EP0084138A2 - Near-zero magnetostrictive glassy metal alloys with high magnetic and thermal stability - Google Patents
Near-zero magnetostrictive glassy metal alloys with high magnetic and thermal stability Download PDFInfo
- Publication number
- EP0084138A2 EP0084138A2 EP82111754A EP82111754A EP0084138A2 EP 0084138 A2 EP0084138 A2 EP 0084138A2 EP 82111754 A EP82111754 A EP 82111754A EP 82111754 A EP82111754 A EP 82111754A EP 0084138 A2 EP0084138 A2 EP 0084138A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- ranges
- atom percent
- alloys
- magnetostriction
- glassy
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 230000005291 magnetic effect Effects 0.000 title abstract description 29
- 229910001092 metal group alloy Inorganic materials 0.000 title abstract description 17
- 239000005300 metallic glass Substances 0.000 title abstract description 17
- 229910045601 alloy Inorganic materials 0.000 claims abstract description 54
- 239000000956 alloy Substances 0.000 claims abstract description 54
- 229910001004 magnetic alloy Inorganic materials 0.000 claims description 12
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 abstract description 27
- 230000006698 induction Effects 0.000 abstract description 21
- 239000000203 mixture Substances 0.000 abstract description 15
- 229910052752 metalloid Inorganic materials 0.000 abstract description 5
- 150000002738 metalloids Chemical class 0.000 abstract description 5
- 229910052742 iron Inorganic materials 0.000 abstract description 4
- 229910052723 transition metal Inorganic materials 0.000 abstract description 3
- 239000010941 cobalt Substances 0.000 abstract description 2
- 229910017052 cobalt Inorganic materials 0.000 abstract description 2
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 abstract description 2
- 150000003624 transition metals Chemical class 0.000 abstract description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 20
- 239000000463 material Substances 0.000 description 13
- 230000035699 permeability Effects 0.000 description 11
- 229910052710 silicon Inorganic materials 0.000 description 10
- 238000002425 crystallisation Methods 0.000 description 8
- 230000008025 crystallization Effects 0.000 description 8
- 238000000034 method Methods 0.000 description 7
- 229910000889 permalloy Inorganic materials 0.000 description 7
- 238000000137 annealing Methods 0.000 description 5
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 4
- 230000005294 ferromagnetic effect Effects 0.000 description 4
- 230000005415 magnetization Effects 0.000 description 4
- 229910052759 nickel Inorganic materials 0.000 description 4
- 229910000702 sendust Inorganic materials 0.000 description 4
- 239000010703 silicon Substances 0.000 description 4
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 229910052750 molybdenum Inorganic materials 0.000 description 3
- 229910000815 supermalloy Inorganic materials 0.000 description 3
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229910052796 boron Inorganic materials 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 239000000696 magnetic material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000000155 melt Substances 0.000 description 2
- 239000011733 molybdenum Substances 0.000 description 2
- 238000004804 winding Methods 0.000 description 2
- 241001279686 Allium moly Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229910020598 Co Fe Inorganic materials 0.000 description 1
- 229910002519 Co-Fe Inorganic materials 0.000 description 1
- 229910001313 Cobalt-iron alloy Inorganic materials 0.000 description 1
- 229910000640 Fe alloy Inorganic materials 0.000 description 1
- MWCLLHOVUTZFKS-UHFFFAOYSA-N Methyl cyanoacrylate Chemical compound COC(=O)C(=C)C#N MWCLLHOVUTZFKS-UHFFFAOYSA-N 0.000 description 1
- 229910000676 Si alloy Inorganic materials 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- 230000005534 acoustic noise Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000004568 cement Substances 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000000113 differential scanning calorimetry Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 229910052732 germanium Inorganic materials 0.000 description 1
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229910052735 hafnium Inorganic materials 0.000 description 1
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 description 1
- UGKDIUIOSMUOAW-UHFFFAOYSA-N iron nickel Chemical compound [Fe].[Ni] UGKDIUIOSMUOAW-UHFFFAOYSA-N 0.000 description 1
- XWHPIFXRKKHEKR-UHFFFAOYSA-N iron silicon Chemical compound [Si].[Fe] XWHPIFXRKKHEKR-UHFFFAOYSA-N 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 239000010955 niobium Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000004080 punching Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000000700 radioactive tracer Substances 0.000 description 1
- 238000007712 rapid solidification Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000000646 scanning calorimetry Methods 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 238000005482 strain hardening Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 229910002058 ternary alloy Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C45/00—Amorphous alloys
- C22C45/04—Amorphous alloys with nickel or cobalt as the major constituent
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/12—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials
- H01F1/14—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys
- H01F1/147—Alloys characterised by their composition
- H01F1/153—Amorphous metallic alloys, e.g. glassy metals
- H01F1/15316—Amorphous metallic alloys, e.g. glassy metals based on Co
Definitions
- This invention relates to glassy metal alloys with near-zero magnetostriction, high magnetic and thermal stability and excellent soft magnetic properties.
- Saturation magnetostriction as is related to the fractional change in length ⁇ l/l that occurs in a magnetic material on going from the demagnetized to the saturated, ferromagnetic state.
- the value of magnetostriction is often given in units of microstrains (i.e., a microstrain is a fractional change in length of one part per million).
- Ferromagnetic alloys of low magnetostriction are desirable for several interrelated reasons:
- Zero magnetostrictive alloys based on the binaries but with small additions of other elements such as molybdenum, copper or aluminum to provide specific property changes.
- These include, for example, 4% Mo, 79% Ni, 17 % Fe (sold under the designation Moly Permalloy) for increased resistivity and permeability; permalloy plus varying amounts of copper (sold under the designation Mumetal) for magnetic softness and improved ductility; and 85 wt.% Fe, 9 wt.% Si, 6 wt.% Al (sold under the designation Sendust) for zero anisotropy.
- the alloys included in category (1) are the most widely used of the three classes listed above because they combine zero magnetostriction with low anisotropy and are, therefore, extremely soft magnetically; that is they have a low coercivity, a high permeability and a low core loss. These permalloys are also relatively soft mechanically and their excellent magnetic properties, achieved by high temperature (above 1000°C) anneal, tend to be degraded by relatively mild mechanical shock.
- Category (2) alloys such as those based on CogoFe l o have a much higher saturation induction (B s about 1.9 Tesla) than the permalloys. However, they also have a strong negative magnetocrystalline anisotropy, which prevents them from being good soft magnetic materials. For example, the initial permeability of Co 90 Fe 10 is only about 100 to 200.
- Category (3) alloys such as Fe/6 wt% Si and the related ternary alloy Sendust (mentioned above) also show higher saturation inductions (B s about 1.8 Tesla and 1.1 Tesla, respectively) than the permalloys.
- these alloys are extremely brittle and have, therefore, found limited use in powder form only.
- compositional dependence of the magnetostriction is very strong in these materials, difficult precise tayloring of the alloy composition to achieve near-zero maganetostriction.
- glassy metal alloys of zero magnetostriction. Such alloys might be found near the compositions listed above. Because of the presence of metalloids which tend to quench the magnetization by the transfer of charge to the transition-metal d-electron states, however, glassy metal alloys based on the 80 nickel permalloys are either non-magnetic at room temperature or have unacceptably low saturation inductions.
- the glassy alloy Fe 40 Ni 40 P 14 B 6 (the subscripts are in atom percent) has a saturation induction of about 0.8 Tesla, while the glassy alloy Ni 49 Fe 29 P l4 B 6 Si 2 has a saturation induction of about 0.46 Tesla and the glassy alloy Ni 80 P 20 is non-magnetic.
- No glassy metal alloys having a saturation magnetostriction approximately equal to zero have yet been found near the iron-rich Sendust composition.
- a number of near-zero magnetostrictive glassy metal alloys based on the Co-Fe crystalline alloy mentioned above in (2) have been reported in the literature. These are, for example, Co 72 Fe 3 P 16 B 6 Al 3 (AIP Conference Proceedings, No. 24, pp.
- a magnetic alloy that is at least 70% glassy, and which has a near-zero magnetostriction, high magnetic and thermal stability and excellent soft magnetic properties.
- the glassy metal alloy has the composition Co a Fe b Ni c MoaB e Si f , where a ranges from about 58 to 70 atom percent, b ranges from about 2 to 7.5 atom percent, c ranges from about 0 to 8 atom percent, d ranges from about 1 to about 2 atom percent, e ranges from about 11 to 15 atom percent and f ranges from about 9 to 14 atom percent, with the proviso that the sum of a, b and c ranges from about 72 to 76 atom percent and the sum of e and f ranges from about 23 to 26 atom percent.
- the glassy alloy has a value of magnetostriction ranging from about -1 x 10-6 to +1 x 10-6 a saturation induction ranging from about 0.6 to 0.8 Tesla, a Curie temperature ranging from about 550 to 670K and a first crystallization temperature ranging from about 790 to 870 K.
- a magnetic alloy that is at least 70% glassy and which has an outstanding combination of properties, including a near-zero magnetostriction, high magnetic and thermal stability and such soft magnetic properties as high permeability, low core loss and low coercivity.
- the glassy metal alloy has the composition C o a Fe b Ni c Mo d B e Si f , where a ranges from about 58 to 70 atom percent, b ranges from about 2 to 7.5 atom percent, c ranges from about 0 to 8 atom percent and d ranges from about 1 to about 2 atom percent, e ranges from about 11 to 15 atom percent and f ranges from about 9 to 14 atom percent, with the proviso that the sum of a, b and c ranges from about 72 to 76 atom percent and the sum of e and f ranges from about 23 to 26 atom percent.
- the glassy alloy has a value of magnetostriction ranging from about -1 x 10-6 to +1 x 10-6 and a saturation induction ranging from about 0.6 to 0.8 Tesla, Curie Temperature, ranging from 550 to 670K and the first crystallization temperature ranging from about 790 to 870 K.
- molybdenum in the alloys of the invention may be replaced by at least one other transition metal element, such as tungsten, niobium, tantalum, titanium, zirconium and hafnium, and up to about 2 atom percent of Si may be replaced by carbon, aluminum or germanium without significantly degrading the desirable magnetic properties of these glassy alloys.
- Examples of essentially zero magnetostrictive glassy metal alloys of the invention include Co 67.4 Fe 4.1 Ni 3.0 Mo 1.5 B 12.5 Si 11.5 . Co 67 .lFe 4 . 4 Ni 3 .o M oi.5 B 12 . 5 Si ll . 5 , Co 64.0 Fe 4.5 Ni 6.0 Mo 1.5 B 12.5 Si 11.5 , C 0 6 7.0 Fe 4.5 Ni 3.0 Mo 1.5 B 12 Si 12 . Co 67.0 Fe 4.5 Ni 3.0 Mo 1.5 B 13 Si 11 and Co 67.5 Fe 4.5 Ni 3.0 Mo 1.0 B 12 Si 12 ⁇ These glassy alloys possess saturation induction between about 0.7 and 0.8 Tesla, Curie temperature between 600 and 670K, the first crystallization temperature of about 800K and excellent ductility. Some magnetic and thermal properties of these and some of other near-zero magnetostrictive glassy alloys of the present invention are listed in Table II. These may be compared with properties listed in Table I for previously-reported glassy metal alloys of zero magnetostriction.
- the activation energy (E a ) for the reorientation of the magnetization is listed in Table III for some representative near-zero magnetostrictive glassy alloys. This table indicates that Si tends to increase E a and also that E a tends to be higher when Si/B ratio is close to 1. The higher values of E a , indicating higher magnetic stability of the system, is desired. Combining these information based Table II and III, preferred Si content is between 9 and 14 atom percent when (Si + B) is between 23 and 26 atom percent.
- the presence of Mo is to increase T cl and hence the thermal stability of the alloy system.
- the content of Mo beyond 2 atom percent reduces the Curie temperature to a level lower than 550 K, which is undesirable in convention magnetic devices.
- Such near-zero magnetostrictive glassy metal alloys are obtained for a, b and c in the ranges of about 58 to 70, 2 to 7.5 and 0 to 8 atom percent respectively, with the provision that the sum of a, b and c ranges between 72 and 76 atom percent.
- of these glassy metal alloys is less than about 1 x 10-6 (i.e., the saturation magnetostriction ranges from about -1 x 10-6 to +1 x 10- 6 , or -1 to +1 microstrains).
- the saturation induction of these glassy alloys ranges between about 0.6 and 0.8 Tesla.
- Values of A s even closer to zero may be obtained for values of a, b and c ranging respectively from about 63 to 69, 3 to 6 and 0 to 6, with the provision that the sum of a, b and c ranges between about 72 and 76 atom percent.
- is less than 0.5 x 10- 6 .
- the glassy metal alloys of the invention are conveniently prepared by techniques readily available elsewhere; see, e.g., U.S. Patents 3,845,805, issued November 5, 1974 and 3,856,513, issued December 24, 1974.
- the glassy alloys, in the form of continuous ribbon, wire, etc. are rapidly quenched from a melt of the desired composition at a rate of at least about 105 K/ sec.
- a metalloid content of boron, and silicon in the range of about 23 to 26 atom percent of the total alloy composition is sufficient for glass formation, with boron ranging from about 11 to 15 atom percent and silicon ranging from about 9 to about 14 atom percent.
- a ratio Si/B close to 1 and a Si content ("f") between 11 and 12 atom percent are most favorable because they lead to higher stability and relative insensitiveness of the magnetostriction value (which is close to zero) to the metalloid composition.
- the rate of change of magnetostriction value with respect to silicon content, dX s/ df, is close to zero for "f" between 11 and 12 atom percent while
- the small amount of Ni is relatively ineffective to alter the magnetostriction values in the present alloy system and Co:Fe ratios essentially determine the resultant maganetostriction values.
- Zero magnetostriction is realized for the Co:Fe ratio of about (14 ⁇ 16.5) to 1 in the present alloy system.
- the ratios are narrowly set at about 14 and 12 respectively.
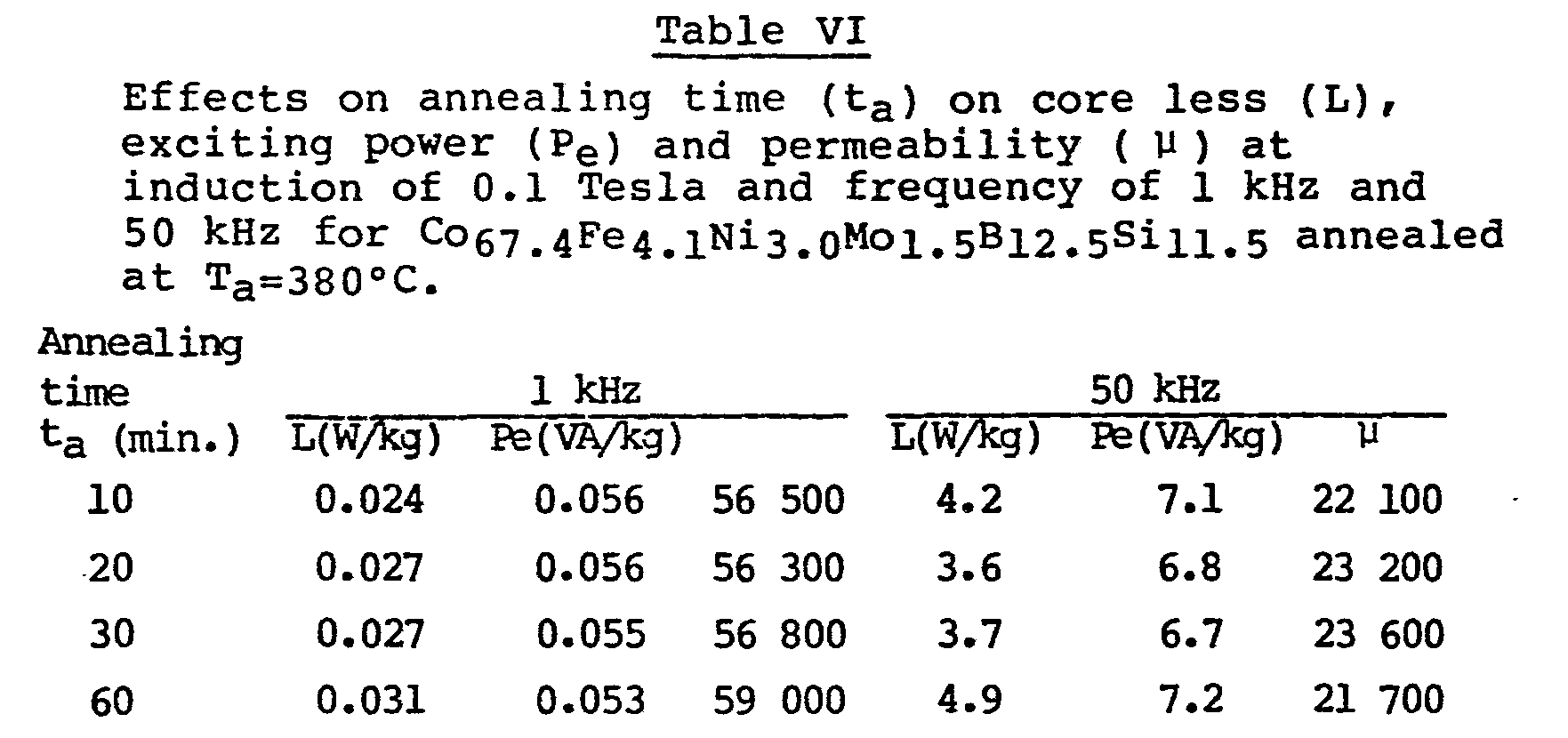
- Table IV gives ac core loss (L), exciting power (P e ) and permeability (P ) at 0.1 Tesla induction and at 50 kHz of the near-zero magnetostrictive glassy alloys of the present invention annealed at different temperatures (T a ).
- Table V shows the effects of the annealing temperature (T a ) and annealing field (H 11 ) applied along the circumferential cirection of the toroidal samples on the dc coercivity (H c ) and remanence (B r ), ac coercivity (H c ') and squareness ratio (B r /B l ), where B 1 is the induction at an applied field of 1 Oe at 50 kHz and ⁇ at 50 kHz and 0.1 T induction for one of the zero magnetostrictive alloys of the present invention.
- Low coercivity and high squareness ratio close to 1 at high frequencies are desirable in some magnetic device applications such as switch-mode power supplies.
- amorphous alloys outside the scope of the invention are set forth in Table VII.
- the advantageous combination of properties provided by the alloys of the present invention cannot be achieved in the prior art nonmagnetostrictive glassy alloys with high saturation induction such as Co 74 Fe 6 B 20 because their Curie temperatures are higher than the first crystallization temperatures and the heat-treatment to improve their properties are not so effective as in those with lower saturation inductions.
- the above properties, achieved in the glassy alloys of the present invention may be obtained in low induction glassy alloys of the prior art.
- these alloys of the prior art such as Co 31.2 Fe 7.8 Ni 39.0 B 14 Si 8 tend to be magnetically unstable at relatively low temperature of about 150°C as pointed earlier.
- Table VII shows the magnetic properties of some of the representative glassy alloys of the composition C Oa Fe b Ni c Mo d B e Si f in which at least one of a, b, c, d, e, and f is outside the composition range defined in the present invention.
- the table indicates that the alloys with at least one of the constituents outside the defined ranges exhibit at least one of the following undesirable properties: (i) The value of
- the glassy alloys listed in Tables II-VII were rapidly quenched (about 106 K/ sec) from the melt following the techniques taught by Chen and Polk in U.S. Patent 3,856,513.
- the resulting ribbons typically 25 to 30u m thick and 0.5 to 2.5 cm wide, were determined to be free of significant crystallinity by X-ray diffractometry (using CuK radiation) and scanning calorimetry. Ribbons of the glassy metal alloys were strong, shiny, hard and ductile.
- Continuous ribbons of the glassy metal alloys prepared in accordance with the procedure described in Example I were wound onto bobbins (3.8 cm O.D.) to form closed-magnetic-path toroidal samples. Each sample contained from 1 to 3 g of ribbon. Insulated primary and secondary windings (numbering at least 10 each) were applied to the toroids. These samples were used to obtain hysteresis loops (coercivity and remanence) and initial permeability with a commercial curve tracer and core loss (IEEE Standard 106-1972).
- the ferromagnetic Curie temperature (Of) was measured by inductance method and also monitored by differential scanning calorimetry, which was used primarily to determine the crystallization temperatures.
- the first or primary crystallization temperature (T cl ) was used to compare the thermal stability of various glassy alloys of the present and prior art inventions.
- Magnetic stability was determined from the reorientation kinetics of the magnetization, in accordance with the method described in Journal of Applied Physics, vol. 49, p. 6510 (1978), which method is incorporated herein by reference thereto.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Electromagnetism (AREA)
- Dispersion Chemistry (AREA)
- Power Engineering (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Soft Magnetic Materials (AREA)
Abstract
Description
- This invention relates to glassy metal alloys with near-zero magnetostriction, high magnetic and thermal stability and excellent soft magnetic properties.
- Saturation magnetostriction as is related to the fractional change in length Δℓ/ℓ that occurs in a magnetic material on going from the demagnetized to the saturated, ferromagnetic state. The value of magnetostriction, a dimensionless quantity, is often given in units of microstrains (i.e., a microstrain is a fractional change in length of one part per million).
- Ferromagnetic alloys of low magnetostriction are desirable for several interrelated reasons:
- 1. Soft magnetic properties (low coercivity, high permeability) are generally obtained when both the saturation magnetostriction λS and the magnetocrystalline anisotropy K approach zero. 'Therefore, given the same anisotropy, alloys of lower magnetostriction will show lower dc coercivities and higher permeabilities. Such alloys are suitable for various soft magnetic applications.
- 2. Magnetic properties of such zero magnetostrictive materials are insensitive to mechanical strains. When this is the case, there is little need for stress-relief annealing after winding, punching or other physical handling needed to form a device from such material. In contrast, magnetic properties of stress-sensitive materials, such as the crystalline alloys, are seriously degraded by such cold working and such materials must be carefully annealed.
- 3. The low dc coercivity of zero magnetostrictive materials carries over to ac operating conditions where again low coercivity and high permeability are realized (provided the magnetocrystalline anisotropy is not too large and the resistivity not too small). Also because energy is not lost to mechanical vibrations when the saturation maganetostriction is zero, the core loss of zero magnetostrictive materials can be quite low. Thus, zero magnetostrictive magnetic alloys (of moderate or low magnetocrystalline anisotropy) are useful where low loss and high ac permeability are required. Such applications include a variety of tape-wound and laminated core devices, such as power transformers, signal transformers, magnetic recording heads and the like.
- 4. Finally, electromagnetic devices containing zero magnetostrictive materials generate no acoustic noise under AC excitation. While this is the reason for the lower core loss mentioned above, it is also a desirable characteristic in itself because it eliminates the hum inherent in many electromagnetic devices.
- There are three well-known crystalline alloys of zero magnetostriction (in atom percent, unless otherwise indicated):
- (1) Nickel-iron alloys containing approximately 80% nickel ("80 nickel permalloys");
- (2) Cobalt-iron alloys containing approximately 90% cobalt; and
- (3) Iron-silicon alloys containing approximately 6 wt. % silicon.
- Also included in these categories are zero magnetostrictive alloys based on the binaries but with small additions of other elements such as molybdenum, copper or aluminum to provide specific property changes. These include, for example, 4% Mo, 79% Ni, 17% Fe (sold under the designation Moly Permalloy) for increased resistivity and permeability; permalloy plus varying amounts of copper (sold under the designation Mumetal) for magnetic softness and improved ductility; and 85 wt.% Fe, 9 wt.% Si, 6 wt.% Al (sold under the designation Sendust) for zero anisotropy.
- The alloys included in category (1) are the most widely used of the three classes listed above because they combine zero magnetostriction with low anisotropy and are, therefore, extremely soft magnetically; that is they have a low coercivity, a high permeability and a low core loss. These permalloys are also relatively soft mechanically and their excellent magnetic properties, achieved by high temperature (above 1000°C) anneal, tend to be degraded by relatively mild mechanical shock.
- Category (2) alloys such as those based on CogoFelo have a much higher saturation induction (Bs about 1.9 Tesla) than the permalloys. However, they also have a strong negative magnetocrystalline anisotropy, which prevents them from being good soft magnetic materials. For example, the initial permeability of Co90Fe10 is only about 100 to 200.
- Category (3) alloys such as Fe/6 wt% Si and the related ternary alloy Sendust (mentioned above) also show higher saturation inductions (Bs about 1.8 Tesla and 1.1 Tesla, respectively) than the permalloys. However these alloys are extremely brittle and have, therefore, found limited use in powder form only. Recently both Fe/6.5 wt.% Si [IEEE Trans. MAG-16, 728 (1980)] and Sendust alloys [IEEE Trans. MAG-15, 1149 (1970)] have been made relatively ductile by rapid solidification. However, compositional dependence of the magnetostriction is very strong in these materials, difficult precise tayloring of the alloy composition to achieve near-zero maganetostriction.
- It is known that magnetocrystalline anisotropy is effectively eliminated in the glassy state. It is therefore, desirable to seek glassy metal alloys of zero magnetostriction. Such alloys might be found near the compositions listed above. Because of the presence of metalloids which tend to quench the magnetization by the transfer of charge to the transition-metal d-electron states, however, glassy metal alloys based on the 80 nickel permalloys are either non-magnetic at room temperature or have unacceptably low saturation inductions. For example, the glassy alloy Fe40Ni40P14B6 (the subscripts are in atom percent) has a saturation induction of about 0.8 Tesla, while the glassy alloy Ni49Fe29Pl4B6Si2 has a saturation induction of about 0.46 Tesla and the glassy alloy Ni80P20 is non-magnetic. No glassy metal alloys having a saturation magnetostriction approximately equal to zero have yet been found near the iron-rich Sendust composition. A number of near-zero magnetostrictive glassy metal alloys based on the Co-Fe crystalline alloy mentioned above in (2) have been reported in the literature. These are, for example, Co72Fe3P16B6Al3 (AIP Conference Proceedings, No. 24, pp. 745-746 (1975)) C070.5Fe4.5Sil5Blo (Vol. 14, Japanese Journal of Applied Physics, pp. 1077-1078 (1975)) Co31.2Fe7.8Ni39.0B14Si8 [proceedings of 3rd International Conference on Rapidly Quenched Metals, p. 183, (1979)] and C074Fe6B20 [IEEE Trans. MAG-12, 942 (1976)]. Table I lists some of the magnetic properties of these materials.
- Clearly desirable are zero magnetostrictive glassy alloys with higher magnetic and thermal stability and a saturation induction as high as possible.
- In accordance with the invention, there is provided a magnetic alloy that is at least 70% glassy, and which has a near-zero magnetostriction, high magnetic and thermal stability and excellent soft magnetic properties. The glassy metal alloy has the composition CoaFebNicMoaBeSif, where a ranges from about 58 to 70 atom percent, b ranges from about 2 to 7.5 atom percent, c ranges from about 0 to 8 atom percent, d ranges from about 1 to about 2 atom percent, e ranges from about 11 to 15 atom percent and f ranges from about 9 to 14 atom percent, with the proviso that the sum of a, b and c ranges from about 72 to 76 atom percent and the sum of e and f ranges from about 23 to 26 atom percent. The glassy alloy has a value of magnetostriction ranging from about -1 x 10-6 to +1 x 10-6 a saturation induction ranging from about 0.6 to 0.8 Tesla, a Curie temperature ranging from about 550 to 670K and a first crystallization temperature ranging from about 790 to 870 K.
- In accordance with the invention, there is provided a magnetic alloy that is at least 70% glassy and which has an outstanding combination of properties, including a near-zero magnetostriction, high magnetic and thermal stability and such soft magnetic properties as high permeability, low core loss and low coercivity. The glassy metal alloy has the composition CoaFebNicModBeSif, where a ranges from about 58 to 70 atom percent, b ranges from about 2 to 7.5 atom percent, c ranges from about 0 to 8 atom percent and d ranges from about 1 to about 2 atom percent, e ranges from about 11 to 15 atom percent and f ranges from about 9 to 14 atom percent, with the proviso that the sum of a, b and c ranges from about 72 to 76 atom percent and the sum of e and f ranges from about 23 to 26 atom percent. The glassy alloy has a value of magnetostriction ranging from about -1 x 10-6 to +1 x 10-6 and a saturation induction ranging from about 0.6 to 0.8 Tesla, Curie Temperature, ranging from 550 to 670K and the first crystallization temperature ranging from about 790 to 870 K.
- The purity of the above composition is that found in normal commercial practice. However, it will be appreciated that molybdenum in the alloys of the invention may be replaced by at least one other transition metal element, such as tungsten, niobium, tantalum, titanium, zirconium and hafnium, and up to about 2 atom percent of Si may be replaced by carbon, aluminum or germanium without significantly degrading the desirable magnetic properties of these glassy alloys.
- Examples of essentially zero magnetostrictive glassy metal alloys of the invention include Co67.4Fe4.1Ni3.0Mo1.5B12.5Si11.5. Co67.lFe4.4Ni3.oMoi.5 B 12.5Sill.5, Co64.0Fe4.5Ni6.0Mo1.5B12.5Si11.5, C067.0 Fe4.5Ni3.0Mo1.5B12Si12. Co67.0Fe4.5Ni3.0Mo1.5B13Si11 and Co67.5Fe4.5Ni3.0Mo1.0B12Si12· These glassy alloys possess saturation induction between about 0.7 and 0.8 Tesla, Curie temperature between 600 and 670K, the first crystallization temperature of about 800K and excellent ductility. Some magnetic and thermal properties of these and some of other near-zero magnetostrictive glassy alloys of the present invention are listed in Table II. These may be compared with properties listed in Table I for previously-reported glassy metal alloys of zero magnetostriction.
- The activation energy (Ea) for the reorientation of the magnetization is listed in Table III for some representative near-zero magnetostrictive glassy alloys. This table indicates that Si tends to increase Ea and also that Ea tends to be higher when Si/B ratio is close to 1. The higher values of Ea, indicating higher magnetic stability of the system, is desired. Combining these information based Table II and III, preferred Si content is between 9 and 14 atom percent when (Si + B) is between 23 and 26 atom percent.
-
- For some applications, it may be desirable or acceptable to use a material with a small positive or a small negative magnetostriction. Such near-zero magnetostrictive glassy metal alloys are obtained for a, b and c in the ranges of about 58 to 70, 2 to 7.5 and 0 to 8 atom percent respectively, with the provision that the sum of a, b and c ranges between 72 and 76 atom percent. The absolute value of saturation magnetostriction |λS| of these glassy metal alloys is less than about 1 x 10-6 (i.e., the saturation magnetostriction ranges from about -1 x 10-6 to +1 x 10-6, or -1 to +1 microstrains). The saturation induction of these glassy alloys ranges between about 0.6 and 0.8 Tesla.
- Values of A s even closer to zero may be obtained for values of a, b and c ranging respectively from about 63 to 69, 3 to 6 and 0 to 6, with the provision that the sum of a, b and c ranges between about 72 and 76 atom percent. For such preferred compositions, |λS| is less than 0.5 x 10-6. Essentially zero values of magnetostriction are obtained for values of a, b and c ranging from about 64 to 68, 4 to 5 and 0 to 6 atom percent respectively with the provision that the sum of a, b and c ranges between about 72 and 76 atom percent and also when f is between 11 and 12 atom percent and (e + f) is close to 24 atom percent and, accordingly, such compositions are most preferred.
- The glassy metal alloys of the invention are conveniently prepared by techniques readily available elsewhere; see, e.g., U.S. Patents 3,845,805, issued November 5, 1974 and 3,856,513, issued December 24, 1974. In general, the glassy alloys, in the form of continuous ribbon, wire, etc., are rapidly quenched from a melt of the desired composition at a rate of at least about 105 K/sec.
- A metalloid content of boron, and silicon in the range of about 23 to 26 atom percent of the total alloy composition is sufficient for glass formation, with boron ranging from about 11 to 15 atom percent and silicon ranging from about 9 to about 14 atom percent. As noted hereinabove, a ratio Si/B close to 1 and a Si content ("f") between 11 and 12 atom percent are most favorable because they lead to higher stability and relative insensitiveness of the magnetostriction value (which is close to zero) to the metalloid composition. For example, the rate of change of magnetostriction value with respect to silicon content, dXs/df, is close to zero for "f" between 11 and 12 atom percent while |dλS/df| is about 0.8x10-6/at.%Si near f=10 or 13 atom percent when a=67.1, b=4.5, c=3.0 and d=1.5 atom percent. The quantity [dλS/df| becomes zero near f=12 atom percent and about 0.1x10-6/at.%Si near f=10 or 13 atom percent when a=67.8, b=3.7,c=3.0 and d=1.5 atom percent.
- The small amount of Ni is relatively ineffective to alter the magnetostriction values in the present alloy system and Co:Fe ratios essentially determine the resultant maganetostriction values. Zero magnetostriction is realized for the Co:Fe ratio of about (14 ~ 16.5) to 1 in the present alloy system. In the prior art glassy metal alloys such as C0 70.5Fe4.5BloSil5 and C074Fe6B20, the ratios are narrowly set at about 14 and 12 respectively. The above range of the Co:Fe ratio between about 14:1 to 16.5:1 and the tolerance of about +0.5 atom percent near f=11.5 atom percent to achieve λs=0 and dλs/df =0 are advantageous from materials synthesis standpoint.
-
- Table V shows the effects of the annealing temperature (Ta) and annealing field (H11) applied along the circumferential cirection of the toroidal samples on the dc coercivity (Hc) and remanence (Br), ac coercivity (Hc') and squareness ratio (Br/Bl), where B1 is the induction at an applied field of 1 Oe at 50 kHz and µ at 50 kHz and 0.1 T induction for one of the zero magnetostrictive alloys of the present invention. Low coercivity and high squareness ratio close to 1 at high frequencies (e.g. 50 kHz) are desirable in some magnetic device applications such as switch-mode power supplies.
-
- The results set forth in Tables IV-VI above indicate that L=4 W/kg, Pe=7 Va/kg and µ =23 000 at 0.1 T and 50 kHz can be achieved for 25-30 µm thick zero magnetostrictive glassy alloys of the present invention. Compared with these values, a prior art crystalline nonmagnetostrictive supermalloy of the similar thickness (25 µm) gives L= 8 W/kg , Pe= 10 VA/kg and u =19 000 at 0.1 T and 50 kHz. It is clear that the properties of the nonmagnetostrictive glassy alloys of the present invention are superior to those of the crystalline supermalloys. Examples of amorphous alloys outside the scope of the invention are set forth in Table VII. The advantageous combination of properties provided by the alloys of the present invention cannot be achieved in the prior art nonmagnetostrictive glassy alloys with high saturation induction such as Co74Fe6B20 because their Curie temperatures are higher than the first crystallization temperatures and the heat-treatment to improve their properties are not so effective as in those with lower saturation inductions. The above properties, achieved in the glassy alloys of the present invention, may be obtained in low induction glassy alloys of the prior art. However, these alloys of the prior art such as Co31.2Fe7.8Ni39.0B14Si8 tend to be magnetically unstable at relatively low temperature of about 150°C as pointed earlier.
- Table VII shows the magnetic properties of some of the representative glassy alloys of the composition COaFebNicModBeSif in which at least one of a, b, c, d, e, and f is outside the composition range defined in the present invention. The table indicates that the alloys with at least one of the constituents outside the defined ranges exhibit at least one of the following undesirable properties: (i) The value of |λs| is larger than 1x10-6, (ii) The Curie temperature (Sf) is higher than the crystallization temperature (Tcl), which makes the post-fabrication field annealing less effective and (iii) The Curie temperature and saturation induction (Bs) become too low to be practical.
- The following examples are presented to provide a more complete understanding of the invention. The specific techniques, conditions, materials, proportions and reported data set forth to illustrate the principles and practice of the invention are exemplary and should not be construed as limiting the scope of the invention.
- The glassy alloys listed in Tables II-VII were rapidly quenched (about 106 K/sec) from the melt following the techniques taught by Chen and Polk in U.S. Patent 3,856,513. The resulting ribbons, typically 25 to 30u m thick and 0.5 to 2.5 cm wide, were determined to be free of significant crystallinity by X-ray diffractometry (using CuK radiation) and scanning calorimetry. Ribbons of the glassy metal alloys were strong, shiny, hard and ductile.
- Continuous ribbons of the glassy metal alloys prepared in accordance with the procedure described in Example I were wound onto bobbins (3.8 cm O.D.) to form closed-magnetic-path toroidal samples. Each sample contained from 1 to 3 g of ribbon. Insulated primary and secondary windings (numbering at least 10 each) were applied to the toroids. These samples were used to obtain hysteresis loops (coercivity and remanence) and initial permeability with a commercial curve tracer and core loss (IEEE Standard 106-1972).
- The saturation magnetization, Ms, of each sample, was measured with a commercial vibrating sample magnetometer (Princeton Applied Research). In this case, the ribbon was cut into several small squares (approximately 2 mm x 2 mm). These were randomly oriented about their normal direction, their plane being parallel to the applied field (0 to 720 kA/m. The saturation induction Bs (=4πMSD) was then calculated by using the measured mass density D.
- The ferromagnetic Curie temperature (Of) was measured by inductance method and also monitored by differential scanning calorimetry, which was used primarily to determine the crystallization temperatures. The first or primary crystallization temperature (Tcl) was used to compare the thermal stability of various glassy alloys of the present and prior art inventions.
- Magnetic stability was determined from the reorientation kinetics of the magnetization, in accordance with the method described in Journal of Applied Physics, vol. 49, p. 6510 (1978), which method is incorporated herein by reference thereto.
- Magnetostriction measurements employed metallic strain gauges (BLH Electronics), which were bonded (Eastman - 910 Cement) between two short lengths of ribbon. The ribbon axis and gauge axis were parallel. The magnetostriction was determined as a function of applied field from the longitudinal strain in the parallel (Δℓ/ℓ)11 and perpendicular(Δℓ/ℓ)1 in-plain fields, according to the formula a =2/3[( Δℓ/ℓ)11 - (Δℓ/ℓ)1].
- Having thus described the invention in rather full detail, it will be understood that this detail need not be strictly adhered to but that further changes and modifications may suggest themselves to one skilled in the art, all falling within the scope of the invention as defined by the subjoined claims.
Claims (9)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US34041382A | 1982-01-18 | 1982-01-18 | |
| US340413 | 1982-01-18 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0084138A2 true EP0084138A2 (en) | 1983-07-27 |
| EP0084138A3 EP0084138A3 (en) | 1985-08-21 |
| EP0084138B1 EP0084138B1 (en) | 1987-02-25 |
Family
ID=23333255
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19820111754 Expired EP0084138B1 (en) | 1982-01-18 | 1982-12-17 | Near-zero magnetostrictive glassy metal alloys with high magnetic and thermal stability |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0084138B1 (en) |
| JP (1) | JPS58123851A (en) |
| CA (1) | CA1222647A (en) |
| DE (1) | DE3275492D1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0240600A1 (en) * | 1986-01-08 | 1987-10-14 | AlliedSignal Inc. | Glassy metal alloys with perminvar characteristics |
| WO1988003699A1 (en) * | 1986-11-03 | 1988-05-19 | Allied Corporation | Near-zero magnetostrictive glassy metal alloys for high frequency applications |
| EP0302747A2 (en) * | 1987-08-07 | 1989-02-08 | Mitsui Petrochemical Industries, Ltd. | Method for assessing insulation conditions |
| EP0303324A1 (en) * | 1987-08-10 | 1989-02-15 | Koninklijke Philips Electronics N.V. | Magnetic material, method of manufacturing this material and a magnetic head provided with this material |
| US4938267A (en) * | 1986-01-08 | 1990-07-03 | Allied-Signal Inc. | Glassy metal alloys with perminvar characteristics |
| WO1998012847A1 (en) * | 1996-09-17 | 1998-03-26 | Vacuumschmelze Gmbh | Pulse transformer for line interfaces operating according to the echo compensation principle |
| US9925653B2 (en) | 2013-07-05 | 2018-03-27 | Black & Decker Inc. | Hammer drill |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0643627B2 (en) * | 1985-07-26 | 1994-06-08 | ユニチカ株式会社 | Amorphous metal wire |
| DE3900946A1 (en) * | 1989-01-14 | 1990-07-26 | Vacuumschmelze Gmbh | MAGNETIC CORE FOR AN INTERFACE TRANSMITTER |
| US6432226B2 (en) * | 1999-04-12 | 2002-08-13 | Alliedsignal Inc. | Magnetic glassy alloys for high frequency applications |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2553003A1 (en) * | 1974-11-29 | 1976-08-12 | Allied Chem | MAGNETIC DEVICES |
| US4038073A (en) * | 1976-03-01 | 1977-07-26 | Allied Chemical Corporation | Near-zero magnetostrictive glassy metal alloys with high saturation induction |
| US4056411A (en) * | 1976-05-14 | 1977-11-01 | Ho Sou Chen | Method of making magnetic devices including amorphous alloys |
| US4150981A (en) * | 1977-08-15 | 1979-04-24 | Allied Chemical Corporation | Glassy alloys containing cobalt, nickel and iron having near-zero magnetostriction and high saturation induction |
-
1982
- 1982-12-17 DE DE8282111754T patent/DE3275492D1/en not_active Expired
- 1982-12-17 EP EP19820111754 patent/EP0084138B1/en not_active Expired
- 1982-12-23 CA CA000418542A patent/CA1222647A/en not_active Expired
-
1983
- 1983-01-18 JP JP58006529A patent/JPS58123851A/en active Granted
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2553003A1 (en) * | 1974-11-29 | 1976-08-12 | Allied Chem | MAGNETIC DEVICES |
| US4038073A (en) * | 1976-03-01 | 1977-07-26 | Allied Chemical Corporation | Near-zero magnetostrictive glassy metal alloys with high saturation induction |
| US4056411A (en) * | 1976-05-14 | 1977-11-01 | Ho Sou Chen | Method of making magnetic devices including amorphous alloys |
| US4150981A (en) * | 1977-08-15 | 1979-04-24 | Allied Chemical Corporation | Glassy alloys containing cobalt, nickel and iron having near-zero magnetostriction and high saturation induction |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4938267A (en) * | 1986-01-08 | 1990-07-03 | Allied-Signal Inc. | Glassy metal alloys with perminvar characteristics |
| EP0240600A1 (en) * | 1986-01-08 | 1987-10-14 | AlliedSignal Inc. | Glassy metal alloys with perminvar characteristics |
| WO1988003699A1 (en) * | 1986-11-03 | 1988-05-19 | Allied Corporation | Near-zero magnetostrictive glassy metal alloys for high frequency applications |
| EP0544646A3 (en) * | 1987-08-07 | 1993-07-21 | Mitsui Petrochemical Industries, Ltd. | Apparatus for assessing insulation conditions |
| EP0302747A3 (en) * | 1987-08-07 | 1990-04-11 | Mitsui Petrochemical Industries, Ltd. | Method and apparatus for assessing insulation conditions |
| EP0544646A2 (en) * | 1987-08-07 | 1993-06-02 | Mitsui Petrochemical Industries, Ltd. | Apparatus for assessing insulation conditions |
| EP0550403A2 (en) * | 1987-08-07 | 1993-07-07 | Mitsui Petrochemical Industries, Ltd. | Apparatus for assessing insulation conditions |
| EP0550403A3 (en) * | 1987-08-07 | 1993-07-14 | Mitsui Petrochemical Industries, Ltd. | Apparatus for assessing insulation conditions |
| EP0302747A2 (en) * | 1987-08-07 | 1989-02-08 | Mitsui Petrochemical Industries, Ltd. | Method for assessing insulation conditions |
| EP0303324A1 (en) * | 1987-08-10 | 1989-02-15 | Koninklijke Philips Electronics N.V. | Magnetic material, method of manufacturing this material and a magnetic head provided with this material |
| WO1998012847A1 (en) * | 1996-09-17 | 1998-03-26 | Vacuumschmelze Gmbh | Pulse transformer for line interfaces operating according to the echo compensation principle |
| US6118365A (en) * | 1996-09-17 | 2000-09-12 | Vacuumschmelze Gmbh | Pulse transformer for a u-interface operating according to the echo compensation principle, and method for the manufacture of a toroidal tape core contained in a U-interface pulse transformer |
| US9925653B2 (en) | 2013-07-05 | 2018-03-27 | Black & Decker Inc. | Hammer drill |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0084138A3 (en) | 1985-08-21 |
| JPH0338334B2 (en) | 1991-06-10 |
| EP0084138B1 (en) | 1987-02-25 |
| DE3275492D1 (en) | 1987-04-02 |
| JPS58123851A (en) | 1983-07-23 |
| CA1222647A (en) | 1987-06-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4038073A (en) | Near-zero magnetostrictive glassy metal alloys with high saturation induction | |
| US4150981A (en) | Glassy alloys containing cobalt, nickel and iron having near-zero magnetostriction and high saturation induction | |
| US4268325A (en) | Magnetic glassy metal alloy sheets with improved soft magnetic properties | |
| JP2013100603A (en) | Magnetic glassy alloy for high frequency application | |
| JP2013168637A (en) | Glassy metal alloy for monitoring electron article | |
| EP0240600B1 (en) | Glassy metal alloys with perminvar characteristics | |
| EP0084138B1 (en) | Near-zero magnetostrictive glassy metal alloys with high magnetic and thermal stability | |
| EP0088244B1 (en) | Cobalt rich manganese containing near-zero magnetostrictive metallic glasses having high saturation induction | |
| EP0329704B1 (en) | Near-zero magnetostrictive glassy metal alloys for high frequency applications | |
| EP0074640B1 (en) | Low-loss amorphous alloy | |
| EP0351051B1 (en) | Fe-based soft magnetic alloy | |
| US4938267A (en) | Glassy metal alloys with perminvar characteristics |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT |
|
| 17P | Request for examination filed |
Effective date: 19840121 |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT |
|
| 17Q | First examination report despatched |
Effective date: 19860523 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB IT |
|
| REF | Corresponds to: |
Ref document number: 3275492 Country of ref document: DE Date of ref document: 19870402 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20001107 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20001204 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20001222 Year of fee payment: 19 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20011217 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020702 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20011217 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020830 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |