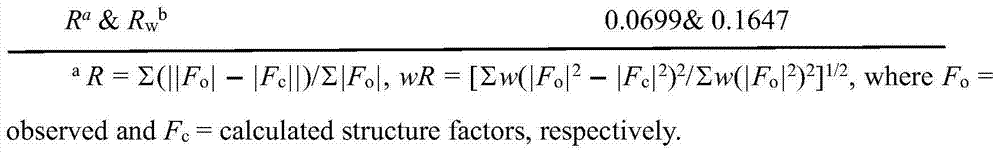CN103709204A - Cobalt complex, preparation method and application thereof - Google Patents
Cobalt complex, preparation method and application thereof Download PDFInfo
- Publication number
- CN103709204A CN103709204A CN201310664449.3A CN201310664449A CN103709204A CN 103709204 A CN103709204 A CN 103709204A CN 201310664449 A CN201310664449 A CN 201310664449A CN 103709204 A CN103709204 A CN 103709204A
- Authority
- CN
- China
- Prior art keywords
- cobalt complex
- hydroxyl
- dtbp
- carboxylic acid
- aqueous solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Catalysts (AREA)
Abstract
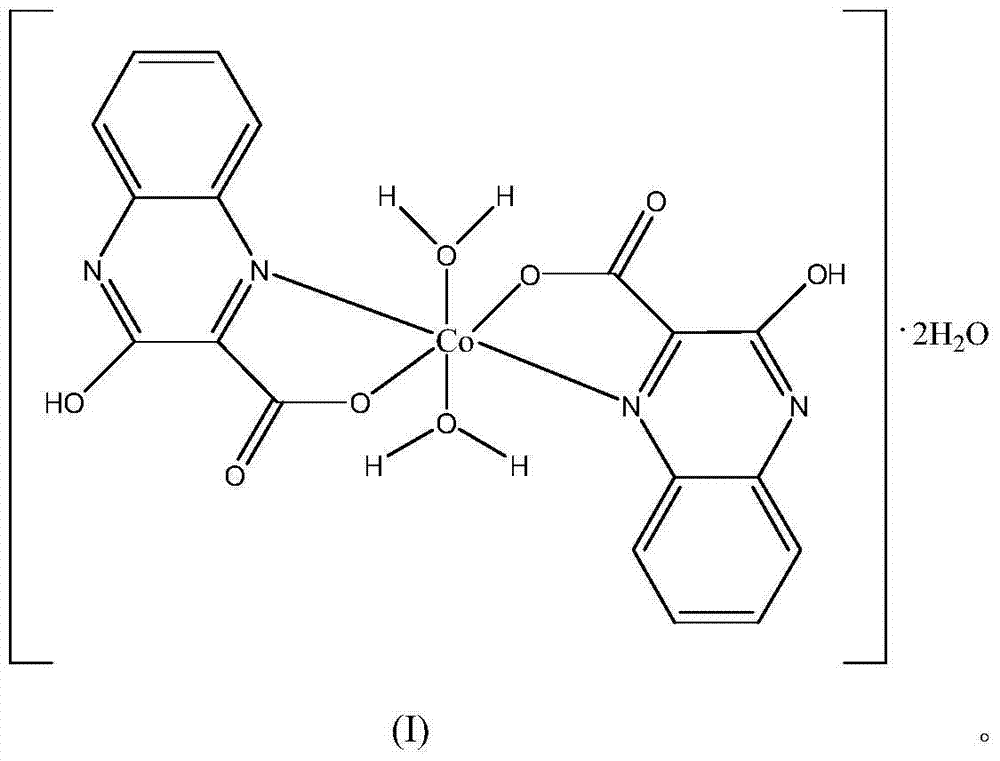
The invention provides a cobalt complex, a preparation method and an application thereof, and belongs to the technical field of metal-organic complex materials. The cobalt complex has a molecular formula of [Co(qc)2(H2O)].2H2O, wherein qc represents 3-hydroxyl-2-quinoxaline carboxylate radicals. The invention also provides a method for preparing the cobalt complex. The cobalt complex is obtained by reacting 3-hydroxyl-2-quinoxaline carboxylic acid and a bivalent cobalt compound in a solution. The invention also provides an application of the cobalt complex in catalyzing an oxidative coupling reaction of 2,4-di-tert-butylphenol. The cobalt complex provided by the invention has a certain granularity and a unique structure, and has relatively high catalytic activity, selectivity and good stability. The preparation method of the cobalt complex is simple, has high yield and has no pollution to the environment. By using the cobalt complex to prepare biphenol, water can be used as a solvent; selectivity is good; yield is high; three wastes are little; and the product is free from pollution.
Description
Technical field
The invention belongs to Metal-organic complex material technology field, be specifically related to a kind of cobalt complex, preparation method and its usage.
Background technology
Title complex is used as catalyzer and has lot of advantages: 1) in the network structure of title complex, often there is the hole of definite shape and volume, can optionally hold guest molecule based on void shape and size, strong raising selectivity of catalyst; 2) under chemically modified, title complex that can synthesis of chiral vesicular structure, this is that traditional organic catalyst is beyond one's reach; 3) organic ligand has occupied part metals hapto, has improved the stability of metal ion, thereby contributes to improve selectivity; 4) synthetic technology of title complex gentleness is conducive to the industrial applications of catalyzer; 5) dispersed as the transition metal ion of catalytic active center, be difficult for assembling; 6) introducing of organic group makes ligand structure present the catalytic selectivity that variation likely changes metal ion.Based on these advantages, it is extremely noticeable that title complex is used as the prospect of catalyzer.
2,2 '-dihydroxybiphenyl is as 2,2 '-dihydroxyl-3,3 ', 5,5 '-tetra-tert biphenyl (TBBP) is a kind of important organic synthesis intermediate, can be used for synthetic organic material stablizer, metal catalyst part, bidentate phosphite ester ligand and as anionic trapping agent etc.2,2 '-dihydroxybiphenyl can or be take ethylene dichloride as solvent with oxygen by corresponding phenol and hydroperoxidation, under copper, cobalt, manganese salt catalyzed oxidation, obtains.External only two patents (WO9946227.1999 and US4380676.1983) have been reported the synthetic method of TBBP.But the method needs with an organic solvent, environment is had to pollution, and product yield is only 68% left and right.
Be accompanied by the growing environmental stress of chemical process, nowadays people more and more advocate Green Chemistry, do not advocate with an organic solvent.So, find take water as solvent carry out 2,4-DTBP (2, the 4-DBP) catalyzer of oxidative coupling reaction, carrying out so-called " cleaning procedure " reaction is current problem chemist to challenge meaning.
Summary of the invention
The object of the invention is for a kind of cobalt complex is provided.
Another object of the present invention is to provide the preparation method of cobalt complex.
A further object of the present invention is to provide the purposes of described cobalt complex.
Object of the present invention adopts following technical scheme to realize.
A cobalt complex, its molecular formula is [Co (qc)
2(H
2o)
2] 2H
2o, wherein qc represents 3-hydroxyl-2-quinoxaline carboxylic acid root, structural formula as shown in the formula (I):
A method of preparing described cobalt complex, adopts 3-hydroxyl-2-quinoxaline carboxylic acid to react in solution with bivalent Co and obtains described cobalt complex.
The method of described cobalt complex, comprises the steps: 3-hydroxyl-2-quinoxaline carboxylic acid's methanol solution to be added drop-wise to Co (ClO
4)
2in the aqueous solution, mix, under room temperature standing 5~10 days, carry out self-assembling reaction, obtain red bulk crystals, then successively with dehydrated alcohol, ether washing, dry, obtain described cobalt complex.
The concentration of described 3-hydroxyl-2-quinoxaline carboxylic acid's methanol solution is 0.01~2molL
-1, described Co (ClO
4)
2co (ClO in the aqueous solution
4)
2concentration be 0.001~1mol L
-1, described 3-hydroxyl-2-quinoxaline carboxylic acid's methanol solution and Co (ClO
4)
2the volume ratio of the aqueous solution is (3~5): (0.4~2).
The present invention also provides the application of described cobalt complex aspect catalysis 2,4-DTBP oxidative coupling reaction.
Described application, comprises the steps: 2,4-DTBP, KOH, sodium lauryl sulphate and water to mix, and adds described cobalt complex under stirring, stirs lower heating; Rise to after 30-60 ℃ to temperature, splash into mass percentage concentration and be 30% H
2o
2the aqueous solution, reaction 3~5h.The mol ratio of described cobalt complex and 2,4-DTBP is 1%~5%; The mol ratio of described KOH, sodium lauryl sulphate and 2,4-DTBP is (1~4): (1~3): 1; The amount of water that every mmole 2,4-DTBP is corresponding is 5-10ml; H corresponding to every mmole 2,4-DTBP
2o
2the add-on of the aqueous solution is 10-30 μ l.
The present invention has the following advantages compared to existing technology:
1, the monocrystalline cobalt complex that the present invention forms through molecular self-assembling, has certain granularity, and unique structure, has higher catalytic activity, selectivity and satisfactory stability.
2. the preparation method of cobalt complex of the present invention is simple, and productive rate is high, environmentally safe.
3, the cobalt complex that uses the present invention to be constructed by quinoxaline carboxylic acid's class part is prepared '-biphenyl diphenol as catalyzer, can water be solvent, H
2o
2for oxygenant, not only selectivity is good, and productive rate is high, and the three wastes are few, pollution-free to product.In reaction, unique by product of oxygenant is water, and this is significant for the greenization of oxidising process and the Separation & Purification of product.
4, cobalt complex of the present invention is easy to reclaim as catalyzer, can reuse.
5, cobalt complex of the present invention can be prepared at normal temperatures and pressures, and mild condition, productive rate is high, reproducibility good, produces pollution-freely, has potential economic benefit, social benefit and environmental benefit.
Accompanying drawing explanation
Fig. 1 is 2,4-DTBP oxidative coupling reaction route map, and wherein But represents the tertiary butyl.
Fig. 2 is the X-ray single crystal diffraction figure of cobalt complex of the present invention.
Embodiment
Below in conjunction with specific embodiment, the present invention is described in detail.
Room temperature in the present invention refers to 25 °.
Embodiment mono-
By 10ml, concentration, be 0.1mol L
-13-hydroxyl-2-quinoxaline carboxylic acid's methanol solution, being dropwise added drop-wise to 5ml concentration is 0.05mol L
-1co (ClO
4)
2.6H
2in the O aqueous solution, shake up, obtain a red limpid solution, in room temperature, within standing 8 days, carry out self-assembling reaction, obtain red bulk crystals, then successively with dehydrated alcohol, ether washing, dry, obtain molecular formula for [Co (qc)
2(H
2o)
2] 2H
2the cobalt complex of O, wherein qc represents 3-hydroxyl-2-quinoxaline carboxylic acid root, structural formula is suc as formula (I), productive rate 54%.Through X-single crystal diffractometer, analyze, cobalt complex prepared by the present embodiment is the title complex of mononuclear structure.Ultimate analysis: by theoretical structural formula C
18h
18coN
4o
10, calculated value: C42.45, H3.56, N11.00%; Measured value: C42.53, H3.37, N10.88%.
The structure determination of title complex:
The monocrystalline of choosing under the microscope suitable size at room temperature carries out the experiment of X – ray diffraction.On Bruker Smart1000CCD diffractometer, use the alpha-ray through the Mo of graphite monochromator monochromatization – K
with
mode is collected diffraction data.Use respectively BrukerSAINT(BrukerAXS, SAINT SoftwareReferenceManual, Madison, WI, 1998) and SHELXTL(G.M.Sheldrick, G.M.SHELXTL NT Version5.1.Program for Solution and Refinemen of Crystal Structures, Universityof
germany, 1997) program is carried out reduction of data and structure elucidation.The diffraction data of part-structure carries out absorption correction by SADABS program.Crystalline structure is solved in conjunction with difference Fourier is synthetic by direct method.All non-hydrogen atom coordinate and anisotropic parameters carry out complete matrix least-squares refinement, and hydrogen atom position is determined by theoretical mode computation.Hydrogen atom on partial solvent water and methyl alcohol is processed by the method for difference Fourier peak-seeking.Detailed axonometry data are in Table 1 and table 2.Crystalline structure is shown in Fig. 2.
The main crystallographic data of table 1 cobalt complex
Symmetrytransformationsusedtogenerateequivalentatoms:(i)-x-1,-y,-z;(ii)x-1,y,z;(iii)-x,-y,-z;(iv)-x-1/2,y-1/2,-z+1/2.
Embodiment bis-
By 8ml, concentration, be 2molL
-13-hydroxyl-2-quinoxaline carboxylic acid's methanol solution, being dropwise added drop-wise to 2ml concentration is 1molL
-1co (ClO
4)
26H
2the aqueous solution of O, shakes up, and obtains a red limpid solution, in room temperature, within standing 9 days, carries out self-assembling reaction, obtains red bulk crystals, then successively with dehydrated alcohol, ether washing, dry, obtains molecular formula for [Co (qc)
2(H
2o)
2] 2H
2the cobalt complex of O, wherein qc represents 3-hydroxyl-2-quinoxaline carboxylic acid root, structural formula is suc as formula (I), productive rate 59%.Through X-single crystal diffractometer, analyze, cobalt complex prepared by the present embodiment is the title complex of mononuclear structure.Axonometry data and crystalline structure are with compound in embodiment 1.
Embodiment tri-
By 12ml, concentration, be 0.05molL
-13-hydroxyl-2-quinoxaline carboxylic acid's methanol solution, be dropwise added drop-wise to 6ml, concentration is 0.02molL
-1co (ClO
4)
26H
2the aqueous solution of O, shakes up, and obtains a red limpid solution, in room temperature, within standing 6 days, carries out self-assembling reaction, obtains red bulk crystals, then successively with dehydrated alcohol, ether washing, dry, obtains molecular formula for [Co (qc)
2(H
2o)
2] 2H
2the cobalt complex of O, wherein qc represents 3-hydroxyl-2-quinoxaline carboxylic acid root, structural formula is suc as formula (I), productive rate 59%.Through X-single crystal diffractometer, analyze, cobalt complex prepared by the present embodiment is the title complex of mononuclear structure.Axonometry data and crystalline structure are with compound in embodiment 1.
Application Example one
Under room temperature, to being equipped with in the 10ml there-necked flask of prolong and thermometer, add 2,4-DI-tert-butylphenol compounds (206mg, 1mmol), potassium hydroxide (56mg, 1mmol) and sodium lauryl sulphate (SDS, 29mg, 1mmol), then add 5mL pure water, stir and add the cobalt complex (0.02mmol) of embodiment 1 preparation as catalyzer.Under stirring, be heated to 50 ℃, the H that is 30% by 10 μ l syringe holder mass percentage concentration
2o
2the aqueous solution slowly splashes in above-mentioned mixing solutions, within every 15 minutes, adds 10 μ l, drips altogether 20 μ l, and reaction 4h(starts to drip H from just
2o
2the aqueous solution starts timing) after stop.By TLC method reaction product isolated on previously prepared thin layer silica gel.Result shows: the transformation efficiency of 2,4-DTBP is 100%, in product 2, and 2 '-dihydroxyl-3,3 ', 5,5 '-tetra-tert biphenyl (TBBP) yield is 76%, benzoquinones yield is 24%.
Application Example two
Under room temperature, to being equipped with in the 10ml there-necked flask of prolong and thermometer, add 2,4-DI-tert-butylphenol compounds (206mg, 1mmol), potassium hydroxide (224mg, 4mmol) and sodium lauryl sulphate (SDS, 87mg, 3mmol), then add 5mL pure water, stir and add the cobalt complex (0.05mmol) of embodiment 2 preparations as catalyzer.Under stirring, be heated to 60 ℃, the H that is 30% by 10 μ l syringe holder mass percentage concentration
2o
2the aqueous solution slowly splashes in above-mentioned mixing solutions, within every 15 minutes, adds 10 μ l, drips altogether 20 μ l, and reaction 4h(starts to drip H from just
2o
2the aqueous solution starts timing) rear stopped reaction.By TLC method reaction product isolated on previously prepared thin layer silica gel.Result shows: the transformation efficiency of 2,4-DTBP is 100%, in product 2, and 2 '-dihydroxyl-3,3 ', 5,5 '-tetra-tert biphenyl (TBBP) yield is 89%, benzoquinones yield is 11%.
Claims (8)
3. a method of preparing cobalt complex described in claim 1 or 2, is characterized in that 3-hydroxyl-2-quinoxaline carboxylic acid reacts in solution with bivalent Co to obtain described cobalt complex.
4. according to the method for the described cobalt complex of claim 3 preparation, it is characterized in that comprising the steps: that the methanol solution by 3-hydroxyl-2-quinoxaline carboxylic acid is added drop-wise to Co (ClO
4)
2in the aqueous solution, mix, under room temperature standing 5~10 days, carry out self-assembling reaction, obtain red bulk crystals, then successively with dehydrated alcohol, ether washing, dry, obtain described cobalt complex.
5. according to the method for the described cobalt complex of claim 4 preparation, it is characterized in that the concentration of described 3-hydroxyl-2-quinoxaline carboxylic acid's methanol solution is 0.01~2molL
-1, described Co (ClO
4)
2co (ClO in the aqueous solution
4)
2concentration be 0.001~1molL
-1, described 3-hydroxyl-2-quinoxaline carboxylic acid's methanol solution and Co (ClO
4)
2the volume ratio of the aqueous solution is (3~5): (0.4~2).
6. the application of cobalt complex aspect catalysis 2,4-DTBP oxidative coupling reaction described in claim 1 or claim 2.
7. application according to claim 6, is characterized in that comprising the steps: 2,4-DTBP, KOH, sodium lauryl sulphate and water are mixed, and adds described cobalt complex under stirring, stirs lower heating; Rise to after 30-60 ℃ to temperature, splash into mass percentage concentration and be 30% H
2o
2the aqueous solution, reaction 3~5h.
8. application according to claim 7, the mol ratio that it is characterized in that described cobalt complex and 2,4-DTBP is 1%~5%; The mol ratio of described KOH, sodium lauryl sulphate and 2,4-DTBP is (1~4): (1~3): 1; The amount of water that every mmole 2,4-DTBP is corresponding is 5-10ml; H corresponding to every mmole 2,4-DTBP
2o
2the add-on of the aqueous solution is 10-30
μl.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201310664449.3A CN103709204B (en) | 2013-12-09 | 2013-12-09 | A kind of cobalt complex, preparation method and its usage |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201310664449.3A CN103709204B (en) | 2013-12-09 | 2013-12-09 | A kind of cobalt complex, preparation method and its usage |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN103709204A true CN103709204A (en) | 2014-04-09 |
| CN103709204B CN103709204B (en) | 2016-06-29 |
Family
ID=50402592
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201310664449.3A Expired - Fee Related CN103709204B (en) | 2013-12-09 | 2013-12-09 | A kind of cobalt complex, preparation method and its usage |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN103709204B (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104926888A (en) * | 2015-05-29 | 2015-09-23 | 南京信息工程大学 | Cobalt complex and preparation method and application thereof |
| CN106928153A (en) * | 2017-03-24 | 2017-07-07 | 宁波大学 | A kind of PARA FORMALDEHYDE PRILLS(91,95) has cobalt complex of electro catalytic activity and preparation method thereof |
| CN106946798A (en) * | 2017-03-24 | 2017-07-14 | 宁波大学 | It is a kind of that there is cobalt complex of electrochemical response and preparation method thereof to hydrogen peroxide |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3725068A (en) * | 1970-04-17 | 1973-04-03 | Ciba Geigy Ag | Process for the photographic development of silver salts |
| CN101260122A (en) * | 2007-03-08 | 2008-09-10 | 中国科学院化学研究所 | 2-(6'-iminopyridyl)quinoxaline metal complex compound and its preparing method and application |
| WO2010123046A1 (en) * | 2009-04-21 | 2010-10-28 | 住友化学株式会社 | Modified metal complex |
-
2013
- 2013-12-09 CN CN201310664449.3A patent/CN103709204B/en not_active Expired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3725068A (en) * | 1970-04-17 | 1973-04-03 | Ciba Geigy Ag | Process for the photographic development of silver salts |
| CN101260122A (en) * | 2007-03-08 | 2008-09-10 | 中国科学院化学研究所 | 2-(6'-iminopyridyl)quinoxaline metal complex compound and its preparing method and application |
| WO2010123046A1 (en) * | 2009-04-21 | 2010-10-28 | 住友化学株式会社 | Modified metal complex |
Non-Patent Citations (1)
| Title |
|---|
| N.KDUTT等,: "THE USE OF QUINQXALINE-2-CARBUXYLIC ACID AND ITS DERIVATIVES AS ANALYTICAL REAGENTS", 《ANATLYTICA CHIMICA ACTA》, vol. 41, 31 December 1968 (1968-12-31), pages 331 - 339 * |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104926888A (en) * | 2015-05-29 | 2015-09-23 | 南京信息工程大学 | Cobalt complex and preparation method and application thereof |
| CN104926888B (en) * | 2015-05-29 | 2017-09-19 | 南京信息工程大学 | A kind of cobalt complex and preparation method and application |
| CN106928153A (en) * | 2017-03-24 | 2017-07-07 | 宁波大学 | A kind of PARA FORMALDEHYDE PRILLS(91,95) has cobalt complex of electro catalytic activity and preparation method thereof |
| CN106946798A (en) * | 2017-03-24 | 2017-07-14 | 宁波大学 | It is a kind of that there is cobalt complex of electrochemical response and preparation method thereof to hydrogen peroxide |
| CN106928153B (en) * | 2017-03-24 | 2019-04-16 | 宁波大学 | A kind of PARA FORMALDEHYDE PRILLS(91,95) has the cobalt complex and preparation method thereof of electro catalytic activity |
| CN106946798B (en) * | 2017-03-24 | 2019-04-16 | 宁波大学 | A kind of pair of hydrogen peroxide has the cobalt complex and preparation method thereof of electrochemical response |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103709204B (en) | 2016-06-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102633821B (en) | copper complex built by pyrimidine carboxylic acid base ligand, preparation method and use of copper complex | |
| CN105233872A (en) | Pd @MIL-101 composite and preparation method and application thereof | |
| CN108654692A (en) | Application of the n-BuLi in catalysis ketone and borine hydroboration | |
| CN107880079B (en) | Cyclic N-heterocyclic bis-carbene-palladium complex and preparation method and application thereof | |
| CN110116024B (en) | Heterogeneous three-dimensional double-valence Cu-MOF catalyst and preparation method and application thereof | |
| CN111763135A (en) | Application of deprotonated phenyl bridged beta-ketimine lithium compound in preparation of alcohol from ester | |
| Aydemir et al. | Novel neutral phosphinite bridged dinuclear ruthenium (II) arene complexes and their catalytic use in transfer hydrogenation of aromatic ketones: X-ray structure of a new Schiff base, N3, N3′-di-2-hydroxybenzylidene-[2, 2′] bipyridinyl-3, 3′-diamine | |
| CN103204882B (en) | A kind of poly-benzimidazole iron complex, its preparation method and application thereof | |
| CN103709204A (en) | Cobalt complex, preparation method and application thereof | |
| CN101704723B (en) | Preparation method of hydroxymethyl substitutent o-alkyl biphenyl and intermediate thereof | |
| CN103570768B (en) | A kind of cobalt nitrogen complex | |
| CN102500418B (en) | Preparation method of magnetic bidentate imide palladium ligand catalyst | |
| CN102294251B (en) | Nano-oxide catalyst for preparing propylene by oxidative dehydrogenation of propane and preparation method thereof | |
| CN110922420B (en) | 5-isonicotinamide pyridylisotitanium cadmium complex and preparation method and application thereof | |
| CN103113417B (en) | Cobalt-amino acid coordination compound catalyzer, preparation method and application thereof | |
| Brunet et al. | Activation of reducing agents. Sodium hydride containing complex reducing agents. 18. Study of the nature of complex reducing agents prepared from nickel and zinc salts | |
| CN115806504B (en) | Asymmetric chiral ligand and preparation method thereof, prepared catalyst, synthesis method and application | |
| EP2383275A1 (en) | Method for synthesizing o-diphenylphosphino benzoic acid | |
| CN101973537A (en) | Method for preparing transition metal phosphide | |
| CN106694045A (en) | 3:1 type Mg/Li bimetallic catalyst, preparation method therefor and application of 3:1 type Mg/Li bimetallic catalyst | |
| CN103304585B (en) | A kind of copper complex and preparation method thereof and application | |
| CN106964403B (en) | A kind of Magnetic phenyl phosphine palladium composite catalyst and its application | |
| CN109608649A (en) | A kind of Cu-Eu heterometallocenes organic backbone and its preparation and application with catalytic activity | |
| CN103804429B (en) | A kind of chirality leucinol cobalt complex | |
| CN102337555A (en) | Method for synthesizing 2,4-dimethylanisole |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20160629 Termination date: 20181209 |
|
| CF01 | Termination of patent right due to non-payment of annual fee |