CN109851703B - Vinylidene fluoride copolymer suitable for adhesive and preparation method and application thereof - Google Patents
Vinylidene fluoride copolymer suitable for adhesive and preparation method and application thereof Download PDFInfo
- Publication number
- CN109851703B CN109851703B CN201711242240.2A CN201711242240A CN109851703B CN 109851703 B CN109851703 B CN 109851703B CN 201711242240 A CN201711242240 A CN 201711242240A CN 109851703 B CN109851703 B CN 109851703B
- Authority
- CN
- China
- Prior art keywords
- formula
- electrode
- independently
- represented
- integer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Battery Electrode And Active Subsutance (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
Abstract
The invention relates to the field of lithium batteries, in particular to a vinylidene fluoride copolymer suitable for a binder and a preparation method and application thereof. The vinylidene fluoride copolymer contains a structural unit represented by the following formula (1), a structural unit represented by the following formula (2) and a structural unit represented by the following formula (3). The novel vinylidene fluoride copolymer provided by the invention can be well dissolved in huge polar and nonpolar solvents; meanwhile, the coating has better tolerance to strong alkaline environment; in addition, the influence of the binder in the pole piece on ion migration can be reduced; therefore, when the lithium ion battery is used as a binder in a lithium battery, the obtained lithium battery has higher specific capacity and cycle performance.
Description
Technical Field
The invention relates to the field of lithium batteries, in particular to a vinylidene fluoride copolymer suitable for a binder and a preparation method and application thereof.
Background
PVDF-based binders are conventional binders in the field of lithium ion battery production and typically include pure polyvinylidene fluoride, modified polymers of polyvinylidene fluoride, or polymers formed from copolymerization of vinylidene fluoride with hexafluoropropylene. The main unit in the structure of the polymer chain in the bonding agents is a vinylidene fluoride unit. However, the existing PVDF binders have the following drawbacks:
(1) the PVDF binder has high raw material cost;
(2) PVDF binders cannot be used in strongly alkaline environments. It is susceptible to defluorination in solutions having a pH > 10 and can lead to binder failure. This phenomenon may be reflected in the formulation of ternary cathode material slurries.
(3) PVDF binders can only be dissolved in limited kinds of organic solvents such as NMP, DMF, DMSO, etc., which limits their application in specific experimental procedures and the corresponding process improvements.
Disclosure of Invention
The invention aims to provide a novel vinylidene fluoride copolymer suitable for an adhesive, a preparation method and application thereof, wherein the vinylidene fluoride copolymer can be well dissolved in a huge polar solvent and a non-polar solvent; meanwhile, the coating has better tolerance to strong alkaline environment; in addition, the influence of the adhesive in the pole piece on the ion migration can be reduced.
In order to achieve the above object, one aspect of the present invention provides a vinylidene fluoride copolymer suitable for use in a binder, the copolymer comprising a structural unit represented by the following formula (1), a structural unit represented by the following formula (2), and a structural unit represented by the following formula (3);
Wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently an integer of 1 to 5, and m being each independently an integer of 1 to 20;
Rfis-ChF2h+1H is an integer of 0 to 10; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 10;
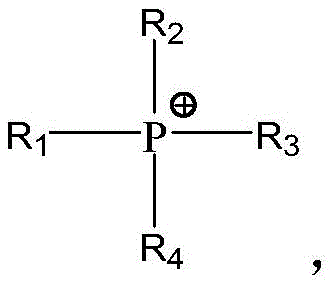
cation Y+Is H+、Li+、Na+、K+、Rb+、Cs+、Mg2+、Ca2+、Sr2+、Ba2+、Al3+A cation represented by the formula (4), a cation represented by the formula (5), a cation represented by the formula (6), a cation represented by the formula (7), a cation represented by the formula (8), and a cation represented by the formula(9) Any one of the cation shown and the cation represented by the formula (10):
formula (4)Formula (5)Formula (6)Formula (7)Formula (8)Formula (9)Formula (10)Wherein R is1、R2、R3And R4Each independently selected from C1-C10 alkyl or- (CH)2CH2O)j-CH3And j is each independently an integer of 1 to 10.
The second aspect of the present invention provides a method for producing a vinylidene fluoride-based copolymer, comprising: copolymerizing a monomer represented by the formula (1-a), a monomer represented by the formula (2-a) and a monomer represented by the formula (3-a) in an organic solvent in the presence of a radical initiator,
Wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently an integer of 1 to 5, and m being each independently an integer of 1 to 20;
Rfis-ChF2h+1H is an integer of 0 to 10; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 10;
cation Y+Is H+、Li+、Na+、K+、Rb+、Cs+、Mg2+、Ca2+、Sr2+、Ba2+、Al3+Any one of a cation represented by formula (4), a cation represented by formula (5), a cation represented by formula (6), a cation represented by formula (7), a cation represented by formula (8), a cation represented by formula (9), and a cation represented by formula (10):
Wherein R is1、R2、R3And R4Each independently selected from C1-C10 alkyl or- (CH)2CH2O)j-CH3And j is each independently an integer of 1 to 10.
The third aspect of the present invention provides a vinylidene fluoride-based copolymer produced by the method of the second aspect described above.
The fourth aspect of the present invention provides the use of the above vinylidene fluoride-based copolymer as a binder in a lithium ion battery.
A fifth aspect of the present invention provides a battery electrode, comprising: the electrode material layer contains an electrode active material and a binder, and the binder contains the vinylidene fluoride copolymer.
A sixth aspect of the present invention provides a method for producing a battery electrode according to the fifth aspect, the method comprising:
(1) providing an electrode slurry containing an electrode active material, optionally a conductive agent and a binder;
(2) and (2) coating the electrode slurry obtained in the step (1) on an electrode current collector, and drying to form an electrode material layer on the electrode current collector.
A seventh aspect of the present invention provides a lithium battery comprising: the battery comprises a positive electrode, a negative electrode, a battery diaphragm and electrolyte, wherein the positive electrode and/or the negative electrode are the battery electrodes.
The novel vinylidene fluoride copolymer provided by the invention can be well dissolved in huge polar and nonpolar solvents; meanwhile, the coating has better tolerance to strong alkaline environment; in addition, the influence of the binder in the pole piece on ion migration can be reduced; therefore, when the lithium ion battery is used as a binder in a lithium battery, the obtained lithium battery has higher specific capacity and cycle performance.
Detailed Description
The endpoints of the ranges and any values disclosed herein are not limited to the precise range or value, and such ranges or values should be understood to encompass values close to those ranges or values. For ranges of values, between the endpoints of each of the ranges and the individual points, and between the individual points may be combined with each other to give one or more new ranges of values, and these ranges of values should be considered as specifically disclosed herein.
One aspect of the present invention provides a vinylidene fluoride copolymer suitable for use in a binder, the copolymer comprising a structural unit represented by the following formula (1), a structural unit represented by the following formula (2), and a structural unit represented by the following formula (3);
Wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently an integer of 1 to 5, and m being each independently an integer of 1 to 20;
Rfis-ChF2h+1H is an integer of 0 to 10; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 10;
cation Y+Is H+、Li+、Na+、K+、Rb+、Cs+、Mg2+、Ca2+、Sr2+、Ba2+、Al3+Any one of a cation represented by formula (4), a cation represented by formula (5), a cation represented by formula (6), a cation represented by formula (7), a cation represented by formula (8), a cation represented by formula (9), and a cation represented by formula (10):
formula (4)Formula (5)Formula (6)Formula (7)Formula (II)Formula (9)Formula (10)Wherein R is1、R2、R3And R4Each independently selected from C1-C10 alkyl or- (CH)2CH2O)j-CH3And j is each independently an integer of 1 to 10.
According to the invention, preferably, each Z is independently chosen from the group consisting of single bonds, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently 1, 2 or 3, and m being each independently an integer from 1 to 10; rfis-ChF2h+1H is an integer of 0 to 5; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 5; r1、R2、R3And R4Each independently selected from C1-C5 alkyl or- (CH)2CH2O)j-CH3And j is each independently an integer of 1 to 5.
More preferably, each Z is independently selected from the group consisting of a single bond, -CH2-、-CH2-CH2-、-CF2-、-CF2-CF2-、-CH2CH2O-、-OCH2CH2-, - (CO) -O-or-O- (CO) -; rfis-F, -CF3or-CF2CF3,Rf1、Rf2And Rf3Each independently is-H, -CH3、-CH2CH3、-F、-CF3or-CF2CF3;R1、R2、R3And R4Each independently selected from methyl, ethyl, n-propyl, isopropyl, n-butyl, -CH2CH2O-CH3Or- (CH)2CH2O)2-CH3。
Wherein, Z is a single bond, which means that Z is only a connecting bond, and groups at two ends of the Z are directly connected.
According to the invention, the structural unit of formula (1) is preferably selected from one or more of the following structural units:
formula (1-1): in the formula (1), Z is a single bond, RfIs F, Rf1And Rf2Is F, Rf3Is H, Y+Is Li+;
Formula (1-2): in the formula (1), Z is a single bond, RfIs F, Rf1And Rf2Is F, Rf3Is H, Y+Is a cation represented by the formula (10), and in the formula (10), R is1Is ethyl, R2Is methyl.
It is understood that for the cation Y+Can convert the unused cation Y+The salt is ion-exchanged with the existing monomer providing the above-mentioned structural unit to obtain the additional cation Y+A structural unit represented by the formula (1).
According to the present invention, the ratio of the structural unit represented by the formula (1), the structural unit represented by the formula (2) and the structural unit represented by the formula (3) may be varied within a wide range, and it is preferable that the molar ratio of the structural unit represented by the formula (1), the structural unit represented by the formula (2) and the structural unit represented by the formula (3) is 0.001 to 999: 1: 0.001 to 9, preferably 0.01 to 100: 1: 0.005 to 5, more preferably 0.1 to 9: 1: 0.01 to 1, more preferably 0.5 to 5: 1: 0.01 to 0.5, preferably 0.5 to 2: 1: 0.01-0.1.
In order to obtain a binder having more excellent properties according to the present invention, it is preferred that the weight average molecular weight of the copolymer is 50,000-2,000,000g/mol, preferably 100,000-1,000,000g/mol, more preferably 200,000-800,000g/mol, still more preferably 250,000-500,000 g/mol. Wherein the molecular weight distribution index of the vinylidene fluoride copolymer is preferably 1.1 to 2, preferably 1.1 to 1.6.
According to the present invention, the vinylidene fluoride-based copolymer of the present invention may be a random copolymer, an alternating copolymer or a block copolymer, preferably a random copolymer, particularly a random copolymer composed of a structural unit represented by formula (1), a structural unit represented by formula (2) and a structural unit represented by formula (3).
The second aspect of the present invention provides a method for producing a vinylidene fluoride-based copolymer, comprising: copolymerizing a monomer represented by the formula (1-a), a monomer represented by the formula (2-a) and a monomer represented by the formula (3-a) in an organic solvent in the presence of a radical initiator,
Wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently an integer of 1 to 5, and m being each independently an integer of 1 to 20;
Rfis-ChF2h+1H is an integer of 0 to 10; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 10;
cation Y+Is H+、Li+、Na+、K+、Rb+、Cs+、Mg2+、Ca2+、Sr2+、Ba2+、Al3+Any one of a cation represented by formula (4), a cation represented by formula (5), a cation represented by formula (6), a cation represented by formula (7), a cation represented by formula (8), a cation represented by formula (9), and a cation represented by formula (10):
formula (4)Formula (5)Formula (6)Formula (7)Formula (8)Formula (9)Formula (10)Wherein R is1、R2、R3And R4Each independently selected from C1-C10 alkyl or- (CH)2CH2O)j-CH3And j is each independently an integer of 1 to 10.
According to the present invention, the groups referred to in the above formulae are as described above and the present invention is not described in detail herein. Among them, the monomer represented by the formula (1-a) can be appropriately selected in accordance with the structural unit represented by the above formula (1).
Preferably, the monomer represented by formula (1-a) is selected from one or more of the following monomers:
formula (1-a-1): in the formula (1-a), Z is a single bond, RfIs F, Rf1And Rf2Is F, Rf3Is H, Y+Is Li+;
Formula (1-a-2): in the formula (1-a), Z is a single bond, RfIs F, Rf1And Rf2Is F, Rf3Is H, Y+Is a cation represented by the formula (10), and in the formula (10), R is1Is ethyl, R2Is methyl.
According to the present invention, the preparation of the monomer represented by the formula (1-a) can be specifically designed according to the structure thereof, and the method in the following embodiment can be specifically referred to, and the present invention is not particularly limited thereto.
According to the present invention, the ratio of the amount of the monomer represented by the formula (1-a), the monomer represented by the formula (2-a) and the monomer represented by the formula (3-a) to be used may be determined depending on the desired ratio of the structural units and the molecular weight of the vinylidene fluoride-based copolymer, wherein the molar ratio of the amount of the monomer represented by the formula (1-a), the amount of the monomer represented by the formula (2-a) and the amount of the monomer represented by the formula (3-a) is preferably 0.001 to 999: 1: 0.001 to 9, preferably 0.01 to 100: 1: 0.005 to 5, more preferably 0.1 to 9: 1: 0.01 to 1, more preferably 0.5 to 5: 1: 0.01 to 0.5, preferably 0.5 to 2: 1: 0.01-0.1.
According to the present invention, the radical initiator may have various options, and preferably, the radical initiator is one or more of di-t-butyl peroxide, cumene peroxide, t-butyl hydroperoxide, cumene hydroperoxide, dibenzoyl peroxide, methyl ethyl ketone peroxide, cyclohexanone peroxide, t-butyl peroxybenzoate, diisopropyl peroxydicarbonate, dicyclohexyl peroxydicarbonate, azobisisobutyronitrile, azobisisoheptonitrile, potassium persulfate, sodium persulfate, and ammonium persulfate.
Wherein the amount of the radical initiator to be used is suitably selected depending on the desired vinylidene fluoride-based copolymer, for example, in order to obtain a vinylidene fluoride-based copolymer having a weight average molecular weight of 50,000-2,000,000g/mol, preferably 100,000-1,000,000g/mol, more preferably 200,000-800,000g/mol, still more preferably 250,000-500,000g/mol, preferably the amount of the radical initiator to be used is 0.001 to 5 mol%, preferably 0.1 to 5 mol%, more preferably 0.2 to 2 mol%, relative to the total molar amount of the monomer represented by the formula (1-a), the monomer represented by the formula (2-a) and the monomer represented by the formula (3-a).
According to the present invention, the organic solvent may have various choices, and preferably, the organic solvent is one or more of N-methylpyrrolidone, ethanol, methanol, acetonitrile, nitromethane, dimethylsulfoxide, N-dimethylformamide, acetone, chloroform, dichloromethane, ethyl acetate, and tetrahydrofuran, preferably one or more of N-methylpyrrolidone, dimethylsulfoxide, ethanol, methanol, N-dimethylformamide, acetone, chloroform, and tetrahydrofuran.
Wherein the amount of the organic solvent to be used may vary within a wide range, and is preferably 10 to 1000mL, preferably 40 to 500mL, relative to 100mmol of the total amount of the monomer represented by formula (1-a), the monomer represented by formula (2-a) and the monomer represented by formula (3-a).
According to the present invention, it is preferred that the copolymerization reaction conditions include: the temperature is 50-90 ℃ and the time is 5-40 h. Preferably, the copolymerization conditions include: the temperature is 60-85 deg.C, and the time is 10-30h (such as 20-25 h).
According to the present invention, the monomer represented by the formula (1-a), the radical initiator and the organic solvent may be mixed, and then the temperature is raised to the temperature of the copolymerization reaction, and then the monomer represented by the formula (2-a) and the monomer represented by the formula (3-a) may be introduced to carry out the copolymerization reaction.
According to the present invention, in order to purify a vinylidene fluoride-based copolymer, the method of the present invention may further comprise: and (3) carrying out solid-liquid separation on the product after the copolymerization reaction, dissolving the obtained solid phase in ethanol, and then recrystallizing by using diethyl ether to obtain the solid, namely the vinylidene fluoride copolymer.
The third aspect of the present invention provides a vinylidene fluoride-based copolymer produced by the method of the second aspect described above.
It is to be understood that the vinylidene fluoride-based copolymer provided by this aspect of the present invention is produced by the above-described method, and although the vinylidene fluoride-based copolymer will not be described herein too much, the structural features thereof can be referred to the above description of the vinylidene fluoride-based copolymer.
The fourth aspect of the present invention provides the use of the above vinylidene fluoride-based copolymer as a binder in a lithium ion battery.
The vinylidene fluoride copolymer obtained by the invention can be well dissolved in huge polar and nonpolar solvents; meanwhile, the coating has better tolerance to strong alkaline environment; in addition, the influence of the binder in the pole piece on ion migration can be reduced; and are therefore particularly suitable as binders in lithium ion batteries. Such binders are generally useful for preparing positive and negative electrodes.
A fifth aspect of the present invention provides a battery electrode, comprising: the electrode material layer contains an electrode active material and a binder, and the binder contains the vinylidene fluoride copolymer.
According to the present invention, the contents of the electrode active material and the binder may vary within a wide range, and preferably, the content of the binder is 1 to 20 parts by weight, preferably 2 to 15 parts by weight, with respect to 100 parts by weight of the electrode active material.
The electrode material layer may further contain a conductive agent, preferably, the content of the conductive agent is 1 to 20 parts by weight, preferably 5 to 15 parts by weight, with respect to 100 parts by weight of the electrode active material.
According to the present invention, preferably, the conductive agent is one or more of acetylene black, superconducting carbon, conductive carbon black, conductive graphite, carbon nanotubes, and carbon nanofibers.
When the electrode is a positive electrode, the electrode active material is a positive electrode active material, and the formed electrode material layer is a positive electrode material layer; when the electrode is a negative electrode, the electrode active material is a negative electrode active material, the formed electrode material layer is a negative electrode material layer, and the electrode active material is a negative electrode material layer.
Wherein the positive electrode active material is preferably LiCoO2、LiNi0.5Mn1.5O4、LiNixCoyMnzO2(NCM is abbreviated as NCM, and commonly known as NCM811, NCM622, NCM523 and NCM333), LiNixCoyAlzO2(NCA is a common example, and LiNi is a common example0.8Co0.15Al0.05O2) And LiMPO4Wherein, 0<x<1,0<y<1,0<z<1, and x + y + z is 1, M is Fe, Co, Ni or Mn.
Wherein the negative active material is preferably one or more of graphite, activated carbon, graphene, silicon and silicon-carbon composite material.
According to the present invention, preferably, the thickness of the electrode material layer is 10 to 200 μm (single-sided thickness).
According to the present invention, the electrode current collector is not particularly limited, and an electrode current collector conventional in the art, for example, a copper foil, an aluminum foil, etc., may be used, and the thickness thereof may be, for example, 1 to 100 μm.
A sixth aspect of the present invention provides a method for producing a battery electrode according to the fifth aspect, the method comprising:
(1) providing an electrode slurry containing an electrode active material, a binder, and optionally a conductive agent;
(2) and (2) coating the electrode slurry obtained in the step (1) on an electrode current collector, and drying to form an electrode material layer on the electrode current collector.
The selection and amounts of the electrode active material, optional conductive agent and binder according to the present invention are as described above and the present invention will not be described herein.
According to the present invention, the solvent used for the electrode slurry may have a very wide choice, and preferably, the solvent used for the electrode slurry is one or more of N-methylpyrrolidone, ethanol, methanol, acetonitrile, nitromethane, dimethyl sulfoxide, N-dimethylformamide, acetone, chloroform, dichloromethane, ethyl acetate, and tetrahydrofuran, and preferably one or more of N-methylpyrrolidone, dimethyl sulfoxide, ethanol, methanol, N-dimethylformamide, acetone, chloroform, and tetrahydrofuran.
The amount of the solvent used is not particularly limited, and for example, the solvent used in the electrode slurry is used in such an amount that the total concentration of the electrode active material, the optional conductive agent, and the binder is 20 to 80% by weight, preferably 30 to 60% by weight.
According to the preparation method of the battery electrode, the electrode slurry is coated on the electrode current collector in the step (2), and the electrode material layer can be formed on the current collector after drying. Wherein, the electrode current collector is as described above, and the drying temperature may be, for example, 50 to 70 ℃, so as to dry.
A seventh aspect of the present invention provides a lithium battery comprising: the battery comprises a positive electrode, a negative electrode, a battery diaphragm and electrolyte, wherein the positive electrode and/or the negative electrode are the battery electrodes.
According to the present invention, it is preferable that the positive electrode is a case where the battery electrode is a positive electrode, and the negative electrode is a case where the battery electrode is a negative electrode.
The battery separator and the electrolyte may be any one conventionally used in the art, and the present invention is not particularly limited thereto.
For example, the battery separator may be a PE or PP separator coated on both sides with PVDF-HFP, which may be commercially available.
For example, the electrolyte may be an organic solution of a lithium salt, and the concentration thereof may be, for example, 0.5 to 2mol/L, preferably 1 to 1.5 mol/L. The lithium salt may be LiClO, for example4(lithium perchlorate) and LiPF6(lithium hexafluorophosphate), LiBF4(lithium tetrafluoroborate), LiBOB (lithium dioxalate borate), LiN (SO)2CF3)2Lithium bistrifluoro (methylsulfonate) imide), LiCF3SO3(lithium trifluoromethanesulfonate) and LiN (SO)2CF2CF3)2One or more of (a). The solvent used may be, for example, one or more of Vinylene Carbonate (VC), Ethylene Carbonate (EC), dimethyl carbonate (DMC), Ethyl Methyl Carbonate (EMC), and diethyl carbonate (DEC), preferably EC: DMC: VC volume ratio is 1: 0.5-2: 0.01-0.05 of mixed solvent.
The present invention will be described in detail below by way of examples.
Preparation example 1
The monomer is prepared according to the above reaction formula, specifically:
(1) 1.9164g (10mmol) of p-chlorobenzenesulfonamide was reacted with 2.3794g (20mmol) of thionyl chloride and 1.3982g (12mmol) of chlorosulfonic acid at 100 ℃ for 12h to give compound 1a (2.6113g, yield 90%);1H NMR(400MHz,CDCl3,ppm),=7.87(d,2×1H)、7.55(d,2×1H)、2.0(s,1H)。
(2) 2.9014g (10mmol) of compound 1a were taken together with 2.1451g (12mmol) of SbF3Reaction at 60 ℃ for 12h gave compound 1b (2.4632g, 90% yield);1H NMR(400MHz,CDCl3,ppm),=7.87(d,2×1H)、7.55(d,2×1H)、2.0(s,1H)。
(3) 2.7369g (10mmol) of compound 1b and 0.7389g (10mmol) of Li were taken2CO3Reaction at 25 ℃ for 2h gave compound 1c (2.7962g, 100% yield);1H NMR(400MHz,CDCl3,ppm),=7.87(d,2×1H)、7.55(d,2×1H)、2.0(s,1H)。
(4) in the case of cooling with an ice salt bath, 2.7962g (10mmol) of Compound 1c was taken and 10mL of a solution of 1.6015g (25mmol) of n-BuLi in tetrahydrofuran was added. After the two were stirred and mixed for 2 hours, 1.4772g (15mmol) of 1, 1-difluoro-2-chloroethylene gas was slowly introduced thereinto to react for 12 hours, giving the compound represented by the formula (1-a-1) (2.7648g, yield 90%);1H NMR(400MHz,CDCl3,ppm),=7.88(d,2×1H)、7.58(d,2×1H)、5.21(m,1H)。
(5) 3.072g (10mmol) of the compound shown in the formula (1-a-1) is reacted with 1.6128g (11mmol) of 1-ethyl-3-methylimidazole chloride at 25 ℃ for 12h to obtain the compound shown in the formula (1-a-2) (3.7028g, yield 90%);1HNMR(400MHz,CDCl3,ppm),=8.94(s,1H)、7.88(d,2×1H)、7.74(s,1H)、7.67(s,1H)、7.58(d,2×1H)、5.21(m,1H)、4.38(q,2H)、4.03(s,3H)、1.56(t,3H)。
example 1
This example is intended to illustrate the vinylidene fluoride-based copolymer of the present invention and the process for producing the same.
6.144g (20mmol) of the compound represented by the formula (1-a-1), 0.1mmol of di-tert-butyl peroxide and 20mL of acetonitrile were mixed well. While a mixed gas (about 20mmol) of vinylidene fluoride and hexafluoropropylene was introduced at a molar ratio of 99:1 with heating and stirring at 70 ℃. After the gas introduction process is finished, continuing the reaction for 24 hours; filtering the obtained product, dissolving the solid with 10mL of ethanol, adding 50mL of diethyl ether for recrystallization, repeatedly recrystallizing for three times, and then drying the obtained solid in vacuum to obtain white powdery vinylidene fluoride copolymer P1;
wherein the weight average molecular weight of the vinylidene fluoride copolymer is 300,000g/mol, the molecular weight distribution index is 1.3, and the molar ratio of the structural unit represented by the formula (1-1), the structural unit represented by the formula (2) and the structural unit represented by the formula (3) is 1.01: 1: 0.01.
example 2
This example is intended to illustrate the vinylidene fluoride-based copolymer of the present invention and the process for producing the same.
8.228g (20mmol) of the compound represented by the formula (1-a-2), 0.2mmol of benzoyl peroxide and 50mL of acetonitrile were mixed well. While a mixed gas (about 30mmol) of vinylidene fluoride and hexafluoropropylene was introduced at a molar ratio of 95:5 with heating and stirring at 80 ℃. After the gas introduction process is finished, continuing the reaction for 20 hours; filtering the obtained product, dissolving the solid with 10mL of ethanol, adding 50mL of diethyl ether for recrystallization, repeatedly recrystallizing for three times, and then drying the obtained solid in vacuum to obtain white powdery vinylidene fluoride copolymer P2;
wherein the weight average molecular weight of the vinylidene fluoride copolymer is 200,000g/mol, the molecular weight distribution index is 1.2, and the molar ratio of the structural unit represented by the formula (1-2), the structural unit represented by the formula (2) and the structural unit represented by the formula (3) is 0.7: 1: 0.05.
example 3
This example is intended to illustrate the vinylidene fluoride-based copolymer of the present invention and the process for producing the same.
The process as described in example 1, except that the amount of di-t-butyl peroxide was 0.05mmol, and the reaction continued for 15 hours after the completion of the supply of the mixed gas of vinylidene fluoride and hexafluoropropylene; thereby obtaining a vinylidene fluoride copolymer P3;
wherein the weight average molecular weight of the vinylidene fluoride copolymer is 100,000g/mol, and the molecular weight distribution index is 1.4.
Example 4
This example is intended to illustrate the vinylidene fluoride-based copolymer of the present invention and the process for producing the same.
The method of embodiment 1, except that the amount of di-tert-butyl peroxide is 2mmol, and the reaction time after the introduction of the mixed gas of vinylidene fluoride and hexafluoropropylene is 35 h; thereby obtaining a vinylidene fluoride copolymer P4;
wherein the weight average molecular weight of the vinylidene fluoride copolymer is 1,000,000g/mol, and the molecular weight distribution index is 1.5.
Comparative example 1
According to the method described in example 1, except that the compound represented by the formula (1-a-1) was not used, the molar amount of the mixed gas of vinylidene fluoride and hexafluoropropylene was adjusted to 40mmol, thereby obtaining a vinylidene fluoride-hexafluoropropylene copolymer DP 1.
Wherein the weight average molecular weight of DP1 is 300,000g/mol, and the molecular weight distribution index is 1.32.
Test example 1
Solubility test the solubility of the vinylidene fluoride copolymer P1-P4 and DP1 obtained in the above examples and the PVDF-HFP type LBG of Achima in a solvent were respectively tested, specifically 0.5g of the polymer was dissolved in 10g of the corresponding solvent, and the dissolution was observed, and the results are shown in Table 1.
TABLE 1
| Solvent(s) | P1 | P2 | P3 | P4 | DP1 | LBG |
| N-methyl pyrrolidone | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Can dissolve |
| Dimethyl sulfoxide | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Can dissolve |
| Methanol | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Is insoluble | Is insoluble |
| Ethanol | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Is insoluble | Is insoluble |
| N, N-dimethylformamide | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Can dissolve |
| Acetone (II) | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Is insoluble | Is insoluble |
| Chloroform | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Is insoluble | Is insoluble |
| Tetrahydrofuran (THF) | Can dissolve | Can dissolve | Can dissolve | Can dissolve | Is insoluble | Is insoluble |
As can be seen from the data in table 1, the vinylidene fluoride copolymer obtained in the present invention can have excellent solubility in a wide variety of solvents.
Test example 2
Alkali resistance test: the vinylidene fluoride copolymer P1-P4 and DP1 obtained in the above examples and LBG type PVDF-HFP of Achima company were each taken in an amount of 0.5g and dissolved in DMF solution containing 0.5g of LiOH, and then the solution was observed for discoloration; the results are shown in Table 2.
TABLE 2
| Sample solution | P1 | P2 | P3 | P4 | DP1 | LBG |
| Whether the solution changes color or not | Whether or not | Whether or not | Whether or not | Whether or not | Is that | Is that |
As can be seen from the data in Table 2, the vinylidene fluoride copolymer obtained by the invention has excellent alkali resistance.
Positive electrode sheet preparation examples 1 to 4
This preparation example is intended to illustrate the battery electrode of the present invention and the preparation method thereof.
(1) 0.5g of the above vinylidene fluoride-based copolymer P1-P4 and 9g of LiCoO were separately added2Uniformly dispersing 0.5g of acetylene black and 10g of ethanol by using a dispersion machine to obtain positive electrode slurry;
(2) the positive electrode slurry was uniformly coated on an aluminum foil (having a thickness of 18 μm) by a coater, and then dried at 60 c, thereby obtaining a positive electrode sheet a1-a4 in which the thickness of one side of the positive electrode material layer was about 200 μm.
Comparative positive plate example 1
According to the method described in production example 1 of a positive electrode sheet, except that an equal weight of the vinylidene fluoride-hexafluoropropylene copolymer DP1 obtained in comparative example 1 was used in place of the vinylidene fluoride-based copolymer P1, and an equal weight of N-methylpyrrolidone was used in place of ethanol, the positive electrode sheet DA1 was obtained.
Comparative positive plate example 2
According to the method described in preparation example 1 of the positive electrode sheet, except that LBG type PVDF-HFP available from arkema was used in place of the vinylidene fluoride-based copolymer P1 in equal weight, and N-methylpyrrolidone was used in place of ethanol in equal weight, the positive electrode sheet DA2 was obtained.
Negative electrode sheet preparation examples 1 to 4
This preparation example is intended to illustrate the battery electrode of the present invention and the preparation method thereof.
(1) Respectively uniformly dispersing 0.5g of the vinylidene fluoride copolymer P1-P4, 9g of graphite, 0.5g of carbon nanotubes (purchased from Qingdao Hao Xin New energy science and technology Co., Ltd.) and 10g of ethanol by using a dispersing machine to obtain cathode slurry;
(2) the negative electrode slurry was uniformly coated on an aluminum foil (having a thickness of 18 μm) using a coater, and then dried at 60 c, thereby obtaining negative electrode sheets B1-B4 in which the thickness of the negative electrode material layer was about 200 μm.
Negative plate comparative example 1
According to the method described in preparation example 1 of the negative electrode sheet, except that the vinylidene fluoride-hexafluoropropylene copolymer DP1 obtained in comparative example 1 was used in place of the vinylidene fluoride-based copolymer P1 in equal weight, and N-methylpyrrolidone was used in place of ethanol in equal weight, the negative electrode sheet DB1 was obtained.
Negative plate comparative example 2
According to the method described in negative electrode sheet preparation example 1, except that an equal weight of aqueous styrene-butadiene rubber of SD332 brand available from basf was used instead of the vinylidene fluoride-based copolymer P1, and an equal weight of water was used instead of ethanol, the negative electrode sheet DB2 was obtained.
Battery production example 1
This preparation example is illustrative of a lithium battery of the invention.
A positive electrode sheet A1, a negative electrode sheet B1, a PVDF-HFP both-side coated PP separator (obtained from Celgard, PP-based film having a thickness of 12 μm, PVDF-HFP coating having a thickness of 2 μm on one side and a total thickness of 16 μm), and LiPF6Liquid electrolyte (LiPF) with concentration of 1mol/L6And preparing the/EC-DMC-VC (volume ratio of 1:1:0.02)) into a flexible package battery C1.
Battery production example 2
This preparation example is illustrative of a lithium battery of the invention.
According to the method described in battery preparation example 1, a pouch battery C2 was prepared, except that the positive electrode tab a2 was used in place of the positive electrode tab a1, and the negative electrode tab B2 was used in place of the negative electrode tab B1.
Battery production example 3
This preparation example is illustrative of a lithium battery of the invention.
According to the method described in battery preparation example 1, a pouch battery C3 was prepared, except that the positive electrode tab A3 was used in place of the positive electrode tab a1, and the negative electrode tab B3 was used in place of the negative electrode tab B1.
Battery production example 4
This preparation example is illustrative of a lithium battery of the invention.
According to the method described in battery preparation example 1, a pouch battery C4 was prepared, except that the positive electrode tab a4 was used in place of the positive electrode tab a1, and the negative electrode tab B4 was used in place of the negative electrode tab B1.
Comparative example of Battery 1
According to the method described in battery preparation example 1, a pouch battery DC1 was prepared, except that the positive electrode tab DA1 was used in place of the positive electrode tab a1, and the negative electrode tab DB1 was used in place of the negative electrode tab B1.
Comparative battery example 2
According to the method described in battery preparation example 1, a pouch battery DC2 was prepared, except that the positive electrode tab DA2 was used in place of the positive electrode tab a1, and the negative electrode tab DB2 was used in place of the negative electrode tab B1.
Test example 3
The rate performance and cycle performance of the above batteries were measured, respectively, and the results are shown in table 3.
Wherein, the multiplying power performance test process: the battery is charged at constant current of 3.0V to 4.2V at a rate of 0.1C, then charged at constant voltage of 4.2V to 0.01C, and then left for 5 minutes, and finally discharged at rates of 0.5C, 1C, 2C, 5C, 8C and 10C to 3.0V.
The cycle performance test process: the cell was first charged at 2C rate from 3.0V to 4.2V with constant current, then left to stand for 5 minutes, then charged at 4.2V with constant voltage to 0.02C cut off, finally discharged at 2C rate to 3.0V, and finally left to stand for 5 minutes. The process is circulated 500 times.
TABLE 3
It can be seen from the data in table 3 that lithium batteries with higher specific capacity and cycle performance can be obtained by using the vinylidene fluoride copolymer of the present invention as a binder.
The preferred embodiments of the present invention have been described above in detail, but the present invention is not limited thereto. Within the scope of the technical idea of the invention, many simple modifications can be made to the technical solution of the invention, including combinations of various technical features in any other suitable way, and these simple modifications and combinations should also be regarded as the disclosure of the invention, and all fall within the scope of the invention.
Claims (34)
1. A vinylidene fluoride copolymer suitable for a binder, characterized in that the copolymer contains a structural unit represented by the following formula (1), a structural unit represented by the following formula (2) and a structural unit represented by the following formula (3);
Wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently an integer of 1 to 5, and m being each independently an integer of 1 to 20;
Rfis-ChF2h+1H is an integer of 0 to 10; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 10;
cation Y+Is H+、Li+、Na+、K+、Rb+、Cs+、Mg2+、Ca2+、Sr2+、Ba2+、Al3+Any one of a cation represented by formula (4), a cation represented by formula (5), a cation represented by formula (6), a cation represented by formula (7), a cation represented by formula (8), a cation represented by formula (9), and a cation represented by formula (10):
Wherein R is1、R2、R3And R4Each independently selected from C1-C10 alkyl or- (CH)2CH2O)j-CH3Each j is independently an integer from 1 to 10;
the molar ratio of the structural unit represented by the formula (1), the structural unit represented by the formula (2) and the structural unit represented by the formula (3) is 0.5-5: 1: 0.01-0.5.
2. The copolymer of claim 1, wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently 1, 2 or 3, and m being each independently an integer from 1 to 10; rfis-ChF2h+1H is an integer of 0 to 5; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 5; r1、R2、R3And R4Each independently selected from C1-C5 alkyl or- (CH)2CH2O)j-CH3And j is each independently an integer of 1 to 5.
3. The copolymer of claim 2, wherein each Z is independently selected from the group consisting of a single bond, -CH2-、-CH2-CH2-、-CF2-、-CF2-CF2-、-CH2CH2O-、-OCH2CH2-, - (CO) -O-or-O- (CO) -; rfis-F, -CF3or-CF2CF3,Rf1、Rf2And Rf3Each independently is-H, -CH3、-CH2CH3、-F、-CF3or-CF2CF3;R1、R2、R3And R4Each independently selected from methyl, ethyl, n-propyl, isopropyl, n-butyl, -CH2CH2O-CH3Or- (CH)2CH2O)2-CH3。
4. The copolymer according to any one of claims 1 to 3, wherein the molar ratio of the structural unit represented by the formula (1), the structural unit represented by the formula (2), and the structural unit represented by the formula (3) is from 0.5 to 2: 1: 0.01-0.1.
5. The copolymer according to any one of claims 1 to 3, wherein the weight average molecular weight of the copolymer is 50,000-2,000,000 g/mol.
6. The copolymer of claim 5, wherein the copolymer has a weight average molecular weight of 100,000-1,000,000 g/mol.
7. The copolymer of claim 6 wherein the weight average molecular weight of the copolymer is 200,000-800,000 g/mol.
8. A method for producing a vinylidene fluoride-based copolymer, comprising: copolymerizing a monomer represented by the formula (1-a), a monomer represented by the formula (2-a) and a monomer represented by the formula (3-a) in an organic solvent in the presence of a radical initiator,
Wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently an integer of 1 to 5, and m being each independently an integer of 1 to 20;
Rfis-ChF2h+1H is an integer of 0 to 10; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 10;
cation Y+Is H+、Li+、Na+、K+、Rb+、Cs+、Mg2+、Ca2+、Sr2+、Ba2+、Al3+Any one of a cation represented by formula (4), a cation represented by formula (5), a cation represented by formula (6), a cation represented by formula (7), a cation represented by formula (8), a cation represented by formula (9), and a cation represented by formula (10):
Wherein R is1、R2、R3And R4Each independently selected from C1-C10 alkyl or- (CH)2CH2O)j-CH3Each j is independently an integer from 1 to 10;
the molar ratio of the amount of the monomer represented by the formula (1-a), the amount of the monomer represented by the formula (2-a), and the amount of the monomer represented by the formula (3-a) is 0.5 to 5: 1: 0.01-0.5.
9. The method of claim 8, wherein each Z is independently selected from the group consisting of a single bond, - (C)mH2m)-、-(CmF2m)-、-(CH2CH2O)m-、-(OCH2CH2)m-, - (CO) -O-or-O- (CO) -, k being each independently 1, 2 or 3, and m being each independently an integer from 1 to 10; rfis-ChF2h+1H is an integer of 0 to 5; rf1、Rf2And Rf3Each independently is-CiH2i+1or-CiF2i+1I is an integer of 0 to 5; r1、R2、R3And R4Each independently selected from C1-C5 alkyl or- (CH)2CH2O)j-CH3And j is each independently an integer of 1 to 5.
10. The method of claim 9, wherein each Z is independently selected from the group consisting of a single bond, -CH2-、-CH2-CH2-、-CF2-、-CF2-CF2-、-CH2CH2O-、-OCH2CH2-, - (CO) -O-or-O- (CO) -; rfis-F, -CF3or-CF2CF3,Rf1、Rf2And Rf3Each independently is-H, -CH3、-CH2CH3、-F、-CF3or-CF2CF3;R1、R2、R3And R4Each independently selected from methyl, ethyl, n-propyl, isopropyl, n-butyl, -CH2CH2O-CH3Or- (CH)2CH2O)2-CH3。
11. The method according to any one of claims 8 to 10, wherein the monomer represented by formula (1-a), the monomer represented by formula (2-a), and the monomer represented by formula (3-a) are used in a molar ratio of 0.5 to 2: 1: 0.01-0.1.
12. The process of any one of claims 8-10, wherein the free radical initiator is one or more of di-t-butyl peroxide, cumene peroxide, t-butyl hydroperoxide, cumene hydroperoxide, dibenzoyl peroxide, methyl ethyl ketone peroxide, cyclohexanone peroxide, t-butyl peroxybenzoate, diisopropyl peroxydicarbonate, dicyclohexyl peroxydicarbonate, azobisisobutyronitrile, azobisisoheptonitrile, potassium persulfate, sodium persulfate, and ammonium persulfate.
13. The method according to claim 12, wherein the radical initiator is used in an amount of 0.001 to 5 mol% relative to the total molar amount of the monomer represented by formula (1-a), the monomer represented by formula (2-a) and the monomer represented by formula (3-a).
14. The process according to claim 13, wherein the free radical initiator is used in an amount of 0.1 to 5 mol%.
15. The process according to claim 14, wherein the free radical initiator is used in an amount of 0.2 to 2 mol%.
16. The method according to any one of claims 8-10, wherein the organic solvent is one or more of N-methylpyrrolidone, ethanol, methanol, acetonitrile, nitromethane, dimethylsulfoxide, N-dimethylformamide, acetone, chloroform, dichloromethane, ethyl acetate, and tetrahydrofuran.
17. The method of claim 16, wherein the organic solvent is one or more of N-methylpyrrolidone, dimethylsulfoxide, ethanol, methanol, N-dimethylformamide, acetone, chloroform, and tetrahydrofuran.
18. The method according to claim 16, wherein the organic solvent is used in an amount of 10 to 1000mL per 100mmol of the total amount of the monomer represented by formula (1-a), the monomer represented by formula (2-a) and the monomer represented by formula (3-a).
19. The process of any one of claims 8-10, wherein the copolymerization reaction conditions include: the temperature is 50-90 ℃ and the time is 5-40 h.
20. A vinylidene fluoride-based copolymer produced by the method of any one of claims 8 to 19.
21. Use of a vinylidene fluoride-based copolymer according to any of claims 1 to 7 and 20 as a binder in a lithium ion battery.
22. A battery electrode, the electrode comprising: an electrode current collector and an electrode material layer attached to a surface thereof, the electrode material layer containing an electrode active material and a binder, the binder containing the vinylidene fluoride-based copolymer according to any one of claims 1 to 7 and 20.
23. The battery electrode according to claim 22, wherein the binder is contained in an amount of 1-20 parts by weight with respect to 100 parts by weight of the electrode active material.
24. The battery electrode according to claim 23, wherein the binder is contained in an amount of 2 to 15 parts by weight, relative to 100 parts by weight of the electrode active material.
25. The battery electrode according to any one of claims 22 to 24, wherein the electrode material layer further contains a conductive agent in an amount of 1 to 20 parts by weight with respect to 100 parts by weight of the electrode active material.
26. The battery electrode according to claim 25, wherein the content of the conductive agent is 5 to 15 parts by weight.
27. The battery electrode of claim 25, wherein the conductive agent is one or more of acetylene black, superconducting carbon, conductive carbon black, conductive graphite, carbon nanotubes, and carbon nanofibers.
28. The battery electrode of any of claims 22-24, wherein when the electrode is a positive electrode, the electrode active material is a positive electrode active material, and the positive electrode active material is LiCoO2、LiNi0.5Mn1.5O4、LiNixCoyMnzO2、LiNixCoyAlzO2And LiMPO4Wherein, 0<x<1,0<y<1,0<z<1, and x + y + z is 1, M is Fe, Co, Ni or Mn; when the electrode is a negative electrode, the electrode active material is a negative electrode active material,the negative active material is one or more of graphite, activated carbon, graphene, silicon and silicon-carbon composite materials.
29. The battery electrode of any of claims 22-24, wherein the electrode material layer has a thickness of 10-200 μ ι η.
30. A method of preparing a battery electrode as claimed in any one of claims 22 to 29, the method comprising:
(1) providing an electrode slurry containing an electrode active material, a binder, and optionally a conductive agent;
(2) and (2) coating the electrode slurry obtained in the step (1) on an electrode current collector, and drying to form an electrode material layer on the electrode current collector.
31. The preparation method according to claim 30, wherein the solvent used for the electrode slurry is one or more of N-methylpyrrolidone, dimethylsulfoxide, ethanol, methanol, N-dimethylformamide, acetone, chloroform and tetrahydrofuran.
32. The production method according to claim 31, wherein the solvent used in the electrode slurry is used in such an amount that the total concentration of the electrode active material, the optional conductive agent, and the binder is 20 to 80% by weight.
33. The production method according to claim 32, wherein the solvent used in the electrode slurry is used in such an amount that the total concentration of the electrode active material, the optional conductive agent, and the binder is 30 to 60% by weight.
34. A lithium battery, the battery comprising: a positive electrode, a negative electrode and an electrolyte, wherein the positive electrode and/or the negative electrode is the battery electrode of any one of claims 22 to 29.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201711242240.2A CN109851703B (en) | 2017-11-30 | 2017-11-30 | Vinylidene fluoride copolymer suitable for adhesive and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201711242240.2A CN109851703B (en) | 2017-11-30 | 2017-11-30 | Vinylidene fluoride copolymer suitable for adhesive and preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109851703A CN109851703A (en) | 2019-06-07 |
| CN109851703B true CN109851703B (en) | 2020-10-23 |
Family
ID=66888639
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201711242240.2A Active CN109851703B (en) | 2017-11-30 | 2017-11-30 | Vinylidene fluoride copolymer suitable for adhesive and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN109851703B (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112500519B (en) * | 2020-09-30 | 2022-05-17 | 氟金(上海)新材料有限公司 | Terpolymer based on polyvinylidene fluoride and preparation method thereof |
| CN112500518B (en) * | 2020-09-30 | 2022-05-13 | 氟金(上海)新材料有限公司 | Binary copolymer based on vinylidene fluoride and preparation method thereof |
| CN113249060B (en) * | 2021-05-11 | 2022-06-28 | 乌海瑞森新能源材料有限公司 | Preparation method of polyvinylidene fluoride modified by lithium ion battery binder |
| WO2023240628A1 (en) * | 2022-06-17 | 2023-12-21 | 宁德时代新能源科技股份有限公司 | Additive and preparation method therefor and use thereof, and positive electrode plate and preparation method therefor |
| CN117638072A (en) * | 2022-08-30 | 2024-03-01 | 宁德时代新能源科技股份有限公司 | Fluoropolymer, method for producing the same, use of the same, binder composition, secondary battery, battery module, battery pack, and electric device |
| CN116715798B (en) * | 2023-08-03 | 2024-02-23 | 宁德时代新能源科技股份有限公司 | Fluorine-containing polymer, preparation method, positive electrode plate, secondary battery and electricity utilization device |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009152276A2 (en) * | 2008-06-10 | 2009-12-17 | University Of North Carolina At Charlotte | Photoacid generators and lithographic resists comprising the same |
| CN103874724A (en) * | 2011-09-05 | 2014-06-18 | 埃克斯-马赛大学 | Block copolymer including a polyanion based on a tfsili anion monomer as a battery electrolyte |
| WO2017158310A1 (en) * | 2016-03-18 | 2017-09-21 | Blue Solutions | Lithium metal polymer battery having a high energy density |
| CN107305950A (en) * | 2016-04-19 | 2017-10-31 | 宁德新能源科技有限公司 | Polymeric protective film, lithium anode piece, lithium secondary battery |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0850933A1 (en) * | 1996-12-30 | 1998-07-01 | Centre National De La Recherche Scientifique (Cnrs) | Salts of pentacyclic or tetrapentaline derived anions, and their uses as ionic conductive materials |
| KR102303831B1 (en) * | 2014-12-26 | 2021-09-17 | 삼성전자주식회사 | Polymer, electrolyte comprising the polymer, and lithium secondary battery comprising the electrolyte |
-
2017
- 2017-11-30 CN CN201711242240.2A patent/CN109851703B/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009152276A2 (en) * | 2008-06-10 | 2009-12-17 | University Of North Carolina At Charlotte | Photoacid generators and lithographic resists comprising the same |
| CN103874724A (en) * | 2011-09-05 | 2014-06-18 | 埃克斯-马赛大学 | Block copolymer including a polyanion based on a tfsili anion monomer as a battery electrolyte |
| WO2017158310A1 (en) * | 2016-03-18 | 2017-09-21 | Blue Solutions | Lithium metal polymer battery having a high energy density |
| CN107305950A (en) * | 2016-04-19 | 2017-10-31 | 宁德新能源科技有限公司 | Polymeric protective film, lithium anode piece, lithium secondary battery |
Also Published As
| Publication number | Publication date |
|---|---|
| CN109851703A (en) | 2019-06-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109851703B (en) | Vinylidene fluoride copolymer suitable for adhesive and preparation method and application thereof | |
| WO2024045471A1 (en) | Polymer with core-shell structure and preparation method therefor and use thereof, positive electrode paste, secondary battery, battery module, battery pack, and electric device | |
| CN105940530A (en) | Slurry for positive electrode of lithium ion secondary cell, positive electrode obtained using said slurry, method for manufacturing said positive electrode, lithium ion secondary cell formed using said positive electrode, and method for manufacturing said cell | |
| WO2017154949A1 (en) | Binder composition for negative electrode, slurry for negative electrode, negative electrode, and lithium ion secondary battery | |
| WO2023093880A1 (en) | Lithium-ion battery | |
| CN114094165A (en) | Lithium ion battery | |
| WO2024045554A1 (en) | Binder, preparation method, positive electrode sheet, secondary battery and electric device | |
| WO2022155830A1 (en) | Binder, and electrochemical apparatus and electronic device using binder | |
| CN113273005A (en) | Secondary battery, device comprising same, method for producing secondary battery, and binder composition | |
| JP7520242B2 (en) | Binder compound and method for producing same | |
| CN115286804B (en) | BAB type block copolymer, preparation method, binder, positive pole piece, secondary battery and electric device | |
| JP2016042408A (en) | Manufacturing method of lithium secondary battery electrode binder, and lithium secondary battery electrode binder | |
| JPWO2018230599A1 (en) | Composition, binder composition for positive electrode | |
| CN109851704B (en) | Polymer diaphragm, preparation method and application thereof, and lithium battery | |
| WO2020162503A1 (en) | Composition, slurry for positive electrode, and battery | |
| WO2020162505A1 (en) | Composition, slurry for positive electrode, and battery | |
| CN109860471B (en) | Polymer diaphragm, preparation method and application thereof, and lithium battery | |
| KR102616597B1 (en) | Slurry for non-aqueous battery electrodes and method for producing non-aqueous battery electrodes and non-aqueous batteries | |
| KR102593568B1 (en) | Composition for positive electrode | |
| CN109980232A (en) | Cathode and lithium ion battery comprising it | |
| WO2022155829A1 (en) | Binder, electrochemical apparatus using said binder, and electronic device | |
| CN114142039B (en) | Adhesive and lithium ion battery comprising same | |
| JP5136946B2 (en) | Binder resin composition for non-aqueous electrolyte system energy device electrode, electrode for non-aqueous electrolyte system energy device using the same, and non-aqueous electrolyte system energy device | |
| CN114316119B (en) | Binder and battery comprising same | |
| CN117143547B (en) | Adhesive and preparation method thereof, negative electrode plate, battery and power utilization device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |