CN116162816A - Method for preparing magnesium-lithium alloy by double-temperature-zone vacuum tube type high-temperature sintering furnace - Google Patents
Method for preparing magnesium-lithium alloy by double-temperature-zone vacuum tube type high-temperature sintering furnace Download PDFInfo
- Publication number
- CN116162816A CN116162816A CN202310132467.0A CN202310132467A CN116162816A CN 116162816 A CN116162816 A CN 116162816A CN 202310132467 A CN202310132467 A CN 202310132467A CN 116162816 A CN116162816 A CN 116162816A
- Authority
- CN
- China
- Prior art keywords
- magnesium
- temperature
- lithium
- lithium alloy
- double
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- GCICAPWZNUIIDV-UHFFFAOYSA-N lithium magnesium Chemical compound [Li].[Mg] GCICAPWZNUIIDV-UHFFFAOYSA-N 0.000 title claims abstract description 66
- 229910000733 Li alloy Inorganic materials 0.000 title claims abstract description 65
- 239000001989 lithium alloy Substances 0.000 title claims abstract description 65
- 238000000034 method Methods 0.000 title claims abstract description 42
- 238000005245 sintering Methods 0.000 title claims abstract description 32
- 239000011777 magnesium Substances 0.000 claims abstract description 57
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 claims abstract description 56
- 229910052749 magnesium Inorganic materials 0.000 claims abstract description 56
- 229910052744 lithium Inorganic materials 0.000 claims abstract description 55
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims abstract description 54
- 229910052751 metal Inorganic materials 0.000 claims abstract description 41
- 239000002184 metal Substances 0.000 claims abstract description 41
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 claims abstract description 40
- 239000001095 magnesium carbonate Substances 0.000 claims abstract description 40
- 235000014380 magnesium carbonate Nutrition 0.000 claims abstract description 40
- 229910000021 magnesium carbonate Inorganic materials 0.000 claims abstract description 40
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 claims abstract description 28
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims abstract description 27
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims abstract description 24
- 239000000843 powder Substances 0.000 claims abstract description 23
- 239000003638 chemical reducing agent Substances 0.000 claims abstract description 22
- 239000007789 gas Substances 0.000 claims abstract description 22
- 229910052786 argon Inorganic materials 0.000 claims abstract description 14
- 239000008188 pellet Substances 0.000 claims abstract description 14
- 229910002092 carbon dioxide Inorganic materials 0.000 claims abstract description 11
- 239000001569 carbon dioxide Substances 0.000 claims abstract description 11
- 239000000956 alloy Substances 0.000 claims abstract description 10
- 238000002156 mixing Methods 0.000 claims abstract description 10
- 229910045601 alloy Inorganic materials 0.000 claims abstract description 9
- 238000010438 heat treatment Methods 0.000 claims abstract description 9
- 238000005275 alloying Methods 0.000 claims abstract description 5
- 238000003825 pressing Methods 0.000 claims abstract description 5
- 239000012300 argon atmosphere Substances 0.000 claims abstract description 4
- 238000002844 melting Methods 0.000 claims abstract description 4
- 230000008018 melting Effects 0.000 claims abstract description 4
- 230000001681 protective effect Effects 0.000 claims abstract description 4
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 claims description 30
- 230000009467 reduction Effects 0.000 claims description 30
- 239000000395 magnesium oxide Substances 0.000 claims description 22
- 229910001947 lithium oxide Inorganic materials 0.000 claims description 16
- 239000002893 slag Substances 0.000 claims description 16
- FUJCRWPEOMXPAD-UHFFFAOYSA-N lithium oxide Chemical compound [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 claims description 14
- XGZVUEUWXADBQD-UHFFFAOYSA-L lithium carbonate Chemical compound [Li+].[Li+].[O-]C([O-])=O XGZVUEUWXADBQD-UHFFFAOYSA-L 0.000 claims description 13
- 229910052808 lithium carbonate Inorganic materials 0.000 claims description 13
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 claims description 13
- 239000012535 impurity Substances 0.000 claims description 11
- 239000002994 raw material Substances 0.000 claims description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 11
- 238000007670 refining Methods 0.000 claims description 10
- 238000006243 chemical reaction Methods 0.000 claims description 8
- 239000000463 material Substances 0.000 claims description 7
- 229910052596 spinel Inorganic materials 0.000 claims description 7
- 239000011029 spinel Substances 0.000 claims description 7
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 claims description 6
- 239000011819 refractory material Substances 0.000 claims description 6
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims description 5
- 238000001035 drying Methods 0.000 claims description 5
- 238000001238 wet grinding Methods 0.000 claims description 5
- 229910018072 Al 2 O 3 Inorganic materials 0.000 claims description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 4
- 229910052799 carbon Inorganic materials 0.000 claims description 4
- 239000003795 chemical substances by application Substances 0.000 claims description 4
- 239000005431 greenhouse gas Substances 0.000 claims description 4
- 238000006555 catalytic reaction Methods 0.000 claims description 3
- 229910010293 ceramic material Inorganic materials 0.000 claims description 3
- 238000007599 discharging Methods 0.000 claims description 3
- 239000012153 distilled water Substances 0.000 claims description 3
- 239000002245 particle Substances 0.000 claims description 3
- 238000006722 reduction reaction Methods 0.000 abstract description 40
- 238000004519 manufacturing process Methods 0.000 abstract description 16
- 230000008569 process Effects 0.000 abstract description 15
- 239000000203 mixture Substances 0.000 abstract 1
- 238000011084 recovery Methods 0.000 description 14
- 238000002360 preparation method Methods 0.000 description 10
- 230000000694 effects Effects 0.000 description 6
- 238000005265 energy consumption Methods 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 239000002699 waste material Substances 0.000 description 5
- 230000008901 benefit Effects 0.000 description 4
- 238000001354 calcination Methods 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 3
- 229910000831 Steel Inorganic materials 0.000 description 3
- 238000002441 X-ray diffraction Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 239000012071 phase Substances 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 239000000376 reactant Substances 0.000 description 3
- 238000004064 recycling Methods 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000010959 steel Substances 0.000 description 3
- 229910004298 SiO 2 Inorganic materials 0.000 description 2
- 238000003723 Smelting Methods 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 239000013065 commercial product Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- 229910000514 dolomite Inorganic materials 0.000 description 2
- 239000010459 dolomite Substances 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- YQNQTEBHHUSESQ-UHFFFAOYSA-N lithium aluminate Chemical compound [Li+].[O-][Al]=O YQNQTEBHHUSESQ-UHFFFAOYSA-N 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 238000000498 ball milling Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- OMKPRTQVLDBJSG-UHFFFAOYSA-J calcium;magnesium;dicarbonate;hydrate Chemical compound [OH-].[Mg+2].[Ca+2].OC([O-])=O.[O-]C([O-])=O OMKPRTQVLDBJSG-UHFFFAOYSA-J 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000005229 chemical vapour deposition Methods 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000013016 damping Methods 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000005868 electrolysis reaction Methods 0.000 description 1
- 239000008151 electrolyte solution Substances 0.000 description 1
- PQVSTLUFSYVLTO-UHFFFAOYSA-N ethyl n-ethoxycarbonylcarbamate Chemical compound CCOC(=O)NC(=O)OCC PQVSTLUFSYVLTO-UHFFFAOYSA-N 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229910001234 light alloy Inorganic materials 0.000 description 1
- GLXDVVHUTZTUQK-UHFFFAOYSA-M lithium hydroxide monohydrate Substances [Li+].O.[OH-] GLXDVVHUTZTUQK-UHFFFAOYSA-M 0.000 description 1
- 229940040692 lithium hydroxide monohydrate Drugs 0.000 description 1
- 238000005272 metallurgy Methods 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000011946 reduction process Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000002910 solid waste Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000012808 vapor phase Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/02—Making non-ferrous alloys by melting
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/24—Binding; Briquetting ; Granulating
- C22B1/2406—Binding; Briquetting ; Granulating pelletizing
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B26/00—Obtaining alkali, alkaline earth metals or magnesium
- C22B26/10—Obtaining alkali metals
- C22B26/12—Obtaining lithium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B26/00—Obtaining alkali, alkaline earth metals or magnesium
- C22B26/20—Obtaining alkaline earth metals or magnesium
- C22B26/22—Obtaining magnesium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
- C22B5/02—Dry methods smelting of sulfides or formation of mattes
- C22B5/04—Dry methods smelting of sulfides or formation of mattes by aluminium, other metals or silicon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
- C22B5/02—Dry methods smelting of sulfides or formation of mattes
- C22B5/16—Dry methods smelting of sulfides or formation of mattes with volatilisation or condensation of the metal being produced
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C23/00—Alloys based on magnesium
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P10/00—Technologies related to metal processing
- Y02P10/20—Recycling
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Geochemistry & Mineralogy (AREA)
- Manufacture And Refinement Of Metals (AREA)
Abstract
The invention provides a method for preparing magnesium-lithium alloy by a double-temperature-zone vacuum tube type high-temperature sintering furnace, which comprises the steps of uniformly mixing microcrystalline magnesite fine powder with magnesium carbonate and reducing agent aluminum powder, and pressing the mixture into pellets; heating and reducing for 2-5 hours at 1170-1200 ℃ and vacuum degree of 10-100Pa to generate vapor of metal magnesium and metal lithium; introducing protective argon into the furnace to bring distilled magnesium/lithium vapor and generated carbon dioxide gas into a low-temperature area, and condensing and alloying the magnesium/lithium vapor to form crude magnesium-lithium alloy; and taking out the crude magnesium-lithium alloy, and heating and melting the crude magnesium-lithium alloy at the temperature of 500-700 ℃ under the argon atmosphere condition. The reduction reaction and alloy collection can be directly completed in two temperature-controllable areas in the double-temperature-area vacuum tube type high-temperature sintering furnace, so that the use of equipment is reduced, the process flow is shortened, and the production cost is reduced.
Description
Technical Field
The invention belongs to the technical field of vacuum metallurgy, and particularly relates to a method for preparing magnesium-lithium alloy by a double-temperature-zone vacuum tube type high-temperature sintering furnace.
Background
The magnesium-lithium alloy is the metal structural material with the lowest density at present, and has the advantages of light weight, high specific strength, high specific rigidity, good radiation resistance, electromagnetic interference resistance, damping performance, deformation performance and the like, so that the magnesium-lithium alloy is widely applied to the fields of aerospace, national defense, military industry, transportation, electronic communication and the like, and the dosage of the magnesium-lithium alloy is increased year by year. The ultra-light magnesium-lithium alloy of the novel magnesium-lithium alloy material is also comprehensively applied to spacecrafts, and plays a guiding and pushing role in promoting the technical development and application of the light alloy material.
At present, magnesium-lithium alloy is produced by adopting a mode of mixing metal magnesium and metal lithium, and the preparation of the metal magnesium and the metal lithium is carried out firstly in consideration of the energy consumption of the industrial process. The metal magnesium is produced by adopting a Pidgeon process, which is the most widely used metal magnesium smelting process with higher energy consumption, and has the problems of high raw material consumption, lower magnesium reduction rate, high waste residue discharge and the like. The molten salt electrolysis process is used mainly in the production of lithium metal, and has high power consumption, electrolyte solution and produced chlorine gas (Cl) 2 ) Both have a corrosive effect on the equipment and the environment. Therefore, smelting of metal magnesium and metal lithium belongs to metallurgical industries with high energy consumption, high pollution and high cost, and the important regulation and improvement of the metal magnesium and the metal lithium are required by the country. The method has the advantages that the burning loss of lithium is high in the production process, and the alloy components are not uniform; magnesium and lithium have a large density difference, and lithium can be seriously segregated; and in the process of miscibility, magnesium and lithium have poor capability of forming compounds with other alloy elements; in addition, each pure metal element is expensive, the energy consumption of the acquisition path is high, and the production cost is increased. This limits the further popularization and application of the blending method for preparing the magnesium-lithium alloy.
The vacuum thermal reduction technology is a novel heat treatment technology capable of regulating and controlling atmosphere and reducing temperature, and can be used for preparing metal magnesium and metal lithium. Because the vacuum aluminothermic reduction temperature of magnesium oxide and lithium oxide is basically the same, the technology can be used for simultaneously reducing magnesium oxide and lithium oxide to obtain magnesium vapor and lithium vapor, and then the vapor phase alloying condensation is carried out to obtain the magnesium-lithium alloy. The method shortens the process flow, reduces the process energy consumption, improves the synthesis efficiency of the magnesium-lithium alloy and reduces the production cost.
Related technical schemes for synchronously preparing magnesium-lithium alloy by vacuum aluminothermic reduction are disclosed, for example, patent document CN102080164A discloses a method for synchronously preparing magnesium-lithium alloy by vacuum aluminothermic reduction by taking magnesium oxide and lithium oxide as raw materials, patent document CN109536751A discloses a method for producing magnesium-lithium alloy by vacuum aluminothermic reduction by taking magnesite and lithium carbonate or lithium hydroxide as raw materials, and reducing slag phases Li of the two patents 2 O·5Al 2 O 3 Resulting in a lower rate of lithium oxide reduction. Patent document CN111286653a discloses a method for producing a magnesium-lithium alloy with high lithium reduction rate by vacuum aluminothermic reduction by using dolomite and lithium hydroxide monohydrate as raw materials, and secondary recycling is performed on refining slag. Patent document CN111097920a discloses a method for producing high purity and high uniformity magnesium-lithium alloy by using a gaseous co-condensation method, but increases the use of a solvent resistance and a catalyst in the raw material. CN110042240a discloses a process for preparing metallic lithium and metallic magnesium simultaneously by vacuum aluminothermic reduction method using lithium aluminate and calcined dolomite as raw materials. The methods for producing the magnesium-lithium alloy have some problems which are difficult to solve, or have low reduction rate or complex process, so that the production cost is very high, and the further popularization and application of the magnesium-lithium alloy are limited.
Disclosure of Invention
The invention provides a method for preparing magnesium-lithium alloy by a double-temperature-zone vacuum tube type high-temperature sintering furnace, which solves the problems of complex preparation process and higher cost of the existing magnesium-lithium alloy.
In order to achieve the technical effects, the invention adopts the following technical scheme:
a method for preparing magnesium-lithium alloy by a double-temperature-zone vacuum tube type high-temperature sintering furnace comprises the following steps:
(1) Crushing microcrystalline magnesite to obtain coarse powder, and then wet-grinding and drying the coarse powder to obtain fine powder; uniformly mixing microcrystalline magnesite fine powder with magnesium carbonate and reducing agent aluminum powder, pressing into pellets, and mixing the raw materials in percentage by mass: 48-68% of microcrystalline magnesite fine powder, 12-31% of lithium carbonate and 20-30% of reducer aluminum powder;
(2) Placing the pellets arranged in the crucible in a high-temperature region of a double-temperature region vacuum tube type high-temperature sintering furnace, and heating and reducing the pellets for 2 to 5 hours at the temperature of 1170 to 1200 ℃ and the vacuum degree of 10 to 100Pa to generate vapor of metal magnesium and metal lithium and carbon dioxide gas;
(3) Introducing protective argon into the furnace to bring distilled magnesium/lithium vapor and generated carbon dioxide gas into a low-temperature area, condensing and alloying the magnesium/lithium vapor to form crude magnesium-lithium alloy, and taking away the carbon dioxide gas by flowing argon;
(4) And taking out the crude magnesium-lithium alloy, heating and melting the crude magnesium-lithium alloy at the temperature of 500-700 ℃ under the condition of argon atmosphere, and discharging refining slag in an alloy solution under the catalysis of a refining agent to obtain the magnesium-lithium alloy.
Preferably, in the step (1), mgO+Loss of the microcrystalline magnesite is >99wt%.
Preferably, in the step (1), the coarse powder is formed by mixing the raw materials in a ball-to-material ratio of zirconia balls: wet milling for 4 hours under distilled water=4:1, and drying to obtain fine powder with particle size in 20-70 microns; the microcrystalline magnesite is uniformly mixed with magnesium carbonate and reducing agent aluminum powder and then pressed into pellets under 50-200 MPa.
Preferably, in the step (2), the pellets are calcined at the high temperature of 700-800 ℃ for 5-10 hours to obtain magnesium oxide/lithium oxide, and then reduced by aluminum powder in the pellets at 1170-1200 ℃ for 2-5 hours to obtain magnesium/lithium vapor.
Preferably, in the step (2), after the reaction is completed, the reducing slag phase in the crucible in the high temperature zone is mainly magnesia-alumina spinel MgO.Al 2 O 3 The reduction rate of the metal magnesium is 85-95%, and the reduction rate of the metal lithium can reach 99%.
Preferably, in the step (3), the generated carbon dioxide gas diffuses upward around and is moved forward by the flowing argon gas flow belt, and is introduced into a filter flask filled with water at an air outlet valve port, and CO 2 Gas and method for producing the sameCarbonic acid is produced by the water, while argon escapes from the filter flask.
Preferably, in the step (4), the prepared magnesium-lithium alloy contains 70-90% of magnesium, 10-30% of lithium and less than or equal to 1% of impurities in percentage by mass.
Preferably, the method further comprises the step (5) of using the collected reducing slag as a refractory material or for preparing a lithium-containing ceramic material; collected CO 2 The greenhouse gases are recycled or made into carbon products.
The beneficial effects of the invention are as follows:
the invention relates to a process for preparing magnesium-lithium alloy by thermal reduction of a double-temperature-zone vacuum tube type high-temperature sintering furnace (CVD method), which combines and synchronously prepares magnesium-lithium alloy by aluminum thermal magnesium preparation and vacuum thermal reduction lithium preparation, and the reduction reaction and alloy collection can be directly completed in two controllable temperature zones in the double-temperature-zone vacuum tube type high-temperature sintering furnace, so that the use of equipment is reduced, the process flow is shortened, and the production cost is reduced.
In addition, the use of high-quality microcrystalline magnesite (MgO+Loss >99 wt%) in the raw material increases MgO in the reduction material, reduces the material-magnesium ratio in the reduction process (3.3/1), further improves the reduction rate and achieves the aim of high-efficiency utilization of resources. The produced magnesia-alumina spinel reducing slag can be used as an important refractory material for the steel industry; the industrial magnesium-lithium alloy can be produced after component adjustment; reduces the generation of waste and improves the economic benefit.
By applying the technology of the invention, in the process of producing the magnesium-lithium alloy, starting from magnesium raw ore, combining lithium carbonate and reducer powder, synchronously completing metal reduction reaction and alloying collection in a double-temperature-zone vacuum tube type high-temperature sintering furnace, and obtaining the magnesium-lithium alloy with uniform components by regulating and controlling a temperature control program. The main component of the generated reducing slag is magnesium lithium spinel which can be used as a refractory material in the steel industry, and the high stability of the magnesium aluminum spinel reducing slag can reduce the free energy of the reduction reaction, so that the reaction is carried out in the direction of producing metal Mg/Li. The whole production process has no waste and waste residue, and is a green and environment-friendly process for producing the magnesium-lithium alloy with low cost and high yield.
The invention has simple process, integrated flow, reduced intermediate energy consumption, uniform components of the prepared magnesium-lithium alloy and reduced production cost; no waste is produced, and the double carbon pressure caused by the emission of solid waste slag and the emission of greenhouse gases is reduced.
Drawings
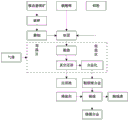
FIG. 1 is a schematic illustration of a process flow for the preparation of the present invention.
FIG. 2 is an X-ray diffraction pattern of the high quality microcrystalline magnesite of the present invention.
Detailed Description
The present invention will be described in further detail with reference to the drawings and examples, in order to make the objects, technical solutions and advantages of the present invention more apparent. It should be understood that the specific embodiments described herein are for purposes of illustration only and are not intended to limit the scope of the invention.
The microcrystalline magnesite used in the examples of the present invention is provided by the Tibetan kamadurapidite region.
The chemical components of the high-quality microcrystalline magnesite in the embodiment of the invention comprise 47.42 percent of MgO, 0.35 percent of CaO and 0.10 percent of SiO 2 、0.04%Fe 2 O 3 、0.02%Al 2 O 3 、0.01%Na 2 O and 52.06% loss. Wherein, the magnesite has higher purity and MgO+loss>99wt%。
The Loss of the microcrystalline magnesite in the embodiment of the invention refers to evaporation of moisture (physical water) of mineral powder, escape of crystal water, decomposition of oxide and the like in the sintering process, so that the weight of the ore is lost.
The lithium carbonate adopted in the embodiment of the invention is a commercial product, the purity is more than 99.5 percent, and the granularity is less than 1mm.
The aluminum powder adopted in the embodiment of the invention is a commercial product, the purity is more than 99 percent, and the granularity is less than 1mm.
The model of the double-temperature-zone vacuum tube type high-temperature sintering furnace in the embodiment of the invention is GSL series.
The argon gas adopted in the embodiment of the invention is a commercially available product, and the flow rate is 25-250sccm.
In the embodiment of the invention, an iron crucible is adopted for heating and refining the crude magnesium-lithium alloy.
In the embodiment of the invention, the mass percentage of impurities in the magnesium-lithium alloy is less than or equal to 1 percent.
The magnesium-lithium alloy in the embodiment of the invention contains 10-30% of lithium by mass percent.
The refining agent in the embodiment of the invention is a multi-element compound (containing LiCl 10-60%, liF 10-60%, KCl 0-50%, caF) 2 :0-30%,MgCl 2 :0-50%,MgF 2 0-30 percent of the alloy, and the dosage of the alloy accounts for 1-5 percent of the total mass of the crude magnesium-lithium alloy.
Example 1
With reference to fig. 1, the present embodiment includes the following steps:
and step 1, crushing the high-quality microcrystalline magnesite by using a small jaw crusher, and then crushing by using a sample preparation crusher to obtain coarse powder. Then wet milling the coarse powder for 4 hours under the condition of ball-to-material ratio (zirconia balls: distilled water) =4:1, and drying to obtain fine powder with the particle size in the range of 20-70 microns, wherein the X-ray diffraction diagram is shown in fig. 2. The purity of the microcrystalline magnesite is higher (MgO+Loss)>99 wt%) and low impurity content (such as Fe 2 O 3 、SiO 2 、Al 2 O 3 Etc.). The granularity of the reducing agent aluminum powder is controlled to be about 100 meshes, and the high activity is maintained; in order to promote the reduction reaction and improve the metal reduction rate, the actual addition amount of the aluminum powder is higher than 50% of the theoretical reducing agent calculated by the stoichiometric ratio. The preparation method comprises the following steps of: 60% of magnesite, 15% of lithium carbonate and 25% of aluminum powder serving as a reducing agent, wherein the sum is 100%.
Step 2, uniformly mixing the wet-ground magnesite, magnesium carbonate and reducing agent powder, pressing into a block under 50-200MPa, arranging the block in a crucible, placing the block in a high-temperature region of a double-temperature region vacuum tube type high-temperature sintering furnace, and then adjusting a temperature control system of the sintering furnace (the two temperature regions can be respectively provided with temperature control programs). And (3) carrying out thermal reduction for 5 hours at the temperature of 1170 ℃ under the vacuum degree of 10-100Pa to generate metal magnesium and metal lithium vapor. The reaction process in the double-temperature-zone vacuum tube type high-temperature sintering furnace comprises the following steps: calcining magnesite/lithium carbonate in a high temperature region at 700-800 ℃ for 5-10 hours to obtain magnesium oxide/lithium oxide, regulating a temperature program, and reducing the magnesium oxide/lithium oxide by aluminum powder in the pellets at 1170 ℃ for 5 hours to obtain magnesium/lithium vapor.
Step 3, introducing protective argon (Ar) into the furnace to bring distilled magnesium/lithium vapor and generated carbon dioxide gas forward to a low-temperature zone, wherein the magnesium/lithium vapor is condensed and alloyed to form crude magnesium-lithium alloy, and CO 2 The gas is taken away by the forward flowing argon gas, and is collected at the outlet valve port for recycling. After the reduction reaction is finished, the reducing slag phase in the crucible in the high temperature zone is mainly magnesia-alumina spinel (MgO.Al) 2 O 3 ) The reduction rate of the metal magnesium is 85-95%, and the reduction rate of the metal theory can be up to 99%. Generated CO 2 The gas diffuses upwards around, the flowing argon gas flow moves forwards, and the gas is introduced into the filtering bottle with water at the outlet valve port. Where CO 2 The gas and water produced carbonic acid, while argon was evolved from the bottle.
And 4, taking out the crude magnesium-lithium alloy, heating and melting the crude magnesium-lithium alloy at 500-700 ℃ under the argon atmosphere condition, and discharging refining slag in an alloy solution under the catalysis of a refining agent to obtain the magnesium-lithium alloy.
Step 5, separating and reutilizing the reducing slag generated in the step 2: magnesia alumina spinel may be used as a refractory material; lithium aluminate is used in the battery industry by purification or for preparing lithium-containing ceramic materials, etc.; CO collected in the step 3 is processed 2 Recycling greenhouse gases or preparing carbon products; and (3) using the refining slag discharged in the step (4) for refractory materials for the steel industry.
As shown in FIG. 2, each diffraction peak in the X-ray diffraction pattern of the microcrystalline magnesite fine powder after ball milling treatment was relatively close to that of a standard PDF card (PDF#08-0479 MgCO) of magnesium carbonate 3 ). This shows that the microcrystalline magnesite raw material participating in the reaction has higher purity and mainly contains magnesium carbonate, which reduces the 'material-to-magnesium ratio' in the reduced material and is beneficial to improving the metal reduction rate.
In this example, the magnesium-lithium alloy (100%) prepared contained about 77% magnesium, 22% lithium, and 1% impurity; in addition, the recovery rate of magnesium can reach 88%, and the recovery rate of lithium can reach 98%.
Example 2
As shown in fig. 1: the process is the same as in example 1, except that: the temperature and time of the reduction reaction controlled by the high temperature region in the double-temperature region vacuum tube type high temperature sintering furnace are different.
And (3) carrying out thermal reduction for 4 hours at the temperature of 1180 ℃ under the vacuum degree of 10-100Pa to generate metal magnesium and metal lithium vapor. The reaction process in the double-temperature-zone vacuum tube type high-temperature sintering furnace comprises the following steps: calcining magnesite/lithium carbonate in a high temperature region at 700-800 ℃ for 5-10 hours to obtain magnesium oxide/lithium oxide, regulating a temperature program, and reducing the magnesium oxide/lithium oxide by aluminum powder at 1180 ℃ for 4 hours to obtain magnesium/lithium vapor.
In this example, the magnesium-lithium alloy (100%) prepared contained about 78% magnesium, 21% lithium, and 1% impurity; in addition, the recovery rate of magnesium can reach 91%, and the recovery rate of lithium can reach 98%.
Example 3
The main method of this embodiment is the same as that of embodiment 1, except that: the temperature and time of the reduction reaction controlled by the high temperature region in the double-temperature region vacuum tube type high temperature sintering furnace are different.
After uniformly mixing the wet-ground magnesite, magnesium carbonate and reducing agent powder, pressing into a block under 50-200MPa, arranging the block in a crucible, placing the block in a high-temperature region of a double-temperature region vacuum tube type high-temperature sintering furnace, and then adjusting a temperature control system of the sintering furnace (the two temperature regions can be respectively provided with temperature control programs). And (3) carrying out thermal reduction for 3 hours at the temperature of 1190 ℃ under the vacuum degree of 10-100Pa to generate metal magnesium and metal lithium vapor. The reaction process in the double-temperature-zone vacuum tube type high-temperature sintering furnace comprises the following steps: calcining magnesite/lithium carbonate in a high temperature region at 700-800 ℃ for 5-10 hours to obtain magnesium oxide/lithium oxide, regulating a temperature program, and reducing the magnesium oxide/lithium oxide by aluminum powder at 1190 ℃ for 3 hours to obtain magnesium/lithium vapor.
In this example, the magnesium-lithium alloy (100%) prepared contains about 79% of magnesium, 20% of lithium and 1% of impurities; in addition, the recovery rate of magnesium can reach 95%, and the recovery rate of lithium can reach 99%.
Example 4
The main method of this embodiment is the same as that of embodiment 1, except that: the temperature and time of the reduction reaction controlled by the high temperature region in the double-temperature region vacuum tube type high temperature sintering furnace are different.
And (3) carrying out thermal reduction for 2 hours at the temperature of 1200 ℃ under the vacuum degree of 10-100Pa to generate metal magnesium and metal lithium vapor. The reaction process in the double-temperature-zone vacuum tube type high-temperature sintering furnace comprises the following steps: calcining magnesite/lithium carbonate in a high temperature region at 700-800 ℃ for 5-10 hours to obtain magnesium oxide/lithium oxide, regulating a temperature program, and reducing the magnesium oxide/lithium oxide by aluminum powder at 1200 ℃ for 2 hours to obtain magnesium/lithium vapor.
In this example, the magnesium-lithium alloy (100%) prepared contained about 78% magnesium, 21% lithium, and 1% impurity; in addition, the recovery rate of magnesium can reach 92%, and the recovery rate of lithium can reach 99%.
Example 5
As shown in fig. 1: the method is the same as in example 3, except that: the ratio of the ingredients of each reactant was different from those of examples 1 to 4 above.
The granularity of the reducing agent aluminum powder is controlled to be about 100 meshes, and the high activity is maintained; in order to promote the reduction reaction and improve the metal reduction rate, the actual addition amount of the aluminum powder is higher than 15% of the theoretical reducing agent calculated by the stoichiometric ratio. The preparation method comprises the following steps of: 68% of magnesite, 12% of lithium carbonate and 20% of aluminum powder serving as a reducing agent, and the sum is 100%.
In this example, the magnesium-lithium alloy (100%) prepared contains about 89% of magnesium, 10% of lithium and 1% of impurities; in addition, the recovery rate of magnesium can reach 95%, and the recovery rate of lithium can reach 99%.
Example 6
As shown in fig. 1: the method is the same as in example 3, except that: the ratio of the ingredients of each reactant was different from those of examples 1 to 4 above.
The granularity of the reducing agent aluminum powder is controlled to be about 100 meshes, and the high activity is maintained; in order to promote the reduction reaction and improve the metal reduction rate, the actual addition amount of the aluminum powder is higher than 20% of the theoretical reducing agent calculated by the stoichiometric ratio. The preparation method comprises the following steps of: 55% of magnesite, 25% of lithium carbonate and 21% of aluminum powder serving as a reducing agent, wherein the sum is 100%.
In this example, the magnesium-lithium alloy (100%) prepared contained about 75% magnesium, 24% lithium, and 1% impurity; in addition, the recovery rate of magnesium can reach 95%, and the recovery rate of lithium can reach 99%.
Example 7
As shown in fig. 1: the method is the same as in example 3, except that: the ratio of the ingredients of each reactant was different from those of examples 1 to 4 above.
The granularity of the reducing agent aluminum powder is controlled to be about 100 meshes, and the high activity is maintained; in order to promote the reduction reaction and improve the metal reduction rate, the actual addition amount of the aluminum powder is higher than 5% of the theoretical reducing agent calculated by the stoichiometric ratio. The preparation method comprises the following steps of: 48% of magnesite, 31% of lithium carbonate and 21% of aluminum powder serving as a reducing agent, and the sum is 100%.
In this example, the magnesium-lithium alloy (100%) prepared contained about 70% magnesium, 29% lithium, and 1% impurity; in addition, the recovery rate of magnesium can reach 95%, and the recovery rate of lithium can reach 98%.
Claims (7)
1. A method for preparing magnesium-lithium alloy by a double-temperature-zone vacuum tube type high-temperature sintering furnace is characterized by comprising the following steps: the method comprises the following steps:
(1) Crushing microcrystalline magnesite to obtain coarse powder, and then wet-grinding and drying the coarse powder to obtain fine powder; uniformly mixing microcrystalline magnesite fine powder with magnesium carbonate and reducing agent aluminum powder, pressing into pellets, and mixing the raw materials in percentage by mass: 48-68% of microcrystalline magnesite fine powder, 12-31% of lithium carbonate and 20-30% of reducer aluminum powder;
(2) Placing the pellets arranged in the crucible in a high-temperature region of a double-temperature region vacuum tube type high-temperature sintering furnace, and heating and reducing the pellets for 2 to 5 hours at the temperature of 1170 to 1200 ℃ and the vacuum degree of 10 to 100Pa to generate vapor of metal magnesium and metal lithium and carbon dioxide gas;
(3) Introducing protective argon into the furnace to bring distilled magnesium/lithium vapor and generated carbon dioxide gas into a low-temperature area, condensing and alloying the magnesium/lithium vapor to form crude magnesium-lithium alloy, and taking away the carbon dioxide gas by flowing argon;
(4) And taking out the crude magnesium-lithium alloy, heating and melting the crude magnesium-lithium alloy at the temperature of 500-700 ℃ under the condition of argon atmosphere, and discharging refining slag in an alloy solution under the catalysis of a refining agent to obtain the magnesium-lithium alloy.
2. The method for preparing the magnesium-lithium alloy by the double-temperature-zone vacuum tube type high-temperature sintering furnace according to claim 1, which is characterized in that: in the step (1), the coarse powder is mixed in the ball-to-material ratio, and the zirconia balls: wet milling for 4 hours under distilled water=4:1, and drying to obtain fine powder with particle size in 20-70 microns; the microcrystalline magnesite is uniformly mixed with magnesium carbonate and reducing agent aluminum powder and then pressed into pellets under 50-200 MPa.
3. The method for preparing the magnesium-lithium alloy by the double-temperature-zone vacuum tube type high-temperature sintering furnace according to claim 1, which is characterized in that: in the step (2), the pellets are calcined at the temperature of 700-800 ℃ for 5-10 hours to obtain magnesium oxide/lithium oxide, and then reduced by aluminum powder in the pellets at the temperature of 1170-1200 ℃ for 2-5 hours to obtain magnesium/lithium vapor.
4. The method for preparing the magnesium-lithium alloy by the double-temperature-zone vacuum tube type high-temperature sintering furnace according to claim 1, which is characterized in that: in the step (2), after the reaction is finished, the reducing slag phase in the crucible in the high temperature area is mainly magnesia-alumina spinel MgO.Al 2 O 3 The reduction rate of the metal magnesium is 85-95%, and the reduction rate of the metal lithium can reach 99%.
5. The method for preparing the magnesium-lithium alloy by the double-temperature-zone vacuum tube type high-temperature sintering furnace according to claim 1, which is characterized in that: in the step (3), the generated carbon dioxide gas diffuses upwards around and is moved forward by the flowing argon gas flow belt, and the carbon dioxide gas is introduced into a filter bottle filled with water at an air outlet valve port to obtain CO 2 The gas and water produced carbonic acid, while argon was evolved from the filter flask.
6. The method for preparing the magnesium-lithium alloy by the double-temperature-zone vacuum tube type high-temperature sintering furnace according to claim 1, which is characterized in that: in the step (4), the prepared magnesium-lithium alloy contains 70-90% of magnesium, 10-30% of lithium and less than or equal to 1% of impurities according to mass percent.
7. The method for preparing the magnesium-lithium alloy by the double-temperature-zone vacuum tube type high-temperature sintering furnace according to claim 1, which is characterized in that: further comprising the step (5) of using the collected reducing slag as a refractory material or for preparing a lithium-containing ceramic material; collected CO 2 The greenhouse gases are recycled or made into carbon products.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310132467.0A CN116162816A (en) | 2023-02-17 | 2023-02-17 | Method for preparing magnesium-lithium alloy by double-temperature-zone vacuum tube type high-temperature sintering furnace |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310132467.0A CN116162816A (en) | 2023-02-17 | 2023-02-17 | Method for preparing magnesium-lithium alloy by double-temperature-zone vacuum tube type high-temperature sintering furnace |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116162816A true CN116162816A (en) | 2023-05-26 |
Family
ID=86416034
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310132467.0A Pending CN116162816A (en) | 2023-02-17 | 2023-02-17 | Method for preparing magnesium-lithium alloy by double-temperature-zone vacuum tube type high-temperature sintering furnace |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116162816A (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109536751A (en) * | 2018-12-04 | 2019-03-29 | 辽宁科技学院 | A kind of method of aluminothermic reduction production magnesium lithium alloy by-product magnesium aluminate spinel |
| CN110042240A (en) * | 2019-04-29 | 2019-07-23 | 安徽工业大学 | A kind of technique that vacuum thermit reduction produces lithium metal and magnesium metal simultaneously |
| CN111097920A (en) * | 2020-01-03 | 2020-05-05 | 四川万邦胜辉新能源科技有限公司 | Method for producing magnesium-lithium alloy by gaseous co-condensation method |
| CN111286653A (en) * | 2020-03-31 | 2020-06-16 | 东北大学 | Method for producing magnesium-lithium alloy by vacuum aluminothermic reduction |
-
2023
- 2023-02-17 CN CN202310132467.0A patent/CN116162816A/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109536751A (en) * | 2018-12-04 | 2019-03-29 | 辽宁科技学院 | A kind of method of aluminothermic reduction production magnesium lithium alloy by-product magnesium aluminate spinel |
| CN110042240A (en) * | 2019-04-29 | 2019-07-23 | 安徽工业大学 | A kind of technique that vacuum thermit reduction produces lithium metal and magnesium metal simultaneously |
| CN111097920A (en) * | 2020-01-03 | 2020-05-05 | 四川万邦胜辉新能源科技有限公司 | Method for producing magnesium-lithium alloy by gaseous co-condensation method |
| CN111286653A (en) * | 2020-03-31 | 2020-06-16 | 东北大学 | Method for producing magnesium-lithium alloy by vacuum aluminothermic reduction |
Non-Patent Citations (1)
| Title |
|---|
| 郑笑芳等: "真空硅热直接还原制备镁锂合金研究", 《真空科学与技术学报》, vol. 32, no. 11, 30 November 2012 (2012-11-30), pages 1023 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101812599B (en) | Method for preparing metal magnesium by using dolomite as raw material | |
| CN111286653B (en) | Method for producing magnesium-lithium alloy by vacuum aluminothermic reduction | |
| CN109536751B (en) | Method for producing magnesium-lithium alloy and by-product magnesium aluminate spinel by aluminothermic reduction | |
| CN110512094B (en) | Process for clean and continuous reduction of metal magnesium | |
| EP3960337B1 (en) | Method for producing magnesium-lithium alloy by means of gaseous co-condensation | |
| CN101891215B (en) | Method for preparing nano titanium diboride polycrystalline powder | |
| CN106498185B (en) | A kind of method of vacuum microwave refining magnesium | |
| CN112111660B (en) | Method for enriching lithium from lithium ore and preparing ferro-silicon alloy and recycling aluminum oxide | |
| CN102080164A (en) | Method for preparing Mg-Li alloy by vacuum synchronous thermal reduction | |
| CN110042240A (en) | A kind of technique that vacuum thermit reduction produces lithium metal and magnesium metal simultaneously | |
| CN102605185A (en) | Comprehensive iron-aluminium paragenetic mineral utilization method | |
| CN109020265A (en) | A kind of air high temperature preheating technique raising light-calcined magnesite product high yield method | |
| WO2021135398A1 (en) | Method for preparing highly pure metallic lithium by vacuum thermal reduction | |
| CN107523700B (en) | A kind of method that vacuum-thermal reduction William stone mine prepares magnesium metal and byproduct | |
| CN104164576B (en) | Method for preparing magnesium | |
| Yuan et al. | Aluminum production by carbothermo-chlorination reduction of alumina in vacuum | |
| CN116162816A (en) | Method for preparing magnesium-lithium alloy by double-temperature-zone vacuum tube type high-temperature sintering furnace | |
| WO2024056107A1 (en) | Green and environmentally friendly method for producing magnesium by means of aluminothermic reduction | |
| CN107904410B (en) | A kind of compound degasser prepares the production method of high temperature alloy and the dedicated high-purity metal chromium of target | |
| CN116102042A (en) | Method for simultaneously preparing metal magnesium and aluminum magnesium spinel from magnesite | |
| CN110195174B (en) | Preparation method of aluminum-lithium intermediate alloy | |
| CA2988445A1 (en) | Direct production of aluminum and silicon from their ore | |
| CN103086718B (en) | Preparation method of in-situ-synthesized composite aluminum nitride powder comprising sintering aid | |
| CN101812703A (en) | Method for preparing metallic titanium by electrolyzing sodium titanate-sodium hydroxide melt | |
| CN101358289A (en) | Method for preparing metal aluminum by coal reduction |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |