DK200100061A - Preparation of citalopram by reaction of a halo-derivative with a cyanide source in the presence of a nickel catalyst - Google Patents
Preparation of citalopram by reaction of a halo-derivative with a cyanide source in the presence of a nickel catalyst Download PDFInfo
- Publication number
- DK200100061A DK200100061A DK200100061A DKPA200100061A DK200100061A DK 200100061 A DK200100061 A DK 200100061A DK 200100061 A DK200100061 A DK 200100061A DK PA200100061 A DKPA200100061 A DK PA200100061A DK 200100061 A DK200100061 A DK 200100061A
- Authority
- DK
- Denmark
- Prior art keywords
- citalopram
- reaction
- preparation
- nickel catalyst
- cyanide source
- Prior art date
Links
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
KRAVREQUIREMENTS
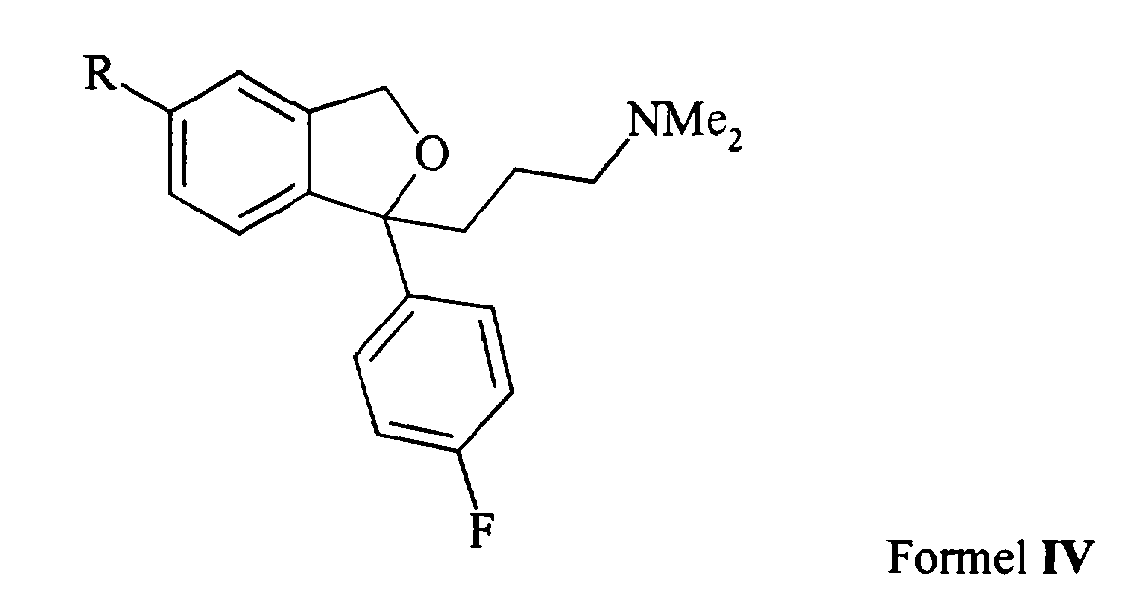
1. En fremgangsmåde til fremstilling af citalopram omfattende reaktion af en forbindelse med formlen IVA process for the preparation of citalopram comprising the reaction of a compound of formula IV
hvori R er Cl eller Br med en cyanidkilde i nærvær af en nikkelkatalysator og isolering af den tilsvarende 5-cyano-forbindelse, dvs. citalopramwherein R is Cl or Br with a cyanide source in the presence of a nickel catalyst and isolation of the corresponding 5-cyano compound, i. citalopram
som basen eller et farmaceutisk acceptabelt salt deraf. 2. Fremgangsmåden ifølge krav 1, hvori R er chlor. 3. Fremgangsmåden ifølge krav 1 eller 2, hvori cyanidkilden er KCN, NaCN, Zn(CN)2eller (R'4N)CN, hvor R'4 indikerer fire grupper, som kan være ens eller forskellige og som vælges blandt hydrogen og ligekædet eller forgrenet C,.6 alkyl. 4. Fremgangsmåden ifølge et hvilket som helst af kravene 1-3, hvori cyanidkilden er NaCN eller KCN. 5. Fremgangsmåden ifølge et hvilket som helst af kravene 1-4, hvori nikkelkatalysatoren er Ni(PPh3)3 eller (a-aryl)-Ni(PPh)2Cl. 6. Fremgangsmåden ifølge et hvilket som helst af kravene 1-5, hvori nikkelkatalysatoren er et nikkel(0)-kompleks fremstillet in situ før cyaneringsreaktionen ved reduktion af en nikkel(II)-precursor såsom NiCl2 med et metal såsom zink, magnesium eller mangan i nærvær af overskud af kompleksligander. 7. Fremgangsmåden ifølge krav 6, hvori nikkel(II) precursoren er NiCl2, metallet er zink, og kompleksligandeme er triphenylphosphiner. 8. Fremgangsmåden ifølge krav 1, hvori en forbindelse med formlen IV, hvori R er Cl, reageres med NaCN eller KCN i nærvær af en Ni(PPh3)3 katalysator. 9. Fremgangsmåden ifølge krav 8, hvori Ni(PPh3)3 fremstilles in situ før cyanerings¬ reaktionen ved reduktion af NiCl2 med zink, i nærvær af et overskud af kompleks- triphenyl- phosphinligander. ..... 10. Fremgangsmåden ifølge et hvilket som helst af kravene 1-9, hvori reaktionen udføres i nærvær af en katalytisk mængde af Cu+, fortrinsvis i form af Cul. 11. Fremgangsmåde ifølge et hvilket som helst af kravene 1 - 9, hvori reaktionen udføres i nærvær af en katalytisk mængde af Zn2+. 12. Fremgangsmåden ifølge et hvilket som helst af kravene 1-11, hvori forbindelsen med formlen IV er S-enatiomeren. 13. En antidepressiv farmaceutisk komposition omfattende citalopram fremstillet ved processen ifølge et hvilket som helst af kravene 1-12.as the base or a pharmaceutically acceptable salt thereof. The process of claim 1, wherein R is chlorine. The method of claim 1 or 2, wherein the cyanide source is KCN, NaCN, Zn (CN) 2 or (R'4N) CN, wherein R'4 indicates four groups which may be the same or different and selected from hydrogen and the straight chain or branched C1-6 alkyl. The method of any one of claims 1-3, wherein the cyanide source is NaCN or KCN. The process of any one of claims 1-4, wherein the nickel catalyst is Ni (PPh3) 3 or (a-aryl) -Ni (PPh) 2Cl. The process of any one of claims 1-5, wherein the nickel catalyst is a nickel (O) complex prepared in situ prior to the cyanation reaction by reduction of a nickel (II) precursor such as NiCl2 with a metal such as zinc, magnesium or manganese. in the presence of excess complex ligands. The method of claim 6, wherein the nickel (II) precursor is NiCl2, the metal is zinc, and the complex ligands are triphenylphosphines. The process of claim 1, wherein a compound of formula IV wherein R is Cl is reacted with NaCN or KCN in the presence of a Ni (PPh 3) 3 catalyst. The process of claim 8, wherein Ni (PPh 3) 3 is prepared in situ prior to the cyanation reaction by reduction of NiCl 2 with zinc, in the presence of an excess of complex triphenylphosphine ligands. The process of any one of claims 1-9, wherein the reaction is carried out in the presence of a catalytic amount of Cu +, preferably in the form of Cul. A process according to any one of claims 1 to 9, wherein the reaction is carried out in the presence of a catalytic amount of Zn 2+. The process of any one of claims 1-11, wherein the compound of formula IV is the S-enantiomer. An antidepressant pharmaceutical composition comprising citalopram prepared by the process of any one of claims 1-12.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DK200100061A DK200100061A (en) | 1999-06-25 | 2001-01-15 | Preparation of citalopram by reaction of a halo-derivative with a cyanide source in the presence of a nickel catalyst |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DKPA199900921 | 1999-06-25 | ||
| PCT/DK1999/000643 WO2000011926A2 (en) | 1999-06-25 | 1999-11-19 | Method for the preparation of citalopram |
| DK200100061A DK200100061A (en) | 1999-06-25 | 2001-01-15 | Preparation of citalopram by reaction of a halo-derivative with a cyanide source in the presence of a nickel catalyst |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| DK200100061A true DK200100061A (en) | 2001-03-22 |
Family
ID=26064909
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| DK200100061A DK200100061A (en) | 1999-06-25 | 2001-01-15 | Preparation of citalopram by reaction of a halo-derivative with a cyanide source in the presence of a nickel catalyst |
Country Status (1)
| Country | Link |
|---|---|
| DK (1) | DK200100061A (en) |
-
2001
- 2001-01-15 DK DK200100061A patent/DK200100061A/en not_active Application Discontinuation
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DK200100060A (en) | Preparation of citalopram in high yields and purity comprises reacting isobenzofuran compound and cyanide in the presence of palladium catalyst and e.g. copper ions | |
| FI108538B (en) | Process for the preparation of citalopram | |
| García-Álvarez et al. | Metal-catalyzed amide bond forming reactions in an environmentally friendly aqueous medium: nitrile hydrations and beyond | |
| CN109776462A (en) | A kind of preparation method of 2,5- dicyan furans | |
| JP5674059B2 (en) | Catalyst for hydrogen transfer reaction containing ruthenium complex and method for producing hydrogen transfer reactant | |
| Katayev et al. | Synthesis of quaternary α-perfluoroalkyl lactams via electrophilic perfluoroalkylation | |
| CN106925349A (en) | A kind of solid supported type metal porphyrin catalyst and its application in terms of maleic acid is prepared | |
| CN110511217B (en) | Catalyst for catalyzing synthesis of indoxacarb key intermediate and application thereof | |
| DK200100061A (en) | Preparation of citalopram by reaction of a halo-derivative with a cyanide source in the presence of a nickel catalyst | |
| CN109422603A (en) | A kind of method of iridium catalysis asymmetric hydrogenation imines synthesis of chiral amine compounds | |
| CN1391566A (en) | Preparation of citalopram | |
| CN109503513A (en) | A kind of " one kettle way " synthetic method of Febustat intermediate | |
| CN110003274A (en) | Phosphonylation dihydro-isoquinoline ketone compounds and preparation method thereof | |
| KR20140134300A (en) | Metal powderdous catalyst for hydrogenation processes | |
| CN109666049B (en) | Chiral amino sulfonamide ligand metal complex and application thereof in catalytic reaction | |
| JP2011111426A (en) | Method for producing bisimidazolidine ligand and catalyst using the same | |
| CN106111132B (en) | A kind of Pd-Zn bimetallic catalyst being used to prepare aryl cyanides | |
| CN105924390B (en) | A kind of synthetic method of Mei Tafeini | |
| CN102617503A (en) | Novel synthetic method of (S)-3-morpholinyl carboxylic acid | |
| CN1492861A (en) | Process for preparation of citalopram | |
| CN113860984A (en) | Magnesium-activated ligand-promoted chromium-catalyzed polycyclic aromatic hydrocarbon and olefin regioselective hydrogenation method | |
| CN113683582A (en) | Photocatalytic synthesis method of N- (2-morpholinoethyl) substituted benzamide compound | |
| CN107459485B (en) | A kind of new method of the imino-diacetic benzylidene derivative aminating reaction of nickel catalysis | |
| Liu et al. | Efficient Synthesis of Oxazolidin‐2‐one via (Chitosan‐Schiff Base) cobalt (II)‐Catalyzed Oxidative Carbonylation of 2‐Aminoalkan‐1‐ols | |
| JPWO2005070864A1 (en) | Enantioselective nucleophilic addition reaction to enamide carbonyl group and synthesis method of optically active α-hydroxy-γ-keto acid ester and hydroxy diketone |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AHS | Application shelved for other reasons than non-payment |