CN112251141A - Graphene modified nano silver-copper-zinc-aluminum composite coating and preparation method and application thereof - Google Patents
Graphene modified nano silver-copper-zinc-aluminum composite coating and preparation method and application thereof Download PDFInfo
- Publication number
- CN112251141A CN112251141A CN202011224323.0A CN202011224323A CN112251141A CN 112251141 A CN112251141 A CN 112251141A CN 202011224323 A CN202011224323 A CN 202011224323A CN 112251141 A CN112251141 A CN 112251141A
- Authority
- CN
- China
- Prior art keywords
- zinc
- copper
- graphene
- silver
- nano silver
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D183/00—Coating compositions based on macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon, with or without sulfur, nitrogen, oxygen, or carbon only; Coating compositions based on derivatives of such polymers
- C09D183/04—Polysiloxanes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/08—Anti-corrosive paints
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/08—Anti-corrosive paints
- C09D5/10—Anti-corrosive paints containing metal dust
- C09D5/103—Anti-corrosive paints containing metal dust containing Al
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/08—Anti-corrosive paints
- C09D5/10—Anti-corrosive paints containing metal dust
- C09D5/106—Anti-corrosive paints containing metal dust containing Zn
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/14—Paints containing biocides, e.g. fungicides, insecticides or pesticides
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/16—Antifouling paints; Underwater paints
- C09D5/1606—Antifouling paints; Underwater paints characterised by the anti-fouling agent
- C09D5/1612—Non-macromolecular compounds
- C09D5/1618—Non-macromolecular compounds inorganic
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/16—Antifouling paints; Underwater paints
- C09D5/1687—Use of special additives
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/02—Elements
- C08K3/08—Metals
- C08K2003/0806—Silver
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/02—Elements
- C08K3/08—Metals
- C08K2003/085—Copper
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K2201/00—Specific properties of additives
- C08K2201/011—Nanostructured additives
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/02—Polymer mixtures characterised by other features containing two or more polymers of the same C08L -group
- C08L2205/025—Polymer mixtures characterised by other features containing two or more polymers of the same C08L -group containing two or more polymers of the same hierarchy C08L, and differing only in parameters such as density, comonomer content, molecular weight, structure
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T70/00—Maritime or waterways transport
- Y02T70/10—Measures concerning design or construction of watercraft hulls
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Plant Pathology (AREA)
- Paints Or Removers (AREA)
Abstract
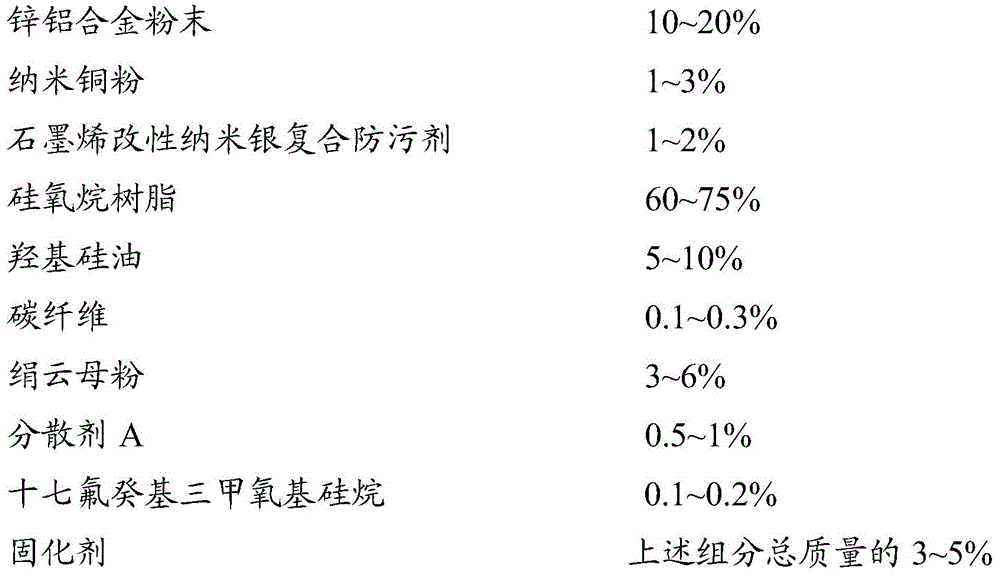
The invention provides a graphene modified nano silver-copper-zinc-aluminum composite coating, which comprises the following components in percentage by weight: 10-20% of zinc-aluminum alloy powder; 1-3% of nano copper powder; 1-2% of graphene modified nano-silver composite antifouling agent; 60-75% of siloxane resin; 5-10% of hydroxyl silicone oil; 0.1-0.3% of carbon fiber; 3-6% of sericite powder; 0.5-1% of a dispersant; 0.1-0.2% of heptadecafluorodecyltrimethoxysilane; curing agent 3-5% of the total mass of the components. The coating has excellent low surface energy effect, marine organisms are not easy to attach, and even if the marine organisms are attached, the marine organisms are easy to remove; the invention also has excellent sterilization and antibacterial properties, and effectively inhibits the attachment and the propagation of microorganisms; the barrier shielding effect of graphene is utilized, seawater corrosion resistance of the coating is effectively improved, the zinc-aluminum alloy is utilized as an internal micro-anode, copper and silver are protected from corrosion, the antifouling effect can be kept for a long time, and in addition, the coating is simple and convenient to construct, environment-friendly and safe, can be coated on various anticorrosive primer, and is good in compatibility.
Description
Technical Field
The invention relates to the technical field of graphene materials, and particularly relates to a graphene modified nano silver-copper-zinc-aluminum composite coating as well as a preparation method and application thereof.
Background
The ship is an important device for human life, and plays an important role in national defense, national economy, ocean development and the like. Since the world of canoes and wooden boats since the former time when the router was used as a boat, the era mainly using steel boats was started after the first steel boat appeared in 1879. The propulsion of ships has also been developed in the 19 th century from the reliance on manpower, animal power and wind power (i.e. punt poles, oars, sculls, lashes and sails) to the use of machine drives.
For plants that are always operated at sea, many organisms in the sea will attach to the bottom of the ship and to the underwater marine facilities, which are extremely harmful to the ship and the underwater marine facilities, and which significantly increase the hull mass (on average up to 20 kg/m)2) The sailing resistance is increased, the sailing speed of the ship is influenced, and therefore fuel consumption is increased. It is reported that for every 10 μm roughness increase of the bottom surface of the ship, the fuel loss will increase by 1%, and the fouling organisms will accelerate the ship corrosion and increase the number of repairs to the approach. The attached organisms also have a serious influence on marine facilities such as marine drilling platforms, seawater pipelines, sonar housings and the like. Among the methods for preventing marine fouling, the use of antifouling paints is the most widespread and effective means.
The history proves that most of the antifouling paints adopted in the early period of offshore equipment contain mercury, arsenic, tin, copper, manganese and other toxic compounds, and have great destructiveness to marine environment.
The research on nontoxic antifouling paint is one of the major technical problems to be solved urgently in the current marine science and technology. The research and development of the marine ecological restoration method are directly related to marine economic development and marine environmental protection, and a large amount of capital is invested for the marine ecological restoration method in many marine countries.
For example, the pollution-free marine antifouling paint, namely 'capsaicin antifouling paint' paint developed and researched by the State oceanic second-institute forest luck researchers, is found in experiments that 7 ships with different tonnages are carried out in different sea areas such as the pacific ocean and the south sea, the developed capsaicin paint has special effects on inhibiting the marine organism barnacles with the largest harm on the ship bottom and the highest occurrence frequency and is also effective on other fouling organisms, but the irritation is too strong during construction, the antifouling period effect depends on the release rate of capsaicin, the release is fast, the effective period is short, the release is slow, the antifouling effect is not good, and the total antifouling period is about 3 years.
With the improvement of the environmental protection requirement of people, the toxic antifouling paint must be eliminated, and the development of the antifouling paint which is nontoxic and can meet the requirements of various ship facilities is the final target. Since 2008 1 month and 1 happy and praised as an antifouling special weapon in the last 70 century, tin acrylate resin (TBT) is regarded as an antifouling special effect weapon and is withdrawn from the historical stage from the polishing antifouling paint, the development of an environment-friendly novel antifouling paint becomes a research and development hotspot of marine paint. Development of antifouling paints depends on advancement of antifouling resin and antifouling agent technologies, and therefore, the antifouling resin and antifouling agent technologies are key technologies for development of antifouling paints. At present, the mainstream international high-end antifouling paint mainly uses copper acrylate resin as a film forming material and cuprous oxide as a main antifouling agent, and the domestic antifouling paint has few research and development units, and although China has considerable investment in the aspect of marine antifouling paint and related research and development make certain progress, domestic related products are yet to be developed.
Disclosure of Invention
The invention provides a graphene modified nano silver-copper-zinc-aluminum composite coating, and a preparation method and application thereof, wherein low surface energy and antibacterial antifouling are combined, so that efficient antifouling of the coating is realized, and the coating has a long-term antifouling effect by utilizing the barrier protection effect of graphene and zinc-aluminum alloy.
In order to achieve the purpose, the invention provides a graphene modified nano silver-copper-zinc-aluminum composite coating, which comprises the following components in percentage by weight:
preferably, the particle size of the zinc-aluminum alloy powder is 600-800 meshes.
Preferably, the particle size of the nano copper powder is 20-50 nm.
Preferably, the siloxane resin is obtained by hydrolyzing and polymerizing alkoxy silane with different functionalities, wherein the functionality of the alkoxy silane is 2-4, and the molecular weight is 1000-10000.
Preferably, the dispersant A is one or more of carboxylate polymer and acrylic polymer.
Preferably, the curing agent is alkyl butyl titanate.
The invention also provides a preparation method of the graphene modified nano silver-copper-zinc-aluminum composite coating, which comprises the following steps:
s1: uniformly mixing siloxane resin and heptadecafluorodecyltrimethoxysilane according to a formula, sequentially adding hydroxyl silicone oil and a dispersing agent, and uniformly stirring to obtain slurry;
s2: adding the graphene modified nano-silver composite antifouling agent, the nano-copper powder, the zinc-aluminum alloy powder, the carbon fiber and the sericite powder into the slurry obtained in the step S1, and uniformly stirring to obtain a mixture;
s3: grinding the mixture obtained in the step S4, and discharging after grinding to obtain coating slurry;
s4: and (3) uniformly mixing the coating slurry and a curing agent to obtain the graphene modified nano silver-copper-zinc-aluminum composite coating.
Preferably, in S3, the grinding method is sanding, and the number of times of grinding is 2 to 3.
Preferably, the fineness of the coating slurry is 30um or less.
Preferably, the mass ratio of the coating slurry to the curing agent is 100: 5.
Preferably, the preparation method of the graphene modified nano-silver composite antifouling agent is as follows:
s11: dissolving silver nitrate in water, and adding ammonia water to form a silver-ammonia solution;
s12: mixing maltose, polyvinylpyrrolidone and a dispersant B, and adding distilled water to dissolve to obtain a transparent liquid;
s13: uniformly mixing the silver-ammonia solution obtained in the step S11 and the transparent liquid obtained in the step S12 to form a mixed solution, and carrying out ultrasonic heat preservation treatment on the mixed solution to obtain nano silver sol;
s14: adding a KH550 silane coupling agent into the nano-silver sol obtained in the step S13, performing ultrasonic dispersion uniformly, adding sepiolite powder and polyhexamethylene guanidine, performing ultrasonic dispersion uniformly, and standing and soaking to obtain a mixed solution;
s15: adding the graphene powder subjected to ultraviolet radiation treatment into distilled water for constant-temperature water bath, adding a KH560 silane coupling agent and a boric acid solution, uniformly dispersing, adding into the mixed solution obtained in S14, and dispersing and grinding to obtain graphene modified sepiolite nano silver slurry;
s16: and (4) carrying out vacuum drying on the graphene modified sepiolite nano silver slurry obtained in the step (S15), taking out the slurry after drying, and carrying out ball milling on the slurry to obtain the graphene nano silver composite antifouling agent.
Preferably, in S12, the dispersant B is one or more of a carboxylate polymer or an acrylate polymer.
Preferably, in the S13, the molar ratio of maltose to silver is 1.0-1.4: 1.
Preferably, in the step S14, before the nano silver sol is added into the sepiolite powder, the heat is preserved for 1-2 hours at 260-300 ℃.
Preferably, in the S15, the molar ratio of graphene to sepiolite powder to polyhexamethylene guanidine to silver is 0.6-0.8: 1-2: 0.01-0.1: 0.001-0.01.
The invention also provides application of the graphene modified nano silver-copper-zinc-aluminum composite coating, and the marine antifouling coating is obtained by coating the composite coating on marine equipment, drying and curing.
The scheme of the invention has the following beneficial effects:
the paint of the present invention has excellent low surface energy effect, and marine organism adhesion is realized through secreting mucus to wet the adhesion surface in the initial stage while the antifouling paint has excellent surface energy<2.5×10–4N/m, i.e. contact angle of coating with liquid>Glycoprotein and polysaccharide in seawater at 98 °The substances are not easy to be adsorbed on the surface, so that marine organisms are difficult to attach or are not firmly attached. The surface free energy of the coating is reduced through modification of the siloxane resin, the contact angle between the formed coating and water is more than 110 degrees, the coating is not easy to be attached by marine organisms, and even if certain marine organisms are attached, the coating can be easily removed by the continuous washing of seawater in the advancing process.
Most of the coating is inorganic components, so that microbial nutrients are difficult to provide, microorganisms are difficult to survive on the surface of the coating, and the adhesion amount of the surface of the coating is indirectly reduced.
The coating disclosed by the invention utilizes the bactericidal effect of the graphene modified nano-silver composite antifouling agent to inhibit the growth of microorganisms, and in addition, the coating contains nano-copper, the nano-copper can effectively prevent the attachment of fungi, algae, marine plants and lower animals, and hydroxyl silicone oil is utilized to enable the coating to slowly release trace copper ions into seawater to effectively kill the microorganisms, so that the bactericidal validity period of the coating is prolonged.
According to the invention, the barrier shielding effect of graphene is utilized, the seawater corrosion resistance of the coating is effectively improved, and meanwhile, the zinc-aluminum alloy is utilized as an internal micro-anode, so that copper and silver are effectively protected from corrosion, and the antifouling effect of silver and copper is maintained for a long time.
The coating disclosed by the invention does not contain a toxic antifouling agent, is harmless to the environment and is an environment-friendly antifouling coating.
The coating formed by the coating has high hardness, good wear resistance, high solid content, simple and convenient construction, environmental protection and safety, can be coated on various anticorrosion priming paints, and has good compatibility.
Drawings
FIG. 1 is a graph of a sample of a coating anti-mold test of the present invention;
FIG. 2 is a graph of the effect of marine coupon half a year after application of the coating of the present invention;
FIG. 3 is a graph of the effect of a marine coupon half a year without the use of the coating of the present invention.
Detailed Description
In order to make the technical problems, technical solutions and advantages of the present invention more apparent, the following detailed description is given with reference to the accompanying drawings and specific embodiments.
The embodiment of the invention provides a graphene modified nano silver-copper-zinc-aluminum composite coating which comprises the following components in percentage by weight:
the preparation method of the graphene modified nano-silver composite antifouling agent comprises the following steps:
(1) weighing 4.8g of silver nitrate, adding distilled water until 26g of silver nitrate is dissolved, and adding a proper amount of ammonia water (30%) and about 4-6 g of ammonia water (30%) to form a transparent silver-ammonia solution;
(2) weighing 12.58g of maltose and 3.4g of PVP, adding 1.35g of dispersant, adding distilled water to 100g, and dissolving into a transparent liquid to form a solution B;
(3) slowly and uniformly mixing the silver ammonia solution and the solution B to form a mixed solution;
(4) carrying out ultrasonic heat preservation treatment on the mixed solution for 2 hours to prepare nano silver sol (about 125g, and storing in a cool and dry environment);
(5) weighing 4g of KH550 silane coupling agent, adding into 100g of nano-silver sol, ultrasonically dispersing uniformly, weighing 100g of high-purity sepiolite powder, keeping the temperature at 300 ℃ for 2 hours, removing water, drying, and adding into the nano-silver sol. Adding 1.5% polyhexamethylene guanidine (the mass concentration is 25%), ultrasonically dispersing uniformly, standing and soaking for more than 24h to obtain a mixed solution;
(6) weighing 10g of graphene powder, radiating the graphene powder for 40 minutes by ultraviolet light, immediately adding 96g of distilled water, adding 4g of KH560 silane coupling agent at the temperature of constant-temperature water bath of 60 ℃, dropwise adding 1.5g of boric acid solution (the mass concentration is 3%) after uniform ultrasonic dispersion, and adding the mixture into the mixed solution obtained in the step (5) after ultrasonic treatment for 30 minutes. Continuing ultrasonic dispersion for 30 minutes, and then carrying out bead milling to obtain graphene modified sepiolite nano-silver antibacterial slurry;
(7) vacuum drying the obtained graphene modified sepiolite nano-silver antibacterial slurry at 120 ℃, taking out the dried powder, ball-milling, and grinding to obtain a powdered graphene modified nano-silver composite antifouling agent universal for water and oil;
the preparation method of the graphene modified nano silver-copper-zinc-aluminum composite coating comprises the following steps:
s1: weighing the solvent-free low-molecular alkoxysilane hydrolyzed polysiloxane resin with different functionalities and the heptadecafluorodecyltrimethoxysilane according to the formula, and uniformly mixing;
s2: adding the hydroxyl silicone oil with the formula amount, and uniformly stirring; adding a dispersant in a formula amount, and uniformly stirring;
s3: adding the graphene modified nano-silver composite antifouling agent, the nano-copper powder, the zinc-aluminum alloy powder, the carbon fiber and the sericite powder according to the formula ratio, and uniformly stirring to obtain a mixture;
s4: sanding the mixture formed in the step S4 twice to achieve the paint fineness of less than 30 micrometers, and discharging to form paint slurry;
s5: and (3) uniformly mixing 100 parts of the coating slurry and 5 parts of butyl alkyl titanate, and coating the mixture on a steel plate or the surface of other coatings to form the marine antifouling coating.
The coatings obtained in this example were tested according to HG/T3950-2007, and their antibacterial properties are shown in Table 1:
TABLE 1 antibacterial Properties of the coatings obtained in this example
As can be seen from Table 1, the antibacterial rate of the coating of the embodiment of the invention is more than 99.9%, and the coating has excellent antibacterial effect and can effectively prevent the growth and reproduction of bacteria.
The coating obtained in this example was tested for anti-fungal properties, and the test strains were: aspergillus niger AS3.4463, Aspergillus terreus AS3.3935, Paecilomyces variotii AS3.4253, Penicillium funiculosum AS3.3875, Aureobasidium pullulans AS3.3984, and Chaetomium globosum AS 3.4254; the test conditions were: the time is 28 days, the humidity is 90% RH, and the temperature is 28 ℃;
the evaluation criteria were:
level 0: no growth, i.e. no growth observed under microscope (magnification 50);
level 1: trace growth, i.e., growth visible to the naked eye, but growth coverage area is less than 10%;
and 2, stage: the growth coverage area is more than 10%.
The test result of the coating of this example is 0 grade, i.e. no growth is observed under a microscope (50 times magnification), as shown in fig. 1, the surface of the coating has no mold adhesion after 28 days of test in the culture solution, which indicates that the coating of this example has excellent mold resistance.
The coating of the present example was applied to a marine coupon, and the antifouling effect after half a year was shown in fig. 2 and 3, respectively, using a marine coupon without the coating of the present example as a comparison.
As shown in FIG. 2, the surface of the marine coupon coated with the paint of the embodiment of the invention is very clean and almost free from fouling, while the surface of the marine coupon not coated with the paint of the embodiment of the invention is completely covered by the fungus, the algae and other dirt. Therefore, the coating has excellent antifouling effect, can protect the marine equipment for a long time, and greatly reduces the maintenance cost of the marine equipment.
While the foregoing is directed to the preferred embodiment of the present invention, it will be understood by those skilled in the art that various changes and modifications may be made without departing from the spirit and scope of the invention as defined in the appended claims.
Claims (10)
2. the graphene-modified nano silver-copper-zinc-aluminum composite coating as claimed in claim 1, wherein the particle size of the zinc-aluminum alloy powder is 600-800 meshes.
3. The graphene-modified nano silver-copper-zinc-aluminum composite coating as claimed in claim 1, wherein the siloxane resin is obtained by hydrolyzing and polymerizing alkoxy silanes with different functionalities, the functionality of the alkoxy silane is 2-4, and the molecular weight is 1000-10000.
4. The graphene-modified nano silver-copper-zinc-aluminum composite coating as claimed in claim 1, wherein the dispersant A is one or more of carboxylate polymer and acrylic acid polymer; the curing agent is alkyl butyl titanate.
5. The preparation method of the graphene modified nano silver-copper-zinc-aluminum composite coating as claimed in any one of claims 1 to 4, characterized by comprising the following steps:
s1: uniformly mixing siloxane resin and heptadecafluorodecyltrimethoxysilane according to a formula, sequentially adding hydroxyl silicone oil and a dispersing agent, and uniformly stirring to obtain slurry;
s2: adding the graphene modified nano-silver composite antifouling agent, the nano-copper powder, the zinc-aluminum alloy powder, the carbon fiber and the sericite powder into the slurry obtained in the step S1, and uniformly stirring to obtain a mixture;
s3: grinding the mixture obtained in the step S4, and discharging after grinding to obtain coating slurry;
s4: and (3) uniformly mixing the coating slurry and a curing agent to obtain the graphene modified nano silver-copper-zinc-aluminum composite coating.
6. The method for preparing the graphene-modified nano silver-copper-zinc-aluminum composite coating according to claim 5, wherein in S3, the grinding mode is sand grinding, and the grinding times are 2-3.
7. The preparation method of the graphene modified nano silver-copper-zinc-aluminum composite coating according to claim 5, wherein the fineness of the coating slurry is below 30 um.
8. The preparation method of the graphene-modified nano silver-copper-zinc-aluminum composite coating according to claim 5, wherein the preparation method of the graphene-modified nano silver composite antifouling agent comprises the following steps:
s11: dissolving silver nitrate in water, and adding ammonia water to form a silver-ammonia solution;
s12: mixing maltose, polyvinylpyrrolidone and a dispersant B, and adding distilled water to dissolve to obtain a transparent liquid;
s13: uniformly mixing the silver-ammonia solution obtained in the step S11 and the transparent liquid obtained in the step S12 to form a mixed solution, and carrying out ultrasonic heat preservation treatment on the mixed solution to obtain nano silver sol;
s14: adding a KH550 silane coupling agent into the nano-silver sol obtained in the step S13, performing ultrasonic dispersion uniformly, adding sepiolite powder and polyhexamethylene guanidine, performing ultrasonic dispersion uniformly, and standing and soaking to obtain a mixed solution;
s15: adding the graphene powder subjected to ultraviolet radiation treatment into distilled water for constant-temperature water bath, adding a KH560 silane coupling agent and a boric acid solution, uniformly dispersing, adding into the mixed solution obtained in S14, and dispersing and grinding to obtain graphene modified sepiolite nano silver slurry;
s16: and (4) carrying out vacuum drying on the graphene modified sepiolite nano silver slurry obtained in the step (S15), taking out the slurry after drying, and carrying out ball milling on the slurry to obtain the graphene nano silver composite antifouling agent.
9. The preparation method of the graphene modified nano silver-copper-zinc-aluminum composite coating according to claim 8, wherein in S12, a dispersant B is one or more of carboxylate high molecular polymers or acrylate polymers; in the S13, the molar ratio of maltose to silver is 1.0-1.4: 1; preserving heat for 1-2 h at 260-300 ℃ before adding the nano silver sol into the sepiolite powder; in the S15, the molar ratio of graphene, sepiolite powder, polyhexamethylene guanidine and silver is 0.6-0.8: 1-2: 0.01-0.1: 0.001-0.01.
10. The application of the graphene modified nano silver-copper-zinc-aluminum composite coating is characterized in that the composite coating as claimed in any one of claims 1 to 4 or the composite coating prepared by the method as claimed in any one of claims 5 to 9 is coated on marine equipment, and the marine antifouling coating is obtained after drying and curing.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011224323.0A CN112251141B (en) | 2020-11-05 | 2020-11-05 | Graphene modified nano silver-copper-zinc-aluminum composite coating and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011224323.0A CN112251141B (en) | 2020-11-05 | 2020-11-05 | Graphene modified nano silver-copper-zinc-aluminum composite coating and preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN112251141A true CN112251141A (en) | 2021-01-22 |
| CN112251141B CN112251141B (en) | 2022-08-16 |
Family
ID=74268922
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202011224323.0A Active CN112251141B (en) | 2020-11-05 | 2020-11-05 | Graphene modified nano silver-copper-zinc-aluminum composite coating and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112251141B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113563714A (en) * | 2021-07-24 | 2021-10-29 | 浙江天源网业有限公司 | Nano silver carbon antibacterial anti-mite honeycomb net material and preparation method thereof |
| CN115433484A (en) * | 2022-10-11 | 2022-12-06 | 袁瑞 | Graphene-based conductive coating and preparation method thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101426958A (en) * | 2006-03-14 | 2009-05-06 | Csl硅树脂公司 | Silicone coating composition for cathodic stress prevention |
| CN106189719A (en) * | 2016-08-31 | 2016-12-07 | 湖南航天新材料技术研究院有限公司 | A kind of Graphene anticorrosive paint and preparation method thereof |
| US20180237659A1 (en) * | 2016-04-05 | 2018-08-23 | Adaptive Surface Technologies, Inc. | Curable polysiloxane compositions and slippery materials and coatings and articles made therefrom |
| CN108587262A (en) * | 2018-05-30 | 2018-09-28 | 中国科学院宁波材料技术与工程研究所 | A kind of anti-corrosion anti-fouling coating and preparation method thereof |
| CN111357765A (en) * | 2020-03-17 | 2020-07-03 | 长沙天源羲王材料科技有限公司 | Preparation method of water-oil universal nano-silver antibacterial agent for antibacterial coating |
-
2020
- 2020-11-05 CN CN202011224323.0A patent/CN112251141B/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101426958A (en) * | 2006-03-14 | 2009-05-06 | Csl硅树脂公司 | Silicone coating composition for cathodic stress prevention |
| US20180237659A1 (en) * | 2016-04-05 | 2018-08-23 | Adaptive Surface Technologies, Inc. | Curable polysiloxane compositions and slippery materials and coatings and articles made therefrom |
| CN106189719A (en) * | 2016-08-31 | 2016-12-07 | 湖南航天新材料技术研究院有限公司 | A kind of Graphene anticorrosive paint and preparation method thereof |
| CN108587262A (en) * | 2018-05-30 | 2018-09-28 | 中国科学院宁波材料技术与工程研究所 | A kind of anti-corrosion anti-fouling coating and preparation method thereof |
| CN111357765A (en) * | 2020-03-17 | 2020-07-03 | 长沙天源羲王材料科技有限公司 | Preparation method of water-oil universal nano-silver antibacterial agent for antibacterial coating |
Non-Patent Citations (1)
| Title |
|---|
| 孙阔腾等: "湿固化型石墨烯改性重防腐涂料的性能研究", 《表面技术》 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113563714A (en) * | 2021-07-24 | 2021-10-29 | 浙江天源网业有限公司 | Nano silver carbon antibacterial anti-mite honeycomb net material and preparation method thereof |
| CN113563714B (en) * | 2021-07-24 | 2023-05-26 | 浙江天源网业有限公司 | Nano silver carbon antibacterial anti-mite honeycomb net material and preparation method thereof |
| CN115433484A (en) * | 2022-10-11 | 2022-12-06 | 袁瑞 | Graphene-based conductive coating and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN112251141B (en) | 2022-08-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Jin et al. | Recent advances in emerging integrated antifouling and anticorrosion coatings | |
| Liu et al. | Research progress of environmentally friendly marine antifouling coatings | |
| CN102807796B (en) | Novel environment-friendly anti-fouling coating paint for ocean | |
| CN109536013B (en) | Preparation method of graft-modified graphene in-situ generated hydrophobic antifouling coating | |
| Liu et al. | Review on formation of biofouling in the marine environment and functionalization of new marine antifouling coatings | |
| CN112251141B (en) | Graphene modified nano silver-copper-zinc-aluminum composite coating and preparation method and application thereof | |
| CN111286273A (en) | Novel long-acting broad-spectrum antibacterial multifunctional water-based building coating and preparation method thereof | |
| CN104693970A (en) | Contact type inshore fishing boat anti-fouling paint and preparation method thereof | |
| Zeng et al. | Fabrication of zwitterionic polymer-functionalized MXene nanosheets for anti-bacterial and anti-biofouling applications | |
| Quan et al. | Antibacterial and antifouling performance of bisphenol-A/Poly (ethylene glycol) binary epoxy coatings containing bromine-benzyl-disubstituted polyaniline | |
| Zhao et al. | Antifouling performance of in situ synthesized chitosan-zinc oxide hydrogel film against alga M. aeruginosa | |
| Sun et al. | Fabrication of an anti-fouling coating based on epoxy resin with a double antibacterial effect via an in situ polymerization strategy | |
| Wang et al. | MXene/Metal–Organic framework based composite coating with photothermal self-healing performances for antifouling application | |
| CN109651907A (en) | Novel tin-free self-polishing type antifouling paint | |
| CN112694833A (en) | Composite low-surface-energy antifouling paint containing basalt fiber powder and scale powder and preparation method thereof | |
| CN116676010B (en) | Nanocellulose-nano cuprous oxide antifouling agent and preparation method and application thereof | |
| CN1616560A (en) | Environment protection anti-foulant material and anti-fouling paint | |
| CN108329783B (en) | Marine antifouling paint and preparation method thereof | |
| CN112111186B (en) | Marine organism fouling resistant coating and preparation method thereof | |
| CN112745731B (en) | Hyperbranched polymer-containing waterborne antifouling composite coating and preparation method thereof | |
| CN115058169A (en) | MXene-based anticorrosive and antifouling composite coating and preparation method and application thereof | |
| CN111217583B (en) | Graphene modified nano coating and preparation method thereof | |
| CN114702879B (en) | Anti-corrosion antifouling coating and preparation method thereof | |
| CN114292589B (en) | Preparation method of antifouling paint for low-modulus fishing net | |
| KR101014964B1 (en) | Tin-free and Cu2O-free Antifouling Paint |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |