CN110959908A - Adsorbing material for cigarette filter stick - Google Patents
Adsorbing material for cigarette filter stick Download PDFInfo
- Publication number
- CN110959908A CN110959908A CN201911126768.2A CN201911126768A CN110959908A CN 110959908 A CN110959908 A CN 110959908A CN 201911126768 A CN201911126768 A CN 201911126768A CN 110959908 A CN110959908 A CN 110959908A
- Authority
- CN
- China
- Prior art keywords
- cigarette filter
- adsorbing material
- diphenyl sulfide
- filter stick
- friedel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 235000019504 cigarettes Nutrition 0.000 title claims abstract description 39
- 239000000463 material Substances 0.000 title claims abstract description 29
- 238000001179 sorption measurement Methods 0.000 claims abstract description 31
- LTYMSROWYAPPGB-UHFFFAOYSA-N diphenyl sulfide Chemical compound C=1C=CC=CC=1SC1=CC=CC=C1 LTYMSROWYAPPGB-UHFFFAOYSA-N 0.000 claims abstract description 22
- NKDDWNXOKDWJAK-UHFFFAOYSA-N dimethoxymethane Chemical compound COCOC NKDDWNXOKDWJAK-UHFFFAOYSA-N 0.000 claims abstract description 21
- 238000005727 Friedel-Crafts reaction Methods 0.000 claims abstract description 17
- 229920000642 polymer Polymers 0.000 claims abstract description 14
- 239000002841 Lewis acid Substances 0.000 claims abstract description 12
- 150000007517 lewis acids Chemical class 0.000 claims abstract description 12
- 229930040373 Paraformaldehyde Natural products 0.000 claims abstract description 7
- 239000012442 inert solvent Substances 0.000 claims abstract description 7
- 239000000178 monomer Substances 0.000 claims abstract description 7
- 229920002866 paraformaldehyde Polymers 0.000 claims abstract description 7
- 239000003431 cross linking reagent Substances 0.000 claims abstract description 4
- 239000013315 hypercross-linked polymer Substances 0.000 claims abstract 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 21
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium chloride Substances Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 claims description 10
- 239000003463 adsorbent Substances 0.000 claims description 4
- 238000000746 purification Methods 0.000 claims description 4
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical group CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 claims description 3
- 229910021578 Iron(III) chloride Inorganic materials 0.000 claims description 3
- 238000000944 Soxhlet extraction Methods 0.000 claims description 2
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 claims description 2
- 239000002904 solvent Substances 0.000 claims description 2
- 238000001514 detection method Methods 0.000 claims 1
- 230000035484 reaction time Effects 0.000 claims 1
- 125000006267 biphenyl group Chemical group 0.000 abstract description 6
- 230000000694 effects Effects 0.000 abstract description 5
- 238000009825 accumulation Methods 0.000 abstract description 4
- 125000000101 thioether group Chemical group 0.000 abstract description 4
- 125000000524 functional group Chemical group 0.000 abstract description 3
- 238000006116 polymerization reaction Methods 0.000 abstract description 2
- -1 alkaline earth metal salts Chemical class 0.000 description 12
- 239000007789 gas Substances 0.000 description 12
- 125000003118 aryl group Chemical group 0.000 description 10
- 239000011148 porous material Substances 0.000 description 9
- 239000000779 smoke Substances 0.000 description 9
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 7
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 239000000376 reactant Substances 0.000 description 6
- 239000012043 crude product Substances 0.000 description 5
- YMWUJEATGCHHMB-UHFFFAOYSA-N dichloromethane Substances ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 241000208125 Nicotiana Species 0.000 description 4
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 239000012467 final product Substances 0.000 description 3
- 229960002715 nicotine Drugs 0.000 description 3
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 3
- 125000004430 oxygen atom Chemical group O* 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- 125000004434 sulfur atom Chemical group 0.000 description 3
- 238000005160 1H NMR spectroscopy Methods 0.000 description 2
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 238000007336 electrophilic substitution reaction Methods 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 150000004005 nitrosamines Chemical class 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 238000000197 pyrolysis Methods 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- BGJSXRVXTHVRSN-UHFFFAOYSA-N 1,3,5-trioxane Chemical group C1OCOCO1 BGJSXRVXTHVRSN-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 1
- FLAQQSHRLBFIEZ-UHFFFAOYSA-N N-Methyl-N-nitroso-4-oxo-4-(3-pyridyl)butyl amine Chemical compound O=NN(C)CCCC(=O)C1=CC=CN=C1 FLAQQSHRLBFIEZ-UHFFFAOYSA-N 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 150000001335 aliphatic alkanes Chemical group 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 239000011218 binary composite Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000005660 chlorination reaction Methods 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- MLUCVPSAIODCQM-NSCUHMNNSA-N crotonaldehyde Chemical compound C\C=C\C=O MLUCVPSAIODCQM-NSCUHMNNSA-N 0.000 description 1
- MLUCVPSAIODCQM-UHFFFAOYSA-N crotonaldehyde Natural products CC=CC=O MLUCVPSAIODCQM-UHFFFAOYSA-N 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical group C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 1
- 239000012039 electrophile Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 150000008282 halocarbons Chemical class 0.000 description 1
- LELOWRISYMNNSU-UHFFFAOYSA-N hydrogen cyanide Chemical compound N#C LELOWRISYMNNSU-UHFFFAOYSA-N 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000013067 intermediate product Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000000034 method Methods 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000000379 polymerizing effect Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012827 research and development Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical class [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 125000000542 sulfonic acid group Chemical group 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000011206 ternary composite Substances 0.000 description 1
- 238000001757 thermogravimetry curve Methods 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24D—CIGARS; CIGARETTES; TOBACCO SMOKE FILTERS; MOUTHPIECES FOR CIGARS OR CIGARETTES; MANUFACTURE OF TOBACCO SMOKE FILTERS OR MOUTHPIECES
- A24D3/00—Tobacco smoke filters, e.g. filter-tips, filtering inserts; Filters specially adapted for simulated smoking devices; Mouthpieces for cigars or cigarettes
- A24D3/06—Use of materials for tobacco smoke filters
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24D—CIGARS; CIGARETTES; TOBACCO SMOKE FILTERS; MOUTHPIECES FOR CIGARS OR CIGARETTES; MANUFACTURE OF TOBACCO SMOKE FILTERS OR MOUTHPIECES
- A24D3/00—Tobacco smoke filters, e.g. filter-tips, filtering inserts; Filters specially adapted for simulated smoking devices; Mouthpieces for cigars or cigarettes
- A24D3/06—Use of materials for tobacco smoke filters
- A24D3/08—Use of materials for tobacco smoke filters of organic materials as carrier or major constituent
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
Abstract
The application discloses an adsorbing material for cigarette filter sticks. The adsorbing material for the cigarette filter stick is a super-crosslinked microporous polymer (HCPs) obtained by carrying out Friedel-crafts reaction by taking diphenyl sulfide as a monomer and dimethoxymethane and/or paraformaldehyde as a crosslinking agent in an inert solvent under the addition of Lewis acid. According to the adsorbing material, the sufficient unit of the diphenyl sulfide contained in the adsorbing material has a ph-S-ph group, the sulfide group is used as a functional group, the p electron effect is good, the breadth of a pi conjugated system with a diphenyl ring can be greatly expanded, the acting force of pi-p electron accumulation is improved, and the adsorption rate of CO is further improved. Therefore, the HCPs obtained by polymerization with the diphenyl sulfide as the monomer have high adsorption rate on CO gas and high selectivity on CO gas adsorption.
Description
Technical Field
The invention relates to the technical field of cigarette filter sticks, in particular to an adsorption material for a cigarette filter stick.
Background
At present, the international cigarette market has a trend of accelerating the development of cigarettes with low tar and low harm, but China clearly proposes that the tar content of nationwide famous cigarettes is averagely reduced to about 12 mg/cigarette in 2005, and cigarettes higher than 15 mg/cigarette are not allowed to enter the market for sale. Meanwhile, the domestic tobacco industry further defines substances in the Hoffman list, and the national bureau indicates that the release amount of 7 harmful components such as CO, HCN, NNK, NH3, phenol and crotonaldehyde can effectively represent the biohazard of the mainstream smoke of the cigarettes. It is necessary to further reduce tar in developing Chinese style cigarettes, but it is more important to selectively reduce the release amount of the 7 harmful ingredients. Therefore, research and development of low tar and low harm become the current primary tasks of cigarette enterprises. The cigarette with the filter tip is invented in the 60 th of the 20 th century, so that the tar content of the cigarette is effectively reduced. The contribution of the cigarette filter to reducing harmful components such as cigarette tar, nicotine and the like is that the filter stick mechanically filters particulate matters in smoke, the smoke passes through the filter and enters a cavity when the cigarette is smoked, and the particulate matters in the smoke are immediately stuck and cannot return to the air flow again after being contacted with filter fiber tows. Therefore, the cigarette filter is most effective in reducing harmful components such as tar and NNK flowers.
The tobacco industry is actively developing the research of cigarette filters, especially in the aspect of enhancing the filtering and adsorbing functions of the filters. At present, aiming at the filtration and adsorption functions of the reinforced filter tip, some adsorption materials with large specific surface area and strong adsorption capacity are selected and added into a filter tip tow to form the composite filter tip with strong adsorption capacity. For example, Chinese patent CN 01122241.7 is to mix diatomite uniformly into a filter plug to prepare a cigarette, which can reduce the tar of the cigarette by 10-60% compared with the common filter cigarette. Chinese patent CN 03119218.1 mentions that granular loess plastic particles adsorbed with tobacco flavor are added into cigarette filter, because the yellow upper fine particle component is porous structure, the surface is large, the adsorption and reactivity are strong, and the harmful components such as tar and nicotine can be effectively removed. There have also been some studies to add alkaline earth metal salts or zeolites to filter rods to effectively remove cyanide from smoke and reduce tobacco specific nitrosamines. Such as the modified molecular sieve mentioned in CN 1879511.
Another composite filter tip commonly used at present isThe active carbon filter tip is characterized in that active carbon is added into cigarettes to be made into a binary composite filter tip or a ternary composite filter tip. The active carbon filter tip has good adsorptivity, can effectively remove oil, nicotine and some volatile substances in the smoke of the cigarette, but can remove tar, CO and N in the smokexOyAnd the harmful gas phase components have no effect. For example, CN1663677 mentions an activated carbon for selectively adsorbing nitrosamines.
The diatomite, loess plastic particles, active carbon and other adsorbing materials are used in cigarette filter rod to raise the filtering and adsorbing capacity of the filter tip. However, the adsorption rate of CO in harmful gas is limited by adopting the adsorption materials, selective adsorption of smoke cannot be realized, and original fragrant gas of the smoke is also adsorbed while the harmful gas is adsorbed, so that the suction quality is reduced.
Disclosure of Invention
In order to solve the problems, the application provides an adsorbing material for cigarette filter sticks, which can adsorb CO contained in harmful gas at a high level, has high selectivity for adsorbing the harmful gas, avoids the adsorption of fragrant gas, and improves the smoking quality.
The inventor unexpectedly finds that the HCPs obtained by polymerizing the diphenyl sulfide serving as the monomer have higher adsorption rate on CO gas and higher selectivity on CO gas adsorption. The reason for the adsorptivity is not only that the polymer material has micropores of a more suitable size, but also that the p-electrons of the sulfur atom of the repeating unit diphenylsulfide group contained in the polymer (the lone pair of electrons thereof is present) are more likely to undergo p-pi electron stacking with the pi-electrons of CO, which is understood to be an electrostatic force. The reason why the p-pi electron stacking can be formed is that the sufficient unit of diphenyl sulfide has a ph-S-ph group, the diphenyl ether group is a p-pi conjugated system formed by the pi orbital of the benzene ring and the p orbital of the lone pair electron of the S atom, and the electron delocalization of the p-pi conjugated system is smaller than that of the pi-pi conjugated system. The force that CO has pi electrons capable of forming with p-pi conjugated systems at such longer distances is called pi-p electron stacking, whereby stacking of molecules occurs, thereby forming a wider area of electron delocalization toward a more stable state. Based on this, the invention of the present application has been completed.
The adsorption material for the cigarette filter stick is a super-crosslinked microporous polymer (HCPs) obtained by taking diphenyl sulfide as a monomer and dimethoxymethane and/or paraformaldehyde as a crosslinking agent, adding Lewis acid and carrying out Friedel-crafts reaction in an inert solvent.
Hypercrosslinked microporous polymers
Hypercrosslinked microporous polymers (HCPs) are a class of organic microporous polymer Materials (MOPs) prepared based on the friedel-crafts reaction. The super-crosslinked polymer was found to use the concept of "crosslinking" used in the synthesis of other materials, the polymer has a large number of pore-like structures, the surface tension of the porous polymer and the supporting force of benzene rings (benzene rings derived from the characteristic units of monomers in the friedel-crafts reaction) as a structural skeleton are mutually offset, and the degree of crosslinking is large, the resulting polymer network exhibits high rigidity, prevents the tight shrinkage of the polymer chains, and thus some voids exist between the molecular chains to form pores.
It is known to the person skilled in the art that the friedel-crafts reaction based on dimethoxymethane and/or paraformaldehyde as cross-linking agent in the present application is not described in the classical textbooks (university textbook organic chemistry) as a well-known technique. Even if the reactants of Friedel-crafts reaction described in these classical texts are halogen-containing substances such as halogenated hydrocarbons or acid halides, alkoxy groups such as dimethoxymethane and/or paraformaldehyde can be easily generated by analogy with this. For convenience, dimethoxymethane is used as an example in this application because alkoxy groups such as dimethoxymethane and/or paraformaldehyde contain C-O bonds.
For the mid-friedel-crafts reaction using dimethoxymethane as a reactant, the approximate reaction process is that under the attack of lewis acid, lewis acid coordinates with oxygen atom of dimethoxymethane to form a complex, which causes dimethoxymethane to separate from the methoxide anion to obtain methoxymethylene positive ion (i.e. the product from which one methoxide anion leaves). The methoxymethylene cation is used as an electrophilic reagent to generate electrophilic substitution with the aromatic ring (the electrophilic substitution is similar to the mechanism of the benzene ring to generate chlorination and sulfonic acid substitution reaction, and the description is omitted here) to obtain the methoxymethylene substituted aromatic compound. Then, the methoxy methylene substituted aromatic is attacked by Lewis acid continuously to generate methylene carbonium ions, and the carbonium ions and other aromatic reactants have aromatic ring substitution reaction to obtain the linear methylene substituted aromatic polymer. Similarly, for two linear methylene-substituted aromatic polymers, dimethoxymethane can be electrophilically substituted with the aromatic rings of the two molecules according to the mechanism described above, thereby achieving crosslinking of the linear methylene-substituted aromatic polymer into a network structure.
It should be added that Friedel-crafts reaction using dimethoxymethane as a reactant is more likely to occur than Friedel-crafts reaction using halogen-containing substances as a reactant, for two reasons. First, dimethoxymethane has an oxygen atom with a lower electronegativity than a halogen atom of a halogen-containing compound, so that the former has a higher coordination capability than the latter, and dimethoxymethane forms a complex with a Lewis acid more easily than the halogen-containing compound; second, from the stability considerations of the intermediate production of carbenium ion after the reactants form a complex with a lewis acid, the carbenium ion carries a methoxy group whose O atom has a higher electron donating effect on the carbenium ion than the halogen atom, resulting in a higher stability of the intermediate product, which has a sufficiently long "survival" time for the aromatic ring substitution to occur as an electrophile.
It is particularly pointed out that in the application, the thioether group of the diphenyl sulfide is taken as a functional group, and the good p electron-donating effect of the thioether group can greatly expand the breadth of a pi conjugated system with a diphenyl ring, so that the acting force of pi-p electron accumulation is improved, and the adsorption rate of CO is further improved. In addition, compared with a single benzene ring, the diphenyl ring has the advantages that the pi conjugated orbit of the diphenyl ring and the p orbit are communicated through the bridge action of the p orbit of the S atom, and compared with the single benzene ring, the electron cloud density is improved to a certain extent, so that the acting force of pi-p electron accumulation is improved, and meanwhile, the Friedel-crafts reaction is promoted to occur more easily. Of course, the diphenyl ring of the present application has a smaller spatial position than the fused aromatic ring, and the biph groups are connected by a sigma bond between S, and the sigma bond has certain rotatability, so that the biph groups have certain rotatability to adjust the positions, and the substituted alkane groups are not hindered by the spatial position of the benzene ring.
The number of the above-mentioned polymers of paraformaldehyde is not limited, and examples thereof include trioxymethylene, and dimers.
The Lewis acid may be AlCl3And/or FeCl3Or other forms.
The inert solvent may be dichloroethane, carbon tetrachloride, DMSO, etc., preferably dichloroethane.
The molar ratio of the dimethoxymethane to the diphenyl sulfide is 1.2 to 3.4: 1, e.g. 1.2: 1. 1.22: 1. 1.28: 1. 1.3: 1. 1.5: 1. 2: 1. 2.3: 1. 2.6: 1. 3: 1. 3.2: 1. 3.3: 1. 3.4: 1.
in a typical embodiment, the molar ratio of the Lewis acid to the diphenyl sulfide may be 1.2 to 3.4: 1, e.g. 1.2: 1. 1.22: 1. 1.28: 1. 1.3: 1. 1.5: 1. 2: 1. 2.3: 1. 2.6: 1. 3: 1. 3.2: 1. 3.3: 1. 3.4: 1.
in a typical embodiment, the ratio of the amount of the inert solvent to the amount of the diphenyl sulfide may be 1.2 to 2.5 ml: 1mmol, e.g. 1.2 ml: 1mmol, 1.25 ml: 1mmol, 1.28 ml: 1mmol, 1.3 ml: 1mmol, 1.4 ml: 1mmol, 1.6 ml: 1mmol, 1.8 ml: 1mmol, 2 ml: 1mmol, 2.2 ml: 1mmol, 2.4 ml: 1mmol, 2.45 ml: 1mmol, 2.5 ml: 1 mmol.
In a typical embodiment, the temperature of the friedel-crafts reaction may be 68-82 ℃, such as 68 ℃, 69 ℃, 72 ℃, 75 ℃, 78 ℃, 80 ℃, 82 ℃ and the like, and the time of the friedel-crafts reaction may be 55-65 h, such as 55h, 58h, 60h, 62h, 64h, 65 h.
The purification solvent used for purifying the product after the Friedel-crafts reaction is methanol or other forms.
The purification is carried out by Soxhlet extraction for 36-60 h, such as 36h, 38h, 40h, 45h, 48h, 54h, 56h, 58h or 60 h.
According to the adsorbing material, the sufficient unit of the diphenyl sulfide contained in the adsorbing material has a ph-S-ph group, the sulfide group is used as a functional group, the p electron effect is good, the breadth of a pi conjugated system with a diphenyl ring can be greatly expanded, the acting force of pi-p electron accumulation is improved, and the adsorption rate of CO is further improved. Therefore, the HCPs obtained by polymerization with the diphenyl sulfide as the monomer have high adsorption rate on CO gas and high selectivity on CO gas adsorption.
Drawings
FIG. 1 is an aperture profile of an embodiment of the present application;
FIG. 2 is a drawing of CO absorption according to an embodiment of the present application;
FIG. 3 shows a CO/N implementation of the present application2Adsorption adsorptivity diagram;
FIG. 4 is a FTIR Spectrometry chart of an embodiment of the present application;
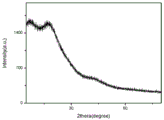
FIG. 5 is an H-NMR chart of an example of the present application;
FIG. 6 is an XRD pattern of an embodiment of the present application;
figure 7 is a TGA profile of an embodiment of the present application.
Detailed Description
The following are specific examples of the present application and further describe the technical solutions of the present application, but the present application is not limited to these examples.
Example 1
In a 100 ml single neck round bottom flask were added 20 ml of 1, 2-dichloromethane, 15 mmol of diphenyl sulfide, 30 mmol of dimethoxymethane and 30 mmol of anhydrous ferric chloride in this order. The whole mixed system was then heated to 80 ℃ and refluxed, after 72 h of reaction, the crude product was filtered 3 times with methanol, soxhlet extracted for 48h, vacuum dried at 60 ℃ for 24 h. 3.245 g of light brown product are finally obtained, yield 91%. BET specific surface area of 167.0581 m2g-1Total pore volume: 0.4589 m3g-1。
Example 2
In turn in a 100 ml single-neck round-bottom flask15 ml of 1, 2-dichloromethane, 10 mmol of diphenyl sulfide, 24 mmol of dimethoxymethane and 24 mmol of anhydrous aluminum chloride are added. The whole mixture was then heated to 68 ℃ and refluxed, after 76 h of reaction, the crude product was filtered 3 times with methanol, soxhlet extracted for 50h, vacuum dried at 55 ℃ for 24 h. The final product was obtained in light brown color with a yield of 85%. BET specific surface area of 155.1265 m2g-1Total pore volume: 0.4029 m3g-1。
Example 3
In a 100 ml single neck round bottom flask were added 15 ml of 1, 2-dichloromethane, 20 mmol of diphenyl sulfide, 24 mmol of dimethoxymethane and 24 mmol of anhydrous aluminum chloride in this order. The whole mixture was then heated to 68 ℃ and refluxed, after 76 h of reaction, the crude product was filtered 3 times with methanol, soxhlet extracted for 50h, vacuum dried at 55 ℃ for 24 h. The final product was obtained as a light brown product in 85% yield. BET specific surface area of 145.3245 m2g-1Total pore volume: 0.4334 m3g-1。
Example 4
Into a 100 ml single-neck round-bottom flask were successively charged 25ml of 1, 2-dichloromethane, 10 mmol of diphenyl sulfide, 34 mmol of dimethoxymethane and 34 mmol of anhydrous aluminum chloride. The whole mixture was then heated to 82 ℃ and refluxed, after 66 h of reaction, the crude product was filtered 3 times with methanol, soxhlet extracted for 43h, vacuum dried at 55 ℃ for 24 h. The final product was obtained in light brown color with a yield of 88%. BET specific surface area of 159.2395 m2g-1Total pore volume: 0.4019 m3g-1。
Example 5
In a 100 ml single neck round bottom flask were added 15 ml of 1, 2-dichloromethane, 10 mmol of diphenyl sulfide, 24 mmol of dimethoxymethane and 24 mmol of anhydrous aluminum chloride in this order. The whole mixture was then heated to 68 ℃ and refluxed, after 76 h of reaction, the crude product was filtered 3 times with methanol, soxhlet extracted for 50h, vacuum dried at 55 ℃ for 24 h. 2.845 g of light brown product are finally obtained, yield 85%. BET specific surface area of 145.3245 m2g-1Total pore volume: 0.3854 m3g-1。
Evaluation of
The adsorbing materials obtained in the examples 1 to 5 are subjected to pore size distribution, CO adsorption and CO/N adsorption in a conventional manner2The adsorption, FTIR Spectrometer, H-NMR, XRD, TGA are shown in 1-7.
As can be seen from figure 1, the pore size distribution of the adsorption material is at the position of 1-5 nm most widely, which also lays a foundation for the completion of molecular-level adsorption.
As can be seen from fig. 2 and 3, the pore diameter of the adsorbent of the present invention is good for CO gas adsorption rate, and is good for CO/N2 for CO selectivity.
As can be seen from FIGS. 4 and 5, the characteristic groups ph-S-ph in the adsorbent material of the present application are retained.
As can be seen from FIG. 6, the adsorption material of the present application shows obvious diffraction peaks at less than 6 degrees and 26 degrees.
As can be seen from figure 7, the heat stability of the adsorption material is good, pyrolysis basically does not occur at about 300 ℃, a large amount of pyrolysis can occur only at 600 ℃, and the adsorption material is completely applicable to the use environment during filter stick suction.
The specific embodiments described herein are merely illustrative of the spirit of the application. Various modifications or additions may be made to the described embodiments or alternatives may be employed by those skilled in the art without departing from the spirit or ambit of the present application as defined by the appended claims.
Claims (9)
1. An adsorption material for cigarette filter sticks is characterized in that diphenyl sulfide is taken as a monomer, dimethoxymethane and/or paraformaldehyde are taken as a cross-linking agent, and Friedel-crafts reaction is carried out in an inert solvent under the addition of Lewis acid to obtain super-crosslinked microporous polymers (HCPs).
2. The adsorbent material for cigarette filter sticks according to claim 1, wherein the Lewis acid is AlCl3And/or FeCl3。
3. The adsorbent material for cigarette filter sticks according to claim 1 or 2, wherein the inert solvent is dichloroethane.
4. The adsorbing material for the cigarette filter stick according to any one of claims 1 to 3, wherein the molar ratio of the dimethoxymethane to the diphenyl sulfide is 1.2 to 3.4: 1.
5. the adsorbing material for the cigarette filter stick according to any one of claims 1 to 3, wherein the molar ratio of the Lewis acid to the diphenyl sulfide is 1.2 to 3.4: 1.
6. the adsorbing material for the cigarette filter stick according to any one of claims 1 to 5, wherein the ratio of the amount of the inert solvent to the amount of the diphenyl sulfide is 1.2 to 2.5 ml: 1 mmol.
7. The adsorbing material for the cigarette filter stick according to any one of claims 1 to 6, wherein the Friedel-crafts reaction temperature is 68-82 ℃, and the Friedel-crafts reaction time is 55-65 hours.
8. The adsorbing material for the cigarette filter stick according to any one of claims 1 to 7, wherein a purification solvent used for purifying a product after the Friedel-crafts reaction is methanol.
9. The detection method according to any one of claims 8, wherein the purification is performed by Soxhlet extraction for 36-60 h.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911126768.2A CN110959908A (en) | 2019-11-18 | 2019-11-18 | Adsorbing material for cigarette filter stick |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911126768.2A CN110959908A (en) | 2019-11-18 | 2019-11-18 | Adsorbing material for cigarette filter stick |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN110959908A true CN110959908A (en) | 2020-04-07 |
Family
ID=70030955
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201911126768.2A Pending CN110959908A (en) | 2019-11-18 | 2019-11-18 | Adsorbing material for cigarette filter stick |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN110959908A (en) |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011042172A1 (en) * | 2009-10-09 | 2011-04-14 | Philip Morris Products S.A. | Coated impregnated porous filter plug |
| CN104738814A (en) * | 2015-02-11 | 2015-07-01 | 浙江中烟工业有限责任公司 | Micro-nano structure filter tip additive reducing phenol in cigarette smoke and preparation method and application thereof |
| CN104923183A (en) * | 2015-05-27 | 2015-09-23 | 河南中烟工业有限责任公司 | Adsorbent for reducing release amount of HCN (hydrogen cyanide) in mainstream smoke of cigarette |
| CN206354422U (en) * | 2016-12-30 | 2017-07-28 | 广东省金叶科技开发有限公司 | It is a kind of that there is the filter stick for selecting adsorption function |
| CN108835709A (en) * | 2018-03-21 | 2018-11-20 | 云南中烟工业有限责任公司 | Reduce the modified molecular screen additive and the preparation method and application thereof of CO burst size in flue gas |
| CN209284314U (en) * | 2018-09-06 | 2019-08-23 | 河南中烟工业有限责任公司 | A kind of binary compound filter candle reducing CO in cigarette mainstream flue gas |
-
2019
- 2019-11-18 CN CN201911126768.2A patent/CN110959908A/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011042172A1 (en) * | 2009-10-09 | 2011-04-14 | Philip Morris Products S.A. | Coated impregnated porous filter plug |
| CN104738814A (en) * | 2015-02-11 | 2015-07-01 | 浙江中烟工业有限责任公司 | Micro-nano structure filter tip additive reducing phenol in cigarette smoke and preparation method and application thereof |
| CN104923183A (en) * | 2015-05-27 | 2015-09-23 | 河南中烟工业有限责任公司 | Adsorbent for reducing release amount of HCN (hydrogen cyanide) in mainstream smoke of cigarette |
| CN206354422U (en) * | 2016-12-30 | 2017-07-28 | 广东省金叶科技开发有限公司 | It is a kind of that there is the filter stick for selecting adsorption function |
| CN108835709A (en) * | 2018-03-21 | 2018-11-20 | 云南中烟工业有限责任公司 | Reduce the modified molecular screen additive and the preparation method and application thereof of CO burst size in flue gas |
| CN209284314U (en) * | 2018-09-06 | 2019-08-23 | 河南中烟工业有限责任公司 | A kind of binary compound filter candle reducing CO in cigarette mainstream flue gas |
Non-Patent Citations (3)
| Title |
|---|
| 姚姝文: "超交联微孔聚合物的制备及性能研究", 《中国优秀硕士学位论文全文数据库 工程科技Ⅰ辑》 * |
| 张雨薇: "多孔有机聚合物骨架的合成及性能研究", 《中国博士学位论文全文数据库 工程科技Ⅰ辑》 * |
| 谭良骁等: "超交联微孔聚合物研究进展", 《化学学报》 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Gan et al. | Oxygen-rich hyper-cross-linked polymers with hierarchical porosity for aniline adsorption | |
| RU2602116C2 (en) | Porous coal and methods for production thereof | |
| FI65444C (en) | FOERFARANDE FOER FRAMSTAELLNING AV CYKLODEXTRIN / POLYVINYLALKOHOL-POLYMERER VILKA BILDAR INKLUSIONSKOMPLEX | |
| CA1332167C (en) | Carbonaceous adsorbents from pyrolyzed polysulfonated polymers | |
| Asadullah et al. | Preparation of microporous activated carbon and its modification for arsenic removal from water | |
| Tiwari et al. | Melamine-formaldehyde derived porous carbons for adsorption of CO2 capture | |
| Li et al. | Naphthalene-based microporous polyimides: adsorption behavior of CO2 and toxic organic vapors and their separation from other gases | |
| CN104193969B (en) | Preparation method, material and application of porous polymer | |
| US20210147600A1 (en) | Porous cyclodextrin polymer | |
| WO2013019865A2 (en) | Porous catalytic matrices for elimination of toxicants found in tobacco combustion products | |
| RU2480407C2 (en) | Activated carbon from microcrystalline cellulose | |
| Shou et al. | Ordered mesoporous carbon preparation by the in situ radical polymerization of acrylamide and its application for resorcinol removal | |
| EP1578457B1 (en) | Carbon nanoball for deodorization | |
| CN110959908A (en) | Adsorbing material for cigarette filter stick | |
| WO2015183330A1 (en) | Improved adsorption process for recovering condensable components from a gas stream | |
| Kang et al. | Engineered Removal of Trace NH3 by Porous Organic Polymers Modified via Sequential Post‐Sulfonation and Post‐Alkylation | |
| Todee et al. | Synthesis and post-polymerization functionalization of a tosylated hyper-crosslinked polymer for fast and efficient removal of organic pollutants in water | |
| CN105622812A (en) | Aminated high-specific surface area polystyrene resin and its preparation method and use | |
| CN112841708B (en) | Application of spherical carbon in smoke adsorption generated by combustion of tobacco products | |
| CN114302769B (en) | Activated carbon for adsorbing perfluoro and polyfluoroalkyl compounds | |
| JPH01236941A (en) | Gaseous ammonia adsorbent | |
| US20130220935A1 (en) | Removal of 1,4-dioxane from water using carbonaceous adsorbents | |
| US20240164432A1 (en) | Materials for extracting toxins from tobacco | |
| JPH0445445B2 (en) | ||
| JP4882588B2 (en) | Gas adsorbent for tobacco filter |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20200407 |
|
| RJ01 | Rejection of invention patent application after publication |