This repository includes data and scripts to analyze structural variations of 25 Chinese samples using nanopore sequencing.
If you use the methods or pipeline in this repository, please cite our paper. Also, you can get detailed information about these methods in the supplementary method of our paper.
Quan, C., Li, Y., Liu, X. et al. Characterization of structural variation in Tibetans reveals new evidence of high-altitude adaptation and introgression. Genome Biol 22, 159 (2021). https://doi.org/10.1186/s13059-021-02382-3
| Description | Format | Location |
|---|---|---|
| SV callsets | vcf.gz | example/merge.ont.genotyped.SURVIVOR.sorted.reheader.corrected.INDELtoSYMBOL.sorted.local.addINFO.addFST.addCIPOS.addCIEND.vcf.gz |
| SV genotypes | vcf.gz | example/merge.genotypes.corrected.delCHR.svtk.vcf.gz |
| Sample list | xlsx | example/samples.xlsx |
| SV annotation | tsv.zip | example/merge.genotypes.corrected.delCHR.svtk.annotsv.public.tsv.zip |
| SV Distribution | tsv | example/merge.genotypes.corrected.delCHR.svtk.dist |
| SV hom&het | tsv | example/merge.genotypes.corrected.delCHR.svtk.gte1ngs.LDpruned.het |
| Fixation index | tsv | example/merge.paragraph.genotypes.TIBvsHAN.20k_5k.windowed.weir.fst |
| Gene annotations | gtf.gz | 0_raw_data/gencode.v32lift37.canonical_annotation.gtf.gz |
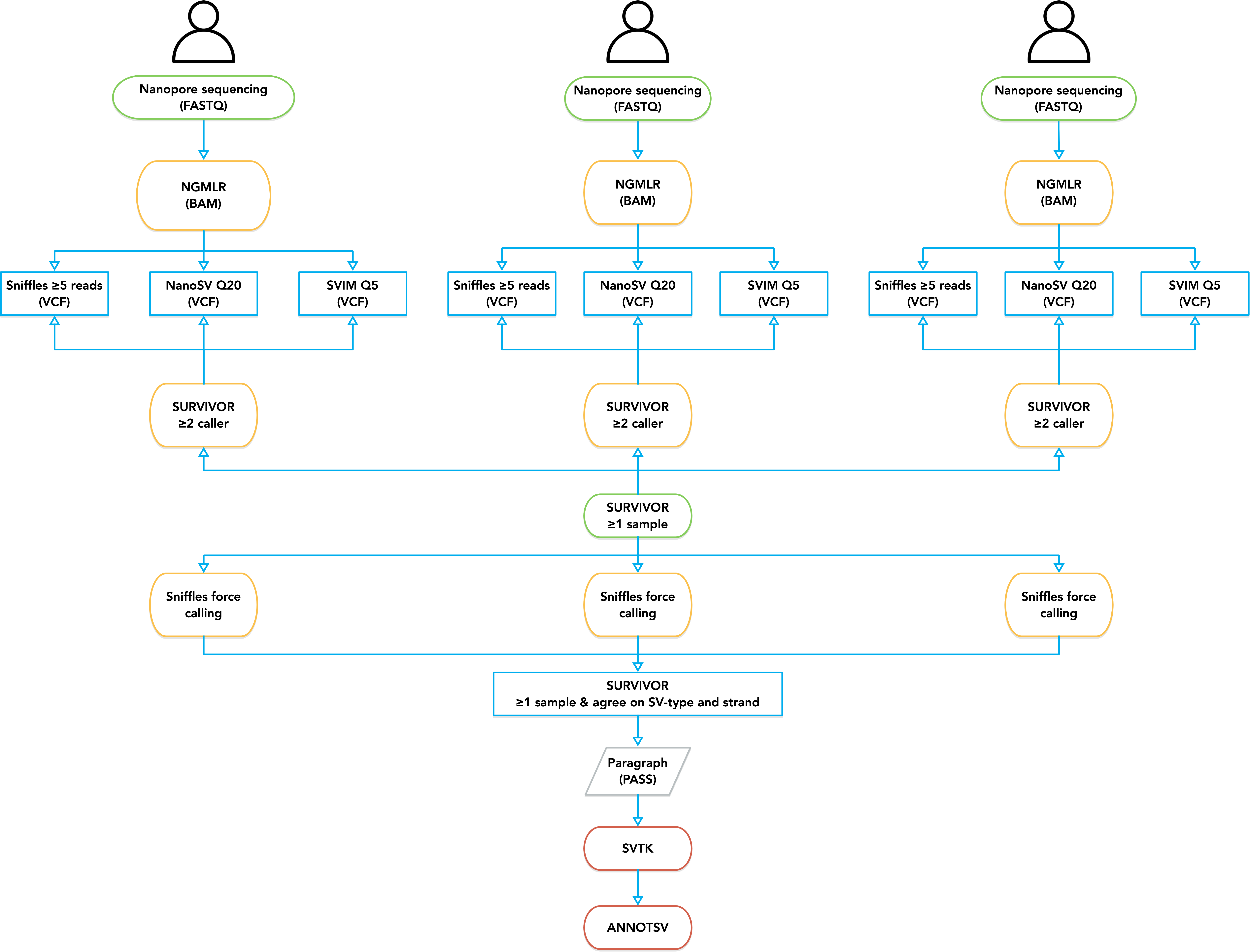
In the bash file pipeline.sv-calling.sh, we use sample data to demonstrate the complete process of detecting and annotating structural variations based on nanopore sequencing technology.
-
Firstly, long reads were mapped to GRCh37 human reference from NCBI without alternate sequences. Mapping was performed with NGMLR with ONT default parameters.
-
Then SV calling was performed on each sample using Sniffles, NanoSV, and SVIM. These tools have been reported to be compatible with NGMLR and show better accuracy and sensitivity than others. Five minimum supporting reads with at least 50 bp length was required. The insertion sequence and read ID was required for each method, and the rest are all default parameters.
-
SURVIVOR was used to merge the SVs per each sample, which are supported by at least two methods with a maximum allowed pairwise distance of 1,000 bp between breakpoints. Meanwhile, SVs obtained from different tools are not necessary to agree on the SV-type or the strand, so that we could capture as many potential breakpoints as possible. Finally, we merged the SVs obtained from all the samples as long as one sample supports it.
-
At last, we need to get a fully genotyped multi-samples dataset. We re-ran Sniffles across all the samples with all these potential regions (--Ivcf) and finally combined SVs with SURVIVOR. This time, we asked SURVIVOR only to report SVs supported by at least one sample, and they have to agree on the SV-type. Furthermore, we used a hard threshold with five minimum supporting reads, and all non-missing genotypes less than this threshold were modified to reference.
-
After SVs were discovered within a small population using long-read sequencing, we then genotyped these SVs with a relatively large amount of NGS data accumulated in previous studies. Paragraph, which is a new graph-based method, was used to genotype each NGS genome. We set the maximum allowed read count for SVs to 20 times the mean genome coverage for each dataset described above. We replaced all genotypes which failed to pass any filters by Paragraph with missing genotypes (./.).
-
We annotated SVs for a range of potential effects on coding sequences using SVTK and AnnotSV.
In the bash file pipeline.demographic_inference.sh, we performed demographic inference by easySFS and make whole-genome simulation by msprime.
-
Firstly, we used SNVs to estimate the demographic history of Tibetans and Hans. An unfolded joint site frequency spectrum (SFS) of non-genic autosomal bases was estimated using easySFS.
-
To explore the alternative demographic models for the population model YRI-TIB-HAN, we used the diffusion approximation method of ∂a∂i to analyze the joint SFS.
-
When the best-fit demographic model was recognized, we used msprime to perform whole-genome coalescent simulations. To approximately account for mutational heterogeneity across the genome, we applied a three-step framework described in a previous study (Hsieh PH, et al. 2019).
In the bash file pipeline.detection_introgression.sh, we applied the D-statistic and fd-statistic for each simualtion.
-
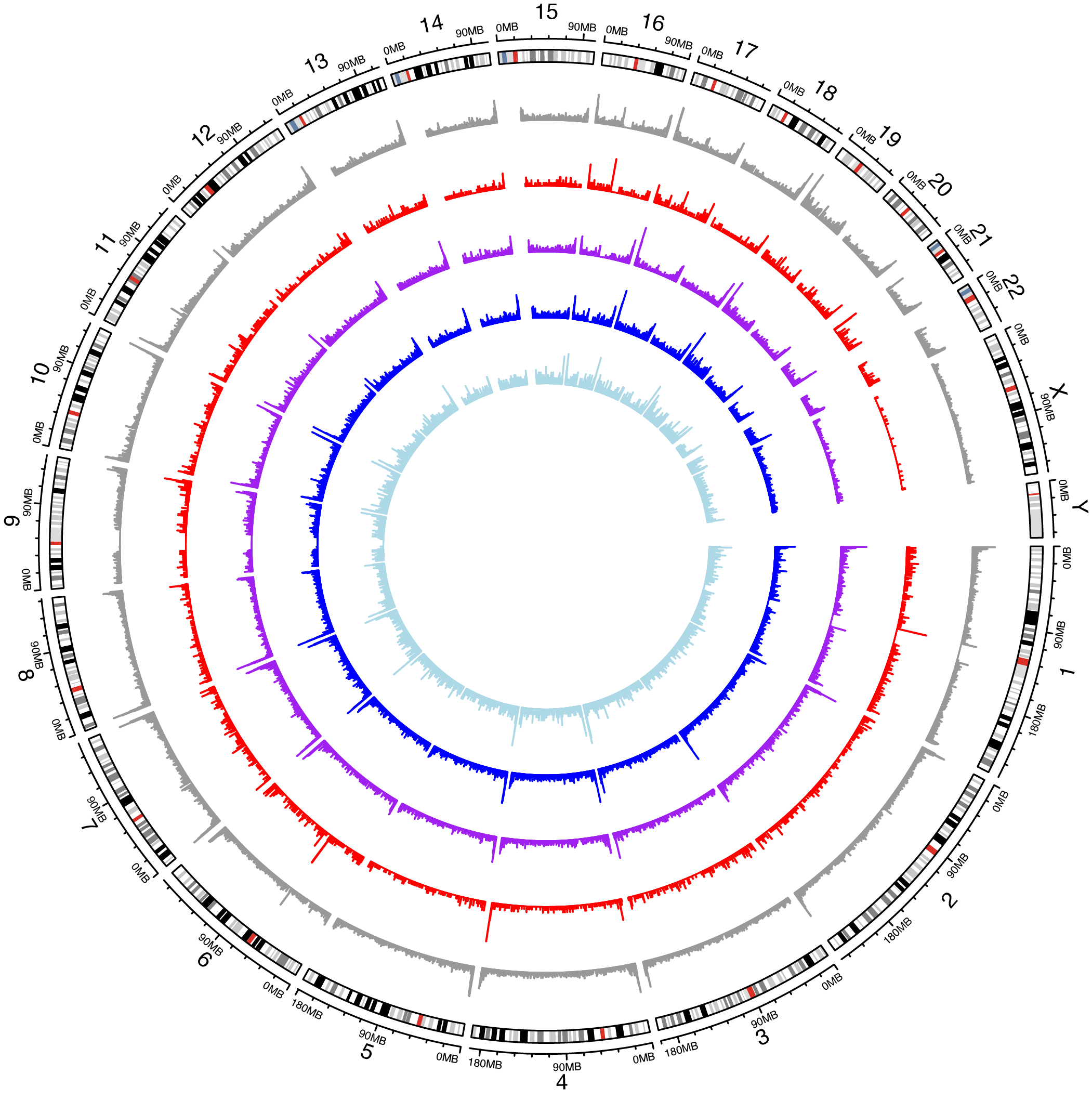
SV distribution
library(circlize) karyo_plot <- as.data.frame(data_sv_details_all_karyo) karyo_plot$SUPP <- as.integer(karyo_plot$SUPP) karyo_plot_BND <- karyo_plot[karyo_plot$SVTYPE=='TRA',] karyo_plot_BND$seqnames <- paste('chr',karyo_plot_BND$seqnames, sep = '') karyo_plot_BND_link <- karyo_plot_BND[,c('CHR2','END','END')] colnames(karyo_plot_BND_link) <- c('seqnames', 'start', 'end') karyo_plot_BND_link$seqnames <- paste('chr', as.character(karyo_plot_BND_link$seqnames), sep = "") karyo_plot_BND_link$start <- as.integer(karyo_plot_BND_link$start) karyo_plot_BND_link$end <- as.integer(karyo_plot_BND_link$end) array_seqnames <- paste('chr', karyo_plot$seqnames,sep = '') circos.initializeWithIdeogram(species = 'hg19') group_shared <- karyo_plot$SUPP==25 group_major <- karyo_plot$SUPP%in%seq(13,24) group_polymorphic <- karyo_plot$SUPP%in%seq(2,12) group_singleton <- karyo_plot$SUPP==1 circos.trackHist(factors=array_seqnames, track.height = 0.1, x=karyo_plot$start,col = "#999999", border = '#999999', bg.border = NA, bin.size = 500000) circos.trackHist(factors=array_seqnames[group_shared], track.height = 0.1, x=karyo_plot[group_shared,]$start,col = "red", border = 'red', bg.border = NA,bin.size = 500000) circos.trackHist(factors=array_seqnames[group_major], track.height = 0.1, x=karyo_plot[group_major,]$start,col = "purple", border = "purple",bg.border = NA,bin.size = 500000) circos.trackHist(factors=array_seqnames[group_polymorphic], track.height = 0.1, x=karyo_plot[group_polymorphic,]$start,col = "blue",border = "blue",bg.border = NA,bin.size = 500000) circos.trackHist(factors=array_seqnames[group_singleton], track.height = 0.1, x=karyo_plot[group_singleton,]$start,col = "light blue",border = "light blue",bg.border = NA,bin.size = 500000)
-
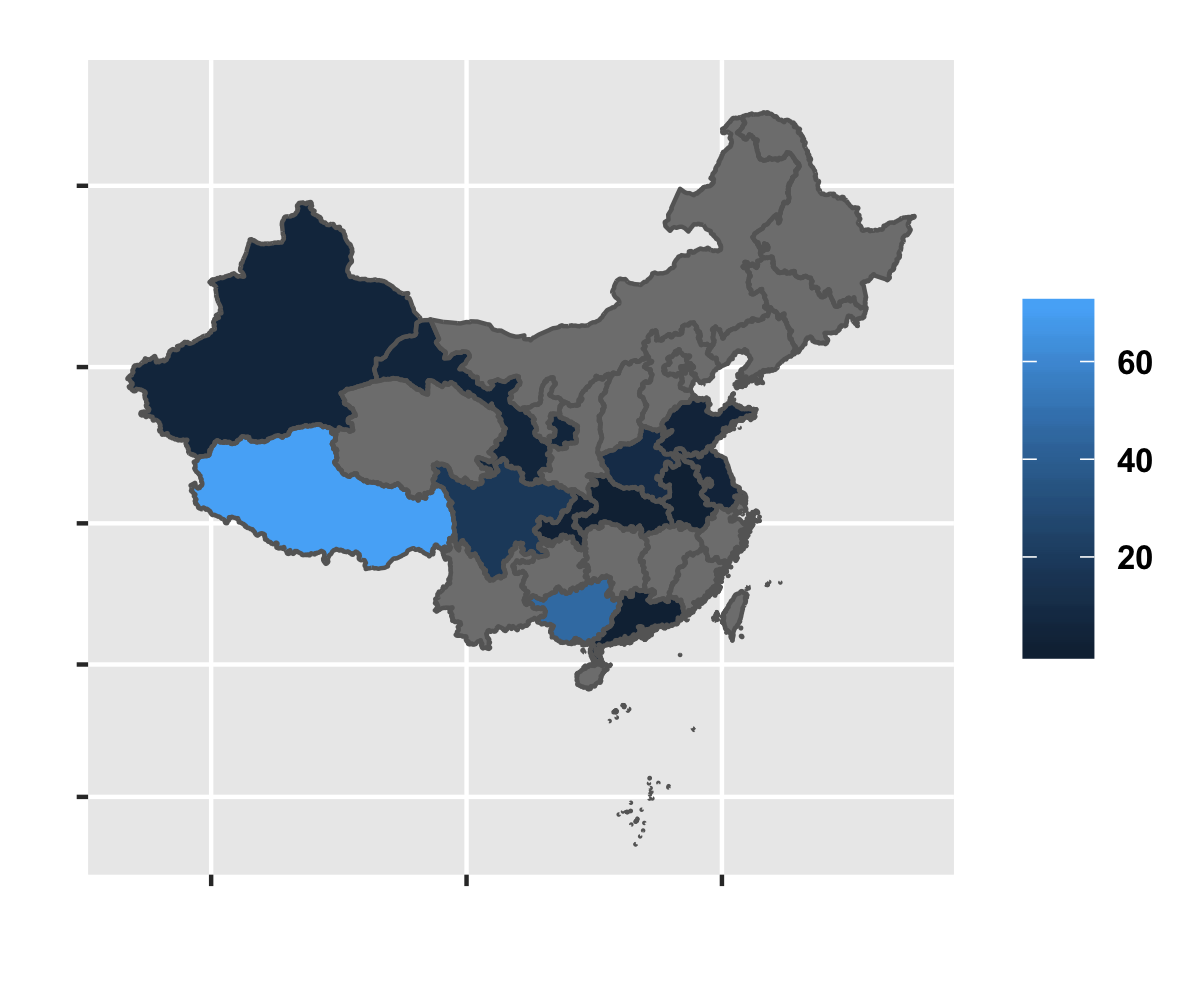
Sample Distribution for NGS data
library(maps) library(mapdata) library(maptools); china_map=readShapePoly('china-province-border-data/bou2_4p.shp'); china_map@data$cName <- iconv(china_map@data$NAME, from = "GBK") x <- china_map@data xs <- data.frame(x,id=seq(0:924)-1) china_map1 <- fortify(china_map) china_map_data <- join(china_map1, xs, type = "full", ) tmp <- summary(factor(data_samples_info[data_samples_info$Platform=='NGS',]$cLocation)) NAME <- names(tmp) pop <- tmp pop <- data.frame(NAME, pop) colnames(pop) <- c('cName', 'pop') china_map_pop <- join(china_map_data, pop, type = "full") ggplot(china_map_pop, aes(x = long, y = lat, group = group, fill = pop)) + geom_polygon() + geom_path(color = "grey40") + coord_map() + xlab('') + ylab("") + theme(legend.title=element_blank(), legend.text = element_text(size = 8,face = "bold"), axis.text.x = element_blank(), axis.text.y = element_blank())
-
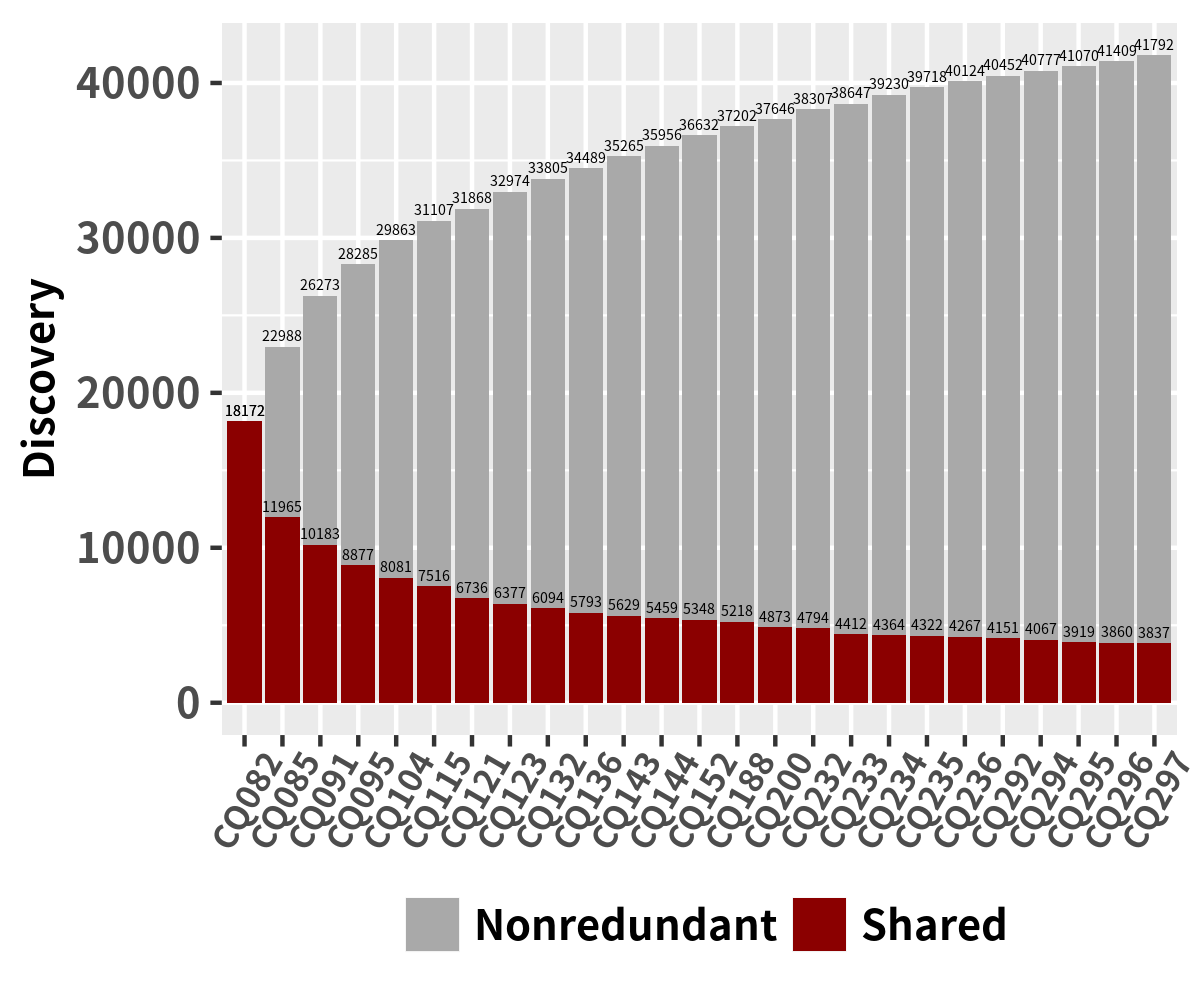
Sample Distribution for NGS data
df_sorted <- arrange(data_sample_details_ONT, id, Type) df_cumsum <- ddply(df_sorted, "id", transform, ypos=cumsum(Discovery) - 0.5*Discovery) ggplot(data=df_cumsum, aes(x=id, y=Discovery, fill = Type)) + geom_bar(stat="identity") + theme_minimal()+ theme(legend.title=element_blank(), legend.direction = 'horizontal', legend.position=c(0.5,0.98), legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold", angle = 60, vjust = 0.6), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(-15,0,0,0))) + scale_fill_manual(values=c('light blue', 'blue', 'purple','dark red')) + xlab("") + ylab("Discovery")
-
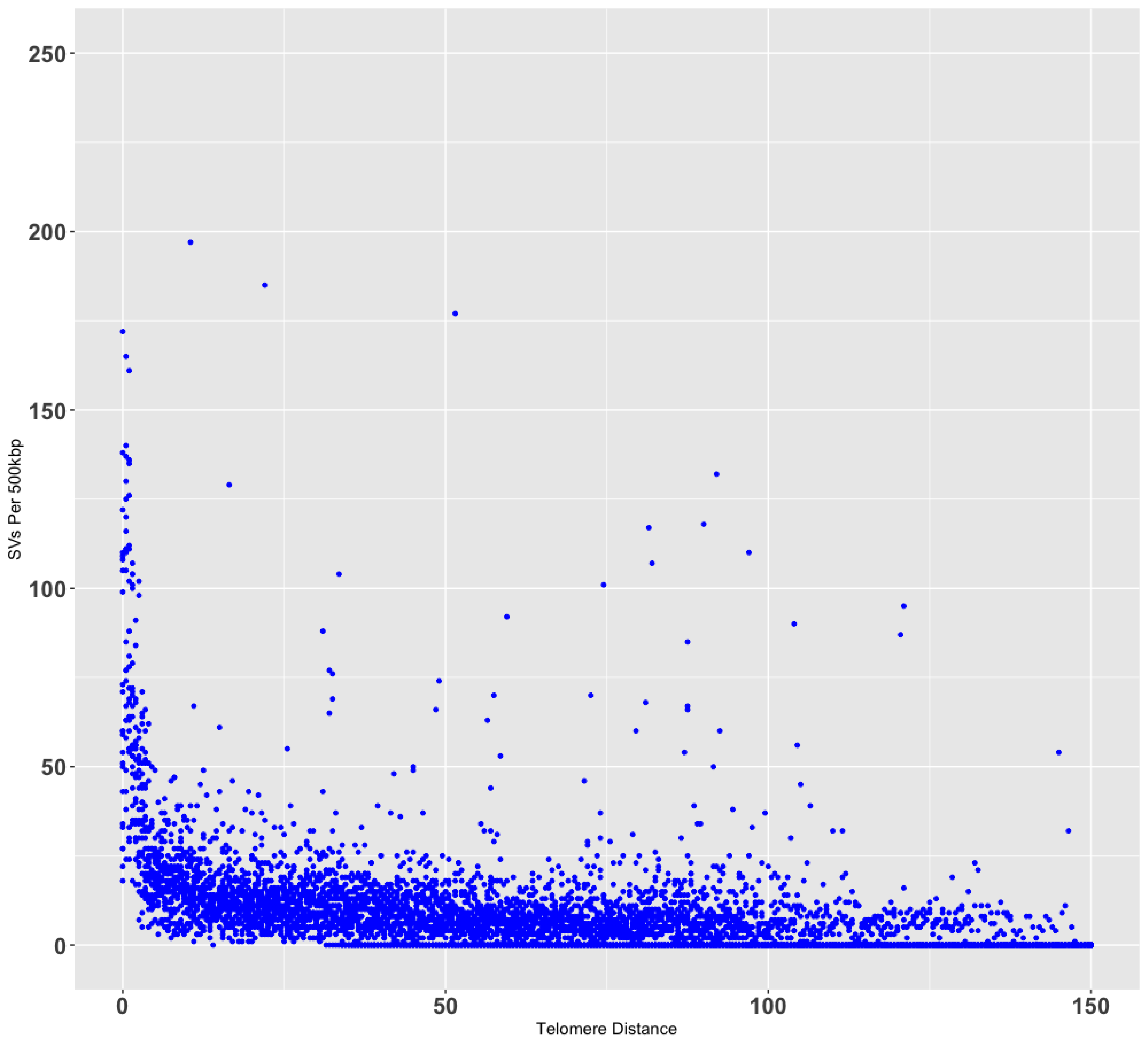
telomere enrichment
ggplot(data = data_sv_dist[data_sv_dist$All!=0,], aes(x = Dist, y = All)) + geom_point(size = 0.05, color="blue") + annotate("rect", fill = "dark gray", alpha = 0.5, xmin = 0, xmax = 5, ymin = -Inf, ymax = Inf)+ xlim(0, 150) + # ylim(0, 250) + ylab('SVs Per 500kbp') + xlab('Telomere Distance') + theme_minimal()+ theme( axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold"))
-
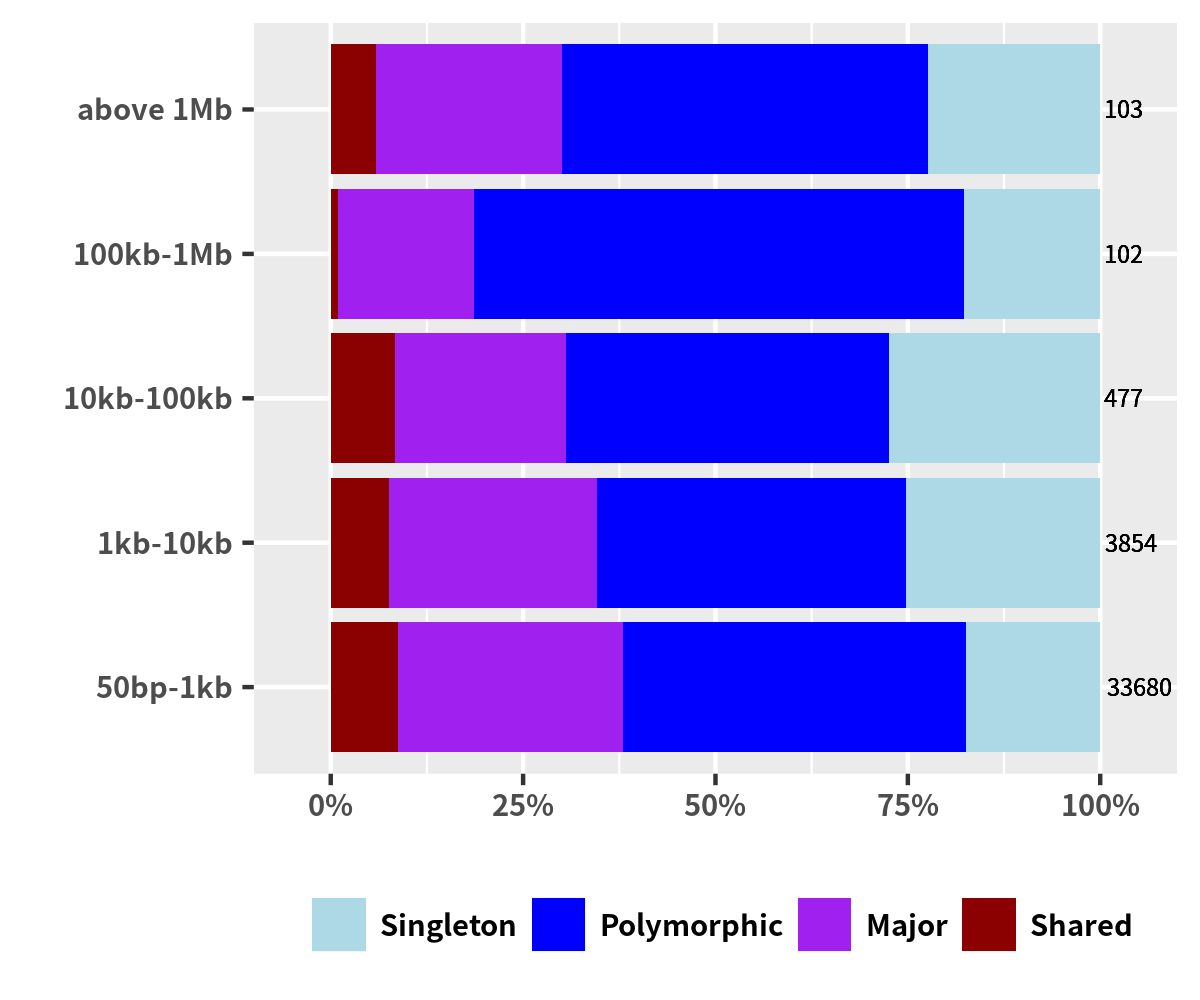
Group Support
data_sv_group <- plyr::count(data_plot,'GROUP_SUPP') data_sv_group$GROUP_SUPP <- factor(data_sv_group$GROUP_SUPP) ggplot(data_sv_group, aes(x="",y=freq,fill=GROUP_SUPP)) + geom_bar(width=1,stat="identity") + coord_polar("y",start=0) + geom_text(aes(y=freq/4+c(0,cumsum(freq)[-length(freq)])), label=percent(data_sv_group$freq/sum(data_sv_group$freq)), size=2, color='white', fontface="bold") + scale_fill_manual(values=config_color_group_supp) + theme_minimal() + theme(legend.position = c(0.5,0), legend.direction = 'horizontal', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.title=element_blank(), legend.text = element_text(size = 6,face = "bold"), panel.border = element_blank(), panel.grid = element_blank(), axis.ticks = element_blank(), axis.text = element_blank(), axis.title.x = element_blank(), axis.title.y = element_blank())
-
SV length
ggplot(data=data_plot[data_plot$SVTYPE!='TRA',], aes(x=SVLEN, color = SVTYPE)) + geom_freqpoly(binwidth=1/5, size=0.8) + scale_x_continuous(trans = 'log10', breaks=c(100,1000,10000,100000,1000000,10000000), labels =c('100bp','1kb','10kb','100kb','1Mb', '10Mb'), limits = c(50, 10000000) ) + scale_y_continuous(trans = 'log10') + theme_minimal() + theme(legend.title=element_blank(), legend.position=c(0.9,0.9), legend.direction = 'vertical', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(-10,0,0,0))) + scale_fill_manual(values=config_color_svtype) + xlab('') + ylab("SV Count")
-
SV length group
data_sv_group <- plyr::count(data_plot,c('GROUP_LEN','GROUP_SUPP')) data_sv_group$label <- 0 for (type_sv in levels(data_sv_group$GROUP_LEN)){ data_sv_group[data_sv_group$GROUP_LEN==type_sv,]$label <- sum(data_sv_group[data_sv_group$GROUP_LEN==type_sv,]$freq) } ggplot(data_sv_group, aes(x = GROUP_LEN, y = freq, fill = GROUP_SUPP)) + geom_bar(position = "fill",stat = "identity") + scale_y_continuous(labels = scales::percent_format(), expand = expand_scale(mult = .1)) + coord_flip() + theme_minimal()+ theme(legend.title=element_blank(), legend.position="bottom", legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(-10,0,0,0))) + scale_fill_manual(values=config_color_group_supp) + xlab("") + ylab("") + geom_text(aes(label = label, y= ..prop..), stat= "count", hjust = -0.1, size=1)
-
SV type group
data_sv_group <- plyr::count(data_plot,c('SVTYPE','GROUP_SUPP')) data_sv_group$SVTYPE <- factor(data_sv_group$SVTYPE) data_sv_group$label <- 0 for (type_sv in levels(data_sv_group$SVTYPE)){ data_sv_group[data_sv_group$SVTYPE==type_sv,]$label <- sum(data_sv_group[data_sv_group$SVTYPE==type_sv,]$freq) } data_sv_group$SVTYPE <- factor(data_sv_group$SVTYPE, levels = c( 'DEL', 'INS', 'DUP', 'INV', 'TRA')) ggplot(data_sv_group, aes(x = SVTYPE, y = freq, fill = GROUP_SUPP)) + geom_bar(position = "fill",stat = "identity") + scale_y_continuous(labels = scales::percent_format(), expand = expand_scale(mult = .1)) + coord_flip() + theme_minimal()+ theme(legend.title=element_blank(), legend.position="bottom", legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(-15,0,0,0))) + scale_fill_manual(values=config_color_group_supp) + xlab("") + ylab("") + geom_text(aes(label = label, y= ..prop..), stat= "count", hjust = -0.1, size=2)
-
SV breakpoints CI
data_tmp <- data_plot data_tmp$GROUP_CI <- NA data_tmp[data_tmp$MAXCI_POS<=1000&data_tmp$MAXCI_END<=1000,]$GROUP_CI <- '500bp-1kb' data_tmp[data_tmp$MAXCI_POS<=500&data_tmp$MAXCI_END<=500,]$GROUP_CI <- '250bp-500bp' data_tmp[data_tmp$MAXCI_POS<=250&data_tmp$MAXCI_END<=250,]$GROUP_CI <- '100bp-250bp' data_tmp[data_tmp$MAXCI_POS<=100&data_tmp$MAXCI_END<=100,]$GROUP_CI <- '0-100bp' data_tmp$GROUP_CI <- factor(data_tmp$GROUP_CI, levels = c('0-100bp', '100bp-250bp', '250bp-500bp', '500bp-1kb')) df.new<-ddply(data_tmp,.(GROUP_SUPP),plyr::summarise, prop=prop.table(table(GROUP_CI)), SUPP=names(table(GROUP_CI))) df.new$SUPP <- factor(df.new$SUPP, levels = c('0-100bp', '100bp-250bp', '250bp-500bp', '500bp-1kb')) ggplot(df.new, aes(SUPP, prop, fill=GROUP_SUPP)) + geom_bar(stat="identity",position = 'dodge') + theme_minimal()+ theme(legend.title=element_blank(), legend.position=c(0.5,0.9), legend.direction = 'horizontal', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold") ) + scale_fill_manual(values=config_color_group_supp) + xlab("Max Interval of Breakpoints") + ylab("Prop")
-
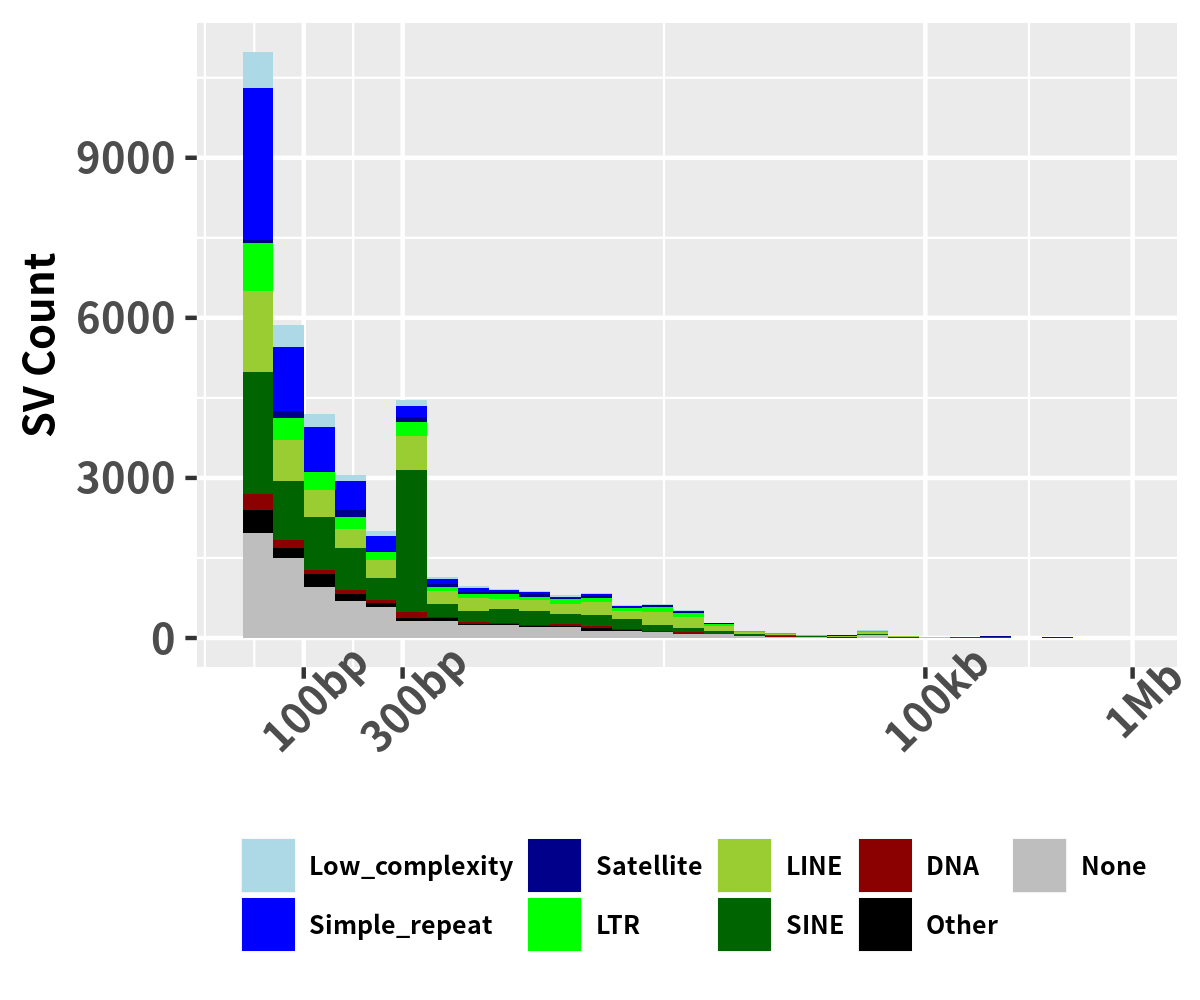
SV breakpoints repeat length
