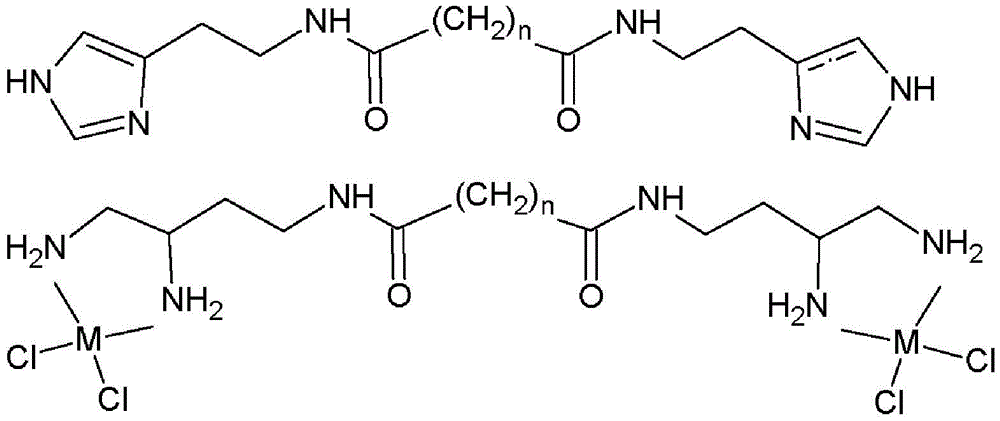
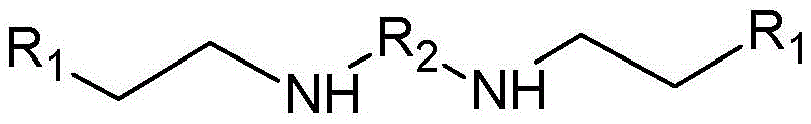
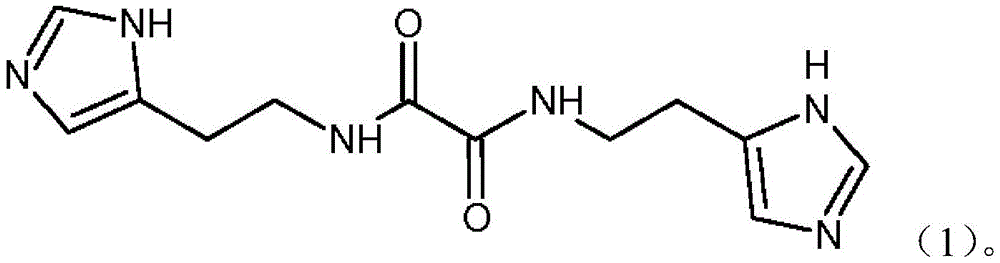
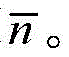CN109096198B - 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 - Google Patents
二羧酸双酰胺衍生物、其用途和基于其的药物组合物 Download PDFInfo
- Publication number
- CN109096198B CN109096198B CN201811167661.8A CN201811167661A CN109096198B CN 109096198 B CN109096198 B CN 109096198B CN 201811167661 A CN201811167661 A CN 201811167661A CN 109096198 B CN109096198 B CN 109096198B
- Authority
- CN
- China
- Prior art keywords
- disease
- general formula
- following general
- compound
- agent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- -1 Dicarboxylic acid bisamide derivatives Chemical class 0.000 title claims abstract description 36
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract description 25
- 150000001875 compounds Chemical class 0.000 claims abstract description 95
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 56
- 201000010099 disease Diseases 0.000 claims abstract description 51
- 239000003814 drug Substances 0.000 claims abstract description 28
- 238000011282 treatment Methods 0.000 claims abstract description 26
- 150000003839 salts Chemical class 0.000 claims abstract description 23
- 229910021645 metal ion Inorganic materials 0.000 claims abstract description 21
- 230000003612 virological effect Effects 0.000 claims abstract description 16
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 14
- 208000015122 neurodegenerative disease Diseases 0.000 claims abstract description 11
- 231100000757 Microbial toxin Toxicity 0.000 claims abstract description 10
- 208000007848 Alcoholism Diseases 0.000 claims abstract description 9
- 201000007930 alcohol dependence Diseases 0.000 claims abstract description 9
- 206010009208 Cirrhosis alcoholic Diseases 0.000 claims abstract description 8
- 208000010002 alcoholic liver cirrhosis Diseases 0.000 claims abstract description 8
- 208000007502 anemia Diseases 0.000 claims abstract description 8
- 206010012601 diabetes mellitus Diseases 0.000 claims abstract description 8
- 208000027866 inflammatory disease Diseases 0.000 claims abstract description 8
- 229910052723 transition metal Inorganic materials 0.000 claims abstract description 8
- 241000097929 Porphyria Species 0.000 claims abstract description 7
- 208000010642 Porphyrias Diseases 0.000 claims abstract description 7
- 231100000572 poisoning Toxicity 0.000 claims abstract description 7
- 230000000607 poisoning effect Effects 0.000 claims abstract description 7
- 230000003111 delayed effect Effects 0.000 claims abstract description 6
- 230000002526 effect on cardiovascular system Effects 0.000 claims abstract description 5
- 238000000034 method Methods 0.000 claims description 39
- 239000003795 chemical substances by application Substances 0.000 claims description 29
- 229910052725 zinc Inorganic materials 0.000 claims description 20
- 229910052751 metal Inorganic materials 0.000 claims description 18
- 239000002184 metal Substances 0.000 claims description 18
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 13
- 150000001412 amines Chemical class 0.000 claims description 12
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 12
- 230000001419 dependent effect Effects 0.000 claims description 12
- 230000002265 prevention Effects 0.000 claims description 12
- 201000011510 cancer Diseases 0.000 claims description 11
- 230000003647 oxidation Effects 0.000 claims description 10
- 238000007254 oxidation reaction Methods 0.000 claims description 10
- 238000002360 preparation method Methods 0.000 claims description 10
- 230000008569 process Effects 0.000 claims description 10
- 150000003254 radicals Chemical class 0.000 claims description 10
- 241000711549 Hepacivirus C Species 0.000 claims description 9
- 238000004519 manufacturing process Methods 0.000 claims description 9
- 208000024172 Cardiovascular disease Diseases 0.000 claims description 8
- 229910052791 calcium Inorganic materials 0.000 claims description 8
- 229910052742 iron Inorganic materials 0.000 claims description 8
- 230000004770 neurodegeneration Effects 0.000 claims description 8
- 241000701806 Human papillomavirus Species 0.000 claims description 7
- 241000700605 Viruses Species 0.000 claims description 7
- 208000024827 Alzheimer disease Diseases 0.000 claims description 6
- 208000005374 Poisoning Diseases 0.000 claims description 6
- 235000012000 cholesterol Nutrition 0.000 claims description 6
- 201000008482 osteoarthritis Diseases 0.000 claims description 6
- 201000001320 Atherosclerosis Diseases 0.000 claims description 5
- 208000002177 Cataract Diseases 0.000 claims description 5
- 201000000628 Gas Gangrene Diseases 0.000 claims description 5
- 208000002972 Hepatolenticular Degeneration Diseases 0.000 claims description 5
- 208000023105 Huntington disease Diseases 0.000 claims description 5
- 208000018737 Parkinson disease Diseases 0.000 claims description 5
- 208000012641 Pigmentation disease Diseases 0.000 claims description 5
- 208000024777 Prion disease Diseases 0.000 claims description 5
- 208000017442 Retinal disease Diseases 0.000 claims description 5
- 208000018839 Wilson disease Diseases 0.000 claims description 5
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 claims description 5
- 239000000824 cytostatic agent Substances 0.000 claims description 5
- 230000001085 cytostatic effect Effects 0.000 claims description 5
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 5
- 208000003508 Botulism Diseases 0.000 claims description 4
- 206010048610 Cardiotoxicity Diseases 0.000 claims description 4
- 206010020772 Hypertension Diseases 0.000 claims description 4
- 241000580858 Simian-Human immunodeficiency virus Species 0.000 claims description 4
- 231100000259 cardiotoxicity Toxicity 0.000 claims description 4
- 229910052802 copper Inorganic materials 0.000 claims description 4
- 241001493065 dsRNA viruses Species 0.000 claims description 4
- 208000032839 leukemia Diseases 0.000 claims description 4
- 229910052749 magnesium Inorganic materials 0.000 claims description 4
- 231100000590 oncogenic Toxicity 0.000 claims description 4
- 230000002246 oncogenic effect Effects 0.000 claims description 4
- 206010022979 Iron excess Diseases 0.000 claims description 3
- 238000010438 heat treatment Methods 0.000 claims description 3
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 3
- 150000002500 ions Chemical class 0.000 claims description 3
- 230000003078 antioxidant effect Effects 0.000 claims 3
- 230000008021 deposition Effects 0.000 claims 3
- 125000002883 imidazolyl group Chemical group 0.000 claims 1
- 230000000626 neurodegenerative effect Effects 0.000 abstract description 3
- 238000011321 prophylaxis Methods 0.000 abstract description 3
- 230000000536 complexating effect Effects 0.000 abstract description 2
- 239000000243 solution Substances 0.000 description 40
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 38
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 25
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 20
- 239000011701 zinc Substances 0.000 description 20
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 18
- 150000001408 amides Chemical class 0.000 description 18
- 239000002738 chelating agent Substances 0.000 description 17
- 238000006243 chemical reaction Methods 0.000 description 16
- 230000000694 effects Effects 0.000 description 16
- 229910052757 nitrogen Inorganic materials 0.000 description 16
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 15
- 239000000047 product Substances 0.000 description 14
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 13
- 229960001340 histamine Drugs 0.000 description 13
- 239000003446 ligand Substances 0.000 description 13
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 12
- 239000011541 reaction mixture Substances 0.000 description 12
- QQVIHTHCMHWDBS-UHFFFAOYSA-N isophthalic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=C1 QQVIHTHCMHWDBS-UHFFFAOYSA-N 0.000 description 11
- 239000002674 ointment Substances 0.000 description 11
- 238000004448 titration Methods 0.000 description 11
- 239000000203 mixture Substances 0.000 description 10
- 238000005160 1H NMR spectroscopy Methods 0.000 description 9
- 238000001962 electrophoresis Methods 0.000 description 9
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 8
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- 125000000217 alkyl group Chemical group 0.000 description 8
- 239000011575 calcium Substances 0.000 description 8
- 230000000875 corresponding effect Effects 0.000 description 8
- 239000002904 solvent Substances 0.000 description 8
- 238000004809 thin layer chromatography Methods 0.000 description 8
- 238000004566 IR spectroscopy Methods 0.000 description 7
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 239000007864 aqueous solution Substances 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 238000009835 boiling Methods 0.000 description 7
- 230000009920 chelation Effects 0.000 description 7
- 238000010494 dissociation reaction Methods 0.000 description 7
- 230000005593 dissociations Effects 0.000 description 7
- 125000000623 heterocyclic group Chemical group 0.000 description 7
- 239000003112 inhibitor Substances 0.000 description 7
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 6
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 6
- 239000002552 dosage form Substances 0.000 description 6
- 125000005842 heteroatom Chemical group 0.000 description 6
- 235000019271 petrolatum Nutrition 0.000 description 6
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 6
- RTBFRGCFXZNCOE-UHFFFAOYSA-N 1-methylsulfonylpiperidin-4-one Chemical compound CS(=O)(=O)N1CCC(=O)CC1 RTBFRGCFXZNCOE-UHFFFAOYSA-N 0.000 description 5
- JFCQEDHGNNZCLN-UHFFFAOYSA-N anhydrous glutaric acid Natural products OC(=O)CCCC(O)=O JFCQEDHGNNZCLN-UHFFFAOYSA-N 0.000 description 5
- 210000004027 cell Anatomy 0.000 description 5
- 238000011161 development Methods 0.000 description 5
- 230000018109 developmental process Effects 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 239000003883 ointment base Substances 0.000 description 5
- 239000003960 organic solvent Substances 0.000 description 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- NWZSZGALRFJKBT-KNIFDHDWSA-N (2s)-2,6-diaminohexanoic acid;(2s)-2-hydroxybutanedioic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O.NCCCC[C@H](N)C(O)=O NWZSZGALRFJKBT-KNIFDHDWSA-N 0.000 description 4
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 4
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 4
- 240000007594 Oryza sativa Species 0.000 description 4
- 235000007164 Oryza sativa Nutrition 0.000 description 4
- 239000004264 Petrolatum Substances 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 4
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 4
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical compound OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 4
- 239000013522 chelant Substances 0.000 description 4
- 238000002655 chelation therapy Methods 0.000 description 4
- 230000009918 complex formation Effects 0.000 description 4
- 239000010949 copper Substances 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- IKDUDTNKRLTJSI-UHFFFAOYSA-N hydrazine monohydrate Substances O.NN IKDUDTNKRLTJSI-UHFFFAOYSA-N 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- PHTQWCKDNZKARW-UHFFFAOYSA-N isoamylol Chemical compound CC(C)CCO PHTQWCKDNZKARW-UHFFFAOYSA-N 0.000 description 4
- 230000014759 maintenance of location Effects 0.000 description 4
- 239000011159 matrix material Substances 0.000 description 4
- 238000002844 melting Methods 0.000 description 4
- 230000008018 melting Effects 0.000 description 4
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 4
- 230000007170 pathology Effects 0.000 description 4
- RCCYSVYHULFYHE-UHFFFAOYSA-N pentanediamide Chemical compound NC(=O)CCCC(N)=O RCCYSVYHULFYHE-UHFFFAOYSA-N 0.000 description 4
- 229940066842 petrolatum Drugs 0.000 description 4
- 235000009566 rice Nutrition 0.000 description 4
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 4
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- IJJWOSAXNHWBPR-HUBLWGQQSA-N 5-[(3as,4s,6ar)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]-n-(6-hydrazinyl-6-oxohexyl)pentanamide Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)NCCCCCC(=O)NN)SC[C@@H]21 IJJWOSAXNHWBPR-HUBLWGQQSA-N 0.000 description 3
- 102000005369 Aldehyde Dehydrogenase Human genes 0.000 description 3
- 108020002663 Aldehyde Dehydrogenase Proteins 0.000 description 3
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N DMSO-d6 Substances [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 3
- UBQYURCVBFRUQT-UHFFFAOYSA-N N-benzoyl-Ferrioxamine B Chemical compound CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN UBQYURCVBFRUQT-UHFFFAOYSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 108010067390 Viral Proteins Proteins 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- ORILYTVJVMAKLC-UHFFFAOYSA-N adamantane Chemical compound C1C(C2)CC3CC1CC2C3 ORILYTVJVMAKLC-UHFFFAOYSA-N 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 235000011037 adipic acid Nutrition 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 238000009833 condensation Methods 0.000 description 3
- 230000005494 condensation Effects 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 239000013078 crystal Substances 0.000 description 3
- 229960000958 deferoxamine Drugs 0.000 description 3
- XTDYIOOONNVFMA-UHFFFAOYSA-N dimethyl pentanedioate Chemical compound COC(=O)CCCC(=O)OC XTDYIOOONNVFMA-UHFFFAOYSA-N 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 230000008020 evaporation Effects 0.000 description 3
- 208000006454 hepatitis Diseases 0.000 description 3
- 231100000283 hepatitis Toxicity 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000011777 magnesium Substances 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 3
- 238000003918 potentiometric titration Methods 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 239000001384 succinic acid Substances 0.000 description 3
- 241001529453 unidentified herpesvirus Species 0.000 description 3
- BMKDZUISNHGIBY-ZETCQYMHSA-N (+)-dexrazoxane Chemical compound C([C@H](C)N1CC(=O)NC(=O)C1)N1CC(=O)NC(=O)C1 BMKDZUISNHGIBY-ZETCQYMHSA-N 0.000 description 2
- WXTMDXOMEHJXQO-UHFFFAOYSA-N 2,5-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC(O)=CC=C1O WXTMDXOMEHJXQO-UHFFFAOYSA-N 0.000 description 2
- VUEOQWDVOXEFDJ-UHFFFAOYSA-N 2-pyridin-2-ylacetic acid;hydrate Chemical compound O.OC(=O)CC1=CC=CC=N1 VUEOQWDVOXEFDJ-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 2
- 108030001720 Bontoxilysin Proteins 0.000 description 2
- 108010087806 Carnosine Proteins 0.000 description 2
- 206010008342 Cervix carcinoma Diseases 0.000 description 2
- QCDFBFJGMNKBDO-UHFFFAOYSA-N Clioquinol Chemical compound C1=CN=C2C(O)=C(I)C=C(Cl)C2=C1 QCDFBFJGMNKBDO-UHFFFAOYSA-N 0.000 description 2
- JPVYNHNXODAKFH-UHFFFAOYSA-N Cu2+ Chemical compound [Cu+2] JPVYNHNXODAKFH-UHFFFAOYSA-N 0.000 description 2
- 241000701022 Cytomegalovirus Species 0.000 description 2
- UDSFAEKRVUSQDD-UHFFFAOYSA-N Dimethyl adipate Chemical compound COC(=O)CCCCC(=O)OC UDSFAEKRVUSQDD-UHFFFAOYSA-N 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- IMQLKJBTEOYOSI-UHFFFAOYSA-N Diphosphoinositol tetrakisphosphate Chemical compound OP(O)(=O)OC1C(OP(O)(O)=O)C(OP(O)(O)=O)C(OP(O)(O)=O)C(OP(O)(O)=O)C1OP(O)(O)=O IMQLKJBTEOYOSI-UHFFFAOYSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 2
- 239000004166 Lanolin Substances 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- JLVVSXFLKOJNIY-UHFFFAOYSA-N Magnesium ion Chemical compound [Mg+2] JLVVSXFLKOJNIY-UHFFFAOYSA-N 0.000 description 2
- 108060004795 Methyltransferase Proteins 0.000 description 2
- CQOVPNPJLQNMDC-UHFFFAOYSA-N N-beta-alanyl-L-histidine Natural products NCCC(=O)NC(C(O)=O)CC1=CN=CN1 CQOVPNPJLQNMDC-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 201000010273 Porphyria Cutanea Tarda Diseases 0.000 description 2
- 206010036186 Porphyria non-acute Diseases 0.000 description 2
- RADKZDMFGJYCBB-UHFFFAOYSA-N Pyridoxal Chemical compound CC1=NC=C(CO)C(C=O)=C1O RADKZDMFGJYCBB-UHFFFAOYSA-N 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 2
- XMYKNCNAZKMVQN-NYYWCZLTSA-N [(e)-(3-aminopyridin-2-yl)methylideneamino]thiourea Chemical compound NC(=S)N\N=C\C1=NC=CC=C1N XMYKNCNAZKMVQN-NYYWCZLTSA-N 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- 239000001361 adipic acid Substances 0.000 description 2
- IBVAQQYNSHJXBV-UHFFFAOYSA-N adipic acid dihydrazide Chemical compound NNC(=O)CCCCC(=O)NN IBVAQQYNSHJXBV-UHFFFAOYSA-N 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 125000003368 amide group Chemical group 0.000 description 2
- 230000033115 angiogenesis Effects 0.000 description 2
- 230000000840 anti-viral effect Effects 0.000 description 2
- 150000001540 azides Chemical class 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- GGZKZGHRTRAMOT-UHFFFAOYSA-N but-1-ene-1,2,4-triamine Chemical compound NC=C(CCN)N GGZKZGHRTRAMOT-UHFFFAOYSA-N 0.000 description 2
- SNCZNSNPXMPCGN-UHFFFAOYSA-N butanediamide Chemical compound NC(=O)CCC(N)=O SNCZNSNPXMPCGN-UHFFFAOYSA-N 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- CQOVPNPJLQNMDC-ZETCQYMHSA-N carnosine Chemical compound [NH3+]CCC(=O)N[C@H](C([O-])=O)CC1=CNC=N1 CQOVPNPJLQNMDC-ZETCQYMHSA-N 0.000 description 2
- 201000010881 cervical cancer Diseases 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 229960005228 clioquinol Drugs 0.000 description 2
- 229940126214 compound 3 Drugs 0.000 description 2
- 150000002019 disulfides Chemical class 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 238000007876 drug discovery Methods 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 229960002885 histidine Drugs 0.000 description 2
- 150000003949 imides Chemical class 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000011835 investigation Methods 0.000 description 2
- 150000002505 iron Chemical class 0.000 description 2
- 229940039717 lanolin Drugs 0.000 description 2
- 235000019388 lanolin Nutrition 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- 229910001425 magnesium ion Inorganic materials 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- FTQWRYSLUYAIRQ-UHFFFAOYSA-N n-[(octadecanoylamino)methyl]octadecanamide Chemical class CCCCCCCCCCCCCCCCCC(=O)NCNC(=O)CCCCCCCCCCCCCCCCC FTQWRYSLUYAIRQ-UHFFFAOYSA-N 0.000 description 2
- 150000003833 nucleoside derivatives Chemical class 0.000 description 2
- 230000036542 oxidative stress Effects 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- MUJIDPITZJWBSW-UHFFFAOYSA-N palladium(2+) Chemical compound [Pd+2] MUJIDPITZJWBSW-UHFFFAOYSA-N 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- LGYJSPMYALQHBL-UHFFFAOYSA-N pentanedihydrazide Chemical compound NNC(=O)CCCC(=O)NN LGYJSPMYALQHBL-UHFFFAOYSA-N 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- FAIAAWCVCHQXDN-UHFFFAOYSA-N phosphorus trichloride Chemical compound ClP(Cl)Cl FAIAAWCVCHQXDN-UHFFFAOYSA-N 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- KNCYXPMJDCCGSJ-UHFFFAOYSA-N piperidine-2,6-dione Chemical class O=C1CCCC(=O)N1 KNCYXPMJDCCGSJ-UHFFFAOYSA-N 0.000 description 2
- HRGDZIGMBDGFTC-UHFFFAOYSA-N platinum(2+) Chemical compound [Pt+2] HRGDZIGMBDGFTC-UHFFFAOYSA-N 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- FGIUAXJPYTZDNR-UHFFFAOYSA-N potassium nitrate Inorganic materials [K+].[O-][N+]([O-])=O FGIUAXJPYTZDNR-UHFFFAOYSA-N 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- WQGWDDDVZFFDIG-UHFFFAOYSA-N pyrogallol Chemical compound OC1=CC=CC(O)=C1O WQGWDDDVZFFDIG-UHFFFAOYSA-N 0.000 description 2
- 150000003232 pyrogallols Chemical class 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 2
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 2
- 235000010344 sodium nitrate Nutrition 0.000 description 2
- 239000004317 sodium nitrate Substances 0.000 description 2
- 235000010288 sodium nitrite Nutrition 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 229960005526 triapine Drugs 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical class [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 239000003871 white petrolatum Substances 0.000 description 2
- ONDPHDOFVYQSGI-UHFFFAOYSA-N zinc nitrate Chemical compound [Zn+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O ONDPHDOFVYQSGI-UHFFFAOYSA-N 0.000 description 2
- 230000004572 zinc-binding Effects 0.000 description 2
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 1
- PHIQHXFUZVPYII-ZCFIWIBFSA-N (R)-carnitine Chemical compound C[N+](C)(C)C[C@H](O)CC([O-])=O PHIQHXFUZVPYII-ZCFIWIBFSA-N 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- LTMRRSWNXVJMBA-UHFFFAOYSA-L 2,2-diethylpropanedioate Chemical compound CCC(CC)(C([O-])=O)C([O-])=O LTMRRSWNXVJMBA-UHFFFAOYSA-L 0.000 description 1
- GJEPMYMUORZPMP-UHFFFAOYSA-N 2-(1h-benzimidazol-2-yl)ethanamine Chemical compound C1=CC=C2NC(CCN)=NC2=C1 GJEPMYMUORZPMP-UHFFFAOYSA-N 0.000 description 1
- KAWIOCMUARENDQ-UHFFFAOYSA-N 2-(4-chlorophenyl)sulfanyl-n-(4-pyridin-2-yl-1,3-thiazol-2-yl)acetamide Chemical compound C1=CC(Cl)=CC=C1SCC(=O)NC1=NC(C=2N=CC=CC=2)=CS1 KAWIOCMUARENDQ-UHFFFAOYSA-N 0.000 description 1
- HZLCGUXUOFWCCN-UHFFFAOYSA-N 2-hydroxynonadecane-1,2,3-tricarboxylic acid Chemical compound CCCCCCCCCCCCCCCCC(C(O)=O)C(O)(C(O)=O)CC(O)=O HZLCGUXUOFWCCN-UHFFFAOYSA-N 0.000 description 1
- OZUBORKYZRYLSQ-UHFFFAOYSA-N 3-nitrosobenzamide Chemical class NC(=O)C1=CC=CC(N=O)=C1 OZUBORKYZRYLSQ-UHFFFAOYSA-N 0.000 description 1
- HLBZWYXLQJQBKU-UHFFFAOYSA-N 4-(morpholin-4-yldisulfanyl)morpholine Chemical compound C1COCCN1SSN1CCOCC1 HLBZWYXLQJQBKU-UHFFFAOYSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 1
- GVNWZKBFMFUVNX-UHFFFAOYSA-N Adipamide Chemical compound NC(=O)CCCCC(N)=O GVNWZKBFMFUVNX-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 206010001497 Agitation Diseases 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 206010059313 Anogenital warts Diseases 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 239000004156 Azodicarbonamide Substances 0.000 description 1
- NKGNREWERBOCEP-UHFFFAOYSA-N CC1=NC=C(CO)C(C23OC4(O)CC(O)(CC(C4)(O)O2)O3)=C1O Chemical compound CC1=NC=C(CO)C(C23OC4(O)CC(O)(CC(C4)(O)O2)O3)=C1O NKGNREWERBOCEP-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- QRYRORQUOLYVBU-VBKZILBWSA-N Carnosic acid Natural products CC([C@@H]1CC2)(C)CCC[C@]1(C(O)=O)C1=C2C=C(C(C)C)C(O)=C1O QRYRORQUOLYVBU-VBKZILBWSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- VVNCNSJFMMFHPL-VKHMYHEASA-N D-penicillamine Chemical compound CC(C)(S)[C@@H](N)C(O)=O VVNCNSJFMMFHPL-VKHMYHEASA-N 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 208000018565 Hemochromatosis Diseases 0.000 description 1
- 241000700588 Human alphaherpesvirus 1 Species 0.000 description 1
- 241000701074 Human alphaherpesvirus 2 Species 0.000 description 1
- 241000701085 Human alphaherpesvirus 3 Species 0.000 description 1
- 241000701024 Human betaherpesvirus 5 Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 241000207919 Hydrophyllum Species 0.000 description 1
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 206010065973 Iron Overload Diseases 0.000 description 1
- 208000000913 Kidney Calculi Diseases 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 201000009906 Meningitis Diseases 0.000 description 1
- 206010027439 Metal poisoning Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 101000905241 Mus musculus Heart- and neural crest derivatives-expressed protein 1 Proteins 0.000 description 1
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 206010029148 Nephrolithiasis Diseases 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 101710163270 Nuclease Proteins 0.000 description 1
- YIKSCQDJHCMVMK-UHFFFAOYSA-N Oxamide Chemical compound NC(=O)C(N)=O YIKSCQDJHCMVMK-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 108020002230 Pancreatic Ribonuclease Proteins 0.000 description 1
- 102000005891 Pancreatic ribonuclease Human genes 0.000 description 1
- 208000009608 Papillomavirus Infections Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 102000029797 Prion Human genes 0.000 description 1
- 108091000054 Prion Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 101710132594 Protein E6 Proteins 0.000 description 1
- 101800001554 RNA-directed RNA polymerase Proteins 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 1
- 229910021607 Silver chloride Inorganic materials 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 240000006394 Sorghum bicolor Species 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 235000021307 Triticum Nutrition 0.000 description 1
- 244000098338 Triticum aestivum Species 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 150000001279 adipic acids Chemical class 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 229940040563 agaric acid Drugs 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 235000011114 ammonium hydroxide Nutrition 0.000 description 1
- 238000002399 angioplasty Methods 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 238000011319 anticancer therapy Methods 0.000 description 1
- 229910052787 antimony Inorganic materials 0.000 description 1
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 230000006793 arrhythmia Effects 0.000 description 1
- 229910052785 arsenic Inorganic materials 0.000 description 1
- RQNWIZPPADIBDY-UHFFFAOYSA-N arsenic atom Chemical compound [As] RQNWIZPPADIBDY-UHFFFAOYSA-N 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 125000000751 azo group Chemical group [*]N=N[*] 0.000 description 1
- XOZUGNYVDXMRKW-AATRIKPKSA-N azodicarbonamide Chemical compound NC(=O)\N=N\C(N)=O XOZUGNYVDXMRKW-AATRIKPKSA-N 0.000 description 1
- 235000019399 azodicarbonamide Nutrition 0.000 description 1
- 230000006399 behavior Effects 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000027455 binding Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 229940053031 botulinum toxin Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 230000003293 cardioprotective effect Effects 0.000 description 1
- 229960004203 carnitine Drugs 0.000 description 1
- 229940044199 carnosine Drugs 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229920001429 chelating resin Polymers 0.000 description 1
- OEUUFNIKLCFNLN-LLVKDONJSA-N chembl432481 Chemical compound OC(=O)[C@@]1(C)CSC(C=2C(=CC(O)=CC=2)O)=N1 OEUUFNIKLCFNLN-LLVKDONJSA-N 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- WORJEOGGNQDSOE-UHFFFAOYSA-N chloroform;methanol Chemical compound OC.ClC(Cl)Cl WORJEOGGNQDSOE-UHFFFAOYSA-N 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000008294 cold cream Substances 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 229940125797 compound 12 Drugs 0.000 description 1
- 229940125782 compound 2 Drugs 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 229910001431 copper ion Inorganic materials 0.000 description 1
- 229910000365 copper sulfate Inorganic materials 0.000 description 1
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- 229960000605 dexrazoxane Drugs 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- WYACBZDAHNBPPB-UHFFFAOYSA-N diethyl oxalate Chemical compound CCOC(=O)C(=O)OCC WYACBZDAHNBPPB-UHFFFAOYSA-N 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 229940042396 direct acting antivirals thiosemicarbazones Drugs 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 150000004662 dithiols Chemical class 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 206010014599 encephalitis Diseases 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- IMBKASBLAKCLEM-UHFFFAOYSA-L ferrous ammonium sulfate (anhydrous) Chemical compound [NH4+].[NH4+].[Fe+2].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O IMBKASBLAKCLEM-UHFFFAOYSA-L 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 210000004211 gastric acid Anatomy 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 150000002311 glutaric acids Chemical class 0.000 description 1
- VANNPISTIUFMLH-UHFFFAOYSA-N glutaric anhydride Chemical compound O=C1CCCC(=O)O1 VANNPISTIUFMLH-UHFFFAOYSA-N 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 208000010501 heavy metal poisoning Diseases 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- 229960004931 histamine dihydrochloride Drugs 0.000 description 1
- PPZMYIBUHIPZOS-UHFFFAOYSA-N histamine dihydrochloride Chemical compound Cl.Cl.NCCC1=CN=CN1 PPZMYIBUHIPZOS-UHFFFAOYSA-N 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000008311 hydrophilic ointment Substances 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 230000003463 hyperproliferative effect Effects 0.000 description 1
- 125000005462 imide group Chemical group 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 229920000554 ionomer Polymers 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000001698 laser desorption ionisation Methods 0.000 description 1
- 230000003859 lipid peroxidation Effects 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000003712 lysosome Anatomy 0.000 description 1
- 230000001868 lysosomic effect Effects 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- WRIRWRKPLXCTFD-UHFFFAOYSA-N malonamide Chemical compound NC(=O)CC(N)=O WRIRWRKPLXCTFD-UHFFFAOYSA-N 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000003990 molecular pathway Effects 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 230000003018 neuroregenerative effect Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 229940053973 novocaine Drugs 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 229940127073 nucleoside analogue Drugs 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 238000001139 pH measurement Methods 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000007918 pathogenicity Effects 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 229960001639 penicillamine Drugs 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 238000012355 ph-metric titration Methods 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- LFGREXWGYUGZLY-UHFFFAOYSA-N phosphoryl Chemical group [P]=O LFGREXWGYUGZLY-UHFFFAOYSA-N 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 125000003367 polycyclic group Chemical group 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 229940116317 potato starch Drugs 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- 229960003581 pyridoxal Drugs 0.000 description 1
- 235000008164 pyridoxal Nutrition 0.000 description 1
- 239000011674 pyridoxal Substances 0.000 description 1
- 229940079877 pyrogallol Drugs 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000008085 renal dysfunction Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 1
- 229960000329 ribavirin Drugs 0.000 description 1
- 229940100486 rice starch Drugs 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 230000006001 small intestine dysfunction Effects 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000002798 spectrophotometry method Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000002511 suppository base Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 150000003584 thiosemicarbazones Chemical class 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 238000000954 titration curve Methods 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 229910001428 transition metal ion Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 229940078499 tricalcium phosphate Drugs 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 229940100445 wheat starch Drugs 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4178—1,3-Diazoles not condensed 1,3-diazoles and containing further heterocyclic rings, e.g. pilocarpine, nitrofurantoin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4184—1,3-Diazoles condensed with carbocyclic rings, e.g. benzimidazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/427—Thiazoles not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/428—Thiazoles condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/18—Antioxidants, e.g. antiradicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/32—Alcohol-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/12—Ophthalmic agents for cataracts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/02—Antidotes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/04—Chelating agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/06—Free radical scavengers or antioxidants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/14—Vasoprotectives; Antihaemorrhoidals; Drugs for varicose therapy; Capillary stabilisers
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/64—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms, e.g. histidine
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/06—Benzimidazoles; Hydrogenated benzimidazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached in position 2
- C07D235/14—Radicals substituted by nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/02—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings
- C07D277/20—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D277/22—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
- C07D277/28—Radicals substituted by nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/60—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings condensed with carbocyclic rings or ring systems
- C07D277/62—Benzothiazoles
- C07D277/64—Benzothiazoles with only hydrocarbon or substituted hydrocarbon radicals attached in position 2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Virology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Oncology (AREA)
- Epidemiology (AREA)
- Communicable Diseases (AREA)
- Diabetes (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Toxicology (AREA)
- Ophthalmology & Optometry (AREA)
- Psychiatry (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Addiction (AREA)
- Biochemistry (AREA)
- Vascular Medicine (AREA)
- Obesity (AREA)
- Psychology (AREA)
- Rheumatology (AREA)
- Endocrinology (AREA)
- AIDS & HIV (AREA)
Abstract
本发明涉及二羧酸双酰胺衍生物、其用途和基于其的药物组合物。该新的生物学活性化合物为通式(I)的二羧酸双酰胺衍生物或其药用盐,其能够复合或螯合金属离子。本发明还涉及其作为用于预防和/或治疗心血管、病毒、肿瘤、神经变性和炎性疾病,糖尿病和与年龄有关的疾病以及由微生物毒素引起的疾病,以及酒精中毒、酒精性肝硬化、贫血、迟发性皮肤卟啉病和过渡金属盐中毒的药剂的用途。
Description
本申请是申请日为2014年4月10日的题为“二羧酸双酰胺衍生物、其用途、基于其的药物组合物和用于生产二羧酸双酰胺衍生物的方法”的中国专利申请No.201480027307.9的分案申请。
技术领域
本发明涉及新生物活性化合物,具体地涉及二羧酸双酰胺衍生物或其药用盐,其能够与金属离子形成复合物(络合物,complex)或螯合金属离子。本发明还涉及所述化合物作为用于预防和/或治疗心血管疾病、病毒疾病、肿瘤疾病、神经变性疾病和炎性疾病、糖尿病、与年龄有关的疾病、由微生物毒素引起的疾病、酒精中毒、酒精性肝硬化、贫血、迟发性皮肤卟啉病和过渡金属盐中毒的药剂(试剂,agent)的用途。
背景技术
金属离子在细胞与整个机体的功能化以及在病理学的发展中均非常重要。
存在有已知的药物,它们的活性通过它们螯合金属离子的能力来确定。在本上下文中,当前正在研究无毒化合物,其能够以高效率和高选择性螯合金属离子并且其适于生物医学应用。
在不同种类的化合物中已经发现了具有螯合能力的化合物,诸如单-和二硫醇、二硫化物、偶氮化合物、亚硝基芳香族化合物、聚氨基羧酸衍生物、缩氨基硫脲、吡哆醛异烟腙、喹啉、金刚烷、邻苯三酚(pyrogallol)、邻菲咯啉、硫代焦磷酸盐等。此外,在其它天然化合物中也发现了它们,诸如例如肌肽、菲汀、果胶。具有多种在复合物形成反应中能够充当电子供体的官能团的化合物最令人感兴趣。因此,此类化合物可以允当特定地与金属离子或金属基团相互作用的配体。
当今广泛已知的复合物形成剂是聚氨基羧酸衍生物(例如EDTA)、D-青霉胺和多环化合物,它们已经被成功地应用在重金属中毒疗法中。在一些铁过量病况和血色素沉着症中,将去铁胺用作铁螯合剂。另外,螯合剂在与高钙水平病况(例如,关节病、动脉粥样硬化和肾结石)有关的病理治疗中可以是有用的。还已知的是,螯合疗法预防胆固醇蓄积并且恢复其在血液中的水平,降低血压,允许避免血管成形术,抑制某些心脏药物的不良副作用,从胆固醇斑块中除去钙,溶解血栓并恢复血管弹性,恢复正常心律不齐,防止衰老,恢复心脏肌肉力量并且改善心功能,增加细胞内钾浓度,调节矿物质代谢,有用于治疗阿耳茨海默氏病,预防癌症,改善记忆,并且具有多种其它积极效果。但是,目前在螯合疗法中使用的强螯合剂通常具有毒性效应,其本身主要表现为小肠的粘膜损伤和肾功能异常。在一些情况下,快速给予大量的已知螯合剂可能损害肌肉应激性和血液凝固。另外,强螯合剂能够与宝贵的生物元素(Na、K、Ca、Mg和Ca)相互作用并且能够改变最为重要的金属酶的活性[Zelenin K.N."Chelators in medicine,"Soros Educational Journal,2001,v.7,No.1,pp.45-50]。
在本上下文中,目前正在研究的新高效螯合剂具有足以实现在体内的生物效应的螯合能力并且无副作用。
诸如HIV、人乳头瘤病毒、疱疹、肝炎等病毒性疾病的螯合疗法的理念是特别实际的。
病毒蛋白的结构中的锌结合位点(所谓的“锌指”)被认为是潜在的目标。
当今,已经发现多种化合物影响这些病毒的重要蛋白质的“锌指”结构。
Andreas J.K.的文章Bioorganic&Medicinal chemistry,2003,v.11,pp.4599-4613公开了一种惯用名Bananin的金刚烷衍生物,其是一种锌离子螯合剂。该化合物被假定为化学适用于除去HIV蛋白NCp7的锌。偶氮二甲酰胺是一种应该表现出上述的作用机制的偶氮衍生物,其目前处于针对进行性AIDS的I/II期临床试验中。
文章Rice W.G.,Schaeffer C.A.,Harten B.,Nature,1993,v.4,pp.473-475公开了3-亚硝基苯甲酰胺,其能够从HIV蛋白NCp7中除去锌,抑制HIV的复制并且压制其在体外和体内的致病性。
人乳头瘤病毒蛋白E6的“锌指”-样位点也被选择为靶标。这种病毒是宫颈癌的病因学中的潜在介体(介导物,mediator)。
文章Beerheide W.,Bernard H.-U.,Tan Y.-J.,Ganesan A.J.,National CancerInstitute,1999,v.91,No.14,pp.1211-1220描述了氮杂化合物、以及二硫化物和亚硝基芳族衍生物的体外研究。4,4’-二硫代二吗啉型化合物已被证明引起锌离子的释放。因此,观察到了病毒蛋白的结构的变化以及与人乳头瘤病毒的生物学和病理学有关的其功能的削弱。但是,该化合物的临床试验尚未完成,并且它们的有效性仅能在体外研究的基础上判定。
以上所指出的化合物被假定为针对宫颈癌、尖锐湿疣和潜伏性生殖器乳头状瘤病毒感染的药物开发的潜在目标。丙型肝炎病毒是人类最常见的病原体之一。丙型肝炎的现代治疗几乎仅基于干扰素及其与核苷类似物利巴韦林的组合的使用[Kozlov M.V.,Polyakov K.M.,Ivanov A.V.,Biochemistry,2006,v.71,No.9,pp.1253-12594]。应该注意的是,该治疗不是很有效。
关于针对丙型肝炎病毒的治疗剂的开发,靶标之一是具有锌位点的NS3-丝氨酸蛋白酶,该锌位点对于维持其结构稳定性是重要的[Andrea Urbani,Renzo Bazzo,MariaChiara Nardi,Daniel Oscar Cicero,Raffaele De Francesco,J.Biol.Chem.,1998,v.273,No.30,pp.18760-18769]。一些文献资料报道,通过使用具有锌去除能力的化合物来抑制或改变其活性是一种有前途的控制丙型肝炎病毒疾病的策略。
文章Timothy L.Tellinghuisen,Matthew S.Paulson,Charles M.Rice,J.Virology,2006,v.80,No.15,pp.7450-7458教导了,相对于NS2/3自动裂解评估的金属螯合剂(EDTA和1,10-菲咯啉)是有效的蛋白酶抑制剂。此外,有资料称,在这种情况下,1,10-菲咯啉的效果与锌的螯合完全相关。
文章Sperandio D.,Gangloff A.R.,Litvak J.Goldsmith R.,Bioorg.Med.Chem.Lett.,2002,v.12,No.21,3129-3133公开了二苯并咪唑化合物的筛选,目的在于探索丙型肝炎病毒NS3/NS4A丝氨酸蛋白酶的抑制剂,并且提到该筛选还产生了相应强大的Zn2+-依赖性抑制剂的识别。
在丙型肝炎的治疗的新方法开发中的另一种吸引人的靶标可以是丙型肝炎病毒RNA依赖性RNA聚合酶(病毒蛋白NS5B),其包含锌结合位点[Timothy L.Tellinghuisen,Matthew S.Paulson,Charles M.Rice,J.Virology,2006,v.80,No.15,p.7450-7458]。
目前已知的丙型肝炎病毒RNA依赖性RNA聚合酶的抑制剂可以常规地分为两个主要类别:核苷衍生物和各种非核苷抑制剂[Maria Bretner,Acta biochemica polonica,2005,v.52,No.1,p.57-70]。此外,已经发现,连苯三酚衍生物抑制所指示的酶的活性。值得注意的是,邻苯三酚衍生物的抑制机制被假定为基于镁离子的螯合作用,该镁离子参与磷酰基转移步骤中的催化作用[Kozlov M.V.,Polyakov K.M.,Ivanov A.V.,Biochemistry,2006,v.71,No.9,pp.1253-12594]。
由疱疹病毒引起的疾病的广泛传播。因此,已知有多种人类疱疹病毒,诸如单纯疱疹病毒1和2(HSV-1和HSV-2)、细胞巨化病毒(CMV)、水痘带状疱疹病毒和埃-巴二氏病毒(Epstein-Barr virus)。它们对中枢神经系统的破坏性行为引起疾病,诸如脑炎和脑膜炎。对锌螯合化合物(例如二亚乙基三氨基五乙酸)在体内对人巨细胞病毒复制的影响的研究存在巨大的兴趣[Kanekiyo M.,Itoh N.,Mano M.,Antiviral Res.,2000,v.47,p.207-214]。
但是,还值得注意的是,类似于上述的病毒,疱疹病毒包含具有“锌指”-样部分的蛋白质。该“锌指”结构的化学修饰(化学改性,chemical modification)可以导致锌的释放以及病毒蛋白的功能改变[Yan Chen,Christine M.Livingston,Stacy D.Carrington-Lawrence,J.of Virology,2007,v.81,No.16,p.8742-8751]。
该“锌指”结构可以作为新一代抗病毒治疗的靶点。已经发现了一些这样的化合物。但是,当今它们的效率仅参基于体外研究进行判定。
上述信息暗示了金属离子对于细胞和作为整体的生物体的正常运作的重要性以及对于病理的发展的重要性。一些化合物螯合金属离子的能力界定了开发有效于治疗各种疾病(包括病毒性疾病)的研究策略。
文章Megan Whitnall,Jonathan Howard,Prem Ponka,“A class of ironchelators with a wide spectrum of potent antitumor activity that overcomesresistance to chemotherapeutics”,PNAS,2006,v.103,No.40,p.14901-14906公开了有效的铁螯合剂,其表现出类似于已知细胞抑制剂的活性的高增殖和抗癌活性,并且其有潜力进行临床试验。
Kik K.,Szmigiero L.,“Dexrazoxane(ICRF-187)-a cardioprotectant andmodulator of some anticancer drugs”,Postepy Hig Med Dosw Online,2006,v.60,p.584-590报道,一些铁螯合剂由于其心脏保护作用而可以在抗癌治疗中用作“辅助剂(helper)”。
铜离子的螯合作用抑制血管生成并且减慢肿瘤的生长[Yu Yu,Jacky Wong,DavidB.Loveioy,“Chelatorsat the cancer coalface:Desferrioxamine to Triapine andBeyond”,Clin.Cancer Res.,2006,v.12,p.6876-6883]。
锌螯合剂氯碘羟喹通过结合Zn2+而引起人类癌细胞的凋亡[Haijun Yu,YunfengZhou,Stuart E.Lind,“Clioquinol targets zinc to lysosomes in human cancercells”,Biochem.J.,2009,v.417,p.133-139]。
螯合剂在酒精中毒的治疗中被用作醛脱氢酶的抑制剂[Shian S.G.,Kao Y.R.,WuF.Y.,Wu C.W.,“Inhibition of invasion and angiogenesis by zinc-chelating agentdisulfiram”,Mol.Pharmacol.,2003,v.64(5),p.1076-84]且在酒精性肝硬化、铁过量贫血和迟发性皮肤卟啉病的治疗中被用作醛脱氢酶的抑制剂[Schroterova L.,Kaiserova H.,Baliharova V.,“The effect of new lipophilic chelators on the activities ofcytosolic reductases and P450cytochromes involved in the metabolism ofanthracycline as antibiotics:studies in vitro”,Physiol.Res.,2004,v.53(6),p.683-691]。
一些抗氧化剂的活性机理是螯合过渡金属离子(Fe、Cu),这会导致减少金属依赖性脂质过氧化作用[Babizhayev M.A.,Seguin Marie-C.,Gueynej J.,Evstigneev R.P.,Ageyeva E.A.,Zheltuchina G.A.,“L-Carnosine(-alanyl-L-histidine)and carcininef-alanylhistamine)act as natural antioxidants with hydroxyl-radical-scavenging and lipid-peroxidase activities”,Biochem.J.,1994,v.304,p.509-516]。
抗氧化剂的使用可导致白内障分辨、消除视网膜疾病、减少糖尿病患者对胰岛素的需求、去除皮肤色素沉着和消除中风后遗症症状。螯合在炎症性疾病(诸如骨性关节炎和类风湿性关节炎)的治疗中也是有用的[Zelenin K.N.,"Chelators in medicine",SorosEducational Journal,2001,v.7,No.1,pp.45-50]。
螯合剂可以作为用于运输和易于从有机体提取砷、汞、锑、钴、锌、铬和镍的螯合剂而在药剂中使用[Zholnin A.V.,"Complex compounds",Chelyabinsk:ChGMA,2000,p.28]。
已知肉毒毒素是通过螯合锌离子而被抑制[Anne C.,Blommaert A.,“Thio-derivede disulfides as potent inhibitors of botulinum neurotoxin B:implications of zinc interaction”,Bioorg.Med.Chem.,2003,v.11(21),pp.4655-60]。此外,螯合在在气性坏疽的治疗中提供保护[Zelenin K.N.,"Chelators in medicine",Soros Educational Journal,2001,v.7,No.1,pp.45-50]。
螯合疗法被用于治疗神经退行性疾病(特别是阿耳茨海默氏病)以改善记忆[Bossy-Wetzel E.,Schwarzenbacher R.,Lipton S.A.,“Molecular pathways toneurodegeneration”,Nat.Med,2004,v.10,p.2-9];帕金森病[Kevin J.Barnham,ColinL.Masters,Ashley I.Bush,“Neurodegenerative diseases and oxidative stress”,Nature Reviews Drug Discovery,2004,v.3,p.205-214];威尔逊氏病[Yu Yu,JackyWong,David B.Lovejoy,“Chelators at the Cancer Coalface:Desferrioxamine toTriapine and Beyond”,Clin.Cancer Res.?2006,v.12,p.6876-6883];亨廷顿病[Whitnall M.,Richardson D.R.,“Iron:a new target for pharmacologicalintervention in neurodegenerative diseases”,Semin Pediatr Neurol,2006,v.13,p.186-197];肌萎缩性侧索硬化[Kevin J.Bernham,Colin L.Masters,Ashley I.Bush,“Neurodegenerative diseases and oxidative stress”,Nature Reviews DrugDiscovery,2004,v.4]以及朊病毒病[Daniel L.Cox,Jianping Pan,Rajiv R.P.Singh,“AMechanism for Copper Inhibition of Infection Prion Conversion”,BiophysicalJournal,2006,v.91,L11-L13]。螯合剂预防癌症的发展[Megan Whitnall,“A class ofiron chelators with a wide spectrum of potent antitumor activity thatovercomes resistance to chemotherapeutics”,PNAS,2006,v.103,No.40,p.14901-14906]。
在已知的螯合剂中,包含酰亚胺基和酰胺基的杂环化合物(例如咪唑)的衍生物尤其重要。
文章M.A.Podyminogin,V.V.Vlassov,“Synthesis RNA-cleaving moleculesmimicking ribonuclease A active center.Design and cleavage of tRNAtranscrints”,Nucleic Acids Research,1993,v.21,No.25,pp.5950-5956教导了戊二酸的双组胺衍生物,其可以用作核酸酶的活性中心的模型并且在RNA分子的消化中表现出弱活性。
Elfriede Schuhmann等(参见“Bis[platinum(II)]and Bis[Palladium(II)]complexes ofα,-Dicarboxylic Acid Bis(1,2,4-triaminobutane-N4)-Amides”,Inorg.Chem.,1995,v.34,p.2316-2322)描述了戊二酸和己二酸的双-组胺衍生物,它们是用于合成铂和钯的复合物的中间体:
n=3-6、8;
M=Pt、Pd。
在文章Elfriede Schuhmann et al.,“Bis[platinum(II)]and Bis[Palladium(II)]complexes ofα,-Dicarboxylic Acid Bis(1,2,4-triaminobutane-N4)-Amides”,Inorg.Chem.,1995,v.34,p.2316-2322中描述了合成N1,N1-戊二酰基-双(组胺)的方法,包含在二甲基甲酰胺中在4倍过量的三乙胺的存在下使组胺二盐酸盐与戊二酸二氯酸酐接触。
本发明人已经发现,戊二酸双组胺衍生物(特别是N1,N1-戊二酰基-双(组胺))能够与金属离子形成复合物。
因此,本发明的目的是提供生物相容性杂环金属离子螯合剂以及通过利用所要求保护的化合物螯合金属离子的能力而将其作为用于治疗和/或预防各种疾病的药物的用途。
本发明的另一目的是通过使用可得到的反应物来提供用于制备此种化合物的简单方法。
发明内容
本发明涉及通式I的二羧酸双酰胺衍生物或其药用盐:
其中
R1为包含1至2个选自N和/或S的杂原子的5-元不饱和杂环基团,其可选地缩合有6-元不饱和环基团;
R2为-C(O)-R3-C(O)-,其中R3为可选地取代有1-2个C1-C6烷基或苯基的-(CH2)n-,
n为0-4的整数。
本发明还涉及通式I的二羧酸双酰胺衍生物,其能够螯合金属离子(Zn、Cu、Fe、Mg、Ca等);以及它们作为用于预防和/或治疗心脏疾病、病毒疾病、癌症疾病、神经变性疾病和炎性疾病、糖尿病、与年龄有关的疾病、由微生物毒素引起的疾病、酒精中毒和酒精性肝硬化、贫血、迟发性皮肤卟啉病和过渡金属盐中毒的药剂的用途。
本发明还涉及用于制备通式I的化合物的方法,包含以下步骤:
在加热下使二羧酸与相应的胺接触;或
在N,N'-二环己基碳二亚胺的存在下使二羧酸与N-羟基琥珀酰亚胺接触以形成相应的二-N-氧代琥珀酰亚胺酯,使该二-N-氧代琥珀酰亚胺酯与胺缩合;或
使二羧酸二酯与水合肼接触,用亚硝酸钠处理所形成的二酰肼以形成相应的叠氮化物,使该叠氮化物与胺缩合;或
加热在有机溶剂中的由二羧酸与相应的胺形成的酰亚胺的溶液;或
在缩合剂的存在下以2:1的摩尔比使相应的胺与二羧酸接触。
所要求保护的用于制备通式I的二羧酸杂环双衍生物的方法实施简单,在相当温和的条件下进行,无副产物,容易重现,且以高产率(高达82%)和高纯度提供目标产物。
具体实施方式
优选的根据本发明的化合物是通式I的化合物:
其中R1是选自下列的基团:
R2是选自-C(O)-(CH2)0-(CO)-、-C(O)-(CH2)1-(CO)-、-C(O)-(CH2)2-(CO)-、-C(O)-(CH2)3-(CO)-、-C(O)-(CH2)4-(CO)-、-C(O)-CH2-CH(CH3)-CH2-C(O)-、-C(O)-CH2-C(CH3)2-CH2-C(O)-的基团或选自的基团。
本发明最优选的化合物是表1中给出的化合物。
表1
根据本发明的化合物的药用盐可以选自有机酸的加成盐(例如甲酸盐、乙酸盐、马来酸盐、酒石酸盐、甲磺酸盐、苯磺酸盐、甲苯磺酸盐等)、无机酸的加成盐(例如盐酸盐、氢溴酸盐、硫酸盐、磷酸盐等)以及与氨基酸的盐(例如天冬氨酸盐、谷氨酸盐等),优选氢氯酸盐和乙酸盐。
可以在根据本发明的药物组合物以及治疗方法中使用的最优选的已知化合物是表2中所示的戊二酰亚胺衍生物。
表2
根据本发明的化合物可以通过包括在加热下可选地在溶剂的存在下使以下通式II的二羧酸:
R4O-C(O)-R3-C(O)-OR4
其中R3为可选地取代有1-2个C1-C6烷基或苯基的-(CH2)n-;
n为0-4的整数,且
R4为氢或C1-C6烷基;
与以下通式III的胺缩合的方法来制备:
NH2-(CH2)2-R1
其中R1为包含1-2个选自N和/或S的杂原子的5-元不饱和杂环基团,其可选地缩合有6-元不饱和环基团。
优选使用二甲醚并且加热至150-170℃;并且更优选在沸腾下进行该缩合。
溶剂可以为二甘醇二甲醚或醇,更优选异戊醇。
用于生产通式I的二羧酸双酰胺衍生物的另一种方法是如下方法,其包括在N,N'-二环己基碳二亚胺的存在下,使以下通式II的二羧酸:
R4O-C(O)-R3-C(O)-OR4
其中R3是可选地取代有1-2个C1-C6烷基或苯基的-(CH2)n-,
n为0-4的整数,且
R4为氢,
与N-羟基琥珀酰亚胺接触以形成相应的以下通式IV的二-N-氧代琥珀酰亚胺酯:
使其与以下通式III的胺缩合:
NH2-(CH2)2-R1
其中R1是包含1至2个选自N和/或S的杂原子的5-元不饱和杂环基团,其可选地缩合有6-元不饱和环基团。
优选地提供冷却至0-5℃的温度。
用于生产通式I的二羧酸双酰胺衍生物的另一种方法是这样的方法,其包括在有机溶剂中使以下通式II的二羧酸酯:
R4O-C(O)-R3-C(O)-OR4
其中R3为可选地取代有1-2个C1-C6烷基或苯基的-(CH2)n-,
n为0-4的整数,且
R4为C1-C6烷基,
与水合肼接触以形成以下通式V的双酰肼:
H2N-NH-C(O)-R3-C(O)-NH-NH2
在约0℃的温度下在酸性介质中用亚硝酸钠处理该双酰肼以形成以下通式VI的双叠氮化物:
N--N+=N-C(O)-R3-C(O)-N=N+-N-
在有机溶剂中使该双叠氮化物与以下通式III的胺缩合:
NH2-(CH2)2-R1
其中R1为包含1-2个选自N和/或S的杂原子的5-元不饱和杂环基团,其可选地缩合有6-元不饱和环基团。
优选将醇用作有机溶剂,且更优选异丙醇。该方法简单,但是在初始的二羧酸的亚甲基单元的数目大于或等于3时才使用,因为由此获得的具有更低亚甲基数目的相应酸的双叠氮化物不稳定。
通式I的二羧酸双酰胺衍生物也可以通过这样的方法来制备,其包括在有机溶剂中在加热下使以下通式VII的酰亚胺:
其中R3为可选地取代有一个或两个C1-C6烷基的-(CH2)n-,且
n为0-4的整数,
与等摩尔量的以下通式III的胺缩合:
NH2-(CH2)2-R1
其中R1为包含1-2个选自N和/或S的杂原子的5-元不饱和杂环基团,其可选地缩合有6-元不饱和环基团。
优选将醇用作有机溶剂,且更优选异丙醇。该缩合优选在沸腾下进行。
本发明的另一种方法是以下用于生产通式I的二羧酸双酰胺衍生物的方法,包括在四氢呋喃溶液中在缩合剂(优选羰基二咪唑)的存在下以1:2-2.5的摩尔比使以下通式II的二羧酸:
R4O-C(O)-R3-C(O)-OR4
其中R3为可选地取代有1-2个C1-C6烷基或苯基的-(CH2)n-,
n为0-4的整数,且
R4为氢,
与以下通式III的胺接触:
NH2-(CH2)2-R1
其中R1为包含1-2个选自N和/或S的杂原子并且可选地缩合有6-元不饱和环基团的5-元不饱和杂环基团。
本发明还涉及包含通式I的二羧酸双酰胺衍生物的药剂及药物组合物,并且涉及以下方法,其包括给予通式I的二羧酸双酰胺衍生物以治疗和/或预防人类和动物的病毒性疾病,诸如由丙型肝炎病毒、人乳头瘤病毒、HIV或致癌RNA病毒(诸如白血病病毒)引起的疾病;心血管疾病,包括由抑制细胞生长的心脏毒性、胆固醇蓄积、和高血压引起的疾病;与金属依赖性自由基氧化反应有关的疾病,包括与年龄相关的疾病,诸如白内障、视黄醛性疾病和皮肤色素沉着;中风后遗症;动脉粥样硬化症;炎症性疾病,诸如骨关节炎、类风湿性关节炎;
糖尿病及其心血管并发症;神经退行性疾病,包括阿耳茨海默氏病、帕金森氏症、威尔逊氏病、亨廷顿氏病、肌萎缩侧索硬化和朊病毒病;
肿瘤疾病;微生物毒素引起的疾病,特别是肉毒中毒或气性坏疽;
酒精中毒与酒精性肝硬化;铁过量贫血、迟发性皮肤卟啉病;和过渡金属盐中毒。
根据本发明的化合物以提供所需的治疗效果的有效量进行给药。
通式(I)的化合物可以以包含无毒的药用载体的单位剂型口服、局部、胃肠外、鼻内、吸入和经直肠给药。本发明中使用的术语“口服给药(oral administration)”是指皮下、静脉内、肌内或胸腔内(intrathoric)注射或输注。
根据本发明的化合物可以以0.1-100mg/kg体重的剂量每日一次向患者给药,优选以0.25-25mg/kg的剂量每日一次或多次。
此外,应该注意的是,用于特定患者的特定剂量取决于许多因素,包括某些化合物的活性、患者的年龄、体重、性别、一般健康状况及饮食、药剂给予的时间和途径以及其从身体排泄的速率、药物的具体组合以及待治疗的个体中的疾病的严重程度。
根据本发明的药物组合物包含达到所需技术效果的有效量的通式(I)的化合物,并且可以联合剂型(unite dosage form)(例如固体、半固体或液体形式)给药,该联合剂型在混合物中包含根据本发明的化合物作为活性剂与适合于肌肉内、静脉内、经口和舌下给药、通过吸入给药、鼻内及直肠内给药的载体或赋形剂。该活性成分可以与适合于制备溶液、片剂、丸剂、胶囊剂、包衣丸剂、乳剂、混悬剂、软膏剂、凝胶剂和任何其它剂型的常规无毒的药用载体一起处于组合物中。
作为赋形剂,可以使用各种化合物,诸如糖类,例如葡萄糖、乳糖、或蔗糖;甘露醇或山梨糖醇;纤维素衍生物;和/或磷酸钙,例如磷酸三钙或磷酸氢钙。作为粘合剂,可以使用下列化合物,诸如淀粉糊(例如,玉米、小麦、大米或马铃薯淀粉)、明胶、黄蓍胶、甲基纤维素、羟丙基甲基纤维素、羧甲基纤维素钠和/或聚乙烯吡咯烷酮。可选使用的崩解剂是上述的淀粉和羧甲基淀粉、交联聚乙烯吡咯烷酮、琼脂、或藻酸或其盐,如藻酸钠。
可以可选使用的添加剂是流动控制剂和润滑剂,诸如二氧化硅、滑石粉、硬脂酸及其盐(诸如硬脂酸镁或硬脂酸钙)和/或丙二醇。
包衣的丸剂的核通常使用耐胃酸作用的层进行包衣。为了这种目的,可以使用糖类的浓缩溶液,其中该溶液可以可选地包含阿拉伯树胶、滑石、聚乙烯吡咯烷酮、聚乙二醇和/或二氧化钛以及合适的有机溶剂或它们的混合物。
稳定剂、增稠剂、着色剂和芳香剂也可以用作添加剂。
作为软膏基质,可以使用烃类软膏基质,诸如白色凡士林和黄色凡士林(分别为白凡士林(Vaselinum album)和黄凡士林(Vaselinum flavum))、凡士林油(液状石蜡)、和白药膏和液体膏剂(分别为白软膏(Unguentum album)和黄软膏)(Unguentum flavum)),其中固体石蜡或蜡可以用作提供更坚实质地的添加剂;吸收软膏基质,诸如亲水性凡士林(Vaselinum hydrophylicum)、羊毛脂(Lanolinum)、和冷霜(Unguentum leniens);水可除去的软膏基质,诸如亲水性软膏(Unguentum hydrophylum);水溶性软膏基质,诸如聚乙二醇软膏(Unguentum Glycolis Polyaethyleni);膨润土基质及其它。
凝胶剂用基质可以选自甲基纤维素、羧甲基纤维素钠、氧基丙基纤维素、聚乙二醇或聚环氧乙烷和卡波普(carbopol)。
栓剂用基质可以是水不溶性基质,诸如可可脂;水溶性或水混溶性基质,诸如明胶甘油或聚环氧乙烷;或组合基质,诸如皂-甘油基质。
在单位剂型的制备中,与载体组合使用的活性剂的量可以根据待治疗的接受者以及治疗剂的特定给药途径而改变。
例如,当将根据本发明的化合物以注射用溶液的形式使用时,该活性剂在这种溶液中的量可以高达5wt.%。稀释剂可以选自0.9%的氯化钠溶液、蒸馏水、注射用奴佛卡因溶液、林格氏液、葡萄糖溶液、和具体的增溶佐剂。当以片剂或栓剂形式给予根据本发明的化合物时,它们的量高达200mg/单位剂型。
根据本发明的剂型是通过常规工序制备的,诸如共混、造粒、成形包衣丸剂、溶解和冻干。
根据本发明的化合物、它们的制备及它们的活性的研究的详细描述公开于下列实施例中,该实施例仅用于说明性用途且不旨在限制本发明的范围。
通式I的戊二酰亚胺衍生物的合成的实施例
装置及方法
通过薄层色谱法(TLC)在板“Kieselgel 60F254”(“Merck”,German)上在吡啶-乙酸-水(20:6:11)(体系A);A:乙酸乙酯(3:1)(1)和氯仿-甲醇(9:1)(2)的溶剂体系中评估所获得的化合物的特性。
在室(150x320x150mm)中使用15V/cm的递度在由吡啶-乙酸-水(12:10:1000)组成的缓冲液(pH 5.1)中进行1.5小时的纸电泳(Kondopozhskii TsBK的电泳纸,120x320mm)。
使用氯四甲苯试剂和Pauly试剂处理色谱图和电泳图谱。
使用熔点测定器(Plant of lab.equipment,Klin,Russia)测量熔点。
在"Magna 750"光谱仪上使用KBr压片(“Nicolet”(US))记录傅里叶IR光谱。
在Bruker AMX-400(German)和DPX-400(German)光谱仪上记录1H NMR光谱。
在UltraFlex质谱仪(“Bruker”,German)上,通过基质激光解吸电离的方法使用2,5-二羟基苯甲酸作为基质在飞行时间辅助的质谱仪上获得高分辨质谱。
在PE SCIEX API 165质谱仪(150)(Canada)上使用岛津分析HPLC SCL10Avp LC/MS系统分析多组分混合物。
在如下条件下在岛津HPLC色谱仪上进行分析尺度的反相HPLC:Luna C18(2)100A柱,250x4.6mm(No.599779-23),磷酸盐缓冲溶液(pH 3.0):甲醇的体系的洗脱梯度(条件A);并且在"System Gold"色谱仪(Beckman,US)上,使用UV-检测器(λ=220nm),Phenomenex"Gemini"C-18柱,150x2,0mm,用0.05M磷酸盐缓冲液(pH 3.0)、90%溶于水的MeCN的流动相流量梯度,洗脱速率0.25ml/min,且注入10μl(条件B)。
实施例1
双-1,5-(Nβ-组胺基)戊二酸;(N,N'-双-[2-(1H-咪唑-4-基)乙基]戊二酰胺(化合 物11)。
将组胺(8g;0.072mol)加入到戊二酸二甲酯(5g;0.031mol)中并且在170℃下加热3.5-4小时直到完全蒸发甲醇。通过TLC或电泳方法来检查反应的完全性。然后将反应混合物悬浮于异丙醇中并且允许在+4℃下静置24小时。分离产物,使用异丙醇洗涤并且干燥。产量为6.8g(69%)。Rf0.42(1)。E+49mm。M.p.166-168℃。LC/MS:在保留时间0.3min处的个体峰,[M+H]+=319。条件A下的HPLC:在保留时间3.58min处的个体峰。1H NMR(400.13MHz,DMSO-d6,δ,m.d.,J/Hz):1.70(五重峰,2H,CH2CH2 CH2,J=7.3Hz),2.03(t,4H,CH 2CH 2 CH 2,J=7.3Hz),2.61(t,4H,CCH2 CH2N,J=7.5Hz),3.26(五重峰,4H,CCH 2 CH 2N,J=7.5Hz),6.76(b.s,2H,CCH),7.50(s,2H,NCHN),7.94(b.t.,2H,NH)。傅立叶-IR光谱(在KBr-片中,ν,cm-1):1651(νC=O酰胺I),1580(δNH酰胺II),1425(-CH2 -CO-)。发现值,%:C,56.40;H,6.86;N,26.31。C15H22N6O2。计算值,%:C,56.59;H,6.96;N,26.40。
实施例2
双-1,4-(Nβ-组胺基)琥珀酸;(N,N'-双-[2-(1H-咪唑-4-基)乙基]丁二酰胺)(化
合物3)
将组胺(12.2g;0.11mol)加入到琥珀酸(5.5g;0.046mol)中并且在170℃加热3.5-4小时直到甲醇蒸发完全。通过TLC或电泳方法检查反应的完全性。然后将反应物质悬浮于50ml水中并且允许在+4℃静置24小时。分离产物,用水洗涤,并且干燥。产量为10.7g(76%)。Rf0.51(1)。E+51mm。M.p.230-231℃。[M+H]+304.95。1H-NMR(D2O):δ,m.d.:2.48(s,4H,CH2-Suc),2.80-2.84(t,4H,β-CH2-HA),3.44-3.48(t,4H,α-CH2-HA),6.97(s,2H,5-CH-Im),7.78(s,2H,2-CH-Im)。傅立叶-IR光谱(在KBr-片中,ν,cm-1):1634(νC=O酰胺I),1583(δNH酰胺II),1429(-CH2 -CO-)。发现值,%:C,55.40;H,6.66;N,27.31。C14H20N6O2。计算值,%:C,55.25;H,6.62;N,27.61。
实施例3
双-1,3-(Nβ-组胺基)丙二酸;(N,N'-双-[2-(1H-咪唑-4-基)乙基]丙二酰胺)(化
合物2)
将组胺(9.0g;0.081mol)加入到丙二酸二乙酯(6.4g;0.039mol)中并明在170℃下加热2.5-3小时直到甲醇蒸发完全。通过TLC或电泳方法检查反应的完全性。然后将反应物质悬浮于20ml水中并且允许在+4℃静置72小时。分离出产物,用异丙醇洗涤,并且干燥。产量为5.0g(44%)。Rf 0.41(1)。E+57mm。M.p.187-188℃。1H-NMR(D2O):δ,m.d.:2.74-2.78(t,4H,β-CH2-HA),3.15(s,2H,CH2-Mal),3.41-3.44(t,4H,α-СН2-НА),6.78(s,2H,5-CH-Im),7.66(s,2H,2-CH-Im)。傅立叶-IR光谱(在KBr-片中,ν,cm-1):1643(νC=O酰胺I),1574(δNH酰胺II),1426(-CH2 -CO-)。发现值,%:C,53.24;H,6.51;N,28.56。C13H18N6O2。计算值,%:C,53.78;H,6.25;N,28.95。
实施例4
双-1,2-(Nβ-组胺基)草酸;(N,N'-双-[2-(1H-咪唑-4-基)乙基]乙二酰胺)(化合
物1)
将组胺(12.2g;0.11mol)加入到草酸二乙酯(7.3g;0.05mol)的异戊醇(35ml)的溶液中并且煮沸4小时。通过TLC或电泳方法检查反应的完全性。使用反应混合物在+4℃下静置16小时。分离出残留物,使用异丙醇洗涤,并且干燥。产量为10g(72%)。Rf0.55(1)。E+55mm。M.p.235-236℃。1H-NMR(DMSO):δ,m.d.:2.66-2.72(t,4H,β-CH2-HA),3.33-3.41(m,4H,α-СН2-НА),6.81(s,2H,5-CH-Im),7.54(s,2H,2-CH-Im),8.78-8.84(t,2H,NH-CO)。傅立叶-IR光谱(在KBr-片中,ν,cm-1):1651(νC=O酰胺I),1566(δNH酰胺II),1425(-CH2 -CO-)。发现值,%:C,52.24;H,5.51;N,29.97。C12H16N6O2。计算值,%:C,52.17;H,5.84;N,30.42。
实施例5
双-1,3-(Nβ-组胺基)间苯二甲酸(化合物6)
将间苯二甲酸(5g;30mmol)和N-羟基琥珀酰亚胺(7.93;69mmol)溶解于二甲基甲酰胺(30ml)中。向冷却至5℃的由此产生的溶液中加入N,N'-二环己基碳二亚胺(14.24g;69mmol)的二甲基甲酰胺(10ml)的冷却溶液,并且使反应混合物在5℃静置过夜。分离出尿素,使用15ml二甲基甲酰胺洗涤,并且干燥。基于所产生的尿素的质量,反应的产率被认为等于80%。将间苯二甲酸N-羟基琥珀酰亚胺二酯的溶液冷却至5℃,然后分批向其中进料熔融组胺(6.84g;61.6mmol)。使反应混合物静置2小时。在真空下除去溶剂。将黄色油状残留物溶解于120ml水中并且通过具有Amberlite IRA-96的柱(27x115)。将包含该产物的馏分合并。分离出所析出的目标产物的晶体。在异丙醇(35ml)和水(10ml)的混合物中重结晶所获得的粗产物。过滤出产物的晶体,使用水(3x30ml)和异丙醇(2x13ml)洗涤,并且干燥。获得白色晶体形式的目标产物。产量为1.55g(基于组胺的18%,或基于间苯二甲酸的14%)。Rf0.42(1)。M.p.218-219℃。E+40mm。MS:[M+1]+353。1H-NMR:(300MHz,DMSO-d6):δ,m.d.:2.74-2.79(t,4H,β-СН2-Ha),3.46-3.52(t,4H,α-СН2-Ha),6.81(s,2H,5-CH-Im),7.52-7.56(m,3H,ArH+2-CH-Im),7.92-7.95(d,2H,ArH),8.28(s,1H,ArH),8.65(b.s.,2H,-C(O)-NH-),11.80(b.s.,2H,-NH-)。在条件B下的HPLC:在保留时间12.5min处的个体峰。
实施例6
双-1,5-(Nβ-组胺基)戊二酸;(N,N'-双-[2-(1H-咪唑-4-基)乙基]戊二酰胺)(化
合物11)
在冷却和搅拌下,将PCl3(8ml)分批加入到戊二酸(13.2g;0.10mol)的甲醇(50ml)溶液中。在真空下从反应混合物中将溶剂除去。在真空下蒸馏除去所得的残余物。所产生的具有110-112℃沸点的戊二酸二甲酯的量为14.7(92%)。
将水合肼(7.5ml)加入到异丙醇(20ml)中,加热至沸点,然后向其中逐滴加入该戊二酸二甲酯(8g;0.05mol),然后使反应混合物在+20℃下静置16小时。分离出析出的产物,使用异丙醇洗涤,并且干燥。具有210-212℃熔点的戊二酸二酰肼的量为7.3(91%)。
在搅拌下将硝酸钠(1.45g;0.021mol)分批加入到戊二酸二酰肼(1.61g;0.010mol)在冰(20g)与盐酸(2.5ml)和预先冷却的氯仿(10ml)的混合物中的溶液中。分离出氯仿层,使用水(10ml)洗涤,冷却至+4℃,然后向冷却的组胺溶液(2.6g;0.023mol)中加入异丙醇(5ml)。使反应混合物在+20℃下静置2小时,然后在真空下除去溶剂。将所得的残留物溶解于水(20ml)中并且通过具有50ml离子交换树脂KU-2-20(H+-形式)的柱。使用0.5%氨溶液洗涤该树脂,收集不含组胺的产物馏分。合并洗脱液,在真空下除去溶剂,并且在异丙醇(2ml)中重结晶所产生的残留物并且干燥。产量为1.8g(55%)。Rf0.42(1)。E+49mm。M.p.166-168℃。1H-NMR(D2O):δ,m.d.:1.73-1.78(m,2H,β-CH2-Glt),2.10-2.15(t,4H,α-СН2-Glt),2.78-2.82(t,4H,β-СН2-HA),3.43-3.48(t,4H,α-СН2-HA),6.94(s,2H,5-CH-Im),7.69(s,2H,2-CH-Im)。傅立叶-IR光谱(在KBr片中,ν,cm-1):1651(νC=O酰胺I),1580(δNH酰胺II),1425(-CH2 -CO-)。发现值,%:C,56.40;H,6.86;N,26.31.C15H22N6O2。计算值,%:C,56.59;H,6.96;N,26.40。
实施例7
双-1,5-(Nβ-组胺基)己二酸;(N,N'-双-[2-(1H-咪唑-4-基)乙基]己二酰胺)(化
合物12)
在冷却下将三氯化磷(4ml)逐滴地加入到己二酸(7.40;0.05mol)的甲醇(25ml)溶液中。在真空下从反应混合物中除去溶剂,并且在真空下蒸馏除去所产生的残留物。所产生的具有115-117℃沸点的己二酸二甲酯的量为7.5(86%)。
将该己二酸二甲酯(7.5g;0.04mol)逐滴加入到异丙醇(20ml)和水合肼(5ml)的混合物中,加热至沸点,然后使反应混合物在+20℃下静置16小时。分离出沉淀物,使用异丙醇洗涤,并且干燥。所产生的具有182-182.5℃熔点的己二酸二酰肼的量为6.2(83%)。
在搅拌下将硝酸钠(1.8g;0.026mol)分批地加入至己二酸二酰肼(2.2g;0.013mol)在冰(30g)与盐酸(3ml)和冷却的氯仿(15ml)的混合物中的溶液中。分离出氯仿层,用水(10ml)洗涤,并且冷却至+4℃,然后向该冷却的组胺溶液(3g;0.027mol)中加入异丙醇(8ml)。通过TLC或电泳方法检查反应的完全性。使反应混合物在+4℃下静置24小时,并且分离出残留物,用异丙醇洗涤,在水中重结晶,并且干燥。产量为2.8g(65%)。Rf0.48(1)。E+45mm。M.p.184-186℃。[M+H]+332.92。1H-NMR(D2O):δ,m.d.:1.37-1.41(m,4H,β-CH2-Аdip),2.11-2.15(m,4H,α-СН2-Adip),2.74-2.78(t,4H,β-СН2-НА),3.40-3.44(t,4H,α-СН2-НА),6.88(s,2H,5-CH-Im),7.63(s,2H,2-CH-Im)。傅立叶-IR光谱(在KBr片中,ν,cm-1):1646(νC=O酰胺I),1581(δNH酰胺II),1425(-CH2 -CO-)。发现值,%:C,57.45;H,7.15;N,24.97.C16H24N6O2。计算值,%:C,57.81;H,7.28;N,25.28。
通过以上公开的方法制备下列化合物:
实施例8
双-1,4-(Nβ-组胺基)琥珀酸;(N,N'-双-[2-(1H-咪唑-4-基)乙基]丁二酰胺)(化
合物3)
将琥珀酸亚胺组胺(0.15;0.78mmol)加入到组胺(0.10g;0.9mmol)的异丙醇(3ml)溶液中并且在沸腾下加热2.5-3小时。通过TLC或电泳方法检查反应的完全性。然后,将反应混合物冷却至室温并且允许在+4℃下静置26小时。分离出产物,用异丙醇洗涤,并且干燥。产量为0.20g(81%)。Rf0.44(1)。E+51mm。M.p.231℃。[M]+304.12。1H-NMR(D2O):δ,m.d.:2.48(s,4H,CH2-Suc),2.80-2.84(t,4H,β-СН2-HA),3.44-3.48(t,4H,α-СН2-НА),6.97(s,2H,5-CH-Im),7.78(s,2H,2-CH-Im)。傅立叶-IR光谱(在KBr片中,ν,cm-1):1634(νC=O酰胺I),1583(δNH酰胺II),1429(-CH2 -CO-)。发现值,%:C,6.57;N,27.18。C14H20N6O2。计算值,%:C,55.25;H,6.62;N,27.61。
实施例9
双-1,5-(N-乙基氨基-2-苯并咪唑基)戊二酸;(N,N'-双-[2-(1–Э-苯并咪唑-2-
基)乙基]戊二酰胺)(化合物7)
将羰基二咪唑(23.4g;0.145mol)加入到戊二酸(8g;0.06mol)的无水四氢呋喃(500ml)溶液中并且搅拌2小时。然后将苯并咪唑基乙胺(21.6g;0.133mol)加入到该反应混合物中。将所得到的溶液搅拌3小时并且允许在室温下静置。分离产物的残留物,用水洗涤,并且干燥。产量为21g(82.9%)。Rf0.21(2)。M.p.170-170.5℃。[M]+419。1H-NMR(DMSO):δ,m.d.:1.61-1.76(m,2H,CH2),1.95-2.07(t,2H,CH2),2.9-3.0(t,4H,2CH2),3.45-3.55(五重峰,4H,2CH2),7.06-7.14(m,4H,Ar),7.39-7.54(m,4H,Ar),7.98-8.08(t,2H,Ar),11.6-12.7(s,2H,NH)。在条件B下的HPLC:在保留时间26.6min处的个体峰。
通过以上公开的方法制备下列化合物:
所要求保护的化合物的螯合能力的研究
下列研究已经显示,所要求保护的通式I的化合物能够与金属离子形成复合物或螯合金属离子。它们包含多个在复合物形成过程中可以用作供体的官能团,例如羧基、亚氨基和酰氨基,并且是特异性结合金属离子的配体,例如锌、铜、铁、钙和镁的离子。
配体形成复合物的重要性质是所研究的化合物在水溶液中的解离常数(pkn)。pkn值是通过电位滴定法测定的。解离常数的计算通过史瓦哲巴赫(Schwarzenbach)方法进行[Grinberg A.A.,"An introduction to the chemistry of complex compounds",fourthed.,Amend.,L.,Khimiya,1971]。
pH-电位滴定法
通过使用实验室离聚物I 120.1在仪器上进行测定。一种经设计的由两个电极特别是指示剂玻璃电极和氯化银比较电极组成的原电池。pH值测量准确度为±0.2。
由锌的硝酸盐(Zn(NO3)2·6H2O)、钙的硝酸盐(Ca(NO3)2·4H2O)、硫酸铜(CuSO4·5H2O)、氯化镁(MgCl2·6H2O)和莫尔盐((NH4)2Fe(SO4)2·6H2O)制备初始0.05M的Mz+金属离子的溶液。通过使用乙二胺四乙酸(trilon B)(Mz+=Zn2+、Cu2+、Mg2+、Ca2+)的络合滴定法和使用邻菲咯啉(Mz+=Fe2+)在490nm的分光光度法来进行Mz+盐初始溶液的标准化。
通过使用pH-计量滴定法的用于确定酸解离步常数(k1,…,kn)的传统方法是史瓦哲巴赫方法。
将研究化合物的样品(3·10-4mol)溶解于15.0ml的0.5M KNO3水溶液中。在搅拌下,用0.05M NaOH或HCl的水溶液滴定所获得的0.2M溶液。在每个滴定点记录所使用的滴定剂的量(V,ml)以及溶液的pH值。在接近等当点处的滴定步骤等于0.5ml和0.2ml。继续滴定直至溶液的pH值达到11.0-11.5。通过滴定曲线pH=f(a)来确定对应于a=0.5和/或a=1.5的溶液的pH值,并且以1摩尔滴定的化合物的方式计算所添加的滴定剂的克当量的量。
表3中给出了通过史瓦哲巴赫方法确定的研究化合物的酸解离常数[pkn]的结果。
表3
通过史瓦哲巴赫方法计算的研究化合物在水溶液中的pk值(C=0.02M;溶液的离子强度=0.5(KNO3);22℃)
| 化合物的编号 | pk<sub>1</sub> | pk<sub>2</sub> |
| 2 | 7.11<sup>(1)</sup> | 11.27<sup>(2)</sup> |
| 3 | 6.88<sup>(1)</sup> | 11.27<sup>(2)</sup> |
| 11 | 7.16<sup>(1)</sup> | 11.89<sup>(2)</sup> |
| 12 | 7.13<sup>(1)</sup> | 11.95<sup>(2)</sup> |
(1)-使用0.05M HCl水溶液的滴定。
(2)-使用0.05M NaOH水溶液的滴定。
考虑到参数,诸如电位滴定所需的研究化合物的量、测试程序和常数计算法,Bjerrum法是最合适的和信息性的[Yu.P.Galaktionov,M.A.Chistyakov,V.G.Sevastiyanov,etc.,"Step Stability Constants of Group IIIB Metal andLanthanide Complexes with Acetylacetone in Aqueous Solution and theSolubility Products of Metal Triacetylacctonates",Journal of physicalchemistry,2004,v.78,No.9,pp.1596-1604]。
该方法基于这样的观点,复合物的形成是逐步的。Bjerrum法的一个主要假设是关于只有多核复合物的形成的假设。复合物形成的方法可以示意性地描述为如下:
根据Bjerrum法,在存在和不存在金属离子的情况下,使用NaOH溶液测定所研究的化合物两次。该滴定是在高恒定浓度的中性电解质下进行的,其为该溶液提供恒定的离子强度。
为了这些目的,使用高可溶性盐,例如钠盐和钾盐。由实验确定浓度步稳常数(step stability constant)(Kn)。此外,该方法提供了关于所形成的复合物的组成的信息,特别是结合配体的最大可能数(n)。
通过pH-计量滴定的Bjerrum法的确定复合物的步稳常数的传统工序如下进行。
将所研究化合物的样品(3·10-4mol)溶解于14.0ml的0.5M的KNO3水溶液中。在剧烈搅拌下加入1ml Mz+盐的0.05M水溶液。以1:6的Mz+/L比研究该体系。为共存的配体和金属离子固定溶液的pH值。使用0.05M NaOH水溶液在宽范围pH值(高达pH 11.0-11.5)内滴定该混合物。记录每个滴定点所使用的滴定剂的量(VNaOH,ml)和溶液的pH值。在形成残留物的时刻,溶液变得混浊,或溶液的颜色改变,记录pH值、改变的性质和用于滴定的NaOH的量。滴定步长为0.25ml。通过Bjerrum曲线为复合物生成的函数的每个半整数值确定lgKn的值。
当今,Bjerrum法被频繁地用于研究复合物形成过程。这种方法提供了双参数的确定:在平衡条件下的游离配体的浓度([L-])和复合物形成的函数其为包含于复合物中的配体的平均数。因此,使用实验数据,可以为滴定的每个时间点计算值[L-](具体地,-lg[L-]=pA)并且可以通过等式(1)计算当配体具有多个解离基团时,等式(1)中的KL值将解离常数的值与最大pk值匹配。
从根据复合物形成的函数的每个半整数值的等式(2)绘制的Bjerrum曲线(复合物形成的曲线)发现该Kn值。因此,该步稳常数将等于在这一点的游离配体的浓度的倒数。因此,Kn值越大,络合化合物(螯合物)越稳定。
其中CL -和CM z+为在溶液中存在的配体和金属的总浓度,mol/g;
[L-]为游离配体离子的浓度,mol/g;
KL为配体的酸解离常数;
nL和nM z+为配体化合物和Mz+盐在滴定过的溶液中的量,mol。
表4和表5中给出了通过Bjerrum法发现的所研究的化合物与二价金属的复合物的lgK1值。1:6的Mz+/L比率基于关于Zn(II)和Fe(II)的最大可能的配位数(等于六)和Cu(II)的最大可能的配位数(等于四)的数据[Claudia A.Blindauer,M.Tahir Razi,SimonParsons,Peter J.Sadler,Polyhedron,2006,v.25,p.513-520]。
表4
由Bjerrum法发现的具有1:1研究化合物与二价金属离子的组成的复合物的第一稳定常数(lgK1)(水溶液,CL=0.02M;Mz+/L=1:6;离子强度=0.5(KNO3),22℃)。
表5
通过Bjerrum法发现的具有1:1研究化合物与锌离子的比率的复合物的步稳常数(水溶液,CL=0.02M;Mz+/L=1:6;离子强度=0.5(KNO3),22℃)。
因此,该研究已经证明,所研究的化合物(特别是二羧酸双酰胺衍生物)是有效的复合物形成剂,已经发现它们的大多数对于过渡金属离子具有lgK1≥5的高值。此外,已经证明,所要求保护的通式I的化合物表现出略有提高的与铁离子形成复合物的能力。
Claims (59)
3.根据权利要求2所述的用途,所述二羧酸双酰胺衍生物能够螯合Zn、Cu、Fe、Mg和Ca的离子。
5.根据权利要求4所述的药剂,其中所述病毒性疾病是由丙型肝炎病毒、人乳头瘤病毒、HIV或致癌RNA病毒引起的。
6.根据权利要求5所述的药剂,其中所述病毒性疾病是由白血病病毒引起的。
8.根据权利要求7所述的药剂,其中所述心血管疾病是由抑制细胞生长的心脏毒性、胆固醇沉积和高血压引起的。
10.根据权利要求9所述的药剂,用于预防和/或治疗与金属依赖性自由基氧化反应有关的疾病。
11.根据权利要求10所述的药剂,其中所述与金属依赖性自由基氧化反应有关的疾病是与年龄有关的疾病;中风后遗症;动脉粥样硬化症或炎症性疾病。
12.根据权利要求10所述的药剂,其中所述与金属依赖性自由基氧化反应有关的疾病是白内障、视黄醛性疾病、皮肤色素沉着、骨关节炎或类风湿性关节炎。
15.根据权利要求14所述的药剂,其中所述神经退行性疾病为阿耳茨海默氏病、帕金森氏症、威尔逊氏病、亨廷顿氏病、肌萎缩侧索硬化或朊病毒病。
18.根据权利要求17所述的药剂,其中所述由微生物毒素引起的疾病是肉毒中毒或气性坏疽。
23.根据权利要求22所述的药物组合物,其中所述病毒性疾病是由丙型肝炎病毒、人乳头瘤病毒、HIV或致癌RNA病毒引起的。
24.根据权利要求22所述的药物组合物,其中所述病毒性疾病是由白血病病毒引起的。
26.根据权利要求25所述的药物组合物,其中所述心血管疾病是由抑制细胞生长的心脏毒性、胆固醇沉积或高血压引起的。
28.根据权利要求27所述的药物组合物,用于预防和/或治疗与金属依赖性自由基氧化反应有关的疾病。
29.根据权利要求28所述的药物组合物,其中所述与金属依赖性自由基氧化反应有关的疾病是与年龄有关的疾病;中风后遗症;动脉粥样硬化症或炎症性疾病。
30.根据权利要求28所述的药物组合物,其中所述与金属依赖性自由基氧化反应有关的疾病是白内障、视黄醛性疾病、皮肤色素沉着、骨关节炎或类风湿性关节炎。
33.根据权利要求32所述的药物组合物,其中所述神经退行性疾病是阿耳茨海默氏病、帕金森氏症、威尔逊氏病、亨廷顿氏病、肌萎缩侧索硬化或朊病毒病。
36.根据权利要求35所述的药物组合物,其中所述由微生物毒素引起的疾病是肉毒中毒或气性坏疽。
39.一种用于预防和/或治疗过渡金属盐中毒的药物组合物,包含有效量的权利要求1所述的衍生物或其药用盐。
41.根据权利要求40所述的用途,其中所述病毒性疾病是由丙型肝炎病毒、人乳头瘤病毒、HIV或致癌RNA病毒引起的。
42.根据权利要求41所述的用途,其中所述病毒性疾病是由白血病病毒引起的。
44.根据权利要求43所述的用途,其中所述心血管疾病是由抑制细胞生长的心脏毒性、胆固醇沉积或高血压引起的。
46.根据权利要求45所述的用途,用于预防和/或治疗与金属依赖性自由基氧化反应有关的疾病。
47.根据权利要求46所述的用途,其中所述与金属依赖性自由基氧化反应有关的疾病是与年龄有关的疾病;中风后遗症;动脉粥样硬化症或炎症性疾病。
48.根据权利要求46所述的用途,其中所述与金属依赖性自由基氧化反应有关的疾病是白内障、视黄醛性疾病、皮肤色素沉着、骨关节炎或类风湿性关节炎。
51.根据权利要求50所述的用途,其中所述神经退行性疾病为阿耳茨海默氏病、帕金森氏症、威尔逊氏病、亨廷顿氏病、肌萎缩侧索硬化或朊病毒病。
54.根据权利要求53所述的用途,其中所述由微生物毒素引起的疾病是肉毒中毒或气性坏疽。
59.根据权利要求58所述的方法,其中加热步骤是在150-170℃的温度进行的。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| RU2013116822 | 2013-04-12 | ||
| RU2013116822A RU2665688C2 (ru) | 2013-04-12 | 2013-04-12 | Производные бисамидов дикарбоновых кислот, их применение, фармацевтическая композиция на их основе, способы их получения |
| CN201480027307.9A CN105358548A (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途、基于其的药物组合物和用于生产二羧酸双酰胺衍生物的方法 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201480027307.9A Division CN105358548A (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途、基于其的药物组合物和用于生产二羧酸双酰胺衍生物的方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109096198A CN109096198A (zh) | 2018-12-28 |
| CN109096198B true CN109096198B (zh) | 2022-03-08 |
Family
ID=51689820
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201811167661.8A Active CN109096198B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
| CN201480027307.9A Pending CN105358548A (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途、基于其的药物组合物和用于生产二羧酸双酰胺衍生物的方法 |
| CN201811168167.3A Active CN109134378B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
| CN201811167688.7A Active CN109096199B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
| CN201811167650.XA Active CN109336819B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
| CN201811167690.4A Active CN109096200B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
Family Applications After (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201480027307.9A Pending CN105358548A (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途、基于其的药物组合物和用于生产二羧酸双酰胺衍生物的方法 |
| CN201811168167.3A Active CN109134378B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
| CN201811167688.7A Active CN109096199B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
| CN201811167650.XA Active CN109336819B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
| CN201811167690.4A Active CN109096200B (zh) | 2013-04-12 | 2014-04-10 | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 |
Country Status (26)
| Country | Link |
|---|---|
| US (3) | US20160031858A1 (zh) |
| EP (1) | EP2985284B1 (zh) |
| JP (1) | JP6760837B2 (zh) |
| KR (1) | KR102218134B1 (zh) |
| CN (6) | CN109096198B (zh) |
| AU (1) | AU2014251443C1 (zh) |
| BR (1) | BR112015025675B1 (zh) |
| CA (1) | CA2909411C (zh) |
| CY (1) | CY1125062T1 (zh) |
| DK (1) | DK2985284T3 (zh) |
| EA (6) | EA202092453A3 (zh) |
| ES (1) | ES2904568T3 (zh) |
| HK (1) | HK1221223A1 (zh) |
| HR (1) | HRP20220103T1 (zh) |
| HU (1) | HUE057209T2 (zh) |
| IL (1) | IL242029B (zh) |
| LT (1) | LT2985284T (zh) |
| MX (2) | MX2015014213A (zh) |
| PL (1) | PL2985284T3 (zh) |
| PT (1) | PT2985284T (zh) |
| RS (1) | RS62889B1 (zh) |
| RU (1) | RU2665688C2 (zh) |
| SG (2) | SG10202008505VA (zh) |
| SI (1) | SI2985284T1 (zh) |
| UA (1) | UA119748C2 (zh) |
| WO (1) | WO2014168523A1 (zh) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2665688C2 (ru) * | 2013-04-12 | 2018-09-04 | Общество С Ограниченной Ответственностью "Фарминтерпрайсез" | Производные бисамидов дикарбоновых кислот, их применение, фармацевтическая композиция на их основе, способы их получения |
| BR122020010204B1 (pt) * | 2013-04-12 | 2022-03-03 | Obschestvo S Ogranichennoi Otvetstvennostiyu "Pharmenterprises" | Composto derivado de glutarimida, seu uso, composiçâo farmacêutica e método de de preparação |
| RU2647438C2 (ru) * | 2015-05-27 | 2018-03-15 | Общество С Ограниченной Ответственностью "Фарминтерпрайсез" | Бисамидное производное дикарбоновой кислоты в качестве средства, стимулирующего регенерацию тканей и восстановление сниженных функций тканей |
| RU2662559C1 (ru) * | 2017-10-27 | 2018-07-26 | Общество С Ограниченной Ответственностью "Фарминтерпрайсез" | Новый ингибитор глутаминилциклаз и его применение для лечения заболеваний легких и дыхательных путей |
| RU2665633C1 (ru) * | 2017-05-26 | 2018-09-03 | Общество С Ограниченной Ответственностью "Фарминтерпрайсез" | Новый ингибитор глутаминилциклаз и его применение |
| KR102714537B1 (ko) * | 2017-05-26 | 2024-10-11 | 엘티디 "발렌타-인텔렉트" | 신규한 글루타미닐 고리화효소 억제제 및 다양한 질환의 치료에서의 이의 용도 |
| US11479532B2 (en) | 2017-06-23 | 2022-10-25 | University Of South Florida | 5-aminolevulinate synthase inhibitors and methods of use thereof |
| RU2685277C1 (ru) * | 2018-05-24 | 2019-04-17 | Общество с ограниченной ответственностью "Фарминтерпрайсез Аллерджи" | Применение бисамидного производного малоновой кислоты для лечения аллергических и других заболеваний человека и животных |
| RU2679636C1 (ru) * | 2018-10-29 | 2019-02-12 | Общество С Ограниченной Ответственностью "Фарминтерпрайсез" | Новый способ получения n,n'-бис[2-(1н-имидазол-4-ил)этил]малонамида |
| RU2745265C2 (ru) * | 2019-09-12 | 2021-03-22 | Общество С Ограниченной Ответственностью "Валента - Интеллект" | Новые составы 2-(имидазол-4-ил)-этанамида пентандиовой-1,5 кислоты для лечения и профилактики вирусных заболеваний |
| RU2746692C1 (ru) * | 2020-04-14 | 2021-04-19 | Общество С Ограниченной Ответственностью "Валента - Интеллект" | Новые составы 2-(имидазол-4-ил)-этанамида пентандиовой-1,5 кислоты для лечения и профилактики вирусных заболеваний |
| WO2021049980A1 (ru) * | 2019-09-12 | 2021-03-18 | Общество С Ограниченной Ответственностью "Валента-Интеллект" | Новые составы для лечения и профилактики вирусных заболеваний |
| WO2023177328A1 (en) | 2022-03-17 | 2023-09-21 | Treamid Therapeutics Gmbh | Bisamide derivative of dicarboxylic acid for use in restoring the external respiratory function after coronavirus infection |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6169759B1 (ja) * | 2016-07-11 | 2017-07-26 | 株式会社山寿セラミックス | 弾性表面波素子用基板及びその製造方法 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6169759A (ja) * | 1984-09-14 | 1986-04-10 | Agency Of Ind Science & Technol | タンパク質の架橋反応用修飾剤及びそれを用いる方法 |
| JPS6344567A (ja) * | 1986-08-08 | 1988-02-25 | Shikoku Chem Corp | 新規なイミダゾール系ジアミド化合物、該化合物の合成方法および該化合物を有効成分とするポリエポキシ樹脂硬化剤 |
| IL86131A0 (en) * | 1987-04-24 | 1988-11-15 | Boehringer Ingelheim Kg | Benzo-and thieno-3,4-dihydropyridine derivatives,their preparation and pharmaceutical compositions containing them |
| AU2001283740A1 (en) * | 2000-08-17 | 2002-02-25 | University Of British Columbia | Chemotherapeutic agents conjugated to p97 and their methods of use in treating neurological tumours |
| RU2287524C1 (ru) * | 2005-03-31 | 2006-11-20 | ООО "Фарминтерпрайсез" | Аспартильные производные гистамина, способ их получения, фармацевтическая композиция и их применение в качестве модуляторов активности ферментов антиоксидантной защиты |
| RU2335495C2 (ru) * | 2005-06-15 | 2008-10-10 | Общество С Ограниченной Ответственностью "Фарминтерпрайсез" | N-ацильные производные аминокислот, их фармацевтически приемлевые соли, фармацевтическая композиция и применение в качестве гиполипидемических средств |
| CN1919884A (zh) * | 2005-08-26 | 2007-02-28 | 建筑技术研究有限公司 | 基于氧化烯二醇烯基醚和不饱和二羧酸衍生物的共聚物 |
| AU2006315467B2 (en) * | 2005-11-14 | 2013-02-07 | Curis, Inc. | Bisamide inhibitors of hedgehog signaling |
| EP1884515A1 (en) * | 2006-07-31 | 2008-02-06 | Laboratorios del Dr. Esteve S.A. | Substituted indanyl sulfonamide compounds, their preparation and use as medicaments |
| JP6079148B2 (ja) * | 2012-11-07 | 2017-02-15 | Jsr株式会社 | 液晶配向剤、液晶配向膜及び液晶表示素子 |
| RU2665688C2 (ru) * | 2013-04-12 | 2018-09-04 | Общество С Ограниченной Ответственностью "Фарминтерпрайсез" | Производные бисамидов дикарбоновых кислот, их применение, фармацевтическая композиция на их основе, способы их получения |
-
2013
- 2013-04-12 RU RU2013116822A patent/RU2665688C2/ru active
-
2014
- 2014-04-10 HU HUE14782759A patent/HUE057209T2/hu unknown
- 2014-04-10 BR BR112015025675-9A patent/BR112015025675B1/pt active IP Right Grant
- 2014-04-10 CA CA2909411A patent/CA2909411C/en active Active
- 2014-04-10 EA EA202092453A patent/EA202092453A3/ru unknown
- 2014-04-10 EA EA202190774A patent/EA202190774A3/ru unknown
- 2014-04-10 JP JP2016507513A patent/JP6760837B2/ja active Active
- 2014-04-10 EA EA202190775A patent/EA202190775A1/ru unknown
- 2014-04-10 CN CN201811167661.8A patent/CN109096198B/zh active Active
- 2014-04-10 PT PT147827596T patent/PT2985284T/pt unknown
- 2014-04-10 HR HRP20220103TT patent/HRP20220103T1/hr unknown
- 2014-04-10 ES ES14782759T patent/ES2904568T3/es active Active
- 2014-04-10 SG SG10202008505VA patent/SG10202008505VA/en unknown
- 2014-04-10 LT LTEPPCT/RU2014/000265T patent/LT2985284T/lt unknown
- 2014-04-10 EA EA201992275A patent/EA039440B1/ru unknown
- 2014-04-10 EP EP14782759.6A patent/EP2985284B1/en active Active
- 2014-04-10 RS RS20220090A patent/RS62889B1/sr unknown
- 2014-04-10 CN CN201480027307.9A patent/CN105358548A/zh active Pending
- 2014-04-10 EA EA201501014A patent/EA036961B1/ru unknown
- 2014-04-10 UA UAA201510873A patent/UA119748C2/uk unknown
- 2014-04-10 KR KR1020157032328A patent/KR102218134B1/ko active IP Right Grant
- 2014-04-10 CN CN201811168167.3A patent/CN109134378B/zh active Active
- 2014-04-10 MX MX2015014213A patent/MX2015014213A/es unknown
- 2014-04-10 SG SG11201508342RA patent/SG11201508342RA/en unknown
- 2014-04-10 CN CN201811167688.7A patent/CN109096199B/zh active Active
- 2014-04-10 PL PL14782759T patent/PL2985284T3/pl unknown
- 2014-04-10 DK DK14782759.6T patent/DK2985284T3/da active
- 2014-04-10 EA EA202092452A patent/EA202092452A3/ru unknown
- 2014-04-10 CN CN201811167650.XA patent/CN109336819B/zh active Active
- 2014-04-10 AU AU2014251443A patent/AU2014251443C1/en active Active
- 2014-04-10 SI SI201431938T patent/SI2985284T1/sl unknown
- 2014-04-10 CN CN201811167690.4A patent/CN109096200B/zh active Active
- 2014-04-10 WO PCT/RU2014/000265 patent/WO2014168523A1/ru active Application Filing
-
2015
- 2015-10-08 MX MX2020013371A patent/MX2020013371A/es unknown
- 2015-10-09 US US14/879,648 patent/US20160031858A1/en not_active Abandoned
- 2015-10-12 IL IL242029A patent/IL242029B/en active IP Right Grant
-
2016
- 2016-08-08 HK HK16109432.9A patent/HK1221223A1/zh unknown
-
2021
- 2021-11-18 US US17/530,143 patent/US20220324843A1/en active Pending
-
2022
- 2022-02-03 CY CY20221100101T patent/CY1125062T1/el unknown
- 2022-12-21 US US18/086,141 patent/US20230203016A1/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6169759B1 (ja) * | 2016-07-11 | 2017-07-26 | 株式会社山寿セラミックス | 弾性表面波素子用基板及びその製造方法 |
Non-Patent Citations (5)
| Title |
|---|
| A zinc chelator inhibiting gelatinases exerts potent in vitro anti-invasive effects;Gilles Ferry et al.;《European Journal of Pharmacology》;19981231;第351卷;第225-233页 * |
| Lipophilic Complexones. Part 6. Synthesis of Di- and Triarmed Ligands containing Pyridine and Imidazole Moieties;Anil Gupta et al.;《J. Chem. Research(s)》;19941231(第6期);第1117-1133页 * |
| Preparation of an Imidazole-Conjugated Oligonucleotide;Marc Beban et al.;《Bioconjugate Chemistry》;20000613;第11卷(第4期);第599-603页 * |
| Preparation of certain bis-compounds derived from β-(2-thienyl)ethylamine and β-(2-thienyl)isopropyllamine;Tadeusz Juliusz Burakowski et al.;《Acta Poloniae Pharmaceutica》;19781231;第35卷(第2期);第175-179页 * |
| Synthetic RNA-cleaving molecules mimicking ribonuclease A active center. Design and cleavage of tRNA transcripts;Mikhail A.Podyminogin et al;《Nucleic Acids Research》;19931231;第21卷(第25期);第5950-5956页 * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109096198B (zh) | 二羧酸双酰胺衍生物、其用途和基于其的药物组合物 | |
| MX2010013773A (es) | Compuestos de 2,4'-bipiridinilo como inhibidores de cinasa d de proteina utiles para el tratamiento de, entre otras, insuficiencia cardiaca ia y cancer. | |
| AU2014225889B2 (en) | CaMKII inhibitors and uses thereof | |
| AU2022231918B2 (en) | Triazole derivative, preparation method therefor, and application thereof | |
| CA2993519C (en) | Fto inhibitors | |
| US20230099089A1 (en) | Antiviral substances with a wide spectrum of activity | |
| RU2719464C2 (ru) | Производное бисамидов дикарбоновых кислот, его применение, фармацевтическая композиция на его основе, способы его получения | |
| RU2709529C1 (ru) | Производные бисамидов дикарбоновых кислот, их применение, фармацевтическая композиция на их основе, способы их получения | |
| RU2725770C2 (ru) | Производное бисамидов дикарбоновых кислот, его применение, фармацевтическая композиция на его основе, способы его получения | |
| RU2721421C2 (ru) | Производное бисамидов дикарбоновых кислот, его применение, фармацевтическая композиция на его основе, способы его получения | |
| RU2725881C2 (ru) | Производное бисамидов дикарбоновых кислот, его применение, фармацевтическая композиция на его основе, способы его получения | |
| RU2815401C2 (ru) | Производное бисамидов дикарбоновых кислот, его применение, фармацевтическая композиция на его основе, способы его получения | |
| EA043024B1 (ru) | Производное бисамидов дикарбоновых кислот и способы его получения | |
| EA045515B1 (ru) | Производные бисамидов дикарбоновых кислот, их применение, фармацевтическая композиция на их основе, способы их получения | |
| Moianos et al. | N-Hydroxypiridinedione: A Privileged Heterocycle for Targeting the HBV RNase H | |
| EA043025B1 (ru) | Производное бисамида дикарбоновой кислоты и способы его получения | |
| EA045675B1 (ru) | Способы получения бисамида дикарбоновой кислоты | |
| EA043680B1 (ru) | Производное бисамидов дикарбоновых кислот, его применение, фармацевтическая композиция на его основе |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 40002625 Country of ref document: HK |
|
| GR01 | Patent grant | ||
| GR01 | Patent grant |