KR102070316B1 - Composition for treating or preventing oral diseases comprising natural complex - Google Patents
Composition for treating or preventing oral diseases comprising natural complex Download PDFInfo
- Publication number
- KR102070316B1 KR102070316B1 KR1020160076736A KR20160076736A KR102070316B1 KR 102070316 B1 KR102070316 B1 KR 102070316B1 KR 1020160076736 A KR1020160076736 A KR 1020160076736A KR 20160076736 A KR20160076736 A KR 20160076736A KR 102070316 B1 KR102070316 B1 KR 102070316B1
- Authority
- KR
- South Korea
- Prior art keywords
- extract
- composition
- present
- clove
- oral
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 78
- 208000025157 Oral disease Diseases 0.000 title abstract description 25
- 208000030194 mouth disease Diseases 0.000 title abstract description 25
- 239000000284 extract Substances 0.000 claims abstract description 175
- 235000016639 Syzygium aromaticum Nutrition 0.000 claims abstract description 54
- 235000000604 Chrysanthemum parthenium Nutrition 0.000 claims abstract description 42
- 235000000802 Leonurus cardiaca ssp. villosus Nutrition 0.000 claims abstract description 42
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical group [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 claims abstract description 19
- 239000004480 active ingredient Substances 0.000 claims abstract description 19
- 229910052733 gallium Inorganic materials 0.000 claims abstract description 19
- 210000000214 mouth Anatomy 0.000 claims abstract description 11
- 244000061408 Eugenia caryophyllata Species 0.000 claims abstract 2
- 240000007890 Leonurus cardiaca Species 0.000 claims abstract 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 6
- 238000000034 method Methods 0.000 claims description 6
- 201000010099 disease Diseases 0.000 claims description 5
- 230000000694 effects Effects 0.000 abstract description 22
- 241000234282 Allium Species 0.000 abstract description 14
- 239000000126 substance Substances 0.000 abstract description 7
- 229930014626 natural product Natural products 0.000 abstract description 3
- 231100000331 toxic Toxicity 0.000 abstract description 2
- 230000002588 toxic effect Effects 0.000 abstract description 2
- 244000223014 Syzygium aromaticum Species 0.000 description 52
- 241000207925 Leonurus Species 0.000 description 40
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 26
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 21
- 230000005415 magnetization Effects 0.000 description 21
- -1 pH adjusters Substances 0.000 description 18
- 239000002904 solvent Substances 0.000 description 18
- 238000002360 preparation method Methods 0.000 description 17
- 239000000606 toothpaste Substances 0.000 description 17
- 229940034610 toothpaste Drugs 0.000 description 17
- 241000894006 Bacteria Species 0.000 description 15
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 15
- 238000009472 formulation Methods 0.000 description 15
- 206010006326 Breath odour Diseases 0.000 description 14
- 235000019441 ethanol Nutrition 0.000 description 14
- 230000002401 inhibitory effect Effects 0.000 description 14
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 13
- 230000000052 comparative effect Effects 0.000 description 12
- 208000002925 dental caries Diseases 0.000 description 11
- 238000000605 extraction Methods 0.000 description 11
- 208000007565 gingivitis Diseases 0.000 description 10
- 235000011187 glycerol Nutrition 0.000 description 10
- 230000002265 prevention Effects 0.000 description 10
- 208000002193 Pain Diseases 0.000 description 9
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 241000194017 Streptococcus Species 0.000 description 8
- 241000194019 Streptococcus mutans Species 0.000 description 8
- 235000013305 food Nutrition 0.000 description 8
- PUZPDOWCWNUUKD-UHFFFAOYSA-M sodium fluoride Chemical compound [F-].[Na+] PUZPDOWCWNUUKD-UHFFFAOYSA-M 0.000 description 8
- 235000013361 beverage Nutrition 0.000 description 7
- 235000014113 dietary fatty acids Nutrition 0.000 description 7
- 239000000194 fatty acid Substances 0.000 description 7
- 229930195729 fatty acid Natural products 0.000 description 7
- 230000036541 health Effects 0.000 description 7
- 235000013402 health food Nutrition 0.000 description 7
- 239000003755 preservative agent Substances 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 239000000725 suspension Substances 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 241000270728 Alligator Species 0.000 description 6
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 6
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 6
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- 239000002537 cosmetic Substances 0.000 description 6
- 210000004268 dentin Anatomy 0.000 description 6
- 230000006806 disease prevention Effects 0.000 description 6
- 235000003599 food sweetener Nutrition 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 239000000377 silicon dioxide Substances 0.000 description 6
- 230000000638 stimulation Effects 0.000 description 6
- 239000003765 sweetening agent Substances 0.000 description 6
- 208000004371 toothache Diseases 0.000 description 6
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 5
- 241000196324 Embryophyta Species 0.000 description 5
- 208000032843 Hemorrhage Diseases 0.000 description 5
- 206010061218 Inflammation Diseases 0.000 description 5
- 241000605894 Porphyromonas Species 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 5
- 230000000740 bleeding effect Effects 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 239000003085 diluting agent Substances 0.000 description 5
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 description 5
- 229960002986 dinoprostone Drugs 0.000 description 5
- 239000003814 drug Substances 0.000 description 5
- 239000000839 emulsion Substances 0.000 description 5
- 239000000796 flavoring agent Substances 0.000 description 5
- 239000004088 foaming agent Substances 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 208000028169 periodontal disease Diseases 0.000 description 5
- 239000008194 pharmaceutical composition Substances 0.000 description 5
- XEYBRNLFEZDVAW-UHFFFAOYSA-N prostaglandin E2 Natural products CCCCCC(O)C=CC1C(O)CC(=O)C1CC=CCCCC(O)=O XEYBRNLFEZDVAW-UHFFFAOYSA-N 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 239000000600 sorbitol Substances 0.000 description 5
- 235000010356 sorbitol Nutrition 0.000 description 5
- 239000004094 surface-active agent Substances 0.000 description 5
- GHXZTYHSJHQHIJ-UHFFFAOYSA-N Chlorhexidine Chemical compound C=1C=C(Cl)C=CC=1NC(N)=NC(N)=NCCCCCCN=C(N)N=C(N)NC1=CC=C(Cl)C=C1 GHXZTYHSJHQHIJ-UHFFFAOYSA-N 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 4
- 239000011230 binding agent Substances 0.000 description 4
- 229960003260 chlorhexidine Drugs 0.000 description 4
- 235000009508 confectionery Nutrition 0.000 description 4
- 239000006071 cream Substances 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 239000000469 ethanolic extract Substances 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 235000019204 saccharin Nutrition 0.000 description 4
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 4
- 229940081974 saccharin Drugs 0.000 description 4
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 4
- 239000011775 sodium fluoride Substances 0.000 description 4
- 235000013024 sodium fluoride Nutrition 0.000 description 4
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 235000015112 vegetable and seed oil Nutrition 0.000 description 4
- 239000000080 wetting agent Substances 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- 208000034619 Gingival inflammation Diseases 0.000 description 3
- 206010020751 Hypersensitivity Diseases 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241000605862 Porphyromonas gingivalis Species 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 3
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 150000001298 alcohols Chemical class 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 230000000844 anti-bacterial effect Effects 0.000 description 3
- 230000000845 anti-microbial effect Effects 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229910000019 calcium carbonate Inorganic materials 0.000 description 3
- 150000001720 carbohydrates Chemical class 0.000 description 3
- 235000014633 carbohydrates Nutrition 0.000 description 3
- 239000003086 colorant Substances 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 235000013355 food flavoring agent Nutrition 0.000 description 3
- 239000003205 fragrance Substances 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 239000002674 ointment Substances 0.000 description 3
- 239000013588 oral product Substances 0.000 description 3
- 239000003002 pH adjusting agent Substances 0.000 description 3
- 201000001245 periodontitis Diseases 0.000 description 3
- 150000002978 peroxides Chemical class 0.000 description 3
- 239000000419 plant extract Substances 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000000344 soap Substances 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 230000009885 systemic effect Effects 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 235000019640 taste Nutrition 0.000 description 3
- 239000002562 thickening agent Substances 0.000 description 3
- 239000008158 vegetable oil Substances 0.000 description 3
- 239000011782 vitamin Substances 0.000 description 3
- 229940088594 vitamin Drugs 0.000 description 3
- 235000013343 vitamin Nutrition 0.000 description 3
- 229930003231 vitamin Natural products 0.000 description 3
- 239000000230 xanthan gum Substances 0.000 description 3
- 229920001285 xanthan gum Polymers 0.000 description 3
- 235000010493 xanthan gum Nutrition 0.000 description 3
- 229940082509 xanthan gum Drugs 0.000 description 3
- CSAVDNHVPJNKTC-UHFFFAOYSA-N 5-methyl-2-propan-2-ylcyclohexan-1-ol;5-methyl-2-propan-2-ylphenol;2,2,4-trimethyl-3-oxabicyclo[2.2.2]octane Chemical compound CC(C)C1CCC(C)CC1O.CC(C)C1=CC=C(C)C=C1O.C1CC2CCC1(C)OC2(C)C CSAVDNHVPJNKTC-UHFFFAOYSA-N 0.000 description 2
- 108010011485 Aspartame Proteins 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- 239000004386 Erythritol Substances 0.000 description 2
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- 240000003271 Leonurus japonicus Species 0.000 description 2
- MJVAVZPDRWSRRC-UHFFFAOYSA-N Menadione Chemical compound C1=CC=C2C(=O)C(C)=CC(=O)C2=C1 MJVAVZPDRWSRRC-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- 241000589516 Pseudomonas Species 0.000 description 2
- 244000046146 Pueraria lobata Species 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 241000194023 Streptococcus sanguinis Species 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- 239000003945 anionic surfactant Substances 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 239000003125 aqueous solvent Substances 0.000 description 2
- 239000000605 aspartame Substances 0.000 description 2
- 235000010357 aspartame Nutrition 0.000 description 2
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 2
- 229960003438 aspartame Drugs 0.000 description 2
- 230000003385 bacteriostatic effect Effects 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 2
- 229920003123 carboxymethyl cellulose sodium Polymers 0.000 description 2
- 229940063834 carboxymethylcellulose sodium Drugs 0.000 description 2
- 239000004359 castor oil Substances 0.000 description 2
- 235000019438 castor oil Nutrition 0.000 description 2
- 239000004568 cement Substances 0.000 description 2
- 239000002738 chelating agent Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 210000003298 dental enamel Anatomy 0.000 description 2
- 210000004262 dental pulp cavity Anatomy 0.000 description 2
- 235000019700 dicalcium phosphate Nutrition 0.000 description 2
- 235000013399 edible fruits Nutrition 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 235000019414 erythritol Nutrition 0.000 description 2
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 2
- 229940009714 erythritol Drugs 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 235000019634 flavors Nutrition 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 235000015203 fruit juice Nutrition 0.000 description 2
- 229940029982 garlic powder Drugs 0.000 description 2
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 2
- 238000000227 grinding Methods 0.000 description 2
- 239000003906 humectant Substances 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 230000000622 irritating effect Effects 0.000 description 2
- 229940076522 listerine Drugs 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- 244000005700 microbiome Species 0.000 description 2
- 239000002736 nonionic surfactant Substances 0.000 description 2
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 2
- 239000004006 olive oil Substances 0.000 description 2
- 235000008390 olive oil Nutrition 0.000 description 2
- 229940023486 oral product Drugs 0.000 description 2
- 238000010422 painting Methods 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- 230000003239 periodontal effect Effects 0.000 description 2
- 239000002831 pharmacologic agent Substances 0.000 description 2
- 239000000049 pigment Substances 0.000 description 2
- 230000000607 poisoning effect Effects 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 230000002335 preservative effect Effects 0.000 description 2
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 239000001488 sodium phosphate Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 235000013599 spices Nutrition 0.000 description 2
- 238000001694 spray drying Methods 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 150000005846 sugar alcohols Polymers 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 235000013616 tea Nutrition 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 239000001993 wax Substances 0.000 description 2
- 239000000811 xylitol Substances 0.000 description 2
- 235000010447 xylitol Nutrition 0.000 description 2
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 2
- 229960002675 xylitol Drugs 0.000 description 2
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 2
- NOOLISFMXDJSKH-UTLUCORTSA-N (+)-Neomenthol Chemical compound CC(C)[C@@H]1CC[C@@H](C)C[C@@H]1O NOOLISFMXDJSKH-UTLUCORTSA-N 0.000 description 1
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 1
- CUNWUEBNSZSNRX-RKGWDQTMSA-N (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexol;(z)-octadec-9-enoic acid Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O CUNWUEBNSZSNRX-RKGWDQTMSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical class CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 1
- POAOYUHQDCAZBD-UHFFFAOYSA-N 2-butoxyethanol Chemical compound CCCCOCCO POAOYUHQDCAZBD-UHFFFAOYSA-N 0.000 description 1
- XGRSAFKZAGGXJV-UHFFFAOYSA-N 3-azaniumyl-3-cyclohexylpropanoate Chemical compound OC(=O)CC(N)C1CCCCC1 XGRSAFKZAGGXJV-UHFFFAOYSA-N 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical compound OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 241000606750 Actinobacillus Species 0.000 description 1
- 241000186046 Actinomyces Species 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- POJWUDADGALRAB-PVQJCKRUSA-N Allantoin Natural products NC(=O)N[C@@H]1NC(=O)NC1=O POJWUDADGALRAB-PVQJCKRUSA-N 0.000 description 1
- XOJVHLIYNSOZOO-SWOBOCGESA-N Arctiin Chemical compound C1=C(OC)C(OC)=CC=C1C[C@@H]1[C@@H](CC=2C=C(OC)C(O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O3)O)=CC=2)C(=O)OC1 XOJVHLIYNSOZOO-SWOBOCGESA-N 0.000 description 1
- 241000208843 Arctium Species 0.000 description 1
- 240000005528 Arctium lappa Species 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 241000208838 Asteraceae Species 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 206010007269 Carcinogenicity Diseases 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- ZAKOWWREFLAJOT-CEFNRUSXSA-N D-alpha-tocopherylacetate Chemical compound CC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C ZAKOWWREFLAJOT-CEFNRUSXSA-N 0.000 description 1
- NOOLISFMXDJSKH-UHFFFAOYSA-N DL-menthol Natural products CC(C)C1CCC(C)CC1O NOOLISFMXDJSKH-UHFFFAOYSA-N 0.000 description 1
- 208000002064 Dental Plaque Diseases 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 208000010201 Exanthema Diseases 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 206010018276 Gingival bleeding Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 238000003794 Gram staining Methods 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 208000035154 Hyperesthesia Diseases 0.000 description 1
- 241000207923 Lamiaceae Species 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- 235000002434 Leonurus sibiricus Nutrition 0.000 description 1
- 241001126925 Lobata Species 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 102000029749 Microtubule Human genes 0.000 description 1
- 108091022875 Microtubule Proteins 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 229920002230 Pectic acid Polymers 0.000 description 1
- 239000004264 Petrolatum Substances 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 241000219780 Pueraria Species 0.000 description 1
- 201000004328 Pulpitis Diseases 0.000 description 1
- 206010037464 Pulpitis dental Diseases 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 244000228451 Stevia rebaudiana Species 0.000 description 1
- 241000194024 Streptococcus salivarius Species 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 208000002697 Tooth Abrasion Diseases 0.000 description 1
- 208000008312 Tooth Loss Diseases 0.000 description 1
- 206010062544 Tooth fracture Diseases 0.000 description 1
- 241001302463 Viola mandshurica Species 0.000 description 1
- 244000137773 Viola philippica Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- YKTSYUJCYHOUJP-UHFFFAOYSA-N [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] Chemical compound [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] YKTSYUJCYHOUJP-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000013334 alcoholic beverage Nutrition 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- POJWUDADGALRAB-UHFFFAOYSA-N allantion Natural products NC(=O)NC1NC(=O)NC1=O POJWUDADGALRAB-UHFFFAOYSA-N 0.000 description 1
- 229960000458 allantoin Drugs 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- 239000010775 animal oil Substances 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 238000003287 bathing Methods 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960004365 benzoic acid Drugs 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 235000008429 bread Nutrition 0.000 description 1
- 230000001680 brushing effect Effects 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- JUNWLZAGQLJVLR-UHFFFAOYSA-J calcium diphosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])(=O)OP([O-])([O-])=O JUNWLZAGQLJVLR-UHFFFAOYSA-J 0.000 description 1
- 229940043256 calcium pyrophosphate Drugs 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229960001631 carbomer Drugs 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 235000014171 carbonated beverage Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 231100000260 carcinogenicity Toxicity 0.000 description 1
- 230000007670 carcinogenicity Effects 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 239000000679 carrageenan Substances 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 229940113118 carrageenan Drugs 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000001364 causal effect Effects 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 230000001055 chewing effect Effects 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 235000019219 chocolate Nutrition 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 235000008504 concentrate Nutrition 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 235000013365 dairy product Nutrition 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000037123 dental health Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 235000019821 dicalcium diphosphate Nutrition 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 235000019800 disodium phosphate Nutrition 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 238000004043 dyeing Methods 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000001700 effect on tissue Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 239000000686 essence Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 201000005884 exanthem Diseases 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000012041 food component Nutrition 0.000 description 1
- 239000005417 food ingredient Substances 0.000 description 1
- 239000010794 food waste Substances 0.000 description 1
- 210000003953 foreskin Anatomy 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 210000000232 gallbladder Anatomy 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 230000000762 glandular Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- BTIJJDXEELBZFS-QDUVMHSLSA-K hemin Chemical compound CC1=C(CCC(O)=O)C(C=C2C(CCC(O)=O)=C(C)\C(N2[Fe](Cl)N23)=C\4)=N\C1=C/C2=C(C)C(C=C)=C3\C=C/1C(C)=C(C=C)C/4=N\1 BTIJJDXEELBZFS-QDUVMHSLSA-K 0.000 description 1
- 229940025294 hemin Drugs 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 235000015243 ice cream Nutrition 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical class OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 235000021374 legumes Nutrition 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000002803 maceration Methods 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000002075 main ingredient Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- 239000001525 mentha piperita l. herb oil Substances 0.000 description 1
- 239000001683 mentha spicata herb oil Substances 0.000 description 1
- 229940041616 menthol Drugs 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 210000004688 microtubule Anatomy 0.000 description 1
- 238000000874 microwave-assisted extraction Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 239000005445 natural material Substances 0.000 description 1
- 235000021096 natural sweeteners Nutrition 0.000 description 1
- 231100000957 no side effect Toxicity 0.000 description 1
- 235000012149 noodles Nutrition 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 229940042125 oral ointment Drugs 0.000 description 1
- 239000000668 oral spray Substances 0.000 description 1
- 229940041678 oral spray Drugs 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- LCLHHZYHLXDRQG-ZNKJPWOQSA-N pectic acid Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)O[C@H](C(O)=O)[C@@H]1OC1[C@H](O)[C@@H](O)[C@@H](OC2[C@@H]([C@@H](O)[C@@H](O)[C@H](O2)C(O)=O)O)[C@@H](C(O)=O)O1 LCLHHZYHLXDRQG-ZNKJPWOQSA-N 0.000 description 1
- 235000019477 peppermint oil Nutrition 0.000 description 1
- 238000005325 percolation Methods 0.000 description 1
- 235000020030 perry Nutrition 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 229940066842 petrolatum Drugs 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 235000013550 pizza Nutrition 0.000 description 1
- 230000007505 plaque formation Effects 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 239000010318 polygalacturonic acid Substances 0.000 description 1
- 229920002503 polyoxyethylene-polyoxypropylene Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 239000003531 protein hydrolysate Substances 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 239000012264 purified product Substances 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- ZUFQODAHGAHPFQ-UHFFFAOYSA-N pyridoxine hydrochloride Chemical compound Cl.CC1=NC=C(CO)C(CO)=C1O ZUFQODAHGAHPFQ-UHFFFAOYSA-N 0.000 description 1
- 229960004172 pyridoxine hydrochloride Drugs 0.000 description 1
- 235000019171 pyridoxine hydrochloride Nutrition 0.000 description 1
- 239000011764 pyridoxine hydrochloride Substances 0.000 description 1
- 206010037844 rash Diseases 0.000 description 1
- HELXLJCILKEWJH-NCGAPWICSA-N rebaudioside A Chemical compound O([C@H]1[C@H](O)[C@@H](CO)O[C@H]([C@@H]1O[C@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)O[C@]12C(=C)C[C@@]3(C1)CC[C@@H]1[C@@](C)(CCC[C@]1([C@@H]3CC2)C)C(=O)O[C@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O HELXLJCILKEWJH-NCGAPWICSA-N 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 235000013580 sausages Nutrition 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 201000000980 schizophrenia Diseases 0.000 description 1
- 239000003352 sequestering agent Substances 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 235000011888 snacks Nutrition 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 229960003885 sodium benzoate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- AQMNWCRSESPIJM-UHFFFAOYSA-M sodium metaphosphate Chemical compound [Na+].[O-]P(=O)=O AQMNWCRSESPIJM-UHFFFAOYSA-M 0.000 description 1
- 229960004711 sodium monofluorophosphate Drugs 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 235000011008 sodium phosphates Nutrition 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 210000004872 soft tissue Anatomy 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 229960005078 sorbitan sesquioleate Drugs 0.000 description 1
- 229960002920 sorbitol Drugs 0.000 description 1
- 235000014347 soups Nutrition 0.000 description 1
- 235000019721 spearmint oil Nutrition 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 230000000475 sunscreen effect Effects 0.000 description 1
- 239000000516 sunscreening agent Substances 0.000 description 1
- 238000000194 supercritical-fluid extraction Methods 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- YUOWTJMRMWQJDA-UHFFFAOYSA-J tin(iv) fluoride Chemical compound [F-].[F-].[F-].[F-].[Sn+4] YUOWTJMRMWQJDA-UHFFFAOYSA-J 0.000 description 1
- 229940042585 tocopherol acetate Drugs 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 210000005239 tubule Anatomy 0.000 description 1
- 238000002137 ultrasound extraction Methods 0.000 description 1
- 239000002966 varnish Substances 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 230000017260 vegetative to reproductive phase transition of meristem Effects 0.000 description 1
- 235000012711 vitamin K3 Nutrition 0.000 description 1
- 239000011652 vitamin K3 Substances 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 229940041603 vitamin k 3 Drugs 0.000 description 1
- 239000000341 volatile oil Substances 0.000 description 1
- 230000002087 whitening effect Effects 0.000 description 1
- 239000011592 zinc chloride Substances 0.000 description 1
- 235000005074 zinc chloride Nutrition 0.000 description 1
- 229960001939 zinc chloride Drugs 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 1
- GFQYVLUOOAAOGM-UHFFFAOYSA-N zirconium(iv) silicate Chemical compound [Zr+4].[O-][Si]([O-])([O-])[O-] GFQYVLUOOAAOGM-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/61—Myrtaceae (Myrtle family), e.g. teatree or eucalyptus
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/28—Asteraceae or Compositae (Aster or Sunflower family), e.g. chamomile, feverfew, yarrow or echinacea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/48—Fabaceae or Leguminosae (Pea or Legume family); Caesalpiniaceae; Mimosaceae; Papilionaceae
- A61K36/488—Pueraria (kudzu)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/53—Lamiaceae or Labiatae (Mint family), e.g. thyme, rosemary or lavender
- A61K36/533—Leonurus (motherwort)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/86—Violaceae (Violet family)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9789—Magnoliopsida [dicotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q11/00—Preparations for care of the teeth, of the oral cavity or of dentures; Dentifrices, e.g. toothpastes; Mouth rinses
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/30—Foods, ingredients or supplements having a functional effect on health
- A23V2200/312—Foods, ingredients or supplements having a functional effect on health having an effect on dental health
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Life Sciences & Earth Sciences (AREA)
- Mycology (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Botany (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Medical Informatics (AREA)
- Alternative & Traditional Medicine (AREA)
- Birds (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Nutrition Science (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Cosmetics (AREA)
- Medicines Containing Plant Substances (AREA)
Abstract
본 발명은 구강용 조성물에 관한 것으로, 보다 상세하게는 구강질환 예방 또는 치료용 조성물에 관한 것이다. 본 발명의 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하는 조성물은 구강질환 예방 또는 치료 효과가 우수하다. 또한, 본 발명의 조성물은 천연물 유래의 물질이어서 독성이 없으며, 인체에 적용하여도 부작용을 유발하지 않아 활용도가 높다.The present invention relates to a composition for oral cavity, and more particularly to a composition for preventing or treating oral disease. Clove extract of the present invention; And a composition of gallium extract, allium extract, self-designated extract and motherwort extract as an active ingredient at least one selected from the group is excellent in preventing or treating oral diseases. In addition, since the composition of the present invention is a substance derived from natural products, it is not toxic and does not cause side effects even when applied to the human body, and thus has high utility.
Description
본 발명은 구강질환 예방 및/또는 치료용 조성물에 관한 것이다.The present invention relates to a composition for preventing and / or treating oral diseases.
치아건강의 개념이 사회생활개념으로 변화되면서 구강건강에 관한 관심이 증대되고 있으며 구강질환의 예방과 치료뿐만 아니라, 사회생활을 하는데 있어서 중요한 대인관계의 장애가 되는 구취에 대한 관심도 높아지고 있는 실정이다.As the concept of dental health is changed to the concept of social life, interest in oral health is increasing, and interest in bad breath, which is an important interpersonal disorder in social life, is increasing as well as prevention and treatment of oral diseases.
치아조직은 에나멜질, 시멘트질 및 상아질로 구성되어 있다. 외부에 노출되어 있는 치아의 바깥쪽은 에나멜질로 덮여 있고, 치조골 내에 위치하는 치아 뿌리 부분의 바깥쪽은 시멘트질로 덮여 있으며, 이들 내부에 상아질이 존재한다. 상아질에는 상아세관이라는 미세한 관이 상아질 전체에 걸쳐 분포하고 있다.Dental tissue is composed of enamel, cement and dentin. The outside of the teeth exposed to the outside is covered with enamel, the outside of the root of the teeth located in the alveolar bone is covered with cement, dentin is present inside them. In dentin, microtubules called dentin tubules are distributed throughout the dentin.
구강질환 중 치아우식과 치주질환은 통증, 저작기능 장애, 치주조직의 파괴, 구취 및 시린이와 같은 다양한 임상적인 증상을 유발하고 치아상실을 초래하는 주된 요인으로 알려져 있으며 식생활의 변화로 치아우식의 원인요소는 더 증가하고 있는 실정이다.Dental caries and periodontal disease are known to cause various clinical symptoms such as pain, masticatory dysfunction, periodontal tissue destruction, bad breath, and rash, and cause tooth loss. The causal factor is increasing.
치면세균막 내에 서식하면서 치아우식, 치주질환, 구취 및 시린이를 유발하는 구강 병원균 증식을 억제하는 방법으로, 이들 세균들에 대한 살균 및 정균작용을 갖는 항생제를 포함한 다양한 종류의 항균 제제가 충치, 치주질환, 치수 및 치근단 감염의 억제 및 치료제로써 개발되어 왔다.Inhibits the growth of oral pathogens that cause dental caries, periodontal disease, bad breath, and syring, while living in the dental plaque, and various types of antimicrobial agents, including antibiotics, have bactericidal and bacteriostatic effects on these bacteria. It has been developed as an inhibitor and treatment for disease, pulp and root canal infection.
하지만, 항생제는 우리몸에 대한 전신적인 부작용과 함께 구강내 내성균의 출현 및 균교대증을 유발할 수 있기 때문에 장기적인 사용이 곤란하여 단지 치료제로만 이용될 수 있는 단점이 있다. 또한, 구강 청정제에 사용되고 있는 항균제제로는 생귀나린(Sangquinarine), 리스테린(Listerine), 퍼옥사이드(Peroxide), 클로르헥시딘(Chlorhexidine) 등이 있는데, 생귀나린은 구강내에서 세균에 대한 효과가 불분명하고 치주질환시 치료효과에 대해서는 더욱 불분명할 뿐만 아니라 가격도 비싼 단점이 있고, 리스테린은 알코올이 주성분으로 실제 구강내에서는 일시적인 효과를 내지만 약간의 정균작용이 있으나 장기간 사용시 조직에 대해 위해작용도 나타날 수 있는 단점이 있으며, 최근 미백효과를 위해 첨가되고 있는 퍼옥사이드는 세균에 대한 독작용이 있으나 동시에 인체조직에도 독작용을 나타내어 안전성에 문제가 있을 뿐 아니라 간혹 세균에서 퍼옥사이드에 대한 내성균이 출현하기도 한다. 또한, 클로르헥시딘은 치태형성 억제와 더불어 치주질환 예방 및 치료제로써 현재까지 알려진 제제 중에서 가장 우수한 것으로 알려져 있으나, 조직에 대한 자극, 조직의 착색 및 변성, 특히 자극적인 맛이 강하고 냄새가 심한 부작용을 나타내는 문제점이 있을 뿐 아니라, 그 외도 균교대증이 유발될 수 있고, 발암성이 있어 임신부의 경우 사용이 제한되는 등 치료나 특히 예방의 목적으로 장기간 사용할 수 없는 단점이 있다.However, since antibiotics can cause the appearance and resistance to mycelial infection and systemic side effects with systemic side effects on our bodies, long-term use is difficult and can be used only as a therapeutic agent. In addition, antibacterial agents used in mouthwashes include Sangquinarine, Listerine, Peroxide, Chlorhexidine, etc. It is not clear about the treatment effect at the time, but it is also expensive. Listerine has alcohol as the main ingredient, but it has a temporary effect in the oral cavity but has a slight bacteriostatic effect. In addition, the peroxide that has been recently added for the whitening effect has a poisoning effect on bacteria, but at the same time has a poisoning effect on human tissues as well as a safety problem, and sometimes a bacterium resistant to peroxides appear in bacteria. In addition, chlorhexidine is known to be the best among the formulations known to date to prevent plaque formation and prevent and treat periodontal disease. However, chlorhexidine has a strong side effect on tissue irritation, staining and degeneration of tissues, especially irritating taste and odor. In addition, there are other disadvantages that can be caused by myocerosis, carcinogenicity is limited in the use of pregnant women and can not be used for a long time for the purpose of treatment or prevention.
이에 따라, 본 발명자들은 상기와 같은 부작용에 대한 우려가 없으면서도 구강질환, 즉 치통, 치아 우식증, 치은염, 치주염, 구취, 시린이 등의 예방 및/또는 치료에 효과가 뛰어난 천연 소재를 도출하기 위해 연구를 수행하였다.Accordingly, the inventors of the present invention provide a natural material that is excellent in the prevention and / or treatment of oral diseases, that is, toothache, dental caries, gingivitis, periodontitis, bad breath, schizophrenia and the like without fear of such side effects. The study was conducted.
본 발명이 해결하고자 하는 과제는 구강질환에 우수한 효과를 발휘하면서도 천연물로부터 유래되어 안정성이 확보되는 성분을 유효성분으로 포함하는 구강질환 예방 및/또는 치료용 조성물을 제공하는 것이다.The problem to be solved by the present invention is to provide a composition for the prevention and / or treatment of oral diseases, including as an active ingredient a component derived from natural products to ensure stability while showing an excellent effect on oral diseases.
상기 과제를 해결하기 위하여, 본 발명은 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하는 구강질환 예방 및/또는 치료용 조성물, 바람직하게는 구강용 조성물, 화장료 조성물, 약학 조성물 또는 건강식품 조성물을 제공한다.In order to solve the above problems, the present invention is a clove extract; And a gallium extract, allium extract, self-designated extract and motherwort extract, any one or more selected from the group consisting of oral disease prevention and / or treatment composition, preferably oral composition, cosmetic composition, pharmaceutical composition or Provide a health food composition.
즉, 본 발명은 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상의 추출물의 구강질환 예방 및/또는 치료용이라는 새로운 용도를 제공한다.That is, the present invention is a clove extract; And it provides a new use for the prevention and / or treatment of oral diseases of any one or more extracts selected from the group consisting of gallium extract, alligator extract, self-designated extract and motherwort extract.
정향(丁香, Syzygium aromaticum Merrill et Perry)은 열대 상록성 아교목 정향 나무의 꽃봉오리 부분을 말하며, 몰루카 제도가 원산지이며 탄자니아의 잔지바르 섬, 인도네시아의 수마트라, 브라질, 말레이시아, 필리핀, 베트남, 중국 남부 등에서 생산된다.Clove (丁香, Syzygium aromaticum Merrill et Perry) is a part of the bud of the tropical evergreen subtilis clove tree. It is native to the Molucca Islands and is produced in Zanzibar Island of Tanzania, Sumatra in Indonesia, Brazil, Malaysia, the Philippines, Vietnam, and South China.
갈화(葛花)는 콩과 식물인 칡(Pueraria lobata Ohwi, P. thunbergiana Bentham 또는 P. hirsuta Matsum)의 꽃을 말린 것이다.Gall flower is a legume, Pueraria. Dried flowers of lobata Ohwi, P. thunbergiana Bentham or P. hirsuta Matsum).
우방자(牛蒡子)는 국화과(Compositae) 식물인 우엉(Arctium lappa Linne)의 열매이며, 악실(惡實)이라고도 한다.The allies are the Arctium , a compositae plant. The fruit of lappa Linne), also referred to as aksil (惡實).
자화지정(紫花地丁)은 제비꽃과의 제비꽃(Viola mandshurica W.Becker) 또는 호제비꽃(Viola yedoensis Makino)의 전초를 말한다. 자화지정은 약간의 특이한 냄새가 있고, 자극성이 있으며 맛은 쓰고 매우며 성질은 차다.The designation of the flower (紫花地丁) refers to the outpost of the violet ( Viola mandshurica W.Becker) or the violet ( Viola yedoensis Makino). Magnetization has a slight peculiar smell, is irritating, tastes very bitter, and is cold.
익모초(益母草, Chinese motherwort)는 꿀풀과(Labiatae)의 익모초(Leonurus sibiricus L)의 꽃이 피었을 때의 지상부를 말린 것이다. 익모초는 약간 특이한 냄새가 있고 맛은 쓰고 매우며 수렴성이 있고 성질은 약간 차다.Chinese motherwort (말린 草) is the ground part of the flowering of Leonurus sibiricus L from the labiatae. Motherwort has a slightly peculiar smell, taste is bitter, very astringent, and slightly cold.
본 발명자들은 정향 추출물과 함께 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상의 추출물을 사용하는 경우 구강 질환에 우수한 효과를 가지며, 바람직하게 정향 추출물, 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물의 혼합 추출물은 가장 우수한 시너지적 생물학적 활성을 가짐을 발견하였다.The present inventors have an excellent effect on oral diseases when using any one or more extracts selected from the group consisting of clove extract, wooja extract, self-specified extract and motherwort extract together with the clove extract, preferably clove extract, hwahwa extract, wooja extract It was found that the mixed extracts of the designated extracts, the designated magnetization extract and motherwort extract had the best synergistic biological activity.
본 발명에 있어서, 용어 "추출물"은 상기 추출처리에 의하여 얻어지는 추출액, 상기 추출액의 희석액이나 농축액, 상기 추출액을 건조하여 얻어지는 건조물, 상기 추출액의 조정제물이나 정제물, 또는 이들의 혼합물 등, 추출액 자체 및 추출액을 이용하여 형성 가능한 모든 제형의 추출물을 포함한다.In the present invention, the term "extract" refers to the extract itself, such as an extract obtained by the extraction process, a diluent or concentrate of the extract, a dried product obtained by drying the extract, a crude or purified product of the extract, or a mixture thereof. And extracts of all formulations that can be formed using extracts.
상기 추출물에는 각각의 식물을 설명하며 특정된 부위 외에도 그의 잎, 줄기, 나무 껍질, 뿌리, 꽃 또는 꽃눈, 과실, 종자, 수액 및 전체 식물이 포함될 수 있다.The extract describes each plant and may include leaves, stems, bark, roots, flowers or flower buds, fruits, seeds, sap and whole plants in addition to the specified sites.
상기 추출물을 제조하기 위하여, 통상의 기술자는 당업계에 공지된 임의의 적합한 방법을 사용할 수 있다. 예를 들어, 증기 추출법 또는 용매 추출법에 의할 수 있다. 식물 전체 또는 임의의 부분을 분쇄한 다음(예를 들어, 블렌더), 추출 용매를 처리하여, 용매 추출물을 수득할 수 있다. 분쇄 전에 건조 과정(예를 들어, 40 내지 70℃에서 15 내지 50시간 동안의 건조)을 거친 다음 분쇄할 수도 있다. 또한, 용매 추출물은 감압 증류 및 동결건조 또는 분무 건조 등과 같은 추가적인 과정에 의해 분말 상태로 제조될 수 있다.To prepare the extract, the skilled person can use any suitable method known in the art. For example, it may be by a vapor extraction method or a solvent extraction method. The whole or any part of the plant may be ground (eg a blender) and then the extraction solvent may be treated to yield a solvent extract. The grinding may be followed by a drying process (for example, drying for 15 to 50 hours at 40 to 70 ° C.) and then grinding. In addition, the solvent extract may be prepared in powder form by additional processes such as distillation under reduced pressure and lyophilization or spray drying.
상기 사용되는 추출 용매의 종류는 특별히 제한되지 아니하며, 당해 기술 분야에서 공지된 임의의 용매를 사용할 수 있다. 상기 추출 용매의 비제한적인 예로는 물; 메탄올, 에탄올, 프로필알코올, 부틸알코올 등의 C1 내지 C4의 저급 알코올; 글리세린, 부틸렌글라이콜, 프로필렌글라이콜 등의 다가 알코올; 및 메틸아세테이트, 에틸아세테이트, 아세톤, 벤젠, 헥산, 디에틸에테르, 디클로로메탄 등의 탄화수소계 용매; 또는 이들의 혼합물을 사용할 수 있으며, 바람직하게, 물, 저급알코올을 단독으로 사용하거나 2종 이상 혼합하여 사용할 수 있다. 상기 용매를 사용하여 1회 이상 추출하여 용매 추출물을 제조할 수 있으며, 상기 용매 추출물을 감압 증류한 후 동결건조 또는 분무 건조하여 얻은 건조 추출물을 제조할 수 있다.The type of extraction solvent used is not particularly limited, and any solvent known in the art may be used. Non-limiting examples of such extraction solvents include water; C1-C4 lower alcohols, such as methanol, ethanol, propyl alcohol, and butyl alcohol; Polyhydric alcohols such as glycerin, butylene glycol, and propylene glycol; And hydrocarbon solvents such as methyl acetate, ethyl acetate, acetone, benzene, hexane, diethyl ether and dichloromethane; Or a mixture thereof can be used, Preferably, water and lower alcohol can be used individually or in mixture of 2 or more types. The solvent extract may be prepared by extracting one or more times using the solvent, and the dried extract obtained by freeze-drying or spray drying the solvent extract under reduced pressure may be prepared.
상기 추출 용매의 양은 이용되는 추출 용매의 종류에 따라 다양할 수 있으나, 예를 들어 대상 식물의 건조 중량에 대하여 1 내지 20배, 또는 5 내지 20배로 사용할 수 있다.The amount of the extraction solvent may vary depending on the type of extraction solvent used, for example, it may be used in 1 to 20 times, or 5 to 20 times with respect to the dry weight of the target plant.
이외에도, 당업계 공지된 다양한 추출 공정, 예를 들어, 온침(maceration), 인퓨전(infusion), 퍼콜레이션(percolation), 소화, 전즙(decoction), 고온 연속 추출(hot continuous extraction), 수성-알코올성 추출, 역류 추출, 마이크로파 보조 추출, 초음파 추출, 초임계 유체 추출, 조직편 추출(phytonic extract) (예를 들어, 하이드로-플루오로-카본 용매) 등) 등을 선택하여 사용할 수 있으며, 이들은 단독으로 수행되거나 2 종 이상의 방법을 병용하여 수행될 수 있다.In addition, various extraction processes known in the art, for example, maceration, infusion, percolation, digestion, decoction, hot continuous extraction, aqueous-alcoholic extraction , Countercurrent extraction, microwave assisted extraction, ultrasonic extraction, supercritical fluid extraction, phytonic extract (e.g., hydro-fluoro-carbon solvent), etc. may be selected and used alone or It can be performed in combination of two or more methods.
본 발명에 따른 조성물은 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하며, 바람직하게는 정향 추출물, 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물을 유효성분으로 포함한다.The composition according to the present invention is a clove extract; And one or more selected from the group consisting of gallium extract, allium extract, magnetization designated extract and motherwort extract as an active ingredient, preferably clove extract, gallium extract, allium extract, magnetized extract and motherwort extract as an active ingredient Include.
본 발명에 있어서 "유효성분으로 포함한다"는 의미는 본 발명에 따른 조성물로부터 구강질환 예방 및/또는 치료 효과를 나타낼 수 있는 정도로, 추출물이 첨가되는 것을 의미하고, 안정화 등을 위하여 다양한 성분을 부성분으로 첨가하여 다양한 형태로 제형화(formulation) 가능함을 포함하는 의미이다.In the present invention, "comprising as an active ingredient" means that the extract is added to the extent that can prevent oral disease and / or treatment effect from the composition according to the present invention, means that various components for stabilization, etc. It means that it can be added to the formulation in various forms (formulation).
본 발명에 따른 조성물은 유효성분(정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상)을 조성물 총 중량 대비 0.00001 내지 1 중량% 포함할 수 있으며, 바람직하게는 0.00005 내지 0.5 중량% 포함할 수 있고, 더욱 바람직하게는 0.0001 내지 0.1 중량% 포함할 수 있다.The composition according to the present invention may comprise 0.00001 to 1% by weight of the active ingredient (clove extract; and at least one selected from the group consisting of gallium extract, allium extract, magnetized extract and motherwort extract) relative to the total weight of the composition, preferably Preferably 0.00005 to 0.5% by weight, and more preferably 0.0001 to 0.1% by weight.
본 발명의 바람직한 일 양태에서, 정향 추출물, 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물을 유효성분으로 포함하는 본 발명에 따른 조성물에 각각의 식물 추출물은 다음과 같은 비율로 포함될 수 있다. 예를 들어, 정향 추출물 : 갈화 추출물 : 우방자 추출물 : 자화지정 추출물 : 익모초 추출물의 중량비는 1 : 0.1 ~ 10 : 0.1 ~ 10 : 0.1 ~ 10 : 0.1 ~ 10으로 포함될 수 있다.In a preferred embodiment of the present invention, each plant extract in the composition according to the present invention comprising a clove extract, a gallbladder extract, a wooja extract, a designated magnetization extract and motherwort extract as an active ingredient may be included in the following ratio. For example, the weight ratio of the clove extract: browning extract: allium extract: magnetization designated extract: motherwort extract may be included as 1: 0.1 ~ 10: 0.1 ~ 10: 0.1 ~ 10: 0.1 ~ 10.
본 발명에 따른 조성물은 구강질환 예방 및/또는 치료용일 수 있다.The composition according to the present invention may be for the prevention and / or treatment of oral diseases.
본 발명에서 상기 구강질환은 예를 들어, 이에 한정되는 것은 아니지만, 치통, 치아 우식증, 치은염, 치주염, 구취, 시린이 등을 포함하는 포괄적 개념이다.In the present invention, the oral disease is, for example, but not limited to, a comprehensive concept including toothache, dental caries, gingivitis, periodontitis, bad breath, shilin and the like.
본 발명에서, 용어 "예방"은 상기 조성물을 이용하여 질환을 억제 또는 지연시키는 모든 행위를 의미한다.In the present invention, the term "prevention" means any action that inhibits or delays a disease using the composition.
또한, 본 발명에서, 용어 "치료"는 상기 조성물을 이용하여 질환의 증세가 완화되거나 완치되는 모든 행위를 의미한다.In addition, in the present invention, the term "treatment" means all the actions that alleviate or cure the symptoms of the disease using the composition.
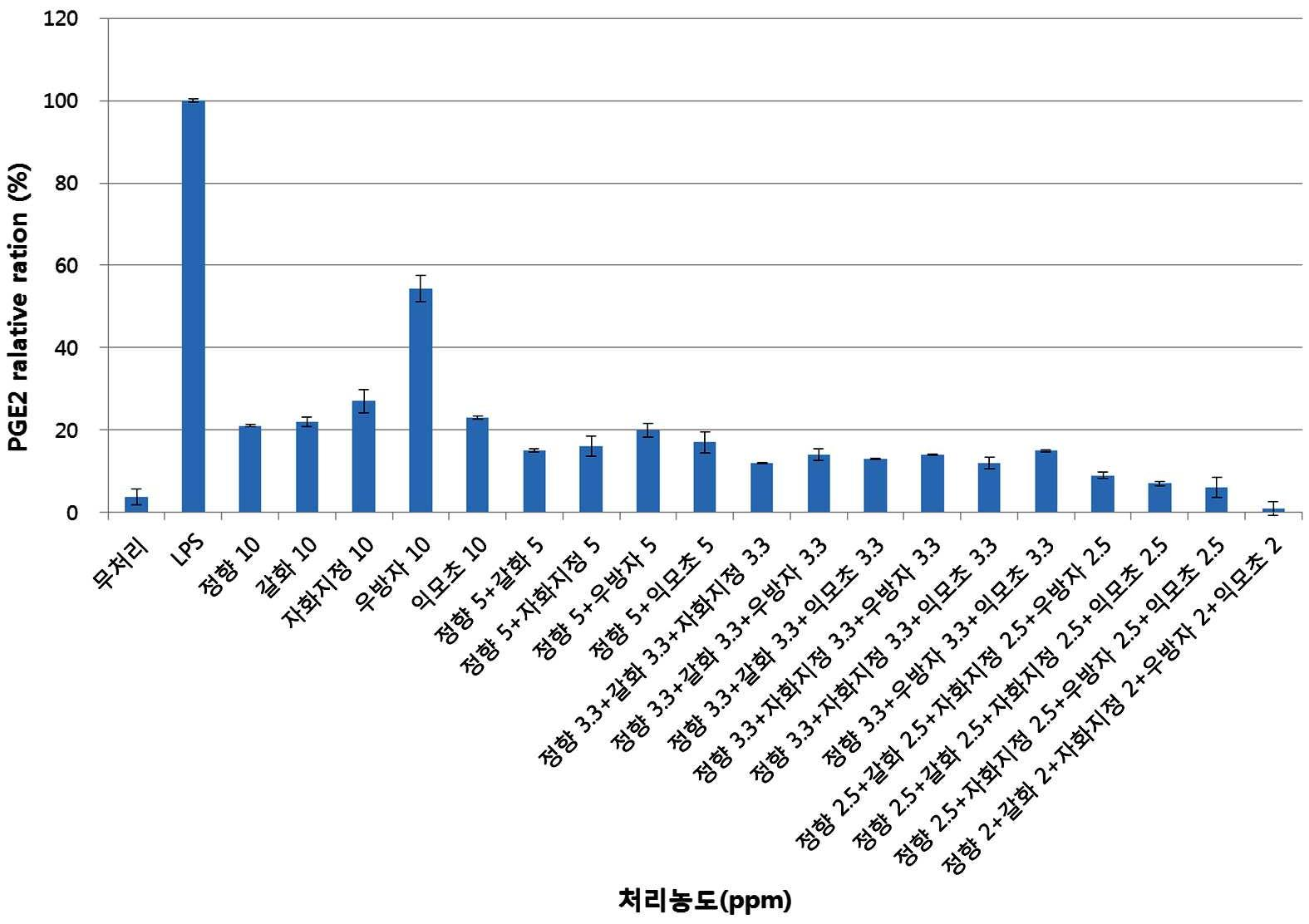
본 발명에서, 용어 "치통"은 치아에 발생하는 통증을 의미하며, 일반적으로 저작시 치아에 발생하는 통증을 의미한다. 치통의 원인으로는 이에 한정되는 것은 아니지만, 치아 우식증, 치수염, 매복치, 치아 파절, 치아 마모증 등이 포함된다. 본 발명자들은 본 발명에 따른 조성물이 치통의 지표가 될 수 있는 우수한 PGE2 억제능 갖는 것을 확인하였다.In the present invention, the term "toothache" refers to pain that occurs in the tooth, and generally refers to pain that occurs in the tooth upon chewing. Causes of toothache include, but are not limited to, dental caries, pulpitis, ambush, tooth fracture, tooth abrasion, and the like. The inventors have found that the compositions according to the invention have excellent PGE2 inhibitory ability which can be an indicator of toothache.
본 발명에서, 용어 "치아 우식증"은 구강 내 박테리아에 의해 치아가 손상되어 충치가 생기는 것을 의미한다. 예를 들어, 치아 표면에 생성된 바이오필름인 플라크(plaque)가 원인일 수 있다. 상기 구강 내 박테리아는 이에 한정되는 것은 아니지만, 스트렙토코커스(Streptococcus) 속, 포피로모나스(Porphyromonas) 속 등일 수 있다.In the present invention, the term "tooth caries" means that the tooth is damaged by bacteria in the oral cavity resulting in caries. For example, plaque, a biofilm produced on the tooth surface, may be the cause. The oral bacteria may be, but are not limited to, Streptococcus genus, Porphyromonas genus, and the like.
본 발명에서, 용어 "치은염" 및 "치주염"은, 치은연하치태내에 존재하는 박테리아, 예를 들어, 스트렙토코커스(Streptococcus) 속, 포피로모나스(Porphyromonas) 속 등의 세균이 적절한 생육조건이 형성되었을 때 치은 부위의 연조직에 감염을 일으켜 치은염 또는 치주염의 치주질환으로 발전하여 잇몸의 염증을 일으키는 구강질환이다.In the present invention, the terms "gingivitis" and "periodonitis", bacteria present in the subgingival plaque, for example, bacteria such as Streptococcus , Porphyromonas genus such that the appropriate growth conditions have been formed It is an oral disease that causes infection of the soft tissues of the gingival area and develops into periodontal disease of gingivitis or periodontitis and causes inflammation of the gums.
본 발명에서, 용어 "구취"는 후천적인 전신질환에 의해서나 타액 중의 단백질, 음식물 찌꺼기 등이 구강 내에서 미생물에 의해 분해되어 생성된 물질이 분해되어 악취를 유발하는 물질을 생성함으로써 발생하는 것을 의미한다. 구취를 유발하는 1차 원인인 구강 내 미생물에는 이에 한정되는 것은 아니지만, 스트렙토코커스(Streptococcus) 속, 포피로모나스(Porphyromonas) 속, 악티노마이세스(Actinomyces) 속, 악티노바실러스(Actinobacillus) 속 등이 있을 수 있다.In the present invention, the term "bad breath" means that by the acquired systemic disease or protein in the saliva, food waste, etc. are generated by the decomposition of the substances produced by decomposition by microorganisms in the oral cavity to produce a substance causing odor do. What are the primary cause of oral microorganisms that cause bad breath are not limited to, Streptococcus (Streptococcus) genus Pseudomonas (Porphyromonas) in a foreskin, evil Tino Mai Seth (Actinomyces), An evil Tino Bacillus (Actinobacillus) in such This can be.
본 발명에서, 용어 "시린이"는 이에 한정되는 것은 아니지만, 충치나 다른 병적인 원인과 별개로, 외부 자극에 대해 예리하고 일시적이거나 지속적인 통증이 나타나는 모든 현상을 의미한다. 상기 외부 자극은 보통 온도 자극을 의미하며, 대개 차가운 온도 자극에 증상을 호소하는데 뜨거운 온도에 통증이 나타나기도 한다. 이와 같은 온도 자극 외에도 치아의 건조, 외부 물질과의 접촉, 달거나 신 음식을 통한 삼투압 등의 자극에 의해서도 통증이 나타날 수 있다. 이는 치아 전체에 전반적으로 나타나기도 하며, 상악이나 하악, 또는 오른쪽이나 왼쪽 등 특정 부위에 한정되어 나타나기도 하고, 상아질 지각과민증(dentine hyperesthesia), 우식, 치수 염증과 동반하기도 한다.In the present invention, the term "single" means, but is not limited to, any phenomenon in which sharp, temporary or persistent pain appears with respect to external stimuli, independent of caries or other pathological causes. The external stimulus usually refers to a temperature stimulus, which usually causes symptoms at a cold temperature stimulus, which may cause pain at a hot temperature. In addition to such temperature stimulation, pain may also be caused by stimulation such as dry teeth, contact with foreign substances, and osmotic pressure through sweet or sour foods. It may appear throughout the entire tooth, or may be confined to the upper or lower jaw, or to specific areas such as the right or left side, and may be accompanied by dentin hyperesthesia, caries, and pulp inflammation.
본 발명의 조성물은 스트렙토코커스(streptococcus) 속 또는 포피로모나스(Porphyromonas) 속, 더욱 상세하게는 스트렙토코커스 뮤탄스(streptococcus mutans), 스트렙토코커스 상귀스(streptococcus sanguis), 스트렙토코커스 상귀니스(Streptococcus sanguinis), 스트렙토코커스 살리바리우스 종 써모필스(Streptococcus salivarius subsp. thermophils), 및 포피로모나스 진지발리스(Porphyromonas gingivalis)로 이루어진 군에서 선택된 하나 이상의 세균에 항균 효과가 탁월하여 구강질환의 예방 및 치료에 우수한 효과가 있음을 확인하였다.The compositions of the present invention Streptococcus Pseudomonas (Porphyromonas) genus, and more particularly, Streptococcus mutans (streptococcus mutans), Streptococcus sanggwi switch (streptococcus sanguis), Streptococcus sanggwi varnish (Streptococcus sanguinis) to (streptococcus) in or wrapping , Streptococcus salivarius subsp. thermophils ), and Porphyromonas gingivalis ( Pirphyromonas gingivalis ) was confirmed that the excellent antibacterial effect on one or more bacteria selected from the group consisting of excellent effect in the prevention and treatment of oral diseases.
더욱 바람직하게, 본 발명의 조성물은 스트렙토코커스 뮤탄스(streptococcus mutans) 또는 포피로모나스 진지발리스(Porphyromonas gingivalis) 세균에 항균 효과가 탁월한 것을 실험적으로 확인하였다.More preferably, the composition of the present invention is streptococcus mutans ( Streptococcus mutans ) or Porphyromonas jinjivalis ( Porphyromonas gingivalis ) It was experimentally confirmed that the antibacterial effect on bacteria is excellent.
본 발명에 따른 조성물은 구강용 조성물일 수 있다.The composition according to the present invention may be a composition for oral cavity.
상기 구강용 조성물은 상기 유효성분 이외에, 그 제형 및 사용 목적에 따라 통상적으로 성분들로서, 습윤제, 연마제, 약효제, 감미제, pH조정제, 방부제, 결합제, 향료, 기포제 등을 더 포함할 수 있다.In addition to the active ingredient, the composition for oral cavity may further include a wetting agent, an abrasive, a medicinal agent, a sweetener, a pH adjuster, a preservative, a binder, a flavoring agent, a foaming agent, etc., according to the formulation and the purpose of use.
습윤제로는 농글리세린, 글리세린, 소르비톨수용액, 비결정성 소르비톨수용액, 폴리에틸렌글리콜류 및 프로필렌글리콜로 이루어진 군중에서 단독 또는 혼합하여 사용하며, 그 사용량은 구강용 조성물 총 중량 대비 1 내지 70중량% 포함될 수 있다.The humectant may be used alone or in a mixture of concentrated glycerin, glycerin, sorbitol aqueous solution, amorphous sorbitol aqueous solution, polyethylene glycol, and propylene glycol, and the amount thereof may be included in an amount of 1 to 70% by weight based on the total weight of the composition for oral cavity. .
연마제로는 침강실리카, 실리카겔, 지르코늄실리케이트, 인산일수소칼슘, 무수인산일수소칼슘, 함수알루미나, 경질탄산칼슘, 중질탄산칼슘, 칼슘피로인산염, 불용성메타인산염 및 알루미늄실리케이트로 이루어진 군중에서 선택된 1종 등을 사용할 수 있다. 이러한 연마제의 사용량은 일반적으로 구강용 조성물 총 중량 대비 1 내지 60 중량% 포함될 수 있다. 또한, 소량 사용되는 첨가제로는 통상 사용되는 성분들로서 약효제, 감미제, pH 조정제, 방부제, 착색제, 기포제, 결합제 등이 포함될 수 있으며 구강용 조성물 총 중량 대비 0.001 내지 3 중량% 포함될 수 있다..The abrasive is selected from the group consisting of precipitated silica, silica gel, zirconium silicate, calcium monohydrogen phosphate, calcium monohydrogen phosphate, hydrous alumina, hard calcium carbonate, heavy calcium carbonate, calcium pyrophosphate, insoluble metaphosphate and aluminum silicate. Etc. can be used. The amount of the abrasive may generally be included in an amount of 1 to 60% by weight based on the total weight of the composition for oral cavity. In addition, the additives used in small amounts may include pharmacological agents, sweeteners, pH adjusters, preservatives, colorants, foaming agents, binders and the like as conventionally used components, and may include 0.001 to 3% by weight relative to the total weight of the composition for oral cavity.
약효제는 불화나트륨, 일불소인산나트륨, 불화나트륨, 불화주석, 클로로헥시딘, 알란토인 클로로히드록시알루미네이트, 아미노카프론산, 염화아연, 염산피리독신, 초산토코페롤, 효소류 등을 단독 또는 2종 이상 혼합하여 사용할 수 있다. Pharmacologic agents include sodium fluoride, sodium monofluorophosphate, sodium fluoride, tin fluoride, chlorohexidine, allantoin chlorohydroxy aluminate, aminocaproic acid, zinc chloride, pyridoxine hydrochloride, tocopherol acetate, enzymes, etc. It can mix and use the above.
결합제로는 카르복시메틸셀룰로오스나트륨, 카보머, 카라기난, 잔탄검, 알지네이트류 등을 사용할 수 있고, 기포제로서 알킬황산나트륨, 라우릴황산나트륨, 자당지방산에스테르, 소르비탄지방산에스테르 등의 음이온 및 비이온 계면 활성제를 단독 또는 2종 이상을 혼합하여 사용할 수 있다.As the binder, carboxymethyl cellulose sodium, carbomer, carrageenan, xanthan gum, alginate and the like can be used.As a foaming agent, anionic and nonionic surfactants such as alkyl sodium sulfate, sodium lauryl sulfate, sucrose fatty acid ester and sorbitan fatty acid ester are used. It can be used individually or in mixture of 2 or more types.
감미제로는 사카린, 자일리톨, 에리스리톨, 아스파탐 등을 사용할 수 있다.Saccharin, xylitol, erythritol, aspartame and the like may be used as the sweetening agent.
pH조정제로는 인산나트륨, 인산이나트륨, 구연산, 트리에탄올아민 등을 사용할 수 있다.Sodium phosphate, disodium phosphate, citric acid, triethanolamine, etc. can be used as a pH adjuster.
방부제로는 안식향산, 메틸파라벤, 프로필파라벤, 안식향산나트륨 등을 사용할 수 있다. 향료로는 페퍼민트 오일, 스피아민트 오일, 멘톨 등을 혼합하여 사용하기도 하며, 기타 첨가제로서 덱스타라나제 등의 효소류를 사용할 수도 있다.As the preservative, benzoic acid, methylparaben, propylparaben, sodium benzoate and the like can be used. As a fragrance, peppermint oil, spearmint oil, menthol, etc. may be mixed and used, and other additives, such as dexteranase, may also be used.
기포제로는 음이온성 계면활성제인 라우릴황산나트륨, 비이온성 계면활성제인 폴리옥시에틸렌폴리옥시프로필렌의 공중합체(폴록사머), 폴리옥시에틸렌경화피마자유, 폴리옥시에틸렌솔비탄 지방산에스테르 등이 사용될 수 있다.Examples of the foaming agent include sodium lauryl sulfate as anionic surfactant, a copolymer of polyoxyethylene polyoxypropylene as a nonionic surfactant (poloxamer), polyoxyethylene hardened castor oil, polyoxyethylene sorbitan fatty acid ester, and the like. .
그밖에 잔량의 물을 포함한다.In addition, it contains a residual amount of water.
또한, 본 발명은 상기 조성물을 포함하는 구강용 제품을 제공한다.The present invention also provides a product for oral cavity comprising the composition.
정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하는 본 발명에 따른 구강용 조성물은 일반적으로 구강 건강을 위해 제조, 시판되는 제품들에 모두 포함될 수 있는 구강용 조성물로서, 적용되는 구강용 제품에는 제한이 없다.Clove extract; And oral extracts, alligator extracts, self-specified extracts, and motherwort extracts, including any one or more selected from the group consisting of an oral composition according to the present invention is generally included in all products manufactured and marketed for oral health. As an oral composition which can be used, there is no limitation on the oral product to be applied.
예를 들어, 상기 구강용 제품으로는 치약, 구강 스프레이, 구강 세정제, 구강용 연고, 구강 청정제, 가글링 제품, 껌 등을 포함하나 이에 한정되지 않는다. 본 발명의 구강용 제품은 액상, 고상, 현탁액, 젤상, 에어로졸 형태일 수 있으나, 이에 한정되는 것은 아니다.For example, the oral product may include, but is not limited to, toothpaste, oral spray, oral cleaner, oral ointment, oral cleanser, gargle product, gum, and the like. Oral products of the present invention may be in the form of liquid, solid, suspension, gel, aerosol, but is not limited thereto.
또한, 본 발명에 따른 조성물은 화장료 조성물일 수 있다.In addition, the composition according to the present invention may be a cosmetic composition.
상기 구강질환 예방 및/또는 치료용 조성물은 용액, 외용연고, 크림, 폼, 영양화장수, 유연화장수, 팩, 유연수, 유액, 메이크업베이스, 에센스, 비누, 액체 세정료, 입욕제, 선 스크린크림, 선오일, 현탁액, 유탁액, 페이스트, 겔, 로션, 파우더, 비누, 계면활성제-함유 클린싱, 오일, 분말 파운데이션, 유탁액 파운데이션, 왁스 파운데이션, 패취 및 스프레이로 구성된 군으로부터 선택되는 제형으로 제조할 수 있으나, 이에 제한되는 것은 아니다.The composition for the prevention and / or treatment of oral diseases is a solution, external ointment, cream, foam, nourishing longevity, softening longevity, pack, softening water, latex, makeup base, essence, soap, liquid detergent, bathing agent, sunscreen cream, sun May be prepared in a formulation selected from the group consisting of oils, suspensions, emulsions, pastes, gels, lotions, powders, soaps, surfactant-containing cleansing, oils, powder foundations, emulsion foundations, wax foundations, patches and sprays. However, the present invention is not limited thereto.
또한, 본 발명의 화장료 조성물은 일반 피부 화장료에 배합되는 화장학적으로 허용 가능한 담체를 1 종 이상 추가로 포함할 수 있으며, 통상의 성분으로 예를 들면 유분, 물, 계면활성제, 보습제, 저급 알코올, 증점제, 킬레이트제, 색소, 방부제, 향료 등을 적절히 배합할 수 있으나, 이에 제한되는 것은 아니다.In addition, the cosmetic composition of the present invention may further include one or more cosmetically acceptable carriers formulated in general skin cosmetics, and as conventional components, for example, oil, water, surfactants, moisturizers, lower alcohols, Thickeners, chelating agents, pigments, preservatives, flavoring agents, and the like may be appropriately blended, but are not limited thereto.
본 발명의 화장료 조성물에 포함되는 화장학적으로 허용 가능한 담체는 제형에 따라 다양하다. 본 발명의 제형이 연고, 페이스트, 크림 또는 겔인 경우에는, 담체성분으로서 동물성 유, 식물성 유, 왁스, 파라핀, 전분, 트라칸트, 셀룰로오스 유도체, 폴리에틸렌 글리콜, 실리콘, 벤토나이트, 실리카, 탈크, 산화아연 또는 이들의 혼합물이 이용될 수 있다.The cosmetically acceptable carrier included in the cosmetic composition of the present invention varies depending on the dosage form. When the formulation of the present invention is an ointment, paste, cream or gel, the carrier component is animal oil, vegetable oil, wax, paraffin, starch, tracant, cellulose derivative, polyethylene glycol, silicone, bentonite, silica, talc, zinc oxide or Mixtures of these may be used.
본 발명의 제형이 파우더 또는 스프레이인 경우에는, 담체 성분으로서 락토오스, 탈크, 실리카, 알루미늄 히드록사이드, 칼슘 실케이트, 폴리아미드 파우더 또는 이들의 혼합물이 이용될 수 있고, 특히 스프레이인 경우에는 추가적으로 클로로플루오로히드로카본, 프로판/부탄 또는 디메틸 에테르와 같은 추진제를 포함할 수 있다.If the formulation of the invention is a powder or a spray, lactose, talc, silica, aluminum hydroxide, calcium silicate, polyamide powder or mixtures thereof may be used, in particular in the case of a spray additionally chloro Propellants such as fluorohydrocarbons, propane / butane or dimethyl ether.
본 발명의 제형이 용액 또는 유탁액인 경우에는, 담체 성분으로서 용매, 용해화제, 또는 유탁화제가 이용되고 예컨대 물, 에탄올, 이소프로판올, 에틸 카보네이트, 에틸 아세테이트, 벤질 벤조에이트, 프로필렌 글리콜, 1,3-부틸글리콜 오일이 있으며, 특히, 목화씨 오일, 땅콩 오일, 옥수수 배종 오일, 올리브 오일, 피마자 오일 및 참깨 오일, 글리세롤 지방족 에스테르, 폴리에틸렌 글리콜 또는 소르비탄의 지방산 에스테르가 있다.If the formulation of the invention is a solution or emulsion, a solvent, solubilizer, or emulsifier is used as the carrier component, for example water, ethanol, isopropanol, ethyl carbonate, ethyl acetate, benzyl benzoate, propylene glycol, 1,3 Butylglycol oils, and in particular, cottonseed oil, peanut oil, corn seed oil, olive oil, castor oil and sesame oil, glycerol aliphatic esters, fatty acid esters of polyethylene glycol or sorbitan.
본 발명의 제형이 현탁액인 경우에는, 담체 성분으로서 물, 에탄올 또는 프로필렌 글리콜과 같은 액상의 희석제, 에톡실화 이소스테아릴 알코올, 폴리옥시에틸렌 소르비톨 에스테르 및 폴리 옥시에틸렌 소르비탄 에스테르와 같은 현탁제, 미소결정성 셀룰로오스, 알루미늄 메타히드록시드, 벤토나이트, 아가 또는 트라칸트 등이 이용될 수 있다.When the formulation of the present invention is a suspension, liquid carrier diluents such as water, ethanol or propylene glycol, suspending agents such as ethoxylated isostearyl alcohol, polyoxyethylene sorbitol esters and polyoxyethylene sorbitan esters, micro Crystalline cellulose, aluminum metahydroxy, bentonite, agar or tracant and the like can be used.
본 발명의 제형이 비누인 경우에는 담체 성분으로서 지방산의 알칼리 금속 염, 지방산 헤미에스테르 염, 지방산 단백질 히드롤리제이트, 이세티오네이트, 라놀린 유도체, 지방족 알코올, 식물성 유, 글리세롤, 당 등이 이용될 수 있다.When the formulation of the present invention is a soap, alkali metal salts of fatty acids, fatty acid hemiester salts, fatty acid protein hydrolyzates, isethionates, lanolin derivatives, aliphatic alcohols, vegetable oils, glycerol, sugars and the like may be used as carrier components. Can be.
또한, 본 발명에 따른 조성물은 약학 조성물일 수 있다.In addition, the composition according to the invention may be a pharmaceutical composition.
본 발명의 또 다른 실시예에 따르면, 본 발명은 상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 함유하는 구강질환 예방 및/또는 치료용 약학 조성물을 제공한다. According to another embodiment of the present invention, the present invention is a clove extract; And it provides a pharmaceutical composition for oral disease prevention and / or treatment containing any one or more selected from the group consisting of gallium extract, wooja extract, self-designated extract and motherwort extract as an active ingredient.
본 발명의 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 포함하는 조성물은 경구 또는 비경구의 여러 가지 제형일 수 있다. 제제화할 경우에는 보통 사용하는 충진제, 증량제, 결합제, 습윤제, 붕해제, 계면활성제 등의 희석제 또는 부형제를 사용하여 조제된다. 경구투여를 위한 고형제제에는 정제, 환제, 산제, 과립제, 캡슐제 등이 포함되며, 이러한 고형제제는 하나 이상의 화합물에 적어도 하나 이상의 부형제 예를 들면, 전분, 탄산칼슘, 수크로오스(sucrose) 또는 락토오스(lactose), 젤라틴 등을 섞어 조제된다. 또한, 단순한 부형제 이외에 스테아린산 마그네슘, 탈크 등과 같은 윤활제들도 사용된다. 경구투여를 위한 액상제제로는 현탁제, 내용액제, 유제, 시럽제 등이 해당되는데 흔히 사용되는 단순 희석제인 물, 리퀴드 파라핀 이외에 여러 가지 부형제, 예를 들면 습윤제, 감미제, 방향제, 보존제 등이 포함될 수 있다. 비경구투여를 위한 제제에는 멸균된 수용액, 비수성용제, 현탁제, 유제가 포함된다. 비수성용제, 현탁용제로는 프로필렌 글리콜(propylene glycol), 폴리에틸렌 글리콜, 올리브 오일과 같은 식물성 기름, 에틸올레이트와 같은 주사 가능한 에스테르 등이 사용될 수 있다.Clove extract of the present invention; And a browning extract, a milky way extract, a designated magnetization extract, and a motherwort extract, the composition including any one or more may be various formulations of oral or parenteral. When formulated, diluents or excipients such as fillers, extenders, binders, wetting agents, disintegrating agents and surfactants are usually used. Solid form preparations for oral administration include tablets, pills, powders, granules, capsules, and the like, which form at least one excipient such as starch, calcium carbonate, sucrose or lactose (at least one compound). lactose) and gelatin. In addition to simple excipients, lubricants such as magnesium stearate, talc and the like are also used. Liquid preparations for oral administration include suspensions, solutions, emulsions, and syrups, and various excipients such as wetting agents, sweeteners, fragrances, and preservatives, in addition to commonly used simple diluents such as water and liquid paraffin, may be included. have. Formulations for parenteral administration include sterile aqueous solutions, non-aqueous solvents, suspensions, emulsions. As the non-aqueous solvent and the suspension solvent, propylene glycol, polyethylene glycol, vegetable oil such as olive oil, injectable ester such as ethyl oleate, and the like can be used.
본 발명은 또한 상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하는 구강질환 예방 및/또는 치료용 피부외용제의 제형으로 제공할 수 있다.The invention also the clove extract; And it can be provided in the formulation of the skin external preparation for oral disease prevention and / or treatment comprising any one or more selected from the group consisting of gallium extract, wooja extract, self-designated extract and motherwort extract as an active ingredient.
상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 포함하는 조성물을 피부외용제로 사용하는 경우, 추가로 지방 물질, 유기 용매, 용해제, 농축제 및 겔화제, 연화제, 항산화제, 현탁화제, 안정화제, 발포제(foaming agent), 방향제, 계면활성제, 물, 이온형 또는 비이온형 유화제, 충전제, 금속이온봉쇄제 및 킬레이트화제, 보존제, 비타민, 차단제, 습윤화제, 필수 오일, 염료, 안료, 친수성 또는 친유성 활성제, 지질 소낭 또는 피부용 외용제에 통상적으로 사용되는 임의의 다른 성분과 같은 피부 과학 분야에서 통상적으로 사용되는 보조제를 함유할 수 있다. 또한, 상기 성분들은 피부 과학 분야에서 일반적으로 사용되는 양으로 도입될 수 있다.The clove extract; And when using a composition comprising any one or more selected from the group consisting of gallium extract, allium extract, self-specified extract and motherwort extract as a skin external preparation, further comprising fatty substances, organic solvents, solubilizers, thickening and gelling agents, emollients , Antioxidants, suspending agents, stabilizers, foaming agents, fragrances, surfactants, water, ionic or nonionic emulsifiers, fillers, metal ion sequestrants and chelating agents, preservatives, vitamins, blockers, wetting agents, It may contain adjuvants commonly used in the field of dermatology, such as essential oils, dyes, pigments, hydrophilic or lipophilic active agents, lipid vesicles or any other ingredients commonly used in external preparations for skin. In addition, the ingredients may be introduced in amounts generally used in the field of dermatology.
상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상이 피부외용제 제형으로 제공될 경우, 이에 제한되는 것은 아니나, 연고, 패취, 겔, 크림 또는 분무제와 같은 제형을 가질 수 있다.The clove extract; And if the one or more selected from the group consisting of gallium extract, allium extract, self-specified extract and motherwort extract is provided as a skin external preparation, but is not limited thereto, have a formulation such as ointment, patch, gel, cream or spray Can be.
본 발명의 약학 조성물은 비경구용 제제로 이용될 수 있으며, 예를 들어, 피부외용제는 바세린, 스테아릴알코올 등의 약제학적으로 허용되는 적당한 기제; 폴리소르베이트, 소르비탄 세스퀴올레이트 등의 약제학적으로 허용되는 적당한 계면활성제; 글리세린 등의 약제학적으로 허용되는 적당한 보습제; 약제학적으로 허용되는 적당한 용제; 및 착향제, 착색제, 안정화제, 점성화제 등을 균질하게 혼합하는 통상의 피부외용제 제조방법에 의해서 제조될 수 있다.The pharmaceutical composition of the present invention can be used as a parenteral preparation, for example, the external skin preparation may be a suitable pharmaceutically acceptable base such as petrolatum, stearyl alcohol, etc .; Pharmaceutically acceptable suitable surfactants such as polysorbate, sorbitan sesquioleate; Pharmaceutically acceptable humectants, such as glycerin; Pharmaceutically acceptable solvents; And it may be prepared by a conventional skin external preparation method of homogeneously mixing a flavoring agent, colorant, stabilizer, viscosity agent and the like.
본 발명의 약학 조성물은 유효량의 상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 포함할 때 바람직한 구강질환 예방 및/또는 치료 효과를 제공할 수 있다. 본 발명에 있어서, '유효량'이라 함은 구강질환 예방 및/또는 치료 효과를 충분히 나타낼 수 있는 양을 의미한다.The pharmaceutical composition of the present invention comprises an effective amount of the clove extract; And it may provide a desirable oral disease prevention and / or therapeutic effect when it comprises any one or more selected from the group consisting of gallium extract, ally extract, self-designated extract and motherwort extract. In the present invention, the term "effective amount" means an amount capable of sufficiently exhibiting oral disease prevention and / or treatment effects.
본 발명의 조성물에 포함되는 상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상의 유효량은 조성물이 제품화되는 형태, 상기 화합물이 피부에 적용되는 방법 및 피부에 머무르는 시간 등에 따라 달라질 것이다. 예컨대, 상기 조성물이 의약품으로 제품화되는 경우에는 일상적으로 피부에 적용하게 되는 화장품으로 제품화되는 경우에 비해 높은 농도로 상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 포함할 수 있을 것이다.The clove extract included in the composition of the present invention; And at least one effective amount selected from the group consisting of gallium extract, alligator extract, designated magnetization extract, and motherwort extract will vary depending on the form in which the composition is commercialized, the method by which the compound is applied to the skin, and the time it stays on the skin. For example, when the composition is commercialized in medicine, the clove extract at a higher concentration than when commercialized in cosmetics that are routinely applied to the skin; And it may include any one or more selected from the group consisting of gallium extract, ally extract, magnetization designated extract and motherwort extract.
본 발명의 또 다른 실시예에 따르면 상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하는 구강질환 예방 및/또는 치료용 건강식품을 제공한다.According to another embodiment of the present invention; And it provides a health food for oral disease prevention and / or treatment comprising any one or more selected from the group consisting of gallium extract, wooja extract, self-designated extract and motherwort extract as an active ingredient.
본 명세서에서 '건강식품'이란, 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 음료, 차류, 향신료, 껌, 과자류 등의 식품소재에 첨가하거나, 캡슐화, 분말화, 현탁액 등으로 제조한 식품으로, 이를 섭취할 경우 건강상 특정한 효과를 가져오는 것을 의미하나, 일반 약품과는 달리 식품을 원료로 하여 약품의 장기 복용 시 발생할 수 있는 부작용 등이 없는 장점이 있다. 이와 같이 하여 얻어지는 본 발명의 건강식품은, 일상적으로 섭취하는 것이 가능하기 때문에 구강질환에 우수한 효과를 기대할 수 있어 매우 유용하다.In the present specification, 'health food', clove extract; And one or more selected from the group consisting of gallium extract, allium extract, self-specified extract and motherwort extract, as an active ingredient to food materials such as beverages, teas, spices, gums, confectionery, or the like, encapsulated, powdered, suspensions, etc. As a manufactured food, it means that when ingested to bring a specific effect on health, unlike general medicine, there is no side effect that can occur when taking a long-term use of the drug as a food raw material. Since the health food of the present invention thus obtained can be consumed on a daily basis, an excellent effect can be expected for oral diseases, which is very useful.
상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 식품첨가물로 사용하는 경우, 상기 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 그대로 첨가하거나 다른 식품 또는 식품 성분과 함께 사용될 수 있고, 통상적인 방법에 따라 적절하게 사용될 수 있다. 유효성분의 혼합양은 그의 사용 목적(예방, 건강 또는 치료적 처치)에 따라 적합하게 결정될 수 있다. 일반적으로, 식품 또는 음료의 제조시에 본 발명의 조성물은 원료에 대하여 15 중량부 이하, 바람직하게는 10 중량부 이하의 양으로 첨가된다. 그러나, 건강 및 위생을 목적으로 하거나 또는 건강 조절을 목적으로 하는 장기간의 섭취의 경우에는 상기 양은 상기 범위 이하일 수 있으며, 안전성 면에서 아무런 문제가 없기 때문에 유효성분은 상기 범위 이상의 양으로도 사용될 수 있다. 상기 식품의 종류에는 특별한 제한은 없다. 상기 물질을 첨가할 수 있는 식품의 예로는 육류, 소세지, 빵, 초콜릿, 캔디류, 스넥류, 과자류, 피자, 라면, 기타 면류, 껌류, 아이스크림류를 포함한 낙농제품, 각종 스프, 음료수, 차, 드링크제, 알코올 음료 및 비타민 복합제 등이 있으며, 통상적인 의미에서의 건강식품을 모두 포함한다. 본 발명의 건강음료 조성물은 통상의 음료와 같이 여러 가지 향미제 또는 천연 탄수화물 등을 추가 성분으로서 함유할 수 있다. 상술한 천연 탄수화물은 포도당, 과당과 같은 모노사카라이드, 말토스, 수크로오스와 같은 디사카라이드, 및 덱스트린, 사이클로덱스트린과 같은 폴리사카라이드, 자일리톨, 소르비톨, 에리트리톨 등의 당알코올이다. 감미제로서는 타우마틴, 스테비아 추출물과 같은 천연 감미제나, 사카린, 아스파르탐과 같은 합성 감미제 등을 사용할 수 있다. 상기 천연 탄수화물의 비율은 본 발명의 조성물 100 mL 당 일반적으로 약 0.01 ∼ 0.04 g, 바람직하게는 약 0.02 ∼ 0.03 g 이다. 상기 외에 본 발명의 건강식품은 여러 가지 영양제, 비타민, 전해질, 풍미제, 착색제, 펙트산 및 그의 염, 알긴산 및 그의 염, 유기산, 보호성 콜로이드 증점제, pH 조절제, 안정화제, 방부제, 글리세린, 알코올, 탄산 음료에 사용되는 탄산화제 등을 함유할 수 있다. 그 밖에 본 발명의 건강식품은 천연 과일주스, 과일주스 음료 및 야채 음료의 제조를 위한 과육을 함유할 수 있다. 이러한 성분은 독립적으로 또는 혼합하여 사용할 수 있다. 이러한 첨가제의 비율은 크게 중요하진 않지만 본 발명의 조성물 100 중량부당 0.01 ~ 0.1 중량부의 범위에서 선택되는 것이 일반적이다.The clove extract; And clove extract, when using any one or more selected from the group consisting of gallium extract, allium extract, self-designated extract and motherwort extract as an active ingredient, the clove extract; And one or more selected from the group consisting of gallium extract, alligator extract, designated magnetization extract, and motherwort extract, as an active ingredient, or may be used together with other food or food ingredients, and may be appropriately used according to a conventional method. The mixed amount of the active ingredient can be suitably determined according to the purpose of use (prevention, health or therapeutic treatment). Generally, in the preparation of food or beverages, the composition of the present invention is added in an amount of up to 15 parts by weight, preferably up to 10 parts by weight based on the raw materials. However, in the case of long-term intake for health and hygiene or health control, the amount may be below the above range, and the active ingredient may be used in an amount above the above range because there is no problem in terms of safety. . There is no particular limitation on the kind of the food. Examples of foods to which the substance may be added include meat, sausages, bread, chocolate, candy, snacks, confectionery, pizza, ramen, other noodles, gums, dairy products including ice cream, various soups, beverages, teas, drinks, Alcoholic beverages and vitamin complexes, and the like and include all of the health foods in the conventional sense. The health beverage composition of the present invention may contain various flavors or natural carbohydrates and the like as additional ingredients, as in the usual beverage. Natural carbohydrates described above are glucose, monosaccharides such as fructose, disaccharides such as maltose and sucrose, and polysaccharides such as dextrin and cyclodextrin, sugar alcohols such as xylitol, sorbitol, and erythritol. As the sweetener, natural sweeteners such as tautin and stevia extract, synthetic sweeteners such as saccharin and aspartame, and the like can be used. The proportion of said natural carbohydrate is generally about 0.01 to 0.04 g, preferably about 0.02 to 0.03 g per 100 mL of the composition of the present invention. In addition to the above, the health food of the present invention includes various nutrients, vitamins, electrolytes, flavors, coloring agents, pectic acid and salts thereof, alginic acid and salts thereof, organic acids, protective colloid thickeners, pH adjusting agents, stabilizers, preservatives, glycerin, alcohols. And carbonation agents used in carbonated beverages. In addition, the health food of the present invention may contain a flesh for preparing natural fruit juice, fruit juice beverage and vegetable beverage. These components can be used independently or in combination. The proportion of such additives is not critical but is usually selected in the range of 0.01 to 0.1 parts by weight per 100 parts by weight of the composition of the present invention.
본 발명의 정향 추출물; 및 갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하는 조성물은 구강질환 예방 또는 치료 효과가 우수하다.Clove extract of the present invention; And a composition of gallium extract, allium extract, self-designated extract and motherwort extract as an active ingredient at least one selected from the group is excellent in preventing or treating oral diseases.
또한, 본 발명의 조성물은 천연물 유래의 물질이어서 독성이 없으며, 인체에 적용하여도 부작용을 유발하지 않아 활용도가 높다.In addition, since the composition of the present invention is a substance derived from natural products, it is not toxic and does not cause side effects even when applied to the human body, and thus has high utility.
도 1은 각 추출물의 통증 및 염증 마커인 PGE2의 억제 효능을 확인한 그래프이다(각 식물 추출물에서 '추출물'은 삭제하고 식물 명칭만 표시함).1 is a graph confirming the inhibitory effect of PGE2, a pain and inflammation marker of each extract ('extracts' are deleted from each plant extract and only the plant names are displayed).
이하, 본 발명의 이해를 돕기 위하여 실시예 등을 들어 상세하게 설명하기로 한다. 그러나 본 발명에 따른 실시예들은 여러 가지 다른 형태로 변형될 수 있으며, 본 발명의 범위가 하기 실시예들에 한정되는 것으로 해석되어서는 안된다. 본 발명의 실시예들은 당업계에서 평균적인 지식을 가진 자에게 본 발명을 보다 완전하게 설명하기 위해 제공되는 것이다.Hereinafter, examples and the like will be described in detail to help the understanding of the present invention. However, embodiments according to the present invention can be modified in many different forms, the scope of the present invention should not be construed as limited to the following examples. Embodiments of the present invention are provided to more fully explain the present invention to those skilled in the art.
재료 준비Material preparation
정향cloves 추출물의 제조 Preparation of Extract
정향을 증기 추출하여 정향 증기 추출물을 얻었다.Cloves were steam extracted to obtain a clove steam extract.
갈화Gall painting 추출물의 제조 Preparation of Extract
갈화를 세절하여 95% 에탄올 용액에 침지하고 48시간 동안 상온에서 추출하여 갈화 에탄올 추출물을 얻었다.Subcutaneous fragmentation was cut and immersed in a 95% ethanol solution, and extracted at room temperature for 48 hours to obtain a brown ethanol extract.
우방자Allies 추출물의 제조 Preparation of Extract
우방자를 세절하여 95% 에탄올 용액에 침지하고 48시간 동안 상온에서 추출하여 우방자 에탄올 추출물을 얻었다.Allies were chopped and immersed in a 95% ethanol solution and extracted at room temperature for 48 hours to obtain allium ethanol extract.
자화지정 추출물의 제조Preparation of magnetized extract
자화지정 전초를 세절하여 95% 에탄올 용액에 침지하고 48시간 동안 상온에서 추출하여 자화지정 에탄올 추출물을 얻었다.The magnetized directed ethanol was cut and immersed in a 95% ethanol solution and extracted at room temperature for 48 hours to obtain a magnetized directed ethanol extract.
익모초 추출물의 제조Preparation of Motherwort Extract
익모초를 세절하여 95% 에탄올 용액에 침지하고 48시간 동안 상온에서 추출하여 익모초 에탄올 추출물을 얻었다.Motherwort was chopped and immersed in 95% ethanol solution and extracted at room temperature for 48 hours to obtain motherwort ethanol extract.
정향cloves , , 갈화Gall painting , , 우방자Allies , 자화지정, 익모초(, Magnetization, motherwort ( 5 가지5 branches ) 혼합 추출물의 제조) Preparation of Mixed Extract
상술한 각각 추출물 형태로 제조한 정향, 갈화, 우방자, 자화지정, 익모초 추출물을 1 : 1 : 1 : 1 : 1의 비율로 주입하여 정향, 갈화, 우방자, 자화지정, 익모초 혼합 추출물을 제조하였다.Clove, browned, allium, magnetized, and motherwort extract prepared in the above-mentioned extract form in a ratio of 1: 1: 1: 1: 1 to prepare a clove, browned, allium, magnetized, and motherwort extract.
실시예Example 및 And 비교예의Comparative Example 제조 Produce
본 발명자들은 구강질환에 대한 효과를 확인하기 위한 실시예 및 이에 대한 비교예 치약을 하기 표 1의 조성으로 제조하였다. 즉, 우선 치약을 제조하기 위해 카르복시메틸셀룰로오스나트륨, 라우릴황산나트륨, 글리세린(농글리세린(98%)), 소르비톨수용액(70%), 실리카류(침강 실리카), 잔탄검, 불화나트륨, 항료, 사카린, 구연산 등을 사용하여 비교예 치약을 만들었고, 여기에 혼합 추출물이 0.01 중량% 함유된 실시예 치약을 제조하였다.The inventors prepared an example and a comparative example toothpaste for confirming the effect on oral diseases with the composition of Table 1 below. That is, to prepare toothpaste, sodium carboxymethylcellulose, sodium lauryl sulfate, glycerin (concentrated glycerin (98%)), sorbitol aqueous solution (70%), silica (precipitated silica), xanthan gum, sodium fluoride, pharmaceuticals, saccharin Comparative Example Toothpaste was prepared using citric acid, and the like, and an example toothpaste containing 0.01 wt% of a mixed extract was prepared.
<< 실험예Experimental Example 1> 1>
항균 효과 확인Confirm antimicrobial effect
본 발명자들은 혼합 추출물의 우수한 항균 효과를 확인하기 위하여, 치아우식과 치주질환 대표 유발 세균인, 그람양성균 스트렙토코커스 뮤탄스와 그람음성균인 포피로모나스 진지발리스를 이용하여 균주를 억제하는 효과가 있는지 페이퍼 디스크(paper disk) 검사법을 사용하여 항균력 테스트를 실시하였다. 상기 각 세균들은 하기 표 2의 최적 배양조건에서 활성을 키운 후, 각 균의 최적 배지에서 4 내지 6시간 정도 배양하여 배양액의 탁도를 Macfarland turbidity No. 0.5(1.5×108)가 되도록 맞춘 후, 0.1ml를 골고루 도말하였다. 멸균한 페이퍼 디스크(Whatman no.5 paper, 8mm diameter)에 각 구분별(표 3) 추출물을 10mg/disc 농도로 접종하여 1시간 동안 흡수 건조시킨 뒤 사용하였다. 상기의 각 세균의 최적온도에서 24 내지 48시간 배양 후 생육 저지환의 크기(직경 mm)를 측정하였다.In order to confirm the excellent antimicrobial effect of the mixed extract, the present inventors have shown that there is an effect of inhibiting the strain by using Gram-positive bacteria Streptococcus mutans and Gram-negative bacteria Popiromonas gingivalis, which are representative bacteria of dental caries and periodontal disease. Antimicrobial testing was conducted using a paper disk test. Each of the bacteria was grown in the optimum culture conditions of Table 2, and then cultured for about 4 to 6 hours in the optimum medium of each bacterium turbidity of the culture medium Macfarland turbidity No. After adjusting to 0.5 (1.5 × 10 8 ), 0.1 ml were evenly spread. The sterilized paper disc (Whatman no.5 paper, 8 mm diameter) was inoculated at 10 mg / disc concentration for each fraction (Table 3) extract, and used after absorption drying for 1 hour. After culturing for 24 to 48 hours at the optimum temperature of each bacterium, the size of the growth resistant ring (diameter mm) was measured.
(Gram staining)Gram Dyeing
Gram staining
그 결과를 하기 표 3에 나타내었다(하기 구분란에서 각 식물 추출물에서 '추출물'은 생략하고 표시하였다).The results are shown in Table 3 (in the following section, 'extracts' are omitted from each plant extract).
그 결과, 상기 표 3에서 확인할 수 있는 바와 같이 각각의 추출물에 비하여 정향 추출물을 포함하는 혼합 추출물에서 생육저지환 직경이 우수한 효과를 나타내는 것을 확인할 수 있으며, 정향, 갈화, 자화지정, 우방자 및 익모초 추출물 모두를 포함하는 것의 생육저지환 직경이 스트렙토코커스 뮤탄스 및 포피로모나스 진지발리스 균에 대하여 가장 넓은 것을 알 수 있었다.As a result, as can be seen in Table 3, it can be seen that the growth inhibitory ring diameter shows an excellent effect in the mixed extract containing the clove extract as compared to the respective extracts, clove, browning, magnetization, allergy and motherwort extract It was found that the growth-lowering ring diameters of all-containing ones were the widest for Streptococcus mutans and Popiromonas jinjivalis.
<< 실험예Experimental Example 2> 2>
PGE2PGE2 억제 효과 확인 Confirm inhibitory effect
본 발명자들은 혼합 추출물의 치통 완화 효과를 확인하기 위해, 통증 및 염증 마커인 PGE2의 억제 효능을 확인하였다. 대식세포를 10% FBS를 포함하는 DMEM 배지에서 1.5×105 cells/ml 농도로 접종하여 37℃, 5% CO2 조건으로 24 well plate에 24시간을 배양하였다. 그리고 염증 유도 자극원인 LPS 1㎍/ml와 각각의 추출물을 처리하여 24시간 배양한 상층액으로 PGE2 억제능을 평가하였다. 그 결과는 도 1에 나타내었다.The present inventors confirmed the inhibitory effect of PGE2, a pain and inflammation marker, in order to confirm the toothache relieving effect of the mixed extract. Macrophages were inoculated at a concentration of 1.5 × 10 5 cells / ml in DMEM medium containing 10% FBS and incubated in a 24 well plate at 37 ° C. and 5% CO 2 for 24 hours. In addition, the inhibitory effect of PGE2 was evaluated in the supernatant cultured for 24 hours by treating LPS, which is an inflammation-induced stimulus, and each extract. The results are shown in FIG.
그 결과, 상기 도 1에서 확인할 수 있는 바와 같이 각각의 추출물에 비하여, 정향 추출물을 포함하는 혼합 추출물이 우수한 효과를 나타내는 것을 확인할 수 있으며, 그 중에서도 정향, 갈화, 자화지정, 우방자 및 익모초 추출물 모두를 포함하는 것의 효과가 가장 우수한 것을 알 수 있었다.As a result, as can be seen in FIG. 1, compared to the respective extracts, it can be seen that the mixed extract containing the clove extract exhibits an excellent effect, and among them, the clove, the browning, the magnetization, the alligator and motherwort extract It turned out that the effect of containing is the best.
<< 실험예Experimental Example 3> 3>
치은염증Gingivitis 형성 억제 효과 확인 Confirmation of Formation Inhibitory Effect
본 발명자들은 치은염증 형성 억제 효과를 확인하기 위하여, 상기 표 1의 실시예 및 비교예 치약을 이용하였다. 실험 대상자는 치열이 고르고 결손 치아가 없는 치은염증 환자를 대상으로 연령별 30세부터 50세까지 10세 간격으로 성별에 따라 30명씩 정밀한 구강검진을 실시하여 120명의 실험대상군을 선별한 후 60명씩 나누어 치은염증 치료효과에 대한 임상실험을 수행하였다.The present inventors used the Example and Comparative Example toothpaste of Table 1 to confirm the inhibitory effect of gingival inflammation formation. Subjects were selected from 120 oral subjects with 30 oral examinations of 30 patients from ages 30 to 50 years old. A clinical trial was conducted on the effect of gingival inflammation.
실험 대상군을 실험군(실시예)과 대조군(비교예)으로 나누어 식후 2시간 경과 후 잠자기 전에 하루 3회 사용하도록 교육시키고, 치면세마를 실시하여 초기 치은염 지수를 점수화하고 각각의 치약 조성물을 사용하도록 하여 1주, 1개월, 3개월 및 6개월 경과 후 구강검진을 실시하여 치은염 지수를 검사하였다. 치은염 지수의 측정방법은 페리오덴탈 프로브(periodontal probe)를 치은열구 내에 삽입하여 힘을 가하지 않은 상태로 각 치아주위를 연소하여 탐침하고 30초가 지난 뒤에 출혈된 상태를 측정하여 하기 표 4와 같은 기준으로 점수를 기록하여 결과를 얻었다.The test subjects were divided into the experimental group (example) and the control group (comparative example), and then trained to use three times a day after sleep 2 hours after eating, and to perform an initial toothpaste to score an initial gingivitis index and to use each toothpaste composition. After 1 week, 1 month, 3 months and 6 months, oral examination was performed to check the gingivitis index. The gingivitis index is measured by inserting a periodontal probe into the gingival bulb and burning the probe around each tooth without applying force and measuring the bleeding state after 30 seconds. The score was recorded and the results obtained.
그 결과는 표 5에 나타내었다.The results are shown in Table 5.
그 결과, 상기 표 5에서 알 수 있는 바와 같이, 실시예 치약을 사용한 실험군의 경우 6개월이 지난 후에도 치은염 지수가 초기와 비교하여 거의 상승하지 않은 것을 확인할 수 있었으며, 비교예 치약을 사용한 대조군의 경우 1주일 경과 후부터 급격히 상승하여 치은염증 형성을 억제하지 못하는 것을 확인할 수 있었다.As a result, as can be seen in Table 5, in the experimental group using the example toothpaste was confirmed that the gingivitis index is almost not increased compared to the initial after 6 months, the control group using the comparative toothpaste It was confirmed that the rapid increase after one week did not inhibit the formation of gingival inflammation.
따라서, 본 발명에 따른 조성물은 우수한 치은염증 형성 억제 효과를 갖는 것을 확인하였다.Therefore, it was confirmed that the composition according to the present invention has an excellent gingivitis inhibitory effect.
<< 실험예Experimental Example 4> 4>
시린이Sirin 억제 효과 확인 Confirm inhibitory effect
본 발명자들은 시린이 억제 효과를 확인하기 위하여, 상기 표 1의 실시예 및 비교예 치약을 이용하였다. 지각과민 치아를 가진 사람으로 이 실험에 참여하기를 동의한 지원자 40명을 연구대상으로 하였으며, 총 연구 대상 치아는 80개다. 또한, 지원자 중 남자가 20명, 여자가 20명이었고, 연령은 20세에서 50세였다. 연구 대상자들은 치약 내용물을 알지 못하도록 하였고, 총 시험기간은 2주로 하였다(실험군: 실시예, 대조군: 비교예).The present inventors used the Example 1 and Comparative Example toothpaste of Table 1 in order to confirm the inhibitory effect of aspirin. The subjects were 40 volunteers who had hypersensitive teeth and agreed to participate in this experiment. A total of 80 teeth were studied. In addition, there were 20 males and 20 females, and the age ranged from 20 to 50 years. The study subjects were not aware of the toothpaste contents and the total test period was 2 weeks (experimental group: Example, control group: comparative example).
온도 자극을 가한 후 환자의 반응을 측정하는 방법으로 시험을 실시하였다. 시험을 실시하기 전에 미리 각 지원자의 지각과민 치아의 과민부위를 체크하였고, 치아의 시린부위에 약 5℃의 차가운 물을 스포이드로 떨어뜨려 평점을 한 후 2주간 각각의 조성물을 1일 3회 사용하게 하고, 2주가 지나 다시 약 5℃의 차가운 물을 스포이드로 떨어뜨려 평점을 하였다. 자극 후 환자의 반응 평점 기준은 다음과 같이 설정하였다. (0점: 아무런 불편감이 없다, 1점: 약간 불편하거나 시리다, 3점: 아프다). 통계처리는 실험실시 전과 2주 후의 자극 점수를 paired student-t test로 검정하였다.The test was conducted by measuring the patient's response after applying the temperature stimulus. Prior to the test, each volunteer's hypersensitivity area was checked beforehand, and each of the compositions was used three times a day for two weeks after dropping the cold water of about 5 ° C with a dropper on the aerobic area of the tooth. After two weeks, the water was dropped again by dropping cold water at about 5 ° C. with a dropper. The response grade criteria of the patient after stimulation were set as follows. (0 points: no discomfort, 1 point: slightly uncomfortable or cold, 3 points: pain). Statistical analysis was performed using a paired student-t test for stimulation scores before and after 2 weeks in the laboratory.
2주 후의 온도 자극에 대한 반응 점수는 하기 표 6에 나타내었다.Response scores for temperature stimulation after 2 weeks are shown in Table 6 below.
그 결과, 상기 표 6에서 알 수 있는 바와 같이, 초기에는 대조군과 실험군의 온도 자극에 대한 반응 점수에 큰 차이가 없으나, 2주 경과 후에 대조군은 차이가 없는 반면, 실험군의 경우 반응 점수가 유의미하게 감소하였다.As a result, as can be seen in Table 6, initially there was no significant difference in the response score for the temperature stimulation of the control group and the experimental group, but after 2 weeks, the control group did not have a difference, whereas the experimental group had a significant response score. Decreased.
즉, 본 발명에 따른 혼합 추출물이 포함된 실시예 치약을 이용하여 우수한 시린이 억제 효과를 확인하였다.In other words, using the Example toothpaste containing the mixed extract according to the present invention was confirmed excellent sirin inhibitory effect.
<< 실험예Experimental Example 5> 5>
구취 제거 효과 확인Determination of bad breath
본 발명자들은 구취 제거 효과를 확인하기 위하여, 상기 표 1의 실시예 및 비교예 치약을 이용하였다. The present inventors used the Example 1 and Comparative Example toothpaste of Table 1 to confirm the bad breath removal effect.
치아우식증이 없는 남녀 50명을 선정하여 대상 음료에 대하여 교차 반복 실험(Cross-over test)을 실시하였다. 시판하는 마늘분을 물에 분산시켜 24시간 방치한 후 희석하여 할리미터(Halimeter) 측정값이 700ppb 이상이 되도록 하여, 상기 희석액을 구취 유발원으로 사용하였다. 마늘분 희석액 15ml로 30초간 가글을 한 후 1분 뒤에 할리미터로 구취정도를 측정한 후, 비교예(대조군), 실시예(실험군) 치약을 사용하여 30초 내지 1분간 양치질하였다. 양치질 후 1분, 5분, 30분 경과 후 할리미터로 구취정도를 측정하여 구취 억제의 지속 여부를 측정하였다.Fifty men and women without dental caries were selected and subjected to a cross-over test. Commercial garlic powder was dispersed in water, left to stand for 24 hours, and diluted to make the Halimeter measurement value of 700 ppb or more, and the diluent was used as a source of bad breath. Gargle was diluted with 15 ml of garlic powder for 30 seconds, and after 1 minute, the degree of bad breath was measured with a halimeter, and then brushed with a toothpaste of Comparative Example (Control) and Example (Experimental Group) for 30 seconds to 1 minute. After 1 minute, 5 minutes, and 30 minutes after brushing the teeth, the degree of bad breath was measured by using a halimeter to determine whether the bad breath was continued.
그 결과는 하기 표 7에 나타내었다.The results are shown in Table 7 below.
그 결과, 상기 표 7에서 알 수 있는 것과 같이 실시예 치약을 사용한 실험군은 비교예 치약을 사용한 대조군과 비교하여 우수한 구취 억제 효과를 나타내는 것을 확인하였다.As a result, it was confirmed that the experimental group using the example toothpaste as shown in Table 7 shows an excellent bad breath inhibitory effect compared to the control group using the comparative example toothpaste.
Claims (7)
갈화 추출물, 우방자 추출물, 자화지정 추출물 및 익모초 추출물로 이루어진 군으로부터 선택된 어느 하나 이상을 유효성분으로 포함하는 치통, 및 시린이로 이루어진 군에서 선택된 1종 이상의 질환 예방 또는 개선을 위한 구강용 조성물.Clove extract; And
Oral composition for preventing or improving at least one disease selected from the group consisting of one or more selected from the group consisting of gallium extract, wooja extract, self-specified extract and motherwort extract as an active ingredient, and sirini.
상기 유효성분은 조성물 총 중량 대비 0.00001 내지 1 중량% 포함되는 것을 특징으로 하는 구강용 조성물.The method of claim 1,
The active ingredient is a composition for oral cavity, characterized in that 0.00001 to 1% by weight based on the total weight of the composition.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020160076736A KR102070316B1 (en) | 2016-06-20 | 2016-06-20 | Composition for treating or preventing oral diseases comprising natural complex |
| CN201710202985.XA CN107260792B (en) | 2016-04-05 | 2017-03-30 | Composition for preventing or treating oral diseases comprising natural complex |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020160076736A KR102070316B1 (en) | 2016-06-20 | 2016-06-20 | Composition for treating or preventing oral diseases comprising natural complex |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20170142740A KR20170142740A (en) | 2017-12-28 |
| KR102070316B1 true KR102070316B1 (en) | 2020-01-28 |
Family
ID=60939804
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020160076736A KR102070316B1 (en) | 2016-04-05 | 2016-06-20 | Composition for treating or preventing oral diseases comprising natural complex |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR102070316B1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102574859B1 (en) * | 2018-02-06 | 2023-09-05 | 주식회사 엘지생활건강 | Composition for prevention or treatment of oral disease comprising Ginkgo biloba extract |
| KR102328308B1 (en) | 2018-02-22 | 2021-11-18 | 주식회사 온엔온 | Oral Composition Containing Natural Botanical Extracts and Manufacturing Method Thereof |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100431170B1 (en) * | 2003-06-11 | 2004-05-12 | 신화제약 (주) | Compositions for Dental Clinic Comprising Extracts of Plant |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20110088978A (en) * | 2010-01-29 | 2011-08-04 | 애경산업(주) | Oral composition containing herbal extract |
| KR101293335B1 (en) * | 2011-11-16 | 2013-08-06 | 한승국 | The liquefied dentifrice making method besides |
| KR101591499B1 (en) | 2013-11-01 | 2016-02-03 | 가천대학교 산학협력단 | Composition for skin whitening comprising amaranthus spp. l. extract or fraction thereof |
-
2016
- 2016-06-20 KR KR1020160076736A patent/KR102070316B1/en active IP Right Grant
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100431170B1 (en) * | 2003-06-11 | 2004-05-12 | 신화제약 (주) | Compositions for Dental Clinic Comprising Extracts of Plant |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20170142740A (en) | 2017-12-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102408893B1 (en) | Composition for prevention or treatment of oral disease comprising Pueraria Extract | |
| KR102070316B1 (en) | Composition for treating or preventing oral diseases comprising natural complex | |
| KR102562835B1 (en) | Composition for prevention or treatment of oral disease comprising ROSAE LAEVIGATAE Extract | |
| KR101881636B1 (en) | Composition for prevention or treatment of oral disease comprising Pueraria Extract | |
| CN107260792B (en) | Composition for preventing or treating oral diseases comprising natural complex | |
| CN110613801B (en) | Oral composition for preventing or improving oral diseases and use thereof | |
| KR20140143063A (en) | Oral care composition comprising Magnolia officinalis extract and bicarbonate | |
| KR102177066B1 (en) | Composition for prevention or treatment of oral disease comprising Epimedium Herb extract | |
| KR102415612B1 (en) | Composition for prevention or treatment of oral disease comprising Melonis Pedicellus Extract | |
| KR102548925B1 (en) | Composition for prevention or treatment of oral disease comprising Scopoletin | |
| KR102283663B1 (en) | Antimicrobial composition comprising extracts of herb | |
| KR102562829B1 (en) | Composition for prevention or treatment of oral disease comprising Alpinia officinarum Extract | |
| KR102562830B1 (en) | Composition for prevention or treatment of oral disease comprising Kochiae Fructus Extract | |
| KR20170103482A (en) | Composition for prevention or treatment of oral disease comprising Glechoma hederacea extract | |
| KR20180047703A (en) | Composition for prevention or treatment of oral disease comprising Nelumbo Nucifera extract | |
| KR102156884B1 (en) | Composition for prevention or treatment of oral disease comprising Meliae toosendan fructus Extract | |
| KR102562831B1 (en) | Composition for prevention or treatment of oral disease comprising Gleditsiae fructus Extract | |
| KR20180047705A (en) | Composition for prevention or treatment of oral disease comprising Forsythiae Fructus extract | |
| KR102580941B1 (en) | Composition for prevention or treatment of oral disease comprising Qucercetin 3-glucoside | |
| KR102417082B1 (en) | Composition for prevention or treatment of oral disease comprising Celosia argentea Extract | |
| KR101818211B1 (en) | Composition for prevention or treatment of oral disease comprising Arctill fructus Extract | |
| KR20240078208A (en) | Composition for teeth whitening and antibacterial for oral microorganisms comprising apple extract | |
| KR102541843B1 (en) | Composition for prevention or treatment of oral disease comprising Arctiin | |
| KR102564564B1 (en) | Composition for prevention or treatment of oral disease comprising 2-methoxycinnamaldehyde | |
| KR20200107917A (en) | Composition for prevention or treatment of oral disease comprising Meliae toosendan fructus Extract |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant |