Symbiodinium
| Symbiodinium | |
|---|---|

| |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Alveolata |
| Phylum: | Myzozoa |
| Superclass: | Dinoflagellata |
| Class: | Dinophyceae |
| Order: | Suessiales |
| Family: | Symbiodiniaceae |
| Genus: | Symbiodinium Freudenthal, 1962 [1] |
| Species | |
Symbiodinium is a genus of dinoflagellates that encompasses the largest and most prevalent group of endosymbiotic dinoflagellates known and have photosymbiotic relationships with many species. These unicellular microalgae commonly reside in the endoderm of tropical cnidarians such as corals, sea anemones, and jellyfish, where the products of their photosynthetic processing are exchanged in the host for inorganic molecules. They are also harbored by various species of demosponges, flatworms, mollusks such as the giant clams, foraminifera (soritids), and some ciliates. Generally, these dinoflagellates enter the host cell through phagocytosis, persist as intracellular symbionts, reproduce, and disperse to the environment. The exception is in most mollusks, where these symbionts are intercellular (between the cells). Cnidarians that are associated with Symbiodinium occur mostly in warm oligotrophic (nutrient-poor), marine environments where they are often the dominant constituents of benthic communities. These dinoflagellates are therefore among the most abundant eukaryotic microbes found in coral reef ecosystems.
Symbiodinium are colloquially called zooxanthellae, and animals symbiotic with algae in this genus are said to be "zooxanthellate". The term was loosely used to refer to any golden-brown endosymbionts, including diatoms and other dinoflagellates. Continued use of the term in the scientific literature is discouraged because of the confusion caused by overly generalizing taxonomically diverse symbiotic relationships.[2]
In 2018, the systematics of Symbiodiniaceae was revised, and the distinct clades have been reassigned into seven genera.[3] Following this revision, the name Symbiodinium is now sensu stricto a genus name for only species that were previously classified as Clade A.[3] The other clades were reclassified as distinct genera (see Molecular Systematics below).
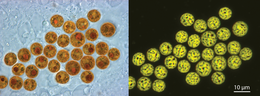
Intracellular symbionts
[edit]
Many Symbiodinium species are known primarily for their role as mutualistic endosymbionts. In hosts, they usually occur in high densities, ranging from hundreds of thousands to millions per square centimeter.[4] The successful culturing of swimming gymnodinioid cells from coral led to the discovery that "zooxanthellae" were actually dinoflagellates.[5][6] Each Symbiodinium cell is coccoid in hospite (living in a host cell) and surrounded by a membrane that originates from the host cell plasmalemma during phagocytosis. This membrane probably undergoes some modification to its protein content, which functions to limit or prevent phago-lysosome fusion.[7][8][9] The vacuole structure containing the symbiont is therefore termed the symbiosome. A single symbiont cell occupies each symbiosome. It is unclear how this membrane expands to accommodate a dividing symbiont cell. Under normal conditions, symbiont and host cells exchange organic and inorganic molecules that enable the growth and proliferation of both partners.
Natural services and economic value
[edit]Symbiodinium is one of the most studied symbionts. Their mutualistic relationships with reef-building corals form the basis of a highly diverse and productive ecosystem. Coral reefs have economic benefits – valued at hundreds of billions of dollars each year – in the form of ornamental, subsistence and commercial fisheries, tourism and recreation, coastal protection from storms, a source of bioactive compounds for pharmaceutical development, and more.[10]
Coral bleaching
[edit]The study of Symbiodinium biology is driven largely by a desire to understand global coral reef decline. A chief mechanism for widespread reef degradation has been stress-induced coral bleaching caused by unusually high seawater temperature. Bleaching is the disassociation of the coral and the symbiont and/or loss of chlorophyll within the alga, resulting in a precipitous loss in the animal's pigmentation. Many Symbiodinium-cnidarian associations are affected by sustained elevation of sea surface temperatures,[11] but may also result from exposure to high irradiance levels (including UVR),[12][13] extreme low temperatures,[14] low salinity[15] and other factors.[16] The bleached state is associated with decreased host calcification,[17] increased disease susceptibility[18] and, if prolonged, partial or total mortality.[19] The magnitude of mortality from a single bleaching event can be global in scale as it was in 2015. These episodes are predicted to become more common and severe as temperatures worldwide continue to rise.[20] The physiology of a resident Symbiodinium species often regulates the bleaching susceptibility of a coral.[21][22] Therefore, a significant amount of research has focused on characterizing the physiological basis of thermal tolerance[23][24][25][26] and in identifying the ecology and distribution of thermally tolerant symbiont species.[27][28][29]
The symbiosis Symbodinium-coral could provide higher resistance to multiple stress (desiccation, high UVR) to the coral holobiont through its mycosporine-like amino acids (MAAs). The concentration of MAAs increases with stress and ROS in Symbodinium.[30] These UV-absorbing MAAs may also support light-harvesting pigments during photosynthesis, be source of nitrogen storage and for reproduction. More than half of the Symbodinium taxa contain MAAs.[31][32][33]
Symbiodinium trenchi is a stress-tolerant species and is able to form mutualistic relationships with many species of coral. It is present in small numbers in coral globally and is common in the Andaman Sea, where the water is about 4 °C (7 °F) warmer than in other parts of the Indian Ocean.[34][self-published source?] In the Caribbean Sea in late 2005, water temperature was elevated for several months and it was found that S. trenchi, a symbiont not normally abundant, took up residence in many corals in which it had not previously been observed. Those corals did not bleach. Two years later, it had largely been replaced as a symbiont by the species normally found in the Caribbean.[28]
S. thermophilum was recently found to make up the bulk of the algal population inside the corals of the Persian Gulf. It is also present in the Gulf of Oman and the Red Sea, at a much lower concentration. Coral that hosted this species was able to tolerate the 35 °C (95 °F) waters of the Persian Gulf, much hotter than the 31 °C (88 °F) of coral reefs globally.[35]
Molecular systematics
[edit]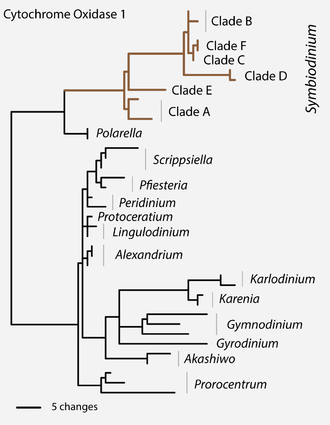
The advent of DNA sequence comparison initiated a rebirth in the ordering and naming of all organisms. The application of this methodology helped overturn the long-held belief that (traditional understood) Symbiodinium comprised a single genus, a process which began in earnest with the morphological, physiological, and biochemical comparisons of cultured isolates. Currently, genetic markers are exclusively used to describe ecological patterns and deduce evolutionary relationships among morphologically cryptic members of this group. Foremost in the molecular systematics of Symbiodinium is to resolve ecologically relevant units of diversity (i.e. species).
Phylogenetic disparity among "clades"
[edit]The earliest ribosomal gene sequence data indicated that Symbiodinium had lineages whose genetic divergence was similar to that seen in other dinoflagellates from different genera, families, and even orders.[36] This large phylogenetic disparity among clades A, B, C, etc. was confirmed by analyses of the sequences of the mitochondrial gene coding for cytochrome c oxidase subunit I (CO1) among Dinophyceae.[37] Most of these clade groupings comprise numerous reproductively isolated, genetically distinct lineages (see ‘Species diversity’), exhibiting different ecological and biogeographic distributions (see ‘Geographic distributions and patterns of ‘diversity’).
Recently (2018), these distinct clades within the family of Symbiodiniaceae have been reassigned, although not exclusively, into seven genera: Symbiodinium (understood sensu stricto, i. e. clade A), Breviolum (clade B), Cladocopium (clade C), Durusdinium (clade D), Effrenium (clade E), Fugacium (clade F), and Gerakladium (clade G).[3]
Species diversity
[edit]
The recognition of species diversity in this genus remained problematic for many decades due to the challenges of identifying morphological and biochemical traits useful for diagnosing species.[38] Presently, phylogenetic, ecological, and population genetic data can be more rapidly acquired to resolve Symbiodinium into separate entities that are consistent with Biological, Evolutionary, and Ecological Species Concepts.[39][40] Most genetics-based measures of diversity have been estimated from the analysis of one genetic marker (e. g. LSU, ITS2, or cp23S[41]), yet in recent studies these and other markers were analyzed in combination. The high concordance found among nuclear, mitochondrial and chloroplast DNA argues that a hierarchical phylogenetic scheme, combined with ecological and population genetic data, can unambiguously recognize and assign nomenclature to reproductively isolated lineages, i.e. species.[3][39][40]
The analysis of additional phylogenetic markers show that some Symbiodinium that were initially identified by slight differences in ITS sequences may comprise members of the same species[40] whereas, in other cases, two or more genetically divergent lineages can possess the same ancestral ITS sequence.[42][43] When analysed in the context of the major species concepts,[44] the majority of ITS2 sequence data provide a reasonable proxy for species diversity.[39][40][45] Currently, ITS2 types number in the hundreds, but most communities of symbiotic cnidaria around the world still require comprehensive sampling. Furthermore, there appears to be a large number of unique species found in association with equally diverse species assemblages of soritid foraminifera,[46] as well as many other Symbiodinium that are exclusively free-living and found in varied, often benthic, habitats.[47] Given the potential species diversity of these ecologically cryptic Symbiodinium, the total species number may never be accurately assessed.[46]
Clone diversity and population genetics
[edit]Through the use of microsatellite markers, multilocus genotypes identifying a single clonal line of Symbiodinium can be resolved from samples of host tissue. It appears that most individual colonies harbor a single multilocus genotype (i.e. clone).[48][49] Extensive sampling within colonies confirms that many colonies harbor a homogeneous (clonal) Symbiodinium population. Additional genotypes do occur in some colonies, yet rarely more than two or three are found. When present in the same colony, multiple clones often exhibit narrow zones of overlap.[49] Colonies adjacent to each other on a reef may harbor identical clones, but across the host population the clone diversity of a particular Symbiodinium species is potentially large and comprises recombinant genotypes that are the product of sexual recombination. A clone tends to remain dominant in a colony over many months and years, but may be occasionally displaced or replaced. The few studies examining clone dispersal find that most genotypes have limited geographic distributions, but that dispersal and gene flow is likely influenced by host life history and mode of symbiont acquisition (e. g. horizontal vs. vertical).[49][48]
Species diversity, ecology, and biogeography
[edit]Geographic distributions and patterns of diversity
[edit]
Symbiodinium are perhaps the best group for studying micro-eukaryote physiology and ecology for several reasons. Firstly, available phylogenetic and population genetic markers allow for detailed examination of their genetic diversity over broad spatial and temporal scales. Also, large quantities of Symbiodinium cells are readily obtained through the collection of hosts that harbor them. Lastly, their association with animals provides an additional axis by which to compare and contrast ecological distributions.[citation needed]
The earliest genetic methods for assessing Symbiodinium diversity relied on low-resolution molecular markers that separated the genus into a few evolutionarily divergent lineages, referred to as "clades". Previous characterizations of geographic distribution and dominance have focused on the clade-level of genetic resolution, but more detailed assessments of diversity at the species level are needed. While members of a given clade may be ubiquitous, the species diversity within each clade is potentially large, with each species often having different ecological and geographic distributions related to their dispersal ability, host biogeography, and external environmental conditions. A small number of species occur in temperate environments where few symbiotic animals occur. As a result, these high latitude associations tend to be highly species specific.[citation needed]

Species diversity assigned to different ecological guilds
[edit]The large diversity of Symbiodinium revealed by genetic analyses is distributed non-randomly and appears to comprise several guilds with distinct ecological habits. Of the many Symbiodinium characterized genetically, most are host-specific, mutualistic, and dominate their host.[50] Others may represent compatible symbionts that remain as low-abundance background populations because of competitive inferiority under the prevailing external environmental conditions (e.g. high light vs. low light).[51] Some may also comprise opportunistic species that may proliferate during periods of physiological stress and displace the normal resident symbiont and remain abundant in the host's tissues for months to years before being replaced by the original symbiont.[28][52][53] There are also those that rapidly infect and establish populations in host juveniles until being replaced by symbionts that normally associate with host adult colonies.[54] Finally, there appears to be another group of Symbiodinium that are incapable of establishing endosymbiosis yet exist in environments around the animal or associate closely with other substrates (i.e. macro-algal surfaces, sediment surface)[47][55] Symbiodinium from functional groups 2, 3, and 4 are known to exist because they culture easily, however species with these life histories are difficult to study because of their low abundance in the environment.
Free-living and "non-symbiotic" populations
[edit]There are few examples of documented populations of free-living Symbiodinium.[47] Given that most host larvae must initially acquire their symbionts from the environment, viable Symbiodinium cells occur outside the host. The motile phase is probably important in the external environment and facilitates the rapid infection of host larvae. The use of aposymbiotic host polyps deployed as "capture vessels" and the application of molecular techniques has allowed for the detection of environmental sources of Symbiodinium.[53][56] With these methods employed, investigators may resolve the distribution of different species on various benthic surfaces[55] and cell densities suspended in the water column.[57] The genetic identities of cells cultured from the environment are often dissimilar to those found in hosts. These likely do not form endosymbioses and are entirely free-living; they are different from "dispersing" symbiotic species.[50] Learning more about the "private lives" of these environmental populations and their ecological function will further our knowledge about the diversity, dispersal success, and evolution among members within this large genus.
Culturing
[edit]Certain Symbiodinium strains and/or species are more easily cultured and can persist in artificial or supplemented seawater media (e.g. ASP–8A, F/2)[58] for decades. The comparison of cultured isolates under identical conditions show clear differences in morphology, size, biochemistry, gene expression, swimming behavior, growth rates, etc.[59][60][61] This pioneering comparative approach initiated a slow paradigm shift in recognizing that the traditional genus sensu lato comprised more than a single real genus.
Culturing is a selective process, and many Symbiodinium isolates growing on artificial media are not typical of the species normally associated with a particular host. Indeed, most host–specific species have yet to be cultured. Samples for genetic analysis should be acquired from the source colony in order to match the resulting culture with the identity of the dominant and ecologically relevant symbiont originally harbored by the animal.[50][62][63]
Life cycle
[edit]
The life cycle of Symbiodinium was first described from cells growing in culture media. For isolates that are in log phase growth, division rates occur every 1–3 days, with Symbiodinium cells alternating between a spherical, or coccoid, morphology and a smaller flagellated motile mastigote stage. While several similar schemes are published that describe how each morphological state transitions to other, the most compelling life history reconstruction was deduced from light and electron microscopy and nuclear staining evidence.[64] During asexual propagation (sometimes referred to as mitotic or vegetative growth), cells undergo a diel cycle of karyokinesis (chromosome/nuclear division) in darkness. The mother cell then divides (cytokinesis) soon after exposure to light and releases two motile cells. The initiation and duration of motility varies among species.[64] Approaching or at the end of the photoperiod the mastigotes cease swimming, release their flagella, and undergo a rapid metamorphosis into the coccoid form. As cultures reach stationary growth phase, fewer and fewer motile cells are observed, indicating slower division rates.
Large tetrads are occasionally observed, particularly when cells in stationary growth phase are transferred to fresh media. However, it is unknown whether this stage is the product of two consecutive mitotic divisions, or perhaps a process that generates sexually competent motile cells (i.e. gametes), or is the end result of meiosis (i. e. as meiotic tetrads) following gamete fusion. There is no cytological evidence for sexual recombination, and meiosis has never been observed, but population genetic evidence supports the view that Symbiodinium periodically undergo events of sexual recombination. How, when, and where the sexual phase in their life history occurs remains unknown.[42][65][66]
Morphology
[edit]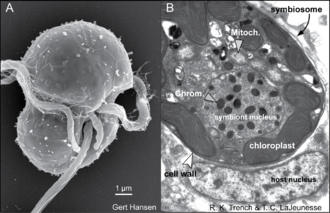


The morphological description of the genus Symbiodinium is originally based on the type species (holotype) S. microadriaticum.[38][67] Because these dinoflagellates possess two major stages in their life history (see above), namely the mastigote (motile) and coccoid (non-motile) stages, the morphology of both is described in order to provide a complete diagnosis of the organism.
Flagellated (mastigote) cell
[edit]The motile flagellated form is gymnodinioid and athecate ("nude").[68] The relative dimensions of the episoma (cell portion above the groove) and hyposoma (cell portion below the groove) differ among species.[38] The alveoli are most visible in the motile phase but lack fibrous cellulosic structures found in thecate ("armored") dinoflagellates. Between the points of origin of the two flagella is an extensible structure of unknown function called the peduncle. In other dinoflagellates, an analogous structure has been implicated in heterotrophic feeding and sexual recombination. In Symbiodinium, it has been suggested that the peduncle may be involved in substrate attachment, explaining why certain cells seem to spin in place.[67] Compared to other Gymnodiniales genera, there is little or no displacement at the sulcus where the ends of the cingulum (or cigulum) groove converge.
The internal organelles of the mastigote are essentially the same as described in the coccoid cell (see below). The transition from mastigote to coccoid stage in Symbiodinium occurs rapidly, but details about cellular changes are unknown. Mucocysts (an ejectile organelle[69]) located beneath the plasmalemma are found in S. pilosum and their function is unknown, but may be involved in heterotrophic feeding.
Coccoid cell
[edit]The coccoid cell of Symbiodinium is spherical and ranges in average diameter from 6 to 13 μm, depending on the species (Blank et al. 1989). This stage is often wrongly interpreted as a dinocyst; hence, in published literature, the alga in hospite is often referred to as a vegetative cyst.[67] The term cyst usually refers to a dormant, metabolically quiescent stage in the life history of other dinoflagellates, initiated by several factors, including nutrient availability, temperature, and day length.[70] Such cysts permit extended resistance to unfavourable environmental conditions. Instead, coccoid Symbiodinium cells are metabolically active, as they photosynthesize, undergo mitosis, and actively synthesize proteins and nucleic acids. While most dinoflagellates undergo mitosis as a mastigote, in Symbiodinium, mitosis occurs exclusively in the coccoid cell.[64]
Cell wall
[edit]The coccoid cell is surrounded by a cellulosic, usually smooth cell wall that contains large-molecular-weight proteins and glycoproteins.[38][71] Cell walls grow thicker in culture than in hospite.[7] The cell membrane (plasmalemma) is located beneath the cell wall, yet little is known about its composition and function in terms of the regulation of trans-membrane transport of metabolites. During karyokinesis and cytokinesis, the cell wall remains intact until the mastigotes escape the mother cell. In culture, the discarded walls accumulate at the bottom of the culture vessel. It is not known what becomes of the walls from divided cells in hospite.[72] One species, S. pilosum, possesses tufts of hair-like projections from the cell wall; this is the only known surface characteristic used to diagnose a species in the genus.
Chloroplast
[edit]Most described species possess a single, peripheral, reticulated chloroplast bounded by three membranes. The volume of the cell occupied by the chloroplast varies among species.[38] The lamellae comprise three closely appressed (stacked) thylakoids, and are attached by two stalks to the pyrenoid[38] surrounded by a starch sheath. In three of the described species, the thylakoids are in parallel arrays, but in S. pilosum, there are also peripheral lamellae. There are no thylakoid membranes invading the pyrenoid, which is unlike other symbiotic dinoflagellates.[73][74] The lipid components of thylakoids include the galactolipids as monogalactosyl-diglycerides (MGDG)[75] and digalactosyl-diglycerides( DGDG),;[76] the sulpholipid sulphoquinovosyl-diglyceride (SQDG),[77] phosphatidyl glycerol,[78] and phosphatidyl choline. Associated with these are various fatty acids.[79] The light-harvesting and reaction centre components in the thylakoid membrane include a water-soluble peridinin-chlorophyll a-protein complex (PCP or PerCP),[80] and a membrane-bound chlorophyll a-chlorophyll c2-peridinin-protein-complex (acpPC),[81] along with typical photosynthetic electron transport systems such as the photosystem II reaction centre and the chlorophyll-a-P700 reaction centre complex of photosystem I.[82][83] Also associated with the thylakoids are the xanthophylls dinoxanthin, diadinoxanthin, diatoxanthin and the carotene, β-Carotene. The pyrenoid contains the nuclear-encoded enzyme type II Ribulose-1,5-bis-phosphate-carboxylase-oxygenase (RuBisCO),[84] which is responsible for the catalysis of inorganic carbon dioxide (CO2) into organic compounds.
All cultured isolates (i.e. strains) are capable of phenotypic adjustment in their capacity for light harvesting (i.e. photoacclimation), such as by altering the cellular Chl. a and peridinin quota, as well as the size and number of photosynthetic units.[85] However, the ability to acclimate is a reflection of genetic differences among species that are differently adapted (evolved) to a particular photic environment.[86][87] For example, S. pilosum is characterized as a high light-adapted species, while others are low light-adapted (S. kawagutii) or adapted to a larger range in varying light fields (S. microadriaticum).

Nucleus
[edit]In general, the nucleus is centrally located and the nucleolus is often associated with the inner nuclear membrane. The chromosomes, as in other dinoflagellates, are seen as ‘permanently super-coiled’ DNA in transmission electron micrographs (TEM).[88] The described species of Symbiodinium possess distinct chromosome numbers (ranging from 26 to 97[38]), which remain constant throughout all phases of the nuclear cycle. However, during M-phase, the volume of each chromosome is halved, as is the volume of each of the two resulting nuclei. Thus, the ratio of chromosome volume to nuclear volume remains constant. These observations are consistent with the interpretation that the algae are haploid, a conclusion supported by molecular genetic data.[89] During S-phase of the nuclear cycle the chromosomes do uncoil to facilitate DNA synthesis, and the volumes of both the chromosomes and the nucleus return to those seen in the G22 phase.[88]
Other cytoplasmic organelles
[edit]There are several additional organelles found in the cytoplasm of Symbiodinium. The most obvious of these is the structure referred to as the "accumulation body". This is a membrane-bound vesicle (vacuole) with contents that are unrecognizable, but appear red or yellow under the light microscope. It may serve to accumulate cellular debris or act as an autophagic vacuole in which non-functional organelles are digested and their components recycled. During mitosis, only one daughter cell appears to acquire this structure. There are other vacuoles that may contain membranous inclusions,[90] while still others contain crystalline material variously interpreted as oxalate crystals or crystalline uric acid.
Species
[edit]
The following species are recognised by the World Register of Marine Species:[1]
- Symbiodinium bermudense R.K.Trench, 1993
- Symbiodinium californium A.T.Banaszak, R.Iglesias-Prieto & R.K.Trench, 1993
- Symbiodinium cariborum R.K.Trench, 1993
- Symbiodinium corculorum R.K.Trench, 1993
- Symbiodinium glynnii D.C.Wham, G.Ning, T.C.LaJeunesse, 2017 [91]
- Symbiodinium goreaui Trench & Blank, 2000
- Symbiodinium kawagutii Trench & Blank, 2000
- Symbiodinium meandrinae R.K.Trench, 1993
- Symbiodinium microadriaticum Freudenthal, 1962
- Symbiodinium minutum T.C.LaJeunesse, J.E. Parkinson & J.D.Reimer, 2012
- Symbiodinium pilosum Trench & Blank, 2000
- Symbiodinium psygmophilum LaJeunesse, T.C., Parkinson, J.E. & Reimer, J.D., 2012
- Symbiodinium pulchrorum R.K.Trench, 1993
- Symbiodinium thermophilum, new species [92]
References
[edit]- ^ a b Guiry, Michael D. (2014). "Symbiodinium Freudenthal, 1962". WoRMS. World Register of Marine Species. Retrieved 2015-01-29.
- ^ Blank, Rudolf J.; Trench, Robert K. (May 1986). "Nomenclature of Endosymbiotic Dinoflagellates". Taxon. 35 (2): 286–94. doi:10.2307/1221270. JSTOR 1221270.
- ^ a b c d e LaJeunesse, Todd C.; Parkinson, John E.; Gabrielson, Paul W.; Jeong, Hae Jin; Reimer, James D.; Voolstra, Christian R.; Santos, Scott R. (2018). "Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts". Current Biology. 28 (16): P2570–2580. doi:10.1016/j.cub.2018.07.008. hdl:10754/630499. PMID 30100341.
- ^ Stimson, J.; Sakai, K.; Sembali, H. (December 2002). "Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality". Coral Reefs. 21 (4): 409–21. doi:10.1007/s00338-002-0264-3. S2CID 43084578.
- ^ Kawaguti, Siro (1944). "On the physiology of reef corals VI. Study on the pigments". Palau Tropical Biological Station Studies. 2: 617–74.
- ^ McLaughlin, John J. A.; Zahl, Paul A. (1959). "Axenic Zooxanthellae from Various Invertebrate Hosts". Annals of the New York Academy of Sciences. 77 (2): 55–72. Bibcode:1959NYASA..77...55M. doi:10.1111/j.1749-6632.1959.tb36892.x. S2CID 85877213.
- ^ a b Colley, Nansi J.; Trench, R. K. (1983). "Selectivity in Phagocytosis and Persistence of Symbiotic Algae by the Scyphistoma Stage of the Jellyfish Cassiopeia xamachana". Proceedings of the Royal Society of London. Series B. 219 (1214): 61–82. Bibcode:1983RSPSB.219...61C. doi:10.1098/rspb.1983.0059. JSTOR 35678. PMID 22470960. S2CID 22673808.
- ^ Wakefield, Timothy S.; Kempf, Stephen C. (2001). "Development of host- and symbiont-specific monoclonal antibodies and confirmation of the origin of the symbiosome membrane in a cnidarian-dinoflagellate symbiosis". The Biological Bulletin. 200 (2): 127–43. doi:10.2307/1543306. JSTOR 1543306. PMID 11341574. S2CID 21833840.
- ^ Peng, Shao-En; Wang, Yu-Bao; Wang, Li-Hsueh; Chen, Wan-Nan Uang; Lu, Chi-Yu; Fang, Lee-Shing; Chen, Chii-Shiarng (2010). "Proteomic analysis of symbiosome membranes in Cnidaria-dinoflagellate endosymbiosis". Proteomics. 10 (5): 1002–16. doi:10.1002/pmic.200900595. PMID 20049864. S2CID 27108503.
- ^ Moberg, Fredrik; Folke, Carl (1999). "Ecological goods and services of coral reef ecosystems". Ecological Economics. 29 (2): 215–33. doi:10.1016/S0921-8009(99)00009-9.
- ^ Jokiel, P. L.; Coles, S. L. (1990). "Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature". Coral Reefs. 8 (4): 155–62. Bibcode:1990CorRe...8..155J. doi:10.1007/BF00265006. S2CID 5871917.
- ^ Lesser, Michael P (1996). "Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates". Limnology and Oceanography. 41 (2): 271–83. Bibcode:1996LimOc..41..271L. doi:10.4319/lo.1996.41.2.0271. S2CID 16428743.
- ^ Fitt, William; Brown, Barbara; Warner, Mark; Dunne, Richard (2001). "Coral bleaching: Interpretation of thermal tolerance limits and thermal thresholds in tropical corals". Coral Reefs. 20 (1): 51–65. doi:10.1007/s003380100146. S2CID 19361052.
- ^ Lajeunesse, Todd C.; Smith, Robin; Walther, Mariana; Pinzon, Jorge; Pettay, Daniel T.; McGinley, Michael; Aschaffenburg, Matthew; Medina-Rosas, Pedro; Cupul-Magana, Amilcar L.; Pérez, Andrés López; Reyes-Bonilla, Hector; Warner, Mark E. (2010). "Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance". Proceedings of the Royal Society B: Biological Sciences. 277 (1696): 2925–34. doi:10.1098/rspb.2010.0385. JSTOR 27862400. PMC 2982020. PMID 20444713.
- ^ Goreau, Thomas F (1964). "Mass Expulsion of Zooxanthellae from Jamaican Reef Communities after Hurricane Flora". Science. 145 (3630): 383–6. Bibcode:1964Sci...145..383G. doi:10.1126/science.145.3630.383. PMID 17816975. S2CID 46191566.
- ^ Brown, Barbara E. (2000). "The significance of pollution in eliciting the 'bleaching' response in symbiotic cnidarians". International Journal of Environment and Pollution. 13 (1–6): 392–415. doi:10.1504/IJEP.2000.002328.
- ^ Colombo-Pallotta et al. 2010
- ^ Brandt, Marilyn E.; McManus, John W. (2009). "Disease incidence is related to bleaching extent in reef-building corals". Ecology. 90 (10): 2859–67. doi:10.1890/08-0445.1. JSTOR 25592820. PMID 19886494. S2CID 3896954.
- ^ Baker, Andrew C.; Glynn, Peter W.; Riegl, Bernhard (2008). "Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook". Estuarine. 80 (4): 435–71. Bibcode:2008ECSS...80..435B. doi:10.1016/j.ecss.2008.09.003.
- ^ Hoegh-Guldberg, O.; Mumby, P. J.; Hooten, A. J.; Steneck, R. S.; Greenfield, P.; Gomez, E.; Harvell, C. D.; Sale, P. F.; Edwards, A. J.; Caldeira, K.; Knowlton, N.; Eakin, C. M.; Iglesias-Prieto, R.; Muthiga, N.; Bradbury, R. H.; Dubi, A.; Hatziolos, M. E. (2007). "Coral Reefs Under Rapid Climate Change and Ocean Acidification". Science. 318 (5857): 1737–42. Bibcode:2007Sci...318.1737H. CiteSeerX 10.1.1.702.1733. doi:10.1126/science.1152509. PMID 18079392. S2CID 12607336.
- ^ Berkelmans, R.; Van Oppen, M. J.H (2006). "The role of zooxanthellae in the thermal tolerance of corals: A 'nugget of hope' for coral reefs in an era of climate change". Proceedings of the Royal Society B: Biological Sciences. 273 (1599): 2305–12. doi:10.1098/rspb.2006.3567. PMC 1636081. PMID 16928632.
- ^ Sampayo, E. M.; Ridgway, T.; Bongaerts, P.; Hoegh-Guldberg, O. (2008). "Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type". Proceedings of the National Academy of Sciences. 105 (30): 10444–9. Bibcode:2008PNAS..10510444S. doi:10.1073/pnas.0708049105. JSTOR 25463173. PMC 2492480. PMID 18645181.
- ^ Robison, Jennifer D.; Warner, Mark E. (2006). "Differential Impacts of Photoacclimation and Thermal Stress on the Photobiology of Four Different Phylotypes of Symbiodinium (Pyrrhophyta)". Journal of Phycology. 42 (3): 568–79. doi:10.1111/j.1529-8817.2006.00232.x. S2CID 84675726.
- ^ Warner, Mark E.; Lajeunesse, Todd C.; Robison, Jennifer D.; Thur, Rebecca M. (2006). "The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: Potential implications for coral bleaching". Limnology and Oceanography. 51 (4): 1887–97. Bibcode:2006LimOc..51.1887W. CiteSeerX 10.1.1.322.1206. doi:10.4319/lo.2006.51.4.1887. S2CID 1240348.
- ^ Ragni, Maria; Airs, Ruth L.; Hennige, Sebastian J.; Suggett, David J.; Warner, Mark E.; Geider, Richard J. (2010). "PSII photoinhibition and photorepair in Symbiodinium (Pyrrhophyta) differs between thermally tolerant and sensitive phylotypes". Marine Ecology Progress Series. 406: 57–70. Bibcode:2010MEPS..406...57R. doi:10.3354/meps08571.
- ^ Takahashi, Shunichi; Whitney, Spencer; Itoh, Shigeru; Maruyama, Tadashi; Badger, Murray (2008). "Heat stress causes inhibition of the de novo synthesis of antenna proteins and photobleaching in cultured Symbiodinium". Proceedings of the National Academy of Sciences. 105 (11): 4203–8. Bibcode:2008PNAS..105.4203T. doi:10.1073/pnas.0708554105. JSTOR 25461395. PMC 2393757. PMID 18322010.
- ^ Lien, Yi-T.; Nakano, Y.; Plathong, S.; Fukami, H.; Wang, Jih-T.; Chen, C. A. (2007). "Occurrence of the putatively heat-tolerant Symbiodinium phylotype D in high-latitudinal outlying coral communities". Coral Reefs. 26 (1): 35–44. Bibcode:2007CorRe..26...35L. doi:10.1007/s00338-006-0185-7. S2CID 19100056.
- ^ a b c Lajeunesse, Todd C.; Smith, Robin T.; Finney, Jennifer; Oxenford, Hazel (2009). "Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral 'bleaching' event". Proceedings of the Royal Society B: Biological Sciences. 276 (1676): 4139–48. doi:10.1098/rspb.2009.1405. JSTOR 40506039. PMC 2821356. PMID 19740874.
- ^ Lajeunesse, Todd C.; Pettay, Daniel T.; Sampayo, Eugenia M.; Phongsuwan, Niphon; Brown, Barbara; Obura, David O.; Hoegh-Guldberg, Ove; Fitt, William K. (2010). "Long-standing environmental conditions, geographic isolation and host-symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium". Journal of Biogeography. 37 (5): 785–800. doi:10.1111/j.1365-2699.2010.02273.x. S2CID 30508571.
- ^ Rosic, Nedeljka N. (November 2019). "Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens". Marine Drugs. 17 (11): 638. doi:10.3390/md17110638. PMC 6891770. PMID 31726795.
- ^ Shoguchi, Eiichi; Beedessee, Girish; Hisata, Kanako; Tada, Ipputa; Narisoko, Haruhi; Satoh, Noriyuki; Kawachi, Masanobu; Shinzato, Chuya (2021-02-03). Phadke, Sujal (ed.). "A New Dinoflagellate Genome Illuminates a Conserved Gene Cluster Involved in Sunscreen Biosynthesis". Genome Biology and Evolution. 13 (2): evaa235. doi:10.1093/gbe/evaa235. ISSN 1759-6653. PMC 7875005. PMID 33146374.
- ^ Shoguchi, Eiichi; Yoshioka, Yuki; Shinzato, Chuya; Arimoto, Asuka; Bhattacharya, Debashish; Satoh, Noriyuki (2020-03-01). McFadden, Geoff (ed.). "Correlation between Organelle Genetic Variation and RNA Editing in Dinoflagellates Associated with the Coral Acropora digitifera". Genome Biology and Evolution. 12 (3): 203–209. doi:10.1093/gbe/evaa042. ISSN 1759-6653. PMC 7144361. PMID 32108224.
- ^ Shoguchi, Eiichi; Beedessee, Girish; Tada, Ipputa; Hisata, Kanako; Kawashima, Takeshi; Takeuchi, Takeshi; Arakaki, Nana; Fujie, Manabu; Koyanagi, Ryo; Roy, Michael C.; Kawachi, Masanobu (2018-06-14). "Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes". BMC Genomics. 19 (1): 458. doi:10.1186/s12864-018-4857-9. ISSN 1471-2164. PMC 6001144. PMID 29898658.
- ^ David DeFranza (2010-02-17). "Andaman Sea Coral May Hold the Secret to Warm Water Reef Survival". Treehugger. Retrieved 2015-02-02.
- ^ "A hot survivor". The Economist. April 9, 2016. ISSN 0013-0613. Retrieved 2016-04-30.
- ^ Rowan, Rob; Powers, Dennis A. (1992). "Ribosomal RNA Sequences and the Diversity of Symbiotic Dinoflagellates (Zooxanthellae)". Proceedings of the National Academy of Sciences of the United States of America. 89 (8): 3639–43. Bibcode:1992PNAS...89.3639R. doi:10.1073/pnas.89.8.3639. JSTOR 2359156. PMC 48924. PMID 1565660.
- ^ Stern, Rowena F.; Horak, Ales; Andrew, Rose L.; Coffroth, Mary-Alice; Andersen, Robert A.; Küpper, Frithjof C.; Jameson, Ian; Hoppenrath, Mona; Véron, Benoît; Kasai, Fumai; Brand, Jerry; James, Erick R.; Keeling, Patrick J. (2010). "Environmental Barcoding Reveals Massive Dinoflagellate Diversity in Marine Environments". PLOS ONE. 5 (11): e13991. Bibcode:2010PLoSO...513991S. doi:10.1371/journal.pone.0013991. PMC 2981561. PMID 21085582.
- ^ a b c d e f g Trench, Robert K.; Blank, Rudolf J. (1987). "Symbiodinium Microadriaticum Freudenthal, S. Goreauii Sp. Nov., S. Kawagutii Sp. Nov. And S. Pilosum Sp. Nov.: Gymnodinioid Dinoflagellate Symbionts of Marine Invertebrates". Journal of Phycology. 23 (3): 469–81. doi:10.1111/j.1529-8817.1987.tb02534.x. S2CID 83712799.
- ^ a b c Sampayo, E. M.; Dove, S.; Lajeunesse, T. C. (2009). "Cohesive molecular genetic data delineate species diversity in the dinoflagellate genus Symbiodinium". Molecular Ecology. 18 (3): 500–19. doi:10.1111/j.1365-294X.2008.04037.x. PMID 19161470. S2CID 24285565.
- ^ a b c d Lajeunesse, Todd C.; Thornhill, Daniel J. (2011). "Improved Resolution of Reef-Coral Endosymbiont (Symbiodinium) Species Diversity, Ecology, and Evolution through psbA Non-Coding Region Genotyping". PLOS ONE. 6 (12): e29013. Bibcode:2011PLoSO...629013L. doi:10.1371/journal.pone.0029013. PMC 3247227. PMID 22216157.
- ^ Christian Renicke: Symbiodinium cp23S RFLP and sequence analysis, Pringle Lab, Grossman Lab, Jul 23, 2018
- ^ a b Santos, S. R.; Shearer, T. L.; Hannes, A. R.; Coffroth, M. A. (2004). "Fine-scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium, Dinophyceae) of the Caribbean". Molecular Ecology. 13 (2): 459–69. doi:10.1046/j.1365-294X.2003.02058.x. PMID 14717900. S2CID 32227215.
- ^ Finney, J. Christine; Pettay, Daniel Tye; Sampayo, Eugenia M.; Warner, Mark E.; Oxenford, Hazel A.; Lajeunesse, Todd C. (2010). "The Relative Significance of Host–Habitat, Depth, and Geography on the Ecology, Endemism, and Speciation of Coral Endosymbionts in the Genus Symbiodinium". Microbial Ecology. 60 (1): 250–63. doi:10.1007/s00248-010-9681-y. JSTOR 40802290. PMID 20502891. S2CID 5890376.
- ^ De Queiroz, Kevin (2007). "Species Concepts and Species Delimitation". Systematic Biology. 56 (6): 879–86. doi:10.1080/10635150701701083. PMID 18027281.
- ^ Thornhill, Daniel J.; Lajeunesse, Todd C.; Santos, Scott R. (2007). "Measuring rDNA diversity in eukaryotic microbial systems: How intragenomic variation, pseudogenes, and PCR artifacts confound biodiversity estimates". Molecular Ecology. 16 (24): 5326–40. doi:10.1111/j.1365-294X.2007.03576.x. PMID 17995924. S2CID 12791319.
- ^ a b Pochon, X.; Garcia-Cuetos, L.; Baker, A. C.; Castella, E.; Pawlowski, J. (2007). "One-year survey of a single Micronesian reef reveals extraordinarily rich diversity of Symbiodinium types in soritid foraminifera" (PDF). Coral Reefs. 26 (4): 867–82. Bibcode:2007CorRe..26..867P. doi:10.1007/s00338-007-0279-x. S2CID 24642825.
- ^ a b c Reimer, James Davis; Shah, Md Mahfuzur Rahman; Sinniger, Frederic; Yanagi, Kensuke; Suda, Shoichiro (2010). "Preliminary analyses of cultured Symbiodinium isolated from sand in the oceanic Ogasawara Islands, Japan". Marine Biodiversity. 40 (4): 237–47. doi:10.1007/s12526-010-0044-1. S2CID 10186027.
- ^ a b Andras, Jason P.; Kirk, Nathan L.; Drew Harvell, C. (2011). "Range-wide population genetic structure of Symbiodinium associated with the Caribbean Sea fan coral, Gorgonia ventalina". Molecular Ecology. 20 (12): 2525–42. doi:10.1111/j.1365-294X.2011.05115.x. PMID 21545573. S2CID 25577087.
- ^ a b c Pettay, Daniel T.; Wham, Drew C.; Pinzón, Jorge H.; Lajeunesse, Todd C. (2011). "Genotypic diversity and spatial-temporal distribution of Symbiodinium clones in an abundant reef coral". Molecular Ecology. 20 (24): 5197–212. doi:10.1111/j.1365-294X.2011.05357.x. PMC 5957298. PMID 22082053.
- ^ a b c LaJeunesse, T. (2002). "Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs". Marine Biology. 141 (2): 387–400. doi:10.1007/s00227-002-0829-2. S2CID 84357806.
- ^ Rowan, Rob; Knowlton, Nancy; Baker, Andrew; Jara, Javier (1997). "Landscape ecology of algal symbionts creates variation in episodes of coral bleaching". Nature. 388 (6639): 265–9. Bibcode:1997Natur.388..265R. doi:10.1038/40843. PMID 9230434.
- ^ Toller, W. W.; Rowan, R; Knowlton, N (2001). "Repopulation of Zooxanthellae in the Caribbean corals Montastraea annularis and M. Faveolata following experimental and disease-associated bleaching". The Biological Bulletin. 201 (3): 360–73. doi:10.2307/1543614. JSTOR 1543614. PMID 11751248. S2CID 7765487.
- ^ a b Thornhill, Daniel J.; LaJeunesse, Todd C.; Kemp, Dustin W.; Fitt, William K.; Schmidt, Gregory W. (2005). "Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion". Marine Biology. 148 (4): 711–22. doi:10.1007/s00227-005-0114-2. S2CID 36226084.
- ^ Coffroth, Mary Alice; Santos, Scott R.; Goulet, Tamar L. (2001). "Early ontogenetic expression of specificity in a cnidarian-algal symbiosis" (PDF). Marine Ecology Progress Series. 222: 85–96. Bibcode:2001MEPS..222...85C. doi:10.3354/meps222085.
- ^ a b Porto, Isabel; Granados, Camila; Restrepo, Juan C.; Sánchez, Juan A. (2008). "Macroalgal-Associated Dinoflagellates Belonging to the Genus Symbiodinium in Caribbean Reefs". PLOS ONE. 3 (5): e2160. Bibcode:2008PLoSO...3.2160P. doi:10.1371/journal.pone.0002160. PMC 2364641. PMID 18478069.
- ^ Coffroth, Mary Alice; Lewis, Cynthia F.; Santos, Scott R.; Weaver, Jessica L. (2006). "Environmental populations of symbiotic dinoflagellates in the genus Symbiodinium can initiate symbioses with reef cnidarians". Current Biology. 16 (23): R985–7. doi:10.1016/j.cub.2006.10.049. PMID 17141602.
- ^ Manning, Mackenzie M.; Gates, Ruth D. (2008). "Diversity in populations of free-living Symbiodinium from a Caribbean and Pacific reef". Limnology and Oceanography. 53 (5): 1853–61. Bibcode:2008LimOc..53.1853M. doi:10.4319/lo.2008.53.5.1853. S2CID 17895332.
- ^ Culturing, Georgia State University, International Genetically Engineered Machine (iGEM)
- ^ Schoenberg, D. A.; Trench, R. K. (1980). "Genetic Variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and Specificity in its Symbiosis with Marine Invertebrates. I. Isoenzyme and Soluble Protein Patterns of Axenic Cultures of Symbiodinium microadriaticum". Proceedings of the Royal Society of London. Series B. 207 (1169): 405–27. Bibcode:1980RSPSB.207..405S. doi:10.1098/rspb.1980.0031. JSTOR 35362. S2CID 84190993.
- ^ Schoenberg, D. A.; Trench, R. K. (1980). "Genetic Variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and Specificity in its Symbiosis with Marine Invertebrates. II. Morphological Variation in Symbiodinium microadriaticum". Proceedings of the Royal Society B: Biological Sciences. 207 (1169): 429–44. Bibcode:1980RSPSB.207..429S. doi:10.1098/rspb.1980.0032. JSTOR 35363. S2CID 84395179.
- ^ Schoenberg, D. A.; Trench, R. K. (1980). "Genetic Variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and Specificity in its Symbiosis with Marine Invertebrates. III. Specificity and Infectivity of Symbiodinium microadriaticum". Proceedings of the Royal Society of London. Series B. 207 (1169): 445–60. Bibcode:1980RSPSB.207..445S. doi:10.1098/rspb.1980.0033. JSTOR 35364. S2CID 83896405.
- ^ Santos, Scott R.; Taylor, Derek J.; Coffroth, Mary Alice (2001). "Genetic Comparisons of Freshly Isolated Versus Cultured Symbiotic Dinoflagellates: Implications for Extrapolating to the Intact Symbiosis". Journal of Phycology. 37 (5): 900–12. doi:10.1046/j.1529-8817.2001.00194.x. S2CID 2335127.
- ^ Goulet, T.; Coffroth, M. (February 2003). "Genetic composition of zooxanthellae between and within colonies of the octocoral Plexaura kuna, based on small subunit rDNA and multilocus DNA fingerprinting". Marine Biology. 142 (2): 233–9. doi:10.1007/s00227-002-0936-0. S2CID 85919438.
- ^ a b c Fitt, W. K.; Trench, R. K. (1983). "The Relation of Diel Patterns of Cell Division to Diel Patterns of Motility in the Symbiotic Dinoflagellate Symbiodinium Microadria Ticum Freudenthal in Culture". New Phytologist. 94 (3): 421–32. doi:10.1111/j.1469-8137.1983.tb03456.x. JSTOR 2432757.
- ^ Baillie, B. K.; Belda-Baillie, C. A.; Silvestre, V.; Sison, M.; Gomez, A. V.; Gomez, E. D.; Monje, V. (2000). "Genetic variation in Symbiodinium isolates from giant clams based on random-amplified-polymorphic DNA (RAPD) patterns". Marine Biology. 136 (5): 829–36. doi:10.1007/s002270000290. S2CID 84351451.
- ^ Lajeunesse, Todd C. (2001). "Investigating the Biodiversity, Ecology, and Phylogeny of Endosymbiotic Dinoflagellates in the Genus Symbiodinium Using the Its Region: In Search of A 'species' Level Marker". Journal of Phycology. 37 (5): 866–80. doi:10.1046/j.1529-8817.2001.01031.x. S2CID 4208350.
- ^ a b c Freudenthal, Hugo D. (1962). "Symbiodinium gen. Nov. And Symbiodinium microadriaticum sp. nov., a Zooxanthella: Taxonomy, Life Cycle, and Morphology". The Journal of Protozoology. 9 (1): 45–52. doi:10.1111/j.1550-7408.1962.tb02579.x.
- ^ Taylor, FJR (1987). "Dinoflagellate morphology". In Taylor, F. J. R. (ed.). The Biology of Dinoflagellates. Botanical Monographs, vol. 21. Oxford: Blackwell Scientific Publications. pp. 24–91. ISBN 978-0-632-00915-2.
- ^ Dodge, JD; Greuet, C (1987). "Dinoflagellate ultrastructure and complex organelles". In Taylor, F. J. R. (ed.). The Biology of Dinoflagellates. Botanical Monographs, vol. 21. Oxford: Blackwell Scientific Publications. pp. 92–142. ISBN 978-0-632-00915-2.
- ^ Lee, Edward Lee (2008). Phycology (4th ed.). New York: Cambridge University Press. ISBN 978-1-139-46987-6.[page needed]
- ^ Markell, DA; Trench, RK; Iglesias-Prieto, R (1992). "Macromolecules associated with the cell-walls of symbiotic dinoflagellates". Symbiosis. 12 (1): 19–31. INIST 5092729.
- ^ Wakefield, Timothy S.; Farmer, Mark A.; Kempf, Stephen C. (August 2000). "Revised description of the fine structure of in situ 'zooxanthellae' genus Symbiodinium". The Biological Bulletin. 199 (1): 76–84. doi:10.2307/1542709. JSTOR 1542709. PMID 10975645.
- ^ Trench, RK; Winsor, H (1987). "Symbiosis with dinoflagellates in two pelagic flatworms, Amphiscolops sp. and Haplodiscus sp". Symbiosis. 3 (1): 1–21. INIST 8265704.
- ^ Banaszak, Anastazia T.; Iglestas-Prieto, Roberto; Trench, Robert K. (1993). "Scrippsiella velellae sp. nov. (Peridiniales) and Gloeokinium viscum sp. nov. (Phytodiniales), Dinoflagellate Symbionts of Two Hydrozoans (Cnidiaria)". Journal of Phycology. 29 (4): 517–28. doi:10.1111/j.1529-8817.1993.tb00153.x. S2CID 84895398.
- ^ Monogalactosyl Diglyceride, biomol
- ^ Digalactosyl diglyceride, Merck Sigma-Aldrich
- ^ Sulfoquinovosyl diglyceride, Karel's Nutrition Blog
- ^ Phosphatidylglycerol and Related Lipids, The LipidWeb
- ^ Díaz-Almeyda, E.; Thomé, P. E.; El Hafidi, M.; Iglesias-Prieto, R. (2011). "Differential stability of photosynthetic membranes and fatty acid composition at elevated temperature in Symbiodinium". Coral Reefs. 30 (1): 217–25. Bibcode:2011CorRe..30..217D. doi:10.1007/s00338-010-0691-5. S2CID 20410939.
- ^ Donatella Carbonera, Marilena Di Valentin, Riccardo Spezia, Alberto Mezzetti: The Unique Photophysical Properties of the Peridinin-Chlorophyll-a-Protein. In: Current Protein & Peptide Science, volume 15, issue 4, Jun 2014, doi:10.2174/1389203715666140327111139, PMC 4030626, PMID 24678668, PMC4030626 (Web Archive July 2021, 10th)
- ^ Dariusz M. Niedzwiedzki, Jing Jiang, Cynthia S. Lo, Robert E. Blankenship: Spectroscopic properties of the Chlorophyll a–Chlorophyll c2–Peridinin-Protein-Complex (acpPC) from the coral symbiotic dinoflagellate Symbiodinium. In: Photosynthesis Research, volume 120, pp. 125–139, (May 2014)/ January 2013, 20th, doi:10.1007/s11120-013-9794-5
- ^ Iglesias-Prieto, R.; Govind, N. S.; Trench, R. K. (1991). "Apoprotein Composition and Spectroscopic Characterization of the Water-Soluble Peridinin–Chlorophyll a–Proteins from Three Symbiotic Dinoflagellates". Proceedings: Biological Sciences. 246 (1317): 275–83. Bibcode:1991RSPSB.246..275I. doi:10.1098/rspb.1991.0155. JSTOR 76745. S2CID 84255943.
- ^ Iglesias-Prieto, R.; Govind, N. S.; Trench, R. K. (1993). "Isolation and Characterization of Three Membrane-Bound Chlorophyll-Protein Complexes from Four Dinoflagellate Species". Philosophical Transactions of the Royal Society B: Biological Sciences. 340 (1294): 381–92. doi:10.1098/rstb.1993.0080. JSTOR 3030171.
- ^ Rowan, Rob; Whitney, Spencer M.; Fowler, Amanda; Yellowlees, David (1996). "Rubisco in marine symbiotic dinoflagellates: Form II enzymes in eukaryotic oxygenic phototrophs encoded by a nuclear multigene family". The Plant Cell Online. 8 (3): 539–53. doi:10.1105/tpc.8.3.539. JSTOR 3870331. PMC 161119. PMID 8721755.
- ^ Hennige, S. J.; Suggett, D. J.; Warner, M. E.; McDougall, K. E.; Smith, D. J. (2009). "Photobiology of Symbiodinium revisited: Bio-physical and bio-optical signatures". Coral Reefs. 28 (1): 179–95. Bibcode:2009CorRe..28..179H. doi:10.1007/s00338-008-0444-x. S2CID 34202181.
- ^ Iglesias-Prieto, Roberto; Trench, Robert K. (1994). "Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density". Marine Ecology Progress Series. 113 (1): 163–75. Bibcode:1994MEPS..113..163I. doi:10.3354/meps113163.
- ^ Iglesias-Prieto, R.; Trench, R. K. (1997). "Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll-protein complexes to different photon-flux densities". Marine Biology. 130 (1): 23–33. doi:10.1007/s002270050221. S2CID 84705656.
- ^ a b Blank, Rudolf J.; Trench, Robert K. (1985). "Speciation and Symbiotic Dinoflagellates". Science. 229 (4714): 656–8. Bibcode:1985Sci...229..656B. doi:10.1126/science.229.4714.656. PMID 17739379. S2CID 45228641.
- ^ Santos, Scott R.; Coffroth, Mary Alice (February 2003). "Molecular genetic evidence that dinoflagellates belonging to the genus Symbiodinium freudenthal are haploid". The Biological Bulletin. 204 (1): 10–20. doi:10.2307/1543491. JSTOR 1543491. PMID 12588740. S2CID 7258554.
- ^ Trench, R. K. (1974). "Nutritional potentials in Zoanthus sociathus (Coelenterata, Anthozoa)". Helgoländer Wissenschaftliche Meeresuntersuchungen. 26 (2): 174–216. Bibcode:1974HWM....26..174T. doi:10.1007/BF01611382.
- ^ Drew C. Wham, Gang Ning, and Todd C. LaJeunesse (2017) [Symbiodinium glynnii sp. nov., a species of stress-tolerant symbiotic dinoflagellates from pocilloporid and montiporid corals in the Pacific Ocean]. Phycologia: 2017, Vol. 56, No. 4, pp. 396-409.
- ^ Algal species helps corals survive in Earth's hottest reefs
External links
[edit]- "Symbiodinium". The Encyclopedia of Life.
- An image of Symbiodinium at Smithsonian Ocean Portal


