KR101990384B1 - A Method for Producing highly pure Allyl Alcohol and Process system for Producing highly pure Allyl Alcohol - Google Patents
A Method for Producing highly pure Allyl Alcohol and Process system for Producing highly pure Allyl Alcohol Download PDFInfo
- Publication number
- KR101990384B1 KR101990384B1 KR1020150068005A KR20150068005A KR101990384B1 KR 101990384 B1 KR101990384 B1 KR 101990384B1 KR 1020150068005 A KR1020150068005 A KR 1020150068005A KR 20150068005 A KR20150068005 A KR 20150068005A KR 101990384 B1 KR101990384 B1 KR 101990384B1
- Authority
- KR
- South Korea
- Prior art keywords
- allyl alcohol
- formic acid
- reaction product
- allyl
- reaction
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/60—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by elimination of -OH groups, e.g. by dehydration
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/74—Separation; Purification; Use of additives, e.g. for stabilisation
- C07C29/76—Separation; Purification; Use of additives, e.g. for stabilisation by physical treatment
- C07C29/80—Separation; Purification; Use of additives, e.g. for stabilisation by physical treatment by distillation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C33/00—Unsaturated compounds having hydroxy or O-metal groups bound to acyclic carbon atoms
- C07C33/02—Acyclic alcohols with carbon-to-carbon double bonds
- C07C33/025—Acyclic alcohols with carbon-to-carbon double bonds with only one double bond
- C07C33/03—Acyclic alcohols with carbon-to-carbon double bonds with only one double bond in beta-position, e.g. allyl alcohol, methallyl alcohol
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/582—Recycling of unreacted starting or intermediate materials
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
Abstract
The present invention relates to a process for producing high purity allyl alcohol and a process for producing high purity allyl alcohol.
Description
More particularly, the present invention relates to a method of reacting glycerol with formic acid to obtain allyl alcohol in a liquid reaction product in high purity.
Allyl alcohol is the simplest unsaturated alcohol represented by CH 2 ═CHCH 2 OH, which is used as an insecticide or as a raw material or intermediate in the preparation of many compounds. It is also used in various fields such as the production of a phthalic acid ester used as a preservative for a polymer or as a raw material for producing a butane-1,4-diol, which is a monomer of a polyester (PBT), or an acrylic acid, have.
In the case of allyl alcohol, studies have been actively made on production methods such as production of allyl acetate by reacting propylene, acetic acid, and oxygen in a petrochemical-based process, and hydrolysis of allyl acetate to produce allyl alcohol. In addition, in a method for producing allylic alcohol using bio-based raw materials as an environmentally friendly manufacturing method compared to a conventional petrochemical-based process, when the glycerol is reacted with formic acid, a method in which allyl alcohol can be obtained at a high yield without a catalyst US Patent No. 8273926 and the like. Glycerol, which is used as a raw material in the production of allyl alcohol in the above-mentioned patent, is mainly used as a raw material, solvent, or lubricant for pharmaceuticals or cosmetics as a by-product from biodiesel manufacturing process. However, as the production amount of biodiesel increases, The supply of glycerol is expected to increase. Therefore, new applications of glycerol are under study. However, in order to maximize the yield of allyl alcohol in the above-mentioned patent, formic acid had to be added in excess of glycerol (for example, 1.45 equivalents). In this case, the yield of allyl alcohol is high, but the reaction time is long and the process steps are complicated. In addition, due to the use of an excess amount of formic acid and a low product selection of allyl alcohol, the gas phase products arising from the prior art are CO 2 , H 2 O (W, bp 100 ° C), allyl formate , AF, bp 80 - 83 ℃), allyl alcohol (AA, ALA bp 97 ℃) and unreacted formic acid (FA, bp 100.8 ℃). Also, the concentration of allyl alcohol in the liquid reaction product passing through the gas separator is low, which leads to an increase in energy and cost for the separation process. Since the boiling points of W, ALA and FA, which are gas phase products, are close to 100 ° C, separation of the products by general distillation is impossible. Furthermore, there is a problem that it is very difficult to separate formic acid from the product because of the existence of an azeotrope of formic acid-water vapor (FA-W) and allyl alcohol-formic acid-water vapor (ALA-FA-W). Therefore, in order to produce allyl alcohol on a commercial scale, it is necessary to study the development of a purification process capable of obtaining highly pure allyl alcohol by effectively separating the by-products contained in the reaction products.
Conventionally, by-products are removed by using a neutralizing agent such as potassium carbonate in an allyl alcohol production method to obtain allyl alcohol. However, this is not economical because not only a large amount of wastewater is generated but also a loss of formic acid due to an acid-base reaction is large.
In order to solve the problems of the prior art as described above, it is an object of the present invention to provide a process for producing allyl alcohol of high purity in the process for producing allyl alcohol from glycerol.
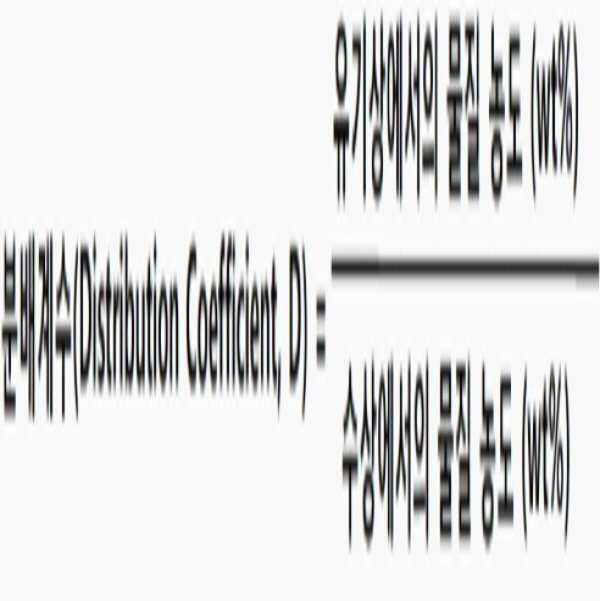
In order to achieve the above object, the present invention provides a method for producing allyl alcohol having high purity from the reaction of glycerol and formic acid, comprising the steps of: 1) adding water and an organic extractant to a liquid reaction product produced by the reaction of glycerol and formic acid, ; 2) separating the organic phase containing the allyl alcohol and the organic extractant from the mixed solution; 3) distilling the organic phase to obtain an effluent containing allyl alcohol; And 4) to obtain an alcohol solution of allyl by distilling the effluent; to comprises, wherein the organic extraction agent is at least the E T N value by using the equation (1) 0.420, provides 0.680 or lower allyl alcohol production process.
[Equation 1]
E T (solvent), E T (TMS) and E T (water) in
Another aspect of the present invention provides a process system for manufacturing high purity allyl alcohol to which the process for producing high purity allyl alcohol of the present invention is applied.
In the step of producing allyl alcohol from glycerol of the present invention, unreacted formic acid and by-products contained in the product can be separated to produce allyl alcohol of high purity. Therefore, the economical efficiency can be improved.
1 is a schematic diagram showing a high purity allyl alcohol production process system according to a preferred embodiment of the present invention.
Figures 2a, 2b and 2c are flow charts of a high purity allyl alcohol production process system according to a preferred embodiment of the present invention.
Hereinafter, the present invention will be described in detail. The following detailed description is merely an example of the present invention, and therefore, the present invention is not limited thereto.
Conventionally, in the step of producing allyl alcohol from glycerol, unreacted formic acid contained in the product was separated using a neutralizing agent such as potassium carbonate. However, this causes not only a large amount of wastewater to be generated but also high purity allyl alcohol, which is uneconomical.
Accordingly, the present inventors have made extensive efforts to confirm that allyl alcohol can be efficiently isolated using a specific organic extractant in the method of producing allyl alcohol, and allyl alcohol can be obtained in a high purity by a multi- Thereby completing the invention.
That is, A method for producing high purity allyl alcohol from the reaction of glycerol and formic acid, comprising the steps of: 1) adding water and an organic extractant to a liquid reaction product produced by the reaction of glycerol and formic acid to produce a mixed solution; 2) separating the organic phase containing the allyl alcohol and the organic extractant from the mixed solution; 3) distilling the organic phase to obtain an effluent containing allyl alcohol; 4) distilling the effluent to obtain a solution containing allyl alcohol, wherein the organic extractant is selected from the group consisting of organic extractants having an E T N value of 0.420 or more and 0.680 or less, Wherein the allyl alcohol is at least one selected from the group consisting of allyl alcohol and allyl alcohol.
According to a preferred embodiment of the present invention, the organic extractant includes at least one selected from a hydrocarbon ring having 4 to 10 carbon atoms or an alcohol having a chain. Preferably, the organic extractant may include at least one selected from 1-octanol, n-butanol, ortho-tert-butyl phenol and 2-ethylhexanol.
In the above-mentioned high-purity allyl alcohol production process, the glycerol can be used without any particular limitation as long as it is usable for producing allyl alcohol. Preferably, glycerol having a purity of 60 to 99.5% can be used. If formic acid is used in the glycerol reaction, it can be used without any particular limitation.
Hereinafter, the method for producing high purity allylic alcohol of the present invention will be described.
First, step 1) will be described.
In this step, water and an organic extractant are added to the resulting liquid reaction product.
According to a preferred embodiment of the present invention, the weight ratio of the water and the organic extractant may be 1:20 to 20: 1, and preferably 1: 5 to 5: 1.
The liquid reaction product of step 1) may be prepared by a) introducing formic acid into glycerol, firstly reacting in an inert gas atmosphere, and then raising the temperature to effect a second reaction; And b) separating the liquid reaction product containing allyl alcohol from the gaseous reaction product generated in the step a) through a condensation process.
In step a), allyl alcohol can be prepared from glycerol in a two-step reaction. According to a preferred embodiment of the present invention, the first reaction of step a) is carried out at 0 to 100 ° C, and the second reaction is carried out at 220 to 240 ° C. That is, the reaction in the second step is a reaction in which 1 mole of allyl alcohol is produced by sequentially removing 2 moles of water and 1 mole of carbon dioxide (CO 2 ) from 1 mole of glycerol. Specifically, in the first reaction step, 1 mole of formic acid is reacted with 1 mole of glycerol under a low-temperature and inert gas atmosphere to remove one mole of water, and glycerol formate, which is a precursor of allyl alcohol, is produced. Subsequently, allyl alcohol is produced by removing 1 mole of water and 1 mole of carbon dioxide from the glycerol formate in the presence of formic acid at a high temperature through the second reaction step. In the second reaction, formic acid acts as a catalyst, not as a reactant. The inert gas may be any one selected from the group consisting of nitrogen, argon, and helium, and may be used for both the first reaction and the second reaction.
According to a preferred embodiment of the present invention, in the method for producing allyl alcohol, the reaction between glycerol and formic acid is carried out at a rate of 2.0 ° C / min or more so as to reach a reaction temperature of 220 to 240 ° C from room temperature have. If the reaction temperature is lower than 220 ° C., the reaction does not proceed to the next step in the intermediate glyceryl formate. If the reaction temperature is higher than 240 ° C., allylformate formation is increased. The rate of temperature rise from the room temperature after the addition of formic acid to the glycerol in the step a) is preferably 2.0 ° C / min or more, more preferably 2.0 to 7.0 ° C / min, more preferably 4.0 to 7.0 ° C / min desirable. When the rate of temperature rise is less than 2.0 DEG C / min, the production of glyceryl diformate is dominant in the one-step reaction, and the amount of allyl formate is increased in the two-step reaction. When the total reaction time is more than 7 hours, the content of allyl alcohol in the liquid reaction product sample may be lowered, . The gas phase reaction product generated in step a) may include at least one selected from the group consisting of carbon dioxide, water vapor, allyl formate, allyl alcohol and unreacted formic acid.
The liquid reaction product may be separated in step b) as long as it is generally usable, and preferably a gas separator may be used. The liquid reaction product separated in step b) may include at least one member selected from the group consisting of allyl alcohol, allyl formate, unreacted formic acid, and water.
Reaction products, such as allyl alcohol, contained in the liquid reaction products obtained as described above have a similar partition coefficient for certain organic extractants. Allyl formate and formic acid, on the other hand, have very high or very low partition coefficients. Thus, in this step, water and a particular organic extractant are added to the liquid reaction product to allow separation of the allyl alcohol from the liquid reaction product.
According to one preferred embodiment of the invention, the particular choice of organic extractant in the can, based on the E N T value indicates the polarity of the solvent, and the E T N has to be defined by the equation (1).
[Equation 1]
E T (solvent), E T (TMS) and E T (water) in
The value E T (solvent) through the equation E 1 T (TMS), and converts the E T (solvent) value to a value between a standard by E T (water) 0 and 1. Set the water T E N value to 1, more by setting the E N T value of TMS to 0. The greater the value of E T N large polarity, smaller the value of N T E relativization thereby the difference in polarity to be small polarity.
More specifically, for example, E T N of water Value is 1.0, and formic acid (FA) of the E N T value of 0.728, the allyl alcohol (ALA) of the E N T value of 0.660, E N T value of 0.543 and 1-octanol is allyl formate (AF) E T The N value is 0.441 or less. Thus, if using an organic extraction agent allyl 1-octanol having a low E N T than the alcohol (ALA) it is possible to remove the allyl alcohol (ALA) in an allyl formate (AF), formic acid (FA).
As another example, E T N of water Value is 1.0, and formic acid (FA) of the E N T value of 0.728, an allyl alcohol (ALA) of the E N T value of E N T value of 0.660, 0.602, and n- butanol is allyl formate (AF) of the N E T The value is 0.441 or less. Thus, if using an organic extraction agent butanol having approximately the same E N T value and allyl alcohol (ALA) it is possible to remove the allyl alcohol (ALA) in an allyl formate (AF), formic acid (FA).
The method of adding the organic extractant to the liquid reaction product as described above may be used in any manner without any particular limitation. Preferably, in the case of two or more stages of multi-stage extraction, it may be carried out by one or more methods selected from cross current, counter current and co-current.
Next, step 2) will be described.
In this step, the mixed solution produced in the step 1) may be separated into an aqueous phase and an organic phase. The separating method may be any method that is generally applicable, Preferably by an extraction method. Specifically, the organic phase comprises allyl alcohol, allyl formate and an organic extractant, and the aqueous phase may comprise unreacted formic acid. However, the organic phase may contain a small amount of a water phase component, and the water phase may also contain a small amount of the organic phase component.
According to a preferred embodiment of the present invention, the mass ratio of the water phase to the organic phase may be 1:20 to 20: 1, and preferably 1: 5 to 5: 1.
The organic phase and the water phase of the present invention are defined as an aqueous phase when the amount of water is larger than that of the organic compound and a phase when the organic compound is more than water. Therefore, the organic phase may contain water, and the water phase may also contain an organic compound.
The allyl alcohol may be 60 to 80 wt%, preferably 70 to 75 wt%, based on the total weight percentage of the organic phase. If the amount of the allyl alcohol is less than 60% by weight, the amount of the impurities may be large, which may require additional purification. And water may be from 30 to 99 wt%, and preferably from 60 to 90 wt%, based on the total weight percentage of the water phase. If the amount of water is less than 30% by weight, the layer may not be separated during extraction or take too long. If the amount of water is more than 99% by weight, an additional water removal process may be required, which may result in poor economical efficiency. In addition, the remaining balance of the aqueous phase may be formic acid, and formic acid may be obtained by a commonly available method. Preferably formic acid, by distillation.
In the step 3), the organic phase is distilled to obtain a distillate containing allyl alcohol contained in the organic phase. According to a preferred embodiment of the present invention, after the organic phase is distilled in the step 3), the residual organic extractant may be recovered. For example, when the organic phase is added to the distillation column, the organic extractant can be recovered from the column and the effluent containing allyl alcohol can be recovered on the column. In addition to the allyl alcohol, the effluent containing the allyl alcohol includes by-products such as allyl formate.
In the next step 4), the effluent containing the allyl alcohol is further distilled again to obtain an allyl alcohol solution from which impurities such as allyl formate have been removed.
The allyl alcohol solution can be obtained by obtaining a high purity allyl alcohol by a commonly used method, and preferably by separating the allyl alcohol by a distillation method.
According to a preferred embodiment of the present invention, the allyl alcohol may be 60 to 80% by weight based on the total weight percentage of the allyl alcohol solution in the step 4) May be 70 to 75% by weight. If the amount of allyl alcohol is less than 60% by weight, the amount of the impurities may be large, which may lead to refining problems.
Consequently, in the present invention, the liquid reaction product can be separated into an aqueous phase and an organic phase by using a specific organic extractant and water, and the high purity allyl alcohol can be sequentially extracted through multi-stage extraction.
Hereinafter, the present invention will be described in more detail with reference to examples. However, the embodiments of the present invention described below are illustrative only and the scope of the present invention is not limited to these embodiments. The scope of the present invention is indicated in the claims, and moreover, includes all changes within the meaning and range of equivalency of the claims. In the following Examples and Comparative Examples, "%" and "part" representing the content are based on weight unless otherwise specified.
Manufacturing example : Obtain a liquid reaction product
A thermometer was installed on the first neck of the 3-neck round bottom flask to measure the reactor internal temperature. A second sphere of a 3-neck round bottom flask was connected to a gas separator separator connected to a 1-neck round bottom flask. Then, glycerol and formic acid were added at a molar ratio of 1: 1.45 to a third sphere of a 3-neck round bottom flask. Thereafter, the reaction was heated up to 230 ° C at a rate of 4.2 ° C / min using a sand bath in a nitrogen atmosphere. At this time, as the reaction proceeded, a gaseous reaction product was generated from the liquid reaction product, which was passed through a gas separator connected to the reactor, and only a liquid reaction product was obtained in a 1-neck round bottom flask installed at the end of the gas separator. After the reaction was completed, the 3-neck round bottom flask reactor was cooled and the liquid reaction product collected in a 1-neck round bottom flask was analyzed by gas chromatography (GC) and high performance liquid chromatography HPLC) analysis, and the composition is shown in Table 1. < tb > < TABLE >
Example One
10 g of the liquid reaction product obtained in the above Production Example was placed in a 250 ml Erlenmeyer flask, and water corresponding to 12.8 times the weight of allyl formate was added thereto to prepare an aqueous solution. 5 g of the aqueous solution was taken and 1-octanol having an E T N value of 0.543 as an extracting agent was added in the same amount as the aqueous solution. Then, it was agitated with a vortex mixer for 1 minute and allowed to stand for 1 hour. Then, the weight of each layer was measured after confirming that the layer separation occurred between the organic phase and the water phase. The components contained in each phase were quantified by gas chromatography (GC) and high performance liquid chromatography (HPLC) on the separated organic phase and water phase. Based on the quantified data, the distribution coefficient of each substance was calculated using the following equation (2). The calculated values are shown in Table 2.
&Quot; (2) "
Example 2
The procedure of Example 1 was repeated except that n-butanol having an E T N value of 0.602 was used as an organic extracting agent which was an extracting agent. The calculated values are shown in Table 2.
Example 3
The procedure of Example 1 was repeated except that ortho-tertiary butylphenol was used as the organic extractant as the extracting agent. The calculated values are shown in Table 2.
Comparative Example One
The same procedure as in Example 1 was repeated except that ethyl acetate having an E T N value of 0.228 was used as an organic extractant as an extractant. The calculated values are shown in Table 2.
Comparative Example 2
The procedure of Example 1 was repeated, except that methyl isobutyl ketone having an E T N value of 0.269 was used as the organic extractant, which is an extractant, and the calculated values are shown in Table 2.
Comparative Example 3
The procedure of Example 1 was repeated, except that methyl alcohol having an E T N value of 0.762 was used as an organic extracting agent. The calculated values are shown in Table 2.
As can be seen from Table 2, Examples 1 to 3 were separated, and allyl alcohol and allyl formate had high concentrations in the organic phase and formic acid had a high concentration in the aqueous phase.
However, it was not observed that all of the comparative examples 1 to 3 were layer-separated.
Example 4
A liquid reaction product having the composition shown in Table 1 was prepared. Based on the results of the extraction experiment of Example 2, n-butanol having an E T N value of 0.602 was used as an extractant and the process simulation as shown in FIG. 1 was carried out using Aspen. The flow of the process is shown in Figs. 2A, 2B, and 2C.
As can be seen from Table 3 in Figs. 2A, 2B and 2C, allyl alcohol of high purity can be produced by using n-butanol.
Claims (12)
1) adding a water and an organic extractant to a liquid reaction product produced by the reaction of glycerol and formic acid to produce a mixture;
2) separating the organic phase containing the allyl alcohol and the organic extractant from the mixed solution;
3) distilling the organic phase to obtain an effluent containing allyl alcohol; And
4) distilling the effluent to obtain an allyl alcohol solution,
Wherein the organic extractant comprises at least one selected from the group consisting of 1-octanol, n-butanol, and ortho-tert-butyl phenol.
Wherein the organic phase is distilled in step 3), and then the remaining organic extractant is recovered.
The weight ratio of water and organic extractant in step 1) is 1: 20 to 20: 1 ≪ / RTI >
Wherein the allyl alcohol in the total weight percentage of the allyl alcohol solution in step 4) is 60 to 80 wt%.
The liquid phase reaction product of step 1)
a) adding formic acid to glycerol, first reacting in an inert gas atmosphere, and then raising the temperature to effect a second reaction; And
and b) separating the liquid reaction product containing allyl alcohol from the gaseous reaction product generated in the step a) through a condensation process.
Wherein the first reaction of step a) is carried out at 0 to 100 ° C, and the second reaction is carried out at 220 to 240 ° C.
Wherein the heating rate in the step a) is 2.0 to 7.0 ° C / min.
Wherein the inert gas is any one selected from the group consisting of nitrogen, argon, and helium.
Wherein the gas phase reaction product comprises at least one member selected from the group consisting of carbon dioxide, water vapor, allyl formate, allyl alcohol and unreacted formic acid.
Wherein the liquid reaction product comprises at least one member selected from the group consisting of allyl alcohol, allyl formate, unreacted formic acid, and water.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20140061369 | 2014-05-22 | ||
| KR1020140061369 | 2014-05-22 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20150135096A KR20150135096A (en) | 2015-12-02 |
| KR101990384B1 true KR101990384B1 (en) | 2019-06-19 |
Family
ID=54883369
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020140079572A KR101641140B1 (en) | 2013-06-27 | 2014-06-27 | A Method for Preparation of Allyl Alcohol |
| KR1020150068004A KR102016732B1 (en) | 2014-05-22 | 2015-05-15 | Formic Acid Separating Method and Formic Acid Separation Process System |
| KR1020150068005A KR101990384B1 (en) | 2014-05-22 | 2015-05-15 | A Method for Producing highly pure Allyl Alcohol and Process system for Producing highly pure Allyl Alcohol |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020140079572A KR101641140B1 (en) | 2013-06-27 | 2014-06-27 | A Method for Preparation of Allyl Alcohol |
| KR1020150068004A KR102016732B1 (en) | 2014-05-22 | 2015-05-15 | Formic Acid Separating Method and Formic Acid Separation Process System |
Country Status (1)
| Country | Link |
|---|---|
| KR (3) | KR101641140B1 (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003267895A (en) * | 2002-03-12 | 2003-09-25 | Solvay Solexis Spa | Method of liquid-liquid extraction of polar organic material |
| WO2008092115A1 (en) * | 2007-01-26 | 2008-07-31 | The Regents Of The University Of California | Conversion of glycerol from biodiesel production to allyl alcohol |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100377034B1 (en) | 1995-03-29 | 2003-09-19 | 글리치인코포레이티드 | How to recover carboxylic acid from aqueous solution |
| KR101634221B1 (en) * | 2013-07-08 | 2016-06-28 | 주식회사 엘지화학 | Method for producing acrylic acid from glycerol |
| KR101679717B1 (en) * | 2013-06-27 | 2016-11-25 | 주식회사 엘지화학 | A Method for Preparation of Allyl alcohol and the Allyl alcohol Prepared by the Same |
-
2014
- 2014-06-27 KR KR1020140079572A patent/KR101641140B1/en active IP Right Grant
-
2015
- 2015-05-15 KR KR1020150068004A patent/KR102016732B1/en active IP Right Grant
- 2015-05-15 KR KR1020150068005A patent/KR101990384B1/en active IP Right Grant
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003267895A (en) * | 2002-03-12 | 2003-09-25 | Solvay Solexis Spa | Method of liquid-liquid extraction of polar organic material |
| WO2008092115A1 (en) * | 2007-01-26 | 2008-07-31 | The Regents Of The University Of California | Conversion of glycerol from biodiesel production to allyl alcohol |
| US20090287004A1 (en) | 2007-01-26 | 2009-11-19 | The Regents Of The University Of California | Method of Converting a Polyol to an Olefin |
Also Published As
| Publication number | Publication date |
|---|---|
| KR102016732B1 (en) | 2019-09-02 |
| KR20150135095A (en) | 2015-12-02 |
| KR20150135029A (en) | 2015-12-02 |
| KR101641140B1 (en) | 2016-07-20 |
| KR20150135096A (en) | 2015-12-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10081584B2 (en) | Process for the separation of glycols | |
| EP3153495A1 (en) | Method for producing carboxylic acid anhydride, and method for producing carboxylic acid ester | |
| KR101679717B1 (en) | A Method for Preparation of Allyl alcohol and the Allyl alcohol Prepared by the Same | |
| EP3015447B1 (en) | Method for preparing allyl alcohol | |
| US9926248B2 (en) | Process for the preparation of 3-heptanol from a mixture containing 2-ehthylhexanal and 3-heptyl formate | |
| JP5848819B2 (en) | Method for producing adamantyl (meth) acrylate | |
| KR101990384B1 (en) | A Method for Producing highly pure Allyl Alcohol and Process system for Producing highly pure Allyl Alcohol | |
| JP2002356451A (en) | Method for manufacturing acetylene diol compound | |
| CN104058960A (en) | Preparation method of methyl 3-methoxyacrylate | |
| CN104053642A (en) | Method for the production of 2-octyl acrylate by means of transesterification | |
| US20130178638A1 (en) | Process for producing dioxolane | |
| US10544077B2 (en) | Process for making formic acid utilizing higher-boiling formate esters | |
| KR20160149440A (en) | Method for separating 3-hydroxypropionic acid and Acrylic Acid | |
| JP4355489B2 (en) | Method for producing high purity 2,2,2-trifluoroethanol | |
| JP6807695B2 (en) | Method for producing 2-methoxyethyl vinyl ether | |
| JP2015017071A (en) | Method for producing ester by using 4-methyltetrahydrofuran as solvent | |
| US9822059B2 (en) | Method for separating high-boiling carboxylic acid vinyl ester/carboxylic acid mixtures | |
| US10570081B2 (en) | Process for making formic acid utilizing lower-boiling formate esters | |
| JP2010138096A (en) | METHOD FOR PRODUCING 2-t-BUTOXYETHYL ACETATE OR 1-(t-BUTOXY)-2-PROPYL ACETATE | |
| KR20170001088A (en) | Method for Producing Acrylic Acid | |
| JP2006008519A (en) | Method for producing triethylene glycol divinyl ether | |
| MX2014011878A (en) | Process for separating monochloroacetic acid and dichloroacetic acid via extractive distillation using an organic solvent. | |
| JP2018104379A (en) | Manufacturing method and composition of 2-methyl-2-hydroxy-1-propyl(meth)acrylate and/or 3-methyl-3-hydroxy-1-butyl(meth)acrylate |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant |