CN112939779A - Terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization and preparation method thereof - Google Patents
Terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization and preparation method thereof Download PDFInfo
- Publication number
- CN112939779A CN112939779A CN202110195940.0A CN202110195940A CN112939779A CN 112939779 A CN112939779 A CN 112939779A CN 202110195940 A CN202110195940 A CN 202110195940A CN 112939779 A CN112939779 A CN 112939779A
- Authority
- CN
- China
- Prior art keywords
- photoinitiator
- photopolymerization
- terephthaloyl
- led
- formate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 title claims abstract description 16
- 238000002360 preparation method Methods 0.000 title claims abstract description 6
- 125000001931 aliphatic group Chemical group 0.000 claims abstract description 3
- 125000003710 aryl alkyl group Chemical group 0.000 claims abstract description 3
- 125000003118 aryl group Chemical group 0.000 claims abstract description 3
- 150000002170 ethers Chemical class 0.000 claims abstract description 3
- 239000000126 substance Substances 0.000 claims abstract description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 45
- 238000006243 chemical reaction Methods 0.000 claims description 20
- 239000012043 crude product Substances 0.000 claims description 15
- 239000002904 solvent Substances 0.000 claims description 13
- 238000000034 method Methods 0.000 claims description 12
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 claims description 10
- 239000004925 Acrylic resin Substances 0.000 claims description 10
- 229920000178 Acrylic resin Polymers 0.000 claims description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 10
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 10
- 239000000203 mixture Substances 0.000 claims description 10
- 239000000178 monomer Substances 0.000 claims description 10
- 238000003756 stirring Methods 0.000 claims description 10
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims description 9
- 238000004440 column chromatography Methods 0.000 claims description 8
- 238000001914 filtration Methods 0.000 claims description 8
- 239000013067 intermediate product Substances 0.000 claims description 8
- 238000004821 distillation Methods 0.000 claims description 7
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 claims description 6
- 230000015572 biosynthetic process Effects 0.000 claims description 6
- 239000012024 dehydrating agents Substances 0.000 claims description 6
- 239000007800 oxidant agent Substances 0.000 claims description 6
- 230000001590 oxidative effect Effects 0.000 claims description 6
- 239000002244 precipitate Substances 0.000 claims description 6
- JPJALAQPGMAKDF-UHFFFAOYSA-N selenium dioxide Chemical group O=[Se]=O JPJALAQPGMAKDF-UHFFFAOYSA-N 0.000 claims description 6
- 238000003786 synthesis reaction Methods 0.000 claims description 6
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical group C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 claims description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 5
- 239000012295 chemical reaction liquid Substances 0.000 claims description 5
- 239000012467 final product Substances 0.000 claims description 5
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims description 5
- 239000011347 resin Substances 0.000 claims description 5
- 229920005989 resin Polymers 0.000 claims description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 5
- SKBBQSLSGRSQAJ-UHFFFAOYSA-N 1-(4-acetylphenyl)ethanone Chemical compound CC(=O)C1=CC=C(C(C)=O)C=C1 SKBBQSLSGRSQAJ-UHFFFAOYSA-N 0.000 claims description 4
- BSKHPKMHTQYZBB-UHFFFAOYSA-N 2-methylpyridine Chemical compound CC1=CC=CC=N1 BSKHPKMHTQYZBB-UHFFFAOYSA-N 0.000 claims description 4
- ITQTTZVARXURQS-UHFFFAOYSA-N 3-methylpyridine Chemical compound CC1=CC=CN=C1 ITQTTZVARXURQS-UHFFFAOYSA-N 0.000 claims description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 claims description 4
- -1 aralkyl alcohol Chemical compound 0.000 claims description 4
- 239000002585 base Substances 0.000 claims description 4
- 238000001816 cooling Methods 0.000 claims description 4
- 239000002274 desiccant Substances 0.000 claims description 4
- 239000012074 organic phase Substances 0.000 claims description 4
- KMUONIBRACKNSN-UHFFFAOYSA-N potassium dichromate Chemical compound [K+].[K+].[O-][Cr](=O)(=O)O[Cr]([O-])(=O)=O KMUONIBRACKNSN-UHFFFAOYSA-N 0.000 claims description 4
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical group [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 claims description 3
- BDNKZNFMNDZQMI-UHFFFAOYSA-N 1,3-diisopropylcarbodiimide Chemical compound CC(C)N=C=NC(C)C BDNKZNFMNDZQMI-UHFFFAOYSA-N 0.000 claims description 2
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 claims description 2
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 claims description 2
- 239000004593 Epoxy Substances 0.000 claims description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 claims description 2
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 claims description 2
- 239000004721 Polyphenylene oxide Substances 0.000 claims description 2
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 claims description 2
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 claims description 2
- 239000003513 alkali Substances 0.000 claims description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 claims description 2
- BGRWYRAHAFMIBJ-UHFFFAOYSA-N diisopropylcarbodiimide Natural products CC(C)NC(=O)NC(C)C BGRWYRAHAFMIBJ-UHFFFAOYSA-N 0.000 claims description 2
- 238000001035 drying Methods 0.000 claims description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 2
- 239000012299 nitrogen atmosphere Substances 0.000 claims description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 2
- 229920000728 polyester Polymers 0.000 claims description 2
- 229920000570 polyether Polymers 0.000 claims description 2
- 239000012286 potassium permanganate Substances 0.000 claims description 2
- 238000010992 reflux Methods 0.000 claims description 2
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 claims 1
- 238000006116 polymerization reaction Methods 0.000 abstract description 18
- 238000010521 absorption reaction Methods 0.000 abstract description 11
- 230000009286 beneficial effect Effects 0.000 abstract description 5
- 230000000977 initiatory effect Effects 0.000 abstract description 5
- 229920000642 polymer Polymers 0.000 abstract 1
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 12
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 11
- 229910052753 mercury Inorganic materials 0.000 description 11
- ZDQNWDNMNKSMHI-UHFFFAOYSA-N 1-[2-(2-prop-2-enoyloxypropoxy)propoxy]propan-2-yl prop-2-enoate Chemical compound C=CC(=O)OC(C)COC(C)COCC(C)OC(=O)C=C ZDQNWDNMNKSMHI-UHFFFAOYSA-N 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 239000011342 resin composition Substances 0.000 description 6
- 238000001228 spectrum Methods 0.000 description 6
- 150000003254 radicals Chemical class 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 238000000576 coating method Methods 0.000 description 4
- 230000007613 environmental effect Effects 0.000 description 4
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 3
- 238000005160 1H NMR spectroscopy Methods 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 238000005481 NMR spectroscopy Methods 0.000 description 3
- 238000000862 absorption spectrum Methods 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000005336 cracking Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 239000003999 initiator Substances 0.000 description 3
- 239000010410 layer Substances 0.000 description 3
- IOLCXVTUBQKXJR-UHFFFAOYSA-M potassium bromide Chemical compound [K+].[Br-] IOLCXVTUBQKXJR-UHFFFAOYSA-M 0.000 description 3
- 230000002194 synthesizing effect Effects 0.000 description 3
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 2
- DAKWPKUUDNSNPN-UHFFFAOYSA-N Trimethylolpropane triacrylate Chemical compound C=CC(=O)OCC(CC)(COC(=O)C=C)COC(=O)C=C DAKWPKUUDNSNPN-UHFFFAOYSA-N 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000010146 3D printing Methods 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000004134 energy conservation Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 239000000976 ink Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000012044 organic layer Substances 0.000 description 1
- 125000005254 oxyacyl group Chemical group 0.000 description 1
- 229920002120 photoresistant polymer Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 238000012719 thermal polymerization Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C69/00—Esters of carboxylic acids; Esters of carbonic or haloformic acids
- C07C69/66—Esters of carboxylic acids having esterified carboxylic groups bound to acyclic carbon atoms and having any of the groups OH, O—metal, —CHO, keto, ether, acyloxy, groups, groups, or in the acid moiety
- C07C69/73—Esters of carboxylic acids having esterified carboxylic groups bound to acyclic carbon atoms and having any of the groups OH, O—metal, —CHO, keto, ether, acyloxy, groups, groups, or in the acid moiety of unsaturated acids
- C07C69/738—Esters of keto-carboxylic acids or aldehydo-carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C51/00—Preparation of carboxylic acids or their salts, halides or anhydrides
- C07C51/16—Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/08—Preparation of carboxylic acid esters by reacting carboxylic acids or symmetrical anhydrides with the hydroxy or O-metal group of organic compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F122/00—Homopolymers of compounds having one or more unsaturated aliphatic radicals each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides or nitriles thereof
- C08F122/10—Esters
- C08F122/1006—Esters of polyhydric alcohols or polyhydric phenols, e.g. ethylene glycol dimethacrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2/00—Processes of polymerisation
- C08F2/46—Polymerisation initiated by wave energy or particle radiation
- C08F2/48—Polymerisation initiated by wave energy or particle radiation by ultraviolet or visible light
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/027—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Spectroscopy & Molecular Physics (AREA)
- General Physics & Mathematics (AREA)
- Polymerisation Methods In General (AREA)
Abstract
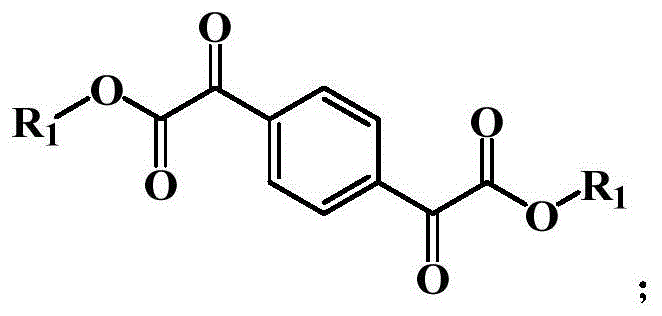
The invention discloses a terephthaloyl formate type photoinitiator suitable for deep photopolymerization of an ultraviolet light emitting diode (UV-LED), which relates to the field of photosensitive polymers and is provided based on the problems that the conventional photoinitiator has poor initiating performance under the irradiation of a UV-LED light source and is limited in application in the field of deep photopolymerization, and the chemical structural general formula of the photoinitiator is as follows:wherein R is1Selected from the group consisting of C1-C16 aliphatic hydrocarbon groups, aralkyl groups, ethers, and aryl groups; the invention also provides a preparation method of the photoinitiator and application of the photoinitiator in a photopolymerization system; the invention has the beneficial effects that: the photoinitiator prepared by the invention has proper absorption capacity in a visible light region, ensures higher photopolymerization efficiency under the action of a UV-LED light source, can be applied to the field of deep polymerization, and is beneficial to the development of UV-LED photopolymerization industry.
Description
Technical Field
The invention belongs to the field of photosensitive high polymer materials, and particularly relates to a terephthaloyl formate photoinitiator suitable for deep photopolymerization of an ultraviolet light emitting diode (UV-LED) and application thereof in the field of photopolymerization.
Background
The photopolymerization technology refers to a technology in which a liquid monomer or oligomer is converted into a solid material under irradiation of light (ultraviolet light, visible light, or infrared light). Compared with the traditional thermal polymerization, the photopolymerization is a green technology and has the advantages of low VOC, high curing speed, energy conservation, environmental protection, low curing temperature and the like. In addition, the photopolymerization technology is widely applied to various fields such as functional coatings, inks, adhesives, photoresists, medical treatment, 3D printing and the like due to its "5E" characteristic, i.e., high Efficiency (Efficiency), wide adaptability (adaptability), economy (economic), Energy Saving (Energy Saving), and Environmental friendliness (Environmental Friendly).
The UV-LED light curing technology appeared in 2008 for the first time, and even now, it is still a hot spot in the field of photo-polymerization, and the advantages of LED light source are as follows: (1) the service life is long, the output energy is concentrated, and the energy conversion efficiency is high; the service life of the LED light source is generally more than 10000h, and in comparison, the service life of the mercury lamp is only about 1000 h. The main peak of the light emitted by the LED light source is narrow and single, and more than 90% of the light output is concentrated in the range of 10nm of the main peak. (2) The working temperature is low; the temperature of the lamp body of the LED light source is below 100 ℃, the temperature of the lamp surface is about 60 ℃, the surface temperature of the lamp body of the mercury lamp can reach 600 ℃, and the temperature of the working surface can also reach about 80 ℃. (3) Emitting light instantly; the LED light source can be used immediately after being turned on, preheating is not needed, and the service life is not influenced by the switching times. The mercury lamp needs to be preheated for 3-5 min, and can be restarted after being cooled for 5-10 min after being turned off, and the service life is influenced by the switching times. (4) The output voltage is low, and the power is adjustable; the LED light source is superior to mercury lamp in light emitting strength, uniformity and stability. And the output power of the LED light source can be adjusted through current, but the mercury lamp cannot be adjusted. (5) No mercury pollution and no ozone generation; mercury is a harmful heavy metal, seriously affects the ecological environment and human health, and inevitably causes mercury pollution by using mercury lamps. The LED light source does not use mercury, the environmental protection effect is obvious, and no ozone is generated, so that the way that the LED light source replaces a mercury lamp light source to be used in the field of photopolymerization is created.
The radiation wavelength of the LED light source is generally above 385nm, and the common wavelengths include 395nm, 405nm, 455nm, and the like. The absorption wavelength of the traditional ultraviolet initiator is difficult to reach the visible region, so that the traditional ultraviolet initiator cannot be well matched with an LED light source, and the popularization and the application of the UV-LED photopolymerization technology are limited. In addition, along with the red shift of the wavelength of the light source, the absorption wavelength of the photoinitiator is inevitably red shifted, so that the common photoinitiator for UV-LED photopolymerization has good absorption capacity in a visible light region, and the common photoinitiator has color; during deep polymerization, because the UV-LED photoinitiator has strong absorption capacity in a visible light region, light irradiated by a light source can be almost completely absorbed by the photoinitiator on the surface layer of a photopolymerization system, so that the photoinitiator in the polymerization system can not effectively absorb light energy, the deep polymerization is influenced, and the application range of the photoinitiator is limited.
Disclosure of Invention
The invention provides a terephthaloyl formate type photoinitiator suitable for deep photopolymerization of an ultraviolet light emitting diode (UV-LED). The photoinitiator can be well matched with common UV-LED light sources (with the emission wavelengths of 385nm, 395nm and 405nm) for use, has good initiation capability, and has weaker absorption capability in a visible light region compared with commercial UV-LED type photoinitiators, so that the photoinitiator has the capability of being applied to the deep polymerization field. The photoinitiator has simple synthesis process and great advantages in preparation.
In order to achieve the purpose, the invention adopts the following technical scheme:
1. a terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization is characterized in that: the chemical structural general formula of the photoinitiator is shown as follows:
wherein R is1Selected from the group consisting of C1-C16 aliphatic hydrocarbon groups, aralkyl groups, ethers, and aryl groups.
2. The terephthaloyl formate-type photoinitiator suitable for deep photopolymerization of UV-LED according to item 1, which is characterizedCharacterized in that: r1Selected from methyl, ethyl and phenyl.
3. Method for preparing the terephthaloyl formate type photoinitiator suitable for deep photopolymerization of UV-LED according to item 1 or 2, characterized in that: the general synthesis process is as follows:
4. the method of item 3, wherein: the preparation method of the photoinitiator comprises the following steps:
(1) in the step a, 1, 4-diacetylbenzene and an oxidant are added into a reaction vessel, an appropriate amount of pyridine is added as a solvent, reflux stirring is carried out for 1h at the reaction temperature of 120 ℃, then the temperature is reduced to 90 ℃ for reaction for 4h, and the reaction is carried out in the nitrogen atmosphere; after the reaction is finished, cooling the reaction liquid to room temperature, pouring the reaction liquid into water, filtering to remove black selenium powder, adding a proper amount of dilute hydrochloric acid until the pH value is about 3, extracting a water layer three times by using a proper amount of ethyl acetate, combining organic phases, drying the organic phases by using a drying agent, removing the ethyl acetate by reduced pressure distillation to obtain a crude product, and purifying the crude product by using a column chromatography to obtain an intermediate product A;
(2) in the step b, adding alcohol or phenol, a dehydrating agent and alkali into a reaction container, adding a proper amount of ethyl acetate as a solvent, dissolving an intermediate product A by using ethyl acetate, slowly dropwise adding the intermediate product A into the reaction container, and stirring at 25 ℃ until the dropwise adding is finished; after the dropwise addition, white precipitates were removed by filtration, the solvent was removed by distillation under reduced pressure to obtain a crude product, and then the crude product was purified by column chromatography to obtain a final product.
5. The method of item 4, wherein: in the step a, the oxidant is selected from selenium dioxide, potassium permanganate, potassium dichromate and hydrogen peroxide; the molar ratio of the oxidant to the 1, 4-diacetylbenzene is 1: 3; the concentration of the dilute hydrochloric acid is 1mol L-1(ii) a The drying agent is selected from anhydrous sodium sulfate and anhydrous magnesium sulfate.
6. The method of claim 4, wherein: in the step b, the alcohol is selected from C1-C16 aliphatic hydrocarbon alcohol, aralkyl alcohol and hydroxy ether; the phenol is phenol; the dehydrating agent is selected from dicyclohexylcarbodiimide, diisopropylcarbodiimide and 1- (3-dimethylaminopropyl) -3-ethylcarbodiimide; the base is selected from pyridine, 3-methylpyridine, 2-methylpyridine, 4-dimethylamino pyridine, triethylamine and diethylamine; the molar ratio of intermediate product a, alcohol or phenol, dehydrating agent and base is 1:3:3: 0.05.
7. A radical photopolymerizable composition, characterized by comprising the terephthaloyl formate type photoinitiator suitable for deep photopolymerization of UV-LED according to item 1 or 2; the composition comprises 1% to 5% of said terephthaloyl formate-type photoinitiator and 95% to 99% of a photocurable resin or monomer, based on the total weight of the composition.
8. The composition according to item 7, wherein the photocurable resin is selected from one or more of epoxy (meth) acrylic resin, polyurethane (meth) acrylic resin, polyester (meth) acrylic resin, polyether (meth) acrylic resin, acrylated poly (meth) acrylic resin; the monomer is one or more of monofunctional, difunctional or multifunctional (methyl) acrylate.
9. Use of the terephthaloyl formate-type photoinitiator suitable for UV-LED deep photopolymerization according to item 1 or 2 in deep photopolymerization.
In the following description of the present invention, numerical values in this application are to be considered modified by the word "about", unless expressly stated otherwise. However, the inventors have reported numerical values in the examples as precisely as possible, although such numerical values inevitably include certain errors.
The invention has the beneficial effects that: compared with the traditional photoinitiator, the photoinitiator prepared by the invention can be applied to the field of UV-LED photopolymerization, overcomes the limitation that the common UV-LED photoinitiator is difficult to apply to the field of deep polymerization, and is beneficial to the development of photopolymerization industry.
Drawings
FIG. 1 is a diagram of the photoinitiation mechanism of the photoinitiator provided by the present invention;
FIGS. 2 and 3 are UV absorption spectra of the phthaloyl formate-based photoinitiators prepared in Synthesis examples 1, 2 and 3;
FIGS. 4 and 5 are real-time IR spectra of the polymerization of monomeric trimethylolpropane triacrylate and tripropylene glycol diacrylate initiated by the terephthaloyl formate type photoinitiator prepared in Synthesis example 1, respectively;
FIG. 6 is a graph comparing the terephthaloyl formate-type photoinitiator prepared in Synthesis example 1 with a commercial photoinitiator 819 to initiate deep layer polymerization of monomeric tripropylene glycol diacrylate.
Detailed Description
In order to make the technical solution and advantages of the present invention more apparent, the present invention is further described in detail by the following embodiments in conjunction with the accompanying drawings, which illustrate the present invention in detail, but do not limit the scope of the present invention.
The photoinitiator can perform hydrolysis under the irradiation of a common UV-LED light source (the emission wavelengths are 385nm, 395nm and 405nm) to initiate polymerization, and the mechanism is shown in the attached figure 1: under the irradiation of light, the photoinitiator firstly undergoes a first-step cracking to generate a molecule of terephthaloyl free radical and two molecules of oxyacyl free radical, then the oxyacyl undergoes a second-step cracking to remove a molecule of carbon dioxide to generate a molecule of free radical, and the free radical and the terephthaloyl free radical generated by the first-step cracking can both initiate the monomer to undergo polymerization reaction.
Example 1:
and (3) synthesizing a photoinitiator DM-BD-F, wherein the structural formula of the DM-BD-F is as follows:
(a) adding 1, 4-diphenylethanone (0.649g, 0.004mol), selenium dioxide (1.553g, 0.012mol) and 5mL of pyridine into a 250mL single-neck flask, heating to 120 ℃ under the protection of nitrogen, stirring at constant temperature for 1h, cooling to 90 ℃, and stirring at constant temperature for 4 h. After the reaction is finished, cooling the reaction liquid to room temperature, filtering to remove black precipitates, combining with 50mL of deionized water, and adding 20mL of diluted hydrochloric acid (1mol/L) until Ph is 3; subsequently, the aqueous layer was extracted three times with ethyl acetate (30mL × 3), the organic layers were combined and dried with anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and then the crude product was purified by column chromatography to obtain intermediate BDFA.
(b) Methanol (0.192g, 0.006mol), dicyclohexylcarbodiimide (1.236g, 0.006mol) and 4-dimethylaminopyridine (12.2mg, 0.1mmol) were added to a 100mL single-neck flask, and 30mL ethyl acetate was added as a solvent; BDFA (0.444g, 0.002mol) was dissolved in 30mL ethyl acetate and added dropwise at a rate of 1 drop per second at 25 ℃ to a 250mL single-neck flask with stirring. After the reaction is finished, white precipitate is removed by filtration, the solvent is removed by reduced pressure distillation to obtain a crude product, then the crude product is purified by column chromatography to obtain a final product DM-BD-F, and the structure identification is carried out by nuclear magnetic resonance spectroscopy.
The hydrogen spectrum data of the photoinitiator DM-BD-F are as follows:1H NMR(400MHz,CDCl3,ppm):δ8.11(s,4H),3.94(s,6H).
the carbon spectrum data of the photoinitiator DM-BD-F are as follows:13C NMR(100MHz,CDCl3,ppm):δ190.31,167.79,141.80,135.54,58.43.
example 2:
and (3) synthesizing a photoinitiator DE-BD-F, wherein the structural formula of the DE-BD-F is as follows:
ethanol (0.276g, 0.006mol), dicyclohexylcarbodiimide (1.236g, 0.006mol) and 4-dimethylaminopyridine (12.2mg, 0.1mmol) were added to a 100mL single-neck flask, and 30mL of ethyl acetate was added as a solvent; the intermediate BDFA (0.444g, 0.002mol) synthesized in example 1 was dissolved in 30mL of ethyl acetate and added dropwise at a rate of 1 drop per second at a temperature of 25 ℃ to a 100mL single-neck flask with stirring. After the reaction is finished, white precipitate is removed by filtration, the solvent is removed by reduced pressure distillation to obtain a crude product, then the crude product is purified by column chromatography to obtain a final product DE-BD-F, and the structure identification is carried out by nuclear magnetic resonance spectroscopy.
The hydrogen spectrum data of the photoinitiator DE-BD-F are as follows:1H NMR(400MHz,CDCl3,ppm):δ8.10(s,4H),4.35(dd,J=8.0,8.0Hz,4H),1.39(t,J=7.6,6H).
the carbon spectrum data of the photoinitiator DE-BD-F are as follows:13C NMR(100MHz,CDCl3,ppm):δ185.63,164.15,138.82,130.56,60.85,13.84.
example 3:
and (3) synthesizing a photoinitiator DP-BD-F, wherein the structural formula of the DP-BD-F is as follows:
phenol (0.564g, 0.006mol), dicyclohexylcarbodiimide (1.236g, 0.006mol) and 4-dimethylaminopyridine (12.2mg, 0.1mmol) were added to a 100mL single-neck flask, and 30mL of ethyl acetate was added as a solvent; the intermediate BDFA (0.444g, 0.002mol) synthesized in example 1 was dissolved in 30mL of ethyl acetate and added dropwise at a rate of 1 drop per second at a temperature of 25 ℃ to a 100mL single-neck flask with stirring. After the reaction is finished, white precipitate is removed by filtration, the solvent is removed by reduced pressure distillation to obtain a crude product, then the crude product is purified by column chromatography to obtain a final product DP-BD-F, and the structure identification is carried out by nuclear magnetic resonance spectroscopy.
The hydrogen spectrum data of the photoinitiator DP-BD-F are as follows:1H NMR(400MHz,CDCl3,ppm):δ8.14(s,4H),7.44(t,J=7.9Hz,4H),7.33(m,6H).
the carbon spectrum data of the photoinitiator DP-BD-F are as follows:13C NMR(100MHz,CDCl3,ppm):δ185.63,157.35,151.38,138.82,130.44,129.12,125.56,121.69.
example 4:
example 4 is intended to illustrate the absorption of the terephthaloyl formate type photoinitiator prepared in examples 1-3 at the LED emission wavelength.
50mL of anhydrous acetonitrile solutions of the photoinitiators synthesized in example 1, example 2 and example 3 were prepared at a concentration of 1X 10-5mol L-1. Make itAn ultraviolet spectrophotometer is used for respectively testing the absorption curves of the three different solutions in the wavelength range of 220-500nm, namely the ultraviolet visible absorption spectrum.
The ultraviolet and visible absorption spectra of the three photoinitiators are shown in fig. 2 and fig. 3; from fig. 2 and fig. 3, it can be seen that the maximum absorption wavelength of the three photoinitiators is less than 300nm, but they have certain absorption capacity at about 400nm, i.e. at the emission wavelength of the LED, and the weak absorption capacity not only can endow the initiators with the initiating capacity at the emission wavelength of the LED, but also is beneficial to deep curing.
Examples 5 to 6:
examples 5 to 6 are intended to illustrate that the terephthaloyl formate type photoinitiator prepared in example 1 can effectively initiate polymerization of monomers under irradiation of a UV-LED light source.
1. Disposed photosensitive resin composition
Two acrylate monomers and the terephthaloyl formate photoinitiator prepared in example 1 were selected respectively, and two photosensitive resin compositions were prepared according to the following proportions:
example 5: trimethylolpropane triacrylate (99 parts by mass), photoinitiator (1 part by mass)
Example 6: tripropylene glycol diacrylate (99 parts by mass), photoinitiator (1 part by mass)
2. Test for polymerization Properties
Uniformly stirring the composition in the dark, uniformly coating the composition on a potassium bromide salt sheet by using a capillary tube to form a coating film with the thickness of about 30 mu m, covering another potassium bromide salt sheet, placing the potassium bromide salt sheet in a real-time infrared instrument (Nicolet 5700, model number Nicolet science and technology Co., Ltd., Shenzhen, Lanspectral Rick science and technology Co., Ltd., model number UVEC-4II, light intensity of 100 mW/cm)2) The coating film was exposed to light at a wavelength of 405nm for a period of 200 s.
The test results of the photosensitive resin composition formulated in example 5 and the photosensitive resin composition formulated in example 6 are shown in fig. 4 and fig. 5, respectively. The photoinitiator prepared by the invention can successfully initiate the photopolymerization reaction of the acrylate monomer under the irradiation of the UV-LED light source with the emission wavelength of 405nm, which shows that the photoinitiator has better applicability under the UV-LED photopolymerization system.
Examples 7 to 8:
the effect of the currently commercially available UV-LED photoinitiator 819 and the terephthaloyl formate type photoinitiator prepared in example 1 on initiating deep photopolymerization under the irradiation of a UV-LED light source was determined:
1. two photosensitive resin compositions were prepared in the following proportions:
example 7: tripropylene glycol diacrylate (99 parts by mass), photoinitiator 819(1 part by mass)
Example 8: tripropylene glycol diacrylate (99 parts by mass), photoinitiator DM-BD-F (1 part by mass)
2. Depth of polymerization test
Two kinds of photosensitive resins were injected into a glass tube having a depth of 7.5cm and a diameter of 0.7cm, and irradiated with the bottom of the tube under a 405nm UV-LED light source at a distance of 4cm from the bottom of the glass tube. After 30 seconds the glass tube was inverted and the depth of the polymerized tripropylene glycol diacrylate in the tube was determined. The test results are shown in FIG. 6. Under the same condition, the polymerization depth of the photosensitive resin composition of the photoinitiator prepared by the invention under the irradiation of a 405nm UV-LED light source is 6.6cm, the deep polymerization of the monomer can be smoothly initiated, while the polymerization depth of the commercial photoinitiator 819 is only 0.8cm and is far lower than that of the photoinitiator DM-BD-F, which shows that the photoinitiator has excellent capability of initiating the deep polymerization under a UV-LED photopolymerization system.
The above is only a preferred embodiment of the present invention, and the protection scope of the present invention is not limited to the above examples, and various process schemes having no substantial difference from the concept of the present invention are within the protection scope of the present invention.
Claims (9)
1. A terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization is characterized in that: the chemical structural general formula of the photoinitiator is shown as follows:
wherein R is1Selected from the group consisting of C1-C16 aliphatic hydrocarbon groups, aralkyl groups, ethers, and aryl groups.
2. The terephthaloyl formate-type photoinitiator for deep photopolymerization of UV-LED according to claim 1, wherein: r1Selected from methyl, ethyl and phenyl.
4. the method of claim 3, wherein: the preparation method of the photoinitiator comprises the following steps:
(1) in the step a, 1, 4-diacetylbenzene and an oxidant are added into a reaction vessel, an appropriate amount of pyridine is added as a solvent, reflux stirring is carried out for 1h at the reaction temperature of 120 ℃, then the temperature is reduced to 90 ℃ for reaction for 4h, and the reaction is carried out in the nitrogen atmosphere; after the reaction is finished, cooling the reaction liquid to room temperature, pouring the reaction liquid into water, filtering to remove black selenium powder, adding a proper amount of dilute hydrochloric acid until the pH value is about 3, extracting a water layer three times by using a proper amount of ethyl acetate, combining organic phases, drying the organic phases by using a drying agent, removing the ethyl acetate by reduced pressure distillation to obtain a crude product, and purifying the crude product by using a column chromatography to obtain an intermediate product A;
(2) in the step b, adding alcohol or phenol, a dehydrating agent and alkali into a reaction container, adding a proper amount of ethyl acetate as a solvent, dissolving an intermediate product A by using ethyl acetate, slowly dropwise adding the intermediate product A into the reaction container, and stirring at 25 ℃ until the dropwise adding is finished; after the dropwise addition, white precipitates were removed by filtration, the solvent was removed by distillation under reduced pressure to obtain a crude product, and then the crude product was purified by column chromatography to obtain a final product.
5. The method of claim 4, wherein: in the step a, the oxidant is selected from selenium dioxide, potassium permanganate, potassium dichromate and hydrogen peroxide; the molar ratio of the oxidant to the 1, 4-diacetylbenzene is 1: 3; the concentration of the dilute hydrochloric acid is 1mol L-1(ii) a The drying agent is selected from anhydrous sodium sulfate and anhydrous magnesium sulfate.
6. The method of claim 4, wherein: in the step b, the alcohol is selected from C1-C16 aliphatic hydrocarbon alcohol, aralkyl alcohol and hydroxy ether; the phenol is phenol; the dehydrating agent is selected from dicyclohexylcarbodiimide, diisopropylcarbodiimide and 1- (3-dimethylaminopropyl) -3-ethylcarbodiimide; the base is selected from pyridine, 3-methylpyridine, 2-methylpyridine, 4-dimethylamino pyridine, triethylamine and diethylamine; the molar ratio of intermediate product a, alcohol or phenol, dehydrating agent and base is 1:3:3: 0.05.
7. A free-radical photopolymerizable composition, comprising a terephthaloyl formate-type photoinitiator according to claim 1 or 2 suitable for deep photopolymerization in UV-LEDs; the composition comprises 1% to 5% of said terephthaloyl formate-type photoinitiator and 95% to 99% of a photocurable resin or monomer, based on the total weight of the composition.
8. The composition according to item 7, wherein the photopolymerizable resin is selected from one or more of epoxy (meth) acrylic resin, urethane (meth) acrylic resin, polyester (meth) acrylic resin, polyether (meth) acrylic resin, acrylated poly (meth) acrylic resin; the monomer is one or more of monofunctional, difunctional or multifunctional (methyl) acrylate.
9. Use of the terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization according to claim 1 or 2 in deep photopolymerization.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110195940.0A CN112939779B (en) | 2021-02-22 | 2021-02-22 | Terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110195940.0A CN112939779B (en) | 2021-02-22 | 2021-02-22 | Terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization and preparation method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN112939779A true CN112939779A (en) | 2021-06-11 |
| CN112939779B CN112939779B (en) | 2024-03-26 |
Family
ID=76245079
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110195940.0A Active CN112939779B (en) | 2021-02-22 | 2021-02-22 | Terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112939779B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023169520A1 (en) * | 2022-03-09 | 2023-09-14 | 湖北固润科技股份有限公司 | Use of arylvinyl a-carbonyl acid ester compound as photoinitiator in led photopolymerization and preparation method therefor |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2041423A5 (en) * | 1969-04-23 | 1971-01-29 | Inst Francais Du Petrole | Thermally stable heterocyclic polymers |
| US4038164A (en) * | 1975-09-18 | 1977-07-26 | Stauffer Chemical Company | Photopolymerizable aryl and heterocyclic glyoxylate compositions and process |
| CN111423394A (en) * | 2020-04-28 | 2020-07-17 | 常州大学 | Synthesis method of 1,3, 4-oxadiazole heterocyclic compound |
| CN111574425A (en) * | 2020-05-20 | 2020-08-25 | 北京化工大学常州先进材料研究院 | Novel benzoyl formic acid methyl ester photoinitiator and preparation method thereof |
| CN111574352A (en) * | 2020-05-12 | 2020-08-25 | 北京化工大学常州先进材料研究院 | Long-wavelength carbonyl alcohol photoinitiator and preparation method thereof |
-
2021
- 2021-02-22 CN CN202110195940.0A patent/CN112939779B/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2041423A5 (en) * | 1969-04-23 | 1971-01-29 | Inst Francais Du Petrole | Thermally stable heterocyclic polymers |
| US4038164A (en) * | 1975-09-18 | 1977-07-26 | Stauffer Chemical Company | Photopolymerizable aryl and heterocyclic glyoxylate compositions and process |
| CN111423394A (en) * | 2020-04-28 | 2020-07-17 | 常州大学 | Synthesis method of 1,3, 4-oxadiazole heterocyclic compound |
| CN111574352A (en) * | 2020-05-12 | 2020-08-25 | 北京化工大学常州先进材料研究院 | Long-wavelength carbonyl alcohol photoinitiator and preparation method thereof |
| CN111574425A (en) * | 2020-05-20 | 2020-08-25 | 北京化工大学常州先进材料研究院 | Novel benzoyl formic acid methyl ester photoinitiator and preparation method thereof |
Non-Patent Citations (3)
| Title |
|---|
| ,ISMATOV, D. N.等: "Synthesis and properties of 1, 4-benzenediketo- and 1, 4-benzenemonoketodicarboxylic acids and their derivatives", KHIMIKO-FARMATSEVTICHESKII ZHURNAL, vol. 25, no. 7, pages 56 - 59 * |
| PAUL GAUSS等: "α-Ketoesters as Nonaromatic Photoinitiators for Radical Polymerization of (Meth)acrylates", MACROMOLECULES, vol. 52 * |
| TAKAHITO ITOH等: "Molecular Oxygen Insertion Polymerization into Crystals ofTetrakis(alkoxycarbonyl)quinodimethanes", MACROMOLECULES, vol. 37, pages 8230 - 8238 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023169520A1 (en) * | 2022-03-09 | 2023-09-14 | 湖北固润科技股份有限公司 | Use of arylvinyl a-carbonyl acid ester compound as photoinitiator in led photopolymerization and preparation method therefor |
Also Published As
| Publication number | Publication date |
|---|---|
| CN112939779B (en) | 2024-03-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Karaca et al. | Mechanistic studies of thioxanthone–carbazole as a one-component type II photoinitiator | |
| Balta et al. | Thioxanthone based water-soluble photoinitiators for acrylamide photopolymerization | |
| CN114478436B (en) | Alpha-aminoketone photoinitiator modified by containing polymerizable itaconic acid group, and preparation method and application thereof | |
| JP7059384B2 (en) | Dibutylfluorenyl derivative and its use as a photoinitiator | |
| WO2023169520A1 (en) | Use of arylvinyl a-carbonyl acid ester compound as photoinitiator in led photopolymerization and preparation method therefor | |
| CN112939779B (en) | Terephthaloyl formate type photoinitiator suitable for UV-LED deep photopolymerization and preparation method thereof | |
| CN114891215B (en) | Three-dimensional near-infrared photoinitiator and preparation method and application thereof | |
| CN112321812A (en) | Polyester type composite photoinitiator and preparation method thereof | |
| CN113072440A (en) | Amphiphilic fluorine-containing benzoyl formate photoinitiator suitable for LED photopolymerization and preparation method thereof | |
| CN110950977B (en) | Acylphosphine oxide photoinitiator and synthesis method thereof | |
| CN110894191B (en) | Phenothiazinyl conjugated benzylidene ketone photosensitizer as well as preparation method and application thereof | |
| Xiao et al. | Synthesis and characterization of copolymerizable one‐component type II photoinitiator | |
| CN113651904B (en) | Photopolymerizable single-component thioxanthone photoinitiator | |
| CN113527138B (en) | Para-fluorobenzoyl oxime ester photoinitiator for preparing photochromic material as well as preparation method and application thereof | |
| CN113683714B (en) | Thioether type naphthalimide derivative photoinitiator containing hydrogen donor and suitable for UV-LED aerobic light curing | |
| CN110317346B (en) | Dendritic fluorescein sodium-iodonium salt visible light initiator and preparation method and application thereof | |
| CN114479112B (en) | Dendritic eosin B-iodonium salt visible light initiator and preparation method and application thereof | |
| CN112961165A (en) | Preparation and application of novel carbazole benzopyran compound | |
| CN115819643B (en) | Application of sulfur-containing cyanine molecules as photoinitiator | |
| CN114656418B (en) | (E) -benzo five-membered ring-styryl sulfonium salt derivative and preparation and application thereof | |
| CN114874260B (en) | Photoinitiator and preparation method and application thereof | |
| CN114196226B (en) | Coumarin dye, composite initiator for ultrasonic curing, preparation method and application thereof | |
| CN113292528B (en) | Thioxanthone photoinitiator with high migration stability, preparation method and application | |
| CN115108959B (en) | Photobleachable visible light initiator containing benzylidene ketone structure, and preparation method and application thereof | |
| CN117683014A (en) | Thioxanthone photoinitiator containing chalcone structure and preparation and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20220929 Address after: 448000 Hubei Jingmen Chemical Recycling Industrial Park (Group 3, Fengmiao Village, Baimiao Sub district Office, Duodao District, Jingmen City) Applicant after: HUBEI GURUN TECHNOLOGY Co.,Ltd. Address before: 100029, No. 15 East Third Ring Road, Chaoyang District, Beijing Applicant before: BEIJING University OF CHEMICAL TECHNOLOGY |
|
| TA01 | Transfer of patent application right | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |