KR102414700B1 - A coating method for plastic using silsesquioxane composite polymer - Google Patents
A coating method for plastic using silsesquioxane composite polymer Download PDFInfo
- Publication number
- KR102414700B1 KR102414700B1 KR1020150031865A KR20150031865A KR102414700B1 KR 102414700 B1 KR102414700 B1 KR 102414700B1 KR 1020150031865 A KR1020150031865 A KR 1020150031865A KR 20150031865 A KR20150031865 A KR 20150031865A KR 102414700 B1 KR102414700 B1 KR 102414700B1
- Authority
- KR
- South Korea
- Prior art keywords
- methyl
- group
- phenyl
- pomma
- glyp
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D183/00—Coating compositions based on macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon, with or without sulfur, nitrogen, oxygen, or carbon only; Coating compositions based on derivatives of such polymers
- C09D183/02—Polysilicates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/0427—Coating with only one layer of a composition containing a polymer binder
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D3/00—Pretreatment of surfaces to which liquids or other fluent materials are to be applied; After-treatment of applied coatings, e.g. intermediate treating of an applied coating preparatory to subsequent applications of liquids or other fluent materials
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D183/00—Coating compositions based on macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon, with or without sulfur, nitrogen, oxygen, or carbon only; Coating compositions based on derivatives of such polymers
- C09D183/04—Polysiloxanes
- C09D183/06—Polysiloxanes containing silicon bound to oxygen-containing groups
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/20—Diluents or solvents
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2300/00—Characterised by the use of unspecified polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2483/00—Characterised by the use of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen, or carbon only; Derivatives of such polymers
- C08J2483/04—Polysiloxanes
- C08J2483/06—Polysiloxanes containing silicon bound to oxygen-containing groups
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Silicon Polymers (AREA)
- Paints Or Removers (AREA)
- Laminated Bodies (AREA)
Abstract
본 발명은 실세스퀴옥산 복합 고분자를 이용한 플라스틱코팅방법에 관한 것으로, 보다 상세하게는 하나의 고분자 내에 특정 구조의 선형 실세스퀴옥산 사슬 및 케이지형 실세스퀴옥산을 포함하는 실세스퀴옥산 복합 고분자를 이용하여 플라스틱표면을 코팅함으로써 코팅공정이 용이할 뿐만 아니라 형성된 코팅층이 매우 높은 표면경도를 가지며, 우수한 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 가지며, 플라스틱 기재와의 접착력이 우수하며, 안료와의 높은 상용성으로 착색이 용이한 실세스퀴옥산 복합 고분자를 이용한 플라스틱코팅방법에 관한 것이다.The present invention relates to a plastic coating method using a silsesquioxane composite polymer, and more particularly, a silsesquioxane composite comprising a linear silsesquioxane chain of a specific structure and a cage-type silsesquioxane in one polymer. By coating the plastic surface using a polymer, the coating process is easy, and the formed coating layer has very high surface hardness, and has excellent transparency, scratch resistance, water repellency, antifouling properties, anti-fingerprint properties, thermal stability and gloss properties. It relates to a plastic coating method using a silsesquioxane composite polymer that has excellent adhesion to plastic substrates and is easy to color due to high compatibility with pigments.
Description
본 발명은 실세스퀴옥산 복합 고분자를 이용한 플라스틱코팅방법에 관한 것으로, 보다 상세하게는 하나의 고분자 내에 특정 구조의 선형 실세스퀴옥산 사슬 및 케이지형 실세스퀴옥산을 포함하는 실세스퀴옥산 복합 고분자를 이용하여 플라스틱표면을 코팅함으로써 코팅공정이 용이할 뿐만 아니라 형성된 코팅층이 매우 높은 표면경도를 가지며, 우수한 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 가지며, 플라스틱 기재와의 접착력이 우수하며, 안료와의 높은 상용성으로 착색이 용이한 실세스퀴옥산 복합 고분자를 이용한 플라스틱코팅방법에 관한 것이다.
The present invention relates to a plastic coating method using a silsesquioxane composite polymer, and more particularly, to a silsesquioxane composite comprising a linear silsesquioxane chain of a specific structure and a cage-type silsesquioxane in one polymer. By coating the plastic surface using a polymer, the coating process is easy, and the formed coating layer has very high surface hardness, and has excellent transparency, scratch resistance, water repellency, antifouling properties, anti-fingerprint properties, thermal stability and gloss properties. It relates to a plastic coating method using a silsesquioxane composite polymer that has excellent adhesion to plastic substrates and is easy to color due to high compatibility with pigments.
일반적으로 플라스틱 제품은 높은 가공성과 유연성으로 인하여 많은 제품에 응용되어 사용되고 있다. 그러나 플라스틱 재질은 표면경도, 내구성, 내오염성, 내스크레치성, 광택특성 및 내열성이 떨어져 플라스틱 제품의 용도에 따라 표면에 기능성을 부여하기 다양한 코팅제를 이중 또는 다중으로 코팅하는 방법이 이용되고 있다.In general, plastic products have been applied and used in many products due to their high processability and flexibility. However, the plastic material has poor surface hardness, durability, stain resistance, scratch resistance, gloss properties and heat resistance, so a method of double or multiple coating of various coatings is used to give functionality to the surface depending on the use of the plastic product.
일예로 대한민국특허공개 제10-2006-0121334호에서는 플라스틱 표면에 유기질 피막을 형성하고, 그 외방에 다시 무기질 피막을 형성한 후, 진공챔버내에서 물리적, 모든 플라스틱의 성형 후 그 표면으로 유기질 피막을 형성하고, 그 외방에 다시 무기질 피막을 형성한 후, 진공챔버내에서 물리적, 화학적 증착을 통해 다양한 색상의 금속질감을 연출할 수 있도록 한 것으로, 플라스틱성형품의 표면에 잔존하는 각종 이물질을 제거하기 위해 압축공기나 기타 용제를 이용하여 세척하여 건조하는 세척공정; 상기 세척공정을 통해 표면을 세정한 플라스틱성형품에 표면경화와 무기질과 접착력이 우수한 유기질을 스프레이 또는 침지에 의한 방법으로 도포하여 피막을 형성하는 유기질피막공정; 상기 유기질피막공정을 통해 표면을 경화한 플라스틱성형품은 가스용출억제를 위해 무기질을 스프레이 또는 침지에 의한 방법으로 도포하여 피막을 형성하는 무기질피막공정; 상기 유기/무기질 피막의 복합피막을 교차형성한 플라스틱성형품에 물리적, 화학적 특성부여와 다양한 색상의 금속질감을 구현하기 위해 진공챔버에서 물리적, 화학적 증착을 수행하여 금속피막을 형성하는 진공증착공정으로 진행되는 것을 특징으로 하는 플라스틱성형품의 표면코팅방법이 개시되어 있으나, 공정이 지나치게 복잡하고 형성된 코팅층도 내구성 및 열안정성이 떨어지는 문제점이 있으며, 대한민국특허공개 제10-2011-0014517호에서는 분자 내에 에틸렌성 불포화기를 갖고, 겔 투과 크로마토그래피로 측정한 질량 평균 분자량이 3,000-200,000이며, 그리고 유리 전이 온도가 30% 이상인 우레탄계 고분자와 에틸렌성 불포화기를 가지는 실리카 입자를 이용하여 표면코팅을 하여 플라스틱을 하드 코팅하는 방법이 개시되어 있으나, 표면경도, 내오염성 및 열안정성이 여전히 만족스럽지 못한 수준인 문제점이 있었다.
For example, in Korean Patent Laid-Open No. 10-2006-0121334 No. 10-2006-0121334, an organic film is formed on the surface of a plastic, an inorganic film is again formed on the outside, and an organic film is formed on the surface after physical and all plastics are molded in a vacuum chamber. After forming an inorganic film on the outside, it is possible to produce metal textures of various colors through physical and chemical vapor deposition in a vacuum chamber. Compressed to remove various foreign substances remaining on the surface of plastic molded products a washing process of washing and drying using air or other solvents; an organic coating process of forming a film by spraying or immersing an organic substance having excellent surface hardening and inorganic substances and adhesion to the plastic molded article whose surface has been cleaned through the washing process; An inorganic coating process for forming a film by applying an inorganic substance by spraying or immersing the plastic molded article having a surface hardened through the organic coating process to suppress gas elution; In order to impart physical and chemical properties to the plastic molded article cross-formed with the composite film of the organic/inorganic film and to realize the metal texture of various colors, physical and chemical vapor deposition is performed in a vacuum chamber to form a metal film. Although a method for surface coating a plastic molded article has been disclosed, the process is too complicated and the formed coating layer also has a problem in that durability and thermal stability are poor. A method of hard coating a plastic by surface coating using silica particles having a group, a mass average molecular weight of 3,000-200,000 measured by gel permeation chromatography, and a urethane-based polymer having a glass transition temperature of 30% or more and an ethylenically unsaturated group Although this has been disclosed, there is a problem in that the surface hardness, stain resistance and thermal stability are still at an unsatisfactory level.
상기와 같은 문제점을 해결하기 위해, 본 발명은 플라스틱 표면 위에 코팅용액을 이용하여 코팅함으로써 코팅공정이 용이할 뿐만 아니라 형성된 코팅층이 매우 높은 표면경도를 가지며, 우수한 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 가지며, 플라스틱 기재와의 접착력이 우수하며, 안료와의 높은 상용성으로 착색이 용이한 실세스퀴옥산 복합 고분자를 이용한 플라스틱코팅방법을 제공하는 것을 목적으로 한다.
In order to solve the above problems, in the present invention, the coating process is easy by coating the plastic surface using a coating solution, and the formed coating layer has very high surface hardness, and excellent transparency, scratch resistance, water repellency, and antifouling properties. To provide a plastic coating method using a silsesquioxane composite polymer that has properties, fingerprint resistance, thermal stability and gloss properties, has excellent adhesion to plastic substrates, and is easy to color due to high compatibility with pigments. do.
또한 본 발명은 플라스틱 표면에 높은 표면경도, 우수한 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 부여할 수 있는 플라스틱코팅조성물을 제공하는 것을 목적으로 한다.
Another object of the present invention is to provide a plastic coating composition capable of imparting high surface hardness, excellent transparency, scratch resistance, water repellency, antifouling properties, anti-fingerprint properties, thermal stability and gloss properties to the plastic surface.
또한 본 발명은 표면에 매우 높은 표면경도를 가지며, 우수한 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 가지며, 플라스틱 기재와의 접착력이 우수한 코팅층을 가지는 플라스틱을 제공하는 것을 목적으로 한다.
In addition, the present invention has a very high surface hardness on the surface, has excellent transparency, scratch resistance, water repellency, antifouling properties, anti-fingerprint properties, thermal stability and gloss properties, and provides a plastic having a coating layer having excellent adhesion to a plastic substrate. aim to do
또한 본 발명은 상기 코팅층을 가지는 플라스틱을 포함하는 것을 특징으로 하는 물품을 제공하는 것을 목적으로 한다.
Another object of the present invention is to provide an article comprising a plastic having the coating layer.
상기 목적을 달성하기 위해 본 발명은 플라스틱 표면 위에 하기 화학식 1 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자를 포함하는 플라스틱코팅조성물을 코팅하고 경화하는 것을 특징으로 하는 플라스틱코팅방법을 제공한다:In order to achieve the above object, the present invention provides a plastic coating method comprising coating and curing a plastic coating composition comprising a silsesquioxane composite polymer represented by any one of the following formulas 1 to 9 on a plastic surface:
[화학식 1][Formula 1]
[화학식 2][Formula 2]
[화학식 3][Formula 3]
[화학식 4][Formula 4]
[화학식 5][Formula 5]
[화학식 6][Formula 6]
[화학식 7][Formula 7]
[화학식 8][Formula 8]
[화학식 9][Formula 9]
상기 화학식 1 내지 9에서,In Formulas 1 to 9,
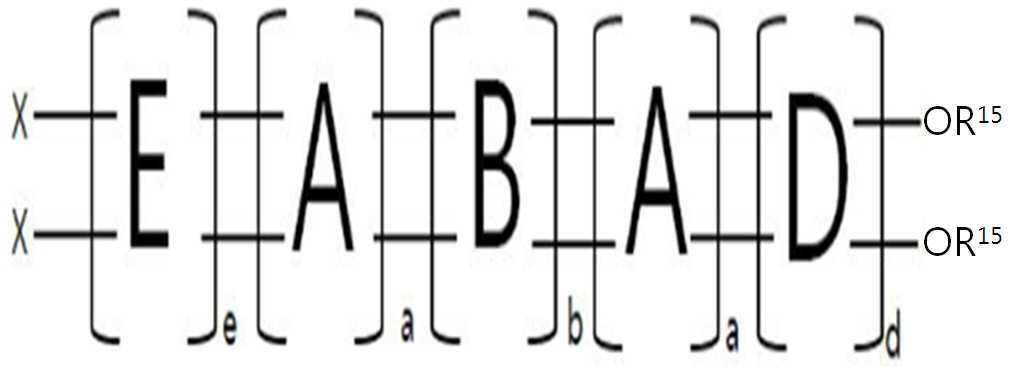
A는 이고, B는 이고, D는 이고, E는 이며,A is and B is and D is and E is is,
Y는 각각 독립적으로 O, NR21 또는 [(SiO3/2R)4+2nO]이며, 적어도 하나는 [(SiO3/2R)4+2nO]이며, Y is each independently O, NR 21 or [(SiO 3/2 R) 4+2n O], at least one is [(SiO 3/2 R) 4+2n O],
X는 각각 독립적으로 R22 또는 [(SiO3/2R)4+2nR]이고, 적어도 하나는 [(SiO3/2R)4+2nR]이고,each X is independently R 22 or [(SiO 3/2 R) 4+2n R], at least one is [(SiO 3/2 R) 4+2n R],
R, R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, R22는 각각 독립적으로 수소; 중수소; 할로겐; 아민기; 에폭시기; 사이클로헥실에폭시기; (메타)아크릴기; 사이올기; 이소시아네이트기; 니트릴기; 니트로기; 페닐기; 중수소, 할로겐, 아민기, 에폭시기, (메타)아크릴기, 사이올기, 이소시아네이트기, 니트릴기, 니트로기, 페닐기로 치환되거나 치환되지 않은 C1~C40의 알킬기, C2~C40의 알케닐기, C1~C40의 알콕시기, C3~C40의 시클로알킬기, C3~C40의 헤테로시클로알킬기, C6~C40의 아릴기, C3~C40의 헤테로아릴기, C3~C40의 아르알킬기, C3~C40의 아릴옥시기, 또는 C3~C40의 아릴사이올기이며, 바람직하기로는 중수소, 할로겐, 아민기, (메타)아크릴기, 사이올기, 이소시아네이트기, 니트릴기, 니트로기, 페닐기, 사이클로헥실 에폭시기로 치환되거나 치환되지 않은 C1~C40의 알킬기, C2~C40의 알케닐기, 아민기, 에폭시기, 사이클로헥실 에폭시기, (메타)아크릴기, 사이올기, 페닐기 또는 이소시아네이트기를 포함하며,R, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , R 22 are each independently hydrogen; heavy hydrogen; halogen; amine group; epoxy group; cyclohexyl epoxy group; (meth)acryl group; thiol group; isocyanate group; nitrile group; nitro group; phenyl group; Deuterium, halogen, amine group, epoxy group, (meth)acrylic group, thiol group, isocyanate group, nitrile group, nitro group, phenyl group or unsubstituted C 1 ~ C 40 alkyl group, C 2 ~ C 40 alkenyl group , C 1 ~ C 40 Alkoxy group, C 3 ~ C 40 Cycloalkyl group, C 3 ~ C 40 Heterocycloalkyl group, C 6 ~ C 40 Aryl group, C 3 ~ C 40 Heteroaryl group, C 3 ~ C 40 aralkyl group, C 3 ~ C 40 aryloxy group, or C 3 ~ C 40 aryl thiol group, preferably deuterium, halogen, amine group, (meth) acryl group, thiol group, isocyanate group , nitrile group, nitro group, phenyl group, cyclohexyl epoxy group or unsubstituted C 1 ~ C 40 alkyl group, C 2 ~ C 40 alkenyl group, amine group, epoxy group, cyclohexyl epoxy group, (meth) acryl group, It contains a thiol group, a phenyl group or an isocyanate group,
a 및 d는 각각 독립적으로 1 내지 100,000의 정수이고, 바람직하기로는 a는 3 내지 1000이고, d는 1 내지 500이며, 더욱 바람직하기로는 a는 5 내지 300이고, d는 2 내지 100이며,a and d are each independently an integer from 1 to 100,000, preferably a is from 3 to 1000, d is from 1 to 500, more preferably a is from 5 to 300, and d is from 2 to 100,
b는 각각 독립적으로 1 내지 500의 정수이며,b is each independently an integer from 1 to 500,
e는 각각 독립적으로 1 또는 2이며, 바람직하기로 1이며,e is each independently 1 or 2, preferably 1,
n은 각각 독립적으로 1 내지 20의 정수이며, 바람직하기로는 3 내지 10이다.
n is each independently an integer of 1 to 20, preferably 3 to 10.
또한 본 발명은 상기 화학식 1 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자를 포함하는 플라스틱코팅조성물을 제공한다.
The present invention also provides a plastic coating composition comprising the silsesquioxane composite polymer represented by any one of Formulas 1 to 9.
또한 본 발명은 표면 위에 상기 화학식 1 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자를 포함하는 플라스틱코팅조성물이 코팅되어 경화된 경화물을 포함하는 것을 특징으로 실세스퀴옥산 복합 고분자 코팅 플라스틱을 제공한다.
In addition, the present invention is a silsesquioxane composite polymer-coated plastic comprising a cured product coated with a plastic coating composition comprising a silsesquioxane composite polymer represented by any one of Formulas 1 to 9 on the surface and cured. to provide.
또한 본 발명은 상기 실세스퀴옥산 복합 고분자 코팅 플라스틱을 포함하는 물품을 제공한다.
The present invention also provides an article comprising the silsesquioxane composite polymer coated plastic.
본 발명에 따른 플라스틱코팅방법은 하나의 고분자 내에 특정 구조의 선형 실세스퀴옥산 사슬 및 케이지형 실세스퀴옥산을 포함하는 실세스퀴옥산 복합 고분자를 이용하여 플라스틱표면을 코팅함으로써 코팅공정이 용이할 뿐만 아니라 형성된 코팅층이 매우 높은 표면경도를 가지며, 우수한 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 가지며, 플라스틱 기재와의 접착력이 우수하여 광학필름, 보호필름, 전자제품 구성용 플라스틱, 안경, 건축외장제, 건축내장제, 플라스틱 배관, 전선피복제, 광학렌즈, 방음벽, 플라스틱 간판, 플라스틱 조형물, 가구, 조명, 썬루프, 헬멧 등의 다양한 제품에 유용하게 적용될 수 있다.
The plastic coating method according to the present invention uses a silsesquioxane composite polymer containing a linear silsesquioxane chain of a specific structure and a cage-type silsesquioxane in one polymer to coat the plastic surface, thereby making the coating process easy. In addition, the formed coating layer has very high surface hardness, has excellent transparency, scratch resistance, water repellency, antifouling properties, anti-fingerprint properties, thermal stability and gloss properties, and has excellent adhesion to plastic substrates, so that optical films, protective films, It can be usefully applied to a variety of products such as plastics for electronic products, glasses, building exteriors, building interiors, plastic piping, wire coverings, optical lenses, soundproof walls, plastic signs, plastic sculptures, furniture, lighting, sunroofs, helmets, etc. have.
이하 본 발명을 상세히 설명한다.
Hereinafter, the present invention will be described in detail.
본 발명의 플라스틱코팅방법은 플라스틱 표면 위에 하기 화학식 1 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자를 포함하는 플라스틱코팅조성물을 코팅하고 경화하는 것을 특징으로 하는 플라스틱코팅방법을 제공한다:The plastic coating method of the present invention provides a plastic coating method comprising coating and curing a plastic coating composition comprising a silsesquioxane composite polymer represented by any one of the following formulas 1 to 9 on a plastic surface:
[화학식 1][Formula 1]
[화학식 2][Formula 2]
[화학식 3][Formula 3]
[화학식 4][Formula 4]
[화학식 5][Formula 5]
[화학식 6][Formula 6]
[화학식 7][Formula 7]
[화학식 8][Formula 8]
[화학식 9][Formula 9]
상기 화학식 1 내지 9에서,In Formulas 1 to 9,
A는 이고, B는 이고, D는 이고, E는 이며,A is and B is and D is and E is is,
Y는 각각 독립적으로 O, NR21 또는 [(SiO3/2R)4+2nO]이며, 적어도 하나는 [(SiO3/2R)4+2nO]이며, Y is each independently O, NR 21 or [(SiO 3/2 R) 4+2n O], at least one is [(SiO 3/2 R) 4+2n O],
X는 각각 독립적으로 R22 또는 [(SiO3/2R)4+2nR]이고, 적어도 하나는 [(SiO3/2R)4+2nR]이고,each X is independently R 22 or [(SiO 3/2 R) 4+2n R], at least one is [(SiO 3/2 R) 4+2n R],
R, R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, R22는 각각 독립적으로 수소; 중수소; 할로겐; 아민기; 에폭시기; 사이클로헥실에폭시기; (메타)아크릴기; 사이올기; 이소시아네이트기; 니트릴기; 니트로기; 페닐기; 중수소, 할로겐, 아민기, 에폭시기, (메타)아크릴기, 사이올기, 이소시아네이트기, 니트릴기, 니트로기, 페닐기로 치환되거나 치환되지 않은 C1~C40의 알킬기, C2~C40의 알케닐기, C1~C40의 알콕시기, C3~C40의 시클로알킬기, C3~C40의 헤테로시클로알킬기, C6~C40의 아릴기, C3~C40의 헤테로아릴기, C3~C40의 아르알킬기, C3~C40의 아릴옥시기, 또는 C3~C40의 아릴사이올기이며, 바람직하기로는 중수소, 할로겐, 아민기, (메타)아크릴기, 사이올기, 이소시아네이트기, 니트릴기, 니트로기, 페닐기, 사이클로헥실 에폭시기로 치환되거나 치환되지 않은 C1~C40의 알킬기, C2~C40의 알케닐기, 아민기, 에폭시기, 사이클로헥실 에폭시기, (메타)아크릴기, 사이올기, 페닐기 또는 이소시아네이트기를 포함하며,R, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , R 22 are each independently hydrogen; heavy hydrogen; halogen; amine group; epoxy group; cyclohexyl epoxy group; (meth)acryl group; thiol group; isocyanate group; nitrile group; nitro group; phenyl group; Deuterium, halogen, amine group, epoxy group, (meth)acrylic group, thiol group, isocyanate group, nitrile group, nitro group, phenyl group or unsubstituted C 1 ~ C 40 alkyl group, C 2 ~ C 40 alkenyl group , C 1 ~ C 40 Alkoxy group, C 3 ~ C 40 Cycloalkyl group, C 3 ~ C 40 Heterocycloalkyl group, C 6 ~ C 40 Aryl group, C 3 ~ C 40 Heteroaryl group, C 3 ~ C 40 aralkyl group, C 3 ~ C 40 aryloxy group, or C 3 ~ C 40 aryl thiol group, preferably deuterium, halogen, amine group, (meth) acryl group, thiol group, isocyanate group , nitrile group, nitro group, phenyl group, cyclohexyl epoxy group or unsubstituted C 1 ~ C 40 alkyl group, C 2 ~ C 40 alkenyl group, amine group, epoxy group, cyclohexyl epoxy group, (meth) acryl group, It contains a thiol group, a phenyl group or an isocyanate group,
a 및 d는 각각 독립적으로 1 내지 100,000의 정수이고, 바람직하기로는 a는 3 내지 1000이고, d는 1 내지 500이며, 더욱 바람직하기로는 a는 5 내지 300이고, d는 2 내지 100이며,a and d are each independently an integer from 1 to 100,000, preferably a is from 3 to 1000, d is from 1 to 500, more preferably a is from 5 to 300, and d is from 2 to 100,
b는 각각 독립적으로 1 내지 500의 정수이며,b is each independently an integer from 1 to 500,
e는 각각 독립적으로 1 또는 2이며, 바람직하기로 1이며,e is each independently 1 or 2, preferably 1,
n은 각각 독립적으로 1 내지 20의 정수이며, 바람직하기로는 3 내지 10이다.
n is each independently an integer of 1 to 20, preferably 3 to 10.
본 발명의 플라스틱코팅방법 및 이에 사용되는 플라스틱코팅조성물은 상기 [A]a와 [D]d의 반복단위를 가지며, 선택적으로 [B]b 또는 [E]e 반복단위를 가지는 특정 구조의 실세스퀴옥산 고분자를 플라스틱의 표면에 코팅하고 경화함으로써, 용액공정을 통한 단일 코팅층의 형성만으로도 플라스틱에 대하여 우수한 표면경도, 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 가지게 할 수 있다.
The plastic coating method of the present invention and the plastic coating composition used therein have a specific structure having repeating units of [A]a and [D]d, and optionally having repeating units [B]b or [E]e By coating and curing the quioxane polymer on the surface of the plastic, excellent surface hardness, transparency, scratch resistance, water repellency, antifouling properties, anti-fingerprint properties, thermal stability and gloss properties for plastics are achieved only by forming a single coating layer through a solution process. can have it
본 발명에 있어서 코팅의 대상이 되는 상기 플라스틱은 플라스틱으로 구성된 것이면 특별히 한정되지 않으며, 일예로 폴리에틸렌(polyethylene, PE), 폴리프로필렌(polypropylene, PP), 폴리스타이렌(polystyrene, PS), 폴리에틸렌 테레프탈레이트(polyethylene terephthalate, PET, 페트), 폴리아미드(polyamides, PA, 나일론), 폴리에스터(polyester, PES), 폴리염화비닐(polyvinyl chloride, PVC), 폴리우레탄(polyurethanes, PU), 폴리카보네이트(polycarbonate, PC), 고경도 폴리카보네이트(고경도 PC), 폴리염화비닐리덴(polyvinylidene chloride, PVDC), 폴리테트라플루오로에틸렌(polytetrafluoroethylene, PTFE), 폴리에테르에테르케톤(polyetheretherketone, PEEK), 폴리에테르이미드(polyetherimide, PEI), 아크릴 등과 같이 단일 플라스틱 재질뿐만 아니라 2종 이상의 플라스틱 재료가 혼합된 것일 수 있으며, 플라스틱과 유리섬유 또는 탄소섬유와 같은 무기섬유가 혼합된 복합플라스틱일 수도 있으며, 형태에 있어서도 용액공정을 사용하기 때문에 시트, 필름, 사출물, 조형물 및 비드를 포함하여 모든 플라스틱 제품에 적용될 수 있다.
The plastic to be coated in the present invention is not particularly limited as long as it is composed of plastic, for example, polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (polyethylene) terephthalate, PET, PET), polyamides (polyamides, PA, nylon), polyester (PES), polyvinyl chloride (PVC), polyurethane (polyurethanes, PU), polycarbonate (PC) , high hardness polycarbonate (high hardness PC), polyvinylidene chloride (PVDC), polytetrafluoroethylene (PTFE), polyetheretherketone (PEEK), polyetherimide (PEI) ), a single plastic material such as acrylic, etc., may be a mixture of two or more plastic materials, and may be a composite plastic in which plastic and inorganic fibers such as glass fibers or carbon fibers are mixed. Therefore, it can be applied to all plastic products including sheets, films, injection moldings, sculptures and beads.
본 발명의 상기 화학식 1로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 1 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합하여 하기 화학식 10을 제조하는 제1단계; 및 상기 제1단계 이후에 화학식 10에 [D]d(OR2)2 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제2단계; 및 상기 2단계 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계를 포함하여 제조될 수 있다.A first step of mixing a basic catalyst and an organic solvent in a reactor, then adding an organic silane compound and condensing to prepare the following formula (10); and a second step of adding an organic silane compound and stirring the reaction solution to acidity by adding an acidic catalyst to the reactor to introduce the [D]d(OR 2 ) 2 structure in Chemical Formula 10 after the first step step; and a third step of converting the reaction solution to basic by adding a basic catalyst to the reactor after the second step to carry out the condensation reaction.
[화학식 10][Formula 10]
상기 식에서 R1, R2, R16, D, a 및 d는 화학식 1 내지 9에서 정의한 바와 같다.
In the above formula, R 1 , R 2 , R 16 , D, a and d are as defined in Formulas 1 to 9.
본 발명의 상기 화학식 2로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 2 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합하여 상기 화학식 10을 제조하는 제1단계; 및 상기 제1단계 이후에 화학식 10에 [D]d(OR3)2 및 [D]d(OR4)2 구조를 화학식 2와 같이 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 과량의 유기 실란 화합물을 첨가하고 교반하는 제2단계; 상기 2단계 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계; 및 제3단계 반응을 거쳐, 단독으로 생성되는 부산물인 cage 구조를 재결정으로 제거하여주는 정제단계를 진행하여 제조될 수 있다.
A first step of mixing a basic catalyst and an organic solvent in a reactor, then adding an organic silane compound and condensing to prepare the above formula (10); And after the first step, in order to introduce [D]d(OR 3 ) 2 and [D]d(OR 4 ) 2 structures in Formula 10 as shown in Formula 2, an acidic catalyst is added to the reactor to acidify the reaction solution. After adjustment, a second step of adding an excess of an organosilane compound and stirring; a third step of performing a condensation reaction by adding a basic catalyst to the reactor after the second step to convert the reaction solution to basic; And through the third step reaction, it can be prepared by proceeding with a purification step in which the cage structure, which is a by-product produced alone, is removed by recrystallization.
본 발명의 상기 화학식 3으로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 3 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합하여 상기 화학식 10을 제조하는 제1단계; 및 상기 제1단계 이후에 화학식 10에 [D]d(OR5)2 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제2단계; 상기 2단계 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계; 및 상기 제3단계 이후에 복합고분자의 말단에 [E]eX2 구조를 도입하여 위하여 반응기에 산성 촉매를 투입하여 반응액을 산성 분위기로 변환하고 유기실란 화합물을 혼합하여 교반하는 제4단계를 포함하여 제조될 수 있다.
A first step of mixing a basic catalyst and an organic solvent in a reactor, then adding an organic silane compound and condensing to prepare the above formula (10); and a second step of adding an organic silane compound and stirring after adjusting the reaction solution to acidity by adding an acid catalyst to the reactor to introduce the [D]d(OR 5 ) 2 structure in Chemical Formula 10 after the first step step; a third step of performing a condensation reaction by adding a basic catalyst to the reactor after the second step to convert the reaction solution to basic; and a fourth step of adding an acidic catalyst to the reactor to introduce the [E]eX 2 structure at the end of the composite polymer after the third step, converting the reaction solution into an acidic atmosphere, mixing an organosilane compound and stirring can be manufactured.
본 발명의 상기 화학식 4로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 4 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합도를 조절하여 상기 화학식 10을 제조하는 제1단계; 및 상기 제1단계 이후에 화학식 10에 [B]b 구조 및 [D]d(OR7)2 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제2단계; 및 상기 2단계 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계를 포함하여 제조될 수 있다.
A first step of preparing Chemical Formula 10 by mixing a basic catalyst and an organic solvent in a reactor, adding an organic silane compound, and controlling the degree of condensation; and after the first step, an acidic catalyst was added to the reactor to introduce the [B]b structure and [D]d(OR 7 ) 2 structure in Chemical Formula 10 to adjust the reaction solution to acidity, and then to prepare an organosilane compound a second step of adding and stirring; and a third step of converting the reaction solution to basic by adding a basic catalyst to the reactor after the second step to carry out the condensation reaction.
본 발명의 상기 화학식 5로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 5 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합하여 상기 화학식 10을 제조하는 제1단계; 및 상기 제1단계 이후에 화학식 10에 [B]b 구조 및 [D]d(OR8)2, [D]d(OR9)2 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 과량의 유기 실란 화합물을 첨가하고 교반하는 제2단계; 및 상기 2단계 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 제3단계; 및 제3단계 이후 재결정과 필터과정을 통하여, 단독 cage 생성 구조를 제거하는 제4단계를 포함하여 제조될 수 있다.
A first step of mixing a basic catalyst and an organic solvent in a reactor, then adding an organic silane compound and condensing to prepare the above formula (10); And after the first step, in order to introduce the [B]b structure and [D]d(OR 8 ) 2 , [D]d(OR 9 ) 2 structure in Chemical Formula 10, an acidic catalyst is added to the reactor to prepare the reaction solution. a second step of adding and stirring an excess of an organosilane compound after adjusting to acidity; and a third step of the condensation reaction by adding a basic catalyst to the reactor after the second step to convert the reaction solution to basic; and a fourth step of removing the single cage-forming structure through recrystallization and filtering after the third step.
본 발명의 상기 화학식 6으로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 6 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합하여 상기 화학식 10을 제조하는 제1단계; 및 상기 제1단계 이후에 화학식 10에 [B]b 구조 및 [D]d(OR10)2 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제2단계; 상기 2단계 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계; 및 상기 제3단계 이후에 복합고분자의 말단에 [E]eX2 구조를 도입하여 위하여 반응기에 산성 촉매를 투입하여 반응액을 산성 분위기로 변환하고 유기실란 화합물을 혼합하여 교반하는 제4단계를 포함하여 제조될 수 있다.
A first step of mixing a basic catalyst and an organic solvent in a reactor, then adding an organic silane compound and condensing to prepare the above formula (10); and after the first step, an acidic catalyst was added to the reactor to introduce the [B]b structure and [D]d(OR 10 ) 2 structure in Chemical Formula 10 to adjust the reaction solution to acidity, and then, an organosilane compound a second step of adding and stirring; a third step of performing a condensation reaction by adding a basic catalyst to the reactor after the second step to convert the reaction solution to basic; and a fourth step of adding an acidic catalyst to the reactor to introduce the [E]eX 2 structure at the end of the composite polymer after the third step, converting the reaction solution into an acidic atmosphere, mixing an organosilane compound and stirring can be manufactured.
바람직하기로 상기 화학식 1 내지 6을 제조하는 방법에서 본 발명의 제1단계의 반응액의 pH는 9 내지 11.5인 것이 바람직하고, 제2단계의 반응액의 pH는 2 내지 4인 것이 바람직하고, 제3단계의 반응액의 pH는 8 내지 11.5인 것이 바람직하고, Ee을 도입하는 제4단계의 반응액의 pH는 1.5 내지 4인 것이 바람직하다. 상기 범위 내인 경우 제조되는 실세스퀴옥산 복합 고분자의 수율이 높을 뿐만 아니라 제조된 실세스퀴옥산 복합 고분자의 기계적 물성을 향상시킬 수 있다.
Preferably, in the method for preparing Formulas 1 to 6, the pH of the reaction solution in the first step of the present invention is preferably 9 to 11.5, and the pH of the reaction solution in the second step is preferably 2 to 4, The pH of the reaction solution in the third step is preferably 8 to 11.5, and the pH of the reaction solution in the fourth step of introducing Ee is preferably 1.5 to 4. When it is within the above range, the yield of the prepared silsesquioxane composite polymer may be high, and mechanical properties of the prepared silsesquioxane composite polymer may be improved.
본 발명의 상기 화학식 7로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 7 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합도가 조절된 두 가지 형태의 상기 화학식 10을 제조하는 1단계; 상기 1단계에서 얻어진 화학식 10에 [B]b 구조 및 [D]d(OR12)2 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제2단계; 상기 각각의 2단계 반응 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계; 및 상기 3단계를 통해 얻어진 2가지 이상의 물질을 염기성 조건에서 축합하여 연결하는 4단계를 포함하여 제조될 수 있다.
A first step of mixing a basic catalyst and an organic solvent in a reactor, adding an organic silane compound, and preparing two types of Chemical Formula 10 with a controlled degree of condensation; In order to introduce the [B]b structure and [D]d(OR 12 ) 2 structure to the formula 10 obtained in step 1, an acidic catalyst was added to the reactor to adjust the reaction solution to be acidic, and then an organosilane compound was added, a second step of stirring; a third step of performing a condensation reaction by adding a basic catalyst to the reactor after each of the two-step reactions to convert the reaction solution to basic; and step 4 of condensing and linking the two or more substances obtained through step 3 under basic conditions.
본 발명의 상기 화학식 8로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 8 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합도가 조절된 두 가지 형태의 상기 화학식 10을 제조하는 1단계; 상기 1단계에서 얻어진 화학식 10에 [B]b 구조, [D]d(OR14)2 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제2단계; 상기 각각의 2단계 반응 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계; 상기 3단계를 통해 얻어진 2가지 이상의 물질을 염기성 조건에서 축합하여 연결하는 4단계; 상기 4단계 이후 [D]d(OR13)2를 도입하기 위한 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제5단계; 및 상기 5단계 반응 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제6단계를 포함하여 제조될 수 있다.
A first step of mixing a basic catalyst and an organic solvent in a reactor, adding an organic silane compound, and preparing two types of Chemical Formula 10 with a controlled degree of condensation; In order to introduce the [B]b structure and [D]d(OR 14 ) 2 structure to Chemical Formula 10 obtained in step 1, an acidic catalyst was added to the reactor to adjust the reaction solution to be acidic, and then an organosilane compound was added, a second step of stirring; a third step of performing a condensation reaction by adding a basic catalyst to the reactor after each of the two-step reactions to convert the reaction solution to basic; a fourth step of condensing and connecting two or more substances obtained through the third step under basic conditions; a fifth step of adding an organic silane compound and stirring after adjusting the reaction solution to acidity by adding an acid catalyst to the reactor for introducing [D]d(OR 13 ) 2 after step 4; and a sixth step of performing a condensation reaction by adding a basic catalyst to the reactor after the five-step reaction to convert the reaction solution to basic.
본 발명의 상기 화학식 9로 표시되는 실세스퀴옥산 복합 고분자는 The silsesquioxane composite polymer represented by Formula 9 of the present invention is
반응기에 염기성 촉매와 유기용매를 혼합한 후 유기 실란 화합물을 첨가하고 축합도가 조절된 두 가지 형태의 상기 화학식 10을 제조하는 1단계; 상기 1단계에서 얻어진 화학식 10에 [B]b 구조를 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제2단계; 상기 각각의 2단계반응 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제3단계; 상기 3단계를 통해 얻어진 2가지 이상의 화합물을 염기성 조건에서 축합하여 연결하는 4단계; 상기 제4단계 이후 [D]d(OR5)2를 도입하기 위한 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 제5단계; 상기 5단계 반응 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 제6단계; 상기 제6단계 이후에 복합고분자의 말단에 [E]eX2 구조를 도입하여 위하여 반응기에 산성 촉매를 투입하여 반응액을 산성 분위기로 변환하고 유기실란 화합물을 혼합하여 교반하는 제7단계를 포함하여 제조될 수 있다.
A first step of mixing a basic catalyst and an organic solvent in a reactor, adding an organic silane compound, and preparing two types of Chemical Formula 10 with a controlled degree of condensation; a second step of adding an organic silane compound and stirring after adjusting the reaction solution to acidity by adding an acid catalyst to the reactor to introduce the [B]b structure to Chemical Formula 10 obtained in step 1; A third step of performing a condensation reaction by adding a basic catalyst to the reactor after each of the two-step reactions to convert the reaction solution to basic; a fourth step of condensing and linking two or more compounds obtained through the third step under basic conditions; After the fourth step [D]d(OR 5 ) 2 A fifth step of adding an organic silane compound and stirring after adjusting the reaction solution to be acidic by adding an acidic catalyst to the reactor for introducing; a sixth step of performing a condensation reaction by adding a basic catalyst to the reactor after the five-step reaction to convert the reaction solution to basic; After the sixth step, an acidic catalyst is introduced into the reactor to introduce the [E]eX 2 structure at the end of the composite polymer, the reaction solution is converted into an acidic atmosphere, and a seventh step of mixing and stirring an organosilane compound. can be manufactured.
바람직하기로 상기 화학식 7 내지 9의 고분자를 제조하는 방법에서 제1단계의 반응액의 pH는 9 내지 11.5인 것이 바람직하고, 제2단계의 반응액의 pH는 2 내지 4인 것이 바람직하고, 제3단계의 반응액의 pH는 8 내지 11.5인 것이 바람직하고, 제4단계의 반응액의 pH는 9 내지 11.5인 것이 바람직하고, 제5단계의 반응액의 pH는 2 내지 4인 것이 바람직하고, 제6단계의 반응액의 8 내지 11.5인 것이 바람직하고, Ee를 도입하는 제7단계의 반응액의 pH는 1.5 내지 4인 것이 바람직하다. 상기 범위 내인 경우 제조되는 실세스퀴옥산 복합 고분자의 수율이 높을 뿐만 아니라 제조된 실세스퀴옥산 복합 고분자의 기계적 물성을 향상시킬 수 있다.
Preferably, in the method for preparing the polymer of Chemical Formulas 7 to 9, the pH of the reaction solution in the first step is preferably 9 to 11.5, and the pH of the reaction solution in the second step is preferably 2 to 4, The pH of the reaction solution in step 3 is preferably 8 to 11.5, the pH of the reaction solution in step 4 is preferably 9 to 11.5, and the pH of the reaction solution in step 5 is preferably 2 to 4, It is preferable that the pH of the reaction solution of the sixth step is 8 to 11.5, and the pH of the reaction solution of the seventh step of introducing Ee is preferably 1.5 to 4. When it is within the above range, the yield of the prepared silsesquioxane composite polymer may be high, and mechanical properties of the prepared silsesquioxane composite polymer may be improved.
또한 필요한 경우 각각의 복합 고분자에 [B]b 구조 및 [D]d(OR)2 구조를 더욱 도입하기 위하여 반응기에 산성 촉매를 첨가하여 반응액을 산성으로 조절한 후, 유기 실란 화합물을 첨가하고 교반하는 단계; 및 상기 단계 이후에 반응기에 염기성 촉매를 첨가하여 반응액을 염기성으로 변환하여 축합반응을 실시하는 단계를 통하여 복합 고분자 내에 [B]b 반복단위를 더욱 포함할 수 있다.
In addition, if necessary, in order to further introduce the [B]b structure and [D]d(OR) 2 structure to each composite polymer, an acidic catalyst is added to the reactor to adjust the reaction solution to acidity, then an organosilane compound is added, stirring; and a [B]b repeating unit may be further included in the composite polymer through the step of adding a basic catalyst to the reactor after the step to convert the reaction solution to basic and performing a condensation reaction.
또한 필요한 경우 각각의 복합 고분자의 말단에 [E]eX2 구조를 도입하기 위하여 반응기에 산성 촉매를 투입하여 반응액을 산성 분위기로 변환하고 유기실란 화합물을 혼합하여 교반하는 단계를 포함하여 복합 고분자의 말단에 [E]e의 반복단위를 더욱 포함할 수 있다.
In addition, if necessary, in order to introduce the [E]eX 2 structure at the end of each composite polymer, an acidic catalyst is put into the reactor to convert the reaction solution into an acidic atmosphere, and an organosilane compound is mixed and stirred. It may further include a repeating unit of [E]e at the end.
상기 실세스퀴옥산 복합 고분자의 제조방법에서는 염기성 촉매로서 바람직하기로는 2종 이상의 염기성 촉매의 혼합촉매를 사용하고, 이를 산성 촉매로 중화 및 산성화하여 재 가수분해를 유도하며, 다시 2종 이상의 염기성 촉매의 혼합촉매를 이용하여 염기성으로 축합을 진행함으로써 하나의 반응기내에서 산도와 염기도를 연속적으로 조절할 수 있다.
In the method for producing the silsesquioxane composite polymer, a mixed catalyst of two or more basic catalysts is preferably used as a basic catalyst, and this is neutralized and acidified with an acidic catalyst to induce re-hydrolysis, and again two or more basic catalysts are used. Acidity and basicity can be continuously controlled in one reactor by carrying out the basic condensation using a mixed catalyst of
이때, 상기 염기성 촉매는 Li, Na, K, Ca 및 Ba 으로 이루어진 군에서 선택된 금속계 염기성 촉매 및 아민계 염기성 촉매에서 선택되는 2종 이상의 물질을 적절히 조합하여 제조될 수 있다. 바람직하게는 상기 아민계 염기성 촉매가 테트라메틸암모늄 하이드록시드(TMAH)이고, 금속계 염기성 촉매가 포타슘 하이드록시드(KOH) 또는 중탄산나트륨 (NaHCO3)일 수 있다. 상기 혼합촉매에서 각 성분의 함량은 바람직하기로는 아민계 염기성 촉매와 금속계 염기성 촉매의 비율이 10 내지 90: 10 내지 90 중량부의 비율에서 임의로 조절할 수 있다. 상기 범위 내인 경우 가수분해시 관능기와 촉매와의 반응성을 최소화시킬 수 있으며, 이로 인해 Si-OH 또는 Si-알콕시 등의 유기 관능기의 결함이 현저히 감소하여 축합도를 자유로이 조절할 수 있는 장점이 있다. 또한, 상기 산성 촉매로는 당분야에서 통상적으로 사용하는 산성 물질이라면 제한 없이 사용될 수 있으며, 예를 들어, HCl, H2SO4, HNO3, CH3COOH 등의 일반 산성물질을 사용할 수 있고, 또한 latic acid, tartaric acid, maleic acid, citric acid 등의 유기계 산성물질도 적용할 수 있다.
In this case, the basic catalyst may be prepared by appropriately combining two or more materials selected from a metal-based basic catalyst and an amine-based basic catalyst selected from the group consisting of Li, Na, K, Ca and Ba. Preferably, the amine-based basic catalyst is tetramethylammonium hydroxide (TMAH), and the metal-based basic catalyst is potassium hydroxide (KOH) or sodium bicarbonate (NaHCO 3 ). The content of each component in the mixed catalyst may be arbitrarily adjusted in a ratio of preferably 10 to 90: 10 to 90 parts by weight of the amine-based basic catalyst and the metal-based basic catalyst. If it is within the above range, it is possible to minimize the reactivity of the functional group with the catalyst during hydrolysis, and this has the advantage that defects of organic functional groups such as Si-OH or Si-alkoxy are significantly reduced, so that the degree of condensation can be freely controlled. In addition, the acid catalyst may be used without limitation as long as it is an acidic material commonly used in the art, for example, a general acidic material such as HCl, H 2 SO 4 , HNO 3 , CH 3 COOH may be used, In addition, organic acidic substances such as latic acid, tartaric acid, maleic acid, and citric acid can be applied.
본 발명의 실세스퀴옥산 복합 고분자의 제조방법에서 상기 유기용매는 당분야에서 통상적으로 사용하는 유기용매라면 제한 없이 사용될 수 있으며, 예를 들어, 메틸알콜, 에틸알콜, 이소프로필알콜, 부틸알콜, 셀로솔브계 등의 알코올류, 락테이트계, 아세톤, 메틸(아이소부틸)에틸케톤 등의 케톤류, 에틸렌글리콜 등의 글리콜류, 테트라하이드로퓨란 등의 퓨란계, 디메틸포름아미드, 디메틸아세트아미드, N-메틸-2-피롤리돈 등의 극성용매 뿐 아니라, 헥산, 사이클로헥산, 사이클로헥사논, 톨루엔, 자일렌, 크레졸, 클로로포름, 디클로로벤젠, 디메틸벤젠, 트리메틸벤젠, 피리딘, 메틸나프탈렌, 니트로메탄, 아크로니트릴, 메틸렌클로라이드, 옥타데실아민, 아닐린, 디메틸설폭사이드, 벤질알콜 등 다양한 용매를 사용할 수 있다.
In the method for producing the silsesquioxane composite polymer of the present invention, the organic solvent may be used without limitation as long as it is an organic solvent commonly used in the art, for example, methyl alcohol, ethyl alcohol, isopropyl alcohol, butyl alcohol, Alcohols such as cellosolve, ketones such as lactate, acetone, and methyl (isobutyl) ethyl ketone, glycols such as ethylene glycol, furan such as tetrahydrofuran, dimethylformamide, dimethylacetamide, N- In addition to polar solvents such as methyl-2-pyrrolidone, hexane, cyclohexane, cyclohexanone, toluene, xylene, cresol, chloroform, dichlorobenzene, dimethylbenzene, trimethylbenzene, pyridine, methylnaphthalene, nitromethane, acro Various solvents such as nitrile, methylene chloride, octadecylamine, aniline, dimethyl sulfoxide, and benzyl alcohol can be used.
또한, 상기 유기 실란계 화합물로는 본 발명의 실세스퀴옥산 복합 고분자인 화학식 1 내지 9의 R, R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, R22를 포함하는 유기 실란이 사용될 수 있으며, 바람직하기로 실세스퀴옥산 복합 고분자의 내화학성을 증가시켜 비팽윤성을 향상시키는 효과가 있는 페닐기 또는 아미노기를 포함하는 유기 실란 화합물, 또는 복합 고분자의 경화 밀도를 증가시켜 경화층의 기계적 강도 및 경도를 향상시키는 효과가 있는 에폭시기 또는 (메타)아크릴기를 포함하는 유기 실란 화합물을 사용할 수 있다.
In addition, as the organosilane compound, R, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , which is a silsesquioxane composite polymer of the present invention, of Formulas 1 to 9, Organosilanes comprising R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , R 22 may be used and , preferably by increasing the chemical resistance of the silsesquioxane composite polymer to improve the non-swelling property of an organosilane compound containing a phenyl group or an amino group, or by increasing the curing density of the composite polymer to increase the mechanical strength and hardness of the cured layer An organic silane compound containing an epoxy group or (meth)acryl group having the effect of improving the silane may be used.
상기 유기 실란계 화합물의 구체적인 예로는 (3-글리시드옥시프로필)트리메톡시실란, (3-글리시드옥시프로필)트리에톡시실란, (3-글리시드옥시프로필)메틸디메톡시실란, (3-글리시드옥시프로필)디메틸에톡시실란, 3-(메타아크릴옥시)프로필트리메톡시실란, 3,4-에폭시부틸트리메톡시실란, 3,4-에폭시부틸트리에톡시실란, 2-(3,4-에폭시시클로헥실)에틸트리메톡시실란, 2-(3,4-에폭시시클로헥실)에틸트리에톡시실란, 아미노프로필트리에톡시실란, 비닐트리에톡시실란, 비닐트리-t-부톡시실란, 비닐트리이소부톡시실란, 비닐트리이소프로폭시실란, 비닐트리페녹시실란, 페닐트리에톡시실란, 페닐트리메톡시실란, 아미노프로필트리메톡시실란, N-페닐-3-아미노프로필트리메톡시실란, 디메틸테트라메톡시실록산, 디페닐테트라메톡시실록산 등을 들 수 있으며, 이들 중 1종 단독으로 또는 2종 이상을 병용하여 사용할 수도 있다. 최종 제조되는 조성물의 물성을 위하여 2종 이상을 혼합하여 사용하는 것이 보다 바람직하다.
Specific examples of the organosilane compound include (3-glycidoxypropyl)trimethoxysilane, (3-glycidoxypropyl)triethoxysilane, (3-glycidoxypropyl)methyldimethoxysilane, (3 -glycidoxypropyl) dimethylethoxysilane, 3-(methacryloxy)propyltrimethoxysilane, 3,4-epoxybutyltrimethoxysilane, 3,4-epoxybutyltriethoxysilane, 2-(3 ,4-epoxycyclohexyl)ethyltrimethoxysilane, 2-(3,4-epoxycyclohexyl)ethyltriethoxysilane, aminopropyltriethoxysilane, vinyltriethoxysilane, vinyltri-t-butoxy Silane, vinyltriisobutoxysilane, vinyltriisopropoxysilane, vinyltriphenoxysilane, phenyltriethoxysilane, phenyltrimethoxysilane, aminopropyltrimethoxysilane, N-phenyl-3-aminopropyltrime oxysilane, dimethyltetramethoxysiloxane, diphenyltetramethoxysiloxane, etc. are mentioned, Among these, it can also be used individually by 1 type or in combination of 2 or more types. It is more preferable to use a mixture of two or more for the physical properties of the final composition.
본 발명에서 상기 화학식들의 반복단위 [D]d에 도입된[(SiO3/2R)4+2nO] 구조의 n은 1 내지 20의 정수로 치환될 수 있으며, 바람직하기로는 3 내지 10이며, 더욱 바람직하기로는 평균 n 값이 4 내지 5이며, 예를 들어, 상기 n이 4일 때 치환된 구조를 표현하면 하기 화학식 11과 같다:In the present invention, n of the [(SiO 3/2 R) 4+2n O] structure introduced into the repeating unit [D]d of the above formulas may be substituted with an integer of 1 to 20, preferably 3 to 10, , more preferably, the average n value is 4 to 5, for example, when n is 4, when expressing a substituted structure, it is as follows:
[화학식 11][Formula 11]
상기 식에서, R은 상기에서 정의한 바와 같다.
In the above formula, R is as defined above.
본 발명에서, 상기 화학식들의 반복단위 [B]b 또는 [E]e에 도입된[(SiO3/2R)4+2nR] 구조의 n은 1 내지 20의 정수로 치환될 수 있으며, 바람직하기로는 3 내지 10이며, 더욱 바람직하기로는 평균 n 값이 4 내지 5이며, 예를 들어, 상기 n이 4일 때 치환된 구조를 표현하면 하기 화학식 12와 같다: In the present invention, n of the [(SiO 3/2 R) 4+2n R] structure introduced into the repeating unit [B]b or [E]e of the above formulas may be substituted with an integer of 1 to 20, preferably It is 3 to 10, and more preferably, the average n value is 4 to 5. For example, when n is 4, a substituted structure is expressed as shown in Formula 12:
[화학식 12][Formula 12]
상기 식에서, R은 상기에서 정의한 바와 같다.
In the above formula, R is as defined above.
구체적인 예로 본 발명에 따른 실세스퀴옥산 고분자는 하기 표 1 내지 18에 고분자일 수 있다. 하기 표 1 내지 9에서 ECHE는 (Epoxycyclohexyl)ethyl, GlyP는 Glycidoxypropyl, POMMA는 (methacryloyloxy)propyl을 의미하며, 두 개 이상이 기재된 경우 혼합사용을 의미한다. n은 각각 독립적으로 1 내지 8이다.
As a specific example, the silsesquioxane polymer according to the present invention may be a polymer shown in Tables 1 to 18 below. In Tables 1 to 9, ECHE means (Epoxycyclohexyl)ethyl, GlyP means Glycidoxypropyl, and POMMA means (methacryloyloxy)propyl, and when two or more are described, it means mixed use. n is each independently 1 to 8;
상기 화학식 1의 실세스퀴옥산 복합고분자는 하기 표 1 또는 2에 기재된 고분자일 수 있다.The silsesquioxane composite polymer of Formula 1 may be a polymer shown in Table 1 or 2 below.
구체적인 예로 상기 화학식 2의 실세스퀴옥산 복합고분자는 하기 표 3 및 4에 기재된 고분자일 수 있다.As a specific example, the silsesquioxane composite polymer of Formula 2 may be the polymers shown in Tables 3 and 4 below.
구체적인 예로 상기 화학식 3의 실세스퀴옥산 복합고분자는 하기 표 5 또는 6에 기재된 고분자일 수 있다.As a specific example, the silsesquioxane composite polymer of Formula 3 may be a polymer shown in Tables 5 or 6 below.
구체적인 예로 상기 화학식 4의 실세스퀴옥산 복합고분자는 하기 표 7 및 8에 기재된 고분자일 수 있다.As a specific example, the silsesquioxane composite polymer of Formula 4 may be the polymers shown in Tables 7 and 8 below.
구체적인 예로 상기 화학식 5의 실세스퀴옥산 복합고분자는 하기 표 9 및 10에 기재된 고분자일 수 있다.As a specific example, the silsesquioxane composite polymer of Formula 5 may be the polymers shown in Tables 9 and 10 below.
구체적인 예로 상기 화학식 6의 실세스퀴옥산 복합고분자는 하기 표 11 및 12에 기재된 고분자일 수 있다.As a specific example, the silsesquioxane composite polymer of Formula 6 may be the polymers shown in Tables 11 and 12 below.
구체적인 예로 상기 화학식 7의 실세스퀴옥산 복합고분자는 하기 표 13 및 14에 기재된 고분자일 수 있다. As a specific example, the silsesquioxane composite polymer of Formula 7 may be the polymers shown in Tables 13 and 14 below.
구체적인 예로 상기 화학식 8의 실세스퀴옥산 복합고분자는 하기 표 15 및 16에 기재된 고분자일 수 있다.As a specific example, the silsesquioxane composite polymer of Formula 8 may be the polymers shown in Tables 15 and 16 below.
구체적인 예로 상기 화학식 9의 실세스퀴옥산 복합고분자는 하기 표 17 및 18에 기재된 고분자일 수 있다.As a specific example, the silsesquioxane composite polymer of Formula 9 may be the polymers shown in Tables 17 and 18 below.
말단 RE's
terminal R
본 발명의 상기 실세스퀴옥산 복합 고분자는 우수한 보관 안정성을 확보하여 폭넓은 응용성을 얻기 위해, 축합도가 1 내지 99.9% 이상으로 조절될 수 있다. 즉, 말단 및 중앙의 Si에 결합된 알콕시 그룹의 함량이 전체 고분자의 결합기에 대해 50%에서 0.01%까지 조절될 수 있다.
The degree of condensation of the silsesquioxane composite polymer of the present invention may be adjusted to 1 to 99.9% or more in order to obtain wide applicability by securing excellent storage stability. That is, the content of the alkoxy group bonded to Si at the ends and the center can be controlled from 50% to 0.01% of the total polymer bonding group.
또한 본 발명에 실세스퀴옥산 복합 고분자의 중량평균분자량은 1,000 내지 1,000,000, 바람직하게는 5,000 내지 100,000이며, 더욱 바람직하게는 7,000 내지 50,000일 수 있다. 이 경우 실세스퀴옥산의 가공성 및 물리적 특성을 동시에 향상시킬 수 있다.
In addition, the weight average molecular weight of the silsesquioxane composite polymer in the present invention may be 1,000 to 1,000,000, preferably 5,000 to 100,000, and more preferably 7,000 to 50,000. In this case, the processability and physical properties of silsesquioxane can be improved at the same time.
본 발명에서 상기 화학식 1 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자를 포함하는 플라스틱코팅조성물은 2종 이상의 복합 고분자를 사용하는 것도 가능하며, 바람직하기로는 화학식 3 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자를 사용하는 것이 좋다. 이 경우 반복단위 [B]b 또는 [E]e를 포함함으로써 표면경도를 포함한 투명기판의 물성을 더욱 향상시킬 수 있다.In the present invention, the plastic coating composition comprising the silsesquioxane composite polymer represented by any one of Formulas 1 to 9 may use two or more composite polymers, preferably represented by any one of Formulas 3 to 9 It is preferable to use a silsesquioxane composite polymer. In this case, physical properties of the transparent substrate including surface hardness can be further improved by including the repeating unit [B]b or [E]e.
본 발명에서 상기 플라스틱코팅조성물은 실세스퀴옥산 복합 고분자가 액상인 경우 무용제 타입으로 단독으로 코팅이 가능하며, 고상인 경우 유기용매를 포함하여 구성될 수 있다. 또한 코팅 조성물은 개시제 또는 경화제를 더욱 포함할 수 있다.
In the present invention, when the silsesquioxane composite polymer is in the liquid phase, the plastic coating composition can be coated alone as a solvent-free type, and in the solid phase, the plastic coating composition may include an organic solvent. In addition, the coating composition may further include an initiator or a curing agent.
바람직하기로 상기 코팅조성물은 상기 화학식 1 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자, 상기 복합 고분자와 상용성이 있는 당분야에서 통상적으로 사용하는 유기용매, 개시제를 포함하는 것을 특징으로 하며, 선택적으로 경화제, 가소제, 자외선 차단제, 기타 기능성 첨가제 등의 첨가제를 추가로 포함하여 경화성, 내열특성, 자외선차단, 가소 효과 등을 향상시킬 수 있다.
Preferably, the coating composition comprises a silsesquioxane composite polymer represented by any one of Formulas 1 to 9, an organic solvent commonly used in the art that is compatible with the composite polymer, and an initiator, , optionally by further including additives such as curing agents, plasticizers, sunscreens, and other functional additives to improve curability, heat resistance, UV protection, and plasticizing effect.
본 발명의 코팅 조성물에 있어서 상기 실세스퀴옥산 복합 고분자는 코팅 조성물 100 중량부에 대하여 적어도 5 중량부 이상으로 포함되는 것이 좋으며, 바람직하게는 5 내지 90 중량부, 더욱 바람직하게는 10 내지 50 중량부의 양으로 포함되는 것이 바람직하다. 상기 범위 내인 경우 코팅 조성물의 경화막의 기계적 물성을 더욱 향상시킬 수 있다.
In the coating composition of the present invention, the silsesquioxane composite polymer is preferably included in an amount of at least 5 parts by weight or more, preferably 5 to 90 parts by weight, more preferably 10 to 50 parts by weight based on 100 parts by weight of the coating composition. It is preferably included in a negative amount. When within the above range, the mechanical properties of the cured film of the coating composition may be further improved.
상기 유기용매로는 메틸알콜, 에틸알콜, 이소프로필알콜, 부틸알콜, 셀로솔브계 등의 알코올류, 락테이트계, 아세톤, 메틸(아이소부틸)에틸케톤 등의 케톤류, 에틸렌글리콜 등의 글리콜 류, 테트라하이드로퓨란 등의 퓨란계, 디메틸포름아미드, 디메틸아세트아미드, N-메틸-2-피롤리돈 등의 극성용매 뿐 아니라, 헥산, 사이클로헥산, 사이클로헥사논, 톨루엔, 자일렌, 크레졸, 클로로포름, 디클로로벤젠, 디메틸벤젠, 트리메틸벤젠, 피리딘, 메틸나프탈렌, 니트로메탄, 아크로니트릴, 메틸렌클로라이드, 옥타데실아민, 아닐린, 디메틸설폭사이드, 벤질알콜 등 다양한 용매를 이용할 수 있으나, 이에 제한되지는 않는다. 상기 유기용매의 양은 복합고분자, 개시제, 및 선택적으로 추가되는 첨가제를 제외한 잔량으로 포함된다.
Examples of the organic solvent include alcohols such as methyl alcohol, ethyl alcohol, isopropyl alcohol, butyl alcohol, and cellosolve; ketones such as lactate, acetone, and methyl (isobutyl) ethyl ketone; glycols such as ethylene glycol; Furan-based solvents such as tetrahydrofuran, polar solvents such as dimethylformamide, dimethylacetamide, and N-methyl-2-pyrrolidone, as well as hexane, cyclohexane, cyclohexanone, toluene, xylene, cresol, chloroform, Various solvents such as dichlorobenzene, dimethylbenzene, trimethylbenzene, pyridine, methylnaphthalene, nitromethane, acrynitrile, methylene chloride, octadecylamine, aniline, dimethyl sulfoxide, and benzyl alcohol may be used, but are not limited thereto. The amount of the organic solvent is included in the remaining amount excluding the complex polymer, the initiator, and optionally added additives.
또한 본 발명의 코팅 조성물에 있어서 상기 개시제 또는 경화제는 실세스퀴옥산 복합 고분자에 포함된 유기관능기에 따라 적절히 선택하여 사용할 수 있다.In addition, in the coating composition of the present invention, the initiator or curing agent may be appropriately selected and used according to the organic functional group contained in the silsesquioxane composite polymer.
구체적인 예로서 상기 유기관능기에 불포화 탄화수소, 사이올계, 에폭시계, 아민계, 이소시아네이트계 등의 후경화가 가능한 유기계가 도입될 경우, 열 또는 광을 이용한 다양한 경화가 가능하다. 이때 열 또는 광에 의한 변화를 고분자 자체 내에서 도모할 수 있지만, 바람직하게는 상기와 같은 유기용매에 희석함으로써 경화공정을 도모할 수 있다.
As a specific example, when an organic type capable of post-curing, such as an unsaturated hydrocarbon, a thiol type, an epoxy type, an amine type, an isocyanate type, etc. is introduced into the organic functional group, various curing using heat or light is possible. At this time, the change by heat or light can be achieved within the polymer itself, but preferably, the curing process can be achieved by diluting it in the organic solvent as described above.
또한 본 발명에서는 복합 고분자의 경화 및 후 반응을 위하여, 다양한 개시제를 사용할 수 있으며, 상기 개시제는 조성물 총중량 100 중량부에 대하여 0.1-20 중량부로 포함되는 것이 바람직하며, 상기 범위 내의 함량으로 포함될 때, 경화 후 투과도 및 코팅안정성을 동시에 만족시킬 수 있다.
In addition, in the present invention, various initiators can be used for curing and post-reaction of the composite polymer, and the initiator is preferably included in an amount of 0.1-20 parts by weight based on 100 parts by weight of the total weight of the composition, and when included in an amount within the above range, After curing, transmittance and coating stability can be satisfied at the same time.
또한 상기 유기관능기에 불포화 탄화수소 등이 도입될 경우에는 라디칼 개시제를 사용할 수 있으며, 상기 라디칼 개시제로는 트리클로로 아세토페논(trichloro acetophenone), 디에톡시 아세토페논(diethoxy acetophenone), 1-페닐-2-히드록시-2-메틸프로판-1-온(1-phenyl-2-hydroxyl-2-methylpropane-1-one), 1-히드록시사이클로헥실페닐케톤, 2-메틸-1-(4-메틸 티오페닐)-2-모르폴리노프로판-1-온(2-methyl-1-(4-methyl thiophenyl)-2-morpholinopropane-1-one), 2,4,6-트리메틸 벤조일 디페닐포스핀 옥사이드(trimethyl benzoyl diphenylphosphine oxide), 캠퍼 퀴논(camphor quinine), 2,2'-아조비스(2-메틸부티로니트릴), 디메틸-2,2'-아조비스(2-메틸 부틸레이트), 3,3-디메틸-4-메톡시-벤조페논, p-메톡시벤조페논, 2,2-디에톡시 아세토페논, 2,2-디메톡시-1,2-디페닐 에탄-1-온 등의 광 래디컬 개시제, t-부틸파옥시 말레인산, t-부틸하이드로퍼옥사이드, 2,4-디클로로벤조일퍼옥사이드, 1,1-디(t-부틸퍼옥시)-3,3,5-트리메틸시클로헥산, N-부틸-4,4'-디(t-부틸퍼옥시)발레레이트 등의 열 라디칼 개시제 및 이들의 다양한 혼합물 등이 사용될 수 있다.
In addition, when an unsaturated hydrocarbon is introduced into the organic functional group, a radical initiator may be used, and the radical initiator includes trichloro acetophenone, diethoxy acetophenone, 1-phenyl-2-hydro Roxy-2-methylpropane-1-one (1-phenyl-2-hydroxyl-2-methylpropane-1-one), 1-hydroxycyclohexylphenyl ketone, 2-methyl-1- (4-methyl thiophenyl) -2-morpholinopropane-1-one (2-methyl-1- (4-methyl thiophenyl)-2-morpholinopropane-1-one), 2,4,6-trimethyl benzoyl diphenylphosphine oxide (trimethyl benzoyl diphenylphosphine oxide), camphor quinone, 2,2'-azobis (2-methylbutyronitrile), dimethyl-2,2'-azobis (2-methyl butyrate), 3,3-dimethyl- Photoradical initiators such as 4-methoxy-benzophenone, p-methoxybenzophenone, 2,2-diethoxy acetophenone, and 2,2-dimethoxy-1,2-diphenyl ethan-1-one, t- Butylparoxy maleic acid, t-butyl hydroperoxide, 2,4-dichlorobenzoyl peroxide, 1,1-di(t-butylperoxy)-3,3,5-trimethylcyclohexane, N-butyl-4, Thermal radical initiators, such as 4'-di(t-butylperoxy)valerate, and various mixtures thereof, etc. can be used.
또한, 상기 유기관능기에 에폭시 등이 포함되는 경우에는, 광중합 개시제(양이온)로서 트리페닐술포늄, 디페닐-4-(페닐티오)페닐술포늄 등의 술포늄계, 디페닐요오드늄이나 비스(도데실페닐)요오드늄 등의 요오드늄, 페닐디아조늄 등의 디아조늄, 1-벤질-2-시아노피리니늄이나 1-(나프틸메틸)-2-시아노프리디늄 등의 암모늄, (4-메틸페닐)[4-(2-메틸프로필)페닐]-헥사플루오로포스페이트 요오드늄, 비스(4-t-부틸페닐)헥사플루오로포스페이트 요오드늄, 디페닐헥사플루오로포스페이트 요오드늄, 디페닐트리플루오로메탄술포네이트 요오드늄, 트리페닐술포늄 테트라풀루오로보레이트, 트리-p-토일술포늄 헥사풀루오로포스페이트, 트리-p-토일술포늄 트리풀루오로메탄술포네이트 및 (2,4-시클로펜타디엔-1-일)[(1-메틸에틸)벤젠]-Fe 등의 Fe 양이온들과 BF4 -, PF6 -, SbF6 - 등의 [BQ4]- 오늄염 조합을 이용할 수 있다(여기서, Q는 적어도 2개 이상의 불소 또는 트리플루오로메틸기로 치환된 페닐기이다.). In addition, when an epoxy etc. are contained in the said organic functional group, as a photoinitiator (cation), sulfonium types, such as triphenylsulfonium, diphenyl-4- (phenylthio) phenylsulfonium, diphenyliodonium, or bis (dode iodonium such as silphenyl) iodonium, diazonium such as phenyldiazonium, ammonium such as 1-benzyl-2-cyanopyrininium and 1-(naphthylmethyl)-2-cyanopridinium, (4- Methylphenyl)[4-(2-methylpropyl)phenyl]-hexafluorophosphate iodonium, bis(4-t-butylphenyl)hexafluorophosphate iodonium, diphenylhexafluorophosphate iodonium, diphenyltrifluoro Romethanesulfonate iodonium, triphenylsulfonium tetrafluoroborate, tri-p-toylsulfonium hexafluorophosphate, tri-p-toylsulfonium trifluoromethanesulfonate and (2,4- A combination of Fe cations such as cyclopentadien-1-yl)[(1-methylethyl)benzene]-Fe and [BQ 4 ] -onium salts such as BF 4 - , PF 6 - , SbF 6 - may be used. (Wherein, Q is a phenyl group substituted with at least two or more fluorine or trifluoromethyl groups).
또한, 열에 의해 작용하는 양이온 개시제로는 트리플산염, 3불화 붕소 에테르착화합물, 3불화 붕소 등과 같은 양이온계 또는 프로톤산 촉매, 암모늄염, 포스포늄염 및 술포늄염 등의 각종 오늄염 및 메틸트리페닐포스포늄 브롬화물, 에틸트리페닐포스포늄 브롬화물, 페닐트리페닐포스포늄 브롬화물 등을 제한 없이 사용할 수 있으며, 이들 개시제 또한 다양한 혼합형태로 첨가할 수 있으며, 상기에 명시한 다양한 라디칼 개시제들과의 혼용도 가능하다.
In addition, as a cationic initiator acting by heat, a cationic or protonic acid catalyst such as triflate, boron trifluoride ether complex, boron trifluoride, etc., various onium salts such as ammonium salt, phosphonium salt and sulfonium salt, and methyltriphenylphosphonium Bromide, ethyltriphenylphosphonium bromide, phenyltriphenylphosphonium bromide, etc. can be used without limitation, and these initiators can also be added in various mixed forms, and can be mixed with the various radical initiators specified above do.
또한, 상기 유기관능기의 종류에 따라, 아민 경화제류인 에틸렌디아민, 트리에틸렌 테트라민, 테트라에틸렌 펜타민, 1,3-디아미노프로판, 디프로필렌트리아민, 3-(2-아미노에틸)아미노-프로필아민, N,N'-비스(3-아미노프로필)-에틸렌디아민, 4,9-디옥사도테칸-1,12-디아민, 4,7,10-트리옥사트리데칸-1,13-디아민, 헥사메틸렌디아민, 2-메틸펜타메틸렌디아민, 1,3-비스아미노메틸시클로헥산, 비스(4-아니모시클로헥실)메탄, 노르보르넨디아민, 1,2-디아미노시클로헥산 등을 이용할 수 있다.
In addition, depending on the type of the organic functional group, amine curing agents ethylenediamine, triethylenetetramine, tetraethylenepentamine, 1,3-diaminopropane, dipropylenetriamine, 3-(2-aminoethyl)amino-propyl amine, N,N'-bis(3-aminopropyl)-ethylenediamine, 4,9-dioxadothecan-1,12-diamine, 4,7,10-trioxatridecane-1,13-diamine, Hexamethylenediamine, 2-methylpentamethylenediamine, 1,3-bisaminomethylcyclohexane, bis(4-animocyclohexyl)methane, norbornenediamine, 1,2-diaminocyclohexane, etc. can be used. .
아울러, 무수프탈산, 무수트리멜리트산, 무수피로멜리트산, 무수말레산, 테트라히드로 무수프탈산, 메틸헥사히드로 무수프탈산, 메틸테트라히드로 무수프탈산, 메틸나드산 무수물, 수소화메틸나드산 무수물, 트리알킬테트라히드로 무수프탈산, 도데세닐 무수숙신산, 무수2,4-디에틸글루타르산 등의 산무수경화제류도 폭넓게 사용될 수 있다.In addition, phthalic anhydride, trimellitic anhydride, pyromellitic anhydride, maleic anhydride, tetrahydrophthalic anhydride, methylhexahydrophthalic anhydride, methyltetrahydrophthalic anhydride, methylnadic anhydride, hydrogenated methylnadic anhydride, trialkyltetra Acid anhydride curing agents such as hydrophthalic anhydride, dodecenyl succinic anhydride, and 2,4-diethyl glutaric anhydride can also be widely used.
상기 경화제는 조성물 100 중량부에 대하여 0.1-20 중량부로 포함되는 것이 좋다.
The curing agent is preferably included in an amount of 0.1-20 parts by weight based on 100 parts by weight of the composition.
또한 상기 경화작용을 촉진하기 위한 경화 촉진제로, 아세토구아나민, 벤조구아나민, 2,4-디아미노-6-비닐-s-트리아진 등의 트리아진계 화합물, 이미다졸, 2-메틸이미다졸, 2-에틸-4-메틸이미다졸, 2-페닐이미다졸, 2-페닐-4-메틸이미다졸, 비닐이미다졸, 1-메틸이미다졸 등의 이미다졸계 화합물, 1,5-디아자비시클로[4.3.0]논엔-5,1,8-디아자비시클로[5.4.0]운데센-7, 트리페닐포스핀, 디페닐(p-트릴)포스핀, 트리스(알킬페닐)포스핀, 트리스(알콕시페닐)포스핀, 에틸트리페닐포스포늄포스페이트, 테트라부틸포스포늄히드록시드, 테트라부틸포스포늄아세테이트, 테트라부틸포스포늄하이드로젠디플루오라이드, 테트라부틸포스포늄디하이드로젠트리플루오르 등도 사용될 수 있다.
In addition, as a curing accelerator for accelerating the curing action, triazine-based compounds such as acetoguanamine, benzoguanamine, 2,4-diamino-6-vinyl-s-triazine, imidazole, and 2-methylimidazole , 2-ethyl-4-methylimidazole, 2-phenylimidazole, 2-phenyl-4-methylimidazole, vinylimidazole, imidazole compounds such as 1-methylimidazole, 1, 5-diazabicyclo[4.3.0]nonene-5,1,8-diazabicyclo[5.4.0]undecene-7, triphenylphosphine, diphenyl(p-triyl)phosphine, tris(alkylphenyl) ) Phosphine, tris (alkoxyphenyl) phosphine, ethyl triphenyl phosphonium phosphate, tetrabutyl phosphonium hydroxide, tetrabutyl phosphonium acetate, tetrabutyl phosphonium hydrogen difluoride, tetrabutyl phosphonium dihydrogen tri Fluorine or the like may also be used.
본 발명에서는 또한 경화공정 또는 후반응을 통한 경도, 강도, 내구성, 성형성 등을 개선하는 목적으로 자외선 흡수제, 산화 방지제, 소포제, 레벨링제, 발수제, 난연제, 접착개선제 등의 첨가제를 추가로 포함할 수 있다. 이러한 첨가제는 그 사용에 있어 특별하게 제한은 없으나 기판의 특성 즉, 유연성, 투광성, 내열성, 경도, 강도 등의 물성을 해치지 않는 범위 내에서 적절히 첨가할 수 있다. 상기 첨가제는 각각 독립적으로 조성물 100 중량부에 대하여 0.01-10 중량부로 포함되는 것이 좋다.
In the present invention, additives such as UV absorbers, antioxidants, defoamers, leveling agents, water repellents, flame retardants, and adhesion improvers are additionally included for the purpose of improving hardness, strength, durability, moldability, etc. through the curing process or post-reaction. can These additives are not particularly limited in their use, but may be appropriately added within a range that does not impair the properties of the substrate, that is, physical properties such as flexibility, light transmittance, heat resistance, hardness, and strength. Preferably, the additives are each independently included in an amount of 0.01-10 parts by weight based on 100 parts by weight of the composition.
본 발명에서 사용가능한 첨가제로는 폴리에테르 디메틸폴리실록산계 (Polyether-modified polydimethylsiloxane, 예를 들어, BYK 사 제품인 BYK-300, BYK-301, BYK-302, BYK-331, BYK-335, BYK-306, BYK-330, BYK-341, BYK-344, BYK-307, BYK-333, BYK-310 등), 폴리에테르 하이드록시 폴리디메틸실록산계 (Polyether modified hydroxyfunctional poly-dimethyl-siloxane, 예를 들어, BYK 사의 BYK-308, BYK-373 등), 폴리메틸알킬실록산계 (Methylalkylpolysiloxane, 예를 들어, BYK-077, BYK-085 등), 폴리에테르 폴리메틸알킬실록산계 (Polyether modified methylalkylpolysiloxane, 예를 들어, BYK-320, BYK-325 등), 폴리에스테르 폴리메틸알킬실록산계 (Polyester modified poly-methyl-alkyl-siloxane, 예를 들어, BYK-315 등), 알랄킬 폴리메틸알킬실록산계 (Aralkyl modified methylalkyl polysiloxane, 예를 들어, BYK-322, BYK-323 등), 폴리에스테르 하이드록시 폴리디메틸실록산계 (Polyester modified hydroxy functional polydimethylsiloxane, 예를 들어, BYK-370 등), 폴리에스테르 아크릴 폴리디메틸실록산계 (Acrylic functional polyester modified polydimethylsiloxane, 예를 들어, BYK-371, BYK-UV 3570 등), 폴리에테르-폴리에스테르 하이드록시 폴리디메틸실록산계 (Polyeher-polyester modified hydroxy functional polydimethylsiloxane, 예를 들어, BYK-375 등), 폴리에테르 폴리디메틸실록산계 (Polyether modified dimethylpolysiloxane, 예를 들어, BYK-345, BYK-348, BYK-346, BYK-UV3510, BYK-332, BYK-337 등), 비이온 폴리아크릴계 (Non-ionic acrylic copolymer, 예를 들어, BYK-380 등), 이온성 폴리아크릴계 (Ionic acrylic copolymer, 예를 들어, BYK-381 등), 폴리아크릴레이트계 (Polyacrylate, 예를 들어, BYK-353, BYK-356, BYK-354, BYK-355, BYK-359, BYK-361 N, BYK-357, BYK-358 N, BYK-352 등), 폴리메타아크릴레이트계 (Polymethacrylate, 예를 들어, BYK-390 등), 폴리에테르 아크릴 폴리디메틸실록산계 (Polyether modified acryl functional polydimethylsiloxane, 예를 들어, BYK-UV 3500, BYK-UV3530 등), 폴리에테르 실록산계 (Polyether modified siloxane, 예를 들어, BYK-347 등), 알코올 알콕시레이트계 (Alcohol alkoxylates, 예를 들어, BYK-DYNWET 800 등), 아크릴레이트계 (Acrylate, 예를 들어, BYK-392 등), 하이드록시 실리콘 폴리아크릴레이트계 (Silicone modified polyacrylate (OH-functional), 예를 들어, BYK-Silclean 3700 등) 등을 들 수 있다.
Additives usable in the present invention include polyether-modified polydimethylsiloxane (for example, BYK-300, BYK-301, BYK-302, BYK-331, BYK-335, BYK-306, manufactured by BYK). BYK-330, BYK-341, BYK-344, BYK-307, BYK-333, BYK-310, etc.), polyether hydroxyfunctional poly-dimethyl-siloxane (Polyether modified hydroxyfunctional poly-dimethyl-siloxane, for example, manufactured by BYK BYK-308, BYK-373, etc.), polymethylalkylpolysiloxane (eg, BYK-077, BYK-085, etc.), polyether modified methylalkylpolysiloxane (eg, BYK- 320, BYK-325, etc.), polyester polymethylalkylsiloxane (Polyester modified poly-methyl-alkyl-siloxane, e.g., BYK-315, etc.), Aralkyl modified methylalkyl polysiloxane, e.g. For example, BYK-322, BYK-323, etc.), polyester hydroxy polydimethylsiloxane-based (Polyester modified hydroxy functional polydimethylsiloxane, for example, BYK-370, etc.), polyester acrylic polydimethylsiloxane-based (Acrylic functional polyester modified polydimethylsiloxane, such as BYK-371, BYK-UV 3570, etc.), polyether-polyester hydroxy polydimethylsiloxane (Polyeher-polyester modified hydroxy functional polydimethylsiloxane, such as BYK-375, etc.), polyether poly Dimethylsiloxane (Polyether modified dimethylpolysiloxane, for example, BYK-345, BYK-348, BYK-346, BYK-UV3510, BYK-332, BYK-337, etc.), Non-ionic acrylic copolymer, for example , BYK-380, etc.), ionic polyacrylic copolymer (eg, BYK-381, etc.), polyacrylate (eg, BYK-353, BYK-356, BYK-354, BYK) -355, BYK-359, BYK-361 N, BYK-357, BYK-358 N, BYK-352, etc.), polymethacrylate-based (Polymethacrylate, such as BYK-390, etc.), polyether acrylic polydimethyl Siloxane-based (Polyether modified acryl functional polydimethylsiloxane, for example, BYK-UV 3500, BYK-UV3530, etc.), polyether siloxane-based (Polyether modified siloxane, for example, BYK-347, etc.), alcohol alkoxylates , for example, BYK-DYNWET 800, etc.), acrylate-based (Acrylate, for example, BYK-392, etc.), hydroxy silicone polyacrylate (OH-functional), such as BYK -Silclean 3700, etc.) and the like.
본 발명에 있어서, 상기 플라스틱코팅조성물을 플라스틱 표면 위에 코팅하는 방법은 스핀코팅, 바코팅, 슬릿코팅, 딥 코팅, 내츄럴 코팅, 리버스 코팅, 롤 코팅, 스핀코팅, 커텐코팅, 스프레이 코팅, 침지법, 함침법, 그라비어 코팅 등 공지된 방법 중에서 당업자가 임의로 선택하여 적용할 수 있음은 물론이며, 경화방법에 있어도 광경화 또는 열경화를 복합고분자의 관능기에 따라 적절하게 선택하여 적용할 수 있음은 물론이다. 바람직하기로 열경화의 경우 경화온도는 80 내지 120 ℃이다.
In the present invention, the method of coating the plastic coating composition on the plastic surface is spin coating, bar coating, slit coating, dip coating, natural coating, reverse coating, roll coating, spin coating, curtain coating, spray coating, dipping method, Of course, those skilled in the art can arbitrarily select and apply from known methods such as impregnation method and gravure coating, and even in the curing method, photocuring or thermal curing can be appropriately selected and applied according to the functional group of the composite polymer. . Preferably, in the case of thermosetting, the curing temperature is 80 to 120 °C.
본 발명에서 상기 코팅 조성물의 코팅 두께는 임의로 조절 가능하며, 바람직하게는 0.01 내지 500 um이며, 더욱 바람직하게는 0.1 내지 300 um, 더더욱 바람직하기로는 1 내지 100 um 범위가 좋다. 상기 범위 내인 경우 7H 이상의 표면경도를 안정적으로 확보할 수 있을 뿐만 아니라 기판 표면 특성에 있어서도 우수한 물성을 나타낸다. 특히 10 um 이상의 두께로 코팅층이 적층된 경우 표면경도가 9H를 안정적으로 나타낼 수 있다.
In the present invention, the coating thickness of the coating composition is arbitrarily adjustable, preferably 0.01 to 500 um, more preferably 0.1 to 300 um, even more preferably 1 to 100 um. When it is within the above range, it is possible to stably secure a surface hardness of 7H or more, and exhibit excellent physical properties in terms of substrate surface properties. In particular, when the coating layer is laminated to a thickness of 10 μm or more, the surface hardness can stably represent 9H.
또한 본 발명은 표면 위에 상기 화학식 1 내지 9 중 어느 하나로 표시되는 실세스퀴옥산 복합 고분자를 포함하는 플라스틱코팅조성물이 코팅되어 경화된 경화물을 포함하는 것을 특징으로 실세스퀴옥산 복합 고분자 코팅 플라스틱과 상기 실세스퀴옥산 복합 고분자 코팅 플라스틱을 포함하는 제품을 제공하는 바, 상기 실세스퀴옥산 복합 고분자 코팅 플라스틱은 상기 플라스틱코팅방법에 의하여 제조될 수 있다.
In addition, the present invention is a silsesquioxane composite polymer coated plastic, characterized in that it comprises a cured product coated with a plastic coating composition containing a silsesquioxane composite polymer represented by any one of Formulas 1 to 9 on the surface of the cured product To provide a product including the silsesquioxane composite polymer coated plastic, the silsesquioxane composite polymer coated plastic may be manufactured by the plastic coating method.
본 발명에 따른 실세스퀴옥산 복합 고분자 코팅 플라스틱은 실세스퀴옥산 복합 고분자 코팅층과 플라스틱과의 접착력이 우수하며, 형성된 실세스퀴옥산 복합 고분자 코팅층은 매우 높은 표면경도를 가지며, 우수한 투명성, 내스크레치성, 발수특성, 방오특성, 내지문성, 열안정성 및 광택특성을 가지며, 플라스틱 기재와의 접착력이 우수하며 광학필름, 보호필름, 전자제품 구성용 플라스틱, 안경, 건축외장제, 건축내장제, 플라스틱 배관, 전선피복제, 광학렌즈, 방음벽, 플라스틱 간판, 플라스틱 조형물, 가구, 조명, 썬루프, 헬멧 등의 다양한 제품에 유용하게 적용될 수 있다.
The silsesquioxane composite polymer coating plastic according to the present invention has excellent adhesion between the silsesquioxane composite polymer coating layer and the plastic, and the formed silsesquioxane composite polymer coating layer has very high surface hardness, excellent transparency, and scratch resistance. It has water-repellent properties, antifouling properties, anti-fingerprint properties, thermal stability and gloss properties, and excellent adhesion to plastic substrates. , wire coverings, optical lenses, soundproof walls, plastic signs, plastic sculptures, furniture, lighting, sunroofs, helmets, etc. can be usefully applied to various products.
이하, 본 발명의 이해를 돕기 위하여 바람직한 실시예를 제시하나, 하기 실시예는 본 발명을 예시하는 것일 뿐 본 발명의 범위가 하기 실시예에 한정되는 것은 아니다. 하기 본 발명의 실시예에서 ECHETMS는 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane, GPTMS는 Glycidoxypropytrimethoxysilane, MAPTMS는 (methacryloyloxy)propyltrimethoxysilane, PTMS는 Phenyltrimethoxysilane, MTMS는 Methyltrimethoxysilane, ECHETMDS는 Di(epoxycyclohexyethyl) tetramethoxy disiloxane, GPTMDS는 Di(glycidoxypropyl) tetramethoxy disiloxane, MAPTMDS는 Di(methacryloyloxy)propy, PTMDS는 Di(phenyl) tetramethoxy disiloxane, MTMDS는 Di(Methyl) tetramethoxy disiloxane을 의미한다.
Hereinafter, preferred examples are presented to help the understanding of the present invention, but the following examples are only illustrative of the present invention and the scope of the present invention is not limited to the following examples. In the examples of the present invention, ECHETMS is 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane, GPTMS is Glycidoxypropytrimethoxysilane, MAPTMS is (methacryloyloxy)propyltrimethoxysilane, PTMS is Phenyltrimethoxysilane, MTMS is Methyltritetramethoxysilane, ECHETMDS is Di(epoxymethoxycyclohexyloxane) tetramethoxysilane, ECHETMDS is Di(epoxymethoxycyclohexyethyl) Di(glycidoxypropyl) tetramethoxy disiloxane, MAPTMDS stands for Di(methacryloyloxy)propy, PTMDS stands for Di(phenyl) tetramethoxy disiloxane, and MTMDS stands for Di(Methyl) tetramethoxy disiloxane.
[실시예 1] 공중합체 1 및 9을 포함하는 코팅조성물의 제조[Example 1] Preparation of coating composition comprising copolymers 1 and 9
합성단계는 아래와 같이, 연속가수분해 및 축합을 단계적으로 진행하였다. As for the synthesis step, continuous hydrolysis and condensation were carried out step by step.
[실시예 1-a] 촉매의 제조[Example 1-a] Preparation of catalyst
염기도 조절을 위하여, Tetramethylammonium hydroxide (TMAH) 25 중량% 수용액에 10 중량% Potassium hydroxide (KOH) 수용액을 혼합하여 촉매 1a를 준비하였다.
For basicity control, catalyst 1a was prepared by mixing 10 wt% potassium hydroxide (KOH) aqueous solution with 25 wt% tetramethylammonium hydroxide (TMAH) aqueous solution.
[실시예 1-b] 선형 실세스퀴옥산 구조의 합성[Example 1-b] Synthesis of linear silsesquioxane structure
냉각관과 교반기를 구비한 건조된 플라스크에, 증류수 5 중량부, 테트라하이드로퓨란 15 중량부, 상기 실시예 1-a에서 제조된 촉매 1 중량부를 적가하고, 1시간 동안 상온에서 교반 한 후, 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane 20중량부를 적가하고, 다시 테트라하이드로류란을 15 중량부 적가하여 5시간 추가 교반 하였다. 교반 중의 혼합용액을 적취하여, 두 차례 세정하는 것으로 촉매와 불순물을 제거하고 필터 한 후, IR 분석을 통하여 말단기에 생성된 SI-OH 관능기를 확인할 수 있었으며(3200 cm-1), 분자량을 측정한 결과, 화학식 4구조와 같은 선형구조의 실세스퀴옥산이 8,000 스티렌 환산 분자량을 가짐을 확인할 수 있었다.
5 parts by weight of distilled water, 15 parts by weight of tetrahydrofuran, and 1 part by weight of the catalyst prepared in Example 1-a were added dropwise to a dried flask equipped with a cooling tube and a stirrer, and after stirring at room temperature for 1 hour, 2 20 parts by weight of -(3,4-epoxycyclohexyl)ethyltrimethoxysilane was added dropwise, and then 15 parts by weight of tetrahydrolurane was added dropwise, followed by further stirring for 5 hours. After removing the catalyst and impurities by collecting the mixed solution during stirring, washing twice, and filtering, the SI-OH functional group generated at the terminal group was confirmed through IR analysis (3200 cm -1 ), and the molecular weight was measured. As a result, it was confirmed that silsesquioxane having a linear structure such as the structure of Chemical Formula 4 had a molecular weight equivalent to 8,000 styrene.
[실시예 1-c] 연속적 cage 구조의 생성[Example 1-c] Creation of a continuous cage structure
상기 실시예 1-b 혼합용액에 0.36 중량% HCl 수용액을 매우 천천히 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 Diphenyltetramethoxydisiloxane 5 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하고, 1시간 교반 후 실시예 1-a에서 제조된 촉매를 7 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, 선형고분자와는 별도로 alkoxy가 열려있는 D구조의 전구체가 형성된다. 소량의 샘플을 적취하여, H-NMR과 IR로 분석하여 methoxy의 잔존율을 확인한 후, 잔존율이 20% 일 때, 0.36 중량% HCl 수용액을 10 중량부 천천히 적가하여, pH를 산성으로 조절해 주었다. 이후 Phenyltrimethoxysilane 1 중량부를 한번에 적가하여 15분간 교반 후, 1-a에서 제조된 촉매 20 중량부를 첨가하였다. 4시간의 혼합교반 이후, 확인결과 고분자내에 cage 형태의 고분자가 생성됨을 확인 할 수 있었다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여, 전체적인 반응물이 수용액 혼합물로 변환되도록 하였다. 4시간의 혼합 교반 이후, 일부를 적취하여 29Si-NMR을 통해 분석한 결과 phenyl기를 이용해 도입된 구조의 분석피크가 날카로운 형태의 2개로 나타나고 별도로 잔존하는 부산물 없이 화학식 1과 같은 A-D 고분자가 50% 이상 제조되었음을 확인할 수 있었다. 또한 스티렌 환산 분자량은 11,000으로 측정되었으며, n 값은 4-6이었다. 29Si-NMR (CDCl3) δ To the mixed solution of Example 1-b, 5 parts by weight of 0.36 wt% HCl aqueous solution was added dropwise very slowly, the pH was adjusted to have acidity, and the mixture was stirred at a temperature of 4 °C for 30 minutes. Then, 5 parts by weight of diphenyltetramethoxydisiloxane was added dropwise at a time to achieve stable hydrolysis, and after stirring for 1 hour, 7 parts by weight of the catalyst prepared in Example 1-a was added again to adjust the pH of the mixed solution to a basic state. At this time, a precursor of the D structure in which the alkoxy is open is formed separately from the linear polymer. After taking a small sample and analyzing it with H-NMR and IR to check the residual ratio of methoxy, when the residual ratio is 20%, 10 parts by weight of 0.36 wt% HCl aqueous solution is slowly added dropwise to adjust the pH to acid. gave. Then, 1 part by weight of Phenyltrimethoxysilane was added dropwise at a time, stirred for 15 minutes, and then 20 parts by weight of the catalyst prepared in 1-a was added. After 4 hours of mixing and stirring, it was confirmed that a cage-type polymer was produced in the polymer. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed by vacuum, so that the entire reactant was converted into an aqueous mixture. After 4 hours of mixing and stirring, a portion was taken and analyzed through 29 Si-NMR. As a result, the analysis peaks of the structure introduced using a phenyl group appeared as two sharp peaks, and the AD polymer as in Formula 1 was 50% It could be confirmed that the above was manufactured. In addition, the molecular weight in terms of styrene was measured to be 11,000, and the n value was 4-6. 29 Si-NMR (CDCl 3 ) δ
[실시예 1-d] 광경화형 수지 조성물 제조[Example 1-d] Preparation of photocurable resin composition
상기 실시예 1-c에서 수득한 실세스퀴옥산 복합 고분자 30 g을 메틸아이소부틸케톤에 30 중량%로 녹여 100 g의 코팅조성물을 제조하였다. 이후, 코팅 조성물 100 중량부에 클로로 아세토페논(chloro acetophenone) 3 중량부와 BYK-347 1 중량부, BYK-UV 3500 1 중량부를 각각 첨가하고 10분간 교반하여 광경화형 코팅 조성물을 제조하였다.
30 g of the silsesquioxane composite polymer obtained in Example 1-c was dissolved in methyl isobutyl ketone at 30 wt % to prepare 100 g of a coating composition. Thereafter, 3 parts by weight of chloro acetophenone, 1 part by weight of BYK-347, and 1 part by weight of BYK-UV 3500 were added to 100 parts by weight of the coating composition, respectively, and stirred for 10 minutes to prepare a photocurable coating composition.
[실시예 1-e] 열경화형 수지 조성물의 제조[Example 1-e] Preparation of thermosetting resin composition
상기 실시예 1-c에서 수득한 실세스퀴옥산 복합 고분자 50 g을 메틸에틸케톤에 50 중량%로 녹여 100 g의 코팅조성물을 제조하였다. 이후, 준비된 코팅 조성물 100 중량부에 1,3-디아미노프로판 3 중량부와 BYK-357 및 BYK-348을 각 1 중량부씩 첨가하고 10분간 교반하여 열경화형 코팅 조성물을 제조하였다.
50 g of the silsesquioxane composite polymer obtained in Example 1-c was dissolved in methyl ethyl ketone at 50 wt % to prepare 100 g of a coating composition. Thereafter, 3 parts by weight of 1,3-diaminopropane and 1 part by weight of BYK-357 and BYK-348 were added to 100 parts by weight of the prepared coating composition and stirred for 10 minutes to prepare a thermosetting coating composition.
[실시예 1-f] 고분자 자체로 구성된 코팅 조성물[Example 1-f] Coating composition composed of polymer itself
실시예 1-c 만으로 별도의 조성 없이 코팅 조성물을 구성하였다.
Only Example 1-c was composed of a coating composition without a separate composition.
또한, 하기 표 19에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자를 제조 및 코팅 조성물을 제조하였다. 이때 제조 방법은 상기 실시예 1-b, 1-c, 1-d, 1-e 및 1-f에서 사용한 방법을 대등하게 적용하였다.In addition, by applying the monomers described in Table 19 below, a silsesquioxane composite polymer was prepared and a coating composition was prepared. At this time, the manufacturing method was equally applied to the methods used in Examples 1-b, 1-c, 1-d, 1-e and 1-f.
방법practice
Way
적용 단량체1-b method
Applicable monomer
적용 단량체1-c method
Applicable monomer
(Mw)Molecular Weight
(Mw)
실시예Example 2 2 : : 실세스퀴옥산Silsesquioxane D-A-D 구조 복합 고분자의 합성 Synthesis of D-A-D Structured Composite Polymers
D-A-D구조의 복합 고분자를 제조하기 위하여 아래의 실시예를 이용하였으며, 상기 실시예 1에 기재된 방법과 대등한 방법으로 코팅 조성물을 제조하였다. 촉매 및 선형구조의 제조는 실시예 1-a 및 1-b의 방법을 동일하게 사용하였으며, 이후 연속적 D-A-D 구조를 생성하기 위하여 아래의 방법으로 제조를 실시하였다.
The following example was used to prepare a composite polymer having a DAD structure, and a coating composition was prepared by a method equivalent to the method described in Example 1 above. For the preparation of the catalyst and the linear structure, the methods of Examples 1-a and 1-b were used in the same manner, and then, the preparation was carried out in the following manner in order to generate a continuous DAD structure.
[실시예 2-a] 과량의 연속적 cage 구조의 생성[Example 2-a] Creation of an excess of continuous cage structure
상기 실시예 1-b 혼합용액에 0.36 중량% HCl 수용액을 매우 천천히 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 실시예 1-b에서 사용된 Diphenyltetramethoxydisiloxane의 5배인 25 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하고, 1시간 교반 후 실시예 1-a에서 제조된 촉매를 7 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, 선형고분자와는 별도로 alkoxy가 열려있는 D구조의 전구체가 형성된다. 소량의 샘플을 적취하여, H-NMR과 IR로 분석하여 methoxy의 잔존율을 확인한 후, 잔존율이 20% 일 때, 0.36 중량% HCl 수용액을 10 중량부 천천히 적가하여, pH를 산성으로 조절해 주었다. 이후 Phenyltrimethoxysilane 1 중량부를 한번에 적가하여 15분간 교반 후, 1-a에서 제조된 촉매 20 중량부를 첨가하였다. 4시간의 혼합교반 이후, 확인결과 고분자내에 cage 형태의 고분자가 생성됨을 확인 할 수 있었다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여, 전체적인 반응물이 수용액 혼합물로 변환되도록 하였다. 4시간의 혼합 교반 이후, 일부를 적취하여 29Si-NMR을 통해 분석한 결과 phenyl기를 이용해 도입된 구조의 분석피크가 날카로운 형태의 2개로 나타나고 별도로 잔존하는 부산물 없이 화학식 1과 같은 A-D 고분자가 제조되었음을 확인할 수 있었다. 또한 스티렌 환산 분자량은 14,000으로 측정되었으며, n 값은 4-6이었다. 또한, Si-NMR 분석에서 A-D구조와는 달리 A구조의 말단에서 보이던 -68ppm 근방의 피크가 사라져, A구조의 말단이 D구조로 모두 변환되어 D-A-D구조로 생성됨을 확인 하였다. 29Si-NMR (CDCl3) δ -72.3(broad), -81.1(sharp), -80.8(sharp), -82.5(broad)
To the mixed solution of Example 1-b, 5 parts by weight of 0.36 wt% HCl aqueous solution was added dropwise very slowly, the pH was adjusted to have acidity, and the mixture was stirred at a temperature of 4 °C for 30 minutes. After that, 25 parts by weight, which is 5 times the amount of Diphenyltetramethoxydisiloxane used in Example 1-b, was added dropwise at a time to achieve stable hydrolysis, and after stirring for 1 hour, 7 parts by weight of the catalyst prepared in Example 1-a was added again to give a basic state to adjust the pH of the mixed solution. At this time, a precursor of the D structure in which the alkoxy is open is formed separately from the linear polymer. After taking a small sample and analyzing it with H-NMR and IR to check the residual ratio of methoxy, when the residual ratio is 20%, 10 parts by weight of 0.36 wt% HCl aqueous solution is slowly added dropwise to adjust the pH to acid. gave. Then, 1 part by weight of Phenyltrimethoxysilane was added dropwise at a time, stirred for 15 minutes, and then 20 parts by weight of the catalyst prepared in 1-a was added. After 4 hours of mixing and stirring, it was confirmed that a cage-type polymer was produced in the polymer. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed by vacuum, so that the entire reactant was converted into an aqueous mixture. After 4 hours of mixing and stirring, a portion was taken and analyzed through 29 Si-NMR. As a result, the analysis peaks of the structure introduced using a phenyl group appeared as two sharp shapes, indicating that an AD polymer as in Formula 1 was prepared without separately remaining by-products. could check In addition, the molecular weight in terms of styrene was measured to be 14,000, and the n value was 4-6. In addition, unlike the AD structure in Si-NMR analysis, the peak near -68ppm that was seen at the end of the A structure disappeared, confirming that the end of the A structure was all converted to the D structure to form a DAD structure. 29 Si-NMR (CDCl 3 ) δ -72.3 (broad), -81.1 (sharp), -80.8 (sharp), -82.5 (broad)
또한, 하기 표 20에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자 및 코팅 조성물을 제조하였다. 이때 제조 방법은 상기 실시예 2에서 사용한 방법을 대등하게 적용하였다.In addition, silsesquioxane composite polymers and coating compositions were prepared by applying the monomers described in Table 20 below. At this time, the manufacturing method was equally applied to the method used in Example 2.
방법practice
Way
적용 단량체1-b method
Applicable monomer
적용 단량체2-a method
Applicable monomer
(Mw)Molecular Weight
(Mw)
실시예 3Example 3 : 실세스퀴옥산 E-A-D 구조 복합 고분자의 합성: Synthesis of silsesquioxane E-A-D structure composite polymer
E-A-D구조의 복합 고분자를 제조하기 위하여 아래의 실시예를 이용하였으며, 상기 실시예 1에 기재된 방법과 대등한 방법으로 코팅 조성물을 제조하였다. 촉매 및 선형구조의 제조는 실시예 1의 방법을 동일하게 사용하였으며, 이후 E-A-D 구조를 생성하기 위하여 아래의 방법으로 제조를 실시하였다.
The following example was used to prepare a composite polymer having an EAD structure, and a coating composition was prepared by a method equivalent to the method described in Example 1 above. The catalyst and the linear structure were prepared in the same manner as in Example 1, and then the EAD structure was prepared by the following method.
[실시예 3-a] 사슬 말단 E구조의 생성[Example 3-a] Generation of chain terminal E structure
실시예 1-c 에서 얻어진 A-D 혼합물에 별도의 정제 없이 메틸렌크로라이드 20 중량부를 적가하고, 0.36 중량% HCl 수용액을 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 dimethyltetramethoxysilane 1 중량부를 한번에 적가하였다. 이때, 아직 분자구조 내에서 가수분해되지 않고 존재하던 부분들이 용매와 분리된 산성 수용액 층에서 가수분해물로 쉽게 변환되며, 생성된 별도의 반응물과 유기용매 층에서 축합되어 말단단위에 E가 도입되었다. 5시간의 교반 후, 반응의 교반을 정지하고 상온으로 반응기의 온도를 조절 하였다.
To the AD mixture obtained in Example 1-c, 20 parts by weight of methylene chloride was added dropwise without separate purification, and 5 parts by weight of a 0.36% by weight aqueous HCl solution was added dropwise, and the pH was adjusted to have acidity, and the pH was adjusted to 30 at a temperature of 4°C. stirred for minutes. Then, 1 part by weight of dimethyltetramethoxysilane was added dropwise at a time. At this time, the parts that have not yet been hydrolyzed in the molecular structure are easily converted into hydrolysates in the acidic aqueous solution layer separated from the solvent, and are condensed with the generated separate reactants in the organic solvent layer to introduce E into the terminal unit. After stirring for 5 hours, the stirring of the reaction was stopped and the temperature of the reactor was adjusted to room temperature.
[실시예 3-b] 말단 E 구조에 cage 도입[Example 3-b] Incorporation of cage into end E structure
상기 실시예 3-a에서 얻어진 결과물의 유기층을 별도의 정제 없이 준비한 후, 3관능 단량체를 이용해서 말단을 cage구조로 변환하였다. 반응이 진행 중인 실시예 3-a 혼합용액에 Methyltrimethoxysilane 3 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하고, 24시간 교반 후 실시예 1-a에서 제조된 촉매를 3 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, E 구조 말단에 cage 형태의 고분자가 도입되며, 반응기 내에서 연속적으로 반응이 진행되어 화학식 3과 같은 고분자가 형성된다. 그러나, 다른 부산물들과 함께 얻어지므로, 별도의 정제가 필요하였다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여 정제를 준비하였다.
After the organic layer obtained in Example 3-a was prepared without further purification, the ends were converted into a cage structure using a trifunctional monomer. 3 parts by weight of Methyltrimethoxysilane was added dropwise to the mixed solution of Example 3-a in which the reaction is in progress, to achieve stable hydrolysis, and after stirring for 24 hours, 3 parts by weight of the catalyst prepared in Example 1-a was added again to give a basic state to adjust the pH of the mixed solution. At this time, a cage-type polymer is introduced at the end of the E structure, and the reaction proceeds continuously in the reactor to form a polymer as shown in Chemical Formula 3. However, since it was obtained together with other by-products, separate purification was required. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed in a vacuum to prepare a tablet.
[실시예 3-c] 침전 및 재결정을 통한 부산물 제거, 결과물의 수득[Example 3-c] Removal of by-products through precipitation and recrystallization, obtaining the result
상기 실시예 3-b에서 반응이 완료된 혼합물을 얻어낸 후, 증류수를 이용하여 세척하고, 증류수 층의 pH가 중성을 나타낼 때, 진공감압으로 용매를 완전히 제거하였다. 이후, 메탄올에 2회 침전하여, 미반응 단량체를 제거하고 테트라하이드로퓨란과 수용액이 9.5:0.5 중량비율로 혼합된 용매에 30 중량부로 녹여 -20 ℃의 온도에서 2일간 보관하였다. 이는 고분자에 도입되지 못하고, cage구조로 닫혀 버린 물질의 재결정을 도모하여, 정제가 쉽게 이루어지도록 하기 위함이다. After obtaining the mixture in which the reaction was completed in Example 3-b, the mixture was washed with distilled water, and when the pH of the distilled water layer was neutral, the solvent was completely removed under reduced pressure. Thereafter, by precipitation twice in methanol, unreacted monomers were removed, and 30 parts by weight was dissolved in a solvent in which tetrahydrofuran and aqueous solution were mixed at a weight ratio of 9.5:0.5 and stored at a temperature of -20 °C for 2 days. This is to promote recrystallization of the material that cannot be introduced into the polymer and is closed in a cage structure, so that purification can be performed easily.
재결정 과정을 마치고 얻어진 고체물질들을 필터 후, 진공감압을 통해 화학식 3의 고분자를 여러 부산물과 함께 얻어짐을 확인하였다. 또한, GPC 결과를 NMR 결과와 비교할 때, 각 단계의 고분자 성장에서 단독으로 얻어지는 저분자가 없이 Sharp한 형태의 Cage 형태가 결과로 도출되는 것으로 미루어 보아, 복합 고분자가 문제없이 얻어질 수 있음을 확인할 수 있었다. 이때, 분자량은 스티렌환산 값으로 17,000이었으며, n 값은 4-6이었으며, 특히 화학식 3의 결과는 다음과 같다. After filtering the solid materials obtained after the recrystallization process, it was confirmed that the polymer of Formula 3 was obtained along with various by-products through vacuum pressure reduction. In addition, when comparing the GPC result with the NMR result, it can be confirmed that a complex polymer can be obtained without problems, considering that a sharp cage form is derived as a result without a small molecule obtained alone in each stage of polymer growth. there was. At this time, the molecular weight was 17,000 in terms of styrene, and the n value was 4-6. In particular, the result of Formula 3 is as follows.
29Si-NMR (CDCl3) δ -68.2, -71.8(sharp). -72.3(broad), -81.1(sharp), -80.8(sharp), -82.5(broad)
29 Si-NMR (CDCl 3 ) δ -68.2, -71.8 (sharp). -72.3(broad), -81.1(sharp), -80.8(sharp), -82.5(broad)
또한, 하기 표 21에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자 및 코팅 조성물을 제조하였다. 이때 제조 방법은 상기 실시예 3에서 사용한 방법을 대등하게 적용하였다.In addition, by applying the monomers described in Table 21 below, a silsesquioxane composite polymer and coating composition were prepared. At this time, the manufacturing method was equally applied to the method used in Example 3.
방법practice
Way
적용 단량체1-b method
Applicable monomer
적용 단량체1-c method
Applicable monomer
방법
적용 단량체3-a
Way
Applicable monomer
방법
적용단량체3-b
Way
Applicable monomer
실시예 4Example 4 : A-B-D 구조 복합 실세스퀴옥산 고분자의 합성: Synthesis of A-B-D structure complex silsesquioxane polymer
합성단계는 아래와 같이, 연속가수분해 및 축합을 단계적으로 진행하여 E-A-D구조의 복합 고분자를 제조하였으며, 상기 실시예 1에 기재된 방법과 대등한 방법으로 코팅 조성물을 제조하였다.In the synthesis step, continuous hydrolysis and condensation were carried out step by step as follows to prepare a composite polymer having an E-A-D structure, and a coating composition was prepared by a method equivalent to the method described in Example 1.
[실시예 4-a] 가수분해 및 축합 반응을 위한 촉매의 제조[Example 4-a] Preparation of catalyst for hydrolysis and condensation reaction
염기도 조절을 위하여, Tetramethylammonium hydroxide (TMAH) 25 wt% 수용액에 10 wt% Potassium hydroxide (KOH) 수용액을 혼합하여 촉매 1a를 준비하였다.
For basicity control, catalyst 1a was prepared by mixing 10 wt% potassium hydroxide (KOH) aqueous solution with 25 wt% tetramethylammonium hydroxide (TMAH) aqueous solution.
[실시예 4-b] 선형 실세스퀴옥산 구조의 합성 (A-B전구체의 합성)[Example 4-b] Synthesis of linear silsesquioxane structure (Synthesis of A-B precursor)
냉각관과 교반기를 구비한 건조된 플라스크에, 증류수 5 중량부, 테트라하이드로퓨란 40 중량부, 상기 실시예 4-a에서 제조된 촉매 0.5 중량부를 적가하고, 1시간 동안 상온에서 교반 한 후, 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane 10 중량부를 적가하고, 다시 테트라하이드로류란을 20 중량부 적가하여 2시간 추가 교반 하였다. 교반 중의 혼합용액을 적취하여, 두 차례 세정하는 것으로 촉매와 불순물을 제거하고 필터 한 후, 1H-NMR 분석을 통하여 잔존하는 alkoxy group이 0.1 mmol/g 이하로 잔존하고 있는 선형 실세스퀴옥산을 얻어 내었고, 이는 이후 cage를 연속반응으로 도입하는데 이용되는 부분이다. 선형 구조의 형태 분석은 XRD 분석을 통해 전체적인 구조가 선형구조체임을 확인하였다. 분자량을 측정한 결과, 선형구조의 실세스퀴옥산이 6,000 스티렌 환산 분자량을 가짐을 확인할 수 있었다. 5 parts by weight of distilled water, 40 parts by weight of tetrahydrofuran, and 0.5 parts by weight of the catalyst prepared in Example 4-a were added dropwise to a dried flask equipped with a cooling tube and a stirrer, and after stirring at room temperature for 1 hour, 2 10 parts by weight of -(3,4-epoxycyclohexyl)ethyltrimethoxysilane was added dropwise, and 20 parts by weight of tetrahydrolurane was added dropwise again, followed by stirring for an additional 2 hours. After removing the catalyst and impurities and filtering the mixed solution while stirring, washing twice to remove linear silsesquioxane in which the residual alkoxy group remains at 0.1 mmol/g or less through 1 H-NMR analysis obtained, which is then used to introduce the cage as a continuous reaction. The morphology analysis of the linear structure confirmed that the overall structure was a linear structure through XRD analysis. As a result of measuring the molecular weight, it was confirmed that silsesquioxane having a linear structure had a molecular weight equivalent to 6,000 styrene.
1H-NMR (CDCl3) δ 3.7, 3.4, 3.3(broad), 3.1, 2.8, 2.6, 1.5(broad), 0.6.
1 H-NMR (CDCl 3 ) δ 3.7, 3.4, 3.3 (broad), 3.1, 2.8, 2.6, 1.5 (broad), 0.6.
[실시예 4-c] 사슬 내 cage 구조의 생성을 위한 pH 변환 반응 (B,D 구조의 도입)[Example 4-c] pH shift reaction for generation of intra-chain cage structure (introduction of B, D structures)
반응이 진행 중인 실시예 4-b 혼합용액에 0.36 wt% HCl 수용액을 매우 천천히 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 DiPhenyltetramethoxydisiloxane 5 중량부를 한번에 적가하여, 1시간 교반 후 실시예 4-a에서 제조된 촉매를 5 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, 선형구조체와는 별도로 cage 형태의 구조체가 생성되어 고분자 사슬에 도입됨을 확인 할 수 있었으며, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여, 전체적인 반응물이 수용액 혼합물로 변환되도록 하였다. 4시간의 혼합교반 이후, 일부를 적취하여 29Si-NMR 및 1H-NMR 을 통해 분석한 결과 B 구조내에 존재하는 alkoxy group의 양이 0.025 mmol/g으로 변화되어 B 와 D의 반복단위가 약 5:5 비율로 도입되었음을 확인할 수 있었다. 또한 스티렌 환산 분자량은 10,000으로 측정되었다. 또한, cage형 구조가 도입되었음에도, 고분자의 GPC 형태에서 단독 cage형 물질의 분자량 분포를 찾아볼 수 없으므로, cage구조가 연속반응을 통해 고분자 사슬에 잘 도입되었음을 확인할 수 있었다.5 parts by weight of 0.36 wt% HCl aqueous solution was very slowly added dropwise to the mixed solution of Example 4-b in which the reaction was in progress, the pH was adjusted to have acidity, and the mixture was stirred at a temperature of 4° C. for 30 minutes. Then, 5 parts by weight of DiPhenyltetramethoxydisiloxane was added dropwise at a time, and after stirring for 1 hour, 5 parts by weight of the catalyst prepared in Example 4-a was added again to adjust the pH of the mixed solution to a basic state. At this time, it was confirmed that a cage-type structure was created separately from the linear structure and introduced into the polymer chain, the temperature was changed to room temperature, and the tetrahydrofuran in the mixed solution was removed in a vacuum, and the entire reactant was converted into an aqueous mixture. made to be After 4 hours of mixing and stirring, a portion was taken and analyzed through 29 Si-NMR and 1 H-NMR. As a result, the amount of alkoxy group in structure B was changed to 0.025 mmol/g, so that the repeating units of B and D were about It was confirmed that it was introduced in a ratio of 5:5. In addition, the molecular weight in terms of styrene was measured to be 10,000. In addition, even though the cage-type structure was introduced, the molecular weight distribution of the single cage-type material was not found in the GPC form of the polymer, so it was confirmed that the cage structure was well introduced into the polymer chain through a continuous reaction.
1H-NMR (CDCl3) δ 7.5, 7.2, 3.7, 3.4, 3.3(broad), 3.1, 2.8, 2.6, 1.5(broad), 0.6. 29Si-NMR (CDCl3) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -82.5(broad)
1 H-NMR (CDCl 3 ) δ 7.5, 7.2, 3.7, 3.4, 3.3 (broad), 3.1, 2.8, 2.6, 1.5 (broad), 0.6. 29 Si-NMR (CDCl 3 ) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -82.5(broad)
[실시예 4-d] B 구조내 X도입 (B,D 구조의 도입)[Example 4-d] Introduction of X in structure B (introduction of structures B, D)
상기 실시예 4-c에서 얻어진 결과물의 유기층을 별도의 정제 없이 준비한 후, 3관능 단량체를 이용해서 말단을 cage구조로 변환하였다. 실시예 4-c에서 얻어진 물질 100 중량부를 50 중량부의 테트라하이드로퓨란에 녹인 후, 5 중량부의 증류수를 넣어 혼합용액을 제조하였다. 이후 제조된 혼합용액에 0.36 wt% HCl 10 중량부를 첨가하고 10분간 교반 후, Methyltrimethoxysilane 3 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하였다. 24시간 교반 후 실시예 4-a에서 제조된 촉매를 3 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, B 구조의 X 부분에 cage 형태의 고분자가 도입되며, 반응기 내에서 연속적으로 반응이 진행되어 화학식 4와 같은 고분자가 형성된다. 그러나, 다른 부산물들과 함께 얻어지므로, 별도의 정제가 필요하였다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여 정제를 준비하였다.
After the organic layer obtained in Example 4-c was prepared without further purification, the ends were converted into a cage structure using a trifunctional monomer. After dissolving 100 parts by weight of the material obtained in Example 4-c in 50 parts by weight of tetrahydrofuran, 5 parts by weight of distilled water was added to prepare a mixed solution. Then, 10 parts by weight of 0.36 wt% HCl was added to the prepared mixed solution, and after stirring for 10 minutes, 3 parts by weight of Methyltrimethoxysilane was added dropwise at once to achieve stable hydrolysis. After stirring for 24 hours, 3 parts by weight of the catalyst prepared in Example 4-a was added again to adjust the pH of the mixed solution to a basic state. At this time, a cage-shaped polymer is introduced into the X portion of the B structure, and the reaction proceeds continuously in the reactor to form a polymer as shown in Chemical Formula 4. However, since it was obtained together with other by-products, separate purification was required. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed in a vacuum to prepare a tablet.
[실시예 4-e] 침전 및 재결정을 통한 부산물 제거, 결과물의 수득[Example 4-e] Removal of by-products through precipitation and recrystallization, obtaining the result
상기 실시예 4-d에서 반응이 완료된 혼합물에 메틸렌크로라이드 200 중량부를 넣어, 증류수와 함께 분별 세척하고, 증류수 층의 pH가 중성을 나타낼 때, 진공감압으로 용매를 완전히 제거하였다. 이후, 메탄올에 2회 침전하여, 미반응 단량체를 제거하고 테트라하이드로퓨란과 수용액이 9.5:0.5 중량비율로 혼합된 용매에 30 중량부로 녹여 -20 ℃의 온도에서 2일간 보관하였다. 이는 고분자에 도입되지 못하고, cage구조로 닫혀 버린 물질의 재결정을 도모하여, 정제가 쉽게 이루어지도록 하기 위함이다. In Example 4-d, 200 parts by weight of methylene chloride was put into the mixture in which the reaction was completed, fractional washing was performed with distilled water, and when the pH of the distilled water layer was neutral, the solvent was completely removed by vacuum. Thereafter, by precipitation twice in methanol, unreacted monomers were removed, and 30 parts by weight was dissolved in a solvent in which tetrahydrofuran and aqueous solution were mixed at a weight ratio of 9.5:0.5 and stored at a temperature of -20 °C for 2 days. This is to promote recrystallization of the material that cannot be introduced into the polymer and is closed in a cage structure, so that purification can be performed easily.
재결정 과정을 마치고 얻어진 고체물질들을 필터 후, 진공감압을 통해 화학식 4의 고분자가 여러 부산물 없이 얻어짐을 확인하였다. 또한, GPC 결과를 NMR 결과와 비교할 때, 각 단계의 고분자 성장에서 단독으로 얻어지는 저분자가 없이 Sharp한 형태의 Cage 형태가 결과로 도출되는 것으로 미루어 보아, 복합 고분자가 문제없이 얻어질 수 있음을 확인할 수 있었다. 이때, 분자량은 스티렌환산 값으로 12,000의 값을 얻을 수 있었으며, X의 n 값은 4-6이었으며, Y의 n 값은 4-6이었으며, 특히 화학식 4의 결과는 다음과 같다. After filtering the solid materials obtained after the recrystallization process, it was confirmed that the polymer of Formula 4 was obtained without various by-products through vacuum pressure reduction. In addition, when comparing the GPC result with the NMR result, it can be confirmed that a complex polymer can be obtained without problems, considering that a sharp cage form is derived as a result without a small molecule obtained alone in each stage of polymer growth. there was. At this time, the molecular weight was 12,000 in terms of styrene, and the n value of X was 4-6, and the n value of Y was 4-6. In particular, the result of Chemical Formula 4 is as follows.
29Si-NMR (CDCl3) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -81.5(sharp), -82.5(broad)
29 Si-NMR (CDCl 3 ) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -81.5(sharp), -82.5(broad)
또한, 하기 표 22에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자 및 코팅 조성물을 제조하였다. 이때 제조 방법은 상기 실시예 4에서 사용한 방법을 대등하게 적용하였다.In addition, silsesquioxane composite polymers and coating compositions were prepared by applying the monomers described in Table 22 below. At this time, the manufacturing method was equally applied to the method used in Example 4.
방법practice
Way
적용 단량체4-b method
Applicable monomer
적용 단량체4-c method
Applicable monomer
적용 단량체4-d method
Applicable monomer
(Mw)Molecular Weight
(Mw)
실시예 5Example 5 : D-A-B-D 구조 복합 실세스퀴옥산 고분자의 합성: Synthesis of D-A-B-D structure complex silsesquioxane polymer
D-A-B-D구조의 복합 고분자를 제조하기 위하여 아래의 방법을 이용하였으며, 상기 실시예 1과 대등한 방법으로 코팅 조성물을 제조하였다.
The following method was used to prepare a composite polymer having a DABD structure, and a coating composition was prepared in the same manner as in Example 1.
[실시예 5-a] D구조의 과량 생성을 위한 pH 변환 반응 (B,D 구조의 도입)[Example 5-a] pH conversion reaction for excessive production of structure D (introduction of structures B, D)
반응이 진행 중인 실시예 4-b 혼합용액에 0.36 wt% HCl 수용액을 매우 천천히 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 Diphenyltetramethoxydisiloxane의 양을 실시예 4-b의 5배인 25 중량부로 준비하여 한번에 적가하고, 1시간 교반 후 실시예 1-a에서 제조된 촉매를 5 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 반응 완료 후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여, 전체적인 반응물이 수용액 혼합물로 변환되도록 하였다. 4시간의 혼합교반 이후, 일부를 적취하여 29Si-NMR 및 1H-NMR 을 통해 분석한 결과 B 구조내에 존재하는 alkoxy group의 양이 0.012 mmol/g으로 변화되고 B 와 D의 반복단위가 약 1:9 비율로 도입되었음을 확인할 수 있었다. 또한 스티렌 환산 분자량은 24,000으로 측정되었다. 또한, cage형 구조가 도입되었음에도, 고분자의 GPC 형태에서 단독 cage형 물질의 분자량 분포를 찾아볼 수 없으므로, cage구조가 연속반응을 통해 고분자 사슬에 잘 도입되었음을 확인할 수 있었다.5 parts by weight of 0.36 wt% HCl aqueous solution was very slowly added dropwise to the mixed solution of Example 4-b in which the reaction was in progress, the pH was adjusted to have acidity, and the mixture was stirred at a temperature of 4° C. for 30 minutes. After that, the amount of Diphenyltetramethoxydisiloxane was prepared in an amount of 25 parts by weight, which is 5 times that of Example 4-b, and added dropwise at once, and after stirring for 1 hour, 5 parts by weight of the catalyst prepared in Example 1-a was again added to the pH of the mixed solution in a basic state was regulated After completion of the reaction, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed by vacuum, so that the entire reactant was converted into an aqueous mixture. After mixing and stirring for 4 hours, a portion was taken and analyzed through 29 Si-NMR and 1 H-NMR. As a result, the amount of alkoxy group present in structure B was changed to 0.012 mmol/g, and the repeating units of B and D were about It was confirmed that it was introduced in a 1:9 ratio. In addition, the molecular weight in terms of styrene was measured to be 24,000. In addition, even though the cage-type structure was introduced, the molecular weight distribution of the single cage-type material was not found in the GPC form of the polymer, so it was confirmed that the cage structure was well introduced into the polymer chain through a continuous reaction.
1H-NMR (CDCl3) δ 7.5, 7.2, 3.7, 3.4, 3.3(broad), 3.1, 2.8, 2.6, 1.5(broad), 0.6. 29Si-NMR (CDCl3) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -82.5(broad)
1 H-NMR (CDCl 3 ) δ 7.5, 7.2, 3.7, 3.4, 3.3 (broad), 3.1, 2.8, 2.6, 1.5 (broad), 0.6. 29 Si-NMR (CDCl 3 ) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -82.5(broad)
[실시예 5-b] B 구조내 X도입 (B,D 구조의 도입)[Example 5-b] Introduction of X in structure B (introduction of structures B, D)
상기 실시예 5-a에서 얻어진 결과물의 유기층을 별도의 정제 없이 준비한 후, 3관능 단량체를 이용해서 말단을 cage구조로 변환하였다. 실시예 5-a에서 얻어진 물질 100 중량부를 50 중량부의 테트라하이드로퓨란에 녹인 후, 5 중량부의 증류수를 넣어 혼합용액을 제조하였다. 이후 제조된 혼합용액에 0.36 wt% HCl 10 중량부를 첨가하고 10분간 교반 후, Methyltrimethoxysilane 3 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하였다. 24시간 교반 후 실시예 4-a에서 제조된 촉매를 3 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, B 구조의 X 부분에 cage 형태의 고분자가 도입되며, 반응기 내에서 연속적으로 반응이 진행되어 화학식 5와 같은 고분자가 형성된다. 그러나, 다른 부산물들과 함께 얻어지므로, 별도의 정제가 필요하였다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여 정제를 준비하였다.
After the organic layer obtained in Example 5-a was prepared without further purification, the ends were converted into a cage structure using a trifunctional monomer. After dissolving 100 parts by weight of the material obtained in Example 5-a in 50 parts by weight of tetrahydrofuran, 5 parts by weight of distilled water was added to prepare a mixed solution. Then, 10 parts by weight of 0.36 wt% HCl was added to the prepared mixed solution, and after stirring for 10 minutes, 3 parts by weight of Methyltrimethoxysilane was added dropwise at once to achieve stable hydrolysis. After stirring for 24 hours, 3 parts by weight of the catalyst prepared in Example 4-a was added again to adjust the pH of the mixed solution to a basic state. At this time, a cage-type polymer is introduced into the X portion of the B structure, and the reaction proceeds continuously in the reactor to form a polymer as shown in Chemical Formula 5. However, since it was obtained together with other by-products, separate purification was required. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed in a vacuum to prepare a tablet.
[실시예 5-c] 침전 및 재결정을 통한 부산물 제거, 결과물의 수득[Example 5-c] Removal of by-products through precipitation and recrystallization, obtaining the result
상기 실시예 5-b에서 반응이 완료된 혼합물에 메틸렌크로라이드 200 중량부를 넣어, 증류수와 함께 분별 세척하고, 증류수 층의 pH가 중성을 나타낼 때, 진공감압으로 용매를 완전히 제거하였다. 이후, 메탄올에 2회 침전하여, 미반응 단량체를 제거하고 테트라하이드로퓨란과 수용액이 9.5:0.5 중량비율로 혼합된 용매에 30 중량부로 녹여 -20 ℃의 온도에서 2일간 보관하였다. 이는 고분자에 도입되지 못하고, cage구조로 닫혀 버린 물질의 재결정을 도모하여, 정제가 쉽게 이루어지도록 하기 위함이다. In Example 5-b, 200 parts by weight of methylene chloride was put into the mixture in which the reaction was completed, fractional washing was performed with distilled water, and when the pH of the distilled water layer was neutral, the solvent was completely removed by vacuum reduction. Thereafter, it was precipitated twice in methanol to remove unreacted monomers, and dissolved in 30 parts by weight in a solvent in which tetrahydrofuran and aqueous solution were mixed in a 9.5:0.5 weight ratio, and stored at a temperature of -20 °C for 2 days. This is to promote recrystallization of the material that cannot be introduced into the polymer and is closed in a cage structure, so that purification can be performed easily.
재결정 과정을 마치고 얻어진 고체물질들을 필터 후, 진공감압을 통해 화학식 5의 고분자가 여러 부산물 없이 얻어짐을 확인하였다. 또한, GPC 결과를 NMR 결과와 비교할 때, 각 단계의 고분자 성장에서 단독으로 얻어지는 저분자가 없이 Sharp한 형태의 Cage 형태가 결과로 도출되는 것으로 미루어 보아, 복합 고분자가 문제없이 얻어질 수 있음을 확인할 수 있었다. 이때, 분자량은 스티렌환산 값으로 16,000의 값을 얻을 수 있었으며, X의 n 값은 4-6이었으며, Y의 n 값은 4-6이었으며, 특히 화학식 5의 결과는 다음과 같다. After filtering the solid materials obtained after the recrystallization process, it was confirmed that the polymer of Chemical Formula 5 was obtained without various by-products through vacuum pressure reduction. In addition, when comparing the GPC result with the NMR result, it can be confirmed that a complex polymer can be obtained without problems, considering that a sharp cage form is derived as a result without a small molecule obtained alone in each stage of polymer growth. there was. At this time, the molecular weight was 16,000 in terms of styrene, and the n value of X was 4-6, and the n value of Y was 4-6. In particular, the result of Chemical Formula 5 is as follows.
29Si-NMR (CDCl3) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -81.5(sharp), -82.5(broad)
29 Si-NMR (CDCl 3 ) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -81.5(sharp), -82.5(broad)
또한, 하기 표 23에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자 및 코팅 조성물을 제조하였다. 이때 제조 방법은 상기 실시예 5에서 사용한 방법을 대등하게 적용하였다.In addition, silsesquioxane composite polymers and coating compositions were prepared by applying the monomers described in Table 23 below. At this time, the manufacturing method was equally applied to the method used in Example 5.
방법practice
Way
적용 단량체4-b method
Applicable monomer
적용 단량체4-a method
Applicable monomer
적용 단량체5-b method
Applicable monomer
(Mw)Molecular Weight
(Mw)
실시예 6Example 6 : 실세스퀴옥산 E-A-B-D 구조 복합 고분자의 합성: Synthesis of silsesquioxane E-A-B-D structure composite polymer
E-A-B-D구조의 복합 고분자를 제조하기 위하여 아래의 방법을 이용하였으며, 상기 실시예 1과 대등한 방법으로 코팅 조성물을 제조하였다.
The following method was used to prepare a composite polymer having an EABD structure, and a coating composition was prepared in the same manner as in Example 1.
[실시예 6-a] 사슬 말단 E구조의 생성[Example 6-a] Generation of chain terminal E structure
실시예 4-c 에서 얻어진 혼합물에 별도의 정제 없이 메틸렌크로라이드 20 중량부를 적가하고, 0.36 중량% HCl 수용액을 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 dimethyltetramethoxysilane 1 중량부를 한번에 적가하였다. 이때, 아직 분자구조 내에서 가수분해되지 않고 존재하던 부분들이 용매와 분리된 산성 수용액 층에서 가수분해물로 쉽게 변환되며, 생성된 별도의 반응물과 유기용매 층에서 축합되어 말단단위에 E가 도입되었다. 5시간의 교반 후, 반응의 교반을 정지하고 상온으로 반응기의 온도를 조절 하였다.
20 parts by weight of methylene chloride was added dropwise to the mixture obtained in Example 4-c without separate purification, and 5 parts by weight of a 0.36% by weight aqueous HCl solution was added dropwise, the pH was adjusted to have acidity, and the temperature was 4° C. for 30 minutes. stirred. Then, 1 part by weight of dimethyltetramethoxysilane was added dropwise at a time. At this time, the parts that have not yet been hydrolyzed in the molecular structure are easily converted into hydrolysates in the acidic aqueous solution layer separated from the solvent, and are condensed with the generated separate reactants in the organic solvent layer to introduce E into the terminal unit. After stirring for 5 hours, the stirring of the reaction was stopped and the temperature of the reactor was adjusted to room temperature.
[실시예 6-b] B구조 및 말단 E 구조의 X에 cage 도입[Example 6-b] Introduction of cage into structure B and structure X at the end E
상기 실시예 6-a에서 얻어진 결과물의 유기층을 별도의 정제 없이 준비한 후, 3관능 단량체를 이용해서 말단을 cage구조로 변환하였다. 반응이 진행 중인 실시예 6-a 혼합용액에 Methyltrimethoxysilane 3 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하고, 24시간 교반 후 실시예 1-a에서 제조된 촉매를 3 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, E 구조 말단에 cage 형태의 고분자가 도입되며, 반응기 내에서 연속적으로 반응이 진행되어 화학식 6과 같은 고분자가 형성된다. 그러나, 다른 부산물들과 함께 얻어지므로, 별도의 정제가 필요하였다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여 정제를 준비하였다.
The organic layer obtained in Example 6-a was prepared without further purification, and the ends were converted into a cage structure using a trifunctional monomer. 3 parts by weight of Methyltrimethoxysilane was added dropwise to the mixed solution of Example 6-a in which the reaction is in progress, to achieve stable hydrolysis, and after stirring for 24 hours, 3 parts by weight of the catalyst prepared in Example 1-a was added again to give a basic state to adjust the pH of the mixed solution. At this time, a cage-type polymer is introduced at the end of the E structure, and the reaction proceeds continuously in the reactor to form the polymer shown in Chemical Formula 6. However, since it was obtained together with other by-products, separate purification was required. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed in a vacuum to prepare a tablet.
[실시예 6-c] 침전 및 재결정을 통한 부산물 제거, 결과물의 수득[Example 6-c] Removal of by-products through precipitation and recrystallization, obtaining the result
상기 실시예 6-b에서 반응이 완료된 혼합물을 얻어낸 후, 증류수를 이용하여 세척하고, 증류수 층의 pH가 중성을 나타낼 때, 진공감압으로 용매를 완전히 제거하였다. 이후, 메탄올에 2회 침전하여, 미반응 단량체를 제거하고 테트라하이드로퓨란과 수용액이 9.5:0.5 중량비율로 혼합된 용매에 30 중량부로 녹여 -20 ℃의 온도에서 2일간 보관하였다. 이는 고분자에 도입되지 못하고, cage구조로 닫혀 버린 물질의 재결정을 도모하여, 정제가 쉽게 이루어지도록 하기 위함이다. After obtaining the mixture in which the reaction was completed in Example 6-b, it was washed with distilled water, and when the pH of the distilled water layer was neutral, the solvent was completely removed under reduced pressure. Then, it was precipitated twice in methanol to remove unreacted monomers, and dissolved in 30 parts by weight in a solvent in which tetrahydrofuran and aqueous solution were mixed in a 9.5:0.5 weight ratio, and stored at a temperature of -20 °C for 2 days. This is to facilitate recrystallization of the material that cannot be introduced into the polymer and is closed in a cage structure.
재결정 과정을 마치고 얻어진 고체물질들을 필터 후, 진공감압을 통해 화학식 6의 고분자를 여러 부산물과 함께 얻어짐을 확인하였다. 또한, GPC 결과를 NMR 결과와 비교할 때, 각 단계의 고분자 성장에서 단독으로 얻어지는 저분자가 없이 Sharp한 형태의 Cage 형태가 결과로 도출되는 것으로 미루어 보아, 복합 고분자가 문제없이 얻어질 수 있음을 확인할 수 있었다. 이때, 분자량은 스티렌환산 값으로 21,000의 값을 얻을 수 있었으며, X의 n 값은 4-6이었으며, Y의 n 값은 4-6이었으며, 특히 화학식 6의 결과는 다음과 같다. After filtering the solid materials obtained after the recrystallization process, it was confirmed that the polymer of Formula 6 was obtained along with various by-products through vacuum pressure reduction. In addition, when comparing the GPC result with the NMR result, it can be confirmed that a complex polymer can be obtained without problems, considering that a sharp cage form is derived as a result without a small molecule obtained alone in each stage of polymer growth. there was. In this case, the molecular weight was 21,000 in terms of styrene, and the n value of X was 4-6, and the n value of Y was 4-6. In particular, the result of Chemical Formula 6 is as follows.
29Si-NMR (CDCl3) δ -68.2, -71.8(sharp). -72.3(broad), -81.1(sharp), -80.8(sharp), -82.5(broad)
29 Si-NMR (CDCl 3 ) δ -68.2, -71.8 (sharp). -72.3(broad), -81.1(sharp), -80.8(sharp), -82.5(broad)
또한, 하기 표 24에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자를 제조하였다. 이때 제조 방법은 상기 실시예 6에서 사용한 방법을 대등하게 적용하였다.In addition, a silsesquioxane composite polymer was prepared by applying the monomers described in Table 24 below. At this time, the manufacturing method was equally applied to the method used in Example 6.
방법practice
Way
적용 단량체4-b method
Applicable monomer
적용 단량체4-c method
Applicable monomer
방법
적용 단량체6-a
Way
Applicable monomer
방법
적용단량체6-b
Way
Applicable monomer
실시예 7Example 7 : 실세스퀴옥산 A-B-A-D 구조 복합 고분자의 합성: Synthesis of silsesquioxane A-B-A-D structure composite polymer
합성단계는 아래와 같이, 연속가수분해 및 축합을 단계적으로 진행하였으며, 상기 실시예 1과 대등한 방법으로 코팅 조성물을 제조하였다.
As for the synthesis step, continuous hydrolysis and condensation were carried out step by step as follows, and a coating composition was prepared in a method equivalent to that of Example 1.
[실시예 7-a] 촉매의 제조[Example 7-a] Preparation of catalyst
염기도 조절을 위하여, Tetramethylammonium hydroxide (TMAH) 25 중량% 수용액에 10 중량% Potassium hydroxide (KOH) 수용액을 혼합하여 촉매 1a를 준비하였다.
For basicity control, catalyst 1a was prepared by mixing 10 wt% potassium hydroxide (KOH) aqueous solution with 25 wt% tetramethylammonium hydroxide (TMAH) aqueous solution.
[실시예 7-b] 선형 실세스퀴옥산 합성 (A 전구체)[Example 7-b] Linear silsesquioxane synthesis (A precursor)
냉각관과 교반기를 구비한 건조된 플라스크에, 증류수 5 중량부, 테트라하이드로퓨란 15 중량부, 상기 실시예 7-a에서 제조된 촉매 1 중량부를 적가하고, 1시간 동안 상온에서 교반 한 후, 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane 20 중량부를 적가하고, 다시 테트라하이드로류란을 15 중량부 적가하여 5시간 추가 교반 하였다. 교반 중의 혼합용액을 적취하여, 두 차례 세정하는 것으로 촉매와 불순물을 제거하고 필터 한 후, IR 분석을 통하여 말단기에 생성된 SI-OH 관능기를 확인할 수 있었으며(3200 cm-1), 분자량을 측정한 결과, 선형구조의 실세스퀴옥산이 6,000 스티렌 환산 분자량을 가짐을 확인할 수 있었다.
5 parts by weight of distilled water, 15 parts by weight of tetrahydrofuran, and 1 part by weight of the catalyst prepared in Example 7-a were added dropwise to a dried flask equipped with a cooling tube and a stirrer, and after stirring at room temperature for 1 hour, 2 20 parts by weight of -(3,4-epoxycyclohexyl)ethyltrimethoxysilane was added dropwise, and then 15 parts by weight of tetrahydrourean was added dropwise, followed by further stirring for 5 hours. After removing the catalyst and impurities by collecting the mixed solution during stirring, washing twice, and filtering, the SI-OH functional group generated at the terminal group was confirmed through IR analysis (3200 cm -1 ), and the molecular weight was measured. As a result, it was confirmed that silsesquioxane having a linear structure had a molecular weight equivalent to 6,000 styrene.
[실시예 7-c] 선형 실세스퀴옥산 구조의 합성 (A-B전구체의 합성)[Example 7-c] Synthesis of linear silsesquioxane structure (Synthesis of A-B precursor)
냉각관과 교반기를 구비한 건조된 플라스크에, 증류수 5 중량부, 테트라하이드로퓨란 40 중량부, 상기 실시예 7-a에서 제조된 촉매 0.5 중량부를 적가하고, 1시간 동안 상온에서 교반 한 후, 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane 10 중량부를 적가하고, 다시 테트라하이드로류란을 20 중량부 적가하여 2시간 추가 교반 하였다. 교반 중의 혼합용액을 적취하여, 두 차례 세정하는 것으로 촉매와 불순물을 제거하고 필터 한 후, 1H-NMR 분석을 통하여 잔존하는 alkoxy group이 0.1 mmol/g 이하로 잔존하고 있는 선형 실세스퀴옥산을 얻어 내었고, 이는 이후 cage를 연속반응으로 도입하는데 이용되는 부분이다. 선형 구조의 형태 분석은 XRD 분석을 통해 전체적인 구조가 선형구조체임을 확인하였다. 분자량을 측정한 결과, 선형구조의 실세스퀴옥산이 8,000 스티렌 환산 분자량을 가짐을 확인할 수 있었다.
5 parts by weight of distilled water, 40 parts by weight of tetrahydrofuran, and 0.5 parts by weight of the catalyst prepared in Example 7-a were added dropwise to a dried flask equipped with a cooling tube and a stirrer, and after stirring at room temperature for 1 hour, 2 10 parts by weight of -(3,4-epoxycyclohexyl)ethyltrimethoxysilane was added dropwise, and 20 parts by weight of tetrahydrolurane was added dropwise again, followed by stirring for an additional 2 hours. After removing the catalyst and impurities and filtering the mixed solution while stirring, washing twice to remove linear silsesquioxane in which the residual alkoxy group remains at 0.1 mmol/g or less through 1 H-NMR analysis obtained, which is then used to introduce the cage as a continuous reaction. The morphological analysis of the linear structure confirmed that the overall structure was a linear structure through XRD analysis. As a result of measuring the molecular weight, it was confirmed that the silsesquioxane having a linear structure had a molecular weight in terms of 8,000 styrene.
[실시예 7-d] 선형 실세스퀴옥산 구조의 합성 (A-B-A전구체의 합성)[Example 7-d] Synthesis of linear silsesquioxane structure (Synthesis of A-B-A precursor)
냉각관과 교반기를 구비한 건조된 플라스크에, 증류수 5 중량부, 테트라하이드로퓨란 5 중량부, 제조된 실시예 7-a 촉매를 10 중량부를 적가하고, 1시간 동안 상온에서 교반 한 후, 실시예 7-b 전구체와 7-c 전구체를 20 중량부씩 각각 적가하고, 다시 테트라하이드로류란을 10 중량부 적가하여 24시간 추가 교반 하였다. 교반 중의 혼합용액을 적취하여, 두 차례 세정하는 것으로 촉매와 불순물을 제거하고 필터 한 후, IR 분석을 통하여 말단기에 생성된 SI-OH 관능기를 확인할 수 있었으며(3200 cm-1), 분자량을 측정한 결과, 선형구조의 실세스퀴옥산이 15,000 스티렌 환산 분자량을 가짐을 확인할 수 있었다.5 parts by weight of distilled water, 5 parts by weight of tetrahydrofuran, and 10 parts by weight of the prepared Example 7-a catalyst were added dropwise to a dried flask equipped with a cooling tube and a stirrer, and after stirring at room temperature for 1 hour, Example 20 parts by weight of the 7-b precursor and the 7-c precursor were added dropwise, respectively, and then 10 parts by weight of tetrahydroleulan was added dropwise, followed by further stirring for 24 hours. After removing the catalyst and impurities by collecting the mixed solution during stirring, washing twice, and filtering, the SI-OH functional group generated at the terminal group was confirmed through IR analysis (3200 cm -1 ), and the molecular weight was measured. As a result, it was confirmed that silsesquioxane having a linear structure had a molecular weight equivalent to 15,000 styrene.
1H-NMR (CDCl3) δ 3.7, 3.4, 3.3(broad), 3.1, 2.8, 2.6, 1.5(broad), 0.6.
1 H-NMR (CDCl 3 ) δ 3.7, 3.4, 3.3 (broad), 3.1, 2.8, 2.6, 1.5 (broad), 0.6.
[실시예 7-e] 연속적 cage 구조의 생성 (D 구조의 도입)[Example 7-e] Creation of continuous cage structure (introduction of D structure)
상기 실시예 7-d 혼합용액에 0.36 중량% HCl 수용액을 매우 천천히 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 Diphenyltetramethoxydisiloxane 5 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하고, 1시간 교반 후 실시예 7-a에서 제조된 촉매를 7 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, 선형고분자와는 별도로 alkoxy가 열려있는 D구조의 전구체가 형성된다. 소량의 샘플을 적취하여, H-NMR과 IR로 분석하여 methoxy의 잔존율을 확인한 후, 잔존율이 10% 일 때, 0.36 중량% HCl 수용액을 10 중량부 천천히 적가하여, pH를 산성으로 조절해 주었다. 이후 Phenyltrimethoxysilane 1 중량부를 한번에 적가하여 15분간 교반 후, 1-a에서 제조된 촉매 20 중량부를 첨가하였다. 4시간의 혼합교반 이후, 확인결과 고분자내에 cage 형태의 고분자가 생성됨을 확인 할 수 있었다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여, 전체적인 반응물이 수용액 혼합물로 변환되도록 하였다. 4시간의 혼합 교반 이후, 일부를 적취하여 29Si-NMR을 통해 분석한 결과 phenyl기를 이용해 도입된 구조의 분석피크가 날카로운 형태의 2개로 나타나고 별도로 잔존하는 부산물 없이 화학식 7과 같은 고분자가 제조되었음을 확인할 수 있었다. 또한 스티렌 환산 분자량은 18,000으로 측정되었다.5 parts by weight of 0.36 wt% HCl aqueous solution was added dropwise very slowly to the mixed solution of Example 7-d, the pH was adjusted to have acidity, and the mixture was stirred at a temperature of 4 °C for 30 minutes. Then, 5 parts by weight of diphenyltetramethoxydisiloxane was added dropwise at once to achieve stable hydrolysis, and after stirring for 1 hour, 7 parts by weight of the catalyst prepared in Example 7-a was added again to adjust the pH of the mixed solution to a basic state. At this time, a precursor of the D structure in which the alkoxy is open is formed separately from the linear polymer. After taking a small sample and analyzing it with H-NMR and IR to check the residual ratio of methoxy, when the residual ratio is 10%, 10 parts by weight of 0.36 wt% HCl aqueous solution is slowly added dropwise to adjust the pH to acid. gave. Then, 1 part by weight of Phenyltrimethoxysilane was added dropwise at a time, stirred for 15 minutes, and then 20 parts by weight of the catalyst prepared in 1-a was added. After 4 hours of mixing and stirring, it was confirmed that a cage-type polymer was produced in the polymer. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed by vacuum, so that the entire reactant was converted into an aqueous mixture. After 4 hours of mixing and stirring, a portion was taken and analyzed through 29 Si-NMR. As a result, the analysis peaks of the structure introduced using a phenyl group appeared as two sharp shapes, and it was confirmed that a polymer such as Formula 7 was prepared without separately remaining by-products. could In addition, the molecular weight in terms of styrene was measured to be 18,000.
29Si-NMR (CDCl3) δ -68.2, -72.3(broad), -81.1(sharp), -80.8(sharp), -82.5(broad)
29 Si-NMR (CDCl 3 ) δ -68.2, -72.3 (broad), -81.1 (sharp), -80.8 (sharp), -82.5 (broad)
[실시예 7-f] B 구조내 X도입 (A-B-A-D구조의 완성)[Example 7-f] Introduction of X in structure B (Completion of structure A-B-A-D)
상기 실시예 7-e에서 얻어진 결과물의 유기층을 별도의 정제 없이 준비한 후, 3관능 단량체를 이용해서 말단을 cage구조로 변환하였다. 실시예 7-e에서 얻어진 물질 100 중량부를 50 중량부의 테트라하이드로퓨란에 녹인후, 5 중량부의 증류수를 넣어 혼합용액을 제조하였다. 이후 제조된 혼합용액에 0.36 wt% HCl 10 중량부를 첨가하고 10분간 교반 후, Methyltrimethoxysilane 3 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하였다. 24시간 교반 후 실시예 7-a에서 제조된 촉매를 3 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, B 구조의 X 부분에 cage 형태의 고분자가 도입되며, 반응기 내에서 연속적으로 반응이 진행되어 화학식 7과 같은 고분자가 형성된다. 그러나, 다른 부산물들과 함께 얻어지므로, 별도의 정제가 필요하였다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여 정제를 준비하였다.
The organic layer of the resultant obtained in Example 7-e was prepared without further purification, and the ends were converted into a cage structure using a trifunctional monomer. After dissolving 100 parts by weight of the material obtained in Example 7-e in 50 parts by weight of tetrahydrofuran, 5 parts by weight of distilled water was added to prepare a mixed solution. Then, 10 parts by weight of 0.36 wt% HCl was added to the prepared mixed solution, and after stirring for 10 minutes, 3 parts by weight of Methyltrimethoxysilane was added dropwise at once to achieve stable hydrolysis. After stirring for 24 hours, 3 parts by weight of the catalyst prepared in Example 7-a was added again to adjust the pH of the mixed solution to a basic state. At this time, a cage-type polymer is introduced into the X portion of the B structure, and the reaction proceeds continuously in the reactor to form a polymer as shown in Chemical Formula 7. However, since it was obtained together with other by-products, separate purification was required. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed in a vacuum to prepare a tablet.
[실시예 7-g] 침전 및 재결정을 통한 부산물 제거, 결과물의 수득[Example 7-g] Removal of by-products through precipitation and recrystallization, obtaining the result
상기 실시예 7-f에서 반응이 완료된 혼합물에 메틸렌크로라이드 200 중량부를 넣어, 증류수와 함께 분별 세척하고, 증류수 층의 pH가 중성을 나타낼 때, 진공감압으로 용매를 완전히 제거하였다. 이후, 메탄올에 2회 침전하여, 미반응 단량체를 제거하고 테트라하이드로퓨란과 수용액이 9.5:0.5 중량비율로 혼합된 용매에 30 중량부로 녹여 -20 ℃의 온도에서 2일간 보관하였다. 이는 고분자에 도입되지 못하고, cage구조로 닫혀 버린 물질의 재결정을 도모하여, 정제가 쉽게 이루어지도록 하기 위함이다. In Example 7-f, 200 parts by weight of methylene chloride was added to the reaction mixture, followed by fractional washing with distilled water, and when the pH of the distilled water layer was neutral, the solvent was completely removed by vacuum. Thereafter, by precipitation twice in methanol, unreacted monomers were removed, and 30 parts by weight was dissolved in a solvent in which tetrahydrofuran and aqueous solution were mixed at a weight ratio of 9.5:0.5 and stored at a temperature of -20 °C for 2 days. This is to facilitate recrystallization of the material that cannot be introduced into the polymer and is closed in a cage structure, so that purification can be performed easily.
재결정 과정을 마치고 얻어진 고체물질들을 필터 후, 진공감압을 통해 화학식 7의 고분자가 여러 부산물 없이 얻어짐을 확인하였다. 또한, GPC 결과를 NMR 결과와 비교할 때, 각 단계의 고분자 성장에서 단독으로 얻어지는 저분자가 없이 Sharp한 형태의 Cage 형태가 결과로 도출되는 것으로 미루어 보아, 복합 고분자가 문제없이 얻어질 수 있음을 확인할 수 있었다. 이때, 분자량은 스티렌환산 값으로 24,000의 값이었으며, X의 n 값은 4-6이었으며, Y의 n 값은 4-6이었다.
After filtering the solid materials obtained after the recrystallization process, it was confirmed that the polymer of Chemical Formula 7 was obtained without various by-products through vacuum reduction. In addition, when comparing the GPC result with the NMR result, it can be confirmed that a complex polymer can be obtained without problems, considering that a sharp cage form is derived as a result without a small molecule obtained alone in each stage of polymer growth. there was. At this time, the molecular weight was a value of 24,000 in terms of styrene, the n value of X was 4-6, and the n value of Y was 4-6.
또한, 하기 표 25에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자를 제조하였다. 이때 제조 방법은 상기 실시예 7에서 사용한 방법을 대등하게 적용하였다.In addition, a silsesquioxane composite polymer was prepared by applying the monomers described in Table 25 below. At this time, the manufacturing method was equally applied to the method used in Example 7.
방법practice
Way
적용 단량체7-b,c method
Applicable monomer
적용 단량체7-e method
Applicable monomer
적용 단량체7-f method
Applicable monomer
(Mw)Molecular Weight
(Mw)
실시예 8Example 8 : D-A-B-A-D 구조 복합 실세스퀴옥산 고분자의 합성: Synthesis of D-A-B-A-D structure complex silsesquioxane polymer
D-A-B-D구조의 복합 고분자를 제조하기 위하여 아래의 실시예를 이용하였으며, 상기 실시예 1과 대등한 방법으로 코팅 조성물을 제조하였다.
The following example was used to prepare a composite polymer having a DABD structure, and a coating composition was prepared in the same manner as in Example 1.
[실시예 8-a] D구조의 과량 생성을 위한 pH 변환 반응 [Example 8-a] pH conversion reaction for excessive production of structure D
반응이 진행 중인 실시예 7-d 혼합용액에 0.36 wt% HCl 수용액을 매우 천천히 15 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 Diphenyltetramethoxydisiloxane의 양을 실시예 7-e의 5배인 25 중량부로 준비하여 한번에 적가하고, 1시간 교반 후 실시예 7-a에서 제조된 촉매를 20 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 반응 완료 후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여, 전체적인 반응물이 수용액 혼합물로 변환되도록 하였다. 4시간의 혼합교반 이후, 일부를 적취하여 29Si-NMR 및 1H-NMR 을 통해 분석한 결과 B 구조내에 존재하는 alkoxy group의 양이 0.006 mmol/g으로 변화되고 B 와 D의 반복단위가 약 5:5 비율로 도입되었음을 확인할 수 있었다. 또한 스티렌 환산 분자량은 32,000으로 측정되었다. 또한, cage형 구조가 도입되었음에도, 고분자의 GPC 형태에서 단독 cage형 물질의 분자량 분포를 찾아볼 수 없으므로, cage구조가 연속반응을 통해 고분자 사슬에 잘 도입되었음을 확인할 수 있었다.15 parts by weight of 0.36 wt% HCl aqueous solution was very slowly added dropwise to the mixed solution of Example 7-d in which the reaction was in progress, the pH was adjusted to have acidity, and the mixture was stirred at a temperature of 4° C. for 30 minutes. Then, the amount of diphenyltetramethoxydisiloxane was prepared in an amount of 25 parts by weight, which is 5 times that of Example 7-e, and added dropwise at a time, and after stirring for 1 hour, 20 parts by weight of the catalyst prepared in Example 7-a was added again by adding 20 parts by weight of the catalyst to a basic state. was regulated After completion of the reaction, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed by vacuum, so that the entire reactant was converted into an aqueous mixture. After 4 hours of mixing and stirring, a portion was taken and analyzed through 29 Si-NMR and 1 H-NMR. As a result, the amount of alkoxy group present in structure B was changed to 0.006 mmol/g, and the repeating units of B and D were about It was confirmed that it was introduced in a ratio of 5:5. In addition, the molecular weight in terms of styrene was measured to be 32,000. In addition, even though the cage-type structure was introduced, the molecular weight distribution of the single cage-type material was not found in the GPC form of the polymer, so it was confirmed that the cage structure was well introduced into the polymer chain through a continuous reaction.
1H-NMR (CDCl3) δ 7.5, 7.2, 3.7, 3.4, 3.3(broad), 3.1, 2.8, 2.6, 1.5(broad), 0.6. 29Si-NMR (CDCl3) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -82.5(broad)
1 H-NMR (CDCl 3 ) δ 7.5, 7.2, 3.7, 3.4, 3.3 (broad), 3.1, 2.8, 2.6, 1.5 (broad), 0.6. 29 Si-NMR (CDCl 3 ) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -82.5(broad)
[실시예 8-b] B 구조내 X도입[Example 8-b] Introduction of X in structure B
상기 실시예 8-a에서 얻어진 결과물의 유기층을 별도의 정제 없이 준비한 후, 3관능 단량체를 이용해서 말단을 cage구조로 변환하였다. 실시예 8-a에서 얻어진 물질 100 중량부를 50 중량부의 테트라하이드로퓨란에 녹인 후, 5 중량부의 증류수를 넣어 혼합용액을 제조하였다. 이후 제조된 혼합용액에 0.36 wt% HCl 10 중량부를 첨가하고 10분간 교반 후, Methyltrimethoxysilane 3 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하였다. 24시간 교반 후 실시예 7-a에서 제조된 촉매를 3 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, B 구조의 X 부분에 cage 형태의 고분자가 도입되며, 반응기 내에서 연속적으로 반응이 진행되어 화학식 8와 같은 고분자가 형성된다. 그러나, 다른 부산물들과 함께 얻어지므로, 별도의 정제가 필요하였다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여 정제를 준비하였다.
After the organic layer obtained in Example 8-a was prepared without further purification, the ends were converted into a cage structure using a trifunctional monomer. After dissolving 100 parts by weight of the material obtained in Example 8-a in 50 parts by weight of tetrahydrofuran, 5 parts by weight of distilled water was added to prepare a mixed solution. Then, 10 parts by weight of 0.36 wt% HCl was added to the prepared mixed solution, and after stirring for 10 minutes, 3 parts by weight of Methyltrimethoxysilane was added dropwise at once to achieve stable hydrolysis. After stirring for 24 hours, 3 parts by weight of the catalyst prepared in Example 7-a was added again to adjust the pH of the mixed solution to a basic state. At this time, a cage-type polymer is introduced into the X portion of the B structure, and the reaction proceeds continuously in the reactor to form a polymer as shown in Chemical Formula 8. However, since it was obtained together with other by-products, separate purification was required. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed in a vacuum to prepare a tablet.
[실시예 8-c] 침전 및 재결정을 통한 부산물 제거, 결과물의 수득[Example 8-c] Removal of by-products through precipitation and recrystallization, obtaining the result
상기 실시예 8-b에서 반응이 완료된 혼합물에 메틸렌크로라이드 200 중량부를 넣어, 증류수와 함께 분별 세척하고, 증류수 층의 pH가 중성을 나타낼 때, 진공감압으로 용매를 완전히 제거하였다. 이후, 메탄올에 2회 침전하여, 미반응 단량체를 제거하고 테트라하이드로퓨란과 수용액이 9.5:0.5 중량비율로 혼합된 용매에 30 중량부로 녹여 -20 ℃의 온도에서 2일간 보관하였다. 이는 고분자에 도입되지 못하고, cage구조로 닫혀 버린 물질의 재결정을 도모하여, 정제가 쉽게 이루어지도록 하기 위함이다. In Example 8-b, 200 parts by weight of methylene chloride was added to the reaction mixture, followed by fractional washing with distilled water, and when the pH of the distilled water layer was neutral, the solvent was completely removed by vacuum. Thereafter, by precipitation twice in methanol, unreacted monomers were removed, and 30 parts by weight was dissolved in a solvent in which tetrahydrofuran and aqueous solution were mixed at a weight ratio of 9.5:0.5 and stored at a temperature of -20 °C for 2 days. This is to facilitate recrystallization of the material that cannot be introduced into the polymer and is closed in a cage structure, so that purification can be performed easily.
재결정 과정을 마치고 얻어진 고체물질들을 필터 후, 진공감압을 통해 화학식 1의 고분자가 여러 부산물 없이 얻어짐을 확인하였다. 또한, GPC 결과를 NMR 결과와 비교할 때, 각 단계의 고분자 성장에서 단독으로 얻어지는 저분자가 없이 Sharp한 형태의 Cage 형태가 결과로 도출되는 것으로 미루어 보아, 복합 고분자가 문제없이 얻어질 수 있음을 확인할 수 있었다. 이때, 분자량은 스티렌환산 값으로 36,000의 값을 얻을 수 있었으며, X의 n 값은 4-6이었으며, Y의 n 값은 4-6이었으며, 특히 화학식 8의 결과는 다음과 같다. After filtering the solid materials obtained after the recrystallization process, it was confirmed that the polymer of Formula 1 was obtained without various by-products through vacuum pressure reduction. In addition, when comparing the GPC result with the NMR result, it can be confirmed that a complex polymer can be obtained without problems, considering that a sharp cage form is derived as a result without a small molecule obtained alone in each stage of polymer growth. there was. At this time, the molecular weight was 36,000 in terms of styrene, the n value of X was 4-6, and the n value of Y was 4-6. In particular, the result of Chemical Formula 8 is as follows.
29Si-NMR (CDCl3) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -81.5(sharp), -82.5(broad)
29 Si-NMR (CDCl 3 ) δ -72.5(broad), -81.1(sharp), -80.8(sharp), -79.9(sharp), -81.5(sharp), -82.5(broad)
또한, 하기 표 26에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자 및 코팅 조성물을 제조하였다. 이때 제조 방법은 상기 실시예 8에서 사용한 방법을 대등하게 적용하였다.In addition, silsesquioxane composite polymers and coating compositions were prepared by applying the monomers described in Table 26 below. At this time, the manufacturing method was equally applied to the method used in Example 8.
방법practice
Way
적용 단량체7-b,c method
Applicable monomer
적용 단량체8-a method
Applicable monomer
적용 단량체8-b method
Applicable monomer
(Mw)Molecular Weight
(Mw)
실시예 9Example 9 : 실세스퀴옥산 E-A-B-A-D 구조 복합 고분자의 합성: Synthesis of silsesquioxane E-A-B-A-D structure composite polymer
E-A-B-A-D구조의 복합 고분자를 제조하기 위하여 아래의 실시예를 이용하였으며, 상기 실시예 1과 대등한 방법으로 코팅 조성물을 제조하였다.
The following example was used to prepare a composite polymer having an EABAD structure, and a coating composition was prepared in the same manner as in Example 1.
[실시예 9-a] 사슬 말단 E구조의 생성[Example 9-a] Generation of chain terminal E structure
실시예 7-g 에서 얻어진 혼합물에 별도의 정제 없이 메틸렌크로라이드 20 중량부를 적가하고, 0.36 중량% HCl 수용액을 5 중량부 적가하고, pH가 산성을 가지도록 조절하였으며, 4 ℃의 온도에서 30분간 교반하였다. 이후 dimethyltetramethoxysilane 1 중량부를 한번에 적가하였다. 이때, 아직 분자구조 내에서 가수분해되지 않고 존재하던 부분들이 용매와 분리된 산성 수용액 층에서 가수분해물로 쉽게 변환되며, 생성된 별도의 반응물과 유기용매 층에서 축합되어 말단단위에 E가 도입되었다. 5시간의 교반 후, 반응의 교반을 정지하고 상온으로 반응기의 온도를 조절 하였다.
20 parts by weight of methylene chloride was added dropwise to the mixture obtained in Example 7-g without separate purification, and 5 parts by weight of a 0.36% by weight aqueous HCl solution was added dropwise, and the pH was adjusted to have acidity, and the pH was adjusted to have acidity, and at a temperature of 4° C. for 30 minutes. stirred. Then, 1 part by weight of dimethyltetramethoxysilane was added dropwise at a time. At this time, the parts that have not yet been hydrolyzed in the molecular structure are easily converted into hydrolysates in the acidic aqueous solution layer separated from the solvent, and are condensed with the generated separate reactants in the organic solvent layer to introduce E into the terminal unit. After stirring for 5 hours, the stirring of the reaction was stopped and the temperature of the reactor was adjusted to room temperature.
[실시예 9-b] B구조 및 말단 E 구조의 X에 cage 도입[Example 9-b] cage introduction into structure B and structure X at the end E
상기 실시예 9-a에서 얻어진 결과물의 유기층을 별도의 정제 없이 준비한 후, 3관능 단량체를 이용해서 말단을 cage구조로 변환하였다. 반응이 진행 중인 실시예 9-a 혼합용액에 Methyltrimethoxysilane 3 중량부를 한번에 적가하여, 안정적인 가수분해를 도모하고, 24시간 교반 후 실시예 7-a에서 제조된 촉매를 3 중량부 다시 첨가해 주어 염기성 상태로 혼합용액의 pH를 조절해 주었다. 이때, E 구조 말단에 cage 형태의 고분자가 도입되며, 반응기 내에서 연속적으로 반응이 진행되어 화학식 9과 같은 고분자가 형성된다. 그러나, 다른 부산물들과 함께 얻어지므로, 별도의 정제가 필요하였다. 이후, 상온으로 온도를 변화시키고, 혼합용액 내 테트라하이드로퓨란을 진공으로 제거하여 정제를 준비하였다.
The organic layer of the resultant obtained in Example 9-a was prepared without further purification, and the ends were converted into a cage structure using a trifunctional monomer. 3 parts by weight of Methyltrimethoxysilane was added dropwise to the mixed solution of Example 9-a in which the reaction is in progress, to achieve stable hydrolysis, and after stirring for 24 hours, 3 parts by weight of the catalyst prepared in Example 7-a was added again to give basic state to adjust the pH of the mixed solution. At this time, a cage-type polymer is introduced at the end of the E structure, and the reaction proceeds continuously in the reactor to form a polymer shown in Chemical Formula 9. However, since it was obtained together with other by-products, separate purification was required. Thereafter, the temperature was changed to room temperature, and tetrahydrofuran in the mixed solution was removed in a vacuum to prepare a tablet.
[실시예 9-c] 침전 및 재결정을 통한 부산물 제거, 결과물의 수득[Example 9-c] Removal of by-products through precipitation and recrystallization, obtaining the result
상기 실시예 9-b에서 반응이 완료된 혼합물을 얻어낸 후, 증류수를 이용하여 세척하고, 증류수 층의 pH가 중성을 나타낼 때, 진공감압으로 용매를 완전히 제거하였다. 이후, 메탄올에 2회 침전하여, 미반응 단량체를 제거하고 테트라하이드로퓨란과 수용액이 9.5:0.5 중량비율로 혼합된 용매에 30 중량부로 녹여 -20 ℃의 온도에서 2일간 보관하였다. 이는 고분자에 도입되지 못하고, cage구조로 닫혀 버린 물질의 재결정을 도모하여, 정제가 쉽게 이루어지도록 하기 위함이다. After obtaining the mixture in which the reaction was completed in Example 9-b, the mixture was washed with distilled water, and when the pH of the distilled water layer was neutral, the solvent was completely removed under reduced pressure. Thereafter, by precipitation twice in methanol, unreacted monomers were removed, and 30 parts by weight was dissolved in a solvent in which tetrahydrofuran and aqueous solution were mixed at a weight ratio of 9.5:0.5 and stored at a temperature of -20 °C for 2 days. This is to promote recrystallization of the material that cannot be introduced into the polymer and is closed in a cage structure, so that purification can be performed easily.
재결정 과정을 마치고 얻어진 고체물질들을 필터 후, 진공감압을 통해 화학식 9의 고분자를 여러 부산물과 함께 얻어짐을 확인하였다. 또한, GPC 결과를 NMR 결과와 비교할 때, 각 단계의 고분자 성장에서 단독으로 얻어지는 저분자가 없이 Sharp한 형태의 Cage 형태가 결과로 도출되는 것으로 미루어 보아, 복합 고분자가 문제없이 얻어질 수 있음을 확인할 수 있었다. 이때, 분자량은 스티렌환산 값으로 28,000의 값을 얻을 수 있었으며, X의 n 값은 4-6이었으며, Y의 n 값은 4-6이었다.
After filtering the solid materials obtained after the recrystallization process, it was confirmed that the polymer of Formula 9 was obtained along with various by-products through vacuum pressure reduction. In addition, when comparing the GPC result with the NMR result, it can be confirmed that a complex polymer can be obtained without problems, considering that a sharp cage form is derived as a result without a small molecule obtained alone in each stage of polymer growth. there was. At this time, the molecular weight could obtain a value of 28,000 in terms of styrene, the n value of X was 4-6, and the n value of Y was 4-6.
또한, 하기 표 27에 기술한 단량체들을 적용하여 실세스퀴옥산 복합 고분자를 제조하였다. 이때 제조 방법은 상기 실시예 9에서 사용한 방법을 대등하게 적용하였다.In addition, a silsesquioxane composite polymer was prepared by applying the monomers described in Table 27 below. At this time, the manufacturing method was equally applied to the method used in Example 9.
방법practice
Way
적용 단량체7-b,c method
Applicable monomer
적용 단량체7-e method
Applicable monomer
방법
적용 단량체9-a
Way
Applicable monomer
방법
적용단량체9-b
Way
Applicable monomer
[실험] [Experiment]
PC(i-components사, Glastic 0.5T), PET(SKC, V5400), PMMA(EVONIK, Plexiglas OF058), PVC(신동아합성(주), 투명 PVC film 1 mm), PU(SONG-STOMER P-7100으로 제조된 필름)에 상기 실시예 1 내지 9에서 제조한 코팅 조성물을 코팅하고, 경화시켜 표면특성을 측정하였다.
PC (i-components, Glastic 0.5T), PET (SKC, V5400), PMMA (EVONIK, Plexiglas OF058), PVC (Shindong-A Synthetic Co., Ltd., transparent PVC film 1 mm), PU (SONG-STOMER P-7100) The coating composition prepared in Examples 1 to 9 was coated on a film prepared by
- 표면경도측정 : 일반적으로 연필경도법(JIS 5600-5-4)은 일반적으로 750 g 하중으로 평가하는데 이보다 가혹조건인 1 kgf 하중으로 코팅면에 45도 각도로 연필을 매초 0.5 mm의 속도로 수평으로 10 mm 이동해서 코팅막을 긁어서 긁힌 흔적으로 평가하였다. 5회 실험 중 3 mm 이상의 긁힌 흔적이 2회 이상 확인되지 않으면 상위 경도의 연필을 선택하여 평가하고, 긁힌 흔적이 2회 이상 확인되면 그 연필경도보다 한단 하위의 연필을 선택하여 해당 코팅막의 연필경도를 평가하여 하기 표 28 및 표 29에 나타내었다. 평가 결과는 10 um 이상의 코팅 두께에서 기판 종류에 상관없이 유리수준의 9H 경도를 확인하였다.- Surface hardness measurement : In general, the pencil hardness method (JIS 5600-5-4) is generally evaluated with a 750 g load. Under a harsher condition, a 1 kgf load, the pencil is moved horizontally at a speed of 0.5 mm per second at a 45 degree angle to the coated surface 10 mm It moved and scraped the coating film and evaluated it as a scratch mark. If scratch marks of 3 mm or more are not confirmed 2 or more times out of 5 experiments, select and evaluate a pencil of higher hardness. was evaluated and shown in Tables 28 and 29 below. As a result of the evaluation, glass-level 9H hardness was confirmed regardless of the substrate type at a coating thickness of 10 μm or more.
- 신뢰성 평가 : 85%, 85℃ 신뢰성 챔버에 240시간 보관하고 휨 특성 평가(YI(ASTMD1925))하였으며, 그 결과를 하기 표 30 및 표 31에 나타내었다. - Reliability evaluation: 85%, stored in a reliability chamber at 85°C for 240 hours, and evaluated for bending characteristics (YI (ASTMD1925)), and the results are shown in Tables 30 and 31 below.
휨평가 기준은 ± 0.1 mm이내: ◎, ± 0.2 mm이내: ○, -0.3 mm 이하 또는 0.3 mm 이상: X이다. 평가 결과는 모든 기재에 있어서 우수하였다.Warpage evaluation criteria are within ±0.1 mm: ◎, within ±0.2 mm: ○, -0.3 mm or less or 0.3 mm or more: X. The evaluation result was excellent in all the base materials.
YI(ASTMD1925)Example (coating thickness 10 um)
YI (ASTMD1925)
YI(ASTMD1925)Example (coating thickness 10 um)
YI (ASTMD1925)
- Scratch test 측정(JIS K5600-5-9): Steel wool에 의한 마모 평가법은 1kg 정도 무게의 쇠망치의 선단에 #0000의 Steel wool을 감아서 15회 왕복 시험편을 문지르고 그 헤이즈를 값을 측정하는데, 이번 평가에서는 이보다 가혹한 조건인 400회 시험편을 문지르고 헤이즈 측정 및 현미경으로 육안 평가 진행하였다. 결과는 헤이즈 증가가 0.05% 이상 증가할 경우 실패로 판단하였다. 코팅두께가 5 um 이상의 코팅에서는 표면에 발생되는 스크레치에 대한 내성이 우수한 것을 확인하였다.
- Scratch test measurement (JIS K5600-5-9): The method for evaluating abrasion by steel wool is to wind the steel wool of #0000 around the tip of a sledgehammer weighing about 1 kg, rub the reciprocating specimen 15 times, and measure the haze. , In this evaluation, the test piece, which is a harsher condition, was rubbed 400 times, and haze was measured and visual evaluation was performed with a microscope. As a result, it was judged as a failure when the haze increase increased by 0.05% or more. It was confirmed that the coating with a coating thickness of 5 μm or more had excellent resistance to scratches on the surface.
- 접착력 평가(JIS K5600-5-6) : 코팅막을 1-5 mm간격으로 컷터날로 긁어서 그 위에 셀로판테이프를 붙이고 붙인 테입을 잡아당겼을 때 이탈된 갯수로 접착성 판단하는데 이때 컷터날로 100개의 칸을 만들어 100개 중 떨어지는 개수로 접착성 판단 시행하였으며, 실시예 6의 광경화성 코팅 조성물에 대한 결과는 하기 표 32 및 표 33에 나타내었다. 표기는 100개중 떨어지지 않은 개수로 "(떨어지지 않은 개수/100)"로 표기 예제로 100개가 떨어지지 않으면 "(100/100)"로 표기 하였다. 접착성은 매우 우수한 것을 확인하였다. 표 32 및 표 33에 기재되지 않았지만 본 발명의 다른 실시예의 코팅 조성물들은 평가결과 접착성은 매우 우수한 것을 확인하였다. - Adhesion evaluation (JIS K5600-5-6): Scratch the coating film at intervals of 1-5 mm with a cutter blade and attach cellophane tape on it. Adhesiveness was judged by the number falling out of 100, and the results for the photocurable coating composition of Example 6 are shown in Tables 32 and 33 below. The notation is "(Number that does not fall/100)" as the number that does not fall out of 100. It was confirmed that the adhesiveness was very good. Although not described in Tables 32 and 33, it was confirmed that the coating compositions of other examples of the present invention had excellent adhesion as a result of evaluation.
(100/100)pass
(100/100)
(100/100)pass
(100/100)
(100/100)pass
(100/100)
(Steel wool, 1kgf하중,
400회)Scratch test
(Steel wool, 1 kg f load,
400 times)
(◎: 0.00 ~ 0.10
○ : 0.11~0.20,
X : 0.21 이상)Haze (%)
(◎: 0.00 to 0.10
○: 0.11 to 0.20,
X: 0.21 or more)
(100/100)pass
(100/100)
(100/100)pass
(100/100)
(Steel wool, 1kgf하중,
400회)Scratch test
(Steel wool, 1 kg f load,
400 times)
(◎: 0.00 ~ 0.10
○ : 0.11~0.20,
X : 0.21 이상)Haze (%)
(◎: 0.00 to 0.10
○: 0.11 to 0.20,
X: 0.21 or more)
상기 표 32 및 표 33에 나타난 바와 같이 본 발명의 플라스틱코팅조성물은 매우 우수한 표면경도를 보일 뿐만 아니라 기타 물성에 있어서도 동시에 우수함을 확인할 수 있다.As shown in Tables 32 and 33, it can be seen that the plastic coating composition of the present invention not only exhibits very excellent surface hardness, but is also excellent in other physical properties.
Claims (14)
[화학식 1]
[화학식 2]
[화학식 3]
[화학식 4]
[화학식 5]
[화학식 6]
[화학식 7]
[화학식 8]
[화학식 9]
상기 화학식 1 내지 9에서,
A는 이고, B는 이고, D는 이고, E는 이며,
Y는 각각 독립적으로 O, NR21 또는 [(SiO3/2R)4+2nO]이며, 적어도 하나는 [(SiO3/2R)4+2nO]이며,
X는 각각 독립적으로 R22 또는 [(SiO3/2R)4+2nR]이고, 적어도 하나는 [(SiO3/2R)4+2nR]이고,
R, R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, R22는 각각 독립적으로 수소; 중수소; 할로겐; 아민기; 에폭시기; 사이클로헥실에폭시기; (메타)아크릴기; 사이올기; 이소시아네이트기; 니트릴기; 니트로기; 페닐기; 중수소, 할로겐, 아민기, 에폭시기, (메타)아크릴기, 사이올기, 이소시아네이트기, 니트릴기, 니트로기, 페닐기로 치환되거나 치환되지 않은 C1~C40의 알킬기, C2~C40의 알케닐기, C1~C40의 알콕시기, C3~C40의 시클로알킬기, C3~C40의 헤테로시클로알킬기, C6~C40의 아릴기, C3~C40의 헤테로아릴기, C3~C40의 아르알킬기, C3~C40의 아릴옥시기, 또는 C3~C40의 아릴사이올기이며,
a 및 d는 각각 독립적으로 1 내지 100,000의 정수이고,
b는 각각 독립적으로 1 내지 500의 정수이며,
e는 각각 독립적으로 1 또는 2이며,
n은 각각 독립적으로 1 내지 20의 정수이다.A plastic coating method comprising coating and curing a plastic coating composition comprising a silsesquioxane composite polymer represented by any one of the following formulas 1 to 9 on a plastic surface:
[Formula 1]
[Formula 2]
[Formula 3]
[Formula 4]
[Formula 5]
[Formula 6]
[Formula 7]
[Formula 8]
[Formula 9]
In Formulas 1 to 9,
A is and B is and D is and E is is,
Y is each independently O, NR 21 or [(SiO 3/2 R) 4+2n O], at least one is [(SiO 3/2 R) 4+2n O],
each X is independently R 22 or [(SiO 3/2 R) 4+2n R], at least one is [(SiO 3/2 R) 4+2n R],
R, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , R 22 are each independently hydrogen; heavy hydrogen; halogen; amine group; epoxy group; cyclohexyl epoxy group; (meth)acryl group; thiol group; isocyanate group; nitrile group; nitro group; phenyl group; Deuterium, halogen, amine group, epoxy group, (meth)acrylic group, thiol group, isocyanate group, nitrile group, nitro group, phenyl group or unsubstituted C 1 ~ C 40 alkyl group, C 2 ~ C 40 alkenyl group , C 1 ~ C 40 Alkoxy group, C 3 ~ C 40 Cycloalkyl group, C 3 ~ C 40 Heterocycloalkyl group, C 6 ~ C 40 Aryl group, C 3 ~ C 40 Heteroaryl group, C 3 ~ C 40 aralkyl group, C 3 ~ C 40 aryloxy group, or C 3 ~ C 40 aryl thiol group,
a and d are each independently an integer from 1 to 100,000,
b is each independently an integer from 1 to 500,
e is each independently 1 or 2,
n is each independently an integer from 1 to 20;
상기 플라스틱은 폴리에틸렌(polyethylene, PE), 폴리프로필렌(polypropylene, PP), 폴리스타이렌(polystyrene, PS), 폴리에틸렌 테레프탈레이트(polyethylene terephthalate, PET, 페트), 폴리아미드(polyamides, PA, 나일론), 폴리에스터(polyester, PES), 폴리염화비닐(polyvinyl chloride, PVC), 폴리우레탄(polyurethanes, PU), 폴리카보네이트(polycarbonate, PC), 고경도 폴리카보네이트(고경도 PC), 폴리염화비닐리덴(polyvinylidene chloride, PVDC), 폴리테트라플루오로에틸렌(polytetrafluoroethylene, PTFE), 폴리에테르에테르케톤(polyetheretherketone, PEEK), 및 폴리에테르이미드(polyetherimide, PEI)로 이루어진 군으로부터 1종 이상 선택되는 것을 특징으로 하는 플라스틱코팅방법.According to claim 1,
The plastic is polyethylene (polyethylene, PE), polypropylene (polypropylene, PP), polystyrene (PS), polyethylene terephthalate (polyethylene terephthalate, PET, PET), polyamides (polyamides, PA, nylon), polyester ( polyester, PES), polyvinyl chloride (PVC), polyurethane (polyurethanes, PU), polycarbonate (PC), high-hardness polycarbonate (high-hardness PC), polyvinylidene chloride (polyvinylidene chloride, PVDC) ), polytetrafluoroethylene (PTFE), polyetheretherketone (polyetheretherketone, PEEK), and polyetherimide (polyetherimide, PEI), characterized in that at least one selected from the group consisting of plastic coating method.
상기 코팅 두께는 0.01 내지 500 um인 것을 특징으로 하는 플라스틱코팅방법.According to claim 1,
The coating thickness is a plastic coating method, characterized in that 0.01 to 500 um.
[화학식 1]
[화학식 2]
[화학식 3]
[화학식 4]
[화학식 5]
[화학식 6]
[화학식 7]
[화학식 8]
[화학식 9]
상기 화학식 1 내지 9에서,
A는 이고, B는 이고, D는 이고, E는 이며,
Y는 각각 독립적으로 O, NR21 또는 [(SiO3/2R)4+2nO]이며, 적어도 하나는 [(SiO3/2R)4+2nO]이며,
X는 각각 독립적으로 R22 또는 [(SiO3/2R)4+2nR]이고, 적어도 하나는 [(SiO3/2R)4+2nR]이고,
R, R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, R22는 각각 독립적으로 수소; 중수소; 할로겐; 아민기; 에폭시기; 사이클로헥실에폭시기; (메타)아크릴기; 사이올기; 이소시아네이트기; 니트릴기; 니트로기; 페닐기; 중수소, 할로겐, 아민기, 에폭시기, (메타)아크릴기, 사이올기, 이소시아네이트기, 니트릴기, 니트로기, 페닐기로 치환되거나 치환되지 않은 C1~C40의 알킬기, C2~C40의 알케닐기, C1~C40의 알콕시기, C3~C40의 시클로알킬기, C3~C40의 헤테로시클로알킬기, C6~C40의 아릴기, C3~C40의 헤테로아릴기, C3~C40의 아르알킬기, C3~C40의 아릴옥시기, 또는 C3~C40의 아릴사이올기이며,
a 및 d는 각각 독립적으로 1 내지 100,000의 정수이고,
b는 각각 독립적으로 1 내지 500의 정수이며,
e는 각각 독립적으로 1 또는 2이며,
n은 각각 독립적으로 1 내지 20의 정수이다.A plastic coating composition comprising a silsesquioxane composite polymer represented by any one of the following Chemical Formulas 1 to 9:
[Formula 1]
[Formula 2]
[Formula 3]
[Formula 4]
[Formula 5]
[Formula 6]
[Formula 7]
[Formula 8]
[Formula 9]
In Formulas 1 to 9,
A is and B is and D is and E is is,
Y is each independently O, NR 21 or [(SiO 3/2 R) 4+2n O], at least one is [(SiO 3/2 R) 4+2n O],
each X is independently R 22 or [(SiO 3/2 R) 4+2n R], at least one is [(SiO 3/2 R) 4+2n R],
R, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , R 22 are each independently hydrogen; heavy hydrogen; halogen; amine group; epoxy group; cyclohexyl epoxy group; (meth)acryl group; thiol group; isocyanate group; nitrile group; nitro group; phenyl group; Deuterium, halogen, amine group, epoxy group, (meth)acrylic group, thiol group, isocyanate group, nitrile group, nitro group, phenyl group or unsubstituted C 1 ~ C 40 alkyl group, C 2 ~ C 40 alkenyl group , C 1 ~ C 40 Alkoxy group, C 3 ~ C 40 Cycloalkyl group, C 3 ~ C 40 Heterocycloalkyl group, C 6 ~ C 40 Aryl group, C 3 ~ C 40 Heteroaryl group, C 3 ~ C 40 aralkyl group, C 3 ~ C 40 aryloxy group, or C 3 ~ C 40 aryl thiol group,
a and d are each independently an integer from 1 to 100,000,
b is each independently an integer from 1 to 500,
e is each independently 1 or 2,
n is each independently an integer from 1 to 20;
a는 3 내지 1000이고, b는 1 내지 500, d는 1 내지 500인 것을 특징으로 하는 플라스틱코팅조성물.5. The method of claim 4,
a is 3 to 1000, b is 1 to 500, d is 1 to 500 plastic coating composition.
n 값의 평균이 4 내지 5인 것을 특징으로 하는 플라스틱코팅조성물.5. The method of claim 4,
A plastic coating composition, characterized in that the average of n values is 4 to 5.
상기 실세스퀴옥산 복합 고분자의 중량평균분자량이 1,000 내지 1,000,000인 것을 특징으로 하는 플라스틱코팅조성물.5. The method of claim 4,
The plastic coating composition, characterized in that the weight average molecular weight of the silsesquioxane composite polymer is 1,000 to 1,000,000.
상기 코팅 조성물은 무용제 타입인 것을 특징으로 하는 플라스틱코팅조성물.5. The method of claim 4,
The coating composition is a plastic coating composition, characterized in that the solvent-free type.
상기 코팅조성물은
상기 실세스퀴옥산 복합 고분자;
개시제; 및
유기용매;
를 포함하는 것을 특징으로 플라스틱코팅조성물.5. The method of claim 4,
The coating composition is
the silsesquioxane composite polymer;
initiator; and
organic solvents;
A plastic coating composition comprising a.
상기 코팅조성물이 안료를 더욱 포함하는 것을 특징으로 하는 플라스틱코팅조성물.5. The method of claim 4,
The plastic coating composition, characterized in that the coating composition further comprises a pigment.
[화학식 1]
[화학식 2]
[화학식 3]
[화학식 4]
[화학식 5]
[화학식 6]
[화학식 7]
[화학식 8]
[화학식 9]
상기 화학식 1 내지 9에서,
A는 이고, B는 이고, D는 이고, E는 이며,
Y는 각각 독립적으로 O, NR21 또는 [(SiO3/2R)4+2nO]이며, 적어도 하나는 [(SiO3/2R)4+2nO]이며,
X는 각각 독립적으로 R22 또는 [(SiO3/2R)4+2nR]이고, 적어도 하나는 [(SiO3/2R)4+2nR]이고,
R, R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, R22는 각각 독립적으로 수소; 중수소; 할로겐; 아민기; 에폭시기; 사이클로헥실에폭시기; (메타)아크릴기; 사이올기; 이소시아네이트기; 니트릴기; 니트로기; 페닐기; 중수소, 할로겐, 아민기, 에폭시기, (메타)아크릴기, 사이올기, 이소시아네이트기, 니트릴기, 니트로기, 페닐기로 치환되거나 치환되지 않은 C1~C40의 알킬기, C2~C40의 알케닐기, C1~C40의 알콕시기, C3~C40의 시클로알킬기, C3~C40의 헤테로시클로알킬기, C6~C40의 아릴기, C3~C40의 헤테로아릴기, C3~C40의 아르알킬기, C3~C40의 아릴옥시기, 또는 C3~C40의 아릴사이올기이며,
a 및 d는 각각 독립적으로 1 내지 100,000의 정수이고,
b는 각각 독립적으로 1 내지 500의 정수이며,
e는 각각 독립적으로 1 또는 2이며,
n은 각각 독립적으로 1 내지 20의 정수이다.A silsesquioxane composite polymer coated plastic comprising a cured product coated with a plastic coating composition containing a silsesquioxane composite polymer represented by any one of the following Chemical Formulas 1 to 9 on the surface thereof:
[Formula 1]
[Formula 2]
[Formula 3]
[Formula 4]
[Formula 5]
[Formula 6]
[Formula 7]
[Formula 8]
[Formula 9]
In Formulas 1 to 9,
A is and B is and D is and E is is,
Y is each independently O, NR 21 or [(SiO 3/2 R) 4+2n O], at least one is [(SiO 3/2 R) 4+2n O],
each X is independently R 22 or [(SiO 3/2 R) 4+2n R], at least one is [(SiO 3/2 R) 4+2n R],
R, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 , R 18 , R 19 , R 20 , R 21 , R 22 are each independently hydrogen; heavy hydrogen; halogen; amine group; epoxy group; cyclohexyl epoxy group; (meth)acryl group; thiol group; isocyanate group; nitrile group; nitro group; phenyl group; Deuterium, halogen, amine group, epoxy group, (meth)acrylic group, thiol group, isocyanate group, nitrile group, nitro group, phenyl group or unsubstituted C 1 ~ C 40 alkyl group, C 2 ~ C 40 alkenyl group , C 1 ~ C 40 Alkoxy group, C 3 ~ C 40 Cycloalkyl group, C 3 ~ C 40 Heterocycloalkyl group, C 6 ~ C 40 Aryl group, C 3 ~ C 40 Heteroaryl group, C 3 ~ C 40 aralkyl group, C 3 ~ C 40 aryloxy group, or C 3 ~ C 40 aryl thiol group,
a and d are each independently an integer from 1 to 100,000,
b is each independently an integer from 1 to 500,
e is each independently 1 or 2,
n is each independently an integer from 1 to 20;
상기 실세스퀴옥산 복합 고분자 코팅 플라스틱은 제1항 기재의 플라스틱코팅방법에 의하여 형성된 것을 특징으로 하는 실세스퀴옥산 복합 고분자 코팅 플라스틱.12. The method of claim 11,
The silsesquioxane composite polymer coated plastic is a silsesquioxane composite polymer coated plastic, characterized in that it is formed by the plastic coating method of claim 1.
상기 물품은 광학필름, 보호필름, 전자제품 구성용 플라스틱, 안경, 건축외장제, 건축내장제, 플라스틱 배관, 전선피복제, 광학렌즈, 방음벽, 플라스틱 간판, 플라스틱 조형물, 가구, 조명, 썬루프, 또는 헬멧인 것을 특징으로 물품.14. The method of claim 13,
The above articles are optical films, protective films, plastics for electronic products, glasses, building exterior materials, building interior materials, plastic piping, electric wire coverings, optical lenses, soundproof walls, plastic signs, plastic sculptures, furniture, lighting, sunroofs, or An article characterized in that it is a helmet.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/KR2015/002217 WO2015133873A1 (en) | 2014-03-07 | 2015-03-06 | Method for coating plastic using silsesquioxane composite polymer |
| TW104107436A TWI669351B (en) | 2014-03-07 | 2015-03-09 | A coating method for plastic using silsesquioxane composite polymer |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20140027159 | 2014-03-07 | ||
| KR1020140027159 | 2014-03-07 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| KR20150105611A KR20150105611A (en) | 2015-09-17 |
| KR102414700B1 true KR102414700B1 (en) | 2022-06-29 |
| KR102414700B9 KR102414700B9 (en) | 2023-05-11 |
Family
ID=54244770
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020150031865A KR102414700B1 (en) | 2014-03-07 | 2015-03-06 | A coating method for plastic using silsesquioxane composite polymer |
Country Status (2)
| Country | Link |
|---|---|
| KR (1) | KR102414700B1 (en) |
| TW (1) | TWI669351B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024143871A1 (en) * | 2022-12-28 | 2024-07-04 | 주식회사 동진쎄미켐 | Composition for colored coating containing silsesquioxane polymer binder |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7311946B2 (en) * | 2016-06-06 | 2023-07-20 | エヌビーディー ナノテクノロジーズ, インコーポレイテッド | Fingerprint Invisibility Coating and Method of Forming The Same |
| KR20200082946A (en) * | 2018-12-31 | 2020-07-08 | 주식회사 동진쎄미켐 | Synthetic resin coating composition and method of manufacturing synthetic resin substrate using the same |
| KR20210111946A (en) * | 2020-03-03 | 2021-09-14 | 삼성디스플레이 주식회사 | Light emitting device |
| US20210341649A1 (en) * | 2020-04-29 | 2021-11-04 | Samsung Display Co., Ltd. | Anti-reflective film and display device including the same |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101067283B1 (en) | 2003-03-11 | 2011-09-23 | 제이엔씨 주식회사 | Polymer obtained with silsesquioxane derivative |
| WO2011118939A2 (en) | 2010-03-22 | 2011-09-29 | 주식회사 엘지화학 | Photocurable and thermocurable resin composition, and dry film solder resist |
| US20130072609A1 (en) | 2011-09-21 | 2013-03-21 | Government Of The United States As Represented By The Secretary Of The Air Force | Sythesis of functional fluorinated polyhedral oligomeric silsesquioxane (f-poss) |
| KR101249798B1 (en) | 2010-08-18 | 2013-04-03 | 한국과학기술연구원 | A Method for Preparing a Controlled Structure of Polysilsesquioxane and Polysilsesquioxane Prepared by the Same |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5425698B2 (en) * | 2010-04-27 | 2014-02-26 | 株式会社日本触媒 | Flexible film, metal foil having the same, and printed wiring board using the same |
| CN102241869B (en) * | 2010-05-14 | 2013-01-09 | 北京仁创科技集团有限公司 | Composition, formed body prepared therefrom and preparation method of formed body |
-
2015
- 2015-03-06 KR KR1020150031865A patent/KR102414700B1/en active IP Right Grant
- 2015-03-09 TW TW104107436A patent/TWI669351B/en active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101067283B1 (en) | 2003-03-11 | 2011-09-23 | 제이엔씨 주식회사 | Polymer obtained with silsesquioxane derivative |
| WO2011118939A2 (en) | 2010-03-22 | 2011-09-29 | 주식회사 엘지화학 | Photocurable and thermocurable resin composition, and dry film solder resist |
| KR101249798B1 (en) | 2010-08-18 | 2013-04-03 | 한국과학기술연구원 | A Method for Preparing a Controlled Structure of Polysilsesquioxane and Polysilsesquioxane Prepared by the Same |
| US20130072609A1 (en) | 2011-09-21 | 2013-03-21 | Government Of The United States As Represented By The Secretary Of The Air Force | Sythesis of functional fluorinated polyhedral oligomeric silsesquioxane (f-poss) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024143871A1 (en) * | 2022-12-28 | 2024-07-04 | 주식회사 동진쎄미켐 | Composition for colored coating containing silsesquioxane polymer binder |
Also Published As
| Publication number | Publication date |
|---|---|
| TWI669351B (en) | 2019-08-21 |
| KR102414700B9 (en) | 2023-05-11 |
| KR20150105611A (en) | 2015-09-17 |
| TW201542719A (en) | 2015-11-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102367173B1 (en) | A coating method for preventing warpage of substrate | |
| KR102708914B1 (en) | Laminate and producing method for it | |
| KR102325278B1 (en) | A thermoplastic resin composion comprising silsesquioxane composite polymer | |
| KR102414700B1 (en) | A coating method for plastic using silsesquioxane composite polymer | |
| KR102363819B1 (en) | Silsesquioxane composite polymer and method for manufacturing thereof | |
| KR102367120B1 (en) | A surface enhanced transparent substrate and method for manufacturing thereof | |
| KR102363818B1 (en) | Silsesquioxane composite polymer and method for manufacturing thereof | |
| CN106029747B (en) | Silsesquioxane composite polymer and method for producing same | |
| KR20150105606A (en) | A coating method for ceramics using silsesquioxane composite polymer | |
| KR20160092507A (en) | Coating Product and Hard Coating Method Having High Hardness | |
| KR102363820B1 (en) | Silsesquioxane composite polymer and method for manufacturing thereof | |
| KR20150105605A (en) | A coating method for metal using silsesquioxane composite polymer | |
| KR20150105608A (en) | A coating method for wood ana pulp using silsesquioxane composite polymer | |
| KR20150105604A (en) | A coating method for fiber using silsesquioxane composite polymer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| E902 | Notification of reason for refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant | ||
| G170 | Re-publication after modification of scope of protection [patent] |