CN107137403B - Application of PI3K/MTOR inhibitor in preparation of medicine for treating pancreatic cancer - Google Patents
Application of PI3K/MTOR inhibitor in preparation of medicine for treating pancreatic cancer Download PDFInfo
- Publication number
- CN107137403B CN107137403B CN201710116510.9A CN201710116510A CN107137403B CN 107137403 B CN107137403 B CN 107137403B CN 201710116510 A CN201710116510 A CN 201710116510A CN 107137403 B CN107137403 B CN 107137403B
- Authority
- CN
- China
- Prior art keywords
- pi3k
- pancreatic cancer
- mtor inhibitor
- compound
- inhibitor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/444—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring heteroatom, e.g. amrinone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/50—Pyridazines; Hydrogenated pyridazines
- A61K31/5025—Pyridazines; Hydrogenated pyridazines ortho- or peri-condensed with heterocyclic ring systems
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The invention relates to application of a PI3K/MTOR inhibitor in preparing a medicament for treating pancreatic cancer. The PI3K/MTOR inhibitor can be used in combination with a Hedgehog inhibitor. The invention also relates to a pharmaceutical composition containing the two inhibitors.
Description
Technical Field
The invention relates to application of a PI3K/MTOR inhibitor in preparing a medicament for treating pancreatic cancer.
Background
Pancreatic cancer is a malignant tumor of the digestive tract that is highly malignant and difficult to diagnose and treat, and about 90% of pancreatic cancers are ductal adenocarcinomas originating from the epithelium of the glandular duct. The incidence and mortality of the Chinese pancreatic cancer are obviously increased in 2000-2011. Survival rate < 1% for 5 years is one of the worst-prognosis malignancies. The early diagnosis rate of pancreatic cancer is low, the operative mortality rate is high, and the cure rate is low. The incidence rate of the disease is higher for men than for women, the ratio of men to women is 1.5-2: 1, male patients are far more common than women before menopause, and the incidence rate of postmenopausal women is similar to that of men.
CN103282363A discloses a PI3K/mTOR dual inhibitor, which has strong inhibition effect on PI3K and mTOR kinase, the compounds are shown in the following formula (I) and have the chemical name of 1-methyl-3- [5- [ 3-methyl-2-oxygen-1- [3- (trifluoromethyl) phenyl ] -2, 3-dihydro-1H-imidazo [4,5-c ] quinolin-8-yl ] pyridin-2-yl ] urea, and the possibility of application of the compounds in treating melanoma, papillary thyroid tumors, cholangiocarcinoma, colon cancer, ovarian cancer, lung cancer, malignant lymphoma, liver, kidney, bladder, prostate, breast and pancreatic cancers and sarcomas, primary and recurrent solid tumors of skin, colon, thyroid, lung and ovary or leukemia is also indicated.
CN103261198A discloses a class of Hedgehog signaling pathway inhibitors, which includes compounds of the following formula (II) with the chemical name N- (2-ethyl-5- (6- (2- (trifluoromethyl) pyridin-3-yl) imidazo [1,2-b ] pyridazin-2-yl) phenyl) -1-methylcyclopropylformamide, and discloses that the Hedgehog signaling pathway may be associated with glioblastoma, basal cell carcinoma and pancreatic cancer.
None of the above documents disclose the use of specific combinations of PI3K/mTOR with Hedgehog inhibitors for the treatment of pancreatic cancer.
Disclosure of Invention
The invention surprisingly discovers that the PI3K/mTOR inhibitor has good treatment effect on pancreatic cancer. In a preferred embodiment of the invention, the PI3K/mTOR inhibitor is a compound of formula (I) as follows or a pharmaceutically acceptable salt thereof. In the present invention, the amount of said PI3K/MTOR inhibitor may be in the range of 0.1 to 1000mg/kg, preferably 1 to 100mg/kg, more preferably 5 to 20mg/kg, said amount being measured as compound (I).
In a preferred embodiment of the present invention, the PI3K/MTOR inhibitor is used in combination with a Hedgehog signaling pathway inhibitor, which is more effective for pancreatic cancer, and particularly preferably, the Hedgehog signaling pathway inhibitor is a compound represented by the following formula (II) or a pharmaceutically acceptable salt thereof. In the present invention, the amount of said Hedgehog signaling pathway inhibitor may be in the range of 1-1000 mg/kg, preferably 5-500 mg/kg, more preferably 10-200 mg/kg, most preferably 20-150 mg/kg, said amount being measured as compound (II). In the present invention, the combined use means that two or more drugs are administered to a subject in the same administration cycle, but whether they are administered simultaneously or not is not limited, and may be preceded and followed by a certain time interval.
The pharmaceutically acceptable salts of the compounds of formula (I) and formula (II) in the present invention may be selected from various inorganic or organic acid salts known in the art.
The compounds of formula (I) and (II) or their pharmaceutically acceptable salts can also be formulated with pharmaceutically acceptable carriers into compositions well known in the art, such as tablets, capsules, granules, injections, etc. The invention also relates to the use of a composition containing a compound selected from formula (I) and formula (II) or a pharmaceutically acceptable salt thereof, for the preparation of a medicament for the treatment of pancreatic cancer as described above.
Drawings
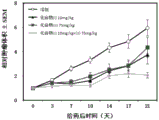
FIG. 1 shows the therapeutic effect of compounds (I), (II) alone or in combination on human pancreatic cancer BXPC-3 nude mouse transplantable tumors.
FIG. 2 shows the therapeutic effect of compounds (I), (II) alone or in combination on human pancreatic cancer BXPC-3 nude mouse transplantable tumors (tumor photographs).
Detailed Description
The present invention will be further described with reference to the following examples, which are not intended to limit the scope of the present invention.
Example 1: evaluating the efficacy of the compound (I) and the compound (II) alone or in combination on human pancreatic cancer BXPC-3 nude mouse transplantable tumors.
1 test drugs
The test compound (I) and the compound (II) were synthesized in accordance with the disclosures of CN103282363A and CN103261198A, respectively. The preparation method comprises the following steps: compound (I) was formulated with 70% PEG 400 and compound (II) was formulated with 30% PEG 400.
2 laboratory animals
BALB/cA-nude mice, 6-7 weeks old, purchased from Shanghai Spiker laboratory animals, Inc. Certificate number: SCXK (Shanghai) 2007 & 0005. A breeding environment: SPF grade.
3 Experimental procedures
The nude mouse is inoculated with BXPC-3 cells of human pancreatic cancer subcutaneously until the tumor grows to 100-200mm3Thereafter, the animals were randomly assigned (D0). The dosage and schedule of administration are shown in table 1. Tumor volumes were measured 2-3 times a week, mice weighed, and data recorded. Tumor volume (V) was calculated as:
V=1/2×a×b2wherein a and b represent length and width, respectively.
T/C(%)=(T-T0)/(C-C0) X 100 where T, C is the tumor volume at the end of the experiment; t is0、C0Tumor volume at the beginning of the experiment.
4 results
The compound (II) (75mg/kg, PO, QD X16) and the compound (I) (10mg/kg, PO, QD X16) have certain inhibition effect on the growth of human pancreatic cancer BxPC-3 nude mouse transplanted tumor, and the inhibition rates are 33% and 40% respectively; the combination of the two drugs has obviously enhanced tumor inhibition effect, and the tumor inhibition rate reaches 77 percent, which is obviously better than the curative effect of the single use of the two drugs).
TABLE 1 curative effects of Compound (I), Compound (II) alone or in combination on human pancreatic cancer BxPC-3 nude mouse transplantable tumor
D0 time to first dose. P-value refers to comparison to control; p <0.05vs compound (I) + compound (II), Student's t' assay. Control group n is 10 and treatment group n is 6.
5 conclusion
The compounds (I) and (II) have certain inhibition effect on the growth of human pancreatic cancer BxPC-3 nude mouse transplantation tumor when used singly, and the inhibition effect is obviously enhanced when the compounds (I) and (II) are used together.
Claims (9)
- Use of a PI3K/MTOR inhibitor and a Hedgehog signaling pathway inhibitor in the preparation of a medicament for treating pancreatic cancer, wherein the PI3K/MTOR inhibitor is a compound represented by formula (I) or a pharmaceutically acceptable salt thereof,the Hedgehog signal pathway inhibitor is a compound shown as a formula (II) or a pharmaceutically acceptable salt thereof,
- 2. the use according to claim 1, wherein the amount of PI3K/MTOR inhibitor is 0.1-1000 mg/kg.
- 3. The use according to claim 2, wherein the amount of PI3K/MTOR inhibitor is 1-100 mg/kg.
- 4. The use according to claim 2, wherein the amount of PI3K/MTOR inhibitor is 5-20 mg/kg.
- 5. The use of claim 1, wherein the Hedgehog signaling pathway inhibitor is present in an amount of 1-1000 mg/kg.
- 6. The use of claim 5, wherein the Hedgehog signaling pathway inhibitor is present in an amount of 5-500 mg/kg.
- 7. The use of claim 5, wherein the Hedgehog signaling pathway inhibitor is present in an amount of 10-200 mg/kg.
- 8. The use of claim 5, wherein the Hedgehog signaling pathway inhibitor is present in an amount of 20-150 mg/kg.
- 9. A pharmaceutical composition for treating pancreatic cancer, comprising a compound of formula (I) or a pharmaceutically acceptable salt thereof as set forth in claim 1 and a compound of formula (II) or a pharmaceutically acceptable salt thereof as set forth in claim 1, and a pharmaceutically acceptable carrier.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610115807 | 2016-03-01 | ||
| CN2016101158079 | 2016-03-01 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107137403A CN107137403A (en) | 2017-09-08 |
| CN107137403B true CN107137403B (en) | 2021-07-02 |
Family
ID=59784087
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201710116510.9A Active CN107137403B (en) | 2016-03-01 | 2017-02-28 | Application of PI3K/MTOR inhibitor in preparation of medicine for treating pancreatic cancer |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN107137403B (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103030637A (en) * | 2011-10-10 | 2013-04-10 | 上海恒瑞医药有限公司 | Imidazole quinoline derivative, and pharmaceutically acceptable salts thereof, preparation method thereof and application thereof on medicines |
| CN103261198A (en) * | 2010-12-22 | 2013-08-21 | 江苏恒瑞医药股份有限公司 | 2-arylimidazo[1,2-]pyridazine, 2-phenylimidazo[1,2-a]pyridine, and 2-phenylimidazo[1,2-a]pyrazine derivatives |
-
2017
- 2017-02-28 CN CN201710116510.9A patent/CN107137403B/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103261198A (en) * | 2010-12-22 | 2013-08-21 | 江苏恒瑞医药股份有限公司 | 2-arylimidazo[1,2-]pyridazine, 2-phenylimidazo[1,2-a]pyridine, and 2-phenylimidazo[1,2-a]pyrazine derivatives |
| CN103030637A (en) * | 2011-10-10 | 2013-04-10 | 上海恒瑞医药有限公司 | Imidazole quinoline derivative, and pharmaceutically acceptable salts thereof, preparation method thereof and application thereof on medicines |
| WO2013053273A1 (en) * | 2011-10-10 | 2013-04-18 | 上海恒瑞医药有限公司 | Imidazo quinoline derivative and medicinal salt thereof, preparation method thereof and use in medicine thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107137403A (en) | 2017-09-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20210100813A1 (en) | Combination therapy for cancer using bromodomain and extra-terminal (bet) protein inhibitors | |
| JP6648040B2 (en) | Use of eribulin and poly (ADP-ribose) polymerase (PARP) inhibitors as combination therapy for cancer treatment | |
| KR101848131B1 (en) | Low-dose antitumor agent including irinotecan hydrochloride hydrate | |
| KR20180048804A (en) | Combination therapy with baritinib and anticancer drugs | |
| KR20170017932A (en) | Intermittent dosing of mdm2 inhibitor | |
| WO2015061832A1 (en) | Pharmaceutical combinations for the treatment of cancer | |
| CN107137407B (en) | Application of VEGFR inhibitor in preparation of medicine for treating pancreatic cancer | |
| WO2016081773A2 (en) | Combination cancer therapy with c-met inhibitors and synthetic oligonucleotides | |
| KR102382771B1 (en) | Combination Therapy for Proliferative Diseases | |
| JP2016520665A (en) | A combination for the treatment of cancer comprising a Mps-1 kinase inhibitor and a mitosis inhibitor | |
| KR101847252B1 (en) | Antitumor agent including irinotecan hydrochloride hydrate | |
| CN107137403B (en) | Application of PI3K/MTOR inhibitor in preparation of medicine for treating pancreatic cancer | |
| TW201821107A (en) | Combination use of VEGFR inhibitor and PARP inhibitor in the preparation of a medicament for the treatment of gastric cancer | |
| CN111514140A (en) | Application of MEK inhibitor and androgen receptor antagonist in preparation of tumor treatment drug | |
| MX2010009795A (en) | Methods to inhibit tumor cell growth by using proton pump inhibitors. | |
| KR101925553B1 (en) | Pharmaceutical composition for anti-cancer comprising a complex composition with PI3-kinase inhibitor and doxorubicin and preparation method of thereof | |
| CN112237586A (en) | Application of rhizoma paridis saponin and sorafenib in preparation of anti-tumor combined medicine | |
| CN111494371B (en) | Application of Pyr3 | |
| CN107137406B (en) | Application of Hedgehog signal pathway inhibitor in preparation of medicine for treating EGFR (epidermal growth factor receptor) over-expression cancer | |
| CN111939165B (en) | Application of non-natural ginsenoside 3 beta-O-Glc-DM in preparation of medicine for preventing or treating glioblastoma | |
| CN106176757B (en) | application of combination of compound and tegafur in preparation of medicine for treating proliferative diseases | |
| WO2009047291A2 (en) | Use of quaternary pyridinium salts for inhibiting cancer metastases | |
| RU2021118927A (en) | COMPOSITIONS AND METHODS FOR REDUCING SIGNIFICANT THROMBOTIC EVENTS IN CANCER PATIENTS | |
| TW201943687A (en) | Composition comprising 4-phenylbutyric acid derivatives & opioids | |
| NZ786609A (en) | Combination therapy with notch and pi3k/mtor inhibitors for use in treating cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |