CN112771043B - 取代吡唑稠环类衍生物及其制备方法和应用 - Google Patents
取代吡唑稠环类衍生物及其制备方法和应用 Download PDFInfo
- Publication number
- CN112771043B CN112771043B CN201980064157.1A CN201980064157A CN112771043B CN 112771043 B CN112771043 B CN 112771043B CN 201980064157 A CN201980064157 A CN 201980064157A CN 112771043 B CN112771043 B CN 112771043B
- Authority
- CN
- China
- Prior art keywords
- methyl
- pyridine
- pyridin
- carbonitrile
- pyrazolo
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000002360 preparation method Methods 0.000 title claims abstract description 71
- NOIXNOMHHWGUTG-UHFFFAOYSA-N 2-[[4-[4-pyridin-4-yl-1-(2,2,2-trifluoroethyl)pyrazol-3-yl]phenoxy]methyl]quinoline Chemical compound C=1C=C(OCC=2N=C3C=CC=CC3=CC=2)C=CC=1C1=NN(CC(F)(F)F)C=C1C1=CC=NC=C1 NOIXNOMHHWGUTG-UHFFFAOYSA-N 0.000 title description 4
- 150000001875 compounds Chemical class 0.000 claims abstract description 115
- 150000003839 salts Chemical class 0.000 claims abstract description 58
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 39
- 201000010099 disease Diseases 0.000 claims abstract description 24
- 239000003814 drug Substances 0.000 claims abstract description 22
- 101000579425 Homo sapiens Proto-oncogene tyrosine-protein kinase receptor Ret Proteins 0.000 claims abstract description 8
- 102100028286 Proto-oncogene tyrosine-protein kinase receptor Ret Human genes 0.000 claims abstract description 8
- 230000001404 mediated effect Effects 0.000 claims abstract description 5
- -1 pyrazol-4-yl Chemical class 0.000 claims description 234
- 125000000217 alkyl group Chemical group 0.000 claims description 98
- 206010028980 Neoplasm Diseases 0.000 claims description 61
- 201000011510 cancer Diseases 0.000 claims description 50
- 125000003545 alkoxy group Chemical group 0.000 claims description 46
- 238000000034 method Methods 0.000 claims description 42
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 claims description 34
- 229910052736 halogen Inorganic materials 0.000 claims description 31
- 150000002367 halogens Chemical class 0.000 claims description 31
- 238000011282 treatment Methods 0.000 claims description 28
- 239000008194 pharmaceutical composition Substances 0.000 claims description 26
- 208000002551 irritable bowel syndrome Diseases 0.000 claims description 20
- 208000035475 disorder Diseases 0.000 claims description 15
- 230000000694 effects Effects 0.000 claims description 15
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 claims description 13
- 230000002401 inhibitory effect Effects 0.000 claims description 11
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 11
- 238000004519 manufacturing process Methods 0.000 claims description 10
- 229940124597 therapeutic agent Drugs 0.000 claims description 7
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 6
- 239000003937 drug carrier Substances 0.000 claims description 5
- 206010027476 Metastases Diseases 0.000 claims description 4
- 208000002193 Pain Diseases 0.000 claims description 4
- 229910052731 fluorine Inorganic materials 0.000 claims description 4
- 230000009401 metastasis Effects 0.000 claims description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 4
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 claims description 3
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 3
- 229910052796 boron Inorganic materials 0.000 claims description 3
- 125000004289 pyrazol-3-yl group Chemical class [H]N1N=C(*)C([H])=C1[H] 0.000 claims description 3
- 125000004497 pyrazol-5-yl group Chemical class N1N=CC=C1* 0.000 claims description 3
- 125000003226 pyrazolyl group Chemical class 0.000 claims description 3
- 125000004076 pyridyl group Chemical group 0.000 claims description 3
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 2
- 238000006069 Suzuki reaction reaction Methods 0.000 claims description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims description 2
- 229910052794 bromium Inorganic materials 0.000 claims description 2
- 239000000460 chlorine Substances 0.000 claims description 2
- 229910052801 chlorine Inorganic materials 0.000 claims description 2
- NLFBCYMMUAKCPC-KQQUZDAGSA-N ethyl (e)-3-[3-amino-2-cyano-1-[(e)-3-ethoxy-3-oxoprop-1-enyl]sulfanyl-3-oxoprop-1-enyl]sulfanylprop-2-enoate Chemical compound CCOC(=O)\C=C\SC(=C(C#N)C(N)=O)S\C=C\C(=O)OCC NLFBCYMMUAKCPC-KQQUZDAGSA-N 0.000 claims description 2
- 239000011737 fluorine Substances 0.000 claims description 2
- 230000008569 process Effects 0.000 claims description 2
- 230000003319 supportive effect Effects 0.000 claims description 2
- HTSGKJQDMSTCGS-UHFFFAOYSA-N 1,4-bis(4-chlorophenyl)-2-(4-methylphenyl)sulfonylbutane-1,4-dione Chemical compound C1=CC(C)=CC=C1S(=O)(=O)C(C(=O)C=1C=CC(Cl)=CC=1)CC(=O)C1=CC=C(Cl)C=C1 HTSGKJQDMSTCGS-UHFFFAOYSA-N 0.000 claims 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims 1
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 abstract description 15
- 229940079593 drug Drugs 0.000 abstract description 2
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 66
- 125000003118 aryl group Chemical group 0.000 description 54
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 50
- 238000006243 chemical reaction Methods 0.000 description 44
- GZPHSAQLYPIAIN-UHFFFAOYSA-N 3-pyridinecarbonitrile Chemical compound N#CC1=CC=CN=C1 GZPHSAQLYPIAIN-UHFFFAOYSA-N 0.000 description 43
- 125000000623 heterocyclic group Chemical group 0.000 description 42
- 125000000392 cycloalkenyl group Chemical group 0.000 description 40
- 150000003254 radicals Chemical class 0.000 description 39
- 230000002829 reductive effect Effects 0.000 description 37
- 239000000203 mixture Substances 0.000 description 36
- 239000012453 solvate Substances 0.000 description 35
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 33
- 239000000047 product Substances 0.000 description 31
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 30
- 125000004432 carbon atom Chemical group C* 0.000 description 29
- 230000000155 isotopic effect Effects 0.000 description 29
- 238000004440 column chromatography Methods 0.000 description 27
- 239000000243 solution Substances 0.000 description 27
- 125000006714 (C3-C10) heterocyclyl group Chemical group 0.000 description 26
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 26
- FEPXZFGQVDIXMZ-UHFFFAOYSA-N 5-fluoropyridine-3-carbaldehyde Chemical compound FC1=CN=CC(C=O)=C1 FEPXZFGQVDIXMZ-UHFFFAOYSA-N 0.000 description 25
- 125000000753 cycloalkyl group Chemical group 0.000 description 25
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 24
- 125000004429 atom Chemical group 0.000 description 24
- 125000001072 heteroaryl group Chemical group 0.000 description 24
- 239000012074 organic phase Substances 0.000 description 24
- 229910052799 carbon Inorganic materials 0.000 description 23
- 125000005133 alkynyloxy group Chemical group 0.000 description 21
- 125000004465 cycloalkenyloxy group Chemical group 0.000 description 21
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 description 19
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 description 18
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 description 18
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical class ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 18
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- 125000000304 alkynyl group Chemical group 0.000 description 18
- 125000005844 heterocyclyloxy group Chemical group 0.000 description 18
- 125000003342 alkenyl group Chemical group 0.000 description 17
- 125000004104 aryloxy group Chemical group 0.000 description 17
- 125000005553 heteroaryloxy group Chemical group 0.000 description 17
- 125000001424 substituent group Chemical group 0.000 description 17
- 229910052739 hydrogen Inorganic materials 0.000 description 16
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 16
- 125000004307 pyrazin-2-yl group Chemical group [H]C1=C([H])N=C(*)C([H])=N1 0.000 description 16
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 14
- 229910052757 nitrogen Inorganic materials 0.000 description 14
- 125000004043 oxo group Chemical group O=* 0.000 description 14
- 238000006467 substitution reaction Methods 0.000 description 14
- 210000004027 cell Anatomy 0.000 description 13
- 125000004122 cyclic group Chemical group 0.000 description 13
- 125000000000 cycloalkoxy group Chemical group 0.000 description 13
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 12
- 125000003320 C2-C6 alkenyloxy group Chemical group 0.000 description 11
- 125000003302 alkenyloxy group Chemical group 0.000 description 11
- 238000000605 extraction Methods 0.000 description 11
- FWJMGAPEKMKBIO-UHFFFAOYSA-N FC(C(=O)O)(F)F.N1=CC(=C2N1C=CC=C2)C#N Chemical compound FC(C(=O)O)(F)F.N1=CC(=C2N1C=CC=C2)C#N FWJMGAPEKMKBIO-UHFFFAOYSA-N 0.000 description 10
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical class CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 10
- 229910052760 oxygen Inorganic materials 0.000 description 10
- QHYZFTNTVXUBOP-UHFFFAOYSA-N pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound C1=CC=CC2=C(C#N)C=NN21 QHYZFTNTVXUBOP-UHFFFAOYSA-N 0.000 description 10
- 239000007787 solid Substances 0.000 description 10
- 238000003756 stirring Methods 0.000 description 10
- 238000012360 testing method Methods 0.000 description 10
- 125000006376 (C3-C10) cycloalkyl group Chemical group 0.000 description 9
- 201000009030 Carcinoma Diseases 0.000 description 9
- 108091000080 Phosphotransferase Proteins 0.000 description 9
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical class C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 9
- 125000002619 bicyclic group Chemical group 0.000 description 9
- HVTICUPFWKNHNG-UHFFFAOYSA-N iodoethane Chemical compound CCI HVTICUPFWKNHNG-UHFFFAOYSA-N 0.000 description 9
- 102000020233 phosphotransferase Human genes 0.000 description 9
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical class [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 8
- 239000004480 active ingredient Substances 0.000 description 8
- 239000002246 antineoplastic agent Substances 0.000 description 8
- 230000004927 fusion Effects 0.000 description 8
- 150000002430 hydrocarbons Chemical group 0.000 description 8
- 229910052740 iodine Inorganic materials 0.000 description 8
- 125000004433 nitrogen atom Chemical group N* 0.000 description 8
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 8
- 108090000623 proteins and genes Proteins 0.000 description 8
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 7
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 7
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 229940041181 antineoplastic drug Drugs 0.000 description 7
- 125000005842 heteroatom Chemical group 0.000 description 7
- 230000005764 inhibitory process Effects 0.000 description 7
- 125000002950 monocyclic group Chemical group 0.000 description 7
- 238000005406 washing Methods 0.000 description 7
- 125000006708 (C5-C14) heteroaryl group Chemical group 0.000 description 6
- IZBHWLYKWKYMOY-UHFFFAOYSA-N 5-chloro-6-methoxypyridine-3-carbaldehyde Chemical compound COC1=NC=C(C=O)C=C1Cl IZBHWLYKWKYMOY-UHFFFAOYSA-N 0.000 description 6
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 6
- 206010009944 Colon cancer Diseases 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 6
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 6
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 6
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 6
- 101150077555 Ret gene Proteins 0.000 description 6
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 239000005457 ice water Substances 0.000 description 6
- 208000023356 medullary thyroid gland carcinoma Diseases 0.000 description 6
- 238000002156 mixing Methods 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 239000012279 sodium borohydride Substances 0.000 description 6
- 229910000033 sodium borohydride Inorganic materials 0.000 description 6
- 229910052717 sulfur Inorganic materials 0.000 description 6
- 208000024891 symptom Diseases 0.000 description 6
- 206010006187 Breast cancer Diseases 0.000 description 5
- 208000026310 Breast neoplasm Diseases 0.000 description 5
- 239000004215 Carbon black (E152) Substances 0.000 description 5
- 206010033701 Papillary thyroid cancer Diseases 0.000 description 5
- 206010035226 Plasma cell myeloma Diseases 0.000 description 5
- 208000006265 Renal cell carcinoma Diseases 0.000 description 5
- 239000002552 dosage form Substances 0.000 description 5
- 229930195733 hydrocarbon Natural products 0.000 description 5
- 239000003112 inhibitor Substances 0.000 description 5
- 201000005202 lung cancer Diseases 0.000 description 5
- 208000020816 lung neoplasm Diseases 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 5
- 230000007170 pathology Effects 0.000 description 5
- 230000037361 pathway Effects 0.000 description 5
- 125000004193 piperazinyl group Chemical group 0.000 description 5
- 239000013641 positive control Substances 0.000 description 5
- 229920006395 saturated elastomer Polymers 0.000 description 5
- 239000003826 tablet Substances 0.000 description 5
- 208000030045 thyroid gland papillary carcinoma Diseases 0.000 description 5
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 4
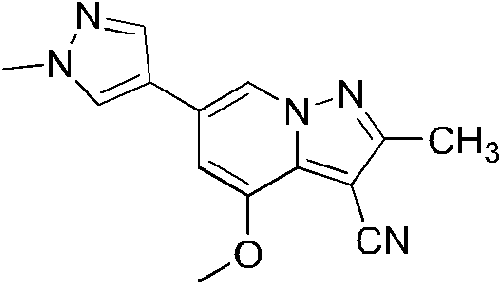
- XBPKVHDCBXBKPB-UHFFFAOYSA-N 2-amino-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)OC)=C1C#N XBPKVHDCBXBKPB-UHFFFAOYSA-N 0.000 description 4
- CTAIEPPAOULMFY-UHFFFAOYSA-N 6-methoxypyridine-3-carbaldehyde Chemical group COC1=CC=C(C=O)C=N1 CTAIEPPAOULMFY-UHFFFAOYSA-N 0.000 description 4
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- 208000014767 Myeloproliferative disease Diseases 0.000 description 4
- 102000001253 Protein Kinase Human genes 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- 208000024770 Thyroid neoplasm Diseases 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 238000002512 chemotherapy Methods 0.000 description 4
- 150000001924 cycloalkanes Chemical class 0.000 description 4
- 208000015799 differentiated thyroid carcinoma Diseases 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 4
- 239000013642 negative control Substances 0.000 description 4
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 4
- 238000007911 parenteral administration Methods 0.000 description 4
- 239000006187 pill Substances 0.000 description 4
- 125000003386 piperidinyl group Chemical group 0.000 description 4
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 4
- 229910000027 potassium carbonate Inorganic materials 0.000 description 4
- 108060006633 protein kinase Proteins 0.000 description 4
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 4
- 102200006099 rs79658334 Human genes 0.000 description 4
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- 201000002510 thyroid cancer Diseases 0.000 description 4
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 4
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 4
- 125000006717 (C3-C10) cycloalkenyl group Chemical group 0.000 description 3
- CFQHDQFLAIUJDP-UHFFFAOYSA-N 1-(5-bromopyridin-2-yl)-4-[(5-fluoropyridin-3-yl)methyl]piperazine Chemical compound BrC=1C=CC(=NC1)N1CCN(CC1)CC=1C=NC=C(C1)F CFQHDQFLAIUJDP-UHFFFAOYSA-N 0.000 description 3
- KXLQQJNAIGTCKE-UHFFFAOYSA-N 2-(piperidin-4-ylmethyl)pyridine;hydrochloride Chemical compound Cl.C=1C=CC=NC=1CC1CCNCC1 KXLQQJNAIGTCKE-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 3
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 3
- 206010008342 Cervix carcinoma Diseases 0.000 description 3
- 206010012735 Diarrhoea Diseases 0.000 description 3
- 208000001976 Endocrine Gland Neoplasms Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 208000018522 Gastrointestinal disease Diseases 0.000 description 3
- 208000017604 Hodgkin disease Diseases 0.000 description 3
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 3
- 206010025323 Lymphomas Diseases 0.000 description 3
- 208000009018 Medullary thyroid cancer Diseases 0.000 description 3
- 208000037196 Medullary thyroid carcinoma Diseases 0.000 description 3
- 208000034578 Multiple myelomas Diseases 0.000 description 3
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 3
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 3
- 206010041067 Small cell lung cancer Diseases 0.000 description 3
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 3
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 150000003863 ammonium salts Chemical class 0.000 description 3
- 125000005605 benzo group Chemical group 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 201000010881 cervical cancer Diseases 0.000 description 3
- 208000006990 cholangiocarcinoma Diseases 0.000 description 3
- 201000010902 chronic myelomonocytic leukemia Diseases 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 201000011523 endocrine gland cancer Diseases 0.000 description 3
- HKSZLNNOFSGOKW-UHFFFAOYSA-N ent-staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(C)O1 HKSZLNNOFSGOKW-UHFFFAOYSA-N 0.000 description 3
- 239000012065 filter cake Substances 0.000 description 3
- 108020001507 fusion proteins Proteins 0.000 description 3
- 102000037865 fusion proteins Human genes 0.000 description 3
- 230000002496 gastric effect Effects 0.000 description 3
- 201000005787 hematologic cancer Diseases 0.000 description 3
- 230000002489 hematologic effect Effects 0.000 description 3
- 238000002868 homogeneous time resolved fluorescence Methods 0.000 description 3
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 3
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 3
- 230000000366 juvenile effect Effects 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 3
- 201000005249 lung adenocarcinoma Diseases 0.000 description 3
- PQIOSYKVBBWRRI-UHFFFAOYSA-N methylphosphonyl difluoride Chemical group CP(F)(F)=O PQIOSYKVBBWRRI-UHFFFAOYSA-N 0.000 description 3
- 229940124303 multikinase inhibitor Drugs 0.000 description 3
- 206010051747 multiple endocrine neoplasia Diseases 0.000 description 3
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 3
- 229910052763 palladium Inorganic materials 0.000 description 3
- 208000028591 pheochromocytoma Diseases 0.000 description 3
- 235000018102 proteins Nutrition 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 125000006413 ring segment Chemical group 0.000 description 3
- 208000000587 small cell lung carcinoma Diseases 0.000 description 3
- 229910000029 sodium carbonate Inorganic materials 0.000 description 3
- HKSZLNNOFSGOKW-FYTWVXJKSA-N staurosporine Chemical compound C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1[C@H]1C[C@@H](NC)[C@@H](OC)[C@]4(C)O1 HKSZLNNOFSGOKW-FYTWVXJKSA-N 0.000 description 3
- CGPUWJWCVCFERF-UHFFFAOYSA-N staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(OC)O1 CGPUWJWCVCFERF-UHFFFAOYSA-N 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- QKXGIQCDHIQVRD-UHFFFAOYSA-N tert-butyl 4-(pyridin-2-ylmethyl)piperidine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)CCC1CC1=CC=CC=N1 QKXGIQCDHIQVRD-UHFFFAOYSA-N 0.000 description 3
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 3
- USFPINLPPFWTJW-UHFFFAOYSA-N tetraphenylphosphonium Chemical compound C1=CC=CC=C1[P+](C=1C=CC=CC=1)(C=1C=CC=CC=1)C1=CC=CC=C1 USFPINLPPFWTJW-UHFFFAOYSA-N 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 2
- DKNUPRMJNUQNHR-UHFFFAOYSA-N 1-[3-(6,7-dimethoxyquinazolin-4-yl)oxyphenyl]-3-[5-(1,1,1-trifluoro-2-methylpropan-2-yl)-1,2-oxazol-3-yl]urea Chemical compound C=12C=C(OC)C(OC)=CC2=NC=NC=1OC(C=1)=CC=CC=1NC(=O)NC=1C=C(C(C)(C)C(F)(F)F)ON=1 DKNUPRMJNUQNHR-UHFFFAOYSA-N 0.000 description 2
- ZHXUWDPHUQHFOV-UHFFFAOYSA-N 2,5-dibromopyridine Chemical compound BrC1=CC=C(Br)N=C1 ZHXUWDPHUQHFOV-UHFFFAOYSA-N 0.000 description 2
- SOWRUJSGHKNOKN-UHFFFAOYSA-N 2,6-difluorobenzaldehyde Chemical group FC1=CC=CC(F)=C1C=O SOWRUJSGHKNOKN-UHFFFAOYSA-N 0.000 description 2
- GNZVAIZIJBXHHD-UHFFFAOYSA-N 2-(5-fluoropyridin-2-yl)acetic acid Chemical group OC(=O)CC1=CC=C(F)C=N1 GNZVAIZIJBXHHD-UHFFFAOYSA-N 0.000 description 2
- HYYYQRNOUNUZOE-UHFFFAOYSA-N 2-(dimethylamino)-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound CN(C1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)OC)=C1C#N)C HYYYQRNOUNUZOE-UHFFFAOYSA-N 0.000 description 2
- HYVYENSUJSOJGI-UHFFFAOYSA-N 2-amino-4-[6-(4-benzylpiperazin-1-yl)pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2CCN(CC2)CC2=CC=CC=C2)=C1C#N HYVYENSUJSOJGI-UHFFFAOYSA-N 0.000 description 2
- QSOMULQDLVRVOE-UHFFFAOYSA-N 2-amino-4-[6-[4-(2,6-difluorobenzoyl)piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2CCN(CC2)C(C2=C(C=CC=C2F)F)=O)=C1C#N QSOMULQDLVRVOE-UHFFFAOYSA-N 0.000 description 2
- USFZMYXVNLETJU-UHFFFAOYSA-N 2-amino-4-[6-[4-[(2,6-difluorophenyl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=C(C=CC=C2F)F)=C1C#N USFZMYXVNLETJU-UHFFFAOYSA-N 0.000 description 2
- DZMOGPUNEMINDL-UHFFFAOYSA-N 2-amino-4-[6-[4-[(2-chlorophenyl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=C(C=CC=C2)Cl)=C1C#N DZMOGPUNEMINDL-UHFFFAOYSA-N 0.000 description 2
- IPVIHBHKRCGTDK-UHFFFAOYSA-N 2-amino-4-[6-[4-[(3,4-difluorophenyl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=CC(=C(C=C2)F)F)=C1C#N IPVIHBHKRCGTDK-UHFFFAOYSA-N 0.000 description 2
- VXDDRSPLNRSJDY-UHFFFAOYSA-N 2-amino-4-[6-[4-[(3-methoxyphenyl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=CC(=CC=C2)OC)=C1C#N VXDDRSPLNRSJDY-UHFFFAOYSA-N 0.000 description 2
- DJNKVEOPFVYQQU-UHFFFAOYSA-N 2-amino-4-[6-[4-[(4-fluorophenyl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=CC=C(C=C2)F)=C1C#N DJNKVEOPFVYQQU-UHFFFAOYSA-N 0.000 description 2
- BMRSKVANRWXSHX-UHFFFAOYSA-N 2-amino-4-[6-[4-[(5-methoxypyridin-2-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=NC=C(C=C2)OC)=C1C#N BMRSKVANRWXSHX-UHFFFAOYSA-N 0.000 description 2
- GJYZXKJTDCRCMF-UHFFFAOYSA-N 2-amino-4-[6-[4-[(6-methoxypyridin-2-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=NC(=CC=C2)OC)=C1C#N GJYZXKJTDCRCMF-UHFFFAOYSA-N 0.000 description 2
- YWWXLHCQWAYSFC-LJQANCHMSA-N 2-amino-6-(1-methylpyrazol-4-yl)-4-[6-[(3R)-3-methyl-4-(pyridin-2-ylmethyl)piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2C[C@H](N(CC2)CC2=NC=CC=C2)C)=C1C#N YWWXLHCQWAYSFC-LJQANCHMSA-N 0.000 description 2
- ROTGNGHPGQHMSS-UHFFFAOYSA-N 2-amino-6-(1-methylpyrazol-4-yl)-4-[6-[4-(pyrazin-2-ylmethyl)piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=NC=CN=C2)=C1C#N ROTGNGHPGQHMSS-UHFFFAOYSA-N 0.000 description 2
- HUXUDIITYRSESD-UHFFFAOYSA-N 2-amino-6-(1-methylpyrazol-4-yl)-4-[6-[4-(pyridin-3-ylmethyl)piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC=CC=2)=C1C#N HUXUDIITYRSESD-UHFFFAOYSA-N 0.000 description 2
- DCLMGIVBMKZDDP-UHFFFAOYSA-N 2-amino-6-(1-methylpyrazol-4-yl)-4-[6-[4-(pyridin-4-ylmethyl)piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC2=CC=NC=C2)=C1C#N DCLMGIVBMKZDDP-UHFFFAOYSA-N 0.000 description 2
- SWEDPSIASDDSGN-UHFFFAOYSA-N 2-amino-6-(2-methoxyethoxy)-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCCOC)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)OC)=C1C#N SWEDPSIASDDSGN-UHFFFAOYSA-N 0.000 description 2
- VRARFYMNVRWWMQ-UHFFFAOYSA-N 2-amino-6-ethoxy-4-[6-[2-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC)C=2C=NC(=CC=2)N2CCNCC2CC=2C=NC(=CC=2)OC)=C1C#N VRARFYMNVRWWMQ-UHFFFAOYSA-N 0.000 description 2
- XUBNCGHJIIMBKA-UHFFFAOYSA-N 2-fluoro-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound FC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)OC)=C1C#N XUBNCGHJIIMBKA-UHFFFAOYSA-N 0.000 description 2
- HGZNNOCVLOVXAO-UHFFFAOYSA-N 2-fluoro-6-(2-methoxyethoxy)-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound FC1=NN2C(C(=CC(=C2)OCCOC)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)OC)=C1C#N HGZNNOCVLOVXAO-UHFFFAOYSA-N 0.000 description 2
- CSDSSGBPEUDDEE-UHFFFAOYSA-N 2-formylpyridine Chemical group O=CC1=CC=CC=N1 CSDSSGBPEUDDEE-UHFFFAOYSA-N 0.000 description 2
- MQKOKBGOHMADAD-UHFFFAOYSA-N 3-(5-bromopyridin-2-yl)-6-(pyridin-2-ylmethyl)-3,6-diazabicyclo[3.1.1]heptane Chemical compound BrC=1C=CC(=NC=1)N1CC2N(C(C1)C2)CC1=NC=CC=C1 MQKOKBGOHMADAD-UHFFFAOYSA-N 0.000 description 2
- RHIUSYHOAJBWGA-UHFFFAOYSA-M 3-bromo-5-methoxypyridin-1-ium-1-amine;2,4,6-trimethylbenzenesulfonate Chemical compound COC1=CC(Br)=C[N+](N)=C1.CC1=CC(C)=C(S([O-])(=O)=O)C(C)=C1 RHIUSYHOAJBWGA-UHFFFAOYSA-M 0.000 description 2
- WMPDAIZRQDCGFH-UHFFFAOYSA-N 3-methoxybenzaldehyde Chemical group COC1=CC=CC(C=O)=C1 WMPDAIZRQDCGFH-UHFFFAOYSA-N 0.000 description 2
- LWDINILXCWWMOH-UHFFFAOYSA-N 4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]-2-(methylamino)-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound CNC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)OC)=C1C#N LWDINILXCWWMOH-UHFFFAOYSA-N 0.000 description 2
- QLKCTQTUEKQDQN-UHFFFAOYSA-N 4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)-2-oxo-1H-pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound OC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)OC)=C1C#N QLKCTQTUEKQDQN-UHFFFAOYSA-N 0.000 description 2
- TYTONLPACKXIAS-UHFFFAOYSA-N 5-bromo-2-[4-(pyridin-2-ylmethyl)piperidin-1-yl]pyridine Chemical compound BrC=1C=CC(=NC1)N1CCC(CC1)CC1=NC=CC=C1 TYTONLPACKXIAS-UHFFFAOYSA-N 0.000 description 2
- BCELHNLIYYAOLV-UHFFFAOYSA-N 5-chloropyridine-3-carbaldehyde Chemical compound ClC1=CN=CC(C=O)=C1 BCELHNLIYYAOLV-UHFFFAOYSA-N 0.000 description 2
- GUXUSCSUCCMCGK-UHFFFAOYSA-N 6-ethoxy-2-fluoro-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound C(C)OC=1C=C(C=2N(C=1)N=C(C=2C#N)F)C=1C=NC(=CC=1)N1CCN(CC1)CC=1C=NC(=CC=1)OC GUXUSCSUCCMCGK-UHFFFAOYSA-N 0.000 description 2
- YDNWTNODZDSPNZ-UHFFFAOYSA-N 6-methoxypyridine-2-carbaldehyde Chemical compound COC1=CC=CC(C=O)=N1 YDNWTNODZDSPNZ-UHFFFAOYSA-N 0.000 description 2
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 2
- 208000036762 Acute promyelocytic leukaemia Diseases 0.000 description 2
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 2
- 206010073478 Anaplastic large-cell lymphoma Diseases 0.000 description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 2
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 2
- 208000005623 Carcinogenesis Diseases 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- XBLVHTDFJBKJLG-UHFFFAOYSA-N Ethyl nicotinate Chemical compound CCOC(=O)C1=CC=CN=C1 XBLVHTDFJBKJLG-UHFFFAOYSA-N 0.000 description 2
- 102000034615 Glial cell line-derived neurotrophic factor Human genes 0.000 description 2
- 108091010837 Glial cell line-derived neurotrophic factor Proteins 0.000 description 2
- 102100035108 High affinity nerve growth factor receptor Human genes 0.000 description 2
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- 208000032004 Large-Cell Anaplastic Lymphoma Diseases 0.000 description 2
- 206010061523 Lip and/or oral cavity cancer Diseases 0.000 description 2
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 206010073148 Multiple endocrine neoplasia type 2A Diseases 0.000 description 2
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 2
- 208000033833 Myelomonocytic Chronic Leukemia Diseases 0.000 description 2
- 201000007224 Myeloproliferative neoplasm Diseases 0.000 description 2
- 206010029260 Neuroblastoma Diseases 0.000 description 2
- 208000033755 Neutrophilic Chronic Leukemia Diseases 0.000 description 2
- DFPAKSUCGFBDDF-UHFFFAOYSA-N Nicotinamide Chemical compound NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- 208000033826 Promyelocytic Acute Leukemia Diseases 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 108700020978 Proto-Oncogene Proteins 0.000 description 2
- 102000052575 Proto-Oncogene Human genes 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 108010017324 STAT3 Transcription Factor Proteins 0.000 description 2
- 206010039491 Sarcoma Diseases 0.000 description 2
- 102100024040 Signal transducer and activator of transcription 3 Human genes 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 208000017733 acquired polycythemia vera Diseases 0.000 description 2
- 208000036676 acute undifferentiated leukemia Diseases 0.000 description 2
- 208000009956 adenocarcinoma Diseases 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 125000005090 alkenylcarbonyl group Chemical group 0.000 description 2
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- CHKQALUEEULCPZ-UHFFFAOYSA-N amino 2,4,6-trimethylbenzenesulfonate Chemical compound CC1=CC(C)=C(S(=O)(=O)ON)C(C)=C1 CHKQALUEEULCPZ-UHFFFAOYSA-N 0.000 description 2
- 229940024606 amino acid Drugs 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 235000011114 ammonium hydroxide Nutrition 0.000 description 2
- 125000005129 aryl carbonyl group Chemical group 0.000 description 2
- 125000002393 azetidinyl group Chemical group 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical class C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 2
- 230000036952 cancer formation Effects 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 231100000504 carcinogenesis Toxicity 0.000 description 2
- 208000002458 carcinoid tumor Diseases 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 201000010903 chronic neutrophilic leukemia Diseases 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 208000029742 colonic neoplasm Diseases 0.000 description 2
- 125000004093 cyano group Chemical group *C#N 0.000 description 2
- NXQGGXCHGDYOHB-UHFFFAOYSA-L cyclopenta-1,4-dien-1-yl(diphenyl)phosphane;dichloropalladium;iron(2+) Chemical compound [Fe+2].Cl[Pd]Cl.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1 NXQGGXCHGDYOHB-UHFFFAOYSA-L 0.000 description 2
- 208000010643 digestive system disease Diseases 0.000 description 2
- 238000006471 dimerization reaction Methods 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000012055 enteric layer Substances 0.000 description 2
- JTBNALXABGMHNR-UHFFFAOYSA-N ethyl 6-bromo-4-methoxy-2-methylpyrazolo[1,5-a]pyridine-3-carboxylate Chemical compound CC1=NN2C(C(=CC(=C2)Br)OC)=C1C(=O)OCC JTBNALXABGMHNR-UHFFFAOYSA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 206010017758 gastric cancer Diseases 0.000 description 2
- 208000018685 gastrointestinal system disease Diseases 0.000 description 2
- 210000004602 germ cell Anatomy 0.000 description 2
- 201000003911 head and neck carcinoma Diseases 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000005223 heteroarylcarbonyl group Chemical group 0.000 description 2
- 125000006517 heterocyclyl carbonyl group Chemical group 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 201000008298 histiocytosis Diseases 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 125000002757 morpholinyl group Chemical group 0.000 description 2
- 201000000050 myeloid neoplasm Diseases 0.000 description 2
- 150000002825 nitriles Chemical class 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 230000002246 oncogenic effect Effects 0.000 description 2
- 201000002528 pancreatic cancer Diseases 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 201000002530 pancreatic endocrine carcinoma Diseases 0.000 description 2
- 201000010279 papillary renal cell carcinoma Diseases 0.000 description 2
- 208000025061 parathyroid hyperplasia Diseases 0.000 description 2
- 229940124531 pharmaceutical excipient Drugs 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- 125000005936 piperidyl group Chemical group 0.000 description 2
- 239000002798 polar solvent Substances 0.000 description 2
- 208000037244 polycythemia vera Diseases 0.000 description 2
- 235000011056 potassium acetate Nutrition 0.000 description 2
- 229910000160 potassium phosphate Inorganic materials 0.000 description 2
- 235000011009 potassium phosphates Nutrition 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 230000002685 pulmonary effect Effects 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 125000004353 pyrazol-1-yl group Chemical group [H]C1=NN(*)C([H])=C1[H] 0.000 description 2
- 125000006514 pyridin-2-ylmethyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 description 2
- QJZUKDFHGGYHMC-UHFFFAOYSA-N pyridine-3-carbaldehyde Chemical compound O=CC1=CC=CN=C1 QJZUKDFHGGYHMC-UHFFFAOYSA-N 0.000 description 2
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 2
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 2
- 230000000306 recurrent effect Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- RLXCSEPIBOWMOJ-MRVPVSSYSA-N tert-butyl (2s)-2-(bromomethyl)morpholine-4-carboxylate Chemical group CC(C)(C)OC(=O)N1CCO[C@H](CBr)C1 RLXCSEPIBOWMOJ-MRVPVSSYSA-N 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 125000001544 thienyl group Chemical group 0.000 description 2
- 208000013818 thyroid gland medullary carcinoma Diseases 0.000 description 2
- 238000002877 time resolved fluorescence resonance energy transfer Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 125000002827 triflate group Chemical group FC(S(=O)(=O)O*)(F)F 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- LSPHULWDVZXLIL-UHFFFAOYSA-N (+/-)-Camphoric acid Chemical class CC1(C)C(C(O)=O)CCC1(C)C(O)=O LSPHULWDVZXLIL-UHFFFAOYSA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- DNISEZBAYYIQFB-PHDIDXHHSA-N (2r,3r)-2,3-diacetyloxybutanedioic acid Chemical compound CC(=O)O[C@@H](C(O)=O)[C@H](C(O)=O)OC(C)=O DNISEZBAYYIQFB-PHDIDXHHSA-N 0.000 description 1
- JUSWZYFYLXTMLJ-JTQLQIEISA-N (2s)-1-(benzenesulfonyl)pyrrolidine-2-carboxylic acid Chemical compound OC(=O)[C@@H]1CCCN1S(=O)(=O)C1=CC=CC=C1 JUSWZYFYLXTMLJ-JTQLQIEISA-N 0.000 description 1
- RQYKQWFHJOBBAO-JTQLQIEISA-N (2s)-1-benzoylpyrrolidine-2-carboxylic acid Chemical compound OC(=O)[C@@H]1CCCN1C(=O)C1=CC=CC=C1 RQYKQWFHJOBBAO-JTQLQIEISA-N 0.000 description 1
- 125000004916 (C1-C6) alkylcarbonyl group Chemical group 0.000 description 1
- 125000006592 (C2-C3) alkenyl group Chemical group 0.000 description 1
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- UWYVPFMHMJIBHE-OWOJBTEDSA-N (e)-2-hydroxybut-2-enedioic acid Chemical class OC(=O)\C=C(\O)C(O)=O UWYVPFMHMJIBHE-OWOJBTEDSA-N 0.000 description 1
- VMFCTZUYOILUMY-UHFFFAOYSA-N 1,1-difluoro-2-iodoethane Chemical compound FC(F)CI VMFCTZUYOILUMY-UHFFFAOYSA-N 0.000 description 1
- 125000005918 1,2-dimethylbutyl group Chemical group 0.000 description 1
- WORJRXHJTUTINR-UHFFFAOYSA-N 1,4-dioxane;hydron;chloride Chemical compound Cl.C1COCCO1 WORJRXHJTUTINR-UHFFFAOYSA-N 0.000 description 1
- MMYKTRPLXXWLBC-UHFFFAOYSA-N 1-bromo-2-ethoxyethane Chemical compound CCOCCBr MMYKTRPLXXWLBC-UHFFFAOYSA-N 0.000 description 1
- NBLDQRVLOJJCCJ-UHFFFAOYSA-N 1-bromo-2-methoxy-2-methylpropane Chemical compound COC(C)(C)CBr NBLDQRVLOJJCCJ-UHFFFAOYSA-N 0.000 description 1
- YZUPZGFPHUVJKC-UHFFFAOYSA-N 1-bromo-2-methoxyethane Chemical compound COCCBr YZUPZGFPHUVJKC-UHFFFAOYSA-N 0.000 description 1
- 125000004972 1-butynyl group Chemical group [H]C([H])([H])C([H])([H])C#C* 0.000 description 1
- 125000006218 1-ethylbutyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- LVYJIIRJQDEGBR-UHFFFAOYSA-N 1-fluoro-2-iodoethane Chemical compound FCCI LVYJIIRJQDEGBR-UHFFFAOYSA-N 0.000 description 1
- QWENRTYMTSOGBR-UHFFFAOYSA-N 1H-1,2,3-Triazole Chemical compound C=1C=NNN=1 QWENRTYMTSOGBR-UHFFFAOYSA-N 0.000 description 1
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Natural products C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 description 1
- GOCCREQJUBABAL-UHFFFAOYSA-N 2,2-dihydroxyacetic acid Chemical compound OC(O)C(O)=O GOCCREQJUBABAL-UHFFFAOYSA-N 0.000 description 1
- LXFQSRIDYRFTJW-UHFFFAOYSA-N 2,4,6-trimethylbenzenesulfonic acid Chemical compound CC1=CC(C)=C(S(O)(=O)=O)C(C)=C1 LXFQSRIDYRFTJW-UHFFFAOYSA-N 0.000 description 1
- PVJZBZSCGJAWNG-UHFFFAOYSA-N 2,4,6-trimethylbenzenesulfonyl chloride Chemical compound CC1=CC(C)=C(S(Cl)(=O)=O)C(C)=C1 PVJZBZSCGJAWNG-UHFFFAOYSA-N 0.000 description 1
- VVVOJODFBWBNBI-UHFFFAOYSA-N 2,5-difluorobenzaldehyde Chemical compound FC1=CC=C(F)C(C=O)=C1 VVVOJODFBWBNBI-UHFFFAOYSA-N 0.000 description 1
- QRHUZEVERIHEPT-UHFFFAOYSA-N 2,6-difluorobenzoyl chloride Chemical compound FC1=CC=CC(F)=C1C(Cl)=O QRHUZEVERIHEPT-UHFFFAOYSA-N 0.000 description 1
- 125000006508 2,6-difluorobenzyl group Chemical group [H]C1=C([H])C(F)=C(C(F)=C1[H])C([H])([H])* 0.000 description 1
- KYKUZTBLYLKPIT-UHFFFAOYSA-N 2-(2-bromoethoxy)propane Chemical compound CC(C)OCCBr KYKUZTBLYLKPIT-UHFFFAOYSA-N 0.000 description 1
- FPZWZCWUIYYYBU-UHFFFAOYSA-N 2-(2-ethoxyethoxy)ethyl acetate Chemical group CCOCCOCCOC(C)=O FPZWZCWUIYYYBU-UHFFFAOYSA-N 0.000 description 1
- SPUABSOFIGYJTJ-UHFFFAOYSA-N 2-(5-bromo-3-chloropyridin-2-yl)acetonitrile Chemical compound ClC1=CC(Br)=CN=C1CC#N SPUABSOFIGYJTJ-UHFFFAOYSA-N 0.000 description 1
- UZYQSNQJLWTICD-UHFFFAOYSA-N 2-(n-benzoylanilino)-2,2-dinitroacetic acid Chemical compound C=1C=CC=CC=1N(C(C(=O)O)([N+]([O-])=O)[N+]([O-])=O)C(=O)C1=CC=CC=C1 UZYQSNQJLWTICD-UHFFFAOYSA-N 0.000 description 1
- RRRGVNWBXDBULK-BBHUYCHWSA-N 2-amino-4-[5-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyrazin-2-yl]-6-[[(2S)-4-methylmorpholin-2-yl]methoxy]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OC[C@@H]2CN(CCO2)C)C2=NC=C(N=C2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N RRRGVNWBXDBULK-BBHUYCHWSA-N 0.000 description 1
- UMHBGTKCPHOKFG-QCXPCDNBSA-N 2-amino-4-[5-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyrazin-2-yl]-6-[[(2S)-morpholin-2-yl]methoxy]pyrazolo[1,5-a]pyridine-3-carbonitrile 2,2,2-trifluoroacetic acid Chemical compound COC1=NC=C(C=C1)CN2C3CC2CN(C3)C4=NC=C(N=C4)C5=CC(=CN6C5=C(C(=N6)N)C#N)OC[C@@H]7CNCCO7.C(=O)(C(F)(F)F)O UMHBGTKCPHOKFG-QCXPCDNBSA-N 0.000 description 1
- WUZVRBHAAKFVBG-UHFFFAOYSA-N 2-amino-4-[6-[4-[2-(5-fluoropyridin-2-yl)acetyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2CCN(CC2)C(CC2=NC=C(C=C2)F)=O)=C1C#N WUZVRBHAAKFVBG-UHFFFAOYSA-N 0.000 description 1
- XUZBFXNVQJVENU-UHFFFAOYSA-N 2-amino-4-[6-[4-[[6-(dimethylamino)pyridin-3-yl]methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C=2)C)C=2C=NC(=CC=2)N2CCN(CC2)CC=2C=NC(=CC=2)N(C)C)=C1C#N XUZBFXNVQJVENU-UHFFFAOYSA-N 0.000 description 1
- CLXQUZAXFIYVBC-UHFFFAOYSA-N 2-amino-4-[6-[6-[(5-chloro-6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]-6-(2-methoxyethoxy)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCCOC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=C(C=2)Cl)OC)=C1C#N CLXQUZAXFIYVBC-UHFFFAOYSA-N 0.000 description 1
- ZOQJGYHOXCCMAG-UHFFFAOYSA-N 2-amino-4-[6-[6-[(5-chloropyridin-2-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]-6-ethoxypyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC2=NC=C(C=C2)Cl)=C1C#N ZOQJGYHOXCCMAG-UHFFFAOYSA-N 0.000 description 1
- FAQAJVYOHZAWFW-UHFFFAOYSA-N 2-amino-4-[6-[6-[(5-chloropyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]-6-ethoxypyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC=C(C=2)Cl)=C1C#N FAQAJVYOHZAWFW-UHFFFAOYSA-N 0.000 description 1
- GGRBIBQQYAZSNL-UHFFFAOYSA-N 2-amino-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]-6-(2-propan-2-yloxyethoxy)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCCOC(C)C)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N GGRBIBQQYAZSNL-UHFFFAOYSA-N 0.000 description 1
- IDXJYPUCFDONAN-UHFFFAOYSA-N 2-amino-4-chloro-6-(2-hydroxy-2-methylpropoxy)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC(C)(C)O)Cl)=C1C#N IDXJYPUCFDONAN-UHFFFAOYSA-N 0.000 description 1
- OTJVVZZQKVALJL-LJQANCHMSA-N 2-amino-6-(1-methylpyrazol-4-yl)-4-[6-[(2R)-2-methyl-4-(pyridin-2-ylmethyl)piperazin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound [C@H]1(C)N(CCN(C1)CC1=CC=CC=N1)C1=NC=C(C2=CC(C=3C=NN(C=3)C)=CN3C2=C(C(=N3)N)C#N)C=C1 OTJVVZZQKVALJL-LJQANCHMSA-N 0.000 description 1
- AQQLTZRIIVLULH-UHFFFAOYSA-N 2-amino-6-(1-methylpyrazol-4-yl)-4-[6-[4-(pyridin-2-ylmethyl)piperidin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2CCC(CC2)CC2=NC=CC=C2)=C1C#N AQQLTZRIIVLULH-UHFFFAOYSA-N 0.000 description 1
- YERKXPOIJRKUPW-UHFFFAOYSA-N 2-amino-6-(1-methylpyrazol-4-yl)-4-[6-[6-(pyridin-2-ylmethyl)-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2CC3N(C(C2)C3)CC3=NC=CC=C3)=C1C#N YERKXPOIJRKUPW-UHFFFAOYSA-N 0.000 description 1
- XEQYSHSXGUNFML-UHFFFAOYSA-N 2-amino-6-(2,2-difluoroethoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC(F)F)C=2C=NC(=CC2)N2CC3N(C(C2)C3)CC=3C=NC(=CC3)OC)=C1C#N XEQYSHSXGUNFML-UHFFFAOYSA-N 0.000 description 1
- AODPIFCEZSGFQI-UHFFFAOYSA-N 2-amino-6-(2-cyclopropyloxyethoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCCOC2CC2)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N AODPIFCEZSGFQI-UHFFFAOYSA-N 0.000 description 1
- GPWQVAHXOBWDAG-UHFFFAOYSA-N 2-amino-6-(2-ethoxyethoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCCOCC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N GPWQVAHXOBWDAG-UHFFFAOYSA-N 0.000 description 1
- SATJMQWCVFWZTF-UHFFFAOYSA-N 2-amino-6-(2-fluoroethoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCCF)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N SATJMQWCVFWZTF-UHFFFAOYSA-N 0.000 description 1
- WJKUYAGCENTUKT-UHFFFAOYSA-N 2-amino-6-(2-methoxy-2-methylpropoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC(C)(C)OC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N WJKUYAGCENTUKT-UHFFFAOYSA-N 0.000 description 1
- BZGFTVCFNYFYFK-UHFFFAOYSA-N 2-amino-6-(2-methoxyethoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCCOC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N BZGFTVCFNYFYFK-UHFFFAOYSA-N 0.000 description 1
- KBQRIXSURAHLDC-UHFFFAOYSA-N 2-amino-6-(difluoromethoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OC(F)F)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N KBQRIXSURAHLDC-UHFFFAOYSA-N 0.000 description 1
- JYSKKDXNSJFTHQ-UHFFFAOYSA-N 2-amino-6-ethoxy-4-[5-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyrazin-2-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC)C2=NC=C(N=C2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N JYSKKDXNSJFTHQ-UHFFFAOYSA-N 0.000 description 1
- UEFFPLMZOGVIHL-UHFFFAOYSA-N 2-amino-6-ethoxy-4-[6-[6-[(5-fluoropyridin-2-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC2=NC=C(C=C2)F)=C1C#N UEFFPLMZOGVIHL-UHFFFAOYSA-N 0.000 description 1
- VEEQOEXOIJIHRH-UHFFFAOYSA-N 2-amino-6-ethoxy-4-[6-[6-[(5-fluoropyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC=2C=NC=C(C=2)F)=C1C#N VEEQOEXOIJIHRH-UHFFFAOYSA-N 0.000 description 1
- XSUQHASHQWFHCS-UHFFFAOYSA-N 2-amino-6-ethoxy-4-[6-[6-[(6-methoxypyridin-2-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound NC1=NN2C(C(=CC(=C2)OCC)C=2C=NC(=CC=2)N2CC3N(C(C2)C3)CC2=NC(=CC=C2)OC)=C1C#N XSUQHASHQWFHCS-UHFFFAOYSA-N 0.000 description 1
- KMGUEILFFWDGFV-UHFFFAOYSA-N 2-benzoyl-2-benzoyloxy-3-hydroxybutanedioic acid Chemical compound C=1C=CC=CC=1C(=O)C(C(C(O)=O)O)(C(O)=O)OC(=O)C1=CC=CC=C1 KMGUEILFFWDGFV-UHFFFAOYSA-N 0.000 description 1
- LDLCZOVUSADOIV-UHFFFAOYSA-N 2-bromoethanol Chemical compound OCCBr LDLCZOVUSADOIV-UHFFFAOYSA-N 0.000 description 1
- IMRWILPUOVGIMU-UHFFFAOYSA-N 2-bromopyridine Chemical compound BrC1=CC=CC=N1 IMRWILPUOVGIMU-UHFFFAOYSA-N 0.000 description 1
- 125000000069 2-butynyl group Chemical group [H]C([H])([H])C#CC([H])([H])* 0.000 description 1
- GQXQZXMCYFHHOO-UHFFFAOYSA-N 2-chloro-5-fluoropyrazine Chemical compound FC1=CN=C(Cl)C=N1 GQXQZXMCYFHHOO-UHFFFAOYSA-N 0.000 description 1
- FPYUJUBAXZAQNL-UHFFFAOYSA-N 2-chlorobenzaldehyde Chemical group ClC1=CC=CC=C1C=O FPYUJUBAXZAQNL-UHFFFAOYSA-N 0.000 description 1
- MTPPJFHIYMURGK-UHFFFAOYSA-N 2-cyclopropyloxyethyl 4-methylbenzenesulfonate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)OCCOC1CC1 MTPPJFHIYMURGK-UHFFFAOYSA-N 0.000 description 1
- 125000006176 2-ethylbutyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(C([H])([H])*)C([H])([H])C([H])([H])[H] 0.000 description 1
- JBLCLEOCTGAZCB-BBHUYCHWSA-N 2-fluoro-4-[5-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyrazin-2-yl]-6-[[(2S)-4-methylmorpholin-2-yl]methoxy]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound FC1=NN2C(C(=CC(=C2)OC[C@H]2OCCN(C2)C)C2=NC=C(N=C2)N2CC3N(C(C2)C3)CC=2C=NC(=CC=2)OC)=C1C#N JBLCLEOCTGAZCB-BBHUYCHWSA-N 0.000 description 1
- RDOILFHCOGJZDJ-AOMIVXANSA-N 2-fluoro-4-[5-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyrazin-2-yl]-6-[[(2S)-morpholin-2-yl]methoxy]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound FC1=NN2C(C(=CC(=C2)OC[C@@H]2CNCCO2)C2=NC=C(N=C2)N2CC3N(C(C2)C3)CC=3C=NC(=CC3)OC)=C1C#N RDOILFHCOGJZDJ-AOMIVXANSA-N 0.000 description 1
- GQVDRNCLLGCFOC-UHFFFAOYSA-N 2-fluoro-6-(2-methoxyethoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound FC1=NN2C(C(=CC(=C2)OCCOC)C=2C=NC(=CC2)N2CC3N(C(C2)C3)CC=3C=NC(=CC3)OC)=C1C#N GQVDRNCLLGCFOC-UHFFFAOYSA-N 0.000 description 1
- ZWDVQMVZZYIAHO-UHFFFAOYSA-N 2-fluorobenzaldehyde Chemical group FC1=CC=CC=C1C=O ZWDVQMVZZYIAHO-UHFFFAOYSA-N 0.000 description 1
- 125000004847 2-fluorobenzyl group Chemical group [H]C1=C([H])C(F)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000004791 2-fluoroethoxy group Chemical group FCCO* 0.000 description 1
- QQLUJYFLORJFTH-UHFFFAOYSA-N 2-methyl-6-(1-methylpyrazol-4-yl)-4-[6-[4-(pyridin-2-ylmethyl)piperidin-1-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound CC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2CCC(CC2)CC2=NC=CC=C2)=C1C#N QQLUJYFLORJFTH-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-M 2-methylbenzenesulfonate Chemical compound CC1=CC=CC=C1S([O-])(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-M 0.000 description 1
- 125000004493 2-methylbut-1-yl group Chemical group CC(C*)CC 0.000 description 1
- 125000005916 2-methylpentyl group Chemical group 0.000 description 1
- BSKHPKMHTQYZBB-UHFFFAOYSA-N 2-methylpyridine Chemical class CC1=CC=CC=N1 BSKHPKMHTQYZBB-UHFFFAOYSA-N 0.000 description 1
- 229940080296 2-naphthalenesulfonate Drugs 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- JPHKMYXKNKLNDF-UHFFFAOYSA-N 3,4-difluorobenzaldehyde Chemical group FC1=CC=C(C=O)C=C1F JPHKMYXKNKLNDF-UHFFFAOYSA-N 0.000 description 1
- GNFTZDOKVXKIBK-UHFFFAOYSA-N 3-(2-methoxyethoxy)benzohydrazide Chemical compound COCCOC1=CC=CC(C(=O)NN)=C1 GNFTZDOKVXKIBK-UHFFFAOYSA-N 0.000 description 1
- GKDWQXUNJXWPTG-UHFFFAOYSA-N 3-(5-bromopyridin-2-yl)-3,6-diazabicyclo[3.1.1]heptane dihydrochloride Chemical compound Cl.Cl.BrC=1C=CC(=NC=1)N1CC2NC(C1)C2 GKDWQXUNJXWPTG-UHFFFAOYSA-N 0.000 description 1
- MFAMQDBLLUVGDP-UHFFFAOYSA-N 3-(5-bromopyridin-2-yl)-6-[(5-chloro-6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptane Chemical compound BrC=1C=CC(=NC=1)N1CC2N(C(C1)C2)CC=1C=NC(=C(C=1)Cl)OC MFAMQDBLLUVGDP-UHFFFAOYSA-N 0.000 description 1
- ZAZHRJCWMGXICW-UHFFFAOYSA-N 3-(5-bromopyridin-2-yl)-6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptane Chemical compound BrC=1C=CC(=NC=1)N1CC2N(C(C1)C2)CC=1C=NC(=CC=1)OC ZAZHRJCWMGXICW-UHFFFAOYSA-N 0.000 description 1
- FMQNZWXNHGGKIG-UHFFFAOYSA-N 3-(5-chloropyrazin-2-yl)-3,6-diazabicyclo[3.1.1]heptane dihydrochloride Chemical compound Cl.Cl.ClC=1N=CC(=NC=1)N1CC2NC(C1)C2 FMQNZWXNHGGKIG-UHFFFAOYSA-N 0.000 description 1
- HIFVEGONPGKERQ-UHFFFAOYSA-N 3-(5-chloropyrazin-2-yl)-6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptane Chemical compound ClC=1N=CC(=NC=1)N1CC2N(C(C1)C2)CC=1C=NC(=CC=1)OC HIFVEGONPGKERQ-UHFFFAOYSA-N 0.000 description 1
- ALKYHXVLJMQRLQ-UHFFFAOYSA-N 3-Hydroxy-2-naphthoate Chemical class C1=CC=C2C=C(O)C(C(=O)O)=CC2=C1 ALKYHXVLJMQRLQ-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- MPIZIIRYHHOHEI-UHFFFAOYSA-N 3-bromo-5-methoxypyridin-1-ium-1-amine Chemical compound COC1=CC(Br)=C[N+](N)=C1 MPIZIIRYHHOHEI-UHFFFAOYSA-N 0.000 description 1
- FZWUIWQMJFAWJW-UHFFFAOYSA-N 3-bromo-5-methoxypyridine Chemical compound COC1=CN=CC(Br)=C1 FZWUIWQMJFAWJW-UHFFFAOYSA-N 0.000 description 1
- NYPYPOZNGOXYSU-UHFFFAOYSA-N 3-bromopyridine Chemical compound BrC1=CC=CN=C1 NYPYPOZNGOXYSU-UHFFFAOYSA-N 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- ZRPLANDPDWYOMZ-UHFFFAOYSA-N 3-cyclopentylpropionic acid Chemical class OC(=O)CCC1CCCC1 ZRPLANDPDWYOMZ-UHFFFAOYSA-N 0.000 description 1
- PIKNVEVCWAAOMJ-UHFFFAOYSA-N 3-fluorobenzaldehyde Chemical group FC1=CC=CC(C=O)=C1 PIKNVEVCWAAOMJ-UHFFFAOYSA-N 0.000 description 1
- 125000006284 3-fluorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(F)=C1[H])C([H])([H])* 0.000 description 1
- BZISNWGGPWSXTK-UHFFFAOYSA-N 3-hydroxypropyl benzoate Chemical compound OCCCOC(=O)C1=CC=CC=C1 BZISNWGGPWSXTK-UHFFFAOYSA-N 0.000 description 1
- 125000003542 3-methylbutan-2-yl group Chemical group [H]C([H])([H])C([H])(*)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000005917 3-methylpentyl group Chemical group 0.000 description 1
- JDYVLWWFVYNMTN-UHFFFAOYSA-N 3-methylpyridine-2-carbaldehyde Chemical group CC1=CC=CN=C1C=O JDYVLWWFVYNMTN-UHFFFAOYSA-N 0.000 description 1
- XMIIGOLPHOKFCH-UHFFFAOYSA-N 3-phenylpropionic acid Chemical class OC(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-N 0.000 description 1
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- BGNGWHSBYQYVRX-UHFFFAOYSA-N 4-(dimethylamino)benzaldehyde Chemical compound CN(C)C1=CC=C(C=O)C=C1 BGNGWHSBYQYVRX-UHFFFAOYSA-N 0.000 description 1
- DUCWXBQSVRRHKK-UHFFFAOYSA-N 4-[5-[2-amino-3-cyano-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridin-4-yl]pyridin-2-yl]-N,N-diethylpiperazine-1-carboxamide Chemical compound NC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=CC(=NC2)N2CCN(CC2)C(=O)N(CC)CC)=C1C#N DUCWXBQSVRRHKK-UHFFFAOYSA-N 0.000 description 1
- BYCQNVNJHGTYHC-UHFFFAOYSA-N 4-[6-[4-[2-(5-fluoropyridin-2-yl)acetyl]piperazin-1-yl]pyridin-3-yl]-2-methyl-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound CC1=NN2C(C(=CC(=C2)C=2C=NN(C2)C)C=2C=NC(=CC2)N2CCN(CC2)C(CC2=NC=C(C=C2)F)=O)=C1C#N BYCQNVNJHGTYHC-UHFFFAOYSA-N 0.000 description 1
- UOQXIWFBQSVDPP-UHFFFAOYSA-N 4-fluorobenzaldehyde Chemical group FC1=CC=C(C=O)C=C1 UOQXIWFBQSVDPP-UHFFFAOYSA-N 0.000 description 1
- UAKMHSRHDUBNJR-UHFFFAOYSA-N 4-methylpyridine-2-carbaldehyde Chemical group CC1=CC=NC(C=O)=C1 UAKMHSRHDUBNJR-UHFFFAOYSA-N 0.000 description 1
- YOVIXSRXKCZJRN-UHFFFAOYSA-N 5-methoxypyridine-3-carbaldehyde Chemical compound COC1=CN=CC(C=O)=C1 YOVIXSRXKCZJRN-UHFFFAOYSA-N 0.000 description 1
- RBYMQCXEHSRTTG-UHFFFAOYSA-N 6-(2-hydroxy-2-methylpropoxy)-2-methoxy-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound OC(COC=1C=C(C=2N(C=1)N=C(C=2C#N)OC)C=1C=NC(=CC=1)N1CC2N(C(C1)C2)CC=1C=NC(=CC=1)OC)(C)C RBYMQCXEHSRTTG-UHFFFAOYSA-N 0.000 description 1
- IRDPUZWWWIDFQX-UHFFFAOYSA-N 6-(dimethylamino)pyridine-3-carbaldehyde Chemical compound CN(C)C1=CC=C(C=O)C=N1 IRDPUZWWWIDFQX-UHFFFAOYSA-N 0.000 description 1
- RMWVNHSTYVTMKD-UHFFFAOYSA-N 6-[6-[(5-chloro-6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]-6-ethoxy-7H-pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound ClC=1C=C(C=NC1OC)CN1C2CN(CC1C2)C2(C=CC=1N(C2)N=CC1C#N)OCC RMWVNHSTYVTMKD-UHFFFAOYSA-N 0.000 description 1
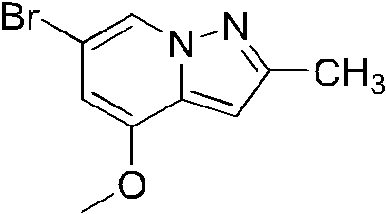
- GQGWGIOHUBDTMW-UHFFFAOYSA-N 6-bromo-4-methoxy-2-methylpyrazolo[1,5-a]pyridine Chemical compound CC1=NN2C(C(=CC(=C2)Br)OC)=C1 GQGWGIOHUBDTMW-UHFFFAOYSA-N 0.000 description 1
- NIPUJJAVHWINFD-UHFFFAOYSA-N 6-bromo-4-methoxy-2-methylpyrazolo[1,5-a]pyridine-3-carbaldehyde Chemical compound CC1=NN2C(C(=CC(=C2)Br)OC)=C1C=O NIPUJJAVHWINFD-UHFFFAOYSA-N 0.000 description 1
- KTSGJTWZMGYZKB-UHFFFAOYSA-N 7-fluoro-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile Chemical compound FC1=C(C=C(C=2N1N=CC2C#N)C=2C=NC(=CC2)N2CCN(CC2)CC=2C=NC(=CC2)OC)C=2C=NN(C2)C KTSGJTWZMGYZKB-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- JBMKAUGHUNFTOL-UHFFFAOYSA-N Aldoclor Chemical class C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NC=NS2(=O)=O JBMKAUGHUNFTOL-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 206010061424 Anal cancer Diseases 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010006143 Brain stem glioma Diseases 0.000 description 1
- 208000011691 Burkitt lymphomas Diseases 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- 125000005914 C6-C14 aryloxy group Chemical group 0.000 description 1
- PHFCAYFTCPEEJP-UHFFFAOYSA-N C=O.FC=1C=NC=CC1 Chemical group C=O.FC=1C=NC=CC1 PHFCAYFTCPEEJP-UHFFFAOYSA-N 0.000 description 1
- NQLFRVRUTMRGLN-UHFFFAOYSA-N C=O.N1=CC(=CC=C1)Cl Chemical group C=O.N1=CC(=CC=C1)Cl NQLFRVRUTMRGLN-UHFFFAOYSA-N 0.000 description 1
- JGLMVXWAHNTPRF-CMDGGOBGSA-N CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O Chemical compound CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O JGLMVXWAHNTPRF-CMDGGOBGSA-N 0.000 description 1
- VGKGSAQORFDKFH-UHFFFAOYSA-N CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC2=C(CN1CCNCC1)C(=CC=C2)F Chemical compound CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC2=C(CN1CCNCC1)C(=CC=C2)F VGKGSAQORFDKFH-UHFFFAOYSA-N 0.000 description 1
- RFPLNZMSWGCOAZ-UHFFFAOYSA-N CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC2=C(CN1CCNCC1)C=C(C=C2)F Chemical compound CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC2=C(CN1CCNCC1)C=C(C=C2)F RFPLNZMSWGCOAZ-UHFFFAOYSA-N 0.000 description 1
- RMZJYFNWBOGPDP-UHFFFAOYSA-N CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC2=C(CN1CCNCC1)C=CC=C2 Chemical compound CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC2=C(CN1CCNCC1)C=CC=C2 RMZJYFNWBOGPDP-UHFFFAOYSA-N 0.000 description 1
- IYJWYMCYQDOLTN-UHFFFAOYSA-N CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC=2C=C(CN1CCNCC1)C=CC2 Chemical compound CN1N=CC(=C1)C=1C=CC=2N(C1)N=CC2C#N.FC=2C=C(CN1CCNCC1)C=CC2 IYJWYMCYQDOLTN-UHFFFAOYSA-N 0.000 description 1
- DRSHXJFUUPIBHX-UHFFFAOYSA-N COc1ccc(cc1)N1N=CC2C=NC(Nc3cc(OC)c(OC)c(OCCCN4CCN(C)CC4)c3)=NC12 Chemical compound COc1ccc(cc1)N1N=CC2C=NC(Nc3cc(OC)c(OC)c(OCCCN4CCN(C)CC4)c3)=NC12 DRSHXJFUUPIBHX-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 206010007275 Carcinoid tumour Diseases 0.000 description 1
- 208000017897 Carcinoma of esophagus Diseases 0.000 description 1
- 229920002284 Cellulose triacetate Polymers 0.000 description 1
- 206010007953 Central nervous system lymphoma Diseases 0.000 description 1
- 101800005151 Cholecystokinin-8 Proteins 0.000 description 1
- 102400000888 Cholecystokinin-8 Human genes 0.000 description 1
- 239000004381 Choline salt Substances 0.000 description 1
- 201000009047 Chordoma Diseases 0.000 description 1
- 208000016718 Chromosome Inversion Diseases 0.000 description 1
- 206010052358 Colorectal cancer metastatic Diseases 0.000 description 1
- 206010010539 Congenital megacolon Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical class C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 206010014967 Ependymoma Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical class CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 1
- 208000006168 Ewing Sarcoma Diseases 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- 102100030708 GTPase KRas Human genes 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 206010064571 Gene mutation Diseases 0.000 description 1
- 208000021309 Germ cell tumor Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 208000004592 Hirschsprung disease Diseases 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000584612 Homo sapiens GTPase KRas Proteins 0.000 description 1
- 101000984753 Homo sapiens Serine/threonine-protein kinase B-raf Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 201000003803 Inflammatory myofibroblastic tumor Diseases 0.000 description 1
- 206010067917 Inflammatory myofibroblastic tumour Diseases 0.000 description 1
- 208000037396 Intraductal Noninfiltrating Carcinoma Diseases 0.000 description 1
- 206010073094 Intraductal proliferative breast lesion Diseases 0.000 description 1
- 206010061252 Intraocular melanoma Diseases 0.000 description 1
- VQTUBCCKSQIDNK-UHFFFAOYSA-N Isobutene Chemical group CC(C)=C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- 102000010638 Kinesin Human genes 0.000 description 1
- 108010063296 Kinesin Proteins 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical class NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 206010024305 Leukaemia monocytic Diseases 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical class CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 206010062038 Lip neoplasm Diseases 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- 102000019149 MAP kinase activity proteins Human genes 0.000 description 1
- 108040008097 MAP kinase activity proteins Proteins 0.000 description 1
- 208000006644 Malignant Fibrous Histiocytoma Diseases 0.000 description 1
- 206010073059 Malignant neoplasm of unknown primary site Diseases 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 206010027406 Mesothelioma Diseases 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical class NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 208000003445 Mouth Neoplasms Diseases 0.000 description 1
- 206010073149 Multiple endocrine neoplasia Type 2 Diseases 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- MJUXVUBMBXHOJK-UHFFFAOYSA-N N-[3-cyano-4-[6-[4-[(6-methoxypyridin-3-yl)methyl]piperazin-1-yl]pyridin-3-yl]-6-(1-methylpyrazol-4-yl)pyrazolo[1,5-a]pyridin-2-yl]acetamide Chemical compound C(#N)C=1C(=NN2C=1C(=CC(=C2)C=1C=NN(C=1)C)C=1C=NC(=CC=1)N1CCN(CC1)CC=1C=NC(=CC=1)OC)NC(C)=O MJUXVUBMBXHOJK-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical class CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 208000034176 Neoplasms, Germ Cell and Embryonal Diseases 0.000 description 1
- 208000032976 Neuroendocrine carcinoma of pancreas Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 208000010505 Nose Neoplasms Diseases 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 206010031096 Oropharyngeal cancer Diseases 0.000 description 1
- 206010057444 Oropharyngeal neoplasm Diseases 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 102000038030 PI3Ks Human genes 0.000 description 1
- 108091007960 PI3Ks Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000008900 Pancreatic Ductal Carcinoma Diseases 0.000 description 1
- 206010061332 Paraganglion neoplasm Diseases 0.000 description 1
- 208000000821 Parathyroid Neoplasms Diseases 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229920002230 Pectic acid Polymers 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- 208000009565 Pharyngeal Neoplasms Diseases 0.000 description 1
- 206010034811 Pharyngeal cancer Diseases 0.000 description 1
- 102000004422 Phospholipase C gamma Human genes 0.000 description 1
- 108010056751 Phospholipase C gamma Proteins 0.000 description 1
- 208000007913 Pituitary Neoplasms Diseases 0.000 description 1
- 208000007452 Plasmacytoma Diseases 0.000 description 1
- 108010076039 Polyproteins Proteins 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 208000026149 Primary peritoneal carcinoma Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 201000008183 Pulmonary blastoma Diseases 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 206010071987 RET gene mutation Diseases 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 108090000873 Receptor Protein-Tyrosine Kinases Proteins 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 208000008938 Rhabdoid tumor Diseases 0.000 description 1
- 206010073334 Rhabdoid tumour Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 206010061934 Salivary gland cancer Diseases 0.000 description 1
- 102100027103 Serine/threonine-protein kinase B-raf Human genes 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 208000021712 Soft tissue sarcoma Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 description 1
- 206010042971 T-cell lymphoma Diseases 0.000 description 1
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 206010043276 Teratoma Diseases 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 206010043515 Throat cancer Diseases 0.000 description 1
- 208000000728 Thymus Neoplasms Diseases 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical class OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 208000015778 Undifferentiated pleomorphic sarcoma Diseases 0.000 description 1
- 201000005969 Uveal melanoma Diseases 0.000 description 1
- 201000003761 Vaginal carcinoma Diseases 0.000 description 1
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- NNLVGZFZQQXQNW-ADJNRHBOSA-N [(2r,3r,4s,5r,6s)-4,5-diacetyloxy-3-[(2s,3r,4s,5r,6r)-3,4,5-triacetyloxy-6-(acetyloxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6s)-4,5,6-triacetyloxy-2-(acetyloxymethyl)oxan-3-yl]oxyoxan-2-yl]methyl acetate Chemical compound O([C@@H]1O[C@@H]([C@H]([C@H](OC(C)=O)[C@H]1OC(C)=O)O[C@H]1[C@@H]([C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O1)OC(C)=O)COC(=O)C)[C@@H]1[C@@H](COC(C)=O)O[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O NNLVGZFZQQXQNW-ADJNRHBOSA-N 0.000 description 1
- GJWAPAVRQYYSTK-UHFFFAOYSA-N [(dimethyl-$l^{3}-silanyl)amino]-dimethylsilicon Chemical compound C[Si](C)N[Si](C)C GJWAPAVRQYYSTK-UHFFFAOYSA-N 0.000 description 1
- IKHGUXGNUITLKF-XPULMUKRSA-N acetaldehyde Chemical compound [14CH]([14CH3])=O IKHGUXGNUITLKF-XPULMUKRSA-N 0.000 description 1
- WDJHALXBUFZDSR-UHFFFAOYSA-M acetoacetate Chemical compound CC(=O)CC([O-])=O WDJHALXBUFZDSR-UHFFFAOYSA-M 0.000 description 1
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 1
- 239000012346 acetyl chloride Substances 0.000 description 1
- 229940081735 acetylcellulose Drugs 0.000 description 1
- 125000000641 acridinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3C=C12)* 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 150000001278 adipic acid derivatives Chemical class 0.000 description 1
- 208000020990 adrenal cortex carcinoma Diseases 0.000 description 1
- 208000007128 adrenocortical carcinoma Diseases 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000005257 alkyl acyl group Chemical group 0.000 description 1
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 1
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 1
- 125000005087 alkynylcarbonyl group Chemical group 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N ammonia Natural products N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- 201000007538 anal carcinoma Diseases 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 125000000637 arginyl group Chemical class N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 125000003289 ascorbyl group Chemical class [H]O[C@@]([H])(C([H])([H])O*)[C@@]1([H])OC(=O)C(O*)=C1O* 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000035578 autophosphorylation Effects 0.000 description 1
- JPNZKPRONVOMLL-UHFFFAOYSA-N azane;octadecanoic acid Chemical class [NH4+].CCCCCCCCCCCCCCCCCC([O-])=O JPNZKPRONVOMLL-UHFFFAOYSA-N 0.000 description 1
- 159000000009 barium salts Chemical class 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- HUMNYLRZRPPJDN-KWCOIAHCSA-N benzaldehyde Chemical group O=[11CH]C1=CC=CC=C1 HUMNYLRZRPPJDN-KWCOIAHCSA-N 0.000 description 1
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzenecarboxaldehyde Natural products O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000004603 benzisoxazolyl group Chemical group O1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000004618 benzofuryl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 1
- 239000012964 benzotriazole Substances 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical class NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 201000001531 bladder carcinoma Diseases 0.000 description 1
- 201000004571 bone carcinoma Diseases 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical class CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- 229960003340 calcium silicate Drugs 0.000 description 1
- 235000012241 calcium silicate Nutrition 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 description 1
- 150000001719 carbohydrate derivatives Chemical class 0.000 description 1
- 208000025046 carcinoma of lip Diseases 0.000 description 1
- 208000022033 carcinoma of urethra Diseases 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 201000003719 cervical neuroblastoma Diseases 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 208000011654 childhood malignant neoplasm Diseases 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical class CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 235000019417 choline salt Nutrition 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 208000037516 chromosome inversion disease Diseases 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 229940114081 cinnamate Drugs 0.000 description 1
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 1
- 150000001860 citric acid derivatives Chemical class 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000012059 conventional drug carrier Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- 201000007241 cutaneous T cell lymphoma Diseases 0.000 description 1
- 125000006254 cycloalkyl carbonyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000006547 cyclononyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 125000004855 decalinyl group Chemical group C1(CCCC2CCCCC12)* 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 125000005959 diazepanyl group Chemical group 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical class CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- YSLFMGDEEXOKHF-UHFFFAOYSA-N difluoro(iodo)methane Chemical compound FC(F)I YSLFMGDEEXOKHF-UHFFFAOYSA-N 0.000 description 1
- 125000004786 difluoromethoxy group Chemical group [H]C(F)(F)O* 0.000 description 1
- 125000005045 dihydroisoquinolinyl group Chemical group C1(NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000005046 dihydronaphthyl group Chemical group 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 229910001873 dinitrogen Chemical group 0.000 description 1
- 230000006806 disease prevention Effects 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 125000005883 dithianyl group Chemical group 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 229940043264 dodecyl sulfate Drugs 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 229940088679 drug related substance Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 208000028715 ductal breast carcinoma in situ Diseases 0.000 description 1
- 201000007273 ductal carcinoma in situ Diseases 0.000 description 1
- 210000001198 duodenum Anatomy 0.000 description 1
- 239000008157 edible vegetable oil Substances 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 208000014616 embryonal neoplasm Diseases 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 201000003914 endometrial carcinoma Diseases 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 125000002587 enol group Chemical group 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 201000005619 esophageal carcinoma Diseases 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- FCJJZKCJURDYNF-UHFFFAOYSA-N ethyl but-2-ynoate Chemical compound CCOC(=O)C#CC FCJJZKCJURDYNF-UHFFFAOYSA-N 0.000 description 1
- 238000013213 extrapolation Methods 0.000 description 1
- 201000009311 eye carcinoma Diseases 0.000 description 1
- 201000001343 fallopian tube carcinoma Diseases 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000020375 flavoured syrup Nutrition 0.000 description 1
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-L fumarate(2-) Chemical class [O-]C(=O)\C=C\C([O-])=O VZCYOOQTPOCHFL-OWOJBTEDSA-L 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 208000024386 fungal infectious disease Diseases 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 201000010175 gallbladder cancer Diseases 0.000 description 1
- 201000007487 gallbladder carcinoma Diseases 0.000 description 1
- 201000005649 gangliocytoma Diseases 0.000 description 1
- 201000008361 ganglioneuroma Diseases 0.000 description 1
- 208000010749 gastric carcinoma Diseases 0.000 description 1
- 201000011243 gastrointestinal stromal tumor Diseases 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 229940014259 gelatin Drugs 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 150000002315 glycerophosphates Chemical class 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical class C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 201000010235 heart cancer Diseases 0.000 description 1
- 208000024348 heart neoplasm Diseases 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical class CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 125000005549 heteroarylene group Chemical group 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical class CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000487 histidyl group Chemical class [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 208000013010 hypopharyngeal carcinoma Diseases 0.000 description 1
- 125000002632 imidazolidinyl group Chemical group 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000010874 in vitro model Methods 0.000 description 1
- 125000003392 indanyl group Chemical group C1(CCC2=CC=CC=C12)* 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000003454 indenyl group Chemical group C1(C=CC2=CC=CC=C12)* 0.000 description 1
- 125000003406 indolizinyl group Chemical group C=1(C=CN2C=CC=CC12)* 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- HVTICUPFWKNHNG-BJUDXGSMSA-N iodoethane Chemical group [11CH3]CI HVTICUPFWKNHNG-BJUDXGSMSA-N 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 150000002505 iron Chemical class 0.000 description 1
- BAUYGSIQEAFULO-UHFFFAOYSA-L iron(2+) sulfate (anhydrous) Chemical compound [Fe+2].[O-]S([O-])(=O)=O BAUYGSIQEAFULO-UHFFFAOYSA-L 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000004491 isohexyl group Chemical group C(CCC(C)C)* 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical class CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 201000005992 juvenile myelomonocytic leukemia Diseases 0.000 description 1
- 238000003674 kinase activity assay Methods 0.000 description 1
- 150000003893 lactate salts Chemical class 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 201000005264 laryngeal carcinoma Diseases 0.000 description 1
- 201000006721 lip cancer Diseases 0.000 description 1
- 239000011344 liquid material Substances 0.000 description 1
- 229910003002 lithium salt Inorganic materials 0.000 description 1
- 159000000002 lithium salts Chemical class 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 201000005296 lung carcinoma Diseases 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 239000012931 lyophilized formulation Substances 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 150000002688 maleic acid derivatives Chemical class 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 150000004701 malic acid derivatives Chemical class 0.000 description 1
- 208000026045 malignant tumor of parathyroid gland Diseases 0.000 description 1
- 150000002690 malonic acid derivatives Chemical class 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 150000002696 manganese Chemical class 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 229940095102 methyl benzoate Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004001 molecular interaction Effects 0.000 description 1
- 201000006894 monocytic leukemia Diseases 0.000 description 1
- 150000002780 morpholines Chemical class 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 201000005962 mycosis fungoides Diseases 0.000 description 1
- 201000006462 myelodysplastic/myeloproliferative neoplasm Diseases 0.000 description 1
- 206010028537 myelofibrosis Diseases 0.000 description 1
- 208000025113 myeloid leukemia Diseases 0.000 description 1
- OFCCYDUUBNUJIB-UHFFFAOYSA-N n,n-diethylcarbamoyl chloride Chemical compound CCN(CC)C(Cl)=O OFCCYDUUBNUJIB-UHFFFAOYSA-N 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- YZMHQCWXYHARLS-UHFFFAOYSA-N naphthalene-1,2-disulfonic acid Chemical class C1=CC=CC2=C(S(O)(=O)=O)C(S(=O)(=O)O)=CC=C21 YZMHQCWXYHARLS-UHFFFAOYSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-M naphthalene-2-sulfonate Chemical compound C1=CC=CC2=CC(S(=O)(=O)[O-])=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-M 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 description 1
- 208000037830 nasal cancer Diseases 0.000 description 1
- 208000018795 nasal cavity and paranasal sinus carcinoma Diseases 0.000 description 1
- 201000011216 nasopharynx carcinoma Diseases 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 150000002814 niacins Chemical class 0.000 description 1
- 235000005152 nicotinamide Nutrition 0.000 description 1
- 239000011570 nicotinamide Substances 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- ZCYXXKJEDCHMGH-UHFFFAOYSA-N nonane Chemical compound CCCC[CH]CCCC ZCYXXKJEDCHMGH-UHFFFAOYSA-N 0.000 description 1
- BKIMMITUMNQMOS-UHFFFAOYSA-N normal nonane Natural products CCCCCCCCC BKIMMITUMNQMOS-UHFFFAOYSA-N 0.000 description 1
- 201000002575 ocular melanoma Diseases 0.000 description 1
- 239000012053 oil suspension Substances 0.000 description 1
- 231100000590 oncogenic Toxicity 0.000 description 1
- 230000000771 oncological effect Effects 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 201000006958 oropharynx cancer Diseases 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- 150000003891 oxalate salts Chemical class 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- 201000008129 pancreatic ductal adenocarcinoma Diseases 0.000 description 1
- 208000003154 papilloma Diseases 0.000 description 1
- 208000029211 papillomatosis Diseases 0.000 description 1
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 description 1
- 208000007312 paraganglioma Diseases 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 125000003538 pentan-3-yl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 201000002524 peritoneal carcinoma Diseases 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 125000001791 phenazinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3N=C12)* 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 125000001484 phenothiazinyl group Chemical group C1(=CC=CC=2SC3=CC=CC=C3NC12)* 0.000 description 1
- 125000001644 phenoxazinyl group Chemical group C1(=CC=CC=2OC3=CC=CC=C3NC12)* 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 125000004592 phthalazinyl group Chemical group C1(=NN=CC2=CC=CC=C12)* 0.000 description 1
- 238000000053 physical method Methods 0.000 description 1
- OXNIZHLAWKMVMX-UHFFFAOYSA-N picric acid Chemical class OC1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O OXNIZHLAWKMVMX-UHFFFAOYSA-N 0.000 description 1
- 125000004194 piperazin-1-yl group Chemical group [H]N1C([H])([H])C([H])([H])N(*)C([H])([H])C1([H])[H] 0.000 description 1
- 150000004885 piperazines Chemical class 0.000 description 1
- 125000004482 piperidin-4-yl group Chemical group N1CCC(CC1)* 0.000 description 1
- 150000003053 piperidines Chemical class 0.000 description 1
- 201000002511 pituitary cancer Diseases 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-M pivalate Chemical compound CC(C)(C)C([O-])=O IUGYQRQAERSCNH-UHFFFAOYSA-M 0.000 description 1
- 125000005547 pivalate group Chemical group 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 238000011085 pressure filtration Methods 0.000 description 1
- 208000016800 primary central nervous system lymphoma Diseases 0.000 description 1
- 208000025638 primary cutaneous T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 208000003476 primary myelofibrosis Diseases 0.000 description 1
- 201000003725 primary thrombocytopenia Diseases 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical class CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical class CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 1
- 125000001042 pteridinyl group Chemical group N1=C(N=CC2=NC=CN=C12)* 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- DXBWJLDFSICTIH-UHFFFAOYSA-N pyrazine-2-carbaldehyde Chemical group O=CC1=CN=CC=N1 DXBWJLDFSICTIH-UHFFFAOYSA-N 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003072 pyrazolidinyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- ILAILUUAUWUQNG-UHFFFAOYSA-N pyridin-1-ium-3-carbonitrile;2,2,2-trifluoroacetate Chemical class OC(=O)C(F)(F)F.N#CC1=CC=CN=C1 ILAILUUAUWUQNG-UHFFFAOYSA-N 0.000 description 1
- CTEFYDWUNZVMNS-UHFFFAOYSA-N pyridin-4-yl trifluoromethanesulfonate Chemical compound FC(F)(F)S(=O)(=O)OC1=CC=NC=C1 CTEFYDWUNZVMNS-UHFFFAOYSA-N 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000001422 pyrrolinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 201000010174 renal carcinoma Diseases 0.000 description 1
- 208000010639 renal pelvis urothelial carcinoma Diseases 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 150000003873 salicylate salts Chemical class 0.000 description 1
- MOODSJOROWROTO-UHFFFAOYSA-N salicylsulfuric acid Chemical class OC(=O)C1=CC=CC=C1OS(O)(=O)=O MOODSJOROWROTO-UHFFFAOYSA-N 0.000 description 1
- 201000003804 salivary gland carcinoma Diseases 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000012056 semi-solid material Substances 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- IZTQOLKUZKXIRV-YRVFCXMDSA-N sincalide Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)NCC(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](N)CC(O)=O)C1=CC=C(OS(O)(=O)=O)C=C1 IZTQOLKUZKXIRV-YRVFCXMDSA-N 0.000 description 1
- 208000037968 sinus cancer Diseases 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 201000002314 small intestine cancer Diseases 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 235000010288 sodium nitrite Nutrition 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000011877 solvent mixture Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 208000037969 squamous neck cancer Diseases 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000012058 sterile packaged powder Substances 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 201000000498 stomach carcinoma Diseases 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 150000003890 succinate salts Chemical class 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- IIACRCGMVDHOTQ-UHFFFAOYSA-M sulfamate Chemical compound NS([O-])(=O)=O IIACRCGMVDHOTQ-UHFFFAOYSA-M 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 230000005062 synaptic transmission Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 150000003892 tartrate salts Chemical class 0.000 description 1
- 238000003419 tautomerization reaction Methods 0.000 description 1
- FMLPQHJYUZTHQS-MRVPVSSYSA-N tert-butyl (3r)-3-methylpiperazine-1-carboxylate Chemical compound C[C@@H]1CN(C(=O)OC(C)(C)C)CCN1 FMLPQHJYUZTHQS-MRVPVSSYSA-N 0.000 description 1
- PDTZMULNKGUIEJ-UHFFFAOYSA-N tert-butyl 4-methylidenepiperidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCC(=C)CC1 PDTZMULNKGUIEJ-UHFFFAOYSA-N 0.000 description 1
- DRDVJQOGFWAVLH-UHFFFAOYSA-N tert-butyl n-hydroxycarbamate Chemical compound CC(C)(C)OC(=O)NO DRDVJQOGFWAVLH-UHFFFAOYSA-N 0.000 description 1
- YBRBMKDOPFTVDT-UHFFFAOYSA-N tert-butylamine Chemical class CC(C)(C)N YBRBMKDOPFTVDT-UHFFFAOYSA-N 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000001412 tetrahydropyranyl group Chemical group 0.000 description 1
- YAPQBXQYLJRXSA-UHFFFAOYSA-N theobromine Chemical class CN1C(=O)NC(=O)C2=C1N=CN2C YAPQBXQYLJRXSA-UHFFFAOYSA-N 0.000 description 1
- 238000011287 therapeutic dose Methods 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 239000003451 thiazide diuretic agent Substances 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000005556 thienylene group Chemical group 0.000 description 1
- 150000003567 thiocyanates Chemical class 0.000 description 1
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 1
- 206010043554 thrombocytopenia Diseases 0.000 description 1
- 208000008732 thymoma Diseases 0.000 description 1
- 201000009377 thymus cancer Diseases 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical class CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M trans-cinnamate Chemical compound [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- 108091008578 transmembrane receptors Proteins 0.000 description 1
- 102000027257 transmembrane receptors Human genes 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- VYIVTFUZGSIQIA-UHFFFAOYSA-N triazolo[1,5-a]pyridine-3-carbonitrile Chemical compound C1=CC=CC2=C(C#N)N=NN21 VYIVTFUZGSIQIA-UHFFFAOYSA-N 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- ILWRPSCZWQJDMK-UHFFFAOYSA-N triethylazanium;chloride Chemical compound Cl.CCN(CC)CC ILWRPSCZWQJDMK-UHFFFAOYSA-N 0.000 description 1
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical class CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical class CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical class OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 125000005455 trithianyl group Chemical group 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- ZDPHROOEEOARMN-UHFFFAOYSA-N undecanoic acid Chemical class CCCCCCCCCCC(O)=O ZDPHROOEEOARMN-UHFFFAOYSA-N 0.000 description 1
- 210000000626 ureter Anatomy 0.000 description 1
- 201000000334 ureter transitional cell carcinoma Diseases 0.000 description 1
- 208000010570 urinary bladder carcinoma Diseases 0.000 description 1
- 208000037965 uterine sarcoma Diseases 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/415—1,2-Diazoles
- A61K31/4162—1,2-Diazoles condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/444—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring heteroatom, e.g. amrinone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/4545—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring hetero atom, e.g. pipamperone, anabasine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4995—Pyrazines or piperazines forming part of bridged ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D519/00—Heterocyclic compounds containing more than one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system not provided for in groups C07D453/00 or C07D455/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
Abstract
涉及药物化学领域,主要涉及式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、或其药学上可接受的盐及其制备方法和在制备用于治疗RET激酶介导的疾病的药物中的用途。
Description
本申请要求享有2018年9月30日向中国国家知识产权局提交的申请号为201811162497.1、发明名称为“取代吡唑稠环类衍生物及其制备方法和应用”的在先专利申请的优先权权益。该在先专利申请的全文以引用的方式结合至本文中。
技术领域
本发明涉及药物化学领域,具体涉及取代吡唑稠环类衍生物及其制备方法和应用。
背景技术
RET(Rearranged During Transfection)原癌基因最初是在1985年,通过NIH3T3(小鼠胚胎成纤维细胞系)细胞与人类淋巴瘤DNA的转染被证实(Cell,1985,42(2):581-588)。RET原癌基因定位于染色体10q11.2,其DNA全长为60kb,含有外显子21个,编码由1100个氨基酸组成的RET蛋白:这种RET蛋白是一种酪氨酸激酶受体,含有一个由半胱氨酸组成的细胞外区、一个跨膜区和一个具有催化酪氨酸激酶作用的细胞内区(Mol CellEndocrinol,2010,322(1-2):2-7)。RET参与细胞增殖、神经传导、细胞迁移和细胞分化,通过配体/复合受体/RET多蛋白复合物的信号,激活各种下游途径,如RAS/RAF/MEK/ERK,PI3K/AKT和STAT通路,诱导细胞增生(J Clin Oncol,2012,30(2):200-202)。
随着研究的逐步进展,现已发现多种疾病的发生与RET基因突变有密切联系,包括甲状腺乳头状癌(papillary thyroid carcinoma,PTC)(Cell,1990,60(4):557-563)、甲状腺髓样癌(medullary thyroid carcinoma,MTC)(hyroid,2009,19(6):565-612)、多发性内分泌腺瘤病2型(multiple endocrineneoplasia type II,MEN2)(Endocr Rev,2006,27(5):535-560)、先天性巨结肠(Proc Natl Acad Sci USA,2000,97(1):268-273)、肺腺癌(Nat Med,2012,18(3):375-377)等。目前只有KIF5B-RET、CCDC6-RET、TRIM33-RET、NCOA4-RET这四种RET融合基因在非小细胞肺癌中被报道,而KIF5B-RET是非小细胞肺癌中最常见的RET融合基因(Cancer,2013,119(8):1486-1494)。KIF5B-RET是KIF5B(kinesin familymember 5B)基因和RET基因的染色体倒置(p11;q11)形成的一种融合基因,通过全基因组和转录组测序,第一次在非吸烟韩国人的腺癌中被证实;KIF5B-RET在肺癌中的比例很低,在非吸烟者和腺癌患者中更常见,并与其它突变,如EGFR、KRAS、BRAF、ErbB2、EML4-ALK相排斥(Genome Res,2012,22(3):436-445)。KIF5B-RET融合蛋白包含马达结构域和KIF5B的卷曲螺旋结构域,通过卷曲螺旋结构域的二聚化作用,该融合蛋白的RET酪氨酸激酶活性可异常活化,从而促进肺肿瘤发生(Cancer,2011,117(12):2709-2718)。在Qian等的研究中(MolCancer,2014,13:176),KIF5B-RET融合激酶被证实在体外和体内都具有显着的致癌活性,STAT3的信号转导途径可能是肿瘤发生的主要下游介质。有证据显示KIF5B-RET可调节STAT3的持续活化。KIF5B-RET融合激酶可以结合STAT3,直接磷酸化和激活STAT3-Tyr705;它也可以通过JAK/STAT3依赖性途径,介导激活STAT3-Tyr705,并通过RAS/RAF/MEK/ERK1途径触发Ser727的磷酸化。
证明RET融合物在一些癌症中是驱动者,这件事促进了已具有RET抑制活性的多激酶抑制剂(multi-kinase inhibitor)的应用,用于治疗c负载RET融合物蛋白质的肿瘤病人。目前尚未有批准药剂可以用来针对性地靶向这一致癌基因,目前RET特异性癌症的治疗方式仅限于多激酶抑制剂和化疗,但这些非特异性治疗临床表现ORR(客观缓释率)不好并且有很大的脱靶毒性。再者,癌症治疗的最大挑战之一是肿瘤细胞对于治疗一定阶段后出现耐药,一旦耐药,病人的治疗方式通常极为有限,且大多数例子中,癌症一直进展、不受抑制。为了能在这方面获得突破,需要一些化合物可以选择性专门靶向致癌RET融合和基因突变。
发明内容
为改善上述问题,本发明提供一种如下式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物:
其中,X1、X2、X3、X4、X5、X6相同或不同,彼此独立地选自CR1、-C-A或N,其中每一个R1相同或不同,彼此独立地选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Ra取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、NR2R3、-NHC(O)R2、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;A选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Rb取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、-OS(O)2R7、-NHC(O)R2;
B选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Rc取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、NR2R3、-C(O)R4、-NHC(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
D选自无取代或任选被一个、两个或更多个Rd取代的C3-40环烷基、C3-40环烯基、C3-40环炔基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C6-20芳基、5-20元杂芳基或3-20元杂环基;
E选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Re取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基或3-20元杂环基氧基;
G选自无取代或任选被一个、两个或更多个Rf取代的下列基团:C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基或3-20元杂环基氧基;
K选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Rg取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基、3-20元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
每一个R2相同或不同,彼此独立地选自H、无取代或任选被OH、NH2取代的如下基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、-C(O)R4、-S(O)2R6;
每一个R3相同或不同,彼此独立地选自H、C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、-C(O)R4、-S(O)2R6;
或者,R2和R3与所连的N原子一起形成5-20元杂芳基或3-20元杂环基;
每一个R4相同或不同,彼此独立地选自H、C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基、3-20元杂环基氧基、NR2R3;
每一个R5相同或不同,彼此独立地选自H、C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基羰基、C2-40烯基羰基、C2-40炔基羰基、C3-40环烷基羰基、C3-40环烯基羰基、C3-40环炔基羰基、C6-20芳基羰基、5-20元杂芳基羰基、3-20元杂环基羰基;
每一个R6相同或不同,彼此独立地选自H、C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基、3-20元杂环基氧基、NR2R3;
每一个R7相同或不同,彼此独立地选自H、C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基;
每一个Ra、Rb、Rc、Rd、Re、Rf、Rg相同或不同,彼此独立地选自卤素、CN、OH、SH、氧代(=O)、NO2,无取代或任选被一个、两个或更多个Rh取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基、3-20元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
每一个Rh相同或不同,彼此独立地选自卤素、CN、OH、SH、氧代(=O)、NO2,无取代或任选被一个、两个或更多个Rj取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基、3-20元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;或者,当环状基团(包括但不限于C3-40环烷基、C3-40环烯基、C3-40环炔基、3-20元杂环基等)的不同位置被两个或更多个取代基取代时,所述取代基中的两个也可以与所述环状基团形成桥环,其中所述桥环中除桥头原子之外的桥原子可以包含1、2、3、4或5个选自CH2、O、NH的二价基团;
每一个Rj相同或不同,彼此独立地选自卤素、CN、OH、SH、氧代(=O)、NO2、无取代或任选被一个、两个或更多个Rh取代的下列基团:C1-40烷基、C2-40烯基、C2-40炔基、C3-40环烷基、C3-40环烯基、C3-40环炔基、C6-20芳基、5-20元杂芳基、3-20元杂环基、C1-40烷基氧基、C2-40烯基氧基、C2-40炔基氧基、C3-40环烷基氧基、C3-40环烯基氧基、C3-40环炔基氧基、C6-20芳基氧基、5-20元杂芳基氧基、3-20元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
或者,当环状基团(包括但不限于C3-40环烷基、C3-40环烯基、C3-40环炔基、3-20元杂环基等)的不同位置被两个或更多个取代基取代时,所述取代基中的两个也可以与所述环状基团形成桥环,其中所述桥环中除桥头原子之外的桥原子可以包含1、2、3、4或5个选自CH2、O、NH的二价基团;
或者,当一个原子(如碳原子)被两个或更多个取代基取代时,所述取代基中的两个也可以与其共同连接的原子形成环状基团(包括但不限于C3-40环烷基、C3-40环烯基、C3-40环炔基、3-20元杂环基等)。
根据本发明的实施方案,式I所示的化合物选自下列式I’所示的化合物:
其中,X1、X2、X3、X4、X5、A、B、D、E、G、K独立地具有上文所述的定义。
根据本发明的实施方案,其中:
X1、X2、X3、X4、X5、X6相同或不同,彼此独立地选自CR1、-C-A或N,其中每一个R1相同或不同,彼此独立地选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Ra取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、NR2R3、-NHC(O)R2、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;A选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Rb取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
B选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Rc取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、NR2R3、-C(O)R4、-NHC(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
D选自无取代或任选被一个、两个或更多个Rd取代的C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基或3-10元杂环基;
E选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Re取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-10芳基氧基、5-10元杂芳基氧基或3-10元杂环基氧基;
G选自无取代或任选被一个、两个或更多个Rf取代的下列基团:C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-10芳基氧基、5-10元杂芳基氧基或3-10元杂环基氧基;
K选自H、卤素、CN、OH,无取代或任选被一个、两个或更多个Rg取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-10芳基氧基、5-10元杂芳基氧基、3-10元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
每一个R2相同或不同,彼此独立地选自H、无取代或任选被OH、NH2取代的如下基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、-C(O)R4、-S(O)2R6;
每一个R3相同或不同,彼此独立地选自H、C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、-C(O)R4、-S(O)2R6;
或者,R2和R3与所连的N原子一起形成5-10元杂芳基或3-10元杂环基;
每一个R4相同或不同,彼此独立地选自H、C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-10芳基氧基、5-10元杂芳基氧基、3-10元杂环基氧基、NR2R3;
每一个R5相同或不同,彼此独立地选自H、C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基羰基、C2-6烯基羰基、C2-6炔基羰基、C3-8环烷基羰基、C3-8环烯基羰基、C3-8环炔基羰基、C6-10芳基羰基、5-10元杂芳基羰基、3-10元杂环基羰基;
每一个R6相同或不同,彼此独立地选自H、C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-10芳基氧基、5-10元杂芳基氧基、3-10元杂环基氧基、NR2R3;
每一个R7相同或不同,彼此独立地选自H、C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基;
每一个Ra、Rb、Rc、Rd、Re、Rf、Rg相同或不同,彼此独立地选自卤素、CN、OH、SH、氧代(=O)、NO2,无取代或任选被一个、两个或更多个Rh取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-10芳基氧基、5-10元杂芳基氧基、3-10元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、-OS(O)2R7;
每一个Rh相同或不同,彼此独立地选自卤素、CN、OH、SH、氧代(=O)、NO2,无取代或任选被一个、两个或更多个Rj取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-10芳基氧基、5-10元杂芳基氧基、3-10元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;或者,当环状基团(包括但不限于C3-8环烷基、C3-8环烯基、C3-8环炔基、3-10元杂环基等)的不同位置被两个或更多个取代基取代时,所述取代基中的两个也可以与所述环状基团形成桥环,其中所述桥环中除桥头原子之外的桥原子可以包含1、2、3、4或5个选自CH2、O、NH的二价基团;
每一个Rj相同或不同,彼此独立地选自卤素、CN、OH、SH、氧代(=O)、NO2、无取代或任选被一个、两个或更多个Rh取代的下列基团:C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-8环烷基氧基、C3-8环烯基氧基、C3-8环炔基氧基、C6-20芳基氧基、5-10元杂芳基氧基、3-10元杂环基氧基、NR2R3、-C(O)R4、-OCR5、-S(O)2R6、OS(O)2R7;
或者,当环状基团(包括但不限于C3-8环烷基、C3-8环烯基、C3-8环炔基、3-10元杂环基等)的不同位置被两个或更多个取代基取代时,所述取代基中的两个也可以与所述环状基团形成桥环,其中所述桥环中除桥头原子之外的桥原子可以包含1、2、3、4或5个选自CH2、O、NH的二价基团;
或者,当一个原子(如碳原子)被两个或更多个取代基取代时,所述取代基中的两个也可以与其共同连接的原子形成环状基团(包括但不限于C3-8环烷基、C3-8环烯基、C3-8环炔基、3-10元杂环基等)。
根据本发明的实施方案,其中X1、X2、X3、X4、X5、X6相同或不同,彼此独立地选自CR1、-C-A或N,R1选自NH2、CH3、N(CH3)2、OH、H、NHC(O)CH3、NHCH3、F、OCH3、NHC2H4OH;例如,X1,X2,X3,X4和X5中的至少一个为N,如1、2、3、4或5个为N;
A可以选自NH2、OH、H、卤素、氰基,无取代或任选被一个、两个或更多个卤素、OH或NH2取代的下列基团:C1-6烷基、-NR2R3、-NH-C1-6烷基-NR2R3、-NHCO-C1-6烷基-NR2R3、-NH-C1-6烷基-CO-NR2R3、-NHCO-C1-6烷基-COO-C1-6烷基、-NH-C3-8环烷基-CO-NR2R3、-NH-C2-8烯基-CONR2R3、-NH-C1-6烷基-CN、-NHCO-NH-R2、-CONR2R3或-CONH-C1-6烷基-NR2R3、-NHCO-R2、-NH-C1-6烷基、C1-6烷基氧基、-NH-C1-6烷基-OH;
根据本发明的实施方案,其中每一个R2和R3相同或不同,彼此独立地选自H、OH、C1-6烷基、5-6元杂环基、C1-6烷基酰基或C1-6烷基磺酰基;或者R2和R3与所连N原子一起形成无取代或任选被氧代的5-6元杂环基;
根据本发明的实施方案,其中B可以选自H、Cl、CN、Br、-COOH,无取代或任选被一个、两个或更多个Rc取代的下列基团:-CH3,-CH2CH3,-环丙基,-COOCH3,-COOCH2CH3,-CONH2和-CONHCH3;
根据本发明的实施方案,其中D可以选自无取代或任选被一个、两个或更多个Rd取代的如下基团:C1-6烷基氧基-、5-10元杂芳基或3-10元杂环基;
根据本发明的实施方案,每一个Rc、Rd相同或不同,彼此独立地选自OH、卤素、C1-6烷基-、C1-6烷基氧基-、C1-6环烷基氧基、羟基C1-6烷基-、单氟代C1-6烷基-、二氟代C1-6烷基-、三氟代C1-6烷基-、氰基C1-6烷基-、(C1-6烷氧基)C1-6烷基-、(C1-6烷氧基)CH2C(=O)-、(C1-6烷氧基)C(=O)C1-6烷基-、C3-8环烷基-、(R2R3N)C1-6烷基-、(R2R3N)C(=O)C1-6烷基-、C1-6烷基S(O)2-C1-6烷基-、C1-6烷基氧基苄基,无取代或任选被下列基团取代的3-10元杂环基:卤素、C1~6烷基、氟代C1~6烷基、二氟代C1-6烷基、三氟代C1-6烷基、(C1-6烷氧基)C1~6烷基、(C1-6烷基)2NCH2C(O)-、(C1-6烷氧基)C(O)-或(C1~6烷氧基)CH2C(O)-、(C1-6烷基)2NC1-6烷基-、(C1-6烷基)2NC(=O)C1-6烷基-;
根据本发明的实施方案,其中E选自H、卤素、CN、无取代或任选被一个、两个或更多个Re取代的下列基团:C1~6烷基、C1-6烷氧基,C1~6环烷基,含N、S和O的1~3个环杂原子的5元或六元杂芳基环,所述5元或6元杂芳基环任选被氧代取代;
根据本发明的实施方案,其中G可以选自无取代或任选被一个、两个或多个Rf取代的下列基团:5-10元杂芳基,3-10元杂环基,其中所述杂环基选自例如吡咯烷基、哌啶基、哌嗪基、吗啉基或吖丁啶基;或者,当所述杂环基的不同位置被两个或更多个取代基取代时,所述取代基中的两个也可以与所述杂环基形成桥环,其中所述桥环中除桥头原子之外的桥原子可以包含1、2、3、4或5个选自CH2、O、NH的二价基团;
根据本发明的实施方案,其中Rf可以选自卤素、CN、OH、SH、氧代(=O)、NO2、无取代或任选被一个、两个或更多个Rh取代的下列基团:C1-6烷基、C3-8环烷基。
根据本发明的实施方案,其中K可以选自H、OH,无取代或任选被一个或多个Rg取代的下列基团:C1-6烷基、C3-8环烷基、C6-10芳基、5-10元杂芳基、3-10元杂环基、C1-6烷基氧基、C3-6环烷基氧基、C6-10芳基氧基、5-10元杂芳基氧基、3-10元杂环基氧基、N(C1-6烷基)2、-C(O)R4、-OCR5、-S(O)2R6、-OS(O)2R7;
根据本发明的实施方案,其中K可以选自无取代或任选被一个、两个或更多个Rg取代的下列基团:C1-6烷基、C3-10环烷基、-C(O)R4、-OCR5、-S(O)2R6、-OS(O)2R7。
根据本发明的实施方案,所述R4、R5、R6、R7相同或不同,彼此独立地选自C1-6烷基、C2-6烯基、C2-6炔基、C3-8环烷基、C3-8环烯基、C3-8环炔基、C1-6烷基氧基。
根据本发明的实施方案,其中K可以选自无取代或任选被一个、两个或更多个Rh取代的下列基团:C6-10芳基C1-6烷基-或3-10元杂环基C1-6烷基-,例如苯基C1-6烷基-或吡啶基C1-6烷基-;
根据本发明的实施方案,其中Rg可以选自卤素、CN、OH、SH、氧代(=O)、NO2,无取代或任选被一个、两个或更多个Rh取代的下列基团:C1-6烷基、C3-10环烷基、C6-10芳基、5-10元杂芳基、3-10元杂环基;
或者,当K中的一个原子(如碳原子)被两个或更多个Rg取代时,两个Rg也可以与其共同连接的原子形成环状基团(包括但不限于C3-6环烷基、C3-6环烯基、C3-6环炔基、4-6元杂环基等);
根据本发明示例性的实施方案,例如:X6选自-C-A或N,A可以选自H,无取代或任选被一个、两个或更多个Rb取代的下列基团:NH2、C1-6烷基、-NH(C1-6烷基)2、OH、F、-NHC(O)C1-6烷基、-NHC1-6烷基、C1-6烷基氧基、-NHC1-6烷基-OH;
每一个Rb相同或不同,彼此独立地选自C1-6烷基、C1-6烷氧基;
B可以选自H、CN、-CONH2,无取代或任选被一个、两个或更多个Rc取代的C1-6烷基;
每一个Rc相同或不同,彼此独立地选自卤素、C1-6烷基;
D可以选自无取代或任选被一个、两个或更多个Rd取代的如下基团:C1-6烷基氧基或5-14元杂芳基,所述Rd选自无取代或任选被一个或多个如下基团取代的C1-6烷基或3-10元杂环基:氧代、卤素、OH、-N(C1-6烷基)2或-S(O)2-C1-6烷基;
E选自H,无取代或任选被一个、两个或更多个Re取代的下列基团:C1-6烷基、C1-6烷氧基;
每一个Re相同或不同,彼此独立地选自OH、F、C1-6烷基;
G选自无取代或任选被氧代的3-10元杂环基,例如无取代或任选被氧代或被C1-6烷基取代的哌嗪基、哌啶基;或者,所述当所述杂环基的间位被两个取代基取代时,所述取代基可以与所述杂环基形成桥环,其中所述桥环中除桥头原子之外的桥原子可以包含1、2、3、4或5个选自CH2的二价基团;
K选自无取代或任选被一个、两个或更多个Rg取代的下列基团:C1-6烷基、-C(O)R4,所述Rg选自氧代、OH、无取代或任选被一个、两个或更多个Rh取代的C1-6烷基、C1-6烷氧基,C3-10环烷基、C6-14芳基、5-14元杂芳基、-SO2-C6-14芳基;每一个R4相同或不同,彼此独立地选自H、C1-6烷基、C2-6烯基、C2-6炔基、C3-10环烷基、C3-10环烯基、C3-10环炔基、C6-14芳基、5-14元杂芳基、3-10元杂环基、C1-6烷基氧基、C2-6烯基氧基、C2-6炔基氧基、C3-10环烷基氧基、C3-10环烯基氧基、C3-10环炔基氧基、C6-14芳基氧基、5-14元杂芳基氧基、3-10元杂环基氧基、-N(C1-6烷基)2;所述Rh选自卤素、C1-6烷氧基、NH2、-N(C1-6烷基)2、-NHC6-14芳基、-NHC1-6烷基;或者,当K中的一个原子(如碳原子)被两个或更多个Rg取代时,两个Rg也可以与其共同连接的原子形成环状基团(包括但不限于C3-10环烷基、C3-10环烯基、C3-10环炔基、3-10元杂环基等);
根据本发明示例性的实施方案,例如:
X6选自-C-A或N,A可以选自NH2、甲基、乙基或丙基、-NH(CH3)2、OH、F、-NH(O)CH3、-NHCH3、甲氧基、-NHC2H4-OH;
B可以选自H、CN或-COCH3;
D可以选自无取代或任选被一个、两个或更多个Rd取代的如下基团:吡唑基、甲氧基或乙氧基,例如吡唑-1-基、吡唑-3-基、吡唑-4-基、吡唑-5-基;
每一个Rd相同或不同,彼此独立地选自OH、F、C1-6烷基、羟基C1-6烷基-、二氟代C1-6烷基-、C1-6烷基S(O)2-C1-6烷基-、(C1-6烷基)2N-C(O)C1-6烷基-,C1-6烷基氧基-、C1-6环烷基氧基、无取代或任选被C1~6烷基取代的5-6元杂环基;
G选自无取代或任选被氧代的5-6元杂环基,例如无取代或任选被氧代或甲基取代的哌嗪基、哌啶基;或者,当所述杂环基的间位被两个取代基取代时,所述取代基可以与所述杂环基形成桥环,其中所述桥环中除桥头原子之外的桥原子可以包含1、2或3个选自CH2的二价基团;
K选自无取代或任选被一个、两个或更多个Rg取代的C1-6烷基、-C(O)C1-6烷基,所述Rg选自氧代、OH、无取代或任选被一个、两个或更多个Rh取代的C1-6烷基、C1-6烷氧基,C3-10环烷基、C6-14芳基、5-14元杂芳基、-S(O)2-C6-14芳基;所述Rh选自卤素、C1-6烷氧基、NH2、-N(C1-6烷基)2、-NHC6-14芳基、-NHC1-6烷基;或者,当K中的一个原子(如碳原子)被两个或更多个Rg取代时,两个Rg也可以与其共同连接的原子形成C3-10环烷基。
作为实例,D可以选自如下基团:
作为实例,G可以选自如下基团:
根据本发明的实施方案,K选自如下基团:
作为实例,所述式I化合物选自如下化合物:
本发明还提供式I化合物的制备方法,包括:
化合物II与化合物III发生铃木反应得到式I化合物;
其中,式II和III中B、D、E、G、K、X1、X2、X3、X4、X5、X6具有如上所述定义;式III中B为硼元素;L选自离去基团如卤素或者OTf。
本领域技术人员应当理解,除非如式III定义中的专门定义,或者根据有机化学反应常识能够确定为硼元素的情况外,其他通式化合物中的B应理解为说明书对于取代基的定义符号。
本发明还提供一种药物组合物,其包含治疗有效量的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物中的至少一种。
根据本发明,所述药物组合物还包括一种或多种药学上可接受的载体或赋形剂。
根据本发明,所述药物组合物还可以进一步含有一种或多种额外的治疗剂。
本文还提供体外或体内抑制细胞增殖的方法,所述方法包括使细胞与有效量的本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物接触。
本发明还提供一种治疗RET激酶介导的疾病的方法,包括给予患者治疗有效量的如式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物中的至少一种。
本文还提供了在有治疗需要的患者中治疗RET相关疾病或病症的方法,所述方法包括向所述患者施用治疗有效量的如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物。
本文还提供了在有治疗需要的患者中治疗癌症和/或抑制与特定癌症相关的转移的方法,所述方法包括向所述患者施用治疗有效量的如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物。
本文还提供了在有治疗需要的患者中治疗肠易激综合征(IBS)和/或与IBS相关的疼痛的方法,所述方法包括向所述患者施用治疗有效量的如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物。
本文还提供了为癌症患者提供支持护理的方法,包括预防或最小化与治疗(包括化疗治疗)相关的胃肠疾病(例如腹泻),所述方法包括给予患者治疗有效量的如本文所限定的化合物或其药物组合物。
本文还提供了用于治疗的如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物,或其药物组合物。
本文还提供用于治疗癌症和/或抑制与特定癌症相关的转移的如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物。
本文还提供用于治疗肠易激综合征(IBS)或与IBS相关的疼痛的如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物。
本发明还提供了如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物,其用于为癌症患者提供支持性护理,包括预防或最小化与治疗(包括化疗治疗)相关的胃肠病症,例如腹泻。
本文还提供了用于抑制RET激酶活性的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物。
本文还提供用于治疗RET相关疾病或病症的如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物或其药物组合物。
本发明还提供式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物中的至少一种在制备用于治疗RET激酶介导的疾病的药物中的用途。
本文还提供如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物在制备用于治疗癌症和/或抑制与特定癌症相关的转移的药物中的用途。
本文还提供如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物在制备用于治疗肠易激综合征(IBS)或与IBS相关的疼痛的药物中的用途。
本文还提供了如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物在制备用于向癌症患者提供支持护理的药物中的用途,所述支持护理包括预防或最小化与治疗(包括化疗治疗)相关的胃肠病症,例如腹泻。
本文还提供了如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物在制备用于抑制RET激酶活性的药物中的用途。
本文还提供了如本文所限定的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物在制备用于治疗RET相关疾病或病症的药物中的用途。
本文还提供用于在有需要的患者中治疗癌症的方法,所述方法包括(a)确定所述癌症是否与下述的失调有关:RET基因、RET激酶、或其中任何一者的表达或活性或水平(例如,RET相关的癌症);(b)如果确定所述癌症与下述的失调有关:RET基因、RET激酶、或其中任何一者的表达或活性或水平(例如,RET相关的癌症),向患者施用治疗有效量的通式I化合物或其药学上可接受的盐或溶剂合物,或其药物组合物。
本文还提供了用于在有需要的患者中治疗癌症(例如,RET相关癌症,如具有一种或多种RET抑制剂抗性突变的RET相关癌症)的药物组合,其包含(a)式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物,(b)其他治疗剂,和(c)任选的至少一种药学上可接受的运载体,其中式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物和所述其他治疗剂被配制成单独的组合物或剂量用于同时、分开或顺序用于治疗癌症,其中式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物和所述治疗剂的量一起有效治疗所述癌症。本文还提供了包含这种组合的药物组合物。本文还提供了这种组合在制备用于治疗癌症的药物中的用途。本文还提供了商业包装或产品,其包含这种组合作为用于同时、单独或顺序使用的组合制剂;并涉及一种治疗有需要的患者的癌症的方法。
本文还提供了用于逆转或预防对抗癌药物的获得性抗性的方法,所述方法包括将治疗有效量的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物给予处于对抗癌药物发展或具有获得性抗性的风险的患者。
本文还提供延迟和/或预防个体中抗癌药抗药性发展的方法,所述方法包括在个体中施用有效量的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物,在此之前、期间或之后施用有效量的抗癌药物。
本文还提供了治疗患有癌症且对抗癌药物发展抗性的可能性增加的个体的方法,其包括对个体伴随施用(a)有效量的式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物;和(b)有效量的抗癌药物。
还提供了治疗患有RET相关癌症的个体的方法,所述癌症具有一种或多种RET抑制剂抗性突变,所述RET抑制剂抗性突变增加所述癌症对不是式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物的RET抑制剂的抗性(例如,在氨基酸位置804处的取代,例如V804M、V804L或V804E),其包括在给予另一种其他的抗癌药物之前、期间或之后,给予式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物。
还提供了治疗患有RET相关癌症的个体的方法,所述方法包括在给予另一种其他的抗癌药物之前、期间或之后,给予式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物。
本文提供了在有需要的患者中治疗癌症(例如RET相关癌症)的方法,所述方法包括向所述患者施用治疗有效量的通式I的化合物或其药学上可接受的盐或溶剂合物或其药物组合物。
在本文所述的任何方法或用途的一些实施方案中,癌症(例如RET相关癌症)是血液学癌症。在本文所述的任何方法或用途的一些实施方案中,癌症(例如RET相关癌症)是实体瘤。在本文所述的任何方法或用途的一些实施方案中,癌症(例如RET相关癌症)是肺癌(例如,小细胞肺癌或非小细胞肺癌),乳头状甲状腺癌,甲状腺髓样癌,分化型甲状腺癌,复发性甲状腺癌,难治性分化型甲状腺癌,肺腺癌,细支气管肺癌,2A或2B型多发性内分泌肿瘤(分别为MEN2A或MEN2B),嗜铬细胞瘤,甲状旁腺增生,乳腺癌,结直肠癌(例如转移性结肠直肠癌),乳头状肾细胞癌,胃肠粘膜的神经节细胞瘤病,炎性肌纤维母细胞瘤或宫颈癌。在本文所述的任何方法或用途的一些实施方案中,癌症(例如RET相关癌症)选自:急性淋巴细胞白血病(ALL),急性髓性白血病(AML),青少年癌症,肾上腺皮质癌,肛门癌、阑尾癌,星形细胞瘤,非典型性畸胎瘤/横纹肌样瘤,基底细胞癌,胆管癌,膀胱癌,骨癌,脑干胶质瘤,脑肿瘤,乳腺癌,支气管肿瘤,伯基特淋巴瘤,类癌瘤,未知原发癌,心脏肿瘤,宫颈癌,儿童癌症,脊索瘤,慢性淋巴细胞白血病(CLL),慢性骨髓性白血病(CML),慢性骨髓增殖性肿瘤,结肠癌,结肠直肠癌,颅咽管瘤,皮肤T细胞淋巴瘤,胆管癌,原位导管癌,胚胎性肿瘤,子宫内膜癌,室管膜瘤,食道癌,成感觉神经细胞瘤,尤因肉瘤,颅外生殖细胞肿瘤,性腺外生殖细胞瘤,肝外胆管癌,眼癌,输卵管癌,骨纤维组织细胞瘤,胆囊癌,胃癌,胃肠类癌瘤,胃肠道间质瘤(GIST),生殖细胞瘤,妊娠滋养细胞疾病,神经胶质瘤,多毛细胞瘤,多毛细胞白血病,头颈癌,心脏癌,肝细胞癌,组织细胞增多症,霍奇金淋巴瘤,下咽癌,眼内黑色素瘤,胰岛细胞瘤,胰腺神经内分泌瘤,卡波西肉瘤,肾癌,朗格汉斯细胞组织细胞增多症,喉癌,白血病,唇和口腔癌,肝癌,肺癌,淋巴瘤,巨球蛋白血症,骨恶性纤维组织细胞瘤,骨癌,黑色素瘤,梅克尔细胞癌,间皮瘤,转移性鳞状颈癌,中线状癌,口癌,多发性内分泌瘤综合征,多发性骨髓瘤,真菌病蕈样肉芽肿,骨髓增生异常综合征,骨髓增生异常/骨髓增殖性肿瘤,髓性白血病,骨髓性白血病,多发性骨髓瘤,骨髓增殖性肿瘤,鼻腔和鼻窦癌,鼻咽癌,成神经细胞瘤,非霍奇金淋巴瘤,非小细胞肺癌,口部癌,口腔癌,唇癌,口咽癌,骨肉瘤,卵巢癌,胰腺癌,乳头状瘤病,副神经节瘤,鼻旁窦和鼻腔癌,甲状旁腺癌,阴茎癌,咽癌,嗜铬细胞瘤,垂体癌,浆细胞瘤,胸膜肺胚细胞瘤,妊娠和乳腺癌,原发性中枢神经系统淋巴瘤,原发腹膜癌,前列腺癌,直肠癌,肾细胞癌,视网膜母细胞瘤,横纹肌肉瘤,唾液腺癌,肉瘤,塞扎里综合征,皮肤癌,小细胞肺癌,小肠癌,软组织肉瘤,鳞状细胞癌,鳞状颈癌,胃癌,T细胞淋巴瘤,睾丸癌,咽喉癌,胸腺瘤和胸腺癌,甲状腺癌症,肾盂和输尿管的移行细胞癌,未知原发癌,尿道癌,子宫癌,子宫肉瘤,阴道癌,外阴癌和威尔姆氏瘤。
在一些实施方案中,血液学癌症(例如,与RET相关的癌症的血液学癌症)选自白血病,淋巴瘤(非霍奇金淋巴瘤),霍奇金病(也称霍奇金淋巴瘤)和骨髓瘤,例如,急性淋巴细胞白血病(ALL),急性骨髓性白血病(AML),急性早幼粒细胞白血病(APL),慢性淋巴细胞白血病(CLL),慢性粒细胞白血病(CML),慢性髓单核细胞白血病(CMML),慢性嗜中性白血病(CNL),急性未分化型白血病(AUL),间变性大细胞淋巴瘤(ALCL),幼淋巴细胞白血病(PML),幼年型单核细胞白血病(JMML),成人T细胞ALL,三联型骨髓发育不良的AML(AML/TMDS),混合谱系白血病(MLL),骨髓增生异常综合征(MDS),骨髓增殖性疾病(MPD)和多发性骨髓瘤(MM)。血液癌症的其它示例包括骨髓增`殖性疾病(MPD),如真性红细胞增多症(PV),原发性血小板减少症(ET)和特发性原发性骨髓纤维化(IMF/IPF/PMF)。在一个实施方案中,血液学癌症(例如,作为与RET相关癌症的血液学癌症)是AML或CMML。
在一些实施方案中,癌症(例如RET相关癌症)是实体瘤。实体瘤(例如,作为与RET相关癌症的实体瘤)的示例包括例如甲状腺癌(例如乳头状甲状腺癌,甲状腺髓样癌),肺癌(例如肺腺癌,小细胞肺癌),胰腺癌,胰腺导管癌,乳腺癌,结肠癌,结直肠癌,前列腺癌,肾细胞癌,头颈肿瘤,神经母细胞瘤和黑素瘤。参见,例如,NatureReviewsCancer,2014,14,173-186。
在一些实施方案中,癌症选自肺癌,乳头状甲状腺癌,甲状腺髓样癌,分化的甲状腺癌,复发性甲状腺癌,难治性分化型甲状腺癌,2A或2B型多发性内分泌瘤(分别为MEN2A或MEN2B),嗜铬细胞瘤,甲状旁腺增生,乳腺癌,结直肠癌,乳头状肾细胞癌,胃肠粘膜神经节细胞瘤和宫颈癌。
在一些实施方案中,所述患者是人。
式I化合物及其药学上可接受的盐和溶剂合物也可用于治疗RET相关的癌症。
本文还提供用于在有需要的患者中治疗肠易激综合征(IBS)的方法,所述方法包括(a)确定IBS是否与下述的失调有关:RET基因、RET激酶、或其中任何一者的表达或活性或水平;(b)如果确定IBS与下述的失调有关:RET基因、RET激酶、或其中任何一者的表达或活性或水平,向患者施用治疗有效量的通式I化合物或其药学上可接受的盐或溶剂合物,或其药物组合物。
本文还提供了用于治疗有需要的患者的肠易激综合征(IBS)的药物组合,其包括施用(a)式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物,(b)其他治疗剂,和(c)任选的至少一种药学上可接受的运载体,用于同时、分开或依次用于治疗IBS,其中式I所示的化合物、其立体异构体、消旋体、互变异构体、同位素标记物、氮氧化物、药学上可接受的盐或溶剂合物的量和其他治疗剂的量在治疗IBS方面共同有效。本文还提供了包含这种组合的药物组合物。本文还提供了这种组合在制备用于治疗IBS的药物中的用途。本文还提供了商业包装或产品,其包含这种组合作为用于同时、单独或顺序使用的组合制剂;并涉及一种治疗有需要的患者的IBS的方法。
作为药物时,可按药物组合物的形式给予本发明化合物。可按药剂领域中熟知的方式制备这些组合物,可通过多种途径给予它们,这取决于是否需要局部或全身治疗和所治疗的区域。可局部(例如,透皮、皮肤、眼和粘膜包括鼻内、阴道和直肠递药)、肺(例如,通过吸入或吹入粉末或气雾剂,包括通过喷雾器;气管内、鼻内)、口服或肠胃外给药。肠胃外给药包括静脉内、动脉内、皮下、腹膜内或肌内注射或输注;或颅内例如鞘内或脑室内给药。可按单次大剂量形式肠胃外给药,或可通过例如连续灌注泵给药。局部给予的药用组合物和制剂可包括透皮贴剂、软膏、洗剂、霜剂、凝胶剂、滴剂、栓剂、喷雾剂、液体剂和散剂。常规药物载体、水、粉末或油性基质、增稠剂等可能是必须的或需要的。包衣避孕套(Coatedcondoms)、手套等也可以是有用的。
在制备本发明的组合物时,通常将活性成分与赋形剂混合,通过赋形剂稀释或装入例如胶囊、小药囊、纸或其它容器形式的这种载体内。当赋形剂用作稀释剂时,它可以是固体、半固体或液体物质,用作溶媒、载体或活性成分的介质。因此,组合物可以是以下形式:片剂、丸剂、散剂、锭剂、小药囊、扁囊剂、酏剂、混悬剂、乳剂、溶液剂、糖浆剂、气雾剂(固体或溶于液体溶媒);含例如高达10%重量活性化合物的软膏剂、软和硬明胶胶囊、栓剂、无菌注射溶液和无菌包装粉末。
适宜的赋形剂的某些实例包括乳糖、葡萄糖、蔗糖、山梨醇、甘露醇、淀粉、阿拉伯胶、磷酸钙、藻酸盐、黄蓍胶、明胶、硅酸钙、微晶纤维素、聚乙烯吡咯烷酮、纤维素、水、糖浆和甲基纤维素。制剂还可含有:润滑剂例如滑石粉、硬脂酸镁和矿物油;湿润剂;乳化剂和悬浮剂;防腐剂例如苯甲酸甲酯和苯甲酸羟基丙酯;甜味剂和矫味剂。可通过使用本领域中已知的方法配制本发明组合物,以便在给予患者后提供速释、缓释或延迟释放活性成分的作用。
可按单位剂型配制组合物,每一剂量含约5~1000mg,更通常约100~500mg活性成分。术语“单位剂型”是指物理上分离的适宜作为用于人患者和其它哺乳动物的单一剂量单位,各单位含有与适宜的药物赋形剂混合的经计算可产生所需疗效的预定量的活性物质。
活性化合物的有效剂量的范围可很大,通常按药用有效量给药。但是,可以理解实际给予的化合物的量通常由医师根据相关情况决定,它们包括所治疗的病症、所选择的给药途径、所给予的实际化合物;患者个体的年龄、重量和反应;患者症状的严重程度等。
对于制备固体组合物例如片剂,将主要的活性成分与药物赋形剂混合,形成含本发明化合物的均匀混合物的固体预制剂组合物。当称这些预制剂组合物为均匀时,是指活性成分通常均匀地分布在整个组合物中,致使该组合物可容易地划分为同等有效的单位剂型例如片剂、丸剂和胶囊剂。然后将该固体预制剂划分为上述类型的含例如约0.1~1000mg本发明活性成分的单位剂型。
可将本发明片剂或丸剂包衣或复合,得到提供长效作用优点的剂型。例如,片剂或丸剂含内剂量和外剂量组分,后者是前者的被膜形式。可通过肠溶层将两种组分隔离,肠溶层用于在胃中阻止崩解,以使内组分完整通过十二指肠或延迟释放。多种物质可用于此类肠溶层或包衣剂,此类物质包括多种高分子酸和高分子酸与此类物质如虫胶、鲸蜡醇和醋酸纤维素的混合物。
其中可掺入本发明化合物和组合物,用于口服或注射给药的液体形式包括水溶液、适当矫味的糖浆剂、水或油混悬液;和用食用油例如棉子油、芝麻油、椰子油或花生油矫味的乳剂;以及酏剂和类似的药用溶媒。
用于吸入或吹入的组合物包括溶于药学上可接受的水或有机溶剂或其混合物的溶液剂和混悬液、散剂。液体或固体组合物可含有如上所述适宜的药学上可接受的赋形剂。在某些实施方案中,通过口服或鼻呼吸途径给予组合物,实现局部或全身作用。可通过使用惰性气体,使组合物成雾化。可直接由雾化装置吸入雾化溶液,或雾化装置可与面罩帷或间歇正压呼吸机连接。可通过口服或由按适当方式递送制剂的装置通过鼻给予溶液、混悬液或粉末组合物。
给予患者的化合物或组合物的量不固定,取决于给予的药物、给药的目的例如预防或治疗;患者的状态、给药的方式等。在治疗应用时,可给予已患疾病的患者足够治愈或至少部分抑制疾病及其并发症症状的量的组合物。有效剂量应取决于所治疗的疾病状态和主治临床医师的判断,该判断取决于例如疾病的严重程度、患者的年龄、体重和一般状况等因素。
给予患者的组合物可以是上述药用组合物形式。可通过常规灭菌技术或可过滤灭菌,将这些组合物灭菌。可将水溶液包装原样使用,或冻干,给药前,将冻干制剂与无菌水性载体混合。化合物制剂的pH通常为3~11,更优选5~9,最优选7~8。可以理解,使用某些前述赋形剂、载体或稳定剂会导致形成药物盐。
本发明化合物的治疗剂量可根据例如以下而定:治疗的具体用途、给予化合物的方式、患者的健康和状态,以及签处方医师的判断。本发明化合物在药用组合物中的比例或浓度可不固定,取决于多种因素,它们包括剂量、化学特性(例如疏水性)和给药途径。例如可通过含约0.1~10%w/v该化合物的生理缓冲水溶液提供本发明化合物,用于肠胃外给药。某些典型剂量范围为约1μg/kg~约1g/kg体重/日。在某些实施方案中,剂量范围为约0.01mg/kg~约100mg/kg体重/日。剂量很可能取决于此类变量,如疾病或病症的种类和发展程度、具体患者的一般健康状态、所选择的化合物的相对生物学效力、赋形剂制剂及其给药途径。可通过由体外或动物模型试验系统导出的剂量-反应曲线外推,得到有效剂量。
术语与定义
除非另有说明,本申请说明书和权利要求书中记载的基团和术语定义,包括其作为实例的定义、示例性的定义、优选的定义、表格中记载的定义、实施例中具体化合物的定义等,可以彼此之间任意组合和结合。这样的组合和结合后的基团定义及化合物结构,应当属于本申请说明书记载的范围内。
除非另有说明,本说明书和权利要求书记载的数值范围相当于至少记载了其中每一个具体的整数数值。例如,数值范围“1-40”相当于记载了数值范围“1-10”中的每一个整数数值即1、2、3、4、5、6、7、8、9、10,以及数值范围“11-40”中的每一个整数数值即11、12、13、14、15、......、35、36、37、38、39、40。应当理解,本文在描述取代基时使用的一个、两个或更多个中,“更多个”应当是指≥3的整数,例如3、4、5、6、7、8、9或10。此外,当某些数值范围被定义为“数”时,应当理解为记载了该范围的两个端点、该范围内的每一个整数以及该范围内的每一个小数。例如,“0~10的数”应当理解为不仅记载了0、1、2、3、4、5、6、7、8、9和10的每一个整数,还至少记载了其中每一个整数分别与0.1、0.2、0.3、0.4、0.5、0.6、0.7、0.8、0.9的和。
术语“卤素”表示氟、氯、溴和碘。
术语“C1-40烷基”应理解为优选表示具有1~40个碳原子的直链或支链饱和一价烃基。例如,“C1-6烷基”表示具有1、2、3、4、5或6个碳原子的直链和支链烷基。所述烷基是例如甲基、乙基、丙基、丁基、戊基、己基、异丙基、异丁基、仲丁基、叔丁基、异戊基、2-甲基丁基、1-甲基丁基、1-乙基丙基、1,2-二甲基丙基、新戊基、1,1-二甲基丙基、4-甲基戊基、3-甲基戊基、2-甲基戊基、1-甲基戊基、2-乙基丁基、1-乙基丁基、3,3-二甲基丁基、2,2-二甲基丁基、1,1-二甲基丁基、2,3-二甲基丁基、1,3-二甲基丁基或1,2-二甲基丁基等或它们的异构体。
术语“C2-40烯基”应理解为优选表示直连或支链的一价烃基,其包含一个或多个双键并且具有2~40个碳原子,优选“C2-6烯基”。“C2-6烯基”应理解为优选表示直连或支链的一价烃基,其包含一个或多个双键并且具有2、3、4、5或6个碳原子,特别是2或3个碳原子(“C2-3烯基”),应理解,在所述烯基包含多于一个双键的情况下,所述双键可相互分离或者共轭。所述烯基是例如乙烯基、烯丙基、(E)-2-甲基乙烯基、(Z)-2-甲基乙烯基、(E)-丁-2-烯基、(Z)-丁-2-烯基、(E)-丁-1-烯基、(Z)-丁-1-烯基、戊-4-烯基、(E)-戊-3-烯基、(Z)-戊-3-烯基、(E)-戊-2-烯基、(Z)-戊-2-烯基、(E)-戊-1-烯基、(Z)-戊-1-烯基、己-5-烯基、(E)-己-4-烯基、(Z)-己-4-烯基、(E)-己-3-烯基、(Z)-己-3-烯基、(E)-己-2-烯基、(Z)-己-2-烯基、(E)-己-1-烯基、(Z)-己-1-烯基、异丙烯基、2-甲基丙-2-烯基、1-甲基丙-2-烯基、2-甲基丙-1-烯基、(E)-1-甲基丙-1-烯基、(Z)-1-甲基丙-1-烯基、3-甲基丁-3-烯基、2-甲基丁-3-烯基、1-甲基丁-3-烯基、3-甲基丁-2-烯基、(E)-2-甲基丁-2-烯基、(Z)-2-甲基丁-2-烯基、(E)-1-甲基丁-2-烯基、(Z)-1-甲基丁-2-烯基、(E)-3-甲基丁-1-烯基、(Z)-3-甲基丁-1-烯基、(E)-2-甲基丁-1-烯基、(Z)-2-甲基丁-1-烯基、(E)-1-甲基丁-1-烯基、(Z)-1-甲基丁-1-烯基、1,1-二甲基丙-2-烯基、1-乙基丙-1-烯基、1-丙基乙烯基、1-异丙基乙烯基。
术语“C2-40炔基”应理解为表示直连或支链的一价烃基,其包含一个或多个三键并且具有2~40个碳原子,优选“C2-C6-炔基”。术语“C2-C6-炔基”应理解为优选表示直连或支链的一价烃基,其包含一个或多个三键并且具有2、3、4、5或6个碳原子,特别是2或3个碳原子(“C2-C3-炔基”)。所述C2-C6-炔基是例如乙炔基、丙-1-炔基、丙-2-炔基、丁-1-炔基、丁-2-炔基、丁-3-炔基、戊-1-炔基、戊-2-炔基、戊-3-炔基、戊-4-炔基、己-1-炔基、己-2-炔基、己-3-炔基、己-4-炔基、己-5-炔基、1-甲基丙-2-炔基、2-甲基丁-3-炔基、1-甲基丁-3-炔基、1-甲基丁-2-炔基、3-甲基丁-1-炔基、1-乙基丙-2-炔基、3-甲基戊-4-炔基、2-甲基戊-4-炔基、1-甲基戊-4-炔基、2-甲基戊-3-炔基、1-甲基戊-3-炔基、4-甲基戊-2-炔基、1-甲基戊-2-炔基、4-甲基戊-1-炔基、3-甲基戊-1-炔基、2-乙基丁-3-炔基、1-乙基丁-3-炔基、1-乙基丁-2-炔基、1-丙基丙-2-炔基、1-异丙基丙-2-炔基、2,2-二甲基丁-3-炔基、1,1-二甲基丁-3-炔基、1,1-二甲基丁-2-炔基或3,3-二甲基丁-1-炔基。特别地,所述炔基是乙炔基、丙-1-炔基或丙-2-炔基。
术语“C3-40环烷基”应理解为表示饱和的一价单环、双环烃环或桥环烷烃,其具有3~40个碳原子,优选“C3-10环烷基”。术语“C3-10环烷基”应理解为表示饱和的一价单环、双环烃环或桥环烷烃,其具有3、4、5、6、7、8、9或10个碳原子。所述C3-10环烷基可以是单环烃基,如环丙基、环丁基、环戊基、环己基、环庚基、环辛基、环壬基或环癸基,或者是双环烃基如十氢化萘环。
术语“3-20元杂环基”意指饱和的一价单环、双环烃环或桥环烷烃,其包含1-5个独立选自N、O和S的杂原子的总成环原子数为3-20(如原子数为3、4、5、6、7、8、9、10等)的非芳族环状基团,优选“3-10元杂环基”。术语“3-10元杂环基”意指饱和的一价单环、双环烃环或桥环烷烃,其包含1-5个,优选1-3个选自N、O和S的杂原子。所述杂环基可以通过所述碳原子中的任一个或氮原子(如果存在的话)与分子的其余部分连接。特别地,所述杂环基可以包括但不限于:4元环,如氮杂环丁烷基、氧杂环丁烷基;5元环,如四氢呋喃基、二氧杂环戊烯基、吡咯烷基、咪唑烷基、吡唑烷基、吡咯啉基;或6元环,如四氢吡喃基、哌啶基、吗啉基、二噻烷基、硫代吗啉基、哌嗪基或三噻烷基;或7元环,如二氮杂环庚烷基。任选地,所述杂环基可以是苯并稠合的。所述杂环基可以是双环的,例如但不限于5,5元环,如六氢环戊并[c]吡咯-2(1H)-基环,或者5,6元双环,如六氢吡咯并[1,2-a]吡嗪-2(1H)-基环。含氮原子的环可以是部分不饱和的,即它可以包含一个或多个双键,例如但不限于2,5-二氢-1H-吡咯基、4H-[1,3,4]噻二嗪基、4,5-二氢恶唑基或4H-[1,4]噻嗪基,或者,它可以是苯并稠合的,例如但不限于二氢异喹啉基。根据本发明,所述杂环基是无芳香性的。所述3-20元杂环基与其它基团相连构成本发明的化合物时,可以为3-20元杂环基上的碳原子与其它基团相连,也可以为3-20元杂环基环上杂环原子与其它基团相连。例如当3-20元杂环基选自哌嗪基时,可以为哌嗪基上的氮原子与其它基团相连。或当3-20元杂环基选自哌啶基时,可以为哌啶基环上的氮原子和其对位上的碳原子与其它基团相连。
术语“C6-20芳基”应理解为优选表示具有6~20个碳原子的一价芳香性或部分芳香性的单环、双环或三环烃环,优选“C6-14芳基”。术语“C6-14芳基”应理解为优选表示具有6、7、8、9、10、11、12、13或14个碳原子的一价芳香性或部分芳香性的单环、双环或三环烃环(“C6-14芳基”),特别是具有6个碳原子的环(“C6芳基”),例如苯基;或联苯基,或者是具有9个碳原子的环(“C9芳基”),例如茚满基或茚基,或者是具有10个碳原子的环(“C10芳基”),例如四氢化萘基、二氢萘基或萘基,或者是具有13个碳原子的环(“C13芳基”),例如芴基,或者是具有14个碳原子的环(“C14芳基”),例如蒽基。当所述C6-20芳基被取代时,其可以为单取代或者多取代。并且,对其取代位点没有限制,例如可以为邻位、对位或间位取代。
术语“5-20元杂芳基”应理解为包括这样的一价单环、双环或三环芳族环系:其具有5~20个环原子且包含1-5个独立选自N、O和S的杂原子,例如“5-14元杂芳基”。术语“5-14元杂芳基”应理解为包括这样的一价单环、双环或三环芳族环系:其具有5、6、7、8、9、10、11、12、13或14个环原子,特别是5或6或9或10个碳原子,且其包含1-5个,优选1-3各独立选自N、O和S的杂原子并且,另外在每一种情况下可为苯并稠合的。特别地,杂芳基选自噻吩基、呋喃基、吡咯基、恶唑基、噻唑基、咪唑基、吡唑基、异恶唑基、异噻唑基、恶二唑基、三唑基、噻二唑基、噻-4H-吡唑基等以及它们的苯并衍生物,例如苯并呋喃基、苯并噻吩基、苯并恶唑基、苯并异恶唑基、苯并咪唑基、苯并三唑基、吲唑基、吲哚基、异吲哚基等;或吡啶基、哒嗪基、嘧啶基、吡嗪基、三嗪基等,以及它们的苯并衍生物,例如喹啉基、喹唑啉基、异喹啉基等;或吖辛因基、吲嗪基、嘌呤基等以及它们的苯并衍生物;或噌啉基、酞嗪基、喹唑啉基、喹喔啉基、萘啶基、蝶啶基、咔唑基、吖啶基、吩嗪基、吩噻嗪基、吩恶嗪基等。当所述5-20元杂芳基与其它基团相连构成本发明的化合物时,可以为5-20元杂芳基环上的碳原子与其它基团相连,也可以为5-20元杂芳基环上的杂原子与其它基团相连。当所述5-20元杂芳基被取代时,其可以为单取代或者多取代。并且,对其取代位点没有限制,例如可以为杂芳基环上与碳原子相连的氢被取代,或者杂芳基环上与杂原子相连的氢被取代。
除非另有说明,杂环基、杂芳基或亚杂芳基包括其所有可能的异构形式,例如其位置异构体。因此,对于一些说明性的非限制性实例,可以包括在其1-、2-、3-、4-、5-、6-、7-、8-、9-、10-、11-、12-位等(如果存在)中的一个、两个或更多个位置上取代或与其他基团键合的形式,包括吡啶-2-基、亚吡啶-2-基、吡啶-3-基、亚吡啶-3-基、吡啶-4-基和亚吡啶-4-基;噻吩基或亚噻吩基包括噻吩-2-基、亚噻吩-2-基、噻吩-3-基和亚噻吩-3-基;吡唑-1-基、吡唑-3-基、吡唑-4-基、吡唑-5-基。
术语“氧代”是指取代基中的碳原子、氮原子或硫原子被氧化后形成的氧基取代(=O)。
除非另有说明,本文中术语的定义同样适用于包含该术语的基团,例如C1-6烷基的定义也适用于C1-6烷基氧基、-N(C1-6烷基)2、-NHC1-6烷基或-S(O)2-C1-6烷基等。
本领域技术人员可以理解,式I所示化合物可以以各种药学上可接受的盐的形式存在。如果这些化合物具有碱性中心,则其可以形成酸加成盐;如果这些化合物具有酸性中心,则其可以形成碱加成盐;如果这些化合物既包含酸性中心(例如羧基)又包含碱性中心(例如氨基),则其还可以形成内盐。本申请中化合物成盐的个数由碱性中心或酸性中心决定。例如当化合物中含有多个成盐位点时,则成盐个数等于成盐位点数。酸加成盐包括但不限于:盐酸盐、氢氟酸盐、氢溴酸盐、氢碘酸盐、硫酸盐、焦硫酸盐、磷酸盐、硝酸盐、甲磺酸盐、乙磺酸盐、2-羟基乙磺酸盐、苯磺酸盐、甲苯磺酸盐、氨基磺酸盐、2-萘磺酸盐、甲酸盐、乙酰乙酸、丙酮酸、月硅酸酯、肉桂酸酯、苯甲酸盐、醋酸盐、二羟乙酸盐、三氟乙酸盐、三甲基乙酸盐、丙酸盐、丁酸盐、己酸盐、庚酸盐、十一酸盐、硬脂酸盐、抗坏血酸盐、樟脑酸盐、樟脑磺酸盐、柠檬酸盐、富马酸盐、苹果酸盐、马来酸盐、羟基马来酸盐、草酸盐、水杨酸盐、琥珀酸盐、葡萄糖酸盐、奎尼酸盐、双羟萘酸盐、甘醇酸盐、酒石酸盐、乳酸盐、2-(4-羟基苯甲酰基)苯甲酸盐、环戊烷丙酸盐、二葡糖酸盐、3-羟基-2-萘甲酸盐、烟酸盐、扑酸盐、果胶酯酸盐、3-苯基丙酸盐、苦味酸盐、特戊酸盐、衣康酸盐、三氟甲磺酸盐、十二烷基硫酸盐、对甲苯磺酸盐、萘二磺酸盐、丙二酸盐、己二酸盐、藻酸盐、扁桃酸盐、葡庚酸盐、甘油磷酸盐、磺基水杨酸盐、半硫酸或硫氰酸盐、天冬氨酸盐等;碱加成盐例如碱金属盐、碱土金属盐和铵盐等,具体包括但不限于:钠盐、锂盐、钾盐、铵盐、铝盐、镁盐、钙盐、钡盐、铁盐、亚铁盐、锰盐、亚锰盐、锌盐、铵盐(包括与NH3和有机胺形成的盐(NH4盐)、甲铵盐、三甲铵盐、二乙铵盐、三乙铵盐、丙铵盐、三丙铵盐、异丙铵盐、叔丁铵盐、N,N′-二苄基乙二铵盐、二环己铵盐、1,6-己二铵盐、苄铵盐、乙醇铵盐、N,N-二甲基乙醇铵盐、N,N-二乙基乙醇铵盐、三乙醇铵盐、氨丁三醇盐、赖氨酸盐、精氨酸盐、组氨酸盐、葡糖铵盐、N-甲基葡糖铵盐、二甲基葡糖铵盐、乙基葡糖铵盐、葡甲铵盐、甜菜碱盐、咖啡因盐、氯普鲁卡因盐、普鲁卡因盐、利多卡因盐、吡啶盐、甲基吡啶盐、哌啶盐、吗啉盐、哌嗪盐、嘌呤盐、可可碱盐、胆碱盐)等。
本发明的化合物可以溶剂合物(如水合物)的形式存在,其中本发明的化合物包含作为所述化合物晶格的结构要素的极性溶剂,特别是例如水、甲醇或乙醇。极性溶剂特别是水的量可以化学计量比或非化学计量比存在。
根据其分子结构,本发明的化合物可以是手性的,因此可能存在各种对映异构体形式。因而这些化合物可以以消旋体形式或光学活性形式存在。本发明的化合物或其中间体可以通过本领域技术人员公知的化学或物理方法分离为对映异构体化合物,或者以此形式用于合成。在外消旋的胺的情况中,通过与光学活性的拆分试剂反应,从混合物制得非对映异构体。适当的拆分试剂的示例是光学活性的酸,例如R和S形式的酒石酸、二乙酰酒石酸、二苯甲酰酒石酸、扁桃酸、苹果酸、乳酸、适当的N-保护的氨基酸(例如N-苯甲酰脯氨酸或N-苯磺酰基脯氨酸)或各种光学活性的樟脑磺酸。借助光学活性的拆分试剂(例如固定在硅胶上的二硝基苯甲酰基苯基甘氨酸、三乙酸纤维素或其它碳水化合物的衍生物或手性衍生化的异丁烯酸酯聚合物),也可有利地进行色谱对映体拆分。用于此目的的适当的洗脱剂是含水或含醇的溶剂混合物,例如,己烷/异丙醇/乙腈。
术语“互变异构体”是指因分子中某一原子在两个位置迅速移动而产生的官能团异构体。本发明化合物可表现出互变异构现象。互变异构的化合物可以存在两种或多种可相互转化的种类。质子移变互变异构体来自两个原子之间共价键合的氢原子的迁移。互变异构体一般以平衡形式存在,尝试分离单一互变异构体时通常产生一种混合物,其理化性质与化合物的混合物是一致的。平衡的位置取决于分子内的化学特性。例如,在很多脂族醛和酮如乙醛中,酮型占优势;而在酚中,烯醇型占优势。本发明包含化合物的所有互变异构形式。
可以根据已知的方法,例如通过萃取、过滤或柱层析来分离相应的稳定异构体。
术语“患者”是指包括哺乳动物在内的任何动物,优选小鼠、大鼠、其它啮齿类动物、兔、狗、猫、猪、牛、羊、马或灵长类动物,最优选人。
本文中使用的短语“治疗有效量”是指研究人员、兽医、医师或其它临床医师正在组织、系统、动物、个体或人中寻找的引起生物学或医学反应的活性化合物或药物的量,它包括以下一项或多项:(1)预防疾病:例如在易感染疾病、紊乱或病症但尚未经历或出现疾病病理或症状的个体中预防疾病、紊乱或病症。(2)抑制疾病:例如在正经历或出现疾病、紊乱或病症的病理或症状的个体中抑制疾病、紊乱或病症(即阻止病理和/或症状的进一步发展)。(3)缓解疾病:例如在正经历或出现疾病、紊乱或病症的病理或症状的个体中缓解疾病、紊乱或病症(即逆转病理和/或症状)。
有益效果
发明人惊讶地发现,本发明制备的化合物具有显著的抑制RET野生型、突变型和融合型抑制活性。并且相对于现有化合物其活性有明显改善。与其他RET抑制剂相比,本发明的代表性实施例化合物还具有特别优异的药代动力学性质,作为活性成分时可以较小的剂量给患者施用,从而降低患者的治疗成本。并且,本申请的化合物制备方法简单,适于规模化生产。
具体实施方式
下文将结合具体实施例对本发明的技术方案做更进一步的详细说明。应当理解,下列实施例仅为示例性地说明和解释本发明,而不应被解释为对本发明保护范围的限制。凡基于本发明上述内容所实现的技术均涵盖在本发明旨在保护的范围内。
除非另有说明,以下实施例中使用的原料和试剂均为市售商品,或者可以通过已知方法制备。
对比例1
上式所示化合物APS001可参考专利文献CN108349969A的实施例342公开的方法,在制备得到的APS001三氟乙酸盐后,在碱性条件下(碳酸氢钠)游离,得到目标化合物APS001。
化合物APS002可参考专利文献CN108349969A实施例570公开的方法进行制备,得到目标化合物APS002。
实施例1
2-氨基-4-(6-(4-(2-(5-氟吡啶-2-基)乙酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS069)
步骤A:叔丁基((甲磺酰基)氧基)氨基甲酸酯
冰浴搅拌下,向盛有2,4,6-三甲基苯磺酰氯(20.0g,91.5mmol)和N-羟基氨基甲酸叔丁酯(12.2g,91.5mmol)的甲基叔丁基醚(500mL)溶液中,恒压滴液漏斗缓慢滴加三乙胺(13.0mL,93.3mmol),滴加过程中保持反应体系温度小于5℃。在冰浴下,反应体系搅拌40小时,减压过滤移去三乙胺盐酸盐,并用甲基叔丁基醚冲洗三次,所有滤液在小于15℃水浴温度下减压浓缩除去大部分甲基叔丁基醚;在冰浴下,向浓缩的残留物中加入正己烷,强烈搅拌10分钟,析出大量白色固体,减压过滤,滤饼用正己烷冲洗两次,真空干燥得到叔丁基((甲磺酰基)氧基)氨基甲酸酯(26.1g,90%收率)。m/z=316[M+1]+。
步骤B:2-[(氨基氧基)磺酰]-1,3,5-三甲基苯
零度下,向三氟乙酸(80mL)中分批次加入叔丁基((甲磺酰基)氧基)氨基甲酸酯(10.0g,31.7mmol),完毕后,反应体系在零度下继续搅拌3小时;TLC点板确认反应完毕,将反应体系倒入大量的冰水中,并搅拌15分钟,析出大量白色固体,减压过滤,大量水洗涤滤饼直到固体PH值为中性,减压抽滤至固体含水量20%左右即可,无须再次纯化,直接用于下一步反应。
步骤C:2,4,6-三甲基苯磺酸1-氨基-3-溴-5-甲氧基吡啶-1-鎓
零度下,向2-[(氨基氧基)磺酰]-1,3,5-三甲基苯(6.8g,31.7mmol)的二氯甲烷(50mL)溶液中加入3-溴-5-甲氧基吡啶(6.0g,32.0mmol),并在零度下继续搅拌3小时,析出大量白色固体;反应完毕后,零度下向反应体系中加入乙醚(50mL)并搅拌10分钟,减压过滤,乙醚冲洗,真空干燥得到2,4,6-三甲基苯磺酸1-氨基-3-溴-5-甲氧基吡啶-1-鎓(12.8g,100%收率),无须再次纯化,直接用于下一步反应。
m/z=204[M+1]+。
步骤D:乙基2-氰基亚氨代乙酸酯盐酸盐
向封管中加入丙二腈(4.9g,74.2mmol)、无水乙醇(3.4g,74.2mmol)和乙酸乙酯(6mL),反应体系冷却至0℃,向反应体系中滴加2M HCl乙醚溶液(41mL,82mmol),加料完毕后升温至室温,析出大量白色固体,室温下反应过夜,TLC点板监测反应完毕,减压过滤,乙醚洗涤,真空干燥得到乙基2-氰基亚氨代乙酸酯盐酸盐(9.0g,82%收率),无须再次纯化,直接用于下一步反应。
步骤E:2-氨基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈
室温下,向装有2,4,6-三甲基苯磺酸1-氨基-3-溴-5-甲氧基吡啶-1-鎓(4.0g,10.0mmol)的DMF(30mL)溶液中加入碳酸钾(4.2g,30.0mmol);反应体系冷却至0℃,分批次加入乙基2-氰基亚氨代乙酸酯盐酸盐(3.0g,20.0mmol),升至室温下搅拌1小时,再90℃下搅拌1小时;反应完毕并冷却至室温,加水淬灭,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-氨基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈(420mg,16%收率)。
1HNMR(400MHz,DMSO-d6)δ8.48(d,J=0.8Hz,1H),7.08(s,1H),6.34(brs,2H),3.95(s,3H)。m/z=267[M+1]+。
步骤F:2-氨基-4-甲氧基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈
室温下,向装有2-氨基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈(220mg,0.824mmol)和1-甲基-4-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)-1H-吡唑(206mg,0.989mmol)的1,4-二氧六环(10mL)溶液中加入碳酸钠(262mg,2.472mmol,2M)水溶液,氮气换气2分钟,随后加入四三苯基膦钯(190mg,0.165mmol),氮气换气2分钟,在80℃下搅拌3小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-氨基-4-甲氧基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(220mg,100%收率)。1HNMR(400MHz,DMSO-d6)δ8.42(s,1H),8.27(s,1H),8.00(s,1H),7.12(s,1H),6.22(s,2H),4.05(s,3H),3.89(d,3H).m/z=269[M+1]+。
步骤G:2-氨基-4-羟基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈
室温下,向装有2-氨基-4-甲氧基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(220mg,0.824mmol)的1,2-二氯乙烷(15mL)的混悬液中加入无水三氯化铝(550mg,4.12mmol),移至80℃下搅拌3小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-氨基-4-羟基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(70mg,33%收率)。m/z=255[M+1]+。
步骤H:2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基三氟甲磺酸酯
室温下,向装有2-氨基-4-羟基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(70mg,0.275mmol)的DMA(5mL)溶液中加入DIEA(71mg,0.55mmol)和N-苯基双(三氟甲烷)磺酰亚胺(148mg,0.413mmol),搅拌反应3小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基三氟甲磺酸酯(26mg,25%收率)。m/z=387[M+1]+。
步骤I:2-(5-氟吡啶-2-基)丙二酸二乙酯
向1000mL单口瓶中加入2-溴-5-氟吡啶(20g,0.114mol),二吡啶甲酸(11.22g,0.091mol),丙二酸二乙酯(73g,0.457mol),碘化亚铜(8.66g,0.046mol),碳酸铯(111.49g,0.342mol),1,4-二氧六环(400mL),100℃反应24小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-(5-氟吡啶-2-基)丙二酸二乙酯(16.6g,57%收率)。m/z=256[M+1]+。
步骤J:2-(5-氟吡啶-2-基)乙酸乙酯
向250mL单口瓶中加入2-(5-氟吡啶-2-基)丙二酸二乙酯(16.6g,0.065mol),氯化钠(15.1g,0.26mol),水(4.68g,0.26mol),DMSO(50mL),150℃下反应48小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品2-(5-氟吡啶-2-基)乙酸乙酯(9.1g,76%收率)。m/z=184[M+1]+。
步骤K:2-(5-氟吡啶-2-基)乙酸
室温下,向250mL单口瓶中加入2-(5-氟吡啶-2-基)乙酸乙酯(5.5g,0.03mol),四氢呋喃(50mL),水(10mL),氢氧化锂(1.44g,0.06mol),60℃反应24小时,TLC检测原料反应完,将反应液浓缩至原反应液的三分之一体积,加乙酸乙酯萃取,分层得水相,水相用1M盐酸调节pH至3左右,再用乙酸乙酯萃取,合并有机相,用无水硫酸钠干燥,过滤,减压浓缩得到产品2-(5-氟吡啶-2-基)乙酸(2.04g,44%收率)。
步骤L:1-(5-溴吡啶)-2-哌嗪
室温下,向250mL单口瓶中加入2,5-二溴吡啶(12.2g,0.052mol),哌嗪(5.0g,0.058mol),加入DMSO(20mL)中,120℃下反应过夜。经LCMS确认反应完全,减压除去DMSO,加入甲醇使其溶解,冰水浴下慢慢加入饱和氢氧化钠溶液,调节pH至13左右,再搅拌2小时。减压浓缩出甲醇,二氯甲烷萃取,无水硫酸钠干燥,减压浓缩,柱层析分离得到产品(4.6g,37%收率)。m/z=242[M+1]+。
步骤M:1-(4-(5-溴吡啶-2)哌嗪-1-基)-2-(5-氟吡啶-2-基)乙酮
向100mL单口瓶中,加入2-(5-氟吡啶-2-基)乙酸(500mg,3.22mmol),HATU(1.34g,3.54mmol),DIEA(830mg,6.44mmol),DMF(20mL),室温搅拌15分钟,加入1-(5-溴吡啶)-2-哌嗪(780mg,3.22mmol),搅拌2小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品1-(4-(5-溴吡啶-2)哌嗪-1-基)-2-(5-氟吡啶-2-基)乙酮(350mg,29%收率)。m/z=379[M+1]+。
步骤N:2-(5-氟吡啶-2-基)-1-(4-(5-(4,4,5,5-四甲基-1,3,2-二恶硼酸-2-基)吡啶-2-基)哌嗪-1-基)乙酮
向10mL封管中,加入1-(4-(5-溴吡啶-2)哌嗪-1-基)-2-(5-氟吡啶-2-基)ethan-1-酮(50mg,0.132mmol),双联频哪醇硼酸酯(50mg,0.198mmol),[1,1′-双(二苯基膦基)二茂铁]二氯化钯(10mg,0.013mmol),醋酸钾(39mg,0.396mmol),1,4-二氧六环(20mL),氮气置换三次,100℃反应3小时,LCMS确认原料反应完,有产品生成,无须后处理,直接进行下一步反应。m/z=427[M+1]+。
步骤O:2-氨基-4-(6-(4-(2-(5-氟吡啶-2-基)乙酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈
向步骤N的反应液中加入2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基三氟甲磺酸酯(26mg,0.067mol),四三苯基磷钯(15mg,0.013mol),碳酸钾(36mg,0.264mol),1,4-二氧六环(2mL),H2O(1mL),氮气置换三次,90℃反应2小时,LCMS确认原料反应完,有产品生成。加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品2-氨基-4-(6-(4-(2-(5-氟吡啶-2-基)乙酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(5mg,13%收率)。
1HNMR(400MHz,DMSO-d6)δ8.79(s,1H),8.49(d,J=3.0Hz,1H),8.35(d,J=2.5Hz,1H),8.28(s,1H),8.02(s,1H),7.80-7.78(m,1H),7.71-7.66(m,1H),7.57-7.54(m,1H),7.42-7.38(m,1H),6.98(d,J=8.8Hz,1H),6.29(s,2H),3.98(s,2H),3.86(s,3H),3.70-3.69(d,J=4.4Hz,2H),3.613-3.604(d,J=3.6Hz,6H)。m/z=537[M+1]+。
实施例2
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡啶-2-基甲基)哌啶-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS070)
步骤A:1-叔丁氧羰基-4-(吡啶-2-基甲基)哌啶
将1-叔丁氧羰基-4-亚甲基哌啶(6.0g,30.4mmol)和9-硼杂双环[3,3,2]壬烷(60.8mL,30.4mmol,形成的THF溶液浓度为2M)的混合物在氮气保护下加热回流3小时,冷却至室温,向反应混合物中加入2-溴吡啶(5.28g,33.44mmol),1,1′-二(二苯膦基)二茂铁二氯化钯(II)(666mg,0.91mmol),碳酸钾(5.04g,36.48mmol),DMF(80mL),H2O(12mL);反应混合物在60℃下搅拌过夜,TLC点板反应完毕,加入水,用10%氢氧化钠水溶液调节反应混合物pH值至11,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品1-叔丁氧羰基-4-(吡啶-2-基甲基)哌啶(5.8g,69%收率)。m/z=277[M+1]+。
步骤B:2-(哌啶-4-基甲基)吡啶盐酸盐
室温下,向装有1-叔丁基氧羰基-4-(吡啶2-基甲基)哌啶(5.66g,20.507mmol)的1,4-二氧六环(21mL)溶液中滴加氯化氢的1,4-二氧六环溶液(21mL,84.000mmol,4M),在室温下搅拌1小时,TLC点板反应完毕,减压浓缩,乙酸乙酯(100mL)打浆得到产品2-(哌啶-4-基甲基)吡啶盐酸盐(6.10g,140%收率),直接用于下一步反应。m/z=177[M+1]+。
步骤C:5-溴-2-(4-(吡啶-2-基甲基)哌啶-1-基)吡啶
室温下,向装有2-(哌啶-4-基甲基)吡啶盐酸盐(7.60g,35.681mmol)的二甲基亚砜(80mL)溶液中加入2,5-二溴吡啶(10.00g,42.195mmol),无水磷酸钾(38.00g,179.245mmol),在120℃下搅拌12小时,TLC点板反应完毕,降至室温,过滤出多余的磷酸钾固体,用乙酸乙酯洗滤饼,滤液加入水洗涤,收集有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得到产品5-溴-2-(4-(吡啶-2-基甲基)哌啶-1-基)吡啶(7.00g,59%收率)。m/z=332[M+1]+。
步骤D:2-(4-(吡啶-2-基甲基)哌啶-1-基)-5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶
室温下,向装有5-溴-2-(4-(吡啶-2-基甲基)哌啶-1-基)吡啶(500mg,1.505mmol)和联硼酸频那醇酯(421mg,1.656mmol)的1,4-二氧六环(4.5mL)溶液中加入乙酸钾(443mg,4.515mmol),氮气换气2分钟,随后加入[1,1′-双(二苯基膦基)二茂铁]二氯化钯(110mg,0.151mmol),氮气换气2分钟,在100℃下搅拌1小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,打浆得到产品2-(4-(吡啶-2-基甲基)哌啶-1-基)-5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶(480mg,84%收率)。m/z=380[M+1]+。
步骤E:2-氨基-6-(1-甲基1-1氢-吡唑-4-基)-4-(6-(4-吡啶-2-基甲基)哌啶-1-基)哌啶-3-基)吡唑[1,5-a]吡啶-3-腈
室温下,向装有2-(4-(吡啶-2-基甲基)哌啶-1-基)-5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶(33mg,0.087mmol)和2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑[1,5-a]吡啶-4-基-三氟甲磺酸盐(28mg,0.073mmol)的1,4-二氧六环(2.5mL)溶液中加入碳酸钠(38mg,0.358mmol,2M)水溶液,氮气换气2分钟,随后加入四三苯基膦钯(4mg,0.003mmol),氮气换气2分钟,在90℃下搅拌1小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,2-氨基-6-(1-甲基-1氢-吡唑-4-基)-4-(6-(4-吡啶-2-基甲基)哌啶-1-基)哌啶-3-基)吡唑[1,5-a]吡啶-3-腈(10mg,28%收率)。
1HNMR(400MHz,DMSO-d6)δ8.77(s,1H),8.50(s,1H),8.30-8.27(t,J=2.4Hz,2H),8.01(s,1H),7.77-7.68(m,2H),7.53(s,1H),7.30-7.19(m,2H),6.96-6.89(m,1H),6.26(brs,2H),4.42-4.31(m,2H),3.86(s,3H),2.90-2.80(m,2H),2.70-2.68(m,2H),2.12-1.99(m,1H),1.70-1.61(m,2H),1.31-1.18(m,2H)。m/z=490[M+1]+。
实施例3
2-甲基-4-(6-(4-(2-(5-氟吡啶-2-基)乙酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS071)
步骤A:2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲酸乙酯
室温下,向装有2,4,6-三甲基苯磺酸1-氨基-3-溴-5-甲氧基吡啶-1-鎓(2.79g,6.91mmol)的DMF(25mL)溶液中加入三乙胺(1.93mL,13.82mmol);反应体系冷却至0℃,分批次加入丁炔酸乙酯(1.62mL,13.82mmol),升至室温下搅拌过夜;反应完毕,加水淬灭,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲酸乙酯(510mg,24%收率)。
1HNMR(400MHz,CDCl3)δ8.13(d,J=1.2Hz,1H),6.58(d,J=13.2Hz,1H),4.31-4.17(m,2H),3.85(t,J=20.8Hz,3H),2.51(s,3H),1.39-1.31(m,3H)。m/z=313[M+1]+。
步骤B:2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶
室温下,向装有2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲酸乙酯(510mg,1.63mmol)的封管中加入48%氢溴酸(10mL),密封好,加热至80℃,并在80℃下搅拌2.0小时,冷却至室温,真空除去溶剂,干法柱层析得到产品2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶(392mg,100%收率),直接投入下一步反应。m/z=241[M+1]+。
步骤C:2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲醛
在0℃下,向2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶(392mg,1.63mmol)的DMF(10mL)溶液中加入三氯氧磷(0.45mL,4.89mmol),升至室温下搅拌4.0小时,TLC点板反应完毕,加入水稀释,混合物用氢氧化钠水溶液(1M)调节PH值为10左右并搅拌1.0小时,真空过滤,滤饼用水洗,甲基叔丁基醚洗,真空干燥得到产品2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲醛(414mg,100%收率),直接投入下一步反应。m/z=269[M+1]+。
步骤D:(E)-2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲醛肟
将2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲醛(414mg,1.63mmol)、盐酸羟胺(170mg,2.45mmol)、水(6mL)和乙醇(3mL)的混合物在50℃下搅拌4.0小时,TLC点板反应完毕,反应体系冷却至室温,减压除去乙醇,饱和碳酸氢钠水溶液中和反应体系,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品(E)-2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-甲醛肟(463mg,100%收率),直接投入下一步反应。m/z=284[M+1]+。
步骤E:2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈
将2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈(463mg,1.63mmol)的乙酸酐(10mL)混合物加热到120℃并搅拌过夜,TLC点板反应完毕,反应体系冷却至室温,减压除去乙酸酐,加水稀释,饱和碳酸氢钠水溶液中和反应体系,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈(300mg,69%收率)。m/z=266[M+1]+。
步骤F:2-甲基-4-甲氧基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈
将2-甲基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈(300mg,1.13mmol),1-甲基-4-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)-1H-吡唑(282mg,1.35mmol),四三苯基膦钯(26mg,0.02mmol),碳酸钾(467mg,3.39mmol),1,4-二氧六环(5mL)和H2O(1mL)放于20mL封管中,氮气置换三次,80℃反应3小时,TLC检测反应完,浓缩反应液,乙酸乙酯稀释,水洗,有机相用无水硫酸钠干燥,减压浓缩,柱层析分离得到产品2-氨基-4-甲氧基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(300mg,99%收率)。m/z=268[M+1]+。
步骤G:2-甲基-4-羟基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈
将2-氨基-4-甲氧基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(300mg,1.12mmol),DCE(20mL),氯化铝(446mg,3.36mmol)放于100mL单口瓶中,氮气保护,80℃加热搅拌,颜色逐渐变深棕色,反应3小时,TLC检测反应完,加水搅拌淬灭,乙酸乙酯萃取,有机相用无水硫酸钠干燥,减压浓缩,柱层析分离得到产品2-甲基-4-羟基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(150mg,53%收率)。m/z=254[M+1]+。
步骤H:2-甲基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基三氟甲磺酸酯
将2-甲基-4-羟基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(150mg,0.59mmol),DMF(15mL),DIEA(152mg,1.17mmol),N-苯基双(三氟甲烷)磺酰亚胺(317mg,0.89mmol)放于100mL单口瓶中,室温搅拌2小时,TLC检测反应完,加水稀释,乙酸乙酯萃取,合并有机层,水洗,饱和食盐水洗,有机相用无水硫酸钠干燥,减压浓缩,柱层析分离得到2-甲基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基三氟甲磺酸酯(180mg,79%收率)。m/z=386[M+1]+。
步骤I:2-(5-氟吡啶-2-基)-1-(4-(5-(4,4,5,5-四甲基-1,3,2-二恶硼酸-2-基)吡啶-2-基)哌嗪-1-基)乙酮
向10mL封管中,加入1-(4-(5-溴吡啶-2)哌嗪-1-基)-2-(5-氟吡啶-2-基)ethan-1-酮(50mg,0.132mmol),双联频哪醇硼酸酯(50mg,0.198mmol),[1,1′-双(二苯基膦基)二茂铁]二氯化钯(10mg,0.013mmol),醋酸钾(39mg,0.396mmol),1,4-二氧六环(20mL),氮气置换三次,100℃反应3小时,LCMS确认原料反应完,有产品生成,无须后处理,直接进行下一步反应。m/z=427[M+1]+。
步骤J:2-甲基-4-(6-(4-(2-(5-氟吡啶-2-基)乙酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈
向步骤I的反应液中加入2-甲基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基三氟甲磺酸酯(50mg,0.13mol),四三苯基磷钯(15mg,0.01mol),碳酸钾(36mg,0.26mol),1,4-二氧六环(4mL),H2O(1mL),氮气置换三次,90℃反应2小时,LCMS确认原料反应完,有产品生成。加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品2-甲基-4-(6-(4-(2-(5-氟吡啶-2-基)乙酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(14mg,20%收率)。
1HNMR(400MHz,DMSO-d6)δ9.12(s,1H),8.49(d,J=1.2Hz,1H),8.39-8.36(m,2H),8.09(s,1H),7.84(brs,1H),7.81-7.65(m,2H),7.48-7.36(m,1H),7.01(d,J=9.2Hz,1H),3.98(s,3H),3.88(s,3H),3.80-3.56(m,8H),3.89(t,J=2.8Hz,2H)。m/z=536[M+1]+。
实施例4
2-甲基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡啶-2-基甲基)哌啶-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS072)
室温下,向装有-(4-(吡啶-2-基甲基)哌啶-1-基)-5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶(45mg,0.12mmol)和2-甲基-3-氰基-6-(1-甲基-1氢-吡唑-4-基)吡唑[1,5-a]吡啶-4-基三氟甲磺酸盐(38mg,0.10mmol)的1,4-二氧六环(3mL)溶液中加入碳酸钠(53mg,0.50mmol,2M)水溶液,氮气换气2分钟,随后加入四三苯基膦钯(12mg,0.01mmol),氮气换气2分钟,在80℃下搅拌3小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得到产品2-甲基-6-(1-甲基1-1氢-吡唑-4-基)-4-(6-(4-吡啶-2-基甲基)哌啶-1-基)哌啶-3-基)吡唑[1,5-a]吡啶-3-腈(12mg,25%收率)。
1HNMR(400MHz,DMSO-d6)δ9.1(s,1H),8.55-8.50(m,1H),8.36-8.34(m,2H),8.09(s,1H),7.79-7.76(m,1H),7.73-7.68(m,2H),7.26-7.19(m,2H),6.95-6.93(d,J=9.2Hz,1H),4.40-4.37(d,J=12.8Hz,2H),3.93(s,3H),2.92-2.79(m,2H),2.75-2.66(m,3H),2.33(s,2H),2.07(brs,1H),1.67-1.64(d,J=12.8Hz,2H),1.29-1.19(m,2H)。m/z=489[M+1]+。
实施例5
2-氨基-4-(6-(4-((5-氟吡啶-3-基)甲基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS014)
步骤A:1-(5-溴吡啶-2-基)-4-((5-氟吡啶-3-基)甲基)哌嗪
向100mL单口瓶中,加入5-氟烟醛(2.4g,19.2mmol),1-(5-溴吡啶)-2-哌嗪(4.6g,19.2mmol),醋酸硼氢化钠(12.2g,57.6mmol),二氯甲烷200mL,室温搅拌12小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品1-(5-溴吡啶-2-基)-4-((5-氟吡啶-3-基)甲基)哌嗪(1.7g,收率25%)。m/z=352[M+1]+。
步骤B:1-((4-氟吡啶-3-基)甲基)-4-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)哌嗪
向10mL封管中,加入1-(5-溴吡啶-2-基)-4-((5-氟吡啶-3-基)甲基)哌嗪(47mg,0.132mmol),双联频哪醇硼酸酯(50mg,0.198mmol),[1,1′-双(二苯基膦基)二茂铁]二氯化钯(10mg,0.013mmol),醋酸钾(39mg,0.396mmol),1,4-二氧六环(20mL),氮气置换三次,100℃反应3小时,LCMS确认原料反应完,有产品生成,无需后处理,直接进行下一步反应。m/z=399[M+1]+。
步骤C:2-氨基-4-(6-(4-((5-氟吡啶-3-基)甲基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈
室温下,向装有1-((4-氟吡啶-3-基)甲基)-4-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)哌嗪(35mg,0.087mmol)和2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑[1,5-a]吡啶-4-基-三氟甲磺酸盐(28mg,0.073mmol)的1,4-二氧六环(2.5mL)溶液中加入碳酸钠(38mg,0.358mmol,2M)水溶液,氮气换气2分钟,随后加入四三苯基膦钯(4mg,0.003mmol),氮气换气2分钟,在90℃下搅拌1小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,2-氨基-4-(6-(4-((5-氟吡啶-3-基)甲基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(17mg,46%收率)。
1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.51(d,1H),8.50(d,1H),8.33(d,1H),8.28(s,1H),8.02(s,1H),7.75-7.78(m,1H),7.72(d,1H),7.55(d,1H),6.95(d,1H),6.29(s,2H),4.08-4.12(m,1H),3.86(s,3H),3.59-3.64(m,6H),3.17(d,2H),2.50(s,3H).m/z=509[M+1]+。
实施例6
2-氨基-4-(6-(4-((2-氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS015)
制备方法同实施例5,将5-氟烟醛替换为2-氟苯甲醛,得2-氨基-4-(6-(4-((2-氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(12mg,31%收率)。1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.33(d,1H),8.28(s,1H),8.02(s,1H),7.75(d,1H),7.55(s,1H),7.42-7.48(m,1H),7.35(s,1H),7.20-7.21(m,2H),6.94(d,1H),6.29(s,2H),3.86(s,3H),3.61(d,6H),3.49(s,1H),1.24(s,3H).m/z=508[M+1]+。
实施例7
4-(5-(2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基)吡啶-2-基)-N,N-二乙基哌嗪-1-甲酰胺(APS016)
制备方法同实施例1,将2-(5-氟吡啶-2-基)乙酸替换为二乙氨基甲酰氯,得4-(5-(2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-4-基)吡啶-2-基)-N,N-二乙基哌嗪-1-甲酰胺(5.1mg,收率14%),1HNMR(400MHz,CDCl3)δ8.79(s,1H),8.39(s,1H),8.30(s,1H),7.74-7.83(s,2H),7.70(s,1H),7.52(s,1H),6.83(d,1H),4.00(d,3H),3.70(s,4H),3.39(d,4H),3.24-3.30(m,4H),1.16(t,6H).m/z=489[M+1]+。
实施例8
2-氨基-4-(6-(4-((3-氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS019)
制备方法同实施例5,将5-氟烟醛替换为3-氟苯甲醛,得2-氨基-4-(6-(4-((3-氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(26.9mg,收率73%)。1HNMR(400MHz,DMSO-d6)δ8.79(s,1H),8.78(d,1H),8.32(s,1H),8.02(s,1H),7.75(d,2H),7.55(d,1H),7.37-7.42(m,1H),7.17-7.21(m,2H),7.08-7.13(m,1H),6.92-6.95(d,1H),6.29(s,2H),4.08-4.12(m,1H),3.86(s,3H),3.57-3.61(m,6H),3.18(d,2H).m/z=508[M+1]+。
实施例9
2-氨基-4-(6-(4-((2,5-二氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS020)
制备方法同实施例5,将5-氟烟醛替换为2,5-二氟苯甲醛,得2-氨基-4-(6-(4-((2,5-二氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(9.9mg,收率26%)。1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.33(d,1H),8.28(s,1H),8.02(s,1H),7.75-7.78(dd,1H),7.55(d,1H),6.95-7.33(m,3H),6.92(d,1H),6.28(s,2H),3.86(s,3H),3.60(s,6H).m/z=526[M+1]+。
实施例10
2-氨基-4-(6-(4-((5-氯吡啶-3-基)甲基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS021)
制备方法同实施例5,将5-氟烟醛替换为5-氯-烟醛,得2-氨基-4-(6-(4-((5-氯吡啶-3-基)甲基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(21mg,收率55%)。1HNMR(400MHz,CDCl3)δ8.39-8.44(m,2H),8.25-8.29(m,2H),7.65-7.71(m,2H),7.54-7.59(m,1H),7.19-7.21(m,4H),6.63-6.71(m,1H),4.45(s,1H),3.90(s,2H),3.53-3.72(d,7H),2.54(s,4H),m/z=525[M+1]+。
实施例11
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-((3-甲基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS022)
制备方法同实施例5,将5-氟烟醛替换为3-甲基-2-吡啶甲醛,得2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-((3-甲基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(5mg,收率14%)。1HNMR(400MHz,DMSO-d6)δ8.8.79(s,1H),8.28-8.78(m,3H),8.02(s,1H),7.74-7.77(m,1H),7.54-7.60(m,2H),7.21-7.24(m,1H),6.93(d,1H),6.28(d,2H),4.08-4.11(m,1H),3.86(s,3H),3.65(s,2H),3.58(s,4H),3.3(s,1H),3.17(d,2H),2.43(s,3H).m/z=505[M+1]+。
实施例12
2-氨基-4-(6-(4-((2,6-二氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS024)
制备方法同实施例5,将5-氟烟醛替换为2,6-二氟苯甲醛,得2-氨基-4-(6-(4-((2,6-二氟苄基)哌嗪-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(3.5mg,收率9%)。1HNMR(400MHz,DMSO-d6)δ8.78(s,1H),8.31(d,2H),8.27-8.31(m,2H),8.01(s,1H),7.73(d,1H),7.54(s,1H),7.42-7.48(m,1H),7.13(t,2H),6.28(s,2H),3.86(s,3H),3.56-3.65(s,7H),3.27(s,1H).m/z=526[M+1]+。
实施例13
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-((4-甲基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS025)
制备方法同实施例5,将5-氟烟醛替换为4-甲基-2-吡啶甲醛得2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-((4-甲基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(3.9mg,收率11%)。1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.29-8.33(m,3H),8.02(s,1H),8.75(d,1H),7.55(d,1H),7.33(s,1H),7.23(d,1H),6.95(d,2H),6.30(s,2H),3.86(s,3H),3.62(d,6H),3.32(s,2H)2.55(s,4H),2.34(s,3H).1.23(s,1H).m/z=505[M+1]+。
实施例14
2-氨基-4-(6-(4-(3-甲氧基苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS026)
制备方法同实施例5,将5-氟烟醛替换为3-甲氧基苯甲醛,得2-氨基-4-(6-(4-(3-甲氧基苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(14.2mg,收率37%)。1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.51(d,1H),8.45(s,1H),8.33(d,1H),8.28(s,1H),8.02(s,1H),7.75-7.78(d,1H),7.72(d,1H),7.55(d,1H),6.95(d,1H),6.29(s,2H),4.11(t,1H),3.86(s,3H),3.59-3.64(m,6H),3.17(d,2H),2.53(s,3H).m/z=520[M+1]+。
实施例15
2-氨基-4-(6-(4-(2-氯苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS027)
制备方法同实施例5,将5-氟烟醛替换为2-氯苯甲醛,得2-氨基-4-(6-(4-(2-氯苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(9.4mg,收率25%)。1HNMR(400MHz,DMSO-d6)δ8.79(s,1H),8.80(d,1H),8.28(s,1H),8.02(s,1H),7.78(d,1H),7.56-7.58(m,3H),7.45-7.47(m,2m/z=524[M+1]+。
实施例16
2-氨基-4-(6-(4-((6-甲氧基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS030)
制备方法同实施例5,将5-氟烟醛替换为6-甲氧基-2-吡啶甲醛,得2-氨基-4-(6-(4-((6-甲氧基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(8.6mg,收率23%)。1HNMR(400MHz,CDCl3)δ8.27(d,2H),7.65-7.68(m,2H),7.45-7.54(m,2H),7.21(s,1H),7.01(s,1H),6.71(d,1H),6.59(d,1H),4.45(s,2H),3.86-3.90(d,6H),3.66(s,6H),2.69(brs,4H),1.54(brs,4H),m/z=521[M+1]+。
实施例17
2-氨基-4-(6-(4-((5-甲氧基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS033)
制备方法同实施例5,将5-氟烟醛替换为5-甲氧基烟醛,得2-氨基-4-(6-(4-((5-甲氧基吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(21.9mg,收率58%).1HNMR(400MHz,DMSO-d6)δ8.77(s,1H),8.32(d,1H),8.27(d,1H),8.22(d,1H),8.01(s,1H),7.77(dd,1H),7.54(s,1H),7.37-7.43(m,2H),6.94(d,1H),6.26(s,2H),4.08(s,1H),3.83-3.86(m,6H),3.60(s,6H),3.28(s,2H),3.18(d,2H).m/z=521[M+1]+。
实施例18
2-氨基-4-(6-(4-(4-(二甲氨基)苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS034)
制备方法同实施例5,将5-氟烟醛替换为4-二甲氨基苯甲醛,得2-氨基-4-(6-(4-(4-(二甲氨基)苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(7.0mg,收率18%),1HNMR(400MHz,DMSO-d6)δ8.78(d,1H),8.28-8.32(m,2H),8.02(s,1H),7.74-7.76(d,1H),7.54(d,1H),7.15(d,2H),6.93(d,1H),6.71(d,1H),6.28(s,2H),4.08-4.11(m,1H),3.86(s,3H),3.56(s,4H),3.41(s,2H),3.17(s,2H),2.88(s,6H),2.44-2.50(m,4H).m/z=533[M+1]+。
实施例19
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(4-(吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS035)
制备方法同实施例5,将5-氟烟醛替换为2-吡啶甲醛,得2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(4-(吡啶-2-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(12.7mg,收率35%),1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.52(d,1H),8.33(d,1H),8.28(s,1H),8.02(s,1H),7.72-8.80(m,2H),7.48-7.51(m,2H),7.24-7.31(m,1H),6.91-6.96(d,1H),6.29(s,2H),3.86(s,3H),3.59-3.67(m,8H),2.56(s,2H).m/z=491[M+1]+。
实施例20
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS038)
步骤A:3-(5-溴吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯
室温下,向250mL单口瓶中加入2,5-二溴吡啶(12.2g,0.052mol),3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯(11.5g,0.058mol),加入DMSO(20mL)中,120℃下反应过夜。经LCMS确认反应完全,减压除去DMSO,加入甲醇使其溶解,冰水浴下慢慢加入饱和氢氧化钠溶液,调节pH至13左右,再搅拌2小时。减压浓缩出甲醇,二氯甲烷萃取,无水硫酸钠干燥,减压浓缩,柱层析分离得到产品(5.2g,28%收率)。m/z=355[M+1]+。
步骤B:3-(5-溴吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷二盐酸盐
向100mL单口瓶中,加入3-(5-溴吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯(5.0g,14mmol),氯化氢二氧六环溶液(15mL),室温搅拌2h,直接浓缩后用于下步反应。m/z=255[M+1]+。
步骤C:3-(5-溴吡啶-2-基)-6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷
向100mL单口瓶中,加入2-吡啶甲醛(0.21g,1.9mmol),3-(5-溴吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷二盐酸盐(0.6g,1.9mmol),醋酸硼氢化钠(1.2g,5.8mmol),二氯甲烷20mL,室温搅拌12小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品3-(5-溴吡啶-2-基)-6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷(0.6g,收率85%)。m/z=346[M+1]+。
步骤D:6-(吡啶-2-基甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷
向10mL封管中,加入3-(5-溴吡啶-2-基)-6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷(46mg,0.132mmol),双联频哪醇硼酸酯(50mg,0.198mmol),[1,1′-双(二苯基膦基)二茂铁]二氯化钯(10mg,0.013mmol),醋酸钾(39mg,0.396mmol),1,4-二氧六环(20mL),氮气置换三次,100℃反应3小时,LCMS确认原料反应完,有产品生成,无须后处理,直接进行下一步反应。
步骤E:室温下,向装有6-(吡啶-2-基甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷(34mg,0.087mmol)和2-氨基-3-氰基-6-(1-甲基-1H-吡唑-4-基)吡唑[1,5-a]吡啶-4-基-三氟甲磺酸盐(28mg,0.073mmol)的1,4-二氧六环(2.5mL)溶液中加入碳酸钠(38mg,0.358mmol,2M)水溶液,氮气换气2分钟,随后加入四三苯基膦钯(4mg,0.003mmol),氮气换气2分钟,在90℃下搅拌1小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(10mg,28%收率)。
1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.46(d,1H),8.39(d,1H),8.29(s,1H),8.03(s,1H),7.76-7.83(m,2H),7.57(d,1H),7.49(d,1H),6.79(d,1H),6.29(s,2H),3.86(s,2H),3.73(d,2H),3.57(s,2H),3.52-3.55(m,2H),3.31(s,1H),1.98-2.00(m,1H),1.74(brs,1H).m/z=503[M+1]+。
实施例21
2-氨基-4-(6-(4-((6-(二甲氨基)吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS041)
制备方法同实施例5,将5-氟烟醛替换为6-二甲氨基烟醛,得2-氨基-4-(6-(4-((6-(二甲氨基)吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(22.2mg,收率56%),1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.28-8.33(m,2H),8.02(d,2H),7.74-7.77(m,1H),7.55(d,1H),7.46-7.49(m,1H),6.94(d,1H),6.64(d,1H),6.28(s,2H),3.86(s,3H),3.57(s,4H),3.40(s,2H),3.30(s,1H),3.02(s,6H),2.50(s,4H).m/z=534[M+1]+。
实施例22
2-氨基-4-(6-(4-(2,6-二氟苯甲酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS062)
制备方法同实施例1,将2-(5-氟吡啶-2-基)乙酸替换为2,6-二氟苯甲酰氯,得2-氨基-4-(6-(4-(2,6-二氟苯甲酰基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(28.7mg,收率73%)。1HNMR(400MHz,DMSO-d6)δ9.25(d,1H),8.66(s,1H),8.40-8.43(m,2H),8.13(s,1H),7.90(d,1H),7.80(d,1H),7.5-7.63(m,1H),7.25-7.29(m,2H),7.04(d,1H),4.08-4.12(m,1H),3.89(s,3H),3.82-3.85(m,2H),3.72-3.75(d,2H),3.61-3.63(m,2H),3.42(d,2H),3.18(d,3H).m/z=539[M+1]+。
实施例23
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡嗪-2-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS064)
制备方法同实施例5,将5-氟烟醛替换为吡嗪-2-甲醛,得2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡嗪-2-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(9.5mg,收率26%)。1HNMR(400MHz,CDCl3)δ8.82(s,1H),8.60(s,1H),8.56(s,1H),8.34-8.37(m,2H),7.75-7.78(m,2H),7.63(s,1H),7.30(s,1H),6.81(d,1H),4.54(s,2H),3.99(s,3H),3.78-3.88(brs,5H),2.76(brs,3H).m/z=492[M+1]+。
实施例24
2-氨基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS068)
制备方法同实施例5,将5-氟烟醛替换为6-甲氧基-3-吡啶甲醛,得2-氨基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(20mg,收率53%)。1HNMR(400MHz,DMSO-d6)δ8.79(s,1H),8.34(d,2H),8.20(d,2H),7.68-7.77(m,2H),7.55(s,1H),6.81-6.94(dd,2H),6.29(s,2H),3.86(d,6H),3.49-3.58(d,6H).m/z=521[M+1]+。
实施例25
(R)-2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(3-甲基-4-(吡啶-2-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS073)
制备方法同实施例20,将3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯替换为(R)-4-N-叔丁氧羰基-2-甲基哌嗪,得(R)-2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(3-甲基-4-(吡啶-2-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(23mg,收率62%)。1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.52(d,1H),8.32(d,1H),8.28(s,1H),8.02(s,1H),7.74-7.81(m,2H),7.50-7.55(m,2H),7.26-7.29(m,1m/z=505[M+1]+。
实施例26
2-氨基-4-(6-(4-苄基哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS074)
制备方法同实施例5,将5-氟烟醛替换为苯甲醛,得2-氨基-4-(6-(4-苄基哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(27mg,收率75%)。1HNMR(400MHz,DMSO-d6)δ8.79(s,1H),8.33(d,1H),8.28(s,1H),8.02(s,1H),7.75-7.77(m,1H),7.55(d,1H),7.37(d,4H),7.29(s,1H),6.95(d,1H),6.28(s,2H).3.86(s,3H),3.55-3.59(m,7H),1.24(s,3H).m/z=490[M+1]+。
实施例27
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡啶-3-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS075)
制备方法同实施例5,将5-氟烟醛替换为烟醛,得2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡啶-3-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(7mg,19%)。1HNMR(400MHz,CDCl3)δ8.62(d,2H),8.36(dd,2H),7.74-7.77(m,2H),7.63(s,1H),7.36(s,1H),7.28(s,1H),6.81(d,1H),4.53(s,2H),3.99(s,3H),3.65(s,6H),2.70(s,4H)。m/z=491[M+1]+。
实施例28
(R)-2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(2-甲基-4-(吡啶-2-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS076)
制备方法同实施例20,将3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯替换为(R)-4-N-叔丁氧羰基-3-甲基哌嗪制备得到(R)-2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(2-甲基-4-(吡啶-2-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(12mg,33%)。1HNMR(400MHz,DMSO-d6)δ8.94(s,1H),8.78(s,1H),8.52(d,2H),8.05(s,1H),7.74-7.84(m,2H),7.569d,2H),7.31(t,1H),6.89(d,1H),6.27(s,1H),3.70(s,3H),3.62-3.86(m,1H),3.30(s,5H),3.12(t,1H),2.95(d,1H),2.74(d,1H),1.23(t,3H).m/z=505[M+1]+。
实施例29
2-氨基-4-(6-(4-(4-氟苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS077)
制备方法同实施例5,将5-氟烟醛替换为4-氟苯甲醛,得2-氨基-4-(6-(4-(4-氟苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(10mg,收率27%)。1HNMR(400MHz,DMSO-d6)δ8.79(d,1H),8.33(d,1H),8.28(s,1H),8.02(s,1H),7.75(d,1H),7.55(d,1H),7.38-7.40(m,1H),7.17-7.20(m,2H),6.95(d,1H),6.28(s,2H),3.86(s,3H),3.53-3.59(m,6H).m/z=508[M+1]+。
实施例30
2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡啶-4-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS078)
制备方法同实施例5,将5-氟烟醛替换为异烟醛,得2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(4-(吡啶-4-基甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(8.4mg,收率23%)。1HNMR(400MHz,DMSO-d6)δ8.79(s,1H),8.53(d,2H),8.29-8.32(m,2H),8.02(s,1H),7.78(d,1H),7.52(s,1H),7.40(d,2H),6.98(d,1H),6.29(s,2H),3.86(s,3H),3.62)d,7H).m/z=491[M+1]+。
实施例31
2-氨基-4-(6-(4-(3,4-二氟苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS079)
制备方法同实施例5,将5-氟烟醛替换为3,4-二氟苯甲醛,得2-氨基-4-(6-(4-(3,4-二氟苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(12.7mg,收率33%)。1HNMR(400MHz,CDCl3)δ8.27(d,1H),7.65(s,1H),7.41(s,1H),7.20(s,4H),7.02-7.08(m,2H),6.70(d,1H),4.45(s,1H),4.07(s,2H),3.59(s,3H),3.45(s,1H),2.50(s,2H),2.01(s,1H)。m/z=526[M+1]+。
实施例32
2-氨基-4-(6-(4-(2,6-二氟苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS024)
制备方法同实施例5,将5-氟烟醛替换为2,6-二氟苯甲醛,得2-氨基-4-(6-(4-(2,6-二氟苄基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(27mg,收率70%)。1HNMR(400MHz,DMSO-d6)δ10.9(brs,1H),9.27(d,1H),8.67(s,1H),8.46(d,1H),8.40(s,1H),8.13(s,1H),7.94-7.97(m,1H),7.80(d,1H),7.75-7.68(m,1H),7.28-7.32(m,2H),7.14(d,1H),7.48-7.56(m,4H),3.89(s,4H),3.25-3.45(m,5H).m/z=526[M+1]+。
实施例33
4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,2,3]三氮唑[1,5-a]吡啶-3-腈(APS081)
步骤A:6-溴-4-氯-[1,2,3]三氮唑[1,5-a]吡啶-3-腈
将2-(5-溴-3-氯吡啶-2-基)乙腈(1.2g,5mmol),对甲苯磺酰叠氮(1.2g,6mmol),碳酸铯(3.3g,10mmol)分别加入到DMF(50mL)中,加热100℃反应12h。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品6-溴-4-氯-[1,2,3]三氮唑[1,5-a]吡啶-3-腈(450mg,收率35%)。m/z=258[M+1]+。
步骤B:4-氯-6-(1-甲基-1H-吡唑-4-基)-[1,2,3]三氮唑[1,5-a]吡啶-3-腈
制备方法同实施例1步骤F,将2-氨基-6-溴-4-甲氧基吡唑并[1,5-a]吡啶-3-腈替换为6-溴-4-氯-[1,2,3]三氮唑[1,5-a]吡啶-3-腈,得4-氯-6-(1-甲基-1H-吡唑-4-基)-[1,2,3]三氮唑[1,5-a]吡啶-3-腈(20mg,收率40%)。m/z=259[M+1]+。
步骤C:制备方法同实施例5,将5-氟烟醛替换为6-甲氧基烟醛,得4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,2,3]三氮唑[1,5-a]吡啶-3-腈(10mg,收率27%)。1HNMR(400MHz,DMSO-d6)δ9.64(d,1H),8.46-8.51(m,2H),8.23(s,1H),8.01(d,1H),7.91(d,1H),7.69(d,2H),7.02(d,1H),6.83(d,1H),3.91(s,3H),3.85(s,3H),3.62(s,4H),3.49(s,2H),3.32(s,1H),2.50(s,3H)。m/z=507[M+1]+。
实施例34
2-(二甲氨基)-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS082)
将2-氨基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS068)(50mg,0.1mmol)溶于DMF(5.0mL)中,冰水冷却至0度,加入60%矿物油NaH(8mg,0.2mmol)搅拌30分钟后,加入碘甲烷(28mg,0.2mmol),自然升至室温反应12h。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品2-(二甲氨基)-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(9.1mg,收率16%)。1HNMR(400MHz,CDCl3)δ8.31-8.37(m,2H),8.09(s,1H),7.68-7.72(m,2H),7.61-7.63(m,2H),7.22(s,1H),6.76(d,2H),3.97(d,6H),3.64(s,3H),3.49(s,2H),3.19(s,6H),2.56(s,3H).m/z=549[M+1]+。
实施例35
2-氟-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS084)
制备方法同实施例40,将2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐替换为2-氨基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈得7-氟-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(20mg,收率47%)。1HNMR(400MHz,DMSO-d6)δ8.79(s,1H),8.37(s,2H),8.10(d,2H),7.94(d,1H),7.70(d,1H),7.55(d,1H),6.98(d,1H),6.81(d,1H),3.93(s,3H),3.85(s,3H),3.60(s,4H),3.49(s,2H),2.51(s,4H).m/z=524[M+1]+。
实施例36
2-羟基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS083)
将2-氟-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS084)(50mg,0.1mmol)和碳酸钾(41mg,0.3mmol)加入DMSO(2.0mL)中,再加入水(0.5mL)加热至100℃反应6h。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品2-羟基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(7.3mg,收率14%)。m/z=522[M+1]+。
实施例37
N-(3-氰基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-2-基)乙酰胺(APS085)
将2-氨基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS068)(20mg,0.038mmol)溶于醋酸酐(2.0mL)加热至80℃反应4h。将反应液加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品N-(3-氰基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-2-基)乙酰胺(14mg,收率65%)。m/z=563[M+1]+。
实施例38
2-甲氨基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(APS086)
制备方法同实施例34,得2-甲氨基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)-6-(1-甲基-1H-吡唑-4-基)吡唑并[1,5-a]吡啶-3-腈(8mg,收率15%)。m/z=535[M+1]+。
实施例39
2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)
步骤A:3-(5-氯吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯
室温下,向250mL单口瓶中加入2-氯-5-氟吡嗪(6.9g,0.052mol),3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯(11.5g,0.058mol),加入DMSO(20mL)中,120℃下反应过夜。经LCMS确认反应完全,减压除去DMSO,加入甲醇使其溶解,冰水浴下慢慢加入饱和氢氧化钠溶液,调节pH至13左右,再搅拌2小时。减压浓缩出甲醇,二氯甲烷萃取,无水硫酸钠干燥,减压浓缩,柱层析分离得到产品(12g,74%收率)。m/z=311[M+1]+。
步骤B:3-(5-氯吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷二盐酸盐
向100mL单口瓶中,加入3-(5-氯吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷-6-碳酸叔丁酯(5.0g,14mmol),氯化氢二氧六环溶液(15mL),室温搅拌2h,直接浓缩后用于下步反应。m/z=211[M+1]+。
步骤C:3-(5-氯吡嗪-2-基)-6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷
向100mL单口瓶中,加入6-甲氧基烟醛(0.21g,1.9mmol),3-(5-氯吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷二盐酸盐(0.4g,1.9mmol),醋酸硼氢化钠(1.2g,5.8mmol),二氯甲烷20mL,室温搅拌12小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品3-(5-氯吡嗪-2-基)-6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷(0.5g,收率80%)。m/z=332[M+1]+。
步骤D:6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷
向10mL封管中,加入3-(5-氯吡嗪-2-基)-6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷(44mg,0.132mmol),双联频哪醇硼酸酯(50mg,0.198mmol),[1,1′-双(二苯基膦基)二茂铁]二氯化钯(10mg,0.013mmol),醋酸钾(39mg,0.396mmol),1,4-二氧六环(20mL),氮气置换三次,100℃反应3小时,LCMS确认原料反应完,有产品生成,无须后处理,直接进行下一步反应。
步骤E:2-氨基-4-氯-6-甲氧基吡唑并[1,5-a]吡啶-3-腈
室温下,向装有2,4,6-三甲基苯磺酸1-氨基-3-氯-5-甲氧基吡啶-1-鎓(4.0g,10.0mmol)的DMF(30mL)溶液中加入碳酸钾(4.2g,30.0mmol);反应体系冷却至0℃,分批次加入乙基2-氰基亚氨代乙酸酯盐酸盐(3.0g,20.0mmol),升至室温下搅拌1小时,再90℃下搅拌1小时;反应完毕并冷却至室温,加水淬灭,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品2-氨基-4-氯-6-甲氧基吡唑并[1,5-a]吡啶-3-腈(89mg,4%收率)。m/z=223[M+1]+。
步骤F:4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-甲氧基吡唑并[1,5-a]吡啶-3-腈
将2-氨基-4-氯-6-甲氧基吡唑并[1,5-a]吡啶-3-腈(80mg,0.35mmol)和邻苯二甲酸酐(78mg,0.53mmol)加入到冰醋酸(5.0mL),加热至100℃反应5h,减压浓缩,柱层析分离得到产品4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-甲氧基吡唑并[1,5-a]吡啶-3-腈(80mg,收率65%)。m/z=353[M+1]+。
步骤G:4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-羟基吡唑并[1,5-a]吡啶-3-腈
将4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-甲氧基吡唑并[1,5-a]吡啶-3-腈(80mg,0.22mmol)和无水三氯化铝(146mg,1.1mmol)加入到乙腈(5.0mL)中,加热至80℃反应2h。反应完毕并冷却至室温,加水淬灭,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-羟基吡唑并[1,5-a]吡啶-3-腈(62mg,收率84%)。m/z=339[M+1]+。
步骤H:(S)-2-(((4-氯-3-氰基-2-(1,3-邻苯二甲酰亚胺-2-基)吡唑并[1,5-a]吡啶-6-基)氧基)甲基)吗啉-4-碳酸叔丁酯
将4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-羟基吡唑并[1,5-a]吡啶-3-腈(60mg,0.17mmol),(2S)-2-(溴甲基)-4-吗啉羧酸叔丁酯(52mg,0.19mmol),碳酸铯(166mg,0.51mmol)加入到DMF(10mL)中,加热60℃反应12h。反应完毕并冷却至室温,加水淬灭,用乙酸乙酯萃取,合并有机相,并用水洗涤,减压浓缩,柱层析分离得到产品(S)-2-(((4-氯-3-氰基-2-(1,3-邻苯二甲酰亚胺-2-基)吡唑并[1,5-a]吡啶-6-基)氧基)甲基)吗啉-4-碳酸叔丁酯(77mg,收率85%).m/z=538[M+1]+。
步骤I:室温下,向装有6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷(37mg,0.087mmol)和(S)-2-(((4-氯-3-氰基-2-(1,3-邻苯二甲酰亚胺-2-基)吡唑并[1,5-a]吡啶-6-基)氧基)甲基)吗啉-4-碳酸叔丁酯(39mg,0.073mmol)的1,4-二氧六环(2.5mL)溶液中加入碳酸钠(38mg,0.358mmol,2M)水溶液,氮气换气2分钟,随后加入四三苯基膦钯(4mg,0.003mmol),氮气换气2分钟,在90℃下搅拌1小时,TLC点板反应完毕。加入氯化氢二氧六环溶液(0.5mL,4.0M)继续反应2h。将至室温,再加入50%氨水室温反应2h。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,反相柱层析分离得到产品2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)哌嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(12mg,25%收率)。1HNMR(400MHz,DMSO-d6)δ9.34(s,1H),8.99(s,2H),8.55-8.65(m,1H),8.25-8.38(m,3H),7.83-7.90(m,1H),7.38-7.44(m,1H),6.91-6.95(m,1H),6.18(s,1H),4.65(d,2H),4.49(d,1H),4.26(s,1H),4.11-4.20(m,5H),4.00-4.11(m,3H),3.93-4.00(m,1H),3.89(s,3H),3.67-3.74(m,1H),3.5-3.26(m,1H),2.97-3.04(m,1H).m/z=569[M+1]+。
实施例40
2-氟-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS088)
将2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)(50mg,0.08mmol)溶于四氟硼酸(5.0mL)中,冰水浴冷却至0℃,将亚硝酸钠(71mg,1.0mmol)加入,自然升至室温反应12h,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得到产品2-氟-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈(3.4mg,收率7.4%)。m/z=572[M+1]+。
实施例41
2-氨基-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS089)
步骤A:4-氯-2-(1,3--邻苯二甲酰亚胺-2-基)-6-(2-羟基-2-甲基丙氧基)吡唑并[1,5-a]吡啶-3-腈
将4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-羟基吡唑并[1,5-a]吡啶-3-腈(250mg,071mmol),氧化异丁烯(500mg,7.1mmol),碳酸钾(300mg,2.13mmol)加入DMF(5.0mL)中,加入60℃反应16h。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得到产品4-氯-2-(1,3--邻苯二甲酰亚胺-2-基)-6-(2-羟基-2-甲基丙氧基)吡唑并[1,5-a]吡啶-3-腈(30mg,收率10%).m/z=411[M+1]+。
步骤B:2-氨基-4-氯-6-(2-羟基-2-甲基丙氧基)吡唑并[1,5-a]吡啶-3-腈
将4-氯-2-(1,3--邻苯二甲酰亚胺-2-基)-6-(2-羟基-2-甲基丙氧基)吡唑并[1,5-a]吡啶-3-腈(30mg,0.073mmol)溶于THF(4.0mL)中,加入0.5mL 50%氨水,室温搅拌1h。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得到产品2-氨基-4-氯-6-(2-羟基-2-甲基丙氧基)吡唑并[1,5-a]吡啶-3-腈(18mg,收率87%)。m/z=281[M+1]+。
步骤C:3-(5-溴吡啶-2-基)-6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷
向100mL单口瓶中,加入6-甲氧基烟醛(0.26g,1.9mmol),3-(5-溴吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷二盐酸盐(0.6g,1.9mmol),醋酸硼氢化钠(1.2g,5.8mmol),二氯甲烷20mL,室温搅拌12小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品3-(5-溴吡啶-2-基)-6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷(356mg,收率50%)。m/z=375[M+1]+。
步骤D:6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷
向10mL封管中,加入3-(5-溴吡啶-2-基)-6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷(49mg,0.132mmol),双联频哪醇硼酸酯(50mg,0.198mmol),[1,1′-双(二苯基膦基)二茂铁]二氯化钯(10mg,0.013mmol),醋酸钾(39mg,0.396mmol),1,4-二氧六环(20mL),氮气置换三次,100℃反应3小时,LCMS确认原料反应完,有产品生成,无须后处理,直接进行下一步反应。
步骤E:室温下,向装有6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷(37mg,0.087mmol)和2-氨基-4-氯-6-(2-羟基-2-甲基丙氧基)吡唑并[1,5-a]吡啶-3-腈(21mg,0.073mmol)的1,4-二氧六环(2.5mL)溶液中加入碳酸钠(38mg,0.358mmol,2M)水溶液,氮气换气2分钟,随后加入四三苯基膦钯(4mg,0.003mmol),氮气换气2分钟,在110℃下搅拌4小时,TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得2-氨基-6-(1-甲基-1H-吡唑-4-基)-4-(6-(6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(11.3mg,28%收率)。1HNMR(400MHz,DMSO-d6)δ8.35(d,1H),8.27(d,1H),8.07(s,1H),7.81(d,1H),7.67(d,1H),7.10(d,1H),6.78(d,2H),6.15(s,2H),4.67(s,1H),3.82(d,5H),3.62-3.68(m,4H),3.48-3.55(m,4H),1.58(s,1H),1.21(s,6H)。m/z=541[M+1]+。
实施例42
2-氟-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS090)
制备方法同实施例40,将2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)替换为2-氨基-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS089)得产品(2.8mg,收率6.8%)1HNMR(400MHz,DMSO-d6)δ8.63(s,1H),8.42(s,1H),8.07(s,1H),7.87(s,1H),7.69(s,1H),7.44(s,1H),6.79(s,2H),4.74(s,1H),3.82-3.88(m,5H),3.68-3.72(m 4H),3.51(brs,5H),1.59(s,1H),1.23(s,6H).m/z=544[M+1]+。
实施例43
6-(2-羟基-2-2甲基丙氧基)-2-甲氧基-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS091)
将2-氟-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS090)(15mg,0.028mmol),碳酸钾(11mg,0.08mmol)加入甲醇(2.0mL)中,60℃封管反应12h。加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得6-(2-羟基-2-2甲基丙氧基)-2-甲氧基-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(14mg,收率89%)。1HNMR(400MHz,DMSO-d6)δ8.55(d,1H),8.39(d,1H),8.07(d,1H),7.83(dd,1H),7.69(dd,1H),7.29(d,1H),6.79(dd,2H),4.70(s,1H),4.05(s,3H),3.85(s,2H),3.82(s,3H),3.62-3.78(m,4H),3.50-3.55(m,4H),1.59(d,1H),1.22(s,6H).m/z=556[M+1]+。
实施例44
2-氨基-6-乙氧基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)吡唑并[1,5-a]吡啶-3-腈(APS092)
制备方法同实施例39,将(2S)-2-(溴甲基)-4-吗啉羧酸叔丁酯替换为碘乙烷得2-氨基-6-乙氧基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)吡唑并[1,5-a]吡啶-3-腈(2.9mg,收率8%)。1HNMR(400MHz,DMSO-d6)δ8.56(s,1H),8.30(d,2H),8.10(s,1H),7.83-8.04(m,1H),7.71(d,1H),7.35(s,1H),6.78(d,1H),6.11(s,2H),4.12(d,2H),3.55-3.82(m,12H),1.63(s,1H),1.36(brs,3H).m/z=498[M+1]+。
实施例45
N-(3-氰基-6-(2-羟基-2-甲基丙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS093)
将2-氨基-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS089)(50mg,0.093mmol),溶于DCM(2.0mL),加入三乙胺(28mg,0.3mmol),冰水浴冷却至0℃,加入乙酰氯(8mg,0.1mmol)自然升至室温反应12h。加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得N-(3-氰基-6-(2-羟基-2-甲基丙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(2.7mg,收率5%)。1HNMR(400MHz,DMSO-d6)δ10.67(s,1H),8.55(d,1H),8.38(d,1H),8.07(s,1H),7.82(dd,1H),7.69(dd,1H),7.27(d,1H),6.80(t,2H),4.72(s,1H),3.87(s,2H),3.82(s,3H),3.67-3.75(m,4H),3.50-3.59(m,5H),2.10(s,3H),1.60(d,1H),1.23(s,6H).m/z=583[M+1]+。
实施例46
6-(2-羟基-2-甲基丙氧基)-2-((2-2-羟基乙基)氨基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS094)
2-氨基-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS089)(100mg,0.17mmol)和2-溴乙醇(21mg,0.17mmol)加入到DMF(2.0mL)中,加热120℃反应12h。加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得6-(2-羟基-2-甲基丙氧基)-2-((2-2-羟基乙基)氨基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(5.0mg,收率5%)。1HNMR(400MHz,DMSO-d6)δ8.38(dd,2H),8.07(s,1H),7.79(dd,1H),7.69(dd,1H),7.12(d,1H),6.78(d,2H),6.36(t,1H),4.66-4.72(m,2H),3.82(d,5H),3.62-3.78(m,4H),3.45-3.61(m,6H),1.60(d,1H),1.21(s,6H).m/z=585[M+1]+。
实施例47
2-氨基-6-乙氧基-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS095)
制备方法同实施例44,将6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷替换为6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷得2-氨基-6-乙氧基-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(9.2mg,收率25%)。1HNMR(400MHz,DMSO-d6)δ8.35(d,1H),8.26(d,1H),8.08(d,1H),7.79(dd,1H),7.70(dd,1H),7.08(d,1H),6.75-6.78(m,2H),6.15(s,2H),4.06-4.12(m,2H),3.82(s,3H),3.67-3.74(m,4H),3.51-3.55(m,4H),2.08(s,1H),1.59(d,1H),1.35(t,3H).m/z=497[M+1]+。
实施例48
6-乙氧基-2-氟-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS096)
制备方法同实施例40,将2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)替换为2-氨基-6-乙氧基-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS095)得6-乙氧基-2-氟-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(7.1mg,收率19%)。m/z=500[M+1]+。
实施例49
2-氨基-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS097)
制备方法同实施例47,将碘乙烷替换为2-溴乙基甲基醚得2-氨基-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(20mg,收率52%)。1HNMR(400MHz,DMSO-d6)δ8.35(d,1H),8.30(d,1H),8.08(d,1H),7.79(dd,1H),7.70(dd,1H),7.10(d,1H),6.77(t,2H),6.16(s,2H),4.19(dd,2H),3.82(s,3H),3.66-3.74(m,6H),3.50(s,4H),1.60(d,1H).m/z=527[M+1]+。
实施例50
2-氟-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS098)
制备方法同实施例40,将2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)替换为2-氨基-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS097)得2-氟-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(5.2mg,收率14%)1HNMR(400MHz,DMSO-d6)δ8.65(d,1H),8.42(d,1H),8.07(d,2H),7.82(dd,1H),7.70(dd,1H),7.45(d,1H),6.81(t,2H),4.25(t,2H),3.82(s,3H),3.68-3.82(m,6H),3.44-3.58(m,5H),1.23(d,1H),1.21(s,1H).m/z=530[M+1]+。
实施例51
2-氨基-6-乙氧基-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS099)
制备方法同实施例47,将6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷替换为1-((6-甲氧基吡啶-3-基)甲基)-4-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)哌嗪得2-氨基-6-乙氧基-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(8.9mg,收率25%)。1HNMR(400MHz,DMSO-d6)δ8.27(dd,2H),8.09(d,1H),7.66-7.72(m,2H),7.06(d,1H),6.91(d,1H),6.82(d,1H),6.14(s,2H),4.05-4.10(m,2H),3.84(s,3H),3.55(brs,4H),3.48(s,2H),2.49(s,4H),1.34(t,3H).m/z=485[M+1]+。
实施例52
2-氨基-6-(2-甲氧基乙氧基)-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS100)
制备方法同实施例49,将6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷替换为1-((6-甲氧基吡啶-3-基)甲基)-4-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)哌嗪得2-氨基-6-(2-甲氧基乙氧基)-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(5.1mg,收率13%)。1HNMR(400MHz,DMSO-d6)δ8.29(dd,2H),8.09(d,1H),7.62-7.71(m,2H),7.90(d,1H),6.89(d,1H),6.82(d,1H),6.16(s,2H),4.16(d,2H),3.84(s,3H),3.66-3.67(m,2H),3.55-3.57(s,4H),3.48(s,2H),3.31(s,3H),2.46(s,3H).m/z=515[M+1]+。
实施例53
6-乙氧基-2-氟-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS101)
制备方法同实施例40,将2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)替换为2-氨基-6-乙氧基-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS099)得6-乙氧基-2-氟-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(3.4mg,收率9%)。1HNMR(400MHz,DMSO-d6)δ8.61(d,1H),8.33(d,1H),8.09(d,1H),7.78(dd,1H),7.68(dd,1H),7.41(d,1H),6.91(d,1H),6.82(d,1H),4.15(d,2H),3.84(s,3H),3.57-3.59(m,4H),3.48(s,2H),1.39(t,3H).m/z=488[M+1]+。
实施例54
2-氟-6-(2-甲氧基乙氧基)-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS102)
制备方法同实施例40,将2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)替换为2-氨基-6-(2-甲氧基乙氧基)-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS100)得2-氟-6-(2-甲氧基乙氧基)-4-(6-(4-((6-甲氧基吡啶-3-基)甲基)哌嗪-1-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(5.3mg,14%)。1HNMR(400MHz,DMSO-d6)δ8.64(d,1H),8.33(d,1H),8.09(d,1H),7.80(dd,1H),7.69(dd,1H),7.44(d,1H),6.95(d,1H),6.82(d,1H),4.24(t,2H),3.84(s,3H),3.68-3.70(m,2H),3.57-3.60(m,4H),3.48(s,4H),2.45(t,3H).m/z=518[M+1]+。
实施例55
N-(3-氰基-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS103)
将2-氨基-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS097)(50mg,0.09mmol)加入到醋酸酐中(2.0mL),加热至80℃反应4h。加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得N-(3-氰基-6-(2-甲氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(30mg,收率58%)。1HNMR(400MHz,DMSO-d6)δ10.65(s,1H),8.57(d,1H),8.37(d,1H),8.07(d,1H),7.79(d,1H),7.67(d,1H),7.28(d,1H),6.76-6.79(m,2H),4.23-4.25(m,2H),3.82(s,3H),3.66-3.71(m,6H),3.50-3.59(m,4H),2.10(s,3H),1.60(d,1H).m/z=569[M+1]+。
实施例56
2-氟-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-4-甲基吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈(APS104)
将2-氟-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS088)(120mg,0.21mmol),37%甲醛水溶液(120mg,1.48mmol),醋酸硼氢化钠(133mg,0.63mmol),二氯甲烷(10mL)室温搅拌12小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品2-氟-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-4-甲基吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈(8.2mg,收率6%)。m/z=586[M+1]+。
实施例57
2-氨基-4-(6-(6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(APS105)
步骤A:3-(5-溴吡啶-2-基)-6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷
向100mL单口瓶中,加入5-氯-6-甲氧基烟醛(0.32g,1.9mmol),3-(5-溴吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷二盐酸盐(0.6g,1.9mmol),醋酸硼氢化钠(1.2g,5.8mmol),二氯甲烷20mL,室温搅拌12小时。TLC点板反应完毕,加入水,用乙酸乙酯萃取,合并有机相,并用水洗涤,无水硫酸钠干燥,过滤,减压浓缩,柱层析分离得到产品3-(5-溴吡啶-2-基)-6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷(0.4g,收率51%)。m/z=409[M+1]+。
步骤B:6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷
向10mL封管中,加入3-(5-溴吡啶-2-基)-6-(吡啶-2-基甲基)-3,6-二氮杂双环[3.1.1]庚烷(53mg,0.132mmol),双联频哪醇硼酸酯(50mg,0.198mmol),[1,1′-双(二苯基膦基)二茂铁]二氯化钯(10mg,0.013mmol),醋酸钾(39mg,0.396mmol),1,4-二氧六环(20mL),氮气置换三次,100℃反应3小时,LCMS确认原料反应完,有产品生成,无须后处理,直接进行下一步反应。
步骤C:4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈
制备方法同实施例39步骤H,将(2S)-2-(溴甲基)-4-吗啉羧酸叔丁酯替换为碘乙烷。得4-氯-2-(1,3-邻苯二甲酰亚胺-2-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(60mg,收率86%)。m/z=367[M+1]+。
步骤D:制备方法同实施例39步骤I,将6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡嗪-2-基)-3,6-二氮杂双环[3.1.1]庚烷替换为6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷得产品2-氨基-4-(6-(6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(14mg,收率36%)。1HNMR(400MHz,CDCl3)δ8.38(d,1H),8.01(d,1H),7.86(d,1H),7.80(dd,2H),7.02(d,1H),6.69(d,1H),4.45(d,2H),4.01-4.42(m,5H),3.82(brs,4H),3.60(brs,4H),2.75(s,1H),1.63-1.67(m,1H),1.47(t,3H).m/z=531[M+1]+。
实施例58
2-氨基-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-甲酰胺(APS106)
将2-氨基-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS089)(40mg,0.074mol)溶于DMSO(2.0mL),加入双氧水(0.2mL),加热65℃反应6h。加入水,用乙酸乙酯萃取,合并有机相,并用饱和食盐水洗涤,减压浓缩,柱层析分离得2-氨基-6-(2-羟基-2-甲基丙氧基)-4-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-甲酰胺(3.5mg,收率8%)。1HNMR(400MHz,DMSO-d6)δ8.27(d,1H),8.17(d,1H),8.08(d,1H),7.63-7.69(m,2H),6.96(d,1H),6.78(d,1H),6.72(d,1H),5.76(s,2H),4.66(s,1H),3.79-3.82(m,5H),3.61-3.68(m,4H),3.48(brs,4H),1.55(d,1H),1.21(s,6H).m/z=559[M+1]+。
实施例59
2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-4-甲基吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈(APS107)
制备方法同实施例56,将2-氟-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS088)替换为2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈三氟乙酸盐(APS087)得产品2-氨基-4-(5-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡嗪-2-基)-6-(((S)-4-甲基吗啉-2-基)甲氧基)吡唑并[1,5-a]吡啶-3-腈(30mg,收率24%)。1HNMR(400MHz,CDCl3)δ8.48(d,1H),8.25(d,1H),8.11(d,1H),7.94(d,1H),7.64(dd,1H),7.33(d,1H),6.73(d,1H),4.53(s,2H),3.93-4.18(m,4H),3.90-3.92(m,3H),3.81-3.90(m,2H),3.71-3.80(m,3H),3.67-3.75(m,2H),3.59-3.62(m,2H),2.86(d,1H),2.68-2.75(m,2H),2.34(s,3H),2.20(t,1H),2.08(t,1H),1.65(d,1H).m/z=583[M+1]+。
实施例60
2-氨基-4-(6-(6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-(2-甲氧基乙氧基)吡唑并[1,5-a]吡啶-3-腈(APS108)
制备方法同实施例52,将6-((6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷替换为6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3-(5-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)吡啶-2-基)-3,6-二氮杂双环[3.1.1]庚烷得产品2-氨基-4-(6-(6-((5-氯-6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-(2-甲氧基乙氧基)吡唑并[1,5-a]吡啶-3-腈(10.6mg,收率26%)。1HNMR(400MHz,CDCl3)δ8.38(d,1H),8.02(s,1H),7.92(d,1H),7.79(dd,2H),7.08(d,1H),6.69(d,1H),4.45(d,2H),4.13-4.15(m,2H),4.03(d,3H),3.78-3.84(m,6H),3.61(brs,4H),3.41(s,3H),2.76(s,1H),1.70(d,1H).m/z=561[M+1]+。
实施例61
2-氨基-6-(2-乙氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS109)
制备方法同实施例47,将碘乙烷替换为2-溴乙基乙基醚得产品2-氨基-6-(2-乙氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(16.2mg,收率41%)。1HNMR(400MHz,DMSO-d6)δ8.30-8.36(m,2H),8.08(s,1H),7.77-7.80(m,1H),7.70(d,1H),7.11(d,1H),6.75-6.77(m,2H),6.17(s,2H),4.16-4.18(m,2H),3.82(s,3H),3.70-3.74(m,6H),3.48-3.53(m,6H),1.60(d,1H),1.15(t,3H).m/z=541[M+1]+。
实施例62
2-氨基-6-(2-甲氧基-2-甲基丙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS110)
制备方法同实施例47,将碘乙烷替换为1-溴-2-甲氧基-2-甲基丙烷得产品2-氨基-6-(2-甲氧基-2-甲基丙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(2.8mg,收率7%).m/z=555[M+1]+。
实施例63
2-氨基-6-乙氧基-4-(6-(6-((5-氟吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS111)
制备方法同实施例57,将5-氯-6-甲氧基烟醛替换为5-氟烟醛得产品2-氨基-6-乙氧基-4-(6-(6-((5-氟吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(3.6mg,收率10%)。1HNMR(400MHz,DMSO-d6)δ8.44(d,2H),8.35(d,1H),8.26(d,1H),7.79(dd,1H),7.71(d,1H),7.07(d,1H),6.77(d,1H),6.16(s,1H),4.10(d,2H),3.72-3.74(m,5H),3.65(s,2H),2.51(brs,1H),2.01(d,1H),1.62(d,1H),1.23(s,3H)。m/z=485[M+1]+。
实施例64
2-氨基-4-(6-(6-((5-氯吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(APS112)
制备方法同实施例57,将5-氯-6-甲氧基烟醛替换为5-氯烟醛得产品2-氨基-4-(6-(6-((5-氯吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(13.6mg,收率37%)。1HNMR(400MHz,DMSO-d6)δ8.40-8.51(m,2H),8.35(d,1H),8.26(d,1H),7.90(s,1H),7.79(dd,1H),7.07(d,1H),6.77(d,1H),6.16(s,2H),4.90(q,2H),3.72-3.74(d,4H),3.63(s,2H),3.52(brs,2H),2.00(s,1H),1.61(d,1H),1.37(t,3H).m/z=501[M+1]+。
实施例65
2-氨基-6-(2-环丙氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS113)
制备方法同实施例47,将碘乙烷替换为对甲苯磺酸2-环丙氧基乙基酯得产品2-氨基-6-(2-环丙氧基乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(31.5mg,收率78%)。1HNMR(400MHz,DMSO-d6)δ8.36(d,1H),8.30(d,1H),8.08(d,1H),7.80(dd,1H),7.70(dd,1H),7.10(d,1H),6.78(dd,2H),6.18(s,2H),4.18(t,2H),3.82(s,3H),3.64-3.78(m,6H),3.50-3.55(m,4H),3.35-3.40(m,1H),1.60(d,1H),0.43-0.52(m,4H).m/z=553[M+1]+。
实施例66
2-氨基-6-(2,2-二氟乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS114)
制备方法同实施例47,将碘乙烷替换为1,1-二氟-2-碘乙烷得产品2-氨基-6-(2,2-二氟乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(3.8mg,收率9%)。1HNMR(400MHz,DMSO-d6)δ8.43(d,1H),8.37(d,1H),8.08(s,1H),7.81(dd,1H),7.70(dd,1H),7.18(d,1H),6.78(d,2H),6.23(s,2H),4.39-4.47(m,2H),3.82(s,3H),3.67-3.74(m,4H),3.50-3.55(m,4H),1.60(d,1H).m/z=533[M+1]+。
实施例67
2-氨基-6-(二氟甲氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS115)
制备方法同实施例47,将碘乙烷替换为二氟碘甲烷得产品2-氨基-6-(二氟甲氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(2.2mg,收率5.8%)。m/z=519[M+1]+。
实施例68
2-氨基-4-(6-(6-((5-氯吡啶-2-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(APS116)
制备方法同实施例57,将5-氯-6-甲氧基烟醛替换为5-氯吡啶甲醛得产品2-氨基-4-(6-(6-((5-氯吡啶-2-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(7.0mg,收率19%)。1HNMR(400MHz,DMSO-d6)δ8.51(d,1H),8.34(d,1H),8.26(d,1H),7.88(dd,1H),7.78(dd,1H),7.53(d,1H),7.08(d,1H),6.75(d,1H),6.17(s,2H),4.10(d,2H),3.72-3.74(m,4H),3.65(s,2H),3.55(brs,2H),2.52(s,1H),2.08(s,3H),2.60(d,1H).m/z=501[M+1]+。
实施例69
2-氨基-4-(6-(6-((5-氟吡啶-2-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(APS117)
制备方法同实施例57,将5-氯-6-甲氧基烟醛替换为5-氟吡啶甲醛得产品2-氨基-4-(6-(6-((5-氟吡啶-2-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)-6-乙氧基吡唑并[1,5-a]吡啶-3-腈(10.8mg,收率30%)。1HNMR(400MHz,DMSO-d6)δ8.46(d,1H),8.34(d,1H),8.26(d,1H),7.78(t,1H),7.67-7.72(m,1H),7.53-7.56(m,1H),7.08(d,1H),6.76(d,1H),6.17(s,2H),4.06-4.11(t,2H),3.71-3.79(m,4H),3.64(s,2H),3.55(d,2H),2.08(s,1H),1.62(d,1H),1.37(t,3H).m/z=485[M+1]+。
实施例70
2-氨基-6-乙氧基-4-(6-(6-((6-甲氧基吡啶-2-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS118)
制备方法同实施例57,将5-氯-6-甲氧基烟醛替换为6-甲氧基吡啶甲醛得产品2-氨基-6-乙氧基-4-(6-(6-((6-甲氧基吡啶-2-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(28mg,收率77%)。1HNMR(400MHz,DMSO-d6)δ8.34(d,1H),8.26(d,1H),7.78(dd,1H),7.65(t,1H),7.02-7.08(m,2H),6.77(d,1H),6.66(d,1H),6.17(s,2H),4.06-4.11(t,2H),3.77-3.83(m,7H),3.60(brs,4H),2.54-2.57(m,1H),1.63(d,1H),1.35(t,3H).m/z=497[M+1]+。
实施例71
2-氨基-6-(2-氟乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS119)
制备方法同实施例47,将碘乙烷替换为1-氟-2-碘乙烷得产品2-氨基-6-(2-氟乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(5.0mg,收率13%)。1HNMR(400MHz,DMSO-d6)8.36(dd,2H),8.08(d,1H),7.80(dd,1H),7.68(dd,1H),7.14(d,1H),6.78(d,2H),6.20(s,2H),4.84(t,1H),4.72(t,1H),4.38(t,1H),4.31(t,1H),3.82(s,3H),3.64-3.74(m,4H),3.50-3.55(m,4H),1.60(d,1H).m/z=515[M+1]+。
实施例72
2-氨基-6-(2-异丙氧乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(APS120)
制备方法同实施例47,将碘乙烷替换为2-(2-溴乙氧基)丙烷得产品2-氨基-6-(2-异丙氧乙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮杂双环[3.1.1]庚烷-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-腈(32.6mg,收率80%)。1HNMR(400MHz,CDCl3)δ8.39(s,1H),8.13(s,1H),7.93(s,1H),7.80(d,1H),7.08(d,1H),6.68-6.76(m,2H),4.44(d,1H),4.14(d,2H),3.86(s,4H),3.80-3.82(m,3H),3.64-3.76(m,3H),1.23-1.25(m,10H)。m/z=555[M+1]+。
参考上述实施例的方法,还制备得到了其他化合物。将这些化合物与上述化合物的表征数据汇总于如下表1中:
表1
激酶活性测试
转染重组基因(RET)是一个已经确认的原癌基因。它编码的单次跨膜受体酪氨酸激酶,是许多组织和细胞类型的发育,成熟和维持所必需的。在正常条件下,神经胶质细胞系衍生的神经营养因子(GDNF)家族配体与细胞表面上的RET的结合导致细胞内酪氨酸残基的二聚化和自磷酸化。这反过来导致下游RAS-MAPK,PI3K-AKT和磷脂酶Cγ(PLCγ)通路的激活,并增加细胞存活和增殖。激活RET突变的实例包括C634W,M918T和关守突变,V804L和V804M。
该试验将肽底物和单一专有单克隆抗体与HTRF技术相结合,HTRF技术是一种高灵敏度和稳定的技术,用于检测蛋白质的分子相互作用。酶将底物磷酸化,然后Eu标记的抗体结合磷酸化底物,链霉抗生物素蛋白-XL665结合所有底物。TR-FRET信号由HTRF原理产生。一旦添加抑制剂(受试化合物),就获得较弱的TR-FRET信号。据此,评估抑制效果。
5.1.试剂和耗材
星形孢菌素
(Staurosporine)
5.2配制溶液
用DMSO将CEP-32496从10mM和1mM分别梯度稀释3倍,共10个浓度。
其他化合物从10mM的原液用DMSO梯度稀释3倍,共10个浓度。
制备1000×阳性对照(1mM CEP-32496和0.2mMStaurosporine)和1000×阴性对照(100%DMSO)。
在平板振荡器上振荡5分钟。
5.3制备1x激酶缓冲液
将4体积蒸馏水加入1体积酶缓冲液5X;5mM MgCl2;1mM DTT。
5.4筛选方法
a)将10nl化合物稀释液(5.2中制备)转移到测试板(784075,Greiner)的每个孔中;
b)在1000g下将化合物板离心1分钟。
c)密封测试板。
d)制备1x激酶缓冲液中的5X Ret wt(0.2ng/μl)和5X Ret V804L(0.5ng/μl)/5XRet V804M(0.5ng/μl)。
e)加入2μl的5X Ret wt或2μl的Ret V804L/RET V804M至384孔测试板(784075,Greiner)。
f)将4μl的1x激酶缓冲液加入测试板的每个孔中,在1000g下离心样品板30秒,在室温放置10分钟。
g)制备激酶缓冲液中的5x TK-底物-生物素(5μM)和激酶缓冲液中的5x ATP(50μM)的溶液。
h)通过加入2μl STK-底物-生物素和2μlATP(步骤g中制备的)开始反应。
i)在1000g下离心样品板30秒。密封测试板,室温放置30分钟。
j)制备在HTRF检测缓冲液中的4X Sa-XL 665(250nM)。
k)将5μl的Sa-XL 665和5μl的TK-antibody-Cryptate(在步骤i中制备的)加入到测试板的每个孔中。
l)在1000g下离心30秒,室温放置1小时。
m)在Envision 2104读板仪上读取615nm(Cryptate)和665nm(XL665)的荧光信号值。
5.5数据分析
计算每个孔的比率(665nm/615nm)。
抑制率%通过如下方式计算:
抑制率%=[1-(受试化合物荧光信号值-阳性对照荧光信号值)/(阴性对照平均比率-阳性对照平均比率)]*100%
比率:由测得的荧光信号值产生
阳性对照平均比率为样品板中阳性对照(200nM AT13148)的平均比率;
阴性对照平均比率为样品板中阴性对照(0.1%DMSO)的平均比率。
利用GraphPad 6.0将抑制率%与化合物浓度的对数拟合为非线性回归(剂量响应-可变斜率)计算IC50值。
表2
细胞抑制活性测试
1、在96孔板中配置90μl的细胞悬液,4000细胞/孔。将培养板在培养箱预培养24小时(37℃,5%CO2)。
2、向每孔细胞中加入10μl不同浓度的待测化合物,每个化合物均有复孔。
3、将培养板在培养箱孵育72小时。
4、向每孔加入10μl CCK8溶液(注意不要在孔中生成气泡,它们会影响OD值的读数)。
5、将培养板在培养箱内孵育1-2小时。
6、用酶标仪测定在450nm处的吸光度。
7、根据化合物浓度及吸光度值,利用GraphpadPrism软件计算每个化合物的IC50。
计算结果如下表所示:
表3
上表中“N/A”表示未测试活性。
如上表所示,本发明实施例化合物具有改善的抑制RET野生型、突变型和/或融合型抑制活性,特别是诸如APS014、APS015、APS019、APS020、APS022、APS024、APS025、APS026、APS027、APS035、APS038、APS068、APS069、APS070、APS087、APS089、APS092、APS095、APS097、APS099、APS100、APS104、APS105、APS107、APS108、APS109、APS111、APS113、APS114、APS119、APS120等化合物取得了优异的活性。
以上,对本发明的实施方式进行了说明。但是,本发明不限定于上述实施方式。凡在本发明的精神和原则之内,所做的任何修改、等同替换、改进等,均应包含在本发明的保护范围之内。
Claims (14)
2.根据权利要求1所述的化合物,其中:
D选自被C1-6烷基取代的吡唑-3-基、吡唑-4-基、吡唑-5-基;
K选自被一个Rg取代的下列基团:甲基、-C(O)CH3;
Rg选自无取代或任选被一个、两个或更多个Rh取代的下列基团:苯基、吡啶-2-基、吡啶-3-基、吡啶-4-基;
Rh选自氟、氯、溴、甲氧基、乙氧基。
6.根据权利要求5所述的方法,其中L选自卤素或者OTf。
7.一种药物组合物,其特征在于,包含治疗有效量的权利要求1-4任一项所述的化合物或其药学上可接受的盐中的至少一种。
8.根据权利要求7所述的药物组合物,其特征在于,所述药物组合物还包括一种或多种药学上可接受的载体或赋形剂。
9.根据权利要求7或8所述的药物组合物,其特征在于,所述药物组合物进一步含有一种或多种额外的治疗剂。
10.权利要求1-4任一项所述的化合物或其药学上可接受的盐中的至少一种在制备用于治疗RET激酶介导的疾病的药物中的用途。
11.根据权利要求10所述的用途,其特征在于,所述RET激酶介导的疾病包括癌症、肠易激综合征或与它们相关的疼痛。
12.根据权利要求10所述的用途,其特征在于,所述药物用于治疗癌症和/或抑制与特定癌症相关的转移;或者用于向癌症患者提供支持护理。
13.权利要求1-4任一项所述的化合物或其药学上可接受的盐中的至少一种在制备用于抑制RET激酶活性的药物中的用途。
14.权利要求1-4任一项所述的化合物或其药学上可接受的盐中的至少一种在制备用于治疗RET相关疾病或病症的药物中的用途。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2018111624971 | 2018-09-30 | ||
| CN201811162497 | 2018-09-30 | ||
| PCT/CN2019/109502 WO2020064009A1 (zh) | 2018-09-30 | 2019-09-30 | 取代吡唑稠环类衍生物及其制备方法和应用 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN112771043A CN112771043A (zh) | 2021-05-07 |
| CN112771043B true CN112771043B (zh) | 2023-04-28 |
Family
ID=69950354
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201980064157.1A Active CN112771043B (zh) | 2018-09-30 | 2019-09-30 | 取代吡唑稠环类衍生物及其制备方法和应用 |
| CN202210125578.4A Active CN114380825B (zh) | 2018-09-30 | 2019-09-30 | 取代吡唑稠环类衍生物及其制备方法和应用 |
| CN201910942753.7A Active CN110964008B (zh) | 2018-09-30 | 2019-09-30 | 取代吡唑稠环类衍生物及其制备方法和应用 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210125578.4A Active CN114380825B (zh) | 2018-09-30 | 2019-09-30 | 取代吡唑稠环类衍生物及其制备方法和应用 |
| CN201910942753.7A Active CN110964008B (zh) | 2018-09-30 | 2019-09-30 | 取代吡唑稠环类衍生物及其制备方法和应用 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20210379056A1 (zh) |
| EP (1) | EP3845531A4 (zh) |
| JP (1) | JP7493251B2 (zh) |
| KR (1) | KR20210070286A (zh) |
| CN (3) | CN112771043B (zh) |
| WO (1) | WO2020064009A1 (zh) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20220008322A (ko) * | 2019-05-14 | 2022-01-20 | 상하이 한서 바이오메디컬 컴퍼니 리미티드 | 2환식 유도체를 포함하는 억제제, 이의 제조방법 및 용도 |
| KR20220042293A (ko) * | 2019-08-05 | 2022-04-05 | 베이징 즈지엔진루이 셩우이야오 커지 요우시엔공스 | 질소 함유 다환 축합 고리계 화합물, 이의 약학 조성물, 제조 방법 및 용도 |
| CN112574235B (zh) * | 2019-09-29 | 2024-01-16 | 广东东阳光药业股份有限公司 | 一种ret抑制剂、其药物组合物及其用途 |
| CN112851664B (zh) * | 2019-11-12 | 2024-03-29 | 浙江海正药业股份有限公司 | 吡唑[1,5-a]吡啶-3-腈化合物及其在医药上的用途 |
| AU2020410900B2 (en) * | 2019-12-27 | 2024-06-13 | Tyk Medicines, Inc. | Compound used as RET kinase inhibitor and application thereof |
| TW202202501A (zh) * | 2020-04-17 | 2022-01-16 | 美商絡速藥業公司 | 結晶ret抑制劑 |
| CN113683611A (zh) * | 2020-05-18 | 2021-11-23 | 广东东阳光药业有限公司 | 新的ret抑制剂、其药物组合物及其用途 |
| CN113683610A (zh) * | 2020-05-18 | 2021-11-23 | 广东东阳光药业有限公司 | 一种ret抑制剂、其药物组合物及其用途 |
| CN115968288B (zh) * | 2020-08-20 | 2024-08-27 | 江苏正大丰海制药有限公司 | 作为ret激酶抑制剂的杂芳环化合物及其制备和应用 |
| WO2022068739A1 (zh) * | 2020-09-29 | 2022-04-07 | 广州白云山医药集团股份有限公司白云山制药总厂 | 吡啶并吡唑类化合物的晶型及其制备方法 |
| WO2022083741A1 (zh) * | 2020-10-23 | 2022-04-28 | 上海辉启生物医药科技有限公司 | 吡唑并吡啶类化合物或其盐及其制备方法和用途 |
| TWI818424B (zh) * | 2021-02-08 | 2023-10-11 | 大陸商北京志健金瑞生物醫藥科技有限公司 | 含氮多環稠環類化合物,其藥物組合物、製備方法和用途 |
| JP2024524601A (ja) * | 2021-07-09 | 2024-07-05 | 北京志健金瑞生物医▲薬▼科技有限公司 | 窒素含有多環式縮合環系化合物の製造方法及びその中間体並びに使用 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TW201825488A (zh) * | 2016-10-10 | 2018-07-16 | 美商亞雷生物製藥股份有限公司 | 作為pet激酶抑制劑之經取代吡唑并[1,5-a]吡啶化合物 |
| WO2018136661A1 (en) * | 2017-01-18 | 2018-07-26 | Andrews Steven W | SUBSTITUTED PYRAZOLO[1,5-a]PYRAZINE COMPOUNDS AS RET KINASE INHIBITORS |
| CN108349969A (zh) * | 2015-07-16 | 2018-07-31 | 阵列生物制药公司 | 作为RET激酶抑制剂的取代的吡唑并[1,5-a]吡啶化合物 |
| WO2019075114A1 (en) * | 2017-10-10 | 2019-04-18 | Mark Reynolds | FORMULATIONS COMPRISING 6- (2-HYDROXY-2-METHYLPROPOXY) -4- (6- (6 - ((6-METHOXYPYRIDIN-3-YL) METHYL) -3,6-DIAZABICYCLO [3.1.1] HEPTAN-3- YL) PYRIDIN-3-YL) PYRAZOLO [1,5-A] pYRIDINE-3-carbonitrile |
| WO2019075108A1 (en) * | 2017-10-10 | 2019-04-18 | Metcalf Andrew T | CRYSTALLINE FORMS |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AR086042A1 (es) * | 2011-04-28 | 2013-11-13 | Galapagos Nv | Compuesto util para el tratamiento de enfermedades degenerativas e inflamatorias y composicion farmaceutica |
| CN102827073A (zh) | 2011-06-17 | 2012-12-19 | 安吉奥斯医药品有限公司 | 治疗活性组合物和它们的使用方法 |
| CN103814020B (zh) * | 2011-06-17 | 2017-07-14 | 安吉奥斯医药品有限公司 | 治疗活性组合物和它们的使用方法 |
| JOP20190077A1 (ar) | 2016-10-10 | 2019-04-09 | Array Biopharma Inc | مركبات بيرازولو [1، 5-a]بيريدين بها استبدال كمثبطات كيناز ret |
| TWI783057B (zh) * | 2017-10-10 | 2022-11-11 | 美商絡速藥業公司 | 製備6-(2-羥基-2-甲基丙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮雜雙環[3.1.1]庚-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-甲腈的方法 |
-
2019
- 2019-09-30 CN CN201980064157.1A patent/CN112771043B/zh active Active
- 2019-09-30 CN CN202210125578.4A patent/CN114380825B/zh active Active
- 2019-09-30 KR KR1020217009429A patent/KR20210070286A/ko not_active Application Discontinuation
- 2019-09-30 JP JP2021542252A patent/JP7493251B2/ja active Active
- 2019-09-30 CN CN201910942753.7A patent/CN110964008B/zh active Active
- 2019-09-30 WO PCT/CN2019/109502 patent/WO2020064009A1/zh unknown
- 2019-09-30 EP EP19865058.2A patent/EP3845531A4/en active Pending
- 2019-09-30 US US17/281,567 patent/US20210379056A1/en active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108349969A (zh) * | 2015-07-16 | 2018-07-31 | 阵列生物制药公司 | 作为RET激酶抑制剂的取代的吡唑并[1,5-a]吡啶化合物 |
| TW201825488A (zh) * | 2016-10-10 | 2018-07-16 | 美商亞雷生物製藥股份有限公司 | 作為pet激酶抑制劑之經取代吡唑并[1,5-a]吡啶化合物 |
| WO2018136661A1 (en) * | 2017-01-18 | 2018-07-26 | Andrews Steven W | SUBSTITUTED PYRAZOLO[1,5-a]PYRAZINE COMPOUNDS AS RET KINASE INHIBITORS |
| WO2019075114A1 (en) * | 2017-10-10 | 2019-04-18 | Mark Reynolds | FORMULATIONS COMPRISING 6- (2-HYDROXY-2-METHYLPROPOXY) -4- (6- (6 - ((6-METHOXYPYRIDIN-3-YL) METHYL) -3,6-DIAZABICYCLO [3.1.1] HEPTAN-3- YL) PYRIDIN-3-YL) PYRAZOLO [1,5-A] pYRIDINE-3-carbonitrile |
| WO2019075108A1 (en) * | 2017-10-10 | 2019-04-18 | Metcalf Andrew T | CRYSTALLINE FORMS |
Non-Patent Citations (1)
| Title |
|---|
| RN 2222758-45-2,RN 2222758-41-8, RN 2222758-39-4,RN 2222758-12-3,RN 2222758-09-8,RN 2222758-07-6,RN 2222758-02-1;《STN REGISTER》;《STN REGISTER》;20180511;第1-7页 * |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20210070286A (ko) | 2021-06-14 |
| CN110964008A (zh) | 2020-04-07 |
| JP2022508533A (ja) | 2022-01-19 |
| US20210379056A1 (en) | 2021-12-09 |
| CN112771043A (zh) | 2021-05-07 |
| WO2020064009A1 (zh) | 2020-04-02 |
| EP3845531A1 (en) | 2021-07-07 |
| JP7493251B2 (ja) | 2024-05-31 |
| CN110964008B (zh) | 2021-12-21 |
| EP3845531A4 (en) | 2021-11-24 |
| CN114380825B (zh) | 2024-02-13 |
| CN114380825A (zh) | 2022-04-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN112771043B (zh) | 取代吡唑稠环类衍生物及其制备方法和应用 | |
| TWI848954B (zh) | 作為hpk1抑制劑的吡咯並[2,3-b]吡啶或吡咯並[2,3-b]吡嗪及其用途 | |
| JP7034084B2 (ja) | Tam阻害剤としてのピロロトリアジン化合物 | |
| CN108699057B (zh) | 5-取代的2-(吗啉-4-基)-1,7-萘啶 | |
| WO2020239077A1 (zh) | 含氮杂环类衍生物调节剂、其制备方法和应用 | |
| JP2023510929A (ja) | ヘテロアリール誘導体、その製造方法およびその使用 | |
| KR20170040300A (ko) | 2-(모르폴린-4-일)-l,7-나프티리딘 | |
| CN113490670B (zh) | 含氮多环稠环类化合物,其药物组合物、制备方法和用途 | |
| TW202039489A (zh) | Mat2a之雜雙環抑制劑及用於治療癌症之使用方法 | |
| EP2481736A1 (en) | Kinase inhibitors useful for the treatment of myleoproliferative diseases and other proliferative diseases | |
| JP2009510161A (ja) | 認知症及び神経変性疾患の治療に用いるためのグリコーゲンシンターゼキナーゼ3阻害剤としての新規なイミダゾ「4,5−b]ピリジン誘導体 | |
| KR20100134693A (ko) | 2-아미노퀴나졸린 유도체 | |
| TWI818424B (zh) | 含氮多環稠環類化合物,其藥物組合物、製備方法和用途 | |
| JP2024516317A (ja) | Shp2リン酸化酵素阻害剤の調製と応用 | |
| WO2023091726A1 (en) | Inhibitors of cyclin‑dependent kinase 12 (cdk12) | |
| CN115151550A (zh) | 外核苷酸焦磷酸酶/磷酸二酯酶1(enpp1)调节剂及其用途 | |
| JP2022540200A (ja) | Lrrk2阻害剤としてのインダゾールおよびアザインダゾール | |
| CN108794484A (zh) | [1,2,4]三唑并[4,3-a]吡嗪衍生物、其药物组合物、制备方法和应用 | |
| JP7233130B2 (ja) | Irak4阻害剤としての新規な三環式化合物 | |
| EP3628669A1 (en) | Novel compounds as nadph oxidase inhibitors | |
| US9249163B2 (en) | PDE10a inhibitors for the treatment of type II diabetes | |
| EA041747B1 (ru) | Ингибиторы g12c kras, содержащая их фармацевтическая композиция и использующий их способ лечения рака |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |