CN112530689A - Method for improving grain boundary diffusion effect of high-abundance cerium magnet - Google Patents
Method for improving grain boundary diffusion effect of high-abundance cerium magnet Download PDFInfo
- Publication number
- CN112530689A CN112530689A CN202011355320.0A CN202011355320A CN112530689A CN 112530689 A CN112530689 A CN 112530689A CN 202011355320 A CN202011355320 A CN 202011355320A CN 112530689 A CN112530689 A CN 112530689A
- Authority
- CN
- China
- Prior art keywords
- powder
- cerium
- product
- diffusion
- magnet
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F41/00—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties
- H01F41/02—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties for manufacturing cores, coils, or magnets
- H01F41/0253—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties for manufacturing cores, coils, or magnets for manufacturing permanent magnets
- H01F41/0293—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties for manufacturing cores, coils, or magnets for manufacturing permanent magnets diffusion of rare earth elements, e.g. Tb, Dy or Ho, into permanent magnets
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/032—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of hard-magnetic materials
- H01F1/04—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of hard-magnetic materials metals or alloys
- H01F1/047—Alloys characterised by their composition
- H01F1/053—Alloys characterised by their composition containing rare earth metals
- H01F1/055—Alloys characterised by their composition containing rare earth metals and magnetic transition metals, e.g. SmCo5
- H01F1/057—Alloys characterised by their composition containing rare earth metals and magnetic transition metals, e.g. SmCo5 and IIIa elements, e.g. Nd2Fe14B
- H01F1/0571—Alloys characterised by their composition containing rare earth metals and magnetic transition metals, e.g. SmCo5 and IIIa elements, e.g. Nd2Fe14B in the form of particles, e.g. rapid quenched powders or ribbon flakes
- H01F1/0573—Alloys characterised by their composition containing rare earth metals and magnetic transition metals, e.g. SmCo5 and IIIa elements, e.g. Nd2Fe14B in the form of particles, e.g. rapid quenched powders or ribbon flakes obtained by reduction or by hydrogen decrepitation or embrittlement
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F41/00—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties
- H01F41/02—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties for manufacturing cores, coils, or magnets
- H01F41/0253—Apparatus or processes specially adapted for manufacturing or assembling magnets, inductances or transformers; Apparatus or processes specially adapted for manufacturing materials characterised by their magnetic properties for manufacturing cores, coils, or magnets for manufacturing permanent magnets
Landscapes
- Engineering & Computer Science (AREA)
- Power Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Chemical & Material Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Hard Magnetic Materials (AREA)
Abstract
The invention relates to the field of sintered neodymium-iron-boron magnetic materials, in particular to a method for improving the grain boundary diffusion effect of a high-abundance cerium magnet, which comprises two-stage diffusion and is characterized by comprising the following steps: carrying out hydrogen crushing on praseodymium and neodymium alloy cast pieces, grinding the crushed praseodymium and neodymium alloy cast pieces into powder, mixing the powder with a solvent, coating the mixture on a cerium-containing magnet, and diffusing the cerium-containing magnet at high temperature; and then crushing the terbium alloy cast sheet, grinding the crushed terbium alloy cast sheet into powder, mixing the powder with a solvent, coating the powder on the surface of a product subjected to primary diffusion, and performing high-temperature secondary diffusion to obtain a final product.
Description
Technical Field
The invention relates to the field of sintered neodymium-iron-boron magnetic materials, in particular to a method for improving the grain boundary diffusion effect of a high-abundance cerium magnet.
Background
The rare earth permanent magnetic material is a key functional material in the national development industry, and is widely applied to the fields of new energy, intelligent equipment, rail transit, electronic information and the like. At present, the rare earth permanent magnet material is the largest consumption market of rare earth resources, and accounts for more than 60 percent of the rare earth application market.
The rare earth permanent magnet material is mainly neodymium iron boron magnet. According to the statistics of the association of rare earth industry, the yield of neodymium iron boron blanks in China in 2015 is about 14 ten thousand tons, and accounts for more than 80% of the global market share. The neodymium-iron-boron magnet generally contains about 30% of praseodymium-neodymium, 1% -2% of dysprosium, terbium and other high-valence heavy rare earth elements.
With the rapid development of the rare-earth permanent magnet motor industry, the demand for neodymium-iron-boron magnets is greatly increased, and the usage amount of rare-earth metals such as praseodymium and neodymium is continuously increased. In rare earth resources, lanthanum and cerium are more than 70%, praseodymium and neodymium are 20%, the medium-weight rare earth element is less than 10%, and the price of praseodymium and neodymium is about 14 times that of lanthanum and cerium.
At present, the existing neodymium iron boron enterprises begin to add cerium to replace praseodymium and neodymium elements in low-performance materials, so that the material cost is reduced. However, the reduction of the overall magnetic performance of the neodymium iron boron by the addition of cerium results in that the application of the conventional cerium magnet only stays in the low-performance fields of N series and below, and how to add cerium into the medium-high performance neodymium iron boron magnet to reduce the production cost of materials also becomes the research and development direction of many neodymium iron boron enterprises.
Disclosure of Invention
The invention discloses a method for improving the grain boundary diffusion effect of a high-abundance cerium magnet, which comprises two-stage diffusion and is characterized by comprising the following steps:
(1) and (3) carrying out cerium magnet grain boundary diffusion treatment: smelting mass ratio PrNd0.86Cu0.1Ga0.04Alloy casting, grinding into powder after hydrogen crushing, mixing the powder with a solvent, coating the mixture on a cerium-containing magnet, placing a product coated with praseodymium and neodymium alloy in a molybdenum box, and diffusing at high temperature;
(2) secondary diffusion treatment of a cerium magnet grain boundary: meltingMass ratio Tb0.88PrNd0.04Fe0.04Al0.02Ga0.02And (2) alloy casting, grinding the alloy casting into powder after hydrogen crushing, mixing the powder with a solvent, coating the mixture on the surface of the product treated in the step (1), placing the product coated with the terbium alloy in a molybdenum material box, and performing high-temperature diffusion to obtain the final product.
Preferably, the smelting PrNd in the step (1)0.86Cu0.1Ga0.04Alloy casting sheet is hydrogen crushed and airflow milled into powder of 3-9 um size.
Preferably, said PrNd of step (1)0.86Cu0.1Ga0.04The mass volume ratio of the powder to the solvent is 1-2 g/ml.
Preferably, the solvent in steps (1) and (2) is one or two selected from diethyl ether or ethanol.
Preferably, the diffusion temperature in the step (1) is 700-850 ℃, preferably 750-800 ℃; the diffusion time is 3-6h, preferably 4-5 h.
Preferably, the product diffused in the step (1) is subjected to secondary diffusion treatment after surface oxide skin is removed by a grinding machine and acid cleaning.
Preferably, the alloy coated in the step (1) is 4-8 mg/cm2Preferably 6 to 8mg/cm2。
Smelting Tb in step (2)0.88PrNd0.04Fe0.04Al0.02Ga0.02Alloy casting sheet is hydrogen crushed and airflow milled to produce powder of 2-6 um size.
Preferably, the smelting in the step (2) is Tb0.88PrNd0.04Fe0.04Al0.02Ga0.02Alloy casting sheet is hydrogen crushed and airflow milled to produce powder of 2-6 um size.
Preferably, step (2) Tb0.88PrNd0.04Fe0.04Al0.02Ga0.02The mass volume ratio of the powder to the solvent is 1-2 g/ml.
Preferably, the diffusion temperature in the step (2) is 870-950 ℃, preferably 880-900 ℃; the diffusion time is 5-11h, preferably 6-8 h; cooling the product to below 60 deg.C, heating to 450-530 deg.C, preferably 480-500 deg.C, and maintaining for 2-4 hr, preferably 3-4 hr.
Preferably, the alloy coated in the step (2) is 4-8 mg/cm2Preferably 6 to 8mg/cm2。
Preferably, the cerium-containing magnet is selected from 5-20% by mass, sliced, sequentially subjected to oil removal and acid washing until the surface of the product is cleaned; the acid reagent is dilute nitric acid with the mass percentage of 3-5%;
preferably, the alloy coated in the steps (1) and (2) is 4-8 mg/cm2。
When cerium is added to neodymium iron boron to replace praseodymium and neodymium, a CeFe2 phase is easily generated in a grain boundary phase, and the CeFe2 phase structure seriously hinders the diffusion of heavy rare earth elements, so that the coercive force expansion amplitude is far less than the coercive force expansion amplitude of a cerium-free neodymium iron boron magnet when the cerium-containing magnet is subjected to grain boundary diffusion, and the vast majority of coercive force expansion amplitude of the cerium-containing magnet subjected to grain boundary diffusion currently stays between 3kOe and 5 kOe.
The invention compares the performance of the base material through two-stage diffusion, and can improve the coercive force expansion amplitude of the cerium-containing magnet to 9.12kOe after the two-stage diffusion.
Detailed Description
The present invention will be further described with reference to the following examples. The described embodiments and their results are only intended to illustrate the invention and should not be taken as limiting the invention described in detail in the claims.
Example 1:
(1) pretreatment of a cerium-containing magnet: selecting a cerium-containing N40 magnet with the mass percentage of 8%, slicing the magnet into products with the sizes of 25mm multiplied by 20mm multiplied by 3mm, sequentially removing oil, and pickling with 3% by mass of dilute nitric acid until the surfaces of the products are clean to be treated;
(2) and (3) carrying out cerium magnet grain boundary diffusion treatment: smelting 1kg of PrNd0.86Cu0.1Ga0.04 (mass ratio) alloy cast sheet, grinding the alloy cast sheet into powder with the granularity of 3-9 um through airflow after hydrogen crushing, mixing the powder with 2000ml of organic solvent such as ether, ethanol and the like, coating the mixture on the surface of the cerium-containing magnet pretreated in the step (1), and coating praseodymium and neodymium alloy (8 mg/cm)2) The product is placed in a molybdenum material box dried at 500 ℃, heated to 700 ℃ at a speed of 5 ℃/min and kept for 4h, and the diffusion surface of the product is taken out of the furnaceRemoving surface oxide skin by a grinding machine and acid washing, and then treating;
(3) secondary diffusion treatment of a cerium magnet grain boundary: smelting 1kg of Tb0.88PrNd0.04Fe0.04Al0.02Ga0.02 (mass ratio) alloy cast sheet, carrying out hydrogen crushing, carrying out airflow milling to prepare powder with the particle size of 2-6 um, mixing the powder with 2000ml of organic solvent such as diethyl ether, ethanol and the like, coating the mixture on the surface of the product treated in the step (2), and coating terbium alloy (8 mg/cm)2) The product is placed in a molybdenum material box dried at 500 ℃, heated to 900 ℃ at a speed of 5 ℃/min and kept warm for 8 hours; when the product is cooled to below 60 ℃, the temperature is raised to 500 ℃ through 5 ℃/min and the temperature is kept for 3 h; cooling to room temperature to obtain the final product;
(4) and (3) testing the magnetic property of the product: the magnetic properties of the product of example 1 were measured out of the furnace and the results of the property tests are shown in Table 1 (see national Standard GB/T-3217-2013):
TABLE 1
Example 2:
(1) pretreatment of a cerium-containing magnet: the conditions were the same as in example 1;
(2) and (3) carrying out cerium magnet grain boundary diffusion treatment: coating praseodymium and neodymium alloy (4 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, heated to 830 ℃ through 5 ℃/min and kept warm for 6h ", and the other conditions are the same as those in the embodiment 1;
(3) secondary diffusion treatment of a cerium magnet grain boundary: the conditions were the same as in example 1;
(4) and (3) testing the magnetic property of the product: the magnetic properties of the product of example 2 were measured out of the furnace and the results of the property tests are shown in Table 2:
TABLE 2
Example 3:
(1) pretreatment of a cerium-containing magnet: the conditions were the same as in example 1;
(2) cerium magnetAnd (3) bulk grain boundary diffusion treatment: coating praseodymium and neodymium alloy (6 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, heated to 750 ℃ at a speed of 5 ℃/min and kept warm for 5h ", and the other conditions are the same as those in the embodiment 1;
(3) secondary diffusion treatment of a cerium magnet grain boundary: coated with terbium (6 mg/cm) alloy2) The other conditions were the same as in example 1;
(4) and (3) testing the magnetic property of the product: the magnetic properties of the product of example 3 were measured out of the furnace and the results of the property tests are shown in Table 3:
TABLE 3
Example 4:
(1) pretreatment of a cerium-containing magnet: the conditions were the same as in example 1;
(2) and (3) carrying out cerium magnet grain boundary diffusion treatment: coating praseodymium and neodymium alloy (8 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, heated to 850 ℃ through 5 ℃/min and kept warm for 6h ", and other conditions are the same as those in the embodiment 1;
(3) secondary diffusion treatment of a cerium magnet grain boundary: the conditions were the same as in example 1;
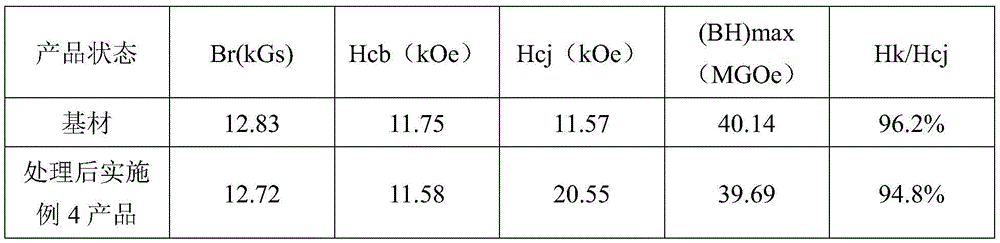
(4) and (3) testing the magnetic property of the product: the magnetic properties of the product of example 4 were measured out of the furnace and the results of the property tests are shown in Table 4:
TABLE 4
Example 5:
(1) pretreatment of a cerium-containing magnet: the conditions were the same as in example 1;
(2) and (3) carrying out cerium magnet grain boundary diffusion treatment: coating praseodymium and neodymium alloy (8 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, the temperature is raised to 830 ℃ at the speed of 5 ℃/min and is kept for 6h ", and other conditions are the same as those in the embodiment 1;
(3) secondary diffusion treatment of a cerium magnet grain boundary: mixing "coated terbium complexGold (4 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, heated to 920 ℃ through 5 ℃/min and kept warm for 11h ", and the other conditions are the same as those in the embodiment 1;
(4) and (3) testing the magnetic property of the product: the magnetic properties of the product of example 5 were measured out of the furnace and the results of the property tests are shown in Table 5:
TABLE 5
Example 6:
(1) pretreatment of a cerium-containing magnet: the conditions were the same as in example 1;
(2) and (3) carrying out cerium magnet grain boundary diffusion treatment: coating praseodymium and neodymium alloy (8 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, the temperature is raised to 830 ℃ at the speed of 5 ℃/min and is kept for 6h ", and other conditions are the same as those in the embodiment 1;
(3) secondary diffusion treatment of a cerium magnet grain boundary: coating a "terbium-coated alloy (8 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, heated to 950 ℃ by 5 ℃/min and kept warm for 12h ", and the other conditions are the same as those in the embodiment 1;
(4) and (3) testing the magnetic property of the product: the magnetic properties of the product of example 6 were measured out of the furnace and the results of the property tests are shown in Table 6:
TABLE 6
Comparative example 1:
(1) pretreatment of a cerium-containing magnet: the conditions were the same as in example 1;
(2) and (3) carrying out cerium magnet grain boundary diffusion treatment: the step (3) of the step 1 is carried out under the same conditions;
(3) and (3) testing the magnetic property of the product: the magnetic properties of the product of comparative example 1 were measured out of the furnace and the results of the property tests are shown in Table 7:
TABLE 7
Comparative example 2:
(1) pretreatment of a cerium-containing magnet: the conditions were the same as in example 1;
(2) and (3) carrying out cerium magnet grain boundary diffusion treatment: coating praseodymium and neodymium alloy (8 mg/cm)2) The product is placed in a molybdenum box dried at 500 ℃, heated to 750 ℃ at a speed of 5 ℃/min and kept warm for 5h ", and the other conditions are the same as those in the embodiment 1;
(3) and (3) testing the magnetic property of the product: the magnetic properties of the product of comparative example 2 were measured out of the furnace and the results of the property tests are shown in Table 8:
TABLE 8
From the example and comparative example data it can be seen that: the coercive force amplitude of the cerium-containing magnet is obviously improved by the secondary diffusion process method, and the amount and depth of cerium in the rare earth-rich phase displaced by primary diffusion have obvious influence on the amplitude of the secondary diffusion coercive force; however, too high a temperature and too long a time for the second-order diffusion also have an effect on the overall increase in coercivity.
Claims (10)
1. A method for improving the grain boundary diffusion effect of a high-abundance cerium magnet comprises two-stage diffusion and is characterized by comprising the following steps:
(1) and (3) carrying out cerium magnet grain boundary diffusion treatment: smelting mass ratio PrNd0.86Cu0.1Ga0.04Alloy casting, grinding into powder after hydrogen crushing, mixing the powder with a solvent, coating the mixture on a cerium-containing magnet, placing a product coated with praseodymium and neodymium alloy in a molybdenum box, and diffusing at high temperature;
(2) secondary diffusion treatment of a cerium magnet grain boundary: melting mass ratio Tb0.88PrNd0.04Fe0.04Al0.02Ga0.02Alloy casting, grinding into powder after hydrogen crushing, mixing the powder with a solvent, coating the mixture on the surface of the product treated in the step (1), and coating the terbium-coated alloyAnd placing the product in a molybdenum material box, and diffusing at high temperature to obtain the final product.
2. The method of claim 1, wherein: the smelting PrNd in the step (1)0.86Cu0.1Ga0.04Alloy casting sheet is hydrogen crushed and airflow milled into powder of 3-9 um size.
3. The method of claim 1, wherein: the PrNd in the step (1)0.86Cu0.1Ga0.04The mass volume ratio of the powder to the solvent is 1-2 g/ml.
4. The method of claim 1, wherein: the solvent in the steps (1) and (2) is one or two of diethyl ether or ethanol.
5. The method of claim 1, wherein: the diffusion temperature in the step (1) is 700-850 ℃, and the diffusion time is 3-6 h.
6. The method of claim 1, wherein: and (3) removing surface oxide scales of the diffused product in the step (1) by a grinding machine and acid washing, and then performing secondary diffusion treatment.
7. The method of claim 1, wherein: smelting Tb in step (2)0.88PrNd0.04Fe0.04Al0.02Ga0.02Alloy casting sheet is hydrogen crushed and airflow milled to produce powder of 2-6 um size.
8. The method of claim 1, wherein: step (2) Tb0.88PrNd0.04Fe0.04Al0.02Ga0.02The mass volume ratio of the powder to the solvent is 1-2 g/ml.
9. The method of claim 1, wherein: in the step (2), the diffusion temperature is 870-950 ℃, and the diffusion time is 5-11 h; cooling the product to below 60 deg.c, raising the temperature to 450-530 deg.c and maintaining for 2-4 hr.
10. The method of claim 1, wherein: selecting a cerium-containing magnet with the mass ratio of 5-20%, slicing, sequentially removing oil, and carrying out acid washing until the surface of the product is cleaned; the acid reagent is dilute nitric acid with the mass percentage of 3-5%; preferably, the alloy coated in the steps (1) and (2) is 4-8 mg/cm2。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011355320.0A CN112530689A (en) | 2020-11-27 | 2020-11-27 | Method for improving grain boundary diffusion effect of high-abundance cerium magnet |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011355320.0A CN112530689A (en) | 2020-11-27 | 2020-11-27 | Method for improving grain boundary diffusion effect of high-abundance cerium magnet |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN112530689A true CN112530689A (en) | 2021-03-19 |
Family
ID=74994111
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202011355320.0A Pending CN112530689A (en) | 2020-11-27 | 2020-11-27 | Method for improving grain boundary diffusion effect of high-abundance cerium magnet |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112530689A (en) |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105957679A (en) * | 2016-07-18 | 2016-09-21 | 江苏东瑞磁材科技有限公司 | Ndfeb permanent magnet material with high magnetic energy product and high coercivity and manufacturing method thereof |
| CN108417380A (en) * | 2018-05-21 | 2018-08-17 | 钢铁研究总院 | A kind of low cost diffusion source alloy and grain boundary decision magnet and preparation method thereof |
| CN109712797A (en) * | 2019-01-03 | 2019-05-03 | 浙江东阳东磁稀土有限公司 | A method of improving neodymium iron boron magnetic body grain boundary decision magnetic property consistency |
| CN110148507A (en) * | 2019-05-23 | 2019-08-20 | 钢铁研究总院 | One kind containing REFe2Grain boundary decision cerium magnet of phase and preparation method thereof |
| CN110931197A (en) * | 2019-11-22 | 2020-03-27 | 宁波同创强磁材料有限公司 | Diffusion source for high-abundance rare earth permanent magnet |
-
2020
- 2020-11-27 CN CN202011355320.0A patent/CN112530689A/en active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105957679A (en) * | 2016-07-18 | 2016-09-21 | 江苏东瑞磁材科技有限公司 | Ndfeb permanent magnet material with high magnetic energy product and high coercivity and manufacturing method thereof |
| CN108417380A (en) * | 2018-05-21 | 2018-08-17 | 钢铁研究总院 | A kind of low cost diffusion source alloy and grain boundary decision magnet and preparation method thereof |
| CN109712797A (en) * | 2019-01-03 | 2019-05-03 | 浙江东阳东磁稀土有限公司 | A method of improving neodymium iron boron magnetic body grain boundary decision magnetic property consistency |
| CN110148507A (en) * | 2019-05-23 | 2019-08-20 | 钢铁研究总院 | One kind containing REFe2Grain boundary decision cerium magnet of phase and preparation method thereof |
| CN110931197A (en) * | 2019-11-22 | 2020-03-27 | 宁波同创强磁材料有限公司 | Diffusion source for high-abundance rare earth permanent magnet |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109192495B (en) | Preparation method of regenerative sintered neodymium-iron-boron permanent magnet | |
| EP3182423B1 (en) | Neodymium iron boron magnet and preparation method thereof | |
| CN104575920B (en) | Rare-earth permanent magnet and preparation method thereof | |
| CN107958760B (en) | Rare earth permanent magnetic material and preparation method thereof | |
| CN108281246B (en) | High-performance sintered neodymium-iron-boron magnet and preparation method thereof | |
| CN104036945A (en) | Method for manufacturing high-temperature stable regenerated sintered neodymium-iron-boron magnet by waste permanent-magnet motor magnet steel | |
| CN104036947A (en) | Method for manufacturing high-coercivity regenerated sintered neodymium-iron-boron magnet by waste permanent-magnet motor magnet steel | |
| CN108922768B (en) | Method for enhancing coercive force of neodymium iron boron magnet by high-pressure heat treatment of grain boundary diffusion | |
| CN114284018A (en) | Neodymium-iron-boron magnet and preparation method and application thereof | |
| CN111383808A (en) | Preparation method of high-remanence high-coercivity neodymium iron boron magnet | |
| JP7170377B2 (en) | Method for producing Nd--Fe--B based sintered magnetic material | |
| CN110648813A (en) | R-T-B series permanent magnetic material, raw material composition, preparation method and application | |
| CN109326404B (en) | Neodymium-iron-boron magnetic material and preparation method thereof | |
| CN112530689A (en) | Method for improving grain boundary diffusion effect of high-abundance cerium magnet | |
| CN113205939B (en) | Zirconium-containing sintered neodymium-iron-boron magnet and preparation method thereof | |
| CN115472372A (en) | Neodymium iron boron magnetic material and preparation method thereof | |
| CN108777228B (en) | Method for improving magnetic property of cerium-rich magnet through mixed material hydrogenation | |
| CN112017833A (en) | Efficient utilization method of neodymium iron boron jet mill base material | |
| CN118248426B (en) | Sintered NdFeB magnet with high magnetic performance and high resistivity, and preparation method and application thereof | |
| CN1229510C (en) | Prepn of Dy-Fe alloy powder | |
| CN112466651B (en) | Preparation method of rare earth-free high-performance composite magnet | |
| CN114068169B (en) | Permanent magnet capable of saving Dy and Tb and improving coercivity of permanent magnet and preparation method of permanent magnet | |
| CN112712955B (en) | Sintered neodymium-iron-boron magnet and preparation method thereof | |
| CN108417372B (en) | Preparation method of cerium-rich magnet for driving motor | |
| CN105234402A (en) | Method for preparing cerium-containing rare earth permanent magnet material by adding nano metal powder into magnet steel scrap |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20210319 |