JP5291129B2 - 細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法 - Google Patents
細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法 Download PDFInfo
- Publication number
- JP5291129B2 JP5291129B2 JP2011005485A JP2011005485A JP5291129B2 JP 5291129 B2 JP5291129 B2 JP 5291129B2 JP 2011005485 A JP2011005485 A JP 2011005485A JP 2011005485 A JP2011005485 A JP 2011005485A JP 5291129 B2 JP5291129 B2 JP 5291129B2
- Authority
- JP
- Japan
- Prior art keywords
- dnazyme
- cells
- mrna
- bet
- specific
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000003814 drug Substances 0.000 title claims description 37
- 229940079593 drug Drugs 0.000 title claims description 30
- 238000004519 manufacturing process Methods 0.000 title claims description 15
- 201000010099 disease Diseases 0.000 title abstract description 40
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 title abstract description 40
- 108091027757 Deoxyribozyme Proteins 0.000 claims abstract description 145
- 108020004999 messenger RNA Proteins 0.000 claims abstract description 77
- 101000713602 Homo sapiens T-box transcription factor TBX21 Proteins 0.000 claims abstract description 65
- 102100036840 T-box transcription factor TBX21 Human genes 0.000 claims abstract description 54
- 230000014509 gene expression Effects 0.000 claims abstract description 43
- 125000003729 nucleotide group Chemical group 0.000 claims description 50
- 239000002773 nucleotide Substances 0.000 claims description 47
- 230000027455 binding Effects 0.000 claims description 24
- 238000009739 binding Methods 0.000 claims description 24
- 239000000758 substrate Substances 0.000 claims description 20
- 208000037976 chronic inflammation Diseases 0.000 claims description 19
- 238000011282 treatment Methods 0.000 claims description 19
- 230000003197 catalytic effect Effects 0.000 claims description 16
- 230000006020 chronic inflammation Effects 0.000 claims description 11
- 238000001727 in vivo Methods 0.000 claims description 11
- 230000004048 modification Effects 0.000 claims description 9
- 238000012986 modification Methods 0.000 claims description 9
- 230000000295 complement effect Effects 0.000 claims description 6
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- -1 nucleotide compound Chemical class 0.000 claims description 4
- CZVCGJBESNRLEQ-UHFFFAOYSA-N 7h-purine;pyrimidine Chemical compound C1=CN=CN=C1.C1=NC=C2NC=NC2=N1 CZVCGJBESNRLEQ-UHFFFAOYSA-N 0.000 claims description 3
- 230000015556 catabolic process Effects 0.000 claims description 3
- 238000006731 degradation reaction Methods 0.000 claims description 3
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 claims description 2
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 claims description 2
- 230000002401 inhibitory effect Effects 0.000 claims description 2
- 229940104230 thymidine Drugs 0.000 claims description 2
- QJAVERMNASGYHO-UHFFFAOYSA-N O=C1C=CNC(=O)N1.C1=NC=C2NC=NC2=N1 Chemical compound O=C1C=CNC(=O)N1.C1=NC=C2NC=NC2=N1 QJAVERMNASGYHO-UHFFFAOYSA-N 0.000 claims 1
- 101001066288 Gallus gallus GATA-binding factor 3 Proteins 0.000 abstract description 50
- 238000000034 method Methods 0.000 abstract description 30
- 230000000694 effects Effects 0.000 abstract description 23
- 108091032973 (ribonucleotides)n+m Proteins 0.000 abstract description 17
- 102000040945 Transcription factor Human genes 0.000 abstract description 11
- 108091023040 Transcription factor Proteins 0.000 abstract description 11
- 230000019491 signal transduction Effects 0.000 abstract description 11
- 238000012360 testing method Methods 0.000 abstract description 6
- 230000002779 inactivation Effects 0.000 abstract description 4
- 230000003110 anti-inflammatory effect Effects 0.000 abstract description 2
- 230000001506 immunosuppresive effect Effects 0.000 abstract description 2
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 abstract 1
- 230000007022 RNA scission Effects 0.000 abstract 1
- 230000003266 anti-allergic effect Effects 0.000 abstract 1
- 230000001088 anti-asthma Effects 0.000 abstract 1
- 230000002682 anti-psoriatic effect Effects 0.000 abstract 1
- 230000003356 anti-rheumatic effect Effects 0.000 abstract 1
- 239000000924 antiasthmatic agent Substances 0.000 abstract 1
- 239000003435 antirheumatic agent Substances 0.000 abstract 1
- 230000009368 gene silencing by RNA Effects 0.000 abstract 1
- 230000010534 mechanism of action Effects 0.000 abstract 1
- 210000004027 cell Anatomy 0.000 description 166
- 229920002477 rna polymer Polymers 0.000 description 42
- 238000003776 cleavage reaction Methods 0.000 description 36
- 230000007017 scission Effects 0.000 description 36
- 108090000623 proteins and genes Proteins 0.000 description 26
- 108020004459 Small interfering RNA Proteins 0.000 description 19
- 210000001519 tissue Anatomy 0.000 description 18
- 238000000338 in vitro Methods 0.000 description 16
- 239000004055 small Interfering RNA Substances 0.000 description 16
- 208000023275 Autoimmune disease Diseases 0.000 description 15
- 102000004169 proteins and genes Human genes 0.000 description 15
- 230000004069 differentiation Effects 0.000 description 14
- 102000004127 Cytokines Human genes 0.000 description 13
- 108090000695 Cytokines Proteins 0.000 description 13
- 210000001744 T-lymphocyte Anatomy 0.000 description 12
- 210000000056 organ Anatomy 0.000 description 12
- 108091028043 Nucleic acid sequence Proteins 0.000 description 10
- 101100013966 Homo sapiens GATA3 gene Proteins 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 208000037893 chronic inflammatory disorder Diseases 0.000 description 8
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 8
- 238000013461 design Methods 0.000 description 8
- 238000001502 gel electrophoresis Methods 0.000 description 8
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 8
- 239000013612 plasmid Substances 0.000 description 8
- 238000011002 quantification Methods 0.000 description 8
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical group CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 8
- 108020004414 DNA Proteins 0.000 description 7
- 238000011161 development Methods 0.000 description 7
- 230000018109 developmental process Effects 0.000 description 7
- 230000005764 inhibitory process Effects 0.000 description 7
- 238000001890 transfection Methods 0.000 description 7
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- 108091008874 T cell receptors Proteins 0.000 description 6
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 6
- 230000001684 chronic effect Effects 0.000 description 6
- 230000012085 chronic inflammatory response Effects 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 150000007523 nucleic acids Chemical class 0.000 description 6
- 102000007469 Actins Human genes 0.000 description 5
- 108010085238 Actins Proteins 0.000 description 5
- 241001482108 Alosa pseudoharengus Species 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 238000010367 cloning Methods 0.000 description 5
- 238000009396 hybridization Methods 0.000 description 5
- 238000003119 immunoblot Methods 0.000 description 5
- 239000013598 vector Substances 0.000 description 5
- 229930024421 Adenine Natural products 0.000 description 4
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 4
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- 241000699670 Mus sp. Species 0.000 description 4
- 229960000643 adenine Drugs 0.000 description 4
- 208000026935 allergic disease Diseases 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 239000000427 antigen Substances 0.000 description 4
- 102000036639 antigens Human genes 0.000 description 4
- 108091007433 antigens Proteins 0.000 description 4
- 208000006673 asthma Diseases 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 229940104302 cytosine Drugs 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 230000028993 immune response Effects 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 230000028709 inflammatory response Effects 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 238000010369 molecular cloning Methods 0.000 description 4
- 238000010839 reverse transcription Methods 0.000 description 4
- 238000010561 standard procedure Methods 0.000 description 4
- 229940113082 thymine Drugs 0.000 description 4
- 238000013518 transcription Methods 0.000 description 4
- 230000035897 transcription Effects 0.000 description 4
- 239000012096 transfection reagent Substances 0.000 description 4
- 206010003210 Arteriosclerosis Diseases 0.000 description 3
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 3
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 3
- 101100026251 Caenorhabditis elegans atf-2 gene Proteins 0.000 description 3
- 206010012442 Dermatitis contact Diseases 0.000 description 3
- 108010003338 GATA3 Transcription Factor Proteins 0.000 description 3
- 102000004610 GATA3 Transcription Factor Human genes 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 206010020751 Hypersensitivity Diseases 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 102000004289 Interferon regulatory factor 1 Human genes 0.000 description 3
- 108090000890 Interferon regulatory factor 1 Proteins 0.000 description 3
- 108090000176 Interleukin-13 Proteins 0.000 description 3
- 102000004388 Interleukin-4 Human genes 0.000 description 3
- 108090000978 Interleukin-4 Proteins 0.000 description 3
- 102000010787 Interleukin-4 Receptors Human genes 0.000 description 3
- 108010038486 Interleukin-4 Receptors Proteins 0.000 description 3
- 108010002616 Interleukin-5 Proteins 0.000 description 3
- 108010002335 Interleukin-9 Proteins 0.000 description 3
- 108010024121 Janus Kinases Proteins 0.000 description 3
- 102000015617 Janus Kinases Human genes 0.000 description 3
- 108090000744 Mitogen-Activated Protein Kinase Kinases Proteins 0.000 description 3
- 102000004232 Mitogen-Activated Protein Kinase Kinases Human genes 0.000 description 3
- 101000707232 Mus musculus SH2 domain-containing protein 2A Proteins 0.000 description 3
- 102000004422 Phospholipase C gamma Human genes 0.000 description 3
- 108010056751 Phospholipase C gamma Proteins 0.000 description 3
- 201000004681 Psoriasis Diseases 0.000 description 3
- 108010018242 Transcription Factor AP-1 Proteins 0.000 description 3
- 102100023132 Transcription factor Jun Human genes 0.000 description 3
- 239000007983 Tris buffer Substances 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 230000000172 allergic effect Effects 0.000 description 3
- 230000003321 amplification Effects 0.000 description 3
- 230000000692 anti-sense effect Effects 0.000 description 3
- 208000011775 arteriosclerosis disease Diseases 0.000 description 3
- 208000010668 atopic eczema Diseases 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 210000002808 connective tissue Anatomy 0.000 description 3
- 208000010247 contact dermatitis Diseases 0.000 description 3
- 230000002950 deficient Effects 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 230000008482 dysregulation Effects 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 238000012744 immunostaining Methods 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 238000011866 long-term treatment Methods 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 238000002844 melting Methods 0.000 description 3
- 230000008018 melting Effects 0.000 description 3
- 210000000653 nervous system Anatomy 0.000 description 3
- 238000003199 nucleic acid amplification method Methods 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 230000009870 specific binding Effects 0.000 description 3
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 3
- 101001110283 Canis lupus familiaris Ras-related C3 botulinum toxin substrate 1 Proteins 0.000 description 2
- 108010045171 Cyclic AMP Response Element-Binding Protein Proteins 0.000 description 2
- 102000005636 Cyclic AMP Response Element-Binding Protein Human genes 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 102100031480 Dual specificity mitogen-activated protein kinase kinase 1 Human genes 0.000 description 2
- 102100023266 Dual specificity mitogen-activated protein kinase kinase 2 Human genes 0.000 description 2
- 102100023275 Dual specificity mitogen-activated protein kinase kinase 3 Human genes 0.000 description 2
- 102100023274 Dual specificity mitogen-activated protein kinase kinase 4 Human genes 0.000 description 2
- 102100023401 Dual specificity mitogen-activated protein kinase kinase 6 Human genes 0.000 description 2
- 102100023332 Dual specificity mitogen-activated protein kinase kinase 7 Human genes 0.000 description 2
- 101100480905 Escherichia phage P1 tec gene Proteins 0.000 description 2
- 108010007457 Extracellular Signal-Regulated MAP Kinases Proteins 0.000 description 2
- 102000007665 Extracellular Signal-Regulated MAP Kinases Human genes 0.000 description 2
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 2
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 2
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 2
- 102100031154 Growth arrest and DNA damage-inducible protein GADD45 gamma Human genes 0.000 description 2
- 101150086785 Hlx gene Proteins 0.000 description 2
- 101001014196 Homo sapiens Dual specificity mitogen-activated protein kinase kinase 1 Proteins 0.000 description 2
- 101001115394 Homo sapiens Dual specificity mitogen-activated protein kinase kinase 3 Proteins 0.000 description 2
- 101001115395 Homo sapiens Dual specificity mitogen-activated protein kinase kinase 4 Proteins 0.000 description 2
- 101000624426 Homo sapiens Dual specificity mitogen-activated protein kinase kinase 6 Proteins 0.000 description 2
- 101000624594 Homo sapiens Dual specificity mitogen-activated protein kinase kinase 7 Proteins 0.000 description 2
- 101001066163 Homo sapiens Growth arrest and DNA damage-inducible protein GADD45 gamma Proteins 0.000 description 2
- 101000997835 Homo sapiens Tyrosine-protein kinase JAK1 Proteins 0.000 description 2
- 101000934996 Homo sapiens Tyrosine-protein kinase JAK3 Proteins 0.000 description 2
- 238000012404 In vitro experiment Methods 0.000 description 2
- 108010002350 Interleukin-2 Proteins 0.000 description 2
- 102000000588 Interleukin-2 Human genes 0.000 description 2
- 108010055717 JNK Mitogen-Activated Protein Kinases Proteins 0.000 description 2
- 108091054455 MAP kinase family Proteins 0.000 description 2
- 102000043136 MAP kinase family Human genes 0.000 description 2
- 101150040099 MAP2K2 gene Proteins 0.000 description 2
- 101100180319 Mus musculus Itk gene Proteins 0.000 description 2
- 101100405128 Mus musculus Nr4a3 gene Proteins 0.000 description 2
- 101100519086 Mus musculus Pcgf2 gene Proteins 0.000 description 2
- 101100480907 Mus musculus Tec gene Proteins 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 101710181935 Phosphate-binding protein PstS 1 Proteins 0.000 description 2
- 238000002123 RNA extraction Methods 0.000 description 2
- 239000012979 RPMI medium Substances 0.000 description 2
- 208000025747 Rheumatic disease Diseases 0.000 description 2
- 108010019992 STAT4 Transcription Factor Proteins 0.000 description 2
- 102000005886 STAT4 Transcription Factor Human genes 0.000 description 2
- 102000001712 STAT5 Transcription Factor Human genes 0.000 description 2
- 108010029477 STAT5 Transcription Factor Proteins 0.000 description 2
- 108010011005 STAT6 Transcription Factor Proteins 0.000 description 2
- 102000013968 STAT6 Transcription Factor Human genes 0.000 description 2
- 238000010818 SYBR green PCR Master Mix Methods 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 101150045565 Socs1 gene Proteins 0.000 description 2
- 101150043341 Socs3 gene Proteins 0.000 description 2
- 108700027336 Suppressor of Cytokine Signaling 1 Proteins 0.000 description 2
- 108700027337 Suppressor of Cytokine Signaling 3 Proteins 0.000 description 2
- 102100024779 Suppressor of cytokine signaling 1 Human genes 0.000 description 2
- 102100024283 Suppressor of cytokine signaling 3 Human genes 0.000 description 2
- 108010009978 Tec protein-tyrosine kinase Proteins 0.000 description 2
- 102100033438 Tyrosine-protein kinase JAK1 Human genes 0.000 description 2
- 102100025387 Tyrosine-protein kinase JAK3 Human genes 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 230000036755 cellular response Effects 0.000 description 2
- 206010009887 colitis Diseases 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- 210000004748 cultured cell Anatomy 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 210000000750 endocrine system Anatomy 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000000899 immune system response Effects 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 238000001638 lipofection Methods 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 101150068383 mkk2 gene Proteins 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 238000003359 percent control normalization Methods 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000000651 prodrug Substances 0.000 description 2
- 238000004393 prognosis Methods 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000007634 remodeling Methods 0.000 description 2
- 108091008146 restriction endonucleases Proteins 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 208000002491 severe combined immunodeficiency Diseases 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 108010087686 src-Family Kinases Proteins 0.000 description 2
- 102000009076 src-Family Kinases Human genes 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 1
- ZIIUUSVHCHPIQD-UHFFFAOYSA-N 2,4,6-trimethyl-N-[3-(trifluoromethyl)phenyl]benzenesulfonamide Chemical compound CC1=CC(C)=CC(C)=C1S(=O)(=O)NC1=CC=CC(C(F)(F)F)=C1 ZIIUUSVHCHPIQD-UHFFFAOYSA-N 0.000 description 1
- 241000238876 Acari Species 0.000 description 1
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 1
- 102000057234 Acyl transferases Human genes 0.000 description 1
- 108700016155 Acyl transferases Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 208000031104 Arterial Occlusive disease Diseases 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 108010077544 Chromatin Proteins 0.000 description 1
- RGJOEKWQDUBAIZ-IBOSZNHHSA-N CoASH Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCS)O[C@H]1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-IBOSZNHHSA-N 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 230000005778 DNA damage Effects 0.000 description 1
- 231100000277 DNA damage Toxicity 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 108010051542 Early Growth Response Protein 1 Proteins 0.000 description 1
- 102100023226 Early growth response protein 1 Human genes 0.000 description 1
- 206010014950 Eosinophilia Diseases 0.000 description 1
- 102100022086 GRB2-related adapter protein 2 Human genes 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- 206010019851 Hepatotoxicity Diseases 0.000 description 1
- 101000687808 Homo sapiens Suppressor of cytokine signaling 2 Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000819111 Homo sapiens Trans-acting T-cell-specific transcription factor GATA-3 Proteins 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102100020792 Interleukin-12 receptor subunit beta-2 Human genes 0.000 description 1
- 101710103840 Interleukin-12 receptor subunit beta-2 Proteins 0.000 description 1
- 101150069380 JAK3 gene Proteins 0.000 description 1
- 102000019145 JUN kinase activity proteins Human genes 0.000 description 1
- 108010000837 Janus Kinase 1 Proteins 0.000 description 1
- 102000002295 Janus Kinase 1 Human genes 0.000 description 1
- 241000215452 Lotus corniculatus Species 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 102000004083 Lymphotoxin-alpha Human genes 0.000 description 1
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 1
- 108010033714 Maf Transcription Factors Proteins 0.000 description 1
- 102000007307 Maf Transcription Factors Human genes 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101100101239 Mus musculus Txk gene Proteins 0.000 description 1
- 102000003945 NF-kappa B Human genes 0.000 description 1
- 108010057466 NF-kappa B Proteins 0.000 description 1
- 206010029155 Nephropathy toxic Diseases 0.000 description 1
- 201000009053 Neurodermatitis Diseases 0.000 description 1
- 102000007999 Nuclear Proteins Human genes 0.000 description 1
- 108010089610 Nuclear Proteins Proteins 0.000 description 1
- 208000011623 Obstructive Lung disease Diseases 0.000 description 1
- 239000012124 Opti-MEM Substances 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 208000001132 Osteoporosis Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108091093037 Peptide nucleic acid Proteins 0.000 description 1
- 102000015439 Phospholipases Human genes 0.000 description 1
- 108010064785 Phospholipases Proteins 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 208000009052 Precursor T-Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 102000001253 Protein Kinase Human genes 0.000 description 1
- 102000007987 Proto-Oncogene Proteins c-maf Human genes 0.000 description 1
- 108010089507 Proto-Oncogene Proteins c-maf Proteins 0.000 description 1
- 102000000850 Proto-Oncogene Proteins c-rel Human genes 0.000 description 1
- 108010001859 Proto-Oncogene Proteins c-rel Proteins 0.000 description 1
- 238000011530 RNeasy Mini Kit Methods 0.000 description 1
- 102100024784 Suppressor of cytokine signaling 2 Human genes 0.000 description 1
- 208000029052 T-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- 210000000447 Th1 cell Anatomy 0.000 description 1
- 206010054094 Tumour necrosis Diseases 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 102000038627 Zinc finger transcription factors Human genes 0.000 description 1
- 108091007916 Zinc finger transcription factors Proteins 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 201000011186 acute T cell leukemia Diseases 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 239000011543 agarose gel Substances 0.000 description 1
- 239000013566 allergen Substances 0.000 description 1
- 201000009961 allergic asthma Diseases 0.000 description 1
- 208000030961 allergic reaction Diseases 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000003092 anti-cytokine Effects 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 208000021328 arterial occlusion Diseases 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 201000004982 autoimmune uveitis Diseases 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000011712 cell development Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 210000003483 chromatin Anatomy 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- RGJOEKWQDUBAIZ-UHFFFAOYSA-N coenzime A Natural products OC1C(OP(O)(O)=O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-UHFFFAOYSA-N 0.000 description 1
- 239000005516 coenzyme A Substances 0.000 description 1
- 229940093530 coenzyme a Drugs 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 108010057085 cytokine receptors Proteins 0.000 description 1
- 102000003675 cytokine receptors Human genes 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000003936 denaturing gel electrophoresis Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- KDTSHFARGAKYJN-UHFFFAOYSA-N dephosphocoenzyme A Natural products OC1C(O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 KDTSHFARGAKYJN-UHFFFAOYSA-N 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000007686 hepatotoxicity Effects 0.000 description 1
- 231100000304 hepatotoxicity Toxicity 0.000 description 1
- 102000050891 human GATA3 Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 229940099472 immunoglobulin a Drugs 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 229960003444 immunosuppressant agent Drugs 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 230000000415 inactivating effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 208000030603 inherited susceptibility to asthma Diseases 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- PGHMRUGBZOYCAA-UHFFFAOYSA-N ionomycin Natural products O1C(CC(O)C(C)C(O)C(C)C=CCC(C)CC(C)C(O)=CC(=O)C(C)CC(C)CC(CCC(O)=O)C)CCC1(C)C1OC(C)(C(C)O)CC1 PGHMRUGBZOYCAA-UHFFFAOYSA-N 0.000 description 1
- PGHMRUGBZOYCAA-ADZNBVRBSA-N ionomycin Chemical compound O1[C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)/C=C/C[C@@H](C)C[C@@H](C)C(/O)=C/C(=O)[C@@H](C)C[C@@H](C)C[C@@H](CCC(O)=O)C)CC[C@@]1(C)[C@@H]1O[C@](C)([C@@H](C)O)CC1 PGHMRUGBZOYCAA-ADZNBVRBSA-N 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000011813 knockout mouse model Methods 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000012160 loading buffer Substances 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 238000010841 mRNA extraction Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 238000007837 multiplex assay Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 230000007694 nephrotoxicity Effects 0.000 description 1
- 231100000417 nephrotoxicity Toxicity 0.000 description 1
- 230000001703 neuroimmune Effects 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 210000004967 non-hematopoietic stem cell Anatomy 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 230000000414 obstructive effect Effects 0.000 description 1
- 101710135378 pH 6 antigen Proteins 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 230000004796 pathophysiological change Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000000751 protein extraction Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 208000023504 respiratory system disease Diseases 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000020183 skimmed milk Nutrition 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 108091006106 transcriptional activators Proteins 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000003151 transfection method Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1136—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against growth factors, growth regulators, cytokines, lymphokines or hormones
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6809—Methods for determination or identification of nucleic acids involving differential detection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/12—Type of nucleic acid catalytic nucleic acids, e.g. ribozymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering N.A.
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Immunology (AREA)
- Biochemistry (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Analytical Chemistry (AREA)
- Pulmonology (AREA)
- Endocrinology (AREA)
- Dermatology (AREA)
- Urology & Nephrology (AREA)
- Epidemiology (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Pain & Pain Management (AREA)
- Transplantation (AREA)
- Rheumatology (AREA)
Description
・自己免疫疾患およびリウマチ性(rheumatischen Formenkreises)の疾患(とりわけ皮膚、肺、腎臓、血管系、神経系、結合組織、運動器官、内分泌系における症状発現)
・アレルギー性急性反応および喘息
・慢性閉塞性肺疾患(COPD)
・動脈硬化症
・乾癬および接触皮膚炎
・臓器移植、骨髄移植後の慢性拒絶反応、
が包含される。
(A)通常は人間にとって無害な抗原に対する過剰な免疫応答が起こる。この抗原は、環境的要素(例えば花粉、動物の毛、食物、ダニ、化学物質(例えば防腐剤、染料、洗剤)のようなアレルゲン)である場合もある。そして患者はアレルギー性反応を示すようになる。例えば能動および受動喫煙の場合に、慢性閉塞性肺疾患(COPD)を発病することがある。一方、免疫系が自己組織の成分に対しても反応する場合があり、自己組織の成分を非自己として認識し、それに対して炎症反応を起こす。この場合に、自己免疫疾患が発病する。いずれの場合においても、無害で非毒性の抗原が非自己または危険なものとして誤って認識され、不適当な炎症反応が引き起こされる。
適応免疫系(重要な成分:Tリンパ球とBリンパ球)の細胞の活性化および分化が起こる。その後、T細胞が、高度に特化したエフェクター細胞に分化することで中心的機能を引き継ぐ。このとき、T細胞は、特に
以下の機能:抗体産生、免疫系のエフェクター細胞(例えば、好中球顆粒球、好塩基球顆粒球、好酸球顆粒球)の機能性制御、先天性免疫系の機能へのフィードバック、例えば上皮細胞、内皮細胞、結合組織、骨および軟骨、特に神経細胞のような非造血性細胞の機能性への影響、
を含む一定のエフェクター機構を活性化および獲得する。この際、免疫系と神経系の間に特別な相互作用が生じ、この相互作用から慢性炎症における神経−免疫相互作用のコンセプトが展開された。
(1)これらの疾患では、患者に特異的な(サブ)−表現型が現れる。従って、薬剤は高い患者または症例特異性を示すものでなくてはならない。
配列1:ヒトGATA−3、データバンクNo.:XM_043124
配列2:ヒトGATA−3、データバンクNo.:X58072
配列3:ヒトGATA−3(プラスミドpCR2.1より配列決定)
塩基が多様な場合を灰色のバックで、GATA−3のクローニング用プライマー位置を下線で示した。DNAzyme hgd40の位置は、太字のアルファベット、灰色のバックおよび下線で強調されている(A=アデニン、G=グアニン、C=シトシン、T=チミン)。
配列1:ヒトT−bet、データバンクNo.:NM_013351
配列2:ヒトT−bet(pBluescript−SKより配列決定)
塩基が多様な場合を灰色のバックで、T−betのクローニング用プライマー位置を下線で示した。LightCyclerにおける半定量化用プライマー位置を囲み線で示す。DNAzyme td54およびtd69の位置は、灰色のバックおよび下線で、またtd70は、更に太字のアルファベットで強調されている(A=アデニン、G=グアニン、C=シトシン、T=チミン)。
・STAT4、STAT5aおよびSTAT1(シグナル伝達物質および転写アクチベーター)
・c−Rel
・CREB2(cAMP response element−binding protein 2:cAMP応答要素結合タンパク質2)
・ATF−2、ATF−2
・Hlx
・IRF−1(インターフェロン制御因子−1)
・c−Maf
・NFAT(Nuclear factor of activated T cells:活性化T細胞核因子)
・NIP45(NF−AT interacting protein 45:NF−AT相互作用タンパク質45)
・AP1(Activator Protein 1:アクチベータータンパク質1)
・Mel−18
・SKAT−2(SCAN box, KRAB domain associated with a Th2phenotype:SCANボックス、Th2表現型関連KRABドメイン)
・CTLA−4(Cytolytic T lymphocyte−associated antigen 4:細胞障害性Tリンパ球抗原4)。
・Srcキナーゼ
・Tecキナーゼ
Rlk(ヒトではTxk)
Itk
Tec
・RIBP(Rlk/Itk結合タンパク質)
・PLCγ(ホスホリパーゼCγ1)
・MAPキナーゼ(マイトジェン活性化タンパク質キナーゼ)
ERK
JNK
P38
・MKK(MAPキナーゼキナーゼ)
MKK1
MKK2
MKK3
MKK4
MKK6
MKK7
・Rac2
・GADD45(増殖停止およびDNA損傷遺伝子45(Growth arrest and DNA damage gene 45))
GADD45β
GADD45γ
・SOCS(サイトカインシグナルのサプレッサー)
CIS(サイトカイン誘導性SH2タンパク質)
SOCS1
SOCS2
SOCS3
・JAK(Janusキナーゼ)
JAK1
JAK3
・NIP45(NF−AT相互作用タンパク質) 。
a)その標的細胞における発現が、コントロール細胞での発現と比較して差異を有するリボ核酸分子の同定
b)段階a)のリボ核酸分子と結合し、それを機能的に不活化する特異的なリボ核酸分子の設計
c)段階b)の特異的なリボ核酸分子の標的細胞への導入
d)段階b)の特異的なリボ核酸分子および/または段階c)の標的細胞の薬剤への製剤化、
を含む、細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法を提供する。
a)その標的細胞における発現が、コントロール細胞での発現と比較して差異を示すリボ核酸分子の同定
i)慢性炎症反応の発症に関与しているナイーブCD4+細胞を標的細胞として使用した。
に記載されている標準的な方法を用いて行った。
なおこれは、Qiagen社製RNeasy Kitを用いる方法、或いは、Qiagen社製Oligotex mRNA Kitを用いて直接CD4+目標細胞からmRNAを単離する方法で、製造者の指示に従って行うこともできる。
図3に、GATA−3 mRNAに特異的なDNAzymeである本発明のプールhgd 1〜hgd 70を示す。該DNAzymeの全長は33ヌクレオチドであるが、中心の触媒ドメインは15ヌクレオチド(小文字)であり、公知の10−23 DNAzyme(図2)の触媒ドメインと一致する。この触媒ドメインは、それぞれ9ヌクレオチドからなる2つの基質結合ドメイン(大文字)に左右から挟まれている。左右の基質結合ドメインのヌクレオチド配列は、DNAzyme hgd 1〜hgd 70において異なっており且つ可変であるため、GATA−3 mRNAへのワトソン・クリック対合による様々な特異的結合が生じる。
1)3’末端に安定化インバース・チミジン(inverses Thymidin)
2)細胞のトランスフェクション効率をFACS分析で評価するための、5’末端のFAMによる標識、
を使用した。
フォワードプライマー GGCGCCGTCTTGATACTTT
リバースプライマー CCGAAAATTGAGAGAGAAGGAA、
を用いて逆転写し、2731ヌクレオチドの長さを持つPCR産物が増幅される(JumpStart Accu Taq DNA Polymerase, Sigma社製)。
高い活性を示したDNAzyme hgd 11、hgd 13、hgd 17およびhgd 40を、上述のように修飾してまたは修飾しないで標的細胞に使用した。
GATA−3特異的な基質結合ドメインを有する種々のDNAzymeの解析により、DNAzyme hgd 40がGATA−3の発現をin vivoにおいて特異的に阻害し、細胞および/または組織および/または疾患フェーズ特異的な薬剤を製造するための特異的なリボ核酸として適していることが示される。ここでは、hgd 40(5’−GTGGATGGAggctagctacaacgaGTCTTGGAG)またはhgd 40で形質転換された細胞を、薬学的に許容可能なキャリアー、例えばリポソームまたは生分解性ポリマーを含む医薬組成物に組み入れる。
a)その標的細胞における発現が、コントロール細胞での発現と比較して差異を示すリボ核酸分子の同定
上述の方法と同様に同定を行った。
T−betの切断部位の同定は、GATA−3に関する記載と同様に行った。
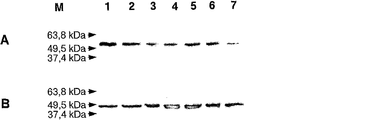
DNAzyme td54、td69およびtd70を、上述のように修飾してまたは修飾しないで標的細胞に使用した。Jurkat E6.1細胞のトランスフェクションは、GATA−3の実施例同様に行った。Jurkat E6.1細胞のトランスフェクション後、GAPDH−mRNAの発現に対する相対的なT−bet−mRNA量を、Real−Time−PCR(LightCycler, Roche社)を用いて定量的に測定し、DNAzymeのin vitro効率を調べた。
様々なT−bet特異的な基質結合ドメインを有するDNAzymeの分析により、DNAzyme td69およびtd70がT−betの発現をin vivoにおいて特異的に阻害し、細胞および/または組織および/または疾患フェーズ特異的な薬剤製造のための特異的なリボ核酸として適していることが示される。
Claims (8)
- 以下:
・ヌクレオチド配列GGCTAGCTACAACGAを有する触媒ドメインであって、それが結合する各々のプリン−ピリミジン結合部位においてT−bet mRNAを切断する触媒ドメイン
・触媒ドメインの3’末端に結合する右側の基質結合ドメイン、および
・触媒ドメインの5’末端に結合する左側の基質結合ドメインであって、前記2つの基質結合ドメインはT−bet mRNAの2つの領域とそれぞれ相補的であるために前記mRNAとハイブリダイズする
・in vivoで活性である、および
・配列td69 GGCAATGAA GGCTAGCTACAACGA TGGGTTTCTまたはtd70 TCACGGCAA GGCTAGCTACAACGA GAACTGGGTを含む、
からなることを特徴とする、DNAzyme。 - T−bet mRNAの触媒ドメインをプリン−ウラシル結合部位で切断することを特徴とする、請求項1記載のDNAzyme。
- 3’−3’インバージョン(Inversion)の導入により、生体における分解に対して安定化されていることを特徴とする、請求項1または2記載のDNAzyme。
- 修飾ヌクレオチドまたはヌクレオチド化合物の導入により、生体における分解に対して安定化されていることを特徴とする、請求項1〜3のいずれか1つに記載のDNAzyme。
- 修飾として、3’末端のインバースチミジン(inverses Thymidin)および/または5’末端のFAM標識を有することを特徴とする、請求項1〜4のいずれか1つに記載のDNAzyme。
- 請求項2〜5のいずれか1つに記載のDNAzymeおよび薬学的に許容可能なキャリアーを含む薬剤。
- 慢性炎症の治療用薬剤の製造のための、請求項1〜5のいずれか1つに記載のDNAzymeの使用。
- 慢性炎症の治療のために患者の標的細胞におけるT−bet発現を特異的に阻害するための薬剤の製造のための、請求項1〜5のいずれか1つに記載のDNAzymeの使用。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE10346487.5 | 2003-10-02 | ||
| DE10346487A DE10346487A1 (de) | 2003-10-02 | 2003-10-02 | Verfahren zur Herstellung eines Zell- und/oder Gewebe- und/oder Krankheitsphasen-spezifischen Arzneimittels |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006529624A Division JP5013870B2 (ja) | 2003-10-02 | 2004-10-01 | 細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011074089A JP2011074089A (ja) | 2011-04-14 |
| JP5291129B2 true JP5291129B2 (ja) | 2013-09-18 |
Family
ID=34399325
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006529624A Expired - Lifetime JP5013870B2 (ja) | 2003-10-02 | 2004-10-01 | 細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法 |
| JP2011005485A Expired - Lifetime JP5291129B2 (ja) | 2003-10-02 | 2011-01-14 | 細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006529624A Expired - Lifetime JP5013870B2 (ja) | 2003-10-02 | 2004-10-01 | 細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法 |
Country Status (14)
| Country | Link |
|---|---|
| US (2) | US8119789B2 (ja) |
| EP (2) | EP1670919B1 (ja) |
| JP (2) | JP5013870B2 (ja) |
| KR (1) | KR101121818B1 (ja) |
| AT (2) | ATE391788T1 (ja) |
| CA (2) | CA2541240C (ja) |
| CY (1) | CY1108161T1 (ja) |
| DE (3) | DE10346487A1 (ja) |
| DK (2) | DK1670919T3 (ja) |
| ES (2) | ES2320706T3 (ja) |
| PL (2) | PL1670919T3 (ja) |
| PT (2) | PT1857555E (ja) |
| SI (1) | SI1670919T1 (ja) |
| WO (1) | WO2005033314A2 (ja) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI322690B (en) * | 2006-05-11 | 2010-04-01 | Flysun Dev Co Ltd | Short interference ribonucleic acids for treating allergic dieases |
| JP2012520685A (ja) * | 2009-03-19 | 2012-09-10 | メルク・シャープ・エンド・ドーム・コーポレイション | 低分子干渉核酸(siNA)を用いたGATA結合タンパク質3(GATA3)遺伝子発現のRNA干渉媒介性阻害 |
| JPWO2010110314A1 (ja) * | 2009-03-27 | 2012-10-04 | 協和発酵キリン株式会社 | 核酸を含有する肺高血圧症治療剤 |
| DE102010007562A1 (de) | 2010-02-10 | 2011-08-11 | sterna biologicals GmbH & Co KG, 35043 | Dermatologische, pharmazeutische Zusammensetzung geeignet für Oligonukleotide |
| DE102010056610A1 (de) * | 2010-12-31 | 2012-07-05 | Volker A. Erdmann | Pharmazeutische Zusammensetzung enthaltend L-DNA |
| DE102011109868A1 (de) * | 2011-08-10 | 2013-02-14 | STERNA BIOLOGICALS GmbH & Co. KG. | Multiple Emulsion |
| EP2708898A1 (de) * | 2012-09-14 | 2014-03-19 | Sterna Biologicals GmbH & Co. Kg | Verfahren zur Diagnose eines molekularen Phänotyps eines an einer mit chronischen Entzündungen einhergehenden Erkrankung leidenden Patienten |
| HUE046190T2 (hu) * | 2015-05-15 | 2020-02-28 | Sterna Biologicals Gmbh & Co Kg | GATA-3 inhibitorok TH2-vezérelt asztma kezelése során történõ alkalmazásra |
| US10647987B2 (en) | 2016-02-26 | 2020-05-12 | Secama Pharmaceuticals GmbH & Co KG | Approach for treating inflammatory disorders |
| EP3211081A1 (en) | 2016-02-26 | 2017-08-30 | Secarna Pharmaceuticals GmbH & Co. KG | Novel approach for treating inflammatory disorders |
| WO2017205506A1 (en) | 2016-05-24 | 2017-11-30 | Emory University | Particles with rna cleaving nucleobase polymers and uses for managing inflammatory disorders |
| EP3501607A1 (de) * | 2017-12-22 | 2019-06-26 | Sterna Biologicals GmbH & Co. KG | Zusammensetzung zur behandlung eines an einer mit chronischen entzündungen einhergehenden atemwegserkrankung leidenden patienten sowie herstellungsverfahren und verwendung der zusammensetzung |
| EP3514235B8 (de) | 2018-01-18 | 2024-02-14 | Sterna Biologicals GmbH | Zusammensetzung zur behandlung eines an colitis ulcerosa leidenden patienten sowie verwendung der zusammensetzung als arzneimittel |
| US20210022324A1 (en) * | 2018-03-05 | 2021-01-28 | Dr. Reddy's Institute Of Life Sciences | Embryonic zebrafish models using dnazyme mediated knockdown |
| EP3603617A1 (de) | 2018-07-30 | 2020-02-05 | Sterna Biologicals GmbH & Co. KG | Aerosolerzeugungseinrichtung zur inhalativen verabreichung einer antisense-molekül-haltigen zusammensetzung |
| EP3805242A1 (en) | 2019-10-07 | 2021-04-14 | Sterna Biologicals GmbH & Co. KG | Method for the production of a catalytically active dna molecule having improved activity and its use in a method of treating asthma |
| US11976282B2 (en) | 2020-06-23 | 2024-05-07 | Qatar University | GATA3 inhibitors for the promotion of subcutaneous fat deposition |
| AU2021411103A1 (en) * | 2020-12-28 | 2023-07-13 | 1E Therapeutics, Ltd. | P21 mrna target areas for silencing |
| AU2021416356A1 (en) | 2020-12-28 | 2023-08-10 | 1E Therapeutics, Ltd. | P21 mrna targeting dnazymes |
| JP2024534653A (ja) | 2021-09-30 | 2024-09-20 | シュテルナ バイオロジカルズ ゲーエムベーハー | Dnaザイムヒドロゲル製剤 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU763135B2 (en) * | 1998-03-27 | 2003-07-17 | Johnson & Johnson Research Pty. Limited | Catalytic nucleic acid-based diagnostic methods |
| CA2340322A1 (en) * | 1998-08-13 | 2000-02-24 | Johnson & Johnson Research Pty Limited | Dnazymes and methods for treating restenosis |
| AUPP810399A0 (en) * | 1999-01-11 | 1999-02-04 | Unisearch Limited | Catalytic molecules |
| JP2002537792A (ja) * | 1999-03-05 | 2002-11-12 | エピジェネシス ファーマシューティカルズ,アイエヌシー | 標的および経路をバリデート/インバリデートする方法 |
| AUPQ201499A0 (en) * | 1999-08-04 | 1999-08-26 | Unisearch Limited | Treatment of inflammatory and malignant diseases |
| WO2002068637A2 (en) * | 2000-10-20 | 2002-09-06 | Ribozyme Pharmaceuticals, Inc. | Nucleic acid-based treatment of diseases or conditions related to west nile virus infection |
| US20030148971A1 (en) * | 2002-02-04 | 2003-08-07 | Handel Malcolm Lovell | Treatment of inflammatory and malignant diseases |
-
2003
- 2003-10-02 DE DE10346487A patent/DE10346487A1/de not_active Withdrawn
-
2004
- 2004-10-01 DK DK04802618T patent/DK1670919T3/da active
- 2004-10-01 JP JP2006529624A patent/JP5013870B2/ja not_active Expired - Lifetime
- 2004-10-01 WO PCT/DE2004/002197 patent/WO2005033314A2/de active IP Right Grant
- 2004-10-01 ES ES07013576T patent/ES2320706T3/es not_active Expired - Lifetime
- 2004-10-01 SI SI200430734T patent/SI1670919T1/sl unknown
- 2004-10-01 EP EP04802618A patent/EP1670919B1/de not_active Expired - Lifetime
- 2004-10-01 KR KR1020067008072A patent/KR101121818B1/ko active IP Right Grant
- 2004-10-01 CA CA2541240A patent/CA2541240C/en not_active Expired - Lifetime
- 2004-10-01 ES ES04802618T patent/ES2304633T3/es not_active Expired - Lifetime
- 2004-10-01 US US10/574,560 patent/US8119789B2/en active Active
- 2004-10-01 DK DK07013576T patent/DK1857555T3/da active
- 2004-10-01 AT AT04802618T patent/ATE391788T1/de active
- 2004-10-01 PT PT07013576T patent/PT1857555E/pt unknown
- 2004-10-01 PL PL04802618T patent/PL1670919T3/pl unknown
- 2004-10-01 PT PT04802618T patent/PT1670919E/pt unknown
- 2004-10-01 PL PL07013576T patent/PL1857555T3/pl unknown
- 2004-10-01 DE DE502004008802T patent/DE502004008802D1/de not_active Expired - Lifetime
- 2004-10-01 DE DE502004006791T patent/DE502004006791D1/de not_active Expired - Lifetime
- 2004-10-01 AT AT07013576T patent/ATE419364T1/de active
- 2004-10-01 EP EP07013576A patent/EP1857555B1/de not_active Expired - Lifetime
- 2004-10-01 CA CA2776997A patent/CA2776997C/en not_active Expired - Lifetime
-
2008
- 2008-06-27 CY CY20081100675T patent/CY1108161T1/el unknown
-
2011
- 2011-01-14 JP JP2011005485A patent/JP5291129B2/ja not_active Expired - Lifetime
- 2011-03-01 US US13/037,411 patent/US8247544B2/en not_active Expired - Lifetime
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5291129B2 (ja) | 細胞および/または組織および/または疾患フェーズ特異的な薬剤の製造方法 | |
| Mor et al. | Species-specific microRNA roles elucidated following astrocyte activation | |
| WO2018067991A1 (en) | Modulation of novel immune checkpoint targets | |
| EP2374884A2 (en) | Human miRNAs isolated from mesenchymal stem cells | |
| US8580562B2 (en) | Inhibitory RNA for modulating the molecular function of ZFAT gene | |
| He et al. | Identification of a Long noncoding RNA TRAF3IP2-AS1 as key regulator of IL-17 signaling through the SRSF10–IRF1–Act1 axis in autoimmune diseases | |
| US20210254097A1 (en) | Engineering of immune cells for ex vivo cell therapy applications | |
| JP2007507438A5 (ja) | ||
| JP2020143123A (ja) | マクロファージ活性化の主要制御因子としてのparp9およびparp14 | |
| US10323067B2 (en) | Methods and compositions for controlling gene expression and treating cancer | |
| JP2009530417A (ja) | 炎症性サイトカインの発現を下方制御し敗血症を治療するためのアポトーシス特異的eIF−5AsiRNAの使用 | |
| JP6566612B2 (ja) | 免疫体質又は免疫応答型を判定するためのバイオマーカー及び免疫応答型制御剤 | |
| EP3635098B1 (en) | T-cells modified to overexpress phf19 | |
| Xu et al. | Upregulated miR-96-5p inhibits cell proliferation by targeting HBEGF in T-cell acute lymphoblastic leukemia cell line | |
| JP2010529852A (ja) | 癌治療のためのNuMAのRNAi媒介ノックダウン | |
| JPWO2009147742A1 (ja) | ヒト・オステオポンチンsiRNA | |
| JP2008211985A (ja) | Il−17産生抑制物質及びそのスクリーニング方法 | |
| WO2020196453A1 (ja) | Il-33関連疾患の予防又は治療剤 | |
| Pickett-Leonard | Identification of novel genes and compounds for the development of precision therapeutics for dystrophic epidermolysis bullosa and associated cutaneous squamous cell carcinoma | |
| Tian | Supplementary Methods Study subjects and genotyping | |
| JP2023013932A (ja) | SARS-CoV-2 RNA配列に基づく新規siRNA及びその利用 | |
| Gamez et al. | Primary Immunodeficiency: 137 Hypogammaglobulinemia in a Boy: Consider Also X-linked Lymphoproliferative Disease | |
| JP2008142011A (ja) | オステオポンチンsiRNA | |
| JP2016158525A (ja) | がんの悪性度、予後及び/又は抗がん剤治療の有効性を判定するためのバイオマーカー、抗がん剤を選択するためのコンパニオン診断薬並びに抗がん剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20110520 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20110520 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121218 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130130 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130528 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130606 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5291129 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |