US20030092912A1 - Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa Receptors - Google Patents
Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa Receptors Download PDFInfo
- Publication number
- US20030092912A1 US20030092912A1 US10/115,361 US11536102A US2003092912A1 US 20030092912 A1 US20030092912 A1 US 20030092912A1 US 11536102 A US11536102 A US 11536102A US 2003092912 A1 US2003092912 A1 US 2003092912A1
- Authority
- US
- United States
- Prior art keywords
- methylbutyl
- methylpropyl
- fluorophenyl
- methylphenyl
- propyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [1*]N1C(CN([3*])C(=O)*[W])=NC2=C1[2H]=CB=*2.[2*]C Chemical compound [1*]N1C(CN([3*])C(=O)*[W])=NC2=C1[2H]=CB=*2.[2*]C 0.000 description 57
- OMFHHTCEJGFUNY-UHFFFAOYSA-N CC(C)(C)CCN1CCCC1 Chemical compound CC(C)(C)CCN1CCCC1 OMFHHTCEJGFUNY-UHFFFAOYSA-N 0.000 description 3
- LNICFFMFQREHQW-UHFFFAOYSA-N CC(C)N(C)[Rn] Chemical compound CC(C)N(C)[Rn] LNICFFMFQREHQW-UHFFFAOYSA-N 0.000 description 3
- YQOPNAOQGQSUHF-UHFFFAOYSA-N CC(C)N1CCCC1 Chemical compound CC(C)N1CCCC1 YQOPNAOQGQSUHF-UHFFFAOYSA-N 0.000 description 3
- UGCLVZNBEBUGBB-UHFFFAOYSA-N C=C(CC)C(C)CC1=CC2=CC=CC=C2C1C.CC Chemical compound C=C(CC)C(C)CC1=CC2=CC=CC=C2C1C.CC UGCLVZNBEBUGBB-UHFFFAOYSA-N 0.000 description 2
- YZAIMLYKRNTJJJ-UHFFFAOYSA-N CCCCN1C2=CC=C(CN(CC)CC)C=C2N=C1CN(CCC)C(=O)C1=CC(F)=CC=C1 Chemical compound CCCCN1C2=CC=C(CN(CC)CC)C=C2N=C1CN(CCC)C(=O)C1=CC(F)=CC=C1 YZAIMLYKRNTJJJ-UHFFFAOYSA-N 0.000 description 2
- UIJWAYOOHACFQD-UHFFFAOYSA-N C.C.C.C.C.C.C1=CCC=C1.C1=CCC=C1.CC.CC.CC.CC.CC.CC1=CC=CC=C1 Chemical compound C.C.C.C.C.C.C1=CCC=C1.C1=CCC=C1.CC.CC.CC.CC.CC.CC1=CC=CC=C1 UIJWAYOOHACFQD-UHFFFAOYSA-N 0.000 description 1
- RIHGVGBTTWHVLU-UHFFFAOYSA-N C1=CCC=C1.C=C(CC)C(C)CC1=CC2=CC=CC=C2C1C.CC.CC Chemical compound C1=CCC=C1.C=C(CC)C(C)CC1=CC2=CC=CC=C2C1C.CC.CC RIHGVGBTTWHVLU-UHFFFAOYSA-N 0.000 description 1
- UCCTYBYRULJXCH-UHFFFAOYSA-N C=C(C1=CC(C)=CC=C1)C(CCC)CC1=CC2=CC=CC=C2C1CC1CC1 Chemical compound C=C(C1=CC(C)=CC=C1)C(CCC)CC1=CC2=CC=CC=C2C1CC1CC1 UCCTYBYRULJXCH-UHFFFAOYSA-N 0.000 description 1
- KPYLYHSURHSNFY-UHFFFAOYSA-N C=C(C1=CC(C)=CC=C1C)C(CCC)CC1=CC2=CC(CC3CCCCC3)=CC=C2C1CCC Chemical compound C=C(C1=CC(C)=CC=C1C)C(CCC)CC1=CC2=CC(CC3CCCCC3)=CC=C2C1CCC KPYLYHSURHSNFY-UHFFFAOYSA-N 0.000 description 1
- GHLJGWUBOXJELD-UHFFFAOYSA-N C=C(C1=CC(C)=CC=C1C)C(CCC)CC1=CC2=CC=CC=C2C1CC1CC1 Chemical compound C=C(C1=CC(C)=CC=C1C)C(CCC)CC1=CC2=CC=CC=C2C1CC1CC1 GHLJGWUBOXJELD-UHFFFAOYSA-N 0.000 description 1
- AKQCWSCMOKTVFS-UHFFFAOYSA-N C=C(CC1=CC=CC=C1)C(C)CC1=CC2=CC=CC=C2C1C.CC.CC Chemical compound C=C(CC1=CC=CC=C1)C(C)CC1=CC2=CC=CC=C2C1C.CC.CC AKQCWSCMOKTVFS-UHFFFAOYSA-N 0.000 description 1
- OEHVNZPQCXBBGM-UHFFFAOYSA-N CC.CC.CC.CC.CC.CC1=CC(C)=C(C)C(C)=C1C.CC1=CC(C)=C(C)C(C)=C1C.CC1=CC(C)=C(C)C(C)=C1C.CC1=CC(C)=C(C)C1.CC1=CC(C)=C(C)C1 Chemical compound CC.CC.CC.CC.CC.CC1=CC(C)=C(C)C(C)=C1C.CC1=CC(C)=C(C)C(C)=C1C.CC1=CC(C)=C(C)C(C)=C1C.CC1=CC(C)=C(C)C1.CC1=CC(C)=C(C)C1 OEHVNZPQCXBBGM-UHFFFAOYSA-N 0.000 description 1
- ARBJMCXQSQMYHD-UHFFFAOYSA-N CCCCCN1C(CN(CCC)C(=O)C2=CC(I)=CC=C2)=NC2=CC=CN=C21 Chemical compound CCCCCN1C(CN(CCC)C(=O)C2=CC(I)=CC=C2)=NC2=CC=CN=C21 ARBJMCXQSQMYHD-UHFFFAOYSA-N 0.000 description 1
- ANXVOJFWQNRJKN-UHFFFAOYSA-N CCCN(CC1=NC2=CC=C(Cl)C=C2N1CCC)C(=O)C1=CC(F)=CC=C1F Chemical compound CCCN(CC1=NC2=CC=C(Cl)C=C2N1CCC)C(=O)C1=CC(F)=CC=C1F ANXVOJFWQNRJKN-UHFFFAOYSA-N 0.000 description 1
- ZTYMLFFKSLVXCD-UHFFFAOYSA-N CCCN1C2=CC=C(F)C=C2N=C1CCl Chemical compound CCCN1C2=CC=C(F)C=C2N=C1CCl ZTYMLFFKSLVXCD-UHFFFAOYSA-N 0.000 description 1
- MPTUHGGDNJEETR-UHFFFAOYSA-N CCCN1C2=CC=C(F)C=C2N=C1CN(C)C(=O)C1=CC=CC=C1 Chemical compound CCCN1C2=CC=C(F)C=C2N=C1CN(C)C(=O)C1=CC=CC=C1 MPTUHGGDNJEETR-UHFFFAOYSA-N 0.000 description 1
- AEWGZJBOKSUSHJ-UHFFFAOYSA-N CCCN1C2=CC=CC=C2N=C1CN(C)C(=O)C1=CC(F)=CC=C1F Chemical compound CCCN1C2=CC=CC=C2N=C1CN(C)C(=O)C1=CC(F)=CC=C1F AEWGZJBOKSUSHJ-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/06—Benzimidazoles; Hydrogenated benzimidazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached in position 2
- C07D235/14—Radicals substituted by nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/06—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
Definitions
- This invention relates to heteroaryl fused aminoalkylimidazole derivatives which when appropriately substituted selectively bind to GABA A receptors.
- This invention also relates to pharmaceutical compositions comprising such compounds and to the use of such compounds in enhancing alertness and treating anxiety, overdoses of benzodiazepine-type drugs, Down Syndrome, depression, sleep, seizure and cognitive disorders both in human as well as domestic pets and livestock.
- the compounds of this invention are also useful as probes for the localization of cell surface receptors.
- the GABA A receptor superfamily represents one of the classes of receptors through which the major inhibitory neurotransmitter, ⁇ -aminobutyric acid, or GABA, acts. Widely, although unequally, distributed through the mammalian brain, GABA mediates many of its actions through a complex of proteins called the GABA A receptor, which causes alteration in chloride conductance and membrane polarization.
- GABA A receptor subunits A number of cDNAs for GABA A receptor subunits have been characterized. To date at least 6 ⁇ , 3 ⁇ , 3 ⁇ , 1 ⁇ , 1 ⁇ and 2 ⁇ subunits have been identified. It is generally accepted that native GABA A receptors are typically composed of 2 ⁇ , 2 ⁇ , and 1 ⁇ subunits (Pritchett & Seeburg Science 1989; 245:1389-1392 and Knight et. al., Recept. Channels 1998; 6:1-18).
- Benzodiazepines exert their pharmacological actions by interacting with the benzodiazepine binding sites associated with the GABA A receptor.
- the GABA A receptor contains sites of interaction for several other classes of drugs. These include a steroid binding site, a picrotoxin site, and the barbiturate site.
- the benzodiazepine site of the GABA A receptor is a distinct site on the receptor complex that does not overlap with the site of interaction for GABA or for other classes of drugs that bind to the receptor (see, e.g., Cooper, et al., The Biochemical Basis of Neuropharmacology, 6 th ed., 1991, pp. 145-148, Oxford University Press, New York).
- GABA A selective ligands may also act to potentiate the effects of certain other CNS active compounds.
- selective serotonin reuptake inhibitors SSRIs
- SSRIs selective serotonin reuptake inhibitors
- This invention relates to heteroaryl fused aminoalkyl-derivatives.
- Preferred compounds of the invention that bind with high affinity to the benzodiazepine site of the GABA A receptor, including human GABA A receptors.
- Preferred compounds of the invention also bind with high selectivity to the benzodiazepine site of the GABA A receptor.
- the invention provides novel compounds of Formula I (shown below), and pharmaceutical compositions comprising compounds of Formula I.
- the invention further comprises methods of treating patients suffering from certain CNS disorders with an effective amount of a compound of the invention.
- the patient may be a human or other mammal. Treatment of humans, domesticated companion animals (pets) or livestock animals suffering such conditions with an effective amount of a compound of the invention is contemplated by the invention.
- the invention provides a method of potentiating the actions of other CNS active compounds. This method comprises administering an effective amount of a compound of the invention with another CNS active compound.
- this invention relates to the use of the compounds of the invention as probes for the localization of GABA A receptors in tissue sections.
- probes are useful for in vitro studies, such as binding assays and autoradiography of tissue sections and for in vivo techniques such as PET and SPECT scans.
- the invention provides a method of potentiating the actions of other CNS active compounds. This method comprises administering an effective amount of a compound of the invention with another CNS active compound.
- the invention furthermore provides methods of using compounds of this invention as positive controls in assays for receptor activity and using appropriately labeled compounds of the invention as probes for the localization of receptors, particularly GABA A receptors, in tissue sections.
- probes are useful for in vitro studies, such as binding assays and autoradiography of tissue sections and for in vivo techniques such as PET and SPECT scans.
- Z is O, or S
- R 1 represents phenyl, C 1 -C 6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- R 3 represents C 1 -C 6 alkyl, allyl, cyclopropylmethyl, cyclopentyl;
- X represents a bond, CH 2 , or CHCH
- A,B,C,D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
- Preferred compounds of the invention are highly selective agonists, antagonists or inverse agonists for GABA A brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors, the benzodiazepine receptor. These compounds are useful in the diagnosis and treatment of anxiety, Down Syndrome, depression, sleep and seizure disorders, cognitive disorders overdose with benzodiazepine drugs, and enhancement of alertness, both in human and non-human animals and domestic pets, especially dogs and cats and farm animals such as sheep, swine and cattle.
- the invention also provides methods and compositions for treating and diagnosing anxiety, Down Syndrome, depression, sleep, cognitive and seizure disorders, and overdose with benzodiazepine drugs.
- the invention encompasses compounds that are intermediates in the synthesis of the compounds of Formula I.
- Z is O, or S
- R 1 represents phenyl, C 1 -C 6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- R 2 represents
- C 1 -C 6 alkyl or C 1 -C 6 alkoxy each of which are optionally substituted with amino, mono or di(C 1 -C 6 ) alkylamino, a C 5 -C 7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion;
- NR 8 R 9 forms a 5-, 6-, or 7-membered heterocyclic ring
- R 3 represents
- C 1 -C 6 alkyl or C 1 -C 6 alkoxy each of which is optionally substituted with amino, mono or di(C 1 -C 6 ) alkylamino, a C 5 -C 7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion;
- NR 8 R 9 forms a 5-, 6-, 7-membered heterocyclic ring
- R 4 , R 5 and R 6 are the same or different and represent
- C 1 -C 6 alkyl or C 1 -C 6 alkoxy each of which is optionally substituted with amino, mono or di(C 1 -C 6 ) alkylamino, a C 5 -C 7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion, C 1 -C 6 alkylthiol, or halogen;
- NR 8 R 9 forms a 5-, 6-, or 7-membered heterocyclic ring
- R 4 and R 5 can form a 1,3-dioxolene ring
- X represents a bond, CH 2 , or CHCH
- A, B, C, and D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
- R 2 may also represent
- R n and R k independently represent C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 1 -C 6 cycloalkyl (C 1 -C 6 ) alkyl, benzoyl where the phenyl portion is optionally substituted with halgoen, C 1 -C 6 alkyl, or C 1 -C 6 alkoxy;
- p, s, and t independently represent 1 or 2;
- J is CH, N, O, S, or a carbon atom substituted with C 1 -C 6 alkyl; or
- NR k R n represents
- Preferred compounds of the invention are represented by Formula II.
- R 1 represents phenyl, C 1 -C 6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- X represents a bond, CH 2 , CHCH
- A,B,C,D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
- Z is O, or S
- R 1 represents phenyl, C 1 -C 6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- X represents a bond, CH 2 , CHCH
- A,B,C,D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
- R 4 , R 5 , and R 6 are as defined above for Formula I;
- R 1 and R 3 are independently C 1 -C 6 alkyl; and R a and R b are independently
- R n and R k independently represent C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 1 -C 6 cycloalkyl (C 1 -C 6 ) alkyl, benzoyl where the phenyl portion is optionally substituted with halgoen, C 1 -C 6 alkyl, or C 1 -C 6 alkoxy;
- p, s, and t independently represent 1 or 2;
- J is CH, N, O, or a carbon atom substituted with C 1 -C 6 alkyl; or
- NR k R n represents
- Preferred compounds of Formula IV include those where R 1 is propyl and R 3 is C 3 -C 5 alkyl, preferably isobutyl. More preferred compounds of IV are those where R b is hydrogen and R a is —NHR n where R n is defined as above or —NR k R n where both R n and R k are allyl or C 1 -C 6 alkyl.
- Preferred —NR k R n groups include diallylamino, dimethylamino, diethylamino, and N-ethyl-N-cyclopropylmethylamino.
- Preferred NHR n groups include those where R n is allyl, C 1 -C 6 alkyl, or a group of IV-a.
- Preferred IV-a groups include pyrrolidinyl, morpholinyl and piperidinyl.
- Particularly preferred compounds of IV are those where R 1 is propyl, R 3 is isobutyl, R b is hydrogen, and R a is
- the compounds of Formula I may contain one or more asymmetric carbon atoms, so that the compounds can exist in different stereoisomeric forms.
- These compounds can be, for example, racemates or optically active forms.
- the single enantiomers, i.e., optically active forms can be obtained by asymmetric synthesis or by resolution of the racemates. Resolution of the racemates can be accomplished, for example, by conventional methods such as crystallization in the presence of a resolving agent, or chromatography, using, for example a chiral HPLC column.
- Representative compounds of the present invention include, but are not limited to the compounds described in the Examples and their pharmaceutically acceptable acid addition salts.
- the free base can be obtained by basifying a solution of the acid salt.
- an addition salt particularly a pharmaceutically acceptable addition salt, may be produced by dissolving the free base in a suitable organic solvent and treating the solution with an acid, in accordance with conventional procedures for preparing acid addition salts from base compounds.
- Non-toxic pharmaceutical salts include salts of acids such as hydrochloric, phosphoric, hydrobromic, sulfuric, sulfinic, formic, toluenesulfonic, methanesulfonic, nitric, benzoic, citric, tartaric, maleic, hydroiodic, alkanoic such as acetic, HOOC—(CH 2 ) n -COOH where n is 0-4, and the like.
- acids such as hydrochloric, phosphoric, hydrobromic, sulfuric, sulfinic, formic, toluenesulfonic, methanesulfonic, nitric, benzoic, citric, tartaric, maleic, hydroiodic, alkanoic such as acetic, HOOC—(CH 2 ) n -COOH where n is 0-4, and the like.
- acids such as hydrochloric, phosphoric, hydrobromic, sulfuric, sulfinic, formic,
- the present invention also encompasses the acylated prodrugs of the compounds of Formula I.
- acylated prodrugs of the compounds of Formula I include those skilled in the art and various synthetic methodologies which may be employed to prepare non-toxic pharmaceutically acceptable addition salts and acylated prodrugs of the compounds encompassed by Formula I.
- alkyl or “lower alkyl” in the present invention is meant C 1 -C 6 alkyl, i.e., straight or branched chain alkyl groups having 1-6 carbon atoms, such as, for example, methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, pentyl, 2-pentyl, isopentyl, neopentyl, hexyl, 2-hexyl, 3-hexyl, and 3-methylpentyl.
- Preferred C 1 -C 6 alkyl groups are methyl, ethyl, propyl, butyl, cyclopropyl or cyclopropylmethyl.
- alkoxy or “lower alkoxy” in the present invention is meant C 1 -C 6 alkoxy, i.e., straight or branched chain alkoxy groups having 1-6 carbon atoms, such as, for example, methoxy, ethoxy, propoxy, isopropoxy, n-butoxy, sec-butoxy, tert-butoxy, pentoxy, 2-pentyl, isopentoxy, neopentoxy, hexoxy, 2-hexoxy, 3-hexoxy, and 3-methylpentoxy.
- (hetero) cyclic ring is meant a ring that is either aliphatic or aromatic and optionally contains at least one hetero atom. Hetero atoms include nitrogen, sulfur, and oxygen. Examples of such (hetero) cyclic rings are cyclohexyl, cyclopenyl, cyclohexyl, piperidinyl, piperazinyl, pyrrolidinyl, morpholinyl, etc.
- heteroaryl in the present invention is meant one or more aromatic ring systems of 5-, 6-, or 7-membered rings containing at least one and up to four hetero atoms selected from nitrogen, oxygen, or sulfur.
- heteroaryl groups include, for example, thienyl, furanyl, thiazolyl, imidazolyl, (is)oxazolyl, pyridyl, pyrimidinyl, imidazolyl, (iso)quinolinyl, naphthyridinyl, benzimidazolyl, and benzoxazolyl.
- heteroaryl groups are the following:
- L is nitrogen or —CR 11 ;
- T is —NR 19 , oxygen, or sulfur
- R 11 and R 11i are the same or different and are selected from hydrogen, halogen, hydroxy, C 1 -C 6 alkyl, (C 1 -C 6 )alkoxy, amino, or mono- or di(C 1 -C 6 )alkylamino;
- R 12 , R 12i , and R 13 are the same or different and are selected from hydrogen, halogen, (C 1 -C 6 )alkyl, (C 1 -C 6 )alkoxy, amino, mono- or di(C 1 -C 6 )alkylamino, hydroxy, or trifluoromethyl; and
- R 19 is hydrogen, lower alkyl having 1-6 carbon atoms.
- the invention encompasses all possible tautomers and rotamers represented by Formula I.
- halogen in the present invention is meant fluorine, bromine, chlorine, and iodine.
- Aryl and heteroaryl fused aminoalkyl-imidazoles of Formula I and their salts are suitable for the diagnosis and treatment of anxiety, Down Syndrome, sleep and seizure disorders, overdoses of benzodiazepine-type drugs, depression and cognitive disorders and for the enhancement of alertness, both in human and non-human animals and domestic pets, especially dogs and cats and farm animals such as sheep, swine and cattle. These interactions result in the pharmacological activites of these compounds.
- the compounds of general Formula I may be administered orally, topically, parenterally, by inhalation or spray or rectally in dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles.
- parenteral as used herein includes subcutaneous injections, intravenous, intramuscular, intrasternal injection or infusion techniques.
- a pharmaceutical formulation comprising a compound of general Formula I and a pharmaceutically acceptable carrier.
- One or more compounds of general Formula I may be present in association with one or more non-toxic pharmaceutically acceptable carriers and/or diluents and/or adjuvants and if desired other active ingredients.
- compositions containing compounds of general Formula I may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsion, hard or soft capsules, or syrups or elixirs.
- compositions intended for oral use may be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents and preserving agents in order to provide pharmaceutically elegant and palatable preparations.
- Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients which are suitable for the manufacture of tablets.
- excipients may be for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example starch, gelatin or acacia, and lubricating agents, for example magnesium stearate, stearic acid or talc.
- the tablets may be uncoated or they may be coated by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay material such as glyceryl monosterate or glyceryl distearate may be employed.
- Formulations for oral use may also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin or olive oil.
- an inert solid diluent for example, calcium carbonate, calcium phosphate or kaolin
- water or an oil medium for example peanut oil, liquid paraffin or olive oil.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions.
- excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydropropylmethylcellulose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents may be a naturally-occurring phosphatide, for example, lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbitan monoo
- the aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- preservatives for example ethyl, or n-propyl p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl p-hydroxybenzoate
- flavoring agents for example ethyl, or n-propyl p-hydroxybenzoate
- sweetening agents such as sucrose or saccharin.
- Oily suspensions may be formulated by suspending the active ingredients in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
- the oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol.
- Sweetening agents such as those set forth above, and flavoring agents may be added to provide palatable oral preparations. These compositions may be preserved by the addition of an anti-oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives.
- a dispersing or wetting agent e.g., sodium EDTA
- suspending agent e.g., sodium EDTA
- preservatives e.g., sodium EDTA, sodium bicarbonate, sodium bicarbonate
- compositions of the invention may also be in the form of oil-in-water emulsions.
- the oily phase may be a vegetable oil, for example olive oil or arachis oil, or a mineral oil, for example liquid paraffin or mixtures of these.
- Suitable emulsifying agents may be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol, anhydrides, for example sorbitan monoleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monoleate.
- the emulsions may also contain sweetening and flavoring agents.
- Syrups and elixirs may be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative and flavoring and coloring agents.
- the pharmaceutical compositions may be in the form of a sterile injectable aqueous or oleaginous suspension. This suspension may be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents which have been mentioned above.
- the sterile injectable preparation may also be sterile injectable solution or suspension in a non-toxic parentally acceptable diluent or solvent, for example as a solution in 1,3-butanediol.
- Suitable vehicles and solvents that may be employed are water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil may be employed including synthetic mono-or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- the compounds of general Formula I may also be administered in the form of suppositories for rectal administration of the drug.
- These compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- Such materials are cocoa butter and polyethylene glycols.
- Compounds of general Formula I may be administered parenterally in a sterile medium.
- the drug depending on the vehicle and concentration used, can either be suspended or dissolved in the vehicle.
- adjuvants such as local anesthetics, preservatives and buffering agents can be dissolved in the vehicle.
- Dosage levels of the order of from about 0.1 mg to about 140 mg per kilogram of body weight per day are useful in the treatment of the above-indicated conditions (about 0.5 mg to about 7 g per patient per day).
- the amount of active ingredient that may be combined with the carrier materials to produce a single dosage form will vary depending upon the host treated and the particular mode of administration. Dosage unit forms will generally contain between from about 1 mg to about 500 mg of an active ingredient.
- Frequency of dosage may also vary depending on the compound used and the particular disease treated. However, for treatment of most disorders, a dosage regimen of 4 times daily or less is preferred. For the treatment of anxiety or depression a dosage regimen of 1 or 2 times daily is particularly preferred. For the treatment of sleep disorders a single dose that rapidly reaches effective concentrations is desirable.
- Preferred compounds of the invention will have certain pharmacological properties. Such properties include, but are not limited to oral bioavailability, low toxicity, low serum protein binding and desirable in vitro and in vivo half-lifes. Penetration of the blood brain barrier for compounds used to treat CNS disorders is necessary, while low brain levels of compounds used to treat periphereal disorders are often preferred.
- Assays may be used to predict these desirable pharmacological properties. Assays used to predict bioavailability include transport across human intestinal cell monolayers, including Caco-2 cell monolayers. Toxicity to cultured hepatocyctes may be used to predict compound toxicity. Penetration of the blood brain barrier of a compound in humans may be predicted from the brain levels of the compound in laboratory animals given the compound intravenously. Serum protein binding may be predicted from albumin binding assays. Such assays are described in a review by Oravcová, et al. (Journal of Chromatography B (1996) volume 677, pages 1-27).
- Compound half-life is inversely proportional to the frequency of dosage of a compound.
- In vitro half-lifes of compounds may be predicted from assays of microsomal half-life as described by Kuhnz and Gieschen (Drug Metabolism and Disposition, (1998) volume 26, pages 1120-1127).
- the present invention also pertains to packaged pharmaceutical compositions for treating disorders responsive to GABA A receptor modulation, e.g., treatment of cognitive deficits, anxiety or depression by GABA A receptor modulation.
- the packaged pharmaceutical compositions include a container holding a therapeutically effective amount of at least one GABA A receptor modulator as described supra and instructions (e.g., labeling) indicating the contained GABA A receptor ligand is to be used for treating a disorder responsive to GABA A receptor modulation in the patient.
- the present invention also pertains to methods for altering the signal-tranducing activity of GABA A receptors, said method comprising exposing cells expressing such receptor to an effective amount of a compound of the invention.
- a method of inhibiting the binding of a benzodiazepine compound to the benzodiazepine site of the GABA A receptor comprising contacting a compound of Formula I with cells expressing such a receptor in the presence of a the benzodiazepine compound, wherein the compound is present at a concentration sufficient to inhibit benzodiazepine compound binding to cells expressing a cloned human GABA A receptor in vitro is provided by a separate aspect of the invention.
- the invention provides a method of potentiating the actions of other CNS active compounds, which comprises administering an effective amount of a compound of the invention in combination with another CNS active compound.
- CNS active compounds include, but are not limited to the following: for anxiety, serotonin receptor (e.g. 5-HT 1A ) agonists and antagonists; for anxiety and depression, neurokinin receptor antagonists or corticotropin releasing factor receptor (CRF 1 ) antagonists; for sleep disorders, melatonin receptor agonists; and for neurodegenerative disorders, such as Alzheimer's dementia, nicotinic agonists, muscarinic agents, acetylcholinesterase inhibitors and dopamine receptor agonists.
- SSRIs selective serotonin reuptake inhibitors
- Combination administration can be carried out in an analogous fashion to that disclosed in Da-Rocha, et al., J. Psychopharmacology (1997) 11(3) 211-218; Smith, et al., Am. J. Psychiatry (1998) 155(10) 1339-45; and Le, et al., Alcohol and Alcoholism (1996) 31 Suppl. 127-132.
- a solution of 150 mL (2.37 mole) of chloroacetonitrile, 139 mL (2.37 mole) of ethanol in 1,200 mL of dry benzene is cooled to 0° C. in an ice/ethanol bath. Dry HCl gas is bubbled through the vigorously stirred solution for approximately 30 min. while the internal temperature is maintained below 10° C. The solution is allowed to stand at rt. overnight. The resulting solid is filtered and washed with 2L of dry ether and allowed to air dry to afford 328 g (88%) of imidate hydrochloride.
- a solution of 11.25 g (0.07 mole) of 2-n-Propyl-5-fluorophenelyenediamine in 200 mL of anhydrous CHCl 13 is treated with 11.06 g (0.07 mole) of imidate at room temperature.
- the heterogeneous reaction mixture is allowed to stir for 45 min. at which time no starting material is detectable by TLC.
- 100 mL of saturated NaHCO 3 is added and extracted 3 ⁇ 50 mL of CH 2 Cl 2 .
- a solution of 20 g (0.095 mole) of [3-nitro-4-(propylamino)phenyl]methan-1-ol and 19.2 g (0.28 mole) of imidazole in 200 mL of anhydrous DMF is treated with 19 g (0.13 mole) of t-butyldimethylsilyl chloride at room temperature for 30 min.
- the resulting mixture is diluted with 400 mL of ethyl acetate and washed 3 ⁇ 200 mL of water and 1 ⁇ 200 mL of brine.
- the resulting orgainc layer is dried over anhydrous Na 2 SO 4 and the solvent removed in vacuo.
- 2,5-difluorobenzoylchloride 1.5 eq is treated with 1.0 eq 1.25 g (3.3 mmole) of propyl ( ⁇ 1-propyl-5-[(1,1,2,2-tetramethyl-1-silapropoxy)methyl]benzimidazol-2-yl ⁇ methyl) amine in dichloromethane at room temperature for 1 hr.
- the reaction is quenched with 1 N NaOH and partitioned between dichloromethane and water.
- the organic layer is dried over anhydrous Na 2 SO 4 and the solvent removed in vacuo.
- a solution of 0.2 mL of 0.2M (2,5-difluorophenyl)-N- ⁇ ([5-(chloromethyl)-1-propylbenzimidazol-2-yl]methyl ⁇ -N-propylcarboxamide in 1-methyl-2-pyrrolidinone is treated at room temperature for 16 hr with 0.3 mL of 0.2M solution of morpholine in toluene.
- the resulting mixture is diluted with 2 mL of ethyl acetate and washed 2 ⁇ 2 mL of water 1 ⁇ 2 mL brine.
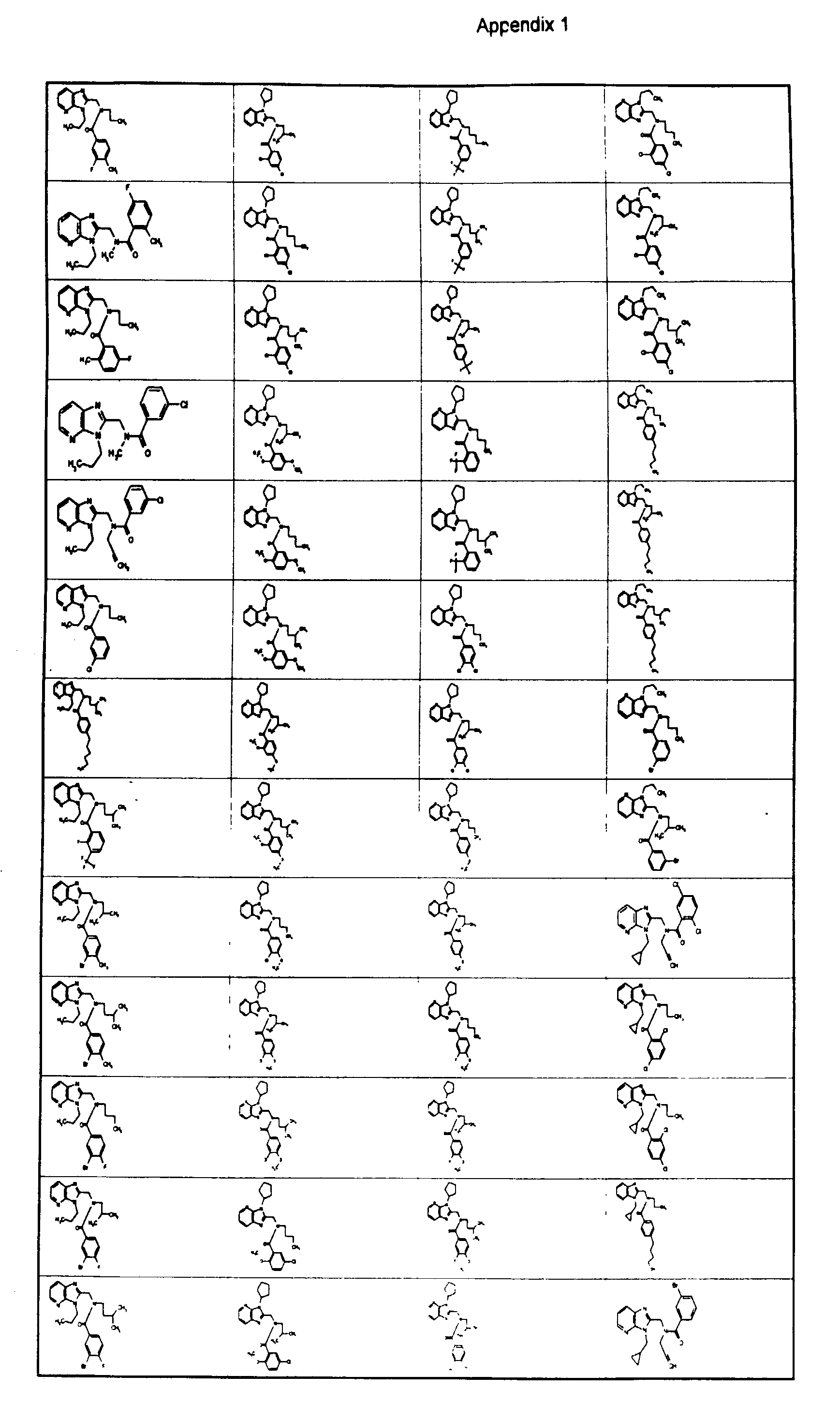
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 1 Methyl 3-Fluorophenyl 2 Allyl 3-Fluorophenyl 3 Propyl 3-Fluorophenyl 4 Allyl 3-Fluorophenyl 5 Propyl 3-Fluorophenyl 6 Propyl 3,4-Difluorophenyl 7 Allyl 2,5-Difluorophenyl 8 Propyl 2,5-Difluorophenyl 9 Propyl 1,3-Benzodioxol-5-yl 10 Allyl 3-Chloro-4-fluorophenyl 11 Propyl 3-Chloro-4-fluorophenyl 12 Methyl 5-Chloro-2-methoxyphenyl 13 3-Methylbutyl 3- ⁇ 2-[(3- Methoxypropyl)amino]ethoxy ⁇ phenyl 14 3-Methylbutyl
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 273 2-Methylpropyl 2,4,6-Trifluorophenyl 274 3-Methylbutyl 2,4,6-Trifluorophenyl 275 2-Methylpropyl 2,3,6-Trifluorophenyl 276 Pentyl 2,3,6-Trifluorophenyl 277 3-Methylbutyl 2,3,6-Trifluorophenyl 278 Pentyl 2-Chloro-6-fluorophenyl 279 3-Methylbutyl 2-Chloro-6-fluorophenyl 280 Pentyl 2-Fluoro-6- trifluoromethylphenyl 281 3-Methylbutyl 2-Fluoro-6- trifluoromethylphenyl 282 Pentyl 3-Bromo-4-fluorophenyl 283 2-Methylpropyl 4-Hexylphen
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 Propyl 3-Chlorophenyl 558 Propyl Phenyl 559 Allyl 2-Fluorophenyl 560 Propyl 2-Fluorophenyl 561 Propyl 3-Fluoro-4- methylphenyl 562 Methyl 2,5-Dichlorophenyl 563 Propyl 2,5-Dichlorophenyl 564 Propyl 4-Pentylphenyl 565 Propyl 3-Bromophenyl 566 Propyl 3-Methyl-2-thienyl
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 683 Allyl 3-Fluorophenyl 684 Allyl 3,4-Difluorophenyl 685 Propyl 1,3-Benzodioxol-5-yl 686 Allyl 5-Chloro-2-methoxyphenyl 687 Propyl 3-Methyl-2-Thienyl 688 Propyl 3-Fluoro-4-methylphenyl 689 Propyl 3-Fluorophenyl 690 Propyl 3,4-Difluorophenyl 691 Allyl 3-Chloro-4-fluorophenyl 692 Propyl 5-Chloro-2-methoxyphenyl 693 Allyl Phenyl 694 Allyl 5-Fluoro-2-methylphenyl 695 Propyl 4-Fluorophenyl 696 Allyl 2,5-Difluorophenyl 697
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 715 Methyl 3-Fluorophenyl 716 Methyl 5-Fluoro-2-methylphenyl 717 Methyl 3-Chlorophenyl 718 Methyl 5-Chloro-2-methoxyphenyl 839 2-Methylpropyl 2,3,6-Trifluorophenyl 840 Pentyl 2,3,6-Trifluorophenyl 841 3-Methylbutyl 2,3,6-Trifluorophenyl 938 Butyl Phenyl 939 2-Methylpropyl Phenyl 940 Pentyl Phenyl 941 3-Methylbutyl Phenyl 942 Butyl 3-Methylphenyl 943 2-Methylpropyl 3-Methylphenyl 944 3-Methylbutyl 3-Methylphenyl 945 2-Met
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 719 Propyl 3-Fluorophenyl 720 Propyl 1,3-Benzodioxol-5-yl 721 Propyl 5-Fluoro-2-methylphenyl 722 Allyl 2-Fluorophenyl 723 Propyl 3-Chloro-4-fluorophenyl 724 Propyl 3-Chlorophenyl 725 Propyl 2-Fluorophenyl 726 Allyl 5-Chloro-2-methoxyphenyl 727 Allyl 3-Chlorophenyl 728 Methyl 3-Fluorophenyl 729 Methyl 2,5-Difluorophenyl 730 Propyl Phenyl 731 Propyl 3-Chlorophenyl 732 Allyl 3-Fluorophenyl 733 Propyl 2,5-Difluorophenyl 734 Propyl
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 800 Propyl Phenyl 801 Methyl 3-Chlorophenyl 802 Allyl 3-Chlorophenyl 803 Propyl 3-Chlorophenyl 804 Propyl 5-Chloro-2-methoxyphenyl 805 Propyl 3-Trifluoromethylphenyl 806 Propyl 2,5-Dichlorophenyl 807 Propyl 3-Bromophenyl 808 Propyl 3-Bromo-4-fluorophenyl 809 Methyl 3-Iodophenyl 810 Allyl 3-Iodophenyl 811 Propyl 3-Iodophenyl 888 Allyl 5-Chloro-2-methoxyphenyl 931 Propyl 3-Fluorophenyl 932 Propyl 2-Fluorophenyl 1092 Propyl 3-Fluorophenyl 1093 Propyl
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 889 Methyl 2,5-Difluorophenyl 890 Methyl 2,5-Dichlorophenyl 891 Propyl 3-Bromophenyl 892 Methyl 3-Iodophenyl 893 Allyl 3-Iodophenyl 894 Propyl 3-Iodophenyl 1126 Propyl 2,5-Dichlorophenyl 1127 Methyl 3-Bromophenyl 1128 Allyl 3-Bromophenyl 1432 Propyl 3-Bromo-4-fluorophenyl 1517 2-Methylpropyl 3-Fluorophenyl 1518 2-Methylpropyl 3,4-Dimethylphenyl 1519 2-Methylpropyl 3-Methoxyphenyl 1520 2-Methylpropyl 3-Fluoro-4-methylphenyl 1521 Cyclo
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 902 Allyl 3-Bromo-4-methylphenyl 903 Propyl 3-Bromo-4-methylphenyl 904 Allyl 3-Bromo-4-fluorophenyl 905 Propyl 3-Bromo-4-fluorophenyl 906 Methyl 3-Iodophenyl 907 Allyl 3-Iodophenyl 908 Propyl 3-Iodophenyl 909 Propyl 3-Iodo-4-methylphenyl 910 Methyl 2-Thienyl 911 Methyl 3-Thienyl 912 Methyl 3-Methyl-2-thienyl 913 Propyl 5-Methyl-2-thienyl 914 Propyl Phenyl 915 Methyl 3-Methylphenyl 916 Propyl 3-Fluorophenyl 917 Propyl 2-Fluor
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 818 Propyl 3-Fluorophenyl 819 Propyl 2-Fluorophenyl 820 Propyl 3,4-Difluorophenyl 821 Methyl 2,5-Difluorophenyl 822 Allyl 2,5-Difluorophenyl 823 Propyl 2,5-Difluorophenyl 824 Propyl 1,3-Benzodioxol-5-yl 825 Propyl 3-Chloro-4-fluorophenyl 826 Methyl 5-Chloro-2-methoxyphenyl 827 Ethyl 5-Chloro-2-methoxyphenyl 828 Allyl 5-Chloro-2-methoxyphenyl 829 Propyl 5-Chloro-2-methoxyphenyl 830 Methyl 2,5-Dichlorophenyl 831 Allyl 2,5
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 955 Methyl Phenyl 956 Propyl Phenyl 957 Methyl 3-Methylphenyl 958 Propyl 3-Methylphenyl 959 Methyl 3-Fluorophenyl 960 Propyl 3-Fluorophenyl 961 Methyl 2-Fluorophenyl 962 Allyl 2-Fluorophenyl 963 Propyl 2-Fluorophenyl 964 Methyl 5-Fluoro-2-methylphenyl 965 Methyl 3-Chlorophenyl 966 Propyl 3-Chlorophenyl 989 Propyl 3-Chloro-4-fluorophenyl 994 Methyl 2-Thienyl 995 Propyl 2-Thienyl 996 Methyl 3-Thienyl 997 Methyl 3-Methyl
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 967 Propyl Phenyl 968 Propyl 3-Methylphenyl 969 Propyl 4-Methylphenyl 970 Propyl 3-Fluorophenyl 971 Propyl 2-Fluorophenyl 972 Propyl 5-Fluoro-2-methylphenyl 973 Ethyl 3-Chlorophenyl 974 Allyl 3-Chlorophenyl 975 Propyl 3-Chlorophenyl 990 Propyl 1,3-Benzodioxol-5-yl 991 Allyl 3-Chloro-4-fluorophenyl 992 Propyl 3-Chloro-4-fluorophenyl 1103 Propyl 5-Chloro-2-methoxyphenyl 1104 Propyl 3-Trifluoromethylphenyl 1105 Propyl 3,4-Dichlorophenyl 1106 Allyl 2,
- R 2 and R 3 are specified in the following table.
- Compound No R 2 R 3 1220 2-Methylpropyl Phenyl 1221 2-Methylpropyl 3-Methylpropyl 1222 2-Methylpropyl 4-Methylpropyl 1223 2-Methylpropyl 2-Fluorophenyl 1224 2-Methylpropyl 4-Ethylphenyl 1225 2-Methylpropyl 3,4-Dimethylphenyl 1227 2-Methylpropyl 2,5-Difluorophenyl 1228 2-Methylpropyl 2,4-Difluorophenyl 1229 2-Methylpropyl 1,3-Benzodioxol-5-yl 1230 2-Methylpropyl 4-Bromophenyl 1251 2-Methylpropyl 3-Bromo-4-methylphenyl 1272 2-Methylpropyl 3-Chloro
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 1226 Propyl 2-Fluorophenyl 1231 Allyl 5-Chloro-2-methoxyphenyl 1232 Propyl 5-Chloro-2-methoxyphenyl 1233 Methyl 2,5-Dichlorophenyl 1234 Allyl 2,5-Dichlorophenyl 1235 Propyl 2,5-Dichlorophenyl 1236 Methyl 3-Bromophenyl 1237 Allyl 3-Bromophenyl 1238 Propyl 3-Bromophenyl 1252 Propyl 3-Iodophenyl 1748 2-Methylpropyl 2,3,6-Trifluorophenyl 1749 3-Methylbutyl 2,3,6-Trifluorophenyl 1750 2-Methylpropyl 3-Chloro-4-phenyl 1751 3-Methylbutyl 3-Chloro-4-phenyl 1751 3-Methylbut
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 1250 Propyl 3-Iodophenyl 1616 2-Methylpropyl 3-Chloro-4-fluorophenyl 1617 2-Methylpropyl 3-Bromophenyl 1618 3-Methylbutyl 3-Bromophenyl 1634 2-Methylpropyl 3-Bromo-4-methylphenyl 1635 2-Methylpropyl 3-Bromo-4-fluorophenyl 1636 3-Methylbutyl 3-Bromo-4-fluorophenyl 1637 2-Methylpropyl 3-Iodophenyl 1638 3-Methylbutyl 3-Bromo-4-fluorophenyl 1639 2-Methylpropyl Phenyl 1640 3-Methylbutyl Phenyl 1641 2-Methylpropyl 3-Methylphenyl 1642 3-Met
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 1276 2-Methylpropyl Phenyl 1277 Pentyl Phenyl 1278 3-Methylbutyl Phenyl 1279 3-Methylbutyl 3-Methylphenyl 1280 2-Methylpropyl 4-Methylphenyl 1281 2-Methylpropyl 3-Fluorophenyl 1282 3-Methylbutyl 3-Fluorophenyl 1283 2-Methylpropyl 4-Fluorophenyl 1284 Butyl 2-Fluorophenyl 1285 2-Methylpropyl 2-Fluorophenyl 1286 Pentyl 2-Fluorophenyl 1287 3-Methylbutyl 2-Fluorophenyl 1288 2-Methylpropyl 3-Methoxyphenyl 1289 3-Methylbut
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 2004 2-Methylpropyl 2-(4-Chlorophenyl)ethenyl
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 2025 3-Pyrrolinyl 2,5-Difluorophenyl 2026 3-Pyrrolinyl 3-Fluorophenyl 2027 Pyrrolidinyl 2,5-Difluorophenyl 2028 Pyrrolidinyl 3-Fluorophenyl 2029 1,2,5,6-Tetrahydro 2,5-Difluorophenyl pyridyl 2030 1,2,5,6-Tetrahydro 3-Fluorophenyl pyridyl 2031 Piperidyl 2,5-Difluorophenyl 2032 Piperidyl 3-Fluorophenyl 2039 Morpholinyl 2,5-Difluorophenyl 2040 Morpholinyl 3-Fluorophenyl 2043 4-Methyl 2,5-Difluorophenyl piperidyl
- R 2 and R 3 are specified in the following table.
- Compound No. R 4 R 3 2033 Pyrrolidinyl 2,5-Difluorophenyl 2034 Pyrrolidinyl 3-Fluorophenyl 2035 1,2,5,6-Tetrahydro 2,5-Difluorophenyl pyridyl 2036 1,2,5,6-Tetrahydro 3-Fluorophenyl pyridyl 2037
- Piperidyl 2,5-Difluorophenyl 2038 Morpholinyl 3-Fluorophenyl 2041
- 4-Methyl 2,5-Difluorophenyl piperidyl 2042 4-Methyl 3-Fluorophenyl piperidyl 2045 Azaperhydro 3-Fluorophenyl Epinyl 2048 1,4-Thiazaper 3-Fluorophenyl hydroin-4-yl 2051 3,3-dimethyl 2,
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 2147 3-Methylbutyl 3-Chlorophenyl 2219 3-Methylbutyl 3-Trifluoromethylphenyl 2220 Butyl 3-Bromophenyl 2221 2-Methylpropyl 3-Bromophenyl 2222 3-Methylbutyl 3-Bromophenyl
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 2186 Butyl 2,5-Dimethoxyphenyl 2187 2-Methylpropyl 2,5-Dimethoxyphenyl 2188 3-Methylbutyl 2,5-Dimethoxyphenyl 2189 Butyl 3-Chloro-4-methoxyphenyl 2190 2-Methylpropyl 3-Chloro-4-methoxyphenyl 2191 3-Methylbutyl 3-Chloro-4-methoxyphenyl 2192 Butyl 5-Chloro-2-methoxyphenyl 2193 2-Methylpropyl 5-Chloro-2-methoxyphenyl 2194 3-Methylbutyl 5-Chloro-2-methoxyphenyl 2195 2-Methylpropyl 4-Chloro-2-methoxyphenyl 2196 Butyl 3-Trifluoromethylphenyl 2197 2-Methylpropyl
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 2380 2-Methylpropyl 2,4-Difluorophenyl 2381 2-Methylpropyl 2H-Benzo[d]1,3-dioxolane 2382 2-Methylpropyl 3-Chloro-4-methylphenyl
- R 2 and R 3 are specified in the following table.
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 2383 2-Methylpropyl 3-Chloro-4-methylphenyl 2384 2-Methylpropyl 2,4-Difluorophenyl 2385 2-Methylpropyl 2H-Benzo [d]1,3-dioxolane
- R 2 and R 3 are specified in the following table.
- Compound No. R 2 R 3 2389 Pentyl 3-Fluoro-4-methylphenyl
- the following assay is a standard assay for GABA A receptor binding.
- Rat cortical tissue is dissected and homogenized in 25 volumes (w/v) of Buffer A (0.05 M Tris HCl buffer, pH 7.4 at 4° C.). The tissue homogenate is centrifuged in the cold (4° C.) at 20,000 ⁇ g for 20 minutes. The supernatant is decanted, the pellet rehomogenized in the same volume of buffer, and centrifuged again at 20,000 ⁇ g. The supernatant of this centrifugation step is decanted and the pellet stored at ⁇ 20° C. overnight.
- Buffer A 0.05 M Tris HCl buffer, pH 7.4 at 4° C.
- the pellet is then thawed and resuspended in 25 volumes of Buffer A (original wt/vol), centrifuged at 20,000 ⁇ g and the supernatant decanted. This wash step is repeated once. The pellet is finally resuspended in 50 volumes of Buffer A.
- Buffer A original wt/vol
- a competition binding curve is obtained with up to 11 points spanning the compound concentration range from 10 ⁇ 12 M to 10 ⁇ 5 M obtained per curve by the method described above for determining percent inhibition.
- K i values are calculated according the Cheng-Prussof equation. When tested in this assay compounds of the invention exihibit K i values of less than 1 uM, preferred compounds of the invention have K i values of less than 500 nM and more compounds of the invention have K i values of less than 100 nM.
- the following assay is used to determine if a compound of the invention act as an agonist, an antagonist, or an inverse agonist at the benzodiazepine site of the GABA A receptor.
- Assays are carried out as described in White and Gurley (NeuroReport 6: 1313-1316, 1995) and White, Gurley, Hartnett, Stirling, and Gregory (Receptors and Channels 3: 1-5, 1995) with modifications. Electrophysiological recordings are carried out using the two electrode voltage-clamp technique at a membrane holding potential of ⁇ 70 mV. Xenopus Laevis oocytes are enzymatically isolated and injected with non-polyadenylated cRNA mixed in a ratio of 4:1:4 for , and subunits, respectively. Of the nine combinations of , and subunits described in the White et al. publications, preferred combinations are 1 2 2, 2 3 2, 3 3 2, and 5 3 2.
- each combination Preferably all of the subunit cRNAs in each combination are human clones or all are rat clones.
- the sequence of each of these cloned subunits is available from GENBANK, e.g., human 1, GENBANK accession no. X14766, human 2, GENBANK accession no. A28100; human 3, GENBANK accession no. A28102; human 5, GENBANK accession no. A28104; human 2, GENBANK accession no. M82919; human 3, GENBANK accession no. Z20136; human 2, GENBANK accession no. X15376; rat 1, GENBANK accession no. L08490, rat 2, GENBANK accession no.
- Compounds are evaluated against a GABA concentration that evokes ⁇ 10% of the maximal evokable GABA current (e.g. 1 M -9 M). Each oocyte is exposed to increasing concentrations of compound in order to evaluate a concentration/effect relationship. Compound efficacy is calculated as a percent-change in current amplitude: 100*((Ic/I) ⁇ 1), where Ic is the GABA evoked current amplitude observed in the presence of test compound and I is the GABA evoked current amplitude observed in the absence of the test compound.
- Specificity of a compound for the benzodiazepine site is determined following completion of a concentration/effect curve. After washing the oocyte sufficiently to remove previously applied compound, the oocyte is exposed to GABA+1 ⁇ M RO15-1788, followed by exposure to GABA+1 ⁇ M RO15-1788+test compound. Percent change due to addition of compound is calculated as described above. Any percent change observed in the presence of RO15-1788 is subtracted from the percent changes in current amplitude observed in the absence of 1 ⁇ M RO15-1788. These net values are used for the calculation of average efficacy and EC 50 values by standard methods. To evaluate average efficacy and EC 50 values, the concentration/effect data are averaged across cells and fit to the logistic equation.
- the compounds of the invention are prepared as radiolabeled probes by carrying out their synthesis using precursors comprising at least one atom that is a radioisotope.
- the radioisotope is preferably selected from of at least one of carbon (preferably 14 C), hydrogen (preferably 3 H), sulfur (preferably 35 S), or iodine (preferably 125 I).
- Such radiolabeled probes are conveniently synthesized by a radioisotope supplier specializing in custom synthesis of radiolabeled probe compounds. Such suppliers include Amersham Corporation, Arlington Heights, Ill.; Cambridge Isotope Laboratories, Inc.
- Tritium labeled probe compounds are also conveniently prepared catalytically via platinum-catalyzed exchange in tritiated acetic acid, acid-catalyzed exchange in tritiated trifluoroacetic acid, or heterogeneous-catalyzed exchange with tritium gas. Such preparations are also conveniently carried out as a custom radiolabeling by any of the suppliers listed in the preceding paragraph using the compound of the invention as substrate. In addition, certain precursors may be subjected to tritium-halogen exchange with tritium gas, tritium gas reduction of unsaturated bonds, or reduction using sodium borotritide, as appropriate.
- Receptor autoradiography (receptor mapping) of NK-3 or GABA A receptors in cultured cells or tissue samples is carried out in vitro as described by Kuhar in sections 8.1.1 to 8.1.9 of Current Protocols in Pharmacology (1998) John Wiley & Sons, New York, using radiolabeled compounds of the invention prepared as described in the preceding Example.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
or the pharmaceutically acceptable non-toxic salts thereof wherein the A, B, C, D, X, R1, R2, R3, R4, R5, and R6, are variables defined herein, which compounds are highly selective agonists, antagonists or inverse agonists for GABAa brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors, and are therefore useful in the diagnosis and treatment of anxiety, Down Syndrome, sleep, cognitive and seizure disorders, depression, overdose with benzodiazepine drugs, and enhancement of memory and alertness.
Description
- This application claims the benefit of U.S. Provisional Application No. 60/127,526, filed Apr. 2, 1999.
- This invention relates to heteroaryl fused aminoalkylimidazole derivatives which when appropriately substituted selectively bind to GABA A receptors. This invention also relates to pharmaceutical compositions comprising such compounds and to the use of such compounds in enhancing alertness and treating anxiety, overdoses of benzodiazepine-type drugs, Down Syndrome, depression, sleep, seizure and cognitive disorders both in human as well as domestic pets and livestock.
- The compounds of this invention are also useful as probes for the localization of cell surface receptors.
- The GABA A receptor superfamily represents one of the classes of receptors through which the major inhibitory neurotransmitter, γ-aminobutyric acid, or GABA, acts. Widely, although unequally, distributed through the mammalian brain, GABA mediates many of its actions through a complex of proteins called the GABAA receptor, which causes alteration in chloride conductance and membrane polarization.
- A number of cDNAs for GABA A receptor subunits have been characterized. To date at least 6α, 3β, 3γ, 1ε, 1δ and 2ρ subunits have been identified. It is generally accepted that native GABAA receptors are typically composed of 2α, 2β, and 1γ subunits (Pritchett & Seeburg Science 1989; 245:1389-1392 and Knight et. al., Recept. Channels 1998; 6:1-18). Evidence such as message distribution, genome localization and biochemical study results suggest that the major naturally occurring receptor combinations are α1β2γ2, α2β3γ2, α3β3γ2, and α5β3γ2 (Mohler et. al. Neuroch. Res. 1995; 20(5): 631-636).
- Benzodiazepines exert their pharmacological actions by interacting with the benzodiazepine binding sites associated with the GABA A receptor. In addition to the benzodiazepine site, the GABAA receptor contains sites of interaction for several other classes of drugs. These include a steroid binding site, a picrotoxin site, and the barbiturate site. The benzodiazepine site of the GABAA receptor is a distinct site on the receptor complex that does not overlap with the site of interaction for GABA or for other classes of drugs that bind to the receptor (see, e.g., Cooper, et al., The Biochemical Basis of Neuropharmacology, 6th ed., 1991, pp. 145-148, Oxford University Press, New York). Early electrophysiological studies indicated that a major action of the benzodiazepines was enhancement of GABAergic inhibition. Compounds that selectively bind to the benzodiazepine site and enhance the ability of GABA to open GABAA receptor channels are agonists of GABA receptors. Other compounds that interact with the same site but negatively modulate the action of GABA are called inverse agonists. Compounds belonging to a third class bind selectively to the benzodiazepine site and yet have little or no effect on GABA activity, but can block the action of GABAA receptor agonists or inverse agonists that act at this site. These compounds are referred to as antagonists.
- The important allosteric modulatory effects of drugs acting at the benzodiazepine site were recognized early and the distribution of activities at different receptor subtypes has been an area of intense pharmacological discovery. Agonists that act at the benzodiazepine site are known to exhibit anxiolytic, sedative, and hypnotic effects, while compounds that act as inverse agonists at this site elicit anxiogenic, cognition enhancing, and proconvulsant effects. While benzodiazepines have a long history of pharmaceutical use as anxiolytics, these compounds often exhibit a number of unwanted side effects. These may include cognitive impairment, sedation, ataxia, potentiation of ethanol effects, and a tendency for tolerance and drug dependence.
- GABA A selective ligands may also act to potentiate the effects of certain other CNS active compounds. For example, there is evidence that selective serotonin reuptake inhibitors (SSRIs) may show greater antidepressant activity when when used in combination with GABAA selective ligands than when used alone.
- This invention relates to heteroaryl fused aminoalkyl-derivatives. Preferred compounds of the invention that bind with high affinity to the benzodiazepine site of the GABA A receptor, including human GABAA receptors. Preferred compounds of the invention also bind with high selectivity to the benzodiazepine site of the GABAA receptor.
- The invention provides novel compounds of Formula I (shown below), and pharmaceutical compositions comprising compounds of Formula I.
- The invention further comprises methods of treating patients suffering from certain CNS disorders with an effective amount of a compound of the invention. The patient may be a human or other mammal. Treatment of humans, domesticated companion animals (pets) or livestock animals suffering such conditions with an effective amount of a compound of the invention is contemplated by the invention.
- In a separate aspect, the invention provides a method of potentiating the actions of other CNS active compounds. This method comprises administering an effective amount of a compound of the invention with another CNS active compound.
- Additionally this invention relates to the use of the compounds of the invention as probes for the localization of GABA A receptors in tissue sections. Such probes are useful for in vitro studies, such as binding assays and autoradiography of tissue sections and for in vivo techniques such as PET and SPECT scans.
- Packaged pharmaceutical compositions including instructions for use of the composition are also included.
- In a separate aspect, the invention provides a method of potentiating the actions of other CNS active compounds. This method comprises administering an effective amount of a compound of the invention with another CNS active compound.
- The invention furthermore provides methods of using compounds of this invention as positive controls in assays for receptor activity and using appropriately labeled compounds of the invention as probes for the localization of receptors, particularly GABA A receptors, in tissue sections. Such probes are useful for in vitro studies, such as binding assays and autoradiography of tissue sections and for in vivo techniques such as PET and SPECT scans.
-
- or the pharmaceutically acceptable non-toxic salts thereof wherein:
-
- where Z is O, or S;
- R 1 represents phenyl, C1-C6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- R 2 represents hydroxyl, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring; or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring;
- R 3 represents C1-C6 alkyl, allyl, cyclopropylmethyl, cyclopentyl;
- or benzyl optionally mono-, di-, or trisubstituted independently with halogen, nitro, trifluoromethyl, trifluoromethoxy, cyano, hydroxyl, C 1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring; or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring, additional substitution on the benzyl ring can be directly bound or O(CH2)n (where n=1,2,3,4) linked SO2R8, NHSO2R8, SO2NHR8, SO2NHCOR8, CONHSO2R8, as well as tetrazole, triazole, imidazole, thiazole, oxazole, thiophene, and pyridyl;
- R 4, R5 and R6 are the same or different and represent hydrogen, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring, C1-C6 alkylthiol, or halogen, or O(CH2)nco2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or straight or branched chain lower alkyl having 1-6 carbon atoms, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring, additionally R4 and R5 can form a 1,3-dioxolene ring;
- X represents a bond, CH 2, or CHCH;
- A,B,C,D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
- Preferred compounds of the invention are highly selective agonists, antagonists or inverse agonists for GABA A brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors, the benzodiazepine receptor. These compounds are useful in the diagnosis and treatment of anxiety, Down Syndrome, depression, sleep and seizure disorders, cognitive disorders overdose with benzodiazepine drugs, and enhancement of alertness, both in human and non-human animals and domestic pets, especially dogs and cats and farm animals such as sheep, swine and cattle.
- Thus, the invention also provides methods and compositions for treating and diagnosing anxiety, Down Syndrome, depression, sleep, cognitive and seizure disorders, and overdose with benzodiazepine drugs.
- In another aspect, the invention encompasses compounds that are intermediates in the synthesis of the compounds of Formula I.
-
- or pharmaceutically acceptable non-toxic salts thereof wherein:
-
- where
- Z is O, or S;
- R 1 represents phenyl, C1-C6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- R 2 represents
- hydroxyl;
- C 1-C6 alkyl or C1-C6 alkoxy, each of which are optionally substituted with amino, mono or di(C1-C6) alkylamino, a C5-C7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion;
- O(CH 2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl; or
- NR 8R9 forms a 5-, 6-, or 7-membered heterocyclic ring;
- R 3 represents
- C 1-C6 alkyl, allyl, cyclopropylmethyl, cyclopentyl; or
- benzyl optionally mono-, di-, or trisubstituted independently with
- halogen, nitro, trifluoromethyl, trifluoromethoxy, cyano, or hydroxy;
- C 1-C6 alkyl or C1-C6 alkoxy, each of which is optionally substituted with amino, mono or di(C1-C6) alkylamino, a C5-C7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion;
- O(CH 2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl;
- NR 8R9 forms a 5-, 6-, 7-membered heterocyclic ring;
- SO 2R8, NHSO2R8, SO2NHR8, SO2NHCOR8, CONHSO2R8 where R8 is defined as above;
- O(CH 2)n-G where n=1,2,3,4 and G is SO2R8, NHSO2R8, SO2NHR8, SO2NHCOR8, or CONHSO2R8, where R8 is as defined above; or
- tetrazole, triazole, imidazole, thiazole, oxazole, thiophene, or pyridyl;
- R 4, R5 and R6 are the same or different and represent
- hydrogen; or
- C 1-C6 alkyl or C1-C6 alkoxy, each of which is optionally substituted with amino, mono or di(C1-C6) alkylamino, a C5-C7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion, C1-C6 alkylthiol, or halogen;
- O(CH 2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl;
- NR 8R9 forms a 5-, 6-, or 7-membered heterocyclic ring; or
- R 4 and R5 can form a 1,3-dioxolene ring;
- X represents a bond, CH 2, or CHCH; and
- A, B, C, and D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
- In formula I, R 2 may also represent
- hydrogen or
-
- where
- R n and Rk independently represent C1-C6 alkyl, C2-C6 alkenyl, C1-C6 cycloalkyl (C1-C6) alkyl, benzoyl where the phenyl portion is optionally substituted with halgoen, C1-C6 alkyl, or C1-C6 alkoxy;
-
- where
- p, s, and t independently represent 1 or 2;
- J is CH, N, O, S, or a carbon atom substituted with C 1-C6 alkyl; or
-
- where s, t, and J are as defined above.
-
- R 1 represents phenyl, C1-C6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- R 2 represents hydroxyl, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring; or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring;
- R 3 represents C1-C6 alkyl, allyl, cyclopropylmethyl, cyclopentyl; or benzyl optionally mono-, di-, or trisubstituted independently with halogen, nitro, trifluoromethyl, trifluoromethoxy, cyano, hydroxyl, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring; or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring, additional substitution on the benzyl ring can be directly bound or O(CH2)n (where n=1,2,3,4) linked SO2R8, NHSO2R8, SO2NHR8, SO2NHCOR8, CONHSO2R8, as well as tetrazole, triazole, imidazole, thiazole, oxazole, thiophene, and pyridyl;
- R 4, R5 and R6 are the same or different and represent hydrogen, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring, C1-C6 alkylthiol, or halogen, or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or straight or branched chain lower alkyl having 1-6 carbon atoms, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring, additionally R4 and R5 can form a 1,3-dioxolene ring;
- X represents a bond, CH 2, CHCH;
- A,B,C,D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
-
- where
- Z is O, or S;
- R 1 represents phenyl, C1-C6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
- R 2 represents hydroxyl, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring; or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring;
- R 3 represents C1-C6 alkyl, allyl, cyclopropylmethyl, cyclopentyl; or benzyl optionally mono-, di-, or trisubstituted independently with halogen, nitro, trifluoromethyl, trifluoromethoxy, cyano, hydroxyl, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring; or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring, additional substitution on the benzyl ring can be directly bound or O(CH2)n (where n=1,2,3,4) linked SO2R8, NHSO2R8, SO2NHR8, SO2NHCOR8, CONHSO2R8, as well as tetrazole, triazole, imidazole, thiazole, oxazole, thiophene, and pyridyl;
- R 4, R5 and R6 are the same or different and represent hydrogen, C1-C6 alkyl or C1-C6 alkoxy, either of which could be substituted with amino or mono or di(C1-C6) alkylamino, additionally the alkyl portion can form a 5,6,7 member ring, C1-C6 alkylthiol, or halogen, or O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or straight or branched chain lower alkyl having 1-6 carbon atoms, additionally R8 and R9 can be a 5,6,7 member heterocyclic ring, additionally R4 and R5 can form a 1,3-dioxolene ring;
- X represents a bond, CH 2, CHCH;
- A,B,C,D are the same or different and represent CH or N with the proviso that not more than two of A,B,C, or D represent N.
-
- where
- R 4, R5, and R6 are as defined above for Formula I;
- R 1 and R3 are independently C1-C6 alkyl; and Ra and Rb are independently
- hydrogen or
-
- where
- R n and Rk independently represent C1-C6 alkyl, C2-C6 alkenyl, C1-C6 cycloalkyl (C1-C6) alkyl, benzoyl where the phenyl portion is optionally substituted with halgoen, C1-C6 alkyl, or C1-C6 alkoxy;
-
- where
- p, s, and t independently represent 1 or 2;
- J is CH, N, O, or a carbon atom substituted with C 1-C6 alkyl; or
-
- where s, t, and J are as defined above.
- Preferred compounds of Formula IV include those where R 1 is propyl and R3 is C3-C5 alkyl, preferably isobutyl. More preferred compounds of IV are those where Rb is hydrogen and Ra is —NHRn where Rn is defined as above or —NRkRn where both Rn and Rk are allyl or C1-C6 alkyl.
- Preferred —NR kRn groups include diallylamino, dimethylamino, diethylamino, and N-ethyl-N-cyclopropylmethylamino.
- Preferred NHR n groups include those where Rn is allyl, C1-C6 alkyl, or a group of IV-a. Preferred IV-a groups include pyrrolidinyl, morpholinyl and piperidinyl.
- Particularly preferred compounds of IV are those where R 1 is propyl, R3 is isobutyl, Rb is hydrogen, and Ra is
- In certain situations, the compounds of Formula I may contain one or more asymmetric carbon atoms, so that the compounds can exist in different stereoisomeric forms. These compounds can be, for example, racemates or optically active forms. In these situations, the single enantiomers, i.e., optically active forms, can be obtained by asymmetric synthesis or by resolution of the racemates. Resolution of the racemates can be accomplished, for example, by conventional methods such as crystallization in the presence of a resolving agent, or chromatography, using, for example a chiral HPLC column.
- Representative compounds of the present invention, which are encompassed by Formula I, include, but are not limited to the compounds described in the Examples and their pharmaceutically acceptable acid addition salts. In addition, if the compound of the invention is obtained as an acid addition salt, the free base can be obtained by basifying a solution of the acid salt. Conversely, if the product is a free base, an addition salt, particularly a pharmaceutically acceptable addition salt, may be produced by dissolving the free base in a suitable organic solvent and treating the solution with an acid, in accordance with conventional procedures for preparing acid addition salts from base compounds.
- Non-toxic pharmaceutical salts include salts of acids such as hydrochloric, phosphoric, hydrobromic, sulfuric, sulfinic, formic, toluenesulfonic, methanesulfonic, nitric, benzoic, citric, tartaric, maleic, hydroiodic, alkanoic such as acetic, HOOC—(CH 2)n-COOH where n is 0-4, and the like. Those skilled in the art will recognize a wide variety of non-toxic pharmaceutically acceptable addition salts.
- The present invention also encompasses the acylated prodrugs of the compounds of Formula I. Those skilled in the art will recognize various synthetic methodologies which may be employed to prepare non-toxic pharmaceutically acceptable addition salts and acylated prodrugs of the compounds encompassed by Formula I.
- By “alkyl” or “lower alkyl” in the present invention is meant C 1-C6 alkyl, i.e., straight or branched chain alkyl groups having 1-6 carbon atoms, such as, for example, methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, pentyl, 2-pentyl, isopentyl, neopentyl, hexyl, 2-hexyl, 3-hexyl, and 3-methylpentyl. Preferred C1-C6 alkyl groups are methyl, ethyl, propyl, butyl, cyclopropyl or cyclopropylmethyl.
- By “alkoxy” or “lower alkoxy” in the present invention is meant C 1-C6 alkoxy, i.e., straight or branched chain alkoxy groups having 1-6 carbon atoms, such as, for example, methoxy, ethoxy, propoxy, isopropoxy, n-butoxy, sec-butoxy, tert-butoxy, pentoxy, 2-pentyl, isopentoxy, neopentoxy, hexoxy, 2-hexoxy, 3-hexoxy, and 3-methylpentoxy.
- By (hetero) cyclic ring is meant a ring that is either aliphatic or aromatic and optionally contains at least one hetero atom. Hetero atoms include nitrogen, sulfur, and oxygen. Examples of such (hetero) cyclic rings are cyclohexyl, cyclopenyl, cyclohexyl, piperidinyl, piperazinyl, pyrrolidinyl, morpholinyl, etc.
- By heteroaryl (aromatic heterocycle) in the present invention is meant one or more aromatic ring systems of 5-, 6-, or 7-membered rings containing at least one and up to four hetero atoms selected from nitrogen, oxygen, or sulfur. Such heteroaryl groups include, for example, thienyl, furanyl, thiazolyl, imidazolyl, (is)oxazolyl, pyridyl, pyrimidinyl, imidazolyl, (iso)quinolinyl, naphthyridinyl, benzimidazolyl, and benzoxazolyl.
-
- wherein
- L is nitrogen or —CR 11;
- T is —NR 19, oxygen, or sulfur;
- R 11 and R11i are the same or different and are selected from hydrogen, halogen, hydroxy, C1-C6 alkyl, (C1-C6)alkoxy, amino, or mono- or di(C1-C6)alkylamino;
- R 12, R12i, and R13 are the same or different and are selected from hydrogen, halogen, (C1-C6)alkyl, (C1-C6)alkoxy, amino, mono- or di(C1-C6)alkylamino, hydroxy, or trifluoromethyl; and
- R 19 is hydrogen, lower alkyl having 1-6 carbon atoms.
- The invention encompasses all possible tautomers and rotamers represented by Formula I.
- By the term “halogen” in the present invention is meant fluorine, bromine, chlorine, and iodine.
- Aryl and heteroaryl fused aminoalkyl-imidazoles of Formula I and their salts are suitable for the diagnosis and treatment of anxiety, Down Syndrome, sleep and seizure disorders, overdoses of benzodiazepine-type drugs, depression and cognitive disorders and for the enhancement of alertness, both in human and non-human animals and domestic pets, especially dogs and cats and farm animals such as sheep, swine and cattle. These interactions result in the pharmacological activites of these compounds.
- The compounds of general Formula I may be administered orally, topically, parenterally, by inhalation or spray or rectally in dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles. The term parenteral as used herein includes subcutaneous injections, intravenous, intramuscular, intrasternal injection or infusion techniques. In addition, there is provided a pharmaceutical formulation comprising a compound of general Formula I and a pharmaceutically acceptable carrier. One or more compounds of general Formula I may be present in association with one or more non-toxic pharmaceutically acceptable carriers and/or diluents and/or adjuvants and if desired other active ingredients. The pharmaceutical compositions containing compounds of general Formula I may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsion, hard or soft capsules, or syrups or elixirs.
- Compositions intended for oral use may be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents and preserving agents in order to provide pharmaceutically elegant and palatable preparations. Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients which are suitable for the manufacture of tablets. These excipients may be for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example starch, gelatin or acacia, and lubricating agents, for example magnesium stearate, stearic acid or talc. The tablets may be uncoated or they may be coated by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period. For example, a time delay material such as glyceryl monosterate or glyceryl distearate may be employed.
- Formulations for oral use may also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin or olive oil.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions. Such excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydropropylmethylcellulose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents may be a naturally-occurring phosphatide, for example, lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbitan monooleate. The aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- Oily suspensions may be formulated by suspending the active ingredients in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin. The oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as those set forth above, and flavoring agents may be added to provide palatable oral preparations. These compositions may be preserved by the addition of an anti-oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives. Suitable dispersing or wetting agents and suspending agents are exemplified by those already mentioned above. Additional excipients, for example sweetening, flavoring and coloring agents, may also be present.
- Pharmaceutical compositions of the invention may also be in the form of oil-in-water emulsions. The oily phase may be a vegetable oil, for example olive oil or arachis oil, or a mineral oil, for example liquid paraffin or mixtures of these. Suitable emulsifying agents may be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol, anhydrides, for example sorbitan monoleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monoleate. The emulsions may also contain sweetening and flavoring agents.
- Syrups and elixirs may be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative and flavoring and coloring agents. The pharmaceutical compositions may be in the form of a sterile injectable aqueous or oleaginous suspension. This suspension may be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents which have been mentioned above. The sterile injectable preparation may also be sterile injectable solution or suspension in a non-toxic parentally acceptable diluent or solvent, for example as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that may be employed are water, Ringer's solution and isotonic sodium chloride solution. In addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose any bland fixed oil may be employed including synthetic mono-or diglycerides. In addition, fatty acids such as oleic acid find use in the preparation of injectables.
- The compounds of general Formula I may also be administered in the form of suppositories for rectal administration of the drug. These compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug. Such materials are cocoa butter and polyethylene glycols.
- Compounds of general Formula I may be administered parenterally in a sterile medium. The drug, depending on the vehicle and concentration used, can either be suspended or dissolved in the vehicle. Advantageously, adjuvants such as local anesthetics, preservatives and buffering agents can be dissolved in the vehicle.
- Dosage levels of the order of from about 0.1 mg to about 140 mg per kilogram of body weight per day are useful in the treatment of the above-indicated conditions (about 0.5 mg to about 7 g per patient per day). The amount of active ingredient that may be combined with the carrier materials to produce a single dosage form will vary depending upon the host treated and the particular mode of administration. Dosage unit forms will generally contain between from about 1 mg to about 500 mg of an active ingredient.
- Frequency of dosage may also vary depending on the compound used and the particular disease treated. However, for treatment of most disorders, a dosage regimen of 4 times daily or less is preferred. For the treatment of anxiety or depression a dosage regimen of 1 or 2 times daily is particularly preferred. For the treatment of sleep disorders a single dose that rapidly reaches effective concentrations is desirable.
- It will be understood, however, that the specific dose level for any particular patient will depend upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diet, time of administration, route of administration, and rate of excretion, drug combination and the severity of the particular disease undergoing therapy.
- Preferred compounds of the invention will have certain pharmacological properties. Such properties include, but are not limited to oral bioavailability, low toxicity, low serum protein binding and desirable in vitro and in vivo half-lifes. Penetration of the blood brain barrier for compounds used to treat CNS disorders is necessary, while low brain levels of compounds used to treat periphereal disorders are often preferred.
- Assays may be used to predict these desirable pharmacological properties. Assays used to predict bioavailability include transport across human intestinal cell monolayers, including Caco-2 cell monolayers. Toxicity to cultured hepatocyctes may be used to predict compound toxicity. Penetration of the blood brain barrier of a compound in humans may be predicted from the brain levels of the compound in laboratory animals given the compound intravenously. Serum protein binding may be predicted from albumin binding assays. Such assays are described in a review by Oravcová, et al. (Journal of Chromatography B (1996) volume 677, pages 1-27).
- Compound half-life is inversely proportional to the frequency of dosage of a compound. In vitro half-lifes of compounds may be predicted from assays of microsomal half-life as described by Kuhnz and Gieschen (Drug Metabolism and Disposition, (1998) volume 26, pages 1120-1127).
- The present invention also pertains to packaged pharmaceutical compositions for treating disorders responsive to GABA A receptor modulation, e.g., treatment of cognitive deficits, anxiety or depression by GABAA receptor modulation. The packaged pharmaceutical compositions include a container holding a therapeutically effective amount of at least one GABAA receptor modulator as described supra and instructions (e.g., labeling) indicating the contained GABAA receptor ligand is to be used for treating a disorder responsive to GABAA receptor modulation in the patient.
- The present invention also pertains to methods for altering the signal-tranducing activity of GABA A receptors, said method comprising exposing cells expressing such receptor to an effective amount of a compound of the invention.
- A method of inhibiting the binding of a benzodiazepine compound to the benzodiazepine site of the GABA A receptor, comprising contacting a compound of Formula I with cells expressing such a receptor in the presence of a the benzodiazepine compound, wherein the compound is present at a concentration sufficient to inhibit benzodiazepine compound binding to cells expressing a cloned human GABAA receptor in vitro is provided by a separate aspect of the invention.
- In a separate aspect, the invention provides a method of potentiating the actions of other CNS active compounds, which comprises administering an effective amount of a compound of the invention in combination with another CNS active compound. Such CNS active compounds include, but are not limited to the following: for anxiety, serotonin receptor (e.g. 5-HT 1A) agonists and antagonists; for anxiety and depression, neurokinin receptor antagonists or corticotropin releasing factor receptor (CRF1) antagonists; for sleep disorders, melatonin receptor agonists; and for neurodegenerative disorders, such as Alzheimer's dementia, nicotinic agonists, muscarinic agents, acetylcholinesterase inhibitors and dopamine receptor agonists. Particularly the invention provides a method of potentiating the antidepressant activity of selective serotonin reuptake inhibitors (SSRIs) by administering an effective amount of a GABA agonist compound of the invention in combination with an SSRI.
- Combination administration can be carried out in an analogous fashion to that disclosed in Da-Rocha, et al., J. Psychopharmacology (1997) 11(3) 211-218; Smith, et al., Am. J. Psychiatry (1998) 155(10) 1339-45; and Le, et al., Alcohol and Alcoholism (1996) 31 Suppl. 127-132. Also see, the discussion of the use of the GABAA receptor ligand 3-(5-methylisoxazol-3-yl)-6-(1-methyl-1,2,3-triazol-4-yl) methyloxy-1,2,4-triazolo [3,4-alphthalzine in combination with nicotinic agonists, muscarinic agonists, and acetylcholinesterase inhibitors, in PCT International publications Nos. WO 99/47142, WO 99/47171, and WO 99/47131, respectively. Also see in this regard PCT International publication No. WO 99/37303 for its discussion of the use of a class of GABAA receptor ligands, 1,2,4-triazolo[4,3-b]pyridazines, in combination with SSRIs.
- The disclosures of all articles and references mentioned in in this application, including patents, are incorporated herein by reference.
-
- Those having skill in the art will recognize that the starting materials may be varied and additional steps employed to produce compounds encompassed by the present invention, as demonstrated by the following examples.
- The following examples illustrate the general procedures for the preparation of compounds of the invention using the reactions outlined above in Schemes I-VI. These examples are not to be construed as limiting the invention in scope or spirit to the specific procedures and compounds described in them.
- Analysis is performed on a Hewlett Packard 6890 GC, equipped with a dual cool on-column inlets and flame ionization detectors or mass spec detectors. All gas flows are regulated via electronic pneumatic control. The analytical column used is a Supelco PTE-5 QTM, 15 m×0.53 mm ID×0.50 μm film. GC instrument control and data collection are handled using a Perkin Elmer TurboChrom Client/Server data system. GC conditions: On-column injector 163 C. for 2.5 min., ramp at 40 C./min to 323 C. Oven program 100 C. for 1 minute, ramp at 40 C./min to 320 C. Detector temperature is set at 325 C. GC conditions: for compounds 7-12 initial temperature 200 C., ramp to 300 C. at 20 C./min on a 12 m, DB-5 column.
- 1. Imidate Hydrochloride:
- A solution of 150 mL (2.37 mole) of chloroacetonitrile, 139 mL (2.37 mole) of ethanol in 1,200 mL of dry benzene is cooled to 0° C. in an ice/ethanol bath. Dry HCl gas is bubbled through the vigorously stirred solution for approximately 30 min. while the internal temperature is maintained below 10° C. The solution is allowed to stand at rt. overnight. The resulting solid is filtered and washed with 2L of dry ether and allowed to air dry to afford 328 g (88%) of imidate hydrochloride.
-
- A solution of 11.25 g (0.07 mole) of 2-n-Propyl-5-fluorophenelyenediamine in 200 mL of anhydrous CHCl 13 is treated with 11.06 g (0.07 mole) of imidate at room temperature. The heterogeneous reaction mixture is allowed to stir for 45 min. at which time no starting material is detectable by TLC. 100 mL of saturated NaHCO3 is added and extracted 3×50 mL of CH2Cl2. The extracts are dried over anhydrous MgSO4, the solvent removed in vacuo, and the residue chromatgraphed (SiO2) with 50% ethyl acetate/hexane to afford 15 g (95%) of 1-n-Propyl-2-(chloromethyl)-5-fluorobenzimidazole.
-
- A solution of 8 mmole of 1-n-Propyl-2-(chloromethyl)-5-fluorobenzimidazole (alternatively named 2-(chloromethyl)-5-fluoro-1-propylbenzimidazole) in 20 mL of dry Acetonitrile is treated with 10 mL of 40% aqueous methylamine for 16 hr at room temperature. The solvent is removed in vacuo and the residue is partitioned between 30 mL of ethyl acetate and 10 mL of 1 N NaOH. The ethyl acetate layer is dried over anhydrous Na 2SO4 and solvent removed in vacuo to afford 1.68 g 95% of 1-n-Propyl-2-(methanamine)-5-fluorobenzimidazole. Benzoylchloride 1.5 eq is treated with of 1-n-Propyl-2-(methanamine)-5-fluorobenzimidazole 1.0 eq in dichloromethane at room temperature for 1 hr. The reaction is quenched with 1 N NaOH and partitioned between dichloromethane and water. The organic layer is dried with Na2SO4 and the solvent removed in vacuo. The residue is chromatographed (SiO2) with ethyl acetate to afford 95% of N-[benzoyl]-N-methyl-1-n-propyl-2-(methanamine)-5-fluorobenzimidazole [alternatively named N-((5-fluorobenzimidazol-2-yl)methyl)-N-methylbenzamide] (Compound A1).
-
- A solution of 20 g (0.095 mole) of [3-nitro-4-(propylamino)phenyl]methan-1-ol and 19.2 g (0.28 mole) of imidazole in 200 mL of anhydrous DMF is treated with 19 g (0.13 mole) of t-butyldimethylsilyl chloride at room temperature for 30 min. The resulting mixture is diluted with 400 mL of ethyl acetate and washed 3×200 mL of water and 1×200 mL of brine. The resulting orgainc layer is dried over anhydrous Na 2SO4 and the solvent removed in vacuo. The resulting oil is column chromatographed 5% ethyl acetate/hexanes to afford 11 g (35%) of {2-nitro-4-[(1,1,2,2-tetramethy-1-silapropoxy)methyl]phenyl} propylamine.
- A solution of 11 g (0.033 mole) of {2-nitro-4-[(1,1,2,2-tetramethy-1-silapropoxy)methyl]phenyl} propylamine in 100 mL of ethanol and 1 g 10% Pd/C is treated with 50 psi of H 2 at room temperature for 2 hr. The resulting mixture is filtered through celite, washed with 200 mL of ethanol and the solvent removed in vacuo. The crude material is treated with 9.7 g (0.06 mole) of imidate hydrochloride in 250 mL of chloroform at room temperature for 1 hr. The reaction mixture is partitioned between 200 mL sat NaHCO3 and 200 mL of chloroform. The organic layer is dried over anhydrous anhydrous Na2SO4 and the solvent removed in vacuo. The resulting oil is column chromatographed 50% ethyl acetate/hexanes to afford 6 g (52% for 2 steps) of 1-{[2-(chloromethyl)-1-propylbenzimidazol-5-yl]methoxy}-1,1,2,2-tetramethyl-1-silapropane.
- A solution of 2.0 g (5.6 mmole) of 1-{[2-(chloromethyl)-1-propylbenzimidazol-5-yl]methoxy}-1,1,2,2-tetramethyl-1-silapropane in 20 mL of anhydrous acetonitrile is treated with 10 mL of propylamine for 16 hr at room temperature. The solvent is removed in vacuo and the residue is partitioned between 30 mL of ethyl acetate and 10 mL of 1 N NaOH. The ethyl acetate layer is dried over anhydrous Na 2SO4 and solvent removed in vacuo to afford 2.1 g (99%) of propyl ({1-propyl-5-[(1,1,2,2-tetramethyl-1-silapropoxy)methyl]benzimidazol-2-yl}methyl)amine.
- 2,5-difluorobenzoylchloride 1.5 eq is treated with 1.0 eq 1.25 g (3.3 mmole) of propyl ({1-propyl-5-[(1,1,2,2-tetramethyl-1-silapropoxy)methyl]benzimidazol-2-yl}methyl) amine in dichloromethane at room temperature for 1 hr. The reaction is quenched with 1 N NaOH and partitioned between dichloromethane and water. The organic layer is dried over anhydrous Na 2SO4 and the solvent removed in vacuo. The residue is chromatographed (SiO2) with ethyl acetate to afford 74% of (2,5-difluorophenyl)-N-propyl-N-({1-propyl-5-[(1,1,2,2-tetramethyl-1-silapropoxy)methyl]benzimidazol-2-yl}methyl)carboxamide.
- A solution 1.25 g (2.4 mmole) of (2,5-difluorophenyl)-N-propyl-N-({1-propyl-5-[(1,1,2,2-tetramethyl-1-silapropoxy)methyl]benzimidazol-2-yl}methyl)carboxamide in 20 mL of THF is treated at room temperature with 3 mL of 1M tetrabutylammonium fluoride for 1 hr. The reaction solution is diluted with 20 mL of sat NaHCO 3 and extracted with 3×100 mL of dichloromethane. The organic extracts are dried over anhydrous Na2SO4 and the solvent removed in vacuo to afford 0.96 g (99%) of (2,5-difluorophenyl)-N-{[5-(hydroxymethyl)-1-propylbenzimidazol-2-yl]methyl}-N-propylcarboxamide.
- (2,5-difluorophenyl)-N-{[5-(hydroxymethyl)-1-propylbenzimidazol-2-yl]methyl}-N-propylcarboxamide 0.96 g (2.3 mmole) is treated with 30 mL of thionyl chloride for 15 min a room temperature. The resulting mixture is concentrated in vacuo and partitioned between 100 mL sat NaHCO 3 and 100 mL of ethyl acetate. The ethyl acetate layer is dried over anhydrous Na2SO4 and concentrated in vacuo. The resulting oil is chroamtoagraphed 50% ethyl acetate/hexanes to afford 0.9 g (93%) of (2,5-difluorophenyl)-N-{[5-(chloromethyl)-1-propylbenzimidazol-2-yl]methyl}-N-propylcarboxamide.
- A solution of 0.2 mL of 0.2M (2,5-difluorophenyl)-N-{([5-(chloromethyl)-1-propylbenzimidazol-2-yl]methyl}-N-propylcarboxamide in 1-methyl-2-pyrrolidinone is treated at room temperature for 16 hr with 0.3 mL of 0.2M solution of morpholine in toluene. The resulting mixture is diluted with 2 mL of ethyl acetate and washed 2×2 mL of water 1×2 mL brine. The ethyl acetate layer is dried over anhydrous Na 2SO4 and concentrated in vacuo to afford 70% of (2,5-difluorophenyl)-N-{[5-(morpholin-4-ylmethyl)-1-propylbenzimidazol-2-yl]methyl}-N-propylcarboxamide.
-
-
-
-
-
-
- (f) N-((3-n-butyl-imidazolo[5,4-b]pyridin-2-yl)methyl](3-iodophenyl)-N-propylcarboxamide (Compound A10); GC retention time=6.12 minutes.
- (g) N-[(7-chloro-1-propylbenzimidazol-2-yl)methyl](3-fluorophenyl)-N-methylcarboxamide M+ 361 amu
- (h) N-[(7-chloro-1-propylbenzimidazol-2-yl)methyl](3-fluorophenyl)-N-propylcarboxamide M+ 389 amu
- (i) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl]{3-[(methylamino)methyl]phenyl}-N-propylcarboxamide M+ 414 amu
- (j) (3-fluorophenyl)-N-[(4-fluoro-1-propylbenzimidazol-2-yl)methyl]-N-propylcarboxamide M+ 372 amu
- (k) (2,5-difluorophenyl)-N-{[1-(cyclopropylmethyl)benzimidazol-2-yl]methyl}-N-propylcarboxamide M+ 384 amu
- (l) N-{[5-(N,N-diethylcarbamoyl)-1-propylbenzimidazol-2-yl]methyl}(3-fluorophenyl)-N-propylcarboxamide M+ 454 amu
- (m) (2,5-difluorophenyl)-N-[(4-fluoro-1-propylbenzimidazol-2-yl)methyl]-N-propylcarboxamide M+ 391 amu
- (n) N-{[6-chloro-1-(cyclopropylmethyl)benzimidazol-2-yl]methyl}(3-fluorophenyl)-N-propylcarboxamide M+ 401 amu
- (o) (2,5-difluorophenyl)-N-({5-[(ethylamino)methyl]-1-propylbenzimidazol-2-yl}methyl)-N-propylcarboxamide M+ 430 amu
- (p) (2,5-difluorophenyl)-N-propyl-N-({1-propyl-5-[(propylamino)methyl]benzimidazol-2-yl}methyl)carboxamide M+ 444 amu
- (q) (2,5-difluorophenyl)-N-({5-[(methylamino)methyl]-1-propylbenzimidazol-2-yl}methyl)-N-propylcarboxamide M+ 416 amu
- (r) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl]{4-[2-(ethylamino)ethoxy]phenyl}-N-(3-methylbutyl)carboxamide M+ 486 amu
- (s) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl]-N-(3-methylbutyl){4-[2-(propylamino)ethoxy]phenyl}carboxamide M+ 500 amu
- (t) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl](2-methyl(1,3-thiazol-4-yl))-N-(2-methylpropyl)carboxamide M+ 406 amu
- (u) (5-bromo(2-thienyl))-N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl]-N-(2-methylpropyl)carboxamide M+ 470 amu
- (v) [3-(2-bromoethoxy)phenyl]-N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl]-N-(2-methylpropyl)carboxamide M+ 508 amu
- (w) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl]-N-(2-methylpropyl){3-[2-(propylamino)ethoxy]phenyl}carboxamide M+ 486 amu
- (x) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl](3-{2-[(2-methoxyethyl)amino]ethoxy}phenyl)-N-(2-methylpropyl)carboxamide M+ 502 amu
- (y) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl](3-{2-[(2-ethoxyethyl)amino]propoxy}phenyl)-N-(2-methylpropyl)carboxamide M+ 530 amu
- (z) N-[(6-chloro-1-propylbenzimidazol-2-yl)methyl](3-(2-{[2-(methylethoxy)ethyl]amino}propoxy)phenyl]-N-(2-methylpropyl)carboxamide M+ 544 amu
- The compounds of Examples 5-41 are prepared essentially according to the procedure described in Examples 1-3, and as shown in Schemes 1-6. These compounds are represented by the formulae presented in each of the examples with the definitions of the substituents found within the table. It is noted for the reader that the R 2 and R3 groups used in these formulae are not the same R2 and R3 groups used in Formula I.
- Structures for the compounds of Examples 5-42 are shown in Appendices 1 and 2 hereto.
-
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 1 Methyl 3-Fluorophenyl 2 Allyl 3-Fluorophenyl 3 Propyl 3-Fluorophenyl 4 Allyl 3-Fluorophenyl 5 Propyl 3-Fluorophenyl 6 Propyl 3,4-Difluorophenyl 7 Allyl 2,5-Difluorophenyl 8 Propyl 2,5-Difluorophenyl 9 Propyl 1,3-Benzodioxol-5-yl 10 Allyl 3-Chloro-4-fluorophenyl 11 Propyl 3-Chloro-4-fluorophenyl 12 Methyl 5-Chloro-2-methoxyphenyl 13 3-Methylbutyl 3-{2-[(3- Methoxypropyl)amino]ethoxy} phenyl 14 3-Methylbutyl 3-{2-[(3- Ethoxypropyl)amino]ethoxy} phenyl 15 3-Methylbutyl 3-{2-[(3- Ethoxypropyl)amino]ethoxy} phenyl 16 3-Methylbutyl 3-[2-(Benzylamino) ethoxy]phenyl 17 3-Methylbutyl 3-[2-(Benzylamino) ethoxy]phenyl 18 2-Methylpropyl 3-{2-[(3-i- Propoxypropyl)amino]ethoxy} phenyl 19 3-Methylbutyl 3-{2-[(3-i- Propoxypropyl)amino]ethoxy} phenyl 20 Benzyl 3-Chloro-2-thienyl 21 4-Fluorobenzyl 3-Chloro-2-thienyl 22 Benzyl 3-Chloro-4-methylphenyl 23 2-Fluorobenzyl 3-Chloro-4-methylphenyl 24 4-Fluorobenzyl 3-Chloro-4-methylphenyl 25 4-Fluorobenzyl 2-Fluoro-6-trifluoromethyl phenyl 26 4-Fluorobenzyl 3,5-Dibromophenyl 27 Pentyl 3-Bromophenyl 28 3-Methylbutyl 3-Bromophenyl 29 2-Methylpropyl 4-Bromophenyl 30 3-Methylbutyl 4-Bromophenyl 31 Butyl 2-Bromophenyl 32 Pentyl 2-Bromophenyl 33 3-Methylbutyl 2-Bromophenyl 34 3-Methylbutyl 3-Methoxyphenyl 35 3-Methylbutyl 2-Methoxyphenyl 36 3-Methylbutyl 3-Chlorophenyl 37 3-Methylbutyl 2-Chlorophenyl 38 3-Methylbutyl 2-Chlorophenyl 39 Ethyl 5-Chloro-2-methoxyphenyl 40 Allyl 5-Chloro-2-methoxyphenyl 41 Propyl 5-Chloro-2-methoxyphenyl 42 Methyl 2,5-Dichlorophenyl 43 Allyl 2,5-Dichlorophenyl 44 Propyl 2,5-Dichlorophenyl 45 Propyl 5-Methyl-2-thienyl 46 Propyl Phenyl 47 Propyl 3-Methylphenyl 48 Propyl 3-Fluoro-4-methylphenyl 49 Allyl 5-Fluoro-2-methylphenyl 50 Propyl 5-Fluoro-2-methylphenyl 51 Benzyl 2,3,5,6-Tetrafluoro phenyl 52 4-Fluorobenzyl 2,3,5,6-Tetrafluoro phenyl 53 Benzyl 2,4,6-Trifluoro phenyl 54 Benzyl 2,3,6-Trifluoro phenyl 55 4-Fluorobenzyl 2,3,6-Trifluoro phenyl 56 4-Fluorobenzyl 2-Chloro-6-fluorophenyl 57 Benzyl 2-Fluoro-6-trifluoromethyl phenyl 58 2-Methylpropyl 3-(2-{[(4-Methylphenyl) methyl]amino} ethoxy)phenyl 59 3-Methylbutyl 3-{2-[(2-Cyclohex-1-enylethyl) amino]ethoxy} phenyl 60 2-Methylpropyl 3-(2-{[(2-Methylphenyl) methyl]amino} ethoxy)phenyl 61 2-Methylpropyl 3-(2-{[(3-Methylphenyl) methyl]amino} ethoxy)phenyl 62 2-Methylpropyl 3-(2-{[(2-Methoxyphenyl) methyl]amino} ethoxy)phenyl 63 2-Fluorobenzyl 3-Iodo-4-methylphenyl 64 4-Fluorobenzyl 3-Iodo-4-methylphenyl 65 4-Fluorobenzyl 2-Thienyl 66 Benzyl 2-Thienyl 67 4-Fluorobenzyl 2-Thienyl 68 Benzyl 3-Methyl-2-thienyl 69 4-Fluorobenzyl 3-Methyl-2-thienyl 70 Benzyl 5-Methyl-2-thienyl 71 2-Fluorobenzyl 5-Methyl-2-thienyl 72 4-Fluorobenzyl 5-Methyl-2-thienyl 73 4-Fluorobenzyl 4,5-Dimethyl-2-furyl 74 2-Methylpropyl 3,4-Dichlorophenyl 75 Pentyl 3,4-Dichlorophenyl 76 3-Methylbutyl 3,4-Dichlorophenyl 77 3-Methylbutyl 3,5-Dichlorophenyl 78 3-Methylbutyl 2,3-Dichlorophenyl 79 Butyl 2,5-Dichlorophenyl 80 2-Methylpropyl 2,5-Dichlorophenyl 81 Pentyl 2,5-Dichlorophenyl 82 3-Methylbutyl 2,5-Dichlorophenyl 83 Butyl 2,4-Dichlorophenyl 84 2-Methylpropyl 2,4-Dichlorophenyl 85 3-Methylbutyl 2,4-Dichlorophenyl 86 Allyl 3-Chlorophenyl 87 Propyl 3-Chlorophenyl 88 Propyl 2,3,6-Trifluorophenyl 89 Methyl 5-Chloro-2-methoxyphenyl 90 Ethyl 5-Chloro-2-methoxyphenyl 91 Allyl 5-Chloro-2-methoxyphenyl 92 Methyl 2,5-Dichlorophenyl 93 Methyl 3-Bromophenyl 94 Ethyl 3-Bromophenyl 95 Propyl 3-Bromophenyl 96 Methyl 3-Bromo-4-fluorophenyl 97 Methyl 3-Iodophenyl 98 3-Methylbutyl 3-(2-{[(2-Methoxyphenyl) methyl]amino} ethoxy)phenyl 99 2-Methylpropyl 3-(2-{[(3-Methoxyphenyl) methyl]amino} ethoxy)phenyl 100 2-Methylpropyl 3-(2-{[(4-Methoxyphenyl) methyl]amino} ethoxy)phenyl 101 2-Methylpropyl 3-(2-{[(2-Chlorophenyl) methyl]amino} ethoxy)phenyl 102 Benzyl 2,5-Dimethoxyphenyl 103 2-Fluorobenzyl 2,5-Dimethoxyphenyl 104 4-Fluorobenzyl 2,5-Dimethoxyphenyl 105 Butyl 4-Pentylphenyl 106 2-Methylpropyl 4-Pentylphenyl 107 3-Methylbutyl 4-Pentylphenyl 108 Butyl 3-Bromophenyl 109 2-Methylpropyl 3-Bromophenyl 110 Pentyl 3-Bromophenyl 111 3-Methylbutyl 3-Bromophenyl 112 2-Methylpropyl 4-Bromophenyl 113 3-Methylbutyl 4-Bromophenyl 114 Butyl 2-Bromophenyl 115 Pentyl 2-Bromophenyl 116 3-Methylbutyl 2-Bromophenyl 117 Ethyl 3-Iodophenyl 118 Allyl 3-Iodophenyl 119 Propyl 3-Chloro-4-methylphenyl 120 Propyl 5-Bromo-2-thienyl 121 Ethyl Phenyl 122 Allyl Phenyl 123 Propyl Phenyl 124 Allyl 3-Methylphenyl 125 Propyl 3-Methylphenyl 126 Propyl 4-Methylphenyl 127 Methyl 3-Fluorophenyl 128 Propyl 3-Fluorophenyl 129 Butyl 3-Chloro-4-methoxyphenyl 130 2-Methylpropyl 3-Chloro-4-methoxyphenyl 131 3-Methylbutyl 3-Chloro-4-methoxyphenyl 132 Butyl 5-Chloro-2-methoxyphenyl 133 2-Methylpropyl 5-Chloro-2-methoxyphenyl 134 Pentyl 5-Chloro-2-methoxyphenyl 135 3-Methylbutyl 5-Chloro-2-methoxyphenyl 136 Butyl 3-Trifluoromethylphenyl 137 Pentyl 3-Trifluoromethylphenyl 138 3-Methylbutyl 3-Trifluoromethylphenyl 139 3-Methylbutyl 2-Trifluoromethylphenyl 140 Butyl 3,4-Dichlorophenyl 141 Propyl 4-Fluorophenyl 142 Methyl 2-Fluorophenyl 143 Allyl 2-Fluorophenyl 144 Propyl 2-Fluorophenyl 145 Propyl 3-Fluoro-4-methylphenyl 146 Methyl 5-Fluoro-2-methylphenyl 147 Propyl 5-Fluoro-2-methylphenyl 148 Methyl 3-Chlorophenyl 149 Allyl 3-Chlorophenyl 150 Propyl 3-Chlorophenyl 151 3-Methylbutyl 4-Hexylphenyl 152 3-Methylbutyl 2-Fluoro-3- trifluoromethylphenyl 153 Butyl 2,5-Dichlorophenyl 154 2-Methylpropyl 2,5-Dichlorophenyl 155 Pentyl 2,5-Dichlorophenyl 156 3-Methylbutyl 2,5-Dichlorophenyl 157 Butyl 2,4-Dichlorophenyl 158 2-Methylpropyl 2,4-Dichlorophenyl 159 3-Methylbutyl 2,4-Dichlorophenyl 160 Butyl 4-Pentylphenyl 161 2-Methylpropyl 4-Pentylphenyl 162 3-Methylbutyl 4-Pentylphenyl 163 Butyl 3-Bromophenyl 164 2-Methylpropyl 3-Bromophenyl 165 2-Methylpropyl 3-Bromo-4-methylphenyl 166 3-Methylbutyl 3-Bromo-4-methylphenyl 167 Butyl 3-Bromo-4-fluorophenyl 168 2-Methylpropyl 3-Bromo-4-fluorophenyl 169 3-Methylbutyl 3-Bromo-4-fluorophenyl 170 Butyl 3-Iodophenyl 171 2-Methylpropyl 3-Iodophenyl 172 Pentyl 3-Iodophenyl 173 3-Methylbutyl 3-Iodophenyl 174 2-Methylpropyl 4-Iodophenyl 175 3-Methylbutyl 3-Iodo-4-methylphenyl 176 Butyl 2-Thienyl 177 Pentyl 2-Thienyl 178 3-Methylbutyl 2-Thienyl 179 Butyl 3-Thienyl 180 Pentyl 3-Thienyl 181 3-Methylbutyl 3-Thienyl 182 3-Methylbutyl Benzyl 183 Butyl 3-Methyl-2-thienyl 184 Pentyl 3-Methyl-2-thienyl 185 3-Methylbutyl 3-Methyl-2-thienyl 186 Pentyl 3-Methyl-5-thienyl 187 3-Methylbutyl 3-Methyl-5-thienyl 188 3-Methylbutyl 3-Methylphenyl 189 2-Methylpropyl 5-Chloro-2-methoxyphenyl 190 Pentyl 5-Chloro-2-methoxyphenyl 191 3-Methylbutyl 5-Chloro-2-methoxyphenyl 192 Butyl 3-Trifluoromethylphenyl 193 Pentyl 3-Trifluoromethylphenyl 194 3-Methylbutyl 3-Trifluoromethylphenyl 195 3-Methylbutyl 2-Trifluoromethylphenyl 196 Butyl 3,4-Dichlorophenyl 197 2-Methylpropyl 3,4-Dichlorophenyl 198 3-Methylbutyl 3,4-Dichlorophenyl 199 3-Methylbutyl 3,5-Dichlorophenyl 200 3-Methylbutyl 2,3-Dichlorophenyl 201 Butyl Phenyl 202 Pentyl Phenyl 203 3-Methylbutyl Phenyl 204 Pentyl 3-Methylphenyl 205 3-Methylbutyl 3-Methylphenyl 206 2-Methylpropyl 4-Methylphenyl 207 3-Methylbutyl 4-Methylphenyl 208 Pentyl 2-Methylphenyl 209 3-Methylbutyl 2-Methylphenyl 210 Butyl 3-Fluorophenyl 211 2-Methylpropyl 3-Fluorophenyl 212 Pentyl 3-Fluorophenyl 213 3-Methylbutyl 3-Fluorophenyl 214 Pentyl 4-Fluorophenyl 215 3-Methylbutyl 4-Fluorophenyl 216 Pentyl 2-Fluorophenyl 217 3-Methylbutyl 2-Fluorophenyl 218 2-Methylpropyl 3,4-Dimethylphenyl 219 3-Methylbutyl 3,4-Dimethylphenyl 220 Pentyl 2,5-Dimethylphenyl 221 3-Methylbutyl 2,5-Dimethylphenyl 222 2-Methylpropyl 2,4-Dimethylphenyl 223 3-Methylbutyl 2,4-Dimethylphenyl 224 2-Methylpropyl 3-Methoxyphenyl 225 Pentyl 3-Methoxyphenyl 226 3-Methylbutyl 3-Methoxyphenyl 227 2-Methylpropyl 4-Methoxyphenyl 228 3-Methylbutyl 4-Methoxyphenyl 229 Pentyl 2-Methoxyphenyl 230 3-Methylbutyl 2-Methoxyphenyl 231 2-Methylpropyl 3-Fluoro-4-methylphenyl 232 Pentyl 3-Fluoro-4-methylphenyl 233 3-Methylbutyl 3-Fluoro-4-methylphenyl 234 3-Methylbutyl 3-Fluoro-2-methylphenyl 235 2-Methylpropyl 5-Fluoro-2-methylphenyl 236 Pentyl 5-Fluoro-2-methylphenyl 237 2-Methylpropyl 3-Chloro-4-fluorophenyl 238 Pentyl 3-Chloro-4-fluorophenyl 239 3-Methylbutyl 3-Chloro-4-fluorophenyl 240 3-Methylbutyl 3,4,5-Trifluorophenyl 241 3-Methylbutyl 4-Butylphenyl 242 Pentyl 4-i-propylphenyl 243 3-Methylbutyl 4-i-propylphenyl 244 Butyl 4-Ethylthiophenyl 245 2-Methylpropyl 4-Ethylthiophenyl 246 3-Methylbutyl 4-Ethylthiophenyl 247 3-Methylbutyl 3-Chloro-4-methoxyphenyl 248 Butyl 5-Chloro-2-methoxyphenyl 249 3-Methylbutyl 5-Fluoro-2-methylphenyl 250 2-Methylpropyl 2-Fluoro-3-methylphenyl 251 Pentyl 2-Fluoro-3-methylphenyl 252 3-Methylbutyl 2-Fluoro-3-methylphenyl 253 2-Methylpropyl 3-Chlorophenyl 254 Pentyl 3-Chlorophenyl 255 3-Methylbutyl 3-Chlorophenyl 256 2-Methylpropyl 4-Chlorophenyl 257 3-Methylbutyl 4-Chlorophenyl 258 3-Methylbutyl 2-Chlorophenyl 259 3-Methylbutyl 3,4-Difluorophenyl 260 3-Methylbutyl 1,2-Difluorophenyl 261 Pentyl 2,5-Difluorophenyl 262 3-Methylbutyl 2,5-Difluorophenyl 263 Pentyl 2,4-Difluorophenyl 264 3-Methylbutyl 2,4-Difluorophenyl 265 3-Methylbutyl 4-Propylphenyl 266 Pentyl 1,3-Benzodioxol-5-yl 267 3-Methylbutyl 1,3-Benzodioxol-5-yl 268 3-Methylbutyl 4-Methylthio phenyl 269 3-Methylbutyl 3-Fluoro-4-methoxyphenyl 270 2-Methylpropyl 4-Chloro-3-methylphenyl 271 3-Methylbutyl 4-Chloro-3-methylphenyl 272 Butyl 3-Chloro-4-fluorophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 273 2-Methylpropyl 2,4,6-Trifluorophenyl 274 3-Methylbutyl 2,4,6-Trifluorophenyl 275 2-Methylpropyl 2,3,6-Trifluorophenyl 276 Pentyl 2,3,6-Trifluorophenyl 277 3-Methylbutyl 2,3,6-Trifluorophenyl 278 Pentyl 2-Chloro-6-fluorophenyl 279 3-Methylbutyl 2-Chloro-6-fluorophenyl 280 Pentyl 2-Fluoro-6- trifluoromethylphenyl 281 3-Methylbutyl 2-Fluoro-6- trifluoromethylphenyl 282 Pentyl 3-Bromo-4-fluorophenyl 283 2-Methylpropyl 4-Hexylphenyl 284 Butyl 4-Pentoxyphenyl 285 2-Methylpropyl 4-Pentoxyphenyl 286 Butyl 2-Fluoro-3- trifluoromethylphenyl 287 2-Methylpropyl 2-Fluoro-3- trifluoromethylphenyl 288 3-Methylbutyl 3-Bromo-4-fluorophenyl 289 2-Methylpropyl 4-heptylphenyl 290 Butyl 3-Iodophenyl 291 2-Methylpropyl 3-Iodophenyl 292 Pentyl 3-Iodophenyl 293 3-Methylbutyl 3-Iodophenyl 294 Butyl 4-Iodophenyl 295 2-Methylpropyl 4-Iodophenyl 296 2-Methylpropyl 4-Pentylphenyl 297 3-Methylbutyl 2-Fluoro-3- trifluoromethylphenyl 298 Butyl 3-Bromo-4-methylphenyl 299 2-Methylpropyl 3-Bromo-4-methylphenyl 300 Pentyl 3-Bromo-4-methylphenyl 301 3-Methylbutyl 3-Bromo-4-methylphenyl 302 Butyl 3-Bromo-4-fluorophenyl 303 2-Methylpropyl 3-Bromo-4-fluorophenyl 304 3-Methylbutyl 3,4-Dichlorophenyl 305 Butyl 2,3-Dichlorophenyl 306 2-Methylpropyl 2,3-Dichlorophenyl 307 3-Methylbutyl 2,3-Dichlorophenyl 308 Butyl 2,5-Dichlorophenyl 309 Butyl 3-Bromophenyl 310 2-Methylpropyl 3-Bromophenyl 311 Pentyl 3-Bromophenyl 312 3-Methylbutyl 3-Bromophenyl 313 Butyl 4-Bromophenyl 314 2-Methylpropyl 4-Bromophenyl 315 3-Methylbutyl 4-Bromophenyl 316 Butyl 2-Bromophenyl 317 Pentyl 2-Bromophenyl 318 3-Methylbutyl 2-Bromophenyl 319 Pentyl 4-Hexylphenyl 320 2-Methylpropyl 4-Chloro-2-methoxyphenyl 321 2-Methylpropyl 2,5-Dichlorophenyl 322 Pentyl 2,5-Dichlorophenyl 323 3-Methylbutyl 2,5-Dichlorophenyl 324 Butyl 2,4-Dichlorophenyl 325 2-Methylpropyl 2,4-Dichlorophenyl 326 Pentyl 2,4-Dichlorophenyl 327 3-Methylbutyl 2,4-Dichlorophenyl 328 2-Methylpropyl 2,5-Dimethoxyphenyl 329 Pentyl 2,5-Dimethoxyphenyl 330 3-Methylbutyl 2,5-Dimethoxyphenyl 331 2-Methylpropyl 2,4-Dimethoxyphenyl 332 3-Methylbutyl 2,4-Dimethoxyphenyl 333 Pentyl 4-Chloro-2-methoxyphenyl 334 3-Methylbutyl 4-Chloro-2-methoxyphenyl 335 Butyl 3-Trifluoromethylphenyl 336 2-Methylpropyl 3-Trifluoromethylphenyl 337 Pentyl 3-Trifluoromethylphenyl 338 3-Methylbutyl 3-Trifluoromethylphenyl 339 2-Methylpropyl 4-Trifluoromethylphenyl 340 Butyl 2-Trifluoromethylphenyl 341 3-Methylbutyl 2-Trifluoromethylphenyl 342 Butyl 3,4-Dichlorophenyl 343 2-Methylpropyl 3,4-Dichlorophenyl 344 Butyl 4-Methylthio phenyl 345 Butyl 3-Chloro-4-methoxyphenyl 346 2-Methylpropyl 3-Chloro-4-methoxyphenyl 347 3-Methylbutyl 3-Chloro-4-methoxyphenyl 348 Butyl 5-Chloro-2-methoxyphenyl 349 2-Methylpropyl 5-Chloro-2-methoxyphenyl 350 Pentyl 5-Chloro-2-methoxyphenyl 351 3-Methylbutyl 5-Chloro-2-methoxyphenyl 352 Butyl 2,5-Difluorophenyl 353 2-Methylpropyl 2,5-Difluorophenyl 354 Pentyl 2,5-Difluorophenyl 355 3-Methylbutyl 2,5-Difluorophenyl 356 Butyl 2,4-Difluorophenyl 357 2-Methylpropyl 4-Methylthio phenyl 358 Butyl 3-Fluoro-4-methoxyphenyl 359 2-Methylpropyl 3-Fluoro-4-methoxyphenyl 360 3-Methylbutyl 3-Fluoro-4-methoxyphenyl 361 2-Methylpropyl 4-Chloro-3-methylphenyl 362 Butyl 3-Chloro-4-fluorophenyl 363 2-Methylpropyl 3-Chloro-4-fluorophenyl 364 Pentyl 3-Chloro-4-fluorophenyl 365 3-Methylbutyl 3-Chloro-4-fluorophenyl 366 2-Methylpropyl 4-Ethylthiophenyl 367 Butyl 2,5-Dimethoxyphenyl 368 Butyl 2-Chlorophenyl 369 2-Methylpropyl 2,4-Difluorophenyl 370 Pentyl 2,4-Difluorophenyl 371 3-Methylbutyl 2,4-Difluorophenyl 372 Butyl 1,3-Benzodioxol-5-yl 373 2-Methylpropyl 1,3-Benzodioxol-5-yl 374 Pentyl 1,3-Benzodioxol-5-yl 375 3-Methylbutyl 1,3-Benzodioxol-5-yl 376 3-Methylbutyl 3-Fluoro-2-methylphenyl 377 Butyl 5-Fluoro-2-methylphenyl 378 2-Methylpropyl 5-Fluoro-2-methylphenyl 379 Pentyl 5 Fluoro-2-methylphenyl 380 3-Methylbutyl 5-Fluoro-2-methylphenyl 381 2-Methylpropyl 2-Chlorophenyl 382 Pentyl 2-Chlorophenyl 383 3-Methylbutyl 2-Chlorophenyl 384 Butyl 3,4-Difluorophenyl 385 2-Methylpropyl 3,4-Difluorophenyl 386 Pentyl 3,4-Difluorophenyl 387 3-Methylbutyl 3,4-Difluorophenyl 388 Butyl 2,3-Difluorophenyl 389 2-Methylpropyl 2,3-Difluorophenyl 390 Pentyl 2,3-Difluorophenyl 391 3-Methylbutyl 2,3-Difluorophenyl 392 2-Methylpropyl 4-Methoxyphenyl 393 Butyl 3-Chlorophenyl 394 2-Methylpropyl 3-Chlorophenyl 395 Pentyl 3-Chlorophenyl 396 3-Methylbutyl 3-Chlorophenyl 397 Butyl 4-Chlorophenyl 398 2-Methylpropyl 4-Chlorophenyl 399 3-Methylbutyl 4-Chlorophenyl 400 Butyl 2,5-Dimethylphenyl 401 2-Methylpropyl 2,5-Dimethylphenyl 402 Pentyl 2,5-Dimethylphenyl 403 3-Methylbutyl 2,5-Dimethylphenyl 404 Butyl 2,4-Dimethylphenyl 405 3-Methylbutyl 4-Methoxyphenyl 406 Butyl 2-Methoxyphenyl 407 2-Methylpropyl 2-Methoxyphenyl 408 Pentyl 2-Methoxyphenyl 409 3-Methylbutyl 2-Methoxyphenyl 410 Butyl 3-Fluoro-4-methylphenyl 411 2-Methylpropyl 3-Fluoro-4-methylphenyl 412 Pentyl 3-Fluoro-4-methylphenyl 413 3-Methylbutyl 3-Fluoro-4-methylphenyl 414 Butyl 3-Fluoro-2-methylphenyl 415 2-Methylpropyl 3-Fluoro-2-methylphenyl 416 Butyl 4-Fluorophenyl 417 2-Methylpropyl 2,4-Dimethylphenyl 418 3-Methylbutyl 2,4-Dimethylphenyl 419 Butyl 3-Methoxyphenyl 420 2-Methylpropyl 3-Methoxyphenyl 421 Pentyl 3-Methoxyphenyl 422 3-Methylbutyl 3-Methoxyphenyl 423 Butyl 4-Methoxyphenyl 424 3-Methylbutyl 3-Methylphenyl 425 Butyl 4-Methylphenyl 426 2-Methylpropyl 4-Methylphenyl 427 Pentyl 4-Methylphenyl 428 3-Methylbutyl 4-Methylphenyl 429 2-Methylpropyl 4-Fluorophenyl 430 Pentyl 4-Fluorophenyl 431 3-Methylbutyl 4-Fluorophenyl 432 Butyl 2-Fluorophenyl 433 2-Methylpropyl 2-Fluorophenyl 434 Pentyl 2-Fluorophenyl 435 3-Methylbutyl 2-Fluorophenyl 436 2-Methylpropyl 4-Ethylphenyl 437 Butyl 3,4-Dimethylphenyl 438 2-Methylpropyl 3,4-Dimethylphenyl 439 3-Methylbutyl 3,4-Dimethylphenyl 440 Butyl 2-Methylphenyl 441 Pentyl 2-Methylphenyl 442 3-Methylbutyl 2-Methylphenyl 443 Butyl 3-Fluorophenyl 444 2-Methylpropyl 3-Fluorophenyl 445 Pentyl 3-Fluorophenyl 446 3-Methylbutyl 3-Fluorophenyl 447 Butyl Phenyl 448 2-Methylpropyl Phenyl 449 Pentyl Phenyl 450 3-Methylbutyl Phenyl 451 Butyl 3-Methylphenyl 452 2-Methylpropyl 3-Methylphenyl 453 Pentyl 3-Methylphenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 454 Allyl 2,5-Dichlorophenyl 455 Propyl 2,5-Dichlorophenyl 456 Propyl 2,4-Dichlorophenyl 457 Propyl 4-Pentylphenyl 458 Allyl 3-Bromophenyl 459 Propyl 3-Bromophenyl 460 Propyl 4-Bromophenyl 461 Propyl 2-Chlorophenyl 462 Methyl Phenyl 463 Propyl Phenyl 464 Methyl 3-Methylphenyl 465 Propyl 3-Methylphenyl 466 Propyl 2-Chlorophenyl 467 Propyl 3,4-Difluorophenyl 468 Methyl 2,3-Difluorophenyl 469 Propyl 2,3-Difluorophenyl 470 Methyl 2,5-Difluorophenyl 471 Allyl 2,5-Difluorophenyl 472 Propyl 2,5-Difluorophenyl 473 Propyl 2,4-Difluorophenyl 474 Allyl 1,3-Benzodioxol-5-yl 475 Propyl 1,3-Benzodioxol-5-yl 476 Propyl 4-Methylthio phenyl 477 Propyl 4-Chloro-3-methylphenyl 478 Propyl 4-Methylphenyl 479 Propyl 3-Fluorophenyl 480 Propyl 4-Fluorophenyl 481 Methyl 2-Fluorophenyl 482 Allyl 2-Fluorophenyl 483 Propyl 2-Fluorophenyl 484 Propyl 3,4-Dimethylphenyl 485 Propyl 3-Fluoro-4-methylphenyl 486 Propyl 2-Fluoro-3-methylphenyl 487 Allyl 3-Chlorophenyl 488 Propyl 3-Chlorophenyl 489 Propyl 4-Chlorophenyl 490 2-Methylpropyl 3-Chloro-2-thienyl 491 Pentyl 3-Chloro-2-thienyl 492 3-Methylbutyl 3-Chloro-2-thienyl 493 Butyl 3-Ethoxy-2-thienyl 494 Pentyl 3-Ethoxy-2-thienyl 495 3-Methylbutyl 2-Methoxybenzyl 496 3-Methylbutyl 2-(2-Fluorophenyl) ethenyl 497 2-Methylpropyl 2-(2-Chlorophenyl) ethenyl 498 3-Methylbutyl 2-(2-Chlorophenyl) ethenyl 499 Pentyl 2-Fluoro-6- trifluoromethylphenyl 500 3-Methylbutyl 3-Ethoxy-2-thienyl 501 Butyl 5-Methylthio-2-thienyl 502 2-Methylpropyl 5-Methylthio-2-thienyl 503 3-Methylbutyl 5-Methylthio-2-thienyl 504 3-Methylbutyl 4-Fluorophenyl 505 3-Methylbutyl 2-Fluorophenyl 506 3-Methylbutyl 3-Methoxyphenyl 507 3-Methylbutyl 2,3,5,6-Tetrafluoro phenyl 508 2-Methylpropyl 2,4,6-Trifluoro phenyl 509 3-Methylbutyl 2,4,6-Trifluoro phenyl 510 Butyl 2,3,6-Trifluoro phenyl 511 2-Methylpropyl 2,3,6-Trifluoro phenyl 512 3-Methylbutyl 2-Fluoro-6- trifluoromethylphenyl 513 2-Methylpropyl 2,4,6-Trichlorophenyl 514 Pentyl 2,5-Dimethyl-3-furyl 515 3-Methylbutyl 4,5-Dimethyl-2-furyl 516 Butyl 3,4-Dimethyl-2-furyl 517 2-Methylpropyl 3,4-Dimethyl-2-furyl 518 Pentyl 3,4-Dimethyl-2-furyl 519 3-Methylbutyl 3,4-Dimethyl-2-furyl 520 Butyl 4-Methoxy-3-thienyl 521 3-Methylbutyl 4-Methoxy-3-thienyl 522 Butyl 3-Chloro-2-thienyl 523 Allyl 3-Bromo-4-fluorophenyl 524 Propyl 3-Bromo-4-fluorophenyl 525 Methyl 3-Iodophenyl 526 Ethyl 3-Iodophenyl 527 Allyl 3-Iodophenyl 528 Propyl 3-Iodophenyl 529 Propyl 3-Methyl-2-thienyl 530 Propyl 3-Fluorobenzyl 531 Pentyl 2,3,6-Trifluoro phenyl 532 3-Methylbutyl 2,3,6-Trifluoro phenyl 533 Butyl 2-Chloro-6-fluorophenyl 534 2-Methylpropyl 2-Chloro-6-fluorophenyl 535 Pentyl 2-Chloro-6-fluorophenyl 536 3-Methylbutyl 2-Chloro-6-fluorophenyl 537 Butyl 2-Fluoro-6- trifluoromethylphenyl 538 3-Methylbutyl 3-Chlorobenzyl 539 2-Methylpropyl 4-Chlorobenzyl 540 3-Methylbutyl 2-Chlorobenzyl 541 Butyl 2,3,5,6-Tetrafluoro phenyl 542 2-Methylpropyl 2,3,5,6-Tetrafluoro phenyl 543 Pentyl 2,3,5,6-Tetrafluoro phenyl 544 Allyl 3-Chloro-4-fluorophenyl 545 Propyl 3-Chloro-4-fluorophenyl 546 Propyl 4-Butylphenyl 547 Propyl 3-Chloro-4-methoxyphenyl 548 Allyl 5-Chloro-2-methoxyphenyl 549 Propyl 5-Chloro-2-methoxyphenyl 550 Propyl 3,4-Dichlorophenyl 551 Propyl 4-Hexylphenyl 552 Methyl 3-Bromo-4-methylphenyl 553 Allyl 3-Bromo-4-methylphenyl 554 Propyl 3-Bromo-4-methylphenyl 555 Methyl 3-Bromo-4-fluorophenyl 556 Butyl 2-Methoxybenzyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 557 Propyl 3-Chlorophenyl 558 Propyl Phenyl 559 Allyl 2-Fluorophenyl 560 Propyl 2-Fluorophenyl 561 Propyl 3-Fluoro-4- methylphenyl 562 Methyl 2,5-Dichlorophenyl 563 Propyl 2,5-Dichlorophenyl 564 Propyl 4-Pentylphenyl 565 Propyl 3-Bromophenyl 566 Propyl 3-Methyl-2-thienyl - Compound No. 567: (5-Chloro-2-methoxyphenyl)-N-({3-[(2-chlorophenyl)methyl]imidazolo[5,4-b]pyridin-2-yl}methyl-N-pentylcarboxamide.
-
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 568 Methyl Phenyl 569 Methyl 3-Chlorophenyl 570 Butyl 2,5-Dimethylphenyl 571 Butyl 5-Fluoro-2-methylphenyl 572 Butyl 2,3-Dimethylphenyl 573 Propyl 3-Fluorophenyl 574 Butyl 3-Methylphenyl 575 Butyl 4-Fluorophenyl 576 Butyl 3-Methoxyphenyl 577 Butyl 2,5-Difluorophenyl 578 Methyl 2-Fluorophenyl 579 Butyl 4-Methylphenyl 580 Butyl 2-Fluorophenyl 581 Butyl 4-Methoxyphenyl 582 Butyl 3-Chlorophenyl 583 Methyl 2,5-Dimethylphenyl 584 Butyl 2-Methylphenyl 585 Butyl 4-Ethylphenyl 586 Butyl 2-Methoxyphenyl 587 Butyl 3-Chlorophenyl 588 Propyl 3-Fluoro-4-methylphenyl 589 Butyl 3-Fluorophenyl 590 Butyl 3,4-Dimethylphenyl 591 Butyl 3-Fluoro-4-methylphenyl 592 Butyl 3,4-Difluorophenyl 593 Propyl 2,4-Dimethoxyphenyl 594 Methyl 2,5-Dichlorophenyl 595 Butyl 5-Chloro-2-methoxyphenyl 596 Butyl 3-Methyl-2-thienyl 597 Butyl 3-Methylphenyl 598 Pentyl 3-Fluorophenyl 599 Pentyl 2,5-Dimethylphenyl 600 Propyl 2,5-Dichlorophenyl 601 Butyl 3-Methyl-2-thienyl 602 Pentyl 3-Methylphenyl 603 Butyl 2-Fluorophenyl 604 Pentyl 3-Methoxyphenyl 605 Methyl 3-Bromophenyl 606 Butyl 3-Iodophenyl 607 Butyl 4-Fluorophenyl 608 2-Methylpropyl 4-Methylphenyl 609 2-Methylpropyl 2-Fluorophenyl 610 2-Methylpropyl 4-Methoxyphenyl 611 Propyl 3-Bromophenyl 612 Allyl 4-Octylphenyl 613 Butyl Phenyl 614 Pentyl 2-Methylphenyl 615 Pentyl 2-Fluorophenyl 616 Butyl 2-Methoxyphenyl 617 Butyl 3-Chloro-4-methoxyphenyl 618 Propyl 4-Octylphenyl 619 Pentyl Phenyl 620 Butyl 3-Fluorophenyl 621 2-Methylpropyl 3,4-Dimethylphenyl 622 Pentyl 2-Methoxyphenyl 623 Butyl 3-Fluoro-4-methylphenyl 624 Butyl 2-Fluoro-3-methylphenyl 625 2-Methylpropyl 4-Chlorophenyl 626 2-Methylpropyl 2,3-Difluorophenyl 627 2-Methylpropyl 1,3-Benzodioxol-5-yl 628 2-Methylpropyl 3-Chloro-4-methoxyphenyl 629 2-Methylpropyl 3-Fluoro-4-methylphenyl 630 Pentyl 2-Fluoro-3-methylphenyl 631 Pentyl 2-Chlorophenyl 632 Pentyl 2,3-Difluorophenyl 633 Butyl 4-Methylthio phenyl 634 2-Methylpropyl 3-Chloro-4-methoxyphenyl 635 Butyl 5-Fluoro-2-methylphenyl 636 Butyl 3-Chlorophenyl 637 Butyl 3,4-Difluorophenyl 638 Butyl 2,5-Difluorophenyl 639 Butyl 3-Chloro-4-fluorophenyl 640 Butyl 5-Chloro-2-methoxyphenyl 641 2-Methylpropyl 5-Fluoro-2-methylphenyl 642 2-Methylpropyl 3-Chlorophenyl 643 2-Methylpropyl 3,4-Difluorophenyl 644 Pentyl 2,5-Difluorophenyl 645 2-Methylpropyl 4-Ethylthiophenyl 646 2-Methylpropyl 5-Chloro-2-methoxyphenyl 647 Pentyl 5-Fluoro-2-methylphenyl 648 Pentyl 3-Chlorophenyl 649 Butyl 2,3-Difluorophenyl 650 2-Methylpropyl 2,4-Difluorophenyl 651 Butyl 3-Chloro-4-methoxyphenyl 652 Pentyl 5-Chloro-2-methoxyphenyl 653 3-Methylbutyl 5-Chloro-2-methoxyphenyl 654 3-Methylbutyl 2,5-Dichlorophenyl 655 2-Methylpropyl 4-Bromophenyl 656 Butyl 2-Thienyl 657 3-Methylbutyl 3-Thienyl 658 2-Methylpropyl 3-Methyl-2-thienyl 659 3-Methylbutyl 3-Trifluoromethylphenyl 660 Butyl 3-Bromophenyl 661 3-Methylbutyl 2-Bromophenyl 662 Pentyl 2-Thienyl 663 Butyl 5-Methyl-2-thienyl 664 3-Methylbutyl 3-Methyl-2-thienyl 665 2-Methylpropyl 3,4-Dichlorophenyl 666 2-Methylpropyl 3-Bromophenyl 667 3-Methylbutyl 3-Bromo-4-fluorophenyl 668 3-Methylbutyl 2-Thienyl 669 Pentyl 5-Methyl-2-thienyl 670 Butyl 3-Fluorophenyl 671 Butyl 2,5-Dichlorophenyl 672 Pentyl 3-Bromophenyl 673 Pentyl 3-Iodophenyl 674 Butyl 3-Thienyl 675 3-Methylbutyl 5-Methyl-2-thienyl 676 3-Methylbutyl 3-Fluorophenyl 677 Pentyl 2,5-Dichlorophenyl 678 3-Methylbutyl 3-Bromophenyl 679 3-Methylbutyl 3-Iodophenyl 680 Pentyl 3-Thienyl 681 Butyl 3-Methyl-2-thienyl 682 2-Methylpropyl 2-Chlorophenyl 815 2-Methylpropyl 3,5-Difluorophenyl 816 3-Methylbutyl 3,5-Difluorophenyl 817 Butyl 3,5-Difluorophenyl 2238 Benzyl 3-Fluorophenyl 2242 Benzyl 2-Fluorophenyl 2253 Benzyl 2-Methoxyphenyl 2257 Benzyl 5-Fluoro-2-methylphenyl 2260 Benzyl 3-Chlorophenyl 2268 Benzyl 2,3-Difluorophenyl 2271 Benzyl 2,5-Difluorophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 683 Allyl 3-Fluorophenyl 684 Allyl 3,4-Difluorophenyl 685 Propyl 1,3-Benzodioxol-5-yl 686 Allyl 5-Chloro-2-methoxyphenyl 687 Propyl 3-Methyl-2-Thienyl 688 Propyl 3-Fluoro-4-methylphenyl 689 Propyl 3-Fluorophenyl 690 Propyl 3,4-Difluorophenyl 691 Allyl 3-Chloro-4-fluorophenyl 692 Propyl 5-Chloro-2-methoxyphenyl 693 Allyl Phenyl 694 Allyl 5-Fluoro-2-methylphenyl 695 Propyl 4-Fluorophenyl 696 Allyl 2,5-Difluorophenyl 697 Propyl 3-Chloro-4-fluorophenyl 698 Methyl 2,5-Dichlorophenyl 699 Propyl Phenyl 700 Propyl 5-Fluoro-2-methylphenyl 701 Allyl 2-Fluorophenyl 702 Propyl 2,5-Difluorophenyl 703 Methyl 5-Chloro-2-methoxyphenyl 704 Allyl 2,5-Dichlorophenyl 705 Allyl 3-Methylphenyl 706 Allyl 3-Chlorophenyl 707 Propyl 2-Fluorophenyl 708 Allyl 1,3-Benzodioxol-5-yl 709 Ethyl 5-Chloro-2-methoxyphenyl 710 Propyl 2,5-Dichlorophenyl 711 Propyl 3-Methylphenyl 712 Propyl 3-Chlorophenyl 713 Propyl 4-Methylthio phenyl 714 Propyl 3-Iodo-4-methylphenyl 887 Propyl 2,3,6-Trifluorophenyl 2306 3-Methylbutyl 2,3,6-Trifluorophenyl 2347 3-Methylbutyl 3-(2-1,2,3,4-Teterahydro isoquinolinyl methyl) phenyl 2348 3-Methylbutyl 3-(Diethylamino methyl)phenyl 2349 3-Methylbutyl 3-(Hexylmethyl amino methyl)phenyl 2351 3-Methylbutyl 3-(Dibutylamino methyl)phenyl 2364 3-Methylbutyl 3-[(1-methylethyl) methylamino methyl]phenyl 2365 3-Methylbutyl 3-(Cyclohexyl ethylamino methyl)phenyl 2367 3-Methylbutyl 3-[bis(2-Methoxyethyl) aminomethyl] phenyl 2369 3-Methylbutyl 3-[(3,3,5-Trimethylaza perhydroepinyl)methyl]phenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 715 Methyl 3-Fluorophenyl 716 Methyl 5-Fluoro-2-methylphenyl 717 Methyl 3-Chlorophenyl 718 Methyl 5-Chloro-2-methoxyphenyl 839 2-Methylpropyl 2,3,6-Trifluorophenyl 840 Pentyl 2,3,6-Trifluorophenyl 841 3-Methylbutyl 2,3,6-Trifluorophenyl 938 Butyl Phenyl 939 2-Methylpropyl Phenyl 940 Pentyl Phenyl 941 3-Methylbutyl Phenyl 942 Butyl 3-Methylphenyl 943 2-Methylpropyl 3-Methylphenyl 944 3-Methylbutyl 3-Methylphenyl 945 2-Methylpropyl 4-Methylphenyl 946 Butyl 3-Fluorophenyl 947 Pentyl 3-Fluorophenyl 948 3-Methylbutyl 3-Fluorophenyl 949 3-Methylbutyl 4-Fluorophenyl 950 Butyl 2-Fluorophenyl 951 2-Methylpropyl 2-Fluorophenyl 952 Pentyl 2-Fluorophenyl 953 3-Methylbutyl 2-Fluorophenyl 954 2-Methylpropyl 3,4-Dimethylphenyl 1002 Butyl 2-Chlorophenyl 1003 Pentyl 2-Chlorophenyl 1004 3-Methylbutyl 2-Chlorophenyl 1005 Butyl 3,4-Difluorophenyl 1006 2-Methylpropyl 3,4-Difluorophenyl 1007 Pentyl 3,4-Difluorophenyl 1008 3-Methylbutyl 3,4-Difluorophenyl 1009 Butyl 2,3-Difluorophenyl 1010 2-Methylpropyl 2,3-Difluorophenyl 1011 Pentyl 2,3-Difluorophenyl 1012 3-Methylbutyl 2,3-Difluorophenyl 1013 Butyl 2,5-Difluorophenyl 1014 2-Methylpropyl 2,5-Difluorophenyl 1015 Pentyl 2,5-Difluorophenyl 1016 3-Methylbutyl 2,5-Difluorophenyl 1017 Butyl 2,4-Difluorophenyl 1018 2-Methylpropyl 2,4-Difluorophenyl 1019 3-Methylbutyl 2,4-Difluorophenyl 1020 2-Methylpropyl 3-Ethoxyphenyl 1021 Butyl 1,3-Benzodioxol-5-yl 1022 2-Methylpropyl 1,3-Benzodioxol-5-yl 1023 3-Methylbutyl 1,3-Benzodioxol-5-yl 1024 2-Methylpropyl 4-Methylthio phenyl 1025 3-Methylbutyl 4-Methylthio phenyl 1026 2-Methylpropyl 3-Fluoro-4-methoxyphenyl 1027 3-Methylbutyl 3-Fluoro-4-methoxyphenyl 1028 2-Methylpropyl 4-Chloro-3-methylphenyl 1029 Butyl 3-Chloro-4-fluorophenyl 1030 2-Methylpropyl 3-Chloro-4-fluorophenyl 1031 Pentyl 3-Chloro-4-fluorophenyl 1032 3-Methylbutyl 3-Chloro-4-fluorophenyl 1033 2-Methylpropyl 3,4,5-Trifluorophenyl 1034 3-Methylbutyl 3,4,5-Trifluorophenyl 1035 2-Methylpropyl 4-Butylphenyl 1036 2-Methylpropyl 4-Ethylthiophenyl 1109 Cyclopropyl Phenyl methyl 1110 Cyclopropyl 3-Methylphenyl Methyl 1111 Cyclopropyl 4-Methylphenyl Methyl 1112 Cyclopropyl 3-Fluorophenyl Methyl 1113 Cyclopropyl 2-Fluorophenyl Methyl 1114 Cyclopropyl 3-Methoxyphenyl Methyl 1115 Cyclopropyl 3-Fluoro-4-methylphenyl Methyl 1116 Cyclopropyl 5-Fluoro-2-methylphenyl methyl 1130 Cyclopropyl 5-Chloro-2-methoxyphenyl Methyl 1131 Cyclopropyl 2,5-Dichlorophenyl Methyl 1132 Cyclopropyl 3-Bromophenyl Methyl 1133 3-Methylbutyl 5-Chloro-2-methoxyphenyl 1134 Butyl 2,5-Dichlorophenyl 1135 2-Methylpropyl 2,5-Dichlorophenyl 1136 Pentyl 2,5-Dichlorophenyl 1137 3-Methylbutyl 2,5-Dichlorophenyl 1138 2-Methylpropyl 2,4-Dichlorophenyl 1139 2-Methylpropyl 4-Pentylphenyl 1140 Butyl 3-Bromophenyl 1141 2-Methylpropyl 3-Bromophenyl 1142 Pentyl 3-Bromophenyl 1143 3-Methylbutyl 3-Bromophenyl 1144 2-Methylpropyl 4-Bromophenyl 1256 Cyclopropyl 3,4-Difluorophenyl Methyl 1257 Cyclopropyl 2,4-Difluorophenyl Methyl 1258 Propyl 1,3-Benzodioxol-5-yl 1259 Cyclopropyl 1,3-Benzodioxol-5-yl Methyl 1260 Cyclopropyl 3-Chloro-4-fluorophenyl Methyl 1261 3-Methylbutyl 3-Iodo-4-methylphenyl 1262 3-Methylbutyl 2-Thienyl 1263 3-Methylbutyl 3-Thienyl 1264 2-Methylpropyl 5-Methyl-2-thienyl 1265 Pentyl 5-Methyl-2-thienyl 1266 3-Methylbutyl 5-Methyl-2-thienyl 1267 3-Methylbutyl 3-Fluorobenzyl 1448 Methyl 2,5-Difluorophenyl 1449 Methyl 2,5-Dichlorophenyl 2005 3-Methylbutyl 5-Bromo-2-thienyl 2239 Benzyl 3-Fluorophenyl 2243 Benzyl 2-Fluorophenyl 2245 Benzyl 3,4-Dimethylphenyl 2251 Benzyl 3-Methoxyphenyl 2254 Benzyl 2-Methoxyphenyl 2258 Benzyl 5-Fluoro-2-methylphenyl 2261 Benzyl 3-Chlorophenyl 2266 Benzyl 3,4-Difluorophenyl 2269 Benzyl 2,3-Difluorophenyl 2272 Benzyl 2,5-Difluorophenyl 2281 Benzyl 5-Chloro-2-methoxyphenyl 2289 Benzyl 2,5-Dichlorophenyl 2292 Benzyl 3-Bromophenyl 2295 Benzyl 2-Bromophenyl 2298 Benzyl 3-Iodophenyl 2302 Benzyl 2,5-Dimethylpyrrol-3-yl 2305 Benzyl 3-Methylbutyl 2320 3-Methylbutyl 3-(Methylamino methyl)phenyl 2321 3-Methylbutyl 3-(Ethylamino methyl)phenyl 2322 3-Methylbutyl 3-(Cyclobutyl amino methyl)phenyl 2323 3-Methylbutyl 3-[(1-Methylpropyl) amino methyl]phenyl 2324 3-Methylbutyl 3-(Cyclopentyl amino methyl)phenyl 2350 3-Methylbutyl 3-(Dibutylamino methyl)phenyl 2366 3-Methylbutyl 3-[bis(2-Methoxyethyl) aminomethyl] phenyl 2368 3-Methylbutyl 3-[(3,3,5-Trimethylaza perhydroepinyl)methyl]phenyl 2391 Methyl 2,5-Difluorophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 719 Propyl 3-Fluorophenyl 720 Propyl 1,3-Benzodioxol-5-yl 721 Propyl 5-Fluoro-2-methylphenyl 722 Allyl 2-Fluorophenyl 723 Propyl 3-Chloro-4-fluorophenyl 724 Propyl 3-Chlorophenyl 725 Propyl 2-Fluorophenyl 726 Allyl 5-Chloro-2-methoxyphenyl 727 Allyl 3-Chlorophenyl 728 Methyl 3-Fluorophenyl 729 Methyl 2,5-Difluorophenyl 730 Propyl Phenyl 731 Propyl 3-Chlorophenyl 732 Allyl 3-Fluorophenyl 733 Propyl 2,5-Difluorophenyl 734 Propyl 3-Fluoro-4-methylphenyl 735 Propyl 4-Methylthio phenyl 736 3-Methylbutyl 3-Fluorophenyl 737 2-Methylpropyl 2-Fluorophenyl 738 Butyl 3,4-Difluorophenyl 739 2-Methylpropyl 2,5-Difluorophenyl 740 3-Methylbutyl 1,3-Benzodioxol-5-yl 741 Butyl 4-Fluorophenyl 742 Pentyl 2-Fluorophenyl 743 2-Methylpropyl 3,4-Difluorophenyl 744 Pentyl 2,5-Difluorophenyl 745 Butyl 3-Chloro-4-fluorophenyl 746 Butyl 3-Fluorophenyl 747 2-Methylpropyl 4-Fluorophenyl 748 3-Methylbutyl 2-Fluorophenyl 749 Pentyl 3,4-Difluorophenyl 750 3-Methylbutyl 2,5-Difluorophenyl 751 2-Methylpropyl 3-Chloro-4-fluorophenyl 752 2-Methylpropyl 3-Fluorophenyl 753 3-Methylbutyl 4-Fluorophenyl 754 2-Methylpropyl 2,5-Dimethylphenyl 755 3-Methylbutyl 3,4-Difluorophenyl 756 Butyl 1,3-Benzodioxol-5-yl 757 Pentyl 3-Chloro-4-fluorophenyl 758 Pentyl 3-Fluorophenyl 759 Butyl 2-Fluorophenyl 760 3-Methylbutyl 2,5-Dimethylphenyl 761 Butyl 2,5-Difluorophenyl 762 2-Methylpropyl 1,3-Benzodioxol-5-yl 763 3-Methylbutyl 3-Chloro-4-fluorophenyl 764 Butyl 5-Chloro-2-methoxyphenyl 765 2-Methylpropyl 2,5-Dichlorophenyl 766 Pentyl 5-Methyl-2-thienyl 767 3-Methylbutyl Phenyl 768 2-Methylpropyl 2-Methylphenyl 769 3-Methylbutyl 5-Fluoro-2-methylphenyl 770 2-Methylpropyl 5-Chloro-2-methoxyphenyl 771 Pentyl 2,5-Dichlorophenyl 772 3-Methylbutyl 5-Methyl-2-thienyl 773 Butyl 3-Methylphenyl 774 3-Methylbutyl 2-Methylphenyl 775 Butyl 3-Chlorophenyl 776 Pentyl 5-Chloro-2-methoxyphenyl 777 3-Methylbutyl 2,5-Dichlorophenyl 778 Butyl Phenyl 779 2-Methylpropyl 3-Methylphenyl 780 2-Methylpropyl 3-Fluoro-4-methylphenyl 781 2-Methylpropyl 3-Chlorophenyl 782 3-Methylbutyl 5-Chloro-2-methoxyphenyl 783 Butyl 5-Methyl-2-thienyl 784 2-Methylpropyl Phenyl 785 Pentyl 3-Methylphenyl 786 3-Methylbutyl 3-Fluoro-4-methylphenyl 787 Pentyl 3-Chlorophenyl 788 Butyl 2,5-Dichlorophenyl 789 2-Methylpropyl 5-Methyl-2-thienyl 790 Pentyl Phenyl 791 3-Methylbutyl 3-Methylphenyl 792 Pentyl 5-Fluoro-2-methylphenyl 793 3-Methylbutyl 3-Chlorophenyl 794 2-Methylpropyl 4-Methylthio phenyl 795 2-Methylpropyl 3-Fluoro-4-methoxyphenyl 796 3-Methylbutyl 3-Fluoro-4-methoxyphenyl 797 2-Methylpropyl 2,4,6-Trifluorophenyl 798 Butyl 2,3,6-Trifluorophenyl 799 2-Methylpropyl 2,3,6-Trifluorophenyl 885 Methyl 2,3,6-Trifluorophenyl 886 Propyl 2,3,6-Trifluorophenyl 933 Propyl Phenyl 934 Propyl 3-Fluorophenyl 935 Propyl 4-Fluorophenyl 936 Allyl 2-Fluorophenyl 937 Propyl 2-Fluorophenyl 1037 Butyl 3-Chlorophenyl 1038 2-Methylpropyl 3-Chlorophenyl 1039 Pentyl 3-Chlorophenyl 1040 3-Methylbutyl 3-Chlorophenyl 1041 Butyl 3,4-Difluorophenyl 1042 2-Methylpropyl 3,4-Difluorophenyl 1043 Pentyl 3,4-Difluorophenyl 1044 3-Methylbutyl 3,4-Difluorophenyl 1045 Butyl 2,3-Difluorophenyl 1046 2-Methylpropyl 2,3-Difluorophenyl 1047 Pentyl 2,3-Difluorophenyl 1048 3-Methylbutyl 2,3-Difluorophenyl 1049 Butyl 2,5-Difluorophenyl 1050 2-Methylpropyl 2,5-Difluorophenyl 1051 Pentyl 2,5-Difluorophenyl 1052 3-Methylbutyl 2,5-Difluorophenyl 1053 Butyl 2,4-Difluorophenyl 1054 2-Methylpropyl 2,4-Difluorophenyl 1055 Pentyl 2,4-Difluorophenyl 1056 3-Methylbutyl 2,4-Difluorophenyl 1057 2-Methylpropyl 4-Propylphenyl 1058 2-Methylpropyl 4-Ethoxyphenyl 1059 Butyl 1,3-Benzodioxol-5-yl 1060 2-Methylpropyl 1,3-Benzodioxol-5-yl 1061 Pentyl 1,3-Benzodioxol-5-yl 1062 3-Methylbutyl 1,3-Benzodioxol-5-yl 1063 Butyl 4-Methylothio phenyl 1064 2-Methylpropyl 4-Methylothio phenyl 1065 Butyl 3-Fluoro-4-methoxyphenyl 1066 2-Methylpropyl 3-Fluoro-4-methoxyphenyl 1067 3-Methylbutyl 3-Fluoro-4-methoxyphenyl 1068 2-Methylpropyl 4-Chloro-3-methylphenyl 1069 3-Methylbutyl 4-Chloro-3-methylphenyl 1070 Butyl 3-Chloro-4-fluorophenyl 1071 2-Methylpropyl 3-Chloro-4-fluorophenyl 1072 Pentyl 3-Chloro-4-fluorophenyl 1073 3-Methylbutyl 3-Chloro-4-fluorophenyl 1074 2-Methylpropyl 3,4,5-Trifluorophenyl 1075 3-Methylbutyl 3,4,5-Trifluorophenyl 1076 2-Methylpropyl 4-Ethylthiophenyl 1077 3-Methylbutyl 2,3,6-Trifluorophenyl 1078 Allyl 5-Chloro-2-methoxyphenyl 1079 Propyl 5-Chloro-2-methoxyphenyl 1080 Propyl 3-Trifluoromethylphenyl 1081 Allyl 2,5-Dichlorophenyl 1082 Propyl 2,5-Dichlorophenyl 1083 Methyl 3-Bromophenyl 1084 Allyl 3-Bromophenyl 1085 Propyl 3-Bromo-4-fluorophenyl 1086 Methyl 3-Iodophenyl 1087 Allyl 3-Iodophenyl 1088 Propyl 3-Iodophenyl 1089 2-Methoxy 2,5-Difluorophenyl ethyl 1090 2-Methoxy 2,5-Dichlorophenyl ethyl 1091 2-Methoxy 3-Bromophenyl ethyl 1145 2-Methylpropyl 3-Chloro-4-methoxyphenyl 1146 3-Methylbutyl 3-Chloro-4-methoxyphenyl 1147 2-Methylpropyl 5-Chloro-2-methoxyphenyl 1148 Pentyl 5-Chloro-2-methoxyphenyl 1149 3-Methylbutyl 5-Chloro-2-methoxyphenyl 1150 Pentyl 3-Trifluoromethylphenyl 1151 3-Methylbutyl 3-Trifluoromethylphenyl 1152 Butyl 2-Trifluoromethylphenyl 1153 3-Methylbutyl 2-Trifluoromethylphenyl 1154 Butyl 3,4-Dichlorophenyl 1155 2-Methylpropyl 3,4-Dichlorophenyl 1156 3-Methylbutyl 3,4-Dichlorophenyl 1157 2-Methylpropyl 2,5-Dichlorophenyl 1158 Pentyl 2,5-Dichlorophenyl 1159 3-Methylbutyl 2,5-Dichlorophenyl 1160 2-Methylpropyl 2,4-Dichlorophenyl 1161 2-Methylpropyl 3-Bromophenyl 1162 Pentyl 3-Bromophenyl 1163 3-Methylbutyl 3-Bromophenyl 1164 2-Methylpropyl 4-Bromophenyl 1165 2-Methylpropyl 2-Bromophenyl 1166 Pentyl 2-Bromophenyl 1167 3-Methylbutyl 2-Bromophenyl 1194 2-Methylpropyl 3-Phenoxyphenyl 1195 2-Methylpropyl 4-Phenoxyphenyl 1196 Butyl 3-Bromo-4-methylphenyl 1197 2-Methylpropyl 3-Bromo-4-methylphenyl 1198 Butyl 3-Bromo-4-fluorophenyl 1199 2-Methylpropyl 3-Bromo-4-fluorophenyl 1200 Pentyl 3-Bromo-4-fluorophenyl 1201 3-Methylbutyl 3-Bromo-4-fluorophenyl 1202 Butyl 3-Iodophenyl 1203 Pentyl 3-Iodophenyl 1204 3-Methylbutyl 3-Iodophenyl 1205 2-Methylpropyl 4-Iodophenyl 1206 Methyl 3-Iodophenyl 1239 Cyclopentyl 4-Methylphenyl 1240 Cyclopentyl 3-Fluoro-4-methylphenyl 1241 Cyclopropyl 5-Chloro-2-methoxyphenyl Methyl 1242 Cyclopropyl 3-Trifluoromethylphenyl Methyl 1243 Cyclopropyl 2,5-Dichlorophenyl Methyl 1244 Cyclopropyl 3-Bromophenyl Methyl 1245 Cyclopentyl 3-Methoxybenzyl 1246 Cyclopentyl 2-(2-Chlorophenyl) ethenyl 1247 Cyclopropyl 3-Bromo-4-methylphenyl Methyl 1248 Cyclopropyl 3-Bromo-4-fluorophenyl Methyl 1249 Cyclopropyl 3-Iodophenyl Methyl 1253 Cyclopentyl 3-Chloro-4-methoxyphenyl 1254 Cyclopropyl 5-Chloro-2-methoxyphenyl Methyl 1255 Cyclopentyl 2,4-Dichlorophenyl 1268 Cyclopentyl 3-Fluorobenzyl 1269 Cyclopentyl 2-(2- Trifluoromethylphenyl)ethenyl 1270 Cyclopentyl 2-(2-Bromophenyl) ethenyl 1271 Cyclopropyl 2,3,6-Trifluorophenyl Methyl 1274 Cyclopentyl 3-Chloro-4-methylphenyl 1275 Cyclopropyl 2,4,5-Trifluorophenyl Methyl 1425 Propyl 3-Fluoro-4-methylphenyl 1426 Propyl 3-Chlorophenyl 1427 Allyl 3-Bromo-4-fluorophenyl 1428 Propyl 3-Bromo-4-fluorophenyl 1429 Allyl 3-Iodophenyl 1430 Propyl 3-Iodophenyl 1431 Propyl 3-Iodo-4-methylphenyl 1433 Propyl 3,4-Difluorophenyl 1434 Propyl 2,3-Difluorophenyl 1435 Propyl 2,4-Difluorophenyl 1436 Propyl 1,3-Benzodioxol-5-yl 1437 Propyl 3-Chloro-4-fluorophenyl 1438 Propyl 5-Chloro-2-methoxyphenyl 1439 Methyl 2,5-Dichlorophenyl 1440 Allyl 2,5-Dichlorophenyl 1441 Propyl 2,5-Dichlorophenyl 1442 Propyl 2,4-Dichlorophenyl 1443 Methyl 3-Bromophenyl 1444 Allyl 3-Bromophenyl 1445 Propyl 3-Bromophenyl 1446 Propyl 5-Methyl-2-thienyl 1447 Propyl 2,6-Difluorophenyl 1977 3-Methylbutyl 4,5-Dimethyl-2-furyl 1978 3-Methylbutyl 3-Chloro-4-methylphenyl 1980 3-Methylbutyl 2,4,5-Trifluorophenyl 1982 3-Methylbutyl 2,6-Difluorophenyl 1983 3-Methylbutyl 2-Bromo-5-methoxyphenyl 1984 3-Methylbutyl 3,5-Difluorophenyl 2006 3-Methylbutyl 5-Bromo-2-thienyl 2008 3-Methylbutyl 3-Bromo-2-thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 800 Propyl Phenyl 801 Methyl 3-Chlorophenyl 802 Allyl 3-Chlorophenyl 803 Propyl 3-Chlorophenyl 804 Propyl 5-Chloro-2-methoxyphenyl 805 Propyl 3-Trifluoromethylphenyl 806 Propyl 2,5-Dichlorophenyl 807 Propyl 3-Bromophenyl 808 Propyl 3-Bromo-4-fluorophenyl 809 Methyl 3-Iodophenyl 810 Allyl 3-Iodophenyl 811 Propyl 3-Iodophenyl 888 Allyl 5-Chloro-2-methoxyphenyl 931 Propyl 3-Fluorophenyl 932 Propyl 2-Fluorophenyl 1092 Propyl 3-Fluorophenyl 1093 Propyl 2-Fluorophenyl 1094 Allyl 2,5-Difluorophenyl 1095 Propyl 2,5-Difluorophenyl 1096 Propyl 1,3-Benzodioxol-5-yl 1097 Methyl 5-Chloro-2-methoxyphenyl 1098 Allyl 5-Chloro-2-methoxyphenyl 1099 Methyl 2,5-Dichlorophenyl 1168 Methyl 5-Chloro-2-methoxyphenyl 1169 Allyl 5-Chloro-2-methoxyphenyl 1170 Propyl 5-Chloro-2-methoxyphenyl 1171 Propyl 3,4-Dichlorophenyl 1172 Allyl 2,5-Dichlorophenyl 1173 Propyl 2,5-Dichlorophenyl 1174 Propyl 2,4-Dichlorophenyl 1175 Methyl 3-Bromophenyl 1176 Allyl 3-Bromophenyl 1177 Propyl 3-Bromophenyl 1178 Cyclopropyl 5-Chloro-2-methoxyphenyl methyl 1179 Cyclopropyl 2,5-Dichlorophenyl methyl 1180 Propyl 3-Bromophenyl 1181 Cyclopropyl 3-Bromophenyl methyl 1182 Pentyl 3-Bromo-4-fluorophenyl 1183 3-Methylbutyl 3-Bromo-4-fluorophenyl 1184 Pentyl 3-Iodophenyl 1185 Cyclopropyl 3-Bromo-4-fluorophenyl Methyl 1186 Cyclopropyl 3-Iodophenyl Methyl 1756 Butyl 2-Thienyl 1757 2-Methylpropyl 2-Thienyl 1758 Pentyl 2-Thienyl 1759 3-Methylbutyl 2-Thienyl 1760 Butyl 3-Thienyl 1761 2-Methylpropyl 3-Thienyl 1762 Pentyl 3-Thienyl 1763 3-Methylbutyl 3-Thienyl 1764 3-Methylbutyl Benzyl 1765 Butyl 5-Methyl-2-thienyl 1766 2-Methylpropyl 5-Methyl-2-thienyl 1767 Pentyl 5-Methyl-2-thienyl 1768 3-Methylbutyl 5-Methyl-2-thienyl 1769 3-Methylbutyl 3-Fluorobenzyl 1770 3-Methylbutyl 4-Fluorobenzyl 1771 3-Methylbutyl 3-Methoxybenzyl 1787 3-Methylbutyl 2,3,6-Trifluorophenyl 1788 2-Methylpropyl 2,3,6-Trifluorophenyl 1789 3-Methylbutyl 2,3,6-Trifluorophenyl 1790 3-Methylbutyl 2-Chloro-6-fluorophenyl 1791 Butyl Phenyl 1792 2-Methylpropyl Phenyl 1793 Pentyl Phenyl 1794 3-Methylbutyl Phenyl 1795 Butyl 3-Methylphenyl 1796 2-Methylpropyl 3-Methylphenyl 1797 Pentyl 3-Methylphenyl 1798 3-Methylbutyl 3-Methylphenyl 1799 Butyl 4-Methylphenyl 1800 2-Methylpropyl 4-Methylphenyl 1801 Butyl 3-Fluorophenyl 1802 2-Methylpropyl 3-Fluorophenyl 1803 Pentyl 3-Fluorophenyl 1804 3-Methylbutyl 3-Fluorophenyl 1805 Butyl 4-Fluorophenyl 1806 3-Methylbutyl 4-Fluorophenyl 1807 Butyl 2-Fluorophenyl 1808 2-Methylpropyl 2-Fluorophenyl 1809 Pentyl 2-Fluorophenyl 1810 3-Methylbutyl 2-Fluorophenyl 1811 2-Methylpropyl 4-Ethylphenyl 1812 2-Methylpropyl 3,4-Dimethylphenyl 1813 3-Methylbutyl 3-Methoxyphenyl 1814 2-Methylpropyl 3-Fluoro-4-methylphenyl 1815 3-Methylbutyl 3-Fluoro-4-methylphenyl 1816 2-Methylpropyl 5-Fluoro-2-methylphenyl 1817 Pentyl 5-Fluoro-2-methylphenyl 1818 3-Methylbutyl 5-Fluoro-2-methylphenyl 1857 Butyl 2,5-Dichlorophenyl 1858 2-Methylpropyl 2,5-Dichlorophenyl 1859 Pentyl 2,5-Dichlorophenyl 1860 3-Methylbutyl 2,5-Dichlorophenyl 1861 2-Methylpropyl 4-Pentylphenyl 1862 Butyl 3-Bromophenyl 1863 2-Methylpropyl 3-Bromophenyl 1864 Pentyl 3-Bromophenyl 1865 3-Methylbutyl 3-Bromophenyl 1866 2-Methylpropyl 4-Bromophenyl 1922 Butyl 3,4-Dimethylphenyl 1923 2-Methylpropyl 3-Iodo-4-methylphenyl 1924 3-Methylbutyl 3-Iodo-4-methylphenyl 1986 Butyl 4,5-Dimethyl-2-furyl 1987 2-Methylpropyl 4,5-Dimethyl-2-furyl 1988 3-Methylbutyl 4,5-Dimethyl-2-furyl 1989 3-Methylbutyl 4-Methoxy-3-thienyl 1990 Butyl 3-Chloro-2-thienyl 1991 2-Methylpropyl 3-Chloro-2-thienyl 1992 Pentyl 3-Chloro-2-thienyl 1993 3-Methylbutyl 3-Chloro-2-thienyl 1994 2-Methylpropyl 3-Chloro-4-methylphenyl 1995 3-Methylbutyl 3-Chloro-4-methylphenyl 1996 3-Methylbutyl 2,4,5-Trifluorophenyl 1997 Pentyl 2,6-Difluorophenyl 1998 3-Methylbutyl 2,6-Difluorophenyl 1999 Pentyl 2-Bromo-5-methoxyphenyl 2000 3-Methylbutyl 2-Bromo-5-methoxyphenyl 2001 3-Methylbutyl 3,5-Difluorophenyl 2002 2-Methylpropyl 5-Bromo-2-thienyl 2003 3-Methylbutyl 5-Bromo-2-thienyl 2009 Butyl 5-Ethyl-2-thienyl 2010 2-Methylpropyl 5-Ethyl-2-thienyl 2011 3-Methylbutyl 5-Ethyl-2-thienyl 2012 2-Methylpropyl 5-Propyl-2-thienyl 2013 2-Methylpropyl 5-Butyl-2-thienyl 2014 2-Methylpropyl 5-Pentyl-2-thienyl 2015 2-Methylpropyl 5-Hexyl-2-thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 889 Methyl 2,5-Difluorophenyl 890 Methyl 2,5-Dichlorophenyl 891 Propyl 3-Bromophenyl 892 Methyl 3-Iodophenyl 893 Allyl 3-Iodophenyl 894 Propyl 3-Iodophenyl 1126 Propyl 2,5-Dichlorophenyl 1127 Methyl 3-Bromophenyl 1128 Allyl 3-Bromophenyl 1432 Propyl 3-Bromo-4-fluorophenyl 1517 2-Methylpropyl 3-Fluorophenyl 1518 2-Methylpropyl 3,4-Dimethylphenyl 1519 2-Methylpropyl 3-Methoxyphenyl 1520 2-Methylpropyl 3-Fluoro-4-methylphenyl 1521 Cyclopentyl 3-Fluoro-4-methylphenyl 1522 2-Methylpropyl 5-Fluoro-2-methylphenyl 1523 2-Methylpropyl 2-Fluoro-3-methylphenyl 1524 2-Methylpropyl 3-Chlorophenyl 1525 2-Methylpropyl 4-Chlorophenyl 1567 2-Methylpropyl 1,3-Benzodioxol-5-yl 1568 Cyclopentyl 4-Methoxyphenyl 1569 Cyclopentyl 4-Butylphenyl 1570 3-Methylbutyl 3-Chloro-4-methoxyphenyl 1571 Cyclopentyl 3-Chloro-4-methoxyphenyl 1572 2-Methylpropyl 3,4-Dichlorophenyl 1573 3-Methylbutyl 2,5-Dichlorophenyl 1574 Cyclopentyl 2,4-Dichlorophenyl 1575 Cyclopentyl 4-Pentylphenyl 1576 3-Methylbutyl 3-Bromophenyl 1619 2-Methylpropyl 4-Hexylphenyl 1620 Cyclopentyl 4-Hexylphenyl 1621 2-Methylpropyl 3-Bromo-4-methylphenyl 1622 2-Methylpropyl 3-Bromo-4-fluorophenyl 1623 3-Methylbutyl 3-Bromo-4-fluorophenyl 1624 2-Methylpropyl 3-Iodophenyl 1625 3-Methylbutyl 3-Iodophenyl 1653 2-Methylpropyl 3-Iodo-4-methylphenyl 1654 3-Methylbutyl 2-Thienyl 1655 3-Methylbutyl Benzyl 1656 2-Methylpropyl 5-Methyl-2-thienyl 1657 3-Methylbutyl 5-Methyl-2-thienyl 1658 3-Methylbutyl 3-Fluorobenzyl 1659 Cyclopentyl 3-Fluorobenzyl 1678 Cyclopentyl 2-Chlorobenzyl 1682 2-Methylpropyl 2-(2-Chlorophenyl) ethenyl 1683 Cyclopentyl 2-(2-Chlorophenyl) ethenyl 1701 2-Methylpropyl 2,3,6-Trifluorophenyl 1702 2-Methylpropyl 4,5-Dimethyl-2-furyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 895 Propyl 5-Bromo-2-thienyl 993 Propyl 1,3-Benzodioxol-5-yl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 896 Propyl 3-Bromo-4-fluorophenyl 897 Allyl 3-Iodophenyl 898 Propyl 3-Iodophenyl 899 Propyl 3-Iodo-4-methylphenyl 900 Methyl 2-Thienyl 901 Methyl 5-Methyl-2-thienyl 923 Propyl 3-Methylphenyl 1117 Propyl 5-Chloro-2-methoxyphenyl 1118 Propyl 2,5-Dichlorophenyl 1119 Propyl 3-Bromophenyl 1979 3-Methylbutyl 3-Chloro-4-methylphenyl 1981 3-Methylbutyl 2,4,5-Trifluorophenyl 1985 3-Methylbutyl 3,5-Difluorophenyl 2007 3-Methylbutyl 5-Bromo-2-thienyl 2386 2-(2- 2,5-Dichlorophenyl Fluorophenyl) ethyl 2387 2-(2- 3-Bromophenyl Fluorophenyl) ethyl 2388 2-(2- 3-Iodophenyl Fluorophenyl) ethyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 902 Allyl 3-Bromo-4-methylphenyl 903 Propyl 3-Bromo-4-methylphenyl 904 Allyl 3-Bromo-4-fluorophenyl 905 Propyl 3-Bromo-4-fluorophenyl 906 Methyl 3-Iodophenyl 907 Allyl 3-Iodophenyl 908 Propyl 3-Iodophenyl 909 Propyl 3-Iodo-4-methylphenyl 910 Methyl 2-Thienyl 911 Methyl 3-Thienyl 912 Methyl 3-Methyl-2-thienyl 913 Propyl 5-Methyl-2-thienyl 914 Propyl Phenyl 915 Methyl 3-Methylphenyl 916 Propyl 3-Fluorophenyl 917 Propyl 2-Fluorophenyl 918 Methyl 5-Fluoro-2-methylphenyl 919 Allyl 5-Fluoro-2-methylphenyl 920 Methyl 3-Chlorophenyl 921 Propyl 3-Chlorophenyl 976 Propyl 2-Chlorophenyl 977 Allyl 3,4-Difluorophenyl 978 Propyl 3,4-Difluorophenyl 979 Methyl 2,3-Difluorophenyl 980 Allyl 2,3-Difluorophenyl 981 Propyl 2,3-Difluorophenyl 982 Methyl 2,5-Difluorophenyl 983 Allyl 2,5-Difluorophenyl 984 Propyl 2,5-Difluorophenyl 985 Propyl 2,4-Difluorophenyl 986 Propyl 1,3-Benzodioxol-5-yl 987 Allyl 3-Chloro-4-fluorophenyl 988 Propyl 3-Chloro-4-fluorophenyl 1120 Allyl 5-Chloro-2-methoxyphenyl 1121 Propyl 5-Chloro-2-methoxyphenyl 1122 Allyl 2,5-Dichlorophenyl 1123 Propyl 2,5-Dichlorophenyl 1124 Allyl 3-Bromophenyl 1125 Propyl 3-Bromophenyl 1516 Methyl 5-Ethoxy-2-thienyl 1706 2-Methylpropyl 2,4,6-Trifluorophenyl 1707 2-Methylpropyl 2,3,6-Trifluorophenyl 1708 3-Methylbutyl 2,3,6-Trifluorophenyl 1709 2-Methylpropyl 4,5-Dimethyl-2-furyl 1710 3-Methylbutyl 4,5-Dimethyl-2-furyl 1711 2-Methylpropyl 3-Chloro-2-thienyl 1712 3-Methylbutyl 3-Chloro-2-thienyl 1713 2-Methylpropyl 5-Methylthio-2-thienyl 1719 2-Methylpropyl 3-Chlorophenyl 1720 3-Methylbutyl 2,4,5-Trifluorophenyl 1725 2-Methylpropyl 2,6-Difluorophenyl 1727 3-Methylbutyl Phenyl 1728 2-Methylpropyl 3-Methylphenyl 1729 3-Methylbutyl 3-Methylphenyl 1730 2-Methylpropyl 4-Methylphenyl 1731 3-Methylbutyl 4-Methylphenyl 1732 2-Methylpropyl 2-Methylphenyl 1733 3-Methylbutyl 2-Methylphenyl 1734 2-Methylpropyl 3-Fluorophenyl 1735 3-Methylbutyl 3-Fluorophenyl 1736 2-Methylpropyl 3-Fluorophenyl 1737 3-Methylbutyl 4-Fluorophenyl 1738 2-Methylpropyl 2-Fluorophenyl 1739 3-Methylbutyl 2-Fluorophenyl 1740 2-Methylpropyl 4-Ethylphenyl 1741 2-Methylpropyl 3,4-Dimethylphenyl 1742 2-Methylpropyl 3-Fluoro-4-methylphenyl 1743 Cyclopentyl 3-Fluoro-4-methylphenyl 1744 2-Methylpropyl 4-Chlorophenyl 1745 Cyclopentyl 4-Methoxyphenyl 1746 3-Methylbutyl 3-Chloro-4-fluorophenyl 1747 3-Methylbutyl 2-Thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 812 Methyl 2,5-Difluorophenyl 813 Propyl 2,5-Dichlorophenyl 814 Propyl 3-Iodophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 818 Propyl 3-Fluorophenyl 819 Propyl 2-Fluorophenyl 820 Propyl 3,4-Difluorophenyl 821 Methyl 2,5-Difluorophenyl 822 Allyl 2,5-Difluorophenyl 823 Propyl 2,5-Difluorophenyl 824 Propyl 1,3-Benzodioxol-5-yl 825 Propyl 3-Chloro-4-fluorophenyl 826 Methyl 5-Chloro-2-methoxyphenyl 827 Ethyl 5-Chloro-2-methoxyphenyl 828 Allyl 5-Chloro-2-methoxyphenyl 829 Propyl 5-Chloro-2-methoxyphenyl 830 Methyl 2,5-Dichlorophenyl 831 Allyl 2,5-Dichlorophenyl 832 Propyl 2,5-Dichlorophenyl 833 Propyl Phenyl 834 Propyl 3-Fluoro-4-methylphenyl 835 Propyl 5-Fluoro-2-methylphenyl 836 Methyl 3-Chlorophenyl 837 Allyl 3-Chlorophenyl 838 Propyl 3-Chlorophenyl 842 Methyl 5-Chloro-2-methoxyphenyl 843 Ethyl 5-Chloro-2-methoxyphenyl 844 Allyl 5-Chloro-2-methoxyphenyl 845 Propyl 5-Chloro-2-methoxyphenyl 846 Methyl 3-Trifluorophenyl 847 Propyl 3-Trifluorophenyl 848 Methyl 2,5-Dichlorophenyl 849 Allyl 2,5-Dichlorophenyl 850 Propyl 2,5-Dichlorophenyl 851 Methyl 3-Bromophenyl 852 Allyl 3-Bromophenyl 853 Propyl 3-Bromophenyl 854 Propyl 3-Bromo-4-fluorophenyl 855 Methyl 3-Iodophenyl 856 Allyl 3-Iodophenyl 857 Propyl 3-Iodophenyl 859 Allyl 2-Fluorophenyl 860 Propyl 2-Fluorophenyl 861 Propyl 2-Chlorophenyl 862 Propyl 3,4-Difluorophenyl 863 Propyl 2,3-Difluorophenyl 864 Methyl 2,5-Difluorophenyl 865 Propyl 4-Methylthiophenyl 866 Propyl 3-Fluoro-4-methoxyphenyl 867 Propyl 4-Chloro-3-methylphenyl 868 Methyl 3-Chloro-4-fluorophenyl 869 Allyl 3-Chloro-4-fluorophenyl 870 Propyl 3-Chloro-4-fluorophenyl 871 Propyl 3,4,5-Trifluorophenyl 872 Propyl 4-Butylphenyl 873 Propyl 4-Methylthiophenyl 1772 Butyl 2-Thienyl 1773 2-Methylpropyl 2-Thienyl 1774 Pentyl 2-Thienyl 1775 3-Methylbutyl 2-Thienyl 1776 Butyl 3-Thienyl 1777 2-Methylpropyl 3-Thienyl 1778 Pentyl 3-Thienyl 1779 3-Methylbutyl 3-Thienyl 1780 3-Methylbutyl Benzyl 1781 Butyl 5-Methyl-2-thienyl 1782 2-Methylpropyl 5-Methyl-2-thienyl 1783 Pentyl 5-Methyl-2-thienyl 1784 3-Methylbutyl 5-Methyl-2-thienyl 1785 3-Methylbutyl 3-Fluorobenzyl 1786 3-Methylbutyl 3-Methoxybenzyl 1819 Butyl Phenyl 1820 2-Methylpropyl Phenyl 1821 Pentyl Phenyl 1822 3-Methylbutyl Phenyl 1823 Butyl 3-Methylphenyl 1824 2-Methylpropyl 3-Methylphenyl 1825 Pentyl 3-Methylphenyl 1826 3-Methylbutyl 3-Methylphenyl 1827 Butyl 4-Methylphenyl 1828 2-Methylpropyl 4-Methylphenyl 1829 3-Methylbutyl 4-Methylphenyl 1830 Butyl 3-Fluorophenyl 1831 2-Methylpropyl 3-Fluorophenyl 1832 Pentyl 3-Fluorophenyl 1833 3-Methylbutyl 3-Fluorophenyl 1834 Butyl 4-Fluorophenyl 1835 2-Methylpropyl 4-Fluorophenyl 1836 Pentyl 4-Fluorophenyl 1837 3-Methylbutyl 4-Fluorophenyl 1838 Butyl 2-Fluorophenyl 1839 2-Methylpropyl 2-Fluorophenyl 1840 Pentyl 2-Fluorophenyl 1841 3-Methylbutyl 2-Fluorophenyl 1842 2-Methylpropyl 4-Ethylphenyl 1843 2-Methylpropyl 3,4-Dimethylphenyl 1844 3-Methylbutyl 2,5-Dimethylphenyl 1845 2-Methylpropyl 2,4-Dimethylphenyl 1846 2-Methylpropyl 3-Methoxyphenyl 1847 3-Methylbutyl 3-Methoxyphenyl 1848 3-Methylbutyl 2-Methoxyphenyl 1849 2-Methylpropyl 3-Fluoro-4-methylphenyl 1850 3-Methylbutyl 3-Fluoro-4-methylphenyl 1851 Butyl 5-Fluoro-2-methylphenyl 1852 2-Methylpropyl 5-Fluoro-2-methylphenyl 1853 Pentyl 5-Fluoro-2-methylphenyl 1854 3-Methylbutyl 5-Fluoro-2-methylphenyl 1855 2-Methylpropyl 4-Chlorophenyl 1856 3-Methylbutyl 4-Chlorophenyl 1867 2-Methylpropyl 2,5-Dichlorophenyl 1868 Pentyl 2,5-Dichlorophenyl 1869 3-Methylbutyl 2,5-Dichlorophenyl 1870 2-Methylpropyl 4-Pentylphenyl 1871 2-Methylpropyl 3-Bromophenyl 1872 Pentyl 3-Bromophenyl 1873 3-Methylbutyl 3-Bromophenyl 1925 2-Methylpropyl 3-Iodo-4-methylphenyl 1926 3-Methylbutyl 3-Iodo-4-methylphenyl 1928 Butyl 2-Chlorophenyl 1929 2-Methylpropyl 2-Chorophenyl 1930 Pentyl 2-Chlorophenyl 1931 Butyl 3,4-Difluorophenyl 1932 2-Methylpropyl 3,4-Difluorophenyl 1933 Pentyl 3,4-Difluorophenyl 1934 3-Methylbutyl 3,4-Difluorophenyl 1935 Butyl 2,3-Difluorophenyl 1936 2-Methylpropyl 2,3-Difluorophenyl 1937 Pentyl 2,3-Difluorophenyl 1938 3-Methylbutyl 2,3-Difluorophenyl 1939 Butyl 2,5-Difluorophenyl 1940 2-Methylpropyl 2,5-Difluorophenyl 1941 Pentyl 2,5-Difluorophenyl 1942 3-Methylbutyl 2,5-Difluorophenyl 1943 Butyl 2,4-Difluorophenyl 1944 2-Methylpropyl 2,4-Difluorophenyl 1945 Pentyl 2,4-Difluorophenyl 1946 3-Methylbutyl 2,4-Difluorophenyl 1947 2-Methylpropyl 4-Propylphenyl 1948 2-Methylpropyl 4-i-Propylphenyl 1949 Butyl 1,3-Benzodioxol-5-yl 1950 2-Methylpropyl 1,3-Benzodioxol-5-yl 1951 Pentyl 1,3-Benzodioxol-5-yl 1952 3-Methylbutyl 1,3-Benzodioxol-5-yl 1953 Butyl 3-Bromo-4-methylphenyl 1954 2-Methylpropyl 3-Bromo-4-methylphenyl 1955 Pentyl 3-Bromo-4-methylphenyl 1956 3-Methylbutyl 3-Bromo-4-methylphenyl 1957 2-Methylpropyl 4-Heptylphenyl 1958 Butyl 3-Iodophenyl 1959 2-Methylpropyl 3-Iodophenyl 1960 Pentyl 3-Iodophenyl 1961 3-Methylbutyl 3-Iodophenyl 1962 2-Methylpropyl 4-Iodophenyl 2016 Butyl 5-Ethyl-2-thienyl 2017 2-Methylpropyl 5-Ethyl-2-thienyl 2018 3-Methylbutyl 5-Ethyl-2-thienyl 2019 2-Methylpropyl 5-Propyl-2-thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 874 Methyl 3-Fluorophenyl 875 Allyl 3-Fluorophenyl 876 Propyl 3-Fluorophenyl 877 Propyl 4-Fluorophenyl 878 Methyl 3-Chloro-4-methylphenyl 879 Allyl 3-Chloro-4-methylphenyl 880 Propyl 3-Chloro-4-methylphenyl 881 Allyl 5-Bromo-2-thienyl 882 Propyl 5-Bromo-2-thienyl 883 Propyl 3-Fluoro-4-methylphenyl 884 Propyl 5-Fluoro-2-methylphenyl 922 Propyl 3-Methoxyphenyl 1450 Propyl 3-Bromo-4-methylphenyl 1451 Allyl 3-Bromo-4-fluorophenyl 1452 Propyl 3-Bromo-4-fluorophenyl 1453 Allyl 3-Iodophenyl 1454 Propyl 3-Iodophenyl 1455 Allyl 5-Chloro-2-methoxyphenyl 1456 Propyl 5-Chloro-2-methoxyphenyl 1457 Propyl 3,4-Dichlorophenyl 1458 Ethyl 2,5-Dichlorophenyl 1459 Allyl 2,5-Dichlorophenyl 1460 Propyl 2,5-Dichlorophenyl 1461 Propyl 2,4-Dichlorophenyl 1462 Ethyl 3-Bromophenyl 1463 Allyl 3-Bromophenyl 1464 Propyl 3-Bromophenyl 1465 Propyl 5-Methyl-2-thienyl 1466 Propyl 4-Chloro-3-methylphenyl 1467 Propyl 3-Chloro-4-fluorophenyl 1526 2-Methylpropyl Phenyl 1527 3-Methylbutyl Phenyl 1528 2-Methylpropyl 3-Methylphenyl 1529 3-Methylbutyl 3-Methylphenyl 1530 2-Methylpropyl 4-Methylphenyl 1531 Cyclopentyl 4-Methylphenyl 1532 2-Methylpropyl 2-Methylphenyl 1533 3-Methylbutyl 2-Methylphenyl 1534 2-Methylpropyl 3-Fluorophenyl 1535 3-Methylbutyl 3-Fluorophenyl 1536 2-Methylpropyl 4-Fluorophenyl 1537 3-Methylbutyl 4-Fluorophenyl 1538 2-Methylpropyl 2-Fluorophenyl 1539 Cyclopentyl 2-Fluorophenyl 1540 2-Methylpropyl 4-Ethylphenyl 1541 2-Methylpropyl 3,4-Dimethylphenyl 1542 2-Methylpropyl 2,3-Dimethylphenyl 1543 2-Methylpropyl 2,5-Dimethylphenyl 1544 3-Methylbutyl 2,5-Dimethylphenyl 1545 2-Methylpropyl 2,4-Dimethylphenyl 1546 3-Methylbutyl 2,4-Dimethylphenyl 1547 Cyclopentyl 2,4-Dimethylphenyl 1548 2-Methylpropyl 3-Methoxyphenyl 1549 3-Methylbutyl 3-Methoxyphenyl 1550 2-Methylpropyl 4-Methoxyphenyl 1551 3-Methylbutyl 4-Methoxyphenyl 1552 Cyclopentyl 4-Methoxyphenyl 1553 2-Methylpropyl 2-Methoxyphenyl 1554 3-Methylbutyl 2-Methoxyphenyl 1555 2-Methylpropyl 3-Fluoro-4-methylphenyl 1556 Cyclopentyl 3-Fluoro-4-methylphenyl 1557 2-Methylpropyl 3-Fluoro-2-methylphenyl 1558 3-Methylbutyl 3-Fluoro-2-methylphenyl 1559 2-Methylpropyl 5-Fluoro-2-methylphenyl 1560 3-Methylbutyl 5-Fluoro-2-methylphenyl 1561 2-Methylpropyl 2-Fluoro-3-methylphenyl 1562 2-Methylpropyl 3-Chlorophenyl 1563 3-Methylbutyl 3-Chlorophenyl 1564 Cyclopentyl 3-Chlorophenyl 1565 2-Methylpropyl 4-Chlorophenyl 1566 Cyclopentyl 4-Chlorophenyl 1577 3-Methylbutyl 2-Chlorophenyl 1578 Cyclopentyl 2-Chlorophenyl 1579 2-Methylpropyl 3,4-Difluorophenyl 1580 3-Methylbutyl 3,4-Difluorophenyl 1581 2-Methylpropyl 2,3-Difluorophenyl 1582 3-Methylbutyl 2,3-Difluorophenyl 1583 Cyclopentyl 2,3-Difluorophenyl 1584 2-Methylpropyl 2,5-Difluorophenyl 1585 3-Methylbutyl 2,5-Difluorophenyl 1586 2-Methylpropyl 2,4-Difluorophenyl 1587 3-Methylbutyl 2,4-Difluorophenyl 1588 Cyclopentyl 2,4-Difluorophenyl 1589 2-Methylpropyl 1,3-Benzodioxol-5-yl 1590 3-Methylbutyl 1,3-Benzodioxol-5-yl 1591 Cyclopentyl 1,3-Benzodioxol-5-yl 1592 2-Methylpropyl 4-Methylthio phenyl 1593 Cyclopentyl 4-Methylthio phenyl 1594 Cyclopentyl 3-Fluoro-4-methoxy 1595 Cyclopentyl 4-Butylphenyl 1596 Cyclopentyl 4-Ethylthiophenyl 1597 2-Methylpropyl 3-Chloro-4-methoxyphenyl 1598 3-Methylbutyl 3-Chloro-4-methoxyphenyl 1599 Cyclopentyl 3-Chloro-4-methoxyphenyl 1600 2-Methylpropyl 2-Trifluoromethylphenyl 1601 3-Methylbutyl 2-Trifluoromethylphenyl 1602 2-Methylpropyl 3,4-Dichlorophenyl 1603 3-Methylbutyl 3,4-Dichlorophenyl 1604 2-Methylpropyl 2,3-Dichlorophenyl 1605 2-Methylpropyl 2,5-Dichlorophenyl 1606 3-Methylbutyl 2,5-Dichlorophenyl 1607 2-Methylpropyl 2,4-Dichlorophenyl 1608 Cyclopentyl 2,4-Dichlorophenyl 1609 2-Methylpropyl 3-Bromophenyl 1610 3-Methylbutyl 3-Bromophenyl 1611 Cyclopentyl 3-Bromophenyl 1612 2-Methylpropyl 4-Bromophenyl 1613 Cyclopentyl 4-Bromophenyl 1614 2-Methylpropyl 2-Bromophenyl 1615 3-Methylbutyl 2-Bromophenyl 1626 2-Methylpropyl 3-Bromo-4-methylphenyl 1627 3-Methylbutyl 3-Bromo-4-methylphenyl 1628 Cyclopentyl 3-Bromo-4-methylphenyl 1629 2-Methylpropyl 3-Bromo-4-fluorophenyl 1630 3-Methylbutyl 3-Bromo-4-fluorophenyl 1631 2-Methylpropyl 3-Iodophenyl 1632 3-Methylbutyl 3-Iodophenyl 1633 2-Methylpropyl 4-Iodophenyl 1660 2-Methylpropyl 3-Iodo-4-methylphenyl 1661 2-Methylpropyl 4-Iodobenzyl 1662 2-Methylpropyl 2-Thienyl 1663 3-Methylbutyl 2-Thienyl 1664 2-Methylpropyl Benzyl 1665 3-Methylbutyl Benzyl 1666 Cyclopentyl Benzyl 1667 2-Methylpropyl 5-Methyl-2-thienyl 1668 3-Methylbutyl 5-Methyl-2-thienyl 1669 Cyclopentyl 5-Methyl-2-thienyl 1670 Cyclopentyl 3-Methylbenzyl 1671 2-Methylpropyl 3-Fluorobenzyl 1672 3-Methylbutyl 3-Fluorobenzyl 1673 Cyclopentyl 3-Fluorobenzyl 1679 3-Methylbutyl 2-Methoxybenzyl 1680 Cyclopentyl 1-(4-Fluorophenyl) ethyl 1681 Cyclopentyl 2-Chlorobenzyl 1684 Cyclopentyl 2-(2-Chlorophenyl) ethenyl 1703 2-Methylpropyl 2,4,6-Trifluorophenyl 1704 2-Methylpropyl 2,3,6-Trifluorophenyl 1705 2-Methylpropyl 2-Chloro-6-fluorophenyl 1714 2-Methylpropyl 3-Chloro-4-methylphenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 923 Propyl Phenyl 924 Propyl 3-Methylphenyl 925 Propyl 4-Methylphenyl 926 Propyl 3-Fluorophenyl 927 Methyl 2-Fluorophenyl 928 Allyl 2-Fluorophenyl 929 Propyl 2-Fluorophenyl 1000 Methyl 2,3-Difluorophenyl 1001 Methyl 2,5-Difluorophenyl 1129 Ethyl 5-Chloro-2-methoxyphenyl 2307 3-Methylbutyl 2,3,6-Trifluorophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 955 Methyl Phenyl 956 Propyl Phenyl 957 Methyl 3-Methylphenyl 958 Propyl 3-Methylphenyl 959 Methyl 3-Fluorophenyl 960 Propyl 3-Fluorophenyl 961 Methyl 2-Fluorophenyl 962 Allyl 2-Fluorophenyl 963 Propyl 2-Fluorophenyl 964 Methyl 5-Fluoro-2-methylphenyl 965 Methyl 3-Chlorophenyl 966 Propyl 3-Chlorophenyl 989 Propyl 3-Chloro-4-fluorophenyl 994 Methyl 2-Thienyl 995 Propyl 2-Thienyl 996 Methyl 3-Thienyl 997 Methyl 3-Methyl-2-thienyl 998 Methyl 5-Methyl-2-thienyl 999 Propyl 5-Methyl-2-thienyl 1100 Propyl 5-Chloro-2-methoxyphenyl 1101 Methyl 3-Bromophenyl 1102 Propyl 3-Bromophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 967 Propyl Phenyl 968 Propyl 3-Methylphenyl 969 Propyl 4-Methylphenyl 970 Propyl 3-Fluorophenyl 971 Propyl 2-Fluorophenyl 972 Propyl 5-Fluoro-2-methylphenyl 973 Ethyl 3-Chlorophenyl 974 Allyl 3-Chlorophenyl 975 Propyl 3-Chlorophenyl 990 Propyl 1,3-Benzodioxol-5-yl 991 Allyl 3-Chloro-4-fluorophenyl 992 Propyl 3-Chloro-4-fluorophenyl 1103 Propyl 5-Chloro-2-methoxyphenyl 1104 Propyl 3-Trifluoromethylphenyl 1105 Propyl 3,4-Dichlorophenyl 1106 Allyl 2,5-Dichlorophenyl 1107 Allyl 3-Bromophenyl 1108 Propyl 3-Bromophenyl 1187 Propyl 3-Bromo-4-methylphenyl 1188 Methyl 3-Bromo-4-fluorophenyl 1189 Allyl 3-Bromo-4-fluorophenyl 1190 Propyl 3-Bromo-4-fluorophenyl 1191 Methyl 3-Iodophenyl 1192 Allyl 3-Iodophenyl 1193 Propyl 3-Iodophenyl 1207 Propyl 3-Bromo-4-fluorophenyl 1208 Methyl 3-Bromo-4-fluorophenyl 1209 Allyl 3-Bromo-4-fluorophenyl 1210 Propyl 3-Bromo-4-fluorophenyl 1211 Methyl 3-Iodophenyl 1212 Ethyl 3-Iodophenyl 1213 Allyl 3-Iodophenyl 1214 Propyl 3-Iodophenyl 1215 Propyl 3-Iodo-4-methylphenyl 1216 Methyl 2-Thienyl 1217 Propyl 2-Thienyl 1218 Allyl 5-Methyl-2-thienyl 1219 Propyl 5-Methyl-2-thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No R2 R3 1220 2-Methylpropyl Phenyl 1221 2-Methylpropyl 3-Methylpropyl 1222 2-Methylpropyl 4-Methylpropyl 1223 2-Methylpropyl 2-Fluorophenyl 1224 2-Methylpropyl 4-Ethylphenyl 1225 2-Methylpropyl 3,4-Dimethylphenyl 1227 2-Methylpropyl 2,5-Difluorophenyl 1228 2-Methylpropyl 2,4-Difluorophenyl 1229 2-Methylpropyl 1,3-Benzodioxol-5-yl 1230 2-Methylpropyl 4-Bromophenyl 1251 2-Methylpropyl 3-Bromo-4-methylphenyl 1272 2-Methylpropyl 3-Chloro-4-methylphenyl 1273 2-Methylpropyl 2,4,5-Trifluorophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 1226 Propyl 2-Fluorophenyl 1231 Allyl 5-Chloro-2-methoxyphenyl 1232 Propyl 5-Chloro-2-methoxyphenyl 1233 Methyl 2,5-Dichlorophenyl 1234 Allyl 2,5-Dichlorophenyl 1235 Propyl 2,5-Dichlorophenyl 1236 Methyl 3-Bromophenyl 1237 Allyl 3-Bromophenyl 1238 Propyl 3-Bromophenyl 1252 Propyl 3-Iodophenyl 1748 2-Methylpropyl 2,3,6-Trifluorophenyl 1749 3-Methylbutyl 2,3,6-Trifluorophenyl 1750 2-Methylpropyl 3-Chloro-4-phenyl 1751 3-Methylbutyl 3-Chloro-4-phenyl 1752 2-Methylpropyl 2,4,5-Trifluorophenyl 1753 3-Methylbutyl 2,4,5-Trifluorophenyl 1754 2-Methylpropyl 2,6-Difluorophenyl 1755 3-Methylbutyl 2,6-Difluorophenyl 1881 Butyl Phenyl 1882 2-Methylpropyl Phenyl 1883 Pentyl Phenyl 1884 3-Methylbutyl Phenyl 1885 Butyl 3-Methylphenyl 1886 2-Methylpropyl 3-Methylphenyl 1887 Pentyl 3-Methylphenyl 1888 3-Methylbutyl 3-Methylphenyl 1889 2-Methylpropyl 4-Methylphenyl 1890 3-Methylbutyl 4-Methylphenyl 1891 Butyl 3-Fluorophenyl 1892 2-Methylpropyl 3-Fluorophenyl 1893 Pentyl 3-Fluorophenyl 1894 3-Methylbutyl 3-Fluorophenyl 1895 2-Methylpropyl 4-Fluorophenyl 1896 3-Methylbutyl 4-Fluorophenyl 1897 Butyl 2-Fluorophenyl 1898 2-Methylpropyl 2-Fluorophenyl 1899 Pentyl 2-Fluorophenyl 1900 3-Methylbutyl 2-Fluorophenyl 1901 2-Methylpropyl 3,4-Dimethylphenyl 1902 Butyl 2-Chlorophenyl 1903 2-Methylpropyl 2-Chlorophenyl 1904 Pentyl 2-Chlorophenyl 1905 3-Methylbutyl 2-Chlorophenyl 1906 Butyl 3,4-Difluorophenyl 1907 2-Methylpropyl 3,4-Difluorophenyl 1908 Pentyl 3,4-Difluorophenyl 1909 3-Methylbutyl 3,4-Difluorophenyl 1910 Butyl 2,3-Difluorophenyl 1911 2-Methylpropyl 2,3-Difluorophenyl 1912 Pentyl 2,3-Difluorophenyl 1913 3-Methylbutyl 2,3-Difluorophenyl 1914 Butyl 2,5-Difluorophenyl 1915 2-Methylpropyl 2,5-Difluorophenyl 1916 Pentyl 2,5-Difluorophenyl 1917 3-Methylbutyl 2,5-Difluorophenyl 1918 2-Methylpropyl 2,4-Difluorophenyl 1919 3-Methylbutyl 2,4-Difluorophenyl 1920 2-Methylpropyl 1,3-Benzodioxol-5-yl 1921 3-Methylbutyl 1,3-Benzodioxol-5-yl 1927 3-Methylbutyl 3-Iodo-4-methylphenyl 1963 2-Methylpropyl 2-(2-Chlorophenyl) ethenyl 1964 Butyl 2-Thienyl 1965 Pentyl 2-Thienyl 1966 3-Methylbutyl 2-Thienyl 1967 Pentyl 3-Thienyl 1968 3-Methylbutyl 3-Thienyl 1969 3-Methylbutyl Benzyl 1970 Butyl 5-Methyl-2-thienyl 1971 2-Methylpropyl 5-Methyl-2-thienyl 1972 Pentyl 5-Methyl-2-thienyl 1973 3-Methylbutyl 5-Methyl-2-thienyl 1974 3-Methylbutyl 3-Fluorobenzyl 1975 3-Methylbutyl 3-Methoxybenzyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 1250 Propyl 3-Iodophenyl 1616 2-Methylpropyl 3-Chloro-4-fluorophenyl 1617 2-Methylpropyl 3-Bromophenyl 1618 3-Methylbutyl 3-Bromophenyl 1634 2-Methylpropyl 3-Bromo-4-methylphenyl 1635 2-Methylpropyl 3-Bromo-4-fluorophenyl 1636 3-Methylbutyl 3-Bromo-4-fluorophenyl 1637 2-Methylpropyl 3-Iodophenyl 1638 3-Methylbutyl 3-Bromo-4-fluorophenyl 1639 2-Methylpropyl Phenyl 1640 3-Methylbutyl Phenyl 1641 2-Methylpropyl 3-Methylphenyl 1642 3-Methylbutyl 3-Methylphenyl 1643 2-Methylpropyl 4-Methylphenyl 1644 2-Methylpropyl 3-Fluorophenyl 1645 3-Methylbutyl 3-Fluorophenyl 1646 2-Methylpropyl 4-Fluorophenyl 1647 2-Methylpropyl 2-Fluorophenyl 1648 3-Methylbutyl 2-Fluorophenyl 1649 2-Methylpropyl 3,4-Dimethylphenyl 1650 2-Methylpropyl 3-Fluoro-4-methylphenyl 1651 2-Methylpropyl 3-Chlorophenyl 1652 3-Methylbutyl 3-Chlorophenyl 1674 2-Methylpropyl 3-Iodo-4-methylphenyl 1675 2-Methylpropyl 5-Methyl-2-thienyl 1676 3-Methylbutyl 5-Methyl-2-thienyl 1677 3-Methylbutyl 3-Fluorobenzyl 1715 2-Methylpropyl 3-Chloro-4-methylphenyl 1716 2-Methylpropyl 2,4,5-Trifluorophenyl 1874 Butyl 3,4-Dimethylphenyl 1875 2-Methylpropyl 3,4-Dimethylphenyl 1876 3-Methylbutyl 3,4-Dimethylphenyl 1877 3-Methylbutyl 2,3-Dimethylphenyl 1878 2-Methylpropyl 2,5-Dimethylphenyl 1879 3-Methylbutyl 2,5-Dimethylphenyl 1880 2-Methylpropyl 1,3-Benzodioxol-5-yl 1976 3-Methylbutyl 3-Methoxybenzyl 2262 Benzyl 3-Chlorophenyl 2282 Benzyl 5-Chloro-2-methoxyphenyl 2293 Benzyl 3-Bromophenyl 2299 Benzyl 3-Iodophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 1276 2-Methylpropyl Phenyl 1277 Pentyl Phenyl 1278 3-Methylbutyl Phenyl 1279 3-Methylbutyl 3-Methylphenyl 1280 2-Methylpropyl 4-Methylphenyl 1281 2-Methylpropyl 3-Fluorophenyl 1282 3-Methylbutyl 3-Fluorophenyl 1283 2-Methylpropyl 4-Fluorophenyl 1284 Butyl 2-Fluorophenyl 1285 2-Methylpropyl 2-Fluorophenyl 1286 Pentyl 2-Fluorophenyl 1287 3-Methylbutyl 2-Fluorophenyl 1288 2-Methylpropyl 3-Methoxyphenyl 1289 3-Methylbutyl 3-Methoxyphenyl 1290 3-Methylbutyl 4-Methoxyphenyl 1291 2-Methylpropyl 3-Fluoro-4-methylphenyl 1292 3-Methylbutyl 2-Fluoro-3-methylphenyl 1293 Butyl 3-Chlorophenyl 1294 2-Methylpropyl 3-Chlorophenyl 1295 Pentyl 3-Chlorophenyl 1296 3-Methylbutyl 3-Chlorophenyl 1297 2-Methylpropyl 3,4-Difluorophenyl 1298 2-Methylpropyl 2,3-Difluorophenyl 1299 3-Methylbutyl 2,3-Difluorophenyl 1300 3-Methylbutyl 2,5-Difluorophenyl 1301 2-Methylpropyl 1,3-Benzodioxol-5-yl 1302 2-Methylpropyl 1,3-Benzodioxol-5-yl 1386 3-Methylbutyl 5-Chloro-2-methoxyphenyl 1387 3-Methylbutyl 3-Bromophenyl 1388 2-Methylpropyl 4-Bromophenyl 1389 2-Methylpropyl 5-Methyl-2-thienyl 1390 3-Methylbutyl 5-Methyl-2-thienyl 1685 3-Methylbutyl 2,3,6-Trifluorophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 1303 Butyl Phenyl 1304 2-Methylpropyl Phenyl 1305 Pentyl Phenyl 1306 3-Methylbutyl Phenyl 1307 Butyl 3-Methylphenyl 1308 2-Methylpropyl 3-Methylphenyl 1309 Pentyl 3-Methylphenyl 1310 3-Methylbutyl 3-Methylphenyl 1311 Butyl 4-Methylphenyl 1312 2-Methylpropyl 4-Methylphenyl 1313 3-Methylbutyl 4-Methylphenyl 1314 3-Methylbutyl 2-Methylphenyl 1315 Butyl 3-Fluorophenyl 1316 2-Methylpropyl 3-Fluorophenyl 1317 Pentyl 3-Fluorophenyl 1318 3-Methylbutyl 3-Fluorophenyl 1319 2-Methylpropyl 4-Fluorophenyl 1320 3-Methylbutyl 4-Fluorophenyl 1321 Butyl 2-Fluorophenyl 1322 2-Methylpropyl 2-Fluorophenyl 1323 Pentyl 2-Fluorophenyl 1324 3-Methylbutyl 2-Fluorophenyl 1325 2-Methylpropyl 4-Ethylphenyl 1326 Butyl 3,4-Dimethylphenyl 1327 2-Methylpropyl 3,4-Dimethylphenyl 1328 3-Methylbutyl 3,4-Dimethylphenyl 1329 2-Methylpropyl 2,4-Dimethylphenyl 1330 Butyl 3-Methoxyphenyl 1331 2-Methylpropyl 3-Methoxyphenyl 1332 Pentyl 3-Methoxyphenyl 1333 3-Methylbutyl 3-Methoxyphenyl 1334 Butyl 4-Methoxyphenyl 1335 2-Methylpropyl 4-Methoxyphenyl 1336 3-Methylbutyl 4-Methoxyphenyl 1337 Pentyl 2-Methoxyphenyl 1338 3-Methylbutyl 2-Methoxyphenyl 1339 Butyl 3-Fluoro-4-methylphenyl 1340 Pentyl 3-Fluoro-4-methylphenyl 1341 3-Methylbutyl 3-Fluoro-4-methylphenyl 1342 3-Methylbutyl 3-Fluoro-2-methylphenyl 1343 Butyl 2-Fluoro-3-methylphenyl 1344 2-Methylpropyl 2-Fluoro-3-methylphenyl 1345 Pentyl 2-Fluoro-3-methylphenyl 1346 3-Methylbutyl 2-Fluoro-3-methylphenyl 1347 Butyl 3-Chlorophenyl 1348 2-Methylpropyl 3-Chlorophenyl 1349 Pentyl 3-Chlorophenyl 1350 3-Methylbutyl 3-Chlorophenyl 1351 2-Methylpropyl 4-Chlorophenyl 1352 Pentyl 4-Chlorophenyl 1353 3-Methylbutyl 4-Chlorophenyl 1354 Butyl 2-Chlorophenyl 1355 2-Methylpropyl 2-Chlorophenyl 1356 Pentyl 2-Chlorophenyl 1357 3-Methylbutyl 2-Chlorophenyl 1358 Butyl 3,4-Difluorophenyl 1359 2-Methylpropyl 3,4-Difluorophenyl 1360 Pentyl 3,4-Difluorophenyl 1361 3-Methylbutyl 3,4-Difluorophenyl 1362 Butyl 2,3-Difluorophenyl 1363 2-Methylpropyl 2,3-Difluorophenyl 1364 Pentyl 2,3-Difluorophenyl 1365 3-Methylbutyl 2,3-Difluorophenyl 1366 Butyl 2,5-Difluorophenyl 1367 2-Methylpropyl 2,5-Difluorophenyl 1368 Pentyl 2,5-Difluorophenyl 1369 3-Methylbutyl 2,5-Difluorophenyl 1370 Butyl 2,4-Difluorophenyl 1371 2-Methylpropyl 2,4-Difluorophenyl 1372 Pentyl 2,4-Difluorophenyl 1373 3-Methylbutyl 2,4-Difluorophenyl 1374 2-Methylpropyl 3-Ethoxyphenyl 1375 3-Methylbutyl 3-Ethoxyphenyl 1376 Butyl 1,3-Benzodioxol-5-yl 1377 2-Methylpropyl 1,3-Benzodioxol-5-yl 1378 Pentyl 1,3-Benzodioxol-5-yl 1379 3-Methylbutyl 1,3-Benzodioxol-5-yl 1380 Butyl 4-Methylthio phenyl 1381 2-Methylpropyl 4-Methylthio phenyl 1382 3-Methylbutyl 3-Fluoro-4-methoxyphenyl 1383 Butyl 3-Chloro-4-fluorophenyl 1384 2-Methylpropyl 3-Chloro-4-fluorophenyl 1385 3-Methylbutyl 3-Chloro-4-fluorophenyl 1391 3-Methylbutyl 3-Chloro-4-methoxyphenyl 1392 Pentyl 5-Chloro-2-methoxyphenyl 1393 3-Methylbutyl 5-Chloro-2-methoxyphenyl 1394 2-Methylpropyl 3,4-Dichlorophenyl 1395 3-Methylbutyl 3,4-Dichlorophenyl 1396 Butyl 2,5-Dichlorophenyl 1397 2-Methylpropyl 2,5-Dichlorophenyl 1398 Pentyl 2,5-Dichlorophenyl 1399 3-Methylbutyl 2,5-Dichlorophenyl 1400 2-Methylpropyl 2,4-Dichlorophenyl 1401 3-Methylbutyl 2,4-Dichlorophenyl 1402 Butyl 3-Bromophenyl 1403 2-Methylpropyl 3-Bromophenyl 1404 Pentyl 3-Bromophenyl 1405 3-Methylbutyl 3-Bromophenyl 1406 2-Methylpropyl 4-Bromophenyl 1407 3-Methylbutyl 4-Bromophenyl 1408 3-Methylbutyl 2-Bromophenyl 1409 3-Methylbutyl 3-Bromo-4-methylphenyl 1410 Butyl 3-Bromo-4-fluorophenyl 1411 2-Methylpropyl 3-Bromo-4-fluorophenyl 1412 Pentyl 3-Bromo-4-fluorophenyl 1413 3-Methylbutyl 3-Bromo-4-fluorophenyl 1414 Butyl 3-Iodophenyl 1415 2-Methylpropyl 3-Iodophenyl 1416 Pentyl 3-Iodophenyl 1417 3-Methylbutyl 3-Iodophenyl 1418 Butyl 5-Methyl-2-thienyl 1419 2-Methylpropyl 5-Methyl-2-thienyl 1420 Pentyl 5-Methyl-2-thienyl 1421 3-Methylbutyl 5-Methyl-2-thienyl 1422 3-Methylbutyl 3-Fluorobenzyl 1423 3-Methylbutyl 3-Methoxybenzyl 1424 3-Methylbutyl 2-Methoxybenzyl 1686 2-Methylpropyl 2,4,6-Trifluorophenyl 1687 Butyl 2,3,6-Trifluorophenyl 1688 2-Methylpropyl 2,3,6-Trifluorophenyl 1689 Pentyl 2,3,6-Trifluorophenyl 1690 3-Methylbutyl 2,3,6-Trifluorophenyl 1691 3-Methylbutyl 2,5-Dimethyl-3-furyl 1692 Butyl 4,5-Dimethyl-2-furyl 1693 2-Methylpropyl 4,5-Dimethyl-2-furyl 1694 Pentyl 4,5-Dimethyl-2-furyl 1695 3-Methylbutyl 4,5-Dimethyl-2-furyl 1696 2-Methylpropyl 2-(3-Thienyl)ethenyl 1697 Pentyl 3-Chloro-2-thienyl 1698 3-Methylbutyl 3-Chloro-2-thienyl 1699 2-Methylpropyl 5-Methylthio-2-thienyl 1700 3-Methylbutyl 5-Methylthio-2-thienyl 1721 Butyl 3-Chloro-4-methylphenyl 1722 2-Methylpropyl 3-Chloro-4-methylphenyl 1723 3-Methylbutyl 3-Chloro-4-methylphenyl 1724 2-Methylpropyl 2,4,5-Trichlorophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 1468 Methyl Phenyl 1469 Allyl Phenyl 1470 Propyl Phenyl 1471 Methyl 3-Methylphenyl 1472 Allyl 3-Methylphenyl 1473 Propyl 3-Methylphenyl 1474 Propyl 4-Methylphenyl 1475 Methyl 3-Fluorophenyl 1476 Allyl 3-Fluorophenyl 1477 Propyl 3-Fluorophenyl 1478 Propyl 4-Fluorophenyl 1479 Methyl 2-Fluorophenyl 1480 Allyl 2-Fluorophenyl 1481 Propyl 2-Fluorophenyl 1482 Propyl 3,4-Dimethylphenyl 1483 Propyl 3-Methoxyphenyl 1484 Propyl 3-Fluoro-4-methylphenyl 1485 Allyl 3-Chlorophenyl 1486 Propyl 3-Chlorophenyl 1487 Propyl 2-Chlorophenyl 1488 Propyl 3,4-Difluorophenyl 1489 Methyl 2,3-Difluorophenyl 1490 Propyl 2,3-Difluorophenyl 1491 Methyl 2,5-Difluorophenyl 1492 Allyl 2,5-Difluorophenyl 1493 Propyl 2,5-Difluorophenyl 1494 Propyl 2,4-Difluorophenyl 1495 Propyl 1,3-Benzodioxol-5-yl 1496 Propyl 3-Chloro-4-fluorophenyl 1497 Methyl 5-Chloro-2-methoxyphenyl 1498 Methyl 3-Trifluoromethylphenyl 1499 Propyl 3-Trifluoromethylphenyl 1500 Methyl 2,5-Dichlorophenyl 1501 Propyl 2,5-Dichlorophenyl 1502 Methyl 3-Bromophenyl 1503 Allyl 3-Bromophenyl 1504 Propyl 3-Bromophenyl 1505 Propyl 3-Bromo-4-methylphenyl 1506 Methyl 3-Bromo-4-fluorophenyl 1507 Allyl 3-Bromo-4-fluorophenyl 1508 Propyl 3-Bromo-4-fluorophenyl 1509 Methyl 3-Iodophenyl 1510 Ethyl 3-Iodophenyl 1511 Allyl 3-Iodophenyl 1512 Propyl 3-Iodophenyl 1513 Propyl 5-Methyl-2-thienyl 1514 Propyl 3-Fluorobenzyl 1515 Methyl 5-Ethoxy-2-thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 1717 Propyl 3-Chloro-4-methylphenyl 1718 Propyl 2,4,5-Trifluorophenyl 2237 Benzyl Phenyl 2240 Benzyl 3-Fluorophenyl 2241 Benzyl 4-Fluorophenyl 2244 Benzyl 2-Fluorophenyl 2246 Benzyl 3,4-Dimethylphenyl 2247 Benzyl 3,5-Dimethylphenyl 2248 Benzyl 2,3-Dimethylphenyl 2249 Benzyl 2,5-Dimethylphenyl 2250 Benzyl 2,4-Dimethylphenyl 2252 Benzyl 3-Methoxyphenyl 2255 Benzyl 2-Methoxyphenyl 2256 Benzyl 3-Fluoro-4-methylphenyl 2259 Benzyl 5-Fluoro-2-methylphenyl 2263 Benzyl 3-Chlorophenyl 2264 Benzyl 4-Chlorophenyl 2265 Benzyl 2-Chlorophenyl 2267 Benzyl 3,4-Difluorophenyl 2270 Benzyl 2,3-Difluorophenyl 2273 Benzyl 2,5-Difluorophenyl 2274 Benzyl 2,4-Difluorophenyl 2275 Benzyl 3-Ethoxyphenyl 2276 Benzyl 1,3-Benzodioxol-5-yl 2277 Benzyl 4-Chloro-3-methylphenyl 2278 Benzyl 3-Chloro-4-fluorophenyl 2279 Benzyl 3,4,5-Trifluorophenyl 2280 Benzyl 2,5-Dimethoxyphenyl 2283 Benzyl 5-Chloro-2-methoxyphenyl 2284 Benzyl 4-Chloro-2-methoxyphenyl 2285 Benzyl 3-Trifluoromethylphenyl 2286 Benzyl 2-Trifluoromethylphenyl 2287 Benzyl 3,4-Dichlorophenyl 2288 Benzyl 2,3-Dichlorophenyl 2290 Benzyl 2,5-Dichlorophenyl 2291 Benzyl 2,4-Dichlorophenyl 2294 Benzyl 3-Bromophenyl 2296 Benzyl 2-Bromophenyl 2297 Benzyl 3-Bromo-4-fluorophenyl 2300 Benzyl 3-Iodophenyl 2301 Benzyl 2-Methoxyphenyl 2303 Benzyl 2,5-Dimethylpyrrol-3-yl 2308 Benzyl 2,3,6-Trifluorphenyl 2309 3-Methylbutyl 2-Chloro-6-fluorophenyl 2325 3-Methylbutyl 3-(Methylamino methyl)phenyl 2326 3-Methylbutyl 3-(Ethylamino methyl)phenyl 2327 3-Methylbutyl 3-(allylamino methyl)phenyl 2328 3-Methylbutyl 3-(propylamino methyl)phenyl 2329 3-Methylbutyl 3-[(Cyclopropyl methyl)amino methyl]phenyl 2330 3-Methylbutyl 3-(butylamino methyl)phenyl 2331 3-Methylbutyl 3-[(2-Methylpropyl) amino methyl]phenyl 2332 3-Methylbutyl 3-(Pentylamino methyl)phenyl 2333 3-Methylbutyl 3-[(3-Methylbutyl) amino methyl]phenyl 2334 3-Methylbutyl 3-[(2-Methylbutyl) amino methyl]phenyl 2335 3-Methylbutyl 3-(Hexylamino methyl)phenyl 2336 3-Methylbutyl 3-(Cyclopropyl amino methyl)phenyl 2337 3-Methylbutyl 3-[(1-Methylethyl) aminomethyl] phenyl 2338 3-Methylbutyl 3-(Cyclobutyl amino methyl)phenyl 2339 3-Methylbutyl 3-[(1-Methylpropyl) aminomethyl] phenyl 2340 3-Methylbutyl 3-[(1,1-Dimethylethyl) aminomethyl] phenyl 2341 3-Methylbutyl 3-(Cyclopentyl amino methyl)phenyl 2342 3-Methylbutyl 3-[(1-Methylbutyl) aminomethyl] phenyl 2343 3-Methylbutyl 3-[(1,2-Dimethylpropyl) aminomethyl] phenyl 2344 3-Methylbutyl 3-[(1-Ethylpropyl) aminomethyl] phenyl 2345 3-Methylbutyl 3-[(1,1-Dimethylpropyl) aminomethyl] phenyl 2346 3-Methylbutyl 3-(Cyclohexyl amino methyl)phenyl 2352 3-Methylbutyl 3-(Piperidyl methyl)phenyl 2353 3-Methylbutyl 3-(Morpholin-4-yl methyl)phenyl 2354 3-Methylbutyl 3-(Azaperhydro epinylmethyl) phenyl 2355 3-Methylbutyl 3-(Azaperhydro ocinylmethyl) phenyl 2356 3-Methylbutyl 3-(2-1,2,3,4-Teterahydro isoquinolinyl methyl) phenyl 2357 3-Methylbutyl 3-(Methylpropyl aminomethyl) phenyl 2358 3-Methylbutyl 3-(i-propylethyl aminomethyl) phenyl 2359 3-Methylbutyl 3-(Diethyl aminomethyl) phenyl 2360 3-Methylbutyl 3-(Butylethyl aminomethyl) phenyl 2361 3-Methylbutyl 3-[(Cyclopropyl methyl)propyl aminomethyl] phenyl 2362 3-Methylbutyl 3-(Hexylmethyl aminomethyl) phenyl 2363 3-Methylbutyl 3-(Dibutyl aminomethyl) phenyl 2370 3-Methylbutyl 3-[(1-methylethyl) methyl aminomethyl] phenyl 2371 3-Methylbutyl 3-[(2-Methyl piperidyl) methyl]phenyl 2372 3-Methylbutyl 3-[Ethyl(2-Methylprop-2-enyl)amino methyl]phenyl 2373 3-Methylbutyl 3-[(2-Ethyl piperidyl) methyl]phenyl 2374 3-Methylbutyl 3-(Cyclohexyl ethyl aminomethyl) phenyl 2375 3-Methylbutyl 3-[bis(2-Methoxyethyl) aminomethyl] phenyl 2376 3-Methylbutyl 3-[(3,3,5-Trimethylaza perhydroepinyl)methyl]phenyl 2377 3-Methylbutyl 3-[(8-Aza-1,4-dioxaspiro[4.5]dec-8- yl)methyl] phenyl 2378 3-Methylbutyl 3-(Dipentylamino methyl)phenyl 2379 3-Methylbutyl 3-(Dihexylamino methyl)phenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2004 2-Methylpropyl 2-(4-Chlorophenyl)ethenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2020 Methyl 3-Thienyl 2021 i-Propyl 3-Methyl-2-thienyl 2022 Methyl 4-Methylbenzyl 2023 Methyl 2-Methylbenzyl 2024 Methyl 3-Fluorobenzyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2025 3-Pyrrolinyl 2,5-Difluorophenyl 2026 3-Pyrrolinyl 3-Fluorophenyl 2027 Pyrrolidinyl 2,5-Difluorophenyl 2028 Pyrrolidinyl 3-Fluorophenyl 2029 1,2,5,6-Tetrahydro 2,5-Difluorophenyl pyridyl 2030 1,2,5,6-Tetrahydro 3-Fluorophenyl pyridyl 2031 Piperidyl 2,5-Difluorophenyl 2032 Piperidyl 3-Fluorophenyl 2039 Morpholinyl 2,5-Difluorophenyl 2040 Morpholinyl 3-Fluorophenyl 2043 4-Methyl 2,5-Difluorophenyl piperidyl 2044 4-Methyl 3-Fluorophenyl piperidyl 2046 Azaperhydro 2,5-Difluorophenyl epinyl 2047 Azaperhydro 3-Fluorophenyl Epinyl 2049 1,4-Thiazaper 2,5-Difluorophenyl hydroin-4-yl 2050 1,4-Thiazaper 3-Fluorophenyl hydroin-4-yl 2053 3,3-dimethyl 2,5-Difluorophenyl piperidyl 2054 3,3-dimethyl 3-Fluorophenyl piperidyl 2057 Azaperhydro 2,5-Difluorophenyl ocinyl 2058 Azaperhydro 3-Fluorophenyl Ocinyl 2061 2-(1,2,3,4-Tetrahydroiso 2,5-Difluorophenyl quinolyl) 2062 2-(1,2,3,4-Tetrahydroiso 3-Fluorophenyl quinolyl) 2065 Methylprop-2-enylamino 2,5-Difluorophenyl 2066 Methylprop-2-enylamino 3-Fluorophenyl 2068 Diethylamino 2,5-Difluorophenyl 2069 Diethylamino 3-Fluorophenyl 2072 Methylpropyl 2,5-Difluorophenyl amino 2073 Methylpropyl 3-Fluorophenyl Amino 2076 Butylmethyl 2,5-Difluorophenyl amino 2077 Butylmethyl 3-Fluorophenyl Amino 2080 i-Propylethyl 2,5-Difluorophenyl amino 2081 i-Propylethyl 3-Fluorophenyl amino 2084 Diallylamino 2,5-Difluorophenyl 2085 Diallylamino 3-Fluorophenyl 2088 Dipropylamino 2,5-Difluorophenyl 2089 Dipropylamino 3-Fluorophenyl 2092 Butylethyl 2,5-Difluorophenyl Amino 2093 Butylethyl 3-Fluorophenyl Amino 2096 (Cyclo 2,5-Difluorophenyl propylmethyl) propylamino 2097 (Cyclo 3-Fluorophenyl propylmethyl) propylamino 2100 Hexylmethyl 2,5-Difluorophenyl amino 2101 Hexylmethyl 3-Fluorophenyl Amino 2104 Dibutylamino 2,5-Difluorophenyl 2105 Dibutylamino 3-Fluorophenyl 2107 Methylamino 2,5-Difluorophenyl 2108 Methylamino 3-Fluorophenyl 2110 Ethylamino 2,5-Difluorophenyl 2111 Ethylamino 3-Fluorophenyl 2114 Allylamino 2,5-Difluorophenyl 2115 Allylamino 3-Fluorophenyl 2118 Propylamino 2,5-Difluorophenyl 2119 Propylamino 3-Fluorophenyl 2122 (Cyclopropyl 2,5-Difluorophenyl methyl)amino 2123 (Cyclopropyl 3-Fluorophenyl methyl)amino 2126 Butyl 2,5-Difluorophenyl 2127 Butyl 3-Fluorophenyl 2130 (2-Methylpropyl) 2,5-Difluorophenyl amino 2131 (2-Methylpropyl) 3-Fluorophenyl amino 2134 Pentylamino 2,5-Difluorophenyl 2135 Pentylamino 3-Fluorophenyl 2138 (3-Methylbutyl) 2,5-Difluorophenyl amino 2139 (3-Methylbutyl) 3-Fluorophenyl amino 2141 (2-Methylbutyl) 2,5-Difluorophenyl amino 2142 (2-Methylbutyl) 3-Fluorophenyl amino 2145 Hexylamino 2,5-Difluorophenyl 2146 Hexylamino 3-Fluorophenyl 2148 [2-(Dimethyl 2,5-Difluorophenyl amino)ethyl] amino 2149 [2-(Dimethyl 3-Fluorophenyl amino)ethyl] amino 2150 [3-(Dimethyl 2,5-Difluorophenyl amino)propyl] amino 2151 [3-(Dimethyl 3-Fluorophenyl amino)propyl] amino 2153 (2-Pyrrolidinyl 2,5-Difluorophenyl ethyl)amino 2154 (2-Pyrrolidinyl 3-Fluorophenyl ethyl)amino 2157 [2-(Diethyl 2,5-Difluorophenyl amino)ethyl] amino 2158 [2-(Diethyl 3-Fluorophenyl amino)ethyl] amino 2161 (2-Piperidyl 2,5-Difluorophenyl ethyl)amino 2162 (2-Piperidyl 3-Fluorophenyl ethyl)amino 2164 [2-(1-Methyl 2,5-Difluorophenyl pyrrolidin-2-yl)ethyl]amino 2165 [2-(1-Methyl 3-Fluorophenyl pyrrolidin-2-yl)ethyl]amino 2168 [2-(Diethyl 2,5-Difluorophenyl amino)propyl] amino 2169 [2-(Diethyl 3-Fluorophenyl amino)propyl] amino 2172 (2-Morpholin-4-yl 2,5-Difluorophenyl ethyl)amino 2173 (2-Morpholin-4-yl 3-Fluorophenyl ethyl)amino 2176 (3-Morpholin-4-yl 2,5-Difluorophenyl propyl)amino 2177 (3-Morpholin-4-yl 3-Fluorophenyl propyl)amino 2180 [3-(2-Methyl 2,5-Difluorophenyl piperidyl) propyl]amino 2181 [3-(2-Methyl 3-Fluorophenyl piperidyl) propyl]amino 2184 [3-(2-Oxo 2,5-Difluorophenyl pyrrolidinyl) propyl]amino 2185 [3-(2-Oxo 3-Fluorophenyl pyrrolidinyl) propyl]amino -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R4 R3 2033 Pyrrolidinyl 2,5-Difluorophenyl 2034 Pyrrolidinyl 3-Fluorophenyl 2035 1,2,5,6-Tetrahydro 2,5-Difluorophenyl pyridyl 2036 1,2,5,6-Tetrahydro 3-Fluorophenyl pyridyl 2037 Piperidyl 2,5-Difluorophenyl 2038 Morpholinyl 3-Fluorophenyl 2041 4-Methyl 2,5-Difluorophenyl piperidyl 2042 4-Methyl 3-Fluorophenyl piperidyl 2045 Azaperhydro 3-Fluorophenyl Epinyl 2048 1,4-Thiazaper 3-Fluorophenyl hydroin-4-yl 2051 3,3-dimethyl 2,5-Difluorophenyl piperidyl 2052 3,3-dimethyl 3-Fluorophenyl piperidyl 2055 Azaperhydro 2,5-Difluorophenyl ocinyl 2056 Azaperhydro 3-Fluorophenyl Ocinyl 2059 2-(1,2,3,4- 2,5-Difluorophenyl Tetrahydroiso quinolyl) 2060 2-(1,2,3,4- 3-Fluorophenyl Tetrahydroiso quinolyl) 2063 Methylprop-2- 2,5-Difluorophenyl enylamino 2064 Methylprop-2- 3-Fluorophenyl enylamino 2067 Diethylamino 3-Fluorophenyl 2070 Methylpropyl 2,5-Difluorophenyl amino 2071 Methylpropyl 3-Fluorophenyl Amino 2074 Butylmethyl 2,5-Difluorophenyl amino 2075 Butylmethyl 3-Fluorophenyl Amino 2078 i-Propylethyl 2,5-Difluorophenyl amino 2079 i-Propylethyl 3-Fluorophenyl amino 2082 Diallylamino 2,5-Difluorophenyl 2083 Diallylamino 3-Fluorophenyl 2086 Dipropylamino 2,5-Difluorophenyl 2087 Dipropylamino 3-Fluorophenyl 2090 Butylethyl 2,5-Difluorophenyl Amino 2091 Butylethyl 3-Fluorophenyl Amino 2094 (Cyclo 2,5-Difluorophenyl propylmethyl) propylamino 2095 (Cyclo 3-Fluorophenyl propylmethyl) propylamino 2098 Hexylmethyl 2,5-Difluorophenyl Amino 2099 Hexylmethyl 3-Fluorophenyl Amino 2102 Dibutylamino 2,5-Difluorophenyl 2103 Dibutylamino 3-Fluorophenyl 2106 Methylamino 3-Fluorophenyl 2109 Ethylamino 3-Fluorophenyl 2112 Allylamino 2,5-Difluorophenyl 2113 Allylamino 3-Fluorophenyl 2116 Propylamino 2,5-Difluorophenyl 2117 Propylamino 3-Fluorophenyl 2120 (Cyclopropyl 2,5-Difluorophenyl methyl)amino 2121 (Cyclopropyl 3-Fluorophenyl methyl)amino 2124 Butyl 2,5-Difluorophenyl 2125 Butyl 3-Fluorophenyl 2128 (2-Methylpropyl) 2,5-Difluorophenyl amino 2129 (2-Methylpropyl) 3-Fluorophenyl amino 2132 Pentylamino 2,5-Difluorophenyl 2133 Pentylamino 3-Fluorophenyl 2136 (3-Methylbutyl) 2,5-Difluorophenyl amino 2137 (3-Methylbutyl) 3-Fluorophenyl amino 2140 (2-Methylbutyl) 3-Fluorophenyl amino 2143 Hexylamino 2,5-Difluorophenyl 2144 Hexylamino 3-Fluorophenyl 2152 (2-Pyrrolidinyl 3-Fluorophenyl ethyl)amino 2155 [2-(Diethyl 2,5-Difluorophenyl amino)ethyl] amino 2156 [2-(Diethyl 3-Fluorophenyl amino)ethyl] amino 2159 (2-Piperidyl 2,5-Difluorophenyl ethyl)amino 2160 (2-Piperidyl 3-Fluorophenyl ethyl)amino 2163 [2-(1-Methyl 3-Fluorophenyl pyrrolidin-2- yl)ethyl]amino 2166 [2-(Diethyl 2,5-Difluorophenyl amino)propyl] amino 2167 [2-(Diethyl 3-Fluorophenyl amino)propyl] amino 2170 (2-Morpholin-4-yl 2,5-Difluorophenyl ethyl)amino 2171 (2-Morpholin-4-yl 3-Fluorophenyl ethyl)amino 2174 (3-Morpholin-4-yl 2,5-Difluorophenyl propyl)amino 2175 (3-Morpholin-4-yl 3-Fluorophenyl propyl)amino 2178 [3-(2-Methyl 2,5-Difluorophenyl piperidyl) propyl]amino 2179 [3-(2-Methyl 3-Fluorophenyl piperidyl) propyl]amino 2182 [3-(2-Oxo 2,5-Difluorophenyl pyrrolidinyl) propyl]amino 2183 [3-(2-Oxo 3-Fluorophenyl pyrrolidinyl) propyl]amino -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2147 3-Methylbutyl 3-Chlorophenyl 2219 3-Methylbutyl 3-Trifluoromethylphenyl 2220 Butyl 3-Bromophenyl 2221 2-Methylpropyl 3-Bromophenyl 2222 3-Methylbutyl 3-Bromophenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2186 Butyl 2,5-Dimethoxyphenyl 2187 2-Methylpropyl 2,5-Dimethoxyphenyl 2188 3-Methylbutyl 2,5-Dimethoxyphenyl 2189 Butyl 3-Chloro-4-methoxyphenyl 2190 2-Methylpropyl 3-Chloro-4-methoxyphenyl 2191 3-Methylbutyl 3-Chloro-4-methoxyphenyl 2192 Butyl 5-Chloro-2-methoxyphenyl 2193 2-Methylpropyl 5-Chloro-2-methoxyphenyl 2194 3-Methylbutyl 5-Chloro-2-methoxyphenyl 2195 2-Methylpropyl 4-Chloro-2-methoxyphenyl 2196 Butyl 3-Trifluoromethylphenyl 2197 2-Methylpropyl 3-Trifluoromethylphenyl 2198 3-Methylbutyl 3-Trifluoromethylphenyl 2199 Butyl 2-Trifluoromethylphenyl 2200 3-Methylbutyl 2-Trifluoromethylphenyl 2201 Butyl 3,4-Dichlorophenyl 2202 2-Methylpropyl 3,4-Dichlorophenyl 2203 3-Methylbutyl 3,4-Dichlorophenyl 2204 Butyl 2,5-Dichlorophenyl 2205 2-Methylpropyl 2,5-Dichlorophenyl 2206 Pentyl 2,5-Dichlorophenyl 2207 3-Methylbutyl 2,5-Dichlorophenyl 2208 Butyl 2,4-Dichlorophenyl 2209 2-Methylpropyl 2,4-Dichlorophenyl 2210 3-Methylbutyl 2,4-Dichlorophenyl 2211 Butyl 3-Bromophenyl 2212 2-Methylpropyl 3-Bromophenyl 2213 Pentyl 3-Bromophenyl 2214 3-Methylbutyl 3-Bromophenyl 2215 2-Methylpropyl 4-Bromophenyl 2216 Butyl 2-Bromophenyl 2217 2-Methylpropyl 2-Bromophenyl 2218 3-Methylbutyl 2-Bromophenyl 2223 2-Methylpropyl 3-Phenoxyphenyl 2224 2-Methylpropyl 4-Phenoxyphenyl 2225 2-Methylpropyl 3-Bromo-4-methylphenyl 2226 Pentyl 3-Bromo-4-methylphenyl 2227 3-Methylbutyl 3-Bromo-4-methylphenyl 2228 Butyl 3-Bromo-4-methylphenyl 2229 2-Methylpropyl 3-Bromo-4-methylphenyl 2230 Pentyl 3-Bromo-4-methylphenyl 2231 3-Methylbutyl 3-Bromo-4-methylphenyl 2232 Butyl 3-Iodophenyl 2233 2-Methylpropyl 3-Iodophenyl 2234 Pentyl 3-Iodophenyl 2235 3-Methylbutyl 3-Iodophenyl 2236 2-Methylpropyl 4-Iodophenyl 2310 2-Methylpropyl 2,3,5,6-Tetrafluoro phenyl 2311 2-Methylpropyl 2,4,6-Trifluorophenyl 2312 Butyl 2,3,6-Trifluorophenyl 2313 2-Methylpropyl 2,3,6-Trifluorophenyl 2314 Pentyl 2,3,6-Trifluorophenyl 2315 3-Methylbutyl 2,3,6-Trifluorophenyl 2316 Butyl 3-Chloro-6-fluorophenyl 2317 Pentyl 3-Chloro-6-fluorophenyl 2318 3-Methylbutyl 3-Chloro-6-fluorophenyl 2319 Butyl 2-Fluoro-6- trifluoromethylphenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2304 2-Methylpropyl 5-Methyl-2-thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2380 2-Methylpropyl 2,4-Difluorophenyl 2381 2-Methylpropyl 2H-Benzo[d]1,3-dioxolane 2382 2-Methylpropyl 3-Chloro-4-methylphenyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2390 2-Methylpropyl 5-Methyl-2-thienyl -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2383 2-Methylpropyl 3-Chloro-4-methylphenyl 2384 2-Methylpropyl 2,4-Difluorophenyl 2385 2-Methylpropyl 2H-Benzo [d]1,3-dioxolane -
- For each compound, the definitions of R 2 and R3 are specified in the following table.
Compound No. R2 R3 2389 Pentyl 3-Fluoro-4-methylphenyl - The following assay is a standard assay for GABA A receptor binding.
- The high affinity and high selectivity of compounds of this invention for the benzodiazepine site of the GABA A receptor is confirmed using the binding assay described in Thomas and Tallman (J. Bio. Chem. 1981; 156:9838-9842, and J. Neurosci. 1983; 3:433-440).
- Rat cortical tissue is dissected and homogenized in 25 volumes (w/v) of Buffer A (0.05 M Tris HCl buffer, pH 7.4 at 4° C.). The tissue homogenate is centrifuged in the cold (4° C.) at 20,000× g for 20 minutes. The supernatant is decanted, the pellet rehomogenized in the same volume of buffer, and centrifuged again at 20,000× g. The supernatant of this centrifugation step is decanted and the pellet stored at −20° C. overnight. The pellet is then thawed and resuspended in 25 volumes of Buffer A (original wt/vol), centrifuged at 20,000× g and the supernatant decanted. This wash step is repeated once. The pellet is finally resuspended in 50 volumes of Buffer A.
- Incubations containi 100 l of tissue homogenate, 100 l of radioligand, (0.5 nM 3H-Rol5-1788 [3H-Flumazenil], specific activity 80 Ci/mmol), and test compound or control (see below), and are brought to a total volume of 500 l with Buffer A. Incubations are carried for 30 min at 4° C. and then rapidly filtered through Whatman GFB filters to separate free and bound ligand. Filters are washed twice with fresh Buffer A and counted in a liquid scintillation counter. Nonspecific binding (control) is determined by displacement of 3H Rol5-1788 with 10 M Diazepam (Research Biochemicals International, Natick, Mass.). Data were collected in triplicate, averaged, and percent inhibition of total specific binding (Total Specific Binding=Total−Nonspecific) was calculated for each compound.
- A competition binding curve is obtained with up to 11 points spanning the compound concentration range from 10 −12M to 10−5M obtained per curve by the method described above for determining percent inhibition. Ki values are calculated according the Cheng-Prussof equation. When tested in this assay compounds of the invention exihibit Ki values of less than 1 uM, preferred compounds of the invention have Ki values of less than 500 nM and more compounds of the invention have Ki values of less than 100 nM.
- The following assay is used to determine if a compound of the invention act as an agonist, an antagonist, or an inverse agonist at the benzodiazepine site of the GABA A receptor.
- Assays are carried out as described in White and Gurley (NeuroReport 6: 1313-1316, 1995) and White, Gurley, Hartnett, Stirling, and Gregory (Receptors and Channels 3: 1-5, 1995) with modifications. Electrophysiological recordings are carried out using the two electrode voltage-clamp technique at a membrane holding potential of −70 mV. Xenopus Laevis oocytes are enzymatically isolated and injected with non-polyadenylated cRNA mixed in a ratio of 4:1:4 for , and subunits, respectively. Of the nine combinations of , and subunits described in the White et al. publications, preferred combinations are 1 2 2, 2 3 2, 3 3 2, and 5 3 2. Preferably all of the subunit cRNAs in each combination are human clones or all are rat clones. The sequence of each of these cloned subunits is available from GENBANK, e.g., human 1, GENBANK accession no. X14766, human 2, GENBANK accession no. A28100; human 3, GENBANK accession no. A28102; human 5, GENBANK accession no. A28104; human 2, GENBANK accession no. M82919; human 3, GENBANK accession no. Z20136; human 2, GENBANK accession no. X15376; rat 1, GENBANK accession no. L08490, rat 2, GENBANK accession no. L08491; rat 3, GENBANK accession no. L08492; rat 5, GENBANK accession no. L08494; rat 2, GENBANK accession no. X15467; rat 3, GENBANK accession no. X15468; and rat 2, GENBANK accession no. L08497. For each subunit combination, sufficient message for each constituent subunit is injected to provide current amplitudes of >10 nA when 1 μM GABA is applied.
- Compounds are evaluated against a GABA concentration that evokes <10% of the maximal evokable GABA current (e.g. 1 M -9 M). Each oocyte is exposed to increasing concentrations of compound in order to evaluate a concentration/effect relationship. Compound efficacy is calculated as a percent-change in current amplitude: 100*((Ic/I)−1), where Ic is the GABA evoked current amplitude observed in the presence of test compound and I is the GABA evoked current amplitude observed in the absence of the test compound.
- Specificity of a compound for the benzodiazepine site is determined following completion of a concentration/effect curve. After washing the oocyte sufficiently to remove previously applied compound, the oocyte is exposed to GABA+1 μM RO15-1788, followed by exposure to GABA+1 μM RO15-1788+test compound. Percent change due to addition of compound is calculated as described above. Any percent change observed in the presence of RO15-1788 is subtracted from the percent changes in current amplitude observed in the absence of 1 μM RO15-1788. These net values are used for the calculation of average efficacy and EC 50 values by standard methods. To evaluate average efficacy and EC50 values, the concentration/effect data are averaged across cells and fit to the logistic equation.
- The compounds of the invention are prepared as radiolabeled probes by carrying out their synthesis using precursors comprising at least one atom that is a radioisotope. The radioisotope is preferably selected from of at least one of carbon (preferably 14C), hydrogen (preferably 3H), sulfur (preferably 35S), or iodine (preferably 125I). Such radiolabeled probes are conveniently synthesized by a radioisotope supplier specializing in custom synthesis of radiolabeled probe compounds. Such suppliers include Amersham Corporation, Arlington Heights, Ill.; Cambridge Isotope Laboratories, Inc. Andover, Mass.; SRI International, Menlo Park, Calif.; Wizard Laboratories, West Sacramento, Calif.; ChemSyn Laboratories, Lexena, Kans.; American Radiolabeled Chemicals, Inc., St. Louis, Mo.; and Moravek Biochemicals Inc., Brea, Calif.
- Tritium labeled probe compounds are also conveniently prepared catalytically via platinum-catalyzed exchange in tritiated acetic acid, acid-catalyzed exchange in tritiated trifluoroacetic acid, or heterogeneous-catalyzed exchange with tritium gas. Such preparations are also conveniently carried out as a custom radiolabeling by any of the suppliers listed in the preceding paragraph using the compound of the invention as substrate. In addition, certain precursors may be subjected to tritium-halogen exchange with tritium gas, tritium gas reduction of unsaturated bonds, or reduction using sodium borotritide, as appropriate.
- Receptor autoradiography (receptor mapping) of NK-3 or GABA A receptors in cultured cells or tissue samples is carried out in vitro as described by Kuhar in sections 8.1.1 to 8.1.9 of Current Protocols in Pharmacology (1998) John Wiley & Sons, New York, using radiolabeled compounds of the invention prepared as described in the preceding Example.
- The invention and the manner and process of making and using it, are now described in such full, clear, concise and exact terms as to enable any person skilled in the art to which it pertains, to make and use the same. It is to be understood that the foregoing describes preferred embodiments of the present invention and that modifications may be made therein without departing from the spirit or scope of the present invention as set forth in the claims. To particularly point out and distinctly claim the subject matter regarded as invention, the following claims conclude this specification.
Claims (26)
1. A compound of the formula:
or pharmaceutically acceptable non-toxic salts thereof wherein:
W represents
where
Z is O, or S;
R1 represents phenyl, C1-C6 alkyl, cyclopentyl, cyclohexyl, benzyl, 3-fluorobenzyl, or cyclopropylmethyl;
R2 represents
hydroxyl;
C1-C6 alkyl or C1-C6 alkoxy, each of which are optionally substituted with amino, mono or di(C1-C6) alkylamino, a C5-C7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion;
O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl; or
NR8R9 forms a 5-, 6-, or 7-membered heterocyclic ring;
or R2 represents
hydrogen or
a group of the formula
where
Rn and Rk independently represent C1-C6 alkyl, C2-C6 alkenyl, C1-C6 cycloalkyl (C1-C6) alkyl, benzoyl where the phenyl portion is optionally substituted with halgoen, C1-C6 alkyl, or C1-C6 alkoxy;
a group of the formula IV-a
where
p, s, and t independently represent 1 or 2;
J is CH, N, O, or a carbon atom substituted with C1-C6 alkyl; or
NRkRn represents
where s, t, and J are as defined above;
R3 represents
C1-C6 alkyl, allyl, cyclopropylmethyl, cyclopentyl; or
benzyl optionally mono-, di-, or trisubstituted independently with halogen, nitro, trifluoromethyl, trifluoromethoxy, cyano, or hydroxy;
C1-C6 alkyl or C1-C6 alkoxy, each of which is optionally substituted with amino, mono or di(C1-C6) alkylamino, a C5-C7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion;
O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl;
NR8R9 forms a 5-, 6-, 7-membered heterocyclic ring;
SO2R8, NHSO2R8, SO2NHR8, SO2NHCOR8, CONHSO2R8 where R8 is defined as above;
O(CH2)n-G where n=1,2,3,4 and G is SO2R8, NHSO2R8, SO2NHR8, SO2NHCOR8, or CONHSO2R8, where R8 is as defined above; or
tetrazole, triazole, imidazole, thiazole, oxazole, thiophene, or pyridyl;
R4, R5 and R6 are the same or different and represent hydrogen; or
C1-C6 alkyl or C1-C6 alkoxy, each of which is optionally substituted with amino, mono or di(C1-C6) alkylamino, a C5-C7 heterocycloalkyl group where the heteroatom is nitrogen and the nitrogen is attached to the parent alkyl portion, C1-C6 alkylthiol, or halogen;
O(CH2)nCO2R8 where n=1,2,3,4, NR8COR9, COR8, CONR8R9 or CO2R8 where R8 and R9 are the same or different and represent hydrogen or C1-C6 alkyl;
NR8R9 forms a 5-, 6-, or 7-membered heterocyclic ring; or
R4 and R5 can form a 1,3-dioxolene ring;
X represents a bond, CH2, or CHCH; and
A, B, C, and D are the same or different and represent CH or N with the proviso that at least one, but not more than two of A,B,C, or D represent N.
2. A compound according to claim 1 , which is N-((3-cyclopropylmethylimidazolo[5,4-b]pyridin-2-yl)methyl)(3-fluorophenyl)-N-propylcarboxamide.
3. A compound according to claim 1 , which is N-[(3-cyclopropylmethylimidazolo[5,4-b]pyridin-2-yl)methyl](2,5-difluorophenyl)-N-propylcarboxamide.
4. A compound according to claim 1 , which is N-((3-n-butyl-imidazolo[5,4-b]pyridin-2-yl)methyl](3-iodophenyl)-N-propylcarboxamide.
5. A compound according to claim 1 for use in therapeutic treatment of a disease or disorder associated with pathogenic agonism, inverse agonism or antagonism of the GABAA receptor.
6. A pharmaceutical composition comprising a compound according to claim 1 combined with at least one pharmaceutically acceptable carrier or excipient.
7. A method for the treatment or prevention of a disease or disorder associated with pathogenic associated with pathogenic agonism, inverse agonism or antagonism of the GABAA receptor, the method comprising administering to a patient in need of such treatment or prevention an effective amount of a compound of claim 1 .
8. The use of a compound according to claim 1 for the manufacture of a medicament for the treatment or prevention of a disease or disorder associated with pathogenic agonism, inverse agonism or antagonism of the GABAA receptor.
9. The use of a compound according to claim 1 for the manufacture of a medicament for the treatment or prevention of anxiety, depression, sleep disorders, or cognitive impairment.
10. A method according to claim 7 wherein the disease or disorder associated with pathogenic agonism, inverse agonism or antagonism of the GABAA receptor is anxiety, depression, a sleep disorder, or cognitive impairment.
11. A method for localizing GABAA receptors in a tissue sample comprising: contacting the sample with a detectably-labeled compound of claim 1 under conditions that permit binding of the compound to GABAA receptors, washing the sample to remove unbound compound, and detecting the bound compound.
12. A method for altering the signal-transducing activity of GABAA receptors, the method comprising exposing cells expressing GABAA receptors to a compound according to claim 1 at a concentration sufficient to inhibit RO15-1788 binding to cells expressing a cloned human GABAA receptor in vitro.
13. A packaged pharmaceutical composition comprising the pharmaceutical composition of claim 6 in a container and instructions for using the composition to treat a patient suffering from a disorder responsive to agonism, inverse agonism or antagonism of the GABAA receptor.
14. The packaged pharmaceutical composition of claim 13 , wherein the patient is suffering from anxiety, depression, a sleep disorder, or cognitive impairment.
15. A compound according to claim 1 wherein the compound exhibits an IC50 of 1 micromolar or less in a standard assay of GABAA receptor binding.
16. A compound according to claim 1 wherein the compound exhibits an IC50 of 100 nanomolar or less in a standard assay of GABAA receptor binding.
17. A compound according to claim 1 wherein the compound exhibits an IC50 of 10 nanomolar or less in a standard assay of GABAA receptor binding.
18. A compound according to claim 1 which has the formula
where R2 and R3 are defined in the following table:
19. A compound according to claim 1 which has the formula
where R2 and R3 are defined in the following table:
20. A compound according to claim 1 which has the formula
where R2 and R3 are defined in the following table:
21. A compound according to claim 1 which has the formula
where R2 and R3 are defined in the following table:
22. A pharmaceutical composition comprising a compound according to claim 1 , together with at least one pharmaceutically acceptable carrier or excipient.
23. A method for the treatment or prevention of physiological disorders associated with modulation of the GABAa receptor complex by selective interaction with the benzodiazepine receptor, the method comprising administering to a patient in need thereof a GABAa receptor complex agonist, antagonist or inverse agonist of a compound according to claim 1 .
24. A method according to claim 23 for the treatment of enhancing alertness and treating anxiety, overdoses of benzodiazepine-type drugs, Down Syndrome, depression, sleep, seizure and cognitive disorders both in human and non-human animals and domestic pets, especially dogs and cats and farm animals such as sheep, swine and cattle.
25. The use of a compound as claimed in claim 1 for the manufacture of a medicament for the treatment of enhancing alertness and treating anxiety, overdoses of benzodiazepine-type drugs, Down Syndrome, depression, sleep, seizure and cognitive disorders both in human and non-human animals and domestic pets, especially dogs and cats and farm animals such as sheep, swine and cattle.
26. A process for the preparation of a compound as claimed in claim 1.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/115,361 US20030092912A1 (en) | 1999-04-02 | 2002-04-03 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa Receptors |
| US10/609,941 US6972293B2 (en) | 1999-04-02 | 2003-06-30 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
| US11/295,173 US7348326B2 (en) | 1999-04-02 | 2005-12-06 | Heteroaryl fused aminoalkyl imidazole derivatives: selective modulators of GABAa receptors |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12752699P | 1999-04-02 | 1999-04-02 | |
| US09/540,454 US6380210B1 (en) | 1999-04-02 | 2000-03-31 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
| US10/115,361 US20030092912A1 (en) | 1999-04-02 | 2002-04-03 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa Receptors |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/540,454 Continuation US6380210B1 (en) | 1999-04-02 | 2000-03-31 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/609,941 Continuation US6972293B2 (en) | 1999-04-02 | 2003-06-30 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030092912A1 true US20030092912A1 (en) | 2003-05-15 |
Family
ID=26825722
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/540,454 Expired - Fee Related US6380210B1 (en) | 1999-04-02 | 2000-03-31 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
| US10/115,361 Abandoned US20030092912A1 (en) | 1999-04-02 | 2002-04-03 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa Receptors |
| US10/609,941 Expired - Fee Related US6972293B2 (en) | 1999-04-02 | 2003-06-30 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/540,454 Expired - Fee Related US6380210B1 (en) | 1999-04-02 | 2000-03-31 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/609,941 Expired - Fee Related US6972293B2 (en) | 1999-04-02 | 2003-06-30 | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
Country Status (1)
| Country | Link |
|---|---|
| US (3) | US6380210B1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007139818A2 (en) | 2006-05-22 | 2007-12-06 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
| US8937087B2 (en) | 2013-04-18 | 2015-01-20 | Astellas Pharma Inc. | Heterocyclic acetamide compound |
| US8946206B2 (en) | 2010-12-17 | 2015-02-03 | The Board Of Trustees Of The Leland Stanford Junior University | Methods for improving cognitive function |
| AU2013204190B2 (en) * | 2006-05-22 | 2016-09-22 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6455734B1 (en) * | 2000-08-09 | 2002-09-24 | Magnesium Diagnostics, Inc. | Antagonists of the magnesium binding defect as therapeutic agents and methods for treatment of abnormal physiological states |
| DE60014339T8 (en) * | 1999-04-02 | 2006-04-27 | Neurogen Corp., Branford | ARYL AND HETEROARYL-CONDENSED AMINOALKYL-IMIDAZOLE DERIVATIVES: SELECTIVE MODULATORS OF GABAA RECEPTORS |
| US6380210B1 (en) * | 1999-04-02 | 2002-04-30 | Neurogen Corporation | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
| WO2002076987A1 (en) | 2001-03-27 | 2002-10-03 | Neurogen Corporation | (oxo-pyrazolo[1,5a]pyrimidin-2-yl)alkyl-carboxamides |
| IL159811A0 (en) | 2001-07-13 | 2004-06-20 | Neurogen Corp | Heteroaryl substituted fused bicyclic heteroaryl compounds as gabaa receptor ligands |
| JP2005526709A (en) | 2002-01-10 | 2005-09-08 | ニューロジェン・コーポレーション | Melanin-concentrating hormone receptor ligand: substituted benzimidazole analogs |
| US7271271B2 (en) * | 2004-06-28 | 2007-09-18 | Amgen Sf, Llc | Imidazolo-related compounds, compositions and methods for their use |
| US20060128777A1 (en) * | 2004-11-05 | 2006-06-15 | Bendall Heather H | Cancer treatments |
| US8436190B2 (en) | 2005-01-14 | 2013-05-07 | Cephalon, Inc. | Bendamustine pharmaceutical compositions |
| DE202005011043U1 (en) * | 2005-07-06 | 2006-11-16 | Mapa Gmbh Gummi- Und Plastikwerke | Sucking and chewing articles for babies or toddlers |
| AR072777A1 (en) | 2008-03-26 | 2010-09-22 | Cephalon Inc | SOLID FORMS OF BENDAMUSTINE CHLORHYDRATE |
| CN104224703A (en) * | 2008-09-25 | 2014-12-24 | 赛福伦公司 | Liquid Formulations Of Bendamustine |
| UA109109C2 (en) * | 2009-01-15 | 2015-07-27 | Сефалон, Інк. | Crystalline forms of bendamustine free base (variants) and pharmaceutical composition for treatment of cancer (variants) |
| WO2020065642A1 (en) * | 2018-09-25 | 2020-04-02 | Pepticom Ltd. | Positive allosteric modulators of gabaa receptor |
Family Cites Families (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3255202A (en) | 1963-08-23 | 1966-06-07 | Union Carbide Corp | Process for the preparation of 2-(acylamidoalkyl)benzimidazoles |
| US3455940A (en) | 1965-12-07 | 1969-07-15 | Herbert C Stecker | Certain halo and dihalo n-substituted salicylamides |
| DE2300018C2 (en) | 1973-01-02 | 1983-01-20 | Knoll Ag, 6700 Ludwigshafen | 1- [N-methyl-N - (β-phenylethyl) -3-aminopropyl] benzimidazole derivatives |
| HU168095B (en) | 1973-05-09 | 1976-02-28 | ||
| DE2428673A1 (en) | 1974-06-14 | 1976-01-02 | Bayer Ag | CARBON ACID AMIDE, THE PROCESS FOR THEIR MANUFACTURING AND THEIR USE AS A MEDICINAL PRODUCT |
| JPH03200961A (en) | 1989-10-03 | 1991-09-02 | Fuji Photo Film Co Ltd | Silver halide color photographic sensitive material |
| JPH04274425A (en) | 1991-03-01 | 1992-09-30 | Fuji Photo Film Co Ltd | Processing method for silver halide color photographic sensitive material |
| US5296493A (en) | 1991-05-23 | 1994-03-22 | Neurosearch A/S | 1-substituted-2-(N-phenyl-N-(phenylmethyl)methanamine)-4,5-dihydro-imidazoles and related compounds and their use in treating calcium overload in brain cells |
| US5877195A (en) | 1992-11-06 | 1999-03-02 | Bayer Aktiengesellschaft | 2-perhalogenalkyl-substituted benzimidazoles, and their use as pesticides |
| AU675484B2 (en) | 1993-03-24 | 1997-02-06 | Neurosearch A/S | Benzimidazole compounds, their use and preparation |
| JPH07133224A (en) | 1993-11-10 | 1995-05-23 | Kyowa Hakko Kogyo Co Ltd | Medicine for arteriosclerosis |
| CZ382496A3 (en) | 1994-06-29 | 1997-12-17 | Smithkline Beecham Corp | Compounds representing antagonists of vitronectin receptors, process of their preparation, intermediates of such process, pharmaceutical composition containing the compounds and their use |
| UA49805C2 (en) | 1994-08-03 | 2002-10-15 | Куміаі Кемікал Індастрі Ко., Лтд | amino acid amide derivative, method of its producing (versions), AGROHORTICULTURAL fungicide and method of fungi DESTRUCtion |
| CA2218552C (en) | 1995-04-21 | 2002-04-16 | Neurosearch A/S | Benzimidazole compounds and their use as modulators of the gabaa receptor complex |
| EE04310B1 (en) | 1995-04-21 | 2004-06-15 | Neurosearch A/S | Benzimidazole compounds, pharmaceutical compositions containing these compounds and their use |
| EP0830359A1 (en) | 1995-06-05 | 1998-03-25 | Neurogen Corporation | Novel substituted aryl and cycloalkyl imidazolones; a new class of gaba brain receptor ligands |
| US5955613A (en) | 1995-10-13 | 1999-09-21 | Neurogen Corporation | Certain pyrrolopyridine derivatives; novel CRF1 specific ligands |
| AU722514B2 (en) | 1995-12-28 | 2000-08-03 | Fujisawa Pharmaceutical Co., Ltd. | Benzimidazole derivatives |
| IL125033A0 (en) | 1995-12-29 | 1999-01-26 | Smithkline Beecham Corp | Vitronectin receptor antagonists |
| NZ334868A (en) | 1996-10-21 | 2001-02-23 | Neurosearch As | 1-Phenyl-benzimidazole compounds and their use as BAGA-a receptor modulators |
| DE19702130C1 (en) | 1997-01-22 | 1998-04-23 | Siemens Ag | Sintered armature design e.g. for contactors |
| GB9801538D0 (en) | 1998-01-23 | 1998-03-25 | Merck Sharp & Dohme | Pharmaceutical product |
| GB9805557D0 (en) | 1998-03-16 | 1998-05-13 | Merck Sharp & Dohme | A combination of therapeutic agents |
| GB9805559D0 (en) | 1998-03-16 | 1998-05-13 | Merck Sharp & Dohme | A combination of therapeutic agents |
| GB9805561D0 (en) | 1998-03-16 | 1998-05-13 | Merck Sharp & Dohme | A combination of therapeutic agents |
| US6380210B1 (en) | 1999-04-02 | 2002-04-30 | Neurogen Corporation | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors |
-
2000
- 2000-03-31 US US09/540,454 patent/US6380210B1/en not_active Expired - Fee Related
-
2002
- 2002-04-03 US US10/115,361 patent/US20030092912A1/en not_active Abandoned
-
2003
- 2003-06-30 US US10/609,941 patent/US6972293B2/en not_active Expired - Fee Related
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007139818A2 (en) | 2006-05-22 | 2007-12-06 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
| US20080009475A1 (en) * | 2006-05-22 | 2008-01-10 | Garner Craig C | Pharmacological treatment of cognitive impairment |
| US8729067B2 (en) | 2006-05-22 | 2014-05-20 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
| EP3050562A1 (en) | 2006-05-22 | 2016-08-03 | The Board of Trustees of the Leland Stanford Junior University | Pharmacological treatment of cognitive impairment using gabaa receptor antagonists |
| AU2013204190B2 (en) * | 2006-05-22 | 2016-09-22 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
| AU2013204190B9 (en) * | 2006-05-22 | 2016-10-06 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
| US10172825B2 (en) | 2006-05-22 | 2019-01-08 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
| US8946206B2 (en) | 2010-12-17 | 2015-02-03 | The Board Of Trustees Of The Leland Stanford Junior University | Methods for improving cognitive function |
| US9789119B2 (en) | 2010-12-17 | 2017-10-17 | The Board Of Trustees Of The Leland Stanford Junior University | Cognitive function |
| US8937087B2 (en) | 2013-04-18 | 2015-01-20 | Astellas Pharma Inc. | Heterocyclic acetamide compound |
| US9708307B2 (en) | 2013-04-18 | 2017-07-18 | Astellas Pharma Inc. | Heterocyclic acetamide compound |
Also Published As
| Publication number | Publication date |
|---|---|
| US20040023993A1 (en) | 2004-02-05 |
| US6972293B2 (en) | 2005-12-06 |
| US6380210B1 (en) | 2002-04-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6380210B1 (en) | Heteroaryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors | |
| EP1165557B1 (en) | ARYL AND HETEROARYL FUSED AMINOALKYL-IMIDAZOLE DERIVATIVES: SELECTIVE MODULATORS OF GABAa RECEPTORS | |
| US6413956B1 (en) | Substituted 4-oxo-quinoline-3-carboxamides | |
| US5130430A (en) | 2-substituted imidazoquinoxaline diones, a new class of gaba brain receptor ligands | |
| US5908932A (en) | Certain pyrroloquinolinones; a new class of GABA brain receptor | |
| US6646124B2 (en) | Substituted 4-oxo-napthyridine-3-carboxamides: GABA brain receptor ligands | |
| US20060247245A1 (en) | Substituted imidazolopyrazine and triazolopyrazine derivatives: gabaa receptor ligands | |
| US6723332B2 (en) | Oxomidazopyridine-carboxamides | |
| US6177569B1 (en) | Oxo-pyridoimidazole-carboxamides: GABA brain receptor ligands | |
| US6194427B1 (en) | Substituted cycloalkyl-4-Oxonicotinic carboxamides; gaba brain receptor ligands | |
| WO2000058313A1 (en) | 4-substituted quinoline derivatives as gaba receptor ligands | |
| JP2001514181A (en) | Substituted 4-oxo-naphthyridine-3-carboxamides as GABA brain receptor ligands | |
| US5637725A (en) | Substituted aryl and cycloalkyl imidazolones; a new class of GABA brain receptor ligands | |
| US6448246B1 (en) | Substituted 4H-1,4-benzothiazine-2-carboxamide: GABA brain receptor ligands | |
| US6310212B1 (en) | 4-substituted quinoline derivatives | |
| US5744602A (en) | Certain imidazoquinoxalines; a new class of GABA brain receptor ligands | |
| US6627624B1 (en) | Aryl fused aminoalkyl-imidazole derivatives: selective modulators of GABAa receptors | |
| US7351826B2 (en) | Aryl acid pyrimidinyl methyl amides, pyridazinyl methyl amides and related compounds | |
| US7030144B2 (en) | Substituted imidazole derivatives: GABAA receptor ligands | |
| US20040077653A1 (en) | Imidazol-1-ylmethyl pyridazine derivatives | |
| US6528649B2 (en) | Imidazoloisoquinolines | |
| US6916827B2 (en) | Substituted ring-fused imidazole derivative: GABAA receptors ligands |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |