JP2023529545A - ポリペプチドアルブミンナノ粒子、およびその調製方法と使用 - Google Patents
ポリペプチドアルブミンナノ粒子、およびその調製方法と使用 Download PDFInfo
- Publication number
- JP2023529545A JP2023529545A JP2022563369A JP2022563369A JP2023529545A JP 2023529545 A JP2023529545 A JP 2023529545A JP 2022563369 A JP2022563369 A JP 2022563369A JP 2022563369 A JP2022563369 A JP 2022563369A JP 2023529545 A JP2023529545 A JP 2023529545A
- Authority
- JP
- Japan
- Prior art keywords
- polypeptide
- albumin
- nanoparticles
- solution
- cationic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000002105 nanoparticle Substances 0.000 title claims abstract description 161
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 148
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 141
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 138
- 102000009027 Albumins Human genes 0.000 title claims abstract description 113
- 108010088751 Albumins Proteins 0.000 title claims abstract description 113
- 238000000034 method Methods 0.000 title claims abstract description 27
- 238000002360 preparation method Methods 0.000 title claims abstract description 19
- 125000002091 cationic group Chemical group 0.000 claims abstract description 64
- 230000002209 hydrophobic effect Effects 0.000 claims abstract description 18
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 53
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 53
- 238000002156 mixing Methods 0.000 claims description 20
- 239000000872 buffer Substances 0.000 claims description 19
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 16
- 235000001014 amino acid Nutrition 0.000 claims description 9
- 229940024606 amino acid Drugs 0.000 claims description 9
- 150000002632 lipids Chemical class 0.000 claims description 9
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 8
- 150000001413 amino acids Chemical class 0.000 claims description 8
- 239000004475 Arginine Substances 0.000 claims description 7
- 239000002246 antineoplastic agent Substances 0.000 claims description 7
- 229940041181 antineoplastic drug Drugs 0.000 claims description 7
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 7
- DEQXHPXOGUSHDX-UHFFFAOYSA-N methylaminomethanetriol;hydrochloride Chemical compound Cl.CNC(O)(O)O DEQXHPXOGUSHDX-UHFFFAOYSA-N 0.000 claims description 7
- 239000008363 phosphate buffer Substances 0.000 claims description 7
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 6
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 claims description 6
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 6
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 claims description 6
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 6
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 6
- 239000008194 pharmaceutical composition Substances 0.000 claims description 6
- 108091003079 Bovine Serum Albumin Proteins 0.000 claims description 5
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 claims description 5
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 5
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 5
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 5
- 229960000310 isoleucine Drugs 0.000 claims description 5
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 5
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 claims description 4
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 4
- 239000004472 Lysine Substances 0.000 claims description 4
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 claims description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 claims description 4
- 239000004474 valine Substances 0.000 claims description 4
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 3
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 claims description 3
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 3
- 235000004279 alanine Nutrition 0.000 claims description 3
- 235000012000 cholesterol Nutrition 0.000 claims description 3
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 3
- 229930195729 fatty acid Natural products 0.000 claims description 3
- 239000000194 fatty acid Substances 0.000 claims description 3
- 150000004665 fatty acids Chemical class 0.000 claims description 3
- 229930182817 methionine Natural products 0.000 claims description 3
- 229940098773 bovine serum albumin Drugs 0.000 claims description 2
- 239000003085 diluting agent Substances 0.000 claims description 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 2
- 210000004881 tumor cell Anatomy 0.000 abstract description 22
- 230000000694 effects Effects 0.000 abstract description 19
- 230000008685 targeting Effects 0.000 abstract description 13
- 230000021603 oncosis Effects 0.000 abstract description 11
- 230000002949 hemolytic effect Effects 0.000 abstract description 9
- 230000001988 toxicity Effects 0.000 abstract description 9
- 231100000419 toxicity Toxicity 0.000 abstract description 9
- 230000005975 antitumor immune response Effects 0.000 abstract description 8
- 230000002147 killing effect Effects 0.000 abstract description 8
- 239000000243 solution Substances 0.000 description 51
- 206010028980 Neoplasm Diseases 0.000 description 38
- 210000004027 cell Anatomy 0.000 description 38
- 239000002245 particle Substances 0.000 description 24
- 108010011110 polyarginine Proteins 0.000 description 21
- 229910021642 ultra pure water Inorganic materials 0.000 description 20
- 239000012498 ultrapure water Substances 0.000 description 20
- 241000699670 Mus sp. Species 0.000 description 14
- 238000012360 testing method Methods 0.000 description 13
- 238000009826 distribution Methods 0.000 description 11
- 210000004369 blood Anatomy 0.000 description 10
- 239000008280 blood Substances 0.000 description 10
- 210000003462 vein Anatomy 0.000 description 10
- 150000001875 compounds Chemical class 0.000 description 9
- 238000011725 BALB/c mouse Methods 0.000 description 8
- 230000005540 biological transmission Effects 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 239000003814 drug Substances 0.000 description 7
- 238000010186 staining Methods 0.000 description 7
- 238000007920 subcutaneous administration Methods 0.000 description 7
- 241000699666 Mus <mouse, genus> Species 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- 235000009697 arginine Nutrition 0.000 description 6
- 210000004443 dendritic cell Anatomy 0.000 description 6
- 210000004013 groin Anatomy 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 230000005909 tumor killing Effects 0.000 description 6
- 238000010586 diagram Methods 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 210000001165 lymph node Anatomy 0.000 description 5
- 238000001000 micrograph Methods 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 108010036176 Melitten Proteins 0.000 description 4
- 238000007385 chemical modification Methods 0.000 description 4
- 238000002296 dynamic light scattering Methods 0.000 description 4
- 230000008030 elimination Effects 0.000 description 4
- 238000003379 elimination reaction Methods 0.000 description 4
- 230000007515 enzymatic degradation Effects 0.000 description 4
- 230000035800 maturation Effects 0.000 description 4
- 239000011259 mixed solution Substances 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 230000004614 tumor growth Effects 0.000 description 4
- AZKSAVLVSZKNRD-UHFFFAOYSA-M 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide Chemical compound [Br-].S1C(C)=C(C)N=C1[N+]1=NC(C=2C=CC=CC=2)=NN1C1=CC=CC=C1 AZKSAVLVSZKNRD-UHFFFAOYSA-M 0.000 description 3
- 206010006187 Breast cancer Diseases 0.000 description 3
- 208000026310 Breast neoplasm Diseases 0.000 description 3
- 206010018910 Haemolysis Diseases 0.000 description 3
- 210000001744 T-lymphocyte Anatomy 0.000 description 3
- 230000002860 competitive effect Effects 0.000 description 3
- 230000001085 cytostatic effect Effects 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 239000012091 fetal bovine serum Substances 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- VDXZNPDIRNWWCW-JFTDCZMZSA-N melittin Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O)CC1=CNC2=CC=CC=C12 VDXZNPDIRNWWCW-JFTDCZMZSA-N 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 150000003904 phospholipids Chemical class 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 125000000393 L-methionino group Chemical group [H]OC(=O)[C@@]([H])(N([H])[*])C([H])([H])C(SC([H])([H])[H])([H])[H] 0.000 description 2
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 230000001268 conjugating effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 230000005284 excitation Effects 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 230000008588 hemolysis Effects 0.000 description 2
- 235000014304 histidine Nutrition 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 229910052741 iridium Inorganic materials 0.000 description 2
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 235000018977 lysine Nutrition 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 108700022109 ropocamptide Proteins 0.000 description 2
- 239000012192 staining solution Substances 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 241000024188 Andala Species 0.000 description 1
- 108700042778 Antimicrobial Peptides Proteins 0.000 description 1
- 102000044503 Antimicrobial Peptides Human genes 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 125000000998 L-alanino group Chemical group [H]N([*])[C@](C([H])([H])[H])([H])C(=O)O[H] 0.000 description 1
- -1 L-form amino acids Chemical class 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 230000005809 anti-tumor immunity Effects 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- QZPSXPBJTPJTSZ-UHFFFAOYSA-N aqua regia Chemical compound Cl.O[N+]([O-])=O QZPSXPBJTPJTSZ-UHFFFAOYSA-N 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 150000001484 arginines Chemical class 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 210000005252 bulbus oculi Anatomy 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000007541 cellular toxicity Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003413 degradative effect Effects 0.000 description 1
- 238000012938 design process Methods 0.000 description 1
- IQDGSYLLQPDQDV-UHFFFAOYSA-N dimethylazanium;chloride Chemical compound Cl.CNC IQDGSYLLQPDQDV-UHFFFAOYSA-N 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000000295 emission spectrum Methods 0.000 description 1
- 238000000695 excitation spectrum Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000007614 genetic variation Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 230000006054 immunological memory Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000029226 lipidation Effects 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 229920001427 mPEG Polymers 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 239000002091 nanocage Substances 0.000 description 1
- 239000002086 nanomaterial Substances 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 210000004882 non-tumor cell Anatomy 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229960002895 phenylbutazone Drugs 0.000 description 1
- VYMDGNCVAMGZFE-UHFFFAOYSA-N phenylbutazonum Chemical compound O=C1C(CCCC)C(=O)N(C=2C=CC=CC=2)N1C1=CC=CC=C1 VYMDGNCVAMGZFE-UHFFFAOYSA-N 0.000 description 1
- IYDGMDWEHDFVQI-UHFFFAOYSA-N phosphoric acid;trioxotungsten Chemical compound O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.OP(O)(O)=O IYDGMDWEHDFVQI-UHFFFAOYSA-N 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 208000011581 secondary neoplasm Diseases 0.000 description 1
- 230000037380 skin damage Effects 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/42—Proteins; Polypeptides; Degradation products thereof; Derivatives thereof, e.g. albumin, gelatin or zein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/12—Cyclic peptides, e.g. bacitracins; Polymyxins; Gramicidins S, C; Tyrocidins A, B or C
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/643—Albumins, e.g. HSA, BSA, ovalbumin or a Keyhole Limpet Hemocyanin [KHL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/645—Polycationic or polyanionic oligopeptides, polypeptides or polyamino acids, e.g. polylysine, polyarginine, polyglutamic acid or peptide TAT
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6927—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores
- A61K47/6929—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores the form being a nanoparticle, e.g. an immuno-nanoparticle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/513—Organic macromolecular compounds; Dendrimers
- A61K9/5169—Proteins, e.g. albumin, gelatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Nanotechnology (AREA)
- Physics & Mathematics (AREA)
- Immunology (AREA)
- Optics & Photonics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Inorganic Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Manufacturing & Machinery (AREA)
- Crystallography & Structural Chemistry (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
それぞれ、カチオン性の両親媒性ポリペプチド溶液とアルブミン溶液を調製し、モル比で前記カチオン性の両親媒性ポリペプチド溶液とアルブミン溶液を混合し、前記ポリペプチドアルブミンナノ粒子を得ることを含む調製方法を提供する。
カチオン性の両親媒性ポリペプチドを、水、40~60mMのトリスヒドロキシメチルアミノメタン塩酸塩緩衝液又は10~20mMのリン酸塩緩衝液に添加し、20~5000μMのカチオン性の両親媒性ポリペプチド溶液を得て、アルブミンを、水又は10~20mMのリン酸塩緩衝液に添加し、20~5000μMのアルブミン溶液を得るステップ(1);
カチオン性の両親媒性ポリペプチドとアルブミンのモル比(0.1~10):1で、カチオン性の両親媒性ポリペプチド溶液とアルブミン溶液を混合し、水又はリン酸塩緩衝液を添加し、5~100℃で0.5~120min混合し、前記ポリペプチドアルブミンナノ粒子を得るステップ(2)を含む。
本実施例は、イリジウム配位オリゴアルギニンポリペプチドおよびヒト血清アルブミン(HSA)により組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本実施例は、イリジウム配位オリゴアルギニンポリペプチドおよびヒト血清アルブミンにより組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本実施例は、イリジウム配位オリゴアルギニンポリペプチドおよびヒト血清アルブミンにより組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本実施例は、CH3CO-FWLFLRRRRRRRR-CONH2(FR)およびヒト血清アルブミンにより組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本実施例は、chol-RRRRRRRR-CONH2(CR)およびヒト血清アルブミンにより組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本実施例は、イリジウム配位オリゴアルギニンポリペプチドおよびヒト血清アルブミンにより組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本実施例は、イリジウム配位オリゴポリペプチドおよびヒト血清アルブミンにより組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本実施例は、イリジウム配位オリゴポリペプチドおよびヒト血清アルブミンにより組付けて形成するポリペプチドアルブミンナノ粒子を提供する。前記ポリペプチドアルブミンナノ粒子の調製方法は、以下のステップを含む。
本試験例は、動的光散乱装置および透過型電子顕微鏡を用いて実施例1~8で調製したポリペプチドアルブミンナノ粒子の粒径を測定した。
本試験例は、カチオン性の両親媒性ポリペプチドの電荷数、およびカチオン性の両親媒性ポリペプチドとヒト血清アルブミンの割合の、ポリペプチドアルブミンナノ粒子の組付けに対する影響を考察した。
本試験例は、イリジウム配位オリゴアルギニンポリペプチドの腫瘍細胞活性に対する抑制能力を測定した。
ヒト血清アルブミンは、一本鎖ポリペプチドであり、AとBの2つのサブドメインに分けられている。各々のサブドメインは、さらにI、II、IIIの3つの部分に分けられてもよい。それには、3種の主な薬物結合サイトがあり、それぞれ、IIAに位置する薬物結合サイト1(Sudlowサイト1とも呼ばれる)、IIIAに位置する薬物結合サイト2(Sudlowサイト2とも呼ばれる)、およびIBに位置する薬物結合サイト3である。本試験例においてIr-cR8のヒト血清アルブミンにおける結合位置が測定されている。10μMのヒト血清アルブミンの水溶液、および10μMの、ヒト血清アルブミンと、ヒト血清アルブミンの薬物結合サイト1、2、3における競合化合物であるフェニルブタゾン、イブプロフェンおよびリドカインとの混合溶液を調製し、5分間混合した後、それぞれ、0、2、4、6、8、および10μMのIr-cR8を添加し、280nmの光で励起し、300~450nmにおける励起スペクトルを走査した。数式(1)から、競合化合物の有無下でのIr-cR8とアルブミンとの結合定数および結合サイトの数を算出した。
本試験例は、Ir-cR8-HSAナノ粒子、Ir-cR5-HSAナノ粒子およびIr-aR8-HSAナノ粒子の腫瘍の殺傷能力および殺傷メカニズムについて検討した。
本試験例は、Ir-cR8-HSAナノ粒子の安定性および安全性について分析した。
本試験例は、Ir-cR8-HSAナノ粒子の腫瘍ターゲティング性、および生体自身の抗腫瘍免疫反応の誘発の効果について検討した。
Claims (13)
- カチオン性の両親媒性ポリペプチドおよびアルブミンにより組付けて形成する、
ポリペプチドアルブミンナノ粒子。 - 前記カチオン性の両親媒性ポリペプチドが、疎水性部分および親水性部分を含む、
請求項1に記載のポリペプチドアルブミンナノ粒子。 - 前記親水性部分が、アルギニン、リジン又はヒスチジンのうちのいずれか1種又は少なくとも2種の組合せを含み、アルギニンであることが好ましい、
請求項1又は2に記載のポリペプチドアルブミンナノ粒子。 - 前記疎水性部分が、[Ir(ppy)2(H2O)2]OTf、疎水性アミノ酸又は脂質のうちのいずれか1種又は少なくとも2種の組合せを含む、
請求項1~3のいずれか一項に記載のポリペプチドアルブミンナノ粒子。 - 前記疎水性アミノ酸が、フェニルアラニン、ロイシン、イソロイシン、トリプトファン、バリン、メチオニン又はアラニンのうちのいずれか1種又は少なくとも2種の組合せを含み、
好ましくは、前記脂質が、コレステロールおよびその誘導体又は脂肪酸およびその誘導体のうちのいずれか1種又は少なくとも2種の組合せを含み、
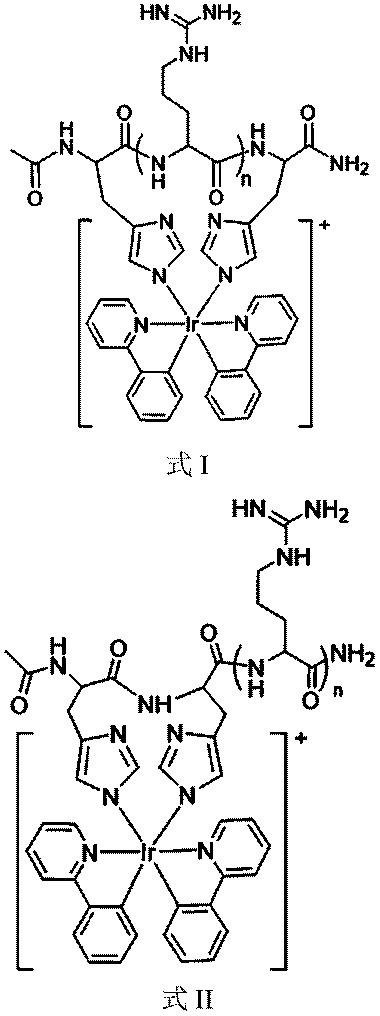
好ましくは、前記カチオン性の両親媒性ポリペプチドの構造式が、式I又は式IIで表され、
好ましくは、前記カチオン性の両親媒性ポリペプチドの一段構造がCH3CO-XRn-CONH2又は脂質-Rn-CONH2であり、ただし、Xは、フェニルアラニン、ロイシン、イソロイシン、トリプトファン、バリン、メチオニン又はアラニンのうちのいずれか1種又は少なくとも2種の組合せを含み、nは、アルギニン残基の数で、1~12の整数である、
請求項1~4のいずれか一項に記載のポリペプチドアルブミンナノ粒子。 - 前記アルブミンが、哺乳動物アルブミンを含み、
好ましくは、前記哺乳動物アルブミンが、ヒト血清アルブミン及び/又は牛血清アルブミンを含む、
請求項1~5のいずれか一項に記載のポリペプチドアルブミンナノ粒子。 - 請求項1~6のいずれか一項に記載のポリペプチドアルブミンナノ粒子の調製方法であって、
カチオン性の両親媒性ポリペプチド溶液とアルブミン溶液をそれぞれ調製し、前記カチオン性の両親媒性ポリペプチド溶液とアルブミン溶液を混合して前記ポリペプチドアルブミンナノ粒子を得ることを含む、
調製方法。 - 前記カチオン性の両親媒性ポリペプチド溶液の濃度が20~5000μMであり、
好ましくは、前記アルブミン溶液の濃度が20~5000μMである、
請求項7に記載の調製方法。 - 前記カチオン性の両親媒性ポリペプチドと前記アルブミンのモル比が(0.1~10):1である、
請求項7又は8に記載の調製方法。 - 前記混合の温度が5~100℃であり、
好ましくは、前記混合の時間が0.5~120minである、
請求項7~9のいずれか一項に記載の調製方法。 - 前記調製方法は、
カチオン性の両親媒性ポリペプチドを、水、40~60mMのトリスヒドロキシメチルアミノメタン塩酸塩緩衝液又は10~20mMのリン酸塩緩衝液に添加し、20~5000μMのカチオン性の両親媒性ポリペプチド溶液を得て、アルブミンを、水又は10~20mMのリン酸塩緩衝液に添加し、20~5000μMのアルブミン溶液を得るステップ(1)と、
カチオン性の両親媒性ポリペプチドとアルブミンのモル比(0.1~10):1で、カチオン性の両親媒性ポリペプチド溶液とアルブミン溶液を混合し、水又はリン酸塩緩衝液を添加し、5~100℃で0.5~120min混合して前記ポリペプチドアルブミンナノ粒子を得るステップ(2)と、を含む、
請求項7~10のいずれか一項に記載の調製方法。 - 請求項1~6のいずれか一項に記載のポリペプチドアルブミンナノ粒子を含む医薬組成物であって、
好ましくは、前記医薬組成物が、薬学的に許容可能な担体、賦形剤又は希釈剤のうちのいずれか1種又は少なくとも2種の組合せをさらに含む、医薬組成物。 - 請求項1~6のいずれか一項に記載のポリペプチドアルブミンナノ粒子又は請求項12に記載の医薬組成物の、抗腫瘍薬物の調製における使用。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011455391 | 2020-12-10 | ||
| CN202011455391.8 | 2020-12-10 | ||
| CN202111151600.4A CN114617974B (zh) | 2020-12-10 | 2021-09-29 | 一种多肽白蛋白纳米粒及其制备方法和应用 |
| CN202111151600.4 | 2021-09-29 | ||
| PCT/CN2021/128313 WO2022121561A1 (zh) | 2020-12-10 | 2021-11-03 | 一种多肽白蛋白纳米粒及其制备方法和应用 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2023529545A true JP2023529545A (ja) | 2023-07-11 |
| JP7523159B2 JP7523159B2 (ja) | 2024-07-26 |
Family
ID=81897400
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2022563369A Active JP7523159B2 (ja) | 2020-12-10 | 2021-11-03 | ポリペプチドアルブミンナノ粒子、およびその調製方法と使用 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20240261365A1 (ja) |
| EP (1) | EP4122496A4 (ja) |
| JP (1) | JP7523159B2 (ja) |
| CN (1) | CN114617974B (ja) |
| AU (1) | AU2021394243A1 (ja) |
| WO (1) | WO2022121561A1 (ja) |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9393396B2 (en) * | 2002-02-14 | 2016-07-19 | Gholam A. Peyman | Method and composition for hyperthermally treating cells |
| JP2012017281A (ja) | 2010-07-07 | 2012-01-26 | Canon Inc | 化合物及び前記化合物を有する造影剤 |
| WO2013084198A1 (en) * | 2011-12-07 | 2013-06-13 | Universidade De Lisboa | Chemical modification and bioconjugation of proteins or peptides using boron compounds |
| WO2013185032A1 (en) * | 2012-06-07 | 2013-12-12 | President And Fellows Of Harvard College | Nanotherapeutics for drug targeting |
| CN103204899A (zh) * | 2013-03-12 | 2013-07-17 | 中国科学院苏州纳米技术与纳米仿生研究所 | 一种基于组氨酸与金属铱配合物结合的多肽环化方法 |
| CN106831997B (zh) * | 2015-12-03 | 2020-04-24 | 中国科学院苏州纳米技术与纳米仿生研究所 | 多肽、脂蛋白样纳米粒子及其应用 |
| US20170290778A1 (en) * | 2016-04-12 | 2017-10-12 | Illustris Pharmaceuticals, Inc. | Compositions for topical application of compounds |
| ES2664584B2 (es) * | 2016-09-19 | 2018-12-13 | Universidade De Santiago De Compostela | Nanoparticulas con interiores protegidos, y métodos de uso de las mismas |
| CN106729755B (zh) * | 2016-12-31 | 2020-08-04 | 中山大学肿瘤防治中心 | 一种miR-214纳米基因复合物及其制备方法、应用 |
| US20200282075A1 (en) | 2017-09-07 | 2020-09-10 | AbbVie Deutschland GmbH & Co. KG | Albumin-modified nanoparticles carrying a targeting ligand |
| CN108078958B (zh) * | 2017-12-28 | 2020-02-07 | 国家纳米科学中心 | 一种抗肿瘤多肽纳米药物及其制备方法和应用 |
| CN108578369B (zh) * | 2018-05-21 | 2021-05-04 | 天津科技大学 | 表面双修饰的靶向人血清白蛋白纳米药物载体的制备与应用 |
| CN110496229B (zh) * | 2019-09-16 | 2022-09-23 | 上海市肺科医院 | 一种具有缓释性的纳米颗粒包被的抗菌肽及其制备方法 |
| CN110548142A (zh) * | 2019-09-19 | 2019-12-10 | 湖北大学 | 一种提高光动力和免疫治疗的纳米粒子及其制备方法 |
| CN111888484B (zh) * | 2020-08-18 | 2021-07-27 | 上海市第一人民医院 | 一种可穿透角膜并靶向视网膜的眼用脂质体及其制备方法和应用 |
-
2021
- 2021-09-29 CN CN202111151600.4A patent/CN114617974B/zh active Active
- 2021-11-03 US US17/998,404 patent/US20240261365A1/en active Pending
- 2021-11-03 JP JP2022563369A patent/JP7523159B2/ja active Active
- 2021-11-03 EP EP21902268.8A patent/EP4122496A4/en active Pending
- 2021-11-03 WO PCT/CN2021/128313 patent/WO2022121561A1/zh active Application Filing
- 2021-11-03 AU AU2021394243A patent/AU2021394243A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| US20240261365A1 (en) | 2024-08-08 |
| CN114617974A (zh) | 2022-06-14 |
| JP7523159B2 (ja) | 2024-07-26 |
| CN114617974B (zh) | 2023-10-03 |
| WO2022121561A1 (zh) | 2022-06-16 |
| AU2021394243A1 (en) | 2022-11-17 |
| EP4122496A1 (en) | 2023-01-25 |
| EP4122496A4 (en) | 2024-10-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11406597B2 (en) | Fusogenic liposome-coated porous silicon nanoparticles | |
| CN111888484B (zh) | 一种可穿透角膜并靶向视网膜的眼用脂质体及其制备方法和应用 | |
| JP2939341B2 (ja) | 赤血球代用剤 | |
| CN108976288B (zh) | 基于野生型穿膜肽penetratin的亲脂性衍生物 | |
| CN104415338B (zh) | 主动靶向型抗肿瘤药物及其制备方法 | |
| CN109908370B (zh) | 一种靶向肿瘤相关成纤维细胞的携载阿霉素的脂质纳米级超声造影剂及其制备方法 | |
| CN110339168B (zh) | 一种负载抗肺纤维化药物和免疫调节剂的纳米制剂及其制备方法 | |
| EP1325739B1 (en) | Liposomes encapsulating anticancer drugs and the use thereof in the treatment of malignant tumors | |
| WO2009062299A1 (en) | Gel-stabilized liposome compositions, methods for their preparation and uses thereof | |
| Bergers et al. | Interleukin-2-containing liposomes: interaction of interleukin-2 with liposomal bilayers and preliminary studies on application in cancer vaccines | |
| JP6238366B2 (ja) | 非極性溶媒に分散性を有する細菌菌体成分を内封する脂質膜構造体およびその製造方法 | |
| CN114848831A (zh) | 包裹型纳米制剂及其载体的制备方法和应用 | |
| JP7523159B2 (ja) | ポリペプチドアルブミンナノ粒子、およびその調製方法と使用 | |
| Yuan et al. | Impact of physicochemical properties on biological effects of lipid nanoparticles: Are they completely safe | |
| CN118059063A (zh) | 一种靶向治疗血管内皮细胞的间充质干细胞仿生纳米囊泡及其制备方法和应用 | |
| CN109568598B (zh) | 用于药物流产的胎盘靶向纳米颗粒及其制备方法和应用 | |
| Li et al. | Research Progress on the Nano-Delivery Systems of Antitumor Drugs | |
| WO2016073548A1 (en) | Multifunctional metallic nanoparticle-peptide bilayer complexes | |
| Denieva et al. | Vesicle Delivery Systems of Biologically Active Compounds: From Liposomes to Cerasomes | |
| JP2005168312A (ja) | 遺伝子類導入方法 | |
| CN116813714A (zh) | 一种注射型药物缓释肽水凝胶及其制备方法 | |
| WO2009131216A1 (ja) | オリゴアルキレングリコールで修飾された脂質膜構造体 | |
| KR101671864B1 (ko) | 요오드화 오일을 함유하는 양이온성 에멀젼 및 바이러스 벡터의 유전자 전달 증진을 위한 이의 용도 | |
| CN116983262A (zh) | 一种pH/磁场双重响应性载药胶束及其制备方法和应用 | |
| CN117624302A (zh) | 一种用于有效递送核酸的分支链结构多肽载体及其变化形式 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20221216 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20221216 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20240116 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20240411 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20240611 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20240708 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7523159 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |