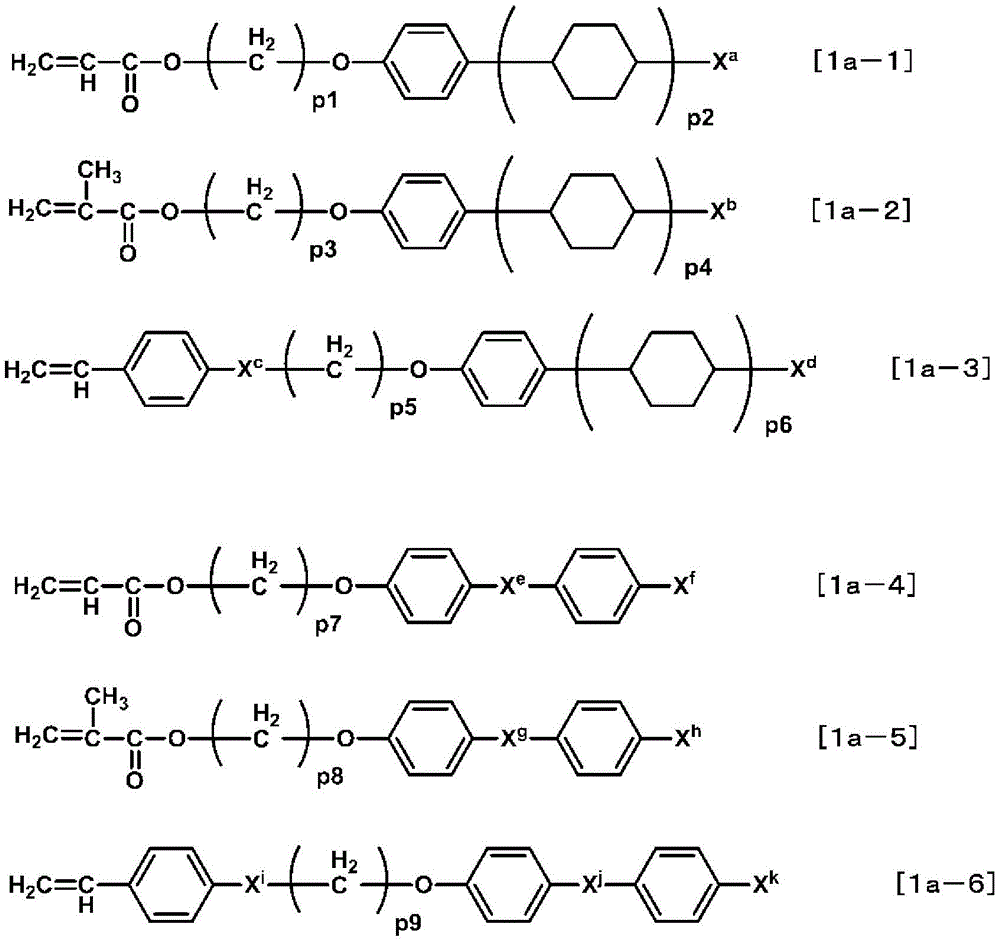CN107003570B - Liquid crystal display element - Google Patents
Liquid crystal display element Download PDFInfo
- Publication number
- CN107003570B CN107003570B CN201580063916.4A CN201580063916A CN107003570B CN 107003570 B CN107003570 B CN 107003570B CN 201580063916 A CN201580063916 A CN 201580063916A CN 107003570 B CN107003570 B CN 107003570B
- Authority
- CN
- China
- Prior art keywords
- liquid crystal
- group
- formula
- carbon atoms
- display element
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2/00—Processes of polymerisation
- C08F2/44—Polymerisation in the presence of compounding ingredients, e.g. plasticisers, dyestuffs, fillers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G73/00—Macromolecular compounds obtained by reactions forming a linkage containing nitrogen with or without oxygen or carbon in the main chain of the macromolecule, not provided for in groups C08G12/00 - C08G71/00
- C08G73/06—Polycondensates having nitrogen-containing heterocyclic rings in the main chain of the macromolecule
- C08G73/10—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G77/00—Macromolecular compounds obtained by reactions forming a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon in the main chain of the macromolecule
- C08G77/04—Polysiloxanes
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1334—Constructional arrangements; Manufacturing methods based on polymer dispersed liquid crystals, e.g. microencapsulated liquid crystals
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1337—Surface-induced orientation of the liquid crystal molecules, e.g. by alignment layers
Landscapes
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Nonlinear Science (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Polymers & Plastics (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Optics & Photonics (AREA)
- Crystallography & Structural Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Mathematical Physics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Dispersion Chemistry (AREA)
- Liquid Crystal (AREA)
- Polymerisation Methods In General (AREA)
- Macromolecular Compounds Obtained By Forming Nitrogen-Containing Linkages In General (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
Abstract
The invention provides a liquid crystal display element which has high vertical alignment of liquid crystal, good transparency when no voltage is applied and scattering property when voltage is applied, and high adhesion with a liquid crystal layer. A liquid crystal display element comprising a liquid crystal layer between a pair of substrates having electrodes, at least one of the substrates having a liquid crystal alignment film for vertically aligning the liquid crystal, wherein the liquid crystal layer is obtained by curing a liquid crystal composition comprising a liquid crystal and a polymerizable compound, which is disposed between the pair of substrates, by irradiating the liquid crystal composition with ultraviolet light, and the liquid crystal composition contains a compound represented by the formula [1]]The compound, wherein the liquid crystal alignment film is obtained from a liquid crystal alignment treatment agent containing a polymer having the formula [2-1]]Or formula [2-2]Side chain structures as shown. (X)1: formula [1-a]-formula [1-g]Etc.; x2、X3、X4And X6: a single bond, etc.; x5And X7: benzene rings, etc.; x8:C1~18Alkyl of (e) (X) and the like)A: h, etc.; xB: benzene rings, etc.; xC:C1~18Alkyl of (e) (Y) and the like) (Y1、Y2And Y3: a single bond, etc.; y is4And Y5: benzene rings, etc.; y is6:C1~18Alkyl of (e) (Y) and the like) (Y7: a single bond, etc.; y is8:C8~22Alkyl groups of (ii), etc.).
Description
Technical Field
The present invention relates to a transmission/scattering type liquid crystal display element which exhibits a transmission state when no voltage is applied and exhibits a scattering state when a voltage is applied.
Background
As a liquid crystal display element using a liquid crystal material, a TN (twisted nematic) mode is actually used. This mode utilizes the optical rotation characteristics of liquid crystal for switching light, and requires the use of a polarizing plate when used as a liquid crystal display element. However, the use efficiency of light becomes low due to the use of the polarizing plate.
As a Liquid crystal display element having high light utilization efficiency without using a polarizing plate, there is a Liquid crystal display element that switches between a transmissive state (also referred to as a transparent state) and a scattering state of a Liquid crystal, and generally, a Liquid crystal display element using a polymer Dispersed Liquid crystal (also referred to as a pdlc (polymer Dispersed Liquid crystal) or a polymer Network Liquid crystal (pnlc) is known.
A liquid crystal display element using these elements is formed by having a liquid crystal layer between a pair of substrates provided with electrodes, and performing the following operations: a liquid crystal composition containing a polymerizable compound that is polymerized by ultraviolet rays is disposed between the pair of substrates, and the liquid crystal composition is cured by irradiation with ultraviolet rays, thereby forming a liquid crystal layer, that is, a cured product composite (for example, a polymer network) of liquid crystal and the polymerizable compound. The liquid crystal display element controls the transmission state and the scattering state of the liquid crystal by applying a voltage.
A conventional liquid crystal display element using PDLC or PNLC is a liquid crystal display element (also referred to as a normal (normal) type element) in which liquid crystal molecules are oriented in random directions to be in a white turbid (scattering) state when no voltage is applied, and liquid crystal molecules are aligned in an electric field direction to transmit light when a voltage is applied, thereby being in a transmissive state. However, since the standard type element requires a constant voltage application in order to obtain a transmissive state, power consumption is increased in many applications in which the element is used in a transparent state, for example, when the element is used as a window glass or the like.
On the other hand, a liquid crystal display element (also referred to as a reverse type element) using PDLC which exhibits a transmissive state when no voltage is applied and exhibits a scattering state when a voltage is applied has been proposed (for example, see patent document 1 or patent document 2).
Documents of the prior art
Patent document
Patent document 1: japanese patent No. 2885116
Patent document 2: japanese patent No. 4132424
Disclosure of Invention
Problems to be solved by the invention
The polymerizable compound in the liquid crystal composition of the reverse cell has a function of forming a polymer network to obtain a desired optical characteristic and a function as a curing agent for improving the adhesion between the liquid crystal layer and the liquid crystal alignment film (also referred to as a vertical liquid crystal alignment film). In order to improve the adhesion to the liquid crystal alignment film, it is necessary to make the polymer network denser, but when the polymer network is made denser, there are problems that the vertical alignment of the liquid crystal is inhibited, and the optical characteristics of the reverse type element, that is, the transparency when no voltage is applied and the scattering characteristics when a voltage is applied are deteriorated. Therefore, the liquid crystal composition used in the reverse type device needs to have high liquid crystal vertical alignment properties when forming a liquid crystal layer.
Further, since the liquid crystal alignment film used in the reverse cell is a film having high hydrophobicity for vertically aligning the liquid crystal, there is a problem that the adhesion between the liquid crystal layer and the liquid crystal alignment film is low. Therefore, a large amount of polymerizable compound having a curing agent action must be introduced into the liquid crystal composition used for the retroreflective element. However, when a large amount of polymerizable compound is introduced, the vertical alignment of the liquid crystal is inhibited, and the transparency when no voltage is applied and the scattering property when a voltage is applied are significantly reduced. Therefore, the liquid crystal alignment film used in the reverse device must have high liquid crystal vertical alignment properties.
The present invention aims to provide a liquid crystal display element having high vertical alignment properties of liquid crystal, good optical properties, that is, good transparency when no voltage is applied and good scattering properties when a voltage is applied, and having high adhesion between a liquid crystal layer and a liquid crystal alignment film.
Means for solving the problems
The present inventors have conducted intensive studies to achieve the above object, and as a result, have completed the present invention.
That is, the present invention is a liquid crystal display element including a liquid crystal layer between a pair of substrates having electrodes, at least one of the substrates including a liquid crystal alignment film for vertically aligning the liquid crystal, the liquid crystal layer being obtained by disposing a liquid crystal composition including a liquid crystal and a polymerizable compound between the pair of substrates and curing the composition by irradiation with ultraviolet rays, the liquid crystal composition being a liquid crystal composition containing a compound represented by the following formula [1], and the liquid crystal alignment film being a liquid crystal alignment film obtained from a liquid crystal alignment treatment agent including a polymer having a side chain structure represented by the following formula [2-1] or formula [2-2 ].
(X1Is represented by a formula [1-a ] selected from]-formula [1-g]At least 1 of the group consisting of. X2Represents a group selected from the group consisting of a single bond, -O-, -NH-, -N (CH)3)-、-CH2O-、-CONH-、-NHCO-、-CON(CH3)-、-N(CH3) At least 1 bonding group selected from the group consisting of CO-, -COO-and-OCO-. X3Represents a single bond or- (CH)2)a- (a is an integer of 1 to 15). X4Represents a group selected from the group consisting of a single bond, -O-, -OCH2-, -COO-and-OCO-are used. X5A benzene ring, a cyclohexane ring, or a C17-51 organic group having a steroid skeleton, wherein any hydrogen atom on the benzene ring or the cyclohexane ring is optionally substituted by a C1-3 alkyl group, a C1-3 alkoxy group, a C1-3 fluoroalkyl group, or a C1-3 fluoroalkyl groupFluoroalkoxy or fluorine atom. X6Represents a group selected from the group consisting of a single bond, -O-, -OCH2-、-CH2At least 1 bonding group selected from the group consisting of O-, -COO-and-OCO-. X7Represents a benzene ring or a cyclohexane ring, and any hydrogen atom in these cyclic groups is optionally substituted with an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluorine-containing alkyl group having 1 to 3 carbon atoms, a fluorine-containing alkoxy group having 1 to 3 carbon atoms or a fluorine atom. p represents an integer of 0 to 4. X8Represents at least 1 selected from the group consisting of C1-18 alkyl, C1-18 fluorinated alkyl, C1-18 alkoxy and C1-18 fluorinated alkoxy. )
(XARepresents a hydrogen atom or a benzene ring. XBRepresents at least 1 cyclic group selected from the group consisting of a benzene ring, a cyclohexane ring and a heterocyclic ring. XCRepresents at least 1 selected from the group consisting of C1-18 alkyl, C1-18 fluorinated alkyl, C1-18 alkoxy and C1-18 fluorinated alkoxy. )
(Y1And Y3Each independently represents a group selected from a single bond, - (CH)2)a- (a is an integer of 1 to 15), -O-, -CH2At least 1 bonding group selected from the group consisting of O-, -COO-and-OCO-. Y is2Represents a single bond or- (CH)2)b- (b is an integer of 1 to 15). Y is4At least 1 2-valent cyclic group selected from the group consisting of benzene ring, cyclohexane ring and heterocycle, or a 2-valent organic group having 17 to 51 carbon atoms and having a steroid skeleton, wherein any hydrogen atom on the cyclic group is optionally substituted by an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluorine-containing alkyl group having 1 to 3 carbon atoms, a fluorine-containing alkoxy group having 1 to 3 carbon atoms or a fluorine atom. Y is5Represents at least 1 cyclic group selected from the group consisting of a benzene ring, a cyclohexane ring and a heterocyclic ring, these ringsAny hydrogen atom in the cyclic group is optionally substituted by an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluoroalkyl group having 1 to 3 carbon atoms, a fluoroalkoxy group having 1 to 3 carbon atoms or a fluorine atom. n represents an integer of 0 to 4. Y is6Represents at least 1 selected from the group consisting of C1-18 alkyl, C1-18 fluorinated alkyl, C1-18 alkoxy and C1-18 fluorinated alkoxy. )
-Y7-Y8[2-2]
(Y7Represents a group selected from the group consisting of a single bond, -O-, -CH2O-、-CONH-、-NHCO-、-CON(CH3)-、-N(CH3) At least 1 bonding group selected from the group consisting of CO-, -COO-and-OCO-. Y is8Represents an alkyl group having 8 to 22 carbon atoms or a fluoroalkyl group having 6 to 18 carbon atoms. )
ADVANTAGEOUS EFFECTS OF INVENTION
According to the present invention, a reverse type element having excellent optical characteristics, that is, excellent transparency when no voltage is applied and excellent scattering characteristics when a voltage is applied, and having high adhesion between a liquid crystal layer and a liquid crystal alignment film can be provided. The mechanism by which the liquid crystal display element having the above-described excellent characteristics can be obtained by the present invention is not clear, and is estimated roughly as follows.
The liquid crystal composition used in the present invention comprises a liquid crystal, a polymerizable compound and the compound represented by the formula [1]]The compounds shown. Formula [1]The specific compound has a site having a rigid structure such as a benzene ring or a cyclohexane ring and the formula [1]]X in (1)1The sites where polymerization reaction occurs by ultraviolet rays are shown. Therefore, when the specific compound is contained in the liquid crystal composition, the portion of the rigid structure of the specific compound improves the vertical alignment property of the liquid crystal, and further, the portion where the polymerization reaction occurs reacts with the polymerizable compound, thereby improving the stability of the vertical alignment property of the liquid crystal. Thus, even when the polymer network is densified to improve adhesion to the liquid crystal alignment film, the vertical alignment of the liquid crystal is not inhibited, and a reverse type element exhibiting good optical characteristics can be obtained.
Further, the liquid crystal alignment film used in the present invention is obtained from a liquid crystal alignment treatment agent containing a polymer having a side chain structure represented by the aforementioned formula [2-1] or formula [2-2 ]. In particular, since the specific side chain structure represented by the formula [2-1] shows a rigid structure, a liquid crystal display element obtained using a liquid crystal alignment film having the side chain structure can obtain a high and stable liquid crystal vertical alignment property. Therefore, when a specific side chain structure represented by the formula [2-1] is used, a reverse type element exhibiting excellent optical characteristics can be obtained.
In this way, the liquid crystal display element provided with the liquid crystal alignment film obtained from the liquid crystal alignment treatment agent of the present invention, which contains a liquid crystal composition and a specific polymer having a specific side chain structure, has good optical properties, that is, good transparency when no voltage is applied and good scattering properties when a voltage is applied, and further has high adhesion between the liquid crystal layer and the liquid crystal alignment film.
Detailed Description
< liquid Crystal display element >
The liquid crystal display element of the present invention is a liquid crystal display element having a liquid crystal layer between a pair of substrates having electrodes, at least one of the substrates having a liquid crystal alignment film for vertically aligning liquid crystal, wherein the liquid crystal layer is obtained by disposing a liquid crystal composition containing a liquid crystal and a polymerizable compound between the pair of substrates and curing the liquid crystal composition by irradiating ultraviolet light to the liquid crystal composition by an ultraviolet light irradiation device, and the liquid crystal display element is suitably used as a converse-type element which exhibits a transmission state when no voltage is applied and a scattering state when a voltage is applied.
The liquid crystal composition of the present invention contains a liquid crystal and a polymerizable compound that is polymerized by ultraviolet rays and plays a role of forming a polymer network (curable resin). The liquid crystal layer is a cured product composite of a liquid crystal and a polymerizable compound, and the cured product composite herein refers to a state in which a liquid crystal is present in a polymer network formed of, for example, a polymerizable compound, as described above.
< specific Compound and liquid Crystal composition >
The liquid crystal composition of the present invention is a liquid crystal composition containing a liquid crystal, a polymerizable compound and a specific compound represented by the following formula [1 ].
The specific compound is a compound represented by the following formula [1 ].
Formula [1]In, X1、X2、X3、X4、X5、X6、X7、X8And p are as defined above, wherein each is preferably as follows.
From the viewpoint of optical characteristics of the liquid crystal display element, X1Preferably the aforementioned formula [1-a]Formula [1-b ]]Formula [1-c ]]Or formula [1-e]. More preferably of the formula [1-a]Formula [1-b ]]Or formula [1-c]。
From the viewpoints of availability of raw materials and ease of synthesis, X2Preferably a single bond, -O-, -CH2O-, -CONH-, -COO-or-OCO-. More preferably a single bond, -O-, -COO-or-OCO-. X3Preferably a single bond or- (CH)2)a- (a is an integer of 1 to 10). More preferably- (CH)2)a- (a is an integer of 1 to 10). From the viewpoints of availability of raw materials and ease of synthesis, X4Preferably a single bond, -O-or-COO-. More preferably-O-.
From the viewpoint of optical characteristics of the liquid crystal display element, X5Preferably a benzene ring, a cyclohexane ring, or a C17-51 organic group having a steroid skeleton. More preferably a benzene ring or a C17-51 2-valent organic group having a steroid skeleton. From the viewpoint of ease of synthesis, X6Preferably a single bond, -O-, -COO-or-OCO-. More preferably a single bond, -COO-or-OCO-.
From the viewpoint of optical characteristics of the liquid crystal display element, X7Preferably a benzene ring or a cyclohexane ring. From the viewpoint of optical characteristics of the liquid crystal display element, X8Preferably an alkyl group having 1 to 18 carbon atoms or an alkoxy group having 1 to 18 carbon atoms. More preferably an alkyl group having 1 to 12 carbon atoms. From the viewpoint of raw material availability and ease of synthesis, p is preferably an integer of 0 to 2.
Formula [1]X in (1)1~X8Preferred combinations with p are shown in tables 1 to 9 below.
[ Table 1]
[ Table 2]
[ Table 3]
[ Table 4]
[ Table 5]
[ Table 6]
[ Table 7]
[ Table 8]
[ Table 9]
Among them, from the viewpoint of optical characteristics of the liquid crystal display element, a combination of (1-1a) to (1-12a), (1-13a), (1-14a), (1-17a), (1-18a), (1-21a), (1-22a), (1-25a) to (1-38a), (1-41a), (1-42a), (1-45a), (1-46a), (1-49a) to (1-96a), or (1-121a) to (1-130a) is preferable.
More preferably a combination of (1-1a) - (1-4a), (1-9a), (1-12a), (1-25a) - (1-28a), (1-33a), (1-36a), (1-49a) - (1-52a), (1-61a) - (1-64a), (1-85a) - (1-88a), (1-121a), (1-122a), (1-125a) or (1-126 a).
Combinations of (1-3a), (1-4a), (1-9a), (1-10a), (1-27a), (1-28a), (1-33a), (1-34a), (1-49a) and (1-52a), (1-61a) and (1-64a), (1-85a) and (1-88a), (1-121a), (1-122a), (1-125a) and (1-126a) are particularly preferred.
More specific compounds include compounds represented by the following formulae [1a-1] to [1a-6], and these compounds are preferably used.
Formula [1a-1]-formula [1a-6]In, Xa~XkAnd p1 to p9 are as defined above, and among them, the following are preferred.
From the viewpoint of optical characteristics of the liquid crystal display element, Xa、Xb、Xd、Xf、XhAnd XkEach independently preferably an alkyl group having 1 to 12 carbon atoms or an alkoxy group having 1 to 12 carbon atoms. More preferably an alkyl group having 1 to 8 carbon atoms or an alkoxy group having 1 to 8 carbon atoms. From the viewpoints of availability of raw materials and ease of synthesis, XcAnd XiEach independently is preferably-O-or-COO-. From the viewpoints of availability of raw materials and ease of synthesis, Xe、XgAnd XjEach independently is preferably-COO-or-OCO-. p1, p3, p5, p7, p8 and p9 are each independently preferably an integer of 1 to 10. From the viewpoint of optical characteristics of the liquid crystal display element, an integer of 1 to 8 is more preferable. p2, p4 and p6 are each independently preferably an integer of 1 or 2.
From the viewpoint of optical characteristics of the liquid crystal display element, the use ratio of the specific compound in the liquid crystal composition is preferably 0.1 to 20 parts by mass with respect to 100 parts by mass of the total of the liquid crystal and the polymerizable compound. More preferably 0.5 to 15 parts by mass, and particularly preferably 1 to 10 parts by mass.
These specific compounds may be used in 1 kind or 2 or more kinds in combination depending on the optical characteristics of the liquid crystal display element and the adhesion characteristics of the liquid crystal layer and the liquid crystal alignment film.
The liquid crystal in the liquid crystal composition may be nematic liquid crystal, smectic liquid crystal or cholesteric liquid crystal. Among them, it preferably has negative dielectric anisotropy. From the viewpoint of low-voltage driving and scattering characteristics, liquid crystals having a large anisotropy of dielectric constant and a large anisotropy of refractive index are preferable. Further, 2 or more kinds of liquid crystals may be mixed and used according to the respective physical property values of the phase transition temperature, the dielectric anisotropy and the refractive index anisotropy.
In order to drive a liquid crystal display element using an active element such as a TFT (Thin Film Transistor), the liquid crystal is required to have high resistance and high voltage holding ratio (also referred to as VHR). Therefore, fluorine-based or chlorine-based liquid crystals having high resistance and a VHR that is not lowered by active energy rays such as ultraviolet rays are preferably used as the liquid crystals.
Further, the liquid crystal display element may be a guest-host type element in which a dichroic dye is dissolved in a liquid crystal composition. In this case, an element which is transparent when no voltage is applied and which absorbs (scatters) when a voltage is applied can be obtained. In this liquid crystal display element, the direction of the director (alignment direction) of the liquid crystal changes by 90 degrees depending on whether or not a voltage is applied. Therefore, the liquid crystal display element can obtain a high contrast by utilizing the difference in light absorption characteristics of the dichromatic dye, as compared with the existing guest-host type element in which switching is performed by random alignment and vertical alignment. In the guest-host type element in which the dichroic dye is dissolved, the liquid crystal becomes colored when oriented in the horizontal direction, and becomes opaque only in the scattering state. Therefore, an element which switches from colorless transparency when no voltage is applied to a colored opaque state to colored transparent state with the application of a voltage can also be obtained.
The polymerizable compound in the liquid crystal composition may be a liquid crystal layer, which is a cured product composite (e.g., a polymer network) of the liquid crystal composition formed by polymerization reaction of ultraviolet rays. In this case, a monomer of a polymerizable compound may be introduced into the liquid crystal composition, or a polymer obtained by polymerizing the monomer in advance may be introduced into the liquid crystal composition. Among them, even when a polymer is produced, it is necessary to have a site where a polymerization reaction occurs by ultraviolet rays. From the viewpoint of handling of the liquid crystal composition, that is, suppression of an increase in viscosity of the liquid crystal composition and solubility in liquid crystal, a method of introducing a monomer into the liquid crystal composition and causing a polymerization reaction by ultraviolet irradiation at the time of producing a liquid crystal display element to form a cured product is more preferable.
The polymerizable compound is preferably a compound dissolved in a liquid crystal. Among them, when the polymerizable compound is dissolved in the liquid crystal, it is preferable that a temperature at which a part or the whole of the liquid crystal composition shows a liquid crystal phase is present. Preferably, even when a part of the liquid crystal composition exhibits a liquid crystal phase, the liquid crystal composition can be visually confirmed to have substantially uniform transparency and scattering characteristics throughout the liquid crystal cell.
The polymerizable compound may be any compound that undergoes a polymerization reaction by ultraviolet light, and in this case, any reaction form may be used to promote polymerization to form a cured product of the liquid crystal composition. Specific reaction forms include radical polymerization, cationic polymerization, anionic polymerization, and addition polymerization. Among them, radical polymerization is preferable. In this case, the following radical type polymerizable compounds (monomers) and oligomers thereof can be used as the polymerizable compound. Further, as described above, a polymer obtained by polymerizing these monomers may be used.
Examples of the monofunctional polymerizable compound (also referred to as a monofunctional monomer) include 2-ethylhexyl acrylate, butyl ethyl acrylate, butoxyethyl acrylate, 2-cyanoethyl acrylate, benzyl acrylate, cyclohexyl acrylate, hydroxyethyl acrylate, 2-hydroxypropyl acrylate, 2-ethoxyethyl acrylate, N-diethylaminoethyl acrylate, N-dimethylaminoethyl acrylate, dicyclopentyl acrylate, dicyclopentenyl acrylate, glycidyl acrylate, tetrahydrofurfuryl acrylate, isobornyl acrylate, isodecyl acrylate, lauryl acrylate, morpholino acrylate, phenoxyethyl acrylate, phenoxydiethylene glycol acrylate, 2,2, 2-trifluoroethyl acrylate, 2,2,3,3, 3-pentafluoropropyl acrylate, 2,3, 3-tetrafluoropropyl acrylate, 2,3,4,4, 4-hexafluorobutyl acrylate, 2-ethylhexyl methacrylate, butyl ethyl methacrylate, butoxyethyl methacrylate, 2-cyanoethyl methacrylate, benzyl methacrylate, cyclohexyl methacrylate, hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, 2-ethoxyethyl acrylate, N-diethylaminoethyl methacrylate, N-dimethylaminoethyl methacrylate, dicyclopentanyl methacrylate, dicyclopentenyl methacrylate, glycidyl methacrylate, tetrahydrofurfuryl methacrylate, isobornyl methacrylate, isodecyl methacrylate, lauryl methacrylate, and, Morpholine methacrylate (morpholino methacrylate), phenoxyethyl methacrylate, phenoxydiethylene glycol methacrylate, 2,2, 2-trifluoroethyl methacrylate, 2,2,3, 3-tetrafluoropropyl methacrylate, or 2,2,3,4, 4-hexafluorobutyl methacrylate, and oligomers thereof, and the like.
Examples of the bifunctional polymerizable compound (also referred to as a bifunctional monomer) include 4,4 ' -biphenyl diacrylate, diethylstilbestrol diacrylate, 1, 4-bisacryloxybenzene, 4 ' -bisacryloxydiphenyl ether, 4 ' -bisacryloxydiphenylmethane, 3,9- [1, 1-dimethyl-2-acryloyloxyethyl ] -2,4,8, 10-tetraoxaspiro [5,5] undecane, α ' -bis [ 4-acryloyloxyphenyl ] -1, 4-diisopropylbenzene, 1, 4-bisacryloxytetrafluorobenzene, 4 ' -bisacryloxytetrafluorobiphenyl, diethylene glycol acrylate, 1, 4-butanediol diacrylate, 1, 3-butanediol diacrylate, dicyclopentanyl diacrylate, glycerin diacrylate, 1, 6-hexanediol diacrylate, neopentyl glycol diacrylate, tetraethylene glycol diacrylate, 1, 9-nonanediol diacrylate, polyethylene glycol diacrylate, polypropylene glycol diacrylate, 1, 9-nonanediol dimethacrylate, polyethylene glycol dimethacrylate, polypropylene glycol dimethacrylate, and oligomers thereof.
Examples of the polyfunctional polymerizable compound (also referred to as a polyfunctional monomer) include trimethylolpropane triacrylate, pentaerythritol tetraacrylate, pentaerythritol triacrylate, ditrimethylolpropane tetraacrylate, dipentaerythritol hexaacrylate, dipentaerythritol monohydroxypentaacrylate, 4 '-diacryloyloxydiphenylene, 4' -diacryloyloxydimethylstyrene, 4 '-diacryloyloxydiethylstilbene, 4' -diacryloyloxydipropylstilbene, 4 '-diacryloyloxydibutylstilbene, 4' -diacryloyloxydiphenyloxydiphenylstilbene, 4, 4' -diacryloyloxydifluorodiphenylstyrene, 2,2,3,3,4, 4-hexafluoropentanediol-1, 5-diacrylate, 1,2,2,3, 3-hexafluoropropyl-1, 3-diacrylate, diethylene glycol dimethacrylate, 1, 4-butanediol dimethacrylate, 1, 3-butanediol dimethacrylate, 1, 6-hexanediol dimethacrylate, neopentyl glycol dimethacrylate, tetraethylene glycol dimethacrylate, trimethylolpropane trimethacrylate, pentaerythritol tetramethacrylate, pentaerythritol trimethacrylate, ditrimethylolpropane tetramethacrylate, dipentaerythritol hexamethacrylate, dipentaerythritol monohydroxypentamethacrylate or 2,2,3,3,4, 4-hexafluoropentanediol-1, 5-dimethacrylate, an oligomer thereof, and the like.
These radical type polymerizable compounds may be used in 1 kind or in a mixture of 2 or more kinds depending on the optical characteristics of the liquid crystal display element and the adhesion characteristics between the liquid crystal layer and the liquid crystal alignment film. In order to accelerate the formation of a cured product composite from the liquid crystal composition, it is preferable to introduce a radical initiator (also referred to as a polymerization initiator) that generates radicals by ultraviolet rays into the liquid crystal composition in order to accelerate radical polymerization of the polymerizable compound.
Examples thereof include tert-butyl peroxyisobutyrate, 2, 5-dimethyl-2, 5-bis (benzoyldioxy) hexane, 1, 4-bis [ alpha- (tert-butyldioxy) isopropoxy ] benzene, di-tert-butyl peroxide, 2, 5-dimethyl-2, 5-bis (tert-butyldioxy) hexylene peroxide, alpha- (isopropylphenyl) isopropyl peroxide, 2, 5-dimethylhexane, tert-butyl peroxide, 1-bis (tert-butyldioxy) -3,3, 5-trimethylcyclohexane, butyl-4, 4-bis (tert-butyldioxy) valerate, cyclohexanone peroxide, 2 ', 5, 5' -tetrakis (tert-butylperoxycarbonyl) benzophenone, 3 ', 4, 4' -tetrakis (tert-butylperoxycarbonyl) benzophenone, tert-butylperoxycarbonyl, Organic peroxides such as 3,3 ', 4, 4' -tetrakis (t-amylperoxycarbonyl) benzophenone, 3 ', 4, 4' -tetrakis (t-hexylperoxycarbonyl) benzophenone, 3 '-bis (t-butylperoxycarbonyl) -4, 4' -dicarboxybenzophenone, t-butyl peroxybenzoate, and di-t-butyl diperoxyiisophthalate; quinones such as 9, 10-anthraquinone, 1-chloroanthraquinone, 2-chloroanthraquinone, octamethylanthraquinone, and 1, 2-benzoanthraquinone; benzoin derivatives such as benzoin methyl ether, benzoin ethyl ether, α -methylbenzoin, and α -phenylbenzoin.
These radical initiators may be used in 1 kind or in combination of 2 or more kinds depending on the optical characteristics of the liquid crystal display element and the adhesion characteristics between the liquid crystal layer and the liquid crystal alignment film.
As the polymerizable compound, the following ionic polymerizable compounds can be used. Specifically, the compound has at least 1 kind of crosslinking-forming group selected from the group consisting of a hydroxyl group, a hydroxyalkyl group and a lower alkoxyalkyl group.
More specifically, examples of the crosslinkable compound having at least 1 group selected from the group consisting of a hydroxyl group, a hydroxyalkyl group and a lower alkoxyalkyl group include, specifically, melamine derivatives and benzoguanamine derivatives described on pages 39 to 40 of WO (international publication (kokai) No. WO) 2013/125595(2013.8.29 publication (the same shall apply hereinafter); a crosslinkable compound represented by the formulae [6-1] to [6-48] described in WO2011/132751 (published 2011.10.27) at pages 62 to 66.
Further, as the ionic polymerizable compound, a compound containing an epoxy group and an isocyanate group and having a crosslinking group may be used. Specifically, examples thereof include crosslinkable compounds having an epoxy group or an isocyanate group as described in WO2013/125595 (published 2013.8.29) on pages 37 to 38.
When an ionic polymerizable compound is used, an ionic initiator that generates an acid or a base by ultraviolet rays may be introduced to accelerate the polymerization reaction.
Specifically, for example, triazine compounds, acetophenone derivative compounds, disulfone compounds, diazomethane compounds, sulfonic acid derivative compounds, diaryliodonium salts, triarylsulfonium salts, triarylphosphonium salts, iron arene complexes, and the like can be used, but the present invention is not limited thereto. More specifically, there may be mentioned, for example, iodine diphenylchloride, iodine diphenyltrifluoromethanesulfonate, iodine diphenylmethanesulfonate, iodine diphenyltoluenesulfonate, iodine diphenylbromide, iodine diphenyltetrafluoroborate, iodine diphenylhexafluoroantimonate, iodine diphenylhexafluoroarsenate, iodine bis (p-tert-butylphenyl) hexafluorophosphate, iodine bis (p-tert-butylphenyl) methanesulfonate, iodine bis (p-tert-butylphenyl) toluenesulfonate, iodine bis (p-tert-butylphenyl) trifluoromethanesulfonate, iodine bis (p-tert-butylphenyl) tetrafluoroborate, iodine bis (p-tert-butylphenyl) chloride, iodine bis (p-chlorophenyl) tetrafluoroborate, triphenyl sulfonium chloride, triphenyl sulfonium bromide, sulfonium tris (p-methoxyphenyl) tetrafluoroborate, sulfonium tris (p-methoxyphenyl) hexafluorophosphate, sulfonium tris (p-ethoxyphenyl) tetrafluoroborate, triphenyl phosphonium chloride, iodine tris (p-chlorophenyl) tetrafluoroborate, triphenyl sulfonium chloride, Triphenyl phosphonium bromide, tris (p-methoxyphenyl) phosphonium tetrafluoroborate, tris (p-methoxyphenyl) phosphonium hexafluorophosphate, tris (p-ethoxyphenyl) phosphonium tetrafluoroborate, bis [ [ (2-nitrobenzyl) oxy ] carbonylhexane-1, 6-diamine ], nitrobenzylcyclohexylcyclohexane carbamate, bis (methoxybenzyl) hexamethylene dicarbamate, bis [ [ (2-nitrobenzyl) oxy ] carbonylhexane-1, 6-diamine ], nitrobenzylcyclohexane carbamate, or bis (methoxybenzyl) hexamethylene dicarbamate, and the like.
< specific side chain Structure >
The specific side chain structure of the present invention is represented by the following formula [2-1] or formula [2-2 ].
Formula [2-1]In, Y1、Y2、Y3、Y4、Y5、Y6And n are as defined above, wherein each is preferably as follows.
From the viewpoints of availability of raw materials and ease of synthesis, Y1Preferably a single bond, - (CH)2)a- (a is an integer of 1 to 15), -O-, -CH2O-or-COO-. More preferably a single bond, - (CH)2)a- (a is an integer of 1 to 10), -O-, -CH2O-or-COO-. Y is2Preferably a single bond or- (CH)2)b- (b is an integer of 1 to 10). From the viewpoint of ease of synthesis, Y3Preferably a single bond, - (CH)2)a- (a is an integer of 1 to 15), -O-, -CH2O-or-COO-. More preferably a single bond, - (CH)2)a- (a is an integer of 1 to 10), -O-, -CH2O-or-COO-. From the viewpoint of ease of synthesis, Y4Preferably a benzene ring, a cyclohexane ring, or an organic group having 17 to 51 carbon atoms and having a steroid skeleton.
Y5Preferably a benzene ring or a cyclohexane ring. Y is6Preferably an alkyl group having 1 to 18 carbon atoms, a fluorinated alkyl group having 1 to 10 carbon atoms, an alkoxy group having 1 to 18 carbon atoms or a fluorinated alkoxy group having 1 to 10 carbon atoms. More preferably an alkyl group having 1 to 12 carbon atoms or an alkoxy group having 1 to 12 carbon atoms. Particularly preferably an alkyl group having 1 to 9 carbon atoms or an alkoxy group having 1 to 9 carbon atoms. From the viewpoint of raw material availability and ease of synthesis, n is preferably 0 to 3, more preferably 0 to 2.
Y1~Y6Preferable combinations with n include the same combinations as (2-1) to (2-629) described in tables 6 to 47 on pages 13 to 34 of WO2011/132751 (disclosed in 2011.10.27). In the tables of International publication, Y of the present invention is shown1~Y6Shown as Y1-Y6, but Y1-Y6 are understood to be Y1~Y6. In addition, (2-605) to (2-629) described in each table of the international publication, the organic group having 17 to 51 carbon atoms of the steroid skeleton of the present invention is shown as an organic group having 12 to 25 carbon atoms of the steroid skeleton, but the organic group having 12 to 25 carbon atoms of the steroid skeleton can be understood as an organic group having 17 to 51 carbon atoms of the steroid skeleton.
Among them, preferred is a combination of (2-25) to (2-96), (2-145) to (2-168), (2-217) to (2-240), (2-268) to (2-315), (2-364) to (2-387), (2-436) to (2-483), (2-603) to (2-615), or (2-624). Particularly preferred combinations are (2-49) to (2-96), (2-145) to (2-168), (2-217) to (2-240), (2-603) to (2-606), (2-607) to (2-609), (2-611), (2-612), or (2-624).
-Y7-Y8[2-2]
Formula [2-2]]In, Y7And Y8As described above, the following are preferred.
Y7Preferably a single bond, -O-, -CH2O-、-CONH-、-CON(CH3) -or-COO-. More preferably a single bond, -O-, -CONH-or-COO-. Y is8Preferably an alkyl group having 8 to 18 carbon atoms.
As described above, the specific side chain structure of the present invention is preferably a specific side chain structure represented by the formula [2-1] from the viewpoint of obtaining a high and stable vertical alignment property of liquid crystal.
< specific Polymer >
The specific polymer having a specific side chain structure is not particularly limited, and is preferably at least 1 polymer selected from the group consisting of an acrylic polymer, a methacrylic polymer, a novolac resin, polyhydroxystyrene, a polyimide precursor, polyimide, polyamide, polyester, cellulose, and polysiloxane. More preferably a polyimide precursor, polyimide or polysiloxane.
When a polyimide precursor or a polyimide (also collectively referred to as a polyimide-based polymer) is used as the specific polymer, it is preferably a polyimide precursor or a polyimide obtained by reacting a diamine component with a tetracarboxylic acid component.
The polyimide precursor has a structure represented by the following formula [ A ].
(R1Represents a 4-valent organic group. R2Represents a 2-valent organic group. A. the1And A2Each independently represents a hydrogen atom or an alkyl group having 1 to 8 carbon atoms. A. the3And A4Each independently represents a hydrogen atom, an alkyl group having 1 to 5 carbon atoms or an acetyl group. n represents a positive integer. )
The diamine component may be a diamine having 2 primary or secondary amino groups in the molecule, and the tetracarboxylic acid component may be a tetracarboxylic acid compound, a tetracarboxylic acid dianhydride, a tetracarboxylic acid dihalide compound, a tetracarboxylic acid dialkyl ester compound, or a tetracarboxylic acid dialkyl ester dihalide compound.
The polyimide polymer is preferably a polyamic acid having a structural formula of a repeating unit represented by the following formula [ D ] or a polyimide obtained by imidizing the polyamic acid, because the polyimide polymer can be obtained relatively easily from a tetracarboxylic dianhydride represented by the following formula [ B ] and a diamine represented by the following formula [ C ].
(R1And R2And formula [ A]The meanings defined in (1) are the same. )
(R1And R2And formula [ A]The meanings defined in (1) are the same. )
Further, the formula [ D ] obtained as described above can also be synthesized by a usual synthesis method]Into the polymer of the formula [ A ]]Shown as A1And A2An alkyl group having 1 to 8 carbon atoms and the formula [ A]Shown as A3And A4An alkyl group or acetyl group having 1 to 5 carbon atoms.
As a method for introducing the specific side chain structure into the polyimide-based polymer, a diamine having a specific side chain structure is preferably used as a part of the raw material. Particularly, a diamine represented by the following formula [2a ] (also referred to as a specific side chain type diamine) is preferably used.
In the formula [2a ], Y represents a structure represented by the formula [2-1] or the formula [2-2 ].
Further, the formula [2-1]Y in (1)1、Y2、Y3、Y4、Y5、Y6And details and preferred combinations of n are as shown above, formula [2-2]Y in (1)7And Y8The details and preferred combinations of (a) are as indicated above.
m represents an integer of 1 to 4. Among them, an integer of 1 is preferable.
As having the formula [2-1]Specific examples of the specific side chain type diamine having the specific side chain structure include those described in WO2013/125595 (published 2013.8.29) on pages 15 to 19 and represented by the formula [2-1]]-formula [2-6]Is of the formula [2-9]-formula [2-36]The diamine compound of (1). In the description of WO2013/125595, the formula [2-1]]-formula [2-3]R in (1)2And formula [2-4 ]]-formula [2-6]R in (1)4Represents at least 1 selected from the group consisting of C1-18 alkyl, C1-18 fluorinated alkyl, C1-18 alkoxy and C1-18 fluorinated alkoxy. Furthermore, the formula [2-13]A in (A)4Represents a linear or branched alkyl group having 3 to 18 carbon atoms. And, formula [2-4 ]]-formula [2-6]R in (1)3Represents a group selected from-O-, -CH2At least 1 of the group consisting of O-, -COO-and-OCO-.
Among them, the diamine is preferably a diamine compound of the formula [2-1] to the formula [2-6], the formula [2-9] to the formula [2-13] or the formula [2-22] to the formula [2-31] described in WO 2013/125595.
Further, from the viewpoints of the vertical alignment properties of liquid crystals when the liquid crystal alignment film is formed and the optical characteristics of a liquid crystal display element, diamines represented by the following formulae [2a-32] to [2a-36] are most preferable.
(R1represents-CH2O-。R2Represents an alkyl group having 3 to 12 carbon atoms. )
(R3Represents an alkyl group having 3 to 12 carbon atoms, and cis-trans isomerization of a1, 4-cyclohexylidene group into a trans isomer. )
As having the aforementioned formula [2-2]Specific examples of the specific side chain type diamine having a specific side chain structure include those described in WO2013/125595 (published 2013.8.29) on page 23 of the formula [ DA 1]]-formula [ DA11]The diamine compound of (1). In the description of WO2013/125595, the formula [ DA 1]]-formula [ DA5]A in (A)1Represents an alkyl group having 8 to 22 carbon atoms or a fluoroalkyl group having 6 to 18 carbon atoms.
The proportion of the specific side chain diamine used is preferably 10 to 80 mol%, more preferably 20 to 70 mol%, based on the whole diamine component, from the viewpoints of the vertical alignment properties of liquid crystal when the liquid crystal alignment film is formed and the adhesion between the liquid crystal layer and the liquid crystal alignment film in the liquid crystal display element.
The specific side chain type diamine may be used in 1 kind or 2 or more kinds in combination depending on the solubility of the polyimide-based polymer in a solvent, the vertical alignment property of liquid crystal when a liquid crystal alignment film is formed, and the optical characteristics of a liquid crystal display element.
As the diamine component used for producing the polyimide-based polymer, a diamine represented by the following formula [2b ] (also referred to as a2 nd diamine) is preferably used.
T represents at least 1 substituent selected from the group consisting of structures represented by the following formulae [2-1b ] to [2-4b ]. r represents an integer of 1 to 4. Among them, an integer of 1 is preferable.
Formula [2-1b ]]Wherein a represents an integer of 0 to 4. Among them, an integer of 0 or 1 is preferable from the viewpoint of availability of raw materials and ease of synthesis. Formula [2-2b ]]Wherein b represents an integer of 0 to 4. Among them, an integer of 0 or 1 is preferable from the viewpoint of availability of raw materials and ease of synthesis. Formula [2-3b ]]In, T1And T2Independently represents a C1-12 hydrocarbon group. Formula [2-4b ]]In, T3Represents an alkyl group having 1 to 5 carbon atoms.
Specific structures of the 2 nd type diamines are listed below, but the diamines are not limited to these examples. Examples thereof include 2, 4-dimethyl-m-phenylenediamine, 2, 6-diaminotoluene, 2, 4-diaminophenol, 3, 5-diaminobenzyl alcohol, 2, 4-diaminobenzyl alcohol, 4, 6-diaminoresorcinol, 2, 4-diaminobenzoic acid, 2, 5-diaminobenzoic acid or 3, 5-diaminobenzoic acid, and diamines having structures represented by the following formulae [2b-1] to [2b-6 ].
Among them, preferred is 2, 4-diaminophenol, 3, 5-diaminobenzyl alcohol, 2, 4-diaminobenzyl alcohol, 4, 6-diaminoresorcinol, 2, 4-diaminobenzoic acid, 2, 5-diaminobenzoic acid, 3, 5-diaminobenzoic acid, a diamine represented by the formula [2b-1], the formula [2b-2] or the formula [2b-3 ]. Particularly preferred are diamines represented by the formula [2b-1] or the formula [2b-2], 2, 4-diaminophenol, 3, 5-diaminobenzyl alcohol, 3, 5-diaminobenzoic acid, and the like, from the viewpoints of solubility of a polyimide polymer in a solvent and optical characteristics of a liquid crystal display element.
The proportion of the 2 nd type diamine used is preferably 1 to 50 mol%, more preferably 1 to 40 mol%, and particularly preferably 5 to 40 mol% based on the whole diamine component, from the viewpoint of adhesion between a liquid crystal layer and a liquid crystal alignment film in a liquid crystal display element.
The 2 nd diamine may be used in 1 kind or 2 or more kinds in combination depending on the solubility of the polyimide polymer in a solvent, the vertical alignment property of liquid crystal when a liquid crystal alignment film is formed, and the optical characteristics of a liquid crystal display element.
As the diamine component used for producing the polyimide-based polymer, diamines other than the specific side chain type diamine and the 2 nd type diamine (also referred to as other diamines) may be used within a range not impairing the effect of the present invention.
Specifically, there may be mentioned other diamine compounds described on pages 19 to 23 of WO2013/125595 (published 2013.8.29), diamine compounds of the formulae [ DA12] and [ DA15] to [ DA20] described on page 24 of the publication, and diamine compounds of the formulae [ DA26] to [ DA28] described on pages 25 to 26 of the publication. Further, other diamines may be used in 1 kind or 2 or more kinds in combination depending on the characteristics.
The tetracarboxylic acid component used for producing the polyimide-based polymer is a tetracarboxylic dianhydride represented by the following formula [3] or a tetracarboxylic acid derivative thereof, and among them, a tetracarboxylic acid dihalide, a tetracarboxylic acid dialkyl ester or a tetracarboxylic acid dialkyl ester dihalide (all of which are also collectively referred to as a specific tetracarboxylic acid component) is preferable.
Z represents at least 1 structure selected from the group consisting of the following formulas [3a ] to [3k ].
In view of ease of synthesis and ease of polymerization in the production of a polymer, Z in the formula [3] is preferably represented by the formula [3a ], the formula [3c ], the formula [3d ], the formula [3e ], the formula [3f ], the formula [3g ] or the formula [3k ]. More preferably, the formula [3a ], the formula [3e ], the formula [3f ], the formula [3g ] or the formula [3k ], and particularly preferably the formula [3a ], the formula [3e ], the formula [3f ] or the formula [3g ] from the viewpoint of optical characteristics of the liquid crystal display element.
The proportion of the specific tetracarboxylic acid component to be used is preferably 1 mol% or more, more preferably 5 mol% or more, and still more preferably 10 mol% or more based on the total tetracarboxylic acid component. Among them, from the viewpoint of optical characteristics of the liquid crystal display element, 10 to 90 mol% is particularly preferable.
When the specific tetracarboxylic acid component of the above formula [3e ], formula [3f ], formula [3g ] or formula [3k ] is used, a desired effect can be obtained by using the specific tetracarboxylic acid component in an amount of 20 mol% or more based on the whole tetracarboxylic acid component. More preferably 30 mol% or more. Further, the tetracarboxylic acid component may be a tetracarboxylic acid component of the formula [3e ], the formula [3f ], the formula [3g ] or the formula [3k ].
The polyimide-based polymer may contain a tetracarboxylic acid component other than the specific tetracarboxylic acid component within a range not impairing the effects of the present invention. Examples of the other tetracarboxylic acid component include tetracarboxylic acids, tetracarboxylic dianhydrides, dicarboxylic acid dihalides, dialkyl dicarboxylates, or dialkyl ester dihalides shown below.
Specifically, there may be mentioned other tetracarboxylic acid components described on pages 27 to 28 of WO2013/125595 (published 2013.8.29). The specific tetracarboxylic acid component and the other tetracarboxylic acid components may be used in 1 kind or in a mixture of 2 or more kinds depending on the characteristics.
The method for synthesizing the polyimide-based polymer is not particularly limited. Usually, the diamine component is reacted with a tetracarboxylic acid component. Specifically, the method described on pages 29 to 30 of WO2013/125595 (published 2013.8.29) is included.
The reaction of the diamine component and the tetracarboxylic acid component is usually carried out in a solvent containing the diamine component and the tetracarboxylic acid component. The solvent used in this case is not particularly limited as long as the polyimide precursor formed is dissolved.
Examples thereof include N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone, γ -butyrolactone, N-dimethylformamide, N-dimethylacetamide, dimethylsulfoxide, and 1, 3-dimethyl-imidazolidinone. When the polyimide precursor has high solubility in the solvent, a solvent represented by methyl ethyl ketone, cyclohexanone, cyclopentanone, 4-hydroxy-4-methyl-2-pentanone, or a solvent represented by the following formulae [ D-1] to [ D-3] can be used.
(D1Represents an alkyl group having 1 to 3 carbon atoms. D2Represents an alkyl group having 1 to 3 carbon atoms. D3Represents an alkyl group having 1 to 4 carbon atoms. )
They may be used alone or in combination. Further, even if the solvent does not dissolve the polyimide precursor, the solvent may be mixed and used within a range where the produced polyimide precursor is not precipitated. Further, the organic solvent is preferably dehydrated and dried for use because moisture in the organic solvent inhibits the polymerization reaction and causes hydrolysis of the polyimide precursor to be produced.
The molecular weight of the polyimide-based polymer is preferably 5,000 to 1,000,000, more preferably 10,000 to 150,000, in terms of the strength of the liquid crystal alignment film obtained therefrom, the workability in forming the liquid crystal alignment film, and the film coatability, as measured by GPC (Gel permeation chromatography).
When a polysiloxane is used as the specific polymer, it is preferably: a polysiloxane obtained by condensation polymerization of an alkoxysilane represented by the following formula [ A1 ]; alternatively, a polysiloxane (also collectively referred to as a polysiloxane polymer) obtained by polycondensing the alkoxysilane represented by the formula [ a1] with the alkoxysilane represented by the formula [ a2] and/or the alkoxysilane represented by the formula [ A3 ].
An alkoxysilane of the formula [ A1 ]:
(A1)mSi(A2)n(OA3)p[A1]
formula [ A1]In (A)1Represents the aforementioned formula [2-1]Or formula [2-2]The structure shown. Further, the formula [2-1]Y in (1)1、Y2、Y3、Y4、Y5、Y6And details and preferred combinations of n are as described above, formula [2-2]Y in (1)7And Y8The details and preferred combinations of (a) are as described above.
In the present invention, from the viewpoints of the vertical alignment properties of liquid crystals when the liquid crystal alignment film is formed and the optical characteristics of the liquid crystal display element, a1Preferably of the formula [2-1]The specific side chain structure shown.
Formula [ A1]In (A)2、A3M, n and p are as defined above. Among them, the following are preferred. A. the2Preferably a hydrogen atom or an alkyl group having 1 to 3 carbon atoms. From the viewpoint of polycondensation reactivity, A3Preferably an alkyl group having 1 to 3 carbon atoms. From the viewpoint of synthesis, m is preferably an integer of 1. n represents an integer of 0 to 2. From the viewpoint of polycondensation reactivity, p is preferably an integer of 1 to 3, more preferably an integer of 2 or 3. m + n + p is an integer of 4.
As having the formula [2-1]Specific examples of the alkoxysilane having a specific side chain structure include those described in WO2014/061779 (published 2014.4.24) on pages 41 to 44 [ A1-1]-formula [ A1-22]And formula [ A1-25]-formula [ A1-32]The alkoxysilane of (4). In the description of WO2014/061779, the formula [ A1-19 ] is]-formula [ A1-22]And formula [ A1-25]-formula [ A1-31]R in (1)2Represents a group selected from-O-, -CH2At least 1 of the group consisting of O-, -COO-and-OCO-.
Among them, the alkoxysilane is preferably represented by the formula [ A1-9] to the formula [ A1-21], the formula [ A1-25] to the formula [ A1-28] or the formula [ A1-32] described in WO2014/061779, from the viewpoints of the vertical alignment of a liquid crystal when a liquid crystal alignment film is formed and the optical characteristics of a liquid crystal display element.
The alkoxysilane represented by the formula [ a1] may be used in 1 kind or in a mixture of 2 or more kinds depending on the solubility of the polysiloxane polymer in a solvent, the vertical alignment property of liquid crystal when a liquid crystal alignment film is formed, and the optical characteristics of a liquid crystal display element.
An alkoxysilane of the formula [ A2 ]:
(B1)mSi(B2)n(OB3)p[A2]
formula [ A2]In (B)1、B2、B3M, n and p are as defined above. Wherein, each is superiorThe following were selected. From the viewpoint of ease of acquisition, B1Organic groups having a vinyl group, an epoxy group, an amino group, a methacryloyl group, an acryloyl group, or a ureido group are preferable. More preferably an organic group having a methacryloyl group, an acryloyl group or a ureido group. B is2Preferably a hydrogen atom or an alkyl group having 1 to 3 carbon atoms.
From the viewpoint of polycondensation reactivity, B3Preferably an alkyl group having 1 to 3 carbon atoms. From the viewpoint of synthesis, m is preferably an integer of 1. n represents an integer of 0 to 2. From the viewpoint of polycondensation reactivity, p is preferably an integer of 1 to 3, more preferably an integer of 2 or 3. m + n + p is 4.
Specific examples of the alkoxysilane represented by the formula [ A2] include alkoxysilanes represented by the formula [ A2] described on pages 45 to 46 of WO2014/061779 (published under 2014.4.24).
Among them, preferred is allyltriethoxysilane, allyltrimethoxysilane, diethoxymethylvinylsilane, dimethoxymethylvinylsilane, triethoxyvinylsilane, vinyltrimethoxysilane, vinyltris (2-methoxyethoxy) silane, 3- (triethoxysilyl) propyl methacrylate, 3- (trimethoxysilyl) propyl acrylate, 3- (trimethoxysilyl) propyl methacrylate, 3- (trimethoxysilyl) propyl 3-glycidoxypropyl (dimethoxy) methylsilane, 3-glycidoxypropyl (diethoxy) methylsilane, 3-glycidoxypropyltrimethoxysilane or 2- (3, 4-epoxycyclohexyl) ethyltrimethoxysilane.
From the viewpoint of adhesion between the liquid crystal layer of the liquid crystal display element and the liquid crystal alignment film, 3- (triethoxysilyl) propyl methacrylate, 3- (trimethoxysilyl) propyl acrylate, 3- (trimethoxysilyl) propyl methacrylate, 3-glycidoxypropyl (dimethoxy) methylsilane, 3-glycidoxypropyl (diethoxy) methylsilane, 3-glycidoxypropyltrimethoxysilane, or 2- (3, 4-epoxycyclohexyl) ethyltrimethoxysilane is particularly preferable. The alkoxysilane represented by the formula [ A2] may be used in 1 kind or in combination of 2 or more kinds.
An alkoxysilane of the formula [ A3 ]:
(D1)nSi(OD2)4-n[A3]
formula [ A3]In (D)1、D2And n are as defined above, wherein each is preferably as follows. D1Preferably a hydrogen atom or an alkyl group having 1 to 3 carbon atoms. From the viewpoint of polycondensation reactivity, D2Preferably an alkyl group having 1 to 3 carbon atoms. n represents an integer of 0 to 3.
Specific examples of the alkoxysilane represented by the formula [ A3] include alkoxysilanes represented by the formula [ A3] described on page 47 of WO2014/061779 (published as 2014.4.24).
Examples of the alkoxysilane in which n is 0 in the formula [ A3] include tetramethoxysilane, tetraethoxysilane, tetrapropoxysilane and tetrabutoxysilane. As the alkoxysilane of the formula [ A3], it is preferable to use these alkoxysilanes. The alkoxysilane represented by the formula [ A3] may be used in 1 kind or in combination of 2 or more kinds.
The polysiloxane polymer is a polysiloxane obtained by polycondensing an alkoxysilane represented by the formula [ A1] or a polysiloxane obtained by polycondensing an alkoxysilane represented by the formula [ A1] with an alkoxysilane represented by the formula [ A2] and/or an alkoxysilane represented by the formula [ A3 ]. That is, the polysiloxane polymer is any one of the following polysiloxanes: a polysiloxane obtained by condensation-polymerizing only an alkoxysilane represented by the formula [ A1 ]; polysiloxane obtained by condensation polymerization of 2 kinds of alkoxysilane represented by the formula [ A1] and the formula [ A2 ]; polysiloxane obtained by condensation polymerization of 2 kinds of alkoxysilane represented by the formula [ A1] and the formula [ A3 ]; and a polysiloxane obtained by condensation polymerization of 3 kinds of alkoxysilanes represented by the formulae [ A1], [ A2] and [ A3 ].
Among these, from the viewpoint of the polycondensation reactivity and the solubility of the polysiloxane polymer in a solvent, a polysiloxane obtained by polycondensing a plurality of alkoxysilanes is preferable. That is, any of the following polysiloxanes is preferably used: polysiloxane obtained by condensation polymerization of 2 kinds of alkoxysilane represented by the formula [ A1] and the formula [ A2 ]; polysiloxane obtained by condensation polymerization of 2 kinds of alkoxysilane represented by the formula [ A1] and the formula [ A3 ]; and a polysiloxane obtained by condensation polymerization of 3 kinds of alkoxysilanes represented by the formulae [ A1], [ A2] and [ A3 ].
When a plurality of alkoxysilanes are used in the preparation of the polysiloxane polymer, the ratio of the alkoxysilane represented by the formula [ a1] to be used is preferably 1 to 40 mol%, more preferably 1 to 30 mol%, based on the total amount of the alkoxysilanes. The ratio of the alkoxysilane represented by the formula [ A2] is preferably 1 to 70 mol%, more preferably 1 to 60 mol% based on the total amount of the alkoxysilanes. Further, the ratio of the alkoxysilane represented by the formula [ A3] is preferably 1 to 99 mol%, more preferably 1 to 80 mol%, based on the total amount of the alkoxysilanes.
The method of polycondensation reaction for producing the polysiloxane polymer is not particularly limited. Specifically, the method described on pages 49 to 52 of WO2014/061779 (published 2014.4.24) can be mentioned.
In the polycondensation reaction for producing the polysiloxane polymer, when a plurality of kinds of alkoxysilanes represented by the formula [ A1], the formula [ A2] or the formula [ A3] are used, a mixture in which a plurality of kinds of alkoxysilanes are mixed in advance may be used for the reaction, or the reaction may be carried out while a plurality of kinds of alkoxysilanes are added in order.
In the present invention, the solution of the polysiloxane polymer obtained by the above-mentioned method may be used as it is as the specific polymer, or the solution of the polysiloxane polymer obtained by the above-mentioned method may be concentrated, diluted by adding a solvent, or replaced with another solvent, if necessary, to be used as the specific polymer.
The solvent (also referred to as an additive solvent) used for dilution may be a solvent used for the polycondensation reaction or another solvent. The solvent to be added is not particularly limited as long as it uniformly dissolves the polysiloxane polymer, and 1 or 2 or more kinds thereof can be selected and used. Examples of the solvent to be added include, in addition to the solvent used in the above polycondensation reaction, ketone solvents such as acetone, methyl ethyl ketone, and methyl isobutyl ketone; ester solvents such as methyl acetate, ethyl acetate, and ethyl lactate. Further, when the polysiloxane polymer and other polymers are used as the specific polymer, it is preferable that the alcohol generated in the polycondensation reaction of the polysiloxane polymer is distilled off under normal pressure or reduced pressure in advance before the other polymers are mixed with the polysiloxane polymer.
< liquid Crystal alignment treating agent >
The liquid crystal alignment treatment agent is a solution for forming a liquid crystal alignment film, and contains a solvent and a specific polymer having a specific side chain structure of the aforementioned formula [2-1] or formula [2-2 ].
The specific polymer is not particularly limited as described above, and is preferably at least 1 polymer selected from the group consisting of acrylic polymers, methacrylic polymers, novolac resins, polyhydroxystyrene, polyimide precursors, polyimide, polyamide, polyester, cellulose, and polysiloxane. More preferably a polyimide precursor, polyimide or polysiloxane. Further, 1 or 2 or more of these polymers may be used as the specific polymer.
All of the polymer components in the liquid crystal aligning agent may be a specific polymer, or other polymers may be mixed. In this case, the content of the other polymer is 0.5 to 15 parts by mass, preferably 1 to 10 parts by mass, based on 100 parts by mass of the specific polymer. Examples of the other polymers include the polymers described above which do not have a specific side chain structure represented by the formula [2-1] or the formula [2-2 ].
The content of the solvent in the liquid crystal aligning agent can be appropriately selected from the viewpoint of the method of applying the liquid crystal aligning agent and obtaining a desired film thickness. Among them, the solvent content in the liquid crystal alignment treatment agent is preferably 50 to 99.9% by mass, more preferably 60 to 99% by mass, from the viewpoint of forming a uniform liquid crystal alignment film by coating. Particularly preferably 65 to 99 mass%.
The solvent used for the liquid crystal aligning agent is not particularly limited as long as it dissolves the specific polymer. Among them, when the specific polymer is a polyimide precursor, polyimide, polyamide or polyester, or when the solubility of an acrylic polymer, methacrylic polymer, novolac resin, polyhydroxystyrene, cellulose or polysiloxane in a solvent is low, it is preferable to use the solvent (also referred to as solvent a).
Examples thereof include N, N-dimethylformamide, N-dimethylacetamide, N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone, dimethyl sulfoxide, γ -butyrolactone, 1, 3-dimethyl-imidazolidinone, methyl ethyl ketone, cyclohexanone, cyclopentanone, and 4-hydroxy-4-methyl-2-pentanone. Among them, N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone or γ -butyrolactone is preferably used. Further, they may be used alone or in combination.
When the specific polymer is an acrylic polymer, a methacrylic polymer, a novolac resin, polyhydroxystyrene, cellulose, or polysiloxane, and further when the specific polymer is a polyimide precursor, polyimide, polyamide, or polyester and the solubility of the specific polymer in a solvent is high, the following solvents (also referred to as solvents B.) can be used.
Specific examples of the solvent B include poor solvents described on pages 35 to 37 of WO2013/125595 (published 2013.8.29).
Among them, 1-hexanol, cyclohexanol, 1, 2-ethylene glycol, 1, 2-propylene glycol, propylene glycol monobutyl ether, ethylene glycol monobutyl ether, dipropylene glycol dimethyl ether, cyclohexanone, cyclopentanone, or a solvent represented by the above-described formulae [ D1] to [ D3] is preferably used.
When these solvents B are used, it is preferable to use N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone or γ -butyrolactone of the above solvents A in combination in order to improve the coatability of the liquid crystal alignment treatment agent. More preferably, gamma-butyrolactone is used in combination.
These solvents B can improve the film coatability and surface smoothness of the liquid crystal alignment film when the liquid crystal alignment treatment agent is applied, and therefore, when a polyimide precursor, a polyimide, a polyamide or a polyester is used as the specific polymer, it is preferable to use them in combination with the above-mentioned solvents a. In this case, the solvent B is preferably 1 to 99% by mass of the entire solvent contained in the liquid crystal aligning agent. Among them, it is preferably 10 to 99% by mass. More preferably 20 to 95 mass%.
In the present invention, at least 1 kind of generating agent (also referred to as a specific generating agent) selected from the group consisting of a photoradical generating agent, a photoacid generator, and a photobase generating agent is preferably introduced into the liquid crystal alignment treatment agent. Among them, the specific generator is preferably a photo radical generator from the viewpoint of adhesion between the liquid crystal layer and the liquid crystal alignment film.
The photo radical generator is not particularly limited as long as it generates radicals by ultraviolet rays. Examples thereof include tert-butyl peroxyisobutyrate, 2, 5-dimethyl-2, 5-bis (benzoyldioxy) hexane, 1, 4-bis [ alpha- (tert-butyldioxy) isopropoxy ] benzene, di-tert-butyl peroxide, 2, 5-dimethyl-2, 5-bis (tert-butyldioxy) hexylene peroxide, alpha- (isopropylphenyl) isopropyl peroxide, 2, 5-dimethylhexane, tert-butyl peroxide, 1-bis (tert-butyldioxy) -3,3, 5-trimethylcyclohexane, butyl-4, 4-bis (tert-butyldioxy) valerate, cyclohexanone peroxide, 2 ', 5, 5' -tetrakis (tert-butylperoxycarbonyl) benzophenone, 3 ', 4, 4' -tetrakis (tert-butylperoxycarbonyl) benzophenone, tert-butylperoxycarbonyl, Organic peroxides such as 3,3 ', 4, 4' -tetrakis (t-amylperoxycarbonyl) benzophenone, 3 ', 4, 4' -tetrakis (t-hexylperoxycarbonyl) benzophenone, 3 '-bis (t-butylperoxycarbonyl) -4, 4' -dicarboxybenzophenone, t-butyl peroxybenzoate, and di-t-butyl diperoxyiisophthalate; quinones such as 9, 10-anthraquinone, 1-chloroanthraquinone, 2-chloroanthraquinone, octamethylanthraquinone, and 1, 2-benzoanthraquinone; benzoin derivatives such as benzoin methyl ether, benzoin ethyl ether, α -methylbenzoin, and α -phenylbenzoin.
The photoacid generator and the photobase generator are not particularly limited as long as they generate an acid or a base by ultraviolet rays, and examples thereof include triazine compounds, acetophenone derivative compounds, disulfone compounds, diazomethane compounds, sulfonic acid derivative compounds, diaryliodonium salts, triarylsulfonium salts, triarylphosphonium salts, and iron arene complexes. More specifically, there may be mentioned, for example, iodine diphenylchloride, iodine diphenyltrifluoromethanesulfonate, iodine diphenylmethanesulfonate, iodine diphenyltoluenesulfonate, iodine diphenylbromide, iodine diphenyltetrafluoroborate, iodine diphenylhexafluoroantimonate, iodine diphenylhexafluoroarsenate, iodine bis (p-tert-butylphenyl) hexafluorophosphate, iodine bis (p-tert-butylphenyl) methanesulfonate, iodine bis (p-tert-butylphenyl) toluenesulfonate, iodine bis (p-tert-butylphenyl) trifluoromethanesulfonate, iodine bis (p-tert-butylphenyl) tetrafluoroborate, iodine bis (p-tert-butylphenyl) chloride, iodine bis (p-chlorophenyl) tetrafluoroborate, triphenyl sulfonium chloride, triphenyl sulfonium bromide, sulfonium tris (p-methoxyphenyl) tetrafluoroborate, sulfonium tris (p-methoxyphenyl) hexafluorophosphate, sulfonium tris (p-ethoxyphenyl) tetrafluoroborate, triphenyl phosphonium chloride, iodine tris (p-chlorophenyl) tetrafluoroborate, triphenyl sulfonium chloride, Triphenyl phosphonium bromide, tris (p-methoxyphenyl) phosphonium tetrafluoroborate, tris (p-methoxyphenyl) phosphonium hexafluorophosphate, tris (p-ethoxyphenyl) phosphonium tetrafluoroborate, bis [ [ (2-nitrobenzyl) oxy ] carbonylhexane-1, 6-diamine ], nitrobenzylcyclohexylcyclohexane carbamate, bis (methoxybenzyl) hexamethylene dicarbamate, bis [ [ (2-nitrobenzyl) oxy ] carbonylhexane-1, 6-diamine ], nitrobenzylcyclohexane carbamate, or bis (methoxybenzyl) hexamethylene dicarbamate, and the like.
In order to improve the adhesion between the liquid crystal layer and the liquid crystal alignment film, the liquid crystal alignment agent of the present invention preferably contains a compound having at least 1 structure selected from the group consisting of the following formulas [ b-1] to [ b-8] (also referred to as a specific adhesion compound).
Formula [ b-4]In (B)aRepresents a hydrogen atom or a benzene ring. Among them, a hydrogen atom is preferable. Formula [ b-8]In (B)bRepresents at least 1 cyclic group selected from the group consisting of a benzene ring, a cyclohexane ring and a heterocyclic ring. B iscRepresents at least 1 kind selected from the group consisting of alkyl, fluorine-containing alkyl, alkoxy and fluorine-containing alkoxy with 1-18 carbon atoms. Among them, preferred is an alkyl group or an alkoxy group having 1 to 12 carbon atoms.
More specifically, the specific adhesion compound is preferably a compound represented by the following formula [7A ].
Formula [7A ]]In, M1Is represented by a formula [ a-1] selected from]~[a-7]At least 1 of the group consisting of. Among them, the formula [ a-1] is preferred from the viewpoint of ease of production]Is of the formula [ a-2]Is of the formula [ a-3]Is of the formula [ a-5]Or formula [ a-6]. More preferably of the formula [ a-1]Is of the formula [ a-3]Is of the formula [ a-5]Or formula [ a-6]。
A1Represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms. Among them, from the viewpoint of ease of production, a hydrogen atom or an alkyl group having 1 to 2 carbon atoms is preferable. More preferably a hydrogen atom or a methyl group.
A2Represents a hydrogen atom or an alkyl group having 1 to 3 carbon atoms. Among them, from the viewpoint of ease of production, a hydrogen atom or an alkyl group having 1 to 2 carbon atoms is preferable. More preferably a hydrogen atom or a methyl group.
A3、A5、A6And A9Independently represents a hydrogen atom or an alkyl group having 1 to 3 carbon atoms. Among them, from the viewpoint of ease of production, a hydrogen atom or an alkyl group having 1 to 2 carbon atoms is preferable. More preferably a hydrogen atom or a methyl group.
A4、A7And A8Independently represents an alkylene group having 1 to 3 carbon atoms. Among them, an alkylene group having 1 to 2 carbon atoms is preferable from the viewpoint of ease of production.
Formula [7A ]]In, M2Is selected from the group consisting of a single bond, -CH2-、-O-、-NH-、-N(CH3)-、-CONH-、-NHCO-、-CH2O-、-OCH2-、-COO-、-OCO-、-CON(CH3) -and-N (CH)3) At least 1 bonding group of the group consisting of CO-. Among them, a single bond, -CH is preferable from the viewpoint of ease of production2-、-O-、-NH-、-CONH-、-NHCO-、-CH2O-、-OCH2-、-COO-、-OCO-、-CON(CH3) -or-N (CH)3) CO-. More preferably a single bond, -CH2-、-O-、-NH-、-CONH-、-CH2O-、-OCH2-, -COO-or-OCO-. In particularPreferably a single bond, -O-, -CONH-, -OCH2-, -COO-or-OCO-.
Formula [7A ]]In, M3Is selected from the group consisting of C1-20 alkylene groups, - (CH)2-CH2-O)p- (p represents an integer of 1 to 10), - (CH)2-O-)q- (q represents an integer of 1 to 10) and an organic group having a carbon number of 6 to 20 and having a benzene ring or a cyclohexane ring. In this case, any-CH of the aforementioned alkylene group2The radical-is optionally substituted by-COO-, -OCO-, -CONH-, NHCO-, -CO-, -S-, -SO2-、-CF2-、-C(CF3)2-、-Si(CH3)2-、-OSi(CH3)2-or-Si (CH)3)2O-substitution, the hydrogen atom bonded to any carbon atom being optionally substituted by a hydroxyl group (OH group), a carboxyl group (COOH group) or a halogen atom. Among them, alkylene group having 1 to 20 carbon atoms, - (CH) is preferable from the viewpoint of ease of production2-CH2-O)p-、-(CH2-O-)q-, or the following formula [ c-1]-formula [ c-5]. More preferably an alkylene group having 1 to 15 carbon atoms, - (CH)2-CH2-O)p-、-(CH2-O-)q-, the following formula [ c-1]Is of the formula [ c-3]Is of the formula [ c-4]Or formula [ c-5]. Particularly preferably C1-15 alkylene, - (CH)2-CH2-O)p-, formula [ c-1]Is of the formula [ c-4]Or formula [ c-5]。
Formula [7A ]]In, M4Is selected from the group consisting of a single bond, -CH2-、-OCH2And O-CH2-CH2-at least 1 bonding group of the group. Among them, a single bond, -CH is preferable from the viewpoint of ease of production2-or-OCH2-the structure shown. M5Is selected from the group consisting of the foregoing formula [ b-1]~[b-8]At least 1 structure of the group. Among them, the formula [ b-1] is preferred from the viewpoint of ease of production]Is of the formula [ b-2]Or formula [ b-6]. More preferably [ b-1]]Or formula [ b-2]. n represents an integer of 1 to 3. Among them, from the viewpoint of ease of production,an integer of 1 or 2 is preferable, and an integer of 1 is particularly preferable. m represents an integer of 1 to 3, and is preferably an integer of 1 or 2 in view of ease of production.
The specific adhesion compound is preferably at least 1 compound selected from the group consisting of the following formulas [7a-1] and [7a-5 ].
(n2 represents an integer of 1 to 10. m2 represents an integer of 1 to 10.)
Further, as the specific adhesion compound, the following compounds can be used.
Examples thereof include compounds having 3 polymerizable unsaturated groups in the molecule, such as trimethylolpropane tri (meth) acrylate, pentaerythritol tri (meth) acrylate, dipentaerythritol penta (meth) acrylate, tris (meth) acryloyloxyethoxy trimethylolpropane, or glycerol polyglycidyl ether poly (meth) acrylate; further, ethylene glycol di (meth) acrylate, diethylene glycol di (meth) acrylate, tetraethylene glycol di (meth) acrylate, polyethylene glycol di (meth) acrylate, propylene glycol di (meth) acrylate, polypropylene glycol di (meth) acrylate, butylene glycol di (meth) acrylate, neopentyl glycol di (meth) acrylate, ethylene oxide bisphenol a type di (meth) acrylate, propylene oxide bisphenol type di (meth) acrylate, 1, 6-hexanediol di (meth) acrylate, glycerin di (meth) acrylate, pentaerythritol di (meth) acrylate, ethylene glycol diglycidyl ether di (meth) acrylate, diethylene glycol diglycidyl ether di (meth) acrylate, phthalic acid diglycidyl ester di (meth) acrylate, hydroxypivalic acid neopentyl glycol di (meth) acrylate, or the like having 2 polymerizable unsaturated groups in the molecule A compound; and compounds having 1 polymerizable unsaturated group in the molecule, such as 2-hydroxyethyl (meth) acrylate, 2-hydroxypropyl (meth) acrylate, 2-hydroxybutyl (meth) acrylate, 2-phenoxy-2-hydroxypropyl (meth) acrylate, 2- (meth) acryloyloxy-2-hydroxypropyl phthalate, 3-chloro-2-hydroxypropyl (meth) acrylate, glycerol mono (meth) acrylate, 2- (meth) acryloyloxyethyl phosphate, or N-methylol (meth) acrylamide.
The content of the specific adhesion compound in the liquid crystal aligning agent is preferably 0.1 to 150 parts by mass per 100 parts by mass of the total polymer components. In order to promote the crosslinking reaction and to exhibit the desired effect, the amount of the crosslinking agent is more preferably 0.1 to 100 parts by mass, and particularly preferably 1 to 50 parts by mass, based on 100 parts by mass of the total polymer components. The specific adhesion compounds may be used in 1 kind or in a mixture of 2 or more kinds.
The liquid crystal aligning agent of the present invention preferably comprises: a compound having an epoxy group, an isocyanate group, an oxetanyl group or a cyclocarbonate group; or a compound having at least 1 group selected from the group consisting of a hydroxyl group, a hydroxyalkyl group and a lower alkoxyalkyl group (also collectively referred to as a specific crosslinkable compound). In this case, these groups are preferably present in the compound in an amount of 2 or more.
Examples of the crosslinkable compound having an epoxy group or an isocyanate group include, specifically, crosslinkable compounds having an epoxy group or an isocyanate group described on pages 37 to 38 of WO2013/125595 (published 2013.8.29).
Specific examples of the crosslinkable compound having an oxetanyl group include crosslinkable compounds represented by the formulae [4a ] to [4k ] described in WO2011/132751 at pages 58 to 59.
Specific examples of the crosslinkable compound having a cyclocarbonate group include crosslinkable compounds represented by the formulae [5-1] to [5-42] described in WO2012/014898 on pages 76 to 82.
Specific examples of the crosslinkable compound having at least 1 group selected from the group consisting of a hydroxyl group, a hydroxyalkyl group and a lower alkoxyalkyl group include melamine derivatives and benzoguanamine derivatives described on pages 39 to 40 of WO2013/125595 (disclosed in 2013.8.29), and crosslinkable compounds represented by formulae [6-1] to [6-48] described on pages 62 to 66 of WO2011/132751 (disclosed in 2011.10.27).
The content of the specific crosslinkable compound in the liquid crystal aligning agent is preferably 0.1 to 100 parts by mass per 100 parts by mass of the total polymer components. In order to promote the crosslinking reaction and to exhibit the desired effect, the amount of the crosslinking agent is more preferably 0.1 to 50 parts by mass, particularly preferably 1 to 30 parts by mass, based on 100 parts by mass of the total polymer components.
In order to promote charge transfer in the liquid crystal alignment film and to promote element charge removal, a nitrogen-containing heterocyclic amine compound represented by the formulae [ M1] to [ M156] described on pages 69 to 73 of WO2011/132751 (disclosed in 2011.10.27) may be added to the liquid crystal alignment treatment agent. The amine compound may be added directly to the liquid crystal aligning agent, or may be added after being dissolved in an appropriate solvent to a concentration of 0.1 to 10% by mass, preferably 1 to 7% by mass. The solvent is not particularly limited as long as it is an organic solvent that dissolves the specific polymer.
In addition, as the liquid crystal aligning agent, a compound which improves the uniformity of the film thickness and the surface smoothness of the liquid crystal alignment film when the liquid crystal aligning agent is applied can be used within a range not to impair the effects of the present invention. Further, a compound or the like which improves the adhesion between the liquid crystal alignment film and the substrate may be used.
Examples of the compound for improving the uniformity of the film thickness and the surface smoothness of the liquid crystal alignment film include a fluorine-based surfactant, a silicone-based surfactant, and a nonionic surfactant. Specifically, the surfactant described on pages 42 to 43 of WO2013/125595 (published 2013.8.29) can be mentioned.
The amount of the surfactant to be used is preferably 0.01 to 2 parts by mass, more preferably 0.01 to 1 part by mass, per 100 parts by mass of the total polymer components contained in the liquid crystal aligning agent.
Specific examples of the compound for improving the adhesion between the liquid crystal alignment film and the substrate include a functional silane-containing compound and an epoxy-containing compound. Specifically, the compounds described on pages 43 to 44 of WO2013/125595 (published 2013.8.29) are exemplified.
The amount of the compound for improving the adhesion between the liquid crystal alignment film and the substrates is preferably 0.1 to 30 parts by mass, more preferably 1 to 20 parts by mass, per 100 parts by mass of the total polymer components contained in the liquid crystal alignment agent. If the amount is less than 0.1 part by mass, the effect of improving adhesion cannot be expected, and if the amount is more than 30 parts by mass, the storage stability of the liquid crystal aligning agent may be deteriorated.
In addition to the above, a dielectric or conductive material for changing electrical characteristics such as permittivity and conductivity of the liquid crystal alignment film may be added to the liquid crystal alignment agent.
< methods for producing liquid Crystal alignment film and liquid Crystal display element >
The substrate used in the liquid crystal display element of the present invention is not particularly limited as long as it is a substrate having high transparency, and a plastic substrate such as an acrylic substrate, a polycarbonate substrate, a PET (polyethylene terephthalate) substrate, or the like, and a film thereof may be used in addition to a glass substrate. When the liquid crystal display element is used for a light control window or the like as a reverse type element, a plastic substrate or a film is preferable. In addition, from the viewpoint of simplifying the process, a substrate on which an ITO (Indium Tin Oxide) electrode, an IZO (Indium Zinc Oxide) electrode, an IGZO (Indium Gallium Zinc Oxide) electrode, an organic conductive film, and the like for driving a liquid crystal are formed is preferable. In the case of a reflection-type reverse type device, a substrate formed with a dielectric multilayer film of a metal such as silicon wafer or aluminum can be used as long as it is a single-sided substrate.
At least one substrate of the liquid crystal display element of the present invention has a liquid crystal alignment film for vertically aligning liquid crystal molecules. The liquid crystal alignment film can be obtained by applying a liquid crystal alignment treatment agent to a substrate, baking the applied liquid crystal alignment treatment agent, and then performing alignment treatment such as brushing treatment or light irradiation. In the case of the liquid crystal alignment film of the present invention, the liquid crystal alignment film can be used without performing such alignment treatment.
The method of applying the liquid crystal alignment treatment agent is not particularly limited, and there are industrial fields such as screen printing, offset printing, flexographic printing, ink jet method, dipping method, roll coating method, slit coating method, spin coating method, and spray method, and it can be appropriately selected depending on the kind of substrate and the target film thickness of the liquid crystal alignment film.
After the liquid crystal alignment treatment agent is coated on the substrate, the solvent is evaporated at a temperature of 30 to 300 ℃, preferably 30 to 250 ℃ depending on the kind of the substrate and the solvent used in the liquid crystal alignment treatment agent by heating means such as a hot plate, a thermal cycle oven, or an IR (infrared ray) oven, thereby forming a liquid crystal alignment film. In particular, when a plastic substrate is used as the substrate, the substrate is preferably treated at a temperature of 30 to 150 ℃.
When the thickness of the liquid crystal alignment film after firing is too large, it is disadvantageous in terms of power consumption of the device, and when the thickness is too small, the reliability of the device may be lowered, and therefore, it is preferably 5 to 500nm, more preferably 10 to 300nm, and particularly preferably 10 to 250 nm.
The liquid crystal composition used in the present invention is a liquid crystal composition as described above, and a spacer for controlling an electrode gap (also referred to as a gap) of the liquid crystal display element may be introduced therein.
The method of injecting the liquid crystal composition is not particularly limited, and examples thereof include the following methods. That is, when a glass substrate is used as the substrate, the following methods can be used: a pair of substrates on which liquid crystal alignment films were formed were prepared, a sealant was applied to all but a part of 4 sides of one substrate, and then the other substrate was attached with the surface of the liquid crystal alignment film facing inward, thereby producing an empty cell. Then, the liquid crystal composition was injected under reduced pressure from a portion where the sealant was not applied, thereby obtaining a cell in which the liquid crystal composition was injected. .
When a plastic substrate or film is used as the substrate, the following methods can be mentioned: a pair of substrates on which liquid crystal alignment films are formed are prepared, a liquid crystal composition is dropped on One substrate by an ODF (One Drop Filling) method, an ink jet method, or the like, and then the other substrate is bonded to obtain a cell in which the liquid crystal composition is injected. In the present invention, since the liquid crystal layer has high adhesion to the liquid crystal alignment film, the sealant need not be applied to the 4 sides of the substrate.
The gap of the liquid crystal display element can be controlled by the aforementioned spacer or the like. The method includes: a method of introducing a spacer of a target size into a liquid crystal composition, a method of using a substrate having a column spacer of a target size, and the like are described above. In addition, when the substrate is bonded by lamination using a plastic or film substrate, the gap can be controlled without introducing a spacer.
The gap size is preferably 1 to 100 μm, more preferably 2 to 50 μm, and particularly preferably 5 to 20 μm. If the gap is too small, the contrast of the liquid crystal display element decreases, and if the gap is too large, the driving voltage of the element increases.
The liquid crystal display element of the present invention is obtained by forming a liquid crystal layer of a cured product composite of a liquid crystal and a polymerizable compound by curing a liquid crystal composition by irradiation with ultraviolet rays. The liquid crystal composition is cured by irradiating the liquid crystal composition injection unit with ultraviolet rays. Examples of the light source of the ultraviolet irradiation device used in this case include a metal halide lamp and a high-pressure mercury lamp. The wavelength of the ultraviolet light is preferably 250 to 400nm, and particularly preferably 310 to 370 nm. After the irradiation with ultraviolet rays, heat treatment may be performed. The temperature at this time is 40 to 120 ℃, particularly preferably 40 to 80 ℃.
Examples
The present invention will be described in more detail with reference to the following examples, but the present invention is not limited thereto. The abbreviation is shown below.
L1: MLC-6608 (made by MERCK CORPORATION)
A1: 1, 3-diamino-4- [4- (trans-4-n-heptylcyclohexyl) phenoxy ] benzene
A2: 1, 3-diamino-4- [4- (trans-4-n-heptylcyclohexyl) phenoxymethyl ] benzene
A3: 1, 3-diamino-4- {4- [ trans-4- (trans-4-n-pentylcyclohexyl) cyclohexyl ] phenoxy } benzene
A4: diamine represented by the following formula [ A4]
A5: 1, 3-diamino-4-octadecyloxybenzene
B1: 3, 5-diaminobenzoic acid
B2: diamine represented by the following formula [ B2]
C1: m-phenylenediamine
(tetracarboxylic dianhydride)
D1: 1,2,3, 4-cyclobutanetetracarboxylic dianhydride
D2: bicyclo [3,3,0] octane-2, 4,6, 8-tetracarboxylic dianhydride
D3: tetracarboxylic dianhydride represented by the following formula [ D3]
D4: tetracarboxylic dianhydride represented by the following formula [ D4]
D5: tetracarboxylic dianhydride represented by the following formula [ D5]
E1: an alkoxysilane monomer represented by the formula [ E1]
E2: octadecyltriethoxysilane
E3: 3-methacryloxypropyltrimethoxysilane
E4: 3-Urea propyl triethoxy silane
E5: tetraethoxysilane
N1: a photo-radical generator represented by the following formula [ N1]
< specific adhesion Compound >
K1: a crosslinkable compound represented by the following formula [ K1]
NMP: n-methyl-2-pyrrolidone
NEP: n-ethyl-2-pyrrolidone
gamma-BL: gamma-butyrolactone
BCS: ethylene glycol monobutyl ether
PB: propylene glycol monobutyl ether
PGME: propylene glycol monomethyl ether
And (3) ECS: ethylene glycol monoethyl ether
EC: diethylene glycol monoethyl ether
"molecular weight measurement of polyimide-based Polymer"
The measurement was carried out by the following procedure using a gel permeation chromatography at room temperature (GPC) apparatus (GPC-101) (manufactured by Showa Denko K.K.) and columns (KD-803, KD-805) (manufactured by Shodex).
Column temperature: 50 deg.C
Eluent: n, N' -dimethylformamide (as additive, lithium bromide monohydrate (LiBr. H)2O) 30mmol/L (liter), phosphoric acid-anhydrous crystal (orthophosphoric acid) 30mmol/L, Tetrahydrofuran (THF) 10ml/L)
Flow rate: 1.0 ml/min
Standard sample for standard curve preparation: TSK standard polyethylene oxides (molecular weight: about 900000, 150000, 100000 and 30000) (manufactured by Tosoh corporation) and polyethylene glycols (molecular weight: about 12000, 4000 and 1000) (manufactured by Polymer laboratories Ltd.).
"measurement of imidization ratio of polyimide-based Polymer"
20mg of the polyimide powder was put into an NMR (nuclear magnetic resonance) sample tube (. phi.5, manufactured by Softweed scientific Co., Ltd.), deuterated dimethyl sulfoxide (DMSO-d6, 0.05 mass% TMS (tetramethylsilane) mixture) (0.53ml) was added thereto, and the mixture was completely dissolved by applying ultrasonic waves. The solution was subjected to proton NMR measurement at 500MHz using an NMR spectrometer (JNW-ECA500) (manufactured by JEOLDATUM). The imidization ratio is determined using a proton derived from a structure that does not change before and after imidization as a reference proton, and is obtained by the following equation using the peak integral value of the proton and the peak integral value of a proton derived from an amic acid NH group appearing in the vicinity of 9.5 to 10.0 ppm.
Imidization ratio (%) - (1-. alpha.x/y). times.100
(x is the peak integral value of the proton derived from the NH group of amic acid, y is the peak integral value of the reference proton, and α is the ratio of the number of reference protons to 1 proton of the NH group of amic acid when polyamic acid (imidization ratio of 0%))
Synthesis of polyimide-based Polymer "
< Synthesis example 1>
D1(3.00g, 15.3mmol), A1(2.95g, 7.75mmol), B1(0.94g, 6.18mmol) and C1(0.17g, 1.57mmol) were mixed in PGME (21.2g) and reacted at 40 ℃ for 24 hours to obtain a polyamic acid solution (1) having a resin solid concentration of 25 mass%. The polyamic acid had a number-average molecular weight (Mn) of 10,100 and a weight-average molecular weight (Mw) of 43,600.
< Synthesis example 2>
D2(3.83g, 15.3mmol), A2(6.11g, 15.5mmol) and B1(2.36g, 15.5mmol) were mixed with NMP (30.6g) and reacted at 80 ℃ for 5 hours, then D1(3.00g, 15.3mmol) and NMP (15.3g) were added and reacted at 40 ℃ for 6 hours to obtain a polyamic acid solution (2) having a resin solid concentration of 25 mass%. The polyamic acid had Mn of 22,500 and Mw of 67,100.
< Synthesis example 3>
To a polyamic acid solution (2) (30.0g) obtained in Synthesis example 2 was added NMP and diluted to 6 mass%, and then acetic anhydride (3.90g) and pyridine (2.40g) were added as an imidization catalyst to react at 60 ℃ for 2 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (3). The polyimide had an imidization ratio of 60%, an Mn of 20,100 and an Mw of 57,100.
< Synthesis example 4>
D2(2.55g, 10.2mmol), A3(4.47g, 10.3mmol), B1(1.57g, 10.3mmol) and B2(1.05g, 5.17mmol) were mixed with NMP (25.3g) and reacted at 80 ℃ for 5 hours, and then D1(3.00g, 15.3mmol) and NMP (12.6g) were added and reacted at 40 ℃ for 6 hours to obtain a polyamic acid solution having a resin solid concentration of 25 mass%.
NMP was added to the obtained polyamic acid solution (30.0g) to dilute the solution to 6 mass%, and acetic anhydride (3.85g) and pyridine (2.42g) were added as an imidization catalyst to react at 50 ℃ for 3 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (4). The polyimide had an imidization rate of 56%, Mn of 18,500 and Mw of 54,000.
< Synthesis example 5>
D2(2.55g, 10.2mmol), A4(3.05g, 6.19mmol), B1(1.57g, 10.3mmol), B2(0.42g, 2.07mmol) and C1(0.22g, 2.03mmol) were mixed with NMP (19.6g) and reacted at 80 ℃ for 5 hours, then D1(2.00g, 10.2mmol) and NMP (9.82g) were added and reacted at 40 ℃ for 6 hours to obtain a polyamic acid solution having a resin solid content of 25 mass%.
NMP was added to the obtained polyamic acid solution (30.0g) to dilute the solution to 6 mass%, and acetic anhydride (3.85g) and pyridine (2.50g) were added as an imidization catalyst to react at 50 ℃ for 2 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (5). The polyimide had an imidization rate of 49%, Mn of 16,100 and Mw of 49,800.
< Synthesis example 6>
D3(5.50g, 24.5mmol), A2(5.88g, 14.9mmol), B1(1.13g, 7.43mmol) and B2(0.51g, 2.51mmol) were mixed in NMP (39.1g) and reacted at 40 ℃ for 12 hours to obtain a polyamic acid solution having a resin solid concentration of 25% by mass.
NMP was added to the obtained polyamic acid solution (30.0g) to dilute the solution to 6 mass%, and acetic anhydride (3.85g) and pyridine (2.48g) were added as an imidization catalyst to react at 60 ℃ for 2.5 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (6). The polyimide had an imidization rate of 63%, Mn of 17,200 and Mw of 49,100.
< Synthesis example 7>
D3(5.50g, 24.5mmol), A4(3.67g, 7.45mmol) and B1(2.65g, 17.4mmol) were mixed with NMP (35.5g) and reacted at 40 ℃ for 12 hours to obtain a polyamic acid solution having a resin solid concentration of 25 mass%.
NMP was added to the obtained polyamic acid solution (30.0g) to dilute the solution to 6 mass%, and acetic anhydride (3.85g) and pyridine (2.50g) were added as an imidization catalyst to react at 50 ℃ for 3 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (7). The polyimide had an imidization rate of 54%, Mn of 17,400 and Mw of 47,800.
< Synthesis example 8>
D4(4.59g, 15.3mmol), A3(6.70g, 15.5mmol), B1(1.89g, 12.4mmol) and B2(0.63g, 3.10mmol) were mixed with NMP (33.6g) and reacted at 50 ℃ for 8 hours, and then D1(3.00g, 15.3mmol) and NMP (16.8g) were added and reacted at 40 ℃ for 6 hours to obtain a polyamic acid solution having a resin solid concentration of 25 mass%.
NMP was added to the obtained polyamic acid solution (30.0g) to dilute the solution to 6 mass%, and acetic anhydride (4.50g) and pyridine (3.30g) were added as an imidization catalyst to react at 70 ℃ for 3 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (8). The polyimide had an imidization rate of 71%, Mn of 17,100 and Mw of 38,800.
< Synthesis example 9>
D5(1.68g, 7.92mmol), A2(2.53g, 6.41mmol), B1(0.49g, 3.22mmol) and B2(1.30g, 6.40mmol) were mixed with PGME (15.1g) and reacted at 50 ℃ for 24 hours, and then D1(1.55g, 7.90mmol) and PGME (7.54g) were added and reacted at 50 ℃ for 12 hours to obtain a polyamic acid solution (9) having a resin solid concentration of 25 mass%. The polyamic acid had Mn of 10,500 and Mw of 48,500.
< Synthesis example 10>
D2(3.83g, 15.3mmol), A5(5.84g, 15.5mmol) and B1(2.36g, 15.5mmol) were mixed with NMP (30.0g) and reacted at 80 ℃ for 5 hours, and then D1(3.00g, 15.3mmol) and NMP (15.0g) were added and reacted at 40 ℃ for 6 hours to obtain a polyamic acid solution having a resin solid concentration of 25 mass%.
NMP was added to the obtained polyamic acid solution (30.0g) to dilute the solution to 6 mass%, and acetic anhydride (3.90g) and pyridine (2.40g) were added as an imidization catalyst to react at 60 ℃ for 2 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (10). The polyimide had an imidization ratio of 61%, Mn of 19,000 and Mw of 58,100.
< Synthesis example 11>
D2(3.83g, 15.3mmol) and B1(4.72g, 31.0mmol) were mixed with NMP (23.1g) and reacted at 80 ℃ for 5 hours, then D1(3.00g, 15.3mmol) and NMP (11.5g) were added and reacted at 40 ℃ for 6 hours to obtain a polyamic acid solution (11) having a resin solid concentration of 25 mass%. The polyamic acid had Mn of 25,900 and Mw of 79,100.
< Synthesis example 12>
To a polyamic acid solution (11) (30.0g) obtained in Synthesis example 11 was added NMP and diluted to 6 mass%, and then acetic anhydride (3.85g) and pyridine (2.40g) were added as an imidization catalyst to conduct a reaction at 60 ℃ for 2 hours. The reaction solution was poured into methanol (460ml), and the resulting precipitate was collected by filtration. The precipitate was washed with methanol and dried under reduced pressure at 100 ℃ to obtain polyimide powder (12). The polyimide had an imidization rate of 59%, Mn of 21,200 and Mw of 60,100.
The polyimide-based polymers obtained in synthesis examples 1 to 12 are shown in Table 10.
[ Table 10]
*1: polyamic acid
Synthesis of polysiloxane Polymer "
< Synthesis example 13>
In a 200ml four-necked reaction flask equipped with a thermometer and a reflux tube, ECS (28.3g), E1(4.10g), E3(7.45g) and E5(32.5g) were mixed to prepare a solution of an alkoxysilane monomer. To this solution, a solution prepared by previously mixing ECS (14.2g), water (10.8g) and oxalic acid (0.70g) as a catalyst was added dropwise at 25 ℃ over 30 minutes, and further stirred at 25 ℃ for 30 minutes. Thereafter, the mixture was heated to reflux for 30 minutes using an oil bath, and then a mixed solution of a methanol solution (1.20g) having an E4 content of 92 mass% and ECS (0.90g) which had been prepared in advance was added. Further refluxing for 30 minutes, and naturally cooling to obtain SiO2Polysiloxane solution (1) having a concentration of 12% by mass as converted.
< Synthesis example 14>
In a 200ml four-necked reaction flask equipped with a thermometer and a reflux tube, EC (25.4g), E1(8.20g), E3(19.9g) and E5(20.0g) were mixed to prepare a solution of an alkoxysilane monomer. To the solution was added dropwise a solution prepared by previously mixing EC (12.7g), water (10.8g) and oxalic acid (1.10g) as a catalyst at 25 ℃ over 30 minutes,further, the mixture was stirred at 25 ℃ for 30 minutes. Thereafter, the mixture was heated to reflux for 30 minutes using an oil bath, and then a previously prepared mixed solution of a methanol solution (1.20g) having an E4 content of 92 mass% and EC (0.90g) was added. Further refluxing for 30 minutes, and naturally cooling to obtain SiO2A polysiloxane solution (2) having a concentration of 12% by mass as converted.
< Synthesis example 15>
A solution of alkoxysilane monomer was prepared by mixing EC (29.2g), E1(4.10g) and E5(38.8g) in a 200ml four-necked reaction flask equipped with a thermometer and reflux tube. To this solution, a solution prepared by previously mixing EC (14.6g), water (10.8g), and oxalic acid (0.50g) as a catalyst was added dropwise at 25 ℃ over 30 minutes, and further stirred at 25 ℃ for 30 minutes. Thereafter, the mixture was heated to reflux for 30 minutes using an oil bath, and then a previously prepared mixed solution of a methanol solution (1.20g) having an E4 content of 92 mass% and EC (0.90g) was added. Further refluxing for 30 minutes, and naturally cooling to obtain SiO2A polysiloxane solution (3) having a concentration of 12% by mass as converted.
< Synthesis example 16>
In a 200ml four-necked reaction flask equipped with a thermometer and a reflux tube, ECS (28.3g), E2(4.07g), E3(7.45g) and E5(32.5g) were mixed to prepare a solution of an alkoxysilane monomer. To this solution, a solution prepared by previously mixing ECS (14.2g), water (10.8g) and oxalic acid (0.70g) as a catalyst was added dropwise at 25 ℃ over 30 minutes, and further stirred at 25 ℃ for 30 minutes. Thereafter, the mixture was heated to reflux for 30 minutes using an oil bath, and then a mixed solution of a methanol solution (1.20g) having an E4 content of 92 mass% and ECS (0.90g) which had been prepared in advance was added. Further refluxing for 30 minutes, and naturally cooling to obtain SiO2A polysiloxane solution (4) having a concentration of 12% by mass as converted.
The polysiloxane polymers obtained in Synthesis examples 13 to 16 are shown in Table 11.
[ Table 11]
Preparation of liquid Crystal composition "
(liquid Crystal composition (1))
L1(2.40g), R1(1.20g), R2(1.20g), S1(0.024g) and P1(0.012g) were mixed to obtain liquid crystal composition (1).
(liquid Crystal composition (2))
L1(2.40g), R1(1.20g), R2(1.20g), S1(0.24g) and P1(0.012g) were mixed to obtain a liquid crystal composition (2).
(liquid Crystal composition (3))
L1(2.40g), R1(1.20g), R2(1.20g), S2(0.048g) and P1(0.012g) were mixed to obtain a liquid crystal composition (3).
(liquid Crystal composition (4))
L1(2.40g), R1(1.20g), R2(1.20g) and P1(0.012g) were mixed to obtain a liquid crystal composition (4).
"production of liquid Crystal display element and evaluation of liquid Crystal alignment Property (glass substrate)"
The liquid crystal aligning agents obtained in examples 4, 11, 12, 16, 17 and 20 and comparative examples 1,3 and 5 were subjected to pressure filtration using a membrane filter having a pore size of 1 μm. The obtained solution was spin-coated on the ITO surface of a 100X 100mm glass substrate (longitudinal: 100mm, transverse: 100mm, thickness: 0.7mm) with an ITO electrode cleaned with pure water and IPA (isopropyl alcohol), and the ITO substrate was heat-treated on a hot plate at 100 ℃ for 5 minutes and a thermal cycle type cleaning oven at 210 ℃ for 30 minutes to obtain an ITO substrate with a liquid crystal alignment film having a film thickness of 100 nm. 2 pieces of the obtained ITO substrates with liquid crystal alignment films were prepared, and a spacer of 6 μm was coated on the liquid crystal alignment film surface of one of the substrates. Then, the liquid crystal composition was dropped on the spacer-coated liquid crystal alignment film surface of the substrate by an odf (one Drop filling) method, and then bonded to the liquid crystal alignment film surface of the other substrate so as to face the liquid crystal alignment film interface, thereby obtaining a liquid crystal display element before treatment.
The liquid crystal display element before the treatment was irradiated with ultraviolet light for 30 seconds using a metal halide lamp with an illuminance of 20mW, with a wavelength of 350nm or less cut off. At this time, the temperature in the irradiation device when the liquid crystal cell was irradiated with ultraviolet rays was controlled to 25 ℃. Thereby, a liquid crystal display element (reverse type element) was obtained.
The liquid crystal display element was used to evaluate the liquid crystal alignment properties. The liquid crystal alignment properties were observed with a polarizing microscope (ECLIPSE E600WPOL) (manufactured by nikon) to confirm whether the liquid crystal was vertically aligned. Specifically, the evaluation was regarded as excellent when the liquid crystal was vertically aligned (as good in tables 15 to 17).
The liquid crystal display element after the evaluation of the liquid crystal alignment was stored in a thermostatic bath at a temperature of 90 ℃ for 96 hours. Then, the liquid crystal alignment was evaluated under the same conditions as described above. Specifically, when no disturbance of the liquid crystal alignment was observed and the liquid crystal was uniformly aligned, the evaluation was regarded as excellent (as good in tables 15 to 17).
"production of liquid Crystal display element and evaluation of liquid Crystal alignment Property (Plastic substrate)"
The liquid crystal aligning agents obtained in examples 1 to 3,5 to 10, 13 to 15, 18 and 19 and comparative examples 2 and 4 were subjected to pressure filtration using a membrane filter having a pore diameter of 1 μm. The obtained solution was applied to an ITO surface of a 150X 150mm PET (polyethylene terephthalate) substrate (longitudinal: 150mm, lateral: 150mm, thickness: 0.2mm) with an ITO electrode, which was cleaned with pure water, by a bar coater, and heat-treated on a hot plate at 100 ℃ for 5 minutes and a heat-circulation type cleaning oven at 120 ℃ for 2 minutes, to obtain an ITO substrate with a liquid crystal alignment film having a film thickness of 100 nm. 2 pieces of the obtained ITO substrates with liquid crystal alignment films were prepared, and a spacer of 6 μm was coated on the liquid crystal alignment film surface of one of the substrates. Thereafter, the liquid crystal composition was dropped on the spacer-coated liquid crystal alignment film surface of the substrate by the ODF method, and then the liquid crystal composition was bonded to the liquid crystal alignment film surface of the other substrate so as to face the liquid crystal alignment film interface of the other substrate, thereby obtaining a liquid crystal display element before treatment
The liquid crystal display element before the treatment was irradiated with ultraviolet light for 30 seconds using a metal halide lamp with an illuminance of 20mW, with a wavelength of 350nm or less cut off. At this time, the temperature in the irradiation device when the liquid crystal cell was irradiated with ultraviolet rays was controlled to 25 ℃. Thereby, a liquid crystal display element (reverse type element) was obtained.
The liquid crystal display element was used to evaluate the liquid crystal alignment properties. The liquid crystal alignment properties were observed with a polarizing microscope (ECLIPSE E600WPOL) (manufactured by nikon) to confirm whether the liquid crystal was vertically aligned. Specifically, the evaluation was regarded as excellent when the liquid crystal was vertically aligned (as good in tables 15 to 17).
The liquid crystal display element after the evaluation of the liquid crystal alignment was stored in a thermostatic bath at a temperature of 90 ℃ for 96 hours. Then, the liquid crystal alignment was evaluated under the same conditions as described above. Specifically, when no disturbance of the liquid crystal alignment was observed and the liquid crystal was uniformly aligned, the evaluation was regarded as excellent (as good in tables 15 to 17).
"evaluation of optical Properties (transparency and Scattering Property)"
The liquid crystal display elements (glass substrates and plastic substrates) obtained by the above-described methods were used to evaluate optical properties (transparency and scattering properties).
The transparency when no voltage was applied was evaluated by measuring the transmittance of the liquid crystal display element (glass substrate and plastic substrate) in a state where no voltage was applied. Specifically, the transmittance of the sample was measured by using a UV-3600 (manufactured by Shimadzu corporation) under the conditions of a temperature of 25 ℃ and a scanning wavelength of 300 to 800 nm. In this case, reference (reference example) was made to the use of the glass substrate with ITO electrodes in the case of a liquid crystal display element (glass substrate), and the use of the PET substrate with ITO electrodes in the case of a liquid crystal display element (plastic substrate). The evaluation was carried out to determine that the higher the transmittance, the better the transparency, based on the transmittance at a wavelength of 450 nm.
Further, in examples 1 to 3,5 to 7, 13 to 17 and 20, in addition to the above-mentioned standard test, as an emphasis test, the transmittance of the liquid crystal display element after storage for 144 hours in a constant temperature bath at a temperature of 90 ℃ was evaluated. The evaluation method is the same as the above. The values of the transmittance (%) are shown in tables 15 to 17.
The scattering characteristics when a voltage was applied were evaluated by applying 30V to the liquid crystal display element (glass substrate and plastic substrate) by alternating current driving and observing the alignment state of the liquid crystal by visual observation. Specifically, when the device was clouded, that is, when scattering characteristics could be obtained, the evaluation was regarded as excellent (shown as good in tables 15 to 17).
"evaluation of adhesion between liquid Crystal layer and liquid Crystal alignment film"
Using the liquid crystal display element (glass substrate and plastic substrate) obtained by the above method, adhesion between the liquid crystal layer and the liquid crystal alignment film was evaluated.
The liquid crystal display elements (glass substrate and plastic substrate) were stored in a constant temperature and humidity chamber at 80 ℃ and a humidity of 90% RH for 24 hours to confirm the presence or absence of air bubbles in the liquid crystal display elements and the peeling of the elements. Specifically, when no bubble was observed in the cell and the cell was not peeled off (the liquid crystal layer and the liquid crystal alignment film were peeled off), the evaluation was regarded as excellent (good in the table).
Further, in examples 1 to 3,5 to 7, 13 to 17 and 20, in addition to the above-mentioned standard test, as an emphasized test, an evaluation was made after storing the steel in a constant temperature and humidity chamber at a temperature of 80 ℃ and a humidity of 90% RH for 48 hours. The evaluation method is the same as the above. The results of adhesion (adhesion) between the liquid crystal layer and the liquid crystal alignment film are shown in tables 15 to 17.
< preparation of liquid Crystal alignment treating agent >
< liquid Crystal alignment treating agent (1) >
PGME (20.7g) and γ -BL (4.38g) were added to a polyamic acid solution (1) (5.50g) having a resin solid content concentration of 25 mass% obtained in Synthesis example 1, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal alignment treatment agent (1). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (2) >
To a polyamic acid solution (1) (10.0g) having a resin solid content concentration of 25% by mass obtained in Synthesis example 1 were added N1(0.125g), M1(0.375g), K1(0.175g), PGME (37.6g), and γ -BL (7.96g), and the mixture was stirred at 25 ℃ for 6 hours to obtain a liquid crystal aligning agent (2). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (3) >
To a polyamic acid solution (2) (5.50g) having a resin solid content concentration of 25% by mass obtained in synthesis example 2 were added NMP (10.5g) and BCS (14.6g), and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (3). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (4) >
To the polyimide powder (3) (1.45g) obtained in Synthesis example 3 were added γ -BL (3.08g) and PGME (27.7g), and the mixture was stirred at 60 ℃ for 24 hours to obtain a liquid crystal aligning agent (4). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (5) >
To the polyimide powder (4) (1.40g) obtained in Synthesis example 4 were added γ -BL (2.97g) and PGME (26.7g), and the mixture was stirred at 60 ℃ for 24 hours to obtain a liquid crystal aligning agent (5). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (6) >
To the polyimide powder (4) (1.40g) obtained in Synthesis example 4 were added γ -BL (2.97g) and PGME (26.7g), and the mixture was stirred at 60 ℃ for 24 hours. Thereafter, N1(0.098g), M1(0.42g) and K1(0.07g) were added thereto, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (6). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (7) >
To the polyimide powder (4) (1.45g) obtained in Synthesis example 4 were added γ -BL (6.15g) and PGME (24.6g), and the mixture was stirred at 60 ℃ for 24 hours. Subsequently, N1(0.073g) and K1(0.145g) were added thereto, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (7). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (8) >
To the polyimide powder (5) (1.45g) obtained in Synthesis example 5 were added γ -BL (6.15g) and PGME (24.6g), and the mixture was stirred at 60 ℃ for 24 hours. Thereafter, N1(0.044g), M1(0.145g) and K1(0.073g) were added thereto, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (8). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (9) >
To the polyimide powder (6) (1.40g) obtained in Synthesis example 6 were added γ -BL (2.97g) and PGME (26.7g), and the mixture was stirred at 60 ℃ for 24 hours. Subsequently, N1(0.07g) and K1(0.098g) were added thereto, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (9). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (10) >
To the polyimide powder (7) (1.45g) obtained in Synthesis example 7 were added γ -BL (13.9g), BCS (7.69g) and PB (9.23g), and the mixture was stirred at 60 ℃ for 24 hours. Thereafter, N1(0.102g), M2(0.073g) and K1(0.073g) were added thereto, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (10). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (11) >
To the polyimide powder (8) (1.45g) obtained in Synthesis example 8 were added NEP (12.3g), BCS (9.23g) and PB (9.23g), and the mixture was stirred at 60 ℃ for 24 hours. Subsequently, N1(0.073g) and K1(0.044g) were added thereto, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (11). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (12) >
PGME (20.7g) and γ -BL (4.38g) were added to a polyamic acid solution (9) (5.50g) having a resin solid content concentration of 25 mass% obtained in Synthesis example 9, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal alignment treatment agent (12). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (13) >
PGME (20.7g) and γ -BL (4.38g) were added to a polyamic acid solution (9) (5.50g) having a resin solid content concentration of 25% by mass obtained in Synthesis example 9, and the mixture was stirred at 25 ℃ for 4 hours. Thereafter, N1(0.069g), M2(0.138g) and K1(0.097g) were added thereto, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (13). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (14) >
To the polyimide powder (10) (1.45g) obtained in Synthesis example 10 were added γ -BL (3.08g) and γ -BL (27.7g), and the mixture was stirred at 60 ℃ for 24 hours to obtain a liquid crystal aligning agent (14). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (15) >
To the polysiloxane solution (1) (12.5g) obtained in Synthesis example 13 were added ECS (1.73g), BCS (9.55g) and PB (9.55g), and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (15). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (16) >
To the polysiloxane solution (2) (12.0g) obtained in Synthesis example 14 were added N1(0.072g), M1(0.288g), EC (0.14g), PB (10.7g) and PGME (9.17g), and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (16). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (17) >
To the polysiloxane solution (3) (12.0g) obtained in Synthesis example 15 were added EC (0.14g), PB (10.7g) and PGME (9.17g), and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal alignment treatment agent (17). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (18) >
To the polysiloxane solution (4) (12.0g) obtained in Synthesis example 16 were added ECS (1.66g), BCS (9.17g) and PB (9.17g), and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal alignment treatment agent (18). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (19) (comparative example) >
NMP (10.5g) and BCS (14.6g) were added to a polyamic acid solution (11) (5.50g) having a resin solid content concentration of 25% by mass obtained in Synthesis example 11, and the mixture was stirred at 25 ℃ for 4 hours to obtain a liquid crystal aligning agent (19). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< liquid Crystal alignment treating agent (20) (comparative example) >
To the polyimide powder (12) (1.45g) obtained in Synthesis example 12 were added γ -BL (3.08g) and PGME (27.7g), and the mixture was stirred at 60 ℃ for 24 hours to obtain a liquid crystal aligning agent (20). The liquid crystal aligning agent was confirmed to be a uniform solution without any abnormality such as turbidity and precipitation.
< examples 1 to 20 and comparative examples 1 to 5>
Liquid crystal display elements for evaluation were produced using any of the above-described liquid crystal compositions (1) to (4) and any of the liquid crystal alignment treatment agents (1) to (20) in the combinations shown in tables 12 to 17 below, and the liquid crystal display elements were evaluated. These results are shown in tables 12 to 14.
In tables 12 to 14, the values "1" to "3" represent the following values, respectively.
*1: the content ratio (parts by mass) of the specific initiator to 100 parts by mass of the total polymer is shown. *2: the content ratio (parts by mass) of the specific adhesion compound to 100 parts by mass of the total polymer is shown. *3: the content ratio (parts by mass) of the specific crosslinkable compound to 100 parts by mass of the total polymer is shown.
In tables 15 to 17, each of the symbols 1 to 5 represents the following.
*1: the liquid crystals are not vertically aligned. *2: disturbance of liquid crystal alignment was observed at several sites in the device. *3: very few bubbles were observed in the cell. *4: a small number of bubbles (more than in x 3) were observed within the cell. *5: the liquid crystal was not vertically aligned, and thus it was not evaluated.
[ Table 12]
[ Table 13]
[ Table 14]
[ Table 15]
[ Table 16]
[ Table 17]
As can be seen from tables 12 to 17: the liquid crystal display elements of the examples were superior in liquid crystal alignment properties after storage in the constant temperature and humidity chamber and transparency when no voltage was applied, and also had high adhesion between the liquid crystal layer and the liquid crystal alignment film, as compared with the comparative examples. In particular, even when a plastic substrate is used as the substrate of the liquid crystal display element, these characteristics are good.
In particular, in the examples containing the specific compound, the liquid crystal alignment property was not disturbed even when the liquid crystal display element was stored in the thermostatic bath, and the transparency of the liquid crystal display element was improved when no voltage was applied, as compared with the comparative examples containing no specific compound in the liquid crystal composition. Specifically, the comparison under the same conditions is a comparison of example 4 with comparative example 3, example 5 with comparative example 4, and example 16 with comparative example 5.
In addition, when the amount of the specific compound in the liquid crystal composition is larger than that in the case where the amount of the specific compound is small, the liquid crystal display element exhibits high transparency when no voltage is applied. Specifically, the comparison under the same conditions is the comparison of example 2 with example 3, and example 16 with example 17.
Further, among the specific side chain structures, the following results were exhibited when the specific side chain structure of the formula [2-1] was used, as compared with the case of using the formula [2-2 ]: the liquid crystal display element had high transparency when no voltage was applied, and the transparency when no voltage was applied was also high after long-term storage in a thermostatic bath (storage in the thermostatic bath at 90 ℃ C. for 144 hours) as an emphasis test. In addition, when the specific side chain structure of the formula [2-1] was used for evaluating the adhesion between the liquid crystal layer and the liquid crystal alignment film, the adhesion was high even after the liquid crystal layer was stored in the constant temperature and humidity chamber for a long period of time (48 hours of storage in the constant temperature and humidity chamber at a temperature of 80 ℃ and a humidity of 90% RH) as an emphasis test. Specifically, it is emphasized that the comparisons under the same conditions in the tests are the comparisons of example 5 with example 15, and of example 16 with example 20.
When the specific generator, the specific adhesive compound, and the specific crosslinkable compound are introduced into the liquid crystal alignment agent, the adhesion between the liquid crystal layer and the liquid crystal alignment film in the liquid crystal display device is further improved as compared with the case where they are not introduced. Specifically, it is emphasized that the comparisons under the same conditions in the tests are the comparisons of example 1 with example 2, example 6 with example 7, and example 13 with example 14.
Industrial applicability
The liquid crystal display element of the present invention is used as a light control window or a shutter element for controlling light transmission and light shielding, particularly as a retroreflective element, in a liquid crystal display for display purposes, and in transportation equipment and transportation machinery such as automobiles, railways, and aircrafts.
In particular, when the element is used for a window glass of a vehicle, the light-capturing efficiency at night is higher than that of a conventional reverse type element, and the effect of preventing glare from external light is also higher. Therefore, safety when driving the vehicle and comfort when riding can be further improved. Further, when the element is produced from a film and used by being stuck to a glass window of a vehicle, defects and deterioration due to low adhesion of the liquid crystal layer to the liquid crystal alignment film are less likely to occur as compared with a conventional reverse type element, and thus, the reliability of the element is improved.
Further, the element is used for a light guide plate of a display device such as LCD and OLED, and a back plate of a transparent display. Specifically, when the transparent display is used as a back plate of a transparent display, light can be prevented from entering from the back surface of the transparent display when the transparent display is displayed on the transparent display together with the element.
The entire contents of the specification, claims and abstract of japanese patent application No. 2014-195601, which was filed on 9/25/2014, are hereby incorporated by reference as the disclosure of the present invention specification.
Claims (13)
1. A liquid crystal display element of a transmission/scattering type having a liquid crystal layer between a pair of substrates having electrodes, at least one of the substrates having a liquid crystal alignment film for vertically aligning liquid crystal, and exhibiting a transmission state when no voltage is applied and a scattering state when a voltage is applied, wherein the liquid crystal layer is obtained by curing a liquid crystal composition containing a liquid crystal and a polymerizable compound disposed between the pair of substrates by irradiating the liquid crystal composition with ultraviolet rays,
the liquid crystal composition contains a compound represented by the following formula [1], the liquid crystal alignment film is obtained from a liquid crystal alignment treatment agent containing a polymer having a side chain structure represented by the following formula [2-1] or formula [2-2],
X1is represented by a formula [1-a ] selected from]-formula [1-g]At least 1 of the group consisting of; x2Represents a group selected from the group consisting of a single bond, -O-, -NH-, -N (CH)3)-、-CH2O-、-CONH-、-NHCO-、-CON(CH3)-、-N(CH3) At least 1 bonding group selected from the group consisting of CO-, -COO-and-OCO-; x3Is represented by- (CH)2)a-, wherein a is an integer of 1 to 15; x4Represents a group selected from the group consisting of a single bond, -O-, -OCH2At least 1 bonding group of the group consisting of-COO-and-OCO-; x5A 2-valent organic group having 17 to 51 carbon atoms and representing a benzene ring, a cyclohexane ring, or a steroid skeleton, wherein any hydrogen atom on the benzene ring or the cyclohexane ring is optionally substituted by an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluorine-containing alkyl group having 1 to 3 carbon atoms, a fluorine-containing alkoxy group having 1 to 3 carbon atoms, or a fluorine atom; x6Represents a group selected from the group consisting of a single bond, -O-, -OCH2-、-CH2At least 1 bonding group selected from the group consisting of O-, -COO-and-OCO-; x7Represents a benzene ring or a cyclohexane ring, and any hydrogen atom on these cyclic groups is optionally substituted by an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluorine-containing alkyl group having 1 to 3 carbon atoms, a fluorine-containing alkoxy group having 1 to 3 carbon atoms or a fluorine atom; p represents an integer of 0 to 4; x8Represents at least 1 kind selected from the group consisting of C1-18 alkyl, C1-18 fluorine-containing alkyl, C1-18 alkoxy and C1-18 fluorine-containing alkoxy,
XArepresents a hydrogen atom or a benzene ring; xBRepresents at least 1 cyclic group selected from the group consisting of a benzene ring, a cyclohexane ring and a heterocyclic ring; xCRepresents at least 1 kind selected from the group consisting of C1-18 alkyl, C1-18 fluorine-containing alkyl, C1-18 alkoxy and C1-18 fluorine-containing alkoxy,
Y1and Y3Each independently represents a group selected from a single bond, - (CH)2)a-、-O-、-CH2At least 1 bonding group selected from the group consisting of O-, -COO-and-OCO-, wherein a is an integer of 1 to 15; y is2Represents a single bond or- (CH)2)b-, wherein b is an integer of 1 to 15; y is4Represents at least 1 2-valent cyclic group selected from the group consisting of a benzene ring, a cyclohexane ring and a heterocycle, or a 2-valent organic group having 17 to 51 carbon atoms and having a steroid skeleton, wherein any hydrogen atom on the cyclic group is optionally substituted by an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluorine-containing alkyl group having 1 to 3 carbon atoms, a fluorine-containing alkoxy group having 1 to 3 carbon atoms or a fluorine atom; y is5Represents at least 1 cyclic group selected from the group consisting of a benzene ring, a cyclohexane ring and a heterocycle, any hydrogen atom on these cyclic groups being optionally substituted by an alkyl group having 1 to 3 carbon atoms, an alkoxy group having 1 to 3 carbon atoms, a fluoroalkyl group having 1 to 3 carbon atoms, a fluoroalkoxy group having 1 to 3 carbon atoms or a fluorine atom; n represents an integer of 0 to 4; y is6Represents at least 1 kind selected from the group consisting of C1-18 alkyl, C1-18 fluorine-containing alkyl, C1-18 alkoxy and C1-18 fluorine-containing alkoxy,
-Y7-Y8[2-2]
Y7represents a group selected from the group consisting of a single bond, -O-, -CH2O-、-CONH-、-NHCO-、-CON(CH3)-、-N(CH3) At least 1 bonding group selected from the group consisting of CO-, -COO-and-OCO-; y is8Represents an alkyl group having 8 to 22 carbon atoms or a fluoroalkyl group having 6 to 18 carbon atoms.
2. The liquid crystal display element according to claim 1, wherein the compound represented by the formula [1] is at least 1 selected from the group consisting of compounds represented by the following formulae [1a-1] to [1a-6],
Xa、Xb、Xd、Xf、Xhand XkEach independently represents an alkyl group having 1 to 18 carbon atoms or an alkoxy group having 1 to 18 carbon atoms; xcAnd XiEach independently represents-O-, -COO-or-OCO-; xe、XgAnd XjEach independently represents-CH2-, -O-, -COO-or-OCO-; p1, p3, p5, p7, p8 and p9 independently represent an integer of 1 to 12; p2, p4 and p6 each independently represent an integer of 1 or 2.
3. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal alignment treatment agent is a liquid crystal alignment treatment agent containing at least 1 polymer selected from the group consisting of an acrylic polymer, a methacrylic polymer, a novolac resin, polyhydroxystyrene, a polyimide precursor, polyimide, polyamide, polyester, cellulose, and polysiloxane.
4. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal aligning agent is a liquid crystal aligning agent containing a polyimide precursor obtained by a reaction of a diamine component containing: a diamine having a side chain structure represented by the formula [2-1] or the formula [2-2 ].
6. The liquid crystal display element according to claim 4, wherein the tetracarboxylic acid component comprises a tetracarboxylic dianhydride represented by the following formula [3],
z represents at least 1 structure selected from the group consisting of structures represented by the following formulas [3a ] to [3k ],
Z1~Z4each independently represents at least 1 selected from the group consisting of a hydrogen atom, a methyl group, a chlorine atom and a benzene ring; z5And Z6Each independently represents a hydrogen atom or a methyl group.
7. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal alignment treatment agent comprises: a polysiloxane obtained by condensation polymerization of an alkoxysilane represented by the following formula [ A1 ]; or a polysiloxane obtained by polycondensing the alkoxysilane represented by the formula [ A1] with the alkoxysilane represented by the formula [ A2] and/or the alkoxysilane represented by the formula [ A3],
(A1)mSi(A2)n(OA3)p[A1]
A1represents said formula [2-1]Or formula [2-2]The side chain structure shown; a. the2Represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms; a. the3Represents an alkyl group having 1 to 5 carbon atoms; m represents an integer of 1 or 2; n represents an integer of 0 to 2; p represents an integer of 0 to 3; wherein m + n + p is 4,
(B1)mSi(B2)n(OB3)p[A2]
B1an organic group having 2 to 12 carbon atoms, which is at least 1 selected from the group consisting of a vinyl group, an epoxy group, an amino group, a mercapto group, an isocyanate group, a methacryloyl group, an acryloyl group, a ureido group and a cinnamoyl group; b is2Represents hydrogenAn alkyl group having 1 to 5 atoms or carbon atoms; b is3Represents an alkyl group having 1 to 5 carbon atoms; m represents an integer of 1 or 2; n represents an integer of 0 to 2; p represents an integer of 0 to 3; wherein m + n + p is 4,
(D1)nSi(OD2)4-n[A3]
D1represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms; d2Represents an alkyl group having 1 to 5 carbon atoms; n represents an integer of 0 to 3.
8. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal alignment treatment agent contains at least 1 kind of generating agent selected from the group consisting of a photoradical generating agent, a photoacid generating agent, and a photobase generating agent.
9. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal alignment treatment agent contains a compound having at least 1 structure selected from the group consisting of structures represented by the following formulae [ b-1] to [ b-8],
Barepresents a hydrogen atom or a benzene ring; b isbRepresents at least 1 cyclic group selected from the group consisting of a benzene ring, a cyclohexane ring and a heterocyclic ring; b iscRepresents at least 1 selected from the group consisting of C1-18 alkyl, C1-18 fluorinated alkyl, C1-18 alkoxy and C1-18 fluorinated alkoxy.
10. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal alignment treatment agent comprises a compound having at least 1 substituent selected from the group consisting of an epoxy group, an isocyanate group, an oxetanyl group, a cyclocarbonate group, a hydroxyl group, a hydroxyalkyl group, and a lower alkoxyalkyl group.
11. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal alignment treatment agent contains at least 1 solvent selected from the group consisting of 1-hexanol, cyclohexanol, 1, 2-ethylene glycol, 1, 2-propylene glycol, propylene glycol monobutyl ether, ethylene glycol monobutyl ether, dipropylene glycol dimethyl ether, cyclohexanone, cyclopentanone, and a compound represented by any one of formulae [ D1] to [ D3],
D1represents an alkyl group having 1 to 3 carbon atoms; d2Represents an alkyl group having 1 to 3 carbon atoms; d3Represents an alkyl group having 1 to 4 carbon atoms.
12. The liquid crystal display element according to claim 1 or 2, wherein the liquid crystal alignment treatment agent contains at least 1 solvent selected from the group consisting of N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone, and γ -butyrolactone.
13. The liquid crystal display element according to claim 1 or 2, wherein the substrate of the liquid crystal display element is a glass substrate or a plastic substrate.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-195601 | 2014-09-25 | ||
| JP2014195601 | 2014-09-25 | ||
| PCT/JP2015/077167 WO2016047770A1 (en) | 2014-09-25 | 2015-09-25 | Lcd element |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107003570A CN107003570A (en) | 2017-08-01 |
| CN107003570B true CN107003570B (en) | 2020-10-09 |
Family
ID=55581286
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201580063916.4A Active CN107003570B (en) | 2014-09-25 | 2015-09-25 | Liquid crystal display element |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP6686889B2 (en) |
| KR (1) | KR102474324B1 (en) |
| CN (1) | CN107003570B (en) |
| TW (1) | TWI695881B (en) |
| WO (1) | WO2016047770A1 (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10241359B2 (en) * | 2014-11-07 | 2019-03-26 | Nissan Chemical Industries, Ltd. | Liquid crystal display device |
| KR102596591B1 (en) * | 2017-02-28 | 2023-10-31 | 닛산 가가쿠 가부시키가이샤 | Compounds, liquid crystal compositions, and liquid crystal display devices |
| JP7198668B2 (en) | 2017-09-01 | 2023-01-04 | 積水化学工業株式会社 | Composite Particles, Composite Particle Powders and Light Modulating Materials |
| WO2019181885A1 (en) * | 2018-03-20 | 2019-09-26 | 日産化学株式会社 | Liquid crystal display element |
| CN111936922B (en) * | 2018-03-28 | 2023-05-26 | 日产化学株式会社 | Liquid crystal aligning agent, liquid crystal alignment film and liquid crystal display element |
| TWI844632B (en) * | 2019-03-08 | 2024-06-11 | 日商日產化學股份有限公司 | Resin composition, resin film and liquid crystal display element |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2885116B2 (en) | 1994-07-05 | 1999-04-19 | 日本電気株式会社 | Liquid crystal optical element and manufacturing method thereof |
| JP4132424B2 (en) | 1999-06-22 | 2008-08-13 | 旭硝子株式会社 | Manufacturing method of liquid crystal optical element |
| JP5017963B2 (en) * | 2006-08-29 | 2012-09-05 | Dic株式会社 | Liquid crystal element |
| JP6459959B2 (en) * | 2013-03-01 | 2019-01-30 | 日産化学株式会社 | Liquid crystal display element, liquid crystal alignment film, and liquid crystal alignment treatment agent |
-
2015
- 2015-09-25 WO PCT/JP2015/077167 patent/WO2016047770A1/en active Application Filing
- 2015-09-25 CN CN201580063916.4A patent/CN107003570B/en active Active
- 2015-09-25 KR KR1020177010503A patent/KR102474324B1/en active IP Right Grant
- 2015-09-25 JP JP2016550406A patent/JP6686889B2/en active Active
- 2015-09-25 TW TW104131845A patent/TWI695881B/en active
Also Published As
| Publication number | Publication date |
|---|---|
| KR102474324B1 (en) | 2022-12-05 |
| TW201627485A (en) | 2016-08-01 |
| TWI695881B (en) | 2020-06-11 |
| JPWO2016047770A1 (en) | 2017-07-06 |
| WO2016047770A1 (en) | 2016-03-31 |
| KR20170060069A (en) | 2017-05-31 |
| CN107003570A (en) | 2017-08-01 |
| JP6686889B2 (en) | 2020-04-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN106662780B (en) | Liquid crystal display element | |
| CN107003570B (en) | Liquid crystal display element | |
| CN107003571B (en) | Liquid crystal display element | |
| CN107533258B (en) | Liquid crystal display element | |
| KR102196272B1 (en) | Liquid crystal display element, liquid crystal alignment film, and liquid crystal alignment treatment agent | |
| KR102411032B1 (en) | Liquid crystal display element, liquid crystal alignment film, and liquid crystal alignment treatment agent | |
| CN113614624B (en) | Resin composition, resin film and liquid crystal display element | |
| KR102217700B1 (en) | Liquid-crystal display element, liquid-crystal alignment agent, and liquid-crystal alignment film | |
| EP3422092B1 (en) | Liquid crystal display device | |
| KR102596591B1 (en) | Compounds, liquid crystal compositions, and liquid crystal display devices | |
| CN110945416B (en) | Resin composition, resin film, and liquid crystal display element | |
| KR20160138184A (en) | Liquid crystal display element, liquid crystal alignment film, and liquid crystal alignment treatment agent | |
| CN111868616A (en) | Liquid crystal display element |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |