CN113151143A - Method and device for inhibiting pathological tissue cell growth by using sensitive frequency electric field - Google Patents
Method and device for inhibiting pathological tissue cell growth by using sensitive frequency electric field Download PDFInfo
- Publication number
- CN113151143A CN113151143A CN202110424833.0A CN202110424833A CN113151143A CN 113151143 A CN113151143 A CN 113151143A CN 202110424833 A CN202110424833 A CN 202110424833A CN 113151143 A CN113151143 A CN 113151143A
- Authority
- CN
- China
- Prior art keywords
- electric field
- frequency
- cells
- sensitive
- sensitive frequency
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M35/00—Means for application of stress for stimulating the growth of microorganisms or the generation of fermentation or metabolic products; Means for electroporation or cell fusion
- C12M35/02—Electrical or electromagnetic means, e.g. for electroporation or for cell fusion
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M41/00—Means for regulation, monitoring, measurement or control, e.g. flow regulation
- C12M41/30—Means for regulation, monitoring, measurement or control, e.g. flow regulation of concentration
- C12M41/36—Means for regulation, monitoring, measurement or control, e.g. flow regulation of concentration of biomass, e.g. colony counters or by turbidity measurements
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N13/00—Treatment of microorganisms or enzymes with electrical or wave energy, e.g. magnetism, sonic waves
Landscapes
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Sustainable Development (AREA)
- Cell Biology (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Electromagnetism (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
The invention provides a method and a device for inhibiting the growth of cells of lesion tissues by using a sensitive frequency electric field, wherein the method comprises the following steps: obtaining and culturing the pathological tissue cells; measuring the cell radius of the diseased tissue cells after said culturing; selecting a sensitive frequency based on the measured cell radius, and treating diseased tissue using an electric field having the sensitive frequency. Meanwhile, the optimal sensitivity frequency can be selected by combining the observation of inhibiting the growth of cells of the pathological tissue. Compared with the method for inhibiting the growth of the pathological tissue by the electric field in the prior art, the method and the device can more quickly and accurately find the sensitive frequency suitable for the specific pathological tissue cells, can also timely track and correct the sensitive frequency according to the development of the pathological tissue cells, have more obvious treatment effect than the traditional fixed frequency, and open up an individualized application scheme.
Description
Technical Field
The present invention is in the field of medical biotechnology, and more particularly, the present invention relates generally to a method and apparatus for inhibiting the growth of diseased tissue cells using a sensitive frequency electric field.
Background
When the malignant lesion enters the development stage, cells of the lesion tissue are in a state of rapid growth, and the number and the time are in an exponential relation. The basic principle of the current tumor electric field therapy is established on the basis that an electric field has the effect of inhibiting and destroying the mitosis of tumor cells, and the electric field generated by using 200KHz electric signals is used for inhibiting the rapid growth of the tumor cells of a patient so as to achieve the treatment effect. It is found in cell experiment that the electric field that the signal of telecommunication produced with different frequencies suppresses many patients 'tumour cell fast growth, the inhibitory effect diverse, some 150KHz is effectual, some 180KHz is effectual, some 200KHz is effectual, some 220KHz is effectual, some 240KHz is effectual, 200KHz is the central frequency who has the inhibitory action frequency section to tumour cell fast growth, but to individual patient, it is not necessarily the best, some patient's tumour cell is insensitive to the electric field that 200KHz signal of telecommunication produced even, lead to the inhibitory effect not to reach the needs of treatment.
Further experiments show that when the disease condition of the same patient progresses to different stages, the sensitivity frequency of the pathological tissue cells of the same patient is different, and the sensitivity frequency of the pathological tissue cells of a plurality of patients tends to become lower along with the progress of the disease condition. The electric field generated by the sensitive frequency is used for inhibiting the pathological tissue cells of the patient, and the effect is better than that of the current uniform fixed frequency. If the sensitive frequency tracking correction can be carried out in time according to the development of the state of an illness, the effect is more obvious. It can be concluded that the electric field generated by the targeted sensitive frequency is used to suppress the diseased tissue of the patient, with better effect than the uniform frequency.
Therefore, based on the current situation, it becomes very important to quickly find the sensitive frequency suitable for different patients in different periods, so that the treatment efficiency can be greatly improved. However, in the prior art, the defects of complex process or inaccurate result still exist in determining the optimal growth inhibition electric field frequency of different lesion tissues. The treatment method for inhibiting the growth of the cells of the lesion tissue by using the sensitive frequency electric field can obviously improve the effect of the tumor electric field therapy after being applied to clinic.
Disclosure of Invention
The invention aims to overcome the defects of poor effect caused by using uniform frequency for inhibiting diseased tissue cells of different individuals and different stages and complex process or inaccurate result and the like when the sensitive frequency of an electric field for inhibiting cell growth is measured in the prior art, thereby providing a novel method for inhibiting the growth of the diseased tissue cells by using a sensitive frequency electric field. The present inventors have found that, even when a cell of different cell lines having the same radius is subjected to a cell proliferation-suppressing electric field sensitivity frequency test, the cells of the different cell lines having the same radius exhibit substantially the same sensitivity frequency, that is, the sensitivity frequency of the cell is related to the radius (or cell volume) of the cell and has no significant relationship with the type of the cell, and have completed the present invention.
To achieve the above object, in one aspect, the present invention provides a method for inhibiting the growth of cells in a diseased tissue using an electric field of sensitive frequency, characterized in that the method comprises the steps of: (1) obtaining and culturing the pathological tissue cells; (2) measuring the cell radius of the diseased tissue cells after said culturing; (3) selecting a sensitivity frequency based on the measured cell radius; and (4) treating the diseased tissue cells with an electric field having the sensitive frequency.
In another aspect, the present invention provides a method for selecting a sensitive frequency at which an electric field inhibits growth of cells in a diseased tissue, the method comprising the steps of: (1) obtaining and culturing the pathological tissue cells; (2) measuring the cell radius of the diseased tissue cells after said culturing; (3) selecting a sensitivity frequency based on the measured cell radius.
With respect to step (1), in a preferred embodiment of the present invention, the diseased tissue cells may be obtained by means of isolation from a diseased tissue sample. According to the present invention, the manner of the separation is not particularly limited, and can be achieved using a cell extraction technique commonly used in the art.
For example, in one embodiment, the diseased tissue sample may be minced as much as possible to form small pieces, however, Trypsin-EDTA (0.25%) is added to the petri dish and incubated at 37 ℃ for 2-3min, followed by addition of media (DMEM + 10% FBS) to stop digestion, the cells are screened to remove excess tissue debris, and the filtered cells are transferred to a centrifuge tube to prepare a cell suspension. Among them, Trypsin-EDTA pancreatin is the most commonly used cell separation reagent in the field, is a single-chain polypeptide consisting of 223 amino acids, and is obtained by cutting amino N-terminal 6 peptide of trypsinogen (zhitrypsinogen) between Lys6 and Ile 7.
In a preferred embodiment of the invention, the culture may include, but is not limited to, primary culture or PDX modeling to obtain the number of cells for which a radius measurement can be made. In one embodiment, the primary culture can obtain cells that best match the performance of cells in the diseased tissue sample, in contrast, the subculture or cell line culture often has different heredity and phenotype from the primary cells, for example, certain genes are increased or decreased in expression, cell surface antigens and the like are likely to change, and the cell line undergoes numerous subculture in vitro, and the heredity characteristics are already obviously changed from the cells in vivo. The number of cells after culture is not particularly limited, and may be used for subsequent measurement of cell radius (or cell volume).
For step (2), in a preferred embodiment of the present invention, the measurement of the cell radius (or cell volume) can be performed by means of flow cytometry estimation, cytometry measurement, or ocular microscopical measurement, but is not limited thereto, and the measurement can be performed by other cell radius measurement methods commonly used in the art. In addition, the measurement of the cell radius may be performed by measuring the radius of a single cell, or by measuring the radius of a plurality of cells, however, divided by the number of cells.
Further, in order to measure a more accurate cell radius, multiple measurements may be taken to obtain a more accurate result. For example, in another preferred embodiment of the present invention, the measurement may be performed two or more times, and an average value of the two or more measurements is calculated as the value of the cell radius.
For step (3), since the inventors found that the sensitivity frequency of a cell is related to the radius of the cell, and has no significant relationship with the kind of the cell, the sensitivity frequency can be selected based on the measured radius of the cell. Specifically, based on the test results of cell radius and sensitive frequency tests, the cell radius and the electric field frequency have a certain inverse proportional relation, and the larger the cell radius is, the lower the sensitive frequency is; conversely, the smaller the cell radius, the higher the frequency of sensitivity.
In a preferred embodiment of the present invention, the selecting the sensitive frequency may include: the sensitive frequency is determined by a graphical or computational method.
FIG. 1 shows a graph of sensitivity frequency versus cell radius obtained according to one embodiment of the present invention. Since the relationship between the sensitivity frequency and the cell radius may also be influenced by different experimenters or devices and other factors, in step (3), the relationship graph and formula as shown in fig. 1 can be used to obtain the selection sensitivity frequency, or standard graphs and formulas showing the relationship between the sensitivity frequency and the cell radius can be obtained again based on the way the experimenters are used currently, and then the sensitivity frequency is selected.
According to the present invention, the graph method represents that a frequency value in an ordinate is found in a curve correspondingly based on a cell radius in an abscissa as a sensitive frequency, and the calculation method represents that a frequency value is directly calculated based on a cell radius and according to a formula of the sensitive frequency and the cell radius in an image as a sensitive frequency.
In a preferred embodiment of the present invention, the calculation method may include substituting the measured cell radius into the formula f ═ k/l to obtain the sensitive frequency, where l denotes the cell radius and f denotes the sensitive frequency, and k is a constant. In a preferred embodiment, k may be 1700-2100KHz μm, 1800-2000KHz μm, more preferably 1900KHz μm. Since the size of the diseased tissue cells of a patient is not uniform and the size of the shape varies from individual to individual, the k values provided by the present invention are merely exemplary and may vary slightly for other tumor species. In addition, the results obtained by using the calculation method and the drawing method may have slight errors, but the results are very close to each other, and the sensitive frequency can be usually an integer intermediate value with the mantissa of 0 or 5 for the convenience of experiment and clinic operation.
In addition, the method for selecting the sensitivity frequency based on the cell radius provided by the invention can also be used in combination with a sensitivity frequency test. For example, specifically, a rough point of the sensitive frequency (or referred to as a pre-sensitive frequency) is first found by the method for selecting the sensitive frequency based on the cell radius provided by the present invention, and then quantitative measurement is performed on frequency values near the pre-sensitive frequency by a sensitive frequency test, so as to more accurately find the sensitive frequency most suitable for the cells of the lesion tissue. For example, in a preferred embodiment of the present invention, the selecting a sensitive frequency may include: the pre-sensitivity frequency is determined by a plotting method or a calculation method, and is determined by a sensitivity frequency test in a frequency range of + -50% of the pre-sensitivity frequency (e.g., pre-sensitivity frequency + 10%, + 20%, + 30%, -10%, -20%, and-30%, etc.) or + -100 KHz (e.g., pre-sensitivity frequency +20KHz, +50KHz, +80KHz, -20KHz, -50KHz, and-80 KHz, etc.).
The sensitivity frequency test according to the present invention indicates that the diseased tissue cells are treated by using a plurality of electric fields having different frequencies, and the sensitivity frequency having the best suppression effect is determined according to the size of the diseased tissue cells after treatment. In a preferred embodiment of the present invention, the sensitive frequency test may include: setting a plurality of electric fields having different frequencies within the frequency range to perform an experiment for suppressing the growth of the cells of the lesion tissue, and selecting, as the sensitive frequency, a frequency having the best effect of suppressing the growth of the cells of the lesion tissue based on the suppression effect. In addition, if when the electric field suppression effects of two adjacent frequencies are comparable, the average of the two adjacent frequencies can be taken as the sensitive frequency.
For the step (4), the cells of the lesion tissue can be treated by using the electric field with the sensitive frequency based on the sensitive frequency obtained by the method as described above, so as to achieve the effect of inhibiting the growth of the cells of the lesion tissue. In a preferred embodiment of the present invention, the treatment may include treatment with an electric field strength of not less than 0.7V/cm (e.g., 1V/cm, 1.5V/cm, 2V/cm, 3V/cm, or the like) for not less than 18 hours (e.g., 20 hours, 22 hours, 24 hours, or the like) per day.
According to the invention, the selection of the sensitivity frequency can be changed periodically, considering that the cells of the pathological tissue may also have a variant behavior during the process of treating the cells of the pathological tissue, and the sensitivity frequency may be changed accordingly. For example, in a preferred embodiment of the present invention, after step (4) is performed for a predetermined time, steps (1) to (4) may be repeatedly performed, and particularly, the predetermined time may be 1 to 10 days (e.g., 2, 3, 4, 5, 6, 7, 8, or 9 days, etc.).
In another aspect, the present invention provides a device for inhibiting the growth of cells in a diseased tissue using an electric field of sensitive frequency, the device comprising: electric field generating means for applying the electric field; an electrical environment culture device for culturing the diseased tissue cells in an electrical environment; a slide glass which is arranged in the electric field generating device and is used for bearing the cultured pathological tissue cells; and a cell radius measuring instrument for measuring a cell radius of the lesion tissue cell.
Specifically, the obtained pathological tissue cells can be placed in an electric environment culture device for culture, cell suspension is obtained through separation after culture, the radius is measured by using a cell radius measurer, the other part of the pathological tissue cells is uniformly smeared on the surface of a glass slide, the glass slide is placed in an electric field generation device, electric fields with different frequencies are applied through the electric field generation device, the cell suspension is collected again after the electric field stimulation, the supernatant is discarded through centrifugation, the cell suspension is evenly mixed in a heavy suspension manner, a new cell suspension is prepared, and cell counting is carried out.
In a preferred embodiment of the present invention, an electric signal applying medium unit capable of applying an electric signal to the culture solution may be provided in the electric field generating device, and one end of the electric signal applying medium unit contacts the culture solution and the other end is a conductive electrode. In another embodiment of the present invention, an electric field adjusting device may be disposed in the electric field generating device, and the electric field adjusting device is used for adjusting the frequency and intensity of the electric field. In another embodiment of the present invention, the cell radius measurer may be selected from a flow cytometer, a cytometer, or a microscope having an ocular microsize, but is not limited thereto. In addition, according to the present invention, the manner of applying the electric field may be various, for example, the applying the electric field may include sequentially applying the electric field in a plurality of different directions, and the order of applying the electric field applied in the plurality of different directions may be any order, without particular limitation.
Compared with the method for inhibiting the growth of the cells of the pathological tissue by using the sensitive frequency electric field in the prior art, the method and the device can more quickly and accurately find the sensitive frequency suitable for the cells of the specific pathological tissue, and can also timely track and correct the sensitive frequency according to the development of the cells of the pathological tissue, so that the treatment effect is more obvious than that under the condition of uniform frequency.
Drawings
The accompanying drawings, which are included to provide a further understanding of the invention and are incorporated in and constitute a part of this specification, illustrate embodiments of the invention and together with the description serve to explain the principles of the invention and not to limit the invention. In the drawings:
FIG. 1 is a graph showing the relationship between radius and sensitivity frequency of diseased tissue cells according to one embodiment of the present invention;
FIG. 2 shows the results of a frequency test of cellular sensitivity of a lesion tissue according to example 1 of the present invention;
FIG. 3 shows the results of a frequency test of cellular sensitivity of a lesion tissue according to example 2 of the present invention; and is
FIG. 4 shows the cell sensitivity frequency test results of the lesion tissue according to example 3 of the present invention.
Detailed Description
The following describes in detail specific embodiments of the present invention. It should be understood that the detailed description and specific examples, while indicating the present invention, are given by way of illustration and explanation only, not limitation.
The endpoints of the ranges and any values disclosed herein are not limited to the precise range or value, and such ranges or values should be understood to encompass values close to those ranges or values. For ranges of values, between the endpoints of each of the ranges and the individual points, and between the individual points may be combined with each other to give one or more new ranges of values, and these ranges of values should be considered as specifically disclosed herein.
The present invention is described in terms of particular embodiments, other advantages and features of the invention will become apparent to those skilled in the art from the following disclosure, and it is to be understood that the described embodiments are merely exemplary of the invention and that it is not intended to limit the invention to the particular embodiments disclosed. All other embodiments, which can be derived by a person skilled in the art from the embodiments given herein without making any creative effort, shall fall within the protection scope of the present invention.
Example 1 selection of sensitive frequencies by means of a graphical method
Obtaining a pathological change (glioma) tissue specimen 1 of a patient, wherein the pathological change tissue specimen 1 comprises pathological change tissue cells obtained through resection operation and pathological change tissue specimens obtained through interventional puncture operation, and culturing the pathological change tissue cells to enable the pathological change tissue cells to grow, divide and proliferate so as to obtain a certain number of pathological change tissue cells.
One flask of T75 flasks cultured to above 80% confluence was first removed and the medium was discarded, however Trypsin-EDTA (0.25%), incubated at 37 ℃ for 2-3min and then medium (DMEM + 10% FBS) was added to stop the digestion. Centrifuging for five minutes in a centrifuge, adding special culture medium, and regulating cell concentration to 1-2 x 105And/ml. Sterilizing the glass slide, placing the glass slide in an electric environment culture device, adding cell suspension into each glass slide,the cell suspension is evenly distributed on the surface of the slide. Placing the slide in an incubator with 37 ℃ saturated humidity for 2-4h, supplementing a special culture medium, and then placing the slide in the incubator with 37 ℃ saturated humidity for culture.
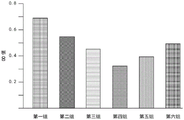
Respectively measuring cells in at least three regions on the same slide by using an ocular micrometer, wherein the number of the cells measured in each region is not less than three, adding the radiuses of the cells, dividing the radiuses by the number of the cells to obtain an average value, calculating to obtain the average value of 9.5 mu m, and obtaining the sensitivity frequency of the cells of the lesion tissue to be 200KHz based on a curve chart shown in figure 1.
Dividing the cells of the lesion tissue into six groups, wherein the first group is a control group without electric field application, the second group applies 160KHz, the third group applies 180KHz, the fourth group applies 200KHz, the fifth group applies 220KHz, the sixth group applies 240KHz, the field intensity of the electric field is set to be 2.2v/cm, and the action time of the electric field is 72h (24 h every day, 3 days). After the cells are treated by an electric field, the cells are digested by pancreatin, the cell suspension is collected into a centrifuge tube and centrifuged, the supernatant is discarded, and the cell suspension is prepared by resuspension and uniform mixing, and then the cells are counted.
The statistics of the results are shown in fig. 2, the OD value of the fourth group is significantly lower than that of the other groups, and the highest value of the control group, which indicates that the frequency used in the fourth group is the optimal frequency for inhibiting the cell growth of the lesion tissue, namely the sensitive frequency, among several frequencies.
Example 2 selection of sensitive frequencies by computational methods
Obtaining a pathological change tissue specimen 2 of a patient, wherein the pathological change tissue specimen comprises pathological change tissue cells obtained through a resection operation and pathological change tissue specimens obtained through an interventional puncture operation, and the pathological change tissue cells are cultured to grow, divide and proliferate the pathological change tissue cells so as to obtain a certain number of pathological change tissue cells.
One flask of T75 flasks cultured to above 80% confluence was first removed and the medium was discarded, however Trypsin-EDTA (0.25%), incubated at 37 ℃ for 2-3min and then medium (DMEM + 10% FBS) was added to stop the digestion. Centrifuging for five minutes in a centrifuge, adding special culture medium, and regulating cell concentration to 1-2 x 105And/ml. Sterilizing the slide, and culturing in electric environmentAnd (4) adding cell suspension into each slide to uniformly distribute the cell suspension on the surface of the slide. Placing the slide in an incubator with 37 ℃ saturated humidity for 2-4h, supplementing a special culture medium, and then placing the slide in the incubator with 37 ℃ saturated humidity for culture.
Respectively measuring cells in not less than three regions on the same slide by an ocular micrometer, wherein the number of the cells measured in each region is not less than three, adding the radiuses of the cells, dividing the sum by the number of the cells to obtain an average value, for example, calculating to obtain the average value of 9.5 micrometers, and calculating to obtain the sensitive frequency of the cells of the lesion tissue to be 200KHz based on the constant k of 1900KHz mum.
Dividing the cells of the lesion tissue into six groups, wherein the first group is a control group without electric field application, the second group applies 160KHz, the third group applies 180KHz, the fourth group applies 200KHz, the fifth group applies 220KHz, the sixth group applies 240KHz, the field intensity of the electric field is set to be 1.8v/cm, and the action time of the electric field is 72h (18 h every day, 4 days). After the cells are treated by an electric field, the cells are digested by pancreatin, the cell suspension is collected into a centrifuge tube and centrifuged, the supernatant is discarded, and the cell suspension is prepared by resuspension and uniform mixing, and then the cells are counted.
Statistics of results as shown in fig. 3, the OD value of the fourth group using 200KHz is significantly lower than that of the other groups, indicating that the frequency used in the fourth group is the optimal frequency for inhibiting the cell growth of the diseased tissue, i.e. the sensitive frequency, among several frequencies. .
Example 3 selection of sensitive frequencies by plotting and calculating
Obtaining a pathological change tissue specimen 3 of a patient, which comprises pathological change tissue cells obtained through a resection operation and pathological change tissue specimens obtained through an interventional puncture operation, culturing the pathological change tissue cells to enable the pathological change tissue cells to grow, divide and proliferate so as to obtain a certain number of pathological change tissue cells.
One flask of T75 flasks cultured to above 80% confluence was first removed and the medium was discarded, however Trypsin-EDTA (0.25%), incubated at 37 ℃ for 2-3min and then medium (DMEM + 10% FBS) was added to stop the digestion. Centrifuging for five minutes in a centrifuge, adding special culture medium, and regulating cell concentration to 1-2 x 105/ml。After the glass slide is sterilized, the glass slide is placed in an electric environment culture device, and cell suspension is added to each glass slide, so that the cell suspension is uniformly distributed on the surface of the glass slide. Placing the slide in an incubator with 37 ℃ saturated humidity for 2-4h, supplementing a special culture medium, and then placing the slide in the incubator with 37 ℃ saturated humidity for culture.
And respectively measuring cells in at least three areas on the same slide by using an ocular micrometer, wherein the number of the cells measured in each area is not less than three, adding the radiuses of the cells, and dividing the sum by the number of the cells to obtain an average value. For example, the average value is calculated to be 9 μm, the sensitivity frequency of the cells of the lesion tissue is 210KHz based on the graph shown in FIG. 1, and the sensitivity frequency of the cells of the lesion tissue is 211KHz based on the constant k of 1900KHz μm. In the case of very close proximity, for experimental and clinical stool manipulation, integers with mantissas of 0 or 5 are taken, thus 210KHz is taken in this example.
Dividing the cells of the lesion tissue into six groups, wherein the first group is a control group without electric field application, the second group applies 170KHz, the third group applies 190KHz, the fourth group applies 210KHz, the fifth group applies 230KHz, the sixth group applies 250KHz electric field intensity is set as 2v/cm, and the electric field action time is 60h (20 h every day, 3 days). After the cells are treated by an electric field, the cells are digested by pancreatin, the cell suspension is collected into a centrifuge tube and centrifuged, the supernatant is discarded, and the cell suspension is prepared by resuspension and uniform mixing, and then the cells are counted.
Statistics of results as shown in fig. 4, the OD value of the fourth group using 210KHz is significantly lower than that of the other groups, indicating that the frequency used in the fourth group is the optimal frequency for inhibiting the cell growth of the lesion tissue, i.e. the sensitive frequency, among several frequencies.
The preferred embodiments of the present invention have been described in detail, however, the present invention is not limited to the specific details of the above embodiments, and various simple modifications may be made to the technical solution of the present invention within the technical idea of the present invention, and these simple modifications are within the protective scope of the present invention.
It should be noted that the various technical features described in the above embodiments can be combined in any suitable manner without contradiction, and the invention is not described in any way for the possible combinations in order to avoid unnecessary repetition.
In addition, any combination of the various embodiments of the present invention is also possible, and the same should be considered as the disclosure of the present invention as long as it does not depart from the spirit of the present invention.
Claims (18)
1. A method for inhibiting the growth of cells in a diseased tissue using an electric field of sensitive frequency, said method comprising the steps of:
(1) obtaining and culturing the pathological tissue cells;
(2) measuring the cell radius of the diseased tissue cells after said culturing;
(3) selecting a sensitivity frequency based on the measured cell radius; and
(4) the diseased tissue cells are treated with an electric field having the sensitive frequency.
2. The method of claim 1, wherein the diseased tissue cells are obtained by isolation from a diseased tissue sample.
3. The method of claim 1, wherein the culturing includes, but is not limited to, primary culturing or PDX modeling, to obtain the number of cells that can be measured for radius.
4. The method of claim 1, wherein the measurement of the cell radius is performed by way of flow cytometry estimation, cytometry measurement, or microscopically with an ocular microstick.
5. The method of claim 1, wherein the measuring is performed two or more times and an average of the two or more measurements is calculated as the value of the cell radius.
6. The method of claim 1, wherein the selecting the sensitive frequency comprises: the sensitive frequency is determined by a graphical or computational method.
7. The method of claim 6, wherein the calculation comprises substituting the measured cell radius into the equation f ═ k/l to obtain the sensitive frequency, wherein l represents the cell radius, f represents the sensitive frequency, and k is a constant.
8. The method of claim 1, wherein the selecting the sensitive frequency comprises: the pre-sensitivity frequency is determined by a graph or a calculation method and the sensitivity frequency is determined by a sensitivity frequency test in a frequency range of ± 50% of the pre-sensitivity frequency.
9. The method of claim 8, wherein the sensitivity frequency test comprises: setting a plurality of electric fields having different frequencies within the frequency range to perform an experiment for suppressing the growth of the cells of the lesion tissue, and selecting, as the sensitive frequency, a frequency having the best effect of suppressing the growth of the cells of the lesion tissue based on the suppression effect.
10. The method according to claim 9, wherein when the electric field suppression effect of two adjacent frequencies is comparable, taking the average of the two adjacent frequencies as the sensitive frequency.
11. The method of claim 1, wherein the treating comprises treating for not less than 18 hours per day with an electric field strength of not less than 0.7V/cm.
12. The method according to claim 1, wherein the steps (1) to (4) are repeatedly performed after the step (4) is performed for a predetermined time.
13. The method of claim 12, wherein the predetermined time is 1-10 days.
14. An apparatus for inhibiting the growth of cells in diseased tissue using an electric field of sensitive frequency, the apparatus comprising:
electric field generating means for applying the electric field;
an electrical environment culture device for culturing the diseased tissue cells in an electrical environment;
a slide glass which is arranged in the electric field generating device and is used for bearing the cultured pathological tissue cells; and
a cell radius measurer for measuring a cell radius of the lesion tissue cell.
15. The device according to claim 14, wherein an electric signal applying medium unit capable of applying an electric signal to the culture solution is provided in the electric field generating device, one end of the electric signal applying medium unit contacts the culture solution, and the other end is a conductive electrode.
16. The device of claim 14, wherein an electric field adjusting device is disposed in the electric field generating device, and the electric field adjusting device is used for adjusting the frequency and intensity of the electric field.
17. The device of claim 14, wherein the cell radius measurer is selected from a flow cytometer, a cytometer, or a microscope with an ocular microscope.
18. The apparatus of claim 14, wherein the applying the electric field comprises sequentially applying the electric field in a plurality of different directions.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110424833.0A CN113151143A (en) | 2021-04-20 | 2021-04-20 | Method and device for inhibiting pathological tissue cell growth by using sensitive frequency electric field |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110424833.0A CN113151143A (en) | 2021-04-20 | 2021-04-20 | Method and device for inhibiting pathological tissue cell growth by using sensitive frequency electric field |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN113151143A true CN113151143A (en) | 2021-07-23 |
Family
ID=76869348
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110424833.0A Pending CN113151143A (en) | 2021-04-20 | 2021-04-20 | Method and device for inhibiting pathological tissue cell growth by using sensitive frequency electric field |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113151143A (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116196554A (en) * | 2023-03-23 | 2023-06-02 | 湖南安泰康成生物科技有限公司 | System for treating tumors by covering tumor cell sensitive frequency with multiple electric fields |
| WO2024093734A1 (en) * | 2022-11-03 | 2024-05-10 | 赛福凯尔(绍兴)医疗科技有限公司 | Electric field energy-focused emitting device and method |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080125772A1 (en) * | 2004-09-10 | 2008-05-29 | Minnow Medical, Inc | Tuned RF energy and electrical tissue characterization for selective treatment of target tissues |
| CN101484245A (en) * | 2006-04-12 | 2009-07-15 | 硅生物系统股份公司 | Methods and apparatus for the selection and/or processing ofparticles, in particular for the selective and/or optimised lysis of cells |
| CN105530862A (en) * | 2013-05-06 | 2016-04-27 | 尤伦·帕提 | Apparatus and methods for treating a tumor with an alternating electric field and for selecting a treatment frequency based on estimated cell size |
| CN210458213U (en) * | 2019-04-26 | 2020-05-05 | 湖南安泰康成生物科技有限公司 | Biological culture system of electricity environment |
| CN112553075A (en) * | 2020-12-28 | 2021-03-26 | 湖南安泰康成生物科技有限公司 | Method and device for inhibiting rapid growth of tumor cells by using sensitive frequency electric field |
-
2021
- 2021-04-20 CN CN202110424833.0A patent/CN113151143A/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080125772A1 (en) * | 2004-09-10 | 2008-05-29 | Minnow Medical, Inc | Tuned RF energy and electrical tissue characterization for selective treatment of target tissues |
| CN101484245A (en) * | 2006-04-12 | 2009-07-15 | 硅生物系统股份公司 | Methods and apparatus for the selection and/or processing ofparticles, in particular for the selective and/or optimised lysis of cells |
| CN105530862A (en) * | 2013-05-06 | 2016-04-27 | 尤伦·帕提 | Apparatus and methods for treating a tumor with an alternating electric field and for selecting a treatment frequency based on estimated cell size |
| CN110638450A (en) * | 2013-05-06 | 2020-01-03 | 尤伦·帕提 | Apparatus and method for treating tumors with alternating electric field and for selecting treatment frequency based on estimated cell size |
| CN210458213U (en) * | 2019-04-26 | 2020-05-05 | 湖南安泰康成生物科技有限公司 | Biological culture system of electricity environment |
| CN112553075A (en) * | 2020-12-28 | 2021-03-26 | 湖南安泰康成生物科技有限公司 | Method and device for inhibiting rapid growth of tumor cells by using sensitive frequency electric field |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024093734A1 (en) * | 2022-11-03 | 2024-05-10 | 赛福凯尔(绍兴)医疗科技有限公司 | Electric field energy-focused emitting device and method |
| CN116196554A (en) * | 2023-03-23 | 2023-06-02 | 湖南安泰康成生物科技有限公司 | System for treating tumors by covering tumor cell sensitive frequency with multiple electric fields |
| CN116196554B (en) * | 2023-03-23 | 2024-03-12 | 湖南安泰康成生物科技有限公司 | System for treating tumors by covering tumor cell sensitive frequency with multiple electric fields |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Qiao et al. | Electrical properties of breast cancer cells from impedance measurement of cell suspensions | |
| CN113151143A (en) | Method and device for inhibiting pathological tissue cell growth by using sensitive frequency electric field | |
| Thielecke et al. | A multicellular spheroid-based sensor for anti-cancer therapeutics | |
| Javani Jouni et al. | Investigation on the effect of static magnetic field up to 15 mT on the viability and proliferation rate of rat bone marrow stem cells | |
| CN112553075A (en) | Method and device for inhibiting rapid growth of tumor cells by using sensitive frequency electric field | |
| JP2017102118A (en) | Fibroblast growth patterns for diagnosis of alzheimer's disease | |
| Özdemir et al. | xCELLigence Real Time Cell Analysis System A New Method for cell proliferation and cytotoxicity | |
| Lin et al. | Prostatic stromal cells derived from benign prostatic hyperplasia specimens possess stem cell like property | |
| Qi et al. | In vitro differentiation of bone marrow stromal cells into neurons and glial cells and differential protein expression in a two-compartment bone marrow stromal cell/neuron co-culture system | |
| Wang et al. | Identification of tumor stem-like cells in admanatimomatous craniopharyngioma and determination of these cells’ pathological significance | |
| Kar et al. | Establishment of primary cell culture from ascitic fluid and solid tumor obtained from epithelial ovarian carcinoma patients | |
| Eswaramoorthy et al. | Regional differentiation of adipose-derived stem cells proves the role of constant electric potential in enhancing bone healing | |
| Holland et al. | Towards non-invasive characterisation of coronary stent re-endothelialisation–An in-vitro, electrical impedance study | |
| Lim et al. | Extracellular signal-regulated kinase involvement in human astrocyte migration | |
| Enríquez et al. | High‐Throughput Magnetic Actuation Platform for Evaluating the Effect of Mechanical Force on 3D Tumor Microenvironment | |
| Antoccia et al. | Cell cycle perturbations and genotoxic effects in human primary fibroblasts induced by low-energy protons and X/γ-rays | |
| JP2009027928A (en) | Method for evaluating cancer metastasis ability and three-dimensional double membrane structure for evaluating cancer metastasis ability | |
| Shalileh et al. | Label-free mechanoelectrical investigation of single cancer cells by dielectrophoretic-induced stretch assay | |
| CN114807115B (en) | Construction method of aging cells and method for evaluating anti-aging effect | |
| CN218879923U (en) | Device for determining optimal electric field frequency for inhibiting tumor cell growth | |
| CN113156123B (en) | Use of EphA2 gene for the preparation of a product for the treatment or diagnosis of breast cancer caused by protein associated with cell apoptosis | |
| CN108653314A (en) | Applications of the MiR-22 in the drug for preparing the migration for inhibiting nasopharyngeal carcinoma | |
| Balasubramanian et al. | GHz ultrasound and electrode chip-scale arrays stimulate and influence morphology of human neural cells | |
| Li et al. | Quantitative characterization of mechano-biological interrelationships of single cells | |
| Jaatinen | The Effect of an Applied Electric Current on Cell Proliferation, Viability, Morphology, Adhesion, and Stem Cell Differentiation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |