US20070287780A1 - Curable Composition - Google Patents
Curable Composition Download PDFInfo
- Publication number
- US20070287780A1 US20070287780A1 US11/579,635 US57963505A US2007287780A1 US 20070287780 A1 US20070287780 A1 US 20070287780A1 US 57963505 A US57963505 A US 57963505A US 2007287780 A1 US2007287780 A1 US 2007287780A1
- Authority
- US
- United States
- Prior art keywords
- group
- component
- carbon atoms
- curable composition
- substituted
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- ZXNGFKPTYFOOCA-FGSKAQBVSA-M C.CC1=CC(C)=O[Ti](C)O1 Chemical compound C.CC1=CC(C)=O[Ti](C)O1 ZXNGFKPTYFOOCA-FGSKAQBVSA-M 0.000 description 6
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G65/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G65/02—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from cyclic ethers by opening of the heterocyclic ring
- C08G65/32—Polymers modified by chemical after-treatment
- C08G65/329—Polymers modified by chemical after-treatment with organic compounds
- C08G65/336—Polymers modified by chemical after-treatment with organic compounds containing silicon
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/01—Use of inorganic substances as compounding ingredients characterized by their specific function
- C08K3/014—Stabilisers against oxidation, heat, light or ozone
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0091—Complexes with metal-heteroatom-bonds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/54—Silicon-containing compounds
- C08K5/544—Silicon-containing compounds containing nitrogen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D171/00—Coating compositions based on polyethers obtained by reactions forming an ether link in the main chain; Coating compositions based on derivatives of such polymers
- C09D171/02—Polyalkylene oxides
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J171/00—Adhesives based on polyethers obtained by reactions forming an ether link in the main chain; Adhesives based on derivatives of such polymers
- C09J171/02—Polyalkylene oxides
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K3/00—Materials not provided for elsewhere
- C09K3/10—Materials in mouldable or extrudable form for sealing or packing joints or covers
Definitions
- the present invention relates to a curable composition
- a curable composition comprising an organic polymer containing a silicon-containing group (hereinafter referred to as a “reactive silicon group”) which has hydroxyl group or a hydrolyzable group bonded to the silicon atom and which is capable of crosslinking by forming siloxane bonds.
- an organic polymer having at least one reactive silicon group in the molecule undergoes crosslinking by formation of siloxane bonds accompanying a hydrolysis reaction of the reactive silicon group with moisture or the like even at room temperature, and a rubber-like cured article can be obtained.
- polymers having a reactive silicon group organic polymers in which the main chain skeleton is a polyoxyalkylene polymer or a (meth)acrylate polymer are disclosed in (Patent Document 1), (Patent Document 2) and the like. Those polymers have already been industrially produced, and are widely used in applications to sealants, adhesives, coatings and the like.
- Curable compositions comprising the above described organic polymers having a reactive silicon group are cured by using silanol condensation catalysts, and usually organotin catalysts having a carbon-tin bond such as dibutyltin bis(acetylacetonate) are widely used.
- organotin catalysts having a carbon-tin bond such as dibutyltin bis(acetylacetonate)
- toxicity of organotin catalysts is pointed out, and development of non-organotin catalysts are demanded.
- Patent Document 4 As described in (Patent Document 4), (Patent Document 5), (Patent Document 6), (Patent Document 7) and the like, as a result of various investigations made, improvement methods such as modification of a silicone-terminated structure, use of a chelate type titanium compound, and the like have been found, and at the present time, dealcoholization type silicone compositions using titanium catalysts are widely used in a variety of applications. Additionally, in (Patent Document 8), a technique for improving curability by using a chelate type methyl titanium is disclosed.
- Patent Document 1 JP-52-73998A
- Patent Document 2 JP-59-74149A
- Patent Document 3 JP-39-27643B (U.S. Pat. No. 3,175,993)
- Patent Document 4 U.S. Pat. No. 3,334,067
- Patent Document 5 JP-56-14701B
- Patent Document 6 JP-55-43119A
- Patent Document 7 JP-2-133490A
- Patent Document 8 JP-2001-302934A
- Patent Document 9 JP-58-17154A
- Patent Document 10 JP-11-209538A
- Patent Document 11 JP-5-311063A
- Patent Document 12 JP-2001-302929A
- Patent Document 13 JP-2001-302930A
- Patent Document 14 JP-2001-302931A
- Patent Document 15 JP-2001-302934A
- Patent Document 16 JP-2001-348528A
- Patent Document 17 JP-2002-249672A
- Patent Document 18 JP-2003-165916A
- Patent Document 19 JP-62-35421B
- a one-component type curable composition which comprises a reactive silicon group-containing organic polymer as a main component, is prepared using a titanium catalyst as a non-tin curing catalyst and contains an amino silane as an adhesion-imparting agent, and have found that mechanical properties of a cured article obtained from the composition having been stored for a given period of time are remarkably lowered as compared with a cured article obtained from the composition before storing.
- a curable composition having practical curability and good adhesion and minimizing lowering of mechanical properties of a cured article after storing can be obtained by use of a silane compound having a nitrogen-substituted group as an adhesion-imparting agent and a chelate type methyl titanate (b1) and/or a chelate type ethyl titanate (b2) as a curing catalyst for the polymer although the catalyst concerned is a non-tin curing catalyst.
- the present invention relates to a one-component type curable composition characterized by comprising, as components,
- the polyoxyalkylene polymer (al) is preferably a polyoxypropylene polymer.
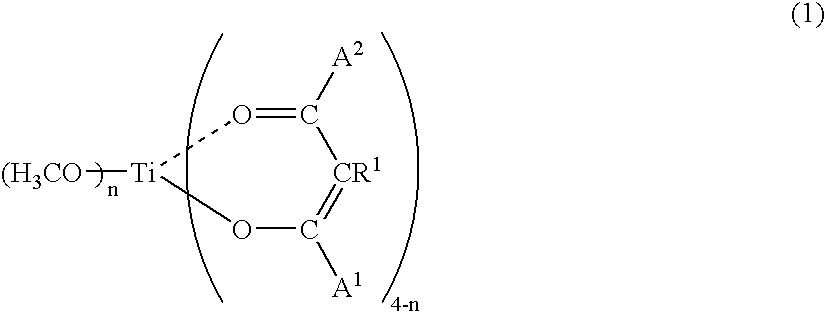
- the chelate type methyl titanate (b1) represented by the general formula (1) is preferable.
- the silicon-containing group contained in the component (A) and the hydrolyzable silicon group contained in the component (C) are groups represented by the following general formula (5) and the general formula (6), respectively: —SiR 5 3-a (OR 6 ) a (5) . . . silicon-containing group of the component (A) —SiR 7 3-b (OR 8 ) b (6) . . .
- each of R 5 and R 7 is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R) 3 SiO— where each of R's is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, R 6 is an alkyl group having 1 to 4 carbon atoms, R 8 is an alkyl group having 1 to 4 carbon atoms, a is 1, 2 or 3, b is 1, 2 or 3, and R 6 is an alkyl group having the number of carbon atoms equal to or more than the number of carbon atoms of R 8 .
- the hydrolyzable silicon group contained in the component (C) is preferably a methoxysilyl group represented by the general formula (7): —SiR 7 3-b (OCH 3 ) b (7) wherein each of R 7 s is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R′) 3 SiO— where each of R's is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, b is 1, 2 or 3.
- curable composition of the present invention are sealants or adhesives comprising any of the above described one-component type curable compositions.
- the curable composition of the present invention is excellent in curability and adhesion by use of a non-tin curing catalyst, and lowering of mechanical properties of a cured article obtained after storing is minimized.
- the polyoxyalkylene polymer (a1) having a reactive silicon group and/or the (meth)acrylate polymer (a2) having a reactive silicon group are used as the component (A).
- organic polymer By using the polyoxyalkylene polymer and/or the (meth)acrylate polymer as a main chain skeleton of the polymer of the component (A), a satisfactory adhesion can be achieved.
- a curable composition is prepared using a titanium catalyst as the component (B) of the present invention, there is a tendency that deep-part curability of the obtained composition is lowered depending on an added amount thereof.
- a polyoxyalkylene polymer and a (meth)acrylate polymer as used for the component (A) of the present invention are preferable because they are high in moisture permeability and excellent in deep-part curability in the case of a one-component type composition.
- the glass transition temperature of the organic polymer of the component (A) is preferably not more than 20° C., more preferably not more than 0° C., particularly preferably not more than ⁇ 20° C. If the glass transition temperature is higher than 20° C., in some cases, a viscosity increases and workability is lowered in wintertime or at a cold district or flexibility and elongation of the cured article are degraded.
- the above described glass transition temperature denotes values measured by DSC.
- the reactive silicon group contained in the polyoxyalkylene polymer (a1) having a reactive silicon group and the (meth)acrylate polymer (a2) having a reactive silicon group is a group which has a hydroxyl group or a hydrolyzable group bonded to a silicon atom, and is capable of cross-linking through a reaction accelerated by a curing catalyst.
- Examples of the reactive silicon groups include groups represented by the general formula (8): —(SiR 9 2-c X c O) m —SiR 10 3-d X d (8) wherein each of R 9 and R 10 is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R′) 3 SiO—, where R′ is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, each of Xs is independently hydroxyl group or a hydrolyzable group, c is 0, 1 or 2, d is 0, 1, 2 or 3, c and d are not 0 at the same time, m is 0 or an integer of 1 to 19.
- the hydrolyzable group may be a hydrolyzable group well known in the art. More specifically, examples of the hydrolyzable group include, for instance, hydrogen atom, a halogen atom, an alkoxy group, an acyloxy group, a ketoximate group, an amino group, an amide group, an acid amide group, an aminooxy group, a mercapto group, an alkenyloxy group and the like.
- an alkoxy group hydrogen atom, an alkoxy group, an acyloxy group, a ketoximate group, an amino group, an amide group, an aminooxy group, a mercapto group and an alkenyloxy group are preferable and an alkoxy group is particularly preferable from the viewpoint that an alkoxy group is moderately hydrolyzable and easily handled.
- One to three hydrolyzable groups and hydroxyl groups can bond to one silicon atom, and (a+ ⁇ c) is preferably within a range from 1 to 5.
- two or more hydrolyzable groups and hydroxyl groups are bonded in the reactive silicon group, they may be the same or different.
- the number of silicon atoms forming the reactive silicon group is one or more, and is preferably not more than 20 in the case of the silicon atoms connected by siloxane bonds.
- R 9 and R 10 in the above described general formulae (8) and (9) include alkyl groups such as a methyl group and an ethyl group, cycloalkyl groups such as a cyclohexyl group, aryl groups such as a phenyl group, aralkyl groups such as a benzyl group, a triorganosiloxy group represented by (R′) 3 SiO— where R′ is a methyl group, a phenyl group or the like and the like group.
- R′ is a methyl group, a phenyl group or the like and the like group.
- a methyl group is particularly preferable.
- the reactive silicon group include a trimethoxysilyl group, a triethoxysilyl group, a triisopropoxysilyl group, a dimethoxymethylsilyl group, a diethoxymethylsilyl group and a diisopropoxymethylsilyl group.
- a trimethoxysilyl group, a triethoxysilyl group and a dimethoxymethylsilyl group are more preferable and a trimethoxysilyl group is particularly preferable because these groups are high in activity and satisfactory curability can be obtained.
- a dimethoxymethylsilyl group is particularly preferable.
- a triethoxysilyl group is particularly preferable because the alcohol produced by the hydrolysis reaction of the reactive silicon group is ethanol and hence a triethoxysilyl group has a high safety. Additionally, a trimethoxysilyl group and a triethoxysilyl group are preferable because lowering of physical properties of a cured article after storing is relatively small.
- the introduction of the reactive silicon group can be carried out by methods well known in the art. More specifically, examples of such methods include the followings.
- an organic polymer having in the molecule functional groups such as hydroxy groups an organic compound having both an active group exhibiting reactivity to the functional groups and an unsaturated group is reacted, to yield an unsaturated group-containing organic polymer.
- an unsaturated group-containing organic polymer is obtained by copolymerization of an epoxy compound having an unsaturated group with an organic polymer having in the molecule functional groups such as hydroxy groups. Then, a reactive silicon group-containing hydrosilane is reacted with the reaction product to be hydrosilylated.
- the method described in (a) or the method described in (c) in which a hydroxy group-terminated polymer is reacted with an isocyanate group- and reactive silicon group-containing compound is preferable because the method provides a high conversion rate at a relatively short reaction time. Additionally, the method described in (a) is particularly preferable because the reactive silicon group-containing organic polymer obtained by the method described in (a) is lower in viscosity and more satisfactory in workability than an organic polymer obtained by the method described in (c), and an organic polymer obtained by the method described in (b) is strong in odor due to mercaptosilane.
- hydrosilane compound used in the method described in (a) examples include halogenated silanes such as trichlorosilane, methyldichlorosilane, dimethylchlorosilane and phenyldichlorosilane; alkoxysilanes such as trimethoxysilane, triethoxysilane, methyldiethoxysilane, methyldimethoxysilane and phenyldimethoxysilane; acyloxysilanes such as methyldiacetoxysilane and phenyldiacetoxysilane; ketoximate silanes such as bis(dimethylketoximate)methylsilane and bis(cyclohexylketoximate)methylsilane and the like.
- halogenated silanes such as trichlorosilane, methyldichlorosilane, dimethylchlorosilane and phenyldichlorosilane
- the hydrosilane compound used in the method described in (a) is not limited to these compounds.
- halogenated silanes and alkoxysilanes are preferable and in particular, alkoxysilanes are most preferable because the obtained curable compositions are moderately hydrolyzable and easily handled.
- alkoxysilanes methyldimethoxysilane is particularly preferable because it is easily available and curability, storage stability, elongation property and tensile strength of the curable composition containing the obtained organic polymer are high.
- Examples of the synthesis method described in (b) include a method in which a mercapto group- and reactive silicon group-containing compound is introduced into the sites on the unsaturated bonds of an organic polymer by means of a radical addition reaction in the presence of a radical initiator and/or a radical generating source; however, the synthesis method concerned is not limited to these methods.
- Examples of the above described mercapto group- and reactive silicon group-containing compound include ⁇ -mercaptopropyltrimethoxysilane, ⁇ -mercaptopropylmethyldimethoxysilane, ⁇ -mercaptopropyltriethoxysilane, ⁇ -mercaptopropylmethyldiethoxysilane, mercaptomethyltrimethoxysilane, mercaptomethyltriethoxysilane and the like; however, the mercapto group- and reactive silicon group-containing compound is not limited to these compounds.
- Examples of the method, of the methods described in (c), in which a hydroxy-terminated polymer is reacted with an isocyanate group- and reactive silicon group-containing compound include a method disclosed in Japanese Patent Laid-Open No. 3-47825; however, the method concerned is not limited to these methods.
- Examples of the above described isocyanate group- and reactive silicon group-containing compound include ⁇ -isocyanatopropyltrimethoxysilane, ⁇ -isocyanatopropylmethyldimethoxysilane, ⁇ -isocyanatopropyltriethoxysilane, ⁇ -isocyanatopropylmethyldiethoxysilane, isocyanatomethyltrimethoxysilane, isocyanatomethyltriethoxysilane, isocyanatomethyldimethoxymethylsilane, isocyanatomethyldiethoxymethylsilane and the like; however, the compound concerned is not limited to these compounds.
- disproportionation reaction proceeds. If disproportionation reaction proceeds, a very dangerous compound like dimethoxysilane is generated. However in the cases of ⁇ -mercaptopropyltrimethoxysilane and ⁇ -isocyanatepropyltrimethoxysilane, such a disproportionation reaction does not proceed. Accordingly, when using, as a silicon-containing group, a group such as a trimethoxysilyl group in which three hydrolyzable groups are bonded to one silicon atom, it is preferable to employ the synthesis method of (b) or (c).
- the reactive silicon group-containing organic polymer may be a straight chain or may have branches, and the number average molecular weight thereof, measured by GPC relative to polystyrene standard, is preferably of the order of 500 to 100,000, more preferably 1,000 to 50,000, particularly preferably 3,000 to 30,000.
- the number average molecular weight is less than 500, it tends to be disadvantageous from the viewpoint of an elongation property of the cured article, while when the number average molecular weight exceeds 100,000, it tends to be disadvantageous from the viewpoint of workability because the viscosity becomes high.
- the number of reactive silicon groups contained in the organic polymer is, on average in one polymer molecule, at least one, and preferably 1.1 to 5.
- the reactive silicon group may be located at the terminal of the main chain or at the terminal of the side chain, or at the both in the organic polymer molecule chain.
- the reactive silicon group when located only at the terminal of the main chain, the effective network content in the organic polymer component contained in the finally formed cured article becomes large, so that it becomes easier to obtain a rubber-like cured article having a high strength, a high elongation property and a low elastic modulus.
- the polyoxyalkylene polymer (a1) is essentially a polymer having the repeating units represented by the general formula (10): —R 11 —O— (10) wherein R 11 is a linear or branched alkylene group having 1 to 14 carbon atoms.
- R 11 is preferably a linear or branched alkylene group having 1 to 14 carbon atoms, and more preferably 2 to 4 carbon atoms.
- repeating units represented by the general formula (10) include: —CH 2 O—, —CH 2 CH 2 O—, —CH 2 CH(CH 3 )O—, —CH 2 CH(C 2 H 5 )O—, —CH 2 C(CH 3 ) 2 O—, —CH 2 CH 2 CH 2 CH 2 O—, and the like.
- the main chain skeleton of the polyoxyalkylene polymer may be formed of either only one type of repeating unit or two or more types of repeating units.
- the main chain skeleton is formed of a polymer containing as the main component a propyleneoxide polymer because a polymer having such a main chain skeleton is amorphous and relatively low in viscosity.
- Examples of the synthesis method of the polyoxyalkylene polymer include a polymerization method in the presence of an alkaline catalyst such as KOH; a polymerization method in the presence of a transition metal compound-porphyrin complex catalyst prepared by reacting an organoaluminum compound with porphyrin, disclosed in Japanese Patent Laid-Open No. 61-215623; polymerization methods in the presence of composite metal cyanide complex catalysts, disclosed in Japanese Patent Examined Publication Nos. 46-27250 and 59-15336, and U.S. Pat. Nos.
- Examples of the preparation method of the reactive silicon group-containing polyoxyalkylene polymer (a1) of the present invention include the methods disclosed in Japanese Patent Examined Publication Nos. 45-36319 and 46-12154, Japanese Patent Laid-Open Nos. 50-156599, 54-6096, 55-13767, 55-13468 and 57-164123, Japanese Patent Examined Publication No. 3-2450, and U.S. Pat. Nos.
- the above described reactive silicon group-containing polyoxyalkylene polymers (a1) may be used either each alone or in combinations of two or more thereof.
- the (meth)acrylate monomers constituting the main chains of the above described (meth)acrylate polymers (a2) and various types can be used.
- the monomers concerned include (meth)acrylic acid monomers such as (meth)acrylic acid, methyl (meth)acrylate, ethyl (meth)acrylate, n-propyl (meth)acrylate, isopropyl (meth)acrylate, n-butyl (meth)acrylate, isobutyl (meth)acrylate, tert-butyl (meth)acrylate, n-pentyl (meth)acrylate, n-hexyl (meth)acrylate, cyclohexyl (meth)acrylate, n-heptyl (meth)acrylate, n-octyl (meth)acrylate, 2-ethylhexyl (meth)acrylate, nonyl (meth)
- (meth)acrylate monomers can be copolymerized with the following vinyl monomers.
- the vinyl monomers concerned include styrene monomers such as styrene, vinyltoluene, ⁇ -methylstyrene, chlorostyrene, styrenesulfonic acid and the salts thereof; fluorine-containing vinyl monomers such as perfluoroethylene, perfluoropropylene and fluorinated vinylidene; silicon-containing vinyl monomers such as vinyltrimethoxysilane and vinyltriethoxysilane; maleic anhydride, maleic acid, and monoalkyl esters and dialkyl esters of maleic acid; fumaric acid, and monoalkyl esters and dialkyl esters of fumaric acid; maleimide monomers such as maleimide, methylmaleimide, ethylmaleimide, propylmaleimide, butylmaleimide, hexyl
- These monomers may be used each alone or two or more of these monomers may be copolymerized.
- polymers formed of styrene monomers and (meth)acrylic acid monomers are preferable. More preferable are the (meth)acryl polymers formed of acrylate monomers and methacrylate monomers, and particularly preferable are the acryl polymers formed of acrylate monomers.
- the butyl acrylate monomers are further preferable because compositions concerned each are required to have physical properties including a low viscosity, and the cured articles each are required to have physical properties including a low modulus, a high elongation property, a weather resistance and a heat resistance.
- copolymers made of ethyl acrylate as the main material are further preferable.
- the copolymers made of ethyl acrylate as the main material are excellent in oil resistance, but slightly tend to be poor in low-temperature property (low-temperature resistance); for the purpose of improving the low-temperature property thereof, a part of ethyl acrylates can be replaced with butyl acrylate.
- the ratio of butyl acrylate is set preferably to not more than 40%, and more preferably to not more than 30%.
- 2-methoxyethyl acrylate and 2-ethoxyethyl acrylate which have side chain alkyl groups containing oxygen atoms introduced for the purpose of improving the low-temperature property and the like without degrading the oil resistance; in this connection, it is to be noted that the introduction of alkoxy groups having an ether bond in the side chains tends to degrade the heat resistance, so that the ratio of such an acrylate is preferably not more than 40% when heat resistance is required. It is possible to obtain appropriate polymers by varying the ratio in consideration of required physical properties such as oil resistance, heat resistance and low-temperature property according to the various applications and the required objectives.
- Examples of the polymers excellent in the balance between the physical properties including the oil resistance, heat resistance, low-temperature property and the like include a copolymer of ethyl acrylate/butyl acrylate/2-methoxyethyl acrylate (40 to 50/20 to 30/30 to 20 in a ratio by weight), this copolymer imposing no constraint on the polymers concerned.
- these preferable monomers can be copolymerized with other monomers, and moreover, block copolymerized with other monomers. In such cases, it is preferable that the preferable monomers are contained in an amount of not less than 40% in a ratio by weight.
- (meth)acrylic acid means acrylic acid and/or methacrylic acid.
- the living radical polymerization methods “the living radical polymerization methods” in which (meth)acrylate monomers are polymerized by use of an organic halogenated compound or a halogenated sulfonyl compound as an initiator and a transition metal complex as a catalyst has, in addition to the features of the above described “living radical polymerization methods,” features such that the obtained polymer has halogen atoms at the terminals relatively favorable for the functional group conversion reaction and freedom for designing the initiator and the catalyst is wide, so that the atom transfer radical polymerization method is further preferable as a method for preparing (meth)acrylate polymers having particular functional groups.
- the atom transfer radical polymerization method include the method reported by Matyjaszewski et al. in Journal of the American Chemical Society (J. Am. Chem. Soc.), Vol. 117, p. 5614 (1995).
- a reactive silicon group-containing (meth)acrylate polymer for example, Japanese Patent Examined Publication Nos. 3-14068 and 4-55444, and Japanese Patent Laid-Open No. 6-211922 and the like disclose preparation methods according to the free radical polymerization methods by using chain transfer agents. Additionally, Japanese Patent Laid-Open No. 9-272714 and the like disclose a preparation method according to the atom transfer radical polymerization method. However, the preparation method concerned is not limited to these methods.
- the above described reactive silicon group-containing (meth)acrylate polymers (a2) may be used either each alone or in combinations of two or more thereof.
- the preparation methods of the organic polymers formed by blending the reactive silicon group-containing polyoxyalkylene polymers (a1) with the reactive silicon group-containing (meth)acrylate polymers (a2) are proposed in Japanese Patent Laid-Open Nos. 59-122541, 63-112642, 6-172631, 11-116763 and the like. However, the preparation method concerned is not limited to these methods.
- a reactive silicon group-containing polyoxyalkylene polymer is blended with a copolymer formed of two (meth)acrylate monomer units: one (meth)acrylate monomer unit has the reactive silicon groups and alkyl groups having 1 to 8 carbon atoms, and the molecular chain is substantially represented by the following general formula (11): —CH 2 —C(R 12 )(COOR 13 )— (11) wherein R 12 represents hydrogen atom or a methyl group, and R 13 represents an alkyl group having 1 to 8 carbon atoms; and the other (meth)acrylate monomer unit has alkyl groups having 10 or more carbon atoms and is represented by the following general formula (12): —CH 2 —C(R 12 )(COOR 14 )— (12) wherein R 12 is the same as defined above, and R
- examples of R 13 include alkyl groups having 1 to 8 carbon atoms, preferably 1 to 4 carbon atoms and further preferably 1 to 2 carbon atoms such as a methyl group, an ethyl group, a propyl group, a n-butyl group, a t-butyl group and a 2-ethylhexyl group. It is also to be noted that the alkyl group of R 13 may represent either one type or admixtures of two or more types.
- examples of R 14 include long chain alkyl groups having 10 or more carbon atoms, usually 10 to 30 carbon atoms, and preferably 10 to 20 carbon atoms such as a lauryl group, a tridecyl group, a cetyl group, a stearyl group and a behenyl group. It is also to be noted that the alkyl group of R 14 may represent, similarly to R 13 , either one type or admixtures of two or more types.
- the molecular chains of the above described (meth)acrylate copolymers are substantially formed of the monomer units represented by formulas (11) and (12): “substantially” as referred to here means that in the copolymer concerned, the sum content of the monomer unit of formula (11) and the monomer unit of formula (12) exceeds 50 wt %.
- the sum content of the monomer units of formulas (11) and (12) is preferably not less than 70 wt %.
- the abundance ratio by weight of the monomer unit of formula (11) to the monomer unit of formula (12) is preferably 95:5 to 40:60, and further preferably 90:10 to 60:40.
- Examples of the monomer units other than the monomer units of formulas (11) and (12) which may be contained in the above described copolymer include acrylic acids such as acrylic acid and methacrylic acid; monomers containing amide groups such as acrylamide, methacrylamide, N-methylolacrylamide and N-methylolmethacrylamide, monomers containing epoxy groups such as glycidylacrylate and glycidylmethacrylate, and monomers containing amino groups such as diethylaminoethyl acrylate, diethylaminoethyl methacrylate and aminoethyl vinyl ether; and monomer units derived from acrylonitrile, styrene, ⁇ -methylstyrene, alkyl vinyl ethers, vinyl chloride, vinyl acetate, vinyl propionate and ethylene.
- acrylic acids such as acrylic acid and methacrylic acid
- monomers containing amide groups such as acrylamide, methacrylamide, N-methylolacrylamide and
- the main chain skeleton of the organic polymer of the present invention may include other components such as binding urethane components as far as such inclusion does not largely impair the effect of the invention.
- binding urethane components include groups (hereinafter referred to as amide segments) formed by a reaction of an isocyanate group with an active hydrogen group.
- amide segments are groups represented by the general formula (13): —NR 15 —C( ⁇ O)— (13) wherein R 15 represents hydrogen atom or a substituted or unsubstituted organic group.
- Examples of the above described amide segments include a urethane group formed by a reaction of an isocyanate group with hydroxyl group; a urea group formed by a reaction of an isocyanate group with an amino group; a thiourethane group formed by a reaction of an isocyanate group with a mercapto group; and the like. Additionally, in the present invention, groups formed by further reaction of an active hydrogen in the urethane group, urea group or thiourethane group with an isocyanate group are included in the groups of the general formula (13).
- Example of an industrially easy method of preparing an organic polymer having both of an amide segment and a reactive silicon group is a method in which after or at the same time of reacting an organic polymer having an active hydrogen-containing group at the terminal with an excessive amount of polyisocyanate compound to yield a polymer having isocyanate groups at the terminals of polyurethane main chains, a part or the whole of isocyanate groups are reacted with a W group of the silicon compound represented by the general formula (14): W—R 16 —SiR 10 3-e X e (14) wherein R 10 , X and e are the same as defined above; R 16 is a divalent organic group, more preferably a substituted or unsubstituted divalent hydrocarbon group having 1 to 20 carbon atoms; W is an active hydrogen-containing group selected from hydroxyl group, a carboxyl group, a mercapto group and an amino group (unsubstituted or mono-substituted).
- Polyether polyols prepared by any of preparation methods can be used, and preferred are polyether polyols having at least 0.7 hydroxyl group per a molecular terminal on average of the whole molecules.
- examples thereof are oxyalkylene polymers prepared by using conventional alkali metal catalysts, oxyalkylene polymers prepared by reacting an initiator such as a polyhydroxy compound having at least two hydroxyl groups with alkylene oxide in the presence of a composite metal cyanide complex and cesium, and the like.
- the method using a composite metal cyanide complex is preferable because oxyalkylene polymers having a lower degree of unsaturation, a narrow Mw/Mn, a lower viscosity and high acid resistance and weather resistance can be obtained.
- Examples of the above described polyacryl polyols include polyols having an alkyl (meth)acrylate (co)polymer skeleton and containing hydroxy groups in the molecule.
- the living radical polymerization method is preferable because this method can lead to narrow molecular weight distributions and low viscosities, and the atom transfer radical polymerization method is further preferable.
- examples thereof include UH-2000 produced by Toagosei Co., Ltd., and the like.
- polyisocyanate compound examples include aromatic polyisocyanates such as toluene (tolylene) diisocyanate, diphenylmethane diisocyanate and xylylene diisocyanate; and aliphatic polyisocyanates such as isophorone diisocyanate and hexamethylene diisocyanate.
- aromatic polyisocyanates such as toluene (tolylene) diisocyanate, diphenylmethane diisocyanate and xylylene diisocyanate
- aliphatic polyisocyanates such as isophorone diisocyanate and hexamethylene diisocyanate.
- examples thereof include silanes having amino group such as ⁇ -aminopropyltrimethoxysilane, N-( ⁇ -aminoethyl)- ⁇ -aminopropyltrimethoxysilane, ⁇ -(N-phenyl)aminopropyltrimethoxysilane, N-ethylaminoisobutyltrimethoxysilane, N-cyclohexylaminomethyltriethoxysilane, N-cyclohexylaminomethyldiethoxymethylsilane and N-phenylaminomethyltrimethoxysilane; silanes having hydroxy group such as ⁇ -hydroxypropyltrimethoxysilane; silanes having mercapto group such as ⁇ -mercaptopropyltrimethoxysilane; and the like.
- the reactive silicon group-containing isocyanate compound of the general formula (15) examples thereof include ⁇ -trimethoxysilylpropylisocyanate, ⁇ -triethoxysilylpropylisocyanate, ⁇ -methyldimethoxysilylpropylisocyanate, ⁇ -methyldiethoxysilylpropylisocyanate, trimethoxysilylmethylisocyanate, diethoxymethylsilylmethylisocyanate, dimethoxymethylsilylmethylisocyanate, diethoxymethylsilylmethylisocyanate and the like.
- a compound obtained by reacting the silicon compound of the general formula (14) with an excessive amount of the above described polyisocyanate compound can be used as the reactive silicon group-containing isocyanate compound of the general formula (15).
- the viscosity of the organic polymer becomes high and forms a composition poor in workability as the case may be.
- curability of the composition of the present invention tends to be enhanced by the amide segments contained in the main chain skeleton of the organic polymer.
- the organic polymer having amide segments in its main skeleton is used as the component (A)
- the composition prepared in a combination of the polymer with the component (B) is preferable because the composition has a quicker curability by use of a non-organotin catalyst.
- the average number of amide segments per molecule is preferably from 1 to 10, more preferably from 1.5 to 7, particularly preferably from 2 to 5.
- the number of amide segments is less than 1, in some cases, curability becomes insufficient, and when the number of amide segments is more than 10, the viscosity of the organic polymer becomes high and forms a composition poor in workability.
- the chelate type methyl titanate (b1) represented by the general formula (1): wherein each of (4-n) R 1 s is independently hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, each of (4-n) A 1 s and (4-n) A 2 s is independently —R 2 or —OR 2 where R 2 is a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, n is 1, 2 or 3, and/or the chelate type ethyl titanate (b2) represented by the general formula (2): wherein R 1 , A 1 , A 2 and n are the same as defined above, are used as the component (B).
- components (B) function as a curing catalyst for the organic polymer of the component (A).
- Organotin compounds such as dibutyltin dilaurate and dibutyltin bis(acetylacetonate) which have a fear of having an influence on environment have been so far used as a curing catalyst for the reactive silicon group-containing organic polymer of the component (A), but by using the titanium catalysts of the component (B) of the present invention, a load on environment is small and a curable composition having practical curing properties can be obtained. Further as compared with the case of using other curing catalysts such as organotin catalysts, adhesion to articles to be hardly adhered such as acrylic resins can be enhanced.
- Tetraalkoxy titanium, halogenated titanium, titanium chelate and the like are known as a titanium catalyst, and particularly when titanium chelate is used, there is a tendency that quick curability can be obtained.
- a one-component type curable composition is prepared using a silane compound having a nitrogen-substituted group like the component (C) as an adhesion-imparting agent and a commonly used titanium diisopropoxidebis(ethylacetoacetate) as a titanium catalyst, there is a tendency that mechanical properties such as a modulus and a strength of a cured article obtained from the stored composition are significantly lowered as compared with mechanical properties before storing.
- the reactive silicon group of the component (A) is a dimethoxymethylsilyl group
- a part or the whole of methoxy groups on silicon are converted to isopropoxy groups, and the reactive silicon group becomes a group having a lower reactivity.
- a titanium chelate in which an alkoxy-substituted group is limited to a methoxy group or an ethoxy group is used as a titanium catalyst like the component (B) of the present invention, lowering of mechanical properties of a cured article after storing can be inhibited. Namely, even if during the storing, an alkoxy group on the reactive silicon group of the component (A) and a methoxy group or an ethoxy group in the component (B) are exchanged with each other by the same mechanism as above, the converted reactive silicon group of the component (A) is a relatively active methoxy group or ethoxy group, and therefore it can be considered that a crosslinking reaction proceeds sufficiently.
- Examples of the chelate type methyl titanate (b1) include titanium dimethoxidebis(acetylacetonate), titanium dimethoxidebis(3-methylacetylacetonate), titanium dimethoxidebis(methylacetoacetate), titanium dimethoxidebis(ethylacetoacetate), titanium dimethoxidebis(ethyl-2-methylacetoacetate), titanium dimethoxidebis(ethyl-2-ethylacetoacetate), titanium dimethoxidebis(t-butylacetoacetate), titanium dimethoxidebis(allylacetoacetate), titanium dimethoxidebis(methyl-3-oxo-4,4-dimethylhexanoate), titanium dimethoxidebis(ethyl-3-oxo-4,4,4-trifluorobutanoate), titanium dimethoxidebis(2,2,6,6-tetramethyl-3,5-heptanedionate), titanium trimethoxide(acetylacetonate
- titanium dimethoxidebis(ethylacetoacetate), titanium dimethoxidebis(ethyl-2-methylacetoacetate) and titanium dimethoxidebis(acetylacetonate) are preferable from the viewpoint of catalytic activity, and titanium dimethoxidebis(ethylacetoacetate) and titanium dimethoxidebis(ethyl-2-methylacetoacetate) are especially preferable.
- examples of the chelate type ethyl titanate (b2) include titanium diethoxidebis(acetylacetonate), titanium diethoxidebis(3-methylacetylacetonate), titanium diethoxidebis(methylacetoacetate), titanium diethoxidebis(ethylacetoacetate), titanium diethoxidebis(ethyl-2-methylacetoacetate), titanium diethoxidebis(ethyl-2-ethylacetoacetate), titanium diethoxidebis(t-butylacetoacetate), titanium diethoxidebis(allylacetoacetate), titanium diethoxidebis(methyl-3-oxo-4,4-dimethylhexanoate), titanium diethoxidebis(ethyl-3-oxo-4,4,4-trifluorobutanoate), titanium diethoxidebis(2,2,6,6-tetramethyl-3,5-heptanedionate), titanium triethoxide(acetylacetonate), titanium triethoxide(
- titanium diethoxidebis(ethylacetoacetate), titanium diethoxidebis(ethyl-2-methylacetoacetate) and titanium diethoxidebis(acetylacetonate) are preferable from the viewpoint of catalytic activity, and titanium dimethoxidebis(ethylacetoacetate) and titanium dimethoxidebis(ethyl-2-methylacetoacetate) are especially preferable.
- the chelate type methyl titanate (b1) as the component (B) from the point that the effect of the present invention, i.e. an effect of inhibiting lowering of physical properties after storing is higher. Additionally, it is preferable to use the chelate type methyl titanate (b1) from the point that higher curability can be obtained. On the other hand, use of the chelate type ethyl titanate (b2) is preferable because a by-product derived from the titanate is ethanol which has less effect on environment.
- the reactive silicon group of the component (A) is an ethoxysilyl group such as a triethoxysilyl group
- Preferred examples of the chelating agents capable of forming chelate ligands of the above described component (B) include ⁇ -diketones such as acetylacetone, 3-methylacetylacetone and 2,2,4,4-tetramethyl-3,5-heptanedion; ⁇ -ketoesters such as methyl acetoacetate, ethyl acetoacetate, t-butyl acetoacetate, allyl acetoacetate, (2-methacryloxyethyl)acetoacetate, methyl 3-oxo-4,4-dimethylhexanoate, ethyl 3-oxo-4,4,4-trifluorobutanoate, 3-methylacetylacetone, ethyl 2-methylacetoacetate and ethyl 2-ethylacetoacetate; ⁇ -diesters such as dimethyl malonate and diethyl malonate; cyclic dicarbonyl compounds such as 2-acetylbutyrol
- ⁇ -Diketones and ⁇ -ketoesters are more preferred from the viewpoint of curability and storage stability, and ⁇ -ketoesters are particularly preferred.
- acetylacetone, methyl acetoacetate, ethyl acetoacetate and ethyl 2-methylacetoacetate are more preferred from the viewpoint of curability and storage stability and because those compounds are easily available, and ethyl acetoacetate and ethyl 2-methylacetoacetate are particularly preferable.
- those chelate ligands may be the same or different.
- the component (B) may be used either each alone or in combinations of two or more thereof.
- the used amount of the component (B) is preferably from 0.1 to 20 parts by weight, and more preferably from 0.5 to 15 parts by weight, especially preferably from 1 to 10 parts by weight with respect to 100 parts by weight of the component (A).
- the blended amount of the component (B) is less than the above described ranges, sometimes the practical curing rate cannot be obtained, and the curing reaction hardly proceeds to a sufficient extent.
- the blended amount of the component (B) exceeds the above described ranges, sometimes the work life becomes too short, and the workability is degraded.
- a titanium catalyst other than the component (B) can be used to an extent not to degrade the effect of the present invention.
- titanium chelates such as titanium diisopropoxidebis(ethylacetoacetate), titanium diisopropoxidebis(methylacetoacetate), titanium diisopropoxidebis(t-butylacetoacetate), titanium diisopropoxidebis(methyl-3-oxo-4,4-dimethylhexanoate), titanium diisopropoxidebis(ethyl-3-oxo-4,4,4-trifluoropentanoate), titanium diisopropoxidebis(acetylacetonate), titanium diisopropoxidebis(2,2,6,6-tetramethyl-3,5-heptanedionate), titanium di-n-butoxidebis(ethylacetoacetate), titanium di-t-butoxidebis(ethylacetoacetate), titanium di-2-ethylhexoxidebis(ethylacetoa)
- the component (C) of the present invention is a kind of so-called silane coupling agent, and acts as an adhesion-imparting agent.
- a marked effect of improving adhesion is exhibited under a non-primer condition or under a condition of treating with a primer when the composition is used on various articles to be adhered, namely, inorganic substrates such as glass, aluminum, stainless steel, zinc, copper and mortar and organic substrates such as polyvinyl chloride, acryl, polyester, polyethylene, polypropylene and polycarbonate.
- inorganic substrates such as glass, aluminum, stainless steel, zinc, copper and mortar
- organic substrates such as polyvinyl chloride, acryl, polyester, polyethylene, polypropylene and polycarbonate.
- hydrolyzable silicon groups of the component (C) are the groups represented by the general formula (8) in which X is a hydrolyzable group. Specifically there are groups exemplified supra as a hydrolyzable group, and a methoxy group and an ethoxy group are preferable from the viewpoint of a hydrolyzing rate.
- the number of hydrolyzable groups is preferably not less than 2, particularly not less than 3.
- the reactive silicon group contained in the component (A) is described as “a silicon-containing group being crosslinkable by forming siloxane bonds”, and the reactive silicon group contained in the component (C) is described as “a hydrolyzable silicon group”, and the both are substantially the same kind of reactive silicon groups.
- Representative examples of those reactive silicon groups are reactive silicon groups represented by the following general formulae (5) and (6), respectively. Those groups have an alkoxy group having 1 to 4 carbon atoms as a hydrolyzable group.
- R 6 is an alkyl group having the number of carbon atoms more than the number of carbon atoms of R 8 , from the viewpoint of curability. This is because when R 6 is an alkyl group having the number of carbon atoms less than that of R 8 , it is presumed that in some cases, inherent curability of the component (A) is lowered.
- the hydrolyzable silicon group of the component (C) is preferably a methoxysilyl group represented by the general formula (7): —SiR 7 3-b (OCH 3 ) b (7) wherein each of R 7 s is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R′) 3 SiO— where each of R's is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, b is 1, 2 or 3.
- the methoxysilyl group are a methoxydimethylsilyl group, a methoxydiphenylsilyl group, a methoxybistrimethylsiloxysilyl group, a dimethoxymethylsilyl group, an ethyldimethoxysilyl group, a dimethoxyphenylsilyl group, a dimethoxytrimethylsiloxysilyl group, a trimethoxysilyl group and the like.
- the methoxysilyl group is not limited to them. From the viewpoint of curability of the obtained curable composition, a dimethoxymethylsilyl group and a trimethoxysilyl group are preferable and a trimethoxysilyl group is particularly preferable.
- the component (C) has the nitrogen-substituted group of the above described general formula (3) or (4), a higher adhesion can be obtained. Since an effect of imparting adhesion is higher, the substituent represented by the general formula (3) is preferable, and an amino group is particularly preferable.
- component (C) include amino group-containing silanes such as ⁇ -aminopropyltrimethoxysilane, ⁇ -aminopropylmethyldimethoxysilane, ⁇ -aminopropyltriethoxysilane, ⁇ -aminopropylmethyldiethoxysilane, ⁇ -aminopropyltriisopropoxysilane, ⁇ -(methylamino)propyltrimethoxysilane, ⁇ -(2-aminoethyl)aminopropyltrimethoxysilane, ⁇ -(2-aminoethyl)aminopropylmethyldimethoxysilane, ⁇ -(2-aminoethyl)aminopropyltriethoxysilane, ⁇ -(2-aminoethyl)aminopropylmethyldiethoxysilane, ⁇ -(2-aminoethyl)aminopropyltriis
- component (C) amino-modified silylpolymer, silylated aminopolymer, unsaturated aminosilane complex, phenylamino-long chain alkylsilane and aminosilylated silicone.
- the reactive silicon group of the component (A) is a methoxysilyl group represented by the general formula (5), it is essential to use the component (C) having a methoxysilyl group among those described above.
- the above described component (C) may be used alone or in a mixture of two or more kinds thereof.
- the used amount of the component (C) is preferably of the order of 0.1 to 20 parts by weight, and more preferably of the order of 0.5 to 10 parts by weight, especially preferably of the order of 2 to 7 parts by weight with respect to 100 parts by weight of the component (A).
- the blended amount of the component (C) is less than the above described ranges, sometimes sufficient adhesion cannot be obtained.
- the blended amount of the component (C) exceeds the above described ranges, sometimes the practical curing rate cannot be obtained, and the curing reaction hardly proceeds to a sufficient extent.
- a silane coupling agent other than the component (C) can be used to an extent not to degrade the effect of the present invention.
- these silane coupling agents are di-substituted amino group-containing silanes such as ⁇ -(dimethylamino)propyltrimethoxysilane; mercapto group-containing silanes such as ⁇ -mercaptopropyltrimethoxysilane, ⁇ -mercaptopropyltriethoxysilane, ⁇ -mercaptopropylmethyldimethoxysilane, ⁇ -mercaptopropylmethyldiethoxysilane, mercaptomethyltrimethoxysilane and mercaptomethyltriethoxysilane; epoxy group-containing silanes such as ⁇ -glycidoxypropyltrimethoxysilane, ⁇ -glycidoxypropyltriethoxysilane, ⁇ -glycidoxypropylmethyldimethoxysilane,
- adhesion-imparting agent for example, epoxy resins, phenolic resins, sulfur, alkyl titanates, aromatic polyisocyanates and the like can be used as an adhesion-imparting agent.
- the adhesion-imparting agent is not limited particularly to them.
- the above described adhesion-imparting agents may be used alone or in a mixture of two or more thereof.
- the component (B) is used, and other curing catalysts can be simultaneously used to an extent not to degrade the effect of the present invention.
- the curing catalyst include metal salts of carboxylic acids such as tin 2-ethylhexanoate, tin versatate and bismuth 2-ethylhexanoate; tetravalent organotin compounds such as dibutyltin dilaurate, dibutyltin maleate, dibutyltin phthalate, dibutyltin dioctanoate, dibutyltin bis(2-ethylhexanoate), dibutyltin bis(methylmaleate), dibutyltin bis(ethylmaleate), dibutyltin bis(butylmaleate), dibutyltin bis(octylmaleate), dibutyltin bis(tridecylmaleate), dibutyltin bis(benzyl
- a filler can be added to the composition of the present invention.
- the fillers include reinforcing fillers such as fumed silica, precipitated silica, crystalline silica, fused silica, dolomite, anhydrous silicic acid, hydrous silicic acid and carbon black; fillers such as ground calcium carbonate, precipitated calcium carbonate, magnesium carbonate, diatomite, sintered clay, clay, talc, titanium oxide, bentonite, organic bentonite, ferric oxide, aluminum fine powder, flint powder, zinc oxide, active zinc white, shirasu balloon, glass microballoon, organic microballoons of phenolic resin and vinylidene chloride resin, and resin powders such as PVC powder and PMMA powder; and fibrous fillers such as asbestos, glass fiber and glass filament.
- the used amount thereof is 1 to 250 parts by weight, and preferably 10 to 200 parts by weight with respect to 100 parts by weight of the polymer of the component (A).
- a highly transparent composition when preparing a highly transparent composition, as described in Japanese Patent Laid-Open No. 11-302527, it is possible to use, as a filler, a polymer powder prepared by using a polymer such as methyl methacrylate as a starting material or a non-crystalline silica. Also as described in Japanese Patent Laid-Open No. 2000-38560, a highly transparent composition can be obtained by using, as a filler, a hydrophobic silica which is a fine powder of silicon dioxide having hydrophobic groups bonded to the surface thereof.
- the surface of a fine powder of silicon dioxide has generally silanol groups (—SiOH), but can be formed into a hydrophobic silica by reacting those silanol groups with halides of organosilicon or alcohols to produce —SiO— hydrophobic group.
- the hydrophobic silica is obtained by reacting silanol groups being present in the surface of the fine powder of silicon dioxide with dimethylsiloxane, hexamethyldisilazane, dimethyldichlorosilane, trimethoxyoctylsilane, trimethylsilane or the like.
- a fine powder of silicon dioxide in which the surface thereof is formed by silanol groups (—SiOH) is called a hydrophilic fine powder of silica.
- a filler mainly selected from fumed silica, precipitated silica, crystalline silica, fused silica, dolomite, anhydrous silicic acid, hydrous silicic acid, carbon black, surface treated fine calcium carbonate, sintered clay, clay and active zinc white; a desirable effect is obtained when such a filler is used within a range from 1 to 200 parts by weight with respect to 100 parts by weight of the reactive silicone group-containing organic polymer (A).
- a desirable effect is obtained by use of a filler mainly selected from titanium oxide, calcium carbonate such as ground calcium carbonate and magnesium carbonate, talc, ferric oxide, zinc oxide and shirasu balloon within a range from 5 to 200 parts by weight with respect to 100 parts by weight of the reactive silicone group-containing organic polymer (A).
- the calcium carbonate exhibits, with increasing specific surface area value thereof, an increasing improvement effect of the tensile strength at break, elongation at break and adhesion of the cured article.
- these fillers may be used either each alone or in admixtures of two or more thereof.
- surface treated fine calcium carbonate in combination with calcium carbonate larger in particle size such as ground calcium carbonate.
- the particle size of surface treated fine calcium carbonate is preferably not more than 0.5 ⁇ m, and the surface treatment is preferably carried out by treating with a fatty acid or a fatty acid salt.
- the calcium carbonate larger in particle size is preferably not less than 1 ⁇ m in particle size, and can be used without being subjected to surface treatment.
- organic balloons and inorganic balloons are preferably added.
- Such fillers can be subjected to surface treatment, and may be used each alone or can be used in admixtures of two or more thereof.
- the particle sizes of these balloons are preferably not more than 0.1 mm.
- the particle sizes are preferably 5 to 300 ⁇ m.
- the composition of present invention is suitably used for joints of housing exterior wall such as sizing boards, in particular, ceramic sizing boards, for an adhesive for exterior wall tiles, for an adhesive for exterior wall tiles remaining in the joints and for the like purposes; in this connection, it is desirable that the design of the exterior wall and the design of the sealant are in harmony with each other.
- posh exterior walls have come to be used by virtue of sputter coating and mixing colored aggregates.
- the composition of the present invention When the composition of the present invention is blended with a scale-like or granulated material having a diameter of not less than 0.1 mm, preferably of the order of 0.1 to 5.0 mm, the cured article comes to be in harmony with such posh exterior walls, and is excellent in chemical resistance, so that the composition concerned comes to be an excellent composition in the sense that the exterior appearance of the cured article remains unchanged over a long period of time.
- Use of a granulated material provides a dispersed sand-like or sandstone-like surface with a rough texture, while use of a scale-like material provides an irregular surface based on the scale-like shape of the material.
- the diameter is not less than 0.1 mm, preferably of the order of 0.1 to 5.0 mm, and there is used a material having an appropriate size in conformity with the material quality and pattern of exterior wall. Materials having a diameter of the order of 0.2 mm to 5.0 mm and materials having a diameter of the order of 0.5 mm to 5.0 mm can also be used.
- the thickness is set to be as thin as the order of 1/10 to 1 ⁇ 5 the diameter (the order of 0.01 to 1.00 mm).
- the scale-like or granulated material is transported to the construction site as a sealant on condition that the material is beforehand mixed in the main component of the sealant, or is mixed in the main component of the sealant at the construction site when the sealant is used.
- the scale-like or granulated material is blended in a content of the order of 1 to 200 parts by weight with respect to 100 parts by weight of a composition such as a sealant composition and an adhesive composition.
- the blended amount is appropriately selected depending on the size of the scale-like or granulated material, and the material quality and pattern of exterior wall.
- the scale-like or granulated material natural products such as silica sand and mica, synthetic rubbers, synthetic resins and inorganic substances such as alumina are used.
- the material is colored in an appropriate color so as to match the material quality and pattern of exterior wall to heighten the design quality when filled in the joints.
- a balloon preferably the mean particle size thereof is not less than 0.1 mm
- the surface is formed to have a dispersed sand-like or sandstone-like surface with a rough texture, and a reduction of weight can be achieved.
- the preferable diameter, blended amount and materials for the balloon are described in Japanese Patent Laid-Open No. 10-251618 as follows.
- the balloon is a sphere-shaped material with a hollow interior.
- the material for such a balloon include inorganic materials such as glass, shirasu and silica; and organic materials such as phenolic resin, urea resin, polystyrene and SaranTM; however, the material concerned is not limited to these examples; an inorganic material and an organic material can be compounded, or can be laminated to form multiple layers.
- An inorganic balloon, an organic balloon, a balloon made of a compounded inorganic-organic material or the like can be used. Additionally, as a balloon to be used, either a type of balloon or an admixture of multiple types of balloons can be used.
- a balloon with the processed surface thereof or with the coated surface thereof can be used, and additionally, a balloon with the surface thereof subjected to treatment with various surface treatment agents can also be used. More specifically, examples are an organic balloon coated with calcium carbonate, talc, titanium oxide and the like, and an inorganic balloon subjected to surface treatment with a silane coupling agent.
- the particle size of the balloon is preferably not less than 0.1 mm.
- a balloon of a particle size of the order of 0.2 mm to 5.0 mm or a balloon of a particle size of the order of 0.5 mm to 5.0 mm can also be used.
- Use of a balloon of a particle size of less than 0.1 mm sometimes only increases the viscosity of the composition, and yields no rough texture, even when the used amount of the balloon is large.
- the blended amount of the balloon can be easily determined in conformity with the desired degree of the dispersed sand-like or sandstone-like rough texture.
- a balloon of not less than 0.1 mm in particle size is blended in a ratio of 5 to 25 vol % in terms of the volume concentration in the composition.
- the volume concentration of the balloon is less than 5 vol %, no rough texture can be obtained, while when the volume concentration of the balloon exceeds 25 vol %, the viscosity of the sealant and that of the adhesive tend to become high to degrade the workability, and the modulus of the cured article becomes high, so that the basic performance of the sealant and that of the adhesive tend to be impaired.
- the preferable volume concentration to balance with the basic performance of the sealant is 8 to 22 vol %.
- thermally expandable fine hollow particles disclosed in Japanese Patent Laid-Open No. 2004-51701 or 2004-66749 can be used.
- the thermally expandable fine hollow particles are plastic spherical particulates produced by surrounding a low boiling point compound such as a hydrocarbon having 1 to 5 carbon atoms with a high molecular weight shell material (vinylidene chloride copolymer, acrylonitrile copolymer or vinylidene chloride-acrylonitrile copolymer).
- a low boiling point compound such as a hydrocarbon having 1 to 5 carbon atoms
- a high molecular weight shell material vinylidene chloride-acrylonitrile copolymer
- the cured article can make irregularities on the surface to improve the design quality.
- the preferable diameter, blended amount and materials of the cured article particle material derived from a sealant is described in Japanese Patent Laid-Open No. 2001-115142 as follows.
- the diameter is preferably of the order of 0.1 mm to 1 mm, and further preferably of the order of 0.2 to 0.5 mm.
- the blended amount is preferably 5 to 100 wt %, and further preferably 20 to 50 wt % in the curable composition.
- the materials include urethane resin, silicone, modified silicone and polysulfide rubber. No constraint is imposed on the materials as long as the materials can be used as sealants; however, modified silicone sealants are preferable.
- an adhesion-imparting agent To the composition of the present invention can be added an adhesion-imparting agent. No particular constraint is imposed on the adhesion-imparting resins, and usual adhesion-imparting resins can be used irrespective of solid form or liquid form at ordinary temperature.
- adhesion-imparting resins include styrene block copolymers, hydrogenated products thereof, phenolic resins, modified phenolic resins (for example, cashew oil-modified phenolic resins, tall oil-modified phenolic resins and the like), terpene phenolic resins, xylene-phenol resins, cyclopentadiene-phenol resins, cumarone-indene resins, rosin resins, rosin ester resins, hydrogenated rosin ester resins, xylene resins, low molecular weight polystyrene resins, styrene copolymer resins, petroleum resins (for example, C5 hydrocarbon resin, C9 hydrocarbon resin, C5C9 hydrocarbon copolymer resin and the like), hydrogenated petroleum resins, terpene resins, DCPD resin-petroleum resin and the like.
- phenolic resins modified phenolic resins (for example, cashew oil-modified
- styrene block copolymers and hydrogenated products thereof include styrene-butadiene-styrene block copolymer (SBS), styrene-isoprene-styrene block copolymer (SIS), styrene-ethylenebutylene-styrene block copolymer (SEBS), styrene-ethylenepropylene-styrene block copolymer (SEPS), styrene-isobutylene-styrene block copolymer (SIBS) and the like.
- SBS styrene-butadiene-styrene block copolymer
- SIS styrene-isoprene-styrene block copolymer
- SEBS styrene-ethylenebutylene-styrene block copolymer
- SEPS styrene-ethylenepropylene-styrene block
- the adhesion-imparting resins are used within a range from 5 to 1,000 parts by weight, preferably from 10 to 100 parts by weight with respect to 100 parts by weight of the organic polymer (A).
- a solvent or a diluent To the composition of the present invention can be added a solvent or a diluent. No particular constraint is imposed on the solvent or the diluent, and aliphatic hydrocarbon, aromatic hydrocarbon, alicyclic hydrocarbon, halogenated hydrocarbon, alcohol, ester, ketone, ether and the like can be used.
- the boiling point of the solvent is preferably not less than 150° C., more preferably not less than 200° C., particularly preferably not less than 250° C. from the viewpoint of a problem with contamination to the air when the composition is used indoor.
- solvents or diluents may be used either each alone or in combinations of two or more thereof.
- a plasticizer can be added to the composition of the present invention. Addition of a plasticizer makes it possible to adjust the viscosity and slump property of the curable composition and the mechanical properties such as tensile strength and elongation of the cured article obtained by curing the composition.
- plasticizer examples include phthalates such as dibutyl phthalate, diheptyl phthalate, bis(2-ethylhexyl)phthalate, diisodecy phthalate, diundecyl phthalate and butyl benzyl phthalate; non-aromatic dibasic acid esters such as dioctyl adipate, dioctyl sebacate, dibutyl sebacate and diisodecyl succinate; aliphatic esters such as butyl oleate and methyl acetyl recinolate; phosphates such as tricresyl phosphate and tributyl phosphate; trimellitates; chlorinated paraffins; hydrocarbon oils such as alkyldiphenyls and partially hydrogenated terphenyls; process oils; epoxy plasticizers such as epoxidized soybean oil and benzyl epoxystearate.
- phthalates such as dibutyl phthalate,
- a polymer plasticizer can be used.
- a polymer plasticizer When a polymer plasticizer is used, there can be obtained effects of hardly causing curing retardation and improving drying property (also referred to as coating property) in the case of coating of an alkyd coating material on the cured article as compared with the case of using a low molecular weight plasticizer containing no polymer component in the molecule.
- polymer plasticizer examples include vinyl polymers obtained by polymerizing vinyl monomers by means of various methods; polyalkylene glycol esters such as diethylene glycol dibenzoate, triethylene glycol dibenzoate and pentaerythritol ester; polyester plasticizers obtained from dibasic acids such as sebacic acid, adipic acid, azelaic acid and phthalic acid and dihydric alcohols such as ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol and dipropylene glycol; polyethers including polyether polyols each having a molecular weight of not less than 500, additionally not less than 1,000 such as polyethylene glycol, polyprolpylene glycol and polytetramethylene glycol, and the derivatives of these polyether polyols in which the hydroxy groups in these polyether polyols are substituted with acyl groups such as acetoxy groups, alkoxy groups such as methoxy groups, ethoxy groups, butoxy groups and allyl
- polymer plasticizers which are compatible with the polymer of component (A) are preferable.
- polyethers and vinyl polymers are preferable.
- polyethers when polyethers are used as plasticizers, the surface curability, deep-part curability and adhesion are improved, and no curing retardation after storage occurs, and hence polyethers are preferable; of polyethers, polypropylene glycol is more preferable.
- polymer plasticizers polyether polyols such as polyethylene glycol and polypropylene glycol in which hydroxyl groups thereof are converted to alkoxy groups or the like because storage stability is increased. Additionally, from the viewpoint of the compatibility, weather resistance and heat resistance, vinyl polymers are preferable.
- acryl polymers and/or methacryl polymers are preferable, and acryl polymers such as polyalkylacrylate are further preferable.
- the living radical polymerization method is preferable because this method can lead to narrow molecular weight distributions and low viscosities, and the atom transfer radical polymerization method is further preferable.
- the number average molecular weight of the polymer plasticizer is preferably 500 to 15,000, more preferably 800 to 10,000, further preferably 1,000 to 8,000, particularly preferably 1,000 to 5,000, and most preferably 1,000 to 3,000.
- the molecular weight is too low, the plasticizer is removed with time due to heat and by rainfall, and hence it is made impossible to maintain the initial physical properties over a long period of time, contamination is caused due to cohesion of dusts, and the coating property with an alkyd coating material cannot be improved.
- the molecular weight is too high, the viscosity becomes high and the workability is degraded. No particular constraint is imposed on the molecular weight distribution of the polymer plasticizer.
- the molecular weight distribution is narrow; the molecular weight distribution is preferably less than 1.80, more preferably not more than 1.70, further preferably not more than 1.60, yet further preferably not more than 1.50, particularly preferably not more than 1.40 and most preferably not more than 1.30.
- the number average molecular weight of a polyether polymer is measured with the terminal group analysis method, and that of other polymers is measured with the GPC method. Additionally, the molecular weight distribution (Mw/Mn) is measured with the GPC method (relative to polystyrene standard).
- the polymer plasticizer either may have no reactive silicon group or may have a reactive silicon group.
- the polymer plasticizer acts as a reactive plasticizer, and can prevent the migration of the plasticizer from the cured article.
- the average number of reactive silicon groups per molecule is not more than 1, and preferably not more than 0.8.
- the reactive silicon group-containing plasticizer in particular, a reactive silicon group-containing oxyalkylene polymer is used, it is necessary that the number average molecular weight thereof is lower than that of the organic polymer (A).
- the plasticizers may be used either each alone or in combinations of two or more thereof. Additionally, a low molecular weight plasticizer and a polymer plasticizer may be used in combination. It is to be noted that these plasticizers can also be blended when the polymer is produced.
- the used amount of the plasticizer is 5 to 150 parts by weight, preferably 10 to 120 parts by weight, and further preferably 20 to 100 parts by weight, with respect to 100 parts by weight of the polymer of the component (A).
- the used amount is less than 5 parts by weight, the effect as the plasticizer is not exhibited, while when the used amount exceeds 150 parts by weight, the mechanical strength of the cured article is insufficient.
- a physical properties-regulating agent for adjusting tensile properties of a produced cured article may be added.
- the physical properties-regulating agent include tetraalkoxysilanes (tetraalkylsilicates) such as tetramethoxysilane, tetraethoxysilane, ethoxytrimethoxysilane, dimethoxydiethoxysilane, methoxytriethoxysilane, tetra-n-propoxysilane, tetra-1-propoxysilane, tetra-n-butoxysilane, tetra-1-butoxysilane and tetra-t-butoxysilane; trialkoxysilanes such as methyltrimethoxysilane, methyltriethoxysilane, methyltriisopropoxysilane, methyltriphenoxysilane, ethy
- the above described physical properties-regulating agent when the composition of the present invention is cured, a hardness can be increased or reversely can be decreased and an elongation at break can be increased.
- the above described physical properties-regulating agent may be used alone or in a mixture of two or more kinds thereof. Additionally, by adding partially hydrolyzed condensates of tetraalkoxysilanes and alkoxysilanes, there can be obtained effects of enhancing water resistant adhesion of the curable composition and enhancing recovery properties of the obtained cured article.
- Partially hydrolyzed condensates of organosilicate compounds are commercially available.
- Examples of such condensates include Methyl silicate 51 and Ethyl silicate 40 (both are products of Colcoat Co., Ltd.).
- the used amount of the physical properties-regulating agent is preferably 0.1 to 20 parts by weight, and more preferably 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- a compound to hydrolytically produce a compound having a monovalent silanol group in the molecule thereof This compound has an effect of decreasing the modulus of the cured article without degrading the stickiness of the surface of the cured article.
- a compound to produce trimethylsilanol is preferable.
- Examples of the compound to hydrolytically produce a compound having a monovalent silanol group in the molecule thereof include a compound described in Japanese Patent Laid-Open No. 5-117521.
- examples of such a compound include a compound which is a derivative of an alkyl alcohol such as hexanol, octanol or decanol, and produces a silicon compound to hydrolytically produce R 3 SiOH such as trimethylsilanol, and a compound described in Japanese Patent Laid-Open No. 11-241029 which is a derivative of a polyhydric alcohol having three or more hydroxy groups such as trimethylolpropane, glycerin, pentaerythritol or sorbitol, and produces a silicon compound to hydrolytically produce R 3 SiOH such as trimethylsilanol.
- the compound to hydrolytically produce a compound having a monovalent silanol group in the molecule thereof is used within a range from 0.1 to 20 parts by weight, and preferably from 0.5 to 10 parts by weight, with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- a thixotropy providing agent may be added for the purpose of preventing sagging and improving workability.
- the antisagging agent include polyamide waxes; hydrogenated castor oil derivatives; and metal soaps such as calcium stearate, aluminum stearate and barium stearate.
- a rubber powder having a particle size of 10 to 500 ⁇ m as described in Japanese Patent Laid-Open No. 11-349916, and an organic fiber as described in Japanese Patent Laid-Open No. 2003-155389 are used, a composition having high thixotropy and satisfactory workability can be obtained.
- thixotropy providing agents may be used either each alone or in combinations of two or more thereof.
- the thixotropy providing agents each are used within a range from 0.1 to 20 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- a compound containing an epoxy group in one molecule can be used.
- Use of an epoxy group-containing compound can increase the recovery properties of the cured article.
- the epoxy group-containing compound include compounds such as epoxidized unsaturated oils and fats, epoxidized unsaturated fatty acid esters, alicyclic epoxy compounds and epichlorohydrin derivatives, and admixtures of these compounds. More specific examples include epoxidized soybean oil, epoxidized flaxseed oil, bis(2-ethylhexyl)-4,5-epoxycyclohexane-1,2-dicarboxylate (E-PS), epoxyoctyl stearate and epoxybutyl stearate. Of these, E-PS is particularly preferable. It is recommended that these epoxy group-containing compounds each are used within a range from 0.5 to 50 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- a photocuring substance can be used for the composition of the present invention.
- Use of a photocuring substance forms a coating film of the photocuring substance on the surface of the cured article to improve the stickiness and the weather resistance of the cured article.
- a photocuring substance means a substance which undergoes a chemical change, caused by action of light, of the molecular structure thereof in a fairly short time to result in changes of the physical properties such as curability.
- organic monomers, oligomers, resins and compositions containing these substances are organic monomers, oligomers, resins and compositions containing these substances, and any commercially available substances concerned can optionally be adopted.
- As representative photocuring substances unsaturated acryl compounds, polyvinyl cinnamates, azidized resins and the like can be used.
- the unsaturated acryl compounds are monomers, oligomers and admixtures of the monomers and the oligomers, the monomers and oligomers each having one or a few acryl or methacryl unsaturated groups; examples of the unsaturated acryl compounds include monomers such as propylene (or butylene, or ethylene)glycol di(meth)acrylate and neopentylglycol di(meth)acrylate, and oligoesters of not more than 10,000 in molecular weight related to these monomers.
- acrylates such as ARONIX M-210, ARONIX M-215, ARONIX M-220, ARONIX M-233, ARONIX M-240 and ARONIX M-245; special acrylates (trifunctional) such as ARONIX M-305, ARONIX M-309, ARONIX M-310, ARONIX M-315, ARONIX M-320 and ARONIX M-325; and special acrylates (multifunctional) such as ARONIX M-400.
- polyvinyl cinnamates examples include photosensitive resins having cinnamoyl groups as photosensitive groups, namely, those compounds obtained by esterification of polyvinyl alcohol with cinnamic acid; and additionally, a large number of derivatives of polyvinyl cinnamates.
- Azidized resins are known as photosensitive resins having azide groups as photosensitive groups; common examples of the azidized resins include a rubber photosensitive solution added with an azide compound as a photosensitive agent, and additionally, the compounds detailed in “photosensitive resins” (published by Insatu Gakkai Shuppanbu, Mar. 17, 1972, p. 93, p. 106 and p.
- the photocuring substance is used within a range from 0.1 to 20 parts by weight and preferably from 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A); when the content of the photocuring substance is less than 0.1 part by weight, no effect to increase the weather resistance is displayed, while when the content exceeds 20 parts by weight, the cured article tends to be too hard and cracked.
- an oxygen-curable substance can be used.
- the oxygen-curable substance include unsaturated compounds reactable with the oxygen in the air, which react with the oxygen in the air and form a cured coating film around the surface of the cured article to act to prevent the surface stickiness and the sticking of dust and grime to the surface of the cured article and to do the like.
- oxygen-curable substance examples include drying oils represented by wood oil, flaxseed oil and the like and various alkyd resins obtained by modifying these compounds; acryl polymers, epoxy resins and silicon resins all modified with drying oils; liquid polymers such as 1,2-polybutadiene and 1,4-polybutadiene obtained by polymerizing or copolymerizing diene compounds such as butadiene, chloroprene, isoprene and 1,3-pentadiene, and polymers derived from C5 to C8 dienes, liquid copolymers such as NBR, SBR and the like obtained by copolymerizing these diene compounds with monomers such as acrylonitrile, styrene and the like having copolymerizability so as for the diene compounds to dominate, and various modified substances of these compounds (maleinized modified substances, boiled oil-modified substances, and the like).
- liquid polymers such as 1,2-polybutadiene and 1,4-polybutadiene obtained by polymer
- These substances can be used either each alone or in combinations of two or more thereof. Of these substances, wood oil and liquid diene polymers are particularly preferable. Additionally, in some cases, when catalysts to accelerate the oxidation curing reaction and metal dryers are used in combination with these substances, the effect is enhanced. Examples of these catalysts and metal dryers include metal salts such as cobalt naphthenate, lead naphthenate, zirconium naphthenate, cobalt octylate and zirconium octylate; and amine compounds.
- the oxygen-curing substance is used within a range from 0.1 to 20 parts by weight and further preferably from 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A); when the used amount is less than 0.1 part by weight, improvement of stain-proof property becomes insufficient, while when the used amount exceeds 20 parts by weight, the tensile property and the like of the cured article tends to be impaired. It is recommended that the oxygen-curing substance is used in combination with a photocuring substance as described in Japanese Patent Laid-Open No. 3-160053.
- an antioxidant antioxidant agent
- Use of an antioxidant can increase the heat resistance of the cured article.
- examples of the antioxidant can include hindered phenol antioxidants, monophenol antioxidants, bisphenol antioxidants and polyphenol antioxidants, and hindered phenol antioxidants are particularly preferable.
- hindered amine photostabilizers can also be used: TINUVIN 622LD, TINUVIN 144; CHIMASSORB944LD and CHIMASSORB119FL (all produced by Ciba-Geigy Japan Ltd.); MARK LA-57, MARK LA-62, MARK LA-67, MARK LA-63 and MARK LA-68 (all produced by Adeka Corporation); and SANOL LS-770, SANOL LS-765, SANOL LS-292, SANOL LS-2626, SANOL LS-1114 and SANOL LS-744 (all produced by Sankyo Co., Ltd.).
- Specific examples of the antioxidants are described also in Japanese Patent Laid-Open Nos. 4-283259 and 9-194731. It is recommended that the antioxidant is used within a range from 0.1 to 10 parts by weight and further preferably from 0.2 to 5 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- a photostabilizer can be used.
- Use of a photostabilizer can prevent the photooxidation degradation of the cured article.
- the photostabilizer include benzotriazole compounds, hindered amine compounds, benzoate compounds and the like; hindered amine compounds are particularly preferable. It is recommended that the photostabilizer is used within a range from 0.1 to 10 parts by weight and further preferably from 0.2 to 5 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A). Specific examples of the photostabilizer are described in Japanese Patent Laid-Open No. 9-194731.
- the photocuring substance is used for the composition of the present invention, in particular, when an unsaturated acryl compound is used, it is preferable to use a tertiary amine-containing hindered amine photostabilizer as a hindered amine photostabilizer as described in Japanese Patent Laid-Open No. 5-70531 for the purpose of improving the storage stability of the composition.
- tertiary amine-containing hindered amine photostabilizer examples include TINUVIN 622LD, TINUVIN 144 and CHIMASSORB119FL (all produced by Ciba-Geigy Japan Ltd.); MARK LA-57, LA-62, LA-67 and LA-63 (all produced by Adeka Corporation); and SANOL LS-765, SANOL LS-292, SANOL LS-2626, SANOL LS-1114 and SANOL LS-744 (all produced by Sankyo Co., Ltd.).
- an ultraviolet absorber can be used.
- Use of an ultraviolet absorber can increase the surface weather resistance of the cured article.
- Examples of the ultraviolet absorber include benzophenone compounds, benzotriazole compounds, salicylate compounds, substituted tolyl compounds and metal chelate compounds; benzotriazole compounds are particularly preferable.
- the ultraviolet absorber is used within a range from 0.1 to 10 parts by weight, and further preferably from 0.2 to 5 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A). It is preferable that a phenol antioxidant, a hindered phenol antioxidant, a hindered amine photostabilizer and a benzotriazole ultraviolet absorber are used in combination.
- an epoxy resin can be added.
- the composition added with an epoxy resin is particularly preferable as an adhesive, in particular, an adhesive for exterior wall tile.
- the epoxy resin include epichlorohydrin-bisphenol A-type epoxy resins, epichlorohydrin-bisphenol F-type epoxy resins, flame resistant epoxy resins such as glycidyl ether of tetrabromobisphenol A, novolac-type epoxy resins, hydrogenated bisphenol A-type epoxy resins, epoxy resins of the type of glycidyl ether of bisphenol A propyleneoxide adduct, p-oxybenzoic acid glycidyl ether ester-type epoxy resins, m-aminophenol epoxy resins, diaminodiphenylmethane epoxy resins, urethane modified epoxy resins, various alicyclic epoxy resins, N,N-diglycidylaniline, N,N-diglycidyl-o-toluidine, trigly
- Epoxy resins having at least two epoxy groups in one molecule are preferable because such compositions are high in reactivity when curing is made, and the cured articles can easily form three dimensional networks.
- further preferable epoxy resins include bisphenol A-type epoxy resins or novolac-type epoxy resins.
- the preferable ratio of the used amounts varies depending on the application of the curable resin composition and hence cannot be unconditionally determined; for example, when the impact resistance, flexibility, toughness, peel strength and the like of the cured article of the epoxy resin are to be improved, it is recommended that with respect to 100 parts by weight of the epoxy resin, 1 to 100 parts by weight of the component (A), further preferably 5 to 100 parts by weight of the component (A) is used. On the other hand, when the strength of the cured article of the component (A) is to be improved, it is recommended that with respect to 100 parts of the component (A), 1 to 200 parts by weight of the epoxy resin, further preferably 5 to 100 parts by weight of the epoxy resin is used.
- a curing agent to cure the epoxy resin can be applied together to the composition of the present invention.
- No particular constraint is imposed on the usable epoxy resin-curing agent, and commonly used epoxy resin-curing agents can be used.
- epoxy resin-curing agent examples include primary and secondary amines such as triethylenetetramine, tetraethylenepentamine, diethylaminopropylamine, N-aminoethylpiperidine, m-xylylenediamine, m-phenylenediamine, diaminodiphenylmethane, diaminodiphenylsulfone, isophoronediamine, and polyether with amine terminal groups; tertiary amines such as 2,4,6-tris(dimethylaminomethyl)phenol and tripropylamine, and salts of those tertiary amines; polyamide resins; imidazoles; dicyandiamides; borontrifluoride complexes; carboxylic anhydrides such as phthalic anhydride, hexahydrophthalic anhydride, tetrahydrophthalic anhydride, dodecynylsuccinic anhydride, pyrom
- the used amount thereof falls within a range from 0.1 to 300 parts by weight with respect to 100 parts by weight of the epoxy resin.
- a ketimine As an epoxy resin-curing agent, a ketimine can be used.
- a ketimine is stable when no moisture is present, but moisture decomposes the ketimine into a primary amine and a ketone; the thus produced primary amine acts as a room temperature curable curing agent to cure the epoxy resin.
- Use of a ketimine makes it possible to obtain a one-component type composition.
- Such a ketimine can be obtained by condensation reaction of an amine compound and a carbonyl compound.
- an amine compound and a carbonyl compound well known in the art can be used.
- the following compounds can be used as such an amine compound: diamines such as ethylenediamine, propylenediamine, trimethylenediamine, tetramethylenediamine, 1,3-diaminobutane, 2,3-diaminobutane, pentamethylenediamine, 2,4-diaminopentane, hexamethylenediamine, p-phenylenediamine and p,p′-biphenylenediamine; polyvalent amines such as 1,2,3-triaminopropane, triaminobenzene, tris(2-amionoethyl)amine and tetrakis(aminomethyl)methane; polyalkylenepolyamines such as diethylenetriamine, triethylenetriamine and tetraethylenepentamine; polyoxyalkylene polyamines; and
- aldehydes such as acetaldehyde, propionaldehyde, n-butylaldehyde, isobutylaldehyde, diethylacetaldehyde, glyoxal and benzaldehyde
- cyclic ketones such as cyclopentanone, trimethylcyclopentanone, cyclohexanone and trimethylcyclohexanone
- aliphatic ketones such as acetone, methyl ethyl ketone, methyl propyl ketone, methyl isopropyl ketone, methyl isobutyl ketone, diethyl ketone, dipropyl ketone, diisopropyl ketone, dibutyl ketone and diisobutyl ketone
- ⁇ -dicarbonyl compounds such as acetylacetone, methyl acetoacetate, ethy
- the imino group can be reacted with styrene oxide; glycidyl ethers such as butyl glycidyl ether and allyl glycidyl ether; and glycidyl esters.
- styrene oxide glycidyl ethers
- glycidyl esters such as butyl glycidyl ether and allyl glycidyl ether
- glycidyl esters glycidyl esters.
- These ketimines may be used either each alone or in combinations of two or more thereof; these ketimines each are used within a range from 1 to 100 parts by weight with respect to 100 parts by weight of the epoxy resin, the used amount of each of the ketimines varies depending on the type of the epoxy resin and the type of the ketimine.
- a phosphorous-based plasticizer such as polyphosphoric acid ammonium or tricresyl phosphate and a flame retardant such as aluminum hydroxide, magnesium hydroxide or thermally expandable graphite.
- a flame retardant such as aluminum hydroxide, magnesium hydroxide or thermally expandable graphite.
- the flame retardant is used within a range from 5 to 200 parts by mass, and preferably from 10 to 100 parts by mass with respect to 100 parts by weight of the component (A).
- additives can be added according to need for the purpose of regulating the physical properties of the curable composition or the cured article.
- additives include curing regulators, radical inhibitors, metal deactivators, antiozonants, phosphorus-based peroxide decomposers, lubricants, pigments, foaming agents, ant-proofing agents and mildewproofing agents.
- curing regulators radical inhibitors, metal deactivators, antiozonants, phosphorus-based peroxide decomposers, lubricants, pigments, foaming agents, ant-proofing agents and mildewproofing agents.
- additives may be used either each alone or in combinations of two or more thereof.
- Specific examples of additives other than the specific examples cited in the present specification are described, for example, in Japanese Patent Examined Publication Nos. 4-69659 and 7-108928, Japanese Patent Laid-Open Nos. 63-254149, 64-22904, 2001-72854 and the like.
- the blended components containing moisture are used after dehydrating and drying, or the components are dehydrated by reducing pressure or the like while being kneaded for blending.
- a thermal drying method is suitable for a powdery solid substance or the like, while a reduced pressure dehydration method or a dehydration method which uses a synthetic zeolite, active alumina, silica gel, quick lime or magnesium oxide is suitable for a liquid substance.
- addition of the following compounds further improves the storage stability: lower alcohols such as methanol and ethanol; and alkoxysilane compounds such as n-propyltrimethoxysilane, vinyltrimethoxysilane, vinylmethyldimethoxysilane, methylsilicate, ethylsilicate, ⁇ -mercaptopropylmethyldimethoxysilane, ⁇ -mercaptopropylmethyldiethoxysilane and ⁇ -glycidoxypropyltrimethoxysilane.
- lower alcohols such as methanol and ethanol
- alkoxysilane compounds such as n-propyltrimethoxysilane, vinyltrimethoxysilane, vinylmethyldimethoxysilane, methylsilicate, ethylsilicate, ⁇ -mercaptopropylmethyldimethoxysilane, ⁇ -mercaptopropylmethyldiethoxysilane and ⁇ -glycidoxypropyltrime
- the used amount of a dehydrating agent in particular, a silicon compound capable of reacting with water such as vinyltrimethoxysilane falls within a range from 0.1 to 20 parts by weight, and preferably 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- the components (A), (B), (C) and other additives are blended together with a mixer, a roll or a kneader, and are subjected to complete dehydration by heating under reduced pressure or the like, so that water content is decreased to an extent substantially not causing a problem.
- the obtained one-component type curable composition is stored in a moisture-proof sealed vessel.
- the composition forms three dimensional networks to be quickly cured from its surface into a solid matter having a rubber-like elasticity.
- the curable composition of the present invention can be used as tackifiers, sealants for buildings, ships, vehicles and road, adhesives, mold forming materials, vibration-proof material, damping materials, soundproof materials, foaming materials, coating materials, spraying materials and the like. It is preferable that the cured article obtained by curing the curable composition of the present invention is used as a sealant and an adhesive because the cured article is excellent in flexibility and adhesion.
- the curable composition of the present invention can be used in various applications as liquid sealants to be used in materials for electric and electronic components such as backside sealants for solar cells, electric insulating materials such as insulating coating materials for use in electric wire and cable, elastic adhesives, contact type adhesives, spray type sealants, crack repairing materials, adhesives for tiling, powdery coating materials, casting materials, medical rubber materials, medical adhesives, medical instrument sealants, food packaging materials, sealants for joints in exterior materials such as sizing boards, coating materials, primers, electromagnetic wave shielding conductive materials, heat conducting materials, hot melt materials, electric and electronic potting agents, films, gaskets, various molding materials, antirust and waterproof sealants for edges (cut portions) of wire glass and laminated glass, vehicle components, electric appliance components, various machinery components and the like.
- electric insulating materials such as insulating coating materials for use in electric wire and cable
- elastic adhesives such as insulating coating materials for use in electric wire and cable
- elastic adhesives such as insulating coating materials for use in electric wire
- the curable composition of the present invention can adhere alone or in combination with a primer to a wide variety of substrates including glass, porcelain, woods, metals and molded resin articles, and accordingly, can be used as various types of sealing and adhesive compositions. Additionally, the curable composition of the present invention can be used as adhesives for interior panels, adhesive for exterior panels, adhesives for tiling, adhesives for stone tiling, adhesives for finishing ceiling, adhesives for finishing floor, adhesives for finishing wall, adhesives for vehicle panels, adhesives for assembling electric, electronic and precision instruments, sealants for direct glazing, sealants for double glazing, sealants for the SSG technique and sealants for working joints of buildings.
- polymerization of propylene oxide was carried out to yield a polypropylene oxide having a number average molecular weight of about 25,500 (a molecular weight relative to polystyrene standard measured by using a HLC-8120 GPC manufactured by Tosoh Corp. as a liquid delivery system, a column of TSK-GEL H-type manufactured by Tosoh Corp., and THF as a solvent).
- a methanol solution of NaOMe was added in an amount of 1.2 equivalents with respect to the hydroxy group of the above hydroxy group-terminated polypropylene oxide, the methanol was distilled off, and allyl chloride was further added to thereby convert the terminal hydroxy group into an allyl group.
- the unreacted allyl chloride was distilled off under reduced pressure.
- the reactive silicon group-containing polyoxyalkylene polymer (A-1) obtained in Synthesis Example 1 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, an adhesion-imparting agent and titanium diisopropoxidebis(ethylacetoacetate) as a curing catalyst were weighed out according to the formulations shown in Table 1, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and the composition was charged in an aluminum cartridge. It is to be noted that the added amount of each adhesion-imparting agent was so set that the number of silyl groups of the respective adhesion-imparting agents became equimolar amounts.
- the curable compositions each were extruded from the cartridge and filled in a molding frame of about 5 mm in thickness with a spatula; the surface of each of the filled compositions was fully flattened, and the planarization completion time was set as the curing starting time.
- the surface of each of the compositions was touched every five minutes with a finger wiped with ethanol, and the tack-free time was measured as the time when the composition no longer stuck to the finger.
- compositions each were extruded so that the compositions each were adhered to various articles to be adhered (anodized aluminum, cold rolled steel sheet, hard polyvinyl chloride, FRP, polycarbonate, acrylic resin sheet), and were aged at 23° C. for 7 days. Thereafter, a 90 degree hand peel test was carried out. The breakdown conditions of the cured articles were observed and the cohesion failure rates (CF rate) were measured.
- CF rate cohesion failure rates
- the curable compositions each were filled in a sheet-like molding frame of about 3 mm in thickness, and the surface of each of the filled compositions was fully flattened, followed by aging at 23° C. for 3 days+50° C. for 4 days, to produce sheet-like cured articles.
- the cured articles were punched to obtain No. 3 dumbbell type test pieces (JIS K6251), and a modulus at 50% tension, a strength at break and an elongation at break (measuring environment: 23° C., tensile speed: 200 mm/min) were measured with an AUTOGRAPH produced by Shimadzu Corporation.
- Table 1 shows curability, adhesion, mechanical properties (modulus, strength, elongation) before and after storing and maintenance factor of physical properties of each curable composition.
- A denotes a CF rate of 100%
- B denotes a CF rate of not less than 50% and less than 100%
- C denotes a CF rate of not less than 10% and less than 50%
- D denotes a CF rate of less than 10%.
- curable compositions prepared using titanium diisopropoxidebis(ethylacetoacetate) as a curing catalyst and aminosilanes of Comparative Examples 1 and 2 and ketimine silane of Comparative Example 6 of the component (C) of the present invention as an adhesion-imparting agent displayed satisfactory adhesion to various articles to be adhered, but after storing, mechanical properties of the cured articles were lowered.
- titanium dimethoxidebis(ethylacetoacetate) (B-1) was obtained at a purity of not less than 95%.
- the purity of the generated product was calculated from a ratio of integrated peak intensities of TC750 and the product (B-1) based on 1 HNMR spectrum.
- Titanium diethoxidebis(ethylacetoacetate) (B-2) was obtained at a purity of not less than 95% in the same manner as in Synthesis Example 2 except that ethanol (produced by Wako Pure Chemicals Co., Ltd.) subjected to dehydration treatment with a molecular sieve was used instead of methanol of Synthesis Example 2.
- the reactive silicon group-containing polyoxyalkylene polymer (A-1) obtained in Synthesis Example 1 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, the component (C) as an adhesion-imparting agent and a titanium catalyst as a curing catalyst were weighed out according to the formulations shown in Table 2, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and the composition was charged in an aluminum cartridge. It is to be noted that the added amount of each curing catalyst was so set as to became equimolar amounts.
- the curable compositions each were extruded from the cartridge and filled in a molding frame of about 5 mm in thickness with a spatula; the surface of each of the filled compositions was fully flattened, and the planarization completion time was set as the curing starting time.
- the surface of each of the compositions was touched with a spatula every five minutes, and the skin formation time was measured as the time when the composition no longer stuck to the spatula.
- the curable compositions each were filled in a sheet-like molding frame of about 3 mm in thickness, and the surface of each of the filled compositions was fully flattened, followed by aging at 23° C. for 3 days+50° C. for 4 days, to produce sheet-like cured articles.
- the cured articles were punched to obtain No. 2 (1 ⁇ 3) dumbbell type test pieces (JIS K7113), and a modulus at 50% tension, a strength at break and an elongation at break (measuring environment: 23° C., tensile speed: 200 mm/min) were measured with an AUTOGRAPH produced by Shimadzu Corporation.
- composition containing no component (C) like Comparative Example 10 is insufficient in adhesion to several kinds of articles to be adhered.
- Titanium dimethoxidebis(ethyl-2-methylacetoacetate) (B-3) of a purity of not less than 95% was obtained by the same procedures as in Synthesis Example 2 except that 27.1 g (60 mmol) of titanium diisopropoxidebis(ethyl-2-methylacetoacetate) (B′-1) obtained in Synthesis Example 4 was used instead of TC750 of Synthesis Example 2.
- Titanium diethoxidebis(ethyl-2-acetoacetate) (B-4) of a purity of not less than 95% was obtained in the same manner as in Synthesis Example 5 except that ethanol (produced by Wako Pure Chemical Industries, Ltd.) subjected to dehydration treatment with a molecular sieve was used instead of methanol of Synthesis Example 5.
- the reactive silicon group-containing polyoxyalkylene polymer (A-1) obtained in Synthesis Example 1 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, the component (C) as an adhesion-imparting agent and a titanium catalyst as a curing catalyst were weighed out according to the formulations shown in Table 3, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and the composition was charged in an aluminum cartridge. It is to be noted that the added amount of each curing catalyst was so set as to became equimolar amounts.
- the curable compositions each were extruded from the cartridge and filled in a molding frame of about 5 mm in thickness with a spatula; the surface of each of the filled compositions was fully flattened, and the planarization completion time was set as the curing starting time.
- the surface of each of the compositions was touched with a spatula every five minutes, and the skin formation time was measured as the time when the composition no longer stuck to the spatula.
- a triethoxysilyl group-terminated polyoxypropylene polymer (A-2) was obtained in the same manner as in Synthesis Example 1 except that 1.3 parts by weight of triethoxysilane was used instead of methyldimethoxysilane. According to measurement based on 1 H-NMR, the number of terminal triethoxysilyl groups per molecule was about 1.1 on average.
- the reactive silicon group-containing polyoxyalkylene polymer (A-2) obtained in Synthesis Example 7 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, the component (C) as an adhesion-imparting agent and a titanium catalyst as a curing catalyst were weighed out according to the formulations shown in Table 4, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and each composition was charged in an aluminum cartridge. It is to be noted that the added amount of each curing catalyst was so set as to be equimolar amounts.
- a skin formation time and adhesion to various articles to be adhered were evaluated in the same manner as above.
- Photostabilizer Sanol LS-765 Sankyo Co., Ltd. 1 1 Antioxidant Irganox 1010 (6) Ciba-Geigy Japan Ltd. 1 1 Dehydrating agent ESi28 (7) Colcoat Co., Ltd. 2 2 Adhesion-imparting KBE603 (8) Shin-Etsu Chemical Co., Ltd. 3.57 3.57 agent Chelate type ethyl Titanium diethoxidebis(ethylacetoacetate) (B-2) 7 titanate (b1) Titanium catalyst Orgatix TC-750 (9) Matsumoto Chemical Industry Co., 7.5 Ltd.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Materials Engineering (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Sealing Material Composition (AREA)
- Adhesives Or Adhesive Processes (AREA)
Abstract
Description
- The present invention relates to a curable composition comprising an organic polymer containing a silicon-containing group (hereinafter referred to as a “reactive silicon group”) which has hydroxyl group or a hydrolyzable group bonded to the silicon atom and which is capable of crosslinking by forming siloxane bonds.
- It is known that an organic polymer having at least one reactive silicon group in the molecule undergoes crosslinking by formation of siloxane bonds accompanying a hydrolysis reaction of the reactive silicon group with moisture or the like even at room temperature, and a rubber-like cured article can be obtained.
- Among the polymers having a reactive silicon group, organic polymers in which the main chain skeleton is a polyoxyalkylene polymer or a (meth)acrylate polymer are disclosed in (Patent Document 1), (Patent Document 2) and the like. Those polymers have already been industrially produced, and are widely used in applications to sealants, adhesives, coatings and the like.
- Curable compositions comprising the above described organic polymers having a reactive silicon group are cured by using silanol condensation catalysts, and usually organotin catalysts having a carbon-tin bond such as dibutyltin bis(acetylacetonate) are widely used. However, in recent years, toxicity of organotin catalysts is pointed out, and development of non-organotin catalysts are demanded.
- With respect to curable silicone compositions prepared using a titanium compound as a non-organotin curing catalyst, so far many investigations have been made, and are disclosed in (Patent Document 3) and the like. At an initial stage of the investigations, when a titanium compound was used as a curing catalyst, there were many problems with curability, storage stability, an extreme increase in viscosity when silicone came into contact with a titanium catalyst, and the like. As described in (Patent Document 4), (Patent Document 5), (Patent Document 6), (Patent Document 7) and the like, as a result of various investigations made, improvement methods such as modification of a silicone-terminated structure, use of a chelate type titanium compound, and the like have been found, and at the present time, dealcoholization type silicone compositions using titanium catalysts are widely used in a variety of applications. Additionally, in (Patent Document 8), a technique for improving curability by using a chelate type methyl titanium is disclosed.
- However there are relatively few examples of adding a titanium catalyst to a reactive silicon group-containing organic polymer. Those examples are disclosed in (Patent Document 9), (Patent Document 10), (Patent Document 11), (Patent Document 12), (Patent Document 13), (Patent Document 14), (Patent Document 15), (Patent Document 16), (Patent Document 17) and (Patent Document 18).
- On the other hand, various properties such as curability, adhesion and mechanical properties such as a modulus, a strength, an elongation and the like are demanded for curable compositions used for a sealant, an adhesive, a coating and the like and rubber-like cured articles obtained by curing the compositions. Also with respect to organic polymers having a reactive silicon group, so far many investigations have been made. As a result, it is already known that a strong adhesion to various articles to be adhered can be imparted by blending a silane coupling agent such as amino silane as proposed in (Patent Document 19).
- Patent Document 1: JP-52-73998A
- Patent Document 2: JP-59-74149A
- Patent Document 3: JP-39-27643B (U.S. Pat. No. 3,175,993)
- Patent Document 4: U.S. Pat. No. 3,334,067
- Patent Document 5: JP-56-14701B
- Patent Document 6: JP-55-43119A
- Patent Document 7: JP-2-133490A
- Patent Document 8: JP-2001-302934A
- Patent Document 9: JP-58-17154A
- Patent Document 10: JP-11-209538A
- Patent Document 11: JP-5-311063A
- Patent Document 12: JP-2001-302929A
- Patent Document 13: JP-2001-302930A
- Patent Document 14: JP-2001-302931A
- Patent Document 15: JP-2001-302934A
- Patent Document 16: JP-2001-348528A
- Patent Document 17: JP-2002-249672A
- Patent Document 18: JP-2003-165916A
- Patent Document 19: JP-62-35421B
- In order to obtain a one-component type curable composition having practical curability and sufficient adhesion, the present inventors have investigated a one-component type curable composition which comprises a reactive silicon group-containing organic polymer as a main component, is prepared using a titanium catalyst as a non-tin curing catalyst and contains an amino silane as an adhesion-imparting agent, and have found that mechanical properties of a cured article obtained from the composition having been stored for a given period of time are remarkably lowered as compared with a cured article obtained from the composition before storing.
- It is an object of the present invention to provide a one-component type curable composition which comprises a reactive silicon group-containing organic polymer as a main component, has practical curability and good adhesion by use of a non-tin curing catalyst, and minimizes lowering of mechanical properties of a cured article after storing.
- As a result of a diligent investigation to solve such problems, the present inventors perfected the present invention by discovering that a curable composition having practical curability and good adhesion and minimizing lowering of mechanical properties of a cured article after storing can be obtained by use of a silane compound having a nitrogen-substituted group as an adhesion-imparting agent and a chelate type methyl titanate (b1) and/or a chelate type ethyl titanate (b2) as a curing catalyst for the polymer although the catalyst concerned is a non-tin curing catalyst.
- Namely, the present invention relates to a one-component type curable composition characterized by comprising, as components,
- (A) a polyoxyalkylene polymer (a1) having a silicon-containing group being capable of crosslinking by forming siloxane bonds and/or a (meth)acrylate polymer (a2) having a silicon-containing group being capable of crosslinking by forming siloxane bonds,
(B) a chelate type methyl titanate (b 1) represented by the general formula (1):
wherein each of (4-n) R1s is independently hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, each of (4-n) A1s and (4-n) A2s is independently —R2 or —OR2 where R2 is a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, n is 1, 2 or 3, and/or a chelate type ethyl titanate (b2) represented by the general formula (2):
wherein R1, A1, A2 and n are the same as defined above, and (C) a compound having, in its molecule, a hydrolyzable silicon group and a nitrogen-substituted group represented by the general formula (3):
—NHR3 (3)
wherein R3 is hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, or the general formula (4):
—N═R4 (4)
wherein R4 is CO or a substituted or unsubstituted divalent hydrocarbon group having 1 to 20 carbon atoms and is bonded to a nitrogen atom by double bonding. - The polyoxyalkylene polymer (al) is preferably a polyoxypropylene polymer.
- As the component (B), the chelate type methyl titanate (b1) represented by the general formula (1) is preferable.
- Additionally, it is preferable that the silicon-containing group contained in the component (A) and the hydrolyzable silicon group contained in the component (C) are groups represented by the following general formula (5) and the general formula (6), respectively:
—SiR5 3-a(OR6)a (5) . . . silicon-containing group of the component (A)
—SiR7 3-b(OR8)b (6) . . . hydrolyzable silicon group of the component (C)
wherein each of R5 and R7 is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R)3SiO— where each of R's is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, R6 is an alkyl group having 1 to 4 carbon atoms, R8 is an alkyl group having 1 to 4 carbon atoms, a is 1, 2 or 3, b is 1, 2 or 3, and R6 is an alkyl group having the number of carbon atoms equal to or more than the number of carbon atoms of R8. - Additionally, the hydrolyzable silicon group contained in the component (C) is preferably a methoxysilyl group represented by the general formula (7):
—SiR7 3-b(OCH3)b (7)
wherein each of R7s is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R′)3SiO— where each of R's is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, b is 1, 2 or 3. - Additionally, preferred embodiments of the curable composition of the present invention are sealants or adhesives comprising any of the above described one-component type curable compositions.
- The curable composition of the present invention is excellent in curability and adhesion by use of a non-tin curing catalyst, and lowering of mechanical properties of a cured article obtained after storing is minimized.
- The present invention is then explained below in detail.
- In the present invention, the polyoxyalkylene polymer (a1) having a reactive silicon group and/or the (meth)acrylate polymer (a2) having a reactive silicon group (hereinafter referred to as “organic polymer”) are used as the component (A). By using the polyoxyalkylene polymer and/or the (meth)acrylate polymer as a main chain skeleton of the polymer of the component (A), a satisfactory adhesion can be achieved. Additionally, when a curable composition is prepared using a titanium catalyst as the component (B) of the present invention, there is a tendency that deep-part curability of the obtained composition is lowered depending on an added amount thereof. Accordingly, a polyoxyalkylene polymer and a (meth)acrylate polymer as used for the component (A) of the present invention are preferable because they are high in moisture permeability and excellent in deep-part curability in the case of a one-component type composition.
- No particular constraint is imposed on the glass transition temperature of the organic polymer of the component (A). The glass transition temperature is preferably not more than 20° C., more preferably not more than 0° C., particularly preferably not more than −20° C. If the glass transition temperature is higher than 20° C., in some cases, a viscosity increases and workability is lowered in wintertime or at a cold district or flexibility and elongation of the cured article are degraded. The above described glass transition temperature denotes values measured by DSC.
- The reactive silicon group contained in the polyoxyalkylene polymer (a1) having a reactive silicon group and the (meth)acrylate polymer (a2) having a reactive silicon group is a group which has a hydroxyl group or a hydrolyzable group bonded to a silicon atom, and is capable of cross-linking through a reaction accelerated by a curing catalyst. Examples of the reactive silicon groups include groups represented by the general formula (8):
—(SiR9 2-cXcO)m—SiR10 3-dXd (8)
wherein each of R9 and R10 is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R′)3SiO—, where R′ is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, each of Xs is independently hydroxyl group or a hydrolyzable group, c is 0, 1 or 2, d is 0, 1, 2 or 3, c and d are not 0 at the same time, m is 0 or an integer of 1 to 19. - No particular constraint is imposed on the hydrolyzable group, and the hydrolyzable group may be a hydrolyzable group well known in the art. More specifically, examples of the hydrolyzable group include, for instance, hydrogen atom, a halogen atom, an alkoxy group, an acyloxy group, a ketoximate group, an amino group, an amide group, an acid amide group, an aminooxy group, a mercapto group, an alkenyloxy group and the like. Among these groups, hydrogen atom, an alkoxy group, an acyloxy group, a ketoximate group, an amino group, an amide group, an aminooxy group, a mercapto group and an alkenyloxy group are preferable and an alkoxy group is particularly preferable from the viewpoint that an alkoxy group is moderately hydrolyzable and easily handled.
- One to three hydrolyzable groups and hydroxyl groups can bond to one silicon atom, and (a+Σc) is preferably within a range from 1 to 5. When two or more hydrolyzable groups and hydroxyl groups are bonded in the reactive silicon group, they may be the same or different.
- The number of silicon atoms forming the reactive silicon group is one or more, and is preferably not more than 20 in the case of the silicon atoms connected by siloxane bonds.
- Particularly reactive silicon groups represented by the general formula (9):
—SiR10 3-eXe (9)
wherein R10 and X are the same as defined above, e is an integer of 1 to 3, are preferable because they are easily available. - Examples of R9 and R10 in the above described general formulae (8) and (9) include alkyl groups such as a methyl group and an ethyl group, cycloalkyl groups such as a cyclohexyl group, aryl groups such as a phenyl group, aralkyl groups such as a benzyl group, a triorganosiloxy group represented by (R′)3SiO— where R′ is a methyl group, a phenyl group or the like and the like group. Of these groups, a methyl group is particularly preferable.
- More specific examples of the reactive silicon group include a trimethoxysilyl group, a triethoxysilyl group, a triisopropoxysilyl group, a dimethoxymethylsilyl group, a diethoxymethylsilyl group and a diisopropoxymethylsilyl group. A trimethoxysilyl group, a triethoxysilyl group and a dimethoxymethylsilyl group are more preferable and a trimethoxysilyl group is particularly preferable because these groups are high in activity and satisfactory curability can be obtained. Also from the viewpoint of storage stability, a dimethoxymethylsilyl group is particularly preferable. Additionally, a triethoxysilyl group is particularly preferable because the alcohol produced by the hydrolysis reaction of the reactive silicon group is ethanol and hence a triethoxysilyl group has a high safety. Additionally, a trimethoxysilyl group and a triethoxysilyl group are preferable because lowering of physical properties of a cured article after storing is relatively small.
- The introduction of the reactive silicon group can be carried out by methods well known in the art. More specifically, examples of such methods include the followings.
- (a) With an organic polymer having in the molecule functional groups such as hydroxy groups, an organic compound having both an active group exhibiting reactivity to the functional groups and an unsaturated group is reacted, to yield an unsaturated group-containing organic polymer. Alternatively, an unsaturated group-containing organic polymer is obtained by copolymerization of an epoxy compound having an unsaturated group with an organic polymer having in the molecule functional groups such as hydroxy groups. Then, a reactive silicon group-containing hydrosilane is reacted with the reaction product to be hydrosilylated.
- (b) With an unsaturated group-containing organic polymer obtained similarly to the method described in (a), a mercapto group- and reactive silicon group-containing compound is reacted.
- (c) With an organic polymer having in the molecule functional groups such as hydroxy groups, epoxy groups and isocyanate groups, a compound having a functional group exhibiting reactivity to the functional groups and a reactive silicon group is reacted.
- Among the above methods, the method described in (a) or the method described in (c) in which a hydroxy group-terminated polymer is reacted with an isocyanate group- and reactive silicon group-containing compound is preferable because the method provides a high conversion rate at a relatively short reaction time. Additionally, the method described in (a) is particularly preferable because the reactive silicon group-containing organic polymer obtained by the method described in (a) is lower in viscosity and more satisfactory in workability than an organic polymer obtained by the method described in (c), and an organic polymer obtained by the method described in (b) is strong in odor due to mercaptosilane.
- Examples of the hydrosilane compound used in the method described in (a) include halogenated silanes such as trichlorosilane, methyldichlorosilane, dimethylchlorosilane and phenyldichlorosilane; alkoxysilanes such as trimethoxysilane, triethoxysilane, methyldiethoxysilane, methyldimethoxysilane and phenyldimethoxysilane; acyloxysilanes such as methyldiacetoxysilane and phenyldiacetoxysilane; ketoximate silanes such as bis(dimethylketoximate)methylsilane and bis(cyclohexylketoximate)methylsilane and the like. However, the hydrosilane compound used in the method described in (a) is not limited to these compounds. Of these compounds, halogenated silanes and alkoxysilanes are preferable and in particular, alkoxysilanes are most preferable because the obtained curable compositions are moderately hydrolyzable and easily handled. Of the alkoxysilanes, methyldimethoxysilane is particularly preferable because it is easily available and curability, storage stability, elongation property and tensile strength of the curable composition containing the obtained organic polymer are high.
- Examples of the synthesis method described in (b) include a method in which a mercapto group- and reactive silicon group-containing compound is introduced into the sites on the unsaturated bonds of an organic polymer by means of a radical addition reaction in the presence of a radical initiator and/or a radical generating source; however, the synthesis method concerned is not limited to these methods. Examples of the above described mercapto group- and reactive silicon group-containing compound include γ-mercaptopropyltrimethoxysilane, γ-mercaptopropylmethyldimethoxysilane, γ-mercaptopropyltriethoxysilane, γ-mercaptopropylmethyldiethoxysilane, mercaptomethyltrimethoxysilane, mercaptomethyltriethoxysilane and the like; however, the mercapto group- and reactive silicon group-containing compound is not limited to these compounds.
- Examples of the method, of the methods described in (c), in which a hydroxy-terminated polymer is reacted with an isocyanate group- and reactive silicon group-containing compound include a method disclosed in Japanese Patent Laid-Open No. 3-47825; however, the method concerned is not limited to these methods. Examples of the above described isocyanate group- and reactive silicon group-containing compound include γ-isocyanatopropyltrimethoxysilane, γ-isocyanatopropylmethyldimethoxysilane, γ-isocyanatopropyltriethoxysilane, γ-isocyanatopropylmethyldiethoxysilane, isocyanatomethyltrimethoxysilane, isocyanatomethyltriethoxysilane, isocyanatomethyldimethoxymethylsilane, isocyanatomethyldiethoxymethylsilane and the like; however, the compound concerned is not limited to these compounds.
- In the case of silane compounds such as trimethoxysilane in which three hydrolyzable groups are bonded to one silicon atom, in some cases, disproportionation reaction proceeds. If disproportionation reaction proceeds, a very dangerous compound like dimethoxysilane is generated. However in the cases of γ-mercaptopropyltrimethoxysilane and γ-isocyanatepropyltrimethoxysilane, such a disproportionation reaction does not proceed. Accordingly, when using, as a silicon-containing group, a group such as a trimethoxysilyl group in which three hydrolyzable groups are bonded to one silicon atom, it is preferable to employ the synthesis method of (b) or (c).
- The reactive silicon group-containing organic polymer may be a straight chain or may have branches, and the number average molecular weight thereof, measured by GPC relative to polystyrene standard, is preferably of the order of 500 to 100,000, more preferably 1,000 to 50,000, particularly preferably 3,000 to 30,000. When the number average molecular weight is less than 500, it tends to be disadvantageous from the viewpoint of an elongation property of the cured article, while when the number average molecular weight exceeds 100,000, it tends to be disadvantageous from the viewpoint of workability because the viscosity becomes high.
- For the purpose of obtaining a rubber-like cured article having a high strength, a high elongation property and a low elastic modulus, it is recommended that the number of reactive silicon groups contained in the organic polymer is, on average in one polymer molecule, at least one, and preferably 1.1 to 5. When the average number of reactive silicon groups contained in the molecule is less than 1, the curability becomes insufficient, and hence a satisfactory rubber elasticity behavior can hardly be exhibited. The reactive silicon group may be located at the terminal of the main chain or at the terminal of the side chain, or at the both in the organic polymer molecule chain. In particular, when the reactive silicon group is located only at the terminal of the main chain, the effective network content in the organic polymer component contained in the finally formed cured article becomes large, so that it becomes easier to obtain a rubber-like cured article having a high strength, a high elongation property and a low elastic modulus.
- The polyoxyalkylene polymer (a1) is essentially a polymer having the repeating units represented by the general formula (10):
—R11—O— (10)
wherein R11 is a linear or branched alkylene group having 1 to 14 carbon atoms. In the general formula (10), R11 is preferably a linear or branched alkylene group having 1 to 14 carbon atoms, and more preferably 2 to 4 carbon atoms. Examples of the repeating units represented by the general formula (10) include:
—CH2O—, —CH2CH2O—, —CH2CH(CH3)O—, —CH2CH(C2H5)O—, —CH2C(CH3)2O—, —CH2CH2CH2CH2O—,
and the like. The main chain skeleton of the polyoxyalkylene polymer may be formed of either only one type of repeating unit or two or more types of repeating units. In particular, in the case where the polymer is used for a sealant and the like, it is preferable that the main chain skeleton is formed of a polymer containing as the main component a propyleneoxide polymer because a polymer having such a main chain skeleton is amorphous and relatively low in viscosity. - Examples of the synthesis method of the polyoxyalkylene polymer include a polymerization method in the presence of an alkaline catalyst such as KOH; a polymerization method in the presence of a transition metal compound-porphyrin complex catalyst prepared by reacting an organoaluminum compound with porphyrin, disclosed in Japanese Patent Laid-Open No. 61-215623; polymerization methods in the presence of composite metal cyanide complex catalysts, disclosed in Japanese Patent Examined Publication Nos. 46-27250 and 59-15336, and U.S. Pat. Nos. 3,278,457, 3,278,458, 3,278,459, 3,427,256, 3,427,334, 3,427,335 and the like; a polymerization method in the presence of a catalyst composed of a polyphosphazene salt disclosed in Japanese Patent Laid-Open No. 10-273512, and a polymerization method in the presence of a catalyst composed of a phosphazene compound disclosed in Japanese Patent Laid-Open No. 11-060722. However, the method concerned is not limited to these methods.
- Examples of the preparation method of the reactive silicon group-containing polyoxyalkylene polymer (a1) of the present invention include the methods disclosed in Japanese Patent Examined Publication Nos. 45-36319 and 46-12154, Japanese Patent Laid-Open Nos. 50-156599, 54-6096, 55-13767, 55-13468 and 57-164123, Japanese Patent Examined Publication No. 3-2450, and U.S. Pat. Nos. 3,632,557, 4,345,053, 4,366,307 and 4,960,844; and the methods of preparing polyoxyalkylene polymers each having a high molecular weight such that the number average molecular weight is not less than 6,000 and a narrow molecular weight distribution such that the Mw/Mn value is not more than 1.6, disclosed in Japanese Patent Laid-Open Nos. 61-197631, 61-215622, 61-215623, 61-218632, 3-72527, 3-47825 and 8-231707. However, the method concerned is not limited to these methods.
- The above described reactive silicon group-containing polyoxyalkylene polymers (a1) may be used either each alone or in combinations of two or more thereof.
- On the other hand, no particular constraint is imposed on the (meth)acrylate monomers constituting the main chains of the above described (meth)acrylate polymers (a2), and various types can be used. Examples of the monomers concerned include (meth)acrylic acid monomers such as (meth)acrylic acid, methyl (meth)acrylate, ethyl (meth)acrylate, n-propyl (meth)acrylate, isopropyl (meth)acrylate, n-butyl (meth)acrylate, isobutyl (meth)acrylate, tert-butyl (meth)acrylate, n-pentyl (meth)acrylate, n-hexyl (meth)acrylate, cyclohexyl (meth)acrylate, n-heptyl (meth)acrylate, n-octyl (meth)acrylate, 2-ethylhexyl (meth)acrylate, nonyl (meth)acrylate, decyl (meth)acrylate, dodecyl (meth)acrylate, phenyl (meth)acrylate, tolyl (meth)acrylate, benzyl (meth)acrylate, 2-methoxyethyl (meth)acrylate, 3-methoxybutyl (meth)acrylate, 2-hydroxyethyl (meth)acrylate, 2-hydroxypropyl (meth)acrylate, stearyl (meth)acrylate, glycidyl (meth)acrylate, 2-aminoethyl (meth)acrylate, γ-(methacryloyloxypropyl)trimethoxysilane, γ-(methacryloyloxypropyl)dimethoxymethylsilane, methacryloyloxymethyltrimethoxysilane, methacryloyloxymethyltriethoxysilane, methacryloyloxymethyldimethoxymethylsilane, methacryloyloxymethyldiethoxymethylsilane, ethylene oxide adduct of (meth)acrylate, trifluoromethylmethyl (meth)acrylate, 2-trifluoromethylethyl (meth)acrylate, 2-perfluoroethylethyl (meth)acrylate, 2-perfluoroethyl-2-perfluorobutylethyl(meth)acrylate, perfluoroethyl(meth)acrylate, trifluoromethyl(meth)acrylate, bis(trifluoromethlymethyl)(meth)acrylate, 2-trifluoromethyl-2-perfluoroethylethyl(meth)acrylate, 2-perfluorohexylethyl(meth)acrylate, 2-perfluorodecylethyl (meth)acrylate and 2-perfluorohexadecylethyl(meth)acrylate. For the above described (meth)acrylate polymers, (meth)acrylate monomers can be copolymerized with the following vinyl monomers. Examples of the vinyl monomers concerned include styrene monomers such as styrene, vinyltoluene, α-methylstyrene, chlorostyrene, styrenesulfonic acid and the salts thereof; fluorine-containing vinyl monomers such as perfluoroethylene, perfluoropropylene and fluorinated vinylidene; silicon-containing vinyl monomers such as vinyltrimethoxysilane and vinyltriethoxysilane; maleic anhydride, maleic acid, and monoalkyl esters and dialkyl esters of maleic acid; fumaric acid, and monoalkyl esters and dialkyl esters of fumaric acid; maleimide monomers such as maleimide, methylmaleimide, ethylmaleimide, propylmaleimide, butylmaleimide, hexylmaleimide, octylmaleimide, dodecylmaleimide, stearylmaleimide, phenylmaleimide and cyclohexylmaleimide; nitrile group-containing vinyl monomers such as acrylonitrile and methacrylonitrile; amide group-containing vinyl monomers such as acrylamide and methacrylamide; vinyl esters such as vinyl acetate, vinyl propionate, vinyl pivalate, vinyl benzoate and vinyl cinnamate; alkenes such as ethylene and propylene; conjugated dienes such as butadiene and isoprene; and vinyl chloride, vinylidene chloride, allyl chloride and allyl alcohol. These monomers may be used each alone or two or more of these monomers may be copolymerized. Among these, from the viewpoint of the physical properties of the products, polymers formed of styrene monomers and (meth)acrylic acid monomers are preferable. More preferable are the (meth)acryl polymers formed of acrylate monomers and methacrylate monomers, and particularly preferable are the acryl polymers formed of acrylate monomers. For general construction applications, the butyl acrylate monomers are further preferable because compositions concerned each are required to have physical properties including a low viscosity, and the cured articles each are required to have physical properties including a low modulus, a high elongation property, a weather resistance and a heat resistance. On the other hand, for applications to vehicles and the like where oil resistance is required, copolymers made of ethyl acrylate as the main material are further preferable. The copolymers made of ethyl acrylate as the main material are excellent in oil resistance, but slightly tend to be poor in low-temperature property (low-temperature resistance); for the purpose of improving the low-temperature property thereof, a part of ethyl acrylates can be replaced with butyl acrylate. However, with the increase of the ratio of butyl acrylate, the satisfactory oil resistance comes to be degraded, so that for the application to the use requiring oil resistance, the ratio of butyl acrylate is set preferably to not more than 40%, and more preferably to not more than 30%. Additionally, it is also preferable to use 2-methoxyethyl acrylate and 2-ethoxyethyl acrylate which have side chain alkyl groups containing oxygen atoms introduced for the purpose of improving the low-temperature property and the like without degrading the oil resistance; in this connection, it is to be noted that the introduction of alkoxy groups having an ether bond in the side chains tends to degrade the heat resistance, so that the ratio of such an acrylate is preferably not more than 40% when heat resistance is required. It is possible to obtain appropriate polymers by varying the ratio in consideration of required physical properties such as oil resistance, heat resistance and low-temperature property according to the various applications and the required objectives. Examples of the polymers excellent in the balance between the physical properties including the oil resistance, heat resistance, low-temperature property and the like include a copolymer of ethyl acrylate/butyl acrylate/2-methoxyethyl acrylate (40 to 50/20 to 30/30 to 20 in a ratio by weight), this copolymer imposing no constraint on the polymers concerned. In the present invention, these preferable monomers can be copolymerized with other monomers, and moreover, block copolymerized with other monomers. In such cases, it is preferable that the preferable monomers are contained in an amount of not less than 40% in a ratio by weight. Incidentally, it is to be noted that in the above form of presentation, for example, “(meth)acrylic acid” means acrylic acid and/or methacrylic acid.
- No particular constraint is imposed on the synthesis methods of the (meth)acrylate polymers, and the methods well known in the art can be applied. However, polymers obtained by the usual free radical polymerization methods using azo compounds and peroxides as polymerization initiators have a problem such that the molecular weight distribution values of the polymers are generally as large as not less than 2 and the viscosities of the polymers are high. Accordingly, it is preferable to apply living radical polymerization methods for the purpose of obtaining (meth)acrylate polymers being narrow in molecular weight distribution and low in viscosity, and moreover, having cross-linking functional groups at the molecular chain terminals in a high ratio.
- Among “the living radical polymerization methods,” “the atom transfer radical polymerization method” in which (meth)acrylate monomers are polymerized by use of an organic halogenated compound or a halogenated sulfonyl compound as an initiator and a transition metal complex as a catalyst has, in addition to the features of the above described “living radical polymerization methods,” features such that the obtained polymer has halogen atoms at the terminals relatively favorable for the functional group conversion reaction and freedom for designing the initiator and the catalyst is wide, so that the atom transfer radical polymerization method is further preferable as a method for preparing (meth)acrylate polymers having particular functional groups. Examples of the atom transfer radical polymerization method include the method reported by Matyjaszewski et al. in Journal of the American Chemical Society (J. Am. Chem. Soc.), Vol. 117, p. 5614 (1995).
- As a preparation method of a reactive silicon group-containing (meth)acrylate polymer (a2), for example, Japanese Patent Examined Publication Nos. 3-14068 and 4-55444, and Japanese Patent Laid-Open No. 6-211922 and the like disclose preparation methods according to the free radical polymerization methods by using chain transfer agents. Additionally, Japanese Patent Laid-Open No. 9-272714 and the like disclose a preparation method according to the atom transfer radical polymerization method. However, the preparation method concerned is not limited to these methods.
- The above described reactive silicon group-containing (meth)acrylate polymers (a2) may be used either each alone or in combinations of two or more thereof.
- The preparation methods of the organic polymers formed by blending the reactive silicon group-containing polyoxyalkylene polymers (a1) with the reactive silicon group-containing (meth)acrylate polymers (a2) are proposed in Japanese Patent Laid-Open Nos. 59-122541, 63-112642, 6-172631, 11-116763 and the like. However, the preparation method concerned is not limited to these methods.
- From the viewpoint of compatibility of (a1) with (a2) and adhesion of an obtained cured article, with respect to a preferable specific example of the component (a2), there is a preparation method in which a reactive silicon group-containing polyoxyalkylene polymer is blended with a copolymer formed of two (meth)acrylate monomer units: one (meth)acrylate monomer unit has the reactive silicon groups and alkyl groups having 1 to 8 carbon atoms, and the molecular chain is substantially represented by the following general formula (11):
—CH2—C(R12)(COOR13)— (11)
wherein R12 represents hydrogen atom or a methyl group, and R13 represents an alkyl group having 1 to 8 carbon atoms; and the other (meth)acrylate monomer unit has alkyl groups having 10 or more carbon atoms and is represented by the following general formula (12):
—CH2—C(R12)(COOR14)— (12)
wherein R12 is the same as defined above, and R14 represents an alkyl group having 10 or more carbon atoms. - In the above general formula (11), examples of R13 include alkyl groups having 1 to 8 carbon atoms, preferably 1 to 4 carbon atoms and further preferably 1 to 2 carbon atoms such as a methyl group, an ethyl group, a propyl group, a n-butyl group, a t-butyl group and a 2-ethylhexyl group. It is also to be noted that the alkyl group of R13 may represent either one type or admixtures of two or more types.
- In the above general formula (12), examples of R14 include long chain alkyl groups having 10 or more carbon atoms, usually 10 to 30 carbon atoms, and preferably 10 to 20 carbon atoms such as a lauryl group, a tridecyl group, a cetyl group, a stearyl group and a behenyl group. It is also to be noted that the alkyl group of R14 may represent, similarly to R13, either one type or admixtures of two or more types.
- The molecular chains of the above described (meth)acrylate copolymers are substantially formed of the monomer units represented by formulas (11) and (12): “substantially” as referred to here means that in the copolymer concerned, the sum content of the monomer unit of formula (11) and the monomer unit of formula (12) exceeds 50 wt %. The sum content of the monomer units of formulas (11) and (12) is preferably not less than 70 wt %.
- Additionally, the abundance ratio by weight of the monomer unit of formula (11) to the monomer unit of formula (12) is preferably 95:5 to 40:60, and further preferably 90:10 to 60:40.
- Examples of the monomer units other than the monomer units of formulas (11) and (12) which may be contained in the above described copolymer include acrylic acids such as acrylic acid and methacrylic acid; monomers containing amide groups such as acrylamide, methacrylamide, N-methylolacrylamide and N-methylolmethacrylamide, monomers containing epoxy groups such as glycidylacrylate and glycidylmethacrylate, and monomers containing amino groups such as diethylaminoethyl acrylate, diethylaminoethyl methacrylate and aminoethyl vinyl ether; and monomer units derived from acrylonitrile, styrene, α-methylstyrene, alkyl vinyl ethers, vinyl chloride, vinyl acetate, vinyl propionate and ethylene.
- Moreover, for the preparation method of the organic polymers formed by blending the (meth)acrylate polymers having the reactive silicon functional groups, there can be used additional methods in which (meth)acrylate monomers are polymerized in the presence of a reactive silicon group-containing polyoxyalkylene polymer. These methods are disclosed specifically in Japanese Patent Laid-Open Nos. 59-78223, 60-228516, 60-228517 and the like. However, the method concerned is not limited to these methods.
- The main chain skeleton of the organic polymer of the present invention may include other components such as binding urethane components as far as such inclusion does not largely impair the effect of the invention.
- No particular constraint is imposed on the binding urethane components. Examples thereof include groups (hereinafter referred to as amide segments) formed by a reaction of an isocyanate group with an active hydrogen group.
- The above described amide segments are groups represented by the general formula (13):
—NR15—C(═O)— (13)
wherein R15 represents hydrogen atom or a substituted or unsubstituted organic group. - Examples of the above described amide segments include a urethane group formed by a reaction of an isocyanate group with hydroxyl group; a urea group formed by a reaction of an isocyanate group with an amino group; a thiourethane group formed by a reaction of an isocyanate group with a mercapto group; and the like. Additionally, in the present invention, groups formed by further reaction of an active hydrogen in the urethane group, urea group or thiourethane group with an isocyanate group are included in the groups of the general formula (13).
- Example of an industrially easy method of preparing an organic polymer having both of an amide segment and a reactive silicon group is a method in which after or at the same time of reacting an organic polymer having an active hydrogen-containing group at the terminal with an excessive amount of polyisocyanate compound to yield a polymer having isocyanate groups at the terminals of polyurethane main chains, a part or the whole of isocyanate groups are reacted with a W group of the silicon compound represented by the general formula (14):
W—R16—SiR10 3-eXe (14)
wherein R10, X and e are the same as defined above; R16 is a divalent organic group, more preferably a substituted or unsubstituted divalent hydrocarbon group having 1 to 20 carbon atoms; W is an active hydrogen-containing group selected from hydroxyl group, a carboxyl group, a mercapto group and an amino group (unsubstituted or mono-substituted). Known methods of preparing organic polymers in relation to this preparation method are disclosed in Japanese Patent Examined Publication No. 46-12154 (U.S. Pat. No. 3,632,557), Japanese Patent Laid-Open Nos. 58-109529 (U.S. Pat. No. 4,374,237), 62-13430 (U.S. Pat. No. 4,645,816), 8-53528 (EP Patent No. 0676403), and 10-204144 (EP Patent No. 0831108), Japanese Patent Laid-Open No. 2003-508561 (U.S. Pat. No. 6,197,912), Japanese Patent Laid-Open Nos. 6-211879 (U.S. Pat. No. 5,364,955), 10-53637 (U.S. Pat. No. 5,756,751), 11-100427, 2000-169544, 2000-169545, and 2002-212415, Japanese Patent No. 3313360, U.S. Pat. No. 4,067,844, U.S. Pat. No. 3,711,445, Japanese Patent Laid-Open No. 2001-323040 and the like. - Additionally, there are methods of preparation by reacting an organic polymer having an active hydrogen-containing group at the terminal (oxyalkylene polymer (polyether polyol) having hydroxyl group at the terminal, polyacryl polyol and the like) with a reactive silicon group-containing isocyanate compound represented by the general formula (15):
O═C═N—R16—SiR10 3-eXe (15)
wherein R10, R16, X and e are the same as defined above. Known methods of preparing organic polymers in relation to this preparation method are disclosed in Japanese Patent Laid-Open Nos. 11-279249 (U.S. Pat. No. 5,990,257), 2000-119365 (U.S. Pat. No. 6,046,270), 58-29818 (U.S. Pat. No. 4,345,053), 3-47825 (U.S. Pat. No. 5,068,304), 11-60724, 2002-155145, 2002-249538, WO03/018658, WO03/059981 and the like. - Polyether polyols prepared by any of preparation methods can be used, and preferred are polyether polyols having at least 0.7 hydroxyl group per a molecular terminal on average of the whole molecules. Specifically, examples thereof are oxyalkylene polymers prepared by using conventional alkali metal catalysts, oxyalkylene polymers prepared by reacting an initiator such as a polyhydroxy compound having at least two hydroxyl groups with alkylene oxide in the presence of a composite metal cyanide complex and cesium, and the like.
- Of the above described polymerization methods, the method using a composite metal cyanide complex is preferable because oxyalkylene polymers having a lower degree of unsaturation, a narrow Mw/Mn, a lower viscosity and high acid resistance and weather resistance can be obtained.
- Examples of the above described polyacryl polyols include polyols having an alkyl (meth)acrylate (co)polymer skeleton and containing hydroxy groups in the molecule. As the synthesis method to produce these polymers, the living radical polymerization method is preferable because this method can lead to narrow molecular weight distributions and low viscosities, and the atom transfer radical polymerization method is further preferable. Additionally, it is preferable to use a polymer based on the so-called SGO process which is obtained by continuously block-polymerizing an alkyl acrylate monomer at a high temperature under a high pressure, as described in Japanese Patent Laid-Open No. 2001-207157. Specifically, examples thereof include UH-2000 produced by Toagosei Co., Ltd., and the like.
- Examples of the polyisocyanate compound include aromatic polyisocyanates such as toluene (tolylene) diisocyanate, diphenylmethane diisocyanate and xylylene diisocyanate; and aliphatic polyisocyanates such as isophorone diisocyanate and hexamethylene diisocyanate.
- No particular constraint is imposed on the silicon compound of the general formula (14). Specifically, examples thereof include silanes having amino group such as γ-aminopropyltrimethoxysilane, N-(β-aminoethyl)-γ-aminopropyltrimethoxysilane, γ-(N-phenyl)aminopropyltrimethoxysilane, N-ethylaminoisobutyltrimethoxysilane, N-cyclohexylaminomethyltriethoxysilane, N-cyclohexylaminomethyldiethoxymethylsilane and N-phenylaminomethyltrimethoxysilane; silanes having hydroxy group such as γ-hydroxypropyltrimethoxysilane; silanes having mercapto group such as γ-mercaptopropyltrimethoxysilane; and the like. Additionally, as described in Japanese Patent Laid-Open Nos. 6-211879 (U.S. Pat. No. 5,364,955), 10-53637 (U.S. Pat. No. 5,756,751), 10-204144 (EP Patent No. 0831108), 2000-169544 and 2000-169545, there can be used Michael addition reaction products of various α,β-unsaturated carbonyl compounds and primary amino group-containing silanes and Michael addition reaction products of various (meth)acryloyl group-containing silanes and primary amino group-containing compounds as the silicon compounds of the general formula (14).
- No particular constraint is imposed on the reactive silicon group-containing isocyanate compound of the general formula (15). Specifically, examples thereof include γ-trimethoxysilylpropylisocyanate, γ-triethoxysilylpropylisocyanate, γ-methyldimethoxysilylpropylisocyanate, γ-methyldiethoxysilylpropylisocyanate, trimethoxysilylmethylisocyanate, diethoxymethylsilylmethylisocyanate, dimethoxymethylsilylmethylisocyanate, diethoxymethylsilylmethylisocyanate and the like. Additionally, as described in Japanese Patent Laid-Open No. 2000-119365 (U.S. Pat. No. 6,046,270), a compound obtained by reacting the silicon compound of the general formula (14) with an excessive amount of the above described polyisocyanate compound can be used as the reactive silicon group-containing isocyanate compound of the general formula (15).
- When amide segments contained in the main chain skeleton of the organic polymer of the present invention are abundant, the viscosity of the organic polymer becomes high and forms a composition poor in workability as the case may be. On the other hand, curability of the composition of the present invention tends to be enhanced by the amide segments contained in the main chain skeleton of the organic polymer. When the organic polymer having amide segments in its main skeleton is used as the component (A), the composition prepared in a combination of the polymer with the component (B) is preferable because the composition has a quicker curability by use of a non-organotin catalyst. Accordingly, when the amide segments are contained in the main chain skeleton of the organic polymer, the average number of amide segments per molecule is preferably from 1 to 10, more preferably from 1.5 to 7, particularly preferably from 2 to 5. When the number of amide segments is less than 1, in some cases, curability becomes insufficient, and when the number of amide segments is more than 10, the viscosity of the organic polymer becomes high and forms a composition poor in workability.
- In the present invention, the chelate type methyl titanate (b1) represented by the general formula (1):
wherein each of (4-n) R1s is independently hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, each of (4-n) A1s and (4-n) A2s is independently —R2 or —OR2 where R2 is a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, n is 1, 2 or 3, and/or the chelate type ethyl titanate (b2) represented by the general formula (2):
wherein R1, A1, A2 and n are the same as defined above, are used as the component (B). - These components (B) function as a curing catalyst for the organic polymer of the component (A). Organotin compounds such as dibutyltin dilaurate and dibutyltin bis(acetylacetonate) which have a fear of having an influence on environment have been so far used as a curing catalyst for the reactive silicon group-containing organic polymer of the component (A), but by using the titanium catalysts of the component (B) of the present invention, a load on environment is small and a curable composition having practical curing properties can be obtained. Further as compared with the case of using other curing catalysts such as organotin catalysts, adhesion to articles to be hardly adhered such as acrylic resins can be enhanced.
- Tetraalkoxy titanium, halogenated titanium, titanium chelate and the like are known as a titanium catalyst, and particularly when titanium chelate is used, there is a tendency that quick curability can be obtained.
- However, like the present invention, when a one-component type curable composition is prepared using a silane compound having a nitrogen-substituted group like the component (C) as an adhesion-imparting agent and a commonly used titanium diisopropoxidebis(ethylacetoacetate) as a titanium catalyst, there is a tendency that mechanical properties such as a modulus and a strength of a cured article obtained from the stored composition are significantly lowered as compared with mechanical properties before storing. The reason for this is not obvious, but it is presumed that when a silane compound having a nitrogen-substituted group like the component (C) is present, during the storing, an alkoxy group constituting the reactive silicon group of the component (A) and an isopropoxy group of titanium diisopropoxidebis(ethylacetoacetate) are exchanged with each other by any mechanism, and as a result, inherent curability of the component (A) is lowered and a crosslinking reaction does not proceed sufficiently. Namely, for example, when the reactive silicon group of the component (A) is a dimethoxymethylsilyl group, a part or the whole of methoxy groups on silicon are converted to isopropoxy groups, and the reactive silicon group becomes a group having a lower reactivity.
- Accordingly, when a titanium chelate in which an alkoxy-substituted group is limited to a methoxy group or an ethoxy group is used as a titanium catalyst like the component (B) of the present invention, lowering of mechanical properties of a cured article after storing can be inhibited. Namely, even if during the storing, an alkoxy group on the reactive silicon group of the component (A) and a methoxy group or an ethoxy group in the component (B) are exchanged with each other by the same mechanism as above, the converted reactive silicon group of the component (A) is a relatively active methoxy group or ethoxy group, and therefore it can be considered that a crosslinking reaction proceeds sufficiently.
- Examples of the chelate type methyl titanate (b1) include titanium dimethoxidebis(acetylacetonate), titanium dimethoxidebis(3-methylacetylacetonate), titanium dimethoxidebis(methylacetoacetate), titanium dimethoxidebis(ethylacetoacetate), titanium dimethoxidebis(ethyl-2-methylacetoacetate), titanium dimethoxidebis(ethyl-2-ethylacetoacetate), titanium dimethoxidebis(t-butylacetoacetate), titanium dimethoxidebis(allylacetoacetate), titanium dimethoxidebis(methyl-3-oxo-4,4-dimethylhexanoate), titanium dimethoxidebis(ethyl-3-oxo-4,4,4-trifluorobutanoate), titanium dimethoxidebis(2,2,6,6-tetramethyl-3,5-heptanedionate), titanium trimethoxide(acetylacetonate), titanium trimethoxide(ethylacetoacetate), titanium trimethoxide(allylacetoacetate), titanium trimethoxide(diethylmalonate), titanium trimethoxide(methacryloxyethylacetoacetate), and the like. Of these chelates, titanium dimethoxidebis(ethylacetoacetate), titanium dimethoxidebis(ethyl-2-methylacetoacetate) and titanium dimethoxidebis(acetylacetonate) are preferable from the viewpoint of catalytic activity, and titanium dimethoxidebis(ethylacetoacetate) and titanium dimethoxidebis(ethyl-2-methylacetoacetate) are especially preferable.
- Additionally, examples of the chelate type ethyl titanate (b2) include titanium diethoxidebis(acetylacetonate), titanium diethoxidebis(3-methylacetylacetonate), titanium diethoxidebis(methylacetoacetate), titanium diethoxidebis(ethylacetoacetate), titanium diethoxidebis(ethyl-2-methylacetoacetate), titanium diethoxidebis(ethyl-2-ethylacetoacetate), titanium diethoxidebis(t-butylacetoacetate), titanium diethoxidebis(allylacetoacetate), titanium diethoxidebis(methyl-3-oxo-4,4-dimethylhexanoate), titanium diethoxidebis(ethyl-3-oxo-4,4,4-trifluorobutanoate), titanium diethoxidebis(2,2,6,6-tetramethyl-3,5-heptanedionate), titanium triethoxide(acetylacetonate), titanium triethoxide(ethylacetoacetate), titanium triethoxide(allylacetoacetate), titanium triethoxide(diethylmalonate), titanium triethoxide(methacryloxyethylacetoacetate), and the like. Of these chelates, titanium diethoxidebis(ethylacetoacetate), titanium diethoxidebis(ethyl-2-methylacetoacetate) and titanium diethoxidebis(acetylacetonate) are preferable from the viewpoint of catalytic activity, and titanium dimethoxidebis(ethylacetoacetate) and titanium dimethoxidebis(ethyl-2-methylacetoacetate) are especially preferable.
- It is preferable to use the chelate type methyl titanate (b1) as the component (B) from the point that the effect of the present invention, i.e. an effect of inhibiting lowering of physical properties after storing is higher. Additionally, it is preferable to use the chelate type methyl titanate (b1) from the point that higher curability can be obtained. On the other hand, use of the chelate type ethyl titanate (b2) is preferable because a by-product derived from the titanate is ethanol which has less effect on environment. Additionally, also when the reactive silicon group of the component (A) is an ethoxysilyl group such as a triethoxysilyl group, it is preferable to use the chelate type ethyl titanate (b2) from the viewpoint of storage stability. Further use of the chelate type ethyl titanate (b2) is preferable because good adhesion can be obtained.
- Preferred examples of the chelating agents capable of forming chelate ligands of the above described component (B) include β-diketones such as acetylacetone, 3-methylacetylacetone and 2,2,4,4-tetramethyl-3,5-heptanedion; β-ketoesters such as methyl acetoacetate, ethyl acetoacetate, t-butyl acetoacetate, allyl acetoacetate, (2-methacryloxyethyl)acetoacetate, methyl 3-oxo-4,4-dimethylhexanoate, ethyl 3-oxo-4,4,4-trifluorobutanoate, 3-methylacetylacetone, ethyl 2-methylacetoacetate and ethyl 2-ethylacetoacetate; β-diesters such as dimethyl malonate and diethyl malonate; cyclic dicarbonyl compounds such as 2-acetylbutyrolactone; and the like from the viewpoint of curability. β-Diketones and β-ketoesters are more preferred from the viewpoint of curability and storage stability, and β-ketoesters are particularly preferred. Specifically acetylacetone, methyl acetoacetate, ethyl acetoacetate and ethyl 2-methylacetoacetate are more preferred from the viewpoint of curability and storage stability and because those compounds are easily available, and ethyl acetoacetate and ethyl 2-methylacetoacetate are particularly preferable. Also when two or more chelate ligands are present, those chelate ligands may be the same or different.
- The component (B) may be used either each alone or in combinations of two or more thereof.
- The used amount of the component (B) is preferably from 0.1 to 20 parts by weight, and more preferably from 0.5 to 15 parts by weight, especially preferably from 1 to 10 parts by weight with respect to 100 parts by weight of the component (A). When the blended amount of the component (B) is less than the above described ranges, sometimes the practical curing rate cannot be obtained, and the curing reaction hardly proceeds to a sufficient extent. On the other hand, when the blended amount of the component (B) exceeds the above described ranges, sometimes the work life becomes too short, and the workability is degraded.
- In the present invention, a titanium catalyst other than the component (B) can be used to an extent not to degrade the effect of the present invention. Examples thereof include titanium chelates such as titanium diisopropoxidebis(ethylacetoacetate), titanium diisopropoxidebis(methylacetoacetate), titanium diisopropoxidebis(t-butylacetoacetate), titanium diisopropoxidebis(methyl-3-oxo-4,4-dimethylhexanoate), titanium diisopropoxidebis(ethyl-3-oxo-4,4,4-trifluoropentanoate), titanium diisopropoxidebis(acetylacetonate), titanium diisopropoxidebis(2,2,6,6-tetramethyl-3,5-heptanedionate), titanium di-n-butoxidebis(ethylacetoacetate), titanium di-t-butoxidebis(ethylacetoacetate), titanium di-2-ethylhexoxidebis(ethylacetoacetate), 1,2-dioxyethanetitaniumbis(ethylacetoacetate), 1,3-dioxypropanetitaniumbis(ethylacetoacetate), 2,4-dimethyl-2,4-dioxypentanetitaniumbis(ethylacetoacetate), titanium diisopropoxidebis(triethanolaminate), titanium bis(trimethylsiloxy)bis(ethylacetoacetate), titanium bis(trimethylsiloxy)bis(acetylacetonate), titanium tetrakis(ethylacetoacetate) and titanium tetrakis(acetylacetonate); titanium alkoxides such as titanium tetramethoxide, titanium tetraethoxide, titanium tetraallyloxide, titanium tetraisopropoxide, titanium tetra-n-butoxide, titanium tetra-t-butoxide, titanium tetracyclohexyloxide, titanium tetrabenzyloxide, titanium tetraoctyloxide, titanium tetrakis(2-ethylhexyloxide), titanium tetrabutoxide dimer, titanium tetrakis(8-hydroxyoctyloxide), titanium tetrakis(trimethylsilyloxide), titanium diisopropoxidebis(2-ethyl-1,3-hexanediolate), titanium tetrakis(2-chloroethoxide), titanium tetrakis(2-methoxyethoxide), titanium tetraphenoxide, titanium tetrakis(o-chlorophenoxide) and titanium tetrakis(m-nitrophenoxide); titanium acylates such as titanium acrylate triisopropoxide, titanium methacrylate triisopropoxide, titanium dimethacrylate diisopropoxide, titanium trimethacrylate isopropoxide, titanium hexanoate triisopropoxide and titanium stearate triisopropoxide; halogenated titanates such as titanium chloride triisopropoxide, titanium dichloride diisopropoxide, titanium isopropoxide trichloride, titanium bromide triisopropoxide, titanium fluoride triisopropoxide, titanium chloride triethoxide and titanium chloride tributoxide; other titanates such as titanium tris(dioctylphosphate)isopropoxide, titanium tris(dodecylbenzenesulfonate)isopropoxide and dihydroxytitaniumbislactate; and the like.
- In the present invention, the compound having, in its molecule, a hydrolyzable silicon group and a nitrogen-substituted group represented by the general formula (3):
—NHR3 (3)
wherein R3 is hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, or the general formula (4):
—N═R4 (4)
wherein R4 is CO or a substituted or unsubstituted divalent hydrocarbon group having 1 to 20 carbon atoms and is bonded to a nitrogen atom by double bonding, is used. The component (C) of the present invention is a kind of so-called silane coupling agent, and acts as an adhesion-imparting agent. - By use of the component (C), a marked effect of improving adhesion is exhibited under a non-primer condition or under a condition of treating with a primer when the composition is used on various articles to be adhered, namely, inorganic substrates such as glass, aluminum, stainless steel, zinc, copper and mortar and organic substrates such as polyvinyl chloride, acryl, polyester, polyethylene, polypropylene and polycarbonate. In the case of use under the non-primer condition, an effect of improving adhesion to various articles to be adhered is exhibited markedly.
- Examples of the hydrolyzable silicon groups of the component (C) are the groups represented by the general formula (8) in which X is a hydrolyzable group. Specifically there are groups exemplified supra as a hydrolyzable group, and a methoxy group and an ethoxy group are preferable from the viewpoint of a hydrolyzing rate. The number of hydrolyzable groups is preferably not less than 2, particularly not less than 3.
- In claims of the instant patent application, the reactive silicon group contained in the component (A) is described as “a silicon-containing group being crosslinkable by forming siloxane bonds”, and the reactive silicon group contained in the component (C) is described as “a hydrolyzable silicon group”, and the both are substantially the same kind of reactive silicon groups. Representative examples of those reactive silicon groups are reactive silicon groups represented by the following general formulae (5) and (6), respectively. Those groups have an alkoxy group having 1 to 4 carbon atoms as a hydrolyzable group.
SiR5 3-a(OR6)a (5) . . . Silicon-containing group of the component (A)
—SiR7 3-b(OR8)b (6) . . . Hydrolyzable silicon group of the component (C)
wherein each of R5 and R7 is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R′)3SiO— where each of R's is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, R6 is an alkyl group having 1 to 4 carbon atoms, R8 is an alkyl group having 1 to 4 carbon atoms, a is 1, 2 or 3, b is 1, 2 or 3. - It is preferable that R6 is an alkyl group having the number of carbon atoms more than the number of carbon atoms of R8, from the viewpoint of curability. This is because when R6 is an alkyl group having the number of carbon atoms less than that of R8, it is presumed that in some cases, inherent curability of the component (A) is lowered.
- Supplementary explanation of the above described presumption is as follows.
- In the results of experiments explained infra, when a polyoxypropylene polymer having a methyldimethoxy group is used as the component (A) and γ-(2-aminoethyl)aminopropyltriethoxysilane and N-(1,3-dimethylbutylidene)-3-(triethoxysilyl)-1-propaneamine are used as the component (C), a tack-free time is extremely long. Though the reason for this is not obvious, it can be considered that this is because an exchange reaction of an alkoxy group occurs between the reactive silicon group of the component (A) and the reactive silicon group of the component (C) and a part of methoxy groups of the component (A) are replaced by ethoxy groups which contain more carbon atoms and have moderate hydrolyzability.
- Accordingly, from the point that the reactive silicon group of the component (A) is selected from a wider range, the hydrolyzable silicon group of the component (C) is preferably a methoxysilyl group represented by the general formula (7):
—SiR7 3-b(OCH3)b (7)
wherein each of R7s is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms or a triorganosiloxy group represented by (R′)3SiO— where each of R's is independently a substituted or unsubstituted hydrocarbon group having 1 to 20 carbon atoms, b is 1, 2 or 3. - Specific examples of the methoxysilyl group are a methoxydimethylsilyl group, a methoxydiphenylsilyl group, a methoxybistrimethylsiloxysilyl group, a dimethoxymethylsilyl group, an ethyldimethoxysilyl group, a dimethoxyphenylsilyl group, a dimethoxytrimethylsiloxysilyl group, a trimethoxysilyl group and the like. The methoxysilyl group is not limited to them. From the viewpoint of curability of the obtained curable composition, a dimethoxymethylsilyl group and a trimethoxysilyl group are preferable and a trimethoxysilyl group is particularly preferable.
- Additionally, since the component (C) has the nitrogen-substituted group of the above described general formula (3) or (4), a higher adhesion can be obtained. Since an effect of imparting adhesion is higher, the substituent represented by the general formula (3) is preferable, and an amino group is particularly preferable.
- Specific examples of the component (C) include amino group-containing silanes such as γ-aminopropyltrimethoxysilane, γ-aminopropylmethyldimethoxysilane, γ-aminopropyltriethoxysilane, γ-aminopropylmethyldiethoxysilane, γ-aminopropyltriisopropoxysilane, γ-(methylamino)propyltrimethoxysilane, γ-(2-aminoethyl)aminopropyltrimethoxysilane, γ-(2-aminoethyl)aminopropylmethyldimethoxysilane, γ-(2-aminoethyl)aminopropyltriethoxysilane, γ-(2-aminoethyl)aminopropylmethyldiethoxysilane, γ-(2-aminoethyl)aminopropyltriisopropoxysilane, γ-(2-(2-aminoethyl)aminoethyl)aminopropyltrimethoxysilane, γ-(6-aminohexyl)aminopropyltrimethoxysilane, 3-(N-ethylamino)-2-methylpropyltrimethoxysilane, 4-amino-3,3-dimethylbutyltrimethoxysilane, 4-amino-3,3-dimethylbutyldimethoxymethylsilane, γ-ureidopropyltrimethoxysilane, γ-ureidopropyltriethoxysilane, N-phenyl-γ-aminopropyltrimethoxysilane, N-benzyl-γ-aminopropyltrimethoxysilane, N-vinylbenzyl-γ-aminopropyltriethoxysilane, N-phenylaminomethyltrimethoxysilane, N-cyclohexylaminomethyltriethoxysilane, N-cyclohexylaminomethyldiethoxymethylsilane, (2-aminoethyl)aminomethyltrimethoxysilane and N,N′-bis[3-(trimethoxysilyl)propyl]ethylenediamine; isocyanate group-containing silanes such as γ-isocyanatepropyltrimethoxysilane, γ-isocyanatepropyltriethoxysilane, γ-isocyanatepropylmethyldiethoxysilane, γ-isocyanatepropylmethyldimethoxysilane, (isocyanatemethyl)trimethoxysilane, (isocyanatemethyl)dimethoxymethylsilane, isocyanatemethyltriethoxysilane, and isocyanatemethyldiethoxymethylsilane; ketimine type silanes such as N-(1,3-dimethylbutylidene)-3-(trimethoxysilyl)-1-propaneamine and N-(1,3-dimethylbutylidene)-3-(triethoxysilyl)-1-propaneamine; and the like. Additionally, partially condensed products of the above described silanes can be used. Further the following derivatives obtained by modifying these compounds can be used as the component (C): amino-modified silylpolymer, silylated aminopolymer, unsaturated aminosilane complex, phenylamino-long chain alkylsilane and aminosilylated silicone. It is to be noted that as described above, when the reactive silicon group of the component (A) is a methoxysilyl group represented by the general formula (5), it is essential to use the component (C) having a methoxysilyl group among those described above.
- The above described component (C) may be used alone or in a mixture of two or more kinds thereof.
- In the present invention, the used amount of the component (C) is preferably of the order of 0.1 to 20 parts by weight, and more preferably of the order of 0.5 to 10 parts by weight, especially preferably of the order of 2 to 7 parts by weight with respect to 100 parts by weight of the component (A). When the blended amount of the component (C) is less than the above described ranges, sometimes sufficient adhesion cannot be obtained. On the other hand, when the blended amount of the component (C) exceeds the above described ranges, sometimes the practical curing rate cannot be obtained, and the curing reaction hardly proceeds to a sufficient extent.
- In the present invention, a silane coupling agent other than the component (C) can be used to an extent not to degrade the effect of the present invention. Examples of these silane coupling agents are di-substituted amino group-containing silanes such as γ-(dimethylamino)propyltrimethoxysilane; mercapto group-containing silanes such as γ-mercaptopropyltrimethoxysilane, γ-mercaptopropyltriethoxysilane, γ-mercaptopropylmethyldimethoxysilane, γ-mercaptopropylmethyldiethoxysilane, mercaptomethyltrimethoxysilane and mercaptomethyltriethoxysilane; epoxy group-containing silanes such as γ-glycidoxypropyltrimethoxysilane, γ-glycidoxypropyltriethoxysilane, γ-glycidoxypropylmethyldimethoxysilane, β-(3,4-epoxycyclohexyl)ethyltrimethoxysilane and β-(3,4-epoxycyclohexyl)ethyltriethoxysilane; carboxysilanes such as β-carboxyethyltriethoxysilane, β-carboxyethylphenylbis(2-methoxyethoxy)silane and N-β-(carboxymethyl)aminoethyl-γ-aminopropyltrimethoxysilane; vinyl-type unsaturated group-containing silanes such as vinyltrimethoxysilane, vinyltriethoxysilane, γ-methacryloyloxypropylmethyldimethoxysilane, γ-acryloyloxypropyltriethoxysilane and methacryloyloxymethyltrimethoxysilane; halogen-containing silanes such as γ-chloropropyltrimethoxysilane; isocyanurate silanes such as tris(3-trimethoxysilylpropyl)isocyanurate, and the like. Additionally, compounds (for example, a compound of epoxysilane and aminosilane, a compound of isocyanatesilane and aminosilane, and the like) obtained by reacting the above described silane coupling agents with each other can be used.
- In addition to the above described component (C) and other silane coupling agents, for example, epoxy resins, phenolic resins, sulfur, alkyl titanates, aromatic polyisocyanates and the like can be used as an adhesion-imparting agent. The adhesion-imparting agent is not limited particularly to them. The above described adhesion-imparting agents may be used alone or in a mixture of two or more thereof.
- In the present invention, as the curing catalyst, the component (B) is used, and other curing catalysts can be simultaneously used to an extent not to degrade the effect of the present invention. Examples of the curing catalyst include metal salts of carboxylic acids such as tin 2-ethylhexanoate, tin versatate and bismuth 2-ethylhexanoate; tetravalent organotin compounds such as dibutyltin dilaurate, dibutyltin maleate, dibutyltin phthalate, dibutyltin dioctanoate, dibutyltin bis(2-ethylhexanoate), dibutyltin bis(methylmaleate), dibutyltin bis(ethylmaleate), dibutyltin bis(butylmaleate), dibutyltin bis(octylmaleate), dibutyltin bis(tridecylmaleate), dibutyltin bis(benzylmaleate), dibutyltin diacetate, dioctyltin bis(ethylmaleate), dioctyltin bis(octylmaleate), dibutyltin dimethoxide, dibutyltin bis(nonylphenoxide), dibutyltin oxide, dibutenyltin oxide, dibutyltin bis(acetylacetonate), dibutyltin bis(ethylacetoacetate), a reaction product of dibutyltin oxide and a silicate compound, and a reaction product of dibutyltin oxide and a phthalic acid ester; organoaluminum compounds such as aluminum tris(acetylacetonate), aluminum tris(ethylacetoacetate) and diisopropoxyaluminumethylacetoacetate; and zirconium compounds such as zirconium tetrakis(acetylacetonate). However, depending on an adding amount of the organotin compound, there is a case where toxicity of the obtained curing composition becomes strong.
- A filler can be added to the composition of the present invention. Examples of the fillers include reinforcing fillers such as fumed silica, precipitated silica, crystalline silica, fused silica, dolomite, anhydrous silicic acid, hydrous silicic acid and carbon black; fillers such as ground calcium carbonate, precipitated calcium carbonate, magnesium carbonate, diatomite, sintered clay, clay, talc, titanium oxide, bentonite, organic bentonite, ferric oxide, aluminum fine powder, flint powder, zinc oxide, active zinc white, shirasu balloon, glass microballoon, organic microballoons of phenolic resin and vinylidene chloride resin, and resin powders such as PVC powder and PMMA powder; and fibrous fillers such as asbestos, glass fiber and glass filament. When a filler is used, the used amount thereof is 1 to 250 parts by weight, and preferably 10 to 200 parts by weight with respect to 100 parts by weight of the polymer of the component (A).
- As described in Japanese Patent Laid-Open No. 2001-181532, it is possible to homogeneously mix the above described filler with a dehydrating agent such as oxidized calcium, put the mixture in a bag made of an air-tight material to be sealed and then allow to stand for a proper period of time to dehydrate and dry previously. The use of this low molecular weight filler makes it possible to improve storage stability particularly in the case of one-component type composition.
- Additionally, when preparing a highly transparent composition, as described in Japanese Patent Laid-Open No. 11-302527, it is possible to use, as a filler, a polymer powder prepared by using a polymer such as methyl methacrylate as a starting material or a non-crystalline silica. Also as described in Japanese Patent Laid-Open No. 2000-38560, a highly transparent composition can be obtained by using, as a filler, a hydrophobic silica which is a fine powder of silicon dioxide having hydrophobic groups bonded to the surface thereof. The surface of a fine powder of silicon dioxide has generally silanol groups (—SiOH), but can be formed into a hydrophobic silica by reacting those silanol groups with halides of organosilicon or alcohols to produce —SiO— hydrophobic group. Specifically, the hydrophobic silica is obtained by reacting silanol groups being present in the surface of the fine powder of silicon dioxide with dimethylsiloxane, hexamethyldisilazane, dimethyldichlorosilane, trimethoxyoctylsilane, trimethylsilane or the like. A fine powder of silicon dioxide in which the surface thereof is formed by silanol groups (—SiOH) is called a hydrophilic fine powder of silica.
- When it is desired to obtain a cured article high in strength by use of these fillers, preferable is a filler mainly selected from fumed silica, precipitated silica, crystalline silica, fused silica, dolomite, anhydrous silicic acid, hydrous silicic acid, carbon black, surface treated fine calcium carbonate, sintered clay, clay and active zinc white; a desirable effect is obtained when such a filler is used within a range from 1 to 200 parts by weight with respect to 100 parts by weight of the reactive silicone group-containing organic polymer (A). Additionally, when it is desired to obtain a cured article low in tensile strength and large in elongation at break, a desirable effect is obtained by use of a filler mainly selected from titanium oxide, calcium carbonate such as ground calcium carbonate and magnesium carbonate, talc, ferric oxide, zinc oxide and shirasu balloon within a range from 5 to 200 parts by weight with respect to 100 parts by weight of the reactive silicone group-containing organic polymer (A). It is to be noted that in general, the calcium carbonate exhibits, with increasing specific surface area value thereof, an increasing improvement effect of the tensile strength at break, elongation at break and adhesion of the cured article. Needless to say, these fillers may be used either each alone or in admixtures of two or more thereof. When calcium carbonate is used, it is desirable to use surface treated fine calcium carbonate in combination with calcium carbonate larger in particle size such as ground calcium carbonate. The particle size of surface treated fine calcium carbonate is preferably not more than 0.5 μm, and the surface treatment is preferably carried out by treating with a fatty acid or a fatty acid salt. The calcium carbonate larger in particle size is preferably not less than 1 μm in particle size, and can be used without being subjected to surface treatment.
- For the purpose of improving the workability (cutting property, etc) of the composition and deglossing the surface of the cured article, organic balloons and inorganic balloons are preferably added. Such fillers can be subjected to surface treatment, and may be used each alone or can be used in admixtures of two or more thereof. For the purpose of improving the workability (cutting property, etc), the particle sizes of these balloons are preferably not more than 0.1 mm. For the purpose of deglossing the surface of the cured article, the particle sizes are preferably 5 to 300 μm.
- On the grounds that the cured article of the composition of the present invention is satisfactory in chemical resistance and the like, the composition of present invention is suitably used for joints of housing exterior wall such as sizing boards, in particular, ceramic sizing boards, for an adhesive for exterior wall tiles, for an adhesive for exterior wall tiles remaining in the joints and for the like purposes; in this connection, it is desirable that the design of the exterior wall and the design of the sealant are in harmony with each other. Particularly, posh exterior walls have come to be used by virtue of sputter coating and mixing colored aggregates. When the composition of the present invention is blended with a scale-like or granulated material having a diameter of not less than 0.1 mm, preferably of the order of 0.1 to 5.0 mm, the cured article comes to be in harmony with such posh exterior walls, and is excellent in chemical resistance, so that the composition concerned comes to be an excellent composition in the sense that the exterior appearance of the cured article remains unchanged over a long period of time. Use of a granulated material provides a dispersed sand-like or sandstone-like surface with a rough texture, while use of a scale-like material provides an irregular surface based on the scale-like shape of the material.
- The preferable diameter, blended amount and materials for the scale-like or granulated material are described in Japanese Patent Laid-Open No. 9-53063 as follows.
- The diameter is not less than 0.1 mm, preferably of the order of 0.1 to 5.0 mm, and there is used a material having an appropriate size in conformity with the material quality and pattern of exterior wall. Materials having a diameter of the order of 0.2 mm to 5.0 mm and materials having a diameter of the order of 0.5 mm to 5.0 mm can also be used. In the case of a scale-like material, the thickness is set to be as thin as the order of 1/10 to ⅕ the diameter (the order of 0.01 to 1.00 mm). The scale-like or granulated material is transported to the construction site as a sealant on condition that the material is beforehand mixed in the main component of the sealant, or is mixed in the main component of the sealant at the construction site when the sealant is used.
- The scale-like or granulated material is blended in a content of the order of 1 to 200 parts by weight with respect to 100 parts by weight of a composition such as a sealant composition and an adhesive composition. The blended amount is appropriately selected depending on the size of the scale-like or granulated material, and the material quality and pattern of exterior wall.
- As the scale-like or granulated material, natural products such as silica sand and mica, synthetic rubbers, synthetic resins and inorganic substances such as alumina are used. The material is colored in an appropriate color so as to match the material quality and pattern of exterior wall to heighten the design quality when filled in the joints.
- A preferable finishing method and the like are described in Japanese Patent Laid-Open No. 9-53063.
- Additionally, when a balloon (preferably the mean particle size thereof is not less than 0.1 mm) is used for a similar purpose, the surface is formed to have a dispersed sand-like or sandstone-like surface with a rough texture, and a reduction of weight can be achieved. The preferable diameter, blended amount and materials for the balloon are described in Japanese Patent Laid-Open No. 10-251618 as follows.
- The balloon is a sphere-shaped material with a hollow interior. Examples of the material for such a balloon include inorganic materials such as glass, shirasu and silica; and organic materials such as phenolic resin, urea resin, polystyrene and Saran™; however, the material concerned is not limited to these examples; an inorganic material and an organic material can be compounded, or can be laminated to form multiple layers. An inorganic balloon, an organic balloon, a balloon made of a compounded inorganic-organic material or the like can be used. Additionally, as a balloon to be used, either a type of balloon or an admixture of multiple types of balloons can be used. Moreover, a balloon with the processed surface thereof or with the coated surface thereof can be used, and additionally, a balloon with the surface thereof subjected to treatment with various surface treatment agents can also be used. More specifically, examples are an organic balloon coated with calcium carbonate, talc, titanium oxide and the like, and an inorganic balloon subjected to surface treatment with a silane coupling agent.
- For the purpose of obtaining a dispersed sand-like or sandstone-like surface with a rough texture, the particle size of the balloon is preferably not less than 0.1 mm. A balloon of a particle size of the order of 0.2 mm to 5.0 mm or a balloon of a particle size of the order of 0.5 mm to 5.0 mm can also be used. Use of a balloon of a particle size of less than 0.1 mm sometimes only increases the viscosity of the composition, and yields no rough texture, even when the used amount of the balloon is large. The blended amount of the balloon can be easily determined in conformity with the desired degree of the dispersed sand-like or sandstone-like rough texture. Usually, it is desirable that a balloon of not less than 0.1 mm in particle size is blended in a ratio of 5 to 25 vol % in terms of the volume concentration in the composition. When the volume concentration of the balloon is less than 5 vol %, no rough texture can be obtained, while when the volume concentration of the balloon exceeds 25 vol %, the viscosity of the sealant and that of the adhesive tend to become high to degrade the workability, and the modulus of the cured article becomes high, so that the basic performance of the sealant and that of the adhesive tend to be impaired. The preferable volume concentration to balance with the basic performance of the sealant is 8 to 22 vol %.
- When a balloon is used, there can be added an antislip agent described in Japanese Patent Laid-Open No. 2000-154368 and an amine compound to make irregular and degloss the surface of the cured article as described in Japanese Patent Laid-Open No. 2001-164237, in particular, a primary amine and/or a secondary amine having a melting point of not less than 35° C.
- Specific examples of the balloon are described in the following publications: Japanese Patent Laid-Open Nos. 2-129262, 4-8788, 4-173867, 5-1225, 7-113073, 9-53063, 10-251618, 2000-154368 and 2001-164237, and WO97/05201 pamphlet.
- Additionally, thermally expandable fine hollow particles disclosed in Japanese Patent Laid-Open No. 2004-51701 or 2004-66749 can be used. The thermally expandable fine hollow particles are plastic spherical particulates produced by surrounding a low boiling point compound such as a hydrocarbon having 1 to 5 carbon atoms with a high molecular weight shell material (vinylidene chloride copolymer, acrylonitrile copolymer or vinylidene chloride-acrylonitrile copolymer). When the adhered portion obtained by using the composition of the present invention is heated, gas pressure inside the shell of the thermally expandable fine hollow particles is increased and the high molecular weight shell material is softened to markedly expand its volume and functions to cause peeling at the adhesion interface. By the addition of the thermally expandable fine hollow particles, it is possible to obtain an adhesive composition which can be peeled easily only by heating without breakage of material when adhesion becomes unnecessary and also can be peeled by heating without using an organic solvent.
- When the composition of the present invention includes the particles of the cured article derived from a sealant, the cured article can make irregularities on the surface to improve the design quality. The preferable diameter, blended amount and materials of the cured article particle material derived from a sealant is described in Japanese Patent Laid-Open No. 2001-115142 as follows. The diameter is preferably of the order of 0.1 mm to 1 mm, and further preferably of the order of 0.2 to 0.5 mm. The blended amount is preferably 5 to 100 wt %, and further preferably 20 to 50 wt % in the curable composition. Examples of the materials include urethane resin, silicone, modified silicone and polysulfide rubber. No constraint is imposed on the materials as long as the materials can be used as sealants; however, modified silicone sealants are preferable.
- To the composition of the present invention can be added an adhesion-imparting agent. No particular constraint is imposed on the adhesion-imparting resins, and usual adhesion-imparting resins can be used irrespective of solid form or liquid form at ordinary temperature. Specific examples of such adhesion-imparting resins include styrene block copolymers, hydrogenated products thereof, phenolic resins, modified phenolic resins (for example, cashew oil-modified phenolic resins, tall oil-modified phenolic resins and the like), terpene phenolic resins, xylene-phenol resins, cyclopentadiene-phenol resins, cumarone-indene resins, rosin resins, rosin ester resins, hydrogenated rosin ester resins, xylene resins, low molecular weight polystyrene resins, styrene copolymer resins, petroleum resins (for example, C5 hydrocarbon resin, C9 hydrocarbon resin, C5C9 hydrocarbon copolymer resin and the like), hydrogenated petroleum resins, terpene resins, DCPD resin-petroleum resin and the like. Those resins may be used either each alone or in combinations of two or more thereof. Examples of the styrene block copolymers and hydrogenated products thereof include styrene-butadiene-styrene block copolymer (SBS), styrene-isoprene-styrene block copolymer (SIS), styrene-ethylenebutylene-styrene block copolymer (SEBS), styrene-ethylenepropylene-styrene block copolymer (SEPS), styrene-isobutylene-styrene block copolymer (SIBS) and the like. The above described adhesion-imparting resins may be used either each alone or in combinations of two or more thereof.
- The adhesion-imparting resins are used within a range from 5 to 1,000 parts by weight, preferably from 10 to 100 parts by weight with respect to 100 parts by weight of the organic polymer (A).
- To the composition of the present invention can be added a solvent or a diluent. No particular constraint is imposed on the solvent or the diluent, and aliphatic hydrocarbon, aromatic hydrocarbon, alicyclic hydrocarbon, halogenated hydrocarbon, alcohol, ester, ketone, ether and the like can be used. When a solvent or a diluent is used, the boiling point of the solvent is preferably not less than 150° C., more preferably not less than 200° C., particularly preferably not less than 250° C. from the viewpoint of a problem with contamination to the air when the composition is used indoor. These solvents or diluents may be used either each alone or in combinations of two or more thereof.
- A plasticizer can be added to the composition of the present invention. Addition of a plasticizer makes it possible to adjust the viscosity and slump property of the curable composition and the mechanical properties such as tensile strength and elongation of the cured article obtained by curing the composition. Examples of the plasticizer include phthalates such as dibutyl phthalate, diheptyl phthalate, bis(2-ethylhexyl)phthalate, diisodecy phthalate, diundecyl phthalate and butyl benzyl phthalate; non-aromatic dibasic acid esters such as dioctyl adipate, dioctyl sebacate, dibutyl sebacate and diisodecyl succinate; aliphatic esters such as butyl oleate and methyl acetyl recinolate; phosphates such as tricresyl phosphate and tributyl phosphate; trimellitates; chlorinated paraffins; hydrocarbon oils such as alkyldiphenyls and partially hydrogenated terphenyls; process oils; epoxy plasticizers such as epoxidized soybean oil and benzyl epoxystearate.
- Additionally, a polymer plasticizer can be used. When a polymer plasticizer is used, there can be obtained effects of hardly causing curing retardation and improving drying property (also referred to as coating property) in the case of coating of an alkyd coating material on the cured article as compared with the case of using a low molecular weight plasticizer containing no polymer component in the molecule. Examples of the polymer plasticizer include vinyl polymers obtained by polymerizing vinyl monomers by means of various methods; polyalkylene glycol esters such as diethylene glycol dibenzoate, triethylene glycol dibenzoate and pentaerythritol ester; polyester plasticizers obtained from dibasic acids such as sebacic acid, adipic acid, azelaic acid and phthalic acid and dihydric alcohols such as ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol and dipropylene glycol; polyethers including polyether polyols each having a molecular weight of not less than 500, additionally not less than 1,000 such as polyethylene glycol, polyprolpylene glycol and polytetramethylene glycol, and the derivatives of these polyether polyols in which the hydroxy groups in these polyether polyols are substituted with acyl groups such as acetoxy groups, alkoxy groups such as methoxy groups, ethoxy groups, butoxy groups and allyloxy groups and the like; polystyrenes such as polystyrene and poly-α-methylstyrene; and polybutadiene, polybutene, polyisobutylene, butadiene-acrylonitrile and polychloroprene. However, the polymer plasticizer concerned is not limited to these examples.
- Of these polymer plasticizers, the polymer plasticizers which are compatible with the polymer of component (A) are preferable. In this regard, polyethers and vinyl polymers are preferable. Additionally, when polyethers are used as plasticizers, the surface curability, deep-part curability and adhesion are improved, and no curing retardation after storage occurs, and hence polyethers are preferable; of polyethers, polypropylene glycol is more preferable. Also it is preferable to use polymer plasticizers; polyether polyols such as polyethylene glycol and polypropylene glycol in which hydroxyl groups thereof are converted to alkoxy groups or the like because storage stability is increased. Additionally, from the viewpoint of the compatibility, weather resistance and heat resistance, vinyl polymers are preferable. Of the vinyl polymers, acryl polymers and/or methacryl polymers are preferable, and acryl polymers such as polyalkylacrylate are further preferable. As the polymerization method to produce acryl polymers, the living radical polymerization method is preferable because this method can lead to narrow molecular weight distributions and low viscosities, and the atom transfer radical polymerization method is further preferable. Additionally, it is preferable to use a polymer based on the so-called SGO process which is obtained by continuously block-polymerizing an alkyl acrylate monomer at a high temperature under a high pressure, as described in Japanese Patent Laid-Open No. 2001-207157.
- The number average molecular weight of the polymer plasticizer is preferably 500 to 15,000, more preferably 800 to 10,000, further preferably 1,000 to 8,000, particularly preferably 1,000 to 5,000, and most preferably 1,000 to 3,000. When the molecular weight is too low, the plasticizer is removed with time due to heat and by rainfall, and hence it is made impossible to maintain the initial physical properties over a long period of time, contamination is caused due to cohesion of dusts, and the coating property with an alkyd coating material cannot be improved. On the other hand, when the molecular weight is too high, the viscosity becomes high and the workability is degraded. No particular constraint is imposed on the molecular weight distribution of the polymer plasticizer. However, it is preferable that the molecular weight distribution is narrow; the molecular weight distribution is preferably less than 1.80, more preferably not more than 1.70, further preferably not more than 1.60, yet further preferably not more than 1.50, particularly preferably not more than 1.40 and most preferably not more than 1.30.
- The number average molecular weight of a polyether polymer is measured with the terminal group analysis method, and that of other polymers is measured with the GPC method. Additionally, the molecular weight distribution (Mw/Mn) is measured with the GPC method (relative to polystyrene standard).
- Additionally, the polymer plasticizer either may have no reactive silicon group or may have a reactive silicon group. When the polymer plasticizer has a reactive silicon group, the polymer plasticizer acts as a reactive plasticizer, and can prevent the migration of the plasticizer from the cured article. When the polymer plasticizer has a reactive silicon group, the average number of reactive silicon groups per molecule is not more than 1, and preferably not more than 0.8. When the reactive silicon group-containing plasticizer, in particular, a reactive silicon group-containing oxyalkylene polymer is used, it is necessary that the number average molecular weight thereof is lower than that of the organic polymer (A).
- The plasticizers may be used either each alone or in combinations of two or more thereof. Additionally, a low molecular weight plasticizer and a polymer plasticizer may be used in combination. It is to be noted that these plasticizers can also be blended when the polymer is produced.
- The used amount of the plasticizer is 5 to 150 parts by weight, preferably 10 to 120 parts by weight, and further preferably 20 to 100 parts by weight, with respect to 100 parts by weight of the polymer of the component (A). When the used amount is less than 5 parts by weight, the effect as the plasticizer is not exhibited, while when the used amount exceeds 150 parts by weight, the mechanical strength of the cured article is insufficient.
- To the curable composition of the present invention, according to need, a physical properties-regulating agent for adjusting tensile properties of a produced cured article may be added. No particular constraint is imposed on the physical properties-regulating agent. Examples thereof include tetraalkoxysilanes (tetraalkylsilicates) such as tetramethoxysilane, tetraethoxysilane, ethoxytrimethoxysilane, dimethoxydiethoxysilane, methoxytriethoxysilane, tetra-n-propoxysilane, tetra-1-propoxysilane, tetra-n-butoxysilane, tetra-1-butoxysilane and tetra-t-butoxysilane; trialkoxysilanes such as methyltrimethoxysilane, methyltriethoxysilane, methyltriisopropoxysilane, methyltriphenoxysilane, ethyltrimethoxysilane, butyltrimethoxysilane and phenyltrimethoxysilane; dialkoxysilanes such as dimethyldimethoxysilane, diethyldimethoxysilane and diphenyldimethoxysilane; monoalkoxysilanes such as trimethylmethoxysilane and triphenylmethoxysilane; alkylisopropenoxysilanes such as dimethyldiisopropenoxysilane and methyltriisopropenoxysilane; the partially hydrolyzed condensates of these silanes; silicone varnishes; polysiloxanes; and the like. By the use of the above described physical properties-regulating agent, when the composition of the present invention is cured, a hardness can be increased or reversely can be decreased and an elongation at break can be increased. The above described physical properties-regulating agent may be used alone or in a mixture of two or more kinds thereof. Additionally, by adding partially hydrolyzed condensates of tetraalkoxysilanes and alkoxysilanes, there can be obtained effects of enhancing water resistant adhesion of the curable composition and enhancing recovery properties of the obtained cured article.
- Partially hydrolyzed condensates of organosilicate compounds are commercially available. Examples of such condensates include Methyl silicate 51 and Ethyl silicate 40 (both are products of Colcoat Co., Ltd.).
- The used amount of the physical properties-regulating agent is preferably 0.1 to 20 parts by weight, and more preferably 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- To the curable composition of the present invention may be added, as case demands, a compound to hydrolytically produce a compound having a monovalent silanol group in the molecule thereof. This compound has an effect of decreasing the modulus of the cured article without degrading the stickiness of the surface of the cured article. Particularly, a compound to produce trimethylsilanol is preferable. Examples of the compound to hydrolytically produce a compound having a monovalent silanol group in the molecule thereof include a compound described in Japanese Patent Laid-Open No. 5-117521. Additionally, examples of such a compound include a compound which is a derivative of an alkyl alcohol such as hexanol, octanol or decanol, and produces a silicon compound to hydrolytically produce R3SiOH such as trimethylsilanol, and a compound described in Japanese Patent Laid-Open No. 11-241029 which is a derivative of a polyhydric alcohol having three or more hydroxy groups such as trimethylolpropane, glycerin, pentaerythritol or sorbitol, and produces a silicon compound to hydrolytically produce R3SiOH such as trimethylsilanol.
- Additionally, there can be cited such a compound as described in Japanese Patent Laid-Open No. 7-258534 which is a derivative of oxypropylene polymer and produces a silicon compound to hydrolytically produce R3SiOH such as trimethylsilanol. Moreover, there can be used a polymer described in Japanese Patent Laid-Open No. 6-279693 which contains a hydrolyzable silicon-containing group capable of cross-linking and a silicon-containing group capable of hydrolytically forming a monosilanol-containing compound.
- The compound to hydrolytically produce a compound having a monovalent silanol group in the molecule thereof is used within a range from 0.1 to 20 parts by weight, and preferably from 0.5 to 10 parts by weight, with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- To the curable composition of the present invention, according to need, a thixotropy providing agent (antisagging agent) may be added for the purpose of preventing sagging and improving workability. No particular constraint is imposed on the antisagging agent; however, examples of the antisagging agent include polyamide waxes; hydrogenated castor oil derivatives; and metal soaps such as calcium stearate, aluminum stearate and barium stearate. Additionally, when a rubber powder having a particle size of 10 to 500 μm as described in Japanese Patent Laid-Open No. 11-349916, and an organic fiber as described in Japanese Patent Laid-Open No. 2003-155389 are used, a composition having high thixotropy and satisfactory workability can be obtained. These thixotropy providing agents (antisagging agents) may be used either each alone or in combinations of two or more thereof. The thixotropy providing agents each are used within a range from 0.1 to 20 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- In the composition of the present invention, a compound containing an epoxy group in one molecule can be used. Use of an epoxy group-containing compound can increase the recovery properties of the cured article. Examples of the epoxy group-containing compound include compounds such as epoxidized unsaturated oils and fats, epoxidized unsaturated fatty acid esters, alicyclic epoxy compounds and epichlorohydrin derivatives, and admixtures of these compounds. More specific examples include epoxidized soybean oil, epoxidized flaxseed oil, bis(2-ethylhexyl)-4,5-epoxycyclohexane-1,2-dicarboxylate (E-PS), epoxyoctyl stearate and epoxybutyl stearate. Of these, E-PS is particularly preferable. It is recommended that these epoxy group-containing compounds each are used within a range from 0.5 to 50 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- For the composition of the present invention, a photocuring substance can be used. Use of a photocuring substance forms a coating film of the photocuring substance on the surface of the cured article to improve the stickiness and the weather resistance of the cured article. A photocuring substance means a substance which undergoes a chemical change, caused by action of light, of the molecular structure thereof in a fairly short time to result in changes of the physical properties such as curability. Among such a large number of compounds known are organic monomers, oligomers, resins and compositions containing these substances, and any commercially available substances concerned can optionally be adopted. As representative photocuring substances, unsaturated acryl compounds, polyvinyl cinnamates, azidized resins and the like can be used. The unsaturated acryl compounds are monomers, oligomers and admixtures of the monomers and the oligomers, the monomers and oligomers each having one or a few acryl or methacryl unsaturated groups; examples of the unsaturated acryl compounds include monomers such as propylene (or butylene, or ethylene)glycol di(meth)acrylate and neopentylglycol di(meth)acrylate, and oligoesters of not more than 10,000 in molecular weight related to these monomers. Specific examples include special acrylates (bifunctional) such as ARONIX M-210, ARONIX M-215, ARONIX M-220, ARONIX M-233, ARONIX M-240 and ARONIX M-245; special acrylates (trifunctional) such as ARONIX M-305, ARONIX M-309, ARONIX M-310, ARONIX M-315, ARONIX M-320 and ARONIX M-325; and special acrylates (multifunctional) such as ARONIX M-400. Those compounds which each have acrylic functional groups are particularly preferable, and additionally, those compounds which each have, on average, three or more acrylic functional groups in one molecule are preferable (all the aforementioned ARONIXs are the products of Toagosei Co., Ltd.).
- Examples of the polyvinyl cinnamates include photosensitive resins having cinnamoyl groups as photosensitive groups, namely, those compounds obtained by esterification of polyvinyl alcohol with cinnamic acid; and additionally, a large number of derivatives of polyvinyl cinnamates. Azidized resins are known as photosensitive resins having azide groups as photosensitive groups; common examples of the azidized resins include a rubber photosensitive solution added with an azide compound as a photosensitive agent, and additionally, the compounds detailed in “photosensitive resins” (published by Insatu Gakkai Shuppanbu, Mar. 17, 1972, p. 93, p. 106 and p. 117); and these compounds can be used each alone or in admixtures thereof, and in combination with sensitizers to be added according to need. It is to be noted that addition of sensitizers such as ketones and nitro compounds and accelerators such as amines sometimes enhances the effect. It is recommended that the photocuring substance is used within a range from 0.1 to 20 parts by weight and preferably from 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A); when the content of the photocuring substance is less than 0.1 part by weight, no effect to increase the weather resistance is displayed, while when the content exceeds 20 parts by weight, the cured article tends to be too hard and cracked.
- For the composition of the present invention, an oxygen-curable substance can be used. Examples of the oxygen-curable substance include unsaturated compounds reactable with the oxygen in the air, which react with the oxygen in the air and form a cured coating film around the surface of the cured article to act to prevent the surface stickiness and the sticking of dust and grime to the surface of the cured article and to do the like. Specific examples of the oxygen-curable substance include drying oils represented by wood oil, flaxseed oil and the like and various alkyd resins obtained by modifying these compounds; acryl polymers, epoxy resins and silicon resins all modified with drying oils; liquid polymers such as 1,2-polybutadiene and 1,4-polybutadiene obtained by polymerizing or copolymerizing diene compounds such as butadiene, chloroprene, isoprene and 1,3-pentadiene, and polymers derived from C5 to C8 dienes, liquid copolymers such as NBR, SBR and the like obtained by copolymerizing these diene compounds with monomers such as acrylonitrile, styrene and the like having copolymerizability so as for the diene compounds to dominate, and various modified substances of these compounds (maleinized modified substances, boiled oil-modified substances, and the like). These substances can be used either each alone or in combinations of two or more thereof. Of these substances, wood oil and liquid diene polymers are particularly preferable. Additionally, in some cases, when catalysts to accelerate the oxidation curing reaction and metal dryers are used in combination with these substances, the effect is enhanced. Examples of these catalysts and metal dryers include metal salts such as cobalt naphthenate, lead naphthenate, zirconium naphthenate, cobalt octylate and zirconium octylate; and amine compounds. It is recommended that the oxygen-curing substance is used within a range from 0.1 to 20 parts by weight and further preferably from 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A); when the used amount is less than 0.1 part by weight, improvement of stain-proof property becomes insufficient, while when the used amount exceeds 20 parts by weight, the tensile property and the like of the cured article tends to be impaired. It is recommended that the oxygen-curing substance is used in combination with a photocuring substance as described in Japanese Patent Laid-Open No. 3-160053.
- For the composition of the present invention, an antioxidant (antiaging agent) can be used. Use of an antioxidant can increase the heat resistance of the cured article. Examples of the antioxidant can include hindered phenol antioxidants, monophenol antioxidants, bisphenol antioxidants and polyphenol antioxidants, and hindered phenol antioxidants are particularly preferable. Similarly, the following hindered amine photostabilizers can also be used: TINUVIN 622LD, TINUVIN 144; CHIMASSORB944LD and CHIMASSORB119FL (all produced by Ciba-Geigy Japan Ltd.); MARK LA-57, MARK LA-62, MARK LA-67, MARK LA-63 and MARK LA-68 (all produced by Adeka Corporation); and SANOL LS-770, SANOL LS-765, SANOL LS-292, SANOL LS-2626, SANOL LS-1114 and SANOL LS-744 (all produced by Sankyo Co., Ltd.). Specific examples of the antioxidants are described also in Japanese Patent Laid-Open Nos. 4-283259 and 9-194731. It is recommended that the antioxidant is used within a range from 0.1 to 10 parts by weight and further preferably from 0.2 to 5 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- For the composition of the present invention, a photostabilizer can be used. Use of a photostabilizer can prevent the photooxidation degradation of the cured article. Examples of the photostabilizer include benzotriazole compounds, hindered amine compounds, benzoate compounds and the like; hindered amine compounds are particularly preferable. It is recommended that the photostabilizer is used within a range from 0.1 to 10 parts by weight and further preferably from 0.2 to 5 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A). Specific examples of the photostabilizer are described in Japanese Patent Laid-Open No. 9-194731.
- When the photocuring substance is used for the composition of the present invention, in particular, when an unsaturated acryl compound is used, it is preferable to use a tertiary amine-containing hindered amine photostabilizer as a hindered amine photostabilizer as described in Japanese Patent Laid-Open No. 5-70531 for the purpose of improving the storage stability of the composition. Examples of the tertiary amine-containing hindered amine photostabilizer include TINUVIN 622LD, TINUVIN 144 and CHIMASSORB119FL (all produced by Ciba-Geigy Japan Ltd.); MARK LA-57, LA-62, LA-67 and LA-63 (all produced by Adeka Corporation); and SANOL LS-765, SANOL LS-292, SANOL LS-2626, SANOL LS-1114 and SANOL LS-744 (all produced by Sankyo Co., Ltd.).
- For the composition of the present invention, an ultraviolet absorber can be used. Use of an ultraviolet absorber can increase the surface weather resistance of the cured article. Examples of the ultraviolet absorber include benzophenone compounds, benzotriazole compounds, salicylate compounds, substituted tolyl compounds and metal chelate compounds; benzotriazole compounds are particularly preferable. The ultraviolet absorber is used within a range from 0.1 to 10 parts by weight, and further preferably from 0.2 to 5 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A). It is preferable that a phenol antioxidant, a hindered phenol antioxidant, a hindered amine photostabilizer and a benzotriazole ultraviolet absorber are used in combination.
- To the composition of the present invention, an epoxy resin can be added. The composition added with an epoxy resin is particularly preferable as an adhesive, in particular, an adhesive for exterior wall tile. Examples of the epoxy resin include epichlorohydrin-bisphenol A-type epoxy resins, epichlorohydrin-bisphenol F-type epoxy resins, flame resistant epoxy resins such as glycidyl ether of tetrabromobisphenol A, novolac-type epoxy resins, hydrogenated bisphenol A-type epoxy resins, epoxy resins of the type of glycidyl ether of bisphenol A propyleneoxide adduct, p-oxybenzoic acid glycidyl ether ester-type epoxy resins, m-aminophenol epoxy resins, diaminodiphenylmethane epoxy resins, urethane modified epoxy resins, various alicyclic epoxy resins, N,N-diglycidylaniline, N,N-diglycidyl-o-toluidine, triglycidyl isocyanurate, polyalkylene glycol diglycidyl ether, glycidyl ethers of polyhydric alcohols such as glycerin, hydantoin-type epoxy resins and epoxidized substances of unsaturated polymers such as petroleum resins; however the epoxy resin is not limited to these examples, and commonly used epoxy resins can be used. Epoxy resins having at least two epoxy groups in one molecule are preferable because such compositions are high in reactivity when curing is made, and the cured articles can easily form three dimensional networks. Examples of further preferable epoxy resins include bisphenol A-type epoxy resins or novolac-type epoxy resins. The ratio of the used amount of each of these epoxy resins to the used amount of the reactive silicon group-containing organic polymer (A) falls, in terms of weight ratio, in the range such that organic polymer (A)/epoxy resin=100/1 to 1/100. When the ratio of organic polymer (A)/epoxy resin is less than 1/100, the effect of improving the impact strength and the toughness of the cured article of the epoxy resin becomes hardly obtainable, while when the ratio of organic polymer (A)/epoxy resin exceeds 100/1, the strength of the cured article of the organic based polymer becomes insufficient. The preferable ratio of the used amounts varies depending on the application of the curable resin composition and hence cannot be unconditionally determined; for example, when the impact resistance, flexibility, toughness, peel strength and the like of the cured article of the epoxy resin are to be improved, it is recommended that with respect to 100 parts by weight of the epoxy resin, 1 to 100 parts by weight of the component (A), further preferably 5 to 100 parts by weight of the component (A) is used. On the other hand, when the strength of the cured article of the component (A) is to be improved, it is recommended that with respect to 100 parts of the component (A), 1 to 200 parts by weight of the epoxy resin, further preferably 5 to 100 parts by weight of the epoxy resin is used.
- When the epoxy resin is added, as a matter of course, a curing agent to cure the epoxy resin can be applied together to the composition of the present invention. No particular constraint is imposed on the usable epoxy resin-curing agent, and commonly used epoxy resin-curing agents can be used. Specific examples of the epoxy resin-curing agent include primary and secondary amines such as triethylenetetramine, tetraethylenepentamine, diethylaminopropylamine, N-aminoethylpiperidine, m-xylylenediamine, m-phenylenediamine, diaminodiphenylmethane, diaminodiphenylsulfone, isophoronediamine, and polyether with amine terminal groups; tertiary amines such as 2,4,6-tris(dimethylaminomethyl)phenol and tripropylamine, and salts of those tertiary amines; polyamide resins; imidazoles; dicyandiamides; borontrifluoride complexes; carboxylic anhydrides such as phthalic anhydride, hexahydrophthalic anhydride, tetrahydrophthalic anhydride, dodecynylsuccinic anhydride, pyromellitic anhydride and chlorendic anhydride; alcohols; phenols; carboxylic acids; and diketone complexes of aluminum and zirconium. However, the epoxy resin-curing agent is not limited to these examples. Additionally, the curing agents may be used either each alone or in combinations of two or more thereof.
- When an epoxy resin-curing agent is used, the used amount thereof falls within a range from 0.1 to 300 parts by weight with respect to 100 parts by weight of the epoxy resin.
- As an epoxy resin-curing agent, a ketimine can be used. A ketimine is stable when no moisture is present, but moisture decomposes the ketimine into a primary amine and a ketone; the thus produced primary amine acts as a room temperature curable curing agent to cure the epoxy resin. Use of a ketimine makes it possible to obtain a one-component type composition. Such a ketimine can be obtained by condensation reaction of an amine compound and a carbonyl compound.
- For the synthesis of a ketimine, an amine compound and a carbonyl compound well known in the art can be used. For example, the following compounds can be used as such an amine compound: diamines such as ethylenediamine, propylenediamine, trimethylenediamine, tetramethylenediamine, 1,3-diaminobutane, 2,3-diaminobutane, pentamethylenediamine, 2,4-diaminopentane, hexamethylenediamine, p-phenylenediamine and p,p′-biphenylenediamine; polyvalent amines such as 1,2,3-triaminopropane, triaminobenzene, tris(2-amionoethyl)amine and tetrakis(aminomethyl)methane; polyalkylenepolyamines such as diethylenetriamine, triethylenetriamine and tetraethylenepentamine; polyoxyalkylene polyamines; and aminosilanes such as γ-aminopropyltriethoxysilane, N-(β-aminoethyl)-γ-aminopropyltrimethoxysilane and N-(β-aminoethyl)-γ-aminopropylmethyldimethoxysilane. Additionally, the following compounds can be used as such a carbonyl compound: aldehydes such as acetaldehyde, propionaldehyde, n-butylaldehyde, isobutylaldehyde, diethylacetaldehyde, glyoxal and benzaldehyde; cyclic ketones such as cyclopentanone, trimethylcyclopentanone, cyclohexanone and trimethylcyclohexanone; aliphatic ketones such as acetone, methyl ethyl ketone, methyl propyl ketone, methyl isopropyl ketone, methyl isobutyl ketone, diethyl ketone, dipropyl ketone, diisopropyl ketone, dibutyl ketone and diisobutyl ketone; and β-dicarbonyl compounds such as acetylacetone, methyl acetoacetate, ethyl acetoacetate, dimethyl malonate, diethyl malonate, methyl ethyl malonate and dibenzoylmethane.
- When an imino group is present in the ketimine, the imino group can be reacted with styrene oxide; glycidyl ethers such as butyl glycidyl ether and allyl glycidyl ether; and glycidyl esters. These ketimines may be used either each alone or in combinations of two or more thereof; these ketimines each are used within a range from 1 to 100 parts by weight with respect to 100 parts by weight of the epoxy resin, the used amount of each of the ketimines varies depending on the type of the epoxy resin and the type of the ketimine.
- To the curable composition of the present invention can be added a phosphorous-based plasticizer such as polyphosphoric acid ammonium or tricresyl phosphate and a flame retardant such as aluminum hydroxide, magnesium hydroxide or thermally expandable graphite. These flame retardants may be used either each alone or in combinations of two or more thereof.
- The flame retardant is used within a range from 5 to 200 parts by mass, and preferably from 10 to 100 parts by mass with respect to 100 parts by weight of the component (A).
- To the curable composition of the present invention, various additives can be added according to need for the purpose of regulating the physical properties of the curable composition or the cured article. Examples of such additives include curing regulators, radical inhibitors, metal deactivators, antiozonants, phosphorus-based peroxide decomposers, lubricants, pigments, foaming agents, ant-proofing agents and mildewproofing agents. These various additives may be used either each alone or in combinations of two or more thereof. Specific examples of additives other than the specific examples cited in the present specification are described, for example, in Japanese Patent Examined Publication Nos. 4-69659 and 7-108928, Japanese Patent Laid-Open Nos. 63-254149, 64-22904, 2001-72854 and the like.
- With respect to the method of preparing the one-component type curable composition of the present invention, all the blended components are blended together beforehand, so that it is preferable that the blended components containing moisture are used after dehydrating and drying, or the components are dehydrated by reducing pressure or the like while being kneaded for blending. As for the methods of dehydration and drying, a thermal drying method is suitable for a powdery solid substance or the like, while a reduced pressure dehydration method or a dehydration method which uses a synthetic zeolite, active alumina, silica gel, quick lime or magnesium oxide is suitable for a liquid substance. Additionally, there can be adopted a method in which a small amount of an isocyanate compound is added to make its isocyanate group react with water for dehydration, or a method in which an oxazolidine compound such as 3-ethyl-2-methyl-2-(3-methylbutyl)-1,3-oxazolidine is added to make it react with water for dehydration. In addition to these dehydration and drying methods, addition of the following compounds further improves the storage stability: lower alcohols such as methanol and ethanol; and alkoxysilane compounds such as n-propyltrimethoxysilane, vinyltrimethoxysilane, vinylmethyldimethoxysilane, methylsilicate, ethylsilicate, γ-mercaptopropylmethyldimethoxysilane, γ-mercaptopropylmethyldiethoxysilane and γ-glycidoxypropyltrimethoxysilane.
- It is particularly preferable that the used amount of a dehydrating agent, in particular, a silicon compound capable of reacting with water such as vinyltrimethoxysilane falls within a range from 0.1 to 20 parts by weight, and preferably 0.5 to 10 parts by weight with respect to 100 parts by weight of the reactive silicon group-containing organic polymer (A).
- With respect to the preparation method, the components (A), (B), (C) and other additives are blended together with a mixer, a roll or a kneader, and are subjected to complete dehydration by heating under reduced pressure or the like, so that water content is decreased to an extent substantially not causing a problem. The obtained one-component type curable composition is stored in a moisture-proof sealed vessel.
- In the thus obtained one-component type curable composition of the present invention, curing does not proceed during the storing, but when taken out of the vessel and exposed to moisture in the air, the composition forms three dimensional networks to be quickly cured from its surface into a solid matter having a rubber-like elasticity.
- The curable composition of the present invention can be used as tackifiers, sealants for buildings, ships, vehicles and road, adhesives, mold forming materials, vibration-proof material, damping materials, soundproof materials, foaming materials, coating materials, spraying materials and the like. It is preferable that the cured article obtained by curing the curable composition of the present invention is used as a sealant and an adhesive because the cured article is excellent in flexibility and adhesion.
- Additionally, the curable composition of the present invention can be used in various applications as liquid sealants to be used in materials for electric and electronic components such as backside sealants for solar cells, electric insulating materials such as insulating coating materials for use in electric wire and cable, elastic adhesives, contact type adhesives, spray type sealants, crack repairing materials, adhesives for tiling, powdery coating materials, casting materials, medical rubber materials, medical adhesives, medical instrument sealants, food packaging materials, sealants for joints in exterior materials such as sizing boards, coating materials, primers, electromagnetic wave shielding conductive materials, heat conducting materials, hot melt materials, electric and electronic potting agents, films, gaskets, various molding materials, antirust and waterproof sealants for edges (cut portions) of wire glass and laminated glass, vehicle components, electric appliance components, various machinery components and the like. Moreover, the curable composition of the present invention can adhere alone or in combination with a primer to a wide variety of substrates including glass, porcelain, woods, metals and molded resin articles, and accordingly, can be used as various types of sealing and adhesive compositions. Additionally, the curable composition of the present invention can be used as adhesives for interior panels, adhesive for exterior panels, adhesives for tiling, adhesives for stone tiling, adhesives for finishing ceiling, adhesives for finishing floor, adhesives for finishing wall, adhesives for vehicle panels, adhesives for assembling electric, electronic and precision instruments, sealants for direct glazing, sealants for double glazing, sealants for the SSG technique and sealants for working joints of buildings.
- In the next place, the present invention is specifically described on the basis of examples and comparative examples, but the present invention is not limited by these examples and comparative examples.
- By use of a polyoxypropylene diol having a molecular weight of about 2,000 as an initiator and zinc hexacyanocobaltate-glyme complex as a catalyst, polymerization of propylene oxide was carried out to yield a polypropylene oxide having a number average molecular weight of about 25,500 (a molecular weight relative to polystyrene standard measured by using a HLC-8120 GPC manufactured by Tosoh Corp. as a liquid delivery system, a column of TSK-GEL H-type manufactured by Tosoh Corp., and THF as a solvent). Then, a methanol solution of NaOMe was added in an amount of 1.2 equivalents with respect to the hydroxy group of the above hydroxy group-terminated polypropylene oxide, the methanol was distilled off, and allyl chloride was further added to thereby convert the terminal hydroxy group into an allyl group. The unreacted allyl chloride was distilled off under reduced pressure. To 100 parts by weight of the obtained crude allyl-terminated polypropylene oxide, 300 parts by weight of n-hexane and 300 parts by weight of water were added. The mixture thus obtained was stirred to mix, and then the water was removed by centrifugal separation. To the hexane solution thus obtained, 300 parts by weight of water was further added, the mixture thus obtained was stirred to mix, the water was again removed by centrifugal separation, and then the hexane was distilled off under reduced pressure. Thus, an allyl group-terminated bifunctional polypropylene oxide having a number average molecular weight of about 25,500 was obtained.
- 100 Parts by weight of the obtained allyl group-terminated polypropylene oxide and 0.9 part by weight of methyldimethoxysilane were reacted at 90° C. for five hours by using, as a catalyst, 150 ppm of isopropanol solution of platinum-vinylsiloxane complex containing 3 wt % platinum to yield a polyoxypropylene polymer (A-1) terminated with methyldimethoxysilyl group. By measurement using 1H-NMR (measured in CDCl3 as a solvent by use of a JNM-LA400 manufactured by JEOL Ltd.), the number of terminal methyldimethoxysilyl groups per molecule was about 1.3 on average.
- The reactive silicon group-containing polyoxyalkylene polymer (A-1) obtained in Synthesis Example 1 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, an adhesion-imparting agent and titanium diisopropoxidebis(ethylacetoacetate) as a curing catalyst were weighed out according to the formulations shown in Table 1, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and the composition was charged in an aluminum cartridge. It is to be noted that the added amount of each adhesion-imparting agent was so set that the number of silyl groups of the respective adhesion-imparting agents became equimolar amounts.
- The curable compositions each were extruded from the cartridge and filled in a molding frame of about 5 mm in thickness with a spatula; the surface of each of the filled compositions was fully flattened, and the planarization completion time was set as the curing starting time. The surface of each of the compositions was touched every five minutes with a finger wiped with ethanol, and the tack-free time was measured as the time when the composition no longer stuck to the finger.
- Further the one-component type curable compositions each were extruded so that the compositions each were adhered to various articles to be adhered (anodized aluminum, cold rolled steel sheet, hard polyvinyl chloride, FRP, polycarbonate, acrylic resin sheet), and were aged at 23° C. for 7 days. Thereafter, a 90 degree hand peel test was carried out. The breakdown conditions of the cured articles were observed and the cohesion failure rates (CF rate) were measured.
- Also by using a cartridge containing one-component type curable composition, after storing at a constant temperature of 23° C. for 7 days (before storing) and further after storing in an oven at 50° C. for 28 days (after storing), a tension test was carried out under the following conditions, and variations of physical properties before and after storing were investigated.
- (Tension Test Method 1)
- First, the curable compositions each were filled in a sheet-like molding frame of about 3 mm in thickness, and the surface of each of the filled compositions was fully flattened, followed by aging at 23° C. for 3 days+50° C. for 4 days, to produce sheet-like cured articles. The cured articles were punched to obtain No. 3 dumbbell type test pieces (JIS K6251), and a modulus at 50% tension, a strength at break and an elongation at break (measuring environment: 23° C., tensile speed: 200 mm/min) were measured with an AUTOGRAPH produced by Shimadzu Corporation.
- Table 1 shows curability, adhesion, mechanical properties (modulus, strength, elongation) before and after storing and maintenance factor of physical properties of each curable composition. In the table, A denotes a CF rate of 100%, B denotes a CF rate of not less than 50% and less than 100%, C denotes a CF rate of not less than 10% and less than 50%, and D denotes a CF rate of less than 10%.
TABLE 1 Comparative Example Composition (parts by weight) 1 2 3 4 Organic polymer (A) A-1 100 100 100 100 Filler Hakuenka CCR (1) Shiraishi Kogyo Kaisha, Ltd. 120 120 120 120 Titanium oxide Tipaque R-820 Ishihara Sangyo Kaisha, Ltd. 20 20 20 20 Plasticizer DIDP (2) Kyowa Hakko Kogyo Co., Ltd. 55 55 55 55 Antisagging agent Disparlon #6500 (3) Kusumoto Chemicals, Ltd. 2 2 2 2 Ultraviolet absorber Sumisorb 400 (4) Sumitomo Chemical Co., Ltd. 1 1 1 1 Photostabilizer Sanol LS-765 (5) Sankyo Co., Ltd. 1 1 1 1 Antioxidant Irganox 1010 (6) Ciba-Geigy Japan Ltd. 1 1 1 1 Dehydrating agent A-171 (7) Nippon Unicar Company Limited 2 2 2 2 Adhesion-imparting A-1120 (8) Nippon Unicar Company Limited 3 agent KBE603 (9) Shin-Etsu Chemical Co., Ltd. 3.57 Y-11597 (10) Nippon Unicar Company Limited 2.8 A-189 (11) Nippon Unicar Company Limited 2.7 A-187 (12) Nippon Unicar Company Limited S340 (13) Chisso Corporation Reaction mixture of aminosilane and epoxysilane (14) Titanium catalyst Orgatix TC-750 (15) Matsumoto Chemical Industry Co., 7.5 7.5 7.5 7.5 Ltd. Curability Tack-free time (hour) 3.2 9.8 1.5 2.8 Tensile properties of Modulus at 50% (MPa) 0.3 0.35 0.32 0.25 dumbbell before tension storing Strength at break (MPa) 1.7 1.79 1.96 1.63 Elongation at break (%) 538 484 479 622 Tensile properties of Modulus at 50% (MPa) 0.22 0.19 0.32 0.23 dumbbell after tension storing at 50° C. Strength at break (MPa) 1.13 0.87 1.68 1.52 for four weeks Elongation at break (%) 472 433 437 705 Maintenance factor Modulus at 50% (%) 73% 54% 100% 92% of physical tension properties Strength at break (%) 66% 49% 86% 93% (after storing/before Elongation at break (%) 88% 89% 91% 113% storing) Adhesion Hand peel Anodized aluminum A A A D at 90 degrees Steel sheet A A D D Hard polyvinyl chloride A A D D FRP A A B C Polycarbonate A A D D Acrylic resin sheet A A A A Comparative Example Composition (parts by weight) 5 6 7 8 Organic polymer (A) A-1 100 100 100 100 Filler Hakuenka CCR (1) Shiraishi Kogyo Kaisha, Ltd. 120 120 120 120 Titanium oxide Tipaque R-820 Ishihara Sangyo Kaisha, Ltd. 20 20 20 20 Plasticizer DIDP (2) Kyowa Hakko Kogyo Co., Ltd. 55 55 55 55 Antisagging agent Disparlon #6500 (3) Kusumoto Chemicals, Ltd. 2 2 2 2 Ultraviolet absorber Sumisorb 400 (4) Sumitomo Chemical Co., Ltd. 1 1 1 1 Photostabilizer Sanol LS-765 (5) Sankyo Co., Ltd. 1 1 1 1 Antioxidant Irganox 1010 (6) Ciba-Geigy Japan Ltd. 1 1 1 1 Dehydrating agent A-171 (7) Nippon Unicar Company Limited 2 2 2 2 Adhesion-imparting A-1120 (8) Nippon Unicar Company Limited agent KBE603 (9) Shin-Etsu Chemical Co., Ltd. Y-11597 (10) Nippon Unicar Company Limited A-189 (11) Nippon Unicar Company Limited A-187 (12) Nippon Unicar Company Limited 3.9 S340 (13) Chisso Corporation 4.1 Reaction mixture of aminosilane and epoxysilane (14) 3.1 Titanium catalyst Orgatix TC-750 (15) Matsumoto Chemical Industry Co., 7.5 7.5 7.5 4 Ltd. Curability Tack-free time (hour) 2.8 >24 2.6 1.7 Tensile properties of Modulus at 50% (MPa) 0.27 0.28 0.28 0.21 dumbbell before tension storing Strength at break (MPa) 1.86 1.59 1.94 1.77 Elongation at break (%) 615 534 579 903 Tensile properties of Modulus at 50% (MPa) 0.28 0.19 0.28 0.18 dumbbell after tension storing at 50° C. Strength at break (MPa) 1.69 0.91 1.53 1.33 for four weeks Elongation at break (%) 608 424 468 794 Maintenance factor Modulus at 50% (%) 104% 68% 100% 86% of physical tension properties Strength at break (%) 91% 57% 79% 75% (after storing/before Elongation at break (%) 99% 79% 81% 88% storing) Adhesion Hand peel Anodized aluminum C A A D at 90 degrees Steel sheet C A C D Hard polyvinyl chloride D D D D FRP C A B D Polycarbonate D D D D Acrylic resin sheet A A A A
(1) Colloidal calcium carbonate
(2) Diisodecyl phthalate
(3) Fatty acid amide wax
(4) 2,4-di-t-butylphenyl-3,5-di-t-butyl-4-hydroxybenzoate
(5) Bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate
(6) Pentaerythrityl-tetrakis[3-(3,5-di-t-butyl-4-hydroxyphenyl)propionate]
(7) Trimethoxyvinylsilane
(8) H2NC2H4NHC3H6Si(OMe)3
(9) H2NC2H4NHC3H6Si(OEt)3
(10) 1,3,5-N-tris(3-trimethoxysilylpropyl)isocyanurate
(11) HSC3H6Si(OMe)3
(12) 3-glycidoxypropyltrimethoxysilane
(13) N-(1,3-dimethylbutylidene)-3-(triethoxysilyl)-1-propaneamine
(14) a mixture obtained by mixing γ-aminopropyltrimethoxysilane and γ-glycidoxypropyltrimethoxysilane at a molar ratio of 1:2 and allowing to stand under conditions of a room temperature of 23° C. and a humidity of 50% for four weeks
(15) Diisopropoxytitaniumbis(ethylacetoacetate)
- The curable compositions prepared using titanium diisopropoxidebis(ethylacetoacetate) as a curing catalyst and aminosilanes of Comparative Examples 1 and 2 and ketimine silane of Comparative Example 6 of the component (C) of the present invention as an adhesion-imparting agent displayed satisfactory adhesion to various articles to be adhered, but after storing, mechanical properties of the cured articles were lowered.
- On the other hand, in the case of using silane coupling agents other than the component (C) as an adhesion-imparting agent like Comparative Examples 3, 4, 5 and 7 and in the case of adding no adhesion-imparting agent like Comparative Example 8, lowering of physical properties was small, but sufficient adhesion could not be obtained. Also like Comparative Examples 2 and 6, the curable compositions prepared by using silane coupling agents having ethoxysilyl groups as a hydrolyzable silicon group exhibited a tendency that curability was inferior.
- At 23° C. in nitrogen gas atmosphere, 100 ml of dehydrated methanol (product of Kanto Chemical Co., Inc.) was poured in a reaction vessel containing 25.5 g (60 mmol) of Orgatix TC750 (titanium diisopropoxidebis(ethylacetoacetate)) produced by Matsumoto Chemical Industry Co., Ltd., followed by stirring for about 30 minutes. Thereafter methanol and 2-propanol generated by an alkoxy exchange reaction of methanol and TC750 were subjected to azeotropic dehydration under reduced pressure. As a result of measurement of 1HNMR spectrum of the obtained reaction product (measured in CDCl3 as a solvent by use of a JNM-LA400 manufactured by JEOL Ltd.), the integrated value of (—OCH)1H peak (4.7 ppm) of an isopropoxy group of TC750 was decreased and a peak (4.2 ppm) of a methoxy group (—OCH3) of the methoxy-substituted titanium compound appeared. Further 100 ml of dehydrated methanol was poured to the reaction product, and the same procedures as above were repeated three times. Lastly, 100 ml of hexane was added, followed by azeotropic dehydration. As a result, titanium dimethoxidebis(ethylacetoacetate) (B-1) was obtained at a purity of not less than 95%. The purity of the generated product was calculated from a ratio of integrated peak intensities of TC750 and the product (B-1) based on 1HNMR spectrum.
- 1HNMR spectrum of TC750:1.0-1.3 ppm (18H), 1.8-2.0 ppm (6H), 3.9-4.2 ppm (4H), 4.7 ppm (2H), 4.9 ppm (2H); 1HNMR spectrum of (B-1): 1.0-1.3 ppm (6H), 1.7-2.0 ppm (6H), 3.9-4.1 ppm (4H), 4.2 ppm (6H), 4.9 ppm (2H)
- Titanium diethoxidebis(ethylacetoacetate) (B-2) was obtained at a purity of not less than 95% in the same manner as in Synthesis Example 2 except that ethanol (produced by Wako Pure Chemicals Co., Ltd.) subjected to dehydration treatment with a molecular sieve was used instead of methanol of Synthesis Example 2.
- 1HNMR spectrum of (B-2): 1.0-1.3 ppm (12H), 1.8-2.0 ppm (6H), 3.9-4.1 ppm (4H), 4.3-4.5 ppm (4H), 4.9 ppm (2H)
- The reactive silicon group-containing polyoxyalkylene polymer (A-1) obtained in Synthesis Example 1 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, the component (C) as an adhesion-imparting agent and a titanium catalyst as a curing catalyst were weighed out according to the formulations shown in Table 2, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and the composition was charged in an aluminum cartridge. It is to be noted that the added amount of each curing catalyst was so set as to became equimolar amounts.
- The curable compositions each were extruded from the cartridge and filled in a molding frame of about 5 mm in thickness with a spatula; the surface of each of the filled compositions was fully flattened, and the planarization completion time was set as the curing starting time. The surface of each of the compositions was touched with a spatula every five minutes, and the skin formation time was measured as the time when the composition no longer stuck to the spatula.
- Also by using a cartridge containing one-component type curable composition, after storing at a constant temperature of 23° C. for 7 days (before storing) and further after storing in an oven at 50° C. for 28 days (after storing), a tension test was carried out under the following conditions, and variations of physical properties before and after storing were investigated.
- (Tension Test Method 2)
- First, the curable compositions each were filled in a sheet-like molding frame of about 3 mm in thickness, and the surface of each of the filled compositions was fully flattened, followed by aging at 23° C. for 3 days+50° C. for 4 days, to produce sheet-like cured articles. The cured articles were punched to obtain No. 2 (⅓) dumbbell type test pieces (JIS K7113), and a modulus at 50% tension, a strength at break and an elongation at break (measuring environment: 23° C., tensile speed: 200 mm/min) were measured with an AUTOGRAPH produced by Shimadzu Corporation.
- Also adhesion to various articles to be adhered (anodized aluminum, polyvinyl chloride-coated steel sheet, FRP, polycarbonate, acrylic resin sheet) was evaluated in the same manner as above.
- Mechanical properties (modulus, strength, elongation) and maintenance factors of physical properties of each curable composition before and after the storing are shown in Table 2. The added amounts of each curing catalyst is so set as to be equimolar amounts.
TABLE 2 Example Composition (parts by weight) 1 2 3 4 Organic polymer (A) A-1 100 100 100 100 Filler Hakuenka CCR (1) Shiraishi Kogyo Kaisha, Ltd. 120 120 120 120 Titanium oxide Tipaque R-820 Ishihara Sangyo Kaisha, Ltd. 20 20 20 20 Plasticizer DIDP (2) Kyowa Hakko Kogyo Co., Ltd. 55 55 55 Allyl group-terminated PPG(3) 55 Antisagging agent Disparlon #6500 (4) Kusumoto Chemicals, Ltd. 2 2 2 2 Ultraviolet absorber Sumisorb 400 (5) Sumitomo Chemical Co., Ltd. 1 1 1 1 Photostabilizer Sanol LS-765 (6) Sankyo Co., Ltd. 1 1 1 1 Antioxidant Irganox 1010 (7) Ciba-Geigy Japan Ltd. 1 1 1 1 Dehydrating agent A-171 (8) Nippon Unicar Company Limited 2 2 2 2 Adhesion-imparting A-1120 (9) Nippon Unicar Company Limited 3 3 3 agent KBE603 (10) Shin-Etsu Chemical Co., Ltd. 3 Chelate type methyl Titanium dimethoxidebis(ethylacetoacetate) (B-1) 6.5 6.5 6.5 titanate (b1) Chelate type ethyl Titanium diethoxidebis(ethylacetoacetate) (B-2) 7 titanate (b2) Titanium catalyst Orgatix TC-750 (11) Matsumoto Chemical Industry Co., Ltd. Curability Skin formation time (hour) 3.5 1.5 8.5 8 Tensile properties of Modulus at 50% (MPa) 0.4 0.4 0.37 0.38 dumbbell before tension storing Strength at break (MPa) 1.83 1.83 1.47 1.8 Elongation at break (%) 486 486 411 499 Tensile properties of Modulus at 50% (MPa) 0.37 0.37 0.32 0.33 dumbbell after tension storing at 50° C. Strength at break (MPa) 1.47 1.47 1.38 1.31 for four weeks Elongation at break (%) 461 461 432 440 Maintenance factor Modulus at 50% (%) 93% 93% 86% 87% of physical tension properties Strength at break (%) 80% 80% 94% 73% (after storing/before Elongation at break (%) 95% 95% 105% 88% storing) Adhesion Hand peel Anodized aluminum A A A A at 90 degrees Polyvinyl chloride resin-coated A A A A steel sheet FRP A A A A Polycarbonate A A A A Acrylic resin sheet A A A A Comparative Example Composition (parts by weight) 9 10 Organic polymer (A) A-1 100 100 Filler Hakuenka CCR (1) Shiraishi Kogyo Kaisha, Ltd. 120 120 Titanium oxide Tipaque R-820 Ishihara Sangyo Kaisha, Ltd. 20 20 Plasticizer DIDP (2) Kyowa Hakko Kogyo Co., Ltd. 55 55 Allyl group-terminated PPG(3) Antisagging agent Disparlon #6500 (4) Kusumoto Chemicals, Ltd. 2 2 Ultraviolet absorber Sumisorb 400 (5) Sumitomo Chemical Co., Ltd. 1 1 Photostabilizer Sanol LS-765 (6) Sankyo Co., Ltd. 1 1 Antioxidant Irganox 1010 (7) Ciba-Geigy Japan Ltd. 1 1 Dehydrating agent A-171 (8) Nippon Unicar Company Limited 2 2 Adhesion-imparting A-1120 (9) Nippon Unicar Company Limited 3 agent KBE603 (10) Shin-Etsu Chemical Co., Ltd. Chelate type methyl Titanium dimethoxidebis(ethylacetoacetate) (B-1) 3.47 titanate (b1) Chelate type ethyl Titanium diethoxidebis(ethylacetoacetate) (B-2) titanate (b2) Titanium catalyst Orgatix TC-750 (11) Matsumoto Chemical Industry Co., 7.5 Ltd. Curability Skin formation time (hour) 2.5 3.7 Tensile properties of Modulus at 50% (MPa) 0.39 0.28 dumbbell before tension storing Strength at break (MPa) 1.65 1.95 Elongation at break (%) 458 775 Tensile properties of Modulus at 50% (MPa) 0.26 0.26 dumbbell after tension storing at 50° C. Strength at break (MPa) 0.97 1.98 for four weeks Elongation at break (%) 362 808 Maintenance factor Modulus at 50% (%) 67% 93% of physical tension properties Strength at break (%) 59% 102% (after storing/before Elongation at break (%) 79% 104% storing) Adhesion Hand peel Anodized aluminum A D at 90 degrees Polyvinyl chloride resin-coated D D steel sheet FRP A D Polycarbonate A A Acrylic resin sheet A A
(1) Colloidal calcium carbonate
(2) Diisodecyl phthalate
(3) Allyl-terminated PPG prepared by subjecting terminal hydroxyl group of PPG3000 to allylation
(4) Fatty acid amide wax
(5) 2,4-di-t-butylphenyl-3,5-di-t-butyl-4-hydroxybenzoate
(6) Bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate
(7) Pentaerythrityl-tetrakis[3-(3,5-di-t-butyl-4-hydroxyphenyl)propionate]
(8) Trimethoxyvinylsilane
(9) H2NC2H4NHC3H6Si(OMe)3
(10) H2NC2H4NHC3H6Si(OEt)3
(11) Diisopropoxytitaniumbis(ethylacetoacetate)
- When titanium diisopropxidebis(ethylacetoacetate) was used as a titanium catalyst like Comparative Example 9, mechanical properties after storing were lowered considerably. On the contrary, lowering of various mechanical properties could be inhibited by using, as a curing catalyst, titanium dimethoxidebis(ethylacetoacetate) and titanium diethoxidebis(ethylacetoacetate) of the component (B) of the present invention.
- Also the composition containing no component (C) like Comparative Example 10 is insufficient in adhesion to several kinds of articles to be adhered.
- Into a reactor containing 42.6 g (150 mmol) of titanium tetraisopropoxide was added dropwise 43.3 g (300 mmol) of ethyl 2-methylacetoacetate over 30 minutes with stirring. During the addition, an exothermic reaction proceeded. After completion of the addition, stirring was carried out further one hour, and then produced 2-propanol was distilled off under reduced pressure. Further hexane was added to the reaction product. Then the mixture was subjected to azeotropic dehydration, and measurement of a spectrum of 1HNMR was carried out. As a result, titanium diisopropoxidebis(ethyl-2-methylacetoacetate) (B′-1) was obtained.
- 1HNMR spectrum of (B′-1): 1.0-1.3 ppm (18H), 1.7 ppm (6H), 1.8-2.1 ppm (6H), 3.9-4.2 ppm (4H), 4.7-4.8 ppm (2H)
- Titanium dimethoxidebis(ethyl-2-methylacetoacetate) (B-3) of a purity of not less than 95% was obtained by the same procedures as in Synthesis Example 2 except that 27.1 g (60 mmol) of titanium diisopropoxidebis(ethyl-2-methylacetoacetate) (B′-1) obtained in Synthesis Example 4 was used instead of TC750 of Synthesis Example 2.
- 1HNMR spectrum of (B-3): 1.1-1.3 ppm (6H), 1.75 ppm (6H), 2.0 ppm (6H), 3.9-4.2 ppm (4H), 4.2 ppm (6H)
- Titanium diethoxidebis(ethyl-2-acetoacetate) (B-4) of a purity of not less than 95% was obtained in the same manner as in Synthesis Example 5 except that ethanol (produced by Wako Pure Chemical Industries, Ltd.) subjected to dehydration treatment with a molecular sieve was used instead of methanol of Synthesis Example 5.
- 1HNMR spectrum of (B-4): 1.1-1.4 ppm (12H), 1.7 ppm (6H), 2.0 ppm (6H), 3.9-4.2 ppm (4H), 4.3-4.5 ppm (4H)
- The reactive silicon group-containing polyoxyalkylene polymer (A-1) obtained in Synthesis Example 1 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, the component (C) as an adhesion-imparting agent and a titanium catalyst as a curing catalyst were weighed out according to the formulations shown in Table 3, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and the composition was charged in an aluminum cartridge. It is to be noted that the added amount of each curing catalyst was so set as to became equimolar amounts.
- The curable compositions each were extruded from the cartridge and filled in a molding frame of about 5 mm in thickness with a spatula; the surface of each of the filled compositions was fully flattened, and the planarization completion time was set as the curing starting time. The surface of each of the compositions was touched with a spatula every five minutes, and the skin formation time was measured as the time when the composition no longer stuck to the spatula.
- Also adhesion to various articles to be adhered (anodized aluminum, polyvinyl chloride-coated steel sheet, FRP, polycarbonate, acrylic resin sheet) was evaluated in the same manner as above.
- Mechanical properties before and after storing were measured by the same test method as the above described (Tension test method 2), and maintenance factors of physical properties were calculated. The results are shown in Table 3.
TABLE 3 Comparative Example Example Composition (parts by weight) 5 6 11 Organic polymer (A) A-1 100 100 100 Filler Hakuenka CCR (1) Shiraishi Kogyo Kaisha, Ltd. 120 120 120 Titanium oxide Tipaque R-820 Ishihara Sangyo Kaisha, Ltd. 20 20 20 Plasticizer DIDP (2) Kyowa Hakko Kogyo Co., Ltd. 55 55 55 Antisagging agent Disparlon #6500 (3) Kusumoto Chemicals, Ltd. 2 2 2 Ultraviolet absorber Sumisorb 400 (4) Sumitomo Chemical Co., Ltd. 1 1 1 Photostabilizer Sanol LS-765 (5) Sankyo Co., Ltd. 1 1 1 Antioxidant Irganox 1010 (6) Ciba-Geigy Japan Ltd. 1 1 1 Dehydrating agent A-171 (7) Nippon Unicar Company Limited 2 2 2 Adhesion-imparting A-1120 (8) Nippon Unicar Company Limited 3 3 3 agent Chelate type methyl Titanium dimethoxidebis(ethyl-2-methylacetoacetate) (B-3) 7 titanate (b1) Chelate type ethyl Titanium diethoxidebis(ethyl-2-methylacetoacetate) (B-4) 7.5 titanate (b2) Titanium catalyst Titanium diisopropoxidebis(ethyl-2-methylacetoacetate) 8 (B′-1) Curability Skin formation time (hour) 1.5 4.5 0.8 Tensile properties of Modulus at 50% (MPa) 0.46 0.4 0.41 dumbbell before tension storing Strength at break (MPa) 1.7 1.53 1.44 Elongation at break (%) 374 373 349 Tensile properties of Modulus at 50% (MPa) 0.38 0.31 0.26 dumbbell after tension storing at 50° C. Strength at break (MPa) 1.55 1.3 0.98 for four weeks Elongation at break (%) 482 453 361 Maintenance factor Modulus at 50% (%) 83% 78% 63% of physical tension properties Strength at break (%) 91% 85% 68% (after storing/before Elongation at break (%) 129% 121% 103% storing) Adhesion Hand peel Anodized aluminum A A A at 90 degrees Polyvinyl chloride-coated A A A steel sheet FRP A A A Polycarbonate A A A Acrylic resin sheet A A A
(1) Colloidal calcium carbonate
(2) Diisodecyl phthalate
(3) Fatty acid amide wax
(4) 2,4-di-t-butylphenyl-3,5-di-t-butyl-4-hydroxybenzoate
(5) Bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate
(6) Pentaerythrityl-tetrakis[3-(3,5-di-t-butyl-4-hydroxyphenyl)propionate]
(7) Trimethoxyvinylsilane
(8) H2NC2H4NHC3H6Si(OMe)3
- When titanium diisopropxidebis(ethyl-2-methylacetoacetate) was used like Comparative Example 11 in the same manner as above, mechanical properties after storing were lowered considerably. On the contrary, lowering of various mechanical properties could be inhibited by using, as a curing catalyst, titanium dimethoxidebis(ethyl-2-methylacetoacetate) and titanium diethoxidebis(ethyl-2-methylacetoacetate) of the component (B) of the present invention.
- A triethoxysilyl group-terminated polyoxypropylene polymer (A-2) was obtained in the same manner as in Synthesis Example 1 except that 1.3 parts by weight of triethoxysilane was used instead of methyldimethoxysilane. According to measurement based on 1H-NMR, the number of terminal triethoxysilyl groups per molecule was about 1.1 on average.
- The reactive silicon group-containing polyoxyalkylene polymer (A-2) obtained in Synthesis Example 7 as the component (A), a filler, a titanium oxide, a plasticizer, an antisagging agent, an ultraviolet absorber, a photostabilizer, an antioxidant, a dehydrating agent, the component (C) as an adhesion-imparting agent and a titanium catalyst as a curing catalyst were weighed out according to the formulations shown in Table 4, then, these ingredients were mixed together with a mixer to yield each one-component type curable composition, and each composition was charged in an aluminum cartridge. It is to be noted that the added amount of each curing catalyst was so set as to be equimolar amounts.
- A skin formation time and adhesion to various articles to be adhered (anodized aluminum, polyvinyl chloride-coated steel sheet, FRP, polycarbonate, acrylic resin sheet) were evaluated in the same manner as above.
- Mechanical properties before and after storing were measured by the same test method as the above described (Tension test method 2), and maintenance factors of physical properties were calculated. The results are shown in Table 4.
TABLE 4 Comparative Example Example Composition (parts by weight) 7 12 Organic polymer (A) A-2 100 100 Filler Hakuenka CCR (1) Shiraishi Kogyo Kaisha, Ltd. 120 120 Titanium oxide Tipaque R-820 Ishihara Sangyo Kaisha, Ltd. 20 20 Plasticizer DIDP (2) Kyowa Hakko Kogyo Co., Ltd. 55 55 Antisagging agent Disparlon #6500 (3) Kusumoto Chemicals, Ltd. 2 2 Ultraviolet absorber Sumisorb 400 (4) Sumitomo Chemical Co., Ltd. 1 1 Photostabilizer Sanol LS-765 (5) Sankyo Co., Ltd. 1 1 Antioxidant Irganox 1010 (6) Ciba-Geigy Japan Ltd. 1 1 Dehydrating agent ESi28 (7) Colcoat Co., Ltd. 2 2 Adhesion-imparting KBE603 (8) Shin-Etsu Chemical Co., Ltd. 3.57 3.57 agent Chelate type ethyl Titanium diethoxidebis(ethylacetoacetate) (B-2) 7 titanate (b1) Titanium catalyst Orgatix TC-750 (9) Matsumoto Chemical Industry Co., 7.5 Ltd. Curability Skin formation time (hour) 3.3 2.8 Tensile properties of Modulus at 50% (MPa) 0.45 0.46 dumbbell before tension storing Strength at break (MPa) 1.83 1.77 Elongation at break (%) 356 338 Tensile properties of Modulus at 50% (MPa) 0.41 0.34 dumbbell after tension storing at 50° C. Strength at break (MPa) 1.72 1.44 for four weeks Elongation at break (%) 395 363 Maintenance factor Modulus at 50% (%) 91% 74% of physical tension properties Strength at break (%) 94% 81% (after storing/before Elongation at break (%) 111% 107% storing) Adhesion Hand peel Anodized aluminum A A at 90 degrees Polyvinyl chloride-coated A B steel sheet FRP A A Polycarbonate A A Acrylic resin sheet A A
(1) Colloidal calcium carbonate
(2) Diisodecyl phthalate
(3) Fatty acid amide wax
(4) 2,4-di-t-butylphenyl-3,5-di-t-butyl-4-hydroxybenzoate
(5) Bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate
(6) Pentaerythrityl-tetrakis[3-(3,5-di-t-butyl-4-hydroxyphenyl)propionate]
(7) Tetraethoxysilane
(8) H2NC2H4NHC3H6Si(OEt)3
(9) Diisopropoxytitaniumbis(ethylacetoacetate)
- When titanium diisopropxidebis(ethylacetoacetate) was used like Comparative Example 12 in the same manner as above, mechanical properties after storing were lowered considerably. On the contrary, lowering of various mechanical properties could be inhibited by using, as a curing catalyst, titanium diethoxidebis(ethylacetoacetate) of the component (B) of the present invention.
Claims (20)
—NHR2 (3)
—N═R4 (4)
—SiR5 3-a(OR6)a (5) . . . silicon-containing group of the component (A)
—SiR7 3-b(OR8)b (6) . . . hydrolyzable silicon group of the component (C)
—SiR7 3-b(OCH3)b (7)
—SiR5 3
—SiR7 3
—SiR5 3
—SiR7 3
—SiR5 3-a(OR6)a (5) . . . silicon-containing group of the component (A)
—SiR7 3-b(OR8)b (6) . . . hydrolyzable silicon group of the component (C)
—SiR7 3-b(OCH3)b (7)
—SiR7 3-b(OCH3)b (7)
—SiR7 3-b(OCH3)b (7)
Applications Claiming Priority (15)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2004-139110 | 2004-05-07 | ||
| JP2004139110 | 2004-05-07 | ||
| JP2004-139111 | 2004-05-07 | ||
| JP2004139111 | 2004-05-07 | ||
| JP2004-146977 | 2004-05-17 | ||
| JP2004-146973 | 2004-05-17 | ||
| JP2004146972 | 2004-05-17 | ||
| JP2004-146976 | 2004-05-17 | ||
| JP2004146976 | 2004-05-17 | ||
| JP2004-146972 | 2004-05-17 | ||
| JP2004146973 | 2004-05-17 | ||
| JP2004146974 | 2004-05-17 | ||
| JP2004146977 | 2004-05-17 | ||
| JP2004-146974 | 2004-05-17 | ||
| PCT/JP2005/007799 WO2005108492A1 (en) | 2004-05-07 | 2005-04-25 | Curable composition |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20070287780A1 true US20070287780A1 (en) | 2007-12-13 |
Family
ID=35320211
Family Applications (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/579,553 Expired - Fee Related US7973108B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition |
| US11/579,630 Expired - Fee Related US7625990B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition |
| US11/579,635 Abandoned US20070287780A1 (en) | 2004-05-07 | 2005-04-25 | Curable Composition |
| US11/579,405 Expired - Fee Related US7763673B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition containing a silicon-containing group polymer, a titanium chelate and an amide wax |
| US11/579,551 Expired - Fee Related US7893170B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition having improved curability and adhesion |
| US11/579,726 Expired - Fee Related US7446158B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition |
| US11/579,587 Expired - Fee Related US7910681B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition having improved adhesion |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/579,553 Expired - Fee Related US7973108B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition |
| US11/579,630 Expired - Fee Related US7625990B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/579,405 Expired - Fee Related US7763673B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition containing a silicon-containing group polymer, a titanium chelate and an amide wax |
| US11/579,551 Expired - Fee Related US7893170B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition having improved curability and adhesion |
| US11/579,726 Expired - Fee Related US7446158B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition |
| US11/579,587 Expired - Fee Related US7910681B2 (en) | 2004-05-07 | 2005-04-25 | Curable composition having improved adhesion |
Country Status (5)
| Country | Link |
|---|---|
| US (7) | US7973108B2 (en) |
| EP (7) | EP1749857B1 (en) |
| JP (7) | JP5225580B2 (en) |
| CN (1) | CN1950459B (en) |
| WO (7) | WO2005108499A1 (en) |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080076878A1 (en) * | 2004-05-07 | 2008-03-27 | Masayuki Wakioka | Curable Composition Having Improved Curability and Adhesion |
| US20100137529A1 (en) * | 2007-05-01 | 2010-06-03 | Akzo Nobel Coatings International B.V. | Antifouling coating composition based on curable polyorganosiloxane polyoxyalkylene copolymers |
| US8785537B2 (en) | 2009-07-20 | 2014-07-22 | Bostik S.A. | Glue for repair or attachment free of organotin |
| US20150007938A1 (en) * | 2012-02-06 | 2015-01-08 | Wacker Chemie Ag | Compositions on the basis of organyloxysilane-terminated polymers |
| US20160160103A1 (en) * | 2013-08-23 | 2016-06-09 | Wacker Chemie Ag | Cross-linkable masses based on organyl-oxysilane-terminated polymers |
| US10040908B2 (en) * | 2014-05-30 | 2018-08-07 | Wacker Chemie Ag | Cross-linkable masses based on organyloxysilane-terminated polymers |
| CN112552703A (en) * | 2020-11-27 | 2021-03-26 | 山东奥斯登房车有限公司 | Light-tight glass fiber reinforced plastic composite material product and application thereof |
| US11090253B2 (en) | 2016-08-03 | 2021-08-17 | Dow Silicones Corporation | Cosmetic composition comprising silicone materials |
| US11254847B2 (en) | 2017-05-09 | 2022-02-22 | Dow Silicones Corporation | Lamination adhesive compositions and their applications |
| US11261350B2 (en) | 2018-02-26 | 2022-03-01 | Nitto Denko Corporation | Double-sided pressure-sensitive adhesive tape |
| US11332581B2 (en) | 2015-01-28 | 2022-05-17 | Dow Silicones Corporation | Elastomeric compositions and their applications |
| US11479022B2 (en) | 2017-05-09 | 2022-10-25 | Dow Silicones Corporation | Lamination process |
| US11485936B2 (en) | 2016-08-03 | 2022-11-01 | Dow Silicones Corporation | Fabric care composition comprising silicone materials |
Families Citing this family (113)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4101632B2 (en) * | 2002-11-01 | 2008-06-18 | 株式会社カネカ | CURABLE COMPOSITION AND METHOD OF IMPROVING RESTORE AND CREEP |
| JP4480457B2 (en) * | 2004-05-17 | 2010-06-16 | 株式会社カネカ | Curable composition |
| DE102005042899A1 (en) * | 2005-09-08 | 2007-03-15 | Ewald Dörken Ag | Weldable corrosion inhibitor and binder therefor |
| JP5226314B2 (en) * | 2005-09-30 | 2013-07-03 | 株式会社カネカ | Curable composition |
| CN101341216B (en) * | 2005-12-02 | 2012-06-13 | 迈图高新材料日本合同公司 | Room temperature curable silicon group-containing polymer composition |
| JP5109147B2 (en) * | 2005-12-21 | 2012-12-26 | 旭硝子株式会社 | Elongation enhancer and curable composition containing the same |
| JP5335178B2 (en) * | 2005-12-22 | 2013-11-06 | モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社 | Method for preparing room temperature curable silicon group-containing polymer composition |
| DE102006006975A1 (en) * | 2006-02-14 | 2007-08-30 | Bostik Gmbh | One-component, solvent-free contact adhesive |
| EP2036938B1 (en) * | 2006-07-03 | 2012-12-26 | Asahi Glass Company, Limited | Process for production of oxyalkylene polymer and curable composition |
| JP2008019361A (en) * | 2006-07-14 | 2008-01-31 | Momentive Performance Materials Japan Kk | Preparation method of polymer containing reactive silicon group and room temperature curable polymer composition containing silicon group |
| JP5040229B2 (en) * | 2006-09-20 | 2012-10-03 | 住友化学株式会社 | UV cut film |
| JP5188698B2 (en) * | 2006-11-06 | 2013-04-24 | モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社 | Curable composition |
| ATE546492T1 (en) * | 2006-11-22 | 2012-03-15 | Kaneka Corp | CURABLE COMPOSITION AND CATALYST COMPOSITION |
| JP2010513608A (en) * | 2006-12-20 | 2010-04-30 | コロプラスト アクティーゼルスカブ | Adhesive composition containing salt |
| DE102006061458B4 (en) | 2006-12-23 | 2014-06-18 | Bostik Gmbh | Use of a self-leveling, anhydrous coating and floor, with laminate or parquet panels |
| JP5398975B2 (en) * | 2007-05-07 | 2014-01-29 | モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社 | Curable composition |
| JP5354511B2 (en) * | 2007-07-12 | 2013-11-27 | 日東化成株式会社 | Curing catalyst for organic polymer and moisture curable organic polymer composition containing the same |
| GB0714257D0 (en) * | 2007-07-23 | 2007-08-29 | Dow Corning | Sealant for insulating glass unit |
| WO2009014077A1 (en) * | 2007-07-24 | 2009-01-29 | Kaneka Corporation | Curable composition |
| JP4460591B2 (en) | 2007-07-30 | 2010-05-12 | 信越化学工業株式会社 | Liquid silicone rubber coating composition, curtain airbag and method for producing the same |
| DE102007038030B4 (en) * | 2007-08-10 | 2009-07-09 | Henkel Ag & Co. Kgaa | Curable compositions of dimethoxysilanes |
| DE102007038661A1 (en) * | 2007-08-15 | 2009-02-19 | Henkel Ag & Co. Kgaa | Silane-crosslinking adhesive or sealant with N-silylalkylamides and its use |
| EP2098548B1 (en) | 2008-03-05 | 2009-10-21 | Sika Technology AG | Compound with improved adhesion to porous substrates |
| US20110023944A1 (en) * | 2008-03-26 | 2011-02-03 | Aica Kogyo Co., Ltd. | Hot-melt composition, sealing material, and solar battery |
| WO2009126186A1 (en) | 2008-04-10 | 2009-10-15 | Cardinal Ig Company | Manufacturing of photovoltaic subassemblies |
| JP5424598B2 (en) * | 2008-09-09 | 2014-02-26 | 日東電工株式会社 | Optical film adhesive composition, optical film adhesive layer, adhesive optical film, and image display device |
| DE102008047362A1 (en) | 2008-09-15 | 2010-04-15 | Henkel Ag & Co. Kgaa | Composition for skin lightening |
| JP2010100839A (en) * | 2008-09-26 | 2010-05-06 | Kaneka Corp | Curable composition for solar cell module and solar cell module |
| JP2010111870A (en) * | 2008-11-07 | 2010-05-20 | Kaneka Corp | Curable composition and sealing material for multilayered glass |
| EP2199351A1 (en) * | 2008-12-19 | 2010-06-23 | Sika Technology AG | Fluid film based on silane-terminated polymers |
| US9640396B2 (en) * | 2009-01-07 | 2017-05-02 | Brewer Science Inc. | Spin-on spacer materials for double- and triple-patterning lithography |
| JP5717320B2 (en) * | 2009-03-12 | 2015-05-13 | リンテック株式会社 | Re-peelable adhesive sheet |
| DE102009026679A1 (en) * | 2009-06-03 | 2010-12-16 | Henkel Ag & Co. Kgaa | Adhesives and sealants based on silane-terminated binders for bonding and sealing flexible solar foils / photovoltaic modules |
| DE102009027357A1 (en) * | 2009-06-30 | 2011-01-05 | Wacker Chemie Ag | Alkoxysilane-terminated polymers containing adhesives or sealants |
| US8952188B2 (en) * | 2009-10-23 | 2015-02-10 | Air Products And Chemicals, Inc. | Group 4 metal precursors for metal-containing films |
| WO2011072056A2 (en) | 2009-12-08 | 2011-06-16 | Dow Corning Coporation | Cure rate control for alkoxysilyl-end-blocked polymers |
| EP2336210B1 (en) * | 2009-12-17 | 2014-03-12 | Sika Technology AG | Silane-functional polymer that does not liberate methanol upon cross-linking |
| JP5789087B2 (en) * | 2010-04-23 | 2015-10-07 | 株式会社カネカ | Non-organotin adhesive composition for interior and cured product thereof |
| JP5975871B2 (en) * | 2010-06-03 | 2016-08-23 | 株式会社カネカ | Moisture curable reactive hot melt adhesive composition |
| DE102010030096A1 (en) * | 2010-06-15 | 2011-12-15 | Wacker Chemie Ag | Silane-crosslinking compositions |
| DE102010041676A1 (en) * | 2010-09-29 | 2012-03-29 | Wacker Chemie Ag | Crosslinkable organopolysiloxane composition |
| TWI540151B (en) * | 2010-10-20 | 2016-07-01 | 可隆股份有限公司 | Photopolymerizable composition and optical sheet |
| JP5887786B2 (en) * | 2010-10-27 | 2016-03-16 | セメダイン株式会社 | Curable composition |
| JP6161103B2 (en) * | 2010-10-27 | 2017-07-12 | セメダイン株式会社 | Method for producing curable composition |
| DE102010062186A1 (en) * | 2010-11-30 | 2012-05-31 | Henkel Ag & Co. Kgaa | Two-component curable composition |
| FR2969638B1 (en) * | 2010-12-22 | 2014-05-02 | Bostik Sa | ADHESIVE COMPOSITION AND CORRESPONDING PARQUET INSTALLATION METHOD WITH IMPROVED DIMENSIONAL STABILITY |
| WO2012099719A1 (en) | 2011-01-18 | 2012-07-26 | Dow Corning Corporation | Method for treating substrates with halosilanes |
| DE102011003425B4 (en) * | 2011-02-01 | 2015-01-08 | Henkel Ag & Co. Kgaa | Use of a curable composition with combined stabilizers |
| EP2698481B1 (en) * | 2011-04-15 | 2017-04-05 | Kaneka Corporation | Cladding material for construction |
| KR101305438B1 (en) | 2011-05-13 | 2013-09-06 | 현대자동차주식회사 | Adhesives for Bonding Polyurethane and Aluminium |
| CN102140268B (en) * | 2011-05-17 | 2013-04-10 | 欧美龙(南通)重防腐涂料有限公司 | Ship ballast tank anticorrosion solventless epoxy coating modifying additive and modified epoxy coating |
| TWI506110B (en) | 2011-05-30 | 2015-11-01 | Cheil Ind Inc | Adhesive composition, optical member and adhesive sheet |
| JP2013032450A (en) * | 2011-08-02 | 2013-02-14 | Kaneka Corp | Pressure-sensitive adhesive composition |
| CN103781850B (en) * | 2011-09-07 | 2016-10-26 | 道康宁公司 | Containing zirconium complex and condensation catalyst, prepare the method for this catalyst and comprise the compositions of this catalyst |
| US9371422B2 (en) | 2011-09-07 | 2016-06-21 | Dow Corning Corporation | Titanium containing complex and condensation reaction catalysts, methods for preparing the catalysts, and compositions containing the catalysts |
| JP2012036397A (en) * | 2011-09-30 | 2012-02-23 | Matsumoto Fine Chemical Co Ltd | Curable composition |
| US9139699B2 (en) | 2012-10-04 | 2015-09-22 | Dow Corning Corporation | Metal containing condensation reaction catalysts, methods for preparing the catalysts, and compositions containing the catalysts |
| JP5834755B2 (en) * | 2011-10-17 | 2015-12-24 | 信越化学工業株式会社 | Method for forming peelable cured film |
| KR101738602B1 (en) * | 2011-10-17 | 2017-06-08 | 신에쓰 가가꾸 고교 가부시끼가이샤 | Silicone Release Coating Composition of Condensation Reaction Curing Type |
| CN102505484B (en) * | 2011-11-04 | 2013-11-06 | 西安康本材料有限公司 | Glue solution for testing tensile property of multi-filament |
| JP5810865B2 (en) * | 2011-11-25 | 2015-11-11 | 信越化学工業株式会社 | Condensation reaction curable primer composition for silicone adhesive |
| US8481626B1 (en) | 2012-01-16 | 2013-07-09 | Itron, Inc. | Wax-based encapsulant/moisture barrier for use with electronics received in water meter pits |
| US8728568B2 (en) * | 2012-01-16 | 2014-05-20 | Itron, Inc. | Method for encapsulation of electronics received in water meter pits with an improved wax-based encapsulant/moisture barrier |
| EP2641935A1 (en) * | 2012-03-19 | 2013-09-25 | Sika Technology AG | Composition based on silane terminated polymers |
| JP5480359B1 (en) * | 2012-12-25 | 2014-04-23 | 古河電気工業株式会社 | Transparent resin composition for sealing organic electroluminescent elements, resin sheet for sealing organic electroluminescent elements, and image display device |
| DE102013101993A1 (en) * | 2013-02-28 | 2014-08-28 | Gerd Hoffmann | Leak seal for a particular oil or oily liquid containing container |
| WO2014141596A1 (en) * | 2013-03-11 | 2014-09-18 | パナソニック株式会社 | Coating for preventing scattering of fragments |
| US20160083524A1 (en) * | 2013-05-16 | 2016-03-24 | Shin-Etsu Chemical Co., Ltd. | Aluminum chelate compound and room temperature-curable resin composition containing same |
| WO2014200100A1 (en) * | 2013-06-14 | 2014-12-18 | 積水フーラー株式会社 | Adhesive composition |
| DE102013213655A1 (en) * | 2013-07-12 | 2015-01-15 | Evonik Industries Ag | Curable silyl group-containing compositions with improved storage stability |
| JP6477473B2 (en) * | 2013-07-31 | 2019-03-06 | 株式会社スリーボンド | Moisture curable composition |
| JP6735668B2 (en) * | 2013-11-26 | 2020-08-05 | モメンティブ パフォーマンス マテリアルズ インコーポレイテッドMomentive Performance Materials Inc. | Moisture-curable composition having metal-arene complex |
| EP3105300B1 (en) | 2014-02-13 | 2019-08-21 | Honeywell International Inc. | Compressible thermal interface materials |
| JP5918908B2 (en) * | 2014-04-01 | 2016-05-18 | 日東化成株式会社 | Curing catalyst for organic polymer or organopolysiloxane, moisture curable composition, cured product and method for producing the same |
| WO2015151787A1 (en) * | 2014-04-01 | 2015-10-08 | 日東化成株式会社 | Curing catalyst for organic polymers or organopolysiloxanes, moisture-curable composition, cured product and method for producing same |
| CN103923583A (en) * | 2014-04-11 | 2014-07-16 | 苏州之诺新材料科技有限公司 | One-component end silane polyacrylate binder and preparation method thereof |
| JP6377415B2 (en) * | 2014-06-03 | 2018-08-22 | 宇部興産建材株式会社 | Surface impregnating material and structure |
| DE102014212291A1 (en) | 2014-06-26 | 2015-12-31 | Henkel Ag & Co. Kgaa | Titanium complexes as vulcanization catalysts |
| JP2016020407A (en) * | 2014-07-11 | 2016-02-04 | 信越化学工業株式会社 | Method for curing fluorine-containing organic silicon compound, method for producing cured film, composition including fluorine-containing organic silicon compound, and surface-treated article with cured product of the composition |
| JP2017534721A (en) * | 2014-10-13 | 2017-11-24 | アベリー・デニソン・コーポレイションAvery Dennison Corporation | Silicone adhesive with weldability and vibration damping |
| PL3048141T3 (en) | 2015-01-26 | 2018-05-30 | Avery Dennison Corporation | Self adhesive fouling release coating composition |
| JP6509650B2 (en) * | 2015-04-02 | 2019-05-08 | アイカ工業株式会社 | Translucent adhesive composition |
| ES2682598T3 (en) | 2015-12-17 | 2018-09-21 | Henkel Ag & Co. Kgaa | Titanium complexes as vulcanization catalysts |
| JP6842469B2 (en) | 2016-03-08 | 2021-03-17 | ハネウェル・インターナショナル・インコーポレーテッドHoneywell International Inc. | Phase change material |
| GB201613414D0 (en) * | 2016-08-03 | 2016-09-14 | Dow Corning | Elastomeric compositions and their applications |
| GB201613412D0 (en) | 2016-08-03 | 2016-09-14 | Dow Corning | Elastomeric compositions and their applications |
| EP3323844A1 (en) | 2016-11-17 | 2018-05-23 | Henkel AG & Co. KGaA | Curable compositions based on silicon-containing polymers using phosphazenes as catalysts |
| EP3645639B1 (en) | 2017-06-26 | 2021-07-21 | Dow Silicones Corporation | Silicone-polyether copolymer, isocyanate-functional silicone-polyether copolymer formed therewith, silicone-polyether-urethane copolymer, sealants comprising same, and related methods |
| KR20190013091A (en) * | 2017-07-31 | 2019-02-11 | 다우 실리콘즈 코포레이션 | Dually-Curable Resin Composition, Cured Body Prepared Therefrom, And Electronic Device Comprising Such Cured Body |
| CN107541170B (en) * | 2017-09-06 | 2021-07-13 | 广州集泰化工股份有限公司 | Single-component silane modified polyether sealant for building and preparation method thereof |
| US11041103B2 (en) | 2017-09-08 | 2021-06-22 | Honeywell International Inc. | Silicone-free thermal gel |
| CN108003804A (en) * | 2017-12-20 | 2018-05-08 | 烟台德邦科技有限公司 | Preparation method of fast-curing environment-friendly adhesive |
| HUE062214T2 (en) | 2017-12-29 | 2023-09-28 | Henkel Ag & Co Kgaa | Acid resistant adhesive composition |
| US11072706B2 (en) | 2018-02-15 | 2021-07-27 | Honeywell International Inc. | Gel-type thermal interface material |
| JP7310047B2 (en) * | 2018-03-12 | 2023-07-19 | リンテック株式会社 | Curable composition, cured product, method for producing cured product, and method for using curable composition |
| CN108754861B (en) * | 2018-04-27 | 2021-02-09 | 安徽索亚装饰材料有限公司 | Production process of non-woven fabric for leather carving |
| US11760841B2 (en) | 2018-12-21 | 2023-09-19 | Dow Silicones Corporation | Silicone-polycarbonate copolymer, sealants comprising same, and related methods |
| US11807775B2 (en) | 2018-12-21 | 2023-11-07 | Dow Silicones Corporation | Silicone-organic copolymer, sealants comprising same, and related methods |
| CN109943271A (en) * | 2019-04-10 | 2019-06-28 | 广东绿洲化工有限公司 | A kind of no-solvent type nail-free glue and preparation method thereof |
| US11373921B2 (en) | 2019-04-23 | 2022-06-28 | Honeywell International Inc. | Gel-type thermal interface material with low pre-curing viscosity and elastic properties post-curing |
| US12104098B2 (en) | 2019-10-10 | 2024-10-01 | Dow Silicones Corporation | Self-sealing tires |
| CN114466903B (en) | 2019-10-10 | 2024-01-02 | 美国陶氏有机硅公司 | Organosilicon-based products and their use |
| TW202118833A (en) * | 2019-11-13 | 2021-05-16 | 美商陶氏有機矽公司 | Room temperature storage-stable uv/vis and moisture dual curable polysiloxane composition |
| WO2021106942A1 (en) * | 2019-11-29 | 2021-06-03 | 日東化成株式会社 | Curing catalyst used for curing of polymer, moisture curable composition, and method for producing cured product |
| CN110845989B (en) * | 2019-12-02 | 2021-09-03 | 苏州太湖电工新材料股份有限公司 | Two-component organic silicon pouring sealant and application method thereof |
| EP4077429A4 (en) * | 2019-12-19 | 2023-12-06 | Henkel AG & Co. KGaA | Moisture curable polyacrylate compositions and uses thereof |
| JP6919010B1 (en) * | 2020-03-18 | 2021-08-11 | 旭化成ワッカーシリコーン株式会社 | Moisture-curable composition and method for producing the moisture-curable composition |
| EP3889222A1 (en) * | 2020-03-30 | 2021-10-06 | Henkel AG & Co. KGaA | Curable potting composition free of substances of very high concern |
| WO2021261383A1 (en) * | 2020-06-22 | 2021-12-30 | 株式会社カネカ | Thermally curable composition and cured product of same |
| CN115461414B (en) * | 2020-06-29 | 2024-06-25 | 日东化成株式会社 | Curing catalyst for curing polymer, method for producing same, moisture-curable composition, and method for producing cured product |
| CA3201435A1 (en) | 2020-12-23 | 2022-06-30 | Kintra Fibers, Inc. | Polyester polymer nanocomposites |
| CN115991875B (en) * | 2023-02-15 | 2023-12-15 | 杭州之江有机硅化工有限公司 | Titanate catalyst for dealcoholized room temperature vulcanized silicone rubber and preparation method thereof |
| CN116786389B (en) * | 2023-08-23 | 2023-10-27 | 淄博大洋阻燃制品有限公司 | Flame-retardant heat-insulating composite material and preparation method thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6642309B2 (en) * | 2001-08-14 | 2003-11-04 | Kaneka Corporation | Curable resin composition |
| US7144953B2 (en) * | 2001-10-23 | 2006-12-05 | Kaneka Corporation | Curable resin composition |
| US20080033087A1 (en) * | 2004-05-07 | 2008-02-07 | Kaneka Corporation | Curable Composition |
| US7351782B2 (en) * | 2002-10-02 | 2008-04-01 | Kaneka Corporation | One-part curable composition |
Family Cites Families (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4439557A (en) * | 1981-05-08 | 1984-03-27 | Toray Industries, Incorporated | Coating compositions |
| JPS62146959A (en) * | 1985-12-19 | 1987-06-30 | Kanegafuchi Chem Ind Co Ltd | Curable composition |
| JPS62252456A (en) * | 1986-04-24 | 1987-11-04 | Toray Silicone Co Ltd | Room temperature-curable organopolysiloxane composition |
| JP2565333B2 (en) * | 1987-04-28 | 1996-12-18 | 東レ・ダウコーニング・シリコーン株式会社 | Room temperature curable organopolysiloxane composition for electrical and electronic equipment |
| JP2557444B2 (en) * | 1988-02-03 | 1996-11-27 | 鐘淵化学工業株式会社 | Curable composition with improved dryness of alkyd paint |
| JP2835400B2 (en) * | 1988-10-07 | 1998-12-14 | 鐘淵化学工業株式会社 | Curable composition |
| JP2813801B2 (en) * | 1989-02-01 | 1998-10-22 | 鐘淵化学工業株式会社 | Bonding method |
| EP0476150B1 (en) * | 1990-04-03 | 1995-12-27 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Curable resin composition |
| DE4019074C1 (en) * | 1990-06-15 | 1991-07-18 | Teroson Gmbh, 6900 Heidelberg, De | |
| JP3304954B2 (en) * | 1991-03-11 | 2002-07-22 | 鐘淵化学工業株式会社 | Curable composition for sealant |
| JP3725178B2 (en) * | 1991-03-22 | 2005-12-07 | 東レ・ダウコーニング株式会社 | Room temperature curable organopolysiloxane composition |
| DE4110796A1 (en) * | 1991-04-04 | 1992-10-08 | Bayer Ag | PROVISIONAL FASTENING MATERIALS |
| US5703146A (en) * | 1991-09-12 | 1997-12-30 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Curable composition containing an oxypropylene polymer and calcium carbonate which has been surface treated with a fatty acid |
| JP3112753B2 (en) * | 1991-09-12 | 2000-11-27 | 鐘淵化学工業株式会社 | Curable composition |
| JPH05311063A (en) * | 1992-05-07 | 1993-11-22 | Sekisui Chem Co Ltd | Sealant composition |
| JPH05331063A (en) * | 1992-05-25 | 1993-12-14 | Takeda Chem Ind Ltd | Antagonist for endothelin receptor |
| US5719249A (en) * | 1993-11-29 | 1998-02-17 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Reactive silicon group-containing polyoxyalkylene-polysiloxane copolymer |
| JPH06166810A (en) * | 1992-11-30 | 1994-06-14 | Toray Dow Corning Silicone Co Ltd | Room-temperature-curable composition |
| DE4329244A1 (en) * | 1993-08-31 | 1995-03-02 | Sandoz Ag | Aqueous wax and silicone dispersions, their preparation and use |
| JP3080835B2 (en) * | 1994-04-05 | 2000-08-28 | 積水化学工業株式会社 | Room temperature curable composition |
| TW325490B (en) * | 1995-06-23 | 1998-01-21 | Ciba Sc Holding Ag | Polysiloxane light stabilizers |
| AU712008B2 (en) * | 1995-10-12 | 1999-10-28 | Kaneka Corporation | Method for glazing motor vehicles |
| JPH09124921A (en) * | 1995-10-30 | 1997-05-13 | Sekisui Chem Co Ltd | Jointing material for external wall |
| US5962074A (en) * | 1996-06-05 | 1999-10-05 | 3M Innovative Properties Company | Wax composition and method of use |
| AU3341297A (en) * | 1996-07-02 | 1998-01-21 | Ciba Specialty Chemicals Holding Inc. | Process for curing a polymerizable composition |
| GB2327425B (en) * | 1996-08-15 | 2000-03-15 | Simson B V | Adhesive composition |
| GB9721132D0 (en) * | 1997-10-07 | 1997-12-03 | Tioxide Specialties Ltd | Polymeric sealant compositions |
| JP3903549B2 (en) * | 1997-10-21 | 2007-04-11 | 旭硝子株式会社 | Room temperature curable composition |
| US6080816A (en) * | 1997-11-10 | 2000-06-27 | E. I. Du Pont De Nemours And Company | Coatings that contain reactive silicon oligomers |
| GB9724055D0 (en) * | 1997-11-15 | 1998-01-14 | Dow Corning Sa | Curable polysiloxane compositions |
| JPH11209538A (en) * | 1998-01-26 | 1999-08-03 | Kanegafuchi Chem Ind Co Ltd | Curing composition |
| JP3636583B2 (en) | 1998-01-29 | 2005-04-06 | 株式会社カネカ | Sealant for double-glazed glass |
| JP2000109676A (en) * | 1998-10-08 | 2000-04-18 | Asahi Glass Co Ltd | Curable composition |
| CA2365171A1 (en) * | 1999-03-23 | 2000-09-28 | Kaneka Corporation | Curable resin compositions |
| ATE272675T1 (en) | 2000-01-06 | 2004-08-15 | Dow Corning Sa | ORGANOSILOXANE COMPOSITIONS |
| WO2001049774A2 (en) * | 2000-01-06 | 2001-07-12 | Dow Corning Corporation | Organosiloxane compositions |
| JP4614499B2 (en) * | 2000-04-20 | 2011-01-19 | ダウ コーニング コーポレーション | Curable composition |
| JP2001302934A (en) * | 2000-04-20 | 2001-10-31 | Dow Corning Asia Ltd | Curable composition excellent in room-temperature curability |
| JP4400983B2 (en) * | 2000-01-06 | 2010-01-20 | 東レ・ダウコーニング株式会社 | Curable composition |
| EP1138732B1 (en) * | 2000-03-31 | 2005-08-31 | JSR Corporation | Coating composition and cured product |
| JP4618843B2 (en) | 2000-04-20 | 2011-01-26 | ダウ コーニング コーポレーション | Curable composition |
| US7176269B2 (en) * | 2000-07-25 | 2007-02-13 | Mitsui Chemicals, Inc. | Curable composition and its use |
| WO2002048118A1 (en) * | 2000-12-12 | 2002-06-20 | Ciba Specialty Chemicals Holding Inc. | Benzophenone uv-absorbers with heterocyclic substituents |
| JP3793031B2 (en) * | 2001-02-23 | 2006-07-05 | 日東化成株式会社 | Moisture curable composition |
| JP4141198B2 (en) * | 2001-08-14 | 2008-08-27 | 株式会社カネカ | Curable resin composition |
| DE60216966T2 (en) * | 2001-11-29 | 2007-10-04 | Kaneka Corp. | HARDENING COMPOSITION |
| JP2004002757A (en) | 2002-03-28 | 2004-01-08 | Kanegafuchi Chem Ind Co Ltd | Moisture curable composition |
| JP3866154B2 (en) * | 2002-05-16 | 2007-01-10 | オート化学工業株式会社 | Curable composition and sealant composition |
| JP2004083895A (en) | 2002-07-05 | 2004-03-18 | Kanegafuchi Chem Ind Co Ltd | Method for production of curable composition |
| JP3789867B2 (en) * | 2002-07-31 | 2006-06-28 | 横浜ゴム株式会社 | Curable resin composition |
| JP4101632B2 (en) * | 2002-11-01 | 2008-06-18 | 株式会社カネカ | CURABLE COMPOSITION AND METHOD OF IMPROVING RESTORE AND CREEP |
| WO2005003230A1 (en) * | 2003-07-08 | 2005-01-13 | Kaneka Corporation | Curing composition |
| JP4480457B2 (en) * | 2004-05-17 | 2010-06-16 | 株式会社カネカ | Curable composition |
-
2005
- 2005-04-25 JP JP2006512949A patent/JP5225580B2/en active Active
- 2005-04-25 EP EP05734309.7A patent/EP1749857B1/en active Active
- 2005-04-25 US US11/579,553 patent/US7973108B2/en not_active Expired - Fee Related
- 2005-04-25 EP EP05734521.7A patent/EP1752496A4/en not_active Withdrawn
- 2005-04-25 EP EP05734515.9A patent/EP1746134B1/en active Active
- 2005-04-25 JP JP2006512952A patent/JP5225582B2/en not_active Expired - Fee Related
- 2005-04-25 WO PCT/JP2005/007804 patent/WO2005108499A1/en active Application Filing
- 2005-04-25 US US11/579,630 patent/US7625990B2/en not_active Expired - Fee Related
- 2005-04-25 EP EP05734516.7A patent/EP1749858B1/en active Active
- 2005-04-25 US US11/579,635 patent/US20070287780A1/en not_active Abandoned
- 2005-04-25 EP EP05734604.1A patent/EP1749859B1/en active Active
- 2005-04-25 WO PCT/JP2005/007798 patent/WO2005108491A1/en active Application Filing
- 2005-04-25 JP JP2006512946A patent/JP5002262B2/en active Active
- 2005-04-25 WO PCT/JP2005/007802 patent/WO2005108498A1/en active Application Filing
- 2005-04-25 US US11/579,405 patent/US7763673B2/en not_active Expired - Fee Related
- 2005-04-25 US US11/579,551 patent/US7893170B2/en not_active Expired - Fee Related
- 2005-04-25 US US11/579,726 patent/US7446158B2/en not_active Expired - Fee Related
- 2005-04-25 JP JP2006512950A patent/JP4898430B2/en not_active Expired - Fee Related
- 2005-04-25 WO PCT/JP2005/007805 patent/WO2005108500A1/en active Application Filing
- 2005-04-25 EP EP05734513.4A patent/EP1746133B1/en not_active Not-in-force
- 2005-04-25 WO PCT/JP2005/007803 patent/WO2005108494A1/en active Application Filing
- 2005-04-25 US US11/579,587 patent/US7910681B2/en not_active Expired - Fee Related
- 2005-04-25 WO PCT/JP2005/007799 patent/WO2005108492A1/en active Application Filing
- 2005-04-25 CN CN2005800145584A patent/CN1950459B/en not_active Expired - Fee Related
- 2005-04-25 WO PCT/JP2005/007800 patent/WO2005108493A1/en active Application Filing
- 2005-04-25 JP JP2006512948A patent/JP4819675B2/en not_active Expired - Fee Related
- 2005-04-25 JP JP2006512951A patent/JP5225581B2/en active Active
- 2005-04-25 EP EP05734518.3A patent/EP1746135B1/en active Active
- 2005-04-25 JP JP2006512947A patent/JP4480719B2/en not_active Expired - Fee Related
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6642309B2 (en) * | 2001-08-14 | 2003-11-04 | Kaneka Corporation | Curable resin composition |
| US7144953B2 (en) * | 2001-10-23 | 2006-12-05 | Kaneka Corporation | Curable resin composition |
| US7351782B2 (en) * | 2002-10-02 | 2008-04-01 | Kaneka Corporation | One-part curable composition |
| US20080033087A1 (en) * | 2004-05-07 | 2008-02-07 | Kaneka Corporation | Curable Composition |
| US20080076878A1 (en) * | 2004-05-07 | 2008-03-27 | Masayuki Wakioka | Curable Composition Having Improved Curability and Adhesion |
| US7446158B2 (en) * | 2004-05-07 | 2008-11-04 | Kaneka Corporation | Curable composition |
| US20080287636A1 (en) * | 2004-05-07 | 2008-11-20 | Katsuyu Wakabayashi | Curable Composition |
| US20080319152A1 (en) * | 2004-05-07 | 2008-12-25 | Toshihiko Okamoto | Curable Composition |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080194773A1 (en) * | 2004-05-07 | 2008-08-14 | Masayuki Wakioka | Curable Composition Having Improved Adhesion |
| US20080319152A1 (en) * | 2004-05-07 | 2008-12-25 | Toshihiko Okamoto | Curable Composition |
| US7893170B2 (en) * | 2004-05-07 | 2011-02-22 | Kaneka Corporation | Curable composition having improved curability and adhesion |
| US7910681B2 (en) * | 2004-05-07 | 2011-03-22 | Kaneka Corporation | Curable composition having improved adhesion |
| US7973108B2 (en) * | 2004-05-07 | 2011-07-05 | Kaneka Corporation | Curable composition |
| US20080076878A1 (en) * | 2004-05-07 | 2008-03-27 | Masayuki Wakioka | Curable Composition Having Improved Curability and Adhesion |
| US20100137529A1 (en) * | 2007-05-01 | 2010-06-03 | Akzo Nobel Coatings International B.V. | Antifouling coating composition based on curable polyorganosiloxane polyoxyalkylene copolymers |
| US8450443B2 (en) * | 2007-05-01 | 2013-05-28 | Akzo Nobel Coatings International B.V. | Antifouling coating composition based on curable polyorganosiloxane polyoxyalkylene copolymers |
| US8785537B2 (en) | 2009-07-20 | 2014-07-22 | Bostik S.A. | Glue for repair or attachment free of organotin |
| US10077386B2 (en) | 2012-02-06 | 2018-09-18 | Wacker Chemie Ag | Compositions on the basis of organyloxysilane-terminated polymers |
| US20150007938A1 (en) * | 2012-02-06 | 2015-01-08 | Wacker Chemie Ag | Compositions on the basis of organyloxysilane-terminated polymers |
| US20160160103A1 (en) * | 2013-08-23 | 2016-06-09 | Wacker Chemie Ag | Cross-linkable masses based on organyl-oxysilane-terminated polymers |
| US9920229B2 (en) * | 2013-08-23 | 2018-03-20 | Wacker Chemie Ag | Cross-linkable masses based on organyl-oxysilane-terminated polymers |
| US10040908B2 (en) * | 2014-05-30 | 2018-08-07 | Wacker Chemie Ag | Cross-linkable masses based on organyloxysilane-terminated polymers |
| US11332581B2 (en) | 2015-01-28 | 2022-05-17 | Dow Silicones Corporation | Elastomeric compositions and their applications |
| US11090253B2 (en) | 2016-08-03 | 2021-08-17 | Dow Silicones Corporation | Cosmetic composition comprising silicone materials |
| US11485936B2 (en) | 2016-08-03 | 2022-11-01 | Dow Silicones Corporation | Fabric care composition comprising silicone materials |
| US11254847B2 (en) | 2017-05-09 | 2022-02-22 | Dow Silicones Corporation | Lamination adhesive compositions and their applications |
| US11479022B2 (en) | 2017-05-09 | 2022-10-25 | Dow Silicones Corporation | Lamination process |
| US11261350B2 (en) | 2018-02-26 | 2022-03-01 | Nitto Denko Corporation | Double-sided pressure-sensitive adhesive tape |
| CN112552703A (en) * | 2020-11-27 | 2021-03-26 | 山东奥斯登房车有限公司 | Light-tight glass fiber reinforced plastic composite material product and application thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1749859B1 (en) | Curable composition | |
| EP1624027B2 (en) | Curing composition | |
| EP2527406B1 (en) | Curable composition | |
| US8415444B2 (en) | Curable composition | |
| JP4480457B2 (en) | Curable composition | |
| JP5210685B2 (en) | Method for producing reactive silicon group-containing organic polymer composition, method for adjusting fluidity, and joint structure using the organic polymer composition | |
| JP5564312B2 (en) | Curable composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: KANEKA CORPORATION, JAPAN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:WAKABAYASHI, KATSUYU;OKAMOTO, TOSHIHIKO;KUSAKABE, MASATO;REEL/FRAME:019694/0204 Effective date: 20061113 |
|
| AS | Assignment |
Owner name: KANEKA CORPORATION, JAPAN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:WAKABAYASHI, KATSUYU;OKAMOTO, TOSHIHIKO;KUSAKABE, MASATO;REEL/FRAME:020002/0257 Effective date: 20061113 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |