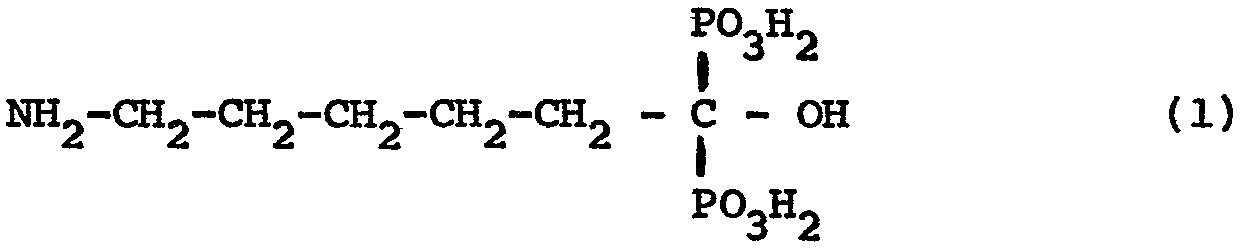FR2489334A1 - 6-Amino-1-hydroxy hexylidene di:phosphonic acid prepn. - from epsilon amino caproic acid with phosphorous acid and phosphorus tri:chloride - Google Patents
6-Amino-1-hydroxy hexylidene di:phosphonic acid prepn. - from epsilon amino caproic acid with phosphorous acid and phosphorus tri:chloride Download PDFInfo
- Publication number
- FR2489334A1 FR2489334A1 FR8019152A FR8019152A FR2489334A1 FR 2489334 A1 FR2489334 A1 FR 2489334A1 FR 8019152 A FR8019152 A FR 8019152A FR 8019152 A FR8019152 A FR 8019152A FR 2489334 A1 FR2489334 A1 FR 2489334A1
- Authority
- FR
- France
- Prior art keywords
- acid
- amino
- hexylidene
- hydroxy
- chloride
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 title abstract 3
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 title abstract 2
- 229960002684 aminocaproic acid Drugs 0.000 title abstract 2
- 229910052698 phosphorus Inorganic materials 0.000 title abstract 2
- 239000011574 phosphorus Substances 0.000 title abstract 2
- -1 6-Amino-1-hydroxy hexylidene Chemical group 0.000 title description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 title description 2
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 title description 2
- ISIJQEHRDSCQIU-UHFFFAOYSA-N tert-butyl 2,7-diazaspiro[4.5]decane-7-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCCC11CNCC1 ISIJQEHRDSCQIU-UHFFFAOYSA-N 0.000 title 1
- 239000002253 acid Substances 0.000 claims abstract description 27
- 150000003839 salts Chemical class 0.000 claims abstract description 12
- 238000000034 method Methods 0.000 claims abstract description 10
- 238000006243 chemical reaction Methods 0.000 claims abstract description 8
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 claims abstract description 8
- FAIAAWCVCHQXDN-UHFFFAOYSA-N phosphorus trichloride Chemical compound ClP(Cl)Cl FAIAAWCVCHQXDN-UHFFFAOYSA-N 0.000 claims abstract description 8
- 150000001408 amides Chemical class 0.000 claims abstract description 5
- 239000003960 organic solvent Substances 0.000 claims abstract description 4
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 22
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 20
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 10
- 239000000203 mixture Substances 0.000 claims description 6
- 150000008044 alkali metal hydroxides Chemical class 0.000 claims description 2
- PUUSSSIBPPTKTP-UHFFFAOYSA-N neridronic acid Chemical compound NCCCCCC(O)(P(O)(O)=O)P(O)(O)=O PUUSSSIBPPTKTP-UHFFFAOYSA-N 0.000 claims description 2
- 125000004122 cyclic group Chemical group 0.000 abstract description 2
- PBWYBJGLSOHAAL-UHFFFAOYSA-N 1-phosphonooxyhexyl dihydrogen phosphate Chemical compound CCCCCC(OP(O)(O)=O)OP(O)(O)=O PBWYBJGLSOHAAL-UHFFFAOYSA-N 0.000 abstract 1
- 229910018828 PO3H2 Inorganic materials 0.000 abstract 1
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 4
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical class [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 125000001931 aliphatic group Chemical group 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 239000005909 Kieselgur Substances 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000006386 neutralization reaction Methods 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- ODTSIPWEPOOBBS-UHFFFAOYSA-N (6-amino-6-hydroxy-1-phosphonohexyl)phosphonic acid Chemical compound NC(O)CCCCC(P(O)(O)=O)P(O)(O)=O ODTSIPWEPOOBBS-UHFFFAOYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 1
- CIUQDSCDWFSTQR-UHFFFAOYSA-N [C]1=CC=CC=C1 Chemical compound [C]1=CC=CC=C1 CIUQDSCDWFSTQR-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 239000000010 aprotic solvent Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000010908 decantation Methods 0.000 description 1
- YWEUIGNSBFLMFL-UHFFFAOYSA-N diphosphonate Chemical compound O=P(=O)OP(=O)=O YWEUIGNSBFLMFL-UHFFFAOYSA-N 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 229960001484 edetic acid Drugs 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 150000008282 halocarbons Chemical class 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 125000002960 margaryl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000010413 mother solution Substances 0.000 description 1
- DLYUQMMRRRQYAE-UHFFFAOYSA-N phosphorus pentoxide Inorganic materials O1P(O2)(=O)OP3(=O)OP1(=O)OP2(=O)O3 DLYUQMMRRRQYAE-UHFFFAOYSA-N 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000012265 solid product Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/28—Phosphorus compounds with one or more P—C bonds
- C07F9/38—Phosphonic acids [RP(=O)(OH)2]; Thiophosphonic acids ; [RP(=X1)(X2H)2(X1, X2 are each independently O, S or Se)]
- C07F9/3804—Phosphonic acids [RP(=O)(OH)2]; Thiophosphonic acids ; [RP(=X1)(X2H)2(X1, X2 are each independently O, S or Se)] not used, see subgroups
- C07F9/3839—Polyphosphonic acids
- C07F9/3873—Polyphosphonic acids containing nitrogen substituent, e.g. N.....H or N-hydrocarbon group which can be substituted by halogen or nitro(so), N.....O, N.....S, N.....C(=X)- (X =O, S), N.....N, N...C(=X)...N (X =O, S)
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
Abstract
Description
L'invention concerne l'acide 6-amino-lhydroxyhexylidéne-diphosphonique, ses sels et un procédé pour la production de ces produits. The invention relates to 6-amino-hydroxyhexylidene-diphosphonic acid, its salts and a process for the production of these products.
La littérature décrit surtout des acides non substitués, de formule générale
dans laquelle R représente un radical alcoyl ou phényl.The literature mainly describes unsubstituted acids, of general formula
in which R represents an alkyl or phenyl radical.
A part les composés à channe carbonée droite, dans lesquels R signifie un groupe méthyl ou heptadecyl, on trouve également décrits des composés à chatne ramifiée. Le représentant le plus connu de ces produits est constitué par l'acide éthylidène-hydroxydiphosphonique. Pour la production de cet acide, il existe une série de procédés, avec cette caractéristiques commune à tous, selon laquelle on fait réagir un acide aliphatique ou un dérivé approprié, avec de l'acide phosphorique ou son precurseur. I1 est par exemple possible d'agir avec un agent d'acylation (chlorure ou anhydride) sur l'acide phosphorique ou bien de chauffer un mélange d'un acide aliphatique et de pentoxyde de phosphore avec de l'acide phosphorique, éventuellement de laisser réagir l'acide approprié avec le trichlorure de phosphore, dans un rapport molaire convenable. Apart from the compounds with a straight carbon chain, in which R signifies a methyl or heptadecyl group, there are also described branched chain compounds. The best known representative of these products is ethylidene hydroxydiphosphonic acid. For the production of this acid, there is a series of processes, with this common characteristic, according to which an aliphatic acid or an appropriate derivative is reacted with phosphoric acid or its precursor. It is for example possible to act with an acylating agent (chloride or anhydride) on phosphoric acid or else to heat a mixture of an aliphatic acid and of phosphorus pentoxide with phosphoric acid, possibly leaving reacting the appropriate acid with phosphorus trichloride, in a suitable molar ratio.
Toutefois, la production d'acide alkylidène hydroxydiphosphonique est plus difficile, car les acides aliphatiques substitués conduisent, dans des conditions réactionnelles semblables, souvent à une autre réaction. Ainsi par exemple on a tenté de produire certains dérivés animés à partir de l'acide éthylène-diamine-tétracétique, sans résultat, car il s'est produit une séparation du carboxyl et une substitution de celui-ci. Dans le cas d'acides aminés, en outre, l'isolation du produit est également difficile, car on ne peut recourir à la distillation pour séparer le mélange réactionnel ; le produit de départ, le produit de la réaction, ainsi que l'ester correspondant, ne sont pas volatils. However, the production of alkylidene hydroxydiphosphonic acid is more difficult, because the substituted aliphatic acids lead, under similar reaction conditions, often to another reaction. Thus, for example, attempts have been made to produce certain animated derivatives from ethylene-diamine-tetracetic acid, without result, because there has been a separation of the carboxyl and a substitution of the latter. In the case of amino acids, moreover, the isolation of the product is also difficult, since distillation cannot be used to separate the reaction mixture; the starting material, the reaction product, as well as the corresponding ester, are not volatile.
Malgré tout, ces derniers temps on a décrit quelques acides aminohydroxyalkylidène-diphosphoniques et leurs dérivés N-alcoylés, avec au plus 4 atomes de carbone dans la charnue. Ces acides comportent, par suite des deux groupes phosphoniques voisins, un groupe amine très faiblement basique, et donnent, avec deux équivalents d'hydroxyde de sodium, des sels neutres. Le sel neutre est donc caractérisé par une teneur élevée en sodium ou en un autre métal, ce qui peut être un inconvénient dans les applications pharmaceutiques. Despite everything, in recent times we have described some aminohydroxyalkylidene-diphosphonic acids and their N-alkylated derivatives, with at most 4 carbon atoms in the flesh. These acids contain, as a result of the two neighboring phosphonic groups, a very weakly basic amine group, and give, with two equivalents of sodium hydroxide, neutral salts. Neutral salt is therefore characterized by a high content of sodium or another metal, which can be a disadvantage in pharmaceutical applications.
Les inconvénients mentionnés sont éliminés dans le cas de l'acide 6-amino-l-hydroxy-hexylidène-diphospho- nique de formule
et de ses sels, selon l'invention.The drawbacks mentioned are eliminated in the case of 6-amino-1-hydroxy-hexylidene-diphosphonic acid of formula
and its salts, according to the invention.
Une partie de l'invention constitue aussi un procédé pour l'obtention de cet acide ; le principe consiste en ce que l'on fait réagir, sur l'acide g -aminocapronique ou sur l'amide correspondant, un mélange d'acide phosphorique et de trichlorure de phosphore, à une température de 50 à 1500C. Part of the invention also constitutes a process for obtaining this acid; the principle consists in reacting, on g -aminocapronic acid or on the corresponding amide, a mixture of phosphoric acid and phosphorus trichloride, at a temperature of 50 to 1500C.
La réaction peut être conduite en présence d'un solvant organique, de préférence le chlorobenzène. The reaction can be carried out in the presence of an organic solvent, preferably chlorobenzene.
On obtient un sel de l'acide selon l'invention, en neutralisant l'acide avec un ou deux équivalents d'un hydroxyde de métal alcalin, de préférence de l'hydroxyde de sodium. An acid salt according to the invention is obtained by neutralizing the acid with one or two equivalents of an alkali metal hydroxide, preferably sodium hydroxide.
Le nouvel acide phosphonique selon l'invention présente un autre caractère et une basicité plus élevée du groupe amine, qui est plus éloigné des groupes acides. I1 forme un sel monosodique neutre et un sel disodique à réaction alcaline. The new phosphonic acid according to the invention has another character and a higher basicity of the amine group, which is further from the acid groups. It forms a neutral monosodium salt and a disodium salt with an alkaline reaction.
Le principe du procédé d'obtention selon ltinvention consiste en ce que laon transforme l'acide g -aminocapronique, par action d'acide phosphorique et de trichlorure de phosphore, éventuellement en présence d'un solvant organique, en l'acide de formule (1), puis on obtient, par neutralisation avec une solution d'hydroxyde de sodium, le sel monosodique ou disodique correspondant. A la place de l'acide g -aminocapronique, on peut utiliser ses amides cycliques ou linéaires, caprolactane, polyamide, lesquels s'hydrolysent au cours de la réaction. The principle of the process for obtaining according to the invention consists in that laon transforms g -aminocapronic acid, by the action of phosphoric acid and phosphorus trichloride, optionally in the presence of an organic solvent, into the acid of formula ( 1), then, by neutralization with a sodium hydroxide solution, the corresponding monosodium or disodium salt is obtained. Instead of g -aminocapronic acid, its cyclic or linear amides, caprolactan, polyamide, which can be hydrolyzed during the reaction, can be used.
Le mode opératoire le plus avantageux pour le procédé, consiste en ce que l'on fait chauffer un mélange d'acide t-aminocapronique, d'acide phosphorique et d'un solvant aprotique, à une température de 1000C, et que l'on ajoute au mélange goutte à goutte, sous agitation, du trichlorure de phosphore. A la place de l'acide phosphorique, on peut utiliser du trichlorure de phosphore et une quantité adéquate d'eau, qui donnent naissance au sein du mélange réactionnel, à l'acide phosphorique. Cette possibilité est moins avantageuse, car une grande quantité d'acide chlorhydrique est libérée. Comme solvant, les hydrocarbures ou hydrocarbures halogénés sont appropriés. The most advantageous procedure for the process consists in heating a mixture of t-aminocapronic acid, phosphoric acid and an aprotic solvent, at a temperature of 1000C, and add to the mixture drop by drop, with stirring, phosphorus trichloride. Instead of phosphoric acid, phosphor trichloride and an adequate amount of water can be used, which give rise to phosphoric acid in the reaction mixture. This possibility is less advantageous because a large amount of hydrochloric acid is released. As the solvent, hydrocarbons or halogenated hydrocarbons are suitable.
Apres mélange de tous les composants, le mélange réactionnel est chauffé à 100 C durant deux à trois heures, et le produit solide qui se sépare est recristallisé dans l'eau chaude. On peut récupérer une autre fraction à partir de la solution merle après addition de méthanol. La forme pure de l'acide n'est que difficilement soluble dans l'eau t on obtient le sel disodique correspondant en dissolvant l'acide dans une quantité équivalente d'hydroxyde de sodium et l'on dose après, ou par addition d'exactement deux équivalents d'hydroxyde de sodium à une suspension aqueuse du sel pur.On prépare le sel monosodique,soit par addition d'un équivalent de hydroxyde de sodium à la suspension du sel dans l'eau, soit par neutralisation de la suspension acide par la soude jusqu'à pH 7,0. After mixing all the components, the reaction mixture is heated at 100 ° C. for two to three hours, and the solid product which separates is recrystallized from hot water. Another fraction can be recovered from the merle solution after addition of methanol. The pure form of the acid is only hardly soluble in water t the corresponding disodium salt is obtained by dissolving the acid in an equivalent amount of sodium hydroxide and dosing after, or by addition of exactly two equivalents of sodium hydroxide to an aqueous suspension of pure salt. The sodium salt is prepared, either by adding one equivalent of sodium hydroxide to the suspension of salt in water, or by neutralization of the acid suspension. with soda until pH 7.0.
L'invention sera mieux comprise en regard des exemples décrits ci-après. The invention will be better understood with reference to the examples described below.
ExemPle 1.EXAMPLE 1.
On fait chauffer un mélange de 13 g d'acide -aminocapronique et de 12,7 g d'acide phosphorique dans îoe ml de chlorobenzène, sous agitation, à 100 C, et l'on y ajoute goutte à goutte, en 30 minutes, 14 ml de trichlorure de phosphore. Après cela, la solution est encore agitée durant 3 heures : durant cette période, une masse blanche insoluble se sépare. Après refroidissement, on sépare le solvant par décantation, on reprend le résidu par 60 ml d'eau et le fait bouillir durant environ 30 minutes, puis le filtre à chaud avec du charbon actif sur une couche de Kieselgur. On lave le charbon actif et le kieselgur avec de l'eau chaude et l'on fait évaporer les filtrats réunis à 400C sous pression réduite. Les cristaux apparus sont filtrés et l'on obtient une fraction supplémentaire par addition de méthanol à la solution mère. On obtient au total 15 g (55 % de la quantité théorique) d'acide cristallisé répondant à la formule (1), ayant un point de fusion de 2450C, et présentant une absorption en spectre IR, à 3,15, 6,15 et 6,45 /u. Pour C6H17NO7P2 (277,2) on a calculé
C = 26 % H = 6,18 % N = 5,05 %
On a trouvé
C = 26,28 % H = 6,45 % N = 4,87 %.A mixture of 13 g of -aminocapronic acid and 12.7 g of phosphoric acid in 10 ml of chlorobenzene is heated, with stirring, to 100 ° C., and is added dropwise over 30 minutes. 14 ml of phosphorus trichloride. After that, the solution is further stirred for 3 hours: during this period, an insoluble white mass separates. After cooling, the solvent is separated by decantation, the residue is taken up in 60 ml of water and boiled for approximately 30 minutes, then the filter is hot with activated charcoal on a layer of Kieselgur. The activated carbon and the kieselgur are washed with hot water and the combined filtrates are evaporated at 400C under reduced pressure. The crystals which have appeared are filtered and an additional fraction is obtained by adding methanol to the mother solution. A total of 15 g (55% of the theoretical amount) of crystallized acid corresponding to formula (1) is obtained, having a melting point of 2450C, and having an absorption in IR spectrum, at 3.15, 6.15 and 6.45 / u. For C6H17NO7P2 (277.2) we calculated
C = 26% H = 6.18% N = 5.05%
We found
C = 26.28% H = 6.45% N = 4.87%.
ExemPle 2.EXAMPLE 2.
Obtention du sel monosodique : on dissout 2,77 g d'acide dans 50 ml d'eau, par addition de 10 ml de NaOH1 N, on filtre la solution et la fait évaporer. Le sel qui se sépare est filtré, lavé au méthanol et séché sous pression réduite. Obtaining the monosodium salt: 2.77 g of acid are dissolved in 50 ml of water, by adding 10 ml of NaOH1 N, the solution is filtered and evaporated. The salt which separates is filtered, washed with methanol and dried under reduced pressure.
ExemPle 3.EXAMPLE 3.
Obtention du sel disodique : on neutralise 2,77 g d'acide avec 20 ml de NaOH 1 N comme dans l'exemple précédent, et l'on évapore la solution. Le sel qui se sépare est séche sous pression réduite. Obtaining the disodium salt: neutralizing 2.77 g of acid with 20 ml of 1N NaOH as in the previous example, and evaporating the solution. The separating salt is dried under reduced pressure.
Claims (3)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8019152A FR2489334A1 (en) | 1980-09-04 | 1980-09-04 | 6-Amino-1-hydroxy hexylidene di:phosphonic acid prepn. - from epsilon amino caproic acid with phosphorous acid and phosphorus tri:chloride |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8019152A FR2489334A1 (en) | 1980-09-04 | 1980-09-04 | 6-Amino-1-hydroxy hexylidene di:phosphonic acid prepn. - from epsilon amino caproic acid with phosphorous acid and phosphorus tri:chloride |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| FR2489334A1 true FR2489334A1 (en) | 1982-03-05 |
Family
ID=9245663
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| FR8019152A Pending FR2489334A1 (en) | 1980-09-04 | 1980-09-04 | 6-Amino-1-hydroxy hexylidene di:phosphonic acid prepn. - from epsilon amino caproic acid with phosphorous acid and phosphorus tri:chloride |
Country Status (1)
| Country | Link |
|---|---|
| FR (1) | FR2489334A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2499408A1 (en) * | 1981-02-12 | 1982-08-13 | Gentili Ist Spa | PHARMACEUTICAL COMPOSITION FOR THE TREATMENT OF OSTEOPATHY, BASED ON 6-AMINO-1-HYDROXYHEXANE-1,1-DIPHOSPHONIC ACID |
| EP0082472A2 (en) * | 1981-12-23 | 1983-06-29 | Henkel Kommanditgesellschaft auf Aktien | Process for the preparation of 3-amino-1-hydroxypropane-1,1-diphosphonic acid |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2130794A1 (en) * | 1971-06-22 | 1973-01-11 | Benckiser Gmbh Joh A | 1-hydroxy-3-aminopropane-1,1-diphosphonic acid - - prepn from beta-alanine and phosphorus trihalide,used as complexant |
| FR2259615A1 (en) * | 1974-02-04 | 1975-08-29 | Henkel & Cie Gmbh | |
| EP0039033A1 (en) * | 1980-04-28 | 1981-11-04 | Henkel Kommanditgesellschaft auf Aktien | Process for preparing omega-amino-1-hydroxyalkylidene-1, 1-bis phosphonic acids |
-
1980
- 1980-09-04 FR FR8019152A patent/FR2489334A1/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2130794A1 (en) * | 1971-06-22 | 1973-01-11 | Benckiser Gmbh Joh A | 1-hydroxy-3-aminopropane-1,1-diphosphonic acid - - prepn from beta-alanine and phosphorus trihalide,used as complexant |
| FR2259615A1 (en) * | 1974-02-04 | 1975-08-29 | Henkel & Cie Gmbh | |
| EP0039033A1 (en) * | 1980-04-28 | 1981-11-04 | Henkel Kommanditgesellschaft auf Aktien | Process for preparing omega-amino-1-hydroxyalkylidene-1, 1-bis phosphonic acids |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2499408A1 (en) * | 1981-02-12 | 1982-08-13 | Gentili Ist Spa | PHARMACEUTICAL COMPOSITION FOR THE TREATMENT OF OSTEOPATHY, BASED ON 6-AMINO-1-HYDROXYHEXANE-1,1-DIPHOSPHONIC ACID |
| EP0082472A2 (en) * | 1981-12-23 | 1983-06-29 | Henkel Kommanditgesellschaft auf Aktien | Process for the preparation of 3-amino-1-hydroxypropane-1,1-diphosphonic acid |
| EP0082472A3 (en) * | 1981-12-23 | 1984-03-21 | Henkel Kommanditgesellschaft Auf Aktien | Process for the preparation of 3-amino-1-hydroxypropane-1,1-diphosphonic acid |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4304734A (en) | 6-Amino-1-hydroxyhexylidene diphosphonic acid, salts and a process for production thereof | |
| KR0137455B1 (en) | Process for preparing 4-amino-1-hydroxy butylidene-1,1-bisphosphonic acid or salts thereof | |
| RU2163233C2 (en) | Method of preparing creatine or creatine monohydrate | |
| CA1212380A (en) | Process for the preparation of new thieno(2,3-b) pyrrole derivatives | |
| FR2460959A1 (en) | PROCESS FOR THE PREPARATION OF N-PHOSPHONOMETHYL-GLYCINE | |
| CA1325423C (en) | Process for the synthesis of azido-3'-deoxy-3'-thiamidine and analogs | |
| FR2648460A1 (en) | PROCESS FOR PRODUCING N-PHOSPHONOMETHYLGLYCIN | |
| FR2489334A1 (en) | 6-Amino-1-hydroxy hexylidene di:phosphonic acid prepn. - from epsilon amino caproic acid with phosphorous acid and phosphorus tri:chloride | |
| EP0018415A1 (en) | Process for producing alkyl 4-(alkoxy methyl phosphinoyl)butyrates and alkyl-(2-amino-4-(alkoxy methyl phosphinoyl)) butyrates | |
| KR900006133B1 (en) | Process for the preparation all - cis - 1,3,5-triamino -2,4,6 - cyclohexanetriol derivatives | |
| CH479552A (en) | Process for the preparation of ketimines | |
| EP0427587B1 (en) | Process for the preparation of AZT (3'-azido-3'-deoxy-thymidine) and related compounds | |
| Stein et al. | Preparation of 1-alkyluramil-7, 7-diacetic acids | |
| FR2503713A1 (en) | 2,3-DI-SUBSTITUTED-5,6-DIHYDRO-IMIDAZO- (2,1-B) THIAZOLES, PROCESS FOR THEIR PREPARATION AND ANTI-INFLAMMATORY AGENTS CONTAINING THEM | |
| CA1146968A (en) | 6-amino-1-hydroxyhexyliden diphosphonic acid, salts and a process for production thereof | |
| BE885139A (en) | 6-AMINO-1-HYDROXYHEXYLIDENE-DISPHOSPHONIC ACID, SALTS OF THIS ACID AND MANUFACTURING METHOD THEREOF | |
| GB2082585A (en) | 6-amino-1-hydroxyhexylidene diphosphonic acid, salts thereof and a method of producing the acid and salts. | |
| JP2907520B2 (en) | Method for producing surfactant | |
| CA2038976C (en) | Industrial process for preparing diethyl (1s)-1-amino-n-(o4-desacetyl-23 vinblastinoyl)-2-methylpropylphosphonate and the salts thereof | |
| Avery | THE ACTION OF SODIUM BENZYL CYANIDE WITH CINNAMIC ESTER. II1 | |
| BE893835A (en) | THIAZOLO (3,2, -A) SUBSTITUTED PYRIMIDINES AND PROCESS FOR THEIR PREPARATION | |
| CH283749A (en) | Process for preparing an aromatic acylamidodiol. | |
| FI66836B (en) | FOERFARANDE FOER FRAMSTAELLNING AV FAST SODIUM- ELLER POTASSIUM P-HYDROXIMANDELATMONOHYDRAT | |
| KR790001639B1 (en) | Method for preparation of new antifungal agent | |
| FR2526021A1 (en) | PROCESS FOR THE PREPARATION OF 3-HYDROXY-BENZODIAZEPINONES |