Atomic clock
| Atomic clock | |
|---|---|
 NIST physicists Steve Jefferts (foreground) and Tom Heavner with the NIST-F2 caesium fountain atomic clock, a civilian time standard for the United States | |
| Classification | Clock |
| Industry | Telecommunications, science |
| Application | TAI, satellite navigation |
| Fuel source | Electricity |
| Powered | Yes |

An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions between such states they interact with a very specific frequency of electromagnetic radiation. This phenomenon serves as the basis for the International System of Units' (SI) definition of a second:
The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency, , the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be 9192631770 when expressed in the unit Hz, which is equal to s−1.
This definition is the basis for the system of International Atomic Time (TAI), which is maintained by an ensemble of atomic clocks around the world. The system of Coordinated Universal Time (UTC) that is the basis of civil time implements leap seconds to allow clock time to track changes in Earth's rotation to within one second while being based on clocks that are based on the definition of the second, though leap seconds will be phased out in 2035.[2]
The accurate timekeeping capabilities of atomic clocks are also used for navigation by satellite networks such as the European Union's Galileo Programme and the United States' GPS. The timekeeping accuracy of the involved atomic clocks is important because the smaller the error in time measurement, the smaller the error in distance obtained by multiplying the time by the speed of light is (a timing error of a nanosecond or 1 billionth of a second (10−9 or 1⁄1,000,000,000 second) translates into an almost 30-centimetre (11.8 in) distance and hence positional error).
The main variety of atomic clock uses caesium atoms cooled to temperatures that approach absolute zero. The primary standard for the United States, the National Institute of Standards and Technology (NIST)'s caesium fountain clock named NIST-F2, measures time with an uncertainty of 1 second in 300 million years (relative uncertainty 10−16). NIST-F2 was brought online on 3 April 2014.[3][4]
History

The Scottish physicist James Clerk Maxwell proposed measuring time with the vibrations of light waves in his 1873 Treatise on Electricity and Magnetism: 'A more universal unit of time might be found by taking the periodic time of vibration of the particular kind of light whose wave length is the unit of length.'[5][6] Maxwell argued this would be more accurate than the Earth's rotation, which defines the mean solar second for timekeeping.[7]
During the 1930s, the American physicist Isidor Isaac Rabi built equipment for atomic beam magnetic resonance frequency clocks.[8][9]
The accuracy of mechanical, electromechanical and quartz clocks is reduced by temperature fluctuations. This led to the idea of measuring the frequency of an atom's vibrations to keep time much more accurately, as proposed by James Clerk Maxwell, Lord Kelvin, and Isidor Rabi.[10] He proposed the concept in 1945, which led to a demonstration of a clock based on ammonia in 1949.[11] This led to the first practical accurate atomic clock with caesium atoms being built at the National Physical Laboratory in the United Kingdom in 1955[12][13] by Louis Essen in collaboration with Jack Parry.[14]

In 1949, Alfred Kastler and Jean Brossel[16] developed a technique called optical pumping for electron energy level transitions in atoms using light. This technique is useful for creating much stronger magnetic resonance and microwave absorption signals. Unfortunately, this caused a side effect with a light shift of the resonant frequency. Claude Cohen-Tannoudji and others managed to reduce the light shifts to acceptable levels.
Ramsey developed a method, commonly known as Ramsey interferometry nowadays, for higher frequencies and narrower resonances in the oscillating fields. Kolsky, Phipps, Ramsey, and Silsbee used this technique for molecular beam spectroscopy in 1950.[17]
After 1956, atomic clocks were studied by many groups, such as the National Institute of Standards and Technology (formerly the National Bureau of Standards) in the USA, the Physikalisch-Technische Bundesanstalt (PTB) in Germany, the National Research Council (NRC) in Canada, the National Physical Laboratory in the United Kingdom, International Time Bureau (French: Bureau International de l'Heure, abbreviated BIH), at the Paris Observatory, the National Radio Company, Bomac, Varian, Hewlett–Packard and Frequency & Time Systems.[18]
During the 1950s, the National Radio Company sold more than 50 units of the first atomic clock, the Atomichron.[19] In 1964, engineers at Hewlett-Packard released the 5060 rack-mounted model of caesium clocks.[10]
Definition of the second
In 1968, the SI defined the duration of the second to be 9192631770 vibrations of the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom. Prior to that it was defined by there being 31556925.9747 seconds in the tropical year 1900.[20] In 1997, the International Committee for Weights and Measures (CIPM) added that the preceding definition refers to a caesium atom at rest at a temperature of absolute zero.[21]: 113 Following the 2019 revision of the SI, the definition of every base unit except the mole and almost every derived unit relies on the definition of the second.
Timekeeping researchers are currently working on developing an even more stable atomic reference for the second, with a plan to find a more precise definition of the second as atomic clocks improve based on optical clocks or the Rydberg constant around 2030.[22][23]
Metrology advancements and optical clocks

Technological developments such as lasers and optical frequency combs in the 1990s led to increasing accuracy of atomic clocks.[24][25] Lasers enable the possibility of optical-range control over atomic states transitions, which has a much higher frequency than that of microwaves; while optical frequency comb measures highly accurately such high frequency oscillation in light.
The first advance beyond the precision of caesium clocks occurred at NIST in 2010 with the demonstration of a "quantum logic" optical clock that used aluminum ions to achieve a precision of 10−17.[26] Optical clocks are a very active area of research in the field of metrology as scientists work to develop clocks based on elements ytterbium, mercury, aluminum, and strontium. Scientists at JILA demonstrated a strontium clock with a frequency precision of 10−18 in 2015.[27] Scientists at NIST developed a quantum logic clock that measured a single aluminum ion in 2019 with a frequency uncertainty of 9.4×10−19.[28][29]
At JILA in September 2021, scientists demonstrated an optical strontium clock with a differential frequency precision of 7.6×10−21 between atomic ensembles[clarification needed] separated by 1 mm.[30][31] The second is expected to be redefined when the field of optical clocks matures, sometime around the year 2030 or 2034.[32] In order for this to occur, optical clocks must be consistently capable of measuring frequency with accuracy at or better than 2×10−18. In addition, methods for reliably comparing different optical clocks around the world in national metrology labs must be demonstrated[clarification needed], and the comparison must show relative clock frequency accuracies at or better than 5×10−18.
Chip-scale atomic clocks
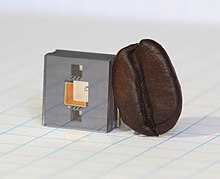
In addition to increased accuracy, the development of chip-scale atomic clocks has expanded the number of places atomic clocks can be used. In August 2004, NIST scientists demonstrated a chip-scale atomic clock that was 100 times smaller than an ordinary atomic clock and had a much smaller power consumption of 125 mW.[33][34] The atomic clock was about the size of a grain of rice with a frequency of about 9 GHz. This technology became available commercially in 2011.[33] Atomic clocks on the scale of one chip require less than 30 milliwatts of power.[35][36]
The National Institute of Standards and Technology created a program NIST on a chip to develop compact ways of measuring time with a device just a few millimeters across.[37]
Metrologists are currently (2022) designing atomic clocks that implement new developments such as ion traps and optical combs to reach greater accuracies.[38]
Measuring time with atomic clocks
Clock mechanism
An atomic clock is based on a system of atoms which may be in one of two possible energy states. A group of atoms in one state is prepared, then subjected to microwave radiation. If the radiation is of the correct frequency, a number of atoms will transition to the other energy state. The closer the frequency is to the inherent oscillation frequency of the atoms, the more atoms will switch states. Such correlation allows very accurate tuning of the frequency of the microwave radiation. Once the microwave radiation is adjusted to a known frequency where the maximum number of atoms switch states, the atom and thus, its associated transition frequency, can be used as a timekeeping oscillator to measure elapsed time.[39]
All timekeeping devices use oscillatory phenomena to accurately measure time, whether it is the rotation of the Earth for a sundial, the swinging of a pendulum in a grandfather clock, the vibrations of springs and gears in a watch, or voltage changes in a quartz crystal watch. However all of these are easily affected by temperature changes and are not very accurate. The most accurate clocks use atomic vibrations to keep track of time. Clock transition states in atoms are insensitive to temperature and other environmental factors and the oscillation frequency is much higher than any of the other clocks (in microwave frequency regime and higher).
One of the most important factors in a clock's performance is the atomic line quality factor, Q, which is defined as the ratio of the absolute frequency of the resonance to the linewidth of the resonance itself . Atomic resonance has a much higher Q than mechanical devices. Atomic clocks can also be isolated from environmental effects to a much higher degree. Atomic clocks have the benefit that atoms are universal, which means that the oscillation frequency is also universal. This is different from quartz and mechanical time measurement devices that do not have a universal frequency.
A clock's quality can be specified by two parameters: accuracy and stability. Accuracy is a measurement of the degree to which the clock's ticking rate can be counted on to match some absolute standard such as the inherent hyperfine frequency of an isolated atom or ion. Stability describes how the clock performs when averaged over time to reduce the impact of noise and other short-term fluctuations (see precision).[40]
The instability of an atomic clock is specified by its Allan deviation .[41] The limiting instability due to atom or ion counting statistics is given by
where is the spectroscopic linewidth of the clock system, is the number of atoms or ions used in a single measurement, is the time required for one cycle, and is the averaging period. This means instability is smaller when the linewidth is smaller and when (the signal to noise ratio) is larger. The stability improves as the time over which the measurements are averaged increases from seconds to hours to days. The stability is most heavily affected by the oscillator frequency . This is why optical clocks such as strontium clocks (429 terahertz) are much more stable than caesium clocks (9.19 GHz).
Modern clocks such as atomic fountains or optical lattices that use sequential interrogation are found to generate type of noise that mimics and adds to the instability inherent in atom or ion counting. This effect is called the Dick effect[42] and is typically the primary stability limitation for the newer atomic clocks. It is an aliasing effect; high frequency noise components in the local oscillator ("LO") are heterodyned to near zero frequency by harmonics of the repeating variation in feedback sensitivity to the LO frequency. The effect places new and stringent requirements on the LO, which must now have low phase noise in addition to high stability, thereby increasing the cost and complexity of the system. For the case of an LO with Flicker frequency noise[43] where is independent of , the interrogation time is , and where the duty factor has typical values , the Allan deviation can be approximated as[44]
This expression shows the same dependence on as does , and, for many of the newer clocks, is significantly larger. Analysis of the effect and its consequence as applied to optical standards has been treated in a major review (Ludlow, et al., 2015)[45] that lamented on "the pernicious influence of the Dick effect", and in several other papers.[46][47]
Tuning and optimization
The core of the traditional radio frequency atomic clock is a tunable microwave cavity containing a gas. In a hydrogen maser clock the gas emits microwaves (the gas mases) on a hyperfine transition, the field in the cavity oscillates, and the cavity is tuned for maximum microwave amplitude. Alternatively, in a caesium or rubidium clock, the beam or gas absorbs microwaves and the cavity contains an electronic amplifier to make it oscillate. For both types, the atoms in the gas are prepared in one hyperfine state prior to filling them into the cavity. For the second type, the number of atoms that change hyperfine state is detected and the cavity is tuned for a maximum of detected state changes.
Most of the complexity of the clock lies in this adjustment process. The adjustment tries to correct for unwanted side-effects, such as frequencies from other electron transitions, temperature changes, and the spreading in frequencies caused by the vibration of molecules including Doppler broadening.[48] One way of doing this is to sweep the microwave oscillator's frequency across a narrow range to generate a modulated signal at the detector. The detector's signal can then be demodulated to apply feedback to control long-term drift in the radio frequency.[49]
In this way, the quantum-mechanical properties of the atomic transition frequency of the caesium can be used to tune the microwave oscillator to the same frequency, except for a small amount of experimental error. When a clock is first turned on, it takes a while for the oscillator to stabilize. In practice, the feedback and monitoring mechanism is much more complex.[50]
Many of the newer clocks, including microwave clocks such as trapped ion or fountain clocks, and optical clocks such as lattice clocks use a sequential interrogation protocol rather than the frequency modulation interrogation described above.[45] An advantage of sequential interrogation is that it can accommodate much higher Q's, with ringing times of seconds rather than milliseconds. These clocks also typically have a dead time, during which the atom or ion collections are analyzed, renewed and driven into a proper quantum state, after which they are interrogated with a signal from a local oscillator (LO) for a time of perhaps a second or so. Analysis of the final state of the atoms is then used to generate a correction signal to keep the LO frequency locked to that of the atoms or ions.
Accuracy

The accuracy of atomic clocks has improved continuously since the first prototype in the 1950s. The first generation of atomic clocks were based on measuring caesium, rubidium, and hydrogen atoms. In a time period from 1959 to 1998, NIST developed a series of seven caesium-133 microwave clocks named NBS-1 to NBS-6 and NIST-7 after the agency changed its name from the National Bureau of Standards to the National Institute of Standards and Technology.[10] The first clock had an accuracy of 10−11, and the last clock had an accuracy of 10−15. The clocks were the first to use a caesium fountain, which was introduced by Jerrod Zacharias, and laser cooling of atoms, which was demonstrated by Dave Wineland and his colleagues in 1978.
The next step in atomic clock advances involves going from accuracies of 10−15 to accuracies of 10−18 and even 10−19.[a] The goal is to redefine the second when clocks become so accurate that they will not lose or gain more than a second in the age of the universe.[b] To do so, scientists must demonstrate the accuracy of clocks that use strontium and ytterbium and optical lattice technology. Such clocks are also called optical clocks where the energy level transitions used are in the optical regime (giving rise to even higher oscillation frequency), which thus, have much higher accuracy as compared to traditional atomic clocks.[52]
The goal of an atomic clock with 10−16 accuracy was first reached at the United Kingdom's National Physical Laboratory's NPL-CsF2 caesium fountain clock[53][54][55] and the United States' NIST-F2.[56][57] The increase in precision from NIST-F1 to NIST-F2 is due to liquid nitrogen cooling of the microwave interaction region; the largest source of uncertainty in NIST-F1 is the effect of black-body radiation from the warm chamber walls.[58][4]
The performance of primary and secondary frequency standards contributing to International Atomic Time (TAI) is evaluated. The evaluation reports of individual (mainly primary) clocks are published online by the International Bureau of Weights and Measures (BIPM).
Comparing atomic clocks
Time standards
A number of national metrology laboratories maintain atomic clocks: including Paris Observatory, the Physikalisch-Technische Bundesanstalt (PTB) in Germany, the National Institute of Standards and Technology (NIST) in Colorado and Maryland, USA, JILA in the University of Colorado Boulder, the National Physical Laboratory (NPL) in the United Kingdom, and the All-Russian Scientific Research Institute for Physical-Engineering and Radiotechnical Metrology. They do this by designing and building frequency standards that produce electric oscillations at a frequency whose relationship to the transition frequency of caesium 133 is known, in order to achieve a very low uncertainty. These primary frequency standards estimate and correct various frequency shifts, including relativistic Doppler shifts linked to atomic motion, the thermal radiation of the environment (blackbody shift) and several other factors. The best primary standards currently produce the SI second with an accuracy approaching an uncertainty of one part in 1016.
It is important to note that at this level of accuracy, the differences in the gravitational field in the device cannot be ignored. The standard is then considered in the framework of general relativity to provide a proper time at a specific point.[59]
The International Bureau of Weights and Measures (BIPM) provides a list of frequencies that serve as secondary representations of the second. This list contains the frequency values and respective standard uncertainties for the rubidium microwave transition and other optical transitions, including neutral atoms and single trapped ions. These secondary frequency standards can be as accurate as one part in 1018; however, the uncertainties in the list are one part in 1014–1016. This is because the uncertainty in the central caesium standard against which the secondary standards are calibrated is one part in 1014–1016.
Primary frequency standards can be used to calibrate the frequency of other clocks used in national laboratories. These are usually commercial caesium clocks having very good long-term frequency stability, maintaining a frequency with a stability better than 1 part in 1014 over a few months. The uncertainty of the primary standard frequencies is around one part in 1013.
Hydrogen masers, which rely on the 1.4 GHz hyperfine transition in atomic hydrogen, are also used in time metrology laboratories. Masers outperform any commercial caesium clock in terms of short-term frequency stability. In the past, these instruments have been used in all applications that require a steady reference across time periods of less than one day (frequency stability of about 1 part in ten[clarification needed] for averaging times of a few hours). Because some active hydrogen masers have a modest but predictable frequency drift with time, they have become an important part of the BIPM's ensemble of commercial clocks that implement International Atomic Time.[59]
Synchronization with satellites
The time readings of clocks operated in metrology labs operating with the BIPM need to be known very accurately. Some operations require synchronization of atomic clocks separated by great distances over thousands of kilometers. Global Navigational Satellite Systems (GNSS) provide a satisfactory solution to the problem of time transfer. Atomic clocks are used to broadcast time signals in the United States Global Positioning System (GPS), the Russian Federation's Global Navigation Satellite System (GLONASS), the European Union's Galileo system and China's BeiDou system.
The signal received from one satellite in a metrology laboratory equipped with a receiver with an accurately known position allows the time difference between the local time scale and the GNSS system time to be determined with an uncertainty of a few nanoseconds when averaged over 15 minutes. Receivers allow the simultaneous reception of signals from several satellites, and make use of signals transmitted on two frequencies. As more satellites are launched and start operations, time measurements will become more accurate.
These methods of time comparison must make corrections for the effects of special relativity and general relativity of a few nanoseconds.
In June 2015, the National Physical Laboratory (NPL) in Teddington, UK; the French department of Time-Space Reference Systems at the Paris Observatory (LNE-SYRTE); the German German National Metrology Institute (PTB) in Braunschweig; and Italy's Istituto Nazionale di Ricerca Metrologica (INRiM) in Turin labs have started tests to improve the accuracy of current state-of-the-art satellite comparisons by a factor of 10, but it will still be limited to one part in 1×1016. These four European labs are developing and host a variety of experimental optical clocks that harness different elements in different experimental set-ups and want to compare their optical clocks against each other and check whether they agree.[60]
International timekeeping

National laboratories usually operate a range of clocks. These are operated independently of one another and their measurements are sometimes combined to generate a scale that is more stable and more accurate than that of any individual contributing clock. This scale allows for time comparisons between different clocks in the laboratory. These atomic time scales are generally referred to as TA(k) for laboratory k.[61]
Coordinated Universal Time (UTC) is the result of comparing clocks in national laboratories around the world to International Atomic Time (TAI), then adding leap seconds as necessary. TAI is a weighted average of around 450 clocks in some 80 time institutions.[62] The relative stability of TAI is around one part in 1016.
Before TAI is published, the frequency of the result is compared with the SI second at various primary and secondary frequency standards. This requires relativistic corrections to be applied to the location of the primary standard which depend on the distance between the equal gravity potential and the rotating geoid of Earth. The values of the rotating geoid and the TAI change slightly each month and are available in the BIPM Circular T publication. The TAI time-scale is deferred by a few weeks as the average of atomic clocks around the world is calculated.
TAI is not distributed in everyday timekeeping. Instead, an integer number of leap seconds are added or subtracted to correct for the Earth's rotation, producing UTC. The number of leap seconds is changed so that mean solar noon at the prime meridian (Greenwich) does not deviate from UTC noon by more than 0.9 seconds.
National metrology institutions maintain an approximation of UTC referred to as UTC(k) for laboratory k. UTC(k) is distributed by the BIPM's Consultative Committee for Time and Frequency. The offset UTC-UTC(k) is calculated every 5 days, the results are published monthly. Atomic clocks record UTC(k) to no more than 100 nanoseconds. In some countries, UTC(k) is the legal time that is distributed by radio, television, telephone, Internet, fiber-optic cables, time signal transmitters, and speaking clocks. In addition, GNSS provides time information accurate to a few tens of nanoseconds or better.
Fiber Optics
In a next phase, these labs strive to transmit comparison signals in the visible spectrum through fibre-optic cables. This will allow their experimental optical clocks to be compared with an accuracy similar to the expected accuracies of the optical clocks themselves. Some of these labs have already established fibre-optic links, and tests have begun on sections between Paris and Teddington, and Paris and Braunschweig. Fibre-optic links between experimental optical clocks also exist between the American NIST lab and its partner lab JILA, both in Boulder, Colorado but these span much shorter distances than the European network and are between just two labs. According to Fritz Riehle, a physicist at PTB, "Europe is in a unique position as it has a high density of the best clocks in the world".[60]
In August 2016 the French LNE-SYRTE in Paris and the German PTB in Braunschweig reported the comparison and agreement of two fully independent experimental strontium lattice optical clocks in Paris and Braunschweig at an uncertainty of 5×10−17 via a newly established phase-coherent frequency link connecting Paris and Braunschweig, using 1,415 km (879 mi) of telecom fibre-optic cable. The fractional uncertainty of the whole link was assessed to be 2.5×10−19, making comparisons of even more accurate clocks possible.[63][64]
In 2021, NIST compared transmission of signals from a series of experimental atomic clocks located about 1.5 km (1 mi) apart at the NIST lab, its partner lab JILA, and the University of Colorado all in Boulder, Colorado over air and fiber optic cable to a precision of 8×10−18.[65][66]
Microwave atomic clocks
Caesium
The SI second is defined as a certain number of unperturbed ground-state hyperfine transitions of the caesium-133 atom. Caesium standards are therefore regarded as primary time and frequency standards.
Caesium clocks include the NIST-F1 clock, developed in 1999, and the NIST-F2 clock, developed in 2013.[67][68]
Caesium has several properties that make it a good choice for an atomic clock. Whereas a hydrogen atom moves at 1,600 m/s at room temperature and a nitrogen atom moves at 510 m/s, a caesium atom moves at a much slower speed of 130 m/s due to its greater mass.[69][10] The hyperfine frequency of caesium (~9.19 GHz) is also higher than other elements such as rubidium (~6.8 GHz) and hydrogen (~1.4 GHz).[10] The high frequency of caesium allows for more accurate measurements. Caesium reference tubes suitable for national standards currently last about seven years and cost about US$35,000. Primary frequency and time standards like the United States Time Standard atomic clocks, NIST-F1 and NIST-F2, use far higher power.[34][70][71][72]
Block diagram

In a caesium beam frequency reference, timing signals are derived from a high stability voltage-controlled quartz crystal oscillator (VCXO) that is tunable over a narrow range. The output frequency of the VCXO (typically 5 MHz) is multiplied by a frequency synthesizer to obtain microwaves at the frequency of the caesium atomic hyperfine transition (about 9192.6317 MHz). The output of the frequency synthesizer is amplified and applied to a chamber containing caesium gas which absorbs the microwaves. The output current of the caesium chamber increases as absorption increases.
The remainder of the circuitry simply adjusts the running frequency of the VCXO to maximize the output current of the caesium chamber which keeps the oscillator tuned to the resonance frequency of the hyperfine transition.[73]
Rubidium

The BIPM defines the unperturbed ground-state hyperfine transition frequency of the rubidium-87 atom, 6 834 682 610.904 312 6 Hz, in terms of the caesium standard frequency. Atomic clocks based on rubidium standards are therefore regarded as secondary representations of the second.
Rubidium standard clocks are prized for their low cost, small size (commercial standards are as small as 1.7×105 mm3)[33] and short-term stability. They are used in many commercial, portable and aerospace applications. Modern rubidium standard tubes last more than ten years, and can cost as little as US$50. Some commercial applications use a rubidium standard periodically corrected by a global positioning system receiver (see GPS disciplined oscillator). This achieves excellent short-term accuracy, with long-term accuracy equal to (and traceable to) the US national time standards.[74]
Hydrogen

The BIPM defines the unperturbed optical transition frequency of the hydrogen-1 neutral atom, 1 233 030 706 593 514 Hz, in terms of the caesium standard frequency. Atomic clocks based on hydrogen standards are therefore regarded as secondary representations of the second.
Hydrogen masers have superior short-term stability compared to other standards, but lower long-term accuracy. The long-term stability of hydrogen maser standards decreases because of changes in the cavity's properties over time. The relative error of hydrogen masers is 5 × 10−16 for periods of 1000 seconds. This makes hydrogen masers good for radio astronomy, in particular for very long baseline interferometry.[6]
Hydrogen masers are used for flywheel oscillators in laser-cooled atomic frequency standards and broadcasting time signals from national standards laboratories, although they need to be corrected as they drift from the correct frequency over time. The hydrogen maser is also useful for experimental tests of the effects of special relativity and general relativity such as gravitational red shift.[6]
Other types of atomic clocks

Quantum clocks
In March 2008, physicists at NIST described a quantum logic clock based on individual ions of beryllium and aluminium. This clock was compared to NIST's mercury ion clock. These were the most accurate clocks that had been constructed, with neither clock gaining nor losing time at a rate that would exceed a second in over a billion years.[75] In February 2010, NIST physicists described a second, enhanced version of the quantum logic clock based on individual ions of magnesium and aluminium. Considered the world's most precise clock in 2010 with a fractional frequency inaccuracy of 8.6×10−18, it offers more than twice the precision of the original.[76][77]
In July 2019, NIST scientists demonstrated such an Al+ quantum logic clock with total uncertainty of 9.4×10−19, which is the first demonstration of such a clock with uncertainty below 10−18.[78][79][80]
Nuclear clock concept
One theoretical possibility for improving the performance of atomic clocks is to use a nuclear energy transition (between different nuclear isomers) rather than the atomic electron transitions which current atomic clocks measure. Most nuclear transitions operate at far too high a frequency to be measured, but the exceptionally low excitation energy of 229m
Th
produces "gamma rays" in the ultraviolet frequency range. In 2003, Ekkehard Peik and Christian Tamm[81] noted this makes a clock possible with current optical frequency-measurement techniques. In 2012, it was shown that a nuclear clock based on a single 229
Th3+
ion could provide a total fractional frequency inaccuracy of 1.5×10−19, which was better than existing 2019 optical atomic clock technology.[82] Although a precise clock remains an unrealized theoretical possibility, efforts through the 2010s to measure the transition energy[83][84][85][86] culminated in the 2024 measurement of the optical frequency with sufficient accuracy (2020407384335±2 kHz = 2.020407384335(2)×1015 Hz[87][88][89][90]) that an experimental optical nuclear clock can now be constructed.[91]
Although neutral 229m
Th
atoms decay in microseconds by internal conversion,[92] this pathway is energetically prohibited in 229m
Th+
ions, as the second and higher ionization energy is greater than the nuclear excitation energy, giving 229m
Th+
ions a long half-life on the order of 103 s.[88] It is the large ratio between transition frequency and isomer lifetime which gives the clock a high quality factor.[82]
A nuclear energy transition offers the following potential advantages:[93]
- Higher frequency. All other things being equal, a higher-frequency transition offers greater stability for simple statistical reasons (fluctuations are averaged over more cycles).
- Insensitivity to environmental effects. Due to its small size and the shielding effect of the surrounding electrons, an atomic nucleus is much less sensitive to ambient electromagnetic fields than is an electron in an orbital.
- Greater number of atoms. Because of the aforementioned insensitivity to ambient fields, it is not necessary to have the clock atoms well-separated in a dilute gas. Current measurements take advantage of the Mössbauer effect and place the thorium ions in a solid, which allows billions of atoms to be interrogated.
Potential for redefining the second
In 2022, the best realisation of the second is done with caesium primary standard clocks such as IT-CsF2, NIST-F2, NPL-CsF2, PTB-CSF2, SU–CsFO2 or SYRTE-FO2. These clocks work by laser-cooling a cloud of caesium atoms to a microkelvin in a magneto-optic trap. These cold atoms are then launched vertically by laser light. The atoms then undergo Ramsey excitation in a microwave cavity. The fraction of excited atoms are then detected by laser beams. These clocks have 5×10−16 systematic uncertainty, which is equivalent to 50 picoseconds per day. A system of several fountains worldwide contributes to International Atomic Time. These caesium clocks also underpin optical frequency measurements.
The advantage of optical clocks can be explained by the statement that the instability , where is the instability, f is the frequency, and S/N is the signal-to-noise ratio. This leads to the equation .
Optical clocks are based on forbidden optical transitions in ions or atoms. They have frequencies around 1015 Hz, with a natural linewidth of typically 1 Hz, so the Q-factor is about 1015, or even higher. They have better stabilities than microwave clocks, which means that they can facilitate evaluation of lower uncertainties. They also have better time resolution, which means the clock "ticks" faster.[94] Optical clocks use either a single ion, or an optical lattice with 104–106 atoms.
Rydberg constant
A definition based on the Rydberg constant would involve fixing the value to a certain value: . The Rydberg constant describes the energy levels in a hydrogen atom with the nonrelativistic approximation .
The only viable way to fix the Rydberg constant involves trapping and cooling hydrogen. Unfortunately, this is difficult because it is very light and the atoms move very fast, causing Doppler shifts. The radiation needed to cool the hydrogen —121.5 nm— is also difficult. Another hurdle involves improving the uncertainty in quantum electrodynamics/QED calculations.[95]
In the Report of the 25th meeting of the Consultative Committee for Units (2021),[96] 3 options were considered for the redefinition of the second sometime around 2026, 2030, or 2034. The first redefinition approach considered was a definition based on a single atomic reference transition. The second redefinition approach considered was a definition based on a collection of frequencies. The third redefinition approach considered was a definition based on fixing the numerical value of a fundamental constant, such as making the Rydberg constant the basis for the definition. The committee concluded there was no feasible way to redefine the second with the third option, since no physical constant is known to enough digits currently to enable realizing the second with a constant.
Requirements
A redefinition must include improved optical clock reliability. TAI must be contributed to by optical clocks before the BIPM affirms a redefinition. A consistent method of sending signals, such as fiber-optics, must be developed before the second is redefined.[95]
Secondary representations of the second
Representations of the second other than the SI cesium standard are motivated by the increasing accuracy of other atomic clocks. In particular the high frequencies and small linewidths of optical clocks promise significantly improved signal-to-noise ratio and instability. Further secondary representations would aid in the preparation of a future redefinition of the second[97]
A list of frequencies recommended for secondary representations of the second is maintained by the International Bureau of Weights and Measures (BIPM) since 2006 and is available online. The list contains the frequency values and the respective standard uncertainties for the rubidium microwave transition and for several optical transitions. These secondary frequency standards are accurate at the level of 10−18; however, the uncertainties provided in the list are in the range 10−14 – 10−15 since they are limited by the linking to the caesium primary standard that currently (2018) defines the second.[59]
| Type | Working frequency (Hz) | Relative Allan deviation (typical clocks) |
Reference |
|---|---|---|---|
| 133Cs | 9.192631770×109 by definition | 10−13 | [98] |
| 87Rb | 6.834682610904324×109 | 10−12 | [99] |
| 1H | 1.4204057517667×109 | 10−15 | [100][101] |
| Optical clock (87Sr) | 4.292280042298734×1014 | 10−17 | [102] |
| Optical clock (27Al+) | 1.12101539320785916×1015 | 10−18 | [103][104] |
| Optical clock (171Yb+, 642 THz) | 6.4212149677264512×1014 | 10−18 | [105][106] |
| Optical clock (171Yb+, 688 THz) | 6.8835897930930824×1014 | 10−16 | [107][108] |
Twenty-first century experimental atomic clocks that provide non-caesium-based secondary representations of the second are becoming so precise that they are likely to be used as extremely sensitive detectors for other things besides measuring frequency and time. For example, the frequency of atomic clocks is altered slightly by gravity, magnetic fields, electrical fields, force, motion, temperature and other phenomena. The experimental clocks tend to continue to improve, and leadership in performance has shifted back and forth between various types of experimental clocks.[109][110][111][112]
Applications
The development of atomic clocks has led to many scientific and technological advances such as precise global and regional navigation satellite systems, and applications in the Internet, which depend critically on frequency and time standards. Atomic clocks are installed at sites of time signal radio transmitters.[113] They are used at some long-wave and medium-wave broadcasting stations to deliver a very precise carrier frequency.[114] Atomic clocks are used in many scientific disciplines, such as for long-baseline interferometry in radio astronomy.[115]
Global navigation satellite systems
The Global Positioning System (GPS) operated by the United States Space Force provides very accurate timing and frequency signals. A GPS receiver works by measuring the relative time delay of signals from a minimum of four, but usually more, GPS satellites, each of which has at least two onboard caesium and as many as two rubidium atomic clocks. The relative times are mathematically transformed into three absolute spatial coordinates and one absolute time coordinate.[116] GPS Time (GPST) is a continuous time scale and theoretically accurate to about 14 nanoseconds.[117] However, most receivers lose accuracy in the interpretation of the signals and are only accurate to 100 nanoseconds.[118][119]
GPST is related to but differs from TAI (International Atomic Time) and UTC (Coordinated Universal Time). GPST remains at a constant offset from TAI (TAI – GPST = 19 seconds) and like TAI does not implement leap seconds. Periodic corrections are performed to the on-board clocks in the satellites to keep them synchronized with ground clocks.[120][121] The GPS navigation message includes the difference between GPST and UTC. As of July 2015, GPST is 17 seconds ahead of UTC because of the leap second added to UTC on 30 June 2015.[122][123] Receivers subtract this offset from GPS Time to calculate UTC.
The GLObal NAvigation Satellite System (GLONASS) operated by the Russian Aerospace Defence Forces provides an alternative to the Global Positioning System (GPS) system and is the second navigational system in operation with global coverage and of comparable precision. GLONASS Time (GLONASST) is generated by the GLONASS Central Synchroniser and is typically better than 1,000 nanoseconds.[124] Unlike GPS, the GLONASS time scale implements leap seconds, like UTC.[125]

The Galileo Global Navigation Satellite System is operated by the European GNSS Agency and European Space Agency. Galileo started offering global Early Operational Capability (EOC) on 15 December 2016, providing the third, and first non-military operated, global navigation satellite system.[126][127] Galileo System Time (GST) is a continuous time scale which is generated on the ground at the Galileo Control Centre in Fucino, Italy, by the Precise Timing Facility, based on averages of different atomic clocks and maintained by the Galileo Central Segment and synchronised with TAI with a nominal offset below 50 nanoseconds.[128][129][130][127] According to the European GNSS Agency, Galileo offers 30 nanoseconds timing accuracy.[131]
The March 2018 Quarterly Performance Report by the European GNSS Service Centre reported the UTC Time Dissemination Service Accuracy was ≤ 7.6 nanoseconds, computed by accumulating samples over the previous 12 months, and exceeding the ≤ 30 ns target.[132][133] Each Galileo satellite has two passive hydrogen maser and two rubidium atomic clocks for onboard timing.[134][135]
The Galileo navigation message includes the differences between GST, UTC and GPST, to promote interoperability.[136][137] In the summer of 2021, the European Union settled on a passive hydrogen maser for the second generation of Galileo satellites, starting in 2023, with an expected lifetime of 12 years per satellite. The masers are about 2 feet long with a weight of 40 pounds.[138]
The BeiDou-2/BeiDou-3 satellite navigation system is operated by the China National Space Administration. BeiDou Time (BDT) is a continuous time scale starting at 1 January 2006 at 0:00:00 UTC and is synchronised with UTC within 100 ns.[139][140] BeiDou became operational in China in December 2011, with 10 satellites in use,[141] and began offering services to customers in the Asia-Pacific region in December 2012.[142] On 27 December 2018 the BeiDou Navigation Satellite System started to provide global services with a reported timing accuracy of 20 ns.[143] The final, 35th, BeiDou-3 satellite for global coverage was launched into orbit on 23 June 2020.[144]
Experimental space clock
In April 2015, NASA announced that it planned to deploy a Deep Space Atomic Clock (DSAC), a miniaturized, ultra-precise mercury-ion atomic clock, into outer space. NASA said that the DSAC would be much more stable than other navigational clocks.[145] The clock was successfully launched on 25 June 2019,[146] activated on 23 August 2019[147] and deactivated two years later on 18 September 2021.[148]
Military usage
In 2022, DARPA announced a drive to upgrade to the U.S. military timekeeping systems for greater precision over time when sensors do not have access to GPS satellites, with a plan to reach precision of 1 part in 1012. The Robust Optical Clock Network will balance usability and accuracy as it is developed over 4 years.[149][150]
Time signal radio transmitters
A radio clock is a clock that automatically synchronizes itself by means of radio time signals received by a radio receiver. Some manufacturers may label radio clocks as atomic clocks,[151] because the radio signals they receive originate from atomic clocks. Normal low-cost consumer-grade receivers that rely on the amplitude-modulated time signals have a practical accuracy uncertainty of ± 0.1 second. This is sufficient for many consumer applications.[151] Instrument grade time receivers provide higher accuracy. Radio clocks incur a propagation delay of approximately 1 ms for every 300 kilometres (186 mi) of distance from the radio transmitter. Many governments operate transmitters for timekeeping purposes.[152]
General relativity
General relativity predicts that clocks tick slower deeper in a gravitational field, and this gravitational redshift effect has been well documented. Atomic clocks are effective at testing general relativity on ever smaller scales. A project to observe twelve atomic clocks from 11 November 1999 to October 2014 resulted in a further demonstration that Einstein's theory of general relativity is accurate at small scales.[153]
In 2021 a team of scientists at JILA measured the difference in the passage of time due to gravitational redshift between two layers of atoms separated by one millimeter using a strontium optical clock cooled to 100 nanokelvins with a precision of 7.6×10−21 seconds.[154] Given its quantum nature and the fact that time is a relativistic quantity, atomic clocks can be used to see how time is influenced by general relativity and quantum mechanics at the same time.[155][156]
Financial systems
Atomic clocks keep accurate records of transactions between buyers and sellers to the millisecond or better, particularly in high-frequency trading.[157][158] Accurate timekeeping is needed to prevent illegal trading ahead of time, in addition to ensuring fairness to traders on the other side of the globe. The current system known as NTP is only accurate to a millisecond.[159]
Transportable Optical Clocks
Many of the most accurate optical clocks are big and only available in large metrology labs. Thus they are not readily useful for space-limited factories or other industrial environments that could use an atomic clock for GPS accuracy.
Researchers have designed a strontium optical lattice clock that can be moved around in an air-conditioned car trailer. They achieved a relative uncertainty of 7.4×10−17 compared to a stationary one.[160]
See also
Explanatory notes
- ^ Researchers at the University of Wisconsin-Madison have demonstrated a clock that will not lose a second in 300 billion years.[51]
- ^ One second in 13.8 billion years, the age of the universe, is an accuracy of 2.3×10−18.
References
- ^ "USNO Master Clock". Archived from the original on 7 December 2010. Retrieved 23 November 2010.
- ^ Brumfiel, Geoff (27 November 2022). "The world is doing away with the leap second". Weekend Edition Sunday. National Public Radio. Retrieved 30 April 2024.
- ^ "NIST Launches a New U.S. Time Standard: NIST-F2 Atomic Clock". NIST. 3 April 2014 – via www.nist.gov.
- ^ a b Thomas P. Heavner; Elizabeth A. Donley; Filippo Levi; Giovanni Costanzo; Thomas E. Parker; Jon H. Shirley; Neil Ashby; Stephan Barlow; Steven R. Jefferts (May 2014). "First Accuracy Evaluation of NIST-F2" (PDF). Metrologia. 51 (3): 174–182. Bibcode:2014Metro..51..174H. doi:10.1088/0026-1394/51/3/174.
Currently, the type B fractional uncertainty in NIST-F1 is 0.31×10−15 and is dominated by the uncertainty in the blackbody radiation (BBR) shift correction, which is 0.28×10−15 (this corresponds to a 1 degree uncertainty in the radiation environment as seen by the atoms in NIST-F1). To improve the performance of the NIST primary frequency standard, we sought to reduce the uncertainty due to the BBR effect. To accomplish this goal and to better understand the accepted model of the BBR shift, we developed NIST-F2, a laser-cooled Cs fountain primary frequency standard in which the microwave cavity structure and flight tube operate at cryogenic temperatures (80 K).
- ^ Ramsey, Norman F. (June 2006). "History of early atomic clocks". Metrologia. 42 (3): S1–S3. doi:10.1088/0026-1394/42/3/s01. ISSN 0026-1394. S2CID 122631200.
- ^ a b c Achard, F. (2005), "James Clerk Maxwell, A treatise on electricity and magnetism, first edition (1873)", Landmark Writings in Western Mathematics 1640–1940, Elsevier, pp. 564–587, doi:10.1016/b978-044450871-3/50125-x, ISBN 9780444508713, retrieved 20 June 2022
- ^ "Milestones:First Atomic Clock, 1948". ETHW. 14 June 2022. Retrieved 20 June 2022.
- ^ Rabi, I. I. (15 April 1937). "Space Quantization in a Gyrating Magnetic Field". Physical Review. 51 (8): 652–654. Bibcode:1937PhRv...51..652R. doi:10.1103/physrev.51.652. ISSN 0031-899X.
- ^ Rabi, I. I.; Zacharias, J. R.; Millman, S.; Kusch, P. (15 February 1938). "A New Method of Measuring Nuclear Magnetic Moment". Physical Review. 53 (4): 318. Bibcode:1938PhRv...53..318R. doi:10.1103/physrev.53.318. ISSN 0031-899X.
- ^ a b c d e Lombardi, M. A.; Heavner, T. P.; Jefferts, S. R. (2007). "NIST Primary Frequency Standards and the Realization of the SI Second" (PDF). Journal of Measurement Science. 2 (4): 74–89. Archived (PDF) from the original on 12 February 2021. Retrieved 24 October 2009.
- ^ Sullivan, D. B. (2001). Time and frequency measurement at NIST: The first 100 years (PDF). IEEE International Frequency Control Symposium. NIST. pp. 4–17. Archived (PDF) from the original on 29 December 2019. Retrieved 1 May 2018.
- ^ Essen, L.; Parry, J. V. L. (1955). "An Atomic Standard of Frequency and Time Interval: A Cæsium Resonator". Nature. 176 (4476): 280–282. Bibcode:1955Natur.176..280E. doi:10.1038/176280a0. S2CID 4191481.
- ^ "60 years of the Atomic Clock". National Physical Laboratory. Archived from the original on 17 October 2017. Retrieved 17 October 2017.
- ^ Essen, L.; Parry, J. V. L. (1955). "An Atomic Standard of Frequency and Time Interval: A Cæsium Resonator". Nature. 176 (4476): 280. Bibcode:1955Natur.176..280E. doi:10.1038/176280a0. S2CID 4191481. p.280.
- ^ "President Piñera Receives ESO's First Atomic Clock". ESO Announcement. 15 November 2013. Archived from the original on 1 April 2014. Retrieved 20 November 2013.
- ^ Ramsey, N. F. (September 1983). "History of Atomic Clocks". Journal of Research of the National Bureau of Standards. 88 (5): 301–320. doi:10.6028/jres.088.015. ISSN 0160-1741. PMC 6768155. PMID 34566107.
- ^ "Paper 1.15: "Experiments with Separated Oscillatory Fields and Hydrogen Masers," (Nobel Lecture), N. F. Ramsey, Les Prix Nobel (1989, The Nobel Foundation) and Rev. Mod. Phys. 62, 541–552 (1990)", Spectroscopy With Coherent Radiation, World Scientific Series in 20th Century Physics, vol. 21, WORLD SCIENTIFIC, pp. 115–127, June 1998, doi:10.1142/9789812795717_0015, ISBN 978-981-02-3250-4, retrieved 20 June 2022
- ^ Hellwig, Helmut; Evenson, Kenneth M.; Wineland, David J. (December 1978). "Time, frequency and physical measurement". Physics Today. 31 (12): 23–30. Bibcode:1978PhT....31l..23H. doi:10.1063/1.2994867. ISSN 0031-9228.
- ^ Forman, Paul (1998). "Atomichron: The Atomic Clock from Concept to Commercial Product". Archived from the original on 21 October 2007. Retrieved 16 February 2022.
- ^ McCarthy, D. D.; Seidelmann, P. K. (2009). TIME—From Earth Rotation to Atomic Physics. Weinheim: Wiley-VCH. pp. 191–195. ISBN 978-3-527-40780-4.
- ^ International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), ISBN 92-822-2213-6, archived (PDF) from the original on 4 June 2021, retrieved 16 December 2021
- ^ Fox, Alex. "New Atomic Clocks May Someday Redefine the Length of a Second". Smithsonian Magazine. Retrieved 16 February 2022.
- ^ Lodewyck, Jérôme (16 September 2019). "On a definition of the SI second with a set of optical clock transitions". Metrologia. 56 (5) 055009. arXiv:1911.05551. Bibcode:2019Metro..56e5009L. doi:10.1088/1681-7575/ab3a82. ISSN 0026-1394. S2CID 202129810.
- ^ Ye, J.; Schnatz, H.; Hollberg, L. W. (2003). "Optical frequency combs: From frequency metrology to optical phase control" (PDF). IEEE Journal of Selected Topics in Quantum Electronics. 9 (4): 1041–1058. Bibcode:2003IJSTQ...9.1041Y. doi:10.1109/JSTQE.2003.819109. Archived (PDF) from the original on 6 March 2016. Retrieved 25 February 2016.
- ^ NIST (31 December 2009). "Optical Frequency Combs". NIST. Retrieved 16 February 2022.
- ^ swenson (4 February 2010). "NIST's Second 'Quantum Logic Clock' Based on Aluminum Ion is Now World's Most Precise Clock". NIST. Retrieved 21 February 2022.
- ^ Nicholson, T. L.; Campbell, S. L.; Hutson, R. B.; Marti, G. E.; Bloom, B. J.; McNally, R. L.; Zhang, W.; Barrett, M. D.; Safronova, M. S.; Strouse, G. F.; Tew, W. L. (21 April 2015). "Systematic evaluation of an atomic clock at 2×10−18 total uncertainty". Nature Communications. 6 (1) 6896. arXiv:1412.8261. Bibcode:2015NatCo...6.6896N. doi:10.1038/ncomms7896. ISSN 2041-1723. PMC 4411304. PMID 25898253.
- ^ sarah.henderson@nist.gov (15 July 2019). "NIST's Quantum Logic Clock Returns to Top Performance". NIST. Retrieved 21 February 2022.
- ^ Brewer, S. M.; Chen, J.-S.; Hankin, A. M.; Clements, E. R.; Chou, C. W.; Wineland, D. J.; Hume, D. B.; Leibrandt, D. R. (15 July 2019). "Al+27 Quantum-Logic Clock with a Systematic Uncertainty below 10−18". Physical Review Letters. 123 (3) 033201. arXiv:1902.07694. doi:10.1103/physrevlett.123.033201. ISSN 0031-9007. PMID 31386450. S2CID 119075546.
- ^ Bothwell, Tobias; Kennedy, Colin J.; Aeppli, Alexander; Kedar, Dhruv; Robinson, John M.; Oelker, Eric; Staron, Alexander; Ye, Jun (16 February 2022). "Resolving the gravitational redshift across a millimetre-scale atomic sample". Nature. 602 (7897): 420–424. arXiv:2109.12238. Bibcode:2022Natur.602..420B. doi:10.1038/s41586-021-04349-7. ISSN 0028-0836. PMID 35173346. S2CID 246902611.
- ^ "An atomic clock measured how general relativity warps time across a millimeter". Science News. 18 October 2021. Retrieved 22 February 2022.
- ^ Dimarcq, Noel; Gertsvolf, Marina; Mileti, Gaetano; Bize, Sebastien; Oates, Christopher; Peik, Ekkehard; Calonico, Davide; Ido, Tetsuya; Tavella, Patrizia; Meynadier, Frédéric (2024). "Roadmap towards the redefinition of the second". Metrologia. 61 (1): 012001. arXiv:2307.14141. Bibcode:2024Metro..61a2001D. doi:10.1088/1681-7575/ad17d2.
- ^ a b c "SA.45s CSAC Chip Scale Atomic Clock (archived version of the original pdf)" (PDF). 2011. Archived from the original (PDF) on 25 May 2013. Retrieved 12 June 2013.
- ^ a b "Chip-Scale Atomic Devices at NIST". NIST. 2007. Archived from the original on 7 January 2008. Retrieved 17 January 2008. Available on-line at: NIST.gov. Archived 7 January 2021 at the Wayback Machine
- ^ Lutwak, Robert (26–29 November 2007). "The Chip-Scale Atomic Clock — Prototype Evaluation". 36th Annual Precise Time and Time Interval (PTTI) Systems and Applications Meeting.
- ^ sarah.henderson@nist.gov (2 December 2020). "Success Story: Chip-Scale Atomic Clock". NIST. Retrieved 20 June 2022.
- ^ sarah.henderson@nist.gov (11 December 2019). "Chip-Scale Clocks". NIST. Retrieved 21 June 2022.
- ^ david.hume@nist.gov (29 October 2016). "Ion Optical Clocks and Precision Measurements". NIST. Retrieved 11 February 2022.
- ^ "How Do Atomic Clocks Work?". www.timeanddate.com. Retrieved 17 February 2022.
- ^ Poli, N (2014). "Optical atomic clocks". La Rivista del Nuovo Cimento. 36 (12): 555. arXiv:1401.2378. Bibcode:2013NCimR..36..555P. doi:10.1393/ncr/i2013-10095-x. S2CID 118430700.
- ^ Allan, David W. Statistics of Atomic Frequency Standards, pp. 221–230. Proceedings of the IEEE, Vol. 54, No 2, February 1966.
- ^ Dick, G. J. (1987). Local oscillator induced instabilities in trapped ion frequency standards (PDF). Precise Time and Time Interval (PTTI) Conference. Redondo Beach.
- ^ J. A. Barnes, A. R. Chi, L. S. Cutler, D. J. Healey, D. B. Leeson, T. E. McGunigal, J. A. Mullen, W. L. Smith, R. Sydnor, R. F. C. Vessot, G. M. R. Winkler: Characterization of Frequency Stability, NBS Technical Note 394, 1970.
- ^ Santarelli, G.; Audoin, C.; Makdissi, A.; Laurent, P.; Dick, G.J.; Clairon, A. (1998). "Frequency stability degradation of an oscillator slaved to a periodically interrogated atomic resonator". IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 45 (4): 887–894. doi:10.1109/58.710548. PMID 18244242. S2CID 12303876.
- ^ a b Ludlow, A. D.; Boyd, M. M.; Ye, Jun; Peik, E.; Schmidt, P. O. (26 June 2015). "Optical atomic clocks". Reviews of Modern Physics. 87 (2): 637–701. arXiv:1407.3493. Bibcode:2015RvMP...87..637L. doi:10.1103/RevModPhys.87.637. S2CID 119116973.
- ^ Quessada, A.; Kovacich, R. P.; Courtillot, I.; Clairon, A.; Santarelli, G.; Lemonde, P. (2 April 2003). "The Dick effect for an optical frequency standard". Journal of Optics B: Quantum and Semiclassical Optics. 5 (2): S150–S154. Bibcode:2003JOptB...5S.150Q. doi:10.1088/1464-4266/5/2/373.
- ^ Westergaard, P. G.; Lodewyck, J.; Lemonde, P. (March 2010). "Minimizing the Dick effect in an optical lattice clock". IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 57 (3): 623–628. arXiv:0909.0909. doi:10.1109/TUFFC.2010.1457. PMID 20211780. S2CID 10581032.
- ^ NIST (December 2007). "NIST Primary Frequency Standards and the Realization of the SI Second" (PDF). NCSL International Measure. 2: 77.
- ^ Jain, Pratik; Priya, Priyanka; Ram, T. V. S.; Parikh, K. S.; Bandi, Thejesh N. (1 December 2021). "Digital lock-in amplifier for space rubidium atomic clock". Review of Scientific Instruments. 92 (12) 124705. Bibcode:2021RScI...92l4705J. doi:10.1063/5.0061727. PMID 34972462. S2CID 245079164.
- ^ Poli, N.; Oates, C. W.; Gill, P.; Tino, G. M. (13 January 2014). "Optical Atomic Clocks" (PDF). Rivista del Nuovo Cimento. 36 (12): 555–624. arXiv:1401.2378. Bibcode:2013NCimR..36..555P. doi:10.1393/ncr/i2013-10095-x. S2CID 118430700.
- ^ University of Wisconsin-Madison. "Ultraprecise atomic clock poised for new physics discoveries".
- ^ "What Are Optical Clocks and Why Are They Important?". Revolutionalized. 20 July 2021. Retrieved 20 July 2021.
- ^ Laboratory, National Physical. "Accuracy of the NPL caesium fountain clock further improved". phys.org. Retrieved 20 February 2022.
- ^ "The atomic clock with the world's best long-term accuracy is revealed after evaluation". EurekAlert!. Retrieved 20 February 2022.
- ^ "2016 Gets Longer with Extra Second Added to New Year Countdown | Sci-News.com". Breaking Science News | Sci-News.com. 23 December 2016. Retrieved 20 February 2022.
- ^ Mann, Adam. "How the U.S. Built the World's Most Ridiculously Accurate Atomic Clock". Wired. ISSN 1059-1028. Retrieved 15 February 2022.
- ^ robin.materese@nist.gov (9 April 2019). "Second: The Future". NIST. Retrieved 20 February 2022.
- ^ "NIST launches a new US time standard: NIST-F2 atomic clock". NIST. nist.gov. 3 April 2014. Archived from the original on 6 April 2014. Retrieved 3 April 2014.
- ^ a b c "Mise en pratique for the definition of the second in the SI" (PDF). Bureau International Poids et Mesures. Consultative Committee for Time and Frequency. 20 May 2019.
- ^ a b Gibney, Elizabeth (2 June 2015). "Hyper-precise atomic clocks face off to redefine time – Next-generation timekeepers can only be tested against each other". Nature. 522 (7554): 16–17. Bibcode:2015Natur.522...16G. doi:10.1038/522016a. PMID 26040875.
- ^ Explanatory Supplement of BIPM Circular T (PDF), International Bureau of Weights and Measures, 12 July 2021, archived (PDF) from the original on 9 October 2022, retrieved 16 June 2022
- ^ BIPM Annual Report on Time Activities (PDF). Vol. 15. International Bureau of Weights and Measures. 2020. p. 9. ISBN 978-92-822-2280-5. ISSN 1994-9405. Archived (PDF) from the original on 14 August 2021. Retrieved 16 June 2022.
- ^ Pottie, Paul-Eric; Grosche, Gesine (19 August 2016). "A clock network for geodesy and fundamental science". Nature Communications. 7: 12443. arXiv:1511.07735. Bibcode:2016NatCo...712443L. doi:10.1038/ncomms12443. PMC 4980484. PMID 27503795.
- ^ "Optical fibre link opens a new era of time-frequency metrology, 19 August 2016". Archived from the original on 14 November 2016. Retrieved 13 November 2016.
- ^ Beloy, Kyle; Bodine, Martha I.; Bothwell, Tobias; Brewer, Samuel M.; Bromley, Sarah L.; Chen, Jwo-Sy; Deschênes, Jean-Daniel; Diddams, Scott A.; Fasano, Robert J.; Fortier, Tara M.; Hassan, Youssef S. (25 March 2021). "Frequency ratio measurements at 18-digit accuracy using an optical clock network". Nature. 591 (7851): 564–569. Bibcode:2021Natur.591..564B. doi:10.1038/s41586-021-03253-4. ISSN 1476-4687. PMID 33762766. S2CID 232355391.
- ^ sarah.henderson@nist.gov (24 March 2021). "NIST Team Compares 3 Top Atomic Clocks With Record Accuracy Over Both Fiber and Air". NIST. Retrieved 16 February 2022.
- ^ swenson (29 December 1999). "NIST-F1 Cesium Fountain Clock". NIST. Retrieved 19 February 2022.
- ^ mweiss (26 August 2009). "NIST-F1 Cesium Fountain Atomic Clock". NIST. Retrieved 19 February 2022.
- ^ "Temperature and Kinetic Energy – Answers". www.grc.nasa.gov. Retrieved 19 February 2022.
- ^ "NIST Launches a New U.S. Time Standard: NIST-F2 Atomic Clock". NIST. 3 April 2014. Archived from the original on 19 August 2016. Retrieved 13 July 2017.
- ^ University, Lancaster (11 May 2021). "Clock Experiment Shows a Fundamental Connection Between Energy Consumption and Accuracy". SciTechDaily. Retrieved 16 February 2022.
- ^ Vleugels, Anouk (23 May 2021). "New experiment: Clocks consuming more energy are more accurate… 'cause thermodynamics". TNW | Science. Retrieved 16 February 2022.
- ^ "A Cesium Beam Frequency Reference for Severe Environment" (PDF). Retrieved 24 February 2022.
- ^ National Physical Laboratory (2019). "OC18". National Physical laboratory.
- ^ Swenson, Gayle (7 June 2010). "Press release: NIST 'Quantum Logic Clock' Rivals Mercury Ion as World's Most Accurate Clock". NIST. Archived from the original on 2 June 2017. Retrieved 27 July 2017.
- ^ NIST's Second 'Quantum Logic Clock' Based on Aluminum Ion is Now World's Most Precise Clock Archived 5 September 2010 at the Wayback Machine, NIST, 4 February 2010
- ^ Chou, C. W.; Hume, D.; Koelemeij, J. C. J.; Wineland, D. J. & Rosenband, T. (17 February 2010). "Frequency Comparison of Two High-Accuracy Al+ Optical Clocks" (PDF). Physical Review Letters. 104 (7) 070802. arXiv:0911.4527. Bibcode:2010PhRvL.104g0802C. doi:10.1103/PhysRevLett.104.070802. PMID 20366869. S2CID 13936087. Archived (PDF) from the original on 21 July 2011. Retrieved 9 February 2011.
- ^ Brewer, S. M.; Chen, J.-S.; Hankin, A. M.; Clements, E. R.; Chou, C. W.; Wineland, D. J.; Hume, D. B.; Leibrandt, D. R. (15 July 2019). "27Al+ Quantum-Logic Clock with a Systematic Uncertainty below 10−18". Physical Review Letters. 123 (3) 033201. arXiv:1902.07694. Bibcode:2019PhRvL.123c3201B. doi:10.1103/PhysRevLett.123.033201. PMID 31386450. S2CID 119075546.
- ^ Wills, Stewart (July 2019). "Optical Clock Precision Breaks New Ground". Archived from the original on 26 August 2019. Retrieved 4 September 2019.
- ^ Dubé, Pierre (15 July 2019). "Viewpoint: Ion Clock Busts into New Precision Regime". Physics. 12 79. doi:10.1103/physics.12.79.
- ^ Peik, E.; Tamm, Chr. (15 January 2003). "Nuclear laser spectroscopy of the 3.5 eV transition in 229Th" (PDF). Europhysics Letters. 61 (2): 181–186. Bibcode:2003EL.....61..181P. doi:10.1209/epl/i2003-00210-x. S2CID 250818523. Archived from the original (PDF) on 16 December 2013. Retrieved 11 September 2019.
- ^ a b Campbell, C.; Radnaev, A.G.; Kuzmich, A.; Dzuba, V.A.; Flambaum, V.V.; Derevianko, A. (2012). "A single ion nuclear clock for metrology at the 19th decimal place". Phys. Rev. Lett. 108 (12) 120802. arXiv:1110.2490. Bibcode:2012PhRvL.108l0802C. doi:10.1103/PhysRevLett.108.120802. PMID 22540568. S2CID 40863227.
- ^ von der Wense, Lars; Seiferle, Benedict; Laatiaoui, Mustapha; Neumayr, Jürgen B.; Maier, Hans-Jörg; Wirth, Hans-Friedrich; Mokry, Christoph; Runke, Jörg; Eberhardt, Klaus; Düllmann, Christoph E.; Trautmann, Norbert G.; Thirolf, Peter G. (5 May 2016). "Direct detection of the 229Th nuclear clock transition". Nature. 533 (7601): 47–51. arXiv:1710.11398. Bibcode:2016Natur.533...47V. doi:10.1038/nature17669. PMID 27147026. S2CID 205248786.
- ^ Thielking, J.; Okhapkin, M.V.; Glowacki, P.; Meier, D.M.; von der Wense, L.; Seiferle, B.; Düllmann, C.E.; Thirolf, P.G.; Peik, E. (2018). "Laser spectroscopic characterization of the nuclear-clock isomer 229mTh". Nature. 556 (7701): 321–325. arXiv:1709.05325. Bibcode:2018Natur.556..321T. doi:10.1038/s41586-018-0011-8. PMID 29670266. S2CID 4990345.
- ^ Masuda, T.; Yoshimi, A.; Fujieda, A.; Fujimoto, H.; Haba, H.; Hara, H.; Hiraki, T.; Kaino, H.; Kasamatsu, Y.; Kitao, S.; Konashi, K.; Miyamoto, Y.; Okai, K.; Okubo, S.; Sasao, N.; Seto, M.; Schumm, T.; Shigekawa, Y.; Suzuki, K.; Stellmer, S.; Tamasaku, K.; Uetake, S.; Watanabe, M.; Watanabe, T.; Yasuda, Y.; Yamaguchi, A.; Yoda, Y.; Yokokita, T.; Yoshimura, M.; Yoshimura, K. (12 September 2019). "X-ray pumping of the 229Th nuclear clock isomer". Nature. 573 (7773): 238–242. arXiv:1902.04823. Bibcode:2019Natur.573..238M. doi:10.1038/s41586-019-1542-3. PMID 31511686. S2CID 119083861.
- ^ Seiferle, B.; von der Wense, L.; Bilous, P.V.; Amersdorffer, I.; Lemell, C.; Libisch, F.; Stellmer, S.; Schumm, T.; Düllmann, C.E.; Pálffy, A.; Thirolf, P.G. (12 September 2019). "Energy of the 229Th nuclear clock transition". Nature. 573 (7773): 243–246. arXiv:1905.06308. Bibcode:2019Natur.573..243S. doi:10.1038/s41586-019-1533-4. PMID 31511684. S2CID 155090121.
- ^ Thirolf, Peter (29 April 2024). "Shedding Light on the Thorium-229 Nuclear Clock Isomer". Physics. Vol. 17. doi:10.1103/Physics.17.71.
- ^ a b
Tiedau, J.; Okhapkin, M. V.; Zhang, K.; Thielking, J.; Zitzer, G.; Peik, E.; et al. (29 April 2024). "Laser Excitation of the Th-229 Nucleus" (PDF). Physical Review Letters. 132 (18) 182501. Bibcode:2024PhRvL.132r2501T. doi:10.1103/PhysRevLett.132.182501. PMID 38759160.
The nuclear resonance for the Th4+ ions in Th:CaF2 is measured at the wavelength 148.3821(5) nm, frequency 2020.409(7) THz, and the fluorescence lifetime in the crystal is 630(15) s, corresponding to an isomer half-life of 1740(50) s for a nucleus isolated in vacuum.
- ^
Elwell, R.; Schneider, Christian; Jeet, Justin; Terhune, J. E. S.; Morgan, H. W. T.; Alexandrova, A. N.; Tran Tan, Hoang Bao; Derevianko, Andrei; Hudson, Eric R. (2 July 2024). "Laser excitation of the 229Th nuclear isomeric transition in a solid-state host". Physical Review Letters. 133 (1) 013201. arXiv:2404.12311. doi:10.1103/PhysRevLett.133.013201. PMID 39042795.
a narrow, laser-linewidth-limited spectral feature at 148.38219(4)stat(20)sys nm (2020407.3(5)stat(30)sys GHz) that decays with a lifetime of 568(13)stat(20)sys s. This feature is assigned to the excitation of the 229Th nuclear isomeric state, whose energy is found to be 8.355733(2)stat(10)</sys> eV in 229Th:LiSrAlF6.
- ^
Zhang, Chuankun; Ooi, Tian; Higgins, Jacob S.; Doyle, Jack F.; von der Wense, Lars; Beeks, Kjeld; Leitner, Adrian; Kazakov, Georgy; Li, Peng; Thirolf, Peter G.; Schumm, Thorsten; Ye, Jun (4 September 2024). "Frequency ratio of the 229mTh nuclear isomeric transition and the 87Sr atomic clock". Nature. 633 (8028): 63–70. arXiv:2406.18719. Bibcode:2024Natur.633...63Z. doi:10.1038/s41586-024-07839-6. PMID 39232152.
The transition frequency between the I = 5/2 ground state and the I = 3/2 excited state is determined as: 𝜈Th = 1/6 (𝜈a + 2𝜈b + 2𝜈c + 𝜈d) = 2020407384335(2) kHz.
- ^ Conover, Emily (4 September 2024). "A nuclear clock prototype hints at ultraprecise timekeeping". ScienceNews.
- ^
Seiferle, Benedict; von der Wense, Lars; Thirolf, Peter G. (2017). "Lifetime measurement of the 229Th nuclear isomer". Physical Review Letters. 118 (4) 042501. arXiv:1801.05205. Bibcode:2017PhRvL.118d2501S. doi:10.1103/PhysRevLett.118.042501. PMID 28186791. S2CID 37518294.
A half-life of 7±1 μs has been measured
- ^ Peik, Ekkehard (25–27 September 2012). Concepts and Prospects for a Thorium-229 Nuclear Clock (PDF). EMMI Workshop: The 229mTh Nuclear Isomer Clock. Darmstadt. Archived (PDF) from the original on 10 October 2021. Retrieved 2 December 2019.
- ^ National Physical Laboratory (2011). "When should we change the definition of the second?". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 369 (1953): 4109–4130. Bibcode:2011RSPTA.369.4109G. doi:10.1098/rsta.2011.0237. PMID 21930568. S2CID 6896025.
- ^ a b Gill, Patrick (28 October 2011). "When should we change the definition of the second?". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 369 (1953): 4109–4130. Bibcode:2011RSPTA.369.4109G. doi:10.1098/rsta.2011.0237. PMID 21930568. S2CID 6896025.
- ^ "Consultative Committee for Units (CCU) Report of the 25th meeting (21-23 September 2021) to the International Committee for Weights and Measures".
- ^ Riehle, Fritz. "On Secondary Representations of the Second" (PDF). Physikalisch-Technische Bundesanstalt, Division Optics. Archived from the original (PDF) on 23 June 2015. Retrieved 22 June 2015.
- ^ "Unit of time (second)". SI Brochure. BIPM. 2014 [2006]. Archived from the original on 19 November 2011. Retrieved 23 June 2015.
- ^ "87Rubidium BIPM document" (PDF). Archived (PDF) from the original on 23 September 2015. Retrieved 22 June 2015.
- ^ Essen, L; Donaldson, R W; Hope, E G; Bangham, M J (July 1973). "Hydrogen Maser Work at the National Physical Laboratory". Metrologia. 9 (3): 128–137. Bibcode:1973Metro...9..128E. doi:10.1088/0026-1394/9/3/004. S2CID 250828528.
- ^ Dupays, Arnaud; Beswick, Alberto; Lepetit, Bruno; Rizzo, Carlo (August 2003). "Proton Zemach radius from measurements of the hyperfine splitting of hydrogen and muonic hydrogen" (PDF). Physical Review A. 68 (5) 052503. arXiv:quant-ph/0308136. Bibcode:2003PhRvA..68e2503D. doi:10.1103/PhysRevA.68.052503. S2CID 3957861. Archived (PDF) from the original on 14 January 2019. Retrieved 26 September 2016.
- ^ "87Strontium BIPM document" (PDF). Archived (PDF) from the original on 4 March 2016. Retrieved 25 June 2015.
- ^ "27Aluminum ion BIPM document". Archived from the original on 2 August 2022. Retrieved 9 December 2022.
- ^ Brewer, S.; Chen, J.-S.; Hankin, A.; Clements, E. (15 July 2019). "27Al+ Quantum-Logic Clock with a Systematic Uncertainty below 10−18". Physical Review Letters. 123 (3) 033201. arXiv:1902.07694. Bibcode:2019PhRvL.123c3201B. doi:10.1103/PhysRevLett.123.033201. PMID 31386450. S2CID 119075546.
- ^ "171Ytterbium 171 ion (642 THz) BIPM document". Archived from the original on 2 August 2022. Retrieved 9 December 2022.
- ^ Huntemann, N.; Sanner, C.; Lipphardt, B.; Tamm, Chr. (8 February 2016). "Single-Ion Atomic Clock with 3×10−18 Systematic Uncertainty". Physical Review Letters. 116 (6) 063001. arXiv:1602.03908. Bibcode:2016PhRvL.116f3001H. doi:10.1103/PhysRevLett.116.063001. PMID 26918984. S2CID 19870627.
- ^ "171Ytterbium 171 ion (688 THz) BIPM document". Archived from the original on 2 August 2022. Retrieved 9 December 2022.
- ^ Leute, J.; Huntemann, N.; Lipphardt, B.; Tamm, Christian (3 February 2016). "Frequency Comparison of 171Yb+ Ion Optical Clocks at PTB and NPL via GPS PPP". IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 63 (7): 981–985. arXiv:1507.04754. doi:10.1109/TUFFC.2016.2524988. PMID 26863657. S2CID 20466105.
- ^ "StackPath". www.laserfocusworld.com. September 2001. Retrieved 11 February 2022.
- ^ Ahmed, Issam. "What the world's most accurate clock can tell us about Earth and the cosmos". phys.org. Retrieved 11 February 2022.
- ^ "New type of atomic clock keeps time even more precisely". MIT News | Massachusetts Institute of Technology. 16 December 2020. Retrieved 11 February 2022.
- ^ Woodward, Aylin (5 October 2017). "The most precise atomic clock ever made is a cube of quantum gas". New Scientist. Retrieved 11 February 2022.
- ^ Ren, Wei; Li, Tang; Qu, Qiuzhi; Wang, Bin; Li, Lin; Lü, Desheng; Chen, Weibiao; Liu, Liang (18 December 2020). "Development of a space cold atom clock". National Science Review. 7 (12): 1828–1836. doi:10.1093/nsr/nwaa215. ISSN 2095-5138. PMC 8288775. PMID 34691520.
- ^ andrew.novick@nist.gov (11 February 2010). "Help with WWVB Radio Controlled Clocks". NIST. Retrieved 15 February 2022.
- ^ McCarthy, D. D.; Seidelmann, P. K. (2009). TIME—From Earth Rotation to Atomic Physics. Weinheim: Wiley-VCH. p. 266. ISBN 978-3-527-40780-4.
- ^ "Global Positioning System". Gps.gov. Archived from the original on 30 July 2010. Retrieved 26 June 2010.
- ^ Allan, David W. (1997). "The Science of Timekeeping" (PDF). Application Note (1289). Hewlett Packard. Archived (PDF) from the original on 25 October 2012.
- ^ Dana, Peter H.; Penro, Bruce M. (July–August 1990). "The Role of GPS in Precise Time and Frequency Dissemination" (PDF). GPSworld. Archived (PDF) from the original on 15 December 2012. Retrieved 27 April 2014.
- ^ "GPS time accurate to 100 nanoseconds". Galleon. Archived from the original on 14 May 2012. Retrieved 12 October 2012.
- ^ "UTC to GPS Time Correction". qps.nl. Archived from the original on 21 March 2017. Retrieved 4 October 2015.
- ^ "NAVSTAR GPS User Equipment Introduction" (PDF). Archived (PDF) from the original on 21 October 2013. Retrieved 4 October 2015. Section 1.2.2
- ^ "NOTICE ADVISORY TO NAVSTAR USERS (NANU)". May 2017. Archived from the original on 28 May 2017. Retrieved 4 October 2015.
- ^ "Notice Advisory to Navstar Users (NANU) 2012034". GPS Operations Center. 30 May 2012. Archived from the original on 8 April 2013. Retrieved 2 July 2012.
- ^ "Time References in GNSS". navipedia.net. Archived from the original on 2 June 2018. Retrieved 2 October 2015.
- ^ "GLONASS Interface Control Document, Navigation radiosignal In bands L1, L2 (ICD L1, L2 GLONASS), Russian Institute of Space Device Engineering, Edition 5.1, 2008" (PDF). Archived (PDF) from the original on 14 April 2016. Retrieved 2 October 2015.
- ^ "Galileo begins serving the globe". European Space Agency. Archived from the original on 13 September 2019. Retrieved 15 December 2016.
- ^ a b "Galileo's contribution to the MEOSAR system". European Commission. Archived from the original on 9 July 2016. Retrieved 30 December 2015.
- ^ "European GNSS (Galileo) Open Service Signal-In-Space Operational Status Definition, Issue 1.0, September 2015" (PDF). Archived from the original (PDF) on 9 January 2017. Retrieved 3 October 2015.
- ^ "1 The Definition and Implementation of Galileo System Time (GST). ICG-4 WG-D on GNSS time scales. Jérôme Delporte. CNES – French Space Agency" (PDF). Archived (PDF) from the original on 6 November 2016. Retrieved 5 October 2015.
- ^ "Galileo's clocks". European Space Agency. Archived from the original on 29 August 2019. Retrieved 16 January 2017.
- ^ "Galileo Goes Live". European GNSS Agency. 15 December 2016. Archived from the original on 15 January 2021. Retrieved 1 February 2017.
- ^ "Galileo Initial Services – Open Service – Quarterly Performance Report Oct–Nov–Dec 2017" (PDF). European GNSS Service Centre. 28 March 2018. Archived (PDF) from the original on 26 August 2019. Retrieved 28 March 2017.
- ^ "Galileo Open Service and Search and Rescue – Quarterly Performance Reports, containing measured performance statistics". Archived from the original on 26 August 2019. Retrieved 3 March 2019.
- ^ "Passive Hydrogen Maser (PHM)". Safran - Navigation & Timing. Archived from the original on 6 March 2019. Retrieved 30 January 2017.
- ^ "Rb Atomic Frequency Standard (RAFS)". safran-navigation-timing.com. Archived from the original on 6 November 2018. Retrieved 30 January 2017.
- ^ "GNSS Timescale Description" (PDF). Archived (PDF) from the original on 28 October 2020. Retrieved 5 October 2015.
- ^ "ESA Adds System Time Offset to Galileo Navigation Message". insidegnss.com. Archived from the original on 28 March 2018. Retrieved 5 October 2015.
- ^ Belcher, David (1 November 2021). "Trying to Get Somewhere? An Atomic Clock May Be Helping". The New York Times. ISSN 0362-4331. Retrieved 15 February 2022.
- ^ China Satellite Navigation Office, Version 2.0, December 2013[permanent dead link]
- ^ "Definition and Realization of the System Time of COMPASS/BeiDou Navigation Satellite System, Chunhao Han, Beijing Global Information Center,(BGIC), Beijing, China" (PDF). Archived (PDF) from the original on 29 October 2020. Retrieved 5 October 2015.
- ^ "China GPS rival Beidou starts offering navigation data". BBC. 27 December 2011. Archived from the original on 3 February 2012. Retrieved 22 June 2018.
- ^ "China's Beidou GPS-substitute opens to public in Asia". BBC. 27 December 2012. Archived from the original on 27 December 2012. Retrieved 27 December 2012.
- ^ Varma, K. J. M. (27 December 2018). "China's BeiDou navigation satellite, rival to US GPS, starts global services". livemint.com. Archived from the original on 27 December 2018. Retrieved 27 December 2018.
- ^ "China puts final satellite for Beidou network into orbit – state media". Reuters. 23 June 2020. Archived from the original on 28 October 2020. Retrieved 23 June 2020.
- ^ Landau, Elizabeth (27 April 2015). "Deep Space Atomic Clock". NASA. Archived from the original on 10 December 2015. Retrieved 29 April 2015.
- ^ Northon, Karen (25 June 2019). "NASA Technology Missions Launch on SpaceX Falcon Heavy". NASA. Retrieved 20 February 2022.
- ^ "NASA Activates Deep Space Atomic Clock". NASA Jet Propulsion Laboratory (JPL). Retrieved 20 February 2022.
- ^ Hartono, Naomi (1 October 2021). "Working Overtime: NASA's Deep Space Atomic Clock Completes Mission". NASA. Retrieved 20 February 2022.
- ^ "DARPA Aims for More Accurate Atomic Clock to Replace GPS". The Defense Post. 1 February 2022. Retrieved 15 February 2022.
- ^ "DARPA to launch programme for creating optical atomic clocks". Airforce Technology. 21 January 2022. Retrieved 15 February 2022.
- ^ a b Michael A. Lombardi, "How Accurate is a Radio Controlled Clock?", Archived 7 January 2021 at the Wayback Machine, National Institute of Standards and Technology, 2010.
- ^ lombardi (24 September 2009). "Radio Station WWV". NIST. Retrieved 16 February 2022.
- ^ Chen, Sophia. "These Physicists Watched a Clock Tick for 14 Years Straight". Wired. ISSN 1059-1028. Retrieved 15 February 2022.
- ^ Bothwell, Tobias; Kennedy, Colin J.; Aeppli, Alexander; Kedar, Dhruv; Robinson, John M.; Oelker, Eric; Staron, Alexander; Ye, Jun (2022). "Resolving the gravitational redshift across a millimetre-scale atomic sample". Nature. 602 (7897): 420–424. arXiv:2109.12238. Bibcode:2022Natur.602..420B. doi:10.1038/s41586-021-04349-7. PMID 35173346. S2CID 237940816.
- ^ sarah.henderson@nist.gov (16 February 2022). "JILA Atomic Clocks Measure Einstein's General Relativity at Millimeter Scale". NIST. Retrieved 17 February 2022.
- ^ "An Ultra-Precise Clock Shows How to Link the Quantum World With Gravity". Quanta Magazine. 25 October 2021. Retrieved 16 February 2022.
- ^ mark.esser@nist.gov (18 June 2020). "Keeping Time at NIST". NIST. Retrieved 16 February 2022.
- ^ "TimeChainZ – Regulatory Reporting For High-Frequency Trading". www.chainzy.com. Retrieved 16 February 2022.
- ^ Geng, Yilong; Liu, Shiyu; Yin, Zi; Naik, Ashish; Prabhakar, Balaji; Rosenblum, Mendel; Vahdat, Amin (2018). Exploiting a Natural Network Effect for Scalable, Fine-grained Clock Synchronization. 15th USENIX Symposium on Networked Systems Design and Implementation. pp. 81–94. ISBN 978-1-939133-01-4.
- ^ Koller, S. B.; Grotti, J.; Vogt, St.; Al-Masoudi, A.; Dörscher, S.; Häfner, S.; Sterr, U.; Lisdat, Ch. (13 February 2017). "Transportable Optical Lattice Clock with 7×10−17 Uncertainty". Physical Review Letters. 118 (7): 073601. arXiv:1609.06183. doi:10.1103/PhysRevLett.118.073601. ISSN 0031-9007. PMID 28256845. S2CID 40822816.
