ggplot(data=data_sv_repeat, aes(x=abs(SVLEN), fill = RepClass)) + geom_histogram() + scale_x_continuous(trans = 'log10',breaks = c(100,300,2000,6000,20000), labels = c('100bp','300bp','2kb','6kb','20kb'),limits = c(50,20000)) + theme_minimal()+ theme(legend.title=element_blank(), legend.position=c(0.8,0.65), legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold", angle = 45), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold")) + scale_fill_manual(values=config_color_repeat) + xlab('') + ylab("SV Count")
-
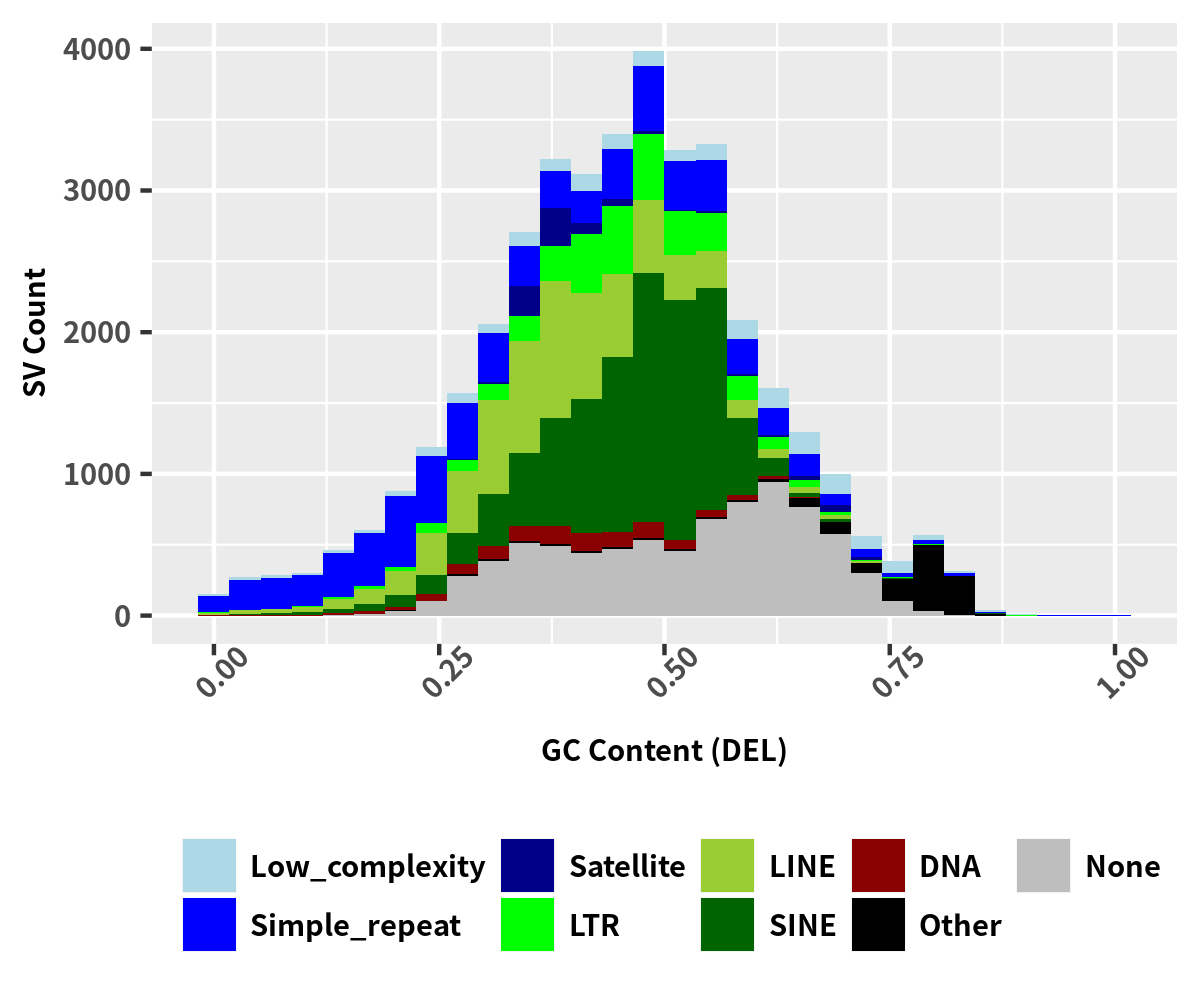
SV breakpoints gc repeat
ggplot(data=data_sv_repeat, aes(x=GCcontent, fill = RepClass)) + geom_histogram() + theme_minimal()+ theme(legend.title=element_blank(), legend.position='bottom', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.5, 'cm'), legend.text = element_text(size = 10,face = "bold"), axis.text.x = element_text(size = 10,face = "bold", angle = 45), axis.text.y = element_text(size = 10,face = "bold"), axis.title.y = element_text(size = 10,face = "bold"), axis.title.x = element_text(size = 10,face = "bold")) + scale_fill_manual(values=config_color_repeat) + xlab('GC Content') + ylab("SV Count")
-
SV Database AF
ggplot(data=data_plot, aes(x=AF_Database, fill = GROUP_SUPP)) + geom_histogram() + theme_minimal()+ # scale_y_continuous(trans = 'log', breaks = c(1000,10000),labels = c('1000','10000')) + theme(legend.title=element_blank(), legend.position='bottom', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.5, 'cm'), legend.text = element_text(size = 10,face = "bold"), axis.text.x = element_text(size = 10,face = "bold", angle = 45), axis.text.y = element_text(size = 10,face = "bold"), axis.title.y = element_text(size = 10,face = "bold"), axis.title.x = element_text(size = 10,face = "bold")) + scale_fill_manual(values=config_color_group_supp) + xlab('AF') + ylab("Discovery")
-
SV Genotyping
data_tmp <- data_plot data_tmp$GROUP_SUPP_NGS <- as.character(data_tmp$GROUP_SUPP_NGS) data_tmp <- data_tmp[!is.na(data_tmp$MR_NGS),] # data_tmp <- data_tmp[!is.na(data_tmp$MR_NGS)&data_tmp$MR_NGS<0.05,] data_tmp[is.na(data_tmp$GROUP_SUPP_NGS),]$GROUP_SUPP_NGS <- 'No Support' data_tmp$GROUP_SUPP_NGS <- factor(data_tmp$GROUP_SUPP_NGS, levels = c('No Support','Singleton', 'Polymorphic', 'Major', 'Shared')) data_tmp_1 <- plyr::count(data_tmp[data_tmp$MR_NGS<0.05,],'GROUP_SUPP_NGS') data_tmp_2 <- plyr::count(data_tmp[data_tmp$MR_NGS<0.05&data_tmp$GROUP_SUPP_NGS=='No Support',], 'GROUP_SUPP') data_tmp_3 <- plyr::count(data_tmp[data_tmp$MR_NGS<0.05&data_tmp$GROUP_SUPP_NGS!='No Support',], 'GROUP_SUPP') ids_pie = c('SV', 'MR_0', 'MR_1', 'No_Support', 'Support') labels_pie = c('SV genotyping', 'MR < 0.05', 'MR >= 0.05', 'genotyped AF = 0', 'genotyped AF > 0') parents_pie = c('', 'SV', 'SV', 'MR_0', 'MR_0') values_pie = c(nrow(data_tmp), sum(data_tmp$MR_NGS<0.05), sum(data_tmp$MR_NGS>=0.05), data_tmp_1[data_tmp_1$GROUP_SUPP_NGS=='No Support',]$freq, sum(data_tmp_1$freq)-data_tmp_1[data_tmp_1$GROUP_SUPP_NGS=='No Support',]$freq) for (label_condition in data_tmp_2$GROUP_SUPP) { ids_pie <- c(ids_pie, paste(label_condition, 'No_Support', sep = '_')) labels_pie <- c(labels_pie, label_condition) parents_pie <- c(parents_pie, 'No_Support') values_pie <- c(values_pie, data_tmp_2[data_tmp_2$GROUP_SUPP==label_condition,]$freq) } for (label_condition in data_tmp_3$GROUP_SUPP) { ids_pie <- c(ids_pie, paste(label_condition, 'Support', sep = '_')) labels_pie <- c(labels_pie, label_condition) parents_pie <- c(parents_pie, 'Support') values_pie <- c(values_pie, data_tmp_3[data_tmp_3$GROUP_SUPP==label_condition,]$freq) } data_sv_group <- subset(data_tmp, GROUP_SUPP_NGS=='No Support' & MR_NGS <= 0.05) data_sv_group$GROUP_REP <- 'None' data_sv_group[data_sv_group$RepClass!='None',]$GROUP_REP <- 'Repclass' data_sv_group[data_sv_group$RepClass=='None'&data_sv_group$SD!='',]$GROUP_REP <- 'SD' data_sv_group[data_sv_group$GROUP_REP=='None'& (data_sv_group$Repeats_type_left!='None'|data_sv_group$Repeats_type_right!='None'),]$GROUP_REP <- 'flanked' data_tmp_4 <- plyr::count(data_sv_group,c('GROUP_SUPP','GROUP_REP')) for (label_group in data_tmp_2$GROUP_SUPP){ for (label_condition in unique(data_tmp_4$GROUP_REP)) { ids_pie <- c(ids_pie, paste(label_condition, label_group, 'No_Support', sep = '_')) labels_pie <- c(labels_pie, label_condition) parents_pie <- c(parents_pie, paste(label_group,'No_Support', sep = '_')) values_pie <- c(values_pie, data_tmp_4[data_tmp_4$GROUP_SUPP==label_group& data_tmp_4$GROUP_REP==label_condition,]$freq) } } if (!require("processx")) install.packages("processx") fig <- plot_ly( ids=ids_pie, labels=labels_pie, parents=parents_pie, values=values_pie, type='sunburst', branchvalues = 'total' ) orca(fig, "../data/result_genotypted/plots/genotyping.pie.svg")
- SV Genotyping HardyWeinberg
library(HardyWeinberg) plot.HWE <- function(dat,pop=NULL,title=NULL,full.legend=F,lab.cex=1){ require(HardyWeinberg,quietly=T) #Gather HW p-values & colors HWE.mat <- dat HW.p <- HWChisqStats(X=HWE.mat,x.linked=F,pvalues=T) HW.cols <- rep("#4DAC26",times=length(HW.p)) HW.cols[which(HW.p<0.05)] <- "#81F850" HW.cols[which(HW.p<0.05/length(HW.p))] <- "#AC26A1" #Generate HW plot frame par(mar=c(1,1,1,1),bty="n") plot(x=1.15*c(-1/sqrt(3),1/sqrt(3)),y=c(-0.15,1.15),type="n", xaxt="n",yaxt="n",xlab="",ylab="",xaxs="i",yaxs="i") segments(x0=c(-1/sqrt(3),0,1/sqrt(3)), x1=c(0,1/sqrt(3),-1/sqrt(3)), y0=c(0,1,0),y1=c(1,0,0)) HWTernaryPlot(X=HWE.mat,n=max(HWE.mat,na.rm=T),newframe=F, vbounds=F,mafbounds=F, region=1,vertexlab=NA, alpha=0.05, curvecols=c("#4DAC26","#81F850",NA,NA),pch=NA) #Add axes text(x=c(-1/sqrt(3),1/sqrt(3)),y=0,labels=c("0/0","1/1"), pos=1,cex=lab.cex,xpd=T,font=2) text(x=0,y=1,labels="0/1",pos=3,cex=lab.cex,xpd=T,font=2) #Finish HW plot HWTernaryPlot(X=HWE.mat,n=max(HWE.mat,na.rm=T),newframe=F, vbounds=F,mafbounds=F, region=1,vertexlab=NA, alpha=0.03/nrow(HWE.mat), curvecols=c("#4DAC26","#AC26A1",NA,NA), pch=21,cex=0.3,signifcolour=F,markercol=NA, markerbgcol=adjustcolor(HW.cols,alpha=0.25)) segments(x0=c(-1/sqrt(3),0,1/sqrt(3)), x1=c(0,1/sqrt(3),-1/sqrt(3)), y0=c(0,1,0),y1=c(1,0,0)) #Add legend n.pass <- length(which(HW.p>=0.05)) print(paste("PASS: ",n.pass/length(HW.p),sep="")) n.nom <- length(which(HW.p<0.05 & HW.p>=0.05/nrow(HWE.mat))) print(paste("NOMINAL FAILS: ",n.nom/length(HW.p),sep="")) n.bonf <- length(which(HW.p<0.05/nrow(HWE.mat))) print(paste("BONFERRONI FAILS: ",n.bonf/length(HW.p),sep="")) legend("right",pch=19,col=c("#4DAC26","#81F850","#AC26A1"),pt.cex=1, legend=c(paste(round(100*(n.pass/nrow(HWE.mat)),0),"%",sep=""), paste(round(100*(n.nom/nrow(HWE.mat)),0),"%",sep=""), paste(round(100*(n.bonf/nrow(HWE.mat)),0),"%",sep="")), bty="n",bg=NA,cex=lab.cex) text(x=par("usr")[2],y=par("usr")[4]-(0.2*(par("usr")[4]-par("usr")[3])),pos=2,cex=lab.cex, labels=paste(title,"\n \n ",sep=""),font=2) text(x=par("usr")[2],y=par("usr")[4]-(0.2*(par("usr")[4]-par("usr")[3])),pos=2,cex=lab.cex, labels=paste(" \n",prettyNum(max(apply(HWE.mat,1,sum),na.rm=T),big.mark=",")," Samples\n ",sep="")) text(x=par("usr")[2],y=par("usr")[4]-(0.2*(par("usr")[4]-par("usr")[3])),pos=2,cex=lab.cex, labels=paste(" \n \n",prettyNum(nrow(HWE.mat),big.mark=",")," SV",sep="")) } data_genotypes_normal <- data_genotypes[ (!is.na(data_sv_details_all$AF_ALL_NGS))& data_sv_details_all$MR_NGS<0.05& data_sv_details_all$AF_ALL_NGS>0.01& data_sv_details_all$AF_ALL_NGS<1, colnames(data_genotypes)%in%data_samples_info[data_samples_info$Platform=='NGS',]$SampleID] data_genotypes_normal[data_genotypes_normal==-1] <- 0 data_genotypes_hw <- data_genotypes_normal data_genotypes_hw$AA <- rowSums(data_genotypes_normal==0) data_genotypes_hw$AB <- rowSums(data_genotypes_normal==1) data_genotypes_hw$BB <- rowSums(data_genotypes_normal==2) data_genotypes_hw <- data_genotypes_hw[,c('AA','AB','BB')] # png(filename = paste(prefix_filename,'genotyping.hardyweinberg.png',sep = '.'), width = 1000, height = 1000, res = val_res) plot.HWE(data_genotypes_hw, lab.cex = 1)
-
SV Genotyping
tmp <- subset(data_plot, SUPP_NGS>0) df.new<-ddply(tmp,.(SVTYPE),plyr::summarise, prop=prop.table(table(GROUP_SUPP_NGS)), SUPP=names(table(GROUP_SUPP_NGS))) df.new$SUPP <- factor(df.new$SUPP, levels = c('Singleton', 'Polymorphic', 'Major', 'Shared')) ggplot(df.new, aes(SUPP, prop, fill=SVTYPE)) + geom_bar(stat="identity",position = 'dodge') + theme_minimal()+ theme(legend.title=element_blank(), legend.position=c(0.9,0.8), panel.border = element_blank(), panel.grid = element_blank(), panel.background = element_blank(), legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(-15,0,0,0))) + scale_fill_manual(values=config_color_svtype) + xlab("") + ylab("Prop")
-
SV Genotyping SUPP
ggplot(data=data_tmp, aes(x=SUPP, fill = GROUP_SUPP_NGS)) + geom_bar() + theme_minimal()+ theme(legend.title=element_blank(), legend.position=c(0.5,0.9), legend.direction = 'horizontal', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold", angle = 45), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold")) + scale_fill_manual(values=config_color_group_supp_han) + xlab('Samples Support') + ylab("Discovery")
-
SV Genotyping LD
ggplot(data_sv_group, aes(x=SVTYPE, y=LD,group=SVTYPE)) + geom_boxplot(aes(fill=SVTYPE),outlier.size = 0.1) + theme_minimal()+ theme(legend.title=element_blank(), legend.position='bottom', # panel.border = element_blank(), # panel.grid = element_blank(), panel.background = element_blank(), legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.5, 'cm'), legend.text = element_text(size = 10,face = "bold"), axis.text.x = element_text(size = 10,face = "bold"), axis.text.y = element_text(size = 10,face = "bold"), axis.title.y = element_text(size = 10,face = "bold"), axis.title.x = element_text(size = 10,face = "bold"), axis.title.x.bottom = element_text(margin = margin(-10,0,0,0))) + scale_fill_manual(values=config_color_svtype) + xlab('') + ylab("max LD")
-
SV gene enrichment
data.gene.background <- read.xlsx('example/enrich.xlsx') for (index_row in seq(nrow(data.gene.background))) { tmp <- fisher.test(matrix(c(data.gene.background[index_row,]$A1, data.gene.background[index_row,]$A0, data.gene.background[index_row,]$N1, data.gene.background[index_row,]$N0),nrow = 2)) data.gene.background[index_row,]$Odds.Ratio <- tmp$estimate data.gene.background[index_row,]$p.value <- tmp$p.value } data.gene.background$Gene.Set <- factor(data.gene.background$Gene.Set, levels = rev(data.gene.background$Gene.Set[order(data.gene.background$Odds.Ratio,decreasing = TRUE)])) ggplot(data=data.gene.background[2:11,],aes(x=Gene.Set, y=A1, fill=p.value)) + geom_bar(stat="identity") + coord_flip() + theme(panel.background=element_rect(fill='transparent',colour = 'black'), legend.key.size=unit(0.2, 'cm'), legend.title=element_blank(), legend.text = element_text(size = 2.5,face = "bold"), axis.text.x = element_text(size = 2.5,face = "bold"), axis.text.y = element_text(color="black",size=2.5,face = "bold"), axis.title.y = element_text(size = 2.5,face = "bold")) + scale_fill_gradient(low="red",high="blue", breaks=c(0.05, 0.1), limits=c(0, 0.15)) + xlab("") + ylab("")
-
SV Genotyping VAF
data_tmp <- data_plot[!is.na(data_plot$AF_ALL_NGS)&data_plot$MR_NGS<0.05&data_plot$AF_ALL_NGS>0&data_plot$AF_ALL_NGS<0.1,] pdf(file = paste(prefix_filename,'genotyping.pop.vaf.group_pop_detail','pdf',sep = '.'), width = 3, height = 3) ggplot(data=data_tmp, aes(AF_ALL_NGS, stat(count), fill = GROUP_POP_DETAIL_NGS)) + geom_density(position = "fill",bw=0.005) + scale_fill_manual(values=config_color_group_pop)+ theme_minimal()+ theme(legend.title=element_blank(), legend.position='bottom', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(5,0,0,0))) + xlab('VAF') + ylab("Proportion") dev.off()
-
SV Genotyping PCA
group_sample <- as.data.frame(data_samples_info[!data_samples_info$Source%in%c('T15H10','HUM'),c('SampleID','Group','Group_Detail','Location')]) group_sample <- group_sample[order(group_sample$SampleID),] rownames(group_sample) <- group_sample$SampleID data_genotypes_normal <- data_genotypes[!is.na(data_sv_details_all$AF_ALL_NGS)&data_sv_details_all$MR_NGS<0.05&data_sv_details_all$AF_ALL_NGS>0.01,colnames(data_genotypes)%in%group_sample$SampleID] data_genotypes_normal <- data_genotypes_normal[, order(colnames(data_genotypes_normal))] data_genotypes_normal[data_genotypes_normal==-1] <- 0 # tmp <- (data_genotypes_normal- rowMeans(data_genotypes_normal))/(1+(2*sqrt(data_sv_details_all$AF_ALL_NGS*(1-data_sv_details_all$AF_ALL_NGS)))) p <- (1 + rowSums(data_genotypes_normal))/(2+2*nrow(group_sample)) tmp <- (data_genotypes_normal- rowMeans(data_genotypes_normal))/2*sqrt(p*(1-p)) data_sv_details_all_matrix.pca <- prcomp(t(as.matrix(tmp))) PCi<-data.frame(data_sv_details_all_matrix.pca$x,Group_Detail=group_sample$Group_Detail) ggplot(PCi,aes(x=PC1,y=PC2,color=Group_Detail))+ geom_point(size=0.8)+ scale_color_manual(values = config_color_group_pop) + theme_minimal()+ theme(legend.title=element_blank(), legend.position=c(0.1,0.9), legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.3, 'cm'), # panel.background = element_blank(), # # panel.border = element_rect(size=0.6, colour = "black"), # axis.line = element_line(size=0.6, colour = "black"), # axis.line.x.top = element_line(size=0.6, colour = "black"), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold"), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(5,0,0,0)) )
-
SV Genotyping PHt
group_sample <- as.data.frame(data_samples_info[!data_samples_info$Source%in%c('T15H10'), c('SampleID','Group','Group_Detail','Location')]) group_sample <- group_sample[order(group_sample$SampleID),] group_sample$PHt <- 0 group_sample$PHot <- 0 rownames(group_sample) <- group_sample$SampleID data_genotypes_normal <- data_genotypes[!is.na(data_sv_details_all$AF_ALL_NGS)&data_sv_details_all$MR_NGS<0.05&data_sv_details_all$AF_ALL_NGS>0.01,colnames(data_genotypes)%in%group_sample$SampleID] data_genotypes_normal <- data_genotypes_normal[, order(colnames(data_genotypes_normal))] data_genotypes_normal[data_genotypes_normal==-1] <- 0 data_genotypes_normal_type <- data_sv_details_all[!is.na(data_sv_details_all$AF_ALL_NGS)&data_sv_details_all$MR_NGS<0.05&data_sv_details_all$AF_ALL_NGS>0.01,]$SVTYPE for (tmp_sample in group_sample$SampleID) { group_sample[group_sample$SampleID==tmp_sample,]$PHt <- sum(data_genotypes_normal[data_genotypes_normal_type%in%c('INS','DEL'),tmp_sample]==1) group_sample[group_sample$SampleID==tmp_sample,]$PHot <- sum(data_genotypes_normal[data_genotypes_normal_type%in%c('INS','DEL'),tmp_sample]==2) } group_sample_svtype <- rbind(group_sample,group_sample) group_sample_svtype$SVTYPE <- c(rep('DEL', nrow(group_sample)), rep('INS', nrow(group_sample))) for (tmp_svtype in unique(group_sample_svtype$SVTYPE)) { for (tmp_sample in group_sample$SampleID) { group_sample_svtype[group_sample_svtype$SampleID==tmp_sample&group_sample_svtype$SVTYPE==tmp_svtype,]$PHt <- sum(data_genotypes_normal[data_genotypes_normal_type==tmp_svtype,tmp_sample]==1) group_sample_svtype[group_sample_svtype$SampleID==tmp_sample&group_sample_svtype$SVTYPE==tmp_svtype,]$PHot <- sum(data_genotypes_normal[data_genotypes_normal_type==tmp_svtype,tmp_sample]==2) } } # group_sample <-group_sample[!group_sample$Group_Detail%in%c('HUM','AFR'),] group_sample <-group_sample[!group_sample$Group_Detail%in%c('HUM'),] group_sample_svtype <-group_sample_svtype[!group_sample_svtype$Group_Detail%in%c('HUM'),] # group_sample <- group_sample[!group_sample$SampleID%in%c('WGC025266D', 'WGC025273D'),] group_sample$Location <- factor(group_sample$Location, levels = unique(c( group_sample[group_sample$Group_Detail=='TIBG',]$Location, group_sample[group_sample$Group_Detail=='TIBL',]$Location, group_sample[group_sample$Group_Detail=='HANN',]$Location, group_sample[group_sample$Group_Detail=='HANS',]$Location, group_sample[group_sample$Group_Detail=='AFR',]$Location ))) pairwise.t.test(group_sample$PHot, g = group_sample$Group_Detail, p.adjust.method = 'bonferroni') pairwise.t.test(group_sample$PHt, g = group_sample$Group_Detail, p.adjust.method = 'bonferroni') p1 <- ggplot(group_sample, aes(x=Group_Detail, y=PHot, fill=Group_Detail)) + geom_violin() + coord_flip() + # geom_signif(comparisons = list(c('TIBG','TIBL')), map_signif_level=TRUE) #geom_signif(comparisons = list(levels(factor(group_sample$Group_Detail))), map_signif_level=TRUE) scale_fill_manual(values=config_color_group_pop) + theme_minimal()+ theme(legend.title=element_blank(), legend.position='none', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.5, 'cm'), legend.text = element_text(size = 6,face = "bold"), panel.border = element_blank(), panel.grid = element_blank(), panel.background = element_blank(), axis.line.x = element_line(size=0.6, colour = "black"), axis.line.y = element_line(size=0.6, colour = "black"), axis.text.x = element_text(size = 6,face = "bold",vjust = 0.6), axis.text.y = element_text(size = 6,face = "bold"), axis.title.y = element_text(size = 6,face = "bold"), axis.title.x = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(5,0,0,0))) + xlab('') + ylab("#hom") p2 <- ggplot(group_sample, aes(x=Group_Detail, y=PHt, fill=Group_Detail)) + geom_violin() + coord_flip() + scale_fill_manual(values=config_color_group_pop) + theme_minimal()+ theme(legend.title=element_blank(), legend.position='none', legend.spacing.x = unit(0.1, 'cm'), legend.key.size=unit(0.5, 'cm'), panel.border = element_blank(), panel.grid = element_blank(), panel.background = element_blank(), axis.line.x = element_line(size=0.6, colour = "black"), axis.text.y = element_blank(), axis.ticks.y = element_blank(), legend.text = element_text(size = 6,face = "bold"), axis.text.x = element_text(size = 6,face = "bold",vjust = 0.6), axis.title.x = element_text(size = 6,face = "bold"), axis.title.x.bottom = element_text(margin = margin(5,0,0,0))) + xlab('') + ylab("#het") # ggarrange(p1, p2,ncol=2,nrow = 1) multiplot(p1,p2,cols = 2)
-
SV Genotyping FST
library(qqman) library(Cairo) Fstfile <- read.table(paste('example/merge.paragraph.genotypes.TIBvsHAN.20k_5k.windowed.weir.fst', sep = ''), header = T, stringsAsFactors = F) SNP <- paste(Fstfile[,1], Fstfile[,2], sep = ':') Fstfile <- cbind(SNP, Fstfile) colnames(Fstfile) <- c('SNP', 'CHR', 'POS','END','Bins', 'Fst','mean') Fstfile[Fstfile$CHR == 'chrX',]$CHR <- 'chr23' Fstfile$CHR <- as.numeric(str_split_fixed(Fstfile$CHR,'chr',2)[,2]) filePNG <-paste(prefix_filename,'genotyping.pop.fst.pdf',sep = '.') CairoPNG(file=filePNG, width = 1500, height = 500) CairoPDF(file = filePNG, width = 8, height = 4, onefile = TRUE, family = "Helvetica") colorset <- c('#FF0000', '#FFD700', '#2E8B57', '#7FFFAA', '#6495ED', '#0000FF', '#FF00FF') manhattan(Fstfile, chr='CHR', bp='POS', p='Fst', snp='SNP', col=colorset, logp=FALSE, suggestiveline=0.15, genomewideline=FALSE, ylab='Fst', ylim=c(0,0.6), font.lab=4, cex.lab=0.8, cex=0.1, chrlabs = c(1:22, "X")) dev.off()
-
SV Genotyping admixture
# Assign the first argument to prefix prefix=paste('example/admixture/merge.paragraph.genotypes.local.reformat.addPopInfo.addFST.LDpruned',sep = '') # Get individual names in the correct order labels <- data.frame('ind' <- colnames(data_sv_details_raw@gt)[27:217], 'pop' <- NA) names(labels)<-c("ind","pop") labels <- labels[labels$ind%in%data_samples_info$SampleID,] index_sample <- 1 while (index_sample <= nrow(labels)) { labels[index_sample,]$pop <- as.character(data_samples_info[data_samples_info$SampleID==labels[index_sample,]$ind,]$Group_Detail) index_sample <- index_sample + 1 } # Add a column with population indices to order the barplots # Use the order of populations provided as the fourth argument (list separated by commas) labels$n<-factor(labels$pop,levels=unlist(strsplit('AFR,HANS,HANN,TIBL,TIBG',","))) levels(labels$n)<-c(1:length(levels(labels$n))) labels$n<-as.integer(as.character(labels$n)) # read in the different admixture output files maxK=7 tbl<-lapply(2:maxK, function(x) read.table(paste0(prefix,".",x,".Q"))) # Prepare spaces to separate the populations/species rep<-as.vector(table(labels$n)) spaces<-0 for(i in 1:length(rep)){spaces=c(spaces,rep(0,rep[i]-1),0.5)} spaces<-spaces[-length(spaces)] # Plot the cluster assignments as a single bar for each individual par(mfrow=c(maxK-1,1),mar=c(0,1,0,0),oma=c(2,1,9,1), mgp=c(0,0.2,0),xaxs="i",cex.lab=1.2,cex.axis=0.8) bp<-barplot(t(as.matrix(tbl[[2]][order(labels$n),])), col=rainbow(n=2), xaxt="n", border=NA, ylab="K=2", yaxt="n", space=spaces) axis(3,at=bp,labels=labels$ind[order(labels$n)],las=2,tick=F,cex=0.6) lapply(3:(maxK-1), function(x) barplot(t(as.matrix(tbl[[x]][order(labels$n),])), col=rainbow(n=x),xaxt="n", border=NA, ylab=paste0("K=",x), yaxt="n", space=spaces)) axis(1,at=c(which(spaces==0.5), bp[length(bp)])-diff(c(1,which(spaces==0.5),bp[length(bp)]))/2, tick = F, labels=unlist(strsplit('AFR,HANS,HANN,TIBL,TIBG',",")))