtain tablets or dragee cores.
- Suitable solid excipients include, but are not limited to, carbohydrate or protein fillers and include, but are not limited to sugars, including lactose, sucrose, mannitol, or sorbitol; starch from corn, wheat, rice, potato, or other plants; cellulose such as methyl cellulose, hydroxypropylmethyl-cellulose or sodium carboxymethylcellulose; and gums including arabic and tragacanth; as well as proteins such as gelatin and collagen.
- disintegrating or solubilizing agents may be added, such as the cross-linked polyvinyl pyrrolidone, agar, alginic acid, or a salt thereof, such as sodium alginate; with optional lubricants, such as talc or magnesium stearate; and if desired, with diluents, buffering agents, moistening agents, preservatives and flavoring agents.
- formulations suitable for oral administration in accordance with the subject invention may be present in discrete units, such as capsules, cachets, lozenges, tablets, and the like, each containing a predetermined amount of the nutritional supplement; as a powder or granules; as a solution or a suspension in a nutritional supplement formulation that may be prepared by any suitable method of pharmacy which includes, but is not limited to, bringing into association the nutritional supplement and a suitable carrier (which may contain one or more optional ingredients as noted above).
- nutritional supplement formulations for use with the subject invention may be prepared by uniformly and intimately admixing the nutritional supplement with a liquid or finely divided solid carrier, or both, and then, if necessary, shaping the resulting mixture.
- a tablet may be prepared by compressing or molding a powder or granules containing the nutritional supplement, optionally with one or more accessory ingredients.
- Compressed tablets may be prepared by compressing, in a suitable machine, the nutritional supplement in a free-flowing form, such as a powder or granules optionally mixed with a binder, lubricant, inert diluent, and/or surface active/dispersing agent(s).
- Molded tablets may be made by molding, in a suitable machine, the powdered nutritional supplement moistened with an inert liquid binder.
- a nutritional supplement employed in the subject invention may be delivered transdermally, by a topical route, formulated as applicator sticks, solutions, suspensions, emulsions, gels, creams, ointments, pastes, jellies, paints, powders, and aerosols.
- implementations may include a nutritional supplement in the form of a discrete patch or film or plaster or the like adapted to remain in intimate contact with the epidermis of the recipient for a period of time.
- such transdermal patches may include a base or matrix layer, e.g., polymeric layer, in which one or more nutritional supplements are retained.
- the base or matrix layer may be operatively associated with a support or backing.
- Nutritional supplement formulations suitable for transdermal administration may also be delivered by iontophoresis and may take the form of an optionally buffered aqueous solution of the nutritional supplement compound.
- Suitable formulations may include citrate or bis/tris buffer (pH 6) or ethanol/water and contain a suitable amount of active ingredient.
- the multi-dose packages of the invention find use in a variety of applications in which it is desired to meet the nutritional needs of a subject.
- nutritional need it is meant a subject's need for a particular nutrient, usually obtained from food, such as a vitamin, ‘mineral’, amino acid, etc., which is necessary to maintain good health.
- the subject is a healthy subject.
- the subject can have a deficiency of an essential nutrient, such as a vitamin.
- a vitamin deficiency can be either ‘primary’ or ‘secondary’.
- a primary deficiency occurs when an insufficient amount of a vitamin is present in the diet.
- a secondary deficiency can be due to an underlying condition that prevents or limits the absorption or use of the vitamin. This can include smoking, excessive alcohol consumption, or other medications that interfere with the absorption or use of the vitamin.
- the subject can have a need for a particular nutrient which varies over time.
- a particular nutrient which varies over time.
- the nutritional requirements of a subject can change over time (e.g., monthly cycles in a female, varying nutritional needs of elderly subjects, athletes, nutritional needs in responses to season changes, etc.).
- the subject can have a condition which increases in severity or occurrence during a season of the year.
- Such conditions can include, for example, seasonal variation in the incidence of allergies, seasonal variation in gastroesophageal reflux disease (GERD) and duodenal ulcers, seasonal variation in variceal bleeding, seasonal variation in the incidence of urolithiasis, seasonal variation in the incidence of hip fractures, seasonal variation in fertility rates, seasonal variation in the incidence of eclampsia, seasonal variation in blood pressure in hypertensive patients, seasonal variation in blood pressure in CAPD (continuous ambulatory peritoneal dialysis) patients, seasonal variation in coronary artery disease and incidence of first hospitalization for myocardial infarction, seasonal variation in paroxysmal atrial fibrillation, seasonal variation in asthma, Seasonal Affective Disorder (SAD), seasonal variation in suicide rates, seasonal variation in vitamin D levels, seasonal variation in hyponatremia, seasonal variation in retinal vein occlusion, seasonal variation in neonatal death rates, seasonal variation in the incidence of congenital hypothyroidism, and seasonal variation in the onset of stage I sarcoidosis.
- Subjects with conditions that have nutritional needs that can be addressed with the multi-dose packages of the subject invention can also include: cardiovascular conditions; neurodegenerative conditions; neuroinflammatory conditions; orthopedic inflammatory conditions; lymphoproliferative conditions; autoimmune conditions; inflammatory conditions; infectious diseases; pulmonary conditions; transplant-related conditions; gastrointestinal conditions; endocrine conditions (e.g., diabetes); genitourinary conditions; skin conditions; aging conditions; neurologic conditions; Th-2 dominant conditions; conditions that cause hypoxia; conditions that cause hypercarbia; conditions that cause hypercapnia; conditions that cause acidosis; conditions that cause acidemia; OB-GYN conditions; sudden death syndromes; menstrual related disorders; fibrosis; chronic pain; trauma; bacterial infections; and fibromyalgia.
- cardiovascular conditions e.g., cardiovascular conditions; neurodegenerative conditions; neuroinflammatory conditions; orthopedic inflammatory conditions; lymphoproliferative conditions; autoimmune conditions; inflammatory conditions; infectious diseases; pulmonary conditions; transplant-related conditions; gastrointestinal conditions; endocrine conditions
- cardiovascular conditions including cardiovascular disease, e.g., atherosclerosis, coronary artery disease, hypertension, hyperlipidemia, cardiomyopathy, volume retention, congestive heart failure, QT interval prolongation, aortic dissection, aortic aneurysm, arterial aneurysm, arterial vasospasm, myocardial infarction, reperfusion syndrome, ischemia, sudden adult death syndrome, arrhythmia, fatal arrythmias, coronary syndromes, coronary vasospasm, sick sinus syndrome, bradycardia, tachycardia, thromboembolic disease, deep vein thrombosis, coagulopathy, disseminated intravascular coagulation (“DIC”), mesenteric ischemia, syncope, venous thrombosis, arterial thrombosis, malignant hypertension, secondary hypertension, primary pulmonary hyper
- DIC disseminated intravascular coagulation
- the multi-dose nutritional supplement packages tailored for a particular subject are particularly useful in avoiding consequences of inadequate nutrition, e.g., insufficient amounts of vitamins.
- consequences of insufficient amounts of vitamins include xerophthalmia or night blindness with vitamin A deficiency, beriberi caused by thiamine deficiency, pellagra caused by niacin deficiency, megaloblastic anemia caused by vitamin B12 deficiency, scurvy caused by vitamin C deficiency, rickets caused by vitamin D deficiency, and impaired coagulation caused by vitamin K deficiency.
- the tailored multi-dose nutritional supplement packages can avoid the problem of doses that are too large, as some vitamins, especially the fat-soluble vitamins, have documented side-effects that tend to be more severe with a large dosage. At high enough dosages, some vitamins cause side-effects such as nausea, diarrhea, and vomiting.
- An appropriate dose of a vitamin for a particular subject can vary, and can in some instances be related to factors such as age and health status.
- Methods of using the multi-dose nutritional supplement packages by a user can include selecting one of a plurality of unit dosage forms of a nutritional supplement contained in the multi-dose package comprising a first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement, wherein the amount of the nutritional supplement in the first unit dosage differs from the amount of the nutritional supplement in the second unit dosage, and ingesting the first unit dosage form.
- the methods further include selecting the second unit dosage form from the multi-dose package and ingesting the second unit dosage form.
- the methods can in some implementations include selecting and ingesting a third, fourth, fifth, etc., unit dosage form of a first nutritional supplement and ingesting the first nutritional supplement.
- the methods can also in some implementations include selecting and ingesting one or more unit dosage forms of a second nutritional supplement and ingesting the second nutritional supplement.
- the nutritional supplement is a vitamin; in other instances the nutritional supplement is a nutraceutical, and in some instances the multi-dose nutritional supplement package can consist of both one or more vitamins, and one or more nutraceuticals.
- more than one nutritional supplement may be administered at the same or different time as another nutritional supplement, where the nutritional supplements administered differ in one or more respects, e.g., may be different types of agents or may be the same type nutritional supplement but that differ in mode of administration, dosage, etc.
- an effective amount of a given nutritional supplement may vary somewhat from subject to subject, and may depend upon factors such as, but not limited to, the age of the subject, the health of the subject, the particular condition being treated, the form of the nutritional supplement, the route and method of delivery, etc., as noted above. Such dosages may be determined in accordance with routine nutritional supplement procedures known to those skilled in the art.
- Nutritional supplements may be administered to a subject in a single oral dose, one time a day or more for weeks, months, years, even as long as a subject's lifetime or as long as the subject experiences the nutritional need.
- an implementation may include administering a given nutritional supplement one time a day over a prolonged period of time, e.g., over a particular time period e.g., up to 1 month, or 2 months, or 3 months, or 9 months, or a year.
- the frequency of administration of a nutritional supplement may vary depending on one or more of the factors described above.
- the frequency of administration of a nutritional supplement may range from 1 time per day to multiple times per day, e.g., 2 times or more per day or as necessary to meet the nutritional needs or nutritional requests of the subject.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Medical Informatics (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
Abstract
Multi-dose packages of nutritional supplements are provided that are designed for regular and continuous use by a specific target (i.e., consumer) over a reoccurring predetermined period of time. The multi-dose packaging of nutritional supplements contains supplements that vary in dosage and that are each intended to be taken at a specific time during the predetermined period of time. Each supplement in the series is tailored to contain unit dosages of nutritional supplements that are designed to address specific needs of the intended consumer during the predetermined period of time at which the supplement is intended to be ingested.
Description
- This application claims priority of U.S. Application Ser. No. 61/451,917, filed on Mar. 11, 2001, titled Multi-Dose Packaging of Nutritional Supplements, which application is incorporated in its entirety by reference in this application.
- 1. Field of the Invention
- The present invention relates to nutritional supplements and, in particular, nutritional supplements having varying dosages over a predetermined time period of time depending upon when the particular supplement is intended to be consumed within such predetermined time period.
- 2. Related Art
- Nutritional supplements are commonly used for the purpose of maintaining good health. Nutritional supplements can include vitamins, ‘minerals’, amino acids, fiber, herbs or other botanicals, etc., which are traditionally sold in packages where all the unit dosages of a particular supplement are the same.
- Nutritional supplements can include vitamins, which are defined as any of various organic substances that are essential in minute quantities to the nutrition of most animals. Vitamins act especially as coenzymes and precursors of coenzymes in the regulation of metabolic processes, but do not provide energy or serve as building units. There are both fat-soluble vitamins (for example, Vitamin E), and water-soluble vitamins (for example, Vitamin C).
- Nutritional supplements can also include “minerals”, which are a chemical element required by living organisms to support the biochemical reactions of metabolism, and maintain optimal health. These required chemical elements can be are naturally present in food (e.g., calcium in milk), or can be added to food (e.g., iodized salt) or included in a dietary supplement.
- Nutritional supplements can also include neutraceuticals, which can be a fortified food that provides health or medical benefits in addition to its basic nutritional value. Nutritional supplements may further include, as vitamins, minerals, neutraceuticals, or in additional to those categories of supplements, soluble and insoluble fiber, fatty acids, herbs, plants, amino acids and digestive enzymes or any other substance or compound having or promoting a health benefit to consumers. As discussed above, nutritional supplements as well as their components, such as vitamins, minerals, neutraceuticals, etc., are traditionally sold in packages where all the unit dosages of a particular supplement are the same.
- While nutritional supplements are traditionally sold in packages where the unit dosages of each supplement are the same, the nutritional needs of consumer vary over predetermined time periods. By way of example only, certain studies have shown that increased calcium assists in decreasing symptoms associated with premenstrual syndrome. It would therefore be beneficial to provide women with a multi-vitamin where a certain number of the vitamins, in the multi-dose package, have increased doses of calcium and where the certain number vitamins are marked for ingested prior to, or during menstruation. Numerous other examples exist citing the benefit of increased supplements during certain times in the natural cycle of an individual's life, whether the consumer may be a man, women or a child. A need therefore exists for nutritional supplements that respond to the various needs of consumers over a predetermined period of time.
- In accordance with the invention, multi-dose packages of nutritional supplements are provided that are designed for regular and continuous use by a specific target (i.e., consumer) over a reoccurring predetermined period of time, such as a day, month, season, year, or other defined time period. The multi-dose packaging of nutritional supplements contains supplements that vary in dosage and that are each intended to be taken at a specific time during the predetermined period of time. Each supplement in the series is tailored to contain unit dosages of nutritional supplements that are designed to address specific needs (e.g., psychological needs) of an individual during the predetermined period of time at which the supplement is intended to be ingested.
- By way of example, the multi-dose package may contain at least a first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement, where the amount of the nutritional supplement in the first unit dosage differs from the amount of the nutritional supplement in the second unit dosage. The multi-dose packages may be prepackages or tailored for the specific needs of an individual with specific conditions having a varying need over time.
- As summarized above, the subject invention provides devices, systems and methods for meeting the nutritional needs of a subject. In some instances, the nutritional needs of a subject may vary over time. In further describing the subject invention, representative implementations of the subject multi-dose nutritional supplement packages are described in detail.
- Other devices, apparatus, systems, methods, features and advantages of the invention will be or will become apparent to one with skill in the art upon examination of the following figures and detailed description. It is intended that all such additional systems, methods, features and advantages be included within this description, be within the scope of the invention, and be protected by the accompanying claims.
- The invention may be better understood by referring to the following figures. The components in the figures are not necessarily to scale, emphasis instead being placed upon illustrating the principles of the invention. In the figures, like reference numerals designate corresponding parts throughout the different views.
-
FIG. 1 illustrates one example of a weekly multi-dose package of the invention. -
FIG. 2 illustrates another example of a weekly multi-dose package of the invention. -
FIG. 3 illustrates one example of a twenty-eight day multi-dose package of the invention. -
FIG. 4 illustrates one example of a six month multi-dose package of the invention. - The foregoing description of various implementations has been presented for purposes of illustration and description. The descriptions are not exhaustive and do not limit the claimed inventions to the precise form disclosed. Modifications and variations are possible in light of the above description or may be acquired from practicing the invention. It is further understood that the terminology used in this application is for the purposes of describing particular implementations of the invention only are is not intended to be limiting. The claims and their equivalents define the scope of the invention.
- As summarized above, the present invention relates to multi-dose packages of nutritional supplements designed for regular and continuous use by a specific target (i.e., consumer) over a reoccurring predetermined period of time (e.g., a day, month, season, year, or other defined time period). The multi-dose packaging of nutritional supplements contains supplements that vary in dosage and that are each intended to be taken at a specific time during the predetermined period of time. Each supplement in the series is tailored to contain unit dosages of nutritional supplements that are designed to address specific needs of an individual during the predetermined period of time at which each supplement is intended to be ingested.
- For purposes of this application, a “supplement” or a “nutritional supplement” is a vitamin, mineral, neutraceutical, enzyme, including digestive enzymes, or other ingestible substance having or promoting a health benefit or providing treatment for a particular condition. A “vitamin” is any of various organic substances that are essential in minute quantities to the nutrition of most animals. Vitamins act especially as coenzymes and precursors of coenzymes in the regulation of metabolic processes, but do not provide energy or serve as building units. Vitamins can include, but are not limited to, Vitamin A (Retinol), Vitamin B1 (Thiamine), Vitamin B2 (Riboflavin), Vitamin B3 (Niacin), Vitamin B5 (Pantothenic acid), Vitamin B6 (Pyridoxine), Vitamin B7 (Biotin), Vitamin B9 (Folic acid), Vitamin B12 (Cobalamins), Vitamin C (Ascorbic acid), Vitamin D (Calciferol), Vitamin E (Tocopherol), and Vitamin K1 (Phylloquinone).
- A “mineral” is a chemical element required by living organisms to support the biochemical reactions of metabolism, and maintain optimal health. These required chemical elements can be are naturally present in food (e.g., calcium in milk), or can be added to food (e.g., iodized salt) or included in a dietary supplement. Minerals can include but are not limited to: potassium, chlorine, sodium, calcium, phosphorus, magnesium, zinc, iron, manganese, copper, iodine, selenium, and molybdenum.
- A “neutraceutical” is a dietary supplement or a fortified food that provides health or medical benefits in addition to its basic nutritional value. A “dietary supplement” is a product taken orally that contains one or more nutrients that are intended to supplement one's diet and are not considered food, specifically according to the Dietary Supplements Health and Education Act. A dietary supplement can contain one or more vitamins, minerals, fiber, fatty acids, herbs or other botanicals, or amino acids, or a concentrate, constituent, metabolite, extract, or combination of these. Fatty acids can include “essential fatty acids,” which are fatty acids that humans and other animals cannot synthesize, but must obtain in the diet because they are required for certain biological processes. The two essential fatty acids for humans include alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid). Other fatty acids that can be used include gamma-linolenic acid (an omega-6 fatty acid), lauric acid (a saturated fatty acid), and palmitoleic acid (a monounsaturated fatty acid).
- Further, a “supplement” or a “nutritional supplement” generally includes substances that do not require a prescription. The multi-dose nutritional supplement packages of the subject invention may, however, in some implementations, include pharmacological agents that require a prescription.
- The multi-dose nutritional supplement package of the invention may contain a plurality of unit dosage forms of a nutritional supplement that meets a nutritional need of the subject. The term “multi-dose” is meant to be a package containing more than one dose of a nutritional supplement. In accordance with the invention, the multi-dose nutritional supplement package of the invention may be sold in unit dosage to be taken over a certain period of time, such as a day, week, a monthly or a given season of the year.
- The term “unit dosage form,” and analogous terms as used in this application refers to physically discrete units of a nutritional supplement(s) suitable as unitary dosages for human and animal subjects, each unit containing a predetermined quantity of a nutritional supplement(s) calculated in an amount sufficient to produce the desired effect. The specifications for the unit dosage forms of a given nutritional supplement employed in the practice of the present invention depend on, for example, the particular nutritional supplement employed and the effect to be achieved, the pharmacodynamics associated with the particular nutritional supplement in the subject, etc.
- The multi-dose package of the invention is tailored for use over a certain period of time by a certain type of consumer, such as a man, woman, child of a certain age. It is well known that the nutritional needs of individuals vary predetermined times depending upon the age and gender of a particular consumer, as well as the consumer's lifestyle. For example, during a given month period, the nutritional needs of both men and women vary based upon hormonal changes that are known to occur in both men and women. Further, the nutritional needs of both genders vary over the course of year depending upon the season and the exposure to sunlight, for example, which may vary by the physical location of the individual consumers (i.e., regions of the country or the world) but also by the local diet of the consumers. Further, sedentary individuals require different types of supplements than active or highly athletic consumer.
- By “tailored,” it is meant that the package is specifically configured to meet one or more specific requests of a target consumer, or to meet one or more specific nutritional needs of a consumer. The tailored multi-dose package can contain unit dosage forms in the appropriate number, in the appropriate dosage(s), and in the appropriate packaging (e.g., daily, monthly packaging, weekly packaging, etc.) for meeting a specific request, a specific nutritional need, or an anticipated nutritional need of a particular subject or target consumer. Parameters that can be used to determine the appropriate dosages for a particular subject can include, in addition the recommended dosages of a nutritional supplement, the subject's age, height, weight, gender, body mass index (BMI), known medical conditions, location of residence, time of the year, local diet, lifestyles and activity levels.
- The appropriate dosage of a nutritional supplement in some instances can be more or in some instances can be less than the “recommended” guidelines, e.g., as published by the FDA, depending upon the parameters. An “effective amount” of a nutritional supplement, or an amount effective for meeting a subject's nutritional needs or nutritional requests may vary somewhat from subject to subject, and may depend upon factors such as, but not limited to, the age of the subject, the health of the subject, the medical conditions of the subject, the form of the nutritional supplement, the route and method of delivery, etc.
- By way of example, a multi-dose package of the invention may be tailored for use by a menstruating woman to be taken over a 28 day period, commencing on the first day of her menses cycle. By way of example only, the dosages of the multi-dose package of may appear as follows, varying in the amount of dosages of each supplement, as illustrated below:
-
DAY SUPPLEMENT DOSAGE 1 Ca 1200 mg; manganese 5 mg; Mg 300 mg; B6 40 mg; E 400IU; Other 2 Ca 1200 mg; manganese 5 mg; Mg 300 mg; B6 40 mg; E 400IU; Other 3 Ca 1200 mg; manganese 5 mg; Mg 300 mg; B6 40 mg; E 400IU; Other 4 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 20 mg; E 100 IU; Other 5 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 6 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 7 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 8 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 9 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 10 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 11 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 12 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 13 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; Other 14 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 15 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 16 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 17 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 18 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 19 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 20 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 21 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 22 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 23 Ca 500 mg; manganese 2 mg; Mg 50 mg; B6 4 mg; E 30 IU; Other 24 Ca 750 mg; manganese 3 mg; Mg 100 mg; B6 4 mg; E 30 IU; Other 25 Ca 1000 mg; manganese 4 mg; Mg 200 mg; B6 4 mg; E 30 IU; Other 26 Ca 1000 mg; manganese 4 mg; Mg 200 mg; B6 20 mg; E 100 IU; Other 27 Ca 1200 mg; manganese 5 mg; Mg 300 mg; B6 40 mg; E 400IU; Other 28 Ca 1200 mg; manganese 5 mg; Mg 300 mg; B6 40 mg; E 400IU; Other - By way of example only, “other” may include constant or varying dosages of any of the following: Vitamin A, C, D, and K, Thiamin (B1), Riboflavin (B2), Niacin (B3), Folic Acid, B12, Biotin, Pantothenic Acid, Iron, Phosphorus, Iodine, Zinc, Selenium, Cooper, Chromium, Molybdenum, Sodium, Chloride, Potassium, Boron, Nickel, Silicon, Tin and Vanadium.
- By way of example, the “other” may include constant dosages in the amounts set for below for each supplement:
-
DOSAGE PER STANDARD SUPPLEMENT SERVING A 2500 IU C 60 MG D 1000 IU K 25 MCG Thiamin (B1) 1.5 MG Riboflavin (B2) 1.7 MG Niacin (B3) 10 MG Folic Acid 400 MCG B12 6 MCG Biotin 300 MCG Pantothenic 10 MG Iron 18 MG Phosphorus 20 MG Iodine 150 MCG Zinc 15 MG Selenium 55 MCG Copper 2 MG Chromium 120 MCG Molybdenum 75 MCG Chloride 72 mg Potassium 80 mg Boron 75 MCG Nickel 5 MCG Silicon 2 MG Tin 10 MCG Vanadium 10 MCG - As illustrated in the above example, the multi-dose package may contain one or more supplements to be taken each day. The package will contain at least a first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement, where the amount of the nutritional supplement in the first unit dosage differs from the amount of the nutritional supplement in the second unit dosage. In some implementations, the package can include a third, fourth, fifth, etc. different dosage of a nutritional supplement. In some implementations, each unit dosage in a package could be different.
- For example, in a monthly package consisting of 30 days, every unit dosage of the nutritional supplement could be different, such that the monthly package contained 30 different unit dosages, with a different unit dosage for each day. The ratio of the difference between the first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement can be any suitable ratio, such as a ratio of from 1:1.1 to 1:1,000, including from 1:2 to 1:10, such as from 1:3 to 1:5.
- The package can also include two or more nutritional supplements. In some implementations, the first nutritional supplement dosage and the second nutritional supplement dosage are formed as separate units (e.g., are in two different pills); in other implementations one or more nutritional supplement dosages can be combined into a single unit or pill.
- Multiple dosage units of one or more nutritional supplements may be packaged in a single container, e.g., a single blister pack, tube, bottle, vial, container, and the like, or one or more dosage units or types of dosage units may be individually packaged and/or individually labeled such that certain packages may have more than one container of a nutritional supplement or of different nutritional supplements. For example, a monthly package of nutritional supplements may be packaged in container with separate sections for each week, and/or separate sections for different nutritional supplements, i.e., a monthly package can have 30 unit dosages, with each unit dosage contained in a separate molded compartment, or blister pack. A seasonal package, for example, can be packaged in a tube with a separate section for each week, or month, etc. Indicia may be further included on or in connection with each unit dosage indicating when, during the predetermined period of time, each supplement should be taken. The unit dosages may be marked by color, by number, or any other means to indicate to a consumer when a particular supplement dosage should be taken.
- Implementations of the multi-dose nutritional supplement package can include multi-dose packages formed for any suitable period of time, such as a daily package, a weekly package, a monthly package, a seasonal package, a 3-month or 90 day package, a yearly package, etc.
- By “season,” it is meant a time of year such as winter, spring, summer or fall. A season of the year can therefore be defined by months of the year (e.g., the winter months can include December, January, and February; the spring can include March, April and May; the summer can include June, July, August; and the fall can include September, October, November). The definition of season is not so limited, however, as conditions typical of a particular season can obviously vary according to the geographic location of a subject (e.g., the winter months are prolonged in the northern regions of the northern hemispheres, and the winter occurs in the “summer” months in the southern hemisphere, etc.) Seasons can be defined by variations in the environment such as the average, minimum or maximum ambient temperature, humidity, barometric pressure, the presence or absence of allergens (e.g., pollen) in the air, mold count, air quality, the average amount of available sunlight or the time of sunrise or sunset, the amount of precipitation, etc. In some implementations, the onset, duration, and termination of a season can be defined by variables that include changes in indoor environments (e.g., inside homes or office buildings), such as changes in humidity, temperature, allergens present in the air, degree of air filtration, etc. Seasons can in other implementations be defined by variations in behavior of a subject, such as variations in the amount of time spent indoors or outdoors, variations in activity level, diet, etc. The onset, duration, and termination of a season can also be defined by measured changes in physiological parameters of a subject, such as but not limited to changes in hormone levels, changes in mood, changes in blood pressure, etc. In some implementations, a season can be defined simply by the period of time in which a subject is noted to have or is suspected to have an exacerbation of a condition.
- A multi-dose nutritional supplement package of the subject invention can include daily discrete unit or continuous unit doses wherein the total number of daily units present in the package may be equal to the total number of days, or a multiple thereof, in implementations in which more than one nutritional supplement is being administered. For example, a seasonal package may include from 30 to 540 doses, such as from 60 to 180 doses, or 90 to 120 doses (
FIG. 1A ). The package may have daily unit doses in the form of oral dosage forms such as tablets, capsules, and the like. In some implementations, a seasonal package can include discrete or continuous unit doses in the form of a transdermal patch, or an injectable dose for parenteral administration, which can have, for example, dosage units which are effective for one or more days, such as for a week, or a month, or more. - The number of unit dosages in a package will vary depending number of days a package is intended to be used, and will vary depending on the number of unit dosages to be administered per day. For example, a multi-dose nutritional supplement package of the subject invention can be a monthly package that includes daily discrete or continuous unit doses wherein the total number of daily units present in the monthly package may be equal to the total number of days in the month, or a multiple thereof, in implementations in which more than one nutritional supplement is being administered. For example, a monthly package may include from 30 to 210 doses, such as from 60 to 180 doses, or 90 to 120 doses. The package may include, for example daily unit doses in the form of tablets, capsules and the like. In some implementations, a monthly package can include discrete or continuous unit doses in the form of a transdermal patch, or an injectable dose, which can have, for example, dosage units which are effective for one or more days, such as for a week, or two weeks, or a month, or more.
- Implementations may include daily discrete or continuous unit doses wherein the total number of daily units may be equal to the total number of days in a given period of time, e.g., a month, 3 months, a given season, a year, and the like, in the form of a package. For example, implementations may include daily discrete or continuous unit doses wherein the total number of daily units may be equal to the total number of days of a season in a year, e.g., in the form of a seasonal package. Such a seasonal package may include a plurality of unit dosage forms having the same or different dosages of a nutritional supplement. For example, implementations may include a monthly nutritional supplement package wherein the dosage of nutritional supplement of certain unit dosage forms of the package to be administered to a subject throughout the year.
- A multi-dose nutritional supplement package can have any suitable shape, e.g., a rectangular or square box, a cylindrical tube, a rectangle, square, or circular blister package, etc. In some implementations, the packages can be formed as a single unit (e.g., one monthly or seasonal pack), organized in sections (e.g., by week, or month, etc.). For example, a monthly or seasonal package can be manufactured as a single unit blister pack with individual months separated by lines, by color, by perforations, etc. A yearly or seasonal package can also be a series of tubes or vials which are connected, such that the package can be manufactured as a single unit. The individual vials can be separated by tabs or links that can be cut as needed. In some implementations, a multi-dose package or can be a series of blister packs, for example, attached by a hinge, etc, or on a detachable ring such that a subject can discard one month's card when the dosages have all been administered. In some implementations, the multi-dose package can have a base to fit a card, e.g., a monthly card, with a flip-top cover. In this implementation, a user can discard one month's card when the dosages have all been administered and replace it with a new card fitted into the base.
- Implementations of the nutritional supplement package can also be enclosed in an outer case of any suitable shape, such as circular, rectangular, square, cylindrical, etc. In some implementations the case can be unitary, i.e., made of a single piece of material. In other implementations a case may be made of more than one separate piece (e.g., a base and a cover). In some implementations, the cover can be attached in any suitable manner such as with a hinge, or snap closure, etc. The case or cover can be made with any suitable material (e.g., plastic).
- In some implementations, a kit can be provided which may include a holder for an individual blister pack, or individual tube, i.e., a blister pack containing medication for a particular month. The holder can be made of any suitable material for holding a portion of a seasonal or yearly pack, e.g., a rectangular holder configured to hold a monthly blister pack in the shape of a rectangle.
- The packages can further be demarcated or labeled to identify the month in which the medication is to be taken, the particular day of the month, the day of the week, time of day, etc. In addition, the packages may have one or more lines or circles indicating the one or more dosages to be taken in a given day, e.g., on some days a subject may take only one medication, and on other days, a subject may take more than one medication. In some implementations, the packages can be color-coded, such that the dose for a particular day or time of day, or week, can be easily determined. For example, a blister package with multiple weeks can have alternating rows of two different colors to allow a subject to readily distinguish one week from another. In another example, a seasonal package may have weeks where the subject takes only one medication, and weeks where two medications are to be taken, and these weeks can be distinguished by printing the background of the package in two different colors to allow a subject to readily distinguish the days and weeks where two medications are to be taken. Multiple variations are possible depending on the complexity of the dosing schedule and number of nutritional supplements to be taken.
- Examples of single dosage packages that may be sold in different predetermined amounts are illustrated in
FIGS. 1-4 .FIGS. 1-4 provide only examples of unit dosages packaged to be taken over a predetermined period of time. The illustrations are not intended to limit the daily dosages to a single dose or single supplement, nor are the figures intended to limit the predetermined time periods over which the dosages are to be taken to those time periods illustrated in the figures. Further, the illustrations offer various optional examples of indicia that may be included on the physical supplements to indicate when the pills are to be taken. The indicia may, for example, include color coding, numbering or indication as to a day of the week. The color coding may indicate changes in dose amounts or a certain time when a given supplement should be taken over the predetermined period. - In particular,
FIG. 1 illustrates one example of a weeklymulti-dose package 100 of the invention. The weeklymulti-dose package 100 as illustrated inFIG. 1 shows sevenunit dosages 102, one to be taken on each day of the week. The sevenunit dosages 102 may be optionally marked by numbers 1 through 7, illustrating which pills should be taken on which day of the week. -
FIG. 2 illustrates another example of a weeklymulti-dose package 200 of the multi-dosenutritional supplement 202 of the invention. As illustrated inFIG. 2 , eachdose 202 may be marked with the day of the week for which therespective pill 202 is to be taken. Optionally, the doses orpills 202 may be color-coded to indicate which day to take inparticular pill 202 or in which order to take thepills 202. In the illustrated example, thedoses 202 are coded by the colors of the rainbow, in which case the user may start with the first color and end with the last color in the series. -
FIG. 3 illustrates one example of a 28-daymulti-dose package 300 of the invention. The example ofFIG. 3 illustrates fourrows pills 302, each row including a week's dose of supplements. In the illustrated example, the target consumer may be a woman and the supplements may be designed to be taken in accordance with her menstrual cycle. The variations in the colors of thepills 302 may illustrate different dosages to be taken at different times. Thepills 302 may be further marked with indicia such as color coding, numbers, or days of the week to indicate on what day a particular pill is intended to be taken during the 28-day pre-determined period. -
FIG. 4 illustrates one example of six monthmulti-dose package 402 of the invention. The example may include any number ofmonths 412 and may, in one implementation, be designed to respond to seasonal changes. Themonths 412 may be perforated around the side edges 420 and thebottom edges 430 so that the individual months may be broken away from theother months 412. As illustrated, themonths 412 may containmultiple rows pills 402 to be taken during each day of themonth 412. Optionally, the pills may be color-coded to indicate when thepills 402 are to be taken during the month or to indicate pills of different dosage amounts. Thepills 402 may also be numbered or may be marked with indicators as to the day upon which eachpill 402 is to be taken. - Depending on the particular nutritional supplement administered to a subject, the nutritional supplement may be incorporated into a variety of formulations for administration. The above example represents only one example of a nutritional supplement that may be administered to a subject. The supplement and types of formulations may vary greatly based upon the target consumer and the specific nutritional needs the supplements are intended to address. For example, the example formulation above could administer additional iron during menstruation. If the supplement is designed to be taken in the winter months, the amount of vitamin D may further vary. Alternatively, the supplement may be further designed to additionally promote colon health, in which case, the supplement could include probiotics. While some nutritional supplements may be tailored for specific medical needs of a target consumer, the nutritional supplements of the invention may be designed for regular and continuous use by health consumers that desire to accommodate the known varying nutritional needs of individuals based upon gender, age, and common and/or naturally reoccurring physiological changes that occur over time based upon hormonal cycles, predetermined time periods, seasonal changes, diet and health and activity levels of certain subjects.
- The nutritional supplement may be formulated into compositions by combination with appropriate, pharmaceutically acceptable carriers. By “pharmaceutically acceptable carrier” is meant a component such as a carrier, diluent, excipient, and the like of a composition that is compatible with the particular nutritional supplement and other optional ingredients of the subject nutritional supplement compositions in that a pharmaceutically acceptable carrier may be combined with the nutritional supplement without eliminating the biological or therapeutically effective activity of the nutritional supplement, and is suitable for use in subjects as provided in this application without undue adverse side effects (such as toxicity, irritation, allergic response, and death). Side effects are “undue” when their risk outweighs the benefit provided by the nutritional supplement. Non-limiting examples of pharmaceutically acceptable components include, but are not limited to, any of the standard pharmaceutical carriers such as phosphate buffered saline solutions, water, emulsions such as oil/water emulsions or water/oil emulsions, microemulsions, and various types of wetting agents. Accordingly, the nutritional supplement employed in the subject methods may be formulated into preparations in solid, semi-solid (e.g., gel), or liquid forms, such as tablets, capsules, powders, granules, solutions. As such, administration of a nutritional supplement may be achieved in various ways, including, but not limited to, oral, buccal (e.g. sub-lingual), topical, or transdermal administration. In certain implementations, a given nutritional supplement may be administered via a transdermal patch or film system such as or analogous to that described, e.g., in U.S. Pat. Nos. 6,503,532; 5,302,395; 5,262,165; 5,248,501; 5,232,702; 5,230,896; 5,227,169; 5,212,199; 5,202,125; 5,173,302; 5,154,922; 5,139,786; 5,122,383; 5,023,252; 4,978,532; 5,324,521; 5,306,503; 5,302,395; 5,296,230; 5,286,491; 5,252,334; 5,248,501; 5,230,896; 5,227,169; 5,212,199; 5,202,125; 5,173,302; 5,171,576; 5,139,786; 5,133,972; 5,122,383; 5,120,546; 5,118,509; 5,077,054; 5,066,494; 5,049,387; 5,028,435; 5,023,252; 5,000,956; 4,911,916; 4,898,734; 4,883,669; 4,882,377; 4,840,796; 4,818,540; 4,814,173; 4,806,341; 4,789,547; 4,786,277; 4,702,732; 4,690,683; 4,627,429; and 4,585,452, the disclosures of which are herein incorporated by reference.
- As noted above, implementations may include formulations for oral administration that may be formulated using pharmaceutically acceptable carriers well known in the art in dosages suitable for oral administration. Such carriers enable the pharmaceutical formulations to be formulated in unit dosage forms as tablets, pills, powder, dragees, capsules, liquids, lozenges, gels, syrups, slurries, suspensions, etc., suitable for ingestion by the patient. Pharmaceutical preparations for oral use may be obtained through combination of at least one nutritional supplement with a solid excipient, optionally grinding a resulting mixture, and processing the mixture of granules, after adding suitable additional compounds, if desired, to obtain tablets or dragee cores. Suitable solid excipients include, but are not limited to, carbohydrate or protein fillers and include, but are not limited to sugars, including lactose, sucrose, mannitol, or sorbitol; starch from corn, wheat, rice, potato, or other plants; cellulose such as methyl cellulose, hydroxypropylmethyl-cellulose or sodium carboxymethylcellulose; and gums including arabic and tragacanth; as well as proteins such as gelatin and collagen. If desired, disintegrating or solubilizing agents may be added, such as the cross-linked polyvinyl pyrrolidone, agar, alginic acid, or a salt thereof, such as sodium alginate; with optional lubricants, such as talc or magnesium stearate; and if desired, with diluents, buffering agents, moistening agents, preservatives and flavoring agents.
- Accordingly, formulations suitable for oral administration in accordance with the subject invention may be present in discrete units, such as capsules, cachets, lozenges, tablets, and the like, each containing a predetermined amount of the nutritional supplement; as a powder or granules; as a solution or a suspension in a nutritional supplement formulation that may be prepared by any suitable method of pharmacy which includes, but is not limited to, bringing into association the nutritional supplement and a suitable carrier (which may contain one or more optional ingredients as noted above). For example, nutritional supplement formulations for use with the subject invention may be prepared by uniformly and intimately admixing the nutritional supplement with a liquid or finely divided solid carrier, or both, and then, if necessary, shaping the resulting mixture. For example, a tablet may be prepared by compressing or molding a powder or granules containing the nutritional supplement, optionally with one or more accessory ingredients. Compressed tablets may be prepared by compressing, in a suitable machine, the nutritional supplement in a free-flowing form, such as a powder or granules optionally mixed with a binder, lubricant, inert diluent, and/or surface active/dispersing agent(s). Molded tablets may be made by molding, in a suitable machine, the powdered nutritional supplement moistened with an inert liquid binder.
- A nutritional supplement employed in the subject invention may be delivered transdermally, by a topical route, formulated as applicator sticks, solutions, suspensions, emulsions, gels, creams, ointments, pastes, jellies, paints, powders, and aerosols. For example, implementations may include a nutritional supplement in the form of a discrete patch or film or plaster or the like adapted to remain in intimate contact with the epidermis of the recipient for a period of time. For example, such transdermal patches may include a base or matrix layer, e.g., polymeric layer, in which one or more nutritional supplements are retained. The base or matrix layer may be operatively associated with a support or backing. Nutritional supplement formulations suitable for transdermal administration may also be delivered by iontophoresis and may take the form of an optionally buffered aqueous solution of the nutritional supplement compound. Suitable formulations may include citrate or bis/tris buffer (pH 6) or ethanol/water and contain a suitable amount of active ingredient.
- The multi-dose packages of the invention find use in a variety of applications in which it is desired to meet the nutritional needs of a subject. By “nutritional need,” it is meant a subject's need for a particular nutrient, usually obtained from food, such as a vitamin, ‘mineral’, amino acid, etc., which is necessary to maintain good health. In some instances, the subject is a healthy subject. In some instances, the subject can have a deficiency of an essential nutrient, such as a vitamin. For example, a vitamin deficiency can be either ‘primary’ or ‘secondary’. A primary deficiency occurs when an insufficient amount of a vitamin is present in the diet. A secondary deficiency can be due to an underlying condition that prevents or limits the absorption or use of the vitamin. This can include smoking, excessive alcohol consumption, or other medications that interfere with the absorption or use of the vitamin.
- In some instances, the subject can have a need for a particular nutrient which varies over time. There are various natural and reoccurring conditions in which the nutritional requirements of a subject can change over time (e.g., monthly cycles in a female, varying nutritional needs of elderly subjects, athletes, nutritional needs in responses to season changes, etc.). In some implementations, the subject can have a condition which increases in severity or occurrence during a season of the year. Such conditions can include, for example, seasonal variation in the incidence of allergies, seasonal variation in gastroesophageal reflux disease (GERD) and duodenal ulcers, seasonal variation in variceal bleeding, seasonal variation in the incidence of urolithiasis, seasonal variation in the incidence of hip fractures, seasonal variation in fertility rates, seasonal variation in the incidence of eclampsia, seasonal variation in blood pressure in hypertensive patients, seasonal variation in blood pressure in CAPD (continuous ambulatory peritoneal dialysis) patients, seasonal variation in coronary artery disease and incidence of first hospitalization for myocardial infarction, seasonal variation in paroxysmal atrial fibrillation, seasonal variation in asthma, Seasonal Affective Disorder (SAD), seasonal variation in suicide rates, seasonal variation in vitamin D levels, seasonal variation in hyponatremia, seasonal variation in retinal vein occlusion, seasonal variation in neonatal death rates, seasonal variation in the incidence of congenital hypothyroidism, and seasonal variation in the onset of stage I sarcoidosis. The subject packages, systems, and methods can be used to treat any suitable condition that is known to be or is at least suspected of being exacerbated during at least one season of the year.
- Subjects with conditions that have nutritional needs that can be addressed with the multi-dose packages of the subject invention can also include: cardiovascular conditions; neurodegenerative conditions; neuroinflammatory conditions; orthopedic inflammatory conditions; lymphoproliferative conditions; autoimmune conditions; inflammatory conditions; infectious diseases; pulmonary conditions; transplant-related conditions; gastrointestinal conditions; endocrine conditions (e.g., diabetes); genitourinary conditions; skin conditions; aging conditions; neurologic conditions; Th-2 dominant conditions; conditions that cause hypoxia; conditions that cause hypercarbia; conditions that cause hypercapnia; conditions that cause acidosis; conditions that cause acidemia; OB-GYN conditions; sudden death syndromes; menstrual related disorders; fibrosis; chronic pain; trauma; bacterial infections; and fibromyalgia.
- The subject packages, systems, and methods can be used in some implementations by subjects with a variety of different conditions, including, but not limited to, cardiovascular conditions including cardiovascular disease, e.g., atherosclerosis, coronary artery disease, hypertension, hyperlipidemia, cardiomyopathy, volume retention, congestive heart failure, QT interval prolongation, aortic dissection, aortic aneurysm, arterial aneurysm, arterial vasospasm, myocardial infarction, reperfusion syndrome, ischemia, sudden adult death syndrome, arrhythmia, fatal arrythmias, coronary syndromes, coronary vasospasm, sick sinus syndrome, bradycardia, tachycardia, thromboembolic disease, deep vein thrombosis, coagulopathy, disseminated intravascular coagulation (“DIC”), mesenteric ischemia, syncope, venous thrombosis, arterial thrombosis, malignant hypertension, secondary hypertension, primary pulmonary hypertension, secondary pulmonary hypertension, Raynaud's, paroxysmal supraventricular tachycardia, and the like; neurodegenerative conditions including neurodegenerative diseases, e.g., Alzheimer's Disease, Pick's Disease, Parkinson's Disease, dementia, delirium, amyotrophic lateral sclerosis, and the like; neuroinflammatory conditions including neuroinflammatory diseases, e.g., viral meningitis, viral encephalitis, fungal meningitis, fungal encephalitis, multiple sclerosis, Charcot joint, schizophrenia, myasthenia gravis, and the like; orthopedic inflammatory conditions including orthopedic inflammatory diseases, e.g., osteoarthritis, inflammatory arthritis, regional idiopathic osteoporosis, reflex sympathetic dystrophy, Paget's disease, osteoporosis, antigen-induced arthritis, juvenile chronic arthritis, and the like; lymphoproliferative conditions including lymphoproliferative diseases, e.g., lymphoma, lymphoproliferative disease, Hodgkin's disease, inflammatory pseudomotor of the liver, and the like; autoimmune conditions including automimmune diseases, e.g., Graves disease, Raynaud's, Hashimoto's, Takayasu's disease, Kawasaki's diseases, arteritis, scleroderma, CREST syndrome, allergies, dermatitis, Henoch-schlonlein purpura, goodpasture syndrome, autoimmune thyroiditis, myasthenia gravis, Reiter's disease, lupus, and the like; inflammatory conditions, e.g., acute respiratory distress syndrome (“ARDS”), multiple sclerosis, rheumatoid arthritis, juvenile rheumatoid arthritis, juvenile chronic arthritis, migraines, chronic headaches, and the like; infectious diseases, e.g., sepsis, viral and fungal infections, diseases of wound healing, wound healing, tuberculosis, infection, AIDS, human immunodeficiency virus, and the like; pulmonary conditions including pulmonary diseases, e.g., tachypnea, fibrotic lung diseases such as cystic fibrosis and the like, interstitial lung disease, desquamative interstitial pneumonitis, non-specific interstitial pneumonitis, lymphocytic interstitial pneumonitis, usual interstitial pneumonitis, idiopathic pulmonary fibrosis, pulmonary edema, aspiration, asphyxiation, pneumothorax, right-to-left shunts, left-to-right shunts, respiratory failure, and the like; transplant-related conditions such as transplant related side effects such as transplant rejection, transplant-related tachycardia, transplant related renal failure, transplant related bowel dysmotility, transplant-related hyperreninemia, and the like; gastrointestinal conditions including gastrointestinal diseases, e.g., hepatitis, xerostomia, bowel mobility, peptic ulcer disease, constipation, ileus, irritable bowel syndrome, post-operative bowel dysmotility, inflammatory bowel disease, typhilitis, cholelethiasis, cholestasis, fecal incontinence, cyclic vomiting syndrome, and the like; endocrine conditions including endocrine diseases, e.g., hypothyroidism, hyperglycemia, diabetes, obesity, syndrome X, insulin resistance, polycystic ovarian syndrome (“PCOS”), and the like; genitourinary conditions including genitourinary diseases, e.g., bladder dysfunction, renal failure, hyperreninemia, hepatorenal syndrome, pulmonary renal syndrome, incontinence, arousal disorder, menopausal mood disorder, premenstrual mood disorder, renal tubular acidosis, pulmonary renal syndrome, and the like; skin conditions including skin diseases, e.g., wrinkles, cutaneous vasculitis, psoriasis, and the like; aging associated conditions including aging associated diseases, e.g., Shy Dragers, multi-system atrophy, age related inflammation conditions, cancer, aging, and the like; neurologic conditions including neurologic or psychiatric diseases such as epilepsy, depression, schizophrenia, seizures, stroke, insomnia, cerebral vascular accident, transient ischemic attacks, stress, bipolar disorder, concussions, post-concussive syndrome, cerebral vascular vasospasm, central sleep apnea, obstructive sleep apnea, sleep disorders, headaches including chronic headaches, migraines, acute disseminated encephalomyelitis (“ADEM”), and the like; Th-2 dominant conditions including Th-2 dominant diseases, e.g., typhilitis, osteoporosis, lymphoma, myasthenia gravis, lupus, and the like; conditions, including diseases, that cause hypoxia, hypercarbia, hypercapnia, acidosis, acidemia, e.g., ventilation/perfusion (V/Q) mismatch, Chronic Obstructive Pulmonary Disease (“COPD”), emphysema, any chronic lung disease that causes acidosis, acute pulmonary embolism, sudden adult death syndrome (“SADS”), chronic pulmonary embolism, pleural effusion, cardiogenic pulmonary edema, non-cardiogenic pulmonary edema, acute respiratory distress syndrome (ARDS), neurogenic edema, hypercapnia, acidemia, asthma, renal tubular acidosis, asthma, acidosis, chronic lung diseases that cause hypoxia, hypercarbia or hypercapnia, and the like; OB-GYN conditions including OB-GYN diseases, e.g., amniotic fluid embolism, menopausal mood disorders, premenstrual mood disorders, cervical incompetence, fetal distress, premenstrual syndrome, dysmenorrhea, endometriosis, fertility and subfertility conditions such as infertility, early pregnancy loss, spontaneous abortion, failure of implantation, amenorrhea, luteal insufficiency, and the like; sudden death syndromes, e.g., sudden adult death syndrome, and the like; menstrual related disorders, e.g., pelvic pain, dysmenorrhea, gastrointestinal disease, nausea, and the like; chronic pain; glaucoma; disorders of thermoregulation; fibromyalgia; and the like.
- The multi-dose nutritional supplement packages tailored for a particular subject are particularly useful in avoiding consequences of inadequate nutrition, e.g., insufficient amounts of vitamins. For example, consequences of insufficient amounts of vitamins include xerophthalmia or night blindness with vitamin A deficiency, beriberi caused by thiamine deficiency, pellagra caused by niacin deficiency, megaloblastic anemia caused by vitamin B12 deficiency, scurvy caused by vitamin C deficiency, rickets caused by vitamin D deficiency, and impaired coagulation caused by vitamin K deficiency.
- Furthermore, the tailored multi-dose nutritional supplement packages can avoid the problem of doses that are too large, as some vitamins, especially the fat-soluble vitamins, have documented side-effects that tend to be more severe with a large dosage. At high enough dosages, some vitamins cause side-effects such as nausea, diarrhea, and vomiting. An appropriate dose of a vitamin for a particular subject can vary, and can in some instances be related to factors such as age and health status.
- While many of the implementations may be manufactured and sold as prepackaged vitamins tailored for consumption by a particular target consumer for general health reasons or tailored to address specific supplemental needs in response to particular conditions or deficiencies, a system may be employed for custom ordering, fabrication and use of the multi-dose nutritional supplement packages of the subject invention.
- Methods of using the multi-dose nutritional supplement packages by a user can include selecting one of a plurality of unit dosage forms of a nutritional supplement contained in the multi-dose package comprising a first unit dosage of a nutritional supplement and a second unit dosage of the nutritional supplement, wherein the amount of the nutritional supplement in the first unit dosage differs from the amount of the nutritional supplement in the second unit dosage, and ingesting the first unit dosage form. In some implementations, the methods further include selecting the second unit dosage form from the multi-dose package and ingesting the second unit dosage form. The methods can in some implementations include selecting and ingesting a third, fourth, fifth, etc., unit dosage form of a first nutritional supplement and ingesting the first nutritional supplement. The methods can also in some implementations include selecting and ingesting one or more unit dosage forms of a second nutritional supplement and ingesting the second nutritional supplement.
- In some instances the nutritional supplement is a vitamin; in other instances the nutritional supplement is a nutraceutical, and in some instances the multi-dose nutritional supplement package can consist of both one or more vitamins, and one or more nutraceuticals.
- In some implementations, more than one nutritional supplement may be administered at the same or different time as another nutritional supplement, where the nutritional supplements administered differ in one or more respects, e.g., may be different types of agents or may be the same type nutritional supplement but that differ in mode of administration, dosage, etc.
- As discussed above, an effective amount of a given nutritional supplement may vary somewhat from subject to subject, and may depend upon factors such as, but not limited to, the age of the subject, the health of the subject, the particular condition being treated, the form of the nutritional supplement, the route and method of delivery, etc., as noted above. Such dosages may be determined in accordance with routine nutritional supplement procedures known to those skilled in the art. Nutritional supplements may be administered to a subject in a single oral dose, one time a day or more for weeks, months, years, even as long as a subject's lifetime or as long as the subject experiences the nutritional need. For example, an implementation may include administering a given nutritional supplement one time a day over a prolonged period of time, e.g., over a particular time period e.g., up to 1 month, or 2 months, or 3 months, or 9 months, or a year.
- The frequency of administration of a nutritional supplement may vary depending on one or more of the factors described above. For example, the frequency of administration of a nutritional supplement may range from 1 time per day to multiple times per day, e.g., 2 times or more per day or as necessary to meet the nutritional needs or nutritional requests of the subject.
- All publications and patent applications cited in this specification are incorporated by reference as if each individual publication or patent application were specifically and individually indicated to be incorporated by reference. The citation of any publication is for its disclosure prior to the filing date and should not be construed as an admission that the present invention is not entitled to antedate such publication by virtue of prior invention.
- Although the foregoing invention has been described in some detail by way of illustration and example for purposes of clarity of understanding, it is readily apparent to those of ordinary skill in the art in light of the teachings of this invention that certain changes and modifications may be made thereto without departing from the spirit or scope of the appended claims.
- Where a range of values is provided, it is understood that each intervening value, to the tenth of the unit of the lower limit unless the context clearly dictates otherwise, between the upper and lower limit of that range and any other stated or intervening value in that stated range is encompassed within the invention. The upper and lower limits of these smaller ranges may independently be included in the smaller ranges is also encompassed within the invention, subject to any specifically excluded limit in the stated range. Where the stated range includes one or both of the limits, ranges excluding either or both of those included limits are also included in the invention.
- Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. Although any methods and materials similar or equivalent to those described herein can also be used in the practice or testing of the present invention, the preferred methods and materials are now described. All publications mentioned herein are incorporated herein by reference to disclose and describe the methods and/or materials in connection with which the publications are cited.
- As will be apparent to those of skill in the art upon reading this disclosure, each of the individual implementations described and illustrated herein has discrete components and features which may be readily separated from or combined with the features of any of the other several implementations without departing from the scope or spirit of the present invention.
Claims (20)
1. A multi-dose package of nutritional supplements designed for regular and continuous consumption by a specific target consumer over a regularly re-occurring predetermined period of time, the multi-dose package of supplements comprising:
a series of supplements to be taken by the target consumer over the predetermined period of time, whereby each supplement in the series is to be taken at a particular time during the predetermined period of time and where each supplement in the series is tailored to contain unit dosages of nutritional supplements that correspond to the nutritional needs of the target consumer for such particular time during which the supplement is to be digested by the target consumer; and
where at least one supplement in the series to be taken at a first particular time contains different dosages of nutritional supplements than at least one other supplement in the series to be taken at a second particular period of time.
2. The multi-dose package of claim 1 where the re-occurring predetermined period of time is a day.
3. The multi-dose package of claim 1 where the re-occurring predetermined period of time is a week.
4. The multi-dose package of claim 1 where the re-occurring predetermined period of time is a 28-days.
5. The multi-dose package of claim 1 where the re-occurring predetermined period of time is a month.
6. The multi-dose package of claim 1 where the re-occurring predetermined period of time is a season.
7. The multi-dose package of claim 1 where the re-occurring predetermined period of time is a year.
8. The multi-dose package of claim 1 where the specific target consumer is a female.
9. The multi-dose package of claim 1 where the specific target consumer is a male.
10. The multi-dose package of claim 1 where the supplements include indicia indicating when each supplement should be taken over the predetermined period of time.
11. A multi-dose package of multi-vitamins designed for consumption by a specific target consumer over a predetermined period of time, the multi-dose package of multi-vitamins comprising a series of supplements to be taken by the target consumer over the predetermined period of time where at least one supplement in the series to be taken at a first particular time contains different dosages of nutritional supplements than at least one other supplement in the series to be taken at a second particular period of time.
12. The multi-dose package of claim 11 where the re-occurring predetermined period of time is a week.
13. The multi-dose package of claim 11 where the re-occurring predetermined period of time is a week.
14. The multi-dose package of claim 11 where the re-occurring predetermined period of time is twenty-days.
15. The multi-dose package of claim 11 where the re-occurring predetermined period of time is a month.
16. The multi-dose package of claim 11 where the re-occurring predetermined period of time is a season.
17. The multi-dose package of claim 11 where the re-occurring predetermined period of time is a year.
18. The multi-dose package of claim 11 where the specific target consumer is a female.
19. The multi-dose package of claim 11 where the specific target consumer is a male.
20. The multi-dose package of claim 11 where the supplements include indicia indicating when each supplement should be taken over the predetermined period of time.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/169,712 US20120228176A1 (en) | 2011-03-11 | 2011-06-27 | Multi-dose nutritional supplements |
| US13/225,064 US20120228190A1 (en) | 2011-03-11 | 2011-09-02 | Multi-dose nutritional supplements for the promotion of joint health |
| US13/225,105 US20120228191A1 (en) | 2011-03-11 | 2011-09-02 | Multi-dose nutritional supplements for the promotion of bone health |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161451917P | 2011-03-11 | 2011-03-11 | |
| US13/169,712 US20120228176A1 (en) | 2011-03-11 | 2011-06-27 | Multi-dose nutritional supplements |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/225,105 Continuation-In-Part US20120228191A1 (en) | 2011-03-11 | 2011-09-02 | Multi-dose nutritional supplements for the promotion of bone health |
| US13/225,064 Continuation-In-Part US20120228190A1 (en) | 2011-03-11 | 2011-09-02 | Multi-dose nutritional supplements for the promotion of joint health |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20120228176A1 true US20120228176A1 (en) | 2012-09-13 |
Family
ID=46794554
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/169,712 Abandoned US20120228176A1 (en) | 2011-03-11 | 2011-06-27 | Multi-dose nutritional supplements |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US20120228176A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8909340B2 (en) | 2011-08-23 | 2014-12-09 | Palo Alto Investors | Methods and devices for treating conditions associated with autonomic dysfunction |
| EP2962578A1 (en) * | 2014-06-30 | 2016-01-06 | Richard Hubertus Verheesen | Method of providing a plurality of nutrition portions to a user and packaging product |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040096522A1 (en) * | 2001-02-14 | 2004-05-20 | Lazarev Mikhail I | Vitamin/mineral complex |
| EP2082717A1 (en) * | 2008-01-28 | 2009-07-29 | Merz Pharma GmbH & Co. KGaA | Titration package |
-
2011
- 2011-06-27 US US13/169,712 patent/US20120228176A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040096522A1 (en) * | 2001-02-14 | 2004-05-20 | Lazarev Mikhail I | Vitamin/mineral complex |
| EP2082717A1 (en) * | 2008-01-28 | 2009-07-29 | Merz Pharma GmbH & Co. KGaA | Titration package |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8909340B2 (en) | 2011-08-23 | 2014-12-09 | Palo Alto Investors | Methods and devices for treating conditions associated with autonomic dysfunction |
| US9326887B2 (en) | 2011-08-23 | 2016-05-03 | Palo Alto Investors | Methods and devices for treating conditions associated with autonomic dysfunction |
| US9821003B2 (en) | 2011-08-23 | 2017-11-21 | Palo Alto Investors | Methods and devices for treating conditions associated with autonomic dysfunction |
| EP2962578A1 (en) * | 2014-06-30 | 2016-01-06 | Richard Hubertus Verheesen | Method of providing a plurality of nutrition portions to a user and packaging product |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Chomba et al. | Zinc absorption from biofortified maize meets the requirements of young rural Zambian children | |
| Yung et al. | Dietary choices and likelihood of abstinence among alcoholic patients in an outpatient clinic | |
| US20040213838A1 (en) | Medical food tablets containing free amino acids | |
| Awuchi et al. | Ready-to-use therapeutic foods (RUTFs) for remedying malnutrition and preventable nutritional diseases | |
| CN106572691A (en) | Preconception/prenatal/postnatal optimal nutrition system, compositions and kits for use therein, and methods of making and using same | |
| JP2007533607A (en) | Micronutrient supplements | |
| US20120228191A1 (en) | Multi-dose nutritional supplements for the promotion of bone health | |
| US20120228190A1 (en) | Multi-dose nutritional supplements for the promotion of joint health | |
| US20120228176A1 (en) | Multi-dose nutritional supplements | |
| Lindzon et al. | Folate during reproduction: the Canadian experience with folic acid fortification | |
| CN110122706A (en) | Hinder the anti-oxidant high fine compound fruity liquid beverage of heat absorption hypoglycemic | |
| CN1835870B (en) | Micronutrient supplement dispensing package which is safe to children | |
| CN102228447B (en) | Zinc-selenium chewable tablet | |
| Wamiti et al. | Effectiveness of leucine supplementation in the management of moderate wasting in children | |
| US20050281889A1 (en) | Nutritional supplement for adults | |
| US11458150B2 (en) | Methylphosphinic acid compositions and methods for reducing aging | |
| CA2505043A1 (en) | Nutritional supplement for infants | |
| Talaulikar et al. | Folic acid in pregnancy | |
| Nair | Contextualizing the principles of iron fortification of foods in India | |
| Vossenaar et al. | Several problem nutrients are identified in the complementary diet of 6 to 11 month old breastfed children in Western Guatemala | |
| Barker et al. | Economy in drug prescribing in Mozambique | |
| Wald | Folic acid and neural tube defects | |
| Poudel | Malnutrition status among children in Nepal | |
| CN107412334B (en) | Compound sugar-controlling mixture and its preparation method | |
| CN114344445B (en) | Composition for improving memory and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: PALO ALTO INVESTORS, CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:YUN, ANTHONY JOONKYOO;MARWAH, RAKESH SINGH;SIGNING DATES FROM 20110712 TO 20110714;REEL/FRAME:026848/0793 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |