Field of the invention
The present invention relates to a photothermographic recording
material comprising a photo-addressable thermally developable
element comprising a species which increases the infrared
sensitivity thereof.
Background of the invention.
Thermal imaging or thermography is a recording process wherein
images are generated by the use of thermal energy.
In thermography three approaches are known:
Thermographic materials of type 1 become photothermographic when
a photosensitive agent is present which after exposure to UV,
visible or IR light is capable of catalyzing or participating in a
thermographic process bringing about changes in colour or optical
density. Examples of photothermographic materials are the so called
"Dry Silver" photographic materials of the 3M Company, which are
reviewed by D.A. Morgan in "Handbook of Imaging Science", edited by
A.R. Diamond, page 43, published by Marcel Dekker in 1991.
In US-P 5,441,866 it is stated that: "While many of such dyes
(dyes which impart spectral sensitivity to a gelatino silver halide
element) provide spectral sensitization in photothermographic
formulations the dye sensitization is often very inefficient and it
is not possible to translate the performance of a dye in gelatino
silver halide elements to photothermographic elements."
US-P 5,441,866 discloses a heat-developable photothermographic
element comprising a preferably hydrophobic binder, supersensitizer,
preferably selected from the group consisting of aromatic,
heterocyclic mercapto or disulfide compounds, and a spectrally
sensitizing amount of an infrared absorbing dye having the central
nucleus:
wherein R
1 represents a (CH
2)
n-COO
- group of from 1-20 carbon atoms,
or an alkyl group of from 1 to 20 carbon atoms; and n is an integer
from 1 to 20; and EP-A 616 014 discloses a heptamethine cyanine dye
characterised in that both nitrogen atoms of the cyanine chromophore
bear a 5 carboxyalkyl substituent comprising an alkyl chain of at
least five carbon atoms, which may be used in conjunction with
supersensitizers such as 2-mercaptobenzimidazoles, metal chelating
agents and pyridine, pyrimidine and triazine derivatives.
US-P 4,873,184 discloses a spectrally sensitized silver halide
photothermographic emulsion layer comprising a reducible silver
source material as 20 to 70% by weight of said emulsion layer,
photosensitive silver halide, and a reducing agent for silver ion,
said silver halide having no latent image therein and being present
as 1.5 to 7.0% by weight of said emulsion layer and said emulsion
layer having a speed increasing effective amount of a metal
complexing agent therein in an amount equal to 0.4 to 40% by weight
of total silver in said emulsion.
WO 96/33442A discloses a heat developable, photothermographic
element comprising a support bearing at least one photosensitive,
image-forming layer comprising: (a) a photosensitive silver halide;
(b) a non-photosensitive, reducible silver source; (c) a reducing
agent for silver ions; (d) a binder; and (e) a spectrally
sensitizing amount of a compound having the central nucleus:
wherein: X is independently a thioalkyl group of from 1 to 20 carbon
atoms; n is independently 0, 1 or 2 with the total of n's being at
least 1; R
1 and R
2 represent an alkyl group of from 1 to 20 carbon
atoms other than carboxy-substituted alkyl; and A
- is an anion.
The detailed descriptions and invention examples of US-P
4,873,184, US-P 5,441,866 and WO 96/33442 are all confined to photo-addressable
thermally developable elements coated from non-aqueous
media. This reflects the standard teaching over such
photothermographic materials, but for economic, safety and
ecological reasons, it is desirable to coat such materials from
aqueous media. However, the extrapolation of materials technology
for photothermographic materials based on organic silver
salts/silver halide/reducing agent-systems coated from non-aqueous
media is by no means self-evident as is borne out by the inventors'
investigation of the spectral sensitization of such
photothermographic materials coated from aqueous media.
Objects of the invention.
It is a first object of the invention to provide a
photothermographic recording material with a high infra-red
sensitivity and excellent image-forming properties.
It is a second object of the invention to provide a
photothermographic recording material comprising a photo-addressable
thermally developable element based on a substantially light-insensitive
organic silver salt, photosensitive silver halide in
catalytic association therewith and an organic reducing agent for
the organic silver salt, which is produceable without necessitating
intermediate drying of the organic silver salt.
It is a third object of the invention to provide a photo-addressable
thermally developable element with excellent image-forming
properties, which can be coated from aqueous media.
It is a yet a still further object of the invention to provide a
recording process for a photothermographic recording material with
the above improved characteristics.
Further objects and advantages of the invention will become
apparent from the description hereinafter.
Summary of the invention
According to the present invention a photothermographic
recording material is provided comprising a support and a photo-addressable
thermally developable element includingprising a
substantially light-insensitive organic silver salt, photosensitive
silver halide in catalytic association with the substantially light-insensitive
organic silver salt and spectrally sensitized to infra-red
light with a dye, a supersensitizer for the dye, a reducing
agent in thermal working relationship with the substantially light-insensitive
organic silver salt and a binder, characterized in that
the binder comprises a water soluble polymer, a water-dispersible
polymer or a water soluble polymer and a water-dispersible polymer
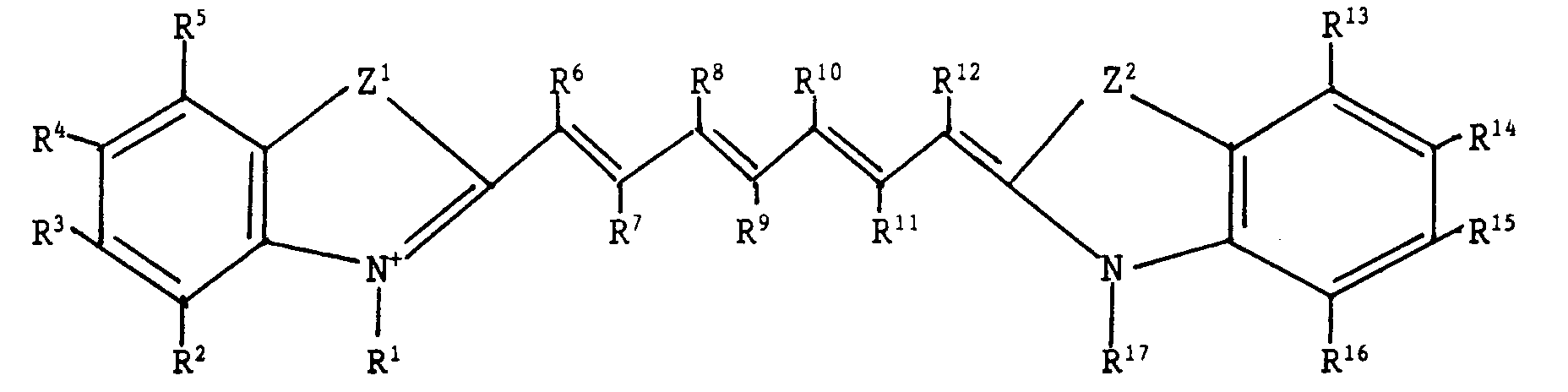
and the dye corresponds to the general formula (I):
wherein Z
1 and Z
2 independently represent S, O or Se; R
1 and R
17 are
independently each an alkyl or sulfo-alkyl group which may be
substituted with at least one fluorine, chlorine, bromine, iodine or
an alkoxy-, aryloxy- or ester-group; R
2, R
3, R
4, R
5, R
13, R
14, R
15 and
R
16 are independently each hydrogen, chlorine, bromine, fluorine, a
nitro-group, a cyano-group or a keto-, sulfo-, carboxy-, ester-,
sulfonamide-, amide-, dialkylamino-, alkyl-, alkenyl-, heteroaromatic,
aryl-, alkoxy- or aryloxy-group, which groups may be
substituted; or each of R
2 together with R
3, R
3 together with R
4, R
4
together with R
5, R
13 together with R
14, R
14 together with R
15 and R
15
together with R
16 may independently constitute the atoms necessary to
complete a benzene ring which may be substituted; R
6, R
7, R
8, R
9, R
10,
R
11 and R
12 independently represent hydrogen, an alkyl group, a
substituted alkyl group, chlorine, fluorine, bromine, iodine, a
disubstituted amino group, wherein the substituents may constitute
the atoms necessary to form a 5-ring atom or 6-ring atom
heterocyclic ring, or each of R
6 together with R
8, R
8 together with
R
10, R
10 together with R
12 and R
9 together with R
11 may independently
constitute the atoms necessary to complete a 5-atom or 6-atom
carbocyclic or heterocyclic ring which may be substituted; R
7
together with R
9 may independently constitute the atoms necessary to
complete a 5-atom heterocyclic ring, a 6-atom heterocyclic ring or a
5-atom carbocyclic ring which may be substituted; each of R
1 together
with R
6 and R
12 together with R
17 may independently constitute the
atoms necessary to complete a 5-atom or 6-atom heterocyclic ring
which may be substituted; and X
- represents an anion.
A process is also provided producing a photothermographic
recording material, as referred to above, comprising the steps of:
(i) producing an aqueous dispersion or aqueous dispersions
comprising the substantially light-insensitive organic silver salt,
the photosensitive silver halide spectrally sensitized to infra-red
light with the dye, the supersensitizer for the dye, the reducing
agent and the binder; (ii) coating the aqueous dispersion or aqueous
dispersions onto a support.
A recording process for a photothermographic recording material
is further provided comprising the steps of: imagewise exposing to
infrared actinic radiation a photothermographic recording material
as referred to above or produced as referred to above and overall
heating of the photothermographic recording material.
Preferred embodiments of the present invention are disclosed in
the detailed description of the invention.
Detailed description of the invention.
Aqueous
The term aqueous for the purposes of the present invention
includes mixtures of water with water-miscible organic solvents such
as alcohols e.g. methanol, ethanol, 2-propanol, butanol, iso-amyl
alcohol, octanol, cetyl alcohol etc; glycols e.g. ethylene glycol;
glycerine; N-methyl pyrrolidone; methoxypropanol; and ketones e.g.
2-propanone and 2-butanone etc.
Spectral sensitizer
According to the present invention the photothermographic
material comprises a photo-addressable thermally developable element
comprising a dye corresponding to the general formula (I).
In a particularly preferred embodiment, according to the present
invention, in formula (I) R1 and R17 each independently represent an
alkyl group consisting of 1 to 6 carbon atoms.
In an especially preferred embodiment, according to the present
invention, the dye corresponds to the formula
Suitable infra-red sensitizing dyes for photosensitive silver
halide, according to the present invention, are the N-alkyl
benzothiazole heptamethine cyanine dyes:
the N-alkylsulfo benzothiazole heptamethine cyanine dyes:
Suitable supersensitizers for use with the dyes, used in the
present invention, are disclosed in EP-A's 559 228 and 587 338 and
in the US-P's 3,877,943 and 4,873,184.
In a particularly preferred embodiment, as used in the present
invention, the supersensitizer is a compound selected from the group
consisting of stilbene compounds, hydrazine compounds and triazine
compounds.
Particularly preferred stilbene supersensitizers, according to
the present invention, are:
According to the present invention the photo-addressable
thermally developable element includes a binder comprising a water-soluble
binder, a water-dispersible binder or a mixture of a water
soluble binder and a water-dispersible binder. An important
prerequisite in the choice of binders and binder-mixtures is their
ability to form a continuous layer with the other ingredients
present.
The water-dispersible binder can be any water-insoluble polymer
e.g. water-insoluble cellulose derivatives, polymers derived from
α,β-ethylenically unsaturated compounds such as polyvinyl chloride,
after-chlorinated polyvinyl chloride, copolymers of vinyl chloride
and vinylidene chloride, copolymers of vinyl chloride and vinyl
acetate, polyvinyl acetate and partially hydrolyzed polyvinyl
acetate, polyvinyl alcohol, polyvinyl acetals that are made from
polyvinyl alcohol as starting material in which only a part of the
repeating vinyl alcohol units may have reacted with an aldehyde,
preferably polyvinyl butyral, copolymers of acrylonitrile and
acrylamide, polyacrylic acid esters, polymethacrylic acid esters,
polystyrene and polyethylene or mixtures thereof. It should be
noted that there is no clear cut transition between a polymer
dispersion and a polymer solution in the case of very small polymer
particles resulting in the smallest particles of the polymer being
dissolved and those slightly larger being in dispersion.
Suitable water-soluble polymers, according to the present
invention, are: polyvinyl alcohol, polyacrylamide, polyacrylic acid,
polymethacrylic acid, polyethyleneglycol, proteins, such as gelatin
and modified gelatins such as phthaloyl gelatin, polysaccharides,
such as starch, gum arabic and dextran and water-soluble cellulose
derivative.
To improve the layer-forming properties of water-soluble and
water-dispersible polymers, plasticizers can be incorporated into
the polymers, water-miscible solvents can be added to the dispersion
medium and mixtures of water-soluble polymers, mixtures of water-dispersible
polymers, or mixtures of water-soluble and water-dispersible
polymers may be used.
Photo-addressable thermally developable element
The photo-addressable thermally developable element, according
to the present invention, comprises a substantially light-insensitive
organic silver salt, photosensitive silver halide in
catalytic association therewith and an organic reducing agent in
thermal working relationship with the substantially light-insensitive
organic silver salt and a water soluble or water-dispersible
binder. The element may include a layer system with the
silver halide in catalytic association with the substantially light-insensitive
organic silver salt, spectral sensitizer optionally
together with a supersensitizer in intimate sensitizing association
with the silver halide particles and the other ingredients active in
the thermal development process or pre- or post-development
stabilization of the element being in the same layer or in other
layers with the proviso that the organic reducing agent and the
toning agent, if present, are in thermal working relationship with
the substantially light-insensitive organic silver salt i.e. during
the thermal development process the reducing agent and the toning
agent, if present, are able to diffuse to the substantially light-insensitive
organic silver salt.
Light-insensitive organic silver salts
Preferred substantially light-insensitive organic silver salts
produced using the process according to the present invention and
used in the photothermographic materials, according to the present
invention, are silver salts of organic carboxylic acids having as
their organic group: aryl, aralkyl, alkaryl or alkyl. For example
aliphatic carboxylic acids known as fatty acids, wherein the
aliphatic carbon chain has preferably at least 12 C-atoms, e.g.
silver laurate, silver palmitate, silver stearate, silver
hydroxystearate, silver oleate and silver behenate, which silver
salts are also called "silver soaps". Silver salts of modified
aliphatic carboxylic acids with thioether group, as described e.g.
in GB-P 1,111,492, may likewise be used to produce a thermally
developable silver image.
In a preferred embodiment, according to the present invention,
the substantially light-insensitive organic silver salt is a silver
salt of a fatty acid.
The term substantially light-insensitive organic silver salt for
the purposes of the present invention also includes mixtures of
organic silver salts.
Binder to organic silver salt ratio
The binder to organic silver salt weight ratio is preferably in
the range of 0.2 to 6, and the thickness of the recording layer is
preferably in the range of 1 to 50 µm.
Production of particles of organic silver salt
Particles of the organic silver salts are prepared by the
reaction of a soluble silver salt with the organic carboxylic acid
or a salt thereof.
According to a process, according to the present invention, the
suspension of particles of a substantially light-insensitive organic
silver salt may be produced by simultaneous metered addition of an
aqueous solution or suspension of an organic carboxylic acid, or its
salt, and an aqueous solution of a silver salt to an aqueous liquid
and the metered addition of the aqueous solution or suspension of
the organic carboxylic acid or its salt; and/or the aqueous solution
of the silver salt is regulated by the concentration of silver ions
or the concentration of anions of the silver salt in the aqueous
liquid as disclosed in EP-A 754 969.
Photosensitive silver halide
The photosensitive silver halide used in the present invention
may be employed in a range of 0.1 to 35 mol percent of substantially
light-insensitive organic silver salt, with the range of 0.5 to 20
mol percent being preferred and the range of 1 to 12 mol percent
being particularly preferred.
The silver halide may be any photosensitive silver halide such
as silver bromide, silver iodide, silver chloride, silver
bromoiodide, silver chlorobromoiodide, silver chlorobromide etc. The
silver halide may be in any form which is photosensitive including,
but not limited to, cubic, orthorhombic, tabular, tetrahedral,
octagonal etc. and may have epitaxial growth of crystals thereon.
The silver halide used in the present invention may be employed
without modification. However, it may be chemically sensitized with
a chemical sensitizing agent such as a compound containing sulphur,
selenium, tellurium etc., or a compound containing gold, platinum,
palladium, iron, ruthenium, rhodium or iridium etc., a reducing
agent such as a tin halide etc., or a combination thereof. The
details of these procedures are described in T.H. James, "The Theory
of the Photographic Process", Fourth Edition, Macmillan Publishing
Co. Inc., New York (1977). Chapter 5, pages 149 to 169.
According to a preferred embodiment used in the present
invention, particles of the photosensitive silver halide are non-aggregating
in the photo-addressable thermally developable element
and are uniformly distributed over and between particles of the
substantially light-insensitive organic silver salt, at least 80% by
number of the particles having a diameter, determined by
transmission electron microscopy, of ≤40nm.
Emulsion of organic silver salt and photosensitive silver halide
The silver halide may be added to the photo-addressable
thermally developable element in any fashion which places it in
catalytic proximity to the substantially light-insensitive organic
silver salt. Silver halide and the substantially light-insensitive
organic silver salt which are separately formed, i.e. ex-situ or
"preformed", in a binder can be mixed prior to use to prepare a
coating solution, but it is also effective to blend both of them for
a long period of time. Furthermore, it is effective to use a process
which comprises adding a halogen-containing compound to the organic
silver salt to partially convert the substantially light-insensitive
organic silver salt to silver halide as disclosed in US-P 3,457,075.
The aqueous emulsion of the organic silver salt optionally
including photosensitive silver halide can, according to the present
invention, also be produced from particles of the organic silver
salt optionally containing photosensitive silver halide by
dispersing the particles in water in the presence of non-ionic or
anionic surfactants or a mixture of non-ionic and anionic
surfactants using any dispersion technique known to one skilled in
the art such as ball milling, dispersion in a impingement mill
(rotor-stator mixer), dispersion in a microfluidizer etc. A
combination of dispersion techniques may also be used, for example
using a first technique to produce a predispersion and a second
technique to produce a fine dispersion.
Onium halides and polyhalides
According to the present invention photosensitive silver halide
particles produced by reacting an aqueous dispersion of particles of
the substantially light-insensitive organic silver salt with at
least one onium salt with halide or polyhalide anions may be
present. Onium cations, according to the present invention, may be
polymeric or non-polymeric. Preferred non-polymeric onium salts for
partial conversion of particles of substantially light-insensitive
organic silver salt into photosensitive silver halides according to
the present invention are:
- PC01 =
- 3-(triphenyl-phosphonium)propionic acid bromide
perbromide
- PC02 =
- 3-(triphenyl-phosphonium)propionic acid bromide
- PC03 =
- 3-(triphenyl-phosphonium)propionic acid iodide
The onium salts are present in quantities of between 0.1 and
35mol % with respect to the quantity of substantially light-insensitive
organic silver salt of organic, with quantities between
0.5 and 20mol% being preferred and with quantities between 1 and
12mol % being particularly preferred.
Organic reducing agent
Suitable organic reducing agents for the reduction of the
substantially light-insensitive organic heavy metal salts are
organic compounds containing at least one active hydrogen atom
linked to O, N or C. Particularly suitable organic reducing agents
for the reduction of the substantially light-insensitive organic
silver salt, an organic reducing agent for the substantially light-insensitive
organic silver salt are non-sulfo-substituted 6-membered
aromatic or heteroaromatic ring compounds with at least three
substituents one of which is a hydroxy group at a first carbon atom
and a second of which is a hydroxy or amino-group substituted on a
second carbon atom one, three or five ring atoms removed in a system
of conjugated double bonds from the first carbon atom in the
compound, in which (i) the third substituent may be part of an
annelated carbocyclic or heterocyclic ring system; (ii) the third
substituent or a further substituent is not an aryl- or oxo-aryl-group
whose aryl group is substituted with hydroxy-, thiol- or
amino-groups; and (iii) the third substituent or a further
substituent is a non-sulfo-electron withdrawing group if the second
substituent is an amino-group.
Particularly preferred reducing agents are substituted catechols
or substitued hydroquinones with 3-(3',4'-dihydroxyphenyl)-propionic
acid, 3',4'-dihydroxy-butyrophenone, methyl gallate, ethyl gallate
and 1,5-dihydroxy-naphthalene being especially preferred.
During the thermal development process the reducing agent must
be present in such a way that it is able to diffuse to the
substantially light-insensitive organic silver salt particles so
that reduction of the substantially light-insensitive organic silver
salt can take place.
Auxiliary reducing agents
The above mentioned reducing agents, regarded as primary or main
reducing agents, may be used in conjunction with so-called auxiliary
reducing agents. Auxiliary reducing agents that may be used in
conjunction with the above mentioned primary reducing agents are
sulfonyl hydrazide reducing agents such as disclosed in US-P
5,464,738, trityl hydrazides and formyl-phenyl-hydrazides such as
disclosed in US-P 5,496,695 and organic reducing metal salts, e.g.
stannous stearate described in US-P 3,460,946 and 3,547,648.
Thermal solvents
The above mentioned binders or mixtures thereof may be used in
conjunction with waxes or "heat solvents" also called "thermal
solvents" or "thermosolvents" improving the reaction speed of the
redox-reaction at elevated temperature.
By the term "heat solvent" in this invention is meant a non-hydrolyzable
organic material which is in a solid state in the
recording layer at temperatures below 50°C, but becomes a
plasticizer for the recording layer where thermally heated and/or a
liquid solvent for at least one of the redox-reactants, e.g. the
reducing agent for the substantially light-insensitive organic
silver salt, at a temperature above 60°C.
Toning agents
In order to obtain a neutral black image tone in the higher
densities and neutral grey in the lower densities,
photothermographic materials according to the present invention may
contain one or more toning agents. The toning agents should be in
thermal working relationship with the substantially light-insensitive
organic silver salts and reducing agents during thermal
processing. Any known toning agent from thermography or
photothermography may be used.
Suitable toning agents are succinimide and the phthalimides and
phthalazinones within the scope of the general formulae described in
US-P 4,082,901 and the toning agents described in US-P 3,074,809,
US-P 3,446,648 and US-P 3,844,797. Particularly useful toning agents
are the heterocyclic toner compounds of the benzoxazine dione or
naphthoxazine dione type as described in GB-P 1,439,478 and US-P
3,951,660.
Stabilizers and antifoggants
In order to obtain improved shelf-life and reduced fogging,
stabilizers and antifoggants may be incorporated into the
photothermographic materials of the present invention. Examples of
suitable stabilizers and antifoggants and their precursors, which
can be used alone or in combination, include the thiazolium salts
described in US-P 2,131,038 and 2,694,716; the azaindenes described
in US-P 2,886,437 and 2,444,605; the urazoles described in US-P
3,287,135; the sulfocatechols described in US-P 3,235,652; the
oximes described in GB-P 623,448; the thiuronium salts described in
US-P 3,220,839; the palladium, platinum and gold salts described in
US-P 2,566,263 and 2,597,915; the tetrazolyl-thio-compounds
described in US-P 3,700,457; the mesoionic 1,2,4-triazolium-3-thiolate
stablizer precursors described in US-P 4,404,390 and
4,351,896; the tribromomethyl ketone compounds described in EP-A 600
587; the combination of isocyanate and halogenated compounds
described in EP-A 600 586; the vinyl sulfone and β-halo sulfone
compounds described in EP-A 600 589; and those compounds mentioned
in this context in Chapter 9 of "Imaging Processes and Materials,
Neblette's 8th edition", by D. Kloosterboer, edited by J. Sturge, V.
Walworth and A. Shepp. page 279, Van Nostrand (1989); in Research
Disclosure 17029 published in June 1978; and in the references cited
in all these documents.
Surfactants
Non-ionic, cationic or anionic surfactants may be used,
according to the present invention, to produce dispersions of
particles of the substantially light-insensitive organic silver salt
in aqueous media and to disperse water-dispersible binders, such as
polymer latexes, in aqueous media. In a preferred embodiment used
in the present invention the surfactant is a sulfonate e.g. alkyl,
aryl, alkaryl or aralkyl sulfonate, with alkyl and alkaryl
sulfonates being particularly preferred e.g.:
Additional ingredients
In addition to the ingredients the photothermographic material
may contain other additives such as free organic carboxylic acids,
surface-active agents, antistatic agents, e.g. non-ionic antistatic
agents including a fluorocarbon group as e.g. in
F3C(CF2)6CONH(CH2CH2O)-H, silicone oil, e.g. BAYSILONE Öl A (tradename
of BAYER AG - GERMANY), ultraviolet light absorbing compounds, white
light reflecting and/or ultraviolet radiation reflecting pigments,
silica, and/or optical brightening agents.
Antihalation dyes
According to a preferred embodiment of the present invention,
the photothermographic recording material further comprises an
antihalation or acutance dye which absorbs light which has passed
through the photosensitive layer, thereby preventing its reflection.
Such dyes may be incorporated into the photo-addressable thermally
developable element or in any other layer comprising the
photothermographic recording material of the present invention. The
antihalation dye may also be bleached either thermally during the
thermal development process or photo-bleached after removable after
the thermal development process and it may be contained in a layer
which can be removed subsequent to the exposure process. Suitable
antihalation dyes for use with infra-red light are described in the
EP-A's 377 961 and 652 473, the EP-B's 101 646 and 102 781 and the
US-P's 4,581,325 and 5,380,635.
Support
The support for the photothermographic recording material
according to the present invention may be transparent, translucent
or opaque, e.g. having a white light reflecting aspect and is
preferably a thin flexible carrier made e.g. from paper,
polyethylene coated paper or transparent resin film, e.g. made of a
cellulose ester, e.g. cellulose triacetate, corona and flame treated
polypropylene, polystyrene, polymethacrylic acid ester,
polycarbonate or polyester, e.g. polyethylene terephthalate or
polyethylene naphthalate as disclosed in GB 1,293,676, GB 1,441,304
and GB 1,454,956. For example, a paper base substrate is present
which may contain white reflecting pigments, optionally also applied
in an interlayer between the recording material and the paper base
substrate.
The support may be in sheet, ribbon or web form and subbed if
needs be to improve the adherence to the thereon coated heat-sensitive
recording layer.
Suitable subbing layers for improving the adherence of the
thermosensitive element and the antistatic layer outermost backing
layer of the present invention for polyethylene terephthalate
supports are described e.g. in GB-P 1,234,755, US-P 3,397,988;
3,649,336; 4,123,278 and US-P 4,478,907 which relates to subbing
layers applied from aqueous dispersion of sulfonated copolyesters,
and further the subbing layers described in Research Disclosure
published in Product Licensing Index, July 1967, p. 6.
Suitable pretreatments of hydrophobic resin supports are, for
example, treatment with a corona discharge and/or attack by
solvent(s), thereby providing a micro-roughening.
Protective layer
According to a preferred embodiment of the photothermographic
recording material of the present invention, the photo-addressable
thermally developable element is provided with a protective layer.
The protective layer preferably comprises a binder, which may be
solvent soluble (hydrophobic), solvent dispersible, water soluble
(hydrophilic) or water dispersible. Among the hydrophobic binders
polycarbonates as described in EP-A 614 769 are particularly
preferred. Suitable hydrophilic binders are, for example, gelatin,
polyvinylalcohol, cellulose derivatives or other polysaccharides,
hydroxyethylcellulose, hydroxypropylcellulose etc., with hardenable
binders being preferred and polyvinylalcohol being particularly
preferred.
A protective layer according to the present invention may be
crosslinked. Crosslinking can be achieved by using crosslinking
agents such as described in WO 95/12495 for protective layers. A
protective layer used in the present invention may include in
addition at least one solid lubricant having a melting point below
150°C and at least one liquid lubricant in a binder, wherein at
least one of the lubricants is a phosphoric acid derivative, further
dissolved lubricating material and/or particulate material, e.g.
talc particles, optionally protruding from the outermost layer. The
lubricant may be applied with or without a polymeric binder. Such
protective layers may also comprise particulate material, e.g. talc
particles, optionally protruding from the protective outermost layer
as described in WO 94/11198. Other additives can also be
incorporated in the protective layer e.g. colloidal particles such
as colloidal silica.
Antistatic layer
In a preferred embodiment the recording material of the present
invention an antistatic layer is applied to the outermost layer on
the side of the support not coated with the photo-addressable
thermally developable element. Suitable antistatic layers therefor
are described in EP-A's 444 326, 534 006 and 644 456, US-P's
5,364,752 and 5,472,832 and DOS 4125758.
Coating techniques
The coating of any layer of the photothermographic materials of
the present invention may proceed by any coating technique e.g. such
as described in Modern Coating and Drying Technology, edited by
Edward D. Cohen and Edgar B. Gutoff, (1992) VCH Publishers Inc. 220
East 23rd Street, Suite 909 New York, NY 10010, U.S.A.
Recording process
Photothermographic materials, according to the present
invention, may be exposed with infrared radiation at wavelengths
between 700 and 1100nm with the image either being obtained by
pixel-wise exposure with a finely focussed light source, such as an
IR wavelength laser or an IR-laser diode, e.g. emitting at 780nm,
830nm or 850nm; or by direct exposure to the object itself or an
image therefrom illuminated with IR light.
For the thermal development of image-wise exposed photothermographic
recording materials, according to the present invention, any
sort of heat source can be used that enables the recording materials
to be uniformly heated to the development temperature in a time
acceptable for the application concerned e.g. contact heating,
radiative heating, microwave heating etc.
According to the present invention a photothermographic
recording process is, in which only heat and the photothermographic
recording material are involved in the thermal development process
and the heat is supplied by conduction, convection or radiation.
Applications
The photothermographic recording materials of the present
invention can be used for both the production of transparencies and
reflection type prints. This means that the support will be
transparent or opaque, e.g. having a white light reflecting aspect.
For example, a paper base substrate is present which may contain
white reflecting pigments, optionally also applied in an interlayer
between the recording material and the paper base substrate. Should
a transparent base be used, the base may be colourless or coloured,
e.g. has a blue colour.
In the hard copy field photothermographic recording materials on
a white opaque base are used, whereas in the medical diagnostic
field black-imaged transparencies are widely used in inspection
techniques operating with a light box.
The following ingredients in addition to those mentioned above
were used in the photothermographic recording materials of the
examples and comparative examples illustrating this invention:
the following supersensitizers in addition to those mentioned above:
- * SS-08:
- 2-mercaptobenzimidazole;
- * SS-09:
- 2-mercaptobenzothiazole-5-[N-(4'-chlorophenyl)]sulfonamide.
the following IR-sensitizing dye according to US-P 5,441,866:
the following IR-sensitizing dyes according to EP-A 616 014:
and the latex binder:
- BINDER 01:
- copolymer consisting of 45% by weight of
methylmethacrylate, 45% by weight of butadiene and 10%
by weight of itaconic acid.
The following examples and comparative examples illustrate the
present invention. The percentages and ratios used in the examples
are by weight unless otherwise indicated.
COMPARATIVE EXAMPLES 1 to 5
Extrapolation of the state of the art regarding photothermographic
materials with IR-spectral sensitizers disclosed in US-P 5,441,866
and EP-A 616 014 to photothermographic materials with photo-addressable
thermally developable elements coated from aqueous
media:
Silver behenate dispersion
Silver behenate was prepared by dissolving 34g (0.1 moles) of
behenic acid in 340mL of 2-propanol at 65°C, converting the behenic
acid to sodium behenate by adding 400mL of 0.25M aqueous sodium
hydroxide to the stirred behenic acid solution and finally adding
250mL of 0.4M aqueous silver nitrate the silver behenate
precipitating out. This was filtered off and then washed with a
mixture of 10% by volume of 2-propanol and 90% by volume of
deionized water to remove residual sodium nitrate.
After drying at 45°C for 12h, the silver behenate was dispersed
in deionized water with the anionic dispersion agents Ultravon™ W
and Mersolat™ H to produce, after rapid mixing using a high speed
impingement mill (rotor-stator mixer) to obtain a paste and
homogenization with a microfluidizer, a finely divided and stable
dispersion containing 20% by weight of silver behenate, 2.1% by
weight of Ultravon™ W and 0.203% by weight of Mersolat™ H. The pH of
the resulting dispersion was adjusted to about 6.5.
The following ingredients were then added with stirring to 3.0g
of the silver behenate dispersion: 2g of a 2.22% by weight aqueous
solution of 3-(triphenyl-phosphonium)propionic acid bromide (PC02),
corresponding to a concentration of 8 mol% of PC02 with respect to
silver behenate, at a pH of 4 to accomplish in situ conversion of
part of the silver behenate to silver bromide. After 10 minutes
further stirring, the supersensitizer was added with stirring as a
solution in water and/or methanol, as specified in table 1,
immediately followed by the IR-spectral sensitizer as a solution or
dispersion in water and/or methanol as specified in table 1. After
stirring for a further 15 minutes 2g of a 30% by weight
concentration of BINDER 01 at a pH of 4 was added with stirring
followed by 2g of a 4.5% by weight aqueous solution of 3-(3',4'-dihydroxyphenyl)propionic
acid.
| Comparative example number | IR-sensitizer | supersensitizer |
| | code | Weight of solution [g] | Conc. of solution [% by wt] | code | Weight of solution [g] | Conc. of solution [% by wt] |
| 1 | SENSI C01 | 0.180 | 0.3 (MeOH) | - | - | - |
| 2 | SENSI C01 | 0.180 | 0.3 (MeOH) | SS-08 | 0.2 | 2 (MeOH) |
| 3 | SENSI C02 | 0.270 | 0.2 (MeOH) | - | - | - |
| 4 | SENSI C02 | 0.270 | 0.2 (MeOH) | SS-08 | 0.4 | 2 (MeOH) |
| 5 | SENSI C02 | 0.270 | 0.2 (MeOH) | SS-09 | 0.8 | 1 (MeOH) |
A subbed polyethylene terephthalate support having a thickness
of 100µm was doctor blade-coated with the silver behenate/silver
bromide dispersion at a blade setting of 90µm. After drying for
several minutes at 40°C on the coating bed, the emulsion layer was
dried for 1 hour in a hot air oven at 40°C.
Image-wise exposure and thermal processing
The photothermographic materials of COMPARATIVE EXAMPLES 1 to 5
were exposed to a beam of a 836nm diode laser type HL 8318G from
HITACHI with a nominal power of 12.8mW focussed to give a spot
diameter (1/e2) of 115µm , scanned at a speed of 5m/s with a pitch of
63µm and an overlap of 30% through a wedge filter with optical
density varying between 0 and 3.3 in optical density steps of 0.15.
The maximum exposure (filter optical density = 0) was about 50J/m2.
Thermal processing was carried out on a heated metal block for 5
to 15s at 105 to 115°C, as specified in table 2. The maximum and
minimum optical densities, Dmax and Dmin, of the images were measured
in transmission with a MacBeth™ TR924 densitometer through a visible
filter.
The D
max- and D
min-values obtained upon image-wise exposure and
thermal processing of the photothermographic recording materials of
COMPARATIVE EXAMPLES 1 to 5 together with the IR-sensitizer,
supersensitizer, molar ratio of supersensitizer to IR-sensitizer and
the thermal processing conditions used are summarized in table 2.
| Comparative example number | IR-sensitizer code | supersensitizer code | moles supersensitizer /mol IR-sensitizer | thermal processing conditions |
| | | | | temperature [°C] | time [s] | Dmax | Dmin |
| 1 | SENSI C01 | - | - | 105 | 15 | 0.20 | 0.20 |
| 2 | SENSI C01 | SS-08 | 36 : 1 | 105 | 15 | 0.24 | 0.14 |
| 3 | SENSI C02 | - | - | 105 | 15 | 0.25 | 0.25 |
| 4 | SENSI C02 | SS-08 | 74 : 1 | 105 | 15 | 0.13 | 0.13 |
| 5 | SENSI C02 | SS-09 | 31 : 1 | 105 | 15 | 0.65 | 0.12 |
A comparison of the results obtained for the photothermographic
recording materials of COMPARATIVE EXAMPLES 1 and 2 coated from
aqueous media, shows that no sensitization was observed with SENSI
C01 and that no supersensitization was observed with the
supersensitizer SS-08. A comparison of the results obtained for the
photothermographic recording materials of COMPARATIVE EXAMPLES 4 and
5 with those for COMPARATIVE EXAMPLE 3, shows that no sensitization
was observed with SENSI C02 and that little supersensitization was
observed with the supersensitizers SS-08 and SS-09.
These results show that the state of the art regarding infra-red
sensitization of photothermographic recording materials as
represented by example 1 of US-P 5,441,586 and example 3 of EP-A 616
014 cannot be readily extrapolated to such materials coated from
aqueous media.
INVENTION EXAMPLES 1 to 4 and COMPARATIVE EXAMPLES 6 to 8
Infra-red sensitization with dyes and supersensitizers according to
the present invention:
A dispersion of silver behenate was prepared as described for
COMPARATIVE EXAMPLES 1 to 5 and the photothermographic emulsion
prepared also as described for COMPARATIVE EXAMPLES 1 to 5 except
that the IR-sensitizer, IR-sensitizer solution, weight of IR-sensitizer
solution, supersensitizer, sensitizer solution and weight
of supersensitizer solution used for each photothermographic
emulsion is as given in table 3.
| Invention example number | IR-sensitizer | supersensitizer |
| | code | Weight of solution [g] | Conc. of solution [% by wt] | code | Weight of solution [g] | Conc. of solution [% by wt] |
| 1 | SENSI 01 | 0.18 | 0.3 (MeOH) | SS-01 | 1.2 | 0.25 (MeOH) |
| 2 | SENSI 01 | 0.18 | 0.3 (MeOH) | SS-09 | 0.8 | 1.0 (MeOH) |
| 3 | SENSI 02 | 0.18 | 0.3 (MeOH) | SS-01 | 1.2 | 0.25 (MeOH) |
| 4 | SENSI 06 | 0.046 | 0.3 (MeOH) | SS-01 | 1.2 | 0.25 (MeOH) |
| Comparative example number |
| 6 | SENSI 01 | 0.18 | 0.3 (MeOH) | - | - | - |
| 7 | SENSI 02 | 0.18 | 0.3 (MeOH) | - | - | - |
| 8 | SENSI 06 | 0.046 | 0.3 (MeOH) | - | - | - |
The photothermographic emulsions of INVENTION EXAMPLES 1 to 4
and COMPARATIVE EXAMPLES 6 to 8 were coated as described for
COMPARATIVE EXAMPLES 1 to 5 and then image-wise exposed, thermally
processed and the resulting images evaluated as described for
COMPARATIVE EXAMPLES 1 to 5.
The D
max- and D
min-values obtained upon image-wise exposure and
thermal processing of the photothermographic recording materials of
INVENTION EXAMPLES 1 to 4 and COMPARATIVE EXAMPLES 6 to 8 together
with the IR-sensitizer, supersensitizer, molar ratio of
supersensitizer to IR-sensitizer and the thermal processing
conditions used are summarized in table 4.
| Invention example number | IR-sensitizer code | supersensitizer code | moles supersensitizer /mol IR-sensitizer | thermal processink conditions |
| | | | | temperature [°C] | time [s] | Dmax | Dmin |
| 1 | SENSI 01 | SS-01 | 3 : 1 | 105 | 15 | 1.59 | 0.17 |
| 2 | SENSI 01 | SS-09 | 25 : 1 | 105 | 15 | 0.89 | 0.12 |
| 3 | SENSI 02 | SS-01 | 4 : 1 | 105 | 15 | 0.75 | 0.15 |
| 4 | SENSI 06 | SS-01 | 18 : 1 | 105 | 15 | 0.58 | 0.15 |
| Comparative example number |
| 6 | SENSI 01 | - | - | 105 | 15 | 0.30 | 0.30 |
| 7 | SENSI 02 | - | - | 105 | 15 | 0.15 | 0.15 |
| 8 | SENSI 06 | - | - | 105 | 15 | 0.25 | 0.25 |
The results in table 4 show that the IR-sensitizer dyes of the
present invention are efficiently supersensitized by stilbene-supersensitizers
(SS-01, SS-02, SS-03, SS-04 and SS-07) and
mercapto-supersensitizers (SS-09), being examples of the general
class of supersensitizers, in photothermographic recording materials
comprising photo-addressable thermally developable elements coated
from aqueous media.
Having described in detail preferred embodiments of the current
invention, it will now be apparent to those skilled in the art that
numerous modifications can be made therein without departing from
the scope of the invention as defined in the following claims.